Talk to our experts
1800-120-456-456

JEE Main Chemistry in Everyday Life Practice Paper FREE PDF Download
- Practice Paper
- Chemistry In Everyday Life

Chemistry in Everyday Life Practice Paper with Solutions and Answer Key For JEE Main
Understanding the role of Chemistry in Everyday Life Practice Paper is essential for JEE Main students, as it explores the practical applications of chemistry in various aspects of our daily lives, including pharmaceuticals, food additives, and environmental chemistry and makes effective competence in JEE Main Chemistry.
Vedantu understands the major role of Chemistry in Everyday Life Practice Paper in JEE Main Chemistry preparation and offers a FREE PDF download for students. This invaluable resource is meticulously prepared for chapter-wise practice, guaranteeing a comprehensive understanding of essential topics. By taking this opportunity at no expense, you can expand your knowledge and bolster your confidence in approaching questions with precision. Detailed solutions and answer keys are included to resolve doubts and guide you through the step-wise process of question-solving. Moreover, practicing Vedantu’s Chemistry in Everyday Life Practice Paper for JEE Main can enhance your question-solving speed.
Prepare to solve 1 or more questions from the Chemistry in Everyday Life chapter in the JEE Main Exam. To understand its importance, find the table detailing the weightage of the Chemistry in Everyday Life chapter in the past five years of the JEE Main Exam.
Practice Papers for JEE Main help you to find and practice the questions that might get asked in the next JEE Main exam. Download the PDF of the Chemistry in Everyday Life Practice Paper today to excel in your JEE Main exams!
Subject-wise Links For JEE Main Practice Paper
In the JEE Main exam, each of the three subjects— Chemistry, Physics, and Maths—holds a weightage of 33%. Hence along with practicing the Chemistry Practice Paper for JEE Main, students have to practice Physics, as well as Maths Practice Papers. This will lead you to score more than 80% in the JEE Main exam. Here are the links for the JEE Main Subject-wise Practice Paper.
Links For JEE Main Chapter-wise Practice Paper
Chemistry Practice Papers need to be worked out after each chapter since the questions from most of the Chemistry chapters can help you to score well in the Chemistry section of the JEE Main exam. This will help you to cover most of the JEE Main Chemistry Syllabus. You can download the Chapter-wise links for the JEE Main Practice Paper .
Important Topics From Chemistry in Everyday Life for JEE Main Practice Paper
It will be easy for you to work out the JEE Main Practice Paper if you have a strong understanding of the Chemistry in Everyday Life. You have to focus more on the important topics to answer most of the questions from the JEE Main Practice Paper of Chemistry in Everyday Life. Here are some of the important topics of Chemistry in Everyday Life.
Acids and Bases in Household Products
Understanding the pH scale and how acids and bases are used in everyday products like cleaning agents and antacids, helps students relate chemistry to their daily lives.
Chemical Reactions in Cooking
Exploring the chemical reactions that occur during cooking, such as caramelization and Maillard reaction, to illustrate the application of chemistry in preparing food.
Polymers in Packaging
Examining the role of polymers in packaging materials, showcasing their versatility and importance in preserving food and reducing environmental impact.
Chemical Kinetics in Medicine
Highlighting the significance of chemical kinetics in drug delivery, emphasizing how the rate of reactions affects the effectiveness and safety of medications.
Environmental Chemistry and Pollution Control
Discussing the chemistry behind air and water pollution, and the role of chemistry in developing solutions for a cleaner environment, fostering an awareness of the importance of environmental chemistry.
Equations To Score More in Practice Paper of JEE Main Chemistry in Everyday Life
Equations are the base for solving the JEE Main Practice Paper. You have to know which equation or formula to use while solving the Practice Paper for JEE Main. Find the Important equations you need to learn while working out the Practice Paper of JEE Main Chemistry in Everyday Life.
1. pH Calculation:
\[\text{pH} = -\log[H^+]\]
2. Molarity (M):
\[M = \frac{\text{moles of solute}}{\text{volume of solution (L)}}\]
3. Mass Percentage:
\[\text{Mass \%} = \frac{\text{Mass of solute}}{\text{Mass of solution}} \times 100\%\]
4. Beer-Lambert Law (Absorption):
\[A = \log\left(\frac{I_0}{I}\right) = \varepsilon \cdot c \cdot l\]
5. Half-life (First-order Reaction):
\[t_{\frac{1}{2}} = \frac{0.693}{k}\]
For more formulas and equations you can refer to Vedantu’s JEE Main Formula page.
What Makes Vedantu’s Practice Paper PDF of JEE Main Chemistry in Everyday Life Different?
Practice Paper for JEE Main serves as a resource for students who prepare for the exam, by offering questions structured in the same manner as the JEE Main exam. Vedantu’s JEE Main Practice Papers stand as the immediate post-chapter learning companion. Now, let’s delve into the distinctive traits of Vedantu’s Chemistry in Everyday Life Practice Paper for JEE Main.
Quality Content: Vedantu's Practice Paper for Chemistry in Everyday Life is curated by experienced educators and subject matter experts, ensuring that the questions are relevant, accurate, and aligned with the latest JEE Main syllabus.
Variety of Questions: They provide a diverse range of questions, covering different difficulty levels and concepts from Chemistry in Everyday Life, allowing students to thoroughly practice and master each topic.
Detailed Solutions: Vedantu offers detailed step-by-step solutions and answer keys for Chemistry in Everyday Life JEE Main Practice Paper, ensuring that students understand not just the final answer but also the underlying concepts and problem-solving techniques.
User-Friendly Interface: Their platform is designed to be user-friendly, making it easy for students to navigate through Practice Paper and access the content they need efficiently.
Accessibility: Vedantu's Practice Papers are often easily accessible online and can be downloaded for FREE, allowing students to practice from the comfort of their homes.
How To Prepare For JEE Main With Chemistry in Everyday Life Practice Paper?
Vedantu’s Chemistry in Everyday Life JEE Main Practice Paper is composed of MCQs and Subjective type questions. At the end of the FREE PDF, you can get the answer keys and detailed solutions for the questions. If you follow the below instructions while working out the Daily Practice Paper you can easily succeed in the JEE Main exam.
Download the Daily Practice Paper of JEE Main Chemistry in Everyday Life.
You can set a timer of 1 hour.
Solve the easy questions first and give time for tough questions.
Note your answers on a sheet of paper and check with the answer key.
Each question carries 4 marks and gives a negative mark of -1 for each question.
Now calculate the score and analyse yourself.
You can take the help of detailed solutions given in the PDF for better clarity of questions and answers.
Learn how to do the incorrect answers and practice the questions again.
Make a note of the time you take for each question to practice.
When to Start Preparing With JEE Main Practice Paper of Chemistry in Everyday Life?
To maximize your JEE Main preparation and increase your chances of success in the exams, it's advisable to start your JEE Main Practice Paper of Chemistry in Everyday Life preparation early in your academic journey. This approach ensures thorough subject mastery and sufficient time for revision. Follow this timeline to effectively utilize the Daily Practice Paper.
Foundation Building (1-2 Years Prior): Start with foundational studies and build a strong understanding of the core concepts in Chemistry in Everyday Life.
Concept Mastery (6-12 Months Prior): About a year before the exam, begin incorporating the Practice Paper of JEE Main Chemistry in Everyday Life into your routine.
Intensive Revision (3-6 Months Prior): As the exam date approaches, intensify your Practice Paper usage. Take a full-length Practice Paper to simulate exam conditions, improve time management, and identify weak areas.
Additional Materials To Cover With JEE Main Chemistry in Everyday Life Practice Paper
After learning the Chemistry in Everyday Life chapter, you need to make sure that you are mastering the contents you learn so that you can perform well in JEE Main. Practice Papers for JEE Main prepared by Vedantu is the best resource for this. Right after your revision of the Chemistry in Everyday Life, you can practice the JEE Main Practice Paper. But this is not enough if you want to score more than 85% in JEE Main exam. Here are some additional materials that you can choose while preparing for JEE Main.
Try Our Online Practice Paper Test For Chemistry
Once you are done with practicing the JEE Main Practice Paper for Chemistry in Everyday Life, you can test your online skills for JEE Main Chemistry. Vedantu is also providing you with an online practice paper test where you can get a real experience of attempting the JEE Main Exam.
Mastering Chemistry in Everyday Life With JEE Main Practice Paper
The JEE Main Chemistry in Everyday Life Practice Paper is a great way to practice for the exam. It covers a lot of important topics, and the solutions and answer keys help you check your work. By practicing with this JEE Main Practice Paper, you can learn the material better and get better at solving problems. You can also learn how to manage your time better and figure out where you need to focus your studies. So, if you're preparing for the JEE Main, be sure to download and practice Vedantu’s Chemistry in Everyday Life Daily Practice Paper for FREE!
JEE Mains Sample Paper: Chemistry in Everyday Life
JEE Mains is a highly competitive exam, and students need to be well-prepared to secure a good score. By practicing with JEE Main 2023 Sample Papers , students can get a feel of the actual exam and identify their strengths and weaknesses. The Chemistry in Everyday Life chapter is a crucial section of the JEE Mains syllabus , and it is essential for students to have a thorough understanding of the concepts covered in this chapter. Our sample papers include questions from all the important topics in this chapter, helping students to assess their preparation level and identify areas where they need more practice.
With the help of JEE Main Model Papers and JEE Model Question Papers , students can also get an idea of the types of questions that are frequently asked in the exam. This can help them to develop effective strategies for answering different types of questions, which can be beneficial in improving their overall score. By practicing with these sample papers, students can boost their confidence and improve their chances of success in the JEE Mains examination.

FAQs on JEE Main Chemistry in Everyday Life Practice Paper FREE PDF Download
1. What is Chemistry in Everyday Life, and why is it important for the JEE Main Practice Paper?
Chemistry in Everyday Life is a chapter in chemistry that explores the applications of chemistry in our daily lives. It is important for the JEE Main exam as it assesses your understanding of real-world applications of chemistry principles, which is essential for a well-rounded knowledge of the subject.
2. How can I effectively prepare for Chemistry in Everyday Life for JEE Main with Practice Paper?
To prepare effectively, start by thoroughly understanding the concepts in the chapter. Practice solving numerical problems and questions related to drug chemistry, pharmaceuticals, and other relevant topics.
3. Can you provide an example question related to Chemistry in Everyday Life for JEE Main?
Sure! Here's an example: "What is the function of an analgesic drug, and can you name a commonly used analgesic? (Hint: Chemistry in Everyday Life, Practice Paper, JEE Main)"
4. What are some key topics covered in Chemistry in Everyday Life for JEE Main Practice Paper?
Some key topics include drug classification, therapeutic action of drugs, drug targets, drug metabolism, drug interactions, and the chemistry behind common pharmaceuticals.
5. Are there any specific guidelines or tips for time management while attempting Chemistry in Everyday Life questions in the JEE Main Practice Paper?
Allocate a specific amount of time to each question, and don't spend too long on any single question. If you're unsure about an answer, mark it for review and move on to easier questions. Manage your time wisely to ensure you attempt all questions.
6. Can I access an answer key for the Chemistry in Everyday Life practice paper for JEE Main?
Yes, practice papers designed for JEE Main will come with an answer key. You can find answer keys at the end of the practice paper PDF.
JEE Practice Papers
Jee main 2024.
- IIT JEE Study Material
Chemistry In Everyday Life

If you are studying chemistry, then you must have wondered about the importance of chemistry in everyday life . Chemistry is the branch of science which deals with the investigation of the properties and changes of matter. From the way how our body exchanges oxygen to how our universe was created, all have a part of chemistry associated with it.
Download Complete Chapter Notes of Chemistry in Everyday Life Download Now
Importance of Chemistry in Everyday Life
- Analgesics Types
- Antibiotics Classification
- Milk of Magnesia
- Slaked Lime
Chemicals of Food in Everyday Life
The following chemicals are widely used in food materials.
- Colouring agents
- Artificial preservatives
- Flow stabilisers
- Binding substance
- Artificial sweetness
- Antioxidants
These substances do not have nutritional value except vitamins.
Also Read: Important Questions on Chemistry in Everyday Life
Artificial Preservatives: They prevent spoilage of food by stopping the growth of microorganisms. For example, sodium benzoate and sodium meta bisulphate.
Artificial Sweetness: They do not impart any calories to the body since these substances are excreted through urine. For example,
- Aspartame: It is used in cool drinks and ice creams.
- Alitame: It is 2000 times sweeter than sucrose.
Antioxidants: They prevent the spoilage of food by preventing the oxidation of food. For example,
- Butylated hydroxyl tolerance (BHT)
- Butylated hydroxyl anisole (BHA)
Dyes are coloured organic compounds that are used to impart colour to various substrates, including paper, leather, fur, hair, drugs and cosmetics. Dyes are classified into natural dyes and s ynthetic dyes.
Chemistry of Cleansing Agents in Everyday Life
What are soap and detergents.
Soaps are sodium or potassium salt of higher carboxylic acid such as stearic acid, palmitic acid and oleic acid, whereas detergents contain a long chain of alkyl groups. Detergents, in comparison to soaps , can also function in hard water.
Saponification: Alkaline hydrolysis of triesters of glycerol to form soap is known as saponification. Soap does not function in hard water since they precipitate in it.
How do soaps work?
Soaps are generally sodium or potassium salts of long-chain fatty acids. Soap molecules have a hydrophobic as well as a hydrophilic part. While the hydrophilic part clings to the water when washing, the hydrophobic end clings to the dirt particles. Thus, when we pour away the water, the dirt particles wash away with the soap molecules.
Also Read: Cleansing Action of Soaps and Detergents
Types of Soaps
- Toilet Soaps: Potassium soaps are softer than sodium soaps.
- Floating Soaps: They can be prepared by beating soap bubbles.
- Transparent Soaps: They contain soap dissolved in excess of alcohol, and it is evaporated.
- Medicated Soaps: They contain soaps by adding little amounts of Dettol, Savlon, etc.
- Laundry Soaps: They mainly contain sodium rosinate and borax.
Types of Detergents
Anionic Detergent: In this type, anions act as detergents. For example, sodium lauryl sulphate
Cationic Detergent: In this type, cations act as detergents. For example, cetyl trimethyl ammonium bromide.
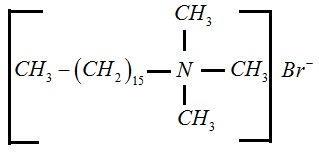
Non-ionic Detergent: They are neutral. The whole molecule acts as a detergent. For example, polyethylene glycol stearate.
Chemistry of Cosmetics in Everyday Life
Cosmetics contain the following categories of chemicals:
- Emulsifiers: They increase the stability of the emulsion . For example, potassium cetyl sulfate.
- Preservatives: They are added to cosmetics to increase their shelf life. For example, benzyl alcohol and salicylic acid.
- Thickeners: They give an appealing consistency. For example, cetyl alcohol and stearic acid.
- Emollients: They soften the skin by preventing water loss. For example, glycerine and zinc oxide .
- Glimmer and Shiners: For example, mica, bismuth oxychloride.
Other Examples of Chemistry in Everyday Life
Let us now discuss some common examples of chemistry in everyday life which most of us never knew about.
The Expiration Date on Bottled Drinking Water
Have you ever wondered why there is an expiration date on a bottle of drinking water? After all, it is just water, isn’t it? Well, most of us haven’t even noticed that there is, in fact, an expiration date on the bottle. The idea behind instilling an expiration on bottled drinking water is to standardise its packaging quality.
What the actual expiration date signifies is if the expiration date is up, the taste of the water will be different as there is a chance that the chemicals in the packaging material may ruin the quality of the water.
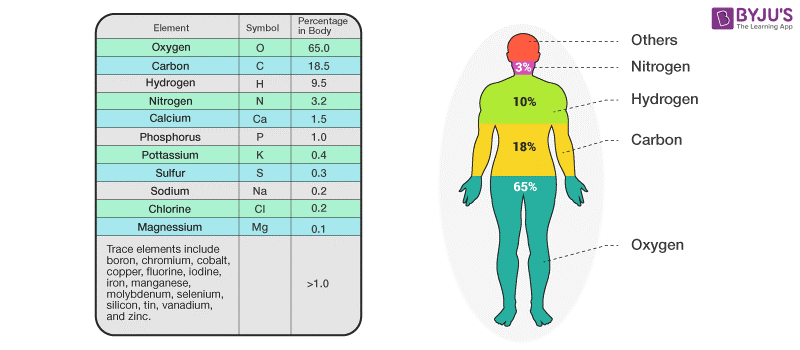
Elements in the Human Body
We all know that our body is about 60% water, but then what composes the rest of it? Carbon, Hydrogen, Nitrogen and Oxygen. These elements compose 96% of the human body. Whereas the rest 4% is composed of about 60 elements. Some of these elements include calcium, phosphorus, potassium and sulphur.
Sunblock and Sunscreen
There are two kinds of rays from the sun which are particularly bad for us, UV-A and UV-B. A sunscreen’s action, as the name suggests, functions as a screen and offers protection from sunburn which is caused by UV-B. Whereas, a sunblock has more of reflective nature and blocks both UV-A and UV-B radiations.
Related Topics
- NCERT Exemplar Questions on Chemistry in Everyday Life
- Revision Notes on Chemistry in Everyday Life
Frequently Asked Questions (FAQs)
Which artificial sweetener is used in cool drinks and ice creams, give an example of an anionic detergent., which chemical is added to increase the shelf life of cosmetics, give an example of cationic detergent., which rays do sunscreens block.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all JEE related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
- Share Share
Register with Aakash BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

Free Notes for All
- IGCSE Notes
- JEE (Mains & Advanced)
- Previous Papers
- Inorganic Chemistry Notes
- Organic Chemistry Notes
- Physical Chemistry Notes

Chemistry In Everyday Life Handwritten Notes PDF

Science stream/Class 12th Chemistry (CY) Chemistry In Everyday Life Class 12th Hand Written Notes in PDF format
After Very Hardworking by contacting Toppers of Twelfth ( 12th ) we have got Chemistry In Everyday Life Class 12th Hand Written Notes Download in PDF . Here We Are Sharing Chemistry In Everyday Life Class 12th Hand Written Notes in PDF.
These Chemistry In Everyday Life Class 12th Hand Written Notes in PDF Class Notes is printed with a high-quality printer so that visible quality should be the best. These Typed/scanned Notes are full of Quick Tips & Tricks Which Are Very Very Important For Your Board Exams .
You Can Download These Best Study Material By liking us on facebook and see the group files in facebook named as chemistryABC free of Cost. These Chemistry In Everyday Life Class XII Hand Written Notes in PDF will help you to prepare different competitive exams like, PSUs and so on. These Topper’s Class typed/scanned notes of Chemistry In Everyday Life Class 12th Hand Written Notes in Pdf will help you to understand all key concepts.
Hurry Up! It’s Time to Start your Upcoming Exam Preparation. If an average student start studying four to six hour / day from Today without losing any single day can qualify Upcoming exam with Excellent marks.
So Don’t Lose This Opportunity. Just Leave Every Minor Thoughts Behind and Concentrate on your Exam.
DOWNLOAD PDF
www.chemistryabc.com Website is Purely Educational. Our Authors Never upload any type of Copyrighted Material and not even host any Copyrighted Contents. We strongly recommend Students to support the real author or publisher of the respective books, study materials and buy all Copyrighted Material from legal sources only.
For any query or suggestion Please Mail to us [email protected]
Important Instruction: Just Prepare only with All Above Listed Typed/scanned Notes and Try to solve previous year’s papers for the last 10 years at least 3 times.
If you follow Our instruction, then you will definitely get selection marks in your Upcoming Exam.
importance of chemistry in everyday life,chemistry in everyday life in tamil,chemistry in everyday life ncert solutions,chemistry in everyday life images,chemistry in everyday life important questions,organic chemistry in everyday life,working model on chemistry in everyday life,chemistry in everyday life for ssc cgl
Related Articles

11th Hydrocarbons Handwritten Notes PDF

States of Matter 11th Handwritten Notes PDF

P Block Elements Handwritten Notes Free Download
Leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.

Chemistry: Atoms First - 2e
(36 reviews)
Paul Flowers, Pembroke, North Carolina
Edward J. Neth, Mansfield, Connecticut
William R. Robinson
Copyright Year: 2019
ISBN 13: 9781947172630
Publisher: OpenStax
Language: English
Formats Available
Conditions of use.
Learn more about reviews.
Reviewed by Pandora White, Visiting Assistant Professor, University of the South on 12/18/23
The text covers all areas expected of a general chemistry book. It does have a great index. read more
Comprehensiveness rating: 5 see less
The text covers all areas expected of a general chemistry book. It does have a great index.
Content Accuracy rating: 5
The book is accurate and appears to be unbiased.
Relevance/Longevity rating: 5
The content is up-to-date. The most recent update was 2023.
Clarity rating: 5
The book is easy to read.
Consistency rating: 5
The book has a framework that allows each chapter to be set up the same way. At the end of the chapter students find, key terns, a summary and practice problems. This is similar to any general chemistry textbook.
Modularity rating: 5
The book has good organization and modules.
Organization/Structure/Flow rating: 5
The book is outlined similarly to any general chemistry book.
Interface rating: 5
I didn't see any issues with the interface. I have been able to embed the book in Canvas courses and it works great.
Grammatical Errors rating: 5
I did not observe any grammatical errors.
Cultural Relevance rating: 3
This book appears unbiased. It doesn't really talk about scientists a lot to pull on different scientists to use as examples.
The book is a great resource for students. There are instructor resources that are available. Although, the PowerPoint slides seem to be mainly composed of images from the book, it is a great place to start when preparing the course materials.
Reviewed by Andrea Verdan, Associate Teaching Professor, Seattle University on 5/23/23
The textbook covers all the necessary topics for a college/university series of general chemistry courses. It provides sufficient depth in explaining concepts and is suitable for STEM majors. The book offers helpful features like clear models and... read more
The textbook covers all the necessary topics for a college/university series of general chemistry courses. It provides sufficient depth in explaining concepts and is suitable for STEM majors. The book offers helpful features like clear models and diagrams, thoroughly worked examples, and chapter summaries that include key terms/equations and end-of-chapter questions. The “atoms first” sequencing of chapters may not be ideal for some, but instructors have the flexibility—as with any textbook—to adapt the order based on their curricular goals and needs. This textbook serves as a comprehensive reference and learning support guide for understanding the concepts of general chemistry.
I have not identified any errors nor bias in the content as presented. As fellow reviewers have mentioned, errors can quickly be amended since this is an online resource.
As the chemistry fundamentals covered throughout this textbook are quite stable and not expected to significantly change in the distant future (unless a paradigm shift occurs), the textbook content should remain relevant. Portions of each chapter that reflect the current context and applications of chemistry are boxed or are given their own sections, i.e., the chapter Introductions, Portrait of a Chemist, and Chemistry in Everyday Life segments. These sections can be easily modified to stay updated.
The textbook was written with students in mind, keeping explanations straightforward and easy to follow. The text, figures, and diagrams are understandable and worked examples are clearly formatted and presented.
The textbook is consistent in use of terms and concepts, formatting of figures, and strategies presented in worked examples.
Modularity rating: 4
Though the textbook chapters are separated into related sections, some sections could be separated further, for example the “Classifying Chemical Reactions” section of Chapter 7. When referring to previously presented content in earlier chapters (i.e., when redox in Chapter 16 references material covered in Chapter 7), the publisher might consider including hyperlinks for readers to easily access and review the previous content.
Organization/Structure/Flow rating: 4
The ideas presented in each chapter start with a foundation that is clearly built upon as the chapter progresses. As previously mentioned, the “atoms first” sequencing of chapters may not be ideal for some, but instructors have the flexibility to adapt the topic order based on their curricular goals and needs.
Interface rating: 4
Having navigated the textbook digitally (both on a laptop and tablet), the sans serif text is easy to read, equations are nicely spaced for clarity, and figures and diagrams are clear and undistorted. As previously mentioned, the publisher might consider including hyperlinks for users to access and review previous content while working through “new” and related content in later chapters.
I have not encountered any usage or grammatical errors in the textbook. The textbook is accessible for both native and non-native English learners.
Cultural Relevance rating: 5
I appreciate the attempts in each chapter to reflect the current context and landscape of the people who “do” chemistry. The chapter introductions and Portrait of a Chemist sections provide—when available—examples of the variety of social identities (races, ethnicities, and backgrounds) that have contributed to the chemistry discipline.
Reviewed by Thiagarajan Soundappan, Associate Professor, Navajo Technical University on 12/18/22
This textbook covers all the essential areas of the introduction to chemistry read more
Comprehensiveness rating: 4 see less
This textbook covers all the essential areas of the introduction to chemistry
Content Accuracy rating: 4
Need more details about the real-time examples
Added contents are highly relevant to the each subject areas
Clarity rating: 4
The textbook is written with enough clarity. There is no jargon/technical terminology.
The textbook is consistent with all the essential chemistry terminologies
This textbook is easily readable, realigned with various sub unites with enough and related images
The topics presented are in the logical order.
Interface rating: 3
Need more attention on images/charts. Some images are not clear enough and not attractive. This includes instructor resource PowerPoint files. More time should be spent creating attractive ppt slides which will help the instructors to select this as their textbook.
There is no grammatical errors. The text has a smooth flow.
Cultural relevancy is not essential. Context is important. Therefore, I don't add this as an important one.
I have attempted to download and embed the blackboard package. The file was downloaded and when I attempted to embed with blackboard the work showed completed. But I couldn't find any question bank in my BB. I don't know whether this error from the Publisher or with BB. Please verify and confirm the BB materials.
Overall this textbook is well written. However, it still lacks in the three sectors. 1) Need high quality images and ppt slides 2) Blackboard QB needs to be fixed 3) There is no integrated lab manual which is mandatory for the gen ed based chemistry courses.
Reviewed by Sen Wang, Lecturer, California State University, Dominguez Hills on 11/1/22
Need more everyday examples and practice questions. read more
Comprehensiveness rating: 3 see less
Need more everyday examples and practice questions.
Generally looks alright. Errors in Table J: BaC2O4 not hydrates. Ba(OH)2 not hydrates CaSO4 not hydrates CaC2O4 not hydrates
Very similar content to the commercial book we use.
Needs more examples in Ch.3, especially 3.1 - Electromagnetic energy is hard for most of our students. You can add daily life examples, such as distance driven = speed x time, that will be similar to light, although the speed of light is faster. Also in Ch.6 (mass percentage for chemicals directly), Ch. 9 (enthalpy), Ch.12 (entropy), and Ch.21.4 (Amides). All are hard for most students.
Consistency rating: 4
Easy to find online.
Nicely written, especially ch.7, ch.14, and ch.16.
Cultural Relevance rating: 4
Reviewed by Emmanuelle Despagnet-Ayoub, Associate Professor, Occidental College on 6/28/22
The content of Chemistry: Atoms First -2e is aligned with any books covering two-sections of general chemistry. The topics are pretty standard. However, I would have appreciated a more detailed definition of Reversible and Irreversible processes... read more
The content of Chemistry: Atoms First -2e is aligned with any books covering two-sections of general chemistry. The topics are pretty standard. However, I would have appreciated a more detailed definition of Reversible and Irreversible processes in Thermodynamics (to be honest, I haven't found it in any General Chemistry books up to now and it's too bad). I guess it is probably considered too advanced for this level.
I haven't found any errors up to now.
I appreciate the relevance of the examples (Solar Thermal Energy Power Plants, Hand Warmer, Decaffeinated Coffee using Supercritical CO2...), for sure the students will identify to them. The use of simulations through the PhET Interactive Simulations websites (platform I have already implemented in my classes) and movies make the book really interactive. In the future, the examples could easily be modulated with up-to-date everyday life examples.
The writing is clear and easy to read. The figures are neat, engaging and well chosen to illustrate the concepts. Each section is well organized with at the beginning a layout of the learning goals and at the end the key term definitions, key equations and the summary. The chapters are well-structured.
The terminology is accurate and the approach of the concepts follows well-known General Chemistry books. The framework is consistent in each chapter with theory, definition, everyday life examples, a decent amount of corrected and uncorrected problems and portrait of chemists, The book is easy to read, and flows well. I also appreciate the Most Challenging Problem section to challenge advanced students.
Each Chapter is divided in reasonable sized sections, so reading assignments could be easily tuned according to the progress of the class. Students have the opportunity to highlight in different colors the content of each Chapter, facilitating the realization of study guide.
The topics are well presented in a logical order. I was comfortable with the concepts flow, it matches the textbook I am currently using.
I haven't encountered any interphase issues, it was straightforward to switch from the text to any links (movies, simulations...). The visualization of a concept with an interactive activity is really pedagogic and matches my teaching style.
I haven't found any grammatical errors.
I appreciate the portrait of famous chemists. It allows the student to learn more about the actors of important concepts in chemistry with a picture and a description, names they may probably have only heard of without an image or a story behind it.
This OER textbook matches my teaching style and I am eager to try it next semester!
Reviewed by Joseph Ngyuen, Professor of Chemistry, Mount Mercy University on 12/25/21
The book covers all areas expected for a general chemistry course. It is also unique for the authors to combine molarity/concentration within the “mole” chapter, being one of the few books to cover the concept this way. However, it works well in... read more
The book covers all areas expected for a general chemistry course. It is also unique for the authors to combine molarity/concentration within the “mole” chapter, being one of the few books to cover the concept this way. However, it works well in the atom’s first approach because the students should have a firmer foundation of dimensional analysis. The index is organized well and the search engine for the book works well.
The book is accurate, error-free and unbiased. The authors do a good job of balancing the concepts and analogies for learning.
The authors do a nice job of utilizing up-to-date examples for the concepts. The examples should be relatable for students, especially in this ever-changing world. It would be difficult for the text to be obsolete because it is an introductory level book; however, it appears that it would be easy to update the examples whenever necessary. The authors do a great job of providing links to videos to visualize some of the concepts. If there was an area that could be updated, it would be to utilize videos that have been created more recently.
The text is clear and written well. It is one of the easier books to read and tries to keep the jargon/technical terminology to a minimum. While I have read other texts with better prose, it is difficult to do to keep the sections to a minimum.
The text is internally consistent with the terms of terminology and framework. It also stays consistent with other texts, making it easier for an educator to utilize while minimally modifying their teaching materials.
The text is split into good, smaller reading sections. While the text sections are good and small, the authors have some rather long chapters. This is generally difficult for educators who like to assign chapter homework assignments, which leads to rather long homework assignments. Even though the homework assignments might have a good number of questions, the students may not have enough “practice” time with the homework. Educators utilizing this book should think about breaking up homework assignments into smaller sections as well.
Overall, the topics are presented in a logical, clear fashion, but there could be some improvements. While it would be fine to cover thermodynamics before equilibrium, having kinetics towards the end is odd. Most books tend to cover the topics in this order: kinetics, equilibrium, thermodynamics, electrochemistry (or electrochemistry then thermodynamics), which works well with first year chemistry students. This flow tends to work better than starting with thermodynamics, which is probably a preferred approach for physical or theoretical chemist.
The text has no interface issues, even with the pdf version. The hyperlinks work well, especially when the text refers to a table or figure somewhere else. The outline for both the web and pdf versions also works well.
There are no noticeable grammatical errors in the text.
Considering the topic is chemistry, it is difficult to find ways to include text that is culturally insensitive or offensive. The authors do bring in a variety of recent and notable chemists throughout the text.
I initially chose this text so my students can utilize their money in other online resources/assessment. The combination of an online homework program and an in class online tool was still cheaper than the vast majority of textbooks, even with “online homework” as part of the package. It has worked out rather well, especially since it is a quality textbook.
Reviewed by Feier Hou, Assistant Professor, Western Oregon University on 6/7/21
The book is very thorough and comprehensive. It includes all the topics typically taught in a general chemistry class. read more
The book is very thorough and comprehensive. It includes all the topics typically taught in a general chemistry class.
The content is very accurate and factually correct.
The contents are up-to-date. I particularly like that the real-life examples given in the textbook, such as cooking, chemistry of a smart phone, etc.. Those examples are likely to be highly relevant to students.
The text is very clear.
Terminology and framework are both very consistent throughout the book.
Overall, the textbook is very easily divisible into smaller reading sections, especially since they have a table of contents list that is clickable and can lead you to various different sections of the book. I did have a few minor problems: first, I had was that a few of the contents should have been under the same topic, but are scattered under different chapters. For example, the formula and molecular mass should be included in the same chapter as moles and molar mass, but they are covered in a much later chapter; second, some very important topics in chemistry, such as moles and net ionic equations, are included in the book but not in the table of contents, so it took me a while to find them, and I imagine that if I were to assign reading on those topics for my students, it would be difficult for them to find those topics, too. I suggest that the authors include at least the key topics in chemistry in the table of contents.
Organization/Structure/Flow rating: 3
Overall, the book is well organized, and flows logically. However, there are several parts of the book that I would suggest the authors to rearrange. For example, molecular and formula masses should be in the same topic as chemical formula and taught before moles and molar mass because they are related; the periodic table, and the related knowledge like atomic number and groups of elements should be taught much earlier in the book, even before the chemical formulas; the net ionic equation should be taught after solubility and acid/base so students would understand why some compounds dissociate into ions while others do not; kinetics should be taught before equilibrium so that students understand that at equilibrium, the forward and reverse rates are equal; molarity should be under the topic "solutions".
It is easy to navigate, especially with the "table of contents" and the feature to search for key words. The quality of the images are high, but more images can be included. For example, it would be helpful and easier for students to read if images of every electron-group and molecular geometries are included when talking about VSEPR theory.
There is no grammatical errors.
Cultural Relevance rating: 2
Thank you for including "portrait of a chemist", and for talking about the contribution by Rosalind Franklin to the DNA structure. However, pretty much all of the chemists included are US/European based, and most are white/"white-passing". I would like to see more inclusion of people from, for example, Asian, African, or Latin American countries, and people from a more diverse racial, ethnic, and cultural backgrounds, which will be a better reflection of students in a general chemistry class.
Overall, this is a good book for teaching general chemistry with an atoms first approach. I am considering adopting this textbook for my general chemistry class. However, I may make a few minor changes or rearrange some topics if I use this textbook.
Reviewed by Aherial Cofield, Chemistry Instructor, Aiken Technical College on 2/12/21
The book covers all areas of a typical general chemistry course. An index, glossary, and appendices are included in the book. They are thorough, easy to navigate, and provide the necessary information needed for students to be successful. read more
The book covers all areas of a typical general chemistry course. An index, glossary, and appendices are included in the book. They are thorough, easy to navigate, and provide the necessary information needed for students to be successful.
No errors in calculations, units, or diagrams were noted.
Relevance/Longevity rating: 4
The book seems to be relevant. I didn't notice any out of date information, nor did I see topics that were too new for students to fully understand. There seems to be a balance.
The language of the book is easy to understand.
All terminology is consistent.
The book has plenty of headers, diagrams, charts, and pictures to keep readers engaged and information organized. I do wish there was a more color on the pages; it may be dull to some.
The sequence of topics in the book is traditional for an atoms first approach.
There are no interface issues.
There are no grammatical errors.
I did not see any insensitive or offensive material.
This is a great source for students that do not have a strong chemistry background. There are examples with explanations and calculations, charts, and diagrams that help guide a student along. The additional sections in the back of the book provide ample information such as constants, values, and definitions to make the material clearer. If the book had more color, it would be near perfect. I'm looking to incorporate this book into my general chemistry courses.
Reviewed by Megan Lazorski, Assistant Professor of Chemistry, Metropolitan State University of Denver on 9/4/20
This book covers the basic principles of chemistry in a mostly comprehensive way. The only thing I would note is that some concepts are covered in a bit too much detail for the point at which the concept would be introduced in the course (e.g.... read more
This book covers the basic principles of chemistry in a mostly comprehensive way. The only thing I would note is that some concepts are covered in a bit too much detail for the point at which the concept would be introduced in the course (e.g. titrations in Ch.7) and some concepts feel covered a bit too sparsely (e.g. combustion analysis doesn't include hydrocarbons with heteroatoms). Specifically, Ch.3 is a critical place for depth of explanation because this is an atom's first text, but there are several places where the explanations are lacking.
The book seems appropriately error free, accurate and unbiased. Conventionally published works are known to also have printed errors and inaccuracies here and there and I think this text is comparable to those texts.
This text does seem up-to-date in a way that seems like it will not quickly become obsolete. I think that is partially a function of the subject matter (general chemistry), since the principles of chemical behavior at the general chemistry level are pretty well-established and it would be difficult to include information from the primary literature without losing the students. However, I also think that the author's did a nice job of keeping true to the idea that science is ever-changing, such that updates could be made easily.
I think the author's did a nice job with the clarity of their writing style. Most concepts are presented clearly and there are only a few places where I have noticed big jumps in logic that may confuse students. The prose is written at an appropriate level for first/second year undergraduates.
I have not noticed any internal inconsistencies in terminology. I have noticed a few small places where it seems like that materials is fractured and presented in chunks at different places in the text, which sometimes feels like the student's could view it as slightly choppy (if they notice_. However, from an instructor standpoint, the decisions made by the authors in the places where these ideas are split seem logical and well-founded.
I have found this text to be very modular and amenable to reorganization where necessary. The length of the sections also feel appropriate and amendable to creating smaller reading assignments for each chapter.
The text is well-organized and the subject matter flows well from one chapter to the next. I think the authors did a good job of arranging the subject matter such that the fundamental principles students need to build a chemical foundation (i.e. classification of matter, simple atomic structure, the mole concept) are presented first, but the book is still appropriately arranged in an "Atom's First" manner.
Interface rating: 2
The images/charts could be improved. For the most part, I think the images/charts/etc are appropriate and to-the-point, but lack some of the visual appeal and clarity of "conventional" texts, however, some of the images/charts seem bigger than necessary with respect to their pedagogical utility (e.g. Figure 2.28 - a spool of copper wire), some of the images/charts do not do a great job of illustrating the point unless you already understood what the point was (e.g. Figure 3.6 - a visual of constructive and destructive interference would help add context to the idea of interference in this figure), some images/charts are not well-explained or are missing critical features (Figure 3.20 - there is no label on the y axis of the graphs, nodes are not labelled, the relevance of the change in color is not discussed in depth). I love the integration of PhET simulation links/suggestions which help students dive deeper into specific concepts than conventional texts, so that is a major plus. I chose several examples from Ch. 3 because this is an atoms first text and I feel like this chapter is critical for the student's understanding.
Grammatical Errors rating: 4
I have not noticed any egregious grammatical errors.
While this is a chemistry text and there are clear issues with finding Nobel laureates in chemistry of various races, ethnicities, and backgrounds for specific breakthroughs in our scientific past, every portrait of a chemist in this text is white or "white-passing." There is a good balance of representation of female vs males, which is good, but it is common in science for white women to take up all of the "underrepresented" space. I think this text could benefit from diversification of examples.
Overall, this text is of sufficiently high-quality for use in undergraduate general chemistry courses. It covers all of the fundamentals of the topic and provides many excellent resources for homework problems and virtual simulations. Figures/charts, and some explanations regarding atomic structure could be improved, but overall this is a great resource for providing accessible, open-educational resources for students. I look forward to seeing how this text progresses!
Reviewed by Begona Bradham, professor, Piedmont Technical College on 8/10/20
The textbook covers everything needed for college chemistry I and II. read more
The textbook covers everything needed for college chemistry I and II.
Did not find any errors, although I would have loved to have more time.
At this level, chemistry is not going to change, so book should be relevant for a long time.
Easy to understand.
Excellent job in consistency.
Great job in subdividing chapters to get smaller reading sections.
I love the order, beginning with the atom. Many chemistry books leave the atoms for last, but I think this makes a whole lot more sense.
Great images
English is not my native tongue, but I did not find obvious grammatical errors.
Not very concerned in chemistry, but it certainly is not offensive in any way.
I am very seriously considering adopting this book for my chemistry classes. I need to have a look at the power points and online assignments, but I like the book.
Reviewed by Benjamen Taber, Adjunct Instructor, Central Oregon Community College on 7/3/20
This book fully covers the General Chemistry sequence, and includes a few "additional" chapter such as ones ones on nuclear chemistry and organic chemistry from which an instructor could add sections (or a student could read to satisfy some... read more
This book fully covers the General Chemistry sequence, and includes a few "additional" chapter such as ones ones on nuclear chemistry and organic chemistry from which an instructor could add sections (or a student could read to satisfy some curiosity). This book compares well to commercial texts, and is aimed at chemistry and science/engineering majors. The index, glossary, and appendixes are organized well, easy to navigate, and comprehensive.
This book appears to be accurate, error-free, and unbiased.
General Chemistry is fairly well-developed, so this book's content will remain up-to-date. The very nature of OpenSTAX allows for updates, and further additions will likely maintain similar electronic formats (and not significantly deviate from the print version).
This book is clear. Chemistry can be a difficult subject for students to grasp, but with lots of examples, plain language, practice problems, and an atoms-first approach, this book does a good job of communicating concepts and placing them in context.
This book is quite consistent, maintaining a cohesive style and flow throughout. I did not find any inconsistencies in abbreviations, terminology, or units.
This book is fairly modular and chapters/sections are for the most part self-contained. It's possible to cover a subset of sections in a chapter, cover sections out-of-order, and cover chapters out-of-order. For example, one could cover electrochemistry (Ch. 16) except for the section on potential, free energy, and equilibrium (16.4) before covering thermodynamics (Ch. 12), and then return to 16.4.
Having taught the General Chemistry sequence using the more traditional flow (stoichiometry early), I believe that the atoms-first approach will do a better job in building the foundations of chemistry. I appreciate how this book builds the foundation of chemistry (atoms, their electrons, and how they interact) in context (developed to explain observations). This gives students a platform upon which to stand when systems become more complicated (ex. chemical reactions in different conditions).
This book's interface is clean. Navigating is easy, especially with the embedded links in the digital versions, and there are no distortions to figures and charts. I did not find any issues with dead or misdirected links, including ones to external sources.
This book utilizes correct grammar throughout.
While not insensitive or offensive, this book is not explicitly inclusive. There's opportunity for the third edition to be more inclusive, helping more students see Chemistry as their domain.
Overall this is a good book that covers the first-year General Chemistry sequence. Having online, downloadable, and print options for the book helps students with different contexts. From my perspective as a new instructor the additional materials--such as powerpoint slides and supplemental test questions--are quite useful in developing course material.
Reviewed by Philip Shivokevich, Visiting Assistant Professor, University of Massachusetts Amherst on 6/30/20
Tis book provides good depth for a range of introductory courses, from basic to more advanced major intro courses. Provides an excellent discussion of every topic covered in courses that I've taught, plus a few more. read more
Tis book provides good depth for a range of introductory courses, from basic to more advanced major intro courses. Provides an excellent discussion of every topic covered in courses that I've taught, plus a few more.
I found no issues with the accuracy of the text,
The book provides a wealth of high-quality images. These are great for use in lectures and activities. It would be great if the authors included more connections to other disciplines and relevant topics (eg. climate change). These topics would help students find the relevance to the readings.
Very accessible and clearly written. Does a good job of defining unfamiliar terms as they are introduced.
I found no problems with consistency.
I have used the text as a companion for several courses, and can assign portions of it without concern for context. Sections are strong for stand alone use.
Overall, I like the organization of topics better than most texts. However, I still find myself mixing things a bit to better suit my particular teaching style.
I use the pdf version, and it provides excellent quality text and images. The images also have high resolution, which is great for incorporating them into lecture slides.
Minimal grammatical errors.
No offensive or insensitive portions. The text was somewhat inclusive, but could improve a bit here.
Overall I think that this is a great resource for students. I have often referred to it for a different spin than the departmental text, and used it for images on my lecture slides. I plan on adding links to the text for my fall classes, as well as the department's required text for the course.
I would likely use this as the main text for my courses if not required to use a paid text by the department. I think that it is of equal, and often of higher quality, than paid texts that I have taught with. I also find it to be much easier to mix and offer sections as stand alone portions than other texts.
Reviewed by Gabriele Backes, Instructor, Portland Community College on 6/22/20
The text is comprehensive and addresses all topics required in a one-year science major’s general chemistry curriculum. The text begins with a nice introduction of how widespread chemistry is and quickly moves into various topics of chemistry.... read more
The text is comprehensive and addresses all topics required in a one-year science major’s general chemistry curriculum. The text begins with a nice introduction of how widespread chemistry is and quickly moves into various topics of chemistry. What follows is a thorough coverage of each topic and this text and could easily be incorporated into an existing curriculum. It contains all the elements of a regular textbook, which includes a table of content, key terms and key equations at the end of each chapter, as well as all tables such as ionization constants, thermodynamic parameters, and many other essential data tables fitting for a general chemistry text. The text is easy to read, though it lacks the rigor compared with the textbooks that are currently on the market. The text could benefit from additional exercises, especially those exercises that are more challenging, and more rigorous.
I did not come across any inaccuracy or misconceptions.
The text is current and its content, for the most, part up-to-date. The text is laid out such that any new discoveries or updates can be easily integrated over time. To that effect, the text would benefit from additional every-day applications in the ‘How Sciences Interconnect’ or ‘Chemistry in Everyday Life’ boxes to add more contemporary material.
The book is easy to read. It is concise and major concepts are easy to follow and easy to understand. It has all the necessary verbiage, definitions and easy to follow explanations. Each chapter ends with ‘Key Terms’, ‘Key Equations’, and a section-by-section ‘Summary’ for easy review. Boxes such as ‘How Sciences Interconnect’ and ‘Chemistry in Everyday Life’ should perhaps be highlighted in a different color than the sample exercises to allow the reader to more readily locate the information as one navigates through the pages.
The text is consistent throughout in its formatting, writing style, and language used. Each chapter highlights illustrations, tables, and practice examples, which are easy to follow and reinforce the concepts. There is some inconsistency in the formatting. Some images, especially Lewis structures in chapter 4, appear to be enormously out of proportion relative to other ones.
This is adequate. Each chapter could very easily be adopted as a stand-alone chapter and the chapters could be moved around without much sacrifice in the content.
This book is very nicely written and easy to follow. The content is accurate, the text comprehensive and it could easily be used in a general chemistry curriculum. There are a couple of formatting issues with superscripts in the introduction of chapter 14. Other than that, I did not come across any major issues. The ‘Chemistry in Everyday Life’ sections add relevance and interest to each chapter.
I opted to review the online version and had no issues navigating through the chapters nor did I have any issues with loading of images, or links to other sites. Each chapter has working links that refer back to previous chapters, and to the tables in the appendices. Links to videos outside of the textbook make a good addition. It would be nice to have an interactive periodic table.
I found no grammatical or spelling errors while reading the text.
I have not found any issues with the text being culturally insensitive.
Overall, this book is nicely written, easy to read and the content is accurate. Even though the text contains all the necessary verbiage, definitions and explanations, some of its chapters are a bit on the short side.
Reviewed by Corinne Deibel, Professor of Chemistry, Earlham College on 3/28/20
I'm impressed with the comprehensiveness of this textbook and how it compares to the published textbook we currently use. Overall, it provides a great alternative. One area that I would like to see expanded is the section on the types of... read more
I'm impressed with the comprehensiveness of this textbook and how it compares to the published textbook we currently use. Overall, it provides a great alternative. One area that I would like to see expanded is the section on the types of reactions, which is folded in the stoichiometry chapter (Ch 7). The concept of complete and net ionic equations should have been integrated within the precipitation and acid/base reactions instead of standing alone in the "balancing equations" section. The acid/base reaction section is also a bit light.
I haven't found any inaccuracy in the textbook
The "Chemistry in everyday life" boxes are very well done. They contain attractive graphics and information relevant to the current student population (ex: Chemistry of Cell Phones).
The text is written clearly and judiciously incorporates figures, examples, external links, etc. Figures, which are used extensively, are clear and convey the important information in a visually appealing way. I like the details in which the calculations are showed. For example, the dimensional analysis section clearly explains each of the steps involved in a calculation and progresses from simpler to more complex problems. Although I do appreciate the "Key Equations" section at the end of each chapter, it would be nice to highlight the main formulas in the text better (draw a box around them for example) so that students can refer to that section more easily if needed. I found that the "link to learning" boxes judiciously placed to help illustrate important concepts. The stoichiometry problems are well explained. The problems are mapped out before any calculations are attempted, which allows the student to better understand the concept before trying the math. There are some small sections with extra information that might confuse some students. For example, the formula to convert between Celcius and Fahrenheit is unnecessarily confusing. You should just give the formula and show the derivation in a link for students who are interested in the math.
All the material is presented in a consistent manner and the format is consistent.
I find this book easy to work with in terms of modularity. As a radiochemist by training, I like to cover some nuclear chemistry as soon as the students learn about the nucleus and Rutherford. I could easily integrate sections from the Nuclear Chemistry chapter (Ch 20) to supplement the atomic structure presented in chapter 2. As we teach a one-semester general chemistry course instead of a more traditional two-semester course, we do cover some later chapters earlier in the course (e.g. kinetics, thermodynamics), and this book would let us do that.
Most of the chapters are organized in a logical fashion for an atom-first textbook. I found a few concepts out of place, such as electrolytes not being mentioned until chapter 11. It would be helpful to introduce that concept in chapter 7 when discussing the types of chemical reactions, most specifically to introduce the strong vs. weak acids and bases concept.
I haven't found any issues with interface. The search engine is very helpful.
No grammatical errors found while reading the textbook.
Could try to reference/portray scientists from a variety of backgrounds.
Those are picky comments from a chemist with a background in nuclear chemistry, but I find some sections of the nuclear chemistry chapter a bit light compared to other textbooks I have used. For example, there is no mention of the term activity (only referred to as deca rate), or no clear explanation as to how the band of stability graph can be used to predict decay modes. I do, however, appreciate the sections on nuclear power, the use of radioisotopes and biological effects of radiation that are well done and increase the relevance of the topic for the students.
Reviewed by Lou Ann Tom, Associate Professor of Chemistry, Susquehanna University on 3/8/20
I did not see anything inaccurate in my review. More details follow: Chapter 1 provided good coverage of scientific method (it is nice that it is in first chapter), and all the basic Ch 1 topics. The example problems are relevant and good... read more
I did not see anything inaccurate in my review. More details follow: Chapter 1 provided good coverage of scientific method (it is nice that it is in first chapter), and all the basic Ch 1 topics. The example problems are relevant and good demonstration of the math. I like how the example problems are set apart in blue. Then the “Check your Learning” problems are good with answers for immediate feedback for students to practice. The 1 click to PHET simulations is extremely useful for those students who learn visually by seeing demos instead of just reading. I also really like the number and types of examples in the chapter. Measurements and significant figures are explained better than in some textbooks and the visual information and explanations are better examples than in some textbooks. Also, the use of conversion factors was shown well. Dimensional analysis examples were very good and I like the way the units are shown as being cancelled. Temperature calculation examples were good and the pictures of thermometers were fine. The Key Terms and Definitions and Key equations at the end of each chapter were good. The summary is OK and hopefully the students won’t just read this! The end of chapter exercises are relevant with many examples. It is nice that they are broken into sections with headings. Ch 2 has nice demo of the laws, including the law of multiple proportions. Also has a nice demo of Millikan and the other scientists’ studies and results. Ch 3 has a lot of info in the chapter but it is all relevant in that chapter. Nice explanation and demo of electromagnetic radiation and all the associated concepts and calcs. Nice useful figures. It is good that goes right into electronic structures and electron configurations and orbital diagrams, and then periodic trends and then introduces ionic and covalent bonds. These are critical for understanding the following chapter concepts. Ch 4 has More in-depth discussion of ionic and covalent compounds and naming. This could use a more extensive list of polyatomic ion names and multivalent cations. Nice demo of VSEPR models. Ch 5 provides pretty standard info which is presented well. I like the info on electron configuration and bond order for molecular orbitals. I like the early intro to valence and conduction band in fig 5.39 because they will see this in many future classes and it is not usually presented in general chemistry. Ch 6 provided a nice intro to solutions and associated calculations. That is followed by balanced equations and stoichiometry in Ch 7. Hopefully it won’t be too much to learn the types of solutions and stoichiometry in one chapter. Chapters 9 (thermochemistry), 14 (acids/bases and buffers), 15 (solubility) and 16 (electrochemistry) all look OK. Chapters 18 and 19 look useful but we usually never get to these by the end of the year. We would try to incorporate some of the sections into the semester following a brief introduction to biochemistry, which is lacking in this textbook. Chapter 20 is a nice presentation of nuclear chemistry, with many modern and relevant and interesting applications. Chapter 21 is a nice chapter on organic chemistry to introduce this next type of chemistry at the end of the semester. I am not sure why there are often duplicates in the index and the only difference is singular vs plural. I am not sure if there is some advantage to this but it seems a bit confusing. The Appendices are helpful. The tables and data look clear and comprehensive. However, in Appendix A, the colors in periodic table are too light. In App B Essential Math is important for many students not strong in math. App C – E, G and J are fine and comprehensive. I would prefer to see Table numbers or letters at the top of the tables rather than at the bottom to be consistent with standard practice. App F is handy for other classes too. I like the Lewis Structures in App H and I. In App L, for the standard reduction potentials, although I think it is unusual to be found alphabetically instead of in order of decreasing potential, I do like that the different oxidation states of the same elements are next to each other to find them easier instead of having to search down through the table. In App M, the isotopes and types of emission for each is nice to have handy.
I did not observe any inaccurate information or any information presented in an inaccurate or biased way.
The content was typical of most general chemistry textbooks used for a one year-2 semester introductory course for chemistry majors. The relation of chemistry to other sciences and Chemistry in Everyday Life is very well done. We use a similar general chemistry textbook, Atoms First approach, to teach chemistry and biochemistry majors as well as majors from other sciences. Especially for those other majors (biology, neuroscience, Earth and Enviromental Science, etc.) it is very important to relate chemistry to current life and to their interests in order to convince them that this information is relevant to them and their fields, to get their interest from the start. I think this information is critical to get buy-in from those students, as well as those interested in chemistry. The Links to Learning are also useful. I find that students will use links to learn the information from websites, and these being right in the electronic textbook are very useful.
The explanations are clear and the examples are well-written. The pictures are clear and relevant.
The chapter styles and sections within each were very consistent and useful.
The modules seem well organized. Specifically, the example problems are relevant and good demonstration of the math. I like how the example problems are set apart in blue. Then the “Check your Learning” problems are good with answers for immediate feedback for students to practice. The 1 click to PHET simulations is extremely useful for those students who learn visually by seeing demos instead of just reading. I also really like the number and types of examples in the chapter. The Key Terms and Definitions and Key equations at the end of each chapter were good. The summary is OK and hopefully the students won’t just read this! The end of chapter exercises are relevant with many examples. It is nice that they are broken into sections with headings. It is nice to have half the answers in the back for students to use and to have half that are not provided. However, the answers that are provided maybe should all either be odd or even for consistency. This is not critical if there is some reason this was done in this way, but it might be easier for faculty to assign if they were more consistent. However, this is not terribly important and would not prevent me from adopting the textbook.
The information in the chapters flows well. The order of chapters seems fairly consistent with other Atoms First textbooks. Ch 8 the gas chapter and CH 10 Liquids and solids and CH 11 solutions look pretty typical and comprehensive although we don’t cover gases and liquids/solids until the second semester, after we have covered equilibrium (Ch 13, good use of ICE tables) and thermochemistry (Ch 9) and Thermodynamics (Ch 12) which all look like they are organized will within the chapters. Ch 17 Kinetics looks good and comprehensive but seems unusual that it is after electrochemistry. We would use this earlier in the year but the chapters seem sufficient that some of the chapters can be taught in an order different from that in the textbook. We would use Ch 20 (nuclear chem) before Chapters 18 and 19. We are teaching organic I and II before the second half of general chemistry (II), so may introduce Ch 21 earlier but it works fine at the end of the textbook for most curricula.
I used the .pdf version and it was fine. I did look at the on-line version but did not have steady internet access so having the .pdf version was very important. Personnally, I like to have a hard copy to prepare lectures. However, the .pdf was easy to read on the computer screen, although I am not usually in favor of this format. On the computer, I like reading from top to bottom and some on-line texts, you have to scroll through pages left to right and you cannot see the whole page at once so you have to scroll down to the bottom of the page before clicking the right arrow. This on-line textbook did not seem to have that problem.
I did not see anything significant but have not taught from it yet so would pick up small errors after one year of use.
I did not see any issues with this.
I am looking forward to trying this textbook for my general chemistry course next year.
Reviewed by Brooke Woolman, Chemistry Lecturer, Metropolitan State University of Denver on 11/20/19
The book covers all the topics found in a full year general chemistry course and is aimed at chemistry majors and other science majors. The chapters and chapter contents are similar to comparable commercial textbooks. read more
The book covers all the topics found in a full year general chemistry course and is aimed at chemistry majors and other science majors. The chapters and chapter contents are similar to comparable commercial textbooks.
This is only my first semester using this textbook. So far, I have not found any errors within the text itself. My only issue that I did come across was with the answers in the back of the book. I noticed on Chapter 8 for the end-of-chapter problems, the answers in the back switch from odd to even which led to some confusion with the homework.
General Chemistry is well enough established that the book will remain relevant.
The book was very clear. Chemistry is a very difficult subject to understand no matter how it is written. I think the multitude of written examples, practice problems with solutions, and external links really help the students.
The text maintains a consistent format from chapter-to-chapter, which will help students in navigating the text. I did not notice any inconsistencies with terminology or abbreviations.
This seems pretty typical for introductory chemistry texts. The fact that there are a lot of modules or sections is advantageous especially when instructors may need to bounce around modules or exclude some for time constraints. I did think that the end-of-chapter problems could be broken down better.
This is my first time teaching using an atoms-first approach, but it seems to be consistent with the other texts out there. The order of topics for a second-semester course (thermochemistry, thermodynamics, equilibrium chemistry, and kinetics) is a little different from my preferred structure (I prefer to cover kinetics before thermodynamics and equilibrium chemistry) but I did not have any difficulty adapting this textbook to that order.
The on-line interface is easy to use and the search box and embedded links are useful. I did not experience any issues related to distorted images or images that would not appear. Also, all of the external links I clicked on worked with no problem.
Correct grammar is used throughout the book.
I feel the book could add more cultural diversity in its examples.
Overall, the quality of this textbook is similar to textbooks I have used in the past. The one area where this textbook could improve, is in the end-of-chapter problems, which include relatively few challenging problems or problems that ask students to draw on multiple concepts within the chapter or between chapters. I do think that the availability of a print copy is advantageous, as some students preferred a paper copy.
Reviewed by David Harvey, Professor of Chemistry & Biochemistry, DePauw University on 10/18/19
This textbook covers all topics typically found in introductory courses in chemistry aimed at chemistry majors and other science majors. The chapters and chapter contents are similar to comparable commercial textbooks. read more
This textbook covers all topics typically found in introductory courses in chemistry aimed at chemistry majors and other science majors. The chapters and chapter contents are similar to comparable commercial textbooks.
After several semesters of use, I have not found any errors within the text itself. I have found occasional errors in the textbook’s answer key to the odd numbered end-of-chapter problems and in the solutions manual available to instructors. There is a link to an errata page that lists all reported errors and their status (in review, reviewed and no correction, and corrected).
Its introductory chemistry! Other than the development of an atoms first approach, there has been no significant change to the basic structure of the courses that will use this textbook. As new external resources become available, the electronic nature of this textbook will allow the authors to provide links to those resources.
The text is easy to read and the worked examples are presented with sufficient details. The figures, particularly, and the tables are well thought out and useful.
The text maintains a consistent format from chapter-to-chapter, which will help students in navigating the text, particularly in its on-line format.
The individual chapters are divided into appropriate sub-sections or reasonable length and make consist use of sections that provide key terms and key equations, a summary of the chapter's contents, and a set of end-of-chapter exercises.
The order of topics for a first-semester course are logical when using an atoms-first approach. The order of topics for a second-semester course (thermochemistry, thermodynamics, equilibrium chemistry, and kinetics matches my preferred structure. Those faculty members who prefer to cover kinetics before thermodynamics and equilibrium chemistry, or between thermodynamics and equilibrium chemistry, should have no difficulty adapting this textbook to that order.
The on-line interface is easy to use and the search box and embedded links are useful. The one limitation to the interface is the amount of text that is displayed. On my monitor the banners at the top of the page, while not large, nevertheless reduces the amount of text that is visible. Many of the figures are sufficiently large that they are not completely visible unless zooming out, which can result in text that is too small. In almost all cases, the figures could be reduced significantly in size without affecting the figures utility to the user.
I did not find any concerns in this area.
The textbook appropriately places its primary focus on the technical details of chemistry. There are several Portraits of Chemists, which highlight the contributions of a reasonably diverse set of chemists; these are few in number, however.
Overall, the quality of this textbook is similar to the commercial textbooks we have used in the past. The one area where this textbook could improve, is in the end-of-chapter problems, which include relatively few challenging problems or problems that ask students to draw on multiple concepts within the chapter or between chapters.
Reviewed by Ann Taylor, Professor, Wabash College on 7/17/19
The book covers all the topics found in a full year general chemistry course. There are additional appendices with help for students who need to review mathematical concepts or dimensional (unit) analysis. read more
The book covers all the topics found in a full year general chemistry course. There are additional appendices with help for students who need to review mathematical concepts or dimensional (unit) analysis.
I did not find any major errors in the book.
The canon for General Chemistry is well enough established that the book will remain relevant.
The examples used are clear, and there is sufficient detail without being overwhelming. I particularly like the chapter on measurement and significant figures; they clearly walk through how to estimate one more place than an analogue instrument is calibrated.
Terms are used consistently throughout the book
We teach "atoms first" general chemistry starting from the nucleus, which meant I taught Chapter 20 relatively early (the first two weeks) of the course. There was assumed knowledge of kinetics, and they acknowledge it ("Since first-order reactions have already been covered in detail in the kinetics chapter...") This is appropriate, because re-deriving kinetics equations would be redundant for those using the book in the published order, and by stating where the information can be found, the reader can easily find that section and learn it.
This is a standard arrangement of topics for an atoms-first Chemistry textbook.
There are several benefits of the book's interface. There are embedded applets/videos/online experiments called "link to learning" that allow students to visualize processes and conduct experiments, some of which are included in the end of chapter questions (for example, Rutherford's gold foil experiment in section 2.2 and questions 8c and 9d in the exercises).
Correct grammar is used throughout.
There are very few images of people in the book, and the ones pictured are the scientists who made substantial discoveries.
I used this online textbook in my General Chemistry course last year. The student reaction was generally positive. The availability of a print copy is adventageous, as some students preferred a paper copy.
Reviewed by Kalani Seu, Associate Professor, Earlham College on 5/14/19
This text covers all of the major topics for an introductory chemistry course with relevant reference tables and review material when needed. read more
This text covers all of the major topics for an introductory chemistry course with relevant reference tables and review material when needed.
From what I have looked at, the material seems current, accurate, and relevant.
The material is relevant for a two-semester (1yr) general chemistry course.
The text is written clearly and concisely. The figures were clear and complimented the text well.
The framework, formatting, and organization all seem to be consistent and easy to follow.
The material was broken up into multiple sub-sections with Key Terms, Key Equations, Summary, and Exercises sections for each chapter.
The material is organized logically similar to most other textbooks I’ve worked with or seen. However, in an atoms first approach, it may make sense to cover Nuclear Chemistry earlier in the text (after Ch.2). Also, I don’t think that the chapter on Organic Chemistry is necessary, as students will see this material when they take an organic chemistry course.
I really like the online interface. The ‘Contents’ button and scroll bar at the top of the page made it really easy to navigate through the textbook and to jump between various section and sub-section. This also makes it easier to link the textbook to a course webpage.
When looking through this text I did not notice any grammatical errors. However, I would not be surprised if some minor errors and/or typos are found, as this is common with any textbook that is used.
The content that I looked at was unbiased and I did not see anything that might be considered culturally insensitive.
I liked the step-by-step diagrams to help students map out how to solve problems and appreciate the use of dimensional analysis. I also liked the “LINK TO LEARNING” sections that provided visualizations or an interactive interface to further learning and exploration of various topics. It’s also nice that the practice problems are separated by the chapter sections from which those problems originated.
Reviewed by Divina Miranda, Adjunct Professor, Southern University on 5/1/19
The textbook is quite comprehensive and covers the major topics in an introductory chemistry course. It provides, index, glossary, and appropriate tables (thermodynamic quantities, equilibrium constants, etc.). read more
The textbook is quite comprehensive and covers the major topics in an introductory chemistry course. It provides, index, glossary, and appropriate tables (thermodynamic quantities, equilibrium constants, etc.).
The book's chemical and mathematical content appears accurate however there are key ideas that are not discussed in enough detail for students to assess their importance. One of these is the exceptions within electron configurations, specifically the difference in the normal pattern of electron configurations of Cu and Cr atoms.
The contents are up-to-date and it also talks about current technologies that can be explained by relevant concepts. Its detailed descriptions of the properties of "interesting" atoms and compounds like hydrogen and ammonia) can provide extra motivation for students to love chemistry!
The written text and the problem-solving strategies are fairly clear and straightforward.
The format of the textbook is fairly consistent: within most topics is a learning objective, text, examples, key equations, summary, and end-of-chapter problems. The book's terminology and voice remain consistent throughout the chapters as well.
The topics are clearly broken into sections and subsections. For topics that can overlap like thermodynamics and thermochemistry, concepts were clearly explained in terms of the topic in each area.
The topics are presented in a logical fashion, beginning with scientific method and measurement, progressing to the elementary structure of the atom, stoichiometry and reactions, energy, more detailed structure of the atom, periodic trends, bonding, molecular shapes, phases of matter, kinetics, equilibria, thermodynamics, electrochemistry, and nuclear chemistry.
Images and tables are clear enough for use.
The text is relatively free of grammatical errors; however, equilibria is generally used as the plural of equilibrium.
The text is not culturally insensitive.
I highly recommend the textbook for a two-semester General Chemistry course. It accurately introduces fundamental concepts in a relatively understandable manner. Step-by-step solutions to problems like balancing chemical equations are included. More colorful diagrams could be added though.
Reviewed by Ray Mohseni, Lab Director/Assistant Professor, East Tennessee State University on 4/22/19
This text book is comprehensive and covers more materials that what needed for two semesters of general chemistry course. The content is well organized and students with a range of chemistry background can benefit from it. read more
This text book is comprehensive and covers more materials that what needed for two semesters of general chemistry course. The content is well organized and students with a range of chemistry background can benefit from it.
Based on a the first four chapters I looked at, the material is very accurate.
The textbook is relevant for a general chemistry course. The different concepts are well organized in details.
It is easy to comprehend with real-world examples that students could easily relate to.
It is very consistent based on the chapters I looked at.
I like the option of moving the topics around within a chapter. This flexibility allows the instructors to modify the chapter based on the student's chemistry background in the classroom.
The organization of the textbook is very similar to the textbooks from the main publishers,
I use the pdf version for the review, and did not find any issues.
Reviewing the first five chapters, I did not found any grammatical errors. But if I do, I will not be surprised. Based on my experience writing a lab manual, I can still find grammatical error after even the third edition.
More diversity can be used in photos and graphics.
I was honestly a little hesitant to review an open chemistry textbook to begin with. I am glad, however, I got a chance to do it. This textbook is as good, if not better, than the textbooks by publisher. I am seriously considering it for my general chemistry sequence courses.
Reviewed by Melissa Rhoten, Professor, Longwood University on 3/20/19
This text covers all of the material normally encountered in a year-long general chemistry course. It also includes relevant tables (equilibrium constants, thermodynamic parameters, etc), review of mathematical concepts, and unit conversions. read more
This text covers all of the material normally encountered in a year-long general chemistry course. It also includes relevant tables (equilibrium constants, thermodynamic parameters, etc), review of mathematical concepts, and unit conversions.
I found no errors associated with the content of the text.
The content is current and representative of knowledge and theories used in teaching chemistry at an introductory level.
The language used is clear. However, the authors used the following style often, " Figure shows a sample of liquid bromine at equilibrium with bromine vapor...", where the word "Figure" is hyperlinked. This example is taken from Chapter 15. In the text there should be a figure number (in this case Figure 4). When you click the word "Figure", you are taken to the appropriate image/graph/table. You can use the back button, which does not alway return you to the exact spot where you were before clicking to see the figure.
I found no issues with the text's use of terminology and framework.
Each chapter is broken down into smaller subsections, which makes it easy to assign small portions of reading at one time.
THis text seems logically organized especially given its focus on an atoms first approach.
I found no interface issues when accessing this text.
I saw no grammatical issues.
I saw no culturally insensitive or offensive references in this text.
Reviewed by Joseph Villa, Associate Professor, Umpqua Community College on 1/24/19
The text was very comprehensive. Chemistry: Atoms First serves as an effective introduction to physical science techniques and manners of thinking. All of the material included in a traditional two-semester general chemistry course is here. read more
The text was very comprehensive. Chemistry: Atoms First serves as an effective introduction to physical science techniques and manners of thinking. All of the material included in a traditional two-semester general chemistry course is here.
Content is accurate, error-free and unbiased. I found no issues with the text.
Fortunately, all OpenStax textbooks are web-based and can be updated periodically when deemed pedagogically necessary. The process to submit corrections is easy to navigate. The reader can find a list of past changes on the specific book page on OpenStax.org. Necessary updates appear to be relatively straightforward to implement.
The text is written in a clear, comprehensible language. When it is necessary to use jargon or technical terminology, appropriate context is provided.
The text is internally consistent in terms of terminology and framework.
The chapters and topics are broken down into small enough sections it makes the text is easily and readily divisible into smaller reading sections which can be assigned at different points within the course. Because the book is openly licensed, the instructor has the freedom to use the entire book or pick and choose the topics most relevant to the needs of their individual course. Additionally, chapters can be taught or read in a slightly modified and preferred order without presenting much disruption to the reader.
This title is an adaptation of the OpenStax Chemistry text. It has been reordered to fit an “atoms first” approach. Atomic and molecular structures are introduced much earlier than in other approaches I have taught. The introduction of more abstract material is delayed so students have time to acclimate to the study of chemistry. Topics are introduced within the context of familiar experiences whenever possible. Chapter sidebars entitled ‘Chemistry in Everyday Life’ tie chemistry concepts to everyday issues and real-world applications of science students encounter in their lives (i.e., cell phones, solar thermal energy power plants, plastics recycling, and measuring blood pressure).
The text is free of significant interface issues, including navigation problems, distortion of images/charts, and any other display features which may distract or confuse the reader. The web-based nature of the title allows instructors to provide a direct link in their syllabus to the sections in the web view of the book. Throughout the text, there are features drawing the students into scientific questioning by taking selected topics a step further. The text has numerous clear, effective illustrations, diagrams, and photographs. Chemistry: Atoms First also has added links to relevant interactive exercises and animations helping bring topics to life through the ‘Link to Learning’ sidebars of the chapters.
I found no grammatical errors in the text.
The text is not culturally insensitive or offensive in any way. It tries to make use of examples that are inclusive of a variety of races, ethnicities, and backgrounds. Sidebars entitled ‘Portrait of a Chemist’ present a short bio and an introduction to the work of prominent figures from history and present day so students can see the “face” of contributors in this field as well as science in action. Many of the historical references lack diversity, but most of the early historical scientists were males of European descent. This is no fault of the publisher or authors.
I will definitely start using this textbook as my adopted text when I start teaching the introductory chemistry course this coming fall. I am very happy with the other OpenStax Chemistry text that I have been using in my General Chemistry class as well. My students like it as well.
Reviewed by Chris Saunders, Clinical Assistant Professor, Boise State University on 1/10/19
This is book is fairly well representative of what you will find in most Atoms First textbooks. There is plenty of coverage of the fundamental topics that you would expect in a full year of chemistry at most institutions. Later chapters provide... read more
This is book is fairly well representative of what you will find in most Atoms First textbooks. There is plenty of coverage of the fundamental topics that you would expect in a full year of chemistry at most institutions. Later chapters provide more specific topics for instructors to use depending on the structure of the course. Ch. 18 and 19 are a nice addition that aren't always found in other textbooks for instructors looking to go a bit more in depth in some topics.
On my first reading, I did not encounter in obvious issue with accuracy or bias.
The book provides a well thought-out framework that will allow for easy updating and adaptations. Much of the fundamental concepts covered haven't drastically changed in many years but the examples put forth in this text are up-to-date and relevant to today's students. It's nice to see a text where the fundamental concepts are solid, but the new ways in which these concepts are being applied is addressed.
I did not encounter any clarity issues. The use of examples throughout the text allows students to read and see examples in a timely manner, rather than referring all practice until the end of a chapter. The end-of-chapter summaries help with understanding new terms presented in that section.
Each chapter feels similar in its overall construction which is important in helping students learn how to effectively use a particular text. There are some times where I noticed that certain chapters treated subjects as new (chemical formulas) while they had been previously introduced (not in as much depth) in a previous chapter. I think a better effort could be put forth to make sure these prior topics are referenced back to more clearly.
The book was well thought out in regards to which topics were organized into each chapters. In many cases, this thoughtful organization improves upon other versions of this type of textbook. This scheme would allow most instructors to easily move around in the text in their own order if they prefer.
Within individual chapters, each section is broken up effectively with useful references and examples.
I think that most instructors of general chemistry would agree that this is one of the areas where textbooks can truly set themselves apart from other textbooks. While there isn't any new organization here that is particular groundbreaking, I did find enough thoughtful changes to appreciate the effort the authors have put into organizing the text. I particular found the organization in the first 5 chapters to be effective. The later half of the book has some chapter ordering that I wouldn't personally use, but the modular nature of the book renders this a moot point.
I have had no issues crop up with using the web version or pdf version.
As with any text of this nature, I am sure there are errors, but I have not found any in my readings so far.
There was nothing about the book that was offensive, however I think there were missed opportunities in some of the choices that were made about which scientists to highlight. More examples or highlights of important scientist of a wider cultural variety would be nice to see.
Reviewed by Alan Szeto, Assistant Professor of Chemistry, Fort Hays State University on 11/30/18
Being a textbook at the "General Chemistry" level, CHEMISTRY: ATOMS FIRST carries the hallmark of any book bearing "General Chemistry" in its title in terms of having 20 or so chapters (which can be easily divided into two halves for a... read more
Being a textbook at the "General Chemistry" level, CHEMISTRY: ATOMS FIRST carries the hallmark of any book bearing "General Chemistry" in its title in terms of having 20 or so chapters (which can be easily divided into two halves for a two-semester course sequence in General Chemistry I and II) covering all of the essential topics that the faculty expect students to have mastered before taking Organic Chemistry. There is no obvious omission or addition of topics in ATOMS FIRST, for those who are very familiar with the General Chemistry curriculum.
Content Accuracy rating: 3
With 20 or so chapters, ATOMS FIRST begins with the "essential ideas" in the definition of matter, physical and chemical changes, significant figures, SI and metric systems of units, and dimensional analysis. Then, a chapter on "atoms, molecules, and ions" is followed by three consecutive chapters on electronic structures and periodicity of the elements, chemical bonding, Lewis structures and molecular geometry, and advanced bonding theories. The focus returns to macroscopic matter immediately in the next chapters in discussing the mole concept, empirical and molecular formulas, molarity, and reaction stoichiometry. The next set of chapters are gases, thermochemistry, solids and liquids, and solutions. While the "switch" from atomic and molecular to macroscopic occurs as the mole concept (the Avogadro number) is introduced to bridge the gap between the "very small atoms and molecules" and "how many of these atoms and molecules would be necessary make up matter that we can see, feel, and touch," other General Chemistry textbooks traditionally do not discuss quantum mechanics until after gases and thermochemistry. It is not clear whether there is a distinct advantage in ATOMS FIRST over the other General Chemistry textbooks to bring home the message that molecular structures (Lewis structures and molecular geometry) do offer further insights into the explanations of different states of matter (solids, liquids, and gases), other physical properties of matter, and chemical reaction mechanisms although placing quantum mechanics earlier in the book does encourage faculty and students to concentrate more on the importance in understanding atomic and molecular structures before all other topics.
The chapters in ATOMS FIRST can be easily re-arranged and taught in various different sequences. Updates can be made and new materials can be incorporated without altering significantly the original presentation. In this sense, the book is robust and should withstand the test of time.
The major concepts of chemistry are introduced with proper amount of contexts.
Consistency rating: 3
Many students find chemistry a difficult subject because of the constant changes of topics from atomic and molecular to macroscopic levels and back. As commented on earlier under "Accuracy," it is not a matter of whether ATOMS FIRST is consistent or not in the use of language but rather it is able to help students overcome the inability (and fear) of following the changes of topics without getting lost. An ideal chemistry textbook "should" help students overcome this challenge so that it becomes natural for them to switch between atomic/molecular and macroscopic levels as various physical and chemical systems are addressed.
The chapters in ATOMS FIRST are individually well organized.
Organization/Structure/Flow rating: 2
Once again, ATOMS FIRST forces the audience to learn quantum mechanics, chemical bonding, and advanced bonding theories before the mole concept and stoichiometry. It is not certain if such organization (or re-organization) without further support in subsequent chapters can make ATOMS FIRST more effective serving as a General Chemistry textbook than others.
The conceptual and quantitative exercises are nicely and appropriately inserted throughout each chapter. Navigation from section-to-section and chapter-to-chapter is easy via the table of contents and the "search" function.
No grammatical errors
Not culturally insensitive
Reviewed by Sathiamoorthy Balasubramanian, Chemistry Instructor, Rochester Community and Technical College on 5/21/18
This text book is adequately comprehensive and covers all chapters for two semester general chemistry course. The content is well organized and explained clearly. This text book will be useful even to those students who don’t have any chemistry... read more
This text book is adequately comprehensive and covers all chapters for two semester general chemistry course. The content is well organized and explained clearly. This text book will be useful even to those students who don’t have any chemistry background.
Appears accurate and error free on basic reading.
This text book is highly relevant for a general chemistry course. The various concepts are well organized and explained in detail. This book can be used by a student to self teach the various fundamental principles.
This text book is easy to understand and is supplemented by excellent examples. I would specially highlight the various examples given in the text book as they are the tools which lead to clear understanding.
Is consistent throughout
The authors have well thought out the various sections in the text book. One can use any of these sections in providing assignments to students.
The highlight of this text book is that the content has been well laid out with relevant diagrams and flowcharts. It’s easy on the eye and one can navigate around comfortably.
I used the pdf version and had no issues navigating the text.
Nothing to comment.
Not applicable
The authors have done an excellent job in presenting a text book which is self explanatory. Would recommend to my general chemistry course students.
Reviewed by Maury Howard, Associate Professor, Virginia Wesleyan University on 2/1/18
Overall, the text covers all of the major areas one would expect in an introductory text book in an atoms first approach. The depth of coverage was appropriate to first year college students even at varied skill levels. There were only minor... read more
Overall, the text covers all of the major areas one would expect in an introductory text book in an atoms first approach. The depth of coverage was appropriate to first year college students even at varied skill levels.
There were only minor issues observed upon a single read-through, primarily in the introductory chapters. For instance: Metallic bonding is not mentioned in bonding chapter (addressed briefly in Ch. 10, then omitted from glossary) also metallic compounds / their properties is not in addressed in early chapters. Applying accuracy and precision to measurement and measuring tools would be valuable.
This text appears to be in agreement with most general chemistry texts. I appreciated the historical perspectives in the earlier chapters and evolution of modern atomic and quantum theories. For the most part the topics were well introduced and thoroughly treated with multiple examples and often supported by engaging visual and interactive links.
However, I had a problems with the treatment of thermal energy in the textbook. Both accuracy and clarity were at issue, a couple of specific instances: I do not agree with referring to absorption and emission of photons as endo and exothermic. Thermal energy is not kinetic energy, it is heat. Heat is thermal energy, not energy flow/transfer (as defined in the text), otherwise, how can an objects release or absorb heat as described later? (p.474)
A few additional, specific errors noted: Fig 8.5 incorrect height is marked on third manometer. Ex 5.2 Is something missing? "...we divide..." by what? Fig 5.3 (typo) - sigma p orbital lower in energy than sigma p (should be pi p).
I thought the examples provided were particularly engaging and well suited to today's students. The content, as written could be readily updated.
For the most part, the text was well written and the concepts broken down well and explained thoroughly for students. The examples worked out in the text are well conceived and descriptive. And, I particularly like the calculation flowcharts provided for the quantitative treatment of reactions and materials.
A few unclear points to mention: What to call the formula of ionic compounds is not addressed (formula unit?)... the text mentions they are not molecular but, also not simply empirical, but suggests no term -- confusing.
The introductory sections for Ch 9 and 13, specifically, are not clearly written; and almost appear to be summaries preceding each chapter. As such, they are difficult to interpret until the chapter has been completed and terms have been identified and defined. Ch 9: Thermochemistry is not as well written as some of the other chapters (the introductory section being particularly flawed), and has a few other specific issues mentioned previously.
Overall, well done. Specific issues have been mentioned previously with regard to thermochemical and thermodynamics sections (including equilibrium).
The chapters will do quite well as standalone, but some of information provided in the earlier introductory chapters is prerequisite knowledge for the more specific/advanced chapters (and a few of them refer back to prior introduction of topics). There are a few examples of out of place references (one example: Ch 3: description of ideal blackbody refers to ideal gas for clarification, but that has not yet been introduced)
Good flow, as arranged for atoms first approach, although I think stoichiometry, balancing reaction equations and reaction types cold be introduced sooner.
I used the pdf version of the text, and aside from a slow download, had no issues. That said, multiple platforms are required for the interactive links which may result in issues for some students, as they may be using different means to access them. (Flashplayer, HTML5 vs Java - sometimes problematic).
I saw no issues with grammar.
Not a wide net for cultural diversity, but likely as wide as readily cast for historical to modern science demographics.
If the issues with Ch 9 (thermochemistry), 12 (thermodynamics) and 13 (equilibrium) can be resolved, and the chapters revised for clarity, I would readily adopt this textbook.
Reviewed by Richard Farrer, Associate Professor - Chemistry, Colorado State University - Pueblo on 2/1/18
The content of the book mirrors that of the majority of general chemistry books on the market. The last few chapters (18-20) are materials that would lead to future courses. The last few chapters (18-21) are associated with focus on specific... read more
The content of the book mirrors that of the majority of general chemistry books on the market. The last few chapters (18-20) are materials that would lead to future courses. The last few chapters (18-21) are associated with focus on specific elements (metals, metalloids, and nonmetals). Chapter 19 deals with transition metal complexes and Chapters 20 and 21 introduce nuclear chemistry and organic chemistry. Additionally, the book has several appendicies that include thermodynamic properties, equilibrium constants, and other fundamental data. The book does not go overboard with extraneous materials that are fond in several, current publisher texts.
While I have not read the entire text, the book appears to have minimal errors. Additionally the content is both accurate and unbiased -- however I have not come across any recent general chemistry texts that lack accuracy or are biased. All of the examples and problems that I encountered while perusing the book, were complete and accurate.
Again, since this is general chemistry text, the book should have sufficient shelf-life, unless there is a major change in the content that is presented in general chemistry. I don't feel that general chemistry textbooks need to be updated every three years. The book covers the relevant topics that have been the relevant topics for several years. There should be no surprised in the content covered by the book, and the book will not lose it relevance as time moves forward. The online version is also very good, and is probably the first online book that I have read that I prefer the ebook to the printed version.
The book is easy to read and is very clear in its used of terminology and chemical references. Clearly it has the necessary 'jargon' required for a chemistry book, but it does not go out-of-the-way to include undefined or incorrect terms.
The text is consistent in its use of scientific terms and vocabulary. Chemical definitions that are required for a specific topic are provided, and, from what I have read, the book does not introduce materials without first defining the necessary terminology that is used in that chapter.
It appears as if the authors put time into development of the text so that chapters could be moved around with vary little issue. However, I have not tried to do this, and I would guess there will be some issues that cannot simply be moved to any other place in the book. Because chemistry builds on itself, there are situations where the order needs to be specific, and trying to change this would not be good. That being said, it is apparent that the authors have tried to produce a book that is flexible in the order in which things are indroduced before others.
I am an "Atom's First" guy. I like introducing atoms and molecules before moving onto calculations, stoichiometry and kinetics. The authors have done a goo job in presenting the material so that students are required to understand that composition and structure are tremendously important for chemistry to know. If you are more of a traditionalist, then check out the other version of the book that takes the traditonal approach amd is available through OpenStax.
As I stated previously, I found the online book to be easier to read than any other online book I have read. I really like the interface of the book and am and honestly preferred the etext to the printed version (I have both). I think the authors did a very good job of writing a book that could work in both formats. I love the scrollable material that is in the etext. There are very few books that I would prefer to read off of a computer screen or a tablet (or phone), but this book was nice to read online.
I have not found grammatical errors.
This is a good question, and I have not done the majority of the problems, and therefore have not seen if the end of the chapter problems present any culturally issues. The parts of the text, that I have read, do not have any culturally relevant issues.
We have been looking for a book that we could provide to the students free of charge. There is so much material out on the internet that, if a student was saavy, he/she would be able to find the find the material posted online as either pdfs or videos. I love the theory of open source books for, at least, the lower division courses. The materials covered in this book are exactly those covered in any descent general chemistry text available today. We have made this text available to our students in an order to keep unnecessary costs to the students to a minimum. I also like that the copyright allows the user significant flexibility in the addition, removal, and reordering of the published material -- it will allow for us to adjust things to fit our students and our faculty.
Reviewed by Alicia Glatfelter, Adjunct Professor, University of North Carolina - Asheville on 8/15/17
All areas typically covered in a two-semester General Chemistry course are included in appropriate detail. read more
All areas typically covered in a two-semester General Chemistry course are included in appropriate detail.
There are a few inaccuracies pointed out by other reviewers that I won't rehash. Chapter 13, in particular, has poor definitions of Q; the entire beginning of Chapter 13 is very poorly done, enough so that I will not use the text, although the rest of the chapters I examined seem quite satisfactory.
Content is up-to-date and should continue to be relevant for quite a long time.
For the most part, the book is very clearly written and easy to follow. There are a few exceptions where terminology is used before the terms have been introduced (noticeably, exothermic/endothermic), likely due to adapting a standard text to the Atoms-First approach. Chapter 13 is an exception - the beginning of it, especially, is very poorly done. My hope is that one of the senior contributing authors will take the time to rework this material.
For the most part, this book is consistent, with a few instances of terminology being used before being properly introduced (for example, endothermic/exothermic).
The textbook is divided into sections within each chapter, each of which would be an appropriate reading assignment. I believe it adapts itself well to using some of the chapters out of order, if desired.
The organization and subdivision of topics in the book is well-done, logical, and clear.
I found no interface issues in either the online version or the pdf.
I didn't read the entire text, but I read enough to convince myself that the authors have a good grasp of the English language - I found no errors in any of the sections that I examined.
Cultural relevance in a subject such as chemistry is fairly difficult to achieve, as chemistry has been mostly dominated by dead white guys. I found no examples of cultural insensitivity.
Overall, the book is well-done. The topics are covered in satisfying clarity and depth. I've been teaching an atoms-first approach from a standard textbook for several years, and now prefer this approach. There are lots of illustrations which I feel are very helpful and informative, and even links to videos and simulations. There are substantial end-of-section problems, some of which include the answers. Two factors are significant enough to prevent me from adopting this text. One is the lack of numbering of worked-out in-text examples and end-of-chapter problems. I don't see how I could assign the end-of-chapter problems when they aren't numbered. This should be a fairly easy change to implement, and I imagine that most users of the textbook would appreciate the examples and problems being numbered. The other problem is Chapter 13. I only paid attention to the chapters I use in teaching the first semester of General Chemistry, and the chapters on equilibrium. All were fine except Chapter 13, which badly needs some attention by one of the senior contributing authors before I would consider adopting the textbook. If these two factors are addressed, I would definitely want to use this book in my course.
Reviewed by Abha Verma, Asst. Prof., Xavier University of Louisiana on 6/20/17
It is indeed a good book for students who are starting out chemistry at college level because a wide range of learners and students of different backgrounds can follow it. Graphic Visualizations along with some other audio tools, make this book... read more
It is indeed a good book for students who are starting out chemistry at college level because a wide range of learners and students of different backgrounds can follow it. Graphic Visualizations along with some other audio tools, make this book interesting and will enhance student learning. This will help understand the concept better.
This is a very well written book with very very few grammatical mistakes. However, I did not solve for all the exercises and problems to see if all the answers were correct. Some statistical figures and periodic tables need to be updated.
Most of the text had enough and to the point information, which is a great asset, most of the time. Some learners, however may feel that the concept was not explained in details.
Clarity rating: 3
The text is written in lucid and understandable language. I would however want to point out that there were certain sections where there was a lot of text and no illustrations to clarify. For example in the periodic table section, a new student of chemistry may get bored by just reading about how elements are classified. The authors may give some tables or visual illustrations to show the relationships.
The text is fairly consistent throughout.
The different sections and topics could be assigned to students individually since most of these are autonomous.
The flow of this text is similar to any other general chemistry. However, it can be customized according to course requirement.
Most of the text has no interface issues, except when calculations are shown and the text size is very different from the rest of the text. Otherwise, the resource links were quite easy to open.
As I have mentioned earlier in accuracy, this is a very well written book with very very few grammatical mistakes.
Based on cultural relevance, this text is as would be any other general chemistry book. Most of the examples are from North American continent and may not be understood by international students.
Overall, this book can be used for learning core concepts of chemistry for a wide audience starting at college level.
Reviewed by Gary Mort, Chemistry Instructor, Lane Community College on 6/20/17
It's fully and adequately comprehensive. The General Chemistry curriculum is nearly de facto standardized. It's rare to find a text that overshoots the mark (unless, and they're labelled as such) for an "Honors" class -- usually, ironically as a... read more
It's fully and adequately comprehensive. The General Chemistry curriculum is nearly de facto standardized. It's rare to find a text that overshoots the mark (unless, and they're labelled as such) for an "Honors" class -- usually, ironically as a "Principles" text. It's somewhat more likely that a text will undershoot either in breadth (rarer) or depth (moderately common). This text falls well within the mainstream for freshman texts. Internal reference materials are adequate (and in any case neither could, nor should, be comprehensive). You don't have to "go somewhere else" for needed material, students should be learning that they will need to do that in any case.
Personally I'd rather work with a text that "goes too far" (within reason) in depth than one that undershoots. Students are generally (nearly universally) accepting/grateful for "don't worry about that", they're less accommodating with "well we'll need to dig deeper on this". (It is, after all, literally, "not in the book" . . .) I'd wind up doing some picking and choosing, omitting a handful of things near the end of the text, as I nearly always do, in favor of slowing down and covering more thoroughly some of the more central/core topics.
It's less a flaw than a question of style, but if I were writing my own book? there would be a bit more depth here and there, and a few less topics overall.
I found no meaningful errors of fact or logic. The presentations are sound. They represent current thinking in both meaning and presentation.
The text takes the "near timeless" approach. The examples are germane, but have long lives -- they're not driven by the news cycle. This seems entirely appropriate/acceptable. It's up to the instructor to provide social context for the value of any particular topic at any particular point in time. The text is a sound summary of the chemical underpinnings of much of the world around us, it is not, and need not be, "Chemical and Engineering News".
Noticeably written "in English" without sacrificing technical clarity or the need for specificity in definitions and terminology.
The book feels seamless. There are no noticeable differences in voice or approach. (Something that is noticeable in a fair number of multi author texts.) And it doesn't feel "over edited" (also common, particularly in high number editions.) While not exactly "fresh" (which can also include "raw" and "grating") the approach is clean, smooth, not mechanical.
It's fundamentally entirely modular. Sections and be used or referenced as needed/desired, and they're in coherent, but digestible chunks. It doesn't lend itself to "read this page/paragraph" or whatever, but in the context of this class a section is a "light" assignment. As with any rearranging done by an instructor (instead of an author) the content editor needs to keep a wary eye on concepts being in place as they become needed.
The topics, while clearly delineated, flow seamlessly together. Enough so that it's entirely possible to read from section to section (ignoring "homework") without disruption, which carries through, where it's appropriate from chapter to chapter. For example you can start reading Chapter 12, and find yourself thinking "this seems like an odd thing to find in the "Thermodynamics" chapter, only to realize that you've been reading Chapter 13 ("Equilibrium") for some time now and hadn't noticed the switch over.
The atoms first approach has appeal and power. Whether or not it would suit the individual's or institutions needs/abilities is a somewhat different question, but done well, and it is here, it's an approach that students seem to flow with better. You start with some broad macro, definitions, math and then start building chemistry from the atom up. The traditional approach is driven in part by history (this is how we figured this out in the first place) and in part by "they need to get into the lab, and understand what they're doing when they get there". The last is a challenge for someone unwilling or unable to respond to "yeah, but they also need to understand that a fair amount of science is a sort of informed (sometimes better, sometimes worse) fumbling around in the dark." We often place great value on that in nonmajors classes, that sense of "this is what it means to do science" isn't well conveyed to the very students we're proposing to send forth to do it.
All interface elements worked well. No distortions were observed. The display worked well (on both a large desktop monitor and midsize laptop). I did not evaluate the "paper" (PDF) version in print or online.
While it's not the intent of the "open" movement, reading the text online made me wish for "jump ability" -- being able to get back to a ICE table (without remembering what it's called) when I need to do so several sections after its use is illustrated. It is a text, not a Wiki, but if you work online much "hyper available" information becomes normal. I don't think that would even occur to the print user (except maybe as "I wish this was online").
It's remarkably smooth and clean.
Chemistry is heavily populated by what I describe as "dead white guys". This text is representational of that. It doesn't seem to reach for examples outside of the mainstream, but maintains a kind of content neutrality. "These people did these things, the things are ultimately what matter."
Unless you've got some particular (and possibly peculiar) approach or goal in mind you can use this text to teach an entirely sound General Chemistry. I could literally switch to this text today -- late in the Gen Chem year, and walk in to the classroom without missing a beat. It isn't "exciting" (rare in any case) but neither is it dull (common). It is both sound and accessible. Homework is more than adequate. The illustration program is good. The structure of the text will be familiar to anyone that's taught Gen Chem. It's certainly more than "good enough".
This is a good book. Worth adoption.
Reviewed by Thomas Sommerfeld, Associate Professor, Southeastern Louisiana University on 6/20/17
The table of context suggests an interesting atoms-first text text for science majors, since the order is a bit more courageous than usual, with thermodynamics very early and kinetics last, but I think this is an approach well worth trying out.... read more
The table of context suggests an interesting atoms-first text text for science majors, since the order is a bit more courageous than usual, with thermodynamics very early and kinetics last, but I think this is an approach well worth trying out. Unfortunately, the authors do not implement their approach, as, for example, core ideas from kinetics are still heavily used in the equilibrium chapter (see below). Another great idea for reordering content is to put the section comparing bond strengths of covalent and ionic substances at the end of the energy chapter. Again, unfortunately, the authors stop, however, with the lattice energy of just one compound and do not bother to convert that into an per cation/anion contact amount so that no real comparison is possible. Be that as it may. A real shortcoming of the book is the incomplete copy-and-paste job. This book started out as a traditional macroscopics-first book, and the authors rearranged the material into a second atoms-first book. There are many problems that remain to be fixed from an incomplete eagle’s eye proofreading (see below). A second serious shortcoming of the book, which becomes especially obvious when one takes a look at the more advanced topics, is that most of the material has been strongly simplified in comparison with other typical texts for science majors. Therefore this text seems to be more of an advanced non-science major book. Since each chapter has a glossary, I do not think that would be a problem, however, Appendix B: Essential Mathematics is poorly written and does not cover the material necessary for text.
Content Accuracy rating: 2
Of course, one only really starts noticing errors once one is teaching out of a book. Here, I flipped through a couple of chapters, and took a brief look. This is far from the careful eye of an editor. Fig. 2.18 should be before Fig. 2.16 and 2.17, and the latter figures need to be extended to indicate that atoms are either represented by letters or by spheres. Section 2.4 is largely incomprehensible as placed in the text. This is probably due to the cut-and-paste job the authors used to rearrange their macroscopics-first into an atoms-first textbook. Page 124, photoelectric effect: “Somehow, at a deep fundamental level still not fully understood, light is both wavelike and particle-like.” Somehow textbook authors seem to keep copying other freshmen texts. The nature of light is fully described, the theory is called quantum chromodynamics, whether that means anyone understands it is as debatable as quantum mechanics for atoms. However, in most advanced quantum chemistry book the photoelectric effect is treated without quantum chromodynamics ever being mentioned. In other words, light is treated as a classical wave, the metal is treated quantum mechanically, and it works. On the other hand, there are wonderful experiments showing that C60 and porphyrin rings also undergo double slit scattering. Why, oh why use totally misleading examples when there are phantastic chemical ones? Fig. 3.20 Highly misleading. Smells of the Bohr model. Fig. 3.21 Many problems. Coloring just the region with the phase information gives a pretty graph, but it needs careful discussion and a clear caption. Some goes for the radial density plots. A common y-axis for all three graphs, potentially with a scale, would be a plus. Page 464 ..., Energy: “Heat is usually released or absorbed, but sometimes the conversion involves light, electrical energy, or some other form of energy.” Brutally misleading sentence. Neither light nor electrical energy are heat, and “some other form of energy” is surely not heat either. “Thermal energy is kinetic energy associated with the random motion of atoms and molecules.” Not so. Thermal energy is the temperature dependent part of the internal energy. This energy may be stored in kinetic energy, but it can also be stored in vibrations, which are half kinetic and half potential, or it may be stored in totally different degrees of freedom such as spin-like components. Fig 9.6 The depicted process is correct only if the two substances have no internal degrees of freedom, for example, one rare gas, one diatomic gas would not work. Entropy Chapter: Page 661: Explanation of S = k ln W for large N incomprehensible, unless “macrostate” is also introduced, and the formulas for the Ws of the most likely and least likely macrostates are derived or at least given. Fig 12.11 right panel is wrong: S should increase as a log function (not linearly) Section 12.4 “Free energy is a state function, and at constant temperature and pressure, the standard free energy change (?G°) may be expressed as the following ...” This is highly misleading. The point is that P=Psys=Psurr and T=Tsys=Tsurr, not, say, simply Psys=constant. Moreover, in the whole chapter and in the subsequent chapter the standard and non-standard quantities are not cleanly distinguished, which may be considered overly pedantic, but is of course crucial on page 711 in the subsection “Free Energy and Equilibrium”. Equilibrium chapter: Kinetics is introduced last, but equilibrium is still motivated with kinetics (Fig 13.2, 13.6). This can, of course, not work. Something has to give. Fig 13.11 Highly misleading. (1) the position of K is not only determined by Delta-G0, but also by the mathematical form of Q itself. (2) The authors leave out Delta-G, which is the slope of the G(reaction progress) curve.
Freshmen chemistry is a pretty settled subject. Thus, one should not think it is among the subjects needing frequent updating, and for most of the text that seems indeed to be true. Alas, most textbook authors present atomic and quantum theory as if we still lived 1935, and the current book is unfortunately no exception. Large quantities of chapters two and three belong into the poison cabinet of a few brief “historical remarks” boxes. Yet, I would support keeping this material only, if there were “historical remarks” boxes equally introduced in the remaining text. That is for gases, energy, acids and bases, redox processes, etc… .
Much of the text is written clearly. However, there are various sentences, which are unclear are even incorrect (see above); paragraphs or sections, where concepts are not sufficiently explained (e.g. section 2.4); and subsequent paragraphs without obvious connection. The latter two issues are, again, most probably the consequence of taking a traditional macroscopics-first textbook, and copy and pasting it into an atoms-first textbook. All three need quite a bit of work before I would use the text in my classroom.
As far as I noticed the text is mostly consistent, but that is far from any guarantee, and owing to the copy-and-paste job into an atoms-first book I would be careful. The only obvious problems that caught my eye were the issues of clearly distinguishing standard versus non-standard thermodynamic functions as well as the energy and the enthalpy occasionally.
Modularity rating: 3
The text can be easily divided into chapters and sections. Yet, most sections have subsections, and those are first of all not numbered, second, not part of the table-of-contents, and therefore-and most importantly-not part of the pdf (or html) index. This is a huge missed opportunity the authors should improve upon.
The flow of topics follows mostly the standard “atom-first” approach, however the authors chose to explore a slightly different order with thermodynamics very early and kinetics last. As I mentioned above, I think this is an approach well worth trying out, but unfortunately the authors do not follow through consequently. For example, the equilibrium chapter still uses kinetics as introduction and in explanations. Similarly, a short introduction to energy is needed before the atomic structure chapter in an atoms-first book; it is missing here.
I used the pdf, and apart from the index, which could have more substructure, it worked fine for me.
Nothing that stood out from the background of content errors and misleading explanations.
The only examples this rubric applies to are the 12 “Portrait of a Chemist” features distributed throughout the text. While it is probably clear to everyone that chemistry as a science has been and is probably still is dominated by white males. Even though we may see a tipping point towards Asia in the future, clearly, if one aims for name recognition, the examples picked by the authors need to reflect that this. However, selecting 10 white, two hispanic, and no asian chemist (provided I counted counted correctly) seems a bit over the top.
Reviewed by Lanette Upshaw, Assistant Professor, Virginia Western Community College on 4/11/17
The book was very comprehensive. The index and glossary were a great asset and reliable. read more
Comprehensiveness rating: 2 see less
The book was very comprehensive. The index and glossary were a great asset and reliable.
The textbook was very accurate. I saw minimal inaccuracies. Most issues were grammatical, which were limited. There were a lot of environmentally related examples, which I love because I use these in class, but not all professors may feel the same.
Examples and images used will work for years to come. Many of the images (both pictures and molecules) are standard that you would see in most any textbook. However, what I like about the online text is that there are external links to many videos, PhET simulations, etc that allow the book to reach a more technologically geared student population. I also like the usage of current examples such as biofuels related to enthalpy, climate change, etc. These may have to be slightly modified over the next decade, but they will still be relevant.
It would be helpful to include more information related to current batteries (fuel cells, batteries in electronic devices, etc).
The book was very clear. Chemistry is a very difficult subject to understand no matter how it is written. I think the multitude of written examples, practice problems, with solutions, and external links will help the students solidify required theories and mathematical equations.
The textbook was very consistent. Every section followed the same outline, which made it very easy to navigate through each chapter. The inserts of pictures and facts related to the topics of the section was very engaging (ex. Gekkos related to intermolecular forces, etc). The extra information included was varied between different disciplines allowing students of most majors to relate to topics (ex. DNA related to biologists and health profession fields, biofuels related to green chemistry and engineering, etc). Hopefully this will help to engage students that otherwise might not normally be interested in the chemistry discipline.
It would be very easy to integrate this online textbook to my current general chemistry courses I teach. Each chapter is also broken down into sections like a traditional textbook.
It would be nice if the solutions were given to all of the problems instead of the first series in each set. Otherwise, the flow of the textbook was logical and consistent.
I did not experience any issues related to distorted images or images that would not appear. Also, all of the external links I clicked on worked with no problem. I did have some issues when I would try to advance to the next section. Occasionally, I would have to go back to the previous page and then click the “next” button to advance to the next section. I had an issue with Figure 1 taking a really long time to load in section 16.4. Not sure if it was from my end related to the internet or an issue within the textbook.
There were minimal grammatical issues. Overall good job on the editing.
Yes. All examples are relatable to a multitude of races and varying disciplines/professions.
The links on certain topics such as SI units and conversions, math review, significant figures, etc will be a great additional resource for students that are struggling with the topic. The PhET simulations were also a very helpful resource for visualizing concepts. It would be very easy to integrate some of these into the lecture linking the online textbook more into the course, hopefully encouraging students to utilize the textbook.
Need to add an expanded table of just polyatomic ions. Many professors use this table to guide students of what polyatomic ions they need to have memorized. I also loved the Eric Brockovich example because I already use this in lecture.
Chapter 6 – There was no summary in the introduction section of chapter 6.
Chapter 7 – I love that the concept of gravimetric analysis is added to the lecture book because we use this as one of three techniques in lab to verify an alum compound synthesized.
Chapter 8 – Great example of the use of diffusion with UF6 gas to enrich uranium.
Chapter 9 – The integration of enthalpy related to combustion reactions and emerging biofuels was excellent.
Chapter 11 – The heating and cooling curve section could be expanded. This is a topic my students often struggle with, and I generally have to work at least two different types of problems to increase their understanding. The expanded section on X-ray crystallography was a great addition to the lattice structure in crystalline solids section. Explanation of the distillation process related to oil was excellent, so students can really understand the process and energy required to refine oil. Also, the section on colloids was excellent because not all current chemistry books include this information.
Chapter 12 – I felt chapter 12 could be slightly expanded to include more information surround entropy, enthalpy, and Gibbs free energy.
Chapter 14 – I like how the information related to acids/bases is in one chapter: weak/strong acids and bases, buffers, and titrations. Many textbooks break this information into multiple chapters so students struggle more to see the connections.
Chapter 16 – The corrosion section was an excellent edition so the students can relate electrochemistry to something they have seen their whole lives.
Chapter 17 – The graph in Figure 5 of section 17.5 will help the students understand the development of the two-point equation.
Chapter 18 – Chapter 18 is generally out of the scope of a general chemistry textbook. However, it does have some great examples of the applications of preparing chemicals or elements, so it would be a nice addition of time allows and/or the professor has a more advanced class.
Chapter 20 – The biological examples related to radioactivity were an excellent addition.
Chapter 21 – Exactly like an organic chemistry chapter in a traditional textbook.
Reviewed by Shoshanna Coon, Associate Professor, University of Northern Iowa on 2/8/17
This text covers all the topics of a typical 2-semester general chemistry sequence for science majors. The order of the topics is designed for an "atoms first" approach, as the title indicates. {This text is a derivative of openstax Chemistry by... read more
This text covers all the topics of a typical 2-semester general chemistry sequence for science majors. The order of the topics is designed for an "atoms first" approach, as the title indicates. {This text is a derivative of openstax Chemistry by three of the same authors, which has the more traditional order of topic coverage, getting into stoichiometry much earlier. } The order of topic coverage after the initial "atoms first" portion is unusual: gases before thermochemistry; thermodynamics before equilibrium; kinetics after electrochemistry. I will reorder the topic coverage if I use the book.
I examined the text in the pdf version (on a PC) and in the online version (on an iPad). Each chapter (in pdf) or subsection (online) ends with a Key Ideas and Equations section and a Glossary (not linked). The index is not as well done--multiple entries for one topics/word for different capitalization or even plural vs. singular. However, the page numbers in the Index are linked (in the pdf anyway).
The Search feature in the online book is case sensitive. For instance, when looking for TED talks mentioned as additional resources, I got no results search for ted or Ted. On the other hand, a search for CO returned results for both CO and Co, which is a problem in my opinion.
Regarding level of coverage, it is largely comparable to other texts I have taught from, including the current "atoms first" commercial book that we are using. Some examples and end of chapter problems do not cover all aspects of a problem, however. For instance, in the section on combustion analysis, there is no example or end of chapter problem for a compound that contains oxygen. Only hydrocarbons are used. In fact, there are no end of chapter problems on combustion analysis at all.
There were also times when I felt a figure could have improved the presentation, such as the initial discussion of diffraction/interference and constructive and destructive interference. There is a very nice photo of an interference pattern, but no figure illustrating constructive and destructive interference, which is an abstract concept that can use some pictorial help for students to understand.
The text has some accuracy problems--mostly in figures or in end of chapter exercises. I did not find any major inaccuracies in the text, outside of a typo here and there. Here are some of the errors I found:
End of section/chapter exercise 1.37c has an incorrect solution given (in the online version). The question asks for SI base units or derived units and the answer: km/s is not an SI base unit or a derived unit using SI base units (as all the other answers were). Should be m/s.
In the discussion of quantum numbers, sometimes the magnetic quantum number was not subscripted.
In the section on successive ionization energies, the text refers to a change of color in the table when the core electrons are being removed. There is no color change in the table (either in online or pdf).
In the section on aqueous reactions, an example is given of the formation of a AgF precipitate and says to refer to the solubility guidelines/rules. But fluoride isn't in any of the solubility rules. How is a student to know that AgF is insoluble? Also in the section on aqueous reactions, gas formation reactions are not included as a class of reactions (or a subclass of acid-base reactions), although there is one example in the chapter of a gas formation reaction.
End of chapter exercise 7.76 refers to phosphorus pentoxide, which is not a substance in the given reaction. Tetraphosphorus decoxide is given in the problem.
Figure 8.5 (pdf) or Fig 4 in Gas Pressure (online): The right-hand illustration is incorrect in showing the "h" that is added to atmospheric pressure in an open-ended manometer.
Figure 8.14 b) in pdf: Axis labels are incorrect. Should be 1/V vs. P or V vs. 1/P rather than V vs. P.
The topics in general chemistry are well-established so the main content of the book will continue to be relevant and have good longevity. One positive feature of the book is the inclusion of connections to other sciences, and connections to technology and the real world. These are current and up-to-date now, but as technology advances and applications change or new applications are found, this is the part of the book that would benefit the most from updating. Since these are set out in boxes within the text, the updating should be fairly easy. The book also includes a "Portrait of a Chemist" feature. As time goes on, new portraits could be introduced. Again, because these are set out in boxes, it is easy to find and update them.
One section that could use a slight expansion of content to be more relevant is Molecular Orbital Theory. The book does not cover anything beyond diatomic molecules, not even with a mention or a figure.
The book is a derivative of openstax Chemistry, by three of the same authors, which has a more traditional order of topic coverage. The authors have paid some attention to making the reordering of topics comprehensible, adding text or bringing sections (such as the mole concept) in early enough to be of use and not confuse the students with concepts that haven't yet been introduced. Despite that, I felt that the topics of electronic structure, chemical formulas, Lewis structures, and bonding theories, showed that they were "out of order". What I mean by that is that they were not written with the level of detail and pedagogy in which early chapters in chemistry books are usually written. They assumed that students were already "up to speed" in their learning process, so to speak. For instance, I think that the relationship of the quantum numbers to the structure of the periodic table could be made more explicit, as could the writing of electron configurations, specifically those of ions. It was here in this section that the reordering showed up in vocabulary--the terms alkali metals, alkaline earth metals, halogens, periods and groups are used in this section, but the section of the text in which those terms are specifically introduced and defined comes later in the book. I suggest that the section on the Periodic Table be moved much earlier in the book--definitely before electronic structure of atoms, but could even come in Chapter 2 right after Atomic Structure and Symbolism.
Another place that I felt the book "got ahead of itself" because of reordering of topics was in Chapter 4, which shows a potential energy curve for the bonding of two hydrogen atoms, but it doesn't explain what 0 potential energy is defined as, or what negative potential energy means. In fact, energy hasn't been addressed much yet at that point (for instance, no discussion yet of potential energy vs. kinetic energy), so there's little context for understanding this figure. There is a section on Energy Basics in the Thermochemistry chapter (chapter 9). I also was not thrilled with the reference in Chapter 3 (in the section on light emission and absorption) to an endothermic process absorbing light and an exothermic process emitting light. This is the FIRST mention of endothermic and exothermic in the book, and to link it with light at this stage is not helpful. Along those same lines, electron affinity is defined so that it's actually Delta U or Delta H, so the EA of Cl is negative, in contrast to other books where EA is the energy released when an atom gains an electron. This may create confusion for those who have had chemistry before. And how to indicate the trend with arrows? Increasing electron affinity means decreasing value of EA (on a number line, anyway).
The discussion of the deBroglie wavelength is ambiguous because of the symbols used. It talks about lambda (sorry, no Greek letters in this text box) meaning something different than it does in light equations, but then talks about v (velocity) vs. nu, which have the same symbol in this particular sentence!). In the online version, the velocity symbol is inconsistent from usage to usage--sometimes it's clearly an italic v and sometimes it could be a nu. In the pdf, v and nu look the same in all usages. So are the authors talking about the lambda symbols being the same, but meaning something different, or are they talking about v and nu?
Another area where some additional attention to topic order would help is in Formula Mass and the Mole Concept, which is in Chapter 6 (Composition of Substances and Solutions). This section is very explicit and should come much earlier in the book. It could be in Chapter 2 along with Chemical Formulas and where the mole concept is first introduced.
The section on Lewis structures was comprehensive, covering basic examples and the important exceptions. However, in the presentation, the authors are working through four examples simultaneously. I think this would be hard to follow for a student who is just learning to draw Lewis structures. I believe it would be better to do those examples individually, highlighting the differences in each one from a simple one--for instance, what do you do when there's a charge on the species?
The discussion of molecular polarity centers on dipole moments and vector cancellation. This is difficult to envision in a trigonal planar and tetrahedral geometry and there was no figure to help with that. There was also no good criteria mentioned for how to decide if a molecule is too symmetrical to be polar. I tell students if the molecule has at least one polar bond and has a definite, unique top, then it's polar. In the Properties of Polar Molecules, it mentions alignment in an electric field and a brief mention (too early?) that polar liquids dissolve in other polar liquids, and nonpolar liquids dissolve in nonpolar liquids. This would be a perfect place to do an early introduction of the concept of intermolecular forces (at least dipole-dipole) to say that polar molecules have higher boiling points than nonpolar molecules of the same size. I did not like the electronegativity difference criteria that the book uses. Their criteria of pure covalent having an electronegativity difference of less than 0.4 means that a C-H bond is considered by them slightly polar covalent. And their cutoff for polar covalent at the high end of 1.8 difference means that HF looks ionic, but it's polar covalent--they mention it as an exception. But a higher cutoff of 1.9 or 2.0 would solve that problem.
One thing that I think would aid clarity throughout the book is more use of bold and italics to emphasize important ideas or statements. Usually only terms are bold. One example that I noticed is the idea that the sum of the formal charges must be equal to the charge on the species. This was buried in a paragraph and not emphasized in any way. It would be easy for a student to miss this important idea.
In general, the layout made it easy to miss a short paragraph sandwiched in between examples. A little more white space to set off the text would be helpful.
I did feel that some topics were done with good clarity. I appreciated the figures of all of the s, p, d and f orbitals. Most general chemistry texts do not include the f orbitals in figures. I also thought the authors did a good job of making an early distinction between ionic and covalent bonds.
The section on nomenclature was good and corresponds well to the way I approach nomenclature. I feel that nomenclature is absolutely foundational to the rest of chemistry, so I was pleased to see the authors spend considerable space on it and yet approach it in a systematic way. I would like to see important binary acid exceptions such as hydrogen cyanide and hydrogen azide mentioned.
The hybridization section stated plainly that hybridization was devised to rationalize experimentally observed molecular geometries--something I heavily emphasize--and makes it fairly plain that the hybridization goes with the VSEPR geometry in a one-to-one correspondence. It does not use the term "promotion", but rather that the electrons distribute themselves among the hybrid (and unhybridized) orbitals. There were good figures--perhaps not as fancy as in some commercial texts, but clear enough.
I was pleased to see the "stoichiometry map" in the Reaction Stoichiometry chapter. I use that concept myself, and by the end of the first semester, have it filled in with heat and volume of gas as well as the quantities shown in their map (entities, grams, moles, volume of solution).
Some of my comments in Clarity relate to this. Because the book was not written from scratch to be atoms first, but rather was derived from a traditional topic order text, there are some issues with vocabulary showing up too early, before it has been defined (such as some column names in the periodic table, or a potential energy curve before potential energy has really been discussed at all).
There seem to be some minor notation differences between the online book and the pdf, particularly with velocity and nu.
I have already mentioned some questionable use of endothermic and exothermic as it relates to the absorption and emission of light. I have never seen this in any other book.
The modularity of this book is quite good. Although some of the sections are longer than others, this is inherent in the topic being covered, or the authors have provided a lot of examples, which is all to the good for students. I found the online book easiest to find a specific subsection I was looking for, but it only displays just that subsection, so for scanning through a chapter, the pdf is easier to work with.
Chemistry is not a subject that can completely avoid self-referencing, because the material builds on itself as the chapters progress, so references to and connections to earlier topics are helpful, not to be avoided. Likewise, the occasional "foreshadowing" can be helpful. For example, it's useful to know why we need to learn how to draw Lewis structures--so that we can predict the shape and polarity of the molecule, which in turn has implications for its physical and chemical properties.
I have addressed this most in Clarity. There are some problems with logical progression of ideas and terminology due to this being a derivative of a traditional-order text. The authors did make a good-faith attempt to present the material in an atoms first approach that students can follow and learn from. There are simply still improvements to be made. I have made several suggestions for some topics that can be moved earlier to help with this. I also would like to see some sort of author's notes about why they chose the order of presentation of topics in the second semester portion of the book--why thermodynamics before equilibrium? Why kinetics after electrochemistry? Some books do kinetics as a lead-in to equilibrium, since the typical definition of equilibrium is that the rate of the forward reaction equals the rate of the reverse reaction.
As mentioned above, the online version is good for quickly getting to a section of the book you want to find, no matter where you are in the book currently. In the pdf version, you have to scroll all the way up to the table of contents to link to a new section. The pdf is easier to scroll through a complete chapter though, and the pdf has all the examples numbered, the figures and tables numbered sequentially, and the end of chapter exercises all numbered. The online version has none of the those numbers, but the appropriate end of chapter exercises are at the end of each subsection.
I ran into several issues with the interface. In Chapter 1, the plasma video did not play on either iPad (online version) or PC (online or pdf).
In the pdf version, it would be helpful to have a reminder to readers/students that when you link out, you need to open the link in a new window. If you fail to do this (as I did many times), when you go back to the pdf you are at the very beginning instead of where you left off.
The online version does not have Example numbers or Exercise numbers that the pdf has. Also the figure numbers do not correspond between the online version and the pdf version. How can the online version be used for teaching--how can I tell students which exercises are assigned or which examples to look at?
Chapter titles are NOT on the first page in the online version, only the Chapter number and "Introduction". You have to go to the Table of Contents to see what the name of the chapter is. Also, on that first screen in the pdf version, there is a "By the end of this section, you will be able to...". This is collapsed into "Summary" in the online version. Why? Why not display it?
In the online version (on iPad at least), when the Table of Contents is used to move to a section, the title of the displayed section goes vertical and does not scale to the width of the frame the way the text does. You have to collapse the Table of Contents in order to be able to read the title of the section well.
When the pdf is first loading, you cannot hotlink in the Table of Contents to sections later in the document (until the pdf gets done loading). Clicking on a link too far down in the Table of Contents too early in the loading process takes you back to the front of the document.
The link to Appendix B in Chapter 1, Measurements section, goes to the preface in the online version on iPad always, or goes to the front of the pdf document if the pdf has not fully loaded.
In the online version, a box came up that told me a newer version was available, and when I clicked on it, it switched me over to openstax Chemistry (not Chemistry Atoms First)!
A few typos here and there, but overall well edited for grammar.
The portraits of a chemist do include some women. I admit I was not able to review all of them, but based on reviews of the original openstax Chemistry, of which this is a derivative, gender diversity seems to be all the diversity that is shown. The book is very US centric and includes some strange examples, such as mentioning by name a 2014 Lambourgini particular sports car in an example problem. Are the authors getting "product placement' gigs? On a more serious note, the colors used on the periodic table are quite pale and I suspect students who are color blind would not be able to distinguish the difference between metalloids and non-metals, in particular, and seeing the difference between metals and non-metals may be tough as well. I also noticed that one figure showed meat on a grill (as an example of a chemical reaction students might have seen) and a later figure showed (and mentioned) a cheeseburger. International students who don't eat beef or other meats for religious reasons might not appreciate these kinds of examples being used when there are others available that are not food-related. One of the special topics boxes is on Climate Change, which may generate some discussion in class. That would be a good opportunity to talk about Evidence for hypotheses. Too often, college chemistry gets presented as "here's what we know from the last 300 years" and there's nothing to discuss.
I am currently teaching from an atoms first commercial book and I really hoped to find that this book would be a good substitute I could recommend to my colleagues. Although there are some quite strong points of the book and some parts are better than our commercial book, at this time (December, 2016), however, it has too many errors and inconsistencies in approach that it is not an improvement over what we are currently using, even to be free to the students. If I have time over the summer, I can edit it myself. I hope, however, that the authors will take another look at it and improve it.
Reviewed by Lisa Smith, Chemistry Instructor (Tenured; Non-Ranked Institution), North Hennepin Community College on 2/8/17
I think this textbook covers all areas and ideas of the subject taught in the first two semesters of a college-level general chemistry course. At the end of each chapter there is a glossary as well as key equations to help the students organize... read more
I think this textbook covers all areas and ideas of the subject taught in the first two semesters of a college-level general chemistry course. At the end of each chapter there is a glossary as well as key equations to help the students organize information.
The book seems to be error-free and accurate upon basic review and skimming. I did not complete every end-of-chapter question nor read every word. I like that the periodic table is updated with the more recent elements that have become official above atomic number 108.
I feel the visuals are modern and can stand the test of time.
I like the way the text is written to be approachable for a wide variety of students. I think balancing chemical reactions could be done in a clearer way, as it is hard to tell which numbers are the co-efficients. Perhaps formatting them differently (bold) would help student identify during the learning phase. The same goes, for example, balancing redox equations in Chapter 16 versus Chapter 7.5. In Chapter 16, it could be laid out a little more clearly. I really like the diagram on page 365 that shows how moles are related to a variety of stoichiometric quantities.
Upon general review and skimming, I think the terminology is internally consistent. I feel that the formatting and delivery of step-wise instructions could be improved. I would add numbers or bullet points instead of having important directions embedded in paragraphs. This could make it hard for students who are mastering English as a secondary language.
One example I see is that there could be more clarify in connecting previous topics. For example, balancing redox reactions in Chapter 16 could have either copy and pasted the same procedure from Chapter 7, with the additional new rules in Chapter 16. This would've added more consistency and clarity. I can see how chunks can be re-organized, which makes it nice.
I feel the book is organized in a manner that matches other printed textbooks. This provides easy reference, especially if moving around material.
It took awhile for the textbook to download, so I see limitations with weak internet access or making a 'quick' reference. I would highly recommend just saving a pdf copy. This flexibility allows readers to search for specific terminology quickly.
I feel the grammar meets requirements.
I feel the book could add more cultural diversity in its examples and visuals.
I look forward to piloting this with my General Chemistry students.
Reviewed by Chad Park, Lecturer, University of Arizona on 2/8/17
The text covers all the typical material in an introductory chemistry text. It is completely useful and relevant to a freshman level general chemistry course. I would like to see a chapter on green chemistry and perhaps a bit more biologically... read more
The text covers all the typical material in an introductory chemistry text. It is completely useful and relevant to a freshman level general chemistry course. I would like to see a chapter on green chemistry and perhaps a bit more biologically relevant chemistry although that may be outside the scope of the book.
Content is great and seems accurate.
Relevance/Longevity rating: 3
The text is nicely updated and goes to great lengths to have relevant examples for general chemistry students. I would think that more examples and a longer text would help.
The section on precision and accuracy uses the classic archery / target example. However, it would be better to look at these terms relevant to measurements.
The text actually covers a great deal of material and is updated compared to some other texts I have seen.
Overall the text is clearly explained. The section on dimensional analysis is both relevant and quite clear and I'm glad to see it written and explained so well. The example problems that are presented are done at a very basic and easily understood level. I think that it would be relevant even as a supplemental text to recommend for struggling students.
The text is quite consistent. It's kept at a constant level. A few of the pictures are lower resolution than the others though.
The consistent use of equations throughout is nice as well. Some texts try to exclude mathematics which is a huge mistake in my opinion.
I think I like that thermodynamics is spaced out throughout the book and introduced in between several chapters. At first that seems a little disconcerting, but it's done in a nice way and probably allows the student to learn a little thermo at a time instead of all at once in a single chapter.
This seems sufficient but is typical for introductory chemistry texts. I would like to see links between sections allowing an interested reader to be able to jump to other relevant portions of the text. The fact that there are a lot of modules or sections is advantageous especially when instructors may need to bounce around modules or exclude some for time constraints. There are a few chapters that seem out of order compared to "classic" chemistry texts but I think the modularity here is a distinct asset.
It follows the classical bottom-up approach to chemistry and I find that reassuring in a way. Taken with the large modularity of the various sections, I think it would be a good book for an intro class to consider. Chapter 3 on waves, radiation, the Bohr model, etc., is detailed and well written. I didn't expect to see it emphasized early like that. However, it's written at an appropriate level for beginning chemists.
I also didn't expect to see the chapter on the gas laws placed so far after descriptions of chemical bonding. In such a modular text it doesn't particularly matter and it may make more sense to talk about, say, molecular orbital theory before Boyle's law.
It's also a little strange that chemical equilibria comes so much later than stoichiometry and balancing equations. I think it may work better this way though. It's just different than the layout of chemistry texts with which I'm familiar.
As mentioned in general comments, the text could benefit somewhat from more example problems and would benefit greatly from more problem sets and exercises. I liked the online connectivity to the "Build A Molecule" website.
It's a little weird that the text will include "Table" and "Figure" without a table or figure number. The fact that it's a hyperlink to the relevant item is great, but it's a little strange to read that way.
If anything, I would like to see an online text have a great deal more hyperlinks to content.
I did not notice any grammatical errors.
This seems like a great choice for many English speaking countries but seems primarily aimed at students in US institutions.
A lot of the sections seem short. The text, especially in it's nicely modular format, would benefit from longer sections. Critically, there would be a vast improvement by supplying many more problems and worked examples. The problems supplied are nice, comprehensive and detailed. And there are, in fact, a fairly large amount of them and I realize it may take away from space for regular material. However, a lot more of them would be extremely useful. It helps instructors write exams and more importantly, it lets students have a vast amount of problems to work for practice. Maybe if there was an online only component that included an expansion on the problem sets.
Many of the figures are well done and at a high quality.
Table of Contents
- Chapter 1: Essential Ideas
- Chapter 2: Atoms, Molecules, and Ions
- Chapter 3: Electronic Structure and Periodic Properties of Elements
- Chapter 4: Chemical Bonding and Molecular Geometry
- Chapter 5: Advanced Theories of Bonding
- Chapter 6: Composition of Substances and Solutions
- Chapter 7: Stoichiometry of Chemical Reactions
- Chapter 8: Gases
- Chapter 9: Thermochemistry
- Chapter 10: Liquids and Solids
- Chapter 11: Solutions and Colloids
- Chapter 12: Thermodynamics
- Chapter 13: Fundamental Equilibrium Concepts
- Chapter 14: Acid-Base Equilibria
- Chapter 15: Equilibria of Other Reaction Classes
- Chapter 16: Electrochemistry
- Chapter 17: Kinetics
- Chapter 18: Representative Metals, Metalloids, and Nonmetals
- Chapter 19: Transition Metals and Coordination Chemistry
- Chapter 20: Nuclear Chemistry
- Chapter 21: Organic Chemistry
- The Periodic Table
- Essential Mathematics
- Units and Conversion Factors
- Fundamental Physical Constants
- Water Properties
- Composition of Commercial Acids and Bases
- Standard Thermodynamic Properties for Selected Substances
- Ionization Constants of Weak Acids
- Ionization Constants of Weak Bases
- Solubility Products
- Formation Constants for Complex Ions
- Standard Electrode (Half-Cell) Potentials
- Half-Lives for Several Radioactive Isotopes
- Chapter 22: Answer Key
Ancillary Material
About the book.
Chemistry: Atoms First 2e is a peer-reviewed, openly licensed introductory textbook produced through a collaborative publishing partnership between OpenStax and the University of Connecticut and UConn Undergraduate Student Government Association.
This text is an atoms-first adaptation of OpenStax Chemistry 2e. The intention of “atoms-first” involves a few basic principles: first, it introduces atomic and molecular structure much earlier than the traditional approach, and it threads these themes through subsequent chapters. This approach may be chosen as a way to delay the introduction of material such as stoichiometry that students traditionally find abstract and difficult, thereby allowing students time to acclimate their study skills to chemistry. Additionally, it gives students a basis for understanding the application of quantitative principles to the chemistry that underlies the entire course. It also aims to center the study of chemistry on the atomic foundation that many will expand upon in a later course covering organic chemistry, easing that transition when the time arrives.
The second edition has been revised to incorporate clearer, more current, and more dynamic explanations, while maintaining the same organization as the first edition. Substantial improvements have been made in the figures, illustrations, and example exercises that support the text narrative.
About the Contributors
Senior Contributing Authors
Paul Flowers , University of North Carolina at Pembroke
Edward J. Neth , University of Connecticut
William R. Robinson , PhD
Contribute to this Page
1.1 Chemistry in Context
Learning objectives.
By the end of this section, you will be able to:
- Outline the historical development of chemistry
- Provide examples of the importance of chemistry in everyday life
- Describe the scientific method
- Differentiate among hypotheses, theories, and laws
- Provide examples illustrating macroscopic, microscopic, and symbolic domains
Throughout human history, people have tried to convert matter into more useful forms. Our Stone Age ancestors chipped pieces of flint into useful tools and carved wood into statues and toys. These endeavors involved changing the shape of a substance without changing the substance itself. But as our knowledge increased, humans began to change the composition of the substances as well—clay was converted into pottery, hides were cured to make garments, copper ores were transformed into copper tools and weapons, and grain was made into bread.
Humans began to practice chemistry when they learned to control fire and use it to cook, make pottery, and smelt metals. Subsequently, they began to separate and use specific components of matter. A variety of drugs such as aloe, myrrh, and opium were isolated from plants. Dyes, such as indigo and Tyrian purple, were extracted from plant and animal matter. Metals were combined to form alloys—for example, copper and tin were mixed together to make bronze—and more elaborate smelting techniques produced iron. Alkalis were extracted from ashes, and soaps were prepared by combining these alkalis with fats. Alcohol was produced by fermentation and purified by distillation.
Attempts to understand the behavior of matter extend back for more than 2500 years. As early as the sixth century BC, Greek philosophers discussed a system in which water was the basis of all things. You may have heard of the Greek postulate that matter consists of four elements: earth, air, fire, and water. Subsequently, an amalgamation of chemical technologies and philosophical speculations was spread from Egypt, China, and the eastern Mediterranean by alchemists, who endeavored to transform “base metals” such as lead into “noble metals” like gold, and to create elixirs to cure disease and extend life ( Figure 1.2 ).
From alchemy came the historical progressions that led to modern chemistry: the isolation of drugs from natural sources, such as plants and animals. But while many of the substances extracted or processed from those natural sources were critical in the treatment of diseases, many were scarce. For example, progesterone, which is critical to women's health, became available as a medicine in 1935, but its animal sources produced extremely small quantities, limiting its availability and increasing its expense. Likewise, in the 1940s, cortisone came into use to treat arthritis and other disorders and injuries, but it took a 36-step process to synthesize. Chemist Percy Lavon Julian turned to a more plentiful source: soybeans. Previously, Julian had developed a lab to isolate soy protein, which was used in firefighting among other applications. He focused on using the soy sterols—substances mostly used in plant membranes—and was able to quickly produce progesterone and later testosterone and other hormones. He later developed a process to do the same for cortisone, and laid the groundwork for modern drug design. Since soybeans and similar plant sources were extremely plentiful, the drugs soon became widely available, saving many lives.
Chemistry: The Central Science
Chemistry is sometimes referred to as “the central science” due to its interconnectedness with a vast array of other STEM disciplines (STEM stands for areas of study in the science, technology, engineering, and math fields). Chemistry and the language of chemists play vital roles in biology, medicine, materials science, forensics, environmental science, and many other fields ( Figure 1.3 ). The basic principles of physics are essential for understanding many aspects of chemistry, and there is extensive overlap between many subdisciplines within the two fields, such as chemical physics and nuclear chemistry. Mathematics, computer science, and information theory provide important tools that help us calculate, interpret, describe, and generally make sense of the chemical world. Biology and chemistry converge in biochemistry, which is crucial to understanding the many complex factors and processes that keep living organisms (such as us) alive. Chemical engineering, materials science, and nanotechnology combine chemical principles and empirical findings to produce useful substances, ranging from gasoline to fabrics to electronics. Agriculture, food science, veterinary science, and brewing and wine making help provide sustenance in the form of food and drink to the world’s population. Medicine, pharmacology, biotechnology, and botany identify and produce substances that help keep us healthy. Environmental science, geology, oceanography, and atmospheric science incorporate many chemical ideas to help us better understand and protect our physical world. Chemical ideas are used to help understand the universe in astronomy and cosmology.
What are some changes in matter that are essential to daily life? Digesting and assimilating food, synthesizing polymers that are used to make clothing, containers, cookware, and credit cards, and refining crude oil into gasoline and other products are just a few examples. As you proceed through this course, you will discover many different examples of changes in the composition and structure of matter, how to classify these changes and how they occurred, their causes, the changes in energy that accompany them, and the principles and laws involved. As you learn about these things, you will be learning chemistry , the study of the composition, properties, and interactions of matter. The practice of chemistry is not limited to chemistry books or laboratories: It happens whenever someone is involved in changes in matter or in conditions that may lead to such changes.
The Scientific Method
Chemistry is a science based on observation and experimentation. Doing chemistry involves attempting to answer questions and explain observations in terms of the laws and theories of chemistry, using procedures that are accepted by the scientific community. There is no single route to answering a question or explaining an observation, but there is an aspect common to every approach: Each uses knowledge based on experiments that can be reproduced to verify the results. Some routes involve a hypothesis , a tentative explanation of observations that acts as a guide for gathering and checking information. A hypothesis is tested by experimentation, calculation, and/or comparison with the experiments of others and then refined as needed.
Some hypotheses are attempts to explain the behavior that is summarized in laws. The laws of science summarize a vast number of experimental observations, and describe or predict some facet of the natural world. If such a hypothesis turns out to be capable of explaining a large body of experimental data, it can reach the status of a theory. Scientific theories are well-substantiated, comprehensive, testable explanations of particular aspects of nature. Theories are accepted because they provide satisfactory explanations, but they can be modified if new data become available. The path of discovery that leads from question and observation to law or hypothesis to theory, combined with experimental verification of the hypothesis and any necessary modification of the theory, is called the scientific method ( Figure 1.4 ).
The Domains of Chemistry
Chemists study and describe the behavior of matter and energy in three different domains: macroscopic, microscopic, and symbolic. These domains provide different ways of considering and describing chemical behavior.
Macro is a Greek word that means “large.” The macroscopic domain is familiar to us: It is the realm of everyday things that are large enough to be sensed directly by human sight or touch. In daily life, this includes the food you eat and the breeze you feel on your face. The macroscopic domain includes everyday and laboratory chemistry, where we observe and measure physical and chemical properties such as density, solubility, and flammability.
Micro comes from Greek and means “small.” The microscopic domain of chemistry is often visited in the imagination. Some aspects of the microscopic domain are visible through standard optical microscopes, for example, many biological cells. More sophisticated instruments are capable of imaging even smaller entities such as molecules and atoms (see Figure 1.5 ( b )).
However, most of the subjects in the microscopic domain of chemistry are too small to be seen even with the most advanced microscopes and may only be pictured in the mind. Other components of the microscopic domain include ions and electrons, protons and neutrons, and chemical bonds, each of which is far too small to see.
The symbolic domain contains the specialized language used to represent components of the macroscopic and microscopic domains. Chemical symbols (such as those used in the periodic table), chemical formulas, and chemical equations are part of the symbolic domain, as are graphs, drawings, and calculations. These symbols play an important role in chemistry because they help interpret the behavior of the macroscopic domain in terms of the components of the microscopic domain. One of the challenges for students learning chemistry is recognizing that the same symbols can represent different things in the macroscopic and microscopic domains, and one of the features that makes chemistry fascinating is the use of a domain that must be imagined to explain behavior in a domain that can be observed.
A helpful way to understand the three domains is via the essential and ubiquitous substance of water. That water is a liquid at moderate temperatures, will freeze to form a solid at lower temperatures, and boil to form a gas at higher temperatures ( Figure 1.5 ) are macroscopic observations. But some properties of water fall into the microscopic domain—what cannot be observed with the naked eye. The description of water as comprising two hydrogen atoms and one oxygen atom, and the explanation of freezing and boiling in terms of attractions between these molecules, is within the microscopic arena. The formula H 2 O, which can describe water at either the macroscopic or microscopic levels, is an example of the symbolic domain. The abbreviations ( g ) for gas, ( s ) for solid, and ( l ) for liquid are also symbolic.
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
- Authors: Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD
- Publisher/website: OpenStax
- Book title: Chemistry 2e
- Publication date: Feb 14, 2019
- Location: Houston, Texas
- Book URL: https://openstax.org/books/chemistry-2e/pages/1-introduction
- Section URL: https://openstax.org/books/chemistry-2e/pages/1-1-chemistry-in-context
© Jan 8, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

ROLE OF CHEMISTRY IN EVERYDAY LIFE

All physical and chemical processes occurring in this universe include chemistry. The living as well as non living substances may be it a goat, a cow, a brick, a stone, a bed sheet, a piece of bread, a biscuit or a drink etc. are made up of chemical compounds. All processes occurring on earth take place as a result of chemical reactions. These reactions may be spontaneous or non-spontaneous i.e. some reactions occur by themselves and for some reactions external agency is required. However in all these reactions chemistry is involved. It means that the chemical reactions or chemistry plays vital role in everyday life. The foods we eat are chemical products and the ways in which they are converted into various substances in the body is greatest chemical mystery. To study the chemistry of these processes will not be possible to cover in the present paper. However knowledge of some chemistry can help a person to take day to day decisions that affects the life processes. In the present paper efforts have been made to summarize the role of chemistry related to the liquids used by us in daily life.
Related Papers
Mumbashu Martin
Dr. Sanjay Roy
CXC CHEMISTRY ROCKELLA
Rockella Samuel
Angewandte Chemie International Edition
Sason Shaik
GAMTAMOKSLINIS UGDYMAS / NATURAL SCIENCE EDUCATION
Jazeps Logins
A concept “Chemistry for life” developed in Germany characterises a number of the-ses generally recognised in modern didactics, from which the principle “from the simple to more difficult” and psychological laws of learning are highlighted. This concept is in par-ticular characterised by the thesis that chemistry studies should be based on student every-day notions. The foundation of chemistry studies is experimentation with everyday substanc-es. The concept “Chemistry for life” can be successfully used for acquiring chemistry in schools in Latvia by intensifying the use of experiments with everyday substances and devel-oping student inquiry skills. Key words: chemistry for life, chemistry learning process, everyday substances, experiments, inquiry skills.
Venkatesh Mohan
Alisher Abdullayev
Minu Bhowon
Nathan Hodges
ASEP WAHYU NUGRAHA
Abstract: Analysis of chemical teaching materials for class X SMA/ MA on the discussion of the role of chemistry in daily life. The purpose of this study was to analyze the concept labels and the suitability of teaching materials to the K13 curriculum on the role of chemistry in everyday life and identify those that would cause misconceptions in chemistry books. The method used in this research is descriptive qualitative method. The instrument used to obtain the data is the Concept Analysis Table which is adapted to the KD in the K13 curriculum syllabus and reviews that will lead to conceptual errors. The results showed that the number of concept labels analyzed was 20 concept labels in total, the concept labels in book A and book B which corresponded to the 2013 curriculum syllabus were 14 and 18 respectively. The same concept labels between book A and book B amounted to 12, and the concept labels that are only found in book B are 5 and all are in accordance with the K13 curriculum...
RELATED PAPERS
agen kain ihram bandung
Access to justice in Eastern Europe
Journal of Gastroenterology and Hepatology
sükrettin Güldütuna
Klára Kovács
Fernando Herrera Gallardo * Fac. Cs. de la Salud *
NATO Science for Peace and Security Series A: Chemistry and Biology
Ibrahim Inanc
SAE International Journal of Alternative Powertrains
Abdullah Al Mamun
IFIP International Federation for Information Processing
Ioannis Mertzanis
Nutrition and Food Processing
Mahendra Pal
Philosophical transactions of the Royal Society of London. Series B, Biological sciences
Kirsten Lyke
European Journal of Medicinal Chemistry
ROBERTA IBBA
Quality Management Journal
Kevin Taaffe
TAZKIYA: Journal of Psychology
Khoirun Nisya
Frontiers in Oncology
Sherley Diaz
Nancy Lawrence
Enfermería Clínica (English Edition)
Alfonso Miguel García Hernández
DergiPark (Istanbul University)
Cansel Türkay
Orbis Scholae
Stanislav Štech
Pesquisa em Educação Ambiental
daniela brusamarelo
Aquatic Living Resources
International Journal of Pharmaceutics: X
Hanne Nielsen
Scientific Drilling
sobhi nasir
Scientific Reports
Michel Cogné
Israeli Oriental Studies Annual
James M Burgin
See More Documents Like This
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
We will keep fighting for all libraries - stand with us!
Internet Archive Audio

- This Just In
- Grateful Dead
- Old Time Radio
- 78 RPMs and Cylinder Recordings
- Audio Books & Poetry
- Computers, Technology and Science
- Music, Arts & Culture
- News & Public Affairs
- Spirituality & Religion
- Radio News Archive

- Flickr Commons
- Occupy Wall Street Flickr
- NASA Images
- Solar System Collection
- Ames Research Center

- All Software
- Old School Emulation
- MS-DOS Games
- Historical Software
- Classic PC Games
- Software Library
- Kodi Archive and Support File
- Vintage Software
- CD-ROM Software
- CD-ROM Software Library
- Software Sites
- Tucows Software Library
- Shareware CD-ROMs
- Software Capsules Compilation
- CD-ROM Images
- ZX Spectrum
- DOOM Level CD

- Smithsonian Libraries
- FEDLINK (US)
- Lincoln Collection
- American Libraries
- Canadian Libraries
- Universal Library
- Project Gutenberg
- Children's Library
- Biodiversity Heritage Library
- Books by Language
- Additional Collections

- Prelinger Archives
- Democracy Now!
- Occupy Wall Street
- TV NSA Clip Library
- Animation & Cartoons
- Arts & Music
- Computers & Technology
- Cultural & Academic Films
- Ephemeral Films
- Sports Videos
- Videogame Videos
- Youth Media
Search the history of over 866 billion web pages on the Internet.
Mobile Apps
- Wayback Machine (iOS)
- Wayback Machine (Android)
Browser Extensions
Archive-it subscription.
- Explore the Collections
- Build Collections
Save Page Now
Capture a web page as it appears now for use as a trusted citation in the future.
Please enter a valid web address
- Donate Donate icon An illustration of a heart shape
Chemistry In Everyday Life
Bookreader item preview, share or embed this item, flag this item for.
- Graphic Violence
- Explicit Sexual Content
- Hate Speech
- Misinformation/Disinformation
- Marketing/Phishing/Advertising
- Misleading/Inaccurate/Missing Metadata
Book Source: Digital Library of India Item 2015.14282
dc.date.accessioned: 2015-06-23T22:44:58Z dc.date.available: 2015-06-23T22:44:58Z dc.identifier.barcode: 5990010155760 dc.identifier.origpath: /data1/upload/0010/517 dc.identifier.copyno: 1 dc.identifier.uri: http://www.new.dli.ernet.in/handle/2015/14282 dc.description.scanningcentre: IIIT, Allahabad dc.description.main: 1 dc.description.tagged: 0 dc.description.totalpages: 731 dc.format.mimetype: application/pdf dc.language.iso: English dc.publisher.digitalrepublisher: Digital Library Of India dc.rights: Copyright Permitted dc.title: Chemistry In Everyday Life
plus-circle Add Review comment Reviews
3 Favorites
DOWNLOAD OPTIONS
For users with print-disabilities
IN COLLECTIONS
Uploaded by Public Resource on January 18, 2017
SIMILAR ITEMS (based on metadata)

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

Everyday Life
- Last updated
- Save as PDF
- Page ID 50973

- Ed Vitz, John W. Moore, Justin Shorb, Xavier Prat-Resina, Tim Wendorff, & Adam Hahn
- Chemical Education Digital Library (ChemEd DL)

IMAGES
VIDEO
COMMENTS
453 Chemistry in Everyday Life Derivatives of barbituric acid viz., veronal, amytal, nembutal, luminal and seconal constitute an important class of tranquilizers. These derivatives are called barbiturates. Barbiturates are hypnotic, i.e., sleep producing agents. Some other substances used as tranquilizers are valium and serotonin. (b) Analgesics
Chemistry in Everyday Life. Utsav Poudel. Department of Civil Engineering. Pulchowk Campus, Tribhuvan University. Lalitpur, Nepal. [email protected]. Abstract. We human beings are ...
Chemistry, as a discipline rooted in science, has broad applications and cultural implications. Everyone can find chemistry in everyday life, especially in the food we consume, the air we breathe ...
Important Concepts and Equations from Chemistry in Everyday Life. 1. pH Calculation: The equation for calculating pH is given by: pH = − log[H +] 2. Buffer Solution: The Henderson-Hasselbalch equation is used for buffer solutions: pH = pKa + log( [ A −] [ HA]) 3.
Download the Daily Practice Paper of JEE Main Chemistry in Everyday Life. You can set a timer of 1 hour. Solve the easy questions first and give time for tough questions. Note your answers on a sheet of paper and check with the answer key. Each question carries 4 marks and gives a negative mark of -1 for each question.
COMMENTS This document was created by the authors of 'Chemistry in Everyday life' Unit and it is. meant for teacher's education purposes only, it being designed and piloted within UAB TED Master's Degree in the academic year 2009-2010. This document has been elaborated with images available on the Internet.
Page ID. Chemistry has been defined as the science that is concerned with the composition, properties, and structure of matter and with the ways in which substances can change from one form to another. But this definition is too broad to be useful. Chemistry isn't the only science that deals with the composition and transformations of matter.
Chemistry in Everyday Life Time & Place: Lectures, Tue & Th, 11.00AM - 12.15 PM. ... The last assignment is graded for content and all instructions will be fully posted on Blackboard. Textbook and Connect Code: Textbook is required for successful completion of the class. You may attempt to go through the class
If you are studying chemistry, then you must have wondered about the importance of chemistry in everyday life. Chemistry is the branch of science which deals with the investigation of the properties and changes of matter. From the way how our body exchanges oxygen to how our universe was created, all have a part of chemistry associated with it.
Chemicals make our clothes clean. We use chemicals when washing utensils. —swetha. Fertilizers are one of the best examples of chemistry in everyday life. —savita. Food is all about chemistry. The ingredients are chemicals. Cooking is a set of chemical reactions. Even the spoon you use is a chemical. —Satya ranjan jena.
These Chemistry In Everyday Life Class XII Hand Written Notes in PDF will help you to prepare different competitive exams like, PSUs and so on. These Topper's Class typed/scanned notes of Chemistry In Everyday Life Class 12th Hand Written Notes in Pdf will help you to understand all key concepts.
The content is accurate, the text comprehensive and it could easily be used in a general chemistry curriculum. There are a couple of formatting issues with superscripts in the introduction of chapter 14. Other than that, I did not come across any major issues. The 'Chemistry in Everyday Life' sections add relevance and interest to each chapter.
Chemistry in our daily lives A discussion on the importance of chemistry in everyday life Introduction Chemistry greatly influence our daily lives. In fact, Chemistry is the study of matter and the changes that matter undergoes. Thus why many of the changes we observe in the world around us, we see that they are caused by chemical reactions.
Figure 1.2 (a) This portrayal shows an alchemist's workshop circa 1580. Although alchemy made some useful contributions to how to manipulate matter, it was not scientific by modern standards. (b) While the equipment used by Alma Levant Hayden in this 1952 picture might not seem as sleek as you might find in a lab today, her approach was highly methodical and carefully recorded.
chemistry in everyday life.pdf - Free download as PDF File (.pdf), Text File (.txt) or read online for free. Scribd is the world's largest social reading and publishing site.
Chemistry in everyday life M.A. Fuentes & E. Hernández, 2010 57 SESSION 1: mute slide show Resources: computer, whiteboard, MS Office (PowerPoint), photocopies, music, beamer. Materials used in this session: ChemistryEverydayLife_ppt1.pdf in ChemistryEverydayLife_sshow folder Page 8 (mute slideshow worksheet 1) Assessment:
students learn the materials more effectively. There will be writing assignments on recent chemistry news or topic, not only to help students build up on their written communication and critical thinking skills, but to keep them up to date on chemistry news that affect their daily life. CHEM 102 COURSE OUTLINE AND TENTATIVE SCHEDULE Spring 2013
All physical and chemical processes occurring in this universe include chemistry. The living as well as non living substances may be it a goat, a cow, a brick, a stone, a bed sheet, a piece of bread, a biscuit or a drink etc. are made up of chemical compounds. All processes occurring on earth take place as a result of chemical reactions.
dc.title: Chemistry In Everyday Life. Addeddate 2017-01-18 12:47:52 Identifier in.ernet.dli.2015.14282 Identifier-ark ark:/13960/t3713b11b Ocr ABBYY FineReader 11.0 Ppi 600 ... PDF WITH TEXT download. download 1 file . SINGLE PAGE PROCESSED JP2 ZIP download. download 1 file ...
Download Page (PDF) Download Full Book (PDF) Resources expand_more. Periodic Table. Physics Constants. Scientific Calculator. Reference expand_more. Reference & Cite. Tools expand_more.
Ana Karine Portela Vasconcelos. Full-text available. Sanjay Roy. PDF | On May 4, 2022, M Jeeva published Chemistry in Everyday Life | Find, read and cite all the research you need on ResearchGate.