Loading metrics
Open Access
Peer-reviewed
Research Article

Patient Adherence to Tuberculosis Treatment: A Systematic Review of Qualitative Research
* To whom correspondence should be addressed. E-mail: [email protected]
Affiliations South African Cochrane Centre, Medical Research Council of South Africa, Cape Town, South Africa , Primary Health Care Directorate, University of Cape Town, Cape Town, South Africa , Health Systems Research Unit, Medical Research Council of South Africa, Cape Town, South Africa
Affiliations Health Systems Research Unit, Medical Research Council of South Africa, Cape Town, South Africa , Department of Public Health and Policy, London School of Hygiene and Tropical Medicine, London, United Kingdom
Affiliation International Health Group, Liverpool School of Tropical Medicine, Liverpool, United Kingdom
Affiliations South African Cochrane Centre, Medical Research Council of South Africa, Cape Town, South Africa , Department of Medicine, University of Cape Town, Cape Town, South Africa
Affiliation Norwegian Knowledge Centre for the Health Services, Oslo, Norway
Affiliations South African Cochrane Centre, Medical Research Council of South Africa, Cape Town, South Africa , University of Stellenbosch, Faculty of Health Sciences, Cape Town, South Africa
- Salla A Munro,
- Simon A Lewin,
- Helen J Smith,
- Mark E Engel,
- Atle Fretheim,
- Jimmy Volmink

- Published: July 24, 2007
- https://doi.org/10.1371/journal.pmed.0040238
- Reader Comments
Tuberculosis (TB) is a major contributor to the global burden of disease and has received considerable attention in recent years, particularly in low- and middle-income countries where it is closely associated with HIV/AIDS. Poor adherence to treatment is common despite various interventions aimed at improving treatment completion. Lack of a comprehensive and holistic understanding of barriers to and facilitators of, treatment adherence is currently a major obstacle to finding effective solutions. The aim of this systematic review of qualitative studies was to understand the factors considered important by patients, caregivers and health care providers in contributing to TB medication adherence.
Methods and Findings
We searched 19 electronic databases (1966–February 2005) for qualitative studies on patients', caregivers', or health care providers' perceptions of adherence to preventive or curative TB treatment with the free text terms “Tuberculosis AND (adherence OR compliance OR concordance)”. We supplemented our search with citation searches and by consulting experts. For included studies, study quality was assessed using a predetermined checklist and data were extracted independently onto a standard form. We then followed Noblit and Hare's method of meta-ethnography to synthesize the findings, using both reciprocal translation and line-of-argument synthesis. We screened 7,814 citations and selected 44 articles that met the prespecified inclusion criteria. The synthesis offers an overview of qualitative evidence derived from these multiple international studies. We identified eight major themes across the studies: organisation of treatment and care; interpretations of illness and wellness; the financial burden of treatment; knowledge, attitudes, and beliefs about treatment; law and immigration; personal characteristics and adherence behaviour; side effects; and family, community, and household support. Our interpretation of the themes across all studies produced a line-of-argument synthesis describing how four major factors interact to affect adherence to TB treatment: structural factors, including poverty and gender discrimination; the social context; health service factors; and personal factors. The findings of this study are limited by the quality and foci of the included studies.
Conclusions
Adherence to the long course of TB treatment is a complex, dynamic phenomenon with a wide range of factors impacting on treatment-taking behaviour. Patients' adherence to their medication regimens was influenced by the interaction of a number of these factors. The findings of our review could help inform the development of patient-centred interventions and of interventions to address structural barriers to treatment adherence.
Citation: Munro SA, Lewin SA, Smith HJ, Engel ME, Fretheim A, Volmink J (2007) Patient Adherence to Tuberculosis Treatment: A Systematic Review of Qualitative Research. PLoS Med 4(7): e238. https://doi.org/10.1371/journal.pmed.0040238
Academic Editor: Barbara Rylko-Bauer, Michigan State University, United States of America
Received: November 28, 2006; Accepted: June 8, 2007; Published: July 24, 2007
Copyright: © 2007 Munro et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: Primary funding for this study was received from the Medical Research Council of South Africa. Further support for the study was received from the Norwegian Knowledge Centre for the Health Services, the GLOBINF Network, the London School of Hygiene and Tropical Medicine, and DFID Effective Health Research Programme Consortium, Liverpool School of Tropical Medicine. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Abbreviations: DOT, direct observation of therapy; DOTS, directly observed treatment, short course; IDU, injecting drug user; TB, tuberculosis
Editors' Summary
Background..
Every year nearly nine million people develop tuberculosis—a contagious infection, usually of the lungs—and about two million people die from the disease. Tuberculosis is caused by Mycobacterium tuberculosis , bacteria that are spread in airborne droplets when people with active tuberculosis sneeze or cough. Tuberculosis can be cured by taking several strong antibiotics daily for at least six months but many patients fail to complete this treatment because the drugs have unpleasant side-effects and the treatment is complicated. In addition, people often feel better soon after starting treatment so they stop taking their tablets before all the bacteria in their body are dead. Poor treatment adherence (poor compliance) means that people remain infectious for longer and are more likely to relapse and die. It also contributes to the emergence of drug-resistant tuberculosis. To help people complete their treatment, the World Health Organization recommends a strategy known as DOTS (directly observed treatment, short course). As part of this strategy, a health worker or a tuberculosis treatment supporter—a person nominated by the health worker and the patient—watches the patient take his/her antibiotics.
Why Was This Study Done?
Although DOTS has contributed to improved tuberculosis control, better patient compliance is needed to halt the global tuberculosis epidemic. Treatment adherence is a complex behavioral issue and improving treatment outcomes for tuberculosis (and for other diseases) requires a full understanding of the factors that prevent people taking medicines correctly and those that help them complete their treatment. In this study, the researchers have done a systematic review (a study in which the medical literature is surveyed and appraised using defined methods to reach a consensus view on a specific question) of qualitative studies that asked patients, carers, and health workers which factors contributed to adherence to tuberculosis treatment. Qualitative studies collect non-quantitative data so, for example, a qualitative study on tuberculosis treatment might ask people how the treatment made them feel whereas a quantitative study might count bacteria in patient samples.
What Did the Researchers Do and Find?
The researchers searched electronic databases and reference lists for qualitative studies on adherence to tuberculosis treatments and also consulted experts on tuberculosis treatment. They carefully read the 44 published papers that met their predefined inclusion criteria and then used a method called “meta-ethnography” to compare the factors (themes) associated with good or bad adherence in the different studies and to synthesize (reach) a consensus view of which factors influence adherence to tuberculosis treatment. The researchers identified eight major factors associated with adherence to treatment. These included: health service factors such as the organization of treatment and care; social context (family, community and household influences); and the financial burden of treatment. Finally, the researchers interpreted the themes that emerged from the studies to build a simple model that proposes that adherence to tuberculosis treatment is influenced by four interacting sets of factors—structural factors (including poverty and gender discrimination), social context factors, health service factors, and personal factors (including attitudes towards treatment and illness).
What Do These Findings Mean?
The findings of this systematic review of qualitative research on patient adherence to tuberculosis treatment are inevitably limited by the quality and scope of the original research. Consequently, further studies into patients' understanding of tuberculosis and its treatment are needed. Nevertheless, the findings and the model proposed by the researchers indicate that patients often take their tuberculosis medications under very difficult conditions and that they cannot control many of the factors that prevent them taking their drugs. So, although current efforts to improve adherence to tuberculosis treatments emphasize instilling a willingness to take their medications into patients, this systematic review suggests that more must be done to address how factors such as poverty and gender affect treatment adherence and to tailor support systems to patients' needs. Most importantly, it indicates that future interventions should involve patients more in the decisions made about their treatment.
Additional Information.
Please access these Web sites via the online version of this summary at http://dx.doi.org/10.1371/journal.pmed.0040238 .
- MedlinePlus has an encyclopedia page on tuberculosis (in English and Spanish)
- See the US National Institute of Allergy and Infectious Disease fact sheet on tuberculosis
- US Centers for Disease Control and Prevention provide a variety of fact sheets and other information resources on tuberculosis
- World Health Organization has produced the 2007 Global Tuberculosis Control report (in English with key findings in French and Spanish), information on DOTS (in English, Spanish, French, Russian, Arabic and Chinese), and A Guide for Tuberculosis Treatment Supporters
- See the brief guide to systematic reviews , published by the British Medical Journal
Introduction
Tuberculosis (TB) is a global health concern, with an estimated 8.9 million new cases worldwide in 2004 and two million deaths each year [ 1 ]. It is a major contributor to the burden of disease, especially in low- and middle-income countries, where it is being fuelled by the HIV/AIDS epidemic [ 2 ].
DOTS (directly observed treatment, short course) is the internationally recommended control strategy for TB [ 3 ]. This strategy includes the delivery of a standard short course of drugs, lasting 6 mo for new patients and 8 mo for retreatment patients, to individuals diagnosed with TB. The delivery includes the direct observation of therapy (DOT), either by a health worker or by someone nominated by the health worker and the patient for this purpose (sometimes called a DOT supporter). The strategy has been promoted widely and implemented globally.
Up to half of all of patients with TB do not complete treatment [ 4 ], which contributes to prolonged infectiousness, drug resistance, relapse, and death [ 5 ]. The difficulty experienced by patients following a particular treatment regimen has raised awareness of adherence as a complex behavioural issue, influenced by many factors [ 6 ], including gender and the impact of HIV/AIDS. WHO has attempted to classify factors that influence adherence to TB treatment based on a cursory review of key papers [ 6 ], but the impact of gender [ 7 ] and HIV status [ 8 ] on adherence are less well documented in the qualitative literature.
Efforts to improve treatment outcomes require a better understanding of the particular barriers to and facilitators of adherence to TB treatment, and of patient experiences of taking treatment [ 9 ]. Qualitative research can contribute to this understanding and help interpret the findings of quantitative studies of the effectiveness of adherence-promoting interventions [ 10 ]. The volume of such qualitative research is growing and we believe that one way to draw useful lessons from this literature is by synthesising the findings of these studies.
Systematic synthesis of relevant qualitative studies of TB treatment adherence can provide more complete knowledge than that derived from individual studies alone. It can assist in the interpretation of findings of single studies; help explain variation or conflicts in study findings; enable the development of new theories; and help inform the design of new interventions. In addition, it may allow the identification of gaps in existing adherence research.
In this review we consider the perspectives of patients, caregivers, and health care providers regarding adherence to TB treatment. The findings of this review will have implications for a range of stakeholders including nongovernment organisations, national policy makers, and international bodies working towards reducing the global health burden of TB.
We followed a meta-ethnographic approach [ 11 ], the steps of which are outlined in Figure 1 , to synthesise findings across included studies. This systematic approach translates ideas, concepts, and metaphors across different studies and is increasingly seen as a favourable approach to synthesising qualitative health research [ 11 , 12 ]. The research team included three social scientists (SM, SL, HS) and three clinical researchers (JV, AF, ME). The social scientists had different disciplinary backgrounds.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pmed.0040238.g001
Inclusion Criteria
We included studies that examined adherence or nonadherence to preventive or curative TB treatments and described the perspectives of patients, care givers, or health care providers. We included studies from any discipline or theoretical tradition that used qualitative methods. We included papers that reported qualitative research only, as well as research using qualitative and quantitative methods (mixed method) that reported qualitative findings. Both published and unpublished studies reported in English were considered. Because of resource limitations, papers published in other languages were not considered.
Search Strategy and Study Selection
Figure 2 maps out the process by which articles were selected for our systematic review. We searched 19 databases, using the keywords: “TB AND (adherence OR concordance OR compliance)” from 1966, where available, until 16 February 2005 (see Table S1 for search results). This process was complemented by reviewing citations, searching in Google Scholar, and expert referrals. Additional articles were included as they became available. We used the search, assessment, and retrieval process outlined by Barroso et al. [ 13 ]. SM scanned more than 7,000 citations identified in the various databases and retrieved abstracts for potentially relevant studies ( n = 2,162). Approximately 10% ( n = 222) of these were also reviewed by JV to validate the selection of articles. Disagreements ( n = 17 papers) were resolved by discussion and reference to the full article. Thereafter, SM screened the titles and abstracts of potentially relevant studies, excluding 1,536 papers and retrieving potentially eligible papers ( n = 626). After scanning the full text, 560 of these articles were not considered eligible and 66 were considered potentially eligible, based on our inclusion criteria. The abstracts of these were assessed by SM and SL, and ineligible and duplicate papers were excluded, leaving 47 that were considered eligible. Two independent reviewers then read the full paper of each study, following which three more papers were excluded because they did not include qualitative data or because they had insufficient descriptions of data collection or analysis methods. The final synthesis therefore involved 44 papers.
https://doi.org/10.1371/journal.pmed.0040238.g002
Quality Assessment
We decided to assess the quality of individual studies using a checklist based on common elements from existing criteria for qualitative study quality assessment [ 10 , 14 – 17 ] ( Table 1 ). These existing checklists are published and peer reviewed, but unlikely to be validated; only the Critical Appraisal Skills Programme criteria [ 17 ] have been used by other meta-ethnographers [ 18 ]. Evaluating study quality allowed us to describe the range of quality across included studies. Two reviewers independently assessed study quality using a pretested form and resolved differences by discussion. No studies were excluded on the basis of quality. This approach was taken for two reasons: first, both the original authors of the meta-ethnographic approach [ 11 ], and other users of the method [ 19 ], have found that poorer-quality studies tend to contribute less to the synthesis. The synthesis therefore becomes “weighted” towards the findings of the better-quality studies. Second, there is currently no consensus among qualitative researchers on the role of quality criteria and how they should be applied [ 10 ], and there is ongoing debate about how study quality should be assessed for the purposes of systematic reviews [ 20 ].
Methodological Quality of Included Studies ( n = 44)
https://doi.org/10.1371/journal.pmed.0040238.t001
Based on the meta-ethnography approach described by Noblit and Hare [ 11 ], we used reciprocal translation, analogous to constant comparison in primary qualitative research, to compare the themes identified in each study. We then conducted a “line-of-argument synthesis,” an approach similar to grounded theory in primary research, to determine a model of factors influencing treatment adherence. From this process we derived hypotheses relating to the reorganisation of treatment and care to improve adherence. The synthesis process is described below and illustrated in Figure 1 .
Identifying themes and concepts.
We identified concepts, themes, and patterns by reading and rereading the included studies. In this process, we understood primary themes or first-order constructs as reflecting participants' understandings, as reported in the included studies (usually found in the results section of an article). Secondary themes or second-order constructs were understood as interpretations of participants' understandings made by authors of these studies (and usually found in the discussion and conclusion section of an article). However, we recognise that all reported data are the product of author interpretation [ 21 ]. One author (SM) extracted first- and second-order constructs from the articles, plus relevant data on study context, participants, treatment type, and methods using a standard form. The rest of the study team independently extracted data from half of the studies, but found no major differences. Although the foci of the studies were not all directly comparable, the study team identified a number of recurring first- and second-order constructs.
Determining how the studies are related.
We used thematic analysis to inductively develop categories from the first-order themes and concepts identified in the included studies. These categories represent related themes and concepts and initially included: family, community, and social support; professional practice and organisation of care; financial burden; personal characteristics as related to treatment adherence; access to services; disease progression; and knowledge, beliefs and attitudes towards treatment. We revised and merged these categories by discussing together as a team how they were related. We followed a similar process for second-order constructs identified from the included studies.
Reciprocal translation of studies.
Following the meta-ethnographic method closely, we compared the concepts and themes in one article with the concepts and themes in others. Translation involves the comparison of themes across papers and an attempt to “match” themes from one paper with themes from another, ensuring that a key theme captures similar themes from different papers (see Britten, et al. for further description [ 12 ]). We approached the reciprocal translation by arranging each paper chronologically, then comparing the themes and concepts from paper 1 with paper 2, and the synthesis of these two papers with paper 3, and so on. We began with the categories identified in the process described above, but incorporated others as they emerged. Two authors conducted the translation independently, returning to the full-text papers frequently throughout. In this review our aim was to explore adherence to TB treatment without confining this variable to a specific population or subgroup, but in doing so we were careful not to inappropriately synthesize the findings of heterogenous studies. In the process of comparing the studies against each other, we looked for explicit differences between the studies in relation to a range of factors including their geographic location, socioeconomic conditions, and the type of treatment programme.
From the reciprocal translation we were able to construct tables showing each theme and related subthemes, and narratives to explain each theme.
Synthesising translations.
We chose to synthesise the results of the translation independently to account for different interpretations by disciplinary background. To develop an overarching framework (or third-order interpretation), we listed our translated themes and subthemes in a table, juxtaposed with secondary themes derived from author interpretations (see Table 2 ). Each member of the research team then independently developed an overarching framework by considering if and how the translations and authors' interpretations linked together. From this we produced a model ( Figure 3 ) and generated hypotheses, in a “line-of-argument” synthesis. Line-of-argument syntheses create new models, theories, or understanding rather than a description of the synthesised papers [ 11 ].
Primary and Secondary Themes Emerging from the Included Studies
https://doi.org/10.1371/journal.pmed.0040238.t002
https://doi.org/10.1371/journal.pmed.0040238.g003
We attempted to explore systematically the influence of socioeconomic status and geographic location on the findings of our synthesis. However, it was difficult to determine many patterns except those highlighted specifically by authors of the primary research. We realised that synthesising studies from a variety of contexts would present challenges, but also felt that including these studies would provide an opportunity in the synthesis to explore the differences between the contexts, if these existed. Similarly, we chose to include studies examining adherence to latent TB treatment as well as adherence by injecting drug users (IDUs) and homeless people, with specific attention being paid to the ways that the issues raised in these studies differed from those focused on active TB in other populations. Again, few differences emerged.
Description of Studies
Forty-four studies published between 1969 and 2006 were included in the review. The studies were conducted in Africa (14), North America (9), South (8) and East Asia (8), Latin America (2), and Europe (2). It was difficult to discern the study setting from the published reports, but most were conducted within a clinic or health service setting (see Table 3 ). Most studies were concerned with curative TB treatment (33); others focused on preventive treatment (8) and some considered both (2). Most of the studies involved TB patients, often also including community members and health care workers. Three studies involved IDUs and homeless individuals. Approximately 3,213 individuals were involved in the included studies. We found few studies that justified their use of a qualitative approach ( n = 13) or specified the underlying theoretical framework ( n = 10), and few authors reported on their role as researcher ( n = 12) ( Table 1 ). In 12 papers the method of analysis was clearly described, but some derivation of thematic analysis appeared to be used in others. Although several studies seemed to have high face validity, they often scored poorly on our quality assessment instrument, possibly due to the instrument's ability to measure only the quality of reporting.
Characteristics of Primary Studies Included in this Review
https://doi.org/10.1371/journal.pmed.0040238.t003
https://doi.org/10.1371/journal.pmed.0040238.ta003
Description of Themes
Eight primary themes (identified from participants' understandings) and six secondary themes (derived from authors' interpretations) arose from the synthesis ( Table 2 ). Each primary theme is described in Boxes 1 – 8 using direct quotes to illustrate meaning.
Box 1: Organisation of Treatment and Care for TB Patients
“The patients do not have the adequate means to go to the health centre to take their drugs. They just have camel, donkey or carts… And sometimes, the state of some patients prevents them from using these” (male family member of TB patient, Burkina Faso) [ 31 ].
“A dirty place can affect the psychology. It makes people lose heart and feel unenthusiastic about continuing treatment” (female participant with TB, Vietnam) [ 26 ].
“It just does not make sense as to why a grown up person should be given medicines by someone else. I felt very awkward, and tried to take my medicines myself” (male TB patient, Pakistan [ 22 ].
“…and I was afraid to go to the doctor, I thought he would scold me because I missed treatment for a day. For this reason, I didn't go back to get more pills. I was afraid…” (female participant, Bolivia) [ 32 ].
“The minute you tell them you're homeless they treat you real snobbish… They treat you like a dog down there once you get past the triage nurse…” (female TB patient, United States) [ 50 ].
‘…It did help, cos I really needed assurance that it was definitely going to be [cured] and doctor spent a lot of time with me. And they were really, really um, they were outstanding there” (male TB patient, United Kingdom) [ 30 ].
Box 2: Interpretations of Illness and Wellness
“…When I feel better, I don't take the tablets. Only when I feel pain” (completer, South Africa) [ 51 ].
“…She said ‘no no no I do not have TB any more' because she no longer has blood in her sputum” (provider, Indonesia) [ 46 ].
“Well, if you know a little bit about the disease and, like we say, if it's latent… you are not sick. It's only.. if it becomes active, then you are liable to be sick and probably very sick. So then you consider taking the medicine that is terribly bad: which is worse? That's when you weigh what is best for you” (provider, United States) [ 35 ].
“I think that I feel healthy, my lungs are good, but I have a bit of fear that the sickness will return… But as I told you, I don't want to take these pills, because they make me sick, they hurt me…. “ (female TB patient, Bolivia) [ 32 ].
Box 3: Financial Burden of TB Treatment
“It's a bit difficult, because, as I told you, the radiography and the control smear cost more than 100B; the consult costs 15B…it will cost me almost 150B to start treatment again. At this moment, I don't even have the money for the trip to the hospital...” (male TB patient, Bolivia) [ 32 ].
“TB here is closely related to social and economic problems. People live in densely populated areas, their income is poor, and they don't understand about TB” (provider, Indonesia) [ 46 ].
“We cannot remain out of a job for long. As soon as we feel better we would like to go to work… If I cannot earn, my whole family will suffer” (male TB patient, South Africa) [ 51 ].
“Typically it [treatment] would be three months.. that's a long time for anyone to be available without any compensation… it's tremendously a matter of economics and economics only…” (male TB patient, Canada) [ 57 ].
Box 4: Knowledge, Attitudes, and Beliefs about TB Treatment
“He believed that he should always use the expensive tablets and not the tablets from [the health care facility]. The … tablets were not correct with the problem inside, and the colour of the tablets doesn't look right” (participant, Indonesia) [ 33 ].
“No doctor is able to cure this” (patient, South Africa) [ 34 ].
“That's just like basic common sense, this is no test… if the doctor says to us take these tablets then that's common sense.” (male TB patient, UK) [ 30 ].
“…And when you take medications, these bugs will die, he told me. The medications kill the bugs. This is what I've been told, but I'm not sure. It seems uncertain to me. Because the pills didn't help me….” (female TB patient, Bolivia) [ 32 ].
“…a lot of people don't take the medicine because they feel that taking it doesn't do any good for their health” (female noncompliant patient on prophylaxis, US) [ 53 ].
Box 5: Law and Immigration
“Because the nurse tells us that here they have a record of people who have TB, and when they go to apply for a job it shows up on the record that they have TB and it was untreated, they need [the completion record] for the job” (male Vietnamese refugee patient, US) [ 53 ].
Box 6. Personal Characteristics and Adherence Behavior
“How would somone who starts drinking early in the morning visit the clinic? Some patients consume alcohol daily. They would rather decide to interrupt their treatment, than discarding their drinking habit” (male respondent, South Africa) [ 40 ].
“…When my husband went back home, he was angry with himself and he was upset about everything. He refused to eat and rejected his medicine. He threw his pills away. He did not take TB medicine at all” (female HIV+ TB patient, Thailand) [64 ].
“[interviewer: ‘Some people don't want to take their pills]’ Stupid people, sorry to say that” (male TB patient, UK) [ 30 ].
“I missed taking some pills because I was drunk or high on drugs” (female TB patient, US) [ 59 ].
Box 7: The Influence of Side Effects on Treatment Adherence
“…Unpleasant metallic taste in his mouth… asked if a non-vegetarian diet would improve this problem. He was laughed at by the [provider] along with a number of others in the clinic and some personal remarks were made…he finally left treatment” (male TB patient, India) [ 24 ].
“I said no wonder they defaulted, many of them defaulted, you know, because it is [side effects] just too much, it is just too much …” (TB patient, UK) [ 30 ].
“These tablets let one's body itches for the whole day. I know someone who interrupted this treatment because of this problem.”(male TB patient, South Africa) [ 38 ].
“…I don't want to take these pills, because they make me sick, they hurt me…” (female TB patient, Bolivia) [ 32 ].
Box 8: Family, Community, and Household Influences
“I arrive early in the morning so that people could not see me. I used to conceal my illness from people… People think that we are the filthiest people… it was really difficult to accept that I have TB” (male patient, South Africa) [ 40 ].
“We are two sisters and marriage arrangements have been made with men from one family. If my (future) family-in-law knows that I have TB they will be sure then to break the engagement...I'm worried for my sister. Her engagement also could break off because of my sickness” (female patient, Pakistan) [ 55 ].
“Just pick up the medication even if you don't use it” (patient advice to another patient on preventive treatment, US) [ 53 ].
“…I must have responsibility to take care of my child… If I die, who will take care of her? …. When I think of my child… I must be cured. This made me feel I must take the medicine” (female HIV-positive TB patient, Thailand) [ 64 ].
“…It was very important, I had my sister and my ex-girlfriend and it was really, really important to have someone, you know, to give you support especially when you don't know much about the disease” (male TB patient, UK) [ 30 ].
“…Since I have three children that I need to support… this worried me more” (male TB patient, Bolivia) [ 32 ].
We found no discernible patterns when we explored the influence of factors such as geographic location, socioeconomic status, latent or active TB, type of treatment programme, or special groups such as IDUs or the homeless. Although some studies differentiated between patients receiving treatment in urban and rural areas, no strong differences emerged between these settings, and we therefore judged it appropriate to synthesize findings across all studies. Any differences that emerged between studies with regard to specific factors are noted in the text below.
Organisation of Treatment and Care for TB Patients
For most patients, access to a health care facility depended on distance and available transport as well as their physical condition. One study indicated that, although the intention was for a DOT supporter to visit the patient's home, in practice the patient had to walk to the supporter's home [ 22 ]. This proved especially difficult for patients with severe symptoms [ 22 – 25 ]. One study noted that access to health care facilities was better in urban areas than rural areas [ 26 ], and both patients [ 27 , 28 ] and providers [ 29 ] noted that adherence was compromised if the distance from patients' homes to the nearest clinic was too great. If patients' homes were close to a clinic, however, the patients could attend regularly [ 30 ]. For patients on DOT, the time needed to present for direct observation of treatment-taking compromised their ability to attend to other daily tasks [ 25 , 31 , 32 ]. In one study, patients found private practitioners more accessible [ 26 ].
Problems manifesting specifically at health facilities included long waiting times, queues, lack of privacy, inconvenient appointment times [ 23 , 26 – 28 , 31 – 35 ], and the poor upkeep of clinics [ 26 , 27 ]. Many studies reported that patients experienced difficulty in accessing treatment at health care facilities because of inconvenient opening hours and provider absenteeism [ 22 , 23 , 31 , 37 – 38 ]. Poor TB medication availability at health care facilities was highlighted by patients [ 23 , 33 , 36 , 38 ] and providers [ 29 ]. For example, one study reported that a health care worker sold TB medication that should have been freely available [ 31 ]. A patient's relationship with the treatment provider also appeared to influence adherence. A large number of studies indicated that poor follow-up by providers [ 33 , 36 , 39 ], and maltreatment by providers [ 23 , 24 , 31 , 39 – 41 ], such as scolding a patient for missing appointments, resulted in nonadherence. In contrast, other studies noted the positive impact of increased provider–patient contact on adherence [ 26 , 39 , 42 , 43 ].
Some studies highlighted how treatment requirements could impact on patient attitudes towards treatment and thus on adherence behaviour. Patients could “become tired” of taking medications [ 26 , 30 , 40 , 44 , 45 ], discontinuing because of the length of treatment [ 38 , 40 , 45 , 46 ], the number of tablets [ 24 ], or fear of painful injections or drugs [ 29 , 47 ], as noted by both providers and patients.
Some patients reported they found it difficult to meet the requirements of DOT [ 24 , 25 , 32 , 39 , 40 ]. In a number of studies conducted with patients being directly observed [ 22 , 24 , 34 , 42 ], adherence to treatment was facilitated by flexibility and patient choice. The continuity of the treatment process was important to patients [ 39 , 42 ], and irregular supervision by a family member sometimes compromised the treatment programme [ 22 , 23 ]. Some patients viewed direct observation negatively [ 22 – 25 , 40 , 45 , 48 ], interpreting it as distrust, and in one study describing the process as “doing time” [ 49 ]. In contrast, a study conducted with IDUs indicated that these patients appreciated the direct observation component of care because they received their treatment together with their methadone from a street nurse [ 50 ].
Interpretations of Illness and Wellness
Studies in our synthesis reported that patients stopped treatment because they felt better and thought that they were cured [ 23 , 24 , 39 , 40 , 45 , 47 , 49 , 51 ] or because their symptoms abated [ 47 , 52 , 53 ]. Some studies noted that patients who felt worse than before treatment [ 23 , 24 , 32 ] or saw no improvement in their condition [ 22 – 24 , 46 ] might be more likely to interrupt treatment. A study conducted in The Gambia reported that migrants arrived in the country to receive TB treatment and returned home once they felt better [ 27 ]. This problem may be linked to patients' conceptions of recovery, and of the aetiology of TB.
Treatment interruption was also reportedly related to perceptions about TB as a disease; some patients did not believe that they had TB, only wanted a cure for their symptoms and ceased treatment once these lessened [ 33 , 43 , 52 ]. Another study reported that patients were motivated to continue treatment as a consequence of symptom relief [ 30 ]. One study conducted in China noted that patients often continued to take medication after the necessary period of six months, and some patients would continue with treatment despite not having any symptoms, because they believed that the “roots” of the disease needed to be removed [ 54 ].
Some patients needed help in taking their medication when they were too weak [ 23 ], while others on preventive treatment and with no symptoms hesitated to even begin treatment, thinking that it could make them ill [ 35 ]. Three studies found that patients experiencing severe symptoms were more likely to adhere [ 39 , 43 , 54 ], possibly due to a fear of becoming more ill.
Financial Burden of TB Treatment
Several studies indicated that having TB had consequences for work [ 22 – 24 , 26 , 27 , 29 , 32 , 34 , 42 , 52 , 54 – 56 ]. Studies suggested that patients hide their disease for fear that employers may discover that they have TB, with consequent effects on adherence. Additional work-related issues included difficulty in obtaining sick leave for treatment; fear of asking for money to purchase TB drugs; and fear of losing work or dismissal [ 26 , 29 , 36 , 55 ].
The reports showed how some patients prioritised work over taking treatment—and for many there appeared to be a “choice” between work and adherence [ 23 , 24 , 26 , 29 , 32 , 34 , 36 , 37 , 42 , 45 , 54 ]. More common in rural areas, this was not a real “choice” but rather a conflict between attending for clinic-based treatment and the need to earn a living. This was manifested in patients feeling “forced” to choose between work and attending treatment [ 26 ]; patients having “no choice” but to abandon treatment because it was too difficult to combine the two [ 29 ]; and patients not being able to afford treatment, but if they sought work, being unable to attend for treatment [ 32 ]. A study with inner-city homeless people on preventive treatment reported that treatment posed an economic barrier for them because they often worked out of town [ 57 ]. Patients also expressed guilt over the impact that the disease had on their family livelihoods [ 31 ]. Several studies found that patients had more pressing issues to attend to in everyday life [ 24 , 29 , 31 , 32 , 40 , 42 , 45 , 56 ], such as taking care of family. Economic constraints were especially noted in rural areas, especially for patients on preventive treatment [ 51 ].
Patients often explained treatment interruption by noting the costs of treatment [ 23 , 26 , 29 , 32 , 33 ]. In some settings, patients reported that drugs were expensive [ 29 , 36 ] and, where treatment itself was free, hidden costs such as hospital stays [ 29 ], reviews of X-ray results, and transport costs could be high. In some cases providers acknowledged patients' financial constraints [ 31 ]. However, there were examples of doctors not accepting that costs caused patients to stop taking treatment because, from the doctors' perspective, treatment was provided at no cost [ 32 ]. Failure to accept patients' reasons for nonadherence may contribute to the negative attitudes sometimes expressed by providers towards defaulting patients, resulting in difficulties in patients returning to treatment following missed appointments.
Conflicts between treatment and work and the hidden costs of treatment, resulting in expenses exceeding resources [ 22 , 26 – 28 , 31 , 32 , 34 , 42 , 43 , 48 , 54 , 55 ], could push people into poverty. This possibility was cited both by health professionals and by patients as a reason for nonadherence [ 23 , 26 , 32 , 37 , 42 , 54 – 56 ]. Males (as head of households and often sole wage earners) tended to cite this reason more frequently than females [ 26 , 37 , 42 , 55 ]. In societies where female or adolescent patients depend on family for financial support (particularly India and Pakistan), poverty was reported as a major reason for nonadherence to treatment [ 22 , 23 , 36 , 51 , 55 ]. For patients living in poverty, the quality of food consumed while on TB treatment was reported to affect adherence [ 22 , 26 , 27 , 29 , 37 , 45 , 54 ]. Patients reported not being able to take medication on an empty stomach, or being unable to remain in hospital due to a lack of free food [ 26 , 29 , 37 , 45 , 54 ].
Knowledge, Attitudes, and Beliefs about TB Treatment
Many studies centred on the influence of patients' understanding of treatment, including its duration and the consequences of defaulting, on adherence to treatment [ 23 , 24 , 26 – 28 , 33 , 34 , 36 , 38 – 40 , 42 , 44 , 46 , 52 , 57 ]. The long treatment period was poorly understood by patients [ 23 , 26 , 28 , 38 – 40 , 46 , 52 ]; and adherence appeared to be facilitated where patients understood the importance of completing treatment [ 24 , 26 , 32 , 36 , 39 , 44 , 55 , 58 , 59 ]. One study on adherence to prophylaxis reported that nonadherent patients had little information on TB as a disease, but were very aware of the potential adverse effects caused by treatment [ 44 ].
Patients' beliefs about the efficacy of treatment, both positive [ 39 , 41 , 52 , 59 ] and negative [ 22 , 23 , 26 , 28 , 32 , 34 , 36 , 39 , 44 , 52 , 54 – 56 ], may impact on adherence. Patients may question the efficacy of the pills or think that only injections are “medicine” [ 22 ], or even question the validity of diagnostic tests that are not considered sophisticated enough for such a dangerous disease [ 52 ]. Belief in treatment efficacy appeared to be related to patient confidence in the medical system [ 25 , 35 , 42 ]; in some cases community-based treatment programmes increased confidence among community members that TB could be cured [ 37 , 55 ]. Another study noted that patients preferred to consult traditional healers [ 34 ].
Fear and denial of diagnosis were common themes across the included studies. Some patients had difficulty accepting their diagnosis, often wanting to hide their disease [ 23 , 29 , 33 , 40 , 42 , 43 , 55 , 56 ]. In other studies, patients' desire to be cured was cited as a motivator for adherence in people presenting with TB symptoms [ 30 , 41 , 43 , 46 , 58 , 59 ], and patients' fear of the negative consequences of irregular treatment was associated with treatment adherence [ 30 , 32 , 39 , 54 ].
Patients could be nonadherent if they were taking other western [ 46 ] or traditional [ 51 , 52 ] medicines and perceived there to be negative consequences if these were taken concurrently with TB medication. Two studies mentioned a relationship between pregnancy and nonadherence [ 54 , 55 ], one of which noted that female patients believed that pregnancy would increase intolerance to drugs and make TB drugs ineffective.
Law and Immigration
In studies with IDUs and homeless people, mainly conducted in the US, legal and immigration requirements had an important influence on whether people adhered to prophylactic regimens. For refugees entering the US with inactive TB, obtaining certification of preventive treatment completion was a motivator for returning to the clinic [ 53 ]. Others also on preventive treatment were concerned that TB would affect their immigration status [ 60 ], that their illegal residence status would be discovered when accessing treatment [ 61 ], or that they would be incarcerated [ 62 ]. Some patients simply stated that they adhered because it was legally required [ 59 ]. In The Gambia, nonadherence was attributed by staff to Senegalese patients coming to the country for free treatment and returning home when feeling better [ 27 ].
Personal Characteristics and Adherence Behaviour
Patients and providers thought that an individual's personal character determined whether they would adhere to treatment or not [ 24 , 25 , 28 , 36 – 38 , 49 , 57 , 63 ]. Substance abuse was noted frequently as a barrier [ 24 , 25 , 28 , 36 – 38 , 49 , 57 , 63 ]. Patients with mental illness [ 49 , 57 ]; particular ethnic groups, such as Hispanic patients in the US [ 49 ]; older and younger age groups [ 42 , 49 ]; and those who were residentially mobile [ 25 , 27 , 49 , 62 ] were considered to be at “high risk” for nonadherence by providers and patients. Religion [ 30 , 49 ] and personal motivation [ 22 , 27 , 37 , 39 , 46 , 54 , 57 ] were regarded as important influences on TB treatment adherence. Female patients were perceived as being more motivated [ 38 , 57 ], but in some countries they required permission from men or heads of household to attend treatment [ 27 , 51 ]. Two studies indicated that female patients who were, or wanted to be, pregnant were less likely to adhere to treatment as they perceived the medication to be harmful [ 54 , 57 ].
Some providers expressed the opinion that difficulties with adherence lay almost entirely with the patients [ 46 ], and used labels such as “difficult cases” for nonadherent patients [ 24 , 27 , 38 , 53 ]. Nonadherent patients were judged to lack interest [ 39 ], to be lazy and not care [ 53 ], or to want to remain sick to qualify for financial support [ 41 ]. Patients were criticised for not actively seeking treatment [ 26 , 29 ], and in one case patient characteristics were used to identify and exclude from treatment those considered at higher risk for nonadherence [ 25 ]. Wealthier, more educated people were deemed more likely to adhere [ 29 ], and illiterate patients more likely to default [ 22 ]. Two studies noted that a structured environment away from home could facilitate adherence [ 28 , 57 ]. Studies involving people living with HIV/AIDS noted the relationship between adherence and coping psychologically with their HIV diagnosis [ 64 , 65 ].
Personal agency was an important aspect of adherence behaviour; self-administering patients [ 22 ] and those who developed their own reminders adhered readily [ 54 ]. It appeared to be easier for male than female patients to be in control of the treatment process, but in one study patients felt the DOT system had transformed them from an adult to a minor, because it prevented them from managing their own treatment [ 42 ].
Treatment Side Effects and Adherence
The influence of side effects—real, anticipated, or culturally interpreted—on adherence to treatment was mentioned in a number of studies [ 24 , 32 , 34 , 38 , 39 , 46 , 53 , 54 , 58 ]. Some patients reported stopping medication because of adverse effects [ 44 , 46 ] while others reported that they were not informed about side effects and what to do to counter them [ 25 , 34 , 58 ]. In some cases, patients had not communicated side effects to providers [ 38 ]; in others, the health care worker had not given attention to the side effects that patients reported [ 24 , 32 , 36 ], or had responded derisively to the patient's attempt to enquire about them [ 24 ]. Few patients acknowledged that side effects had influenced their decision to abandon treatment [ 51 , 54 ]. Cultural interpretations of side effects varied. For example, Vietnamese refugees with inactive TB interpreted treatment side effects as “hot” or “non-hot” and countered these effects differently [ 36 ].
Family, Community, and Household Influences
A main theme across the included studies was the influence of community members or peers on treatment-taking behaviour [ 33 , 53 , 58 ], and the strong influence of stigma among family and friends [ 22 , 26 – 28 , 34 , 36 , 40 , 42 , 46 , 52 , 55 , 56 , 58 , 59 , 61 , 64 ]. TB patients may hide their diagnosis [ 26 , 27 , 29 , 34 , 37 , 38 , 40 , 42 , 56 ], and feel guilt and shame because of the disease [ 26 , 31 , 33 , 34 , 42 , 52 ]. Stigma may also make patients afraid to ask for support from their employer to purchase medication, thereby reducing adherence [ 29 , 65 ].
Sometimes a patient's role and responsibilities in the family could motivate them to adhere to treatment in order to recover and resume those duties [ 22 , 40 , 43 , 58 , 64 , 65 ]. But responsibilities in the home, such as providing income and caring for children, also reduced the likelihood of adherence for some [ 32 ].
Family support, including financial assistance, collecting medication, and emotional support, appeared to be a strong influence on patient adherence to treatment [ 22 , 26 , 27 , 29 , 34 , 36 , 38 , 40 , 42 , 52 , 55 , 56 , 58 , 59 , 61 , 64 ]. In some cases patients on treatment became increasingly demoralised and more likely to become nonadherent as family support weakened [ 23 ]. Providers in a study in Vietnam noted that support for the patients seemed to exist only in the family [ 29 ]. Having family members observe treatment taking was considered important for some patients, especially if the observer was a decision maker in the family [ 53 ], or a respected family member [ 48 ]. Husbands and other males' support was considered important for female patients [ 53 ]. Providers in one study noted that patients also could support each other through their treatment course [ 45 ].
Several studies reported that TB status could affect marriage [ 22 , 27 , 34 , 36 , 42 , 44 , 55 , 56 ]. In some cultures, females diagnosed with TB are at risk of divorce, of their husband taking a second wife, or of being sent to their natal homes [ 27 , 36 , 43 , 55 ]. In South Africa, red urine (a side effect of medication) was interpreted as harmful to the partner, causing abstinence from sex and thus familial disharmony and consequently potential nonadherence [ 34 ]. In Pakistan, parents' perceptions of marriage prospects influence treatment taking or avoidance among unmarried children [ 22 , 43 , 55 ].
The themes identified in this interpretive review were intricately linked and likely to have a combined effect on patient adherence to TB treatment. Secondary interpretations (by authors of included papers) allude to the complex, dynamic nature of adherence to TB treatment. One author suggested that patients experienced three layers of barriers to adherence: attending the health care facility initially, attending repeatedly, and experiences while there [ 31 ]. The layers were considered to be interlinked and exacerbated by geographic, economic, and gender inequalities; and patient decisions in relation to treatment taking were thought likely to shift for various reasons during the treatment course. Other authors considered adherence a chain of responsibilities including patients' behaviour, health care workers' conduct, and decision makers' and society's outlook [ 58 ]. These secondary (author) interpretations influenced our approach towards a higher-order interpretation (third-order interpretation), which distilled the translations into a whole, more complete interpretation. Based on the translated themes and secondary interpretations, we developed a model to depict our understanding of the main influences on adherence ( Figure 3 ). Components of the model include structural, personal, and health service factors influencing adherence, as well as social context. We have presented structural factors and health service factors separately, instead of as a single “health systems” category, because we felt that some interventions could be directed towards wider society-level factors while others could intend to influence the person and the health care service.
Structural Factors: Poverty, Gender, and Discrimination
Structural factors are those factors present in society that influence treatment-taking behaviour, but over which a patient has little personal control. Structural factors have been defined as barriers or facilitators that relate to economic, social, policy, organisational, or other aspects of the environment [ 66 ]. Factors such as gender and poverty determine individual responses to treatment and subsequent behaviour; and they interact with a patient's social context, their personal characteristics, and the health care service. TB programme managers frequently assume that a willingness to adhere must be instilled in patients in order to improve adherence rates. Our synthesis has found that even where patients are willing to adhere, structural factors such as poverty and gender discrimination may prevent them from doing so. It is recognised that incorporating patients' views in medical practice often obscures the real constraints on agency that some patients experience [ 9 ]. In our synthesis, structural factors were discussed in various ways, with poverty remaining one of the most important of these for treatment taking, especially when linked to health care service factors, such as poorly accessible, poorly equipped, and distant clinics. Our findings support the assertion that interventions to increase adherence should focus not only on the patient but also on the wider context and the health care system [ 67 ]. There is a need for a shift in perspective to give greater attention to both the social and economic environment in relation to TB infection, of which the beginnings can already be seen in the international policy arena [ 68 ].
Patient Factors: Motivation, Knowledge, Beliefs, and Attitudes and Interpretations of Illness and Wellness
Patient choice in taking treatment is framed by the physiological and psychological impacts of the disease and also by the social and cultural structures in which the person is immersed [ 68 ]. Patient motivation and willingness, and the effect of incentives on treatment taking, have received some attention [ 69 ]. However, it remains unclear whether the incentive, or the attention received by the patient, serves as the primary source of motivation [ 67 ]. Caution should therefore be exercised when attributing adherence solely to “personal motivation” [ 22 , 27 , 37 , 39 , 46 , 54 , 57 ], because not only can important influences be ignored, but this factor is difficult to modify or even operationalise.
We found that personal and social factors, including poverty and social marginalisation, may be used by some providers to identify patients at risk of nonadherence to their medication regimen. However, it cannot be assumed that all individuals sharing a particular characteristic face the same barriers to adherence. Nonadherence can be a product of programme failures, such as an inadequate supply of drugs, rather than patient-related problems or failures [ 24 ]. Our synthesis also found that patient knowledge, attitudes, and beliefs about the disease TB, TB treatment, and patient interpretations of illness and wellness, can act as a “filter” for the information and treatment offered by the health services. The influence of patients' interpretation of various illnesses on their adherence behaviour is well documented, and it is recognised that patients may interpret the themes of illness, wellness, and disease differently from health professionals [ 70 – 73 ], highlighting the distinctions between lay and biomedical understandings of TB [ 10 ]. This is unlikely to be the only influence on treatment taking, however, and patient interpretations can interact with structural and health care service factors as well as with social context.
Social Context
The influence of social context on treatment adherence was apparent in all included studies. The community, household, and health care service helped in countering the shame and guilt that patients with TB experienced, and also offered support in maintaining treatment taking. Social support can help patients overcome structural and personal barriers, and may influence their knowledge, attitudes, and beliefs. Conversely, community and family members' attitudes may influence a patient's decision to stop taking TB treatment. In such circumstances, community-based TB treatment programmes and stronger involvement of local social networks to support TB patients may be justified [ 6 ].
Health Care Service Factors
Factors related to the provision of health care services emerged strongly in the synthesis. Flexibility and choice in treatment, and options that maintain patient autonomy in treatment taking, appeared to run contrary to the traditional organisation of many TB services [ 6 , 10 ]. These problems were exacerbated by programme failures, such as inadequate supplies of drugs [ 23 , 33 , 36 , 38 ] and difficulties in consulting providers [ 22 , 23 , 31 , 36 – 38 ]. DOT at a health care facility often meant that a patient had to give up part of their working day to attend [ 22 , 23 ]. However, responsibilities in the home, including providing for their family, may be given priority over treatment adherence by patients. Other health care service factors, such as long waiting times and inconvenient opening times in clinics, add to economic discomfort and social disruption for patients [ 49 ], and negatively influence adherence. The studies suggest that patients often face a choice between employment and taking medication for TB; and there is evidence that patients consciously estimate the opportunity costs of taking treatment.
Study Limitations
The majority of studies included in this synthesis were conducted in developing countries; the findings are therefore most applicable to low- and middle-income countries that carry the greatest burden of TB disease and where interventions to improve treatment completion are needed urgently. The findings may also be applicable to countries with better resources; indeed, a meta-ethnography of treatment taking in high-income countries showed findings similar in many ways to those of our study [ 74 ]. The clustering of studies by region may have been due to the difficulties of locating primary studies, and may have produced some of the similarities between issues described by participants.
Studies often included participants from several socioeconomic strata; did not always contain a detailed description of the treatment regimen; and did not explicitly consider gender in treatment adherence. Therefore it was not always possible to tease out similarities or differences in the identified themes based on these characteristics. We identified some patterns relating to the type of treatment intervention—for example, direct observation versus patient-administered treatment—but the majority of studies did not describe adequately interventions or treatment regimens. Our observations regarding gender differences in taking TB treatment are dependent largely on the information provided by original authors. Collecting author (secondary) interpretations proved difficult; most authors maintained a descriptive style in presenting their findings and so the distinction between findings and interpretation was often not clear.
It is important to consider the effect on the review findings of combining studies from different theoretical traditions, and this is widely debated. We found that the level of interpretation in the included studies was fairly basic—most were descriptive studies that used thematic analysis to identify key themes and did not draw extensively on theory or on a particular theoretical tradition. While this made it more feasible to combine the study findings, it also meant we were unable to explore any differences in interpretation of factors affecting adherence in studies conducted within different theoretical frameworks.
Implications for Policy and Practice
Using the reconceptualised model of factors influencing adherence to TB treatment ( Figure 3 ), we consider it important that policy makers, practitioners, and patient support groups acknowledge: patient autonomy in the treatment process; the importance of patient-centred interventions that encourage shared decision-making regarding treatment; the role of support systems tailored to patient needs; the role of informal, societal structures in reinforcing adherence through patient support; and the influence of poverty and gender on patients and their treatment adherence.
New interventions to promote treatment adherence could be designed with these factors in mind. For example, when known barriers to adherence are mapped against the currently available interventions to promote adherence, it is interesting to note that very few interventions are designed to build on social and family support mechanisms. Most are targeted at overcoming barriers to health care delivery to the individual [ 75 ].
Based on our third-order interpretation, we identified a number of hypotheses that may guide policy makers and practitioners in developing and implementing specific measures to improve adherence, including influencing the behaviour of practitioners, the organisation of services, and the behaviour of individuals ( Box 9 ). This review shows the usefulness of qualitative synthesis in informing policies for health interventions. Through bringing together data from multiple primary studies, and looking for commonalities across these studies, the approach provides fresh insights into the reasons for poor adherence and guidance on where the development of more patient-centred interventions to improve adherence could be useful. Such insights can be useful to both programme managers at local and national levels and also in facilitating the development of more appropriate international policies for the management of TB.
Box 9. Factors Likely to Improve TB Treatment Adherence
- Increase the visibility of TB programmes in the community, which may increase knowledge and improve attitudes towards TB
- Provide more information about the disease and treatment to patients and communities
- Increase support from family, peers, and social networks
- Minimize costs and unpleasantness related to clinic visits and increase flexibility and patient autonomy
- Increase flexibility in terms of patient choice of treatment plan and type of support
- Increase the patient centredness of interactions between providers and clients
- Address “structural” and “personal” factors, for example through micro-financing and other empowerment initiatives
- Provide more information about the effects of medication to reduce the risk of patients becoming nonadherent when experiencing treatment side effects
Implications for Research
Based on the findings of this synthesis we believe that further research is needed both to understand people's experience of TB and its treatment and to develop more patient-centred approaches to improving treatment adherence among people with TB. By “patient-centred approaches” we mean interventions that focus on sharing decisions about interventions or the management of health problems with patients and that view the patient as a whole person who has individual preferences situated within a wider social context [ 76 ].
Key issues to be explored in this research include how gender shapes experiences of treatment taking and how differing gender roles may influence adherence. This aspect was reported less frequently than expected in the primary studies in this review and would benefit from further exploration. Patient experiences of side effects of treatment, and how these influence decisions to stop taking treatment, also warrant further research since the existing literature reports vary as to the influence of side effects on treatment adherence [ 77 , 78 ].
There is also little published evidence on the experiences of patients living with HIV/AIDS and taking treatment for TB or receiving concurrent treatment for both diseases; our review included only three reports of qualitative research in this area [ 51 , 63 , 64 ]. The small number of studies is surprising, given the high rates of TB–HIV coinfection, especially in sub-Saharan Africa [ 79 ]; the complex treatment regimens involved; and the need for high rates of treatment adherence for both diseases. There is also some evidence that where coinfection is common, a diagnosis of TB may be seen as a diagnosis of HIV and this “form” of TB may be seen as incurable, with consequent impacts on patient adherence to treatment [ 80 ]. Managing treatment for both HIV and TB is therefore likely to present unique challenges to patients, providers, and the health care system, and further research on the particular experiences of patients taking antiretroviral and anti-TB treatment would be very helpful.
The process of data extraction and quality assessment identified a number of lacunae in the included study reports. Studies frequently failed to report the details of how treatment was delivered, for example whether direct observation of treatment was used; the treatment regimens used; and the sociodemographics of the included study populations. Greater attention to these areas would improve understanding of research findings and facilitate assessment of their transferability to other contexts. The reporting of a number of study quality issues also needs to be addressed in future reports, including the theoretical orientation of the research and sampling and analysis approaches (see Table 1 ).
Finally, lay conceptualisations of illness and wellness, particularly of TB and its treatment, are not well understood. The TB treatment literature is almost entirely conceptualised from a biomedical perspective, and even studies of patient experiences are largely conducted with the aim of improving treatment adherence. Understanding lay conceptualisations will help in comprehending why people may stop taking treatment at particular times. This would involve acknowledging that patients have agency and are active [ 71 ] in shaping their own treatment decisions rather than seeing poor adherence simply as “irresponsible” behaviour. Research approaching TB adherence from a nonbiomedical perspective is required to further understand the impact of traditional beliefs [ 81 ] and perceptions of illness and wellness on adherence to treatment. Any further work on patient experiences of TB adherence should also acknowledge and explore the social, economic, and geographical contexts in which a patient is located.
There are suggestions that the growing interest in the subjective experiences of health care consumers may result in these experiences being used as simply another tool with which to better promote treatment adherence. In addition, this focus, and its attendant notions of shared responsibility for treatment between consumers and providers, could be seen as acting to expand the surveillance of treatment taking from health care workers to consumers and the wider community [ 82 , 83 ]. We therefore believe it is important that this kind of evidence is used carefully by decision makers and practitioners. The extent to which new interventions come from biomedical rather than lay perspectives should be recognised to ensure that structural factors, as well as individual patient responsibilities in treatment taking, are considered.
This synthesis indicates that patients often take their TB medication under difficult circumstances and experience significant challenges, many of which are outside of their direct control. Taking a lengthy course of medication is not straightforward and frequently involves difficult decisions, sometimes at substantial personal and social cost to the patient. Adherence is a complex, dynamic phenomenon; a wide range of interacting factors impact on treatment-taking behaviour, and patient behaviour may change during the course of treatment. More patient-centred interventions, and far greater attention to structural barriers, are needed to improve treatment adherence and reduce the global disease burden attributable to TB.
Supporting Information
Alternative language abstract s1. translation of the abstract into norwegian by atle fretheim.
https://doi.org/10.1371/journal.pmed.0040238.sd001
(48 KB PDF)
Table S1. Search Results
https://doi.org/10.1371/journal.pmed.0040238.st001
(35 KB DOC)
Acknowledgments
The authors would like to thank Sylvia Louw and Anna Gaze for their administrative support.
Author Contributions
SAM, SAL, MEE, AF, and JV developed the protocol and conceptualised the study. All authors participated in data extraction, quality assessment tool development, quality assessment and in retrieving the studies. SAM, SAL, and HJS analysed the data, with later analysis supported by all authors. SAM wrote the initial draft; SAM, SAL, and HJS edited the manuscript; and all authors contributed to the final draft.
- View Article
- Google Scholar
- 3. WHO (2002) An expanded DOTS framework for effective tuberculosis control. WHO/CDS/TB/2002.297. Geneva: World Health Organization. 23 p.
- 5. Volmink J, Garner P (2006) Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev. 2. CD003343. doi: https://doi.org/10.1002/14651858.CD003343.pub2 .
- 6. WHO (2003) Adherence to long term therapies: Evidence for action. Geneva: World Health Organization. Available at: http://www.who.int/chp/knowledge/publications/adherence_full_report.pdf . Accessed: 6 February 2006.
- 11. Noblit GW, Hare RD (1988) Meta-ethnography: Synthesizing qualitative studies. Newbury Park (CA): Sage. 88 p.
- 17. Critical Appraisal Skills Programme (2002) Ten questions to help you make sense of qualitative research. Available: http://www.phru.nhs.uk/learning/casp_qualitative_tool.pdf . Accessed 17 January 2005.
- 21. Schutz A (1971) Collected papers vol 1. The Hague: Martinus Nijhoff. 361 p.
- 28. George LJ (2003) Compliance with medication and directly observed therapy in the treatment of TB in Lesotho. Philadelphia (PA): Faculty of the School of Social Work, University of Pennsylvania. 300 p. [PhD dissertation].
- 30. Gleissberg VG (2001) Patient views on tuberculosis: Is compliance with treatment the key to success or beside the point? Uxbridge (United Kingdom): Department of Anthropology. Brunel University. 55 p. [MSc thesis.].
- 38. Matebesi Z (2004) Living with TB: The career of the tuberculosis patient in the free state, SA . Bloemfontein (South Africa): Department of Sociology, University of the Free State. 216 p. [PhD dissertation].
- 43. Asamoa K (1998) Social counselling and tuberculosis treatment adherence at Bethania hospital, Sialkot, Pakistan. Heidelberg (Germany): University of Heidelberg. 64 p. [Master's thesis].
- 45. Allen S (2006) The feasibility of implementing brief motivational interviewing in the context of tuberculosis treatment in South Africa. Stellenbosch (South Africa): University of Stellenbosch. 165 p. [Master's thesis].
- 49. Klink WB (1969) Problems of regimen compliance in tuberculosis treatment. New York (NY): Columbia University. 275 p. [PhD dissertation].
- 54. Fong C (2004) Gender and access to DOTS program (Directly Observed Treatment, Short Course) in a poor rural and minority area of Gansu province, China. Baltimore (MD): Johns Hopkins University. 138 p. [PhD dissertation].
- 57. de Vos PF (2002) Tuberculosis, adherence behaviour the inner city. Edmonton (Alberta): University of Alberta. 221 p. [Master's thesis].
- 60. Wyss L (2004) Beliefs about medication compliance in a migrant population diagnosed with TB. Akron (OH): University of Akron. 232 p. [PhD dissertation].
- 68. Lienhardt C, Ogden J, Sow O (2003) Rethinking the social context of illness: Interdisciplinary approaches to tuberculosis control. In: Gandy M, Zumla A, editors. The return of the White Plague: Global Poverty and the “new” tuberculosis. London: Verso. pp. 195–291.
- 76. Lewin SA, Skea ZC, Entwistle V, Zwarenstein M, Dick J (2001) Interventions for providers to promote a patient-centred approach in clinical consultations. Cochrane Database of Systematic Reviews. 4. CD003267. doi: https://doi.org/10.1002/14651858.CD003267 .
- 80. Nyblade L, Pande R, Mathur S, McQuarrie K, Kidd R, et al. (2003) Disentangling HIV and stigma in Ethiopia, Tanzania and Zambia. Washington (D.C.): International Center for Research on Women. Available at: http://www.lshtm.ac.uk/dfid/tb/Stigmareport-final-sept17.pdf . Accessed: 18 January 2007.
- 82. Armstrong D (2000) From clinical gaze to regime of total health. In: Heller T, Muston R, Sidell M, Lloyd C, editors. Working for health. London: Sage: pp. 16–27.
- Journal club
- Subscriptions
- Advanced search

Advanced Search
New developments in tuberculosis diagnosis and treatment
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- ORCID record for Cara M. Gill
- For correspondence: [email protected]
- Figures & Data
- Info & Metrics
Tuberculosis (TB) is a major cause of morbidity and mortality worldwide. It is estimated that 25% of the world's population are infected with Mycobacterium tuberculosis , with a 5–10% lifetime risk of progression into TB disease. Early recognition of TB disease and prompt detection of drug resistance are essential to halting its global burden. Culture, direct microscopy, biomolecular tests and whole genome sequencing are approved methods of diagnosis; however, their widespread use is often curtailed owing to costs, local resources, time constraints and operator efficiency. Methods of optimising these diagnostics, in addition to developing novel techniques, are under review. The selection of an appropriate drug regimen is dependent on the susceptibility pattern of the isolate detected. At present, there are 16 new drugs under evaluation for TB treatment in phase I or II clinical trials, with an additional 22 drugs in preclinical stages. Alongside the development of these new drugs, most of which are oral medications, new shorter regimes are under evaluation. The aim of these shorter regimens is to encourage patient adherence, and prevent relapse or the evolution of further drug resistance. Screening for TB infection, especially in vulnerable populations, provides an opportunity for intervention prior to progression towards infectious TB disease. New regimens are currently under evaluation to assess the efficacy of shorter durations of treatment in this population. In addition, there is extensive research into the use of post-exposure vaccinations in this cohort. Worldwide collaboration and sharing of expertise are essential to our ultimate aim of global eradication of TB disease.
Educational aims
Differentiate between TB infection and TB disease.
Understand the different methods of diagnosing TB disease and resistance.
Recognise the different drugs and regimens currently in use for TB disease.
Be able to discuss risk of TB disease in TB infection, and assist patients in making an informed decision on treatment for TB infection.
Early detection of drug resistance is essential to our goal of global eradication of TB. Tolerable drugs and shorter regimens promote patient adherence. Treating TB infection in vulnerable groups will prevent further global spread of TB disease. https://bit.ly/3oUW0SN
- Introduction
Tuberculosis (TB) is a major cause of morbidity and mortality worldwide. TB is caused by the bacillus Mycobacterium tuberculosis (Mtb ), which is spread via airborne droplets. Approximately one in four people worldwide demonstrate an immunological response to Mtb infection, which can remain dormant or progress into active disease forms [ 1 ]. Patients infected with TB who have no active signs or symptoms of disease were previously deemed to have latent TB, more recently changed to TB infection [ 2 ]. Whereas patients with active disease are termed to have TB disease. Patients with TB infection have a 5–10% lifetime risk of developing TB disease, which increases in varying states of immunodeficiency up to a 16% annual risk of activation of TB infection into TB disease in HIV patients [ 3 ]. In 2019, there were an estimated 10 million new incident cases of active TB disease worldwide [ 1 ]. Approximately two-thirds of all cases arise in eight countries alone, the vast majority of which have overwhelmed health services with limited resources [ 1 ]. This significant global burden of disease has been recognised by the World Health Organization (WHO) who launched the End TB initiative in 2016. Their aim is to reduce incidence, morbidity and mortality of this disease by improving diagnostic and therapeutic practices, as well as developing preventative strategies, through innovative research and education. By 2035, the goal is to reduce TB mortality by 95% and reduce overall incidence of TB by 90% worldwide [ 4 ]. Owing to the work of our predecessors, it has been estimated that 60 million lives have been saved globally in the 21st century so far [ 5 ].
Effective TB treatment is dependent on:
Prompt diagnosis of TB and recognition of drug resistance;
Promoting and ensuring patient adherence to regimens;
Robust contact tracing and prophylactic treatment of contacts; and
Screening for TB infection in high-risk groups.
There is ongoing extensive research into developing accurate, timely methods of detecting drug resistance, even in resource poor settings. Many effective, less toxic medications are under development. Furthermore, methods of promoting and ensuring drug adherence are being reviewed. In addition, there is vital research ongoing in proactive areas of TB prevention, such as screening for, and treatment of, TB infection and developing efficacious vaccines to halt the spread of this killer disease.
The aim of this article is to: review current practice in the diagnosis and treatment of TB; outline new diagnostic techniques under development; discuss new drug therapies and treatment regimens under review; and review the evidence for vaccination.
Improving the efficiency and accuracy of TB diagnosis contributes to treatment efficacy. Pulmonary TB should be suspected when patients present with classical symptoms such as non-resolving cough, haemoptysis, fevers, night sweats and weight loss. Extrapulmonary TB, including TB lymphadenitis, TB meningitis, laryngeal TB, Pott's disease and abdominal TB, presents in a variety of manners. Special consideration should always be given to patients who have potential TB exposure, as well as immunocompromised patients who may present atypically. The diagnosis must be made by confirming the presence of the causative pathogen, Mtb . A variety of methods are employed to confirm the diagnosis. In addition, it is essential that there is emphasis on early detection of potential drug resistance.
Drug resistance is a growing issue that threatens TB care worldwide. Traditionally this was categorised into rifampicin-resistant TB (RR-TB), multidrug-resistant TB (MDR-TB) or extensively drug-resistant TB (XDR-TB). MDR-TB is resistant to both rifampicin (RIF) and isoniazid (INH). Recently definitions have been updated to include pre-XDR-TB, which is TB that fulfils the definition for MDR-TB and RR-TB that is also resistant to any fluoroquinolone (FLQ). The updated definition for XDR-TB is strains that fulfil the definition for MDR-TB/RR-TB which are also resistant to any group A drug (namely levofloxacin (LFX), moxifloxacin (MFX), bedaquiline (BDQ) and linezolid (LZD)) [ 6 ]. Replacing the old XDR-TB definition referencing second-line injectable drugs (SLID), it highlights the trend towards use of oral regimens comprising recently developed or repurposed drugs. Despite the importance of early recognition, only 61% of patients with a new diagnosis of bacteriologically confirmed TB disease in 2019 were tested for RIF resistance [ 1 ]. This is in part related to access to diagnostics in resource-limited settings. There are numerous methods currently available, and under development, to determine drug resistance. For these diagnostics to be beneficial on a global scale they need to provide timely, accurate, cost-effective results in centres where access to power, equipment and technical expertise remains limited.
Culture of Mtb in a suitable medium remains the gold standard diagnostic test. The specimen can be cultured in solid ( e.g. Löwenstein–Jensen or Middlebrook 7H11) or liquid media ( e.g. for use with the BACTEC Mycobacterium Growth Indicator Tube (MGIT) 960 system). Sensitivity, specificity, contamination rates and time to detection vary widely amongst both media, with the WHO advocating for dual use of systems where practical. The major benefit of the advent of liquid-based systems is the rapid time to detection, often reducing time to growth by half with a mean time to detection of 12.8 days compared with 25.1–25.5 days for the previously mentioned solid media [ 7 ]. However, sub-optimal laboratory facilities in resource-limited settings often restrict its practical use [ 8 ]. While culture is not recommended for use as a first-line test, it remains an important part of TB diagnostics where persistent culture positivity can predict likelihood of relapse [ 9 ].
Direct microscopy
Direct microscopy is a fast and inexpensive method to identify acid-fast bacilli (AFB), the majority of which are mycobacteria [ 10 ]. Traditionally, Ziehl–Neelsen (ZN) stain was applied and the sample termed “smear positive” or “smear negative”, depending on the presence or absence of AFB. Efficacy is operator dependent, resulting in a broad range of sensitivities and specificities reported in international studies, 25.3–81.6% and 83.4–99%, respectively [ 11 , 12 ]. It is even less sensitive in high-risk groups, such as patients with HIV, and children [ 1 ]. Methods to improve efficacy include use of mercury vapour fluorescence and light-emitting diode (LED) microscopy, which have largely replaced traditional ZN staining [ 13 ]. Education and quality assurance for laboratory technicians is one of the most useful ways to ensure accurate diagnosis, as direct microscopy often remains the only method of diagnosis available in resource-limited settings [ 14 ]. Similar to culture, direct microscopy remains an integral part of monitoring response to treatment, measuring infectiousness, and predicting likelihood of relapse in patients who are smear positive at diagnosis.
Molecular tests
Given the limitations of culture and direct microscopy, the WHO recommends a biomolecular test as the initial diagnostic test in a suspect patient [ 1 ]. Current molecular tests endorsed by WHO include: Xpert MTB/RIF and Xpert MTB/RIF Ultra assays (Cepheid, Sunnyvale, USA); loop-mediated isothermal amplification test (TB-LAMP; Eiken Chemical, Tokyo, Japan); Truenat MTB, MTB Plus and MTBRIF Dx tests (Molbio Diagnostics, Goa, India) and lateral flow urine lipoarabinomannan assay (LF-LAM; Alere Determine TB LAM Ag, Abbott, San Diego, USA).
The WHO currently recommends Xpert (MTB/RIF or MTB/RIF Ultra) or Truenat (MTB or MTB Plus) as the initial diagnostic test of choice in suspected pulmonary TB [ 1 ]. They are cartridge based nucleic acid amplification tests (NAAT) that detect the presence of TB DNA, as well as common mutations associated with RIF resistance along the rpoB gene, within 2 h [ 15 ]. The Xpert MTB/RIF and Xpert MTB/RIF Ultra assays are also endorsed by the WHO for diagnosing extrapulmonary TB and TB in children [ 1 ]. When compared with culture diagnosis, the Xpert assays have demonstrated 89% sensitivity and 99% specificity at diagnosing pulmonary TB in adults [ 16 ]. The Xpert MTB/RIF Ultra assay has a higher sensitivity but lower specificity than the Xpert MTB/RIF assay, owing to its inability to accurately differentiate between dormant and active TB DNA [ 17 , 18 ]. While recommended for use, it is important to remember these assays have reduced sensitivity in certain populations such as children and patients coinfected with HIV, as well as in extrapulmonary TB [ 16 , 19 ]. Moreover, this technology is expensive and requires laboratory facilities with continuous access to power. To overcome this obstacle in resource-limited settings, there are a number of smaller, battery-operated technologies in development. To date, the GeneXpert Omni (Omni; Cepheid) appears to be the most promising potential candidate for widespread use. In a real-world analysis, it has been shown to be a cost-effective method when used in peripheral healthcare settings [ 20 ]. It allows diagnosis to be at/near the point of care, and thus avoids further delays and costs associated with transporting samples to specialised centres.
As well as the Omni, Cepheid is also developing the Xpert MTB/XDR assay. It aims to also detect resistant to INH, FLQ, ethionamide (ETH) and SLID. Similar to other Xpert assays, it is a NAAT that detects 16 clinically relevant mutations associated with resistance in under 90 min [ 21 ]. When compared with phenotypic drug sensitivity testing (pDST), it has a 94% sensitivity and 100% specificity at detecting drug resistance [ 21 ]. There are large scale multicentre clinical trials ongoing to establish its real-world efficacy as a follow-on test to current Xpert MTB/RIF and MTB/RIF Ultra assays, prior to consideration for WHO recommendation. This assay is of paramount importance as the early recognition of drug resistance is a prerequisite to shorter drug regimens, which will be discussed in further detail elsewhere in this review.
While most biomolecular tests are NAAT detecting the presence of Mtb DNA, the LF-LAM test detects a lipopolysaccharide present in mycobacterial cell walls. While not in use in most countries in the developed world, the LF-LAM assay has been recommended for use in HIV-coinfected patients. It is a urinary antigen test that is often employed in resource-limited settings, and is of particular benefit in cases where a sputum sample cannot be obtained. It has a 42% sensitivity in HIV patients with TB symptoms [ 22 ]. However, it cannot distinguish between mycobacterial species, and can cross react with other fungal diseases. As such, it is used as an initial test in peripheral primary care centres in areas of high TB endemicity only, to determine whether symptomatic patients with HIV should be referred for further confirmatory testing [ 23 ].
Line probe assays
Another method of molecular detection of Mtb resistance is line probe assay (LPA). Genotype MTBDR plus and Genotype MTBDR sl (Hain LifeScience GmbH, Nehren, Germany) are used for the detection of Mtb and its associated drug resistance. The WHO approved Genotype MTBDR plus employs a series of steps to detect Mtb and mutations in rpoB and katG , which confer RIF and INH resistance, respectively [ 24 ]. Additionally, it can detect the presence of inhA promoter genes that confer resistance to low dose INH, which are also typically associated with ETH and prothionamide resistance [ 25 ]. This in vitro test delivers results in <6 h [ 26 ]. When compared with traditional culture-based drug sensitivity, it is 78.5% sensitive and 100% specific at detecting RIF and INH resistance [ 27 ]. The WHO endorsed Genotype MTBDR sl 2.0 assay can also detect resistance conferring mutations of FLQ ( gyrA and gyrB ) and SLID ( rrs and eis ) [ 28 ]. Reported sensitivity and specificity are 100% and 98.9% for FLQ, and 89.2% and 98.5% for SLID [ 29 ]. Even more sensitive than NAAT at detecting FLQ resistance, this rapid test could allow for use of FLQ in patients that might otherwise have faced a lengthier regime that potentially required the interim use of SLID. However, these tests are not without limitations including low sensitivity for detecting ethambutol (ETM) and aminoglycoside resistance as demonstrated in a real-world analysis [ 30 ]. Similar in aim to the Xpert MTB/XDR assay, these LPAs provide prompt recognition of drug resistance, so patients can be started on the appropriate regimen and further drug resistance does not have an opportunity to develop while awaiting standard culture-based susceptibility results, nor are patients exposed to burdensome, longer drug regimens with higher potential for toxicity.
Whole genome sequencing (WGS)
While NAAT and LPA tests are rapid, accessible diagnostics, their efficacy at detecting drug resistance is hindered by the inability to detect clinically relevant mutations outside the rifampicin resistance determining region (RRDR) of the rpoB gene [ 31 ]. While 95% of resistant cases arise from mutations in this region, there have been a number of public health crises emerging from missed diagnosis of outbreaks that have arisen from mutations outside it [ 32 ]. One such example is the I491F mutation that has been responsible for an outbreak of MDR-TB in Eswatini and remains a grave public health concern [ 33 ]. Another limitation is the inability to differentiate silent mutations from those that hinder drug efficacy, thus delivering a higher rate of false positive resistance results [ 34 ]. WGS provides a comprehensive review of the entire Mtb genotype with a 96% concordance for culture-based sensitivity testing [ 35 ]. It provides genotypic sensitivity to most drugs required for treatment of MDR-TB [ 36 ]. While full clarification on clinical correlation between genotypic and phenotypic sensitivities remains to be shown, progress has been made in assigning probability of pDST based on genotypic results [ 37 ]. Utility was initially limited in low-income countries by cost and requirement for robust facilities and technical expertise [ 38 ]. However, with ongoing technological advancements in the microfluidic approaches to TB diagnosis, WGS is likely to be available at point of care on a global basis [ 39 ]. For some countries, it remains an important tool not only in case diagnosis, but in formulating public health policy by assisting in tracing TB contact cases in outbreaks [ 40 ]. In the future, with improved knowledge of the genomics involved in TB resistance, WGS is likely to prove revolutionary in tailoring TB treatment to each individual patient based on the particular genome identified by the Mtb strain they have contracted.
Culture-based drug sensitivity testing (DST)
As previously mentioned, the major advantage of liquid culture is rapidity of growth, which has led to more widespread use of liquid broth-based methods such as the MGIT. BACTEC MGIT 960 is a fully automated system that delivers results within 2 weeks [ 41 ]. Culture-based DST remains the gold standard for determining drug resistance at present [ 1 ]. The two approaches currently in use are the critical concentration and minimum inhibitory concentration (MIC). Classically, critical concentration was defined as the lowest concentration of a drug that inhibits growth of 95% of Mtb strain present. Owing to ongoing research, these critical concentrations are regularly updated with a recent reduction in the critical concentration required to determine RIF resistance, allowing for greater concordance between genotypic and phenotypic sensitivity results [ 42 ]. Alternatively, the MIC method is defined as the lowest concentration of a drug that results in complete inhibition of visual growth of the Mtb strain in vitro . Following extensive work completed by national reference laboratories, and international discussion and agreement, a new reference MIC protocol has been set and validated by European consortia [ 43 ].
Computer aided detection for chest radiographs
Given the limitations, in terms of time, cost and infrastructure, to the above testing methods, it has become clear that there need to be affordable, accessible methods of screening available in high-burden areas to assist with risk stratification for allocating further testing. One such proposed method is the use of computer software to digitally interpret chest radiographs, and assign a score indicating the likelihood of TB. The most commonly studied software is CAD4TB, currently on version 6. When compared with NAAT, CAD4TB has been shown to have 90–100% sensitivity, and 23–45% specificity at detecting TB disease [ 44 ]. It performs similarly to expert clinicians and radiologists, with similar pitfalls including disease obscured by musculoskeletal findings and differentiating old scarring from new disease. Its use is intended for high-burden areas, that may lack readily accessible radiological expertise on site to interpret chest radiographs in a timely fashion [ 45 ]. It may assist peripheral health centres to determine which patients require further molecular testing.
Serum biomarkers
Another potential method for triage testing is serum biomarkers. Devising an accurate biomarker that upholds sensitivity across different ethnicities, HIV status and site of TB has proven difficult. However, a nine protein biosignature has recently been discovered which appears to remain efficacious in all of these cohorts. Using fibrinogen, α 2 -macroglobulin, C-reactive protein, matrix metalloproteinase-9, transthyretin, complement factor H, interferon-γ, interferon-γ inducible protein-10 and tumour necrosis factor-α as a host biosignature demonstrated 92% sensitivity and 72% specificity for determining TB from other diseases [ 46 ]. If available on a commercial level, this serum assay could rapidly and effectively determine which patients warranted further testing. It is important to note that most of these biomarkers are markers of inflammation, and as such are widely variable amongst patients and their differing metabolic and disease states. Evaluating serum biomarkers as predictors of response to treatment, potential for relapse and predictors of TB infection versus active disease will be discussed elsewhere.
Alongside research into obtaining accurate and timely diagnostics, there is tremendous work ongoing in developing safe, efficacious, tolerable treatment regimens. The goals of treatment are not only to eradicate disease, but to prevent long-term morbidity arising from either the disease itself or as an adverse effect of the drugs in use. Successful treatment of drug-sensitive TB (DS-TB) has been reported in 85% of patients [ 1 ]. Efficacy in drug-resistant forms is lower at 57% and is likely multifactorial [ 1 ]. To reflect this, there has been a trend towards oral drug regimens, where possible, given research highlighting patient preference and cost-effectiveness of these drugs [ 47 ]. We need to deliver a regimen that will not only aid our global goal of TB eradication, but in a manner that reflects our patients’ wishes, and in doing so, promotes their compliance.
Current treatment
The current medications approved for use in TB treatment, and their notable side-effects are summarised in table 1 .
- View inline
Current medications in use for TB treatment and their notable side-effects
DS-TB tends to follow a standard 6-month regime. This comprises an intensive phase with 2 months treatment consisting of RIF, INH, pyrazinamide (PZA) and ETM, followed by a continuation phase with 4 months treatment of RIF and INH [ 48 ]. If the isolate is susceptible to both RIF and INH, ETM can be stopped. The continuation phase should be extended to 7 months in the presence of: cavitation on the initial chest radiograph; persistent sputum growth at 2 months; or if PZA cannot be used due to monoresistance or drug side-effects. Consideration should also be given to extending this phase to 7 months in patients who are otherwise immunosuppressed, such as patients with HIV, diabetes mellitus, malignancy or medications associated with immunosuppression [ 48 ]. Unfavourable outcomes are most associated with high grade smear positivity (at least 3+) and dependent on the size of cavities, as well as extent of disease on chest radiographs [ 49 ].
Current treatment of drug-resistant TB is more complex and is summarised in table 2 . Most notable is the longer duration of treatment involving combinations of drugs that are often poorly tolerated. There is also minor discordance between the two major international advisory bodies (the WHO and the joint ATS/CDC/ERS/IDSA clinical practice guideline) concerning optimum drug selection and durations. While the WHO recommends only four drugs need to be used in the intensive phase of treatment, the ATS/CDC/ERS/IDSA propose continuing to use five drugs in this phase. The ATS/CDC/ERS/IDSA have proposed this recommendation based on higher success rates in the five-drug group (93.9% versus 89.7%; adjusted odds ratio (aOR) 3.0 versus 1.2; risk difference 8% in both groups). Additionally, they suggest it is likely that one of the drugs may need to be withdrawn due to toxicity [ 50 ]. However, given equivocal risk differences in both groups, the WHO maintain four drugs should be sufficient, providing susceptibilities are known and toxicity is unlikely. De-escalation to a continuation phase comprising three or four drugs is based on similar evidence. Traditionally, MDR-TB required treatment for a total duration of 15–21 months [ 50 ]. Alternatively, it does allow for a shorter 9–12 month all oral regimen for patients who have not previously had more than 1 month of treatment with second-line medications, and in whom FLQ resistance has been ruled out. Additionally, patients should not have extensive disease [ 51 ]. This shorter regimen involves 4 months of six drugs (FLQ, clofazimine (CFZ), ETH, PZA, INH (high dose)), followed by 5 months of FLQ, CFZ, ETH and PZA. BDQ is used concurrently for the first 6 months of this regimen. This conditional recommendation of low certainty evidence was proposed owing to improved success and adherence rates, when compared with shorter regimens containing injectable agents (aOR 1.9 (95% CI 1.6–2.4)) [ 52 ]. (Note, INH is used regardless of susceptibility status).
Current ATS/CDC/ERS/IDSA consolidated guidelines on treating drug-resistant TB
At present, the WHO recommends treatment for RR-TB in line with MDR-TB.
Pre-XDR- and XDR-TB are more difficult to treat, owing to varying patterns of drug resistance and advice should always be sought from national and international expert TB consortia prior to commencing treatment.
New treatment: drugs
At present, there are 16 new drugs in phase I or II clinical trials, and 22 other drugs in discovery or preclinical phases of development, as outlined in figure 1 . Of those drugs undergoing clinical trial, there are 11 drugs of new chemical classes. Of the remaining drugs, TBAJ-587 and TBAJ-876 are diarylquinolines, similar to BDQ, while delpazolid, sutezolid and TBI-223 are oxazolidinones, similar to LZD and cycloserine. At the time of publication, no new drugs have reached phase III trials or been approved for market regulation since the approval of pretomanid (Pa) in 2019. A promising candidate from a new drug class is telacebec. It induces bacterial cell death by inhibiting the mycobacterial cytochrome bc1 complex responsible for ATP synthesis. A proof-of-concept trial has shown increased rates of sputum clearance, with comparable levels of adverse events to currently approved drugs. If results from ongoing clinical trials continue to reflect this, it is likely to be approved as a third new modern drug class with anti-tuberculous activity [ 53 ]. This would be an important achievement as many of the other drugs in development are classified similarly to existing drugs, and as such their use in additive or substitutive places for their relative counterparts will be precluded due to concerns regarding toxicity or resistance. It is also interesting to note that these drugs in development are largely oral preparations, owing to patient preference and thus potential for greater adherence and cure.
- Download figure
- Open in new tab
- Download powerpoint
New anti-tuberculous drugs currently in development. Data from [ 104 ].
New treatment: routes of delivery
While not a novel idea, interest in inhalation routes has been re-ignited. Numerous methods of drug delivery have been shown to be effective in animals, and additional advantages include reduced dosage and systemic toxicity. However, it would likely have no benefit on extrathoracic disease, nor would it be likely to achieve adequate therapeutic serum concentrations. Similar to the use of nebulised aminoglycosides in non- Mycobacterium tuberculosis , the potential for inhalational therapies to augment TB therapy likely lies as an adjunctive therapy to oral or injectable drug regimens. To date, there has been no data published from similar trials investigating its efficacy in sputum clearance from Mtb disease.
New treatment: regimes
In a disease that has the potential to affect one quarter of the world's population, it is astounding that no advances have been made in progressing the regimen for DS-TB since the mid-20th century. At present, international consensus guidelines continue to endorse standard 6-month regimes for the majority of cases of DS-TB, with varying longer regimens requiring expert opinion for drug-resistant cases. However, much research is being done into assessing shorter regimens with the aim of improving patient adherence and reducing risk of relapse and evolution of drug resistance, as seen in table 3 .
A shorter 4-month regimen of rifapentine (RFP) in combination with MFX has recently been shown to be non-inferior to the current standard 6-month regimen, as determined by negative smear or culture at 12 months, with no increase in major adverse events [ 54 ].
The SimpliciTB group have evaluated a 4-month regimen comprising BDQ, Pa, MFX and PZA, in place of the standard 6-month regimen. While it has been said that no drug should ever be kept in reserve, it is unlikely that this regimen will be recommended as first-line therapy for DS-TB, given the need to preserve efficacious drug options for resistant cases [ 55 , 56 ].
Shorter again with a 2-month regime, the TRUNCATE-TB trial is at recruitment phase. This multi-armed approach will assess combinations of 4–5 currently approved oral anti-tuberculous medications given daily for 8 weeks, with the potential to extend to 12 weeks [ 57 ].
The RIFASHORT and ReDEFINe studies are evaluating the risk–benefit ratio of higher doses of RIF in DS-TB [ 58 , 59 ]. The evidence base for these ongoing trials has been provided by the HIRIF trial which found an increased rate of sputum clearance, with no associated increase in toxicity, in patients on higher doses of RIF than currently recommended by the WHO [ 60 ].
2) Drug-resistant TB
• The current recommendations for RR-TB have been considered contentious for quite some time. These longer regimens likely expose patients with mono-resistance to unnecessarily long and toxic drug regimens, and also exclude the benefits of INH therapy [ 61 ]. BEAT TB is at the enrolment stage assessing the efficacy of 6 months of BDQ, LZD, delamanid (DLM), LFX and CFZ in comparison to current practices in South Africa [ 62 ].
• The updated STREAM2 study is evaluating a shorter regimen for RR-TB and MDR-TB in a simultaneous multi-armed approach. Their four regimens are based on: current WHO practice; the Bangladesh regime; a 40-week all oral regimen; and a 28-week oral regimen after an 8 week intense regime that also involves INH and kanamycin (Kan) [ 63 , 64 ].
• Results from the NEXT trial completed in December 2020 are awaited. This group compared 6–9 months of LZD, BDQ, LFX, PZA and ETH or INH (high dose) to current standards of care [ 65 ].
• TB-PRACTECAL stopped early due to superior outcomes in the intervention arm, consisting of a 6-month regimen of BDQ, Pa, LZD and MFX. Full results are awaited [ 66 , 67 ].
• SimpliciTB are also evaluating a regimen for RR-TB/MDR-TB consisting of the same drugs as the DS-TB protocol (BDQ, PZA, MFX, Pa) but for 6 months [ 56 ].
• DELIBERATE are completing a phase II safety trial reviewing the safety and pharmacokinetics of combined BDQ and DLM therapy. Given the updated consensus guidelines, these drugs will often be given together and it is essential we have an evidence base for potential harms that may arise throughout the course of treatment [ 68 ].
• endTB, run by Médecins Sans Frontières, are evaluating a multi-armed approach combining varying combinations of an all oral regimen for 39 weeks. Similar to the STREAM2 study, this is the only other multi-armed trial reviewing multiple combinations of novel drugs simultaneously [ 69 ].
• The ZeNix trial is the only trial, at present, that is reviewing treatment regimens for patients with pre-XDR- or XDR-TB. Using BDQ, Pa and either LZD (BPaL) or placebo for a total duration of 26 weeks, their aim is to assess rates of sputum conversion. This trial is also one of the few to follow patients for a significant period post-treatment, and patients with be reviewed for 78 weeks following the end of treatment [ 70 ]. Data from its predecessor the Nix-TB trial has shown 88% favourable outcomes at 24 months following treatment in patients with either MDR- or XDR-TB [ 71 ]. This BPaL regime can currently be used under operational research conditions in patients with MDR-TB, in accordance with WHO guidance [ 51 ].
New drug regimens under evaluation
While many of these trials demonstrate promise for an improved approach to TB treatment, it is essential that we see long-term data on their efficacy and relapse rates prior to implementing them on a global scale. The fear is that these patients may have excellent short-term results, but disease recurs soon after with the added potential for drug resistance to develop.
New treatment: adjuncts
In addition to shorter regimens, with new or re-purposed drugs, there is research into methods of modifying the host immune response to improve treatment outcomes and prevent permanent morbidity from TB disease. As previously discussed, upon infection with Mtb the host can either suppress bacillary replication into a latent state, or the host is overwhelmed and active disease develops [ 72 ]. Both deficient and hyperinflammatory states have been associated with TB disease morbidity and mortality, suggesting that tailoring a balanced immune response is of paramount importance to survival [ 73 ]. With evolving knowledge of the pathways and subcellular responses involved, new therapeutic targets are being developed to assist with bacillary quiescence in the so called “host directed therapy” approach [ 74 ]. Numerous drug targets have been suggested, largely centred on modulating macrophage activity [ 75 ]. Proposed adjunctive therapies include vitamin D, everolimus, auranofin and CC-11050, a novel anti-inflammatory compound. Preliminary results from trial data suggest none of these compounds improve rates of sputum conversion; however, patients in receipt of CC-11050 or everolimus had increased recovery of FEV 1 (forced expiratory volume in 1 s) post-treatment, perhaps solidifying the role of a balanced immune response to infection [ 76 ].
New treatment: the future
Going forward, with a combination of new drugs, altered durations and more effective testing of response to treatment, it is likely that each patient will have a tailored approach to TB treatment [ 49 ]. With studies like PredictTB, aiming to determine biomarkers and radiographic appearances that predict response and likelihood of relapse, we will be able to devise a drug combination and duration with greater specificity for each patient [ 77 ]. Similar technology may even assist with developing even more efficacious drugs in early-stage clinical trials [ 78 ]. Additionally, it is essential that any new drug or technology developed is affordable and available to all institutions, most importantly hospitals in low-resource environments, where the majority of the global TB burden persists.
Despite ongoing research, treatment for DS-TB has remained unchanged for decades. This highly effective regimen is often poorly tolerated by patients, and “drug holidays” are frequent during treatment. This, of course, increases the likelihood of relapse and evolution of drug resistance. Moreover, patients with resistant TB have to endure longer regimens with their own associated side-effects. While awaiting the development and approval of less toxic regimens, there are a number of measures we can take to ameliorate adverse effects of treatment and promote patient adherence. It has been shown that comprehensive patient-centred approaches, involving nutritional, financial and psychological support, have higher rates of completion. In addition, patients with increased contact with healthcare workers tended to have lower drop-out rates during treatment [ 79 ]. The evidence base for this is provided by systematic reviews of mostly observational case studies and case cohorts, and as such randomised research in this area is required to determine a formal link.
Directly observed therapy
Directly observed therapy (DOT) has been a standard of care in TB treatment for several years. The premise is that patients are more likely to comply if medication ingestion is witnessed multiple times per week. Current recommendations are that it should be implemented in MDR- or XDR-TB cases, or for patients with complex or vulnerable care needs, such as homelessness, comorbid psychiatric illness or addiction [ 80 ]. There have been conflicting results from systematic reviews on the efficacy of DOT [ 81 , 82 ]. What is known, is that community-based DOT appears to be the most effective strategy, as it is less disruptive for patients and thus their adherence is more likely to be maintained [ 83 ]. In recent years, attention has switched towards the use of smartphone technology. Video observed therapy (VOT) has been suggested as an even less disruptive form of monitoring adherence [ 84 ]. Patients can either upload videos of medication ingestion to a secure platform to be watched at a later date, or it can be taken while on a live feed with their healthcare team. VOT has been shown to have a higher uptake rate and patient preference rating [ 85 ]. While plausible that this will improve adherence, and thus relapse should be less likely, this study was not sufficiently powered to assess this, nor did it follow up on relapse rates at an appropriate interval. A real-world efficacy and cost-effectiveness study is ongoing in a tertiary hospital in Ireland at present [ 86 ].
- Prophylaxis
Undoubtedly, a burden of TB infection will persist for years to come. However, we have a chance to prevent many of these patients from progressing to active disease. Screening for TB infection in groups at high risk of progressing to TB disease remains a cost-effective and essential component to the global initiative. Screening via either of the endorsed interferon-γ release assays (QuantiFERON-TB Gold In-Tube and T-SPOT.TB) or traditional tuberculin skin testing is recommended in certain populations. The WHO has advised that clinical judgement is paramount in interpreting these tests, and cautions that a higher rate of false negatives occurs in the most vulnerable populations [ 87 ]. Another essential component of the sustainable development goals is robust public health policy to assist in contact tracing of index cases and early treatment of contacts. In addition, prior to any prophylactic treatment being commenced, it is essential that due caution is taken to rule out the presence of active TB disease.
Currently the WHO advocates for treatment with 4 months of RIF or 6–9 months of INH in cases where the index case is known to be drug sensitive [ 87 ]. A 3-month combination of RIF and INH is also approved, although rarely used due to potential toxicity. Additionally, weekly INH and RFP for 3 months has been shown to demonstrate equal efficacy and toxicity in comparison to 6 months of INH therapy, while higher levels of adherence were noted in the INH/RFP arm [ 88 ]. Moreover, a 1-month regimen of RFP/INH therapy was non-inferior to 9 months INH monotherapy in preventing TB in HIV-infected patients [ 89 ]. However, this regimen has yet to be endorsed by major international consortia.
The recommendations for TB contacts of DS-TB cases who demonstrate evidence of TB infection are as per those above. For contacts of MDR-TB cases, the current recommendation is for 6–12 months treatment with a FLQ with or without a second drug. If a FLQ cannot be used due to resistance in the index case, treatment with ETM and PZA is to be considered [ 87 ]. Regardless of the regimen in use, it is vital that strict adherence is maintained to ensure efficacy and prevent resistance.
At present, the decision to treat is based on the potential for progression to active disease based on similar case profiles. Going forward, we could vastly improve the cost efficacy of this intervention by being able to determine exactly which patients were going to progress to active TB disease or not. It had been hoped the answer would lie in serum transcriptional biomarkers and host response-based gene signatures [ 90 , 91 ]. Recently, a four-protein biomarker panel has shown 67.3% sensitivity and 96.3% specificity at determining active from latent TB [ 92 ]. This subclinical phase of TB disease can be difficult to interpret due to its lower inflammatory profile and person specific confounding factors that influence our immune response. Recent results from transcriptomic studies have been disappointing overall, but may potentially suggest a role for these panels in symptomatic patients with known TB infection and their risk of progression to TB disease in an imminent 6-month period [ 93 ].
- Vaccination
Given the current prevalence of TB infection, with the associated lifetime risk of progressing to active disease, it is paramount that we protect future generations from this burden by halting transmission entirely. With greater understanding of the cellular processes involved in Mtb susceptibility and pathogenesis, scientists have been able to identify various potential targets with a role in vaccination. Central to this is the cellular immune response, with a need to upregulate T-helper cell (Th)1, and downregulate Th2 and regulatory T-cell responses [ 94 ]. It appears that Mtb has also recognised the need to adapt to this hypo-inflammatory phenotype with more modern strains displaying shorter latency and higher virulence than previously seen [ 95 ].
The only worldwide approved vaccine against TB remains bacillus Calmette–Guérin (BCG), effectively reducing the risk of severe childhood disease from TB, with an 85% reduction in TB meningitis and miliary TB in those <10 years of age [ 96 ]. It has also been noted that infants innoculated with BCG have increased survival and lower rates of other childhood infections. This observation is likely secondary to BCG's ability to prime innate immunity through epigenetic modification of innate immune cells [ 97 ].
Vaccination can be categorised into preventive pre-exposure, preventive post-exposure or therapeutic [ 98 ]. Vaccines can alternatively be classified according to their biochemical forms: live attenuated, inactivated, protein subunit or recombinant [ 99 ]. With each of these forms, the aim is to target various cells or subcellular components of TB pathogenesis.
MTBVAC, a pre-exposure live attenuated vaccine, has shown promising results from preclinical trials with a higher protection against TB than BCG [ 100 ]. This live vaccine is based on a genetically modified mutant Mtb strain containing deletions in transcription factors important for Mtb growth in macrophages and subsequent virulence.
VPM1002, another live recombinant BCG vaccine, is undergoing phase III studies at present to evaluate its efficacy at not only preventing infection, but in preventing active disease in those already affected [ 101 ]. This vaccine can modify T-cell immune response and enhance Th1 immunity, important in TB disease pathogenesis.
Another promising post-exposure candidate is M72/AS01E, a subunit vaccine, that prevents pulmonary TB in adults already infected with Mtb in 54% of patients, and thus could be a potentially life-saving intervention for one quarter of the world's population [ 102 ]. Also known as Mtb72F this vaccine comprises two immunogenic proteins that promote T-cell proliferation and interferon-γ release [ 103 ].
Further randomised control trials are warranted in a timely manner if the END TB strategy is to be achieved.
The future is bright for TB treatment. Never before has there been such a global effort to develop new technologies and treatment for TB patients. Combining these advancements, it is possible that we will base each patient's treatment on their own protein biosignatures in conjunction with the genomic expression of mutations in the Mtb strain they have been affected with. If we are to achieve our goal of global eradication of TB, it is essential that we continue to collaborate and share our expertise on an international scale to ensure each patient gets the appropriate treatment and support to overcome their TB diagnosis without significant morbidity.
Self- evaluation questions
1. What proportion of the world's population are estimated to have TB infection ( i.e. demonstrate immunological evidence of prior TB exposure)?
2. Which of the following is a WHO recommended first-line test in the diagnosis of TB disease?
b) Biomolecular test ( e.g. Xpert MTB/RIF or Truenat MTB)
c) Line probe assay ( e.g. Genotype MTBDR plus )
d) Serum interferon-γ release assay
3. Pre-XDR-TB is defined as TB that is resistant to rifampicin, isoniazid and what other drug(s)?
a) Linezolid
b) Second-line injectable drugs ( e.g. amikacin)
c) Fluoroquinolones ( e.g. moxifloxacin)
d) Bedaquiline
4. Which of the following regimens are not currently recommended by the WHO for preventive treatment of TB disease ( i.e. treatment of TB infection)?
Rifampicin (10 mg·kg −1 up to 600 mg maximum) daily for 4 months
Isoniazid (5 mg·kg −1 up to 300 mg maximum) daily for 6–9 months
Rifampicin (10 mg·kg −1 up to 600 mg maximum) and Isoniazid (5 mg·kg −1 up to 300 mg maximum) daily for 3 months
Rifapentine (900 mg if weight >50 kg, adjusted if less) and Isoniazid (15 mg·kg −1 up to 900 mg maximum) weekly for 1 month
Suggested answers
Conflict of interest: C.M. Gill has nothing to disclose.
Conflict of interest: L. Dolan has nothing to disclose.
Conflict of interest: L.M. Piggott has nothing to disclose.
Conflict of interest: A.M. McLaughlin has nothing to disclose.
- Received October 1, 2021.
- Accepted November 16, 2021.
- Copyright ©ERS 2022
Breathe articles are open access and distributed under the terms of the Creative Commons Attribution Non-Commercial Licence 4.0.
- World Health Organisation
- Selwyn PA ,
- Lewis VA , et al.
- Uplekar M ,
- Lonnroth K , et al.
- Centers for Disease Control and Prevention
- Drancourt M
- Gooze L , et al.
- Sotgiu G , et al.
- Sharma PP , et al.
- Ga S-P , et al.
- Singhal R ,
- Lumb R , et al.
- Bates M , et al.
- Steingart KR ,
- Schiller I ,
- Horne DJ , et al.
- Dorman SE ,
- Schumacher SG ,
- Alland D , et al.
- van Soolingen D
- Kendall E , et al.
- Gaur RL , et al.
- Bjerrum S ,
- Dendukuri N , et al.
- Streicher EM ,
- Hoek KG , et al.
- Somoskovi A ,
- Dormandy J ,
- Rivenburg J , et al.
- Kuo YM , et al.
- HAIN LifeScience
- Dreyer AW ,
- Koornhof HJ , et al.
- Pleń M , et al.
- Lau TC , et al.
- Sanchez-Padilla E ,
- Beckert P , et al.
- Palacios JJ ,
- Herranz M , et al.
- Nikolayevskyy V ,
- Kranzer K ,
- Niemann S , et al.
- McNerney R ,
- Preston MD , et al.
- Allix-Béguec C ,
- Arandjelovic I , et al.
- CRyPTIC Consortium and the 100,000 Genomes Project
- Eddabra R ,
- Ait Benhassou H
- Harrison J ,
- McGrath JS , et al.
- Roycroft E ,
- Fitzgibbon MM ,
- Kelly DM , et al.
- Tortoli E ,
- Cichero P ,
- Piersimoni C , et al.
- Werngren J ,
- Machado D , et al.
- Jeagal L , et al.
- Zaidi SMA , et al.
- Morris TC ,
- Hoggart CJ ,
- Chegou NN , et al.
- Migliori GB ,
- Zumla A , et al.
- Alipanah N , et al.
- Imperial MZ ,
- Phillips PPJ , et al.
- Migliori GB , et al.
- Mirzayev F ,
- Linh NN , et al.
- de Jager VR ,
- van Niekerk C , et al.
- Kurbatova EV , et al.
- ClinicalTrials.Gov
- Velasquez GE ,
- Brooks MB ,
- Coit JM , et al.
- Malenfant JH ,
- Van Deun A ,
- Salim MA , et al.
- Medecins San Frontieres
- Conradie F ,
- Ngubane N , et al.
- Oh SF , et al.
- Palucci I ,
- Whitworth LJ ,
- Redmond S , et al.
- Wallis RS ,
- Ginindza S ,
- Beattie T , et al.
- Daftary A ,
- O'Donnell M , et al.
- National Institute of Health and Care Excellence
- Volmink J ,
- Bachmann MO , et al.
- Garfein RS ,
- Hayward A , et al.
- Aldridge RW ,
- Smith CM , et al.
- Health Innovation Hub Ireland
- Sterling TR ,
- Villarino ME ,
- Borisov AS , et al.
- Chaisson RE ,
- Ramchandani R ,
- Swindells S
- Warsinske H ,
- Vashisht R ,
- Cobelens F ,
- Liu P , et al.
- Scriba TJ ,
- Fiore-Gartland A ,
- Penn-Nicholson A , et al.
- Mangtani P ,
- Abubakar I ,
- Ariti C , et al.
- Kleinnijenhuis , J ,
- Quintin J ,
- Preijers F , et al.
- Das G , et al.
- Whitlow E ,
- Mustafa AS ,
- Gonzalo-Asensio J ,
- Marinova D ,
- Martin C , et al.
- Nieuwenhuizen NE ,
- Kulkarni PS ,
- Shaligram U , et al.
- Ottenhoff THM
- Nabavinia MS ,
- Naderi Nasab M ,
- Meshkat Z , et al.
- Working Group on New TB Drugs

- Table of Contents
- Index by author
Thank you for your interest in spreading the word on European Respiratory Society .
NOTE: We only request your email address so that the person you are recommending the page to knows that you wanted them to see it, and that it is not junk mail. We do not capture any email address.
Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager

- Respiratory infections and tuberculosis
- Tweet Widget
- Facebook Like
- Google Plus One
More in this TOC Section
- Increasing exercise capacity and physical activity in the COPD patient
- Exercise limitation and physical inactivity in COPD
- Factors behind exercise limitations and physical inactivity in COPD
Related Articles
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 24 June 2024
Evaluating the diagnostic accuracy of QIAreach QuantiFERON-TB compared to QuantiFERON-TB Gold Plus for tuberculosis: a systematic review and meta-analysis
- Shima Mahmoudi 1 , 2 &
- Sadra Nourazar 3 , 4
Scientific Reports volume 14 , Article number: 14455 ( 2024 ) Cite this article
Metrics details
- Microbiology
Accurate tuberculosis (TB) diagnosis remains challenging, especially in resource-limited settings. This study aims to assess the diagnostic performance of the QIAreach QuantiFERON-TB (QFT) assay, with a specific focus on comparing its diagnostic performance with the QuantiFERON-TB Gold Plus (QFT-Plus). We systematically reviewed relevant individual studies on PubMed, Scopus, and Web of Science up to January 20, 2024. The focus was on evaluating the diagnostic parameters of the QIAreach QFT assay for TB infection, which included sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), negative likelihood ratio (NLR), and concordance with the QFT-Plus assay. QIAreach QFT demonstrated strong diagnostic performance with a pooled sensitivity of 99% (95% CI 95–100%) and specificity of 94% (95% CI 85–97%). Additionally, it showed a PLR of 15.6 (95% CI 6.5–37.5) and NLR of 0.01 (95% CI 0–0.03). The pooled PPV and NPV were 88% (95% CI 70–98%) and 100% (95% CI 99–100%), respectively. Concordance analysis with QFT-Plus revealed a pooled positive percent agreement of 98% (95% CI 88–100%) and pooled negative percent agreement of 91% (95% CI 81–97%), with a pooled overall percent agreement of 92% (95% CI 83–98). In conclusion, QIAreach QFT has shown promising diagnostic performance, with a strong concordance with QFT-Plus. However, further studies are needed to comprehensively evaluate its diagnostic performance in the context of TB infection.
Similar content being viewed by others
Comparative performance evaluation of QIAreach QuantiFERON-TB and tuberculin skin test for diagnosis of tuberculosis infection in Viet Nam

Diagnostic accuracy of interferon-gamma-induced protein 10 for differentiating active tuberculosis from latent tuberculosis: A meta-analysis

Factors associated with false negative interferon-γ release assay results in patients with tuberculosis: A systematic review with meta-analysis
Introduction.
Despite considerable advancements in preventing, diagnosing, and treating tuberculosis (TB), it persists as a significant global public health challenge. To effectively address this widespread problem, a comprehensive strategy integrating research, prevention, diagnosis, and treatment efforts is essential for a globally impactful approach to TB management 1 .
Over the past decade, the conventional understanding of TB has faced increasing challenges 2 , 3 , 4 . In recent years, there has been a growing demand for advanced point-of-care diagnostic assays driven by the need for rapid and simultaneous detection in diverse samples. These assays provide the unique advantage of delivering comprehensive and timely diagnostic information, making them invaluable tools across a spectrum of healthcare settings 5 , 6 , 7 .
Given the limitations of traditional Tuberculin Skin Tests (TSTs), an innovative diagnostic approach known as Interferon-Gamma (IFN-γ) Release Assay (IGRA) has been developed. The continuous evolution and enhancement of IGRA tests contribute significantly to global TB infection diagnostics, offering a more precise and targeted alternative to traditional TSTs 8 , 9 .
As of now, the World Health Organization (WHO) recommends three IGRA tests for detecting tuberculosis (TB). These include the T-SPOT.TB test by Oxford Immunotec, UK, QuantiFERON-TB Gold Plus (QFT-Plus) by Qiagen, USA, and Wantai TB-IGRA by Wantai, China. Moreover, the landscape of TB diagnostics is evolving with the introduction and development of several new assays. These include the QIAreach™ QuantiFERON-TB (QIAreach QFT) by Qiagen, USA, Standard E TB-Feron by SD Biosensor, Korea, LIOFeron TB/LTBI by LIONEX Diagnostics & Therapeutics GmbH, Germany, VIDAS™ TB-IGRA by bioMérieux, France, and AdvanSure™ TB-IGRA enzyme-linked immunosorbent assay (ELISA) by LG Life Sciences, Seoul, Korea. Additionally, three other IGRAs are currently in the development phase, namely ichroma™ IGRA-TB by Boditech Med Inc., Korea, T-Track® TB by Mikrogen GmbH, Neuried, Germany, and interferon protein-10 (IP-10) IGRA ELISA/lateral flow by rBioPharm, Germany 10 , 11 .
The lateral flow assay (LFA) is a versatile paper-based platform employed for the rapid detection and quantification of analytes within complex mixtures. This method involves placing the sample on a test device, with results typically visible within a short timeframe of 5–30 min. LFAs are characterized by their low development costs and ease of production, leading to widespread applications in various fields requiring rapid testing. LFAs accommodate a range of biological samples, including urine, saliva, sweat, serum, plasma, whole blood, and other fluids, making them adaptable to diverse diagnostic scenarios 5 .
The ichroma™ IGRA-TB assay 12 , and QIAreach QFT present promising prospects as point-of-care screening methods for the diagnosis of latent tuberculosis infection (LTBI). The QIAreach QFT test represents a noteworthy advancement in global TB infection diagnostics 13 . This innovative solution combines the advantages of IGRA technology with the convenience of a point-of-care test, making it suitable for use in non-traditional laboratory settings 14 .
The QIAreach QFT (QIAreach; QIAGEN GmbH, Hilden, Germany) is a semi-automated LFA that mimics the QFT-Plus mechanism. It measures IFN-γ levels in plasma released from CD4 + and CD8 + T-cells, utilizing a single blood collection tube (BCT) corresponding to the QTF-Plus TB2 tube 15 . Instead of employing the complex Enzyme-linked Immunosorbent Assay (ELISA) method for IFN-γ detection, QIAreach QFT utilizes digital fluorescence lateral flow nanoparticle technology. This technology condenses the IFN-γ detection process into a single cartridge (eStick) processed on a portable platform (eHub), providing a binary result (positive/negative) within 20 min 16 .
The emergence of new tests for TB infection underscores the need for a comprehensive review of the diagnostic landscape 11 . Identifying any gaps in this context is essential for facilitating the development and adoption of these assays. This review aimed to assess the diagnostic accuracy of QIAreach QFT for TB infection compared to the QFT-Plus.
We adhered to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for conducting diagnostic tests systematic reviews and meta-analyses 17 . The protocol was published on PROSPERO (CRD42024508008).
Search method
We systematically searched for relevant individual studies on PubMed, Scopus, and Web of Science until January 20, 2024, without restricting language or time. The following search terms were utilized:
Tuberculosis” [Title/Abstract] OR “TB”[Title/Abstract] OR “mycobacterium tuberculosis infection” [Title/Abstract] OR “quantiferon tb gold plus” [Title/Abstract] OR “QFT-Plus” [Title/Abstract] OR “QFT”[Title/Abstract] AND “LFA” [Title/Abstract] OR “lateral flow assay” [Title/Abstract] OR “QIAreach” [Title/Abstract] OR “QIAreach QuantiFERON-TB assay” [Title/Abstract].
We explored the grey literature through Google and Google Scholar. Furthermore, we identified additional studies by examining the references cited in the selected papers. Relevant articles linked to the provided keywords were subsequently included in our analysis.
Study selection
Initially, titles and abstracts were screened to identify papers suitable for further examination. Subsequently, two reviewers (SM and SN) independently conducted a thorough assessment of the full texts of the chosen publications to determine eligibility. Any discrepancies were resolved through consensus.
Inclusion criteria included studies meeting the following conditions: (1) use of QFT-Plus for detecting TB infection/disease; (2) availability of data on positive or negative results using both QFT-Plus and QIAreach QFT test.
SM and SN independently evaluated study eligibility, with disagreements resolved through consensus. Studies were eligible for inclusion in the analysis only if they provided comprehensive details in a binary classification format, as represented by a 2 × 2 table(s) showcasing true-positive, false-positive, true-negative, and false-negative results; and/or the availability of data on positive, negative, and overall agreement.
Exclusion criteria were applied based on: (1) inclusion of only positive QFT-Plus individuals at baseline; (2) utilization of QIAreach QFT test compared Standard F TB-Feron FIA Assay.
Data extraction
Two researchers (SM and SN) independently acquired the following information from each study: initial author, country, publication year, sample size, demographic details such as sex and age of participants, participant numbers, counts of positive and negative results for both tests, and values for true-positive, false-positive, true-negative, and false-negative. Furthermore, data on positive, negative, and overall agreement were collected.
Quality assessment
To assess the methodological quality of eligible studies, we employed the Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool 18 . Specific yes/no signaling questions were generated for each QUADAS-2 domain.
Statistical analysis
The analysis was conducted using Stata (version 14; Stata Corporation, TX, USA). Data from individual studies were pooled using a random-effect model to derive values for various measures of test accuracy, including sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), positive, negative, and overall agreement, diagnostic odds ratio (DOR) with corresponding 95% CI. A hierarchical summary receiver operating characteristic (HSROC) model was constructed to summarize the overall diagnostic accuracy. Additionally, the calculation of Positive Predictive Value (PPV) and Negative Predictive Value (NPV) provided further insights into the diagnostic accuracy of the test across the included studies. Concordance analysis between the QIAreach QFT and QFT-Plus diagnostic assays was performed, evaluating Positive Percent Agreement (PPA), Negative Percent Agreement (NPA), and Overall Percent Agreement (OPA).
Heterogeneity was assessed using the I 2 statistic, with I 2 > 0.75 indicating high heterogeneity 19 . Deeks’ funnel plot was used to visually assess publication bias, with a P value ≤ 0.10 indicating significant asymmetry, thereby suggesting the presence of bias.
Figure 1 provides an overview of the selection process for included studies. Initial searches in databases yielded 3701 citations, and after removing duplicates, 2224 unique articles remained. Following the screening of titles and abstracts, 23 papers were selected for full-text reviews, and ultimately, six of those articles met the inclusion criteria for this study 15 , 20 , 21 , 22 , 23 , 24 . One study was excluded from the analysis due to its focus on the comparison of STANDARD F TB-Feron FIA vs. QIAreach QFT, which did not align with the criteria set for inclusion in the study 25 . In the Ntshiqa et al . study, our access was restricted to abstracts from a poster presentation. We had only results for true positive ( tp ) and true negative ( tn ) outcomes, along with information on the agreement of the test 24 .

Study selection.
Characteristics of the included studies
Table 1 provides a summary of selected studies. These studies were conducted in different countries such as the USA 15 , Japan 21 , Italy 23 , Malaysia 22 , Vietnam 20 , Lesotho, South Africa, and Tanzania 24 . Saluzzo et al . evaluated the clinical performance of QIAreach QFT in detecting TB infection in the HIV-negative population with microbiologically confirmed pulmonary TB and healthy low-risk volunteers 23 . Fukushima et al . focused on adult patients with active pulmonary TB, alongside healthy low-TB-risk participants 21 . Vo et al. included individuals aged 18 or older meeting specific inclusion criteria related to TB exposure, comorbidities, and socioeconomic circumstances in the high TB burden country 20 . Ntshiqa et al . targeted household contacts of TB patients in three high-burden countries 24 , while Stieber’s research encompassed individuals aged 18 or older with and without varying risk factors for TB infection 15 .
The diagnostic performance of QIAreach QFT
Table 2 presents an overview of the diagnostic performance of QIAreach QFT across diverse studies. In Ntshiqa’s study, a moderate concordance of QIAreach QFT against QFT-Plus was reported 24 . In this study, among household contacts with paired results, 42% (197 out of 465) tested positive, and 34% (156 out of 465) tested negative on both assays. Additionally, 24% (112 out of 465) had discordant results between the two assays.
The comparative evaluation of QIAreach QFT demonstrates varying specificity across studies, ranging from 72 to 98%. This indicates differences in the test’s ability to accurately exclude individuals without TB in diverse research settings. Regarding sensitivity, the range spans from 96 to 100%, demonstrating high performance in correctly identifying individuals with TB across the studies. The comparative evaluation of QIAreach QFT demonstrates varying specificity across studies, ranging from 72 to 98%. This indicates differences in the test’s ability to accurately exclude individuals without TB in diverse research settings.
The analysis indicates that QIAreach QFT demonstrates a high pooled sensitivity of 99% (95% CI 97–100, I 2 : 61.55%), and a pooled specificity of 94% (95% CI 85–97, I 2 : 97.16%) (Fig. 2 ). Additionally, it demonstrated a PLR of 15.6 (95% CI 6.5–37.5) and a NLR of 0.01 (95% CI 0–0.03), suggesting its accuracy in confirming or excluding TB infections (Supplementary Fig. 1 ). The DOR was 1341 (95% CI 272–6602), reflecting the overall robustness of the test. The AUC of the SROC curve was 0.99 (95% CI 0.98–1.00) (Fig. 3 ).
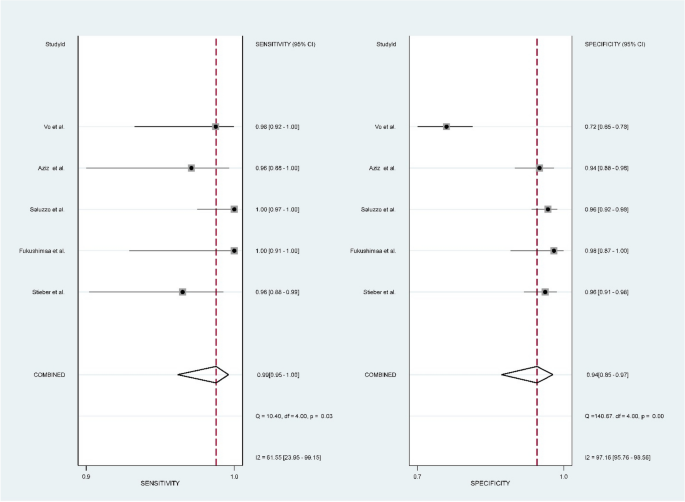
Pooled sensitivity and specificity along with 95% CIs for TB detection using QIAreach QFT.

Summary receiver operating curve of the diagnostic performance of QIAreach QFT.
The comparison of diagnostic performance among the studies reveals a range of values across key parameters. In terms of PPV, the studies exhibit a range from 54.6 to 97.6%, reflecting substantial variability in the ability of the tests to accurately identify true positive cases. NPV demonstrates a narrower range, from 98.3 to 100%, suggesting a high accuracy in ruling out true negative cases (Table 2 ). The pooled analysis showed a PPV of 88% (95% CI 70–98) and NPV of 100% (95% CI 99–100), respectively (Fig. 4 ).

Pooled PPV and NPV along with 95% CIs for TB detection using QIAreach QFT; ( a ) PPV, ( b ) NPV.
In total, 6 studies, encompassing a participant pool of 1495 individuals, were incorporated to evaluate the agreement between the QIAreach QFT and the QFT-Plus diagnostic tests (Table 2 ). PPA varied from 77.3 to 100% across the studies. NPA across the studies demonstrated a range from 72.3 to 97.6%. The OPA values provide a comprehensive measure of the overall performance of the diagnostic tests across all participants in each study, considering both true positive and true negative results. OPA across the studies exhibited a range from 78.3 to 98.8%, emphasizing the diverse levels of agreement between the two tests. The meta-analysis demonstrated a pooled PPA of 98% (95% CI 88–100%) and pooled NPA of 91% (95% CI 81–97%), with a pooled OPA of 92% (95% CI 83–98) (Fig. 5 ).

Concordance between the QIAreach QFT and QFT-Plus diagnostic assay: ( a ) Positive Percent Agreement (PPA); ( b ) Negative Percent Agreement (NPA); ( c ) Overall Percent Agreement (OPA).
The quality assessment of studies was conducted using QUADAS-2. According to the assessment, the risk of bias was considered unclear in four studies and high in three studies, primarily due to patient selection issues. Additionally, a high risk of bias was identified in four studies related to flow and timing issues. It’s noteworthy that applicability concerns were deemed low across all studies (Supplementary Fig. 2 ).
Publication bias
Publication bias was tested by linear regression, and Deeks funnel plot was drawn. The results showed no obvious publication bias ( P = 0.63) (Fig. 6 ).

Deeks’ funnel plot asymmetry test for publication bias.
Our analysis revealed promising diagnostic characteristics through the comparative performance evaluation of QIAreach QFT and QFT-Plus. QIAreach QFT exhibits a high sensitivity of 99% (95% CI 97–100%), indicating its strong ability to correctly identify true positive cases. Additionally, its specificity of 94% (95% CI 85–97%) underscores the test’s capability to accurately recognize true negative cases. However, we observed relatively high heterogeneity ( I 2 = 97.16% for specificity) among the eligible studies. The PLR of 15.6 and NLR of 0.01 further emphasize the test’s effectiveness in confirming or ruling out TB infections. The DOR, calculated at 1341 (95% CI 272–6602), reinforces the overall robustness of the QIAreach QFT. In addition, the analysis revealed a pooled PPV of 88% (95% CI 70–98%), indicating the overall accuracy of the tests in correctly identifying individuals with true positive results across multiple studies. Conversely, the pooled NPV of 100% (95% CI 99–100%) emphasizes the high overall accuracy of the tests in excluding individuals correctly with true negative results.
The meta-analysis indicated strong concordance between the QIAreach QFT and QFT-Plus. It showed a PPA of 98% (95% CI 88–100%) for correctly identifying true positive cases and a pooled NPA of 91% (95% CI 81–97%) for accurately recognizing true negative cases. The Overall OPA was 92% (95% CI 83–98%). Discordant results in LTBI testing pose challenges in accurately categorizing individuals as either positive or negative, which can significantly impact subsequent clinical management decisions. Understanding the reasons behind discordant results is crucial for improving the reliability and accuracy of LTBI diagnostics. However, the absence of a definitive gold standard for LTBI complicates the interpretation of discordant results. To address this issue, further research and larger-scale studies are needed to establish a more standardized approach for interpreting LTBI test results.
The QIAreach QFT is an entirely new test, so independent evaluations of their sensitivity and specificity compared to other IGRAs are limited 26 . Most previous reports have compared QIAreach with QFT-Plus. Only one study has compared the performance of STANDARD F TB-Feron FIA with QIAreach QFT. While QIAreach QFT shows promising diagnostic performance, characterized by high sensitivity and specificity compared to QFT-Plus, its efficacy must be understood within the broader context of TB diagnostics and other IGRA tests.
A notable advantage of QIAreach QFT is its efficiency, offering rapid results within just 20 min. This expedites the testing process, making it more convenient and applicable across diverse clinical settings 20 .
On the other hand, QIAreach QFT, despite its benefits of providing standardized and automated results, encounters challenges related to blood volume requirements and potential interference, particularly seen in a strong interferon response in negative control tubes 27 . QIAreach QFT utilizes only one blood collection tube, lacking the negative (Nil) control, whereas QFT-Plus uses four tubes (TB1, TB2, Nil, and mitogen) for a thorough analysis. This discrepancy is particularly significant if QIAreach QFT positivity was calibrated using QFT-Plus thresholds 20 . Another concern arises from uncertainties and biases in interpreting test results when using QIAreach QFT, particularly compared to QFT-Plus, especially in cases with indeterminate QFT-Plus results and high IFN-γ response in negative control tubes.
IGRAs have become valuable tools for detecting LTBI, presenting advantages over traditional methods like the TST. However, it is crucial to acknowledge and address certain limitations associated with IGRAs, particularly in the context of LTBI diagnosis. One significant limitation is the incapacity of IGRAs to differentiate between latent TB infection and active TB disease. Additionally, immunocompromised individuals, such as those with HIV or undergoing immunosuppressive therapy, may experience reduced sensitivity in IGRA results.
Recognizing the urgent need for diagnostic tests with improved performance and predictive value in identifying individuals at risk of progressing to active TB, it is essential to evaluate the performance of TB infection tests across diverse risk groups 27 . The spectrum of TB encompasses a range of disease states, including minimal (non-infectious), subclinical (asymptomatic but infectious), and clinical (symptomatic and infectious) 2 . Understanding this spectrum is vital for comprehensive disease management. Evaluating this spectrum in different demographic and risk groups is essential to tailor effective interventions and improve outcomes. Addressing the specific diagnostic needs at different disease stages is crucial for effective patient management and treatment strategies. Recognizing the current gaps in existing diagnostic approaches can pave the way for the development and implementation of stage-specific diagnostic tools. Continuous monitoring of disease progression is an area where current tools may have limitations, highlighting the ongoing need for innovation and improvement in TB diagnostics.
Despite the promising results, this research has limitations. The heterogeneity in study characteristics, such as sample populations and study designs, may influence the generalizability of the findings. Additionally, the limited number of studies evaluating the QIAreach QFT underscores the need for more extensive research to establish its diagnostic capabilities comprehensively. Another notable limitation is the absence of a gold standard for LTBI diagnosis, complicating the evaluation of IGRA performance. In our study, we lacked information regarding the performance of QIAreach QFT in cases with indeterminate QFT-Plus tests. Therefore, caution should proceed, and further studies are necessary to validate its performance across diverse epidemiological settings and different population groups, both in low and high-burden countries.
In conclusion, the QIAreach QFT demonstrated favorable diagnostic performance, with high sensitivity and specificity and a high concordance against QFT-Plus. However, the limited number of studies evaluating the QIAreach QFT indicates the need for further research to comprehensively understand its diagnostic capabilities in the context of TB infection.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Rezaei, N. et al. Tuberculosis: integrated studies for a complex disease 2050. In Tuberculosis: Integrated Studies for a Complex Disease (eds Rezaei, N. et al. ) (Springer, 2023).
Chapter Google Scholar
Zaidi, S. M. A. et al. Beyond latent and active tuberculosis: A scoping review of conceptual frameworks. EClinicalMedicine 66 , 102332. https://doi.org/10.1016/j.eclinm.2023.102332 (2023).
Article PubMed PubMed Central Google Scholar
Emery, J. C. et al. Estimating the contribution of subclinical tuberculosis disease to transmission: An individual patient data analysis from prevalence surveys. Elife. https://doi.org/10.7554/eLife.82469 (2023).
Drain, P. K. et al. Incipient and subclinical tuberculosis: A clinical review of early stages and progression of infection. Clin. Microbiol. Rev. https://doi.org/10.1128/cmr.00021-18 (2018).
Koczula, K. M. & Gallotta, A. Lateral flow assays. Essays Biochem. 60 (1), 111–120. https://doi.org/10.1042/ebc20150012 (2016).
Kobashi, Y. Current status and future landscape of diagnosing tuberculosis infection. Respir. Investig. 61 (5), 563–578. https://doi.org/10.1016/j.resinv.2023.04.010 (2023).
Article CAS PubMed Google Scholar
Mahmoudi, S., Pourakbari, B. & Mamishi, S. Immunodiagnostics of tuberculosis: Recent discoveries. In Tuberculosis: Integrated Studies for a Complex Disease (eds Mahmoudi, S. et al. ) (Springer, 2023).
Google Scholar
Pourakbari, B. et al. Can interferon-γ release assays be useful for monitoring the response to anti-tuberculosis treatment?: A systematic review and meta-analysis. Arch. Immunol. Ther. Exp. (Warsz) 68 (1), 4. https://doi.org/10.1007/s00005-020-00568-4 (2020).
Mamishi, S., Pourakbari, B., Marjani, M. & Mahmoudi, S. Diagnosis of latent tuberculosis infection among immunodeficient individuals: Review of concordance between interferon-gamma release assays and the tuberculin skin test. Br. J. Biomed. Sci. 71 (3), 115–124. https://doi.org/10.1080/09674845.2014.11669976 (2014).
Li, L. S. et al. From immunology to artificial intelligence: Revolutionizing latent tuberculosis infection diagnosis with machine learning. Mil. Med. Res. 10 (1), 58. https://doi.org/10.1186/s40779-023-00490-8 (2023).
Article CAS PubMed PubMed Central Google Scholar
Hamada, Y. et al. Tests for tuberculosis infection: Landscape analysis. Eur. Respir. J. https://doi.org/10.1183/13993003.00167-2021 (2021).
Lee, H. H. et al. Evaluation of a lateral flow assay-based IFN-γ release assay as a point-of-care test for the diagnosis of latent tuberculosis infection. Clin. Rheumatol. 40 , 3773–3781 (2021).
Article PubMed Google Scholar
Kaaba, C. et al. Assessing usability of QIAreach QuantiFERON-TB platform in a high tuberculosis prevalence, low-resource setting. ERJ Open Res. https://doi.org/10.1183/23120541.00511-2021 (2021).
Rickman, H. M. et al. Know your tuberculosis epidemic—Is it time to add Mycobacterium tuberculosis immunoreactivity back into global surveillance? PLoS Glob. Public Health 2 (10), e0001208. https://doi.org/10.1371/journal.pgph.0001208 (2022).
Stieber, F. et al. Evaluation of a lateral-flow nanoparticle fluorescence assay for TB infection diagnosis. Int. J. Tuberc. Lung Dis. 25 (11), 917–922. https://doi.org/10.5588/ijtld.21.0391 (2021).
Pagaduan, J. V. & Altawallbeh, G. Advances in TB testing. Adv. Clin. Chem. 115 , 33–62. https://doi.org/10.1016/bs.acc.2023.03.003 (2023).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 6 (7), e1000100. https://doi.org/10.1371/journal.pmed.1000100 (2009).
Whiting, P. F. et al. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 155 (8), 529–536. https://doi.org/10.7326/0003-4819-155-8-201110180-00009 (2011).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327 (7414), 557–560. https://doi.org/10.1136/bmj.327.7414.557 (2003).
Vo, L. N. Q. et al. Comparative performance evaluation of QIAreach QuantiFERON-TB and tuberculin skin test for diagnosis of tuberculosis infection in Viet Nam. Sci. Rep. 13 (1), 15209. https://doi.org/10.1038/s41598-023-42515-1 (2023).
Fukushima, K. et al. First clinical evaluation of the QIAreach(TM) QuantiFERON-TB for tuberculosis infection and active pulmonary disease. Pulmonology 28 (1), 6–12. https://doi.org/10.1016/j.pulmoe.2021.07.003 (2022).
Aziz, Z. A., Noordin, N. M., Wan Mohd, W. M. & Kasim, M. A. First evaluation of the performance of portable IGRA, QIAreach® QuantiFERON®-TB in intermediate TB incidence setting. PLoS ONE 18 (2), e0279882. https://doi.org/10.1371/journal.pone.0279882 (2023).
Saluzzo, F., Mantegani, P., Poletti de Chaurand, V. & Cirillo, D. M. QIAreach QuantiFERON-TB for the diagnosis of Mycobacterium tuberculosis infection. Eur. Respir. J. https://doi.org/10.1183/13993003.02563-2021 (2022).
Ntshiqa, T. et al. PA-738 evaluation of QIAreach QuantiFERON-TB lateral-flow nanoparticle fluorescence assay for TB infection testing among TB household contacts in three high-burden settings. BMJ Spl. J. 1 , 1 (2023).
Saint-Pierre, G. et al. Comparison of two tuberculosis infection tests in a South American tertiary hospital: STANDARD F TB-Feron FIA vs. QIAreach(TM) QuantiFERON-TB. Diagnostics (Basel). https://doi.org/10.3390/diagnostics13061162 (2023).
Ortiz-Brizuela, E. et al. Assessing the diagnostic performance of new commercial interferon-γ release assays for mycobacterium tuberculosis infection: A systematic review and meta-analysis. Clin. Infect. Dis. 76 (11), 1989–1999. https://doi.org/10.1093/cid/ciad030 (2023).
World Health Organization. WHO Consolidated Guidelines on Tuberculosis. Module 3: Diagnosis. Tests for TB Infection (World Health Organization, 2022).
Download references
Acknowledgements
The work was supported by Horizon Europe (HORIZON), as a research project under the “HORIZON-WIDERA-2022-TALENTS-04-01” program [Project Number: 101130873- DET-TB]. The work of SM received partial support from the European Commission-European Research Executive Agency (REA) under Grant Agreement No. 101130873.

Author information
Authors and affiliations.
Biotechnology Centre, Silesian University of Technology, Krzywoustego 8, 44-100, Gliwice, Poland
Shima Mahmoudi
Pediatric Infectious Disease Research Center, Tehran University of Medical Sciences, Tehran, Iran
InPedia Association, Students’ Scientific Research Center, Tehran University of Medical Sciences, Tehran, Iran
Sadra Nourazar
School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
You can also search for this author in PubMed Google Scholar
Contributions
Shima Mahmoudi: conceptualization, visualization, methodology, validation, data curation, writing-original draft, writing-review and editing. Sadra Nourazar: visualization, methodology, validation, data curation, writing-review and editing.
Corresponding author
Correspondence to Shima Mahmoudi .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary figures., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Mahmoudi, S., Nourazar, S. Evaluating the diagnostic accuracy of QIAreach QuantiFERON-TB compared to QuantiFERON-TB Gold Plus for tuberculosis: a systematic review and meta-analysis. Sci Rep 14 , 14455 (2024). https://doi.org/10.1038/s41598-024-65663-4
Download citation
Received : 14 March 2024
Accepted : 22 June 2024
Published : 24 June 2024
DOI : https://doi.org/10.1038/s41598-024-65663-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- QIAreach QuantiFERON-TB
- QuantiFERON-TB gold plus
- Diagnostic accuracy
- Tuberculosis
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Tuberculosis: a survey and review of current literature
Affiliation.
- 1 Kings County Hospital, Brooklyn, New York, USA.
- PMID: 9363058
Despite being a treatable and preventable disease, tuberculosis will kill an estimated 30 million people during the current decade. Tuberculosis is a global problem, and increases in case rates are occurring not only in the developing countries of the world but also in several industrialized nations, such as the United States. Coincident with the resurgence of tuberculosis in the United States, there has also been an alarming increase in the number and proportion of cases caused by strains of Mycobacterium tuberculosis that are resistant to multiple first-line drugs. The increase in multiple-drug resistant tuberculosis has re-taught physicians about the importance of pursuing and ensuring treatment until cure. The HIV epidemic is playing a pivotal and permissive role in the resurgence of tuberculosis morbidity and mortality in those populations where tuberculosis and HIV are prevalent and overlap. Co-infection with HIV distorts the natural history and clinical expression of tuberculosis. Molecular biology has yielded important insights into the mechanisms of drug resistance and provided powerful tools for the rapid diagnosis and epidemiologic study of this disease.
PubMed Disclaimer
Similar articles
- The TB pandemic: an old problem seeking new solutions. Meya DB, McAdam KP. Meya DB, et al. J Intern Med. 2007 Apr;261(4):309-29. doi: 10.1111/j.1365-2796.2007.01795.x. J Intern Med. 2007. PMID: 17391107 Review.
- [Development of antituberculous drugs: current status and future prospects]. Tomioka H, Namba K. Tomioka H, et al. Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese.
- Tuberculosis in Louisiana: an update. George RB, Farley TA, DeGraw CF, Owens MW. George RB, et al. J La State Med Soc. 1998 Dec;150(12):587-95. J La State Med Soc. 1998. PMID: 9926697
- Drug resistance in Mycobacterium tuberculosis. Cole ST, Telenti A. Cole ST, et al. Eur Respir J Suppl. 1995 Sep;20:701s-713s. Eur Respir J Suppl. 1995. PMID: 8590570 Review.
- The changing face of tuberculosis. Huebner RE, Castro KG. Huebner RE, et al. Annu Rev Med. 1995;46:47-55. doi: 10.1146/annurev.med.46.1.47. Annu Rev Med. 1995. PMID: 7598480 Review.
- Combating Tuberculosis Infection: A Forbidding Challenge. Rawal T, Butani S. Rawal T, et al. Indian J Pharm Sci. 2016 Jan-Feb;78(1):8-16. doi: 10.4103/0250-474x.180243. Indian J Pharm Sci. 2016. PMID: 27168676 Free PMC article. Review.
- Use of NMR metabolomics to analyze the targets of D-cycloserine in mycobacteria: role of D-alanine racemase. Halouska S, Chacon O, Fenton RJ, Zinniel DK, Barletta RG, Powers R. Halouska S, et al. J Proteome Res. 2007 Dec;6(12):4608-14. doi: 10.1021/pr0704332. Epub 2007 Nov 3. J Proteome Res. 2007. PMID: 17979227 Free PMC article.
- Determination of N-acetylation phenotyping in a Greek population using caffeine as a metabolic probe. Asprodini EK, Zifa E, Papageorgiou I, Benakis A. Asprodini EK, et al. Eur J Drug Metab Pharmacokinet. 1998 Oct-Dec;23(4):501-6. doi: 10.1007/BF03190002. Eur J Drug Metab Pharmacokinet. 1998. PMID: 10323334 Clinical Trial.
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- Ovid Technologies, Inc.
- Genetic Alliance
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Fact sheets
- Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- HIV and AIDS
- Hypertension
- Mental disorders
- Top 10 causes of death
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- Data collection tools
- Global Health Observatory
- Insights and visualizations
- COVID excess deaths
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment case
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Publications detail /
Framework for conducting reviews of tuberculosis programmes

The review of a formal national tuberculosis (TB) programme (or the efforts that countries make to control the disease regardless of the existence of a formal “programme”) is an important exercise to evaluate the implementation and impact of TB prevention, care and control. It should be jointly undertaken by the government together with the relevant national and international partners that are involved in TB efforts.
A TB programme review assesses the performance of the strategy implemented to fight TB and identifies the strengths and weaknesses of interventions that have been put in place. An appropriate review must, then, describe specific recommendations on the strategic orientations that need to be adopted and developed to overcome the gaps identified in the way that TB prevention, care and control are being implemented. These recommendations provide the foundation for improving the strategy adopted to control TB and for revising or developing a national strategic plan. Moreover, the review of a TB programme provides an important opportunity to advocate for TB prevention, care and control among policy makers, to strengthen the engagement of national health authorities and key stakeholders, and to enhance the mobilization of resources from both domestic and international sources.
The first WHO guidelines on how to review a TB programme were published in 1998, and were designed to support the assessment of, and improvements to, the implementation of the DOTS strategy. Since then, there have been major evolutions in the WHO strategy for prevention, care and control of TB. Important new interventions have been defined, developed and implemented: for example, collaborative TB/HIV activities and the programmatic management of drug-resistant TB. Therefore, this new guidance takes into consideration all strategic approaches that are part of the current WHO strategy for TB control. In 2013, WHO began developing a post-2015 global tuberculosis strategy. Thus, this guidance will be further updated once the new strategy is fully translated into operational language.
The main purpose of this document is therefore to provide guidance on how to organize a review of a national TB programme. It identifies the keys steps needed to plan and prepare the review and specifies how to carry out field visits. It also describes the process of using the findings of the field visits, formulating recommendations and developing a review report. The document also includes, in annexes and in web-based format, checklists that can be adapted and used to assess key areas of TB prevention, care and control such as TB surveillance system, the management of the TB programme, and the process of TB case finding.
We strongly encourage national TB programmes, as well as agencies and organizations involved in TB control, to use the guidance included in this document to organize and implement the national programme reviews they are planning. The outcomes of the reviews should significantly contribute to improving the TB control situation in countries, revising or developing high-quality national strategic plans, and mobilizing the required resources.
Dr Mario Raviglione
Director, Global TB Programme
World Health Organization
French Version
Annexes can be downloaded as a pdf, or a word document
1. assessing the management of the national tb programme.
Assessing the management of the national TB programme pdf, 63kb
Assessing the management of the national TB programme doc, 477kb
2. Assessing the national strategic plan for TB prevention, care and control
Assessing the national strategic plan for TB prevention, care and control pdf, 39kb
Assessing the national strategic plan for TB prevention, care and control doc, 465kb
3. Assessing TB case-finding
Assessing TB case-finding pdf, 68kb
Assessing TB case-finding doc, 493kb
4. Assessing quality-assured diagnoses made by TB laboratories
Assessing quality-assured diagnoses made by TB laboratories pdf, 65kb
Assessing quality-assured diagnoses made by TB laboratories doc, 495kb
5. Assessing the quality of TB diagnoses
Assessing the quality of TB diagnoses pdf, 34kb
Assessing the quality of TB diagnoses doc, 458kb
6. Assessing the management of TB cases
Assessing the management of TB cases pdf, 67kb
Assessing the management of TB cases doc, 476kb
7. Assessing the programmatic management of drug-resistant TB
Assessing the programmatic management of drug-resistant TB pdf, 58kb
Assessing the programmatic management of drug-resistant TB doc, 470kb
8. Assessing TB/HIV collaborative activities
Assessing TB/HIV collaborative activities pdf, 71kb
Assessing TB/HIV collaborative activities doc, 475kb
9. Assessing patients’ adherence to TB
Assessing patients’ adherence to TB pdf, 55kb
Assessing patients’ adherence to TB doc, 464kb
10. Assessing the management of anti-TB medicines and supplies
Assessing the management of anti-TB medicines and supplies pdf, 48kb
Assessing the management of anti-TB medicines and supplies doc, 479kb
11. Assessing recording and reporting
Assessing recording and reporting pdf, 130kb
Assessing recording and reporting doc, 468kb
12. Assessing activities to address childhood TB
Assessing activities to address childhood TB pdf, 39kb
Assessing activities to address childhood TB doc, 467kb
13. Assessing infection control
Assessing infection control pdf, 61kb
Assessing infection control doc, 470kb
14. Assessing public–public and public–private mix approaches
Assessing public–public and public–private mix approaches pdf, 40kb
Assessing public–public and public–private mix approaches doc, 466kb
15. Assessing the implementation of the practical approach to lung health (PAL)
Assessing the implementation of the practical approach to lung health (PAL) pdf, 43kb
Assessing the implementation of the practical approach to lung health (PAL) doc, 472kb
16. Assessing the engagement of civil society, nongovernmental and community organizations
Assessing the engagement of civil society, nongovernmental and community organizations pdf, 38kb
Assessing the engagement of civil society, nongovernmental and community organizations doc, 462kb
17. Assessing human resources development
Assessing human resources development pdf, 41kb
Assessing human resources development doc, 468kb
18. Assessing the integration of TB activities within the health system
Assessing the integration of TB activities within the health system pdf, 37kb
Assessing the integration of TB activities within the health system doc, 464kb
- Open access
- Published: 25 June 2024
Magnitude and determinants of undernutrition among tuberculosis patients in Ethiopia: systematic review and meta-analysis
- Jira Wakoya Feyisa 1 ,
- Robera Demissie Berhanu 2 ,
- Matiyos Lema 1 ,
- Markos Desalegn 1 ,
- Emiru Merdassa 1 ,
- Keno Melkamu Kitila 3 ,
- Wase Benti Hailu 1 ,
- Sidie Debelo Beyena 1 &
- Adisu Tafari Shama 1
BMC Public Health volume 24 , Article number: 1698 ( 2024 ) Cite this article
Metrics details
Undernutrition increases the risk of TB infection to be active TB, death and relapse of the disease. Undernutrition also disturbs the management process of tuberculosis. Therefore, this study aimed to estimate the pooled magnitude and determinants of undernutrition among TB patients in Ethiopia.
From August 20, 2022 to January 6, 2023, the research articles were identified via the search engines Google Scholar, Medline, Pub Med, Cochrane Library, and Web of Science. Stata version 14 was used for analysis, along with a standardized data extraction checklist. The Cochrane Q test statistic and I2 statistics were used to determine heterogeneity. A random-effect model was used to assess the extent of undernutrition among TB patients. OR with a 95% CI was used to report the relationship between undernutrition and independent factors. A funnel plot and Egger’s test were used to examine publication bias.
A total of 720 research articles were identified via several databases and 21 studies were included in the systematic review and meta-analysis. The pooled magnitude of undernutrition among TB patients was 48.23% (95% CI 42.84, 53.62). The current meta-analysis revealed that patients who had no formal education (OR = 2.11(95%CI: 1.09, 4.06), average monthly income < 1800 ETB (OR = 2.32 (95CI: 1.33, 4.04), unable to work (OR = 2.61(95CI:1.99, 3.43), patients who had eating disorder (OR = 2.73 (95CI: 2.09, 3.56), patients who had intestinal parasite (OR = 3.77 (95CI: 2.39, 5.94), patients of > 5 family size (OR = 3.79 (95CI: 1.06, 14.93), and patients who drank alcohol (OR = 1.47(95CI: 1.06, 2.05) were significantly associated with undernutrition.
This meta-analysis examined the high magnitude of undernutrition among TB patients in Ethiopia. Strategic and police-oriented intervention to prevent factors contributing to the problem is mandatory.
Peer Review reports
Introduction
Tuberculosis (TB) is an immune-compromising disease caused by Mycobacterium Tuberculosis complex and other related species and usually affects the lungs but almost all organs can be affected [ 1 ]. Thus it is conveniently classified into; Pulmonary TB (PTB) and Extra-pulmonary TB (EPTB) [ 1 ]. Early diagnosis of infectious TB cases and treating them effectively are the keystones of global TB control programs [ 2 ]. Despite the exhaustive strategies of the World Health Organization for controlling this disease, millions of people are still being infected annually [ 3 ].
Globally, TB is a common cause of mortality and morbidity which is estimated to be 10.6 million TB patients in 2021 increasing by 4.5% from 10.1 million in 2020 whereas, the estimated number of deaths from TB was 1.6 million which is common in developing countries [ 4 ]. Undernutrition increases the risk of TB infection to active TB, death, and relapse of the disease [ 5 ]. Undernutrition also disturbs the management process of tuberculosis [ 6 ]. Poor feeding and dietary practices inhibit the fight against TB especially in low-income countries as well as the body of a person suffering from TB has an increased request for calories, which often leads a TB patient to significant weight loss and this can aggravate acute undernutrition [ 7 ]. TB patients who are malnourished have more severe diseases, which increases the chance of mortality and severe acute undernutrition [ 8 ]. Evidence put forward that undernutrition among TB patients became associated factors for two- to four-fold times more likely in increasing mortality and a five-fold risk of drug-induced hepatotoxicity [ 9 ].
Identification of the pooled magnitude and determinants of undernutrition among TB patients in Ethiopia could have a great role for appropriate intervention to track the problem, which is a very common problem throughout the country. Thus, this systematic review and meta-analysis helps to have pooled evidence for the implementation strategy and to get policy attention.
Search strategy
From the very beginning, the PROSPERO database and the database of abstracts of reviews of effects (DARE) ( http://www.library.UCSF.edu ) were searched to see whether there were any published or ongoing projects related to the topic. This systematic review and meta-analysis study protocol has been submitted to the International Prospective Register of Systematic Reviews (PROSPERO) and has been assigned the registration ID, which is CRD42023422305. The research articles search strategy, selection of studies, data extraction, and result reporting were done in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [ 10 , 11 ]. A PICO principle was adapted for searching terms. The research articles used for this systematic review and meta-analysis were identified through Google Scholar, Medline/Pub Med, Cochrane Library, the Web of Science, Hinari, Science Direct, ProQuest, African Journals Online, and online university repositories (University of Gondar, Addis Ababa, Jimma, and Haramaya University) search engines by developing search strategies. Boolean operators such as OR, AND, and NOT were used with search terms such as prevalence”, “magnitude”, “proportion”, “burden”, “undernutrition”, “malnutrition”, “malnourishment”, “underweight”, “tuberculosis”, “factors”, “determinants”, “Predictors”, “adults”, “Ethiopia. Identified research articles were screened to make sure that all relevant literature was included. Literature was downloaded to Endnote (version X7.8) to maintain and manage citations, and facilitate the review process [ 12 ].
Eligibility criteria
In this systematic review and meta-analysis, we included all studies that were conducted on undernutrition among adult TB patients and/or associated factors in Ethiopia. The participants were Adult TB patients. We included all types of articles that were published in the form of journal articles, master theses, and dissertations in the English language. Full research articles, which were not accessed after at least two email contacts of the primary author, were not included because of the failure to assess the quality of articles in the absence of full text. All studies conducted in Ethiopia were included.
Outcome measurement
There were two main outcomes. The first outcome of interest was the magnitude of undernutrition (BMI < 18.5 kg/m2) among adult tuberculosis patients which was determined by dividing the number of patients having undernutrition by the total number of study subjects included in the final analysis. From the primary studies, undernutrition was operationalized as a BMI < 18.5 kg/m2. The second outcome was the factors associated with undernutrition, which was determined using the odds ratio (OR) and calculated based on binary outcomes from the included primary studies.
Data collection and quality assessment
To assess the quality of the included study, the Joanna Briggs Institute (JBI) quality appraisal checklist for Observational studies quality assessment tool was used [ 13 ]. Five data extractors (JW, RD, AT, MD, and ML) used a Microsoft Excel standardized data extraction checklist for data extraction. Reference management software (Endnote version X7.8) was used to combine search results from databases and to remove duplicate articles initially. Then, research articles were screened and excluded by their abstracts and titles. Full-text articles or reports were assessed for the remaining research articles. Based on the preset inclusion and exclusion criteria, the eligibility of the primary studies was evaluated. For the first outcome (magnitude of undernutrition among TB patients), the data extraction checklist included the authors’ name, Year of publication, region (an area where studies were conducted), study design, sample size, response rate, and the number of participants with undernutrition. For the second outcome (factors associated with undernutrition among TB patients), data were extracted in two-by-two-tables format, and then the log OR was computed based on the findings of the primary articles. Inconsistencies between independent reviewers were fixed by including other reviewers (EM, and KM) after discussion for possible agreement. Corresponding authors of the research articles were contacted via email when the included primary articles did not have sufficient data.
Data analysis and synthesis
The necessary information from each original study was retrieved using a format created in a Microsoft Excel spreadsheet. The data were then exported to STATA version 14.0 and used to determine the pooled effect size with 95% confidence interval. The Cochran Q test (Chi-squared statistic) and I2 statistic on forest plots were computed to assess heterogeneity among the included studies. At p 0.05, Cochran’s Q statistical heterogeneity test is declared statistically significant. I2 statistics range from 0 to 100%, with I2 statistic values of 0, 25, 50, and 75% indicating no, low, moderate, and high degrees of heterogeneity [ 14 , 15 ]. When there was a high degree of heterogeneity for the first and second outcomes, a random-effects model was used to determine the pooled magnitude of undernutrition among TB patients and the pooled effect size of determinants. To determine the source of potential random variation, subgroup analysis was performed depending on the region of the studies and the type of study design. Meta-regression was used to determine the presence of statistically significant heterogeneity based on sample size and year of publication. A funnel plot was used to evaluate publication bias.
A total of 720 studies were identified from various electronic databases and library catalogs. Of these studies, 400 articles records were identified and removed due to duplication. Reviewing titles and abstracts resulted in the exclusion of 270 irrelevant research articles for our study. After assessing the full texts of the remaining articles, 29 studies were excluded as they did not meet the preset eligibility criteria. The remaining twenty-one (21) studies were included in the final systematic review and meta-analysis (Fig. 1 ).

PRISMA flow diagram of included studies in the systematic review and meta-analysis of the magnitude and determinants of undernutrition among tuberculosis patients in Ethiopia, 2023
Characteristics of included studies
All of the twenty-two studies included in this study were published from 2006 to 2023 in peer review journals [ 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 ]. A total of 8001 study participants were included in the current systematic review and meta-analysis. The smallest sample size was 95 from a study conducted in the Dire Dawa [ 27 ], and the largest sample size was 1681 from a study conducted in the Amhara region [ 29 ]. Fifteen included studies were cross-sectional in study design [ 16 , 17 , 18 , 19 , 20 , 21 , 24 , 25 , 26 , 28 , 29 , 30 , 31 , 32 , 36 ], three were retrospective cohort studies [ 22 , 23 , 34 ], and three were case-controls [ 27 , 33 , 35 ]. Regarding study setting, six studies were conducted in the Amhara region [ 17 , 25 , 28 , 29 , 34 , 36 ] two studies in the SNNPR [ 22 , 30 ], one study was from the Somali region [ 18 ], six studies were conducted in the Oromia region [ 16 , 18 , 20 , 26 , 31 , 35 ], two studies were conducted in Tigray [ 21 , 34 ], two studies were conducted in Addis Ababa [ 23 , 32 ] and two studies were from Dire Dawa [ 24 , 27 ]. The summary of the included articles is described in (Table 1 ).
Magnitude of undernutrition among tuberculosis patients in Ethiopia
High heterogeneity was observed across the included studies (I2 = 95.9, p < 0.001) thus, a random-effects model was used to estimate the pooled magnitude of undernutrition among tuberculosis patients in Ethiopia. The pooled magnitude of undernutrition among tuberculosis patients was 48.23% (95% CI 42.84, 53.62). The highest 83.16% (95% CI 75.63, 90.68) magnitude was observed in a study conducted in Dire Dawa [ 27 ] and the lowest 26.67% (95% CI 22.1 31.24) magnitude was observed in a study conducted in Addis Ababa [ 32 ] (Fig. 2 ).

Forest plot of the pooled estimate of the magnitude of undernutrition among tuberculosis patients in Ethiopia, 2023
To check for underlying heterogeneity, meta-regression models were done by using sample size and year of publication, but there was statistically insignificant underlying heterogeneity ( p = 0.861) and ( p = 0.158), respectively (Table 2 ).
Subgroup analysis
To realize heterogeneity among the included articles, subgroup analysis was undertaken by study setting and study design. According to where the studies conducted, the highest magnitude of undernutrition among tuberculosis patients 59.02% (95% CI 38.97, 79.08) was observed in the eastern part (Dire Dawa, Harari and Somali) [ 19 , 24 , 27 ] and the lowest magnitude of undernutrition among tuberculosis patients was 33.20% (95% CI 22.06, 44.34) was reported in SNNPR [ 22 , 30 ] (Fig. 3 ). According to the study design of the studies, the magnitude of undernutrition among tuberculosis patients among cross-sectional, case-control and cohort studies were 47.07% (95% CI 41.45, 52.69), 67.54% (95% CI 50.62, 84.47) and 35.60% (95%CI 25.14, 46.07) respectively (Fig. 4 ).

Subgroup analysis of the magnitude of undernutrition among tuberculosis patients in Ethiopia based on the region, 2023

Subgroup analysis of the magnitude of undernutrition among tuberculosis patients in Ethiopia based on the study design, 2023
Publication bias
To test the existence of publication bias, the graphical funnel plot and Egger’s test at a 5% significance level were executed. The visual examination of the funnel plot presented symmetrically which is an indicator for the absence of publication bias (Fig. 5 ). Egger’s and begg test also showed the absence of publication bias at a 5% significance level at p- value (0.785) and (0.131) respectively.

Funnel plot with 95% confidence limit of the magnitude of undernutrition among tuberculosis patients in Ethiopia, 2023
Sensitivity analysis
Sensitivity analysis was done to see any outliers and it showed there was no single study influence on the overall included studies (Fig. 6 ).

Result of sensitivity analysis of the magnitude of undernutrition among tuberculosis patients in Ethiopia, 2023
Factors associated with undernutrition among tuberculosis patients
Association between education and undernutrition among tuberculosis patients.
To investigate the association between educational status and undernutrition among tuberculosis patients, three studies were included in the analysis [ 16 , 19 , 24 ]. A random-effects model was utilized to evaluate the association between education and undernutrition among tuberculosis patients (I2 = 84.9%, P-value = 0.001). The result of the analysis showed that the association between education and undernutrition among tuberculosis patients was statistically significant (OR = 2.11(95%CI: 1.09, 4.06). TB patients those who had no formal education were 2.11 times more likely to develop undernutrition than who had formal education (Fig. 7 ).

Forest plot of the pooled estimate of the association between education and undernutrition among tuberculosis patients in Ethiopia, 2023
Association between age and undernutrition among tuberculosis patients
To test the association between age and undernutrition among tuberculosis patients, six studies were included in the analysis [ 16 , 18 , 20 , 21 , 23 , 29 ]. The pooled association between age and undernutrition among tuberculosis patients was assessed using a random-effects model (I2 = 91.9%, P-value < 0.001). The result of the analysis indicated that the association between age and undernutrition among tuberculosis patients was statistically insignificant (OR = 1.20 (95%CI: 0.65, 2.19) (Fig. 8 ).

Forest plot of the pooled estimate of the association between age and undernutrition among tuberculosis patients in Ethiopia, 2023
Association between alcohol drinking and undernutrition among tuberculosis patients
To investigate the association between alcohol drinking and undernutrition among tuberculosis patients, two studies were included in the analysis [ 17 , 29 ]. A random-effects model was utilized to estimate the pooled association between alcohol drinking and undernutrition among tuberculosis patients (I2 = 57.6%). The result of the analysis indicated that the association between alcohol and undernutrition among tuberculosis patients was statistically significant (OR = 1.47(95CI: 1.06, 2.05). patients who drank alcohol were 1.47 times more likely to develop undernutrition than those who did not drink alcohol (Fig. 9 ).

Forest plot of the pooled estimate of the association between alcohol drinking and undernutrition among tuberculosis patients in Ethiopia, 2023
Association between family size and undernutrition among tuberculosis patients
To detect the association between family size and undernutrition among tuberculosis patients, three studies were included in the analysis [ 16 , 29 , 30 ]. The pooled association between family size and undernutrition among tuberculosis patients was examined by random-effects model (I2 = 96.6%, p-value < 0.001). The pooled result of the analysis indicated that the association between family size and undernutrition among tuberculosis patients was statistically significant (OR = 3.79 (95CI: 1.06, 14.93). patients of > 5 family size were 3.97 times more likely to develop undernutrition than those whose family size < = 5 (Fig. 10 ).

Forest plot of the pooled estimate of the association between family size and undernutrition among tuberculosis patients in Ethiopia, 2023
Association between income and undernutrition among tuberculosis patients
To investigate the association between income and undernutrition among tuberculosis patients, four studies were included in the analysis [ 18 , 21 , 25 , 32 ]. A random-effects model was utilized to determine the pooled association between income and undernutrition among tuberculosis patients (I2 = 87.6%, p-value < 0.001). The pooled result of the analysis indicated that the association between average monthly income and undernutrition among tuberculosis patients was statistically significant (OR = 2.32 (95CI: 1.33, 4.04). patients who got < 800 ETB average monthly income were 2.32 times more likely to develop undernutrition than their counterparts (Fig. 11 ).

Forest plot of the pooled estimate of the association between average monthly income and undernutrition among tuberculosis patients in Ethiopia, 2023
Association between functional status and undernutrition among tuberculosis patients
To detect the association between functional status and undernutrition among tuberculosis patients, four studies were included in the analysis [ 17 , 19 , 25 , 32 ]. The pooled association between functional status and undernutrition among tuberculosis patients was detected using fixed effect model (I2 9.9, p-value = 0.344). The analysis result indicated that the association between functional status and undernutrition among tuberculosis patients was statistically significant (OR = 2.61 (95CI: 1.99, 3.43). patients who were unable to work were 2.61 times more likely to develop undernutrition than their counterparts (fig. 12 ).

Forest plot of the pooled estimate of the association between functional status and undernutrition among tuberculosis patients in Ethiopia, 2023
Association between eating disorder and undernutrition among tuberculosis patients
To test the association between eating disorder and undernutrition among tuberculosis patients, three studies were included in the analysis [ 18 , 21 , 24 ]. The pooled association between eating disorder and undernutrition among tuberculosis patients was examined using fixed effect model (I2 0.0%, p-value = 0.437). The pooled result of the analysis indicated that the association between eating disorder and undernutrition among tuberculosis patients was statistically significant (OR = 2.73 (95CI: 2.09, 3.56). patients who had eating disorder were 2.73 times more likely to develop undernutrition than their counterparts (Fig. 13 ).

Forest plot of the pooled estimate of the association between eating disorder and undernutrition among tuberculosis patients in Ethiopia, 2023
Association between intestinal parasite and undernutrition among tuberculosis patients
To examine the association between intestinal parasite (IP) and undernutrition among tuberculosis patients, two studies were included in the analysis [ 29 , 37 ]. A random effects model was used to estimate the pooled association between IP and undernutrition among tuberculosis patients (I2 61.7%, p-value = 0.106). The pooled result of the analysis indicated that the association between IP and undernutrition among tuberculosis patients was statistically significant (OR = 3.77 (95CI: 2.39, 5.94). patients who had IP were 3.77 times more likely to develop undernutrition than their counterparts (Fig. 14 ) while the pooled results of other independent variables were statistically insignificant [Figs. 15 , 16 , 17 , 18 , 19 and 20 ].

Forest plot of the pooled estimate of the association between IP and undernutrition among tuberculosis patients in Ethiopia, 2023
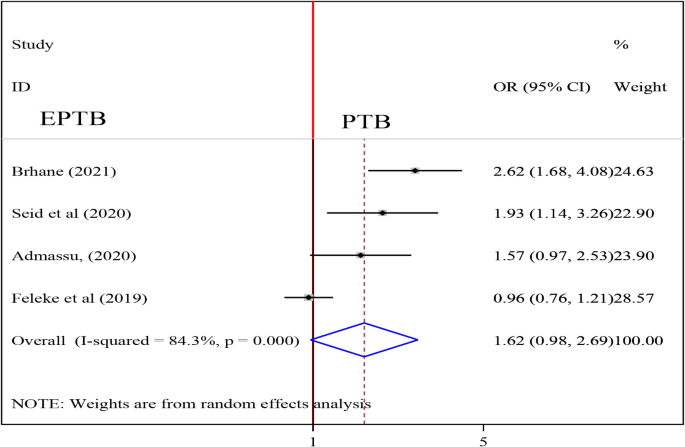
Forest plot of the pooled estimate of the association between type of TB and undernutrition among tuberculosis patients in Ethiopia, 2023

Forest plot of the pooled estimate of the association between sex and undernutrition among tuberculosis patients in Ethiopia, 2023

Forest plot of the pooled estimate of the association between residency and undernutrition among tuberculosis patients in Ethiopia, 2023

Forest plot of the pooled estimate of the association between occupation and undernutrition among tuberculosis patients in Ethiopia, 2023

Forest plot of the pooled estimate of the association between HIV status and undernutrition among tuberculosis patients in Ethiopia, 2023

Forest plot of the pooled estimate of the association between dietary counseling and undernutrition among tuberculosis patients in Ethiopia, 2023
Both undernourishment and TB are serious health issues that affect people in middle- and low-income nations. This systematic review and meta-analysis, to the best of our knowledge, is the first of its type to assess the combined magnitude and associated factors of undernutrition among patients with tuberculosis in Ethiopia. To enhance the nutritional condition of patients, which will boost the effectiveness of TB therapy and reduce TB patient morbidity and death, it is imperative to estimate the total magnitude and associated factors of undernutrition among TB patients. The results of this meta-analysis revealed that 48.23% (95% CI 42.84, 53.62) of TB patients in Ethiopia were malnourished. The finding of this study is consistent with the study conducted in Kenya (50.15%) [ 38 ], Ghana (51%) [ 39 ] and Nepal (50%) [ 40 ]. The proportional socioeconomic status of the two countries may be the cause of this parallelism.
The result of this review is higher than those of studies carried out in Bangladesh (36%) and the United States of America (11.2%) [ 41 , 42 ]. This variation may be explained by disparities in the socioeconomic standing of the two countries. Ethiopia is classified as a low-income nation, the United States as a high-income nation, and Bangladesh as a middle-income nation. Additionally, this distinction could result from changes in the study.
TB patients who had no formal education were 2.11 times more likely to develop undernutrition than those who had formal education. This is because education allows people to better read and understand nutritional issues [ 43 ]. Furthermore, education provides nutritional guidance and support for tuberculosis patients [ 44 ]. The finding is supported by a study conducted in Ghana, which found that TB patients with basic and secondary education were more likely to have normal nutritional status than those who did not attend school [ 45 ]. A low maternal educational level was found to be significantly associated with a lower prevalence of overnutrition in a Colombian study (overweight or obesity). The prevalence of wasting, stunting, and anemia was higher in the lowest maternal educational categories [ 46 ]. Another study conducted in Sekondi, Ghana, found a link between educational status and malnutrition [ 47 ]. Other studies in Bangladesh found a link between education level and nutritional status, indicating that children whose mothers have a secondary or higher education have a lower risk of childhood stunting, underweight, and wasting than children whose mothers do not go to school [ 43 ].
Patients of > 5 family size were 3.97 times more likely to develop undernutrition than those whose family size < = 5. A study in Ghana also found a link between nutritional status and immediate family size [ 47 ]. Furthermore, an Indonesian study found a negative correlation between family size and undernutrition. Leaving a large and extended family was found to be associated with undernutrition in adult TB patients [ 48 ]. Another study conducted in India found that family size was significantly related to undernutrition [ 49 ]. Possible explanations for these associations include the fact that increased family size may have a negative impact on the nutritional status of every member of the household, including preschool children, because it is associated with lower per capita human inputs. Acceptance of lower quality and quantity models of fertility decisions is also implied by increased household size.
The pooled result of the analysis indicated that the association between alcohol and undernutrition among tuberculosis patients was statistically significant (OR = 1.47(95CI: 1.06, 2.05). patients who drank alcohol were 1.47 times more likely to develop undernutrition than those who did not drink alcohol. This finding is similar to that of the Ghana study, which discovered significant associations between normal nutritional status and alcohol intake status. Patients with tuberculosis who had previously or currently used alcohol were less likely to have normal nutritional status than those who had never used alcohol [ 45 ]. Many alcoholics are malnourished, either because they consume insufficient amounts of certain essential nutrients (for example, carbohydrates, proteins, and vitamins) or because alcohol and its metabolism prevent the body from properly absorbing, digesting, and utilizing those nutrients. As a result, drunkards frequently suffer from nutrient deficiencies. Again, irregular feeding habits have been linked to heavy alcohol consumption, and it has been shown that irregular feeding habits in alcoholics lead to malnutrition [ 50 , 51 ].
The odds of being undernourished was about 2.3 times more likely for TB patients who got low average monthly income (< 800 ETB) than their counterparts. This finding is consistent with another study in Sri Lanka [ 52 ]. Although not a significant factor in this review, being employed was found to be a guarantee to access nutritious food in the study of Ghana [ 53 ]. The observed association could be attributed to the fact that TB diseases by itself are the disease which mainly affects poor people because of their low standard of living [ 54 ]. Those people with low income could not afford food and this might contribute for food insecurity, reduced intake, and deficiency of nutrients.
Functionality status was another factor that influenced the likelihood of undernutrition in TB patients. When compared to those TB patients who were able to work, patients who were unable to work were 2.61 times more likely to be undernourished. The possible reason for this observed association might be that a person who is unable to work has a low chance of farming, being productive and generating income which might in turn reduce the probability of getting and/or affording a variety of foodstuffs rich with nutrients. Besides, those patients who are unable to function even might be bedridden and have reduced appetite which would end with the undernutrition consequence. It was observed in the literature that appetite loss and generalized weakness that could affect working ability might lead to low dietary intake in TB patients [ 55 ]. In another way, the inability to work by itself could result from the deficiency of nutrients in the body and might be an explanation for the observed significant association between undernutrition and functionality status. In this regard, Gupta et al. pointed out that macronutrient supplementation improves functionality status in TB patients [ 56 ].
The pooled odds ratio of the included studies in this review shows that eating disorder is the significant factor for undernutrition among TB patients. Accordingly, TB patients with eating disorder had more chance of being undernourished as compared to those without eating disorder. The eating disorder could be due to loss of appetite which is one of the typical symptoms of TB disease [ 57 ]. This association can be explained by the fact that nutrient absorption is preceded by the intake and digestion of foods and any disorder to this biological process might end up with the problem of undernutrition. Patients with eating disorder might not intake, and/or digest the foods and this will contribute to inadequacy of available nutrients in the body. Because inadequate dietary intake and infections are the immediate causes of undernutrition and the association between undernutrition and TB disease is bidirectional [ 58 , 59 ].
Presence of intestinal parasites is another important factor that showed significant association with the undernutrition among TB patients in this systematic review and meta-analysis. Infection with intestinal parasites was found to have 3.77 times more odds of undernutrition in TB affected patients. The study in Ethiopia confirmed that there is co-occurrence of IP and TB [ 60 ]. Other studies reported similar result that infection with intestinal parasites can affect the absorption of both macronutrients such as proteins and fats and micronutrients like zinc and Iron [ 61 ]. IP might also reduce the food intake as they are manifested with gastrointestinal disturbances including nausea [ 62 ]. These might be among the mechanisms by which IP contributes to the development of undernutrition among TB patients.
Strength and limitation
This systematic review and meta-analysis has strengths; various databases were used to search for literature, both published and unpublished studies were searched and relevant studies were included after intensive quality assessment. Because 21 studies were included in the final analysis, it is appropriate for the exact estimation of publication bias from the funnel plot.
This systematic review and meta-analysis examined the substantial magnitude of undernutrition among adult TB patients in Ethiopia. This information is crucial for understanding the scale of the problem and identifying priority areas for intervention. By knowing the prevalence rates, policymakers can allocate resources and design programs to address the specific needs of the affected population. Undernutrition was associated with a low average monthly income, Eating disorder, inability to work, having no formal education, intestinal parasite, having family size > 5 and alcohol drinking. Furthermore, a discrepancy was also found in different regions of the country. So, policy-based interventions to track these contributing factors are mandatory. The findings of this meta-analysis can guide policymakers and program developers in understanding the underlying causes and vulnerabilities related to undernutrition in TB patients.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Confidence interval
Southern Nation nationality and people region
Intestinal parasite
Tuberculosis
E F. Guideline for clinical and programmatic management of TB, leprosy and TB/HIV in Ethiopia. Ethiopia: FMOH Addis Ababa; 2015.
Google Scholar
WHO. The global plan to stop TB 2011–2015: transforming the fight towards elimination of tuberculosis. 2010. 2010.
WHO. GlobalTuberculosis Report: World Health Organization. 2018 [ https://www.who.int/tb/publications/global_report/en/ . 2018.
WHO. Global tuberculosis report, https://www.who.int/teams/global-tuberculosis-programme/tb-reports . Geneva; 2022.
Simon Schaaf H, Cilliers K, Willemse M, Labadarios D, Kidd M, Donald PR. Nutritional status and its response to treatment of children, with and without HIV infection, hospitalized for the management of tuberculosis. Paediatrics Int Child Health. 2012;32(2):74–81.
Article Google Scholar
Napier Ruth J, Rafi W, Cheruvu M, Powell Kimberly R, Zaunbrecher MA, Bornmann W, et al. Imatinib-Sensitive Tyrosine Kinases regulate mycobacterial pathogenesis and represent therapeutic targets against tuberculosis. Cell Host Microbe. 2011;10(5):475–85.
Article CAS PubMed PubMed Central Google Scholar
Abdus Salam PO. Nutritional status in sputum positive and sputum negative cases of pulmonary tuberculosis. Natl J Physiol Pharm Pharmacol. 2018;8(4).
Padmapriyadarsini C, Shobana M, Lakshmi M, Beena T, Swaminathan S. Undernutrition & Tuberculosis in India: Situation analysis & the way forward. Indian J Med Res. 2016;144(1):11–20.
WHO. G. Nutritional care and support for patients with tuberculosis. Geneva: World Health Organization; 2013.
Moher D, Liberati A, Tetzlaff J, Altman DG. Guidelines and Guidance-Preferred reporting items for systematic reviews and Meta-analyses: the PRISMA Statement. PLoS Med. 2009;6(7):698.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and Meta-analyses: the PRISMA Statement. PLoS Med. 2009;6(7):e1000097.
Article PubMed PubMed Central Google Scholar
Institute JB. Meta-analysis of statistics: Assessment and review instrument (JBI MASTARI). Volume 20032007. Adelaide: Joanna Briggs Institute; 2006.
Luchini C, Stubbs B, Solmi M, Veronese N. Assessing the quality of studies in meta-analyses: advantages and limitations of the Newcastle Ottawa Scale. World J Meta-Anal. 2017;5(4):80–4.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Article PubMed Google Scholar
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
Article CAS PubMed Google Scholar
Tadesse F, Mitiku H, Girma S, Kenay A. Magnitude of undernutrition and associated factors among adult tuberculosis patients attending public health facilities in Haramaya District, Eastern Ethiopia. BMC Pulm Med. 2023;23(1):42.
Endalkachew K, Ferede YM, Derso T, Kebede A. Prevalence and associated factors of undernutrition among adult TB patients attending Amhara National Regional State hospitals, Northwest Ethiopia. J Clin Tuberculosis Other Mycobact Dis. 2022;26:100291.
Article CAS Google Scholar
Tesfaye Anbese A, Egeta G, Mesfin F, Arega Sadore A. Determinants of Undernutrition among Adult Tuberculosis Patients Receiving Treatment in Public Health Institutions in Shashemane Town, Southern Ethiopia. J Nutr Metabolism. 2021;2021:4218023.
Muse AI, Osman MO, Ibrahim AM, Wedajo GT, Daud FI, Abate KH. Undernutrition and Associated Factors Among Adult Tuberculosis Patients in Jigjiga Public Health Facilities, Somali Region, East, Ethiopia. Research and reports in tropical medicine. 2021;12:123 – 33.
Hussien B, Ameni G. A cross-sectional study on the magnitude of undernutrition in tuberculosis patients in the Oromia Region of Ethiopia. J Multidisciplinary Healthc. 2021;14:2421–8.
Brhane T, Merga H, Ayele L, Gemeda DH. Undernutrition among Tuberculosis patients on directly observed short-course therapy: an epidemiological study from Northern Ethiopia. Nutr Diet Supplements. 2021;13:83–9.
Bade AB, Mega TA, Negera GZ. Malnutrition is associated with delayed sputum culture conversion among patients treated for MDR-TB. Infection and drug resistance. 2021:1659–67.
Seid G, Ayele M. Undernutrition and Mortality among adult tuberculosis patients in Addis Ababa, Ethiopia. Advances in preventive medicine. 2020;2020:5238010.
Admassu T. Undernutrition and associated factors among adult tuberculosis patients in public health faciities of dire dawa administartion and harari region, eastern Ethiopia 2020.
Abate H. Prevalence of undernutrition and associated factors among adult tuberculosis patients in public health institution in bahirdar town, north west, ethiopia. 2020.
Hussien B, Hussen MM, Seid A, Hussen A. Nutritional deficiency and associated factors among new pulmonary tuberculosis patients of Bale Zone hospitals, southeast Ethiopia. BMC Res Notes. 2019;12(1):751.
Hassen JK, Gizaw A, Mohamed S. Determinants of pulmonary tuberculosis in public health facilities of dire Dawa City, Eastern Ethiopia: unmatched case–control study. Int J Mycobacteriology. 2019;8(2):118.
Gashaw F, Bekele S, Mekonnen Y, Medhin G, Ameni G, Erko B. High helminthic co-infection in tuberculosis patients with undernutritional status in northeastern Ethiopia. Infect Dis Poverty. 2019;8(05):52–62.
Feleke BE, Feleke TE, Biadglegne F. Nutritional status of tuberculosis patients, a comparative cross-sectional study. BMC Pulm Med. 2019;19(1):182.
Geberemeskel T, Woldeyohannes D, Demisie M, Demisie M. Undernutrition and associated factors among adult tuberculosis patients in hossana town public health facilities, Southern Ethiopia. J Trop Dis. 2018;6(01):253.
Guadie FF, Assaminew B. Assessment of nutritional status and associated factors among adult TB patients on directly observed treatment of short course in health facilities at Adama Town, East Shewa Zone, Ethiopia. Scholar Pract Pract J. 2016;1(1).
Dargie B, Tesfaye G, Worku A. Prevalence and associated factors of undernutrition among adult tuberculosis patients in some selected public health facilities of Addis Ababa, Ethiopia: a cross-sectional study. BMC Nutr. 2016;2(1):7.
Ephrem T, Mengiste B, Mesfin F, Godana W. Determinants of active pulmonary tuberculosis in Ambo Hospital, West Ethiopia. Afr J Prim Health care Family Med. 2015;7(1):e1–8.
Wassie M, Shamil F, Worku A. Weight gain and associated factors among adult tuberculosis patients on treatment in northwest Ethiopia: a longitudinal study. J Nutr Disorders Ther. 2014;4(143):2.
Fisseha G, Etana B, Haileslassie K, Alemayehu M. Determinant factors of treatment failure among tuberculosis patients under directly observed therapy in Tigray regional state public hospitals, north Ethiopia: a case–control study. Global J Med Research: F Dis. 2014;2104(14):5.
Kassu A, Yabutani T, Mahmud ZH, Mohammad A, Nguyen N, Huong BTM, et al. Alterations in serum levels of trace elements in tuberculosis and HIV infections. Eur J Clin Nutr. 2006;60(5):580–6.
Tigist G. Assesment of Weight Gain and Associated factors among adults of tuberculosis patients during the first two months of follow up in Public Health Facilities of Bahir Dar City Northwest Ethiopia. Cross-Sectional Study; 2020.
Nthiga I, et al. The nutritional status of pulmonary tuberculosis patients aged 25–44 years attending tuberculosis clinic at Lodwar County and Referral Hospital. Kenya: Turkana County; 2017.
Dodor E. Evaluation of nutritional status of new tuberculosis patients at the effia-nkwanta regional hospital. Ghana Med J. 2008;42(1):22.
PubMed PubMed Central Google Scholar
SanchitaSubedi RSM, PushpaParajuli GayanandMandal, Yadav DK. Nutritional status of patients with pulmonary tuberculosis receiving anti-tuberculosis treatment at bp koirala institute of health sciences, Nepal. J Nurs Health Sci 2019;8(6).
Rahaman MFU, et al. Nutritional status of patients with tuberculosis attending at tertiary medical center in Bangladesh. Bangladesh J Med. 2019;30(2):53–7.
Phan MN, Guy ES, Nickson RN, Kao CC. Predictors and patterns of weight gain during treatment for tuberculosis in the United States of America. Int J Infect Dis. 2016;53:1–5. https://doi.org/10.1016/j.ijid.2016.09.006 .
Hasan T, Magalhaes RJS, Williams GM, Mamun AA. Original article the role of maternal education in the 15-year trajectory of malnutrition in children under 5 years of age in Bangladesh 2016;(Unicef 2013):929–39.
Aslam M, Safdar M, Khalid S, Sharmeen Z, Irfan T. The Effect of Nutrition Education on Nutritional Status of Tuberculosis Patients 2021;(February):1–6.
Kubi P, Id A, Osei B, Id HA. Factors associated with nutritional status, knowledge and attitudes among tuberculosis patients receiving treatment in Ghana : A cross-sectional study in the Tema Metropolis. 2021;1–12. https://doi.org/10.1371/journal.pone.0258033 .
Cediel G, Perez E, Gaitán D, Sarmiento OL, Gonzalez L. Association of all forms of malnutrition and socioeconomic status, educational level and ethnicity in Colombian children and non-pregnant women. 2020;23.
Dodor EA. Evaluatio of utritio al status of ew tuberculosis patie ts at the effia- kwa ta regio al hospital. 2008;42(1).
Town H, Health P. Undernutrition and Associated Factors among Adult Tuberculosis Patients in Undernutrition and Associated Factors among Adult Tuberculosis Patients in Hossana Town Public Health Facilities, Southern Ethiopia. 2018;(January).
Mahanta LB, Roy TD, Dutta RG. Ecology of Food and Nutrition Nutritional Status and the impact of socioeconomic factors on pregnant women in Kamrup District of Assam. 2012;(June 2014).
Lieber CS. Relationships Between Nutrition, Alcohol Use, and Liver Disease.
Santolaria F, Pe L, Milena A, Gonza E, Martı A. Nutritional assessment in alcoholic patients. Its relationship with alcoholic intake, feeding habits, organic complications and social problems. 2000;59:295–304.
Galgamuwa LS, Iddawela D, Dharmaratne SD, Galgamuwa GLS, Appiah PK, Osei B, Amu H. Nutritional status and correlated socio-economic factors among preschool and school children in plantation communities, Sri Lanka. BMC Public Health. 2017;17:377. https://doi.org/10.1186/s12889-017-4311-y .
Appiah PK, Osei B, Amu H. Factors associated with nutritional status, knowledge and attitudes among tuberculosis patients receiving treatment in Ghana: A cross-sectional study in the Tema Metropolis. Plos One. 2021;16 (10). e0258033.
WHO, Guideline. Nutritional care and support for patients with tuberculosis. Geneva: World Health Organization; 2013. https://apps.who.int/iris/bitstream/handle/10665/94836/9789241506410_eng.pdf . Accessed on March 4, 2023.
Meena BP, Taparia P. Association of Tuberculosis and biochemical nutritional status: a case-control study. Int Multispecialty J Health (IMJH). 2016.
Gupta KB, Harshita Gupta, Kant S, Gupta K. Nutrition and Tuberculosis. National Medicos Organisation Journal (ISSN-2348-3806). 2020;12(01), 43–46. Retrieved from https://nmojournal.org/index.php/nmojournal/article/view/24 .
Centers for disease control and prevention (CDC). Tuberculosis (Signs and symptoms). https://www.cdc.gov/ . Accessed on 3/3/2023.
Sablah M. Causes and Impacts of Undernutrition over the Life Course. UNICEF – HQ – PD - Nutrition. UNDESA EXPERT GROUP MEETING Sept. 16–17, 2019 | New York. https://www.un.org/en/development/desa/population/events/pdf/expert/30/presentations/Tuesday/Session4/Causes%20-%20Consequences%20of%20Undernutrition%20ICPD%20-%20UNICEF.pdf . Accessed on 3/3/2023.
Gupta D. Nutrition Can Be A Gamechanger In Addressing TB. https://planet.outlookindia.com/opinions/nutrition-can-be-a-gamechanger-in-addressing-tb-news-413951 . Accessed on 3/3/2023. 2022.
Alemu A, Bitew ZW, Worku T. Intestinal parasites co-infection among tuberculosis patients in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2020;20:510. https://doi.org/10.1186/s12879-020-05237-7 .
Stephenson LS, Latham MC, Ottesen EA. Malnutrition and parasitic helminth infections. Parasitology. 2000;121(Suppl):S23–38. [PubMed] [CrossRef] [Google Scholar].
Koethe JR, Von Reyn CF, Int J. Tuberc Lung Dis. 2016;20(7):857–63. https://doi.org/10.5588/ijtld.15.0936 . [PubMed] [CrossRef] [Google Scholar].
Download references
Acknowledgements
We would like to thank all authors of the studies included in this systematic review and meta-analysis.
No funding was obtained for this study.
Author information
Authors and affiliations.
Department of Public Health, Institute of Health Sciences, Wollega University, P.O.BOX: 395, Nekemte, Ethiopia
Jira Wakoya Feyisa, Matiyos Lema, Markos Desalegn, Emiru Merdassa, Wase Benti Hailu, Sidie Debelo Beyena & Adisu Tafari Shama
School of Nursing and Midwifery, Institute of Health Sciences, Wollega University, Nekemte, Ethiopia
Robera Demissie Berhanu
Department of Public Health, College of Health Sciences, Mettu University, Mettu, Ethiopia
Keno Melkamu Kitila
You can also search for this author in PubMed Google Scholar
Contributions
JW, RD, ML, EM, MD, KM, WB, SD, AT, and were involved in the design, statistical analysis, and manuscript writing, and participated in the selection of articles and data extraction. All authors were involved in developing the initial drafts of the manuscript, revising subsequent drafts, and preparing the final draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Jira Wakoya Feyisa .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Feyisa, J.W., Berhanu, R.D., Lema, M. et al. Magnitude and determinants of undernutrition among tuberculosis patients in Ethiopia: systematic review and meta-analysis. BMC Public Health 24 , 1698 (2024). https://doi.org/10.1186/s12889-024-19220-3
Download citation
Received : 02 May 2023
Accepted : 21 June 2024
Published : 25 June 2024
DOI : https://doi.org/10.1186/s12889-024-19220-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Undernutrition
- Meta-analysis
BMC Public Health
ISSN: 1471-2458
- General enquiries: [email protected]
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Higa2 (rv2021c) is a transcriptional regulator with multiple regulatory targets in mycobacterium tuberculosis.

1. Introduction
2. materials and methods, 2.1. bacterial strains, plasmids, and growth conditions, 2.2. construction of mc 2 155 heterologous expression vector, 2.3. higb2-higa2 neutralization assay, 2.4. protein purification, 2.6. rt-pcr assays, 2.7. dna pull-down, 3.1. higa2 exhibits toxicity in m. smegmatis, 3.2. higa2 binding site identifications, 3.3. identification of conserved higa2 recognition motifs, 3.4. higa2 regulating sites exploration on the genome scale, 3.5. exploration of upstream regulatory genes of higba2, 4. discussion, 4.1. higba2 remains uncleared for ta system activity, 4.2. downstream regulatory genes of higa2 may be involved in the establishment of persistence, 4.3. higba2 may be regulated by multiple upstream proteins in response to external stresses, 5. conclusions, supplementary materials, author contributions, data availability statement, conflicts of interest.
- World Health Organization (WHO). Global Tuberculosis Report 2023 ; World Health Organization: Geneva, Switzerland, 2023. [ Google Scholar ]
- Boldrin, F.; Provvedi, R.; Cioetto Mazzabò, L.; Segafreddo, G.; Manganelli, R. Tolerance and Persistence to Drugs: A Main Challenge in the Fight Against Mycobacterium tuberculosis . Front. Microbiol. 2020 , 11 , 1924. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Balaban, N.Q. Persistence: Mechanisms for triggering and enhancing phenotypic variability. Curr. Opin. Genet. Dev. 2011 , 21 , 768–775. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhang, Y. Persisters, persistent infections and the Yin-Yang model. Emerg. Microbes Infect. 2014 , 3 , e3. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fasani, R.A.; Savageau, M.A. Molecular mechanisms of multiple toxin–antitoxin systems are coordinated to govern the persister phenotype. Proc. Natl. Acad. Sci. USA 2013 , 110 , e2528–e2537. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tanaka, M.M.; Akarsu, H.; Bordes, P.; Mansour, M.; Bigot, D.-J.; Genevaux, P.; Falquet, L. TASmania: A bacterial Toxin-Antitoxin Systems database. PLOS Comput. Biol. 2019 , 15 , e1006946. [ Google Scholar ]
- Ziemski, M.; Leodolter, J.; Taylor, G.; Kerschenmeyer, A.; Weber-Ban, E. Genome-wide interaction screen for Mycobacterium tuberculosis ClpCP protease reveals toxin-antitoxin systems as a major substrate class. FEBS J. 2021 , 288 , 111–126. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kamruzzaman, M.; Wu, A.Y.; Iredell, J.R. Biological Functions of Type II Toxin-Antitoxin Systems in Bacteria. Microorganisms 2021 , 9 , 1276. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Soo, V.W.; Wood, T.K. Antitoxin MqsA represses curli formation through the master biofilm regulator CsgD. Sci. Rep. 2013 , 3 , 3186–3192. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sala, A.; Bordes, P.; Genevaux, P. Multiple toxin-antitoxin systems in Mycobacterium tuberculosis . Toxins 2014 , 6 , 1002–1020. [ Google Scholar ] [ CrossRef ]
- Mansour, M.; Giudice, E.; Xu, X.; Akarsu, H.; Bordes, P.; Guillet, V.; Bigot, D.J.; Slama, N.; D’Urso, G.; Chat, S.; et al. Substrate recognition and cryo-EM structure of the ribosome-bound TAC toxin of Mycobacterium tuberculosis . Nat. Commun. 2022 , 13 , 2641–2654. [ Google Scholar ] [ CrossRef ]
- Turkarslan, S.; Peterson, E.J.R.; Rustad, T.R.; Minch, K.J.; Reiss, D.J.; Morrison, R.; Ma, S.; Price, N.D.; Sherman, D.R.; Baliga, N.S. A comprehensive map of genome-wide gene regulation in Mycobacterium tuberculosis . Sci. Data 2015 , 2 , 150010. [ Google Scholar ] [ CrossRef ]
- Keren, I.; Minami, S.; Rubin, E.; Lewis, K. Characterization and transcriptome analysis of Mycobacterium tuberculosis persisters. mBio 2011 , 2 , 100–111. [ Google Scholar ] [ CrossRef ]
- Betts, J.C.; Lukey, P.T.; Robb, L.C.; McAdam, R.A.; Duncan, K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 2002 , 43 , 717–731. [ Google Scholar ] [ CrossRef ]
- Bähler, J.; Rustad, T.R.; Harrell, M.I.; Liao, R.; Sherman, D.R. The Enduring Hypoxic Response of Mycobacterium tuberculosis . PLoS ONE 2008 , 3 , e1502. [ Google Scholar ]
- Gupta, A.; Venkataraman, B.; Vasudevan, M.; Gopinath Bankar, K. Co-expression network analysis of toxin-antitoxin loci in Mycobacterium tuberculosis reveals key modulators of cellular stress. Sci. Rep. 2017 , 7 , 5868–5881. [ Google Scholar ] [ CrossRef ]
- Richardson, W.; Kang, G.W.; Lee, H.J.; Kwon, K.M.; Kim, S.; Kim, H.J. Chasing the structural diversity of the transcription regulator Mycobacterium tuberculosis HigA2. IUCrJ 2021 , 8 Pt 5 , 823–832. [ Google Scholar ] [ CrossRef ]
- Chi, X.; Chang, Y.; Li, M.; Lin, J.; Liu, Y.; Li, C.; Tang, S.; Zhang, J. Biochemical characterization of mt-PemIK, a novel toxin-antitoxin system in Mycobacterium tuberculosis . FEBS Lett. 2018 , 592 , 4039–4050. [ Google Scholar ] [ CrossRef ]
- Agarwal, S.; Tiwari, P.; Deep, A.; Kidwai, S.; Gupta, S.; Thakur, K.G.; Singh, R. System-Wide Analysis Unravels the Differential Regulation and In Vivo Essentiality of Virulence-Associated Proteins B and C Toxin-Antitoxin Systems of Mycobacterium tuberculosis . J. Infect. Dis. 2018 , 217 , 1809–1820. [ Google Scholar ] [ CrossRef ]
- Gou, L.; Han, T.; Wang, X.; Ge, J.; Liu, W.; Hu, F.; Wang, Z. A Novel TetR Family Transcriptional Regulator, CalR3, Negatively Controls Calcimycin Biosynthesis in Streptomyces chartreusis NRRL 3882. Front. Microbiol. 2017 , 8 , 2371. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gao, C.H.; Yang, M.; He, Z.G. An ArsR-like transcriptional factor recognizes a conserved sequence motif and positively regulates the expression of phoP in Mycobacteria . Biochem. Biophys. Res. Commun. 2011 , 411 , 726–731. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Juréen, P.; Werngren, J.; Toro, J.-C.; Hoffner, S. Pyrazinamide Resistance and pncA Gene Mutations in Mycobacterium tuberculosis . Antimicrob. Agents Chemother. 2008 , 52 , 1852–1854. [ Google Scholar ] [ CrossRef ]
- Sun, X.; Zhang, L.; Jiang, J.; Ng, M.; Cui, Z.; Mai, J.; Ahn, S.K.; Liu, J.; Zhang, J.; Liu, J.; et al. Transcription factors Rv0081 and Rv3334 connect the early and the enduring hypoxic response of Mycobacterium tuberculosis . Virulence 2018 , 9 , 1468–1482. [ Google Scholar ] [ CrossRef ]
- Kumar, A.; Phulera, S.; Rizvi, A.; Sonawane, P.J.; Panwar, H.S.; Banerjee, S.; Sahu, A.; Mande, S.C. Structural basis of hypoxic gene regulation by the Rv0081 transcription factor of Mycobacterium tuberculosis . FEBS Lett. 2019 , 593 , 982–995. [ Google Scholar ] [ CrossRef ]
- Villela, A.D.; Eichler, P.; Pinto, A.F.M.; Rodrigues-Junior, V.; Yates Iii, J.R.; Bizarro, C.V.; Basso, L.A.; Santos, D.S. Gene replacement and quantitative mass spectrometry approaches validate guanosine monophosphate synthetase as essential for Mycobacterium tuberculosis growth. Biochem. Biophys. Rep. 2015 , 4 , 277–282. [ Google Scholar ] [ CrossRef ]
- Zhang, L.; Ma, H.; Wan, S.; Zhang, Y.; Gao, M.; Liu, X. Mycobacterium tuberculosis latency-associated antigen Rv1733c SLP improves the accuracy of differential diagnosis of active tuberculosis and latent tuberculosis infection. Chin. Med. J. 2022 , 135 , 63–69. [ Google Scholar ] [ CrossRef ]
- Eweda, G.; Suzuki, D.; Nagata, T.; Tsujimura, K.; Koide, Y. Identification of murine T-cell epitopes on low-molecular-mass secretory proteins (CFP11, CFP17, and TB18.5) of Mycobacterium tuberculosis . Vaccine 2010 , 28 , 4616–4625. [ Google Scholar ] [ CrossRef ]
- Shi, W.; Chen, J.; Zhang, S.; Zhang, W.; Zhang, Y. Identification of Novel Mutations in LprG (rv1411c), rv0521, rv3630, rv0010c, ppsC, and cyp128 Associated with Pyrazinoic Acid/Pyrazinamide Resistance in Mycobacterium tuberculosis . Antimicrob. Agents Chemother. 2018 , 62 , e00430-18. [ Google Scholar ] [ CrossRef ]
- Moreno-Del Alamo, M.; Marchisone, C.; Alonso, J.C. Antitoxin ε Reverses Toxin zeta-Facilitated Ampicillin Dormants. Toxins 2020 , 12 , 801. [ Google Scholar ] [ CrossRef ]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 2019 , 17 , 441–448. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- De Bruyn, P.; Girardin, Y.; Loris, R. Prokaryote toxin-antitoxin modules: Complex regulation of an unclear function. Protein Sci. 2021 , 30 , 1103–1113. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Schuessler, D.L.; Cortes, T.; Fivian-Hughes, A.S.; Lougheed, K.E.A.; Harvey, E.; Buxton, R.S.; Davis, E.O.; Young, D.B. Induced ectopic expression of HigB toxin in Mycobacterium tuberculosis results in growth inhibition, reduced abundance of a subset of mRNAs and cleavage of tmRNA. Mol. Microbiol. 2013 , 90 , 195–207. [ Google Scholar ] [ CrossRef ]
- Bordes, P.; Cirinesi, A.-M.; Ummels, R.; Sala, A.; Sakr, S.; Bitter, W.; Genevaux, P. SecB-like chaperone controls a toxin–antitoxin stress-responsive system in Mycobacterium tuberculosis . Proc. Natl. Acad. Sci. USA 2011 , 108 , 8438–8443. [ Google Scholar ] [ CrossRef ]
- Gupta, A. Killing activity and rescue function of genome-wide toxin-antitoxin loci of Mycobacterium tuberculosis . FEMS Microbiol. Lett. 2009 , 290 , 45–53. [ Google Scholar ] [ CrossRef ]
- Hua, C.; Huang, J.; Wang, T.; Sun, Y.; Liu, J.; Huang, L.; Deng, X.; Chang, Y.-F. Bacterial Transcription Factors Bind to Coding Regions and Regulate Internal Cryptic Promoters. mBio 2022 , 13 , e0164322. [ Google Scholar ] [ CrossRef ]
- Baddam, R.; Kumar, N.; Wieler, L.H.; Lankapalli, A.K.; Ahmed, N.; Peacock, S.J.; Semmler, T. Analysis of mutations in pncA reveals non-overlapping patterns among various lineages of Mycobacterium tuberculosis . Sci. Rep. 2018 , 8 , 4628–4636. [ Google Scholar ] [ CrossRef ]
- Mahmood, N.; Bhatti, S.; Abbas, S.N.; Shahid, S.; Nasir, S.B. The pncA gene mutations of Mycobacterium tuberculosis in multidrug-resistant tuberculosis. Biotechnol. Appl. Biochem. 2021 , 69 , 2195–2204. [ Google Scholar ] [ CrossRef ]
- Hameed, H.M.A.; Tan, Y.; Islam, M.M.; Lu, Z.; Chhotaray, C.; Wang, S.; Liu, Z.; Fang, C.; Tan, S.; Yew, W.W.; et al. Detection of Novel Gene Mutations Associated with Pyrazinamide Resistance in Multidrug-Resistant Mycobacterium tuberculosis Clinical Isolates in Southern China. Infect. Drug Resist. 2020 , 13 , 217–227. [ Google Scholar ] [ CrossRef ]
- Bacon, J.; James, B.W.; Wernisch, L.; Williams, A.; Morley, K.A.; Hatch, G.J.; Mangan, J.A.; Hinds, J.; Stoker, N.G.; Butcher, P.D.; et al. The influence of reduced oxygen availability on pathogenicity and gene expression in Mycobacterium tuberculosis . Tuberculosis 2004 , 84 , 205–217. [ Google Scholar ] [ CrossRef ]
- Berney, M.; Greening, C.; Conrad, R.; Jacobs, W.R.; Cook, G.M. An obligately aerobic soil bacterium activates fermentative hydrogen production to survive reductive stress during hypoxia. Proc. Natl. Acad. Sci. USA 2014 , 111 , 11479–11484. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Berney, M.; Cook, G.M. Unique flexibility in energy metabolism allows Mycobacteria to combat starvation and hypoxia. PLoS ONE 2010 , 5 , e8614. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cordero, P.R.F.; Bayly, K.; Man Leung, P.; Huang, C.; Islam, Z.F.; Schittenhelm, R.B.; King, G.M.; Greening, C. Atmospheric carbon monoxide oxidation is a widespread mechanism supporting microbial survival. ISME J. 2019 , 13 , 2868–2881. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mohiuddin, S.G.; Massahi, A.; Orman, M.A. High-Throughput Screening of a Promoter Library Reveals New Persister Mechanisms in Escherichia Coli . Microbiol. Spectr. 2022 , 10 , e0225321. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hauryliuk, V.; Atkinson, G.C.; Murakami, K.S.; Tenson, T.; Gerdes, K. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat. Rev. Microbiol. 2015 , 13 , 298–309. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Batt, S.M.; Burke, C.E.; Moorey, A.R.; Besra, G.S. Antibiotics and resistance: The two-sided coin of the mycobacterial cell wall. Cell Surf. 2020 , 6 , 100044–100061. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hicks, N.D.; Giffen, S.R.; Culviner, P.H.; Chao, M.C.; Dulberger, C.L.; Liu, Q.; Stanley, S.; Brown, J.; Sixsmith, J.; Wolf, I.D.; et al. Mutations in dnaA and a cryptic interaction site increase drug resistance in Mycobacterium tuberculosis . PLoS Pathog. 2020 , 16 , e1009063. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Johnson, R.M.; McDonough, K.A. Cyclic nucleotide signaling in Mycobacterium tuberculosis : An expanding repertoire. Pathog. Dis. 2018 , 76 , fty048. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bu, L.; Brooks, C.L., 3rd. De novo prediction of the structures of M. tuberculosis membrane proteins. J. Am. Chem. Soc. 2008 , 130 , 5384–5385. [ Google Scholar ]
- Sable, S.B.; Kumar, R.; Kalra, M.; Verma, I.; Khuller, G.K.; Dobos, K.; Belisle, J.T. Peripheral Blood and Pleural Fluid Mononuclear Cell Responses to Low-Molecular-Mass Secretory Polypeptides of Mycobacterium tuberculosis in Human Models of Immunity to Tuberculosis. Infect. Immun. 2005 , 73 , 3547–3558. [ Google Scholar ] [ CrossRef ]
- Zhang, W.; Jiang, H.; Bai, Y.L.; Kang, J.; Xu, Z.K.; Wang, L.M. Construction and Immunogenicity of the DNA Vaccine of Mycobacterium Tuberculosis Dormancy Antigen Rv1733c. Scand. J. Immunol. 2014 , 79 , 292–298. [ Google Scholar ] [ CrossRef ]
- Haydel, S.E.; Malhotra, V.; Cornelison, G.L.; Clark-Curtiss, J.E. The prrAB Two-Component System Is Essential for Mycobacterium tuberculosis Viability and Is Induced under Nitrogen-Limiting Conditions. J. Bacteriol. 2012 , 194 , 354–361. [ Google Scholar ] [ CrossRef ]
- Subbian, S.; Gautam, U.S.; Mehra, S.; Kaushal, D. In-Vivo Gene Signatures of Mycobacterium tuberculosis in C3HeB/FeJ Mice. PLoS ONE 2015 , 10 , e0135208. [ Google Scholar ]
- Gao, L.-Y.; Groger, R.; Cox, J.S.; Beverley, S.M.; Lawson, E.H.; Brown, E.J. Transposon Mutagenesis of Mycobacterium marinumIdentifies a Locus Linking Pigmentation and Intracellular Survival. Infect. Immun. 2003 , 71 , 922–929. [ Google Scholar ] [ CrossRef ]
- Mendoza Lopez, P.; Golby, P.; Wooff, E.; Garcia, J.N.; Garcia Pelayo, M.C.; Conlon, K.; Gema Camacho, A.; Hewinson, R.G.; Polaina, J.; Suárez García, A.; et al. Characterization of the transcriptional regulator Rv3124 of Mycobacterium tuberculosis identifies it as a positive regulator of molybdopterin biosynthesis and defines the functional consequences of a non-synonymous SNP in the Mycobacterium bovis BCG orthologue. Microbiology 2010 , 156 , 2112–2123. [ Google Scholar ]
- Raghunandanan, S.; Ramachandran, R.; Gomez, R.L.; Devanarayanan, S.; Bommakanti, A.; Kondapi, A.K.; Varadarajan, R.; Kumar, R.A. Rv0474 is a copper-responsive transcriptional regulator that negatively regulates expression of RNA polymerase β subunit in Mycobacterium tuberculosis . FEBS J. 2018 , 285 , 3849–3869. [ Google Scholar ] [ CrossRef ]
- Li, X.; Chen, F.; Liu, X.; Xiao, J.; Andongma, B.T.; Tang, Q.; Cao, X.; Chou, S.H.; Galperin, M.Y.; He, J. Clp protease and antisense RNA jointly regulate the global regulator CarD to mediate mycobacterial starvation response. eLife 2022 , 11 , e73347. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| Gene | Location | Feature | Illustration |
|---|---|---|---|
| Rv2043c | 5′-UTR | pncA: Pyrazinamidase (PZase) | |
| Rv2044c | CDS | relative with pyrazinamide resistance | |
| Rv0086 | CDS | hycQ: hydrogenase HycQ | |
| Rv0087 | 5′-UTR | hycE: Possible formate hydrogenase | |
| Rv3396c | CDS | guaA: GMP synthase | |
| Rv1733c | CDS | transmembrane protein; surface antigen | |
| Rv2433c | 5′-UTR | Secretory protein; T cell antigen | |
| Rv2434c | CDS | transmembrane protein | |
| Rv0010c | 5′-UTR | relative with pyrazinamide resistance | |
| Rv0258c | 5′-UTR | hypothetical protein | |
| higB2 | CDS | higB2: hypothetical protein |
| Protern | Feature |
|---|---|
| Rv0903c | essential gene prrA, transcriptional regulator of the two component system PrrA/PrrB |
| Rv0744c | Possible transcriptional regulatory protein, similar to a two-component sensor |
| Rv3574 | KstR, probable TetR-family transcriptional regulator involved in lipid metabolism |
| Rv3833 | Probable AraC-family transcriptional regulatory protein |
| Rv2488c | Probable LuxR-family transcriptional regulatory protein |
| Rv3676 | Crp, cAMP-activated global transcriptional regulator |
| Rv1909c | FurA, ferric uptake regulation protein, transcriptional regulator |
| Rv2166c | essential gene MraZ, transcriptional regulator |
| Rv3124 | MoaR1, transcriptional regulator |
| Rv0474 | Cu responsive transcriptional regulator |
| Rv2603c | Probable transcriptional regulator |
| Rv3583c | essential gene CarD, RNA polymerase-binding transcription factor |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Xu, M.; Liu, M.; Liu, T.; Pan, X.; Ren, Q.; Han, T.; Gou, L. HigA2 (Rv2021c) Is a Transcriptional Regulator with Multiple Regulatory Targets in Mycobacterium tuberculosis . Microorganisms 2024 , 12 , 1244. https://doi.org/10.3390/microorganisms12061244
Xu M, Liu M, Liu T, Pan X, Ren Q, Han T, Gou L. HigA2 (Rv2021c) Is a Transcriptional Regulator with Multiple Regulatory Targets in Mycobacterium tuberculosis . Microorganisms . 2024; 12(6):1244. https://doi.org/10.3390/microorganisms12061244
Xu, Mingyan, Meikun Liu, Tong Liu, Xuemei Pan, Qi Ren, Tiesheng Han, and Lixia Gou. 2024. "HigA2 (Rv2021c) Is a Transcriptional Regulator with Multiple Regulatory Targets in Mycobacterium tuberculosis " Microorganisms 12, no. 6: 1244. https://doi.org/10.3390/microorganisms12061244
Article Metrics
Article access statistics, supplementary material.
ZIP-Document (ZIP, 208 KiB)
Further Information
Mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Tuberculosis.
Ellis H. Tobin ; Debbie Tristram .
Affiliations
Last Update: March 20, 2024 .
- Continuing Education Activity
More lives have been lost as a result of tuberculosis (TB) than any other human disease. The current yearly estimate of worldwide mortality due to TB exceeds 1 million people. TB is a potentially curable disease, yet it is a challenge to diagnose, treat, and prevent. This activity introduces the epidemiology, pathophysiology, diagnostic approaches, and treatments of latent and active TB. It empowers healthcare professionals with an understanding of the myriad symptoms and signs of TB, the limitations in the accuracy of diagnostic modalities, the challenges associated with anti-TB drug treatment, and the limited resources available to prevent transmission. It describes how the landscape of TB has been altered by the advent of HIV, as well as the emergence of drug resistance.
- Screen high-risk populations for tuberculosis infection using evidence-based screening tools and protocols.
- Implement current World Health Organization guidelines for the diagnosis of tuberculosis.
- Assess the effectiveness of tuberculosis treatment regimens through regular monitoring of patient progress and response to therapy.
- Collaborate with an interprofessional healthcare team and public health authorities to ensure timely reporting and management of tuberculosis cases.
- Introduction
Tuberculosis (TB) encompasses a vast amount of information about a common disease that is challenging to diagnose, treat, and prevent. The following quote by S.T. Cole provides a perspective on the importance of this disease: “ More human lives have been lost to tuberculosis than to any other disease .” [1] Before the SARS-CoV-2 pandemic, Mycobacterium tuberculosis ( Mtb ) was the most prevalent human pathogen in the world. Unlike SARS-CoV-2, Mtb has existed as a human pathogen for millennia. Robert Koch reported his discovery of the Mtb bacterium in 1882, and its complete genome sequence was mapped over 100 years later. [2]
The diagnostic Mantoux skin test, developed in 1909, remains in use today with minor modifications in reagents and interpretive criteria. Interferon-gamma release assay (IGRA), developed in 2014, offers another approach to diagnosis. [3] Both tests possess diagnostic, predictive limitations that require a sophisticated understanding of interpretive criteria. The bacterium is slow-growing, frequently sparse, and often difficult to identify in sputum and tissue samples. These historic challenges in confirmatory TB diagnostics have been mitigated somewhat by the recent introduction of molecular nucleic acid amplification tests (NAATs). [4] However, NAATs are not readily available in many parts of the world where TB is most prevalent. Furthermore, when and how to best implement these tests in practice remains a work in progress. New tests that may enhance diagnostic accuracy are gradually emerging. [5]
The regimens required to treat TB are a challenge to administer. The most commonly used anti-TB antibiotics, all developed in the mid-20th century, remain the mainstay of therapy today. For the first time in over 40 years, 2 new anti-TB antibiotics have recently been approved for treatment. [6] Anti-TB regimens will vary depending on the stage and anatomic location of the infection, the immune status of the host, the age of the host, the presence of comorbidities, the development of toxicities, drug-drug interactions, and resistance patterns of the bacterium. Resistance is on the rise and often requires the administration of novel antibiotic combination strategies that have undergone limited testing in clinical trials. The prolonged duration of therapy required to eradicate the organism represents an additional challenge. With respect to latent TB infection, shortened treatment duration strategies have recently been developed to minimize the adverse effects of antibiotics and maximize patient compliance. [7]
TB prevention remains a worldwide challenge. Mtb is easily transmitted, and conditions that favor poverty, overcrowding, and lack of public health infrastructure contribute to its communicability. Moreover, nonspecific symptoms such as persistent cough often go unnoticed, resulting in high transmission rates. In the case of active TB, the multiple antibiotics required to eliminate the disease, along with their prolonged course of administration, represent a challenge even in those regions that possess robust public health infrastructure. Those parts of the world with the highest TB prevalence often lack resources to prevent it. In resource-rich parts of the world where TB is relatively uncommon, many clinicians rarely encounter it, are unfamiliar with its myriad clinical manifestations, and lack experience in approaches to diagnosis and management.
This activity provides an introduction to and overview of TB. Related activities will present details of diagnosis, clinical manifestations, treatment, and prevention.
Members of the Mtb family, also known as the Mtb complex, capable of causing human disease include M tuberculosis ( sensu stricto ), M bovis, M africanum, and M canetii. These are closely related species; M tuberculosis being the predominant human pathogen worldwide. Mtb is aerobic, non–spore-forming, and nonmotile. Its cell wall contains a uniquely high concentration of lipids that confer a characteristic acid-fast staining property and likely contribute to immunomodulation and virulence. [8] Mtb are slow-growing organisms with a generation time of approximately 20 hours. Visible growth on solid media usually takes from 3 to 8 weeks, and this characteristic contributes to the challenge of establishing a diagnosis. Humans are the only known reservoir of M tuberculosis, although other animals can become infected. Genetic variability exists among isolates from around the world and may confer differences in virulence. [9]
Mtb are intracellular pathogens capable of causing subacute and progressive disease, also referred to as active TB. In addition, the bacteria can remain dormant within infected cells where they may or may not cause disease. The molecular and immunologic mechanisms responsible for dormancy and reactivation remain unknown and represent an important area of Mtb research. [2]
- Epidemiology
TB data derived for the year 2022 from the World Health Organization (WHO) includes the following:
- 1.3 million deaths, including 167,000 patients with HIV
- 10.6 million people with active TB
- 5.8 million men
- 3.5 million women
- 1.3 million children
- Approximately 25% of the world’s population is TB-infected
- 5% to 10% will develop active TB disease
- TB is the leading cause of mortality in people with HIV
- The incidence of new TB cases in 2022 was as follows:
- 46% in South-East Asia
- 23% in Africa
- 18% in Western Pacific [10]
Risk factors associated with the development of active TB are as follows:
- Immunocompromise
- Immune senescence of older age
- Genetic diseases causing immunodeficiency
- HIV infection
- Prolonged corticosteroid use
- Cytoreductive chemotherapy
- Transplantation
- Tumor necrosis factor (TNF) antagonists
- Malnutrition
- Diabetes
- Tobacco
- Alcohol abuse
Recent data from the Centers for Disease Control (CDC) provide a perspective on TB in the United States (US):
- 8,300 cases of TB in 2022 (approximately 2.5 cases per 100,000 persons)
- TB cases have been reported in every state
- >80% of reported cases were associated with untreated latent TB or TB reactivation
- 73% of cases were among non–US-born persons
- Highest TB rates occurred in ethnic minorities
- Approximately 5% of persons with TB were HIV co-infected
- Drug-resistant TB is a serious public health concern [11]
- Pathophysiology
TB is spread from person to person by airborne droplet nuclei that can remain suspended in the air for several hours. The fate of the suspended droplet nuclei depends on environmental conditions: the bacilli can be destroyed by exposure to ultraviolet light, the droplet nuclei may innocuously land on inanimate surfaces, or they can be inhaled into a person’s airway, where they may or may not establish infection. The longer one is exposed to an enclosed space where TB droplet nuclei are present, the greater the likelihood of transmission. In modern times, TB is acquired most often via the respiratory route. It can also be acquired via ingestion of contaminated milk, and while this route of infection is of historical importance, it is a rare occurrence today. Also, it is exceedingly rare for TB to be contracted through contact with nonintact skin.
Once inhaled, droplet nuclei can land on upper airway mucosa where infection is unlikely to be established or reach the alveoli where the infectious processes may begin (see Image. Tuberculosis Pathophysiolgy Flow Chart). Depending on complex and poorly understood pathogen virulence factors in concert with host immunomodulatory mechanisms, the bacillus can either be killed, persist in a latent state or progress to active tuberculosis disease. These discreet categorical stages likely represent an oversimplification of a complex and dynamic host-pathogen relationship. [12] [13] Moreover, aspects of the long-held conceptual pathophysiological model, described in some detail below, have been questioned as knowledge about immune mechanisms has evolved. [14]
Alveolar macrophages play a central role in the immunomodulatory process. The bacilli are internalized by the macrophages where they are either killed or establish the primary infection. In the latter case, the bacilli gain access to lung parenchyma and can migrate to pulmonary lymph nodes, where they prime T cells. The primed T cells orchestrate the recruitment of T cells, B cells, monocytes, multinucleated giant cells, dendritic cells, and fibroblasts, forming a granuloma that surrounds infected macrophages within the lung parenchyma. The immunologic mechanisms that govern granuloma formation and the life cycle of the tubercle bacillus within the granuloma are poorly understood and represent areas of intensive research. [15] [16] [17] [13] Occasionally, this primary granuloma, referred to as a Ghon focus, and the associated draining hilar or mediastinal lymph nodes can calcify and reach a size visible on a chest x-ray; this finding is termed the Ranke complex. In children, the intrathoracic lymph nodes can enlarge, obstruct, and erode into bronchi. In immunocompromised adults and immunocompetent children, the Ghon focus may evolve into pneumonia, a form of progressive primary TB disease, primarily in the lower lung zones where cavitation is uncommon. In young children, progressive primary disease can rapidly disseminate within the lung itself and to other organs, most notably the central nervous system, where it can cause life-threatening Mtb meningitis.
In most immunocompetent adults, the bacilli will be contained by the granuloma and establish a latent infection. It may escape immunologic controls and disseminate lymphohematogenously within the lung and to virtually any other organ. Dissemination within the lung favors location in apical posterior segments. The reason for preferential dissemination to apical lung segments is speculative and has been attributed to regional differences in oxygen tension, differences in lymphatic flow, and differences in regional pulmonary immune function. The organs most commonly associated with extrapulmonary dissemination include pleura, lymph nodes, kidneys, long bones, vertebrae, and meninges. [18] Mtb bacilli will grow within the organs to which they have disseminated until cellular immunity or tuberculin reactivity is established, at which time the bacilli become dormant; this is latent TB infection. This occurs 3 to 8 weeks after infection in immunocompetent people. CD4 and CD8 T cells appear to play a central role in latency. [14]
Latent TB is not necessarily synonymous with Mtb dormancy. People labeled as having latent TB may cycle between periods of dormancy and subclinical TB disease. [13] This concept is supported by surveillance studies conducted in regions of high TB endemicity. [19] [20]
If innate and acquired immunity fails to contain Mtb , people will develop active TB disease. Granulomas can undergo caseation necrosis, erode into an airway, and form a cavity within which Mtb bacilli proliferate. [21] The cavity communicates with the airway and is the source of TB transmission. Because of its high concentration of proliferating bacilli and poorly vascularized inner contents, the cavity represents an environment promoting drug resistance development. Areas of cavitation no longer carry out respiratory functions and are a nidus for opportunistic bacteria and fungi. Lung parenchyma adjacent to cavities becomes fibrotic. Pulmonary blood vessels may erode into cavities and cause massive hemoptysis; this clinical finding is termed a Rasmussen aneurysm. Not all granuloma cavitate; they can involute and heal due to poorly understood immunological mechanisms.
Approximately 5% of recently infected people with TB will develop active disease within the first 2 years after infection. An additional 5% will develop active TB at a later time in their lives. Expressed differently, 90% of people infected with TB will not develop active disease. The risk factors associated with the development of active disease are noted above in the epidemiology section.
People with active TB disease can be asymptomatic at 1 end of the clinical spectrum or severely ill at the other end of the spectrum. Specific disease manifestations are a function of the organs involved; the apical posterior segments of the lung are the most commonly involved structures in adults and adolescents. In young children and older individuals, pneumonia involving the lower lobes is common. Constitutional symptoms are nonspecific and often include cough, fever, weight loss, night sweats, and malaise. Asymptomatic TB disease is well described, and prevalence has been reported to be quite high when active case finding is performed among high-risk populations. [22] [12]
Endobronchial TB represents a unique complication resulting from the spread of organisms from a pulmonary cavity, a pneumonic focus, or an adjacent lymph node into the airway. The endobronchial inflammatory process can produce mucosal ulcerations, granulation tissue, edema, and airway narrowing. [23]
Reactivation of a latent focus of infection represents the most common mechanism leading to active disease, and previously infected people are generally immune to exogenous reinfection. However, it is possible to become reinfected when exposed to a large Mtb inocula or if there is significant underlying immunocompromise. [24] This has important therapeutic and epidemiologic implications since it can be a challenge to determine whether a person with a prior history of TB has relapsed due endogenous reinfection, which is failure to eradicate their infection, or due to exogenous reinfection via a newly acquired infection.
Tuberculosis and HIV Coinfection
The HIV epidemic heralded a new era in the long history of TB and deserves a separate discussion of pathogenesis. [25] [26] [13] [27] Data from the WHO indicates that people living with HIV (PLHIV) are approximately 19 times more likely to develop active TB disease than those without HIV. [28] The initiation of antiretroviral therapy (ART) does not completely restore Mtb immunity to baseline. [27] In addition, the return of TB-specific CD4 T cells after initiating ART can lead to TB-immune reconstitution syndrome (IRIS). Globally, TB is the leading cause of death in PLHIV; those in developing countries bear the highest burden. Both increased rates of reactivation and increased susceptibility to Mtb following exposure appear to contribute to the high rate of active TB disease in the HIV population. The risks of developing active TB disease increase as CD4 T lymphocyte counts decline. [13] Within the first year of Mtb primary infection, PLHIV develop significantly higher rates of progressive primary TB disease. Both primary infection and exogenous reinfection appear to contribute to the TB burden in PLHIV. [25]
The underlying alterations in immune function that account for these findings in the HIV-TB co-infected population are not well understood. [26] Several hypotheses exist as follows:
- Selective depletion of Mtb antigen-specific CD4 T cells
- Dysfunction of CD8 T cells
- Increased production of TNF
- HIV alterations in macrophage function
Observational studies suggest that TB infection accelerates the progression from HIV infection to AIDS. [26] [25] Once again, the mechanisms involved are unknown, and several hypotheses exist, including:
- Active TB disease is associated with an accelerated loss of CD4 T cells
- The immune response to TB increases HIV replication in blood and tissues
- Mtb infection induces the production of proinflammatory cytokines that upregulate HIV replication
TB clinical manifestations in PLHIV with high CD4 T lymphocyte counts are similar to those not infected with HIV. Reactivation TB is often associated with upper lobe infiltrates and cavitation. Data suggest a correlation between CD4 T lymphocyte counts and cavitation due to TB; the higher the CD4 T lymphocyte count, the more likely there will be cavitation. [25] Atypical chest x-ray findings are common in people co-infected with TB and HIV when CD4 T lymphocyte counts fall below 200 cells/μL. These findings include:
- Normal chest x-rays
- Interstitial nodules
- Lower and middle lobe infiltrates
- Intrathoracic lymphadenopathy
- Pleural effusions
PLHIV are at greater risk of developing disseminated TB. Moreover, based on postmortem studies, disseminated TB in the HIV population is often undiagnosed. [13] [29] To reduce complications and transmission, it is of paramount importance to promptly identify and treat HIV-TB co-infected individuals. [29]
- Histopathology
Nonspecific appearing necrotizing and non-necrotizing granuloma may be identified in Mtb- infected tissue sections processed by hematoxylin and eosin (H&E) staining. The granuloma consists of an outer rim of lymphocytes and plasma cells surrounding a peripheral rim of epithelioid histiocytes and multinucleated giant cells. A central region of necrosis, if present, can have a caseous consistency on gross inspection. If abundantly present, acid-fast bacilli may be identified using the Ziehl-Neelsen stain. Greater sensitivity in identifying the organisms may be achieved by fluorescent microscopy using the auramine-rhodamine stain.
- History and Physical
Regarding TB, the first rule of taking a history is to think about it. This rule is likely given great consideration in countries with a high prevalence of TB. In those countries where TB is uncommon, it may not be afforded initial consideration by clinicians during the evaluation of patients presenting with nonspecific symptoms such as cough, fever, malaise, or weight loss. The diagnosis is even more challenging when patients present with extrapulmonary TB. Thus, routine exploration of risk factors such as a prior TB history, known TB contacts, country of origin, foreign travel, family history, occupational and residential exposures, immunosuppression, and immunocompromise, are key components of the history. These aspects of history taking should be performed for all initial patient contacts, given the high worldwide prevalence of TB.
Patients with latent TB are asymptomatic, and those with early active TB disease are often asymptomatic and will have no specific physical findings. In the latter instance, as the disease progresses, patients may experience the insidious onset of cough, fever, night sweats, weight loss, and hemoptysis before seeking medical evaluation. Depending on the extent of the disease, the physical findings on lung examination may be normal or demonstrate areas of consolidation, airway inflammation, or the presence of cavities. Those with chronic, extensive, destructive cavities with surrounding fibrosis may develop chest wall deformities due to loss of underlying lung volume. [30]
Since TB can involve any organ, details of the myriad of physical findings are beyond the scope of this introductory activity (see Image. Clinical Features of Extrapulmonary Tuberculosis).
The approach to TB diagnosis depends on whether one is evaluating a patient for latent, active pulmonary, or extrapulmonary TB disease. A combination of immunologic responses to provocative tests, radiology, microbiology, molecular methods, and biomarkers are used to establish a diagnosis. Most importantly, the approach to diagnosis will depend on TB prevalence within a population and the resources available to the public health care system within a specific geographic region. [31] [5] TB is often a diagnostic challenge. Signs and symptoms, if present, are nonspecific. The sensitivity and specificity of existing diagnostic tests can vary significantly depending on several factors that include pre-test probability, immunological status and age of the patient, timing of the test, adherence to proper test procedures, adequacy of specimen collection, and the ability to interpret test results in the context of the patient's risk factors and immune function. Radiological images can range from normal to nonspecific markedly abnormal findings. In the absence of a confirmatory culture or molecular assay, the clinical diagnosis of TB is presumptive and based on the strengths of clinical suspicion in concert with surrogate markers of infection. This can lead to both under- and over-diagnosis depending on the epidemiological setting. In-depth details of TB diagnostic testing are beyond the scope of this article, and the reader is referred to excellent reviews on the topic. [32] [33] [34] [31] [15] [35]
Latent Tuberculosis
Populations that should undergo screening for latent TB infection include the following:
- Contacts of individuals with active TB disease
- Individuals initiating anti-TNF therapy
- Dialysis patients with end-stage renal disease
- Patients anticipating organ or bone marrow transplants
- Patients with silicosis
In low- and middle-income countries (LMIC), the WHO endorses the use of tuberculin skin tests (TSTs) or interferon-gamma release assays (IGRAs). [5] The traditional TSTs, such as the Mantoux or purified protein derivative (PPD), can suffer from low specificity due to the combination of cross-reactivity with the Bacillus Calmette-Guerin (BCG) vaccine and, to a lesser extent, exposure to nontuberculous mycobacteria (NTMB). This is particularly problematic in parts of the world where high TB prevalence has led to the widespread use of BCG vaccination in children. The IGRA possesses greater specificity since it does not cross-react with BCG or most NTMB strains. Recently, TB skin tests have been developed using specific Mtb antigens. [36] The WHO has concluded that these Mtb antigen-based skin tests (TBSTs) have similar diagnostic accuracy to that of IGRAs. [5] Thus, the choice of TST or IGRA for detecting latent TB comes down to resources and ease of use at the point of care.
The IGRA is a blood test that requires the availability of a laboratory and technical personnel; it may be cost-prohibitive in LMIC regions. It does have the advantage of not requiring a return visit for result interpretation. The traditional TST is simple to perform at the point of care and requires fewer resources, but it suffers from less specificity and requires a return visit for interpretation. The new TBSTs are performed similarly but have the advantage of specificity equivalent to that of IGRAs. In the US, if resources allow, IGRAs are the preferred testing modality for the detection of latent TB. [33] TSTs are an acceptable alternative if IGRAs are unavailable or deemed too costly.
TB skin tests have limitations. They can produce false-negative results, particularly in individuals with compromised immunity, including those at the extremes of age, PLHIV, and people receiving immunosuppressive drugs, or those tested within several weeks of TB infection. False-negative results can also occur due to errors made during intradermal injection and in the interpretation of the skin test reaction. False-positive results can be due to prior BCG vaccination, exposure to NTMB, and misinterpretation of the skin test reaction.
While IGRAs are more sensitive than TSTs in individuals co-infected with HIV, false-negative results can occur in those severely immunocompromised. In addition, technical imprecision in processing the blood sample can result in erroneous results. Indeterminate IGRA results may occur more frequently in children younger than 5 years and in PLHIV with CD4 T lymphocyte counts less than 200 cells/μL. [33] Discordant results between skin tests and IGRAs can occur; refer to "BCG Vaccine" in the Treatment/Management section for more information about discordant results. In this case, repeat testing may be considered, and the decision to initiate anti-TB therapy will ultimately depend on the strength of clinical suspicion.
Neither TSTs nor IGRAs can distinguish latent from active TB, nor can they predict who will evolve from latent infection to active disease. When a decision is made to initiate therapy for latent TB, efforts must be made to exclude the presence of active TB disease. Failure to do so will promote the emergence of resistant organisms and result in inadequate treatment outcomes. To further complicate the matter, individuals who have successfully eradicated their TB infection can continue to test positive by TSTs and IGRAs. Conversely, people with a remote history of untreated latent TB can have a false negative test. [37] There are excellent reviews that explore detailed recommendations on latent TB testing strategies in different at-risk populations. [33] [5] In addition, a user-friendly, web-based, interactive program called the Online TST/IGRA Interpreter can provide an individual estimate of the risk of TB infection based on TST and IGRA results. [38] The algorithm incorporates individual parameters and calculates a positive predictive value.
Active Tuberculosis Disease
A diagnosis of active TB is confirmed by culture. However, confirmation is often elusive due to practical difficulties in obtaining adequate sputum, fluid, and tissue specimens, the frequent paucity of organisms present, and their slow growth characteristics. The diagnosis is often presumptive and based on pretest parameters in association with supporting evidence that includes radiological images, molecular assay, and biomarkers.
A helpful way to introduce this topic is to consider imaging techniques in the context of the stage of TB. However, these are dynamic pathological processes with overlapping clinical and radiologic features. Moreover, any single or combination of image findings is possible. Conventional chest x-ray remains the initial modality employed for screening and diagnosis. Computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography–computed tomography (PET-CT) are employed when necessary to better define anatomic involvement with active TB disease. [39] Imaging features of TB are nonspecific and can mimic other diseases. This activity will focus on imaging of the lungs. A detailed presentation of imaging modalities for the evaluation of extrapulmonary TB can be found in excellent reviews. [39] [35]
- Parenchymal infiltrate or atelectasis
- Usually, in middle and lower lungs
- Identified in <10% of cases
- Intrathoracic lymphadenopathy
- Often seen in children and PLHIV
- Ghon focus and Ranke complex
- Often heals with residual calcification
- Pleural effusion (usually unilateral)
- Generally uncommon; seen more commonly in PLHIV
- Miliary pattern (progressive primary disease)
- Uncommon; seen predominantly in infants, older individuals, and severely immunocompromised patients
- Normal chest x-ray
- CT may identify subtle changes not seen on chest x-ray
- Post-primary TB, including reactivation and reinfection
- Patchy consolidation of the apical and posterior upper lobe and superior segments of lower lobe
- Cavitation is seen in 20% to 45% of adult cases but is rare in young children and those who are severely immunocompromised
- Adenopathy is uncommon in adults but common in young children and PLHIV
- Tree-in-bud nodular distribution if endobronchial spread; seen best with CT
- Pleural effusions are uncommon
- Miliary pattern is generally uncommon and seen predominantly in infants, older individuals, and severely immunocompromised patients
- CT may identify subtle changes not seen on chest x-ray
- CD4 T lymphocyte count less than 200 cells/μL
- Intrathoracic lymphadenopathy is common
- Cavitation is uncommon
- Extrapulmonary disease is common
- Bronchiectasis
- Bronchopleural fistula is an uncommon finding
In the majority of primary TB cases, there will be resolution of parenchymal lesions, and occasionally, a Ranke complex will be the only residual clue to a diagnosis of remote TB. Normal chest x-rays can occur in people with active TB disease. When pathologic lung images due to TB are identified with chest x-rays, it is always a challenge to distinguish active from inactive disease. While there are clues such as cavity wall thickness, presence or absence of consolidation, fibrosis, pleural effusion, and calcifications, no definitive findings can exclude active disease. In general, CT images provide significantly improved sensitivity. A small number of reports suggest that fludeoxyglucose F 18 (FDG) PET-CT may be useful in assessing response to therapy. [39]
Microscopy, Culture, and Molecular analysis
Clinical suspicion of active TB requires further microbiological investigation. [40] Analysis of sputum and, if indicated, fluid and tissue samples using staining and culture techniques have, until recently, been the only available confirmatory diagnostic tests. Automated real-time NAATs and biomarker probes have become an important addition to the TB diagnostic landscape. [41] [42] [43]
Acid-Fast Bacilli Smear Microscopy
Sputum smears and culture are the conventional and most commonly applied approach to diagnosis. Not everyone can produce a deep expectorated sputum sample, and the frequent paucibacillary nature of TB limits the sensitivity of this application. It is important to note that sensitivity values are a function of pretest probability; higher sensitivity will be achieved in regions with high TB prevalence. Notably, the application and interpretation of staining methods have not been standardized and are likely subject to significant variations in practice patterns. [44] Improved sensitivity can presumably be achieved by collecting expectorated sputum samples on 3 separate days, although the value of this practice has been debated. [45] Moreover, in resource-poor parts of the world, this practice can be quite burdensome. Staining sensitivity can be increased using fluorochrome stains, specimen concentration techniques, and at least 5 ml of sputum volume. Improved sensitivity may also be achieved when respiratory specimens are obtained by sputum induction and interventional methods such as bronchoscopy and possibly gastric lavage. [40] The sensitivity of sputum acid-fast bacilli (AFB) smears ranges from 34% to 80%. [34]
NAATs should be performed on all AFB smear-positive specimens and smear-negative specimens when the clinical suspicion is intermediate or high. Refer to the "Molecular Detection Methods" for more information. In the latter case, a negative NAAT does not rule out a diagnosis of TB. [33]
Culture sensitivity ranges from 80% to 93%, and specificity is approximately 93%. [34] Broth media can detect growth within 2 weeks, whereas solid media can take up to 8 weeks for visible growth. When Mtb grows in broth media, it can then be subjected to rapid drug susceptibility tests (DSTs) and identification by DNA probe methods. Conventional phenotypic DST methods are time-consuming and resource-intensive.
Molecular Detection Methods
The past 2 decades have experienced a remarkable evolution in the approach to TB diagnosis and drug-resistance detection. Molecular methods using NAATs and lateral flow biomarker detection provide rapid results that impact immediate public health and direct patient care. Several of these tests are capable of simultaneous Mtb detection and drug resistance identification. [28]
The application of these tests continues to evolve; when, where, and how they are best utilized require consideration of several variables that include prevalence of TB and drug resistance, population screening, individual diagnosis of active pulmonary and extrapulmonary cases, applicability to adults, children, and PLHIV, stand-alone testing, follow-on testing, costs, ease of use, and available resources. Moreover, their application must account for pretest probabilities, sensitivity, specificity, and interpretation when there is discordance with conventional methods. In the latter case, repeat testing may or may not resolve the discrepancy, and decisions regarding the presence or absence of active TB must be made on the strength of clinical suspicion. The assays play an important role in complementing conventional smear and culture methods and, in certain circumstances, can stand alone as diagnostic tools. In 2021, the WHO published a detailed, consolidated, evidence-based report that describes the various technologies and provides recommendations for how best to apply them in practice (see Image. Classes of Technologies and Associated Products). [28] The following is a summary of the assays and WHO recommendations; the reader is referred to the WHO report for a detailed discussion. [28]
The Xpert ® MTB/RIF (Xpert ® ) and Xpert ® MTB/RIF Ultra (Xpert Ultra ® ) (Cepheid, Sunnyvale, CA, USA) are automated, cartridge-based NAATs that have gained widespread use. They detect Mtb and rifampin (RIF) resistance mutations directly from specimens within 2 hours; RIF resistance mutations are a surrogate marker for multidrug resistance. The Xpert ® sensitivity for Mtb detection is approximately 70% when a single smear-negative/culture-positive sputum specimen is tested and increases to approximately 90% when 3 consecutive sputum specimens are tested; specificity is approximately 98%. [28] The sensitivity for detecting RIF resistance is approximately 96%, and the specificity for excluding resistance is approximately 98%. [28] The corresponding sensitivity ofXpert Ultra ® increases to approximately 77%, but specificity decreases to approximately 96%. [28] The decrease in specificity is likely due to an increased rate of false positives resulting from the detection of nonviable Mtb that can occur in people treated for TB within 2 to 5 years of Xpert Ultra ® testing. [46] The sensitivity for detecting RIF resistance is approximately 94%, and the specificity for excluding resistance is approximately 99%. [28] The sensitivity of Xpert ® and Xpert Ultra ® assays in detecting pulmonary TB in children is less than that in adults, and this is likely due to the difficulty of obtaining adequate sputum samples in this population.
Both Xpert ® and Xpert Ultra ® assays are valuable in detecting Mtb from samples obtained at extrapulmonary sites, most notably cerebrospinal fluid (CSF) and lymph node aspirates. Details of sensitivity and specificity on various extrapulmonary specimens can be found in the WHO 2021 update document. [28]
The WHO recommends using Xpert ® and Xpert Ultra ® assays as initial tests in adults and children, including PLHIV, with signs and symptoms of pulmonary TB. [28] This includes analysis of gastric aspirate, nasopharyngeal aspirate, and stool specimens in children. The WHO also recommends their use as an initial diagnostic test in adults and children suspected of having extrapulmonary TB. [28] For PLHIV suspected of having disseminated TB, the WHO recommends using Xpert ® and Xpert Ultra ® assays on blood.
The TrueNat ® MTB Plus assay (Molbio Diagnostics, India) is a rapid, automated NAAT endorsed by the WHO in 2020. [47] The NAAT accuracy appears similar to that of Xpert ® and Xpert Ultra ® assays. [28] [47] The TrueNat ® MTB Plus assay is portable and battery-powered, making it advantageous as a point-of-use technology. However, it is a relatively new platform that has not been as extensively evaluated as Xpert ® .
Globally, approximately 13% of new cases and 17% of previously treated cases of TB are isoniazid (INH)-resistant and RIF-susceptible. [28] Several moderate-complexity NAATs are capable of detecting Mtb as well as both INH and RIF resistance. The WHO has endorsed the tests as initial pulmonary TB detection methods, given their speed and relative ease of use compared to conventional culture-based drug susceptibility tests. [28]
TB loop-mediated isothermal amplification (TB-LAMP) is rapid, relatively simple to perform, and can detect Mtb without the need for sophisticated equipment. [48] The WHO endorses using the TB-LAMP assay (Eiken Chemical Company, Tokyo, Japan) as a replacement for sputum-smear microscopy and as a follow-on test for sputum-smear–negative specimens in adults for whom TB is suspected. [28] The assay does not appear to provide added accuracy to sputum-smear microscopy in PLHIV.
Lipoarabinomannan (LAM), a cell wall component of Mtb, is excreted in the urine of people with active TB. [49] The ability to detect LAM in urine samples using a simple lateral flow assay is obviously appealing. In practice, the LAM assay sensitivity is relatively low in the general population. However, sensitivity improves in individuals with HIV infection as immunosuppression increases. The WHO endorses the use of the Determine™ TB LAM lateral flow test (Alere Determine™ TB LAM Ag Alere, MA, USA) for adults and children infected with HIV with CD4 T lymphocyte counts of less than 200 cells/μL and with signs and symptoms of pulmonary or extrapulmonary TB ( see Image. Classes of Technologies and Associated Products). [28] In addition, the WHO endorses its use in PLHIV with CD4 counts of <100 cells/μL irrespective of signs and symptoms of TB. A detailed tabulation of pooled sensitivity and specificity data based on CD4 count is available. [28]
The group of low-complexity NAATs for detecting resistance to isoniazid (INH) and second-line anti-TB agents are used as follow-on assays in settings where multidrug-resistant TB (MDR-TB) is encountered. Compared to conventional culture-based phenotypic DSTs, the NAATs are automated and rapid. They are not currently capable of determining resistance to some of the new and repurposed agents, such as bedaquiline and linezolid. [28] The assays appear to be applicable to sputum and extrapulmonary specimens.
Line probe assays (LPAs) are rapid molecular diagnostic tests that can detect Mtb and resistance to several first- and second-line anti-TB agents ( see Image. Classes of Technologies and Associated Products). [28] [50] LPAs require instrumentation and technical expertise that lend themselves to regional and reference laboratory settings. As reflex tests when RIF resistance is detected, the LPAs are used to identify the presence of resistance genotypes to other anti-TB agents. Compared with the conventional phenotypic culture-based DST, which can take several weeks to perform, the LPAs can obtain a result within several hours. LPAs can be performed on culture isolates as well as sputum specimens. When MDR-TB is potentially identified, the LPAs should be considered initial DST tests and are not meant to replace conventional phenotypic culture-based DST. The strength of the WHO recommendations on using LPAs and all of the molecular detection methods will evolve as more evidence of their utility in many settings is generated.
Tuberculous pleural effusions (TPE) are challenging to diagnose. The detection of adenosine deaminase (ADA) in pleural fluid supports the diagnosis with a pooled sensitivity and specificity of approximately 92% and 90%, respectively. [51] ADA detection is simple and inexpensive to perform and provides a rapid result. Detection of ADA in CSF has been applied to the diagnosis of Mtb meningitis with a pooled sensitivity and specificity of approximately 85% and 90%, respectively. [52] A small number of studies have suggested the value of obtaining ADA levels of peritoneal and pericardial fluid when TB is suspected at these sites. [53]
- Treatment / Management
Latent Infection
TSTs and IGRAs are unable to predict the progression from latent infection to active disease. Several observational studies have demonstrated that the risk of developing active TB is highest during the first 2 years after acquiring infection and that reactivation is rare beyond 10 years after infection. [37] It has been estimated that the number needed to treat (NNT) to prevent 1 case of active TB ranges from 36 in recently infected contacts to 179 in those remotely infected. [37] Thus, while evidence clearly demonstrates that treatment of latent TB reduces the likelihood of developing TB disease in populations at high risk, the evidence is less clear that treatment of those at low or intermediate risk reduces the incidence. [33] The rationale to treat those with latent TB is best defined by the WHO; it is “premised upon the probability that the condition will progress to active TB disease in specific risk groups, on the underlying epidemiology and burden of TB, the feasibility of the intervention, and the likelihood of a broader public health impact.” [54]
The preferred 3-month regimen of once-weekly INH plus rifapentine (3HP) is attractive due to its short duration and decreased incidence of liver toxicity relative to the 6- and 9-month INH regimens (see Image. Recommendations for Regimens to Treat Latent TB infection). Reports of a flu-like syndrome occurring in recipients of the 3HP regimen have raised concerns. [55] However, a large, multinational trial identified that 11% of participants experienced symptoms of a systemic drug reaction (SDR), most frequently within the first month of therapy. Of those experiencing SDR, 48% were able to complete treatment, and serious adverse events were rare. [55]
The WHO guidelines on TB preventive therapy align with those of the CDC. [54] In addition, the WHO guidelines include an alternative regimen consisting of 1 month of daily 3HP plus INH. [54] Moreover, the WHO recommends that “in settings with high TB transmission, adults and adolescents living with HIV who have an unknown or a positive TST or IGRA and are unlikely to have active TB disease should receive at least 36 months of daily INH preventive therapy.” [54] The latter recommendation is made regardless of immune status and whether or not ART is being administered.
Currently, there are no studies providing robust data to help guide TB preventive therapy for individuals having close contact with people infected with MDR-TB. [56] The WHO guidelines suggest a targeted approach based on individual risk assessment. [54] Trials to ascertain the efficacy and safety of fluoroquinolones and delamanid are currently in progress. However, trials will require long follow-ups to assess the absence of disease as the clinical endpoint. It will be challenging to devise TB preventive therapy protocols that can be applied to various drug resistance patterns and assess safety and efficacy in different populations (eg, children, adults, PLHIV, the immunosuppressed, and those with existing comorbidities). [57] [56] [58] See StatPearls' companion topic, "Latent Tuberculosis," for a complete discussion of the treatment of this disease process.
Active Tuberculosis Infection
The goal of anti-TB therapy is to eradicate disease and eliminate transmission in all cases. While the goal seems unambiguous, it has proven exceedingly difficult to achieve in practice. Historically, anti-TB regimens have required many months of treatment and patient adherence has been a challenge. While these challenges exist everywhere, they are particularly problematic in parts of the world with the highest TB prevalence and where public health infrastructure and literacy about TB are lacking. From a global public health perspective, TB treatment guidelines are designed to be straightforward and somewhat standardized. [59] This “one-size-fits-all” approach has been questioned since a standard 6-month regimen may be too long for some and not long enough for others. [60] [59] It is conceivable that in the future, the application of a pretreatment risk stratification algorithm could inform optimized treatment duration schedules on an individualized basis. [60]
Detailed recommendations on dosing, first- and second-line anti-TB drugs, drug-drug interactions, management of treatment interruption, adverse effects of treatment, culture-negative TB, extrapulmonary TB, HIV coinfection, children, advanced age, pregnancy, breastfeeding, and comorbidities are beyond the scope of this activity and may be found in the referenced guidelines (see Image. Drug Regimens for Microbiologically Confirmed Pulmonary TUberculosis Caused by Drug-Susceptible Organisms). [61] In addition, the management of TB is understood to be very complex, and for that reason, the CDC has established an exceptionally useful online resource, TB Centers of Excellence for Training, Education, and Medical Consultation. ( http://www.cdc.gov/tb/education/rtmc/default.htm )
The treatment for drug-susceptible pulmonary TB disease had not, until 2022, changed in 50 years. The long-held standard regimen involves the use of 4 drugs: isoniazid (INH), rifampin (RIF), ethambutol (ETH), and pyrazinamide (PZA). Before the development of that therapeutic combination, the first anti-TB drug, streptomycin, was used as monotherapy in the 1940s. Streptomycin initially provided significant benefit when administered for 6 months but ultimately failed due to the emergence of resistance. [62] The resistance to monotherapy, along with a better understanding of both the pathophysiology of Mtb infection and drug toxicities, informed decisions that led to the current standard of treatment. [61]
There are 2 phases of treatment – an initial intensive phase that provides bactericidal activity directed at rapidly replicating organisms and the continuation phase that is meant to sterilize slowly replicating and dormant tubercle bacilli. [62] The intensive phase of therapy requires the use of INH, RIF, ETH, and PZA, and the continuation phase uses INH and RIF. In treating pulmonary TB, the intensive phase usually extends for 2 months, during which it is hoped that there will be significant reductions in mortality, lung inflammation, rapidly replicating mycobacteria, and transmission. Should drug susceptibility testing indicate that the isolate is sensitive to both INH and RIF, the EMB can be discontinued. In that case, the intensive phase would consist of INH, RIF, and PZA. [61] The continuation phase extends for an additional 4 months. The rationale for the 4-drug combination is that bactericidal killing of rapidly replicating organisms will reduce the chances of emerging resistance. [62]
Patients receiving anti-TB therapy require monitoring to assess the efficacy and safety of the regimens. Sputum smears and cultures should be evaluated monthly until 2 consecutive cultures are negative. In patients with chest x-ray evidence of cavitation and who remain culture positive at 2 months, the recommendation is to extend the continuation phase for an additional 3 months (ie, a total of 9 months of therapy). [62] Extending the continuation phase should also be considered for the following individuals: PLHIV, the malnourished, active smokers, the immunosuppressed, and those with extensive pulmonary disease.
Centers for Disease Control 2022 Interim Guidance
In 2022, the CDC announced interim guidance on a 4-month anti-TB regimen for drug-susceptible pulmonary tuberculosis. [63] The regimen consists of an intensive phase of 8 weeks of daily rifapentine (RPT), INH, PZA, and moxifloxacin (MOX), followed by a continuation phase of 9 weeks of daily RPT, INH, and MOX. [64] This treatment option is available for patients older than 12 years, including PLHIV with CD4 T lymphocyte counts greater than 100 cells/μ L and anticipating an ART regimen that includes efavirenz in the absence of any other known potential drug-drug interaction. Due to a lack of clinical trial data, the regimen is not intended for pregnant or breastfeeding patients, treatment of extrapulmonary TB, those with a history of prolonged QT syndrome or use of QT-prolonging medications, and those with body weight less than 40 kg.
Drug-Resistant Tuberculosis
The vast majority of patients with drug-susceptible pulmonary TB who are able to complete the standard anti-TB regimens will be cured. Unfortunately, drug-resistant TB poses a much greater risk of treatment failure and requires alternative and often more prolonged regimens. [65] There are several categories of drug resistance, including:
- Rifampicin-resistant TB (RR-TB)
- May be resistant to isoniazid
- May be resistant to other TB drugs
- Rifampicin-susceptible, isoniazid-resistant TB
- Multidrug-resistant TB (MDR-TB)
- Resistant to both RIF and INH
- Extensively drug-resistant TB (XDR-TB)
- Resistant to RIF, INH, a fluoroquinolone, and at least 1 of the second-line injectable drugs, such as capreomycin, kanamycin, and amikacin
Drug-resistant TB poses a threat to global public health control efforts. In 2018, the global estimate of MDR-TB was approximately half a million new cases, of which only 30% were started on second-line therapy. The complexity of treatment and management led to the establishment of programmatic strategies to intensify treatment programs. [66] Extended treatment regimens can range from 18 to 21 months and incorporate a variety of first-line, second-line, new, and repurposed agents during both the intensive and continuation phases of therapy. When prolonged regimens and injectable second-line agents are required, the risks of drug toxicities and poor patient compliance are very high. Adherence interventions are being employed and include psychological counseling and patient education, financial and material incentives, and mobile phone text reminders. [66]
New and repurposed agents including but not limited to delamanid, bedaquiline, pretomanid, linezolid, amoxicillin-clavulanate, meropenem-clavulanate, imipenem-cilastatin, cotrimoxazole, and macrolides have recently been employed and may permit shorter durations of treatment in some circumstances. [65] [67] [68] In addition, adjuvant surgical excision may be necessary in efforts to eradicate cavities and nonviable lung tissue. [65] [69]
Details on the management of drug-resistant TB are beyond the scope of this activity and the reader is directed to both WHO and American Thoracic Society/European Respiratory Society/Infectious Diseases Society of America guidelines. [65] [70] Moreover, it is advisable to seek assistance from government health department experts when caring for an individual with suspected or confirmed drug-resistant TB. Experts can be found at ( http://www.cdc.gov/tb/education/rtmc/default.htm ) and ( http://mdrtb.brit-thoracic.org.uk/ ).
Bacille Calmette-Guerin Vaccine
BCG, a live attenuated strain of M bovis, has been recommended by the WHO as a vaccine for infants and children since 1974. [71] It is intended for use in countries with high TB incidence to prevent miliary TB and Mtb meningitis to which infants are most susceptible. It is the most widely used vaccine worldwide. [72] While the vaccine appears somewhat protective when administered to infants and children, its protection wanes over several years. It does not provide significant protection when administered to adults. [73] As previously discussed, BCG vaccine can cause false-positive TSTs.
A commonly encountered clinical question is whether to initiate TB preventive drugs in patients who anticipate anti-TNF therapy or transplantation, have a possible history of having received the BCG vaccine, and are IGRA-negative and TST-positive. This complex scenario becomes particularly troublesome when one considers that the patient comes from a high TB prevalence region, denies a history of known TB infection, lacks radiological signs of latent or active TB, and could be at risk of developing reactivation TB once anti-TNF therapy or transplant commences. Discordant results, such as TST-positive/IGRA-negative, are common in people who received the BCG vaccine. There are no firm cutoff values for the size of TST induration in this particular setting. Moreover, initiating TB preventive therapy is not without risks of adverse drug reactions.
Unfortunately, there is no way to address this clinical situation with absolute certainty. [74] The consensus guidelines approach this scenario somewhat indirectly and state “ Performing a second diagnostic test when the initial test is negative is one strategy to increase sensitivity. While this strategy to increase sensitivity may reduce the specificity of diagnostic testing, this may be an acceptable tradeoff in situations in which it is determined that the consequences of missing latent TB infection (i.e., not treating individuals who may benefit from therapy) exceed the consequences of inappropriate therapy (i.e. hepatotoxicity).” [74] In this complicated situation, the consensus among most experts is to begin a regimen of TB preventive therapy several weeks before the start of the immunosuppressing intervention. [74]
- Differential Diagnosis
As a result of its myriad manifestations over prolonged periods of time, the differential diagnosis of TB is limitless. [31] A list of the more common diseases that TB can easily be confused with is:
- Any infections causing constitutional symptoms
- Fevers of unknown origin
- Pulmonary infections
- Nontuberculous mycobacteria
- Hematologic
- Metastatic tumors
- Renal
- Peritoneal
- Gastrointestinal
- Autoimmune diseases
- Sarcoidosis
- Drug reactions.
- Toxicity and Adverse Effect Management
The anti-TB drugs are associated with potential toxicities ranging from mild to life-threatening. [62] [75] Patients must be educated about early signs and symptoms of drug toxicity, instructed about when to discontinue their use, and seek immediate evaluation should they occur. There are detailed guidelines for assessing treatment responses, patient monitoring, adverse drug reactions, and management. [76] [77] The following is a list of commonly occurring adverse events and their associated anti-TB drugs:
- Hepatitis (malaise, fatigue, fever, anorexia, nausea, dark urine)
- INH; Bedaquiline; RIF; PZA
- Peripheral neuropathy
- INH; Linezolid
- Ocular toxicity
- PZA; ETH; Fluoroquinolones; Amikacin; Beta-lactams; INH; Streptomycin; Para-aminosalicylic acid
- Cranial nerve VIII dysfunction and renal dysfunction
- Amikacin; Streptomycin; Capreomycin; Kanamycin
- Gastrointestinal reactions
- All of the anti-TB drugs are capable of causing gastrointestinal upset
- Myalgias-arthralgias
- Bedaquiline; PZA
- Anxiety, confusion, psychosis
- Cycloserine; Fluoroquinolones
- Hypoglycemia
- Fluoroquinolones
People with TB and HIV coinfection receiving ART present additional management challenges. Both drug-drug interactions, as well as co-administration of anti-TB and antiretroviral agents, can pose significant risks. [78] [79] [80] Rifamycins cause decreased plasma concentrations of protease inhibitors (PIs) and nonnucleoside reverse-transcriptase inhibitors (NNRTIs). Co-administration of anti-TB drugs and ART can cause significant adverse reactions. In those coinfected individuals, the restoration of immunity associated with ART may result in clinical deterioration due to IRIS. A detailed discussion of the management of these pharmacological challenges is beyond the scope of this activity and is presented in excellent references. [78] [25]
More than 80% of TB-associated mortality occurs in LMICs. TB is the leading cause of death in PLHIV. In 2022, the WHO estimated that there were 1.13 million deaths among HIV-negative people and 167,000 deaths among PLHIV. [10] The current estimate of the prognosis of untreated TB is difficult to calculate; it would have to account for regional differences in healthcare resources, those who have either failed or never initiated treatment, those with drug-resistant strains, and people with different underlying comorbidities. [81] Estimates based on prechemotherapy-era data may be unreliable due to heterogeneity in case definition, patient selection, and reporting. The study by Tiemersma et al estimated a 70% lifetime case fatality among untreated HIV-negative individuals. [81]
The following global estimates of successful treatment outcomes derived by WHO in 2018 are:
- 85% success for people with new and relapsed TB
- 76% success for HIV-coinfected people
- 57% success for people with MDR-TB [82]
The WHO estimates that 15% of patients with MDR-TB die of disease, and 26% of those deaths are due to XDR-TB. [65] For those individuals with drug-susceptible TB who adhere to a full therapeutic regimen, the cure rate can exceed 95%. Variables such as extent of disease, presence of comorbidities, age, and adverse drug reactions influence the therapy outcome. Novel regimens will very likely improve outcomes in people treated for drug-resistant TB. [83]
- Complications
Clinical complications of TB and those resulting from adverse drug events have been presented in previous sections of this activity. TB is theoretically a curable and preventable disease for which the WHO has ambitiously established a goal of 90% reduction in incidence between 2015 and 2035. [84] It is this author’s prerogative to use this section to present complications that impede the attainment of that goal.
TB is predominantly a disease associated with poverty, overcrowding, lack of awareness, limitations in public health resources, lack of political commitment, and lack of clinical expertise. None of these complicating factors are easy to remedy. Adherence to multiple-pill, prolonged, and occasionally unpleasant drug regimens often leads to truncated treatment, resulting in failure to eradicate infection and the emergence of drug resistance. Most active TB cases result from the progression of latent infection rather than community transmission. Active community surveillance, interpreting the results of existing testing modalities, and treating latent TB are all very complicated. Outreach and follow-up of patients with latent and active TB are equally very complex. In resource-rich countries, many new cases of TB occur in recent immigrants and marginalized groups; lack of access to expert medical care remains a significant complication in those segments of society. [85] Finding and allocating funds to achieve the WHO global strategy to eliminate TB is perhaps the major complication. TB, a disease of antiquity, continues to be responsible for the death of millions of people each year. Hopefully, the future of TB will be met with robust vaccine technology, sensitive and specific point-of-use diagnostics, safe and highly active anti-TB drugs, and well-funded public health programs across the globe.
- Deterrence and Patient Education
Deterrence to TB eradication includes:
- Social conditions that favor communicability, particularly poverty and overcrowding
- Lack of political commitment
- Nonspecific signs and symptoms of disease
- Asymptomatic disease
- Failure to consider TB in a differential diagnosis
- Diagnostic tests that may lack sensitivity and specificity
- TB illiteracy
- Pill burden, prolonged duration of treatment, adverse drug effects
- Poor adherence to treatment regimens
- Lack of public health resources
- Absence of robust immunizations that prevent TB infection
- Enhancing Healthcare Team Outcomes
TB is a preventable and curable disease that impacts all segments of humankind. Its diagnosis, treatment, and prevention require coordination between front-line public health officials, adult and pediatric primary care physicians, advanced care practitioners, clinical laboratory technologists, pulmonologists, infectious diseases physicians, pharmacists, nurses, and other healthcare professionals. Skillful management of TB demands expertise in history-taking, physical examination, and interpreting diagnostic tests, coupled with proficiency in implementing evidence-based treatment regimens tailored to individual patient needs. The strategy revolves around developing comprehensive care plans integrating TB diagnosis, treatment, and prevention alongside targeted public health interventions to mitigate transmission risks. Ethically, upholding patient autonomy, confidentiality, and principles of beneficence and non-maleficence underpin TB care, ensuring patients are actively involved in decision-making while safeguarding their well-being. Responsibilities are shared among team members, from timely diagnosis to coordinated care transitions and patient education. Effective interprofessional communication fosters collaboration, optimizing care outcomes by exchanging information and promoting shared decision-making. Care coordination, both within healthcare settings and with community resources, ensures seamless continuity of care and addresses patients' psychosocial needs, ultimately enhancing patient-centered care, outcomes, safety, and team performance in managing tuberculosis.
The complexities of TB care and prevention require the expertise of government officials with specific training to help guide front-line health professionals caring for at-risk and affected patients. Efforts are needed to educate clinicians practicing in low TB endemic areas who are unfamiliar with strategies to diagnose and treat patients with TB. Clinics specializing in TB should be made available to marginalized segments of the population who are at the highest risk of having TB.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Clinical Features of Extrapulmonary Tuberculosis Contributed by Dr. Ellis Tobin
Classes of Technologies and Associated Products Adapted from WHO consolidated guidelines on tuberculosis. Module 3: Diagnosis Rapid diagnosis for tuberculosis detection, 2021 update, World Health Organization. [PMID: 34314130]
Recommendations for Regimens to Treat Latent TB Infection Adapted from [PMID: 32053584]
Drug Regimens for Microbiologically Confirmed Pulmonary Tuberculosis Caused by Drug-Susceptible Organisms Adapted from [PMID: 27516382]
Tuberculosis Pathophysiology Flow Chart Contributed and created by Ellis Tobin, MD
Disclosure: Ellis Tobin declares no relevant financial relationships with ineligible companies.
Disclosure: Debbie Tristram declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Tobin EH, Tristram D. Tuberculosis. [Updated 2024 Mar 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review Tuberculosis. [Major Infectious Diseases. 2017] Review Tuberculosis. Bloom BR, Atun R, Cohen T, Dye C, Fraser H, Gomez GB, Knight G, Murray M, Nardell E, Rubin E, et al. Major Infectious Diseases. 2017 Nov 3
- Active Tuberculosis. [StatPearls. 2024] Active Tuberculosis. Jilani TN, Avula A, Zafar Gondal A, Siddiqui AH. StatPearls. 2024 Jan
- Review The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. [Lancet Respir Med. 2018] Review The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. Floyd K, Glaziou P, Zumla A, Raviglione M. Lancet Respir Med. 2018 Apr; 6(4):299-314.
- Fighting the tuberculosis epidemic in the Western Pacific region: current situation and challenges ahead. [Kekkaku. 2010] Fighting the tuberculosis epidemic in the Western Pacific region: current situation and challenges ahead. van Maaren PJ. Kekkaku. 2010 Jan; 85(1):9-16.
- Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. [JAMA. 1999] Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. JAMA. 1999 Aug 18; 282(7):677-86.
Recent Activity
- Tuberculosis - StatPearls Tuberculosis - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

IMAGES
VIDEO
COMMENTS
Abstract and Figures. Tuberculosis Is a major bluster to humanity resist to progress in health-care systems and the widespread weapon of TB control programs. The World Health Organization (WHO ...
1. Introduction. Tuberculosis (TB) is a leading global public health problem, with high morbidity and mortality in humans. Until the COVID-19 pandemic, TB was still the leading cause of death from a single infectious agent, ranking above HIV/acquired immune deficiency syndrome [].The number of people newly diagnosed with TB fell from 7.1 million in 2019 to 5.8 million in 2020, and reduced ...
Prevention, Diagnosis and Management of Tuberculosis. MOH Singapore 14. 2016. Guidelines were produced by a committee experts, including physicians, infectious disease experts, and the ministry of health. The guidelines were developed by adapting the existing guidelines, a review of the relevant literature, and expert clinical consensus. Not ...
Prevention, Diagnosis and Management of Tuberculosis. MOH Singapore 17. 2016. Guidelines were produced by a committee experts, including physicians, infectious disease experts, and the ministry of health. The guidelines were developed by adapting the existing guidelines, a review of the relevant literature, and expert clinical consensus. Not ...
In 2011, there were 8.7 million new cases of active tuberculosis worldwide (13% of which involved coinfection with the human immunodeficiency virus [HIV]) and 1.4 million deaths, including 430,000 ...
Tuberculosis remains the leading cause of death from an infectious disease among adults worldwide, with more than 10 million people becoming newly sick from tuberculosis each year. Advances in diagnosis, including the use of rapid molecular testing and whole-genome sequencing in both sputum and non-sputum samples, could change this situation. Although little has changed in the treatment of ...
Tuberculosis is highly prevalent among the low socioeconomic section of the population and marginalized sections of the community. In India, National strategic plan (2017-2025) has a national goal of elimination of tuberculosis by 2025. It requires increased awareness and understanding of Tuberculosis. In this review article history, taxonomy ...
New diagnostic tests, regimens and drugs have emerged. With a background of milestones in the management of tuberculosis, we review the advances made in the diagnosis and treatment of pulmonary tuberculosis. Since India accounts for 27% of word's burden of tuberculosis, the changes in RNTCP have been highlighted. 1.
In this study, the researchers have done a systematic review (a study in which the medical literature is surveyed and appraised using defined methods to reach a consensus view on a specific question) of qualitative studies that asked patients, carers, and health workers which factors contributed to adherence to tuberculosis treatment.
There is growing recognition that tuberculosis (TB) infection and disease exists as a spectrum of states beyond the current binary classification of latent and active TB. Our aim was to systematically map and synthesize published conceptual frameworks for TB states. We searched MEDLINE, Embase and EMcare for review articles from 1946 to September 2023. We included 40 articles that explicitly ...
Tuberculosis (TB) is a major cause of morbidity and mortality worldwide. It is estimated that 25% of the world's population are infected with Mycobacterium tuberculosis , with a 5-10% lifetime risk of progression into TB disease. Early recognition of TB disease and prompt detection of drug resistance are essential to halting its global burden. Culture, direct microscopy, biomolecular tests ...
The care of multi and extensively drug-resistant tuberculosis is based on a combination of pyrazinamide and second-line drugs. These regimens are lengthy, partially effective and poorly tolerated. The challenge is to re-evaluate the use of existing molecules and to develop new agents more effective against resistant strains with shorter ...
Extrapulmonary tuberculosis is a severe disease in liver transplant recipients. Systematic pre-transplant screening of latent tuberculosis may prevent most of them. ... Characteristics, management, and outcome of tuberculosis after liver transplant: A case series and literature review Infect Dis Now. 2024 Feb 23;54(3) ...
Introduction. Tuberculosis (TB) is a major cause of morbidity and mortality worldwide. TB is caused by the bacillus Mycobacterium tuberculosis (Mtb), which is spread via airborne droplets. Approximately one in four people worldwide demonstrate an immunological response to Mtb infection, which can remain dormant or progress into active disease forms []. ...
Current Guideline Review. We conducted a literature search in PubMed to identify available clinical studies and reviews describing the TB incidence associated with each of the studied biologic medications. ... Tuberculosis screening and management of latent tuberculosis infection prior to biologic treatment in patients with immune-mediated ...
Accurate tuberculosis (TB) diagnosis remains challenging, especially in resource-limited settings. This study aims to assess the diagnostic performance of the QIAreach QuantiFERON-TB (QFT) assay ...
This review article has been written after extensive literature study in view of better understanding and to increase awareness regarding tuberculosis, as a sincere effort that will help eliminate ...
Mycobacterium tuberculosis (M. tuberculosis) is the etiological agent of TB and currently more than one-third of the world population is suffering from TB. For the treatment of TB, administration ...
We report a rare case of a 9-year-old male diagnosed with right knee tuberculosis after enduring severe symptoms for several months. Despite multiple negative biopsies and aspirates during initial debridement surgeries, a biopsy taken 6 months later confirmed the presence of Mycobacterium tuberculosis (MTB). The patient was subsequently treated ...
Abstract. Despite being a treatable and preventable disease, tuberculosis will kill an estimated 30 million people during the current decade. Tuberculosis is a global problem, and increases in case rates are occurring not only in the developing countries of the world but also in several industrialized nations, such as the United States ...
Introduction. Disseminated tuberculosis (TB) is defined as the presence of two or more noncontiguous sites resulting from hematogenous dissemination of Mycobacterium tuberculosis, occurring as a result of progressive primary infection, reactivation of a latent focus with subsequent spread,[] or rarely through iatrogenic origin.[] Nowadays, the term miliary TB also refers to progressive and ...
Tuberculosis: An Overview and Review of Literature. Acta Scientific Pharmacology. Volume 2 Issue 8 August 2021. Tuberculosis: An Overview and Review of Literature. Surya Kant1, Veenita Mitta2, Anil Kumar Mavi3, Rachna Chaturvedi4, Pooja Singh1*. 1Department of Respiratory Medicine, KGMU, Lucknow, India.
The review of a formal national tuberculosis (TB) programme (or the efforts that countries make to control the disease regardless of the existence of a formal "programme") is an important exercise to evaluate the implementation and impact of TB prevention, care and control. It should be jointly undertaken by the government together with the relevant national and international partners that ...
Background Undernutrition increases the risk of TB infection to be active TB, death and relapse of the disease. Undernutrition also disturbs the management process of tuberculosis. Therefore, this study aimed to estimate the pooled magnitude and determinants of undernutrition among TB patients in Ethiopia. Methods From August 20, 2022 to January 6, 2023, the research articles were identified ...
Osteoarticular tuberculosis (OA-TB) symptoms are not specific; therefore, many differential diagnoses should be in mind before confirming the diagnosis, especially those with suppurative etiologies like pathological fractures, non-unions, dislocations, arthritis, ankylosis, instability, limb deformity, osteomyelitis, neurological deficits, avascular necrosis, and infections such as fungal ...
Abstract. Tuberculosis (TB) is one of the most ancient diseases of mankind, with molecular evidence going back to over 17,000 years. In spite of newer modalities for diagnosis and treatment of TB, unfortunately, people are still suffering, and worldwide it is among the top 10 killer infectious diseases, second only to HIV.
Risk Factors of Pulmonary T uberculosis and Countermeasures: A Literature Review. Edza Aria Wikurendra 1,2 *, Novera Herdiani, Y enni Gustiani T arigan, Arie Arizandi Kurnianto. 1 Doctoral School ...
Toxin-antitoxin (TA) systems are the major mechanism for persister formation in Mycobacterium tuberculosis (Mtb). Previous studies found that HigBA2 (Rv2022c-Rv2021c), a predicted type II TA system of Mtb, could be activated for transcription in response to multiple stresses such as anti-tuberculosis drugs, nutrient starvation, endure hypoxia, acidic pH, etc. In this study, we determined the ...
Tuberculosis (TB) encompasses a vast amount of information about a common disease that is challenging to diagnose, treat, and prevent. The following quote by S.T. Cole provides a perspective on the importance of this disease: "More human lives have been lost to tuberculosis than to any other disease."[1] Before the SARS-CoV-2 pandemic, Mycobacterium tuberculosis (Mtb) was the most ...
By focusing on literature review in business, management, marketing and emerging business practices, this special issue aims to enhance our understanding of the role literature review plays in driving research excellence, shaping theoretical frameworks, informing practical applications and bridging the knowledge gaps in these dynamic fields. ...