- Português Br
- Journalist Pass

Mayo Clinic researchers’ new tool links Alzheimer’s disease types to rate of cognitive decline
Lynda De Widt
Share this:

Mayo Clinic researchers have discovered a series of brain changes characterized by unique clinical features and immune cell behaviors using a new corticolimbic index tool for Alzheimer's disease , a leading cause of dementia . Their findings are published in JAMA Neurology . The tool categorizes Alzheimer's disease cases into three subtypes according to the location of brain changes and continues the team's prior work , demonstrating how these changes impact people differently. Uncovering the microscopic pathology of the disease can help researchers pinpoint biomarkers that may affect future treatments and patient care. The new "corticolimbic index" tool assigns a score to the location of toxic tau protein tangles that damage cells in brain regions associated with Alzheimer's disease. In the study, differences in where the tangles accumulated affected the disease progression.
"Our team found striking demographic and clinical differences among sex, age at symptomatic onset and rate of cognitive decline," says Melissa E. Murray, Ph.D. , a translational neuropathologist at Mayo Clinic in Florida and senior author of the study.
The team analyzed brain tissue samples from a multi-ethnic group of nearly 1,400 patients with Alzheimer's disease, donated from 1991 to 2020. The samples are part of the Florida Autopsied Multi-Ethnic (FLAME) cohort housed at the Mayo Clinic Brain Bank . The FLAME study cohort is derived from a partnership with the state of Florida's Alzheimer's Disease Initiative . The sample population included Asian, Black/African American, Hispanic/Latino American, Native American and non-Hispanic white people who received care at memory disorder clinics in Florida and donated their brains for research.
To verify the clinical value of the tool, researchers further investigated study participants from Mayo Clinic who underwent neuroimaging while alive. In collaboration with a Mayo Clinic team led by Prashanthi Vemuri, Ph.D. , the researchers found that the corticolimbic index scores were consistent with the changes in the hippocampus detected via MRI and tau positron emission tomography (tau-PET) changes in the cortex.
"By combining our expertise in the fields of neuropathology, biostatistics, neuroscience, neuroimaging and neurology to address Alzheimer's disease from all angles, we've made significant strides in understanding how it affects the brain," says Dr. Murray. "The corticolimbic index is a score that could encourage a paradigm shift toward understanding the individuality of this complex disease and broaden our perspective. This study marks a significant step toward personalized care, offering hope for more effective future therapies."
The research team's next step is to translate the findings into clinical practice, giving radiologists and other medical specialists access to the corticolimbic index tool. Dr. Murray says the tool could help physicians determine the progression of Alzheimer's disease in patients and enhance clinical management. The team is also planning more research using the tool to identify areas of the brain resistant to the toxic tau protein's effects.
For a full list of authors, funding and disclosures, see the paper .
- Mayo Clinic, Nemours Children’s Health, Jacksonville collaborate for pediatric care, medical education, research Exploring the exposome
Related Articles

Study Suggests Treatments that Unleash Immune Cells in the Brain Could Help Combat Alzheimer’s
Posted on April 25th, 2024 by Dr. Monica M. Bertagnolli
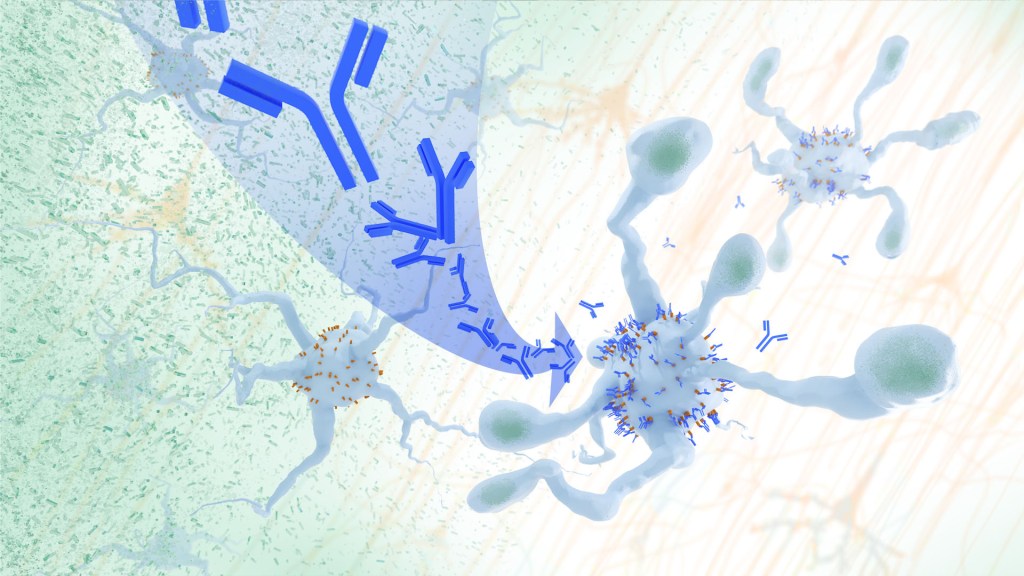
In Alzheimer’s disease, a buildup of sticky amyloid proteins in the brain clump together to form plaques, causing damage that gradually leads to worsening dementia symptoms. A promising way to change the course of this disease is with treatments that clear away damaging amyloid plaques or stop them from forming in the first place. In fact, the Food and Drug Administration recently approved the first drug for early Alzheimer’s that moderately slows cognitive decline by reducing amyloid plaques. 1 Still, more progress is needed to combat this devastating disease that as many as 6.7 million Americans were living with in 2023.
Recent findings from a study in mice, supported in part by NIH and reported in Science Translational Medicine , offer another potential way to clear amyloid plaques in the brain. The key component of this strategy is using the brain’s built-in cleanup crew for amyloid plaques and other waste products: immune cells known as microglia that naturally help to limit the progression of Alzheimer’s. The findings suggest it may be possible to develop immunotherapies—treatments that use the body’s immune system to fight disease—to activate microglia in the brains of people with Alzheimer’s and clear amyloid plaques more effectively. 2
In their report, the research team—including Marco Colonna , Washington University School of Medicine in St. Louis, and Jinchao Hou, now at Children’s Hospital of Zhejiang University School of Medicine in Zhejiang Province, China—wrote that microglia in the brain surround plaques to create a barrier that controls their spread. Microglia can also destroy amyloid plaques directly. But how microglia work in the brain depends on a fine-tuned balance of signals that activate or inhibit them. In people with Alzheimer’s, microglia don’t do their job well enough.
The researchers suspected this might have something to do with a protein called apolipoprotein E (APOE). This protein normally helps carry cholesterol and other fats in the bloodstream. But the gene encoding the protein is known for its role in influencing a person’s risk for developing Alzheimer’s, and in the Alzheimer’s brain, the protein is a key component of amyloid plaques. The protein can also inactivate microglia by binding to a receptor called LILRB4 found on the immune cells’ surfaces.
Earlier studies in mouse models of Alzheimer’s showed that the LILRB4 receptor is expressed at high levels in microglia when amyloid plaques build up. This suggested that treatments targeting this receptor on microglia might hold promise for treating Alzheimer’s. In the new study, the research team looked for evidence that an increase in LILRB4 receptors on microglia plays an important role in the brains of people with Alzheimer’s.
To do this, the researchers first studied brain tissue samples from people who died with this disease and discovered unusually high amounts of the LILRB4 receptor on the surfaces of microglia, similar to what had been seen in the mouse models. This could help explain why microglia struggle to control amyloid plaques in the Alzheimer’s brain.
Next, the researchers conducted studies of mouse brains with accumulating amyloid plaques that express the LILRB4 receptor to see if an antibody targeting the receptor could lower amyloid levels by boosting activity of immune microglia. Their findings suggest that the antibody treatment blocked the interaction between APOE proteins and LILRB4 receptors and enabled microglia to clear amyloid plaques. Intriguingly, the team’s additional studies found that this clearing process also changed the animals’ behavior, making them less likely to take risks. That’s important because people with Alzheimer’s may engage in risky behaviors as they lack memories of earlier experiences that they could use to make decisions.
There’s plenty more to learn. For instance, the researchers don’t know yet whether this approach will affect the tau protein , which forms damaging tangles inside neurons in the Alzheimer’s brain. They also want to investigate whether this strategy of clearing amyloid plaques might come with other health risks.
But overall, these findings add to evidence that immunotherapies of this kind could be a promising way to treat Alzheimer’s. This strategy may also have implications for treating other neurodegenerative conditions characterized by toxic debris in the brain, such as Parkinson’s disease, amyotrophic lateral sclerosis (ALS), and Huntington’s disease. The hope is that this kind of research will ultimately lead to more effective treatments for Alzheimer’s and other conditions affecting the brain.
References:
[1] FDA Converts Novel Alzheimer’s Disease Treatment to Traditional Approval . U.S. Food and Drug Administration (2023).
[2] Hou J, et al . Antibody-mediated targeting of human microglial leukocyte Ig-like receptor B4 attenuates amyloid pathology in a mouse model . Science Translational Medicine . DOI: 10.1126/scitranslmed.adj9052 (2024).
NIH Support: National Institute of General Medical Sciences, National Institute on Aging
Share this:
- Click to share on Facebook (Opens in new window)
- Click to share on X (Opens in new window)
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Posted In: Health , News , Science
Tags: Alzheimer's treatment , Alzheimer’s disease , amyloid , basic research , brain , microglia , neurons , neuroscience
so, whats next? is it available? how to obtain this??
Keep up the good work
Leave a Comment Cancel reply
@nihdirector on x, nih on social media.
Kendall Morgan, Ph.D.
Comments and Questions
If you have comments or questions not related to the current discussions, please direct them to Ask NIH .
You are encouraged to share your thoughts and ideas. Please review the NIH Comments Policy

- Visitor Information
- Privacy Notice
- Accessibility
- No Fear Act
- HHS Vulnerability Disclosure
- U.S. Department of Health and Human Services
- USA.gov – Government Made Easy
Discover more from NIH Director's Blog
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading
Innovative microscopy demystifies metabolism of Alzheimer's
Alzheimer's disease causes significant problems with memory, thinking and behavior and is the most common form of dementia, affecting more than 50 million people around the world each year. This number is expected to triple by the year 2050.
Using their own state-of-the art imaging technologies, scientists at the University of California San Diego have now revealed how the metabolism of lipids, a class of molecule that includes fats, oils and many hormones, is changed in Alzheimer's disease. They also revealed a new strategy to target this metabolic system with new and existing drugs. The findings are published in Cell Metabolism .
"Lipids have been associated with Alzheimer's for as long as we've known about the disease," said senior and co-corresponding author Xu Chen, Ph.D., an assistant professor in the Department of Neurosciences at UC San Diego School of Medicine, referring to the original 1907 report by Alois Alzheimer that described the unusual presence of fat deposits in the brain of the first person to be diagnosed with the disease. "So much of the emphasis since then has been placed on tau and other proteins that the research community has, until the last decade or so, largely overlooked this important aspect of the disease."
"Driven by a keen interest in lipid droplet functions in aging and disease, we initiated this fruitful collaboration to harness cutting-edge SRS technology for studying lipid metabolism in tauopathy mouse brains." Said Yajuan Li, M.D., Ph.D., a postdoctoral researcher in the Shu Chien-Gene Lay Department of Bioengineering at UC San Diego Jacobs School of Engineering. SRS imaging is an approach that analyzes the way molecules in a sample interact with laser light.
In the brain, lipids come in the form of tiny droplets that control a variety of processes, such as energy storage and cellular responses to stress. These processes are tightly regulated in typical brains, but in Alzheimer's or similar diseases, lipid droplet metabolism can malfunction. While scientists understand that there is a relationship between Alzheimer's and lipid metabolism, exactly how they influence one another has remained a mystery.
To answer this question, the team looked directly at lipid droplets in the brains of mice with excess tau protein. They used a state-of-the-art SRS imaging platform developed in Lingyan Shi's lab at the Jacobs School of Engineering. The platform makes it possible to take microscopic images of lipid droplets within cells without the use chemical dyes, which can alter the delicate molecules and compromise the results.
"Intriguingly, the inert lipid droplets observed in tauopathy brains exhibit similar behavior to those found in aging brains," said co-corresponding author Lingyan Shi, Ph.D., assistant professor of bioengineering at the Jacobs School. "We are now focusing on understanding the underlying mechanisms by combing SRS imaging with other utilizing multidisciplinary techniques. Our approach is biologically neutral, so we're able to observe what's happening in the brain at the molecular level with as little interference as possible."
Shi and her team, including Li, pioneered the new approach, which uses a specially modified version of water, called heavy water, as a metabolic probe.
"Instead of using a typical chemical dye to stain lipids, we use heavy water that is naturally participating in the metabolic activities we're interested in," added Shi. "This gives us a much clearer picture of how lipids are formed spatiotemporally, which would not be possible with other approaches. Our current focus is on comprehending the underlying mechanisms of these dynamic changes of lipid metabolism in the context of aging and diseases."
The researchers discovered that in brains with tauopathy, neurons accumulate excess lipids as a result of stress or damage. This influx forces neurons to offload the excess to immune cells in the brain, called microglia. These microglia then mount an inflammatory response that causes further stress to neurons, triggering a repeating and worsening cycle.
In addition to characterizing this process, they were also able to identify a critical enzyme, called adenosine monophosphate-activated protein kinase (AMPK) that orchestrates the cycle. According to the researchers, breaking this cycle could unlock new treatment options for Alzheimer's disease. Chen is particularly optimistic about the possibility of repurposing existing drugs that modify lipid metabolism.
"We don't think this is an incidental phenomenon," said Chen. "The evidence suggests that lipid metabolism is a driving mechanism for Alzheimer's disease. There are many drugs that target lipid metabolism in other body systems, such as in the liver, so we might be able to change this system quite dramatically using tools we already have."
- Alzheimer's Research
- Healthy Aging
- Gene Therapy
- Diseases and Conditions
- Alzheimer's
- Disorders and Syndromes
- Alzheimer's disease
- Dementia with Lewy bodies
- Confocal laser scanning microscopy
- Transmission electron microscopy
- Psychotherapy
- Urinary incontinence
- Erectile dysfunction
- Biological tissue
Story Source:
Materials provided by University of California - San Diego . Original written by Miles Martin. Note: Content may be edited for style and length.
Journal Reference :
- Yajuan Li, Daniel Munoz-Mayorga, Yuhang Nie, Ningxin Kang, Yuren Tao, Jessica Lagerwall, Carla Pernaci, Genevieve Curtin, Nicole G. Coufal, Jerome Mertens, Lingyan Shi, Xu Chen. Microglial lipid droplet accumulation in tauopathy brain is regulated by neuronal AMPK . Cell Metabolism , 2024; DOI: 10.1016/j.cmet.2024.03.014
Cite This Page :
Explore More
- Random Robots Are More Reliable
- Significant Discovery in Teleportation Research
- Orangutan Treats Wound With Pain-Relieving Plant
- 75,000-Year-Old Neanderthal from Burial Cave
- Anticoagulant With an On-Off Switch
- Sleep Resets Brain Connections -- At First
- Far-Reaching Effects of Exercise
- Hidden Connections Between Brain and Body
- Novel Genetic Plant Regeneration Approach
- Early Human Occupation of China
Trending Topics
Strange & offbeat.
2024 Alzheimer's disease facts and figures
- PMID: 38689398
- DOI: 10.1002/alz.13809
This article describes the public health impact of Alzheimer's disease (AD), including prevalence and incidence, mortality and morbidity, use and costs of care and the ramifications of AD for family caregivers, the dementia workforce and society. The Special Report discusses the larger health care system for older adults with cognitive issues, focusing on the role of caregivers and non-physician health care professionals. An estimated 6.9 million Americans age 65 and older are living with Alzheimer's dementia today. This number could grow to 13.8 million by 2060, barring the development of medical breakthroughs to prevent or cure AD. Official AD death certificates recorded 119,399 deaths from AD in 2021. In 2020 and 2021, when COVID-19 entered the ranks of the top ten causes of death, Alzheimer's was the seventh-leading cause of death in the United States. Official counts for more recent years are still being compiled. Alzheimer's remains the fifth-leading cause of death among Americans age 65 and older. Between 2000 and 2021, deaths from stroke, heart disease and HIV decreased, whereas reported deaths from AD increased more than 140%. More than 11 million family members and other unpaid caregivers provided an estimated 18.4 billion hours of care to people with Alzheimer's or other dementias in 2023. These figures reflect a decline in the number of caregivers compared with a decade earlier, as well as an increase in the amount of care provided by each remaining caregiver. Unpaid dementia caregiving was valued at $346.6 billion in 2023. Its costs, however, extend to unpaid caregivers' increased risk for emotional distress and negative mental and physical health outcomes. Members of the paid health care and broader community-based workforce are involved in diagnosing, treating and caring for people with dementia. However, the United States faces growing shortages across different segments of the dementia care workforce due to a combination of factors, including the absolute increase in the number of people living with dementia. Therefore, targeted programs and care delivery models will be needed to attract, better train and effectively deploy health care and community-based workers to provide dementia care. Average per-person Medicare payments for services to beneficiaries age 65 and older with AD or other dementias are almost three times as great as payments for beneficiaries without these conditions, and Medicaid payments are more than 22 times as great. Total payments in 2024 for health care, long-term care and hospice services for people age 65 and older with dementia are estimated to be $360 billion. The Special Report investigates how caregivers of older adults with cognitive issues interact with the health care system and examines the role non-physician health care professionals play in facilitating clinical care and access to community-based services and supports. It includes surveys of caregivers and health care workers, focusing on their experiences, challenges, awareness and perceptions of dementia care navigation.
Keywords: Alzheimer's dementia; Alzheimer's disease; Biomarkers; COVID‐19; Care navigation; Care navigator; Caregivers; Dementia; Dementia care navigation; Dementia workforce; Diagnostic criteria; Family caregiver; Health care costs; Health care expenditures; Health care professional; Health care utilization; Home and community‐based services; Incidence; Long‐term care costs; Long‐term care utilization; MCI due to Alzheimer's disease; Medicaid spending; Medicare spending; Mild cognitive impairment; Morbidity; Mortality; Navigator; Prevalence; Primary care physician; Risk factors.
© 2024 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- NEWS FEATURE
- 04 April 2023
Conquering Alzheimer’s: a look at the therapies of the future
- Alison Abbott 0
Alison Abbott is a writer based in Munich, Germany.
You can also search for this author in PubMed Google Scholar
A brain scan reveals the extent of damage caused by Alzheimer’s disease. Credit: Zephyr/Science Photo Library
When neurologist Reisa Sperling stepped up to receive her lifetime achievement award at an international Alzheimer’s conference last December, she was more excited about the future than about celebrating the past.
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Nature 616 , 26-28 (2023)
doi: https://doi.org/10.1038/d41586-023-00954-w
Van Dyck, C. H. et al. N. Engl. J. Med. 388 , 9–21 (2023).
Article PubMed Google Scholar
Imbimbo, B. P., Balducci, C., Ippati, S. & Watling, M. Neural Regen. Res. 18 , 117–118 (2023).
Aisen, P. S. et al. J. Prev. Alzheimers Dis. 8 , 306–312 (2021).
Bazzari, F. H. & Bazzari, A. H. Molecules 27 , 8823 (2022).
McDade, E. et al. Nature Rev. Neurol. 17 , 703–714 (2021).
Rynearson, K. D. et al. J. Exp. Med. 218 , e20202560 (2021).
Hannestad, J. et al. J. Alzheimers Dis. 81 , 1649–1662 (2021).
Download references
Reprints and permissions
Related Articles

- Alzheimer's disease
- Neuroscience
- Drug discovery
Magnetic field effects on behaviour in Drosophila
Matters Arising 01 MAY 24
A body–brain circuit that regulates body inflammatory responses
Article 01 MAY 24
Magnetic field responses in Drosophila

UTIs make life miserable — scientists are finding new ways to tackle them
News 02 MAY 24

Plastic pollution: three numbers that support a crackdown
News Explainer 24 APR 24

Refining the impact of genetic evidence on clinical success
Analysis 17 APR 24
W2 Professorship with tenure track to W3 in Animal Husbandry (f/m/d)
The Faculty of Agricultural Sciences at the University of Göttingen invites applications for a temporary professorship with civil servant status (g...
Göttingen (Stadt), Niedersachsen (DE)
Georg-August-Universität Göttingen
Postdoctoral Associate- Cardiovascular Research
Houston, Texas (US)
Baylor College of Medicine (BCM)
Faculty Positions & Postdocs at Institute of Physics (IOP), Chinese Academy of Sciences
IOP is the leading research institute in China in condensed matter physics and related fields. Through the steadfast efforts of generations of scie...
Beijing, China
Institute of Physics (IOP), Chinese Academy of Sciences (CAS)
Director, NLM
Vacancy Announcement Department of Health and Human Services National Institutes of Health DIRECTOR, NATIONAL LIBRARY OF MEDICINE THE POSITION:...
Bethesda, Maryland
National Library of Medicine - Office of the Director
Call for postdoctoral fellows in Molecular Medicine, Nordic EMBL Partnership for Molecular Medicine
The Nordic EMBL Partnership is seeking postdoctoral fellows for collaborative projects in molecular medicine through the first NORPOD call.
Helsinki, Finland
Nordic EMBL Partnership for Molecular Medicine
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Clinical Trials
Alzheimer's disease.
Displaying 78 studies
The purpose of this study is to identify potential biomarkers that may predict the development of Alzheimer's disease in people who carry an Alzheimer's mutation.
There is evidence that high blood pressure can cause changes in memory and thinking as people get older. When the SPRINT study intervention was stopped in September of 2015, important data on long term rate of Alzheimer’s, change in cognition, and impact on the kidneys were not able to be collected. Participants from the SPRINT study will undergo a single SPRINT ASK visit approximately two years after their SPRINT closeout visit to assess blood pressure, cognitive and kidney function.
To test the idea that solanezumab will slow the cognitive and functional decline of Alzheimer's Disease (AD) as compared with placebo in participants with mild AD.
The investigators' goal is to determine if certain tests of memory and attention, performed while sleepiness is induced by a single dose of lorazepam, can predict whether or not an individual is at risk for developing Alzheimer's disease.
The purpose of this study is to learn more about amyloid and tau burden in the brain of patients with Atypical Alzheimer's Disease and how that burden may change over a two-year period.
5.4 million Americans have Alzheimer's disease (AD) costing $185 billion annually, while 15 million caregivers look after these individuals. AD is the sixth leading cause of death, but the only one in the top 10 causes that cannot be prevented.
This study may demonstrate exercise in an amount attainable by many will be preventative in asymptomatic individuals including those with brain Abeta deposition already that are at impending risk of the disease. Sperling and colleagues(1) coined the research term AD-pathophysiological process (abbreviated AD-P) for use in studies such as the intervention in this proposal.
Our long term goal is to assess whether ...
An urgent need exists to find effective treatments for Alzheimer's disease (AD) that can arrest or reverse the disease at its earliest stages. The emotional and financial burden of AD to patients, family members, and society is enormous, and is predicted to grow exponentially as the median population age increases. Current FDA-approved therapies are modestly effective at best. This study will examine a novel therapeutic approach using intranasal insulin (INI) that has shown promise in short-term clinical trials. If successful, information gained from the study has the potential to move INI forward rapidly as a therapy for AD. The study ...
The purpose of this study is to test whether an investigational drug called solanezumab can slow the progression of memory problems associated with brain amyloid (protein that forms plaques in the brains of people with Alzheimer Disease [AD]).
Aging is associated with a loss of brain function and conditions such as dementia and Alzheimer's disease. It is likely that decreased brain metabolism is contributing to the progression of age related degenerative diseases. Aerobic exercise training can increase brain volumes and is associated with decreased risk for degenerative brain conditions. However, little is know about the changes that occur to brain metabolism with aerobic training and aging.
The primary objective is to evaluate the long-term safety and tolerability of aducanumab after a wash-out period imposed by discontinuation of feeder studies in participants who had previously received aducanumab (i.e., previously treated participants) or who had previously received placebo (i.e, treatment-naïve participants).
The purpose of this study is to determine whether Alzheimer's Disease related cognitive trajectories can be identified in midlife and disambiguated from normal aging using serial cognitive measurement.
Additionally, to determine the biofluid and imaging biomarker profiles associated with cognitive trajectories and with eventual conversion to dementia; to determine the effect of genetic characteristics on cognitive decline, biomarkers and disease and health status; to determine the influence of lifestyle and health features that confers risk and resilience to AD; to make data available to local, national and international researchers and to facilitate WRAP participation in linked studies including imaging ...
The purpose of this study is to evaluate the effectiveness of LY3314814 in the treatment of people who have mild Alzheimer's disease dementia.
The study is designed to assess the demographic, clinical and imaging associations with the presence of microbleeds in atypical Alzheimer's disease. The primary hypothesis is that cognitive and functional performance will be poorer in atypical Alzheimer's subjects with microbleeds compared to those without microbleeds.
The purpose of this study is to test whether two investigational drugs called CAD106 and CNP520, administered separately, can slow down the onset and progression of clinical symptoms associated with Alzheimer's disease (AD) in participants at the risk to develop clinical symptoms based on their age and genotype.
McLean hospital, Mayo Clinic, Emory University, LIJ/Northwell, and Pine Rest Mental Health are conducting a research study using Electroconvulsive therapy (ECT) to treat agitation in dementia. ECT is a treatment done under general anesthesia, in which brief electric currents are passed through the brain to trigger a brief seizure. It is a safe and highly effective treatment for depression.
Agitation is common in nearly 60% of patients with dementia, increases caretaker burden, creates safety risk for individuals with dementia and others and increases risk for hospitalization and nursing home placement.
While ECT ...
The purpose of this study is to investigate how abrupt loss of ovarian hormones following bilateral oophorectomy affects overall aging, physical performance, and cognitive function, including the risk for Alzheimer’s disease in women who had this procedure performed prior to natural menopause for benign conditions.
The purpose of this research is to iteratively develop an natural language (NL) web-based app tool using information learned from stakeholders on design and content, especially as it relates to the needs and usability for persons with dementia and/or their caregivers.
The primary objective of the study is to evaluate the efficacy of monthly doses of aducanumab in slowing cognitive and functional impairment as measured by changes in the Clinical Dementia Rating-Sum of Boxes (CDR-SB) score as compared with placebo in participants with early AD. Secondary objectives are to assess the effect of monthly doses of aducanumab as compared with placebo on clinical progression as measured by Mini-Mental State Examination (MMSE), AD Assessment Scale-Cognitive Subscale (13 items) [ADAS-Cog 13], and AD Cooperative Study-Activities of Daily Living Inventory (Mild Cognitive Impairment version) [ADCS-ADL-MCI].
The purpose of this study is to measure target engagement in cerebrospinal fluid (CSF) and blood, and to establish the feasibility and safety of Dasatinib plus Quercetin treatment in adults with early stage but symptomatic Alzheimer's Disease (AD) to inform and select the best blood, CSF, urine, and other analyses to conduct in banked samples from a larger Phase 2b clinical trial.
The purpose of this study is to identify lifestyle and health variable associated with abnormal cognitive aging and the development of Alzheimer's Disease (AD) and to use this information to develop interventions that will slow disease progressiong in asymptomatic persons.
The purposes of this study are to determine if neuroinflammation, as measured by PET imaging, is associated with Ab plaques in cognitively impaired vs. cognitively unimpaired participants, to determine if neuroinflammation, as measured by neuroinflammation PET imaging, is associated with the rate of cognitive in the 5 years preceding PET imaging, and to determine if neuroinflammation, as measured by PET imaging, is associated with plasma biomarkers of inflammation.
This study consists of two parts, Part I and Part II. The purpose of Part I of the study is to assess the efficacy and safety of MK-8931 compared with placebo administered for 78 weeks in the treatment of Alzheimer's Disease (AD). The primary study hypotheses for Part I are that at least one MK-8931 dose is superior to placebo at 78 weeks of treatment with respect to change from Baseline in Alzheimer's Disease Assessment Scale Cognitive Subscale (ADAS-Cog) score and that at least one MK-8931 dose is superior to placebo at 78 weeks of treatment with respect to change ...
The purpose of this study is to determine safety of plasma infusion in APOE 44 patients.
The purpose of this study is to evaluate whether treatment with JNJ-54861911 slows cognitive decline compared with placebo treatment, as measured by a composite cognitive measure, the Preclinical Alzheimer Cognitive Composite (PACC), in amyloid-positive participants who are asymptomatic at risk for developing Alzheimer's dementia.
The purpose of this study is to determine the effects of CNP520 on cognition, global clinical status, and underlying AD pathology, as well as the safety of CNP520, in people at risk for the onset of clinical symptoms of AD based on their age, APOE genotype and elevated amyloid.
The primary purpose of this study is to evaluate acceptability of a combination compensation development and lifestyle modification program for brain health in those with subjective cognitive complaint without objective impairment measured by: a) the proportion of invited participants who chose to enroll; and b) quantitative and qualitative program satisfaction ratings.
The purpose of this study is to evaluate the safety and effectiveness of MABT5102A in patients who have mild to moderate Alzheimer's Disease.
This study aims to obtain EEG data on individuals at all stages of AD from preclinical through severe dementia.
The purpose of this study is to validate the Alzheimer Disease Biomarkers assays (Phospho-Tau/Total-Tau/Ab42) being implemented at Mayo to compare them to the referral tests in support of test validation efforts.
This randomized, placebo-controlled, double-blind, parallel-arm study will evaluate the safety and tolerability of at least two dose levels of intravenous (IV) crenezumab in 24-72 participants with mild to moderate Alzheimer disease (AD) (mini-mental state examination [MMSE] 18 to 28 points, inclusive). An optional open-label extension (OLE) will be offered after the completion of initial double-blind stage.
The purpose of this study is to evaluate the rate of cognitive change in clinically normal older individuals who "screen-failed" for the A4 trial on the basis of their screening PET imaging not demonstrating evidence of elevated amyloid accumulation ( were Aβ negative) but met all other A4 study eligibility criteria. While long term data suggests that older individuals with elevated Aβ burden are at increased risk of cognitive decline, it is important to demonstrate the different rate of clinical decline between Aβe ("Aβ elevated") and Aβne ("Aβ not elevated") individuals.
The overall purpose of this research is to understand how ADAD develops in order to eventually provide treatments for this disorder. Each biological child of a person with an ADAD mutation has a 50% risk of inheriting the mutation, and thus of developing ADAD. This study will develop a registry of families with a known ADAD mutation and will collect, analyze and bank data, tissue, and brain images from the members who participate in the DIAN research study. The data and tissue collected are available to all qualified researchers who wish to determine what changes occur before and after ADAD ...
The primary objective of this application is to establish the feasibility of widespread clinical use of advanced MRS technology for early AD diagnosis in a strategic alliance between MR physicists at the UMN and physician scientists at Mayo Clinic.
This study is designed to determine the effectiveness of florbetapir (18F) in changing patient management and to evaluate the association between scan status and cognitive decline.
The purpose of this study is to develop a large, well-characterized, biomarker-confirmed, trial-ready cohort to facilitate rapid enrollment into AD prevention trials utilizing the APT Webstudy and subsequent referral to in-clinic evaluation and biomarker confirmation. Participants with known biomarker status may have direct referral to the Trial-Ready Cohort. If you are interested in being selected for the TRC-PAD study, you should first enroll in the APT Webstudy (https://www.aptwebstudy.org/welcome).
The purpose of this study is to determine whether reduced genetic diversity as reflected in lower heterozygosity reduces our adaptability to stressors and thus influences human susceptibility to age-related cognitive decline and dementia. This will be examined in an established cohort of individuals at genetically defined risk for Alzheimer’s disease based on APOE genotype who have been undergoing longitudinal neuropsychological assessment.
A brain PET scan is recognized as "reasonable and necessary" for some patients with "a recently established diagnosis of dementia" (Centers for Medicare and Medicaid Services, Decision Memo CAG-00088R, 2004), but evidence is less clear for patients having less severe cognitive problems. A substantial portion of such patients will develop Alzheimer's disease and other forms of dementia, which affect millions of people in the U.S., costing us over $100 billion annually. This project employs a prospective randomized protocol to determine whether PET scanning can help distinguish those patients with early Alzheimer's changes in their brains from those having other causes ...
The purpose of this study is to determine whether treatment with BAN2401 is superior to placebo on change from baseline of the Preclinical Alzheimer’s disease (AD) Cognitive Composite 5 (PACC5) at 216 weeks of treatment.
The purpose of this study is to compare different ways of measuring tau both in the brain and in the blood over time in healthy controls and individuals with cognitive impairment. We will collect images in healthy elderly people, individuals with mild cognitive impairment and those diagnosed with Alzheimer’s disease to help us learn how the buildup of amyloid and tau proteins may contribute to developing the disease and in normal aging. Healthy young individuals will be used as controls.
This study is composed of 2 timepoints, separated by approximately 18 months. Each timepoint will require visits to the ...
This study aims to establish, use, and extensively share a comprehensive longitudinal resource of genetic, non-genetic, and cognitive data, brain imaging and fluid biomarker measurements of amyloid-β (Aβ), tau pathophysiology, neurodegeneration, and inflammation (“A,T,N,I”), and biological samples to advance the study of cognitively unimpaired older adults at six levels of genetic risk for Alzheimer’s disease (AD) due to their apolipoprotein E (APOE) genotype, including understudied APOE2 and APOE4 homozygotes (HMs) at the lowest and highest risk and those APOE4 HMs and heterozygotes (HTs) who remain unimpaired at older ages due to unknown protective factors and spared pathophysiological effects ...
The purpose of this study is to examine the safety and effectiveness of suvorexant (MK-4305) to improve sleep in individuals who have Alzheimer's disease.
The purpose of this study is to assess the long-term safety and tolerability of ABBV-8E12 in subjects with early Alzheimer's disease (AD).
There is evidence that neurodegenerative changes precede clinical symptoms in Alzheimer’s disease by two decades (Villemagne et al, 2013). Early detection is critical for development of interventions to halt, slow, or even reverse these pathological processes. The promise of plasma biomarkers to identify early pathology is growing rapidly (Palmqvist et al, 2020), however it is likely that multiple converging biomarkers will be necessary to identify the earliest pathological changes, as subtle differences from healthy controls may fall within the margin of error for any given single biomarker measure. Here we propose that the evaluation of speech and language for both ...
The purpose of this study is to provide subjects who have completed participation in a Phase 2 or Phase 3 trial with TRx0237 continued access to therapy and to evaluate the long-term safety of TRx0237.
The purpose of this study is to explore the various retinal modalities to determine if they may provide a non-invasive method of identifying populations at risk for developing Alzheimer’s Disease (AD) and predict disease progression.
The purpose of this study is to further characterize the serum and Cerebral Spinal Fluid (CSF) biomarker profile of idiopathic normal pressure hydrocephalus, both before and after VP shunt placement, and help differentiate this profile from Alzheimer’s disease.
The purpose of this study is to evaluate the effectiveness and safety of gantenerumab versus placebo in participants with early (prodromal to mild) Alzheimer's Disease (AD). All participants must show evidence of beta-amyloid pathology. Eligible participants will be randomized 1:1 to receive either subcutaneous (SC) injection of gantenerumab or placebo. The primary efficacy assessment will be performed at the end of the double blind period at week 104. Participants will then be offered to enter into an open-label extension (OLE). Participants not willing to go to the OLE will participate in a long term follow-up period for up to 50 weeks ...
Automobile driving is a crucial aspect of everyday life, but driving safety problems including car crashes or speeding violations are a serious public health problem. Alzheimer’s Disease (AD) affects the ability to safely drive and raises crash risk. Mild cognitive impairment (MCI) raises the risk of dementia, and people with MCI have been shown to have problems with memory, decision making, and the ability to concentrate that could lead to unsafe driving, even before obvious dementia begins. Whether MCI patients who continue to drive are safe drivers or not is unknown.
The purpose of this study is to evaluate the impact of the BeWell360 coaching care model to improve functional and experience-related outcomes of informal caregivers (CGs) of patients living with mild cognitive impairment (MCI) due to Alzheimer’s Disease (AD) to create a foundational framework for future evaluation and implementation.
This study seeks to validate and further develop the NIH Toolbox for Assessment of Neurological and Behavioral Function® (NIHTB) for use in studies of cognitive aging beginning with normal cognition through progression into amnestic Mild Cognitive Impairment (aMCI) and into dementia of the Alzheimer’s Type, early stage (DAT).
Since its launch in 2004, the overarching aim of the Alzheimer's Disease Neuroimaging Initiative (ADNI) has been realized in informing the design of therapeutic trials in AD. ADNI3 continues the previously funded ADNI-1, ADNI-GO, and ADNI-2 studies that have been combined public/private collaborations between academia and industry to determine the relationships between the clinical, cognitive, imaging, genetic and biochemical biomarker characteristics of the entire spectrum of Alzheimer's disease (AD). The overall goal of the study is to continue to discover, optimize, standardize, and validate clinical trial measures and biomarkers used in AD research.
The study is designed to determine whether there are clinical features that can be used as biomarkers to predict whether underlying Alzheimer's pathology is the cause of a speech and language based dementia. The primary hypothesis is that the proportion of patients who test positive for beta-amyloid deposition will vary across different speech and language based dementias.
The purpose of this trial is to assess the efficacy and safety of MK-8931 compared with placebo in the treatment of amnestic mild cognitive impairment (aMCI) due to Alzheimer's Disease (AD), also known as prodromal AD. Participants will be randomized to receive placebo, or 12 mg or 40 mg MK-8931, once daily. The primary study hypothesis is that at least one MK-8931 dose is superior to placebo with respect to the change from baseline in the Clinical Dementia Rating scale-Sum of Boxes (CDR-SB) score at 104 weeks.
This study seeks to evaluate the efficacy and safety of ABBV-8E12 in subjects with Early Alzheimer's Disease.
This study is being done to learn more about specialized systems in the brain and how they relate to the disease processes in aMCI and AD.
This study is being done to collect skin samples from people with and without neurodegenerative and vascular disorders including Parkinson’s disease (PD), Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), stroke and many others. We will use these skin samples to make and bank (store) a group of cells (cell line) called inducible pluripotent stem (iPS) cells.
The purpose of this study is to collect blood samples for DNA analysis from patients clinically diagnosed with Alzheimers disease, Lewy Body disease, and Frontotemporal degeneration.
This study consists of two parts, Part I and Part II. The purpose of Part I of the study is to assess the efficacy and safety of verubecestat (MK-8931) compared with placebo administered for 104 weeks in the treatment of amnestic mild cognitive impairment (aMCI) due to Alzheimer's Disease (AD), also known as prodromal AD. Participants will be randomized to receive placebo, or 12 mg or 40 mg verubecestat, once daily. The primary study hypothesis for Part I is that at least one verubecestat dose is superior to placebo with respect to the change from baseline in the Clinical Dementia ...
This is a cross-sectional and longitudinal study that will evaluate imaging characteristics of 18F-AV-1451 in cognitively healthy volunteers, mild cognitive impairment (MCI) and Alzheimer's disease (AD) subjects.
The intent of this research protocol is to test the equivalency of two amyloid imaging drugs (C11 Pittsburgh Compound B and F18 Flutemetamol). The investigators hypothesize that there will be no significant difference in the distribution of the agents to areas of amyloid deposition in the brain or to other normal brain structures. Recent data have shown similarity in the distribution of the drugs in subjects with AD or mild cognitive impairment (MCI). No comparison data of the two PET drugs in normal subjects has been published. It is important to understand differences in the images and biodistribution from the ...
The purpose of this study is to develop and test the effectiveness of an investigational imaging technique called magnetic resonance elastography (MRE) to measure the stiffness (mechanical properties) of tissues.
The purpose of this study is to facilitate focus groups to assess/identify important qualities and characteristics that dementia caregivers are looking for in a supportive person, and to design a prototype website for matching current and former caregivers.
The purpose of this study is to optimize profile questionnaire and matching algorithm developed in Phase I and implement in final website design, and to determine if algorithmically matched participants have statistically significant increase in match satisfaction and self-reported sense of resiliency and quality of life over randomly matched caregivers.
The investigators propose using DaTscan in patients with mild cognitive impairment (MCI), Parkinson's disease (PD), dementia with Lewy bodies (DLB), Alzheimer's disease (AD), and other neurodegenerative syndromes and disorders, to test several hypotheses - some confirmatory, and some novel. Such use will provide new data on the potential clinical and research utility of DaTscan in neurodegenerative diseases. The findings on DaTscan will be correlated with clinical diagnoses and other multimodal imaging studies (e.g., MRI, MRS, FDG-PET, and amyloid-PET) to enhance our understanding of neurodegenerative diseases.
The purpose of this study is to evaluate changes in CSF dynamics (e.g., velocity, flow rate) between patients with normal pressure hydrocephalus and healthy controls, as well as patients with other dementia disorders.
This study is being done to learn more about normal memory and aging, mild memory and thinking problems, Alzheimer's disease and other forms of dementia.
This is an interview study to understand the views of people with the lived experience of 10 different genetic conditions on gene modification therapies, and specifically on prenatal gene editing. Prenatal gene editing is not happening now, but it is possible that prenatal gene editing will be available in the next few years, at least in a research setting, and we want to know your thoughts about the direction this technology is going. We to hope speak with many different stakeholders (patients and their families, clinicians, scientists) with diverse perspectives to understand values and priorities for prenatal gene ...
This study is being done to learn more about normal memory and aging, mild memory and thinking problems, Alzheimer's disease and other forms of dementia. This study will help us determine how often memory problems occur in people in our community, and help to identify factors that may influence changes in memory and thinking skills.
The main goal of this study is to evaluate whether a certain set of memory and thinking tests that are in English also work in other languages after they are translated. The measures will test your memory, thinking, problem solving, and everyday function abilities.
The purpose of this Longitudinal Early-onset Alzheimer's Disease Study (LEADS) is designed to look at disease progression in individuals with early onset cognitive impairment . Clinical/cognitive, imaging, biomarker, and genetic characteristics will be assessed across three cohorts: (1) early onset Alzheimer's Disease (EOAD) participants, (2) early onset non-Alzheimer's Disease (EO-nonAD) participants,and (3) cognitively normal (CN) control participants.
The purpose of this study is to characterize and study the relationship of the clinical risk factors and predictors of seizures and epilepsy in patients with Early Onset Alzheimer's Disease (EOAD) using a 48-hour CAA-EEG.
The purpose of this study is to identify the association between untreated OSA and chronic insomnia and their association with cognitive decline, increased Alzheimer's Disease (AD) and vascular pathology.
To further investigate biomarkers in CSF as possible predictors for mild cognitive impairment and dementia
The goal of this study is to gain a better understanding of the status of advanced care planning among caregivers of patients with dementia and examine how this differs by race and disease stage.
The purpose of this study is to study the structure and biochemistry of the brain and/or bodily fluid and tissue after death. Comparison of specimens from normal and diseased individuals provide essential clues that lead to a greater understanding of the diseased state which, in turn, will lead to new ideas for therapy.
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
Appointments at Mayo Clinic
Alzheimer's treatments: what's on the horizon.
Despite many promising leads, new treatments for Alzheimer's are slow to emerge.
Current Alzheimer's treatments temporarily improve symptoms of memory loss and problems with thinking and reasoning.
These Alzheimer's treatments boost the performance of chemicals in the brain that carry information from one brain cell to another. They include cholinesterase inhibitors and the medicine memantine (Namenda). However, these treatments don't stop the underlying decline and death of brain cells. As more cells die, Alzheimer's disease continues to progress.
Experts are cautious but hopeful about developing treatments that can stop or delay the progression of Alzheimer's. Experts continue to better understand how the disease changes the brain. This has led to the research of potential Alzheimer's treatments that may affect the disease process.
Future Alzheimer's treatments may include a combination of medicines. This is similar to treatments for many cancers or HIV / AIDS that include more than one medicine.
These are some of the strategies currently being studied.
Taking aim at plaques
Some of the new Alzheimer's treatments target clumps of the protein beta-amyloid, known as plaques, in the brain. Plaques are a characteristic sign of Alzheimer's disease.
Strategies aimed at beta-amyloid include:
Recruiting the immune system. Medicines known as monoclonal antibodies may prevent beta-amyloid from clumping into plaques. They also may remove beta-amyloid plaques that have formed. They do this by helping the body clear them from the brain. These medicines mimic the antibodies your body naturally produces as part of your immune system's response to foreign invaders or vaccines.
In 2023, the U.S. Food and Drug Administration (FDA) approved lecanemab (Leqembi) for people with mild Alzheimer's disease and mild cognitive impairment due to Alzheimer's disease.
A phase 3 clinical trial found that the medicine slowed cognitive decline in people with early Alzheimer's disease. The medicine prevents amyloid plaques in the brain from clumping. The phase 3 trial was the largest so far to study whether clearing clumps of amyloid plaques from the brain can slow the disease.
Lecanemab is given as an IV infusion every two weeks. Your care team likely will watch for side effects and ask you or your caregiver how your body reacts to the drug. Side effects of lecanemab include infusion-related reactions such as fever, flu-like symptoms, nausea, vomiting, dizziness, changes in heart rate and shortness of breath.
Also, people taking lecanemab may have swelling in the brain or may get small bleeds in the brain. Rarely, brain swelling can be serious enough to cause seizures and other symptoms. Also in rare instances, bleeding in the brain can cause death. The FDA recommends getting a brain MRI before starting treatment. It also recommends being monitored with brain MRI s during treatment for symptoms of brain swelling or bleeding.
People who carry a certain form of a gene known as APOE e4 appear to have a higher risk of these serious complications. The FDA recommends being tested for this gene before starting treatment with lecanemab.
If you take a blood thinner or have other risk factors for brain bleeding, talk to your health care professional before taking lecanemab. Blood-thinning medicines may increase the risk of bleeds in the brain.
More research is being done on the potential risks of taking lecanemab. Other research is looking at how effective lecanemab may be for people at risk of Alzheimer's disease, including people who have a first-degree relative, such as a parent or sibling, with the disease.
Another medicine being studied is donanemab. It targets and reduces amyloid plaques and tau proteins. It was found to slow declines in thinking and functioning in people with early Alzheimer's disease.
The monoclonal antibody solanezumab did not show benefits for individuals with preclinical, mild or moderate Alzheimer's disease. Solanezumab did not lower beta-amyloid in the brain, which may be why it wasn't effective.
Preventing destruction. A medicine initially developed as a possible cancer treatment — saracatinib — is now being tested in Alzheimer's disease.
In mice, saracatinib turned off a protein that allowed synapses to start working again. Synapses are the tiny spaces between brain cells through which the cells communicate. The animals in the study experienced a reversal of some memory loss. Human trials for saracatinib as a possible Alzheimer's treatment are now underway.
Production blockers. These therapies may reduce the amount of beta-amyloid formed in the brain. Research has shown that beta-amyloid is produced from a "parent protein" in two steps performed by different enzymes.
Several experimental medicines aim to block the activity of these enzymes. They're known as beta- and gamma-secretase inhibitors. Recent studies showed that the beta-secretase inhibitors did not slow cognitive decline. They also were associated with significant side effects in those with mild or moderate Alzheimer's. This has decreased enthusiasm for the medicines.
Keeping tau from tangling
A vital brain cell transport system collapses when a protein called tau twists into tiny fibers. These fibers are called tangles. They are another common change in the brains of people with Alzheimer's. Researchers are looking at a way to prevent tau from forming tangles.
Tau aggregation inhibitors and tau vaccines are currently being studied in clinical trials.
Reducing inflammation
Alzheimer's causes chronic, low-level brain cell inflammation. Researchers are studying ways to treat the processes that lead to inflammation in Alzheimer's disease. The medicine sargramostim (Leukine) is currently in research. The medicine may stimulate the immune system to protect the brain from harmful proteins.
Researching insulin resistance
Studies are looking into how insulin may affect the brain and brain cell function. Researchers are studying how insulin changes in the brain may be related to Alzheimer's. However, a trial testing of an insulin nasal spray determined that the medicine wasn't effective in slowing the progression of Alzheimer's.
Studying the heart-head connection
Growing evidence suggests that brain health is closely linked to heart and blood vessel health. The risk of developing dementia appears to increase as a result of many conditions that damage the heart or arteries. These include high blood pressure, heart disease, stroke, diabetes and high cholesterol.
A number of studies are exploring how best to build on this connection. Strategies being researched include:
- Current medicines for heart disease risk factors. Researchers are looking into whether blood pressure medicines may benefit people with Alzheimer's. They're also studying whether the medicines may reduce the risk of dementia.
- Medicines aimed at new targets. Other studies are looking more closely at how the connection between heart disease and Alzheimer's works at the molecular level. The goal is to find new potential medicines for Alzheimer's.
- Lifestyle choices. Research suggests that lifestyle choices with known heart benefits may help prevent Alzheimer's disease or delay its onset. Those lifestyle choices include exercising on most days and eating a heart-healthy diet.
Studies during the 1990s suggested that taking hormone replacement therapy during perimenopause and menopause lowered the risk of Alzheimer's disease. But further research has been mixed. Some studies found no cognitive benefit of taking hormone replacement therapy. More research and a better understanding of the relationship between estrogen and cognitive function are needed.
Speeding treatment development
Developing new medicines is a slow process. The pace can be frustrating for people with Alzheimer's and their families who are waiting for new treatment options.
To help speed discovery, the Critical Path for Alzheimer's Disease (CPAD) consortium created a first-of-its-kind partnership to share data from Alzheimer's clinical trials. CPAD 's partners include pharmaceutical companies, nonprofit foundations and government advisers. CPAD was formerly called the Coalition Against Major Diseases.
CPAD also has collaborated with the Clinical Data Interchange Standards Consortium to create data standards. Researchers think that data standards and sharing data from thousands of study participants will speed development of more-effective therapies.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.

Sorry something went wrong with your subscription
Please, try again in a couple of minutes
- Treatments and research. Alzheimer's Association. https://www.alz.org/alzheimers-dementia/research_progress/treatment-horizon. Accessed March 23, 2023.
- Cummings J, et al. Alzheimer's disease drug development pipeline: 2022. Alzheimer's and Dementia. 2022; doi:10.1002/trc2.12295.
- Burns S, et al. Therapeutics of Alzheimer's disease: Recent developments. Antioxidants. 2022; doi:10.3390/antiox11122402.
- Plascencia-Villa G, et al. Lessons from antiamyloid-beta immunotherapies in Alzheimer's disease. Handbook of Clinical Neurology. 2023; doi:10.1016/B978-0-323-85555-6.00019-9.
- Brockmann R, et al. Impacts of FDA approval and Medicare restriction on antiamyloid therapies for Alzheimer's disease: Patient outcomes, healthcare costs and drug development. The Lancet Regional Health. 2023; doi:10.1016/j.lana. 2023.100467 .
- Wojtunik-Kulesza K, et al. Aducanumab — Hope or disappointment for Alzheimer's disease. International Journal of Molecular Sciences. 2023; doi:10.3390/ijms24054367.
- Can Alzheimer's disease be prevented? Alzheimer's Association. http://www.alz.org/research/science/alzheimers_prevention_and_risk.asp. Accessed March 23, 2023.
- Piscopo P, et al. A systematic review on drugs for synaptic plasticity in the treatment of dementia. Ageing Research Reviews. 2022; doi:10.1016/j.arr. 2022.101726 .
- Facile R, et al. Use of Clinical Data Interchange Standards Consortium (CDISC) standards for real-world data: Expert perspectives from a qualitative Delphi survey. JMIR Medical Informatics. 2022; doi:10.2196/30363.
- Imbimbo BP, et al. Role of monomeric amyloid-beta in cognitive performance in Alzheimer's disease: Insights from clinical trials with secretase inhibitors and monoclonal antibodies. Pharmacological Research. 2023; doi:10.1016/j.phrs. 2022.106631 .
- Conti Filho CE, et al. Advances in Alzheimer's disease's pharmacological treatment. Frontiers in Pharmacology. 2023; doi:10.3389/fphar. 2023.1101452 .
- Potter H, et al. Safety and efficacy of sargramostim (GM-CSF) in the treatment of Alzheimer's disease. Alzheimer's and Dementia. 2021; doi:10.1002/trc2.12158.
- Zhong H, et al. Effect of peroxisome proliferator-activated receptor-gamma agonists on cognitive function: A systematic review and meta-analysis. Biomedicines. 2023; doi:10.3390/biomedicines11020246.
- Grodstein F. Estrogen and cognitive function. https://www.uptodate.com/contents/search. Accessed March 23, 2023.
- Mills ZB, et al. Is hormone replacement therapy a risk factor or a therapeutic option for Alzheimer's disease? International Journal of Molecular Sciences. 2023; doi:10.3390/ijms24043205.
- Custodia A, et al. Biomarkers assessing endothelial dysfunction in Alzheimer's disease. Cells. 2023; doi:10.3390/cells12060962.
- Overview. Critical Path for Alzheimer's Disease. https://c-path.org/programs/cpad/. Accessed March 29, 2023.
- Shi M, et al. Impact of anti-amyloid-β monoclonal antibodies on the pathology and clinical profile of Alzheimer's disease: A focus on aducanumab and lecanemab. Frontiers in Aging and Neuroscience. 2022; doi:10.3389/fnagi. 2022.870517 .
- Leqembi (approval letter). Biologic License Application 761269. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761269. Accessed July 7, 2023.
- Van Dyck CH, et al. Lecanemab in early Alzheimer's disease. New England Journal of Medicine. 2023; doi:10.1056/NEJMoa2212948.
- Leqembi (prescribing information). Eisai; 2023. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=761269. Accessed July 10, 2023.
Products and Services
- A Book: Mayo Clinic on Alzheimer's Disease
- Assortment of Products for Independent Living from Mayo Clinic Store
- A Book: Day to Day: Living With Dementia
- A Book: Mayo Clinic on Healthy Aging
- Give today to find Alzheimer's cures for tomorrow
- Alzheimer's sleep problems
- Alzheimer's 101
- Understanding the difference between dementia types
- Alzheimer's disease
- Alzheimer's genes
- Alzheimer's drugs
- Alzheimer's prevention: Does it exist?
- Alzheimer's stages
- Antidepressant withdrawal: Is there such a thing?
- Antidepressants and alcohol: What's the concern?
- Antidepressants and weight gain: What causes it?
- Antidepressants: Can they stop working?
- Antidepressants: Side effects
- Antidepressants: Selecting one that's right for you
- Antidepressants: Which cause the fewest sexual side effects?
- Anxiety disorders
- Atypical antidepressants
- Caregiver stress
- Clinical depression: What does that mean?
- Corticobasal degeneration (corticobasal syndrome)
- Depression and anxiety: Can I have both?
- Depression, anxiety and exercise
- What is depression? A Mayo Clinic expert explains.
- Depression in women: Understanding the gender gap
- Depression (major depressive disorder)
- Depression: Supporting a family member or friend
- Diagnosing Alzheimer's
- Did the definition of Alzheimer's disease change?
- How your brain works
- Intermittent fasting
- Lecanemab for Alzheimer's disease
- Male depression: Understanding the issues
- MAOIs and diet: Is it necessary to restrict tyramine?
- Marijuana and depression
- Mayo Clinic Minute: 3 tips to reduce your risk of Alzheimer's disease
- Mayo Clinic Minute: Alzheimer's disease risk and lifestyle
- Mayo Clinic Minute: New definition of Alzheimer's changes
- Mayo Clinic Minute: Women and Alzheimer's Disease
- Memory loss: When to seek help
- Monoamine oxidase inhibitors (MAOIs)
- Natural remedies for depression: Are they effective?
- Nervous breakdown: What does it mean?
- New Alzheimers Research
- Pain and depression: Is there a link?
- Phantosmia: What causes olfactory hallucinations?
- Positron emission tomography scan
- Posterior cortical atrophy
- Seeing inside the heart with MRI
- Selective serotonin reuptake inhibitors (SSRIs)
- Serotonin and norepinephrine reuptake inhibitors (SNRIs)
- Sundowning: Late-day confusion
- Treatment-resistant depression
- Tricyclic antidepressants and tetracyclic antidepressants
- Video: Alzheimer's drug shows early promise
- Vitamin B-12 and depression
- Young-onset Alzheimer's
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
- Alzheimer s treatments What s on the horizon
Make twice the impact
Your gift can go twice as far to advance cancer research and care!
Call our 24 hours, seven days a week helpline at 800.272.3900

- Professionals

- Younger/Early-Onset Alzheimer's
- Is Alzheimer's Genetic?
- Women and Alzheimer's
- Creutzfeldt-Jakob Disease
- Dementia with Lewy Bodies
- Down Syndrome & Alzheimer's
- Frontotemporal Dementia
- Huntington's Disease
- Mixed Dementia
- Normal Pressure Hydrocephalus
- Posterior Cortical Atrophy
- Parkinson's Disease Dementia
- Vascular Dementia
- Korsakoff Syndrome
- Traumatic Brain Injury (TBI)
- Know the 10 Signs
- Difference Between Alzheimer's & Dementia
- 10 Steps to Approach Memory Concerns in Others
- Medical Tests for Diagnosing Alzheimer's
- Why Get Checked?
- Visiting Your Doctor
- Life After Diagnosis
- Stages of Alzheimer's
- Earlier Diagnosis
- Part the Cloud
- Research Momentum
- Our Commitment to Research
- TrialMatch: Find a Clinical Trial
- What Are Clinical Trials?
- How Clinical Trials Work
- When Clinical Trials End
- Why Participate?
- Talk to Your Doctor
- Clinical Trials: Myths vs. Facts
- Can Alzheimer's Disease Be Prevented?
- Brain Donation
- Navigating Treatment Options
- Lecanemab Approved for Treatment of Early Alzheimer's Disease
- Aducanumab Discontinued as Alzheimer's Treatment
- Medicare Treatment Coverage
- Donanemab for Treatment of Early Alzheimer's Disease — News Pending FDA Review
- Questions for Your Doctor
- Medications for Memory, Cognition and Dementia-Related Behaviors
- Treatments for Behavior
- Treatments for Sleep Changes
- Alternative Treatments
- Facts and Figures
- Assessing Symptoms and Seeking Help
- Now is the Best Time to Talk about Alzheimer's Together
- Get Educated
- Just Diagnosed
- Sharing Your Diagnosis
- Changes in Relationships
- If You Live Alone
Treatments and Research
- Legal Planning
- Financial Planning
- Building a Care Team
- End-of-Life Planning
- Programs and Support
- Overcoming Stigma
- Younger-Onset Alzheimer's
- Taking Care of Yourself
- Reducing Stress
- Tips for Daily Life
- Helping Family and Friends
- Leaving Your Legacy
- Live Well Online Resources
- Make a Difference
- Daily Care Plan
- Communication and Alzheimer's
- Food and Eating
- Art and Music
- Incontinence
- Dressing and Grooming
- Dental Care
- Working With the Doctor
- Medication Safety
- Accepting the Diagnosis
- Early-Stage Caregiving
- Middle-Stage Caregiving
- Late-Stage Caregiving
- Aggression and Anger
- Anxiety and Agitation
- Hallucinations
- Memory Loss and Confusion
- Sleep Issues and Sundowning
- Suspicions and Delusions
- In-Home Care
- Adult Day Centers
- Long-Term Care
- Respite Care
- Hospice Care
- Choosing Care Providers
- Finding a Memory Care-Certified Nursing Home or Assisted Living Community
- Changing Care Providers
- Working with Care Providers
- Creating Your Care Team
- Long-Distance Caregiving
- Community Resource Finder
- Be a Healthy Caregiver
- Caregiver Stress
- Caregiver Stress Check
- Caregiver Depression
- Changes to Your Relationship
- Grief and Loss as Alzheimer's Progresses
- Home Safety
- Dementia and Driving
- Technology 101
- Preparing for Emergencies
- Managing Money Online Program
- Planning for Care Costs
- Paying for Care
- Health Care Appeals for People with Alzheimer's and Other Dementias
- Social Security Disability
- Medicare Part D Benefits
- Tax Deductions and Credits
- Planning Ahead for Legal Matters
- Legal Documents
- ALZ Talks Virtual Events
- ALZNavigator™
- Veterans and Dementia
- The Knight Family Dementia Care Coordination Initiative
- Online Tools
- Asian Americans and Pacific Islanders and Alzheimer's
- Native Americans and Alzheimer's
- Black Americans and Alzheimer's
- Hispanic Americans and Alzheimer's
- LGBTQ+ Community Resources for Dementia
- Educational Programs and Dementia Care Resources
- Brain Facts
- 50 Activities
- For Parents and Teachers
- Resolving Family Conflicts
- Holiday Gift Guide for Caregivers and People Living with Dementia
- Trajectory Report
- Resource Lists
- Search Databases
- Publications
- Favorite Links
- 10 Healthy Habits for Your Brain
- Stay Physically Active
- Adopt a Healthy Diet
- Stay Mentally and Socially Active
- Online Community
- Support Groups
- Find Your Local Chapter
- Any Given Moment
- New IDEAS Study
- Bruce T. Lamb, Ph.D., Chair
- Christopher van Dyck, M.D.
- Cynthia Lemere, Ph.D.
- David Knopman, M.D.
- Lee A. Jennings, M.D. MSHS
- Karen Bell, M.D.
- Lea Grinberg, M.D., Ph.D.
- Malú Tansey, Ph.D.
- Mary Sano, Ph.D.
- Oscar Lopez, M.D.
- Suzanne Craft, Ph.D.
- RFI Amyloid PET Depletion Following Treatment
- About Our Grants
- Andrew Kiselica, Ph.D., ABPP-CN
- Arjun Masurkar, M.D., Ph.D.
- Benjamin Combs, Ph.D.
- Charles DeCarli, M.D.
- Damian Holsinger, Ph.D.
- David Soleimani-Meigooni, Ph.D.
- Donna M. Wilcock, Ph.D.
- Elizabeth Head, M.A, Ph.D.
- Fan Fan, M.D.
- Fayron Epps, Ph.D., R.N.
- Ganesh Babulal, Ph.D., OTD
- Hui Zheng, Ph.D.
- Jason D. Flatt, Ph.D., MPH
- Jennifer Manly, Ph.D.
- Joanna Jankowsky, Ph.D.
- Luis Medina, Ph.D.
- Marcello D’Amelio, Ph.D.
- Marcia N. Gordon, Ph.D.
- Margaret Pericak-Vance, Ph.D.
- María Llorens-Martín, Ph.D.
- Nancy Hodgson, Ph.D.
- Shana D. Stites, Psy.D., M.A., M.S.
- Walter Swardfager, Ph.D.
- ALZ WW-FNFP Grant
- Capacity Building in International Dementia Research Program
- ISTAART IGPCC
- Alzheimer’s Disease Strategic Fund: Endolysosomal Activity in Alzheimer’s (E2A) Grant Program
- Imaging Research in Alzheimer’s and Other Neurodegenerative Diseases
- Zenith Fellow Awards
- National Academy of Neuropsychology & Alzheimer’s Association Funding Opportunity
- Part the Cloud-Gates Partnership Grant Program: Bioenergetics and Inflammation
- Pilot Awards for Global Brain Health Leaders (Invitation Only)
- Robert W. Katzman, M.D., Clinical Research Training Scholarship
- Funded Studies
- How to Apply
- Portfolio Summaries
- Supporting Research in Health Disparities, Policy and Ethics in Alzheimer’s Disease and Dementia Research (HPE-ADRD)
- Diagnostic Criteria & Guidelines
- Annual Conference: AAIC
- Professional Society: ISTAART
- Alzheimer's & Dementia
- Alzheimer's & Dementia: DADM
- Alzheimer's & Dementia: TRCI
- International Network to Study SARS-CoV-2 Impact on Behavior and Cognition
- Alzheimer’s Association Business Consortium (AABC)
- Global Biomarker Standardization Consortium (GBSC)
- Global Alzheimer’s Association Interactive Network
- International Alzheimer's Disease Research Portfolio
- Alzheimer’s Disease Neuroimaging Initiative Private Partner Scientific Board (ADNI-PPSB)
- Research Roundtable
- About WW-ADNI
- North American ADNI
- European ADNI
- Australia ADNI
- Taiwan ADNI
- Argentina ADNI
- WW-ADNI Meetings
- Submit Study
- RFI Inclusive Language Guide
- Scientific Conferences
- AUC for Amyloid and Tau PET Imaging
- Make a Donation
- Walk to End Alzheimer's
- The Longest Day
- RivALZ to End ALZ
- Ride to End ALZ
- Tribute Pages
- Gift Options to Meet Your Goals
- Founders Society
- Fred Bernhardt
- Anjanette Kichline
- Lori A. Jacobson
- Pam and Bill Russell
- Gina Adelman
- Franz and Christa Boetsch
- Adrienne Edelstein
- For Professional Advisors
- Free Planning Guides
- Contact the Planned Giving Staff
- Workplace Giving
- Do Good to End ALZ
- Donate a Vehicle
- Donate Stock
- Donate Cryptocurrency
- Donate Gold & Sterling Silver
- Donor-Advised Funds
- Use of Funds
- Giving Societies
- Why We Advocate
- Ambassador Program
- About the Alzheimer’s Impact Movement
- Research Funding
- Improving Care
- Support for People Living With Dementia
- Public Policy Victories
- Planned Giving
- Community Educator
- Community Representative
- Support Group Facilitator or Mentor
- Faith Outreach Representative
- Early Stage Social Engagement Leaders
- Data Entry Volunteer
- Tech Support Volunteer
- Other Local Opportunities
- Visit the Program Volunteer Community to Learn More
- Become a Corporate Partner
- A Family Affair
- A Message from Elizabeth
- The Belin Family
- The Eliashar Family
- The Fremont Family
- The Freund Family
- Jeff and Randi Gillman
- Harold Matzner
- The Mendelson Family
- Patty and Arthur Newman
- The Ozer Family
- Salon Series
- No Shave November
- Other Philanthropic Activities
- Still Alice
- The Judy Fund E-blast Archive
- The Judy Fund in the News
- The Judy Fund Newsletter Archives
- Sigma Kappa Foundation
- Alpha Delta Kappa
- Parrot Heads in Paradise
- Tau Kappa Epsilon (TKE)
- Sigma Alpha Mu
- Alois Society Member Levels and Benefits
- Alois Society Member Resources
- Zenith Society
- Founder's Society
- Joel Berman
- JR and Emily Paterakis
- Legal Industry Leadership Council
- Accounting Industry Leadership Council
Find Local Resources
Let us connect you to professionals and support options near you. Please select an option below:
Use Current Location Use Map Selector
Search Alzheimer’s Association
- I Have Alzheimer's Homepage
- Younger-Onset Alzheimer's

FDA-approved drugs for Alzheimer's
- Alternative treatments and supplements
Research into future treatments
Participate in clinical trials.
The U.S. Food and Drug Administration (FDA) has approved medications that fall into two categories: drugs that change disease progression in people living with Alzheimer’s, and drugs that may temporarily mitigate some symptoms of the disease. Treatments may be available in different forms (pill, patch or other). When considering any treatment, it is important to have a conversation with a health care professional to determine whether it is appropriate. A physician who is experienced in using these types of medications should monitor people who are taking them and ensure that the recommended guidelines are strictly observed.
Drugs that change disease progression
Drugs in this category slow disease progression by going after the underlying biology of the disease process. They aim to slow the decline of memory and thinking, as well as function, in people living with Alzheimer's disease.
Amyloid-targeting approaches
Anti-amyloid treatments work by attaching to and removing beta-amyloid, a protein that accumulates into plaques, from the brain. Each works differently and targets beta-amyloid at a different stage of plaque formation. These treatments change the course of the disease in a meaningful way for people in the early stages, giving them more time to participate in daily life and live independently. Clinical trial participants who received anti-amyloid treatments experienced reduction in cognitive decline observed through measures of cognition and function. Examples of cognition measures include:
- Orientation.
Examples of functional measures include:
- Conducting personal finances.
- Performing household chores such as cleaning.
Anti-amyloid treatments do have side effects. These treatments can cause serious allergic reactions. Side effects can also include amyloid-related imaging abnormalities (ARIA), infusion-related reactions, headaches and falls. ARIA is a common side effect that does not usually cause symptoms but can be serious. It is typically a temporary swelling in areas of the brain that usually resolves over time. Some people may also have small spots of bleeding in or on the surface of the brain with the swelling, although most people with swelling in areas of the brain do not have symptoms. Some may have symptoms of ARIA such as headache, dizziness, nausea, confusion and vision changes. Some people have a genetic risk factor (ApoE ε4 gene carriers) that may cause an increased risk for ARIA. The FDA encourages that testing for ApoE ε4 status should be performed prior to initiation of treatment to inform the risk of developing ARIA. Prior to testing, doctors should discuss with patients the risk of ARIA and the implications of genetic testing results. These are not all the possible side effects, and individuals should talk with their doctors to develop a treatment plan that is right for them, including weighing the benefits and risks of all approved therapies. Aducanumab (Aduhelm®) Aducanumab (Aduhelm ) is an anti-amyloid antibody intravenous (IV) infusion therapy that is delivered every month. It has received accelerated approval from the FDA to treat early Alzheimer's disease, including people living with mild cognitive impairment (MCI) or mild dementia due to Alzheimer's disease who have confirmation of elevated beta-amyloid in the brain. Aducanumab was the first therapy to demonstrate that removing beta-amyloid from the brain reduces cognitive and functional decline in people living with early Alzheimer's. Aducanumab is being discontinued by its manufacturer, Biogen. The company stated that people who are now receiving the drug as part of a clinical trial will continue to have access to it until May 1, 2024, and that people who are now receiving it by prescription will have it available to them until Nov. 1, 2024. Lecanemab (Leqembi®) Lecanemab (Leqembi) is an anti-amyloid antibody intravenous (IV) infusion therapy that is delivered every two weeks. It has received traditional approval from the FDA to treat early Alzheimer's disease, including people living with mild cognitive impairment (MCI) or mild dementia due to Alzheimer's disease who have confirmation of elevated beta-amyloid in the brain. There is no safety or effectiveness data on initiating treatment at earlier or later stages of the disease than were studied. Lecanemab was the second therapy to demonstrate that removing beta-amyloid from the brain reduces cognitive and functional decline in people living with early Alzheimer's. Learn more: Medications for Memory
*To be discontinued on Nov. 1, 2024. Please connect with your provider on treatment options.
Drugs that treat symptoms
Cognitive (memory and thinking) symptoms These medications are prescribed to treat symptoms related to memory and thinking. While these drugs cannot stop the damage Alzheimer’s causes to brain cells, they may help lessen or stabilize symptoms for a limited time by affecting certain chemicals involved in carrying messages between the brain's nerve cells. The drugs currently approved to treat cognitive symptoms are cholinesterase inhibitors and glutamate regulators. Learn more: Medications for Memory
Tips from people living with Alzheimer's: Medication safety
- Keep a calendar and check off each dose as it's taken.
- Set up a pill box each night for use the next day.
- Set the alarm on your cell phone or schedule dosing around meal times.
- See More Tips
Alternative treatments and supplements
Research suggests that lifestyle habits, such as eating a healthy diet, may reduce a person’s risk for cognitive decline and dementia. However, there isn’t a single food, ingredient or supplement that has been shown to prevent, treat or cure Alzheimer’s or other dementias. There are remedies, supplements and “medical foods” that are often referred to as alternative treatments. Alternative treatments are not regulated and do not need to adhere to the same standards as FDA-approved treatments. Claims about their safety and effectiveness are based largely on testimonials, tradition or a small body of scientific research. If you are considering taking an alternative treatment or dietary supplement, it’s important to talk to your physician. He or she can provide you with the best possible advice for your situation and make you aware of any risks. Even if advertised as “natural,” alternative treatments can involve potentially powerful substances that have not met FDA standards for effectiveness or safety, and some alternative medicines can cause unintended reactions when taken with prescription medications. Learn more: Alternative Therapies Here is a list of questions to ask when considering an alternative treatment or supplement: Has the FDA approved this product for the treatment of Alzheimer’s or dementia symptoms? The FDA may have reviewed the data on a product, but found it to be ineffective for the intended purpose. In this instance, the company may still release the product as a medical food, either with or without changes. In the United States, a product can only be considered a medical food if it is designed to treat a condition that has a “distinctive nutritional requirement.” According to the FDA, Alzheimer’s, as currently understood, does not have distinctive nutritional requirements, and therefore, in the United States, no product can legitimately be described as a medical food for Alzheimer’s. Is there independent research to support the safety and effectiveness of this product for treating Alzheimer's or other dementia? If the testing entity has a vested interest in the outcome (e.g., testing done by the company developing the product), the results may not be reliable. To best serve individuals living with Alzheimer’s and their families, the Alzheimer’s Association strongly encourages makers of products that claim to be beneficial for those with Alzheimer’s or other dementia to conduct definitive clinical trials. Does the developer of the product or the person recommending it to you have a potential financial gain from the use of the medication? If so, use extreme caution. Check with your care team to see if they have any questions or concerns with your plan to use it. Does the FDA oversee how dietary supplements are manufactured? No. It is up to each manufacturer and distributer of dietary supplements to meet all safety and labeling requirements of the Dietary Supplement Health and Education Act of 1994 (DSHEA) and the FDA. Most in the industry act responsibly, but some adulterated or misbranded products have made it to market. Therefore, people with Alzheimer’s and their families have no absolute guarantee that supplements contain the ingredients listed on the label in the specified amounts. Is the product compatible with the other medications you are taking or with your diagnoses? Be sure to check with your doctor or pharmacist to find out whether the product could cause negative outcomes given your diagnoses and any FDA-approved medications you are taking. The lack of rigorous research for these products means little (or nothing) is known about the effects, both when taken alone or in combination with approved drugs. We often don’t know whether the products will interact with, and possibly decrease, the effectiveness of approved drugs taken for Alzheimer’s and other dementia.
Researchers are conducting studies to find new interventions and treatments for Alzheimer’s. Because the disease is complex and not fully understood — with a multitude of factors that may contribute to risk — today’s research focuses on several areas of study. Many drugs and medical devices in development aim to interrupt the disease process by impacting one or more of the brain changes associated with Alzheimer’s. These changes offer potential "targets" for new drugs or devices to slow or stop the progress of the disease. These promising targets include the buildup of beta-amyloid and tau protein (hallmarks of Alzheimer's), neuroinflammation, immune response, metabolic changes and more. Researchers believe that future treatments will involve a combination of medications or devices aimed at several targets, along with risk reduction strategies similar to current treatments for many cancers and AIDS. As the leading nonprofit funder of Alzheimer’s research, the Alzheimer’s Association has played a vital role in every significant development in dementia science. Learn more: Research and Progress , Part the Cloud
Recruiting and retaining clinical trial participants is now the greatest obstacle, other than funding, to developing the next generation of Alzheimer's treatments. Individuals with dementia, caregivers and healthy volunteers are all needed to participate in clinical studies focused on Alzheimer's and all other dementia. If you are interested in participating in a current clinical study, Alzheimer's Association TrialMatch® is a free, easy-to-use clinical studies matching service that generates customized lists of studies based on user-provided information. The TrialMatch database includes:
- Trials for new drugs or non-drug-based dementia treatments.
- Studies on new tests or procedures for diagnosis.
- Trials that investigate ways to prevent the onset of diseases.
- Studies exploring ways to improve quality of life for individuals living with a chronic illness, their caregivers and family members.
- Online studies.
Learn more: Clinicial Trials and Alzheimer's Association TrialMatch®
Help Advance Research
At the heart of all medical advances are clinical trials. You can participate in clinical research, helping to find new treatments, and, ultimately, a cure.
.teaseBox.overlay::after { content: ''; display: block; background: rgba(255, 255, 255, 0); top: 0; left: 0; right: 0; bottom: 0; position: absolute; z-index: 0; } Connect with others in our online community.
.teasebox.overlay::after { content: ''; display: block; background: rgba(255, 255, 255, 0); top: 0; left: 0; right: 0; bottom: 0; position: absolute; z-index: 0; } #p_lt_webpartzone2_content_pageplaceholder_p_lt_webpartzone4_widgets_tripleteaserrow__rptteaser_ctl03__divteasebox::before { width: 0; } call our 24/7 helpline anytime: 800.272.3900., volunteer for a clinical trial through trialmatch. .
Find a Trial

Why We Need Alzheimer’s Research and What to Do About It
We need to work to increase the funding for research on alzheimer’s disease..
Posted October 22, 2020
The projected numbers of individuals in this country with Alzheimer’s disease and related disorders is alarming. We are facing a tsunami of dementia . Today, there are approximately 5.8 million Americans living with Alzheimer’s disease—1 in 10 people aged 65 and older. However, in part due to the increased numbers of older adults in our society (Figure 1), that number rises to 13.8 million by 2050 (Figure 2).
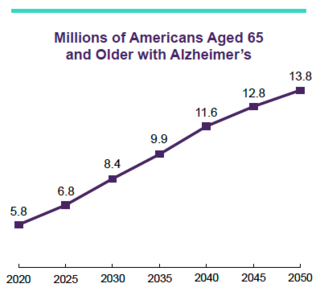
We desperately need to find disease-modifying treatments that can slow down and eventually cure this terrible disease. By “disease-modifying,” I mean treatments that can affect the underlying disease process—the plaques and tangles that build up in the brain and destroy brain cells in Alzheimer’s disease. These treatments have been the major research goal for the past 20 years, which is appropriate, as they will provide the first steps toward a cure.
Potential disease-modifying treatments that are currently under investigation in clinical trials include drugs that use monoclonal antibodies grown in a laboratory that are given intravenously (by vein) to the person monthly. Antibodies are normally what the body uses to fight infections, but these antibodies are designed to stick to either the beta-amyloid plaques or the tau neurofibrillary tangles. Once these antibodies are in place, the body’s immune system will see the antibody and remove the plaques or tangles.
But disease-modifying therapies are not enough. Because any treatment that we can envision today will only slow down the disease—not cure it—we are likely to see individuals living longer with Alzheimer’s disease. For this reason, we also desperately need pharmacologic and non-pharmacologic therapies that can help individuals with memory problems perform better in daily life, keeping them independent and out of nursing homes and other institutions.
And because we need to keep individuals living with Alzheimer’s disease and related dementias living at home longer, we need to support those who support them, that is, the caregivers. We need more research on how to help family members be more effective caregivers so that they do not burn out , and so that they can continue to manage their other responsibilities, whether that means their own families or the work they do outside the home.
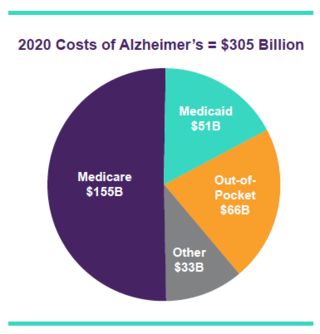
Some would argue that we cannot afford to pursue Alzheimer’s research. I would say we cannot afford not to pursue Alzheimer’s research. Figure 3 shows that, right now, caring for individuals with Alzheimer’s disease costs society approximately $305 billion dollars—that billion, with a B. If there is a proportional increase in cost, that means that in 2050 caring for individuals with Alzheimer’s disease will cost more than $725 billion—and the Alzheimer’s Association is predicting $1.1 trillion. It will bankrupt the country.
Currently, the National Institutes of Health (NIH) is expected to spend approximately $2.8 billion dollars on Alzheimer’s disease in 2020. This is a large amount, but still less than 1% of the amount that we, as a society, are spending caring for individuals with this disease each year!
We need to work to increase the funding for research on Alzheimer’s disease. There are many promising grants submitted to NIH every year that are not funded—not because they are not good, but simply because there are not enough funds. Currently, fewer than one in four grants are funded, with the overall NIH funding rate being 18%.
The bottom line is that we need a president who will increase funding of NIH, not cut it. We need a president who understands the economic and human costs of terrible diseases like Alzheimer’s disease.
© Andrew E. Budson, MD, 2020, all rights reserved.
https://www.alz.org/aaic/downloads2020/2020_Facts_and_Figures_Fact_Shee…
https://www.census.gov/library/visualizations/2018/comm/century-of-chan…
https://www.alz.org/get-involved-now/advocate/research-funding
https://en.wikipedia.org/wiki/NIH_grant

Andrew Budson, M.D. , is a professor of neurology at Boston University, as well as a lecturer in neurology at Harvard Medical School.
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- United States
- Brooklyn, NY
- Chicago, IL
- Houston, TX
- Los Angeles, CA
- New York, NY
- Portland, OR
- San Diego, CA
- San Francisco, CA
- Seattle, WA
- Washington, DC
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Therapy Center NEW
- Diagnosis Dictionary
- Types of Therapy

Understanding what emotional intelligence looks like and the steps needed to improve it could light a path to a more emotionally adept world.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience
Alzheimer’s Research Aimed at Prevention

August 27, 2014
What research is being done to try to prevent Alzheimer’s disease?
The research focus now is turning to prevention trials, and a number of studies are underway to test the effectiveness of various therapies in people without symptoms or who have only slight memory problems. Under scrutiny in these studies are further examination of estrogen and studies of various classes of anti-inflammatory drugs and antioxidants.
The first large-scale Alzheimer’s disease prevention trial, sponsored by the NIH, is comparing the effects of vitamin E, an antioxidant, and one of the drugs currently approved for Alzheimer’s, donepezil (brand name Aricept), in preventing the development of the disease in people diagnosed with mild cognitive impairment, or MCI, a population at high risk. Ongoing trials are also examining the effectiveness of naproxen and celecoxib (anti-inflammatory drugs) in reducing Alzheimer’s risk in persons with a family history of dementia, the effect of estrogen replacement therapy in preventing Alzheimer’s in women with a family history of the disease, and whether treatment with a variety of agents, such as aspirin, vitamin E, antioxidants, or combined folate/B6/B12 supplementation can prevent older women from developing age-related memory impairment or Alzheimer’s.
As scientists test these currently available medications, the next generation of drugs is being developed, targeting specific abnormal cellular pathways uncovered by recent discoveries, including plaque and tangle formation and death of brain cells. Prevention trials are among the most costly of research projects, but, if successful, the payoff in terms of reduced disease and disability will be significant.
Click here to watch other useful videos on Alzheimer’s Disease
What is the NIA Alzheimer’s Disease Prevention Initiative?
The Alzheimer’s Prevention Initiative is an intensive, coordinated effort spearheaded by the National Institute on Aging (NIA) to accelerate basic research and help translate basic research findings into the development of novel compounds that delay or slow the progress of Alzheimer’s or to prevent it entirely. Potentially promising strategies are being identified, based on new information about the initial stages and events in the brain that lead to Alzheimer’s, as well as data from studies of genetic and environmental risk factors. To follow up on these leads, NIA has implemented a five-year research initiative to speed the development of vaccine and other novel approaches for preventing AD. Another research initiative will examine changes in immune function with age, including the response to different vaccination protocols. Along with the prevention initiative, other studies will continue to look at the many similarities in the biological mechanisms underlying neurodegenerative diseases such as Alzheimer’s, Parkinson’s disease, and other dementias, and will help to characterize age-related change in the normal, healthy brain.
Recent Alzheimer’s Disease Prevention Articles:
Certain Foods May Protect Against Alzheimer’s Disease
Can certain foods help ward off the onset of Alzheimer’s disease? A growing body of research suggests that they might…
Some Blood Pressure Drugs May Help Protect Against Alzheimer’s
Certain medications commonly used to treat high blood pressure may help protect against Alzheimer’s disease…
Regular Exercise and Resistance Training Are Good for the Brain
Ongoing physical activity has been linked to a longer life and all kinds of benefits for the body, including less heart disease, fewer falls and broken bones, greater lung function and a trimmer physique…
Does estrogen replacement therapy prevent Alzheimer’s?
A few large studies have investigated whether taking estrogen replacement therapy may help slow the progression of Alzheimer’s in women with the disease.
So far, the largest and best-designed studies have found that estrogen does NOT have a beneficial effect on women who already have Alzheimer’s. However, these studies do not answer the question of whether normally aging women who take estrogen after menopause will be protected from developing Alzheimer’s or age-related cognitive decline.
Two gold-standard studies published in May 2003, part of the ongoing Women’s Health Initiative Memory Study, found that the widely popular duo of estrogen and progestin (Prempro) did nothing to ward off Alzheimer’s when the hormones were begun in women starting at age 65 and older. In one of the reports, the risk of dementia actually slightly increased in women who took the hormones.
The findings also showed that combination hormone replacement did not protect against a milder form of memory loss called mild cognitive impairment (MCI). MCI is characterized by memory lapses that can accompany aging and, in some cases, eventually lead to Alzheimer’s.
These findings are only one additional component to be weighed when women are deciding whether to start or continue on hormone replacement therapy. The study, for example, did not look at younger women who were taking these drugs, some of whom may only take it for a year or two.
It is also important to note that the possibility remains that estrogen-only replacement might afford protection against Alzheimer’s, especially if administered at or around the onset of menopause rather than many years later. However, unopposed estrogen replacement is only available for women who have had hysterectomies. If these women are indeed found to have a diminished risk of Alzheimer’s, that will fuel the search for brain-specific estrogen-like molecules that can protect the brain but spare the side effects on the reproductive tissues.
Additional study on possible preventive measures during normal aging is now under way. One multi-site clinical trial, which is supported by the National Institute on Aging, seeks to determine whether the use of estrogen in cognitively normal older women with a family history of Alzheimer’s (and who therefore have two to three times the risk of developing the disease) may prevent its development.
Can anti-inflammatory drugs prevent Alzheimer’s disease?
One of the hallmarks of Alzheimer’s disease is inflammation in the brain, but whether it is a cause or an effect of the disease is not yet known. Epidemiologic evidence strongly suggests that anti-inflammatory agents, such as prednisone (a steroid) and the popular pain relievers known as non-steroidal anti-inflammatory drugs (NSAIDs), including ibuprofen and indomethacin are associated with a decreased risk of Alzheimer’s. Studies in animal models of Alzheimer’s suggest that an NSAID can limit plaque production in the mouse brain.
However, results of a study that compared the effects of prednisone versus a placebo (inactive pill) on people who had been diagnosed with Alzheimer’s found no difference in cognitive decline between the prednisone and placebo treatment groups. Thus, a low-dose regimen of prednisone does not seem to be useful in treating Alzheimer’s disease.
A report in the Journal of the American Medical Association found that two popular NSAIDs, naproxen (Aleve) and the prescription arthritis painkiller rofecoxib (Vioxx), did nothing to slow the progression of Alzheimer’s disease in people with mild to moderate decline. However, previous studies suggest that NSAIDs may help to prevent Alzhiemer’s disease.
There is evidence that these or related drugs can reduce the risk of developing the illness in the first place if given to people before the onset of symptoms. A large study that followed nearly 7,000 patients for an average of 6.8 years found that people who did not use NSAIDs had a nearly five times greater risk of developing the disease than those who used NSAIDs long-term (24 months or more of cumulative use). People who used NSAIDs for more than one but less than 24 months also had a decreased risk of developing Alzheimer’s. The risk did not vary according to age. These data provide perhaps the most convincing evidence to date that NSAIDs may be useful in the prevention of Alzheimer’s disease.
However, a large government study designed to test whether the anti-inflammatory drugs naproxen (an NSAID sold as Aleve) or Celebrex (a pain reliever related to Vioxx and known as a COX-2 inhibitor) was halted after researchers noted that these drugs may cause an increased risk of heart attacks and strokes. Researchers had long known that NSAIDs are associated with gastrointestinal problems, including bleeding ulcers and with kidney problems, but the heart complications present an additional potential danger. These drugs must be used with caution, and only under a doctor’s supervision.
More research is needed on the safety of the various anti-inflammatory drugs and their possible benefits for treating or preventing Alzheimer’s disease. It is possible that those with Alzheimer’s who take different anti-inflammatories or different doses might show benefits. More studies of NSAIDs are under way. Drugs that work against toxic amyloid, the substance that contributes to plaque buildup and that is thought to be key to Alzheimer’s, are also under investigation.
Use of Non-Steroidal Anti-Inflammatory Drugs Suspended in Large Alzheimer’s Disease Prevention Trial. National Institutes of Health, www.nih.gov
Can taking cholesterol-lowering drugs prevent Alzheimer’s disease?
Scientific evidence is growing for a link between the use of a class of cholesterol-lowering drugs called statins and reduced risk of Alzheimer’s disease. Statins, which are prescribed to millions of Americans for lowering blood levels of low-density lipoprotein (LDL) cholesterol – the “bad” cholesterol – have been the subject of intense focus in Alzheimer’s research ever since a series of epidemiologic studies found that people who took them have a lower incidence of Alzheimer’s.
Here’s what some of the latest studies have found:
- Researchers at Boston University School of Medicine found that people taking statins reduced their risk of developing Alzheimer’s by 29 percent. The study, the largest to date on this subject, tracked more than 2,300 participants and included African-Americans. Alzheimer’s incidence was reduced equally in both whites and African-Americans.
- A team at St. George’s Hospital Medical School in London found that using statins to reduce cholesterol levels dramatically lowered the production of beta amyloid in laboratory experiments. Beta amyloid is a protein that abnormally accumulates in the brain in Alzheimer’s disease. The group has also shown that raising cholesterol levels increases beta amyloid production. A Tokyo-based research group found similar results, and identified a specific pathway through which statins may exert their effect on beta amyloid.
- Scientists at Georgetown University found that high cholesterol levels increase the rate at which beta amyloid breaks off from its “parent” protein and accumulates into the plaques found in Alzheimer’s disease. They also showed that high cholesterol increases the production of another protein, called APOE, which contributes to nerve cell toxicity when overproduced.
Other scientists are looking at the biochemical effects statins have within the brain. A team at the University of South Florida Health Sciences Center discovered that statins were able to block the toxic effects beta amyloid has on nerve cells, and seemed to act through an anti-inflammatory action. Inflammatory processes are thought to contribute to Alzheimer’s in ways that are not completely understood.
A large, prospective clinical trial is currently underway to try to determine conclusively if statins can prevent or delay the onset of Alzheimer’s disease.
Related Links
- Feeling Like Your Life Has Purpose May Protect Against Alzheimer’s
- Doing Crossword Puzzles May Help Delay Alzheimer’s Onset
- Lack of Sleep May Increase Risk of Alzheimer’s
- The Clinical Stages of Alzheimer’s disease
- Find Alzheimer’s resources near you
- Donate to Research

Alzheimer's Articles
Add impact to your inbox.
" * " indicates required fields
Preserving Your Memory ® Magazine

- Name * First Last

WE CAN END ALZHEIMER'S
We support the pioneering research of the late Nobel Laureate Dr. Paul Greengard under the leadership of Dr. Nathaniel Heintz and his team as they continue pursuing the quest for a cure.

Connect with Us
Mailing Address: The Fisher Center for Alzheimer's Research Foundation FDR Station, PO Box 220 New York, NY 10150
1-800-ALZINFO (259-4636) Phone: 1-212-915-1328 Email: [email protected]
About Fisher
Information program, 7 clinical stages of alzheimer’s brochure.

At the Australian Alzheimer’s Research Foundation, we currently support research in four areas of Alzheimer’s disease:
- understanding the pathology of the disease
- developing treatments
- identifying factors to defer or prevent the onset of the disease
- discovering an early diagnosis
Our current research utilises memory tests, medical and neuropsychological assessments; brain imaging; and highly specialised blood tests to help find an early diagnosis. We are also developing lifestyle interventions to delay or prevent the onset of symptoms, and are working to develop better treatments for those already diagnosed.
As part of our research into a diagnosis, we undertake the latest, highly specialised brain imaging, known as PET amyloid imaging. This allows detection of the accumulation of beta amyloid in the living brain.
While beta amyloid is a diagnostic marker, it is not sufficiently reliable on its own. We are therefore collaborating with researchers in Melbourne, the USA, Germany and other parts of the world to develop a suite of bio-markers and an accurate and reliable diagnostic tool for Alzheimer’s disease.
Another major study relates to the preventative value of specific nutritional supplements for Alzheimer’s disease. This study will focus on the potential of antioxidants and poly phenols to defer the onset of the disease.
The major research projects currently supported by the Foundation include:
- Blood-Based Protein and Lipid Biomarkers for Diagnosis of Alzheimer Disease
- Programs investigating the role of genetics in Alzheimer’s disease
- Developing agents that selectively target the enzyme responsible for beta amyloid generation
- The role of diet in the prevention of Alzheimer’s Disease
- Identification and validation of peptide agents that neutralise beta amyloid toxicity
- Molecular and neuropsychological predictive markers of cognitive decline
- The role of testosterone in Alzheimer’s Disease
If you would like further information about any of these projects please contact us
WA Memory Study (WAMS)
Memory changes are common among the general adult population as we age. Dementia, which is primarily caused by Alzheimer’s disease, is not a normal part of ageing and currently there is no cure. Current medications only treat symptoms but do not halt progression of the disease.
It is vital to differentiate early changes that are consistent with dementia from those we see in normal ageing so we can identify those at risk or diagnosed with dementia as early as possible, start preventive measures or treatment when such interventions become available. However, early diagnosis is difficult as early symptoms associated with dementia are not well established. Doctors do not have a reliable means of establishing which individuals with early memory loss will develop dementia due to Alzheimer’s disease.
The WAMS commenced in 1996 and involves baseline measurements and follow-up assessments at 18-month intervals. Currently, 326 participants are enrolled as at June 2019 and over 500 assessments have been made including 18 month and 36 month assessments.
The aims of the WAMS are:
- To identify those factors that may influence memory changes
- To follow the longitudinal trajectory of cognitive change and see who will or will not develop dementia
- To identify amongst individuals with memory loss what characteristics are specifically associated with Alzheimer’s disease, to enable identification of individuals at a higher than average risk of developing dementia
The WAMS baseline measurements include:
- Medical history
- Tests of cognition (e.g., memory, language abilities, thinking skills and so on), mental and psychological health and quality of life
- Blood tests
- Body composition x-ray looking for association between body fat and Alzheimer’s disease-related proteins
- Assessment of peripheral and central hearing, as hearing loss has been associated with decline in memory and other mental abilities and social activities
- Olfactory “smell” examination to examine the link between the loss of smell and Alzheimer’s disease
In addition to the research procedures described above there are other optional sub-studies which includes the following:
- Neuroimaging to identify the changes in the brain caused by Alzheimer’s disease
- Donation of a cerebrospinal fluid (CSF) sample
- Clinical Assessment by a medical doctor specialised in identifying dementia using clinical methods
- Eye imaging
WAMS Outcomes:
Over the next few years, the WAMS is aiming to develop 3 novel assessments of Alzheimer’s disease that have the potential to be used in clinical practice to identify those at higher risk of dementia.
These 3 novel measurements in development are:
- The McCusker Subjective Cognitive Impairment Inventory (McSCI): the primary aim of this test is to be used in research and clinical practice to screen those at risk of future dementia.
- Prospective Memory Test (WA-PROM): This measure will inform research on how forgetting future tasks and events (e.g., taking medication, future medical appointment, and etc.) may indicate higher risk of dementia.
- Olfactory Memory Test (WA-OMT): The WA Olfactory Memory Test assesses ability to remember odours correctly. Impaired olfactory ability is seen in different dementia-related conditions including Parkinson disease and Alzheimer’s. As memory impairment is a common sign of dementia due to Alzheimer’s disease, the WA-OMT is believed differentiate those who will progress to Alzheimer’s disease form other types of dementia.
Current PhD and Master Students:
WAMS provides important support for our future scientists in Alzheimer’s disease research. There are currently 4 PhD Candidates and Master Students working on the WAMS:
- Rasingi Seneviratne (UWA): Olfactory Memory
- Rachal Mumme (UWA): Early detection of Alzheimer’s disease
- Pamela Lam (ECU): Depression and risk of dementia
- Hadeel Tarawneh (UWA): Age-related hearing and dementia
If you would like to be involved in the WA Memory study, please contact Jo Shaw on [email protected]
Why Research is Essential
With an aging population a far larger proportion of our community will be affected by Alzheimer’s and dementia in the coming years. Ongoing and committed research will play a vital role in the continuing journey towards an Alzheimer’s free world for the benefit of our whole community.
Alzheimer’s disease is occurring at an increased pace. The Australian Alzheimer’s Research Foundation is dedicated to ensuring research continues on an international level. Millions will be condemned to a demeaning and frightening end to their lives if treatments are not discovered.
The reality is that despite currently being the second largest cause of death, research into Alzheimer’s disease in Australia is underfunded relative to the current and projected costs and the scope for huge savings from investment in research for cause, prevention and treatment. Urgent action is essential.
Identifying a means of early intervention is a priority as the effectiveness of any treatments will be limited by the current inability to diagnose Alzheimer’s disease until significant neurological damage has already been sustained.
As a result of past research we are now aware of a number of mechanisms implicated in the body developing abnormal levels of beta amyloid in the blood and its deposition on the brain.
Our knowledge of beta amyloid is increasing all the time. We now know that beta amyloid is a commonly occurring protein which has a beneficial role in normal bodily functioning. There are different forms of beta amyloid, some being beneficial, others destructive. We know that in some people there is an increased production of the destructive forms.
We also know that deposition of beta amyloid is widespread among the population, even for those who do not develop the condition. With some people, there is increased production of beta amyloid which in itself may contribute to increased deposition. The problem could also be due to a reduced ability of the body to remove the amyloid from the brain.

New look, same cause
The Australian Alzheimer’s Research Foundation has a new name, Alzheimer’s Research Australia.
We remain resolutely focused on changing the future of Alzheimer’s disease through leading-edge medical research.
Click the links below to visit our new website:
Home | Alzheimer’s | Get Involved | Research | About Us | Donate
Alzheimer's drug adoption in US slowed by doctors' skepticism
- Medium Text

'SIGNIFICANT RISKS, MARGINAL BENEFIT'
'your enemy is nihilism', 'peace and quiet'.
Sign up here.
Reporting by Julie Steenhuysen in Chicago; editing by Caroline Humer and Suzanne Goldenberg
Our Standards: The Thomson Reuters Trust Principles. New Tab , opens new tab

Business Chevron

Expedia lowers full-year revenue forecast on slow B2C growth
Online travel agency Expedia cut its 2024 revenue growth forecast on Thursday, as gross bookings were hit by poor performance in its business-to-consumer segment.


IMAGES
COMMENTS
NIH has released Advancing Alzheimer's Disease and Related Dementias Research for All Populations: Prevent. Diagnose. Treat. Care. (PDF, 17M), a 2022 scientific progress report. The report features science advances and related efforts made between March 2021 and early 2022 in areas including drug development, lifestyle interventions ...
November 8, 2022. Alzheimer's Disease. NIH has released Advancing Alzheimer's Disease and Related Dementias Research for All Populations: Prevent. Diagnose. Treat. Care. (PDF, 17M), a 2022 scientific progress report. The report features science advances and related efforts made between March 2021 and early 2022 in areas including drug ...
Research and Progress This is a time of unprecedented promise in the quest to end Alzheimer's. Today, we are growing philanthropic support for Alzheimer's research, fostering a dynamic community of Alzheimer's scientists and securing increased federal funding for research - all of which are instrumental to finding new treatments to stop, slow and prevent Alzheimer's disease.
In studies of potential disease-modifying drugs for Alzheimer's disease, there has always been a tension between being able to produce a treatment effect and being able to measure it, says ...
Office of the Director (OD). NIH has released Advancing Alzheimer's Disease and Related Dementias Research for All Populations: Prevent. Diagnose. Treat. Care (PDF, 17.5M), a 2022 scientific progress report. This report provides a comprehensive overview of the meaningful progress researchers made from April 2021 through March 2022 to address ...
The tool categorizes Alzheimer's disease cases into three subtypes according to the location of brain changes and continues the team's prior work, demonstrating how these changes impact people differently. Uncovering the microscopic pathology of the disease can help researchers pinpoint biomarkers that may affect future treatments and patient care.
Alzheimer's disease is a progressive neurodegenerative disease that impairs memory and cognitive judgment and is often accompanied by mood swings, disorientation and eventually delirium. It is the ...
The advent of plaque-clearing antibodies to the amyloid-β as the first disease-modifying treatment for Alzheimer's disease will change the course of this disease, the most common type of dementia.
Alzheimer's and dementia research - find the latest information on research funding, grants, clinical trials and global research news. ... Learn how Alzheimer's disease affects the brain. Take the Brain Tour. Don't just hope for a cure. Help us find one. Volunteer for a clinical trial.
In Alzheimer's disease, a buildup of sticky amyloid proteins in the brain clump together to form plaques, causing damage that gradually leads to worsening dementia symptoms. A promising way to change the course of this disease is with treatments that clear away damaging amyloid plaques or stop them from forming in the first place.
1. Introduction. Alzheimer's disease (AD) is a polygenic and multifactorial disease characterized by the deposition of amyloid-β (Aβ) fibrils in the brain, leading to the formation of plaques and neurofibrillary tangles (NFTs), and ultimately resulting in dendritic dysfunction, neuronal cell death, memory loss, behavioral changes, and organ shutdown [1,2,3,4,5].
1. THE CURRENT STATE OF THE ALZHEIMER'S DISEASE THERAPEUTICS AND DIAGNOSTIC PIPELINES. The Senate Committee on Finance, Subcommittee on Health Care, held a hearing titled, "The Alzheimer's Crisis: Examining, Testing, and Treatment Pipelines and Fiscal Implications," on December 16, 2020. 1 The committee convened this hearing because of growing concern about the impact of Alzheimer's ...
Dementia is a general term that refers to memory loss and decline of other cognitive abilities that limit independence in day-to-day function. Alzheimer's is the most common brain disease that causes dementia among older adults, accounting for 60%-80% of cases. It affects an estimated one in nine people age 65 and older — 6.2 million ...
Alzheimer's disease causes significant problems with memory, thinking and behavior and is the most common form of dementia, affecting more than 50 million people around the world each year.
This article describes the public health impact of Alzheimer's disease (AD), including prevalence and incidence, mortality and morbidity, use and costs of care and the ramifications of AD for family caregivers, the dementia workforce and society. ... Official counts for more recent years are still being compiled. Alzheimer's remains the fifth ...
ADNI is a public-private partnership established to develop a multi-site longitudinal, prospective, naturalistic study of normal cognitive aging, mild cognitive impairment, and early Alzheimer's disease. Now in its 13 th year, ADNI continues to develop and integrate new technologies to achieve these goals. For example, research from ADNI led ...
Despite the fact that the prevalence of Alzheimer's disease is growing, with 6 million cases in the United States alone, expected to rise to 13 million by 2050, it is still unclear to scientists ...
Alzheimer's disease is by far the most common type of dementia, representing about 60-80% of all diagnosed cases of cognitive disorders [ 10 ]. In 2020, around 6.2 million Americans aged 65 or older had Alzheimer's disease, and this number is predicted to increase to 12.7 million Americans by 2050. Figure 5.
A similar, US-based, trial 3 is being planned for sporadic, late-onset Alzheimer's disease, which affects older people and accounts for the overwhelming majority of cases. The US National ...
Arizona Alzheimer's Disease Research Center (ADRC-001) Scottsdale/Phoenix, AZ ... This study is being done to collect skin samples from people with and without neurodegenerative and vascular disorders including Parkinson's disease (PD), Alzheimer's disease (AD), amyotrophic lateral sclerosis (ALS), stroke and many others. ...
These Alzheimer's treatments boost the performance of chemicals in the brain that carry information from one brain cell to another. They include cholinesterase inhibitors and the medicine memantine (Namenda). However, these treatments don't stop the underlying decline and death of brain cells. As more cells die, Alzheimer's disease continues to ...
Alzheimer's affects more than just memory and thinking. A person's quality of life may be impacted by a variety of behavioral and psychological symptoms that accompany dementia, such as sleep changes or agitation. It is important to try non-drug strategies to manage non-cognitive symptoms before adding medications.
We are facing a tsunami of dementia. Today, there are approximately 5.8 million Americans living with Alzheimer's disease—1 in 10 people aged 65 and older. However, in part due to the ...
What research is being done to try to prevent Alzheimer's disease? The research focus now is turning to prevention trials, and a number of studies are underway to test the effectiveness of various therapies in people without symptoms or who have only slight memory problems. Under scrutiny in these studies are furth ...
Therapeutic research into Alzheimer's disease (AD) has been dominated by the amyloid cascade hypothesis (ACH) since the 1990s. However, targeting amyloid in AD patients has not yet resulted in highly significant disease-modifying effects. ... There is also increasing research being done with dementia patients in a co-research role in ...
Share on Pinterest AI may help reveal new treatment pathways for Alzheimer's disease. Image credit: Constantinis/Getty Images. Research has suggested that the gut microbiome is disrupted in ...
The reality is that despite currently being the second largest cause of death, research into Alzheimer's disease in Australia is underfunded relative to the current and projected costs and the scope for huge savings from investment in research for cause, prevention and treatment.
Dr. Nathaniel Chin, a geriatrician with the University of Wisconsin's Alzheimer's Disease Research Center, said he was the target of negative comments on social media after he urged geriatricians ...
Introduction. Alzheimer disease (AD) is one of the greatest medical care challenges of our century and is the main cause of dementia. In total, 40 million people are estimated to suffer from dementia throughout the world, and this number is supposed to become twice as much every 20 years, until approximately 2050. 1 Because dementia occurs mostly in people older than 60 years, the growing ...