

Human Research Violations By UCSD Eye Doctor Showcase A National Problem

Tens of millions of people have volunteered their time and bodies to help create breakthroughs in medicine. You see the results with the pain relievers in your medicine cabinet, the vaccines that protect you from disease, the pacemakers that keep your heart beating and the innovations happening now with stem cells.
Yet the systems meant to protect those volunteers from harm are far from perfect, and research violations by Dr. Kang Zhang , an eye doctor at the University of California San Diego, show just how easily that well-intentioned framework can collapse.
Zhang is the chief of eye genetics at UCSD and has a lab named after him at the university. He receives millions of dollars in federal grants and presents at symposiums around the world.
A few years ago, he helped develop a way to remove cataracts from infants and regenerate their lenses using their own stem cells. He also built a tool that scanned over a million patient records and diagnosed illnesses with more than 90% accuracy.
But several of Zhang’s studies were riddled with violations of basic human research standards. A U.S. Food and Drug Administration warning in 2017 and a UCSD audit that followed reveal a pattern that put patients in harm’s way for years.
The 56-year-old doctor enrolled people he shouldn’t have for his medical trials, failed to document what happened to 25 units of a study drug, performed HIV tests on participants without their permission, kept poor records on his patients and didn’t complete necessary ethics training for a stem cell study.

When asked by the FDA to create a plan to prevent more violations from happening in the future, Zhang didn’t provide one.
“There ought to be some serious penalties for this sort of thing,” said Spencer Hey, a Harvard Medical School expert in biomedical research ethics.
inewsource reached out to Zhang, the director of the eye institute where he works and the director of UCSD’s human research protection program for interviews. In response, UCSD sent a statement that said the university had “implemented a comprehensive management plan to address these issues” and suspended Zhang indefinitely from serving as a primary researcher overseeing human research studies at UCSD. He may continue to apply for federal grants, publish in medical journals and train the next generation of scientists.
UCSD later told inewsource , “Zhang’s research had undergone multiple audits since 2012,” which prompted his suspension. When asked if that meant the university had known about Zhang’s violations for five years before taking action, a UCSD spokeswoman would not comment further.
After speaking with five ethics experts for this story, it appears Zhang’s violations — and what happened after they were discovered — are symptomatic of larger problems impacting the field of human subject research in the U.S. Those include patchwork oversight and poor communication between watchdog agencies, a lack of transparency and dialogue with the public, and a combination of money and prestige that sometimes safeguard an institution’s reputation more than patient welfare.
“Science is accelerating always,” said Stacey Springs , Harvard University’s research integrity officer. “But it feels that it’s accelerating in ways that are pushing on regulatory compliance like never before. So we really need to focus now, and learn from each other, and apply these best practices – because it's coming fast.”
An early warning
Zhang received his medical degree and doctorate in genetics from Harvard, then taught at Johns Hopkins University and the University of Utah before founding UCSD’s Institute for Genomic Medicine in 2009.
His accomplishments have landed him on CBS’ “60 Minutes” and in The New York Times, The Wall Street Journal and the Los Angeles Times. He’s received dozens of honors from national and international associations and universities, published or co-authored more than 100 peer-reviewed manuscripts in top journals and recruited human research subjects from around the world – including from the San Diego VA and UCSD’s Shiley Eye Institute on the La Jolla campus.
It was during Zhang’s time at the institute, in the summer of 2016, when the FDA inspected one of his ongoing human trials to ensure the “rights, safety, and welfare” of his patients were protected.
For five years, Zhang had been testing a drug to reverse the effects of a common age-related eye disease. He received approval to enroll patients whose vision had already started to decline – to see if the drug could restore their sight.
Zhang’s research team injected ranibizumab once a month into each test subject’s eyes, 12 times total for each patient. The drug can produce side effects that include eye haemorrhages, pain, inflammation and spots in a field of vision. In rare cases, it can prompt serious cataracts or blindness.

The FDA letter prompted UCSD to suspend enrollment in all of Zhang’s active research projects at the time, pending the results of an internal audit.
Amy Caruso Brown is an assistant professor of bioethics, humanities and pediatrics at New York’s Upstate Medical University. She also is a member of an institutional review board – a safety committee that approves and oversees projects like Zhang’s.
Brown spoke to inewsource after reading the UCSD audit and said, “I have not seen this number of issues in the five years that I've been on an IRB.”Twelve people had participated in the study by the time the FDA stepped in and found five of them were ineligible because they didn’t have the vision problems Zhang outlined for participants. Another patient’s eyesight wasn’t correctly evaluated before the person was injected with the drug.
“If it had been one out of a hundred, we could probably chalk that up to an error that doesn’t reflect a pattern of misconduct,” said Michael Carome , a former associate director at the U.S. Office for Human Research Protections, one of many federal agencies that protects human research subjects.
“But to enroll half the subjects not meeting enrollment criteria – that is more than just an occasional error. That suggests something systematically wrong with how they’re doing the research,” said Carome, who spent years investigating these kind of violations while at the agency.
He left the Office for Human Research Protections in 2010 during a decade of decline, when the office all but stopped using its enforcement tools in favor of “a more friendly approach toward institutions,” he said.
Carome is now a director of the health research group at Public Citizen , a consumer advocacy nonprofit based in Washington, D.C.
He told inewsource that research on otherwise healthy patients needs to be performed carefully, because they aren’t sick enough to justify taking chances.
“Both from a scientific standpoint and ethical human subjects standpoint, not complying with the enrollment criteria is a big deal,” Carome said, calling the guidelines “crucial in terms of ensuring that human subjects are protected.”
The FDA agreed. It issued Zhang a warning letter in January 2017 that called out his use of ineligible patients and his failure to perform required screenings and procedures, poor recordkeeping and lack of documentation about what happened to 25 units of the unused study drug (which Zhang said were destroyed).
Though not mentioned in the letter, the UCSD audit that followed said Zhang also enrolled patients while the study was suspended.
The FDA uses warning letters to document serious research problems and mandate corrective actions. Three times in the letter, it told Zhang his actions raised “concerns about the validity and integrity of the data collected,” and three times it told Zhang he didn’t have an adequate plan to keep his patients safe moving forward.
“We are unable to undertake an informed evaluation of your written response because you did not provide a corrective action plan that, if properly carried out, would prevent this type of violation in the future,” the FDA wrote.
The study was eventually shut down, and inewsource could find no articles published based on the research.

‘Major league science’
Zhang’s work at UCSD should be viewed in context.
He is one of more than 1,600 faculty members in the schools of medicine and pharmacy, one part of a healthcare system at a university ranked among the top research institutions in the country.
UCSD secured $1.2 billion in sponsored research support in 2018 – with $686 million going toward UC Health Sciences – and had more than 7,000 patients participating in clinical trials. Its scientists have made breakthroughs in diabetes research, understanding cancer genes, identifying early signs of autism and treating Alzheimer’s disease. It counts 16 Nobel laureates among current and former faculty.
All of that makes UCSD’s investigation of Zhang unique. The university has published 249 internal audits since July 2010, and the Zhang report is the only one inewsource could find specific to an individual researcher.
Auditors reviewed Zhang’s training records, enrollment logs, regulatory binders and files for ongoing projects that had enrolled human research patients. They found problems everywhere they looked: Zhang failed to get the proper consent from all patients; didn’t report problems to UCSD’s institutional review board; lost documents; kept inaccurate records; wrongly billed patients; and didn’t complete the training required to work with human embryonic stem cells.
In one study, Zhang’s staff tested patients’ blood for HIV and AIDS without telling them, against federal policy.
“He’s lucky there weren’t any major patient harms,” Brown said. ”But if you act like this all the time, eventually you will hurt someone.”
The auditors found Zhang’s actions may have “negatively impacted the rights, welfare, and safety of human subjects in clinical research.” One of his studies, sponsored by the California Institute for Regenerative Medicine , collected tissue from donors with blinding eye disease for a stem cell bank. California voters created the institute in 2004 to fund this type of research.
The study’s rules stated no one under 50 years old was allowed to enroll. UCSD audited files for 50 of the more than 400 patients and found seven were too young, including a minor.
Carome, the former federal director, laughed when he read that section.
“That’s not a subtle mistake,” he said.
Another finding noted the importance of “credible and valid data” when describing a study that was missing 25 of 50 patient progress reports.
“This isn’t minor league stuff. This is major league science,” said Hey, the Harvard bioethicist, who added, “this sort of pattern should raise questions about the validity of (Zhang’s) published work.”
Zhang did publish a correction in January, but not for a study included in the audit. It involved gene editing in animals, and Zhang’s paper said UCSD supervised and approved the research. That wasn’t true – it was overseen by a university and medical center in China.
inewsource couldn’t find any published articles based on the six studies reviewed by the FDA and UCSD. Yet during the audits and study suspensions, Zhang is listed as having continued a genetics research project at the San Diego VA, which did result in 10 published articles.
UCSD doctors often work as attending physi cians at the San Diego VA and share funding, research samples, data and lab space. The VA Hospital is less than a mile from the Shiley Eye Institute and on the same campus. Yet the VA said it was never notified of the UCSD or FDA reports until inewsource asked about them in March.

A VA spokeswoman would not answer questions about whether Zhang was still practicing at the VA, enrolling patients in trials or proposing new research at the institution. The San Diego VA has one of the largest research programs in the national VA network.
Nor were other related federal or state regulators notified. That includes the federal Office for Human Research Protections, which protects research subjects from harm; the National Institutes of Health , which funds many of Zhang’s studies; the Office of Research Integrity, which oversees research misconduct cases; or the California State Medical Board, which gave Zhang a license to practice medicine.
“Communication is fraught with complexity in compliance matters,” said Springs, Harvard’s research integrity officer. Things sometimes happen on a need-to-know basis, she said, and confidentiality plays a big part.
But, she said, “If there are active studies and participants are in danger, the confidentiality is out the window.”
Symptoms of a larger problem
Picture the framework for protecting research subjects as a house.
The foundation consists of a study, planned with sound scientific and ethical principles, and a responsible, ethical researcher. If and when things go wrong or change, they are communicated and addressed immediately. That didn’t happen in this case.
Institutional review boards, the first floor, are often composed of expert volunteers who spend countless hours poring over hundreds of pages of research protocols, guidelines and regulations while also working their regular jobs. They rely on researchers to keep them updated, alert them to problems and speak the truth, but they may also have the power and responsibility to audit ongoing studies. It’s often a proactive system, but it wasn’t in Zhang’s case.
And the institutional review board system is “vulnerable to unethical manipulation, particularly by companies or individuals who intend to abuse the system or to commit fraud,” according to an undercover federal investigation from 2009.
Higher-ups at an institution – the second floor – may have reasons and methods to keep violations quiet and away from public scrutiny. The Zhang audit is a perfect example: It was published more than two years ago but didn’t reach the VA across campus, never made the news, and likely wouldn’t have shocked anyone who stumbled upon it because it never named Zhang as the researcher under scrutiny. The only place his name appears is on the report’s cover page – as one of 16 people copied on its transmittal within the UC system, including UCSD’s chief ethics and compliance officer, vice chancellors, various directors and others.
“It doesn’t look good for the university to be calling out these high-profile faculty for these kinds of violations and making a big deal out of it and applying sanctions, because that’s not the kind of attention you want to draw to your researchers,” Harvard’s Hey said.
Problems with internal probes
An opinion article published by three ethics experts last year in The Journal of the American Medical Association said when internal investigations are completed, the reports are often not standardized, not peer-reviewed, have limited oversight and contain conflicts of interest. “Even when institutions act, the information they release to the public is often limited and unhelpful,” they wrote.
Calling out lucrative researchers can also result in lost funding. Last fiscal year, federal agencies including the National Institutes of Health, National Science Foundation and U.S. Department of Defense gave UCSD $681 million in research funding. UCSD prides itself on how much grant money flows its way each year and has defended that stream aggressively: It sued the University of Southern California in 2015 for poaching one of its most lucrative researchers.
And as quickly as those funding agencies give, they can take away – or even require repayment if serious violations happened using taxpayer dollars.
At the very top of the house, the roof is made up of agencies that regulate human subject protection across the country. The big one – the Office for Human Research Protections – protects those involved in research funded or conducted by the U.S. Health and Human Services Department. It can investigate allegations of wrongdoing but often does not, choosing instead to refer investigations back to the institutions themselves. It can also revoke an entire institution’s ability to perform human research, though that hasn’t happened since 2007.
At Harvard, Springs said she often uses these agencies as a lever in talks with researchers to get them to understand and follow rules. She said the patchwork of institutional, academic and statewide regulations can be messy, so there’s a hope that the feds will act consistently, “because they really are the heavy in these conversations.”
But, she added, “Then they aren't predictable, and you're like, ‘Wow, OK. So they have these enforcement mechanisms and they're not using them. Or they're using them selectively.’”
The Office for Human Research Protections never took action in the Zhang case, which isn’t surprising for two reasons. One, they weren’t informed by the FDA or UCSD of his violations. Two, the agency has drastically cut down on enforcement over the past decade.
For example, it charged institutions with investigating misconduct allegations 94 times in 2002. In 2015, it did that three times. For the same period, the agency went from issuing 146 “determination letters” – an important tool for communicating findings of misconduct – to issuing five.

It’s not due to a lack of funding or a drop in complaints. In fact, institutional misconduct reports sent to the office jumped 400 percent from 2002 to 2014.
Carome said his former agency’s current leadership is “less interested in issuing harsh findings and embarrassing institutions.”
The problem with that, he said, is enforcement actions are “one of the more important tools the office has to change behavior.”
The Office of Research Integrity , another federal agency that oversees research misconduct investigations, has also been criticized for slowing its enforcement. It recently went nearly 10 months without issuing a single finding of research misconduct, though the agency has since stepped up its actions.
One medical ethicist quoted in an industry publication in 2017 called the lack of oversight by the Office of Research Integrity and the Office for Human Research Protections “nothing short of appalling.”
Even if oversight agencies were operating at full speed, a lack of transparency and data sharing would still be major flaws in safeguarding patients that could rip the roof apart.
Compliance data at the Office for Human Research Protections is kept in-house and offline, and getting at it requires a public records request, weeks to months of wait time and then skilled data analysis. The California Medical Board publishes doctor information online , but it’s more concerned with things like medical malpractice judgments, physician substance abuse and negligence in the course of routine health care than monitoring human research and clinical trials. Its database isn’t designed to incorporate audit findings, FDA warning letters or federal databases.
Academic investigations – at least in California – are kept far from Google’s reach, and typically require a public records request and the knowledge they exist. The same goes for institutional review board investigations, reviews and audits.
And none of these systems communicate with each other in any meaningful way.
Carome added one more problem plaguing the field: “Much noncompliance goes undetected or unnoticed.”
Zhang’s audit likely would have gone unnoticed if inewsource weren’t digging into the risks associated with human research, yet Springs said this may prove an opportunity for UCSD.
“When these cases emerge in the press, or people come to hear about them in some way, it highlights our failures – and that should happen,” she said.
But even then, Springs said, it doesn’t always “turn into constructive discussion and dialogue around how we can fix it.”
Audits like Zhang’s are often unintelligible to the community, to patients enrolled and even to academics, even though they are the people who should be providing feedback and criticism, she said.
“These are opportunities where we can actually promote transparency,” she said, “and say, ‘Hey, this is what happened. This is how we approached it,’ and model effective dialogue with communities on how we can work better on this.”
Correction: An earlier version of this story cited a 2017 industry publication that said the Office of Research Integrity had gone a year without making a research misconduct finding. Since the story published, the office has clarified that it went nine months and 21 days without a finding of research misconduct between 2016 and 2017.


Case Summaries
2008 and older.
Email Updates
Annual Review of Ethics Case Studies
What are research ethics cases.
For additional information, please visit Resources for Research Ethics Education
Research Ethics Cases are a tool for discussing scientific integrity. Cases are designed to confront the readers with a specific problem that does not lend itself to easy answers. By providing a focus for discussion, cases help staff involved in research to define or refine their own standards, to appreciate alternative approaches to identifying and resolving ethical problems, and to develop skills for dealing with hard problems on their own.
Research Ethics Cases for Use by the NIH Community
- Theme 23 – Authorship, Collaborations, and Mentoring (2023)
- Theme 22 – Use of Human Biospecimens and Informed Consent (2022)
- Theme 21 – Science Under Pressure (2021)
- Theme 20 – Data, Project and Lab Management, and Communication (2020)
- Theme 19 – Civility, Harassment and Inappropriate Conduct (2019)
- Theme 18 – Implicit and Explicit Biases in the Research Setting (2018)
- Theme 17 – Socially Responsible Science (2017)
- Theme 16 – Research Reproducibility (2016)
- Theme 15 – Authorship and Collaborative Science (2015)
- Theme 14 – Differentiating Between Honest Discourse and Research Misconduct and Introduction to Enhancing Reproducibility (2014)
- Theme 13 – Data Management, Whistleblowers, and Nepotism (2013)
- Theme 12 – Mentoring (2012)
- Theme 11 – Authorship (2011)
- Theme 10 – Science and Social Responsibility, continued (2010)
- Theme 9 – Science and Social Responsibility - Dual Use Research (2009)
- Theme 8 – Borrowing - Is It Plagiarism? (2008)
- Theme 7 – Data Management and Scientific Misconduct (2007)
- Theme 6 – Ethical Ambiguities (2006)
- Theme 5 – Data Management (2005)
- Theme 4 – Collaborative Science (2004)
- Theme 3 – Mentoring (2003)
- Theme 2 – Authorship (2002)
- Theme 1 – Scientific Misconduct (2001)
For Facilitators Leading Case Discussion
For the sake of time and clarity of purpose, it is essential that one individual have responsibility for leading the group discussion. As a minimum, this responsibility should include:
- Reading the case aloud.
- Defining, and re-defining as needed, the questions to be answered.
- Encouraging discussion that is “on topic”.
- Discouraging discussion that is “off topic”.
- Keeping the pace of discussion appropriate to the time available.
- Eliciting contributions from all members of the discussion group.
- Summarizing both majority and minority opinions at the end of the discussion.
How Should Cases be Analyzed?
Many of the skills necessary to analyze case studies can become tools for responding to real world problems. Cases, like the real world, contain uncertainties and ambiguities. Readers are encouraged to identify key issues, make assumptions as needed, and articulate options for resolution. In addition to the specific questions accompanying each case, readers should consider the following questions:
- Who are the affected parties (individuals, institutions, a field, society) in this situation?
- What interest(s) (material, financial, ethical, other) does each party have in the situation? Which interests are in conflict?
- Were the actions taken by each of the affected parties acceptable (ethical, legal, moral, or common sense)? If not, are there circumstances under which those actions would have been acceptable? Who should impose what sanction(s)?
- What other courses of action are open to each of the affected parties? What is the likely outcome of each course of action?
- For each party involved, what course of action would you take, and why?
- What actions could have been taken to avoid the conflict?
Is There a Right Answer?
Acceptable solutions.
Most problems will have several acceptable solutions or answers, but it will not always be the case that a perfect solution can be found. At times, even the best solution will still have some unsatisfactory consequences.
Unacceptable Solutions
While more than one acceptable solution may be possible, not all solutions are acceptable. For example, obvious violations of specific rules and regulations or of generally accepted standards of conduct would typically be unacceptable. However, it is also plausible that blind adherence to accepted rules or standards would sometimes be an unacceptable course of action.
Ethical Decision-Making
It should be noted that ethical decision-making is a process rather than a specific correct answer. In this sense, unethical behavior is defined by a failure to engage in the process of ethical decision-making. It is always unacceptable to have made no reasonable attempt to define a consistent and defensible basis for conduct.
This page was last updated on Friday, July 7, 2023
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 05 June 2019
Japanese hospital uncovers flood of research ethics violations
- Mark Zastrow
You can also search for this author in PubMed Google Scholar
A brain and heart hospital and research centre in Japan has found 158 cases of studies being done in violation of ethics standards since 2013.
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
doi: https://doi.org/10.1038/d41586-019-01747-w
Reprints and permissions
Related Articles
University says prominent Japanese cell biologist committed misconduct
Japan fails to settle university dispute
Research integrity guidelines in Japan

Seed-stashing chickadees overturn ideas about location memory
News & Views 23 MAY 24
Neural pathways for reward and relief promote fentanyl addiction
News & Views 22 MAY 24
AI networks reveal how flies find a mate

Guidelines for academics aim to lessen ethical pitfalls in generative-AI use
Nature Index 22 MAY 24

Are robots the solution to the crisis in older-person care?
Outlook 25 APR 24

Lethal AI weapons are here: how can we control them?
News Feature 23 APR 24
Postdoctoral Research Associate- Oncology
Memphis, Tennessee
St. Jude Children's Research Hospital (St. Jude)
Group Leader in Immunology
The Institut Necker Enfants Malades (https://www.institut-necker-enfants-malades.fr/) in Paris seeks a group leader in Immunology.
Paris, Ile-de-France (FR)
INSERM DR IdF PARIS CENTRE
Postdoc Erdmann Group, Structural Biology Research Center
Job description APPLICATION CLOSING DATE: 12/06/2024 Human Technopole (HT) is an interdisciplinary life science research institute, created and sup...
Human Technopole
The recruitment for Earth Science High-talent in IDSSE, CAS
Seeking global talents in the field of Earth Science and Ocean Engineering.
Sanya, Hainan, China
Institute of Deep-sea Science and Engineering, Chinese Academy of Sciences
Faculty(Group Leaders or Principal Investigators) and Postdoc positions
Faculty and Postdoc positions are open all year.
Hangzhou, Zhejiang, China
The Stomatology Hospital, School of Stomatology, Zhejiang University School of Medicine(ZJUSS)
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Research article
- Open access
- Published: 30 April 2021
A scoping review of the literature featuring research ethics and research integrity cases
- Anna Catharina Vieira Armond ORCID: orcid.org/0000-0002-7121-5354 1 ,
- Bert Gordijn 2 ,
- Jonathan Lewis 2 ,
- Mohammad Hosseini 2 ,
- János Kristóf Bodnár 1 ,
- Soren Holm 3 , 4 &
- Péter Kakuk 5
BMC Medical Ethics volume 22 , Article number: 50 ( 2021 ) Cite this article
26 Citations
28 Altmetric
Metrics details
The areas of Research Ethics (RE) and Research Integrity (RI) are rapidly evolving. Cases of research misconduct, other transgressions related to RE and RI, and forms of ethically questionable behaviors have been frequently published. The objective of this scoping review was to collect RE and RI cases, analyze their main characteristics, and discuss how these cases are represented in the scientific literature.
The search included cases involving a violation of, or misbehavior, poor judgment, or detrimental research practice in relation to a normative framework. A search was conducted in PubMed, Web of Science, SCOPUS, JSTOR, Ovid, and Science Direct in March 2018, without language or date restriction. Data relating to the articles and the cases were extracted from case descriptions.
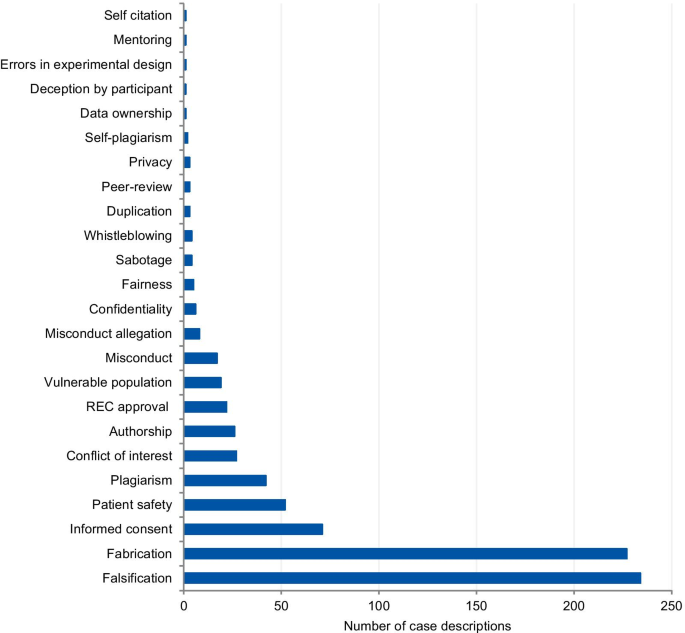
A total of 14,719 records were identified, and 388 items were included in the qualitative synthesis. The papers contained 500 case descriptions. After applying the eligibility criteria, 238 cases were included in the analysis. In the case analysis, fabrication and falsification were the most frequently tagged violations (44.9%). The non-adherence to pertinent laws and regulations, such as lack of informed consent and REC approval, was the second most frequently tagged violation (15.7%), followed by patient safety issues (11.1%) and plagiarism (6.9%). 80.8% of cases were from the Medical and Health Sciences, 11.5% from the Natural Sciences, 4.3% from Social Sciences, 2.1% from Engineering and Technology, and 1.3% from Humanities. Paper retraction was the most prevalent sanction (45.4%), followed by exclusion from funding applications (35.5%).
Conclusions
Case descriptions found in academic journals are dominated by discussions regarding prominent cases and are mainly published in the news section of journals. Our results show that there is an overrepresentation of biomedical research cases over other scientific fields compared to its proportion in scientific publications. The cases mostly involve fabrication, falsification, and patient safety issues. This finding could have a significant impact on the academic representation of misbehaviors. The predominance of fabrication and falsification cases might diverge the attention of the academic community from relevant but less visible violations, and from recently emerging forms of misbehaviors.
Peer Review reports
There has been an increase in academic interest in research ethics (RE) and research integrity (RI) over the past decade. This is due, among other reasons, to the changing research environment with new and complex technologies, increased pressure to publish, greater competition in grant applications, increased university-industry collaborative programs, and growth in international collaborations [ 1 ]. In addition, part of the academic interest in RE and RI is due to highly publicized cases of misconduct [ 2 ].
There is a growing body of published RE and RI cases, which may contribute to public attitudes regarding both science and scientists [ 3 ]. Different approaches have been used in order to analyze RE and RI cases. Studies focusing on ORI files (Office of Research Integrity) [ 2 ], retracted papers [ 4 ], quantitative surveys [ 5 ], data audits [ 6 ], and media coverage [ 3 ] have been conducted to understand the context, causes, and consequences of these cases.
Analyses of RE and RI cases often influence policies on responsible conduct of research [ 1 ]. Moreover, details about cases facilitate a broader understanding of issues related to RE and RI and can drive interventions to address them. Currently, there are no comprehensive studies that have collected and evaluated the RE and RI cases available in the academic literature. This review has been developed by members of the EnTIRE consortium to generate information on the cases that will be made available on the Embassy of Good Science platform ( www.embassy.science ). Two separate analyses have been conducted. The first analysis uses identified research articles to explore how the literature presents cases of RE and RI, in relation to the year of publication, country, article genre, and violation involved. The second analysis uses the cases extracted from the literature in order to characterize the cases and analyze them concerning the violations involved, sanctions, and field of science.
This scoping review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and PRISMA Extension for Scoping Reviews (PRISMA-ScR). The full protocol was pre-registered and it is available at https://ec.europa.eu/research/participants/documents/downloadPublic?documentIds=080166e5bde92120&appId=PPGMS .
Eligibility
Articles with non-fictional case(s) involving a violation of, or misbehavior, poor judgment, or detrimental research practice in relation to a normative framework, were included. Cases unrelated to scientific activities, research institutions, academic or industrial research and publication were excluded. Articles that did not contain a substantial description of the case were also excluded.
A normative framework consists of explicit rules, formulated in laws, regulations, codes, and guidelines, as well as implicit rules, which structure local research practices and influence the application of explicitly formulated rules. Therefore, if a case involves a violation of, or misbehavior, poor judgment, or detrimental research practice in relation to a normative framework, then it does so on the basis of explicit and/or implicit rules governing RE and RI practice.
Search strategy
A search was conducted in PubMed, Web of Science, SCOPUS, JSTOR, Ovid, and Science Direct in March 2018, without any language or date restrictions. Two parallel searches were performed with two sets of medical subject heading (MeSH) terms, one for RE and another for RI. The parallel searches generated two sets of data thereby enabling us to analyze and further investigate the overlaps in, differences in, and evolution of, the representation of RE and RI cases in the academic literature. The terms used in the first search were: (("research ethics") AND (violation OR unethical OR misconduct)). The terms used in the parallel search were: (("research integrity") AND (violation OR unethical OR misconduct)). The search strategy’s validity was tested in a pilot search, in which different keyword combinations and search strings were used, and the abstracts of the first hundred hits in each database were read (Additional file 1 ).
After searching the databases with these two search strings, the titles and abstracts of extracted items were read by three contributors independently (ACVA, PK, and KB). Articles that could potentially meet the inclusion criteria were identified. After independent reading, the three contributors compared their results to determine which studies were to be included in the next stage. In case of a disagreement, items were reassessed in order to reach a consensus. Subsequently, qualified items were read in full.
Data extraction
Data extraction processes were divided by three assessors (ACVA, PK and KB). Each list of extracted data generated by one assessor was cross-checked by the other two. In case of any inconsistencies, the case was reassessed to reach a consensus. The following categories were employed to analyze the data of each extracted item (where available): (I) author(s); (II) title; (III) year of publication; (IV) country (according to the first author's affiliation); (V) article genre; (VI) year of the case; (VII) country in which the case took place; (VIII) institution(s) and person(s) involved; (IX) field of science (FOS-OECD classification)[ 7 ]; (X) types of violation (see below); (XI) case description; and (XII) consequences for persons or institutions involved in the case.
Two sets of data were created after the data extraction process. One set was used for the analysis of articles and their representation in the literature, and the other set was created for the analysis of cases. In the set for the analysis of articles, all eligible items, including duplicate cases (cases found in more than one paper, e.g. Hwang case, Baltimore case) were included. The aim was to understand the historical aspects of violations reported in the literature as well as the paper genre in which cases are described and discussed. For this set, the variables of the year of publication (III); country (IV); article genre (V); and types of violation (X) were analyzed.
For the analysis of cases, all duplicated cases and cases that did not contain enough information about particularities to differentiate them from others (e.g. names of the people or institutions involved, country, date) were excluded. In this set, prominent cases (i.e. those found in more than one paper) were listed only once, generating a set containing solely unique cases. These additional exclusion criteria were applied to avoid multiple representations of cases. For the analysis of cases, the variables: (VI) year of the case; (VII) country in which the case took place; (VIII) institution(s) and person(s) involved; (IX) field of science (FOS-OECD classification); (X) types of violation; (XI) case details; and (XII) consequences for persons or institutions involved in the case were considered.
Article genre classification
We used ten categories to capture the differences in genre. We included a case description in a “news” genre if a case was published in the news section of a scientific journal or newspaper. Although we have not developed a search strategy for newspaper articles, some of them (e.g. New York Times) are indexed in scientific databases such as Pubmed. The same method was used to allocate case descriptions to “editorial”, “commentary”, “misconduct notice”, “retraction notice”, “review”, “letter” or “book review”. We applied the “case analysis” genre if a case description included a normative analysis of the case. The “educational” genre was used when a case description was incorporated to illustrate RE and RI guidelines or institutional policies.
Categorization of violations
For the extraction process, we used the articles’ own terminology when describing violations/ethical issues involved in the event (e.g. plagiarism, falsification, ghost authorship, conflict of interest, etc.) to tag each article. In case the terminology was incompatible with the case description, other categories were added to the original terminology for the same case. Subsequently, the resulting list of terms was standardized using the list of major and minor misbehaviors developed by Bouter and colleagues [ 8 ]. This list consists of 60 items classified into four categories: Study design, data collection, reporting, and collaboration issues. (Additional file 2 ).
Systematic search
A total of 11,641 records were identified through the RE search and 3078 in the RI search. The results of the parallel searches were combined and the duplicates removed. The remaining 10,556 records were screened, and at this stage, 9750 items were excluded because they did not fulfill the inclusion criteria. 806 items were selected for full-text reading. Subsequently, 388 articles were included in the qualitative synthesis (Fig. 1 ).
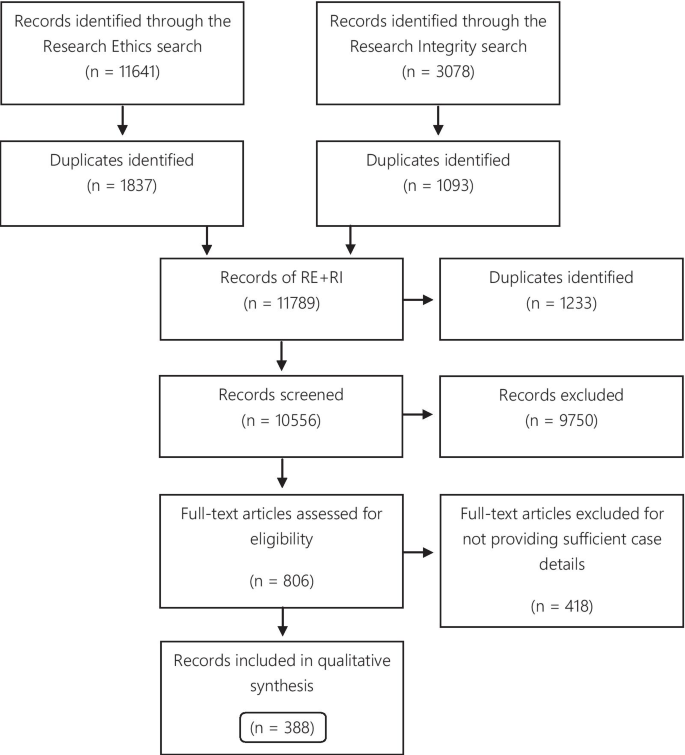
Flow diagram
Of the 388 articles, 157 were only identified via the RE search, 87 exclusively via the RI search, and 144 were identified via both search strategies. The eligible articles contained 500 case descriptions, which were used for the analysis of the publications articles analysis. 256 case descriptions discussed the same 50 cases. The Hwang case was the most frequently described case, discussed in 27 articles. Furthermore, the top 10 most described cases were found in 132 articles (Table 1 ).
For the analysis of cases, 206 (41.2% of the case descriptions) duplicates were excluded, and 56 (11.2%) cases were excluded for not providing enough information to distinguish them from other cases, resulting in 238 eligible cases.
Analysis of the articles
The categories used to classify the violations include those that pertain to the different kinds of scientific misconduct (falsification, fabrication, plagiarism), detrimental research practices (authorship issues, duplication, peer-review, errors in experimental design, and mentoring), and “other misconduct” (according to the definitions from the National Academies of Sciences and Medicine, [ 1 ]). Each case could involve more than one type of violation. The majority of cases presented more than one violation or ethical issue, with a mean of 1.56 violations per case. Figure 2 presents the frequency of each violation tagged to the articles. Falsification and fabrication were the most frequently tagged violations. The violations accounted respectively for 29.1% and 30.0% of the number of taggings (n = 780), and they were involved in 46.8% and 45.4% of the articles (n = 500 case descriptions). Problems with informed consent represented 9.1% of the number of taggings and 14% of the articles, followed by patient safety (6.7% and 10.4%) and plagiarism (5.4% and 8.4%). Detrimental research practices, such as authorship issues, duplication, peer-review, errors in experimental design, mentoring, and self-citation were mentioned cumulatively in 7.0% of the articles.
Tagged violations from the article analysis
Analysis of the cases
Figure 3 presents the frequency and percentage of each violation found in the cases. Each case could include more than one item from the list. The 238 cases were tagged 305 times, with a mean of 1.28 items per case. Fabrication and falsification were the most frequently tagged violations (44.9%), involved in 57.7% of the cases (n = 238). The non-adherence to pertinent laws and regulations, such as lack of informed consent and REC approval, was the second most frequently tagged violation (15.7%) and involved in 20.2% of the cases. Patient safety issues were the third most frequently tagged violations (11.1%), involved in 14.3% of the cases, followed by plagiarism (6.9% and 8.8%). The list of major and minor misbehaviors [ 8 ] classifies the items into study design, data collection, reporting, and collaboration issues. Our results show that 56.0% of the tagged violations involved issues in reporting, 16.4% in data collection, 15.1% involved collaboration issues, and 12.5% in the study design. The items in the original list that were not listed in the results were not involved in any case collected.
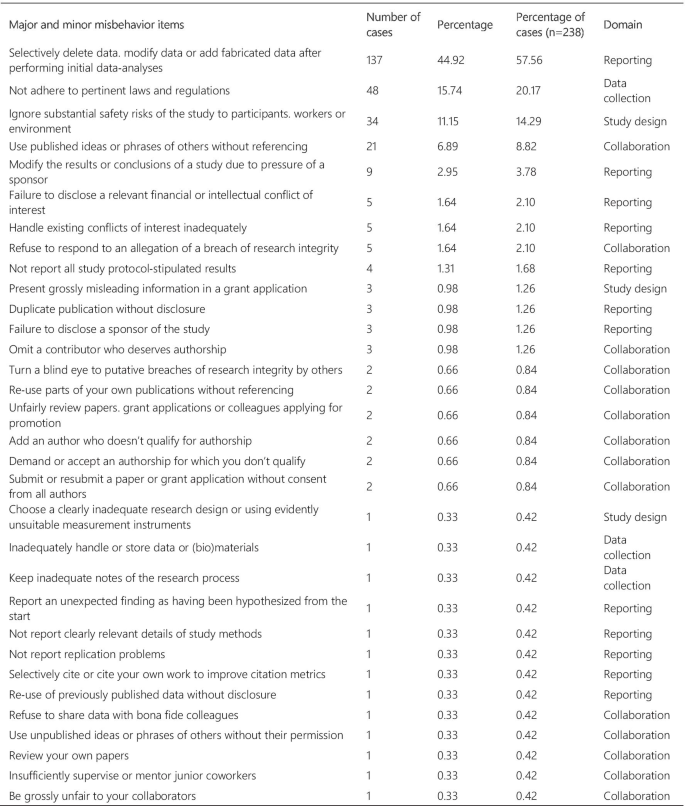
Major and minor misbehavior items from the analysis of cases
Article genre
The articles were mostly classified into “news” (33.0%), followed by “case analysis” (20.9%), “editorial” (12.1%), “commentary” (10.8%), “misconduct notice” (10.3%), “retraction notice” (6.4%), “letter” (3.6%), “educational paper” (1.3%), “review” (1%), and “book review” (0.3%) (Fig. 4 ). The articles classified into “news” and “case analysis” included predominantly prominent cases. Items classified into “news” often explored all the investigation findings step by step for the associated cases as the case progressed through investigations, and this might explain its high prevalence. The case analyses included mainly normative assessments of prominent cases. The misconduct and retraction notices included the largest number of unique cases, although a relatively large portion of the retraction and misconduct records could not be included because of insufficient case details. The articles classified into “editorial”, “commentary” and “letter” also included unique cases.
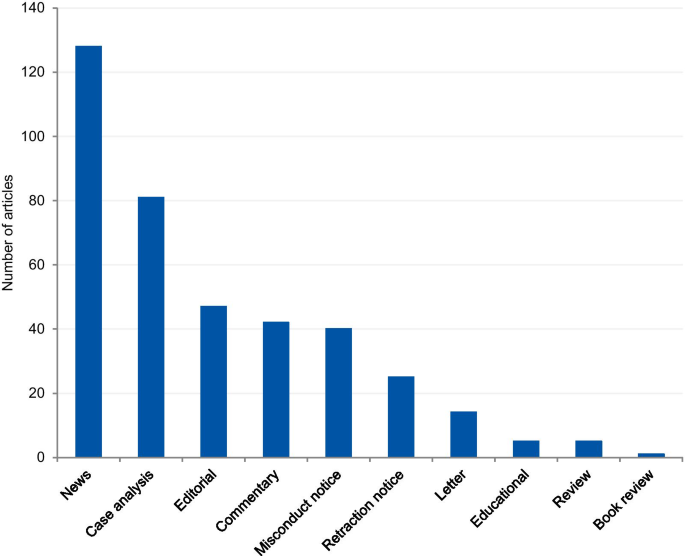
Article genre of included articles
Article analysis
The dates of the eligible articles range from 1983 to 2018 with notable peaks between 1990 and 1996, most probably associated with the Gallo [ 9 ] and Imanishi-Kari cases [ 10 ], and around 2005 with the Hwang [ 11 ], Wakefield [ 12 ], and CNEP trial cases [ 13 ] (Fig. 5 ). The trend line shows an increase in the number of articles over the years.
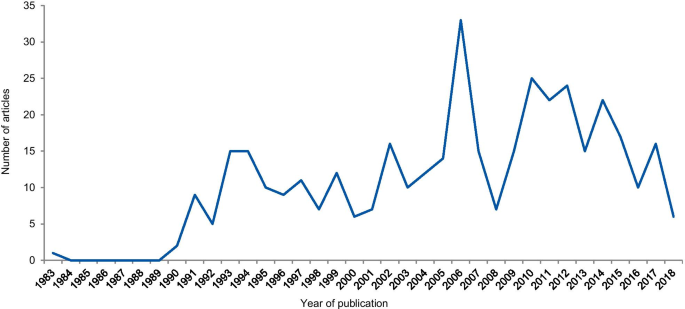
Frequency of articles according to the year of publication
Case analysis
The dates of included cases range from 1798 to 2016. Two cases occurred before 1910, one in 1798 and the other in 1845. Figure 6 shows the number of cases per year from 1910. An increase in the curve started in the early 1980s, reaching the highest frequency in 2004 with 13 cases.
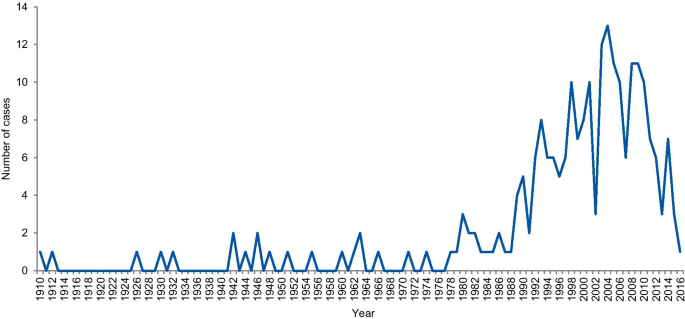
Frequency of cases per year
Geographical distribution
The first analysis concerned the authors’ affiliation and the corresponding author’s address. Where the article contained more than one country in the affiliation list, only the first author’s location was considered. Eighty-one articles were excluded because the authors’ affiliations were not available, and 307 articles were included in the analysis. The articles originated from 26 different countries (Additional file 3 ). Most of the articles emanated from the USA and the UK (61.9% and 14.3% of articles, respectively), followed by Canada (4.9%), Australia (3.3%), China (1.6%), Japan (1.6%), Korea (1.3%), and New Zealand (1.3%). Some of the most discussed cases occurred in the USA; the Imanishi-Kari, Gallo, and Schön cases [ 9 , 10 ]. Intensely discussed cases are also associated with Canada (Fisher/Poisson and Olivieri cases), the UK (Wakefield and CNEP trial cases), South Korea (Hwang case), and Japan (RIKEN case) [ 12 , 14 ]. In terms of percentages, North America and Europe stand out in the number of articles (Fig. 7 ).
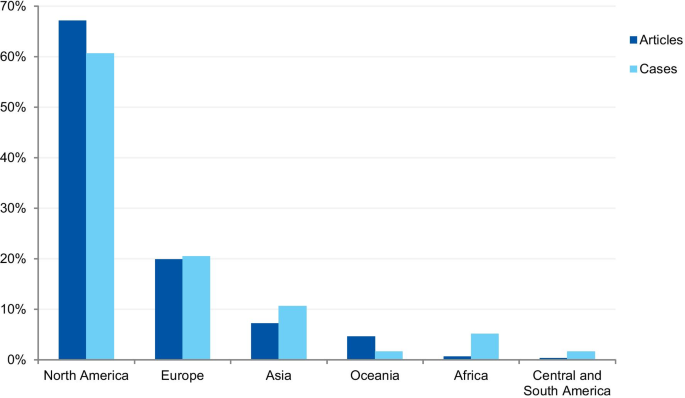
Percentage of articles and cases by continent
The case analysis involved the location where the case took place, taking into account the institutions involved in the case. For cases involving more than one country, all the countries were considered. Three cases were excluded from the analysis due to insufficient information. In the case analysis, 40 countries were involved in 235 different cases (Additional file 4 ). Our findings show that most of the reported cases occurred in the USA and the United Kingdom (59.6% and 9.8% of cases, respectively). In addition, a number of cases occurred in Canada (6.0%), Japan (5.5%), China (2.1%), and Germany (2.1%). In terms of percentages, North America and Europe stand out in the number of cases (Fig. 7 ). To enable comparison, we have additionally collected the number of published documents according to country distribution, available on SCImago Journal & Country Rank [ 16 ]. The numbers correspond to the documents published from 1996 to 2019. The USA occupies the first place in the number of documents, with 21.9%, followed by China (11.1%), UK (6.3%), Germany (5.5%), and Japan (4.9%).
Field of science
The cases were classified according to the field of science. Four cases (1.7%) could not be classified due to insufficient information. Where information was available, 80.8% of cases were from the Medical and Health Sciences, 11.5% from the Natural Sciences, 4.3% from Social Sciences, 2.1% from Engineering and Technology, and 1.3% from Humanities (Fig. 8 ). Additionally, we have retrieved the number of published documents according to scientific field distribution, available on SCImago [ 16 ]. Of the total number of scientific publications, 41.5% are related to natural sciences, 22% to engineering, 25.1% to health and medical sciences, 7.8% to social sciences, 1.9% to agricultural sciences, and 1.7% to the humanities.
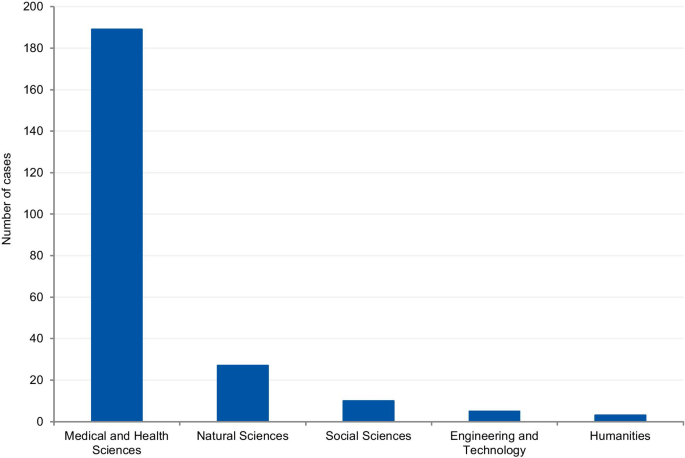
Field of science from the analysis of cases
This variable aimed to collect information on possible consequences and sanctions imposed by funding agencies, scientific journals and/or institutions. 97 cases could not be classified due to insufficient information. 141 cases were included. Each case could potentially include more than one outcome. Most of cases (45.4%) involved paper retraction, followed by exclusion from funding applications (35.5%). (Table 2 ).
RE and RI cases have been increasingly discussed publicly, affecting public attitudes towards scientists and raising awareness about ethical issues, violations, and their wider consequences [ 5 ]. Different approaches have been applied in order to quantify and address research misbehaviors [ 5 , 17 , 18 , 19 ]. However, most cases are investigated confidentially and the findings remain undisclosed even after the investigation [ 19 , 20 ]. Therefore, the study aimed to collect the RE and RI cases available in the scientific literature, understand how the cases are discussed, and identify the potential of case descriptions to raise awareness on RE and RI.
We collected and analyzed 500 detailed case descriptions from 388 articles and our results show that they mostly relate to extensively discussed and notorious cases. Approximately half of all included cases was mentioned in at least two different articles, and the top ten most commonly mentioned cases were discussed in 132 articles.
The prominence of certain cases in the literature, based on the number of duplicated cases we found (e.g. Hwang case), can be explained by the type of article in which cases are discussed and the type of violation involved in the case. In the article genre analysis, 33% of the cases were described in the news section of scientific publications. Our findings show that almost all article genres discuss those cases that are new and in vogue. Once the case appears in the public domain, it is intensely discussed in the media and by scientists, and some prominent cases have been discussed for more than 20 years (Table 1 ). Misconduct and retraction notices were exceptions in the article genre analysis, as they presented mostly unique cases. The misconduct notices were mainly found on the NIH repository, which is indexed in the searched databases. Some federal funding agencies like NIH usually publicize investigation findings associated with the research they fund. The results derived from the NIH repository also explains the large proportion of articles from the US (61.9%). However, in some cases, only a few details are provided about the case. For cases that have not received federal funding and have not been reported to federal authorities, the investigation is conducted by local institutions. In such instances, the reporting of findings depends on each institution’s policy and willingness to disclose information [ 21 ]. The other exception involves retraction notices. Despite the existence of ethical guidelines [ 22 ], there is no uniform and a common approach to how a journal should report a retraction. The Retraction Watch website suggests two lists of information that should be included in a retraction notice to satisfy the minimum and optimum requirements [ 22 , 23 ]. As well as disclosing the reason for the retraction and information regarding the retraction process, optimal notices should include: (I) the date when the journal was first alerted to potential problems; (II) details regarding institutional investigations and associated outcomes; (III) the effects on other papers published by the same authors; (IV) statements about more recent replications only if and when these have been validated by a third party; (V) details regarding the journal’s sanctions; and (VI) details regarding any lawsuits that have been filed regarding the case. The lack of transparency and information in retraction notices was also noted in studies that collected and evaluated retractions [ 24 ]. According to Resnik and Dinse [ 25 ], retractions notices related to cases of misconduct tend to avoid naming the specific violation involved in the case. This study found that only 32.8% of the notices identify the actual problem, such as fabrication, falsification, and plagiarism, and 58.8% reported the case as replication failure, loss of data, or error. Potential explanations for euphemisms and vague claims in retraction notices authored by editors could pertain to the possibility of legal actions from the authors, honest or self-reported errors, and lack of resources to conduct thorough investigations. In addition, the lack of transparency can also be explained by the conflicts of interests of the article’s author(s), since the notices are often written by the authors of the retracted article.
The analysis of violations/ethical issues shows the dominance of fabrication and falsification cases and explains the high prevalence of prominent cases. Non-adherence to laws and regulations (REC approval, informed consent, and data protection) was the second most prevalent issue, followed by patient safety, plagiarism, and conflicts of interest. The prevalence of the five most tagged violations in the case analysis was higher than the prevalence found in the analysis of articles that involved the same violations. The only exceptions are fabrication and falsification cases, which represented 45% of the tagged violations in the analysis of cases, and 59.1% in the article analysis. This disproportion shows a predilection for the publication of discussions related to fabrication and falsification when compared to other serious violations. Complex cases involving these types of violations make good headlines and this follows a custom pattern of writing about cases that catch the public and media’s attention [ 26 ]. The way cases of RE and RI violations are explored in the literature gives a sense that only a few scientists are “the bad apples” and they are usually discovered, investigated, and sanctioned accordingly. This implies that the integrity of science, in general, remains relatively untouched by these violations. However, studies on misconduct determinants show that scientific misconduct is a systemic problem, which involves not only individual factors, but structural and institutional factors as well, and that a combined effort is necessary to change this scenario [ 27 , 28 ].
Analysis of cases
A notable increase in RE and RI cases occurred in the 1990s, with a gradual increase until approximately 2006. This result is in agreement with studies that evaluated paper retractions [ 24 , 29 ]. Although our study did not focus only on retractions, the trend is similar. This increase in cases should not be attributed only to the increase in the number of publications, since studies that evaluated retractions show that the percentage of retraction due to fraud has increased almost ten times since 1975, compared to the total number of articles. Our results also show a gradual reduction in the number of cases from 2011 and a greater drop in 2015. However, this reduction should be considered cautiously because many investigations take years to complete and have their findings disclosed. ORI has shown that from 2001 to 2010 the investigation of their cases took an average of 20.48 months with a maximum investigation time of more than 9 years [ 24 ].
The countries from which most cases were reported were the USA (59.6%), the UK (9.8%), Canada (6.0%), Japan (5.5%), and China (2.1%). When analyzed by continent, the highest percentage of cases took place in North America, followed by Europe, Asia, Oceania, Latin America, and Africa. The predominance of cases from the USA is predictable, since the country publishes more scientific articles than any other country, with 21.8% of the total documents, according to SCImago [ 16 ]. However, the same interpretation does not apply to China, which occupies the second position in the ranking, with 11.2%. These differences in the geographical distribution were also found in a study that collected published research on research integrity [ 30 ]. The results found by Aubert Bonn and Pinxten (2019) show that studies in the United States accounted for more than half of the sample collected, and although China is one of the leaders in scientific publications, it represented only 0.7% of the sample. Our findings can also be explained by the search strategy that included only keywords in English. Since the majority of RE and RI cases are investigated and have their findings locally disclosed, the employment of English keywords and terms in the search strategy is a limitation. Moreover, our findings do not allow us to draw inferences regarding the incidence or prevalence of misconduct around the world. Instead, it shows where there is a culture of publicly disclosing information and openly discussing RE and RI cases in English documents.
Scientific field analysis
The results show that 80.8% of reported cases occurred in the medical and health sciences whilst only 1.3% occurred in the humanities. This disciplinary difference has also been observed in studies on research integrity climates. A study conducted by Haven and colleagues, [ 28 ] associated seven subscales of research climate with the disciplinary field. The subscales included: (1) Responsible Conduct of Research (RCR) resources, (2) regulatory quality, (3) integrity norms, (4) integrity socialization, (5) supervisor/supervisee relations, (6) (lack of) integrity inhibitors, and (7) expectations. The results, based on the seven subscale scores, show that researchers from the humanities and social sciences have the lowest perception of the RI climate. By contrast, the natural sciences expressed the highest perception of the RI climate, followed by the biomedical sciences. There are also significant differences in the depth and extent of the regulatory environments of different disciplines (e.g. the existence of laws, codes of conduct, policies, relevant ethics committees, or authorities). These findings corroborate our results, as those areas of science most familiar with RI tend to explore the subject further, and, consequently, are more likely to publish case details. Although the volume of published research in each research area also influences the number of cases, the predominance of medical and health sciences cases is not aligned with the trends regarding the volume of published research. According to SCImago Journal & Country Rank [ 16 ], natural sciences occupy the first place in the number of publications (41,5%), followed by the medical and health sciences (25,1%), engineering (22%), social sciences (7,8%), and the humanities (1,7%). Moreover, biomedical journals are overrepresented in the top scientific journals by IF ranking, and these journals usually have clear policies for research misconduct. High-impact journals are more likely to have higher visibility and scrutiny, and consequently, more likely to have been the subject of misconduct investigations. Additionally, the most well-known general medical journals, including NEJM, The Lancet, and the BMJ, employ journalists to write their news sections. Since these journals have the resources to produce extensive news sections, it is, therefore, more likely that medical cases will be discussed.
Violations analysis
In the analysis of violations, the cases were categorized into major and minor misbehaviors. Most cases involved data fabrication and falsification, followed by cases involving non-adherence to laws and regulations, patient safety, plagiarism, and conflicts of interest. When classified by categories, 12.5% of the tagged violations involved issues in the study design, 16.4% in data collection, 56.0% in reporting, and 15.1% involved collaboration issues. Approximately 80% of the tagged violations involved serious research misbehaviors, based on the ranking of research misbehaviors proposed by Bouter and colleagues. However, as demonstrated in a meta-analysis by Fanelli (2009), most self-declared cases involve questionable research practices. In the meta-analysis, 33.7% of scientists admitted questionable research practices, and 72% admitted when asked about the behavior of colleagues. This finding contrasts with an admission rate of 1.97% and 14.12% for cases involving fabrication, falsification, and plagiarism. However, Fanelli’s meta-analysis does not include data about research misbehaviors in its wider sense but focuses on behaviors that bias research results (i.e. fabrication and falsification, intentional non-publication of results, biased methodology, misleading reporting). In our study, the majority of cases involved FFP (66.4%). Overrepresentation of some types of violations, and underrepresentation of others, might lead to misguided efforts, as cases that receive intense publicity eventually influence policies relating to scientific misconduct and RI [ 20 ].
Sanctions analysis
The five most prevalent outcomes were paper retraction, followed by exclusion from funding applications, exclusion from service or position, dismissal and suspension, and paper correction. This result is similar to that found by Redman and Merz [ 31 ], who collected data from misconduct cases provided by the ORI. Moreover, their results show that fabrication and falsification cases are 8.8 times more likely than others to receive funding exclusions. Such cases also received, on average, 0.6 more sanctions per case. Punishments for misconduct remain under discussion, ranging from the criminalization of more serious forms of misconduct [ 32 ] to social punishments, such as those recently introduced by China [ 33 ]. The most common sanction identified by our analysis—paper retraction—is consistent with the most prevalent types of violation, that is, falsification and fabrication.
Publicizing scientific misconduct
The lack of publicly available summaries of misconduct investigations makes it difficult to share experiences and evaluate the effectiveness of policies and training programs. Publicizing scientific misconduct can have serious consequences and creates a stigma around those involved in the case. For instance, publicized allegations can damage the reputation of the accused even when they are later exonerated [ 21 ]. Thus, for published cases, it is the responsibility of the authors and editors to determine whether the name(s) of those involved should be disclosed. On the one hand, it is envisaged that disclosing the name(s) of those involved will encourage others in the community to foster good standards. On the other hand, it is suggested that someone who has made a mistake should have the right to a chance to defend his/her reputation. Regardless of whether a person's name is left out or disclosed, case reports have an important educational function and can help guide RE- and RI-related policies [ 34 ]. A recent paper published by Gunsalus [ 35 ] proposes a three-part approach to strengthen transparency in misconduct investigations. The first part consists of a checklist [ 36 ]. The second suggests that an external peer reviewer should be involved in investigative reporting. The third part calls for the publication of the peer reviewer’s findings.
Limitations
One of the possible limitations of our study may be our search strategy. Although we have conducted pilot searches and sensitivity tests to reach the most feasible and precise search strategy, we cannot exclude the possibility of having missed important cases. Furthermore, the use of English keywords was another limitation of our search. Since most investigations are performed locally and published in local repositories, our search only allowed us to access cases from English-speaking countries or discussed in academic publications written in English. Additionally, it is important to note that the published cases are not representative of all instances of misconduct, since most of them are never discovered, and when discovered, not all are fully investigated or have their findings published. It is also important to note that the lack of information from the extracted case descriptions is a limitation that affects the interpretation of our results. In our review, only 25 retraction notices contained sufficient information that allowed us to include them in our analysis in conformance with the inclusion criteria. Although our search strategy was not focused specifically on retraction and misconduct notices, we believe that if sufficiently detailed information was available in such notices, the search strategy would have identified them.
Case descriptions found in academic journals are dominated by discussions regarding prominent cases and are mainly published in the news section of journals. Our results show that there is an overrepresentation of biomedical research cases over other scientific fields when compared with the volume of publications produced by each field. Moreover, published cases mostly involve fabrication, falsification, and patient safety issues. This finding could have a significant impact on the academic representation of ethical issues for RE and RI. The predominance of fabrication and falsification cases might diverge the attention of the academic community from relevant but less visible violations and ethical issues, and recently emerging forms of misbehaviors.
Availability of data and materials
This review has been developed by members of the EnTIRE project in order to generate information on the cases that will be made available on the Embassy of Good Science platform ( www.embassy.science ). The dataset supporting the conclusions of this article is available in the Open Science Framework (OSF) repository in https://osf.io/3xatj/?view_only=313a0477ab554b7489ee52d3046398b9 .
National Academies of Sciences E, Medicine. Fostering integrity in research. National Academies Press; 2017.
Davis MS, Riske-Morris M, Diaz SR. Causal factors implicated in research misconduct: evidence from ORI case files. Sci Eng Ethics. 2007;13(4):395–414. https://doi.org/10.1007/s11948-007-9045-2 .
Article Google Scholar
Ampollini I, Bucchi M. When public discourse mirrors academic debate: research integrity in the media. Sci Eng Ethics. 2020;26(1):451–74. https://doi.org/10.1007/s11948-019-00103-5 .
Hesselmann F, Graf V, Schmidt M, Reinhart M. The visibility of scientific misconduct: a review of the literature on retracted journal articles. Curr Sociol La Sociologie contemporaine. 2017;65(6):814–45. https://doi.org/10.1177/0011392116663807 .
Martinson BC, Anderson MS, de Vries R. Scientists behaving badly. Nature. 2005;435(7043):737–8. https://doi.org/10.1038/435737a .
Loikith L, Bauchwitz R. The essential need for research misconduct allegation audits. Sci Eng Ethics. 2016;22(4):1027–49. https://doi.org/10.1007/s11948-016-9798-6 .
OECD. Revised field of science and technology (FoS) classification in the Frascati manual. Working Party of National Experts on Science and Technology Indicators 2007. p. 1–12.
Bouter LM, Tijdink J, Axelsen N, Martinson BC, ter Riet G. Ranking major and minor research misbehaviors: results from a survey among participants of four World Conferences on Research Integrity. Res Integrity Peer Rev. 2016;1(1):17. https://doi.org/10.1186/s41073-016-0024-5 .
Greenberg DS. Resounding echoes of Gallo case. Lancet. 1995;345(8950):639.
Dresser R. Giving scientists their due. The Imanishi-Kari decision. Hastings Center Rep. 1997;27(3):26–8.
Hong ST. We should not forget lessons learned from the Woo Suk Hwang’s case of research misconduct and bioethics law violation. J Korean Med Sci. 2016;31(11):1671–2. https://doi.org/10.3346/jkms.2016.31.11.1671 .
Opel DJ, Diekema DS, Marcuse EK. Assuring research integrity in the wake of Wakefield. BMJ (Clinical research ed). 2011;342(7790):179. https://doi.org/10.1136/bmj.d2 .
Wells F. The Stoke CNEP Saga: did it need to take so long? J R Soc Med. 2010;103(9):352–6. https://doi.org/10.1258/jrsm.2010.10k010 .
Normile D. RIKEN panel finds misconduct in controversial paper. Science. 2014;344(6179):23. https://doi.org/10.1126/science.344.6179.23 .
Wager E. The Committee on Publication Ethics (COPE): Objectives and achievements 1997–2012. La Presse Médicale. 2012;41(9):861–6. https://doi.org/10.1016/j.lpm.2012.02.049 .
SCImago nd. SJR — SCImago Journal & Country Rank [Portal]. http://www.scimagojr.com . Accessed 03 Feb 2021.
Fanelli D. How many scientists fabricate and falsify research? A systematic review and meta-analysis of survey data. PLoS ONE. 2009;4(5):e5738. https://doi.org/10.1371/journal.pone.0005738 .
Steneck NH. Fostering integrity in research: definitions, current knowledge, and future directions. Sci Eng Ethics. 2006;12(1):53–74. https://doi.org/10.1007/PL00022268 .
DuBois JM, Anderson EE, Chibnall J, Carroll K, Gibb T, Ogbuka C, et al. Understanding research misconduct: a comparative analysis of 120 cases of professional wrongdoing. Account Res. 2013;20(5–6):320–38. https://doi.org/10.1080/08989621.2013.822248 .
National Academy of Sciences NAoE, Institute of Medicine Panel on Scientific R, the Conduct of R. Responsible Science: Ensuring the Integrity of the Research Process: Volume I. Washington (DC): National Academies Press (US) Copyright (c) 1992 by the National Academy of Sciences; 1992.
Bauchner H, Fontanarosa PB, Flanagin A, Thornton J. Scientific misconduct and medical journals. JAMA. 2018;320(19):1985–7. https://doi.org/10.1001/jama.2018.14350 .
COPE Council. COPE Guidelines: Retraction Guidelines. 2019. https://doi.org/10.24318/cope.2019.1.4 .
Retraction Watch. What should an ideal retraction notice look like? 2015, May 21. https://retractionwatch.com/2015/05/21/what-should-an-ideal-retraction-notice-look-like/ .
Fang FC, Steen RG, Casadevall A. Misconduct accounts for the majority of retracted scientific publications. Proc Natl Acad Sci USA. 2012;109(42):17028–33. https://doi.org/10.1073/pnas.1212247109 .
Resnik DB, Dinse GE. Scientific retractions and corrections related to misconduct findings. J Med Ethics. 2013;39(1):46–50. https://doi.org/10.1136/medethics-2012-100766 .
de Vries R, Anderson MS, Martinson BC. Normal misbehavior: scientists talk about the ethics of research. J Empir Res Hum Res Ethics JERHRE. 2006;1(1):43–50. https://doi.org/10.1525/jer.2006.1.1.43 .
Sovacool BK. Exploring scientific misconduct: isolated individuals, impure institutions, or an inevitable idiom of modern science? J Bioethical Inquiry. 2008;5(4):271. https://doi.org/10.1007/s11673-008-9113-6 .
Haven TL, Tijdink JK, Martinson BC, Bouter LM. Perceptions of research integrity climate differ between academic ranks and disciplinary fields: results from a survey among academic researchers in Amsterdam. PLoS ONE. 2019;14(1):e0210599. https://doi.org/10.1371/journal.pone.0210599 .
Trikalinos NA, Evangelou E, Ioannidis JPA. Falsified papers in high-impact journals were slow to retract and indistinguishable from nonfraudulent papers. J Clin Epidemiol. 2008;61(5):464–70. https://doi.org/10.1016/j.jclinepi.2007.11.019 .
Aubert Bonn N, Pinxten W. A decade of empirical research on research integrity: What have we (not) looked at? J Empir Res Hum Res Ethics. 2019;14(4):338–52. https://doi.org/10.1177/1556264619858534 .
Redman BK, Merz JF. Scientific misconduct: do the punishments fit the crime? Science. 2008;321(5890):775. https://doi.org/10.1126/science.1158052 .
Bülow W, Helgesson G. Criminalization of scientific misconduct. Med Health Care Philos. 2019;22(2):245–52. https://doi.org/10.1007/s11019-018-9865-7 .
Cyranoski D. China introduces “social” punishments for scientific misconduct. Nature. 2018;564(7736):312. https://doi.org/10.1038/d41586-018-07740-z .
Bird SJ. Publicizing scientific misconduct and its consequences. Sci Eng Ethics. 2004;10(3):435–6. https://doi.org/10.1007/s11948-004-0001-0 .
Gunsalus CK. Make reports of research misconduct public. Nature. 2019;570(7759):7. https://doi.org/10.1038/d41586-019-01728-z .
Gunsalus CK, Marcus AR, Oransky I. Institutional research misconduct reports need more credibility. JAMA. 2018;319(13):1315–6. https://doi.org/10.1001/jama.2018.0358 .
Download references
Acknowledgements
The authors wish to thank the EnTIRE research group. The EnTIRE project (Mapping Normative Frameworks for Ethics and Integrity of Research) aims to create an online platform that makes RE+RI information easily accessible to the research community. The EnTIRE Consortium is composed by VU Medical Center, Amsterdam, gesinn. It Gmbh & Co Kg, KU Leuven, University of Split School of Medicine, Dublin City University, Central European University, University of Oslo, University of Manchester, European Network of Research Ethics Committees.
EnTIRE project (Mapping Normative Frameworks for Ethics and Integrity of Research) has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement N 741782. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and affiliations.
Department of Behavioural Sciences, Faculty of Medicine, University of Debrecen, Móricz Zsigmond krt. 22. III. Apartman Diákszálló, Debrecen, 4032, Hungary
Anna Catharina Vieira Armond & János Kristóf Bodnár
Institute of Ethics, School of Theology, Philosophy and Music, Dublin City University, Dublin, Ireland
Bert Gordijn, Jonathan Lewis & Mohammad Hosseini
Centre for Social Ethics and Policy, School of Law, University of Manchester, Manchester, UK
Center for Medical Ethics, HELSAM, Faculty of Medicine, University of Oslo, Oslo, Norway
Center for Ethics and Law in Biomedicine, Central European University, Budapest, Hungary
Péter Kakuk
You can also search for this author in PubMed Google Scholar
Contributions
All authors (ACVA, BG, JL, MH, JKB, SH and PK) developed the idea for the article. ACVA, PK, JKB performed the literature search and data analysis, ACVA and PK produced the draft, and all authors critically revised it. All authors have read and approved the manuscript.
Corresponding author
Correspondence to Anna Catharina Vieira Armond .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
. Pilot search and search strategy.
Additional file 2
. List of Major and minor misbehavior items (Developed by Bouter LM, Tijdink J, Axelsen N, Martinson BC, ter Riet G. Ranking major and minor research misbehaviors: results from a survey among participants of four World Conferences on Research Integrity. Research integrity and peer review. 2016;1(1):17. https://doi.org/10.1186/s41073-016-0024-5 ).
Additional file 3
. Table containing the number and percentage of countries included in the analysis of articles.
Additional file 4
. Table containing the number and percentage of countries included in the analysis of the cases.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Armond, A.C.V., Gordijn, B., Lewis, J. et al. A scoping review of the literature featuring research ethics and research integrity cases. BMC Med Ethics 22 , 50 (2021). https://doi.org/10.1186/s12910-021-00620-8
Download citation
Received : 06 October 2020
Accepted : 21 April 2021
Published : 30 April 2021
DOI : https://doi.org/10.1186/s12910-021-00620-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Research ethics
- Research integrity
- Scientific misconduct
BMC Medical Ethics
ISSN: 1472-6939
- General enquiries: [email protected]
- OpenBU
- BU Open Access Articles
Cases of ethical violation in research publications: through editorial decision making process

Date Issued
Publisher version.
Export Citation
Permanent link, citation (published version), collections.
- BU Open Access Articles [6228]
- MET: Scholarly Works [167]
Deposit Materials
McCombs School of Business
- Español ( Spanish )
Videos Concepts Unwrapped View All 36 short illustrated videos explain behavioral ethics concepts and basic ethics principles. Concepts Unwrapped: Sports Edition View All 10 short videos introduce athletes to behavioral ethics concepts. Ethics Defined (Glossary) View All 58 animated videos - 1 to 2 minutes each - define key ethics terms and concepts. Ethics in Focus View All One-of-a-kind videos highlight the ethical aspects of current and historical subjects. Giving Voice To Values View All Eight short videos present the 7 principles of values-driven leadership from Gentile's Giving Voice to Values. In It To Win View All A documentary and six short videos reveal the behavioral ethics biases in super-lobbyist Jack Abramoff's story. Scandals Illustrated View All 30 videos - one minute each - introduce newsworthy scandals with ethical insights and case studies. Video Series
Case Studies UT Star Icon
Case Studies
More than 70 cases pair ethics concepts with real world situations. From journalism, performing arts, and scientific research to sports, law, and business, these case studies explore current and historic ethical dilemmas, their motivating biases, and their consequences. Each case includes discussion questions, related videos, and a bibliography.

A Million Little Pieces
James Frey’s popular memoir stirred controversy and media attention after it was revealed to contain numerous exaggerations and fabrications.

Abramoff: Lobbying Congress
Super-lobbyist Abramoff was caught in a scheme to lobby against his own clients. Was a corrupt individual or a corrupt system – or both – to blame?

Apple Suppliers & Labor Practices
Is tech company Apple, Inc. ethically obligated to oversee the questionable working conditions of other companies further down their supply chain?

Approaching the Presidency: Roosevelt & Taft
Some presidents view their responsibilities in strictly legal terms, others according to duty. Roosevelt and Taft took two extreme approaches.

Appropriating “Hope”
Fairey’s portrait of Barack Obama raised debate over the extent to which an artist can use and modify another’s artistic work, yet still call it one’s own.

Arctic Offshore Drilling
Competing groups frame the debate over oil drilling off Alaska’s coast in varying ways depending on their environmental and economic interests.

Banning Burkas: Freedom or Discrimination?
The French law banning women from wearing burkas in public sparked debate about discrimination and freedom of religion.

Birthing Vaccine Skepticism
Wakefield published an article riddled with inaccuracies and conflicts of interest that created significant vaccine hesitancy regarding the MMR vaccine.

Blurred Lines of Copyright
Marvin Gaye’s Estate won a lawsuit against Robin Thicke and Pharrell Williams for the hit song “Blurred Lines,” which had a similar feel to one of his songs.

Bullfighting: Art or Not?
Bullfighting has been a prominent cultural and artistic event for centuries, but in recent decades it has faced increasing criticism for animal rights’ abuse.

Buying Green: Consumer Behavior
Do purchasing green products, such as organic foods and electric cars, give consumers the moral license to indulge in unethical behavior?

Cadavers in Car Safety Research
Engineers at Heidelberg University insist that the use of human cadavers in car safety research is ethical because their research can save lives.

Cardinals’ Computer Hacking
St. Louis Cardinals scouting director Chris Correa hacked into the Houston Astros’ webmail system, leading to legal repercussions and a lifetime ban from MLB.

Cheating: Atlanta’s School Scandal
Teachers and administrators at Parks Middle School adjust struggling students’ test scores in an effort to save their school from closure.

Cheating: Sign-Stealing in MLB
The Houston Astros’ sign-stealing scheme rocked the baseball world, leading to a game-changing MLB investigation and fallout.

Cheating: UNC’s Academic Fraud
UNC’s academic fraud scandal uncovered an 18-year scheme of unchecked coursework and fraudulent classes that enabled student-athletes to play sports.
Cheney v. U.S. District Court
A controversial case focuses on Justice Scalia’s personal friendship with Vice President Cheney and the possible conflict of interest it poses to the case.

Christina Fallin: “Appropriate Culturation?”
After Fallin posted a picture of herself wearing a Plain’s headdress on social media, uproar emerged over cultural appropriation and Fallin’s intentions.

Climate Change & the Paris Deal
While climate change poses many abstract problems, the actions (or inactions) of today’s populations will have tangible effects on future generations.

Cover-Up on Campus
While the Baylor University football team was winning on the field, university officials failed to take action when allegations of sexual assault by student athletes emerged.

Covering Female Athletes
Sports Illustrated stirs controversy when their cover photo of an Olympic skier seems to focus more on her physical appearance than her athletic abilities.

Covering Yourself? Journalists and the Bowl Championship
Can news outlets covering the Bowl Championship Series fairly report sports news if their own polls were used to create the news?

Cyber Harassment
After a student defames a middle school teacher on social media, the teacher confronts the student in class and posts a video of the confrontation online.

Defending Freedom of Tweets?
Running back Rashard Mendenhall receives backlash from fans after criticizing the celebration of the assassination of Osama Bin Laden in a tweet.

Dennis Kozlowski: Living Large
Dennis Kozlowski was an effective leader for Tyco in his first few years as CEO, but eventually faced criminal charges over his use of company assets.

Digital Downloads
File-sharing program Napster sparked debate over the legal and ethical dimensions of downloading unauthorized copies of copyrighted music.

Dr. V’s Magical Putter
Journalist Caleb Hannan outed Dr. V as a trans woman, sparking debate over the ethics of Hannan’s reporting, as well its role in Dr. V’s suicide.

East Germany’s Doping Machine
From 1968 to the late 1980s, East Germany (GDR) doped some 9,000 athletes to gain success in international athletic competitions despite being aware of the unfortunate side effects.
Ebola & American Intervention
Did the dispatch of U.S. military units to Liberia to aid in humanitarian relief during the Ebola epidemic help or hinder the process?


Edward Snowden: Traitor or Hero?
Was Edward Snowden’s release of confidential government documents ethically justifiable?

Ethical Pitfalls in Action
Why do good people do bad things? Behavioral ethics is the science of moral decision-making, which explores why and how people make the ethical (and unethical) decisions that they do.
Ethical Use of Home DNA Testing
The rising popularity of at-home DNA testing kits raises questions about privacy and consumer rights.

Flying the Confederate Flag
A heated debate ensues over whether or not the Confederate flag should be removed from the South Carolina State House grounds.

Freedom of Speech on Campus
In the wake of racially motivated offenses, student protests sparked debate over the roles of free speech, deliberation, and tolerance on campus.

Freedom vs. Duty in Clinical Social Work
What should social workers do when their personal values come in conflict with the clients they are meant to serve?

Full Disclosure: Manipulating Donors
When an intern witnesses a donor making a large gift to a non-profit organization under misleading circumstances, she struggles with what to do.

Gaming the System: The VA Scandal
The Veterans Administration’s incentives were meant to spur more efficient and productive healthcare, but not all administrators complied as intended.

German Police Battalion 101
During the Holocaust, ordinary Germans became willing killers even though they could have opted out from murdering their Jewish neighbors.
Head Injuries & American Football
Many studies have linked traumatic brain injuries and related conditions to American football, creating controversy around the safety of the sport.
Head Injuries & the NFL
American football is a rough and dangerous game and its impact on the players’ brain health has sparked a hotly contested debate.
Healthcare Obligations: Personal vs. Institutional
A medical doctor must make a difficult decision when informing patients of the effectiveness of flu shots while upholding institutional recommendations.

High Stakes Testing
In the wake of the No Child Left Behind Act, parents, teachers, and school administrators take different positions on how to assess student achievement.

In-FUR-mercials: Advertising & Adoption
When the Lied Animal Shelter faces a spike in animal intake, an advertising agency uses its moral imagination to increase pet adoptions.

Krogh & the Watergate Scandal
Egil Krogh was a young lawyer working for the Nixon Administration whose ethics faded from view when asked to play a part in the Watergate break-in.

Limbaugh on Drug Addiction
Radio talk show host Rush Limbaugh argued that drug abuse was a choice, not a disease. He later became addicted to painkillers.

U.S. Olympic swimmer Ryan Lochte’s “over-exaggeration” of an incident at the 2016 Rio Olympics led to very real consequences.

Meet Me at Starbucks
Two black men were arrested after an employee called the police on them, prompting Starbucks to implement “racial-bias” training across all its stores.

Myanmar Amber
Buying amber could potentially fund an ethnic civil war, but refraining allows collectors to acquire important specimens that could be used for research.

Negotiating Bankruptcy
Bankruptcy lawyer Gellene successfully represented a mining company during a major reorganization, but failed to disclose potential conflicts of interest.

Pao & Gender Bias
Ellen Pao stirred debate in the venture capital and tech industries when she filed a lawsuit against her employer on grounds of gender discrimination.

Pardoning Nixon
One month after Richard Nixon resigned from the presidency, Gerald Ford made the controversial decision to issue Nixon a full pardon.

Patient Autonomy & Informed Consent
Nursing staff and family members struggle with informed consent when taking care of a patient who has been deemed legally incompetent.
Prenatal Diagnosis & Parental Choice
Debate has emerged over the ethics of prenatal diagnosis and reproductive freedom in instances where testing has revealed genetic abnormalities.

Reporting on Robin Williams
After Robin Williams took his own life, news media covered the story in great detail, leading many to argue that such reporting violated the family’s privacy.

Responding to Child Migration
An influx of children migrants posed logistical and ethical dilemmas for U.S. authorities while intensifying ongoing debate about immigration.
Retracting Research: The Case of Chandok v. Klessig
A researcher makes the difficult decision to retract a published, peer-reviewed article after the original research results cannot be reproduced.
Sacking Social Media in College Sports
In the wake of questionable social media use by college athletes, the head coach at University of South Carolina bans his players from using Twitter.

Selling Enron
Following the deregulation of electricity markets in California, private energy company Enron profited greatly, but at a dire cost.

Snyder v. Phelps
Freedom of speech was put on trial in a case involving the Westboro Baptist Church and their protesting at the funeral of U.S. Marine Matthew Snyder.

Something Fishy at the Paralympics
Rampant cheating has plagued the Paralympics over the years, compromising the credibility and sportsmanship of Paralympian athletes.

Sports Blogs: The Wild West of Sports Journalism?
Deadspin pays an anonymous source for information related to NFL star Brett Favre, sparking debate over the ethics of “checkbook journalism.”

Stangl & the Holocaust
Franz Stangl was the most effective Nazi administrator in Poland, killing nearly one million Jews at Treblinka, but he claimed he was simply following orders.

Teaching Blackface: A Lesson on Stereotypes
A teacher was put on leave for showing a blackface video during a lesson on racial segregation, sparking discussion over how to teach about stereotypes.

The Astros’ Sign-Stealing Scandal
The Houston Astros rode a wave of success, culminating in a World Series win, but it all came crashing down when their sign-stealing scheme was revealed.

The Central Park Five
Despite the indisputable and overwhelming evidence of the innocence of the Central Park Five, some involved in the case refuse to believe it.

The CIA Leak
Legal and political fallout follows from the leak of classified information that led to the identification of CIA agent Valerie Plame.

The Collapse of Barings Bank
When faced with growing losses, investment banker Nick Leeson took big risks in an attempt to get out from under the losses. He lost.

The Costco Model
How can companies promote positive treatment of employees and benefit from leading with the best practices? Costco offers a model.

The FBI & Apple Security vs. Privacy
How can tech companies and government organizations strike a balance between maintaining national security and protecting user privacy?

The Miss Saigon Controversy
When a white actor was cast for the half-French, half-Vietnamese character in the Broadway production of Miss Saigon , debate ensued.

The Sandusky Scandal
Following the conviction of assistant coach Jerry Sandusky for sexual abuse, debate continues on how much university officials and head coach Joe Paterno knew of the crimes.

The Varsity Blues Scandal
A college admissions prep advisor told wealthy parents that while there were front doors into universities and back doors, he had created a side door that was worth exploring.

Providing radiation therapy to cancer patients, Therac-25 had malfunctions that resulted in 6 deaths. Who is accountable when technology causes harm?

Welfare Reform
The Welfare Reform Act changed how welfare operated, intensifying debate over the government’s role in supporting the poor through direct aid.

Wells Fargo and Moral Emotions
In a settlement with regulators, Wells Fargo Bank admitted that it had created as many as two million accounts for customers without their permission.
Stay Informed
Support our work.

On Being a Scientist: Responsible Conduct in Research, Second Edition (1995)
Chapter: responding to violations of ethical standards.
investigated and adjudicated through different channels. Regulations adopted by the National Science Foundation and the Public Health Service define misconduct to include "other serious deviations from accepted research practices," in addition to falsification, fabrication, and plagiarism, leaving open the possibility that other actions could be considered misconduct in science. The problem with such language is that it could allow a scientist to be accused of misconduct for using novel or unorthodox research methods, even though such methods are sometimes needed to proceed in science. Federal officials respond by saying that this language is needed to prosecute ethical breaches that do not strictly fall into the categories of falsification, fabrication, or plagiarism and that no scientist has been accused of misconduct on the basis of using unorthodox research methods. This area of science policy is still evolving.
Another category of behaviors—including sexual or other forms of harassment, misuse of funds, gross negligence in a person's professional activities, tampering with the experiments of others or with instrumentation, and violations of government research regulations—are not necessarily associated with scientific conduct. Institutions need to discourage and respond to such behaviors. But these behaviors are subject to generally applicable legal and social penalties and should be dealt with using the same procedures that would be applied to anyone.
RESPONDING TO VIOLATIONS OF ETHICAL STANDARDS
One of the most difficult situations that a researcher can encounter is to see or suspect that a colleague has violated the ethical standards of the research community. It is easy to find excuses to do nothing, but someone who has witnessed misconduct has an unmistakable obligation to act. At the most immediate level, misconduct can seriously obstruct or damage one's own research or the research of colleagues. More broadly, even a single case of misconduct can malign scientists and their institutions, result in the imposition of counterproductive regulations, and shake public confidence in the integrity of science.
To be sure, raising a concern about unethical conduct is rarely an easy thing to do. In some cases, anonymity is possible-but not always. Reprisals by the accused person and by skeptical colleagues have occurred in the past and have had serious consequences. Any allegation of misconduct is a very important charge that needs to be
taken seriously. If mishandled, an allegation can gravely damage the person charged, the one who makes the charge, the institutions involved, and science in general.
Someone who is confronting a problem involving research ethics usually has more options than are immediately apparent. In most cases the best thing to do is to discuss the situation with a trusted friend or advisor. In universities, faculty advisors, department chairs, and other senior faculty can be invaluable sources of advice in deciding whether to go forward with a complaint.
An important consideration is deciding when to put a complaint in writing. Once in writing, universities are obligated to deal with a complaint in a more formal manner than if it is made verbally. Putting a complaint in writing can have serious consequences for the career of a scientist and should be undertaken only after thorough consideration.
The National Science Foundation and Public Health Service require all research institutions that receive public funds to have procedures in place to deal with allegations of unethical practice. These procedures take into account fairness for the accused, protection for the accuser, coordination with funding agencies, and requirements for confidentiality and disclosure.
In addition, many universities and other research institutions have designated an ombudsman, ethics officer, or other official who is available to discuss situations involving research ethics. Such discussions are carried out in strictest confidence whenever possible. Some institutions provide for multiple entry points, so that complainants can go to a person with whom they feel comfortable.
Government agencies, including the National Science Foundation and Public Health Service, enforce laws and regulations that deal with misconduct in science. At the Public Health Service in Washington, D.C., complaints can be referred to the appropriate office through the Office of Research Integrity. At the National Science Foundation in Arlington, Virginia, complaints can be directed to the Office of the Inspector General. Within universities, research grant officials can provide guidance on whether federal rules may be involved in filing a complaint.
Many institutions have prepared written materials that offer guidance in situations involving professional ethics. Volume II of Responsible Science: Ensuring the Integrity of the Research Process (National Academy Press, Washington, D.C., 1993) reprints a number of these documents. Sigma Xi, a national society of research scientists headquartered in Research Triangle Park, North Carolina, the American Association for the Advancement of Science in Washington, D.C., and other scientific and engineering professional organizations also are prepared to advise scientists who encounter cases of possible misconduct.
The research system exerts many pressures on beginning and experienced researchers alike. Principal investigators need to raise funds and attract students. Faculty members must balance the time spent on research with the time spent teaching undergraduates. Industrial sponsorship of research introduces the possibility of conflicts of interest.
All parts of the research system have a responsibility to recognize and respond to these pressures. Institutions must review their own policies, foster awareness of research ethics, and ensure that researchers are aware of the policies that are in place. And researchers should constantly be aware of the extent to which ethically based decisions will influence their success as scientists.
THE SCIENTIST IN SOCIETY
This booklet has concentrated on the responsibilities of scientists for the advancement of science, but scientists have additional responsibilities to society. Even scientists conducting the most fundamental research need to be aware that their work can ultimately have a great impact on society. Construction of the atomic bomb and the development of recombinant DNA—events that grew out of basic research on the nucleus of the atom and investigations of certain bacterial enzymes, respectively—are two examples of how seemingly arcane areas of science can have tremendous societal consequences.
The occurrence and consequences of discoveries in basic research are virtually impossible to foresee. Nevertheless, the scientific community must recognize the potential for such discoveries and be prepared to address the questions that they raise. If scientists do find that their discoveries have implications for some important aspect of public affairs, they have a responsibility to call attention to the public issues involved. They might set up a suitable public forum involving experts with different perspectives on the issue at hand. They could then seek to develop a
Since the first edition of On Being a Scientist was published in 1989, more than 200,000 copies have been distributed to graduate and undergraduate science students. Now this well-received booklet has been updated to incorporate the important developments in science ethics of the past 6 years and includes updated examples and material from the landmark volume Responsible Science (National Academy Press, 1992).
The revision reflects feedback from readers of the original version. In response to graduate students' requests, it offers several case studies in science ethics that pose provocative and realistic scenarios of ethical dilemmas and issues.
On Being a Scientist presents penetrating discussions of the social and historical context of science, the allocation of credit for discovery, the scientist's role in society, the issues revolving around publication, and many other aspects of scientific work. The booklet explores the inevitable conflicts that arise when the black and white areas of science meet the gray areas of human values and biases.
Written in a conversational style, this booklet will be of great interest to students entering scientific research, their instructors and mentors, and anyone interested in the role of scientific discovery in society.
READ FREE ONLINE
Welcome to OpenBook!
You're looking at OpenBook, NAP.edu's online reading room since 1999. Based on feedback from you, our users, we've made some improvements that make it easier than ever to read thousands of publications on our website.
Do you want to take a quick tour of the OpenBook's features?
Show this book's table of contents , where you can jump to any chapter by name.
...or use these buttons to go back to the previous chapter or skip to the next one.
Jump up to the previous page or down to the next one. Also, you can type in a page number and press Enter to go directly to that page in the book.
Switch between the Original Pages , where you can read the report as it appeared in print, and Text Pages for the web version, where you can highlight and search the text.
To search the entire text of this book, type in your search term here and press Enter .
Share a link to this book page on your preferred social network or via email.
View our suggested citation for this chapter.
Ready to take your reading offline? Click here to buy this book in print or download it as a free PDF, if available.
Get Email Updates
Do you enjoy reading reports from the Academies online for free ? Sign up for email notifications and we'll let you know about new publications in your areas of interest when they're released.
Advertisement
- Publications
This site uses cookies to enhance your user experience. By continuing to use this site you are agreeing to our COOKIE POLICY .
Grab your lab coat. Let's get started
Create an account below to get 6 c&en articles per month, receive newsletters and more - all free., it seems this is your first time logging in online. please enter the following information to continue., as an acs member you automatically get access to this site. all we need is few more details to create your reading experience., not you sign in with a different account..
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Already have an ACS ID? Log in here
The key to knowledge is in your (nitrile-gloved) hands
Access more articles now. choose the acs option that’s right for you..
Already an ACS Member? Log in here
$0 Community Associate
ACS’s Basic Package keeps you connected with C&EN and ACS.
- Access to 6 digital C&EN articles per month on cen.acs.org
- Weekly delivery of the C&EN Essential newsletter
$80 Regular Members & Society Affiliates
ACS’s Standard Package lets you stay up to date with C&EN, stay active in ACS, and save.
- Access to 10 digital C&EN articles per month on cen.acs.org
- Weekly delivery of the digital C&EN Magazine
- Access to our Chemistry News by C&EN mobile app
$160 Regular Members & Society Affiliates $55 Graduate Students $25 Undergraduate Students
ACS’s Premium Package gives you full access to C&EN and everything the ACS Community has to offer.
- Unlimited access to C&EN’s daily news coverage on cen.acs.org
- Weekly delivery of the C&EN Magazine in print or digital format
- Significant discounts on registration for most ACS-sponsored meetings

Your account has been created successfully, and a confirmation email is on the way.
Your username is now your ACS ID.
- Are lab safety violations research misconduct?
New paper suggests they are, given their connection to the research enterprise
By dalmeet singh chawla, special to c&en, may 23, 2024 | a version of this story appeared in volume 102, issue 16.
- Highly-cited chemist is suspended for claiming to be affiliated with Russian and Saudi universities
- Former chemistry professor jailed for making meth
- Essay criticizing efforts to increase diversity in organic synthesis deleted after backlash from chemists
- UAE university breaks ties with beleaguered nanoscientist

The US Office of Research Integrity defines research misconduct as “fabrication, falsification, or plagiarism in proposing, performing, or reviewing research, or in reporting research results.”
But should that definition also include violations of safety in the lab? That’s what Bor Luen Tang, a biochemist at the National University of Singapore, suggests in a paper published last month in the Journal of Academic Ethics (2024, DOI: 10.1007/s10805-024-09531-w ).
“Lab safety violations should be considered as such because these transgressions occur within the context of research, and can negatively impact research,” Tang tells C&EN in an email.
Researchers with adequate training and knowledge are obliged to follow safety rules and protocols, Tang says. “Thus, if someone deliberately violate[s] or deviate[s] markedly from safety rules/protocols and an accident/incident occurs, that person is potentially culpable.”
Finding a researcher guilty of violating lab safety rules, as with allforms of research misconduct, requires a preponderance of evidence regarding intent, Tang says. For instance, investigators should consider whether the violation occurred involuntarily, perhaps because of physical or mental illness or natural mishap, he adds.
In Tang’s opinion, researchers who are found guilty of lab safety violations should be sanctioned, with their institution’s health and safety office being the first authority to take action. Depending on the laws within a country, governmental agencies should also be involved, as well as funding agencies that supported the work, if they specify oversight of their grantees’ actions, Tang says.
But Craig Merlic, an organic chemist at the University of California, Los Angeles, and director of the UC Center for Laboratory Safety, says he doesn’t think lab safety violations should be labeled as research misconduct, though he does agree that egregious breaches of safety policies should have consequences.
“While the severity of any falsification, fabrication, and plagiarism can vary greatly, the defining actions are fairly straightforward,” he says. But it’s harder to determine what a violation of lab safety is, Merlic says. “Is it an action that results in an incident such as a chemical spill, or an accident that results in an injury? Or can it be merely not following best lab practices set by a lab?”
In his paper, Tang suggests that everyone, regardless of their endowment, status, or power, should be held accountable for breaches of lab safety. “But we all know from root cause analyses that culpability rarely stops at the immediate players,” Merlic says.
Merlic, who just wrote UCLA’s policy for student noncompliance with safety rules, which is not yet posted, thinks simple compliance should not be the end goal of safety programs. “Instead, compliance should be the natural outcome of an institution’s robust culture of safety that goes well beyond regulatory compliance,” he says.
You might also like...

Sign up for C&EN's must-read weekly newsletter
Contact us to opt out anytime
- Share on Facebook
- Share on Linkedin
- Share on Reddit
This article has been sent to the following recipient:
Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter
The power is now in your (nitrile gloved) hands
Sign up for a free account to get more articles. or choose the acs option that’s right for you..
Already have an ACS ID? Log in
Create a free account To read 6 articles each month from
Join acs to get even more access to.
Main Navigation
- Contact NeurIPS
- Code of Ethics
- Code of Conduct
- Create Profile
- Journal To Conference Track
- Diversity & Inclusion
- Proceedings
- Future Meetings
- Exhibitor Information
- Privacy Policy
NeurIPS Code of Ethics
The Code of Ethics aims to guide the NeurIPS community towards higher standards of ethical conduct as it pertains to elements of research ethics and the broader societal and environmental impact of research submitted to NeurIPS. It outlines conference expectations about the ethical practices that must be adopted by the submitting authors, members of the program and organizing committees. The Code of Ethics complements the NeurIPS Code of Conduct , which focuses on professional conduct and research integrity issues, including plagiarism, fraud and reproducibility concerns. The points described below also inform the NeurIPS Submission Checklist, which outlines more concrete communication requirements.
Potential Harms Caused by the Research Process
Research involving human subjects or participants:
- Fair Wages: all human research subjects or participants must receive appropriate compensation. If you make use of crowdsourcing or contract work for a particular task as part of your research project, you must respect the minimum hourly rate in the region where the work is carried out.
- Research involving human participants: if the research presented involves direct interactions between the researchers and human participants or between a technical system and human participants, authors are required to follow existing protocols in their institutions (e.g. human subject research accreditation, IRB) and go through the relevant process. In cases when no formal process exists, they can undergo an equivalent informal process (e.g. via their peers or an internal ethics review).
Data-related concerns:
The points listed below apply to all datasets used for submissions, both for publicly available data and internal datasets.
- Privacy: Datasets should minimize the exposure of any personally identifiable information, unless informed consent from those individuals is provided to do so.
- Consent: Any paper that chooses to create a dataset with real data of real people should ask for the explicit consent of participants, or explain why they were unable to do so.
- Deprecated datasets: Authors should take care to confirm with dataset creators that a dataset is still available for use. Datasets taken down by the original author (ie. deemed obsolete, or otherwise discontinued), should no longer be used, unless it is for the purposes of audit or critical assessment. For some indication of known depreciated datasets, please refer to the NeurIPS list of deprecated datasets.
- Copyright and Fair Use: While the norms of fair use and copyright in machine learning research are still evolving, authors must respect the terms of datasets that have defined licenses (e.g. CC 4.0, MIT, etc).
- Representative evaluation practice: When collecting new datasets or making decisions about which datasets to use, authors should assess and communicate the degree to which their datasets are representative of their intended population. Claims of diverse or universal representation should be substantiated by concrete evidence or examples.
Societal Impact and Potential Harmful Consequences
Authors should transparently communicate the known or anticipated consequences of research: for instance via the paper checklist or a separate section in a submission.
The following specific areas are of particular concern:
- Safety: Contributors should consider whether there are foreseeable situations in which their technology can be used to harm, injure or kill people through its direct application, side effects, or potential misuse. We do not accept research whose primary goal is to increase the lethality of weapons systems.
- Security: Researchers should consider whether there is a risk that applications could open security vulnerabilities or cause serious accidents when deployed in real world environments. If this is the case, they should take concrete steps to recommend or implement ways to protect against such security risks.
- Discrimination: Researchers should consider whether the technology they developed can be used to discriminate, exclude, or otherwise negatively impact people, including impacts on the provision of services such as healthcare, education or access to credit.
- Surveillance: Researchers should consult on local laws or legislation before collecting or analyzing any bulk surveillance data. Surveillance should not be used to predict protected categories, or be used in any way to endanger individual well-being.
- Deception & Harassment: Researchers should communicate about whether their approach could be used to facilitate deceptive interactions that would cause harm such as theft, fraud, or harassment, and whether it could be used to impersonate public figures and influence political processes, or as a tool to promote hate speech or abuse.
- Environment: Researchers should consider whether their research is going to negatively impact the environment by, e.g., promoting fossil fuel extraction, increasing societal consumption or producing substantial amounts of greenhouse gasses.
- Human Rights: We prohibit circulation of any research work that builds upon or facilitates illegal activity, and we strongly discourage any work that could be used to deny people rights to privacy, speech, health, liberty, security, legal personhood, or freedom of conscience or religion.
- Bias and fairness: Contributors should consider any suspected biases or limitations to the scope of performance of models or the contents of datasets and inspect these to ascertain whether they encode, contain or exacerbate bias against people of a certain gender, race, sexuality, or other protected characteristics.
Impact Mitigation Measures
We propose some reflection and actions taken to mitigate potential harmful consequences from the research project.
- Data and model documentation: Researchers should communicate the details of the dataset or the model as part of their submissions via structured templates.
- Data and model licenses: If releasing data or models, authors should also provide licenses for them. These should include the intended use and limitations of these artifacts, in order to prevent misuse or inappropriate use.
- Secure and privacy-preserving data storage & distribution : Authors should leverage privacy protocols, encryption and anonymization to reduce the risk of data leakage or theft. Stronger measures should be employed for more sensitive data (e.g., biometric or medical data).
- Responsible release and publication strategy: Models that have a high risk for misuse or dual-use should be released with necessary safeguards to allow for controlled use of the model, e.g. by requiring that users adhere to a code of conduct to access the model. Authors of papers exposing a security vulnerability in a system should follow the responsible disclosure procedures of the system owners.
- Allowing access to research artifacts: When releasing research artifacts, it is important to make accessible the information required to understand these artifacts (e.g. the code, execution environment versions, weights, and hyperparameters of systems) to enable external scrutiny and auditing.
- Disclose essential elements for reproducibility: Any work submitted to NeurIPS should be accompanied by the information sufficient for the reproduction of results described. This can include the code, data, model weights, and/or a description of the computational resources needed to train the proposed model or validate the results.
- Ensure legal compliance: Ensure adequate awareness of regional legal requirements. This can be done, for instance, by consulting with law school clinics specializing in intellectual property and technology issues. Additional information is required from authors where legal compliance could not be met due to human rights violations (e.g. freedom of expression, the right to work and education, bodily autonomy, etc.).
Violations to the Code of Ethics should be reported to [email protected] . NeurIPS reserves the right to reject the presentation of scientific works that violate the Code of Ethics. Notice that conference contributors are also obliged to adhere to additional ethical codes or review requirements arising from other stakeholders such as funders and research institutions.
Further reading
UNDERSTANDING LICENSES
- Towards Standardization of Data Licenses: The Montreal Data License
- Behavioral Use Licensing for Responsible AI
- Choose an open source license
MODEL AND DATA DOCUMENTATION TEMPLATES
- Model Cards for Model Reporting
- Datasheets for Datasets
- Using AI Factsheets for AI Governance
- ML Lifecycle Documentation Practices
SOCIETAL IMPACT
- Safety: Key Concepts in AI Safety: An Overview
- Security: SoK: Security and Privacy in Machine Learning
- Discrimination: Bias in algorithms – Artificial intelligence and discrimination ; What about fairness, bias and discrimination?
- Surveillance: The Human Right to Privacy in the Digital Age
- Deception & Harassment: Generative Language Models and Automated Influence Operations: Emerging Threats and Potential Mitigations
- Environment: Quantifying the Carbon Emissions of Machine Learning
- Human Rights: Technology and Rights | Human Rights Watch
- Bias and fairness: Fairness and machine learning
- Dual use problem: Dual use of artificial-intelligence-powered drug discovery
- Data Enrichment: Responsible Sourcing for Data Enrichment
- Synthetic Media: PAI’s Responsible Practices for Synthetic Media
RELATED ENDEAVORS
- ACM Code of Ethics
- ACL Ethics FAQ
- ICLR Code of Ethics
- Responsible Conduct of Research Training
RELATED RESEARCH COMMUNITIES
- IEEE SaTML 2023
- Aies Conference
- FORC 2022
Live Nation Faces Consumer Class Action as Well as DOJ Lawsuit
By Tre’Vaughn Howard

Live Nation Entertainment Inc. is facing a proposed consumer class action that alleges unfair predatory ticket pricing in the live music event market space, following the Justice Department’s pursuit to break up the company over antitrust violations.
Abraham Leifer claims that since the 2010 merger of Live Nation and Ticketmaster, the entities have collectively built a monopoly and “juggernaut trust” over three major economic markets in the US within the live concert and event space: concert promotion for major concert venues, as well as primary and secondary ticketing services for major concert venues.He filed the complaint in the US District Court for the Southern District of New York on Thursday.
Live Nation—which controls hundreds of venues through leaseholds, ownership, or equity interests—requires venues to use Ticketmaster, which controls the primary ticket sale of up to 80% of all major concert venues in the US.
This class action comes as the US Department of Justice sued Live Nation on Thursday in the same court. The DOJ’s suit, joined by 29 states and Washington DC, accuses Live Nation of locking venues into exclusive, long-term ticketing contracts—among other anti-competitive violations. Live Nation is also facing additional pending litigation brought in 2022 by ticket-buyers.
Thursday’s consumer suit alleges that customers “pay supracomeptitive prices because Ticketmaster levies excessively high fees in the primary market on tickets that it sells which are then passed down to consumers in the secondary market.”
The face value of tickets sold in the primary market is supracompetitively priced as well, the complaint says.
The complaint includes two class definitions: The first is a nationwide class of end-user purchasers who bought secondary tickets on the secondary ticket exchanges from a secondary seller who had purchased a primary ticket or secondary ticket to resell it for an event with Ticketmaster or one of its Live Nation affiliates, since the 2010 merger; the second class is is tailored to various US states and territories.
“This exclusive, anti-competitive and unlawful tying eliminates significant competition, as it boxes all other primary ticketing agents out from servicing any concerts or events where Live Nation is the promoter,” the complaint says.
Live Nation acts as the promoting agent for at least 80 of the top 100 largest concert venues, while controlling more than 265 concert venues in North America and managing more than 400 musical artists. It has a market share in the promotion of major concert venues that exceeds 60%, the complaint also says.
The latest complaint asserts two violations of the Sherman Antitrust Act: monopolization and restraints of trade. The complaint also alleges violations of state antitrust laws in almost 30 US states and territories, including Florida and Washington DC.
Leifer is seeking class certification; injunctive and equitable relief; statutory, actual, compensatory, consequential, treble, punitive, and nominal damages; restitution and/or disgorgement of profits; pre- and post-judgment interest; attorneys’ fees; and costs.
Live Nation and Ticketmaster didn’t immediately respond to a request for comment.
Israel David LLC represents Leifer and the proposed class.
The case is Leifer v. Live Nation Entertainment Inc. , S.D.N.Y., No. 1:24-cv-03994, 5/23/24.
To contact the reporter on this story: Tre'Vaughn Howard at [email protected]
To contact the editor responsible for this story: Blair Chavis at [email protected]
Learn more about Bloomberg Law or Log In to keep reading:
Learn about bloomberg law.
AI-powered legal analytics, workflow tools and premium legal & business news.
Already a subscriber?
Log in to keep reading or access research tools.
- Work & Careers
- Life & Arts
- Currently reading: Business school teaching case study: Unilever chief signals rethink on ESG
- Business school teaching case study: can green hydrogen’s potential be realised?
- Business school teaching case study: how electric vehicles pose tricky trade dilemmas
- Business school teaching case study: is private equity responsible for child labour violations?
Business school teaching case study: Unilever chief signals rethink on ESG

- Business school teaching case study: Unilever chief signals rethink on ESG on x (opens in a new window)
- Business school teaching case study: Unilever chief signals rethink on ESG on facebook (opens in a new window)
- Business school teaching case study: Unilever chief signals rethink on ESG on linkedin (opens in a new window)
- Business school teaching case study: Unilever chief signals rethink on ESG on whatsapp (opens in a new window)
Gabriela Salinas and Jeeva Somasundaram
Roula Khalaf, Editor of the FT, selects her favourite stories in this weekly newsletter.
In April this year, Hein Schumacher, chief executive of Unilever, announced that the company was entering a “new era for sustainability leadership”, and signalled a shift from the central priority promoted under his predecessor , Alan Jope.
While Jope saw lack of social purpose or environmental sustainability as the way to prune brands from the portfolio, Schumacher has adopted a more balanced approach between purpose and profit. He stresses that Unilever should deliver on both sustainability commitments and financial goals. This approach, which we dub “realistic sustainability”, aims to balance long- and short-term environmental goals, ambition, and delivery.
As a result, Unilever’s refreshed sustainability agenda focuses harder on fewer commitments that the company says remain “very stretching”. In practice, this entails extending deadlines for taking action as well as reducing the scale of its targets for environmental, social and governance measures.
Such backpedalling is becoming widespread — with many companies retracting their commitments to climate targets , for example. According to FactSet, a US financial data and software provider, the number of US companies in the S&P 500 index mentioning “ESG” on their earnings calls has declined sharply : from a peak of 155 in the fourth quarter 2021 to just 29 two years later. This trend towards playing down a company’s ESG efforts, from fear of greater scrutiny or of accusations of empty claims, even has a name: “greenhushing”.
Test yourself
This is the fourth in a series of monthly business school-style teaching case studies devoted to the responsible business dilemmas faced by organisations. Read the piece and FT articles suggested at the end before considering the questions raised.
About the authors: Gabriela Salinas is an adjunct professor of marketing at IE University; Jeeva Somasundaram is an assistant professor of decision sciences in operations and technology at IE University.
The series forms part of a wider collection of FT ‘instant teaching case studies ’, featured across our Business Education publications, that explore management challenges.
The change in approach is not limited to regulatory compliance and corporate reporting; it also affects consumer communications. While Jope believed that brands sold more when “guided by a purpose”, Schumacher argues that “we don’t want to force fit [purpose] on brands unnecessarily”.
His more nuanced view aligns with evidence that consumers’ responses to the sustainability and purpose communication attached to brand names depend on two key variables: the type of industry in which the brand operates; and the specific aspect of sustainability being communicated.
In terms of the sustainability message, research in the Journal of Business Ethics found consumers can be less interested when product functionality is key. Furthermore, a UK survey in 2022 found that about 15 per cent of consumers believed brands should support social causes, but nearly 60 per cent said they would rather see brand owners pay taxes and treat people fairly.
Among investors, too, “anti-purpose” and “anti-ESG” sentiment is growing. One (unnamed) leading bond fund manager even suggested to the FT that “ESG will be dead in five years”.
Media reports on the adverse impact of ESG controversies on investment are certainly now more frequent. For example, while Jope was still at the helm, the FT reported criticism of Unilever by influential fund manager Terry Smith for displaying sustainability credentials at the expense of managing the business.
Yet some executives feel under pressure to take a stand on environmental and social issues — in many cases believing they are morally obliged to do so or through a desire to improve their own reputations. This pressure may lead to a conflict with shareholders if sustainability becomes a promotional tool for managers, or for their personal social responsibility agenda, rather than creating business value .
Such opportunistic behaviours may lead to a perception that corporate sustainability policies are pursued only because of public image concerns.
Alison Taylor, at NYU Stern School of Business, recently described Unilever’s old materiality map — a visual representation of how companies assess which social and environmental factors matter most to them — to Sustainability magazine. She depicted it as an example of “baggy, vague, overambitious goals and self-aggrandising commitments that make little sense and falsely suggest a mayonnaise and soap company can solve intractable societal problems”.
In contrast, the “realism” approach of Schumacher is being promulgated as both more honest and more feasible. Former investment banker Alex Edmans, at London Business School, has coined the term “rational sustainability” to describe an approach that integrates financial principles into decision-making, and avoids using sustainability primarily for enhancing social image and reputation.
Such “rational sustainability” encompasses any business activity that creates long-term value — including product innovation, productivity enhancements, or corporate culture initiatives, regardless of whether they fall under the traditional ESG framework.
Similarly, Schumacher’s approach aims for fewer targets with greater impact, all while keeping financial objectives in sight.
Complex objectives, such as having a positive impact on the world, may be best achieved indirectly, as expounded by economist John Kay in his book, Obliquity . Schumacher’s “realistic sustainability” approach means focusing on long-term value creation, placing customers and investors to the fore. Saving the planet begins with meaningfully helping a company’s consumers and investors. Without their support, broader sustainability efforts risk failure.
Questions for discussion
Read: Unilever has ‘lost the plot’ by fixating on sustainability, says Terry Smith
Companies take step back from making climate target promises
The real impact of the ESG backlash
Unilever’s new chief says corporate purpose can be ‘unwelcome distraction ’
Unilever says new laxer environmental targets aim for ‘realism’
How should business executives incorporate ESG criteria in their commercial, investor, internal, and external communications? How can they strike a balance between purpose and profits?
How does purpose affect business and brand value? Under what circumstances or conditions can the impact of purpose be positive, neutral, or negative?
Are brands vehicles by which to drive social or environmental change? Is this the primary role of brands in the 21st century or do profits and clients’ needs come first?
Which categories or sectors might benefit most from strongly articulating and communicating a corporate purpose? Are there instances in which it might backfire?
In your opinion, is it necessary for brands to take a stance on social issues? Why or why not, and when?
Climate Capital

Where climate change meets business, markets and politics. Explore the FT’s coverage here .
Are you curious about the FT’s environmental sustainability commitments? Find out more about our science-based targets here
Promoted Content
Explore the series.

Follow the topics in this article
- Sustainability Add to myFT
- Impact investing Add to myFT
- Corporate governance Add to myFT
- Corporate social responsibility Add to myFT
- Business school case Add to myFT
International Edition
Advertisement
In Boston, schools test ways to target student absences with sports, raffles and Saturday schedules
Copy the code below to embed the wbur audio player on your site.
<iframe width="100%" height="124" scrolling="no" frameborder="no" src="https://player.wbur.org/news/2024/05/28/massachusetts-chronic-absenteeism-students-pilot-program"></iframe>
At New Mission High in Hyde Park, many students jump into the school day by first hitting the gymnasium.
Shawn Polk, the school's health and physical education teacher, typically opens the gym doors around 7 a.m., cranking up catchy pop music over a loudspeaker as kids trickle inside to play basketball or volleyball.
On a recent Friday, she greets nearly two dozen students who've come to play. They quickly begin dribbling basketballs, shooting some hoops and laughing with their friends. In about 40 minutes, Polk blows a whistle to signal it's time for them to head to their homerooms.
“The morning is a great opportunity for them to get physically active prior to school,” Polk said. “The incentive to have a physical activity they’re interested in gets them in, gets them out of bed.”

The effort gets some students out of bed and to class on time, but New Mission instructors say a little fitness and fun also helps kids stay in school. The high school is one of several in Boston to adopt the district's initiative to address spikes in absences, especially among older students, by adding new extracurriculars and other programs.
The district launched its "Win the Day" campaign in March, and students at New Mission say the early morning sports routine at their school has so far been well-received.
“Most of the time the gym is really packed," said ninth grader Jesdian Vargas, 15, as he changed out of basketball shoes to begin walking to class. "There’s a lot of good vibe going on throughout the gym. ... It’s like a fun experience.”
Saturday school, check-ins and sports
School leaders across the district not only wanted to improve attendance this spring semester, but also better identify why students don't show up. The issue took on more gravity as "chronic absenteeism" — defined here, the state says, as students who miss 10% or more of days they're enrolled for — soared across the U.S. after the pandemic. And, for some districts, student attendance was already a concern before COVID.

Statewide, 22% of students were considered chronically absent in the 2022-'23 school year, compared to 13% in 2017-'18, according to state education data .
In Boston, nearly 40% of public school students were chronically absent last year, versus 25% in 2017-'18.
Research shows students who regularly miss school by middle school years are more likely to drop out of high school. Chronic absenteeism by ninth grade is also an indicator of whether a student is likely to graduate on time or not, studies suggest. Data reveals certain groups, including low-income students and students of color, also disproportionately have higher levels of absences.
Within Boston Public Schools, Region 8 — a cluster of secondary schools grouped by geography — rates of chronic absenteeism were “unusually high,” said Cory McCarthy, chief of student support for the district, ranging from 40% to 74% last year.
“I thought that we could sort of use this pilot to really unearth some of the root causes as to why some of our kids aren't coming, and staying,” he said.
Students across Region 8 are a diverse group, and a lot of them face a range of challenges with ties to socio-economic problems: from academic struggles to transportation headaches to mental health issues. Many live in households that primarily speak languages other than English, and in some cases, their families depend on them to work part-time while attending school.
McCarthy said economic hardships created by the pandemic impacted school attendance for many kids because "they need jobs to provide for their family."
As part of the district campaign, schools offered weekend tutoring sessions to drop recorded absences. Students could work with a tutor to complete assignments on Saturdays, and if they worked there for at least three and a half hours, McCarthy said, they could “earn back” an absence.
Another campaign initiative called "tag a friend" designated some students as "diplomats" who were asked to call or text absent classmates to check in. Those check-in serve as a way to encourage students to come to school or offer avenues for peer support.
The district also entered partnerships with local businesses to offer kids free services as a reward for good attendance. For example, students could win an attendance “chip” to redeem at a local barbershop or salon for a free haircut.
And like New Mission High, several other schools, including Boston Community Leadership Academy-McCormack, Another Course to College and the Henderson K-12 Inclusion School, began hosting intramural sports before the start of the school day.
“Sports culture in buildings is something that’s underutilized,” McCarthy, who also used to coach basketball for the district, said. "Sports brings so much community to our schools.”
A spokesman said the district was working to compile data on whether the pilots succeeded at improving attendance.
Still, McCarthy touted how relationship-building acts as a core focus of the campaign, improving how districts take a greater interest in students' lives by asking peers and staffers to reach out more to students and their families — or even conduct home visits to find out what families need.

At an April school committee meeting , Boston Public Schools Superintendent Mary Skipper lauded the efforts and stressed the importance of improving attendance among district students.
“We're tapping in, incentivizing with kids, but also making connection with the parents,” she said, adding, “This is one of the biggest issues nationally, statewide, and with us that we just, we have to continue to work on.”
Skipper said the district wants to expand the pilot to more schools in September, noting that it began with Region 8 schools to identify which strategies work and which don't.
Prizes for good attendance
At the Boston Community Leadership Academy-McCormack in Dorchester, school counselor Justine Grace paced around before delivering morning announcements on a rainy Tuesday last month. She explained that many students miss school for a variety of reasons. Some have to help with family matters, she said, acting as translators at medical appointments or helping with child care at home. Others have long distances to travel to campus.
"It could be they have to take two trains, two buses," to reach the school, Grace said.

The school serves students in the seventh to 12th grade, and its ninth graders struggle the most with attendance.
In the spirit of the "Win the Day" pilot, the school organized a raffle this month to award prizes, like movie tickets or field trips, for good attendance.
The effort clicked for 15-year-old Essence Loving, a ninth grader, who said she came to school more often to win a class field trip to Apex, an arcade-type entertainment complex and also to receive a “perfect attendance award.”
"Kids want to come to school and have fun," Grace said. "It's a social aspect, and we know it's academic driven, but we need to figure out other ways to get them in the building."
Ninth grader Nathalie Medina said her attendance improved since January after she sat with the school counselor and figured out why she was struggling: it was a matter of adjusting her morning routine and waking up earlier. They formed an attendance contract.
“I was slacking off really bad last year,” Medina said. “I love school and having motivation, and like people [caring] about me being present made me want to come even more.”
Plus, she said if she was late or didn’t come to school, her younger brother in the grade below hers wouldn’t either.
“He would have to wait for me, and that affected him, too,” Medina said. “So he also motivated me to get here on time so that I don't mess up his education.”
This segment aired on May 28, 2024.
- State reports decline in chronically absent students
- Mass. students are absent from school at 'staggering' rates, says state education official
- State education secretary takes to airwaves with a message: make school attendance ‘a priority’

Suevon Lee Assistant Managing Editor, Education Suevon Lee leads WBUR's education coverage.
More from WBUR

At Justice Alito’s House, a ‘Stop the Steal’ Symbol on Display
An upside-down flag, adopted by Trump supporters contesting the Biden victory, flew over the justice’s front lawn as the Supreme Court was considering an election case.
A photo obtained by The Times shows an inverted flag at the Alito residence on Jan. 17, 2021, three days before the Biden inauguration. Credit...
Supported by
- Share full article

By Jodi Kantor
Jodi Kantor, who has been reporting on the Supreme Court, including the behind-the-scenes story of how the justices overturned the right to abortion, welcomes tips at nytimes.com/tips .
- May 16, 2024
After the 2020 presidential election, as some Trump supporters falsely claimed that President Biden had stolen the office, many of them displayed a startling symbol outside their homes, on their cars and in online posts: an upside-down American flag.
One of the homes flying an inverted flag during that time was the residence of Supreme Court Justice Samuel A. Alito Jr., in Alexandria, Va., according to photographs and interviews with neighbors.
The upside-down flag was aloft on Jan. 17, 2021, the images showed. President Donald J. Trump’s supporters, including some brandishing the same symbol, had rioted at the Capitol a little over a week before. Mr. Biden’s inauguration was three days away. Alarmed neighbors snapped photographs, some of which were recently obtained by The New York Times. Word of the flag filtered back to the court, people who worked there said in interviews.
While the flag was up, the court was still contending with whether to hear a 2020 election case, with Justice Alito on the losing end of that decision. In coming weeks, the justices will rule on two climactic cases involving the storming of the Capitol on Jan. 6, including whether Mr. Trump has immunity for his actions. Their decisions will shape how accountable he can be held for trying to overturn the last presidential election and his chances for re-election in the upcoming one.
“I had no involvement whatsoever in the flying of the flag,” Justice Alito said in an emailed statement to The Times. “It was briefly placed by Mrs. Alito in response to a neighbor’s use of objectionable and personally insulting language on yard signs.”
Judicial experts said in interviews that the flag was a clear violation of ethics rules, which seek to avoid even the appearance of bias, and could sow doubt about Justice Alito’s impartiality in cases related to the election and the Capitol riot.
The mere impression of political opinion can be a problem, the ethics experts said. “It might be his spouse or someone else living in his home, but he shouldn’t have it in his yard as his message to the world,” said Amanda Frost, a law professor at the University of Virginia.
This is “the equivalent of putting a ‘Stop the Steal’ sign in your yard, which is a problem if you’re deciding election-related cases,” she said.
Interviews show that the justice’s wife, Martha-Ann Alito, had been in a dispute with another family on the block over an anti-Trump sign on their lawn, but given the timing and the starkness of the symbol, neighbors interpreted the inverted flag as a political statement by the couple.
The longstanding ethics code for the lower courts, as well as the recent one adopted by the Supreme Court, stresses the need for judges to remain independent and avoid political statements or opinions on matters that could come before them.
“You always want to be proactive about the appearance of impartiality,” Jeremy Fogel, a former federal judge and the director of the Berkeley Judicial Institute, said in an interview. “The best practice would be to make sure that nothing like that is in front of your house.”
The court has also repeatedly warned its own employees against public displays of partisan views, according to guidelines circulated to the staff and reviewed by The Times. Displaying signs or bumper stickers is not permitted, according to the court’s internal rule book and a 2022 memo reiterating the ban on political activity.

Asked if these rules also apply to justices, the court declined to respond.
The exact duration that the flag flew outside the Alito residence is unclear. In an email from Jan. 18, 2021, reviewed by The Times, a neighbor wrote to a relative that the flag had been upside down for several days at that point.
In recent years, the quiet sanctuary of his street, with residents who are Republicans and Democrats, has tensed with conflict, neighbors said. Around the 2020 election , a family on the block displayed an anti-Trump sign with an expletive. It apparently offended Mrs. Alito and led to an escalating clash between her and the family, according to interviews.
Some residents have also bridled at the noise and intrusion brought by protesters, who started showing up outside the Alito residence in 2022 after the Supreme Court overturned the federal right to abortion. Other neighbors have joined the demonstrators, whose intent was “to bring the protest to their personal lives because the decisions affect our personal lives,” said Heather-Ann Irons, who came to the street to protest.
The half-dozen neighbors who saw the flag, or knew of it, requested anonymity because they said they did not want to add to the contentiousness on the block and feared reprisal. Last Saturday, May 11, protesters returned to the street, waving flags of their own (“Don’t Tread on My Uterus”) and using a megaphone to broadcast expletives at Justice Alito, who was in Ohio giving a commencement address . Mrs. Alito appeared in a window, complaining to the Supreme Court security detail outside.
Turning the American flag upside down is a symbol of emergency and distress, first used as a military S.O.S., historians said in interviews. In recent decades, it has increasingly been used as a political protest symbol — a controversial one, because the flag code and military tradition require the paramount symbol of the United States to be treated with respect.
Over the years, upside-down flags have been displayed by both the right and the left as an outcry over a range of issues, including the Vietnam War, gun violence , the Supreme Court’s overturning of the constitutional right to abortion and, in particular, election results. In 2012, Tea Party followers inverted flags at their homes to signal disgust at the re-election of President Barack Obama. Four years later, some liberals advised doing the same after Mr. Trump was elected.
During Mr. Trump’s quest to win, and then subvert, the 2020 election, the gesture took off as never before, becoming “really established as a symbol of the ‘Stop the Steal’ campaign,” according to Alex Newhouse, a researcher at the University of Colorado Boulder.
A flood of social media posts exhorted Trump supporters to flip over their flags or purchase new ones to display upside down.
“If Jan. 6 rolls around and Biden is confirmed by the Electoral College our nation is in distress!!” a poster wrote on Patriots.win, a forum for Trump supporters, garnering over a thousand “up” votes. “If you cannot go to the DC rally then you must do your duty and show your support for our president by flying the flag upside down!!!!”
Local newspapers from Lexington, Ky. , to Sun City, Ariz., to North Jersey wrote about the flags cropping up nearby. A few days before the inauguration, a Senate candidate in Minnesota flew an upside-down flag on his campaign vehicle .
Hanging an inverted flag outside a home was “an explicit signifier that you are part of this community that believes America has been taken and needs to be taken back,” Mr. Newhouse said.
This spring, the justices are already laboring under suspicion by many Americans that whatever decisions they make about the Jan. 6 cases will be partisan. Justice Clarence Thomas has declined to recuse himself despite the direct involvement of his wife, Virginia Thomas, in efforts to overturn the election.
Now, with decisions in the Jan. 6 cases expected in just a few weeks, a similar debate may unfurl about Justice Alito, the ethics experts said. “It really is a question of appearances and the potential impact on public confidence in the court,” Mr. Fogel said. “I think it would be better for the court if he weren’t involved in cases arising from the 2020 election. But I’m pretty certain that he will see that differently.”
If Justice Alito were on another court, Mr. Fogel said, the flag could also trigger some sort of review to determine if there was any misconduct. But because the Supreme Court serves as the arbiter of its own behavior, “you don’t really have anywhere to take it,” he said.
Aric Toler contributed reporting. Julie Tate contributed research.
Jodi Kantor is a Pulitzer Prize-winning investigative reporter and co-author of “She Said,” which recounts how she and Megan Twohey broke the story of sexual abuse allegations against Harvey Weinstein, helping to ignite the #MeToo movement. Instagram • More about Jodi Kantor
Advertisement
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMC Med Ethics

Violation of ethical principles in clinical research. Influences and possible solutions for Latin America
Borys alberto cornejo moreno.
1 Research Coordinator Medical Associate, Planning and Institutional Liaison Coordination, Healthcare Services Management, The Mexican Institute of Social Security, Hidalgo Delegation, Delegación Hidalgo, Boulevard Luis Donaldo Colosio n° 516, Pachuca de Soto, Hidalgo, CP 42070, Mexico
Gress Marissell Gómez Arteaga
2 Planning and Institutional Liaison Coordinator, Healthcare Services Management, The Mexican Institute of Social Security, Hidalgo Delegation, Boulevard Luis Donaldo Colosio n° 516, Canutillo, Pachuca de Soto, Hidalgo, CP 42070, Mexico
Even though we are now well into the 21st century and notwithstanding all the abuse to individuals involved in clinical studies that has been documented throughout History, fundamental ethical principles continue to be violated in one way or another.
Here are some of the main factors that contribute to the abuse of subjects participating in clinical trials: paternalism, improper use of informed consent, lack of strict ethical supervision, pressure exerted by health institutions to increase the production of scientific material, and the absence of legislation regarding ethics in terms of health care and research. Are researchers ready to respect fundamental ethical principles in light of the ample window of information provided by individual genomes, while defending the rights of the subjects participating in clinical studies as a major priority?
As one of the possible solutions to this problem, education regarding fundamental ethical principles is suggested for participants in research studies as an initial method of cognitive training in ethics, together with the promotion of ethical behavior in order to encourage the adoption of reasonable policies in the field of values, attitudes and behavior.
Ethical principles such as autonomy, beneficence, nonmaleficence and justice [ 1 ] are useful for both researchers and participants in a clinical trial as an overarching frame of reference. They are also helpful for making decisions concerning each individual case, as a means of avoiding abusive situations in experimental processes that involve human beings. Nevertheless, close supervision of both researchers and research subjects is necessary to achieve compliance with ethical principles.
Unfortunately, some of the populations that are most exposed to abuse are vulnerable groups (i.e. confined or chronically ill individuals, elderly patients, pregnant women, single mothers, economically disadvantaged populations), whose main weakness is a latent need to be treated, receive medical care or be assisted in some other way. These people are an easy target for abuses related to their own rights, as their situation forces them to look for help without having many options and generally being less informed about their rights. Some questions arise when considering this situation in a generation that should be familiar with the ethical principles of scientific investigation: Are we ready to act ethically in the genomics era, when a significant number of illnesses and their prognoses are directly related to genome analysis? Would it be a better option to make the principles known in order to empower vulnerable populations, helping them to be aware of their rights and educating them on the ways in which they could suffer abuse? Would this prevent manipulation and promote a change of attitude in terms of values, such as respect and tolerance, in our generation?
Factors that promote noncompliance with ethical principles
Lack of strict ethical surveillance.
There is currently no evidence of strict ethical surveillance in many countries of Latin America that participate in clinical investigation, thus making the abuse of ethical principles very likely, as well as factual. The lack of strict ethical supervision encourages the researcher not to inform the participants of their rights. This enables the researcher to have more freedom for the development of the trial. Although it might be assumed that all the necessary information concerning individual rights will be provided by him, in fact this rarely occurs.
Paternalism
An additional factor is paternalism, a situation that allows the treating physician or investigator to perform actions without consulting the patient, and in some cases even with a certain level of concealment that allows them, according to their own judgment, to act in the most convenient manner [ 2 ]. In this setting, the patients see the physician/researcher as their savior, voluntarily giving up their ability to make decisions and subtly losing their autonomy throughout the process. A frequent mindset in participant subjects that are simultaneously being treated in clinical trials is to think of the physician as the person responsible for deciding the treatment, since they have the necessary training and a better capability for making illness-related decisions. This blind trust in the physician/researcher allows him to act almost without limits, minimizing what patients might understand about their illness or what their genetic information might represent. The only thing needed from the patients from this moment on will be their signatures on a document that they rarely understand but acknowledge as a requirement for being treated [ 3 ], the informed consent form.
Informed consent (IC)
The IC form was created to protect the rights of research subjects. However, it currently appears to be used more as way for investigators to protect themselves from any legal situation in which they could be indicted for suspicion of abuse. It has been done as a routine procedure without regard to its real meaning. There are currently very few studies analyzing the effectiveness of the IC form 3 in Latin America or verifying whether the advantages and disadvantages of a trial are fully explained to the patient –i.e. whether or not there are benefits or if these benefits will be direct or long-term-. These kinds of circumstances are frequently covered or not explicitly explained in the IC form, due to the repercussions they might have on recruitment. In an effort to avoid unnecessary difficulties in the research process, only positive expectations are presented, such as: "this study will help discover the cure for your illness", without a clarifying statement to explaining that the probability of success could be extremely low.
The Informed Consent form was created to help the patients understand all the information related to every aspect of the study they would participate in and −particularly important in our times− full disclosure by the investigator of every aspect concerning the experiment, including risks, benefits and interests; as well as complete comprehension of the information by the patient. Where the research involves studies in genetics, it is essential to add the assurance of confidentiality for the obtained information, since this provides substantial and valuable data for both treatment and prognosis [ 4 ].
The concealment of information or the omission of any part of this document also constitutes a form of abuse, even though it may well be an everyday practice in some Latin American countries. When there is no interest in verifying the level of understanding of the patients concerning the study in which they participate, autonomy and justice are voluntarily left out of the equation and the principle of “nonmaleficence " is at risk. Autonomy is also threatened when, not being aware of all the aspects of a particular situation, patients are asked to make decisions that make them responsible for the direct consequences of an action. When only a partial truth is revealed, other people make the actual decisions and autonomy becomes a mere simulation. Justice is placed at risk because the subjects do not receive what they are entitled to when participating in a study, which is to know the reality concerning their situation, the process and the information currently available in the field regarding their entity. The principle of nonmaleficence is also endangered when there is a risk of damaging the subjects while deciding on their behalf, since we do not know if our decision and the decision of the participant are equal.
Lack of healthcare legislation to promote the protection of vulnerable subjects
The General Healthcare Law of Mexico, as updated on June 7, 2012 does explicitly mention the risk of incurring these circumstances. The lack of attention to the aforementioned factors in different countries contributes to an inadequate prevention of abuse [ 5 ].
Other related factors
There is pressure exerted by healthcare institutions on investigators in terms of the productivity of scientific material. Since a large portion of the research funds in both developed and developing countries come from government budgets and from non-governmental organizations, productivity goals are always considered. This is one of the reasons why scientists are pressured to produce; and to do so, they are tempted to abridge the information given to subjects, leaving out what they consider irrelevant for the knowledge of patients, thus accelerating the process of recruitment and production. In cases where transparency complicates the research process, the truth could not be prioritized, which in turn limits value-driven actions.
Are these kinds of acts justifiable?
Notwithstanding the fact that everyone is accountable for their actions, responsibility mostly lies with decision makers who know the consequences of previously noticed actions. Regardless of the motivation, this is not justifiable to the point of leaving ethical principles aside and adopting purely utilitarian stances where "the means are justified by the end," without regard to beneficence, justice and autonomy of the subjects. This is analogous to what happened in the past when experiments were performed on human beings without any consent or benefits and by infringing on their rights.
Article 4 of the Political Constitution of Mexico, in the first item referring to personal rights, states that: "every person has the right to health care protection" [ 6 ] in addition to stating the right to social security and physical and mental healthcare, as second-generation human rights [ 7 ]. When omissions of information happen, healthcare is not protected, nor is social security provided.
Possible solutions
Ethics committees, established for the supervision of international treaties such as the Declaration of Helsinki [ 8 ], could be overhauled in terms of surveillance measures to allow a stricter monitoring of patients participating in clinical trials. For instance, accessibility to ethics committees could be useful for subjects in trials, who would be interviewed and asked to answer questionnaires adapted to their academic training; aiming to explore their level of understanding about the study they are involved in and to assess their actual perception. Despite the fact that in recent years the level of comprehension of participants in clinical trials has been a controversial subject, it is necessary to acknowledge that anyone who voluntarily participates in a research study and signs an IC form deserves, and has a right to an explanation according to their own cognitive capabilities. Likewise, it is essential to design and build a strategic plan for the promotion of values, attitudes and ethical principles as a short or medium-term plan of action to educate the general population, particularly vulnerable groups. This promotion of values could be the cornerstone of a comprehensive and effective educational plan running alongside an established program, to avoid losing resources, that is, unnecessary expenses in a budget focused on an educational campaign to prevent abuse. If the necessary education is not provided, the risk of standing outside of an ethical context is high; a context where moral values are reduced to the memorization of concepts that are not practiced or to a merely ethical positivism where our random emotions lead our actions. An actual knowledge has to be pursued for the implementation of principles with intelligence, specificity and consideration.
There is no current evidence to support the contention that fundamental ethical principles are applied in many countries that carry out clinical investigations, particularly in Latin America and regarding aspects related to safeguarding the rights of individuals and the privacy of confidential information such as data proceeding from the human genome. So long as concrete measures are not taken, vulnerable groups will continue to be the most affected populations. Abuse-related factors are predictable and can be prevented by working hard and collectively committing to the solution. In the advent of the genomics era, it is urgent to start closely monitoring compliance with fundamental ethical principles for research to prevent having massive leaks of information along with mistreatment, harm and violation of fundamental ethical principles, particularly in vulnerable groups.
Abbreviations
IC: Informed Consent.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
BC Design and writing of the manuscript, performance of data research. GG Coordination and help in drafting the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
http://www.biomedcentral.com/1472-6939/13/35/prepub
Acknowledgements
We thank the Healthcare Services Management of the Mexican Institute of Social Security at Hidalgo Delegation for the support in the preparation and publication of this manuscript.
- Beauchamp TL, Childress JF. Principles of Biomedical Ethics. 5th. USA: Oxford University Press; 2008. [ Google Scholar ]
- Gillon R. Paternalism and medical ethics. Br Med J. 1985; 290 :1971–1972. doi: 10.1136/bmj.290.6486.1971. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Verástegui E. Consenting of the vulnerable: the informed consent procedure in advanced cancer patients in Mexico. BMC Med Ethics. 2006; 7 :13. doi: 10.1186/1472-6939-7-13. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Silva-Zolezzi I, Hidalgo-Miranda A, Estrada-Gil J, Fernández-López JC, Uribe-Figueroa L. et al. Analysis of genomic diversity in Mexican mestizo population to develop genomic medicine in Mexico. PNAS. 2009; 106 :8611–8616. doi: 10.1073/pnas.0903045106. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- de Salud LG, Titulo Q. Investigación para la Salud. México; 2012. Available at: http://www.salud.gob.mx/unidades/cdi/legis/lgs/index-t5.htm . [ Google Scholar ]
- Political Constitution of the Mexican United States. Available from: http://www.diputados.gob.mx/LeyesBiblio/pdf/1.pdf .
- United Nations Human Rights. http://www.ohchr.org .
- Leaning J. War crimes and medical science. BMJ. 1996; 313 :1413–1415. doi: 10.1136/bmj.313.7070.1413. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
The study's rules stated no one under 50 years old was allowed to enroll. UCSD audited files for 50 of the more than 400 patients and found seven were too young, including a minor. Carome, the ...
This page contains cases in which administrative actions were imposed due to findings of research misconduct. The list only includes those who CURRENTLY have an imposed administrative actions against them. ... Each case is categorized according to the year in which ORI closed the case. 2024. Case Summary: Brigidi, Gian-Stefano. Case Summary ...
This paper represents the culmination of seven years of research on serious ethical violations in medicine. Serious ethical violations are acts that not only disregard codes of medical ethics, but also risk directly harming patients and subjecting the wrongdoer to criminal, tort, or medical board actions.
It was therefore in violation of French law and the Declaration of Helsinki, an international ethics document that guides clinical research. ... Drug safety agency says IHU studies appear to have violated research ethics laws, confirms it has referred case to prosecutor. ... Part of the failure lies with France's law on research ethics, Amiel ...
Research Ethics Cases for Use by the NIH Community. Theme 23 - Authorship, Collaborations, and Mentoring (2023) ... For example, obvious violations of specific rules and regulations or of generally accepted standards of conduct would typically be unacceptable. However, it is also plausible that blind adherence to accepted rules or standards ...
Elizabeth A. Gulleen, MD and Margaret Lubwama, MBChB, MMed. Patients living in low-and middle-income countries (LMICs) shoulder the greatest burden of infections caused by antimicrobial-resistant pathogens. AMA J Ethics. 2024;26 (5):E373-379. doi: 10.1001/amajethics.2024.373. Case and Commentary.
Several studies have already documented research ethics violations among indigenous populations [5,6,7,8,9,10 ... informed consent and a violation of civil rights in addition to unapproved genetic research with DNA samples in prior research also. The case raised awareness of cultural and political sensitivities around what constitutes ...
Japanese hospital uncovers flood of research ethics violations. In nearly 160 cases, researchers failed to give patients a way to opt out of studies. By. Mark Zastrow. A brain and heart hospital ...
The areas of Research Ethics (RE) and Research Integrity (RI) are rapidly evolving. Cases of research misconduct, other transgressions related to RE and RI, and forms of ethically questionable behaviors have been frequently published. The objective of this scoping review was to collect RE and RI cases, analyze their main characteristics, and discuss how these cases are represented in the ...
The areas of Research Ethics (RE) and Research Integrity (RI) are rapidly evolving. Cases of research misconduct, other transgressions related to RE and RI, and forms of ethically questionable behaviors have been frequently published. The objective of this scoping review was to collect RE and RI cases, analyze their main characteristics, and ...
By egregious violations, we mean clear violations of codes of medical ethics and law that directly harm patients. The working group meeting followed four years of research on cases of violations led by a team at Washington University School of Medicine (WUSM) with funding from the National Institute of Aging (NIA).
Advancing psychology to benefit society and improve lives. Find resources on research misconduct, publication ethics, protecting research participants, ethics of online research, and guidance from various agencies and organizations, such as the NIH.
The purpose of this paper is to identify different cases of ethical violation in research and inform and educate researchers to avoid any violations in publication and research ethics. Furthermore, this article will demonstrate how KODISA journals identify and penalize ethical violations and strengthens its publication ethics and practices.
An ongoing increase in the number of cases of alleged research misconduct in universities has been observed (NRF Citation 2020 ... which offers a clear definition of research misconduct in its federal laws, in Taiwan, violations of research ethics and guidelines for case handling are mainly included in the executive orders issued by government ...
More than 70 cases pair ethics concepts with real world situations. From journalism, performing arts, and scientific research to sports, law, and business, these case studies explore current and historic ethical dilemmas, their motivating biases, and their consequences. Each case includes discussion questions, related videos, and a bibliography.
Industrial sponsorship of research introduces the possibility of conflicts of interest. All parts of the research system have a responsibility to recognize and respond to these pressures. Institutions must review their own policies, foster awareness of research ethics, and ensure that researchers are aware of the policies that are in place.
List of medical ethics cases. ... was an American psychologist and psychotherapist best known for his unconventional 24-hour therapy as well as ethical violations concerning his treatment of Beach Boys co ... University of Minnesota research participant Dan Markingson committed suicide in May 2004 while enrolled in an industry-sponsored ...
Research ethics matter for scientific integrity, human rights and dignity, and collaboration between science and society. ... In extreme cases of self-plagiarism, entire datasets or papers are sometimes duplicated. These are major ethical violations because they can skew research findings if taken as original data.
Multiple examples of unethical research studies conducted in the past throughout the world have cast a significant historical shadow on research involving human subjects. Examples include the Tuskegee Syphilis Study from 1932 to 1972, Nazi medical experimentation in the 1930s and 1940s, and research conducted at the Willowbrook State School in the 1950s and 1960s.[1] As the aftermath of these ...
The US Office of Research Integrity defines research misconduct as "fabrication, falsification, or plagiarism in proposing, performing, or reviewing research, or in reporting research results ...
Research involving human participants: if the research presented involves direct interactions between the researchers and human participants or between a technical system and human participants, authors are required to follow existing protocols in their institutions (e.g. human subject research accreditation, IRB) and go through the relevant ...
Live Nation Entertainment Inc. is facing a proposed consumer class action that alleges unfair predatory ticket pricing in the live music event market space, following the Justice Department's pursuit to break up the company over antitrust violations. Abraham Leifer claims that since the 2010 merger of Live Nation and Ticketmaster, the entities have collectively built a monopoly and ...
Abstract. ARABIC/ اللغة العربية. Background: Despite the potential for ethical violations when research is conducted with conflict-affected populations, there is limited information on how and the extent to which ethical considerations specific to doing research with these populations are integrated into national and international ethics guidelines and, in turn, how these ...
The change in approach is not limited to regulatory compliance and corporate reporting; it also affects consumer communications. While Jope believed that brands sold more when "guided by a ...
Responding to Violations of Ethical Standards. One of the most difficult situations that a researcher can encounter is to see or suspect that a colleague has violated the ethical standards of the research community. It is easy to find excuses to do nothing, but someone who has witnessed misconduct has an unmistakable obligation to act.
Boston Public Schools' new campaign to tackle absenteeism seeks to reward students for improved attendance while identifying why some kids "chronically" miss school.
A research project should achieve its objectives by using proven methods that, in the case of inductive research, are credible, reliable, and transferable or, in the case of deductive research, generalizable, objective, representative, and valid (Drolet & Ruest, accepted). Participants discussing these issues noted that researchers who adopt a ...
Judicial experts said in interviews that the flag was a clear violation of ethics rules, which seek to avoid even the appearance of bias, and could sow doubt about Justice Alito's impartiality ...
Discussion. Here are some of the main factors that contribute to the abuse of subjects participating in clinical trials: paternalism, improper use of informed consent, lack of strict ethical supervision, pressure exerted by health institutions to increase the production of scientific material, and the absence of legislation regarding ethics in terms of health care and research.