

See All Patient & Visitor Information
- All Specialties & Services
- Gastroenterology (GI)
- Heart & Vascular
- LGBTQ+ Care & Services
- Neurology & Neurosurgery
- Orthopaedics
- Patient Information
- Billing & Financial Assistance
- Classes & Events
- Insurance Information
- International Patients
- Visiting Us From Out of State?
- Online Second Opinions
- Price Transparency
- Request Medical Records
- Video Visits
- Visitor Information
- Dining Options
- Locations & Directions
- Our Neighborhood & City
- Visitor's Guide
- Magnet Site Visit: Public Notice
Schedule an Appointment
We offer online appointment scheduling for adult and pediatric primary care and many specialties.
Patient Portal
Communicate with your doctor, view test results, schedule appointments and more.
See All Healthcare Professionals Information
- Referring Physicians
- About our Physicians
- Physician Relations Team
- Refer a Patient
- Molecular and Genomic Diagnostic Laboratories
- Nurse Residency Program
- Nursing Careers at UChicago Medicine
- Nursing Careers at Ingalls Memorial
- Nursing Programs
- Nursing Research
- Nursing Student Opportunities
- Employee Resources
- Continuing Medical Education
- Continuing Nursing Education
- Employee Login
- Graduate Medical Education
- Password Reset Self-Service
UChicago Medicine and Ingalls Memorial offer a broad range of challenging clinical and non-clinical career opportunities doing work that really matters.
Research & Clinical Trials
- Clinical Trials
Find a Clinical Trial
- Clinical Trial FAQs
- Clinical Trial Resources
- Office of Clinical Research
- Clinical Trials at Ingalls
- Our Research
- Firsts at the Forefront
- Research & Innovation
- Our Nobel Laureates
- The Forefront: Health & Science News
- Research & Discoveries News
- Biological Science News
Learn more about clinical trials and find a trial that might be right for you.
Breaking down barriers to breast cancer screening for high-risk individuals
October 30, 2020
Written By Jane Kollmer
- Breast Cancer
- Mammography
- Hematology and Oncology (Cancer)
- Prevention and Screening
- Women's Health
- Research and Discoveries
- Call Us At 1-888-824-0200
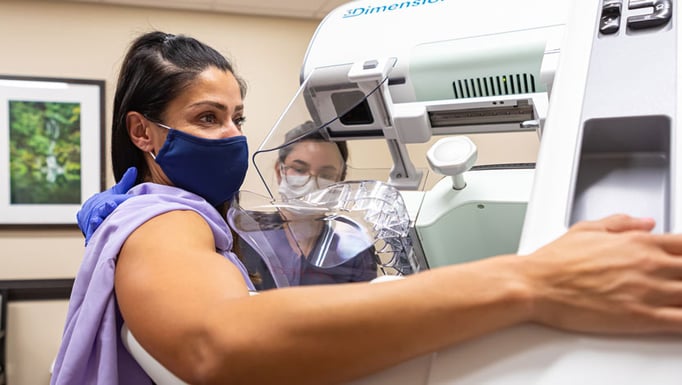
The chance of developing breast cancer among American women is one in eight, or 13%. Screening offers the opportunity to catch breast cancers early, before they have progressed, allowing for better lifetime outcomes. Still, for some groups of women, their chances of getting breast cancer – and especially deadly, aggressive breast cancer – are higher than for others. The group that bears the most disproportionate burden of breast cancer is, by far, Black women.
The most recent statistics show that breast cancer is the most commonly diagnosed cancer among Black women, with 33,840 new cases estimated to have been diagnosed in 2019 alone.
What is more troubling is that the death rate from breast cancer is 39% higher for Black women compared with white women. Moreover, Black women are also more likely to develop breast cancers in their 40s — and to have more fast-growing forms of the disease that often prove difficult to treat.
The most susceptible women are ones with a personal or family history of cancer or who have an ancestry associated with BRCA1/2 gene mutations. These at-risk individuals should receive genetic counseling and genetic testing . Testing rates, however, are surprisingly low. They are far lower for Black women than for white women, and for uninsured patients than for insured patients.
To make matters worse, the COVID-19 pandemic is expected to have a devastating impact on cancer rates. Experts predict an unprecedented increase in the numbers of cancer cases and deaths because of delays in screening and care, intensifying the disparities already felt by under-served communities of color.
In order to tackle racial and socioeconomic disparities head-on, researchers at the UChicago Medicine Comprehensive Cancer Center have developed several cancer prevention studies. Each study looks for ways to make leading advances in breast cancer screening available to all women in the community, especially those who are most at risk of dying from breast cancer.
UChicago Medicine is one of the sites chosen to better understand the safest and most effective guidelines for breast cancer screening through a national study called WISDOM , which stands for “ W omen I nformed to S creen D epending O n M easures of risk.”
Participants can be healthy women between the ages of 40 and 74. Each participant gets to select one of two screening methods: a personalized, risk-based approach informed by genetic testing or the standard annual mammogram. This study of 100,000 women will contribute needed evidence to support guidelines for when and how often women should be screened according to individual risk factors.
In the C hicago A lternate P revention S tudy (CAPS), women at ultra-high risk for breast cancer — as a result of either genetics or dense breasts — are offered two screening scans per year using a new, ultra-fast MRI method developed by UChicago Medicine researchers paired with an annual mammogram. The study aims to show that the biannual 6-minute MRI scans are a safe alternative to prophylactic double mastectomy.
With funding from the American Hospital Association Institute for Diversity and Health Equity and Blue Cross Blue Shield of Illinois, the SC reening O ut R each and E ngagement (SCORE) initiative offers free genetic testing for uninsured women who have never been screened. The women will also be provided free mammograms, help with registering for Medicaid, and transportation to get their mammograms. Community organizations, such as Equal Hope , Sisters Working It Out and Chicago Family Health , are helping to recruit community members.
One group, called Screen to Succeed , is led by Trisha Mondal, a sophomore at Adlai E. Stevenson High School in Lincolnshire, Illinois. As an aspiring oncologist, Mondal was devastated to learn about the lack of cancer screening among low-income and minority groups. In response, she built a website to raise awareness, funds and collaborate with local organizations to help battle healthcare disparities in cancer.
“There are many underutilized resources the community can take advantage of to get screened for breast cancer, but they are unaware or face barriers to getting help,” said Mondal. "By connecting them with resources, we can not only make it easier for them to get screened, but also educate them about cancer prevention.”
Technology’s Potential to Automate Screening
At-risk individuals are now at even higher risk because of COVID-19. The public health emergency has created safety challenges for patients to come in for screening or treatment.
UChicago Medicine researchers are partnering with the hospital’s genetics oncology group and using technology to streamline the process of risk assessment. When patients schedule mammograms through UChicago Medicine’s patient portal, MyChart , they automatically get a message asking them to answer questions about family history.
If a high-risk individual is identified, she can have virtual genetic counseling and receive a testing kit through the mail. This no-contact approach safely connects high-risk women with personalized cancer prevention strategies.
Even during COVID-19, our team is making breast cancer trials and screening accessible to women across socioeconomic and racial groups.
Altogether, the breast cancer screening initiatives and community-based efforts at UChicago Medicine are making prevention and risk reduction strategies accessible for at-risk women. The researchers hope these studies will improve breast care in a way that saves lives while closing the large gap in death rates between Black and white women, thereby making healthcare more equitable.
For more information about breast screening opportunities or ongoing research trials, email [email protected] .

The WISDOM study is a national research effort to determine if breast cancer screening can be made better by personalizing each woman's mammogram schedule. Learn about the study and important facts about breast cancer risk and prevention.
On average, every two minutes, a woman is diagnosed with breast cancer in the United States. Today on At the Forefront Live, we're talking about the WISDOM Study. It's a National Research effort to determine if breast cancer screening can be made better by personalizing each woman's mammogram schedule. Breast cancer specialty oncologist and researchers Dr. Funmi Olopade of UChicago Medicine and Dr. Laura Esserman of UC San Francisco will join us to discuss the study and its goal to enroll 100,000 participants. We will also have physician's assistant, Sarah Bazzetta on to discuss breast cancer risks and prevention and as always, we'll take your questions live. That's coming up right now on At the Forefront Live.
And we want to remind our viewers that today's program is not designed to take the place of a visit with your physician and let's start off with having each of you introduce yourselves and tell us a little bit about what you do here use UChicago Medicine specifically Dr. Olopade, Dr. Esserman, you're obviously joining us from the West Coast but let's start with Dr. Olopade.
Thank you for inviting me to this panel. I'm a breast medical oncologist but my main focus of research is to use genetics to improve care for all women and I serve as director of cancer risk clinic where we do risk assessments so that we can really optimize prevention for every man and woman at risk for cancer.
And Dr. Esserman, if you can tell us a little bit about yourself and what you do at the UC, is it UC San Francisco? Is that correct?
Correct, California San Francisco, yes. So I'm a professor of surgery in radiology. I actually direct our multidisciplinary breast program, our breast cancer program and I am focused on precision medicine approaches to both treatment, screening and prevention and really particularly interested in developing clinical trials and ways in which we can take some of the exciting new advances forward and all that we've learned about breast cancer biology and make sure that we can get the right treatment or screening and prevention strategy to every woman at the time that they need it to make sure that over the next 10 years, we really dramatically reduce the chance that anyone will die of this disease.
And that is a certainly a fantastic effort and I would be remiss if I did not say that I think both of you have been on the program before, about a year ago I think it was and we talked a little bit about the WISDOM Study back then and now we're into it a little bit more and the goal is to have 100,000 participants in the study, which seems like a pretty tremendous goal. But could you start us off by telling us about the WISDOM Study and Dr. Olopade if you can kick us off in just why this is so important?
Yeah, so October is when we ask every woman to come in and get a mammogram. The reason why Laura and I came together to talk about the WISDOM Study, which by the way means women informed to screen based on measures of risk, is because as a medical oncologist, sometimes we find women coming in with advanced breast cancer even when they have been having their mammograms every year and then Laura has a different problem where women come into her because they have been over diagnosed with cancer that would never have killed them, but because they got a mammogram alleged to fear and over diagnosis.
So we know that breast cancer is not one disease, that some women are going to get breast cancer that's very aggressive and some would get breast cancer that even if they didn't do anything about it, they may die of all that causes but we don't know who is going to get what. And I'm a Black woman. I don't know how to screen, when to screen, what to screen. I just hear that I'm at risk for triple negative breast cancer. So I'm asking myself OK, when I'm going to get that first mammogram, I need to do a little bit more questioning about what's my risk for cancer.
And since this is the work out that I have done at the University of Chicago for decades, I think it's about time for every woman, when you go in, when you start your screening mammogram at age 40 or any time, the study is open to women from age 40 to 75 and what we want you to do is ask can I participate in the WISDOM Study, may I choose a personalized arm or choose whatever I want to do? But let me be part of the solution so that we can know when a woman needs to screen, how they should be screened and so this study really is coming from California to the Midwest and I'm very proud that the University of Chicago has sponsored it and I'm participating in it and I'm asking all my friends to participate and because we live in a diverse community, I want Black, Brown, White, all women in Chicago to participate because together we can get the wisdom we need to know when to screen, how to screen and personalize screening for every woman.
So Dr. Esserman, I've got a two parter for you and this isn't based in part on what Dr. Olopade just told us and I've never thought of this before but so you have to, I'm certain, what doesn't surprise me is under reporting or under reacting but I hadn't ever thought of something that might overreact to a diagnosis. Can you talk a little bit about that? And then the second part of the question is if somebody wants to be part of this WISDOM Study, which it sounds like a great thing, how do they go about that?
OK, I'll answer both of those questions. Yes, actually overdiagnosis is really a problem because it can lead to overtreatment. As Dr. Olopade said, as Phil may said, every cancer is not the same and if you've got a cancer that's small and unaggressive, you don't need a lot of extra treatment and some of these cancers, especially some of these preinvasive cancers are never going to amount to anything. So we don't want to be doing mastectomies or giving people toxic therapy that they don't need. On the other hand, we want to make sure that we find that people with the more aggressive cancers and some people get these cancers that come up between screens and it's not their fault that they come in with a large cancer.
It's not because they've neglected it, it's because the cancer grew too fast. So is there a way that we can find those people through the study of genetics and looking at breast density, all those other factors that Dr. Olopade was talking about. That's what we're trying to answer in the WISDOM Study right and so the only way to do better is to know better as Maya Angelou says and so the only way all of us as women can make the future better is to join together and be part of the study. Women will spend 30 to 35 years of their life doing screening. Spend five years with us, help us find those right answers and this is just the beginning.
We not only want to find out when you start, when you stop, how often you screen but take the first part of that knowledge and then start changing and adapting and making it even more specific and more personalized. So over 30,000 women have now joined the study. We're a third of the way there. So you can be one of 100,000, everyone can. Tell all your friends and family. All you have to do is go to wisdomstudy.org, it's that simple. You can do everything online. The only one piece of information we need to know is your mammographic density from your mammogram if you've had one, we'll get that put in and everything else you can do from the comfort of your home.
And if you think about it, now more than ever at the time of COVID, wouldn't it be good to know? How important it is to go in? That's why if you know your risk, we believe that it's going to be better. But we're not just doing it, we're testing it so that our results can apply to everyone, so that guidelines can change, so that everyone can have a better option and that we're not only just screening, we're reaching out to people at high risk to see whether we can improve their chances and decrease their risk. So this is really a very important landmark study that again, all of us women need to stick together and be part of it and as Phoebe says we are both participating, so we put our money where our mouth is so you can do it too. If we all do it, we'll get better answers and we'll get them much sooner.
So wisdomstudy.org that's where people need to go if they want to participate in this study and we also encourage viewers as they're watching the program, if you have any questions for our experts today, please write them in the comments section. We'll try to get to as many as we can during the course of the show. So 100,000 would be the sample size that you're looking for and Dr. Olopade, we now know that you're about a third of the way there. When will we know the results or the outcome of the study? This obviously is a multi-year effort.
Yeah, so the reason why we really want to make this a national study and when my colleague called me, I call her my best science and research partner, Laura. The study at the University of California group, they had organized together across California to really do this study and so they formed the Athena Breast Network and then I was thinking why aren't we doing this in the Midwest? Why aren't we organizing in Chicago? And so as we have learned through this pandemic, we're all in it together and by being able to have testing available, by being able to share information that is really important to our health, I think we would learn how to do better with breast cancer.
So I totally agree with Laura when she said look, we're in a pandemic, we're all at home and we're wondering oh well, I don't want to go to the hospital, I don't want to go and get a mammogram or maybe I don't need it. Well cancer can be deadly if in fact you are at risk for an aggressive breast cancer. So I'm hoping that we will use this opportunity to just say yes, we can do both things, we can shelter in place, we can wear a mask, we can wash our hands and then those of us in the hospital, we're ready for you to come in and get your screening. So I know that there may be some women out there who do not have access to the internet, they cannot fill out the form in their own home.
Well when you come into your screening mammography, we would have a kiosk ready for you. We would have a way for you to be able to just put in the information so that we can then begin the process and that the process will get easier if we can just get all of us engaged in the process. Every woman is at risk for breast cancer and what we're really asking is let's all come together and join this study so we can get information that will inform all women, Black, White, Brown, mid-women in the Midwest, women on the coast and I'm looking for all my friends in Chicago to join, as well as all of our patients at the University of Chicago, other people will walk at the University of Chicago because together we can get the solution that we need.
And Dr. Esserman, you had a comment you wanted to jump in with?
I was just going to say it's just that easy. If you have a smartphone, go to wisdomstudy.org. That easy, you can sign up and importantly, we're trying to, by random, assign people to either the personalized or the annual arm right? But if you feel struggling, you want the personalized arm or you want to say oh, I want to get my annual mammograms, please, you can do that too. Just choose I want to choose, I want to choose my arm. Actually it's just so simple for everyone to join but we need everyone then to stay with us and make sure you keep in touch with us and let us know what's happening because that's how the study works and again all of us together, we can make the future better for sure.
Well it's so important. Dr. Olopade I appreciate you saying what you said a moment ago, get your screenings even with the pandemic, you still need to get your screenings and you still need to get medical care because first of all, come to the hospital. It's very safe but we don't want people to not do those things because it's just, it's not worth it. Get your screenings, you'll be safe, we make sure that people are very well taken care of. So doctor Dr. Esserman, question for you. Now when we talk about personalized mammograms, what exactly does that mean and how does somebody get their screening personalized?
Well so as Dr. Olopade said, we know that breast cancer isn't one disease and today, we don't treat everybody like they have the same cancer. So it may be for screening and prevention, that one size doesn't fit all. Instead of telling everybody get a mammogram at 40 and do it every year, maybe there are some people who really are at very low risk and they can start screening when they're 50. But there are other people whose risk is quite a bit higher and in fact, there's a combination of things that we can learn. It's not only what your family history is and what your, and what other risk factors you might have, but we can learn about your genetics and what does that mean?
Just with the spitting in a tube, we can understand what your DNA is made of. We can find out if you happen to have one of the very uncommon errors in one of the genes that puts you at very high risk for breast cancer. If you have some background genes that when combined together, really do either increase or decrease your risk. Well we may be able to say hey, you are really at risk and you're probably at risk for a hormone cause of breast cancer, we have something we can do to help you reduce that chance of getting a cancer and it's safe and work with you on that.
Maybe if you're a very high risk, you should come in, get a mammogram alternating with an MRI every year and so if you're in that very high risk group. So again, it's about when to start, how often to screen and with what type of screening you should use and would you benefit from prevention and if so, is that prevention working? That's how we're going to move the field forward. We believe that that's better but we're testing it. We want to find out if it's just as safe as the annual screening, if it leads to fewer false positives and scares like Dr. Olopade was talking about earlier.
Whether women prefer that and can follow that protocol and whether at the end of the day, we do better with prevention. That's what the study is all about and if we show the personalized screening is a better way forward, we can just continue to make it better and better. That's the way that we're going to prevent breast cancer.
Yeah, I just want to say that in October, we celebrate Breast Cancer Awareness Month. But we also remember that there's so many women out there now living with metastatic breast cancer who are looking for us to get new cures, new drugs and bring new drugs to help them beat or live longer with breast cancer. So I just want to take a moment to say that because of genetic testing and because of the research that we have done, we have more chances that women would live even longer with advanced breast cancer and that's why we're really seeing yes, we start with prevention before you ever get that first mammogram.
Help us look at when to begin screening or how to screen you. But even after you are diagnosed with breast cancer, we know that using targeted therapy, using genetics to inform how you get treated has changed the games for so many women and that's why at the University of Chicago when we talk about being at the forefront of medicine, it's because I research benefits, our population benefits women and I couldn't be prouder of what we have done during this COVID because we know yes, if you get COVID, you're more likely to die, if you are Black or Brown. Well guess what? If you have the right medication, if you get into the right clinical trial, you'll survive.
And so beyond COVID, we're talking about survivorship, giving yourself a chance to have early diagnosis and the only way that we get that is where you go in, get your mammogram and get good doctors to take care of you. So I hope that you will be part of that movement by signing up to become part of WISDOM. We hope we can follow you for the rest of your life because technology enables that to happen and we can share with you our research, we can share with you the latest findings, the latest kiosk so that those of you who are watching, who may be living with breast cancer now, think about your family members, think about daughters, think about sons, there's a lot we can do together. So that's why we're asking everybody to join this movement.
All right.
And just a follow on that I was to say that one thing that we want to do in this study is do a special genetic profile of each tumor. So that's another benefit of being in the study. It will return that information to you right because this is our opportunity to figure out who is at risk for what kind of cancer and then really start to think differently about prevention.
All right, we need to take a quick break. When we come back, we'll have Sarah bizet to join us to answer questions about genetics and preventative oncology. We will be right back.
Thanks so much for having us.
All right, welcome back. We have a lot of good information about Janette or about the WISDOM Study in the first half the show. Now we're going to talk a little bit about genetics and prevention and Sarah Bazetta joins us to discuss that along with Dr. Olapade. Sarah, thanks for being with us and can you start us off just tell us a little bit about role here at UChicago Medicine?
Thank you for having me today. So I work with Dr. Olapade in the preventive oncology department. We work with whole families to make sure that they can do prevention and that we do early detection of cancers.
Great, well let's talk a little bit about preventative screening and how that works with genetic results because I know a lot of what we're seeing today and obviously with the WISDOM Study as well as looking at those genetic profiles of people that can help folks down the road. So Sarah, if you can start us off and talk about genetic screening with women and really how that changes the game actually?
Yes, so our department in preventive oncology really looks at whole family and family history and based on the family history, many patients benefit from knowing more about their genetic makeup because some genetic makeup have a higher risk of cancer and so those patients can benefit from, as we said, in the WISDOM Study, earlier screening, different types of screening and you can get involved multiple types of cancer prevention beyond even breast cancer.
So Sarah, can you talk to us a little bit about the preventative oncology clinic? I think that's what Dr. Olopade called it and the second part of that question would be how much of this can be done via telehealth if people are trying not to get out too much?
That's a great question. So almost all of it outside of the imaging can be done by telehealth. So our patients come to us through a call like we're doing right now and we talk about their family history, their personal history, their genetics and we talk about how to plan a personalized screening plan, whether that includes mammogram, ultrasound, et varieties and visits with us. So they come in and they see me. As part of our preventive oncology clinic, we have genetic counselors, we have oncologists, we have gynecologists and we have GI specialist because so much of cancer is a whole body of approach.
We also talk a lot about prevention outside of the hospital, diet and exercise. Being healthy is really important. Limiting alcohol and smoking and all of this is a whole body approach to prevention that we can get to patients before they have a diagnosis with cancer.
And Sarah, part of what you do also is really provide hope to folks because I know when you get a cancer diagnosis. It's a scary, scary event and I think for a lot of people initially there is that fear and then they wonder what happens next or how can I help myself down the road. So a lot of what your work does is, I would assume, is to help people realize that you can put together a game plan.
Yeah, that's such an important thing. Dr. Olopade's work for the last 30 years has really advanced what we can offer patient, both those that have cancer and those family members that want to prevent cancer. And so this game plan, whether it's by telehealth and then coming into the hospital to have your screenings can be proactive and can take an active role in their health.
So Dr. Olopade, we have another question from a viewer and that is can men participate in the WISDOM Study?
Oh, absolutely. The reason why how men can participate in WISDOM Study is they can get their wives and daughters to be participants. Unfortunately because men, the risk of breast cancer in men is very low, we cannot really learn how much we need to learn by having men participate in WISDOM. But the good thing about WISDOM is that you get your genes from both your father's side and your mother's side. Knowing that you're BRCA2 mutation carrier only increases your risk for prostate cancer is a big deal. Knowing that you're a BRCA2 carrier and you have a risk for all that cancers, can you help men also actually help their daughters.
So that's why we say we're in this together, men and women should know about the WISDOM Study but only women can participate and if we can use the genetic testing to reach men who may be carrying genes that increase the risk for their daughters or their sisters, that's really why we want everybody to be part of the movement. So yes, men can participate by urging their wives or their daughters or their mothers to be part of the solution.
Sounds great. Sarah, this is--
Dr. Olopade brings up a really good point that breast cancer risk comes from both mom's side of the family and dad's side of the family. So you want to see all of those patients at our clinic to talk about their families.
So Sarah, there's some practical just everyday things that can be done to help prevent breast cancer?
Yes, so the most important thing that you can do is maintain a healthy lifestyle. So exercising every day is good for both your physical health and your emotional health during this pandemic. So we really want everyone to stay active even in the upcoming winter month, get outside and make sure that you're staying active every day. And then eating a healthy diet is really important because we want everyone to maintain a healthy body weight so that we can reduce the risk of cancer.
So Dr. Olopade, we have another question from a viewer and this will have to be our last one. We're about out of time but I think this is an important one and the question is I was just diagnosed with breast cancer this year. Would this study be a good idea for my sisters to participate in?
Absolutely, absolutely. And the good news is we have more survivors and I hope you listen to that ad from the University of Chicago. It's not just that you a survivor, you are a thriver. So congratulations to you. But now the job that you have to do is get all your friends, get your sisters, get everybody in your family to feel empowered. There's a lot we can do. Even if you don't get to prevent cancer, you can give your sisters the gift of having their breast cancer diagnosed early. It's a game changer when your breast cancer is diagnosed early. So let's thrive beyond a diagnosis of breast cancer by sharing with our friends, with our family, everyone in your network, they can help us through that WISDOM Study.
So our personal challenge to everyone who's watching this is go to wisdomstudy.org. Sign up, encourage your wife, your girlfriend, your daughter to sign up if you're a guy watching this. It's very important. This is a great information and I know you need 100,000 people for that sample size. So we need to get a few more people signed up. We are out of time. You all were fantastic and shared just a lot of great information with our audience today. So thank you very much and thank you to our viewers for your great questions and please remember to check out our Facebook page for our schedule of programs that are coming up in the future.
Also, if you want more information about UChicago Medicine, take a look at our website at uchicagomedicine.org. If you need an appointment, you can give us a call at 888-824-0200 and remember, you can schedule your video visit by going to the website. Thanks again for being with us today and I hope you have a great week.
Convenient Locations for Breast Cancer Screening Mammography
Hyde park - chicago.
- Get Directions
River East - Chicago
Calumet city, orland park, tinley park, breast cancer articles.

I'd Like to
- Make an Appointment
- Find Classes & Events
- Make a Donation
- Apply for a Job
- Patients & Visitors
- Healthcare Professionals
- Comer Children's Hospital
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Screening for breast cancer in 2018—what should we be doing today?
* Department of Medical Imaging, The Ottawa Hospital, Ottawa, ON.
T. Alhassan
Although screening mammography has delivered many benefits since its introduction in Canada in 1988, questions about perceived harms warrant an up-to-date review. To help oncologists and physicians provide optimal patient recommendations, the literature was reviewed to find the latest guidelines for screening mammography, including benefits and perceived harms of overdiagnosis, false positives, false negatives, and technologic advances.
For women 40–74 years of age who actually participate in screening every 1–2 years, breast cancer mortality is reduced by 40%. With appropriate corrections, overdiagnosis accounts for 10% or fewer breast cancers. False positives occur in about 10% of screened women, 80% of which are resolved with additional imaging, and 10%, with breast biopsy. An important limitation of screening is the false negatives (15%–20%). The technologic advances of digital breast tomosynthesis, breast ultrasonography, and magnetic resonance imaging counter the false negatives of screening mammography, particularly in women with dense breast tissue.
INTRODUCTION
Breast cancer ( bc a) is the leading cause of cancer death in women worldwide. It is the main cause of cancer-related death in women in developing countries (where many have advanced disease at presentation), and it is the second-leading cause in women in developed countries 1 – 3 . In Canada, cancer is also the leading cause of premature mortality, as measured by potential years of life lost. Breast cancer has one of the highest potential years of life lost: almost 137,000 years, reflecting the burden of bc a in younger women 4 . Since the 1988 peak in the bc a mortality rate, estimates suggest that 32,000 bc a deaths have been avoided in Canada for a variety of reasons, including early detection with screening and advances in bc a treatment 4 . Screening mammography is the method most commonly used worldwide for the detection of early bc a in asymptomatic women, and it is the only imaging modality proven to significantly lower bc a mortality 5 .
In the present review, we cover screening for average-risk women, who represent 80% of those diagnosed with bc a. It has been well established that women at high risk of bc a, including carriers of gene mutations (for example, BRCA1 and BRCA2 ) or those with a lifetime risk of 25% or greater calculated using the ibis or boadicea risk assessment tools, benefit from annual screening with breast magnetic resonance imaging in addition to mammography 6 .
BENEFITS OF SCREENING MAMMOGRAPHY
In 2014, because of concerns about overdiagnosis with mammography, 29 experts in epidemiology, surgical oncology, oncology, radiology, pathology, physics, and genetics from 16 countries met at the International Agency for Research on Cancer as a Working Group to reassess the cancer-preventive and adverse effects of various methods of screening for bc a 7 . All available high-quality observational cohort and case–control studies from 1989–2014 (approximately 40) were assessed and debated until a consensus was reached. A meta-analysis was not performed, but the greatest weight was given to cohort studies with the longest follow-up period and more robust designs. A distinction was made between women invited to screen, which results in only 60% participation in screening, and those who actually participate and undergo mammography. Results showed that women 50–69 years of age who were invited to attend mammographic screening experienced a 23% reduction in the risk of death from bc a and that women who attended mammographic screening had a higher reduction in risk of 40%. Fewer studies have assessed the effectiveness of screening in women 40–44 or 45–49 years of age, and the risk reduction in those studies was less pronounced 7 . In addition to randomized controlled trials ( rct s), many observational studies from modern service-based screening (that is, organized population-based screening) show pooled mortality reductions of 25% [relative risk ( rr ): 0.75; 95% confidence interval ( ci ): 0.69 to 0.81] among women invited to screening and 38% ( rr : 0.62; 95% ci : 0.56 to 0.69) among those attending screening 8 .
The 2014 Pan-Canadian observational study examined the effect of mammographic screening on bc a mortality given the variability of findings from observational studies in different countries where screening was implemented 9 . Of 12 Canadian breast screening programs, 7 programs representing 85% of the Canadian population participated in the study. Data about screens and bc a diagnoses and deaths from 1990 to 2009 were obtained for 2.8 million participants in the screening programs and from the corresponding cancer registries (20.2 million person–years of observation in total). The average bc a mortality among participants was 40% (95% ci : 33% to 48%), which is lower than the mortality for women who did not participate in screening as determined by provincial cancer registry data linked to screening program databases. The bc a mortality reduction observed in the participating provinces was in the 27%–59% range. Age at entry into screening (40 years vs. 50 years) did not affect the magnitude of the average reduction in mortality (between 35% and 44%). The population’s awareness of bc a and trends in treatment efficacy did not influence the results. The study concluded that participation in population-based mammography screening programs in Canada was associated with substantially reduced bc a mortality for women 40–74 years of age.
Benefits: Number Needed to Invite Compared With Number Needed to Screen
Absolute benefit can be measured as the number needed to invite to screening ( nni ) or the number needed to screen ( nns ) to prevent 1 death 10 . The magnitude of the absolute benefit is influenced by the rr , the duration of follow-up, the underlying mortality risks in the population from which the estimate is derived, and whether the estimate is the nni or the nns .
The nni is based on rct s and is not a measure of who is actually screened, only who is invited to screening. Only 50%–70% participate when invited to screen 11 . The nni can be estimated from observational studies or rct s, but should not be used because the numbers will be inflated by deaths among women invited to screening who never attended screening 12 . That distinction was not made by the Canadian Task Force on Preventive Health Care 13 .
The nns is equivalent to the number needed to participate and indicates the actual number needed to be screened or to participate to see a benefit. It is the more accurate assessment of the benefit of screening and is increasingly being used in the literature.
Variable estimates of absolute benefit have been noted in the literature depending on whether the nni , nns , or other model inputs were used. As Table i shows, the nns estimates from the U.K. Independent Review and the Cochrane systematic review differed by a factor of almost 10: 180 compared with 2000 5 , 19 . That difference is attributed to the Cochrane systematic review having used the nni rather than the nns and being based on a less-favourable mortality reduction ( rr : 0.85 vs. 0.80) over a shorter screening program duration (10 years vs. 20 years), with follow-up limited to the period of the screening program. It is important to use long-term follow-up to estimate the nns . That factor is most evident in the Swedish Two-County Trial, in which it was observed that 922 women had to be screened 2–3 times during a 7-year period to prevent 1 bc a death at 10 years of follow-up; that number declined to 414 women at 29 years of follow-up 20 . The latter estimate is similar to the American Cancer Society ( acs ) nns estimate of 462 for women 50–59 years of age at 15 years of follow-up, with a 40% mortality reduction 10 .
Screening recommendations, by organization
NA = not available.
Other benefits to screening include the reduction in costs associated with treatment. Treatment for individuals diagnosed at an earlier stage is less invasive and costly, which might reduce patient anxiety and improve prognosis 21 . From the patient’s perspective, breast-conservation surgery instead of mastectomy, a decreased need for chemotherapy, and less time off work are all huge benefits associated with earlier detection. A decreased likelihood of axillary lymph node metastases with screening can also result in fewer axillary lymph node dissections and reduced risk of lymphedema. A study from 1996 demonstrated that the cumulative costs of treatment for late-stage bc a were US$50,000 to US$60,000 per patient, compared with US$18,000 to US$25,000 for treating early-stage bc a 22 . Montero and colleagues 23 estimated the costs of treating metastatic bc a to be much higher at US$250,000, likely because of increased drug-related costs 20 years later and the increased costs of the medical delivery system. A Canadian study showed that the average undiscounted lifetime cost per case of treating women diagnosed with bc a varied by stage, from $36,340 for stage iv or metastatic disease to $23,275 for stage i disease 24 .
Guidelines for Screening to Maximize Benefit
Most national screening guidelines suggest that there is value in mammography screening for women in their 40s 10 , 15 , 17 , 18 . An informed, personal choice for women in their early 40s is widely supported by the U.S. Preventive Services Task Force, the acs , and the Canadian Task Force on Preventive Health Care 25 , 26 . Several other North American medical societies recommend screening for women starting at age 40 ( Table i ). The acs recommends annual screening for women 45–54 years of age; women 55 years of age and older should then transition to biennial screening 10 . Because the bc a growth rate is faster in premenopausal women, the optimal recommended screening interval for those women is annual 27 . In postmenopausal women, although the maximal benefit is achieved with annual screening, the incremental benefit of that approach compared with biennial screening is less marked, and in the relevant age group, most programs recommend biennial screening for maximal cost-effectiveness 28 .
Breast Cancer Screening in Young Women
An often-touted reason not to screen women 40–49 years of age is that most bc as occur in women more than 50 years of age. However, 17% of bc as are diagnosed in women less than 50 years of age 4 , with fewer than 5% occurring in those less than 40 years of age 10 . It is more informative to express the incidence per decade, with 18% of bc as occurring in women 40–49, 23% in those 50–59, 26% in those 60–69, and 28% in those 70 and older according to U.S. Surveillance, Epidemiology, and End Results data 29 . No abrupt increase occurs at the age of 50. The incidence of bc a can be further subdivided into 5-year age categories, as the acs has done 10 , with the most marked increase in bc a incidence being seen in the 45–49 age category. Hence, the strong recommendation of the acs to begin screening at 45 years of age ( Figure 1 , Table i ).

Breast cancer (BCa) burden by age at diagnosis, 2007–2011. (A) Distribution of invasive female BCa cases ( n = 292,369) by age at diagnosis. (B) Distribution of BCa deaths ( n = 16,789, patients followed for up to 20 years) by age at diagnosis. (C) Distribution of person–years of life lost to BCa ( n = 326,560, patients followed for up to 20 years) by age at diagnosis. Source: Oeffinger et al., 2015 10 .
Limited studies have evaluated screening mammography for women 40–49 years of age. Many of the rct s were designed to include women 50–69 years of age. Although the Canadian National Breast Screening Study evaluated women 40–59 years of age 30 , it has been challenged because of poor-quality mammography and because the rct allocations were not blinded, with an excess of advanced bc as allocated to the screening arm 31 . The Canadian National Breast Screening Study is an outlier among the 8 rct s for screening mammography; it was the only study to show no bc a mortality reduction from screening mammography.
In the Pan-Canadian study, which used data from the 3 provinces that perform screening in women 40–49 years of age, the relative bc a mortality reduction with screening was 44% 9 . The U.K. Age rct reported the effect on bc a mortality of mammographic screening for women 40–49 years of age at 17.7 years of follow-up 32 . From 1990 to 1997, 160,921 women 39–41 years of age in the Breast Screening Programme of the National Health Service were randomly assigned to either an intervention group that was offered annual screening by mammography or to a control group (1:2 allocation) that received usual medical care (screening starting at age 50). Results showed a 25% reduction in bc a mortality in the intervention group compared with the control group in the first 10 years after diagnosis ( rr : 0.75; 95% ci : 0.58 to 0.97), but not thereafter, once they started regular screening at age 50 ( rr : 1.02; 95% ci : 0.80 to 1.30). The overall bc a incidence during the 17-year follow-up was similar in the intervention and control groups. The authors concluded that their results supported an early reduction in bc a mortality with annual screening in women 40–49 years of age.
HARMS OF BREAST CANCER SCREENING
False positives.
A false positive is defined as recall for additional testing after an abnormal mammogram, in which further evaluation determines that the initial abnormal finding is not cancer. False-positive results are one of the most common adverse effects of screening. Most will be resolved with further noninvasive imaging work-up, but a percentage will require further tissue diagnosis (for example, a core biopsy), with the findings being mostly benign. False-positive results invariably lead to some level of anxiety for screening participants. The variability in the recall rate is a result of many factors, including use of postmenopausal hormone therapy, greater mammographic density, first mammogram, longer intervals between screens, lack of previous mammograms for comparison 33 , and differences in performance and training of the interpreting radiologists 34 .
In Canada, data about abnormal recalls from screening programs are publicly available from the Canadian Partnership Against Cancer 11 . These quality indicators help to demonstrate the performance and effectiveness of provincial organized screening programs, summarized in Table ii . Most women who receive an abnormal screening result do not go on to be diagnosed with bc a; however, additional assessment is required to reach a definitive diagnosis. The assessment process can include additional imaging with diagnostic mammographic views, breast ultrasonography, or core or fine-needle aspiration biopsy. Approximately 80% of women with an abnormal screen require only additional imaging; the remaining 20% require a biopsy for diagnosis 11 . Among women who require a breast biopsy, the expected rate of a malignant finding is less than 50% (30%–50%) 11 .
Summary of quality indicators for women 50–69 years of age in organized breast cancer screening programs across Canada, 2011–2012 screen years a
Overdiagnosis
“Overdiagnosis” is the diagnosis, as a result of screening, of a cancer (either invasive or in situ ) that would never have been identified clinically or caused a problem in the individual’s lifetime. Several autopsy studies have demonstrated the frequent presence of breast malignancy in women with no diagnosis before death. Overdiagnosis can result in unnecessary worry, additional imaging or diagnostic work-up, and overtreatment. Reports of overdiagnosis in the literature range widely, from 0% to 57% 35 – 38 , which should call into question their scientific validity.
To obtain an accurate estimate for overdiagnosis, it is important that the screened and unscreened populations studied have similar risk factors for bc a and that adjustments be made for any confounders. Lead-time bias—the time between detection of the disease as a result of screening and the time at which the diagnosis would normally have been made when the patient presented with symptoms—must be accounted for. Because of lead time, an excess incidence of bc a is expected when screening starts. After the end of screening, a reduction in the incidence of bc a should occur because of the earlier diagnosis of cancers during screening. If no overdiagnosis occurs, then the initial increase in bc a in screened women should be fully compensated by a similar decline in bc a in older women who no longer screen, called the “compensatory drop.” An interval of at least 5 years of follow-up is required to observe that drop. If follow-up is insufficient, then the compensatory drop will overestimate any overdiagnosis. If no adjustment is made for the compensatory drop, then estimates of overdiagnosis are much higher, on the order of 57% for in situ and invasive cancers 39 .
The estimation of overdiagnosis requires accurate correction for changes in the baseline incidence of bc a. The problem is that the incidence of bc a has changed over time 40 . Use of an incorrect assumption about the incidence of bc a could inflate the estimate of the magnitude of overdiagnosis. For example, Bleyer and Welch 41 reported that the incidence of bc a increased by 0.25% per year between 1975 and 2008, and they estimated overdiagnosis to be 31%. But, 4 years later, Welch et al. 42 reported that the incidence of bc a was stable during the same time period. Those authors argued that the flat incidence line for metastatic bc a was evidence for massive overdiagnosis from screening mammography. However, if the incidence of bc a had risen steadily, then the flat incidence rate for metastatic bc as was, in reality, evidence of the benefit of screening and a low rate of overdiagnosis. In fact, the Connecticut registry documented a steady increase in the incidence of bc a, by 1% per year, between 1940 and 1980, before screening mammography 43 . Then, between 1980 and 1987, an increase of 32% was reported by the U.S. Surveillance, Epidemiology, and End Results program, attributed to the advent of widespread screening mammography 43 . A recent study that appropriately adjusted for pre-screening trends found a 37% reduction in late-stage disease, with a reciprocal increase in early-stage disease, approximating the bc a mortality reduction seen among women from 1990 through 2009 44 .
Puliti and colleagues undertook a literature review of observational studies to estimate a range for overdiagnosis of bc a, including carcinoma in situ, in 7 mammographic screening programs in Western Europe 39 . Studies were critically reviewed for the methods used to estimate counterfactual rates (what would have happened without screening) and to adjust for lead-time bias. The studies were then categorized as having “adequate” or “not adequate” adjustment for those two factors. The thirteen studies that satisfied the eligibility criteria reported 16 estimates of overdiagnosis. The literature review showed that the unadjusted overdiagnosis estimates ranged widely (from 0% to 54%), but concluded that the most plausible estimates of overdiagnosis ranged from 1% to 10%, the higher estimates being attributed to lack of correction for lead time bias or bc a risk, or both. Data from long-term studies such as the Malmo rct after 15 years of follow-up confirm a similar rate of overdiagnosis of 10% 45 .
Overdetection and Ductal Carcinoma In Situ
It has been argued that the term “overdiagnosis” is not correct, with the correct term being “overdetection,” because the actual diagnosis of bc a is performed by a pathologist after a lesion is detected, usually after an imaging work-up 46 . The overtreatment that accompanies overdetection is what causes the harm. Most overdetection is driven by the diagnosis of ductal carcinoma in situ ( dcis ). The literature contains much debate about the value of screen detection of dcis and subsequent treatment of the disease.
Before the widespread use of screening mammography in the United States, 6 cases of dcis were detected annually per 100,000 women; after the introduction of screening, 37 cases of dcis were detected per 100,000 women 47 . According to the acs , carcinoma in situ accounts for 20% of all new bc a cases, the vast majority (83%) being dcis , a true (non-obligatory) cancer precursor 48 .
On mammography, dcis is most often detected as new microcalcifications ( Figure 2 ), although it can present as a palpable mass. It can also be both mammographically and clinically occult. Breast magnetic resonance imaging ( mri ) has been shown to be more sensitive than mammography for detecting high nuclear grade dcis 49 . The main goal of bc a screening is to detect bc a early and thus to lower the incidence of locally advanced bc a.

Locally advanced breast cancer in a 56-year-old woman, with calcifications seen at the same site 5 years earlier, likely an evolution from ductal carcinoma in situ (DCIS). (A) Bilateral digital mammograms demonstrate heterogeneously dense breasts (American College of Radiology, BI-RADS C), with a large spiculated mass in the central left breast causing left nipple retraction corresponding to the palpable mass. An ultrasound-guided breast biopsy (not shown) confirmed invasive ductal carcinoma, with axillary node metastases. (B) Maximal-intensity projection image from magnetic resonance imaging shows tumour occupying most of the left breast, measuring more than 5 cm. (C) Photographic enlargement of the left breast mass shows fine pleomorphic calcifications within the mass, characteristic for DCIS. (D) Photographic enlargement of the left breast from a screening mammogram 2 years earlier shows a smaller cluster of calcifications within the same area, not detected at screening. (E) Photographic enlargement of the left breast from a screening mammogram 5 years earlier shows a very small group of fine pleomorphic calcifications, likely DCIS, identified only in retrospect.
Does detecting dcis reduce the rate of invasive cancer? Currently, no tools are available to predict which dcis will progress and which will not. In the United Kingdom, Duffy et al. 50 conducted a retrospective population-based study that set out to estimate the association between detection of dcis at screening and the incidence of subsequent invasive interval bc as. Data were obtained for 5.2 million women 50–64 years of age who attended mammographic breast screening through the National Health Service during 2003–2007. Interval cancers diagnosed symptomatically within 36 months after the relevant screen were recorded. The average detection frequency of dcis was 1.6 per 1000 women screened. A significant negative association was observed for screen-detected dcis and the rate of invasive interval cancers; for every 3 screen-detected cases of dcis , 1 fewer invasive interval cancer occurred in the subsequent 3 years. The study concluded that detection and treatment of dcis was worthwhile for the prevention of future invasive disease. To mitigate the harm of overdiagnosis, women should be involved in the decision-making for dcis treatment, based on information about the risks of treatment compared with watchful waiting.
False Negatives
The overall sensitivity of mammography is 80%. Of bc as, 20% are not detected by mammography, but are detected by clinical symptoms such as a palpable mass or suspicious nipple discharge. False negatives are more likely with certain bc as—in particular, lobular carcinomas that tend to grow along the normal breast architecture in a lepidic pattern, making them more difficult to detect. False negatives are also more likely in patients with dense breast tissue, which masks bc a. Breast tissue density is most commonly reported using the American College of Radiology’s 4-category Breast Imaging—Reporting and Data System. Sensitivity is highest in the lowest density category and lowest in the highest density category, with one study showing sensitivity decreased from 87% in fatty breasts to 63% in women with the densest breasts 51 .
TECHNOLOGIC ADVANCES AND DIGITAL BREAST TOMOSYNTHESIS
One technologic advance in screening mammography was the transition from film screen to digital mammography. The dmist trial showed that, in women with dense breasts, the sensitivity of digital mammography was significantly increased 52 . Another recent major technologic advance is digital breast tomosynthesis ( dbt ), a pseudo “three-dimensional” mammography technique in which multiple low-dose mammographic images are acquired of compressed breast from multiple angles and are then reconstructed into overlapping thin slices that can be displayed either individually or in a cine loop. Increasingly, dbt is being used as an adjunct screening tool for the detection of bc a. Two-dimensional (2D) mammography and tomosynthesis can be obtained in a single compression, and synthesized 2D projection images can also be reconstructed from the dbt data 53 . The radiation dose received when dbt is combined with conventional 2D mammography is nearly double that of digital mammography alone, but within the established and acceptable safe dose limits 53 – 56 .
When combined with digital mammography, dbt helps to improve bc a screening and diagnosis. Multiple studies have demonstrated that bc a detection rates are improved by 33%–53% (sensitivity) and that false-positive recall rates are simultaneously reduced by 30%–40% (specificity) 57 – 66 . Several screening studies have shown incremental invasive cancer detection rates of 1.2–2 per 1000 screened women, with no increase in the detection of dcis 59 , 62 , 63 .
The main advantage of tomosynthesis is its ability to diminish the masking effect of tissue overlap and structure noise usually encountered with 2D mammography. That feature is particularly useful in the setting of dense breasts 60 , 67 and helps to improve the radiologist’s reading confidence, with better characterization of masses 68 – 70 . If dbt is used in the screening setting, the marginal definition is equal to that of spot magnification, and so women with masses detected at screening can forego additional mammographic views and attend just for ultrasonography.
Few studies have investigated the long-term sustainability of the improved screening outcomes with dbt . A retrospective analysis looked at outcomes data from 3 years of dbt screening of an entire population at an academic centre. The results showed that dbt screening outcomes were sustainable, with a significant recall reduction, an increase in the cancer cases identified in recalled patients, and a decline in interval cancers 71 . The tmist trial is the first large randomized multicentric study to assess whether, compared with conventional mammography alone, dbt combined with digital mammography is more effective at lowering the incidence of advanced bc as (see {"type":"clinical-trial","attrs":{"text":"NCT03233191","term_id":"NCT03233191"}} NCT03233191 at http://ClinicalTrials.gov ). In the United States and Canada, 165,000 asymptomatic women between the ages of 45 and 74 years will be enrolled. The study aims to provide a modern basis for implementation of the combination technology for bc a screening. The Canadian Lead-in Study began recruitment in 2014, and the full study opened in 2017.
Currently, no widely accepted view for the supplemental screening of women with dense breasts has been reached, even though the sensitivity of screening mammography is recognized to be reduced in such women. No rct s have determined any mortality benefit from supplemental screening. Multiple studies have shown increased detection (3–4 per 1000) of small, invasive, node-negative cancers when supplementary screening is performed for women with dense breasts 72 , 73 . The j-start prospective rct of ultrasonography has shown favourable preliminary results for detecting early-stage cancers, with fewer interval cancers 74 . Currently, 32 U.S. states report on breast tissue density, and many recommend supplemental screening. Personalized screening could become more of a reality in the future, whereby, depending on risk and density, supplemental screening might be offered. That approach has been proposed in Quebec with the international Perspective Project 75 . Recently, studies of contrast-enhanced mammography have shown promise in improving the detection of bc a by relying on its enhanced vascularity 76 , 77 . Although still experimental and currently used only in the diagnostic setting, that type of screening could have future applications. Breast mri has also recently been proposed as a method of screening for average-risk women: a recent study showed a high supplemental cancer detection rate of 15.5 per 1000 in 2120 average-risk women screened with mri 78 . In the latter study, more biologically active tumours were found with mri . However, given the higher cost, the requirement for intravenous contrast, and the lower specificity, breast mri has not become a part of routine screening.
Attending screening mammography has the benefit of reducing bc a mortality by 40% in average-risk women 40–74 years of age. Of the 10% false positives that occur in mammography, 8 of 10 are resolved by taking additional views or obtaining ultrasound images, with the remaining 2 being resolved by biopsy. For women who undergo biopsy, only 1 in 3 will be diagnosed with a malignancy. Overdiagnosis occurs in about 10% of screened women, represented mostly by the detection of dcis . False negatives with mammography are an important limitation, often being related to bc as hidden by dense breast tissue. Digital breast tomosynthesis has the potential to simultaneously increase cancer detection and lower the rate of false positives. In addition, supplemental screening with breast ultrasonography, breast mri , and contrast-enhanced mammography shows promise for further increasing the detection of biologically significant bc as in women at higher risk of bc a. In 2018, based on the best available current evidence, screening mammography should be recommended every 1–2 years for women 40–74 years of age at average risk. In future, as assessment of risk and breast tissue density becomes a reality, more personalized screening will likely be added to that screening mammography regimen.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’ s policy on disclosing conflicts of interest, and we declare that we have none.

MUNEEZA KHAN, MD, AND ANNA CHOLLET, MD, MPH
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2021;103(1):33-41
Patient information: See related handout on mammogram screening for breast cancer , written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Breast cancer is the most common nonskin cancer in women and accounts for 30% of all new cancers in the United States. The highest incidence of breast cancer is in women 70 to 74 years of age. Numerous risk factors are associated with the development of breast cancer. A risk assessment tool can be used to determine individual risk and help guide screening decisions. The U.S. Preventive Services Task Force (USPSTF) and American Academy of Family Physicians (AAFP) recommend against teaching average-risk women to perform breast self-examinations. The USPSTF and AAFP recommend biennial screening mammography for average-risk women 50 to 74 years of age. However, there is no strong evidence supporting a net benefit of mammography screening in average-risk women 40 to 49 years of age; therefore, the USPSTF and AAFP recommend individualized decision-making in these women. For average-risk women 75 years and older, the USPSTF and AAFP conclude that there is insufficient evidence to recommend screening, but the American College of Obstetricians and Gynecologists and the American Cancer Society state that screening may continue depending on the woman's health status and life expectancy. Women at high risk of breast cancer may benefit from mammography starting at 30 years of age or earlier, with supplemental screening such as magnetic resonance imaging. Supplemental ultrasonography in women with dense breasts increases cancer detection but also false-positive results.
Breast cancer is the most common nonskin cancer in women and accounts for 30% of all new cancers in the United States. 1 From 2001 to 2016, more than 2.3 million women in the United States were diagnosed with breast cancer. 2 The incidence of breast cancer increases after 25 years of age, peaking between 70 and 74 years. 2 Approximately one in eight women will develop invasive breast cancer (12.8% lifetime risk). 1
WHAT'S NEW ON THIS TOPIC
Breast Cancer Screening
A 2016 meta-analysis calculated that per 10,000 women screened with mammography, three breast cancer deaths are avoided over 10 years in women 40 to 49 years of age, eight deaths are avoided in women 50 to 59 years, 21 deaths are avoided in women 60 to 69 years, and 13 deaths are avoided in women 70 to 74 years. [ corrected ]
One out of every eight women 40 to 49 years of age who has a screening mammogram will subsequently undergo additional imaging, and for every case of invasive breast cancer detected by screening mammography in this age group, 10 women will have had a biopsy.
In a large, multicenter trial, women with dense breasts and a negative standard mammogram result had two-year screening with MRI or standard mammography. The interval cancer rate was lower in the MRI group than in the mammography group; however, MRI had a high false-positive rate with hundreds of negative breast biopsy results among the 4,738 women who underwent MRI screening.
MRI = magnetic resonance imaging.
The overall mortality rate in U.S. women with breast cancer is about 20 per 100,000. Mortality rates are highest in women 85 years and older (170 per 100,000). 2 White women have the highest rate of breast cancer diagnosis, whereas Black women have the highest rate of breast cancer–related death. 2 Breast cancer is also the most common cause of cancer-related death in Hispanic women and the second leading cause of cancer-related death behind lung cancer among all women. 2
Cancer screening recommendations are determined by the patient's current anatomy. Transgender females with breast tissue and transgender males who have not undergone complete mastectomy should receive screening mammography based on guidelines for cisgender persons (see https://www.aafp.org/afp/2018/1201/p645.html#sec-4 ).
What Are the Risk Factors for Breast Cancer?
The strongest risk factors are a history of childhood chest radiation, older age, increased breast density, family history of breast cancer, and certain genetic mutations ( Table 1 ). 3 – 16 However, most women who develop invasive breast cancer do not have any of these risk factors . 3
EVIDENCE SUMMARY
A retrospective cohort study demonstrated a standardized incidence ratio (i.e., the ratio of observed to expected number of cases) of 21.9 for breast cancer in women who received chest radiation during childhood. 4 Higher doses of radiation were associated with higher risk, and the highest risk was in those who received whole lung radiation (standardized incidence ratio = 43.6). The overall cumulative risk of developing breast cancer by 50 years of age was 30%. 4
Increasing age is another strong risk factor. Invasive breast cancer will be diagnosed in one out of 42 women 50 to 59 years of age, and this rate increases to one out of 14 in women 70 years and older. 5
Breast density is the amount of glandular and stromal tissue compared with adipose tissue shown on a mammogram. A systematic review and meta-analysis found that compared with women who do not have dense breasts, the relative risk of developing breast cancer is 1.79 for women with breast density between 5% and 24% and 4.64 for those with breast density of 75% or higher. 6
Data from the Breast Cancer Surveillance Consortium and the Collaborative Breast Cancer Study showed that having a first-degree relative with breast cancer increases a woman's personal risk by a hazard ratio of 1.61 and odds ratio of 1.64. 7 For patients with BRCA mutations, the risk of developing breast cancer by 80 years of age is 60% to 63%, regardless of family history. 8
How Can Physicians Estimate the Risk of Developing Breast Cancer?
Several validated risk assessment tools are available to stratify breast cancer risk ( Table 2 ). 17 These tools can assist physicians and patients in developing individualized plans regarding screening, genetic testing, or chemoprevention .
A large retrospective cohort study compared the six-year accuracy of five validated risk assessment tools among 35,921 women 40 to 84 years of age who underwent screening mammography in the United States from 2007 to 2009. 17 The models were BRCAPRO ( https://projects.iq.harvard.edu/bayesmendel/bayesmendel-r-package ); Breast Cancer Risk Assessment Tool, or Gail model ( https://bcrisktool.cancer.gov , https://www.mdcalc.com/gail-model-breast-cancer-risk ); Tyrer-Cuzick model, or International Breast Cancer Intervention Study model ( http://www.ems-trials.org/riskevaluator ); Breast Cancer Surveillance Consortium model ( https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm ); and Claus model (computer program).
Based on overall performance, the positive predictive values were 2.6% for BRCAPRO and the Tyrer-Cuzick model, 2.9% for the Breast Cancer Risk Assessment Tool and Breast Cancer Surveillance Consortium model, and 3.9% for the Claus model. The negative predictive values were high at 97% or more for all of the models. 17
Does Screening Mammography Reduce Breast Cancer–Related Mortality?
Screening mammography reduces breast cancer–related mortality, with larger reductions as women get older .
Modeling studies estimate that in women 40 to 49 years of age, the number needed to screen (NNS) with annual mammography to prevent one breast cancer death is 746. The NNS decreases to 351 in women 50 to 59 years and to 233 in women 60 to 69 years. The NNS is 377 in women 70 to 79 years of age. 18 However, randomized controlled trials have demonstrated a substantially higher NNS. A meta-analysis performed for the U.S. Preventive Services Task Force (USPSTF) calculated that per 10,000 women screened with mammography, only three breast cancer deaths are avoided over 10 years in women 40 to 49 years of age, eight deaths are avoided in women 50 to 59 years, 21 deaths are avoided in women 60 to 69 years, and 13 deaths are avoided in women 70 to 74 years. 19 [ corrected ]
Between 2008 and 2017, yearly rates of newly diagnosed breast cancer increased by 0.3%, and rates of breast cancer death fell by 1.5%. 20 This may be partly attributable to early detection of small, curable breast cancers that have a five-year relative survival rate of 98.8% posttreatment. 20 Studies have shown a reduction in the incidence of large tumors, which is also likely because of early detection of smaller tumors by mammography. 21
Lower death rates, however, may also reflect improved treatments. With older treatments, the reduction in mortality after screening mammography was approximately 12 deaths per 100,000 women. With improved treatments, the reduction in mortality after screening mammography is now about eight deaths per 100,000 women. 21
What Are the Potential Harms of Breast Cancer Screening?
False-positive results are common with screening mammography, especially in younger women, leading to further imaging and radiation exposure and subsequent breast biopsies that can be painful, can cause anxiety, and usually yield benign results. Furthermore, screening can lead to overdiagnosis and overtreatment of cancers that may never have become symptomatic or life-threatening .
According to the USPSTF, the false-positive rate of mammography is highest in women 40 to 49 years of age at 121 per 1,000 and decreases with age to 69.6 per 1,000 women 70 to 79 years of age. 22 About one of every eight women 40 to 49 years of age who has a screening mammogram will subsequently undergo additional imaging, and for every case of invasive breast cancer detected by screening mammography in this age group, 10 women will have had a biopsy, compared with only three women in their 70s. 22
False-positive results are associated with increased antidepressant and anxiolytic prescriptions, with a relative risk of 1.13 to 1.19. 23 Women at highest risk of needing antidepressant and anxiolytic therapy are those 40 to 49 years of age who underwent multiple tests, including a biopsy, and who had to wait more than one week to be told the results were false-positive. 23
Systematic reviews have found that screening mammography leads to an overdiagnosis rate of 10% to 30%. 24 , 26 [ corrected ] Overdiagnosis can lead to unnecessary treatments for screening-detected breast cancers. Sometimes this involves treating ductal carcinoma in situ that would have been inconsequential over a woman's lifetime. 3 A study based on a large U.S. cancer registry reported that out of 297,000 women 40 years and older who had a mastectomy in 2013, 18% may not have needed one. 25 Thus, the USPSTF concludes that there is no strong evidence supporting mammography screening of average-risk women in their 40s. 26
What Are the Screening Recommendations for Patients at Average Risk?
Recommendations for breast self-examinations, clinical breast examinations, and mammography vary among organizations . Table 3 summarizes recommendations from the USPSTF, the American Academy of Family Physicians (AAFP), the American College of Obstetricians and Gynecologists (ACOG), the American College of Radiology (ACR), the American Cancer Society (ACS), and the National Comprehensive Cancer Network (NCCN) . 3 , 26 – 33
Breast Self-Examination . The USPSTF and AAFP recommend against teaching patients to perform breast self-examinations because of a lack of supporting evidence. 26 , 27 ACOG, the NCCN, and the ACS encourage breast self-awareness (i.e., patient familiarity with how her breasts usually feel and look) and advise women to seek medical attention if they notice breast changes. 3 , 31 , 33 There may be some rationale for breast self-awareness based on the frequency of self-detection cited in some studies. For example, out of 361 breast cancer survivors who participated in the 2003 National Health Interview Survey, 43% reported detecting their own cancers. 34
Clinical Breast Examination . The USPSTF and AAFP state that there is insufficient evidence to assess the benefits and harms of clinical breast examinations. 26 , 28 The ACS recommends against these examinations because of insufficient evidence of benefit and a high rate of false-positive results (55 false-positives for every breast cancer detected). 31 , 35 For average-risk women 40 years and older, ACOG says that annual clinical breast examinations may be offered, and the NCCN recommends annual clinical breast examinations. 3 , 33
Mammography . Evidence of benefit varies with a woman's age. The USPSTF found lower mortality rates and a reduced risk of advanced breast cancer in women 50 years and older who had mammography screening (relative risk = 0.62; 95% CI, 0.46 to 0.83) but not in women 39 to 49 years of age (relative risk = 0.98; 95% CI, 0.74 to 1.37). 19 The number of breast cancer deaths prevented with screening over 10 years was 12.5 per 10,000 women 50 years and older but only 2.9 per 10,000 women in their 40s. 19 Overall, women 50 to 59 years of age have the best balance of risks and benefits from mammography. 3 , 19
ACS data, however, showed improved mortality benefit across all age groups, although the benefit was lower in younger women. The NNS to reduce mortality rates by 20% was 1,770 for women in their 40s, 1,087 for women in their 50s, and 835 for women in their 60s. 31
The USPSTF recommends biennial screening mammography for women 50 to 74 years of age. 26 This recommendation excludes women 40 to 49 years of age because the number needed to invite (NNI) of 1,904 and the NNS of 1,034 to detect one case of breast cancer with screening mammography were considered too high. The NNI of 1,339 and NNS of 455 in women 50 to 59 years of age and the NNI of 377 and NNS of 233 for women 60 to 69 years of age were considered acceptable. 18 The AAFP supports the USPSTF recommendation. 29
The ACS recommends annual screening mammography starting at 45 years of age and transitioning to biennial screening at 55 years of age. 31 This recommendation is based on multivariable analyses suggesting that women in the younger age group are more likely than older women to have advanced stage cancer when screened biennially rather than annually. 31
The NCCN recommends annual screening mammography. 33 , 36 ACOG recommends shared decision-making based on a discussion of benefits and harms when deciding between annual and biennial screening intervals. 3
At What Age Should Breast Cancer Screening Be Discontinued?
Women at average risk should continue screening mammography through 74 years of age . 3 , 26 , 29 – 31 , 33 Starting at 75 years of age, women should be involved in shared decision-making based on overall health status and life expectancy according to ACOG recommendations . 3 The ACS and NCCN recommend continued screening after 75 years of age if life expectancy is at least 10 years, and the ACR recommends continued screening if life expectancy is at least five to seven years . 30 , 31 , 33 The USPSTF states that there is insufficient evidence to assess the benefits and harms of screening past 74 years of age, and the AAFP supports this finding . 26 , 29
Randomized controlled trials have shown that when mammography screening prevents a death, the death would have occurred within five to seven years after screening; thus, screening women with limited life expectancy is not warranted. 36 In addition, the number of life-years gained from screening decreases from 7.8 to 11.4 per 1,000 mammograms at 74 years of age to 4.8 to 7.8 per 1,000 at 80 years and to 1.4 to 2.4 per 1,000 at 90 years. 37 When adjusted for quality of life, the number of life-years gained decreases even further, and by 90 to 92 years of age, all life-years gained are counter-balanced by a loss in quality of life, presumably because of treatment adverse effects. 37 Yet, despite these data and the corresponding recommendations, 62% of women 75 to 79 years of age and 50% of women 80 years or older get mammograms, and 70% to 86% of physicians recommend mammography for 80-year-old women. 38 , 39
What Are the Screening Recommendations for Patients at Increased Risk?
ACS recommends that women with a 20% or higher lifetime risk of breast cancer (assessed using a risk assessment tool [ Table 2 17 ] ) be offered annual mammography and magnetic resonance imaging (MRI), typically starting at 30 years of age . 32 For high-risk women 25 to 29 years of age, ACOG recommends a clinical breast examination every six to 12 months and annual breast MRI with contrast. For patients 30 years and older, ACOG recommends annual mammography and MRI with contrast . 40 The NCCN recommends that women with a lifetime risk of more than 20% have breast self-awareness and receive a clinical breast examination every six to 12 months starting at 21 years of age. Annual breast MRI is recommended starting at 25 years of age with annual screening mammography starting at 30 years . 33 Women younger than 25 years with a history of chest radiation should have breast self-awareness and receive a clinical breast examination every six to 12 months starting 10 years after radiation therapy. Once these women are 25 years old, annual breast MRI is recommended, then screening mammography starting at 30 years of age . 33 The USPSTF states that there is insufficient evidence to assess the benefits and harms of using MRI for breast cancer screening, and the AAFP supports this finding . 26 , 29
The evidence for adding annual MRI screening to mammography and clinical breast examinations in women with more than a 20% lifetime risk of breast cancer is based on nonrandomized screening trials and observational studies from the 1990s. 32 These studies showed that MRI has a sensitivity of 71% to 100% for detecting breast cancer in high-risk women vs. mammography's sensitivity of 16% to 40% in the same population. However, MRI is less specific (81% to 99%) compared with mammography (93% to more than 99%), resulting in higher rates of false-positives, subsequent medical appointments, and biopsies, with a positive predictive value of 20% to 40%. No data were collected on survival rates with MRI screening or on the optimal MRI screening interval. 32
Does Supplemental Imaging Have a Role in Evaluating Dense Breasts?
Almost 50% of women 40 to 74 years of age have dense breasts, which is a risk factor for breast cancer and for false-negative results on standard mammography . 41 Ultrasonography, MRI, and digital breast tomosynthesis (also known as 3D mammography) have been proposed as methods to detect breast cancers that might be missed on mammography in women with dense breasts .
The ACR recommends considering ultrasonography in addition to screening mammography based on a randomized multicenter trial showing improved cancer detection rates compared with mammography alone (1.9 vs. 4.2 per 1,000). 30 , 42 Ultrasonography may be particularly useful for women who have a 15% to 20% lifetime risk of breast cancer and dense breasts but no additional risk factors. 43
Data from the Connecticut Experiments showed an additional 2.3 cancers detected per 1,000 women with dense breasts who were screened with ultrasonography in addition to mammography. 43 By the fourth year of the study, the positive predictive value had increased from 7.3% to 20.1%, indicating an improved learning curve for the radiologists regarding which lesions to biopsy. Another study, involving 2,662 women with dense breasts plus one other risk factor for breast cancer, showed that adding ultrasonography to mammography increased the sensitivity of breast cancer detection compared with mammography alone (52% vs. 76%). 42
It is important to note, however, that the increased sensitivity comes at the cost of increasing false-positives. An observational cohort study of 6,081 women with varying risk of breast cancer showed that the false-positive rate was 22.2 per 1,000 screens for mammography alone vs. 52 per 1,000 screens for mammography plus ultrasonography (relative risk = 2.23). 44
MRI has also been studied as a screening option in women with dense breasts. A large multicenter trial randomized women with dense breasts and a negative result on standard mammography to two-year screening with either MRI or standard mammography. 45 The cancer detection rate during the two years was lower in the MRI group than in the mammography group (2.5 vs. 5 per 1,000 screens). More than 90% of MRI-detected cancers, however, were stage 0 or 1, and MRI screening resulted in a high false-positive rate (79.8 per 1,000 screens) with hundreds of negative breast biopsy results among the 4,738 women who underwent MRI screening.
MRI has also been compared with digital breast tomosynthesis. There were higher rates of cancer detection with MRI (11.8 per 1,000 screens) than with digital breast tomosynthesis (4.8 per 1,000 screens), but no data are available on long-term outcomes. 46 A study comparing standard mammography with digital breast tomosynthesis is underway. 47
The long-term survival of women whose breast cancers were detected with supplemental imaging modalities has not been studied.
This article updates previous articles on this topic by Tirona , 48 Knutson and Steiner , 49 and Apantaku . 50
Data Sources: A PubMed search was completed in Clinical Queries using the key terms breast cancer, breast cancer screening, risk factors for breast cancer, breast cancer risk assessment tools, breast cancer screening recommendations, breast density, mammography, supplemental screening. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were the Agency for Healthcare Research and Quality Effective Healthcare Reports, the Cochrane database, and Essential Evidence Plus. Search date: April 2020.
American Cancer Society. Cancer facts and figures 2020. Accessed April 4, 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf
Centers for Disease Control and Prevention. United States cancer statistics: data visualizations. Accessed August 2, 2020. https://gis.cdc.gov/Cancer/USCS/DataViz.html
American College of Obstetricians and Gynecologists. Practice bulletin no. 179. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130(1):e1-e16.
Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32(21):2217-2223.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30.
McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1159-1169.
Shiyanbola OO, Arao RF, Miglioretti DL, et al. Emerging trends in family history of breast cancer and associated risk. Cancer Epidemiol Biomarkers Prev. 2017;26(12):1753-1760.
Metcalfe KA, Lubinski J, Gronwald J, et al.; Hereditary Breast Cancer Clinical Study Group. The risk of breast cancer in BRCA1 and BRCA2 mutation carriers without a first-degree relative with breast cancer. Clin Genet. 2018;93(5):1063-1068.
Chen S, Iversen ES, Friebel T, et al. Characterization of BRCA1 and BRCA2 mutations in a large United States sample. J Clin Oncol. 2006;24(6):863-871.
American Cancer Society. Breast cancer facts and figures 2019–2020. Accessed August 2, 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2019-2020.pdf
Schacht DV, Yamaguchi K, Lai J, et al. Importance of a personal history of breast cancer as a risk factor for the development of subsequent breast cancer. AJR Am J Roentgenol. 2014;202(2):289-292.
Dyrstad SW, Yan Y, Fowler AM, et al. Breast cancer risk associated with benign breast disease. Breast Cancer Res Treat. 2015;149(3):569-575.
Liu Y, Colditz GA, Rosner B, et al. Alcohol intake between menarche and first pregnancy. J Natl Cancer Inst. 2013;105(20):1571-1578.
Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019;394(10204):1159-1168.
Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk. Lancet Oncol. 2012;13(11):1141-1151.
Colditz GA, Rosner B. Cumulative risk of breast cancer to age 70 years according to risk factor status. Am J Epidemiol. 2000;152(10):950-964.
McCarthy AM, Guan Z, Welch M, et al. Performance of breast cancer risk-assessment models in a large mammography cohort. J Natl Cancer Inst. 2020;112(5):489-497.
Hendrick RE, Helvie MA. Mammography screening. AJR Am J Roentgenol. 2012;198(3):723-728.
Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Ann Intern Med. 2016;164(4):244-255.
National Cancer Institute. Female breast cancer. Accessed August 2, 2020. https://seer.cancer.gov/statfacts/html/breast.html
Welch HG, Prorok PC, O'Malley AJ, et al. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375(15):1438-1447.
Nelson HD, O'Meara ES, Kerlikowske K, et al. Factors associated with rates of false-positive and false-negative results from digital mammography screening. Ann Intern Med. 2016;164(4):226-235.
Segel JE, Balkrishnan R, Hirth RA. The effect of false-positive mammograms on antidepressant and anxiolytic initiation. Med Care. 2017;55(8):752-758.
Monticciolo DL, Helvie MA, Hendrick RE. Current issues in the overdiagnosis and overtreatment of breast cancer. AJR Am J Roentgenol. 2018;210(2):285-291.
Harding C, Pompei F, Burmistrov D, et al. Use of mastectomy for overdiagnosed breast cancer in the United States. J Cancer Epidemiol. ;2019:5072506.
U.S. Preventive Services Task Force. Breast cancer: screening. January 11, 2016. Accessed July 20, 2019. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-screening
American Academy of Family Physicians. Breast cancer, breast self exam (BSE). Accessed July 20, 2019. https://www.aafp.org/patient-care/clinical-recommendations/all/breast-cancer-self-bse.html
American Academy of Family Physicians. Breast cancer, clinical breast examination (CBE). Accessed July 20, 2019. https://www.aafp.org/patient-care/clinical-recommendations/all/breast-cancer-cbe.html
American Academy of Family Physicians. Breast cancer. Accessed May 31, 2020. https://www.aafp.org/patient-care/clinical-recommendations/all/breast-cancer.html
Lee CH, Dershaw DD, Kopans D, et al. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol. 2010;7(1):18-27.
Oeffinger KC, Fontham ETH, Etzioni R, et al.; American Cancer Society. Breast cancer screening for women at average risk [published correction appears in JAMA . 2016;315(13):1406]. JAMA. 2015;314(15):1599-1614.
Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography [published correction appears in CA Cancer J Clin . 2007;57(3):185]. CA Cancer J Clin. 2007;57(2):75-89.
Bevers TB, Helvie M, Bonaccio E, et al. NCCN clinical practice guidelines in oncology. Breast cancer screening and diagnosis. Version 3.2019. May 17, 2019. Accessed May 31, 2019. https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf
Roth MY, Elmore JG, Yi-Frazier JP, et al. Self-detection remains a key method of breast cancer detection for U.S. women. J Womens Health (Larchmt). 2011;20(8):1135-1139.
Meyers ER, Moorman P, Gierisch JM, et al. Benefits and harms of breast cancer screening: a systematic review [published correction appears in JAMA . 2016;315(13):1406]. JAMA. 2015;314(15):1615-1634.
Helvie MA, Bevers TB. Screening mammography for average-risk women. J Natl Compr Canc Netw. 2018;16(11):1398-1404.
van Ravesteyn NT, Stout NK, Schechter CB, et al. Benefits and harms of mammography screening after age 74 years. J Natl Cancer Inst. 2015;107(7):djv103.
Bellizzi KM, Breslau ES, Burness A, et al. Prevalence of cancer screening in older, racially diverse adults. Arch Intern Med. 2011;171(22):2031-2037.
Leach CR, Klabunde CN, Alfano CM, et al. Physician over-recommendation of mammography for terminally ill women. Cancer. 2012;118(1):27-37.
American College of Obstetricians and Gynecologists. Hereditary breast and ovarian cancer syndrome. Practice bulletin no. 182. September 2017. Accessed August 2, 2020. https://bit.ly/37Kl2M4
Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10):dju255.
Berg WA, Zhang Z, Lehrer D, et al.; ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394-1404.
Thigpen D, Kappler A, Brem R. The role of ultrasound in screening dense breasts. Diagnostics (Basel). 2018;8(1):20.
Lee JM, Arao RF, Sprague BL, et al. Performance of screening ultrasonography as an adjunct to screening mammography in women across the spectrum of breast cancer risk [published correction appears in JAMA Intern Med . 2019;179(5):733]. JAMA Intern Med. 2019;179(5):658-667.
Bakker MF, de Lange SV, Pijnappel RM, et al.; DENSE Trial Study Group. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381(22):2091-2102.
Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening [published correction appears in JAMA . 2020;323(12):1194]. JAMA. 2020;323(8):746-756.
National Cancer Institute. TMIST (Tomosynthesis Mammographic Imaging Screening Trial). Accessed January 10, 2020. https://www.cancer.gov/about-cancer/treatment/clinical-trials/nci-supported/tmist
Tirona MT. Breast cancer screening update. Am Fam Physician. 2013;87(4):274-278. Accessed September 15, 2020. https://www.aafp.org/afp/2013/0215/p274.html
Knutson D, Steiner E. Screening for breast cancer. Am Fam Physician. 2007;75(11):1660-1666. Accessed September 15, 2020. https://www.aafp.org/afp/2007/0601/p1660.html
Apantaku LM. Breast cancer diagnosis and screening. Am Fam Physician. 2000;62(3):596-602. Accessed September 15, 2020. https://aafp.org/afp/2000/0801/p596.html
Continue Reading

More in AFP
More in pubmed.
Copyright © 2021 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
- Introduction
- Conclusions
- Article Information
ICD-10 indicates International Statistical Classification of Diseases and Related Health Problems, Tenth Revision ; OCR, Ontario Cancer Registry; OHIP, Ontario Health Insurance Plan; RPDB, Registered Persons Database.
FFS indicates fee-for-service; FHG, Family Health Group; FHO, Family Health Organization; FHT, Family Health Team; OR, odds ratio.
eTable 1. Databases Used in Study
eTable 2. Definitions of Primary Care Models
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
O’Neill B , Yusuf A , Lofters A, et al. Breast Cancer Screening Among Females With and Without Schizophrenia. JAMA Netw Open. 2023;6(11):e2345530. doi:10.1001/jamanetworkopen.2023.45530
Manage citations:
© 2024
- Permissions
Breast Cancer Screening Among Females With and Without Schizophrenia
- 1 MAP Centre for Urban Health Solutions, Li Ka Shing Knowledge Institute, St Michael’s Hospital, Unity Health Toronto, Ontario, Canada
- 2 Department of Family and Community Medicine, St Michael’s Hospital, Toronto, Ontario, Canada
- 3 Department of Family and Community Medicine, Temerty Faculty of Medicine, University of Toronto, Ontario, Canada
- 4 Women’s College Research Institute, Toronto, Ontario, Canada
- 5 ICES, Toronto, Ontario, Canada
- 6 Department of Family and Community Medicine, North York General Hospital, Toronto, Ontario, Canada
- 7 School of Medicine, Sir James Mackenzie Institute for Early Diagnosis, Population and Behavioural Science Division, University of St Andrews, St Andrews, Scotland
- 8 Institute for Mental Health Policy Research and Campbell Family Mental Health Research Institute, Centre for Addiction and Mental Health, Toronto, Ontario, Canada
Question How does breast cancer screening completion in Ontario, Canada, differ between females with and without schizophrenia, and how does it compare among those who access care from clinicians who work under different primary care payment models?
Findings In this case-control study of 127 590 females with schizophrenia (cases) and without (matched controls) schizophrenia, fewer cases had a mammogram within 2 years of their 50th birthday compared with controls. A higher proportion of cases whose clinicians were enrolled in a blended capitation payment model completed mammograms compared with cases whose clinicians were enrolled in fee-for-service or enhanced fee-for-service payment models.
Meaning Findings of this study suggest that females with schizophrenia tend to undergo less breast cancer screening compared with females without schizophrenia; some of these differences are associated with differences in primary care payment models.
Importance Breast cancer screening with mammography is recommended in Ontario, Canada, for females 50 years or older. Females with schizophrenia are at higher risk of breast cancer, but in Ontario it is currently unknown whether breast cancer screening completion differs between those with vs without schizophrenia and whether primary care payment models are a factor.
Objective To compare breast cancer screening completion within 2 years after the 50th birthday among females with and without schizophrenia, and to identify the association between breast cancer screening completion and different primary care payment models.
Design, Setting, and Participants This case-control study analyzed Ontario-wide administrative data on females with and without schizophrenia who turned 50 years of age between January 1, 2010, and December 31, 2019. Those with schizophrenia (cases) were matched 1:10 to those without schizophrenia (controls) on local health integration network, income quintile, rural residence, birth dates, and weighted Aggregated Diagnosis Group score. Data analysis was performed from November 2021 to February 2023.
Exposures Exposures were schizophrenia and primary care payment models.
Main Outcomes and Measures Outcomes included breast cancer screening completion among cases and controls within 2 years after their 50th birthday and the association with receipt of care from primary care physicians enrolled in different primary care payment models, which were analyzed using logistic regression and reported as odds ratios (ORs) and 95% CIs.
Results The study included 11 631 females with schizophrenia who turned 50 years of age during the study period and a matched cohort of 115 959 females without schizophrenia, for a total of 127 590 patients. Overall, 69.3% of cases and 77.1% of controls had a mammogram within 2 years after their 50th birthday. Cases had lower odds of breast cancer screening completion within 2 years after their 50th birthday (OR, 0.67; 95% CI, 0.64-0.70). Cases who received care from a primary care physician in a fee-for-service (OR, 0.57; 95% CI, 0.53-0.60) or enhanced fee-for-service (OR, 0.79; 95% CI, 0.75-0.82) payment model had lower odds of having a mammogram than cases whose physicians were paid under a Family Health Team model.
Conclusions and Relevance This case-control study found that, in Ontario, Canada, breast cancer screening completion was lower among females with schizophrenia, and differences from those without schizophrenia may partially be explained by differences in primary care payment models. Widening the availability of team-based primary care for females with schizophrenia may play a role in increased breast cancer screening rates.
People with schizophrenia experience markedly earlier mortality than the general population, dying 10 to 25 years sooner than those without the condition. 1 , 2 Multiple studies of premature mortality among this population have identified cancer as an important factor. 3 , 4 Schizophrenia is 1 of the top 5 mental health conditions with the largest implications for the health of people in Ontario, Canada. 5
People with schizophrenia may be at higher risk of developing breast cancer. A study in Finland reported that females with schizophrenia had higher rates of breast cancer, especially those with antipsychotic medication use for at least 5 years. 6 A meta-analysis of 125 760 patients showed that those with schizophrenia had a 31% increased risk of developing breast cancer (standardized incidence ratio, 1.31; 95% CI, 1.14-1.50). 7 The association between schizophrenia and breast cancer may be partially attributable to a shared genetic cause between the 2 diseases. 8
Cancer screening, including for cervical and colorectal cancers, is a factor in reduced mortality. 9 , 10 Although uncertainty exists about the effectiveness of mammography to reduce breast cancer–specific or all-cause mortality, 11 - 13 it is recommended by Cancer Care Ontario and the Canadian Task Force on Preventive Health Care. 14 , 15 In Ontario, the standard of care and guideline recommendation for patients with an average risk of breast cancer is screening with mammography every 2 years from age 50 to 74 years. 16
Many jurisdictions, including Ontario, have health system–level cancer screening programs, which are known to have differential access by socioeconomic status. 17 Some studies have shown lower cancer screening rates among people with severe mental illness, including 2 Ontario studies: 1 reporting lower cervical cancer screening rates among people with psychosis in a Toronto Family Health Team, 18 and another reporting lower cervical cancer screening among people with schizophrenia from provincewide data. 19 An international systematic review found that females with schizophrenia across multiple countries were half as likely to be screened for breast cancer than the general population, but the study did not include subgroup or sensitivity analyses of the characteristics of the study settings, such as different features of how health systems were organized or funded that may be associated with screening completion. 20 Another systematic review found that people with psychosis had a higher risk of breast cancer and were 22% more likely to have had metastasized cancer at the time of diagnosis. 21 Studies from the US 22 - 24 and the UK 25 , 26 and 2 systematic reviews 27 , 28 found lower cancer screening among people with serious mental illness. Studies from Manitoba, Canada, identified lower breast cancer screening with mammography 29 and lower cervical cancer screening with Papanicolaou tests among people with schizophrenia. 30 Although these studies reported differences in cancer screening rates between people with and without schizophrenia, they did not focus on aspects of health system delivery, such as primary care payment models or care organization, that could play a role in increased cancer screening among this population.
There are differences between the health systems in previous studies and the Ontario setting that highlight the importance of investigating screening rates among people with schizophrenia in the Ontario setting. Starting in 2002, Ontario family physicians (who provide most primary care in the province) have had the option to enter a series of new primary care payment models. These models included enhanced fee-for-service (FFS), known as Family Health Groups (FHGs) and comprehensive care models, whereby physicians receive pay-for-performance financial incentives for preventive care, such as completion of cancer screening. Another available model, known as Family Health Organization (FHO), provides compensation mostly through blended capitation rather than FFS payments in addition to pay-for-performance preventive care incentives. 31 In a FHO, specific pay-for-performance financial incentives were instituted starting in 2006 for preventive care, such as cervical, breast, and colon cancer screening. Some FHOs are part of Family Health Teams (FHTs), with additional team members such as nurses, social workers, dietitians, and other allied health professionals. In 2016, 29.1% of Ontario family physicians were in an FFS model, 23.8% were in enhanced FFS models, and 23.7% were in FHO-FHT models. 32 Research comparing cancer screening rates between patients who accessed care from physicians in these capitation-based models and those in the traditional FFS model did not find a difference in rates. 33 Additionally, there were no substantial differences between these models in quality of care for other conditions among the general population, such as those with diabetes, 34 and 1 study 35 suggested that timely access to care might be worse for people whose clinicians were under the capitated models. However, among those with schizophrenia, there is evidence of better guideline-congruent diabetes care favoring capitated models. 36 Therefore, it is important to understand the extent to which these capitation and team-based payment initiatives may be beneficial for cancer screening among high-risk populations, such as those with schizophrenia.
The present study aimed to compare breast cancer screening (mammogram) completion within 2 years after the 50th birthday among females with and without schizophrenia and to identify the association between breast cancer screening completion and different primary care payment models in Ontario, Canada. We investigated differences in breast cancer screening completion among those with schizophrenia who accessed care from a physician practicing in a capitated model vs an FFS model, and differences in rates between capitated models. We hypothesized that a capitated model would have patients with higher breast cancer screening completion, whereas a team-based capitated model would have patients with the highest breast cancer screening completion.
This retrospective matched case-control study obtained data from ICES, which securely houses and provides facility for analyzing health administrative data from Ontario, including data cleaning and linkage. ICES is a prescribed entity under the Section 45 provision in the Ontario Personal Health Information Protection Act, which authorizes health information custodians to transfer personal health information for evaluation of health services for resource allocation planning. In accordance with the Section 45 provision, this study was exempt from research ethics board approval and informed consent requirement. We followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) and Reporting of Studies Conducted Using Observational Routinely Collected Data ( RECORD ) reporting guidelines.
Ontario is Canada’s most populous province, with a population of 15 007 816 as of 2022 (approximately 40% of Canada’s population). 37 All necessary physician visits, medical tests, hospital services, and cancer screenings (including mammograms) are fully insured by the Ontario Health Insurance Plan (OHIP) for all Ontario permanent residents, with no payment at the point of care. Primary care physician (PCP) services are paid for by OHIP through several primary care payment models; reform of these models was instituted between 2002 and 2007. 38
As described, the 3 primary care payment models in Ontario are as follows: FFS, in which PCPs receive payment per visit, without pay-for-performance incentives; enhanced FFS (FHG), in which most compensation is from payment per visit, with some pay-for-performance incentives; and capitation (FHO-FHT), in which most compensation is from a per-patient per-year payment, with pay-for-performance incentives for preventive care. 39 The FHT model includes additional per-patient funding for hiring nonphysician staff, such as nurses, dietitians, and social workers.
The study population included all Ontario residents who were documented as female in the Registered Persons Database, who had continuous OHIP coverage throughout the study period (January 1, 2010, to December 31, 2019), and who turned 50 years of age during the study period. The primary analysis compared breast cancer screening completion between females with schizophrenia and those without that condition. To identify schizophrenia status, we used the algorithm developed by Kurdyak et al, 40 including data from outpatient physician visits and hospitalizations to identify documentation at multiple time points and settings of a schizophrenia diagnosis.
We excluded patients who were diagnosed with breast cancer before age 50 years, as identified through relevant OHIP codes in the Ontario Cancer Registry, a structured database in which all cancer diagnoses in Ontario are documented. Additionally, we excluded those who had mastectomy prior to age 50 years and received breast implants. We excluded females with particularly high risk for breast cancer, as identified from breast cancer screening that was organized through the High Risk Ontario Breast Screening Program for females with a known personal or first-degree family history of a gene variant associated with breast cancer, who were previously assessed by a genetics clinic as having a greater than 25% lifetime risk, those with a personal or family history of a cancer suggestive of a hereditary breast cancer syndrome, and those with a personal history of chest radiation before age 30 years. 41
The primary outcome was completion of breast cancer screening within 2 years after the 50th birthday. We identified this status from the Ontario Breast Screening Program, 16 which facilitates breast cancer screening completion for females aged 50 to 74 years with average risk (excluding those with a history of breast cancer, with a high risk of breast cancer, or with breast implants). We also identified completion of breast cancer screening from physician billing codes in the OHIP database indicating that a radiologist had read and reported the results of a screening mammogram; there are different codes for diagnostic mammograms.
Cases (females with schizophrenia) and controls (females without schizophrenia) were matched 1:10 on the following variables: local health integration network (the region in which the person lives in the province, as of January 1, 2010), 42 income quintile (1-5, with 1 indicating the lowest income and 5 indicating the highest income), rural residence (residential address in a community with <10 000 people as of January 1, 2010), birth dates within 180 days of each other, and weighted Aggregated Diagnosis Group (ADG) score. 43
Data about age and rurality were obtained from the Registered Persons Database. Income levels were ascertained by using Canadian Census data and by assigning residential-address forward sortation areas to income quintiles using the Statistics Canada Postal Code Conversion File Plus. 44 , 45 Health status was assessed using ADGs (Johns Hopkins ACG System). 43 These ADGs allocated related diseases and reasons for presentation to health care to individual ADGs according to the following characteristics: duration, severity, diagnostic certainty, cause, and specialty care involvement. Data used to calculate ADGs were generated when patients interacted with any part of the health system, including primary, specialty outpatient, and hospital and community care. These groupings were associated with different levels of future health service use and represented a measure of patient complexity. Health service use was assessed from OHIP physician billing codes related to the type of service, and data were obtained from the Discharge Abstract Database, 46 National Ambulatory Care Reporting System, 47 and the Ontario Mental Health Reporting System. 48
Data on primary care payment models were obtained from the Client Agency Program Enrolment data set. 49 Cases and controls were attributed to a physician if they were formally enrolled (rostered) or, for those receiving care from physicians who were not under capitation models, were assigned to the family physician who billed the largest dollar amount for primary care services for that patient during the study period. 50 We considered the following primary care payment models in this study: team-based capitation (FHT), non–team-based capitation (FHO), enhanced FFS/FHG, physician not in a patient enrollment model (FFS physicians), and no physician (patient did not have any primary care visits during the study period and were not designated as rostered to a PCP in a capitated payment model). More information on the variables extracted from each database is provided in eTables 1 and 2 in Supplement 1 .
The accuracy of matching cases to controls was assessed using weighted SD of differences between groups. Baseline characteristics (such as income quintiles) were reported with descriptive statistics for both cases and controls. The outcome of breast cancer screening completion for cases and controls was analyzed using logistic regression and reported with odds ratios (ORs). Furthermore, using logistic regression and reported with ORs, we conducted an unadjusted analysis to compare breast cancer screening completion among people with schizophrenia across primary care payment models.
Significance testing was performed with 2-sided tests. P < .05 was used to indicate statistical significance. Data analysis was performed from November 2021 to February 2023 using SAS, version 9.4 (SAS Institute Inc).
This study included 11 631 females with schizophrenia (cases) who turned 50 years of age during the study period and were matched to 115 959 without schizophrenia (controls), for a total of 127 590 participants ( Figure 1 ). Matching was adequate, with SDs close to 0 ( Table 1 ). Overall, 34.8% of cases and 34.9% of controls were in the lowest income quintile, and 8.7% of cases and 8.6% of controls lived in rural communities. The largest proportion of both cases (13.1%) and controls (8.6%) lived in the Toronto Central region, and 1.9% of cases and controls lived in the rural Northwest region of Ontario. Most females with schizophrenia (46.2%) had a weighted ADG score of 10 or higher, suggesting substantial comorbidity and future health service use.
For the primary outcome of breast cancer screening completion, 69.3% of cases and 77.1% of controls had a mammogram within 2 years of their 50th birthday. Those with schizophrenia had lower odds of having a mammogram compared with those with schizophrenia (OR, 0.67; 95% CI, 0.64-0.70; P < .001) ( Table 2 ).
There were differences in breast cancer screening completion among cases who received care from PCPs in different primary care payment models ( Table 3 ; Figure 2 ). Most cases were enrolled with a physician either in an FHG model (30.8%) or an FHT model (24.8%) ( Table 1 ). Among females with schizophrenia, 5.9% were found to have no physician visits during the study period. These patients also had lower odds of having a mammogram while being enrolled with a physician in an FFS vs an FHT model (OR, 0.57; 95% CI, 0.53-0.60; P < .001). The odds of having a mammogram while enrolled with a physician in an FHG model were lower compared with an FHT model for females with schizophrenia (OR, 0.79; 95% CI, 0.75-0.82; P < .001).
Most of the total study population (62.5%) had a mammogram before age 50 years. Furthermore, 55.6% of cases had a mammogram before age 50 years. The proportion of controls who had a mammogram before age 50 years was higher than the proportion of cases (63.2%).
This case-control study of breast cancer screening among females in Ontario, Canada, found lower odds of undergoing mammograms among those with schizophrenia. The overall pattern of lower completion of breast cancer screening among patients with schizophrenia was consistent with findings in other settings.
We explored differences in breast cancer screening completion between patients who accessed care from physicians in different primary care payment models to identify associations between these models and breast cancer screening completion. We found higher odds of mammogram completion among those receiving care under capitated models, in which most of the payments were per patient per year (rather than per visit) and there were pay-for-performance incentives for high proportions of breast cancer screening completion. We were unable to assess the relative implications of these 2 aspects of compensation for breast cancer screening completion, but the fact that patients of physicians in capitation models had higher odds of having mammograms suggests an association with 1 or both aspects of of these models. This association with breast cancer screening was not seen in the general Ontario population in the year after the pay-for-performance initiative was instituted 51 (ie, 63.2% of eligible patients had a mammogram within 30 months of March 31, 2010). In the present study, we found a higher proportion of breast cancer screening completion (77.1% of those without schizophrenia and 69.3% of those with schizophrenia). One possible explanation for this higher mammogram completion may be the use of different definitions or may be the improvement, over time, in breast cancer screening completion within capitation models, specifically patients with higher barriers to screening completion, such as those with schizophrenia. We believe the higher breast cancer screening completion in this study among females with schizophrenia receiving care from PCPs in capitation and team-based capitation models compared with 2010 data may be associated with different allocation of resources (eg, physician or allied health professional time); this resource allocation may be particularly beneficial for patients with complex care needs, such as those with schizophrenia. A recent study in Ontario comparing primary care enrollment of adults with and without serious mental illness found lower enrollment in those models among people with serious mental illness. 52 The finding that a mammogram was higher among those with schizophrenia in capitation models (which require enrollment) suggests that ensuring people with schizophrenia have access to these models is warranted. Total health care costs have been shown to be lower among patients of physicians in capitation models than FFS models, further supporting this point, 53 although a specific comparison between costs for people with schizophrenia between those models has not been reported.
One finding, which to our knowledge has not been reported previously, was the proportion of people in both the case and control cohorts who had mammograms before the age of 50 years. Ontario guidelines recommend the completion of breast cancer screening for people with average risk after age 50 years, noting that before age 50 years mammograms can be ordered for screening purposes on a case-by-case basis and in consultation between patients and clinicians. We found that 55.6% of cases and 63.2% of controls received mammograms before age 50 years. This finding represents a deviation from the Ontario guidelines and is likely associated with patient preference or concern about a family history of breast cancer leading to mammogram ordering at a younger age than at the age when routine screening is recommended. Since mammography has a lower positive predictive value for cancer detection among younger people given their lower prevalence of breast cancer, 54 it is important that clinicians discuss the benefits and risks of this approach with patients.
Limitations of this study include the nature of observational data, which prevented our assessment of causality. We included only patients with valid Ontario health coverage and who were permanent residents of Ontario. Some variables were neighborhood level rather than individual level, such as income quintile, and thus we were unable to account for some potential confounders, such as race and ethnicity. Our definition of completing a screening mammogram was different from that used in other studies. We chose within 2 years after the 50th birthday because that was consistent with Ontario guidelines of starting breast cancer screening with mammography at age 50 years.
This case-control study found that females with schizophrenia had lower breast cancer screening completion in Ontario, Canada, than those without schizophrenia. Among the cases, higher odds of mammography completion were seen in those who accessed care from PCPs who were paid under capitation rather than FFS; mammogram completion was highest among those who received care from PCPs working under team-based capitation models. Given that cancer mortality is one of the most substantial factors of mortality in people with schizophrenia, efforts to increase breast cancer screening rates are essential. Widening the availability of team-based, capitated primary care payment model may be a way to achieve this goal.
Accepted for Publication: October 19, 2023.
Published: November 29, 2023. doi:10.1001/jamanetworkopen.2023.45530
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 O’Neill B et al. JAMA Network Open .
Corresponding Author: Braden O’Neill, MD, DPhil, CCFP, MAP Centre for Urban Health Solutions, Li Ka Shing Knowledge Institute, St Michael’s Hospital, 209 Victoria St, Toronto ON M5B 1T8, Canada ( [email protected] ).
Author Contributions: Dr O’Neill and Ms Huang had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: O'Neill, Lofters, Kiran, Greiver, Sullivan, Kurdyak.
Acquisition, analysis, or interpretation of data: O'Neill, Yusuf, Huang, Ekeleme.
Drafting of the manuscript: O'Neill, Yusuf, Ekeleme.
Critical review of the manuscript for important intellectual content: O'Neill, Lofters, Huang, Ekeleme, Kiran, Greiver, Sullivan, Kurdyak.
Statistical analysis: Huang, Ekeleme, Kurdyak.
Obtained funding: O'Neill.
Administrative, technical, or material support: Lofters, Ekeleme, Sullivan, Kurdyak.
Supervision: O'Neill, Sullivan, Kurdyak.
Conflict of Interest Disclosures: Dr O'Neill reported receiving salary support as a clinician scientist from the Department of Family and Community Medicine at the University of Toronto and St Michael’s Hospital and being a member of the Ontario Health Centre for Excellence in Mental Health and Addictions Schizophrenia and Psychosis Advisory Table outside the submitted work. Dr Lofters reported receiving personal fees from Ontario Health and grants from Pfizer/ReThink Breast Cancer outside the submitted work. No other disclosures were reported.
Funding/Support: This study was supported by the Medical Psychiatry Alliance and the Li Ka Shing Knowledge Institute, St Michael’s Hospital, Unity Health Toronto.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Association between sociodemographic factors and health beliefs related to breast cancer screening behavior among Northern Thai women: a hospital-based study
Affiliations.
- 1 Department of Family Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
- 2 Global Health and Chronic Conditions Research Group, Chiang Mai University, Chiang Mai, 50200, Thailand.
- 3 Department of Psychiatry, Faculty of Medicine, Chiang Mai University, 110 Inthawarorot Rd., Sriphum, Muang, Chiang Mai, 50200, Thailand.
- 4 School of Nursing, Indiana University, Indianapolis, IN, 46202, USA.
- 5 Melvin and Bren Simon Comprehensive Cancer Center, Indiana University, Indianapolis, IN, 46202, USA.
- 6 Department of Radiology, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
- 7 Department of Psychiatry, Faculty of Medicine, Chiang Mai University, 110 Inthawarorot Rd., Sriphum, Muang, Chiang Mai, 50200, Thailand. [email protected].
- PMID: 38556539
- PMCID: PMC10982300
- DOI: 10.1038/s41598-024-58155-y
Early diagnosis of breast cancer is crucial for reducing mortality rates. The purpose of this study is to determine the impact of demographics/social determinants of health on beliefs about the practice of self-breast examination, using mammogram and ultrasound in the context of breast cancer screening among Thai women in a hospital-based setting for implying program planning and future research. A cross-sectional study was conducted in two health centers in Chiang Mai Province from August 2021 to December 2021, involving 130 Thai women ages 40 to 70 years. Data were collected by a survey using a questionnaire to gather sociodemographic information, and health beliefs about breast cancer and screening behavior utilizing the modified Thai version of Champion's Health Belief Model Scale (MT-CHBMS). Descriptive statistics, t-tests, ANOVA, and linear regression models were employed for examining association between sociodemographic factors and health beliefs about the practice of self-breast examination (BSE), using mammogram (MG) and ultrasound (UTS). Health insurance schemes were associated with Benefit-MG, Barrier-BSE, Barrier-MG and Barrier-UTS subscales. Additionally, monthly income was associated with Barrier-MG and Barrier-UTS subscales. The most common barriers reported were "embarrassment", "worry", and "takes too much time". To enhance breast cancer screening in Thailand, program planning and future research should focus on health insurance schemes, especially women with social security schemes, as they may be the most appropriate target group for intervention.
Keywords: Breast cancer; Health beliefs; Perception; Screening.
© 2024. The Author(s).
- Breast Neoplasms* / diagnosis
- Cross-Sectional Studies
- Early Detection of Cancer
- Health Behavior
- Health Knowledge, Attitudes, Practice
- Sociodemographic Factors
- Surveys and Questionnaires

Study: Protein helps prevent breast cancer metastasis
W hile better screening and improved treatments are leading to better outcomes for patients with breast cancer, 90% of breast cancer deaths are a result of metastasis, or the cancer growing and spreading to other parts of the body.
University of Cincinnati Cancer Center researchers in the lab of Jun-Lin Guan, Ph.D., have identified a new protein that helps prevent metastasis of a subset called HER2-positive breast cancer. About 20% of patients have HER2-positive breast cancer, and these cancers tend to be more aggressive than other types.
The study findings were published in the journal Cell Reports.
Autophagy background
Guan's lab has focused on how autophagy, or the cell's "recycling" function, affects cancer metastasis.
"Autophagy can be likened to a self-cleansing mechanism within cells," said Mingang Hao, Ph.D., first author of the study and research scientist in Guan's lab. "It allows them to eliminate undesirable or harmful components and emerge stronger and unharmed. In the context of cancer, dysfunctional autophagy has been linked to the development and progression of tumors."
Research published in 2021 led by Hao found that blocking autophagy in HER2-positive breast cancer cells helped eliminate cancer development in an animal model of the disease.
However, it was not clear whether the autophagy blockade also inhibited the process of cancer metastasis, as the lack of metastasis could be due to the elimination of the primary tumor growth in the model.
Very few studies in the field have directly examined the role of autophagy in metastasis, and most studies focused on genes thought to only play a role in autophagy (so-called "core" autophagy genes).
"In addition to these primary autophagy genes, which have been a major focus of research on cancer development and progression, there are many other proteins that regulate autophagy within cells," Hao said.
A genetic library
Using CRISPR gene-editing technology, Hao and his colleagues created a specialized genetic library that targeted 171 different genes that are involved in autophagy regulation. By "turning off" each gene, the researchers aimed to identify specific genes that prevented the spread of breast cancer cells.
Using this technique, Hao said they identified a protein called p47 that prevents breast cancer metastasis.
"It does this by affecting different cellular pathways that are crucial for tumor cell movement," Hao said. "These findings help us better understand the mechanisms behind cancer metastasis and may eventually lead to new strategies for preventing or treating the spread of breast cancer."
In human breast cancer samples, lower p47 expression was correlated with higher breast cancer metastasis.
"This is one of the first few studies that links a particular autophagy regulatory gene with cancer metastasis with clear mechanisms that can potentially lead to the development of new therapies," said Guan, professor and former chair of the Department of Cancer Biology at UC's College of Medicine.
"I view this as one of the most important findings from my lab and a culmination of a lot of research expertise as well as unique reagents we generated over the years."
Guan said cancer drugs are often developed to inhibit a gene that helps cancer cells grow, but in the case of p47, a potential therapy would seek to increase the functions of the protein so that it can prevent metastasis.
"I would argue it's more powerful than inhibiting something," he said, noting popular immunotherapy drug pembrolizumab similarly works by boosting a pathway the body uses to fight cancer.
Moving forward, the research team will seek to learn more about p47's mechanism of action and potential to be developed as a therapeutic. The researchers identified additional genes involved in autophagy that appear to affect metastasis and could become further targets for new treatments.
The gene library Hao custom-developed for this study is also available for other researchers at the Cancer Center and other institutions to use to identify potential targets in other cancers.
Hao began this particular line of research in 2018 and said patience, time, effort, and genuine interest in your work are essential for cancer researchers.
Guan said many researchers focus on one protein or gene at a time, but it takes specific expertise to conduct systematic, impactful research on many genes at one time.
"Mingang's previous work laid the foundation for this, so this is something very unique that not many labs would be able to perform," Guan said. "When I recruited him, this was the kind of thing I hoped he could do."
More information: Mingang Hao et al, In vivo CRISPR knockout screen identifies p47 as a suppressor of HER2+ breast cancer metastasis by regulating NEMO trafficking and autophagy flux, Cell Reports (2024). DOI: 10.1016/j.celrep.2024.113780
Provided by University of Cincinnati
Breast Cancer Screening (PDQ®)–Patient Version
What is screening.
Screening is looking for signs of disease, such as breast cancer , before a person has symptoms . The goal of screening tests is to find cancer at an early stage when it can be treated and may be cured . Sometimes a screening test finds cancer that is very small or very slow growing. These cancers are unlikely to cause death or illness during the person's lifetime.
Scientists are trying to better understand which people are more likely to get certain types of cancer. For example, they look at the person's age, their family history , and certain exposures during their lifetime. This information helps doctors recommend who should be screened for cancer, which screening tests should be used, and how often the tests should be done.
It is important to remember that your doctor does not necessarily think you have cancer if he or she suggests a screening test. Screening tests are done when you have no cancer symptoms. Women who have a strong family history or a personal history of cancer or other risk factors may also be offered genetic testing .
If a screening test result is abnormal , you may need to have more tests done to find out if you have cancer. These are called diagnostic tests , rather than screening tests.
For more information about cancer screening, see Cancer Screening Overview .
General Information About Breast Cancer
Breast cancer is a disease in which malignant (cancer) cells form in the tissues of the breast., breast cancer is the second leading cause of death from cancer in american women., different factors increase or decrease the risk of breast cancer..
Each breast also has blood vessels and lymph vessels . The lymph vessels carry an almost colorless, watery fluid called lymph . Lymph vessels carry lymph between lymph nodes . Lymph nodes are small, bean-shaped structures that filter lymph and store white blood cells that help fight infection and disease. Groups of lymph nodes are found near the breast in the axilla (under the arm), above the collarbone , and in the chest.
For more information about breast cancer , see the following:
- Breast Cancer Prevention
- Breast Cancer Treatment (Adult)
- Male Breast Cancer Treatment
- Genetics of Breast and Gynecologic Cancers
Women in the United States get breast cancer more than any other type of cancer except for skin cancer .
Breast cancer is more likely to occur as a woman ages. It occurs more often in White women than in Black women, but Black women die from breast cancer more often than White women.
Breast cancer rarely occurs in men. Because men with breast cancer usually have a lump that can be felt, screening tests are not likely to be helpful.
For information about risk factors and protective factors for breast cancer, see Breast Cancer Prevention .
Breast Cancer Screening
Tests are used to screen for different types of cancer when a person does not have symptoms., mammography is the most common screening test for breast cancer., magnetic resonance imaging (mri) may be used to screen women who have a high risk of breast cancer., whether a woman should be screened for breast cancer and the screening test to use depends on certain factors., breast exam, thermography, tissue sampling, screening tests for breast cancer are being studied in clinical trials..
Scientists study screening tests to find those with the fewest harms and most benefits. Cancer screening trials also are meant to show whether early detection (finding cancer before it causes symptoms ) helps a person live longer or decreases a person’s chance of dying from the disease. For some types of cancer, the chance of recovery is better if the disease is found and treated at an early stage .
A mammogram is a picture of the inside of the breast . Mammography may find tumors that are too small to feel. It may also find ductal carcinoma in situ (DCIS). In DCIS, abnormal cells line the breast duct , and in some women may become invasive cancer .
There are different types of mammograms:
- Film mammography is an x-ray picture of the breast.
- Digital mammography (DM) is a computer picture of the breast.
- Digital breast tomosynthesis (DBT) uses x-rays to take a series of pictures of the breast from many different angles. A computer is used to make 3-D pictures of the breast from these x-rays.
- 2-dimensional mammography (S2D) uses x-rays to take pictures of the inside of the breast, usually from two different angles. A computer or x-ray film is used to make 2-D pictures of the breast.
Digital breast tomosynthesis (DBT) was approved by the U.S. Food and Drug Administration (FDA) in 2018 and is now used in 3 out of 4 facilities. One recent study found that 2-dimensional mammography (S2D) combined with DBT improved tumor detection rates and lowered mammogram callbacks, radiation dose , and overall costs. More studies are being done to compare different types of breast cancer screening.
Many factors affect whether mammography is able to detect (find) breast cancer:
- The age and weight of the patient.
- The size and type of tumor.
- Where the tumor has formed in the breast.
- How sensitive the breast tissue is to hormones .
- How dense the breast tissue is.
- The timing of the mammography within the woman's menstrual cycle .
- The quality of the mammogram picture.
- The skill of the radiologist in reading the mammogram.
Women aged 50 to 69 years who have screening mammograms have a lower chance of dying from breast cancer than women who do not have screening mammograms.
Fewer women are dying of breast cancer in the United States, but it is not known whether the lower risk of dying is because the cancer was found early by screening or whether the treatments were better.
MRI may be used as a screening test for women who have a high risk of breast cancer. Factors that put women at high risk include the following:
- Certain gene changes, such as changes in the BRCA1 or BRCA2 genes.
- A family history ( first degree relative , such as a mother, daughter or sister) with breast cancer.
- Certain genetic syndromes , such as Li-Fraumeni or Cowden syndrome .
An MRI is more likely than mammography to find a breast mass that is not cancer.
Women with dense breasts who have supplemental screening (for example, an MRI) show higher rates of breast cancer detection, but there is limited evidence about whether this leads to better health outcomes.
Women with risk factors for breast cancer, such as certain changes in the BRCA1 or BRCA2 gene or certain genetic syndromes may be screened at a younger age and more often.
Women who have had radiation treatment to the chest, especially at a young age, may start routine breast cancer screening at an earlier age. The benefits and risks of mammograms and MRIs for these women have not been studied.
Breast cancer screening has not been shown to benefit the following women:
- Elderly women who, if diagnosed with breast cancer through screening, will usually die of other causes. Screening mammograms for those aged 66 to 79 years may find cancer in a very small percentage of women, but most of these cancers are low risk.
- In women with an average risk of developing breast cancer, screening mammography before age 40 has not shown any benefit.
- In women who are not expected to live for a long time and have other diseases or conditions , finding and treating early stage breast cancer may reduce their quality of life without helping them live longer.
Other screening tests have been or are being studied in clinical trials.
Studies have been done to find out if the following breast cancer screening tests are useful in finding breast cancer or helping women with breast cancer live longer.
A clinical breast exam is an exam of the breast by a doctor or other health professional. He or she will carefully feel the breasts and under the arms for lumps or anything else that seems unusual. It is not known if having clinical breast exams decreases the chance of dying from breast cancer.
Breast self-exams may be done by women or men to check their breasts for lumps or other changes. If you feel any lumps or notice any other changes in your breasts, talk to your doctor. Doing regular breast self-exams has not been shown to decrease the chance of dying from breast cancer.
Thermography is a procedure in which a special camera that senses heat is used to record the temperature of the skin that covers the breasts. Tumors can cause temperature changes that may show up on the thermogram.
There have been no randomized clinical trials of thermography to find out how well it detects breast cancer or the harms of the procedure.
Breast tissue sampling is taking cells from breast tissue to check under a microscope . Breast tissue sampling as a screening test has not been shown to decrease the risk of dying from breast cancer.
Information about clinical trials supported by NCI can be found on NCI’s clinical trials search webpage. Clinical trials supported by other organizations can be found on the ClinicalTrials.gov website.
Harms of Breast Cancer Screening
Screening tests can have harms., false-positive test results can occur., false-positive results can lead to extra testing and cause anxiety., false-negative test results can delay diagnosis and treatment., finding breast cancer may lead to breast cancer treatment and side effects, but it may not improve a woman's health or help her live longer., mammography exposes the breast to low doses of radiation., there may be pain or x-ray discomfort during a mammogram., talk to your doctor about your risk of breast cancer and your need for screening tests..
Not all breast cancers will cause death or illness in a woman's lifetime, so they may not need to be found or treated.
Decisions about screening tests can be difficult. Not all screening tests are helpful and most have harms. Before having any screening test, you may want to discuss the test with your doctor. It is important to know the harms of the test and whether it has been proven to reduce the risk of dying from cancer .
The harms of mammography include the following:
Screening test results may appear to be abnormal even though no cancer is present. A false-positive test result (one that shows there is cancer when there really isn’t) is usually followed by more tests (such as biopsy ), which also have risks.
When a breast biopsy result is abnormal, getting a second opinion from a different pathologist may confirm a correct breast cancer diagnosis .
Most abnormal test results turn out not to be cancer. False-positive results are more common in the following:
- Younger women (under age 50).
- Women who have had previous breast biopsies.
- Women with a family history of breast cancer.
- Women who take hormones for menopause .
False-positive results are more likely the first time screening mammography is done than with later screenings. For every ten women who have a single mammogram , one will have a false-positive result. The chance of having a false-positive result goes up the more mammograms a woman has. Comparing a current mammogram with a past mammogram lowers the risk of a false-positive result.
The skill of the radiologist also can affect the chance of a false-positive result.
If a mammogram is abnormal, more tests may be done to diagnose cancer. Women can become anxious during the diagnostic testing. Even if it is a false-positive test and cancer is not diagnosed, the result can lead to anxiety anywhere from a few days to years later.
Several studies show that women who feel anxiety after false-positive test results are more likely to schedule regular breast screening exams in the future.
Screening test results may appear to be normal even though breast cancer is present. This is called a false-negative test result . A woman who has a false-negative test result may delay seeking medical care even if she has symptoms . About one in 5 cancers are missed by mammography.
The chance of a false-negative test result is more common in women who:
- Are younger.
- Have dense breast tissue .
- Have cancer that is not dependent on hormones ( estrogen and progesterone ).
- Have cancer that is fast growing.
Some breast cancers found only by screening mammography may never cause health problems or become life-threatening. Finding these cancers is called overdiagnosis . When these cancers are found, having treatment may cause serious side effects and may not lead to a longer, healthier life.
Being exposed to high radiation doses is a risk factor for breast cancer. The radiation dose with a mammogram is very low. Women who start getting mammograms after age 50 have very little risk that the overall exposure to radiation from mammograms throughout their lives will cause harm. Women with large breasts or with breast implants may be exposed to slightly higher radiation doses during screening mammography.
During a mammogram, the breast is placed between two plates that are pressed together. Pressing the breast helps to get a better of the breast. Some women have pain or discomfort during a mammogram. The amount of pain may also depend on the following:
- The phase of the woman's menstrual cycle .
- The woman's anxiety level.
- How much pain the woman expected.
Talk to your doctor or other care provider about your risk of breast cancer, whether a screening test is right for you, and the benefits and harms of the screening test. You should take part in the decision about whether you want to have a screening test, based on what is best for you. For more information, see Cancer Screening Overview .
About This PDQ Summary
Physician Data Query (PDQ) is the National Cancer Institute's (NCI's) comprehensive cancer information database. The PDQ database contains summaries of the latest published information on cancer prevention, detection, genetics, treatment, supportive care, and complementary and alternative medicine. Most summaries come in two versions. The health professional versions have detailed information written in technical language. The patient versions are written in easy-to-understand, nontechnical language. Both versions have cancer information that is accurate and up to date and most versions are also available in Spanish .
PDQ is a service of the NCI. The NCI is part of the National Institutes of Health (NIH). NIH is the federal government’s center of biomedical research. The PDQ summaries are based on an independent review of the medical literature. They are not policy statements of the NCI or the NIH.
Purpose of This Summary
This PDQ cancer information summary has current information about breast cancer screening. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care.
Reviewers and Updates
Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary ("Updated") is the date of the most recent change.
The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Screening and Prevention Editorial Board .
Clinical Trial Information
A clinical trial is a study to answer a scientific question, such as whether one treatment is better than another. Trials are based on past studies and what has been learned in the laboratory. Each trial answers certain scientific questions in order to find new and better ways to help cancer patients. During treatment clinical trials, information is collected about the effects of a new treatment and how well it works. If a clinical trial shows that a new treatment is better than one currently being used, the new treatment may become "standard." Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Clinical trials can be found online at NCI's website . For more information, call the Cancer Information Service (CIS), NCI's contact center, at 1-800-4-CANCER (1-800-422-6237).
Permission to Use This Summary
PDQ is a registered trademark. The content of PDQ documents can be used freely as text. It cannot be identified as an NCI PDQ cancer information summary unless the whole summary is shown and it is updated regularly. However, a user would be allowed to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks in the following way: [include excerpt from the summary].”
The best way to cite this PDQ summary is:
PDQ® Screening and Prevention Editorial Board. PDQ Breast Cancer Screening. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/breast/patient/breast-screening-pdq . Accessed <MM/DD/YYYY>. [PMID: 26389160]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use in the PDQ summaries only. If you want to use an image from a PDQ summary and you are not using the whole summary, you must get permission from the owner. It cannot be given by the National Cancer Institute. Information about using the images in this summary, along with many other images related to cancer can be found in Visuals Online . Visuals Online is a collection of more than 3,000 scientific images.
The information in these summaries should not be used to make decisions about insurance reimbursement. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s E-mail Us .
- Open access
- Published: 02 April 2024
Population-based BRCA germline mutation screening in the Han Chinese identifies individuals at risk of BRCA mutation-related cancer: experience from a clinical diagnostic center from greater Shanghai area
- Zhiyuan Wu 1 na1 ,
- Qingyun Zhang 2 na1 ,
- Yiting Jin 3 na1 ,
- Xinju Zhang 2 ,
- Yanli Chen 1 ,
- Can Yang 1 ,
- Xuemei Tang 2 ,
- Haowen Jiang 4 ,
- Xiaoyi Wang 5 ,
- Xinli Zhou 6 ,
- Feng Yu 7 ,
- Bing Wang 7 &
- Ming Guan 1 , 2
BMC Cancer volume 24 , Article number: 411 ( 2024 ) Cite this article
Metrics details
Deleterious BRCA1 / 2 ( BRCA ) mutation raises the risk for BRCA mutation-related malignancies, including breast, ovarian, prostate, and pancreatic cancer. Germline variation of BRCA exhibits substantial ethnical diversity. However, there is limited research on the Chinese Han population, constraining the development of strategies for BRCA mutation screening in this large ethnic group.
We profile the BRCA mutational spectrum, including single nucleotide variation, insertion/deletion, and large genomic rearrangements in 2,080 apparently healthy Chinese Han individuals and 522 patients with BRCA mutation-related cancer, to determine the BRCA genetic background of the Chinese Han population, especially of the East Han. Incident cancer events were monitored in 1,005 participants from the healthy group, comprising 11 BRCA pathogenic/likely pathogenic (PLP) variant carriers and 994 PLP-free individuals, including 3 LGR carriers.
Healthy Chinese Han individuals demonstrated a distinct BRCA mutational spectrum compared to cancer patients, with a 0.53% (1 in 189) prevalence of pathogenic/likely pathogenic (PLP) variant, alongside a 3 in 2,080 occurrence of LGR. BRCA1 c. 5470_5477del demonstrated high prevalence (0.44%) in the North Han Chinese and penetrance for breast cancer. None of the 3 LGR carriers developed cancer during the follow-up. We calculated a relative risk of 135.55 (95% CI 25.07 to 732.88) for the development of BRCA mutation-related cancers in the BRCA PLP variant carriers (mean age 42.91 years, median follow-up 10 months) compared to PLP-free individuals (mean age 48.47 years, median follow-up 16 months).
The unique BRCA mutational profile in the Chinese Han highlights the potential for standardized population-based BRCA variant screening to enhance BRCA mutation-related cancer prevention and treatment.
Key message of article
There is significant ethnical diversity in the prevalence and spectrum of BRCA germline variants. While previous studies of regional and preliminary national BRCA mutation screening have contributed to our knowledge of BRCA germline mutation in China, our research has unveiled a distinctive mutational profile in the Han Chinese across major regions of the country, representing 20% of the world’s population. It also demonstrated the potential of BRCA mutation screening in the general healthy population for identifying individuals at higher risk of BRCA mutation-related cancer, a risk often overlooked by family history-based screening strategies. These findings offer initial insights into the potential benefits of population-based screening for preventing BRCA mutation-related cancer in the Chinese Han. Further investigation is warranted, including multi-center, long-term prospective trials, cost-effectiveness analysis, and psychosomatic medical research.
Peer Review reports
Introduction
Deleterious germline variants of BReast CAncer susceptibility genes BRCA1 and BRCA2 ( BRCA) significantly increase the risk of developing “ BRCA mutation”-related tumors, including breast, ovarian, pancreatic, and prostate cancer [ 1 ]. Screening for these variants in those with a family cancer history has enhanced the early prevention and intervention among high-risk individuals [ 2 , 3 ].
Large-scale genome databases have expanded our understanding of BRCA ’s genetic background in the major populations [ 4 ], highlighting ethnic diversity in both the prevalence and mutational spectrum of germline BRCA variation across Caucasians, Ashkenazi Jews, Hispanics, African Americans, and Asian [ 5 , 6 ]. In addition, it has brought to light the surprising observation that population-based screening can identify nearly twice as many deleterious variant carriers compared to conventional family history-based screening [ 7 , 8 ].
In the last five years, there have been over 40 published studies profiling the mutational spectrum in patients with BRCA mutation-related cancers in China [ 9 , 10 , 11 , 12 , 13 ]. While some of them revealed the mutational landscape in the healthy controls of case-control studies for breast cancer [ 9 , 14 ] and ovarian cancer [ 10 ], investigations into the prevalence of mutations in the major population (Chinese Han) and the subsequent research on whether variant screening can yield benefits remains limited due to the extensive geographical landscape of China and the significant genetic diversity within the Han ethnic group [ 15 ]. Regional studies have documented varying prevalence of single nucleotide variations (SNVs) and small insertion and deletion events (InDels) in areas like Taiwan [ 16 ] and Macau [ 17 ]. There was also nationwide variant screening conducted, but the participants predominately originates from the North Han and Lingnan Han [ 18 ]. Besides, large genomic rearrangements (LGRs), another contributor to the silence of BRCA function, have been less reported in this population. It remains uncertain whether broadening BRCA screening in this demographic offers more benefits in identifying high-risk individuals [ 19 , 20 ]. These gaps in our knowledge of BR CA variants’ genomic and functional aspects have impeded the establishment and standardization of BRCA mutation screening strategy for the Chinese Han population, which constitutes over 20% of the global population.
In this descriptive study, we integrated next-generation sequencing (NGS) data of BRCA1 and BRCA2 exons from 2,080 apparently healthy individuals and 522 patients with BRCA mutation-related cancer, to reveal the unique genetic pattern of deleterious BRCA variants, including SNVs, InDels, and LGRs, in the general Chinese Han population, with a special focus in the East Han, which account for 25% population of the Chinese Han population. Additionally, with clinical follow-up data spanning up to 24 months in the healthy population, we demonstrate that BRCA germline mutation screening can aid in the risk stratification and early detection of BRCA mutation-related cancer in the apparently healthy Chinese Han population.
Participants and methods
Apparently healthy population and patients with brca mutation related-cancer.
From June 2021 to February 2023, 2,080 apparently healthy participants who denied either a personal or family history of cancer were enrolled from the health management center of Huashan Hospital, Fudan University. All the participants were over 18 years old, and their medical records were blindly reviewed by two physicians to confirm the tumor-free status at enrollment. Besides, 121 patients with triple-negative breast cancer (TNBC), 181 with metastatic castration-resistant prostate cancer (mCRPC), 215 with pancreatic ductal adenocarcinoma (PDAC), and 5 with high-grade ovarian cancer (HGOC) seen in Huashan Hospital, Fudan University were enrolled as the BRCA mutation related-cancer group. All the cancer patients were enrolled to undergo BRCA mutation screening with the aim of formulating surgery and chemo-/radio-therapy strategies guided by their genotypes [ 21 ]. The cancer diagnosis were established based on blind review of biopsy or mastectomy slides by 2 certificated pathologists, in accordance to the World Health Organization tumour classification blue book [ 22 , 23 , 24 , 25 ]. The Han ethnicity and place of birth were confirmed in the electronic healthcare registration system. Written informed consent was received from all participants. In compliance with the Helsinki Declaration of 1975, as revised in 1996, this study was approved by the Institutional Review Board of Huashan Hospital of Fudan University (2023 − 812).
Germline mutation profiling of BRCA1 and BRCA2 by next generation sequencing
Genomic DNA was extracted from ethylenediaminetetraacetic acid anti-coagulated blood using the QIAamp DNA blood mini kit (Qiagen, #51,104). Sequencing library was construction with the BRCA1 and BRCA2 gene mutation detection V2 kit (Amoy Diagnostics, #8.06.0092) and sequenced using the MiSeqDx system (Illumina Inc, CA) with a minimum coverage of 200×, uniformity of 95%, and Q30 for over 85% bases.
The germline mutation was called and filtered using the commercial software SSBC-VarScanv1.1.0 developed by Amoy Diagnostics (Xiamen, China). All candidate SNVs or InDels were hard filtered and further confirmed in Integrative Genomics Viewer (IGV). The germline variants in BRCA1 (MANE NM_007294.4) and BRCA2 (MANE NM_000059.4) were classified into five categories, including benign, likely benign, variants of uncertain significance, likely pathogenic, and pathogenic following the American College of Medical Genetics (ACMG) guideline (for detailed variant classification protocol, refer to Supplementary File 1 and 2 ) [ 26 ]. BRCA databases, including BIC, ClinVar, BRCA Exchange, and LOVD3.0 were used for the population comparative analysis.
Detection of large genomic rearrangements and confirmation by multiplex ligation probe amplification (MLPA)
The germline copy number variation (CNV) was identified by the AmpliconCnvCaller software from Amoy Diagnostics. Samples with significant CNV in two or more regions of one gene were considered as candidates harboring BRCA LGRs and subjected to SALSA MLPA assays (MRC Holland, #P002 for BRCA1 and #P090 for BRCA2 ) on a PRISM 3500 DNA analyzer (Applied Biosystems, MA) and further validated by the independent kits (MRC Holland, #P087 for BRCA1 and #P077 for BRCA2 ).
Follow-up of the apparently healthy participants
The apparently healthy participants received detailed BRCA mutation test results through post-test counseling. Those with BRCA pathogenic/likely pathogenic (PLP) variants received guidance from the clinical oncologist on self-examination and health follow-ups. From June 2021 to June 2023, 1,005 out of the 2,080 healthy individuals visited to the health management center every 6 to 14 months for tumor risk screening, which included mammography/MRI, breast physical examination (for breast cancer risk), transvaginal ultrasound and CA125 (for ovarian cancer risk), abdominal CT/MRI, CA199 (for pancreatic cancer risk, imaging test was only performed in the individuals with PLP variant), and digital rectal examination, prostate-specific antigen (PSA) (for prostate cancer risk). Over the 24 months during project period, 412 individuals underwent one examination, 375 individuals underwent two, and 218 individuals underwent three follow-ups.
Association for clinical genomic science (ACGS) classification and computational scoring of variants of unknown significance (VUS)
The VUS obtained by the ACMG criteria were further classified into six categories of pathogenicity: hot, warm, tepid, cool, cold, and ice cold, according to the ACGS classification guideline [ 27 ]. Given that the P/LP variants in BRCA have emerged in recent human history, rather than deriving from non-human species [ 28 ], the evolution conservation-based function prediction tools such as SIFT and polyphen2, were not suitable for annotating missense VUS [ 29 ]. Accordingly, these VUS were analyzed for functional pathogenicity with the predictive scoring data from the DNA/protein sequence machine learning-based software iMutant [ 30 ], MutaionTaster [ 31 ], VEST [ 32 ], EVE [ 33 ] and REVEL [ 34 ].

Statistical analysis
Statistical analysis and data visualization was performed with R (v4.0.2). Comparison of continuous values was performed using a two-sample t -test or Mann-Whitney U test if appropriate. Categorical values were compared with Fisher’s exact test. Statistical significance was defined as a two-sided P < 0.05.
Demographic and genetic background of the participants
The median age for healthy participants was 49.05 (18 to 88) years and 59.77 (19 to 82) years for cancer patients. No gender bias was observed in the healthy group and PDAC patients (Table 1 ).
Among all the 2,080 apparently healthy individuals, there were ten pairs of self-reported first-degree relatives. Three self-reported first-degree relatives were enrolled among all the 522 cancer carriers. The geographic constitution for both the healthy group and cancer cohorts was illustrated in Fig. 1 , with the healthy individuals from 33 out of all 34 administrative regions of China, except for Macau Special Administrative Region (SAR), and cancer patients from 25 of these regions. Most of the study population was from the Greater Shanghai area (for healthy group, 17.02% from Shanghai Municipality, 18.7% from Jiangsu Province, 12.99% from Zhejiang Province; for tumor patients, 34.99% from Shanghai Municipality, 21.03% from Jiangsu Province, 14.72% from Zhejiang Province). 97.63% (1960/2020) of the enrolled healthy individuals and 98.45% (509/517) cancer patients are from the region east of the Hu-line, which covered 93% of the population of China. According to the report from ChinaMAP [ 15 ], we also subdivided the participants into seven distinguished population clusters, including Northwest Han, North Han, East Han, Central Han, Southeast Han, South Han, and Lingnan Han (Fig. 1 ). In short, the top three large subpopulation of this study are the East Han (56.40% of healthy individuals and 77.20% of cancer patients), North Han (21.92% of healthy individuals and 9.77% of cancer patients), and South Han (10.14% of healthy individuals and 6.51% of cancer patients). The detailed composition of participants was listed in Supplementary File 3 .
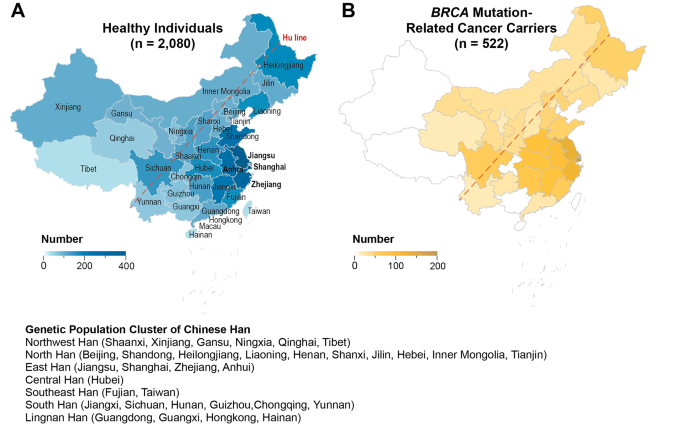
Geographic distribution of the 2,080 healthy individuals and 522 patients with BRCA mutation-related cancer. (A) Birthplace of the healthy individuals across 33 out the 34 provinces, municipalities and autonomous regions in China (except for Macau SAR), with the majority from east coast and central region (48.71%). (B) The major cancer patients are from eastern region of China, represented by the Greater Shanghai area (70.74%)
BRCA germline variations in the general Chinese Han population and BRCA mutation related-cancer cohorts
We gathered 352 distinct germline variants (127 for BRCA1 and 225 for BRCA2 ) from 2,080 Han Chinese healthy individuals and 522 patients with BRCA mutation-related cancer. Among these variations, 211 were specifically identified in healthy individuals and 62 in cancer patients, while 79 variants were present in both two groups (Fig. 2 ). Over a quarter (134 out of 352) of the variants were recurrent (carriers ≥ 2). Among them, 4 were PLPs, 29 were VUS, and 101 were benign/likely benign (BLB) variants, with 51 healthy cohort-specific and 4 cancer cohort specific variations.
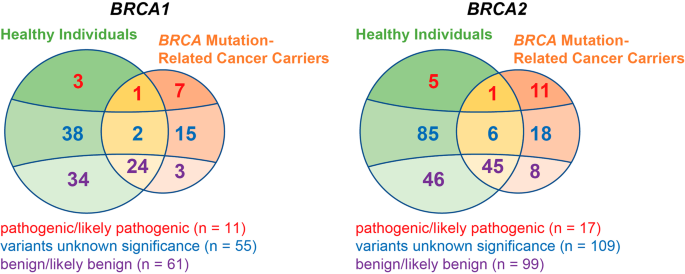
BRCA variants identified in the 2,080 healthy individuals and 522 cancer patients. Vien’s diagram illustrates the distribution differences of clinical classified BRCA variants between healthy individuals and BRCA mutation related-cancer carriers
On average, one healthy individual carried 12.09 BRCA variants ( BRCA1 : 4.80, BRCA2 : 7.29), and one cancer patient harbored 12.20 variants ( BRCA1 : 4.97, BRCA2 : 7.23). No significant difference was observed in the variant burden between the healthy and cancer groups, either for BRCA1 ( P = 0.36) or BRCA2 ( P = 0.52). Among the healthy population, there was no statistically significant differences in the variant burden among different genders ( P = 0.72) and among different age groups ( P = 0.85). There was also no significant regional or cancer species aggregation ( P = 0.65) of high variation burden. This homogeneity of BRCA variant burden across different demographic and pathogenic factors demonstrated a uniform and stable baseline for BRCA germline variations in the Chinese Han population.
BRCA1/2 pathogenic/likely pathogenic SNV and InDels
Ten PLP variants were identified in the apparently healthy individuals (Table 2 ) and 20 in the cancer patients (Table 3 ). There is a 0.53% (11/2080) chance for an individual to harbor the BRCA germline PLP variants within our Chinese Han cohort. There was no significant difference between genders [0.33% (4/1198) in males and 0.79% (7/882) in females, P = 0.22] and age groups [0.57% (10/1751) for < 60 years old vs. 0.30% (1/329) for ≥ 60 years old, P = 1.00] in the incidence of carrying BRCA PLP variants.
The eight healthy individual-specific PLP variants included one frameshift duplication ( BRCA2 c.7409dup), four frameshift deletions ( BRCA1 c.869del, BRCA1 c.5521del, BRCA2 c.8650del, BRCA2 c.9753del), and three nonsense variants ( BRCA1 c.2934T > G, BRCA2 c.47 C > T, BRCA2 c.3599_3600del). Any of these variants was not observed in the 1000 genome resource or gnomAD, except for the BRCA2 c.3599_3600del, which is incorporated in gnomAD with a frequency of 1.09 × 10 − 4 (1/9,197) in East Asian and 5.29 × 10 − 5 (3/56,761) in non-Finnish European. Moreover, to our knowledge, the BRCA1 c.869del, c.2934T > G, c.5521del, and BRCA2 c.3523 C > T, c.8650del, c.9753del have not been reported by any general population screening study in China. All these eight variants were reported in the ClinVar, BIC, BRCA Exchange, or LOVD database as pathogenic, demonstrating that conducting germline BRCA mutation screening in the general Chinese Han population can identify the individuals carrying deleterious variants.
Two nonsense variants, specifically BRCA1 c.5470_5477del and BRCA2 c.5682 C > G, were identified in both healthy individuals and cancer patients. Of note, the BRCA1 c.5470_5477del was present in 5 unrelated individuals − 2 healthy individuals and 3 TNBC patients, all hailing from the North China provinces (Shandong, Hebei, Henan). This variant, previously reported as a founder mutation in the Chinese Han breast cancer patients [ 35 ], demonstrated a significant North Han enrichment in both the cancer patients [3/51 (North Han) vs. 0/471 (non-North Han), P = 8.84 × 10 − 4 ] and healthy individuals [2/456 (North Han) vs. 0/1624 (non-North Han, P = 0.048). The BRCA2 c.5682 C > G was found in 2 unrelated individuals: 1 healthy person and 1 TNBC, both originating from the East Han (Zhejiang and Shanghai). This mutation has been collected in the gnomAD non-Finnish European population, albeit at a low frequency of 1.77 × 10 − 5 (1/56,574), but it was absent in the other gnomAD population or 1000 genome. Heterozygotes made up all bearers of the PLP variants. Additionally, the recurrent BRCA2 c.3847_3848del variant was identified exclusively in PDAC patients from the East (Shanghai and Anhui).This variant has also been reported in previous regional studies in the East and Southeast Han (1/2769 unselected breast cancer patient in Zhejiang [ 36 ], 1/316 prostate cancer patient in Shanghai [ 37 ], and 1/6,314 normal Macan [ 17 ]).
Gene-level analysis of variant prevalence revealed no significant enrichment of PLP variants in specific genes when comparing the healthy individuals (4 for BRCA1 , 6 for BRCA2 ) and cancer patients (8 for BRCA1 , 12 for BRCA2 ) (Fisher’s Exact P = 0.745). The frequency distribution of PLP variants in the gene structures, including UTR, intron, and exon, was similar among healthy individuals and tumor patients for both BRCA1 and BRCA2 (Supplementary File 4 ). However, there was a significant aggregation of PLP variants in the BRC repeats ( P = 0.019) and DNA binding domain ( P = 0.015) of BRCA2 in cancer patients, whereas in healthy individuals, PLP variants were scattered across functional domains (Fig. 3 ).
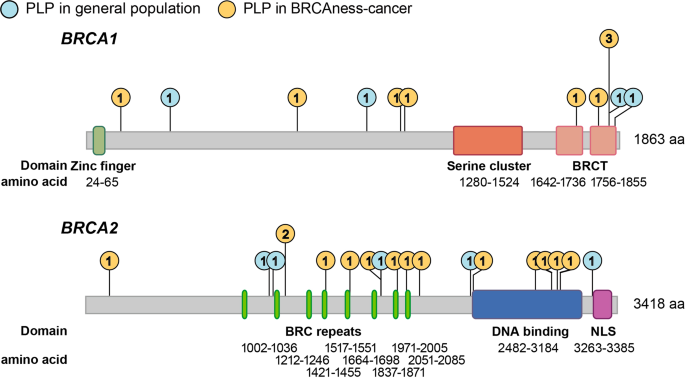
Distribution of PLP variants in the functional domains of BRCA1 and BRCA2 . Lollipop plot illustrating the frequency of PLP variants across the functional domain of BRCA1 [zinc finger, serine cluster, BRCA1 C-terminus (BRCT)] and BRCA2 [BRC repeats, DNA binding, and nuclear localization signals (NLS)]. The blue circles denote the frequency of PLPs found in the healthy individuals and the orange circles denote PLPs found in BRCA mutation related-cancer carriers. PLP variants were clustered in the BRC repeats and DNA binding domain in cancer patients in comparison to the healthy individuals, but not in NLS domain of BRCA2 and any functional domain of BRCA1
Geographically, we observed a significant agglomeration of healthy individuals carrying PLP variants in Yancheng City, with a prevalence of 3.39% (2/59) (Fischer’s Exact, P = 0.039). Yancheng City, with a population of 6.69 million, did not exhibit a significantly higher total BRCA variant load compared to other cities in China, leading to a unique geographical clustering of PLP variants in this city in the northeastern coastal region of China. We believe that a more extensive screening in local population is necessary to elucidate the interaction between genetics and the environment for cancer risk.
Case study of recurrent PLP variants’ carriers and incident cancer cases during follow-up
Among all the 2,080 normal individuals, we detected a recurrent pathogenic variant, BRCA1 c. 5470-5477del, in 2 independent subjects: a 51-year-old female and a 30-year-old female. This variant was also found in three of the 122 TNBC patients, specifically in a 42-year-old female, a 38-year-old female, and a 34-year-old female. Notably, the 51-year-old female carrying BRCA1 c. 5470-5474del variant developed bilateral breast lesions [Breast imaging-reporting and data system (BI-RAD) 4c, measuring 7 mm × 4 mm for the left and 8 mm × 6 mm for the right] during the follow-up ultrasound examination 16 months after her positive BRCA variant screening test. These breast lesions were surgically removed via lumpectomy and confirmed as regional invasive ductal carcinoma (basal-like) by pathology. No other sign of cancer was observed in this patient after the surgical operation.
Additionally, we identified one healthy individual and one TNBC patient sharing the BRCA2 c. 5682 C > G variant. Over the course of a 7-month follow-up, the 50-year-old female healthy carrier exhibited no clinical manifestation and yielded negative cancer examination results.
In the apparently healthy group, a 70-year-old male carrying BRCA2 c.3599_3600del nonsense variant was diagnosed with PDAC measuring 36 mm × 34 mm × 21 mm in the head-hook region 9 months after his BRCA testing. Furthermore, a 33-year-old female with BRCA2 c.3523 C > T variant developed invasive mucinous carcinoma in the left breast (measuring 22 mm × 15 mm × 10 mm) during her second annual examination (10 months) after BRCA mutation scanning. Additionally, we identified new tumors in two PLP variant-free individuals. One case involved a 59-year-old female diagnosed with left breast TNBC (measuring 24 mm × 15 mm × 15 mm) in the 11th month of her follow-up, and the other case was a 71-year-old male diagnosed with pancreatic body-tail PDAC (measuring 33 mm × 32 mm × 27 mm) in the 24th month of follow-up.
Similarity and difference of VUS between the general Chinese Han population and cancer cohorts
We also identified 131 VUS in the general Han Chinese population and 41 VUS in the cancer cohorts (Supplementary File 5 ). Among the 2,080 apparently healthy individuals, we observed 20 recurrent VUS. The most frequently occurring VUS was BRCA1 c.2726 A > T, found in 8 individuals from major areas of South China, including Shanghai (2 individuals), Zhejiang (2 individuals), Jiangsu (1 individual), Fujian Province (1 individual), and Guangdong Province (1 individual). This VUS was also observed in one patient bearing PDAC, a 71-year-old male from Jiangsu Province.
There is no significant VUS enrichment across the gene structures in the cancer group compared to the healthy group, which differs from the splicing mutations clustering in the tumor group among PLPs ( P = 0.03). According to the ACGS classification criteria, we observed no significant enrichment of hot/warm variants in the tumor patients compared to the healthy individuals (Fischer’s exact, P = 0.96). The reclassification and scoring of VUS by computational prediction tools also revealed that the VUS harbored by PLP variant-free tumor patients and apparently healthy persons did not differ significantly according to the current machine learning algorithm including iMutant ( P = 0.12), Mutation Taster ( P = 0.20) and VEST ( P = 0.81), EVE ( P = 0.50) and REVEL ( P = 0.17). This indicates that there should be more extensive research into the pathogenicity of VUS, for example, utilizing the large-scale clinical follow-up data.
In the cancer cohorts, we observed two recurrent VUS in BRCA2 : c.2186T > C (found in a 69-year-old male from Zhejiang with mCRPC and a 74-year-old female from Shanghai with PDAC) and c.8971 C > T (found in a 28-year-old female from Hebei Province with TNBC and a 52-year-old male from Anhui Province with PDAC). The c. 2186T > C variant also appeared in three healthy individuals (a 76-year-old male from Shanghai, a 43-year-old male from Heilongjiang Province, and a 53-year-old male from Zhejiang), while c.8971 C > T was exclusive to cancer cases. Worth noting is that BRCA1 c. 3524 C > T was another cancer-specific VUS observed in a PLP-free 61-year-old female patient bearing primary PDAC and TNBC. We also identified BRCA1 c.548-15G > A in two unrelated healthy individuals, which was previously reported to induce the abnormal transcript splicing [ 38 ].
BRCA LGR in the general Chinese Han population and BRCA mutation-related cancer cohorts
LGR is another genomic contributor to BRCA inactivation beyond SNV and InDels. Using NGS data, we comprehensively analyzed CNV in BRCA1 and BRCA2 at amplicon level. After the MLPA experiment, we confirmed the presence of LGR in three healthy individuals, including two relatives with BRCA2 exon 22 to exon 24 deletion and one subject with BRCA2 exon 12 to exon 13 duplication. Interestingly, we did not identify any BRCA LGR among the 522 cancer patients. These evidence suggest the presence of BRCA LGRs in the general Chinese population, although the pathogenicity of these variations needs further validation with longer-term follow-up and broader population cohorts.
BRCA mutation screening identified individuals at BRCA mutation-related cancer risk in the general Chinese Han population
To assess whether BRCA screening can effectively discriminate individuals at elevated risk of BRCA mutation-related cancers from the general population, we conducted a prospective follow-up for tumor risk assessment in 1,005 individuals (11 BRCA PLP carriers and 994 BRCA PLP-free individuals) out of the apparently healthy group after their BRCA mutation test.
Throughout the 24-month follow-up period, we identified three new cases of BRCA mutation-related cancers (comprising 2 TNBC cases and 1 PDAC case) among the 11 BRCA PLP carriers. In the group of 994 BRCA PLP-free individuals, there were two new cases (1 TNBC and 1 PDAC). There were no statistically significant differences between the PLP carriers and PLP-free individuals in terms of gender distribution [36.36% (4/11) male (PLP carriers) vs. 56.74% (564/994) male (PLP-free), P = 0.23], age [42.91 ± 13.03 years (PLP carriers) vs. 48.47 ± 11.46 years (PLP-free), P = 0.11], or follow-up duration [median of 10 months (25th to 75th percentile: 7 to 20 months, PLP carriers) vs. median of 16 month (25th to 75th percentile: 10 to 19 months, PLP-free), P = 0.15]. Therefore, the relative risk for developing BRCA mutation-related cancer in the exposure to a positive BRCA germline mutation test is 135.55 (95% CI 25.07 to 732.88), with an absolute risk increasement = 27.07% (95% CI = 23.24–30.90%).
BRCA germline mutation carriers face a high risk for BRCA mutation-related cancers. While BRCA variant screening effectively aids risk classification and prevention in those with familial history of breast/ovarian cancer [ 2 , 39 , 40 ], the mutational spectrum shifts across ethnicities [ 5 , 6 ], causing debates about population-wide screening and its implementation [ 20 ].
Prior studies have explored the BRCA germline variants in the Chinese population, but challenges remain unaddressed: (1) most studies have concentrated on patients already diagnosed with BRCA mutation-related cancer [ 11 , 14 , 41 ]; (2) population-based studies on healthy individuals are regionally restricted (Taiwan [ 16 ], Macau [ 17 ], North China [ 18 ]; 3) there has been a lack of post-test follow-up to determine whether screening in the general population identifies high cancer risk individuals. The functional landscape of BRCA germline variation in the world’s largest genetic population, the Han Chinese, remains inadequately understood.
This study presents our experience in BRCA germline variant screening involving 2,080 apparently healthy population and 522 BRCA mutation-related cancer patients. It covered 33 of the 34 administrative regions in China, except Macau SAR, offering a diverse genetic representation of the Chinese Han Population. With a centralized recruitment, testing, and follow-up process, our pipeline ensured consistent and reliable conclusions.
We found an incidence of 0.53% (one in 189) for a Han Chinese to carry germline BRCA pathogenic or likely pathogenic variants. By consolidating our findings with those of previous studies in China, such as Dong et al. (0.53%, n = 11,386 normal Chinese) [ 18 ], Qin et al. (0.38%, n = 6,314 normal Macanese) [ 17 ], Chain et al. (0.53%, n = 1,517 Taiwanese) [ 16 ], Liu et al. (1.10%, n = 6,434 normal control for breast cancer) [ 9 ], Lang et al. (0.38%, n = 1,043 normal control for breast cancer) [ 14 ], and Li et al. (0.34%, n = 1,763 normal control for ovarian cancer), we estimated a 0.52% (95% CI = 0.30–0.84%) prevalence of deleterious BRCA mutations in the Chinese Han. This is lower than the established rate in Ashkenazi Jews (2%) [ 42 ], similar to the American and British populations (0.5%) [ 43 ], and slightly higher than other East Asian populations, including Japanese and Korean (0.2%) [ 44 ]. The consistent variant frequency across various Chinese studies underscores the stable baseline of BRCA germline variations in this demographic. However, we also discovered significant regional differences in the mutational spectrum. For example, the founder mutation BRCA1 c.5470_5477del is specifically harbored by the North Han in our study (0.44% in healthy individuals and 5.89% in cancer patients), and this variant has not been reported in previous BRCA variant screening studies conducted in the south region of China [ 45 , 46 , 47 ]. Furthermore, the PLP variants found in our healthy group, including BRCA1 c.2934, c.5521del, c.869del, and BRCA2 c.3523 C > T, c.8650del, c.9753del have not been reported in previous screenings of the normal Chinese population [ 9 , 10 , 14 , 16 , 17 , 18 ].
Of note, all identified PLP carriers denied a family history of cancer during the pre-test genetic counseling, and there were no serological or radiological indications of tumors. These oversights emphasized the limitations of family history-based screening strategy: it mandates the presence of a family member with cancer diagnosis and a well-documented family history of disease.
The mutational spectrum of PLP variants differs between healthy individuals and cancer patients. Among the 11 BRCA1 PLPs identified in our study, only one (c. 5470_5477del) was common to both healthy individuals and cancer patients. Similarly, these two groups shared only one of the 17 BRCA2 PLPs (c. 5682 C > G). However, the presence of these PLPs in healthy individuals does not negate their pathogenicity. Actually, three out of the 11 individuals carrying these PLPs developed BRCA mutation-related cancer during follow-up. For instance, BRCA1 c.5470_5477del showed a relatively high prevalence (0.26%, 3/1151) in the North Han and demonstrated penetrance for TNBC [ 35 ]. In addition, more efforts should be encouraged on further categorizing the pathogenicity of VUS, such as the recurrent BRCA2 c.8971 C > T in the cancer cohort and the potential splicing abnormalities causing BRCA1 c.548-15G > A in the healthy group. Long-term phenotypic follow-up will provide evidence-based medicine level insight beyond the current machine-learning approach.
In contrast to the aggregation of PLP variants within the functional domains of BRCA ( BRCA2 BRC repeat and DNA-binding) in cancer patients [ 48 , 49 ], we observed a uniformed distribution of PLPs across BRCA1 and BRCA2 sequence in the healthy individuals. This supports the hypothesis that BRCA pathogenic variants originated relatively recently in human history [ 28 ]; however, further disease penetrating restricted the complexity of the variants into a specific genomic region. These findings highlight the necessity of employing NGS for germline mutation screening in BRCA .
Apart from SNV and small insertion/deletion, we observed two types of LGRs in three out of the 2,080 healthy individuals. These included two individuals with kinship harboring the same exon 22 to exon 24 deletion in BRCA2 [ 50 ]. Notably, LGRs were not observed in the 522 cancer patients. All three individuals with LGRs have not shown any sign of developing malignancies so far, even after follow-ups at the ages of 52, 55, and 78. This explains the relatively low penetrance of LGR (~ 1%) in BRCA mutation-related cancers in China [ 51 , 52 ], compared to European patients [ 53 , 54 ]. However, long-term follow-up beyond the 24-month shall be encouraged to elucidate the pathogenicity of these structure variants. It also highlights the need for a sensitive and specific algorithm for LGR calling using NGS data.
Our follow-up on 1,005 healthy Chinese Han individuals showed that those with positive BRCA variant tests had significantly increased BRCA mutation-related cancer risks (RR = 135.55, 95% CI 25.07 to 732.88) after accounting for potential confounders, including age, gender, and duration of follow-up. While the relatively small sample size in the PLP carrier group might cause overestimation, it highlights the value of BRCA mutation screening in the general Chinese Han population.
There is emerging evidence that population-wide screening is a better approach for the prevention of BRCA mutation-related cancer since family history-based screening misses a significant portion of individuals carrying the BRCA variant [ 7 ]. Considering the relatively high prevalence and mutational profile background in this context, the unique “small family” structure within the major Chinese populations, and the widespread culture of “medical stigmatization” in East Asia, we suggest broader BRCA variant screening, accompanied by detailed comprehensive genetic counseling.
One limitation of this study is the relatively short follow-up period, which may not adequately reflect the relative risk of cancer development in PLP carriers compared to PLP-free individuals. Additionally, since the participation of follow-up is voluntary other than mandatory, healthy individuals with the PLP-free results from BRCA mutation screening may have limited willingness to participate in the follow-up, leading to a 50% follow-up rate in this study. Furthermore, although our study included 2,080 healthy individuals, the regional sampling bias limited our findings primarily to the East Han population, and does not fully represent the situation within the 1.4 billion Chinese Han population. Moreover, While the single-center design enhanced the comparability of the testing and follow-up data, it also introduced sampling bias and other unexpected confounders. Therefore, a multi-center prospective study is encouraged to elucidate the medical benefits of population-based screening of BRCA germline variants in the Chinese Han population. Further cost-effectiveness studies comprehending the balance between variant screening and financial expenditure [ 55 , 56 ], and psychosocial studies [ 57 , 58 ] on the impact of genetic test results, will facilitate devising the optimal screening strategy in the Chinese Han population.
By integrating NGS data from 2,080 apparently healthy individuals, we have characterized the genetic landscape of germline BRCA variants, including SNVs, small InDels and LGRs, in the Chinese Han, with a special focus on the East Han subpopulation. The mutational spectrums are of significant difference between the healthy individuals and cancer patients. Furthermore, we conducted a short-term follow-up involving 1,005 individuals from the healthy group, confirming that individuals identified as PLP carriers by population-based screening face a significantly elevated risk of developing BRCA mutation-related cancer compared to those without PLPs.
Our study highlights the utility of BRCA germline variant screening for risk stratification and early cancer detection in the apparently healthy Chinese Han individuals. We advocate for multi-center prospective studies to assess the medical benefits of population-based BRCA germline variant screening compared to conventional family history-based screening in the Chinese Han population. Additionally, we anticipate that our research, along with investigations into financial considerations and psychosocial impact of genetic test results, will contribute to the development of an optimal screening strategy for the Chinese Han population.
Data availability
The datasets used and/or analysed during the current study are available in the main text and supplementary files of the manuscript.
Abbreviations
Single nucleotide variation
Insertion and deletion
Large genomic rearrangement
Next-generation sequencing
Triple-negative breast cancer
Metastatic castration-resistant prostate cancer
Pancreatic ductal adenocarcinoma
High-grade ovarian cancer
American College of Medical Genetics
Copy number variation
Pathogenic/likely pathogenic
Association for Clinical Genomic Science
Variant of unknown significance
benign/likely benign
Breast imaging-reporting and data system
Relative risk
Lord CJ, Ashworth A. BRCAness revisited. Nat Rev Cancer. 2016;16(2):110–20.
Article CAS PubMed Google Scholar
Paluch-Shimon S, Cardoso F, Sessa C, Balmana J, Cardoso MJ, Gilbert F, Senkus E, Committee EG. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice guidelines for cancer prevention and screening. Ann Oncol. 2016;27(suppl 5):v103–10.
Daly MB, Pilarski R, Yurgelun MB, Berry MP, Buys SS, Dickson P, Domchek SM, Elkhanany A, Friedman S, Garber JE, et al. NCCN guidelines insights: Genetic/Familial High-Risk Assessment: breast, ovarian, and pancreatic, Version 1.2020. J Natl Compr Canc Netw. 2020;18(4):380–91.
Article PubMed Google Scholar
Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alfoldi J, Wang Q, Collins RL, Laricchia KM, Ganna A, Birnbaum DP, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581(7809):434–43.
Article CAS PubMed PubMed Central Google Scholar
Rebbeck TR, Friebel TM, Friedman E, Hamann U, Huo D, Kwong A, Olah E, Olopade OI, Solano AR, Teo SH, et al. Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum Mutat. 2018;39(5):593–620.
Bhaskaran SP, Chandratre K, Gupta H, Zhang L, Wang X, Cui J, Kim YC, Sinha S, Jiang L, Lu B, et al. Germline variation in BRCA1/2 is highly ethnic-specific: evidence from over 30,000 Chinese hereditary breast and ovarian cancer patients. Int J Cancer. 2019;145(4):962–73.
Manchanda R, Sun L, Patel S, Evans O, Wilschut J, De Freitas Lopes AC, Gaba F, Brentnall A, Duffy S, Cui B et al. Economic evaluation of Population-based BRCA1/BRCA2 mutation testing across multiple countries and Health systems. Cancers (Basel) 2020, 12(7).
Kemp Z, Turnbull A, Yost S, Seal S, Mahamdallie S, Poyastro-Pearson E, Warren-Perry M, Eccleston A, Tan MM, Teo SH, et al. Evaluation of Cancer-based Criteria for Use in Mainstream BRCA1 and BRCA2 genetic testing in patients with breast Cancer. JAMA Netw Open. 2019;2(5):e194428.
Article PubMed PubMed Central Google Scholar
Liu Y, Wang H, Wang X, Liu J, Li J, Wang X, Zhang Y, Bai Z, Zhou Q, Wu Y, et al. Prevalence and reclassification of BRCA1 and BRCA2 variants in a large, unselected Chinese Han breast cancer cohort. J Hematol Oncol. 2021;14(1):18.
Li A, Xie R, Zhi Q, Deng Y, Wu Y, Li W, Yang L, Jiao Z, Luo J, Zi Y, et al. BRCA germline mutations in an unselected nationwide cohort of Chinese patients with ovarian cancer and healthy controls. Gynecol Oncol. 2018;151(1):145–52.
Yin L, Wei J, Lu Z, Huang S, Gao H, Chen J, Guo F, Tu M, Xiao B, Xi C, et al. Prevalence of germline sequence variations among patients with pancreatic Cancer in China. JAMA Netw Open. 2022;5(2):e2148721.
Zhu Y, Wei Y, Zeng H, Li Y, Ng CF, Zhou F, He C, Sun G, Ni Y, Chiu PKF, et al. Inherited mutations in Chinese men with prostate Cancer. J Natl Compr Canc Netw. 2021;20(1):54–62.
Lei H, Zhang M, Zhang L, Hemminki K, Wang XJ, Chen T. Overview on population screening for carriers with germline BRCA mutation in China. Front Oncol. 2022;12:1002360.
Lang GT, Shi JX, Hu X, Zhang CH, Shan L, Song CG, Zhuang ZG, Cao AY, Ling H, Yu KD, et al. The spectrum of BRCA mutations and characteristics of BRCA-associated breast cancers in China: screening of 2,991 patients and 1,043 controls by next-generation sequencing. Int J Cancer. 2017;141(1):129–42.
Cao Y, Li L, Xu M, Feng Z, Sun X, Lu J, Xu Y, Du P, Wang T, Hu R, et al. The ChinaMAP analytics of deep whole genome sequences in 10,588 individuals. Cell Res. 2020;30(9):717–31.
Chian J, Sinha S, Qin Z, Wang SM. BRCA1 and BRCA2 variation in Taiwanese General Population and the Cancer Cohort. Front Mol Biosci. 2021;8:685174.
Qin Z, Kuok CN, Dong H, Jiang L, Zhang L, Guo M, Leong HK, Wang L, Meng G, Wang SM. Can population BRCA screening be applied in non-ashkenazi jewish populations? Experience in Macau population. J Med Genet. 2021;58(9):587–91.
Dong H, Chandratre K, Qin Y, Zhang J, Tian X, Rong C, Wang N, Guo M, Zhao G, Wang SM. Prevalence of BRCA1/BRCA2 pathogenic variation in Chinese Han population. J Med Genet. 2021;58(8):565–9.
Yurgelun MB, Hiller E, Garber JE. Population-wide screening for germline BRCA1 and BRCA2 mutations: too much of a good thing? J Clin Oncol. 2015;33(28):3092–5.
Ficarazzi F, Vecchi M, Ferrari M, Pierotti MA. Towards population-based genetic screenings for breast and ovarian cancer: a comprehensive review from economic evaluations to patient perspectives. Breast. 2021;58:121–9.
Daly MB, Pal T, Berry MP, Buys SS, Dickson P, Domchek SM, Elkhanany A, Friedman S, Goggins M, Hutton ML, et al. Genetic/Familial High-Risk Assessment: breast, ovarian, and pancreatic, Version 2.2021, NCCN Clinical Practice guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(1):77–102.
Tan PH, Ellis I, Allison K, Brogi E, Fox SB, Lakhani S, Lazar AJ, Morris EA, Sahin A, Salgado R, et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology. 2020;77(2):181–5.
Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of Tumours of the urinary system and male genital organs-Part B: prostate and bladder tumours. Eur Urol. 2016;70(1):106–19.
Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA, Board WHOCTE. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–8.
McCluggage WG, Singh N, Gilks CB. Key changes to the World Health Organization (WHO) classification of female genital tumours introduced in the 5th edition (2020). Histopathology. 2022;80(5):762–78.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24.
Chen D. [A Chinese interpretation for the ACGS Best Practice guidelines for variant classification in Rare Disease 2020]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2023;40(8):915–21.
PubMed Google Scholar
Li J, Zhao B, Huang T, Qin Z, Wang SM. Human BRCA pathogenic variants were originated during recent human history. Life Sci Alliance 2022, 5(5).
Poon KS. In silico analysis of BRCA1 and BRCA2 missense variants and the relevance in molecular genetic testing. Sci Rep. 2021;11(1):11114.
Capriotti E, Fariselli P, Casadio R. I-Mutant2.0: predicting stability changes upon mutation from the protein sequence or structure. Nucleic Acids Res. 2005;33(Web Server issue):W306–310.
Schwarz JM, Rodelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7(8):575–6.
Carter H, Douville C, Stenson PD, Cooper DN, Karchin R. Identifying mendelian disease genes with the variant effect scoring tool. BMC Genomics. 2013;14(Suppl 3):S3.
Frazer J, Notin P, Dias M, Gomez A, Min JK, Brock K, Gal Y, Marks DS. Disease variant prediction with deep generative models of evolutionary data. Nature. 2021;599(7883):91–5.
Ioannidis NM, Rothstein JH, Pejaver V, Middha S, McDonnell SK, Baheti S, Musolf A, Li Q, Holzinger E, Karyadi D, et al. REVEL: an Ensemble Method for Predicting the pathogenicity of rare missense variants. Am J Hum Genet. 2016;99(4):877–85.
Meng H, Yao L, Yuan H, Xu Y, Ouyang T, Li J, Wang T, Fan Z, Fan T, Lin B, et al. BRCA1 c.5470_5477del, a founder mutation in Chinese Han breast cancer patients. Int J Cancer. 2020;146(11):3044–52.
Deng M, Chen HH, Zhu X, Luo M, Zhang K, Xu CJ, Hu KM, Cheng P, Zhou JJ, Zheng S, et al. Prevalence and clinical outcomes of germline mutations in BRCA1/2 and PALB2 genes in 2769 unselected breast cancer patients in China. Int J Cancer. 2019;145(6):1517–28.
Wei Y, Wu J, Gu W, Qin X, Dai B, Lin G, Gan H, Freedland SJ, Zhu Y, Ye D. Germline DNA repair Gene Mutation Landscape in Chinese prostate Cancer patients. Eur Urol. 2019;76(3):280–3.
Dong Z, Wang Y, Zhang J, Zhu F, Liu Z, Kang Y, Lin M, Shi H. Analyzing the effects of BRCA1/2 variants on mRNA splicing by minigene assay. J Hum Genet. 2023;68(2):65–71.
Valencia OM, Samuel SE, Viscusi RK, Riall TS, Neumayer LA, Aziz H. The role of genetic testing in patients with breast Cancer: a review. JAMA Surg. 2017;152(6):589–94.
Sessa C, Balmaña J, Bober SL, Cardoso MJ, Colombo N, Curigliano G, Domchek SM, Evans DG, Fischerova D, Harbeck N, et al. Risk reduction and screening of cancer in hereditary breast-ovarian cancer syndromes: ESMO Clinical Practice Guideline. Ann Oncol. 2023;34(1):33–47.
Wu X, Wu L, Kong B, Liu J, Yin R, Wen H, Li N, Bu H, Feng Y, Li Q, et al. The First Nationwide Multicenter Prevalence Study of Germline BRCA1 and BRCA2 mutations in Chinese ovarian Cancer patients. Int J Gynecol Cancer. 2017;27(8):1650–7.
Hartge P, Struewing JP, Wacholder S, Brody LC, Tucker MA. The prevalence of common BRCA1 and BRCA2 mutations among Ashkenazi jews. Am J Hum Genet. 1999;64(4):963–70.
Manchanda R, Blyuss O, Gaba F, Gordeev VS, Jacobs C, Burnell M, Gan C, Taylor R, Turnbull C, Legood R, et al. Current detection rates and time-to-detection of all identifiable BRCA carriers in the Greater London population. J Med Genet. 2018;55(8):538–45.
Maxwell KN, Domchek SM, Nathanson KL, Robson ME. Population frequency of germline BRCA1/2 mutations. J Clin Oncol. 2016;34(34):4183–5.
Wang Q, Wu H, Lan Y, Zhang J, Wu J, Zhang Y, Li L, Liu D, Zhang J. Changing patterns in clinicopathological characteristics of breast Cancer and prevalence of BRCA mutations: analysis in a rural area of Southern China. Int J Gen Med. 2021;14:7371–80.
Luo Y, Wu H, Huang Q, Rao H, Yu Z, Zhong Z. The features of BRCA1 and BRCA2 germline mutations in Hakka Ovarian Cancer patients: BRCA1 C.536 A > T maybe a founder mutation in this Population. Int J Gen Med. 2022;15:2773–86.
Shui L, Li X, Peng Y, Tian J, Li S, He D, Li A, Tian B, Li M, Gao H, et al. The germline/somatic DNA damage repair gene mutations modulate the therapeutic response in Chinese patients with advanced pancreatic ductal adenocarcinoma. J Transl Med. 2021;19(1):301.
Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer. 2011;12(1):68–78.
Heramb C, Wangensteen T, Grindedal EM, Ariansen SL, Lothe S, Heimdal KR, Maehle L. BRCA1 and BRCA2 mutation spectrum - an update on mutation distribution in a large cancer genetics clinic in Norway. Hered Cancer Clin Pract. 2018;16:3.
Hua D, Tian Q, Wang X, Bei T, Cui L, Zhang B, Bao C, Bai Y, Zhao X, Yuan P. Next-generation sequencing based detection of BRCA1 and BRCA2 large genomic rearrangements in Chinese cancer patients. Front Oncol. 2022;12:898916.
Cao WM, Zheng YB, Gao Y, Ding XW, Sun Y, Huang Y, Lou CJ, Pan ZW, Peng G, Wang XJ. Comprehensive mutation detection of BRCA1/2 genes reveals large genomic rearrangements contribute to hereditary breast and ovarian cancer in Chinese women. BMC Cancer. 2019;19(1):551.
Su L, Zhang J, Meng H, Ouyang T, Li J, Wang T, Fan Z, Fan T, Lin B, Xie Y. Prevalence of BRCA1/2 large genomic rearrangements in Chinese women with sporadic triple-negative or familial breast cancer. Clin Genet. 2018;94(1):165–9.
Riahi A, Chabouni-Bouhamed H, Kharrat M. Prevalence of BRCA1 and BRCA2 large genomic rearrangements in Tunisian high risk breast/ovarian cancer families: implications for genetic testing. Cancer Genet. 2017;210:22–7.
Bozsik A, Pocza T, Papp J, Vaszko T, Butz H, Patocs A, Olah E. Complex characterization of Germline large genomic rearrangements of the BRCA1 and BRCA2 genes in high-risk breast Cancer patients-Novel variants from a large National Center. Int J Mol Sci 2020, 21(13).
Manchanda R, Legood R, Burnell M, McGuire A, Raikou M, Loggenberg K, Wardle J, Sanderson S, Gessler S, Side L, et al. Cost-effectiveness of population screening for BRCA mutations in Ashkenazi jewish women compared with family history-based testing. J Natl Cancer Inst. 2015;107(1):380.
Manchanda R, Patel S, Gordeev VS, Antoniou AC, Smith S, Lee A, Hopper JL, MacInnis RJ, Turnbull C, Ramus SJ, et al. Cost-effectiveness of Population-based BRCA1, BRCA2, RAD51C, RAD51D, BRIP1, PALB2 mutation testing in Unselected General Population women. J Natl Cancer Inst. 2018;110(7):714–25.
Halbert CH, Stopfer JE, McDonald J, Weathers B, Collier A, Troxel AB, Domchek S. Long-term reactions to genetic testing for BRCA1 and BRCA2 mutations: does time heal women’s concerns? J Clin Oncol. 2011;29(32):4302–6.
Watson M, Foster C, Eeles R, Eccles D, Ashley S, Davidson R, Mackay J, Morrison PJ, Hopwood P, Evans DG, et al. Psychosocial impact of breast/ovarian (BRCA1/2) cancer-predictive genetic testing in a UK multi-centre clinical cohort. Br J Cancer. 2004;91(10):1787–94.
Download references
Acknowledgements
Not applicable.
This study was supported by the National Natural Science Foundation of China [82272415 to Z.W], Shanghai Municipal Health Commission (grant number 2022YQ045 to Z.W.), Shanghai “Rising Stars of Medical Talents”–Clinical Laboratory Practitioner Program (grant number 2022-065 to Z.W), and the Innovation Group Project of Shanghai Municipal Health Commission (grant number 2019CXJQ03 to M.G.).
Author information
Zhiyuan Wu, Qingyun Zhang and Yiting Jin contributed equally to this work.
Authors and Affiliations
Department of Laboratory Medicine, Huashan Hospital, Fudan University, 200040, Shanghai, China
Zhiyuan Wu, Yanli Chen, Can Yang & Ming Guan
Central Laboratory, Huashan Hospital, Fudan University, 200040, Shanghai, China
Qingyun Zhang, Xinju Zhang, Xuemei Tang & Ming Guan
Department of General Surgery, Huashan Hospital, Fudan University, 200040, Shanghai, China
Department of Urology, Huashan Hospital, Fudan University, 200040, Shanghai, China
Haowen Jiang
Department of Pancreatic Surgery, Huashan Hospital, Fudan University, 200040, Shanghai, China
Xiaoyi Wang
Department of Oncology, Huashan Hospital, Fudan University, 200040, Shanghai, China
Health Management Center, Huashan Hospital, Fudan University, 200040, Shanghai, China
Feng Yu & Bing Wang
You can also search for this author in PubMed Google Scholar
Contributions
Zhiyuan Wu and Ming Guan organized the project and wrote the paper. Zhiyuan Wu, Yiting Jin, Qingyun Zhang and Can Yang analyzed and interpreted the data. Qingyun Zhang, Xinju Zhang, Xuemei Tang and Yanli Chen performed the experiment. Yiting Jin, Haowen Jiang, Xiaoyi Wang, Xinli Zhou, Feng Yu, Bing Wang recruited patients, provided clinical information, obtained informed consent. All authors contributed to and approved the final manuscript. The work reported in the paper has been performed by the authors.
Corresponding authors
Correspondence to Bing Wang or Ming Guan .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Huashan Hospital of Fudan University (2023 − 812). Written informed consent was received from all participants.
Consent for publication
Additional information, publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, supplementary material 3, supplementary material 4, supplementary material 5, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wu, Z., Zhang, Q., Jin, Y. et al. Population-based BRCA germline mutation screening in the Han Chinese identifies individuals at risk of BRCA mutation-related cancer: experience from a clinical diagnostic center from greater Shanghai area. BMC Cancer 24 , 411 (2024). https://doi.org/10.1186/s12885-024-12089-w
Download citation
Received : 01 November 2023
Accepted : 06 March 2024
Published : 02 April 2024
DOI : https://doi.org/10.1186/s12885-024-12089-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- BRCA mutation related-cancer
- Germline mutation
- Population screening
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 31 March 2024
Association between sociodemographic factors and health beliefs related to breast cancer screening behavior among Northern Thai women: a hospital-based study
- Surin Jiraniramai 1 , 2 ,
- Kanokporn Pinyopornpanish 1 , 2 ,
- Nahathai Wongpakaran 3 ,
- Chaisiri Angkurawaranon 1 , 2 ,
- Victoria L. Champion 4 , 5 ,
- Imjai Chitapanarux 6 ,
- Wichuda Jiraporncharoen 1 , 2 &
- Tinakon Wongpakaran 3
Scientific Reports volume 14 , Article number: 7596 ( 2024 ) Cite this article
54 Accesses
1 Altmetric
Metrics details
- Health services
- Public health
Early diagnosis of breast cancer is crucial for reducing mortality rates. The purpose of this study is to determine the impact of demographics/social determinants of health on beliefs about the practice of self-breast examination, using mammogram and ultrasound in the context of breast cancer screening among Thai women in a hospital-based setting for implying program planning and future research. A cross-sectional study was conducted in two health centers in Chiang Mai Province from August 2021 to December 2021, involving 130 Thai women ages 40 to 70 years. Data were collected by a survey using a questionnaire to gather sociodemographic information, and health beliefs about breast cancer and screening behavior utilizing the modified Thai version of Champion's Health Belief Model Scale (MT-CHBMS). Descriptive statistics, t-tests, ANOVA, and linear regression models were employed for examining association between sociodemographic factors and health beliefs about the practice of self-breast examination (BSE), using mammogram (MG) and ultrasound (UTS). Health insurance schemes were associated with Benefit-MG, Barrier-BSE, Barrier-MG and Barrier-UTS subscales. Additionally, monthly income was associated with Barrier-MG and Barrier-UTS subscales. The most common barriers reported were “embarrassment”, “worry”, and “takes too much time”. To enhance breast cancer screening in Thailand, program planning and future research should focus on health insurance schemes, especially women with social security schemes, as they may be the most appropriate target group for intervention.
Similar content being viewed by others
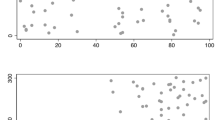
Impact of a risk based breast screening decision aid on understanding, acceptance and decision making
Jocelyn Lippey, Louise Keogh, … Laura Elenor Forrest

Willingness to decrease mammogram frequency among women at low risk for hereditary breast cancer
Yue Guan, Eric Nehl, … Colleen M. McBride
Psychological impact of risk-stratified screening as part of the NHS Breast Screening Programme: multi-site non-randomised comparison of BC-Predict versus usual screening (NCT04359420)
David P. French, Lorna McWilliams, … D. Gareth Evans
Introduction
Female breast cancer is the most commonly diagnosed cancer, with approximately 2.3 million new cases and 685,000 deaths reported in 2020 1 . It is the leading or second leading cause of female cancer-related deaths in 95% of countries worldwide 2 . In 2022, breast cancer in Thailand accounted for 38,559 cases 3 , making it the most prevalent female cancer, and accounting for 32.64% of the top five cancers in the northern region of Thailand 4 . This region has been predicted to have the highest age-standardized incidence rate (ASR) and proportion of female cancer cases by 2025 5 . However, early diagnosis and treatment can significantly reduce breast cancer mortality rates and improve women's overall health 6 .
Globally, high-income countries have adopted mammography as the standard screening method for early diagnosis of breast cancer, which helps reduce rates of advanced and fatal breast cancer 7 . In contrast, low to moderate-income countries, including Thailand 8 , 9 , 10 , often rely on breast self-examination (BSE) due to its insufficient mammography resources, although it is considered less reliable 11 , 12 , 13 . Therefore, it is recommended that women regularly and accurately perform BSE and consult with their physicians, who might recommend mammography and/or ultrasound if a lump is found 9 . It's important to note that BSE alone is not an effective method for reducing breast cancer mortality 14 . However, a recent population-based study of 1,906,697 women participating in a breast cancer awareness program in Thailand reported that women who regularly practiced BSE had better survival rates compared to non-practicing women. Additionally, a significantly higher proportion of smaller tumor sizes and earlier stages of breast cancer were observed in the group that regularly performed BSE. This positive outcome was attributed to the strong collaboration between village health volunteers and the use of BSE record booklets. Village health volunteers played a vital role in reminding women to perform BSE consistently, while the BSE record booklets helped women accurately follow the instructions and document their BSE practices 15 . Many countries of low to moderate-income countries have BSE practice as the first line screening because it is easy, convenient, private safe and no specific equipment requirement. Its purpose is to make women familiar with both the appearance and feel of their breasts as early as possible, so that they will be able to easily detect changes in their breast 13 , 16 . The more practice of BSE, the more empower women health 8 , 13 , 17 Based on these evidence, initial BSE is deemed appropriate for Thailand as a low to moderate-income country. The practice of BSE among women is influenced by their knowledge and beliefs about breast cancer and screening methods 17 .
In Thailand, the current guidelines for breast cancer screening 18 include breast cancer screening according to age. For ages 20–39 years old, it is recommended that breast self-examination should be performed once a month. Women between 40 and 69 years should be examined by a doctor annually. If abnormalities are identified, a mammogram will be scheduled. For the age of 70 years old and over, mammography for breast cancer screening should be weighed in terms of benefits and risks based on individual’s life expectancy and preference. However, in the voluntary case of populations who wish to have breast cancer screening by mammogram in the first place, recommendations for screening have been added that are similar to those recommended by the American Cancer Society. This recommendation was caused by public health policy and public finance management in Thailand.
In some resource-limited areas, breast ultrasound has been proposed as a possible alternative for mammography in breast cancer screening because it is portable, less expensive than mammography, and versatile across a wider range of clinical applications. The use of ultrasound as an effective primary detection tool for breast cancer may be beneficial in low-resource settings where mammography is unavailable 19 . Furthermore, according to the findings of a multi-center randomized trial comparing ultrasound vs. mammography for screening breast cancer in high-risk Chinese women, ultrasound was superior to mammography for screening breast cancer in this group 19 . In Thailand, mammography is not available in most rural areas. Similarly, Thai women, like Chinese women, have smaller and denser breasts than Western women 20 . Additionally, ultrasound yields less pain or discomfort than a mammogram, which is one of the main problems preventing women from breast cancer screening. 21 .
In real-world practice, BSE is not widely adopted among most Thai women. From secondary data of the 2007 Health and Welfare Survey that comprised 18,474 women aged 20 years and older and the 2009 Reproductive Health Survey that comprised 26,951 women aged 30 to 59 years show that only 18.4% of women practice monthly BSE 21 , indicating a low level of knowledge and awareness about breast cancer and the importance of BSE, mammography, and ultrasound screening that are the steps for increasing diagnosis of breast cancer. Before planning effective interventions to motivate the use of these screening methods, it is important to understand Thai women's knowledge and beliefs about breast cancer screening. Previous studies have shown that the Health Belief Model is a reliable and valid tool for measuring individuals' knowledge and beliefs about breast cancer and screening methods 22 . This model predicts the behaviors of people who take action to prevent, screen for, or control illness conditions based on their personal beliefs or perceptions about a disease 23 . Champion's Health Belief Model Scale (CHBMS) is the first and most widely used tool in the literature across continents, countries, cultures, and ethnicities to measure women's beliefs about breast cancer screening 8 , 24 , 25 , 26 , 27 , 28 , 29 .
The CHBMS comprises six main constructs: susceptibility, seriousness, benefits, barriers, health motivation, and confidence (self-efficacy). This scale has also been developed to assess perceived benefits and barriers of BSE and mammogram screening 25 , 26 , 27 , 29 , 30 , 31 . Recently, a modified Thai version of Champion's Health Belief Model Scale (MT-CHBMS) 32 incorporated ultrasound items for breast cancer screening. The primary reason for this addition is that ultrasound can effectively detect small and dense tissue tumors, particularly in younger Asian women who tend to have denser breast tissue compared to Western women 19 . In terms of advanced technology, techniques such as artificial intelligence (e.g., deep-learning-enabled clinical decision support systems) and classification of ultrasound images have demonstrated superior accuracy in detecting breast cancers compared to various screening tools currently available 33 , 34 . The MT-CHBMS has been found to be valid and reliable among Thai women 32 . This scale can be comparing perceived benefits and barriers of BSE, mammogram and ultrasound screening from associate predictors of sociodemographic factors. These predictors could be implying the program design for increasing breast cancer screening.
Numerous studies have demonstrated the significant impact of sociodemographic factors on women's breast cancer screening behaviors, with results varying across cultures and values. For instance, research conducted in Middle Eastern countries revealed notable associations between age, title, giving birth, BC screening in the last 6 months, BSE training, chronic disease, mental illness, and BSE practice 35 . Conversely, a study in a similar cultural context showed that BSE and mammography practices among women were influenced by the only level of their knowledge about breast cancer 36 . In an African country, a study found significant associations between income status, marital status, age of first childbirth in the family, and perceived susceptibility, health motivation, convenience, perceived benefits, and self-efficacy for BSE 37 .
Despite these findings, there is currently a lack of information regarding the health perception of Thai women, the scope of their health beliefs, and how demographics/social determinants impact these beliefs. Additionally, these results have been integrated to plan for detecting and managing for breast cancer in primary care of hospital that is the one of strategic in Thailand’s sustainable development goals 38 , 39 . Therefore, the objective of this study is to determine the impact of demographics/social determinants of health on beliefs about the practice of self-breast examination, using mammogram and ultrasound in the context of breast cancer screening among Thai women in a hospital-based setting for implying program planning and future research.
Study design and participants
A cross-sectional study was conducted in Chiang Mai province, Kingdom of Thailand, from August 2021 to December 2021. One hundred and thirty participants recruited with convenience sampling method for the study, consisting of women from two health centers: Maharaj Nakorn Chiang Mai Hospital, located in an urban area, and San Pa Tong Hospital, situated in a rural area. A comprehensive description of the development of the MT-CHBMS has been previously published 32 .
Inclusion and exclusion criteria
The inclusion criteria for the study were as follows: individuals between the ages of 40 and 70 years (the recommended age for mammograms), no prior history of breast cancer or any other types of cancer, and not currently pregnant or breastfeeding. The exclusion criteria included individuals who were unable to communicate effectively due to language barriers and those who expressed unwillingness to complete the questionnaires.
Sample size
Sample size is calculated based on the following criteria.
Anticipated effect size ( f 2 ) was 0.15 (small). The desired statistical power level was 0.8
The number of predictors was 5. Therefore, the minimum required sample was 91. We recruited 130 participants for this study, indicating that it was sufficient.
The data collection tools
To collect data at the outpatient clinic, the researchers gathered socio-economic information by structured interviewing. The questions included items such as age, religion, marital status, education level, healthcare insurance schemes (including the three main public health insurance schemes: government or state enterprise officer, social security scheme, and universal coverage scheme), income, and residential area. Then paper questionnaires were provided to all participants. Prior to completing the questionnaires, all participants provided written informed consent.
The questionnaire addressing beliefs was the MT-CHBMS. The CHBMS was translated into Thai, validated by a panel of experts, back translated, modified by adding content about ultrasound for screening breast cancer, and pretested. Confirmatory factor analysis was used with a sample of 130 Thai women aged 40 to 70 years old. The scales were measured with an ordinal scale using a five-point Likert type 1: “Strongly disagree”, to 5: “Strongly agree”. Each subscale can be used independently. In the case of overall assessment of the awareness of breast cancer and screening methods, the total score can be adopted but the questions concerning barriers must be reversed before summing up.
The MT-CHBMS’s Cronbach’s alphas values were acceptable, ranging from 0.74 to 0.93 for the scales)and valid(Content validity using the CVI index from 3 experts showed that the average Item-CVI was 1.00, all factor loading coefficients in the confirmatory factor analysis were significant(p < 0.001) and ranged from 0.413 to 1.029) tool for measuring the Health Belief Model related to the practice of breast self-examination (BSE), as well as investigating attitudes towards mammograms and ultrasounds 32 . The confirmatory factor analysis results of the CHBMS and MT-CHBMS. Each item had sufficient factor loadings (estimated coefficients) on the designated factor. All factor loading coefficients were significant ( p < 0.001) and ranged from 0.413 to 1.029. The fit statistics were assessed to demonstrate how well the CFA model fitted the data. For the model MT-CHBM: chi-square = 2488.868, df = 1879, chi-square/df = 1.324, TLI = 0.961, CFI = 0.964, and RMSEA (90% CI) = 0.050(0.045–0.055). Except for the motivation subscale, 21 pairs of error terms in each subscale of T-CHBMS and 23 pairs of error terms of MT-CHBMS were correlated. All these error terms suggested a high correlation between items and became the potential sources of the model misfit.
The questionnaire consisted of 64 items distributed among 10 subscales: susceptibility (5 items), seriousness (7 items), benefits of BSE (6 items), barriers to BSE (6 items), benefits of mammogram (6 items), barriers to mammogram (5 items), benefits of ultrasound (6 items), barriers to ultrasound (5 items), confidence (11 items), and health motivation (7 items). All items were formatted using an ordinal scale with a 5-point Likert scale response: 1 = "Strongly disagree," 2 = "Disagree," 3 = "Neutral," 4 = "Agree," and 5 = "Strongly agree" for positive statements. Each subscale can be utilized independently. However, when conducting an overall assessment of awareness regarding breast cancer and screening methods, the total score may be used. It's important to note that questions pertaining to barriers must be reversed before summing up the scores.
Statistical analysis
The data were analysed using Stata version 15.0. Descriptive statistics, including mean, standard deviation (SD), frequency, and percentages, were used to describe the data. Internal consistency of the items within the health belief subscales was assessed using Cronbach's alpha. The association and comparison of items within the health belief subscales and across other variables were analysed using t-tests, analysis of variance (ANOVA), and linear regression models.
Ethical approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and under the review and approval of the Institutional Research Ethics Committee of the Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand (No. FAM 2564-08138) and Sanpatong Hospital Ethics Committee (No. SPT/REC 012/2564). All procedures were conducted following the relevant institutional guidelines and regulations.
Distribution of sociodemographic factors of women (n = 130)
The sociodemographic characteristics of the 130 participants are presented in Table 1 . The average age of the participants was 52.33 years (SD = 7.28). The majority of participants were single (61.54%). About 37.69% of the participants had attained a college-level education, while 51.54% had a monthly income exceeding 10,000 Baht (270 US dollars). Additionally, 41.54% of the participants had health insurance schemes through government or state enterprise officers.
Distribution statistical data and Cronbach’s alphas for MT-CHBMS
Table 2 presents the mean ranged from 2.46 to 4.35 and SD ranged from 3.56 to 8.00. The overall Cronbach's alphas for the health belief model subscales were found to be within an acceptable range (0.70 or higher), indicating good internal consistency 40 .
Comparison of sociodemographic factors with MT-CHBMS
Table 3 presents the results of the statistical analyses conducted on various sociodemographic factors and their associations with the Health Belief Model subscales.
Participants with education less than secondary school exhibited higher scores in the Seriousness subscale compared to other education level groups ( F = 3.44, p = 0.035). Participants with a college educational level had higher scores in the Barrier-BSE subscale compared to other education level groups ( F = 5.32, p = 0.006).
In terms of monthly income, participants in the lower 10,000 Baht income group demonstrated higher scores in the Seriousness subscale compared to the more than 10,000 Baht income group ( t = 2.43, p = 0.017). Conversely, the more than 10,000 Baht income group had higher scores in the Barrier-BSE and Barrier-UTS subscales compared to the lower 10,000 Baht income group ( t = − 2.71, p = 0.008 and t = − 2.64, p = 0.009).
Participants with health insurance schemes through government or state enterprise officer schemes exhibited higher scores in the Barrier-BSE and Barrier-UTS subscales compared to other groups ( F = 8.50, p = 0.001 and F = 6.85, p = 0.002). Additionally, participants with health insurance schemes through government or state enterprise officer schemes and those covered under the universal coverage scheme had higher scores in the Barrier-MG subscale compared to the social security scheme group ( F = 5.94, p = 0.003).
Multiple linear regression model of MT-CHBMS
Table 4 presents the results of multiple linear regression analysis. None of the factors were found to be significant associated with of Seriousness subscale. However, health insurance schemes were found to be a significant associated with of the Benefit-MG and Barrier-BSE (β m = − 2.48, P = 0.023 and β m = − 3.38, P = 0.008, respectively). Both monthly income and health insurance schemes were significant associated with of the Barrier-MG and Barrier-UTS (β m = 2.65, P = 0.008, β h = − 3.11, P = 0.002 and β m = 2.49, P = 0.013, β h = − 3.40, P = 0.001, respectively).
BM= item from benefit to mammogram, BARB = item from barrier to breast self-examination, BARM = item from barrier to mammogram, BAU = item from barrier of ultrasound.
Comparison of monthly income and health insurance schemes with the significant subscales of MT-CHBMS
To delve deeper into the specifics, each subscale item, including those related to the benefits and barriers of mammograms, breast self-examination, and ultrasound, was compared among different monthly income groups and health insurance schemes using t-tests and ANOVA analyses (Table 5 ). For the Barrier-BSE subscale, the group with an income of 10,000 Baht or more demonstrated higher scores in Barrier-BSE compared to the less than 10,000 Baht income group across the BARB1 (funny), BARB3 (embarrassing), and BARB5 (unpleasant) items. Additionally, participants with health insurance schemes through government or state enterprise officer schemes exhibited higher scores in Barrier-BSE compared to other groups across all BARB (1–6) items.
Regarding the Barrier-MG subscale, participants with health insurance schemes through government or state enterprise officer schemes had higher scores in Barrier-MG compared to other groups across the BARM1 (worry), BARM2 (embarrassing), and BARM3 (take too much time) items.
In terms of the Barrier-UTS subscale, the group with an income of 10,000 Baht or more demonstrated higher scores in barrier-UTS compared to the less than 10,000 Baht income group across the BAU1 (worry), BAU2 (embarrassing), and BAU5 (cost too much money) items. Additionally, participants with health insurance schemes through government or state enterprise officer schemes had higher scores in Barrier-UTS compared to other groups across the BAU2 (embarrassing), BAU3 (take too much time), and BAU4 (painful) items.
The objective of the study was to investigate differences in beliefs related to breast examination among various sociodemographic variables in Thai women, and the results have confirmed their presence.
Using multiple linear regression analysis with the MT-CHBMS, the results indicated several findings. Health insurance schemes were associated with Benefit-MG, Barrier-BSE, Barrier-MG and Barrier-UTS subscales. Additionally, monthly income showed associations with the Barrier-MG and Barrier-UTS subscales. The most common barriers reported by participants were feeling “embarrassed”, “worry”, and feeling that it “takes too much time”.
Unlike population-based studies, the current study reveals a distinct finding: health beliefs were not associated with age, marital status, and education. This contrasts with findings from other related studies, such as those involving Turkish and Iranian women, where age, marital status, and education were significantly correlated with health beliefs scales. 41 , 42 .
Interestingly, our study observed that distinct income groups were associated with varying outcomes in the Barriers-MG and Barriers-UTS subscales. Notably, there is a dearth of similar literature available for direct comparison. However, Kirag and Kizilkaya et al. 35 reported correlations between income levels and Benefit-BSE, Barriers-BSE, Self-efficacy, and Benefit MG, while Altunkurek and Hassan Mohamed 37 also identified a relationship between income status and the Susceptibility and Health Motivation subscales. The connection between lower income and barriers to BSE is not easily explained. It is possible that there are intermediary variables requiring further investigation.
According to the Health Belief Model, perceived barriers have consistently been identified as the most influential predictor in various studies for practicing BSE and mammography 43 . Recent studies have also shown that perceiving more benefits, having higher confidence, and experiencing fewer barriers are positively associated with BSE practice 16 , 44 , 45 . Similarly, perceiving more benefits and fewer barriers is positively associated with mammography 44 . In this study, it was found that the social security scheme associated with Barrier-BSE, Barrier-MG and Barrier-UTS. In addition, the social security scheme had lower scores than the government or state enterprise officer and universal coverage scheme in the barrier to BSE, barrier to mammogram, and barrier to ultrasound subscales. It is to note that the government or state enterprise officer scheme beneficiaries benefit from a higher level of healthcare coverage compared to the other two schemes. It offers a high level of coverage and includes access to government hospitals and medical facilities. This scheme beneficiaries typically have access to a comprehensive range of medical services, often with little or no out-of-pocket expenses. The scheme provides coverage for both routine healthcare and specialized treatments, including access to government-run healthcare facilities. The social security scheme members often enjoy relatively comprehensive healthcare benefits, and the quality of care is generally good. However, it is limited to formal sector employees and their dependents, which means that informal sector workers and those not covered by formal employment arrangements are not eligible. The universal coverage scheme aims to provide equitable access to healthcare for all, emphasizing the principle of social justice. The scheme may have limitations on specialized or high-cost medical treatments, and there may be variations in the quality of care among different facilities.
The impact of the healthcare scheme type on barriers to BSE, MG, or UTS may be influenced by numerous factors. Nevertheless, the results suggests that women who have health coverage through the social security scheme may benefit from targeted interventions to improve detection. Evidence for program planning should be implement in health insurance schemes groups such as health education, skill training and confidence in performing for BSE, reminders to perform BSE, regular use of BSE record booklets 15 , 46 .
One of the general barriers observed in this study is the lack of knowledge and awareness of breast cancer among the participants, as evidenced by their low scores in the Susceptibility, Seriousness, and Confidence scales. Knowledge is identified as the most influential barrier affecting the engagement of participants in BSE, particularly in low to middle-income countries and rural areas where resources are limited 47 . Participants in this study perceived their ability to perform the BSE technique as low, indicating a lack of knowledge or a lack of regular practice. Susceptibility refers to participants' perception of their chances of being at risk for a disease. In this study, participants perceived their chances of having a risk or disease as low, indicating a potential lack of knowledge regarding the risk factors of breast cancer, such as young age, no family history of cancer, and the absence of breast lumps. Seriousness pertains to participants' perception of the severity of the consequences associated with the disease. In this study, participants may perceive breast cancer as not causing pain, exhibiting no symptoms or signs, and not posing a significant threat. This suggests a lack of knowledge or the use of defence mechanisms such as denial or rationalization, similar to behaviours observed in smokers and alcohol drinkers 48 , 49 . Consistent with many Thai studies, interventions focusing on health education and skill training for BSE are recommended to address these knowledge gaps 17 , 21 , 46 .
One of the most common barriers to early screening detection identified in this study is the feeling of “embarrassment” and “worry”. Similar to Amin MN et al. 50 , this study conducted a hospital survey. The feeling of embarrassment can be considered a cultural barrier, where women may feel too embarrassed to have their breasts examined by a male doctor. This cultural aspect can hinder their willingness to seek medical attention for abnormalities. Worry, on the other hand, is associated with feelings of anxiety. Women may experience worry related to breast lumps, the potential consequences of breast cancer, and concerns about health professionals and healthcare facilities. Additionally, the perception that screening “takes too much time” can be a deterrent. Women may feel that they are too busy, have limited time, or believe that they lack sufficient time to perform BSE and undergo screening procedures 47 . Interventions should focus on problem-solving approaches and aim to improve healthcare services in order to overcome barriers faced by the participants. By addressing these barriers and concerns, healthcare providers can create a more supportive and comfortable environment for women to engage in early screening and detection practices. Apart from the issue of “embarrassment”, “worry”, and “takes too much time”, which should be considered as one of the barriers to BSE, mammograms, and ultrasounds, there could be other contributing factors. Future research should incorporate qualitative studies to explore additional causal factors influencing the practice or non-practice of BSE, as well as the utilization or non-utilization of mammograms and ultrasounds. Additionally, it is recommended to compare interventions using a before-and-after study design involving the three main public health insurance schemes: government or state enterprise officer, social security scheme, and universal coverage scheme. This examination is necessary to identify effective interventions for women within each health insurance scheme who may face different barriers.
Participants in this study are to be more empowering their health. They have the highest score of Health Motivation and comparing Benefit-MG and Benefit-UTS more than Benefit-BSE. Conversely, Barrier-BSE when comparing Barrier-MG and Barrier-UTS is inverse. This is show that they would like to take investigate accuracy screening tools more than their manual. As health practitioners’ perspective of Thai study would like to drive a policy of national cancer act to enable women’s rights for accessing standardized screening tools 10 .
Evidence for planning and future research
There is associated between a monthly income and perceived Barriers-MG and Barriers-UTS. This predictor may be sensitive and difficult to approach regarding their monthly income when implementing intervention strategies targeting MG and UTS promotion. However, there is health insurance schemes which associated with Benefit-MG, Barrier-BSE, Barrier-MG and Barrier-UTS subscale. Also, health insurance schemes in the social security scheme is the predictor of perceived Barrier-BSE, Barrier-MG and Barrier-UTS. Specifically, the perceived barriers subscale can help identify the problems of implementation. Furthermore, attitudes toward BSE, mammograms, and ultrasounds can be compared in terms of their benefits and barriers. Such comparisons can yield valuable insights for the development of targeted interventions and approaches aimed at increasing breast cancer screening among Northern Thai women in a hospital-based setting. The design of programs and future research should take this evidence into account during implementation. Future research could employ a before-and-after study design, integrating health education and skill training for BSE, and incorporating qualitative studies to explore the additional causal factors influencing the practice or non-practice of BSE, using or non-using mammogram/ultrasound. Moreover, investigating how to improve healthcare services to ensure women's satisfaction would be beneficial.
Strength and limitations
This study is the first research project known to utilize the MT-CHBMS to study the association between sociodemographic factors and health beliefs of breast cancer and screening behaviors. Additionally, the inclusion of new items related to ultrasound in the MT-CHBMS holds promise for the assessment of breast cancer beliefs among Thai women with dense breast masses and the potential integration of advanced technologies such as artificial intelligence in the future.
However, it is important to acknowledge the limitations of this study. Firstly, the cross-sectional design employed cannot establish causal relationships between beliefs and screening practices. Secondly, the results may not be generalizable to the entire population due to the selection of participants from a single geographic area and hospital setting in Northern Thailand. Thirdly, convenience sampling may cause these study results to only generalize to this research's sampling group. Fourthly, small sample size may cause low statistical power, increased error rate, and less precise information. Fifthly, structured interviews may be subject to interviewer or social desirability bias. Sixthly, no external validation, e.g., concurrent validity, was conducted along with the construct validity. Test–retest reliability and predictive validity were not examined and should be included in future research. Lastly, certain factors such as family history of breast cancer and other breast masses were not specifically excluded from the study, which could potentially influence participants' beliefs regarding breast cancer and their practices related to screening methods.
This study marked the first use of the MT-CHBMS to investigate the association between sociodemographic factors and health beliefs related to breast cancer screening. The findings provide evidence for program design and future research aimed at increasing breast cancer screening among women in Northern Thailand in a hospital-based setting. By successfully implementing the interventions, the ssocial security scheme represents the most targeted interventions can serve as role models for other health insurance schemes and contribute to enhancing the effectiveness of screening among women.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. https://doi.org/10.3322/caac.21660 (2021).
Article PubMed Google Scholar
Global breast cancer initiative implementation framework: assessing, strengthening and scaling up of serviecs for the early detection and management of breast cancer: executive summary eGeneva (World Health Organizaion) (2023).
Department of Health MoPH. The most Thai female cancer is breast cancer, suggested to breast self-examination every month. https://multimedia.anamai.moph.go.th/news/140366/ . Accessed 26 May 2023.
Department of Medical Service. Report of the Ministry of Public Health, Budget year 2023. Reducing illness, reducing death and health security, Cancer branch, Chiang Mai Province, Round 1/2023. http://www.chiangmaihealth.go.th/document/230305167799917158.pdf . Accessed 1 July 2023.
Virani, S. et al. National and subnational population-based incidence of cancer in Thailand: Assessing cancers with the highest burdens. Cancers (Basel) 9 (8), 108 (2017).
Duffy, S. et al. Annual mammographic screening to reduce breast cancer mortality in women from age 40 years: Long-term follow-up of the UK Age RCT. Health Technol. Assess. 24 (55), 1–24. https://doi.org/10.3310/hta24550 (2020).
Article PubMed PubMed Central Google Scholar
Tabár, L. & Dean, P. B. Recommendations for breast cancer screening. Lancet Oncol. 21 (11), e511. https://doi.org/10.1016/S1470-2045(20)30495-2 (2020).
Dewi, T. K. Validation of the Indonesian version of Champion’s Health Belief Model Scale for breast self-examination. Psychol. Res. Behav. Manag. 11 , 433–438. https://doi.org/10.2147/prbm.S177124 (2018).
Erbil, N. & Bölükbaş, N. Beliefs, attitudes, and behavior of Turkish women about breast cancer and breast self-examination according to a Turkish version of the Champion Health Belief Model Scale. Asian Pac. J. Cancer Prev. 13 (11), 5823–5828 (2012).
Pongthavornkamol, W. N. & Khuhaprema, T. Breast cancer prevention and screening system in Thailand in health practitioners’ perspectives. Thai Cancer J. 39 (3), 77–92 (2019).
Google Scholar
Birhane, N., Mamo, A., Girma, E. & Asfaw, S. Predictors of breast self - examination among female teachers in Ethiopia using health belief model. Arch. Public Health. 73 (1), 39. https://doi.org/10.1186/s13690-015-0087-7 (2015).
Nafissi, N., Saghafinia, M., Motamedi, M. H. & Akbari, M. E. A survey of breast cancer knowledge and attitude in Iranian women. J. Cancer Res. Ther. 8 (1), 46–49. https://doi.org/10.4103/0973-1482.95173 (2012).
Nde, F. P., Assob, J. C., Kwenti, T. E., Njunda, A. L. & Tainenbe, T. R. Knowledge, attitude and practice of breast self-examination among female undergraduate students in the University of Buea. BMC Res. Notes 8 , 43. https://doi.org/10.1186/s13104-015-1004-4 (2015).
Hackshaw, A. K. & Paul, E. A. Breast self-examination and death from breast cancer: A meta-analysis. Br. J. Cancer 88 (7), 1047–1053. https://doi.org/10.1038/sj.bjc.6600847 (2003).
Article CAS PubMed PubMed Central Google Scholar
Thaineua, V. et al. Impact of regular Breast Self-Examination on breast cancer size, stage, and mortality in Thailand. Breast J. 26 (4), 822–824. https://doi.org/10.1111/tbj.13611 (2020).
Dewi, T. K., Massar, K., Ruiter, R. A. C. & Leonardi, T. Determinants of breast self-examination practice among women in Surabaya, Indonesia: An application of the health belief model. BMC Public Health 19 (1), 1581. https://doi.org/10.1186/s12889-019-7951-2 (2019).
Pengpid, S. & Peltzer, K. Knowledge, attitude and practice of breast self-examination among female university students from 24 low, middle income and emerging economy countries. Asian Pac. J. Cancer Prev. 15 (20), 8637–8640. https://doi.org/10.7314/apjcp.2014.15.20.8637 (2014).
Thailand NCIo. Recommendations for Breast Cancer Screening in Thailand . National Cancer Institute; (2012).
Shen, S. et al. A multi-centre randomised trial comparing ultrasound vs mammography for screening breast cancer in high-risk Chinese women. Br. J. Cancer 112 (6), 998–1004. https://doi.org/10.1038/bjc.2015.33 (2015).
Office TNHS. Mammography and ultrasound breast cancer screening to be accessible for Thai women with high-risk. 2024. 07 Feb 2024. https://eng.nhso.go.th/view/1/DescriptionNews/Mammography-and-ultrasound-breast-cancer-screening-to-be-accessible-for-Thai-women-with-high-risk/592/EN-US . Accessed 04 March 2024.
Mukem, S., Sriplung, H., McNeil, E. & Tangcharoensathien, V. Breast cancer screening among women in Thailand: Analyses of population-based household surveys. J. Med. Assoc. Thai. 97 (11), 1106–1118 (2014).
PubMed Google Scholar
Liu, N., Wang, J., Chen, D. D., Sun, W. J. & Zhang, W. Tools for the assessment of breast cancer screening beliefs in women: A literature review. J. Comp. Eff. Res. 8 (9), 645–655. https://doi.org/10.2217/cer-2018-0142 (2019).
Rosenstock, I. M. Why people use health services. Milbank Mem. Fund Q. 44 (3), 94–127 (1966).
Article Google Scholar
Champion, V. L. Instrument development for health belief model constructs. ANS Adv. Nurs. Sci. 6 (3), 73–85. https://doi.org/10.1097/00012272-198404000-00011 (1984).
Article CAS PubMed Google Scholar
Lee, E. H., Kim, J. S. & Song, M. S. Translation and validation of champion’s health belief model scale with Korean women. Cancer Nurs. 25 (5), 391–395. https://doi.org/10.1097/00002820-200210000-00010 (2002).
Marmarà, D., Marmarà, V. & Hubbard, G. Maltese translation and adaptation of champion’s health belief model scale and the revised illness perception questionnaire for breast screening among Maltese women. J .Nurs. Meas. 25 (3), 486–503. https://doi.org/10.1891/1061-3749.25.3.486 (2017).
Medina-Shepherd, R. & Kleier, J. A. Spanish translation and adaptation of Victoria Champion’s Health Belief Model Scales for breast cancer screening–mammography. Cancer Nurs. 33 (2), 93–101. https://doi.org/10.1097/NCC.0b013e3181c75d7b (2010).
Noman, S. et al. Factor structure and internal reliability of breast cancer screening Champion’s Health Belief Model Scale in Yemeni women in Malaysia: A cross-sectional study. BMC Womens Health 21 (1), 437. https://doi.org/10.1186/s12905-021-01543-7 (2021).
Secginli, S. & Nahcivan, N. O. Reliability and validity of the breast cancer screening belief scale among Turkish women. Cancer Nurs. 27 (4), 287–294. https://doi.org/10.1097/00002820-200407000-00005 (2004).
Champion, V. L. Revised susceptibility, benefits, and barriers scale for mammography screening. Res. Nurs. Health 22 (4), 341–348. https://doi.org/10.1002/(sici)1098-240x(199908)22:4%3c341::aid-nur8%3e3.0.co;2-p (1999).
Parsa, P., Kandiah, M., Mohd Nasir, M. T., Hejar, A. R. & Nor Afiah, M. Z. Reliability and validity of Champion’s Health Belief Model Scale for breast cancer screening among Malaysian women. Singapore Med. J. 49 (11), 897–903 (2008).
CAS PubMed Google Scholar
Suriyong, P. et al. Translation, adaptation, and validation of the modified Thai version of champion’s health belief model scale (MT-CHBMS). Healthcare (Basel) 11 (1), 128. https://doi.org/10.3390/healthcare11010128 (2022).
Ragab, M., Albukhari, A., Alyami, J. & Mansour, R. F. Ensemble deep-learning-enabled clinical decision support system for breast cancer diagnosis and classification on ultrasound images. Biology (Basel) 11 (3), 439. https://doi.org/10.3390/biology11030439 (2022).
The, L. O. Can artificial intelligence improve cancer care?. Lancet Oncol. 24 (6), 577. https://doi.org/10.1016/s1470-2045(23)00240-1 (2023).
Kirag, N. & Kızılkaya, M. Application of the Champion Health Belief Model to determine beliefs and behaviors of Turkish women academicians regarding breast cancer screening: A cross sectional descriptive study. BMC Womens Health 19 (1), 132. https://doi.org/10.1186/s12905-019-0828-9 (2019).
Dundar, P. E. et al. The knowledge and attitudes of breast self-examination and mammography in a group of women in a rural area in western Turkey. BMC Cancer 6 , 43. https://doi.org/10.1186/1471-2407-6-43 (2006).
Altunkurek, ŞZ. & Hassan, M. S. Determine knowledge and belief of Somalian young women about breast cancer and breast self-examination with champion health belief model: A cross-sectional study. BMC Med. Inform. Decis. Mak. 22 (1), 326. https://doi.org/10.1186/s12911-022-02065-4 (2022).
Library UND. The future we want : outcome of the Conference on Sustainable Development, Rio de Janeiro, Brazil, 20–22 June 2012. https://digitallibrary.un.org/record/3826773?ln=en . Accessed 4 March 2024.
Organization WH. WHO package of essential noncommunicable (PEN) disease interventions for primary health care. https://www.who.int/publications/i/item/9789240009226 . Accessed 4 March 2024.
Danial, W. W. C. C. BIOSTATISTICS A Foundation for Analysis in the Health Sciences 10th edn. (Wiley, 2013).
Gumus, A. B., Cam, O. & Malak, A. T. Socio-demographic factors and the practice of breast self examination and mammography by Turkish women. Asian Pac. J. Cancer Prev. 11 (1), 57–60 (2010).
Esna-Ashari, F., Saffari, N., Parsapour, H. & Rezapur-Shahkolai, F. Factors associated with breast cancer mammographic screening behavior among Iranian Women. Asian Pac. J. Cancer Prev. 23 (12), 4073–4078. https://doi.org/10.31557/apjcp.2022.23.12.4073 (2022).
Champion, V. L. & Skinner, C. S. The health belief model. In Health Behavior and Health Education Theory, Research, and Practice 4th edn (eds Karen Glanz, B. K. R. & Viswanath, K.) (Jossey-Bass, 2008).
Darvishpour, A., Vajari, S. M. & Noroozi, S. Can health belief model predict breast cancer screening behaviors?. Open Access Maced. J. Med. Sci. 6 (5), 949–953. https://doi.org/10.3889/oamjms.2018.183 (2018).
Febriyanti, N. M. A., Lubis, D. S., Wirawan, D. N., Suariyani, N. L. P. & Karmaya, M. The Determinants of Early Breast Cancer Detection via Breast Self-Examination (BSE) in Denpasar (Public Health and Preventive Medicine Archive, 2018).
Satitvipawee, P. S. S., Pitiphat, W., Kalampakorn, S. & Parkin, D. M. Factors associated with breast self-examination among Thai women living in rural areas in Northeastern Thailand. J. Med. Assoc. Thai 92 , 29 (2009).
Francks, L., Murray, A. & Wilson, E. Barriers and facilitators to breast self-examination in women under 50 in an international context: A qualitative systematic review. Int. J. Health Promot. Educ. 03 (02), 1–18. https://doi.org/10.1080/14635240.2023.2185804 (2023).
Jiraniramai, S. et al. Functional beliefs and risk minimizing beliefs among Thai healthcare workers in Maharaj Nakorn Chiang Mai hospital: Its association with intention to quit tobacco and alcohol. Subst. Abuse Treat. Prev. Policy 12 (1), 34. https://doi.org/10.1186/s13011-017-0118-1 (2017).
Jiraniramai, S. et al. Risk-minimizing belief: Its association with smoking and risk of harm from smoking in Northern Thailand. J. Ethn. Subst. Abuse 14 (4), 364–378. https://doi.org/10.1080/15332640.2014.991468 (2015).
Amin, M. N. et al. A hospital based survey to evaluate knowledge, awareness and perceived barriers regarding breast cancer screening among females in Bangladesh. Heliyon 6 (4), e03753. https://doi.org/10.1016/j.heliyon.2020.e03753 (2020).
Download references
This research was self-funded.
Author information
Authors and affiliations.
Department of Family Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
Surin Jiraniramai, Kanokporn Pinyopornpanish, Chaisiri Angkurawaranon & Wichuda Jiraporncharoen
Global Health and Chronic Conditions Research Group, Chiang Mai University, Chiang Mai, 50200, Thailand
Department of Psychiatry, Faculty of Medicine, Chiang Mai University, 110 Inthawarorot Rd., Sriphum, Muang, Chiang Mai, 50200, Thailand
Nahathai Wongpakaran & Tinakon Wongpakaran
School of Nursing, Indiana University, Indianapolis, IN, 46202, USA
Victoria L. Champion
Melvin and Bren Simon Comprehensive Cancer Center, Indiana University, Indianapolis, IN, 46202, USA
Department of Radiology, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
Imjai Chitapanarux
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to this study’s conceptualization and methodology. Validation, S.J., K.P.,V.L.C., N.W. and T.W.; Formal analysis,S.J.,K.P.,T.W.,N.W.and C.A.; investigation, all.; data curation, S.J., C.A.;The original draft was written by Surin Jiraniramai and reviewed and edited by all authors. Resources, S.J.,K.P.,C.A.,W.J.,and T.W.; supervision, T.W. and N.W.
Corresponding author
Correspondence to Tinakon Wongpakaran .
Ethics declarations
Competing interests.
Profs. Wongpakaran are the editorial board members of Scientific Reports, and all the rest of the authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Jiraniramai, S., Pinyopornpanish, K., Wongpakaran, N. et al. Association between sociodemographic factors and health beliefs related to breast cancer screening behavior among Northern Thai women: a hospital-based study. Sci Rep 14 , 7596 (2024). https://doi.org/10.1038/s41598-024-58155-y
Download citation
Received : 13 October 2023
Accepted : 26 March 2024
Published : 31 March 2024
DOI : https://doi.org/10.1038/s41598-024-58155-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
- Health beliefs
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- 25 year trends in...
25 year trends in cancer incidence and mortality among adults aged 35-69 years in the UK, 1993-2018: retrospective secondary analysis
Linked editorial.
Cancer trends in the UK
- Related content
- Peer review
- Jon Shelton , head of cancer intelligence 1 ,
- Ewa Zotow , visiting lecturer (statistics) 2 ,
- Lesley Smith , senior research fellow 3 ,
- Shane A Johnson , senior data and research analyst 1 ,
- Catherine S Thomson , service manager (cancer and adult screening) 4 ,
- Amar Ahmad , principal statistician 1 ,
- Lars Murdock , data analysis and research manager 1 ,
- Diana Nagarwalla , data analysis and research manager 1 ,
- David Forman , visiting professor of epidemiology 5
- 1 Cancer Research UK, London, UK
- 2 University College London, London, UK
- 3 Leeds Institute of Clinical Trials Research, University of Leeds, Leeds, UK
- 4 Public Health Scotland, Edinburgh, UK
- 5 Faculty of Medicine and Health, University of Leeds, Leeds, UK
- Correspondence to: J Shelton jon.shelton{at}cancer.org.uk
- Accepted 19 January 2024
Objective To examine and interpret trends in UK cancer incidence and mortality for all cancers combined and for the most common cancer sites in adults aged 35-69 years.
Design Retrospective secondary data analysis.
Data sources Cancer registration data, cancer mortality and national population data from the Office for National Statistics, Public Health Wales, Public Health Scotland, Northern Ireland Cancer Registry, NHS England, and the General Register Office for Northern Ireland.
Setting 23 cancer sites were included in the analysis in the UK.
Participants Men and women aged 35-69 years diagnosed with or who died from cancer between 1993 to 2018.
Main outcome measures Change in cancer incidence and mortality age standardised rates over time.
Results The number of cancer cases in this age range rose by 57% for men (from 55 014 cases registered in 1993 to 86 297 in 2018) and by 48% for women (60 187 to 88 970) with age standardised rates showing average annual increases of 0.8% in both sexes. The increase in incidence was predominantly driven by increases in prostate (male) and breast (female) cancers. Without these two sites, all cancer trends in age standardised incidence rates were relatively stable. Trends for a small number of less common cancers showed concerning increases in incidence rates, for example, in melanoma skin, liver, oral, and kidney cancers. The number of cancer deaths decreased over the 25 year period, by 20% in men (from 32 878 to 26 322) and 17% in women (28 516 to 23 719); age standardised mortality rates reduced for all cancers combined by 37% in men (−2.0% per year) and 33% in women (−1.6% per year). The largest decreases in mortality were noted for stomach, mesothelioma, and bladder cancers in men and stomach and cervical cancers and non-Hodgkin lymphoma in women. Most incidence and mortality changes were statistically significant even when the size of change was relatively small.
Conclusions Cancer mortality had a substantial reduction during the past 25 years in both men and women aged 35-69 years. This decline is likely a reflection of the successes in cancer prevention (eg, smoking prevention policies and cessation programmes), earlier detection (eg, screening programmes) and improved diagnostic tests, and more effective treatment. By contrast, increased prevalence of non-smoking risk factors are the likely cause of the observed increased incidence for a small number of specific cancers. This analysis also provides a benchmark for the following decade, which will include the impact of covid-19 on cancer incidence and outcomes.
Introduction
The availability of comprehensive cancer registration data across the UK since 1993 makes comparison of cancer incidence and mortality trends over 25 years possible. We examined UK trends in cancer incidence and mortality for men and women, aged 35-69 years, for all cancers combined and for the most common sites (or site groups) of cancer between 1993 and 2018.
This study focuses on the 35-69 years age group because cancer trend data are more reliable and easier to interpret in this age range. 1 Diagnostic accuracy is better in this age range than in older patients who have a greater proportion of clinical and uncertain diagnoses, as evidenced by the relatively low proportion of microscopically verified tumours, 2 especially in the earlier part of the period analysed. By the age of 35 years, the pattern of cancer broadly represents the usual adult profiles because specific cancers that are observed in childhood, adolescence, and young people would not impact on the overall pattern. Trends in the 35-69 years age group are also reflective of causal factors in the more recent and medium term past rather than in the longer term and, therefore, will be more indicative of future patterns of cancer in the older populations.
This time period has also seen the introduction of three population screening programmes across the UK, which have affected trends by diagnosing some cancers at an earlier stage, preventing cancers, but also had the potential for diagnosing some cancers that would not have otherwise caused harm to the individual, particularly breast cancer. 3 4 Cervical smear tests have been used since the 1960s and the national screening programme was introduced in 1988, with over 85% coverage of the target population (women and people with a cervix aged 25-64 years) in the UK by 1994. 5 The breast screening programme was introduced in 1988 and covered all UK countries by the mid-1990s, with women aged 50-70 years being invited. 6 The bowel screening programme was introduced from 2006 and took a number of years to reach full roll-out. Currently, people aged 60-74 across England, Wales, and Northern Ireland, and 50-74 for Scotland are eligible. Prostate specific antigen testing is not part of the national screening programme. Anyone older than 50 years with a prostate can request a prostate specific antigen test from their family doctor (general practitioner).
The past 25 years have seen differing trends in cancer risk factors, with the two most important risk factors displaying trends in opposing directions. In one direction, smoking prevalence is reducing due to introductions of tax rises on tobacco products, further advertising bans, and smokefree policies, including education and encouraging quitting, and, in the other direction, the proportion of the population classified as overweight or obese is increasing, of which diet and exercise contribute to, as well as being independent risk factors for cancer. 7
Cancer registration data are currently collected by four national registries in the UK. These organisations collect detailed information on newly diagnosed primary tumours, referred to as registrations. Prior to 2013, cancer registrations in England were collected by eight regional registries and compiled by the Office for National Statistics, 8 with these regional registries producing complete population coverage for England since 1971. 9 Cancer Research UK aggregate these data from the UK registries, with incidence, mortality, and corresponding national population data provided by the Office for National Statistics, Public Health Wales, 10 Public Health Scotland, 11 the Northern Ireland Cancer Registry, 12 NHS England, 13 and the General Register Office for Northern Ireland. 14 Coding of cancer registrations is consistent between countries of the UK, using internationally accepted codes from the International Classification of Diseases 10th revision (ICD-10) and collaboration through the UK and Ireland Association of Cancer Registries. 15
Cancer sites (for single sites) or site groups (with multiple sites, such as oral) included in these analyses were selected as the most common causes of cancer incidence or death. These cancer sites are: all cancers combined (excluding non-melanoma skin cancer for incidence) (C00-C97, excluding C44); bladder (C67); bowel (C18-C20); brain and central nervous system (C70-C72, C75.1-C75.3, D32-D33, D35.2-D35.4, D42-D43, D44.3-D44.5); breast (women only) (C50); cervix (C53); Hodgkin lymphoma (C81); kidney (C64-C66, C68); larynx (C32); leukaemia (C91-C95); liver (C22); lung (C33-C34); melanoma skin(C43); mesothelioma (C45); myeloma (C90); non-Hodgkin lymphoma (C82-C86); oesophagus (C15); lip, oral cavity, and pharynx (oral) (C00-C06, C09-C10, C12-C14); ovary (C56-C57.4); pancreas (C25); prostate (C61); stomach (C16); testis (C62); and uterus (C54-C55). In addition, sex specific all cancer groups are also presented without breast and prostate cancers to inspect the overall trends in the absence of the most common cancer site for each sex. Sex is reported as recorded by the cancer registries at the time of registration. Mesothelioma was a new specific code introduced in ICD-10 and no reliable mortality data are available for this site before 2001, hence, we have not included this type of cancer prior to then. Non-malignant brain and central nervous system codes (ICD-10 D codes) are included despite their benign nature because they can cause mortality due to their location in the cranial cavity. The codes included for the brain and central nervous system have been chosen following clinical engagement and discussion with cancer registries across the UK. Non-melanoma skin cancer is excluded for incidence data because of the lack of completeness in the recording of these cancers and therefore unreliability of the data; this process is standard practice among UK cancer registries. 16 A proportion of non-melanoma skin cancer cases can be diagnosed and treated within primary care and have not consistently been captured within cancer registration data. 17
To overcome yearly variation for sites with low numbers of cases, we calculated three-year rolling average age standardised rates per 100 000 population. 18 These rates were based on the European standard population 2013 for men and women separately for each cancer site or site group for both incidence and mortality, restricted to the 35-69 years age group. 19
The estimated annual percentage change is commonly computed using a generalised linear regression model with Gaussian or Poisson link function. 18 20 In this analysis, a generalised linear model was performed with quasi-Poisson link function as overdispersion is very common when modelling rates and count data. 21 The outcome was the age standardised cancer (incidence or mortality) rate per 100 000 and the independent variable was the period variable, which was defined as the three year period for each data point, starting from 1993-95 and ending with 2016-18. Estimated annual percentage change was estimated as (exp (β^−1)' 100, where β^ is the estimated slope of the period variable, with corresponding 95% confidence interval, which is derived from the fitted quasi-Poisson regression model. 22 The determination of trends was based on the following criteria: firstly, an increasing trend was identified when the estimated annual percentage change value and its 95% confidence interval were greater than zero. This value suggests a statistically significant increase in the age standardised rate over time. Secondly, a decreasing trend was indicated when both the estimated annual percentage change value and its 95% confidence interval were less than zero, signifying a statistically significant decline in the age standardised rate over the period considered. Finally, in cases where these conditions were not met, the age standardised rate was concluded to have remained relatively stable. This designation means that no significant change in the age standardised rate over the period examined was noted. These criteria ensure a thorough and precise interpretation of the estimated annual percentage change values and their corresponding trends. These analyses were carried out for each sex and site or site group separately. Statistical analysis was performed using R version 4.0.2. 23
Patient and public involvement
This work uses aggregated and non-identifiable routine data that have been provided by patients and collected by the health services of the UK as part of their care and support. Given the aggregated nature of the data, attempts to identify or involve any of the patients whose data are included is not possible nor permitted. Although patients and the public were not involved in the design and conduct of this research, the aim of this research is to provide an assessment of trends in cancer incidence and mortality and the impacts of treatment and policy changes to improve outcomes for cancer patients across the UK. Dissemination to the public will include a press release and a summary published online, written using layman’s terms, and a webinar to discuss the results.
Table 1 and table 2 show the percentage of all newly diagnosed cancer cases and deaths by age group in 1993 and 2018. For male registrations, around 43% of all registrations were in the 35-69 years age group in 1993 and 2018, while for female registrations, between 47% and 48% of all registrations were in this age group in 1993 and 2018, respectively. For mortality, around 40% of male cancer deaths occurred in the 35-69 years age group in 1993 and this value was lower at 30% in 2018. For female cancer deaths, a slightly smaller reduction was noted, from 38% in the 35-69 years age group in 1993 to 31% in 2018.
Number of newly diagnosed cancer cases (% of total) in the UK for all cancers, excluding non-melanoma skin cancer, (ICD-10 C00-C97 excluding C44) by sex and age group in 1993 and 2018
- View inline
Number of deaths (% of total) in the UK for all cancers, (ICD-10 C00-C97) by sex and age group in 1993 and 2018
Figure 1 shows the number of newly diagnosed cancer cases and deaths in the 35-69 years age group between 1993 and 2018 by sex. Across the UK, of cancer registrations in 2018, 83% were from England, and 5.1% from Wales, 9.2% from Scotland, and 2.7% from Northern Ireland; for deaths in 2018, 81.4%, 5.3%, 10.4%, and 2.9% were from England, Wales, Scotland, and Northern Ireland, respectively. These proportions remained relatively stable over the study period. For men, the number of cancer registrations increased by 57% from 55 014 cases registered in 1993 to 86 297 cases registered in 2018, while for women, cases increased by 48% from 60 187 in 1993 to 88 970 in 2018. The rate of increase in the number of cases of cancer was more marked between 2003 and 2013 for both sexes than in other time periods in the study.

Number of newly diagnosed cancer cases and deaths in the UK for all cancers, excluding non-melanoma skin cancer for incidence (International Classification of Diseases (10th revision) codes C00-C97 (excluding C44 for incidence)), men and women, 35-69 years, 1993 to 2018. An interactive version of this graphic is available at https://bit.ly/4acPDjP
- Download figure
- Open in new tab
- Download powerpoint
The number of cancer deaths in men and women aged 35-69 years decreased: by 20% in men from 32 878 in 1993 to 26 322 deaths in 2018 and by 17% in women from 28 516 in 1993 to 23 719 deaths in 2018. The main decrease in the number of deaths per year occurred before the year 2000 ( fig 1 ) with a decrease of 14% in males and 11% in females between 1993 and 2000. Since 2000, the number of deaths each year in both men and women has remained fairly constant ( fig 1 ).
Table 3 , table 4 , figure 2 and figure 3 , and figure 4 and figure 5 show the trends over time in both incidence and mortality rates by sex and cancer site or site group. The tables only include specific age standardised incidence and mortality rates for the first (1993-95) and last (2016-18) period to give an indication of the change over the 25 year period. The trends in incidence and mortality age standardised rates for all years are shown in the figures. Figure 6 and figure 7 show the age adjusted average annual percentage change in the rates. Between 1993-95 and 2016-18, the age standardised incidence rate for all cancers (excluding non-melanoma skin cancer) increased slightly in men and women with age adjusted annual increases of 0.8% for both sexes. The trends in prostate and breast cancer, as the two largest cancer sites in men and women, respectively, substantially contribute to the overall all sites trends for cancer incidence. Figure 3 shows the trends for each sex without the largest cancer site. In contrast to the male age standardised incidence rate for all cancers, which showed a general increase, the incidence trend for men for all cancers excluding non-melanoma skin and prostate cancer, showed a decrease before 2000, but very little change in the following period. For women, an increase in age standardised incidence rates for all cancers excluding non-melanoma skin and breast cancer is still observed but the rate of increase is lower, at 0.7% per annum on average, over the 25 year period. Over the same period reductions in age standardised mortality for all cancers, including non-melanoma skin cancer, were −2.0% per year in men and −1.6% in women. Exclusion of prostate cancer from the mortality trends for men had a negligible effect on the average annual percentage change. For women, the exclusion of breast cancer from mortality trends led to a smaller decrease in mortality of −1.3% per annum.
Age standardised* incidence and mortality rates in 1993-95 and 2016-18 and percentage change by cancer type, men aged 35-69 years, UK
Age standardised* incidence and mortality rates in 1993-95 and 2016-18 and percentage change by cancer type, women aged 35-69 years, UK

European 2013 population age standardised incidence and mortality rates in the UK for all cancers, 19 excluding non-melanoma skin cancer for incidence (International Classification of Diseases (10th revision) codes C00-C97 excluding C44 for incidence), men and women, 35-69 years, 1993-95 to 2016-18. An interactive version of this graphic is available at https://bit.ly/4a484aE

European 2013 population age standardised incidence and mortality rates in the UK for all cancers in men and women aged 35-69 years during 1993-95 to 2016-18, 19 excluding non-melanoma skin cancer for incidence, and breast cancer in women and prostate cancer in men were excluded for incidence and mortality (International Classification of Diseases (10th revision) codes C00-C97 excluding C44 for incidence, C50, C61). An interactive version of this graphic is available at https://bit.ly/3vakQoX

European 2013 age standardised incidence and mortality rates by year, 19 in the UK, for men and women aged 35-69 years from 1993-95 to 2016-18, by cancer site. An interactive version of this graphic is available at https://bit.ly/49a6ovn

Relative European 2013 age standardised incidence and mortality rates by year, 19 in the UK, for men and women aged 35-69 years from 1993-95 to 2016-18 (the reference year is 1993-95=100), by cancer site. CNS=central nervous system. An interactive version of this graphic is available at https://bit.ly/3PiKGOk

Average annual percentage change in incidence and mortality rates, in the UK, for men aged 35-69 years from 1993-95 to 2016-18 by cancer site. An interactive version of this graphic is available at https://bit.ly/3wMR6yU

Average annual percentage change in incidence and mortality rates, in the UK, for women aged 35-69 years, from 1993-95 to 2016-18, by cancer site. An interactive version of this graphic is available at https://bit.ly/3v0QdT7
Incidence rates varied over time across the different cancer sites and site groups. The largest average annual percentage increases over time for cancer incidence rates for men aged 35-69 years were for cancers of the liver (4.7%), prostate (4.2%), and melanoma skin cancer (4.2%). Increases of 1% or more per annum were also seen for oral cancer (3.4%), kidney cancer (2.7%), myeloma (1.6%), Hodgkin lymphoma (1.5%), testicular cancer (1.3%), non-Hodgkin lymphoma (1.0%), and leukaemia (1.0%). The largest annual decreases over the two decades were seen for stomach (−4.2%), bladder (−4.1%), and lung cancers (−2.1%), with decreases of more than 1% per annum also observed for mesothelioma (−1.9% from 2001 onwards) and laryngeal cancer (−1.5%).
For women, the largest average annual percentage increases in incidence rates were noted for liver (3.9%), melanoma skin (3.5%), and oral (3.3%) cancers with increases in incidence of more than 1% per annum also observed for kidney (2.9%), uterus (1.9%), brain and central nervous system cancers (1.8%), Hodgkin lymphoma (1.6%), myeloma (1.1%), and non-Hodgkin lymphoma (1.0%). The largest annual decreases were reported for bladder (−3.6%) and stomach (−3.1%) cancers while the only other site showing a decrease of more than 1% per annum was cervical cancer (−1.3%). Although breast cancer represents the largest individual cancer site for women and therefore plays a large part in all cancer trends, the average annual increase was only 0.9%. All the incidence changes mentioned, for both men and women, and most incidence changes shown in table 3 and table 4 and in figure 6 and figure 7 were statistically significant (P<0.05) even when the size of change was relatively small.
Mortality rates mainly decreased over time in both sexes. For men, the cancer sites that showed average annual percentage reductions in mortality rates of more than 1% per annum were stomach (−5.1%), mesothelioma (–4.2% from 2001), bladder (–3.2%), lung (–3.1%), non-Hodgkin lymphoma (–2.9%), testis (–2.8%), Hodgkin lymphoma (–2.6%), bowel (–2.5%), larynx (–2.5%), prostate (–1.8%), myeloma (–1.7%), and leukaemia (–1.6%). Only liver (3.0%) and oral (1.1%) cancers showed an average annual increase in mortality of 1% or more with melanoma skin cancer (0.3%) the only other site showing an increase. For women, the cancer sites with average annual decreases in mortality per year of 1% or more were stomach (–4.2%), cervix (–3.6%), non-Hodgkin lymphoma (–3.2%), breast (–2.8%), Hodgkin lymphoma (–2.8%), ovary (–2.8%), myeloma (–2.3%), bowel (–2.2%), leukaemia (–2.1%), larynx (–2.0%), mesothelioma (–2.0% since 2001), bladder (–1.6%), oesophagus (–1.2%), and kidney (1.0%). As with men, liver (2.7%) and oral (1.2%) cancers showed average annual increases of more than 1%, in addition to uterine cancer (1.1%). For both men and women, the mortality changes mentioned previously and most mortality changes shown in table 3 and table 4 and in figure 6 and figure 7 were statistically significant (P<0.05), even when the size of change was relatively small.
Principal findings
The most striking finding in this analysis of UK cancer trends among the 35-69 years age group is the substantial decline in cancer mortality rates observed in both sexes (37% decline in men and 33% decline in women) across the period examined. A decrease in mortality was reported across nearly all the specific types of cancer examined (23 in total), with only liver, oral, and uterine cancers showing an increase together with melanoma skin cancer in men and pancreatic cancer in women, both showing small increases. By contrast, the incidence trends in this age group showed varying patterns with some sites increasing, some decreasing and some remaining relatively constant. Over all sites, a modest increase was noted in cancer incidence rates of around 0.8% per annum in both sexes, amounting to an increase of 15% in men and 16% in women over the 25 year time frame.
The increase in prostate cancer incidence over this period, especially in the 35-69 years age group considered here, is very likely to be a direct result of the uptake of prostate specific antigen testing, which results in the detection of early stage disease and, to an unknown extent, indolent disease that may otherwise never have been regarded as clinically significant. 24 25 The results do, however, affect people diagnosed and represent a large increase in workload for clinical staff. The fact that the overall mortality trends for men show no difference whether prostate cancer is included or excluded in the analysis indicates that the incidence increase for this cancer has largely been of non-fatal disease. That the specific mortality rates for prostate cancer showed an appreciable rate of decline during this time (–1.8% per annum) also indicates improved clinical treatment of the disease or an increase in the proportion of men diagnosed with a favourable prognosis, or both. 24 26 However, the increase in prostate cancer incidence still results in thousands of men each year dealing with the concerns of a cancer diagnosis and the impact this may have on their lives.
Breast cancer comprehensively dominated incidence and mortality trends in female cancer. Even though the average annual incidence increase of breast cancer over this period (0.9%) was modest in comparison to the prostate cancer increase in men (4.2%), breast cancer incidence rates remained substantially higher than those for any other cancer site in either sex. Inspection of figure 4 shows that breast cancer incidence rates (age standardised) increased at a faster rate until around 2003-05 (from 194.7 in 1993-95 to 229.9 in 2003-05), a slower rate from then until 2013-15 (240.8) but have levelled off in the most recent years analysed (238.0 in 2016-18). These changes in the incidence trend likely reflect a reduced effect of the initial incidence increases brought about by mammography screening in the UK introduced from the late 1980s or a possible effect of a decline in usage of hormone replacement treatment. 27 28 However, the effect of hormone replacement treatment on breast cancer risk is small in comparison to other risk factors, 7 and trends in this treatment has varied over time, such as changes in preferred formulations, doses, and treatment durations, 29 30 31 which may impact breast cancer risk levels. 32 33 As has been reported elsewhere, 34 35 36 mortality for breast cancer has declined substantially despite the incidence increase, which is indicative of improvements in early detection (including through screening 37 ) and improved treatment.
The other two major sites of cancer in men apart from prostate cancer, namely lung and bowel cancers, showed substantial reductions in mortality. These results are likely from primary prevention (historical reduction in smoking rates) 38 39 40 41 for lung cancer and earlier detection (including screening) and improved treatment for bowel cancer. 42 43 44 While lung cancer incidence substantially decreased, the incidence rates of bowel cancer remained unchanged. However, closer inspection of the bowel cancer incidence trends over the full period shows an increase from the point the bowel screening programme was first introduced from 2006 in the UK. This rate, however, has now decreased back to the observed level prior to the introduction of the screening programme. As others have shown, the introduction of bowel screening leads to an initial short-term increase in cancer incidence due to detection of as-yet undiagnosed cancer cases, followed by a decrease because of removal of adenomas. 42 45 46 Therefore, bowel cancer incidence trends can reasonably be assumed to decrease further over the coming years, unless other preventable risk factors for bowel cancer affect the trend.
Similarly, lung and bowel were the other two major cancer sites for women (alongside breast cancer), and both showed reductions in mortality. The decline in lung cancer mortality was, however, not as extensive as that for men (–0.5% compared with –3.1% per annum) likely reflecting the different demographic pattern in smoking rates that led to peak smoking prevalence in women occurring around 30 years later than men, albeit at around half the peak prevalence observed in men. 40 47 Smoking prevalence in women has always been lower than in men. 39 48 The lung cancer incidence trends showed a significant increase in women of 0.8% per annum as opposed to the –2.1% per annum decrease in men. That the incidence rate in 2016-18 was still higher in men than in women again is almost certainly a reflection of historical differences in smoking patterns. 39 49 50 Bowel cancer incidence in women followed a similar pattern to men and is equally reflective of the introduction of the bowel screening programme. Bowel cancer mortality in women has declined at a similar rate to men (–2.2% compared with –2.5% per annum), indicative of the same improvements in early detection and improved treatment.
These reductions in mortality across the most common cancers in both sexes are likely a representation of considerable success in cancer prevention, diagnosis, and treatment. Further improvements are likely to be realised from the continued reduction in smoking prevalence, of which smoking prevention policies continue to contribute, 51 alongside the recent move to faecal immunochemical testing in the bowel screening programme adopted throughout the UK during 2019. 52 The recommended rollout of targeted lung screening is expected to further help with the earlier diagnosis of lung cancer where surgery is a viable treatment option and outcomes are vastly improved. 53 54
Although four major sites influenced the overall pattern of cancer incidence and mortality, increases in rates among some of the less common sites do raise concerns. Four cancers showed substantial increases in incidence (more than 2% per annum) in both sexes: liver, melanoma skin, oral, and kidney cancers. All have strong associations with established risk factors: alcohol consumption, smoking, and HPV for oral cancer; 7 55 56 overweight and obesity, smoking, alcohol, and hepatitis B and C for liver cancer; 7 57 58 ultraviolet light for melanoma; 59 60 and obesity and smoking for kidney cancer. 61 62 63 Increases in liver cancer incidence and mortality for both men and women are very concerning, with nearly one in two attributable to modifiable risk factors. 7 With high prevalence of overweight and obesity and diabetes in the general population, other studies expect the rates to remain high. 64 For oral and kidney cancer, despite the association with smoking, incidence rates have not followed the decrease seen for lung cancer incidence in men. This is likely to be due to the smaller proportion of cases attributable to smoking in these two sites. Whilst smoking accounts for around 17% of oral cancers, over one in three are attributed to alcohol consumption. 7 For kidney cancer, smoking is attributable to 13% of cases whereas obesity causes around 25%, however, increasing trends in kidney mortality are shown for this age group and period. 7 Therefore, the increasing incidence trends could potentially have been worse, especially in men, if the reduction in smoking prevalence had not occurred. The increased incidence of melanoma skin cancer is likely to be caused by the increased sunlight and ultraviolet exposure caused by the availability of cheaper air travel to countries with a warmer climate and insufficient regulation of tanning beds until 2010. 65 66
In women, uterine cancer incidence increased by 1.9% per annum; although, this increase was predominantly seen over the period 1993-2007 and since then incidence trends have increased at a slower rate. One of the main risk factors for uterine cancer is the use of oestrogen-based hormone replacement therapy, 67 68 and since around 2000, use has substantially declined. 27 Around a third of uterine cancers in the UK are also attributed to overweight and obesity, but the increase in incidence is also likely to be caused by a decrease in the number of women undergoing hysterectomies for menorrhagia, in favour of endometrial ablation. 69
Other cancers that showed increases in incidence were cancers of the pancreas, brain, and central nervous system, together with Hodgkin and non-Hodgkin lymphoma, myeloma, and leukaemia in both sexes, and oesophageal and testicular cancers in men. With the exception of pancreatic cancer, which only decreased in women, all these cancers also showed a reduction in mortality in both sexes, indicating improving treatment or earlier detection, or both. Generally, the causes of these cancers are not well understood although obesity is associated with the adenocarcinoma histological subtype of oesophageal cancer, 70 especially in men, 7 while a combination of smoking and alcohol is implicated in the squamous cell carcinoma subtype. 71 The considerable male excess in oesophageal adenocarcinoma in comparison with squamous cell carcinoma rates, 72 possibly underlined by the higher incidence of gastroesophageal reflux disease in men 73 and the protective effect of oestrogen, 74 75 may explain the differing trends now observed between men and women.
Several cancer sites showed decreases in both incidence and mortality rates over the time period, notably stomach, larynx, and bladder cancer in both sexes, as well as cervical and ovarian cancers in women and mesothelioma in men. The changes in stomach cancer rates were of a similar magnitude and represented the largest percentage mortality decline in both sexes. This decline can probably be attributed to a combination of a reduction in the prevalence of Helicobacter pylori infection and an increase over time in fruit and vegetable consumption reducing the dependency on preserved foods. 76 77 Challenges in coding of stomach and oesophageal cancer before 2000 may also have had a role in shaping these trends. Laryngeal cancer is associated with tobacco use and alcohol consumption as well as occupational exposures, 56 78 79 and the decline in rates is most likely to be related to the decrease in smoking prevalence as well as decreases in occupational exposure. 80 The refinement of understanding pathology for bladder cancer during this period, in which previously diagnosed malignant disease is now categorised as benign, 81 is likely to have resulted in an artificial decline in incidence rates. 82 83 This artefact should not, however, have affected the decline in mortality rates given the benign nature of these tumours that do not cause death. 81 This decline in mortality, although not as marked as that for incidence, remained appreciable. The changes in cervical cancer rates, which showed the largest percentage mortality decline amongst gynaecological cancers, are almost certainly attributed to the success of the cytological screening programme during the whole of the time period considered. 84 85 With the introduction of the HPV vaccination programme for girls in 2008 86 and the subsequent expansion to boys in 2019, 87 rates of cervical cancer are expected to fall substantially over the following decades as the first cohort of vaccinated women reaches the peak age for cervical cancer incidence (aged 30-34 years). A reduction has already been shown for women aged 20-24. 88 The absolute incidence rates of mesothelioma in women were small in magnitude in 1993-95 (0.8 per 100 000 per annum) and remained similar over time (0.7 per 100 000 per annum in 2016-18). The incidence rates of mesothelioma in men were considerably greater, especially in 1993-95 (around 6.3 per 100 000 per annum), due largely to occupational asbestos exposure, 89 but a significant decrease was noted over time (to 3.6 per 100 000 per annum in 2016-18) resulting from both the decline in asbestos exposure and the decline in heavy industries, such as coal mining. Mortality decreased substantially in both sexes over the period for which data are available (2001-03 to 2016-18).
The conclusions that can be drawn from this analysis are, overall, positive and reassuring. Within the 35-69 year age group, cancer mortality rates have shown a substantial overall decline during the last quarter of a century in both men and women. The most probable causes are a combination of changes in the underlying risk of disease for some cancers (notably lung and stomach), in increased levels of early detection (notably breast 37 and cervix 90 ) and improved treatment (notably breast and bowel) for others. The specific circumstances leading to the increased incidence of breast cancer, of which risk factors are complex, need to be better understood and controlled. Similar results have been shown for incidence within Great Britain and mortality in the UK for some cancer sites. 91 Speculated overdiagnosis, where tumours are detected that would not have caused the patient any harm during their lifetimes, has been thought to increase rates for breast and prostate cancers in particular, of which prostate is especially affected by the widespread use of prostate specific antigen testing. 4 92 However, given the decreases in mortality across the wide set of cancer sites analysed here, improvements in early diagnosis, treatment, or both are having a positive effect for most cancer patients, although cancer mortality in this age group still needs reducing.
After accounting for the major two sites in men and women, the increase in overall incidence rates disappeared in men while it remained significant in women. This difference between sexes is due to a decrease in cancers with substantially higher initial incidence rates in men, such as lung, stomach, and bladder, resulting in a higher overall impact on male incidence, combined with an increase in incidence in uterine cancer, one of the most common cancers in women.
Strengths and limitations
This study benefits from high quality cancer registry data collected by all four cancer registries in each country across the UK, which allows for the inspection of a wide range of cancer sites over 25 years. ICD-10 coding changes have been minimal, only affecting trends in cancer incidence for bladder and ovarian cancers and cancer mortality for mesothelioma, whereas challenges in coding stomach and oesophageal cancer may have affected trends for these sites. Changes in registration practice may well have had a small effect on certain cancer sites. By focusing only on the 35-69 age range, we present a clear and reliable comparative picture of cancer incidence across 25 years within the UK, which provides a reliable indicator regarding future cancer incidence trends. Understanding cancer in older people and changes in the trends of different cancers is also of interest, but subject to a different study given the increasing life expectancy over this period, impact of comorbidities, and differing interaction with health services in this age group.
Limitations include the absence of staging data to substantiate any improvements in earlier diagnosis. Due to the number of sites analysed, we also have not broken down sites by histological type, which could be beneficial in certain sites to understand the trends within cancer sites—eg, small cell and non-small cell lung cancer or oestrogen receptor-positive and oestrogen receptor-negative breast cancer. In focusing on the age group selected, we are excluding older ages where rates of cancer are higher. Although this exclusion reduces the number of cases included, providing a smaller cohort for each year, the age group selected provides a more reliable comparator for future trends given the accuracy of incidence recording and also focuses on the cancers that lead to a larger number of years of life lost. The age range included in this study has been well defined; however, other studies are indicating potentially different trends worldwide in young adults with potential increases in risk factors such as dietary risk factors playing a role. 93 94 The data captured across the UK registries provides a basis for further understanding to see whether different trends are observed across younger age groups and whether the causes of this can be determined. Additionally, although we have included a broad range of cancer sites, cancers that have not been included in this study could well be showing different trends, such as a more recent increase in thyroid cancer in the UK. 95
This study also provides a baseline covering a 25 year period uninterrupted by covid-19. Trends in cancer incidence and mortality beyond these years will be affected and therefore understanding the causes of trends will be more complicated. Having a 25 year baseline provides the observed trend for which expected cases can be assessed against observed. This benchmark will present a comparison for the following decade as the presentation, diagnosis, and treatment of cancer have been hugely affected by rules and regulations affecting public and health service staff. Mortality trends will also be impacted with decision making regarding coding of deaths with covid-19 likely to be the underlying cause of death for people with cancer if that has directly led to the patient dying, rather than their cancer.
This study focuses on the overall sex specific trends for cancer incidence and mortality in the specified age group to observe and understand trends over the 25 year period across the entire UK. Further breakdowns have not been possible. Paucity of numbers for less common cancers precluded separate analyses for the individual UK nations while data limitations precluded analyses by other demographic characteristics, for example, ethnic group and deprivation. The main obstacle to analysing data by ethnic group is the completeness of recordings in hospitals. In England, completeness improved substantially in 2012, but prior to this, the proportion of cases with unknown ethnic group renders results over time to be incomparable. In other UK countries, completeness of ethnic group recording is still not good enough to conduct country-wide cancer incidence or mortality analyses by ethnicity. For deprivation, the measures currently available are derived within each UK nation, and a specific validated UK-wide deprivation measure does not yet exist. Given the obvious importance of looking at variation in UK trends within ethnic groups and deprivation categories, such analyses represent a priority for further research and highlights the importance of data collection across all UK nations.
Conclusions
Overall, these results substantiate the view that in this age group there is no generalised increase in cancer incidence, while there is a substantial decrease in cancer mortality in the UK over the 25 year study period. Specific concerns about individual cancer sites identified were raised, of which the most important numerically, apart from the increases in breast and prostate cancer incidence, was the need to accelerate the decrease in female lung cancer. After which, concerns about oral cancer, liver cancer, kidney cancer, uterine cancer, and melanoma skin cancer present the most pressing issues. There are also several cancer sites that showed decreases in both incidence and mortality, notably, stomach, larynx, bladder, and cervical.
What is already known on this topic
No recent studies have investigated cancer incidence and mortality rates over such a long time frame within the 35-69 year age group in the UK
Short term trends for specific cancer sites are related to known risk factors, screening programmes, and improved treatment
Trends in the 35-69 years age group can be indicative of future patterns of cancer in older people
What this study adds
Decreased rates of many cancers, including lung and laryngeal, is positive, and likely to be driven by the decrease in smoking prevalence across the UK
An increase in rates of other cancer sites, including uterine and kidney, was noted, which may be a result of the increasing prevalence of overweight/obesity and other risk factors
Organised population screening programmes have led to an increase in cancer incidence but also look to have contributed to a reduction in cancer mortality across the UK
Ethics statements
Ethical approval.
Ethics approval for this work was not required as the study used publicly available data.
Data availability statement
Data sharing may be possible for additional analyses. All code used for analyses in this paper are also available from the Cancer Research UK website and GitHub. Information on how to access the data used in this analysis are available from the Cancer Research UK website.
Acknowledgments
This work uses data that has been provided by patients and collected by the health services as part of their care and support. The data is collated, maintained, and quality assured by NHS England, Public Health Wales, Public Health Scotland, and the Northern Ireland Cancer Registry.
Contributors: All authors participated in study conception and design, and/or the analysis and interpretation of results. Conception and design: DF, LS, and CT. Analysis and interpretation: all authors. Writing manuscript: all authors. Supervision and guarantor: JS and DF. All authors critically reviewed drafts of the manuscript, read and approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The manuscript’s guarantor (DF) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported, that no important aspects of the study have been omitted and that any discrepancies from the study as planned have been explained.
Dissemination to participants and related and public communities: study results will be disseminated to the public and health professionals by a press release written using layman’s terms; findings will also be shared through mass media communications and social media postings. A webinar produced alongside a patient advocacy group is also planned to accompany the publication of this study, a recording of which will be made available on the Cancer Research UK website. Since the study analyses cancer registry data collected during routine care, and provided in aggregated form, we are unable to specifically disseminate results to study participants beyond the usual channels of publication.
Provenance and peer review: Not commissioned; externally peer reviewed.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
- Queen’s University Belfast, Northern Ireland Cancer Registry
- van Seijen M ,
- Thompson AM ,
- PRECISION team
- Independent UK Panel on Breast Cancer Screening
- Kitchener H ,
- ↵ Cancer Research UK. Screening for cancer. https://www.cancerresearchuk.org/about-cancer/screening
- Office for National Statistics
- Public Health Wales
- Public Health Scotland
- NHS Digital
- NI Direct Government Services
- United Kingdom and Ireland Association of Cancer Registries
- UK Health Security Agency
- National Cancer Intelligence Network
- Benhamou E ,
- Laversanne M ,
- Gardner W ,
- Mulvey EP ,
- Hankey BF ,
- Kosary CL ,
- R Core Team
- Pashayan N ,
- Pharoah P ,
- Martin RM ,
- Donovan JL ,
- Turner EL ,
- CAP Trial Group
- Neuberger MM ,
- Djulbegovic M ,
- Johnson A ,
- Bromley SE ,
- de Vries CS ,
- Burkard T ,
- Alsugeir D ,
- Adesuyan M ,
- Vinogradova Y ,
- Coupland C ,
- Hippisley-Cox J
- Collaborative Group on Hormonal Factors in Breast Cancer
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG)
- Coleman DA ,
- Swerdlow AJ ,
- Youlden DR ,
- Cancer Research UK
- ↵ International Agency for Research on Cancer, World Health Organization. Tobacco Smoke and Involuntary Smoking. 2004. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; vol. 83. https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Tobacco-Smoke-And-Involuntary-Smoking-2004
- McClements PL ,
- Madurasinghe V ,
- Thomson CS ,
- Ait Ouakrim D ,
- Niikura R ,
- Brenner H ,
- Schrotz-King P ,
- Holleczek B ,
- Katalinic A ,
- Hoffmeister M
- Lieberman DA ,
- McFarland B ,
- American Cancer Society Colorectal Cancer Advisory Group ,
- US Multi-Society Task Force ,
- American College of Radiology Colon Cancer Committee
- Silcocks P ,
- Whitley E ,
- Malvezzi M ,
- Bertuccio P ,
- Lortet-Tieulent J ,
- Renteria E ,
- UK National Screening Committee
- Royal College of Physicians
- McLaughlin JK ,
- Hashibe M ,
- Brennan P ,
- Chuang SC ,
- Armstrong GL ,
- Farrington LA ,
- Hutin YJF ,
- Miyamura T ,
- Gilchrest BA ,
- Geller AC ,
- Berwick M ,
- van der Hel OL ,
- McMillan GP ,
- Boffetta P ,
- Zeegers MPA ,
- van Den Brandt PA
- Driver RJ ,
- HCC-UK/BASL/NCRAS Partnership Steering Group
- Doherty VR ,
- Brewster DH ,
- UK government
- Gebretsadik T ,
- Kerlikowske K ,
- Ernster V ,
- Million Women Study Collaborators
- Mukhopadhaya N ,
- Manyonda IT
- Lagergren J
- Rubenstein JH
- Czanner G ,
- Chandanos E ,
- Roberts SE ,
- Morrison-Rees S ,
- Samuel DG ,
- Williams JG
- Rothenbacher D ,
- Talamini R ,
- Bosetti C ,
- La Vecchia C ,
- Menvielle G ,
- Goldberg P ,
- National Institute for Health and Care Excellence
- Vaccarella S ,
- Plummer M ,
- Franceschi S ,
- University of Oxford
- Castanon A ,
- Windridge P ,
- Hodgson JT ,
- Matthews FE ,
- Castañón A ,
- O’Sullivan JW ,
- Nicholson BD
- Bjurlin MA ,
- Nicholson J ,
- Sasamoto N ,

IMAGES
COMMENTS
The introduction of screening mammography in the United States has been associated with a doubling in the number of cases of early-stage breast cancer that are detected each year, from 112 to 234 ...
Two population-based screening studies in Europe and one large retrospective study in the U.S. have shown significantly increased cancer detection rates and decreased false-positive ... To T, Baines CJ, Wall C. The Canadian National Breast Screening Study-1: breast cancer mortality after 11 to 16 years of follow-up. A randomized screening trial ...
A few large observational studies have been conducted on the effectiveness of mammography screening and indicated a reduction in breast cancer mortality in screened women. 10-12 To date, however, there has been no observational study on this research question using the novel principle of target trial emulation, which specifically aims to ...
Several Asian studies have shown that US can improve the detection rate of breast cancer for women with dense breasts [53,54]. However, there is limited evidence for US in breast cancer screening to reduce mortality. ... Wall C. Canadian National Breast Screening Study: 1. Breast cancer detection and death rates among women aged 40 to 49 years ...
Most countries recommend that women receive breast cancer screening every 2 years between ages 50 and 69 years. 1 In 2009 and 2016, the US Preventive Services Task Force (USPSTF) updated its recommendations to advise that women receive screening mammography every 2 years between ages 50 and 74 years, with the decision to begin screening earlier (ie, ages 40-49 years) based on individual ...
Recent evidence of supplemental abbreviated MRI in women at average risk with dense breasts and negative digital tomosynthesis results appears to increase the prevalent cancer detection rate (up ...
Definition of cases and controls. As the main objective was to evaluate the effect of mammography screening on breast cancer mortality, cases were defined as women whose primary cause of death was ...
It is a randomized breast cancer screening study that compares two types of Food and Drug Administration (FDA)-approved digital mammograms for their ability to reduce advanced breast cancer: standard digital mammograms (2-D) and a newer technology called tomosynthesis mammograms (3-D). 2-D mammograms take pictures from two sides of the breast ...
Credit: BSIP/Universal Images Group/Getty. Radiologists using an artificial-intelligence (AI) assistant during breast-cancer screening had better chances of detecting cancer than those who did not ...
Early Detection of Breast Cancer. Breast cancer is one of a few cancers for which an effective screening test, mammography, is available. MRI (magnetic resonance imaging) and ultrasound are also used to detect breast cancer, but not as routine screening tools for people with average risk.. Ongoing studies are looking at ways to enhance current breast cancer screening options.
Conclusions and Relevance In this qualitative study of women of breast cancer screening age, family history was perceived as the primary breast cancer risk factor. Most interviewees did not identify breast density as a risk factor and did not feel confident about actions to mitigate breast cancer risk. ... because prior studies 11,14 have shown ...
ABSTRACT: Breast cancer is the most commonly diagnosed cancer in women in the United States and the second leading cause of cancer death in American women 1.Regular screening mammography starting at age 40 years reduces breast cancer mortality in average-risk women 2.Screening, however, also exposes women to harm through false-positive test results and overdiagnosis of biologically indolent ...
Screening for breast cancer reduces breast cancer-related mortality and earlier detection facilitates less aggressive treatment. Unfortunately, current screening modalities are imperfect, suffering from limited sensitivity and high false-positive rates. Novel techniques in the field of breast imaging may soon play a role in breast cancer screening: digital breast tomosynthesis, contrast ...
Breast cancer screening most often includes mammography but can also include ultrasound, MRI, and other tests. Get detailed information about the potential benefits and harms of the tests used to screen for breast cancer in this summary for clinicians. ... All of these studies were designed to study breast cancer mortality rather than all-cause ...
WISDOM Study and Breast Cancer Prevention: Q & A. The WISDOM study is a national research effort to determine if breast cancer screening can be made better by personalizing each woman's mammogram schedule. Learn about the study and important facts about breast cancer risk and prevention. Watch Video Watch Video With Transcript
Screening & Early Detection Breast Cancer Studies. Treatment & Reducing Metastasis Breast Cancer Studies. Survivorship Breast Cancer Studies. Excess Weight Shortens Life (M Gaudet) Women 65+ & Genetic Tests for Breast Cancer Risk (L Teras) Cancer Disparities ACS Research Highlights. Childhood Cancer Research Highlights.
The Canadian National Breast Screening Study is an outlier among the 8 rct s for screening mammography; it was the only study to show no bc a mortality reduction from screening mammography. In the Pan-Canadian study, which used data from the 3 provinces that perform screening in women 40-49 years of age, the relative bc a mortality reduction ...
The evidence for adding annual MRI screening to mammography and clinical breast examinations in women with more than a 20% lifetime risk of breast cancer is based on nonrandomized screening trials ...
Cancer screening, including for cervical and colorectal cancers, is a factor in reduced mortality. 9,10 Although uncertainty exists about the effectiveness of mammography to reduce breast cancer-specific or all-cause mortality, 11-13 it is recommended by Cancer Care Ontario and the Canadian Task Force on Preventive Health Care. 14,15 In ...
Early diagnosis of breast cancer is crucial for reducing mortality rates. The purpose of this study is to determine the impact of demographics/social determinants of health on beliefs about the practice of self-breast examination, using mammogram and ultrasound in the context of breast cancer screening among Thai women in a hospital-based setting for implying program planning and future research.
What's New in Breast Cancer Research? Breast Cancer Risk and Prevention. Breast Cancer Risk Factors You Cannot Change; ... or you're ready to start breast cancer screening, the topics below can help you know what to expect. Getting a mammogram. Find out what a mammogram is, why it's done, what doctors look for, and what it's like to get one.
W hile better screening and improved treatments are leading to better outcomes for patients with breast cancer, 90% of breast cancer deaths are a result of metastasis, or the cancer growing and ...
Objectives: The purpose of this study was to understand women's comparative health beliefs about breast and colorectal cancers and screening tests, and their decisions to participate or not in fecal immunochemical testing (FIT) following completion of mammography. ... Keywords: cancer prevention, breast cancer screening, colorectal cancer ...
WESTPORT, CT / ACCESSWIRE / April 2, 2024 / CYduct Diagnostics, Inc. (OTC PINK:CYDX) ("CYduct", or the "Company"), a precision medicine-based women's health company focused on the development of breast cancer risk assessment and diagnostic tools, today announced positive results from its recent research on breast cancer biomarkers. The pilot study, utilizing liquid biopsy samples, met its ...
Breast cancer screening is performed using mammogram, clinical breast exam (CBE), and MRI (magnetic resonance imaging) tests. Learn about these and other tests that have been studied to detect or screen for breast cancer in this expert-reviewed and evidence-based summary. ... Scientists study screening tests to find those with the fewest harms ...
Deleterious BRCA1/2 (BRCA) mutation raises the risk for BRCA mutation-related malignancies, including breast, ovarian, prostate, and pancreatic cancer. Germline variation of BRCA exhibits substantial ethnical diversity. However, there is limited research on the Chinese Han population, constraining the development of strategies for BRCA mutation screening in this large ethnic group.
This study is the first research project known to utilize the MT-CHBMS to study the association between sociodemographic factors and health beliefs of breast cancer and screening behaviors.
Objective To examine and interpret trends in UK cancer incidence and mortality for all cancers combined and for the most common cancer sites in adults aged 35-69 years. Design Retrospective secondary data analysis. Data sources Cancer registration data, cancer mortality and national population data from the Office for National Statistics, Public Health Wales, Public Health Scotland, Northern ...