- More from M-W
- To save this word, you'll need to log in. Log In
photosynthesis

Definition of photosynthesis
Did you know.
Photosynthesis Has Greek Roots
The Greek roots of photosynthesis combine to produce the basic meaning "to put together with the help of light". Photosynthesis is what first produced oxygen in the atmosphere billions of years ago, and it's still what keeps it there. Sunlight splits the water molecules (made of hydrogen and oxygen) held in a plant's leaves and releases the oxygen in them into the air. The leftover hydrogen combines with carbon dioxide to produce carbohydrates, which the plant uses as food—as do any animals or humans who might eat the plant.
Examples of photosynthesis in a Sentence
These examples are programmatically compiled from various online sources to illustrate current usage of the word 'photosynthesis.' Any opinions expressed in the examples do not represent those of Merriam-Webster or its editors. Send us feedback about these examples.
Word History
1898, in the meaning defined above
Dictionary Entries Near photosynthesis
photosynthate
photosynthetic ratio
Cite this Entry
“Photosynthesis.” Merriam-Webster.com Dictionary , Merriam-Webster, https://www.merriam-webster.com/dictionary/photosynthesis. Accessed 18 Apr. 2024.
Kids Definition
Kids definition of photosynthesis, medical definition, medical definition of photosynthesis, more from merriam-webster on photosynthesis.
Nglish: Translation of photosynthesis for Spanish Speakers
Britannica.com: Encyclopedia article about photosynthesis
Subscribe to America's largest dictionary and get thousands more definitions and advanced search—ad free!

Can you solve 4 words at once?
Word of the day.
See Definitions and Examples »
Get Word of the Day daily email!
Popular in Grammar & Usage
Your vs. you're: how to use them correctly, every letter is silent, sometimes: a-z list of examples, more commonly mispronounced words, how to use em dashes (—), en dashes (–) , and hyphens (-), absent letters that are heard anyway, popular in wordplay, a great big list of bread words, the words of the week - apr. 12, 10 scrabble words without any vowels, 12 more bird names that sound like insults (and sometimes are), 9 superb owl words, games & quizzes.

ENCYCLOPEDIC ENTRY
Photosynthesis.
Photosynthesis is the process by which plants use sunlight, water, and carbon dioxide to create oxygen and energy in the form of sugar.
Loading ...
Learning materials, instructional links.
- Photosynthesis (Google doc)
Most life on Earth depends on photosynthesis .The process is carried out by plants, algae, and some types of bacteria, which capture energy from sunlight to produce oxygen (O 2 ) and chemical energy stored in glucose (a sugar). Herbivores then obtain this energy by eating plants, and carnivores obtain it by eating herbivores.
The process
During photosynthesis, plants take in carbon dioxide (CO 2 ) and water (H 2 O) from the air and soil. Within the plant cell, the water is oxidized, meaning it loses electrons, while the carbon dioxide is reduced, meaning it gains electrons. This transforms the water into oxygen and the carbon dioxide into glucose. The plant then releases the oxygen back into the air, and stores energy within the glucose molecules.
Chlorophyll
Inside the plant cell are small organelles called chloroplasts , which store the energy of sunlight. Within the thylakoid membranes of the chloroplast is a light-absorbing pigment called chlorophyll , which is responsible for giving the plant its green color. During photosynthesis , chlorophyll absorbs energy from blue- and red-light waves, and reflects green-light waves, making the plant appear green.
Light-dependent Reactions vs. Light-independent Reactions
While there are many steps behind the process of photosynthesis, it can be broken down into two major stages: light-dependent reactions and light-independent reactions. The light-dependent reaction takes place within the thylakoid membrane and requires a steady stream of sunlight, hence the name light- dependent reaction. The chlorophyll absorbs energy from the light waves, which is converted into chemical energy in the form of the molecules ATP and NADPH . The light-independent stage, also known as the Calvin cycle , takes place in the stroma , the space between the thylakoid membranes and the chloroplast membranes, and does not require light, hence the name light- independent reaction. During this stage, energy from the ATP and NADPH molecules is used to assemble carbohydrate molecules, like glucose, from carbon dioxide.
C3 and C4 Photosynthesis
Not all forms of photosynthesis are created equal, however. There are different types of photosynthesis, including C3 photosynthesis and C4 photosynthesis. C3 photosynthesis is used by the majority of plants. It involves producing a three-carbon compound called 3-phosphoglyceric acid during the Calvin Cycle, which goes on to become glucose. C4 photosynthesis, on the other hand, produces a four-carbon intermediate compound, which splits into carbon dioxide and a three-carbon compound during the Calvin Cycle. A benefit of C4 photosynthesis is that by producing higher levels of carbon, it allows plants to thrive in environments without much light or water. The National Geographic Society is making this content available under a Creative Commons CC-BY-NC-SA license . The License excludes the National Geographic Logo (meaning the words National Geographic + the Yellow Border Logo) and any images that are included as part of each content piece. For clarity the Logo and images may not be removed, altered, or changed in any way.
Media Credits
The audio, illustrations, photos, and videos are credited beneath the media asset, except for promotional images, which generally link to another page that contains the media credit. The Rights Holder for media is the person or group credited.
Production Managers
Program specialists, last updated.
March 20, 2024
User Permissions
For information on user permissions, please read our Terms of Service. If you have questions about how to cite anything on our website in your project or classroom presentation, please contact your teacher. They will best know the preferred format. When you reach out to them, you will need the page title, URL, and the date you accessed the resource.
If a media asset is downloadable, a download button appears in the corner of the media viewer. If no button appears, you cannot download or save the media.
Text on this page is printable and can be used according to our Terms of Service .
Interactives
Any interactives on this page can only be played while you are visiting our website. You cannot download interactives.
Related Resources
Photosynthesis
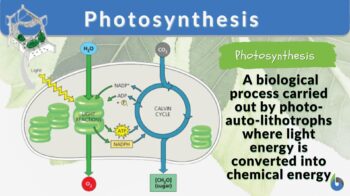
Photosynthesis n., plural: photosyntheses [ˌfŏʊ.ɾoʊ.ˈsɪn̪.θə.sɪs] Definition: the conversion of light energy into chemical energy by photolithorophs
Table of Contents
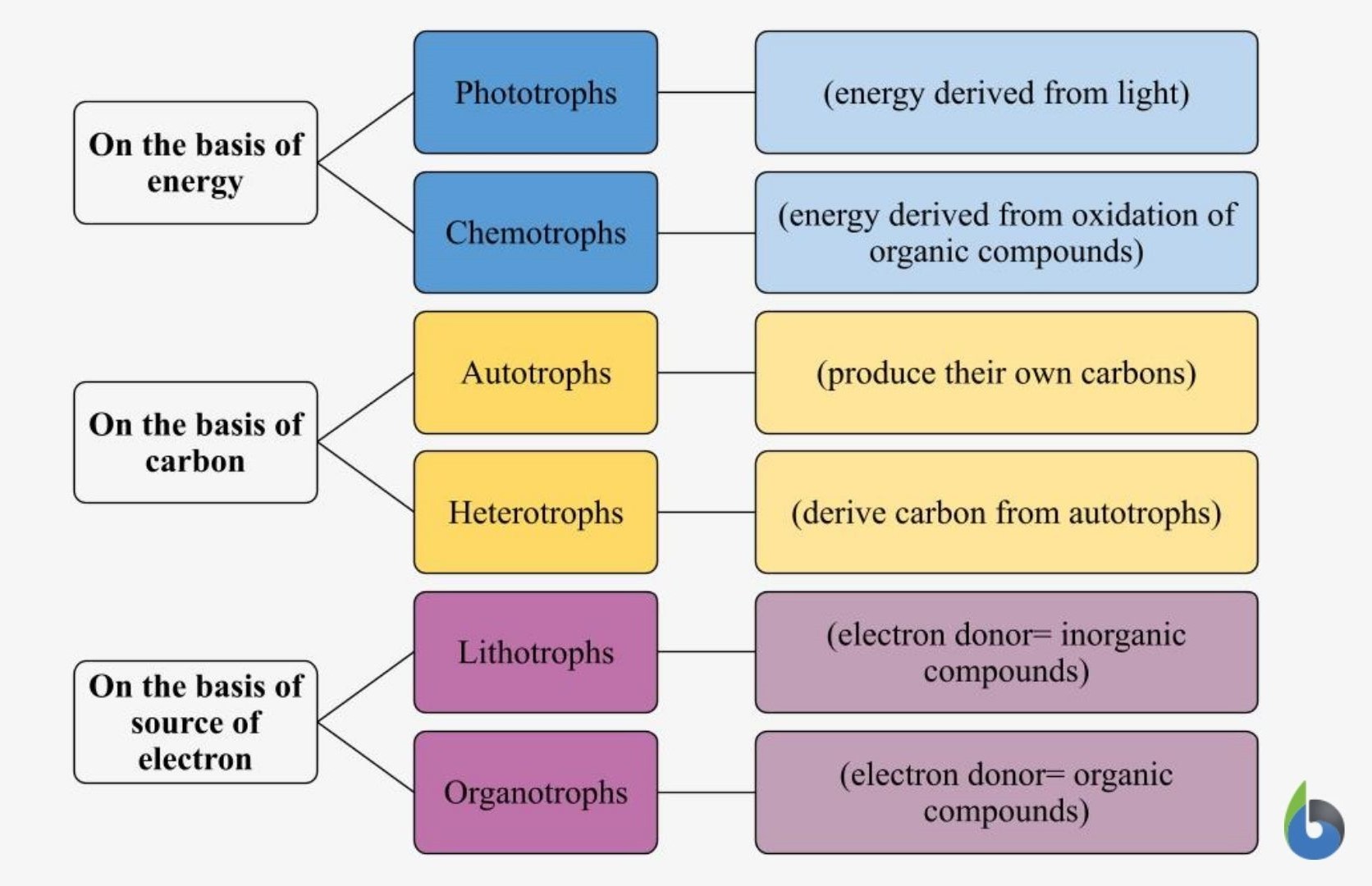
Photosynthesis is a physio-chemical process carried out by photo-auto-lithotrophs by converting light energy into chemical energy . Among the endless diversity of living organisms in the world, producers are a unique breed.
Unlike consumers ( herbivores , carnivores , omnivores , or decomposers ) that rely upon other living organisms for their nutritional requirements and nourishment, producers have been distinguished by their ability to synthesize their own food. This is the reason that we call producers “autotrophic or self-reliable” in nature while consumers of all the different categories are called “heterotrophic or dependent” in nature.
Now among producers, there are different categories of producers, i.e. different mechanisms via which they produce their own food.
- Photo-auto-litho-trophs: Since these organisms tend to derive their nutrition by channeling the sun’s light energy, they are termed phototrophic in nature. Also, since they utilize inorganic carbon and translate it into organic carbon atoms, i.e. their means of deriving food becomes autotrophic. Additionally, since the source of electrons (electron donors) here are inorganic compounds, they are specified as lithotrophic . In totality, they can be called photo-auto-litho-trophic in nature. Example : Green plants are nature’s brilliant entities that come under this category. They carry out a photosynthesis cycle by taking in carbon dioxide and fixing it into carbohydrates (energy storage molecule). Some of them also give out oxygen gas that’s vital for the other life forms to survive in the earth’s atmosphere.
- Chemo-auto-lithotrophs: Many of us might be unaware of the fact that there are some autotrophs that don’t utilize sunlight. Rather they derive their energy stored from a different energy source like oxidation of inorganic compounds.
The scope of today’s discussion is limited to photosynthesis and photoautotrophs. So, let’s get started and get to know the answers to these common questions: what is the photosynthesis process, what are the 3 stages of photosynthesis, what does photosynthesis produce, what is a byproduct of photosynthesis, what is the purpose of photosynthesis, is photosynthesis a chemical change, the various inputs and outputs of photosynthesis, which organisms perform photosynthesis , and many other more questions!!!
What is Photosynthesis?
Photosynthesis definition: Photosynthesis is a physio-chemical process carried out by photo-auto-lithotrophs . In simpler language, photosynthesis is the process by which green plants convert light energy into ‘chemical energy’.
This energy transformation is only possible due to the presence of the miraculous pigment molecule chlorophyll in photosynthesis. The chemical energy as referred to before is the fixed carbon molecules generated during photosynthesis.
Green plants and algae have the ability to utilize carbon dioxide molecules and water and produce food (carbohydrates) for all life forms on Earth. There’s no doubt in the fact that life is impossible and unimaginable without green plants that photosynthesize and sustain the cycles of life.
Let’s give you a brief outline of the topic before we head forward.
- Etymology: The photosynthesis process finds its origin in 2 Greek words, firsts one being “phōs (φῶς)” meaning ‘light’ and the second one being “sunthesis (σύνθεσις)” meaning ‘putting together’ . The process of photosynthesis aids the conversion of light energy to chemical energy in varied forms of carbohydrate molecules like sugar molecules and starches.
- Organisms that perform photosynthesis: The organisms are called photo-auto-litho-trophs or simply photoautotrophs.
- Atmospheric gas consumed: Photosynthesizing organisms utilize carbon dioxide in photosynthesis (CO 2 ).
- Atmospheric gas released by “some” photosynthetic organisms (MIND IT-Not all): Some photosynthesizing organisms convert carbon dioxide and aid the process of producing oxygen gas (O2).
- Examples of photosynthesizing organisms: Green plants, cyanobacteria (earlier termed as blue-green algae), and different types of algae that essentially carry out phytoplankton photosynthesis.
- Why is photosynthesis important? The important function of photosynthesis: Food supply for the organisms on Earth, Oxygen supply for the survival of all organisms.
- Site of photosynthesis: Leaves and green tissues. (So when asked where photosynthesis takes place, we can tell that it is this site.)
- What are the reactants of photosynthesis: Carbon dioxide molecules + Water molecules + Light energy
- Products of photosynthesis: Fixed carbon (carbohydrates) + Oxygen (some cases) + Water
Watch this vid about photosynthesis:
Biology Definition: Photosynthesis is the synthesis of complex organic material using carbon dioxide , water , inorganic salts , and light energy (from sunlight) captured by light-absorbing pigments , such as chlorophyll and other accessory pigments . Photosynthesis may basically be simplified via this equation: 6CO 2 +12H 2 O+energy=C 6 H 12 O 6 +6O 2 +6H 2 O, wherein carbon dioxide (CO 2 ), water (H 2 O), and light energy are utilized to synthesize an energy-rich carbohydrate like glucose (C 6 H 12 O 6 ). Other products are water and oxygen .
- Photosynthesis occurs in plastids (e.g. chloroplasts ), which are membrane-bounded organelles containing photosynthetic pigments (e.g. chlorophyll ), within the cells of plants and algae .
- In photosynthetic bacteria ( cyanobacteria ) that do not have membrane-bounded organelles, photosynthesis occurs in the thylakoid membranes in the cytoplasm .
Etymology: from the Greek photo-, “light”, and synthesis, “putting together” Related forms: photosynthetic (adjective) Compare: chemosynthesis See also: photoautotroph
Types of Photosynthesis
Plant photosynthesis and photosynthetic organisms can be classified under different categories on the basis of some characteristic features. They are:
- Types of organisms that carry out photosynthesis on the basis of “cellular structure” Both prokaryotic and eukaryotic organisms carry out photosynthesis.
- Photosynthetic prokaryotes: for example, cyanobacteria
- Eukaryotic: for example, protists ( diatoms , dinoflagellates , Euglena) and green plants. In particular, algae photosynthesis can be observed in green algae , red algae , brown algae , & land plants, like bryophytes , pteridophytes, gymnosperms , and angiosperms .
- Prokaryotic ONLY (anoxygenic photosynthetic bacteria, green sulfur bacteria and purple bacteria)
Photosynthesis: a two-stage process
Photosynthesis is an example of a metabolic process with 2 stages. Both the stages need light (direct or indirect sunlight). Hence, the long-claimed notion of the 2 processes being ‘absolute LIGHT and DARK reactions’ isn’t apt.
Scientific studies have pointed out that even the 2nd stage of photosynthesis requires indirect sunlight. Therefore, rather than classifying the stages as light and dark photosynthesis reactions, we’ll like to classify the 2 stages as follows:
- Photochemical Reaction Process: Light energy is converted to ATP ; photophosphorylation process (light-dependent reactions)
- Through Calvin cycle: In oxygenic photosynthesis as well as anoxygenic photosynthesis
- Through Non-Calvin cycle: Only is some anoxygenic photosynthesis
Evolution of Photosynthesis Process
It is postulated that the very first photosynthetic beings and photosynthesis evolved quite early down the evolutionary timescale of life.
It is also believed that the first photosynthetic beings would have initially resorted to other available reducing agents like hydrogen ions or hydrogen sulfide in contrast to the modern-day photosynthetic organisms that utilize water as the “prime and only sources of electrons”.
It is believed that cyanobacteria would have appeared on the surface of Earth much later than the first photosynthetic beings. Once appeared they must have saturated the Earth’s atmosphere with oxygen gas and led to its oxygenation. Only after the Earth was oxygenated, the more complex forms of life would have later evolved.
When we compare photosynthesis to other metabolic processes like respiration, we can clearly notice that these two processes are almost opposite to each other. But another point to note is that both the processes in synchrony sustain life on Earth.
You cannot separate respiration from photosynthesis or photosynthesis from respiration and expect life to run normally. It is not possible that way. Let’s try to compare and list some characteristic features of photosynthesis and cellular respiration processes.
Photosynthesis vs. Respiration
- Photosynthesis: Anabolic process
- Cellular respiration: Catabolic process By anabolic, we mean the photosynthesis process “utilizes energy to build biomolecules” like carbohydrates, starch, and sugars. These biomolecules are further utilized by both the plants and the organisms dependent on plants for their nutritional needs. On the other hand, respiration is a catabolic process. This energy is utilized to break down complex molecules to derive nutrition out of them.
- Photosynthesis: In the chloroplasts of the eukaryotic phototrophic cells.
- Respiration: Primarily in the mitochondria of the cell.
- Photosynthesis: Carbon dioxide molecules + Water molecules + Light energy
- Respiration: Glucose + Oxygen
- Photosynthesis: Fixed carbon (carbohydrates) + Oxygen (some cases) + Water
- Respiration: Carbon dioxide + Water +energy (ATP)
- Photosynthesis: Endergonic and endothermic
- Respiration: Exergonic and exothermic Just note that these terms endergonic and endothermic both convey the same meaning of “absorbing heat”. And the terms exergonic and exothermic also convey the same meaning of “releasing heat”. The only difference is that –gonics relates to “the relative change in the free energy of the system” while –thermic relates to “the relative change in enthalpy of the system”.
- Photosynthesis: 6CO 2 + 6H 2 O → C6H 12 O 6 + 6O 2
- Respiration: C 6 H 12 O 6 6 + 6O 2 → 6CO 2 + 6H 2 O
Photosynthetic Membranes and Organelles
When we begin the discussion on this topic, it’s important that we know that no photosynthesis is possible without the pigment molecules that absorb light. The absorption of sunlight is the most vital step of photosynthesis.
We should also note that the energy of photons is different for every light of different wavelengths. And the energy needed for the photosynthesis to be conducted is of “a very specific wavelength range”.
For the absorption of lights of desired wavelengths, phototrophs organize their pigment molecules in the form of reaction center proteins . These proteins are located in the membranes of the organisms. Let’s learn how these pigment molecules reside inside the organism and how they make the membranes photosynthetic in nature.
- Prokaryotic photosynthetic organisms: These organisms have their pigment systems or photosystems located in the cell membranes or the thylakoid membranes in the cytosol itself. There are no special organelles called chloroplasts in the prokaryotes.
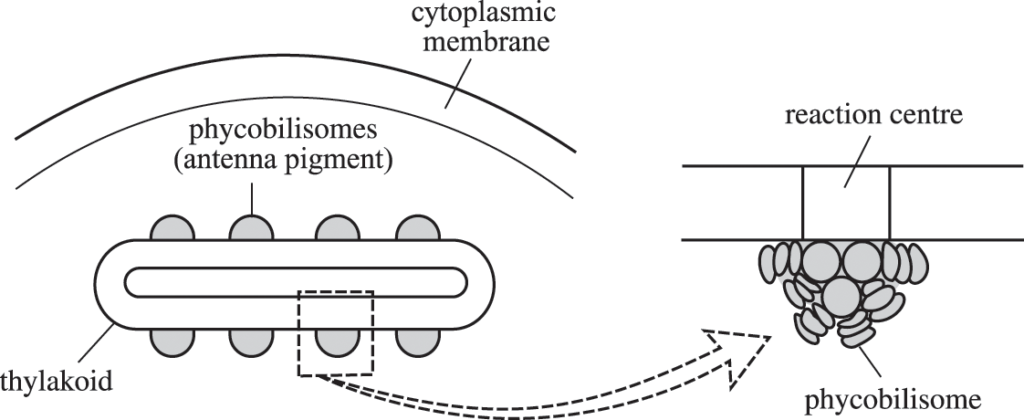
- Eukaryotic photosynthetic organisms (like green plants): These organisms have their pigment systems or photosystems located in the thylakoids of the chloroplast membranes. Eukaryotes have specialized organelles called chloroplasts (chlorophyll-containing plastids) in their cells.
Photosynthetic Pigments
There are 2 types of photosynthetic pigments in the oxygenic photosynthesizing organisms . They are as follows:
- Porphyrin-derivatives (Chlorophyll in plants and Phycobilin)
Carotenoids
Chlorophyll.
Chlorophyll is the green-colored pigment essential for photosynthesis. Let’s try to list its major characteristic features and roles of it.
- Nature: Lipid
- Location: Embedded in the thylakoid membrane
- Types: 9 types as identified by Arnoff and Allen in 1966 (chlorophyll-a, b, c, d, e, bacteriochlorophyll a, b, chlorobium chlorophyll-650,666). Bacteriochlorophylls are present in the anoxygenic photosynthetic organisms.
- Primary photosynthetic pigment: Chlorophyll-a
- Presence: In all oxygenic photosynthetic organisms
- Absorption range: Visible (blue and red) and IR (Infra-red)
- Ion important for its biological functioning: Magnesium ion (Mg 2+ )
- Structure: Chlorophyll-a, b, and d are “ chlorin ” derivatives; c is a “ porphyrin ” derivative.
- Chlorophyll Tail: Oxygenic photosynthetic organisms have a “ phytol ” tail in their chlorophyll; anoxygenic photosynthetic organisms have a “ geranyl ” tail in their bacteriochlorophylls.
- Main pigment for capturing and storing solar energy
- Photochemical reaction (chlorophyll-a is present in the photochemical reaction center i.e. PCRC. Chlorophyll a, b, c, and d play a role in resonance energy transfer.)
Carotenoid is the photosynthetic pigment essential for working in conjunction with chlorophyll. Let’s try to list its major characteristic features and roles of it.
- Nature: Lipid-soluble
- Types: More than 150
- Absorption range: 400-500nm
- Forms: Carotene (simple hydrocarbon, for example, beta carotene) and xanthophyll (oxygenated hydrocarbon, for example, lutein)
- In excitation and resonance energy transfer
- Photo-protection (work as a free-radical scavenger as well as a quencher)
Phycobilins
Phycobilins aren’t present in all the oxygenic photosynthetic organisms. They have a tetrapyrrole structure (no need for magnesium ion).
- Types: Phycoerythrobilin, Phycocyanobilins, Allophycocyanobilins When these pigment molecules combine with a water-soluble protein, they form the pigment-protein complex (phycobiliproteins, like phycoerythrin and phycocyanin).
- Location: Since these phycobiliproteins are water-soluble, they can’t exist in the membranes like chlorophyll and carotenoids. Therefore, phycobilin pigments as their pigment-protein complex aggregate into clusters and adhere to the membrane. These clusters are called phycobilisomes .
- Exceptional Note: These are the only pigments that are associated with protein molecules.
- Role: Resonance energy transfer
Organelle for Photosynthesis
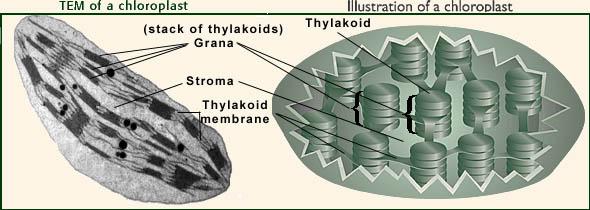
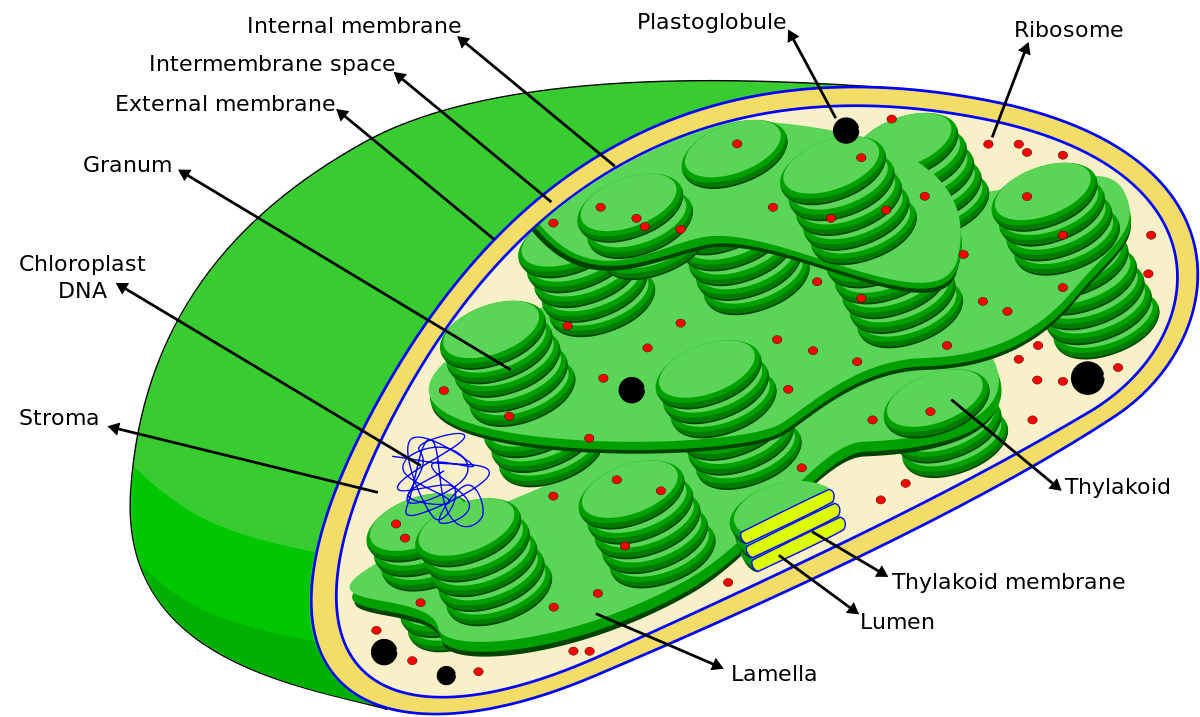
What is chloroplast? In eukaryotes, photosynthesis occurs in chloroplasts as they are the designated organelles for the photosynthesis process. There are nearly 10-100 chloroplasts in a typical plant cell .
Inside chloroplasts are the thylakoids; the very specific site for the light capturing. The structure of this very unique part of the chloroplasts is briefly discussed here.
Thylakoid is a membrane-bound compartment in the chloroplasts of eukaryotic organisms. They are also present as such in the cytosol of cyanobacteria (cyanobacteria don’t have chloroplasts but they have simply thylakoids).
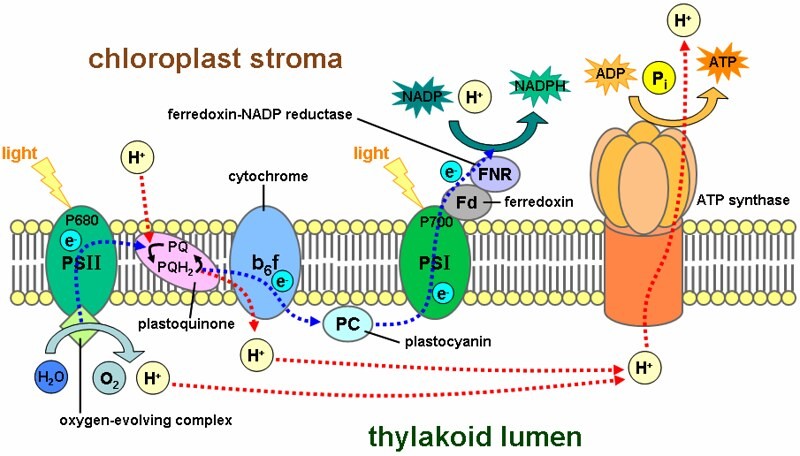
These thylakoids are the “primary site of the 1st stage of photosynthesis. i.e. “photochemical reaction” or popularly called “light-dependent reactions of photosynthesis”. The main components of the thylakoid are membrane, lumen, and lamellae. The chlorophyll molecules are present inside these thylakoid membranes.
Light-dependent Reactions
The first stage of photosynthesis is popularly called “light-dependent reactions” . We choose to call this stage the “1st stage: PHOTOCHEMICAL REACTION STAGE”. It is also called the “thylakoid reaction stage” or “hill’s reaction” .
This stage is marked by 3 essential steps of photosynthesis: Oxidation of water , reduction of NADP + , and ATP formation . The site where these reactions occur is the lamellar part of the chloroplast. The units of light-dependent reactions are quantosomes .
Let’s discuss this stage under some subheadings:
Wavelengths of light involved and their absorption
The white light that reaches Earth has subparts of different wavelengths together constituting the visible spectrum (390-760nm). But the photosynthetic organisms specifically use a subpart called PAR ( P hotosynthetically A ctive R adiation).
PAR ranges from 400-760nm. Blue light is 470-500nm while red light is 660-760nm). The green light (500-580nm) is reflected back by the plants and this is the reason that plants appear green in color. Blue-green light is not used, only blue light is used.
Absorption spectrum and action spectrum
- Absorption Spectrum: This is a pigment-specific entity or terminology. To find the absorption spectrum of a pigment, you need to plot “the amount of absorption of different wavelengths of light by that particular pigment” . The graph has the “wavelengths of light (in nanometers/nm)” on the X-axis and the “percentage of light absorption” on the Y-axis.
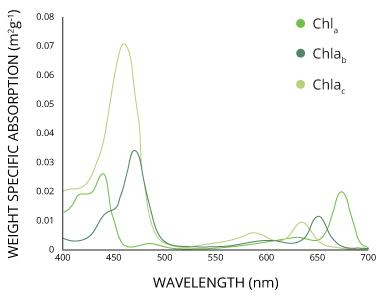
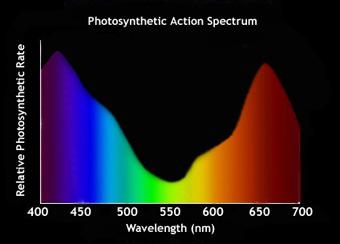
- Action Spectrum: To find the action spectrum of a pigment, you need to plot the “effectiveness of the different wavelengths of light in stimulating photosynthesis process” . The graph has the “wavelengths of light (in nanometers/nm)” on the X-axis and the “rate of photosynthesis (measured as oxygen released)” on the Y-axis. When you superimpose the action spectrum of photosynthesis with the absorption spectrum of the specific pigment, you can find the contribution of each different wavelength in the photosynthesis rate, photosynthetic efficiency, and photosynthetic productivity.
IMPORTANT NOTE: The absorption spectrum is calculated for any of the many pigments involved in photosynthesis. Contrastingly, the action spectrum is calculated only for the photochemical reaction performing pigment i.e. chlorophyll-a present at the reaction center. We identify the progress of photochemical reactions as the “evolution of oxygen gas” that primarily happens at the reaction center where only chlorophyll-a is present. Since the action is directly correlated to the specific excitation of chlorophyll-a molecule, the action spectrum is scientifically calculated only for this chlorophyll-a.
- Absorption spectrum of chlorophyll- a : 430 nm (blue), 660nm (red) {more absorbance at 660 nm)
- Absorption spectrum of chlorophyll-b: 430 nm (blue), 660nm (red) {more absorbance at 430 nm)
What actually happens in the Light-dependent reaction
Let’s briefly describe what actually happens here.
- 1 photon is absorbed by 1 molecule of the chlorophyll (P680) and simultaneously 1 electron is lost here.
- The electron flow of the photochemical reaction begins here.
- The electron is transferred to D1/D2 protein, then to a modified form of chlorophyll and “pheophytin”.
- After that, it’s transferred to plastoquinone A and then B.
- Initiates an electron flow down an electron transport chain.
- Ultimately aids the NADP reduction to NADPH.
- Creation of a proton gradient across the chloroplast membrane.
- Further on this proton gradient is exploited by the ATP synthase for the generation of ATP molecules.
Water photolysis
Now, if you are wondering how the first electron lost by the 1st chlorophyll is replenished to keep this cycle going, read on. The answer to this query is “photolysis of water molecules” . The chlorophyll molecule regains the lost electron when the “oxygen-evolving complex” in the thylakoid membrane carries out the photolysis of water. The chlorophyll molecule ultimately regains the electron it lost when a water molecule is split in a process called photolysis, which releases oxygen.
Many scientists had a doubt about the source of oxygen in photosynthesis. Some speculated the oxygen atom of the CO 2 gas is the source of oxygen post-photosynthesis. But it was the collective contribution of some 4 scientists that gave clarity on this topic.
C.B. Van Niel worked on purple photosynthetic bacteria ( Chromatium vinosum ) and found out that the source of oxygen is the oxidation of water molecules (‘indirect evidence’). While Ruben, Hassid, and Kamen carried out an isotopic study that gave ‘direct evidence’ of oxygen-evolving from H 2 O molecules and not CO 2 molecules.
Hydrolysis of 2 molecules of water leads to the evolution of 1 molecule of oxygen gas. The photosynthesis equation for light-dependent reactions (non-cyclic electron flow) or the chemical formula for photosynthesis:
2 H 2 O + 2 NADP+ + 3 ADP + 3 Pi + light → 2 NADPH + 2 H+ + 3 ATP + O 2
The photochemical reaction (or the light-dependent reactions) can be classified as:
- Cyclic reaction: Only 1 photosystem ( PS1 ) is involved. (Photon excites P700 in PS1, electron reaches Fe-S, then Ferredoxin, then Plastoquinone and then Cyt b6f complex and then Plastocyanin). Since in the solo involvement of PS1 here, the electron flow becomes cyclic. And this phosphorylation process is called cyclic phosphorylation. It happens in the stroma lamellae when light beyond 680nm is available.
- Non-cyclic reaction: Both photosystems (PS1 and PS2 ) are involved. (Photon excites P680 in PS2, the electron is lost and transferred to pheophytin, then sent on a roller coaster (Z-scheme). Within the z-scheme, the final redox reaction enables the reduction of NADP+ to NADPH. And the chemiosmotic potential generation via proton pumping proton across the membrane and into the thylakoid lumen ensures ATP synthesis.
Data Source: Akanksha Saxena of Biology Online
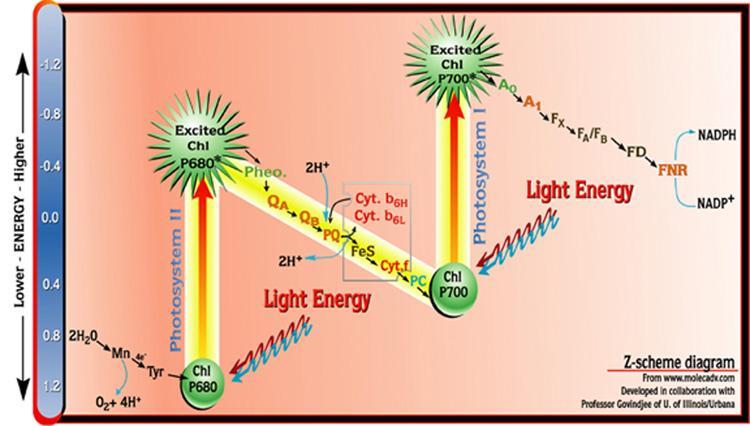
Light-Independent Reactions (Carbon-fixation Reaction)
Also called the carbon fixation process, the “light-independent reactions” is a misnomer as Science has now already proved that the second stage of photosynthesis isn’t really light-independent reactions. Though it doesn’t need direct light, indirect light is involved even in this process. We choose to label this stage of photosynthesis as the “2nd stage: CARBON-FIXATION REACTION STAGE ”, which is also called:
- Calvin Cycle or “stromal reaction” as it manifests in the stroma part of the chloroplast
- “C3 Cycle” or the “reductive pentose phosphate cycle”
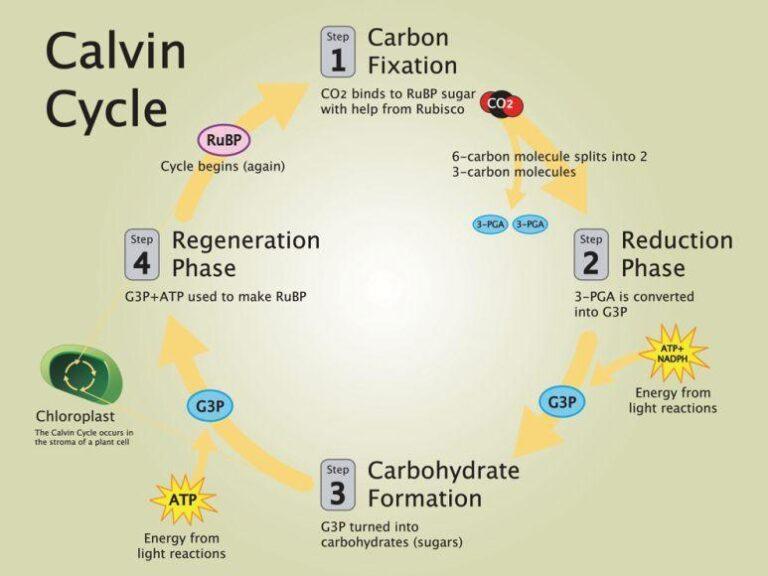
Calvin cycle
The inputs for the Calvin cycle in most plants come from the previously occurred photochemical reaction. In this cycle, the carbon dioxide produced is fixed to a glucose molecule. To be very specific, the Calvin cycle directly doesn’t produce glucose, rather it produces glyceraldehydes-5-phosphate (G-3-P). Glucose is formed after these G-3-P molecules move into the cytosol from the chloroplast .
It consists of primarily 3 steps as follows:
- Carboxylation: Acceptance of CO 2 by RuBP which is a 5-carbon compound and the CO2-acceptor). 2 molecules of 3-phosphoglycerate are generated as the result of the carboxylation process.
- Reduction: Generation of 3C/4C/5C/6C/7C molecules.
- Regeneration of RUBP: 3 molecules of RuBP are regenerated.
In totality, 3 molecules of CO 2 produce 1 molecule of G-3-P. This uses 9 ATPs and 6 NADPHs. And, 6 molecules of CO 2 produce 2 molecules of G-3-P which further produce 1 molecule of glucose. This uses 18ATPs and 12 NADPHs.
The main enzyme is RuBisCo . It’s a multi-enzyme complex with 8 large and 8 small subunits. The substrates for this enzyme are CO 2 , O 2 , and RuBP. An essential ion for the biological functioning of this enzyme: Mg 2+ . The role of RuBisCo is that it captures carbon dioxide gas from the atmosphere and utilizes the NADPH from the 1st stage (photochemical reaction/light-dependent reaction stage) to fix the CO 2 .
The equation of dark reaction of photosynthesis/light-independent reaction stage/2nd stage is: 3 CO 2 + 9 ATP + 6 NADPH + 6 H + → C 3 H 6 O 3 -phosphate + 9 ADP + 8 Pi + 6 NADP+ + 3 H 2 O
The simple carbon sugars formed via the C3 cycle are utilized by the biological systems to form complex organic compounds like cellulose, precursors for amino acids synthesis and thereby proteins, precursors for lipids, and the source of fuel for respiration.
Important Point To Note: It happens in all the photosynthetic organisms as the basic carbon-fixation step.
Carbon concentrating mechanisms
There are many carbon concentrating mechanisms to increase the carbon dioxide levels and the carbon fixation process like C4, CAM, etc.
- Doesn’t happen in all photosynthetic organisms. Rather it happens in conjunction with the C3 cycle in some 4% of angiosperm families.
- Most commonly angiosperm families that witness C4 cycle: Poaceae, Cyperaceae.
- First explained by: Hatch and Slack (hence also called the Hatch and Slack cycle). They worked on the maize plant.
- Role: Endow the ability to efficiently conduct photosynthesis in plants of the semi-arid regions by making them well adapted.
- Mechanism: By separation of photosynthesis stages in 2 types of cells (mesophyll cells and bundle sheath cells). The light reaction is restricted to the mesophyll cells and the CO 2 fixation happens in the bundle sheath cells. This phenomenon is also termed as “chloroplast dimorphism” in C4 plants. The Kranz anatomy is visible here.
- Why does the need arise in the first place? – In semi-arid regions or regions with very hot and dry environmental conditions, plants are forced to close their stomata in order to limit water loss. Under such harsh conditions, the intake of CO 2 decreases during the day as the stomata are forced closed. This might lead to no CO 2 intake and hence no CO 2 fixation (2nd stage of photosynthesis). But the 1st stage of photosynthesis keeps running as it doesn’t depend on stomata opening or closure. This means that a continuous oxygen evolution happens which can lead to oxygen saturation. As we know that RuBisCo enzymes use O 2 gas as substrate too, and this can lead to an increased rate of photorespiration by the oxygenase activity of RuBisCo. This further decreases the carbon fixation. This is a very big issue if not resolved. Hence, for situations like these, carbon concentrating mechanisms have evolved in some families of plants to concentrate and enrich the CO 2 concentration in the leaves of these plants under such conditions.
- Important enzyme for CO 2 concentration: PEP carboxylase
- CO 2 is first added to a three-carbon compound called phosphoenolpyruvate (PEP) in this cycle. This leads to the formation of a four-carbon (4C) molecule called oxaloacetic acid or malate. This step happens in the mesophyll cells of the leaves.
- After that, these 4C compounds are transferred to the bundle sheath cells where the normal C3 cycle fixes them into glucose molecules.
- This CO 2 concentrating mechanism works on the “principle of separating the RuBisCo enzyme from the O 2 -generating photochemical reactions” in order to reduce the rates of photorespiration and simultaneously increase the rates of CO 2 fixation.
- This increases the photosynthetic capacity of the leaf/leaf photosynthesis.
- When the high light and high-temperature conditions are dominant, C4 plants prove more photosynthetically efficient than C3 plants as they produce more sugar molecules in such conditions.
- Examples of C4 plants: Many crop plants like wheat, maize, rice, sorghum, millet, and sugarcane.
- Number of ATPs required: 12 (for C-enrichment) + 18 (for C-fixation)= 30 ATPS for 1 glucose production
- Number of NADPH required: 18 NADPH for 1 glucose production
- Some plants resort to another mechanism called the CAM cycle in conjunction with the C3 cycle to fix carbon dioxide.
- Examples: xerophytes like cactus photosynthesis, and most succulents.
- Around 16,000 species of plants utilize the CAM mechanism
- Mechanism: Utilize PEP carboxylase to capture carbon dioxide. In contrast to the C4 cycle where there is a “spatial separation of the 2 processes of CO 2 reduction to PEP and PEP fixation to glucose”, CAM plants display a “temporal separation of the 2 listed processes”.
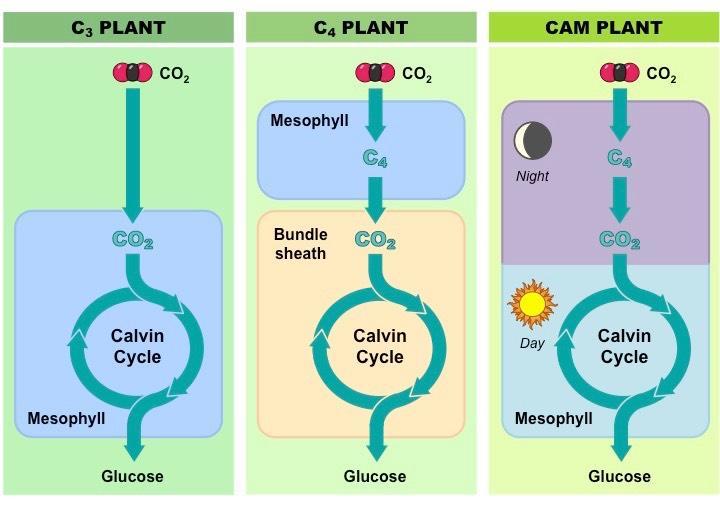
Land plants display different types of photosynthesis based on their requirements and environmental constraints. They are C3, C4 +C3, and CAM+ C3 types of photosynthesis.
Aquatic plants and algae display some extra features in the photosynthetic machinery. These features further refine and define the smooth functioning and efficiency of photosynthesis.
Example: Cyanobacteria photosynthesis – cyanobacteria have carboxysomes that help in enriching the concentration of carbon dioxide around the RuBisCO enzyme. This directly increases the photosynthetic rates. The distinguished and specially enabled enzyme in the carboxysomes is called “carbonic anhydrase”. The carbonic anhydrase possesses the ability to evolve and release CO 2 from the dissolved hydrocarbonate ions (HCO-). As soon as the CO 2 is released, RuBisCo takes care that it doesn’t go to waste.
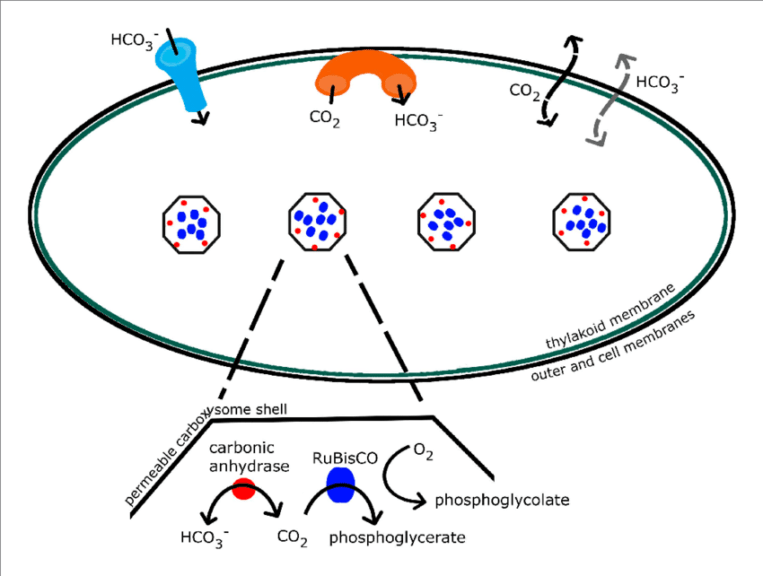
Order and Kinetics
There are innumerable reactions and processes involved in the biological mechanism of photosynthesis. Besides the normal flow of photosynthesis, there are some plant-specific and condition-specific additional steps that further complicate the mechanism.
Since every biological mechanism has a lot of enzymes, factors, cofactors, substrates, and entities involved, photosynthesis is no different.
Let’s try to list some kinetics-specific pointers that may help.
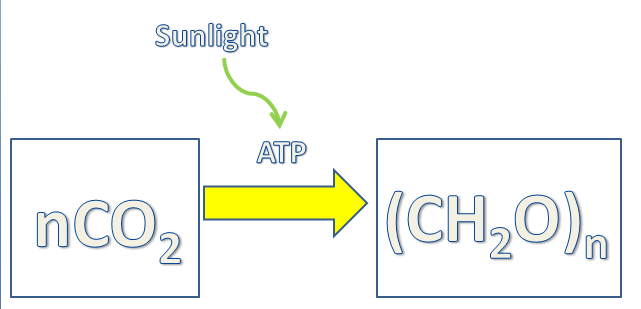
As discussed in the overview and starting of this article, the early photosynthetic organisms must have been primarily “anoxygenic” in nature. These bacteria used some other source than water molecules as their primary electron donors. Even the geological evidence aligns with this fact as the early atmosphere of Earth was highly reducing in nature. Some speculated organisms of the early evolutionary phase are :
- Green sulfur bacteria (Electron donor= hydrogen and sulfur)
- Purple sulfur bacteria (Electron donor= hydrogen and sulfur)
- Green nonsulfur bacteria (Electron donor= various amino and other organic acids)
- Purple nonsulfur bacteria (Electron donor= variety of nonspecific organic molecules)
After this, some filamentous photosynthetic organisms are expected to have evolved. This is scaled to be an occurrence of some 3.4 billion years old timeline. It is around 2 million years ago that oxygenic photosynthesis is believed to have evolved.
The modern and more commonly known photosynthesis in plants and most of the photosynthetic prokaryotes= Oxygenic (Electron donor= Water molecules)
Symbiosis and the origin of chloroplasts
There are some animal groups that have the ability to form and establish symbiotic relationships with photosynthetic organisms. By establishing such a relationship, these organisms can directly rely upon their photosynthetic partner for energy and food requirements. Some examples of such animal groups are:
- Sea anemones
- Marine mollusks (example: Elysia viridis & Elysia chlorotica )
- Fungi photosynthesis (Lichens)
When such symbiotic relationships are established, it’s sometimes observed that some genes of the plant cell’s nucleus get transferred to the animal cell . (Observed in some slugs).

Origin of Chloroplasts
Such symbiosis is popularly claimed to be the source of chloroplast evolution. As we notice many similarities between the photosynthetic bacteria and chloroplasts, the evolution of chloroplasts is often hinted to have occurred from these bacteria. Some of the common features between the 2 are:
- Circular chromosome
- Prokaryotic-type ribosome
- A similar set of proteins in the photosynthetic reaction center
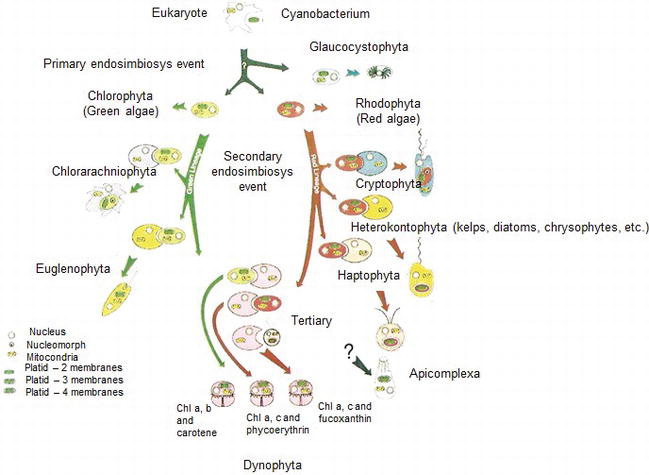
It is for all these commonalities the “ endosymbiotic theory ” had been proposed for the evolution of chloroplasts and mitochondria in the eukaryotic cells. According to the endosymbiotic theory, the early eukaryotic cells are believed to have acquired the photosynthetic bacteria by the process of endocytosis). Those early eukaryotic cells after acquiring the photosynthetic bacteria transformed to be self-sustainable and became the “first plant cells”. (Mitochondria photosynthesis is true, they are associated with respiration!)
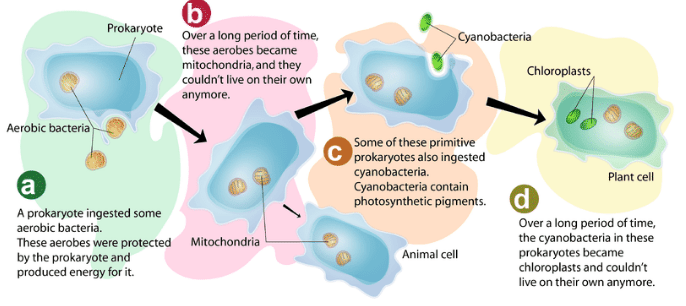
Photosynthetic eukaryotic lineages
Photosynthetic eukaryotic lineages include:
- Glaucophytes
- Chlorophytes
- Rhodophytes
- Cryptophytes (some clades)
- Haptophytes (some clades)
- Dinoflagellates & chromerids
- Euglenids—clade Excavata (unicellular)
Cyanobacteria and the evolution of photosynthesis
Almost all the prokaryotes carry out anoxygenic photosynthesis in contrast to cyanobacteria, which perform oxygenic photosynthesis. This ability to carry out oxygenic photosynthesis is speculated to have evolved at least 2450–2320 million years ago. The first photosynthetic cyanobacteria might not have been oxygenic as Earth’s atmosphere had no oxygen then.
This topic still requires more scientific study to bring out conclusive results. From the paleontological evidence, it is claimed that the 1st cyanobacteria evolved around 2000 Ma.
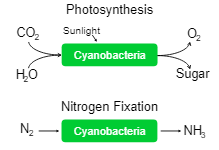
For the initial years of the Earth’s oxygen-rich environment (after the oxygen-evolving mechanism evolved), cyanobacteria are claimed to be the “principal primary producers of oxygen”. Even to date, cyanobacteria have been proven vital for marine ecosystems. They’re the primary producers of oxygen in oceans.
Cyanobacteria also fix nitrogen electrons fixation and play a role in biological nitrogen cycles.
Experimental History
We will list the long experimental history in deciphering the extensive photosynthesis process through the ages.
Discovery, Refinements, and Development of the concept
Find out the discovery, refinements, and development of photosynthesis as summarized in the table below:
C3 : C4 photosynthesis research
Several studies were conducted using isotopes of radioactive elements to identify the various aspects of the photosynthetic process. A number of organisms like Chlorella , Stellaria media, Cladophora, Spirogyra, Rhodopseudomonas , sulfur bacteria, green plants like maize, etc have been used to understand the photosynthesis process over the years. Gas exchange studies, isotopic studies, light spectrum studies, radioactive studies, plant anatomical and physiological studies, studies involving roles of carbon dioxide and water, etc have all together opened the gates for our deeper understanding of this topic.
The 3 main factors that directly affect the photosynthesis process are:
- Light irradiance and wavelength
- Carbon dioxide concentration
Temperature
Although there are many more corollary factors, these 3 are the most important ones.
Light intensity (irradiance), wavelength, and temperature
Light is an essential factor for photosynthesis. It directly affects the rate of it. There are 3 different parameters that we should look into:
- Sciophytes : Grow under “diffuse” light. Example: Oxalis
- Heliophytes: Grow under “direct: light. Example: Dalbergia
- Light quality: PAR as previously discussed is the quality or the fraction of light energy that is ‘photosynthetically active’ in nature. It ranges from 400-700nm in wavelength.
- Duration of light: This parameter doesn’t affect the rate of photosynthesis but affects the total photosynthetic output.
Carbon dioxide levels and photorespiration
Carbon dioxide concentration is the major factor in determining the rate of photosynthesis. There is no carbon-dioxide enriching system in C3 plants like the C4 plants. So, if you increase the concentration of CO 2 in the system, the photosynthetic rate of C3 plants will increase as the CO 2 concentration increases. On the other hand, the photosynthetic yield of the C4 plant won’t increase in such a scenario.
- CO 2 Compensation Point: A stage in CO 2 concentration when there’s no absorption of CO 2 by the illuminated plant part.
Featuring… “The curious case of RuBisCO and PEP Carboxylase”
Imagine an equal concentration (50-50%) of the two isotopes of carbon, C-12 and C-13, in the form of 12CO 2 and 13CO 2 , made available to both C3 and C4 plants. Now, can you tell which isotope of the carbon will be fixed more or less by the two types of photosynthetic organisms? Can you guess if there would be a “preferable” isotope between the two? Do you think C3 plants will fix the 12CO 2 and 13CO 2 equally or unequally? Or do you think the 12CO 2 and 13CO 2 incorporation would have a biased ratio in any of the two (C3/C4 plants)????
The answer to this lies in the major carbon fixing enzyme involved.
- C3 plants: Major C-fixing enzyme is RuBisCo and RuBisCo has a “discriminatory ability” to preferably fix 12CO 2 and not 13CO2. Hence, you will find more 12CO 2 fixed than 13CO 2 in the C3 plants.
- C4 plants: Major C-fixing enzyme is not RuBisCo but PEP Carboxylase . PEP Carboxylase has “no discriminatory ability”. So, you’ll find an almost equal proportion of 12CO 2 and 13CO 2 getting fixed in C4 plants. So, in comparison to C3 plants, the chances of getting 13CO 2 fixed are more in C4 plants.
Choose the best answer.
Send Your Results (Optional)

- Rutherford, A.W., Faller, P. (Jan 2003). “Photosystem II: evolutionary perspectives”. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 358 (1429): 245–253. doi:10.1098/rstb.2002.1186. PMC 1693113. PMID 12594932.
- Arnon, D.I., Whatley, F.R., Allen, M.B. (1954). “Photosynthesis by isolated chloroplasts. II. Photophosphorylation, the conversion of light into phosphate bond energy”. Journal of the American Chemical Society. 76 (24): 6324–6329. doi:10.1021/ja01653a025.
- Ehrenberg, R. (2017-12-15). “The photosynthesis fix”. Knowable Magazine. Annual Reviews. doi:10.1146/knowable-121917-115502. Retrieved 2018-04-03.
- El-Sharkawy, M.A., Hesketh, J.D. (1965). “Photosynthesis among species in relation to characteristics of leaf anatomy and CO 2 diffusion resistances”. Crop Sci. 5 (6): 517–521. doi:10.2135/cropsci1965.0011183x000500060010x.
- Earl, H., Said Ennahli, S. (2004). “Estimating photosynthetic electron transport via chlorophyll fluorometry without Photosystem II light saturation”. Photosynthesis Research. 82 (2): 177–186. doi:10.1007/s11120-004-1454-3. PMID 16151873. S2CID 291238.
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.
Last updated on July 15th, 2022
You will also like...
Photosynthesis – Photolysis and Carbon Fixation
Plant Metabolism

Lights’ Effect on Growth

Freshwater Communities & Lentic Waters

Plant Water Regulation
Related articles....

Macrophytes

Unusual Plants
This page has been archived and is no longer updated
Photosynthetic Cells
Cells get nutrients from their environment, but where do those nutrients come from? Virtually all organic material on Earth has been produced by cells that convert energy from the Sun into energy-containing macromolecules. This process, called photosynthesis, is essential to the global carbon cycle and organisms that conduct photosynthesis represent the lowest level in most food chains (Figure 1).
What Is Photosynthesis? Why Is it Important?
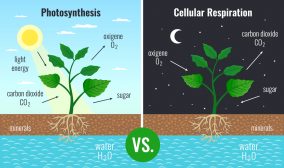
Most living things depend on photosynthetic cells to manufacture the complex organic molecules they require as a source of energy. Photosynthetic cells are quite diverse and include cells found in green plants, phytoplankton, and cyanobacteria. During the process of photosynthesis, cells use carbon dioxide and energy from the Sun to make sugar molecules and oxygen. These sugar molecules are the basis for more complex molecules made by the photosynthetic cell, such as glucose. Then, via respiration processes, cells use oxygen and glucose to synthesize energy-rich carrier molecules, such as ATP, and carbon dioxide is produced as a waste product. Therefore, the synthesis of glucose and its breakdown by cells are opposing processes.
However, photosynthesis doesn't just drive the carbon cycle — it also creates the oxygen necessary for respiring organisms. Interestingly, although green plants contribute much of the oxygen in the air we breathe, phytoplankton and cyanobacteria in the world's oceans are thought to produce between one-third and one-half of atmospheric oxygen on Earth.
What Cells and Organelles Are Involved in Photosynthesis?
Chlorophyll A is the major pigment used in photosynthesis, but there are several types of chlorophyll and numerous other pigments that respond to light, including red, brown, and blue pigments. These other pigments may help channel light energy to chlorophyll A or protect the cell from photo-damage. For example, the photosynthetic protists called dinoflagellates, which are responsible for the "red tides" that often prompt warnings against eating shellfish, contain a variety of light-sensitive pigments, including both chlorophyll and the red pigments responsible for their dramatic coloration.
What Are the Steps of Photosynthesis?
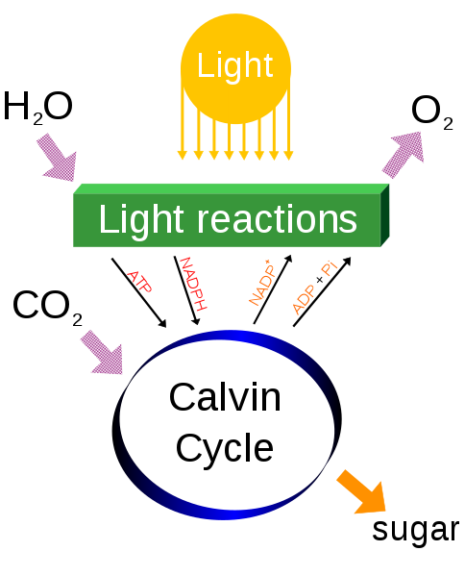
Photosynthesis consists of both light-dependent reactions and light-independent reactions . In plants, the so-called "light" reactions occur within the chloroplast thylakoids, where the aforementioned chlorophyll pigments reside. When light energy reaches the pigment molecules, it energizes the electrons within them, and these electrons are shunted to an electron transport chain in the thylakoid membrane. Every step in the electron transport chain then brings each electron to a lower energy state and harnesses its energy by producing ATP and NADPH. Meanwhile, each chlorophyll molecule replaces its lost electron with an electron from water; this process essentially splits water molecules to produce oxygen (Figure 5).
Once the light reactions have occurred, the light-independent or "dark" reactions take place in the chloroplast stroma. During this process, also known as carbon fixation, energy from the ATP and NADPH molecules generated by the light reactions drives a chemical pathway that uses the carbon in carbon dioxide (from the atmosphere) to build a three-carbon sugar called glyceraldehyde-3-phosphate (G3P). Cells then use G3P to build a wide variety of other sugars (such as glucose) and organic molecules. Many of these interconversions occur outside the chloroplast, following the transport of G3P from the stroma. The products of these reactions are then transported to other parts of the cell, including the mitochondria, where they are broken down to make more energy carrier molecules to satisfy the metabolic demands of the cell. In plants, some sugar molecules are stored as sucrose or starch.
This page appears in the following eBook
Topic rooms within Cell Biology

Within this Subject (25)
- Basic (25)
Other Topic Rooms
- Gene Inheritance and Transmission
- Gene Expression and Regulation
- Nucleic Acid Structure and Function
- Chromosomes and Cytogenetics
- Evolutionary Genetics
- Population and Quantitative Genetics
- Genes and Disease
- Genetics and Society
- Cell Origins and Metabolism
- Proteins and Gene Expression
- Subcellular Compartments
- Cell Communication
- Cell Cycle and Cell Division
© 2014 Nature Education
- Press Room |
- Terms of Use |
- Privacy Notice |

Visual Browse
What is photosynthesis?
Photosynthesis is the process plants, algae and some bacteria use to turn sunlight, carbon dioxide and water into sugar and oxygen.

- Photosynthetic processes
- Photosynthesis equation
- The carbon exchange
- How do plants absorb sunlight?
- Location of photosynthesis
Light-dependent reactions
- The Calvin cycle
Types of photosynthesis
Additional resources.
Photosynthesis is the process used by plants, algae and some bacteria to turn sunlight into energy. The process chemically converts carbon dioxide (CO2) and water into food (sugars) and oxygen . The chemical reaction often relies on a pigment called chlorophyll, which gives plants their green color. Photosynthesis is also the reason our planet is blanketed in an oxygen-rich atmosphere.
Types of photosynthetic processes
There are two types of photosynthesis: oxygenic and anoxygenic. They both follow very similar principles, but the former is the most common and is seen in plants, algae and cyanobacteria.
During oxygenic photosynthesis, light energy transfers electrons from water (H2O) taken up by plant roots to CO2 to produce carbohydrates . In this transfer, the CO2 is "reduced," or receives electrons, and the water is "oxidized," or loses electrons. Oxygen is produced along with carbohydrates.
This process creates a balance on Earth, in which the carbon dioxide produced by breathing organisms as they consume oxygen in respiration is converted back into oxygen by plants, algae and bacteria.
Anoxygenic photosynthesis, meanwhile, uses electron donors that are not water and the process does not generate oxygen, according to "Anoxygenic Photosynthetic Bacteria" by LibreTexts . The process typically occurs in bacteria such as green sulfur bacteria and phototrophic purple bacteria.
The Photosynthesis equation
Though both types of photosynthesis are complex, multistep affairs, the overall process can be neatly summarized as a chemical equation.
The oxygenic photosynthesis equation is:
6CO2 + 12H2O + Light Energy → C6H12O6 + 6O2 + 6H2O
Here, six molecules of carbon dioxide (CO2) combine with 12 molecules of water (H2O) using light energy. The end result is the formation of a single carbohydrate molecule (C6H12O6, or glucose) along with six molecules each of oxygen and water.
Similarly, the various anoxygenic photosynthesis reactions can be represented as a single generalized formula:
CO2 + 2H2A + Light Energy → [CH2O] + 2A + H2O
The letter A in the equation is a variable, and H2A represents the potential electron donor. For example, "A" may represent sulfur in the electron donor hydrogen sulfide (H2S), according to medical and life sciences news site News Medical Life Sciences .
How is carbon dioxide and oxygen exchanged?
Plants absorb CO2 from the surrounding air and release water and oxygen via microscopic pores on their leaves called stomata.
When stomata open, they let in CO2; however, while open, the stomata release oxygen and let water vapor escape. Stomata close to prevent water loss, but that means the plant can no longer gain CO2 for photosynthesis. This tradeoff between CO2 gain and water loss is a particular problem for plants growing in hot, dry environments.
How do plants absorb sunlight for photosynthesis?
Plants contain special pigments that absorb the light energy needed for photosynthesis.
Chlorophyll is the primary pigment used for photosynthesis and gives plants their green color, according to science education site Nature Education . Chlorophyll absorbs red and blue light and reflects green light. Chlorophyll is a large molecule and takes a lot of resources to make; as such, it breaks down towards the end of the leaf's life, and most of the pigment's nitrogen (one of the building blocks of chlorophyll) is resorbed back into the plant, When leaves lose their chlorophyll in the fall, other leaf pigments such as carotenoids and anthocyanins begin to show. While carotenoids primarily absorb blue light and reflect yellow, anthocyanins absorb blue-green light and reflect red light, according to Harvard University's The Harvard Forest .
Pigment molecules are associated with proteins, which allow them the flexibility to move toward light and toward one another. A large collection of 100 to 5,000 pigment molecules constitutes an "antenna," according to an article by Wim Vermaas , a professor at Arizona State University. These structures effectively capture light energy from the sun, in the form of photons.
The situation is a little different for bacteria. While cyanobacteria contain chlorophyll, other bacteria, for example, purple bacteria and green sulfur bacteria, contain bacteriochlorophyll to absorb light for anoxygenic photosynthesis, according to " Microbiology for Dummies " (For Dummies, 2019).
Related: What if humans had photosynthetic skin?
Where in the plant does photosynthesis take place?

Photosynthesis occurs in chloroplasts, a type of plastid (an organelle with a membrane) that contains chlorophyll and is primarily found in plant leaves.
Chloroplasts are similar to mitochondria , the energy powerhouses of cells, in that they have their own genome, or collection of genes, contained within circular DNA. These genes encode proteins that are essential to the organelle and to photosynthesis.
Inside chloroplasts are plate-shaped structures called thylakoids that are responsible for harvesting photons of light for photosynthesis, according to the biology terminology website Biology Online . The thylakoids are stacked on top of each other in columns known as grana. In between the grana is the stroma — a fluid containing enzymes, molecules and ions, where sugar formation takes place.
Ultimately, light energy must be transferred to a pigment-protein complex that can convert it to chemical energy, in the form of electrons. In plants, light energy is transferred to chlorophyll pigments. The conversion to chemical energy is accomplished when a chlorophyll pigment expels an electron, which can then move on to an appropriate recipient.
The pigments and proteins that convert light energy to chemical energy and begin the process of electron transfer are known as reaction centers.
When a photon of light hits the reaction center, a pigment molecule such as chlorophyll releases an electron.
The released electron escapes through a series of protein complexes linked together, known as an electron transport chain. As it moves through the chain, it generates the energy to produce ATP (adenosine triphosphate, a source of chemical energy for cells) and NADPH — both of which are required in the next stage of photosynthesis in the Calvin cycle. The "electron hole" in the original chlorophyll pigment is filled by taking an electron from water. This splitting of water molecules releases oxygen into the atmosphere.
Light-independent reactions: The Calvin cycle
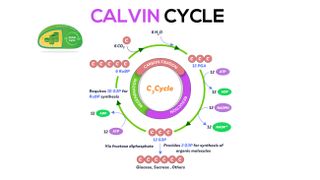
The Calvin cycle is the three-step process that generates sugars for the plant, and is named after Melvin Calvin , the Nobel Prize -winning scientist who discovered it decades ago. The Calvin cycle uses the ATP and NADPH produced in chlorophyll to generate carbohydrates. It takes plate in the plant stroma, the inner space in chloroplasts.
In the first step of this cycle, called carbon fixation, an enzyme called RuBP carboxylase/oxygenase, also known as rubiso, helps incorporate CO2 into an organic molecule called 3-phosphoglyceric acid (3-PGA). In the process, it breaks off a phosphate group on six ATP molecules to convert them to ADP, releasing energy in the process, according to LibreTexts.
In the second step, 3-PGA is reduced, meaning it takes electrons from six NADPH molecules and produces two glyceraldehyde 3-phosphate (G3P) molecules.
One of these G3P molecules leaves the Calvin cycle to do other things in the plant. The remaining G3P molecules go into the third step, which is regenerating rubisco. In between these steps, the plant produces glucose, or sugar.
Three CO2 molecules are needed to produce six G3P molecules, and it takes six turns around the Calvin cycle to make one molecule of carbohydrate, according to educational website Khan Academy.
There are three main types of photosynthetic pathways: C3, C4 and CAM. They all produce sugars from CO2 using the Calvin cycle, but each pathway is slightly different.

C3 photosynthesis
Most plants use C3 photosynthesis, according to the photosynthesis research project Realizing Increased Photosynthetic Efficiency (RIPE) . C3 plants include cereals (wheat and rice), cotton, potatoes and soybeans. This process is named for the three-carbon compound 3-PGA that it uses during the Calvin cycle.
C4 photosynthesis
Plants such as maize and sugarcane use C4 photosynthesis. This process uses a four-carbon compound intermediate (called oxaloacetate) which is converted to malate , according to Biology Online. Malate is then transported into the bundle sheath where it breaks down and releases CO2, which is then fixed by rubisco and made into sugars in the Calvin cycle (just like C3 photosynthesis). C4 plants are better adapted to hot, dry environments and can continue to fix carbon even when their stomata are closed (as they have a clever storage solution), according to Biology Online.
CAM photosynthesis
Crassulacean acid metabolism (CAM) is found in plants adapted to very hot and dry environments, such as cacti and pineapples, according to the Khan Academy. When stomata open to take in CO2, they risk losing water to the external environment. Because of this, plants in very arid and hot environments have adapted. One adaptation is CAM, whereby plants open stomata at night (when temperatures are lower and water loss is less of a risk). According to the Khan Academy, CO2 enters the plants via the stomata and is fixed into oxaloacetate and converted into malate or another organic acid (like in the C4 pathway). The CO2 is then available for light-dependent reactions in the daytime, and stomata close, reducing the risk of water loss.
Discover more facts about photosynthesis with the educational science website sciencing.com . Explore how leaf structure affects photosynthesis with The University of Arizona . Learn about the different ways photosynthesis can be measured with the educational science website Science & Plants for Schools .
This article was updated by Live Science managing editor Tia Ghose on Nov. 3, 2022.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.

Daisy Dobrijevic joined Space.com in February 2022 as a reference writer having previously worked for our sister publication All About Space magazine as a staff writer. Before joining us, Daisy completed an editorial internship with the BBC Sky at Night Magazine and worked at the National Space Centre in Leicester, U.K., where she enjoyed communicating space science to the public. In 2021, Daisy completed a PhD in plant physiology and also holds a Master's in Environmental Science, she is currently based in Nottingham, U.K.
Are kale, broccoli and Brussels sprouts really all the same plant?
390 million-year-old fossilized forest is the oldest ever discovered
Pluto's huge white 'heart' has a surprisingly violent origin, new study suggests
Most Popular
- 2 Underwater mountain range off Easter Island hosts creatures unknown to science, expedition reveals
- 3 'Gambling with your life': Experts weigh in on dangers of the Wim Hof method
- 4 Eclipse from space: See the moon's shadow race across North America at 1,500 mph in epic satellite footage
- 5 Superfast drone fitted with new 'rotating detonation rocket engine' approaches the speed of sound
- 2 32 astonishing ancient burials, from 'vampire' decapitations to riches for the afterlife
- 3 World's fastest camera captures footage at 156 trillion frames per second

- Why Does Water Expand When It Freezes
- Gold Foil Experiment
- Faraday Cage
- Oil Drop Experiment
- Magnetic Monopole
- Why Do Fireflies Light Up
- Types of Blood Cells With Their Structure, and Functions
- The Main Parts of a Plant With Their Functions
- Parts of a Flower With Their Structure and Functions
- Parts of a Leaf With Their Structure and Functions
- Why Does Ice Float on Water
- Why Does Oil Float on Water
- How Do Clouds Form
- What Causes Lightning
- How are Diamonds Made
- Types of Meteorites
- Types of Volcanoes
- Types of Rocks
Photosynthesis
What is photosynthesis.
It is the process by which green plants, algae, and certain bacteria convert light energy from the sun into chemical energy that is used to make glucose. The word ‘photosynthesis’ is derived from the Greek word phōs, meaning ‘light’ and synthesis meaning ‘combining together.’
Jan Ingenhousz, the Dutch-born British physician and scientist, discovered the process of photosynthesis.
Where does Photosynthesis Occur
Photosynthesis takes place mainly in the leaves of green plants and also in the stems of herbaceous plants as they also contain chlorophyll. Sometimes it also occurs in roots that contain chlorophyll like in water chestnut and Heart-leaved moonseed. Apart from plants, photosynthesis is also found to occur in blue-green algae.
What Happens During Photosynthesis
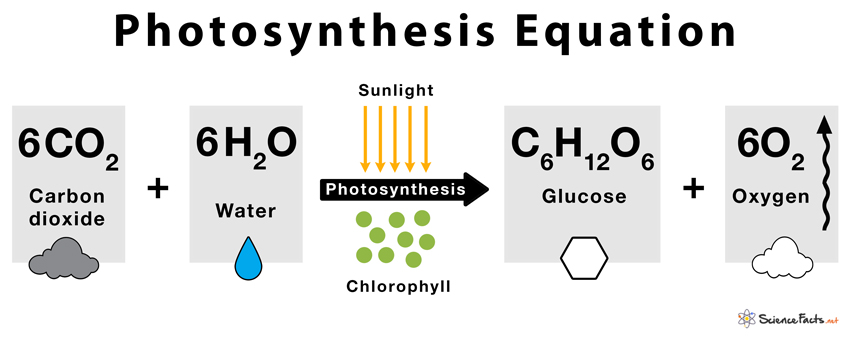
It involves a chemical reaction where water, carbon dioxide, chlorophyll, and solar energy are utilized as raw materials (inputs) to produce glucose, oxygen, and water (outputs).
Stages of the Process
Photosynthesis occurs in two stages:
1) The Light-dependent Reaction
- Takes place in the thylakoid membranes of chloroplasts only during the day in the presence of sunlight
- High-energy phosphate molecules adenosine triphosphate ( ATP ) and the reducing agent NADPH are produced with the help of electron transport chain
2) The Light-independent or Dark Reaction ( Calvin cycle )
- Takes place in the stroma of chloroplast in the absence of light that helps to fix carbon
- ATP and NADPH produced in the light reaction are utilized along with carbon dioxide to produce sugar in the form of glucose
Factors Affecting the Rate of Photosynthesis
- Intensity of Light: The higher intensity of light increases the rate of photosynthesis
- Temperature: Warmer the temperature, higher the rate of photosynthesis. The rate is highest between the temperatures of 25° to 35° C, after which it starts to decrease
- Concentration of Carbon dioxide: Higher concentration of carbon dioxide increases the rate of photosynthesis until it reaches a certain point, beyond which no further effects are found
Although all the above factors together interact to affect the rate of photosynthesis, each of them individually is also capable of directly influencing the process without the other factors and thus called limiting factors.
Importance of Photosynthesis
It serves two main purposes that are essential to support life on earth:
- Producing food for organisms that depend on others for their nutrition such as humans along with all other animals
- Synthesizing oxygen by replacing carbon dioxide in the atmosphere
Ans. Photosynthesis is an endothermic reaction because it absorbs the heat of the sun to carry out the process.
Ans. The oxygen in photosynthesis comes from splitting the water molecules.
Ans. Chlorophyll is the main light-absorbing pigment in photosynthesis.
Ans. The role of water is to provide oxygen in the form of oxygen gas to the atmosphere.
Ans. Sunlight is the source of energy that drives photosynthesis.
Ans. The easiest way to measure the rate of photosynthesis is to quantify the carbon dioxide or oxygen levels using a data logger. The rate of photosynthesis can also be measured by determining the increase in the plant ’s biomass (weight).
Ans. Photosynthesis is an energy-requiring process occurring only in green plants, algae, and certain bacteria that utilizes carbon dioxide and water to produce food in the form of carbohydrates. In contrast, cellular respiration is an energy-releasing process found in all living organisms where oxygen and glucose are utilized to produce carbon dioxide and water.
Ans. Glucose produced in photosynthesis is used in cellular respiration to make ATP.
Article was last reviewed on Tuesday, April 21, 2020
Related articles
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Popular Articles

Join our Newsletter
Fill your E-mail Address
Related Worksheets
- Privacy Policy
© 2024 ( Science Facts ). All rights reserved. Reproduction in whole or in part without permission is prohibited.
- Biology Article
Photosynthesis
Photosynthesis is a process by which phototrophs convert light energy into chemical energy, which is later used to fuel cellular activities. The chemical energy is stored in the form of sugars, which are created from water and carbon dioxide.

Table of Contents
- What is Photosynthesis?
- Site of photosynthesis
Photosynthesis definition states that the process exclusively takes place in the chloroplasts through photosynthetic pigments such as chlorophyll a, chlorophyll b, carotene and xanthophyll. All green plants and a few other autotrophic organisms utilize photosynthesis to synthesize nutrients by using carbon dioxide, water and sunlight. The by-product of the photosynthesis process is oxygen.Let us have a detailed look at the process, reaction and importance of photosynthesis.
What Is Photosynthesis in Biology?
The word “ photosynthesis ” is derived from the Greek words phōs (pronounced: “fos”) and σύνθεσις (pronounced: “synthesis “) Phōs means “light” and σύνθεσις means, “combining together.” This means “ combining together with the help of light .”
Photosynthesis also applies to other organisms besides green plants. These include several prokaryotes such as cyanobacteria, purple bacteria and green sulfur bacteria. These organisms exhibit photosynthesis just like green plants.The glucose produced during photosynthesis is then used to fuel various cellular activities. The by-product of this physio-chemical process is oxygen.
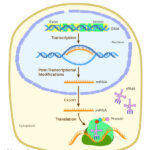
A visual representation of the photosynthesis reaction
- Photosynthesis is also used by algae to convert solar energy into chemical energy. Oxygen is liberated as a by-product and light is considered as a major factor to complete the process of photosynthesis.
- Photosynthesis occurs when plants use light energy to convert carbon dioxide and water into glucose and oxygen. Leaves contain microscopic cellular organelles known as chloroplasts.
- Each chloroplast contains a green-coloured pigment called chlorophyll. Light energy is absorbed by chlorophyll molecules whereas carbon dioxide and oxygen enter through the tiny pores of stomata located in the epidermis of leaves.
- Another by-product of photosynthesis is sugars such as glucose and fructose.
- These sugars are then sent to the roots, stems, leaves, fruits, flowers and seeds. In other words, these sugars are used by the plants as an energy source, which helps them to grow. These sugar molecules then combine with each other to form more complex carbohydrates like cellulose and starch. The cellulose is considered as the structural material that is used in plant cell walls.
Where Does This Process Occur?
Chloroplasts are the sites of photosynthesis in plants and blue-green algae. All green parts of a plant, including the green stems, green leaves, and sepals – floral parts comprise of chloroplasts – green colour plastids. These cell organelles are present only in plant cells and are located within the mesophyll cells of leaves.
Also Read: Photosynthesis Early Experiments
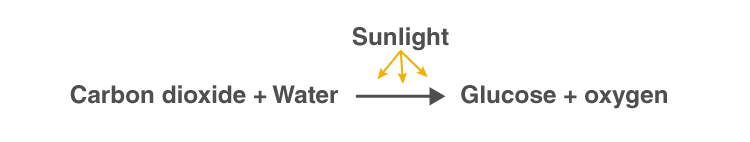
Photosynthesis Equation
Photosynthesis reaction involves two reactants, carbon dioxide and water. These two reactants yield two products, namely, oxygen and glucose. Hence, the photosynthesis reaction is considered to be an endothermic reaction. Following is the photosynthesis formula:
Unlike plants, certain bacteria that perform photosynthesis do not produce oxygen as the by-product of photosynthesis. Such bacteria are called anoxygenic photosynthetic bacteria. The bacteria that do produce oxygen as a by-product of photosynthesis are called oxygenic photosynthetic bacteria.
Structure Of Chlorophyll
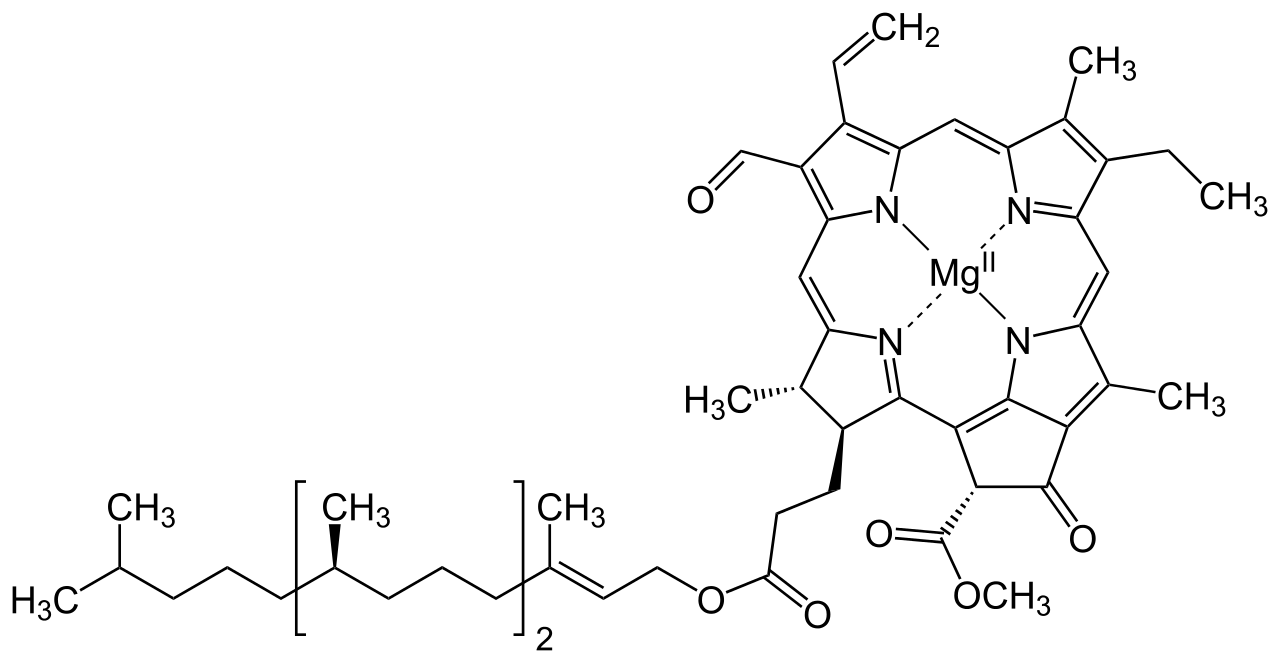
The structure of Chlorophyll consists of 4 nitrogen atoms that surround a magnesium atom. A hydrocarbon tail is also present. Pictured above is chlorophyll- f, which is more effective in near-infrared light than chlorophyll- a
Chlorophyll is a green pigment found in the chloroplasts of the plant cell and in the mesosomes of cyanobacteria. This green colour pigment plays a vital role in the process of photosynthesis by permitting plants to absorb energy from sunlight. Chlorophyll is a mixture of chlorophyll- a and chlorophyll- b .Besides green plants, other organisms that perform photosynthesis contain various other forms of chlorophyll such as chlorophyll- c1 , chlorophyll- c2 , chlorophyll- d and chlorophyll- f .
Also Read: Biological Pigments
Process Of Photosynthesis
At the cellular level, the photosynthesis process takes place in cell organelles called chloroplasts. These organelles contain a green-coloured pigment called chlorophyll, which is responsible for the characteristic green colouration of the leaves.
As already stated, photosynthesis occurs in the leaves and the specialized cell organelles responsible for this process is called the chloroplast. Structurally, a leaf comprises a petiole, epidermis and a lamina. The lamina is used for absorption of sunlight and carbon dioxide during photosynthesis.
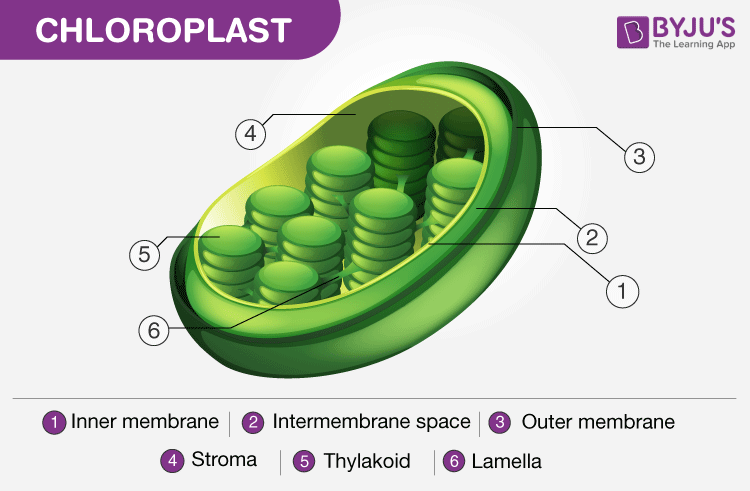
Structure of Chloroplast. Note the presence of the thylakoid
“Photosynthesis Steps:”
- During the process of photosynthesis, carbon dioxide enters through the stomata, water is absorbed by the root hairs from the soil and is carried to the leaves through the xylem vessels. Chlorophyll absorbs the light energy from the sun to split water molecules into hydrogen and oxygen.
- The hydrogen from water molecules and carbon dioxide absorbed from the air are used in the production of glucose. Furthermore, oxygen is liberated out into the atmosphere through the leaves as a waste product.
- Glucose is a source of food for plants that provide energy for growth and development , while the rest is stored in the roots, leaves and fruits, for their later use.
- Pigments are other fundamental cellular components of photosynthesis. They are the molecules that impart colour and they absorb light at some specific wavelength and reflect back the unabsorbed light. All green plants mainly contain chlorophyll a, chlorophyll b and carotenoids which are present in the thylakoids of chloroplasts. It is primarily used to capture light energy. Chlorophyll-a is the main pigment.
The process of photosynthesis occurs in two stages:
- Light-dependent reaction or light reaction
- Light independent reaction or dark reaction
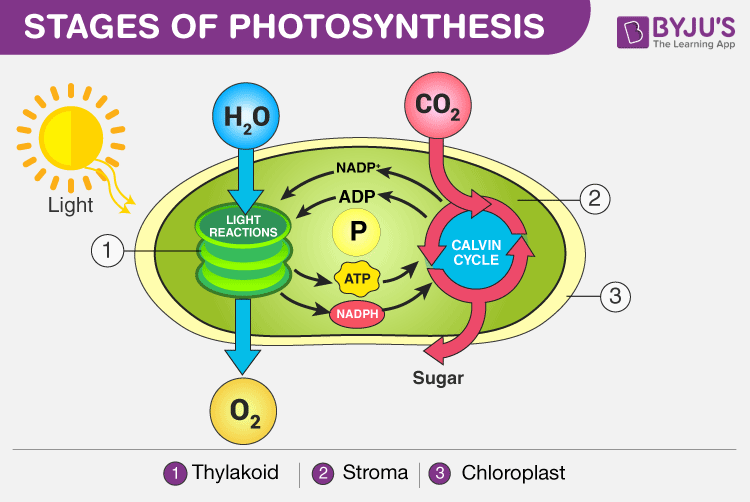
Stages of Photosynthesis in Plants depicting the two phases – Light reaction and Dark reaction
Light Reaction of Photosynthesis (or) Light-dependent Reaction
- Photosynthesis begins with the light reaction which is carried out only during the day in the presence of sunlight. In plants, the light-dependent reaction takes place in the thylakoid membranes of chloroplasts.
- The Grana, membrane-bound sacs like structures present inside the thylakoid functions by gathering light and is called photosystems.
- These photosystems have large complexes of pigment and proteins molecules present within the plant cells, which play the primary role during the process of light reactions of photosynthesis.
- There are two types of photosystems: photosystem I and photosystem II.
- Under the light-dependent reactions, the light energy is converted to ATP and NADPH, which are used in the second phase of photosynthesis.
- During the light reactions, ATP and NADPH are generated by two electron-transport chains, water is used and oxygen is produced.
The chemical equation in the light reaction of photosynthesis can be reduced to:
2H 2 O + 2NADP+ + 3ADP + 3Pi → O 2 + 2NADPH + 3ATP
Dark Reaction of Photosynthesis (or) Light-independent Reaction
- Dark reaction is also called carbon-fixing reaction.
- It is a light-independent process in which sugar molecules are formed from the water and carbon dioxide molecules.
- The dark reaction occurs in the stroma of the chloroplast where they utilize the NADPH and ATP products of the light reaction.
- Plants capture the carbon dioxide from the atmosphere through stomata and proceed to the Calvin photosynthesis cycle.
- In the Calvin cycle , the ATP and NADPH formed during light reaction drive the reaction and convert 6 molecules of carbon dioxide into one sugar molecule or glucose.
The chemical equation for the dark reaction can be reduced to:
3CO 2 + 6 NADPH + 5H 2 O + 9ATP → G3P + 2H+ + 6 NADP+ + 9 ADP + 8 Pi
* G3P – glyceraldehyde-3-phosphate
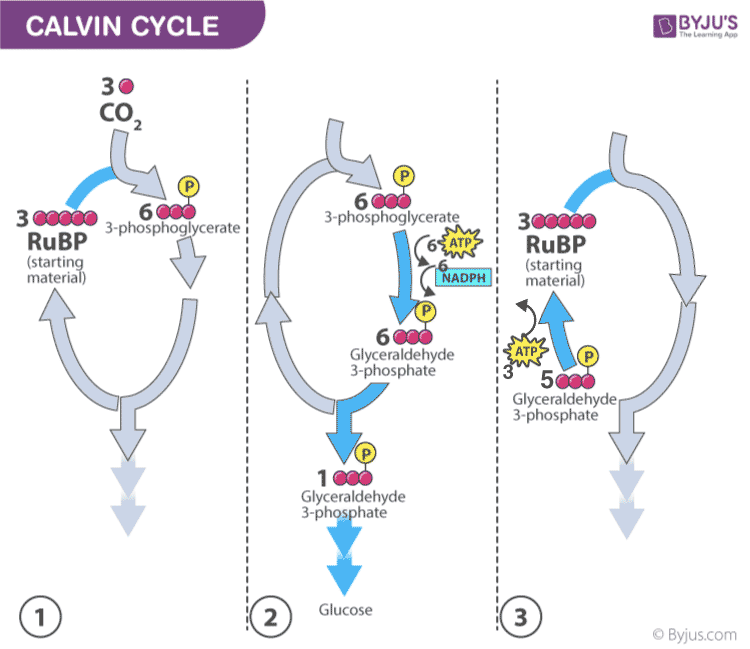
Calvin photosynthesis Cycle (Dark Reaction)
Also Read: Cyclic And Non-Cyclic Photophosphorylation
Importance of Photosynthesis
- Photosynthesis is essential for the existence of all life on earth. It serves a crucial role in the food chain – the plants create their food using this process, thereby, forming the primary producers.
- Photosynthesis is also responsible for the production of oxygen – which is needed by most organisms for their survival.
Frequently Asked Questions
1. what is photosynthesis explain the process of photosynthesis., 2. what is the significance of photosynthesis, 3. list out the factors influencing photosynthesis., 4. what are the different stages of photosynthesis, 5. what is the calvin cycle, 6. write down the photosynthesis equation..

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Biology related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
very useful
It’s very helpful ☺️
Please What Is Meant By 300-400 PPM
PPM stands for Parts-Per-Million. It corresponds to saying that 300 PPM of carbon dioxide indicates that if one million gas molecules are counted, 300 out of them would be carbon dioxide. The remaining nine hundred ninety-nine thousand seven hundred are other gas molecules.
Thank you very much Byju’s! I couldn’t find the answer anywhere. But luckily I hit upon this website. Awesome explanation and illustration.
byjus = Wow!
It helps me a lot thank you
Thanks in a million I love Byjus!
Super Byjus
Thanks helped a lot
Very interesting and helpful site.
Nice it is very uesful
It’s very useful 👍 Thank you Byju’s
Thank you very much Byju’s! I couldn’t find the answer anywhere. But luckily I hit upon this website. Awesome explanation and illustration.
Thank you BYJU’S for helping me in further clarifying my concepts
Excellent material easy to understand
Indeed, it’s precise and understandable. I like it.
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

- Distance Learning
- Director's Circle
- Sustainability
- Smithsonian Science for the Classroom
- Smithsonian Science Stories
- STC Curriculum
- Smithsonian Science for Global Goals
- Explore Smithsonian
- Free Resources
- Smithsonian Science for Makerspaces
- Girls and Women in STEM
- Smithsonian Science for Computational Thinking
- Women in STEM eBook Series
- Space STEM Resources
- Space STEM Career Resources
- Smithsonian Science for NC and SC Classrooms
- Smithsonian Science Education Academies for Teachers
- Good Thinking!
- Quick Tips for Teachers
- English Learners in STEM
- Upcoming Events
- Action Planning Institute
- Building Awareness for Science Education
- Strategic Planning Institute
- Next Steps Institute
- STEM Diversity
- Zero Barriers in STEM
- Network for Emergent Socio-Scientific Thinking (NESST)
- Where We Are
- Smithsonian Science for Summer School (S4)
- Always Thinking Like A Scientist (ATLAS)
- STEM Literacy Project
- Success Stories
- France in Focus
- Smithsonian Science for Global Goals Research
- Smithsonian Youth STEM Exchange
- STEMvisions Blog
- What is Photosynthesis
When you get hungry, you grab a snack from your fridge or pantry. But what can plants do when they get hungry? You are probably aware that plants need sunlight, water, and a home (like soil) to grow, but where do they get their food? They make it themselves!
Plants are called autotrophs because they can use energy from light to synthesize, or make, their own food source. Many people believe they are “feeding” a plant when they put it in soil, water it, or place it outside in the Sun, but none of these things are considered food. Rather, plants use sunlight, water, and the gases in the air to make glucose, which is a form of sugar that plants need to survive. This process is called photosynthesis and is performed by all plants, algae, and even some microorganisms. To perform photosynthesis, plants need three things: carbon dioxide, water, and sunlight.
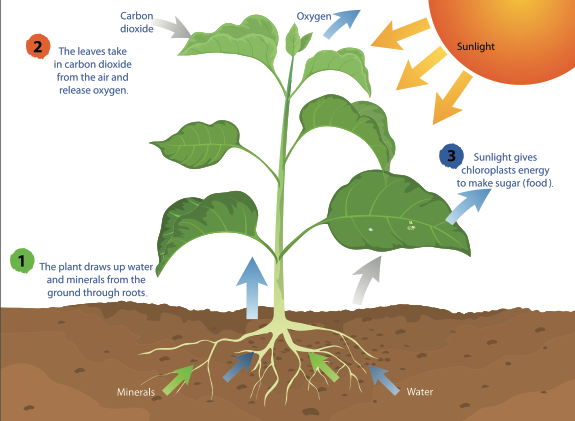
Just like you, plants need to take in gases in order to live. Animals take in gases through a process called respiration. During the respiration process, animals inhale all of the gases in the atmosphere, but the only gas that is retained and not immediately exhaled is oxygen. Plants, however, take in and use carbon dioxide gas for photosynthesis. Carbon dioxide enters through tiny holes in a plant’s leaves, flowers, branches, stems, and roots. Plants also require water to make their food. Depending on the environment, a plant’s access to water will vary. For example, desert plants, like a cactus, have less available water than a lilypad in a pond, but every photosynthetic organism has some sort of adaptation, or special structure, designed to collect water. For most plants, roots are responsible for absorbing water.
The last requirement for photosynthesis is an important one because it provides the energy to make sugar. How does a plant take carbon dioxide and water molecules and make a food molecule? The Sun! The energy from light causes a chemical reaction that breaks down the molecules of carbon dioxide and water and reorganizes them to make the sugar (glucose) and oxygen gas. After the sugar is produced, it is then broken down by the mitochondria into energy that can be used for growth and repair. The oxygen that is produced is released from the same tiny holes through which the carbon dioxide entered. Even the oxygen that is released serves another purpose. Other organisms, such as animals, use oxygen to aid in their survival.
If we were to write a formula for photosynthesis, it would look like this:
6CO 2 + 6H 2 O + Light energy → C 6 H 12 O 6 (sugar) + 6O 2
The whole process of photosynthesis is a transfer of energy from the Sun to a plant. In each sugar molecule created, there is a little bit of the energy from the Sun, which the plant can either use or store for later.
Imagine a pea plant. If that pea plant is forming new pods, it requires a large amount of sugar energy to grow larger. This is similar to how you eat food to grow taller and stronger. But rather than going to the store and buying groceries, the pea plant will use sunlight to obtain the energy to build sugar. When the pea pods are fully grown, the plant may no longer need as much sugar and will store it in its cells. A hungry rabbit comes along and decides to eat some of the plant, which provides the energy that allows the rabbit to hop back to its home. Where did the rabbit’s energy come from? Consider the process of photosynthesis. With the help of carbon dioxide and water, the pea pod used the energy from sunlight to construct the sugar molecules. When the rabbit ate the pea pod, it indirectly received energy from sunlight, which was stored in the sugar molecules in the plant.

Humans, other animals, fungi, and some microorganisms cannot make food in their own bodies like autotrophs, but they still rely on photosynthesis. Through the transfer of energy from the Sun to plants, plants build sugars that humans consume to drive our daily activities. Even when we eat things like chicken or fish, we are transferring energy from the Sun into our bodies because, at some point, one organism consumed a photosynthetic organism (e.g., the fish ate algae). So the next time you grab a snack to replenish your energy, thank the Sun for it!
This is an excerpt from the Structure and Function unit of our curriculum product line, Science and Technology Concepts TM (STC). Please visit our publisher, Carolina Biological , to learn more.
[BONUS FOR TEACHERS] Watch "Photosynthesis: Blinded by the Light" to explore student misconceptions about matter and energy in photosynthesis and strategies for eliciting student ideas to address or build on them.
Related Tags
View the discussion thread.
- Behind the Scenes
Popular Posts
- It’s All About the Tilt: Seasons Misconceptions Debunked
- Are All Snowflakes Really Different? The Science of Winter
- What Are Clouds?
- What is the Winter Solstice?
Featured Authors
- Brian Mandell, PhD
- Ashley Deese
- Kate Echevarria
- Katya Vines, PhD
- Jean Flanagan
- October (1)
- November (1)
- December (2)
- January (1)
- February (2)
- September (3)
- October (7)
- November (4)
- December (3)
- January (4)
- February (4)
- September (4)
- October (2)
- November (2)
- December (1)
- January (3)
- September (6)
- October (3)
- January (2)
- October (6)
- November (5)
- December (4)
- February (6)
- September (2)
- February (1)
- December (5)
- September (1)
- October (5)
- February (5)
- Terms of Use
- Google Plus

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

8.1: Overview of Photosynthesis - The Purpose and Process of Photosynthesis
- Last updated
- Save as PDF
- Page ID 13202

Learning Objectives
- Describe the process of photosynthesis
The Importance of Photosynthesis
The processes of all organisms—from bacteria to humans—require energy. To get this energy, many organisms access stored energy by eating food. Carnivores eat other animals and herbivores eat plants. But where does the stored energy in food originate? All of this energy can be traced back to the process of photosynthesis and light energy from the sun.
Photosynthesis is essential to all life on earth. It is the only biological process that captures energy from outer space (sunlight) and converts it into chemical energy in the form of G3P ( Glyceraldehyde 3-phosphate) which in turn can be made into sugars and other molecular compounds. Plants use these compounds in all of their metabolic processes; plants do not need to consume other organisms for food because they build all the molecules they need. Unlike plants, animals need to consume other organisms to consume the molecules they need for their metabolic processes.
The Process of Photosynthesis
During photosynthesis, molecules in leaves capture sunlight and energize electrons, which are then stored in the covalent bonds of carbohydrate molecules. That energy within those covalent bonds will be released when they are broken during cell respiration. How long lasting and stable are those covalent bonds? The energy extracted today by the burning of coal and petroleum products represents sunlight energy captured and stored by photosynthesis almost 200 million years ago.
Plants, algae, and a group of bacteria called cyanobacteria are the only organisms capable of performing photosynthesis. Because they use light to manufacture their own food, they are called photoautotrophs (“self-feeders using light”). Other organisms, such as animals, fungi, and most other bacteria, are termed heterotrophs (“other feeders”) because they must rely on the sugars produced by photosynthetic organisms for their energy needs. A third very interesting group of bacteria synthesize sugars, not by using sunlight’s energy, but by extracting energy from inorganic chemical compounds; hence, they are referred to as chemoautotrophs.

The importance of photosynthesis is not just that it can capture sunlight’s energy. A lizard sunning itself on a cold day can use the sun’s energy to warm up. Photosynthesis is vital because it evolved as a way to store the energy in solar radiation (the “photo-” part) as high-energy electrons in the carbon-carbon bonds of carbohydrate molecules (the “-synthesis” part). Those carbohydrates are the energy source that heterotrophs use to power the synthesis of ATP via respiration. Therefore, photosynthesis powers 99 percent of Earth’s ecosystems. When a top predator, such as a wolf, preys on a deer, the wolf is at the end of an energy path that went from nuclear reactions on the surface of the sun, to light, to photosynthesis, to vegetation, to deer, and finally to wolf.
- Photosynthesis evolved as a way to store the energy in solar radiation as high-energy electrons in carbohydrate molecules.
- Plants, algae, and cyanobacteria, known as photoautotrophs, are the only organisms capable of performing photosynthesis.
- Heterotrophs, unable to produce their own food, rely on the carbohydrates produced by photosynthetic organisms for their energy needs.
- photosynthesis : the process by which plants and other photoautotrophs generate carbohydrates and oxygen from carbon dioxide, water, and light energy in chloroplasts
- photoautotroph : an organism that can synthesize its own food by using light as a source of energy
- chemoautotroph : a simple organism, such as a protozoan, that derives its energy from chemical processes rather than photosynthesis
- Cambridge Dictionary +Plus
Meaning of photosynthesis in English
Your browser doesn't support HTML5 audio
- chasmogamous
- cleistogamous
- efflorescence
- in flower idiom
- multi-headed
- palynological
Related word
Photosynthesis | american dictionary, examples of photosynthesis, translations of photosynthesis.
Get a quick, free translation!

Word of the Day
have your hands full
to be so busy that you do not have time to do anything else

Binding, nailing, and gluing: talking about fastening things together

Learn more with +Plus
- Recent and Recommended {{#preferredDictionaries}} {{name}} {{/preferredDictionaries}}
- Definitions Clear explanations of natural written and spoken English English Learner’s Dictionary Essential British English Essential American English
- Grammar and thesaurus Usage explanations of natural written and spoken English Grammar Thesaurus
- Pronunciation British and American pronunciations with audio English Pronunciation
- English–Chinese (Simplified) Chinese (Simplified)–English
- English–Chinese (Traditional) Chinese (Traditional)–English
- English–Dutch Dutch–English
- English–French French–English
- English–German German–English
- English–Indonesian Indonesian–English
- English–Italian Italian–English
- English–Japanese Japanese–English
- English–Norwegian Norwegian–English
- English–Polish Polish–English
- English–Portuguese Portuguese–English
- English–Spanish Spanish–English
- English–Swedish Swedish–English
- Dictionary +Plus Word Lists
- English Noun
- American Noun
- Translations
- All translations
Add photosynthesis to one of your lists below, or create a new one.
{{message}}
Something went wrong.
There was a problem sending your report.
Photosynthesis
Part of Science Biology

What is photosynthesis?
This video can not be played
To play this video you need to enable JavaScript in your browser.
Animals need to eat food to get their energy. All animals, including humans, eat food that was, or is, a plant or an animal.
But green plants and algae can use light energy to make their own food! This process called photosynthesis .
Almost all life on Earth depends upon this process.

A leaf usually has a large surface area, so that it can absorb a lot of light. It's top surface is protected from water loss, disease and weather damage by a waxy cuticle, which does not stop light entering the leaf.
The upper part of the leaf is where the light falls, and it contains many cells called palisade cells. This has many chloroplasts, with lots of chlorophyll to trap as much light as possible. It is shaped like a tall box which helps pack them closely together.
Carbon dioxide
Plants get the carbon dioxide they need from the air through their leaves. It moves by diffusion through small holes in the underside of the leaf called stomata. Guard cells control the size of the stomata so that the leaf does not lose too much water in hot, windy or dry conditions.
The lower part of the leaf is a spongy layer with loose-fitting cells. Between the cells in this layer there are 'air spaces' - a bit like a sponge. These allow the gases to diffuse through the leaf.Stomata let carbon dioxide enter the leaf, and let the oxygen produced in photosynthesis leave the leaf easily. In many plants, stomata are open during the day and closed at night.
The water needed for photosynthesis is absorbed through the roots and transported through tubes to the leaf.The roots have a type of cell called a root hair cell. These project out from the root into the soil, and have a big surface area and thin walls. This lets water pass into them easily.Note that root cells do not contain chloroplasts, as they are normally in the dark and cannot carry out photosynthesis.
Results Only the areas of the leaf that were originally green tested positive for starch. The discoloured areas tested negative. As the green areas contained chlorophyll and the white did not, this proves that chlorophyll is needed for photosynthesis.
Investigating the production of oxygen
Food Chains
- count 1 of 11
Gas Exchange and Respiration
- count 2 of 11
- count 3 of 11
Photosynthesis
Photosynthesis is the process of converting light into energy, conducted by plants and other organisms. this expedition explains how it works..
This story was created for the Google Expeditions project by Vida Systems, now available on Google Arts & Culture.
Photosynthesis by Vida Systems
Flora, which is what we call the kingdom of plant life on Earth, generates oxygen through this exact process. Photosynthesis is crucial for all life forms on our planet.
Basics of photosynthesis
Photosynthesis is the way plants, algae, and certain bacteria transform light energy into chemical energy. The absorbing of light waves gives plants energy, which they store in carbohydrate molecules, so they can later release it and use it for their growth.
Common photosynthesis maintains life on Earth with the oxygen it provides for the atmosphere, while giving plants nutrients.
Oxygenic Photosynthesis
The type you can explore while observing plants, algae and cyanobacteria, it supplies the air with oxygen and is most common in nature. It functions as a counterbalance to the carbon dioxide produced by all breathing organisms.
Oxygen being born
In oxygenic photosynthesis, energy that comes from light transfers electrons from water to carbon dioxide, resulting in carbohydrates. During this process, water becomes “oxidized” by losing an electron, so that oxygen is produced along with carbs and emitted as a waste product.
Pigments and Absorption of Light
Do you wonder why there are so many different colored flowers and vegetables? The answer is simple – pigments. Pigments are molecules that trap sunlight and give plants their color. The 3 main pigment varieties are chlorophylls, carotenoids, and phycobilins.
Chlorophylls absorb blue and red but reflect green light and are the reason we instinctively connect Flora with the color green. Carotenoids get along well with green light but reflect red to yellow-colored pigments. Phycobilins predominantly reflect red or blue light.
Pigments are the molecules responsible for color in nature. They trap sunlight and reflect certain wavelengths. The color spectrum of the waves that are not absorbed by the plant is observed externally as color.
Chlorophyll
The green pigments in the chloroplasts of algae and plants is known as chlorophyll. This biomolecule is crucial for absorbing light energy and is the reason we see so many green plants in nature.
Carotenoids
Carotenoid pigments absorb bluish-green light and give plants a red, orange, or yellowish dye. They are the magic ingredient in carrots and pumpkins.
Phycobilins
Phycobilins can be seen in red algae or in cyanobacteria. They reflect red or blue light.
Have you noticed that usually a plant’s leaves and stem are green, its flower buds and fruits are dyed by nature in a really catchy color, and its root is either colorless or really pale? Well, for that we should blame plant’s plastids.
Plastids are organelles that store vital chemical compounds used by the plant cell. The pigments that they contain can determine the cell’s color. Different plastids have different roles in the photosynthetic process and can transform into one another in certain circumstances.
Chloroplasts
Chloroplasts contain chlorophyll, which makes them green. Although present in all cells of a plant’s green parts, they are predominantly concentrated in the leaves or stems.
Leaves are specially adapted structures for catching light, however cacti possess a different leaf anatomy and many conduct photosynthesis through their stems.
Chromoplasts
Chromoplasts synthesize and store pigment. You can find this plastid type in fruits, flowers, or roots as well as leaves, but mainly in autumn when they start to age and lose their green color. Chromoplasts accumulate a lot of carotenoid pigments and are quite orange in hue.
Leucoplasts
Unlike other plastids, leucoplasts are non-pigmented and lack photosynthetic pigments.
Located in roots, bulbs, and seeds, these plastids have the genetic potential of developing photosynthesis because if exposed to sunlight they will be transformed into chloroplasts or chromoplasts.
Light-dependent Reactions
Light-dependent (or simply “light”) reactions require, as the name suggests, a source of light. During the reaction 1 molecule of the pigment chlorophyll takes in 1 photon, while losing 1 electron. Freed electrons travel through a special electron transport chain.
The “electron hole” in the chlorophyll molecule regains an electron by a process called photolysis. Light reactions give the plant a lot of energy and are vital for the development and metabolism of its cells.
Water Photolysis
During photosynthesis in light-dependent reactions, by adopting 1 photon, the molecule of chlorophyll loses 1 electron and becomes oxidized. Water brings balance into the equation by giving away electrons and producing oxygen.
Photosystems I and II
The process of light absorption happens within 2 specialized units called photosystem I and II. Each photosystem has an “antennae” that capture light energy in the form of photons, a reaction center that converts light into chemical energy as well as some other components.
Non-cyclic Reaction
The missing electron from photosystem II is replaced by an electron from photosystem I, which is similarly freed from the reaction center of photosystem I after a photon adoption. The lack of an electron in photosystem I is compensated by an electron that comes from photolysis.
Cyclic Reaction
The cyclic reaction happens only at photosystem I. The freed electron from the chlorophyll molecule is passed down the electron acceptor molecules and eventually returns back to the same photosystem, unlike the more adventurous scenario observed in the non-cyclic reaction.
Light-independent Reactions
After light, there’s darkness, or at least that’s the scenario with the photosynthetic process, performed in plants. These so called “dark” reactions use the energy stored during the previous (light) phase of photosynthesis in order to synthesize carbohydrates.
They begin with a carbon fixation, which brings CO2 to the table in a succession of chemical reactions in which carbon dioxide is transformed into sugars such as sucrose and starch.
Carbon Fixation
This is the natural process of assimilating carbon from carbon dioxide in the atmosphere. Photosynthesis produces simple carbon sugars, which are then used in the creation of other organic compounds, or as a fuel in cellular respiration.
Calvin’s Cycle
Carbon fixation is just the first step of a cyclic process called Calvin’s cycle. What happens is a chain of biochemical reactions performed by plant’s chloroplasts. The Calvin-Benson cycle synthesizes sugars using carbon dioxide.
Early photosynthetic systems were apparently anoxygenic, using various other molecules as electron donors rather than water. Fossils that may have contained photosynthetic organisms have been dated at more than 3 billion years old. Today’s photosynthesis is mostly oxygenic.
That is good news for us, since we are breathing the fine air of Earth’s atmosphere precisely because oxygenic photosynthesis is all over the place. Or, should we say, all over the planet!
The Great Oxygen Event
This event, known by many names, such as Oxygen Catastrophe, or The Great Oxidation, is an evolutionary cornerstone for all life forms. Dioxygen (O2) appears in Earth’s atmosphere, as a result of biological evolution, that is, life forms created oxygen.
Some scientists believe that chloroplasts are photosynthetic bacteria that adapted to life during symbiotic relationships with plants. Chloroplasts have their own DNA, different than the nuclear DNA of the plant’s cells.
The genes in chloroplasts’ DNA are similar to those found in cyanobacteria.
Evolutionary Role of Cyanobacteria
It is not scientifically proven when oxygenic photosynthesis evolved. Nevertheless, we can “blame” a common ancestor of still present cyanobacteria for developing the biological capacity of using water as the source for electrons in photosynthesis.
It is unlikely though that the first photosynthetic cyanobacteria generated oxygen because the atmosphere back then contained almost no O2.
The Oriental Hornet
A pigment called xanthopterin is used by the Oriental hornet (Vespa orientalis) for converting sunlight into electrical power. This is an important evolutionary step by members of the animal kingdom towards engaging in photosynthesis.
Artificial Photosynthesis
The term sums up all the possible schemes for capturing and accumulating energy from sunlight in the chemical bonds of a fuel, hence the name “solar fuel.”
Artificial photosynthesis is a chemical process that replicates the natural one, converting light, water, and CO2 into carbs.
Welcome to South Africa's Oldest and Largest Game Park
Learning with google arts & culture, western cape, south africa, mountains, oceans and animals - south africa's western cape has it all, trip to the moon 1966, california state parks, the route of cervantes (1/2) 1547-1580, sir isaac newton.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.126(4); 2020 Sep 14
Photosynthesis: basics, history and modelling
Alexandrina stirbet.
1 Anne Burras Lane, Newport News, VA, USA
Dušan Lazár
2 Department of Biophysics, Center of the Region Haná for Biotechnological and Agricultural Research, Faculty of Science, Palacký University, Šlechtitelů 27, 783 71 Olomouc, Czech Republic
3 Key Laboratory of Advanced Process Control for Light Industry (Ministry of Education), Jiangnan University, Wuxi, China
4 University of Missouri, Columbia, MO, USA
Govindjee Govindjee
5 Department of Biochemistry, Department of Plant Biology, and Center of Biophysics & Quantitative Biology, University of Illinois at Urbana-Champaign, Urbana, IL, USA
With limited agricultural land and increasing human population, it is essential to enhance overall photosynthesis and thus productivity. Oxygenic photosynthesis begins with light absorption, followed by excitation energy transfer to the reaction centres, primary photochemistry, electron and proton transport, NADPH and ATP synthesis, and then CO 2 fixation (Calvin–Benson cycle, as well as Hatch–Slack cycle). Here we cover some of the discoveries related to this process, such as the existence of two light reactions and two photosystems connected by an electron transport ‘chain’ (the Z-scheme), chemiosmotic hypothesis for ATP synthesis, water oxidation clock for oxygen evolution, steps for carbon fixation, and finally the diverse mechanisms of regulatory processes, such as ‘state transitions’ and ‘non-photochemical quenching’ of the excited state of chlorophyll a.
In this review, we emphasize that mathematical modelling is a highly valuable tool in understanding and making predictions regarding photosynthesis. Different mathematical models have been used to examine current theories on diverse photosynthetic processes; these have been validated through simulation(s) of available experimental data, such as chlorophyll a fluorescence induction, measured with fluorometers using continuous (or modulated) exciting light, and absorbance changes at 820 nm (ΔA 820 ) related to redox changes in P700, the reaction centre of photosystem I.
Conclusions
We highlight here the important role of modelling in deciphering and untangling complex photosynthesis processes taking place simultaneously, as well as in predicting possible ways to obtain higher biomass and productivity in plants, algae and cyanobacteria.
‘ Complexity is the prodigy of the world. Simplicity is the sensation of the universe. Behind complexity, there is always simplicity to be revealed. Inside simplicity, there is always complexity to be discovered.’ Gang Yu
INTRODUCTION
With limited agricultural land and increasing human population, it is essential to enhance photosynthetic activities. Oxygenic photosynthesis is a very important process, not only because it is the source of our food, fibre and many useful substances, but also because almost all life on the Earth depends on it, either directly or indirectly. Plants, algae and cyanobacteria are oxygenic photosynthetizers that use light energy to generate organic molecules [e.g. glucose (C 6 H 12 O 6 ), sugars, starch] from carbon dioxide (CO 2 ) and water (H 2 O), and release molecular oxygen (O 2 ) into the atmosphere (for a background on photosynthesis see, Eaton-Rye et al ., 2012 ; Blankenship, 2014 ; Shevela et al. , 2019 ):
Note that the above global equation of photosynthesis emphasizes that the oxygen molecules released into the atmosphere originate from water oxidation, not from carbon dioxide, as established using 18 O-labelled water ( Ruben et al. , 1941 ).
This process starts in the thylakoid membrane (TM) with two light reactions taking place simultaneously at photosystem (PS) II and PSI reaction centres (RCs; for PSII and PSI, see the review by Nelson and Junge, 2015 ). The light energy absorbed by pigment–protein antenna complexes of the PSs is converted, with very high efficiency, into redox chemical energy; a small part is, however, dissipated as heat (internal conversion), and as chlorophyll (Chl) fluorescence (2–10 %, Latimer et al. , 1956 ). Furthermore, water is oxidized to oxygen, and NADP + is reduced to NADPH, and, in addition, ATP is produced ( Rabinowitch and Govindjee, 1969 ; Blankenship, 2014 ; Shevela et al. , 2019 ). Both NADPH and ATP are then used for CO 2 assimilation in the stroma (for a historical background of the Calvin–Benson cycle, see, Bassham, 2005 ; Benson, 2005 ); here, Rubisco (ribulose 1,5-bisphosphate carboxylase/oxygenase) is a key enzyme, which catalyses the fixation of CO 2 on a five-carbon compound, RuBP (ribulose 1,5- bis phosphate). A diagram of the photosynthetic apparatus and the electron transport (ET) reactions is shown in Fig. 1 .

Diagram of the photosynthetic apparatus and electron transport (ET) pathways in plants and algae. Four major protein complexes in the thylakoid membrane (TM) participate in the production of ATP and nicotinamide adenine dinucleotide phosphate in reduced form (NADPH), needed for the Calvin–Benson cycle to fix CO 2 to produce sugars: two photosystems (PSII and PSI) connected in series via the cytochrome (Cyt) b 6 /f, and the ATP synthase. Light is absorbed simultaneously by pigments in the light harvesting complexes of PSI and PSII (LHCI and LHCII); excitation energy is transferred to reaction centre (RC) P700 (in PSI) and P680 (in PSII), where primary charge separation takes place, initiating a chain of redox reactions. PSII functions as a water/PQ (photo)-oxidoreductase, which has a manganese complex [Mn 4 O 5 Ca], and a tyrosine-161 (Y Z ), located on D1 protein on the electron donor side, as well as pheophytin (Pheo), plastoquinones Q A and Q B , and a non-haem (heme) iron binding a bicarbonate ion (HCO 3 ‒ ) on the electron acceptor side. By contrast, PSI is a plastocyanin (PC)/ferredoxin (Fd) (photo)-oxidoreductase; it uses reduced PC as an electron donor, and a particular Chl a molecule (A 0 ), vitamin K 1 (A 1 ), and three non-haem iron–sulfur centres (shown in the figure as Fe-S) are on the acceptor side of PSI. The Cyt b 6 /f complex includes a Cyt f, a Rieske iron–sulfur protein (Fe-S), two cytochromes b (Cyt b p and Cyt b n ) that participate in the oxidation and reduction of PQH 2 and PQ: PQH 2 is oxidized at the Q p -site by Cyt b p , while PQ is reduced at the Q n -site by Cyt b n . The Q p - and Q n -sides are also called Q o - and Q i -sides, respectively. Besides the linear ET flow from water to NADP + , there are several pathways leading to electron donation to alternative electron acceptors: cyclic electron flow (CEF) around PSI mediated by Fd (involving Fd-NADP + -reductase, FNR, and a proton gradient regulator, PGR5), or NADPH (via NADPH dehydrogenase, NDH); water–water cycle (WWC); chlororespiration (through the plastid terminal oxidase, PTOX); and the malate valve (through malate dehydrogenase, MDH). The proton motive force ( pmf ) [consisting of the proton concentration difference (ΔpH) and the electric potential (ΔΨ) across TM] is used by ATP synthase to produce ATP from ADP and phosphate (P i ); in the pmf formula, R is the gas constant, F is the Faraday constant, and T is the absolute temperature (in K). Modified from Alric (2010) .
The availability of high-performance computers and detailed knowledge of the various steps of photosynthesis have provided new opportunities to use mathematical modelling to better understand the dynamics of this process (see reviews by Lazár and Schansker, 2009 ; Jablonsky et al. , 2011 ; Stirbet et al. , 2014 ). In addition, several studies ( Zhu et al. , 2010 ; Long et al ., 2006 , 2015 ; Ort et al. , 2015 ; South et al. , 2018 ; Simkin et al. , 2019 ) strongly support the idea that the photosynthetic processes can be improved through genetic engineering to increase the yield potential of various crops (see also Rosenthal et al. , 2011 ; Simkin et al ., 2015 , 2017 ; Kromdijk et al. , 2016 ; McGrath and Long, 2016 ). Furthermore, mathematical modelling can be used to predict opportunities for specific genetic modifications and devise optimized engineering designs to improve photosynthesis ( Zhu et al. , 2007 ).
In this review, we first provide a background of oxygenic photosynthesis that forms the basis of its modelling. We then discuss a few selected studies on mathematical models describing photosynthetic processes. Partial reactions of photosynthesis have been often modelled separately, such as: (1) the primary photochemical reactions (e.g. Schatz et al. , 1988 ; Roelofs et al. , 1992 ); (2) water ‘splitting’ reactions (e.g. Kok et al. , 1970 ; Mar and Govindjee, 1972 ; Jablonsky and Lazár, 2008 ; Shen, 2015 ); (3) reduction of Q B , the secondary plastoquinone (PQ) acceptor of PSII (e.g. Velthuys and Amesz, 1974 ; Petrouleas and Crofts, 2005 ); and (4) the redox reactions of the PQ pool at the Cyt b 6 /f complex (which may include the Q-cycle; see e.g. Mitchell, 1975 ; Cramer et al. , 2011 ). However, in this review we mainly discuss larger models, which include several steps, providing information on complex photosynthetic processes.
PHOTOSYNTHESIS IN PLANTS, ALGAE AND CYANOBACTERIA: SOME BASICS
Early discoveries.
Not much was known about photosynthesis before the 20th century; for earlier discoveries in photosynthesis see chapter 2 in Rabinowitch (1945) and the timeline in Govindjee and Krogmann (2004) . The key discoveries were as follows (see chapter 1 in Rabinowitch and Govindjee, 1969 ): Jan van Helmont (1648) showed that plant growth was mainly from the water that plants had absorbed; it was only later that Nicolas Théodore de Saussure (1804) clearly showed that water was an essential reactant of photosynthesis. Joseph Priestley (1776) showed, in elegant experiments, that plants produced ‘oxygen’ (then called de-phlogisticated air) needed by a mouse to live, whereas Jan Ingen-Housz (1773) convincingly established that light was necessary for photosynthesis. The role of CO 2 in photosynthesis was shown by Jean Senebier (1782), whereas the synthesis of starch was shown by Julius von Sachs (1862, 1864). However, the involvement of chlorophyll (Chl) in this process has a long history. For some of the earliest concepts, we must remember to mention Pierre Joseph Pelletier and Joseph Bienaimé Caventou (1817, 1818), and René Joachim Henri Dutrochet (1837). However, Theodor Engelmann (1882) provided the first action spectrum of photosynthesis, showing that red and blue light, absorbed by Chl, produce oxygen (see figure 1.1 and its description in Shevela et al. , 2019 ).
Physiological and biochemical advances
An understanding of how photosynthesis functions began only after 1900, but by 1960 a basic model at the molecular level, including generation of NADPH and ATP as well as the steps leading to the assimilation of CO 2 to produce carbohydrates, was established (see Govindjee and Krogmann, 2004 ; Govindjee et al ., 2005; Nickelsen, 2016 ).
By measuring photosynthesis as a function of light intensity, Frederick Frost Blackman (1905) suggested that photosynthesis consists of two separate phases: a light-dependent phase (i.e. so-called ‘light’ reactions), and a temperature-dependent biochemical phase (so-called ‘dark’ reactions, or ‘Blackman reaction’; see Warburg and Uyesugi, 1924 ). However, because CO 2 fixation uses NADPH and ATP, formed in the light phase, these so-called ‘dark’ reactions are also light-dependent. Moreover, many enzymes, involved in CO 2 assimilation reactions, function only when they are ‘light-activated’, being controlled through the ferredoxin:thioredoxin reductase (FTR) system (see reviews by Buchanan et al. , 2002 ; Nikkanen and Rintamäki, 2019 ). Therefore, the term ‘dark phase’ is inappropriate; Buchanan (2016) has proposed the use of ‘carbon reactions’ for ‘dark reactions’. Furthermore, the true ‘light reactions’ end after the primary charge separation steps in the RCs; both the electron transfer and the proton transfer reactions, in principle, can occur in darkness.
Cornelis B. van Niel (1931, 1941) showed that certain photosynthetic bacteria use H 2 S instead of H 2 O as an electron donor, producing sulfur instead of oxygen, and the global reaction of photosynthesis is:
where A is sulfur in sulfur bacteria and oxygen in plants, algae and cyanobacteria. By analogy with photosynthetic bacteria, van Niel suggested that O 2 released by plants is derived from H 2 O rather than CO 2 . This was confirmed by Sam Ruben, Merle Randall, Martin Kamen and James Logan Hyde (see Ruben et al. , 1941 ), based on results using 18 O-labelled water.
Chlorophyll a fluorescence
As mentioned earlier, in addition to primary photochemistry, photosynthetic organisms lose some energy as heat (internal conversion) and as light (fluorescence). Fluorescence is radiative deactivation of (usually) the first singlet excited state of a molecule to the ground state. Kautsky and Hirsch (1931) discovered what others later called the ‘Kautsky effect’, which is Chl a fluorescence induction (ChlFI; see Govindjee, 1995 ). Kautsky and Hirsch observed (visually) transitory variations in Chl a fluorescence (ChlF) emitted by samples that were illuminated after a period of darkness; this ChlF has an increasing phase (peak, ~1 s) followed by a slower (5–10 min) decreasing phase. McAlister and Myers (1940) made an important observation by showing an inverse relationship between ChlF emission and CO 2 uptake. These ChlF transients were then studied, among other places, in the Photosynthesis Laboratory at the University of Illinois, Urbana-Champaign (beginning in the 1950s; see Govindjee and Papageorgiou, 1971 ; Papageorgiou, 1975 ; Govindjee and Satoh, 1986 ; Papageorgiou et al. , 2007 ). Because ChlF has been shown to be directly or indirectly affected by complex physical and biochemical processes taking place during photosynthesis, analysis of ChlFI curves is of importance in photosynthesis research (see reviews by Krause and Weis, 1991 ; Lazár, 1999 , 2015 ; Strasser et al. , 2004 ; Stirbet and Govindjee, 2011 ; Stirbet et al. , 2018 ).
Photosynthetic unit (antenna and reaction centres): excitation energy transfer
An essential concept related to the light phase of photosynthesis is ‘photosynthetic unit’. It was developed based on the crucial discovery by Emerson and Arnold (1932 a , b ) that ~2400 Chl molecules cooperate to evolve one molecule of O 2 , while the minimum quantum requirement for the evolution of one O 2 molecule was 8–10 ( Emerson, 1958 ; for the history of this discovery, see Nickelsen and Govindjee, 2011 ; Nickelsen, 2016 ). Gaffron and Wohl (1936) suggested the existence of ‘photosynthetic units’, where light energy absorbed by any antenna molecule is transferred as excitation energy among the pigment molecules, until finally it is trapped with high efficiency by a limiting enzyme (a ‘photoenzyme’, as implied by Emerson and Arnold, 1932 b ), which is equivalent to what we now call reaction centre (RC), a term introduced by Duysens (1952) . Here, the primary charge separation (i.e. photochemistry) takes place (see e.g. Myers, 1994 ; Govindjee and Krogmann, 2004 ). Experimental evidence for excitation energy transfer (EET) between photosynthetic pigments was initially obtained by comparing action spectra of photosynthesis and of sensitized ChlF in green, brown and red algae (see chapters 10–12 in Rabinowitch and Govindjee, 1969 ). We now have much more detailed knowledge on the molecular mechanisms of electronic EET in antenna, as well as on exciton trapping by the RCs (e.g. Croce and van Amerongen, 2013 ; van Amerongen and Croce, 2013 ; Roden et al. , 2016 ; Mirkovic et al. , 2017 ; Chan et al. , 2018 ).
Taking things apart
Robert Hill (1937) found that the ‘light phase’ of photosynthesis can operate independently from the ‘dark phase’ (the carbon reaction phase), since isolated chloroplasts can evolve O 2 in the presence of artificial electron acceptors [this reaction is called the ‘Hill-reaction’ in honor of Robert (Robin) Hill], even in the absence of CO 2 . This concept led to a ‘modularization’ in the study of photosynthesis ( Nickelsen, 2016 ), since even if these two partial processes are interrelated, the tendency after 1940 was to investigate them separately. Note that Mehler (1951) had found that molecular oxygen is also a Hill electron acceptor, and this reaction, called the ‘Mehler reaction’, has been shown to play an important role in photoprotection of photosynthetic organisms ( Miyake, 2010 ).
The carbon reactions
The long-lived form of radioactive carbon, 14 C, was discovered by Samuel Ruben and Martin Kamen (1941) . This radioactive isotope was used to decipher the major pathway of CO 2 reduction by photosynthetic organisms, by Andrew Benson (who did most of the early pioneering work, using 14 C), Melvin Calvin, James A. Bassham and co-workers (see Calvin et al. , 1950 ; Calvin, 1989 ; Bassham, 2005 ; Benson, 2005 ). For example, they found that ribulose 1,5-bisphosphate (RuBP; a 5-C sugar) was the acceptor of CO 2 ; the first stable product of CO 2 reduction was 3-phosphoglyceraldehyde (G3P; a triose phosphate); and that there was a cycle to regenerate the RuBP. Melvin Calvin received the Nobel Prize in Chemistry in 1961 for these discoveries; we are of the opinion that Andrew Benson should have been a co-recepient.
Photophosphorylation
Daniel Arnon et al . (1954 a , b ) showed that isolated chloroplasts can produce ATP in light; in addition, they showed that intact isolated chloroplasts can even perform complete photosynthesis (i.e. CO 2 fixation). Furthermore, Allen et al. (1958) found that photophosphorylation can be ‘cyclical’ (i.e. ATP is produced when there is a cyclic ET, which was shown to involve cyclic electron flow around PSI via Cyt b 6 /f, CEF-PSI), or when there is ‘non-cyclic’ [i.e. during linear electron flow (LEF) from PSII to PSI) (see also Arnon, 1984 ; Tagawa et al. , 1963 ). A third pathway, labelled as ‘pseudo-cyclic photophosphorylation’, was also established, in which molecular oxygen plays the role of a terminal electron acceptor (i.e. the Mehler reaction; Mehler, 1951 ; Heber, 2002 ). Furthermore, a coupling mechanism between ATP synthesis and the ET, also in chloroplasts, was demonstrated by Dave Krogmann, Mordhay Avron and André Jagendorf (see Krogmann et al. , 1959 ). Note that the chloroplast coupling factor (CF1) for photophosphorylation, today known as ATP synthase, was discovered by Avron (1963) .
The two-light reaction and the two-pigment system concept
The idea of two light reactions and two types of PSs had its beginning in the 1943 experiments of Robert Emerson and Charleton Lewis on the ‘red drop’ in the action spectrum of the quantum yield of photosynthesis ( Emerson and Lewis, 1943 ) and in the 1957 ‘Emerson enhancement’ effect, that is when the rate of photosynthesis in two lights given together was higher than the sum of the rates of photosynthesis measured when the two lights were given separately ( Emerson et al. , 1957 ; also see: Govindjee and Rabinowitch, 1960 ); this discovery led to the well-known ‘Z’-scheme of photosynthesis ( Hill and Bendall, 1960 ; for the evolution of the Z-scheme, see Govindjee et al. , 2017 ). The very first Chl electron donors in the two PSs are P700 for PSI (identified also by an absorbance change around 705 nm; see Kok, 1956 ; Govindjee and Renger, 1993 ), and P680 in PSII, first suggested by Krey and Govindjee (1964) and shown to exist by Döring et al. (1969) . Key experiments proving the Z-scheme were provided by Duysens et al. (1961) on the red alga Porphyridium cruentum , who showed the antagonistic effect of light 1 and light 2 on the redox state of cytochrome (Cyt). (Here, light absorbed by PSI was ~680 nm, and that absorbed by PSII was ~562 nm.) Furthermore, based on flashing light experiments, Witt et al . (1961 a , b ) provided evidence for the kinetics and on the existence of other intermediate steps in the Z-scheme; details of the ET components involved in the photosynthetic electron transport chain (PETC) are given in Fig. 1 . However, of course, the physical confirmation for the existence and organization of the two PSs was the isolation and characterization via X-ray crystallography of the high-resolution spatial structure of PSII (e.g. Zouni et al. , 2001 ) and PSI (e.g. Jordan et al. , 2001 ).
Evidence from Chl a fluorescence measurements
Additional evidence for the two-pigment-system/two-light-reaction scheme in oxygenic photosynthesis was obtained by Govindjee et al. (1960) on Chlorella cells, using ChlF measurements. They showed an antagonistic effect of light 1 (i.e. predominantly absorbed by PSI) and light 2 (i.e. predominantly absorbed by PSII) on ChlF: addition of far-red light (light 1) to a shorter wavelength light (light 2) caused a decline (rather than an enhancement) of ChlF yield, compared to that produced by the two beams separately. As an explanation of this effect, Duysens and Sweers (1963) proposed that light 2 reduces a quencher Q, while light 1 oxidizes Q ‒ back to Q. The quencher theory of Duysens and Sweers was based not only on ChlF data published by Govindjee et al. (1960) , but also by Butler (1962) , who showed that variable fluorescence is mostly from PSII, and far-red light, absorbed by PSI, gives a smaller amount of PSI fluorescence. The quencher Q (named X-320, but now labelled Q A ) was identified using single turnover flashes, and has an absorption spectrum with maximal spectral changes in the UV, at 270 and 320 nm ( Stiehl and Witt, 1968 ). In several experimental studies ( Stiehl and Witt, 1969 ; van Gorkom, 1974 ; see also Witt, 2004 ), plastoquinone difference spectra in the near UV (300–350 nm) were similar to light-minus-dark spectra of the first plastoquinone acceptor of PSII (i.e. Q A −• − Q A ). According to Duysens and Sweers (1963) , ChlF is proportional to the fraction of the reduced quencher ([Q A − ]/[Q A ] total ; see a discussion in Stirbet and Govindjee, 2012 ; for other views see, Schansker et al ., 2011 , 2014 ; Magyar et al. , 2018 ). Later, it was shown that several non-photochemical quenching (NPQ) processes take place in parallel with the photochemical quenching (i.e. by Q A ) during the so-called slow (~10 min) phase of the ChlF transient, and the proportionality of the fluorescence yield with [Q A − ]/[Q A ] total , observed during the initial (<1 s) Chl fluorescence rise, is lost (see below the section On NPQ of the excited state of Chl). Real advances in the study of these NPQ processes became possible only after Ulrich Schreiber developed a pulse-amplitude modulated (PAM) fluorescence instrument (Walz, Effeltrich, Germany) that could be used on leaves in the presence or the absence of actinic light ( Schreiber, 1986 ; Schreiber et al. , 1986 ).
Vredenberg and Duysens (1963) observed that closure of RCs is accompanied by an increase in fluorescence yield of bacteriochlorophyll in Rhodospirillum rubrum , a purple anoxygenic photosynthetic bacterium, and concluded that several RCs share the same antenna. In an oxygenic photosynthesizer, the green alga Chlorella , Anne and Pierre Joliot ( Joliot and Joliot, 1964 ) measured the rate of steady-state oxygen evolution, and correlated it with the fraction of active PSIIs (see also Joliot and Joliot, 2003 ). Joliot and Joliot (1964) observed that both the oxygen yield and the fluorescence yield are related, in a hyperbolic manner, to the fraction of closed PSII centres; this suggested that there is an energetic connectivity within PSIIs, that is an excitation visiting a closed PSII (i.e. with Q A reduced) is redirected to another PSII. In this manner, the trapping cross-section of the open PSIIs increases as their neighbouring PSIIs become closed (see a review on PSII excitonic connectivity by Stirbet, 2013 ). Joliot and Joliot (1964) also derived theoretical equations describing the dependence of the ChlF yield (Φ F ) and the photochemical yield (Φ P ) on the fraction of open PSIIs, which included a connectivity parameter ( p ) for the probability of excitation energy transfer from a closed PSII to a neighbouring PSII (either closed or open). This was followed by publication of detailed papers on PSII excitonic connectivity by Paillotin (1976) , Strasser (1978) and Butler (1980) , the last two describing the process, using bipartite and tripartite PSII models of Butler and co-workers ( Butler and Kitajima, 1975 ; Butler and Strasser, 1977 ). Later, Lavergne and Trissl (1995) and Trissl and Lavergne (1995) extended the concept of PSII excitonic connectivity, using an exciton–radical pair equilibrium model. The latter is equivalent to the reversible radical pair (RRP) model of Schatz et al. (1988) ; it assumes rapid exciton equilibration between all PSII pigments, including P680, and describes primary photochemistry (charge separation, recombination and stabilization) leading to closed PSII RCs. The major feature of the RRP model is equilibrium , i.e. reversibility of charge separation, meaning fast charge separation followed by fast charge recombination, in both the open and the closed PSII centres (see Fig. 2 ).

Scheme showing the RRP (reversible radical pair) model and related reactions. The original RRP model is represented by the reactions on lines I and II, which are reactions occurring in an open PSII RC (when Q A is initially oxidized) and a closed PSII RC (when Q A is initially reduced), respectively. (L–P680)* denotes Chls in the light harvesting antenna of PSII (L) plus P680, which are in ultrafast excitation kinetic equilibrium, the asterisk (*) indicating the excited state. The rates constants are: k L , overall rate constant of antenna excitation; k 3 , overall rate constant of the excited state deactivation through heat dissipation and ChlF emission; k 1 o and k 1 c , rate constants of the primary charge separation in open and closed PSIIs, respectively; k -1 o and k -1 c , rate constants of the radiative (i.e. to the excited state) charge recombination between P680 + and Pheo − in open and closed PSIIs, respectively; k 2 o , rate constant of charge stabilization in an open PSII, i.e. the ET from Pheo ‒ to Q A ; k 2 c , rate constant of non-radiative (i.e. to the ground state) charge recombination between P680 + and Pheo ‒ in a closed PSII. The scheme presented here also includes excitation energy transfer (the energetic connectivity) between open and closed PSIIs (rate constant k UU ) and reversible reduction of P680 + by Y Z (rate constants k Pred and k Pox ), as well as the reduction of Y Z + by the manganese cluster of the oxygen-evolving complex (OEC; rate constant k Yred ), which produces an S-state transition from S i to S i+1 , where S i and S i+1 represent particular S-states. Modified from Lazár and Schansker (2009) .
ATP synthesis
Peter Mitchell (1961 a , b ) proposed a chemiosmotic theory for phosphorylation, which suggests that a ‘proton motive force’ ( pmf ), i.e. the electrochemical potential of protons, couples the ET reactions with ATP synthesis (from ADP and inorganic phosphate, P i ). Mitchell received the Nobel Prize in Chemistry in 1978 for this hypothesis. Later, Paul Boyer and John E. Walker received the Nobel Prize in Chemistry in 1997 for their work on the structure of F1 mitochondrial ATPase and the mechanism of ATP synthesis (see e.g. Boyer, 2002 ). Hind and Jagendorf (1963) (see also Jagendorf and Uribe, 1966 ) showed how photosynthetic cells convert light energy into free energy stored in the ATP molecule on the basis of the chemiosmotic theory, particularly the ΔpH component. The pmf has two components, one due to the trans-thylakoid electric potential difference (i.e. the membrane potential, ΔΨ), and the other due to the trans-thylakoid difference in proton concentration (ΔpH), which builds up during water splitting reactions on the lumen side of PSII, and the translocation of stroma protons to the lumen during PQ pool reduction by PSII, and by Cyt b 6 /f (including the Q-cycle; Mitchell, 1975 ) in relation to both the linear and the cyclic photosynthetic ET (see Fig. 1 , and a historical review by Jagendorf, 2002 ). We remind the readers that just as André Jagendorf’s work proved the importance of the ΔpH component (of pmf ) for ATP synthesis, Wolfgang Junge’s work proved the importance of ΔΨ in making ATP (see mini-review by Junge, 2004 ). However, a high ∆Ψ component of the pmf was also shown to affect the equilibrium of redox reactions within PSII, and has been linked to higher rates of PSII charge recombination in vivo , and subsequent photodamage due to increased production of singlet oxygen ( Davis et al. , 2016 ). On the other hand, low pH has been shown to inactivate oxygen evolution ( Schlodder and Meyer, 1987 ); furthermore, release of Ca 2+ from the oxygen evolving complex (OEC) has also been suggested to be the cause of this inactivation ( Ono and Inoue, 1988 ; Krieger and Weis, 1993 ). For recent research (and reviews) on ΔΨ and ΔpH across the TM see, Strand and Kramer (2014) , Kaňa and Govindjee (2016) , and Lyu and Lazár (2017 a , b ).
Oxygen evolution
The key experiments that preceded the discovery of the water splitting mechanism, leading to O 2 evolution and P680 + reduction in PSII, were done by Pierre Joliot and co-workers ( Joliot, 1965 ; Joliot et al. , 1969 ). Joliot et al. (1969) discovered period 4 oscillations in oxygen evolution in algal suspensions when they were exposed to a sequence of single turnover (ST) saturating light flashes. These results were explained by Bessel Kok et al. (1970) , who proposed a model (now known as Kok’s oxygen clock model, or the Kok–Joliot model to many), in which the formation of oxygen requires sequential accumulation of four positive charges on the OEC, which cycles through five redox states, labelled as S 0 , S 1 , S 2 , S 3 and S 4 (see Fig. 3 ). For the history of this discovery, see Renger and Govindjee (1993) and Joliot (2003) . The first evidence for the participation of Mn in the S-states was obtained by Chuck Dismukes and Yona Siderer (1980) , who obtained electron paramagnetic resonance (EPR) signals for the same. For a review on the functioning of the OEC, see Najafpour et al . (2012) . For a recent review on oxygen evolution, see Lubitz et al. (2019) .

Highly simplified scheme of Kok’s oxygen clock model; misses and double hits are not shown. S i (i = 0, 1, 2, 3, 4) represent the particular S-states of the manganese cluster of OEC. The S 4 -state is assumed to be kinetically indistinguishable from the S 0 -state. During an S-state transition, Y Z + (formed through PSII reactions) is reduced (with rate constants k 01 , k 12 , k 23 and k 30 ). Modified from Lazár and Schansker (2009) . For a review, including the involvement of manganese, see Najafpour et al. (2012) .
Mechanistic models for early events in photosynthesis
Bay and Pearlstein (1963) provided one of the first mathematical models of the exciton kinetics and trapping in a photosynthetic system; it was based on electronic excitation transfer, FRET (Förster resonance energy transfer; see Förster 1946 , 1948 ; also see a historical review by Clegg, 2006 ). According to this model, the electronic excitation energy moves in a so-called ‘random walk’, hopping from one Chl to another Chl in the antenna, until it is trapped by an RC, or is dissipated as heat or fluorescence (also see: Govindjee, 2004 ). Starting from FRET, other more complex and elegant theories have now been developed to characterize the exciton dynamics in antenna (e.g. Engel et al ., 2007 ; Ishizaki and Fleming, 2009 ; Clegg et al. , 2010 ; Fassioli et al. , 2014 ).
On ‘state transition’ for regulation of balanced excitation in the two photosystems
State transition, a light-adaptive phenomenon that optimizes photosynthesis by synchronizing the turnover rates of PSII RCs and of PSI RCs, when there is an excitation imbalance between their antenna, was discovered by Cecilia Bonaventura and Jack Myers (1969) in Chlorella and, independently, by Norio Murata (1969 a , b ) in the red alga Porphyridium cruentum and spinach chloroplasts. The equilibration of PSII and PSI activities takes place through adjustment of the relative size of their antenna: During a transition from ‘state 1’ to ‘state 2’, the absorption cross-section (CS) of PSII antenna (which provides information on the PSII-specific rates of light absorption and represents an ‘apparent’ measure of PSII antenna size in situ , in units of Å 2 per PSII centre; see Osmond et al. , 2017 ) decreases and that of PSI antenna increases, while the opposite occurs during transition from ‘state 2’ to ‘state 1’. The result is: the overall ChlF yield decreases in ‘state 2’ and increases in ‘state 1’, because, at room temperature, PSI has a much lower ChlF yield than PSII ( Butler, 1962 ). State transitions have been shown by John Allen and collaborators to be regulated by the redox state of the PQ pool ( Allen et al. , 1981 ; see Allen, 2002 ): the transition from ‘state 1’ to ‘state 2’ is triggered by the reduction of the PQ pool, and the transition from ‘state 2’ to ‘state 1’ is triggered by the oxidation of the PQ pool. In plants and algae, the controlling events take place at the Qp site of Cyt b 6 /f (i.e. the binding site of PQH 2 ; see Zito et al. , 1999 ), where the PQ redox-state is sensed, which triggers the activation or inactivation of a protein kinase ( Allen et al. , 1981 ): PQ pool reduction activates the protein kinase, and thus induces phosphorylation of mobile light harvesting complex (LHC) II, followed by its attachment to PSI antenna, while PQ pool oxidation inhibits the protein kinase, followed by dephosphorylation of the mobile LHCIIs by a phosphatase, and their re-attachment to PSII antenna (see Fig. 4 and reviews by Papageorgiou and Govindjee, 2011 ; Rochaix, 2014 ). For background on PSII, see Wydrzynski and Satoh (2005) , on PSI, see Golbeck (2006) , and on the Cyt b6f complex, see Cramer and Kallas (2016) . Note that extensive dynamic changes in the organization and structure of the TMs are associated with state transitions, which include PSII antenna dissociation after LHCII phosphorylation by Stt7/STN7 kinases, or association with PSII after dephosphorylation by PPHI/TAP38 phosphatases (see above, and Iwai et al. , 2010 ). However, new research suggests that these protein kinases and phosphatases can also affect the likelihood of cyclic ET around PSI (see Wood et al. , 2019 ). On the other hand, Pribil et al. (2018) have shown that the changes in the shape of grana stacks are mediated by the CURVATURE THYLAKOID1 (CURT1) protein complexes, which were shown to facilitate adjustments in membrane curvature at the grana margins in a dose-dependent manner.

Diagram of the mechanism of state transitions in plants and algae. In the diagram, the system is shown to be initially in ‘state 1’, with the absorption cross section (CS) of photosystem (PS) II being larger than that of PSI (it will have high Chl fluorescence yield because Chl in PSII is much more fluorescent than in PSI). During illumination, the plastoquinone (PQ) pool will be reduced by PSII because of higher absorption there. This is sensed by the Cyt b 6 /f (via its PQH 2 -oxidizing site, Q p ), and leads to activation of a kinase ( Stt7/STN7 ) and phosphorylation of the mobile light harvesting complexes of PSII (LHCII), which then associate with the PSI antenna. The reverse occurs when the system is in ‘state 2’ initially, with the absorption CS of PSI being larger than that of PSII. Here, oxidation of the PQ pool by PSI during illumination will be sensed by the Cyt b 6 /f, which leads to the inactivation of kinases, followed by de-phosphorylation of the mobile LHCIIs (by the phosphatases Pph1/TAB38 ) and their relocation to PSII. Abbreviations: A 0 and A 1 , a particular Chl a molecule and a vitamin K1 molecule, respectively; Fe-S, three non-haem (heme) iron–sulfur centres; Fd, ferredoxin; Q A and Q B , plastoquinone electron acceptors of PSII; NADP + and NADPH, nicotinamide adenine dinucleotide phosphate in oxidized and reduced state; P680 and P700, reaction centre chlorophylls/primary electron donors of PSII and PSI; PC, plastocyanin. Figure modified from Allen (2003) and Rochaix (2014) .
Two-electron gate on the electron acceptor side of PSII, and the requirement of bicarbonate
Bernadette Bouges-Bocquet (1973) and Bruno Velthuys and Jan Amesz (1974) independently discovered the two-electron gate (TEG) mechanism on the electron acceptor side of PSII in plants; it describes ET from Q A to Q B (see also Robinson and Crofts, 1983 ). As mentioned above, both Q A and Q B are PQs, but Q A is a one-electron acceptor, and is permanently bound to the D2 protein of PSII. By contrast, Q B is a two-electron acceptor that is bound to the D1 protein of PSII; it is strongly bound only when it is in Q B − -state, but is weakly bound in its fully oxidized state (Q B ), and very weakly bound when in the fully reduced state (Q B H 2 ). Following the primary charge separation: (1) Q A is reduced to Q A − (via pheophytin, Pheo; discovered by Vyacheslav Klimov et al. , 1977 ); (2) Q A − then reduces Q B to Q B − , and the latter remains tightly bound to D1; (3) after another light reaction, Q B − is then further reduced by Q A − , becoming fully reduced to Q B H 2 (PQH 2 ), after the addition of two protons; and finally (4) because Q B H 2 is loosely bound to D1, it is released in the membrane and replaced by another PQ molecule from the PQ pool (see Fig. 5 , and reviews discussing light-induced PQ pool reduction by PSII by Cardona et al. , 2012 ; Müh et al. , 2012 ). A bicarbonate ion has been shown to have a very important role in the functioning of the TEG and Q B H 2 formation ( Wydrzynski and Govindjee, 1975 ; see reviews by Govindjee and van Rensen, 1978 ; van Rensen, 2002 ; Shevela et al. , 2012 ). A similar TEG was also discovered in bacteria, independently by Colin Wraight and André Vermeglio (see Vermeglio, 2002 ), but there is no bicarbonate effect there (see Wang et al. , 1992 , and references therein). Note that the TEG model, the Kok model and the RRP model are important partial models that are used in more complex (or complete) models describing the photosynthetic ET (e.g. Nedbal et al. , 2009 ).

Scheme of the two-electron gate (TEG) model and related reactions. The two-electron gate mechanism, by which electrons are transferred from Q A to Q B , is represented by the reactions on line II. The rate constants are: k L , overall rate constant of Q A reduction; k AB1 and k AB2 , rate constants of ET from the reduced Q A to Q B and Q B ‒ , respectively; k BA1 and k BA2 , rate constants of backward ET from Q B ‒ and Q B 2‒ to Q A , respectively. The reactions above and below line II describe the reversible exchange of doubly reduced Q B (after its double protonation, which is implicitly assumed) with a PQ molecule from the PQ pool (rate constants k (B/PQ)exch and k (PQ/B)exch ); the reversible oxidation of the plastoquinol (rate constants k ox and k red ) is implicitly assumed to be the result of chlororespiration and cyclical electron flow around PSI. Modified from Lazár and Schansker (2009) .
On NPQ of the excited state of Chl
In general, NPQ processes can be defined as processes that decrease ChlF through mechanisms other than photochemical quenching (i.e. Q A quenching; e.g. Müller et al. , 2001 ; for a time line, see Papageorgiou and Govindjee, 2014 ). In this sense, the avoidance movement of chloroplasts in the leaf under high light conditions (i.e. qM; Dall’Osto et al. , 2014 ; Baránková et al. , 2016 ; Semer et al ., 2018 , 2019 ), the state 1 to state 2 transition (qT 12 ; see above), as well as the photoinhibition (qI), initiated by the photodamage of PSII ( Tyystjärvi et al. , 2005 ; Murata et al. , 2012 ; Tyystjärvi, 2013 ), would all be considered to be NPQ processes. However, according to Papageorgiou and Govindjee (2014) , it is preferable to consider as NPQ processes only those in which the excess energy accumulated as singlet excited Chl a ( 1 Chl a *) in PSII antenna is dissipated as heat (see Kitajima and Butler, 1975 ), such as the quickly reversible ‘high-energy non-photochemical quenching’ (qE), which develops in a few seconds and relaxes in 1–2 min (see Jahns and Holzwarth, 2012 ; and chapters in Demmig-Adams et al ., 2014 ), or other less clearly elucidated sustained forms of ChlF quenching processes (such as qH; Malnoë, 2018 ). This type of NPQ is induced by low lumen pH, being fully activated only after the pmf is established across the TM, when the TM is in a ‘high-energy’ state; it regulates the utilization of the light energy in PSII antenna in order to reduce photo-oxidative events that can damage the RCs. The exact relationship between lumen pH and NPQ is not fully understood; however, see discussions by Johnson (2011) and Zaks et al. (2013) . There are three main requirements for qE activation: (1) a trans-thylakoid ΔpH formed in light ( Wraight and Crofts, 1970 ; Briantais et al. , 1979 ); (2) the xanthophyll (VAZ) cycle, particularly the conversion of the carotenoid violaxanthin (V) to antheraxanthin (A) and zeaxanthin (Z) ( Yamamoto et al. , 1962 ; Yamamoto and Higashi, 1978 ); and (3) the PSII protein subunit S (PsbS) ( Li et al. , 2000 ; Brooks et al. , 2014 ). Barbara Demmig-Adams et al. (1989) (see also a historical review by Demmig-Adams, 2003 ) were the first to demonstrate that the extent of qE is proportional to the Z content of leaves; Demmig et al. (1987) further showed a correlation between Z and a form of qI manifested as a dark-sustained NPQ. Thus, they proposed that Z, which is derived from V in the xanthophyll cycle, is the link between the high energy state of the membrane and the heat dissipation of excess excitation energy of Chl a (see also Rees et al. , 1989 , 1992 ). In the xanthophyll cycle, the content of V decreases during illumination and is restored in darkness: Light ↝V⇄A⇄Z⇐ Dark . Violaxanthin deepoxidase (VDE) has a higher affinity for A than for V ( Yamamoto and Higashi 1978 ), and binds on the lumen side of the membrane, at pH ≈ 5.0 ( Hager and Holocher, 1994 ), which induces qE. Also, the NPQ kinetics was shown to depend on [Z], its induction being faster and its relaxation being slower when Z is present (see Johnson et al. , 2008 ). Adam Gilmore made an important contribution to the field, which included a successful collaboration with one of us (G) on the effects of intrathylakoid pH and VAZ cycle pigments on Chl a lifetime distributions and intensity in thylakoids ( Gilmore et al ., 1995 , 1998 ; Gilmore, 1997 ). On the other hand, the role of PsbS protein in qE is that of a pH sensor and quenching amplifier, as its amount in plant modulates the maximal qE level, but the underlying event is not yet fully understood ( Horton et al. , 2008 ; Holzwarth et al. , 2009 ; Brooks et al. , 2014 ). However, there is also evidence that qE can be induced in the absence of PsbS ( Johnson et al. , 2011 ), or even xanthophylls ( Johnson et al. , 2012 ), if the lumenal pH is sufficiently low (i.e. lower than the value assumed by the ‘moderate lumen pH paradigm’; see Kramer et al. , 1999 ). Finally, qE in algae is much more species-dependent than in plants. In unicellular green algae, or other algal groups (e.g. diatoms), the qE extent depends on the Light-Harvesting Complex Stress-Related (LHCSR) proteins ( Peers et al. , 2009 ). In most organisms, the LHCSR level is strongly light-dependent, and in some species, such as Chlamydomonas reinhardtii , acclimation to low light leads to very low NPQ levels ( Peers et al. , 2009 ).
Recently, Schreiber et al. (2019) have described a rapidly induced NPQ process during a pulse of high-light intensity in a dilute suspension of Chlorella vulgaris ; they called this process HIQ [high (light) intensity quenching]. The amplitude of the HIQ increases linearly with the effective rate of quantum absorption by PSII, reaching ~8 % of F M (i.e. the maximum Chl fluorescence measured in dark-adapted samples). This quenching rapidly relaxed after the pulse, and was shown to be caused by annihilation of 1 Chl* a by 3 Car* (excited state of a carotenoid in triplet state).
MODELLING CHL FLUORESCENCE INDUCTION IN PLANTS, ALGAE AND CYANOBACTERIA
ChlF emitted by plants and algae has little involvement in the process of photosynthesis, being one of the pathways in which excess excitation energy is dissipated by photosynthetic organisms. However, ChlFI kinetics is well recognized to have an intricate connection with many processes taking place during the conversion of light energy into a stable chemical form. Because it is a non-destructive measurement, although indirect, the ChlFI has numerous applications in the study of photosynthesis (see chapters in Papageorgiou and Govindjee, 2004 ), while its modelling is a straightforward way to verify various theories regarding different photosynthetic processes. Note that ChlFI in cyanobacteria is in part affected in different ways by the activity of the photosynthetic apparatus than in plants and algae, and this is due to their structural differences (see Stirbet et al. , 2019 ), but its modelling is not described in this review.
The ChlFI curve has been labeled O-J-I-P-S-(M)-T, where O-J-I-P represents the first fast (<1 s) phase, also known as the fast ChlF rise, and P-S-(M)-T the slower (5–10 min) phase (see Fig. 6 , and a review by Govindjee, 1995 ). Level O (origin) is the first measured minimum fluorescence level; J and I are intermediate inflections; P is the peak; S is the semi-steady state; M is a maximum, which, in plants, at room temperature is often seen only at low light intensities, but has been observed in Arabidopsis thaliana under low (freezing) temperature conditions ( Mishra et al. , 2019 ); and T is a terminal steady state level.

Chlorophyll a fluorescence induction curves measured in leaves of 10-d-old barley ( Hordeum vulgare L.) plants kept in darkness for 20 min before the measurement, shown on a logarithmic time scale (A), and on a linear time scale (B); a.u., arbitrary units. The O, J, I, P, S, M and T steps marked in the figure represent: O, the origin (minimum fluorescence F O ); J and I, intermediary fluorescence levels at 2 and 30 ms (F J and F I ); P, the peak (F P ); S, a semi-steady state level; M, a maximum; and T, the terminal steady state. Measurements were made under continuous red (650 nm) light of 2500 μmol photons m –2 s –1 with a Plant Efficiency Analyser (Hansatech, UK). Modified from Stirbet et al. (2018) .
The fast phase was labelled OIDP ( Munday and Govindjee, 1969 ), as OI 1 I 2 P ( Schreiber, 1986 ) and then OJIP ( Strasser and Govindjee, 1991 ); the O-J-I-P curves are measured only under a high intensity of excitation light. At low light the J step is missing, so that only an O-I-P curve is observed ( Strasser et al. , 1995 ; Tomek et al. , 2001 ). Below, we briefly discuss several models for the O-J-I-P fluorescence rise, as well as for the entire O-J-I-P-S-(M)-T transient or just the slow P-S-(M)-T phase (see also reviews by Lazár, 1999 ; Lazár and Schansker, 2009 ; Stirbet et al. , 2014 ).
Modelling strategy, definition of the Chl fluorescence signal, and some selected partial models of PSII
Mathematical modelling is an essential part of modern biology and can have several purposes. In any experimental study, the measured data provide information about how the explored system works, and based on these, we formulate hypotheses about how the explored system is functioning. By converting the hypotheses into a mathematical model, running the model and comparing the calculated results with experimental data, we can judge if the model describes the data well or not. In this case, the structure of the model (i.e. the hypotheses as such) and also the values of model parameters can cause agreement/disagreement between the results obtained with the model and the measured data. Regarding the values of the model parameters, we can run the model with fixed parameter values, taken from the literature, or we can fit the values to get the best agreement between the model results and experimental data. However, in the latter case, we may find a perfect agreement, but only by using unrealistic values of the model parameters (based on the literature), which usually rules out the correctness of the model. On the other hand, when values of system variables are not known from the litrrature and/or are not directly accessible from experiments, the fitting can provide this information, assuming the model structure is correct.
Furthermore, a so-called metabolic control analysis (MCA) can be performed, which quantifies the extent to which a given process (hypothesis) affects a given result (for a review see Visser and Heijnen, 2002 ). Sometimes, this quantification can be made easy only by using modelling rather than by doing experiments, because it is not always possible to infer the desired (initial) state of the experimental system, or to experimentally modify the parameters of the system, as needed to perform MCA.
Finally, if we have a robust model that describes well the various measured data, we can modify the model parameters and track the results, or in other words, we can perform ‘experiments’ without measuring anything – i.e. biological experiments in silico . These in silico experiments are very useful in making predictions that allow us to determine the role of model parameters, or to design experiments to prove or refute certain predictions. Concerning the modelling of ChlFI discussed below, it is important to keep in mind that a qualitative agreement between experiment and theory is a useful goal. The ChlFI is a manifestation of a very complex biological system, and therefore describing it correctly and comprehensively is difficult – this is quite different from modelling technical systems, which can be described correctly, and where a quantitative agreement between experiments and theory is strictly required.
Several approaches have been used for the formulation of a fast ChlF rise model, or for the entire ChlFI. The variable ChlF is emitted mostly from PSII (reviewed by Krause and Weis, 1991 ; Dau, 1994 ; Govindjee, 1995 ; Lazár, 1999 , 2006 ; Stirbet and Govindjee, 2011 , 2012 ). The basic strategy for modelling the fast ChlF rise has been to first use a model of the ET reactions occurring only in PSII, but then later add ET reactions beyond PSII, especially for the modelling of the entire ChlFI. The formulation of a ChlFI model also depends on the specific ET components considered, and then, on the way, the variable ChlF emitted during the transient is defined. The basic approach in the definition of the variable ChlF is based on the early work of Duysens and Sweers (1963) and the quencher theory defined there, later identified to be due to Q A (see above the subsection Evidence from Chl a fluorescence measurements). According to this theory, if Q A is oxidized, ChlF is low and if Q A is reduced, ChlF is high, and the variable ChlF is proportional to the fraction of Q A − . Moreover, the energetic PSII connectivity (mentioned earlier) can be also considered in modelling the variable ChlF.
Taken together, the most basic approach used to model the fast ChlF rise has been to define a PSII model that describes the redox changes of Q A during reduction of the PQ pool. These redox changes are modulated by Q B , the second PQ electron acceptor of PSII, which unlike Q A is a two-electron PQ acceptor of the PSII RC; originally, it came from the PQ pool, transiently binding to the Q B -site. The reduction of Q B to plastoquinol is described by the TEG model ( Bouges-Bocquet, 1973 ; Velthuys and Amesz, 1974 ), which is the fundamental partial model used in ChlFI modelling (see discussion earlier, and Fig. 5 ). Thus, one group of models describing the fast ChlF rise, including the first ever models (see below the subsection Modelling the fast Chl fluorescence rise by using only models of PSII reactions), are based on the TEG model. The charge stabilization on Q A (i.e. the reduction of Q A by Pheo − ) means that the PSII RC is closed and thus the ChlF is high. However, this charge stabilization is preceded by the formation of P680 + Pheo − (see Fig. 2 ). Thus, when either P680 + and/or Pheo − are present, the PSII RC is closed, but the ChlF decreases in their presence, as both P680 + and Pheo − are quenchers of ChlF (for P680 + , see Okayama and Butler, 1972 ; Shinkarev and Govindjee, 1993 ; Steffen et al ., 2001 , 2005 ; for Pheo − , see Klimov et al. , 1977 ). Quenching of Chl fluorescence by P680 + accumulation has been considered in several models of the fast ChlF rise (e.g. Lazár, 2003 ; Laisk and Oja, 2018 ). Accumulation of reduced Pheo was shown to take place only under illumination at 200–220 K ( Klimov et al. , 1980 ; Breton, 1982 ). Nonetheless, Vredenberg (2000 , 2008 , 2011 ) has assumed, in his O-J-I-P model, not only that Pheo ‒ accumulates at room temperature, but also that ChlF is higher when both Q A and Pheo are reduced than when only Q A is reduced. Strasser and Stirbet (2001) have also simulated and fitted a fast ChlF rise with a simple TEG-based model, but considering three different PSII redox states that contribute to the fluorescence signal: (1) with Q A ‒ ; (2) with Pheo ‒ ; and (3) with PheoQ A ‒ and Pheo ‒ Q A ‒ ; ChlF in the presence of Pheo ‒ Q A ‒ was considered to be two-fold larger than that when PheoQ A ‒ was present. The experimental O-J-I-P curve was fitted quite well by all three models, but the parameters of the models and the kinetics of the PSII redox states were different in each case. Thus, overparametrized models cannot be validated by fitting one experimental curve, and other approaches must be also used to reach firm conclusions. These can be, for example, measurements of the kinetics of the redox states of PSII during the ChlF transient, as well as through in silico experiments, in which the basic parameters of the model are kept constant.
On the other hand, ChlF yield during ChlFI has also been defined by using ratios of the rate constants related to fluorescence emission, heat dissipation and photochemistry ( Goltsev and Yordanov, 1997 ; Laisk et al. , 2006 ; Ebenhöh et al. , 2014 ; Stirbet and Govindjee, 2016 ). A better estimation of the ChlF signal, in models used to simulate the ChlFI, is obtained by considering fluorescence as a radiative deactivation of the singlet excited state of Chl (i.e. 1 Chl*); this was used in the modelling of the fast ChlF rise by Baake and Schlöder (1992) (see also Lebedeva et al. , 2002 ; Lazár, 2003 ; Belyaeva, 2004 ). If the ChlF signal is defined by the redox states of Q A or by the concentration of 1 Chl*, the model must include these entities. The reactions among the excited states of Chl a in PSII antenna that include P680 and Pheo, besides Q A , have been described by the RRP model of Schatz et al. (1988) ; it was based on measurements of ChlF decay in the picosecond range after excitation by a short laser pulse. In the RRP model, charge separation between P680 and Pheo is reversible and is followed by charge stabilization (ET from Pheo − to Q A ) in the open PSII RCs, and by non-radiative charge recombination (to the ground state) in closed PSII RCs (see Fig. 2 ). Thus, the RRP model is the second fundamental partial model, in addition to the TEG model, which must be considered in modelling the ChlFI.
If the formation of P680 + is considered in a model, then the reduction of P680 + must be also included, i.e. reactions on the donor side of PSII, as well as the recombination reactions between P680 + and Pheo ‒ or Q A ‒ . The P680 + is reduced by tyrosine 161 (i.e. Y Z ; Debus et al. , 1988 ), which is, in turn, reduced by OEC. Electrons are donated to Y Z + , by OEC, as it undergoes the S-state cycle ( Kok et al. , 1970 ; Fig. 3 ). Kok’s model of OEC is the third fundamental partial model for the description of PSII function. This model also includes parameters called ‘misses’ (when the light flash used does not lead to an S-state advancement) and ‘double hits’ (when the flash leads to an advancement by two S-states). Kok’s model has been modified by Jablonsky and Lazar (2008) by including the so-called intermediate S-states, which enable omission of the misses and double hits in the model.
Modelling of the fast Chl fluorescence rise measured after treatment with a herbicide
Because many photosynthetic processes affect ChlFI, herbicides that interrupt the ET from Q A to Q B have been used to simplify the observed curves. Note that many different herbicides are employed to kill weeds, and this can be achieved by using different substances that operate through various other mechanisms, but here we discuss only those that block the Q B -pocket of PSII. DCMU (3-(3′,4′-dichlorophenyl)-1,1-dimethylurea) is a herbicide that has been frequently used in such studies; it binds to the Q B -pocket, blocking ET beyond PSII (e.g. Oettmeier et al. , 1980 ), which leads to a faster closure of PSII RCs during illumination and to a faster accumulation of Q A ‒ . Binding of DCMU at the Q B -pocket results in a faster sigmoidal ChlF rise to its maximal value (F M ), which is reached approximately at the J step (~2 ms) of the ChlF rise, measured (under saturating light) with an untreated sample. The gradual binding of DCMU to the Q B -pocket of PSII, and thus the gradual closure of PSII, as reflected in changes in the O-J-I-P transient, was modelled by Lazár et al. (1998) . Here, the diffusion of DCMU was described using Fick’s laws, and the reaction of DCMU at the Q B -binding site of PSII, by second-order kinetics. From this work, Lazár et al. (1998) provided values of the diffusion coefficient of DCMU, and the second-order rate constant of DCMU binding to the Q B -pocket of PSII.
The sigmoidal shape of the fast ChlF rise measured with DCMU has been suggested to reflect energetic connectivity ( p ) between the PSII units ( Joliot and Joliot, 1964 ; also see above for discussion). This concept is tightly connected with a type of PSII heterogeneity, namely PSII α/β antenna heterogeneity ( Melis and Homann, 1975 ). The PSIIα units, the main PSIIs, have a large and energetically connected light-harvesting antenna. The size of the antenna is reflected in the rate constant of the fast ChlF rise, measured with DCMU, and PSII connectivity is reflected in the value of the parameter p ; the PSIIβ units have smaller antenna and a lower energetic connectivity. Several different procedures have been used to obtain quantitative information on this PSII heterogeneity (see Hsu et al. , 1989 ). To increase the reliability and accuracy in the determination of PSII antenna heterogeneity, Lazár et al. (2001) have fitted the values of rate constants, the parameter p and the fractions of particular PSII types to several curves of fast ChlF rise in the presence of DCMU, measured at different light intensities, by using just one fitting procedure; results from this work were in good agreement with those in the literature.
The fast ChlF rise measured with DCMU has also been explored using the RRP model by Trissl et al. (1993) , Lavergne and Trissl (1995) , and Trissl and Lavergne (1995) , with PSII energetic connectivity included. The RRP model has been further improved by Lazár and Pospíšil (1999) by the addition of P680 + reduction step(s) on the (electron) donor side of PSII; for this, they had used the fast ChlF rise in the presence of DCMU measured at high temperatures. Decreases in PSII energetic connectivity and in the rate of P680 + reduction by Y Z were suggested to occur in the photosynthetic samples kept at high temperatures (e.g. 47 °C for 5 min; Guissé et al. , 1995 ; Srivastava et al. , 1997 ), but these conclusions were based on results on samples, without DCMU. By contrast, Lazár and Pospíšil (1999) have simulated the fast ChlF rise, in the presence of DCMU, at high temperatures by omitting PSII energetic connectivity, and by decreasing the rate constants related to the electron donation to P680 + .
To study photoinhibition in DCMU-treated samples, Vavilin et al. (1998) and Lazár et al. (2005) have simulated fast ChlF rise curves by using the RRP model. Lazár et al. (2005) further extended the RRP model by considering a possible protective function of Cyt b 559 against photoinhibition, as proposed by Thompson and Brudvig (1988) and by Nedbal et al. (1992) . Cyt b 559 is indeed reduced by Pheo − , which then donates electrons to P680 + , involving a CEF around PSII. However, an argument against such an ET may be in the crystal structure of PSII (e.g. Zouni et al. , 2001 ; Kamiya and Shen, 2003 ), which shows that the distance from the Pheo in the active D1 branch of PSII and the Cyt b 559 is too long (~45 Å) to allow an ET between them. However, the distance between Pheo in the inactive D2 branch of PSII and the Cyt b 559 is shorter (22 Å), and ET by tunnelling has been reported for such distances ( Page et al. , 1999 ). Thus, the Pheo in the model of Lazár et al. (2005) could be Pheo in the D2 branch of PSII.
Modelling the fast Chl fluorescence rise by using only models of PSII reactions
Mathematical analyses of the fast ChlF rise were published in the 1960s ( Malkin and Kok, 1966 ; Malkin, 1966 ; Munday and Govindjee 1969 ). Munday and Govindjee (1969) measured the O-I-D-P (where D is for a dip) ChlF rise curve in Chlorella pyrenoidosa and related it successfully to variations in the fraction of reduced Q A . In their paper, the dip was analysed by studying the transient oxidation of Q A − by PSI.
In all likelihood, the first ‘real’ model of the fast ChlF rise [i.e. a scheme of ET reactions and a related set of coupled ordinary differential equations (ODEs)] was that of Holzapfel and Bauer (1975) . This model was rather complex: it described the complete ET chain in the TM, including the formation of NADPH and ATP. On the other hand, some details of the photosynthetic ET were not included in the model, due to limited knowledge of the photosynthesis process at that time. In this model, the ChlF was assumed to be proportional to the amount of Q A − . Holzapfel and Bauer (1975) were able to qualitatively simulate the rate of oxygen evolution at different light intensities, the fast ChlF rise of control samples, and of those treated with DCMU and/or 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone (DBMIB, which blocks the electron flow between PQ and Cyt b 6 /f; cf. Trebst and Reimer, 1973 ), as well as of samples that were dark-adapted under anaerobic conditions. This model was further used by Holzapfel (1978) , where the effect of ΔΨ across the TM was included. It is unclear why these models were missed by others. However, several models on the fast O-I-P ChlF rise, measured using light intensities lower than 1200 µmol photons m −2 s −1 , are available ( Renger and Schulze, 1985 ; Hsu, 1992 a , b ; Goltsev and Yordanov, 1997 ; Tomek et al. , 2003 ); these models were based on the TEG model, where ChlF signal was assumed to be proportional to the amount of reduced Q A (for an exception, see Goltsev and Yotdanov, 1997). Tomek et al. (2003) have further used the amplitude of the I step to estimate the fraction of ‘Q B -non-reducing centres’ (i.e. PSIIs which cannot reduce Q B ).
Different TEG models, and PSII redox states with reduced Q A to calculate the ChlF signal, were also used in modelling the O-J-I-P ChlF rise measured under saturating light (~3000 µmol photons m −2 s −1 ; Stirbet and Strasser, 1995 , 1996 ; Lazár et al. , 1997 ; Stirbet et al. , 1998 , 2001 ; Strasser and Stirbet, 2001 ; Tomek et al. , 2001 ; Sušila et al. , 2004 ). In these studies, the authors mainly showed how selected parameters of the models (e.g. initial concentrations and values of the rate constants) affect the shape of the O-J-I-P curves. However, Stirbet and Strasser (1996) showed that consideration of second-order kinetics for the reactions between Q A and Q B in the TEG model gives different simulated O-J-I-P curves compared to those obtained in the simulation where first-order kinetics is used. Strasser and Stirbet (1998) have also simulated O-J-I-P ChlF transients with a TEG model, by taking into account the heterogeneity of the PSII population in relation to PSII antenna, PSII energetic connectivity, and the ability of PSII to reduce Q B (‘Q B -reducing’ vs. ‘Q B -non-reducing’ RCs).
Sušila et al. (2004) considered a hypothetical sample divided into ten layers of the same thickness, and calculated the light intensity in each layer, based on the Lambert–Beer attenuation law, in order to determine the light gradient inside the sample. They then simulated the fast ChlF rise curve for each layer, by using the same model as in Lazár et al. (1997) and Tomek et al. (2001) , and summed the ChlF signal from all the layers to obtain the total ChlF signal. Their results showed that the light gradient inside a sample can significantly affect the shape of the fast ChlF transient. We note that in all the above models for the O-J-I-P ChlF rise, with the exception of those used by Stirbet et al . (1998 , 2001 ) and Strasser and Stirbet (1998, 2001 ), the presence of an unknown component X that accepts electrons from the Q B ‒ was assumed to exist.
Guo and Tan (2011) have extended the TEG model by taking in account the existence of a light-harvesting antenna system. Later, Feng et al. (2018) extended the above model by including the pH-dependent NPQ process, which allows the fitting of the decrease of the ChlF signal from the peak ‘P’ to ‘S’ and/or the ‘T’ level. To fit the O-J-I-P ChlF curves measured at different temperatures (20, 25, 30 °C), the rate constants in the model of Guo and Tan (2011) were assumed to be dependent on the temperature according to the Arrhenius law ( Xia et al. , 2018 ). Because the formation of 1 Chl* during illumination was included in the models used in all three studies above, the ChlF signal was defined as radiative deactivation of 1 Chl* in the PSII antenna.
In some of the models just mentioned, the function of the PSII donor side was implicitly included. By contrast, in the models of Stirbet et al . (1998 , 2001 ), Chernev et al. (2006) , Lazár and Jablonský (2009) , and Laisk and Oja (2018) , the function of the PSII donor side was included explicitly, and that too in combination with the TEG model. Stirbet et al . (1998 , 2001 ) not only included the S-states of OEC, but also the PSII energetic connectivity, and the quenching of the ChlF signal by P680 + and by the oxidized PQ molecules. Stirbet et al . (1998 , 2001 ) then simulated (or fitted) the O-J-I-P ChlF transient by defining the ChlF signal to be proportional to the amount of reduced Q A , and by considering different initial fractions of Q B and Q B ‒ , or of the S 1 and S 0 states of OEC. In the model of Lazár and Jablonský (2009) , all the S-state transitions of OEC were taken into account, as well as the redox states of P680 + that were explicitly considered in combination with the TEG model, which was then used for simulation of the O-J-I-P ChlF transient. In their study, the effect on the simulated fast ChlF curve was described by using (1) first- or second-order reaction kinetics for electron donation from the OEC to P680 + ; (2) one second-order reaction or two subsequent reactions for the Q B 2‒ /PQ exchange; and (3) all possible reactions between the ET components, or of fewer ‘logical’ reactions.
Other models used for simulation of the fast ChlF rise are those that include, in addition to the TEG model, the description of the fast events in the PSII RC (i.e. charge separation, recombination and stabilization) described by the RRP model. Models by Baake and Schlőder (1992) and Belyaeva et al. (2011) belong to this group, where reduction of P680 + by Y Z (via OEC) was implicitly included. Other authors ( Lazár, 2003 ; Zhu et al. , 2005 ; Matsuoka et al. , 2015 ) have explicitly included Y Z and the S-state transitions of OEC.
Lazár (2003) provided a detailed analysis of how values of particular rate constants and initial conditions affect the simulated fast O-J-I-P ChlF curves. An important aspect of the ChlFI curves analysed by simulations in this work is the origin of the minimal ChlF level (F O ) which is the initial ChlF, when all PSII RCs have all Q A in the oxidized state; F O originates from the radiative deactivation of the excited PSII state [(antenna-P680)*PheoQ A Q B ; see Fig. 7 ]. Interestingly, although the model of Lazár (2003) is one of the most detailed models of PSII reactions (consisting of a set of 44 coupled ODEs), yet it was not able to simulate typical O-J-I-P ChlF transients, as the ChlF signal increased from the J step to a maximum, which was reached at the I step position in the experimental curves ( Fig. 7 ).

Simulations of the O-J-I(=P) ChlF rise (see text) and of the model forms of photosystem (PS) II in the excited state, which mainly contribute to the (chlorophyll a ) fluorescence transient, are shown on a logarithmic time scale. Abbreviations: (L-P)*, the excited state of the PSII antenna, which is equilibrated among all light harvesting Chls, including P680; Ph, pheophytin; A and B, the first and second plastoquinone acceptors of PSII (Q A and Q B ). The time course of the PSII model form (L-P)*PhAB at the beginning of the transient, which represents excited open PSII RCs (i.e. with oxidized Q A ), is at the origin of the minimal ChlF, F O . Modified from Lazár (2003) .
The inability to simulate the proper time-dependence of the ChlF signal by the detailed model based only on PSII redox states is one of the arguments that a proper model for the O-J-I-P ChlF rise should also describe ET reactions occurring beyond the PQ pool, as already inferred by Munday and Govindjee (1969) and later confirmed in other studies (i.e. Schreiber et al. , 1989 ; Schansker et al ., 2003 , 2005 ).
Modelling the fast Chl a fluorescence rise with models that consider electron transport in and around the TM
The last group of models used in simulation of the O-J-I-P ChlF transients are those that include ET reactions occurring in and around the TM ( Lebedeva et al. , 2002 ; Kroon and Thoms, 2006 ; Lazár, 2009 ; Makarov et al. , 2012 ; Belyaeva et al ., 2016 , 2019 ), or even the metabolic reactions in the stroma (e.g. the Calvin–Benson cycle; see Laisk et al ., 2006 , 2009 ; Zhu et al. , 2013 ). Given the all-inclusive nature of these models, some of them were also used for modelling of the the entire ChlFI (see below). A diagram of the reactions considered in the model proposed by Lazár (2009) is shown in Fig. 8 . This model consists of a set of 42 coupled ODEs, and the ChlF emission is defined as being proportional to the amount of reduced Q A . In addition, the ΔA 820 signal, describing redox changes of P700 and plastocyanin (PC), was also modelled. To show that the ET reactions beyond the PQ pool affect the shape of the simulated fast ChlF transients, Lazár (2009) also analysed in silico the effects of DBMIB and MV [methylviologen, which accepts electrons from both the iron–sulfur cluster of PSI and ferredoxin (Fd); Sétif, 2015 ]. The shapes of the simulated fast ChlF transients and of ΔA 820 signal were qualitatively in agreement with the experimental curves (see Fig. 8 ). This model is also a part of e-photosynthesis.org ( Šafránek et al. , 2011 ), which is a web-based platform for modelling complex photosynthetic processes.

Diagram of the ET reactions used in the model of Lazár (2009) (A), the O-J-I-P ChlF transients measured on control (= untreated) leaves, as well as on leaves treated with 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone (DBMIB, which inhibits ET in the cytochrome b 6 /f, see A) or with methyl-viologen (MV, which accepts electrons from the iron–sulfur cluster of PSI and ferredoxin, Fd, see A) (B), and the respective curves simulated with the model (C), the Δ A 820 curves measured under the same conditions (D), and the respective curves simulated by the model (E). The curves are shown on a logarithmic time scale. Abbreviations: S i , the S-states of the oxygen-evolving complex (OEC); f, b L , b H … c, cytochrome f, low/potential cytochrome b 6 , and high-potential cytochrome b 6 in kinetic equilibrium with the haem c of cytochrome b 6 /f complex; PC, plastocyanin. Modified from Lazár (2009) .
In all the models mentioned above, the variable ChlF signal was assumed to originate from the PSII antenna. The problem with direct measurement of the variable ChlF from PSI in vivo (not from isolated PSI complexes) is that it overlaps spectrally with the PSII ChlF. However, some experimental results, presented in the literature (see Lazár, 2013 ), show the existence of a variable ChlF originating in PSI, at least under certain conditions. Lazár (2013) presented a very detailed model of the ET reactions in PSI (i.e. a set of 106 coupled ODEs), and simulated fast ChlF transients originating only from PSI. The ChlF signal was defined as the radiative deactivation of 1 Chl*. PSI was further shown to emit variable ChlF, and its contribution to the total maximal variable ChlF signal from the two PSI and PSII was ~8–17 % ( Lazár, 2013 ). Future studies are needed to quantitatively assess these findings.
Rule-based modelling of the fast Chl fluorescence rise
All the models of the fast ChlF rise discussed thus far have described the photosynthetic processes by using sets of coupled ODEs. Each ODE was used to describe the time-change of a particular PSII redox form (i.e. state variable) of the model. This approach is deterministic, because in any run of the model, the same solution is obtained.
If too many state variables (coupled ODEs) are considered in a model, it becomes difficult to obtain model results, due to high requirements of computational time and hardware; this is because all ODEs must be solved simultaneously at each time of system evolution. While there are ways (specific for each model) to decrease the number of equations, this problem can be better overcome by employing a rule-based modelling approach, where rules are defined that are equivalent to the particular ET reactions. Furthermore, random numbers are generated, and these determine (using internal decision process) which rules should be considered in each particular step of the model run, i.e. in each ‘evolvement’ of the system in time. Thus, a time course of the system behaviour would be described by a sequence of particular rules, which are slightly different in different model runs, i.e. small differences between solutions are obtained after different runs of the model. Thus, this approach would be stochastic (i.e. random). The rule-based stochastic approach by means of kinetic Monte Carlo simulations has been applied for modelling of the O-J-I-P ChlF transient by Xin et al. (2013) , Guo and Tan (2014) , Maslakov et al. (2016) and Antal et al. (2018) . However, in all these cases, the shapes of simulated ChlFI curves were the same (except for the noise) as when using the deterministic approach. Similarly, the O-J-I-P curve was also simulated using stochastic π-calculus ( Tokarčík, 2012 ) and rule-based language-simplified Kappa ( Nižnan, 2014 ). Much further work is needed to obtain conclusive results from this approach.
Modelling the slow PS(M)T phase of the Chl a fluorescence induction curve
The nomenclature of P-S-(M)-T for the slow phase of the ChlFI was first used by Papageorgiou and Govindjee (1968 a , b ). Compared with the fast ChlF rise, this phase is much more complex and less well understood, as the fluorescence yield is modulated by an increasing number of processes triggered during this phase, besides the photochemical quenching by Q A (see above), such as: (1) the NPQ of excited singlet 1 Chl* a in PSII antenna, induced by low pH in the lumen (i.e. the high-energy NPQ qE; Horton et al. , 1996 ; Rochaix, 2014 ); (2) state transitions (i.e. qT 12 or qT 21 ) that regulate the absorption CS of PSI and PSII (with ‘state 1’ being more fluorescent than ‘state 2’; see Papageorgiou and Govindjee, 2011 , 2014 ); (3) photoinactivation processes (qI) due to the photodamage of PSII (e.g. Tyystjärvi, 2013 ); (4) cyclic electron flow around PSI (e.g. Miyake, 2010 ; Buchert et al. , 2018 ), chlororespiration ( Bennoun, 1982 ) and electron flow to molecular oxygen ( Mehler, 1951 ; Asada, 1999 ); as well as (5) activation of the Calvin–Benson cycle. Therefore, besides the partial models necessary for modelling the fast ChlF rise discussed in the previous section (e.g. RRP, Kok’s oxygen clock, TEG, the Q-cycle at the Cyt b 6 /f complex), the processes listed above are fundamental for modelling the whole ChlFI; however, qT and qI, with a few exceptions, have been usually neglected by most authors.
Laisk et al. (1997) were the first to model the qE process, which they used later to simulate successfully the slow PS(M)T phase of ChlFI ( Laisk et al. , 2006 ). This qE model was later adapted by Zhu et al. (2013) for C 3 photosynthesis, but the descending M-T phase is missing in their simulated ChlFI curve. Note that these two papers were centred on the detailed description of metabolic reactions.
The transmembrane pmf , i.e. both ΔpH and ΔΨ, was modelled by Lebedeva et al. (2002) , which predicts that a sufficiently large transmembrane electric potential (positive inside) would slow the rate of PQH 2 oxidation by the Cyt b 6 /f (the so-called backpressure effect; see van Kooten et al. , 1986 ), and consequently the ET rate from PSII to PSI (see also comments in Stirbet et al. , 2014 ). This pmf model was further used by, for example, Rubin et al. (2009) and Belyaeva et al . (2016 , 2019 ) to model the complete ChlFI curve, with a TM model that describes the electron/proton transfer reactions between membrane protein complexes: PSII, PSI, Cyt b 6 /f, mobile PQ pool in the TM, PC in lumen and Fd in stroma, CEF-PSI, and reduction of NADP + via Fd-NADP + -oxidoreductase (FNR) (see Fig. 1 ). Belyaeva et al. (2016) used the TM model to fit both ChlFI data and P700 redox changes (Δ A 810 ), measured in pea leaves, from 20 μs to 20 s. Belyaeva et al. (2019) added to their earlier TM model partial models for the light-induced activation of FNR and qE, with the goal to simulate the ChlFI and Δ A 810 kinetics on the time scale from 40 μs to 30 s. Their results showed that the time-dependent rate constants changed substantially upon the release of ET on the (electron) acceptor side of PSI and during qE induction. Belyaeva et al. (2019) also discussed differences between the parameters related to FNR activation and qE induction evaluated for dark-adapted and pre-illuminated pea leaves, and also analysed the transition between CEF-PSI and LEF modes.
Because the photosynthetic organisms are exposed continuously to fluctuations in the environmental conditions, the activity of their photosynthetic apparatus is dynamic, being feedback-regulated by several processes that reduce imbalances between the rate of energy trapping by the PSs and CO 2 assimilation. These serve to optimize the photosynthetic ET to, for example, light-induced pH changes in the lumen and in the stroma (see Tikhonov, 2013 ; Rochaix, 2014 ; Strand and Kramer, 2014 ), or changes in the PQ pool redox state, as modulated by variations in light irradiance, ATP/ADP ratio and the ambient CO 2 level ( Rochaix, 2014 , 2016 ). Light-induced acidification of the lumen slows down PQH 2 oxidation by the Cyt b 6 /f (the backpressure effect), and also decreases PSII activity by inducing excitonic energy dissipation as heat in PSII antenna through qE ( Jahns and Holzwarth, 2012 ; Rochaix, 2014 , 2016 ). This reduces the excess of input energy in the system, and thus oxidative damage ( Nishiyama et al. , 2006 ), which occurs when singlet excited 1 Chl* forms triplet-state Chl ( 3 Chl) ( Durrant et al. , 1990 ) that interacts with ground state oxygen, generating ‘noxious’ reactive oxygen species (ROS), such as singlet oxygen ( 1 O 2 ). Furthermore, the alkalization of stroma activates the Calvin–Benson cycle, which stimulates the consumption of NADPH and ATP ( Werdan et al. , 1975 ; Noctor and Foyer, 2000 ). As shown earlier, state transitions re-equilibrate PSI and PSII activities through changes in their absorption CS, which are triggered by PQ pool redox state changes (for plants and algae, see reviews by Rochaix, 2014 , 2016 ; Goldschmidt-Clermont and Bassi, 2015 ), and involve phosphorylation/dephosphorylation of the PSII mobile antenna by kinases and phosphatases (i.e. STN7/TAP38 in Arabidopsis thaliana , or Stt7/Pph1 in Chlamydomonas reinhardtii ; Rochaix et al. , 2012 ). Furthermore, during induction of the Calvin–Benson cycle, changes in illumination, or anaerobiosis, photosynthetic electron fluxes are optimally redistributed between the linear electron transport (LET) from water to NADP + , and alternative electron pathways, i.e. cyclic electron flows, pseudocyclic O 2 -dependent electron flows and the malate valve ( Backhausen et al. , 2000 ; Miyake, 2010 ; Hemschemeier and Happe, 2011 ).
Modelling the state transition process
Ebenhöh et al. (2014) were the first to model state transitions in plants and algae based on a mechanism, described by Allen et al. (1981) ; they investigated the dynamics and regulation of state transitions by simulating experimental PAM-SP curves from Chlamydomonas reinhardtii cells, grown under dim light, and thus with little capacity for qE, having a low LHCSR3 content ( Peers et al. , 2009 ). Here, a simplified mathematical model (based on eight coupled ODEs) was used, where the most relevant ET routes, necessary for modelling state transitions in this green alga, were used: LEF, CEF-PSI, and chlororespiration through the plastid terminal oxidase PTOX (see Fig. 1 ; and Bennoun, 1982 ; McDonald et al. , 2011 ). Individual reactions of the Calvin–Benson cycle were treated implicitly, using steady-state consumption of NADPH and ATP, and a quasi-steady state approximation for the dynamics of oxygen evolution and charge separation in PSII. For simplicity, in the partial model of state transitions, it was assumed that the PSII mobile antennas phosphorylated by the kinase Stt7 (activated by the PQ pool reduction) are relocated directly to PSI (i.e. state 1 to state 2 transition, qT 12 ); also, after the Stt7 inhibition (triggered by the PQ pool oxidation), the PSII mobile antennas are dephosphorylated by the phosphatase Pph1, and directly re-associate with PSII (i.e. state 2 to state 1 transition, qT 21 ) (see Fig. 4 ). Finally, the ChlF signal is defined by the ratios of rate constants related to fluorescence emission, heat dissipation and photochemistry, which include changes in the absorption CS of PSI and PSII (due to state transition). Ebenhöh et al. (2014) successfully simulated with their model the main features of the experimental fluorescence signal measured with a PAM instrument from dark-adapted wild-type Chlamydomonas cells illuminated for 10 min with low light (100 μmol photons m −2 s −1 ). The saturating F M ′ peaks during illumination reflect changes in the antenna CS of PSII (i.e. a partial state transition to ‘state 2’), which take place in parallel with the establishment of a stationary redox poise of the PQ pool.
State transitions were also modelled by Stirbet and Govindjee (2016) , with the goal to simulate the slow PS(M)T phase of the ChlFI, in order to determine the origin of the S–M rise of Chlamydomonas reinhardtii cells (see Kodru et al. , 2015 ; Zhou et al. , 2015 ). Here, the photosynthesis model of Ebenhöh et al. (2014) was adapted for the simulation of ChlF data obtained by using a Plant Efficiency Analyser (PEA; Hansatech, UK). Stirbet and Govindjee (2016) confirmed that, under anaerobic conditions, in darkness, the PQ pool reduction through chlororespiration triggers a state 1 to state 2 transition (see Fig. 9A ), when the relative CS of PSII (CSII) is lower than that of PSI (see Bulté et al. , 1990 ). Next, it was shown that, during the subsequent illumination, the hypothetical sample undergoes a transition from this ‘state 2’ to a ‘state 1’, which is the origin of the slow S-M fluorescence rise (see Fig. 9B ). However, if the dark-adaptation period is too short, and the transition to ‘state 2’ in darkness is not complete, the subsequent illumination triggers a state 1 to state 2 transition (see Fig. 9C ). We note, however, that the M-T fluorescence decline observed experimentally ( Kodru et al. , 2015 ; see also Fig. 6B ) is missing in the simulated curves, and, thus, further research is needed to determine its origin.

Simulated kinetics of the fraction of the oxidized plastoquinone (PQ) pool (i.e. PQ/PQ tot ) and the relative absorption cross-section of photosystem (PS) II (i.e. CSII) during dark adaptation under anoxic conditions of a hypothetical sample of Chlamydomonas reinhardtii cells (see A), as well as simulated time courses of PQ/PQ tot , CSII and Chl fluorescence induction (F) during illumination in the presence of oxygen of the hypothetical sample after 600 s (see B) and 200 s (see C) anoxic dark adaptation. Note that a decrease in CSII reflects a state 1 to state 2 transition, while an increase reflects a state 2 to state 1 transition. Modified from Stirbet and Govindjee (2016) .
Stirbet and Govindjee (2016) also examined in silico the influence of different factors on the amplitude of the S-M fluorescence rise under low light conditions (~100 to 300 μmol photons m −2 s −1 ). For example, they found that, under conditions that trigger a qT 21 during a dark-to-light transition (i.e. reduced PQ pool, and CSII < 0.5 at the beginning of illumination), an increase in the CEF-PSI rate leads to a lower CSII increase at the end of the state transition, and a smaller amplitude of the S-M fluorescence rise (see Fig. 10A ). This simulation also confirmed that, when the CEF-PSI is much more rapid, the ATP level increases, while the NADPH level decreases. When the light intensity is higher, the simulations also showed a decrease in the S-M fluorescence rise. This result is in agreement with the experimental ChlFI data on Chlorella published by Papageorgiou and Govindjee (1968 a ), who showed that the slow S-M fluorescence rise is larger at lower exciting light intensities. By contrast, under other conditions taken into account by Stirbet and Govindjee (2016) , such as the increase in NADPH and ATP consumption by the Calvin–Benson cycle, or an increase in the rate of the Mehler reaction, the S-M amplitude increased, due to a larger increase in the PSII CS during the qT 21 (see Fig. 10B ). However, the increase in the S-M rise becomes saturated by further increasing these rate constants. The conclusion is that the factors reducing the PQ pool (e.g. higher light intensity, or more rapid CEF-PSI) decrease the S-M amplitude, and those that oxidize further the PQ pool (e.g. more rapid NADPH consumption or Mehler reaction) increase the S-M amplitude.

Simulated kinetics of the fraction of the oxidized plastoquinone (PQ) pool (i.e. PQ/PQ tot ), the relative absorption cross-section of photosystem (PS) II (i.e. CSII), and of chlorophyll (Chl) fluorescence induction (F) during illumination in the presence of oxygen of a hypothetical sample of Chlamydomonas reinharditii cells dark-adapted for 600 s under anoxic conditions, by considering that: (1) the illumination is equivalent to 100 μmol photons m −2 s −1 , and the rate constant of the cyclic electron flow (CEF) around PSI is k Cyc = 1 or 5 s −1 (see A); and (2) the illumination is equivalent to 300 μmol photons m −2 s −1 , the rate constant of CEF-PSI is 1 s −1 , and that of the Mehler reaction (i.e. ET from ferredoxin to O 2 ) k O2 = 0 or 11 s −1 (see B). Note that an increase in CSII reflects a state 2 to state 1 transition. These simulations show that the S-M fluorescence rise decreases when light intensity increases or when CEF-PSI is faster, but increases when the Mehler reaction is also functioning. Modified from Stirbet and Govindjee (2016) .
Modelling the qE component of NPQ
Because NPQ in plants and algae is associated with LHCs of PSII (see Horton et al. , 1996 ; Tian et al. , 2017 ), models simulating qE usually include reactions around PSII, and focus on describing the ChlFI (see reviews by Zaks et al. , 2013 ; Matuszyńska and Ebenhöh, 2015 ). Different photosynthesis models have been used to simulate either ChlFI curves measured with instruments using direct light (e.g. PEA), or with PAM-SP fluorometers (for a review see Stirbet et al. , (2014) . But, of course, the main phenomenon under analysis with either of these instruments is the same. Besides measurements of ChlF lifetime (e.g. Gilmore et al ., 1995 , 1998 ; Sylak-Glassman et al. , 2016 ), measurements of Chl fluorescence yield with PAM-SP fluorometers are especially suitable for the study of NPQ processes ( Müller et al. , 2001 ). It is clear that models that simulate experimental PAM data are valuable tools to analyse the qE component of NPQ.
Several original qE models have been proposed by, for example, Ebenhöh et al. (2011) and Zaks et al. (2012) ; these have been used for the simulation of the dynamics of ChlF quenching, as measured by PAM-SP instruments (see review by Stirbet et al. , 2014 ). Now, photosynthesis models that include qE are available ( Ebenhöh et al. , 2014 ; Matuszyńska et al ., 2016 , 2019 ; Snellenburg et al. , 2017 ; Morales et al ., 2018 a , b ).
The qE model of Ebenhöh et al. (2014) takes into account the induction of qE by low pH in the lumen (see above), but it is based on the simplifying assumption that the xanthophyll cycle is the only component involved in qE-dependent quenching. Thus, it was assumed that the decrease in lumenal pH leads directly to the formation of ‘Z’ through the xanthophyll cycle (i.e. the de-epoxidation of V via A), which then acts as a fluorescence quencher in the PSII antenna; the quencher acts by increasing the rate constant of the non-radiative deactivation of the 1 Chl*. Furthermore, the qE is reversed in darkness as Z is epoxidized to V by an active epoxidase. The results of the simulations, obtained with this qE model, showed that high light illumination leads to a plateau of the PQ pool redox state, which is relatively constant for a range of CSII values. Based on these theoretical results, Ebenhöh et al. (2014) concluded that, due to qE induction, the requirement to adjust the antenna CS through state transition under high light is much lower than under low light conditions. Indeed. Allorent et al. (2013) showed that the phosphorylation of LHCII antenna, mainly mediated by the STN7/Stt7 kinase in low light, is inhibited by high light, due either to a negative regulation of the kinase through the thioredoxin pathway under high light (see e.g. Lemeille and Rochaix, 2010 ), or to a conformational change in the PSII antenna ( Vink et al. , 2004 ).
To avoid the harmful effects of over-excitation, plants optimize their photosynthetic performance based on their illumination history through a process in which Z seems to play a key role (e.g. Ruban et al. , 2012 ). Matuszyńska et al. (2016) used a combined experimental and theoretical approach in the study of qE, particularly designed to determine if plants have a ‘memory’ of their recent (minutes to hours) light exposure, similar to what occurs after really long (days, months) periods of stress ( Demmig et al. , 1987 ; Adams and Demmig-Adams, 2004 ). In these studies, fluorescence measurements were made on Epipremnum aureum (a shadow (shade)-tolerant, ornamental plant) by PAM-SP. Here, F M ′ was used instead of NPQ, as suggested by Holzwarth et al. (2013) , to avoid mathematical distortion of the ChlF quenching kinetics. Additionally, the pigment composition was measured at the end of each phase of the experiment, in order to determine the contribution of Z to the ‘memory’ effect. These data confirmed the presence of a short-term ‘memory’ effect, which is influenced by both light intensity and the period of dark-relaxation between two light exposures. Matuszyńska et al. (2016) concluded that the ‘memory’ of recent light exposure related to qE can be assigned to dynamic changes in pigment composition, being due to a slower conversion of Z to V, as observed by, for example, Demmig et al. (1987) and Reinhold et al. (2008) . By implementing a qE model based on the ‘4 state-2-site quenching’ system ( Holzwarth and Jahns, 2014 ) in the photosynthesis model of Ebenhöh et al. (2014) (but without state transitions), Matuszyńska et al. (2016) were able to simulate successfully changes in the quantum yield of ChlF during the PAM-SP experiments, discussed above. In these simulations, the ChlF signal was also calculated using ratios of rate constants related to fluorescence emission, heat dissipation and photochemistry, where the rate constant of the heat dissipation was assumed to be modulated by the concentration of a quencher (Q), which was, in turn, calculated by taking into account the concentrations at any time of both Z and the protonated PsbS protein. [Note that Snellenburg et al. (2017) and Morales et al . (2018 a , b ) have used similar qE models, depending on the relative concentrations of Z and protonated PsbS.]
Modelling alternative electron flows
Besides LEF, which provides the Calvin–Benson cycle with NADPH and ATP, other ET routes function during oxygenic photosynthesis (see Fig. 1 ; Alric and Johnson, 2017 ; Shikanai and Yamamoto, 2017 ): (1) CEF-PSI via ferredoxin-plastoquinone reductase, or NADPH dehydrogenase (NDH); and (2) ‘alternative’ non-cyclic pathways that involve reduction of electron acceptors such as O 2 [the water–water cycle (WWC); see a model by Valero et al. (2009) ], or oxaloacetate [by malate dehydrogenase (MDH); see a model by Fridlyand et al. (1998) ]. The main role of CEF-PSI is to increase the ATP/NADPH ratio, as ‘required’ by the metabolic reactions in stroma or other energy-dependent processes in the chloroplast; furthermore, the pH difference, which induces qE, protects PSI and PSII against photoinhibition ( Strand et al , 2016 , 2017 ). The electron pathway to molecular oxygen (Mehler reaction, WWC), besides contributing to the acidification of the lumen and to the reduction of the excitation pressure on PSs, is also important in chloroplast redox signalling during abiotic stress, and in the regulation of CEF-PSI ( Miyake, 2010 ). The respective contributions of alternative electron pathways to the total ET is strictly regulated, depending on environmental conditions, but further research is needed to understand how these diverse pathways and their regulatory mechanisms function (see Yamori et al. , 2016 ; Nawrocki et al. , 2019 ).
Comprehensive dynamic C 3 photosynthesis models, such as those by Laisk et al . (2006 , 2009 ) and Zhu et al. (2013) , include light reactions, proton and electron transport, detailed carbon metabolism reactions, exchange of intermediates between cytosol and stroma, photorespiration, amino acid synthesis, and regulatory mechanisms. However, because these models involve a large number of model parameters, simplified photosynthesis models are much more suitable, and practical, for the study of dynamic responses of the photosynthetic apparatus to diverse changes of environmental factors. Indeed, a number of simplified photosynthesis models have been used in several studies to analyse PETC regulation in silico , through simulation of experimental data measured with a variety of methods ( Ebenhöh et al. , 2011 ; Zaks et al. , 2013 ; Tikhonov and Vershubskii, 2014 ; Stirbet and Govindjee, 2016 ; Snellenburg et al. , 2017 ; Morales et al ., 2018 a , b ; Matuszyńska et al ., 2016 , 2019 ). According to Morales et al. (2018 b ), the term ‘regulation’ means: reaching simultaneously, during environmental fluctuations, a suitable redox state of PETC, dissipation of excess excitation energy and ATP/NADPH ratio through adjustments of NPQ processes, CEF-PSI and reduction of alternative electron acceptors (also including the reduction of NO 2 ‒ during NH 4 + assimilation, NiR), as well as pmf optimization through changes in ATP synthase activity.
We have reviewed above results obtained in studies of photosynthesis regulation through state transitions and qE, based on simulations of ChlFI data. By contrast, Morales et al. (2018 b ) used, for simulations, several types of experimental data on Arabidopsis thaliana , such as PAM-SP ChlF data (for effective quantum yield of PSII and NPQ), Δ A 820 (for the P700 redox state, which is related to LET and alternative ET pathways), the electrochromic shift in A 520 (for pmf and its components), and net CO 2 assimilation ( A n , for the Calvin–Benson cycle and CO 2 diffusion). The results of these simulations have shown that CEF-PSI and alternative ET pathways are strongly interacting, and, thus, changes in FQR- or NDH-dependent CEF-PSI kinetics indirectly influence WWC, NiR and MDH activities, due to changes in the redox state of Fd. It is also known that the steady-state pH in the lumen cannot be controlled only by CEF-PSI and alternative ET, because it is also greatly affected by the pH sensitivity of qE, Cyt b 6 /f and ATP synthase. Additionally, Morales et al. (2018 b ) have examined the influence of the ADP/ATP ratio in stroma on the metabolic regulation of ATP synthesis, and their simulations showed that there is a coordination between the regulation of Rubisco, NPQ and PETC over a large range of light intensities and CO 2 concentrations. These are important observations for programming plants for better productivity.
MODELLING THE REGULATORY DEPENDENCE BETWEEN THE LIGHT REACTIONS AND THE CARBON REACTIONS
The slow part of the ChlFI induction also reflects changes due to the induction of the Calvin–Benson cycle during a dark to light transition. The activation and gradual increase in CO 2 assimilation during this phase leads to a parallel activation of ATP synthesis and an increase in the rate of LEF, which decreases the initial excitation pressure. As a result: (1) the level of Q A reduction decreases and photochemical quenching increases; and (2) qE decreases, because, due to a faster synthesis of ATP, the ΔpH decreases. Therefore, only models that include the induction of the Calvin–Benson cycle are suitable for correctly modelling the slow part of the ChlFI induction (see Laisk et al ., 2006 , 2009 ; Zhu et al. , 2013 ).
The Calvin–Benson cycle is one of the best-studied plant metabolic processes. Besides photosynthesis models, which include both the light and carbon reactions (e.g. Laisk et al ., 2006 , 2009 ; Zhu et al. , 2013 ; Belassio, 2019 ; Matuszyńska et al. , 2019 ), the carbon assimilation was often modelled separately, by considering a simplified relationship for NADPH and ATP supply (see review by Jablonsky et al. , 2011 ). In these models, carbon metabolism was analysed either by taking into account the kinetic properties of the enzymes involved, i.e. dynamic modelling ( Pettersson and Ryde-Pettersson, 1988 ; Zhu et al. , 2007 ), or without the need to use these, i.e. stoichiometric modelling ( Boyle and Morgan, 2009 ). In addition, a combination of both the above approaches has also been used ( Fleming et al. , 2010 ). In many models for the Calvin–Benson cycle, the steady-state behaviour of the photosynthetic apparatus has been analysed based on the equations of Farquhar et al . (1980) . Here we briefly mention some recent results on (short-term) regulation of photosynthesis obtained with the photosynthesis models of Morales et al. (2018 a ), Belassio (2019) and Matuszyńska et al. (2019) .
Fluctuating irradiances, which were shown to limit the performance of photosynthesis ( Pearcy, 1990 ), can be due to transient sun exposure, penumbra effects, shading by clouds, gaps in the canopy that produce ‘light (sun) flecks’, or movements of the leaves in the wind. Morales et al. (2018 a ) used a simplified dynamic model of CO 2 assimilation in a leaf to analyse the effects of fluctuating irradiances. In this study, they extended the canonical steady-state model by adding original empirical (phenomenological) partial models for the effects of chloroplast movement (qM; Dall’Osto et al. , 2014 ; Baránková et al. , 2016 ; Semer et al ., 2018 , 2019 ), qE, qI, regulation of enzyme activity in the Calvin–Benson cycle, metabolite concentrations, and the dynamic CO 2 diffusion through different leaf compartments. Changes in qE were assumed to follow PsbS protonation and Z generation, as was the case with the approach used by Matuszyńska et al. (2016) . With their model, Morales et al. (2018 a ) analysed potential improvements in CO 2 assimilation that may result after removing the kinetic limitation of different regulatory processes. Their simulations predicted that the most limiting steps in the carbon reactions are the activation rates of the Calvin–Benson cycle enzymes and stomatal opening (up to 17 % improvement), followed by the rate of qE relaxation and chloroplast movement (up to 10 % improvement), depending on the frequency of light fluctuations. However, up to 32 % improvement in CO 2 assimilation has been predicted, when all the kinetic limitations were simultaneously removed. Belassio (2019) has presented a dynamic photosynthesis model which also includes both light and carbon reactions, coupled to a mechanistic hydro-mechanical partial model for stomatal behaviour. This model successfully simulates responses to rapid changes in light intensity (light flecks), as well as in atmospheric CO 2 and O 2 concentrations. This model is freely available (as a supplement to the paper), and runs as a stand-alone workbook in Microsoft Excel.
Finally, Matuszyńska et al. (2019) have proposed a dynamic photosynthetic model describing the light reactions and the Calvin–Benson cycle in C 3 plants, for which they have used their earlier models [for light reactions: Ebenhöh et al. (2014) and Matuszyńska et al. (2016) ; for carbon reactions: Pettersson and Ryde-Pettersson (1988) and Poolman et al. (2000) ]. This newly merged model is based on nine coupled ODEs for the PETC, and 15 coupled ODEs for the carbon reactions. Analysis of this model shows the need for a ‘stand-by’ mode of the Calvin–Benson cycle in darkness, so that it can be restarted after prolonged dark periods; in this sense, the oxidative pentose phosphate pathway can play this function. Matuszyńska et al. (2019) have also used MCA (e.g. Visser and Heijnen, 2002 ) and metabolic supply–demand analysis ( Hofmeyr and Cornish-Bowden, 2000 ) to investigate the regulatory dependence between the PETC and the Calvin–Benson cycle, and to quantify the ‘control distribution’ of supply and demand under different light conditions and CO 2 assimilation rates. Th results obtained with MCA have indicated that, when CO 2 is saturating, the demand reactions control the flux under light-saturating conditions (with seduheptulose-1,7- bis phosphatase maintaining the highest overall flux control; see Poolman et al. , 2000 ), while the supply reactions display higher overall flux control under light-limited conditions, with PSII and PSI activities sustaining the highest overall flux control.
CONCLUSIONS
In this review, we have shown the important role played by models in deciphering and untangling different less well-understood and complex processes of photosynthesis, emphasizing the necessity and importance of modelling in the analysis of hypotheses developed from experimental studies. One major example, used in this review, is the ChlFI, which is simultaneously influenced by various photosynthetic processes affecting different segments of the fluorescence transient. As shown here, this process has been simulated by many modellers, who were focused either on understanding the dynamics of the redox states of different PETC components (see also reviews by Lazár, 1999 ; Lazár and Schansker, 2009 ; Stirbet et al. , 2014 ), or that of more complex, regulatory mechanisms involved in processes such as state transitions and qE, or of the relative contributions of alternative ET pathways, as well as their relationship with the CO 2 assimilation (the Calvin–Benson cycle) (see also Stirbet et al ., 2014 ). From the examples discussed in this review, it is evident that correctly simplified but complete dynamic models of photosynthesis are well suited to obtaining information about how the photosynthetic organisms cope with variable environmental conditions (see also Matuszyńska and Ebenhöh, 2015 ). Indeed, modelling is a very efficient method to identify important morphological and physiological parameters of a biological system and to find their optimal values. In addition, by using a larger variety of experimental data to verify such models, the simulations can lead to much more meaningful information about the organizational principles of the photosynthetic apparatus, which can also reveal original ways and means to improve the photosynthetic efficiency of plant crops ( Zhu et al. , 2007 ; Rosenthal et al. , 2011 ; Kromdijk et al. , 2016 ), besides being of theoretical interest. Moreover, multi-scale plant models (also known as plant system models), which quantitatively integrate physical, biochemical and physiological processes at different organizational levels (e.g. molecular, cell, organ, plant, population, or ecosystem), are able to predict physiological and growth properties of plants beyond photosynthetic metabolism, and they represent the future challenge in plant modelling (see Zhu et al. , 2016 ; Marshall-Colón et al. , 2017 ; Chang et al. , 2019 ; Marshall-Colón and Kliebenstein, 2019 ).
D.L. was supported by European Regional Development Fund project ‘Plants as a tool for sustainable global development’ [No. CZ.02.1.01/0.0/0.0/16_019/0000827].
ACKNOWLEDGEMENTS
Govindjee is grateful for IT support provided by the UIUC Life Sciences Office of Information Technology (Andrew Debevec, Karl Schlipf, Thomas Uebele, Jeffrey Haas), and the staff of the Department of Plant Biology and of the Department of Biochemistry, University of Illinois at Urbana-Champaign; he encourages all readers to visit his web site ( http://www.life.illinois.edu/govindjee/ ) to download available educational material on photosynthesis for personal use.
LITERATURE CITED
- Adams WW III, Demmig-Adams B. 2004. Chlorophyll fluorescence as a tool to monitor plant response to the environment. In: George C, Papageorgiou GC, Govindjee eds: Chlorophyll a fluorescence: a signature of photosynthesis. Advances in photosynthesis and respiration . Vol. 19 Dordrecht: Springer, 583–604. [ Google Scholar ]
- Allen JF. 2002. Plastoquinone redox control of chloroplast thylakoid protein phosphorylation and distribution of excitation energy between photosystems: discovery, background, implications . Photosynthesis Research 73 : 139–148. [ PubMed ] [ Google Scholar ]
- Allen JF. 2003. State transitions – a question of balance . Science 299 : 1530–1532. [ PubMed ] [ Google Scholar ]
- Allen JF, Bennett J, Steinback KE, Arntzen CJ. 1981. Chloroplast protein phosphorylation couples plastoquinone redox state to distribution of excitation-energy between photosystems . Nature 291 : 25–29. [ Google Scholar ]
- Allen MB, Whatley FR, Arnon DI. 1958. Photosynthesis with isolated chloroplasts. VI. Rates of conversion of light into chemical energy in photosynthetic phosphorylation . Biochimica et Biophysica Acta 27 : 16–23. [ PubMed ] [ Google Scholar ]
- Allorent G, Tokutsu R, Roach T, et al.. 2013. A dual strategy to cope with high light in Chlamydomonas reinhardtii . Plant Cell 25 : 545–557. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Alric J. 2010. Cyclic electron flow around photosystem I in unicellular green algae , Photosynthesis Research 106 : 47–56. [ PubMed ] [ Google Scholar ]
- Alric J, Johnson X. 2017. Alternative electron transport pathways in photosynthesis: a confluence of regulation . Current Opinion in Plant Biology 37 : 78–86. [ PubMed ] [ Google Scholar ]
- Antal TK, Maslakov A, Yakovleva OV, Krendeleva TE, Riznichenko GY, Rubin AB. 2018. Simulation of chlorophyll fluorescence rise and decay kinetics, and P 700 -related absorbance changes by using a rule-based kinetic Monte-Carlo method . Photosynthesis Research 138 : 191–206. [ PubMed ] [ Google Scholar ]
- Arnon DI. 1984. The discovery of photosynthetic phosphorylation . Trends in Biochemical Sciences 9 : 258–262. [ Google Scholar ]
- Arnon DI, Allen MB, Whatley FR. 1954 a Photosynthesis by isolated chloroplasts . Nature 174 : 394–396. [ PubMed ] [ Google Scholar ]
- Arnon DI, Whatley FR, Allen MB. 1954 b Photosynthesis by isolated chloroplasts. II. Photosynthetic phosphorylation, the conversion of light energy into phosphate bond energy . Journal of the American Chemical Society 76 : 6324–6329. [ Google Scholar ]
- Asada K. 1999. The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons . Annual Review of Plant Physiology and Plant Molecular Biology 50 : 601–639. [ PubMed ] [ Google Scholar ]
- Avron M. 1963. A coupling factor in photophosphorylation . Biochimica et Biophysica Acta 77 : 699–702. [ Google Scholar ]
- Baake E, Schlöder JP. 1992. Modelling the fast fluorescence rise of photosynthesis . Bulletin of Mathematical Biology 54 : 999–1021. [ Google Scholar ]
- Backhausen JE, Kitzmann C, Horton P, Scheibe R. 2000. Electron acceptors in isolated intact spinach chloroplasts act hierarchically to prevent over-reduction and competition for electrons . Photosynthesis Research 64 : 1–13. [ PubMed ] [ Google Scholar ]
- Baránková B, Lazár D, Nauš J. 2016. Analysis of the effect of chloroplast arrangement on optical properties of green tobacco leaves . Remote Sensing of Environment 174 : 181–196. [ Google Scholar ]
- Bassham JA. 2005. Mapping the carbon reduction cycle: a personal perspective. In: Govindjee, Beatty JT, Gest H, Allen JF, eds. Discoveries in photosynthesis. Advances in photosynthesis and respiration . Dordrecht: Springer, 815–832. [ Google Scholar ]
- Bay Z, Pearlstein RM. 1963. A theory of energy transfer in the photosynthetic unit . Proceedings of the National Academy of Sciences of the USA 50 : 1071–1078. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Belassio C. 2019. A generalised dynamic model of leaf-level C3 photosynthesis combining light and dark reactions with stomatal behavior . Photosynthesis Research 141 : 99–118. [ PubMed ] [ Google Scholar ]
- Belyaeva NE. 2004. Generalized model of primary photosynthetic processes in chloroplasts . PhD thesis, Lomonosov Moscow State University. [ Google Scholar ]
- Belyaeva NE, Bulychev AA, Riznichenko GYu, Rubin AB. 2011. A model of photosystem II for the analysis of fast fluorescence rise in plant leaves . Biophysics 56 : 464–477. [ PubMed ] [ Google Scholar ]
- Belyaeva NE, Bulychev AA, Riznichenko GYu, Rubin AB. 2016. Thylakoid membrane model of the Chl a fluorescence transient and P700 induction kinetics in plant leaves . Photosynthesis Research 130 : 491–515. [ PubMed ] [ Google Scholar ]
- Belyaeva NE, Bulychev AA, Riznichenko GYu, Rubin AB. 2019. Analyzing both the fast and the slow phases of chlorophyll a fluorescence and P700 absorbance changes in dark-adapted and preilluminated pea leaves using a thylakoid membrane model . Photosynthesis Research 140 : 1–19. [ PubMed ] [ Google Scholar ]
- Benson AA. 2005. Following the path of carbon in photosynthesis: a personal story. In: Govindjee, Beatty JT, Gest H, Allen JF, eds. Discoveries in photosynthesis. Advances in photosynthesis and respiration . Dordrecht: Springer, 793–813. [ PubMed ] [ Google Scholar ]
- Bennoun P. 1982. Evidence for a respiratory chain in the chloroplast . Proceedings of the National Academy of Sciences of the USA 79 : 4352–4356. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Blackman FF. 1905. Optima and limiting factors . Annals of Botany 19 : 281–295. [ Google Scholar ]
- Blankenship RE. 2014. Molecular Mechanisms of Photosynthesis , 2nd edn.Oxford: Wiley-Blackwell. [ Google Scholar ]
- Bonaventura C, Myers J. 1969. Fluorescence and oxygen evolution from Chlorella pyrenoidosa . Biochimica et Biophysica Acta 189 : 366–383. [ PubMed ] [ Google Scholar ]
- Bouges-Bocquet B. 1973. Electron transfer between two photosystems in spinach chloroplasts . Biochimica et Biophysica Acta 31 : 250–256. [ PubMed ] [ Google Scholar ]
- Boyer PD. 2002. A research journey with ATP synthase . The Journal of Biological Chemistry 277 : 39045–39061. [ PubMed ] [ Google Scholar ]
- Boyle NR, Morgan JA. 2009. Flux balance analysis of primary metabolism in Chlamydomonas reinhardtii . BMC Systems Biology 3 : 4. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Breton J. 1982. The 692 nm fluorescence (F695) of chloroplasts at low temperature is emitted from the primary acceptor of photosystem II . FEBS Letters 147 : 16–20. [ Google Scholar ]
- Briantais J-M, Vernotte C, Picaud M, Krause GH. 1979. A quantitative study of the slow decline of chlorophyll a fluorescence in isolated chloroplasts . Biochimica et Biophysica Acta 548 : 128–138. [ PubMed ] [ Google Scholar ]
- Brooks MD, Jansson S, Niyogi KK. 2014. PsbS-dependent non-photochemical quenching. In: Demmig-Adams B, Garab G, Adams WW III, Govindjee, Sharkey TD, eds. Nonphotochemical quenching and energy dissipation in plants, algae and cyanobacteria. Advances in photosynthesis and respiration , vol. 40. Dordrecht: Springer, 297–314. [ Google Scholar ]
- Buchanan BB, 2016. The carbon (formerly dark) reactions of photosynthesis . Photosynthesis Research 128 : 215–217. [ PubMed ] [ Google Scholar ]
- Buchanan BB, Schürmann P, Wolosiuk RA, Jacquot JP. 2002. The ferredoxin/thioredoxin system: from discovery to molecular structures and beyond . Photosynthesis Research 73 : 215–222. [ PubMed ] [ Google Scholar ]
- Buchert F, Hamon M, Gäbelein P, Scholz M, Hippler M, Wollman F. 2018. The labile interactions of cyclic electron flow effector proteins . Journal of biological chemistry 293 : 17559–17573. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bulté L, Gans P, Rebeillé F, Wollman FA. 1990. ATP control on state transitions in vivo in Chlamydomonas reinhardtii . Biochimica et Biophysica Acta 1020 : 72–80. [ Google Scholar ]
- Butler WL. 1962. Effects of red and far-red light on the fluorescence yield of chlorophyll in vivo . Biochimica et Biophysica Acta 64 : 309–317. [ PubMed ] [ Google Scholar ]
- Butler WL. 1980. Energy transfer between photosystem II units in a connected package model of the photochemical apparatus of photosynthesis (photochemical model) . Proceedings of the National Academy of Sciences of the USA 77 : 4697–4701. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Butler WL, Kitajima M. 1975. Fluorescence quenching in photosystem II of chloroplasts . Biochimica et Biophysica Acta 376 : 116–125. [ PubMed ] [ Google Scholar ]
- Butler WL, Strasser RJ. 1977. Tripartite model for the photochemical apparatus of green plant photosynthesis . Proceedings of the National Academy of Sciences of the USA 74 : 3382–3385. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Calvin M. 1989. Forty years of photosynthesis and related activities . Photosynthesis Research 23 : 3–16. [ PubMed ] [ Google Scholar ]
- Calvin M, Bassham JA, Benson AA. 1950. Chemical transformations in photosynthesis . Federation Proceedings 9 : 524–534. [ PubMed ] [ Google Scholar ]
- Cardona T, Sedoud A, Cox N, Rutherford AW. 2012. Charge separation in Photosystem II: a comparative and evolutionary overview . Biochimica et Biophysica Acta 1817 : 26–43. [ PubMed ] [ Google Scholar ]
- Chan HCH, Gamel OE, Fleming GR, Whaley KB. 2018. Single-photon absorption by single photosynthetic light-harvesting complexes . Journal of Physics B: Atomic, Molecular and Optical Physics 51 : 054002. [ Google Scholar ]
- Chang TG, Chang S, Song QF, Perveen S, Zhu XG. 2019. Systems models, phenomics and genomics: three pillars for developing high-yielding photosynthetically efficient crops . In silico Plants 1 : diy003. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chernev P, Goltsev V, Zaharieva I, Strasser RJ. 2006. A highly restricted model approach quantifying structural and fuctional parameters of photosystem II probed by the chlorophyll a fluorescence rise . Ecological Engineering and Environment Protection 2 : 19–29. [ Google Scholar ]
- Clegg RM. 2006. The history of FRET. In: Geddes CD, Lakowicz JR, eds. Reviews in fluorescence . New York: Springer, 1–45. [ Google Scholar ]
- Clegg RM, Sener M, Govindjee. 2010. From Forster resonance energy transfer (FRET) to coherent resonance energy transfer (CRET) and back – A wheen o’mickles mak’s a muckle. In: Alfano RR, eds. Optical biopsy VII , vol. 7561. Proceedings of SPIE, 7561–7572. [ Google Scholar ]
- Cramer WA, Kallas T, eds. 2016. Cytochrome complexes: evolution, structures, energy transduction, and signaling. Advances in photosynthesis and respiration , Vol. 41. Dordrecht: Springer. [ Google Scholar ]
- Cramer WA, Hasan SS, Yamashita E. 2011. The Q cycle of cytochrome bc complexes: a structure perspective . Biochimica et Biophysica Acta 1807 : 788–802. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Croce R, van Amerongen H. 2013. Light-harvesting in photosystem I . Photosynthesis Research 116 : 153–166. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dall’Osto L, Cazzaniga S, Wada M, Bassi R. 2014. On the origin of a slowly reversible fluorescence decay component in the Arabidopsis npq4 mutant . Philosophical Transactions of the Royal Society B: Biological Sciences 369 , 20130221. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dau H. 1994. Molecular mechanism and quantitative models of variable photosystem II fluorescence . Photochemistry and Photobiology 60 : 1–23. [ Google Scholar ]
- Davis GA, Kanazawa A, Schöttler MA, et al.. 2016. Limitations to photosynthesis by proton motive force-induced photosystem II photodamage . eLife 5 , e16921. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Debus RJ, Barry BA, Sithole I, Babcock GT, McIntosh L. 1988. Direct mutagenesis indicates that the donor to P680+ in photosystem II is tyrosine-161 of the D1 polypeptide . Biochemistry 27 : 9071–9074. [ PubMed ] [ Google Scholar ]
- Demmig-Adams B. 2003. Linking the xanthophyll cycle with thermal energy dissipation . Photosynthesis Research 76 : 73–80. [ PubMed ] [ Google Scholar ]
- Demmig B, Winter K, Krüger A, Czygan F-C. 1987. Photoinhibition and zeaxanthin formation in intact leaves: a possible role of the xanthophyll cycle in the dissipation of excess light energy . Plant Physiology 84 : 218–224 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Demmig-Adams B, Winter K, Krüger A, Czygan F-C. 1989. Zeaxathin and the induction and relaxation kinetics of the dissipation of excess excitation energy in leaves in 2% O 2 , 0% CO 2 . Plant Physiology 90 : 887–893. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Demmig-Adams B, Garab G, Adams WW III, Govindjee, Sharkey TD, eds. 2014. Nonphotochemical quenching and energy dissipation in plants, algae and cyanobacteria. Advances in photosynthesis and respiration , vol. 40. Dordrecht: Springer. [ Google Scholar ]
- Dismukes GC, Siderer Y. 1980. EPR spectroscopic observations of a manganese center associated with water oxidation in spinach chloroplasts . FEBS Letters 121 : 78–80. [ Google Scholar ]
- Döring G, Renger G, Vater J. Witt HT. 1969. Properties of the photoactive chlorophyll-aII in photosynthesis . Zeitschrift für Naturforschung 24 : 1139–1143. [ PubMed ] [ Google Scholar ]
- Durrant J, Giorgi L, Barber J, Klug D, Porter G. 1990. Characterisation of triplet states in isolated photosystem II reaction centres: oxygen quenching as a mechanism for photodamage . Biochimica et Biophysica Acta 1017 : 167–175. [ Google Scholar ]
- Duysens LNM. 1952. Transfer of excitation energy in photosynthesis . PhD Thesis, Leiden University. [ Google Scholar ]
- Duysens LMN, Amesz J, Kamp BM. 1961. Two photochemical systems in photosynthesis . Nature 190 : 510–511. [ PubMed ] [ Google Scholar ]
- Duysens LMN, Sweers HT. 1963. Mechanism of the two photochemical reactions in algae as studied by means of fluorescence. In: Japanese Society of Plant Physiologists, ed. Studies on microalgae and photosynthetic bacteria . Tokyo: University of Tokyo Press, 353–372. [ Google Scholar ]
- Eaton-Rye JJ, Tripathy BC, Sharkey TD, eds. 2012. Photosynthesis: plastid biology, energy conversion and carbon assimilation . Dordrecht: Springer. [ Google Scholar ]
- Ebenhöh O, Houwaart T, Lokstein H, Schlede S, Tirok K. 2011. A minimal mathematical model of nonphotochemical quenching of chlorophyll fluorescence . Biosystems 103 : 196–204. [ PubMed ] [ Google Scholar ]
- Ebenhöh O, Fucile G, Finazzi GG, Rochaix J-D, Goldschmidt-Clermont M. 2014. Short-term acclimation of the photosynthetic electron transfer chain to changing light: a mathematical model . Philosophical Transactions of the Royal Society B: Biological Sciences 369 : 20130223. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Edwards GE, Black CC Jr. 1971. Isolation of mesophyll cells and bundle sheath cells from Digitaria sanguinalis (L.) Scop. leaves and a scanning microscopy study of the internal leaf cell morphology . Plant Physiology 47 : 149–156. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Emerson R. 1958. The quantum yield of photosynthesis . Annual Review of Plant Physiology 9 : 124. [ Google Scholar ]
- Emerson R, Arnold W. 1932 a A separation of the reactions in photosynthesis by means of intermittent light . Journal of General Physiology 15 : 391–420. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Emerson R, Arnold W. 1932 b The photochemical reaction in photosynthesis . The Journal of General Physiology 16 : 191–205. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Emerson R, Lewis CM. 1943. The dependence of the quantum yield of Chlorella photosynthesis on wavelength of light . American Journal of Botany 30 : 165–178. [ Google Scholar ]
- Emerson R, Chalmers RV, Cederstrand CN. 1957. Some factors influencing the long wave limit of photosynthesis . Proceedings of the National Academy of Sciences of the USA 43 : 133–143. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Engel GS, Calhoun TR, Read EL. et al.. 2007. Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems . Nature 446 : 782–786 [ PubMed ] [ Google Scholar ]
- Farquhar GD, von Caemmerer S, Berry JA. 1980. A biochemical model of photosynthetic CO 2 assimilation in leaves of C3 species . Planta 149 : 78–90. [ PubMed ] [ Google Scholar ]
- Fassioli F, Dinshaw R, Arpin PC, Scholes GD. 2014. Photosynthetic light harvesting: excitons and coherence . Journal of the Royal Society Interface 11 : 20130901. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Feng S, Fu L, Xia Q, Tan J, Jiang Y, Guo Y. 2018. Modelling and simulation of photosystem II chlorophyll fluorescence transition from dark-adapted state to light-adapted state . IET Systems Biology 12 : 289–293. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fleming RMT, Thiele I, Provan G, Nasheuer HP. 2010. Integrated stoichiometric, thermodynamic and kinetic modelling of steady state metabolism . Journal of Theoretical Biology 264 : 683–692. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Förster T. 1946. Energiewanderung und fluoreszenz . Naturwissenschaften 6 : 166–175. [ Google Scholar ]
- Förster T. 1948. Zwischenmolekulare energiewanderung und fluoreszenz . Annalen der Physik 2 : 55–75. [ Google Scholar ]
- Fridlyand LE, Backhausen JE, Scheibe R. 1998. Flux control of the malate valve in leaf cells . Archives of Biochemistry and Biophysics 349 : 290–298. [ PubMed ] [ Google Scholar ]
- Gaffron H, Wohl K. 1936. Zür theorie der assimilation . Naturwissenschaften 24 : 103–107. [ Google Scholar ]
- Gilmore AM. 1997. Mechanistic aspects of xanthophyll cycle dependent energy dissipation in higher plant chloroplasts and leaves . Physiologia Plantarum 99 : 197–209. [ Google Scholar ]
- Gilmore AM, Hazlett TL, Govindjee. 1995. Xanthophyll cycle dependent quenching of Photosystem II chlorophyll a fluorescence: formation of a quenching complex with a short fluorescence lifetime . Proceedings of the National Academy of Sciences of the USA 92 : 2273–2277. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gilmore AM, Shinkarev VP, Hazlett TL, Govindjee. 1998. Quantitative analysis of the effects of intra-thylakoid pH and xanthophylls cycle pigments on chlorophyll a lifetime distributions and intensity in thylakoids . Biochemistry 37 : 13582–13593. [ PubMed ] [ Google Scholar ]
- Golbeck JH, ed. 2006. Photosystem I. The light-driven plastocyanin:ferredoxin oxidoreductase. Advances in photosynthesis and respiration , Vol. 24. Dordrecht: Springer. [ Google Scholar ]
- Goldschmidt-Clermont M, Bassi R. 2015. Sharing light between two photosystems: mechanism of state transitions . Current Opinion in Plant Biology 25 : 71–78. [ PubMed ] [ Google Scholar ]
- Goltsev V, Yordanov I. 1997. Mathematical model of prompt and delayed chlorophyll fluorescence induction kinetics . Photosynthetica 33 : 571–586. [ Google Scholar ]
- Govindjee 1995. Sixty-three years since Kautsky: chlorophyll a fluorescence . Australian Journal of Plant Physiology 22 : 131–160. [ Google Scholar ]
- Govindjee 2004. Chlorophyll a fluorescence: a bit of basics and history. In: Papageorgiou GC, Govindjee, eds. Chlorophyll a fluorescence: a signature of photosynthesis . Advances in photosynthesis and respiration , Vol 19. Dordrecht: Springer, 1–41. [ Google Scholar ]
- Govindjee, Krogmann DW. 2004. Discoveries in oxygenic photosynthesis (1727–2003): a perspective: dedicated to the memories of Martin Kamen (1920–2002) and William A. Arnold (1904–2001) . Photosynthesis Research 80 : 15–57. [ PubMed ] [ Google Scholar ]
- Govindjee, Papageorgiou GC. 1971. Chlorophyll fluorescence and photosynthesis: fluorescence transients. In: Giese AC, ed. Photophysiology . New York: Academic Press, 1–46. [ Google Scholar ]
- Govindjee, Rabinowitch EI. 1960. Two forms of chlorophyll a in vivo with distinct photochemical function . Science 132 : 355–356. [ PubMed ] [ Google Scholar ]
- Govindjee, Renger G. 1993. In appreciation of Bessel Kok . Photosynthesis Research 38 : 211–213. [ Google Scholar ]
- Govindjee, Satoh K. 1986. Fluorescence properties of chlorophyll b- and chlorophyll c-containing algae. In: Govindjee, Amesz J, Fork DC eds. Light emission by plants and bacteria . Orlando: Academic Press, 497–537. [ Google Scholar ]
- Govindjee, van Rensen JJS. 1978. Bicarbonate effects on the electron flow in isolated broken chloroplasts . Biochimica et Biophysica Acta 505 : 183–213. [ PubMed ] [ Google Scholar ]
- Govindjee, Ichimura S, Cederstrand C, Rabinowitch EI. 1960. Effect of combining far-red light with shorter wave light on the excitation of fluorescence in Chlorella . Archives of Biochemistry and Biophysics 89 : 322–323. [ PubMed ] [ Google Scholar ]
- Govindjee, Barber J, Cramer WA, et al. , eds. 1986. Excitation and electron transfer in photosynthesis – special issue dedicated to Warren L Butler . Photosynthesis Research 10 : 147–518. [ Google Scholar ]
- Govindjee, Beatty JT, Gest H, Allen JF, eds. 2005. Discoveries in photosynthesis. Advances in photosynthesis and respiration . Dordrecht: Springer. [ Google Scholar ]
- Govindjee, Kern JF, Messinger J, Whitmarsh J. 2010. Photosystem II. In: Encyclopedia of Life Sciences (ELS) . Chichester: John Wiley & Sons, Ltd, 1–15. [ Google Scholar ]
- Govindjee, Shevela D, Björn L. 2017. Evolution of the Z-scheme of photosynthesis: a perspective . Photosynthesis Research 133 : 5–15. [ PubMed ] [ Google Scholar ]
- Guissé B, Srivastava A, Strasser RJ. 1995. The polyphasic rise of the chlorophyll a fluorescence (O-K-J-I-P) in heat stressed leaves . Archives des Sciences - Université de Genève 48 : 147–160. [ Google Scholar ]
- Guo Y, Tan J. 2011. Modeling and simulation of the initial phases of chlorophyll fluorescence from photosystem II . BioSystems 103 : 152–157. [ PubMed ] [ Google Scholar ]
- Guo Y, Tan J. 2014. Kinetic Monte Carlo simulation of the initial phases of chlorophyll fluorescence from photosystem II . BioSystems 115 : 1–4. [ PubMed ] [ Google Scholar ]
- Hager A, Holocher K. 1994. Localization of the xanthophyll-cycle enzyme violaxanthin de-epoxidase within the thylakoid lumen and abolition of its mobility by a (light-dependent) pH decrease . Planta 192 : 581–589. [ Google Scholar ]
- Harbinson J, Woodward FI. 1987. The use of light-induced absorbance changes at 820 nm to monitor the oxidation state of P-700 in leaves . Plant, Cell & Environment 10 : 131–140. [ Google Scholar ]
- Heber U. 2002. Irrungen, Wirrungen? The Mehler reaction in relation to cyclic electron transport in C3 plants . Photosynthesis Research 73 : 223–231. [ PubMed ] [ Google Scholar ]
- Hemschemeier A, Happe T. 2011. Alternative photosynthetic electron transport pathways during anaerobiosis in the green alga Chlamydomonas reinhardtii . Biochimica et Biophysica Acta 1807 : 919–926. [ PubMed ] [ Google Scholar ]
- Hill R. 1937. Oxygen evolution by isolated chloroplasts . Nature 139 : 881–882. [ Google Scholar ]
- Hill R, Bendall F. 1960. Function of the two cytochrome components in chloroplasts: a working hypothesis . Nature 186 : 136–140. [ Google Scholar ]
- Hind G, Jagendorf AT. 1963. Separation of light and dark stages in photophosphorylation . Proceedings of the National Academy of Sciences of the USA 49 : 715–722. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hofmeyr JHS, Cornish-Bowden A. 2000. Regulating the cellular economy of supply and demand . FEBS Letters 476 : 47–51. [ PubMed ] [ Google Scholar ]
- Holzapfel C. 1978. Analysis of the prompt fluorescence induction by means of computer simulation of the primary photosynthetic reactions . Zeitschrift für Naturforschung 33c : 402–407. [ Google Scholar ]
- Holzapfel C, Bauer R. 1975. Computer simulation of primary photosynthetic reactions – Compared with experimental results on O 2 -exchange and chlorophyll fluorescence of green plants . Zeitschrift für Naturforschung 30c : 489–498. [ Google Scholar ]
- Holzwarth A, Jahns P. 2014. Non-photochemical quenching mechanisms in intact organisms as derived from ultrafast-fluorescence kinetic studies. In: Demmig-Adams B, Garab G, Adams WW III, Govindjee, Sharkey TD, eds. Nonphotochemical quenching and energy dissipation in plants, algae and cyanobacteria. Advances in photosynthesis and respiration , vol. 40. Dordrecht: Springer, 129–156. [ Google Scholar ]
- Holzwarth AR, Miloslavina Y, Nilkens M, Jahns P. 2009. Identification of two quenching sites active in the regulation of photosynthetic light-harvesting studied by time-resolved fluorescence . Chemical Physics Letters 483 : 262–267. [ Google Scholar ]
- Holzwarth AR, Lenk D, Jahns P. 2013. On the analysis of non-photochemical chlorophyll fluorescence quenching curves: I. Theoretical considerations . Biochimica et Biophysica Acta 1827 : 786–792. [ PubMed ] [ Google Scholar ]
- Horton P, Ruban A, Rees D, Pascal A, Noctor G, Young A. 1991. Control of the light-harvesting function of chloroplast membranes by aggregation of the LHCII chlorophyll protein complex . FEBS Letters 292 : 1–4. [ PubMed ] [ Google Scholar ]
- Horton P, Ruban V, Walters RG. 1996. Regulation of light harvesting in green plants . Annual Review of Plant Physiology and Plant Molecular Biology 47 : 655–684. [ PubMed ] [ Google Scholar ]
- Horton P, Johnson MP, Perez-Bueno ML, Kiss AZ, Ruban AV. 2008. Photosynthetic acclimation: does the dynamic structure and macro-organisation of photosystem II in higher plant grana membranes regulate light harvesting states? The FEBS Journal 275 : 1069–1079. [ PubMed ] [ Google Scholar ]
- Hsu B-D. 1992 a A theoretical study on the fluorescence induction curve of spinach thylakoids in the absence of DCMU . Biochimica et Biophysica Acta 1140 : 30–36. [ Google Scholar ]
- Hsu B-D. 1992 b The active photosystem II centers can make a significant contibution to the initial fluorescence rise from F 0 to F i . Plant Science 81 : 169–174. [ Google Scholar ]
- Hsu B-D, Lee Y-S, Jang Y-R. 1989. A method for analysis of fluorescence induction curve from DCMU-poisoned chloroplasts . Biochimica et Biophysica Acta 975 : 44–49. [ Google Scholar ]
- Ishizaki A, Fleming GR. 2009. United treatment of quantum coherent and incoherent hopping dynamics in electronic energy transfer: reduced hierarchy equation approach . The Journal of Chemical Physics 130 : 234111. [ PubMed ] [ Google Scholar ]
- Iwai M, Yokono M, Inada N, Minagawa J. 2010. Live-cell imaging of photosystem II antenna dissociation during state transitions . Proceedings of the National Academy of Sciences of the USA 107 : 2337–2342. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jablonsky J, Lazar D. 2008. Evidence for intermediate S-states as initial phase in the process of oxygen-evolving complex oxidation . Biophysical Journal 94 : 2725–2736. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jablonsky J, Bauwe H, Wolkenhauer O. 2011. Modeling the Calvin–Benson cycle . BMC Systems Biology 5 : 185. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jagendorf AT. 2002. Photophosphorylation and the chemiosmotic perspective . Photosynthesis Research 73 : 233–241. [ PubMed ] [ Google Scholar ]
- Jagendorf AT, Uribe E. 1966. ATP formation caused by acid-base transition of spinach chloroplasts . Proceedings of the National Academy of Sciences of the USA 55 : 170–177. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jahns P, Holzwarth AR. 2012. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II . Biochimica et Biophysica Acta 1817 : 182–193. [ PubMed ] [ Google Scholar ]
- Johnson MP, Ruban AV. 2011. Restoration of rapidly reversible photoprotective energy dissipation in the absence of PsbS protein by enhanced ΔpH . Journal of Biological Chemistry 286 : 19973–19981. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Johnson MP, Davison P, Ruban A, Horton P. 2008. The xanthophyll cycle pool size controls the kinetics of non-photochemical quenching in Arabidopsis thaliana . FEBS Letters 582 : 259–263. [ PubMed ] [ Google Scholar ]
- Johnson MP, Zia A, Ruban AV. 2012. Elevated ΔpH restores rapidly reversible photoprotective energy dissipation in Arabidopsis chloroplasts deficient in lutein and xanthophyll cycle activity . Planta 235 : 193–204. [ PubMed ] [ Google Scholar ]
- Joliot A, Joliot P. 1964. Étude cinétique de la réaction photochimique libérant l’oxygène au cours de la photosynthèse . Comptes Rendus de l’Académie des Sciences (Paris) 258 : 4622–4625. [ PubMed ] [ Google Scholar ]
- Joliot P. 1965. Cinétiques de réactions liées à l’émission d’oxygène photosynthétique . Biochimica et Biophysica Acta 102 : 116–134. [ PubMed ] [ Google Scholar ]
- Joliot P. 2003. Period-four oscillations of the flash-induced oxygen formation in photosynthesis . Photosynthesis Research 76 : 65–72. [ PubMed ] [ Google Scholar ]
- Joliot P, Joliot A. 2003. Excitation transfer between photosynthetic units: the 1964 experiment . Photosynthesis Research 76 : 241–245. [ PubMed ] [ Google Scholar ]
- Joliot P, Barbieri G, Chabaud R. 1969. Un nouveau modèle des centres photochimiques du système II . Photochemistry and Photobiology 10 : 309–329. [ Google Scholar ]
- Jordan P, Fromme P, Witt HT, Klukas O, Saenger W, Krauss N. 2001. Three dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution . Nature 411 : 909–917. [ PubMed ] [ Google Scholar ]
- Junge W. 2004. Protons, proteins and ATP . Photosynthesis Research 80 : 197–221. [ PubMed ] [ Google Scholar ]
- Kaiser E, Morales A, Harbinson J, Kromdijk J, Heuvelink E, Marcelis LFM. 2015. Dynamic photosynthesis in different environmental conditions . Journal of Experimental Botany 66 : 2415–2426. [ PubMed ] [ Google Scholar ]
- Kaiser E, Kromdijk J, Harbinson J, Heuvelink E, Marcelis LFM. 2017. Photosynthetic induction and its diffusional, carboxylation and electron transport processes as affected by CO 2 partial pressure, temperature, air humidity and blue irradiance . Annals of Botany 119 : 191–205. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kamen MD. 1947. Radioactive tracers in biology . New York: Academic Press. [ Google Scholar ]
- Kamiya N, Shen R-J. 2003. Crystal structure of oxygen-evolving photosystem II from Thermosynechococcus vulcanus at 3.7-Å resolution . Proceedings of the National Academy of Sciences of the USA 100 : 98–103. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kaňa R, Govindjee. 2016. Role of ions in the regulation of light harvesting . Frontiers in Plant Science 7 : 1849. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kautsky H, Hirsch A. 1931. Neue versuche zur kohlensaureassimilation . Naturwissenschaften 19 : 964. [ Google Scholar ]
- Kitajima M, Butler WL. 1975. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone . Biochimica et Biophysica Acta 723 : 105–115. [ PubMed ] [ Google Scholar ]
- Klimov VV, Klevanik AV, Shuvalov VA, Krasnovsky AA. 1977. Reduction of pheophytin in primary light reaction of photosystem II . FEBS Letters 82 : 183–186. [ PubMed ] [ Google Scholar ]
- Klimov VV, Dolan E, Ke B. 1980. EPR properties of an intermediary electron acceptor (pheophytin) in photosystem II reaction centers at cryogenic temperatures . FEBS Letters 112 : 97–100. [ Google Scholar ]
- Klughammer C, Schreiber U. 1994. An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P700+-absorbance changes at 830 nm . Planta 192 : 261–268. [ Google Scholar ]
- Kodru S, Malavath T, Devadasu E, et al.. 2015. The slow S to M rise of chlorophyll a fluorescence induction reflects transition from state 2 to state 1 in the green alga Chlamydomonas reinhardtii . Photosynthesis Research 125 : 219–231. [ PubMed ] [ Google Scholar ]
- Kok B. 1956. On the reversible absorption change at 705 μm in photosynthetic organisms . Biochimica et Biophysica Acta 22 : 399–401. [ PubMed ] [ Google Scholar ]
- Kok B, Forbush B, McGloin M. 1970. Cooperation of charges in photosynthetic O 2 evolution. 1. A linear four step mechanism . Photochemistry and Photobiology 11 : 457–475. [ PubMed ] [ Google Scholar ]
- Kramer DM, Sacksteder CA, Cruz JA. 1999. How acidic is the lumen? Photosynthesis Research 60 : 151–163. [ Google Scholar ]
- Krause H, Weis W. 1991. Chlorophyll fluorescence and photosynthesis: the basics . Annual Review of Plant Physiology and Plant Molecular Biology 42 : 313–349. [ Google Scholar ]
- Krey A, Govindjee 1964. Fluorescence changes in Porphyridium exposed to green light of different Intensity: A new emission band at 693 nm and its significance to photosynthesis . Proceedings of the National Academy of Sciences of the USA 52 : 1568–1572. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Krieger A, Weis E. 1993. The role of calcium in the pH-dependent control of photosystem II . Photosynthesis Research 37 : 117–130. [ PubMed ] [ Google Scholar ]
- Krogmann DW, Jagendorf AT, Avron M. 1959. Uncouplers of spinach chloroplast photosynthetic phosphorylation . Plant Physiology 34 : 272–277. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kromdijk J, Głowacka K, Leonelli L, et al.. 2016. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection . Science 354 : 857–861. [ PubMed ] [ Google Scholar ]
- Kroon BMA, Thoms S. 2006. From electron to biomass: a mechanistic model to describe phytoplankton photosynthesis and steady-state growth mates . Journal of Phycology 42 : 593–609. [ Google Scholar ]
- Kuvykin IV, Vershubskii AV, Priklonskii VI, Tikhonov AN. 2009. Computer simulation study of pH-dependent regulation of electron transport in chloroplasts . Biophysics 54 : 455–464. [ PubMed ] [ Google Scholar ]
- Laisk A, Oja V. 2018. Kinetics of photosystem II electron transport: a mathematical analysis based on chlorophyll fluorescence induction . Photosynthesis Research 136 : 63–82. [ PubMed ] [ Google Scholar ]
- Laisk A, Oja V, Rasulov B, Eichelmann H, Sumberg A. 1997. Quantum yields and rate constants of photochemical and nonphotochemical excitation quenching. Experiment and model . Plant Physiology 115 : 803–815. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Laisk A, Eichelmann H, Oja V. 2006. C3 photosynthesis in silico . Photosynthesis Research 90 : 45–66. [ PubMed ] [ Google Scholar ]
- Laisk A, Eichelmann H, Oja V. 2009. Leaf C 3 photosynthesis in silico : Integrated carbon/nitrogen metabolism. In: Laisk A, Nedbal AL, Govindjee, eds. Photosynthesis in silico: understanding complexity from molecules to ecosystems. Advances in photosynthesis and respiration , Vol. 29. Dordrecht: Springer, 295–322. [ Google Scholar ]
- Latimer P, Bannister TT, Rabinowitch E. 1956. Quantum yields of fluorescence of plant pigments . Science 124 : 585–586. [ PubMed ] [ Google Scholar ]
- Lavergne J, Trissl H-W. 1995. Theory of fluorescence induction in photosystem II: Derivation of analytical expressions in a model including exciton-radical-pair equilibrium and restricted energy transfer between photosynthetic units . Biophysical Journal 68 : 2474–2492. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lazár D. 1999. Chlorophyll a fluorescence induction . Biochimica et Biophysica Acta 1412 : 1–28. [ PubMed ] [ Google Scholar ]
- Lazár D. 2003. Chlorophyll a fluorescence rise induced by high light illumination of dark-adapted plant tissue studied by means of a model of photosystem II and considering photosystem II heterogeneity . Journal of Theoretical Biology 220 : 469–503. [ PubMed ] [ Google Scholar ]
- Lazár D. 2006. The polyphasic chlorophyll a fluorescence rise measured under high intensity of exciting light . Functional Plant Biology 33 : 9–30. [ PubMed ] [ Google Scholar ]
- Lazár D. 2009. Modelling of light-induced chlorophyll a fluorescence rise (O-J-I-P transient) and changes in 820 nm-transmittance signal of photosynthesis . Photosynthetica 47 : 483–498. [ Google Scholar ]
- Lazár D. 2013. Simulations show that a small part of variable chlorophyll a fluorescence originates in photosystem I and contributes to overall fluorescence rise . Journal of Theoretical Biology 335 : 249–264. [ PubMed ] [ Google Scholar ]
- Lazár D. 2015. Parameters of photosynthetic energy partitioning . Journal of Plant Physiology 175 : 131–147. [ PubMed ] [ Google Scholar ]
- Lazár D, Jablonský J. 2009. On the approaches applied in formulation of a kinetic model of photosystem II: different approaches lead to different simulations of the chlorophyll a fluorescence transients . Journal of Theoretical Biology 257 : 260–269. [ PubMed ] [ Google Scholar ]
- Lazár D, Pospíšil P. 1999. Mathematical simulation of chlorophyll a fluorescence rise measured with 3-(3′,4′-dichlorophenyl)-1,1-dimethylurea-treated barley leaves at room and high temperatures . European Biophysics Journal 28 : 468–477. [ PubMed ] [ Google Scholar ]
- Lazár D, Schansker G. 2009. Models of chlorophyll a fluorescence transients. In: Laisk A, Nedbal L, Govindjee, eds. Photosynthesis in silico: understanding complexity from molecules to ecosystems. Advances in photosynthesis and respiration , Vol. 29. Dordrecht: Springer, 85–123. [ Google Scholar ]
- Lazár D, Nauš J, Matoušková M, Flašarová M. 1997. Mathematical modeling of changes in chlorophyll fluorescence induction caused by herbicides . Pesticide Biochemistry and Physiology 57 : 200–210. [ Google Scholar ]
- Lazár D, Brokeš M, Nauš J, Dvořák L. 1998. Mathematical modelling of 3-(3′,4′-dichlorophenyl)-1,1-dimethylurea action in plant leaves . Journal of Theoretical Biology 191 : 79–86. [ PubMed ] [ Google Scholar ]
- Lazár D, Tomek P, Ilík P, Nauš J. 2001. Determination of the antenna heterogeneity of Photosystem II by direct simultaneous fitting of several fluorescence rise curves measured with DCMU at different light intensities . Photosynthesis Research 68 : 247–257. [ PubMed ] [ Google Scholar ]
- Lazár D, Ilík P, Kruk J, Strzalka K, Nauš J. 2005. A theoretical study on effect of the initial redox state of cytochrome b559 on maximal chlorophyll fluorescence level (F M ). Implications for photoinhibition of photosystem II . Journal of Theoretical Biology 233 : 287–300. [ PubMed ] [ Google Scholar ]
- Lebedeva GV, Belyaeva NE, Demin OV, Riznichenko GYu, Rubin AB. 2002. Kinetic model of primary photosynthetic processes in chloroplasts. Description of the fast phase of chlorophyll fluorescence induction under different light intensities . Biophysics (Russia) 47 : 968–980. [ Google Scholar ]
- Lemeille S, Rochaix JD. 2010. State transitions at the crossroad of thylakoid signalling pathways . Photosynthesis Research 106 : 33–46. [ PubMed ] [ Google Scholar ]
- Li X-P, Björkman O, Shih C, et al.. 2000. A pigment-binding protein essential for regulation of photosynthetic light harvesting . Nature 403 : 391–395. [ PubMed ] [ Google Scholar ]
- Long SP, Zhu XG, Naidu SL, Ort DR. 2006. Can improvement in photosynthesis increase crop yields? Plant, Cell and Environment 29 : 315–330. [ PubMed ] [ Google Scholar ]
- Long SP, Marshall-Colon A, Zhu XG. 2015. Meeting the global food demand of the future by engineering crop photosynthesis and yield potential . Cell 161 : 56–66. [ PubMed ] [ Google Scholar ]
- Lubitz W, Chrysina M, Cox N. 2019. Water oxidation in photosystem II . Photosynthesis Research 142 : 105–125. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lyu H, Lazár D. 2017 a Modeling the light-induced electric potential difference (ΔΨ), the pH difference (ΔpH) and the proton motive force across the thylakoid membrane in C3 leaves . Journal of Theoretical Biology 413 : 11–23. [ PubMed ] [ Google Scholar ]
- Lyu H, Lazár D. 2017 b Modeling the light-induced electric potential difference ΔΨ across the thylakoid membrane based on the transition state rate theory . Biochimica et Biophysica Acta 1858 : 239–248. [ PubMed ] [ Google Scholar ]
- Magyar M, Sipka G, Kovács L, et al.. 2018. Rate-limiting steps in the dark-to-light transition of Photosystem II - revealed by chlorophyll- a fluorescence induction . Scientific Reports 8 : 2755. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Makarov S, Grachev EA, Antal TK. 2012. Mathematical modeling of photosynthetic electron transport chain considering spatial heterogeneity of the thylakoid membrane . Mathematical Biology and Bioinformatics 7 : 508–528. [ Google Scholar ]
- Malkin S. 1966. Fluorescence induction studies in isolated chloroplasts. II. Kinetic analysis of the fluorescence intensity dependence on time . Biochimica et Biophysica Acta 123 : 433–442. [ PubMed ] [ Google Scholar ]
- Malkin S, Kok B. 1966. Fluorescence induction studies in isolated chloroplasts. I. Number of components involved in the reaction and quantum yields . Biochimica et Biophysica Acta 123 : 413–432. [ PubMed ] [ Google Scholar ]
- Malnoë A. 2018. Photoinhibition or photoprotection of photosynthesis? Update on the (newly termed) sustained quenching component qH . Environmental and Experimental Botany 154 : 123–133. [ Google Scholar ]
- Mar T, Govindjee. 1972. Kinetic models of oxygen evolution in photosynthesis . Journal of Theoretical Biology 36 : 427–446. [ PubMed ] [ Google Scholar ]
- Marshall-Colón A, Kliebenstein DJ. 2019. Plant networks as traits and hypotheses: moving beyond description . Trends of Plant Science 24 : 840–852. [ PubMed ] [ Google Scholar ]
- Marshall-Colón A, Long SP, Douglas K, et al.. 2017. Crops in silico: generating virtual crops using an integrative and multi-scale modeling platform . Frontiers in Plant Science 8 : 786. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Maslakov AS, Antal TK, Riznichenko GY, Rubin AB. 2016. Modeling of primary photosynthetic processes using the kinetic Monte Carlo method . Biophysics 61 : 387–399. [ Google Scholar ]
- Matsuoka T, Tanaka S, Ebina K. 2015. Systems approach to excitation-energy and electron transfer reaction networks in photosystem II complex: model studies for chlorophyll a fluorescence induction kinetics . Journal of Theoretical Biology 380 : 220–237. [ PubMed ] [ Google Scholar ]
- Matuszyńska A, Ebenhöh O. 2015. A reductionist approach to model photosynthetic self-regulation in eukaryotes in response to light . Biochemical Society Transactions 43 : 1133–1139. [ PubMed ] [ Google Scholar ]
- Matuszyńska A, Heidari S, Jahns P, Ebenhöh O. 2016. A mathematical model of non-photochemical quenching to study short-term light memory in plants . Biochimica et Biophysica Acta 1857 : 1860–1869. [ PubMed ] [ Google Scholar ]
- Matuszyńska A, Saadat NP, Ebenhöh O. 2019. Balancing energy supply during photosynthesis - a theoretical perspective . Physiologia Plantarum 166 : 392–402. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- McAlister ED, Myers J. 1940. Time course of photosynthesis and fluorescence . Science 92 : 241–243. [ PubMed ] [ Google Scholar ]
- McDonald AE, Ivanov AG, Bode R, Maxwell DP, Rodermel SR, Hüner NP. 2011. Flexibility in photosynthetic electron transport: the physiological role of plastoquinol terminal oxidase (PTOX) . Biochimica et Biophysica Acta 1807 : 954–967. [ PubMed ] [ Google Scholar ]
- McGrath JM, Long SP. 2016. Can the cyanobacterial carbon-concentrating mechanism increase photosynthesis in crop species? A theoretical analysis . Plant Physiology 164 : 2247–2261. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mehler AH. 1951. Studies on reactions of illuminated chloroplasts. I. Mechanisms of the reduction of oxygen and other Hill reagents . Archives of Biochemistry and Biophysics 33 : 65–77. [ PubMed ] [ Google Scholar ]
- Melis A, Homann PH. 1975. Kinetic analysis of the fluorescence induction in 3-(3′,4′-dichlorophenyl)-1,1-dimethylurea poisoned chloroplasts . Photochemistry and Photobiology 21 : 431–437. [ Google Scholar ]
- Mirkovic T, Ostroumov, Anna JM, van Grondelle R, Govindjee, Scholes GD. 2017. Light absorption and energy transfer in the antenna complexes of photosynthetic organisms . Chemical Reviews 117 : 249–293. [ PubMed ] [ Google Scholar ]
- Mishra KB, Mishra A, Kubásek J, Urban O, Heyer AG, Govindjee. 2019. Low temperature induced modulation of photosynthetic induction in non-acclimated and cold-acclimated Arabidopsis thaliana : chlorophyll a fluorescence and gas-exchange measurements . Photosynthesis Research 139 : 123–143. [ PubMed ] [ Google Scholar ]
- Mitchell P. 1961 a Coupling of phosphorylation to electron and hydrogen transfer by a chemiosmotic type of mechanism . Nature 191 : 144–148. [ PubMed ] [ Google Scholar ]
- Mitchell P. 1961 b Chemiosmotic coupling in oxidative and photosynthetic phosphorylation . Bodmin: Glynn Research. [ Google Scholar ]
- Mitchell P. 1975. Protonmotive Q-cycle-general formulation . FEBS Letters 59 : 137–139. [ PubMed ] [ Google Scholar ]
- Miyake C. 2010. Alternative electron flows (water–water cycle and cyclic electron flow around PSI) in photosynthesis: molecular mechanisms and physiological functions . Plant and Cell Physiology 51 : 1951–1963. [ PubMed ] [ Google Scholar ]
- Morales A, Kaiser E, Yin X, et al.. 2018. a Dynamic modelling of limitations on improving leaf CO 2 assimilation under fluctuating irradiance . Plant, Cell & Environment 41 : 589–604. [ PubMed ] [ Google Scholar ]
- Morales A, Yin X, Harbinson J, et al.. 2018. b In silico analysis of the regulation of the photosynthetic electron transport chain in C3 plants . Plant Physiology 176 : 1247–1261. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Müh F, Glöckner C, Hellmich J, Zouni A. 2012. Light-induced quinone reduction in photosystem II . Biochimica et Biophysica Acta 1817 : 44–65. [ PubMed ] [ Google Scholar ]
- Müller P, Li X, Niyogi KK. 2001. Non-photochemical quenching. A response to excess light energy . Plant Physiology 125 : 1558–1566. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Munday JC, Govindjee. 1969. Light-induced changes in the fluorescence yield of chlorophyll a in vivo. III. The dip and the peak in the fluorescence transient of Chlorella pyrenoidosa . Biophysical Journal 9 : 1–21. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Murata N. 1969 a Control of excitation transfer in photosynthesis. I. Light-induced change of chlorophyll a fluorescence in Porphyridium cruentum . Biochimica et Biophysica Acta 172 : 242. [ PubMed ] [ Google Scholar ]
- Murata N. 1969 b Control of excitation transfer in photosynthesis. II. Magnesium ion-dependent distribution of excitation energy between two pigment systems in spinach chloroplasts . Biochimica et Biophysica Acta 189 : 171–181. [ PubMed ] [ Google Scholar ]
- Murata N, Allakhverdiev SI, Nishiyama Y. 2012. The mechanism of photoinhibition in vivo: re-evaluation of the roles of catalase, α-tocopherol, non-photochemical quenching, and electron transport . Biochimica et Biophysica Acta 1817 : 1127–1133. [ PubMed ] [ Google Scholar ]
- Myers J. 1994. The 1932 experiments . Photosynthesis Research 40 : 303–310. [ PubMed ] [ Google Scholar ]
- Najafpour MM, Moghaddam NA, Allakhverdiev SI, Govindjee 2012. Biological water oxidation: Lessons from nature . Biochimica et Biophysica Acta 1817 : 1110–1121. [ PubMed ] [ Google Scholar ]
- Nawrocki WJ, Bailleul B, Picot D, et al.. 2019. The mechanism of cyclic electron flow . Biochimica et Biophysica Acta 1860 : 433–438. [ PubMed ] [ Google Scholar ]
- Nedbal L, Samson G, Whitmarsh J. 1992. Redox state of one-electron component controls the rate of photoinhibition of photosystem II . Proceedings of the National Academy of Sciences of the USA 89 : 7929–7933. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nedbal L, Červený J, Schmidt H. 2009. Scaling and integration of kinetic models of photosynthesis: towards comprehensive e-photosynthesis. In: Laisk A, Nedbal AL, Govindjee, eds. Photosynthesis in silico: understanding complexity from molecules to ecosystems. Advances in photosynthesis and respiration , Vol. 29. Dordrecht: Springer, 17–29. [ Google Scholar ]
- Nelson N, Junge W. 2015. Structure and energy transfer in photosystems of oxygenic photosynthesis . Annual Review of Biochemistry 84 : 659–683. [ PubMed ] [ Google Scholar ]
- Nickelsen K. 2016. Explaining photosynthesis: models of biochemical mechanisms, 1840–1960 . Dordrecht: Springer. [ Google Scholar ]
- Nickelsen K, Govindjee. 2011. The maximum quantum yield controversy: Otto Warburg and the Midwest Gang . Bern Studies in the History and Philosophy of Science, Switzerland: Berne: University of Bern, Institute für Philosophie. [ Google Scholar ]
- Nikkanen L, Rintamäki E. 2019. Chloroplast thioredoxin systems dynamically regulate photosynthesis in plants . Biochemical Journal 476 : 1159–1172. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nishiyama Y, Allakhverdiev SI, Murata N. 2006. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II . Biochimica et Biophysica Acta 1757 : 742–749. [ PubMed ] [ Google Scholar ]
- Nižnan J. 2014. Compact representation of photosynthesis biochemical processes . Diploma Thesis, Masaryk University. [ Google Scholar ]
- Noctor G, Foyer CH. 2000. Homeostasis of adenylate status during photosynthesis in a fluctuating environment . Journal of Experimental Botany 51 : 347–356. [ PubMed ] [ Google Scholar ]
- Oettmeier W, Masson K, Johanningmeier U. 1980. Photoaffinity labelling of the photosystem II herbicide binding protein . FEBS Letters 118 : 267–270. [ Google Scholar ]
- Okayama S, Butler WL. 1972. The influence of cytochrome b 559 on the fluorescence yield of chloroplasts at low temperature . Biochimica et Biophysica Acta 267 : 523–527. [ PubMed ] [ Google Scholar ]
- Ono T, Inoue Y. 1988. Discrete extraction of the Ca atom functional for O 2 evolution in higher plant Photosystem II by a simple low pH treatment . FEBS Letters 227 : 147–152. [ Google Scholar ]
- Ort DR, Merchant SS, Alric J, et al.. 2015. Redesigning photosynthesis to sustainably meet global food and bioenergy demand . Proceedings of the National Academy of Sciences of the USA 112 : 8529–8536. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Osmond B, Chow WS, Wyber R, et al.. 2017. Relative functional and optical absorption cross-sections of PSII and other photosynthetic parameters monitored in situ, at a distance with a time resolution of a few seconds, using a prototype light induced fluorescence transient (LIFT) device . Functional Plant Biology 44 : 985–1006. [ PubMed ] [ Google Scholar ]
- Page CC, Moser CC, Chen X, Dutton PL. 1999. Natural engineering principles of electron tunnelling in biological oxidation-reduction . Nature 402 : 47–52. [ PubMed ] [ Google Scholar ]
- Paillotin G. 1976. Movement of excitations in the photosynthetic domains of photosystem II . Journal of Theoretical Biology 58 : 237–252. [ PubMed ] [ Google Scholar ]
- Papageorgiou GC. 1975. Chlorophyll fluorescence: an intrinsic probe of photosynthesis. In: Govindjee, ed. Bioenergetics of photosynthesis . New York: Academic Press, 319–372. [ Google Scholar ]
- Papageorgiou GC, Govindjee. 1968 a Light-induced changes in the fluorescence yield of chlorophyll a in vivo. I. Anacystis nidulans . Biophysical Journal 8 : 1299–1315. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Papageorgiou GC, Govindjee. 1968 b Light induced changes in the fluorescence yield of chlorophyll a in vivo. II. Chlorella pyrenoidosa . Biophysical Journal 8 : 1316–1328. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Papageorgiou GC, Govindjee, eds. 2004. Chlorophyll a fluorescence: a signature of photosynthesis. Advances in photosynthesis and respiration , Vol. 19. Dordrecht: Springer. [ Google Scholar ]
- Papageorgiou GC, Govindjee. 2011. Photosystem II fluorescence: slow changes–scaling from the past . Journal of Photochemistry and Photobiology B: Biology 104 : 258–270. [ PubMed ] [ Google Scholar ]
- Papageorgiou GC, Govindjee. 2014. The non-photochemical quenching of the electronically excited state of chlorophyll a in plants: definitions, timelines, viewpoints, open questions. In: Demmig-Adams B, Garab G, Adams WW III, Govindjee, Sharkey TD, eds. Nonphotochemical quenching and energy dissipation in plants, algae and cyanobacteria. Advances in photosynthesis and respiration , vol. 40. Dordrecht: Springer, 1–44. [ Google Scholar ]
- Papageorgiou GC, Tsimilli-Michael M, Stamatakis K. 2007. The fast and slow kinetics of chlorophyll a fluorescence induction in plants, algae and cyanobacteria: a viewpoint . Photosynthesis Research 94 : 275–290. [ PubMed ] [ Google Scholar ]
- Pearcy RW. 1990. Sunflecks and photosynthesis in plant canopies . Annual Review of Plant Biology 41 : 421–453. [ Google Scholar ]
- Peers G, Truong TB, Ostendorf E, et al.. 2009. An ancient light-harvesting protein is critical for the regulation of algal photosynthesis . Nature 462 : 518–521. [ PubMed ] [ Google Scholar ]
- Pettersson G, Ryde-Pettersson U. 1988. A mathematical model of the Calvin photosynthesis cycle . European Journal of Biochemistry 175 : 661–672. [ PubMed ] [ Google Scholar ]
- Petrouleas V, Crofts AR. 2005. The quinone iron acceptor complex. In: Wydrzynski T, Satoh K eds. Photosystem II: the light-driven water: plastoquinone oxidoreductase. Advances in photosynthesis and respiration , Vol. 22. Dordrecht: Springer, 177–206. [ Google Scholar ]
- Pfündel E. 1998. Estimating the contribution of photosystem I to total leaf chlorophyll fluorescence . Photosynthesis Research 56 : 185–195. [ Google Scholar ]
- Poolman MG, Fell DA, Thomas S. 2000. Modelling photosynthesis and its control . Journal of Experimental Botany 51 : 319–328. [ PubMed ] [ Google Scholar ]
- Pribil M, Sandoval-Ibáñez O, Xu W. et al.. 2018. Fine-tuning of photosynthesis requires CURVATURE THYLAKOID1-mediated thylakoid plasticity . Plant Physiology 176 : 2351–2364. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rabinowitch EI. 1945. Photosynthesis and related processes, vol 1. Chemistry of photosynthesis, chemosynthesis and related processes in vitro and in vivo . New York: Interscience Publishers. [ Google Scholar ]
- Rabinowitch EI, Govindjee. 1969. Photosynthesis . Chichester: Wiley & Sons. [ Google Scholar ]
- Rees D, Young A, Noctor G, Britton G, Horton P. 1989. Enhancement of the pH-dependent dissipation of excitation energy in spinach chloroplasts by light-activation: correlation with the synthesis of zeaxanthin . FEBS Letters 256 : 85–90. [ Google Scholar ]
- Rees D, Noctor G, Ruban AV, Crofts J, Young A, Horton P. 1992. pH dependent chlorophyll fluorescence quenching in spinach thylakoids from light treated or dark adapted leaves . Photosynthesis Research 31 : 11–19. [ PubMed ] [ Google Scholar ]
- Reinhold C, Niczyporuk S, Beran K, Jahns P. 2008. Short-term down-regulation of zeaxanthin epoxidation in Arabidopsis thaliana in response to photo-oxidative stress conditions . Biochimica et Biophysica Acta 1777 : 462–469. [ PubMed ] [ Google Scholar ]
- Renger G, Schulze A. 1985. Quantitative analysis of fluorescence induction curves in isolated spinach chloroplasts . Photobiochemistry and Photobiophysics 9 : 79–87. [ Google Scholar ]
- Renger G, Govindjee, eds. 1993. How plants and cyanobacteria make oxygen: 25 years of period four oscillations . Photosynthesis Research 38 : 211–469. [ Google Scholar ]
- Robinson HH, Crofts AR, 1983. Kinetics of the changes in oxidation-reduction reactions of the photosystem II quinone acceptor complex, and the pathway for deactivation . FEBS Letters 153 : 221–226. [ Google Scholar ]
- Rochaix J-D. 2014. Regulation and dynamics of the light-harvesting system . Annual Review of Plant Biology 65 : 287–309. [ PubMed ] [ Google Scholar ]
- Rochaix J-D. 2016. The dynamics of the photosynthetic apparatus in algae. In: Najafpour MM, ed. Applied photosynthesis - new progress . London: IntechOpen,23–52. [ Google Scholar ]
- Rochaix JD, Lemeille S, Shapiguzov A, et al.. 2012. Protein kinases and phosphatases involved in the acclimation of the photosynthetic apparatus to a changing light environment . Philosophical Transactions of the Royal Society B: Biological Sciences 367 : 3466–3474. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Roden JJJ, Bennett DIG, Whaley KB. 2016. Long-range energy transport in photosystem II . The Journal of Chemical Physics 144 : 245101. [ PubMed ] [ Google Scholar ]
- Roelofs TA, Lee C-H, Holzwarth AR. 1992. Global target analysis of picosecond fluorescence kinetics from pea chloroplasts. A new approach to the characterization of the primary processes in photosystem II α- and β-units . Biophysical Journal 61 : 1147–1163. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rosenthal DM, Locke AM, Khozaei M, Raines CA, Long SP, Ort DR. 2011. Over-expressing the C3 photosynthesis cycle enzyme sedoheptulose-1–7 bisphosphatase improves photosynthetic carbon gain and yield under fully open air CO 2 fumigation (FACE) . BMC Plant Biology 11 : 123. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ruban AV, Johnson MP, Duffy CD. 2012. The photoprotective molecular switch in the photosystem II antenna . Biochimica et Biophysica Acta 1817 : 167–181. [ PubMed ] [ Google Scholar ]
- Ruben S, Kamen MD. 1941. Long-lived radioactive carbon: C14 . Physics Reviews 59 : 349–354. [ Google Scholar ]
- Ruben S, Hassid WZ, Kamen MD. 1939. Radioactive carbon in the study of photosynthesis . Journal of the American Chemical Society 61 : 661–663. [ Google Scholar ]
- Ruben S, Randall M, Kamen M, Logan Hyde JL. 1941. Heavy oxygen ( 18 O) as a tracer in the study of photosynthesis . Journal of the American Chemical Society 63 : 877–879. [ Google Scholar ]
- Rubin A, Riznichenko G. 2009. Modeling of the primary processes in a photosynthetic membrane. In: Laisk A, Nedbal AL, Govindjee, eds. Photosynthesis in silico: understanding complexity from molecules to ecosystems. Advances in photosynthesis and respiration , Vol. 29. Dordrecht: Springer, 151–176. [ Google Scholar ]
- Šafránek D, Červený J, Klement M, Pospíšilová J, Brim L, Lazár D, Nedbal L. 2011. E-photosynthesis: Web-based platform for modeling of complex photosynthetic processes . BioSystems 103 : 115–124. [ PubMed ] [ Google Scholar ]
- Sapozhnikov DI, Krasnovskaya TA, Maevskaya AN. 1957. Change in the interrelationship of the basic carotenoids of the plastids of green leaves under the action of light . Doklady Akademii Nauk SSSR 113 : 465–467. [ Google Scholar ]
- Schansker G, Srivastava A, Govindjee, Strasser RJ. 2003. Characterization of the 820-nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves . Functional Plant Biology 30 : 785–796 [ PubMed ] [ Google Scholar ]
- Schansker G, Tóth SZ, Strasser RJ. 2005. Methylviologen and dibromorhymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP . Biochimica et Biophysica Acta 1706 : 250–261. [ PubMed ] [ Google Scholar ]
- Schansker G, Tóth SZ, Kovács L, Holzwarth AR, Garab G. 2011. Evidence for a fluorescence yield change driven by a light-induced conformational change within photosystem II during the fast chlorophyll a fluorescence rise . Biochimica et Biophysica Acta 1807 : 1032–1043. [ PubMed ] [ Google Scholar ]
- Schansker G, Tóth SZ, Holzwarth AR, Garab G. 2014. Chlorophyll a fluorescence: beyond the limits of the Q A model . Photosynthesis Research 120 : 43–58. [ PubMed ] [ Google Scholar ]
- Schatz GH, Brock H, Holzwarth AR. 1988. Kinetic and energetic model for the primary processes in photosystem II . Biophysical Journal 54 : 397–405. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Schlodder E, Meyer B. 1987. pH-dependence of oxygen evolution and reduction kinetics of photooxidized chlorophyll a II (P-680) in Photosystem II particles from Synechococcus sp . Biochimica et Biophysica Acta 890 : 23–31. [ Google Scholar ]
- Schreiber U. 1986. Detection of rapid induction kinetics with a new type of high-frequency modulated chlorophyll fluorometer . Photosynthesis Research 9 : 261–272. [ PubMed ] [ Google Scholar ]
- Schreiber U, Schliwa U, Bilger W. 1986. Continuous recording of photochemical and nonphotochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer . Photosynthesis Research 10 : 51–62. [ PubMed ] [ Google Scholar ]
- Schreiber U, Neubauer C, Klughammer C. 1989. Devices and methods for room-temperature fluorescence analysis . Philosophical Transactions of the Royal Society B 323 : 241–251. [ Google Scholar ]
- Schreiber U, Klughammer C, Schansker G. 2019. Rapidly reversible chlorophyll fluorescence quenching induced by pulses of supersaturating light in vivo . Photosynthesis Research 142 : 35–50. [ PubMed ] [ Google Scholar ]
- Semer J, Štroch M, Špunda V, Navrátil M. 2018. Partitioning of absorbed light energy within photosystem II in barley can be affected by chloroplast movement . Journal of Photochemistry and Photobiology B: Biology 186 : 98–106. [ PubMed ] [ Google Scholar ]
- Semer J, Navrátil M, Špunda V, Štroch M. 2019. Chlorophyll fluorescence parameters to assess utilization of excitation energy in photosystem II independently of changes in leaf absorption . Journal of Photochemistry and Photobiology B: Biology 197 : 111535. [ PubMed ] [ Google Scholar ]
- Sétif P. 2015. Electron-transfer kinetics in cyanobacterial cells: Methyl viologen is a poor inhibitor of linear electron flow . Biochimica et Biophysica Acta 1847 : 212–222. [ PubMed ] [ Google Scholar ]
- Shen JR. 2015. The structure of photosystem II and the mechanism of water oxidation in photosynthesis . Annual Review of Plant Biology 66 : 23–48. [ PubMed ] [ Google Scholar ]
- Shevela D, Eaton-Rye JJ, Shen JR, Govindjee. 2012. Photosystem II and the unique role of bicarbonate: a historical perspective . Biochimica et Biophysica Acta 1817 : 1134–1151. [ PubMed ] [ Google Scholar ]
- Shevela D, Björn L, Govindjee. 2019. Photosynthesis: solar energy for life . Singapore: World Scientific. [ Google Scholar ]
- Shikanai TI, Yamamoto H. 2017. Contribution of cyclic and pseudo-cyclic electron transport to the formation of proton motive force in chloroplasts . Molecular Plant 10 : 20–29. [ PubMed ] [ Google Scholar ]
- Shinkarev VP, Govindjee. 1993. Insight into the relationship of chlorophyll a fluorescence yield to the concentration of its natural quenchers in oxygenic photosynthesis . Proceedings of the National Academy of Sciences of the USA 90 : 7466–7469. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Simkin AJ, McAusland L, Headland LR, Lawson T, Raines CA. 2015. Multigene manipulation of photosynthetic carbon assimilation increases CO 2 fixation and biomass yield in tobacco . Journal of Experimental Botany 66 : 4075–4090. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Simkin AJ, McAusland L, Lawson T, Raines CA. 2017. Overexpression of the RieskeFeS protein increases electron transport rates and biomass yield . Plant Physiology 175 : 134–145. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Simkin AJ, López-Calcagno PE, Raines CA. 2019. Feeding the world: improving photosynthetic efficiency for sustainable crop production . Journal of Experimental Botany 70 : 1119–1140. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Snellenburg J, Johnson MP, Ruban AV, van Grondelle R, van Stokkum IHM. 2017. A four state parametric model for the kinetics of the non-photochemical quenching in Photosystem II . Biochimica et Biophysica Acta 1858 : 854–864. [ PubMed ] [ Google Scholar ]
- South PF, Cavanagh AP, Lopez-Calcagno PE, Raines CA, Ort DR. 2018. Optimizing photorespiration for improved crop productivity . Journal of Integrative Plant Biology 60 : 1217–1230. [ PubMed ] [ Google Scholar ]
- Srivastava A, Guissé B, Greppin H, Strasser RJ. 1997. Regulation of antenna structure and electron transport in photosystem II of Pisum sativum under elevated temperature probed by the fast polyphasic chlorophyll a fluorescence transient: OKJIP . Biochimica et Biophysica Acta 1320 : 95–106. [ Google Scholar ]
- Steffen R, Christen G, Renger G. 2001. Time-resolved monitoring of flash-induced changes of fluorescence quantum yield and decay of delayed light emission in oxygen-evolving photosynthetic organisms . Biochemistry 40 :173–180. [ PubMed ] [ Google Scholar ]
- Steffen R, Eckert H-J, Kelly AA, Dörmann P, Renger G. 2005. Investigation on the reaction pattern of photosystem II in leaves from Arabidopsis thaliana by time resolved fluorometric analysis . Biochemistry 44 : 3123–3133. [ PubMed ] [ Google Scholar ]
- Stiehl HH, Witt HT. 1968. Die kurzzeitigen ultravioletten Differenzspektren bei der Photosynthese . Zeitschrift für Naturforschung 23b : 220–224. [ PubMed ] [ Google Scholar ]
- Stiehl HH, Witt HT. 1969. Quantitative treatment of the function of plastoquinone in photosynthesis . Zeitschrift für Naturforschung 24b : 1588–1598. [ PubMed ] [ Google Scholar ]
- Stirbet A. 2013. Excitonic connectivity between photosystem II units: what is it, and how to measure it? Photosynthesis Research 116 189–214. [ PubMed ] [ Google Scholar ]
- Stirbet A, Govindjee. 2011. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: basics and applications of the OJIP fluorescence transient . Journal of Photochemistry and Photobiology B: Biology 104 : 236–257. [ PubMed ] [ Google Scholar ]
- Stirbet A, Govindjee. 2012. Chlorophyll a fluorescence induction: a personal perspective of the thermal phase, the J–I–P rise . Photosynthesis Research 113 : 15–61. [ PubMed ] [ Google Scholar ]
- Stirbet A, Govindjee. 2016. The slow phase of chlorophyll a fluorescence induction in silico: origin of the S-M fluorescence rise . Photosynthesis Research 130 : 193–213. [ PubMed ] [ Google Scholar ]
- Stirbet A, Strasser RJ. 1995. Numerical simulation of the fluorescence induction in plants . Archives des Sciences – Université de Genève 48 : 41–60. [ Google Scholar ]
- Stirbet A, Strasser RJ. 1996. Numerical simulation of the in vivo fluorescence in plants . Mathematics and Computers in Simulation 42 : 245–253. [ Google Scholar ]
- Stirbet A, Govindjee, Strasser BJ, Strasser RJ. 1998. Chlorophyll a fluorescence induction in higher plants: modeling and numerical simulation . Journal of Theoretical Biology 193 : 131–151. [ Google Scholar ]
- Stirbet A, Rosenau P, Strőder AC, Strasser RJ. 2001. Parameter optimisation of fast chlorophyll fluorescence induction model . Mathematics and Computers in Simulation 56 : 443–450. [ Google Scholar ]
- Stirbet A, Riznichenko GY, Rubin AB, Govindjee. 2014. Modeling chlorophyll a fluorescence transient: relation to photosynthesis . Biochemistry (Mosc.) 79 : 291–323. [ PubMed ] [ Google Scholar ]
- Stirbet A, Lazár D, Kromdijk J, Govindjee. 2018. Chlorophyll a fluorescence induction: can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 56 : 86–104. [ Google Scholar ]
- Stirbet A, Lazár D, Papageorgiou CG, Govindjee. 2019. Chlorophyll a fluorescence in cyanobacteria: relation to photosynthesis; In: Mishra AK, Tiwari DN, Rai AN eds. Cyanobacteria – from basic science to applications . London: Academic Press, 79–130. [ Google Scholar ]
- Strand DD, Kramer DM. 2014. Control of non-photochemical exciton quenching by the proton circuit of photosynthesis. In: Demmig-Adams B, Garab G, Adams WW III, Govindjee, Sharkey TD, eds. Nonphotochemical quenching and energy dissipation in plants, algae and cyanobacteria. Advances in photosynthesis and respiration , vol. 40. Dordrecht: Springer, 387–408. [ Google Scholar ]
- Strand DD, Fisher N, Davis GA, Kramer DM. 2016. Redox regulation of the antimycin A sensitive pathway of cyclic electron flow around photosystem I in higher plant thylakoids . Biochimica et Biophysica Acta 1857 : 1–6 [ PubMed ] [ Google Scholar ]
- Strand DD, Fisher N, Kramer DM. 2017. The higher plant plastid NAD(P)H dehydrogenase-like complex (NDH) is a high efficiency proton pump that increases ATP production by cyclic electron flow . The Journal of Biological Chemistry 292 : 11850–11860. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Strasser RJ. 1978. The grouping model of plant photosynthesis. In: Argyroudi-Akoyunoglou JH, Akoyunoglou G, eds. Chloroplast development . Amsterdam: Elsevier Biomedical, 513–515. [ Google Scholar ]
- Strasser RJ, Govindjee. 1991. The F 0 and the O-J-I-P fluorescence rise in higher plants and algae. In: Argyroudi-Akoyunoglou JH, ed. Regulation of chloroplast biogenesis . New York: Plenum Press, 423–426. [ Google Scholar ]
- Strasser RJ, Stirbet A, 1998. Heterogeneity of photosystem II probed by the numerically simulated chlorophyll a fluorescence rise (O-J-I-P) . Mathematics and Computers in Simulation 48 : 3–9. [ Google Scholar ]
- Strasser RJ, Stirbet A. 2001. Estimation of the energetic connectivity of PS II centres in plants using the fluorescence rise O-J-I-P. Fitting of experimental data to three different PS II models . Mathematics and Computers in Simulation 56 : 451–461. [ Google Scholar ]
- Strasser RJ, Srivastava A, Govindjee. 1995. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria . Photochemistry and Photobiology 61 : 32–42. [ Google Scholar ]
- Strasser RJ, Tsimilli-Michael M, Srivastava A. 2004. Analysis of the chlorophyll fluorescence transient. In: Papageorgiou GC, Govindjee eds. Chlorophyll a fluorescence: a signature of photosynthesis. Advances in photosynthesis and respiration , vol. 19. Dordrecht: Springer, 321–362. [ Google Scholar ]
- Sušila P, Lazár D, Ilík P, Tomek P and Nauš J. 2004. The gradient of exciting radiation within a sample affects relative heights of steps in the fast chlorophyll a fluorescence rise . Photosynthetica 42 : 161–172. [ Google Scholar ]
- Sylak-Glassman EJ, Zaks J, Amarnath K, Leuenberger M, Fleming GR. 2016. Characterizing non-photochemical quenching in leaves through fluorescence lifetime snapshots . Photosynthesis Research 127 : 69–76. [ PubMed ] [ Google Scholar ]
- Tagawa K, Tsujimoto HY, Arnon DI. 1963. Role of chloroplast ferredoxin in the energy conversion process of photosynthesis . Proceedings of the National Academy of Sciences of the USA 49 : 567–572. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Thompson LK, Brudvig GW. 1988. Cytochrome b-559 may function to protect photosystem II from photoinhibitin . Biochemistry 27 : 6653–6658. [ PubMed ] [ Google Scholar ]
- Tian L, Xu P, Chukhutsina VU, Holzwarth AR, Croce R. 2017. Zeaxanthin-dependent nonphotochemical quenching does not occur in photosystem I in the higher plant Arabidopsis thaliana . Proceedings of the National Academy of Sciences of the USA 114 : 4828–4832. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tikhonov AN. 2013. pH-Dependent regulation of electron transport and ATP synthesis in chloroplasts . Photosynthesis Research 116 : 511–534. [ PubMed ] [ Google Scholar ]
- Tikhonov AN, Vershubskii AV. 2014. Computer modeling of electron and proton transport in chloroplasts . Biosystems 121 : 1‒ 21. [ PubMed ] [ Google Scholar ]
- Tokarčík A. 2012. Rewriting complex biological models in stochastic process algebras. A case study . Bachelor Thesis, Masaryk University. [ Google Scholar ]
- Tomek P, Lazár D, Ilík P, Nauš J. 2001. On the intermediate steps between the O and P steps in chlorophyll a fluorescence rise measured at different intensities of exciting light . Australian Journal of Plant Physiology 28 : 1151–1160. [ Google Scholar ]
- Tomek P, Ilík P, Lazár D, Štroch M, Nauš J. 2003. On the determination of Q B -non-reducing photosystem II centers from chlorophyll a fluorescence induction . Plant Science 164 : 665–670. [ Google Scholar ]
- Trebst A, Reimer S. 1973. Energy conservation in photoreductions by Photosystem II. Reversal of dibromothymoquinone inhibition of Hill-reactions by phenylenediamines . Zeitschrift für Naturforschung 28c : 710. [ Google Scholar ]
- Trissl H-W, Lavergne J. 1995. Fluorescence induction from photosystem II: analytical equations for the yields of photochemistry and fluorescence derived from analysis of a model including exciton-radical pair equilibrium and restricted energy transfer between units . Australian Journal of Plant Physiology 22 : 183–193. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Trissl H-W, Gao Y, Wulf K. 1993. Theoretical fluorescence induction curves derived from coupled differential equations describing the primary photochemistry of photosystem II by an exciton-radical pair equilibrium . Biophysical Journal 64 : 974–988. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tyystjärvi E. 2013. Photoinhibition of photosystem II . International Review of Cell and Molecular Biology 300 : 243–303. [ PubMed ] [ Google Scholar ]
- Tyystjärvi E, Hakala M, Sarvikas P. 2005. Mathematical modelling of the light response curve of photoinhibition of photosystem II . Photosynthesis Research 84 : 21–27. [ PubMed ] [ Google Scholar ]
- Valero E, Gonzalez-Sanchez MI, Macia H, Garcıa-Carmona F. 2009. Computer simulation of the dynamic behavior of the glutathione-ascorbate redox cycle in chloroplasts . Plant Physiology 149 : 1958–1969. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- van Amerongen H, Croce R. 2013. Light-harvesting in photosystem II . Photosynthesis Research 116 : 251–263. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- van Gorkom HJ. 1974. Identification of the reduced primary electron acceptor of Photosystem II as a bound semiquinone anion . Biochimica et Biophysica Acta 347 : 439–442. [ PubMed ] [ Google Scholar ]
- van Gorkom HJ, Pulles MPJ, Etienne A-L. 1978. Fluorescence and absorbance changes in Tris-washed chloroplasts. In: Metzner H, ed. Photosynthetic oxygen evolution . London: Academic Press, 135–145. [ Google Scholar ]
- van Kooten O, Snel JFH, Vredenberg VJ. 1986. Photosynthetic free energy transduction related to the electric potential changes across the thylakoid membrane . Photosynthesis Research 9 : 211–227. [ PubMed ] [ Google Scholar ]
- van Rensen JJS. 2002. Role of bicarbonate at the acceptor side of Photosystem II . Photosynthesis Research 73 : 185–192. [ PubMed ] [ Google Scholar ]
- Vavilin DV, Tyystjärvi E, Aro E-M. 1998. Model for the fluorescence induction curve of photoinhibited thylakoids . Biophysical Journal 75 : 503–512. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Velthuys BR, Amesz J. 1974. Charge accumulation at the reducing side of Photosystem 2 of photosynthesis . Biochimica et Biophysica Acta 333 : 85–94. [ PubMed ] [ Google Scholar ]
- Vermaas WFJ. 2007. Targeted genetic modification of cyanobacteria: new biotechnological applications. In: Richmond A, ed. Handbook of microalgal culture . Oxford: Blackwell Science, 457–470. [ Google Scholar ]
- Vermeglio A. 2002. The two-electron gate in photosynthetic bacteria . Photosynthesis Research 73 : 83–86. [ PubMed ] [ Google Scholar ]
- Vink M, Zer H, Alumot N, et al.. 2004. Light modulated exposure of the light-harvesting complex II (LHCII) to protein kinase(s) and state transition in Chlamydomonas reinhardtii xanthophyll mutants . Biochemistry 43 : 7824–7833. [ PubMed ] [ Google Scholar ]
- Visser D, Heijnen J. 2002. The mathematics of metabolic control analysis revisited . Metabolic Engineering 4 : 114–123. [ PubMed ] [ Google Scholar ]
- Vredenberg WJ. 2000. A three-state model for energy trapping and chlorophyll fluorescence in photosystem II incorporating radical pair recombination . Biophysical Journal 79 : 26–38. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Vredenberg WJ. 2008. Algorithm for analysis of OJDIP fluorescence induction curves in terms of photo- and electrochemical events in photosystems of plant cells. Derivation and application . Journal of Photochemistry and Photobiology B: Biology 91 : 58–65. [ PubMed ] [ Google Scholar ]
- Vredenberg WJ. 2011. Kinetic analyses and mathematical modeling of primary photochemical and photoelectrochemical processes in plant photosystems . BioSystems 103 : 138–151. [ PubMed ] [ Google Scholar ]
- Vredenberg WJ, Duysens LNM. 1963. Transfer of energy from bacteriochlorophyll to a reaction centre during bacterial photosynthesis . Nature 197 : 355–357. [ PubMed ] [ Google Scholar ]
- Wang X, Cao J, Maroti P, et al.. 1992. Is bicarbonate in photosystem II the equivalent of the glutamate ligand to the iron atom in bacterial reaction centers? Biochimica et Biophysica Acta 1100 : 1–8. [ PubMed ] [ Google Scholar ]
- Warburg O, Uyesugi T. 1924. Über die Blackmansche Reaktion . Biochemische Zeitschrift 146 : 486–492. [ Google Scholar ]
- Werdan K, Heldt HW, Milovancev M. 1975. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO 2 fixation in the light and dark . Biochimica et Biophysica Acta 396 : 276–292. [ PubMed ] [ Google Scholar ]
- Wientjes E, Croce R. 2012. PMS: photosystem I electron donor or fluorescence quencher . Photosynthesis Research 111 : 185–191. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Willstätter R, Stoll A. 1913. Untersuchungen über Chlorophyll . Berlin: Justus Springer. [ Google Scholar ]
- Witt HT. 2004. Steps on the way to building blocks, topologies, crystals and X-ray structural analysis of Photosystems I and II of water-oxidizing photosynthesis . Photosynthesis Research 80 : 85–107. [ PubMed ] [ Google Scholar ]
- Witt HT, Müller A, Rumberg B. 1961 a Experimental evidence for the mechanism of photosynthesis . Nature 191 : 194–195. [ PubMed ] [ Google Scholar ]
- Witt HT, Müller A, Rumberg B. 1961 b Oxidized cytochrome and chlorophyll in photosynthesis . Nature 192 : 967–969. [ PubMed ] [ Google Scholar ]
- Wood WH, Barnett SFH, Flannery S, Hunter CN, Johnson MP. 2019. Dynamic thylakoid stacking is regulated by LHCII phosphorylation but not its interaction with photosystem I . Plant Physiology 180 : 2152–2166. [ Google Scholar ]
- Wraight CA, Crofts AR. 1970. Energy-dependent quenching of chlorophyll a fluorescence in isolated chloroplasts . European Journal of Biochemistry 17 : 319–327. [ PubMed ] [ Google Scholar ]
- Wydrzynski T, Govindjee. 1975. A new site of bicarbonate effect in Photosystem II of photosynthesis; evidence from chlorophyll fluorescence transients in spinach chloroplasts . Biochimica et Biophysica Acta 387 : 403–408. [ PubMed ] [ Google Scholar ]
- Wydrzynski T, Satoh K, eds. 2005. Photosystem II: the light-driven water-plastoquinone oxidoreductase. Advances in photosynthesis and respiration , Vol. 22. Dordrecht: Springer. [ Google Scholar ]
- Xia Q, Tan J, Ji X, Jiang Y, Guo Y. 2018. Modelling and simulation of chlorophyll fluorescence from photosystem II as affected by temperature . IET Systems Biology 12 : 304–310. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Xin CP, Yang J, Zhu XG. 2013. A model of chlorophyll a fluorescence induction kinetics with explicit description of structural constraints of individual photosystem II units . Photosynthesis Research 117 : 339–354. [ PubMed ] [ Google Scholar ]
- Yamamoto HY, Higashi RM. 1978. Violaxanthin deepoxidase. Lipid composition and substrate specificity . Archives of Biochemistry and Biophysics 190 : 514–522. [ PubMed ] [ Google Scholar ]
- Yamamoto HY, Nakayama T, Chichester C. 1962. Studies on the light and dark interconversions of leaf xanthophylls . Archives of Biochemistry and Biophysics 97 : 168–173. [ PubMed ] [ Google Scholar ]
- Yamori W, Makino A, Toshiharu Shikanai T. 2016. A physiological role of cyclic electron transport around photosystem I in sustaining photosynthesis under fluctuating light in rice . Scientific Reports 6 : 20147. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yin X, Harbinson J, Struik PC. 2009. A model of the generalized stoichiometry of electron transport limited C3 photosynthesis: development and applications. In: Laisk A, Nedbal AL, Govindjee, eds. Photosynthesis in silico: understanding complexity from molecules to ecosystems. Advances in photosynthesis and respiration , Vol. 29. Dordrecht: Springer, 247–273. [ Google Scholar ]
- Zaks J, Amarnath K, Kramer DM, Niyogi KK, Fleming GR. 2012. A kinetic model of rapidly reversible nonphotochemical quenching . Proceedings of the National Academy of Sciences of the USA 109 : 15757–15762. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zaks J, Amarnath K, Sylak-Glassman EJ, Fleming GR. 2013. Models and measurements of energy-dependent quenching . Photosynthesis Research 116 : 389–409. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zhou Y, Schideman LC, Park DS, et al.. 2015. Characterization of a Chlamydomonas reinhardtii mutant strain with improved biomass production under low light and mixotrophic conditions . Algal Research 11 : 134–147. [ Google Scholar ]
- Zhu XG, Govindjee, Baker NR, deSturler E, Ort DR, Long SP. 2005. Chlorophyll a fluorescence induction kinetics in leaves predicted from a model describing each discrete step of excitation energy and electron transfer associated with photosystem II . Planta 223 : 114–133. [ PubMed ] [ Google Scholar ]
- Zhu XG, de Sturler E, Long SP. 2007. Optimizing the distribution of resources between enzymes of carbon metabolism can dramatically increase photosynthetic rate: a numerical simulation using an evolutionary algorithm . Plant Physiology 145 : 513–526. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zhu XG, Long SP, Ort DR. 2010. Improving photosynthetic efficiency for greater yield . Annual Review of Plant Biology 61 : 235–261. [ PubMed ] [ Google Scholar ]
- Zhu XG, Wang Y, Ort DR, Long SP. 2013. e-Photosynthesis: a comprehensive dynamic mechanistic model of C3 photosynthesis: from light capture to sucrose synthesis . Plant, Cell & Environment 36 : 1711–1727. [ PubMed ] [ Google Scholar ]
- Zhu XG, Lynch JP, LeBauer DS, Millar AJ, Stitt M, Long SP. 2016. Plants in silico: why, why now and what? – an integrative platform for plant systems biology research . Plant, Cell & Environment 39 : 1049–1057. [ PubMed ] [ Google Scholar ]
- Zito F, Finazzi G, Delosme R, Nitschke W, Picot D, Wollman FA. 1999. The Qo site of cytochrome b6f complexes controls the activation of the LHCII kinase . EMBO Journal 18 : 2961–2969. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zouni A, Witt HT, Kern J, et al.. 2001. Crystal structure of photosystem II from Synechococcus elongatus at 3.8 Å resolution . Nature 409 , 739–743. [ PubMed ] [ Google Scholar ]
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Biology library
Course: biology library > unit 13.
- Conceptual overview of light dependent reactions
- Light dependent reactions actors
- Photosynthesis: Overview of the light-dependent reactions
Light and photosynthetic pigments
- The light-dependent reactions
Introduction
What is light energy, pigments absorb light used in photosynthesis, chlorophylls, carotenoids, what does it mean for a pigment to absorb light, attribution:.
- “ The light-dependent reactions of photosynthesis ,” by OpenStax College ( CC BY 3.0 ). Download the original article for free at http://cnx.org/contents/f829b3bd-472d-4885-a0a4-6fea3252e2b2@11 .
- " Bis2A 06.3 Photophosphorylation: the light reactions of photosynthesis ," by Mitch Singer ( CC BY 4.0 ). Download the original article for free at http://cnx.org/contents/c8fa5bf4-1af7-4591-8d76-711d0c1f05f9@2 .
Works cited:
- Chlorophyll a. (2015, October 11). Retrieved October 22, 2015 from Wikipedia: https://en.wikipedia.org/wiki/Chlorophyll_a .
- Speer, B.R., (1997, July 9) Photosynthetic pigments. In UCMP glossary . Retrieved from http://www.ucmp.berkeley.edu/glossary/gloss3/pigments.html .
- Bullerjahn, G. S. and A. F. Post. (1993). The prochlorophytes: are they more than just chlorophyll a/b-containing cyanobacteria? Crit. Rev. Microbiol. 19(1), 43. http://dx.doi.org/10.3109/10408419309113522 .
- Reece, J. B., Urry, L. A., Cain, M. L., Wasserman, S. A., Minorsky, P. V., and Jackson, R. B. (2011). Photosynthesis. In Campbell biology (10th ed.). San Francisco, CA: Pearson, 193.
Additional references:
Want to join the conversation.
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page

You are using an outdated browser. Please upgrade your browser or activate Google Chrome Frame to improve your experience.
- Account Home
Activities and Experiments to Explore Photosynthesis in the Classroom

Photosynthesis can be a difficult concept to grasp, that’s why we’ve compiled a selection of hands-on activities and experiments to help show students some of the concepts in action.
In addition to the ideas below, PLT’s new Explore Your Environment: K-8 Activity Guide and PLT’s PreK-8 Environmental Education Activity Guide both offer a wealth of hands-on, creative activities and resources for lessons about photosynthesis. Each guide includes a comprehensive Topic Index to help you quickly find a list of relevant activities that fit your needs and every activity includes a background section for educators that gives a science-based introduction to the activity’s content. We also have a few abridged versions related to the physiology of trees and photosynthesis for families to try out together at home, for example, How Plants Grow and Tree Factory .
Introduction to Photosynthesis
The word “photosynthesis” comes from Greek root words that combine to mean “to put together with the help of light.”
All plants, algae, and some microorganisms like bacteria photosynthesize to make their own food. This makes them part of a group of organisms called autotrophs. Unlike heterotrophs, which include animals that feed off other living organisms, autotrophs make nutritional organic substances from simple inorganic substances. What a superpower!
To undergo photosynthesis, plants need carbon dioxide from the air, water from the soil, and sunlight. These elements combine in a chemical reaction that takes place inside of a plant’s leaves to create glucose and oxygen.
Absorbing Carbon Dioxide and Water
Carbon dioxide can be produced naturally from the decomposition of living things and events like volcanic eruptions, and from human activity like burning fossil fuels.
Animals respirate by inhaling gases in the air, retaining oxygen, and releasing carbon dioxide. However, when plants breathe, they take in carbon dioxide, which is a key ingredient required for photosynthesis. Carbon dioxide enters a plant through its stomata, tiny pores that are usually located on the underside of leaves and sometimes stems. Most plants also soak up another substance through their roots that they need for photosynthesis: water.
Adding Energy
Once a plant has carbon dioxide and water, it needs energy to enable these two substances to chemically react with each other. It gets energy from a steady stream of sunlight hitting its leaves. Chlorophyll, a green pigment found in tiny structures called chloroplasts within leaves, absorbs energy from blue and red light waves from the sun. The sunlight’s energy is then transferred to two types of energy-storing molecules within the plant.
The energy already stored from the sun fuels a reaction in the leaves’ chloroplasts that splits water molecules (H 2 0) into pure hydrogen (H) and oxygen (O 2 ). The hydrogen reacts with carbon dioxide (CO 2 ) to produce glucose, a type of sugar. The full chemical equation of photosynthesis looks like this:
6CO 2 + 6H 2 0 + Sunlight → C 6 H 12 O 6 + 6O 2
In other words, the carbon dioxide and water that go into the plant combine with energy from sunlight to produce glucose, and also oxygen.
Storing and Using Glucose
Once this sugar is made, it can be stored as energy (food) that the plant uses for growth and repair. Plants also use the energy from nutrients in the soil along with glucose to grow and develop leaves, flowers, and fruits.
Students often wonder how a gas like carbon dioxide that you can’t see helps form a giant tree or the apple they eat for lunch. It’s because a chemical reaction doesn’t have to start with a solid (like soil) to end with a solid (like a tree or apple). It helps for students to understand the carbon cycle – and PLT has a variety of content to support this.
Glucose is a carbohydrate, which is simply a molecule containing carbon, hydrogen, and oxygen. Smaller glucose molecules can build bigger carbohydrates like cellulose or starch.
Similar to a human skeleton, cellulose is the main component of plant cell walls that help strengthen the plant. Humans can’t digest cellulose, but the fiber found in cellulose-heavy foods like celery and broccoli aids with digestion and can lower the risk of diseases like cancer. These strong fibers are also used to make clothes and paper. Animals like cows, horses, and sheep can digest cellulose, so it makes sense that they eat grass for quick energy and nutrients.
Plants can also convert glucose into starch, which is a larger carbohydrate molecule that can store its energy. Humans break down starches found in foods like potatoes and rice into glucose, and it, in turn, gives them energy.
Though you may not use sunlight to create your food, when you eat something like chicken or rice, you take in energy plants used from the sun. And not only does a plant produce food animals need for their energy as a result of photosynthesis, but it also releases oxygen as a byproduct through its stomata into the atmosphere.
Photosynthesis is critical for the survival of all living organisms — not just plants.
Hands-On Photosynthesis Activities
Photosynthesis can be a difficult concept to grasp, especially for younger learners. That’s why we’ve compiled these interactive activities and experiments that show some of the concepts in action.
Photosynthesis Visuals
These photosynthesis modeling activities will help students visualize and better understand what a plant needs to undergo photosynthesis and what it produces as a result. The 3D and 2D representations will also help them absorb some of the vocabulary associated with photosynthesis.
3D Photosynthesis: Tree Leaf Model
Older students can create these more complex 3D models of a leaf’s front and backside where all of the photosynthesis action takes place, like on its stomata and chloroplasts. They will attach labels to the leaf that describe the different substances involved.
The Ins and Outs of Photosynthesis
Younger learners will enjoy this less complex visual activity that involves a leaf with “IN” and “OUT” envelopes into which they’ll place the respective chemical reactants or products of photosynthesis.
Photosynthesis Paper Craft
Take your lesson in an artistic direction by letting students create these bright and fun paper flower and sun displays, complete with the basic photosynthesis terms.
Exploring Leaves with STEM
These STEM experiments requiring real leaves will spark valuable critical thinking when students observe leaf structure, stomata, plant respiration, and more.
Respirating Leaves
The invisible chemical process of a leaf exchanging carbon dioxide, water, and sunlight for oxygen will become visible when your class observes what happens when they submerge leaves in water.
Stomata Microscope Investigation
Students will use microscopes to explore the structure of a leaf that makes the exchange of gasses during photosynthesis possible. They can also explore other parts of leaves and how plants gain mass.
Stomata Microscope Comparison
Compare the stomata sizes and numbers of different plant species under a microscope and examine leaf texture by creating cool “nail polish imprints.”
Exploring Plants and Sunlight
Plants need sunlight for survival, so it makes sense that their behavior or appearance would change if their access to sunlight is altered. These activities explore this concept.
Measuring Plant Growth with Sunlight
This activity takes a couple of weeks but will give your students valuable insight into how a plant’s growth and green coloration is affected by varying levels of sunlight over time. They’ll flex their critical thinking skills as they take daily notes and conclude what happens to a seed under different light conditions.
Rotating Plants
Track how plants bend towards the sun wherever they are with this great exercise that introduces young students to just how active plants can be when it comes to gaining precious sun energy. You can grow seedlings or even experiment with a larger plant you have and see how its color or growth is affected when you rotate or move closer or further from the sun.
Fun with Plant Pigmentation
There’s a lot of fun that can be had with the chlorophyll in leaves, including art and color experimentation!
Chlorophyll Paintings
Chlorophyll pigment not only turns plants green – it makes leaves great mediums for “green” art projects! Kids will love this out-of-the-box painting style, learn about chlorophyll firsthand, and expand their creativity all at once.
Leaf Color Chemistry Experiment
When the school year begins, recreate how leaves change color in autumn with green leaves, rubbing alcohol, coffee filters, and other easy-to-find items. The pigments of chlorophyll will fade and leave behind hidden pigments that demonstrate why leaves change color in the fall – which is also when your class can reflect back on this eye-opening experiment.
Let Project Learning Tree Be Your Guide
Introduce students to photosynthesis with these PLT activities from the new Explore Your Environment: K-8 Activity Guide :
- Here We Grow Again (for grades K-2), Every Tree for Itself , and Signs of Fall (for grades 3-5) in PLT’s Explore Your Environment: K-8 Activity Guide
- How Plants Grow and Sunlight and Shades of Green (Activities 41 and 42 in PLT’s PreK-8 Environmental Education Activity Guide ), and
- Power Plants (Activity 4 in PLT’s Energy & Ecosystems E-Unit).
Watch an example of an activity! This video walks viewers through PLT’s activity Signs of Fall. In this activity, participants are introduced to different leaf pigments and use chromatography to pull out leaf pigments using simple household items. It helps answer the question, “Why do leaves change color?”.
For further guidance on how to relay the essential concepts of photosynthesis to your classroom and more great activities, check out this Unit of Instruction by Project Learning Tree. It suggests linking select PLT activities to help students learn more about the topic of photosynthesis using a storyline technique. Storylines ensure connectivity and continuity between individual activities and can serve as the “instructional glue” that bind many areas of knowledge and skills. The Unit of Instruction includes a guiding question, concepts addressed, and connections to the Next Generation Science Standards (NGSS) and PLT’s Forest Literacy Framework.
To boost your teaching with 50 field-tested, hands-on multidisciplinary activities that educate and connect elementary students with nature in powerful ways, and more suggested Units of Instruction , look no further than Project Learning Tree’s new Explore Your Environment: K-8 Activity Guide .
Rebecca Reynandez
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
You may use these HTML tags and attributes: <a href="" title=""> <abbr title=""> <acronym title=""> <b> <blockquote cite=""> <cite> <code> <del datetime=""> <em> <i> <q cite=""> <s> <strike> <strong>
Save my name, email, and website in this browser for the next time I comment.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Digital Tools to Connect Your Students to Wildlife
One of the best ways to ensure future generations can enjoy our planet’s rich biodiversity is to raise awareness about the importance of conserving our natural world. World Wildlife Day aims to make that happen with an annual day of observance and call for wildlife conservation and education.

Environmental Education Resources
Every month we carefully select new educational apps, videos, interactive websites, books, careers information, and teacher-generated materials that support PLT lessons.
STEM: Tale of the Sun
Ideas to engage elementary and middle school students in science, technology, engineering, and math as they learn about history, tradition, and storytelling.
PreK-8 Environmental Education Activity Guide – Activity 51, Make Your Own Paper
Students investigate the papermaking process by trying it themselves. Students are thrilled to find that they can make paper and that their product is practical, as well as beautiful. Watch a video of the paper-making process used in this activity.
MAKE LEARNING FUN
ATTEND A TRAINING
Get our educational materials and professional development by participating in an in-person workshop or an online course.
CONTACT YOUR COORDINATOR
Get information relevant to your state, plus local assistance and connections to resources and professionals in your community.
EDUCATOR TIPS
Get a wealth of up-to-date resources, support, and ideas from teachers and other educators.
SUBSCRIBE TO OUR NEWSLETTER, The Branch
Sign up for our monthly e-newsletter for free tools and resources, new lesson plans, professional development and grant opportunities, and tips from educators for teaching about the environment.
- Email Address *
- First Name * First

IMAGES
VIDEO
COMMENTS
In chemical terms, photosynthesis is a light-energized oxidation-reduction process. (Oxidation refers to the removal of electrons from a molecule; reduction refers to the gain of electrons by a molecule.) In plant photosynthesis, the energy of light is used to drive the oxidation of water (H 2 O), producing oxygen gas (O 2 ), hydrogen ions (H ...
The meaning of PHOTOSYNTHESIS is synthesis of chemical compounds with the aid of radiant energy and especially light; especially : formation of carbohydrates from carbon dioxide and a source of hydrogen (such as water) in the chlorophyll-containing cells (as of green plants) exposed to light. Photosynthesis Has Greek Roots
Photosynthesis ( / ˌfoʊtəˈsɪnθəsɪs / FOH-tə-SINTH-ə-sis) [1] is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their activities.
Photosynthesis (Google doc) Most life on Earth depends on photosynthesis .The process is carried out by plants, algae, and some types of bacteria, which capture energy from sunlight to produce oxygen (O 2) and chemical energy stored in glucose (a sugar). Herbivores then obtain this energy by eating plants, and carnivores obtain it by eating ...
Photosynthesis Definition. Photosynthesis is the biochemical pathway which converts the energy of light into the bonds of glucose molecules. The process of photosynthesis occurs in two steps. In the first step, energy from light is stored in the bonds of adenosine triphosphate (ATP), and nicotinamide adenine dinucleotide phosphate (NADPH).
Photosynthesis is the process in which light energy is converted to chemical energy in the form of sugars. In a process driven by light energy, glucose molecules (or other sugars) are constructed from water and carbon dioxide, and oxygen is released as a byproduct. The glucose molecules provide organisms with two crucial resources: energy and ...
Etymology: The photosynthesis process finds its origin in 2 Greek words, firsts one being "phōs (φῶς)" meaning 'light' and the second one being "sunthesis (σύνθεσις)" meaning 'putting together'. The process of photosynthesis aids the conversion of light energy to chemical energy in varied forms of carbohydrate ...
The chloroplast is involved in both stages of photosynthesis. The light reactions take place in the thylakoid. There, water (H 2 O) is oxidized, and oxygen (O 2) is released. The electrons that ...
Photosynthesis is a vital process that converts light energy into chemical energy and produces organic molecules and oxygen for living things. In this article, you will learn how photosynthesis works in different ecosystems, how it affects the carbon cycle, and how it interacts with other biogeochemical cycles. Khan Academy is a free online platform that offers high-quality education for ...
Photosynthesis is powered by energy from sunlight. This energy is used to rearrange atoms in carbon dioxide and water to make oxygen and sugars. Carbon dioxide and water are inputs of photosynthesis. These inputs come from the environment. Oxygen and sugars are outputs of photosynthesis. The oxygen is released into the environment.
Photosynthesis is the process used by plants, algae and some bacteria to turn sunlight into energy. The process chemically converts carbon dioxide (CO2) and water into food (sugars) and oxygen ...
Some organisms can carry out photosynthesis, whereas others cannot. An autotroph is an organism that can produce its own food. The Greek roots of the word autotroph mean "self" (auto) "feeder" (troph). Plants are the best-known autotrophs, but others exist, including certain types of bacteria and algae (Figure \(\PageIndex{1}\)).
Stages of the Process. Photosynthesis occurs in two stages: 1) The Light-dependent Reaction. Takes place in the thylakoid membranes of chloroplasts only during the day in the presence of sunlight. High-energy phosphate molecules adenosine triphosphate ( ATP) and the reducing agent NADPH are produced with the help of electron transport chain.
The word "photosynthesis" is derived from the Greek words phōs (pronounced: "fos") and σύνθεσις (pronounced: "synthesis")Phōs means "light" and σύνθεσις means, "combining together."This means "combining together with the help of light." Photosynthesis also applies to other organisms besides green plants. These include several prokaryotes such as ...
The whole process of photosynthesis is a transfer of energy from the Sun to a plant. In each sugar molecule created, there is a little bit of the energy from the Sun, which the plant can either use or store for later. Imagine a pea plant. If that pea plant is forming new pods, it requires a large amount of sugar energy to grow larger.
5.1: Overview of Photosynthesis. All living organisms on earth consist of one or more cells. Each cell runs on the chemical energy found mainly in carbohydrate molecules (food), and the majority of these molecules are produced by one process: photosynthesis. Through photosynthesis, certain organisms convert solar energy (sunlight) into chemical ...
photosynthesis: the process by which plants and other photoautotrophs generate carbohydrates and oxygen from carbon dioxide, water, and light energy in chloroplasts. photoautotroph: an organism that can synthesize its own food by using light as a source of energy. chemoautotroph: a simple organism, such as a protozoan, that derives its energy ...
PHOTOSYNTHESIS definition: 1. the process by which a plant uses carbon dioxide from the air, water from the ground, and the…. Learn more.
Photosynthesis is really important for the plant because it provides the plant with food: some of the glucose is used immediately, to give the plant energy in the process of respiration. some of ...
Photosynthesis is the process of converting light into energy, conducted by plants and other organisms. This expedition explains how it works. This story was created for the Google Expeditions project by Vida Systems, now available on Google Arts & Culture. Photosynthesis by Vida Systems. Flora, which is what we call the kingdom of plant life ...
Unlike photosynthesis, aerobic respiration is an exergonic process (negative ΔG°) with the energy released being used by the organism to power biosynthetic processes that allow growth and renewal, mechanical work (such as muscle contraction or flagella rotation) and facilitating changes in chemical concentrations within the cell (e.g. accumulation of nutrients and expulsion of waste).
Note that the above global equation of photosynthesis emphasizes that the oxygen molecules released into the atmosphere originate from water oxidation, not from carbon dioxide, as established using 18 O-labelled water (Ruben et al., 1941).. This process starts in the thylakoid membrane (TM) with two light reactions taking place simultaneously at photosystem (PS) II and PSI reaction centres ...
In photosynthesis, the sun's energy is converted to chemical energy by photosynthetic organisms. However, the various wavelengths in sunlight are not all used equally in photosynthesis. Instead, photosynthetic organisms contain light-absorbing molecules called pigments that absorb only specific wavelengths of visible light, while reflecting ...
Photosynthesis Visuals. These photosynthesis modeling activities will help students visualize and better understand what a plant needs to undergo photosynthesis and what it produces as a result. The 3D and 2D representations will also help them absorb some of the vocabulary associated with photosynthesis. 3D Photosynthesis: Tree Leaf Model