- Our Program Divisions
- Our Three Academies
- Government Affairs
- Statement on Diversity and Inclusion
- Our Study Process
- Conflict of Interest Policies and Procedures
- Project Comments and Information
- Read Our Expert Reports and Published Proceedings
- Explore PNAS, the Flagship Scientific Journal of NAS
- Access Transportation Research Board Publications
- Coronavirus Disease 2019 (COVID-19)
- Diversity, Equity, and Inclusion
- Economic Recovery
- Fellowships and Grants
- Publications by Division
- Division of Behavioral and Social Sciences and Education
- Division on Earth and Life Studies
- Division on Engineering and Physical Sciences
- Gulf Research Program
- Health and Medicine Division
- Policy and Global Affairs Division
- Transportation Research Board
- National Academy of Sciences
- National Academy of Engineering
- National Academy of Medicine
- Publications by Topic
- Agriculture
- Behavioral and Social Sciences
- Biography and Autobiography
- Biology and Life Sciences
- Computers and Information Technology
- Conflict and Security Issues
- Earth Sciences
- Energy and Energy Conservation
- Engineering and Technology
- Environment and Environmental Studies
- Food and Nutrition
- Health and Medicine
- Industry and Labor
- Math, Chemistry, and Physics
- Policy for Science and Technology
- Space and Aeronautics
- Surveys and Statistics
- Transportation and Infrastructure
- Searchable Collections
- New Releases
VIEW LARGER COVER

Evidence Review of the Adverse Effects of COVID-19 Vaccination and Intramuscular Vaccine Administration
Vaccines are a public health success story, as they have prevented or lessened the effects of many infectious diseases. To address concerns around potential vaccine injuries, the Health Resources and Services Administration (HRSA) administers the Vaccine Injury Compensation Program (VICP) and the Countermeasures Injury Compensation Program (CICP), which provide compensation to those who assert that they were injured by routine vaccines or medical countermeasures, respectively. The National Academies of Sciences, Engineering, and Medicine have contributed to the scientific basis for VICP compensation decisions for decades.
HRSA asked the National Academies to convene an expert committee to review the epidemiological, clinical, and biological evidence about the relationship between COVID-19 vaccines and specific adverse events, as well as intramuscular administration of vaccines and shoulder injuries. This report outlines the committee findings and conclusions.
RESOURCES AT A GLANCE
- Press Release
- Digital Resource: Evidence Review of the Adverse Effects of COVID-19 Vaccination
- Digital Resource: Evidence Review of Shoulder Injuries from Intramuscular Administration of Vaccines
- Health and Medicine — Health Sciences
- Health and Medicine — Public Health and Prevention
- Health and Medicine — Policy, Reviews and Evaluations
Suggested Citation
National Academies of Sciences, Engineering, and Medicine. 2024. Evidence Review of the Adverse Effects of COVID-19 Vaccination and Intramuscular Vaccine Administration . Washington, DC: The National Academies Press. https://doi.org/10.17226/27746. Import this citation to: Bibtex EndNote Reference Manager
Publication Info
- Prepublication: 978-0-309-71829-5
- Paperback (forthcoming): 978-0-309-71832-5
What is skim?
The Chapter Skim search tool presents what we've algorithmically identified as the most significant single chunk of text within every page in the chapter. You may select key terms to highlight them within pages of each chapter.
Copyright Information
The National Academies Press (NAP) has partnered with Copyright Clearance Center's Marketplace service to offer you a variety of options for reusing NAP content. Through Marketplace, you may request permission to reprint NAP content in another publication, course pack, secure website, or other media. Marketplace allows you to instantly obtain permission, pay related fees, and print a license directly from the NAP website. The complete terms and conditions of your reuse license can be found in the license agreement that will be made available to you during the online order process. To request permission through Marketplace you are required to create an account by filling out a simple online form. The following list describes license reuses offered by the NAP through Marketplace:
- Republish text, tables, figures, or images in print
- Post on a secure Intranet/Extranet website
- Use in a PowerPoint Presentation
- Distribute via CD-ROM
Click here to obtain permission for the above reuses. If you have questions or comments concerning the Marketplace service, please contact:
Marketplace Support International +1.978.646.2600 US Toll Free +1.855.239.3415 E-mail: [email protected] marketplace.copyright.com
To request permission to distribute a PDF, please contact our Customer Service Department at [email protected] .
What is a prepublication?
An uncorrected copy, or prepublication, is an uncorrected proof of the book. We publish prepublications to facilitate timely access to the committee's findings.
You can purchase the uncorrected copy now, or pre-order the final version of the book. Prepublication sales are final. Due to the nature of this type of publication, it is not returnable.
What happens when I pre-order?
The final version of this book has not been published yet. You can pre-order a copy of the book and we will send it to you when it becomes available. We will not charge you for the book until it ships. Pricing for a pre-ordered book is estimated and subject to change. All backorders will be released at the final established price. As a courtesy, if the price increases by more than $3.00 we will notify you. If the price decreases, we will simply charge the lower price. Applicable discounts will be extended.
Downloading and Using eBooks from NAP
What is an ebook.
An ebook is one of two file formats that are intended to be used with e-reader devices and apps such as Amazon Kindle or Apple iBooks.
Why is an eBook better than a PDF?
A PDF is a digital representation of the print book, so while it can be loaded into most e-reader programs, it doesn't allow for resizable text or advanced, interactive functionality. The eBook is optimized for e-reader devices and apps, which means that it offers a much better digital reading experience than a PDF, including resizable text and interactive features (when available).
Where do I get eBook files?
eBook files are now available for a large number of reports on the NAP.edu website. If an eBook is available, you'll see the option to purchase it on the book page.
View more FAQ's about Ebooks
Types of Publications
Consensus Study Report: Consensus Study Reports published by the National Academies of Sciences, Engineering, and Medicine document the evidence-based consensus on the study’s statement of task by an authoring committee of experts. Reports typically include findings, conclusions, and recommendations based on information gathered by the committee and the committee’s deliberations. Each report has been subjected to a rigorous and independent peer-review process and it represents the position of the National Academies on the statement of task.

- < Previous
Home > COMMUNITIES > STUDENTWORK > HONORSTHESIS > 201
Honor Scholar Theses
Attitudes towards covid-19 vaccination: literature review and attitudes of individuals who delayed vaccination.
Sydney Hornberger '22 , DePauw University
Date of Award
Document type, first advisor.
Dr. Ted Bitner
Second Advisor
Dr. Alicia Suarez
Third Advisor
Dr. Jeffrey Jones
This thesis examines attitudes towards and ethics of receiving one of the fastest vaccines ever developed— the COVID-19 vaccine. The Food and Drug Administrations (FDA) in the U.S. has granted either Emergency Use Authorization or full approval to three vaccines: the Pfizer-BioNTech, Johnson & Johnson, and Moderna-NIAID vaccines. However, although the FDA approved and the Center for Disease Control and Prevention (CDC) recommends getting the vaccines, that does not necessarily mean people have an ethical responsibility or a positive attitude towards getting vaccinated against COVID-19; this current paper explores both of these ideas as related to COVID-19 vaccination. First, it surveys sources highlighting the utility of vaccines to control infectious diseases and pandemics. Next, it questions whether getting vaccinated against any disease, and specifically COVID-19, is the ethical action to take. Then, there is a literature review of research into attitudes towards the COVID-19 vaccine, determining the most prevalent attitudes across all people and within specific demographics such as women, people belonging to certain political and religious groups, racial and ethnic minorities, and children. Finally, the results of a study conducted at DePauw University to investigate attitudes, attitude changes, and motivations of recently vaccinated individuals are reported in order to elucidate certain factors that may be useful to understand vaccine decision making.
Recommended Citation
Hornberger, Sydney '22, "Attitudes Towards COVID-19 Vaccination: Literature Review and Attitudes of Individuals Who Delayed Vaccination" (2022). Honor Scholar Theses . 201, Scholarly and Creative Work from DePauw University. https://scholarship.depauw.edu/studentresearch/201
Since June 07, 2022
Included in
Public Health Commons
Advanced Search
- Notify me via email or RSS
- Collections
- Disciplines
Author Corner
Library links.
- DePauw Libraries
Home | About | FAQ | My Account | Accessibility Statement
Privacy Copyright
- Research article
- Open access
- Published: 17 August 2020
A systematic review of studies that measure parental vaccine attitudes and beliefs in childhood vaccination
- Amalie Dyda ORCID: orcid.org/0000-0003-2806-4834 1 , 2 ,
- Catherine King 3 , 4 ,
- Aditi Dey 3 , 5 ,
- Julie Leask 6 , 3 &
- Adam G. Dunn 7 , 1
BMC Public Health volume 20 , Article number: 1253 ( 2020 ) Cite this article
17k Accesses
47 Citations
12 Altmetric
Metrics details
Acceptance of vaccines is an important predictor of vaccine uptake. This has public health implications as those who are not vaccinated are at a higher risk of infection from vaccine preventable diseases. We aimed to examine how parental attitudes and beliefs towards childhood vaccination were measured in questionnaires through a systematic review of the literature .
We systematically reviewed the literature to identify primary research studies using tools to measure vaccine attitudes and beliefs, published between January 2012 and May 2018. Studies were included if they involved a quantitative survey of the attitudes and beliefs of parents about vaccinations recommended for children. We undertook a synthesis of the results with a focus on evaluating the tools used to measure hesitancy.
A total of 116 studies met the inclusion criteria, 99 used a cross sectional study design, 5 used a case control study design, 4 used a pre-post study design and 8 used mixed methods study designs. Sample sizes of included studies ranged from 49 to 12,259. The most commonly used tool was the Parent Attitudes about Childhood Vaccines (PACV) Survey ( n = 7). The most common theoretical framework used was the Health Belief Model ( n = 25). Questions eliciting vaccination attitudes and beliefs varied widely.
Conclusions
There was heterogeneity in the types of questionnaires used in studies investigating attitudes and beliefs about vaccination in parents. Methods to measure parental attitudes and beliefs about vaccination could be improved with validated and standardised yet flexible instruments. The use of a standard set of questions should be encouraged in this area of study.
Peer Review reports
Childhood vaccination rates vary widely by country and region, and the reasons for these variations are likely to be context-specific [ 1 , 2 , 3 ]. While access to vaccination is a perennial challenge, acceptance also remains an issue of importance to uptake which is affected by an individual’s feelings, attitudes and beliefs about vaccination [ 4 ]. There is a spectrum of attitudes towards vaccination, including those who are pro-vaccination and accept all vaccines, those who have many concerns but may fully or partially vaccinate, and those who refuse all vaccines [ 5 ]. Those who have questions and concerns have been shown to have lower levels of vaccination uptake [ 6 ] which may have a substantial impact on vaccination coverage and increases the risk of outbreaks [ 7 ]. Not only are unvaccinated individuals at higher risk of infection and adverse health outcomes, but under-vaccinated populations are at higher risk of more severe outbreaks [ 8 , 9 , 10 ].
A range of questionnaires have been developed and tested for measuring vaccination attitudes and beliefs [ 11 ]. The largest recent questionnaires in the area include The Vaccine Confidence Project [ 12 ] which collected 65,819 responses across 67 countries [ 13 ], and the Wellcome Global Monitor 2018 [ 14 ], which collected more than 140,000 responses from 140 countries. Both were based on the same set of questions, which included items about vaccine importance, effectiveness, safety, and religious compatibility.
Studies using questionnaires to understand vaccine attitudes and beliefs often modify existing items to incorporate the local context of a specific country or region. There is high variability with respect to use of behavioural theories to inform constructs and items and the comprehensiveness of validation, such as whether the items predict vaccination uptake. Moreover, high variability in how constructs such as vaccine confidence are measured between different questionnaires makes it difficult to assess how attitudes and beliefs vary globally.
Our aim was to examine how parental attitudes and beliefs towards childhood vaccination were measured in questionnaires through a systematic review of the literature.
Inclusion criteria
Studies were included if they were quantitative primary studies investigating parental vaccine attitudes and/or beliefs, regardless of whether they considered one or a combination of vaccines or vaccine-preventable diseases. For the purpose of this review studies on vaccine hesitancy were included, with vaccine hesitancy defined as “a motivational state of being conflicted about, or opposed to, getting vaccinated” [ 15 ]. Vaccine hesitancy can result in “a delay in acceptance or refusal of vaccines despite availability of vaccination services” [ 16 ]. Studies published after January 2012 were included. Studies were excluded if they investigated vaccination barriers not associated with attitudes or beliefs (e.g. measuring access other than as a factor affecting convenience), adult and adolescent vaccination, or if they were not reported in English. We applied no geographical constraints.
Search strategy
This review was developed in line with the PRISMA guidelines [ 17 ]. Key bibliographic databases were searched to identify relevant articles. The 19 databases searched included: OVID Medline, PsycINFO and Database of Systematic Reviews (see Additional File 1 for the full list of databases searched) Search terms included thesaurus terms (where available) such as ‘Immunization’, ‘Immunization programs’, ‘Vaccines’, ‘Decision Making’, ‘Decision Theory’, ‘Attitude to Health’, ‘Health Behavior’, ‘Risk Assessment’, ‘Trust’, ‘Uncertainty’, ‘Vaccination Refusal’, ‘Anti-Vaccination movement’, ‘Child, Preschool’ and ‘Infant’ These were used with relevant associated text terms. Truncation was utilised to ensure all variant spelling endings of text words were retrieved. The searches were limited to items published from 2012 and ‘Humans’. (see Additional File 1 for the full search strategy). The last search was conducted on 19 May 2018. Articles reviewed for inclusion were limited from January 2012 to May 2018 to avoid duplicating the findings of a 2014 systematic review that reviewed the global literature on vaccine hesitancy [ 5 ].
All titles and abstracts or executive summaries found through the search strategy were screened independently by two authors (Adam Dunn and Amalie Dyda) to determine if they were relevant to the review. The full text of those articles that appeared to meet the inclusion criteria were retrieved and reviewed for relevance independently by the same two authors. The reference lists of all included items were searched to identify any additional items for inclusion.
Data extraction and synthesis
Data were extracted by one author (Amalie Dyda) and confirmed by a second author (Adam Dunn). A standard data extraction form developed by the authors was used. For each study, study design information extracted from the articles included the method of recruitment and the location and type of participants, the number of participants recruited (and completing the study, where appropriate), the vaccine or set of vaccines of relevance to the study, and details of the questions used to measure attitudes and belief about vaccination including any description of behavioural theories used to inform the questionnaire design, and whether the questions were taken directly or adapted from existing instruments. We defined validated questionnaires as those that followed “the process of establishing that a survey item or measure serves the intended purpose. This process can include establishing whether it measures the intended construct using qualitative means (advice from experts, cognitive testing with lay people) and quantitative means (convergent, discriminant, predictive validity)” [ 18 ]. Data extracted from each study were tabulated and grouped by study type and study characteristics including sample size, recruitment method, and location.
The initial search strategy returned 41,570 titles and abstracts, of which 23,201 were removed as duplicates. Title and abstract screening identified 673 full text items for review. Of these, 116 met the inclusion criteria (Fig. 1 ). A review of the reference lists of included articles did not identify any additional items for inclusion.
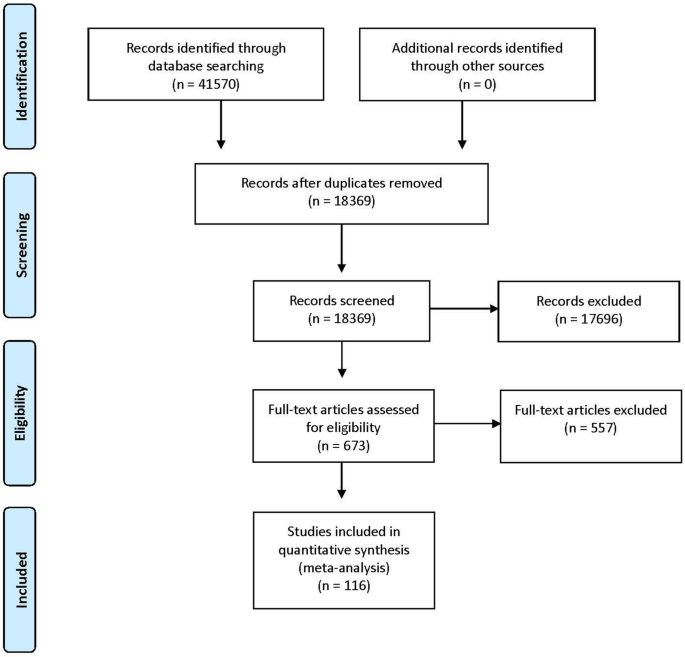
Summary of the search strategy results and set of included studies
Summary of included studies
Of the included studies, 99 (85.3%) used a cross sectional study design (Additional File 2 ). Sample sizes across all 116 included studies ranged from 49 to 12,259 participants, with a median of 455 participants. Parental attitudes and beliefs about childhood vaccines in general were studied in 57 (49.1%) studies, and attitudes and beliefs about influenza vaccination (including pandemic H1N1 influenza) in 35 (30.2%). The other 24 (20.7%) studies asked participants about attitudes and beliefs for other specific vaccines, such as polio and rotavirus vaccines.
Thirty-four countries were represented in the included studies (Fig. 2 ). The most common country in which studies were conducted was the United States ( n = 36), followed by Canada ( n = 9) and the United Kingdom ( n = 8). When aggregated by the number of participants, the United States included the largest number (40,155 participants), followed by Canada (7200 participants), and the United Kingdom (3273 participants).
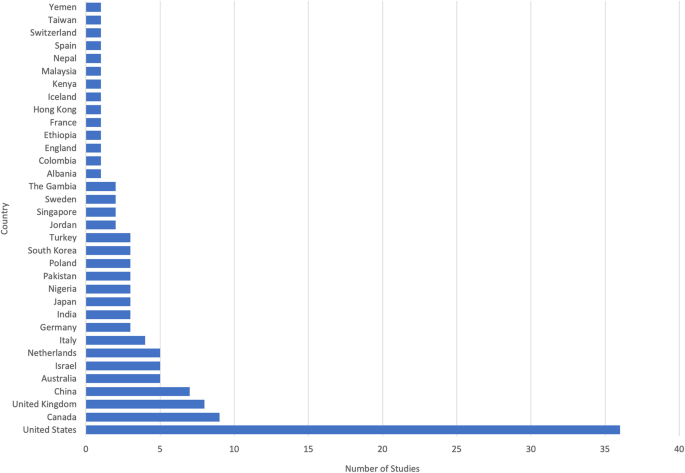
Among the set of 116 included studies, 34 countries were represented
Questionnaires and survey instruments
One hundred and fourteen studies used a survey design, with the two remaining studies using interviews. The questions asked of participants varied substantially across the set of included studies. There was heterogeneity both in terms of the specific questions asked of participants as well as the provenance of those questions in theory or from standardised questionnaire sets. Sixty three studies reported at least one aspect of validation.
The most commonly used standard questionnaire was the Parent Attitudes about Childhood Vaccines (PACV) Survey Tool ( n = 7), used in 4 studies with its full format with 15 questions [ 19 , 20 , 21 , 22 ]. In some studies, the PACV questions were adapted to match the local context or study population, such as in Malaysia [ 21 ] and for expectant parents in the United States [ 19 ]. In 3 studies, a subset of the PACV questions were used [ 23 , 24 , 25 ]. Other questionnaires used included 6 studies based on national immunisation surveys or health department questionnaires [ 26 , 27 , 28 , 29 , 30 , 31 ], 1 study based on the Parental Attitudes toward MMR Vaccine and Trust in Medical Authority questionnaire [ 32 ], and 1 that used the Vaccine Safety, Attitudes, Training and Communication measures [ 33 ].
A total of 62 (53.4%) included studies developed questionnaires using previous literature or previously developed questionnaires, 7 developed questionnaires with experts in the field, 1 used a self-developed scale, and 6 conducted a qualitative data to elicit appropriate questions. The remaining 40 studies did not report having used previous examples as the basis for the designs of their questionnaires.
A variety of theoretical frameworks were used to inform the design of the questionnaires used in the studies. The most common was the Health Belief Model (HBM), which was explicitly stated as having been used to inform the questions in 25 (19.0%) studies [ 30 , 32 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 ], followed by the Theory of Planned Behaviour, which was used in 5 (4.3%) studies [ 58 , 59 , 60 , 61 , 62 , 63 ]. Other studies that were adapted from existing questionnaires may have implicitly been based on these or other theoretical frameworks as a consequence of having adapted from other questionnaires but did not explicitly claim the theoretical framework as a basis for their questions.
Questions about intention to vaccinate
Of the 116 included studies, 38 (32.8%) included questions in which parents were directly asked about their vaccination intentions for one or more antigens. The specific questions that were asked varied across the set of studies. Examples included, “If you had another infant today, would you want him or her to get all the recommended shots?, “I would get a flu vaccine for my child under 5, every year, if it was free?”, and “If your child were offered it at some point in the future, would you vaccinate them against swine flu?”. This variation precluded a synthesis of the results, and the proportion of participants responding in the affirmative varied substantially across the set of studies.
Of the 38 studies which asked about vaccination intentions for one or more antigens, 16 (13.8%) of these specifically asked about whether they would have children vaccinated for all childhood vaccines. The percentages in these studies ranged from 75% in a study involving 200 parents in the United States [ 64 ] to 98% in a study involving 54 parents in Canada [ 35 ]. For the 9 (7.8%) studies that asked about intentions in relation to influenza vaccination, the percentages ranged from 29% in a study involving 236 parents in Canada [ 65 ] to 92% in a before and after study at a clinic involving 5284 and 5755 different groups of parents in rural Kenya [ 66 ].
A substantial number of studies quantitatively examine the childhood vaccination attitudes and beliefs of parents across a broad range of countries. A large number of studies did not report using a validated questionnaire. The countries in which the highest number of studies were conducted were the United States, Canada and the United Kingdom, with most other countries having either none or only a small number of studies. There were significant differences in the way in which questionnaires were developed and the questions asked in each of the studies, making synthesis or comparison of findings a challenge. The use of standardised questionnaires globally would allow findings across countries to be compared and help track longitudinal trends.
The geographical distribution of primary studies included in the review was generally consistent with a previous review on attitudes and beliefs regarding vaccination [ 5 ], in which most included studies were conducted in North America and Europe. Among the subset of studies that used standardised questionnaires, there was no clear difference in rates of vaccine hesitancy between countries, nor any clear pattern in the attitudes and beliefs that exhibited the strongest associations with intention. Given that only a relatively small subset used standardised questionnaires, this result is a reflection of the small number of studies rather than evidence of consistency in what matters most to parents exhibiting vaccine hesitancy.
There was little consistency in the provenance of the questions used to measure attitudes and beliefs across studies. A number of studies did not report how the questionnaire or survey instrument was developed, making comparison of these studies difficult. The majority of studies reported construct and item development methods such as basing the questionnaire on previous literature, expert opinion or the use of previously developed surveys.
The use of qualitative evidence is best practice for forming constructs [ 67 ] and the use of a previously validated questionnaire is the most appropriate methodology as this ensures that items have content, construct and predictive validity. Previously developed questionnaires which are not validated may not accurately capture information, which is then repeated if these questionnaires are reused [ 18 ]. However, as there is no agreed upon gold standard survey instrument, a wide range of sources were used for development, resulting in heterogeneity of questionnaires. The most commonly used standard questionnaire was the PACV Survey Tool, which has been validated in two different settings and been shown to identify vaccine hesitant parents. The questionnaire focuses on the domains of ‘Safety and efficacy’, ‘General attitudes’ and ‘Behaviour’ [ 68 , 69 ]. The use of this questionnaire for studies investigating vaccine hesitancy should be encouraged to better allow for comparison across studies.
For theoretical frameworks, we found that the HBM was most commonly used to support the development of questionnaires, which was consistent with previous reviews [ 5 ]. The HBM posits that perceptions of susceptibility, severity, benefit and barriers, cues to action and self-efficacy predict behaviour. This and other models place emphasis on risk appraisals as important predictors of vaccination. Use of the HBM is complicated by the fact that all related perceptions could apply to vaccination uptake as much as disease outcomes. Since these models look at individual psychological factors by design, they are weaker at measuring other factors like false contraindications, social influence, or access to services or vaccines, which are more likely to be effective in increasing uptake, if they are addressed [ 15 ]. Further, many models fail to measure trust, yet trust in vaccination arises as a relevant phenomenon in both qualitative accounts of under-vaccination and the influence of vaccine safety scares [ 15 ]. Trust is often “ill-defined and a loosely measured concept” [ 70 ]. Recent work on the moral foundations of behaviour suggests that measuring constructs such as contamination and liberty are also relevant [ 71 , 72 ]. Further work is needed to incorporate moral foundations, other feelings and attitudes and beliefs and trust into a single model of vaccination behaviour and test its robustness.
Future studies in this area may benefit from considering standardised questions on vaccine attitudes and beliefs and other barriers or facilitators [ 11 ]. Large international surveys based on a standardised set of questions may be useful for providing international comparisons with context-specific additional questions. To consider the local context, qualitative investigations could supplement the broad based quantitative knowledge from surveys. Both forms of data collection are useful but are also resource intensive and relatively slow to report.
Current outbreaks of measles in the US highlight the importance of monitoring and measuring attitudes and beliefs about vaccinations. From 1st January to 18th July 2019 there were a total of 1148 cases of measles identified in the US which is the largest number of infections reported since 1992. Outbreaks are occurring across a number of states, with an outbreak in Rockland County, reporting the majority (78.4%) of cases have not been vaccinated [ 73 ].
The development of the internet has increased the speed with which information and misinformation can spread in the community. This may outpace our ability to measure and report on attitudes and beliefs using current survey methods which are time and resource intensive. Due to the time lag involved, using these methods may limit the ability to support the rapid design of evidence-informed and localised interventions for debunking or mitigating the impact of misinformation.
There were several limitations to the review approach and conduct. The first limitation was that the geographical distribution of the studies included in the review may be biased by the exclusion of studies not written in English. In addition, parental beliefs and attitudes towards influenza vaccination often differ from routine childhood vaccinations [ 74 ]. This childhood vaccine was included as some countries recommend annual influenza vaccination, but this is unlikely to affect the findings regarding tools used to monitor attitudes and beliefs about vaccination.
Despite the number of studies investigating parental attitudes and beliefs about childhood vaccination which were conducted in at least 36 countries, there was heterogeneity in survey designs. Methods to measure parental attitudes and beliefs about vaccination could be improved with validated and standardised yet flexible instruments, supplemented with qualitative investigations. The use of a standard set of validated questions should be encouraged in this area of study to identify, track, and monitor longitudinal trends using quality data.
Availability of data and materials
Not applicable.
Abbreviations
Health belief model
Parent attitudes about childhood vaccines
Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kang Y. Vaccination coverage among children aged 19-35 months - United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(40):1123–8.
Article PubMed PubMed Central Google Scholar
International Institute for Population Sciences (IIPS) and ICF. National family health survey (nfhs-4), 2015–16: India. Mumbai: IIPS; 2017.
Google Scholar
National Centre for Immunisation Research and Surveillance. Coverage data and reports 2019 [Available from: http://www.ncirs.org.au/health-professionals/coverage-data-and-reports .
Larson HJ. The biggest pandemic risk? Viral misinformation. Nature. 2018;562:309.
Article CAS PubMed Google Scholar
Larson HJ, Jarrett C, Eckersberger E, Smith DM, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007-2012. Vaccine. 2014;32(19):2150–9.
Article PubMed Google Scholar
Damnjanović K, Graeber J, Ilić S, Lam WY, Lep Ž, Morales S, et al. Parental decision-making on childhood vaccination. Front Psychol. 2018;9:735.
Smith LE, Amlot R, Weinman J, Yiend J, Rubin GJ. A systematic review of factors affecting vaccine uptake in young children. Vaccine. 2017;35(45):6059–69.
Phadke VK, Bednarczyk RA, Salmon DA, Omer SB. Association between vaccine refusal and vaccine-preventable diseases in the United States: a review of measles and pertussis. JAMA. 2016;315(11):1149–58.
Article CAS PubMed PubMed Central Google Scholar
Omer SB, Enger KS, Moulton LH, Halsey NA, Stokley S, Salmon DA. Geographic clustering of nonmedical exemptions to school immunization requirements and associations with geographic clustering of pertussis. Am J Epidemiol. 2008;168(12):1389–96.
Salathe M, Bonhoeffer S. The effect of opinion clustering on disease outbreaks. J R Soc Interface. 2008;5(29):1505–8.
Betsch C, Schmid P, Heinemeier D, Korn L, Holtmann C, Böhm R. Beyond confidence: Development of a measure assessing the 5c psychological antecedents of vaccination. Plos One. 2018;13(12):e0208601-e.
Larson HJ. The state of vaccine confidence. Lancet. 2018;392(10161):2244–6.
Larson HJ, de Figueiredo A, Xiahong Z, Schulz WS, Verger P, Johnston IG, et al. The state of vaccine confidence 2016: global insights through a 67-country survey. EBioMedicine. 2016;12:295–301.
Gallup (2019) wellcome global monitor– first wave findings. 2019.
Brewer NT, Chapman GB, Rothman AJ, Leask J, Kempe A. Increasing vaccination: putting psychological science into action. Psychol Sci Public Interest. 2017;18(3):149–207.
World Health Organization. Report of the SAGE working group on vaccine hesitancy. Geneva: World Health Organization; 2014.
Prisma- preferred reporting items for systematic reviews and meta-analyses 2013 [Available from: http://www.prisma-statement.org/ .
Boateng GO, Neilands TB, Frongillo EA, Melgar-Quiñonez HR, Young SL. Best practices for developing and validating scales for health, social, and behavioral research: a primer. Front Public Health. 2018;6:149.
Cunningham RM, Minard CG, Guffey D, Swaim LS, Opel DJ, Boom JA. Prevalence of vaccine hesitancy among expectant mothers in Houston, Texas. Acad Pediatr. 2018;18(2):154–60.
Henrikson NB, Anderson ML, Opel DJ, Dunn J, Marcuse EK, Grossman DC. Longitudinal trends in vaccine hesitancy in a cohort of mothers surveyed in washington state, 2013–2015. Public Health Rep. 2017;132(4):451–4 Available from: http://cochranelibrary-wiley.com/o/cochrane/clcentral/articles/885/CN-01400885/frame.html .
Mohd Azizi FS, Kew Y, Moy FM. Vaccine hesitancy among parents in a multi-ethnic country, Malaysia. Vaccine. 2017;35(22):2955–61.
Orr C, Beck AF. Measuring vaccine hesitancy in a minority community. Clin Pediatr. 2017;56(8):784–8.
Article Google Scholar
Oladejo O, Allen K, Amin A, Frew PM, Bednarczyk RA, Omer SB. Comparative analysis of the parent attitudes about childhood vaccines (pacv) short scale and the five categories of vaccine acceptance identified by gust et al. Vaccine. 2016;34(41):4964–8.
Schoeppe J, Cheadle A, Melton M, Faubion T, Miller C, Matthys J, et al. The immunity community: a community engagement strategy for reducing vaccine hesitancy. Health Promot Pract. 2017;18(5):654–61.
Cataldi JR, Dempsey AF, O'Leary ST. Measles, the media, and mmr: impact of the 2014-15 measles outbreak. Vaccine. 2016;34(50):6375–80.
LaVail KH, Kennedy AM. The role of attitudes about vaccine safety, efficacy, and value in explaining parents' reported vaccination behavior. Health Educ Behav. 2013;40(5):544–51.
Luthy KE, Beckstrand RL, Meyers CJH. Common perceptions of parents requesting personal exemption from vaccination. J Sch Nurs. 2013;29(2):95–103.
Schönberger K, Ludwig MS, Wildner M, Kalies H. Timely mmr vaccination in infancy: influence of attitudes and medical advice on the willingness to vaccinate. Klin Padiatr. 2012;224(7):437–42.
Shrestha S, Shrestha M, Wagle RR, Bhandari G. Predictors of incompletion of immunization among children residing in the slums of Kathmandu valley, Nepal: a case-control study. BMC Public Health. 2016;16:970.
Smith PJ, Marcuse EK, Seward JF, Zhao Z, Orenstein WA. Children and adolescents unvaccinated against measles: geographic clustering, parents' beliefs, and missed opportunities. Public Health Rep. 2015;130(5):485–504.
Walsh S, Thomas DR, Mason BW, Evans MR. The impact of the media on the decision of parents in south wales to accept measles-mumps-rubella (mmr) immunization. Epidemiol Infect. 2015;143(3):550–60.
Leonard W. Parental Confidence in U.S. Government and Medical Authorities, Measles (Rubeloa) Knowledge, and MMR Vaccine Compliance (2015). Walden Dissertations and Doctoral Studies; 1718.
Umeh GC, Nomhwange TI, Shamang AF, Zakari F, Musa AI, Dogo PM, et al. Attitude and subjective wellbeing of non-compliant mothers to childhood oral polio vaccine supplemental immunization in northern Nigeria. BMC Public Health. 2018;18(1):231.
Armitage ET, Camara J, Bah S, Forster AS, Clarke E, Kampmann B, et al. Acceptability of intranasal live attenuated influenza vaccine; influenza knowledge and vaccine intent in the Gambia. Vaccine. 2018;36(13):1772–80.
Atkinson KM, Ducharme R, Westeinde J, Wilson SE, Deeks SL, Pascali D, et al. Vaccination attitudes and mobile readiness: a survey of expectant and new mothers. Hum Vaccin Immunotherapeutics. 2015;11(4):1039–45.
Ben Natan M, Kabha S, Yehia M, Hamza O. Factors that influence israeli muslim Arab parents' intention to vaccinate their children against influenza. J Pediatr Nurs-Nurs Care Children Fam. 2016;31(3):293–8.
Chen CH, Chiu PJ, Chih YC, Yeh GL. Determinants of influenza vaccination among young taiwanese children. Vaccine. 2015;33(16):1993–8.
Cheung S, Wang HL, Mascola L, El Amin AN, Pannaraj PS. Parental perceptions and predictors of consent for school-located influenza vaccination in urban elementary school children in the United States. Influenza Other Respir Viruses. 2015;9(5):255–62.
Chun Chau JP, Lo SHS, Chow Choi K, Kin Chau MH, Tong DWK, Kwong T, et al. Factors determining the uptake of influenza vaccination among children with chronic conditions. Pediatr Infect Dis J. 2017;36(7):e197-e202.
He L, Liao QY, Huang YQ, Feng S, Zhuang XM. Parents' perception and their decision on their children's vaccination against seasonal influenza in Guangzhou. Chin Med J. 2015;128(3):327–41.
Hwang JH, Lim CH, Kim DH, Eun BW, Jo DS, Song YH, et al. A survey of parental perception and pattern of action in response to influenza-like illness in their children: including healthcare use and vaccination in Korea. J Korean Med Sci. 2017;32(2):204–11.
Janks M, Cooke S, Odedra A, Kang H, Bellman M, Jordan RE. Factors affecting acceptance and intention to receive pandemic influenza a h1n1 vaccine among primary school children: A cross-sectional study in Birmingham, UK. Influenza Res Treat. 2012;2012:182565.
Kempe A, Daley MF, Pyrzanowski J, Vogt TM, Campagna EJ, Dickinson LM, et al. School-located influenza vaccination with third-party billing: what do parents think? Acad Pediatr. 2014;14(3):241–8.
Lau JT, Mo PK, Cai YS, Tsui HY, Choi KC. Coverage and parental perceptions of influenza vaccination among parents of children aged 6 to 23 months in hong kong. BMC Public Health. 2013;13:1026.
Malosh R, Ohmit SE, Petrie JG, Thompson MG, Aiello AE, Monto AS. Factors associated with influenza vaccine receipt in community dwelling adults and their children. Vaccine. 2014;32(16):1841–7.
Mergler MJ, Omer SB, Pan WKY, Navar-Boggan AM, Orenstein W, Marcuse EK, et al. Association of vaccine-related attitudes and beliefs between parents and health care providers. Vaccine. 2013;31(41):4591–5.
Morin A, Lemaître T, Farrands A, Carrier N, Gagneur A. Maternal knowledge, attitudes and beliefs regarding gastroenteritis and rotavirus vaccine before implementing vaccination program: which key messages in light of a new immunization program? Vaccine. 2012;30(41):5921–7.
O'Leary ST, Barnard J, Lockhart S, Kolasa M, Shmueli D, Dickinson LM, et al. Urban and rural differences in parental attitudes about influenza vaccination and vaccine delivery models. J Rural Health. 2015;31(4):421–30.
Paek HJ, Shin KA, Park K. Determinants of caregivers' vaccination intention with respect to child age group: A cross-sectional survey in south korea. BMJ Open. 2015;5:e008342.
Saitoh A, Sato I, Shinozaki T, Kamiya H, Nagata S. Improved parental attitudes and beliefs through stepwise perinatal vaccination education. Hum Vaccin Immunother. 2017;13(11):2639–45.
Tsuchiya Y, Shida N, Machida K. Flu vaccination acceptance among children and awareness of mothers in japan. In: Spier R, editor. 7th Vaccine & ISV Annual Global Congress, vol. 8: Procedia in Vaccinology. 2014. p. 12–7.
Wagner AL, Boulton ML, Sun X, Mukherjee B, Huang Z, Harmsen IA, et al. Perceptions of measles, pneumonia, and meningitis vaccines among caregivers in shanghai, China, and the health belief model: a cross-sectional study. BMC Pediatr. 2017;17.
Wu CST, Kwong EWY, Wong HT, Lo SH, Wong ASW. Beliefs and knowledge about vaccination against ah1n1pdm09 infection and uptake factors among chinese parents. Int J Environ Res Public Health. 2014;11(2):1989–2002.
Tsuchiya Y, Shida N, Izumi S, Ogasawara M, Kakinuma W, Tsujiuchi T, et al. Factors associated with mothers not vaccinating their children against mumps in Japan. Public Health. 2016;137:95–105.
Schollin Ask L, Hjern A, Lindstrand A, Olen O, Sjögren E, Blennow M, et al. Receiving early information and trusting swedish child health Centre nurses increased parents’ willingness to vaccinate against rotavirus infections. Acta Paediatr. 2017;106(8):1309–16.
Scheuerman O, Zilber E, Davidovits M, Chodick G, Levy I. Nephrologists need to play a key role in improving annual influenza vaccination rates in children with kidney disease. Acta Paediatr. 2017;106(5):812–8.
Peleg N, Zevit N, Shamir R, Chodick G, Levy I. Seasonal influenza vaccination rates and reasons for non-vaccination in children with gastrointestinal disorders. Vaccine. 2015;33(1):182–6.
Dubé E, Bettinger JA, Halperin B, Bradet R, Lavoie F, Sauvageau C, et al. Determinants of parents' decision to vaccinate their children against rotavirus: results of a longitudinal study. Health Educ Res. 2012;27(6):1069–80.
Dube E, Gagnon D, Ouakki M, Bettinger JA, Witteman HO, MacDonald S, et al. Measuring vaccine acceptance among Canadian parents: a survey of the Canadian immunization research network. Vaccine. 2018;36(4):545–52.
Fadel CW, Colson ER, Corwin MJ, Rybin D, Heeren TC, Wang CL, et al. Maternal attitudes and other factors associated with infant vaccination status in the united states, 2011–2014. J Pediatr. 2017;185:136.
Harmsen IA, Lambooij MS, Ruiter RAC, Mollema L, Veldwijk J, van Weert Y, et al. Psychosocial determinants of parents' intention to vaccinate their newborn child against hepatitis b. Vaccine. 2012;30(32):4771–7.
MacDougall DM, Halperin BA, Langley JM, MacKinnon-Cameron D, Li L, Halperin SA, et al. Knowledge, attitudes, beliefs, and behaviors of parents and healthcare providers before and after implementation of a universal rotavirus vaccination program. Vaccine. 2016;34(5):687–95.
Thorpe EL, Zimmerman RK, Steinhart JD, Lewis KN, Michaels MG. Homeschooling parents' practices and beliefs about childhood immunizations. Vaccine. 2012;30(6):1149–53.
Weiner JL, Fisher AM, Nowak GJ, Basket MM, Gellin BG. Childhood immunizations first-time expectant mothers' knowledge, beliefs, intentions, and behaviors. Vaccine. 2015;33:D92–D8.
Dubé E, Gagnon D, Huot C, Paré R, Jacques S, Kossowski A, et al. Influenza immunization of chronically ill children in pediatric tertiary care hospitals. Hum Vaccin Immunotherapeutics. 2014;10(10):2935–41.
Oria PA, Arunga G, Lebo E, Wong JM, Emukule G, Muthoka P, et al. Assessing parents' knowledge and attitudes towards seasonal influenza vaccination of children before and after a seasonal influenza vaccination effectiveness study in low-income urban and rural Kenya, 2010-2011. BMC Public Health. 2013;13.
Opel DJ, Mangione-Smith R, Taylor JA, Korfiatis C, Wiese C, Catz S, et al. Development of a survey to identify vaccine-hesitant parents: the parent attitudes about childhood vaccines survey. Hum Vaccin. 2011;7(4):419–25.
Opel DJ, Taylor JA, Mangione-Smith R, Solomon C, Zhao C, Catz S, et al. Validity and reliability of a survey to identify vaccine-hesitant parents. Vaccine. 2011;29(38):6598–605.
Abd Halim H, Abdul-Razak S, Md Yasin M, Isa MR. Validation study of the parent attitudes about childhood vaccines (PACV) questionnaire: the Malay version. Hum Vaccin Immunother. 2020;16(5):1040–9.
Larson HJ, Clarke RM, Jarrett C, Eckersberger E, Levine Z, Schulz WS, et al. Measuring trust in vaccination: a systematic review. Hum Vaccin Immunotherapeutics. 2018;14(7):1599–609.
Amin AB, Bednarczyk RA, Ray CE, Melchiori KJ, Graham J, Huntsinger JR, et al. Association of moral values with vaccine hesitancy. Nat Hum Behav. 2017;1(12):873–80.
Luz PM, Brown HE, Struchiner CJ. Disgust as an emotional driver of vaccine attitudes and uptake? A mediation analysis. Epidemiol Infect. 2019;147:e182-e.
Centers for Disease Control and Prevention. Measles cases and outbreaks. 2019.
C.S. Mott Children’s Hospital. Inferiority complex? Parents rate flu lower than other vaccines. Mott Poll Rep. 2016;26(1).
Download references
Acknowledgements
This project was funded by the Australian National Health and Medical Research Council (NHMRC) Project Grant APP1128968. The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and affiliations.
Centre for Health Informatics, Australian Institute of Health Innovation, Macquarie University, Sydney, NSW, Australia
Amalie Dyda & Adam G. Dunn
Department of Health Systems and Populations, Faculty of Medicine, Health and Human Sciences, Macquarie University, Sydney, NSW, Australia
Amalie Dyda
National Centre for Immunisation Research & Surveillance, Sydney, NSW, Australia
Catherine King, Aditi Dey & Julie Leask
The University of Sydney, Children’s Hospital at Westmead Clinical School, Faculty of Medicine and Health, Sydney, NSW, Australia
Catherine King
The University of Sydney, School of Medicine, Faculty of Medicine and Health, Sydney, NSW, Australia
The University of Sydney, Susan Wakil School of Nursing and Midwifery, Sydney, NSW, Australia
Julie Leask
The University of Sydney, Discipline of Biomedical Informatics and Digital Health, School of Medical Sciences, Faculty of Medicine and Health, Sydney, NSW, Australia
Adam G. Dunn
You can also search for this author in PubMed Google Scholar
Contributions
A.Dyda led the design and coordination of the review. CK designed and conducted the literature searches and was a contributor in writing the manuscript. A. Dey assisted in the design of the review and provided critical intellectual content throughout. JL was a major contributor to the design of the review and provided critical intellectual content throughout. A. Dunn was also was a major contributor to the design of the review, and assisted with removing duplicates and screening of titles, abstracts and full review of papers for inclusion. All authors contributed to the revision of the manuscript and provided intellectual content. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Amalie Dyda .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1..
Search strategy. Detailed description of search strategy used for review.
Additional file 2: Table 1.
Summary of included studies. Summary table of each included study with details about study characteristics.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Dyda, A., King, C., Dey, A. et al. A systematic review of studies that measure parental vaccine attitudes and beliefs in childhood vaccination. BMC Public Health 20 , 1253 (2020). https://doi.org/10.1186/s12889-020-09327-8
Download citation
Received : 16 June 2020
Accepted : 02 August 2020
Published : 17 August 2020
DOI : https://doi.org/10.1186/s12889-020-09327-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Immunization/immunisation
- Vaccination
- Vaccines, questionnaire
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Thesis Helpers
Find the best tips and advice to improve your writing. Or, have a top expert write your paper.
170 Vaccination Research Paper Topics For Stellar Students

Research papers are a monumental highlight in your academic journey. They are a critical milestone in your studies that must be tackled with the utmost care and stellar diligence. Vaccination topics are susceptible as you have to show complete mastery of all details.
If you are pursuing a medicine course, then vaccination research topics might be an excellent area of interest. A good research paper starts with a great topic, and we are here to help you nail that. We understand the significance of research papers, and that is why we have handpicked 170 out-of-the-box vaccination research paper topics, titles, and ideas to make your work seamless.
Debate Topics About Vaccination
- What is reverse vaccinology?
- Look at the ways of harnessing the participation of dendritic cells in tolerance and immunity
- What are some of the approaches to advance cancer vaccines to clinical utility?
- Highlight innovative therapeutic and vaccine approaches against respiratory pathogens
- Examine immunity to malaria and vaccine strategies
- Assess molecular vaccines against pathogens in the post-genomic era
- Comprehending the limitations of today’s influenza vaccine strategies and further development of more efficient therapeutic and preventative interventions
- Study HIV-associated persistent inflammation and immune activation
- Analyze recent advances in respiratory virus infection
- What is the novel approach for anti-tumor vaccines
- Unravel the challenges and progress in the development of a B cell-based hepatitis C virus vaccine
- What is the functional relevance of Tatraspanins in the immune system?
- Look at advanced immunization technologies for next-generation vaccines
- Evaluate epitope discovery and synthetic vaccine design
- In what ways can tuberculosis be treated by targeting host immunity
- What are the immunomodulatory effects of drugs in the treatment of immune-related diseases
- Highlight natural antibodies in health and disease
- Discuss different influenza virus vaccines and immunotherapy
- What are some of the shadows of cancer immunotherapy
- Understanding the therapeutical potential of extracellular vesicles
- A review of the ethical theories and problems associated with vaccination in America
- Do vaccines love the Darwinian fitness of immune cells
Vaccination Behavior Research Topics
- Unraveling demand and supply effects on the up-take of influenza vaccinations
- Point out new approaches to the seasonal flu vaccine
- Exploring the impact of vaccination
- Investigating patient experience with, and the use of, an electronic monitoring system to assess vaccination responses
- A meta-analysis of interventions that enhance the use of adult immunization and cancer screening services
- Do vaccines seem to work against bacterial and viral infections, and are they effective?
- Gathering the evidence for the introduction of typhoid vaccine: worldwide vaccine testing
- Explore molecular mimicry to broaden the immune response to carbohydrate antigens for vaccine development
- Tumor-associated glycan and immune surveillance
- Rational design and application of idiotope vaccines
- Assessing the effects of vaccines on immune-deficient people
- What are the impacts of rapid growth and deployment of high-volume vaccines for pandemic response
Anti-vaccination Research Paper Topics
- Should the state impose vaccinations, or should the choice be left up to the child’s parents?
- What is the connection between vaccination and autism?
- Is natural immunity better than immunity through immunization?
- Examining cultural perspectives on vaccination
- Are they worth it? adverse effects of vaccination on children
- To vaccinate or not against HPV? A content analysis of vocabularies of motives
- Vaccines: religious and cultural arguments from an Islamic perspective
- Anti-science populism or biomedicine’s unresolved knots? Comparing views on the movements against mandatory pediatric vaccines
- An anthropological commentary on vaccine hesitancy, decision-making, and interventionism among religious minorities
- Understanding attitudes to vaccination
Research Topics For Covid-19 Vaccination
- Medical mistrust in the context of Covid-19: implications for intended care-seeking and quarantine policy support in the United States
- What is the acceptability of the potential COVID-19 vaccine among smokers and non-smokers?
- COVID-19 vaccine hesitancy in healthcare personnel: are there any differences among classifications
- Discuss various options that one can use to convince people to get the covid-19 vaccine
- Examining COVID-19 vaccine efficacy after the first dose: Pfizer, Moderna, AstraZeneca
- Discuss the impacts of herd immunity during the covid-19 pandemic
- What are some of the effects of covid-19 vaccination on transmission of disease?
- Discuss whether antibodies generated through vaccination recognize all-new variants of covid-19
- Investigate how the intensity of lockdowns accelerate or influence mutation of the COVID virus
- Examine how the new covid-19 strain identified in England will affect the available vaccines.
- Outline which immunoglobulin types can be used as the markers for covid-19 vaccination
- Which is the best way to deal with swaps after completing vaccinations in nursing homes
- How do we curb vaccine hesitancy among healthcare providers?
- Which one is the more dangerous, covid-19 or covid-19 vaccine? What must be the individual decision?
- Analyzing Ebola and the evolving ethics of quarantine
- Break down some of the side effects of covid-19 vaccination
- How long will immunity last after receiving the covid-19 vaccination?
- Will, a covid-19 vaccine work for everyone? Are there people who cannot get vaccinated?
- Is bivalent OPV immunization capable of mitigating the impact of covid-19?
- What are the expected long-term side effects of the vaccination for covid-19?
- Evaluate differences between the first and second doses of the covid-19 mRNA vaccine?
- Examine the ingredients in the covid-19 mRNA vaccine
- Can a person’s DNA change through mRNA vaccines?
- Factors that stops the body from continuing to produce COVID-19 spike protein after getting a COVID-19 mRNA
- Discuss whether a person vaccinated against covid-19 will be able to spread the virus to susceptible people
- Investigating vaccination adverse outcomes and costs of vaccine injury claims(VICs): In the past, present, and during COVID-19.
- Who gets cured: Covid-19 and the development of critical sociology and anthropology of cure
- Development of perception and attitude scales related to COVID-19 pandemic
- Does the mutation of the coronavirus affect the capacity of the vaccines to prevent disease?
- A case-control study: finding a link between pre-existing antibodies got after the childhood vaccinations or past infections and COVID-19?
- Queue questions: ethics of COVID-19 vaccine prioritization
- Disparities between Black and White in H1N1 vaccination among adults in the U.S. in 2009: A cautionary tale for the COVID-19 pandemic
- Autonomy and refusal in pandemics: What to do with those who refuse COVID-19 vaccines
- Knowledge, attitude, and acceptance of a COVID-19 vaccine: a global cross-sectional study
- Prospects of COVID-19 vaccine implementation in the U.S.: Challenges and potential solutions
- What are the effects of COVID-19 vaccines on pregnant women?
- Compare and contrast the efficacy of different covid-19 vaccines.
- Ways to improve covid-19 vaccine acceptance
- Determination of causation between COVID-19 vaccines and potential adverse effects
Vaccination Of Children Topics
- What is the essence of increasing HPV vaccination among children?
- Analyze the primary diseases that vaccines prevent in children
- What will happen if a child’s vaccination schedule is delayed
- Look at the vaccination schedule for children in the U.S.
- Can children receive more than one vaccine at a time?
- Examine revaccination outcomes of children with proximate vaccine seizures
- What are the impacts of measles-containing vaccination in children with the severe underlying neurologic disease?
- Evaluate the challenges involved in measuring immunization activity coverage among measles zero-dose children
- What is the connection between the polio vaccine and the risk of cancer among children?
- Do multiple vaccines affect babies’ health and immune system in an adverse war, or can their bodies handle them?
- What are the various vaccination options available for children, and are they harmful to children’s overall health?
- The case for further research and development: assessing the potential cost-effectiveness of microneedle patches in childhood measles vaccination programs
- Evaluate the accuracy of parental recall of child immunization in an inner-city population
- Evaluating maternal acculturation and childhood immunization levels among children in African-American families in Florida
- Policy analysis: the impact of the vaccine for children’s program on child immunization delivery
- The effect of managed care: investigating access of infant immunizations for poor inner-city families
- Who takes up free flu shots? Investigating the effects of an expansion in coverage
- What are the societal and parental values for the risks and benefits of childhood combination vaccines?
- Looking into trends in vaccination intentions and risk perceptions: a longitudinal study of the first year of the H1N1 pandemic
Healthcare Topics About Vaccination
- Conscious consideration of herd immunity in influenza vaccination decisions
- A case study of ethnic or racial differences in Medicare experiences and immunization
- What preservatives are used in vaccines
- Discuss the relationship between vaccines and autism
- What is the role of epidemiology in infection control?
- How t design and select the most relevant immunogenic peptide sequences
- Discuss why the Zika virus has not had a significant impact in Africa as compared to America
- What are the advantages of using the phage display technology of antibodies versus hybridism technology?
- Analyzing the impact and cost-effectiveness of vaccination programs in a country using mathematical models
- Malaria vaccines: progress and problems
- Malaria: cloning genes for antigens of plasmodium falciparum
- Fighting profits on the pandemic: The fight for vaccines in today’s economic and geopolitical context
- Molecular and biotechnological approaches to fish vaccines
- Immunogenicity of a whole-cell pertussis vaccine with low lipopolysaccharide content in infants
- Immunogrid: an integrative environment for large-scale simulation of the immune system for vaccine discovery, design, and optimization
Thesis Topics In Vaccination
- Investigating challenges and opportunities in vaccine delivery, discovery, and development
- Discuss classic methods of vaccine development
- What are some of the current problems in vaccinology?
- Assess some of the latest tools for vaccine development
- Using cost-effectiveness analysis to support research and development portfolio prioritization for product innovations in measles vaccination
- Communicating vaccine safety during the introduction and development of vaccines
- Highlighting viral vectors for use in the development of biodefense vaccines
- What is the role of US. military research programs in the invention of USA-approved vaccines for naturally occurring infectious diseases
- Curbing outbreaks: utilizing international governmental risk pools to fund research and development of infectious disease medicines and vaccines
- Vaccine stabilization: research commercialization and likely impacts
- Exam the unequal interactions of the role of patient-centered care in the inequitable diffusion of medical innovation, the human papillomavirus(HPV) vaccine
- A case study of the status of development of vaccines and vaccine research for malaria
- Enteric infections vs vaccines: a public health and clinical research agenda for developing countries
- A review of research and vaccine development for industry animals in third world countries
- How the research-based industry approaches vaccine development and establishes priorities
- A look at the status of vaccine research and development of a vaccine for HIV-1
- Modeling a cost-effective vaccination strategy for the prevention of herpes zoster infection
- Using an adequate T.B. vaccination regiment to identify immune responses associated with protection in the murine model
- A systematic analysis of the link between vaccines and atopic dermatitis
- Do vaccines provide better immunity than natural infections?
- Is there a need to be vaccinated against a disease that is not available in your country or community
- How to strengthen adult immunization via coordinated action
- Using the general equilibrium method to assess the value of a malaria vaccine: An application to African countries
- Who should take up free flu shots?
- Evaluate the impact of vaccination among health care personnel
- Retail clinics and their impact on vaccination in the U.S.
- Discuss the societal values for the benefits and risks of childhood combination vaccines
- How safe and effective is the synovial vaccine for people above 60 years
- Evaluating vaccination effectiveness of group-specific fractional-dose strategies
Law Research Topics On Vaccination
- Explain why there are age restrictions for Rotavirus vaccination?
- Vaccination or hygiene : Which factor contributes to the decline of infectious diseases?
- Outline the main factors that cause vaccine failure
- Discuss why HIV is so hard to vaccinate in uninfected people?
- In what ways do maternal vaccinations affect the fetal nervous system development
- How to deliver malaria vaccine effectively and efficiently
- Highlight the vaccines that are specifically licensed in the U.S. for pregnant women
- How does an immune genetic algorithm work?
- Evaluate the relationship between the success of artificial insemination and vaccination
- Outline the reasons why vaccines underperform in low-income countries
- Discuss U.S. immigration and vaccination policy
- Assessing the effectiveness of compelled vaccination
Vaccination Ethical Topics
- What are the requirements for a strain to be used as a vaccine?
- What is the best way to administer vaccines in children?
- Assessing the benefits of maternal vaccination on breastfed infants
- Evaluating the pros and cons of intraperitoneal vaccination
- Examine ways to measure the pattern of vaccination acceptance
- Investigate Covid-19 transmission, vaccination rate, and the fate of resistant strains
- Look into dark web marketplaces and covid-19 vaccines.
- A close look at covid-19 vaccines and kidney diseases
- Contextualizing the impact of covid-19 vaccine misinformation on vaccination intent in the U.S.
- Examining behaviors and attitudes of medical students towards covid-19 vaccines
From the exceptional vaccination debate topics, titles, and other ideas above, you can quickly tell that we have your best interest at heart. We believe that all students deserve decent scores on their papers. As a result, we are more than willing to recommend affordable online help with research papers from the best-rated sites.
Whether you are overwhelmed by other assignments, dealing with a tight deadline, or lack the spark to pull it off, you can count on cheap yet professional thesis help from trusted assignment help sites. They are ready to deliver high-quality papers, and you can confirm that from any sample or example on the platforms.

Make PhD experience your own
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Evidence Review of the Adverse Effects of COVID-19 Vaccination and Intramuscular Vaccine Administration
Vaccines are a public health success story, as they have prevented or lessened the effects of many infectious diseases. To address concerns around potential vaccine injuries, the Health Resources and Services Administration (HRSA) administers the Vaccine Injury Compensation Program (VICP) and the Countermeasures Injury Compensation Program (CICP), which provide compensation to those who assert that they were injured by routine vaccines or medical countermeasures, respectively. The National Academies of Sciences, Engineering, and Medicine have contributed to the scientific basis for VICP compensation decisions for decades.
HRSA asked the National Academies to convene an expert committee to review the epidemiological, clinical, and biological evidence about the relationship between COVID-19 vaccines and specific adverse events, as well as intramuscular administration of vaccines and shoulder injuries. This report outlines the committee findings and conclusions.
Read Full Description
- Digital Resource: Evidence Review of the Adverse Effects of COVID-19 Vaccination
- Digital Resource: Evidence Review of Shoulder Injuries from Intramuscular Administration of Vaccines
- Press Release
Recent News
NAS Launches Science and Innovation Fund for Ukraine
Science Academies Issue Statements to Inform G7 Talks
Supporting Family Caregivers in STEMM
A Vision for High-Quality Preschool for All
- Load More...
Prevention of HIV transmission
Add to collection, downloadable content.
- March 22, 2019
- Affiliation: School of Medicine, Department of Microbiology and Immunology
- The human immunodeficiency virus (HIV) infects and replicates within individuals for the duration of their life. Initial infection results in little to no symptoms for years or even decades. These individuals are infectious and capable of further spreading HIV while completely unaware of their own status. This ability to transmit without detection is what lead to the unknown and thereby unopposed global spread of HIV type one (HIV-1). Once a test was developed to detect HIV, the virus was found in nearly all major countries around the world. Development of a cure has proven to be exceedingly difficult. So far, the only successful tactic to reduce the number of infected HIV individuals has been to prevent HIV transmission. Traditionally, the most effective way to prevent viral transmission is with a vaccine, but an effective HIV vaccine for wide spread use has not been developed. Therefore, alternative HIV transmission prevention strategies have been used. These strategies largely depend on behavioral modifications and include: 1) utilization of universal precautions in medical settings, 2) increased emphasis on HIV testing and self awareness of infection status, 3) encouraged use of protective measures such as condoms and male circumcision, 4) prophylactic use of antiretroviral drugs to limit mother to child transmission MTCT, and 5) the newly available oral pre-exposure prophylactic use of Truvada in HIV negative individuals. Together, these strategies have contributed to nearly eliminating HIV transmission in medical settings and greatly reduced MTCT. Unfortunately, HIV continues to spread globally largely due to sexual transmission. While condom usage is highly efficient to prevent transmission, their use is limited by acceptability and consent. In this dissertation, I evaluated the potential of topically applied interventions to be a novel, effective form of protection against HIV transmission as well as established a novel line of investigation evaluating microbial contributions to HIV transmission. Using humanized mice as a model of HIV transmission, I evaluated two antiretroviral drug based topical pre-exposure prophylaxis (PrEP) for efficacy; tenofovir and maraviroc. In both instances, these drugs were found to be protective when used prior to HIV exposure. Concerns regarding the dual use of tenofovir for treatment as well as PrEP prompted me to evaluate transmission of a tenofovir resistant strain of HIV. This study demonstrated a surprisingly large defect in transmission for tenofovir resistant HIV, which suggests that use of Tenofovir for PrEP may not result in a significant increase of circulating tenofovir resistant strains of HIV. I also utilized the humanized mouse model to start a completely novel line of investigation evaluating the effect of microbial populations on HIV transmission. While these studies are ongoing, preliminary results have shown a clear effect of microbiome composition on rectal HIV transmission. These results are significant in two ways. First, this is the first evidence that humanized mice are viable tools for microbiome based studies. Second, commensal microbiota does affect HIV transmission efficiency. Taken together, the following dissertation supports further efforts to curb the HIV epidemic by development of topical interventions (microbicides) and lends credence toward interventions based on commensal microbiome manipulations.
- https://doi.org/10.17615/36aw-gy12
- Dissertation
- In Copyright
- Garcia, J. Victor
- Doctor of Philosophy
- University of North Carolina at Chapel Hill
This work has no parents.
Select type of work
Master's papers.
Deposit your masters paper, project or other capstone work. Theses will be sent to the CDR automatically via ProQuest and do not need to be deposited.
Scholarly Articles and Book Chapters
Deposit a peer-reviewed article or book chapter. If you would like to deposit a poster, presentation, conference paper or white paper, use the “Scholarly Works” deposit form.
Undergraduate Honors Theses
Deposit your senior honors thesis.
Scholarly Journal, Newsletter or Book
Deposit a complete issue of a scholarly journal, newsletter or book. If you would like to deposit an article or book chapter, use the “Scholarly Articles and Book Chapters” deposit option.
Deposit your dataset. Datasets may be associated with an article or deposited separately.
Deposit your 3D objects, audio, images or video.
Poster, Presentation, Protocol or Paper
Deposit scholarly works such as posters, presentations, research protocols, conference papers or white papers. If you would like to deposit a peer-reviewed article or book chapter, use the “Scholarly Articles and Book Chapters” deposit option.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 18, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
DNA vaccine against Zika performs well in tests on mice
by Maria Fernanda Ziegler, FAPESP

In Brazil, researchers at the University of São Paulo (USP) and the Pernambuco division of Oswaldo Cruz Foundation (FIOCRUZ) are developing a Zika vaccine. The formulation was tested on mice and found to be efficacious, inducing an immune response against the virus and protecting the animals appropriately. The results are reported in an article published in Frontiers in Immunology .
"Most vaccines use an attenuated or inactivated form of the virus they're designed to combat. DNA vaccines are more advanced. The technology has evolved over the past 30 years to become a powerful therapeutic platform.
In this study, we designed four DNA vaccine formulations encoding part of the viral envelope's protein complex and selected the one that proved most efficacious," said Maria Sato, a professor at the University of São Paulo's Medical School (FM-USP) and corresponding author of the article.
In addition to being technologically more advanced, DNA vaccines tend to be cheaper and potentially more efficient than attenuated or inactivated virus vaccines.
"It's a low-cost technology and relatively easy to use because you can design a formulation by taking the key parts of the virus and adding [adjuvant] substances that boost the immune response. However, achieving sufficient immunogenicity [by triggering a robust immune response ] is a challenge for DNA vaccines," said Franciane Teixeira, first author of the article. The study it describes was part of her Ph.D. research at FM-USP.
DNA vaccines
With the aid of molecular biology techniques, the researchers selected Zika virus genes that encode two of its structural proteins —the pre-membrane/membrane (prM) protein and the envelope (E) protein—and deleted specific parts of the viral envelope.
To keep the selected genetic sequences stable, they inserted each one into a plasmid. Plasmids are small circular DNA molecules obtained from bacteria that do not cause disease in humans and are widely used as vectors in genetic engineering.
When the vaccine is injected, the plasmid (the DNA vaccine proper) enters the host organism's cell nuclei, where the encoded sequence is deciphered, and proteins equal to Zika's are produced, leading the host's defense cells to recognize them as viral particles, produce antibodies against the virus and trigger other protective mechanisms.
"It's important to bear in mind that, like the messenger RNA vaccines produced by Pfizer and Moderna against SARS-CoV-2, DNA vaccines don't alter the host organism's genetic code, create a new species, or cause autoimmune disease. The technology is safe, despite all the fake news and disinformation," said Isabelle Viana, a researcher at FIOCRUZ Pernambuco and Teixeira's co-supervisor.
"Humans are the result of billions of years of evolution and constant interaction with other DNAs, as happens when we're infected by a pathogen, for example."
Envelope protein
The target chosen by the researchers for the Zika vaccine is the protein complex that comprises the outer surface of the virus, including the envelope protein, which is the primary trigger for the production of neutralizing antibodies. "We aimed at modulating the regions that make up the envelope protein, and to do so, we removed the regions of this protein that bind it to the cell membrane , which are known as the stem and transmembrane portions," Teixeira explained.
According to the authors of the article, the approach facilitated the enhanced expression of these Zika proteins by the organism after immunization, leading to increased production of antibodies against the virus.
The formulation called ZK_ΔSTP, where the membrane-anchoring regions were completely removed, proved to be far more immunogenic than the other three designed by the research group.
"This strategy corresponds to the removal of the Zika envelope protein cell membrane-anchoring region and promotion of extracellular protein expression. The vaccine induced a strong response by the adaptive immune system in adult mice, with high levels of neutralizing antibodies [in the humoral response] and production of T and B lymphocytes [in the cellular response]," Teixeira said.
The addition of aluminum hydroxide adjuvants led to a sustained neutralizing response, protecting the mice after they were exposed to the virus. "The results show that the formulation is efficacious and deserves to be developed further through more translational studies," Teixeira said.
Despite scientific progress since the last Zika outbreak in the Americas, there are no approved vaccines or treatments for the disease. Besides economic issues , a peculiarity of the pathogen makes the development of a vaccine especially challenging: Zika virus is very similar to all four serotypes of the dengue virus .
"There's a risk of what we call a cross-reaction, where antibodies produced by the Zika vaccine recognize dengue virus. This may seem positive at first glance, but it can be a hazard without proper interpretation," Viana said, adding that a second dengue infection is typically more severe than the first for several reasons.
"The patient will have produced antibodies against dengue in the first infection, and if they aren't potent enough to prevent a second infection by a different dengue serotype, the opposite effect occurs: the antibodies bind to the virus, and it penetrates cells more easily. In other words, the organism helps the virus infect its own cells."
According to Viana, previous research by the group showed that practically the entire population of Brazil has immunity against at least one dengue serotype. "So in formulating a Zika vaccine, it's crucial to make sure this cross-reaction doesn't occur in people who have had dengue. In our tests on mice, the vaccine we formulated induced neutralization only of zika, showing that it doesn't recognize any dengue serotypes and doesn't produce a cross-reaction," he said.
Explore further
Feedback to editors

Immune cells carry a long-lasting 'memory' of early-life pain
4 minutes ago

Cannabis legalization and rising sales have not contributed to increase in substance abuse, study finds

No negative impact from prolonged eye patching on child's development or family stress levels
5 minutes ago


COVID-19 booster immunity lasts much longer than primary series alone, study shows
27 minutes ago

Study finds that human neuron signals flow in one direction

A common pathway in the brain that enables addictive drugs to hijack natural reward processing identified
2 hours ago

Scientists identify airway cells that sense aspirated water and acid reflux

Environment may influence metacognitive abilities more than genetics

Contracting RSV before age two can cause long-term lung changes and impairment

New therapy shows promise for a rare childhood dementia
Related stories.
Scientists reveal details of antibodies that work against Zika virus
Feb 25, 2021
Fighting Zika? Call in the T cells
Nov 4, 2020

Discovery of a Zika antibody offers hope for a vaccine
May 4, 2017
Scientists discover how dengue vaccine fails to protect against disease
Jun 24, 2021
How antibodies offer protection against an infection with HIV
Oct 12, 2023

Researchers discover how body temperature wrecks potential dengue, Zika vaccine
May 18, 2018
Recommended for you

Study shows metabolic health before vaccination determines effectiveness of anti-flu response
5 hours ago

Long COVID patients show immunological improvement two years after infection
6 hours ago

Human cases of bird flu 'an enormous concern': WHO
4 hours ago

Health improvements occurred worldwide since 2010 despite COVID-19 pandemic, but progress was uneven: Study
21 hours ago
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Perspectives
- Published: 01 April 2002
Science and society – vaccines
Ethical issues for vaccines and immunization
- Jeffrey B. Ulmer 1 &
- Margaret A. Liu 2
Nature Reviews Immunology volume 2 , pages 291–296 ( 2002 ) Cite this article
14k Accesses
30 Citations
9 Altmetric
Metrics details
Vaccination is the only type of medical intervention that has eliminated a disease successfully. However, both in countries with high immunization rates and in countries that are too impoverished to protect their citizens, many dilemmas and controversies surround immunization. This article describes some of the ethical issues involved, and presents some challenges and concepts for the global community.
Similar content being viewed by others

A guide to vaccinology: from basic principles to new developments
Andrew J. Pollard & Else M. Bijker
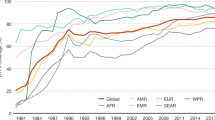
Immunization: vital progress, unfinished agenda
Peter Piot, Heidi J. Larson, … Beate Kampmann
A systematic literature review to clarify the concept of vaccine hesitancy
Daphne Bussink-Voorend, Jeannine L. A. Hautvast, … Marlies E. J. L. Hulscher
Vaccines stand out as being among the most efficacious and cost-effective of global medical interventions 1 ( Box 1 ). Vaccines have saved millions of lives, prevented significant morbidity and suffering, and even eradicated a disease. This last accomplishment, the eradication of smallpox, highlights what can be achieved by vaccination. However, unfortunately, the inequalities in the distribution and use of vaccines are also striking. If vaccines can be deployed so successfully worldwide that a disease can be eliminated, why do millions of children still die each year from other vaccine-preventable diseases? From ethical and humanitarian perspectives, this should be unacceptable. However, despite the medical evidence of the benefits of vaccines, both immunization itself as a goal, as well as the actual efforts for global immunization, are often debated and criticized. So, the ethics of whether, and how, to vaccinate all of the world's population are much more complex than it would seem at first glance. Some of these issues will be examined and a call for action raised in this article.
Current state of immunization rates
At present, in industrialized countries such as the United States, infants routinely ( ∼ 80–95% coverage) receive vaccines against diphtheria, pertussis (whooping cough), tetanus, measles, mumps, rubella, polio, Haemophilus influenzae type b, hepatitis B, varicella, pneumococcus and, often, hepatitis A. The efficacy of these vaccines and the success of routine immunizations are such that in the United States, for example, the morbidity from previously routine childhood diseases has been reduced by 90–100% since the introduction of standard immunization 2 ( Table 1 ). In developed countries, the near disappearance of these diseases from normal childhood has removed much of the fear of the illnesses that they cause. The result is that the consequences of not vaccinating against these diseases are sometimes not fully appreciated by the public.
In developing countries, one in every four children born annually will not be vaccinated 3 . In many of the impoverished countries in which these children reside, the concomitant lack of health care, inadequate nutrition 4 , higher prevalence of disease, decreased hygiene and over-crowding conspire to increase the incidence of, as well as the morbidity and mortality from, both vaccine-preventable and other infectious diseases. Six children die every minute as a result of infectious diseases that could be prevented by existing vaccines, and for measles alone, nearly one million children die each year 3 . So, each day, 4,000–8,000 people 3 , mainly children, die from vaccine-preventable diseases ( Table 2 ).
Various efforts to increase global immunization rates have protected many children successfully. For example, in 1974, before the Expanded Program on Immunization (EPI) was established, an estimated 8 million children died each year from measles 5 . Since then, the EPI has targeted six diseases: tuberculosis, measles, diphtheria, tetanus, pertussis and polio ( Fig. 1 ). By 1980, the incidence of measles had been reduced by ∼ 50% ( Fig. 2 ). In that year, the World Summit for Children set a goal of 80% coverage for these six basic immunizations to be achieved by 1990. Laudably, those efforts succeeded in raising global immunization rates from less than 10% to almost 80% in that decade 6 , with a concomitant reduction in disease burden (for example, see Fig. 2 ). But, a combination of complacency, competition for health-care resources with other diseases (such as HIV) and other factors, such as an inadequate and ageing health-care infrastructure, resulted in a subsequent plateau 5 or decline in average immunization rates in the developing world 6 . This trend is worrying because of what it represents in terms of the commitment and sustainability of immunization programmes. Maintenance of a high level of coverage for immunization is crucial — unless a certain minimum percentage of the population is immune, the benefits of herd immunity (when sufficient people are immune such that a pathogen cannot easily persist and spread in a population) will not be achieved.
As shown, the number of vaccines ( y axis) that are given to children has risen steadily since 1985 in industrialized countries, but not in developing countries. DPT is a combination vaccine against three separate pathogens: diphtheria (D), pertussis (P) and tetanus (T). BCG is the Mycobacterium bovis bacillus Calmètte–Guerin vaccine against tuberculosis. The loss of life due to lack of infant immunization in developing countries is due to the smaller number of vaccines that are routinely used, as well as to lower immunization rates. Reproduced, with permission, from the World Health Organization 6 .
The global incidence of measles has declined as immunization coverage has increased. The level of immunization coverage plateaued in the 1990s, leaving a significant number of cases of measles still occurring each year. Reproduced, with permission, from the World Health Organization 22 .
Ethics of immunization and vaccines
The economic and human benefits of vaccination are clear for many vaccines. But, economic and political realities, along with philosophical questions, raise certain ethical issues concerning the use and distribution of vaccines 7 . What are the rights of individuals in deciding about vaccination versus the rights of, and risks to, society? Should different standards for the efficacy and safety of a particular vaccine be set for different populations for which the risk:benefit ratio might be different? How should priorities be set for different areas between and within countries? Which regulatory bodies have the right to make decisions at the individual or national level? Although few would argue against the responsibility of developed nations to help poorer countries and peoples to develop and use the necessary vaccines, what are the precise responsibilities of both developed and developing countries? Here, we examine several of these issues to highlight the complexities of the ethics of vaccine development and use.
Whose choice is it? During the period when polio epidemics struck terror into the hearts of the population, the public clamoured for, supported the development of (for example, The March of Dimes in the United States, a national public compaign to raise donations to fund the research and development of polio vaccines) and welcomed a vaccine against polio. The live attenuated vaccine made possible a global mass-immunization campaign; on a single day of the various National Immunization Days, 83–147 million children were immunized 8 , 6 . Now, at a time when wild-type polio has been eliminated from the Western Hemisphere and nearly eradicated globally, the only cases of polio in the Western Hemisphere are caused by reversion mutants of the live-attenuated vaccine strain of the virus. For society as a whole, the total elimination of polio seems the obvious and correct path. But, as the probability of an individual being exposed to the virus decreases, and as the devastation of the historic polio epidemics fades in our memories, the rare cases of disease due to reversion of the vaccine itself become more prominent. The benefit of immunization for any given individual decreases (due to a lower probability of contracting the disease), whereas the perception of risk might increase 9 . So, what is best for the individual might be seen as potentially different from what will benefit society as a whole. However, it has only been through the participation of hundreds of millions of individuals in vaccination programmmes that unimmunized individuals have the luxury of an altered risk:benefit ratio. Interestingly, in 1980, the year that smallpox was officially declared to be eradicated, prominent individuals in the international health-planning community were critical of the smallpox campaign. They felt that the approach of 'top–down' (mandated and orchestrated by global bodies) programmes was wrong and contrary to more-localized, primary health care 10 , 11 . Even this pre-eminent example of the beneficial power of immunization was not without controversy.
In the same manner, other vaccines have become victims of their own success. As diseases disappear from the general population after successful vaccination campaigns, the real risk of an individual contracting the disease decreases and the perception of the seriousness of the disease, even if contracted, is reduced. Concomitantly, concerns about the real or imagined adverse effects of the vaccines increase. As a result, individuals might disagree with government mandates for population-wide vaccination. For example, before the introduction of a vaccine against pertussis, there were approximately 2 million cases annually in the United States. In Great Britain, in the mid-1970s, some adverse reactions to the vaccine were widely publicized, and immunization rates declined from 80–90% to 30%. As a consequence, the population was vulnerable to two subsequent severe outbreaks of whooping cough, which resulted in more than 120,000 recorded instances of disease, hundreds of cases of serious complications and 28 deaths 12 . More recently, heightened fears of the perceived adverse effects of other vaccines (such as measles and hepatitis B), even if unproven, have had an impact on immunization rates and the incidence of disease 6 , 13 , 14 , 15 . A greater awareness of the consequences of failure to vaccinate, through better education, might be the best tool to combat this problem 16 .
Poverty and priorities. In wealthier countries, the ethical issues that surround vaccination tend to focus on the rights of individuals versus government or society. In poorer countries, the fundamental issue is the lack of access to basic necessities for health, such as adequate nutrition, clean water, medicines or vaccines. Although poverty is clearly the main cause of these deficiencies, other factors contribute, such as the low priority given to health and preventive measures, the disenfranchisement and lack of political and economic power of the people most affected (children and women), corruption and regional warfare.
In the year 2000, only 10% of global medical-research funds were directed towards the 90% of diseases that affect the world's poorest people 6 . It would seem obvious from the economic benefits that prevention of disease (and health in general) should be a priority for poor countries. It would seem equally obvious that helping to ensure such health should be a high priority for wealthy nations, even if simply to protect their own populations. Not only can newly emerging diseases spread rapidly across the globe, but pathogens eliminated from one population can be 're-imported' (or new strains introduced) by travellers or immigrants 17 .
At present, only about 1% of contributions to overseas development are directed towards immunizations 6 . The hurdle is not simply the purchase price or availability of vaccines, but for many poor countries, there is a lack of infrastructure for health care in general, and vaccine delivery specifically. The needs are so great and so widespread, both geographically and technologically, that assigning priority is difficult. This prioritization is considered by some to be an ethical issue, but in reality, it might be simply that the pie is not sufficiently large, rather than that the pie should be sliced differently. The trade-off of protecting children now from disease versus an emphasis on the development of new vaccines to protect children in the future is not a debate that can be resolved even by Solomonic wisdom. Neither trade-off is ethically defensible, and the world should, instead, work constructively to increase the resources devoted to health, nutrition, prevention and specifically immunization, to make vaccines available to all people as required. But, how is this to be accomplished?
'Trickle-down' or simultaneous introduction. A marked effort is required to introduce vaccines into all necessary areas of the globe in a more timely fashion. The average time lag between licensing of a new vaccine for industrialized countries and its use in less developed countries is 10–20 years 6 . There are many reasons for this, including the lack of manufacturing capability for vaccines that require new technology in their production, return on investment and the cost of manufacturing newer technology-based vaccines. For example, when the recombinant hepatitis B virus vaccine was first introduced, there was not sufficient capacity worldwide for its production. Moreover, the cost of manufacturing such a 'high-tech' vaccine put it beyond the reach of the existing purchasing programmes at the time. Although the technology that supports recombinant protein vaccines is now available worldwide, it took time and effort to develop that capacity, even in developed countries.
People are created equal, but different
The simultaneous introduction of vaccines into developed and developing countries is an important goal. However, this is easier said than done. In addition to the issues of availability and economics, it cannot be assumed that disease burden, strain prevalence, vaccine efficacy and effectiveness (how well a vaccine works in clinical trials and real-world settings, respectively), immunization schedules or risk:benefit ratio will be standard or will justify the use of a vaccine in all areas. Differences in genotype and health status of individuals could affect how their immune system responds to a given immune stimulus. Environmental influences on the immune system (for example, indigenous parasitic infections that affect the predominant cytokine-secretion profile of helper T cells, or bacterial infections that facilitate or hinder immunity against closely related pathogens) might affect the immunological outcome of a particular type of vaccine.
Just as a vaccine that works in one population might not be as effective in another population, so might adverse effects of a vaccine be specific to one population. Hence, a vaccine company might be reluctant to simultaneously test a vaccine in two populations, for fear that an adverse effect in one population (due to genetic, nutritional or other health factors, or limitations of the infrastructure for vaccine delivery) would halt the development of a vaccine that could be both useful and commercially viable in another population. More importantly, the higher background rates of morbidity and mortality in certain developing countries might cause problems when presenting vaccines for licensure in more developed countries.
A counter-example to illustrate why vaccines might need to be tested simultaneously in developed and developing countries is provided by a rotavirus vaccine. Rotavirus is a major cause of diarrhoea in infants. In the United States, 20 children die each year from rotavirus infection, and worldwide, the virus kills 600,000 children under the age of five annually 18 . In 1998, a new rotavirus vaccine was licensed in the United States. But by 1999, reports emerged from American clinics of the occurrence, after immunization, of a small number of cases of intestinal intussusception — a 'telescoping' of the small intestine that often requires emergency surgery 18 . The number of cases was small, and the aetiological link has been debated, but the vaccine was withdrawn from the market. Clinical trials that were testing the vaccine in developing countries were stopped. So, we do not yet know whether the vaccine would have been effective or caused side effects in children from developing countries, for whom the risk:benefit ratio might well be quite different. More than half a million children continue to die each year due to rotavirus infection (although it is hoped that other rotavirus vaccines that are under development will be approved for use). The issue of how to speed up vaccine deployment for developing countries and how to develop vaccines that specifically address the needs of those countries should not be oversimplified. Different vaccines (for example, for different virus strains) might be required to prevent the same disease in different parts of the world. The needs of people in developing countries must be specifically, rapidly and directly addressed to develop appropriate vaccines. To make this happen in a timely manner, more thought and effort is required at early stages of vaccine development. But, by whom and at whose expense?
A farewell to arms
In the face of the unacceptable and gaping inequalities in access to vaccines, the temptation has been to 'point the finger' at various countries or segments of society. A chief target for many of these denouncements has been large vaccine manufacturers, as well as the people and governments of their home countries. It has been easy to stir up indignation, although a thoughtful evaluation of the real contributions, roles and responsibilities of all parties has been more productive ( Box 2 ). The cries for companies to lower the prices of their vaccines has reinforced the unwarranted notion that vaccines should be inexpensive — an idea that perpetuates the vicious cycle of the low valuation of health care, prevention and children's lives. Obviously, low cost might be an important means of increasing access to vaccines, but the fundamental problem is the low priority given to children, health and prevention.
Most vaccines that are in use today were developed fully and are manufactured by industry, rather than the public sector. Commercial companies have responsibilities to their shareholders and are driven by profits. But, it is these profits that have enabled the companies to take the huge economic risks that are required for the long process of discovery, research and development of vaccines. If all economic incentive were removed, or made too small, even fewer companies would bother to make vaccines 19 , and, instead, would concentrate solely on therapeutic agents, because people will pay more for drugs that treat, rather than prevent, disease.
So, companies should not be expected to be philanthropic, although they should be expected to be generous global citizens. Indeed, industry-driven scientific and technological advances have had an important impact on improving global health. Combination vaccines — whereby several vaccines can be given with a single injection — were developed in part for the market advantage that they would provide but have been an important tool for increasing global vaccination rates. In addition, industry and academia are both contributing to the continuing development of vaccines that do not require refrigeration or needles and can be given orally or nasally — a true 'farewell to arms'.
In most walks of life, philanthropy usually comes as a consequence of success, and the same is true for medicines. So, the profit-driven priorities of vaccine companies are not unethical per se ; rather, they are not driven primarily by ethical concerns, and the challenge is how to increase the efforts of industry that are directed at improving health on a global basis. The fact that only 1% of pharmaceuticals that reached the market-place between 1975 and 1997 were approved specifically for diseases of the developing world 6 shows that, as a society, we need to re-evaluate our priorities and our paradigms, not that the economic model of financial incentive driving technical advances is wrong.
The challenge
We should challenge each economic sector, population and government to appropriately fulfil its responsibility to fellow humans or its own citizens; in other words, they should treat all of the world's children as their own, rather than denouncing particular groups as causing these inequities. Further support must be given rapidly to those whose efforts will result in vaccines that are better tailored for developing countries, both in terms of the disease focus and the development of technologies that will facilitate vaccine access and sustainability. New paradigms, including novel public–private partnerships ( Box 2 ) and alliances that are designed to engage local governments and manufacturers at early stages of research and development, are required. In this way, each group can contribute what they do best to the common goals of improving access to existing vaccines, developing new vaccines and technologies for existing diseases (such as HIV and malaria), and ensuring that increases in immunization rates are sustainable. Perhaps most difficult of all will be to change the mindset of people all over the globe. We need to place a higher priority on health and disease prevention, and above all, to value the lives of all people, no matter where they live — even if they are impoverished and powerless.
Box 1 | The economic benefits and cost-effectiveness of vaccines
The statistics for the eradication of smallpox illustrate the benefits and economics of vaccines and disease eradication. The eradication of smallpox has probably saved 40 million lives over the past two decades 6 , at a cost of US $25 million per year during the eradication campaign. The equivalent of US $275 million is saved annually in terms of quarantine and treatment because of the eradication of smallpox 3 .
The eradication of polio is also within reach. Between 1988 and 2000, coordinated public-health efforts, with a large contribution from Rotary International, have decreased the number of polio cases from approximately 350,000 to 3,500 per annum 6 . The potential future cost savings of polio eradication have been estimated at US $1.5 billion per year 6 , although the actual savings will probably be less than this owing to the need to continue immunization after eradication.
The cost of immunizing a child with the six core vaccines that are recommended by the Expanded Program on Immunization (tuberculosis, measles, diphtheria, tetanus, pertussis and polio) is US $17 (Ref. 6 ). Of this amount, only a few dollars are for the cost of the vaccines, with the rest paying, for example, for personnel and transportation of the vaccine (including refrigeration).
The benefits of vaccines are obvious in terms of the lives that are saved and the morbidity that is prevented or reduced, but the economic benefits extend beyond the benefits to the individuals that are protected. The economic benefits of immunization campaigns include savings in health-care costs for the treatment of illness and quarantine, the economic productivity of having a healthy workforce (or of not having parents removed from the workforce to care for an ill child) and coincident benefits that result from the immunization programme (such as an improved health-care infrastructure).
Box 2 | Potential mechanisms to achieve equity in health
The unequal access to existing vaccines and the relative lack of effort being applied to develop and produce vaccines for diseases that predominate in developing countries is part of the larger problem of extreme poverty in many nations. Although the roots and other manifestations of poverty must also be addressed, as Jorge Jiminez of the World Health Organization has said, “Health is seen more and more as preceeding development and not only as a consequence of wealth” 20 . Rather than relying on governments or international bodies as the sole means to address these problems, new models of public–private partnerships (PPPs) have arisen.
The fundamental idea of a PPP is to bring together entities from both the public sector (government or intergovernmental agencies) and the private sector (for example, industries, such as vaccine companies) to address a particular need, with each partner undertaking specific activities on the basis of their particular expertise and capacity.
Participation by industry might be in the form of donating a product, or applying research and development expertise to diseases (or types of health intervention) for which the market alone would not provide sufficient incentive. The motivation of the pharmaceutical industry might range from pure philanthropy (global citizenship and garnering goodwill) to economies of scale (if the product has a market elsewhere that will allow for higher prices to offset the lower prices in developing countries) 21 .
Recent PPPs have been established to tackle the challenges of developing and producing vaccines for diseases such as HIV and malaria. The input of effort or intellectual property from a 'for-profit' company and the potential loss due to 'opportunity costs' (that is, the possible revenue from a highly recompensed product if the effort had been directed towards that product rather than a vaccine for a developing country) can be strong disincentives for companies to participate in PPPs. Another approach that might become increasingly useful is the provision of early access to new vaccines and technologies for developing countries by the transfer of technology from a pharmaceutical company in an industrialized country to a local manufacturer in a developing country. Key roles for governmental and intergovernmental agencies include the regulation of products, and testing and manufacturing processes, and the prevention of reflow of products produced for lower pricing in developing countries into countries in which companies anticipate higher pricing to recoup the costs of development and make profits for reinvestment in future products.
Ten great public health achievements — United States, 1900–1999. MMWR Morb. Mortal. Wkly Rep. 48 , 241–243 (1999).
Impact of vaccines universally recommended for children — United States, 1990–1998. MMWR Morb. Mortal. Wkly Rep. 48 , 243–248 (1999).
Global Alliance for Vaccines and Immunizations. Global immunization challenges [online], 〈 http://www.vaccinealliance.org/reference/globalimmchallenges.html 〉
Rice, A. L., Sacco, L., Hyder, A., & Black, R. E. Malnutrition as an underlying cause of childhood deaths associated with infectious diseases in developing countries. Bull. World Health Organ. 78 , 1207–1221 (2000).
CAS PubMed PubMed Central Google Scholar
Hinman, A. Eradication of vaccine-preventable diseases. Annu. Rev. Public Health 20 , 211–229 (1999).
Article CAS Google Scholar
World Health Organization. State of the World's Vaccine and Immunizations (in the press)
Feudtner, C. & Marcuse, E. K. Ethics and immunization policy: promoting dialogue to sustain consensus. Pediatrics 107 , 1158–1164 (2001).
Foege, W. in New Generation Vaccines (eds Levin, M., Woodrow, G., Kaper, J. & Cobon, G.) 97 (Marcel Dekker, New York, 1997).
Google Scholar
Wilson, C. B. & Marcuse, E. K. Vaccine safety–vaccine benefits: science and the public's percepion. Nature Rev Immunol 1 , 160–165 (2001).
Henderson, D. A. Eradication: lessons from the past. Bull. World Health Organ. 76 , 17–21 (1998).
PubMed PubMed Central Google Scholar
Henderson, D. A. Primary health care as a practical means for measles control. Rev. Infect. Dis. 5 , 606–607 (1983).
Article Google Scholar
Ada, G. in New Generation Vaccines 2nd edn (eds Levine, M. et al.) 13–24 (Marcel Dekker, New York, 1997).
Wakefield, A. et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis and pervasive developmental disorder in children. Lancet 351 , 637–641 (1998).
Birmingham, K. UK immunization highs and lows. Nature Med. 7 , 135 (2001).
Duffell, E. Attitudes of parents towards measles and immunisation after a measles outbreak in an anthroposophical community. J. Epidemiol. Community Health 55 , 685–686 (2001).
Bazin, H. The ethics of vaccine usage in society: lessons from the past. Curr. Opin. Immunol. 13 , 505–510 (2001).
Instussusception among recipients of rotavirus vaccine— United States, 1998–1999. MMWR Morb. Mortal. Wkly Rep. 48 , 577–581 (1999).
Wilde, H. What are today's orphaned vaccines? Clin. Infect. Dis. 33 , 648–650 (2001).
Jimenez, J. Vaccines — a wonderful tool for equity in health. Vaccine 19 , 2201–2205 (2001).
Vandermissen, W. WHO expectation and industry goals. Vaccine 19 , 1611–1615 (2001).
Department of Vaccines and Biologicals. WHO Vaccine-Preventable Diseases: Monitoring System 2001 Global Summary (World Health Organization, Geneva, 2001) 〈 http://www.who.int/vaccines-documents/GlobalSummary/GlobalSummary.pdf 〉
Download references
Acknowledgements
We would like to thank C. Wiley and, in particular, R. Ingrum for their assistance.
Author information
Authors and affiliations.
Vaccines Research, Chiron Corporation, 4560 Horton Street, Emeryville, 94608, California, USA
Jeffrey B. Ulmer
Transgene, 11 rue de Molshelm, Strasbourg Cedex, 67082, France
Margaret A. Liu
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Margaret A. Liu .
Related links
Further reading, public–private partnerships.
Global Polio Eradication Initiative
Global Program to Eliminate Lymphatic Filariasis
International AIDS Vaccine Initiative
Mectizan Donation Program
Medicines for Malaria Venture
The Global Alliance for TB Drug Development
Governmental and professional groups
Global Alliance for Vaccines & Immunization
National Network for Immunization information
World Health Organization
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Ulmer, J., Liu, M. Ethical issues for vaccines and immunization. Nat Rev Immunol 2 , 291–296 (2002). https://doi.org/10.1038/nri780
Download citation
Issue Date : 01 April 2002
DOI : https://doi.org/10.1038/nri780
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Three harm-based arguments for a moral obligation to vaccinate.
- Viktor Ivanković
- Lovro Savić
Health Care Analysis (2022)
A novel vehicle routing problem for vaccine distribution using SIR epidemic model
- Nafiseh Shamsi Gamchi
- S. Ali Torabi
- Fariborz Jolai
OR Spectrum (2021)
Modeling Optimal Age-Specific Vaccination Strategies Against Pandemic Influenza
- Michael Golinski
- Gerardo Chowell
Bulletin of Mathematical Biology (2012)
Präventionsmaßnahmen im Spannungsfeld zwischen individueller Autonomie und allgemeinem Wohl
- Georg Marckmann
Ethik in der Medizin (2010)
Impfprogramme im Spannungsfeld zwischen individueller Autonomie und allgemeinem Wohl
Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz (2008)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

- High contrast
- Press Centre
Search UNICEF
Gavi and unicef welcome approval of new oral cholera vaccine.
GENEVA/NEW YORK, 18 April 2024 – Gavi, the Vaccine Alliance and UNICEF welcome the news that a new oral cholera vaccine (OCV), Euvichol-S, has now received WHO prequalification and can be made available to countries around the world. The prequalification of this new product will help EuBiologics, the manufacturer, produce more volumes of vaccine, faster, and at a lower cost – a key step to expanding supply amidst the on-going acute global upsurge of cholera outbreaks.
Today’s approval will help increase the overall supply of oral cholera vaccines available in 2024, with approximately 50 million doses now forecasted to be available to the global stockpile this year, compared to 38 million in 2023. Euvichol-S is an important product innovation: a simplified formulation of Euvichol-Plus that reduces the number of vaccine components – delivering a vaccine that studies have shown remains equally effective against key cholera serogroups while lowering production cost and complexity – thus allowing for larger volumes to be produced faster.
Cholera has been surging globally since 2021, with high case fatality rates despite availability of simple, effective and affordable treatment. The large number of outbreaks has led to unprecedented demand for vaccines from impacted countries. While global oral cholera vaccine supply has increased eighteen-fold between 2013 and 2023, the large and sustained spike in demand has put a strain on the global stockpile of cholera vaccines. Partners and countries are working urgently on cholera response, prevention and control measures in the face of this crisis, and have called on countries, manufacturers and others to support. Most recently, Gavi, UNICEF and partners announced the largest ever global deployment of cholera diagnostics to support surveillance and response.
“Prequalification of Euvichol-S represents a lifeline for vulnerable communities around the world,” said Dr Derrick Sim, Managing Director of Vaccine Markets and Health Security at Gavi. “Every vaccine dose delivered through Gavi programmes today represents years of planning and investment to shape the market so supply matches countries’ needs. The approval of this new product could not have come at a more important time given the acute upsurge of cholera outbreaks we are seeing worldwide. We commend EuBiologics for their role in ensuring countries around the world have access to cholera vaccine as part of their response toolkit.”
EuBiologics is currently the only supplier of OCV to the global stockpile, although other manufacturers are expected to have products available in the coming years. Gavi, the Vaccine Alliance works to shape the OCV market, and funds the global stockpile of OCV doses, along with transport and vaccination activities in lower-income countries. UNICEF leads on procurement and delivery of doses to countries. Use of the stockpile for emergency response is overseen by the International Coordination Group for Vaccine Provision (ICG), led by WHO.
“Despite cholera being preventable and easily treatable, children continue to suffer from this potentially fatal disease. UNICEF has already secured access to all the available doses of the just-approved vaccine and will deliver these to the countries at the highest possible speed,” said Leila Pakkala, Director of UNICEF Supply Division. “The approval means that UNICEF can increase the procurement and delivery of cholera vaccines by more than 25 per cent, pushing back harder on deadly cholera outbreaks.”
Notes to editors:
- The development of Euvichol-S is a collaboration between EuBiologics, the International Vaccine Institute (IVI), and the Bill and Melinda Gates Foundation .
- Find out more about the science behind Euvichol-S via the Lancet.
- Statement by ICG on current cholera upsurge.
- Download b-roll related to cholera here.
Media contacts
About unicef.
UNICEF works in some of the world’s toughest places, to reach the world’s most disadvantaged children. Across more than 190 countries and territories, we work for every child, everywhere, to build a better world for everyone.
Follow UNICEF on Twitter , Facebook , Instagram and YouTube
About Gavi, the Vaccine Alliance
Gavi, the Vaccine Alliance is a public-private partnership that helps vaccinate more than half the world’s children against some of the world’s deadliest diseases. The Vaccine Alliance brings together developing country and donor governments, the World Health Organization, UNICEF, the World Bank, the vaccine industry, technical agencies, civil society, the Bill & Melinda Gates Foundation and other private sector partners. View the full list of donor governments and other leading organisations that fund Gavi’s work here .
Since its inception in 2000, Gavi has helped to immunise a whole generation – over 1 billion children – and prevented more than 17.3 million future deaths, helping to halve child mortality in 78 lower-income countries. Gavi also plays a key role in improving global health security by supporting health systems as well as funding global stockpiles for Ebola, cholera, meningococcal and yellow fever vaccines. After two decades of progress, Gavi is now focused on protecting the next generation, above all the zero-dose children who have not received even a single vaccine shot. The Vaccine Alliance employs innovative finance and the latest technology – from drones to biometrics – to save lives, prevent outbreaks before they can spread and help countries on the road to self-sufficiency. Learn more at www.gavi.org and connect with us on Facebook and Twitter .
Related topics
More to explore, teachers wanted.
Empowering teachers at the forefront of the learning crisis
Global deployment of rapid diagnostic tests to boost fight against cholera
Six grave violations against children in times of war.
How children have become frontline targets in armed conflicts
Millions at risk from cholera due to lack of clean water, soap and toilets, and shortage of cholera vaccine
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Perspect Clin Res
- v.4(1); Jan-Mar 2013
Current topics in research ethics in vaccine studies
Prasad s. kulkarni.
Serum Institute of India Ltd, Pune, India
About 7.6 million children under the age of five die every year, according to 2010 figures,[ 1 ] out of these 2.4 million children die from vaccine preventable diseases.[ 2 ] The problem is compounded by the absence of effective therapies for many infectious diseases. Obviously, new, more cost-effective and improved vaccines are needed today and in the future.
Vaccines have some distinct features than drugs. Unlike therapeutic molecules, vaccines have preventive role against specific infectious diseases. The target population is healthy people, mostly children and infants; as a result, tolerability of adverse events is less. Additionally, vaccines are highly complex substances derived from living microorganisms and their quality and safety needs to be demonstrated on a lot-to-lot basis. Naturally, these factors have some bearing on the clinical trials of vaccines. Here we discuss some of the current ethical issues in vaccine clinical trials.
Pediatric trials
Most of the vaccine studies are conducted in children, some of them in infants and even in newborns because that is where you want to catch them for prevention of an infection. However, children by themselves are unable to consent, and the vaccinator has to accept a legal guardian's agreement. Also, one would expect children to experience more adverse reactions than adults. For these and many other reasons, it is generally agreed that vaccine studies are, at least primarily, unethical in children if the relevant investigation can be done among adults. The main problem here is, however, that many infections are characteristically only pediatric diseases, or at least, those infections are specially harmful to the youngest.
One therefore needs to seek for a difficult balance between the true and ostensible need of a vaccine in the pediatric population. The CIOMS rightly states that “Before undertaking research involving children, the investigator must ensure that-the research might not be equally well be carried out in adults; and the purpose of the research is to obtain knowledge relevant to the health needs of children.”[ 3 ]
Parental consent
More in developing countries than elsewhere, parents or guardians of children may have little or no understanding of research trials. They may be unfamiliar with concepts such as “informed consent” and “confidentiality” and may not understand the scientific terms and processes involved in trials, including the use of randomization and placebos. Yet these parents will be called upon to give consent on behalf of their small children, or to explain to their older heirs (children) what is happening in the trial.
Another concern is consent of an appropriate legal representative in the absence of parental consent. Recently a demonstration project on a vaccine was conducted in India. An investigation was prompted after press reports of some deaths. Though the deaths were not found caused by the vaccine, consent obtained from hostel wardens in some subjects living in hostels was questioned.[ 4 ]
Need for the trial
Before launching a trial in children one must show that there is compelling need to use children to establish safety, immunogenicity, effectiveness or efficacy of the vaccine. Such a trial would not be justified if the child comes from population in which that particular disease is not a problem. Malaria vaccine cannot be tested soon in Europe or North America.
An absolute care must be taken to ensure that socioeconomic inequalities between industrialized and developing countries are not exploited i.e., that children in a poor country are not asked to undertake risks to produce a vaccine that, for economic or other reasons, would primarily benefit their counterparts in industrialized countries. At the same time, research should not be impeded that aims to reduce the inequality of health care and to benefit pediatric populations in need in developing countries.
Selection of control
If a good vaccine is already in use in some other country or community which is more or less comparable to site where the trial is planned, that vaccine should be used as the comparator. If such a vaccine does not exist, a placebo “vaccine” may be used, provided the set-up is thoroughly explained to the participants, their families and the community. Placebo controls are ethically acceptable when there is no proven vaccine for the indication for which the candidate vaccine is to be tested.[ 5 , 6 ]
A modification of this setting is that the placebo recipients receive the true vaccine later–but all this has to be explained in understandable words to the participants.
An alternative to the use of placebo is to give another vaccine that provides comparable benefit against another disease, or more willingly, against similar disease caused by different agents. This was the approach in Finland in the 1970s, when the first vaccines against bacterial meningitis (due to Neisseria meningitidis and H. influenza) were tested in children.[ 7 ] Here it was important these two types of meningitis were equally common in that community. For some vaccines, the choice is not difficult since there are no effective interventions so far, e.g., malaria or HIV vaccines.
In Indonesia, an exceptional approach was taken on 1998-2002.[ 8 ] Half of children received traditional DTP (diphtheria-tetanus-pertussis) vaccine, whereas the other half of children got DTP with H. influenza type B (Hib) component. Thus, all children were not in an equivalent position, but the setup was considered justified because in the absence of disease burden data and vaccine efficacy data in the region, the trial was deemed helpful for the decision whether or not to introduce Hib vaccination in Indonesia and the whole region.
When, instead of clinical efficacy, “only” immunogenicity (antibody production) is measured, the rules of equipoise are looser. The comparator vaccine may function more as “compensation” to the child in the trial's control arm. For instance, meningococcal C conjugate vaccine in a pneumococcal vaccine trial, or rabies vaccine in a Japanese encephalitis vaccine trial does not restore equipoise but benefit the child who would not otherwise receive that vaccine.
Age de-escalation
Age de-escalation means that phase I and II trials are conducted first in adults, then in older children, and finally, if relevant, in small children. Epidemiology of the disease, the risks/benefits of the vaccine for each age group, and the safety profile are all factors to be taken into account in de-escalation.
However, if a new vaccine is only for infants, trials in older children may expose them to unnecessary risks without giving any benefit to these too “old” vaccinees. Rotavirus vaccines are good examples in this category. Sometimes adult participants can be used in the first trials, although they are of no help in the efficacy trials.
Sometimes there are grounds to use child participants already in phase I trials. This is the case if the new vaccine would likely cause problems in adults (but not in children) because of prior immunity in adults e.g., DTP vaccine.
Participation of adolescents
Only a few vaccines as targeted just for adolescents: examples are human papillomavirus (HPV) and herpes virus (HSV) vaccines. However, adolescents may be used in the de-escalation studies before progressing to small children. The participation of adolescents often involves complex legal, ethical issues and operational issues.
Informed consent is problematic, because adolescents often have the intellectual and emotional capacity to provide consent, but do not have the legal right to consent. Also their views may not be the same as their parents’ views, and appropriate confidentiality can be difficult to maintain. An extreme would be a situation in which the youth disagrees but the parents agree the trial, or in which a willing adolescent would be included in the absence of parental consent.
The participation of adolescent girls is further complicated by the potential or soon materializing pregnancy. Not only would it perhaps risk the young mother and the fetus, but also raise complex issues regarding the consent, confidentiality and legal liability. Routine pregnancy testing of adolescent girls prior to the inclusion in a trial would also have its cultural problems.
Limitations of informed consent
Obtaining informed consent in a developing country has its own problems and should be seen as a process which begins from the voluntary decision to participate in the study. The decision should be based on sufficient information prior to the trial entry. The informed consent form should be simple enough to be understood by the often not-too-educated individual, or in case of a child, by parents or legal guardian, but still comprehensive to explain the concepts, potential risks and benefits, implications of the use of a placebo or other comparator, care that will be provided, and the indemnity for injury or death arising from the trial. Importantly, it must be stated clearly that a withdrawal from study is allowed at any time without giving an explanation for the decision. If the circumstances of the trial change significantly, the consent form is to be changed accordingly, and the whole study warrant discussion with the already enrolled participants. Another consent is then to be obtained.
The problems in getting valid consent are heightened in developing countries where people may be unfamiliar with scientific research, concepts and vocabulary. Thus, the expectations may be unrealistic. Also the individual's full autonomy might become endangered because of the society's cultural and/or gender norms, or the family or spousal pressure. All of these challenges are further complicated when the trial deals with children.
Child's assent
In the case of a child, every effort should be made to explain to him/her also, in language that is understandable to the child, what the participation means, as regards to potential risks (discomfort, time spent, etc.) and benefits, The investigators should document the child's assent.
Community consent
Since an informed consent may be culturally sensitive, family or community discussions are sometimes necessary, albeit the community consent should not be considered as a substitute for the individual consent. There may also be tension between the ethical responsibility to maintain individual confidentiality, and cultural norms that press for “shared confidentiality”. Within appropriate boundaries of confidentiality, it may be useful to have an impartial witness/observer present during an oral consent particularly if verbal rather than signed consent is sought. Such witnessed consent must be recorded in the trial files.
Potential for inducement
The improved medical care provided during the trial may constitute an inducement and may impact on the willingness to participate. Indeed, trial participants often accept the trial in the belief that they will receive improved treatment. It is important to explain that participation will not necessarily ensure protection against disease. In case of a study using placebo, the entire set-up and the meaning of randomization should be explained, including the fact that the participant might fall in the placebo group. Any care or other benefits that perhaps are offered should be described.
Another concern is if the parents see an opportunity for economic benefits, they may encourage enrolling their and perhaps other children in trials in which those should not necessarily be included. All efforts should be made to avoid any exploitation, and to minimize all mental, emotional and physical harm.
Standard of care
In case of vaccine trials in developing countries, the situation is tricky because of a high burden of disease and low standards of health care in that community. With the contribution of local authorities, a standard of care should be offered. This means an improvement in the health conditions of participants, and that it is sustainable. These efforts need an approval from the local ethics committees.
Duration of follow-up
An active follow-up should extend at least to the end of the trial. In case of an adverse effect, the, follow-up should be continued for an additional six months. In high mortality populations, it may be desirable to analyse long-term mortality changes and to follow-up participants for a number of years. Passive follow-up is advisable even longer, and if existing mechanisms can be used for this purpose.
Long-term follow-up may complicate a trial substantially and greatly increase the costs. Therefore, gathering only passive data may suffice. Creative follow-up should be contemplated, both for safety and long-term protection. The high titer measles vaccine was studied in some African countries, however on a long term follow up, it was discovered that female mortality was higher following the vaccine,[ 9 ] which resulted in abandoning the use of the vaccine. This important finding was detected only because of long-term follow-up.
Screening of subjects
Vaccine trials need to be conducted in healthy people and hence, the screening for inclusion/exclusion criteria is very critical. Enrolment of children with underlying medical conditions can complicate the safety outcomes. A recent vaccine trial in India brought forth this issue. A death was reported in the study after an infant had received a licensed vaccine used as a control. The investigation revealed that the infant who died had a pre-existing medical condition.[ 10 ] It is recognized that physical screening of young infants has limitations; however, every effort should be made to ascertain the health status. In case of suspicious cases, it is better to err on the safer side.
CONCLUSIONS
Vaccine clinical research needs to deal with certain ethical issues because of the inherent nature of these trials. The issues are more complicated since the research mostly happens in pediatric populations in developing countries. Keeping in mind these issues while designing research on vaccines is critical.
Source of Support: Nil
Conflict of Interest: None declared
A one-shot vaccine for COVID, flu and future viruses? Researchers say it's coming
New rna-based vaccine strategy could be a breakthrough: no boosters, no needles and far more rapid effects, by nicole karlis.
At the beginning of the pandemic, many people hoped that infections with SARS-CoV-2, the virus that causes COVID-19 — or vaccines against the virus — would provide durable lifetime immunity, as is the case with diseases like measles or mumps. Instead, the COVID virus is more akin to the influenza virus, which mutates constantly and confers only short-term immunity . Both COVID and the flu require new and different vaccine formulas aimed at defeating newly circulating variants of the viruses. The inevitable result of this has been, for most of us, increasing vaccine fatigue.
But what if it were possible to protect against COVID and the flu, and other unknown viruses that haven't yet emerged, with just one shot? If that became reality, seasonal or annual boosters would be part of the past. And what if such vaccinations didn't even require a needle?
While those possibilities may sound far in the future, scientists at the University of California, Riverside, believe they could become reality relatively soon — perhaps within the next five to 10 years. As illustrated in a paper just published in the Proceedings of the National Academy of Sciences , a new, RNA-based vaccine strategy could be effective against any viral strain to emerge in the future. This next generation of vaccines would theoretically offer protection against viruses we aren’t even aware of yet, and could be used safely on infants and people with compromised immune systems, who today must often opt out of vaccination to protect their health.
This new RNA-based technology, the research paper reports, would target a part of the viral genome that is common to all strains of any virus and would depend on a “second immune system” response.
“We have a very strong reason to believe that all these other human viruses, like dengue virus and COVID-19, produce a protein that we can target to make a vaccine,” Shouwei Ding, distinguished professor of microbiology at UC Riverside and lead author of the paper, told Salon in a phone interview. With any future virus, "all we need to do is to identify the protein that can suppress RNAi.”
Here's what Ding was talking about. Traditional vaccines work by training the body to recognize and combat specific molecules found on a particular pathogen. For example, live-attenuated vaccines often use a weakened form of a virus to train the immune system. Once the weakened form of the virus is in the body, the immune system learns to recognize the antigen and develop immunity to it.
Another type of vaccine, based on "viral vectors," uses DNA and RNA to give cells a blueprint, rather than a piece of the pathogen itself, to build immunity. MRNA vaccines, like the best-known vaccines against COVID-19, use a synthetic version of single-stranded RNA to create a bespoke version of the mRNA within the body. This creates cells that can produce proteins like those found in a virus, and which then train the immune system to fight a disease before it enters a person’s bloodstream.
We need your help to stay independent
The new vaccine technology proposed in this paper would still use a live, modified version of a virus. But its effectiveness would not depend on the body's traditional immune response, which produces T-cells and memory B-cells. Instead, it would produce proteins that block a pathogen’s RNAi response, which is something all viruses create.
Researchers tested their theory in mice with a virus called Nodamura. The mice lacked T and B cells, but after one injection with the test vaccine, the mice were protected against the virus for at least 90 days.
This new vaccine tech could be key to fighting bird flu, researchers say: "We are actively seeking funding to do just that.”
In 2013, this same group of researchers at UC Riverside published a paper showing that flu infections also cause people to produce RNAi molecules. Ding said their next step will be to generate a universal, one-time-use influenza vaccine that would be safe for very young infants. Current flu vaccines are only recommended for infants over the age of six months. Furthermore, this new vaccine would likely be delivered as a spray. As Salon has previously reported, vaccines that don’t require needles may one day become standard .
This intriguing report arrives at a moment when the bird flu virus, known as H5N1, has reportedly begun to spread among cattle. There has also been at least one confirmed human case. As Salon has reported , infectious disease experts do not expect bird flu to become a pandemic this year, that's a definite possibility in the future. One virologist told Salon she would recommend public vaccination against bird flu right now. No avian flu vaccines have yet been approved for use in humans, however, although several are under development , none have been approved for use in humans yet.
Want more health and science stories in your inbox? Subscribe to Salon's weekly newsletter Lab Notes .
Ding said the vaccine his team is developing could be a contender: “That's what we're aiming for. We are actively seeking funding to do just that.”
Among the additional implications of this new vaccine technology, Ding said, could be more rapid protection than is now typical. “What we find is that two days after the shot, you are already fully protected,” he said. With current vaccines, "it will often take two weeks or more to be effective, and that's not very good for an emerging infection.”
Ding said his team anticipates having a vaccine candidate ready for human clinical trials in about a year. After that, the traditional regulatory would likely take 5 to 10 years — although a new public health emergency, like the COVID pandemic, could speed that up considerably.
about vaccines and viruses
- Do COVID-19 vaccines really have worse side effects than other vaccines? Here's what experts say
- US ranks last among peer nations for COVID-19 mortality: study
- Does your immune system need a workout? The bad science behind "immunity debt," explained
Nicole Karlis is a senior writer at Salon, specializing in health and science. Tweet her @nicolekarlis .
Related Topics ------------------------------------------
Related articles.
- Work & Careers
- Life & Arts
Become an FT subscriber
Try unlimited access Only $1 for 4 weeks
Then $75 per month. Complete digital access to quality FT journalism on any device. Cancel anytime during your trial.
- Global news & analysis
- Expert opinion
- Special features
- FirstFT newsletter
- Videos & Podcasts
- Android & iOS app
- FT Edit app
- 10 gift articles per month
Explore more offers.
Standard digital.
- FT Digital Edition
Premium Digital
Print + premium digital, weekend print + standard digital, weekend print + premium digital.
Today's FT newspaper for easy reading on any device. This does not include ft.com or FT App access.
- 10 additional gift articles per month
- Global news & analysis
- Exclusive FT analysis
- Videos & Podcasts
- FT App on Android & iOS
- Everything in Standard Digital
- Premium newsletters
- Weekday Print Edition
- FT Weekend Print delivery
- Everything in Premium Digital
Essential digital access to quality FT journalism on any device. Pay a year upfront and save 20%.
- Everything in Print
Complete digital access to quality FT journalism with expert analysis from industry leaders. Pay a year upfront and save 20%.
Terms & Conditions apply
Explore our full range of subscriptions.
Why the ft.
See why over a million readers pay to read the Financial Times.
International Edition

IMAGES
VIDEO
COMMENTS
Thesis Summary . Vaccines are one of the world's most impactful medical therapies. They are cost-effective, successfully proven, and one of the quickest treatment options available today (Clark ... others of health-related topics and empower them to make their own informed health decisions. This passion directly translates into the topic of ...
Two doses of the vaccine or placebo were given 21 days apart to the respective groups. 21 The mean age of the participants was 45.3 years, and the majority of participants were Caucasian ... Studies on these topics are rapidly being conducted and published on a global scale, and scientific communities are working on the clock to produce as much ...
Vaccines work by exposing a subject to part of or a whole pathogen, thus activating the immune system described above. They play a critical role in global health by preventing infection and transmission of multiple diseases worldwide [4]. The World Health Organization estimates that vaccines prevent the death of 2-3 million people every year [5].
As with hepatitis and H. influenzae vaccines 26,27, interchangeability has been an active topic of debate with coronavirus mRNA vaccines which require a second shot for full immunity. Our research ...
Frontline health care personnel (HCP) were among the first to receive the COVID-19 vaccines. Physicians, nurses, allied health professionals, and others with direct patient contact or who handle biological materials are at high risk of exposure and illness and have duties of care and protection to patients, coworkers, and communities. Yet well into 2021, some HCP remain vaccine hesitant, an ...
Article. Vaccine hesitancy (VH) is considered a top-10 global health threat. The concept of VH has been described and applied inconsistently. This systematic review aims to clarify VH by analysing ...
uncertainty around the virus and consequently the vaccine. This thesis aims to explore nurses' decision-making around COVID-19 vaccination, with a specific focus on the role of uncertainty both about the pandemic and available vaccines. In what follows, this study first reviews the state of the COVID-19 pandemic, and specifically the
Vaccines are a public health success story, as they have prevented or lessened the effects of many infectious diseases. To address concerns around potential vaccine injuries, the Health Resources and Services Administration (HRSA) administers the Vaccine Injury Compensation Program (VICP) and the Countermeasures Injury Compensation Program (CICP), which provide compensation to those who assert ...
Vaccine hesitancy is a long-known issue, and defined by the World Health Organization (WHO) SAGE Working Group(1): "Vaccine hesitancy refers to delay in acceptance or refusal of vaccination despite availability of vaccination services. Vaccine hesitancy is complex and context specific, varying across time, place, and vaccines.
With the vaccine for COVID-19, different anti-vaccine narratives are being created and are probably being adopted by large population groups with critical consequences. Assuming full adherence to ...
Brief History of Vaccine Development. Human use of preparations to prevent specific infections have been described since 1500 AD, beginning in China (Needham, 2000) where smallpox was prevented by variolation, which is the introduction of material from scabs into the skin.In 1796 in the United Kingdom, Edward Jenner observed the immunity to smallpox of milkmaids having previously had natural ...
A study of how people perceive the COVID-19 vaccine compared to other historic vaccines, based on surveys of University of South Dakota students and faculty.
This thesis examines attitudes towards and ethics of receiving one of the fastest vaccines ever developed— the COVID-19 vaccine. The Food and Drug Administrations (FDA) in the U.S. has granted either Emergency Use Authorization or full approval to three vaccines: the Pfizer-BioNTech, Johnson & Johnson, and Moderna-NIAID vaccines. However, although the FDA approved and the Center for Disease ...
Vaccine mandates risk treating individuals as a means to an end and risk running afoul of the second categorical imperative. Utilitarian advocates would argue for a vaccine mandate as it provides the greatest well-being for the most people possible. The utilitarian argument that vaccine mandates are doing the best for the most falls flat when ...
Acceptance of vaccines is an important predictor of vaccine uptake. This has public health implications as those who are not vaccinated are at a higher risk of infection from vaccine preventable diseases. ... Walden Dissertations and Doctoral Studies; 1718. Umeh GC, Nomhwange TI, Shamang AF, Zakari F, Musa AI, Dogo PM, et al. Attitude and ...
Vaccination Behavior Research Topics. Unraveling demand and supply effects on the up-take of influenza vaccinations. Point out new approaches to the seasonal flu vaccine. Exploring the impact of vaccination. Investigating patient experience with, and the use of, an electronic monitoring system to assess vaccination responses.
This dissertation employed state-of-the-art machine learning models to collect the first dataset of vaccine misinformation from Twitter that was disseminated from January 2018 to April 2019. Out of 1,721,528 vaccine-related tweets, it was estimated that 15% were not credible, 11% were not supported by evidence, and 18% were considered propaganda.
misinformation corrections, this dissertation also explored the effects of correcting both Covid-19 and flu vaccine misinformation on Twitter using a field experiment approach. This dissertation aims to advance both the knowledge on the most effective correction methodologies and the mechanisms of how corrections work in the field of vaccine
CDC revealed that HPV vaccination coverage among females ages 18 to 26 years was. 17.1% (CDC, 2011). In 2013, girls ages 13 to 17 years who received at least one dose of. HPV vaccine was 57.3%, whereas 91.3% of girls age 13 years would have received at.
WASHINGTON — A new report from the National Academies of Sciences, Engineering, and Medicine reviews evidence for 19 potential harms of the COVID-19 vaccines, and for nine potential shoulder injuries from intramuscular administration of vaccines more broadly. The committee that conducted the review identified sufficient evidence to draw 20 conclusions about whether these vaccines could cause ...
Scroll down the page to view all COVID-19 articles, stories, and resources from across NIH. You can also select a topic from the list to view resources on that topic. - Any -. Aging. Cancer. Children. Clinical Trials. Immune Responses. Long COVID.
Discussion: HPV vaccine offers unprecedented potential to prevent HPV cancers but is underutilized in the US. Policy-makers seeking to reduce cancer disparities by area poverty should prioritize increasing HPV vaccine uptake as part of a multi-component strategy including addressing care access and social determinants of HPV cancer.
Traditionally, the most effective way to prevent viral transmission is with a vaccine, but an effective HIV vaccine for wide spread use has not been developed. Therefore, alternative HIV transmission prevention strategies have been used. These strategies largely depend on behavioral modifications and include: 1) utilization of universal ...
"Most vaccines use an attenuated or inactivated form of the virus they're designed to combat. DNA vaccines are more advanced. The technology has evolved over the past 30 years to become a powerful ...
In wealthier countries, the ethical issues that surround vaccination tend to focus on the rights of individuals versus government or society. In poorer countries, the fundamental issue is the lack ...
Some people with deep-rooted beliefs on a wide range of health topics - from COVID vaccination to mental health stigma to foods including genetically modified organisms - could be persuaded to ...
GENEVA/NEW YORK, 18 April 2024 - Gavi, the Vaccine Alliance and UNICEF welcome the news that a new oral cholera vaccine (OCV), Euvichol-S, has now received WHO prequalification and can be made available to countries around the world. The prequalification of this new product will help EuBiologics, the manufacturer, produce more volumes of vaccine, faster, and at a lower cost - a key step to ...
Current topics in research ethics in vaccine studies - PMC. Journal List. Perspect Clin Res. v.4 (1); Jan-Mar 2013. PMC3601712. As a library, NLM provides access to scientific literature. Inclusion in an NLM database does not imply endorsement of, or agreement with, the contents by NLM or the National Institutes of Health.
The new vaccine technology proposed in this paper would still use a live, modified version of a virus. ... Related Topics -----Covid-19 Flu Medicine Public Health Reporting Vaccines Virus. Related ...
Sales of its Covid-19 mRNA vaccine, its only approved product, are projected to total $3.9bn this year, according to a consensus of analysts' estimates, down 79 per cent from peak sales of $18 ...