- Member Login
- About The Project
- Case Studies

Case Study 3 – Palliative and End-of-Life Care
Click here to review the draft palliative and end-of-life care – interactive case study..
The following case vignette provides key concepts that could be considered when developing a plan of care for a patient who may require a controlled substance to manage their health concerns. As with any clinical situation, there are many patient variables that must be considered, including comorbid conditions, social determinants of health and their personal choices. You may choose to include different or additional health history and physical examination points, diagnostic tests, differential diagnoses and treatments depending on your patient’s context however this case vignette focuses on the aspects relevant to controlled substances.
Danny Kahan NP-Adult, specialty is palliative care Joshi Kamakani – 70 year old male with metastatic prostate cancer June Kamakani – patient’s wife Kelli Kamakani – patient’s 40 year old daughter
Danny is reviewing the patient history outside the house or in the car before visiting the patient.
Joshi Kamakani is a 70 year old retired engineer that the Palliative Care home care team and I have been looking after at home for the last two months. Joshi was diagnosed with inoperable prostate cancer three years ago and has been treated with ablative hormone therapy. Six months ago, Joshi started to have pain in his hips. His oncologist ordered a CT scan and found he had metastases in his ribs, pelvis and lumbar spine. Joshi and his wife June had a meeting with the team at the cancer centre and decided not to go ahead with any further cancer treatment. Our team has been involved since. June called me yesterday and asked me to make a home visit. Joshi has been having more pain this week and has been spending most of his time on the couch. He cannot get around without assistance and is very fatigued.
Joshi’s past medical history includes hypertension and reflux. He is taking Predisone 5 mg PO BID, Leuprorelin Depot 22.5mg IM every 3 months, hydrochlorothiazide 25 mg daily and pantoprazole 40 mg daily.
For pain, Joshi takes Morphine slow release 100 mg q12h and has not needed additional medication for breakthrough pain so far.
Takes place in the home. Patient is seen reclining on couch in first floor living room. Wife and daughter present.
Danny rings the doorbell and June lets him in.
June: Hi Danny. I’m so glad you’ve come.
Danny takes off his coat and shoes and walks into the living room. Kelli is sitting with her father who is covered up with a blanket on a couch in the main living area – he is awake but obviously drowsy. He smiles at Danny and holds out his hand. Danny shakes it a sits down in a chair opposite.
June: His pain killers just are not working any more – he’s uncomfortable when he is resting and it’s worse when he has to move around. It’s been happening for the last few weeks. He hasn’t had a fall but he is unsteady on his feet – especially soon after he get up. Joshi: I tried some acetaminophen from the drug store a few days ago but it really didn’t work. Kelli: Danny, you have to do something. He’s so uncomfortable. Danny: OK let’s talk about this a bit more. Joshi, were you sleepy after we increased the morphine 2 weeks ago? You were at 80 mg for each dose and now you are at 100 mg. Joshi: I was a bit sleepy for a few days and I had a bit of a weak stomach but that is gone now. I am a bit constipated though. Danny: when did you have your last bowel movement? Joshi: 4 days ago. Danny: OK we will have to address that today. I’d like to use the scale that I used at our last visit, it’s called the PPS, to assess your level of activity. ( Edmonton symptom assessment scale and Palliative Performance Score). Your PPS is 40% – last time I visited you were at 60%. June: yes, he is definitely having more trouble. I think the pain is preventing him from moving and that’s just making everything worse.
Danny: Joshi, your pain interference score tells me that the pain is severely interfering with your activity and I see that you are rating your current pain at rest at 6/10 and at 10/10 when you move. When I examined you, I did not note any changes from my last visit except for some new swelling over your left hip. June: Yes that’s where it is most sore – and before you ask, I am not going to the hospital for an xray. Kelli: Why can’t you just double his dose?
Danny [THINKS]: I will also add a bowel regime to address Joshi’s constipation and provide an order for a PRN anti-nauseant like metoclopramide or ondansetron. Joshi and his family will need to have education about the timeline of the peak benefit of the change in the regular dose, keeping track of PRN use, proper use of breakthrough medications (before care or any activity that causes pain), any other interventions we can include to help with his pain including adding other medications.
June: Danny, can I speak to you in private for a moment? June and Danny move to a private area of the house. Kelli and Joshi remain on the sofa. June: Danny, I have some concerns about having extra medication in the house and I need some advice on how to deal with this. My daughter had a real problem with drugs when she was in high school. She had to have treatment and as far as I know, she has been clean for the past 2 years. I have talked to her about having medication in the house and she tells me she’s not tempted but I really want to be sure we don’t have any incidents. I trust my daughter but I do worry that some things are beyond her control. Danny: Well June, it is always a good practice to have a plan for safe storage of medications. Here is some information about where you can purchase a locked box. I recommend you keep a key and have the hospice nurse take the other and have it numbered and controlled at the hospice office for the use of the nurses that care for Joshi. In the meantime, keep the medications in a place that you and Joshi can monitor and please keep a count of the medication in the containers and continue to write down when medication is given. June: Thanks Danny – I don’t want my daughter to think I don’t trust her. This should help.
Two weeks later – Danny is back in his office reviewing Joshi’s file with a Nurse Practitioner student…
Follow-up case question by Danny.
Student: Next up is Joshi Kamakani for review… Danny: Well, I’ve just been to see Joshi and his family. It has been two weeks since we increased his dose of morphine SR. We also added a neuropathic pain agent to help with his pain which has made him a bit more drowsy. He continues to take 20-30 mg breakthrough morphine/day and I noticed today that he has some myoclonus. Joshi’s pain is still in the moderate range with activity and now nausea is a problem.
Opioid rotation and opioid equianalgesia from NOUGG (McMaster Guidelines).
Danny: I think a rotation of opioid is the next step. Student: What medication should Danny consider and at what dose?
Danny: Joshi is using 270 mg oral morphine equivalents per day. To convert this dose to hydromorphone, the medication I have chosen to rotate to, we multiply by 0.2. Morphine 270 mg x 0.2 = 54 mg hydromorphone/day. We will want to convert 60% of the total daily dose so 54mg x .6 = 32mg. I want to give Joshi the new dose in a slow release form. It is most practical to provide Joshi with hydromorphone SR 15mg q12h and also provide him an additional 2-3 mg of hydromorphone immediate release for breakthrough pain. Providing him with the breakthrough dosing will be sure Joshi can have additional medication to help him until we are sure we have a stable, effective dose in 48-72 hours.
Learning Outcome
This interactive case study covered the following information:
- Opiate Titration
- Opiate Rotation
- Pain Assessment
- Assessment of adverse effects
- Safety Assessment
- Collaboration
- Family centred care
Building public engagement and access to palliative care and advance care planning: a qualitative study
Affiliations.
- 1 Institute of Nursing and Health Research, Ulster University, Belfast, BT15 1AD, Northern Ireland.
- 2 Institute of Nursing and Health Research, Ulster University, Belfast, BT15 1ED, Northern Ireland.
- 3 Institute of Nursing and Health Research, Ulster University, Belfast, BT15 1AD, Northern Ireland. [email protected].
- PMID: 38605315
- DOI: 10.1186/s12904-024-01420-8
Background: Research evidence suggests that a lack of engagement with palliative care and advance care planning could be attributed to a lack of knowledge, presence of misconceptions and stigma within the general public. However, the importance of how death, dying and bereavement are viewed and experienced has been highlighted as an important aspect in enabling public health approaches to palliative care. Therefore, research which explores the public views on strategies to facilitate engagement with palliative care and advance care planning is required.
Methods: Exploratory, qualitative design, utilising purposive random sampling from a database of participants involved in a larger mixed methods study. Online semi-structured interviews were conducted (n = 28) and analysed using reflexive thematic analysis. Thematic findings were mapped to the social-ecological model framework to provide a holistic understanding of public behaviours in relation to palliative care and advance care planning engagement.
Results: Three themes were generated from the data: "Visibility and relatability"; "Embedding opportunities for engagement into everyday life"; "Societal and cultural barriers to open discussion". Evidence of interaction across all five social ecological model levels was identified across the themes, suggesting a multi-level public health approach incorporating individual, social, structural and cultural aspects is required for effective public engagement.
Conclusions: Public views around potential strategies for effective engagement in palliative care and advance care planning services were found to be multifaceted. Participants suggested an increase in visibility within the public domain to be a significant area of consideration. Additionally, enhancing opportunities for the public to engage in palliative care and advance care planning within everyday life, such as education within schools, is suggested to improve death literacy and reduce stigma. For effective communication, socio-cultural aspects need to be explored when developing strategies for engagement with all members of society.
Keywords: ACP; Advance care planning; Death and dying.; Death literacy; Palliative care; Public engagement; Public health; Social Ecological Model.
© 2024. The Author(s).
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMC Health Serv Res

What is case management in palliative care? An expert panel study
Annicka g m van der plas.
1 Department of Public and Occupational Health, EMGO Institute for Health and Care Research, VU University Medical Center, Amsterdam, the Netherlands
2 Palliative Care Center of Expertise, VU University Medical Center, Amsterdam, the Netherlands
Bregje D Onwuteaka-Philipsen
Marlies van de watering.
3 Kennemer Gasthuis, Haarlem, the Netherlands
Wim J J Jansen
4 Department of Anaesthesiology, VU University Medical Center, Amsterdam, the Netherlands
5 Agora, National Support Center for Palliative Terminal Care, Bunnik, the Netherlands
Kris C Vissers
6 Department of Anaesthesiology, Pain, and Palliative Medicine, Radboud University Nijmegen Medical Center, Nijmegen, the Netherlands
Luc Deliens
7 End-of-Life Care Research Group, Ghent University and Vrije Universiteit Brussel, Brussels, Belgium
Associated Data
Case management is a heterogeneous concept of care that consists of assessment, planning, implementing, coordinating, monitoring, and evaluating the options and services required to meet the client's health and service needs. This paper describes the result of an expert panel procedure to gain insight into the aims and characteristics of case management in palliative care in the Netherlands.
A modified version of the RAND®/University of California at Los Angeles (UCLA) appropriateness method was used to formulate and rate a list of aims and characteristics of case management in palliative care. A total of 76 health care professionals, researchers and policy makers were invited to join the expert panel, of which 61% participated in at least one round.
Nine out of ten aims of case management were met with agreement. The most important areas of disagreement with regard to characteristics of case management were hands-on nursing care by the case manager, target group of case management, performance of other tasks besides case management and accessibility of the case manager.
Conclusions
Although aims are agreed upon, case management in palliative care shows a high level of variability in implementation choices. Case management should aim at maintaining continuity of care to ensure that patients and those close to them experience care as personalised, coherent and consistent.
Patients facing a life-threatening illness are likely to experience palliative care needs [ 1 , 2 ]. According to the World Health Organization (WHO), palliative care aims at improving the quality of life of patients and their families, through the prevention and relief of suffering by means of early identification and impeccable assessment and treatment of pain and other problems, physical, emotional, and spiritual [ 3 ]. Palliative care is complex care. Firstly because it demands attention to and knowledge of not only disease, pain and symptom management, but also a range of other non-medical issues from reimbursement structures to availability of social services and spiritual care [ 3 ]. Gaps in the general and specialist knowledge required by the health care provider must be filled by access to reliable knowledge from others. Secondly, communication plays a pivotal role; several professionals and informal caregivers across settings can be involved and round-the-clock continuity of information is necessary to deliver consistent care sensitive to rapidly changing needs. In 98% of their palliative care patients, Dutch General Practitioners (GPs) cooperate with at least one other caregiver, with a mean number of four [ 4 ]. In the Netherlands, about half of patients experience one or more transfers in their last month of life [ 5 ], implying the need for communication across settings at least at the start of the transfer period. This will probably be even more true in future with increasing life expectancy and a growing number of patients with multiple chronic diseases [ 6 ] resulting in, among other things, more health care needs and more need for the coordination of care.
Case management has developed as a means of ensuring continuity of care for patients with complex care needs. It is a heterogeneous term for care that consists of assessment, planning, implementation, coordination, monitoring and evaluation of the options and services required to meet the client's health and service needs [ 7 ]. It has been used for many years in psychiatry [ 8 ], among frail elderly people [ 9 ] and many other populations. There have been varying research results on its effectiveness. There are numerous models of and variations in ways of delivering case management [ 10 ]. Adding to the confusion is the multitude of names given to case management; care management, care coordination, disease management, and managed care being some of the most common in the nursing field. Most studies compare one application of case management with care as usual, there is little research comparing different models or applications of case management. It is difficult to compare studies due to differing methodologies and outcome measures, and unclear definitions and descriptions of case management [ 11 - 13 ]. Therefore, we conclude that based on current research, for most medical conditions there is no way of identifying the best model for delivering case management.
The same can be said for case management in palliative care. No reviews on case management in palliative care were found and there is no definitive evidence of its effectiveness in palliative care. Some positive results are reported. In a randomised trial among patients with advanced chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF) or cancer, case management resulted in increased patient satisfaction with care and the earlier development of advance directives [ 14 ]. In patients with advanced illness (mostly cancer) receiving case management, compared with a matched historical control group, hospice use and number of hospice days increased [ 15 ]. There appear to be variations in the application of case management in palliative care. Differences can be seen in target populations (e.g. cancer only [ 16 ] or a range of diagnoses [ 17 ]), whether principles of disease management should be integrated [ 18 ] or focus should be solely on terminal care [ 19 ], whether case management should be delivered by a multidisciplinary team [ 20 ] or not [ 15 ] and a broad range of other variations. Again, these studies cannot be compared, therefore, no conclusions can be drawn as to which application of case management should be preferred.
The question of how case management should best be delivered in palliative care is unanswered. The purpose of this study was to formulate the aims of case management and describe essential characteristics of case management in palliative care in the Netherlands, as perceived by experts. The expert panel procedure also gave insight into which topics there is consensus between experts and what are the main differences in opinion between them.
The RAND® / University of California at Los Angeles (UCLA) appropriateness method is developed to combine scientific evidence with the collective judgment of experts to yield a statement regarding the appropriateness of performing a procedure at the level of patient-specific symptoms, medical history and test results [ 21 ]. The aim of this method is to reach consensus on which medical procedures are appropriate in certain medical conditions and circumstances. With a modified version of this method it is possible to investigate whether there is consensus or disagreement for a diverse range of topics. In three written rounds we consulted experts to formulate and rate aims and characteristics of case management in palliative care. Purpose of round 1 and 2 was to formulate a list of aims and characteristics, in the third round experts rated the aims and characteristics on importance for successful implementation of case management in palliative care.
Expert panel
We invited 73 experts with experience in palliative care to participate in the expert panel: general practitioners, coordinators of palliative care networks, case managers working in palliative care, researchers and policy makers in palliative care. The perspective of district nurses was included in the expert panel through case managers and scientists in the field of nursing. Two experts declined but proposed four others to take their places and the colleague of another was added leading to the questionnaire being sent to 76 experts. Of those, 46 (61%) participated in at least one round. Twenty-four experts gave their reasons for not participating: lack of time (n = 13), lack of knowledge about case management (n = 7), prolonged illness (n = 4). Four reactions in the first round and two in the second were not traceable because they were returned anonymously. This study is exempt from approval from an ethics committee.
Selection of aims and characteristics
We drafted a first list of aims and characteristics of case management in palliative care based on information from existing initiatives, literature and previous research. We used four headings to partition our list of aims and characteristics: aims of case management in palliative care, characteristics of content of case management in palliative care, characteristics of structure of case management in palliative care and general conditions. The 16 characteristics in the fourth section, general conditions, related so commonly to care in general (e.g. 'the caseload is in ratio with the terms of employment of the case manager and the necessary time investment for individual patients') that they were omitted for the purposes of this paper.
For the aims of case management in palliative care, we made use of the conceptual framework of continuity of care by Bachrach [ 22 ]. She identified seven dimensions in continuity of complex care. The dimensions put together describe an ideal model for care in situations where several health care providers, settings and/or needs are involved. Case management does not necessarily incorporate all elements in itself, but its task is to make sure the patient receives continuity of care. Bachrach listed these dimensions specifically for people with long-term mental disorders, and we hypothesised that they would be useful as a starting point in identifying the aims of case management in palliative care. We reformulated the characteristics to reflect palliative terminology and discourse. Additional to the seven characteristics derived from Bachrach, we added two more, one specifically on palliative care (care or coordination of care is aimed at quality of life and death) and the other because the literature suggests that continuity of care across settings is problematic in palliative care [ 23 , 24 ] and we hypothesised that case management should pay special attention to that aspect. In Table 1 the dimensions of Bachrach and the aims of case management are reported.
Transformation of dimensions of continuity of care to aims of case management in palliative care sent to the expert panel for feedback in round 1
In three written rounds the experts were asked to formulate and rate aims and characteristics of case management in palliative care. In the first round we presented the first draft of the list of aims and characteristics and the expert was asked to add and remove some, give textual feedback and feedback on the aims and characteristics included. For readability characteristics were clustered around themes within the sections; aims of case management in palliative care, characteristics of content of case management, characteristics of structure of case management, and general conditions. In the second round we sent a new draft based on the respondents' feedback, with the same question. No reaction was required if the participant agreed with the content and formulation. In order to be rated independently in the third round, the clusters were then divided into separate characteristics (see Table 2 for an example). Thus, a list of 41 clustered aims and characteristics was divided into 104 separate aims and characteristics. In the third round the expert panel rated all aims and characteristics on a nine-point scale, a score of one indicating that the aim or characteristic was 'not important for successful implementation' and of nine that it was 'very important for successful implementation' of case management in palliative care.
Example of a clustered characteristic in round 2 and division into separate characteristics for round 3
Data analysis
We calculated the mean, standard deviation, median and median absolute deviation (M.A.D.) for all aims and characteristics. Agreement was calculated according to the procedure described by the RAND Corporation specifically designed for expert panels with more than nine participants [ 21 ]. Thus, according to the RAND criteria, for an aim or characteristic to be considered important for successful implementation of case management two requirements for agreement had to be met:
1) the expert panel agreed with the aim or characteristic, meaning that an aim or characteristic was scored 7 to 9 by 80% of participants,
2) the expert panel agreed with each other, meaning that the Interpercentile Range Adjusted for Symmetry (IPRAS) is larger than the Interpercentile Range (IPR). We used .30 and .70 percentile scores to calculate the lower and upper limit of the IPR.
All other results are categorised as 'disagreement'. We used the M.A.D. as an estimator of dispersion to assess the level of disagreement within the expert panel. This measure is less susceptible to outliers than the standard deviation. To distinguish between a high and a moderate level of disagreement we used a cut off score of M.A.D. = 2.0.
Round 1 and 2
In the first round we received 35 reactions on the aims and characteristics. In Table 3 the response is shown differentiated by the discipline of the participants. Main topics addressed by the experts on the first draft were: inclusion of informal caregivers (family, partner) in case management, communication and role delineation between the case manager and other health care professionals and the necessity of tailoring care to individual needs and wishes. Also, wording of the aims and characteristics was altered accordingly to feedback from the expert panel. This resulted in an adapted draft sent around for round two. In the second round we received 12 reactions on the adapted draft. The feedback on this draft mainly concerned suggestions for improvements in detail. The complete list of aims and characteristics for case management in palliative care formulated after round two is reported in Additional file 1 .
Background characteristics of respondents per round
1 Some responses could not be traced, we are not certain whether the two unknown respondents from round two did or did not respond in round one. The total number may be between 4 and 6.
2 Some responses could not be traced, we are not certain whether the two unknown respondents from round two are unique, so the number of persons with one or more responses is between 46 and 48.
In the third round we received 34 reactions from the expert panel. Table 4 shows that agreement was reached on 35 aims and characteristics. Overall, about a third of the aims and characteristics met with agreement (34%), almost half with a moderate level of disagreement (49%), and less than a fifth (17%) with a high level of disagreement. Both aims and characteristics which are met with agreement and with a high level of disagreement are marked in the Additional file 1 . There were no notable differences between experts from different backgrounds on rating the aims and characteristics (see the Additional file 1 for mean and median scores).
Scoring of the aims and characteristics by the expert panel
Aims of case management in palliative care
In section one on aims almost all aims were met with agreement (90%) and none with a high level of disagreement. The one aim with a moderate level of disagreement (Additional file 1 , aim 1.2) used the term ‘care on demand’ ('vraaggestuurd'), which is used by Dutch policy makers to indicate that the patient is central to care as opposed to 'care as supplied' ('aanbod gestuurd') which prioritises the habits, rules and regulations of the institution delivering it. This characteristic was added at the request of some of the experts because they felt that aim 1.4 on individual care did not adequately cover the aspect of care on demand. However, we received questions on this term (e.g. 'does this mean that care should not be proactive?') that made clear that the denotation of the term is not well known among the expert panel. At the same time we received feedback indicating that the expert panel agrees that the patient should be at the centre of care and that it should be tailored to the individual needs of the patient and aim 1.4 was met with a high level of agreement.
Content of case management in palliative care
In section two on content of case management most characteristics were met with a moderate level of disagreement (44%), while another 40% were met with agreement and a small proportion with a high level of disagreement (17%). Within this section the highest level of disagreement (M.A.D. = 2.33) was on nursing care tasks (characteristic 2.1.a). This stems from the opinion of some experts that the number of health care providers surrounding the patient should be kept as low as possible. The district nurse can perform case management next to other duties. Others believe that district nurses, due to their busy schedules, do not have time to offer patients adequate comfort, reassurance and information and this will take second place to their nursing tasks. Comfort, reassurance and information may also be needed by patients who are not yet using care from a district nurse.
Structure of case management in palliative care
In section three on structure of case management most characteristics were met with a moderate level of disagreement (63%), while 22% encountered a high level of disagreement and only 15% were met with agreement. Within this section there were three characteristics with a joint highest level of disagreement (M.A.D. = 2.24): whether the case manager should combine case management with other tasks (e.g. consultation) (characteristic 3.5.b), whether she or he should be accessible 24 hours a day, seven days a week (characteristic 3.8.a), and if the target group she or he works for includes all patients with a life-threatening disease (characteristic 3.7.c).
This study shows that agreement was high on the aims of case management. However, how case management should be implemented, and exactly which elements of care it should include, is more open for debate. Disagreement was highest on topics regarding whether the case manager should perform hands-on nursing care themselves or not, on the target group, on accessibility of the case manager and on performance of other tasks besides case management.
Strengths and limitations
This is the first study using a structured procedure to report on the importance of the aims and characteristics of case management in palliative care. The expert panel reflects the opinions of case managers, coordinators of palliative care networks, general practitioners and other physicians, researchers and policy makers. There were no marked differences between experts from different backgrounds on rating the aims and characteristics. However, these opinions not necessarily reflect practice and we lack information on how often and how case management is implemented in the Netherlands. Also, our results may only be representative for mixed public-private health care systems with a strong primary care gatekeeper that resemble the Dutch system. The characteristics of case management may be different in other health care systems.
The aims that met agreement are in accordance with the general principles of palliative care and also reflect the patient advocacy model of case management [ 25 ]. This model offers comprehensive coordination of services aimed at quality of care and is distinguished from the interrogative model, which is more focused on clinical decision-making and emphasises cost-effectiveness. The aims also underline the importance of the seven dimensions of continuity of care formulated by Bachrach for psychiatric care [ 22 ]. This conceptual framework appears to be valid for complex continuous care in general, whether it is psychiatric care or palliative care.
Translation from aims to content of care is apparently relatively straightforward, with 40% agreement and only 17% strong disagreement on what care should be included. Offering information and support, identifying needs and adjusting care to match the patient's needs are the main tasks of the case manager. This can also be seen in descriptions of case management in palliative care [ 20 , 26 ], for cancer patients [ 27 ] and in a Delphi study on case management for patients with dementia [ 28 ]. Delivery of hands-on patient care is the most important area of disagreement within the expert panel. As mentioned in the results section, this stems from task alignment between the district nurse and case manager and whether these should be two different people or not. Besides, this also touches on the discussion whether palliative care should be part of primary (generalist) care, delivered by specialised palliative care providers, or in a cooperation between the two [ 29 ]. Case management could be delivered in a multidisciplinary team taking over all care, or case management can be guiding and assisting the primary health care providers (GP and district nurse) in their care for the patient. Another notable topic of disagreement is whether case management should stop before bereavement support is provided. The panel agrees that bereavement support is part of palliative care, reflected in agreement with characteristic 2.18.c. and aim 3. Whether there can be other endpoints for case management may be related to the target group, which is also a point of disagreement for the expert panel (reflected by characteristics 3.7 a, b and c). In a mixed-method study on case management for cancer patients, there are two distinct case management trajectories for patients receiving curative care and those receiving palliative care [ 27 ]. For curative patients case management can be short-term and stops when information needs are met. The discussion on bereavement support may also be a reflection of the Dutch reimbursement system, where it is not financed by public means and therefore any time the case manager spends on delivering it is not compensated.
Translation from aims to structure of case management is apparently less straightforward, with only 17% agreement and 22% strong disagreement. Characteristics such as the target group and the accessibility of the case manager may reflect the scope and depth of the case manager’s task: when can she or he work with the patient themselves and at what point does she or he refer to another professional? In the aforementioned Delphi study on case management for patients with dementia, no agreement could be reached on similar topics [ 28 ]. Apparently, in correspondence with applications of case management in cancer [ 11 ], CHF [ 12 ] and dementia [ 13 , 28 ], also in palliative care there is no unique best way to deliver case management according to experts.
Case management in palliative care should aim at maintaining continuity of care to ensure that patients and those close to them experience palliative care as personalised, coherent and consistent. There is a high level of agreement about the underlying dimensions of continuity of care [ 22 ]. The most important issues in implementation preferences are defining the target group of case management, the performance of other tasks besides case management, accessibility of the case manager and delivery of hands-on nursing care by the case manager. Research into the feasibility of different options and their effects on implementation could help health care planners make informed decisions on the best way to deliver case management.
Competing interests
The authors declare that they have no competing of interest.
Authors’ contributions
AvdP participated in the design of the study, carried out the measurements, analysed and interpreted the data, and drafted the manuscript. BO-P conceived of the study, participated in its design and coordination and made substantial contributions to the data interpretation and writing of the paper. MvdW, WJ, KV and LD participated in design of the study, interpretation of the data and critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
http://www.biomedcentral.com/1472-6963/12/163/prepub
Supplementary Material
Aims and characteristics of case management in palliative care. This file contains a full list of all aims and characteristics of case management in palliative care, as formulated and rated by the expert panel. (PDF 129 kb)
Acknowledgements
The authors wish to thank ZONMw (grant number 80-82100-98-066) for their financial support. The funders had no role in data collection and analysis, selection of respondents, decision to publish, or preparation of the manuscript.
- Jaul E, Rosin A. Planning care for non-oncologic terminal illness in advanced age. IMAJ. 2005; 7 :5–8. [ PubMed ] [ Google Scholar ]
- McIlfatrick S. Assessing palliative care needs: views of patients, informal carers and healthcare professionals. JAN. 2007; 57 :77–86. doi: 10.1111/j.1365-2648.2006.04062.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sepulveda C, Marlin A, Yoshida T, Ullrich A. Palliative care: the World Health Organization's global perspective. J Pain Symptom Manag. 2002; 24 :91–96. doi: 10.1016/S0885-3924(02)00440-2. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Borgsteede SD, Deliens L, van der Wal G, Francke AL, Stalman WA, van Eijk JT. Interdisciplinary cooperation of GPs in palliative care at home: a nationwide survey in The Netherlands. Scand J Prim Health Care. 2007; 25 :226–231. doi: 10.1080/02813430701706501. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Abarshi E, Echteld M, Van den Block L, Donker G, Deliens L, Onwuteaka-Philipsen B. Transitions between care settings at the end of life in the Netherlands: results from a nationwide study. Palliat Med. 2010; 24 :166–174. doi: 10.1177/0269216309351381. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Van den Akker M, Buntinx F, Metsemakers JF, Roos S, Knottnerus JA. Multimorbidity in general practice: prevalence, incidence, and determinants of co-occurring chronic and recurrent diseases. J Clin Epidemiol. 1998; 51 :367–375. doi: 10.1016/S0895-4356(97)00306-5. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Commission for Case Manager Certification. Definition of Case management. Accessed 14-6-2010. http://www.cmsa.org/Home/CMSA/WhatisaCaseManager/tabid/224/Default.aspx .
- Dixon L, Goldberg R, Iannone V, Lucksted A, Brown C, Kreyenbuhl J. et al. Use of a critical time intervention to promote continuity of care after psychiatric inpatient hospitalization. Psychiatr Serv. 2009; 60 :451–458. doi: 10.1176/appi.ps.60.4.451. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bernabei R, Landi F, Gambassi G, Sgadari A, Zuccala G, Mor V. et al. Randomised trial of impact of model of integrated care and case management for older people living in the community. BMJ. 1998; 316 :1348–1351. doi: 10.1136/bmj.316.7141.1348. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Huber DL. The diversity of case management models. Lippincotts Case Manag. 2002; 7 :212–220. doi: 10.1097/00129234-200211000-00002. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wulff CN, Thygesen M, Sondergaard J, Vedsted P. Case management used to optimize cancer care pathways: a systematic review. BMC Health Serv Res. 2008; 8 :227–234. doi: 10.1186/1472-6963-8-227. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Whellan DJ, Hasselblad V, Peterson E, O'Connor CM, Schulman KA. Metaanalysis and review of heart failure disease management randomized controlled clinical trials. Am Heart J. 2005; 149 :722–729. doi: 10.1016/j.ahj.2004.09.023. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pimouguet C, Lavaud T, Dartigues JF, Helmer C. Dementia case management effectiveness on health care costs and resource utilization: a systematic review of randomized controlled trials. J Nutr Health Aging. 2010; 8 :669–676. [ PubMed ] [ Google Scholar ]
- Engelhardt JB, Clive-Reed KP, Toseland RW, Smith TL, Larson DG, Tobin DR. Effects of a program for coordinated care of advanced illness on patients, surrogates, and healthcare costs: a randomized trial. Am J Manag Care. 2006; 12 :93–100. [ PubMed ] [ Google Scholar ]
- Spettell CM, Rawlins WS, Krakauer R, Fernandes J, Breton ME, Gowdy W. et al. A comprehensive case management program to improve palliative care. J Palliat Med. 2009; 12 :827–832. doi: 10.1089/jpm.2009.0089. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Seow H, Piet L, Kenworthy CM, Jones S, Fagan PJ, Dy SM. Evaluating a palliative care case management program for cancer patients: the Omega Life Program. J Palliat Med. 2008; 11 :1314–1318. doi: 10.1089/jpm.2008.0140. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Head BA, Lajoie S, Augustine-Smith L, Cantrell M, Hofmann D, Keeney C. et al. Palliative care case management: increasing access to community-based palliative care for medicaid recipients. Prof Case Manag. 2010; 15 :206–217. [ PubMed ] [ Google Scholar ]
- Aiken LS, Butner J, Lockhart CA, Volk-Craft BE, Hamilton G, Williams FG. Outcome evaluation of a randomized trial of the PhoenixCare intervention: program of case management and coordinated care for the seriously chronically ill. J Palliat Med. 2006; 9 :111–126. doi: 10.1089/jpm.2006.9.111. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Back AL, Li YF, Sales AE. Impact of palliative care case management on resource use by patients dying of cancer at a Veterans Affairs medical center. J Palliat Med. 2005; 8 :26–35. doi: 10.1089/jpm.2005.8.26. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Holley APH, Gorawara-Bhat R, Dale W, Hemmerich J, Cox-Hayley D. Palliative access through care at home: experiences with an urban, geriatric home palliative care program. J Am Geriatr Soc. 2009; 57 :1925–1931. doi: 10.1111/j.1532-5415.2009.02452.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fitch K, Bernstein SJ, Aguilar MS, Burnand B, LaCalle JR, Lazaro P, The RAND/UCLA Appropriateness Method User's Manual. 2001.
- Bachrach LL. Continuity of care for chronic mental patients: a conceptual analysis. Am J Psychiatry. 1981; 138 :1449–1456. [ PubMed ] [ Google Scholar ]
- Hauser JM. Lost in transition: the ethics of the palliative care handoff. J Pain Symptom Manag. 2009; 37 :930–933. doi: 10.1016/j.jpainsymman.2009.02.231. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Meier DE, Beresford L. Palliative care's challenge: facilitating transitions of care. J Palliat Med. 2008; 11 :416–421. doi: 10.1089/jpm.2008.9956. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Long MJ, Marshall BS. What price an additional day of life? A cost-effectiveness study of case management. Am J Manag Care. 2000; 6 :881–886. [ PubMed ] [ Google Scholar ]
- Head BA, Cantrell M, Pfeifer M. Mark's journey: a study in medicaid palliative care case management. Prof Case Manag. 2009; 14 :39–45. [ PubMed ] [ Google Scholar ]
- Howell D, Sussman J, Wiernikowski J, Pyette N, Bainbridge D, O'Brien M. et al. A mixed-method evaluation of nurse-led community-based supportive cancer care. Support Care Cancer. 2008; 16 :1343–1352. doi: 10.1007/s00520-008-0416-2. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Verkade PJ, van Meijel B, Brink C, van Os-Medendorp H, Koekkoek B, Francke AL. Delphi research exploring essential components and preconditions for case management in people with dementia. BMC Geriatr. 2010; 10 :54. doi: 10.1186/1471-2318-10-54. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gott M, Seymour J, Ingleton C, Gardiner C, Bellamy G. 'That's part of everybody's job': the perspectives of health care staff in England and New Zealand on the meaning and remit of palliative care. Palliat Med. 2012; 26 :232–241. doi: 10.1177/0269216311408993. [ PubMed ] [ CrossRef ] [ Google Scholar ]
Case-based learning: palliative and end-of-life care in community pharmacy
Community pharmacists encounter patients at all stages in their life; however, patients who require palliative care require dedicated time and special consideration.

JL / Shutterstock.com
The World Health Organization (WHO) defines palliative care as an approach that improves the quality of life of patients and their families facing the problem associated with life-threatening illness. This is achieved through the prevention and relief of suffering by means of early identification and impeccable assessment, and treatment of pain and other problems [1] .
Palliative care is often considered to be only for patients with cancer, but it also incl udes patients with conditions such as organ failure (e.g. heart failure , COPD, renal and hepatic failure), neurological conditions (e.g. multiple sclerosis , Parkinson’s disease and motor neurone disease), dementia, frailty , stroke and HIV/AIDS [2] , [3] , [4] .
The same can be said when considering symptoms (see Table) [5] . Being symptom-free is one of the most important factors for patients when considering end-of-life care [6] . Pharmacists have much to offer, not only in providing medicines required for symptom management, but also supporting patients and their carers to receive the right care at the right time [2] , [7] , [8] .
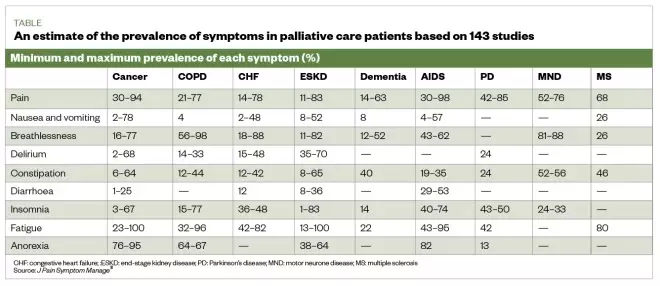
Table: an estimate of the prevalence of physical symptoms in palliative patients based on results from 143 studies
Source: J Pain Symptom Manage [5]
Symptom management
How symptoms are treated may change over time and may depend on many factors, including the symptom being treated, the patient’s ability to swallow (owing to disease process causing fatigue and weakness), and level of consciousness. Although most symptoms can be treated with oral preparations, it is likely that the oral route will become less available and a switch to parenteral preparations may be required.
The Gold Standards Framework recommends considering anticipatory prescribing of subcutaneous formulations for pain, nausea and vomiting, agitation/restlessness and excess respiratory secretions (also known as ‘death rattle’) [9] , [10] . The anticipatory prescribing of these medicines is part of advance care planning (i.e. the conversation between the patient’s families and carers about their future wishes and priorities for care) [11] , [12] .
The way symptoms are treated in patients receiving palliative care may change over time; however, several factors may influence this. For example, the Gold Standards Framework recommends considering subcutaneous formulations for:
- Nausea and vomiting;
- Agitation/restlessness;
- Excess respiratory secretions [12] .
Pharmacy schemes across the UK exist to act as stockists for locally selected anticipatory medicines, including alternative anti-emetics, opiates and steroids. See the Local Pharmaceutical Committee website for details (see Useful resources ). Be aware of the local pharmacies enrolled in these schemes, to ensure the pharmacy team can advise patients or carers if needed.
The Gold Standards Framework also suggests five steps for healthcare professionals to consider when advance care planning for patients, which are:
- Thinking about the future and what is important to the patient;
- Talking with family and friends about the patient’s choices so they are aware of them and can act as a spokesperson should the patient become unable to do this for themselves;
- Recording the patient’s wishes;
- Discussing plans with healthcare teams involved in the patient’s care (this may include conversations about resuscitation);
- Sharing information with the people who will need to be aware of it [12] .
An advance care plan can include many details, such as the desired extent of active treatment; the patient’s preferred place of care at the end of life; preferences for symptom management (e.g. the level of sedation a patient finds acceptable); people they would like to be present at end of life; spiritual and religious needs; and thoughts around funeral planning.
The Palliative Care Formulary provides excellent information and guidance on dosing and routes, which are often outside of the product license [10] . The National Institute for Health and Care Excellence (NICE) has also published several documents to support healthcare professionals in their approach to palliative care [13] (see Useful resources ).
Role of the pharmacist
Towards the end of a patient’s life, drug treatments should be as minimised when possible, with pharmacists reviewing and advising on deprescribing [7] , [14] . Pharmacists should also support patients in fulfilling their wishes to die in the place they choose with their symptoms well managed [8] .
The role of pharmacists in palliative care should be much greater than a supply function, although the importance of stocking anticipatory medicines should not be underestimated. Patients and their carers value more than the basic level of service from the pharmacy, and appreciate friendliness from staff who are familiar with them and can provide support with medicines and symptom management [15] .
The complex and often changing medicines regimens for palliative care patients can be challenging for medicines optimisation. Providing clear instructions on regimens and general communication can help (see Box 1 ) [10] . It is important to establish what medicines the patient is taking and when, and support and simplify their regimen if possible. For example, a patient is instructed to take paracetamol 1,000mg four times a day at 08:00, 12:00, 16:00 and 20:00, but wakes during the night with pain. Instead, the patient could be advised to take the paracetamol at equal intervals throughout the 24-hour period, (e.g. every six hours) [10] . However, this decision would need to take into consideration the patients waking hours and preferences.
The following case describes how pharmacists can assist patients nearing end of life with management of pain, nausea and constipation, and anticipatory prescribing.
Box: Considerations to take into account when communicating with palliative care patients and carers
Aims of communication :
- Information sharing;
- Reducing uncertainty;
- Facilitating choices and joint decision making;
- Creating, developing and maintaining relationships .
Getting started:
- Make time for the conversation, even if it is only for a few uninterrupted minutes;
- Ensure privacy;
- Introduce yourself;
- Indicate clearly that you have time to listen (e.g. offer to sit down with them);
- Use open questions to obtain information and allow the patient to talk (e.g. “what do you think this medicine is for?”);
- Engage using active listening (e.g. nodding and summarising to check information).
Be aware of non-verbal communication, such as:
- Facial expression;
- Eye contact;
- Posture (whether sitting or standing);
- Pitch and pace of voice;
- Use of appropriate touch.
Try to recognise barriers to good communication, for example:
- Language (patient’s own language or the use of jargon);
- Poor hearing, feeling generally unwell, fatigued, distracted by symptoms, fear and anxiety;
- Poor transfer of care information;
- Lack of time and privacy.
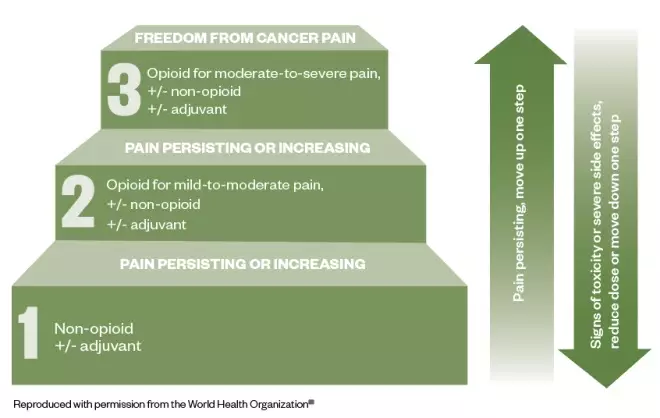
Figure: the three-step analgesic ladder
Source: World Health Organization [16]
This cancer pain management ladder provides a general guide to treatment based on the severity of pain
Palliative care part 1: pain management
Mary*, aged 54 years, comes into the pharmacy and asks to speak to the pharmacist about her prescribed medicines. She was recently diagnosed with metastatic pancreatic cancer, and her GP and palliative care team are working to manage her pain.
Two weeks ago, Mary was started on paracetamol 1,000mg four times per day and morphine sulphate liquid 5mg (2.5mL) every two to four hours as required. She was previously prescribed codeine 30–60mg four times per day, but this did not help to relieve her symptoms. On average, she has been taking six doses of morphine sulphate 5mg in each 24-hour period, and although this helps, it does not completely alleviate her pain.
Today, Mary has been prescribed morphine sulphate (MST; Napp Pharmaceuticals) 15mg twice daily and has been told to continue with her paracetamol and morphine sulphate liquid (for breakthrough pain). Mary explains that her GP said there may be a need to add a medicine for nerve pain. However, Mary goes on to say that she is worried that she is taking “too much” medicine and she is reluctant to take all of these medicines, especially if another may be added for nerve pain.
Actions and recommendations
The pharmacist should consider the following during the consultation:
- Communication — open questions should be used to determine how much has been explained to Mary about pain management. It may be that she has been given an explanation, but it is often worth covering these again;
- World Health Organization pain ladder — explain the use of the ladder (see Figure), including the need to use regular paracetamol;
- Analgesia — patients are often concerned by using more than one opiate. Patients may need to be encouraged to use their breakthrough analgesia when required, explaining how the dose is determined by the background analgesia may help [17] ;
- Keeping a diary — Mary should be encouraged to keep a pain diary. This can be a benefit as keeping a record of breakthrough doses may help when it comes to dose titration;
- Addressing patient concerns — explain to Mary that neuropathic agents may be used as an adjuvant if opiates fail to control the pain [18] ;
- Resources — recommend available online resources to Mary through the British Pain Society and the National Institute for Health and Care Excellence (see Useful resources) [17] , [19] .
Explain to Mary that as her opiate dose is increased, breakthrough doses will also increase. These should be one-sixth of the total daily opiate dose. For example, a dose of MST 30mg twice daily (60mg/24 hours), will require morphine sulphate (liquid or tablet) 10mg every 2–4 hours as required for breakthrough pain [10] , [14] .
When to refer
Mary should be referred back to her GP or the palliative care team if she requires frequent prescriptions for breakthrough medicines, which would indicate that her pain is not adequately managed, or she is experiencing unacceptable side effects (e.g. nausea or severe itch).
Palliative care part 2: nausea and constipation
Mary returns to the pharmacy ten days after starting on MST and her background dose is currently 30mg twice daily. She says that her pain is controlled, but she has been experienced nausea since the MST dose was increased two days ago. She also explains that she has not had “a proper bowel movement” for five days. Mary says she feels generally uncomfortable and has a feeling of fullness in her abdomen.
As a result, Mary is considering stopping the MST capsules. When questioned on her diet, Mary says she understands that dietary intake influences her bowels; however, she explains that her appetite is reduced owing to the abdominal discomfort. Mary would like to know if there is anything she can purchase that will help.
Constipation is the most commonly reported side effect of opiate treatment (up to 80% of patients) and can result in reduced quality of life and discontinuation of the offending medicine, which could result in a resurgence of pain [10] , [20] . When speaking with Mary about management, consider the following:
- Ascertain bowel pattern — the Bristol Stool chart can be used to support the consultation, but it is important to determine what is ‘normal’ for the patient and any other symptoms (e.g. colic) [21] . Mary confirms that her bowels feel sluggish and she is passing small, hard stools, which requires a lot of effort.;
- Maintaining a healthy diet — Mary’s dietary intake is reduced, but with resolution of constipation, this may improve. Encourage a healthy diet that includes whole grains, fruit and vegetables, and a good fluid intake [22] ;
- Laxatives — regular administration of laxatives to avoid constipation is advised [14] . The National Institute for Health and Care Excellence recommends the use of: stimulant laxatives (e.g. Senna [7.5–15mg at night, increasing to 30mg maximum in 24 hours] and sodium picosulphate [5–10mg at night]) and osmotic laxatives (e.g. lactulose [15–45mL daily in three divided doses] and macrogol compound [1–3 sachets daily in divided doses]) [22] . Docusate (up to 500mg daily in divided doses) is an alternative as both a softener and weak stimulant [10] . If tolerance to constipation does not occur, treatment with laxatives is advised for the duration of opiate use [20] . If titration of laxative is required then this occurs every 1–2 days according to response before switching to an alternative [10] ;
- Nausea — this common side effect occurs on initiation and dose increase, but is normally transient, lasting only a few days [15] , [23] , [24] . If patients are aware of this, they may feel more prepared and willing to accept it for a short period of time making the use of an antiemetic unnecessary [20] ;
- Non-pharmacological measures — pharmacy teams can recommend removal of certain stimuli (e.g. sight and smell of nausea-provoking foods) that lead to nausea and massage to help manage constipation [25] , [26] .
It is important to explain to Mary that opioid analgesics are a common cause of constipation, alongside other contributing factors, such as poor fluid, and dietary intake [27] .
Compliance with laxatives may be limited by patient factors, such as palatability, volume required and undesirable effects (e.g. flatulence and colic). Mary’s preference should be considered, especially as there is limited evidence for the use of laxatives, and management is generally dictated by best practice and expert opinion [14] .
The underlying aetiology of nausea and/or vomiting should be considered; gastric dysmotility and stimulation of the chemoreceptor trigger zone are most common with opiates, and can be managed with prokinetics (e.g. domperidone, metoclopramide) or a D2 receptor antagonist (e.g. haloperidol) [10] , [18] , [19] .
When the maximum licensed dose of laxatives is reached without adequate result, then there is a need to refer the patient.
If an antiemetic is considered necessary (e.g. owing to persistent or intolerable nausea ), the patient requires referral for thorough assessment [25] .
Palliative care part 3: Anticipatory prescribing
Mary’s husband, John*, brings a prescription for anticipatory medicines into the pharmacy. He is visibly upset and asks to speak to the pharmacist. He explains that the GP surgery called and asked for this prescription to be collected following a home visit from Mary’s community nurse.
During the visit, the nurse asked difficult questions about where Mary would like to be cared for when she becomes less well, and whether she would want to be resuscitated. The nurse had also suggested that Mary can stop her simvastatin, which she has been taking for the past 17 years.
John’s concerns
Although John is concerned because he knows Mary is unwell, he thinks these conversations suggest that Mary is nearing the end of her life.
When speaking with John, be empathetic while providing information. For example, explain:
- That anticipatory prescribing is part of advance care planning and does not signify that a patient is imminently dying. Medicines are prescribed in anticipation of symptoms, and should be put in place well in advance to enable rapid symptom relief [13] , [28] ;
- The possible use for each of the medicines (pain, nausea and vomiting, excess respiratory secretions, agitation and restlessness) [29] . It is unlikely that all would be needed, and possibly none at all. These medicines can be used for a more acute symptom, such as nausea or pain owing to infection, rather than only for use in the last few days of life;
- Stopping unnecessary medicines (i.e. those for long-term risk, such as statins for cardiovascular disease) should be considered to reduce tablet burden and potential side effects [7] ;
- These conversation can be distressing, but it provides an opportunity to consider serious issues during a time that is less critical and that some patients and carers may find comfort in the planning [30] .
After speaking with and explaining these points to John he appears to be more content and less worried. It is important to explain to him that the pharmacy team is available to speak to him or his wife if they have any further concerns.
*Case study is fictional
Useful resources
- Local Pharmaceutical Committee: lpc-online.org.uk/
[1] World Health Organization. WHO definition of palliative care. 2019. Available at: https://www.who.int/cancer/palliative/definition/en/ (accessed October 2019)
[2] World Health Organization. Palliative care. 2018. Available at: https://www.who.int/news-room/fact-sheets/detail/palliative-care (accessed October 2019)
[3] The Gold Standards Framework. PIG – Proactive identification guidance registration form. 2016. Available at: http://www.goldstandardsframework.org.uk/PIG (accessed October 2019)
[4] Marie Curie. Triggers for palliative care. 2015. Available at: https://www.mariecurie.org.uk/globalassets/media/documents/policy/policy-publications/june-2015/triggers-for-palliative-care-full-report.pdf (accessed October 2019)
[5] Moens K, Higginson IJ & Harding R. Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review. J Pain Symptom Manag 2014;48(4):660–677. doi: 10.1016/j.jpainsymman.2013.11.009
[6] Kobewka D, Ronksley P, McIssac D et al. Prevalence of symptoms at the end of life in an acute care hospital: a retrospective cohort study. CMAJ Open 2017;5(1):E222–E228. doi: 10.9778/cmajo.20160123
[7] Walker KA, Scarpaci L & McPherson ML. Fifty reasons to love your palliative care pharmacist. American J Hosp Palliat Care 2010;27(8):511–513. doi: 10.1177/1049909110371096
[8] Macmillan Cancer Support. The Final Injustice: variation in end of life care in England. 2017. Available at: https://www.macmillan.org.uk/_images/MAC16904-end-of-life-policy-report_tcm9-321025.pdf (accessed October 2019)
[9] The Gold Standards Framework. Examples of Good Practice Resource Guide. Just in Case Boxes. 2006. Available at: https://www.goldstandardsframework.org.uk/cd-content/uploads/files/Library%2C%20Tools%20%26%20resources/ExamplesOfGoodPracticeResourceGuideJustInCaseBoxes.pdf (accessed October 2019)
[10] Twycross R, Wilcock A & Howard P. Palliative Care Formulary 6th Ed (PCF6). 2016. Available at: https://about.medicinescomplete.com/publication/palliative-care-formulary (accessed October 2019)
[11] National Palliative and End of Life Care Partnership. Ambitions for palliative and end of life care. 2015. Available at: http://endoflifecareambitions.org.uk/wp-content/uploads/2015/09/Ambitions-for-Palliative-and-End-of-Life-Care.pdf (accessed October 2019)
[12] Gold Standard Framework. Advance Care Planning. 2018. Available at: https://www.goldstandardsframework.org.uk/advance-care-planning (accessed October 2019)
[13] National Institute for Health and Care Excellence (NICE). Care of dying adults in the last days of life. NICE guideline [NG31]. 2015. Available at: https://www.nice.org.uk/guidance/ng31 (accessed October 2019)
[14] Royal Pharmaceutical Society. British National Formulary 76 . London: Pharmaceutical Press; 2001
[15] Edwards Z, Blenkinsopp A, Ziegler L & Bennett MI. How do patients with cancer pain view community pharmacy services? An interview study. Health Soc Care Community 2018;26(4):507–518. doi: 10.1111/hsc.12549
[16] World Health Organization. WHO’s cancer pain ladder for adults. 2019. Available at: https://www.who.int/cancer/palliative/painladder/en/ (accessed October 2019)
[17] National Institute for Health and Care Excellence (NICE). Palliative care for adults: strong opioids for pain relief. NICE guideline [CG140] 2016. Available at: https://www.nice.org.uk/guidance/cg140/ifp/chapter/Managing-side-effects (accessed October 2019)
[18] The British Pain Society. Cancer pain management. 2010. Available at: https://www.britishpainsociety.org/static/uploads/resources/files/book_cancer_pain.pdf (accessed October 2019)
[19] The British Pain Society. Patient publications. Available at: https://www.britishpainsociety.org/british-pain-society-publications/patient-publications/ (accessed October 2019)
[20] Rogers E, Mehta S, Shengelia R, & Carrington Reid M. Four strategies for managing opioid-induced side effects in older adults. Clin Geriatr 2013;21(4). PMID: 25949094
[21] National Institute for Health and Care Excellence (NICE). Clinical knowledge summary. Constipation in adults. 2019. Available at: https://cks.nice.org.uk/constipation#!scenarioRecommendation:1 (accessed October 2019)
[22] National Institute for Health and Care Excellence (NICE). Constipation in children and young people: diagnosis and management. Clinical guideline [CG99]. 2017. Available at: https://www.nice.org.uk/guidance/cg99/resources/cg99-constipation-in-children-and-young-people-bristol-stool-chart-2 (accessed October 2019)
[23] NHS. Morphine. 2018. Available at: https://www.nhs.uk/medicines/morphine/# (accessed October 2019)
[24] Cherny N, Ripamonti C, Pereira J et al. Strategies to manage the adverse effects of oral morphine: An evidence-based report. J Clin Oncol 2001;19(9):2542–2554. doi: 10.1200/JCO.2001.19.9.2542
[25] Chand S. Nausea and vomiting in palliative care. Pharm J 2014;292(7799)240. doi: 10.1211/PJ.2014.11135047
[26] National Institute for Health and Care Excellence (NICE). Clinical Knowledge Summary. Palliative care — nausea and vomiting. 2016. Available at: https://cks.nice.org.uk/palliative-care-nausea-and-vomiting (accessed October 2019)
[27] Watson M, Lucas C, Hoy A & Back I. Oxford Handbook of Palliative Care. Oxford University Press; 2005.
[28] National Institute for Health and Care Excellence (NICE). Care of dying adults in the last days of life. Quality standard [QS144]. 2017. Available at: https://www.nice.org.uk/guidance/qs144 (accessed October 2019)
[29] National Institute for Health and Care Excellence (NICE). Medicines guidance: prescribing in palliative care. Available at: https://bnf.nice.org.uk/guidance/prescribing-in-palliative-care.html (accessed October 2019)
[30] National Institute for Health and Care Excellence (NICE). End of life care for adults. 2017. Quality Standard [QS13]. Available at: https://www.nice.org.uk/guidance/qs13 (accessed October 2019)
You might also be interested in…

PJ view: Restore pharmacy opening hours to improve end-of-life care

Controlled drug stock licensing is working against high-quality palliative care for care home patients

How palliative care pharmacists in community practice are improving access to medicines

5 case studies: When is it time for palliative care versus hospice?
Never hesitate to say “I need help” if you’re struggling to cope with the pain and distress of a life-limiting illness. Hospice and palliative care providers are specially trained to hear your plea and will offer comfort, compassion and support.
“Just because you ask to speak with a palliative care or hospice care provider doesn’t mean you have to start service,” says Lisa Wasson, RN, clinical educator for HopeHealth.
Palliative care and hospice care are two different sets of services, although you might hear people use the terms interchangeably.
- Palliative care is for patients with a serious illness who are still receiving curative treatments, such as chemotherapy or dialysis. Palliative care providers offer medical relief from the symptoms or stress caused by either the illness itself or the treatment. They also help patients understand their options and establish goals of care.
- Hospice care is for patients with a life-limiting illness who have decided to stop curative treatments or have been given no further treatment options for cure or to prolong life. A full team of doctors, nurses, social workers, spiritual chaplains, hospice aides, grief support professionals and volunteers offer comfort and support to the patient and family.
To learn more about these differences, read The ABC’s of curative, palliative and hospice care .
5 case studies: Is palliative care or hospice care more appropriate?
Below are five fictional stories to give you a sense of when it could be helpful to ask for a palliative care or hospice care consultation. (Every medical case is unique, and only your health care provider can advise on your care.)
Case 1: An 86-year-old with Alzheimer’s disease is repeatedly hospitalized
Janet was diagnosed with Alzheimer’s disease nine years ago and lives at home in the care of her husband. She cannot make her needs known, is incontinent and depends on her husband to feed her. She has lost 20 pounds in six months and been hospitalized three times.
Palliative care or hospice? Janet would likely qualify for hospice care given how far along her disease has advanced.
Case 2: A man wishes to stop dialysis despite family’s wishes
Robert is 64 years old and has kidney failure, coronary artery disease and diabetes. He receives dialysis three times per week but wants to stop treatment. Today he was hospitalized after skipping two dialysis appointments. Robert’s family is concerned he is giving up, and they don’t know what to do.
Palliative care or hospice? Robert and his family need to get on the same page regarding his options and wishes. A good first step would be to ask a palliative care provider to guide that conversation with skill and sensitivity. Ultimately, Robert does have to the right to stop dialysis and choose hospice if he wishes.
“We deserve as much beauty, care and respect from the health care system at the end of life as we receive at the beginning when we are born” —Lisa Wasson, RN, CHPN
Case 3: A 30-year-old with breast cancer and her mother need support
Imani has undergone two rounds of chemotherapy and radiation for breast cancer. She has severe nausea and is losing weight due to poor appetite. Her mother, who works full time, is her primary caregiver.
Palliative care or hospice? Imani is actively fighting her disease with curative treatment and might qualify for palliative care. She would receive symptom management, support services to help her mother, and a conversation about her goals of care.
Case 4: A woman with autoimmune disorders battles depression
Cindy, age 52, has multiple autoimmune disorders, fibromyalgia pain and depression. She takes antidepressant medication, is self-isolating and cannot hold a job due to taking too many sick days.
Palliative care or hospice? Cindy is not a candidate for either palliative care or hospice care because she does not have a life-limiting disease. She still needs support, though, and would be referred to a case manager or social worker.
Case 5: A man with advanced ALS requests a do-not-resuscitate order
Carter has ALS, or amyotrophic lateral sclerosis, a progressive neurodegenerative disease. He has been hospitalized with infection five times and is on a ventilator. Carter is alert and told doctors he wants to return home and sign a do-not-resuscitate order (DNR). His family is upset about his decision.
Palliative care or hospice? While in the hospital, Carter can request to speak with a palliative care or hospice care provider to guide this sensitive conversation with his family. If he wishes to start hospice, a team will help him return home, tend to life-closure tasks and die in peace and comfort surrounded by his family.
Lisa Wasson hopes more patients and their families will seek to understand the benefits of hospice and palliative care. “We deserve as much beauty, care and respect from the health care system at the end of life as we receive at the beginning when we are born,” she says.
Questions about hospice care or palliative care? Contact us at (844) 671-HOPE or [email protected] .
Privacy overview.
- Open access
- Published: 22 October 2022
Stories of paediatric palliative care: a qualitative study exploring health care professionals’ understanding of the concept
- Kirsti Riiser 1 ,
- Heidi Holmen 2 ,
- Anette Winger 2 ,
- Simen A. Steindal 3 ,
- Charlotte Castor 4 ,
- Lisbeth Gravdal Kvarme 2 ,
- Anja Lee 5 ,
- Vibeke Bruun Lorentsen 6 ,
- Nina Misvaer 2 &
- Elena Albertini Früh 2
BMC Palliative Care volume 21 , Article number: 187 ( 2022 ) Cite this article
4464 Accesses
1 Citations
10 Altmetric
Metrics details
By sharing patient stories, health care professionals (HCPs) may communicate their attitudes, values and beliefs about caring and treatment. Previous qualitative research has shown that HCPs usually associate paediatric palliative care (PPC) with death or dying and that they find the concept challenging to understand and difficult to implement. Attending to HCPs’ stories may provide a richer account of their understanding of PPC. Thus, the aim of this study was to explore PPC stories narrated by HCPs to gain increased insight into their understanding of what PPC entails.
This qualitative study collected data from four focus group interviews with 21 HCPs from different units in two Norwegian hospitals. Stories told by the HCPs to illustrate their comprehension of PPC were analysed following thematic analysis procedures.
Four themes were identified illustrating what PPC meant to the participants: creating spaces for normality, providing tailored support for the family, careful preparations for saying goodbye and experiencing dilemmas and distress. The stories centred on family care, particularly relating to dramatic or affective situations when the death of a child was imminent.
The stories reflect how the HCPs view PPC as a specific field of health care that requires particular professional sensitivity, including good communication, collaboration and planning. Thus, the HCPs in this study demonstrated knowledge about the core qualities needed to succeed in PPC. However, similar to previous research, the stories illustrate that how HCPs speak about PPC is strongly associated with end-of-life care, and by that the HCPs do not capture the breadth of the PPC concept. The findings highlight the importance of increasing knowledge about the meaning and content of PPC among HCPs in order to maintain quality of life for all children with life-limiting or life-threatening conditions throughout their illness trajectory.
Peer Review reports
Storytelling is a fundamental part of being human. Through stories information can be shared in a way that creates an emotional connection and by that provides a deeper understanding of the storyteller’s experiences. Thus, storytelling has the potential to bring new perspectives to the Table [ 1 ]. When telling stories about specific events from patients´ lives, health care professionals (HCPs) not only describe the patients’ medical situations. By sharing patient stories, HCPs also communicate their attitudes, values and beliefs about caring and treatment [ 2 ]. The present study used storytelling as a methodological approach to investigate HCPs’ understanding of paediatric palliative care (PPC). According to the World Health Organization (WHO), PPC should be the standard of care for children with life-threatening or life-limiting conditions and should be integrated with prevention, early diagnosis and treatment [ 3 , 4 ]. PPC is defined as “the active total care of the child’s body, mind and spirit, and [this] also involves giving support to the family” [ 5 ]. Children (0–18 years) and their families should be provided with interdisciplinary care aiming to promote their physical, psychological, social and spiritual well-being [ 4 ]. Early implementation of PPC through active and integrated support is endorsed as beneficial to the well-being of the child and the family [ 3 , 6 , 7 ]. HCPs may display good knowledge of the principles of palliative care, but their comprehension of the concept, their way of thinking and their reported reasons for referrals to PPC demonstrate that they associate PPC with death and dying [ 8 , 9 ]. Research reports that HCPs find the concept challenging to understand and implement and that it evokes negative emotions [ 10 , 11 , 12 ]. Consequently, early integration of PPC may be delayed and many children are not referred to PPC until late in their illness trajectory [ 12 ]. Among the barriers reported for implementing PPC at an early stage are uncertainty and confusion about the concept, as well as stigma related to the term [ 10 , 13 ]. In our recent study of interdisciplinary HCPs’ understanding and implementation of PPC, we found that the participants viewed the concept as unfamiliar and not meaningful [ 11 ]. HCPs associated PPC with death and dying and used words like scary, burdensome, dramatic and lack of active treatment to describe their comprehension of the concept [ 11 ].
Attending to stories of HCPs´ clinical experience with children and their families may provide further insights than solely through answering questions and thus be a valuable approach to gain an even richer account of their understanding of PPC. Thus, the aim of this study was to explore stories of PPC narrated by HCPs to enhance our knowledge of their understanding of what PPC entails.
This study included qualitative data obtained through focus group interviews with HCPs and emerged from a project exploring HCPs’ understanding of PPC [ 11 ]. The project was not originally designed to elicit narratives; however, during the focus groups, the HCPs told stories or gave specific examples to illustrate their comprehension of PPC. We decided to subtract these stories and all the utterances made from the initial material to provide examples or illustrate points when reflecting on what PPC is. Thus, the data was not a part of the material analysed in the first article. We explored the stories in more depth, with the assumption that this may provide an enhanced understanding of what HCPs comprehend when they talk about PPC. The reporting of the study was guided by the consolidated criteria for reporting qualitative research [ 14 ].
Participants
HCPs from three paediatric units in two hospitals were recruited using purposeful sampling, including a variety of professions. Both hospitals are located in Eastern Norway. The units include different departments for children aged 0–18 with a wide range of diagnoses. A total of 21 participants, of whom 19 (90%) were female, participated in 4 focus groups including 2–9 participants (Table 1 ). Most of the participants had worked with children with life-threatening and life-limiting conditions for more than 10 years. Only two had fewer than two years of experience and some had more than 20 years. Without giving any reason, four HCPs from one of the units withdrew their consent, which is why one of the groups ended up including only two participants. The other three groups included four, six and nine participants. We have no information about the HCPs who declined invitations to participate.
Data collection
The four focus group meetings were convened between November 2019 and February 2020 in a suitable location at the hospital where the participants worked. The focus groups were moderated by pairs of researchers (AL and KR or LGK and EAF): one researcher led the conversations, and the other assisted and took notes during the focus group. All the moderators are female HCPs with a PhD or a master’s degree. None had any prior professional or private relationship with the participants. All the focus groups started with a short presentation of the aim of the study and the researchers’ background and research interests. A semi-structured interview guide was used to focus the conversation and keep it within relevant topics. The guide was developed based on previous research and included open-ended questions related to the concepts of PPC, alleviation and end-of-life care. The participants were also encouraged to illustrate these concepts by sharing their experiences from working with the children and their parents [ 10 ]. The interview guide was not piloted, but the questions were discussed with a reference group including HCPs, service user representatives and researchers to facilitate relevant and clear questions. The focus groups lasted from 45 to 90 min and were audio-recorded.
Data analysis
The focus group interviews were transcribed verbatim by a professional transcriber and imported into the qualitative software package NVivo 12 to aid the analysis. The data analysis was undertaken following a thematic analysis procedure [ 15 ]. We applied an inductive approach, meaning that the data were coded without trying to fit a pre-existing coding frame [ 15 ]. First, the entire data set was screened for stories of PPC. In addition to the stories shared on request, we included all utterances made to provide an example or illustrate a point when reflecting on what PPC is. The transcripts were read by all the authors to obtain an overall first impression of their content. Next, three authors (KR, HH and AW) jointly coded the transcripts section by section aiming to capture the essence of the data before grouping the codes into semantically related themes determined by perceived patterns. A preliminary set of themes with codes was presented to the other authors, who all provided their comments. Based on the feedback, the first and last authors (KR and EAF) reviewed and refined the themes and validated them against the data set. To tell the story of each theme, a detailed analysis of each theme was written. Lastly, the themes were named with the aim of providing an understanding of each theme’s content [ 15 ]. Quotations from the focus groups used in the article were translated into English by the researchers in collaboration. Minor alterations were made to the text to ensure anonymity.
Dependability was enhanced by being explicit about the data analysis process and how this was systematically carried out. Credibility was enhanced through investigator triangulation in the data analysis [ 16 ]. All the researchers were involved in one or more steps of the analysis, and we challenged each other to provide alternative interpretations and reach intersubjectivity. All the authors are skilled researchers, and most have extensive experience in qualitative research.
Ethical considerations
The project was reviewed by the Norwegian Data Protection Official for research, who concluded that it was in accordance with the Personal Data Act (reference number 935,944). The local data protection officers at the two participating hospitals approved the study. The study participants were HCPs, and the project did not collect data about health and illness. Thus, the project did not require permission from a regional committee for research ethics. The participating HCPs received information about the aim of the study and the procedure for data collection and gave written consent to participate after being reassured about confidentiality issues and their right to withdraw.
Following the analysis, four themes were identified illustrating what PPC entails to the participants: creating spaces for normality , providing tailored support for the family , careful preparations for saying goodbye and experiencing dilemmas and distress (Table 2 ).
Creating spaces for normality
The HCPs seemed attentive to having the child with a life-threatening or life-limiting illness at the centre of their care. Several participants spoke about the importance of supporting a sense of normality and mentioned that the parents as well as the child aimed for ordinary days at home, school or kindergarten: “I believe that most parents want their child’s days to be normal and good until the end and that it’s not going to be something else”. (Group 3)
Stories were told that illustrate how the HCPs managed to create spaces for play and fun for the child despite working in a hospital and being responsible for clinical procedures. They gave examples of how they used medical equipment as props for play and how these playful situations opened up conversations with the child about sensitive and important topics. In one story, the nurse explained that they made the hospital stay so engaging that the child looked forward to treatment and felt sorry for leaving. Another nurse elaborated on a playful moment with a little girl with a life-limiting illness engaging in imaginary play:
…and then the girl says: “At my prom, I want a dress like that”, and I said “Really? Wow, that’s cool”, and then she kind of realizes that she’s never going to a prom and then she says: “Well, well…it would have been cool, right?” and I responded “Yeah, it would have been really cool” (Group 2).
The nurse reflected on the importance of being present in the moment and allowing the child to enjoy the thought of adolescence even though her life expectancy was very limited.

Providing tailored support for the family
The participants spoke about different ways of providing support through adjusted information and tailored care. In their stories, they emphasized the importance of sufficient, timely information. One participant described how a mother once said that she did not understand that her child could die while being on a ventilator and that the lungs could still collapse. The participant had carried this mother’s utterance with her ever since, and she pointed out that making sure that parents understand why their child actually dies is significant. Another participant described how important it is to be able to recognize when the parents communicate a need for more knowledge and to provide accurate information when it is called for. However, the participants also referred to challenging situations in which the parents and HCPs were not in step with each other. One participant admitted that if HCPs believe the parents are informed about the child’s situation and suddenly realize that they are not, this may cause delays in procedures and irritation:
I try to be observant to this, both in myself and others. It’s important to avoid a moralizing attitude: ‘Do they [the parents] still not understand?’ Because it creates hassle and annoyance when the parents are not sufficiently informed and up to date on the child’s situation. (Group 2)
The participants gave several examples of how they adjust and tailor their care for families to make them feel safe in acute as well as more stable situations. They spoke about the importance of working as a team around the family. One participant described it as creating layers upon layers of love around the child. This expression derived from a particular story about a child who was going to be placed in foster care but was too sick to leave the hospital. With help from the HCP, the child’s birth mother and foster mother moved into the children’s ward. With support from the multidisciplinary team, they cared for the child together during the last weeks of the child’s life.
…and the foster mother stepped aside to let the birth mother hold the child close, day and night (…) and the baby received so many layers of love (…) And the HCPs were amazingly professional and put things aside to let it happen. (Group 2)
Later, the birth mother explained that, despite the great tragedy, these had been the finest weeks of her life. This situation was resource-demanding for the unit, but all the HCPs stood out as highly professional and were willing to make an extra effort in the extraordinary situation.
Going that extra mile for children and their families was a recurring theme in the HCPs’ stories. Witnessing severe and distressing situations seemed to make the HCPs mobilize to make the days, weeks and months as good as possible for the child, their parents and their siblings. Many of the participants’ stories were about creating safe spaces for families at the hospital, giving the child, parents and siblings moments to breathe. There were also examples of systems failing to support the families adequately and lack of coordination resulting in disorganized care; however, most stories were examples of how HCPs managed to work together, emphasizing the importance of shared information. One participant said:
One of the finest moments that I’ve witnessed was when a nurse who knew the family well took the initiative to play with an older sibling of a child who would soon die. The nurse took the sibling outside in the hallway, and they talked and played it out. They had a good relationship. The nurse knew very well what was about to happen because she had been in the room when the message about the child’s impending death was given. The more people working around the family, the more important it is that we have the same information and work as a team. (Group 4)
Careful preparations for saying goodbye
Most of the stories emerged from critical situations or when the child’s illness was worsening, especially about initiating advance care planning with the end of the child’s life in mind. Some described the importance of seizing that particular moment when the parents and the child are ready and receptive to these difficult conversations. The HCPs gave examples of when the parents themselves took the initiative, but in most of the stories, the HCPs took the lead. However, as underlined by one participant, engaging in such dialogue requires that the HCPs are present and that they tread carefully: “If you’ve established a good relationship, moments will occur if you’re observant and willing to enter these conversations. For me, this is part of palliative care”. (Group 2)
With the children, such conversations could take place during play, and difficult topics could be touched upon without needing to make eye contact. It became evident through the HCPs’ stories that these conversations were tough and that it was sometimes difficult to control their own feelings.
These conversations are really hard, it is as if you are about to start crying yourself. But I think it is really, really important [to talk about it], because we have reached the moment when it might happen. (Group 3)
Through their stories, the HCPs emphasized the need for advance care plans when the risk of severe incidents increased. The advantages of such plans are to secure continuity and ensure that all important information is available for all the HCPs involved in a possible emergency situation. However, these plans should also be made for the family members so that they know how to act if critical events occur. In several stories, the HCPs described how the care plans included strategies and measures for staying at home as much as possible. One example was given of how specialist care and primary care cooperated, where the child was provided with advanced home care, and the child and the family shifted between being at the hospital and being at home. The family in this story expressed a strong wish to let the child die at home, and the HCPs made great efforts to accommodate this wish. The participant described how all the necessary equipment was installed in the family’s house:
It was arranged for him to stay at home. He received pain relief, and I believe the community nurses cared for him, and he spent his last 2–3 weeks in the living room together with his siblings and parents and they all slept together in the same bed. They were kind of… they were together.” (Group 1)
Making practical arrangements to facilitate a proper farewell in a safe environment together was a prioritized task. The parents and siblings could focus on being there for the child and for each other while the medical team provided care and pain relief for the child.
Experiencing dilemmas and distress
While many stories and examples evolved from cases where the HCPs explained that they managed to provide support and good care, there were also stories about conflicts with parents and distressful experiences. Some HCPs referred to conversations about how to handle a dramatic worsening of the child’s condition (e.g. decisions about not putting the child on a ventilator or stopping medications) and how this had provoked anger and frustration among parents. One HCP described one such situation:
... and then there were discussions about withdrawing antibiotics, and we had to tell the parents, and one of them was really devastated and said, ‘You ’ re giving up on my child!’ whereupon the doctor kindly responded, ‘No, we’re not, we are comforting her during her last days’”. (Group 2)
In some cases, the participants noted that it was the HCPs and not the parents who pushed too hard by suggesting a new treatment, which turned out to result in complications or negative side effects. Another story illustrated the tension that can occur between HCPs and parents when the child is expected to die within a short time. In this case, one HCP accused a parent of hastening the baby’s death by withholding nutrition. This resulted in a deep conflict among the staff, where some supported the nurse and some sympathized with the parents. Eventually, external counselling was required to resolve the dispute among the staff. After the baby died, the parents reported that the whole situation had added even more to their grief. The participant described this as an ethically demanding situation, and, in retrospect, she was struck by how good intentions can lead to conflict and how emotionally involved one may become.
Examples were also given of parents who refused to acknowledge that their child had a life-limiting disease and instead of planning for the end spent all their time searching for a cure. The HCPs experienced it as particularly hard to witness how false hope was being sold through experimental treatment and that parents were willing to squander that precious, limited time left of their child’s life:
There was a family that I was never in a position to speak with, because their attention was always directed at something else. It was impossible to talk to them about anything, because they were always focusing on going off somewhere to find a cure. (Group 2)
In these stories, the parents searched for opportunities to travel far with their child to try experimental treatment, resulting in long periods away from home and separation between family members. The participant found it challenging that the parents were never available to make plans for good palliative care.
As a means of enhancing our knowledge of HCPs’ understanding of what PPC entails, the present study aimed to explore HCPs’ stories about PPC. Our findings are in line with the findings of previous studies that HCPs associate PPC with advanced illness or more acute phases towards the end of the child’s illness trajectory [ 8 , 10 , 11 ]. The HCPs’ stories circled around family care, particularly relating to dramatic or affective situations when the death of a child was imminent. The stories reflected how the HCPs view PPC as a specific field of health care that requires particular professional sensitivity, including good communication, collaboration and planning. Thus, the HCPs in this study demonstrated knowledge about the core qualities needed to succeed in PPC [ 17 ].
The potentially complicated illness trajectories in children with life-limiting and life-threatening conditions make early integration of palliative care particularly beneficial [ 3 , 4 ]. The stories in this study rarely touched upon topics related to earlier palliative phases like living everyday life with a life-limiting disease or coordinating follow-up at home or school. Possible consequences for late referral to palliative care are that symptoms may be unaddressed for longer and psychological needs may be neglected [ 18 ]. Bearing in mind that the participants in the present study all worked in specialist healthcare, it is reasonable that most stories revolved around acute and life-threatening situations at a hospital. However, according to the WHO’s guidelines and the guidelines for PPC in Norway, specialist health care and primary health care services should be involved in the entire process from diagnosis onwards, assuring overall and coordinated follow-up of the child and the family [ 3 , 4 ]. The national guidelines emphasize the importance of continuous mapping, evaluation and adjustment to the family’s needs and that this is a shared responsibility between primary and specialist health care [ 3 ]. Over the last years interdisciplinary PPC-teams have been established in children’s wards throughout the country, aiming at provision of care regardless of whether the child is at home, in institutions or in hospitals. In some of the larger cities in Norway, the ‘Hospital at home’ provides specialized health care in the child’s home. Many families express a strong wish for home care [ 19 ]. Only a small number of the stories included examples of cooperation with community care, enabling the families to stay at home. This indicates that the HCPs either had few such experiences of collaboration outside the hospital or that the collaboration they engaged in did not fall within their conception of PPC and that they did not regard such work as palliative care. This demonstrates a need for clarification of the distinction between basic and specialized PPC. Basic PPC is to some degree integrated into standard care of children with life-limiting and life-threatening conditions in Norway, but specialized PPC is a relatively new field of medical expertise that was brought to public attention with the first national guidelines in 2016 [ 3 ].
While few stories in this study exemplified how HCPs work cooperatively outside the hospital, several stories described the necessity and value of working as a team within the hospital. PPC is defined by the WHO as total care of the child’s body, mind and spirit, which also includes care of the family [ 4 ]. Thus, interdisciplinary teamwork is essential for the provision of PPC. To evaluate and alleviate the distress of the child and the family and promote their well-being throughout the illness trajectory, multiprofessional teams need to work in an interdisciplinary way. Some participants emphasized the importance of equal access to information and shared understanding of a child’s and family’s situation within the health care team. Although each team member has a particular scope of practice and responsibility, a mutual understanding based on shared experiences and information enables mutual decision-making [ 20 ], which can be particularly important in acute or stressful situations. An interdisciplinary team approach, which includes the child and/or the parents, is essential for achieving holistic and comprehensive care encompassing the complex needs of the patient and the family [ 21 ]. One study suggests that HCPs in palliative care teams enhance their ability to see the patient from a holistic perspective by sharing impressions and discussing the patient and family’s situation within the team [ 22 ].
Even though the HPCs’ stories were not about early PPC stages, they do illustrate important elements of later phases, as described in national and international guidelines [ 3 , 4 ]. Many of the stories accompanied the categories identified in our previous paper relating to family-centred care and preparing for the death of a child [ 11 ]. The stories show that the participants recognize the importance of having a family-centred care perspective. The HCPs’ attention is very much drawn to how they can best support the family. In palliative care, family-centred care is defined as seamless continuity addressing patient, family and community needs related to terminal conditions through interdisciplinary collaboration [ 23 ]. Family-centred care has been described as containing the principles of information sharing, respecting and honouring differences, partnership and collaboration, negotiation and care in the context of family and community [ 24 ]. A child’s and family’s needs and preferences vary. Thus, it is not possible to deliver family-centred PPC with a one-size-fits-all approach. Among the stories told were examples of how HCPs stood out as creative, flexible and responsive to the child’s or the parents’ specific needs through adjusting their information and care for the child and the family. Meanwhile, the participants also emphasized the need for structured advance care planning, particularly when death is approaching. Engaging in advance care planning requires that future scenarios be discussed with families [ 25 ]. Coming to a realization that death is inevitable and close is a process for those involved, and families as well as HCPs reach this understanding at different times and paces [ 26 , 27 ]. Several stories evinced that having these conversations is demanding for HCPs, that timing is key and that insufficient communication may have severe consequences for the parties involved. Conversations about future care and end-of-life planning were mostly initiated by HCPs and less often by the parents in the stories we collected, which corresponds with previous research findings [ 26 ]. Several frameworks and tools have been developed to address advance care and end-of-life planning, some of which are specifically developed for PPC [ 28 ]. However, no such tools were mentioned by the participants. Instead, their stories indicated that they relied on their experience and professional tact when engaging in dialogues about palliative care. This may reflect the status and development of PPC in Norway at the time of this study. Advance care planning as a specific method has just recently been integrated into the guidelines of the Norwegian Association of Paediatricians [ 29 ].
The overall aim of PPC is to ensure a child’s quality of life throughout the illness trajectory [ 4 ]. Cherishing moments of joy and aiming for days of normality for the children were spoken of as important by the participants. The support of HCPs in achieving this, has been shown to be of great importance to parents as well [ 30 ]. For children, normality equals playing. The participants’ stories of how they provided children at the hospital with time and opportunities to play illustrate that the participants recognize its value. Playing moves the child out of time, allowing them to be absorbed in the moment and making room for absolute presence while at the same time giving the child an increased understanding of their existence [ 31 ]. This was beautifully illustrated in the story about the girl who knew she would not live to experience her prom but still allowed herself to joyfully engage in play about it. As death approaches, time increases in value. Witnessing how children capture time in play may help parents as well as HCPs broaden their understanding of what PPC comprises.
Strengths and limitations
Using stories as a basis for analysis is a strength of the present study. When we tell stories we use language available and familiar to us and to our audience [ 1 ]. While HCPs’ understanding and experience of PPC has been investigated through qualitative interview studies, few, if any, have used storytelling as a research tool to explore their insights. Compared to other qualitative research methods, stories can generate more nuanced, contextualized and culturally reflective information [ 32 ]. However, participating in focus groups instead of individual interviews may have limited the HCPs’ time and opportunity to elaborate on their stories. In only one of the four focus group interviews did the interviewer explicitly ask the participants to tell a story or give an example from practice. This is an important limitation. If the same request had been given to the remaining groups, we might have collected richer data. Nevertheless, the participants in all the focus groups spontaneously used their experiences to elaborate on their comprehension of PPC through stories and examples, with little prompting required from the interviewers. Some chose to supplement their colleagues’ stories or follow up with their own version of the same case, while at other times listening to a story served as a cue for a new story mirroring or contrasting with the former. The stories stood out as significant to the storyteller, and it was noticeable that they had been told and reflected over before. This underlines the significant potential of stories to capture the participants’ lived experiences. One limitation to be noted is that the sample was predominantly female and thus the results may be less representative for male HCPs.
In this study, we aimed to explore stories of PPC as narrated by HCPs to enhance our knowledge of their understanding of what PPC is. In our previous paper, we discussed that although the HCPs were hesitant to use the term PPC, they found themselves responsible for delivering PPC [ 11 ]. The HCPs’ stories supported this by highlighting key elements of PPC, such as interdisciplinary teamwork family-centred care and advance care planning. However, similar to findings in our first paper [ 11 ] and previous research [ 8 , 9 , 10 ], the stories illustrated that HCPs’ reflections about PPC did not capture the entire concept of PPC, as they were strongly associated with end-of-life care. This underscores the importance of increasing knowledge about PPC among HCPs in Norway. A consolidated recognition of the meaning and content of PPC is necessary to maintain quality of life for all children living with life-limiting or life-threatening conditions.
Data availability
The datasets generated and analysed during the current study are not publicly available due to legal restrictions. Data are however available from the authors upon reasonable request and with permission of the Norwegian Centre for Research Data (NSD) and the hospitals’ Data Protection Officers.
Abbreviations
- Paediatric palliative care
Health care professional
Michael W. Some Thoughts on Storytelling, Science, and Dealing with a Post-Truth World. Storytelling Self Society. 2017;13(1):120–37.
Article Google Scholar
Lehna C. Storytelling in Practice: Part Two—Professional Storytelling. J Hospice Palliat Nurs. 1999;1(1):27–30.
The Norwegian Directorate of Health. [Palliasjon til barn og unge. Nasjonal faglig retningslinje] 2016 [Available from: https://www.helsedirektoratet.no/retningslinjer/palliasjon-til-barn-og-unge .
World Health Organization. Integrating palliative care and symptom relief into paediatrics. A WHO guide for health care planners, implementers and managers. Geneva; 2018.
World Health Organization. Cancer pain relief and palliative care in children. Geneva: WHO; 1998.
Google Scholar
Kaye EC, Rubenstein J, Levine D, Baker JN, Dabbs D, Friebert SE. Pediatric palliative care in the community. CA Cancer J Clin. 2015;65(4):316–33.
Goldman A, Hain R, Liben S. Oxford Textbook of Palliative Care for Children. Oxford University Press; 2012. pp. 2012–07.
Twamley K, Craig F, Kelly P, Hollowell DR, Mendoza P, Bluebond-Langner M. Underlying barriers to referral to paediatric palliative care services: Knowledge and attitudes of health care professionals in a paediatric tertiary care centre in the United Kingdom. J Child Health Care. 2013;18(1):19–30.
Bergstraesser E. Article Commentary: Pediatric Palliative Care: A Reflection on Terminology. Palliative Care: Research and Treatment. 2013;7:PCRT.S12800.
De Clercq E, Rost M, Rakic M, Ansari M, Brazzola P, Wangmo T, et al. The conceptual understanding of pediatric palliative care: a Swiss healthcare perspective. BMC Palliat Care. 2019;18(1):55.
Winger A, Früh EA, Holmen H, Kvarme LG, Lee A, Lorentsen VB, et al. Making room for life and death at the same time – a qualitative study of health and social care professionals’ understanding and use of the concept of paediatric palliative care. BMC Palliat Care. 2022;21(1):50.
Kaye EC, Friebert S, Baker JN. Early Integration of Palliative Care for Children with High-Risk Cancer and Their Families. Pediatr Blood Cancer. 2016;63(4):593–7.
Shen MJ, Wellman JD. Evidence of palliative care stigma: The role of negative stereotypes in preventing willingness to use palliative care. Palliat Support Care. 2019;17(4):374–80.
Consolidated criteria for reporting qualitative research (COREQ). a 32-item checklist for interviews and focus groups (COREQ). [Available from: https://www.equator-network.org/reporting-guidelines/coreq/ .
Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Res Psychol. 2006;3(2):77–101.
Twining P, Heller RS, Nussbaum M, Tsai C-C. Some guidance on conducting and reporting qualitative studies. Comput Educ. 2017;106:A1–9.
Sreedhar SS, Kraft C, Friebert S. Primary palliative care: Skills for all clinicians. Curr Probl Pediatr Adolesc Health Care. 2020;50(6):100814.
De Clercq E, Rost M, Pacurari N, Elger BS, Wangmo T. Aligning guidelines and medical practice: Literature review on pediatric palliative care guidelines. Palliat Supportive Care. 2017;15(4):474–89.
Winger A, Kvarme LG, Løyland B, Kristiansen C, Helseth S, Ravn IH. Family experiences with palliative care for children at home: a systematic literature review. BMC Palliat Care. 2020;19(1):165.
Edwards A. Building common knowledge at the boundaries between professional practices: Relational agency and relational expertise in systems of distributed expertise. Int J Educational Res. 2011;50(1):33–9.
Leclerc B-S, Blanchard L, Cantinotti M, Couturier Y, Gervais D, Lessard S, et al. The effectiveness of Interdisciplinary Teams in End-Of-Life Palliative Care: A Systematic review of Comparative Studies. J Palliat Care. 2014;30(1):44–54.
Klarare A, Hagelin C, Fürst C, Fossum B. Team Interactions in Specialized Palliative Care Teams: A Qualitative Study. J Palliat Med. 2013;16(9):1062–9.
Gilmer MJ. Pediatric palliative care. Crit Care Nurs Clin. 2002;14(2):207–14.
Kuo DZ, Houtrow AJ, Arango P, Kuhlthau KA, Simmons JM, Neff JM. Family-centered care: current applications and future directions in pediatric health care. Matern Child Health J. 2012;16(2):297–305.
Harmoney K, Mobley EM, Gilbertson-White S, Brogden NK, Benson RJ. Differences in Advance Care Planning and Circumstances of Death for Pediatric Patients Who Do and Do Not Receive Palliative Care Consults: A Single-Center Retrospective Review of All Pediatric Deaths from 2012 to 2016. J Palliat Med. 2019;22(12):1506–14.
Verberne LM, Fahner JC, Sondaal SFV, Schouten–van Meeteren AYN, de Kruiff CC, van Delden JJM, et al. Anticipating the future of the child and family in pediatric palliative care: a qualitative study into the perspectives of parents and healthcare professionals. Eur J Pediatrics. 2021;180(3):949–57.
Davies B, Sehring SA, Partridge JC, Cooper BA, Hughes A, Philp JC, et al. Barriers to Palliative Care for Children: Perceptions of Pediatric Health Care Providers. Pediatrics. 2008;121(2):282–8.
Myers J, Cosby R, Gzik D, Harle I, Harrold D, Incardona N, et al. Provider Tools for Advance Care Planning and Goals of Care Discussions: A Systematic Review. Am J Hospice Palliat Medicine®. 2018;35(8):1123–32.
The Norwegian Association of Paediatricians. [Pediatriveiledere: Kommunikasjon i barnepalliative forløp] [Available from: https://www.helsebiblioteket.no/retningslinjer/pediatri/barnepalliasjon/kommunikasjon-i-barnepalliative-forlop .
Ribbers S, Wager J, Hartenstein-Pinter A, Zernikow B, Reuther M. Core outcome domains of pediatric palliative care for children with severe neurological impairment and their families: A qualitative interview study. Palliat Med. 2020;34(3):309–18.
Steinsholt K. [Lett som en lek?]. Trondheim: Tapir forlag; 1998.
McCall B, Shallcross L, Wilson M, Fuller C, Hayward A. Storytelling as a Research Tool Used to Explore Insights and as an Intervention in Public Health: A Systematic Narrative Review. International journal of public health. 2021;66.
Download references
Acknowledgements
We thank the participating health care professionals for their valuable contributions to this study. We are also grateful to the staff who assisted in the recruitment.
The study did not receive any external funding.
Author information
Authors and affiliations.
Department of Rehabilitation Science and Health Technology, Faculty of Health Sciences, OsloMet - Oslo Metropolitan University, St. Olavs plass, NO-0130, Oslo, PO Box 4, Norway
Kirsti Riiser
Department of Nursing and Health Promotion, Faculty of Health Sciences, OsloMet - Oslo Metropolitan University, St. Olavs plass, NO-0130, Oslo, PO Box 4, Norway
Heidi Holmen, Anette Winger, Lisbeth Gravdal Kvarme, Nina Misvaer & Elena Albertini Früh
Lovisenberg Diaconal University College, Lovisenberggt. 15b, NO-0456, Oslo, Norway
Simen A. Steindal
Department of Health Sciences, Faculty of Medicine, Lund University, SE-221 00, Lund, Box 157, Sweden
Charlotte Castor
Division of Paediatric and Adolescent Medicine, Oslo University Hospital, NO-0424, Ullevål, Nydalen, Oslo, PO Box 4950, Norway
Department of Nursing, Faculty of Health Studies, VID Specialized University, NO-0319, Vinderen, Oslo, PO Box 184, Norway
Vibeke Bruun Lorentsen
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed in the design of the study. KR, AL, LGK and EAF conducted the focus group interviews. KR, EAF, HH and AW analysed and interpreted the data, while KR and EAF wrote the manuscript. HH, SAS and AW gave major input to the analysis and results. All authors contributed with critical revisions and editions of the manuscript. The final manuscript has been read and approved by all authors.
Corresponding author
Correspondence to Kirsti Riiser .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the Norwegian Centre for Research Data (reference number 935944) and the local data protection officers/ethical board at the two hospitals (reference number 19/21909 and 21/10389). The Norwegian Centre for Research Data as well as the protection officers have the authority to assess whether ethical principles have been safeguarded in projects that do not fall under the Health Research Act. The project did not require permission from the Regional Committee for Research Ethics Since the study participants are healthcare professionals, and the project did not collect data about health and illness. All participants received verbal and written information about the study and were ensured that their participation was voluntary, that anonymity and confidentiality would be safeguarded and that they could withdraw at any time without giving any reason. Written informed consent was obtained from all participants prior to data collection. The study meets all necessary ethical requirements and guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Riiser, K., Holmen, H., Winger, A. et al. Stories of paediatric palliative care: a qualitative study exploring health care professionals’ understanding of the concept. BMC Palliat Care 21 , 187 (2022). https://doi.org/10.1186/s12904-022-01077-1
Download citation
Received : 21 July 2022
Revised : 04 October 2022
Accepted : 10 October 2022
Published : 22 October 2022
DOI : https://doi.org/10.1186/s12904-022-01077-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Palliative care
- Health care professionals
- Life-threatening conditions
- Life-limiting conditions
- Storytelling
- Thematic analysis
BMC Palliative Care
ISSN: 1472-684X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Palliative Care Costs in Different Ambulatory-Based Settings: A Systematic Review
- Systematic Review
- Open access
- Published: 27 December 2023
- Volume 42 , pages 301–318, ( 2024 )
Cite this article
You have full access to this open access article
- Ana Helena Perea-Bello ORCID: orcid.org/0000-0002-4899-8389 1 ,
- Marta Trapero-Bertran ORCID: orcid.org/0000-0002-9233-1776 2 &
- Christian Dürsteler ORCID: orcid.org/0000-0003-0840-2787 3 , 4
1342 Accesses
Explore all metrics
Cost-of-illness studies in palliative care are of growing interest in health economics. There is no standard methodology to capture direct and non-direct healthcare and non-healthcare expenses incurred by health services, patients and their caregivers in the course of the ambulatory palliative care process.
We aimed to describe the type of healthcare and non-healthcare expenses incurred by patients with cancer and non-cancer patients and their caregivers for palliative care in ambulatory-based settings and the methodology used to capture the data.
We conducted a systematic review of studies on the costs of ambulatory-based palliative care in patients with cancer (breast, lung, colorectal) and non-cancer conditions (chronic heart failure, chronic obstructive pulmonary disease, dementia) found in six bibliographic databases (PubMed, EMBASE [via Ovid], Cochrane Database of Systematic Reviews, EconLit, the National Institute for Health Research Health Technology Assessment Database and the National Health Service Economic Evaluation Database at the University of York, and Google Scholar). The studies were published between January 2000 and December 2022. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology for study selection and assessed study quality using the Quality of Health Economic Studies instrument. The study was registered in PROSPERO (CRD42021250086).
Of 1434 identified references, 43 articles met the inclusion criteria. The primary data source was databases. More than half of the articles presented data from public healthcare systems (65.12%) were retrospective (60.47%), and entailed a bottom-up costing analysis (93.2%) made from a healthcare system perspective (53.49%). The sociodemographic characteristics of patients and families/caregivers were similar across the studies. Cost outcomes reports were heterogeneous; almost all of the studies collected data on direct healthcare costs (97.67%). The main driver of costs was inpatient care (55.81%), which increased during the end-of-life period. Nine studies (20.97%) recorded costs due to productivity losses for caregivers and three recorded such costs for patients. Caregiving costs were explored through an opportunity cost analysis in all cases, based on interviews conducted with and questionnaires administered to patients and caregivers, mainly via telephone calls (23.23%).
Conclusions
This systematic review reveals that studies on the costs of ambulatory-based palliative care are increasing. These studies are mostly conducted from a healthcare system perspective, which leaves out costs related to patients’/caregivers’ economic burden. There is a need for prospective studies to assess this financial burden and evaluate, with strong evidence, the interventions and actions designed to improve the quality of life of palliative care patients. Future studies should propose cost calculation approaches using a societal perspective to better estimate the economic burden imposed on patients in ambulatory-based palliative care.
Similar content being viewed by others

A systematic review of quality of life research in medicine and health sciences
K. Haraldstad, A. Wahl, … the LIVSFORSK network

Palliative Care in the Intensive Care Unit: Not Just End-of-life Care
Hongyan Pan, Weihua Shi, … Pengfei Pan

Primary, allied health, selected specialists, and mental health service utilisation by home care recipients in Australia before and after accessing the care, 2017–2019
Kailash Thapaliya, Gillian E. Caughey, … Maria C. Inacio
Avoid common mistakes on your manuscript.
1 Introduction
Since the hospice movement started in the UK in 1967, the conceptual framework around palliative care (PC) has changed. Currently, healthcare systems worldwide must care for an older and sicker population receiving effective, long-lasting and expensive therapeutics. The need for universal coverage with an individualised medicine approach to achieve the best health-related quality of life stands in contrast to the scarcity of economic resources available for that purpose.
The idea that PC should be started only when patients with cancer are near death has changed. Palliative care can be initiated to alleviate symptoms in various cancer and non-cancer conditions several months before the patient’s death. Healthcare services and patients have changed, and both curative and palliative interventions, previously restricted to in-hospital settings, can be delivered in ambulatory-based settings such as primary care clinics, hospital outpatient services or even at home (the option most preferred by patients and their families/caregivers [ 1 , 2 , 3 , 4 , 5 , 6 ]), all depending on the patient’s health status, the system resources, and the health and social network that provides support [ 7 , 8 , 9 ].
The World Health Organization has defined PC as “an approach that improves the quality of life of patients and their families (adults and children) who are facing problems associated with life-threatening illness. It prevents and relieves suffering through the early identification, correct assessment and treatment of pain and other problems, whether physical, psychosocial, or spiritual” [ 10 ]. The European Association of Palliative Care defines PC as “active, total care of patients whose disease is not responsive to curative treatment. Palliative care takes a holistic approach, addressing physical, psychosocial, and spiritual care, including the treatment of pain and other symptoms. Palliative care is interdisciplinary in its approach and encompasses the care of the patient and their family, and should be available in any location, including hospital, hospice, and community …” [ 11 ]. These definitions encompass several scenarios for the provision of inpatient and ambulatory care.
Palliative care is now a part of early treatment and is provided in a broad spectrum of situations, not restricted to cancer inpatient settings. The variety of conditions and the care needed by patients have turned PC into a complex intervention for which the record-keeping process is not well defined. The resources consumed and the billing process are not standardised or clear-cut, and consequently our knowledge of healthcare costs related to PC is far from complete.
When PC is delivered as part of inpatient care (in a hospital setting), the billing process is standardised and the cost calculation is relatively straightforward. When PC is provided as an outpatient service or in a primary care or home setting; however, the billing process changes and numerous direct, indirect and non-tangible costs are left out of the final calculation.
In ambulatory scenarios, the fact that PC expenditure is shared by the healthcare system and patients/caregivers may lead to the misconception that PC is less costly. From a health system stakeholder’s perspective, it represents a monetary benefit to the system. However, if viewed from a societal perspective, it becomes evident that the money that is not being invested by the system in inpatient care is being spent by patients and families/caregivers to keep patients comfortable, physically, psychosocially and spiritually, while at home [ 5 , 12 , 13 , 14 ].
When the ambulatory approach is included in the calculations, non-paid out-of-pocket expenditures, loss of productivity of patients and caregivers, time devoted to care, leisure time lost by caregivers, and the psychological and emotional burden placed on patients and caregivers must be factored in and quantified. When all these factors are considered, ambulatory PC may be even more expensive than inpatient PC, depending on the population studied and the analysis performed, but this has not been adequately addressed [ 15 , 16 , 17 , 18 , 19 ]. Palliative care in ambulatory-based settings needs to be appropriately measured.
The three most common cancer conditions in Europe are breast, lung and colorectal neoplasms, which account for more than 25% of cancer cases. Chronic heart failure (CHF) and chronic obstructive pulmonary disease (COPD) are the most frequent non-cancer conditions, accounting for almost 15% of long-standing chronic diseases, [ 20 ] and it is estimated that by 2050 dementia (including Alzheimer’s disease [AD]) will rank third in terms of total patient care costs [ 21 ]. A large part of the healthcare budget allocated to PC is currently for the management of these pathologies. Still, clear information on the distribution of resources, the costs and the billing process for the services delivered is lacking, which offers a field for research.
Cost-of-illness studies aim to identify, measure, and appraise the healthcare and non-healthcare costs resulting from illness, premature death, or disability due to a condition and its related comorbidities and the associated billing process. Palliative care is an area of medical practice that looks after patients and caregivers in complex health, social, and emotional situations, and studies of costs and billing in this area are lacking. Identifying the most appropriate methodology to follow in economic studies in this area is complex, and a standardised approach has yet to be devised [ 22 , 23 , 24 ]. The lack of adequate information on costing and billing of the PC services exposes a knowledge gap that needs to be explored. There is thus a need for a systematic literature review (SLR) to describe the most relevant costs in ambulatory-based settings and the most appropriate methodology for appraising the existing data. This review aims to identify, describe and summarise the most common costs of ambulatory PC for patients with cancer and non-cancer patients and their caregivers and the different methodologies used to appraise and calculate them.
2.1 Literature Search
We conducted a SLR following the Preferred Reported Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology, [ 25 ] with a protocol registered in PROSPERO (CRD42021250086). Search terms combined variants of “palliative care”, “cost” and various types of disease. The search strategy included general and Medical Subject Heading terms combined with the Boolean connectors AND OR (Table S1 of the Electronic Supplementary Material [ESM]) and was conducted on PubMed, EMBASE, Cochrane, EconLit, and the National Institute for Health Research Health Technology Assessment Database and the National Health Service Economic Evaluation Database using the Ovid platform. The search was restricted to studies published from 2000 to 2022 that included adult patients. Grey literature was searched in Google Scholar, and article references were hand searched for additional papers.
2.2 Study Selection
We included studies on the costs of PC for patients with various types of cancer (breast, lung or colorectal) and non-cancer patients (mainly those with CHF, COPD or dementia/AD). There was no language restriction. Studies that focused mainly on hospital costs, complete economic evaluations, posters, letters to the editor, case reports and case series were excluded.
To include studies in the SLR, we operationalised the following terms based on other authors’ definitions:
Outpatient/ambulatory services: specialist-level PC services, either as a brief consultation or as a concurrent care model, provided in collaboration with the patient’s primary treating physician (and nurse). The aim is to provide immediate post-discharge follow-up, continuity of care according to plans developed in the hospital, medication reconciliation, and responsiveness to patients’ and family members’ questions and concerns after the patient returns home from the hospital [ 26 ].
Inpatient services: those provided in hospitals (intensive care, inpatient care, emergency department visits, ambulance services and day case treatments) [ 6 ].
Home-based services: pain management, symptom control and psychosocial support delivered by a specialised trained team. Nurses or physicians provide PC with or without connection to a hospital or hospice. The team also provides psychosocial support to family members [ 27 ].
Hospice services: community-based care that is offered, ideally, by a multidisciplinary team that supports patients with advanced disease and their families, [ 28 ] and can be provided in a patient’s home setting or in palliative and hospice facilities. Hospice care may be provided to outpatients or inpatients [ 29 ].
Caregivers: individuals (e.g. adult children, spouses, parents, friends and neighbours) who provide care that is typically uncompensated and usually at home, which involves significant amounts of time and energy for months or years, and that requires the performance of tasks that may be physically, emotionally, socially or financially demanding [ 30 ].
2.3 Data Extraction
A predesigned data collection database was used to extract relevant information from the selected papers: data on general characteristics, design and methodology, clinical data from patients, and sociodemographic information from patients and caregivers, in addition to data on the type of costs recorded and the methods used to measure them. The general conclusions of every study were of interest to establish the utility of the data collected (Table S2 of the ESM). The first screening was conducted by the lead author (AHPB). Each abstract and paper selected was reviewed by two investigators (MTB and CD), and data extraction was performed independently. The decision for inclusion in the review was made by two investigators (AHPB and MTB). Whenever there was a disagreement, the papers were reviewed by a third investigator (CD). Microsoft Excel ® was used to summarise and Stata 14.2 ® to analyse the results from the SLR. After articles that met the inclusion criteria were collected, summary descriptive statistics were used to describe individuals’ demographic and clinical characteristics, the studies’ methodological characteristics and the type of costs analysed. A quantitative analysis of costs was not performed because of the heterogeneity of data units reported; therefore, the results do not show mean unit or annual costs but rather the main types of costs included in the calculations, considering the different settings and types of disease. All descriptive results are expressed in percentages, but the actual number of studies is always indicated to avoid overstating the results, in view of the limited number of studies found.
2.4 Risk of Bias
Study quality, based on the Quality of Health Economic Studies (QHES) grading system [ 31 , 32 ], was double reviewed and rated by two investigators (AHPB, MTB). The QHES criterion (quality categories [QC]) checklist was constructed and validated to evaluate cost-minimisation, cost-effectiveness and cost-utility analyses. The QHES grading system emphasises appropriate methods, valid and transparent results, and comprehensive reporting of results of each study [ 31 ] and has been used previously to evaluate the risk of bias in cost analysis and economic burden studies [ 33 ]. In the QHES grading system, items 1, 2, 7, and 12–16 (Fig. 1 , grey vertical bars) reflect the extent to which studies reported data in the original publication, and items 3–6 and 8–11 reflect study quality (Fig. 1 , blue vertical bars) [ 32 ]. The QHES instrument, like other instruments applied to cost studies, such as the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement [ 34 ], serves as a checklist for the reporting of items. The more items included, the more complete the information. Hence, the use of the QHES instrument allowed us to assess the quality of reporting of a study and, partially, the quality of the way in which the studies had been conducted. During the QHES criterion validation process, the authors proposed four quality categories as a result of the process of weighting the scores obtained (QC1-QC2-QC3-QC4) and suggested that the higher the category, the greater the reporting of data and the higher the quality of the methodology [ 31 ]. We classified every article included in one of these categories to show its overall completeness.
Frequency of each Quality of Health Economic Studies (QHES) criterion met by included studies. Quality categories (QC): QC1 (total score: 0–25) [ red rectangle ], QC2 (total score: >25–50) [ orange rectangle ], QC3 (total score: >50–75) [ yellow rectangle ], QC4 (total score: >75–100) [ green rectangle ]
The search identified 1434 papers. After removing duplicates and documents that did not meet the inclusion criteria (1324 in total), and following a hand search of papers’ references, 117 articles were thoroughly read. Of those, 43 studies were ultimately included in the SLR (Fig. 2 ).
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart
3.1 Risk of Bias Assessment
Only one study was categorised as QC1 and three studies (6.98%) as QC2; 34 studies (79.07%) had scores of between 51 and 75 (QC3), and five studies (11.63%) scored 76 or more (QC4) [Table S3 of the ESM]) (Fig. 3 ). None of the studies was excluded because of the QC score, and therefore all are included in the final count.
Number of studies in each Quality of Health Economic Studies (QHES) criterion interval. QC quality categories
In this SLR, none of the included studies was a “complete” economic evaluation (see Sect. 2.2 ), and therefore items Q12 and Q13 (8 and 7 points, respectively) were not mandatory in any of the included papers. Few scored points for Q12 [ 13 , 14 , 35 , 36 , 37 , 38 ] and only one scored points for Q13 [ 37 ] because it was proposed as a modelling study.
In the case of high scores related to data reporting (Fig. 1 , grey bars), the highest number of studies falling into category QC4 were those in which the study objective was clearly reported (Q1) [reported by all except one study [ 39 ]], followed by those reporting the method of data abstraction (Q7) [reported by all except [ 2 , 39 , 40 , 41 , 42 , 43 ]], those presenting conclusions/recommendations based on study results (Q15) [reported by all except one study [ 44 ]] and those disclosing the source of funding (Q16) [reported by all except [ 1 , 3 , 7 , 35 , 39 , 40 , 42 , 45 ]].
With regard to items relating to study methodology quality (Fig. 1 , blue bars), only one study rated as QC1 (Fig. 3 , red bar), based on the low number of items reported (14). A subgroup analysis (Q4) was mentioned by only three studies [ 46 ], all of which rated as QC3; performance of incremental analysis between alternatives (Q6) was reported only by two studies [ 1 ], which rated as QC3 and QC4, respectively. Item Q4 (1 point) is virtually mandatory in the case of a randomised controlled trial, but few of the included studies were randomised controlled trials. Item Q6 has more weight in the QHES (6 points) and is mandatory in the case of complete economic evaluations, but in the case of cost studies there are no specific suggestions in any guidelines. The highest number of studies reporting items relating to methodology (QC4, Fig. 3 , green bar) were those reporting the source of variable estimates (Q3) [reported in all studies except [ 39 ]]; analytic horizon, outcomes and justification of discount rate (when reported) (Q8) [reported in 37 studies, but not in [ 5 , 15 , 39 , 40 , 42 , 45 ]]; methodology for measurement of costs and estimation of quantities/unit costs (Q9) [reported in 36 studies, but not in [ 2 , 3 , 4 , 39 , 40 , 42 , 43 ]]; primary outcome measure (Q10) [reported in 42 studies, but not in [ 40 ]]; and valid health outcomes measures/scales (Q11) [reported in all studies except [ 39 ]]. In all cases, the QHES score raised as the completeness of reporting in both domains (data report and study quality) increased (Table S6 of the ESM).
3.2 General Characteristics of Studies
The data collected from the selected studies are from the databases created and the patients/caregivers interviewed from April 1993 [ 41 ] to September 2018 [ 47 ]. More than half of the studies (32 [69.77%]) were published from 2010 to 2022.
Most of the studies were conducted in the Americas, mainly in North America (the USA and Canada) [25 (58.14%)], and one in Latin America (Argentina). Seven were conducted in Europe (Finland, Germany, Spain, Italy, Greece and Ireland), four in the UK, four in Asia (India, China and Japan), one in Africa (Ethiopia) and one was multinational (USA, Ireland and the UK). The main characteristics of the included studies are summarised in Table S2 of the ESM.
More than half of the studies presented data on exclusively public healthcare programmes in the Americas, Asia and Europe (28 [65.12%]). Public-private services were mentioned in seven studies from Canada [ 9 , 13 , 15 , 16 , 17 , 18 , 48 ], one from the USA [ 49 ], and one from the UK [ 47 ]; one study did not provide data on the health services provider [ 12 ]. Solely private care was the modality in two studies from the USA [ 2 , 3 ], one from Greece [ 1 ], one from Italy [ 46 ] and one from Ethiopia [ 50 ]. Several studies (27 [62.79%]) reported data on patients receiving in-hospital care combined with different types of ambulatory care, and 37.20% (16 studies) reported mainly ambulatory or home-based care (Table 1 ).
Table 2 describes patients’ and caregivers’ main clinical and sociodemographic characteristics. All the studies included patients with cancer. Twenty-five studies (58.14%) reported cancer as a sole condition in the included patients, and 18 (41.86%) reported data on patients with cancer and patients with other non-oncologic diseases for which PC was provided. Out of all the studies with non-cancer patients requiring PC included as part of the sample, 12 (27.91%) had CHF and COPD, the main non-cancer conditions, and 7 (16.28%) reported dementia/AD. The severity of the conditions and the presence of comorbidities, illnesses unrelated to the one that led to the provision of PC, were also reported (for more details, see Table S2 of the ESM).
The study time frame (follow-up time) was variable, with range from up to a month in ten studies (23.81%) to at least 1 year in 18 studies (41.86%) and more than 2 years in one study [ 35 ]. Seven studies reported several follow-up and cost-measuring periods (i.e. repeated measures, related to the type of data analysis) [ 2 , 5 , 6 , 15 , 44 , 49 , 51 ]. Mean survival time after starting PC was reported in 12 (27.91%) of the studies, and median survival time was reported in seven studies (16.28%); in all cases, it was less than 1 year (Table 2 ).
All papers reported data on the patients’ characteristics, and ten (23.26%) reported data related to caregivers. Patient ethnicity was diverse and reported in nine studies; five were from the USA [ 2 , 3 , 7 , 38 , 52 ], one was from the UK [ 47 ], one was from China (Hong Kong) [ 45 ] and one was multinational (UK, Ireland, USA) [ 6 ]. The caregivers’ ethnicity was not mentioned in any paper.
The clinical professionals in the PC teams assigned to patients were diverse. Some had a specialist physician as a team leader and worked with other physicians, nurses, social workers and other healthcare personnel (e.g. various types of therapists). Others had nurses as team leaders [ 43 ]. There was no homogeneity in the composition of the teams (Table S4 of the ESM).
3.3 Cost Methodology
Thirty-three studies (76.74%) were of costs only, ten (23.26%) were analyses of resource use (three cohort and six descriptive studies) and one used a decision model to estimate costs over longer time horizons [ 37 ] (Table 3 ). The predominant cost methodology was bottom-up (88.37%), and the main study design was retrospective (60.47%). The most common perspective for the analysis was that of the national health system (financer) (53.49%), and direct healthcare costs were the most commonly measured and evaluated costs. The follow-up period was reported in all 43 studies and was up to a year in 33 of them (76.74%).
All studies except for one [ 39 ] reported data on direct healthcare costs, and 12 (27,6%) reported data on direct non-healthcare costs [ 6 , 9 , 13 , 14 , 15 , 16 , 17 , 18 , 47 , 53 , 54 , 55 ]; 12 (27.91%) studies explored direct and indirect costs borne by patients and caregivers; three studies (6.99%) explored patients’ productivity losses [ 12 , 45 , 56 ] and ten (23.26%) caregivers’ productivity losses [ 9 , 12 , 13 , 15 , 16 , 17 , 18 , 45 , 55 , 56 ], all from a societal perspective (Table 3 ). Studies focusing on a societal perspective were mainly prospective.
Data on costs for a 1-year time horizon were collected through questionnaires administered to patients’ caregivers, using the opportunity costs method and collecting data on out-of-pocket expenditures, such as travel expenses, medications needed because of being a caregiver, and time and/or income loss. Only one study [ 17 ] measured the direct healthcare costs for carers. No study conducted a regression analysis to analyse the social and economic factors associated with caregivers’ income losses.
The time horizon and its linked data and discount rate were explicitly reported only in one study [ 57 ]. A regression analysis to define the factors that determine cost drivers was conducted in almost half of the studies (19 [44.19%]); a sensitivity analysis to test the robustness of the final result was conducted in six studies, but only one reported the type of analysis used (deterministic) [ 35 ] [details on regression variables in Table S5 of the ESM].
Twenty studies reported an increase in care intensity and, consequently, an increase in care costs in the end-of-life (EOL) period. Of these, 11 (25.63%) were studies made from the financer perspective (public or private) [ 4 , 5 , 40 , 41 , 42 , 44 , 46 , 48 , 58 , 59 ], seven (16.31%) from the societal perspective [ 12 , 13 , 14 , 15 , 16 , 17 , 55 ] and two (4.66%) from the provider perspective [ 38 , 49 ]. The periods of time of increased care intensity ranged from 1 to 24 months.
Original (primary) data were reported in five studies (11.65%) [ 6 , 17 , 42 , 45 , 55 ]. Data from administrative databases were reported in 26 studies (60.58%), and a mixture of data sources was reported in 12 (27.96%) studies.
Of the five studies that used primary data, one study was conducted from a financer perspective [ 42 ], three studies from a societal perspective [ 17 , 45 , 55 ] and one study did not report the perspective [ 6 ]. Only one study that collected data from administrative data sources applied a societal perspective [ 50 ]; the rest (25 [58.25%]) used a financer or provider perspective.
Of 12 studies conducted from a societal perspective, 11 used a questionnaire to collect data [ 9 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 45 , 55 , 56 ]. Four of those studies used a validated questionnaire to collect data from patients/caregivers (Ambulatory and Home Care Record [AHCR © ]), and those four studies were conducted by the same research team [ 13 , 15 , 16 , 17 ]. Of the remaining studies, three reported using the same non-validated questionnaire [ 9 , 14 , 18 ], and the other mentioned the use of a national-level survey [ 55 ]. In the cases where data were collected from patients/caregivers (13 studies), the questionnaire was administered in a face-to-face scenario in two studies [ 45 , 56 ] and in written form only in one study [ 12 ]. A face-to-face interview and a telephone call was the method in four of the studies [ 9 , 14 , 18 , 55 ] and a phone call only in the remaining four studies [ 13 , 15 , 16 , 17 ]. There were no data related to the interview in one study [ 6 ].
The valuation method for measuring informal care costs (provided by family/caregivers) was the opportunity cost in all ten studies reported; these costs related to diverse caregiving activities (e.g. accompanying patients to appointments, changing dressings, picking up medications [ 17 ]), assisting with basic activities of daily living (personal care, eating and drinking, going to the toilet, mobility indoors) and instrumental activities of daily living (taking medications, household and administrative tasks) [ 55 ]. Direct questions were used to ascertain time spent in most cases [ 9 , 13 , 14 , 15 , 16 , 17 , 18 , 55 ] (Table 3 ).
3.4 Cost Outcomes
The costs during the follow-up period were variable and collected during periods that ranged from 1 to 24 months; some of the costs were measured over several periods for the same patients and caregivers in order to determine unit cost variations (see above, Table 2 ). The most common scenario was the multiple-setting scenario (46.51%), followed by the home-based scenario (30.23%). In six studies (13.95%) in which patients’ care transitioned from in-hospital care to home care, data on costs were collected in both stages. Four studies (9.30%) collected data mainly on the ambulatory use of resources [ 14 , 35 , 47 , 56 ].
Inpatient care (acute hospitalisation and visits to the emergency department) was found to be the main source of billing in 24 studies (55.81%); of those, the use of resources from more than one setting but with a dominance of hospitalisation costs was seen in 15 studies (34.95%) [ 3 , 6 , 12 , 37 , 38 , 41 , 43 , 44 , 48 , 51 , 55 , 57 , 58 , 59 ]. Four studies (9.32%) [ 14 , 35 , 47 , 56 ] reported direct costs covered by patients or unpaid caregivers in mainly ambulatory settings, 12 studies (27.96%) [ 3 , 4 , 13 , 16 , 17 , 18 , 40 , 46 , 49 , 54 , 60 , 61 ] in exclusively home-based settings, and 26 studies (60.58%) in situations where care was provided in the home setting at some point (Table 1 ). Other drivers of resource use worth mentioning were blood transfusion, mentioned in two studies (4.66%) [ 1 , 46 ], and non-quantified volunteer support, mentioned in one study (2.33%) [ 47 ].
Almost half of the studies (20 [46.51%]) included data on the augmented use of resources and costs of healthcare in the EOL period. In 12 studies (27.96%), the main driver was the use of in-hospital resources; in three (6.99%) — conducted from a societal perspective — it was the unpaid workload taken on by caregivers [ 13 , 16 , 17 ]; in the remaining four (9.32%), the drivers varied. The period of more intense use of resources ranged from 1 to 12 months; the proportion of change in the use of resources was highly variable, and ranged from 37% in one study [ 12 ] to 450% in two others [ 13 , 44 ] and more than 600% in one study [ 4 ]. This change was mainly because of hospitalisation. No studies included modelling to estimate costs over longer time horizons.
A regression analysis was conducted in 19 studies (47.5%) to determine the main drivers of costs/expenditures (see Table S5 of the ESM). Because of the distribution of data results, the independent variable was transformed in 13 studies (32.5%). Of the studies analysed with regression models, the most frequently cited cost drivers were hospitalisation/inpatient care, mentioned in ten studies (25%), and costs owing to unpaid caregiving time, mentioned in four studies (10%) [data not shown]. The independent variable used in the models was heterogeneous across studies (Table S5 of the ESM). Only six studies out of 40 included a sensitivity analysis. In all cases, uncertainty was evaluated with a deterministic univariate or multivariate sensitivity analysis.
3.5 Costs Included
Direct healthcare costs covered by the healthcare system were reported in almost all studies (42 [97.67%]). In contrast, direct non-healthcare costs covered by healthcare systems and direct costs covered by the patient were reported in 13 (30.23%) and 12 studies (27.91%), respectively. To manage the heterogeneity of the data reported, the types of costs were assigned to groups according to the setting (outpatient/ambulatory, inpatient, home-based, multiple care settings) and type of disease (cancer and cancer plus other chronic terminal diseases) (Table 4 ). The settings where the productivity losses of patients and caregivers were recorded varied, without any discernible pattern. Almost half of the studies (18 [45%]) included a mix of patients with different diagnoses, some with cancer and others with non-cancer conditions, as the main trigger for initiating ambulatory-based PC.
In the case of studies using a societal perspective (11 [25.58%]), productivity losses for patients were reported in two studies (6.99%) [ 12 , 45 ] and productivity losses of caregivers were reported in nine studies (20.97%) [ 9 , 12 , 13 , 15 , 16 , 17 , 18 , 45 , 55 ]. In terms of patient diagnoses, in the same group of studies conducted from a societal perspective, two studies with cancer as the only condition requiring PC reported productivity losses for patients [ 12 , 45 ]; four studies (9.32%) including both patients with cancer and patients with cancer and other terminal conditions reported productivity losses for caregivers [ 9 , 13 , 18 , 55 ] (Table 4 ).
The reporting of costs by ambulatory-based setting and disease shows that the most common type of cost included was direct healthcare costs (97.67% of studies) (Table 4 ). Data on costs related to productivity losses for patients or their families/caregivers in ambulatory-based settings are lacking. Data related to patients were collected in an inpatient/home setting in two cases [ 12 , 45 ], a home-based setting in two cases [ 16 , 17 ] and an outpatient/ambulatory setting in one case [ 56 ]. Productivity loss for caregivers was reported in nine studies (20.97%), four home-based settings [ 13 , 16 , 17 , 18 ], three in hospital/home settings [ 9 , 15 , 45 ] and two in all possible settings [ 12 , 55 ]; this information was not reported in the exclusively ambulatory setting.
The most commonly included costs are shown in Table 5 . Reported as direct healthcare costs, inpatient care (hospitalisation), physician visits and home care were included in more than 70% of the studies. Physician visits, emergency department visits, medication and nurse visits followed these. Among direct non-healthcare costs, out-of-pocket expenditures and informal spiritual care were the most frequently included and measured costs, followed by professional care at home, medications, private transportation and equipment not covered by the national healthcare system. Patient productivity losses were included far less than caregiver productivity losses. Temporary occupational leave and loss of leisure time were the two most commonly included and quantified costs when the societal perspective was used.
4 Discussion
The current path for PC support is the ambulatory-based setting. Some decades ago, PC services were part of the hospital and hospice setting, and ambulatory or home-based care was considered an exceptional form of service from a logistic point of view. However, patients’ and their caregivers’ medical and social needs have evolved over time, driving a transformation in healthcare systems organisation. To determine the costs and the methodology followed by researchers in these areas to capture data, we proposed an SLR.
To evaluate the methodological quality and transparency of data reporting from the studies included in this SLR, we used the QHES grading system (QC) (Figs. 1 and 3 , Table S3 of the ESM). During the process, because of the scarcity of studies related only to costs in ambulatory/home-based PC, we unanimously decided to include all the studies that passed the second complete examination during the literature search (Fig. 2 ); although several had a low QC score (QC1 [ 39 ] and QC2 [ 2 , 40 , 42 ]), they were not excluded. The score was used not as a criterion for removing studies from the SLR but as a method to determine the thoroughness of each study. Hence, even the four studies with the lowest scores (QC1 [0–25] and QC2 [>25–50]) were reviewed and their data were included.
In the final analysis, only one study had very low scores (2.33%) and three studies had low scores (2.33%); a majority had high scores (QC3 [79.07%]) and very high scores (QC4 [11.63%]). This was interpreted as evidence that, even with failings, the quality of cost studies is high, although it could be better. When we looked at which QC domain — reporting of data or study of methodological quality — determined the change in scores among the studies, we found that both contributed: scores were higher as the number of items related to reporting and those related to methodology increased (Table S6 of the ESM). We did not find any pattern that would explain a low or high result related to the QC score as a whole.
Other SLRs on PC costs, including both complete and incomplete economic evaluations, have been published in recent decades. We double checked that all studies included in the four SLRs identified were found in our study. No previous SLR has been conducted specifically on the costs of PC.
Gomes et al. [ 62 ] published a Cochrane review of 23 studies (from 1978 to 2012) on the effectiveness and cost effectiveness of home PC services, but not specifically on cost data. The results provide evidence of the effectiveness of home-based PC in terms of increasing the patient’s chance of dying at home and reducing the symptom burden for patients, but no significant impact on the caregiver’s grief. Results related to cost effectiveness were not precise, especially in relation to non-oncologic conditions. Data on the effect on (only) the costs of home-based PC, as opposed to usual care, indicated lower expenses in the home-based PC groups, except in one study from 1986, Greer et al. [ 38 ]. However, the differences in costs were statistically significant in just one study (also included in this SLR [Brumley et al. [ 2 ]]). Gomes et al. do not report more data on significant differences in costs that would enable us to compare our results with theirs.
In 2017, Gardiner et al. [ 63 ] published an SLR (1995–2015) whose results proposed the relevant cost components for economic evaluations on PC and approaches for measuring these costs. The paper suggested a framework for determining PC costs rather than describing cost results. This article was identified by our SLR, but was discarded because it failed to meet the inclusion criteria. However, it could help to define some domains and items for the forthcoming questionnaire for cost collection that will follow this publication.
Yadav et al. [ 64 ] published an SLR on healthcare costs for patients with cancer, including 16 studies conducted in the USA between 2008 and 2018. They reported costs in different scenarios (inpatient-based, outpatient/inpatient, home-based and multiple settings) and concluded that the provision of PC after a cancer diagnosis is a cost-saving strategy for the healthcare system, especially when comparing inpatient and outpatient strategies. They report that a home-based approach can save on hospital costs, but there were no data on savings with respect to non-hospital costs. In contrast to this SLR, we have stated the perspective adopted by cost studies, extended the number of years included in the SLR and included studies from around the world. Similarly to our review, the Yadav et al. review concluded that the healthcare system can save money when promoting home-based PC.
Gonzalez-Jaramillo et al. [ 65 ] published an SLR that calculated the effectiveness of home-based PC in terms of reducing hospital visits and whether home-based care lowered healthcare costs. They concluded that in patients with cancer and non-cancer patients, home-based PC consistently reduced the number of hospital visits and their length, as well as hospitalisation and overall healthcare costs, findings that reinforced our results. The authors mention that the higher costs of outpatient care are offset by the hospital expenses saved. However, as in the case of Yadav et al., the authors do not specify the perspective used in collecting cost data or the amount of expenses transferred from the healthcare system to the patient and family/caregivers.
Currently, ambulatory and home-based care are frequently used daily services. However, they are not used independently from hospitalisation. Outpatient (or ambulatory) and home-based care can be used as the primary setting, but will always be shared with hospitalisation periods. We found that the delivery of ambulatory services is not uniform; only three studies reported mainly ambulatory services: first, a modelling study to explore the use of resources from the beginning of a strong opioid prescription (EOL) [ 35 ]; second, a study collecting data on patients in EOL care in urban areas of Canada [ 14 ]; and third, a study exploring PC day services in England, Scotland and Northern Ireland [ 47 ]. The rest of the studies concerned patients being treated with a predominantly ambulatory care model and variations of admittance to hospital, emergency department or hospice if needed. More than half of the studies referred to costs related to hospitalisation services as the main cost driver (24 studies [55.81%]). Thus, most of the resources used for ambulatory management of patients are linked to hospital care at some point, a finding that supports our previous statement. Our findings also highlight the fact that the ambulatory approach needs to be integrated or coordinated with other healthcare system levels [ 13 , 16 , 17 ] and support the proposal of classifying ambulatory and home-based PC settings under the ambulatory-based PC label, which we considered more precise.
Ambulatory-based PC services are increasing in PC practice for several reasons. The first is requests from patients who prefer to die at home [ 62 ]. Such requests have prompted healthcare systems to change and align their outcomes with those of patients [ 66 , 67 ], offering ambulatory and home-based PC when possible.
The second reason is the limited number and the high cost of hospital beds available in hospital acute-care settings for patients with chronic cancer and non-cancer conditions when they move from active treatment to PC [ 68 ]. These beds are mostly needed by acute patients who require inpatient care. Still, even when patients are in better condition and are able to return home, they are considered to have been transferred to home-based hospitalisation and are still regarded as inpatients. These clinical decisions are supported by studies that report home-based care as a safe and good practice with adequate clinical outcomes and lower costs [ 13 , 69 ]. Similar studies of ambulatory-based PC are lacking.
The third and no less important reason is billing. The ambulatory approach has been used broadly in elective surgery scenarios. Large studies of acute ambulatory surgery programmes reveal fewer expenses after medium- and high-complexity surgical procedures (e.g. hernia repair, laparoscopic cholecystectomy, laparoscopic appendicectomy, thyroidectomy, total shoulder, hip or knee replacement) [ 70 , 71 ]. Even some acute medical situations (e.g. pneumonia) can be stabilised during a short inpatient stay, after which the patient is sent home to continue and finish treatment [ 72 ]. Because of their short duration, these acute services are provided by paid healthcare personnel who assist the patient at home, allowing both the patient and family to be well assisted [ 16 , 17 ], a similar approach to PC.
For the reasons mentioned above, ambulatory PC services are being implemented in various healthcare systems worldwide [ 68 ]. These PC programmes complement, rather than replace, classical hospital or hospice-based PC services, with excellent economic results from the point of view of healthcare providers: it is far less expensive to deliver this EOL care modality to the patient at home rather than at the hospital. However, what needs to be recognised is that when a PC patient is moved to home-based management, the burden and most of the costs are transferred to patients and their caregivers/families. The indirect costs of productivity loss, out-of-pocket expenditures, loss of leisure time and burn-out of caregivers, for example, are not currently being quantified. Conceptually, ambulatory PC represents a shift of the economic burden from the provider to the patient without substantial support from health systems for patients’ families [ 9 , 12 , 13 , 14 , 18 , 51 , 64 ]. This shifting leaves out of the equation the non-tangible healthcare costs transferred to the family, in particular the contribution made by caregivers [ 12 , 13 , 64 ].
In the inclusion criteria, we proposed to include studies involving patients with breast, colon and pulmonary malignancies, CHF and COPD. These criteria were based on disease prevalence [ 20 , 73 ]. However, during the screening, we detected two studies conducted with a different sample — patients with haematological neoplasms [ 1 , 46 ]. We included them in the review to explore the cost approach in these oncologic conditions. We saw that ambulatory PC for these patients was based mainly on transfusions, and the reports barely mentioned the role of caregivers [ 38 ] or the burden that the care of patients implies. We also found more studies than expected that reported data on the costs of PC for patients with dementia/AD, and although the PC path was not very clear in those reports, we decided to mention them and examine and include their data [ 4 , 8 , 41 , 46 , 58 , 59 , 69 ] (Table 4 ). What we found reveals that the organisation and the delivery of PC may differ depending on the disease; we also found that our inclusion criteria and the diseases considered may have been too narrow and may have limited our results, making it difficult to extrapolate them. Still, the funnel approach is necessary to deepen the data and to provide more specific results.
The included studies reported cost results using heterogeneous outcome/measure units such as overall cost, mean cost, cost per patient, total cost, average cost and cost per week. Lack of transparency in calculating the different costs and the definition of some types of costs did not facilitate pooling or quantitative summarisation of cost data or the development of a meta-analysis (not a primary aim of this SLR). We suspected this would be the case, based on other studies that mentioned the wide variety of reporting unit costs. Moreover, the appropriate unit for measuring PC in the early stages of cancer or in the case of non-oncological conditions could be annual cost, but as soon as the patients’ health status changes because of the disease’s progression, a more appropriate unit would probably be monthly or weekly costs. Better data pooling and perhaps a meta-analysis could be conducted in the future if more homogeneous methods of reporting unit cost results/outcomes are developed [ 74 ].
The follow-up time was variable and diverse, and ranged from less than a month to more than a year, and in some studies, several time periods were examined [ 2 , 5 , 15 , 37 , 41 ]. We did not find any relationship between follow-up time and diagnosis in the included studies, nor were the criteria for deciding the follow-up time clear in most of them, although there were seven studies that planned a repeated-measures analysis [ 2 , 5 , 15 , 37 , 41 , 42 ].
The follow-up time has cost consequences. Clinically, survival rates of patients with non-cancer conditions included in a PC programme are higher than those of patients with cancer receiving the same PC modality. However, all the evidence in terms of the economic burden or costs of PC patients has been calculated by averaging data on patients with non-cancer diagnoses with those of patients with cancer, and no independent estimations linked to the condition that triggers the PC have been done as yet.
Patients with end-stage COPD, CHF or AD can experience acute episodes of deterioration of their condition, but in most cases, treatment of such deterioration can rapidly ameliorate patients’ health status. Such an outcome is not frequently seen in patients with cancer, in which a severe deterioration in their clinical condition, once the last line of oncological treatment has been completed, invariably progresses to the patients’ death. Thus, the survival expectancy that would define a follow-up time can vary from a few weeks in terminal lung or breast cancer to several years in the case of CHF, COPD or AD.
Calculating a mean or average cost for PC patients and combining patients with a cancer diagnosis and patients with a non-cancer diagnosis is probably an inaccurate method to obtain cost data because of the different evolutions of illnesses requiring PC and the differences in survival rates. The scarcity of cost studies relating exclusively to PC in non-cancer patients, comparing the costs of PC programmes for patients with these different conditions, is remarkable (the initial search included only one complete economic evaluation on CHF [ 75 ], which was excluded because it failed to meet the inclusion criteria). There is therefore an urgent need for such studies.
From the cost perspective, we could not find clarity related to the time horizons used for the cost calculations. All studies reported a follow-up period, but the time horizon was not explicitly mentioned. These two points should be kept in mind when planning future research, and both a follow-up time in relation to the clinical outcome and a time horizon concerning cost calculation should be defined. New approaches might have to be created to consolidate PC costs, as suggested by other authors concerning quality-adjusted life-years in a cost-utility analysis for PC [ 74 ].
Nineteen studies reported increased use of resources during the EOL period. Among these studies, the main source of augmented costs was inpatient care in studies made from a health system perspective (52%) [ 5 , 34 , 36 , 38 , 41 , 44 , 54 , 55 , 56 , 57 ]. When studies were made from a societal perspective, the outcome was not too different; in more than half of such studies, the increase in the use of resources was owing to inpatient care provided at some point [ 12 , 14 , 15 , 51 ] and in the rest it was related to care provided by unpaid caregivers [ 13 , 16 , 17 ]. This finding confirms what we previously pointed out: hospitalisation is the main driver of patients’ care costs, even in ambulatory-based PC [ 9 , 12 , 14 , 15 , 18 , 45 , 51 ].
Most cost studies that have applied a healthcare system perspective have concluded that using ambulatory PC services as an alternative to hospital-based care diminishes costs for the healthcare system and, therefore, is an efficient alternative. In contrast, all studies applying a societal perspective reveal that ambulatory services are costlier and less efficient than inpatient hospital care [ 9 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 39 , 45 , 51 , 52 ]. The economic burden borne by family and informal caregivers is explained by the unpaid costs of productivity and leisure time loss. Thus, although the societal perspective entails challenges in terms of approaching patients and caregivers, it should be considered the ideal for evaluating PC services in an ambulatory-based setting (i.e. one that combines hospitalisation and any type of ambulatory care) in order to include economic impacts that are frequently not considered in PC cost studies but that are relevant from an efficiency point of view [ 13 , 16 , 17 ].
This SLR identified the most common costs related to ambulatory-based PC for patients with cancer or non-cancer terminal illnesses. Direct healthcare costs in home-based or multiple care settings have been those most analysed and evaluated, inpatient care (hospitalisation), physician visits and home care being the most common costs included to measure the economic burden of PC. It is worrying that little evidence exists regarding productivity losses for patients receiving PC and their family members or caregivers, especially for patients in an outpatient/ambulatory setting.
The need for ambulatory PC is clear. It is desired by both patients and the healthcare system, and the changing scenario of healthcare makes it possible to provide PC in an ambulatory setting. However, adequate implementation of ambulatory PC has a cost that the healthcare system should bear in partnership with society, and society should receive support for being part of the healthcare team [ 14 , 15 , 18 ].
5 Conclusions
This SLR reveals that studies on the costs of ambulatory PC are increasing. These studies are mostly conducted from a healthcare system perspective, which leaves out costs related to patients’/caregivers’ economic burden; these costs are mainly driven by productivity and leisure time losses. The available evidence shows a lack of studies that measure and evaluate average patient and caregiver expenses, and there is therefore a need for better evidence on the scale and patterns of costs.
Future studies should preferentially propose cost calculation approaches using societal and patient perspectives to better estimate the economic burden imposed on patients and caregivers in ambulatory-based PC. There is a need for prospective studies to calculate this financial burden more precisely and evaluate, with better quality evidence, interventions and actions designed to improve the quality of life of PC patients. Based on the evidence found, productivity losses for PC patients and their carers have been the least studied cost impact, so future research should endeavour to measure and economically quantify such losses.
The results reported in this SLR should serve as guidance for future cost collection studies and questionnaires in ambulatory-based settings from any perspective, but especially those designed to measure and evaluate the costs from a societal perspective, including the carer’s economic burden. The methodology should include a clear plan for collecting administrative data based on its primary structure and organisation and a well-structured, respectful and comprehensive approach to the collection of data from patients and caregivers. An effort to standardise a methodology would be desirable in order to produce more homogeneous biomedical literature on this subject.
Tzala S, Lord J, Ziras N, Repousis P, Potamianou A, Tzala E. Cost of home palliative care compared with conventional hospital care for patients with haematological cancers in Greece. Eur J Health Econ. 2005;6:102–6.
PubMed Google Scholar
Brumley R, Enguidanos S, Jamison P, Seitz R, Morgenstern N, Saito S, et al. Increased satisfaction with care and lower costs: results of a randomized trial of in-home palliative care. J Am Geriatr Soc. 2007;55:993–1000.
Enguidanos S, Cherin D, Brumley R. Home-based palliative care study: site of death, and costs of medical care for patients with congestive heart failure, chronic obstructive pulmonary disease, and cancer. J Soc Work End Life Palliat Care. 2005;1:37–56.
Cassel JB, Kerr KM, McClish DK, Skoro N, Johnson S, Wanke C, et al. Effect of a home-based palliative care program on healthcare use and costs. J Am Geriatr Soc. 2016;64:2288–95.
Google Scholar
Li Z, Pan Z, Zhang L, He R, Jiang S, Xu C, et al. End-of-life cost and its determinants for cancer patients in urban China: a population-based retrospective study. BMJ Open. 2019;9: e026309.
PubMed PubMed Central Google Scholar
Yi D, Johnston BM, Ryan K, Daveson BA, Meier DE, Smith M, et al. Drivers of care costs and quality in the last 3 months of life among older people receiving palliative care: a multinational mortality follow-back survey across England, Ireland and the United States. Palliat Med. 2020;34:513–23. https://doi.org/10.1177/0269216319896745 .
Article PubMed Google Scholar
Wang H, Qiu F, Boilesen E, Nayar P, Lander L, Watkins K, et al. Rural-urban differences in costs of end-of-life care for elderly cancer patients in the United States. J Rural Health. 2016;32:353–62.
CAS PubMed Google Scholar
Terada T, Nakamura K, Seino K, Kizuki M, Inase N. Cost of shifting from healthcare to long-term care in later life across major diseases: analysis of end-of-life care during the last 24 months of life. J Rural Med. 2018;13:40–7.
Dumont S, Jacobs P, Turcotte V, Turcotte S, Johnston G. Palliative care costs in Canada: a descriptive comparison of studies of urban and rural patients near end of life. Palliat Med. 2015;29:908–17.
World Health Organization. WHO definition of palliative care. 2013. Available from: http://www.who.int/cancer/palliative/definition/en . [Accessed 17 Nov 2023].
European Association for Palliative Care. 2020. Available from: https://www.eapcnet.eu/ . [Accessed 28 Feb 2020].
Haltia O, Färkkilä N, Roine RP, Sintonen H, Taari K, Hänninen J, et al. The indirect costs of palliative care in end-stage cancer: a real-life longitudinal register- and questionnaire-based study. Palliat Med. 2018;32:493–9. https://doi.org/10.1177/0269216317729789 .
Guerriere DN, Zagorski B, Fassbender K, Masucci L, Librach L, Coyte PC. Cost variations in ambulatory and home-based palliative care. Palliat Med. 2010;24:523–32.
Dumont S, Jacobs P, Turcotte V, Anderson D, Harel F. The trajectory of palliative care costs over the last 5 months of life: a Canadian longitudinal study. Palliat Med. 2010;24:630–40.
Yu M, Guerriere DN, Coyte PC. Societal costs of home and hospital end-of-life care for palliative care patients in Ontario, Canada. Health Soc Care Community. 2015;23:605–18.
Chai H, Guerriere DN, Zagorski B, Coyte PC. The magnitude, share and determinants of unpaid care costs for home-based palliative care service provision in Toronto, Canada. Health Soc Care Community. 2014;22:30–9.
Chai H, Guerriere DN, Zagorski B, Kennedy J, Coyte PC. The size, share, and predictors of publicly financed healthcare costs in the home setting over the palliative care trajectory: a prospective study. J Palliat Care. 2013;29:154–62. https://doi.org/10.1177/082585971302900304 .
Dumont S, Jacobs P, Turcotte V, Turcotte S, Johnston G. Distribution and sharing of palliative care costs in rural areas of Canada. J Palliat Care. 2014;30:90–8. https://doi.org/10.1177/082585971403000204 .
Jacobs P, Dumont S, Turcotte V, Anderson D. Evaluating the economic loss of caregiving for palliative care patients. J Palliat Care. 2011;27:210–5. https://doi.org/10.1177/082585971102700305 .
Anguita Sánchez M, Crespo Leiro MG, De Teresa GE, Jiménez Navarro M, Alonso-Pulpón L, Muñiz GJ. Prevalencia de la insuficiencia cardiaca en la población general Española mayor de 45 años. Estudio PRICE Revista Espanola de Cardiologia. 2008;61:1041–9.
Colucci L, Bosco M, Fasanaro AM, Gaeta GL, Ricci G, Amenta F. Alzheimer’s disease costs: what we know and what we should take into account. J Alzheimers Dis. 2014;42:1311–24.
Guerriere DN, Coyte PC. The ambulatory and home care record: a methodological framework for economic analyses in end-of-life care. J Aging Res. 2011;2011: 374237.
Gardiner C, Ingleton C, Ryan T, Ward S, Gott M, Gomersall JS, et al. What cost components are relevant for economic evaluations of palliative care, and what approaches are used to measure these costs? A systematic review. Palliat Med. 2015;31:170–8. https://doi.org/10.1177/0269216317729789 .
Article Google Scholar
Gardiner C, McDermott C, Hulme C. Costs of Family Caregiving in Palliative Care (COFAC) questionnaire: development and piloting of a new survey tool. BMJ Support Palliat care. 2019;9:300–6.
Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6: e1000097.
Meier DE, Beresford L. Outpatient clinics are a new frontier for palliative care. J Palliat Med. 2008;11:823–8.
Nordly M, Vadstrup ES, Sjogren P, Kurita GP. Home-based specialized palliative care in patients with advanced cancer: a systematic review. Palliat Support Care. 2016;14:713–24.
Currow DC, Agar MR, Phillips JL. Role of hospice care at the end of life for people with cancer. J Clin Oncol. 2020;38:937–43. https://doi.org/10.1200/JCO.18.02235 .
Frasca M, Galvin A, Raherison C, Soubeyran P, Burucoa B, Bellera C, et al. Palliative versus hospice care in patients with cancer: a systematic review. BMJ Support Palliat Care. 2021;11:188–99.
Biegel DE, Sales ESR. Family caregiving in chronic illness: Alzheimer’s disease, cancer, heart disease, mental illness, and stroke. Thousand Oaks: Sage Publications; 1991.
Chiou CF, Hay JW, Wallace JF, Bloom BS, Neumann PJ, Sullivan SD, et al. Development and validation of a grading system for the quality of cost-effectiveness studies. Med Care. 2003;41:32–44.
Ofman JJ, Sullivan SD, Neumann PJ, Chiou CF, Henning JM, Wade SW, et al. Examining the value and quality of health economic analyses: implications of utilizing the QHES. J Manag Care Pharm. 2003;9:53–61.
Norum J, Nieder C. Treatments for metastatic prostate cancer (mPC): a review of costing evidence. Pharmacoeconomics. 2017;35:1223–36.
Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health. 2022;25:3–9. https://doi.org/10.1016/j.jval.2021.11.1351 .
Guest JF, Ruiz FJ, Greener MJ, Trotman IF. Palliative care treatment patterns and associated costs of healthcare resource use for specific advanced cancer patients in the UK. Eur J Cancer Care. 2006;15:65–73.
CAS Google Scholar
Walker H, Anderson M, Farahati F, Howell D, Librach SL, Husain A, et al. Resource use and costs of end of life/palliative care: Ontario adult cancer patients dying during 2002–2003. J Palliat Care. 2011;27:79–88.
McBride T, Morton A, Nichols A, Van Stolk C. Comparing the costs of alternative models of end-of-life care. J Palliat Care. 2011;27:126–33. https://doi.org/10.1177/082585971102700208 .
Greer JA, Tramontano AC, McMahon PM, Pirl WF, Jackson VA, El-Jawahri A, et al. Cost analysis of a randomized trial of early palliative care in patients with metastatic nonsmall-cell lung cancer. J Palliat Med. 2016;19:842–8.
Wenk R, Bertolino M, Pussetto J. Direct medical costs of an Argentinean domiciliary palliative care model. J Pain Symptom Manage. 2000;20:162–5.
Serra-Prat M, Gallo P, Picaza JM. Home palliative care as a cost-saving alternative: evidence from Catalonia. Palliat Med. 2001;15:271–8.
Fassbender K, Fainsinger R, Brenneis C, Brown P, Braun T, Jacobs P. Utilization and costs of the introduction of system-wide palliative care in Alberta, 1993–2000. Palliat Med. 2005;19:513–20.
Gómez-Batiste X, Tuca A, Corrales E, Porta-Sales J, Amor M, Espinosa J, et al. Resource consumption and costs of palliative care services in Spain: a multicenter prospective study. J Pain Symptom Manage. 2006;31:522–32.
Yosick L, Crook RE, Gatto M, Maxwell TL, Duncan I, Ahmed T, et al. Effects of a population health community-based palliative care program on cost and utilization. J Palliat Med. 2019;22:1075–81.
Hollander MJ. Costs of end-of-life care: findings from the province of Saskatchewan. Healthc Q. 2009;12:50–8.
Chan ATC, Jacobs P, Yeo W, Lai M, Hazlett CB, Mok TSK, et al. The cost of palliative care for hepatocellular carcinoma in Hong Kong. Pharmacoeconomics. 2001;19:947–53.
Cartoni C, Brunetti GA, D’Elia GM, Breccia M, Niscola P, Marini MG, et al. Cost analysis of a domiciliary program of supportive and palliative care for patients with hematologic malignancies. Haematologica. 2007;92:666–73.
Mitchell PM, Coast J, Myring G, Ricciardi F, Vickerstaff V, Jones L, et al. Exploring the costs, consequences and efficiency of three types of palliative care day services in the UK: a pragmatic before-and-after descriptive cohort study. BMC Palliat Care. 2020;19:1–9.
Cheung MC, Earle CC, Rangrej J, Ho TH, Liu N, Barbera L, et al. Impact of aggressive management and palliative care on cancer costs in the final month of life. Cancer. 2015;121:3307–15.
Kerr CW, Donohue KA, Tangeman JC, Serehali AM, Knodel SM, Grant PC, et al. Cost savings and enhanced hospice enrollment with a home-based palliative care program implemented as a hospice–private payer partnership. J Palliat Med. 2014;17:1328–35.
Reid EA, Abathun E, Diribi J, Mamo Y, Wondemagegnhu T, Hall P, et al. Early palliative care in newly diagnosed cancer in Ethiopia: feasibility randomised controlled trial and cost analysis. BMJ Support Palliat Care. 2022. https://doi.org/10.1136/spcare-2022-003996 .
Look Hong NJ, Liu N, Wright FC, MacKinnon M, Seung SJ, Earle CC, et al. Assessing the impact of early identification of patients appropriate for palliative care on resource use and costs in the final month of life. JCO Oncol Pract. 2020;16:e688-702.
May P, Garrido MM, Cassel JB, et al. Palliative care teams’ Cost-saving effect is larger for cancer patients with higher numbers of comorbidities. Health Aff (Millwood). 2016;35:44–53.
Dzingina MD, Reilly CC, Bausewein C, Jolley CJ, Moxham J, McCrone P, et al. Variations in the cost of formal and informal health care for patients with advanced chronic disease and refractory breathlessness: a cross-sectional secondary analysis. Palliat Med. 2017;31:369–77.
Seow H, Salam-White L, Bainbridge D. Community-based specialist palliative care teams and health system costs at end of life: a retrospective matched cohort study. CMAJ Open. 2019;7:E73-80.
Brick A, Smith S, Normand C, O’Hara S, Droog E, Tyrrell E, et al. Costs of formal and informal care in the last year of life for patients in receipt of specialist palliative care. Palliat Med. 2017;31:356–68. https://doi.org/10.1177/0269216316686277 .
Harsvardhan R, Arora T, Singh S, Lal P. Cost analysis on total cost incurred (including out-of-pocket expenditure and social cost) during palliative care in cases of head-and-neck cancer at a government regional cancer centre in North India. Indian J Palliat Care. 2022;28:419–27.
Emmert M, Pohl-Dernick K, Wein A, Dörje F, Merkel S, Boxberger F, et al. Palliative treatment of colorectal cancer in Germany: cost of care and quality of life. Eur J Health Econ. 2013;14:629–38.
Bremner KE, Krahn MD, Warren JL, Hoch JS, Barrett MJ, Liu N, et al. An international comparison of costs of end-of-life care for advanced lung cancer patients using health administrative data. Palliat Med. 2015;29:918–28.
Huo J, Hong Y-R, Turner K, Diaby V, Chen C, Bian J, et al. Timing, costs, and survival outcome of specialty palliative care in Medicare beneficiaries with metastatic non-small-cell lung cancer. JCO Oncol Pract. 2020;16:e1532–42. https://doi.org/10.1200/OP.20.00298 .
Klinger CA, Howell D, Marshall D, Zakus D, Brazil K, Deber RB. Resource utilization and cost analyses of home-based palliative care service provision: the Niagara West End-of-Life Shared-Care Project. Palliat Med. 2013;27:115–22.
Chen CY, Naessens JM, Takahashi PY, McCoy RG, Borah BJ, Borkenhagen LS, et al. Improving value of care for older adults with advanced medical illness and functional decline: cost analyses of a home-based palliative care program. J Pain Symptom Manage. 2018;56:928–35. https://doi.org/10.1016/j.jpainsymman.2018.08.015 .
Gomes B, Calanzani N, Curiale V, Mccrone P, Higginson IJ. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev. 2013;2013(6): CD007760.
Gardiner C, Ingleton C, Ryan T, Ward S, Gott M. What cost components are relevant for economic evaluations of palliative care, and what approaches are used to measure these costs? A systematic review. Palliat Med. 2017;31:323–37.
Yadav S, Heller IW, Schaefer N, Salloum RG, Kittelson SM, Wilkie DJ, et al. The health care cost of palliative care for cancer patients: a systematic review. Support Care Cancer. 2020;28:4561–73.
Gonzalez-Jaramillo V, Fuhrer V, Gonzalez-Jaramillo N, Kopp-Heim D, Eychmüller S, Maessen M. Impact of home-based palliative care on health care costs and hospital use: a systematic review. Palliat Support Care. 2021;19:474–87.
Porter I, Gonçalves-Bradley D, Ricci-Cabello I, Gibbons C, Gangannagaripalli J, Fitzpatrick R, et al. Framework and guidance for implementing patient-reported outcomes in clinical practice: evidence, challenges and opportunities. J Comp Eff Res. 2016;5:507–19.
Saenger P, Federman AD, DeCherrie LV, Lubetsky S, Catalan E, Leff B, et al. Choosing inpatient vs home treatment: why patients accept or decline hospital at home. J Am Geriatr Soc. 2020;68:1579–83.
Rimar A, Friedman MI, Quinteros MG, Gooch RA, Masick KD, DaCosta N, et al. Changing the care paradigm for patients: advanced illness beds care model. Am J Hosp Palliat Med. 2021;38:1336–41.
Gardiner C, Robinson J, Connolly M, Hulme C, Kang K, Rowland C, et al. Equity and the financial costs of informal caregiving in palliative care: a critical debate. BMC Palliat Care. 2020;19:1–7.
Friedlander DF, Krimphove MJ, Cole AP, Marchese M, Lipsitz SR, Weissman JS, et al. Where is the value in ambulatory versus inpatient surgery? Ann Surg. 2021;273:909–16.
Pollock M, Somerville L, Firth A, Lanting B. Outpatient total hip arthroplasty, total knee arthroplasty, and unicompartmental knee arthroplasty: a systematic review of the literature. JBJS Rev. 2016;4:1–15.
Butt S, Swiatlo E. Treatment of community-acquired pneumonia in an ambulatory setting. Am J Med. 2011;124:297–300.
International Agency for Research on Cancer, World Health Organization. Global Cancer Observatory. 2022. https://gco.iarc.fr/ . [Accessed 5 Nov 2022].
Hughes J. Palliative care and the QALY problem. Health Care Anal. 2005;13:289–301.
Sahlen KG, Boman K, Brännström M. A cost-effectiveness study of person-centered integrated heart failure and palliative home care: based on a randomized controlled trial. Palliat Med. 2016;30:296–302.
Download references
Author information
Authors and affiliations.
Faculty of Medicine and Health Sciences, Universitat de Barcelona, Barcelona, Spain
Ana Helena Perea-Bello
Department of Economics and Business, Faculty of Law, Economics and Tourism, Universitat de Lleida, Lleida, Spain
Marta Trapero-Bertran
Pain Medicine Section, Department of Anaesthesiology, Hospital Clínic de Barcelona, Barcelona, Spain
Christian Dürsteler
Department of Surgery. Faculty of Medicine and Health Sciences, Universitat de Barcelona, Barcelona, Spain
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Ana Helena Perea-Bello .
Ethics declarations
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Acknowledgements
Universitat de Barcelona supports Open Access publishing through the CRUE-CSIC Agreement.
Conflicts of Interest/Competing Interests
Ana Helena Perea-Bello, Marta Trapero-Bertran and Christian Dürsteler have no conflicts of interest that are directly relevant to the content of this article. Disclosure forms provided by the authors are available upon request.
Ethics Approval
This SLR is part of a project approved by the following ethical research committees: Comité de Ética de la Investigación con Medicamentos del Hospital Clínic de Barcelona, 8 October 2021/Reg. HCB/2021/0986; Comitè d’ètica d’Investigació amb Medicaments, IDIAPJordiGol, 23 February 2022/Codi CEIm: 22/006-P; and registered in: PROSPERO, ID: CRD42021250086 (created 4 April 2021) and ClinicalTrials.gov ID: NCT05412784 (created 9 June 2022).
Consent to Participate
Not applicable.
Consent for Publication
Availability of data and material.
The final dataset is available on request.
Code Availability
Authors’ contributions.
All authors were involved in the conception and planning of the SLR. AHPB conducted the literature search, and AHPB, MTB and CD performed the screening. AHPB interpreted and synthesised the review results and wrote the paper’s first draft. MTB and CD contributed to the critical review, revisions and final draft corrections. All authors approved the final submitted version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 19 KB)
Supplementary file2 (docx 51 kb), supplementary file3 (docx 63 kb), supplementary file4 (docx 41 kb), supplementary file5 (docx 18 kb), supplementary file6 (docx 14 kb), rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/ .
Reprints and permissions
About this article
Perea-Bello, A.H., Trapero-Bertran, M. & Dürsteler, C. Palliative Care Costs in Different Ambulatory-Based Settings: A Systematic Review. PharmacoEconomics 42 , 301–318 (2024). https://doi.org/10.1007/s40273-023-01336-w
Download citation
Accepted : 31 October 2023
Published : 27 December 2023
Issue Date : March 2024
DOI : https://doi.org/10.1007/s40273-023-01336-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
own decisions about their health care. The five case studies presented in this issue of Healing Hands will highlight issues and challenges that clinicians may confront while providing palliative care to patients experiencing homelessness. Case #1: Mr. J Case study Mr. J was a man in his mid-50s who was admitted to a
Case Studies in Palliative and End-of-Life Care CCampbell_ffirs.indd iampbell_ffirs.indd i 77/27/2012 12:18:06 PM/27/2012 12:18:06 PM. Case Studies in ... Case Studies In Palliative and End-of-Life Care is written for clinicians caring for patients and their families across diagnoses, illness trajectories, settings for care, and models of care ...
Case Studies in Palliative and End-of-Life Care uses a case-based approach to provide students and practitioners with an important learning tool to improve critical thinking skills and encourage discussion toward improving experiences for patients and their families. The book is organized into three sections covering subjects related to communication, symptom management, and family care.
The UPMC Palliative and Supportive Institute shares monthly cases on areas such as managing pain, dealing with grief, and other palliative care topics. If you have questions or insights about a case, feel free to contact the institute. Browse the extensive archive of palliative and supportive care cases of the month, compiled and maintained by ...
One of the goals of the UPMC Palliative and Supportive Institute is to advance the practice of our specialty by sharing our knowledge with students and health care professionals around the world. To this end, we share monthly synopses of notable or particularly interesting cases in which palliative care has played a role. If you have any ...
Many randomized, controlled trials and case-control studies of palliative care interventions to date have shown reductions in patients' symptoms and health care utilization and improvements in ...
The VOICE Study was designed to assess the acceptability and feasibility of providing planned "Meetings" in specialist inpatient palliative care. 21 "Patient-centered" care provided the conceptual framework for this Study. The proposed intervention—a planned patient-centered family meeting ("Meeting") was designed to be patient ...
Organisational case study methodology will be used to guide the study design. In total, 50 cases will be conducted in five European countries (10 per country). A case involves a semi-structured interview with a relative and an HCP closely involved in the care of a deceased patient who received some type of palliative sedation at the end-of-life ...
A case study approach was conducted. Semi-structured interviews were held between 2012 and 2013, with pioneers (n = 11) of the EoLC in Macao. Thematic analysis was adopted to analyse the interviews. ... establishing hospice and palliative care as a knowledge-based discipline . To a certain extent, these studies have offered a theoretical ...
Series. Case Studies in Palliative and End-of-Life Care uses a case-based approach to provide students and practitioners with an important learning tool to improve critical thinking skills and encourage discussion toward improving experiences for patients and their families. The book is organized into three sections covering subjects related to ...
Study design and participants. We used an embedded case study approach as described by Yin [23, 24] to analyse and synthesize three sources of qualitative data collected from participating primary health care teams: monthly reflection survey data, open text survey data, and focus group transcriptions.A case study approach is highly applicable to program evaluation, allowing for the explanation ...
Care dilemma. The following is a case study of a patient who resided in the CCU for over 8 months. ... Most people approaching end of life die in the hospital and 20% of those die in a CCU. 2 Palliative and end-of-life care are realistic and ethical options that should be considered much sooner for many patients in the CCU. 3 Clinical team ...
In one (Tate, Citation 2022), a single case was examined; the others analyzed multiple cases. Most studies have focused on the initial stages of these conversations, in which people attempt to raise matters relating to the patient's dying to the interactional surface. ... An applied conversation analysis study. BMC palliative care. https ...
Palliative Care. The Case for Hospital Palliative Care—Improving Quality. Reducing Cost. 3 Hallmarks of a Vital Trend Based on need, not prognosis, palliative care responds to the episodic, complex, and long-term nature of serious illness. The pillars of palliative care are: → Improved quality leading to lower costs—of hospital care.
Introduction. Palliative care interventions have increased worldwide. Studies have predicted the future global burden of serious health-related suffering will increase (Sleeman et al., 2019), 87% patients will need palliative care interventions by 2060 (Clark et al., 2020).Palliative care is critically important for the world's aging population and can change illness trajectories and promote ...
Hospice Case Studies Case 1 . Ms. Wamser is an 87-year-old woman with a hx of hypertension, hypothyroidism and advanced dementia consistent with Alzheimer's type dementia. She experienced a gradual functional decline and lost her ability to ambulate (FAST 7c) over the past 3 months and is non-verbal and dependent for all
Click here to review the draft Palliative and End-of-Life Care - Interactive Case Study. The following case vignette provides key concepts that could be considered when developing a plan of care for a patient who may require a controlled substance to manage their health concerns. As with any clinical situation, there are many patient ...
Wilmot, J Palliative Care Med 2013, 3:3. DOI: 10.4172/2165-7386.1000149. Cas e Re port Open Access. Case Studies in Palliative Care. Clare Wilmot*. Medical Director of North Country Home Health ...
Background: Research evidence suggests that a lack of engagement with palliative care and advance care planning could be attributed to a lack of knowledge, presence of misconceptions and stigma within the general public. However, the importance of how death, dying and bereavement are viewed and experienced has been highlighted as an important aspect in enabling public health approaches to ...
Palliative Care Impacts Value. Palliative care teams working in hospitals: Improve patient and family satisfaction with care [1] Reduce 30-day readmission rates [2] Reduce ICU utilization [3] Can save 9-25% of costs for each inpatient stay [4] through a mixture of shorter length of stay and reduced cost per day.
2. Explain the importance of honoring Advance Directives. 3. Demonstrate influence of multicultural background of health care providers and the care perspective. 4. Explain how an interdisciplinary Palliative Care Team utilizing a transdisciplinary approach impacts patient outcomes.
The purpose of this study was to formulate the aims of case management and describe essential characteristics of case management in palliative care in the Netherlands, as perceived by experts. The expert panel procedure also gave insight into which topics there is consensus between experts and what are the main differences in opinion between them.
The Palliative Care Formulary provides excellent information and guidance on dosing and routes, which are often outside of the product license. The National Institute for Health and Care Excellence ... Case study. Palliative care part 1: pain management. Mary*, aged 54 years, comes into the pharmacy and asks to speak to the pharmacist about her ...
Case 5: A man with advanced ALS requests a do-not-resuscitate order. Carter has ALS, or amyotrophic lateral sclerosis, a progressive neurodegenerative disease. He has been hospitalized with infection five times and is on a ventilator. Carter is alert and told doctors he wants to return home and sign a do-not-resuscitate order (DNR).
Background Palliative care (PC) contributes to improved end-of-life care for patients with hematologic malignancies (HM) and solid tumors (ST) by addressing physical and psychological symptoms and spiritual needs. Research on PC in HM vs. ST patients is fragmented and suggests less use. Methods We analyzed claims data of all deceased members of a large German health insurance provider for the ...
Background By sharing patient stories, health care professionals (HCPs) may communicate their attitudes, values and beliefs about caring and treatment. Previous qualitative research has shown that HCPs usually associate paediatric palliative care (PPC) with death or dying and that they find the concept challenging to understand and difficult to implement. Attending to HCPs' stories may ...
Cost-of-illness studies in palliative care are of growing interest in health economics. There is no standard methodology to capture direct and non-direct healthcare and non-healthcare expenses incurred by health services, patients and their caregivers in the course of the ambulatory palliative care process. ... In the case of studies using a ...
Nursing students desire more training and experience in palliative care due to a need for more skills and knowledge. This descriptive qualitative study explored nursing students' experiences in participating in a student death doula service-learning program in palliative care settings. Fourteen final-year undergraduate nursing students ...
An article published in CriticalCareNurse discusses how a critical care team at a community hospital identified the need to improve the process of integrating palliative care upon admission into the ICU. After implementing a screening process, the authors found many benefits: early identification, improved efficiency with consultations, a reduction in readmission rates, and a decrease in the ...
Background: Long-term neurological conditions include multiple sclerosis, Parkinson's-related diseases, and motor neurone disease. National and international guidelines recommend a palliative approach for advancing neurological disease, but there is little research describing and comparing the palliative care needs of these patients side by side. Objective: The aim of this study was to ...