- CRM Software
- Email Marketing Software
- Help Desk Software
- Human Resource Software
- Project Management Software
- Browse All Categories
- Accounting Firms
- Digital Marketing Agencies
- Advertising Agencies
- SEO Companies
- Web Design Companies
- Blog & Research

Electronic Data Capture Software
Capterra lists all providers across its website—not just those that pay us—so that users can make informed purchase decisions. Capterra is free for users. Software and service providers pay us for sponsored profiles to receive web traffic and sales opportunities. Sponsored profiles include a link-out icon that takes users to the provider’s website. Learn more.
Capterra carefully verified over 2 million reviews to bring you authentic software and services experiences from real users. Our human moderators verify that reviewers are real people and that reviews are authentic. They use leading tech to analyze text quality and to detect plagiarism and generative AI. Learn more.
Capterra’s researchers use a mix of verified reviews, independent research and objective methodologies to bring you selection and ranking information you can trust. While we may earn a referral fee when you visit a provider through our links or speak to an advisor, this has no influence on our research or methodology.
Sponsored: Vendors bid for placement within our listings. This option sorts the directory by those bids, highest to lowest. Vendors who bid for placement can be identified by the orange “Visit Website” button on their listing.
Highest Rated: Sorts products as a function of their overall star rating, normalized for recency and volume of reviews, from highest to lowest.
Most Reviews: Sorts listings by number of user reviews, most to least.
Alphabetical: Sorts listings from A to Z.
Related Software Category:
Why is capterra free, i'm looking for electronic data capture software that is:.

Access PeopleHR

Nintex Process Platform

TrueContext

Lucky Orange

Fulcrumapp.com

123FormBuilder

Seamless.AI

Forms On Fire

ResearchManager

Centreviews

Bright Data

- News & Highlights
- Publications and Documents
- Postgraduate Education
- Browse Our Courses
- C/T Research Academy
- K12 Investigator Training
- Harvard Catalyst On-Demand
- Translational Innovator
- SMART IRB Reliance Request
- Biostatistics Consulting
- Regulatory Support
- Pilot Funding
- Informatics Program
- Community Engagement
- Diversity Inclusion
- Research Enrollment and Diversity
- Harvard Catalyst Profiles

REDCap (Research Electronic Data Capture)
Free, web-based electronic data capture tools to support clinical and research studies
- REDCap Creation Process
- Frequently Asked Questions
REDCap: Data Management and Survey Tool
The following Harvard-affiliated institutions have adopted REDCap. If you’re from an institution outside Harvard, please contact REDCap directly .
Beth Israel Deaconess Medical Center Boston Children’s Hospital Harvard T.H. Chan School of Public Health Joslin Diabetes Center (accessible only via Joslin Workstation or VPN) Mass General Brigham
REDCap is a free, secure, web-based application designed to support data capture for research studies. The system was developed by a multi-institutional consortium initiated at Vanderbilt University. Data collection is customized for each study or clinical trial by the research team with guidance from Harvard Catalyst EDC Support Staff. REDCap is designed to comply with HIPAA regulations. REDCap is not 21 CFR Part 11 compliant.
- Free, web-based, and user-friendly electronic data capture (EDC) tools for research studies
- Databases can be quickly developed and customized for studies’ needs
- Collecting and tracking information and data from research studies
- Scheduling study events (e.g., patient visits)
- Conducting surveys
- Investigators at Harvard-affiliated institutions that have adopted REDCap
Using REDCap’s stream-lined process for rapidly developing projects, you may create and design databases and surveys using:
- Online Designer and/or
- Offline method of constructing a “data dictionary” file in Microsoft Excel and uploading it to REDCap
REDCap provides user-friendly web-based case report forms, real-time data entry validation (e.g. for data types and range checks), audit trails, and the ability to set up a calendar to schedule and track critical study events such as blood-draws, participant visits, etc. Also, designated users can assign different levels of access for each member of the research team.
REDCap also provides a powerful tool for creating and managing surveys in your web browser. Collect anonymous responses or track and identify responses from survey participants by:
- sending a link to your survey via email
- entering data manually, and/or
- posting a link on your website
For more information, visit: http://project-redcap.org/
Advantages of REDCap:
- Secure and web-based. Input data from anywhere in the world with secure web authentication, data logging, and Secure Sockets Layer (SSL) encryption.
- Fast and flexible. Conception to production-level database in less than one day.
- Multisite access. Projects can be used by researchers from multiple sites and institutions.
- Fully customizable. You are in total control of shaping your database or survey.
- Advanced question features. Auto-validation, branching logic, and stop actions.
- Mid-study modifications. You may modify the database or survey at any time during the study.
- Data import functions. Data may be imported from external data sources to begin a study or to provide mid-study data uploads.
- Data comparison functions. Double data entry / Blinded data entry.
- Export survey results to common data analysis packages. Export your data to Microsoft Excel, SAS, STATA, R, or SPSS for analysis.
- Save your survey or forms as PDFs. Generate a PDF version for printing in order to collect responses offline.

More than a Quality Management System: Tools for the entire MedTech Lifecycle.
Featured Capabilities:

Experience the #1 QMS software for medical device companies first-hand. Click through an interactive demo.
Data collection and management designed for MedTech clinical trials.

Get a personalized demo of Greenlight Guru Clinical today.
- By Initiative
- Migrating From Paper
- Managing and Assessing Risk
- Preparing for Regulatory Submissions
- Becoming Audit Ready
- Managing Postmarket Quality
- Post-Market
- By Function
- Product / R&D
- ROI Calculator
- Customer Success
- Case Studies
- Checklists & Templates
- eBooks & Guides
- Content Hub
- Live & Virtual Events
- True Quality Roadshow
- Quality Pricing
- Clinical Pricing

Everything You Need to Know about Electronic Data Capture (EDC) for Clinical studies
%20Systems%20(new).png)
The purpose of a clinical investigation (or clinical study) is to determine the safety and effectiveness of a novel treatment, such as a medical device. Biostatisticians make determinations about the results of a clinical investigation by analyzing data from Case Report Forms (CRFs) completed by investigators for each patient during the investigation.
In the past, CRFs from a clinical study would be completed on paper, packed into boxes, and shipped from the study location to a secondary location for analysis. But today, a growing number of medical device companies are going paperless and using web-based Electronic Data Capture (EDC) Systems to collect, store, and manage clinical data .
Software-based EDC systems give clinical study managers the ability to streamline the data collection process, capture more accurate data, and enhance data security and accessibility - all while saving time and reducing the cost of completing a clinical study.
In this blog, we’re breaking down everything you need to know about EDC Systems and the transition from paper-based to digital CRF record-keeping for medical device clinical studies.
BONUS RESOURCE: Click here to download our 15-in-1 clinical investigations content bundle to help you run studies and collect clinical data more efficiently.
What is an Electronic Data Capture (EDC) system for clinical trials?
An Electronic Data Capture (EDC) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. Nowadays, many clinical EDC systems are distributed in the Software-as-a-Service (SaaS) business model and accessed by sites and sponsors over the Internet.
Clinical EDC systems allow the sponsor of a clinical study to create customized electronic case report forms (eCRFs) that can be completed by the researchers performing the study. Clinical Electronic data capture systems can be configured and programmed by data managers to validate user inputs, ensuring that any data collected is both accurate and complete.
After sufficient data has been collected, clinical EDC software allows clinical study managers to export the data to external tools for analysis.
What is 'Direct Data Capture' (DDC)?
“Direct data capture” (DDC) is the process of entering data on the spot into an electronic tool. For example, one can input data directly into an eCRF. To know how to make better decisions that will inform your design and implementation of an eCRF, check out our “10 best practices for eCRF in Medical Device Trials.”
In Greenlight Guru Clinical’s electronic data capture (EDC) software, medical device manufacturers can enter clinical data contemporaneously (during visits) or at a later point in time. Designed to fit MedTech needs, you don’t need technical skills to get started. Our ready-to-use templates, modules, and features will help you to easily design the optimal clinical study.
Greenlight Guru Clinical’s EDC software allows clinicians to collect patient reported outcomes electronically on any device, thanks to bring-your-own-device (BYOD) capabilities. Our clients make full use of the Greenlight Guru Clinical ePRO capabilities by tailoring it to their clinical study needs. It can fulfill your post-market requirements as well as product and market research.
Learn more about Greenlight Guru Clinical ePRO.
What is eSource?
We're taking a moment here to define what eSource is, in the context of collecting and managing clinical trial data. eSource is a term that you might have encountered in the clinical trial space. Several online sources portray eSource as a modern solution, outweighing electronic data capture.
EDC systems of today however include the real-time capabilities of eSource. While there used to be differences between when and what data an electronic data capture system collects, nowadays there is no clear divide.
By definition, eSource (electronic Source) collects data at the source. Examples of source data can be visit-based data inputs (e.g. blood pressure, concomitant medication, weight etc.) That is also what most electronic data capture systems can do nowadays. One can say, EDC systems are a type of eSource.
Greenlight Guru Clinical is a modern EDC system made for medical devices & diagnostics that is able to collect and store both clinical and compliance data. It can be used as a hybrid solution, to collect data during visits, or input data at a later time. One of the greatest advantages of SMART-TRIAL EDC compared to others on the market, is that it considers the needs and workflow of clinical teams.
EDC vs eCRF - what’s the difference?
The terms EDC and eCRF come up often when we’re discussing digital clinical trial data management for medical devices, so it’s important to define each one and understand how they’re different.
An Electronic Data Capture (EDC) System is a software application that helps streamline the process of collecting, storing, and securing data from clinical studies, while an electronic Case Report Form (eCRF) is the digital version of a Case Report Form (CRF) used by researchers to record data from about patients participating in a clinical trial.
Stated differently, the term “ eCRF ” refers to the digital forms that researchers will complete when using EDC software for a clinical trial, while the term “ EDC ” refers to the software itself.
What kind of data can you collect in an EDC system for clinical trials?
Clinical EDC systems are purpose-built to collect and organize data from clinical trials/studies, including eCRF documents and readings from medical devices and other instruments.
An eCRF can include information such as:
Patient characteristics and demographic data,
Clinical study site and patient’s treatment group,
Patient health status, history, and vital sign measurements,
Treatment effects/use
Patient lab reports and test results,
Readings from patient-attached medical devices (e.g. blood pressure, heart rate, oxygen saturation, blood glucose level, etc.)
The purpose of a clinical study is to gather data regarding variables that are relevant to the research hypothesis. For medical device companies, the purpose of a clinical study is to evaluate the safety and efficacy of a medical device for treating a specified disease or condition in patients.
Because each clinical study is different, data collection practices vary significantly between clinical studies. EDC systems allow clinical trial sponsors to design customized eCRF forms to ensure that researchers collect the necessary data as per the stated research hypothesis and data collection plan.
How is data collected in clinical EDC software?
For clinical studies following a paper-based data collection strategy, there’s only one real option for data collection: researchers complete paper CRFs, store them, then box and ship them to clinical trial sponsors where biostatisticians will analyze the results.
To better understand how electronic data capture fits into the clinical study workflow, let’s look at three methods researchers use to input data into clinical EDC software:
Direct Data Entry
The first option for EDC data collection is direct data entry. Researchers can login to the EDC software using secure access credentials, open the relevant eCRF for the study, and enter clinical data into the system where it immediately becomes available for data reviewers and other stakeholders.
Transcription from Paper or Electronic Sources
Researchers can also choose to complete CRF forms the traditional way, using paper, then transcribe those paper CRFs into the EDC system at a later time.
Having said that, we do not recommend simply copying the paper-based CRF to an electronic CRF inside an EDC system for a number of reasons. The most important being, previous challenges encountered on paper, will be transferred to the electronic format.
Check out or free eBook ''7 Principles to Designing an eCRF'' to learn best practices to designing an eCRF to reduce costs and save time.
Transcription-based data collection may also take place when data from patient-reported outcomes (PRO) or electronic health records (EHR) is entered into the EDC system.
Automatic Transmission
An important advantage of using electronic data capture (EDC) instead of paper-based data collection is the ability to capture data via automatic transmission. Modern EDC systems can receive data transmissions from ePRO instruments (which are sometimes a part of the EDC software system), and connected medical devices (DHT)s, automating those portions of the data collection process.
What are the most important features of a clinical EDC system?
Electronic Case Report form (eCRF) Builder
Electronic Patient Reported Outcomes (ePRO)
Adverse Events Reporting Modules
Medication Modules
Connected devices and wearables integration
Integration options via application programming interfaces (APIs)
Case-based Data Collection
Survey Data Collection
Integration with Clinical Trial Management System (CTMS)
6 advantages of using EDC software in medical device clinical investigations/studies
The most efficient medical device companies in 2023 are using EDC software to collect, store, and secure the data they collect in clinical investigations . Below, we summarize six major advantages of EDC software and why it makes sense to move away from paper-based data collection.
1. Streamlined data collection process
Using a software-based EDC system for clinical trials helps streamline the data collection process throughout the medical device lifecycle.
With traditional paper-based data collection, physical CRFs must be completed by researchers, shipped to the study sponsor, and transferred by data entry teams into a computer database before analysis can occur. Using EDC software for clinical data collection bypasses this lengthy process by empowering researchers to create the data in a digital format with an eCRF.
EDC in clinical trials also streamlines data collection by enabling the automatic transmission of clinical data from PRO instruments and medical devices.
2. Improved data quality
Improved data quality is another advantage of deploying an EDC system for data collection in clinical trials.
By streamlining the data collection process, clinical study managers cut down on the possibility for errors in data entry and transcription that occur at higher rates in the convoluted paper-based data collection process.
EDC systems can also incorporate software-based mechanisms for improving data quality, including edit checks, numerical data validation, and remote monitoring, to ensure that data inputs are accurate and correctly formatted before they reach the trial database.
These EDC system features give researchers the opportunity to correct data entry mistakes and discrepancies before they can influence the outcome of the study.
3. Enhanced data authenticity and security
Collecting clinical trial data in an EDC system is a great way to ensure the security, authenticity, and reliability of the data.
While paper-based CRFs can easily be lost, stolen, or damaged - either on-site or in transit, the eCRFs created in a software EDC system are immediately uploaded to secure cloud storage where there’s zero risk of data loss. EDC systems can also incorporate security features, such as role-based access controls (RBAC), multi-factor authentication, and more, to ensure that only authorized persons may access the data.
4. More accessible clinical data
Another advantage of recording clinical data in an EDC system is that the data is more accessible. Once an eCRF has been completed by researchers and saved in your EDC system, it can be accessed immediately from anywhere in the world with an Internet connection by anyone with the appropriate authorization.
Instead of waiting for a box of paper CRF forms to arrive by mail, data reviewers can start reviewing and analyzing eCRF data just moments after it has been created. With near real-time access to data, it becomes easier for clinical trial managers to detect trends and make well-informed decisions.
5. Accelerated completion of clinical studies
When it comes to analyzing clinical data from paper-based CRF forms, the most time-consuming tasks include transcribing, cleaning, preparing, and transforming the data, and defining the database structure prior to analysis.
Entering data directly into an EDC system with eCRFs standardizes the data entry process and reduces the need to invest massive time and effort in data transcription and preparation after data has already been collected. As a result, clinical data collected in EDC software can be analyzed much sooner after it is collected by researchers.
6. Reduced clinical operations costs
Collecting data with an EDC system reduces the overall cost of conducting clinical trials. By streamlining the data collection process, leveraging automation to ensure high-quality data, and reducing the need for data cleaning and preparation, EDC systems reduce the total cost of data collection and analysis needed to complete a study.
The ability to execute clinical studies more quickly means that medical device companies can fast-track their pathway to market authorization and start realizing profits even sooner than expected.
Manage data collection for your medical device clinical investigation with Greenlight Guru Clinical
Greenlight Guru Clinical is the first and only data gathering and management system that’s purpose-built for medical devices and diagnostics.
With Greenlight Guru Clinical, medical device companies can create customized eCRFs and digitize data collection for clinical investigations, in-human studies, and post-market surveillance activities.
Greenlight Guru Clinical is out-of-the-box compliant with ISO 14155:2020 and includes templates for data collection planning and regulatory compliance that help medical device companies execute organized, cost-effective, and compliant studies that bring products to market faster.
Ready to Learn More? Contact us for a customized demo .
Páll Jóhannesson
Páll Jóhannesson, M.Sc. in Medical Market Access, is the founder and Managing Director of Greenlight Guru Clinical (formerly SMART-TRIAL). Páll was previously the CEO of Greenlight Guru Clinical where he led the team to create the only EDC specifically made for medical devices.
Read More Posts
Mdr and ivdr: challenges and opportunities of the new regulations, clinical evidence: the key to market adoption, paper systems are the riskiest way to manage medical device projects, subscribe to our blog.
Join 200,000+ other medical device professionals outperforming their peers.

Get your free PDF
Change impact analysis checklist.
- Checklists/Templates
- Request a Demo
- Content Title Description
News: Teamscope joins StudyPages 🎉
Data collection in the fight against COVID-19
Data Collection
Electronic data capture software: 5 apps for clinical research.
My first encounter with clinical research was as a junior dentist at Al-Sheikh Zayed Al-Nahyan Hospital in Cairo. I conducted several clinical studies to measure the effects of oral and dental diseases in patients in an underdeveloped setting.
.png)
Dear Diary, I have been struggling with an eating disorder for the past few years. I am afraid to eat and afraid I will gain weight. The fear is unjustified as I was never overweight. I have weighed the same since I was 12 years old, and I am currently nearing my 25th birthday. Yet, when I see my reflection, I see somebody who is much larger than reality. I told my therapist that I thought I was fat. She said it was 'body dysmorphia'. She explained this as a mental health condition where a person is apprehensive about their appearance and suggested I visit a nutritionist. She also told me that this condition was associated with other anxiety disorders and eating disorders. I did not understand what she was saying as I was in denial; I had a problem, to begin with. I wanted a solution without having to address my issues. Upon visiting my nutritionist, he conducted an in-body scan and told me my body weight was dangerously low. I disagreed with him. I felt he was speaking about a different person than the person I saw in the mirror. I felt like the elephant in the room- both literally and figuratively. He then made the simple but revolutionary suggestion to keep a food diary to track what I was eating. This was a clever way for my nutritionist and me to be on the same page. By recording all my meals, drinks, and snacks, I was able to see what I was eating versus what I was supposed to be eating. Keeping a meal diary was a powerful and non-invasive way for my nutritionist to walk in my shoes for a specific time and understand my eating (and thinking) habits. No other methodology would have allowed my nutritionist to capture so much contextual and behavioural information on my eating patterns other than a daily detailed food diary. However, by using a paper and pen, I often forgot (or intentionally did not enter my food entries) as I felt guilty reading what I had eaten or that I had eaten at all. I also did not have the visual flexibility to express myself through using photos, videos, voice recordings, and screen recordings. The usage of multiple media sources would have allowed my nutritionist to observe my behaviour in real-time and gain a holistic view of my physical and emotional needs. I confessed to my therapist my deliberate dishonesty in completing the physical food diary and why I had been reluctant to participate in the exercise. My therapist then suggested to my nutritionist and me to transition to a mobile diary study. Whilst I used a physical diary (paper and pen), a mobile diary study app would have helped my nutritionist and me reach a common ground (and to be on the same page) sooner rather than later. As a millennial, I wanted to feel like journaling was as easy as Tweeting or posting a picture on Instagram. But at the same time, I wanted to know that the information I provided in a digital diary would be as safe and private as it would have been as my handwritten diary locked in my bedroom cabinet. Further, a digital food diary study platform with push notifications would have served as a constant reminder to log in my food entries as I constantly check my phone. It would have also made the task of writing a food diary less momentous by transforming my journaling into micro-journaling by allowing me to enter one bite at a time rather than the whole day's worth of meals at once. Mainly, the digital food diary could help collect the evidence that I was not the elephant in the room, but rather that the elephant in the room was my denied eating disorder. Sincerely, The elephant in the room
I would visit each site with paper forms and collect all of my data with pen and paper. Once a week, I would sit down for hours to transcribe all of the data I had on paper to Excel.
Although my experience doing research data collection sounds like a monumental task, I wasn't alone, and although that was a few years ago, most clinicians doing research still rely on pen and paper.
Electronic Data Capture (EDC) solutions simplify data gathering in clinical research by improving data quality, security and making data collection be as easy as possible. In this article I share 5 EDC platforms that I could have used during my clinical studies to eliminate the burden of paper data collection and embrace the benefits of capturing data digitally.
What is Electronic Data Capture?
Electronic Data Capture (EDC) is software for collecting and managing clinical research electronically. The main benefits of EDCs over paper forms is that they increase the reliability of data by eliminating transcription from paper to database, and they increase data security with automatic backups.
If you are a medical researcher looking for ways to capture data for clinical trials to benefit your research significantly, Electronic Data Capture (EDC) may be your answer.
The Difference Between EDC Software and Online Forms
You are likely to have come across survey software such as Google Forms, Survey Monkey, or Qualtrics.
And you might be thinking: if I want to replace paper forms with digital forms, then can't I use something like Google Forms for my clinical research?
The short answer is, it depends .
When is Google Forms ok for collecting for clinical research data?
In the following cases Google Forms is a suitable platform for collecting clinical research data:
- You have simple forms , with basic branching logic.
- The data that you will be collecting is entirely anonymous . In other words, you will not be collecting any personally identifiable information.
- Your data collection is cross-sectional , in other words you won't be doing case management or longitudinal data collection.
If you are planning to do a clinical study and the 3 cases above are true, then Google forms is a great solution for your data collection, and best of all, it's free. Although online survey solutions can be a quick step to eradicate paper-based data collection, there are 5 showstoppers.
Below is a table of 5 main points and showstoppers of what separates online surveys from validated EDCs:
| Example: Teamscope, REDCap, Castor EDC | Example: Survey Monkey, Qualtrics, Google Forms | |
|---|---|---|
| A case in a clinical study represents a subject or patient. Case management is at the core of any EDC and it allows researchers to collect data longitudinally (subjects are followed up over time with continuous or repeated monitoring). | The biggest difference between EDC and online surveys, is that online surveys don't offer case management. The lack of this feature can be fine if the data that you are collecting is on-off (i.e. cross-sectional), but if you need to follow up on subjects over time, then case management is a must have. | |
| Data in healthcare and clinical research is always highly sensitive. For that reason, all validated EDCs offer a feature to grant and revoke access to team members and limit their access based on a least privilege principle. | While role-based access management (also known as permission management) used to be reserved to validated EDC, in the last few years online surveys have been catching up. Today, you can find granular and robust permission management on survey software like Qualtrics. | |
| An audit trail makes it possible to see a history of changes in your data. This feature is at the core of an Electronic Data Capture solution since being able to pinpoint who entered what data in a clinical trial is so crucial. | The audit trail capabilities of online surveys can be very limited or simply non-existent. | |
| In clinical research, after data has been entered into the EDC it must then be verified that everything is in order. This is usually done with the study admin. After that check, also called Source Data Verification, the admin will lock that record to indicate that it has been checked and no further changes should be made. | In online surveys data locking is unfortunately not possible. The verification that each entry is in order has to be done on Excel or Google Sheets. | |
| Branching logic can make completing a form much easier by only showing the relevant questions depending on the data that is entered. In the case of a validated Electronic Data Capture solution, the branching logic capabilities that the software supports should be limitless. | Online surveys nowadays can offer different levels of branching logic. On Google Forms, for example, you can allow respondents to advance to a different question depending on the value entered. If your form needs basic branching conditions then with something like Google Forms, you might be fine. If you need complex branching rules then an Electronic Data Capture (EDC) could be your solution. |
If you have read this far, then the main takeaway is that online forms and surveys are used for more general data. At the same time, Electronic Data Capture solutions are more suitable for healthcare and clinical trials.
The following section compares 5 of the most popular EDC platforms to help you, as a researcher, decide the most suitable option for your human research.
5 EDC Platforms to consider in your following clinical study
EDC platforms are not a one size fits all solution. These platforms come in different packages and flavours. For a researcher to choose the best EDC platform for a project, education on the varieties that exist is key to understanding the subtle nuances between them and selecting the most suitable option.
1. Teamscope: Best for Mobile Data Collection
.png)
Teamscope is a data collection platform for research teams that need a secure solution to capture and analyse data.
Unique selling point
Teamscope's unique selling point is its easy-to-use Android and iOS app for data entry, which can be particularly useful when working in offline or remote settings. Research teams can also use the web interface to collect data from any browser and build powerful reports and graphs.
Teamscope offers pricing plans for different team sizes and access to various features, depending on the study need. The monthly price ranges from €49/month billed annually to €249/month billed annually. Some of the main differences between each plan are the level of features and the number of users, cases and amount of file storage it includes. Further, all plans come with a 30-day money-back guarantee and a 7-day free trial.
Practical features
With Teamscope, research is a collaborative effort. Team management features such as allowing users to edit each other's entries can enhance the user experience by expediting data analysis. This functionality is permitted through a customisable permissions manager that enables the researcher to give predefined functions such as View All, Add Entry, or Export to individual users or groups created.
Another unique feature of Teamscope is the prioritisation of data security and the integration of features to store and protect data, such as the Teamscope Passcode Lock Screen. To unlock the app, users must enter a 4-digit code, thereby adding a layer of security to the sensitive user information on the app.
Point of Improvement
Teamscope does not offer randomisation, which can be a deal-breaker for randomised control trials (RCT). The platform also offers minimal data exporting capabilities, only Excel and CSV. No, SPSS or Stata data exporting capabilities.
Testimonial
"Using Teamscope has been a game-changer for me. It has turned the difficult and stressful process of data collection in the field (and being paramedics - a challenging and often chaotic field at that) into a painless process." Aidan Baron Paramedic Researcher
2. Castor EDC: Best for complex and multi-centre studies
Castor EDC is a cloud-based clinical data management platform that enables researchers to capture and integrate data from any source. Data sources include clinicians, patients, devices, wearables and Electronic Health Record (EHR) systems.
Cost is tailored to the needs of a study. Prices vary for non-commercial and commercial studies.
Non-commercial studies carried out by non-profit organisations are free. There is, however, a maximum of 5 free studies per academic institute. Additionally, this does not include custom contracting or extensive compliance validation.
Commercial fees apply in case a commercial organisation is carrying out a study. Fees also apply if the study is funded commercially or non-commercially. Finally, varying commercial fees apply if a study is run by Clinical Trial Units, Clinical Research Facilities, Academic Research Organisations and similar organisations.
Castor's cloud-based EDC system also enables researchers to capture high quality, reusable data easily.
Castor makes the building of studies within hours using pre-built templates and user-friendly technology. Its EDC interface is designed to be intuitive and researcher-friendly.
To help researchers set up their studies, online video training, monthly webinars and prompt customer service facilitate setting up a study. In the case of more complex analyses, Castor assists through study experts.
Castor is tailored specifically for use in large academic, medical device, biotech, and pharmaceutical research. The platform has everything you need to build a study that matches precisely with your study design. It is a platform that is compliant with patient data privacy laws and Good Clinical Research Practice (GCP).
Point of improvement
The EDC data entry is only possible through the web browser, making it dependent on an internet connection. Castor recently released an ePRO mobile app, which makes it possible for patients to complete surveys from an Android and iOS device without the need for an internet connection.
Castor's new mobile app for patients is exciting news and a sign that the company could be offering a fully mobile and offline experience for their EDC data entry.
"Without Castor EDC, we would never have been able to collect such a large amount of data across all centers with the limited amount of local funding they received. And a paper CRF of this length would certainly not have been manageable." – Dr De Waele, Ghent University Hospital in Belgium
3. Smart Trial: Best for Medical Devices and Post Market Clinical Follow-Up (PMCF)

Smart Trial is a complete data collection toolbox for Post-Market Clinical Follow-Up (PMCF) and clinical investigations. This EDC platform is built to empower clinical teams to be their best and fully control their clinical data without compromising on features, design, or compliance.
Although pricing is not public on the website, they offer a free trial to give a hands-on experience of their solution. For more information on product pricing and custom quotes, email [email protected]
Practical features
Has ready to use templates, modules, and features that help MedTech companies comply with industry regulations.
Unique Selling Point
Ease of use and customer experience are two of the key selling points. Also, Smart Trial has a valuable feature which is the reuse of forms for all the investigators. This feature allows for the easy scalability of the study for multi-sites and multi-phase trials.
No mobile app is available nor support for offline data collection.
''As a chief investigator, I've found SmartTrials to be an incredibly efficient piece of software. It is intuitive to use for all research team members. The functionality of setting up a trial is very easy to navigate. Being able to view data and troubleshoot when running a multicentre trial remotely is invaluable.''
Rob P. Consultant Anaesthetist
4. REDCap: Best for Academic Research

REDCap is a software solution designed to rapidly develop electronic data capture tools (EDC) to support clinical research.
Academic institutions may use REDCap at no charge but need internal IT staff to support its implementation. REDCap is also limited in redistribution because Vanderbilt University (the multi-institutional consortium that initiated the system) is the sole distributor. If you are in academia, there is a high chance that your organisation is part of the REDcap consortium, and you may be able to use it for your clinical study.
The security and web-based feature of REDCap allows users to input data from anywhere globally with secure web authentication, data logging, and Secure Sockets Layer (SSL) Secure Socket Layer (SSL) provides security to the transferred data between web browser and server. SSL encrypt the link between a web server and a browser which ensures that all data passed between them remain private and free from attack. Multi-site access enables the researcher to use projects from multiple sites and multiple institutions.
Unique selling points
REDCap is free and web-based. It allows for databases to be quickly developed and customised for studies' needs. REDCap is a widely used EDC in the academic research community. The REDcap consortium has more than 2400 institutional partners using the software for more than 450,000 research studies.
Technological knowledge is required for the usage of REDcap. Using the REDcap web interface is easy but getting started requires your research organisation to have a specific infrastructure (e.g. PHP web server, MySQL database server) and the technical expertise to install the software on a web server. Technical support provided to users by REDCap is subject to each organisation. The REDcap software is provided for free by the University of Vanderbilt, but each institution is responsible for providing support to users.
"It (REDCap) is relatively easy to use once you get the hang of how it works. The commands are relatively straightforward, with nothing too involved or complex. It is reliable, and I have been using it over the course of a few years. Overall, it is a good way to keep information organized in a way that makes it easy to look up. I work with a patient information database that I need to pull information from and put data into regularly. It has helped me locate records with ease to have access to the information as soon as I need it." Brittany Ann S Student Researcher
5. Medrio: Best for Therapeutics & Drug-based Clinical Trials

Medrio is a cloud-hosted eClinical platform that empowers researchers to accelerate their clinical trials, reduce study costs, and bring life-saving products to market. This EDC platform is tailor-made for pharma, biotech, animal health, medical devices, and diagnostic studies.
Cost is provided by the vendor through scheduling a demo https://medrio.com/request-demo/; however, the website states that the price is 63% lower than two other large EDC providers.
Data on the Medrio software is easy to share, download, and view even with unfamiliar programming and database structures.
Allows users to control the setup, programming, launch and administration of the databases themselves. This advantage enables considerable savings in terms of time and cost. The platform is user friendly. Most of the training needed is on the study-building end, but for the data entry personnel (study sites), Medrio can be used quickly and easily. Further, the customer service is helpful and accommodating to client needs.
Due to this EDC system being user-friendly, it lacks some of the bells and whistles that other EDC systems have. These features include complex custom function query logic and dynamic search list fields. Additionally, the server latency can be improved for this software.
''I have done about twenty clinical studies with Medrio. It is easy to configure a study, design the forms, and deploy. If mid-study changes are required, they are easy to accomplish in Medrio''. Michelle Harden Clinical Data Analyst
Online forms such as Google forms are excellent and free tools for collecting general data such as data on user experience. 5 main differences between online forms and EDCs are:
- Case Management: Not available with online forms.
- Permission Management: More advanced with EDCs.
- Audit Trails: More advanced with EDCs.
- Data Locking: Not possible with online forms.
- Branching/Skip Logic: Limited with online forms.
If you deal with human research where more advanced features are required, such as case management, data privacy, and sensitive data, your best bet is an EDC.
5 of the best EDCs to consider are as follows depending on the needs of your study:
- Teamscope: Best for Working On-the-Go
- Castor EDC: Best for Complex Multi-centre Studies
- Smart Trial: Best for Medical Devices and PMCF
- REDCap: Best for Academic Research
- Medrio: Best for Therapeutics and Drug-based Clinical Trials.
Traditional EDC systems are not one-size-fits-all. While this article compares five of the best and most commonly used EDC systems highlighting their features, many more EDC systems are available.
There are no perfect EDC systems. There will always be missing features, and a great EDC will always be a work in progress. Concerning that, if you are a researcher, you must keep sharing feature requests with EDC developers through honest reviews and contacting support teams. This feedback ensures that your recommendations are taken into account by developers for further software enhancement.
Until then, while choosing your EDC system, do your homework to find the most suitable option for your research. Most of them offer a free demo or a call with their team where you can share your study design and if their tools are indeed the best fit for the kind of research you have in mind.
If appropriately used in the 21st century, data could save us from lots of failed interventions and enable us to provide evidence-based solutions towards tackling malaria globally. This is also part of what makes the ALMA scorecard generated by the African Leaders Malaria Alliance an essential tool for tracking malaria intervention globally. If we are able to know the financial resources deployed to fight malaria in an endemic country and equate it to the coverage and impact, it would be easier to strengthen accountability for malaria control and also track progress in malaria elimination across the continent of Africa and beyond.
Odinaka Kingsley Obeta
West African Lead, ALMA Youth Advisory Council/Zero Malaria Champion
There is a smarter way to do research.
Build fully customizable data capture forms, collect data wherever you are and analyze it with a few clicks — without any training required.
ESM/EMA data collection, made unbelievable easy.
Build customisable mobile forms, create flexible reminders and invite participants with a few clicks — without any training required.
Dear Digital Diary, I realized that there is an unquestionable comfort in being misunderstood. For to be understood, one must peel off all the emotional layers and be exposed. This requires both vulnerability and strength. I guess by using a physical diary (a paper and a pen), I never felt like what I was saying was analyzed or judged. But I also never thought I was understood. Paper does not talk back.Using a daily digital diary has required emotional strength. It has required the need to trust and the need to provide information to be helped and understood. Using a daily diary has needed less time and effort than a physical diary as I am prompted to interact through mobile notifications. I also no longer relay information from memory, but rather the medical or personal insights I enter are real-time behaviours and experiences. The interaction is more organic. I also must confess this technology has allowed me to see patterns in my behaviour that I would have otherwise never noticed. I trust that the data I enter is safe as it is password protected. I also trust that I am safe because my doctor and nutritionist can view my records in real-time. Also, with the data entered being more objective and diverse through pictures and voice recordings, my treatment plan has been better suited to my needs. Sincerely, No more elephants in this room
Rawan is a PRINCE2-certified project manager and a Public Health professional with 3 years of experience managing development projects. She recently served as a Programme Analyst with UNDP in Zambia providing project support across topics such as Inclusive Cities, Climate Action, and Economic Growth. She was part of the inaugural cohort of a 16-month fellowship titled the African Young Women Leaders . Rawan aspires to rise as a development expert with the United Nations.
More articles on
Digital health and human rights: the 3 challenges we face.
.jpeg)
Research Authorship: The Complete Guide for Young Researchers

Home-Based Care: The Top 3 Challenges and Solutions
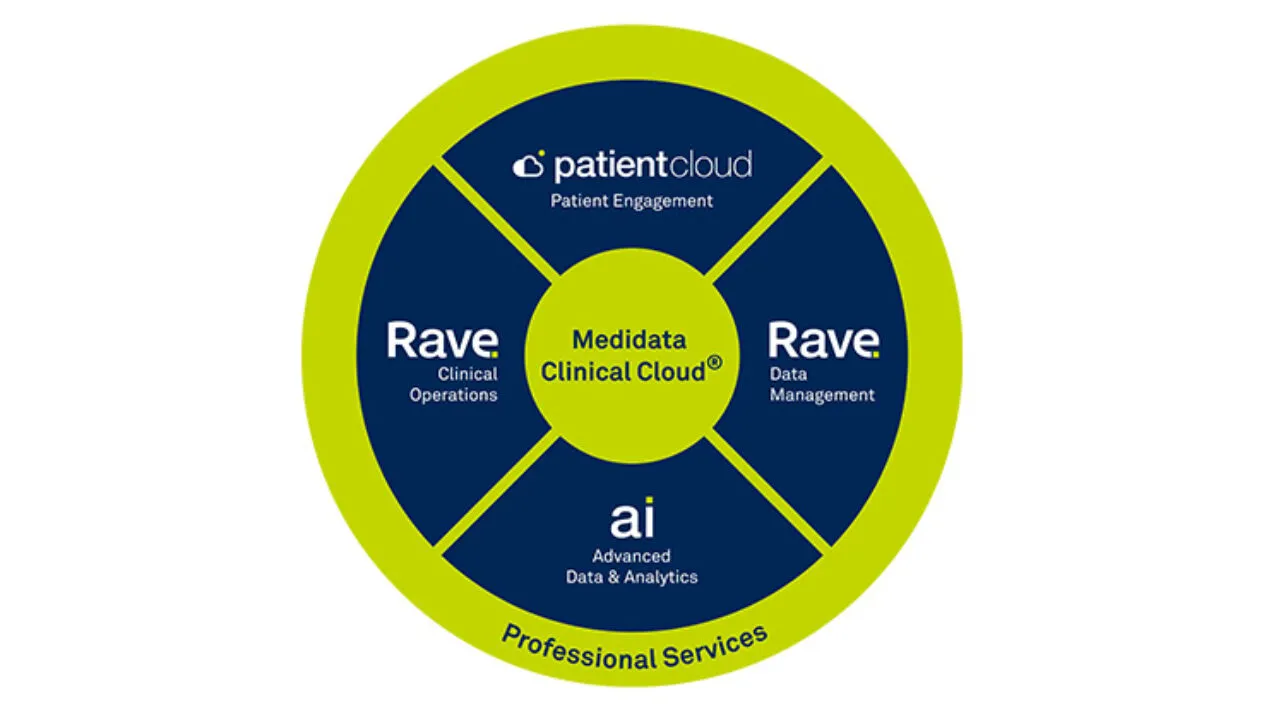
Medidata’s Rave EDC (Electronic Data Capture) is the most advanced, robust, and secure EDC system for clinical trial site, patient, and lab data capture and management.
Rave EDC is the cornerstone of the Medidata Platform – the unified clinical research platform that connects processes, eliminates data reconciliation, and delivers cross-functional and cross-study data insights.
Customer Testimonials
Choosing Rave EDC —The Common Denominator Amongst Sites

The Medidata Platform Simplifies the Site Experience

Rave EDC: Unmatched Efficiency and Ease
Why choose the rave edc system.

Unparalleled Experience and Expertise in EDC
Medidata is the pioneer in Electronic Data Capture (EDC), trusted by you to run over 30,000 clinical trials, capturing data from millions of patients.
In the EDC Benchmarking and Market Dynamics report (June 2023, Industry Standard Research), Rave EDC was rated the most preferred, most recently used, and the leader among EDC providers.

Flexibility and Scalability for All Your Studies
Rave EDC has the flexibility and scalability to run all your biopharmaceutical or medical device studies, regardless of size, phase or therapeutic areas.
Our study design capabilities implement all protocol designs, no matter how simple or complex. And our infrastructure easily hosts your largest study populations and datasets.

Mid-Study Changes with No Downtime
Medidata and our customers perform an average of over 30,000 mid-study changes per year, including for studies with tens of thousands of patients. The combination of our technology and experienced Professional Services team ensures smooth implementation of mid-study changes, with no downtime, for even the most complex, large-scale, and time-critical trials.

Best in Class Data Security, Privacy, and Quality
Ensure the security and privacy of your critical clinical trial data and the privacy of your patients.
Medidata’s data security, privacy and quality processes, systems and certifications are second-to-none in the Life Sciences industry.
Accelerated Decision-Making for Clinical Operations
Rave EDC is unified with Clinical Operations capabilities on the Medidata Platform.
Through automatic data population from Rave EDC to Rave CTMS and Medidata Detect , your CRAs and study managers realize more efficient workflows and immediate data oversight. Detect compiles complete patient profiles and delivers automated, intelligent insights into data trends and anomalies to alert your data managers and central monitors to issues before they become systemic.

“The best qualities of Rave EDC are how user-friendly [it is] for the sites to enter data…, how the query management is very efficient, and [the ease of] getting the data out.”
– Vijay Chundru, Senior Director, EDC Programming Team, Global Clinical Data Operations, Jazz Pharmaceuticals
“We had a study with 6000 patients on it. [With Rave EDC] You could change 6000 patients in hours, whereas another platform took four months to adapt their CRF [Case Report Form] because the platform just wasn’t capable. I know when you say to people, ‘How long is that going to take to update the CRF?’……. ‘Once it’s all signed off, it’ll be an hour maximum,” Other platforms……definitely do take a lot longer.”
– Ian Howson, Senior Manager, Database Programming, Parexel
Contact an Expert
We would love to hear from you.
Want to learn more about our products and solutions or need a demo? Fill out this form to have a Sales representative reach out to you.
If you need technical support, please contact our Helpdesk .
Key Features of Rave EDC

Make Data Entry Easier and Faster For Your Sites
Rave Companion reduces clinical trial data entry efforts for sites by making it simpler and faster to get source data from any system (e.g., EHR – electronic health record) or document (e.g., lab values in a spreadsheet) into Rave EDC.
Centralized Administration and Master Data
Centrally manage your users, roles, studies, and sites across all Rave EDC (and other products on the Medidata Platform ) studies.
Eliminate study master data duplication and inconsistencies (e.g., different IDs for the same sites in different applications).
Give users a single place to login to all systems with the same username and password.
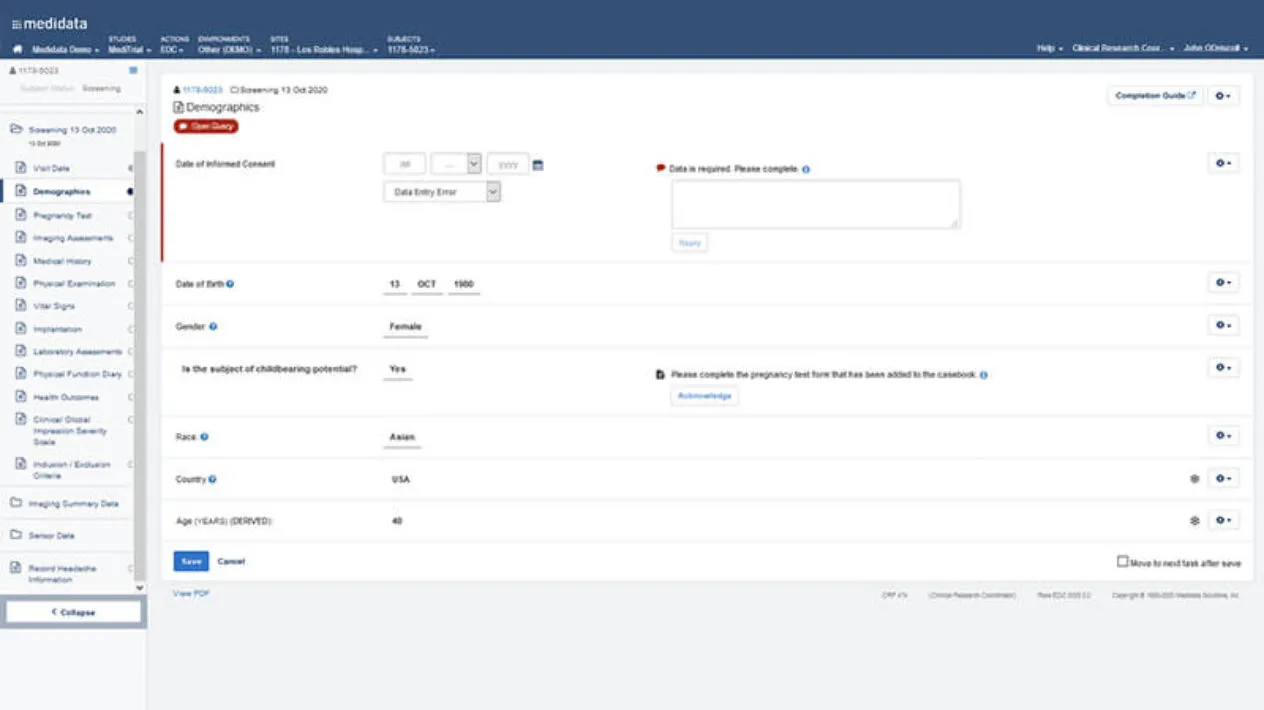
Real-time Data Validation
Make sure the right data is entered at the time of entry, not after the case report form is saved.
Improve your sites’ data entry efficiency and data quality with Rave EDC’s real-time edit checks.
Clinical Data Studio
Medidata’s Clinical Data Studio is the latest innovation to Rave EDC, combining two powerful data management solutions.The first solution is Patient Profiles which enables intuitive safety and medical reviews. The second solution is Data Reviewer, which aggregates patient data from almost any source for review, interrogation, and reconciliation, all from a single location.
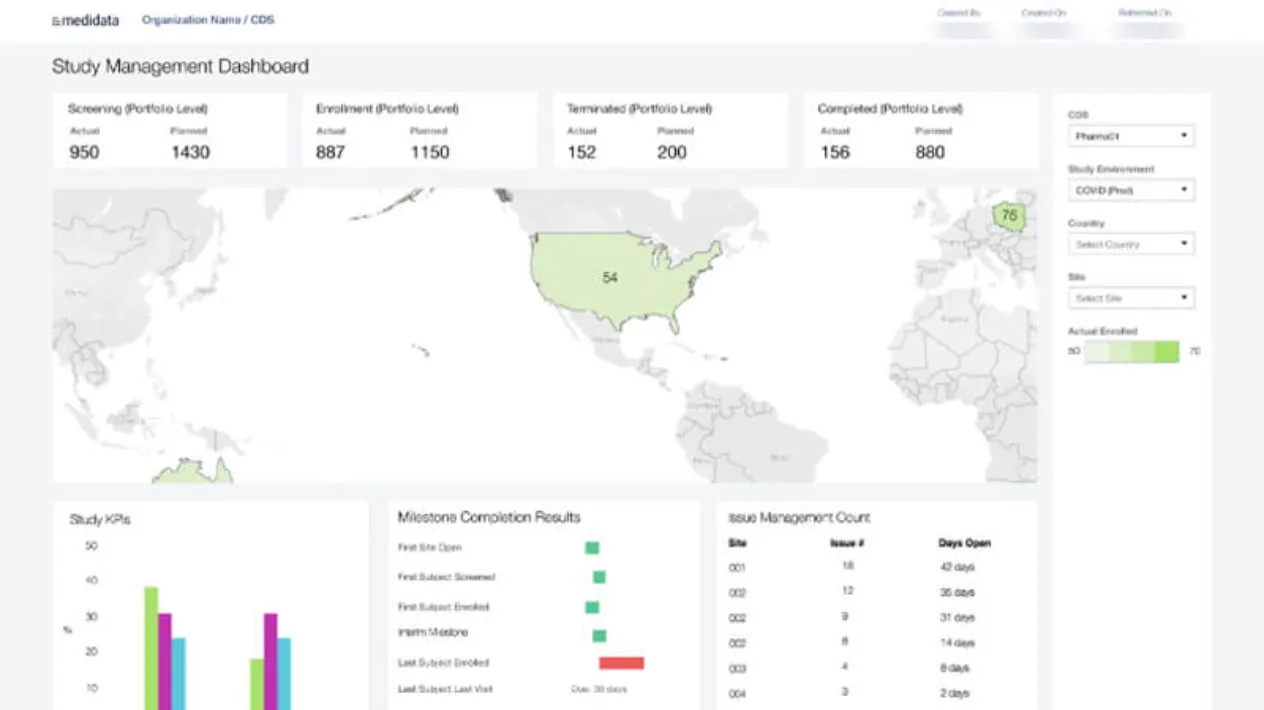
Reporting and Analytics
Make informed decisions with real-time study insights through dashboards and standard/ad-hoc reports, and one-click access from reports to relevant forms.
Standard reports include study data monitoring, study admin, lab admin, and dictionary coding. Visualize your key study metrics such as enrollment tracking and data cleaning progress.
Ad-hoc reports use industry-standard software to let you perform a deeper analysis of your study data.
“This is where working with a partner like Medidata, that builds solutions such as Rave EDC and others, … helps us really leverage the extended expertise over years that they’ve built, to have reassurance and work on a robust platform.”
– Hassan Kadhim, Global Head of Clinical Trial Business Capabilities, BMS
Related Solutions

Rave Data Management

Rave Coder – Medical Coding for Clinical Trials

Rave Safety Gateway – Safety Data Transmission

Modernizing Clinical Data Management to Be Scalable, Flexible, and Intelligent
This white paper provides a summary of why Clinical Data Management (CDM) must quickly adapt to the mounting data pressures in modern clinical trials and discusses the three pillars that form the foundation of a modern intelligent CDM platform that is needed to succeed in an increasingly complex clinical trial world.

TissueTech: Launching a Phase 3 Pivotal Trial in a Pandemic with the Medidata Platform
Learn how during the COVID-19 pandemic, the Medidata Platform equipped TissueTech to immediately pivot to a remote and centralized monitoring model.

The Next-Generation of Clinical Data Management
The clinical data landscape continues to evolve, but the way data is managed (reviewed and cleaned) has not kept pace with the growth in data sources, types, volume, and velocity. This infographic explores the challenges and proposes a solution to modernize clinical data capture and management.

Medidata Explorer
What are your biggest challenges and priorities for clinical data capture and management in your studies?
Walk through our interactive tool to discover how Medidata’s solutions can help you address issues specific to your role, and receive a personalized action plan.
You decide what cookies Medidata will use
What are cookies.
Necessary cookies are essential and are used to provide you with services available through Medidata website. For instance, these cookies allow Medidata to remember your choices about cookies preferences, to record your interface customization trackers e.g. for the choice of language used by the website. Necessary cookies are enabled by default and cannot be switched off. To see the list of the cookies used for this purpose, click here .
Functional cookies are used to provide you with contents and proposals that correspond to your interactions. They may consist of information logged on your device or recorded as you navigate through Medidata website. These cookies also allow us to analyze site usage so we can measure and improve performance. To see the list of the cookies used for these purposes, click here .
Advertising cookies are used to enable Medidata and its trusted Medidata business stakeholders to serve ads that are relevant to your interests. The intention is to display ads that are relevant to you.
- Microsoft Clarity
- Microsoft Bing
- Oracle / Eloqua
To see the list of the cookies used for this purpose, click here .
- Office of Clinical Trials
- Office of Research Integrity
- Office of Sponsored Programs
- International Gaming Institute
- National Supercomputing Institute
- Cannabis Policy Institute
- Policies & Forms
- Faculty Awards
- Councils & Committees
- Faculty/Staff Directory
- Directories
Quick Links
- Directories Home
- Colleges, Schools, and Departments
- Administrative Units
- Research Centers and Institutes
- Resources and Services
- Employee Directory
- Contact UNLV
- Social Media Directory
- UNLV Mobile Apps
- Research Home
- Division Units
- Research Electronic Data Capture
Research Electronic Data Capture (REDCap)
UNLV researchers now have access to REDCap, a server software for building and managing online surveys and databases. It is designed to support online and offline data capture for research studies and operations.
REDCap Features
Data security.
REDCap is a secure web-based platform for data collection and management. REDCap data is encrypted in transit and in storage. Access to REDCap is done via SSL transfer to your web browser. Additional security steps ensure that access to the REDCap site is verified and logins are further secured by two factor authentication through your ACE account.
Optimized for Use Across Devices
REDCap is compatible with desktop computers, laptops, tablets (including iPads), smart phones (Android and Apple), and any other device having an internet connection. Since REDCap is entirely web based, you can access it from any browser, anywhere, at any time. No separate app, download, or software installation is needed.
Track Project Status Levels
There are four different status levels (development, production, inactive, archived) that you can use to track progress of your project.
How to Use REDCap
Request access to redcap.
Using your UNLV email, send a request to [email protected] that includes a completed copy of the REDCap Access Request Form and REDCap End User Agreement . Once received, a research administrator will request access on your behalf from the OIT Help Desk and will notify you when your account has been created.
Where to Access the New REDCap AWS Instance
If you have already received access to REDCap via submission of access and end user forms to [email protected] , please use the following link to sign into the database .
Add Additional People to Your REDCap Projects
If you would like other persons to work with you on a project, make sure they have independently obtained a REDCap account. If they have not done so, they will not be able to log in.
Once they have created an account, you can give them access by adding them as a user of the project. You can also restrict each user’s access to the project as desired.
Receive IRB Approval (If Needed)
You do not need IRB approval to utilize REDCap. However, if you are conducting research that involves human subjects, you must receive IRB approval before moving your project into production status and start collecting data.
Watch Training Videos
There are several informational videos available to help you get started and gain a better understanding of the REDCap application and its functionality. Note that new versions of REDCap are released frequently, so the videos and other training resources may reflect earlier software versions and thus may look slightly different.
Understand Project Status Levels
Development status.
All projects start at development status, which is where you should build and thoroughly test the project. The project is fully functional, and you can use all of the REDCap facilities to modify and check the project, such as sending out survey invitations and downloading the response data. You should only collect or enter test data while in this status.
While a project is in development status, be sure to test every phase of your project before you move it to the next level (production status) and collect “real” data. After a project is moved to production status, it is more challenging to make changes to the project.
Production Status
After you have created, modified, and thoroughly tested your project, and you are ready to collect or enter “real” data, you should move it to production status. This can be done by selecting the “Move Project to Production” button located on the "Project Setup" tab in REDCap.
Important: When you move the project to production status, you will be given the option of deleting all data that has been collected to that point. This option is provided because you usually would have only been collecting test data in development status. If that is the case, you would typically want to delete all that test data before collecting real data.
Inactive Status
If your project is in production status and you are finished using it, you may shut it down and take it offline by moving it to inactive status. To do so, select the “Move to an Inactive Status” button on the "Other Functionality" tab in REDCap.
Once a project is inactive, no responses can be entered or collected. Anyone trying to take a survey will be informed that the survey is no longer active. An inactive project may be brought back to production status by selecting the “move project to production" button, located on the "Other Functionality" tab.
Archived Projects
If you wish to remove a project from your account, you can archive it. After it is archived, you will no longer have access to the project, including its data. To archive a project, select the “Archive the Project” button located on the "Other Functionality" tab.
Deleted Projects
A project (including all its data) may also be deleted by selecting the "Delete the Project" button, located on the "Other Functionality" tab. Deletion is permanent, and a deleted project cannot be retrieved.
Use Data Entry Forms and Surveys
The primary entity in REDCap is a project, and projects use two types of data collection instruments:
- Data Entry Form: This form requires an administrator to enter all the data. To create a data entry form, you must first create a new project by logging in to REDCap and selecting the "New Project" tab.
- Survey: Surveys can be accessed by others on web browsers to provide information. To create a survey, you must first create a new project by logging in to REDCap and selecting the "New Project" tab.
Use Public Survey Links and Participant Lists to Aid Anonymous Data Collection
Note: REDCap records the date and time of day that each survey response is collected. Therefore, if you knew the date and time that someone took your survey, you could potentially identify their answers. Please plan your procedures for anonymous data collection carefully.
Whether or not REDCap collects any identifying information along with the user’s survey answers depends on how you invite your survey takers. When you click "Manage Survey Participants" in the left-hand panel, the resulting screen has two different tabs:
- Public Survey Link Tab: This tab will provide a web link for users to complete a survey. If you provide that link to someone via email, web page, or other means, and the person uses that link to access the survey, then no identifying information will be collected with their survey responses.
- Participant List Tab: This tab will create a participant list of email addresses and have REDCap send those individuals invitations to take the survey. In your participant list, if you provide a participant identifier for an address in the list, then that identifier will be collected along with the person’s survey answers, and you could use it to identify their answers. If you provide no identifier, then no identifying information will be collected (assuming that you have not included a survey question that asks for the respondent’s name or other identifying information).

Use REDCap Without Internet Connection
If you don’t have internet access, you cannot use the online version of REDCap. In such a situation, you may download the REDCap Mobile App onto your device. The app has the same primary functionality as the online version of REDCap, just offline. The app can sync (mass import) your offline data back to your online REDCap project when you return to internet connectivity.
Alternate Solutions
- Store Data in Another Format: Data can be stored in another format and then uploaded into REDCap all at once or incrementally when the internet connection is reliable. Though not ideal, you use another program offline to collect data in areas of low internet coverage. By entering your data into REDCap at a later time, you use REDCap as an electronic record and to prepare the data for analysis.
- Purchase a Portable Wireless Router: A portable wireless router can act as an internet hotspot to enable you to enter data online in the field. This will allow you full use of the application from any low-coverage area. That way, you can access the online REDCap platform from your device’s browser. By providing your own internet access, the data could be stored securely (and directly in REDCap) from the start and there’s no need to transfer it from hard copies later.
Cite REDCap For Your Publications
When citing REDCap for data collection and management in study manuscripts, we recommend using the following boilerplate language:
Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Nevada Las Vegas. REDCap is a secure, web-based software platform designed to support data capture for research studies, providing:
- An intuitive interface for validated data capture;
- Audit trails for tracking data manipulation and export procedures;
- Automated export procedures for seamless data downloads to common statistical packages; and
- Procedures for data integration and interoperability with external sources.
Transfer Your REDCap Project
Transferring existing projects.
- From “My Projects,” select “Existing Project”
- From “Project Home and Design,” select “Dictionary,” which will redirect you to the Data Dictionary tab
- Select “Download the Current Data Dictionary”
- From “Applications,” select “Data Exports, Reports, and Stats.” This will redirect you to the “My Reports & Exports” tab
- Select the blue “Export Data” button (CSV raw data)
Transferring In The New REDCap
- You might be able to upload an XML file (same export procedure as the CSV); however, if you get error messages, use the CSV method
- Note that REDCap advises the project be moved into production status before importing real data
- From “Project Home and Design,” select “Project Set-Up”
- Select “Design your data collection instruments,” and then choose “Data Dictionary”
- Select “Choose File” and upload your CSV
- From “Applications,” select “Data Import Tool”
- If any issues are detected, correct them in the CSV and retry (see “Fix Project Transferring Errors” below)
Fix Project Transferring Errors
Finding the accepted value for editing errors in the csv.
- Note which values need to be adjusted
- From “Project Home and Design,” select “Designer” in the Online Designer tab
- Select the instrument
- Scroll to the item and select “Edit” to view the accepted data values
- Edit the CSV to correct unaccepted values
Email [email protected] if you have any questions about REDCap.
Electronic Data Capture Software
Find the best Electronic Data Capture Software
Popular comparisons, buyers guide, filter products, company size.
- Self-Employed
Pricing Options
- # of User Reviews
- Average Rating
- Alphabetically (A-Z)
Compare Products
Showing 1 - 20 of 152 products

Forms On Fire
Forms On Fire is a mobile app for capturing and dispatching mobile forms such as inspections, audits, safety reports, orders, and many other tasks. Designed for businesses in construction, agriculture, field service management, fo... Read more about Forms On Fire
4.7 ( 127 reviews )

Jotform is a cloud-based form automation solution that enables users to publish online forms and record customer responses. It helps users to generate leads, collect order payments, conduct customer surveys, manage job application... Read more about Jotform
4.7 ( 1999 reviews )

Seamless.AI
Seamless.AI is a lead management solution that helps businesses streamline processes related to contact search, market research, lead conversion, data importing, and more on a centralized platform. With the pitch research tools, t... Read more about Seamless.AI
3.8 ( 150 reviews )

Dacima Clinical Suite
Dacima Clinical Suite is a cloud-based electronic data capture (EDC) and study management (CTMS) system designed to streamline clinical trial data collection processes. This solution includes interactive dashboards, a CDISC-compli... Read more about Dacima Clinical Suite
5.0 ( 5 reviews )

At Medrio, our vision is to improve 100 million lives by enabling faster, more efficient, higher-quality clinical trials. We empower sponsors and CROs through our proven, scalable solutions, unrivaled customer support, and industr... Read more about Medrio
4.6 ( 51 reviews )

Castor EDC is a secure Electronic Data Capture system designed to manage clinical studies and trials. This solution can be used by various organizations including medical device development, biotechnology researchers, pharmaceutic... Read more about Castor EDC
4.7 ( 158 reviews )

OpenClinica
OpenClinica is a clinical data management and electronic data capture solution designed to help medical institutions capture electronic data and streamline clinical trials using electronic case report forms (eCRFs) and a drag-and-... Read more about OpenClinica
4.7 ( 37 reviews )

Grooper was built from the ground up by BIS, a company with 35 years of continuous experience developing and delivering new technology. Grooper is an intelligent document processing and digital data integration solution that empo... Read more about Grooper
4.6 ( 24 reviews )

Fluix is a no-code, simple-to-use software solution that helps companies to digitize documents and automate routine tasks, all in one place. We solve the pain points of office and field teams who want to deliver faster and gather... Read more about Fluix
4.8 ( 45 reviews )

Fulcrumapp.com
Fulcrum is an industrial-strength field data collection and process management platform trusted by organizations worldwide to help them capture and share reliable data about any field activity, in far less time than paper or other... Read more about Fulcrumapp.com
4.8 ( 187 reviews )

Powered by AI-based technology, Rossum is an intelligent cloud-based data extraction plugin solution that helps businesses capture required information from invoices, receipts, purchase orders, and bills of lading to streamline ac... Read more about Rossum
4.6 ( 12 reviews )

Device Magic
Device Magic is a data collection mobile app that helps businesses automate and manage the process of creating forms for mobile devices. Key features include collaboration, version control, text editing and remote access. Des... Read more about Device Magic
4.5 ( 38 reviews )

Prelude EDC
Designed for clinical research, Prelude EDC is a CFR, Annex 11, and HIPAA compliant EDC solution that streamlines the data collection process, automates data cleaning efforts, and monitors the state of electronic case reports form... Read more about Prelude EDC
5.0 ( 12 reviews )

Designed as a comprehensive clinical data management solution, Ennov EDC allows clinical research personnel to capture and collect subject data for clinical trials of any size including post-marketing trials, cohort trails, health... Read more about Ennov EDC
No reviews yet

ResearchManager
ResearchManager offers a sophisticated, flexible, and cost-effective eClinical platform encompassing Clinical Data Management Tools (EDC, ePRO, RTSM, eConsent) and Clinical Operations (CTMS, eTMF, LIMS, RIMS). Backed by a decade ... Read more about ResearchManager
4.2 ( 110 reviews )

ClinCapture
Captivate EDC is a cloud-based electronic data capture solution that assists medical researchers and practitioners with data capture and forms management. Key features include WYSIWYG form editor, risk-based monitoring, activity d... Read more about ClinCapture
4.8 ( 15 reviews )

iMednet is a cloud-based electronic data capture platform designed to help the healthcare industry capture, clean, adjudicate and manage data across clinical trials. The application enables research teams to build and implement st... Read more about iMednet
5.0 ( 2 reviews )

123FormBuilder
123FormBuilder is a cloud-based web form builder that enables businesses of all sizes to create surveys, forms, quizzes and polls. The platform comprises templates for job applications, donations, event registration, online bookin... Read more about 123FormBuilder
4.4 ( 171 reviews )

GoCanvas is a cloud-based mobile business management system suitable for field service companies, including electrical, HVAC, pest control and plumbing. The solution is designed to work on any smartphone or tablet and can also be ... Read more about GoCanvas
4.4 ( 200 reviews )

Centreviews
Centreviews is a cloud-based business intelligence suite providing solutions that include accounts receivable, accounts payable and document management. The payables solutions provides users with an automated workflow system for a... Read more about Centreviews
4.4 ( 68 reviews )

Access PeopleHR

Nintex Process Platform

TrueContext

Lucky Orange

Collecting accurate data is a priority for businesses such as research firms and healthcare organizations that deal with large volumes of data. Data needs to be captured, documented, and compiled for analysis, but trying to complete this process manually is a tough job. Manual paper-based methods are not only time-consuming but also increase the chances of human errors.
Electronic data capture (EDC) software digitalizes the data capture process for field teams, surveyors, researchers, and study coordinators, so they can do away with manual ways of collecting data. It validates the captured data and makes it readily available for analysis and reporting.
A wide range of EDC software options is available on the market, and choosing the one that best meets your needs and budget is important. This guide will help you understand the different factors you must consider when shortlisting an EDC solution.
Here's what we'll cover:
What is EDC software?
Common features of edc software, what type of buyer are you, benefits of edc software, key considerations when buying edc software, market trends to understand.
EDC software is an electronic data capture solution that lets surveyors, clinical researchers, and other field teams collect data via electronic forms. It streamlines data management and helps replace traditional paper-based forms. Data captured by the tool is stored at a centralized location and can be fed into other software applications for analysis or reporting.
EDC software can work with mobile devices, devices that have a built-in camera for scanning paper forms, or form builder tools. It’s most commonly used in pharmaceutical and clinical research companies to capture large volumes of patient and clinical trial data.
Capturing data through a mobile device in Fulcrum ( Source )
Different vendors offer different features as part of their EDC system. Selecting software with the right features is easier when you know what the most common features are and what they do. Here’s a look at some of the common features of EDC tools.
| Use desktops, mobile devices, tablets, etc., to enter data into specific fields on electronic forms. |
| Use built-in customizable templates to create electronic data capture forms. You can also add various data fields to the forms. |
| Set up automated data validation checks to ensure captured data is accurate and can be processed further for analysis. |
| Export captured data in file formats such as Excel and CSV. Use the reporting function to extract business insights from the collected data. |
| Integrate the tool with other software solutions such as data analysis systems to ensure smooth exchange of data for further analysis or reporting. |
Before you start evaluating EDC software options, you'll want to know which buyer category you belong to. Most buyers belong to one of the below categories.
Small and midsize businesses (up to 500 employees): These buyers typically include small and midsize companies with expertise in a specific field. Their data collection teams have fewer members who focus on a single area of research. These buyers should adopt an EDC platform that aligns with their field of specialization. Accurate data capture, data validation, and creation of electronic forms are some features they should look for.
Large enterprises (over 500 employees): These buyers include large companies that have multiple projects on their annual agendas. Thus, they have multiple data collection teams that need to collect huge volumes of data. These buyers should opt for an EDC tool that not only captures and validates data accurately but also supports integration with third-party analysis tools to derive insights from collected data.
While some benefits of EDC solutions may be clear from our discussion above, we've listed the most notable ones in this section.
Better data quality: EDC software validates data for accuracy based on user-defined rules. It immediately flags manual errors or illegible data to ensure correct details are collected. It also automatically calculates field values in electronic forms, thus increasing accuracy and reducing manual work for the data collection team.
Prevention of data loss: EDC software helps store data in a digital format instead of paper-based forms that can get misplaced, lost, or damaged. Also, collected data is kept at a centralized location and backed up regularly. In case of accidental data loss, backed-up data is readily available for use.
Listed below are some points to consider when purchasing an EDC platform.
Mobile app availability: Check if the EDC software you’re planning to purchase offers mobile apps for iOS and Android devices, such as smartphones and tablets. A mobile app will let you input data into the system even if you’re offline or have limited access to the internet. Data collected offline will sync automatically once the internet connectivity is restored.
Customer support: Inquire about the type of support provided by your shortlisted vendors: 24/7, 24/5, or only during business hours. Also, verify if they offer your preferred support channels, such as email, phone, or live chat. Support services will help fix any software issues that can lead to compromise or loss of confidential data.
Here’s a recent trend in the EDC software market that you should be aware of:
Healthcare companies are integrating wearable devices with EDC software: A growing number of healthcare and clinical research companies are integrating patients' wearable devices, such as glucometers and oxygen sensors, with electronic data capture software to automatically collect patient data. Integration is helping these companies capture data on time as well as reduce the number and length of patient visits to clinical sites.
Note: The application selected in this article is an example to show a feature in context and isn’t intended as an endorsement or a recommendation. It has been obtained from sources believed to be reliable at the time of publication.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
The use of Research Electronic Data Capture (REDCap) software to create a database of librarian-mediated literature searches
Affiliation.
- 1 a University of Florida Health Science Center Libraries , Gainesville , Florida , USA.
- PMID: 25023012
- PMCID: PMC4339087
- DOI: 10.1080/02763869.2014.925379
Expert-mediated literature searching, a keystone service in biomedical librarianship, would benefit significantly from regular methodical review. This article describes the novel use of Research Electronic Data Capture (REDCap) software to create a database of literature searches conducted at a large academic health sciences library. An archive of paper search requests was entered into REDCap, and librarians now prospectively enter records for current searches. Having search data readily available allows librarians to reuse search strategies and track their workload. In aggregate, this data can help guide practice and determine priorities by identifying users' needs, tracking librarian effort, and focusing librarians' continuing education.
Keywords: Data capture; REDCap; evidence-based librarianship; expert literature searching; mediated literature searching.
PubMed Disclaimer
Screenshot from data form 3:…
Screenshot from data form 3: Search strategies and databases.
Screenshot showing a REDCap-generated form…
Screenshot showing a REDCap-generated form representing the number of searches by college.
Screenshot showing a REDCap-generated form representing the searching times.
Screenshot showing a REDCap-generated form representing the number of searches by the purpose…
Screenshot showing a REDCap-generated form representing the number of searches by database searched.
Screenshot showing the built-in data…
Screenshot showing the built-in data quality tools of REDCap.
Similar articles
- Multisite collaboration using REDCap to capture library data. Grinstead C, Schwartz A. Grinstead C, et al. J Med Libr Assoc. 2019 Oct;107(4):601-602. doi: 10.5195/jmla.2019.768. Epub 2019 Oct 1. J Med Libr Assoc. 2019. PMID: 31607820 Free PMC article.
- Enhancing Clinical Librarian Work Processes and Communicating Service Impact with REDCap and Data Visualization. Daniels K, Lawson E. Daniels K, et al. Med Ref Serv Q. 2019 Apr-Jun;38(2):187-196. doi: 10.1080/02763869.2019.1588051. Med Ref Serv Q. 2019. PMID: 31173566
- A new hat for librarians: providing REDCap support to establish the library as a central data hub. Read K, LaPolla FWZ. Read K, et al. J Med Libr Assoc. 2018 Jan;106(1):120-126. doi: 10.5195/jmla.2018.327. Epub 2018 Jan 2. J Med Libr Assoc. 2018. PMID: 29339942 Free PMC article.
- New activities and changing roles of health sciences librarians: a systematic review, 1990-2012. Cooper ID, Crum JA. Cooper ID, et al. J Med Libr Assoc. 2013 Oct;101(4):268-77. doi: 10.3163/1536-5050.101.4.008. J Med Libr Assoc. 2013. PMID: 24163598 Free PMC article. Review.
- Expert searching in health librarianship: a literature review to identify international issues and Australian concerns. Lasserre K. Lasserre K. Health Info Libr J. 2012 Mar;29(1):3-15. doi: 10.1111/j.1471-1842.2011.00974.x. Health Info Libr J. 2012. PMID: 22335285 Review.
- Absenteeism Costs Due to COVID-19 and Their Predictors in Non-Hospitalized Patients in Sweden: A Poisson Regression Analysis. Kisiel MA, Lee S, Janols H, Faramarzi A. Kisiel MA, et al. Int J Environ Res Public Health. 2023 Nov 10;20(22):7052. doi: 10.3390/ijerph20227052. Int J Environ Res Public Health. 2023. PMID: 37998283 Free PMC article.
- Clustering Analysis Identified Three Long COVID Phenotypes and Their Association with General Health Status and Working Ability. Kisiel MA, Lee S, Malmquist S, Rykatkin O, Holgert S, Janols H, Janson C, Zhou X. Kisiel MA, et al. J Clin Med. 2023 May 23;12(11):3617. doi: 10.3390/jcm12113617. J Clin Med. 2023. PMID: 37297812 Free PMC article.
- A suggested data structure for transparent and repeatable reporting of bibliographic searching. Haddaway NR, Rethlefsen ML, Davies M, Glanville J, McGowan B, Nyhan K, Young S. Haddaway NR, et al. Campbell Syst Rev. 2022 Nov 23;18(4):e1288. doi: 10.1002/cl2.1288. eCollection 2022 Dec. Campbell Syst Rev. 2022. PMID: 36908843 Free PMC article.
- Predictors of post-COVID-19 and the impact of persistent symptoms in non-hospitalized patients 12 months after COVID-19, with a focus on work ability. Kisiel MA, Janols H, Nordqvist T, Bergquist J, Hagfeldt S, Malinovschi A, Svartengren M. Kisiel MA, et al. Ups J Med Sci. 2022 Aug 9;127. doi: 10.48101/ujms.v127.8794. eCollection 2022. Ups J Med Sci. 2022. PMID: 35991464 Free PMC article.
- Retromode Imaging Modality of Epiretinal Membranes. Savastano A, Ripa M, Savastano MC, Caporossi T, Bacherini D, Kilian R, Rizzo C, Rizzo S. Savastano A, et al. J Clin Med. 2022 Jul 6;11(14):3936. doi: 10.3390/jcm11143936. J Clin Med. 2022. PMID: 35887700 Free PMC article.
- Role of Expert Searching in Health Sciences Libraries: Policy Statement by the Medical Library Association adopted September 2003. Journal of the Medical Library Association. 2005 Jan;93(1):42–44. - PMC - PubMed
- Holst R, Funk CJ. State of the Art of Expert Searching: Results of a Medical Library Association Survey. Journal of the Medical Library Association. 2005 Jan;93(1):45–52. - PMC - PubMed
- Lasserre K. Expert Searching in Health Librarianship: A Literature Review to Identify International Issues and Australian Concerns. Health Information and Libraries Journal. 2012 Mar;29(1):3–15. - PubMed
- Bredderman PJ. Paradox for Online Service Statistics and Reports. Database. 1991 Jun;14(3):31–38.
- Vardell E, Loper K, Vaidhyanathan V. Capturing Every Patron Interaction: The Move from Paper Statistics to an Electronic System to Track the Whole Library. Medical Reference Services Quarterly. 2012 May;31(2):159–170. - PubMed
Publication types
- Search in MeSH
Related information
Grants and funding.
- UL1 TR000064/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full text sources.
- Europe PubMed Central
- PubMed Central
- Taylor & Francis
Other Literature Sources
- scite Smart Citations
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

Efficient Data Collection Solution
Electronic Data Capture
Experience the power of modern data collection with ResearchManager EDC. Optimize and streamline the data collection process, gain quick insights, and ensure data integrity at the highest and most secure level.

Modern and Efficient Data Collection for Any Type of Research
ResearchManager EDC provides an innovative alternative to data collection by replacing traditional (paper-based) methods with an electronic Case Report Form (eCRF). With EDC, we simplify and expedite the data collection process. Additionally, we reduce manual errors, enhance data entry, validation, and reporting efficiency.
Real-time Access to Research Data and Status
With real-time access and insights into all aspects of the clinical research process, research teams can securely view and monitor data. This enhances remote collaboration and increases efficiency. Additionally, ResearchManager EDC provides comprehensive functionality for query management, allowing the generation and resolution of data queries to address inconsistencies or missing information. Every action and modification is recorded in an audit trail, ensuring complete traceability and regulatory compliance.

Increased Integrity of Research Data and Focus on Compliance
ResearchManager EDC, thanks to flexible APIs, can integrate with external systems and other tools within the Clinical Research Suite. This enables data exchange among various tools, such as ePRO, RTSM, and CTMS, as well as with other external databases and wearables. Thus, we create an integrated and efficient research environment where data can seamlessly flow between various processes in the workflow. This not only enhances data management efficiency but also promotes collaboration among different teams and systems, resulting in improved data quality and faster insights.
Highlighted features
User-friendly template builder.
A user-friendly template builder that allows for the quick and intuitive creation of custom forms and questionnaires. This enables researchers to design study-specific data collection instruments up to 50% faster without requiring technical skills.
User Management and Role-Based Access
Advanced user management with role-based access rights to ensure the appropriate access levels for different users.
Study Dashboards
Study dashboards provide customizable views, from individual study tasks to advanced graphical representations, offering insights into metadata.
Real-Time Data Validation
Real-time validations to reduce input errors and ensure data quality by offering immediate feedback.
Query Management
This feature enables efficient tracking, assignment, and resolution of queries raised during data entry or review, ensuring data accuracy and integrity throughout the study.
Notifications
The notifications feature keeps users informed and engaged by providing timely updates on important events, such as data submissions, Adverse Events, task assignments, and study milestones.
Integration Capabilities
In addition to the ability to export data, an API facilitates efficient integration and synchronization of data with other systems, wearables, and platforms.
Audit Trail
Complete audit trail functionality to document all modifications to the data, including the user who made the change, the timestamp of the change, and a detailed description of the alteration.
Users and Partners of ResearchManager

Want to Learn More About Pricing?
Compliance and technical information, researchmanager is compliant.
- Offices in the Netherlands (HQ) and in the USA.
- ISO 27001 certified
- NEN 7510 certified
- ISO 14155 compliant
- 21 CFR PART 11 compliant
- GCP compliant
- GDPR compliant
- HIPAA compliant
- Secure connection with Comodo’s EV SSL certificate
Information about Our Data Centers
- TIER 3+ data centers in the EU
- SOC 2 security standards
- ISO 9001 certified
- ISO 14155 certified
- Dedicated backup server, AES 256 encrypted

Saskia van Gastel
Accountmanager
Ready to see more?
Book a free demonstration with one of our specialists using the button below. We'll guide you through and demonstrate how our solutions can support you.
Clinical Research Suite
Partner Programs
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Research Electronic Data Capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support
Paul a. harris.
1 Department of Biomedical Informatics, Vanderbilt University
Robert Taylor
2 Office of Research, Vanderbilt University
Robert Thielke
3 General Clinical Research Center, Medical College of Wisconsin
Jonathon Payne
4 Biomedical Research Education and Training, Vanderbilt University
Nathaniel Gonzalez
5 Center for Information Architecture in Research, University of Puerto Rico
Jose G. Conde
REDCap is a novel workflow methodology and software solution designed for rapid development and deployment of electronic data capture tools to support clinical and translational research. We present: 1) a brief description of the REDCap metadata-driven software toolset; 2) detail concerning the capture and use of study-related metadata from scientific research teams; 3) measures of impact for REDCap; 4) details concerning a consortium network of domestic and international institutions collaborating on the project; and 5) strengths and limitations of the REDCap system. REDCap is currently supporting 286 translational research projects in a growing collaborative network including 27 active partner institutions.
II. INTRODUCTION
The R01 funding mechanism may be the cornerstone of America’s biomedical research program, but individual scientists often require informatics and other multidisciplinary team expertise that cannot easily be obtained or developed in the independent research environment.( 1 ) The National Center for Research Resources has stated that the future of biomedical research will involve collaborations by many scientists in diverse locations linked through high-speed computer networks that enable submission, analysis, and sharing of data.( 2 ) However, the need to collect and share data in a secure manner with numerous collaborators across academic departments or even institutions is a formidable challenge. This manuscript presents a metadata-driven software application and novel metadata-gathering workflow used to successfully support translational research projects in the academic research environment. REDCap (Research Electronic Data Capture) was initially developed and deployed at Vanderbilt University, but now has collaborative support from a wide consortium of domestic and international partners.
III. METHODS
The REDCap project was developed to provide scientific research teams intuitive and reusable tools for collecting, storing and disseminating project-specific clinical and translational research data. The following key features were identified as critical components for supporting research projects: 1) collaborative access to data across academic departments and institutions; 2) user authentication and role-based security; 3) intuitive electronic case report forms (CRFs); 4) real-time data validation, integrity checks and other mechanisms for ensuring data quality (e.g. double-data entry options); 5) data attribution and audit capabilities; 6) protocol document storage and sharing; 7) central data storage and backups; 8) data export functions for common statistical packages; and 9) data import functions to facilitate bulk import of data from other systems. Given the quantity and diversity of research projects within academic medical centers, we also determined two additional critical features for the REDCap project: 10) a software generation cycle sufficiently fast to accommodate multiple concurrent projects without the need for custom project-specific programming; and 11) a model capable of meeting disparate data collection needs of projects across a wide array of scientific disciplines.
REDCap accomplishes key functions through use of a single study metadata table referenced by presentation-level operational modules. Based on this abstracted programming model, studies are developed in an efficient manner with little resource investment beyond the creation of a single data dictionary. The concept of metadata-driven application development is well established, so we realized early in the project that the critical factor for success would lie in creating a simple workflow methodology allowing research teams to autonomously develop study-related metadata in an efficient manner.( 3 – 5 ) In the following sections, we describe the workflow process developed for REDCap metadata creation and provide a brief overview of the user interface and underlying architecture.
A. Study Creation Workflow
Figure 1 provides a schematic representation of the workflow methodology for building a REDCap database for an individual study. The process begins with a request from the research team to the Informatics Core (IC) for database services. A meeting is scheduled between the research team and an IC representative for a formal REDCap demonstration. Key program features (intuitive user interface, data security model, distributed work environment, data validation procedures, statistical package-ready export features, and audit trail logging) are stressed during the initial meeting in addition to providing project-specific data collection strategy consultation. Researchers are given a Microsoft Excel spreadsheet file providing detailed instructions for completing required metadata information (ex. field name, end-user label, data type, data range, etc) about each measurement in each case report form. They are asked to spend time over the next several days working with the spreadsheet template to define data elements for their specific project, and then return the worksheet to the IC via electronic mail. The returned worksheet is used to build and populate the study-specific database tables feeding a working web-based electronic data collection (EDC) application for the project. A web link to the prototype application is given to the research team along with instructions for testing and further iteration of the metadata spreadsheet until the study data collection plan is complete. The system is then placed in production status for study data collection. The workflow process typically includes several members of the research group and allows the entire team to devise and test every aspect of study data collection requirements before study initiation.

Study-specific databases are created using data dictionaries provided by the research team. After an initial demonstration, research teams use a custom MS-Excel file template to provide project metadata. The informatics team uses this file to create a prototype web application that researchers can test while revising their data dictionary. Once consensus is reached by the research team on the entire data collection CRF package, the application is moved to production status for study initiation.
B. User Interface
The REDCap user interface provides an intuitive method to securely and accurately input data relating to research studies. Figure 2 shows a typical CRF view. Each form is accessible only to users who have sufficient access privileges set by the research team. Forms contain field-specific validation code sufficient to ensure strong data integrity. In addition to checking for mandatory field type (e.g. numeric entry for systolic blood pressure), embedded functions also check data ranges (e.g. 70–180 mmHg) and alert the end-user whenever entered data violates specified limits. Data fields may be populated using text fields or through embedded pull-down boxes or radio buttons where the end-user is shown one value and a separate code is stored in the database for later statistical analysis (e.g. 0=No, 1=Yes).

Case report forms are accessible to users who have sufficient access rights and contain field-specific client-side validation code sufficient to ensure data integrity. In addition to checking for mandatory field type (e.g. numeric entry for systolic blood pressure), validity functions also check data ranges (e.g. 70–180 mmHg) and alert the end-user whenever entered data violates specified limits. CRF pages and REDCap functional modules are accessible to end users by clicking links on the right-side application menu of each project.
Clickable project menu items are shown on the right side of the screen in Fig. 2 . All menu items in the Data Entry tab point to CRFs specific to the scientific project, while the Applications tab contains menu items pointing to general REDCap functions. Researchers use the “Data Export Tool” to push collected data out of the REDCap system for external analysis and may select entire forms and/or individual fields for export. The module returns downloadable raw data files (comma-delimited format) along with syntax script files used to automatically import data and all context information (data labels, coded variable information) into common statistical packages (SPSS, R/S+, SAS, Stata). The “Data Import Tool” module allows bulk upload of data from existing files with automatic validation of data and audit trail creation similar to those created when using CRF data entry methods. The “Data Comparison Tool” module provides a mechanism to view and reconcile data for those studies employing double-data entry or blinded-data entry methods. The “Data Logging” module provides users a view of all data transactions for the duration of the project. The “File Repository” module allows end-users to store individual supporting files for the project and retrieve wherever and whenever necessary. The “Data Dictionary” module allows researchers to download a clean copy of the project metadata during the iterative study data planning process and the “User Rights” module is used by project administrators to add or remove research personnel and set individual security rights.
C. Architecture
The REDCap project uses PHP + JavaScript programming languages and a MySQL database engine for data storage and manipulation.( 6 ; 7 ) Hardware and software requirements are modest and the system runs in Windows/IIS and Linux/Apache web server environments. In keeping with the goal of creating a rapid development methodology and easily maintainable resource for multiple concurrent studies, we devised a set of similar database tables for use in each study. The standard REDCap project requires five distinct tables stored in a single MySQL database: 1) a METADATA table containing all study data pertaining to database storage (data field types and naming used for automatic creation of separate data storage table) and CRF presentation (form names and security levels, field-specific display and validation rules); 2) a LOGGING table used to store all information about data changes and exports; 3) a DOCS table used to store uploaded (ex. consent forms, analysis code) or generated export files (SAS, SPSS, R, Stata, Excel); 4) a RIGHTS table containing specific researcher rights and expiration settings; and 5) a flat DATA table used to store all collected data for the study (one row per subject with all collected data fields stored in columns). In studies requiring greater than 1500 data fields per subject, multiple DATA tables are used with a 1:1 relationship between tables linked on the subject identifier field. Although simplistic, this data model is easy to reproduce and tailor for individual research studies during the project creation process and has proven adequate for a wide variety of clinical and translational research studies seen in multiple academic research environments.
IV. RESULTS
A. research utilization.
The first REDCap project was placed in production at Vanderbilt University in August, 2004. REDCap was successfully deployed and gained a wide reputation within the institution as a toolset and workflow for providing secure, web-based data collection services. Informatics investment has varied during the time of operation, but has typically not exceeded one person performing new module development work, supporting investigators and working with project consortium partners.
Figure 3 shows activity for the REDCap project at Vanderbilt University and our CTSA partner institution, Meharry Medical College, from August, 2004 through September, 2008. REDCap was initially created to support clinical research studies, but we now also use the project to support basic science and operational databases for our CTSA organization (e.g. process database for managing REDCap projects, accounting activity for CTSA Community Engagement core). The total number of projects is currently 204 (clinical=189; basic=7; operational=8), including 156 active projects and 48 projects in prototype (pre-production) status. The project creation process may take from less than a day to over a year to move from prototype to production status and depends entirely on the motivation and readiness of the research team in defining CRF requirements and launching the study. Projects are allowed to remain in prototype status as long as researchers are interested in continuing development.

Local use of the REDCap project has grown steadily since introduction in August, 2004 at Vanderbilt University and our CTSA partner Meharry Medical College. September 11, 2008 statistics indicated 204 total projects, including 156 active and 48 in the prototype development process. Projects are allowed to remain in prototype status as long as researchers are interested in continuing development.
The total subjects in all Vanderbilt and Meharry production-level clinical research study databases currently numbers 17,959 and demonstrates active use for the REDCap system in the clinical domain. The number of registered end-users for all clinical, basic and operational projects is currently 722 unique individuals, including 363 users known through audit logs to have actively participated in data entry or export. An analysis of the number of registered users per project conveys information about the size and diversity of supported studies (median = 4, mean = 5.1, range = 1 to 77). An analysis of the number of projects per registered user conveys information about the diversity of supported users (median = 1, mean = 1.5, range = 1 to 14).
B. Multi-Institutional Consortium – Software Availability and Collaborative Network
In August, 2006, we launched a pilot initiative to study the process surrounding developing and sharing research informatics resources across academic institutions ( Figure 4 ). The REDCap consortium now consists of 27 partners (26 domestic, 1 international) collaborating on new development and providing support for REDCap operations. Using this consortium approach, we have successfully added new REDCap modules, created documentation and support processes for adopting centers, added language abstraction (English, Spanish and Japanese versions currently available) and also regularly define and refine near- and long-term plans for improvement and added functionality. We provide software and support at no charge to institutions, but ask for contributions to the overall project through member participation in weekly all-hands meetings and other initiatives. We are encouraged by progress to date and encourage partnership with other academic institutions who would like to join the project. Information concerning joining the consortium may be found at the consortium public website ( http://www.project-redcap.org ).

Geographical representation of 27 active domestic and international collaborating institutions. Locations depicted do not include sites where software is used, but hosted at another institution (example Venezuela, Argentina, Peru, Mexico, Honduras, Haiti, Chile, and Brazil data entry partner sites).
V. DISCUSSION
Several factors contribute to REDCap’s successful implementation in the clinical and translational research enterprise. Most important, the need for researcher-controlled data services and secure data collection, storage and export is a universal need for any single- or multi-center research study. The initial REDCap demonstration meeting provides an opportunity for project-specific informatics consultation concerning data collection and storage, data validation procedures, data security and logging requirements, and forms layout. The prototype testing and refinement workflow process ensures a team-based data definition strategy prior to study initiation, thereby improving the timeliness and overall quality of study data. The startup time required to launch a new project is short and almost entirely determined by researcher input in defining a structured study-specific data collection plan. After the initial researcher demonstration meeting, IC support for a single project typically requires less than 60 minutes over the life of a study (including allowances for mid-study CRF modifications when necessary). The Vanderbilt, University Office of Research Informatics offers REDCap services at no charge for scientific research teams and considers the modest personnel investment (< 0.5 FTE for universal training and support) a sound investment ensuring researchers have ready access to centralized resources compliant with HIPAA best practices.
A number of limitations should be recognized with the REDCap project. The flat-table structure used for collected research data is inefficient from a data storage standpoint, but very efficient from the perspective of study setup and ideal for embedded data export procedures. The researcher-led metadata creation process is fast and flexible, but does not encourage the use of data naming standards, with the exception that data dictionary templates are typically reused by researcher teams across studies. We are considering methods for adding standards-based data identifiers to the metadata process using IC expert guidance in studies where this approach would add value.
VI. CONCLUSION
The cost of purchasing and supporting major vendor solutions for clinical data management systems can be prohibitive.( 8 ) Although costs can be justified in large organizations running multiple sponsored clinical trials, the academic research environment is typically home to many preclinical investigator-initiated research studies requiring a fraction of the subjects needed for larger trials. In these cases, traditional EDC setup time for CRFs and software expense may outweigh benefits for the study.( 9 ) The REDCap project provides a rapid-development and flexible informatics systems-based approach to supporting studies in the translational research enterprise. The project benefits from a consortium of academic institutions and we encourage others to consider joining the group of adopting and collaborating sites.
Acknowledgments
We gratefully acknowledge: Jerry Zhao (Vanderbilt University) for assistance with Vanderbilt computing resources; Maricruz Silva-Ramos (University of Miami) for assistance with data export procedures, Dr. Andrew Cucchiara (University of Pennsylvania) and Dale Plummer (Vanderbilt University) for consultative assistance with biostatistics package syntax procedures; and the following individuals for general participation and leadership within the REDCap consortium enterprise: Mark Oium and Jim VerDuin (Medical College of Wisconsin), Robert Schuff, Hannah Howard, David Brown, and Ethan Seifert (Oregon Health Sciences University), Ronald Sanders and Lori Sloane (University of New Mexico), Jessie Lee, Elizabeth Wood and Jihad Obeid (Cornell University), Brenda Nieves (University of Puerto Rico), Michael Lin and Rob Pawluk (Mayo Clinic), Judith Hartman (University of Pittsburgh), Sheree Hemphill (Case Western Reserve University), Alexandre Peshansky (University of Medicine & Dentistry of New Jersey), Mitsuhiro Isozaki (Tokai University), Adrian Nida, Bernie Caraviello, and Matthew Gregg (Medical University of South Carolina), Knut Wittkowski (The Rockefeller University), Joseph Wu (University of California, Irvine), Tim Morris and Neeta Shenvi (Emory University), Liz Chen (Harbor-UCLA Medical Center), Charles Lu and Pradeep Mutalik (Yale University), Thomas McKibben (Northwestern University), Kent Anderson and Ayan Patel (University of California, Davis), Clarence Potter (University of North Carolina), Joshua Franklin and Jim Brinkley (University of Washington), Bernie LaSalle (University of Utah), Ted Kalbfleisch (University of Louisville), Todd Ferris, Tanya Podchiyska and Gomathi Krishnan (Stanford University), and Fatima Barnes (Meharry Medical College). This work was supported by NCRR grants 5M01-RR00095, G12RR03051, 5M01RR000058-45, and 1 UL1 RR024975 from NCRR/NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This paper is in the following e-collection/theme issue:
Published on 25.6.2024 in Vol 12 (2024)
User Preferences and Needs for Health Data Collection Using Research Electronic Data Capture: Survey Study
Authors of this article:

Original Paper
- Hiral Soni 1 , PhD ;
- Julia Ivanova 1 , PhD ;
- Hattie Wilczewski 1 , BS ;
- Triton Ong 1 , PhD ;
- J Nalubega Ross 1 , PhD ;
- Alexandra Bailey 1 , MS ;
- Mollie Cummins 1, 2 , PhD ;
- Janelle Barrera 1, 3 , MPH ;
- Brian Bunnell 1, 3 , PhD ;
- Brandon Welch 1, 4 , PhD
1 Doxy.me Research, Doxy.me Inc, Charleston, SC, United States
2 College of Nursing, University of Utah, Salt Lake City, UT, United States
3 Department of Psychiatry and Behavioral Neurosciences, University of South Florida, Tampa, FL, United States
4 Department of Public Health Sciences, Medical University of South Carolina, Charleston, SC, United States
Corresponding Author:
Hiral Soni, PhD
Doxy.me Research
Doxy.me Inc
18 Broad Street, 3rd Floor
Suite 6 and 7
Charleston, SC, 29401
United States
Phone: 1 8444369963
Email: [email protected]
Background: Self-administered web-based questionnaires are widely used to collect health data from patients and clinical research participants. REDCap (Research Electronic Data Capture; Vanderbilt University) is a global, secure web application for building and managing electronic data capture. Unfortunately, stakeholder needs and preferences of electronic data collection via REDCap have rarely been studied.
Objective: This study aims to survey REDCap researchers and administrators to assess their experience with REDCap, especially their perspectives on the advantages, challenges, and suggestions for the enhancement of REDCap as a data collection tool.
Methods: We conducted a web-based survey with representatives of REDCap member organizations in the United States. The survey captured information on respondent demographics, quality of patient-reported data collected via REDCap, patient experience of data collection with REDCap, and open-ended questions focusing on the advantages, challenges, and suggestions to enhance REDCap’s data collection experience. Descriptive and inferential analysis measures were used to analyze quantitative data. Thematic analysis was used to analyze open-ended responses focusing on the advantages, disadvantages, and enhancements in data collection experience.
Results: A total of 207 respondents completed the survey. Respondents strongly agreed or agreed that the data collected via REDCap are accurate (188/207, 90.8%), reliable (182/207, 87.9%), and complete (166/207, 80.2%). More than half of respondents strongly agreed or agreed that patients find REDCap easy to use (165/207, 79.7%), could successfully complete tasks without help (151/207, 72.9%), and could do so in a timely manner (163/207, 78.7%). Thematic analysis of open-ended responses yielded 8 major themes: survey development, user experience, survey distribution, survey results, training and support, technology, security, and platform features. The user experience category included more than half of the advantage codes (307/594, 51.7% of codes); meanwhile, respondents reported higher challenges in survey development (169/516, 32.8% of codes), also suggesting the highest enhancement suggestions for the category (162/439, 36.9% of codes).
Conclusions: Respondents indicated that REDCap is a valued, low-cost, secure resource for clinical research data collection. REDCap’s data collection experience was generally positive among clinical research and care staff members and patients. However, with the advancements in data collection technologies and the availability of modern, intuitive, and mobile-friendly data collection interfaces, there is a critical opportunity to enhance the REDCap experience to meet the needs of researchers and patients.
Introduction
Accurate and complete health outcome data directly from patients or study participants (hereon referred to as patients ) are critical for health care and research [ 1 - 3 ]. Unfortunately, it can be burdensome to extract patient-reported health data that researchers or providers need [ 4 , 5 ]. Collecting patient-reported outcomes data is becoming increasingly important in clinical research and care [ 6 , 7 ]. Self-administered web-based questionnaires, which patients can complete at a clinic or at home, are becoming a conventional approach to collect data for clinical research. Web-based questionnaires have advantages of being low-cost and easy to deploy at scale. A variety of clinical research electronic data capture (EDC) tools exist to streamline remote data collection and management. These systems comply with privacy regulations, integrate with different tools (such as electronic health records [EHRs]) for efficient data collection, and reduce the effort of sharing data [ 8 ]. However, user experience, cost, and maintenance of such commercial EDC systems are often prohibitive. An understanding of user experiences and preferences regarding EDC tools is critical in assessing stakeholder needs, satisfaction, and challenges in clinical and research settings.
REDCap (Research Electronic Data Capture; Vanderbilt University) is a global, secure web application for building and managing EDC for clinical research [ 9 , 10 ]. Developed by Vanderbilt University, REDCap is freely available for its consortium members (ie, network of nonprofit collaborators and supporters), who have an established agreement with the university. REDCap is compliant with global privacy regulations (such as the Health Insurance Portability and Accountability Act [HIPAA] of 1996) and used by more than 2.2 million researchers in more than 140 countries [ 9 ]. REDCap allows researchers to build and conduct electronic surveys, track and manage study information, schedule visits, and manage databases that are fully customizable and at no cost [ 11 ]. REDCap is designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages, and (4) procedures for data integration and interoperability with external sources.
Although REDCap is widely used, user needs and preferences of EDC via REDCap have rarely been studied [ 12 , 13 ]. For example, 1 usability study of a REDCap-based patient-facing intervention reported that patient participants found REDCap useful and easy to use but showed concerns about wordiness and inconsistent visual design [ 13 ]. Researchers have reported frequently on the implementation, use, and interventions using REDCap [ 10 , 14 - 20 ]. Understanding the preferences and needs of REDCap administrators and researchers using REDCap to capture data could help enhance existing features and EDC processes in general. While REDCap is a robust clinical research data management system, this study solely focuses on the experience of REDCap as an EDC tool. To the best of our knowledge, such preferences have not yet been studied.
The aim of this study was to survey REDCap administrators and researchers in the United States to assess their experience with REDCap, including perspectives on advantages, challenges, and suggestions for enhancement.
Study Settings and Respondents
We conducted a web-based survey with representatives of member organizations listed as REDCap Partners on the REDCap website [ 21 ]. The roles of the listed members were unclear at the time of invitation sent via email. The email communication included information related to the study goals, voluntary participation, and a link to the REDCap survey. Respondents were compensated with a US $10 electronic gift card for completing the survey.
Ethical Considerations
This study was reviewed and approved as exempt human subjects research by the Medical University of South Carolina Institutional Review Board (Pro00082875).
Survey Design
We developed a web-based survey with multiple-choice and free-response questions ( Multimedia Appendix 1 ) to capture the perspectives of researchers and administrators from participating REDCap consortium organizations. Our research team includes experts in biomedical informatics, behavioral sciences, mixed methods research, and user experience. The survey included 4 sections, as follows:
- Demographics : multiple-choice questions capturing participant role in their respective organization (Q1) and organization use of REDCap (Q2)
- Quality of patient-reported data collected via REDCap : Likert-scale questions capturing perspectives (ranging from 1=strongly agree to 5=strongly disagree) on the accuracy, reliability and completeness of data reported using REDCap (Q3)
- Patient experience with REDCap : Likert-scale question focusing on perspectives (ranging from 1=strongly agree to 5=strongly disagree) on REDCap usability, including ease of use, success rate, and completion time (Q4).
- Data collection experience : Free-response questions asking about the advantages (Q5), challenges (Q6), and suggestions of enhancements related to data collection, patient experience, and engagement (Q7).
Data Collection and Analysis
We collected and managed study data using REDCap EDC tools hosted at the Medical University of South Carolina [ 22 , 23 ]. We generated plots and univariate statistics to summarize the data (eg, frequencies, means, SDs, and percentages). We conducted 1-way ANOVA tests to determine differences in data quality and patient experience variables by participant role and REDCap use duration. For the ANOVAs, the primary role variable was restructured to include “Educators” in the “Other” category due to the low sample size (n=1). Excel (Microsoft Corp) and SPSS (version 29; IBM Corp) were used for analyses. Free-response questions were qualitatively analyzed to identify emerging themes related to REDCap data collection experience [ 24 ]. We randomly selected 15% of the responses for initial coding and codebook development. The coding unit was done by the entirety of the participant entry. Thematic analysis of all qualitative data was done over 4 iterations using MAXQDA, during which emergent themes were identified. While the research team reviewed and honed the codes and codebook, 1 team member coded and finalized thematic coding. Discrepancies were resolved through consensus. Emergent themes were organized by frequency and topic, allowing for further qualitative analysis using complex coding query to determine concurrent themes. We reported the total frequencies per code, which may not align with the number of participants. For example, 1 participant may report a code multiple times throughout their response [ 25 ]. While thematic analysis allows us to identify principle emergent themes, it also can help identify uncommon trends that may be significant but would require further investigation in follow-up research [ 26 ]. Responses from incomplete surveys with missing quantitative or qualitative responses were excluded from the analysis
Demographics
Between October and November 2020, 3058 representatives from 1676 REDCap member organizations in the United States were invited to complete the survey. In total, 285 (9.3%) invitees started the survey, of which 207 completed the survey. Most (150/207, 72.5%) respondents were REDCap administrators, followed by researchers (25/207, 12.1%). Furthermore, 1 (0.5%) respondent was an educator and 31 (15%) respondents served in other roles, including IT directors and managers, research coordinators and managers, program managers, project managers, director of research, library directors, and data analysts. Respondents reported that their organization had used REDCap for <5 years (92/207, 44.4%), 5 to 10 years (83/207, 40.1%), or >10 years (32/207, 15.5%).
Quality of Patient-Reported Data Collected via REDCap
We asked respondents about their perspectives of the quality of the survey data, including the accuracy, reliability, and completeness of the data collected using REDCap ( Figure 1 ). Most respondents strongly agreed or agreed that the data collected via REDCap are accurate (188/207, 90.8%), reliable (182/207, 87.9%), and complete (166/207, 80.2%). We observed no statistically significant group differences in accuracy ( F 2,204 =1.003; P =.37), completeness ( F 2,204 =0.243; P =.78), or reliability ( F 2,204 =0.245; P =.78) among respondent role groups. Furthermore, we observed no statistically significant group differences in accuracy ( F 2,204 =0.672; P =.51), completeness ( F 2,204 =0.045; P =.96), or reliability ( F 2,204 =1.712; P =.18) among REDCap use groups.

Patient Experience With REDCap
We also asked respondents about their perspectives on patient experiences with completing surveys using REDCap. Figure 2 summarizes their responses. More than half of respondents strongly agreed or agreed that patients find REDCap easy to use (165/207, 79.7%), could successfully complete tasks without help (151/207, 72.9%), and could do so in a timely manner (163/207, 78.7%). We observed no statistically significant group differences in ease ( F 2,204 =2.025; P =.13), successful task completion ( F 2,204 =0.671; P =.51), or timely task completion ( F 2,204 =2.303; P =.10) among respondent role groups. Furthermore, we observed no statistically significant group differences in ease ( F 2,204 =0.711; P =.49), successful task completion ( F 2,204 =1.851; P =.16), or timely task completion ( F 2,204 =2.000; P =.13) among REDCap user groups.

REDCap Advantages, Challenges, and Enhancement Suggestions
We asked respondents about the advantages, challenges, and suggestions for future enhancements using free-response questions. The analysis yielded 8 primary codes: survey development, user experience, survey distribution, survey results, training and support, technology, security, and platform features. Within each of these themes, responses were further categorized at secondary and tertiary levels. Multimedia Appendix 2 shows the qualitative codebook with illustrative examples for each code. Table 1 shows the frequencies response classification of advantages, disadvantages, and enhancements for each code category based on respondents’ responses.
| Code | Advantages, n (%) | Challenges, n (%) | Enhancements, n (%) | |||||
| 52 (50.5) | 59 (47.6) | 40 (47.1) | ||||||
| Survey design | 10 (9.7) | 39 (31.5) | 21 (24.7) | |||||
| Response and logic | 13 (12.6) | 22 (17.7) | 18 (21.2) | |||||
| Survey setup | 20 (19.4) | 2 (1.6) | 0 (0) | |||||
| Flexibility | 7 (6.8) | 1 (0.8) | 3 (3.5) | |||||
| Organization | 1 (1) | 1 (0.8) | 0 (0) | |||||
| Testing | 0 (0) | 0 (0) | 3 (3.5) | |||||
| 7 (100) | 17 (65.4) | 15 (65.2) | ||||||
| Language support | 0 (0) | 9 (34.6) | 8 (34.8) | |||||
| Project interactions | 0 (0) | 3 (100) | 6 (100) | |||||
| Feature suggestions | 1 (100) | 16 (100) | 49 (100) | |||||
| 105 (55.9) | 29 (61.7) | 8 (53.3) | ||||||
| Ease of use | 57 (30.3) | 7 (14.9) | 1 (6.7) | |||||
| Accessibility | 1 (0.5) | 1 (2.1) | 3 (20) | |||||
| Intuitiveness | 3 (1.6) | 2 (4.3) | 1 (6.7) | |||||
| User-friendliness | 11 (5.9) | 4 (8.5) | 2 (13.3) | |||||
| Reliability | 3 (1.6) | 0 (0) | 0 (0) | |||||
| Simplicity | 8 (4.3) | 4 (8.5) | 0 (0) | |||||
| 9 (52.9) | 27 (50.9) | 33 (52.4) | ||||||
| Visual interface | 2 (11.8) | 5 (9.4) | 29 (46) | |||||
| Devices | 5 (29.4) | 9 (17) | 0 (0) | |||||
| Functionality | 1 (5.9) | 8 (15.1) | 0 (0) | |||||
| Design configuration | 0 (0) | 4 (7.5) | 1 (1.6) | |||||
| 20 (60.6) | 16 (64) | 21 (50) | ||||||
| Ease of use | 3 (9.1) | 0 (0) | 0 (0) | |||||
| Interface | 4 (12.1) | 5 (20) | 3 (7.1) | |||||
| Mobile friendly | 5 (15.2) | 0 (0) | 3 (7.1) | |||||
| Mobile app | 1 (3) | 4 (16) | 15 (35.7) | |||||
| 34 (55.7) | 13 (48.1) | 5 (62.5) | ||||||
| Convenience | 10 (16.4) | 0 (0) | 0 (0) | |||||
| Engagement | 9 (14.8) | 6 (22.2) | 3 (37.5) | |||||
| Patient input | 3 (4.9) | 8 (29.6) | 0 (0) | |||||
| Patient log-in | 3 (4.9) | 0 (0) | 0 (0) | |||||
| Efficiency | 1 (1.6) | 0 (0) | 0 (0) | |||||
| Empowerment | 1 (1.6) | 0 (0) | 0 (0) | |||||
| Researcher experience | 8 (100) | 0 (0) | 0 (0) | |||||
| 20 (52.6) | 25 (53.2) | 25 (54.3) | ||||||
| Automated scheduling and messaging | 5 (13.2) | 1 (2.1) | 2 (4.3) | |||||
| Save and return | 3 (7.9) | 15 (31.9) | 9 (19.6) | |||||
| Invitation approaches | 9 (23.7) | 6 (12.8) | 5 (10.9) | |||||
| Calendar integration | 1 (2.6) | 0 (0) | 3 (6.5) | |||||
| Patient opt out | 0 (0) | 0 (0) | 2 (4.3) | |||||
| 7 (87.5) | 7 (87.5) | 7 (63.6) | ||||||
| Email text | 0 (0) | 0 (0) | 1 (9.1) | |||||
| Follow-up with patients | 1 (12.5) | 1 (12.5) | 3 (27.3) | |||||
| Easy distribution | 11 (100) | 0 (0) | 3 (100) | |||||
| Results view | 5 (100) | 0 (0) | 4 (100) | |||||
| Data sharing | 8 (100) | 0 (0) | 3 (100) | |||||
| Data quality | 3 (100) | 1 (100) | 0 (0) | |||||
| Education and training | 7 (100) | 7 (100) | 8 (100) | |||||
| Support | 8 (100) | 15 (100) | 18 (100) | |||||
| Patient support | 2 (100) | 12 (100) | 16 (61.5) | |||||
| Patient education and communication | 0 (0) | 0 (0) | 10 (38.5) | |||||
| Patient feedback | 1 (100) | 0 (0) | 6 (100) | |||||
| User misunderstanding and error | 3 (100) | 8 (100) | 1 (100) | |||||
| Consent | 5 (100) | 0 (0) | 3 (100) | |||||
| Technology integration | 11 (100) | 1 (100) | 12 (100) | |||||
| Technology access | 17 (100) | 51 (100) | 0 (0) | |||||
| Technology literacy | 1 (100) | 33 (100) | 0 (0) | |||||
| Privacy and compliance | 15 (100) | 2 (40) | 2 (100) | |||||
| Trust in technology | 0 (0) | 3 (60) | 0 (0) | |||||
| 14 (56) | 4 (50) | 5 (50) | ||||||
| Comprehensive | 5 (20) | 0 (0) | 0 (0) | |||||
| Data administration | 3 (12) | 1 (12.5) | 3 (30) | |||||
| Offline access | 1 (4) | 3 (37.5) | 2 (20) | |||||
| Familiarity | 2 (8) | 0 (0) | 0 (0) | |||||
| Cost | 10 (100) | 0 (0) | 0 (0) | |||||
| Comparison with other platforms | 3 (100) | 5 (100) | 0 (0) | |||||
| No input | 7 (100) | 22 (100) | 52 (100) | |||||
a Due to the coding process (eg, double coding), the total number of secondary and tertiary codes may not add up to the primary code or 100%. The percentages are calculated based on the total number of codes in secondary and tertiary categories.
Survey Development and Customization
Respondents perceived that REDCap surveys were generally easy (20 codes) and quick (2 codes) to set up, build, organize, and maintain (2 codes). One participant commented on these topics, “Easy to build surveys Easy to make questions easy to answer Easy to build branching questions.”
However, respondents also noted that incorrect setup by the study staff and limited default formatting options and flexibility could be challenging in developing and completing surveys (3 codes).
While some respondents pointed out that REDCap provides continuous releases with new features (2 codes) and various design and automation options to ask a variety of questions for efficient data collection (8 codes), respondents frequently pointed out the value of well-designed survey instruments in gathering high-quality information and engaging patients. They reported that complex, poorly designed surveys and ambiguous instructions (39 codes) could result in poor patient experience, potentially impacting the survey response rate and quality of data gathered. Respondents provided suggestions for enhancing survey design capabilities to streamline survey design and layout for the patients (including simplifying survey formatting, survey nesting abilities, and use of embedded fields). Respondents also suggested pilot testing of surveys before sending them out to patients (3 codes) and for study teams to follow best practices and guidelines to be more informed in survey methodologies and development. For example, 1 respondent commented:
Study teams following best practices with survey methodology and design, which can involve keeping surveys short & sweet, choosing appropriate field types for the question at hand, phrasing questions and response options well to reduce mental burden and make it easier for patients to answer questions.
Respondents also reported that the availability of various response types, data validation, and branching logic ensure high-quality data collection (13 codes). One respondent commented on this advantage, “The wide array of validations can help patients enter data correctly.”
Another respondent noted similarly, “Data validation and branching logic make participants conform to data standards and allows researchers to obtain higher quality data.”
While data validation was discussed positively, respondents more frequently noted the challenges with response and logic types (22 codes), often pointing out that the actual response and logic types available from REDCap are not conducive to good survey design. One respondent made a clear reference to this issue saying, “It all depends on who sets up the survey, but until recently it has been a challenge to create grids of disparate data entry fields.”
In addition, some respondents noted that due to the logic types, patients can make critical mistakes affecting the completeness of the data:
...branching logic at a very question to determine if they qualify or not. Sometimes, they accidently select different value in a hurry, and the survey gets completed. It is hard for them to change the response or refill the survey without admin help.
Respondents noted many enhancement potentials within this category, such as voice input (4 codes), superior data entry experience (5 codes), use of a more conversational approach in response types (1 code), more effective multimedia (5 codes), and gamification of survey (2 codes). While REDCap offers multimedia options, respondents often suggested that options become more interactive and effective:
...more visual aids in questions, and the ability to answer with images. For example, by painting the areas afflicted on an image.
One respondent explained how multimedia may be further useful:
...ability to add images to response options. Especially when working with minorities (traffic lights, or smiley faces).
In addition to the design of the surveys, respondents noted that while REDCap surveys are readily customizable (7 codes), there are far more reported challenges (17 codes) and need for enhancements (15 codes). Respondents noted customization was not possible in some cases: “Default formatting options are limited.”
However, many respondents focused on the lack of multi-language support (9 codes) as the critical challenge:
...multi-linguistic support. This is always a challenge for any software system/platform, and REDCap is no different...
They frequently suggested enhancements to include multi-language support (8 codes) and customizations in forms’ appearance (6 codes). For example, 1 respondent mentioned, “Allow for some more customization of the overall look/feel of surveys.”
With respect to challenges with survey interactions, respondents reported that REDCap capabilities at the time did not send new surveys or allow patients to complete future surveys if previous surveys were incomplete (2 codes). One respondent mentioned the following:
...[t]he longitudinal design functionality in REDCap requires a participant to take each form before moving to the next, but our experiment design does not require this, and sometimes people will miss sessions and need to move on to the form for the next one. But if we stack all of the forms in one event, we cannot direct people to an individual form, only to the queue.
One participant commented on REDCap’s “ inability to provide staff log-in status .” (1 code). Respondents requested features for internal messaging or chat between study staff (2 codes), enhancing flow and cross-linking between projects (2 codes), ability to easily add study staff members outside of the organization (1 code), and ability for patients to skip longitudinal surveys (1 code).
User Experience
Respondents perceived REDCap to be easy to use for both patients (ie, to take surveys) and the study staff (ie, to build and distribute surveys; 57 codes). One respondent commented as follows:
REDCap is the easiest way to survey patients, families, and staff who are not part of our study team. We would not be able to conduct these surveys without it!
They also perceived REDCap to be user-friendly (11 codes), simple (8 codes), intuitive (3 codes), timely (2 codes), and reliable (3 codes). Although some respondents reported REDCap allows for quick data collection (7 codes), they perceived that lengthy or poorly designed surveys (eg, too many clicks and not enough instructions) could lead to fatigue and poor participation (15 codes). While the usability perceptions were generally positive, respondents reported that the platform was not as user-friendly or outdated as other commercial data collection platforms (4 codes), unintuitive (2 codes), and clunky for study staff (4 codes). They reported that “ REDCap is not the simplest tool to learn how to use ” for study staff (4 codes) and patients (3 codes). Respondents suggested the need to enhance accessibility features, such as the ability to change font size, screen reader view, and text-to-voice, among others (3 codes). In total, 8 (3.9%) of 207 respondents reported that the REDCap interface was advantageous for study staff considering its consistent interface and automated features, which reduce burden.
Respondents generally reported REDCap’s visual user interface as challenging to use. Although some respondents perceived the interface to be clean or simple looking (9 codes) and optimized for various devices (5 codes), other respondents perceived that REDCap’s interface was not modern looking (7 codes) or appealing (5 codes). One respondent mentioned, “The web interface of our survey pages are very basic, and narrow,” whereas another respondent said, “[REDCap has] Very set layout of each item, can’t make it look more ‘modern’ like other websites are at this time.”
Respondents considered REDCap as not having a configurable design (4 codes) and some noted the user interface’s poor functionality (8 codes). One respondent described both issues when explaining the challenges of the user interface:
REDCap is simply not user friendly in any way. The data structures are often too rigid and frankly outdated in being an effective tool for data collection.
Respondents suggested the redesign of the REDCap user interface to be consistent with modern data collection platforms (27 codes), options to change the visual appearance and formatting of the surveys (3 codes), adding progress tracking aids (such as an automatic progress bar) for patients (2 codes), and a more flexible interface (1 code).
Some respondents appreciated REDCap’s mobile access (4 codes), availability of mobile apps for study staff (REDCap mobile app; 2 codes) and patients (MyCap; 6 codes) supporting offline data collection, and perceived REDCap to be easy to use on mobile devices (3 codes) and mobile friendly (5 codes). While respondents appreciated the mobile interface, they reported that the mobile experience is affected by poor and suboptimal mobile user interface and scaling on smaller screens (5 codes). One participant reported the following:
We design our surveys on a computer, but many of our participants use their phones. We try to check how answers scale when the screen size changes, but some phones rescale to a different aspect ratio leading to challenges.
They also reported that although the REDCap mobile app is available for study staff, it is not ideal and is difficult for study staff to set up the app (4 codes). One respondent mentioned the following:
I think that the REDCap mobile app is a bit too far separated from the web version, in as much as there is no access to external modules and other important features.
Respondents suggested a need for an enhanced mobile app and interface (21 codes), including advanced capabilities for the study staff to view study records and perform analysis (2 codes) and push notifications (2 codes). One respondent mentioned the following:
[They need] better workflows with mobile phones, like notifications instead of just text messages. Something like an App except not the current one which is focus on asymmetric internet access.
Respondents also commented on patient experience with REDCap. Overall, respondents noted that REDCap makes it easier for patients to complete the surveys at their convenience (10 codes), all while increasing engagement levels (9 codes). They saw REDCap as a way to make data collection more efficient and empowered (2 codes), especially as patients did not need to register or remember usernames or passwords to use the platform (3 codes). One participant said, “[Survey] Can be done at the patient’s convenience from any digital device.” A common challenge reported was the patient’s desire and motivation to complete the surveys, being able to use the platform, and fatigue with lengthy surveys (13 codes). Suggestions for improving patient experience included maintaining engagement using visual aids and gamification (3 codes), a patient dashboard to keep them up to date on status of longitudinal studies (1 code) and making the platform more patient friendly (1 code). One respondent commented as follows:
For longer surveys, having a way of maintaining engagement by making the surveys more interactive (e.g. fun feedback to participants as they progress) would be nice. Some periodic messages of encouragement like “Great job!” “Keep it up!”
Survey Distribution and Reminders
Respondents found it advantageous that REDCap included multiple ways to invite patients, such as emails or embedded links (4 codes). REDCap surveys were easy to distribute (11 codes) and could be automated and scheduled on a timeline easily. One participant commented on this aspect, “It can send surveys to participants directly, and on a schedule when the project is longitudinal.” REDCap’s ability to send patients custom links was an advantage respondents liked (3 codes): “For online surveys: able [to] send individualized email links...automated email with message that has piping upon completion.” One respondent pointed out that there was “ no scheduling component for visits ” and suggested this feature. One respondent suggested the ability to send attachments with automatic notifications.
In addition, the ability to send completion reminder emails to patients was reported to reduce the burden on clinic staff while engaging patients (7 codes). Reminders also allowed the study staff members to follow up on incomplete surveys but 1 respondent mentioned that this was challenging while respondents suggested for improvements in customizing reminders and enhanced tracking for incomplete surveys longitudinally (3 codes):
If there was a more efficient way to upload and manage patient invitations, as well as identify which patients have completed the survey within previous xx months therefore a new survey invitation does not need to be sent.
Respondents noted patients sometimes missed invitations and reminders because email service providers blocked the emails (2 codes): “We have had email providers block REDCap emails, specifically Yahoo.com email.” There was also confusion about the email sender as the emails were “from” REDCap instead of the study staff (2 codes):
From my experience... The emails that are sent out to respondents are not user friendly. The ‘From’ text box comes from REDCap, not from my email address.
In addition, this respondent noted the emails were not user-friendly, sometimes arriving with broken links going to patient’s junk mail, and requiring patients create a completely new log-in to complete a survey. One respondent suggested REDCap may “make it easier to send mass emails that are individually linked with the patient’s profile; create a prettier or more visually appealing interface for patients.” Furthermore, integrations to link communications to personal calendars were thought to be beneficial (3 codes). Respondents wanted a way to automatically opt out patients from surveys that were being distributed over a period (2 codes). One participant stated they, “would really like to be able to set a flag for opt-out subject [s] when distributing surveys over a period of time. We currently have to remove their emails to prevent future distribution.”
Respondents commented on the “Save and Return” feature (25 codes), which allows patients to leave and return using a unique code to complete the survey at a later time. Although REDCap’s Save and Return feature existed, respondents noted that this feature was often difficult to use (15 codes). They reported that patients may forget or not save their return code or may not know how to return to the survey, resulting in incomplete data or delay in data collection. One respondent commented, “It is not always obvious how to ‘save and return later’ if that is an option or even be aware that that is an option.” Respondents suggested improvements (9 codes) to send the unique save and return code via emails, with reminders and save in invitation logs such that the study staff could provide it to patients if needed. In addition, respondents suggested that improving user-friendliness and patient awareness of this feature could increase response rates and data completion. A participant noted the following:
If they [patients] don’t complete the survey the first time they often forget their return code and lose it. It would really help if the reminder emails had the return code, of if it could be included on the survery [sic] invitation log page that would make it much easier to find and give to the patient.
Results and Data
Respondents liked that REDCap made data exportation easy for storage and analysis purposes (8 codes). Not only was it easy to export data out of the REDCap survey tool, it also made the analysis of the data much easier for the study staff, even those with minimal statistics training. As 1 respondent put it, “[REDCap has a] good translation into a dataset [and] easy statistics for those with minimal statistical training.” Respondents (4 codes) pointed out the need for improving data exports and seamless communication with third-party solutions to send and receive information:
Being able to send to communicate and receive information from other software programs like Clinical Conductor for Demographic information and seamless data uploads.
Respondents perceived that it is easy to create reports and monitor patient responses on REDCap and review specific data points (5 codes). Some respondents provided suggestions to edit charts and graphics as well as being able to share user- or survey-specific data (3 codes). For example, 1 respondent mentioned the following:
Ability for researchers to edit/modify graphics that can be automatically displayed with reports within redcap. This would facilitate researchers’ ability to use those charts.”
Another respondent mentioned, “built in tools to share summary-level data (you vs the whole study) or findings.” While respondents perceived that REDCap allows capturing accurate and complete high-quality data (3 codes), 1 respondent mentioned the following:
As with every self-service data entry portal accuracy of self-service data entry is wildly unreliable. There is real value to having a trained rep assisting the client enter information, when possible.
Training and Support
Respondents reported that REDCap’s active online community and support allowed REDCap users (including administrators and researchers) to find information and answers on how to manage, design, and conduct surveys (8 codes): “...it has a huge user base and a great consortium full of all the information you need to begin administering [surveys].” Respondents mentioned needing REDCap or IT support for patients to complete consent forms or surveys (12 codes). Although support existed for survey designers and administrators, it did not extend to patients completing surveys. Respondents suggested REDCap needed a way to educate or support patients in completing surveys (10 codes) and obtain help via on-demand messaging to study staff members (2 codes). As 1 participant suggested, REDCap should allow patients to “Click icon and get video explaining any information on a field . ” Another participant asked that REDCap have the following:
Dedicated instrument defined support button at the top that takes participants to a page made by the study team where we can put in a zoom room link monitored by study staff, phone numbers, or some pointers on definitions/examples on the instrument.
Respondents also suggested a need for obtaining standardized patient feedback surveys to better engage them and understand their experience (6 codes).
Although some respondents mentioned REDCap required minimal training to get started (7 codes), some respondents (especially REDCap administrators) mentioned the need for training survey designers to set up REDCap tools and surveys to design high-quality surveys (7 codes). When asked about challenges, 1 participant mentioned the following:
Lack of resources for support (in person- phone) and functionality. It is not always easy and takes a lot of time to build tools. Not able to use to its fullest capacity or correctly—basically training ourselves. Library or community network does not help either. Not knowing how to set up properly more complicating functions inhibits usage.
Respondents suggested more information and mandatory training for survey builders, including better guidelines and training videos to enhance builder and patient experience (8 codes). Respondents also perceived that patients taking surveys often do not understand how to fill out surveys or certain questions (8 codes) and having expert survey designers and well-designed surveys could alleviate these concerns (1 code).
Respondents often noted challenges of access to the internet and devices (51 codes) as well as technology literacy (33 codes):
Patients [without] a computer, device, or smart phone may not be able to use REDCap.
As REDCap is web based, data collection could be difficult in rural and low-resource areas due to lack of access to technology (4 codes), such as a computer or reliable internet connection. Another participant noted, “I do work in global health, so our colleagues in resource-limited settings have challenges with the internet connection.”
They also noted REDCap’s ability to integrate with other technologies, such as messaging tools (eg, Twilio) as well as open application programmable interface to be beneficial (11 codes). In comparison, 1 participant noted as a challenge that, “integrating the ReCap [sic] extract with Epic [EHR] data. But once the system is setup it’s easy to maintain.” Respondents suggested integrations with other clinical trial management systems for seamless data transfers and EHRs to conduct surveys or autopopulate patient medical information:
The only other thing that would be super cool is if it could blow surveys into EPIC for documentation when needed.
Respondents also referred to the informed consent capabilities of REDCap (8 codes). Even though they noted the consent module to be advantageous to obtain remote informed consents especially after the COVID-19 pandemic (5 codes), respondents suggested more enhancements, such as a 1-step consent process (3 codes).
Respondents commented positively on the security and compliance of REDCap (15 codes). They reported that HIPAA compliance and the ability to store patient data securely are important advantages of REDCap. One participant commented that “all client data can be stored in one HIPAA compliant platform.”
Respondents mentioned mistrust of technology (3 codes) could make patients feel uncomfortable sharing medical information on web-based platforms. One respondent commented that surveys requiring password protection are difficult for patients. They also provided enhancement suggestions (2 codes) related to maintaining HIPAA compliance, enhancing security, and assuring patients that their health information is safe and secure with REDCap.
Platform Features
Respondents found REDCap advantageous in enabling researchers to collect and patients to provide health data remotely (23 codes):“It has made it much easier for patients to submit their questionnaires and information using an online platform,” especially during and after the COVID-19 pandemic.
Respondents perceived REDCap as a comprehensive or versatile (5 codes) data collection solution noting the following: “It provides us a comprehensive tool for collecting, tracking, and managing patient data and outreach.” They also noted administration and maintenance (3 codes) to be advantageous as REDCap allows “ being able to maintain administrative research tasks together with the data collection .” They noted REDCap’s offline data collection (using REDCap mobile apps) to be challenging (3 codes) and suggested that the offline feature should be improved for better data collection experience (2 codes). In addition, respondents noted the familiarity with REDCap among researchers (2 codes) and seamlessness (1 code) for the study personnel to be advantageous.
In addition, REDCap being available for free to REDCap consortium members was sought to be beneficial (10 codes). While some respondents noted REDCap being simpler and easier than other commercial platforms and paper forms (3 codes), some also noted that REDCap’s interface was not easy to use or user-friendly compared to modern data collection tools (5 codes).
Respondents did not provide inputs with respect to advantages, disadvantages, and enhancement suggestions stating lack of experience or ability to provide inputs or not using REDCap for patient data collection (81 codes). Some nonsensical or unrelated comments lacking information context or irrelevant responses were excluded from the analysis. For example, when asked about enhancement suggestions for REDCap, 1 participant responded, “To REDCap or??.”
This study aimed to identify the advantages, challenges, and future opportunities for enhancements from the perspectives of REDCap administrators and researchers. To the best of our knowledge, this is one of the early studies of user perspectives on REDCap services and features. We believe that the findings of this study will aid REDCap developers and consortium users in better understanding stakeholder needs to enhance and customize REDCap features as well as researchers in improved survey development and data collection.
Principal Findings
Respondents had overwhelmingly positive perceptions of REDCap’s survey design and data collection interface. The vast majority of respondents agreed or strongly agreed that data collected via REDCap were accurate (188/207, 90.8%), reliable (182/207, 87.9%), and complete (166/207, 80.2%). They found REDCap advantageous as it is free for its consortium members, secure, and easy to use. Respondents also perceived REDCap as easy and flexible to create and customize surveys including a variety of response and validation options, which make data collection easier for survey takers. However, respondents pointed out that poor survey design—often attributed to human factors (eg, lengthy forms and lack of knowledge among study staff) or technology limitations (eg, restrictions in survey and visual formatting in REDCap)—could result in poor patient experience and, ultimately, response and completion rates. Optimal design of survey forms is critical for assuring patient comprehension of the forms and accurate data collection [ 27 , 28 ]. Furthermore, direct investigations of REDCap user experiences and preferences could allow better understanding of the need for study staff and patient education. In addition, further research related to user needs for survey development and optimization can lead to enhancing their experience of developing high-quality surveys. One respondent pointed out the following:
It [REDCap] needs a much better understanding of how users engage the questions on a form (e.g., sit and watch users and staff try to figure out acceptable data type entries!). Needs a solid revamping in how it works “out front” and to run a series of user groups—patient and staff.
Although respondents appreciated the availability of REDCap’s community support for administrators and study staff, they pointed out that REDCap has room for improvement in this realm: the tool is not simple to learn, and there is a need for more training of study staff to help develop efficient, unambiguous survey instruments that can enhance patient experience. Poorly designed surveys and questions could potentially lead to incomplete responses and inaccurate data. Respondents pointed out the need for supporting patients, especially to ensure they understand the questions and can obtain help when needed in filling out surveys. Direct help from study staff members to fill out surveys or having the ability to directly send a message to study staff could alleviate misunderstandings and errors in completing surveys. Previous research suggests that the ability to obtain clarifications about survey questions can enhance response accuracy [ 28 ]. Further research and availability of resources are necessary to guide study staff members in creating well-designed instruments. In addition, understanding the factors affecting patients’ experience in completing REDCap surveys and reasons for misunderstanding and errors could also enhance the REDCap experience and health data collection processes.
Opinions on patient experience and usability were more mixed. Most respondents agreed or strongly agreed that patients found REDCap easy to use (90.4%), able to be completed without assistance (79.8%), and able to be completed in a timely manner (87.5%). These strongly positive perceptions of REDCap usability are consistent with a prior study in which 6 out of 7 participants needed no help using REDCap, achieved 71% to 100% task completion, and provided 89% positive reaction words [ 13 ]. Qualitative outcomes showed that respondents perceived REDCap made it convenient for patients to provide data remotely without having to log in or remember credentials. Although they commented that patients can complete REDCap surveys using a device of choice (such as a laptop or mobile), technology access and technology literacy appeared to be a concern. Living in rural or low-income areas also presented issues for survey access. Respondents noted low-resource areas without stable internet access meant data collection was not reliable. Lack of internet access not only meant surveys could not be accessed but also meant the data collection process could be interrupted. REDCap’s MyCap and REDCap Mobile app can allow study staff and patients to complete the collection of data offline, but they were also deemed challenging due to the lack of features compared with the web interface. In a study by Doyle et al [ 19 ], the REDCap mobile interface was less favorably received by participants. Similarly, REDCap’s Save and Return feature allows users to complete surveys at a later time, which could be helpful during poor internet access; however, respondents recommended enhancements in the feature to improve patient experience, specifically an easier way for patients to remember and retrieve the return code. One participant noted this difficulty that patients face in attempting to use the feature:
If they don’t complete the survey the first time, they often forget their return code and lose it. It would really help if the reminder emails had the return code, or if it could be included on the survey invitation [log-in] page...
It is imperative to better understand patient and research participant experience with REDCap in completing surveys via larger and direct studies.
This study identified opportunities to improve the usability of REDCap. Respondents suggested enhancements in the patient-facing survey user interface to be in line with present EDC tools on the market, wanting a sleeker, modern, and cleaner looking interface. A variety of EDC tools are available for health care and non–health care data collection providing modern, device-friendly, and intuitive user interfaces to promote patient engagement [ 29 - 32 ]. In recent years, virtual conversational agents or chatbots have emerged as intuitive and engaging mediums for data collection. Modern data collection tools allow survey designers to develop chatbot-based interactions to collect health data mimicking human-to-human conversations. Studies have shown that individuals prefer chatbot-based conversational data collection experience in comparison to traditional web-based forms [ 33 , 34 ]. Visual and graphical enhancements in REDCap appearance of surveys, patient communication, and researcher interface could support modernization of REDCap-based surveys, thus providing study staff and patients with clear and effective experience of health data collection.
Respondents wanted the mobile interface updated to look more like other commercial products, such as Qualtrics or SurveyMonkey. As more individuals are using mobile devices to obtain health information, it is of great importance to enhance their experience with mobile data collection [ 35 ]. They also suggested that the mobile apps have similar features as the web-based REDCap. Other requests included REDCap to support more languages or a translation service, where surveys could be translated to patients’ preferred languages. Though it has some language capabilities, including Spanish, respondents wanted more language options built into REDCap. In addition, there was concern about the literacy of patients leading to suggestions for REDCap to include tools allowing patients with various literacy levels to access surveys. Respondents suggested inclusion of voice capabilities and more multimedia and gamification features in response options, such as a picture interface where patients could locate their pain visually for researchers. Inclusion of these features could further enhance the experience among patients with higher accessibility needs and low literacy. We also noted that some respondents suggested features that were available within REDCap at the time of conducting the survey. Suggestions included availability of REDCap’s mobile version, embedded fields for responses, and integrations with messaging services such as Twilio. This again points out the need for education among study staff and organizational administrators to enable the optimal and effective use of REDCap features.
Limitations
This study is not without limitations. Although we recruited over 200 respondents, the sample size is small in comparison with the existing user base. We recruited fewer researchers (25/207, 12.1%) than administrators (150/207, 72.5%) who may be more directly involved in survey design and data collection. We also did not ask for participants’ training and experience with REDCap. Future studies should focus on better understanding user perspectives (especially researchers) while also considering the type and amount of REDCap training received by the user. We asked individuals’ opinions that are valuable but may be subject to bias, incomplete recall, or lack of information. For example, we asked information about their institution’s REDCap use, but we did not include a response option or decline responding if they did not have accurate information. We also used REDCap as the platform to conduct our survey, which may have potentially biased responses by familiarizing participants with REDCap more than necessary. Participants’ free-ended responses may have been influenced by how our study was designed or how the features were used. Future, more direct studies are warranted to better understand preferences. We recruited respondents from current REDCap consortium members, who may be more likely to believe REDCap is highly usable, as they may act as REDCap champions within institutions. We may be missing critical information by not capturing the perspectives of people who are not frequent users or consortium members. Future research should capture opinions of novice or past REDCap users. We also did not ask for information about participants’ institutions, REDCap versions and plug-ins used, or institutional policies and customizations. It is possible that participant feedback may be related to institutional requirements or policies. Furthermore, we asked researcher and administrator opinions on patient experience. However, we did not directly assess the patient or research participant experience. Understanding patient experience is important to study in future research. In addition, a comparison of the REDCap experience with other EDC platforms could provide a better understanding of study staff and patient needs. A recent study compared individuals’ experience in completing health forms using REDCap versus a chatbot platform. The results revealed that over 69% of participants preferred a chatbot for data collection with higher usability and net promotor scores for the chatbot [ 33 ]. The chatbot provided superior engagement and interactivity and was perceived as more intuitive than a standard, web-based REDCap interface. Future studies should look into better understanding study staff and patient needs to optimize survey development and data collection experience.
Conclusions
This pilot study aimed to assess stakeholder perspectives on experience with REDCap as an electronic health data collection tool. The findings revealed researchers and administrators perceive REDCap as a valued, low-cost resource that enables them to remotely collect and report health data in a secure and easy way. They also indicated a generally positive health data collection experience by clinical research and care staff members and patients. Although, with the advancements in data collection technologies and availability of interactive and intuitive user interfaces, there is a critical opportunity to enhance the REDCap experience to meet the needs of its vast user base of researchers and patients.
Acknowledgments
Research reported in this publication was supported by the National Library of Medicine of the National Institutes of Health (award number 1R41LM013419-01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. BB was funded by the National Institute of Mental Health (grant K23MH118482). BW was funded by the National Library of Medicine (grant R41LM013419).
Conflicts of Interest
BW is a shareholder of Doxy.me Inc and Dokbot LLC. All other authors are employees of Doxy.me Inc, a commercial telemedicine company. All authors declare no other conflicts of interest.
Qualitative codebook.
- Caron-Flinterman JF, Broerse JE, Bunders JF. The experiential knowledge of patients: a new resource for biomedical research? Soc Sci Med. Jun 2005;60(11):2575-2584. [ CrossRef ] [ Medline ]
- Hanley B, Truesdale A, King A, Elbourne D, Chalmers I. Involving consumers in designing, conducting, and interpreting randomised controlled trials: questionnaire survey. BMJ. Mar 03, 2001;322(7285):519-523. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Saczynski JS, McManus DD, Goldberg RJ. Commonly used data-collection approaches in clinical research. Am J Med. Nov 2013;126(11):946-950. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Milinovich A, Kattan MW. Extracting and utilizing electronic health data from Epic for research. Ann Transl Med. Feb 2018;6(3):42. [ FREE Full text ] [ CrossRef ] [ Medline ]
- van Velthoven MH, Mastellos N, Majeed A, O'Donoghue J, Car J. Feasibility of extracting data from electronic medical records for research: an international comparative study. BMC Med Inform Decis Mak. Jul 13, 2016;16(1):90. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Sacristan JA, Aguaron A, Avendaño C, Garrido P, Carrion J, Gutierrez A, et al. Patient involvement in clinical research: why, when, and how. Patient Prefer Adherence. Apr 2016;10:631-640. [ CrossRef ]
- van der Scheer L, Garcia E, van der Laan AL, van der Burg S, Boenink M. The benefits of patient involvement for translational research. Health Care Anal. Sep 24, 2017;25(3):225-241. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Summary of the HIPAA privacy rule. U.S. Department of Health and Human Services. URL: https://www.hhs.gov/hipaa/for-professionals/privacy/laws-regulations/index.html [accessed 2021-12-09]
- REDCap homepage. REDCap. URL: https://www.project-redcap.org/ [accessed 2022-05-09]
- Patridge EF, Bardyn TP. Research electronic data capture (REDCap). J Med Libr Assoc. Jan 12, 2018;106(1). [ FREE Full text ] [ CrossRef ]
- REDCap (Research Electronic Data Capture) - Harvard Catalyst. Harvard Catalyst. URL: https://catalyst.harvard.edu/redcap/ [accessed 2024-06-05]
- Reichold M, Heß M, Kolominsky-Rabas P, Gräßel E, Prokosch HU. Usability evaluation of an offline electronic data capture app in a prospective multicenter dementia registry (digiDEM Bayern): mixed method study. JMIR Form Res. Nov 03, 2021;5(11):e31649. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Stambler DM, Feddema E, Riggins O, Campeau K, Breuch LA, Kessler MM, et al. REDCap delivery of a web-based intervention for patients with voice disorders: usability study. JMIR Hum Factors. Mar 25, 2022;9(1):e26461. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kianersi S, Luetke M, Ludema C, Valenzuela A, Rosenberg M. Use of research electronic data capture (REDCap) in a COVID-19 randomized controlled trial: a practical example. BMC Med Res Methodol. Aug 21, 2021;21(1):175. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Tamuhla T, Tiffin N, Allie T. An e-consent framework for tiered informed consent for human genomic research in the global south, implemented as a REDCap template. BMC Med Ethics. Nov 24, 2022;23(1):119. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Wong TC, Captur G, Valeti U, Moon J, Schelbert EB. Feasibility of the REDCap platform for single center and collaborative multicenter CMR research. J Cardiovasc Magn Reson. Jan 16, 2014;16(Supplement 1):P89. [ CrossRef ]
- Chen C, Turner SP, Sholle ET, Brown SW, Blau VL, Brouwer JP, et al. Evaluation of a REDCap-based workflow for supporting federal guidance for electronic informed consent. AMIA Jt Summits Transl Sci Proc. 2019;2019:163-172. [ FREE Full text ] [ Medline ]
- Lee CA, Gamino D, Lore M, Donelson C, Windsor LC. Use of research electronic data capture (REDCap) in a sequential multiple assignment randomized trial (SMART): a practical example of automating double randomization. BMC Med Res Methodol. Jul 06, 2023;23(1):162. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Doyle S, Pavlos R, Carlson SJ, Barton K, Bhuiyan M, Boeing B, et al. Efficacy of digital health tools for a pediatric patient registry: semistructured interviews and interface usability testing with parents and clinicians. JMIR Form Res. Jan 17, 2022;6(1):e29889. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Garcia KK, Abrahão AA. Research development using REDCap software. Healthc Inform Res. Oct 2021;27(4):341-349. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Partners - REDCap. REDCap. URL: https://projectredcap.org/partners/ [accessed 2023-04-21]
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. Jul 2019;95:103208. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. Apr 2009;42(2):377-381. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Braun V, Clarke V. Thematic analysis. In: Cooper H, Camic PM, Long DL, Panter AT, Rindskopf D, Sher KJ, editors. APA Handbook of Research Methods in Psychology, Vol. 2: Research Designs: Quantitative, Qualitative, Neuropsychological, and Biological. Washington, DC. American Psychological Association; 2012:57-71.
- Bernard HR. Research Methods in Anthropology: Qualitative and Quantitative Approaches. Lanham, MD. Rowman & Littlefield Publishers; 2018.
- Bernard HR, Wutich A, Ryan GW. Analyzing Qualitative Data: Systematic Approaches. Thousand Oaks, CA. SAGE Publications; 2016.
- Bargas-Avila JA, Brenzikofer O, Roth SP, Tuch AN, Orsini S, Opwis K. Simple but crucial user interfaces in the world wide web: introducing 20 guidelines for usable web form design. In: Mátrai R, editor. User Interfaces. London, UK. IntechOpen; May 1, 2010.
- Conrad FG, Schober MF. Clarifying survey questions when respondents don't know they need clarification. National Center for Education Statistics. 2001. URL: https://nces.ed.gov/FCSM/pdf/2001FCSM_Conrad.pdf [accessed 2023-04-24]
- ClinCapture homepage. ClinCapture. URL: https://www.clincapture.com/ [accessed 2023-05-09]
- Pawelek J, Baca-Motes K, Pandit JA, Berk BB, Ramos E. The power of patient engagement with electronic health records as research participants. JMIR Med Inform. Jul 08, 2022;10(7):e39145. [ FREE Full text ] [ CrossRef ] [ Medline ]
- SurveyMonkey homepage. SurveyMonkey. URL: https://www.surveymonkey.com/ [accessed 2023-05-09]
- Qualtrics XM: the leading experience management software. Qualtrics XM. URL: https://www.qualtrics.com/ [accessed 2023-05-09]
- Soni H, Ivanova J, Wilczewski H, Bailey A, Ong T, Narma A, et al. Virtual conversational agents versus online forms: patient experience and preferences for health data collection. Front Digit Health. Oct 13, 2022;4:954069. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Ponathil A, Ozkan F, Welch B, Bertrand J, Chalil Madathil K. Family health history collected by virtual conversational agents: an empirical study to investigate the efficacy of this approach. J Genet Couns. Dec 03, 2020;29(6):1081-1092. [ CrossRef ] [ Medline ]
- Heimlich R. More use cell phones to get health information. Pew Research Center. Nov 14, 2012. URL: https://www.pewresearch.org/fact-tank/2012/11/14/more-use-cell-phones-to-get-health-information/ [accessed 2022-04-18]
Abbreviations
| electronic data capture |
| electronic health record |
| Health Insurance Portability and Accountability Act |
| Research Electronic Data Capture |
Edited by C Lovis; submitted 09.06.23; peer-reviewed by S Wang, C Chen; comments to author 12.03.24; revised version received 10.04.24; accepted 04.05.24; published 25.06.24.
©Hiral Soni, Julia Ivanova, Hattie Wilczewski, Triton Ong, J Nalubega Ross, Alexandra Bailey, Mollie Cummins, Janelle Barrera, Brian Bunnell, Brandon Welch. Originally published in JMIR Medical Informatics (https://medinform.jmir.org), 25.06.2024.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in JMIR Medical Informatics, is properly cited. The complete bibliographic information, a link to the original publication on https://medinform.jmir.org/, as well as this copyright and license information must be included.
Our Partners
- Industry Events
Schematic capture software for electronics engineers
Schematic capture software is an indispensable tool for engineers engaged in the creation of electronic circuits and systems .
This software facilitates the process of designing and documenting electronic circuits by allowing engineers to create and manage schematic diagrams digitally.
What is schematic capture software?
Schematic capture software, also known as schematic entry software, is a specialised application used to create electronic circuit diagrams. These diagrams are essential blueprints for designing and understanding electronic circuits, serving as a visual representation of the components and connections within a circuit.
The primary function of schematic capture software is to provide a user-friendly interface for drawing and managing schematic diagrams.
Key features typically include:
- Component libraries: These libraries contain a vast array of pre-designed electronic components, such as resistors, capacitors, diodes, transistors, and integrated circuits, which engineers can readily incorporate into their designs.
- Symbol creation and editing: Engineers can create custom symbols for components not found in the standard libraries or modify existing symbols to suit specific design requirements.
- Connectivity and netlisting: The software ensures accurate connections between components and generates netlists, which are essential for further stages of the design process, such as PCB layout and simulation.
- Design rule checks (DRC): Automated checks help identify errors and ensure that the schematic adheres to predefined design rules, reducing the risk of issues in later stages of development.
Integration with other tools: Schematic capture software often integrates seamlessly with PCB layout tools, simulation software, and other engineering applications, facilitating a smooth workflow from schematic design to final product.
Who uses schematic capture software?
Schematic capture software is primarily used by electronics engineers and designers involved in various stages of electronic product development. This includes:
- Design engineers: They use the software to create and refine circuit designs, ensuring functionality and reliability.
- Test engineers: They utilise schematics to develop testing protocols and identify potential points of failure in a circuit.
- Research and development teams: These teams rely on schematic capture software to prototype and experiment with new electronic concepts and technologies.
- Educational institutions: The software is also widely used in academic settings to teach students about electronic circuit design and analysis.
The use of schematic capture software offers numerous advantages that make it an essential tool for electronics engineers:
- Accuracy and precision: Digital schematics reduce the likelihood of errors compared to hand-drawn diagrams, ensuring that designs are accurate and reliable.
- Efficiency: The software streamlines the design process, allowing engineers to create and modify schematics quickly and efficiently.
- Documentation: Schematic diagrams serve as detailed documentation for the design and manufacturing process, aiding in troubleshooting, maintenance, and future upgrades.
- Collaboration: Digital schematics can be easily shared and reviewed among team members, fostering collaboration and communication within development teams.
- Integration: The seamless integration with other design tools and software ensures a coherent workflow, from initial design to final production.
Schematic capture software is a vital component in the toolkit of electronics engineers. It enhances the accuracy, efficiency, and collaborative potential of electronic circuit design, making it an indispensable resource in the development of modern electronic devices and systems.
Product Spotlight
PORTENTA HAT CARRIER
| SKU: | 1050-ASX00049-ND |
|---|---|
| Stock: | 942 |
| Cost: | $35.51 |
BeagleBoard

Single Board Computer (SBC), BeagleY-AI
| SKU: | 2820-102991834-ND |
|---|---|
| Stock: | 0 |
| Cost: | $57.23 |
Raspberry Pi
Raspberry Pi 5 – 8GB RAM
| SKU: | 2648-SC1112-ND |
|---|---|
| Stock: | 2228 |
| Cost: | $62.99 |
Tech Videos
Upcoming Events
Further reading
A selection of Design articles for further reading
Social Login
Electronic Data Capture Systems Global Market Report 2024 by The Business Research Company | International Business Machines Corporation (IBM); Oracle Corporation; IQVIA Inc.; Parexel International Corporation; Veeva Systems Inc.; Clario Medical Imaging;
Press release from: the business research company.

Electronic Data Capture Systems Market Size
Permanent link to this press release:
You can edit or delete your press release Electronic Data Capture Systems Global Market Report 2024 by The Business Research Company | International Business Machines Corporation (IBM); Oracle Corporation; IQVIA Inc.; Parexel International Corporation; Veeva Systems Inc.; Clario Medical Imaging; here
Delete press release Edit press release
More Releases from the business research company

All 5 Releases
More Releases for Capture
- Categories Advertising, Media Consulting, Marketing Research Arts & Culture Associations & Organizations Business, Economy, Finances, Banking & Insurance Energy & Environment Fashion, Lifestyle, Trends Health & Medicine Industry, Real Estate & Construction IT, New Media & Software Leisure, Entertainment, Miscellaneous Logistics & Transport Media & Telecommunications Politics, Law & Society Science & Education Sports Tourism, Cars, Traffic RSS-Newsfeeds
- Order Credits
- About Us About / FAQ Newsletter Terms & Conditions Privacy Policy Imprint
Redgrave Data Achieves Top Rankings from Chambers and Partners in Its 2024 Litigation Services and eDiscovery Guides
News provided by
Jun 27, 2024, 09:00 ET
Share this article
Redgrave Data's Tara Emory also achieves high recognition in the guide for sixth consecutive year
DALLAS , June 27, 2024 /PRNewswire/ -- Redgrave Data, an elite group of former practicing attorneys, award-winning data scientists, and IT professionals who solve the world's most complex problems in litigation, investigations, information governance, data privacy, and other regulatory matters, and Tara Emory , the company's senior vice president of AI legal strategy, have been recognized by Chambers and Partners for their expertise in litigation support and eDiscovery. London -based Chambers and Partners annually ranks the leading U.S. firms and attorneys based on in-depth research and interviews with clients and peers.
The guide honored Redgrave Data with elite ratings including a Band 1 (Top Tier) ranking globally in NewLaw Litigation Services, and a Band 2 national rating for Litigation Support in eDiscovery. Redgrave Data was described by Chambers as a prominent player in the U.S. eDiscovery market and attributed their recognition to its ability to help clients address comprehensive data analysis issues when facing litigation, its commitment to product development, as well as its willingness to help clients implement document review processes and protocols.
Chambers NewLaw guide identifies and ranks the most innovative organizations in the alternative legal services and legal tech markets, delivering indispensable insights and enabling decision-makers to secure the talent that is right for them. In recognizing Redgrave Data, Chambers cited the company's handling of technological approaches to complex eDiscovery issues, as well as its expertise in redesigning in-house legal operations related to data. Chambers highlighted the deep experience of Redgrave Data's specialists, who design and implement software solutions, develop process workflows, and create data visualizations to generate data-based insights for its clients, which include both in-house legal teams and law firms.
"We are honored to learn that Chambers and Partners has recognized our unique combination of legal and technological expertise and the significant impact we've had on the industry," said Mollie Nichols , CEO of Redgrave Data. "We will continue to commit ourselves to tackling the world's most intricate challenges in eDiscovery, litigation, investigations, information governance, and more. We look forward to what the future holds."
Tara Emory was recognized as a top litigation support professional with a national Band 1 rating in eDiscovery. Emory is a highly experienced legal technology consultant and lawyer, with a focus on guiding legal teams in efficient and compliant uses of artificial intelligence. Her expertise bridges the areas of law, data compliance, and technical aspects of data and software. In litigation, she is an expert on search methodologies, technology assisted review (TAR/machine learning), data preservation and collection approaches, discovery protocols, and strategies for resolving discovery issues with litigation adversaries, government regulators, and the courts. Tara works closely with the Redgrave Data team, provider partners, and clients to develop custom solutions for litigation and investigation matters, including reporting, data visualizations, analytics and machine learning strategies, workflows, and validation procedures. This is the sixth consecutive year Emory has been recognized in this guide.
"It's an immense honor to be recognized again by Chambers and Partners and to know that the work we're doing for our customers is really making a difference," said Tara. "We recognize that not all customer problems are the same, and we are determined to provide a solution that is unique to each one in this constantly evolving marketplace."
Chambers ranks companies and individuals in Bands 1 (highest) to 6, with all Band rankings considered a significant achievement. Chambers assesses attorneys and service providers on attributes most valued by clients, including technical legal ability, professional conduct, client service, commercial astuteness, diligence, and commitment. The annual Chambers guides are widely read by industry-leading companies and organizations across the world.
About Redgrave Data
Established in 2022, Redgrave Data is multi-disciplinary legal technology services company consisting of an elite group of attorneys, acclaimed data scientists, and seasoned software developers and technologists. Together, we tackle the world's most intricate challenges in litigation, investigations, information governance, data privacy, and regulatory matters. Leveraging our deep-seated proficiency at the crossroads of technology, science, and law, we wield a formidable array of off-the-shelf enterprise, legal tech, AI, and machine learning tools, as well as our own proprietary solutions. We are capable of rapid software development on-the-fly in the heat of litigation as clients are confronted with unique challenges that off-the-shelf technology cannot address. This empowers us to craft highly tailored applications quickly, playbooks for the longer game, and strategic roadmaps for critical areas like eDiscovery and information governance, ensuring they align perfectly with our clients' needs. The luminaries within the Redgrave Data team are recognized as leading authorities in the eDiscovery and legal domains. For further insights into our company, please visit https://www.redgravedata.com/ .
SOURCE Redgrave Data
Modal title

IMAGES
VIDEO
COMMENTS
REDCap (Research Electronic Data Capture) is a free and flexible tool for online or offline project design, data input, and analysis. It offers many features, such as multi-site access, audit trails, automated export, and interoperability with health records.
1 review. AnyDoc is an automated data capture software product. It identifies and captures data from incoming documents and streamlines key processes. It is used for: Minimizing manual data entry: AnyDoc uses optical character recognition (OCR) technology to automatically capture data - including…. 9.
Researched and written by Dominick Duda. Electronic data capture (EDC) software replaces the traditional and very manual process of managing clinical trial data with an electronic system. At its core, EDC software streamlines the collection, review, and processing of clinical trial data. Clinical trial management software is bolstered by EDC ...
REDCap is a web-based tool for data capture, management, and analysis in research studies and operations. It is used in over 150 countries and supports compliance with various regulations and standards.
Find the top Electronic Data Capture software of 2024 on Capterra. Based on millions of verified user reviews - compare and filter for whats important to you to find the best tools for your needs. ... Capterra's researchers use a mix of verified reviews, independent research and objective methodologies to bring you selection and ranking ...
REDCap is a secure, user-friendly application that supports data collection and management for clinical and research studies. It is available to investigators at Harvard-affiliated institutions that have adopted REDCap and can be customized for each study's needs.
The software evolved into a neutral data collection platform - able to capture any type of data, for any purpose. REDCap is now used for just about any type of data collection you can imagine, across thousands of non-profit and government organizations. Each system is independently maintained and supported. So consortium partner sites truly ...
iMednet is a cloud-based electronic data capture platform designed to help the healthcare industry capture, clean, adjudicate and manage data across clinical trials. The application enables research teams to build and implement st... Read more. 5.0 ( 2 reviews) Visit Website.
Introduction. High-quality data are crucial for obtaining medical research results [] and successful machine learning applications [].To collect and manage structured data in digital format, researchers can use computer programs called electronic data capture (EDC) systems [].There is a consensus that EDC leads to improved data quality as well as cost and time efficiency compared with paper ...
An Electronic Data Capture (EDC) system for clinical trials or studies is a software solution that makes it easier for medical device companies to digitally collect, store, and manage the patient data collected. Nowadays, many clinical EDC systems are distributed in the Software-as-a-Service (SaaS) business model and accessed by sites and ...
Research Electronic Data Capture (REDCap) is a web-based application developed by Vanderbilt University to capture data for clinical research and create databases and projects. It is Health Insurance Portability and Accountability Act (HIPAA)-compliant, highly secure, and intuitive to use. The databases use instruments such as surveys and ...
REDCap (Research Electronic Data Capture) is a secure web-based application for data capture and study management. Developed at Vanderbilt. in 2004 by Paul A. Harris and. colleagues. Clinical and Translational Science Award (CTSA) funding. REDCap Consortium: >6,000 institutions in >150 countries around the world.
Research Electronic Data Capture (REDCap) A modern, secure software application for building and managing online clinical research databases and survey forms. REDCap has the required safeguards for research data security and privacy (compliant with HIPAA). The application allows users to create research databases and data entry web-based forms ...
2. Castor EDC: Best for complex and multi-centre studies. Castor EDC is a cloud-based clinical data management platform that enables researchers to capture and integrate data from any source. Data sources include clinicians, patients, devices, wearables and Electronic Health Record (EHR) systems.
I. Introduction. High-quality clinical research is dependent on adequate design, methodology, and data collection [].A common part of clinical research and health surveillance is the dissemination of the information produced throughout the process [2,3].Electronic data capture methods and systems have been used in clinical research to improve data collection, storage, and analysis, and proper ...
Rave EDC. Medidata's Rave EDC (Electronic Data Capture) is the most advanced, robust, and secure EDC system for clinical trial site, patient, and lab data capture and management. Rave EDC is the cornerstone of the Medidata Platform - the unified clinical research platform that connects processes, eliminates data reconciliation, and delivers ...
Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Nevada Las Vegas. REDCap is a secure, web-based software platform designed to support data capture for research studies, providing: An intuitive interface for validated data capture;
Electronic data capture (EDC) software digitalizes the data capture process for field teams, surveyors, researchers, and study coordinators, so they can do away with manual ways of collecting data. It validates the captured data and makes it readily available for analysis and reporting. A wide range of EDC software options is available on the ...
This article describes the novel use of Research Electronic Data Capture (REDCap) software to create a database of literature searches conducted at a large academic health sciences library. An archive of paper search requests was entered into REDCap, and librarians now prospectively enter records for current searches.
Looking to understand the utilization of the resource. REDCap (Research Electronic Data Capture) is a secure web-based application for data capture and study management. Developed at Vanderbilt. in 2004 by Paul A. Harris and. colleagues. Clinical and Translational Science Award (CTSA) funding. REDCap Consortium: >6,000 institutions in >150 ...
Study data were collected and managed using REDCap electronic data capture tools hosted at [YOUR INSTITUTION]. 1,2 REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies, providing 1) an intuitive interface for validated data capture; 2) audit trails for tracking ...
Book a free demonstration with one of our specialists using the button below. We'll guide you through and demonstrate how our solutions can support you. Book demonstration Explore other tools. ResearchManager Electronic Data Capture: ISO 27001 & NEN 7510 certified GCP & GDPR compliant Contact us or request a demo.
REDCap is a novel workflow methodology and software solution designed for rapid development and deployment of electronic data capture tools to support clinical and translational research. ... (Research Electronic Data Capture) was initially developed and deployed at Vanderbilt University, but now has collaborative support from a wide consortium ...
Background: Self-administered web-based questionnaires are widely used to collect health data from patients and clinical research participants. REDCap (Research Electronic Data Capture; Vanderbilt University) is a global, secure web application for building and managing electronic data capture. Unfortunately, stakeholder needs and preferences of electronic data collection via REDCap have ...
Research and development teams: These teams rely on schematic capture software to prototype and experiment with new electronic concepts and technologies. Educational institutions: The software is also widely used in academic settings to teach students about electronic circuit design and analysis.
The electronic data capture systems market size has grown rapidly in recent years. It will grow from $1.75 billion in 2023 to $2.02 billion in 2024 at a compound annual growth rate (CAGR) of 15.5%.
Redgrave Data's Tara Emory also achieves high recognition in the guide for sixth consecutive year . DALLAS, June 27, 2024 /PRNewswire/ -- Redgrave Data, an elite group of former practicing ...