A top consulting firm told employees get vaxxed or don't get paid. Every company considering mandates should follow its lead.
- Alvarez & Marsal's new vaccine mandate requires employees to get vaccinated or go on unpaid leave.
- The policy is a case study in how employers should craft their mandates, workplace experts said.
- Employer mandates are the strongest incentive the US has to encourage workers to get the shot.

One consulting firm is introducing a unique policy to get workers vaccinated: Get the jab or don't get paid.
Alvarez & Marsal, the corporate-turnaround firm that handled Lehman Brother's high-profile bankruptcy in 2008, said that it would be requiring all workers to provide proof of vaccination by September 7. Unvaccinated employees have 75 days to get the shot or they'll be placed on an unpaid leave of absence starting October 31 for up to six months. Alvarez & Marsal did not immediately respond to a request for comment.
More employers are expected to take this type of approach as the FDA gives full approval to COVID-19 vaccines. The Pfizer vaccine received FDA approval Monday.
"One out of every 27,700 vaccinated Americans have been hospitalized," A&M said in a release . "These facts make it clear that vaccinations are extremely effective."
A&M joins a growing number of employers such as IBM and Uber that are requiring employees to get vaccinated if they want to return to the office. Although the firm's policy may appear to be taking a strong stance on vaccination, workplace experts say it's a good example of a nuanced approach to mandates. It provides a timeline for employees to get the shot, and allows exceptions for those who are pregnant. Anyone with a deeply held religious belief or another medical reason can be considered for an exception, though there are no guarantees, the company said.
Related stories
The policy "seems to be grounded in both federal agency guide guidance and the law," Walter Foster, a labor attorney at Eckert Seamans Cherin & Mellott LLC, told Insider. "It's saying, 'We want to accommodate our employees to the degree that we can.'"

Mandates like Alvarez & Marsal's may be the only way to reach universal vaccination in the US
About 92% of Alvarez & Marsal's workforce is vaccinated, the company reported . This policy will likely push the final 8%, who are either unvaccinated or chose not to report their vaccine status, to either get the vaccine or show proof of it, Foster said. And it provides a flexible enough timeline for them to do that, he added.
"It's a way to manage the workforce," Foster said.
If employees are put on unpaid leave and don't get vaccinated, they could run the risk of losing their job, Foster said.
"It definitely holds out the promise that the person could be separated if they're not in compliance by that point," he said. This creates a major incentive for most employees to get the vaccine. Possible downsides, like retention and tracking will be minimal, experts said.
Data shows that most workers are supportive of these kinds of policies. A recent Gallup poll of over 1,800 US adults found that 52% of respondents favor or strongly favor a COVID-19 vaccine mandate at their place of employment.
This is a good thing, said Patricia Toro, a senior director of health management at Willis Towers Watson, because employer mandates might be the strongest incentive the US has to encourage people to get the shot.
"These mandates go beyond incentives, but are likely the only way to move toward near-universal vaccination rates, with the subsequent improved health of the local community," Toro told Insider in an email.
Employers have a clear business interest to help reach near-universal vaccination levels, Foster said. If COVID-19 cases continue to rise, the cost of having to shut down again could create more financial strain.
Foster added, "It's a balanced and incremental approach that I think a number of employers are looking toward."
Shana Lebowitz contributed reporting.
Watch: What it's like to get the COVID-19 vaccine, according to some of the first healthcare workers to receive it
- Main content
- Empowered consumers
- Healthier humans
- Future organisations
- Business for better
- Safer societies
- Sustainable world
- Consumer and manufacturing
- Defence and security
- Energy and utilities
- Financial services
- Government and public sector
- Life sciences
- Set growth strategy
- Build brands, products, and services
- Reimagine AI, digital, and data
- Improve organisational performance
- Deliver complex programmes
- Empower your people

UK Vaccine Taskforce
Steering a world-leading programme to secure COVID-19 vaccines in record time
In spring 2020, as a deadly coronavirus began to race around the globe, the UK Government established its Vaccine Taskforce (VTF). Its mission – to drive development and manufacture of life-saving vaccines and make them available to the public as quickly as possible. At this point, no-one knew if anyone would be able to develop a safe and effective vaccine – but if they were, the speed of roll-out would have a direct impact on the number of lives saved. The pressure to take decisions at pace and simultaneously retain control of the billions in public funding committed to the mission was intense. But by far the biggest pressure was to deliver.
We were an integral part of the team from the start – bringing to the mission deep expertise in complex programme delivery, business cases and life sciences, and experience of working across government. Over more than 18 months, we collaborated with the VTF to shape, lead and, ultimately, deliver a range of projects that enabled the UK to launch one of the first and fastest vaccine rollouts in the world. And we continue to be part of an extraordinary national effort that has saved tens of thousands of lives to date and will save many more in years to come.
Key successes
- Supported the mobilisation of a world-leading vaccine delivery programme allowing over 80 per cent of UK adults to receive at least one dose within seven months of regulatory approval
- Applied commercial and business case expertise to enable the VTF to agree contracts for 400 million doses of seven different vaccines within six months of set-up
- Managed critical vaccines supply projects to deliver effective vaccines faster than ever before
- Supported the programme and helped secure funding for the onshoring of vaccine manufacture in the UK to save lives for years to come
A once-in-a-century global health emergency
The COVID-19 pandemic has claimed millions of lives and decimated economies around the world. Within weeks of the World Health Organisation declaring the pandemic, the UK Government recognised that vaccines would be the way out. So, as cases climbed towards their first peak in the UK, the Department of Business, Energy & Industrial Strategy (BEIS) set up the Vaccine Taskforce (VTF). Its challenge was to build a promising portfolio of vaccines and make them available to the public without delay.
The race is on
The urgency was immense. It typically takes up to ten years to develop new vaccines and bring them to market. But with the number of deaths associated with the virus rising inexorably – and the UK’s first lockdown sending the economy into reverse – there was no time to waste. The VTF need to dramatically accelerate every engagement with the companies racing to develop new vaccines, and with those gearing up to manufacture them.
The need for speed was intense, yet the requirement to retain control was equally powerful. Billions of pounds of public money were going into securing the vaccines. The VTF needed to be fastidious in demonstrating that rigorous governance was in place – all without slowing down decision-making. Any delay or hesitation would jeopardise the UK’s ambition to secure supplies of an effective vaccine as soon as one was available.
Against this backdrop, a raft of challenges lay in wait: from building the business cases to secure funding for procurement contracts, to developing strategy for supplying vaccines into the UK’s roll-out programme and dealing with the complexities of Brexit.
Bringing together the best of the best
To support this unprecedented national effort, the VTF brought together a team with all the relevant skills from a range of sectors. We quickly became integral to the programme’s development and – ultimately – its success.
As the true scale of the pandemic became apparent, global demand for vaccines went through the roof. Our ability to move fast and deliver critical projects at pace was instrumental in enabling the UK to compete successfully and procure millions of doses.”
With our wide experience of working across government to support the delivery of large-scale, complex and high-risk programmes, we could hit the ground running and work as an effective delivery partner from day one. We had already proved our ability to deliver at speed and under pressure with our work on the UK Ventilator Challenge .
We brought an extraordinary breadth of expertise to the challenge. Our wealth of experience in complex programme delivery and our pedigree in the health and life sciences sector were invaluable, giving us important credibility as we engaged with stakeholders across the vaccines space. Our experts in programme and project management and delivery played a key role in leading engagement on initiatives to deliver effective vaccines faster than ever before.
The need to bring together a range of skills and expertise at incredible pace was the perfect challenge for PA. It was an opportunity to bring all our experience, insight and understanding to a national challenge on which lives depended.”
Designing the delivery plan from the outset
As the VTF’s work took off, vaccine developers around the world were launching hundreds of vaccine initiatives and new stakeholders were emerging by the week. We took this shifting landscape and worked closely with the VTF to establish a clear delivery plan and structure for the programme to manage the portfolio of the most promising candidate vaccines. We also worked jointly with the VTF to set up effective governance to enable rapid decision-making.
The programme relied on an open and collaborative approach, and PA embodies this. It was instrumental in building relationships and enabled the rapid and confident decision-making that needed to happen on the programme. It speaks volumes for PA’s programme management expertise.”
As experts in programme management, we knew how important it was to set the right tone at this early stage. As we supported and facilitated dialogue among senior government stakeholders and leading medical and public health experts, we ensured absolute transparency around risks – for example, that contracts for supply would come with no definite date for delivery.
Making the case for investment in candidate vaccines
At this point, no-one knew which initiatives would produce a viable vaccine – if at all. On the advice of VTF chair Kate Bingham, the Government had determined to back a wide range of projects. Our business case expertise was instrumental in informing the decisions on which ones and securing the necessary funding.
Working at pace, we translated scientific recommendations, clinical and commercial advantages, negotiating positions and funding requirements into a set of high-quality business cases. This was a shifting landscape. As different vaccines made their way through clinical trials, and safety and efficacy results came out, the risk profile for each potential vaccine shifted. Similarly, demand for individual vaccines surged or dropped away on the back of trial results.
The Vaccine Task Force is a rainbow team, calling upon skills from industry, public sector and consultancies. PA were responsive, and the speed and quality of their work was key to us, says Ruth Todd (CBE), Programme Director, VTF. At every pressure point – and there were many – we could count on their commitment, energy and expert insight. PA were an essential part of our team.”
In this high-stakes environment, the team compressed the usual timeline from business case development to ministerial approval from months into weeks, and then from days into hours. We didn’t miss a beat.
Leading engagement on four separate vaccine initiatives
By autumn 2020, we had already supported the VTF in agreeing contracts for 400 million doses of seven different vaccines for the UK. Working together, we established a portfolio approach to manage engagement on the different initiatives, going on to lead key projects with four vastly different vaccine developers, including world-leading biopharma Pfizer and biotechnology rising star Moderna.
As part of the VTF, we set up a joint working plan with each of the four companies and started to build the close relationships necessary to secure supplies for the UK. To compete effectively for developers’ attention, we emphasised the UK’s commitment to purchase and its plan to develop a world-leading vaccine roll-out
Preparing for roll-out
There was no approval for any of these vaccines yet, but we were already developing the plan to link up supply with the NHS roll-out. We worked in the interface between the VTF and the deployment community, and together adopted a whole system approach to align every part of the operation and get them ready to receive and use the vaccines.
We worked with the VTF to develop the critical path to ensure readiness, working backwards day by day from an as-yet undetermined launch date to 12 weeks out. Each vaccine required a different pathway, depending on its transport and storage requirements, and on where it was destined for use.
PA’s thorough and proactive approach was impressive. PA were fastidious in searching out and clearing any barriers that had the potential to slow or disrupt the vaccine roll-out”
To ensure there would be no surprises, we assumed nothing. Together, we were proactive in unearthing and resolving challenges – from making sure the right arrangements were in place to import vaccines without barriers, to keeping pace with the vaccines’ evolving characteristics ahead of regulatory approval.
Meeting the moment as jabs approved
Then, in December 2020, a breakthrough. The UK’s medicines regulator gave approval for the Pfizer vaccine, based on revolutionary mRNA technology. The approval was for emergency use, a first for the UK.
The VTF hit ‘go’ on the plans to get vaccines to the roll-out programme. In less than a week, the first patient received a life-saving jab, and the UK became the first country in the world to administer the vaccine to the public.
In the weeks that followed, the Medicines and Healthcare products Regulatory Agency (MRHA) granted approval for vaccines from AstraZeneca and then Moderna. The process we jointly developed for getting supplies of the Pfizer vaccine out to the roll-out became the template for getting these and other future vaccines to the public.
Over the following months, our work proved critical in securing enough COVID-19 vaccines to support one of the fastest vaccines rollouts anywhere in world. By the end of June 2021, over 80 per cent of all UK adults had received at least one dose of a COVID-19 vaccine. By the end of August, the programme was estimated to have saved over 100,000 lives and prevented over 143,000 hospital admissions in England alone.
Bringing vaccine manufacturing capacity offshore
As vaccines started to drive down deaths and hospitalisations in the UK, the challenge of preparing the UK to live with COVID-19 and handle future pandemics was coming into focus. Global competition for vaccines, including the materials involved in their manufacture and the consumables required to develop them safely, had been off the scale since the pandemic began. Brexit had also changed processes involved in importing. So, the next challenge for the VTF was to set up an onshoring manufacturing programme to increase domestic capability and improve supply chain resilience.
PA brought the perfect mix of skills to the onshoring programme. PA’s ability to understand the long-term ambitions for the onshoring programme and to work flexibly proved a valuable counterpoint to their rigorous programme management.”
From the start, the programme comprised a wide range of initiatives. These included exploring opportunities to improve the UK’s capabilities to manufacture vaccines using four different production technologies. The programme also aimed to establish reliable supplies of raw ingredients, such as the lipids that introduce mRNA vaccines into human cells, and essential consumables, such as pipettes and tubing. Already, there were more than 30 investment proposals under review.
In an uncertain environment where strategy was still evolving, we supported the VTF to shape these many themes into a focused and coherent programme, define individual initiatives and introduce the controls and governance needed to move initiatives effectively through development. We also managed the proposal pipeline, supporting the evaluation of new proposals from potential partners.
Protecting tomorrow
By September 2021, we had already enabled the VTF to agree its first offer with a UK supplier to reserve essential capacity for the nation. With experts warning that the world is likely to experience further pandemics in the decades ahead, this is the first of many such agreements that will benefit the UK years from now. The UK’s enhanced vaccine manufacturing capacity and more resilient supply chains will strengthen the nation’s defence against the potentially devastating impact of new global health emergencies.
PA is incredibly proud to be at the heart of the UK’s response to the COVID-19 pandemic. PA’s work to support the procurement of vaccines and enable their roll-out, to develop a template for sharing doses with the world and to bring manufacturing capacity onshore will have an impact on lives nationwide for decades to come.”

Clinical trials transformation
Explore more.

Equimetrics V-Pro

Whittington Health NHS Trust

Nuclear Decommissioning Authority
Contact the team.
Get actionable insight straight to your inbox via our monthly newsletter.

- Harvard Business School →
- Faculty & Research →
- HBS Case Collection
- Format: Video
- | Language: English
About The Authors
Marco Iansiti
Karim R. Lakhani
Related work.
- Faculty Research
Moderna (A) Case Supplement
Moderna (b) case supplement.
- Moderna (A) Case Supplement By: Karim R. Lakhani, Allison J. Wigen and Dave Habeeb
- Moderna (B) Case Supplement By: Karim R. Lakhani, Allison J. Wigen and Dave Habeeb
- Research & IP Evaluation
- Preclinical Strategy & Execution
- Clinical Development
- Regulatory Affairs
- CMC Consulting
- Interim Chief Medical Officer
- Opportunity Mapping
- Business Plans & Fundraising Support
- New Product Planning
- Launch Planning
- Due Diligence
- In-Licensing & Asset Scouting
- Out-Licensing, Partnering & Deal-making
- Expert Witness Services
- Virtual & Semi-Virtual Biotechs
- Pharmaceutical Companies
- Tech Transfer Offices
- Antibodies, ADCs
- Cell Therapies
- Drug Discovery Platforms
- Gene Therapies
- Microbiome Drugs
- Peptides & Proteins
- Small Molecules
- Other Product Modalities
- Digestive & GI
- Endocrine & Metabolic
- Respiratory
- Other Disease Areas
- Anthony Walker
- Simon Turner
- Alastair Southwell
- Saadia Basharat
- Chris Owens
- Jess Hearn-Messenger
- Mark Philip
- Pierre Dodion
- Nigel Burns
- Gary Mansfield
- Christine McCarthy
- Stuart Hendry
- Consulting Network
- Case Studies
- Whitepapers
Pharma and Biotech Consulting
These selected case studies illustrate the scope of our work, the level of expertise we’re able to offer, and our consistent record of successful engagements.
- Location: Resources
Case Study Index
By Service Area
- Business Development
- Product Development
- Strategy & Commercialization
By Functional Discipline
- Asset Scouting
- Business Strategy
- Expert Witness
- Partnering and deal-making
- Pharma Licensing
- Preclinical Development
- Research and IP evaluation
By Modality
- Cell Therapy
- Gene Therapy
- Immunotherapy
- Radiopharmaceuticals
By Disease Area
- Autoimmune / Immunology
- Cardiovascular
- Digestive / GI
- Research & IP evaluation
- Partnering & deal-making
- Antibodies / ADCs
- Central Nervous System (CNS)
- Healthcare Investing
- Biotech Consulting
- Discovery Platforms
- Cell & Gene Therapy
- Pharma Consulting
- Technology Transfer
- Drug Repurposing
- Clinical Development Strategy
- Supply Chain
- Virtual Biotech
Interim medical director for MAA submission
Developing vaccine products, applying to early access to medicines (eams) at the mhra, due diligence on the developability & investability of a cancer vaccine, investor due diligence on preclinical stage vaccine platform technology, clinical strategies for a novel peptide-based cancer vaccine, cancer vaccine clinical strategy, valuation and business planning, reach out today.
Speak with us about our pharma & biotech consulting services.
- Our Services
Alacrita, UK, Europe & Asia 2 Royal College St London, UK NW1 0NH Registered in England & Wales. No. 10530608
Alacrita, US One Broadway Floor 14 Cambridge, MA 02142 Registered in Massachusetts.
© 2009 - 2024 Alacrita Holdings Limited
Privacy Policy | Pharmaceutical Consulting
Vaccitech and the Oxford-AZ COVID-19 Vaccine: Spearheading global health innovation

A landmark partnership between academia and industry. A commitment to equity and fair access. A vaccine developed in under a year. An estimated 6 million lives saved globally in 2021 alone.
The amazing and well-known story of the Oxford-AstraZeneca COVID-19 vaccine and its protagonists is the story of one of the most significant health breakthroughs in recent history.
Quick Facts
COVID-19 vaccine: more than 3 billion doses delivered to 180 countries by end of 2022
COVID-19 vaccine: up to an estimated 122.4 million hospitalisations prevented
9 programmes in Vaccitech’s pipeline across infectious diseases, cancer and autoimmunity

The vaccine was co-developed by Oxford spinout Vaccitech and the University’s Jenner Institute (where Vaccitech has its academic origins through co-founders Professor Sarah Gilbert and Professor Adrian Hill).
Vaccitech’s ChAdOx platform uses a replication deficient chimpanzee version of the common cold virus as a vector for the key ingredient – in COVID’s case the genetic material of the SARS-CoV-2 spike protein – that primes the recipient’s immune system to fight off an infection if or when it arrives.
While best known for its role in the battle against COVID, Vaccitech’s technology can also be turned towards a host of other diseases and disease areas, harnessing the power of the immune system’s T cells.
“Vaccitech’s current pipeline includes novel T cell immunotherapeutics, at various stages of development, for hepatitis B, HPV, coeliac disease, prostate cancer and MERS.”
With Vaccitech’s proven scientific expertise and a broad, high-value portfolio, the company listed on the NASDAQ stock exchange in 2021 and acquired Avidea Technologies at the beginning of 2022. The Avidea acquisition added the SNAPvax™ platform to Vaccitech’s portfolio; a self-assembling, fully synthetic platform that co-delivers multiple antigens and immunomodulators, and gives the company opportunities to advance into the autoimmune space.
Vaccitech’s current pipeline includes novel T cell immunotherapeutics, at various stages of development, for hepatitis B, HPV, coeliac disease, prostate cancer and MERS. Beyond Vaccitech itself, researchers at universities worldwide are using its proprietary technologies to develop vaccines for deadly and debilitating viruses such as rabies, malaria, HIV and Zika (to name just a few).
The societal and economic impacts of Vaccitech and the Oxford-AZ vaccine are extraordinary. Beyond the immediate benefits of reducing COVID-19’s spread and severity, the vaccine has helped revive economies, reopen societies, and restore a sense of safety and normality to billions of citizens worldwide.
Case Studies

BibliU: Enabling equitable access to textbooks

Mind Foundry: AI for high-stakes applications

OrganOx: Pioneering the future of organ transplantation

OUI Consulting Services: Creating instant impact

Oxford University Innovation: A conduit for impact

The Oxford Knee Score: Innovating patient-centric healthcare assessments

Wise Responder: Empowering companies to tackle poverty
- Deutschland
- North America
170+ million vaccines administered: The UK Vaccine Taskforce’s digital supply planning transformation
Launched rapidly to tackle challenges of unparalleled magnitude – namely, delivering COVID vaccines to the UK’s population of 67+ million and overseas during a global pandemic – the Vaccine Taskforce (VTF) faced a myriad of supply and logistical challenges that could only be addressed through a comprehensive data-driven Integrated Business Planning (IBP) function.
Efficio was engaged in May 2021 as an end-to-end supply chain partner with significant public sector expertise to address these challenges and custom-build an inventory-balancing Scenario Modelling Tool, optimising the VTF’s life-saving work.
The VTF (now the COVID Vaccines Unit, or CVU) was set up in April 2020 in response to the COVID-19 outbreak, bringing experts from industry, academia, and government together. Its objectives were to:
- Secure access to promising vaccine(s) for the UK population and achieve lasting immunity.
- Make provisions for the international distribution of vaccines.
- Strengthen the UK’s onshore capacity and capability in vaccine development, manufacturing, and supply chain to provide resilience to this and future pandemics.
Efficio’s Objectives
As supply chain specialists, Efficio was engaged to help the VTF adapt its supply chain to the changing demands of the vaccine deployment programme. This involved setting up:
- An Integrated Business Planning (IBP) function: Joining the Taskforce in May 2021, Efficio helped the VTF prepare for the shift from a “push” 1 to a “pull” 2 supply model, which would enable the organisation to make longer-term strategic decisions.
- A Scenario Modelling Tool (SMT): Efficio identified the need for a custom-built tool that generates complex demand-supply inventory balancing scenarios, enabling the IBP’s data-driven decisions.
Efficio helped develop and implement a weekly IBP process, powered by our custom-built SMT, enabling:
- Clear stakeholder alignment on objectives and timelines within the VTF and with its suppliers and customers
- Proactive management of any opportunities and risks to the programme
- Longer-term decision making enabled by a data-driven “single source of truth” approach
Efficio’s digital, supply chain, and public sector expertise, combined with its embedding into the VTF, enabled the Taskforce to run over 600 supply and demand scenarios in 12 months to navigate a rapidly evolving COVID-19 landscape.
Key Challenges and Solutions
Transforming the VTF’s decision-making processes
While organisations typically have lengthy IBP cycles (quarterly or monthly), the VTF needed a more dynamic process suited to its unique situation: deploying an extremely high volume of an unusually small range of products with an unprecedented level of international demand amidst a global health crisis and extreme supply chain shortages, ultimately delivering the largest vaccination programme in the UK’s history.
Efficio’s deep supply chain expertise meant it was well-placed to help the VTF rapidly tackle the challenge of implementing a weekly IBP cycle that addressed:
- Strategy reviews to align on the latest Joint Committee on Vaccination and Immunisation’s advice (JCVI), regulatory developments, and planning assumptions
- Demand reviews with each of the UK’s nations (England, Scotland, Wales, Northern Ireland) and it’s Crown Dependencies and Overseas Territories, to standardise, challenge, and validate short- to medium- term demand profiles
- Supply reviews with vaccine developers to agree on clear short-term and long-term delivery plans
- Supply-demand balancing through the Scenario Modelling Tool, modelling varying Supply Chain and inventory risks and opportunities
- Pre-executive reviews with VTF Deputy Directors to refine the outputs and propose recommendations based on scenario modelling
- Executive reviews with the VTF’s leadership team to formalise recommendations for the vaccine programme
The resulting IBP process has become the main framework for senior stakeholder agreements, with regular, transparent, and aligned communication with a streamlined decision-making process. This has brought a high level of confidence in the VTF’s work, setting it up for success to continue delivering millions of life-saving vaccines.
The Vaccine Taskforce has stood at the forefront of accelerating the development, manufacturing, and distribution of vaccines. Efficio’s subject matter expertise, digital capabilities, and on-the-ground “can-do” attitude have been key to building a sustainable data-driven IBP function and Scenario Modelling Tool. This has helped us to continue supplying the country with the vaccines they need, at scale, and at the right time. David Edwards, Deputy Director, Supply Chain Planning and Control, COVID Vaccine Unit
Delivering vaccines with the right data
One of the major challenges the VTF initially faced was that different teams used different data sources, analysed them in different ways, and therefore came to different conclusions. As a part of developing the IBP process, Efficio custom-built an inventory forecasting and modelling tool for the VTF, which enables informed decisions on complex matters including:
- Deliveries : Ensuring stock comes in on time to match demand or deciding what future procurement should look like
- Expiry : Identifying supply batches that would not be used before they arrived, arming the VTF with knowledge on which deliveries could be delayed or cancelled
- Donations : Allowing the UK to respond to donation requests by identifying which deliveries we could afford to divert to other countries, whilst minimising their impact on the UK’s vaccination campaign
The tool takes a supply and a demand profile (pre-agreed with stakeholders through the IBP cycle) and balances one against the other, offering a host of capabilities:
- Detailed supply planning, isolating individual vaccine deliveries at batch level with individual expiries
- Complex demand planning, prioritising vaccines for the most vulnerable population groups
- Defining which vaccines will be used when, based on JCVI or VTF planning assumptions
- A bespoke logic aligned to the VTF’s inventory management rules
- UK-wide operational capacity restrictions
- A granular week-by-week analysis highlighting any supply or demand shortfalls and identifying whether future deliveries will be needed, years in advance
- An interactive set of dashboards which highlights any risks, which leadership may use to scrutinise the outputs
With this level of flexibility comes significant complexity. On top of this, Efficio implemented the tool in a complex government environment, navigating a plethora of cybersecurity risk assessments, and led its digital migration from BEIS to UKHSA infrastructure in 2023.
Another key to the project’s success was Efficio’s management of the SMT throughout its entire lifecycle: identifying the challenges, proposing and designing a solution, creating and testing a custom-built tool, embedding it into the VTF’s IBP cycle, using the tool to run scenarios, and refining it over time based on learnings achieved from usage. Efficio consultants both developed and used the SMT, which gave them a real understanding of the end-users’ needs and challenges.
Overall, the tool brought a significant time saving to the VTF, increasing the number of models that could be run, reducing modelling work by a minimum of 30 hours per week, and ultimately allowing for greater focus on other high-value tasks.
How we can help
If you’re looking for a specialist partner to provide you end-to-end support in your bespoke digital and supply chain endeavours, no matter the scale or complexity of the challenge, please get in touch via the “Contact Us” link on our Supply Chain Improvement page.
We also have a strong track record of helping public sector clients deliver on their procurement and supply chain targets. Please visit our Public Sector page to find out more.
1. Vaccine scarcity during the pandemic meant vaccines were deployed as soon as they were received, with a more reactive system.
2. once vaccines were more readily available, it was less risky to make longer term supply decisions and plan future vaccination campaigns. .
clock This article was published more than 2 years ago
How the U.S. vaccination drive came to rely on an army of consultants
Private contractors cost taxpayers millions while demonstrating few clear results and papering over weaknesses in the country’s public health system

When Gavin Newsom outsourced key components of California’s vaccine rollout to the private sector during the pandemic’s darkest days last winter, the Democratic governor promised the changes would benefit the most vulnerable.
His “number one” reason for handing the reins to Blue Shield of California, an Oakland-based health insurance company, was “equity” — delivering vaccine doses to those at greatest risk, many in communities of color, he said in February.
But the $15 million contract with Blue Shield, plus another $13 million for McKinsey, did not deliver on that promise, according to state and county officials, as well as public health experts.
“Equity is a useful catchphrase, but the work was either nonexistent or completely nontransparent,” said Kim Rhoads, a health advocate and physician researcher at the University of California San Francisco. About 45 percent of eligible Black residents and Latino residents are fully vaccinated in California, according to state data , compared to nearly 60 percent of the eligible White population. “The numbers speak for themselves,” Rhoads said.
California wasn’t alone in using private contractors to manage the vaccination campaign. At least 25 states, along with federal agencies and many cities and counties, hired consulting firms, according to a Washington Post tally. The American vaccination drive came to rely on global behemoths such as McKinsey and Boston Consulting Group (BCG), with downsized state and local health departments and even federal health agencies relying on the private sector to make vaccines available to their citizens, according to hundreds of pages of contract documents, emails and text messages obtained through public records requests.
McKinsey’s role extended beyond California to other states, including Ohio and New Jersey. Deloitte worked in 10 states. BCG received millions of dollars from the federal government to coordinate vaccine planning, while at least 11 states also worked with the company, in some cases paying it to address gaps in federal planning.
Consultants say they helped save lives by supporting overextended public servants with specialized expertise. “Our work helped state decision-makers quickly size up key factors impacting the effective distribution of vaccines,” said McKinsey spokesman Neil Grace. “All our work was based on state-defined priorities, and the data we analyzed was provided by state and local public health authorities.”
But critics question whether such contracts improve government performance, arguing the arrangements are costly and difficult to oversee. Taxpayers have no way to know what precisely they are getting under no-bid contracts worth millions of dollars because the internal documents of private consultancies are not subject to public records laws.
“California has chosen to pay Blue Shield and other consultants millions of dollars to do the job that their public health workforce was already doing, while on reduced salaries due to pandemic-related budget cuts,” said a state health official, who spoke on the condition of anonymity to avoid professional reprisal.
Complicating matters, some contractors contributed to the political campaigns and projects of elected officials who then became clients, prompting allegations of favoritism. Such questions have surrounded the no-bid contract Newsom gave to Blue Shield, which helped finance his political campaigns and signature housing initiative. Aides to Newsom, who is battling a recall election , did not respond to inquiries about previous financial backing from the company. Blue Shield spokesman Matthew Yi said the insurer did not profit from the contract, using the $15 million to cover costs. “Our reward is to help save lives,” he said.
A year and a half into a crisis with no end in sight, experts say the continued dependence on private consultants leaves the nation poorly prepared for other health threats, including the spread of new pathogens that could be more deadly than this coronavirus .
By farming out vital health services, from disease surveillance to contact tracing to vaccine distribution, state and local governments have eroded their own capacity, experts argue, making Americans more reliant on private companies to safeguard their health. The weaknesses are all the more glaring with the delta variant’s devastating march through the United States , enabled partly by insufficient penetration of vaccines .
In some instances, current and former health authorities said consultants gave elected leaders political cover while taking on few substantive tasks. But the deeper problem is when private firms are entrusted with too much, rather than contributing too little, said Robin Taylor Wilson, a former chair of the American Public Health Association’s epidemiology section and an associate professor at Temple University in Philadelphia.
“The contractors leave and we’re not retaining that expertise,” she said. “So the next time an emergency hits, we’re going to have another delayed reaction.”
‘Farming things out … to the wrong folks’
The early rollout of coronavirus vaccines was so chaotic that President Biden insisted there was “no plan to vaccinate most of the country” when he took office in January.
Yet there was a plan, or at least the promise of one, and it relied on Boston Consulting Group. For $4.9 million, the Centers for Disease Control and Prevention made BCG responsible for “driving planning for vaccine distribution and administration,” according to a contract with the firm signed in September 2020 and extended this March for another $4.7 million.
The contract called for the creation of a “robust central infrastructure” supported by “accountability mechanisms” to coordinate federal, state and commercial immunization plans. But as shots moved slowly from points of delivery into people’s arms, and as state and federal officials traded blame, these goals went unrealized — part of a bungled early rollout that dissatisfied two-thirds of Americans, according to Gallup polling .
Neither officials from the federal government nor representatives from BCG would say whether the centralized infrastructure or accountability mechanisms were put into place. Senior CDC leaders did not respond or declined to comment on the record about why they enlisted BCG and whether they were satisfied with the firm’s performance. But the arrangement fit a pattern. Since the pandemic began, BCG has won contracts with the Department of Health and Human Services, the CDC’s parent agency, worth more than $165 million, according to a federal contracting database .
A CDC spokeswoman, Kristen Nordlund, compared BCG to a “counselor in that they have been integral in listening to the needs of the states and helping distill that down so CDC can take action.” The firm’s services focused on data analysis and program management, BCG spokeswoman Nidhi Sinha said in an email.
The lack of clarity about the services rendered under a nearly $10 million contract points to the opacity and overpromising that some current and former officials say undermined the first several months of the vaccination campaign — and that also characterized outsourcing at the state level, according to The Post’s examination of those contracts and of extensive email correspondence.
“It’s another example of the government farming things out, and often to the wrong folks,” said Jeffrey P. Koplan, a former CDC director who now serves as vice president for global health at Emory University.
Others said it was reasonable for the CDC to use consultants given the demands on the agency, whose budget has shrunk by about 10 percent over the past decade, according to a 2019 report from the nonprofit Trust for America’s Health. Likewise, the CDC’s funding for state and local preparedness has been cut by a third since 2003. “Career scientists were really busy,” said Helen Keipp Talbot, a member of the CDC’s Advisory Committee on Immunization Practices.
But some of the consultants lacked expertise in logistics and immunization and would often seek out tasks, creating pressure for CDC staff to “find things for them to do,” said one federal health official who, like others not authorized to discuss the issue, spoke on the condition of anonymity. Another found the consultants essential given the volume of the work, but said not all CDC staff had the management skills to deploy them effectively, causing internal tensions.
Instead of the “targeted program management support” promised in the contract, consultants often performed rudimentary services, such as taking notes during calls between states and the CDC, and then organizing that information in PowerPoint slides for presentations, agency officials said.
Sinha disputed that characterization, saying the CDC project was co-led by an expert with a PhD in infectious-disease epidemiology and an emergency medicine physician. But she declined to discuss the firm’s specific contributions, saying the company doesn’t comment on client work.
While promising to streamline the CDC’s vaccine planning, BCG also dispatched consultants to states responsible for executing delayed and confusing guidance from the federal government. It was not federal authorities, but BCG consultants, who convened officials from seven Northeastern states on Zoom last winter to discuss policies ranging from immunizing people from out of state to accelerating inoculations at nursing homes , according to emails.
The vaccine working group served two aims, Selin Zalma, a BCG consultant with no public health expertise noted on her LinkedIn profile, said in an email to the Massachusetts assistant public health commissioner — “part to learn from each other, part therapy.”
BCG’s vaccine footprint extended across more than a fifth of states, planted not just in those seven states — New York, New Jersey, Connecticut, Massachusetts, Pennsylvania, Delaware and Rhode Island — but also in Idaho, Illinois, Kansas and Washington State, officials confirmed.
The services were expensive, especially for states that spend little on public health.
Pennsylvania, which contracted with BCG for nearly $13 million, needed help because of what Keara Klinepeter, executive deputy health secretary, called the “chronic underfunding” of public health in the state, which ranks 47th in the nation for such spending per capita. BCG examined data to help “tee up” decisions about vaccine eligibility, Klinepeter said in an interview, and instilled a “common-sense approach,” for instance not sending more doses than a provider could handle.
Consultants also kept health authorities abreast of national rankings showing how they compared to other states on vaccine administration, which became a point of pride for elected officials. “Metrics are gradually improving … now at #40,” a BCG partner wrote to Klinepeter and others in early March. “Onwards and upwards we go!”
Fear of the political fallout from those rankings led Ohio officials to rely on consultants. Gov. Mike DeWine (R) was “getting pummeled by the media,” the state’s health director, Stephanie McCloud, told her staff in a Jan. 28 email, “about where Ohio falls nationally and in the Midwest.”
For answers, the state turned to McKinsey, signing a $6 million contract later extended for an additional $2.6 million. To “help us tell our stories,” McCloud wrote, the firm needed access to internal data — “visibility into our numbers: how they are arrived at and what they mean.” She directed staff to “prioritize McKinsey requests.”
Some states succeeded by keeping vaccine planning in-house.
Vermont, the first state to deliver a dose to 80 percent of eligible residents, did not join the Zoom confabs or hire consultants. Neither did Colorado, the state performing the best in the Rocky Mountains, or New Mexico, the leader in the Southwest. These states spend more per capita on public health than does Pennsylvania or Ohio.
Vermont Gov. Phil Scott, a Republican and former construction executive, built trust in the rollout by relying on public officials to manage it, his top aide said.
“Our system has at least one major advantage over a contracted system: Executive branch leaders, at the highest elected and appointed level, accepted the responsibility and the accountability of managing the response,” said Jason Gibbs, Scott’s chief of staff.
Gibbs added, “Our approach is scalable to any size state. I simply do not buy the ‘Vermont was successful because it is small’ hogwash.”
‘Glad they all got a state bailout’
No state went as far as California in handing over the reins to contractors — putting Blue Shield in charge of the network of providers administering shots, with assistance from Kaiser Permanente and McKinsey.
Inside the state’s public health department, some officials first learned these duties were being outsourced when they were instructed to transmit vaccine allocation data to McKinsey consultants, according to a state health official. “We were just told today about this, but have no details about how it’s going to work,” the official told The Post in late January.
Newsom announced the shake-up on Jan. 27, saying doses shipped to the state weren’t reaching residents fast enough.
But health officials in Sacramento and throughout the state were baffled by his response, saying the contract was a solution in search of a problem.
They traced the initial delay to limited vaccine supply and data problems with the statewide vaccine registry, which created the appearance of unused doses. Time spent this spring adjusting to a new allocation system, they said, could have been devoted to building vaccine confidence.
“It’s a political decision, not an operational decision, that was made,” said Santa Clara County Executive Jeff Smith, who is also a physician.
Leaders of the state’s academic health centers — among the state’s biggest vaccine providers — were especially irate. They balked at the requirement that all providers use a state-approved appointment system plagued by glitches, which they said undermined equity. “The most vulnerable and least technologically connected patients will be left behind,” Michael Condrin, chief operating officer for ambulatory care at the University of California at Davis, warned in a February email.
Condrin could not fathom why an insurance company was selected. “Blue Shield?” he wrote to colleagues. “They needed to pick Fed Ex, UPS, or Amazon.” Ann Boynton, a former state official who managed California’s nearly $10 billion health benefits program for public employees and now serves as a top administrator at UC Davis, agreed, noting of Blue Shield, “they’re not experts in distribution and delivery.”
Details of the contract also shocked UC San Diego Health’s associate chief medical officer, Chris Longhurst, who said the health center had managed to run the state’s first mega-site without support from the insurer or from McKinsey.
“Glad they all got a state bailout,” he wrote in an email to colleagues.
Newsom spokeswoman Erin Mellon declined to make the governor available for an interview but said the new setup “allowed for a single solution that was easier for the public to understand when signing up for an appointment, and it allowed for vaccines to be allocated to disproportionately impacted communities in an accountable and measurable way.”
Blue Shield had also played a leading role in earlier chapters of California’s pandemic response, with CEO Paul Markovich co-chairing the state’s testing task force.
A year before the company secured the contract to take over the state’s vaccine rollout, Blue Shield of California Foundation, a philanthropy funded exclusively by the insurer, gave $20 million to support Newsom’s signature housing initiative, Project Homekey, which funds the conversion of hotels into housing for the homeless.
When Newsom ran for governor in 2018, Blue Shield partnered with labor unions to sponsor a committee supporting him that was not subject to spending limits. After Newsom won, Blue Shield contributed $100,000 for his inauguration, while giving $50,000 on his behalf to a charity for firefighters, according to filings with the state.
Mellon, Newsom’s spokeswoman, did not respond to an inquiry about the contributions. But she accused those critical of Blue Shield’s role in the immunization effort of seeking to “rewrite history.”
“The crisis needed a solution and lives depended on us moving faster,” she said.
The solution involved outsourcing not just oversight of the vaccine network, but also the work of building confidence in it — a task that depended on McKinsey. In emails, Marta Green, a state official who coordinated with the contractors, requested that “public-facing docs” devised by McKinsey be “pretty and glossy like their project docs.”
But the public outreach faltered.
Company executives developed a list of outside advisers on vaccine equity listed by racial, ethnic and other categories, according to emails. But in a meeting in February, some of those advisers raised concerns that Blue Shield mainly served affluent residents and was “not known for equity,” asking the state to give money instead to community groups capable of distributing the vaccines, said Rhoads, the UCSF researcher representing the “Black/African American” community.
Sami Gallegos, a state public health spokeswoman, did not address criticisms of the lower vaccine uptake by Black and Latino residents but said the state sent more doses to hard-hit communities and partnered with local groups to boost access. Riverside County’s health director, Kim Saruwatari, who was put forward by Gallegos, said Blue Shield improved data collection and “provided benefit overall.” But the insurer added little in the crucial “ last mile ” of “getting vaccine to the people who are hardest to reach,” she acknowledged.
Now, Blue Shield is in the midst of transitioning to an advisory role, returning oversight of “day-to-day operations” to public officials, according to a recent state notice to providers.
Logistical decisions that were supposed to be facilitated by the contractors sometimes became more confused, according to emails and text messages. In several instances, vaccine allocation errors by McKinsey were caught by public health officials, who declined to speak on the record. But internal messages reflect their alarm about the risk of wasting doses or delaying distribution due to those errors.
“This is truly unacceptable,” a state official messaged colleagues one night in April, as they waited for corrected figures from consultants.
“It’s just crazy how much they’re being paid and for such little benefit,” another replied.
Grace, the McKinsey spokesman, declined to address the criticisms.
Coronavirus: What you need to know
Covid isolation guidelines: Americans who test positive for the coronavirus no longer need to routinely stay home from work and school for five days under new guidance planned by the Centers for Disease Control and Prevention. The change has raised concerns among medically vulnerable people .
New coronavirus variant: The CDC said it is monitoring a variant called KP.2 and does not see evidence it causes more severe illness than other strains. It also identified a second emergent variant, KP.1.1. But it is KP.2 that is leading the pack. Both new variants belong to a group of coronavirus variants dubbed “FLiRT” by scientists.
Latest coronavirus booster: The CDC recommends that anyone 6 months or older gets an updated coronavirus shot , but the vaccine rollout has seen some hiccups , especially for children . Here’s what you need to know about the latest coronavirus vaccines , including when you should get it.

Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Clinical Immunization Safety Assessment (CISA) Project
Vaccine Safety Monitoring - CISA
Current CISA Project Sites
Cisa current activities.
CDC’s Clinical Immunization Safety Assessment (CISA) Project was established in 2001 to address the unmet vaccine safety clinical research needs of the United States.
CISA is a national collaborating network of vaccine safety experts from the CDC’s Immunization Safety Office (ISO), eight medical research centers, and other partners. CDC established the CISA Project to improve the understanding of adverse events following immunization at the individual patient level. CISA provides consultations for U.S. healthcare providers with complex vaccine safety questions about their patients and conducts vaccine safety clinical research.
Healthcare providers or health departments in the United States can request a consultation from CISA COVIDvax for a complex COVID-19 vaccine safety question that is (1) about an individual patient residing in the United States or vaccine safety issue and (2) not readily addressed by CDC or Advisory Committee on Immunization Practices (ACIP) guidelines.
This request can be made through CDC-INFO by:
- Calling 800-CDC-INFO (800-232-4636), or
- Submitting a request via CDC-INFO webform
When making the request, indicate that the request is for CISA Project consultation and it will be forwarded to the CISA COVIDvax clinicians for review.
Provide the following in the form:
- Your name and phone number
- Your health professional training category (i.e., medical doctor, nurse practitioner, pharmacist)
- Indication that a CISA COVIDvax consultation is requested
- Enough clinical background information to enable CDC CISA clinical staff to evaluate the inquiry properly. Do not include patient names or personal identifying health information in the request.
In case of an emergency clinical COVID-19 vaccine safety inquiry , healthcare providers and health department staff can call the CDC Emergency Operations Center (EOC) Watch Desk at (770)-488-7100. The EOC Watch Desk will route emergency inquiries to the CISA COVIDvax on-call staff.

If you are a U.S. healthcare provider with a vaccine safety question unrelated to COVID-19 vaccines about a specific patient residing in the U.S., you can contact CISA at [email protected] to request a case evaluation. This service is provided free of charge. View here for more information.
In case of an emergency clinical vaccine safety inquiry , healthcare providers and health department staff can call the CDC Emergency Operations Center (EOC) Watch Desk at (770)-488-7100. The EOC Watch Desk will route emergency inquiries to the on-call staff.
Collaboration between CDC and eight medical research centers
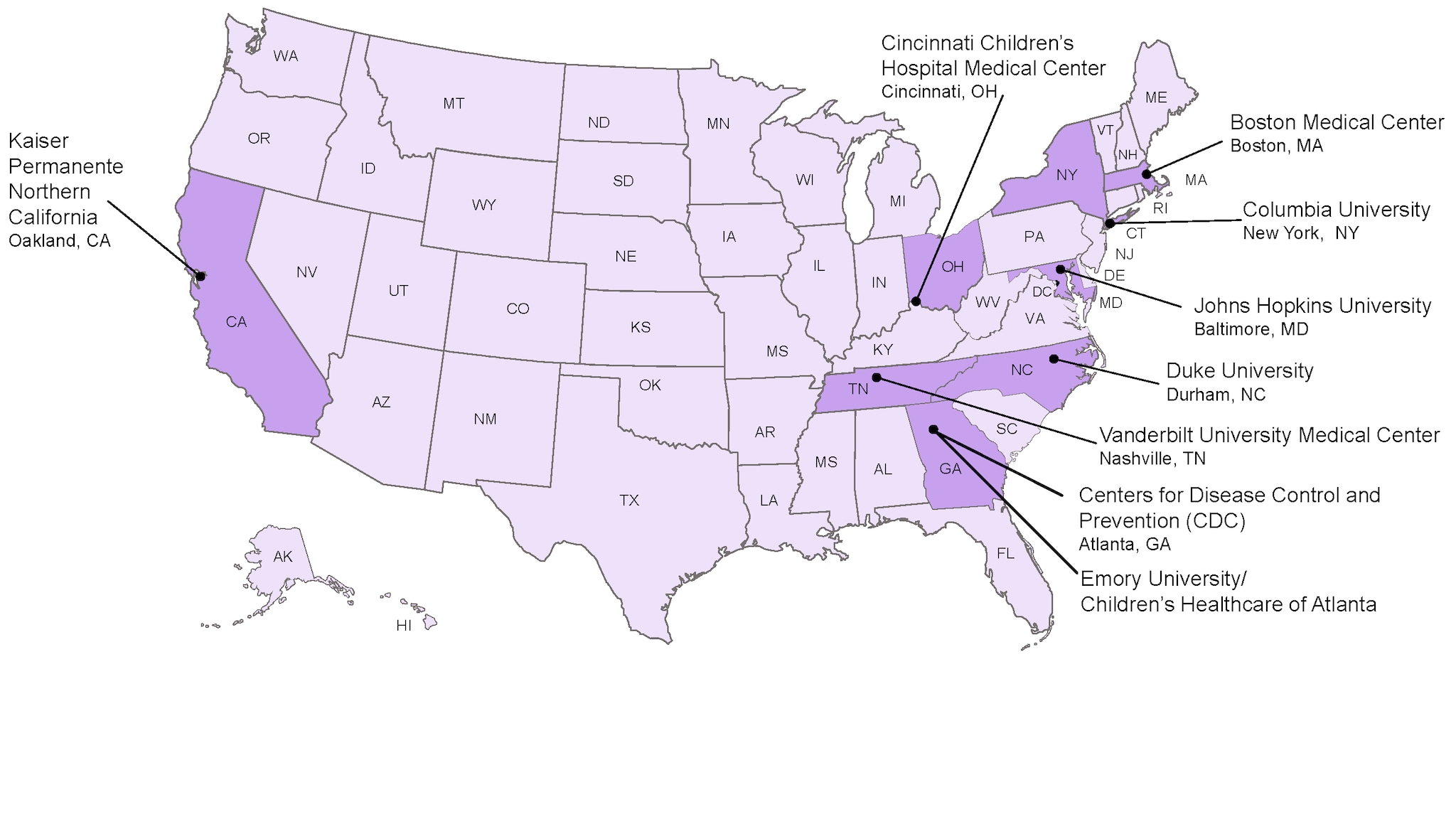
The eight medical research centers that are involved in the CISA network include:
- Boston Medical Center Boston, MA
- Cincinnati Children’s Hospital Medical Center Cincinnati, OH
- Columbia University New York, NY
- Duke University Durham, NC
- Emory University / Children’s Healthcare of Atlanta , Atlanta, GA
- Johns Hopkins University Baltimore, MD
- Kaiser Permanente Northern California Oakland, CA
- Vanderbilt University Medical Center Nashville, TN
The goals of CISA are to:
- Serve as a vaccine safety resource for U.S. healthcare providers with complex vaccine safety questions about a specific patient to assist with immunization decision-making
- Assist CDC and its partners in evaluating emerging vaccine safety issues
- Conduct clinical research studies to better understand vaccine safety and identify preventive strategies for adverse events following immunization
Clinical Case Reviews: CISA provides a clinical case consultation service for U.S. healthcare providers who have vaccine safety questions about a specific patient residing in the United States. CISA provides clinical expertise in various disciplines, including infectious diseases, neurology, allergy, immunology, pediatrics, hematology, and obstetrics/gynecology.
Expert Evaluation of Vaccine Safety Issues: CISA experts provide advice that has led to a broader understanding of vaccine safety issues . CISA has also contributed to clinical guidance and Advisory Committee on Immunization Practices (ACIP) recommendations pertinent to vaccine safety.
Research: To advance knowledge of vaccine safety and inform clinical and public health practice clinical research is essential. CISA has published and continues to develop research studies that address vaccine safety questions.
Current priority areas for CISA research studies include COVID-19 and influenza vaccine safety, and vaccine safety in pregnant women. CISA complements other vaccine safety systems and focuses its efforts on scalable, prospective studies for U.S.-licensed or authorized vaccines. These studies are designed to address clinical vaccine safety questions in targeted or special populations that are often excluded from pre-licensure clinical trials. CISA is well suited to study more common, non-medically attended events (e.g., fever) and to collect biological specimens after vaccination. CISA investigators also have access to special populations (e.g., pregnant women) and specialists who care for these patients.
Public Health Response : CISA has procedures in place to assist in response to vaccine safety emergencies, such as a pandemic, and to assist state health departments during an emergency. Since December 2020, CISA has provided consultation to U.S. healthcare providers and health departments about COVID-19 vaccine safety. Request a COVID-19 CISA Clinical Consultations .
To receive email updates about this page, enter your email address:
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 12 April 2021
Vaccine development for emerging infectious diseases
- Jean-Louis Excler ORCID: orcid.org/0000-0002-6462-5101 1 ,
- Melanie Saville 2 ,
- Seth Berkley 3 &
- Jerome H. Kim ORCID: orcid.org/0000-0003-0461-6438 1
Nature Medicine volume 27 , pages 591–600 ( 2021 ) Cite this article
72k Accesses
205 Citations
92 Altmetric
Metrics details
- Viral infection
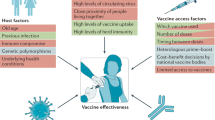
Examination of the vaccine strategies and technical platforms used for the COVID-19 pandemic in the context of those used for previous emerging and reemerging infectious diseases and pandemics may offer some mutually beneficial lessons. The unprecedented scale and rapidity of dissemination of recent emerging infectious diseases pose new challenges for vaccine developers, regulators, health authorities and political constituencies. Vaccine manufacturing and distribution are complex and challenging. While speed is essential, clinical development to emergency use authorization and licensure, pharmacovigilance of vaccine safety and surveillance of virus variants are also critical. Access to vaccines and vaccination needs to be prioritized in low- and middle-income countries. The combination of these factors will weigh heavily on the ultimate success of efforts to bring the current and any future emerging infectious disease pandemics to a close.
Similar content being viewed by others
Looking beyond COVID-19 vaccine phase 3 trials
Leveraging lessons learned from the COVID-19 pandemic for HIV
Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape
Newly emerging and reemerging infectious viral diseases have threatened humanity throughout history. Several interlaced and synergistic factors including demographic trends and high-density urbanization, modernization favoring high mobility of people by all modes of transportation, large gatherings, altered human behaviors, environmental changes with modification of ecosystems and inadequate global public health mechanisms have accelerated both the emergence and spread of animal viruses as existential human threats. In 1918, at the time of the ‘Spanish flu’, the world population was estimated at 1.8 billion. It is projected to reach 9.9 billion by 2050, an increase of more than 25% from the current 2020 population of 7.8 billion ( https://www.worldometers.info ). The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) responsible for the coronavirus disease 2019 (COVID-19) pandemic 1 , 2 , 3 engulfed the entire world in less than 6 months, with high mortality in the elderly and those with associated comorbidities. The pandemic has severely disrupted the world economy. Short of lockdowns, the only means of control have been limited to series of mitigation measures such as self-distancing, wearing masks, travel restrictions and avoiding gatherings, all imperfect and constraining. Now with more than 100 million people infected and more than 2 million deaths, it seems that the addition of vaccine(s) to existing countermeasures holds the best hope for pandemic control. Taken together, these reasons compel researchers and policymakers to be vigilant, reexamine the approach to surveillance and management of emerging infectious disease threats, and revisit global mechanisms for the control of pandemic disease 4 , 5 .
Emerging and reemerging infectious diseases
The appearance of new infectious diseases has been recognized for millennia, well before the discovery of causative infectious agents. Despite advances in development of countermeasures (diagnostics, therapeutics and vaccines), world travel and increased global interdependence have added layers of complexity to containing these infectious diseases. Emerging infectious diseases (EIDs) are threats to human health and global stability 6 , 7 . A review of emerging pandemic diseases throughout history offers a perspective on the emergence and characteristics of coronavirus epidemics, with emphasis on the SARS-CoV-2 pandemic 8 , 9 . As human societies grow in size and complexity, an endless variety of opportunities is created for infectious agents to emerge into the unfilled ecologic niches we continue to create. To illustrate this constant vulnerability of populations to emerging and reemerging pathogens and their respective risks to rapidly evolve into devastating outbreaks and pandemics, a partial list of emerging viral infectious diseases that occurred between 1900 and 2020 is shown in Table 1 .
Although nonemerging infectious diseases (not listed in Table 1 ), two other major mosquito-borne viral infections are yellow fever and dengue. Yellow fever, known for centuries and an Aedes mosquito-borne disease, is endemic in more than 40 countries across Africa and South America. Since 2016, several yellow fever outbreaks have occurred in Angola, Democratic Republic of Congo, Nigeria and Brazil to cite a few 10 , raising major concerns about the adequacy of yellow fever vaccine supply. Four live attenuated vaccines derived from the live attenuated yellow fever strain (17D) 11 and prequalified by the WHO (World Health Organization) are available 12 .
Dengue is an increasing global public health threat with the four dengue virus types (DENV1–4) now cocirculating in most dengue endemic areas. Population growth, an expansion of areas hospitable for Aedes mosquito species and the ease of travel have all contributed to a steady rise in dengue infections and disease. Dengue is common in more than 100 countries around the world. Each year, up to 400 million people acquire dengue. Approximately 100 million people get sick from infection, and 22,000 die from severe dengue. Most seriously affected by outbreaks are the Americas, South/Southeast Asia and the Western Pacific; Asia represents ~70% of the global burden of disease ( https://www.cdc.gov/dengue ). Several vaccines have been developed 13 . A single dengue vaccine, Sanofi Pasteur’s Dengvaxia based on the yellow fever 17D backbone, has been licensed in 20 countries, but uptake has been poor. A safety signal in dengue-seronegative vaccine recipients stimulated an international review of the vaccine performance profile, new WHO recommendations for use and controversy in the Philippines involving the government, regulatory agencies, Sanofi Pasteur, clinicians responsible for testing and administering the vaccine, and the parents of vaccinated children 14 .
Two bacterial diseases, old scourges of humanity, are endemic and responsible for recurrent outbreaks and are increasingly antimicrobial resistant. Cholera, caused by pathogenic strains of Vibrio cholerae , is currently in its seventh global pandemic since 1817; notably, the seventh pandemic started in 1961 15 . Global mortality due to cholera infection remains high, mainly due to delay in rehydrating patients. The global burden of cholera is estimated to be between 1.4 and 4.3 million cases with about 21,000–143,000 deaths per year, mostly in Asia and Africa. Tragic outbreaks have occurred in Yemen and Haiti. Adding to rehydration therapy, antibiotics have been used in the treatment of cholera to shorten the duration of diarrhea and to limit bacterial spread. Over the years, antimicrobial resistance developed in Asia and Africa to many useful antibiotics including chloramphenicol, furazolidone, trimethoprim-sulfamethoxazole, nalidixic acid, tetracycline and fluoroquinolones. Several vaccines have been developed and WHO prequalified; these vaccines constitute a Gavi-supported global stockpile for rapid deployment during outbreaks 16 .
Typhoid fever is a severe disease caused by the Gram-negative bacterium Salmonella enterica subsp. enterica serovar Typhi ( S . Typhi). Antimicrobial-resistant S . Typhi strains have become increasingly common. The first large-scale emergence and spread of a novel extensively drug-resistant (XDR) S . Typhi clone was first reported in Sindh, Pakistan 17 , 18 , and has subsequently been reported in India, Bangladesh, Nepal, the Philippines, Iraq and Guatemala 19 , 20 . The world is in a critical period as XDR S . Typhi has appeared in densely populated areas. The successful development of improved typhoid vaccines (conjugation of the Vi polysaccharide with a carrier protein) with increased immunogenicity and efficacy including in children less than 2 years of age will facilitate the control of typhoid, in particular in XDR areas by decreasing the incidence of typhoid fever cases needing antibiotic treatment 21 , 22 .
A model of vaccine development for emerging infectious diseases
The understanding of emerging infectious diseases has evolved over the past two decades. A look back at the SARS-CoV outbreak in 2002 shows that—despite a small number of deaths and infections—its high mortality and transmissibility caused significant global disruption (see Table 1 ). The epidemic ended as work on vaccines was initiated. Since then, the disease has not reappeared—wet markets were closed and transmission to humans from civets ceased. Consequently, work on vaccines against SARS-CoV ended and its funding was cut. Only a whole inactivated vaccine 23 and a DNA vaccine 24 were tested in phase 1 clinical trials.
Following a traditional research and development pipeline, it takes between 5 and 10 years to develop a vaccine for an infectious agent. This approach is not well suited for the needs imposed by the emergence of a new pathogen during an epidemic. Figure 1 shows a comparison of the epidemic curves and vaccine development timelines between the 2014 West African Ebola outbreak and COVID-19. The 2014 Ebola epidemic lasted more than 24 months with 11,325 deaths and was sufficiently prolonged to enable the development and testing of vaccines for Ebola, with efficacy being shown for one vaccine (of several) toward the end of the epidemic 25 , 26 . What makes the COVID-19 pandemic remarkable is that the whole research and development pipeline, from the first SARS-CoV-2 viral sequenced to interim analyses of vaccine efficacy trials, was accomplished in just under 300 days 27 . Amid increasing concerns about unmitigated transmission during the 2013–2016 Western African Ebola outbreak in mid-2014, WHO urged acceleration of the development and evaluation of candidate vaccines 25 . To ensure that manufacturers would take the Ebola vaccine to full development and deployment, Gavi, the Vaccine Alliance, publicly announced support of up to US$300 million for vaccine purchase and followed that announcement with an advance purchase agreement. Ironically, there had been Ebola vaccines previously developed and tested for biodefense purposes in nonhuman primates, but this previous work was neither ‘ready’ for clinical trials during the epidemic nor considered commercially attractive enough to finish development 28 .
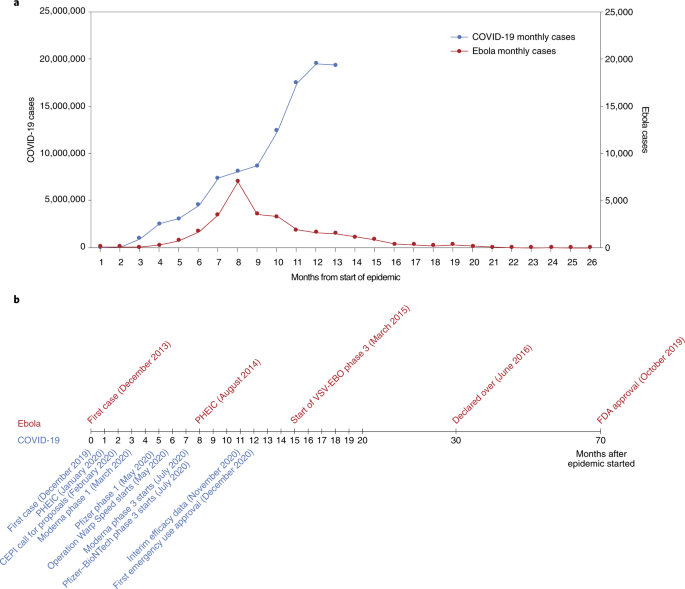
a , The number of months from the onset of the epidemic is shown against the number of reported cases per day. Note that the COVID-19 (left) and Ebola (right) axes are scaled differently. b , Vaccine development timelines for COVID-19 versus Ebola in the context of particular events during the respective outbreaks. PHEIC, public health emergency of international concern.
From these perceived shortcomings in vaccine development during public health emergencies arose the Coalition for Epidemic Preparedness Innovations (CEPI), a not-for-profit organization dedicated to timely vaccine development capabilities in anticipation of epidemics 29 , 30 . CEPI initially focused on diseases chosen from a list of WHO priority pathogens for EIDs—Middle East respiratory syndrome (MERS), Lassa fever, Nipah, Rift Valley fever (RVF) and chikungunya. The goal of CEPI was to advance candidate vaccines through phase 2 and to prepare stockpiles of vaccine against eventual use/testing under epidemic circumstances. CEPI had also prepared for ‘disease X’ by investing in innovative rapid response platforms that could move from sequence to clinical trials in weeks rather than months or years, such as mRNA and DNA technology, platforms that were useful when COVID-19 was declared a global health emergency in January 2020, and a pandemic in March 2020 31 , 32 .
CEPI has been able to fund several vaccine development efforts, among them product development by Moderna, Inovio, Oxford–AstraZeneca and Novavax. Providing upfront funding helped these groups to advance vaccine candidates to clinical trials and develop scaled manufacturing processes in parallel, minimizing financial risk to vaccine developers. The launch of the larger US-funded Operation Warp Speed 33 further provided companies with funding—reducing risks associated with rapid vaccine development and securing initial commitments in vaccine doses.
Vaccine platforms and vaccines for emerging infectious diseases
Vaccines are the cornerstone of the management of infectious disease outbreaks and are the surest means to defuse pandemic and epidemic risk. The faster a vaccine is deployed, the faster an outbreak can be controlled. As discussed in the previous section, the standard vaccine development cycle is not suited to the needs of explosive pandemics. New vaccine platform technologies however may shorten that cycle and make it possible for multiple vaccines to be more rapidly developed, tested and produced 34 . Table 2 provides examples of the most important technical vaccine platforms for vaccines developed or under development for emerging viral infectious diseases. Two COVID-19 vaccines were developed using mRNA technology (Pfizer–BioNTech 35 and Moderna 36 ), both showing safety and high efficacy, and now with US Food and Drug Administration (FDA) emergency use authorization (EUA) 37 , 38 and European Medicines Agency (EMA) conditional marketing authorization 39 , 40 . While innovative and encouraging for other EIDs, it is too early to assert that mRNA vaccines represent a universal vaccine approach that could be broadly applied to other EIDs (such as bacterial or enteric pathogens). While COVID-19 mRNA vaccines are a useful proof of concept, gathering lessons from their large-scale deployment and effectiveness studies still requires more work and time.
While several DNA vaccines are licensed for veterinary applications, and DNA vaccines have shown safety and immunogenicity in human clinical trials, no DNA vaccine has reached licensure for use in humans 41 . Recombinant proteins vary greatly in design for the same pathogen (for example, subunit, virus-like particles) and are often formulated with adjuvants but have longer development times. Virus-like particle-based vaccines used for hepatitis B and human papillomavirus are safe, highly immunogenic, efficacious and easy to manufacture in large quantity. The technology is also easily transferable. Whole inactivated pathogens (for example, SARS-CoV-2, polio, cholera) or live attenuated vaccines (for example, SARS-CoV-2, polio, chikungunya) are unique to each pathogen. Depending on the pathogen, these vaccines also may require biosafety level 3 manufacturing (at least for COVID-19 and polio), which may limit the possibility of technology transfer for increasing the global manufacturing capacity.
Other vaccines are based on recombinant vector platforms, subdivided into nonreplicating vectors (for example, adenovirus 5 (Ad5), Ad26, chimpanzee adenovirus-derived ChAdOx, highly attenuated vectors like modified vaccinia Ankara (MVA)) and live attenuated vectors such as the measles-based vector or the vesicular stomatitis virus (VSV) vector. Either each vector is designed with specific inserts for the pathogen targeted, or the same vector can be designed with different inserts for the same disease. The development of the Merck Ebola vaccine is an example. ERVEBO is a live attenuated, recombinant VSV-based, chimeric-vector vaccine, where the VSV envelope G protein was deleted and replaced by the envelope glycoprotein of Zaire ebolavirus . ERVEBO is safe and highly efficacious, now approved by the US FDA and the EMA, and WHO prequalified, making VSV an attractive ‘platform’ for COVID-19 and perhaps for other EID vaccines 26 although the −70 °C ultracold chain storage requirement still presents a challenge.
Other equally important considerations are speed of development, ease of manufacture and scale-up, ease of logistics (presentation, storage conditions and administration), technology transfer to other manufacturers to ensure worldwide supply, and cost of goods. Viral vectors such as Ad5, Ad26 and MVA have been used in HIV as well as in Ebola vaccines 42 . Finally, regulatory authorities do not approve platforms but vaccines. Each vaccine is different. However, with each use of a specific technology, regulatory agencies may, over time, become more comfortable with underlying technology and the overall safety and efficacy of the vaccine platform, allowing expedited review and approvals in the context of a pandemic 43 . With COVID-19, it meant that the regulatory authorities could permit expedited review of ‘platform’ technologies, such as RNA and DNA, that had been used (for other conditions) and had safety profiles in hundreds of people.
A heterologous prime–boost (HPB) vaccine approach has been extensively explored for HIV 44 and Ebola vaccines 42 . It is being investigated for COVID-19 vaccines with the Oxford–AstraZeneca AZD1222 and Gamaleya Sputnik V COVID-19 vaccines 45 or with the Pfizer–BioNTech vaccine ( https://www.comcovstudy.org.uk ). Other HPB combinations might be considered involving mRNA, DNA, viral vector-based and protein-based vaccines. This may offer the potential benefit of improving the immune response and avoiding mutlidose reactogenicity or anti-vector immune responses. Additionally, people previously vaccinated with the standard regimen (for example, single or two dose) could be offered a booster immunization with a different vaccine. This might mitigate current shortages in vaccines, particularly in low- and middle-income countries (LMICs). Such a matrix of HPB possibilities deserves further consideration by manufacturers, funders and regulators supported by clinical trial studies and assessment of implementation challenges.
Important improvements could speed up availability. Standardized labeling of vaccines so that they can be interchanged across countries and regions, date of production rather than expiration so that shelf life can be tracked, three-dimensional bar coding to allow critical information to be updated, standard indemnification and liability language that would allow agreement with all manufacturers, a no-fault compensation mechanism for serious adverse events related to vaccine administration, and regulatory harmonization are all critical and being worked on as part of the COVID-19 vaccine response and must be optimized for future outbreaks.
The pathway to EUA, licensure and beyond
Big pharmaceutical or biotechnology companies supported by organizations such as CEPI or efforts such as Operation Warp Speed have conducted efficacy trials in countries or regions with the highest SARS-CoV-2 incidence rates. The same groups have also committed funding for large-scale manufacturing at risk. With more than 60 vaccine candidates in clinical trials and another 170 in preclinical development (WHO COVID-19 vaccine landscape) 46 , it is uncertain whether vaccine candidates not in the first wave of testing/approvals will be able to progress to EUA and licensure based solely on results of randomized clinical efficacy trials with clinical endpoints. Regulators and ethics committees may decide that noninferiority clinical trials against comparator vaccines with proven clinical efficacy will be needed for further approvals. Would the demonstration of equivalence between immune responses generated by a new vaccine and those of a clinically proven efficacious vaccine (bridging studies) 47 be accepted by regulatory authorities and replace the need for noninferiority clinical endpoint studies? For that to occur there must be agreement on what are immune correlates of protection (ICP) to COVID-19, and these have yet to be identified. Moreover, it is not yet clear that ICP will translate equally between different vaccine platforms; for example, are immune responses generated by chimpanzee adenovirus the same as those generated by proteins or whole inactivated virus? As incidence rates of a disease decrease over time due to sustained mitigation measures and implementation of vaccination, larger sample sizes in multicountry trials, additional participant accrual time and complex logistics will likely be required for future approvals, compromising the speed of clinical development and increasing cost. Early deployment of scarce doses of still-investigational vaccines (under emergency use listing (EUL) or similar regulatory mechanisms) could bring additional public health benefits if accompanied by firm commitment to maintaining blinded follow-up of participants in ongoing or future placebo-controlled trials until a licensed vaccine is fully deployed in the population 48 .
Randomized controlled trials might underestimate the protective effect of vaccines at the population level. This would occur if the COVID-19 vaccine, in addition to conferring direct protection to individuals, reduces transmission of COVID-19 between individuals, providing protection to unvaccinated individuals and enhanced protection of vaccinated individuals in contact with vaccinated individuals. Vaccine-induced herd protection, which might be crucial to the public health value of a vaccine, will be missed when trials are individually randomized and analyses fail to take account of the geographical distribution of individuals in the population 49 . For these reasons, other clinical trial designs have been proposed once COVID-19 vaccines have achieved licensure via current phase 3 trials to assess how useful the vaccines will be in practice and addressing vaccine effectiveness, including the level of protection of both vaccinated and nonvaccinated individuals in targeted populations 50 .
In the particular context of the COVID-19 pandemic, whether regulatory authorities would require clinical endpoints in future efficacy trials or would consider ICP remains unclear. Clinical endpoints provide increased accuracy with regard to definitive clinical outcomes where outcome-related analyses using ICP are inferential. ICP will contribute to our understanding of viral pathogenesis and immunity, be useful for future approval of vaccines, and help in our understanding of waning of protective immunity following vaccination or infection. The paradox is that the higher the efficacy, the more difficult it will be to identify these correlates because there may not be enough infected vaccine recipients to compare with uninfected vaccine recipients. The analysis of ICP may be possible only in clinical trials showing a lower vaccine efficacy 50 . They would also not provide a rigorous evaluation of long-term safety and the potential for vaccine-associated enhanced respiratory disease 51 .
Pharmacovigilance and surveillance
In May 2020, the 42nd Global Advisory Committee on Vaccine Safety addressed pharmacovigilance preparedness for the launch of the future COVID-19 vaccines 52 . One of their recommendations was that infrastructure and capacity for surveillance of the safety of COVID-19 vaccines should be in place in all countries and engaged before a vaccine is introduced. The WHO’s COVID-19 vaccine safety surveillance manual develops the monitoring and reporting of adverse events following immunization and adverse events of special interest, data management systems and safety communication, and the need for postauthorization safety surveillance studies 53 . One critical element of this surveillance is the duration of the observation period. The implementation of this surveillance will require local, national, regional and global collaboration. While countries should include preparedness plans for COVID-19 vaccine safety in their overall plans for vaccine introduction, building on WHO guidance, it is imperative that the COVID-19 Vaccines Global Access (COVAX) initiative (coordinated by CEPI, Gavi, the Vaccine Alliance, and WHO) works with partners on capacity building and the practical aspects of implementation with technical and training support tailored to the settings.
In view of the public health urgency and the extensive vaccination campaigns foreseen worldwide, the EMA and the national competent authorities in EU member states have prepared themselves for the expected high data volume by putting pharmacovigilance plans specific for COVID-19 vaccines in place. Good pharmacovigilance practices include detailed requirements and guidance on the principles of a risk management plan (RMP) and requirements for vaccines. In addition, core RMP requirements for COVID-19 vaccines have been developed to facilitate and harmonize the preparation of RMPs by companies and their evaluation by assessors. The RMP preparation addresses the planning of the postauthorization safety follow-up of COVID-19 vaccines by marketing authorization holders, while acknowledging uncertainties in the pandemic setting and recommending ways to prepare for pharmacovigilance activities 54 . Similarly, the US Advisory Committee on Immunization Practices (ACIP) initially convened the COVID-19 Vaccine Safety Technical Working Group in June 2020 to advise the ACIP COVID-19 Vaccine Workgroup and the full ACIP on the safety monitoring of COVID-19 vaccines under development and pharmacovigilance postapproval 55 .
Key lessons could be learnt from past situations where new vaccines were introduced in response to pandemic and epidemic emergencies. For the 2009 H1N1 influenza pandemic, few countries had a pandemic preparedness plan that comprehensively addressed vaccine deployment and monitoring of adverse events. The African Vaccine Regulatory Forum, a regional network of regulators and ethics committees, working closely with regulators from other parts of the world, participated in the review of clinical trial protocols and results, the joint monitoring of trials and the joint authorization and deployment of vaccines 56 . Such models can be used to guide pharmacovigilance for the deployment of COVID-19 vaccines, particularly in LMICs with limited resources. The introduction of the first licensed dengue vaccine, while not in the context of an international public health emergency, illustrated a number of lessons for the pharmacovigilance of newly introduced vaccines, particularly the vaccine-associated enhanced disease that was observed 13 , 14 . Due to the significant sequence homology between SARS-CoV-2 and SARS-CoV, antibody-dependent enhancement (ADE) and vaccine-associated enhanced respiratory disease (VAERD) were raised as potential safety issues 57 , 58 . VAERD and ADE have not been described in current reports of SARS-CoV-2 vaccine phase 3 trials. Similarly, VAERD has not been reported in animal challenge studies with SARS-CoV-2 vaccines that conferred protection 50 . With ADE the effect of waning antibody titers after vaccination (or after infection) and potential safety signals are unknown, which emphasizes the importance of follow-up monitoring 57 .
Pregnant women seem to be disproportionately affected during pandemics and emerging pathogen outbreaks 59 , 60 . The Pregnancy Research Ethics for Vaccines, Epidemics, and New Technologies (PREVENT) Working Group has published a roadmap to guide the inclusion of the interests of pregnant women in the development and deployment of vaccines against emerging pathogens 61 , 62 .
Equally important is the surveillance on SARS-CoV-2 circulating strains as well as of other coronaviruses (MERS, seasonal) 63 . SARS-CoV-2 is evolving, with new lineages being reported all over the world. Amongst previous lineages, D614G was shown to have faster growth in vitro and enhanced transmission in small animals, and has subsequently become globally dominant 64 , 65 , 66 . Other variants of concern have been described in the UK (B.1.1.7) 67 and in Brazil (B.1.1.28.1/P1) 68 with higher capacity for transmission and, potentially, lethality. N501Y (B.1.351) isolated in South Africa has an increased affinity for the human ACE2 receptor, which together with the repeated and independent evolution of 501Y-containing lineages 69 strongly argues for enhanced transmissibility. The B.1.351 variant has nine spike alterations; it rapidly emerged in South Africa during the second half of 2020 and has shown resistance to neutralizing antibodies elicited by infection and vaccination with previously circulating lineages. The AstraZeneca COVID-19 vaccine rollout in South Africa was recently halted after the analysis showed minimal efficacy against mild and moderate cases due to B.1.351, which accounts for 90% of the cases in this country 70 . The Novavax vaccine efficacy is 86% against the variant identified in the UK and 60% against the variant identified in South Africa 71 . The efficacy of a single dose of Johnson & Johnson’s Ad26 was 57% against moderate to severe COVID-19 infection in South Africa 72 .
For the many people who have already been infected with SARS-CoV-2 globally and are presumed to have accumulated some level of immunity, new variants such as B.1.351 pose a significant reinfection risk, although vaccine-induced cell-mediated immune responses might mitigate this risk. Scientists do not know how variant lineages will evolve under vaccine-induced immune pressure during the vaccination rollout or whether choices that alter the schedule or dose may impact virus evolution. Whether vaccines efficacious against current circulating strains including the variants identified in the UK and Brazil will keep their efficacy against emerging variants is unknown and deserves enhanced global COVID-19 surveillance in both humans and animals, similar to those developed for influenza. Global influenza surveillance has been conducted through WHO’s Global Influenza Surveillance and Response System since 1952. The Global Influenza Surveillance and Response System is a global mechanism of surveillance, preparedness and response for seasonal, pandemic and zoonotic influenza, a global platform for monitoring influenza epidemiology and disease, and a global alert system for novel influenza viruses and other respiratory pathogens 73 . The Global Initiative on Sharing Avian Influenza Database ( https://www.gisaid.org ) promotes the rapid sharing of data from all influenza viruses and the coronavirus causing COVID-19. These include genetic sequence and related clinical and epidemiological data associated with human viruses, and geographical as well as species-specific data associated with avian and other animal viruses. This molecular epidemiology surveillance should be expanded to all EIDs, particularly the deadliest and most transmissible, as recently described for Ebola 25 . As with influenza, preparations for SARS-CoV-2 vaccine variants should be proactive, with a view that platforms such as mRNA could generate new vaccine strains very rapidly. A clear regulatory pathway for strain change needs discussion with the regulators.
Approval process for licensure and EUA and the risk of speed
Vaccines are classically approved by the country’s national regulatory authority such as the US FDA or by a centralized procedure through the EMA. Once approved for licensure by a stringent or functional national regulatory authority in the country of manufacture, the manufacturing company can submit a dossier for WHO prequalification. However, for SARS-CoV-2 vaccines intended for COVAX, WHO prequalification is not required for initial use if they have received WHO EUL. COVAX is one of three pillars of the Access to COVID-19 Tools Accelerator, which was launched in April 2020 by the WHO, the EC (European Commission) and France. Vaccines receiving WHO EUL can be purchased by UNICEF (United Nations International Children’s Emergency Fund), the largest purchaser of vaccines for Gavi, the Vaccine Alliance. Countries participating in COVAX can access the vaccines through the COVAX Facility either as 1 of the 98 self-financing countries or, for the 92 LMICs, funded through the Gavi COVAX advance market commitment (AMC; https://www.gavi.org ).
In the current pandemic situation, the US FDA is using the EUA process to allow initial use of the vaccines from Pfizer, Moderna and Johnson & Johnson 74 . EMA is taking the approach of conditional approval 75 . The WHO emergency use assessment and listing (EUAL) procedure was developed in the wake of the Ebola virus disease outbreak in Africa to expedite the availability of vaccines. The EUAL was intended as guidance for national regulatory authorities in circumstances when the “community may be more willing to tolerate less certainty about the efficacy and safety of products, given the morbidity and/or mortality of the disease and the shortfall of treatment and/or prevention options” 76 . In early 2020, the WHO issued a revised EUL procedure to assess whether submitted data demonstrate a reasonable likelihood that a vaccine’s quality, safety and performance are acceptable and that the benefits outweigh the foreseeable risks and uncertainties in the context of a public health emergency of international concern 77 . It is intended that vaccines approved through EUAL would eventually go to full review and receive prequalification. WHO member states have the prerogative through their national regulatory authority to use the EUL procedure to authorize the use of unlicensed vaccines.
Some countries have used their national regulatory authorities to secure approval of nationally produced vaccines. The Russian government approved the Ad26 and Ad5 combination-based COVID-19 vaccine, Sputnik V, produced by the Gamaleya Research Institute, for use by individuals aged 60 years and above 78 , 79 . China’s National Medical Products Agency has given conditional approval to the whole inactivated virus BBIBP-CorV COVID-19 vaccine developed by the Beijing Institute of Biological Products, a Sinopharm subsidiary 80 . The authorization allows the general public’s use of the inoculation and comes after the company announced that its vaccine proved 79.3% effective in phase 3 trials 81 . Although the interim results are not yet published, they must have been reviewed and approved by the Chinese Center for Disease Control and Prevention and National Medical Products Agency. The United Arab Emirates was first to approve the Sinopharm vaccine for EUA in early December 2020 based on interim analysis results 82 . The Sinovac CoronaVac vaccine was recently granted conditional approval on the basis of interim efficacy results 83 . The CanSinoBIO COVID-19 vaccine achieved 65.7% efficacy in preventing symptomatic cases in clinical trials (unpublished). The vaccine also showed a 90.98% success rate in stopping severe disease in one of its interim analysis. The vaccine was granted EUA in Mexico and Pakistan 84 .
Manufacturing—how to make more, faster
Production and distribution of hundreds of millions of doses of COVID-19 vaccine within a year of identification of the pandemic pathogen is unprecedented, and while the principles are straightforward, the manufacturing equation is complex and prone to delay. The technical platform utilized to make a vaccine (mRNA, whole inactivated virus, vector, protein with or without adjuvant), the dosage (low, mid, high), the schedule of vaccination (single or two dose) and the manufacturer capability, capacity and reputation are all important considerations for regulators and the WHO. The initial phase of manufacturing scale-up will be a key regulator of vaccine access initially. This could potentially be impacted by vaccine nationalism and the announced bilateral agreements between manufacturers and high-income countries. Companies such as Sinopharm, the Serum Institute of India or Bharat have a huge capacity for production but must supply the gigantic markets of China and India. Delays in the production of several western 85 and Chinese COVID-19 vaccines 86 have already been reported.
The Developing Countries Vaccine Manufacturers Network (DCVMN) was established in 2000 with the mission to increase the availability and affordability of quality vaccines to protect against known and emerging infectious diseases 87 . About 70% of the global EPI vaccine supplies and about 75% procured by UN (United Nations) agencies are produced by DCVMN members 88 . Several technology transfers to DCVMN members have occurred over the past decades to significantly contribute to global health. Following an initial collaboration on the oral cholera vaccine between Sweden and VABIOTECH in Vietnam, the International Vaccine Institute improved the vaccine and then transferred the technology back to VABIOTECH and to several DCVMN members, including Shantha Biotechnics (Shanchol), India; EuBiologics (Euvichol), Republic of Korea; and Incepta (Cholvax), Bangladesh. Shanchol, Euvichol and Euvichol Plus are WHO prequalified and the major contributors to the Gavi-supported global stockpile 16 while Cholvax is marketed in Bangladesh.
For COVID-19 vaccines, several companies have licensed or contracted vaccine production to other manufacturers—AstraZeneca and Novavax with the Serum Institute (India) and SK Bioscience (Korea); Moderna with Lonza (Switzerland), Johnson & Johnson with Biological E (India); and Chinese Sinovac with Butantan (Brazil) and BioFarma (Indonesia). Hopefully the license and contract manufacturing arrangements will allow the production of sufficient doses of vaccines to provide equitable access to at-risk populations globally 89 .
Under the pressures of the pandemic, and with the need for accelerated development of COVID-19 vaccines, optimization of more practical aspects of vaccine implementation, supply and dosing was secondary to the need for rapid proof of concept. COVID-19 mRNA vaccines and the VSV-EBO Ebola vaccine from Merck have a similar requirement for ultracold chain storage. While that might be overcome by relatively simple technology, the scalability of these technologies for universal vaccination is unknown. Additional development is needed to establish the stability of vaccines at higher temperatures (Pfizer mRNA). There is evidence to suggest the presence of some protection against COVID-19 after the first dose; this is critical information not only for COVID-19 but also to frame thinking around other EID vaccines.
Leave no one behind, or the unequal access to vaccines and treatments
The 2030 Agenda for Sustainable Development has the vision to leave no one behind, particularly low-income countries. COVID-19 has seen exceptionalism at either extreme. On the one hand, COVAX aims to provide at least 2 billion doses of WHO-approved vaccine to participating countries by the end of 2021—roughly 20% of each country’s vaccination needs. A total of 92 LMICs will receive vaccine largely through an AMC arranged by Gavi 90 . It now appears that the USA will join COVAX, which recently announced that it had secured agreements for sufficient doses to meet the 2021 target 50 .
Critically, vaccinating people in LMICs will require additional vaccine purchases, at a cost estimated in billions of dollars. In purely economic terms, it appears that such an investment could have substantial benefit for the global economy 91 . On the other hand, COVAX is on track to achieve its goals and poised to start delivering vaccines, and yet no AMC countries had yet been vaccinated when tens of millions of people were already being vaccinated in high-income countries. Among high-income countries, billions of doses have been preordered, several times more than justified by their populations. Can COVAX achieve its target of providing 2 billion doses by 2021, or will manufacturing bottlenecks lead to delay that will allow the coronavirus to continue to circulate in poorer countries and prolong the pandemic? If unable to access COVID-19 vaccines in a timely manner, the 2030 Agenda for Sustainable Development, especially Sustainable Development Goal 3 focusing on health, will be difficult to achieve, and low-income countries will be under extraordinary pressure as the COVID-19 pandemic forces them further into poverty and deeper inequality.
UN Secretary-General António Guterres has again stressed that COVID-19 vaccines must be a global public good, available to everyone, everywhere. “Vaccinationalism is self-defeating and would delay a global recovery” 92 . Modeling studies suggest that if high-income countries take the first 2 billion doses of available COVID-19 vaccines without regard to equity, global COVID-19 deaths will double 93 . Ensuring that all countries have rapid, fair and equitable access to COVID-19 vaccines is the promise of COVAX.
Final remarks
The lessons of the COVID-19 pandemic need to be compiled and applied to the development of future vaccines against emerging infectious diseases and novel pandemic pathogens. The permanent threat of emerging pathogens calls for vigilance, surveillance and preparedness for vaccine development and deployment, all crosscutting activities to be conducted flawlessly between epidemiologists, scientists, developers, human and veterinary health authorities, regulators and funders. Global health stakeholders have learned something about developing vaccines efficiently: they still have much to learn about making and using them with due regard to equity and access.
Zhu, N. et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 382 , 727–733 (2020).
CAS PubMed PubMed Central Google Scholar
Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395 , 497–506 (2020).
Wang, D. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323 , 1061–1069 (2020).
Friedler, A. Sociocultural, behavioural and political factors shaping the COVID-19 pandemic: the need for a biocultural approach to understanding pandemics and (re)emerging pathogens. Glob. Public Health 16 , 17–35 (2021).
Article PubMed Google Scholar
Gully, P. R. Pandemics, regional outbreaks, and sudden-onset disasters. Healthc. Manage. Forum 33 , 164–169 (2020).
Morens, D. M. & Fauci, A. S. Emerging infectious diseases: threats to human health and global stability. PLoS Pathog. 9 , e1003467 (2013).
Article CAS PubMed PubMed Central Google Scholar
Marston, H. D., Folkers, G. K., Morens, D. M. & Fauci, A. S. Emerging viral diseases: confronting threats with new technologies. Sci. Transl. Med. 6 , 253ps210 (2014).
Article Google Scholar
Morens, D. M. & Fauci, A. S. Emerging pandemic diseases: how we got to COVID-19. Cell 182 , 1077–1092 (2020).
Morens, D. M., Daszak, P. & Taubenberger, J. K. Escaping Pandora’s box—another novel coronavirus. N. Engl. J. Med. 382 , 1293–1295 (2020).
Article CAS PubMed Google Scholar
Sacchetto, L., Drumond, B. P., Han, B. A., Nogueira, M. L. & Vasilakis, N. Re-emergence of yellow fever in the neotropics—quo vadis? Emerg. Top. Life Sci. 4 , 399–410 (2020).
PubMed Google Scholar
World Health Organization. Yellow fever. https://www.who.int/biologicals/vaccines/yellow_fever/en/ (2015).
Juan-Giner, A. et al. Immunogenicity and safety of fractional doses of yellow fever vaccines: a randomised, double-blind, non-inferiority trial. Lancet 397 , 119–127 (2021).
Prompetchara, E., Ketloy, C., Thomas, S. J. & Ruxrungtham, K. Dengue vaccine: global development update. Asian Pac. J. Allergy Immunol. 38 , 178–185 (2020).
PubMed CAS Google Scholar
Thomas, S. J. & Yoon, I. K. A review of Dengvaxia ® : development to deployment. Hum. Vaccin. Immunother. 15 , 2295–2314 (2019).
Article PubMed PubMed Central Google Scholar
Hu, D. et al. Origins of the current seventh cholera pandemic. Proc. Natl Acad. Sci. USA 113 , E7730–E7739 (2016).
Shaikh, H., Lynch, J., Kim, J. & Excler, J. L. Current and future cholera vaccines. Vaccine 38 , A118–A126 (2020).
Klemm, E. J. et al. Emergence of an extensively drug-resistant Salmonella enterica serovar Typhi clone harboring a promiscuous plasmid encoding resistance to fluoroquinolones and third-generation cephalosporins. mBio 9 , e00105–18 (2018).
Yousafzai, M. T. et al. Ceftriaxone-resistant Salmonella Typhi outbreak in Hyderabad City of Sindh, Pakistan: high time for the introduction of typhoid conjugate vaccine. Clin. Infect. Dis. 68 , S16–S21 (2019).
Qamar, F. N. et al. Antimicrobial resistance in typhoidal salmonella: surveillance for enteric fever in Asia project, 2016–2019. Clin. Infect. Dis. 71 , S276–S284 (2020).
Marchello, C. S., Carr, S. D. & Crump, J. A. A systematic review on antimicrobial resistance among Salmonella Typhi worldwide. Am. J. Trop. Med. Hyg. 103 , 2518–2527 (2020).
Andrews, J. R. et al. Typhoid conjugate vaccines: a new tool in the fight against antimicrobial resistance. Lancet Infect. Dis. 19 , e26–e30 (2019).
D’Souza, M. P. & Frahm, N. Adenovirus 5 serotype vector-specific immunity and HIV-1 infection: a tale of T cells and antibodies. AIDS 24 , 803–809 (2010).
Lin, J. T. et al. Safety and immunogenicity from a phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antivir. Ther. 12 , 1107–1113 (2007).
Martin, J. E. et al. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial. Vaccine 26 , 6338–6343 (2008).
Jacob, S. T. et al. Ebola virus disease. Nat. Rev. Dis. Primers 6 , 13 (2020).
Wolf, J. et al. Applying lessons from the Ebola vaccine experience for SARS-CoV-2 and other epidemic pathogens. NPJ Vaccines 5 , 51 (2020).
Ball, P. The lightning-fast quest for COVID vaccines—and what it means for other diseases. Nature 589 , 16–18 (2021).
Feldmann, H., Feldmann, F. & Marzi, A. Ebola: lessons on vaccine development. Annu. Rev. Microbiol. 72 , 423–446 (2018).
Gouglas, D., Christodoulou, M., Plotkin, S. A. & Hatchett, R. CEPI: driving progress toward epidemic preparedness and response. Epidemiol. Rev. 41 , 28–33 (2019).
Rottingen, J. A. et al. New vaccines against epidemic infectious diseases. N. Engl. J. Med. 376 , 610–613 (2017).
Sandbrink, J. B. & Shattock, R. J. RNA vaccines: a suitable platform for tackling emerging pandemics? Front. Immunol. 11 , 608460 (2020).
Jackson, N. A. C., Kester, K. E., Casimiro, D., Gurunathan, S. & DeRosa, F. The promise of mRNA vaccines: a biotech and industrial perspective. NPJ Vaccines 5 , 11 (2020).
US Department of Health and Human Health Services. Explaining Operation Warp Speed. https://health.mo.gov/living/healthcondiseases/communicable/novel-coronavirus-lpha/pdf/fact-sheet-operation-warp-speed.pdf 2020).
van Riel, D. & de Wit, E. Next-generation vaccine platforms for COVID-19. Nat. Mater. 19 , 810–812 (2020).
Article PubMed CAS Google Scholar
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383 , 2603–2615 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384 , 403–416 (2021).
US Food and Drug Administration. FDA takes key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19 (2020).
US Food and Drug Administration. FDA takes additional action in fight against COVID-19 by issuing emergency use authorization for second COVID-19 vaccine. https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid (2020).
European Medicines Agency. EMA recommends first COVID-19 vaccine for authorisation in the EU. https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-authorisation-eu (2020).
European Medicines Agency. EMA recommends COVID-19 Vaccine Moderna for authorisation in the EU. https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-moderna-authorisation-eu (2021).
Liu, M. A. A Comparison of plasmid DNA and mRNA as vaccine technologies. Vaccines 7 , 37 (2019).
Article CAS PubMed Central Google Scholar
Pollard, A. J. et al. Safety and immunogenicity of a two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Europe (EBOVAC2): a randomised, observer-blind, participant-blind, placebo-controlled, phase 2 trial. Lancet Infect. Dis . https://doi.org/10.1016/S1473-3099(20)30476-X (2020).
Adalja, A. A., Watson, M., Cicero, A. & Inglesby, T. Vaccine platform technologies: a potent tool for emerging infectious disease vaccine development. Health Secur. 18 , 59–60 (2020).
Excler, J. L. & Kim, J. H. Novel prime-boost vaccine strategies against HIV-1. Expert Rev. Vaccines 18 , 765–779 (2019).
European Pharmaceutical Review. AstraZeneca to test combination of AZD1222 and Sputnik V vaccines. https://www.europeanpharmaceuticalreview.com/news/136683/astrazeneca-to-test-combination-of-azd1222-and-sputnik-v-vaccines/ (2020).
World Health Organization. Draft landscape and tracker of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (2021).
Fritzell, B. Bridging studies. Dev. Biol. Stand. 95 , 181–188 (1998).
CAS PubMed Google Scholar
WHO Ad Hoc Expert Group on the Next Steps for Covid-19 Vaccine Evaluation. Placebo-controlled trials of Covid-19 vaccines—why we still need them. N. Engl. J. Med . 384 , e2 (2021).
Clemens, J., Brenner, R., Rao, M., Tafari, N. & Lowe, C. Evaluating new vaccines for developing countries. Efficacy or effectiveness? JAMA 275 , 390–397 (1996).
Kim, J. H., Marks, F. & Clemens, J. D. Looking beyond COVID-19 vaccine phase 3 trials. Nat. Med. 27 , 205–211 (2021).
Follmann, D. et al. Assessing durability of vaccine effect following blinded crossover in COVID-19 vaccine efficacy trials. Preprint at https://www.medrxiv.org/content/10.1101/2020.12.14.20248137v1 (2020).
World Health Organization. Global Advisory Committee on Vaccine Safety, 27–28 May 2020. https://www.who.int/vaccine_safety/committee/reports/May_2020/en/ (2020).
World Health Organization. COVID-19 vaccines: safety surveillance manual. https://apps.who.int/iris/handle/10665/338400 (2020).
European Medicines Agency. Pharmacovigilance plan of the EU Regulatory Network for COVID-19 Vaccines. https://www.ema.europa.eu/en/documents/other/pharmacovigilance-plan-eu-regulatory-network-covid-19-vaccines_en.pdf (2020).
Lee, G. M., Romero, J. R. & Bell, B. P. Postapproval vaccine safety surveillance for COVID-19 vaccines in the US. JAMA 324 , 1937–1938 (2020).
Kieny, M. P. & Rago, L. Regulatory policy for research and development of vaccines for public health emergencies. Expert Rev. Vaccines 15 , 1075–1077 (2016).
Lambert, P. H. et al. Consensus summary report for CEPI/BC March 12–13, 2020 meeting: assessment of risk of disease enhancement with COVID-19 vaccines. Vaccine 38 , 4783–4791 (2020).
Zellweger, R. M., Wartel, T. A., Marks, F., Song, M. & Kim, J. H. Vaccination against SARS-CoV-2 and disease enhancement - knowns and unknowns. Expert Rev. Vaccines 19 , 691–698 (2020).
Creanga, A. A. et al. Severity of 2009 pandemic influenza A (H1N1) virus infection in pregnant women. Obstet. Gynecol. 115 , 717–726 (2010).
Menendez, C., Lucas, A., Munguambe, K. & Langer, A. Ebola crisis: the unequal impact on women and children’s health. Lancet Glob. Health 3 , e130 (2015).
The PREVENT Working Group. Pregnant women and vaccines against emerging epidemic threats: ethics guidance on preparedness, research and response. http://vax.pregnancyethics.org/prevent-guidance (2018).
Krubiner, C. B. et al. Pregnant women and vaccines against emerging epidemic threats: ethics guidance for preparedness, research, and response. Vaccine 39 , 85–120 (2021).
Edridge, A. W. D. et al. Seasonal coronavirus protective immunity is short-lasting. Nat. Med. 26 , 1691–1693 (2020).
Plante, J. A. et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature https://doi.org/10.1038/s41586-020-2895-3 (2020).
Korber, B. et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell 182 , 812–827 (2020).
Hou, Y. J. et al. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science 370 , 1464–1468 (2020).
Volz, E. et al. Evaluating the effects of SARS-CoV-2 spike mutation D614G on transmissibility and pathogenicity. Cell 184 , 64–75.e11 (2021).
Faria, N. R. Genomic characterisation of an emergent SARS-CoV-2 lineage in Manaus: preliminary findings. Virological https://virological.org/t/586 (2020).
Wibmer, C. K. et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Preprint at bioRxiv https://doi.org/10.1101/2021.01.18.427166 (2021).
BBC. Covid: South Africa halts AstraZeneca vaccine rollout over new variant. https://www.bbc.com/news/world-africa-55975052 (2021).
Mahase, E. Covid-19: Novavax vaccine efficacy is 86% against UK variant and 60% against South African variant. BMJ 372 , n296 (2021).
Johnson & Johnson. Johnson & Johnson announces single-shot Janssen COVID-19 vaccine candidate met primary endpoints in interim analysis of its phase 3 ENSEMBLE trial. https://www.jnj.com/johnson-johnson-announces-single-shot-janssen-covid-19-vaccine-candidate-met-primary-endpoints-in-interim-analysis-of-its-phase-3-ensemble-trial (2021).
World Health Organization. Global Influenza Surveillance and Response System (GISRS). https://www.who.int/influenza/gisrs_laboratory/en/
US Food and Drug Administration. Emergency use authorization. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization (2021).
European Medicines Agency. Conditional marketing authorization. https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/conditional-marketing-authorisation (2020).
Smith, M. J., Ujewe, S., Katz, R. & Upshur, R. E. G. Emergency use authorisation for COVID-19 vaccines: lessons from Ebola. Lancet 396 , 1707–1709 (2020).
World Health Organization. Emergency use listing procedure. https://www.who.int/publications/m/item/emergency-use-listing-procedure (2020).
Pharmaceutical Technology. Russia approves Sputnik V Covid-19 vaccine for senior citizens. https://www.pharmaceutical-technology.com/news/russia-sputnik-senior-citizens/ (2021).
Logunov, D. Y. et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet https://doi.org/10.1016/S0140-6736(21)00234-8 (2021).
Xinhuanet. China approves first self-developed COVID-19 vaccine. http://www.xinhuanet.com/english/2020-12/31/c_139632402.htm (2020).
Sinopharm. China grants conditional market approval for Sinopharm CNBG’s COVID-19 vaccine. http://www.sinopharm.com/en/s/1395-4173-38862.html (2021).
CNBC. Dubai is offering the Pfizer vaccine to residents for free in addition to China’s Sinopharm shot. https://www.cnbc.com/2020/12/23/dubai-offering-pfizer-sinopharm-covid-vaccines-to-residents-for-free.html (2020).
Reuters. China approves Sinovac Biotech COVID-19 vaccine for general public use. https://www.reuters.com/article/us-health-coronavirus-vaccine-sinovac-idUSKBN2A60AY (2021).
Reuters. Pakistan approves Chinese CanSinoBIO COVID vaccine for emergency use. https://www.reuters.com/article/us-health-coronavirus-pakistan-vaccine/pakistan-approves-chinese-cansinobio-covid-vaccine-for-emergency-use-idUSKBN2AC1FG (2021).
Euronews. Coronavirus: 15m people in the UK have now had their first COVID jab—what about wider Europe? https://www.euronews.com/2021/02/14/coronavirus-15m-people-in-the-uk-have-now-had-their-first-covid-jab-what-about-wider-europ (2021).
Deng, C. & Malsin, J. China’s Covid-19 vaccine makers struggle to meet demand. The Wall Street Journal https://www.wsj.com/articles/chinas-covid-19-vaccine-makers-struggle-to-meet-demand-11612958560 (10 February 2021).
Pagliusi, S. et al. Developing countries vaccine manufacturers network: doing good by making high-quality vaccines affordable for all. Vaccine 31 , B176–B183 (2013).
Jadhav, S., Gautam, M. & Gairola, S. Role of vaccine manufacturers in developing countries towards global healthcare by providing quality vaccines at affordable prices. Clin. Microbiol. Infect. 20 , 37–44 (2014).
Kim, J. H. SARS-CoV-2 vaccine development, access, and equity. J. Exp. Med. 217 , e20201288 (2020).
Nhamo, G., Chikodzi, D., Kunene, H. P. & Mashula, N. COVID-19 vaccines and treatments nationalism: challenges for low-income countries and the attainment of the SDGs. Glob. Public Health 16 , 319–339 (2020).
World Bank. Global economy to expand by 4% in 2021; vaccine deployment and investment key to sustaining the recovery. https://www.worldbank.org/en/news/press-release/2021/01/05/global-economy-to-expand-by-4-percent-in-2021-vaccine-deployment-and-investment-key-to-sustaining-the-recovery (2021).
UN News. WHO chief warns against ‘catastrophic moral failure’ in COVID-19 vaccine access. https://news.un.org/en/story/2021/01/1082362 (18 January 2021).
Chinazzi, M. et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science 368 , 395–400 (2020).
Download references
Author information
Authors and affiliations.
International Vaccine Institute, Seoul, Republic of Korea
Jean-Louis Excler & Jerome H. Kim
Coalition for Epidemic Preparedness Innovations (CEPI), London, UK
Melanie Saville
Gavi, the Vaccine Alliance, Geneva, Switzerland
Seth Berkley
You can also search for this author in PubMed Google Scholar
Contributions
J.-L.E., M.S., S.B. and J.H.K. equally contributed to the synopsis of the manuscript. J.-L.E. and J.H.K. wrote the text and tables of the manuscript. J.H.K. provided the figure. M.S. and S.B. edited the manuscript.
Corresponding authors
Correspondence to Jean-Louis Excler or Jerome H. Kim .
Ethics declarations
Competing interests.
J.-L.E. is a consultant for vaccine safety for the Brighton Collaboration, Johnson & Johnson and the US Military HIV Research Program. J.H.K. is a consultant to SK Bioscience. M.S. has a financial interest in Sanofi (shares). S.B. does not have any financial or nonfinancial conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peer review information Nature Medicine thanks Robert Carnahan and David Morens for their contribution to the peer review of this work. Joao Monteiro was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Excler, JL., Saville, M., Berkley, S. et al. Vaccine development for emerging infectious diseases. Nat Med 27 , 591–600 (2021). https://doi.org/10.1038/s41591-021-01301-0
Download citation
Received : 27 January 2021
Accepted : 01 March 2021
Published : 12 April 2021
Issue Date : April 2021
DOI : https://doi.org/10.1038/s41591-021-01301-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Parental hesitancy toward children vaccination: a multi-country psychometric and predictive study.
- Hamid Sharif-Nia
- Fatemeh Khoshnavay Fomani
BMC Public Health (2024)
Nanotechnology’s frontier in combatting infectious and inflammatory diseases: prevention and treatment
- Yujing Huang
- Xiaohan Guo
Signal Transduction and Targeted Therapy (2024)
BCG-booster vaccination with HSP90-ESAT-6-HspX-RipA multivalent subunit vaccine confers durable protection against hypervirulent Mtb in mice
- Kee Woong Kwon
- Han-Gyu Choi
- Sung Jae Shin
npj Vaccines (2024)
A novel epidemic network model incorporating infections induced by exposed individuals and environments
- Jingyuan Li
- Yijun Zhang
- Weiwei Xing
Nonlinear Dynamics (2024)
Potentiating humoral and cellular immunity using a novel hybrid polymer-lipid nanoparticle adjuvant for HBsAg-VLP vaccine
Journal of Nanobiotechnology (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Europe PMC Author Manuscripts
Case-control vaccine effectiveness studies: Preparation, design, and enrollment of cases and controls
Jennifer r. verani.
a National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, 1600 Clifton Rd., Atlanta, GA, USA
Abdullah H. Baqui
b International Center for Maternal and Newborn Health, Johns Hopkins Bloomberg School of Public Health, 615 N Wolfe St, Baltimore, MD, USA
Claire V. Broome
c Rollins School of Public Health Emory University, 1518 Clifton Rd, Atlanta, GA, USA
Thomas Cherian
d Department of Immunizations, Vaccines and Biologicals, World Health Organization, 20 Avenue Appia, 1211 Geneva, Switzerland
Cheryl Cohen
e Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases, 1 Modderfontein Road, Sandringham, Johannesburg, South Africa
Jennifer L. Farrar
Daniel r. feikin.
f International Vaccine Access Center, Johns Hopkins Bloomberg School of Public Health, 615 N Wolfe St, Baltimore, MD, USA
Michelle J. Groome
g Respiratory and Meningeal Pathogens Unit, University of Witwatersrand, Richard Ward, 1 Jan Smuts Ave, Braamfontein, Johannesburg, South Africa
Rana A. Hajjeh
Hope l. johnson.
h Monitoring & Evaluation, Policy & Performance, GAVI Alliance, Chemin des Mines 2, 1202 Geneva, Switzerland
Shabir A. Madhi
Kim mulholland.
i Murdoch Children’s Research Institute, Royal Children’s Hospital, 50 Flemington Rd, Parkville, VIC 3052, Australia
j Department of Infectious Disease Epidemiology, Faculty of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, Keppel St, London WC1E 7HT, UK
Katherine L. O’Brien
Umesh d. parashar, manish m. patel, laura c. rodrigues, mathuram santosham, j. anthony scott.
k KEMRI-Wellcome Trust Research Programme, P.O. Box 230-80108, Kilifi, Kenya
Peter G. Smith
l MRC Tropical Epidemiology Group, London School of Tropical Medicine and Hygiene, London, UK
Halvor Sommerfelt
m Centre of Intervention Science in Maternal and Child Health and Centre for International Health, University of Bergen, P.O. Box 7800, Bergen, Norway
n Department of International Public Health, Norwegian Institute of Public Health, PO Box 4404, Nydalen, Oslo, Norway
Jacqueline E. Tate
J. chris victor.
o PATH, 2201 Westlake Avenue, Seattle, WA, USA
Cynthia G. Whitney
Anita k. zaidi.
p Aga Khan University, Stadium Rd, Karachi, Pakistan
Elizabeth R. Zell
Case-control studies are commonly used to evaluate effectiveness of licensed vaccines after deployment in public health programs. Such studies can provide policy-relevant data on vaccine performance under ‘real world’ conditions, contributing to the evidence base to support and sustain introduction of new vaccines. However, case-control studies do not measure the impact of vaccine introduction on disease at a population level, and are subject to bias and confounding, which may lead to inaccurate results that can misinform policy decisions. In 2012, a group of experts met to review recent experience with case-control studies evaluating the effectiveness of several vaccines; here we summarize the recommendations of that group regarding best practices for planning, design and enrollment of cases and controls. Rigorous planning and preparation should focus on understanding the study context including healthcare-seeking and vaccination practices. Case-control vaccine effectiveness studies are best carried out soon after vaccine introduction because high coverage creates strong potential for confounding. Endpoints specific to the vaccine target are preferable to non-specific clinical syndromes since the proportion of non-specific outcomes preventable through vaccination may vary over time and place, leading to potentially confusing results. Controls should be representative of the source population from which cases arise, and are generally recruited from the community or health facilities where cases are enrolled. Matching of controls to cases for potential confounding factors is commonly used, although should be reserved for a limited number of key variables believed to be linked to both vaccination and disease. Case-control vaccine effectiveness studies can provide information useful to guide policy decisions and vaccine development, however rigorous preparation and design is essential.
1. Introduction
Many new vaccines have been introduced into public health programs over the past decade and others are under development. Vaccines are generally licensed based on safety and efficacy as measured in randomized controlled trials. Once vaccines are introduced into public health programs, their performance under “real world” conditions also needs assessment [ 1 ], including among populations with subgroups that may have been excluded from pre-licensure trials (e.g., malnourished or HIV-infected), with more variable dosing schedules (e.g. age at administration, interval between doses, number of doses), against outcomes not included in randomized clinical trials (e.g. strain-specific protection or mortality), and over more extended periods of time.
Furthermore, some vaccines are licensed based on immunologic correlates of protection [ 2 ], and post-licensure evaluations provide important information about protection against disease endpoints. After vaccines have been introduced, conducting placebo-controlled trials is generally not considered ethical [ 3 ]. Observational post-licensure evaluations are important to underpin policy decisions on vaccine introduction, to optimize the vaccine program implementation, and to provide evidence for sustaining vaccine use and investment from governments and donors.
2. Efficacy, effectiveness and impact
‘Efficacy’, ‘effectiveness’ and ‘impact’ are sometimes used interchangeably in everyday language, but in the context of vaccine studies the terms have come to be used with distinctly different meanings (although not entirely consistently) [ 4 – 7 ]. Their usage in this document is defined below:
Efficacy is the percentage by which the rate of the target disease among those who are vaccinated according to the recommended schedule is reduced compared to the rate in similar unvaccinated persons. This is generally measured in the context of a placebo-controlled randomized trial as the “per protocol” efficacy (i.e. excluding persons who did not receive the recommended schedule), because the intention is to establish the biologic performance capacity of the product under optimal conditions.
Effectiveness measures the same percent reduction in the rate of disease as efficacy, but in the context of routine, real-world use of the vaccine. Vaccine effectiveness may be similar to the efficacy as measured in clinical trials. However, it often differs in magnitude because in routine use the population vaccinated includes some who may have a less robust immune response, and program implementation (e.g. cold-chain maintenance, dosing schedules) is more variable than in clinical trial settings.
Impact quantifies the reduction in disease at a population level following introduction of the vaccine [ 7 ]. Impact can be expressed as a percentage decline or as an absolute change in the rate of disease. It is determined by a combination of vaccine effectiveness, vaccine coverage in the population, and any herd effect (i.e. vaccination of part of the population leading to reduced transmission of the infection in the community, and thus lowered risk of disease in both vaccinated and unvaccinated persons) [ 8 ].
Studies of vaccine efficacy, effectiveness, and impact may use non-disease outcomes such as colonization as endpoints; however disease endpoints are more commonly used.

3. Observational methods to assess vaccine effectiveness and impact
Several observational epidemiologic methods are used to assess the impact of vaccination programs and the effectiveness of vaccines in routine use [ 4 , 5 , 9 ]. Examination of trends in disease incidence before and after vaccine introduction measures vaccination program impact. However this approach requires a stable, unchanged disease surveillance system before and after the introduction of vaccine. Interpretation of such studies can be challenging because of changes in measured disease incidence or the true disease incidence unrelated to vaccination. For example, changes in healthcare seeking behaviors can increase or decrease measured disease incidence, concomitant implementation of non-vaccine interventions can reduce disease risk, and natural temporal variation in disease incidence unrelated to vaccination can also occur.
Vaccine effectiveness is generally measured through either cohort or case-control approaches. Cohort studies estimate effectiveness by comparing the incidence of disease among vaccinated and unvaccinated persons. Cohort studies require large samples, may be costly, and accurate data on the vaccination status and potential confounding variables for an entire population are often not available, especially in resource-poor settings. The cohort design may not be practical for diseases with low incidence. Case-control studies assess effectiveness by comparing the odds of antecedent vaccination among individuals who develop the target disease (cases) and among a group of individuals without the disease (controls) who are representative of the population from which the cases arise [ 10 , 11 ]. Because efforts are focused on accurately ascertaining disease status and vaccination history for a relatively small number of cases and controls (compared to cohort studies), the method can be resource-efficient and particularly useful for diseases or outcomes that are relatively uncommon. The screening method, in which the vaccination status of cases is compared to population-level vaccine coverage, is another approach for assessing vaccine effectiveness [ 12 ]; however accurate data on the proportion of the population vaccinated is often not available in resource-poor settings.
In recent years, case-control studies have been conducted to evaluate the effectiveness of Haemophilus influenzae (Hib) [ 13 – 24 ], pneumococcal [ 25 – 32 ], influenza [ 33 ], rotavirus [ 34 – 47 ], and cholera [ 48 – 50 ] vaccines. Despite being widely used to evaluate vaccine performance, the case-control methodology is susceptible to bias and confounding [ 51 , 52 ]. Because vaccine effectiveness estimates often impact policy decisions and donor support for vaccines their validity is important. In November 2012, a group of experts met to review recent experience with case-control studies evaluating effectiveness of several vaccines. We summarize the recommendations from that group regarding best practices for the preparation and design of such studies, as well as the enrollment of cases and controls. (Data collection, vaccine status ascertainment, analysis and reporting of results are discussed in a separate paper [insert reference for paired manuscript].) While discussions of case-control methodology in general can be found elsewhere [ 52 , 53 ], here we focus on the application of these methods to evaluate vaccine effectiveness in resource-constrained settings.
4. Methodological aspects of case-control vaccine effectiveness studies
4.1. preparation for case-control vaccine effectiveness studies.
Although data collection for case-control vaccine effectiveness studies begins after vaccine implementation, rigorous study planning and preparation, focused on understanding the local study context, should begin well before cases and controls are recruited, ideally a year or more beforehand. In the preparatory period, it is important to assess factors that may affect case ascertainment, such as healthcare-seeking behavior, barriers to care, determinants of hospitalization and diagnostic capacities. Different potential sources of control groups should be considered to identify the group least likely to lead to bias; for example, if cases are identified from a source population that includes large slum areas and controls are recruited only from more wealthy areas, the controls may be very different from cases in ways that could bias effectiveness estimation. Preparation should also include assessing vaccine coverage, factors associated with non-vaccination, and the ability to obtain valid, complete data on vaccination status among the intended study population. Identifying key potential confounders and the most accurate ways to measure them are also essential components of study preparation.
Prior studies of the outcome of interest in the local study context may inform case definitions and strategies for recruitment. For example, a “vaccine-probe” study in South Africa found that a widely used case definition for likely bacterial pneumonia, based on standardized interpretations of pediatric chest radiographs, underestimated the burden of pneumonia that could be prevented with the pneumococcal conjugate vaccine [ 54 ]; therefore a subsequent case-control vaccine effectiveness study used a modified case definition aimed at better capturing probable pneumococcal pneumonia cases in that setting [ 32 ]. Health care utilization studies provide important information on where cases might be identified for a case-control vaccine effectiveness study, as well as cases that may be missed by health facility-based studies [ 55 – 57 ]. Vaccine coverage surveys or analysis of immunization data from Demographic and Health Surveys or Multiple Indicator Cluster Surveys can offer insight on the completeness and timeliness of routine immunization in the intended study population, the availability of documented vaccine histories, and factors associated with non-vaccination that may be important confounders for a vaccine effectiveness study [ 58 ]. In the context of a Hib vaccine study in the Ukraine it was noted that providers considered underlying immunocompromising conditions to be a contraindication for receiving the vaccine; thus children at increased risk for Hib disease were less likely to receive the vaccine, potentially leading to an overestimation of the actual effectiveness in the full population [ 23 ]. Identifying important factors that influence likelihood of vaccination during the planning phase can help avoid bias during study implementation.
4.2. Sample size and feasibility
During the preparatory phase, the feasibility of achieving adequate enrollment during the planned study timeline must be assessed. The desired study size is determined by the expected effectiveness (with lower effectiveness requiring larger sample sizes), anticipated vaccine coverage in the study population, and the number of controls enrolled per case [ 9 ]. Study size may be based on statistical “power” (i.e. testing the hypothesis that the vaccine is significantly protective) or precision-based (i.e. targeting a certain width of confidence interval) [ 59 ]. Sample size calculations should allow for missing data, adjustment for confounding, and the expected prevalence of incomplete vaccination (e.g. one or two doses of a three-dose schedule). Once the desired sample size is determined, an assessment of the ability to enroll that target number must take into account the potential for declining incidence of disease over time following vaccine rollout, refusals, age-eligibility for vaccination, and ability to collect vaccination histories. Thus, simple sample size calculations should be considered as the minimum necessary number needed to assess the primary outcome, but enrollment beyond that minimum is likely required for a robust analysis and the ability to address secondary objectives (e.g. effectiveness in subgroups, effectiveness of incomplete schedule, and strain-specific effectiveness).
Several planned case-control studies of Hib vaccine effectiveness were not completed because of lower than anticipated enrollment attributable to rapid declines in invasive Hib disease burden following vaccine introduction (R. Hajjeh, personal communication, November 16, 2012). Case-control studies may have limited power if the number of available cases is small, which can occur following introduction of highly efficacious vaccines, in settings that achieve rapid, high coverage and significant herd effects.
4.3. Timing of study and vaccine coverage
Case-control studies are most likely to be useful when the vaccine coverage is between 20 and 80% [ 9 ]. At either very low or very high coverage, unvaccinated persons are likely to differ from the general population in ways that may be associated with increased or decreased risk of disease, independent of vaccination. These differences may be more pronounced where coverage levels are driven by individual factors (e.g. lack of access to care, mistrust of medical system) rather than programmatic factors (e.g. vaccine stock-outs). Results of several rotavirus case-control studies were difficult to interpret due to high coverage (>90%) soon after vaccine introduction [ 43 , 60 ]. Settings with high vaccine coverage (e.g. greater than 85–90%) are not suitable for case-control vaccine effectiveness studies because of the strong potential for confounding. High coverage also increases sample size requirements because more observations are required to detect a significant difference in vaccination between cases and controls. Furthermore, high coverage can lead to a rapid decline in cases of the disease of interest if vaccine efficacy is high. Thus, in contexts where the coverage is expected to increase quickly following vaccine introduction, it may be preferable to conduct a study in a short time period after introduction rather than a prolonged study with a slower rate of enrolment.
4.4. Study endpoints
Endpoints for case-control vaccine effectiveness studies range from highly specific for the vaccine target (e.g. invasive pneumococcal disease caused by a serotype included in the vaccine or rotavirus diarrhea) to non-specific (e.g. clinical syndromes such as pneumonia or acute gastroenteritis). Pathogen-specific endpoints have precise case definitions that are generally not open to interpretation or variability in the field application. Non-specific outcomes, however, may be of greater interest from a policy perspective because of the larger associated burden of disease, albeit the fraction of that disease preventable by the vaccine may be low. Yet effectiveness estimates from case-control studies of non-specific outcomes can be confusing or misleading. For example, a systematic review of Hib vaccine effectiveness noted that case-control studies using radiologically confirmed pneumonia endpoints may have overestimated effectiveness (compared to clinical trial estimates of efficacy against that same endpoint), although the reason for the high point estimates is unclear [ 61 ].
Protection against a non-specific endpoint depends on the proportion of the endpoint that is attributable to the pathogen targeted by the vaccine; this may vary over time or seasonally, be higher or lower in certain sub-groups (e.g. young infants, malnourished children) or be affected by outbreaks of other pathogens with overlapping clinical symptoms. Such variability can result in inconsistent estimates of effectiveness against non-specific endpoints between studies. For vaccines that lead to herd effect (e.g. Hib or pneumococcal conjugate vaccine), the proportion of a non-specific endpoint (e.g. pneumonia) attributable to the vaccine-preventable pathogen decreases among both vaccinated and non-vaccinated populations; thus as herd effects increase, effectiveness estimates for non-specific endpoints will decline. The risk for developing non-specific clinical syndromes such as all-cause pneumonia or diarrhea may also be strongly affected by individual-level non-vaccine risk factors (e.g. poverty, maternal education, crowding); such factors are difficult to measure well and may be associated with vaccination status. Furthermore, non-specific endpoints require enrolling larger numbers of participants, since effectiveness against non-specific endpoints is lower than that against specific endpoints [ 10 ]. Because of variability in the vaccine-preventable portion and the strong potential for bias, case-control vaccine effectiveness studies using non-specific endpoints must be interpreted with care, and are best conducted only when accompanied by analyses of disease trends over time or by a nested or parallel evaluation of a more specific endpoint in the same study setting.
4.5. Identification and enrollment of cases
Once the study endpoint is decided, the endpoint case definition must be clearly defined to avoid variable inclusion of cases during study implementation. It is not necessary to enroll all individuals who develop the disease in a given area or time period for a case-control study [ 10 ]. However, studies should report the proportion of eligible cases enrolled, since low enrollment may result in selection bias. Some vaccine effectiveness studies focus on a specific subset of cases because the effectiveness of the vaccine against the outcome is of particular public health interest (e.g. hospitalized or severe cases). The generalizability of the vaccine effectiveness will be limited to the types of cases included, and such restrictions must be taken into account in the interpretation of study findings [ 51 ]. Whenever possible and culturally acceptable, cases among children who have died should be included in case-control vaccine effectiveness studies, since they represent the most severe spectrum of disease and failing to include them could bias the effectiveness estimate if their likelihood of vaccination differs than that of cases who survive.
4.6. Sources of controls
In all case-control studies, controls should be representative of the source population from which the cases are selected [ 51 , 62 – 64 ]. A way of exploring this is to ask “If this control had developed the disease of interest, would he or she have been identified and included in this study as a case?” If the answer is no, then the control selection method is probably not appropriate. This question should be asked at the study design phase, when the source of potential controls is being determined.
4.6.1. Community controls
In many contexts, it is good practice to seek controls in the community in which the case resides, since those living in the community are most reflective of those who would be identified as cases if they were to fall ill [ 62 ]. The community from which the cases are derived from can be defined in various ways, depending on the study context and the available options for identifying controls. Population-based lists, such as birth registries or population-based databases in which the cases are included, can be used to randomly select potential controls [ 65 ]. For example, in studies of the pneumococcal conjugate vaccine in the US [ 17 ] and Brazil [ 66 ], birth registries were used to select potential controls, and in a study in Canada, controls were selected from a health insurance registry that included all residents in a province [ 27 ]. Such lists should be comprehensive and inclusive, since selecting children from an incomplete list may limit generalizability [ 62 ]. The basis for the list must not be associated with receipt of vaccines (e.g. immunization registries that include only vaccinated children). Lists with the appropriate characteristics often do not exist or are incomplete in many resource-poor settings, obviating this method for control selection. If such a list is used to identify controls, then cases not appearing in the list should be excluded.
Alternatively, community controls may be sought geographically, for example, around a case’s place of residence. Children from the same geographic area often tend to be comparable with respect to underlying risk of disease and access to vaccination, and it is possible to match on, or adjust for, distance to healthcare facilities if there is concern about differential access to care. Matching by neighborhood can also help control for a variety of potential confounding factors that may be difficult to measure, such as socio-economic status or other barriers to vaccination [ 65 ]. Geomapping of the population in an area can provide a sample frame from which to select geographically-matched controls, as was done for a Hib vaccine case-control study in Bangladesh [ 67 ]. A less sophisticated, but more commonly employed, strategy is to identify the household of the case, and then walk in a random direction (e.g. by spinning a bottle) from that residence, seeking a suitable control from the nearest neighboring house. This method is based on the approach developed for vaccine coverage surveys [ 15 , 21 , 58 ]. Having standardized procedures for visiting potential control households is essential for reducing selection bias [ 62 ]. Procedures should include the requirement to visit non-responsive households multiple times and at different times of day before excluding their residents as potential controls, since children whose parents are not at home might be more or less likely to be vaccinated than children whose parents are at home. Enrolling community controls can be logistically challenging and resource-intensive, particularly when tight age-matching criteria are used. Security concerns can also interfere with recruitment of community controls; investigators of Hib vaccine effectiveness in Colombia and Pakistan had to alter control recruitment strategies due to the safety risks associated with seeking neighborhood controls [ 24 , 68 ]. Conducting control recruitment in locations that are safer or more convenient can induce substantial biases in the vaccine effectiveness measure if residence in those areas is associated with higher likelihood of vaccination.
4.6.2. Hospital or clinic controls
Another common source of controls for case-control vaccine effectiveness studies are children hospitalized with illnesses other than the outcome of interest [ 62 ]. The specific inclusion criteria for hospitalized children to serve as controls must be carefully considered in the design phase of each study since the local conditions influence the risk of bias. Children who are hospitalized, particularly those with frequent or prolonged hospitalization, may differ in important ways, including vaccination history, from the general population; recruiting from among recently admitted children may avoid overenrolling children with severe prolonged illness as controls. Children hospitalized with vaccine-preventable diseases should be excluded as controls, as they are probably less likely to be vaccinated in general, including with the vaccine under study [ 51 , 62 ]. Where vaccines that protect against the most common childhood illnesses (e.g. gastroenteritis, pneumonia) are in routine use, and therefore children hospitalized with these illnesses cannot serve as controls, it may be challenging to identify enough eligible hospital controls [ 31 ]. In settings where access to health care is limited or hospitalization is largely restricted to certain subsets of children (e.g. children with malnutrition), then hospital controls may have the advantage of being relatively comparable to hospitalized cases with regards to access to care [ 62 ]. However, results of a study in such a context are only generalizable to children who would be hospitalized when ill.
Controls can also be identified in out-patient clinics that cases would attend if ill [ 65 ], an approach used for Hib vaccine effectiveness studies in Colombia [ 68 ] and Ukraine [ 23 ]. However, if immunizations are delivered at the clinic, then controls attending the clinic would be more likely to be vaccinated than the general population, as was found in a study of tuberculosis vaccination in Brazil [ 69 ]. Thus, if outpatient clinics are to be used as a source of controls, they should be clinics where immunizations are not routinely provided.
4.6.3. Controls with same clinical syndrome who are ‘test-negative’ for the pathogen of interest
Another potential source of controls is children who become ill with the same clinical syndrome as those with the outcome of interest, but whose illness is shown to have an etiologic pathogen not targeted by the vaccine under evaluation [ 70 – 72 ]. Examples of this approach include: rotavirus-negative gastroenteritis as controls for cases of rotavirus [ 36 , 41 , 43 ], influenza-negative respiratory infection for cases of influenza [ 71 ], pneumococcal or non-purulent/Hib-negative meningitis as controls for Hib meningitis cases [ 18 , 20 ], and non-vaccine serotype invasive pneumococcal disease for cases of vaccine-type invasive disease (also known as the ‘indirect cohort’ or ‘Broome’ method) [ 73 – 77 ]. This approach requires accurate diagnostic testing and sample collection at an appropriate time to diagnose the pathogen of interest in order to avoid misclassification. Imperfect test sensitivity and specificity leads to an underestimation of effectiveness using test-negative controls [ 78 ]. Some tests, such as culture of blood or cerebrospinal fluid, are too insensitive to reliably identify test-negative controls; however when such tests detect an etiology that is not preventable by vaccines included in the national schedule (e.g. pneumococcal meningitis for evaluation of Hib vaccine [before introduction of pneumococcal vaccine], or non-vaccine-type pneumococcal bacteremia for evaluation of pneumococcal conjugate vaccine), such individuals can serve as controls. The validity of using test-negative controls has been demonstrated by re-analyses of data from randomized clinical trials of influenza [ 70 ] and rotavirus [ 79 ] vaccines that yielded effectiveness estimates very similar to the efficacy measured by the original trials.
One major advantage of the test-negative approach is a high degree of comparability between cases and controls, since controls would have been enrolled as cases if they had the vaccine-preventable outcome of interest. It also offers logistical and cost advantages, since cases and controls can be recruited from within a single surveillance system. Also, since test results are often not available at the time of recruitment, bias in ascertainment of vaccination through knowledge of case-control status is less likely. A limitation to this method is that it assumes the vaccine being evaluated has no effect on the incidence of test-negative cases who will serve as controls. For pneumococcal conjugate vaccines, this assumption may not be valid, since their widespread use has been associated with increases in non-vaccine type pneumococcal carriage and disease incidence [ 80 ]. However, modeling work conducted in conjunction with indirect cohort analyses indicates that while increases in non-vaccine type disease (and carriage) among vaccinated individuals compared with the non-vaccinated can lead to overestimates of VE, the magnitude of the overestimation is relatively small, particularly if conducted before vaccine coverage is very high [ 74 , 76 ]. For influenza vaccine, models have similarly shown that even if influenza infection is presumed to provide transient non-specific immunity to all respiratory infections (and thus individuals vaccinated against influenza, who would not benefit from this immunity, would be over-represented among non-influenza respiratory infection cases) the impact on effectiveness estimates derived from case-control studies using the test-negative design is minor [ 72 , 81 ].
4.6.4. Multiple control groups
In some case-control vaccine effectiveness studies two or more types of control group are enrolled [ 17 , 19 , 40 , 42 ]. However, when the estimates of effectiveness differ by the control group used, the disparate results are difficult to interpret and communicate [ 63 ]. Multiple control groups may be useful for evaluation of study methods and identifying bias in different groups. In general, however, it is preferable to understand the study context well, to consider carefully the best control group before conducting the study, and then to use one source of controls [ 62 ].
4.7. Matching
Matching of cases and controls is often used in vaccine effectiveness studies to increase the statistical efficiency of the analysis and to attempt to control for unmeasured confounders [ 64 , 65 , 82 ]. However, overmatching, which occurs when the matching variable is strongly associated with vaccination but not (or only weakly) with the illness, results in a loss of statistical power [ 83 ]. Matching also greatly increases the operational complexity of enrolling controls. Matching in case-control vaccine effectiveness studies is most commonly done at an individual level, where each enrolled control is matched to a specific case based on certain criteria (e.g. date of birth or geographic region). An alternative approach is ‘frequency’ or ‘stratum’ matching, in which the group of controls is enrolled based on the frequency of certain characteristics among all cases (e.g. if 20% of cases are from a certain neighborhood, then controls are enrolled so that 20% are from that same neighborhood) [ 10 ]. Matching, if used, should be reserved for a limited number of important variables believed to be linked to both vaccination and disease (i.e. confounding), since unnecessary matching can lead to reduced efficiency in the analysis and substantially increases the complexity of study implementation [ 65 , 82 , 84 ].
4.8. Control to case ratio
The preferred ratio of controls to cases depends upon the relative ease (and cost) of enrolling cases and controls. The statistically most efficient approach is equal numbers of cases and controls, if they are equally easy to enroll. If the number of cases is limited, increasing the number of controls per case will increase statistical power, but generally little additional power is gained by enrolling more than four controls per case [ 62 ]. However, for studies using individual matching in contexts where vaccine coverage is very high or very low, more than four controls per case should be considered, since case-control sets in which all cases and controls have the same vaccination status will not contribute to the estimates of effectiveness. In contexts where controls are easy to enroll, for example from a population-based registry [ 29 ], then a higher control to case ratio may be used.
4.9. Timing of control enrollment
For individually matched case-control vaccine effectiveness studies, controls should be enrolled concurrently (i.e. for each incident case enrolled, one or more new matched controls are enrolled from the population at-risk). Rapid enrollment of matched controls can reduce recall bias, minimize difficulty obtaining compatible vaccination histories for cases and controls, and help ensure comparability between cases and controls with respect to unmeasured temporal factors that may affect the risk of developing the outcome of interest (e.g. outbreaks of viral respiratory infections increasing the risk for pneumococcal pneumonia). However, rapid enrollment of matched controls is not always feasible and risks regarding vaccine history can be mitigated if there is written documentation, with dates of administration, of vaccination status so that only doses received before the corresponding case became ill are considered.
5. Conclusions
Evidence of the protection afforded by new vaccines in the context of real-world immunization programs is important for accelerating and sustaining their uptake globally [ 85 , 86 ]. Case-control vaccine effectiveness studies, if carefully conducted, can provide such evidence, complementing data from randomized controlled trials as well as findings from other observational approaches, such as analyses of trends in disease incidence over time or cohort studies. Relative to other observational methods for vaccine evaluation, case-control studies have some advantages. They do not require a stable baseline of disease surveillance data prior to vaccine introduction and are often considerably less expensive to perform than cohort studies. Case-control vaccine effectiveness studies do not measure the actual impact of vaccine introduction on disease at a population level. However, when combined with data on prevaccine burden of disease and vaccine coverage, they can be used to provide insight into the public health impact of vaccines.
The belief that case-control studies are quick and easy to carry out is misplaced. Case-control vaccine effectiveness studies are complex and require rigorous planning and implementation. They are susceptible to various types of bias and, if not conducted rigorously and with careful planning, can produce invalid and potentially misleading results. It is imperative that investigators understand the study context well to minimize bias and correctly interpret results. Case-control vaccine effectiveness studies are most likely to provide reliable information when assessing outcomes specific to the vaccine being evaluated (e.g. Hib meningitis rather than all clinical meningitis). Studies using nonspecific outcomes are particularly challenging and prone to misleading results; such studies should not be undertaken unless the investigators ensure a high level of rigor and complementary data assessing other more specific outcomes are available from the same or comparable population. Selection of an appropriate control group and close attention to potential sources of bias during control enrollment are crucial. While case-control studies can provide useful information to guide vaccine policy decisions and vaccine development, they must be thoughtfully planned and rigorously conducted.
Acknowledgements
The authors would like to acknowledge Claudia DaSilva for organizing the meeting which formed the basis for this paper. We would also like to thank Dr. Jill Ferdinand and Tamara Pilishvili for their contributions to the scientific content of the meeting and subsequent discussions.
Funds from the GAVI Alliance covered the cost of an expert meeting held in November 2012 to discuss the case-control method for evaluating vaccine effectiveness.
Author contributions
AHB: Conceptualization, Writing: Review and editing.
CVB: Conceptualization, Writing: Review and editing.
TC: Conceptualization, Writing: Review and editing.
CC: Conceptualization, Methodology, Writing: Review and editing.
JLF: Writing: Review and editing, Project administration.
DRF: Conceptualization, Writing: Original draft preparation, Writing: Review and editing.
MG: Conceptualization, Writing: Review and editing.
RAH: Conceptualization, Methodology, Writing: Review and editing, Funding acquisition.
HLJ: Conceptualization, Writing: Review and editing.
SAM: Conceptualization, Writing: Review and editing.
KM: Conceptualization, Writing: Review and editing.
KLO: Conceptualization, Methodology, Writing: Review and editing, Supervision.
UDP: Conceptualization, Writing: Original draft preparation, Writing: Review and editing.
MMP: Conceptualization, Writing: Original draft preparation, Writing: Review and editing.
LCR: Conceptualization, Writing: Review and editing.
MS: Conceptualization, Writing: Review and editing.
JAS: Conceptualization, Methodology, Writing; Review and editing.
PGS: Conceptualization, Methodology, Writing: Review and editing.
HS: Conceptualization, Methodology, Writing: Review and editing.
JET: Conceptualization, Writing: Review and editing.
JCV: Conceptualization, Writing: Review and editing.
CGW: Conceptualization, Methodology, Writing: Review and editing, Supervision.
AKZ: Conceptualization, Writing: Review and editing.
ERZ: Conceptualization, Writing: Review and editing.
Disclaimers
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Thomas Cherian is a staff member of the World Health Organization. He alone is responsible for the views expressed in this publication, which may not necessarily represent the decisions or the policies of the World Health Organization
Author disclosures of potential conflict of interest
CC reports having received grant funds from Sanofi Pasteur that were awarded to the National Institute for Communicable Diseases, South Africa
- Introduction
- Conclusions
- Article Information
“Syndrome-negative” participants were persons hospitalized without signs or symptoms consistent with acute COVID-19 and who tested negative for SARS-CoV-2 by molecular testing. They were included as a secondary control group because of the theoretical risk of case misclassification in test-negative controls.
An adjusted odds ratio (aOR) less than 1.0 indicated that COVID-19 hospitalization was associated with being unvaccinated compared with being fully vaccinated. Vaccine effectiveness for prevention of COVID-19 hospitalization can be estimated from the aORs presented here with the following equation: vaccine effectiveness = (1 − aOR) × 100%. BNT162b2 is the vaccine produced by Pfizer-BioNTech and mRNA-1273 is the vaccine produced by Moderna. mRNA indicates messenger RNA.
a Models were mixed-effects logistic regression models with vaccination status (fully vaccinated vs unvaccinated) as the primary independent variable, case-control status (hospitalized with COVID-19 vs hospitalized without it) as the dependent variable, enrolling site as a random effect, and the following covariables: admission date (biweekly intervals), age group (18-49, 50-64, and ≥65 years), sex, and self-reported race and ethnicity. Models stratified by age group were adjusted for continuous age in years.
b Immunocompromising conditions are defined in the Table.
c Alpha estimates restricted to March to June illness-onset dates (Alpha period); Delta estimates restricted to July to August illness-onset dates (Delta period).
An adjusted odds ratio (aOR) less than 1.0 indicated that progression to death or invasive mechanical ventilation after hospital admission for COVID-19 was associated with being unvaccinated compared with being vaccinated.
a Models were adjusted for age group (18-49, 50-64, and ≥65 years), sex, self-reported race and ethnicity, and number of chronic medical comorbidities (0, 1, 2, 3, and ≥4). Models stratified by age group were adjusted for continuous age in years.
c Analysis restricted to COVID-19 case patients with hypoxemia within 24 hours of admission, defined as receiving supplemental oxygen or having an oxygen saturation less than 92% as measured by pulse oximetry.
Cumulative incidence of hospital discharge by vaccination status (fully vaccinated with a 2-dose series of mRNA vaccine vs unvaccinated) is shown for patients not immunocompromised (A), those immunocompromised (B), those aged 18 to 64 years (C), and those aged 65 years or older (D). The event of interest was discharge from the hospital before day 28 in the presence of the competing event of death. Patients who remained hospitalized more than 28 days were censored at 28 days. Competing risk models were adjusted for age group (18-49, 50-64, and ≥65 years), sex, self-reported race and ethnicity, and number of medical comorbidities (0, 1, 2, 3, and ≥4). Models by age group were adjusted for continuous age in years. mRNA indicates messenger RNA; and SHR, subdistribution hazard ratio.
eAppendix 1. Investigators and Collaborators
eAppendix 2. Supplementary Tables and Figures
eTable 1. Modified WHO COVID-19 Clinical Progression Scale Used in This Analysis to Assess Disease Severity Among Adults Hospitalized With COVID-19
eTable 2. Underlying Medical Conditions Obtained Through Medical Record Review
eTable 3. Association Between Hospitalization for COVID-19 and Prior Receipt of Full Vaccination With a Two-Dose Series of a mRNA Vaccine, Restricted to Patients Without Immunocompromising Conditions
eTable 4. Association Between Hospitalization for COVID-19 and Prior Receipt of Full Vaccination With a Two-Dose Series of a mRNA Vaccine, Restricted to Patients Without Immunocompromising Conditions and Aged 18-64 Years
eTable 5. Association Between Hospitalization for COVID-19 and Prior Receipt of Full Vaccination With a Two-Dose Series of a mRNA Vaccine, Restricted to Patients Without Immunocompromising Conditions and Aged ≥65 Years
eTable 6. Treatments, Outcomes, and Severity of Illness Among Hospitalized COVID-19 Cases by Vaccination Status
eTable 7. Odds Prior mRNA COVID-19 Vaccination Among COVID-19 Cases Who Received In-Hospital COVID-19 Therapeutics and Those Who Did Not
eFigure 1. Whole Genome Sequencing SARS-CoV-2 Lineage Determination Among COVID-19 Cases by Admission Week
eFigure 2. Highest Severity Level Experienced on the WHO COVID-19 Clinical Progression Scale During the First 28 Days of Hospitalization Among Vaccine Breakthrough COVID-19 Cases and Unvaccinated COVID-19 Cases
- Association of Prior SARS-CoV-2 Infection With Risk of Breakthrough Infection Following mRNA Vaccination in Qatar JAMA Original Investigation November 16, 2021 This cohort study assesses protection from SARS-CoV-2 breakthrough infection after mRNA vaccination among persons with vs without prior SARS-CoV-2 infection. Laith J. Abu-Raddad, PhD; Hiam Chemaitelly, MSc; Houssein H. Ayoub, PhD; Hadi M. Yassine, PhD; Fatiha M. Benslimane, PhD; Hebah A. Al Khatib, PhD; Patrick Tang, MD, PhD; Mohammad R. Hasan, PhD; Peter Coyle, MD; Zaina Al Kanaani, PhD; Einas Al Kuwari, MD; Andrew Jeremijenko, MD; Anvar Hassan Kaleeckal, MSc; Ali Nizar Latif, MD; Riyazuddin Mohammad Shaik, MSc; Hanan F. Abdul Rahim, PhD; Gheyath K. Nasrallah, PhD; Mohamed Ghaith Al Kuwari, MD; Adeel A. Butt, MBBS, MS; Hamad Eid Al Romaihi, MD; Mohamed H. Al-Thani, MD; Abdullatif Al Khal, MD; Roberto Bertollini, MD, MPH
- Understanding Breakthrough Infections Following mRNA SARS-CoV-2 Vaccination JAMA Editorial November 23, 2021 Michael Klompas, MD, MPH
- Durability of Antibody Levels After SARS-CoV-2 Vaccine in Individuals With or Without Prior Infection JAMA Research Letter December 28, 2021 This study examines antibody durability in individuals who received an mRNA SARS-CoV-2 vaccine with prior SARS-CoV-2 infection vs those without infection. Diana Zhong, MD; Shaoming Xiao, MSPH; Amanda K. Debes, PhD, MS; Emily R. Egbert, MPH, MAT; Patrizio Caturegli, MD, MPH; Elizabeth Colantuoni, PhD, ScMs; Aaron M. Milstone, MD, MHS
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
- CME & MOC
Tenforde MW , Self WH , Adams K, et al. Association Between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity. JAMA. 2021;326(20):2043–2054. doi:10.1001/jama.2021.19499
Manage citations:
© 2024
- Permissions
Association Between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity
- 1 CDC COVID-19 Response Team, Atlanta, Georgia
- 2 Vanderbilt Institute for Clinical and Translational Research, Department of Emergency Medicine, Vanderbilt University Medical Center, Nashville, Tennessee
- 3 Baylor Scott & White Health, Texas A&M University College of Medicine, Temple
- 4 Department of Emergency Medicine, University of Colorado School of Medicine, Aurora
- 5 Department of Anesthesiology, University of Colorado School of Medicine, Aurora
- 6 Departments of Medicine and Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee
- 7 Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee
- 8 Department of Emergency Medicine, University of Iowa, Iowa City
- 9 Department of Emergency Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts
- 10 Department of Medicine, Wake Forest School of Medicine, Winston-Salem, North Carolina
- 11 Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland
- 12 Departments of Emergency Medicine and Medicine, Hennepin County Medical Center, Minneapolis, Minnesota
- 13 Department of Medicine, Hennepin County Medical Center, Minneapolis, Minnesota
- 14 Department of Medicine, The Ohio State University, Columbus
- 15 Department of Medicine, Montefiore Health System, Albert Einstein College of Medicine, Bronx, New York
- 16 Department of Medicine, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, New York
- 17 Department of Emergency Medicine, University of Washington, Seattle
- 18 Department of Medicine, Baystate Medical Center, Springfield, Massachusetts
- 19 Department of Medicine, Intermountain Medical Center, Murray, Utah; and University of Utah, Salt Lake City
- 20 School of Public Health, University of Michigan, Ann Arbor
- 21 Department of Medicine, Oregon Health & Science University, Portland
- 22 Department of Medicine, Emory University, Atlanta, Georgia
- 23 Emory Critical Care Center, Emory Healthcare, Atlanta, Georgia
- 24 Department of Medicine, Cleveland Clinic, Cleveland, Ohio
- 25 Department of Emergency Medicine, Stanford University School of Medicine, Stanford, California
- 26 Department of Medicine, University of California–Los Angeles, Los Angeles
- 27 Department of Medicine, University of Miami, Miami, Florida
- 28 Department of Medicine, Washington University, St Louis, Missouri
- 29 Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee
- 30 Departments of Internal Medicine and Microbiology and Immunology, University of Michigan, Ann Arbor
- 31 Department of Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee
- 32 Department of Emergency Medicine, Vanderbilt University Medical Center, Nashville, Tennessee
- 33 Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville, Tennessee
- 34 Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee
- Editorial Understanding Breakthrough Infections Following mRNA SARS-CoV-2 Vaccination Michael Klompas, MD, MPH JAMA
- Original Investigation Association of Prior SARS-CoV-2 Infection With Risk of Breakthrough Infection Following mRNA Vaccination in Qatar Laith J. Abu-Raddad, PhD; Hiam Chemaitelly, MSc; Houssein H. Ayoub, PhD; Hadi M. Yassine, PhD; Fatiha M. Benslimane, PhD; Hebah A. Al Khatib, PhD; Patrick Tang, MD, PhD; Mohammad R. Hasan, PhD; Peter Coyle, MD; Zaina Al Kanaani, PhD; Einas Al Kuwari, MD; Andrew Jeremijenko, MD; Anvar Hassan Kaleeckal, MSc; Ali Nizar Latif, MD; Riyazuddin Mohammad Shaik, MSc; Hanan F. Abdul Rahim, PhD; Gheyath K. Nasrallah, PhD; Mohamed Ghaith Al Kuwari, MD; Adeel A. Butt, MBBS, MS; Hamad Eid Al Romaihi, MD; Mohamed H. Al-Thani, MD; Abdullatif Al Khal, MD; Roberto Bertollini, MD, MPH JAMA
- Research Letter Durability of Antibody Levels After SARS-CoV-2 Vaccine in Individuals With or Without Prior Infection Diana Zhong, MD; Shaoming Xiao, MSPH; Amanda K. Debes, PhD, MS; Emily R. Egbert, MPH, MAT; Patrizio Caturegli, MD, MPH; Elizabeth Colantuoni, PhD, ScMs; Aaron M. Milstone, MD, MHS JAMA
Question Does prior COVID-19 vaccination reduce hospitalizations for COVID-19, and among patients hospitalized for COVID-19, does prior vaccination reduce disease severity?
Findings In a case-control study that included 4513 hospitalized adults in 18 US states, hospitalization for a COVID-19 diagnosis compared with an alternative diagnosis was associated with an adjusted odds ratio (aOR) of 0.15 for full vaccination with an authorized or approved mRNA COVID-19 vaccine. Among adults hospitalized for COVID-19, progression to death or invasive mechanical ventilation was associated with an aOR of 0.33 for full vaccination; both ORs were statistically significant.
Meaning Vaccination with an mRNA COVID-19 vaccine was significantly less likely among patients with COVID-19 hospitalization and with disease progression, consistent with risk reduction among vaccine breakthrough infections.
Importance A comprehensive understanding of the benefits of COVID-19 vaccination requires consideration of disease attenuation, determined as whether people who develop COVID-19 despite vaccination have lower disease severity than unvaccinated people.
Objective To evaluate the association between vaccination with mRNA COVID-19 vaccines—mRNA-1273 (Moderna) and BNT162b2 (Pfizer-BioNTech)—and COVID-19 hospitalization, and, among patients hospitalized with COVID-19, the association with progression to critical disease.
Design, Setting, and Participants A US 21-site case-control analysis of 4513 adults hospitalized between March 11 and August 15, 2021, with 28-day outcome data on death and mechanical ventilation available for patients enrolled through July 14, 2021. Date of final follow-up was August 8, 2021.
Exposures COVID-19 vaccination.
Main Outcomes and Measures Associations were evaluated between prior vaccination and (1) hospitalization for COVID-19, in which case patients were those hospitalized for COVID-19 and control patients were those hospitalized for an alternative diagnosis; and (2) disease progression among patients hospitalized for COVID-19, in which cases and controls were COVID-19 patients with and without progression to death or mechanical ventilation, respectively. Associations were measured with multivariable logistic regression.
Results Among 4513 patients (median age, 59 years [IQR, 45-69]; 2202 [48.8%] women; 23.0% non-Hispanic Black individuals, 15.9% Hispanic individuals, and 20.1% with an immunocompromising condition), 1983 were case patients with COVID-19 and 2530 were controls without COVID-19. Unvaccinated patients accounted for 84.2% (1669/1983) of COVID-19 hospitalizations. Hospitalization for COVID-19 was significantly associated with decreased likelihood of vaccination (cases, 15.8%; controls, 54.8%; adjusted OR, 0.15; 95% CI, 0.13-0.18), including for sequenced SARS-CoV-2 Alpha (8.7% vs 51.7%; aOR, 0.10; 95% CI, 0.06-0.16) and Delta variants (21.9% vs 61.8%; aOR, 0.14; 95% CI, 0.10-0.21). This association was stronger for immunocompetent patients (11.2% vs 53.5%; aOR, 0.10; 95% CI, 0.09-0.13) than immunocompromised patients (40.1% vs 58.8%; aOR, 0.49; 95% CI, 0.35-0.69) ( P < .001) and weaker at more than 120 days since vaccination with BNT162b2 (5.8% vs 11.5%; aOR, 0.36; 95% CI, 0.27-0.49) than with mRNA-1273 (1.9% vs 8.3%; aOR, 0.15; 95% CI, 0.09-0.23) ( P < .001). Among 1197 patients hospitalized with COVID-19, death or invasive mechanical ventilation by day 28 was associated with decreased likelihood of vaccination (12.0% vs 24.7%; aOR, 0.33; 95% CI, 0.19-0.58).
Conclusions and Relevance Vaccination with an mRNA COVID-19 vaccine was significantly less likely among patients with COVID-19 hospitalization and disease progression to death or mechanical ventilation. These findings are consistent with risk reduction among vaccine breakthrough infections compared with absence of vaccination.
The COVID-19 pandemic, caused by SARS-CoV-2, continues to be a global public health crisis. 1 The messenger RNA (mRNA) COVID-19 vaccines, including mRNA-1273 (Moderna) and BNT162b2 (Pfizer-BioNTech), are highly effective for preventing SARS-CoV-2 infections and COVID-19 hospitalizations. 2 - 5 However, vaccine breakthrough COVID-19 (defined as development of COVID-19 despite prior full vaccination) is now being reported throughout the world. 6 Because vaccine effectiveness is less than 100%, breakthrough cases are expected, and as vaccine coverage increases in the population, the ratio of vaccinated to unvaccinated cases will increase.
A full interpretation of the protective benefits of COVID-19 vaccines must account for protection against SARS-CoV-2 infection, as well as against progression of disease severity after breakthrough infection. 7 , 8 To date, evaluations of COVID-19 vaccines have primarily focused on prevention of symptomatic infection and hospitalizations. 2 , 3 , 9 - 11 Once hospitalized, patients with COVID-19 can progress to more severe disease, including respiratory failure and death. SARS-CoV-2 infection in vaccinated persons is expected to trigger memory antibody and cellular responses owing to prior vaccination; these immune responses could mitigate disease progression, possibly preventing life-threatening organ failure and death. 12 , 13 However, the association between prior vaccination and disease progression to the most severe forms of COVID-19 is not well understood.
To estimate the benefits of mRNA vaccination against severe COVID-19, this study examined the association between prior vaccination and hospitalization for COVID-19, as well as the association between prior vaccination and progression to death or invasive mechanical ventilation among patients hospitalized for COVID-19.
This program was conducted by the Influenza and Other Viruses in the Acutely Ill (IVY) Network, a collaboration among 21 US hospitals in 18 states and the Centers for Disease Control and Prevention (investigators and collaborators are listed in eAppendix 1 in the Supplement ). 4 , 14 A total of 4513 patients hospitalized at the network hospitals between March 11, 2021, and August 15, 2021, were included. Data on hospitalizations were included for patients enrolled through August 15, 2021, and data on 28-day outcomes after hospitalization were included for patients enrolled through July 14, 2021. This analysis was an update to information previously published using earlier versions of the program’s data. 4 , 15 , 16 STROBE guidelines for reporting were followed. This case-control study was determined to be a public health surveillance program, with waiver of participant informed consent by all participating institutions and the Centers for Disease Control and Prevention (CDC).
We used a test-negative case-control design to assess the association between hospitalization for COVID-19 and prior vaccination with an mRNA COVID-19 vaccine. In this analysis, case patients were those hospitalized with COVID-19 and control patients were those hospitalized for other reasons. 17 - 19 In a second analysis among only patients hospitalized with COVID-19, we assessed the association between COVID-19 disease progression and prior vaccination with an mRNA vaccine. In the second analysis, cases and controls were patients hospitalized with COVID-19 with and without progression to death or invasive mechanical ventilation, respectively.
Sites screened hospitalized adults aged 18 years and older for potential eligibility through daily review of hospital admission logs and electronic medical records (eAppendix 2 in the Supplement ). COVID-19 cases included patients hospitalized with a clinical syndrome consistent with acute COVID-19 and a positive molecular or antigen test result for SARS-CoV-2 within 10 days after symptom onset. 20 We included 2 control groups: “test-negative” controls were persons hospitalized with signs or symptoms consistent with acute COVID-19 but who tested negative for SARS-CoV-2 by molecular testing; and “syndrome-negative” controls were persons hospitalized without signs or symptoms consistent with acute COVID-19 and who tested negative for SARS-CoV-2 by molecular testing and were included as a secondary control group because of the theoretic risk of case misclassification in test-negative controls. 21 Sites attempted to capture all cases admitted to the hospital during the surveillance period and targeted a case-control ratio of approximately 1:1 for each group of controls. Controls were selected from lists of eligible participants hospitalized within 2 weeks of enrollment of cases. Information on vaccination status was not collected until after patients were enrolled.
Demographic, clinical, and laboratory data were collected by trained personnel through standardized participant (or proxy) interviews and medical record reviews. Data on race and ethnicity were collected because the association between vaccination and COVID-19 may vary by race or ethnicity. Information on race and ethnicity was reported by participants during interviews conducted by research personnel using fixed categories.
Details of COVID-19 vaccination, including dates and location, vaccine product, and lot number, were ascertained through a systematic process including patient or proxy interview and source verification. Sources of documentation included vaccination cards, hospital records, state vaccine registries, and vaccine records requested from clinics and pharmacies. Vaccine doses were classified as administered if source documentation was identified or if the patient or proxy reported a vaccine dose with a plausible date and location of vaccination.
Upper respiratory specimens were collected from enrolled patients, frozen, and shipped to a central laboratory at Vanderbilt University Medical Center (Nashville, Tennessee). Specimens underwent reverse transcriptase–polymerase chain reaction testing for SARS-CoV-2 nucleocapsid gene targets with standardized methods and interpretive criteria. 22 Specimens positive for SARS-CoV-2 with a cycle threshold less than 32 were shipped to the University of Michigan (Ann Arbor, Michigan) for viral whole-genome sequencing using the ARTIC Network version 3 protocol on an Oxford Nanopore Technologies instrument (GridION). 23 SARS-CoV-2 lineages were assigned with greater than 80% coverage with Phylogenetic Assignment of Named Global Outbreak Lineages (PANGOLIN). 24
During this study period, the mRNA COVID-19 vaccines were administered as a series of at least 2 doses; participants were considered fully vaccinated 14 days after receipt of the second dose. 25 Vaccination status was classified according to vaccine receipt before a reference date, which was the date of symptom onset for cases and test-negative controls and 4 days before hospital admission for syndrome-negative controls. Participants were classified as unvaccinated if they had received no vaccine doses before the reference date and fully vaccinated if they had received 2 or more mRNA vaccine doses at least 14 days before the reference date. Patients were excluded if they had previously received at least 1 dose of an mRNA vaccine but were not fully vaccinated, if they received a different type of COVID-19 vaccine, such as AD26.COV2.S (Janssen/Johnson & Johnson), or if they were vaccinated with a mixed vaccine schedule (ie, BNT1262b2 vaccine for 1 dose and mRNA-1273 vaccine for 1 dose).
We collected data on severity for patients hospitalized with COVID-19. These outcome data were collected until the earlier of hospital discharge or 28 days after hospital admission. The primary classification of disease severity was a binary measure that divided patients into those who experienced death or invasive mechanical ventilation (progression to high disease severity) and those who did not (no progression to high disease severity).
As a secondary assessment, we classified COVID-19 severity using a modified version of the World Health Organization COVID-19 Clinical Progression Scale, a commonly used ordinal scale for assessing COVID-19 severity that ranges from uninfected (level 0) and infected but asymptomatic (level 1) to death (level 9) (eAppendix 2 [eTable 1] in the Supplement ). We classified severity according to the highest ordinal level that the patient experienced during the first 28 days of hospitalization. In this analysis of hospitalized patients, the highest severity level experienced could range from level 4 to 9, including hospitalized without supplemental oxygen (level 4), with standard supplemental oxygen (level 5), with high-flow nasal cannula or noninvasive ventilation (level 6), with invasive mechanical ventilation (level 7), or with mechanical ventilation and additional organ support (extracorporeal membrane oxygenation, vasopressors, or new kidney replacement therapy; level 8); and in-hospital death (level 9).
We also evaluated in-hospital receipt of treatments used for severe COVID-19 (corticosteroids, remdesivir, COVID-19 convalescent plasma, tocilizumab, or baricitinib) according to a binary category of no COVID-19 treatments vs 1 or more.
We also characterized COVID-19 severity by hospital length of stay while accounting for the competing risk of death.
For the association between COVID-19 hospitalization and prior vaccination, we compared the odds of being fully vaccinated with an mRNA vaccine (exposed) vs being unvaccinated (unexposed) in cases hospitalized with COVID-19 vs controls hospitalized with other conditions. Patients from the test-negative and syndrome-negative control groups were pooled for this analysis in accordance with a prior analysis showing similar results with each control group individually. 4 , 15 A mixed-effects logistic regression model was generated, treating enrolling site as a random effect and with the following covariables: admission date (biweekly intervals), age, sex, and race and ethnicity. In this model, an adjusted odds ratio (aOR) less than 1.0 indicated that COVID-19 hospitalization was associated with reduced likelihood of vaccination. The aOR was used to estimate vaccine effectiveness for the prevention of COVID-19 hospitalizations via the following equation: vaccine effectiveness = (1 − aOR) × 100%. 4 , 26
The association between COVID-19 hospitalization and prior vaccination was also evaluated in stratified secondary analyses, including by immunocompetent vs immunocompromised, age group (18-49, 50-64, or ≥65 years), vaccine type (mRNA-1273 or BNT162b2 vaccine), and time between second vaccine dose and illness onset (14-120 days; >120 days). Additional models were constructed to evaluate interactions between vaccination and exposure variable group. For immunocompetent vs immunocompromised status and age groups, we added interaction terms between vaccination and the stratifying variable to the regression model. For vaccine type and time since vaccination, the vaccination exposure variable was replaced with a product variable (unvaccinated, vaccinated with mRNA-1273, and vaccinated with BNT162b2) or time variable (unvaccinated, vaccinated 14-120 days before illness onset, and vaccinated >120 days before illness onset). P values for comparisons across subgroups were calculated for the interaction term between the exposure variable and vaccination status (immunocompetent vs immunocompromised; age group) or with the pwcompare Stata function for pairwise comparisons (vaccine product; time between second vaccine dose and illness onset). In addition, separate assessments were conducted evaluating the association between hospitalization with SARS-CoV-2 variants (B.1.1.7 [Alpha]; B.1617.2 or AY [Delta]) and period of illness onset (March to June 2021, which had predominant Alpha variant circulation; July to August 2021, which had predominant Delta variant circulation). 27
Among patients hospitalized with COVID-19 through July 14, 2021, the association between progression to death or invasive mechanical ventilation and prior mRNA vaccination was calculated with multivariable logistic regression adjusted for the following covariables: age, sex, race and ethnicity, and number of medical comorbidities by category (eTable 2 in the Supplement ). The association between death alone and prior vaccination was similarly calculated with multivariable logistic regression evaluating the odds of vaccination among patients with COVID-19 who died vs survived.
In a subgroup analysis to assess disease progression among COVID-19 patients admitted with hypoxemia, the association between death or invasive mechanical ventilation and prior vaccination was calculated among the subgroup of patients who received oxygen therapy or had oxygen saturation less than 92% as measured by pulse oximetry within 24 hours of hospital admission.
A multivariable proportional odds model was used to compare the highest severity level experienced on the World Health Organization COVID-19 Clinical Progression Scale between patients with vaccine breakthrough COVID-19 and unvaccinated patients with COVID-19, using the same covariables described earlier. An aOR less than 1.0 for this model indicated lower odds of vaccinated patients’ experiencing higher severity levels on the ordinal scale compared with unvaccinated patients.
For receipt of in-hospital COVID-19 treatments, a multivariable logistic regression model was constructed to calculate the aOR of vaccination among patients who did vs did not receive at least 1 COVID-19 treatment.
To assess hospital length of stay, we calculated the probability of hospital discharge within 28 days after admission in vaccinated vs unvaccinated patients with COVID-19, using a Fine-Gray time-to-event analysis. We developed cumulative incidence function curves, with discharge from the hospital as the event of interest, death as the competing event, and patients who remained hospitalized censored at 28 days. 28 Point estimates of subdistribution adjusted hazard ratios were reported.
Missing data for illness onset was imputed according to the median number of days between illness onset and hospital admission for study patients within the same participant group. Imputation was not used for other variables; the number of patients with missing data was reported. Because of the potential for type I error owing to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. Statistical significance was indicated by 95% CIs not containing the null or a 2-sided P < .05. Stata version 16 was used for statistical analysis.
During March 11, 2021, to August 15, 2021, 5479 patients were enrolled from 21 hospitals; 966 patients were excluded from this analysis, with the most common reasons for exclusion being receipt of at least 1 mRNA vaccine but not being fully vaccinated (n = 547) and receipt of a COVID-19 vaccine other than an mRNA vaccine (n = 194) ( Figure 1 ). The analytic population included 4513 patients (median age, 59 years [IQR, 45-69]; 2202 [48.8%] women; 23.0% non-Hispanic Black individuals, 15.9% Hispanic individuals, and 20.1% with an immunocompromising condition), including 1983 cases with COVID-19 and 2530 controls without it (1359 test-negative controls and 1171 syndrome-negative controls).
Among 1983 COVID-19 case patients, vaccine breakthrough patients compared with unvaccinated patients tended to be older (median age 67 vs 53 years), were more likely to be White non-Hispanic (64.0% vs 43.0%), and were more likely to be immunocompromised (40.8% vs 11.5%) ( Table ). Among 1700 fully vaccinated patients (including both COVID-19 cases and controls), 1036 (60.9%) received the BNT162b2 vaccine and 664 (39.1%) received the mRNA-1273 vaccine; 1666 (98.0%) vaccinated patients had source documentation of vaccine doses and 34 (2.0%) had plausible self-report only. Full vaccination was less common in COVID-19 case patients (15.8%) than controls without COVID-19 (54.8%) (absolute difference, −39.0%; 95% CI, −41.5% to −36.4%).
Among 730 COVID-19 case patient specimens that had SARS-CoV-2 lineage determined, 245 (33.6%) were identified as B.1.1.7 (Alpha) variant, 335 (45.9%) as B.1.617.2 or AY group (Delta) variant, and 150 (20.5%) as other variants. The predominant variant shifted from Alpha to Delta in mid-June 2021 (eFigure 1 in the Supplement ), and in this analysis the period between July 1, 2021, and August 15, 2021, was considered dominated by Delta variant circulation.
Overall, COVID-19 hospitalization was strongly associated with a lower likelihood of vaccination, with an aOR of 0.15 (95% CI, 0.13-0.18) ( Figure 2 ). Effect modification was observed by immunocompromised status, with a greater magnitude of association for patients without immunocompromising conditions (aOR, 0.10; 95% CI, 0.09-0.13) than with immunocompromising conditions (aOR, 0.49; 95% CI, 0.35-0.69) ( P < .001) ( Figure 2 ; eTable 3 in the Supplement ). The magnitude of association was higher for the mRNA-1273 vaccine (aOR, 0.11; 95% CI, 0.08-0.14) than the BNT162b2 vaccine (aOR, 0.19; 95% CI, 0.16-0.23) ( P < .001), with this difference largely because of a lower aOR for patients at more than 120 days since vaccination with the mRNA-1273 vaccine (aOR, 0.15; 95% CI, 0.09-0.23; median 141 days from vaccine dose 2 to illness onset) than with the BNT162b2 vaccine (aOR, 0.36; 95% CI, 0.27-0.49; median 143 days from vaccine dose 2 to illness onset) ( P < .001). A lower aOR for the mRNA-1273 vaccine compared with the BNT162b2 vaccine for patients with illness onset greater than 120 days after vaccination was observed after restricting to patients without immunocompromising conditions and further stratifying these patients into younger (18-64 years) and older (≥65 years) groups (eTables 3-5 in the Supplement ). By SARS-CoV-2 variants sequenced, COVID-19 hospitalization was strongly associated with lower likelihood of vaccination for both the B.1.1.7 (Alpha) variant (aOR, 0.10; 95% CI, 0.06-0.16) and B.1.617.2 or AY (Delta) variant (aOR, 0.14; 95% CI, 0.10-0.21).
Among 1197 patients hospitalized with COVID-19 between March 11, 2021, and July 14, 2021, 142 (11.9%) were vaccinated breakthrough cases and 1055 (88.1%) were unvaccinated. Compared with unvaccinated cases, vaccine breakthrough cases were older and had more chronic medical conditions ( Table ). Compared with unvaccinated cases, vaccine breakthrough cases less commonly received ICU-level care (24.6% vs 40.1%; absolute difference, −15.5%; 95% CI, −23.1% to −7.8%; P < .001) and invasive mechanical ventilation (7.7% vs 23.0%; absolute difference, −15.3%; 95% CI, −20.4% to −10.2%; P < .001) (eTable 6 in the Supplement ).
Unvaccinated patients accounted for 93.9% (261/278) of cases with disease progression to death or invasive mechanical ventilation. The composite of death or mechanical ventilation was experienced by 17 of 142 (12.0%) vaccine breakthrough cases and 261 of 1055 (24.7%) unvaccinated cases. Among patients hospitalized with COVID-19, death or invasive mechanical ventilation was associated with a lower likelihood of vaccination (aOR, 0.33; 95% CI, 0.19-0.58) ( Figure 3 ). Restricting to cases admitted with hypoxemia (n = 902, 75.4% of cases), death or mechanical ventilation was also associated with a lower likelihood of vaccination (aOR, 0.30; 95% CI, 0.16-0.58). Receipt of 1 or more COVID-19–related therapeutics during hospitalization was also associated with a lower likelihood of vaccination (aOR, 0.32; 95% CI, 0.20-0.52) (eTable 7 in the Supplement ).
Unvaccinated patients accounted for 91.0% (91/100) of deaths among patients with COVID-19 in this study. Death occurred in 9 of 142 (6.3%) vaccine breakthrough cases and 91 of 1055 (8.6%) unvaccinated patients with COVID-19. Progression to death after COVID-19 hospitalization was associated with a lower likelihood of vaccination (aOR, 0.41; 95% CI, 0.19-0.88).
According to the World Health Organization COVID-19 Clinical Progression Scale, the highest level of disease severity experienced was significantly lower among vaccine breakthrough cases than unvaccinated cases (aOR, 0.36; 95% CI, 0.25-0.51) (eFigure 2 in the Supplement ). Hospital discharge alive within 28 days of hospital admission was experienced by 125 of 142 (88.0%) vaccine breakthrough cases and 814 of 1055 (77.2%) unvaccinated cases ( P = .003). In the competing risk model evaluating time to hospital discharge with a competing risk of death, vaccine breakthrough cases had a higher rate of hospital discharge (ie, shorter length of stay) than unvaccinated cases (subdistribution adjusted hazard ratio, 1.73; 95% CI, 1.42-2.10). These findings remained consistent when stratified by age and immunocompromised status ( Figure 4 ).
In this analysis of adults hospitalized in 21 US hospitals between mid-March and mid-August 2021, vaccination with an mRNA COVID-19 vaccine was significantly less likely among patients with COVID-19 than other conditions and among those with COVID-19 who progressed to death or mechanical ventilation than those with COVID-19 who did not have disease progression. These findings are consistent with risk reduction of developing severe COVID-19 among patients with vaccine breakthrough infections compared with absence of vaccination.
The aOR in this analysis corresponds to an estimated overall vaccine effectiveness of 85% for mRNA vaccines to prevent COVID-19 hospitalizations. The findings also correspond to an estimated vaccine effectiveness of 90% for the immunocompetent population and 86% for COVID-19 hospitalizations caused by the Delta variant. When the mRNA-1273 and BNT162b2 vaccines were compared, estimated vaccine effectiveness was similar within 120 days of vaccination. In contrast, beyond 120 days, the results corresponded to an estimated effectiveness of 85% for the mRNA-1273 and 64% for the BNT162b2 vaccine to prevent COVID-19 hospitalizations.
Among patients hospitalized with COVID-19, the outcome of death or invasive mechanical ventilation was associated with a lower likelihood of vaccination. These data suggest that the COVID-19 mRNA vaccines may attenuate disease severity among patients who develop COVID-19 despite vaccination, and the total benefits of vaccination exceed those estimated from the prevention of hospitalization alone.
These data complement vaccine trials and emerging postmarketing data that suggest receipt of mRNA vaccination is associated with risk reduction of severe COVID-19. The mRNA vaccine clinical trials were not powered to address severe disease, including complications after hospitalization. 29 , 30 Observational postmarketing studies, including this analysis, have consistently demonstrated a strong association between vaccination and risk reductions in COVID-19 hospitalization in immunocompetent individuals, suggesting that the high efficacy observed in mRNA clinical trials translates into beneficial effects in the community setting. 2 , 3 , 10 As vaccine coverage increases, breakthrough cases are also expected to increase. Concerns about vaccine failure against severe disease are especially likely among patients with complicated comorbidities who are overrepresented in inpatient settings compared with the general population. This analysis demonstrated a strong association between hospitalization for COVID-19 and lower likelihood of vaccination. Moreover, disease progression to critical illness after hospital admission was associated with a lower likelihood of vaccination among a population representing typical hospitalized patients in the US, which included high prevalence of medical comorbidities and multimorbidity.
Recent surges of COVID-19 cases from the SARS-CoV-2 Delta variant and signs of potential waning protection over time from a 2-dose mRNA vaccine series have prompted policy discussions about additional vaccine doses. 16 , 31 , 32 Several findings from this study could help inform ongoing policy discussions on implementing booster vaccination and guiding future research. First, the association between mRNA vaccination and reduced risk of COVID-19 hospitalization was substantially weaker in the immunocompromised population than the immunocompetent one, supporting recent recommendations for additional vaccine doses among immunocompromised persons. 33 Second, vaccine breakthrough COVID-19 hospitalization appeared to be more common with the BNT162b2 than the mRNA-1273 mRNA vaccine in this analysis. Third, the association between vaccination with the BNT162b2 vaccine and reduced risk of COVID-19 hospitalization declined after 4 months from vaccination, potentially indicating clinically important waning of protection over time, including for severe COVID-19.
Similar product-specific differences between the mRNA-1273 and BNT162b2 vaccines have also been reported in other recent observational studies in inpatient and outpatient settings. 34 Furthermore, recent immunologic studies have shown higher antibody responses after vaccination with mRNA-1273 compared with BNT162b2. 16 , 35 These differences may be related to higher antigen content in the mRNA-1273 vaccine, a longer recommended interval between vaccine doses (4 weeks for mRNA-1273 and 3 weeks for BNT162b2), or both. However, differences in the population vaccinated with the mRNA-1273 and BNT162b2 vaccines could also contribute to observed differences in vaccine breakthrough. Differences in memory B- and T-cell responses between COVID-19 vaccines have not been assessed and may be robust and similar for both mRNA vaccine products. 36
An unresolved question is whether observed differences by product and time since vaccination are due to declining immunity, evasion of immunity by the Delta variant, or a combination of the 2. Disentangling the mechanism of decline in protection could inform decisions on whether improving protection would be better achieved through booster vaccine dosing of the same products or administration of new vaccine formulations with a strain change, as is done with seasonal influenza vaccines. 37 These issues are epidemiologically challenging to disentangle with certainty because the Alpha variant preceded Delta circulation, and thus patients infected with the Delta variant also were more likely to have been vaccinated longer ago. The association between prior vaccination and COVID-19 hospitalization was strong for sequenced Alpha and Delta variants. Because the numbers sequenced were smaller than the full cohort numbers, the report also evaluated the magnitude of association by time since vaccination between March and June when the Alpha variant circulated vs July and August when the Delta variant predominated. The association between mRNA vaccination and reduced risk for COVID-19 hospitalization observed during the Delta variant circulation was high for participants with illness onset within 120 days of vaccination and lower for participants with illness onset after that period. This suggests that waning immunity rather than primary evasion by the Delta variant may be a driving mechanism of reduced vaccine protection observed. Whether this decline is restricted to specific high-risk subpopulations or vaccine types or is due to unmeasured confounding warrants further investigation.
This study has several limitations. First, although several relevant confounders were controlled for, unmeasured confounding in this observational case-control study could have occurred. Second, progression of COVID-19 to high severity was measured with multiple outcomes that considered death, organ failures, oxygen use, and duration of hospitalization. Although these measures do not comprehensively characterize disease severity, they capture life-threatening complications of COVID-19. Third, this analysis included only hospitalized patients and cannot inform whether vaccination attenuates COVID-19 severity among outpatients. Fourth, if vaccine breakthrough cases were systematically more likely to be hospitalized for COVID-19 of lesser severity than unvaccinated patients, our analyses of the association between vaccination and severe disease could be confounded by an admission bias. However, lower risk of progression to severe disease among vaccine breakthrough cases was sustained after the population was limited to patients who were admitted with hypoxemia. Fifth, sample size limitations prevented assessments of disease attenuation stratified by vaccine type, SARS-CoV-2 variant, and time since vaccination.
Vaccination with an mRNA COVID-19 vaccine was significantly less likely among patients with COVID-19 hospitalization and with disease progression to death or invasive mechanical ventilation. These findings are consistent with risk reduction of developing severe COVID-19 among vaccine breakthrough infections compared with absence of vaccination.
Corresponding Author: Mark W. Tenforde, MD, PhD, Centers for Disease Control and Prevention, 1600 Clifton Rd, Mailstop 24/7, Atlanta, GA 30329 ( [email protected] ).
Accepted for Publication: October 13, 2021.
Published Online: November 4, 2021. doi:10.1001/jama.2021.19499
Author Contributions: Dr Tenforde had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs Tenforde and Self and Ms Adams contributed equally to this work as lead authors.
Concept and design : Tenforde, Self, Adams, Ginde, Talbot, Hager, Exline, Gong, Peltan, Brown, Busse, Qadir, Grijalva, Rice, Kobayashi, Verani, Patel.
Acquisition, analysis, or interpretation of data : Tenforde, Self, Adams, Gaglani, Ginde, McNeal, Ghamande, Douin, Talbot, Casey, Mohr, Zepeski, Shapiro, Gibbs, Files, Hager, Shehu, Prekker, Erickson, Exline, Gong, Mohamed, Henning, Steingrub, Peltan, Brown, Martin, Monto, Khan, Hough, Busse, ten Lohuis, Duggal, Wilson, Gordon, Qadir, Chang, Mallow, Rivas, Babcock, Kwon, Halasa, Chappell, Lauring, Grijalva, Rice, Jones, Stubblefield, Baughman, Womack, Rhoads, Lindsell, Hart, Zhu, Olson, Patel.
Drafting of the manuscript : Tenforde, Self, Adams, Mohamed, Khan, Mallow, Patel.
Critical revision of the manuscript for important intellectual content : Tenforde, Self, Adams, Gaglani, Ginde, McNeal, Ghamande, Douin, Talbot, Casey, Mohr, Zepeski, Shapiro, Gibbs, Files, Hager, Shehu, Prekker, Erickson, Exline, Gong, Henning, Steingrub, Peltan, Brown, Martin, Monto, Khan, Hough, Busse, ten Lohuis, Duggal, Wilson, Gordon, Qadir, Chang, Mallow, Rivas, Babcock, Kwon, Halasa, Chappell, Lauring, Grijalva, Rice, Jones, Stubblefield, Baughman, Womack, Rhoads, Lindsell, Hart, Zhu, Olson, Kobayashi, Verani, Patel.
Statistical analysis : Tenforde, Adams, Talbot, Casey, Monto, Grijalva, Lindsell, Zhu, Olson.
Obtained funding : Self, Steingrub, Verani, Patel.
Administrative, technical, or material support : Self, Mohr, Files, Hager, Exline, Gong, Khan, ten Lohuis, Duggal, Wilson, Gordon, Qadir, Mallow, Babcock, Jones, Baughman, Womack, Rhoads, Lindsell, Hart, Kobayashi, Verani, Patel.
Supervision : Self, Mohr, Shapiro, Exline, Henning, Martin, Monto, Khan, ten Lohuis, Wilson, Gordon, Halasa, Chappell, Grijalva, Hart, Patel.
Operational support for enrollment : McNeal.
Data cleaning, data management : Olson.
Supervision of staff at study site : Kwon.
Conflict of Interest Disclosures: Dr Self reported receiving grants from the Centers for Disease Control and Prevention (CDC) (principal investigator of the primary funding contract from CDC for this work) during the conduct of the study. Dr Gaglani reported receiving grants from CDC, Vanderbilt University Medical Center, Baylor Scott & White Health (BSWH), and the IVY study during the conduct of the study; grants from CDC–BSWH HAIVEN influenza/COVID-19 vaccine effectiveness study, CDC–BSWH ambulatory US influenza/COVID-19 vaccine effectiveness study, CDC–Abt Associates BSWH RECOVER COVID-19/influenza study, and CDC–Westat BSWH VISION COVID-19/influenza study outside the submitted work; and Pfizer BSWH Independent Grants for Learning & Change for meningococcal vaccination of adolescents and an institutional contract with Janssen (BSWH Observational RSV Study in infants). Dr Ginde reported receiving grants from CDC during the conduct of the study; and grants from the National Institutes of Health (NIH), Department of Defense, and AbbVie outside the submitted work. Dr McNeal reported receiving grants from the CDC HAIVEN study group that become the IVY-3 study group during the conduct of the study. Dr Talbot reported receiving grants from CDC during the conduct of the study. Dr Casey reported receiving grants from NIH K23HL153584 outside the submitted work. Dr Mohr reported receiving grants from CDC during the conduct of the study. Dr Shapiro reported receiving grants from CDC during the conduct of the study. Dr Files reported receiving grants from CDC during the conduct of the study and personal fees from Cytovale and Medpace outside the submitted work. Dr Prekker reported receiving grants from CDC during the conduct of the study. Dr Exline reported receiving a speaking honorarium from Abbott Laboratories outside the submitted work. Dr Gong reported receiving grants from CDC during the conduct of the study; grants from NIH to conduct clinical trials on COVID-19 and non–COVID-19 outside the submitted work; and data and safety monitoring board fees for participating in Regeneron trials outside the submitted work. Dr Henning reported receiving grants from CDC during the conduct of the study. Dr Peltan reported receiving grants from CDC during the conduct of the study; grants from NIH, Intermountain Research and Medical Foundation, and Janssen Pharmaceuticals outside the submitted work; and payment to Intermountain Medical Center for subject enrollment from Regeneron and Asahi Kasei Pharma outside the submitted work. Dr Brown reported receiving grants from CDC during the conduct of the study. Dr Martin reported receiving grants from CDC during the conduct of the study and personal fees from Pfizer outside the submitted work. Dr Khan reported receiving grants from United Therapeutics, Actelion Pharmaceuticals, Eli Lilly, Johnson & Johnson, Regeneron Pharmaceuticals, and Gilead Sciences outside the submitted work. Dr Hough reported receiving grants from CDC during the conduct of the study and grants from NIH outside the submitted work. Dr Wilson reported receiving grants from CDC/Vanderbilt during the conduct of the study. Dr Chang reported receiving personal fees from La Jolla Pharmaceuticals and PureTech Health outside the submitted work. Dr Babcock reported receiving grants from CDC during the conduct of the study. Dr Kwon reported receiving grants from NIH National Institute of Allergy and Infectious Diseases (award 1K23 AI137321-01A1) outside the submitted work. Dr Halasa reported receiving grants from CDC during the conduct of the study; grants from Sanofi outside the submitted work; and hemagglutination inhibition and microneutralization testing, vaccine donation, and grants from Quidel outside the submitted work. Dr Chappell reported receiving grants from CDC during the conduct of the study. Dr Lauring reported receiving consulting fees from Sanofi for an influenza antiviral and fees from Roche as a member of an influenza antiviral trial steering committee outside the submitted work. Dr Grijalva reported receiving a contract from CDC during the conduct of the study; consulting fees from Pfizer, Merck, and Sanofi; a contract from CDC, Campbell Alliance, and the Food and Drug Administration outside the submitted work; and grants from NIH and the Agency for Healthcare Research and Quality outside the submitted work. Dr Rice reported receiving grants from CDC during the conduct of the study and personal fees from Cumberland Pharmaceuticals, Sanofi, and Cytovale outside the submitted work. Dr Lindsell reported receiving grants from CDC to Vanderbilt University during the conduct of the study; grants from NIH to institution, grants from Department of Defense to institution, contracts to Vanderbilt University for research services from bioMérieux, Endpoint Health, and Entegrion. In addition, he had a patent for risk stratification in sepsis and septic shock, issued to Cincinnati Children's Hospital Medical Center. Dr Zhu reported receiving grants from CDC during the conduct of the study. No other disclosures were reported.
Funding/Support: Primary funding for this study was provided by the CDC (75D30121F00002).
Role of the Funder/Sponsor: Investigators from CDC were involved in all aspects of the study, including the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The CDC had the right to control decisions about publication via the CDC publication clearance process.
Group Information: A full list of investigators and collaborators in the Influenza and Other Viruses in the Acutely Ill (IVY) Network is available in eAppendix 1 in the Supplement .
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Case-control vaccine effectiveness studies: Preparation, design, and enrollment of cases and controls
Affiliations.
- 1 National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, 1600 Clifton Rd., Atlanta, GA, USA. Electronic address: [email protected].
- 2 International Center for Maternal and Newborn Health, Johns Hopkins Bloomberg School of Public Health, 615 N Wolfe St, Baltimore, MD, USA.
- 3 Rollins School of Public Health Emory University, 1518 Clifton Rd, Atlanta, GA, USA.
- 4 Department of Immunizations, Vaccines and Biologicals, World Health Organization, 20 Avenue Appia, 1211 Geneva, Switzerland.
- 5 Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases, 1 Modderfontein Road, Sandringham, Johannesburg, South Africa.
- 6 National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, 1600 Clifton Rd., Atlanta, GA, USA.
- 7 National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, 1600 Clifton Rd., Atlanta, GA, USA; International Vaccine Access Center, Johns Hopkins Bloomberg School of Public Health, 615 N Wolfe St, Baltimore, MD, USA.
- 8 Respiratory and Meningeal Pathogens Unit, University of Witwatersrand, Richard Ward, 1 Jan Smuts Ave, Braamfontein, Johannesburg, South Africa.
- 9 Monitoring & Evaluation, Policy & Performance, GAVI Alliance, Chemin des Mines 2, 1202 Geneva, Switzerland.
- 10 Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases, 1 Modderfontein Road, Sandringham, Johannesburg, South Africa; Respiratory and Meningeal Pathogens Unit, University of Witwatersrand, Richard Ward, 1 Jan Smuts Ave, Braamfontein, Johannesburg, South Africa.
- 11 Murdoch Children's Research Institute, Royal Children's Hospital, 50 Flemington Rd, Parkville, VIC 3052, Australia; Department of Infectious Disease Epidemiology, Faculty of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, Keppel St, London WC1E 7HT, UK.
- 12 International Vaccine Access Center, Johns Hopkins Bloomberg School of Public Health, 615 N Wolfe St, Baltimore, MD, USA.
- 13 Department of Infectious Disease Epidemiology, Faculty of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, Keppel St, London WC1E 7HT, UK.
- 14 Department of Infectious Disease Epidemiology, Faculty of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, Keppel St, London WC1E 7HT, UK; KEMRI-Wellcome Trust Research Programme, P.O. Box 230-80108, Kilifi, Kenya.
- 15 MRC Tropical Epidemiology Group, London School of Tropical Medicine and Hygiene, London, UK.
- 16 Centre of Intervention Science in Maternal and Child Health and Centre for International Health, University of Bergen, P.O. Box 7800, Bergen, Norway; Department of International Public Health, Norwegian Institute of Public Health, PO Box 4404, Nydalen, Oslo, Norway.
- 17 PATH, 2201 Westlake Avenue, Seattle, WA, USA.
- 18 Aga Khan University, Stadium Rd, Karachi, Pakistan.
- PMID: 28442231
- PMCID: PMC7007298
- DOI: 10.1016/j.vaccine.2017.04.037
Case-control studies are commonly used to evaluate effectiveness of licensed vaccines after deployment in public health programs. Such studies can provide policy-relevant data on vaccine performance under 'real world' conditions, contributing to the evidence base to support and sustain introduction of new vaccines. However, case-control studies do not measure the impact of vaccine introduction on disease at a population level, and are subject to bias and confounding, which may lead to inaccurate results that can misinform policy decisions. In 2012, a group of experts met to review recent experience with case-control studies evaluating the effectiveness of several vaccines; here we summarize the recommendations of that group regarding best practices for planning, design and enrollment of cases and controls. Rigorous planning and preparation should focus on understanding the study context including healthcare-seeking and vaccination practices. Case-control vaccine effectiveness studies are best carried out soon after vaccine introduction because high coverage creates strong potential for confounding. Endpoints specific to the vaccine target are preferable to non-specific clinical syndromes since the proportion of non-specific outcomes preventable through vaccination may vary over time and place, leading to potentially confusing results. Controls should be representative of the source population from which cases arise, and are generally recruited from the community or health facilities where cases are enrolled. Matching of controls to cases for potential confounding factors is commonly used, although should be reserved for a limited number of key variables believed to be linked to both vaccination and disease. Case-control vaccine effectiveness studies can provide information useful to guide policy decisions and vaccine development, however rigorous preparation and design is essential.
Keywords: Case-control studies; Evaluation studies; Vaccines.
Published by Elsevier Ltd.
Publication types
- Research Support, Non-U.S. Gov't
- Case-Control Studies*
- Control Groups
- Immunization Programs*
- Immunogenicity, Vaccine
- Treatment Outcome
- Vaccination
Grants and funding
- 001/WHO_/World Health Organization/International
- 203077/WT_/Wellcome Trust/United Kingdom
- MR/K012126/1/MRC_/Medical Research Council/United Kingdom
- U01 IP001048/IP/NCIRD CDC HHS/United States
Study shows no link between stillbirths, COVID-19 vaccines
A new study from Yale researchers in Obstetrics and Gynecology shows no link between stillbirth and COVID-19 vaccines. Moreover, pregnant women who had received COVID-19 vaccines in pregnancy were at a decreased risk of preterm birth.
The authors say the findings should offer further reassurance that COVID-19 vaccination is safe and useful in pregnancy.
"The results of this robust case-control study can be used to reassure both pregnant patients and health care professionals that COVID-19 vaccination in pregnancy is not associated with an increased risk of pregnancy loss," said study author Anna Denoble, MD, in a press release.
The results of this robust case-control study can be used to reassure both pregnant patients and health care professionals
The study was based on outcomes seen in the Vaccine Safety Datalink, and compared receipt of the vaccine among women who had stillbirths or fetal death after 20 weeks gestation and those who had live births.
Vaccines not associated with any negative birth outcomes
A total of 55,591 people were included, and 23,517 (42.3%) received one or two mRNA COVID-19 vaccine doses during pregnancy.
Overall, 38.4% of patients experiencing stillbirth received a COVID-19 vaccine in pregnancy compared with 39.3% of those with live births.
For pre-term birth, vaccinated women had a rate of 6.4 per 100, compared to 7.7 per 100 for unvaccinated. COVID-19 vaccines were not associated with preeclampsia, gestational hypertension, or small-for-gestational age neonates.
"Results from this study provide additional evidence regarding the safety of mRNA COVID-19 vaccine administration during pregnancy, the authors concluded.
Quick Takes: US COVID-19 levels, H5N1, and Polio
- The latest data from the Centers for Disease Control and Prevention (CDC) show upticks in some markers the agency uses to track COVID-19, though overall activity remains muted. According to the CDC's COVID Data Tracker, test positivity is up 0.4% from the previous week and now at 4.5%, while the percentage of emergency department visits diagnosed with COVID rose by 16.2% and is at 0.5% overall. No changes were seen in the two severity indicators, hospitalizations and deaths, which both remain at low levels. COVID wastewater levels also remain low, but high levels are being reported in the West and in Florida. Variant proportion estimates show the JN.1 offshoots KP.3 and KP.2 now account for 47% of sequenced samples, up from 41.2% the previous week, while LB.1 makes up 14.9%.
- The Michigan Department of Agriculture and Rural Development today announced the detection of H5N1 avian influenza in a dairy herd in Clinton County, the 25 th effected dairy herd in the state. The outbreak total now stands at 87 herds in 11 states. Also today, the CDC posted updated recommendations for symptom monitoring in people exposed to H5N1-infected birds, cattle, or other animals. The guidelines call for local or state health departments to monitor symptoms in exposed farm workers for 10 days after exposure. "Monitoring exposed individuals can help to rapidly identify human cases, provide appropriate treatment, prevent onward spread, and help understand the scope of human risk," the agency said.
- New polio cases were reported in three countries this week, according to the latest update from the Global Polio Eradication Initiative. Afghanistan and Pakistan each recorded one wild poliovirus type (WPV1) case, the fourth WPV1 case reported in each country this year. Also, Niger reported one circulating vaccine-derived poliovirus type 2 (cVDPV2) case, its second case this year.
Study ties prevalence of drug-resistant organisms to socioeconomic conditions
A study in Texas found higher rates of antimicrobial-resistant (AMR) organisms in areas with higher levels of economic deprivation, researchers reported today in Clinical Infectious Diseases.
Using electronic health records from two large healthcare systems in the Dallas-Fort Worth metropolitan area, a team led by researchers from the University of Texas Southwestern Medical Center collected select patient bacterial culture results from 2015 to 2020. They chose five AMR organisms to represent potential community- and healthcare-associated acquisition, used residential addresses to geocode cultures and link them to socioeconomic index values, and identified geographic clusters of high and low AMR organism prevalence.
Among the 43,677 unique cultures collected, 43.5% were identified as methicillin-resistant Staphylococcus aureus (MRSA), 31% as extended-spectrum beta-lactamase (ESBL)–producing organisms, 11.3% as carbapenem-resistant Enterobacterales, 8.2% as vancomycin-resistant Enterococcus , and 5.9% as AmpC beta-lactamase producers. Significant clusters of all five organisms were found in areas with high levels of economic deprivation, as measured by the area deprivation index (ADI), and there was significant spatial autocorrelation between ADI and AMR prevalence.
The strongest correlation was observed for MRSA and AmpC, with 14% and 13%, respectively, of the variability in prevalence rates attributed to their relationship with the ADI values of the neighboring locations.
Tailored interventions may be needed
The study authors say several factors could explain why AMR organisms are more prevalent in areas with greater socioeconomic disparities. For example, people in areas with higher ADI may have poorer sanitation due to utility disruptions or poor plumbing and therefore be more likely to be exposed to bacteria, or may live in overcrowded settings that can contribute to the spread of infection. The practice of sharing "leftover" antibiotics with friends and family could also select for resistant organisms.
While further research is needed to delineate the relationship, they say the findings highlight the importance of addressing these disparities.
"This finding that ADI is associated with higher prevalence of AMR supports the need for tailored, local interventions for small neighborhoods including social programs that increase access to health insurance, along with public health campaigns discussing proper hygiene techniques to prevent the spread of disease within a household," they wrote.
In case you missed it
This week's top reads, h5 influenza wastewater dashboard launches.
Though the test can't pinpoint the virus subtype or source, most of the detections are from states hard hit by H5N1 in dairy herds.

High-path avian flu strikes Iowa layer farm as USDA reports more mammal detections
The USDA's updated list of H5N1 detections in mammals includes domestic cats in 4 states.
Study reveals persistent risk of death, symptoms in COVID survivors at 3 years
"A brief, seemingly innocuous or benign encounter with the virus can still lead to health problems years later," one study author says.

Man dies from H5N2 avian flu in Mexico; Minnesota reports first case in dairy cow
Minnesota, Iowa report infected dairy herds.

N95 respirator gets top billing in stopping SARS-CoV-2 viral leakage into the air
The "duckbill" N95 stopped 98% of the virus that causes COVID-19, the authors say.

Report: More than 200 symptoms tied to long COVID
The report "offers a comprehensive review of the evidence base for how Long COVID may impact a patient's ability to engage in normal activities."

Report describes emerging sexually transmitted fungal infection
The infection is caused by Trichophyton mentagrophytes type VII, a fungus that may spread via sexual contact.
FDA panel supports switch to JN.1 for fall COVID vaccines
Though the vote was unanimous, discussions were complicated by the diversity of JN.1 viruses.
Study: Truthful yet misleading Facebook posts drove COVID vaccine reluctance much more than outright lies did
Unflagged vaccine-skeptical content cut vaccination intent by 2.28 percentage points per user, versus −0.05 percentage points for flagged content.

Second dairy farm worker infected with H5 avian flu in Michigan
Unlike similar earlier cases in the United States, the newly reported patient had respiratory symptoms.

Our underwriters
Unrestricted financial support provided by.

- Antimicrobial Resistance
- Chronic Wasting Disease
- All Topics A-Z
- Resilient Drug Supply
- Influenza Vaccines Roadmap
- CIDRAP Leadership Forum
- Roadmap Development
- Coronavirus Vaccines Roadmap
- Antimicrobial Stewardship
- Osterholm Update
- Newsletters
- About CIDRAP
- CIDRAP in the News
- Our Director
- Osterholm in the Press
- Shop Merchandise
To revisit this article, visit My Profile, then View saved stories .
- Backchannel
- Newsletters
- WIRED Insider
- WIRED Consulting
Emily Mullin
The Case for MDMA’s Approval Is Riddled With Problems

Only two drugs are formally approved for post-traumatic stress disorder, or PTSD , and they don’t help everyone. A lack of effective treatment options has led some patients to seek out the psychedelic drug MDMA, also known as ecstasy, to help relieve their symptoms when traditional medications and therapy don’t work.
In the US, momentum has been growing to legalize MDMA and other psychedelics. Lykos Therapeutics, which has been testing MDMA alongside psychotherapy in clinical trials for years, had a chance this week to prove that the combination is effective at treating PTSD. But at a June 4 meeting , a panel of advisers to the US Food and Drug Administration overwhelmingly voted that there wasn’t enough evidence to recommend its approval. Just two of the 11 committee members were convinced that the treatment was effective, and only one said its benefits outweighed the risks.
Though trial results were generally positive, FDA advisers cited a long list of problems with the data Lykos presented. For one, because it’s not hard to tell whether or not you’re experiencing the effects of a psychedelic drug, participants likely knew whether they had received MDMA or placebo, which could have biased how they reported feeling afterward. The psychotherapy administered during the trial was also under fire, with advisers saying it wasn’t standardized across providers and pointing to misconduct allegations as cause for concern. And many participants had tried MDMA before, potentially making them more likely to report a positive experience. Experts also raised questions about how long symptom relief lasts and whether there was enough monitoring of the drug’s side effects, among other issues.
The FDA is expected to decide by August 11 on the approval of MDMA plus psychotherapy for PTSD, and while the agency typically goes along with advisory committee recommendations, it doesn’t always. The US has yet to approve a psychedelic drug for medical use, so a green light from the agency would represent a major milestone for a class of drugs that has been banned since the 1970s. But now that approval is anything but certain.
“I absolutely agree that we need new and better treatments for PTSD,” said committee member Paul Holtzheimer, deputy director for research at the National Center for PTSD, at the meeting. “However, I also note that premature introduction of a treatment can actually stifle development and stifle implementation and lead to premature adoption of treatments that are either not completely known to be safe, not fully effective, or not being used at their optimal efficacy.”
Advocates of MDMA-assisted therapy were crushed by the recommendation. “My life was saved by psychedelic-assisted therapy,” says Juliana Mercer, director of veteran advocacy and public policy at Healing Breakthrough, a nonprofit working to make MDMA-assisted therapy available to veterans with PTSD. “I think they lost the big picture as to why we need this. We don't have another solution to the veteran suicide epidemic.”
The committee based its decision on data from two trials of nearly 200 individuals with PTSD. The company behind the trials, Lykos, is a spinoff of the Multidisciplinary Association for Psychedelic Studies, a nonprofit based in San Jose, California, that has been working to legalize MDMA since it was founded in 1986. Data from one of the studies concluded that 67 percent of participants who had MDMA and psychotherapy no longer met the criteria for PTSD, compared to 32 percent who received a placebo and therapy. In the other study , 71 percent of participants in the MDMA group no longer met those criteria, versus 48 percent in the placebo group.

By Matt Burgess

By Andy Greenberg

By Matthew Gault
The results sounds promising, but Michael Ostacher, a professor of psychiatry and behavioral sciences at Stanford University who’s not involved with Lykos and wasn’t on the FDA panel, says there’s a big problem: “It's unclear whether or not the participation in the study and anticipation of the effect is what makes people better, rather than the impact of the drug itself.”
In medical research, a double-blinded placebo controlled trial—in which neither the participants nor the researchers know who gets a placebo or the experimental treatment—is considered the gold standard. But the effects of psychedelics are so well known that it’s easy for volunteers and therapists alike to guess whether they’d received it. Ostacher says the growing hype over psychedelics has created certain expectations for people enrolled in these studies.
“My main concern is that people in the trial who did not receive the MDMA would experience a lot of disappointment about not getting the drug, and that would have a large influence on how they reported their symptoms,” he says. “In the same fashion, the people who did get the MDMA, which has been advertised by advocates as a life-changing treatment, would be inclined to feel positive about their experience.”
This “unblinding” effect is a well known conundrum not just for Lykos, but for the entire field of psychedelics research also, and scientists are currently considering alternative ways of designing trials to account for this potential bias.
Another issue surrounds the psychotherapy, or talk therapy, administered during the trial sessions. When participants took MDMA or placebo, two therapists were in the room to help them express and process their memories and emotions during eight-hour sessions. Lykos describes this therapy as a “personalized experience,” but FDA committee members had concerns with the variability in therapy approaches and how much of patient outcomes could be attributed to the drug itself versus the therapy.
Natalie Gukasyan, an assistant professor of psychiatry at Columbia University who studies psychedelics, says “there’s a lot of wiggle room” in the Lykos manual on how the therapy session can be conducted. “If the treatment is medication-assisted psychotherapy, maybe a little bit more of a prescribed therapy is appropriate,” she says.
The FDA does not regulate therapy, nor is it involved with credentialing of psychotherapists, which raises the question of how therapy would be standardized. Gukasyan wonders whether it’s appropriate for Lykos to be involved with training therapists because it could present a conflict of interest.
While some of the participants who testified on Tuesday or submitted written comments to the committee described positive stories of their sessions and lasting benefits, others had negative experiences. In one particularly troubling report, former trial participant Meaghan Buisson testified that her therapists, a married couple, pinned her down on a bed while stroking and cuddling her. (New York Magazine reported on Buisson’s experiences two years ago, publishing a video of this incident.)
Another volunteer, Sarah McNamee, wrote that during her MDMA session, her therapists told her she was “helping make history” and that she was “part of a movement.” According to McNamee, they encouraged her to give a positive report of the experience, saying her responses during and after the trial could jeopardize the drug’s legalization. When her mental health symptoms worsened, she was told she would feel better in six months.
Committee members also noted that Lykos was missing data on the drug’s effects on liver function and whether participants went on to abuse MDMA after the trial.
Many of the points raised at Tuesday’s meeting were echoed in a report released earlier this year by the Institute for Clinical and Economic Review, a nonprofit that evaluates drug efficacy and prices, which said it had “substantial concerns about the validity of the results” of the Lykos trials.
Lykos stands by its research and representatives for the company said during Tuesday’s hearing that many patients enrolled in its trials showed meaningful benefits from MDMA-assisted therapy. Kelley O’Donnell, director of clinical training at the NYU Langone Center for Psychedelic Medicine, said she saw patients “visibly brighten over the course of treatment.”
In a statement after Tuesday's meeting , Lykos CEO Amy Emerson said the company remains committed to finding a path forward for MDMA-assisted therapy and is working with the FDA to address outstanding questions.
And while patients’ positive experiences can’t be discounted, the FDA will be more interested in data rather than anecdotes. The agency’s decision could either pave the way for a whole new class of psychiatric medicines or send Lykos and other companies back to the drawing board to figure out how best to prove the efficacy of psychedelics.
“In many ways we’re creating a new field of medicine,” said Lykos’ senior medical director, Alia Lilienstein, in Tuesday’s meeting. But in order for patients to benefit, Lykos and other companies will first have to prove that they actually work.
You Might Also Like …
In your inbox: Get Plaintext —Steven Levy's long view on tech
Inside the biggest FBI sting operation in history
The WIRED AI Elections Project : Tracking more than 60 global elections
Ecuador is literally powerless in the face of drought
Rest assured: Here are the best mattresses you can buy online

Max G. Levy
Beth Mole, Ars Technica
- Open access
- Published: 03 June 2024
STING agonists as promising vaccine adjuvants to boost immunogenicity against SARS-related coronavirus derived infection: possible role of autophagy
- Aysa Rezabakhsh 1 ,
- M. Reza Sadaie 2 ,
- Alireza Ala 3 ,
- Yousef Roosta 4 ,
- Solomon Habtemariam 5 ,
- Adeleh Sahebnasagh 6 &
- Mohammad Rafi Khezri ORCID: orcid.org/0000-0002-4280-0378 7
Cell Communication and Signaling volume 22 , Article number: 305 ( 2024 ) Cite this article
223 Accesses
2 Altmetric
Metrics details
As a major component of innate immunity and a positive regulator of interferons, the Stimulator of interferon gene (STING) has an immunotherapy potential to govern a variety of infectious diseases. Despite the recent advances regarding vaccines against COVID-19, nontoxic novel adjuvants with the potential to enhance vaccine efficacy are urgently desired. In this connection, it has been well-documented that STING agonists are applied to combat COVID-19. This approach is of major significance for boosting immune responses most likely through an autophagy-dependent manner in susceptible individuals against infection induced by severe acute respiratory syndrome Coronavirus (SARS‑CoV‑2). Given that STING agonists exert substantial immunomodulatory impacts under a wide array of pathologic conditions, these agents could be considered novel adjuvants for enhancing immunogenicity against the SARS-related coronavirus. Here, we intend to discuss the recent advances in STING agonists’ recruitment to boost innate immune responses upon vaccination against SARS-related coronavirus infections. In light of the primordial role of autophagy modulation, the potential of being an antiviral vaccine adjuvant was also explored.
Introduction
The stimulator of interferon genes (STINGs), encoding the transmembrane protein 173, plays a critical role in innate immunity instigation against a wide variety of infections [ 1 , 2 , 3 ]. Recently, the STING pathway has been proposed as a cancer vaccine adjuvant primarily due to the fact that endogenous activation of STING results in the modulation of cellular immunity mainly mediated by cytotoxic CD8 + T cells [ 4 ]. In coronavirus infection, stimulation of the innate and adaptive immune systems is mediated by interferons (IFNs), containing IFN-α and –β subunits [ 5 ]. Of note, both IFN-I and -III are defined as cytokines that prompt the first-line defense against pathogens, particularly viruses [ 6 ]. Similar to other viruses, the severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) recruits advanced mechanisms for evading both host immune response and antiviral functions mediated by IFN-I and –III at multiple stages [ 7 ]. However, the potential clinical utility of STING agonists warrants further investigation.
Calling attention, activation of STING concurrently stimulates a multifaceted IFN-I-mediated immune response that further promotes the maturation and migration of dendritic cells (DCs), primes cytotoxic T cells, and natural killer (NK) cells for spontaneous immune responses [ 8 ]. Presumably, either persistent or aberrant activity of STING can suppress the immune response by engaging regulatory T cells (Tregs), infiltration of myeloid-derived suppressor cells (MDSC), and upregulation of the Programmed Cell Death 1 (PDCD1) gene [ 9 , 10 ]. This can further restrict the antigen-presenting function of DCs through an indoleamine 2, 3-dioxygenase (IDO)-kynurenine-dependent immune tolerance and apoptosis induction [ 11 , 12 ]. In COVID-19, IFN-I dysregulation is supposedly critical in disease pathogenesis [ 13 ]. In support of this hypothesis, either IFN-I pre-treatment or administration at the onset of the disease effectively prevents COVID-19 progression toward the severe form due to the autonomous antiviral state by diminishing viral load [ 14 ]. Regardless of autoimmune reactions and genetic disorders, IFN-I’s aberrance progressively promotes immunopathology, which can develop the severe form of COVID-19 [ 13 ]. Here, we aimed to discuss STING agonists’ and regulated autophagy effects in modulating immune responses upon the SARS-related coronavirus infections and COVID-19 vaccination.
Published Research and Data interpretations
Sting-related mechanism of action.
At the initial stage of the STING pathway, the cyclic guanosine monophosphate-adenosine monophosphate (cyclic GMP-AMP, cGAMP) synthase (cGAS) enzyme can sense cytosolic nucleic acid contents as a danger signal to instigate the STING-dependent IFN immune response [ 15 , 16 ]. A proof-of-concept study demonstrated that viral infections induced by DNA and RNA viruses exhibit two distinct STING pathways [ 17 ], as follows:
First, upon binding to cytosolic DNA, cGAS increases the levels of 2’ 3’-cGAMP and canonical cyclic dinucleotide (CDN) [ 18 ], two secondary messengers synthesized from adenosine triphosphates (ATP) and guanosine triphosphates (GTP), in turn, promotes STING activation in the endoplasmic reticulum (ER) during a length-dependent manner. In better words, the IFN immune response in the presence of cytosolic DNA can emerge at the short lengths of DNA, containing at least 20–40 base pairs (bp), which is entirely dependent on cGAS irrespective of DNA and its broad length span, ranging from the least stimulatory length to several kilobases [ 19 ].
The TANK-binding kinase 1 (TBK1) then activates IFNα/β by phosphorylation of IFN regulatory factor 3 (IRF3), a key effector of STING downstream, and non-canonical nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) [ 20 , 21 ]. Ultimately, activated IFN-β mainly released from Plasmacytoid DCs, T lymphocytes (Th1, CD 4+ T cells), and NK cells emerge in the target sites to promote the desirable antiviral effects (Fig. 1 ).
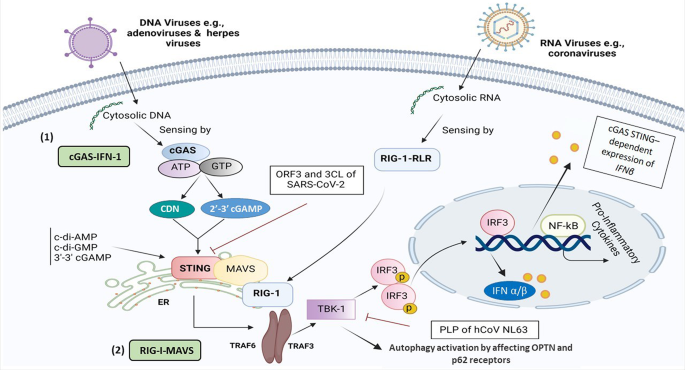
A schematic representation of STING-related cGAS and RIG-1-dependent signaling pathways in the presence of both DNA and RNA-based viruses, respectively. The drawing illustrates a comparison and shared molecular interplays and mechanisms involved in the stimulation and suppression of the immune system, in particular SARS-CoV-2 influence in the induction of inflammation and cytopathic effects through RIG-I, MAVS, TRIF, TBK1, TRAF3/6, and IRF3 signaling axis. This figure was created with BioRender.com. Abbreviations CDN, cyclic dinucleotide; cGAS, cyclic guanosine monophosphate-adenosine monophosphate (GMP-AMP) synthase; ER, endoplasmic reticulum; IFN-I, type I interferon, IRF3; interferon regulatory factor 3; MAVS, mitochondrial antiviral-signaling protein; NFκB, nuclear factor-κB; RIG-I, retinoic Acid-inducible gene I; RLR, RIG-like receptors; STING, stimulator of interferon response cGAMP; TBK1, TANK-binding kinase 1; TRAF3/6, Tumor necrosis factor receptor (TNFR) associated factors 3/6
Second, in comparison to DNA viruses that STING deploys desirable effects through a cGAS-IFN-I dependent manner, STING could also hamper replication of the RNA virus at the translation, but not transcription level, to prevent viral protein synthesis. This appears to occur through the synthetic retinoic acid-inducible gene I (RIG-I) pathway, demonstrated using transfection of RIG-like receptors (RLRs) ligands, and antiviral gene expression stimuli [ 17 ]. In addition, STING and mitochondrial antiviral-signaling protein (MAVS) can coordinate their gene expression as co-regulators through positive feedback [ 22 ]. Mechanistically, there is a cross-link between RIG-I-MAVS and cGAS-STING of RNA and DNA sensing pathways, respectively, for more improvement of the evolutionarily conserved innate immune responses (Fig. 1 ). As illustrated in Fig. 1 , the human coronavirus (hCoV) NL63, through its papain-like protease (PLP) domain, can interrupt STING-TBK1 interaction [ 23 ], leading to the suppression of IFNβ production. PLP impedes STING dimerization, K63-linked polyubiquitination of IFN-I, and negatively regulates IRF3 activation via interaction with STING- TNF receptor-associated factor (TRAF3)-TBK1 complex [ 24 , 25 ]. TRAF6 is a crucial adaptor protein related to the interleukin (IL)-1 receptor/ toll-like receptor (TLR) family and TNF-receptor superfamily [ 26 ]. Both TRAF3 and − 6 are predominately involved in virus-triggered signaling by connecting upstream adaptor proteins to downstream protein kinases and transcription factors [ 27 ].
Whether or not PLP in the SARS-CoV-2 virus can affect the STING-TRAF3-TBK1 complex is yet to be determined.
The cGAS-STING pathway in DNA/RNA-based viruses
Unlike SARS-CoV-2, other coronavirus infections induced by human coronavirus OC43 (HCoV-OC43) could not stimulate the STING pathway, while pharmacological stimulation of the STING-IRF3 pathway substantially hindered the HCoV-OC43 infection [ 28 ]. In this regard, diABZI exerted a desirable anti-coronavirus activity against both HCoV-OC43 and SARS-CoV-2 strains [ 28 ]. In contrast, other RNA viruses, such as flaviviruses exhibit a distinct characteristic of suppressing IFN-I production using NS2B/3 protease activity and cleavage of STING at residues 93–96 (LRRG) [ 29 ]. Furthermore, some enveloped RNA viruses, such as influenza A, can also instigate the STING-IFN axis independent of cGAS as a primary target for viral hemagglutinin glycoprotein or NS2B3 protease [ 30 ]. Also, infection by West Nile virus, an ssRNA virus, leads to higher viral loads and mortality rates in cGAS knockout mice. This was attributed to cGAS-mediated modulation of STING in the absence of direct sensing of the pathogen nucleic acid by cGAS [ 31 ]. In addition, some negative-strand RNA paramyxoviruses could trigger the cGAS-STING pathway. The last findings demonstrate that silencing the cGAS or STING gene reduced IFN-I production with a simultaneous enhanced paramyxoviral infection in vivo [ 32 ]. These observations advocate the feasibility assessments of developing virus-specific STNG adjuvants.
The outcomes of a comprehensive review have also corroborated that the RIG-I-MAVS pathway is a key cytosolic pathogen recognition receptor (PRR) in combating RNA viruses. The findings of this study highlighted that STING-RIG-1 agonists are taken into account as effective antiviral agents besides being vaccine adjuvants [ 22 ]. In parallel to these findings, other studies have also demonstrated the potent antiviral effect of STING induction through a RIG-1 agonist, namely 5’ triphosphorylated RNA (5’ pppRNA), which can increase STING induction both at transcriptional and translational levels following herpes simplex virus 1 (HSV), an enveloped dsDNA virus, infection [ 33 ]. In contrast to RNA, hepatitis B virus (HBV) DNA is also able to stimulate the innate immune response mediated by the cGAS–STING pathway. In addition, a sufficient amount of DNA transfected to hepatocytes in culture demonstrated reduced levels of DNA sensors compared to myeloid immune cells, resulting in HBV evasion of cGAS–STING sensing [ 34 ].
It can be also postulated that the STING plays a core effector role in priming downstream cascades in the interplay between distinct DNA and RNA sensing signaling pathways. Inhibition of the RIG-1-RLR-STING axis induced by evolving viral mechanisms should also be considered in host innate immune system failure, particularly in SARS-CoV-2 infection. Based on the results of a recent study, STING inhibition can be achieved through two distinct pathways:
1) A viral accessory protein named open reading frame (ORF) can prevent nuclear accumulation of p65 and subsequently inhibit the NFκB signaling,
2) The inhibitory effect of 3 C-like protease (3CL), as one of the main proteases targeted for therapeutic COVID-19 antiviral intervention, on K63 ubiquitination and potent suppression of STING-mediated NF-κB signaling [ 35 ].
As the STING mode of action following RNA/DNA viral infection, recent literature reported the elevated levels of monocytes with CD16 positive transcription factor T-box expressed in T cells (TBET) and CD14 positive IRF1, as well as SARS-CoV-2-specific CD8 + T cells in the COVID-19 convalescent plasma. These findings further demonstrate the genomic changes (chromatin remodeling) observed in adaptive immune cells to derive trained immunity by engaging the STING downstream effectors, i.e., IRFs, following COVID-19 infection [ 36 ].
STING agonists
To date, booster doses of COVID-19 vaccines are recommended to maintain the proper immune responses against the emerging variants of SARS-CoV-2 [ 37 ]. As a novel approach, it has been thought that STING agonists could be developed as adjuvants to optimize the immunogenicity and efficacy of the vaccines [ 38 ]. For instance, 2’3’- and 3’3’-cGAMP, as well-known ligands of STING, can prime the innate immune response during de novo antigen-specific CD8 + T cells and IFN-I induction against RNA-based viral infection [ 39 ]. It has also been revealed that the nanoparticles harboring 2′3′-cGAMP isoforms could elicit protective CD8 + T cell-based antiviral responses against human immunodeficiency viruses-1 (HIV-1). Besides vaccination, this novel approach is also applicable to immunotherapy and prophylactics [ 39 ]. Another study also reported that flaviviruses such as yellow fever virus and dengue virus, possessing non-structural protein NS4B, can co-localize with STING and inhibit the STING-RIG-I-dependent signaling pathway [ 29 ]. Therefore, RLRs are responsible for the exclusive detection of cytosolic ss/ds RNA in the course of virus infection to further stimulate innate immune responses.
Given that STING agonists have poor bioavailability, Jang et al. designed exoSTING as a novel and distinct drug delivery system for exclusively delivering CDN into the target sites. This approach of engineered extracellular vesicles, extracted from HEK293 cells, is loaded with STING agonists, which simultaneously augments the CDN potency and the CD8 + T cells response. In addition, the wide therapeutic window of exoSTING also allows effective dose optimization [ 40 ]. Although the cGAS-STING pathway is well-established and elucidated in viral DNA sensing, it can also exhibit paramount functions in host innate immunity against distinct positive-sense single-stranded (+ ss) RNA viruses (e.g., human flaviviruses and coronavirus) with no DNA involvement in their life cycle [ 29 ]. The list and characteristics of various STING agonists are summarized in Table 1 .
Definition of the autophagy
Autophagy, is defined as a tightly preserved cellular process involvedin the turnover of worn-out components, i.e., damaged organelles, aggregated/unfolded proteins, and pathogen particles, resulting in cellular homeostasis, survival, and regulation of cell functions under various stress conditions and viral infection [ 41 , 42 ]. According to the degradation mechanism and delivery route toward the lysosomes, autophagy is classified into three types: macroautophagy (formally defined as autophagy), microautophagy, and chaperon-mediated autophagy (selective autophagy) [ 43 ]. Moreover, the autophagy process consists of some sequential stages, including initiation and membrane nucleation, double-membrane autophagosome formation, lysosomal fusion (autophagolysosome formation), and lysosome-dependent degradation [ 43 ]. To regulate autophagy flux, various autophagy-related genes ( Atgs ) and specific marker proteins such as ATG3, ATG7, microtubule-associated protein 1 A/1B-light chain 3 (LC3), Becline-1, and SQSTM1 (P62) mediate different stages of autophagy [ 44 , 45 ].
Beyond the housekeeping activity, autophagy functions within the immune cells, e.g., T lymphocytes, to further modulate pro-inflammatory processes following a viral invasion [ 46 , 47 ]. Besides, autophagy can contribute to antigen processing and presentation in dendritic cells (DCs) [ 48 ]. In this sense, autophagy has been considered a defensive machinery against viral infection via degrading the pathogens into autolysosomes. As defense machinery, autophagy can be induced to antagonize viral infections by conveying cargo (either cytoplasmic virions or viral components) to lysosomes for targeted degradation. Subsequently, this mechanism provokes the innate immune response, antigen presentation, and clearance of recognized pathogens. Not surprisingly, the supremacy of autophagy has been harnessed to boost the efficacy of vaccination and to date, several autophagy inducers have emerged benefits as vaccine adjuvants [ 49 , 50 , 51 ].
In this connection, several proteins involved in IFN-related signaling pathways are associated with autophagy regulation. For instance, the cGAS–STING pathway can trigger the autophagy flux following dsDNA sensing. As shown in Fig. 2 , upon activation RIG-1-MAVS axis, STING transfers from the ER to the Golgi apparatus through the ER–Golgi intermediate compartment (ERGIC) [ 52 ], where STING triggers autophagosome formation by serving as a membrane source for LC3 lipidation (Fig. 2 ) [ 53 ].
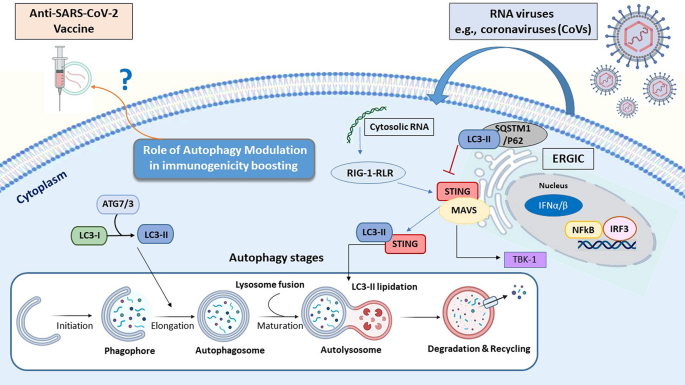
The interplay between autophagy flux and STING pathway following viral infection. Autophagy (macroautophagy) consists of four sequential stages: [ 1 ] initiation followed by membrane nucleation (to form phagophore) [ 2 ], phagosome formation and expansion (named elongation) [ 3 ], the lysosomal fusion to form autolysosome, and [ 4 ] final stage refers to the cargo degradation. The autophagy process is activated by numerous physiologic and pathologic stress conditions such as starvation, pathogen attacks. Following DNA/RNA viruses’ entry into the host cells both autophagy and STING pathways can activate to promote innate immune response. Beyond the interruption in lysosome fusion to promote LC3 lipidation induced by STING, some specific autophagy proteins such as LC3 and SQSTM1/P62 also block the STING-related pathway during different viral infections. Abbreviation : ATG, autophagy-related gene; ERGIC, ER–Golgi intermediate compartment; ER, endoplasmic reticulum; IFN-I, type I interferon, IRF3; interferon regulatory factor 3; LC3, Microtubule-associated protein 1 A/1B-light chain 3; STING, stimulator of interferon response cGAMP; NFκB, nuclear factor-κB
Antiviral impact of STING Signaling mediated by Autophagy
As a signaling adaptor protein, STING directly binds to cyclic di-GMP (c-di-GMP) and orchestrates pro-inflammatory cytokines secretion [ 54 ]. Released IFN-I is able to target multiple viral infections through a STAT-dependent manner and selective autophagy induction to eliminate pathogen particles [ 55 ]. Therefore, STING not only can stimulate the immune response, but it can also promote autophagy activity in a non-cGAMP (non-immune) manner to tune immune responses following viral infections [ 56 ]. In this regard, the STING pathway affects the autophagy flux through the activation of related receptors optineurin (OPTN) and adaptor protein p62 (SQSTM) in a TBK1-dependent manner [ 57 , 58 ].
Available evidence indicated that African swine fever virus (ASFV), SARS-CoV-2, and porcine circovirus type 2 (PCV2) might regulate autophagy stages. For instance, the SARS-CoV-2 ORF7a protein activates the autophagy process via the AKT-mTOR-ULK1 pathway [ 43 ]. Following Zika virus (ZIKV) infection, STING also triggers autophagy by converting LC3-I into LC3-II [ 43 ]. Some viruses evade autophagy by targeting autophagolysosome formation in host cells. During SARS-CoV-2 replication, a viral protein named ORF7a hinders vesicular trafficking and subsequent fusion of autophagosome vesicles to and lysosomes by activating caspase 3 to degrade synaptosomal-associated protein 29 (SNAP29) [ 43 ]. For instance, Epstein–Barr virus (EBV) and Kaposi’s sarcoma-associated herpesvirus inhibit autophagy flux by selectively antagonizing the receptor SQSTM1/p62 [ 43 ].
The role of ORF Protein in Autophagy Flux
Recent literature also demonstrated that SARS-CoV-2-related ORF3a enables to interruption of the fusion of autophagosomes to the lysosomes through binding with a homotypic fusion and protein sorting (HOPS) component, VPS39, in the lysosome site. This simultaneously blocks autophagy flux via cessation of the STX17-SNAP29-VAMP8 SNARE assembly to evade viral degradation into the autophagosomal-lysosomal compartment [ 59 , 60 ]. In addition, it has been well-established that SARS-CoV-2 ORF9b negatively governs STING-mediated antiviral immunity and thus accelerates viral replication. In this respect, inhibition of IFN-I and -III induced immune response mediated by SARS-CoV-2 ORF9b can emerge through blocking RIG-I, MAVS, TRIF, TBK1, and IKKε signaling axis [ 61 ].
Upon screening twenty-nine SARS-CoV-2-encoded viral proteins, Han et al. also recognized that SARS-CoV-2-related ORF10 overexpression has the potential to antagonize cGAS–STING signaling and STING-mediated innate antiviral immunity [ 62 ]. In detail, ORF10 impairs the STING–TBK1 association, blunts ER-to-Golgi trafficking of STING, and ultimately impedes STING-induced autophagy [ 62 ].
Overall, adjuvants promoting the STING-related pathways may shift the immune response in favor of a robust protective antiviral neutralization. These novel strategies and technologies should mitigate the pathological outcomes of SARS-CoV-2-related infections and relapses, by activating the autophagy and STING-mediated antiviral efficacy of viral vaccines. In Fig. 2 depicts sensing viral cytosolic RNA pathways, the interplay between autophagy flux and STING agonists.
This evidence further suggests that employing STING agonists, as vaccine adjuvants, may augment the efficacy of the SARS-CoV-2 vaccine and variants of concern.
Conflicting effects of Autophagy in SARS-CoV-2 infection
Numerous studies explored autophagy (virophagy) modulation and its possible effect on SARS-CoV-2 infection [ 63 ]. Since autophagy influences viral cell entry, transcription, and translation, two scenarios have been represented based on the dual function of autophagy:
[ 1 ] Antiviral effect (regulated autophagy): disrupting the viral replication cycle followed by sequestration of viral particles and subsequent encompassing through autophagosomes and final degradation by lysosomes’ hydrolytic enzymes.
[ 2 ] Pre-viral effect (dysregulated autophagy): following the blocking of the autolysosome formation via viral particles most likely through ORF3a protein, the viral proteins are released from the host cells to the extracellular space [ 64 ].
Noticeably, a prominent role of autophagy in the inflammatory response (especially thrombotic immune-inflammatory syndrome) follows SARS-CoV-2 infection [ 65 ]. Autophagy induction could impede the consecutive robust inflammatory response triggered by SARS-CoV-2 infection, leading to a multi-organ failure [ 65 ]. Calling attention, cells exposed to the SARS-CoV-2 infection can escape immune response mediated by IFN-I induction (markedly increase of green fluorescent protein (GFP)-LC3 positive autophagosomes) in vitro [ 66 ]. Consequently, blocking the excessive autophagy flux using selective inhibitors, like 3-methyadenin (3-MA) and non-selective inhibitor chloroquine leads to the inhibition of viral replication and the reduction of viral load [ 44 , 45 ]. However, another study revealed that increased autophagy flux using the mammalian target of rapamycin (mTOR) inhibitors, e.g., metformin can exert an antiviral effect through the PI3K-AKT-mTOR axis, and hinder the interaction of SARS-CoV-2 proteins, including non-structural protein and ORFs with mTORC1, La-related protein 1 (LARP1), and 4E-BP in host cells [ 67 ].
Modulation of autophagy seems a promising target against SARS-CoV-2 spike protein (S-protein) related diseases to shed light on developing novel therapeutic platforms [ 68 ]. Recent research found that autophagy-induced peptide C5 using human adenovirus (HAd) vector-based vaccine results in better cell-mediated immune response against SARS-CoV-2 when compared to the HAd vector-based vaccine with S protein alone [ 69 ].
The cGAS-STING pathway in the setting of COVID-19 severity
Regarding the COVID-19 pathogenesis, subsequent viral cytopathic effects, and host immunopathology, it has been well-established that the pneumocyte fusion ensues through cleavage of the S-protein via specific proteases at the S1/S2 and the S2’ sites [ 70 ]. After that, binding with the angiotensin-converting enzyme 2 (ACE2) receptor, in part activates the cGAS-STING pathway and related IFN-I response [ 71 ], which can contribute to worsening the COVID-19 severity [ 70 ]. Indeed, viral PLP mainly interacts with the STING pathway to block downstream IFN secretion in the early stage of the SARS-CoV-2 infection [ 72 ]. While in the late phase of infection, damaged DNA (i.e., oxidized mitochondrial DNA) potentially activates the cGAS-STING pathway as a result of the micronuclei and syncytia formation in the infected cells [ 73 ], leading to excessive release of IFN-β and substantial cytokine storm phenomenon during IRF-3 and NFκB activation [ 70 , 72 , 74 ]. In parallel with this, a recent study corroborated that the cGAS-STING pathway is a leading cause of aberrant IFN-I-induced immune responses in COVID-19, via mitochondrial DNA release in the endothelial cells, while pharmacological blocking of the STING pathway significantly reduced inflammatory status, particularly in the respiratory system [ 71 ]. Others advocate that designing STING inhibitors, such as H-151and VS-X4, could serve as novel therapeutic options in the late phase of SARS-CoV-2-like infections to alleviate the hyper-inflammation, as a result of the persistent STING activation, particularly in severe or critically ill patients [ 71 , 75 , 76 ].
Feasibilities of developing these technologies await controlled clinical investigations. However, as a safety issue, it should be pointed out that the detrimental effect of IFN (STING overstimulation) can potentially occur by enhancing the delayed innate immunity, followed by the excessive influx of pathogenic monocytes-macrophages with a worse prognosis [ 74 ].
The impacts of delayed activation of the STING pathway in SARS-CoV-2
SARS-CoV-2 can induce a delayed innate immune response in respiratory epithelial cells, which likely leads to the virus establishment in the respiratory tract [ 77 ]. On this basis, both in vivo and in vitro studies highlighted that early treatments with STING agonists, e.g., diABZI, are promising therapeutic options to govern viral infection by limiting viral replication and inflammatory response in an IFN-dependent manner [ 77 ]. A more recent study also noted that the pharmacological antiviral activity of STING agonists (e.g., CDNs) toward the SARS-CoV-2 infection is highly appreciable [ 78 ]. In addition, the small-molecule STING agonist, diamidobenzimidazole (diABZI) compound, exhibited a desirable anti-SARS-CoV-2 effect against a broad array of variants, especially beta COVID-19 variant (B.1.351), by hampering the virus replication via an IFN-I independent manner under the experimental settings [ 78 ]. Interestingly, this small molecule can further mitigate the ability of SARS-CoV-2 to evade immunity via boosting IFN signaling and the TBK1/IRF-3 in primary human airway epithelial cultures [ 79 ].
In parallel with this finding, Humphries and colleagues also emphasized that either prophylactic or therapeutic administration of diABZI-4 on the onset of the disease completely restricted SARS-CoV-2 replication through transient pro-inflammatory cytokines production and myeloid and lymphocyte activation, particularly in the lung epithelial cells in both in vitro and in vivo settings and exerted a predominant antiviral effect [ 80 ]. Furthermore, they showed that intranasal administration of diABZI-4 in K18-ACE2-transgenic mice infected with SARS-CoV-2 can induce rapid short-lived activation of STING, as a host-directed therapy, through either IFN-dependent or IFN-independent manners in K18-ACE2-transgenic mice infected with SARS-CoV-2 [ 81 ].
In a clinical setting, delayed activation of the STING pathway, T cell delayed excessive responses, and delayed cytokine over-secretion were recently observed in patients with severe COVID-19, most likely due to DNA damages, highlighting the dichotomous role of STING in the case of SARS-CoV-2 [ 82 ]. To further ascertain the delayed activation of the STING pathway was demonstrated in an experimental model of SARS-CoV-2-induced hyperinflammatory immune response in the lung epithelial cells in culture [ 83 ]. Beyond the upregulation of inflammatory cytokines, the authors found the distinct activation of NF-κB and suppression of IRF3 in the infected cells [ 83 ]. Despite early-stage induction, type I IFNs can restrict SARS-CoV-2 infection. A persistent cGAM-STING-dependent type I IFN signature is mainly associated with excessive inflammation and subsequent adverse clinical outcomes [ 84 ]. Moreover, a lung-on-chip model demonstrated that the release of damaged mitochondrial DNA is involved in STING signaling-dependent type I IFN production and endothelial cell death, which could be reversed via pharmacological inhibition [ 84 ]. However, further assessment of STING polymorphism would be valuable toward better management of severe COVID-19.
Applied STING agonists against SARS-CoV-2
Some synthetic adjuvants, including modified emulsions (such as Essai O/W 1,849,101, AS03, AS37, CpG1018 alum) and alum combined with SARS-CoV-2 spike protein receptor-binding domain nanoparticle (RBD–NP) are being studied in multiple ongoing clinical trials to augment the neutralizing-antibody responses [ 85 ]. Notably, the results of an experimental study revealed that the intranasal delivery of a cGAMP nanoparticle as an immune-antiviral agent, named NanoSTING, exerted a broad-spectrum antiviral property and profoundly elicited as a prophylactic and therapeutic aid against neutralization-resistant SARS-CoV-2 variants, e.g., Omicron. Noteworthy, it can also confer durable protection against variants with high prevalence, such as Alpha and Delta in the animal model [ 86 ].
Advances in STING agonist-adjuvant vaccines against COVID-19
Although various platforms of vaccines were designed to combat SARS-CoV-2 infection, due to the limited potency of the available vaccines, and the propensity of coronaviruses to mutate can evade the highly protective immune response, thus precipitating an enduring infection and compromise the effectiveness of the vaccine distribution globally. The necessity of using strong adjuvants may in part complement these limitations.
To design more effective anti-COVID platforms, it has been thought that the involvement of cGAMP within viral vaccine vectors enhances immunogenicity [ 87 ]. Regarding the immunogenicity potential of adjuvant STING agonists, Liu and co-workers recently designed a novel STING agonist, including an IgG fragment crystallizable region (Fc)-conjugated RBD vaccine combined with CF501, as a vaccine’s adjuvant in a pre-clinical setting. The results demonstrate that it is more potent than Alum- and cGAMP-adjuvanted RBD-Fc [ 88 ]. Moreover, CF501 adjuvanted SARS-CoV-2 RBD-Fc (CF501/RBD-Fc) vaccine candidate elicited higher titers of neutralizing Ab (nAb) and durable humoral/cellular immune (T cell) responses accompanied by a lower virus load in the respiratory tract [ 89 , 90 ].
Other approaches demonstrated that colloidal manganese (Mn 2+ ) salt can be applied as an immune booster and delivery system. This adjuvant provoked humoral/cellular immunity through intramuscular and intranasal routes in a cGAS-STING and NLRP3-dependent manner. Therefore, Mn 2+ salt is a promising candidate for both cancer and viral infection vaccines [ 91 ]. Consistent with this, nanoparticle Mn 2+ was considered an optimal and potent adjuvant among proposed inactive vaccine ingredients, which could enhance the immunogenicity and potency of the protein-based COVID-19 subunit vaccines, i.e., RBD-Fc, RBD, and S-trimer. The mechanisms of improved protection are attributable to the cGAS-STING pathway, mainly mediated by the Mn 2+ -adjuvanted RBD-Fc, the highest levels of nAb, and RBD-specific IgG, IgG1, and IgG2a when compared with aluminum and MF59 adjuvants (Fig. 3 Table 2 ) [ 92 ].
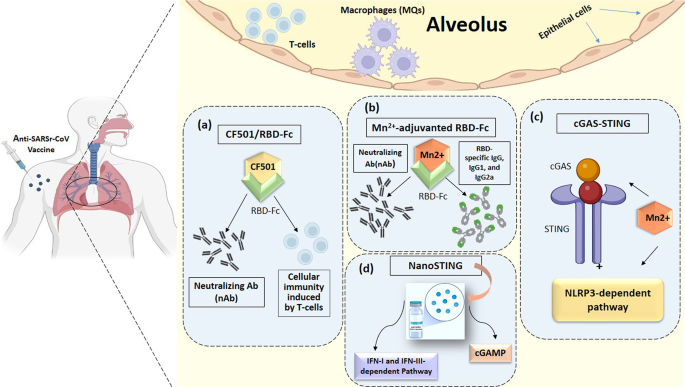
Various STING-agonist adjuvants platforms. All proposed anti-SARS-CoV-2 vaccine adjuvants, including CF501/RBD-Fc ( a ), Mn 2+ - adjuvant RBC-Fc ( b ), cGAS-STING ( c ), and NanoSTING ( d ) are represented in this schematic drawings. Abbreviation CF501/RBD-Fc, CF501-adjuvanted SARS-CoV-2 RBD-Fc; Fc, Freund’s complete; IFN-I and -III, Type I and III Interferon; Mn 2+ , Manganese; NanoSTING, Nanoparticle STING agonists; nAb, Neutralizing Antibodies; RBD, receptor binding domain;
The pan-sarbecovirus (SARS Betacoronavirus) vaccine by design could offer protection against infectious conditions induced by all sarbecovirus strains, especially SARS-CoV-1 and SARS-CoV-2 [ 88 ]. Considering newly emerging SARS-CoV-2, Liu et al. designed an RBD-binding Ab, XG014, which can potently neutralize β-coronavirus lineage B (β-CoV-B) with a unique schedule during distinct targeting of conserved epitopes located outside the ACE2 binding.
site by completely blocking RBD in the non-functional “down” conformation, but not in the “up” position [ 89 ]. Therefore, this conserved antigenic epitope was supposedly considered a promising.
target for developing ultra-potent vaccines, namely the pan-β-CoV-B therapeutics. Due to enhancing the immunogenicity of the conserved epitopes in RBD, a novel STING agonist adjuvant in the pan-sarbecovirus vaccine, CF501/RBD-Fc, could also induce potent immunogenicity even against SARS-CoV-2 circulating variants [ 88 , 93 ]. Of note, CF501 facilitates immunity to SARS-COV-2-related RBD that cross-react with other RBDs from different sarbecovirus subtypes [ 94 ].
Considering evolving COVID-19 variants, recent advances unveiled the development of vaccines formulated by various adjuvants targeting mucosal immunity to impede SARS-CoV-2 infection. For this purpose, SARS-CoV-2 spike trimer conjugated with a unique adjuvant LP-GMP was designed, consisting of TLR2 and STING agonists by generating potent specific IgG, IgA, and memory T cells (tissue-resident memory T-cells) in both lungs and nasal mucosa in human ACE-2 transgenic (K18-hACE) mice [ 95 ]. Another promising STING agonist (c-di-AMP)-based vaccine was designed using monomeric RBD along with CDG SF , displaying an enhanced immunogenicity with appreciated neutralizing antibody and Th1-biased immune responses when compared with aluminum hydroxide (Al (OH) 3) [ 96 , 97 ].
Alternatively, a ternary adjuvant vaccine, comprising of Alum + c-GAMP + poly (I: C) with STING agonist 3, 3′-c-GAMP (c-GAMP) and TLR3 agonist poly (I: C) combined with S1 protein was introduced. This approach represented a significant synergistic impact in favor of eliciting immune responses against live viruses and all variants of concern [ 98 ]. More recently, an Mn 2+ -silica nanoplatform (MnO x @HMSN) multi-potent vaccine was also developed, amplifying the adjuvant effect of CDN by engaging the STING-I IFN pathway with extended humoral immune response and neutralizing antibodies capability [ 99 ]. Therefore, it can be concluded that targeting the cGAS-STING pathway is a promising approach to designing a new generation of RBD/metal/NP-based engineered vaccines against new emerging SARS-CoV-2 variants.
A growing body of literature has investigated the STING pathway in various RNA/DNA viruses. As shown in Fig. 1 , although distinct upstream factors have been reported, they end up in a common pathway for activating the STING signaling pathway. Beyond the favorable effects under various conditions, STING agonists have recently emerged as potential prophylactic/therapeutic agents exploited for patients with SARS-CoV-2 infection. In turn, mounting evidence also supports the fact that the modulation of STING-dependent pathways through various adjuvants provides novel therapeutic targets against immunopathology and immune dysregulation induced by SARS-related coronavirus. Despite multiple engineered carriers and drug-delivery systems, employing both the STING agonists and autophagy modulation in the clinical setting remains to be deciphered in further research.
Limitations of studies and future prospective
Despite plenty of efforts, data is lacking about the safety and effectiveness of the STING agonists as adjuvants used for SARS-CoV-2 vaccines. On the other hand, according to the appreciated effect of regulated autophagy in developing novel antitumor immunotherapy, designed as a microneedle-assisted vaccination [ 100 ], special attention can be also paid in the context of novel antiviral vaccines to combat the threat posed by SARS-related coronavirus.
The compelling evidence highlights that STING agonists can be repurposed as booster adjuvants in SARS-CoV-2 vaccines. This is of particular value for active immunization of susceptible communities, patients with predisposing conditions, senior people, and immunosuppressed recipients against SARS-CoV-2 infection. Considering the magnitude of the well-known detrimental impacts of SARS-CoV-2 pandemics on global health on one hand, and limited data to support this hypothesis that STING agonists can be considered a novel booster adjuvant following the vaccination in the clinical setting, on the other hand, in-depth clinical investigations are greatly recommended.
Data availability
No datasets were generated or analysed during the current study.
Petrovic M, Borchard G, Jordan O. Considerations for the delivery of STING ligands in cancer immunotherapy. J Controlled Release. 2021;339:235–47.
Article CAS Google Scholar
Jiang M, Chen P, Wang L, Li W, Chen B, Liu Y, et al. cGAS-STING, an important pathway in cancer immunotherapy. J Hematol Oncol. 2020;13(1):1–11.
Article Google Scholar
Barber GN. STING: infection, inflammation and cancer. Nat Rev Immunol. 2015;15(12):760–70.
Article CAS PubMed PubMed Central Google Scholar
Gravekamp C, Chandra D. Targeting STING pathways for the treatment of cancer. Oncoimmunology. 2015;4(12):e988463.
Article PubMed PubMed Central Google Scholar
Portela Sousa C, Brites C. Immune response in SARS-CoV-2 infection: the role of interferons type I and type III. Braz J Infect Dis. 2020;24(5):428–33.
Robertsen B. The role of type I interferons in innate and adaptive immunity against viruses in Atlantic salmon. Dev Comp Immunol. 2018;80:41–52.
Article CAS PubMed Google Scholar
Kim Y-M, Shin E-C. Type I and III interferon responses in SARS-CoV-2 infection. Exp Mol Med. 2021;53(5):750–60.
Li S, Luo M, Wang Z, Feng Q, Wilhelm J, Wang X, et al. Prolonged activation of innate immune pathways by a polyvalent STING agonist. Nat Biomedical Eng. 2021;5(5):455–66.
Haist M, Stege H, Grabbe S, Bros M. The functional crosstalk between Myeloid-Derived Suppressor Cells and Regulatory T Cells within the immunosuppressive Tumor Microenvironment. Cancers (Basel). 2021;13(2):210.
Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol. 2021;21(8):485–98.
Lemos H, Mohamed E, Huang L, Ou R, Pacholczyk G, Arbab AS, et al. STING promotes the growth of tumors characterized by low antigenicity via IDO activation. Cancer Res. 2016;76(8):2076–81.
Huang L, Li L, Lemos H, Chandler PR, Pacholczyk G, Baban B, et al. Cutting edge: DNA sensing via the STING adaptor in myeloid dendritic cells induces potent tolerogenic responses. J Immunol. 2013;191(7):3509–13.
Lopez L, Sang PC, Tian Y, Sang Y. Dysregulated interferon response underlying severe COVID-19. Viruses. 2020;12(12):1433.
Jhuti D, Rawat A, Guo CM, Wilson LA, Mills EJ, Forrest JI. Interferon treatments for SARS-CoV-2: challenges and opportunities. Infect Dis Ther. 2022;11(3):953–72.
Decout A, Katz JD, Venkatraman S, Ablasser A. The cGAS–STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol. 2021;21(9):548–69.
Wan D, Jiang W, Hao J. Research advances in how the cGAS-STING pathway controls the cellular inflammatory response. Front Immunol. 2020;11:615.
Franz KM, Neidermyer WJ, Tan Y-J, Whelan SP, Kagan JC. STING-dependent translation inhibition restricts RNA virus replication. Proceedings of the National Academy of Sciences. 2018;115(9):E2058-E67.
Li Y, Wilson HL, Kiss-Toth E. Regulating STING in health and disease. J Inflamm. 2017;14(1):1–21.
Luecke S, Holleufer A, Christensen MH, Jønsson KL, Boni GA, Sørensen LK, et al. cGAS is activated by DNA in a length-dependent manner. EMBO Rep. 2017;18(10):1707–15.
Basit A, Cho M-G, Kim E-Y, Kwon D, Kang S-J, Lee J-H. The cGAS/STING/TBK1/IRF3 innate immunity pathway maintains chromosomal stability through regulation of p21 levels. Exp Mol Med. 2020;52(4):643–57.
Yum S, Li M, Fang Y, Chen ZJ. TBK1 recruitment to STING activates both IRF3 and NF-κB that mediate immune defense against tumors and viral infections. Proceedings of the National Academy of Sciences. 2021;118(14).
Zevini A, Olagnier D, Hiscott J. Crosstalk between cytoplasmic RIG-I and STING sensing pathways. Trends Immunol. 2017;38(3):194–205.
Sun L, Xing Y, Chen X, Zheng Y, Yang Y, Nichols DB, et al. Coronavirus papain-like proteases negatively regulate antiviral innate immune response through disruption of STING-mediated signaling. PLoS ONE. 2012;7(2):e30802.
Clementz MA, Chen Z, Banach BS, Wang Y, Sun L, Ratia K, et al. Deubiquitinating and interferon antagonism activities of coronavirus papain-like proteases. J Virol. 2010;84(9):4619.
Chen X, Yang X, Zheng Y, Yang Y, Xing Y, Chen Z. SARS coronavirus papain-like protease inhibits the type I interferon signaling pathway through interaction with the STING-TRAF3-TBK1 complex. Protein Cell. 2014;5(5):369–81.
Lv H, Dong W, Cao Z, Li X, Wang J, Qian G, et al. TRAF6 is a novel NS3-interacting protein that inhibits classical swine fever virus replication. Sci Rep. 2017;7(1):6737.
Lin D, Zhang M, -X Zhang M, Ren Y, Jin J, Zhao Q, et al. Induction of USP25 by viral infection promotes innate antiviral responses by mediating the stabilization of TRAF3 and TRAF6. Proc Natl Acad Sci. 2015;112(36):11324–9.
Liu W, Reyes HM, Yang JF, Li Y, Stewart KM, Basil MC, et al. Activation of STING signaling pathway effectively blocks human coronavirus infection. J Virol. 2021;95(12):e00490–21.
Ma Z, Damania B. The cGAS-STING defense pathway and its counteraction by viruses. Cell Host Microbe. 2016;19(2):150–8.
Holm CK, Rahbek SH, Gad HH, Bak RO, Jakobsen MR, Jiang Z, et al. Influenza a virus targets a cGAS-independent STING pathway that controls enveloped RNA viruses. Nat Commun. 2016;7(1):10680.
Schoggins JW, MacDuff DA, Imanaka N, Gainey MD, Shrestha B, Eitson JL, et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature. 2014;505(7485):691–5.
Iampietro M, Dumont C, Mathieu C, Spanier J, Robert J, Charpenay A, et al. Activation of cGAS/STING pathway upon paramyxovirus infection. iScience. 2021;24(6):102519.
Liu Y, Goulet M-L, Sze A, Hadj SB, Belgnaoui SM, Lababidi RR, et al. RIG-I-mediated STING upregulation restricts herpes simplex virus 1 infection. J Virol. 2016;90(20):9406.
Lauterbach-Rivière L, Bergez M, Mönch S, Qu B, Riess M, Vondran FWR, et al. Hepatitis B Virus DNA is a substrate for the cGAS/STING pathway but is not sensed in infected hepatocytes. Viruses. 2020;12(6):592.
Rui Y, Su J, Shen S, Hu Y, Huang D, Zheng W, et al. Unique and complementary suppression of cGAS-STING and RNA sensing-triggered innate immune responses by SARS-CoV-2 proteins. Signal Transduct Target Therapy. 2021;6(1):1–11.
Google Scholar
You M, Chen L, Zhang D, Zhao P, Chen Z, Qin E-Q, et al. Single-cell epigenomic landscape of peripheral immune cells reveals establishment of trained immunity in individuals convalescing from COVID-19. Nat Cell Biol. 2021;23(6):620–30.
Mai AS, Lee A, Tay RYK, Shapiro L, Thakkar A, Halmos B, et al. Booster doses of COVID-19 vaccines for patients with haematological and solid cancer: a systematic review and individual patient data meta-analysis. Eur J Cancer. 2022;172:65–75.
Peng H, Fu Y-X. Innovative adjuvant augments potency of a SARS-CoV-2 subunit vaccine. Cell Res. 2022;32(4):331–2.
Gutjahr A, Papagno L, Nicoli F, Kanuma T, Kuse N, Cabral-Piccin MP et al. The STING ligand cGAMP potentiates the efficacy of vaccine-induced CD8 + T cells. JCI Insight. 2019;4(7).
Jang SC, Economides KD, Moniz RJ, Sia CL, Lewis N, McCoy C, et al. ExoSTING, an extracellular vesicle loaded with STING agonists, promotes tumor immune surveillance. Commun Biology. 2021;4(1):1–17.
Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol. 2014;112:24–49.
Rezabakhsh A, Ahmadi M, Khaksar M, Montaseri A, Malekinejad H, Rahbarghazi R, et al. Rapamycin inhibits oxidative/nitrosative stress and enhances angiogenesis in high glucose-treated human umbilical vein endothelial cells: role of autophagy. Biomed Pharmacother. 2017;93:885–94.
Zhai H, Wang T, Liu D, Pan L, Sun Y, Qiu H-J. Autophagy as a dual-faced host response to viral infections. Front Cell Infect Microbiol. 2023;13.
Rezabakhsh A, Rahbarghazi R, Malekinejad H, Fathi F, Montaseri A, Garjani A. Quercetin alleviates high glucose-induced damage on human umbilical vein endothelial cells by promoting autophagy. Phytomedicine. 2019;56:183–93.
Rezabakhsh A, Fathi F, Bagheri HS, Malekinejad H, Montaseri A, Rahbarghazi R, et al. Silibinin protects human endothelial cells from high glucose-induced injury by enhancing autophagic response. J Cell Biochem. 2018;119(10):8084–94.
Germic N, Frangez Z, Yousefi S, Simon H-U. Regulation of the innate immune system by autophagy: monocytes, macrophages, dendritic cells and antigen presentation. Cell Death Differ. 2019;26(4):715–27.
Germic N, Frangez Z, Yousefi S, Simon HU. Regulation of the innate immune system by autophagy: neutrophils, eosinophils, mast cells, NK cells. Cell Death Differ. 2019;26(4):703–14.
Romao S, Münz C. LC3-associated phagocytosis. Autophagy. 2014;10(3):526–8.
Tao S, Drexler I. Targeting autophagy in Innate Immune cells: Angel or Demon during infection and vaccination? Front Immunol. 2020;11(460).
Khan A, Bakhru P, Saikolappan S, Das K, Soudani E, Singh CR, et al. An autophagy-inducing and TLR-2 activating BCG vaccine induces a robust protection against tuberculosis in mice. NPJ Vaccines. 2019;4:34.
Khan A, Bakhru P, Saikolappan S, Das K, Soudani E, Singh CR, et al. An autophagy-inducing and TLR-2 activating BCG vaccine induces a robust protection against tuberculosis in mice. npj Vaccines. 2019;4(1):34.
Zhang K, Wang S, Gou H, Zhang J, Li C. Crosstalk between Autophagy and the cGAS–STING signaling pathway in type I Interferon Production. Front Cell Dev Biology. 2021;9.
Gui X, Yang H, Li T, Tan X, Shi P, Li M, et al. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature. 2019;567(7747):262–6.
Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478(7370):515–8.
Tao J, Zhou X, Jiang Z, cGAS-cGAMP‐STING. The three musketeers of cytosolic DNA sensing and signaling. IUBMB Life. 2016;68(11):858–70.
Motedayen Aval L, Pease JE, Sharma R, Pinato DJ. Challenges and opportunities in the Clinical Development of STING agonists for Cancer Immunotherapy. J Clin Med. 2020;9(10):3323.
Liang Q, Seo GJ, Choi YJ, Kwak MJ, Ge J, Rodgers MA, et al. Crosstalk between the cGAS DNA sensor and Beclin-1 autophagy protein shapes innate antimicrobial immune responses. Cell Host Microbe. 2014;15(2):228–38.
Sharma M, Rajendrarao S, Shahani N, Ramírez-Jarquín UN, Subramaniam S. Cyclic GMP-AMP synthase promotes the inflammatory and autophagy responses in Huntington disease. Proceedings of the National Academy of Sciences. 2020;117(27):15989-99.
Zhang Y, Sun H, Pei R, Mao B, Zhao Z, Li H, et al. The SARS-CoV-2 protein ORF3a inhibits fusion of autophagosomes with lysosomes. Cell Discovery. 2021;7(1):31.
Miao G, Zhao H, Li Y, Ji M, Chen Y, Shi Y, et al. ORF3a of the COVID-19 virus SARS-CoV-2 blocks HOPS complex-mediated assembly of the SNARE complex required for autolysosome formation. Dev Cell. 2021;56(4):427–e425.
Han L, Zhuang MW, Deng J, Zheng Y, Zhang J, Nan ML, et al. SARS-CoV-2 ORF9b antagonizes type I and III interferons by targeting multiple components of the RIG-I/MDA-5-MAVS, TLR3-TRIF, and cGAS-STING signaling pathways. J Med Virol. 2021;93(9):5376–89.
Han L, Zheng Y, Deng J, Nan ML, Xiao Y, Zhuang MW, et al. SARS-CoV-2 ORF10 antagonizes STING-dependent interferon activation and autophagy. J Med Virol. 2022;94(11):5174–88.
Sargazi S, Sheervalilou R, Rokni M, Shirvaliloo M, Shahraki O, Rezaei N. The role of autophagy in controlling SARS-CoV-2 infection: an overview on virophagy-mediated molecular drug targets. Cell Biol Int. 2021;45(8):1599–612.
Samimi N, Farjam M, Klionsky DJ, Rezaei N. The role of autophagy in the pathogenesis of SARS-CoV-2 infection in different cell types. Autophagy. 2022;18(7):1728–31.
Resnik R, Lopez Mingorance F, Rivera F, Mitchell F, Gonzalez CD, Vaccaro MI. Autophagy in inflammatory response against SARS-CoV-2. Int J Mol Sci. 2023;24(5):4928.
Hui X, Zhang L, Cao L, Huang K, Zhao Y, Zhang Y, et al. SARS-CoV-2 promote autophagy to suppress type I interferon response. Signal Transduct Target Therapy. 2021;6(1):180.
Khalid T, Hasan A, Fatima Je, Faridi SA, Khan AF, Mir SS. Therapeutic role of mTOR inhibitors in control of SARS-CoV-2 viral replication. Mol Biol Rep. 2023;50(3):2701–11.
Halma MT, Marik PE, Saleeby YM. Exploring autophagy in treating SARS-CoV-2 spike protein-related pathology. Endocr Metabolic Sci. 2024:100163.
Sayedahmed EE, Araújo MV, Silva-Pereira TT, Chothe SK, Elkashif A, Alhashimi M, et al. Impact of an autophagy-inducing peptide on immunogenicity and protection efficacy of an adenovirus-vectored SARS-CoV-2 vaccine. Mol Therapy-Methods Clin Dev. 2023;30:194–207.
Liu X, Wei L, Xu F, Zhao F, Huang Y, Fan Z, et al. SARS-CoV-2 spike protein–induced cell fusion activates the cGAS-STING pathway and the interferon response. Science Signaling. 2022;15(729):eabg8744.
Domizio JD, Gulen MF, Saidoune F, Thacker VV, Yatim A, Sharma K, et al. The cGAS–STING pathway drives type I IFN immunopathology in COVID-19. Nature. 2022;603(7899):145–51.
Berthelot J-M, Lioté F. COVID-19 as a STING disorder with delayed over-secretion of interferon-beta. EBioMedicine. 2020;56:102801.
Ren H, Ma C, Peng H, Zhang B, Zhou L, Su Y, et al. Micronucleus production, activation of DNA damage response and cGAS-STING signaling in syncytia induced by SARS-CoV-2 infection. Biol Direct. 2021;16(1):1–10.
Li H, Zhou F, Zhang L. STING, a critical contributor to SARS-CoV-2 immunopathology. Signal Transduct Target Therapy. 2022;7(1):1–3.
Haag SM, Gulen MF, Reymond L, Gibelin A, Abrami L, Decout A, et al. Targeting STING with covalent small-molecule inhibitors. Nature. 2018;559(7713):269–73.
Xiao R, Zhang A. Involvement of the STING signaling in COVID-19. Front Immunol. 2022;13:1006395.
Li M, Ferretti M, Ying B, Descamps H, Lee E, Dittmar M, et al. Pharmacological activation of STING blocks SARS-CoV-2 infection. Sci Immunol. 2021;6(59):eabi9007.
Li M, Ferretti M, Ying B, Descamps H, Lee E, Dittmar M, et al. Pharmacological activation of STING blocks SARS-CoV-2 infection. Sci Immunol. 2021;6:59.
Bernard NJ. A STING in the tail for SARS-CoV-2. Nat Immunol. 2021;22(7):800.
Humphries F, Shmuel-Galia L, Jiang Z, Wilson R, Landis P, Ng S-L, et al. A diamidobenzimidazole STING agonist protects against SARS-CoV-2 infection. Sci Immunol. 2021;6(59):eabi9002.
Humphries F, Shmuel-Galia L, Jiang Z, Wilson R, Landis P, Ng SL et al. A diamidobenzimidazole STING agonist protects against SARS-CoV-2 infection. Sci Immunol. 2021;6(59).
Berthelot J-M, Lioté F, Maugars Y, Sibilia J. Lymphocyte changes in severe COVID-19: delayed over-activation of STING? Front Immunol. 2020:3129.
Neufeldt CJ, Cerikan B, Cortese M, Frankish J, Lee J-Y, Plociennikowska A, et al. SARS-CoV-2 infection induces a pro-inflammatory cytokine response through cGAS-STING and NF-κB. Commun Biology. 2022;5(1):1–15.
Di Domizio J, Gulen MF, Saidoune F, Thacker VV, Yatim A, Sharma K, et al. The cGAS-STING pathway drives type I IFN immunopathology in COVID-19. Nature. 2022;1:9.
Arunachalam PS, Walls AC, Golden N, Atyeo C, Fischinger S, Li C et al. Adjuvanting a subunit COVID-19 vaccine to induce protective immunity. Nature. 2021:1–6.
Leekha A, Saeedi A, Kumar M, Sefat SR, Paniagua MM, Fathi M et al. An intranasal nanoparticle STING agonist has broad protective immunity against respiratory viruses and variants. bioRxiv. 2022.
Chauveau L, Bridgeman A, Tan TK, Beveridge R, Frost JN, Rijal P, et al. Inclusion of cGAMP within virus-like particle vaccines enhances their immunogenicity. EMBO Rep. 2021;22(8):e52447.
Liu Z, Zhou J, Xu W, Deng W, Wang Y, Wang M et al. A novel STING agonist-adjuvanted pan-sarbecovirus vaccine elicits potent and durable neutralizing antibody and T cell responses in mice, rabbits and NHPs. Cell Res. 2022:1–19.
Liu Z, Xu W, Chen Z, Fu W, Zhan W, Gao Y, et al. An ultrapotent pan-β-coronavirus lineage B (β-CoV-B) neutralizing antibody locks the receptor-binding domain in closed conformation by targeting its conserved epitope. Protein Cell. 2022;13(9):655–75.
Liu Z, Xu W, Xia S, Gu C, Wang X, Wang Q, et al. RBD-Fc-based COVID-19 vaccine candidate induces highly potent SARS-CoV-2 neutralizing antibody response. Signal Transduct Target Therapy. 2020;5(1):282.
Zhang R, Wang C, Guan Y, Wei X, Sha M, Yi M, et al. Manganese salts function as potent adjuvants. Cell Mol Immunol. 2021;18(5):1222–34.
Zhang N, Ji Q, Liu Z, Tang K, Xie Y, Li K et al. Effect of different adjuvants on Immune responses elicited by protein-based subunit vaccines against SARS-CoV-2 and its Delta variant. Viruses. 2022;14(3).
Liu Z, Chan JF, Zhou J, Wang M, Wang Q, Zhang G, et al. A pan-sarbecovirus vaccine induces highly potent and durable neutralizing antibody responses in non-human primates against SARS-CoV-2 Omicron variant. Cell Res. 2022;32(5):495–7.
Liu Z, Chan JF-W, Zhou J, Wang M, Wang Q, Zhang G, et al. A pan-sarbecovirus vaccine induces highly potent and durable neutralizing antibody responses in non-human primates against SARS-CoV-2 Omicron variant. Cell Res. 2022;32(5):495–7.
Diallo BK, Ní Chasaide C, Wong TY, Schmitt P, Lee KS, Weaver K, et al. Intranasal COVID-19 vaccine induces respiratory memory T cells and protects K18-hACE mice against SARS-CoV-2 infection. npj Vaccines. 2023;8(1):68.
Germanó MJ, Giai C, Cargnelutti DE, Colombo MI, Blanco S, Konigheim B, et al. Receptor-binding domain-based SARS-CoV-2 vaccine adjuvanted with cyclic di-adenosine monophosphate enhances humoral and cellular immunity in mice. J Med Virol. 2023;95(2):e28584.
Article PubMed Google Scholar
Wu J-J, Zhao L, Han B-B, Hu H-G, Zhang B-D, Li W-H, et al. A novel STING agonist for cancer immunotherapy and a SARS-CoV-2 vaccine adjuvant. Chem Commun. 2021;57(4):504–7.
Zhang R-Y, Yin X-G, Zhou S-H, Zhang H-W, Lu J, He C-B, et al. A protein vaccine with Alum/c-GAMP/poly(I:C) rapidly boosts robust immunity against SARS-CoV-2 and variants of concern. Chem Commun. 2022;58(24):3925–8.
Xu C, Dobson HE, Yu M, Gong W, Sun X, Park KS, et al. STING agonist-loaded mesoporous manganese-silica nanoparticles for vaccine applications. J Control Release. 2023;357:84–93.
Yang D, Chen M, Sun Y, Shi C, Wang W, Zhao W, et al. Microneedle-assisted vaccination combined with autophagy regulation for antitumor immunotherapy. J Controlled Release. 2023;357:641–54.
Download references
Acknowledgements
We appreciate Dr. Saeid Ghavami, Dr. Mehdi Eshraghi, and Seyyed-Mohammad Nabavi for their great advice and collaboration to improve this article, as well as all practitioners, healthcare providers, and researchers who are trying to health promotion and fight against the new emerging viral infections.
Not applicable.
Author information
Authors and affiliations.
Cardiovascular Research Center, Shahid Madani specialized Heart Hospita, Tabriz University of Medical Sciences, University St, Tabriz, 5166615573, Iran
Aysa Rezabakhsh
NovoMed Consulting, Biomedical Sciences, Germantown, Maryland, USA
M. Reza Sadaie
Emergency and Trauma Care Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Alireza Ala
Hematology, Immune Cell Therapy, and Stem Cells Transplantation Research Center, Clinical Research Institute, Urmia University of Medical Sciences, Urmia, Iran
Yousef Roosta
Pharmacognosy Research and Herbal Analysis Services UK, University of Greenwich, Kent, UK
Solomon Habtemariam
Clinical Research Center, Department of Internal Medicine, North Khorasan University of Medical Sciences, Bojnurd, Iran
Adeleh Sahebnasagh
Reproductive Health Research Center, Clinical Research Institute, Urmia University of Medical Sciences, Urmia, 5715799313, Iran
Mohammad Rafi Khezri
You can also search for this author in PubMed Google Scholar
Contributions
A.R. was responsible for the conception and design of the review. A.S. performed a bibliography and collected the literature. A.R., A.A., and Y.R. drafted the manuscript. A.R. designed the figures. M.R.S. and M.R.K. and S.H. revised the final version of the manuscript.
Corresponding authors
Correspondence to Aysa Rezabakhsh or Mohammad Rafi Khezri .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declared that they have no conflicts of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Rezabakhsh, A., Sadaie, M.R., Ala, A. et al. STING agonists as promising vaccine adjuvants to boost immunogenicity against SARS-related coronavirus derived infection: possible role of autophagy. Cell Commun Signal 22 , 305 (2024). https://doi.org/10.1186/s12964-024-01680-0
Download citation
Received : 23 January 2024
Accepted : 26 May 2024
Published : 03 June 2024
DOI : https://doi.org/10.1186/s12964-024-01680-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Immunogenicity
- Stimulator of interferon gene
- Vaccine adjuvant
Cell Communication and Signaling
ISSN: 1478-811X
- General enquiries: [email protected]
Bird Flu (H5N1) Explained: Study Finds First Known Case Of Mammal-To-Mammal Transnational Transmission
- Share to Facebook
- Share to Twitter
- Share to Linkedin
Here’s the latest news about a global outbreak of H5N1 bird flu that started in 2020, and recently spread among cattle in U.S. states and marine mammals across the world, which has health officials closely monitoring it and experts concerned the virus could mutate and eventually spread to humans, where it has proven rare but deadly.
A sign warns of a outbreak of bird flu.
June 5 A new study examining the 2023 bird flu outbreak in South America that killed around 17,400 elephant seal pups and 24,000 sea lions found the disease spread between the animals in several countries, the first known case of transnational virus mammal-to-mammal bird flu transmission.
May 30 Another human case of bird flu has been detected in a dairy farm worker in Michigan—though the cases aren’t connected—and this is the first person in the U.S. to report respiratory symptoms connected to bird flu, though their symptoms are “resolving,” according to the Centers for Disease Control and Prevention.
May 23 A new study with mice suggests that drinking infected milk can spread the disease—and that a certain type of pasteurization may not always be effective in killing the virus.
May 22 Michigan reported bird flu in a farmworker—the second U.S. human case tied to transmission from dairy cows—though the worker had a mild infection and has since recovered.
May 21 Australia reported its first human case of bird flu after a child became infected in March after traveling to India, though the child has since recovered after suffering from a “severe infection,” according to the Victorian Department of Health.
May 16 The USDA conducted a study, and discovered that after high levels of the virus was injected into beef, no trace was left after the meat was cooked medium to well done, though the virus was found in meat cooked to lower temperatures.
May 14 The Centers for Disease Control and Prevention released influenza A waste water data for the weeks ending in April 27 and May 4, and found several states like Alaska, California, Florida, Illinois and Kansas had unusually high levels, though the agency isn’t sure if the virus came from humans or animals, and isn’t able to differentiate between influenza A subtypes, meaning the H5N1 virus or other subtypes may have been detected.
May 10 The Food and Drug Administration announced it will commit an additional $8 million to ensure the commercial milk supply is safe, while the Department of Agriculture said it will pay up to $28,000 per farm to help mitigate the spread of the disease, totaling around $98 million in funds.
May 9 Some 70 people in Colorado are being monitored for bird flu due to potential exposure, and will be tested for the virus if they show any symptoms, the Colorado Department of Public Health told Forbes—it was not immediately clear how or when the people were potentially exposed.
May 1 The Department of Agriculture said it tested 30 grocery store ground beef products for bird flu and they all came back negative, reaffirming the meat supply is safe.
May 1 The Food and Drug Administration confirmed dairy products are still safe to consume, announcing it tested grocery store samples of products like infant formula, toddler milk, sour cream and cottage cheese, and no live traces of the bird flu virus were found, although some dead remnants were found in some of the food—though none in the baby products.
April 30 Wenqing Zhang, head of the World Health Organization's Global Influenza Programme, said during a news briefing "there is a risk for cows in other countries to be getting infected," with the bird flu virus, since it’s commonly spread through the movement of migratory birds.
April 29 The Department of Agriculture told Forbes it will begin testing ground beef samples from grocery stores in states with cow outbreaks, and test ground beef cooked at different temperatures and infected with the virus to determine if it's safe to eat.
April 24 The USDA said cow-to-cow transmission may be occurring due to the cows coming into contact with raw milk—and warned against humans and other animals, including pets, consuming unpasteurized milk to prevent potential infection.
April 18 Jeremy Farrar, chief scientist for the World Health Organization, said during a press conference the threat of bird flu spreading between humans was a “great concern,” since it’s evolved and has increasingly been infecting mammals (on land and sea), which means it could possibly spread to humans.
April 1 The CDC reported the second U.S. human case of bird flu in a Texas dairy farmer who became infected after contracting the virus from infected dairy cows, but said the person was already recovering.
Get Forbes Breaking News Text Alerts: We’re launching text message alerts so you'll always know the biggest stories shaping the day’s headlines. Sign up here .
Can Bird Flu Spread Between Humans?
Bird flu doesn’t “transmit easily from person-to-person,” according to the World Health Organization. Bird flu rarely affects humans, and most previous cases came from close contact with infected poultry, according to the CDC. Because human-to-human spread of bird flu poses “pandemic potential,” each human case is investigated to rule out this type of infection. Though none have been confirmed, there are a few global cases—none in the U.S.—where human-to-human transmission of bird flu was thought to be “probable,” including in China , Thailand , Indonesia and Pakistan .
Is Bird Flu Fatal To Humans?
It is very deadly. Between January 2003 and March 28, 2024 there have been 888 human cases of bird flu infection in humans, according to a report by the World Health Organization. Of those 888 cases, 463 (52%) died. To date, only two people in the U.S. have contracted H5N1 bird flu, and they both were infected after coming into contact with sick animals. The most recent case was a dairy worker in Texas who became ill in March after interacting with sick dairy cows, though he only experienced pink eye. The first incident happened in 2022 when a person in Colorado contracted the disease from infected poultry, and fully recovered.
Is It Safe To Drink Milk Infected With Bird Flu?
Raw, unpasteurized milk is unsafe to drink, but pasteurized milk is fine, according to the FDA. Bird flu has been detected in both unpasteurized and pasteurized milk, but the FDA recommends manufacturers against making and selling unpasteurized milk since there’s a possibility consuming it may cause bird flu infection. However, the virus remnants in pasteurized milk have been deactivated by the heat during the pasteurization process , so this type of milk is still believed safe to consume.
Is It Safe To Consume Meat Infected With Bird Flu?
The CDC warns against eating raw meat or eggs from animals “confirmed or suspected” of having bird flu because of the possibility of transmission. However, no human has ever been infected with bird flu from eating properly prepared and cooked meat, according to the agency. The possibility of infected meat entering the food supply is “extremely low” due to rigorous inspection, so properly handled and cooked meat is safe to eat, according to the USDA. To know when meat is properly cooked, whole beef cuts must be cooked to an internal temperature of 145 degrees Fahrenheit, ground meat must be 160 degrees and poultry must be cooked to 165 degrees. Rare and medium rare steaks fall below this temperature. Properly cooked eggs with an internal temperature of 165 degrees Fahrenheit kills bacteria and viruses including bird flu, according to the CDC. “It doesn’t matter if they may or may not have [avian] influenza… runny eggs and rare pieces of meat” are never recommended, Francisco Diez-Gonzalez, director and professor for the Center for Food Safety at the University of Georgia, told Forbes. To “play it safe,” consumers should only eat fully cooked eggs and make sure “the yolks are firm with no runny parts,” Daisy May, veterinary surgeon with U.K.-based company Medivet, said .
What Are Bird Flu Symptoms In Humans?
Symptoms of bird flu include a fever, cough, headache, chills, shortness of breath or difficulty breathing, runny nose, congestion, sore throat, nausea or vomiting, diarrhea, pink eye, muscle aches and headache. However, the CDC advises it can’t be diagnosed based on symptoms alone, and laboratory testing is needed. This typically includes swabbing the nose or throat (the upper respiratory tract), or the lower respiratory tract for critically ill patients.
How Is Bird Flu Affecting Egg Prices?
This year’s egg prices have increased as production decreased due to bird flu outbreaks among poultry, according to the USDA. A dozen large, grade A eggs in the U.S. costed around $2.99 in March, up almost a dollar from the fall. However, this price is down from a record $4.82 in January 2023, which was also spiked by bird flu outbreaks . Earlier this month, Cal-Maine Foods—the country’s largest egg producer—temporarily halted egg production after over one million egg-laying hens and chickens were killed after being infected with bird flu.
Why Do Poultry Farmers Kill Chickens With Bird Flu?
Once chickens have been infected with bird flu, farmers quickly kill them to help control the spread of the virus, since bird flu is highly contagious and fatal in poultry. The USDA pays farmers for all birds and eggs that have to be killed because of bird flu, as an incentive to responsibly try and curb the spread of the disease. The USDA has spent over $1 billion in bird flu compensation for farmers since 2022, according to the nonprofit Food & Environment Reporting Network.
Is There A Vaccine For The Bird Flu (h5n1)?
The FDA has approved a few bird flu vaccines for humans. The U.S. has a stockpile of vaccines for H5N1 bird flu, but it wouldn’t be enough to vaccinate all Americans if an outbreak were to happen among humans. If a human outbreak does occur, the government plans to mass produce vaccines, which can take at least six months to make enough for the entire population. CSL Seqirus, the maker of one of the approved vaccines, expects to have 150 million vaccines ready within six months of an announcement of a human bird flu pandemic. Although there are approved vaccines for other variants designed for birds, there are none for the H5N1 variant circulating. However, the USDA began trials on H5N1 animal-specific vaccines in 2023.
Key Background
As of May 30, more than 92 million poultry (primarily chickens) in 48 states have been euthanized because of bird flu since 2022, and 57 dairy cow herds across nine states have tested positive, according to data from the CDC (unlike chickens, cows appear to recover from the virus). The USDA believes wild migratory birds are the original source of the cow outbreaks that recently has experts concerned it may mutate and spread more easily in humans, though the CDC said its risk to the public remains low . Farrar called the cattle infections in the U.S. a “huge concern,” urging public health officials to continue closely monitoring the situation “because it may evolve into transmitting in different ways.” The increased number of mammal bird flu infections since 2022 “could indicate that the virus is looking for new hosts, and of course, moving closer to people,” Andrea Garcia, vice president of science, medicine and public health for the American Medical Association, said . The first report of a walrus dying from bird flu was detected in April on one of Norway’s Arctic Islands, and the first U.S. dolphin infected with bird flu died back in 2022, according to a report published April 18. More than 10 human bird flu cases were reported to the World Health Organization in 2023, and all but one survived. Bird flu has devastated bird populations, and 67 countries reported the deaths of 131 million poultry in 2022 alone. Although bird flu typically infects wild birds and poultry, it’s spread to other animals during the outbreak, and at least 10 countries have reported outbreaks in mammals since 2022. Around 17,400 elephant seal pups died from bird flu in Argentina in 2023, and at least 24,000 sea lions died in South America the same year. Besides cattle, bird flu has been detected in over 200 other mammals—like seals, raccoons and bears—in the U.S. since 2022. Although rare, even domestic pets like dogs and cats are susceptible to the virus, and the FDA warns against giving unpasteurized milk to cats to avoid possible transmission.
On June 5, WHO confirmed the first human death of a strain of bird flu that’s never before been seen in humans and is separate from H5N1. A 59-year-old man in Mexico contracted H5N2, and died on April 24 after being hospitalized and developing a fever, diarrhea, nausea, shortness of breath and general discomfort. Cases of H5N2 have been reported in poultry in Mexico, but the man had no history with poultry or animals, WHO said. It’s unclear how he became infected. He was bedridden for weeks prior to the infection, and suffered from several other health conditions.
Further Reading
Another Bird Flu Variant Reaches Humans: What To Know About H5N2—After First-Ever Confirmed Death
WHO Warns Threat Of Bird Flu Spreading To Humans Is ‘Great Concern’ (Forbes)
One In Five Milk Samples From Across US Had Traces Of Bird Flu Virus, FDA Says (Forbes)
Can Pets Get Bird Flu? Here’s What To Know (Forbes)
Avian H5N1 (Bird) Flu: Why Experts Are Worried—And What You Should Know (Forbes)

- Editorial Standards
- Reprints & Permissions
Join The Conversation
One Community. Many Voices. Create a free account to share your thoughts.
Forbes Community Guidelines
Our community is about connecting people through open and thoughtful conversations. We want our readers to share their views and exchange ideas and facts in a safe space.
In order to do so, please follow the posting rules in our site's Terms of Service. We've summarized some of those key rules below. Simply put, keep it civil.
Your post will be rejected if we notice that it seems to contain:
- False or intentionally out-of-context or misleading information
- Insults, profanity, incoherent, obscene or inflammatory language or threats of any kind
- Attacks on the identity of other commenters or the article's author
- Content that otherwise violates our site's terms.
User accounts will be blocked if we notice or believe that users are engaged in:
- Continuous attempts to re-post comments that have been previously moderated/rejected
- Racist, sexist, homophobic or other discriminatory comments
- Attempts or tactics that put the site security at risk
- Actions that otherwise violate our site's terms.
So, how can you be a power user?
- Stay on topic and share your insights
- Feel free to be clear and thoughtful to get your point across
- ‘Like’ or ‘Dislike’ to show your point of view.
- Protect your community.
- Use the report tool to alert us when someone breaks the rules.
Thanks for reading our community guidelines. Please read the full list of posting rules found in our site's Terms of Service.
The state of AI in early 2024: Gen AI adoption spikes and starts to generate value
If 2023 was the year the world discovered generative AI (gen AI) , 2024 is the year organizations truly began using—and deriving business value from—this new technology. In the latest McKinsey Global Survey on AI, 65 percent of respondents report that their organizations are regularly using gen AI, nearly double the percentage from our previous survey just ten months ago. Respondents’ expectations for gen AI’s impact remain as high as they were last year , with three-quarters predicting that gen AI will lead to significant or disruptive change in their industries in the years ahead.
About the authors
This article is a collaborative effort by Alex Singla , Alexander Sukharevsky , Lareina Yee , and Michael Chui , with Bryce Hall , representing views from QuantumBlack, AI by McKinsey, and McKinsey Digital.
Organizations are already seeing material benefits from gen AI use, reporting both cost decreases and revenue jumps in the business units deploying the technology. The survey also provides insights into the kinds of risks presented by gen AI—most notably, inaccuracy—as well as the emerging practices of top performers to mitigate those challenges and capture value.
AI adoption surges
Interest in generative AI has also brightened the spotlight on a broader set of AI capabilities. For the past six years, AI adoption by respondents’ organizations has hovered at about 50 percent. This year, the survey finds that adoption has jumped to 72 percent (Exhibit 1). And the interest is truly global in scope. Our 2023 survey found that AI adoption did not reach 66 percent in any region; however, this year more than two-thirds of respondents in nearly every region say their organizations are using AI. 1 Organizations based in Central and South America are the exception, with 58 percent of respondents working for organizations based in Central and South America reporting AI adoption. Looking by industry, the biggest increase in adoption can be found in professional services. 2 Includes respondents working for organizations focused on human resources, legal services, management consulting, market research, R&D, tax preparation, and training.
Also, responses suggest that companies are now using AI in more parts of the business. Half of respondents say their organizations have adopted AI in two or more business functions, up from less than a third of respondents in 2023 (Exhibit 2).
Gen AI adoption is most common in the functions where it can create the most value
Most respondents now report that their organizations—and they as individuals—are using gen AI. Sixty-five percent of respondents say their organizations are regularly using gen AI in at least one business function, up from one-third last year. The average organization using gen AI is doing so in two functions, most often in marketing and sales and in product and service development—two functions in which previous research determined that gen AI adoption could generate the most value 3 “ The economic potential of generative AI: The next productivity frontier ,” McKinsey, June 14, 2023. —as well as in IT (Exhibit 3). The biggest increase from 2023 is found in marketing and sales, where reported adoption has more than doubled. Yet across functions, only two use cases, both within marketing and sales, are reported by 15 percent or more of respondents.
Gen AI also is weaving its way into respondents’ personal lives. Compared with 2023, respondents are much more likely to be using gen AI at work and even more likely to be using gen AI both at work and in their personal lives (Exhibit 4). The survey finds upticks in gen AI use across all regions, with the largest increases in Asia–Pacific and Greater China. Respondents at the highest seniority levels, meanwhile, show larger jumps in the use of gen Al tools for work and outside of work compared with their midlevel-management peers. Looking at specific industries, respondents working in energy and materials and in professional services report the largest increase in gen AI use.
Investments in gen AI and analytical AI are beginning to create value
The latest survey also shows how different industries are budgeting for gen AI. Responses suggest that, in many industries, organizations are about equally as likely to be investing more than 5 percent of their digital budgets in gen AI as they are in nongenerative, analytical-AI solutions (Exhibit 5). Yet in most industries, larger shares of respondents report that their organizations spend more than 20 percent on analytical AI than on gen AI. Looking ahead, most respondents—67 percent—expect their organizations to invest more in AI over the next three years.
Where are those investments paying off? For the first time, our latest survey explored the value created by gen AI use by business function. The function in which the largest share of respondents report seeing cost decreases is human resources. Respondents most commonly report meaningful revenue increases (of more than 5 percent) in supply chain and inventory management (Exhibit 6). For analytical AI, respondents most often report seeing cost benefits in service operations—in line with what we found last year —as well as meaningful revenue increases from AI use in marketing and sales.
Inaccuracy: The most recognized and experienced risk of gen AI use
As businesses begin to see the benefits of gen AI, they’re also recognizing the diverse risks associated with the technology. These can range from data management risks such as data privacy, bias, or intellectual property (IP) infringement to model management risks, which tend to focus on inaccurate output or lack of explainability. A third big risk category is security and incorrect use.
Respondents to the latest survey are more likely than they were last year to say their organizations consider inaccuracy and IP infringement to be relevant to their use of gen AI, and about half continue to view cybersecurity as a risk (Exhibit 7).
Conversely, respondents are less likely than they were last year to say their organizations consider workforce and labor displacement to be relevant risks and are not increasing efforts to mitigate them.
In fact, inaccuracy— which can affect use cases across the gen AI value chain , ranging from customer journeys and summarization to coding and creative content—is the only risk that respondents are significantly more likely than last year to say their organizations are actively working to mitigate.
Some organizations have already experienced negative consequences from the use of gen AI, with 44 percent of respondents saying their organizations have experienced at least one consequence (Exhibit 8). Respondents most often report inaccuracy as a risk that has affected their organizations, followed by cybersecurity and explainability.
Our previous research has found that there are several elements of governance that can help in scaling gen AI use responsibly, yet few respondents report having these risk-related practices in place. 4 “ Implementing generative AI with speed and safety ,” McKinsey Quarterly , March 13, 2024. For example, just 18 percent say their organizations have an enterprise-wide council or board with the authority to make decisions involving responsible AI governance, and only one-third say gen AI risk awareness and risk mitigation controls are required skill sets for technical talent.
Bringing gen AI capabilities to bear
The latest survey also sought to understand how, and how quickly, organizations are deploying these new gen AI tools. We have found three archetypes for implementing gen AI solutions : takers use off-the-shelf, publicly available solutions; shapers customize those tools with proprietary data and systems; and makers develop their own foundation models from scratch. 5 “ Technology’s generational moment with generative AI: A CIO and CTO guide ,” McKinsey, July 11, 2023. Across most industries, the survey results suggest that organizations are finding off-the-shelf offerings applicable to their business needs—though many are pursuing opportunities to customize models or even develop their own (Exhibit 9). About half of reported gen AI uses within respondents’ business functions are utilizing off-the-shelf, publicly available models or tools, with little or no customization. Respondents in energy and materials, technology, and media and telecommunications are more likely to report significant customization or tuning of publicly available models or developing their own proprietary models to address specific business needs.
Respondents most often report that their organizations required one to four months from the start of a project to put gen AI into production, though the time it takes varies by business function (Exhibit 10). It also depends upon the approach for acquiring those capabilities. Not surprisingly, reported uses of highly customized or proprietary models are 1.5 times more likely than off-the-shelf, publicly available models to take five months or more to implement.
Gen AI high performers are excelling despite facing challenges
Gen AI is a new technology, and organizations are still early in the journey of pursuing its opportunities and scaling it across functions. So it’s little surprise that only a small subset of respondents (46 out of 876) report that a meaningful share of their organizations’ EBIT can be attributed to their deployment of gen AI. Still, these gen AI leaders are worth examining closely. These, after all, are the early movers, who already attribute more than 10 percent of their organizations’ EBIT to their use of gen AI. Forty-two percent of these high performers say more than 20 percent of their EBIT is attributable to their use of nongenerative, analytical AI, and they span industries and regions—though most are at organizations with less than $1 billion in annual revenue. The AI-related practices at these organizations can offer guidance to those looking to create value from gen AI adoption at their own organizations.
To start, gen AI high performers are using gen AI in more business functions—an average of three functions, while others average two. They, like other organizations, are most likely to use gen AI in marketing and sales and product or service development, but they’re much more likely than others to use gen AI solutions in risk, legal, and compliance; in strategy and corporate finance; and in supply chain and inventory management. They’re more than three times as likely as others to be using gen AI in activities ranging from processing of accounting documents and risk assessment to R&D testing and pricing and promotions. While, overall, about half of reported gen AI applications within business functions are utilizing publicly available models or tools, gen AI high performers are less likely to use those off-the-shelf options than to either implement significantly customized versions of those tools or to develop their own proprietary foundation models.
What else are these high performers doing differently? For one thing, they are paying more attention to gen-AI-related risks. Perhaps because they are further along on their journeys, they are more likely than others to say their organizations have experienced every negative consequence from gen AI we asked about, from cybersecurity and personal privacy to explainability and IP infringement. Given that, they are more likely than others to report that their organizations consider those risks, as well as regulatory compliance, environmental impacts, and political stability, to be relevant to their gen AI use, and they say they take steps to mitigate more risks than others do.
Gen AI high performers are also much more likely to say their organizations follow a set of risk-related best practices (Exhibit 11). For example, they are nearly twice as likely as others to involve the legal function and embed risk reviews early on in the development of gen AI solutions—that is, to “ shift left .” They’re also much more likely than others to employ a wide range of other best practices, from strategy-related practices to those related to scaling.
In addition to experiencing the risks of gen AI adoption, high performers have encountered other challenges that can serve as warnings to others (Exhibit 12). Seventy percent say they have experienced difficulties with data, including defining processes for data governance, developing the ability to quickly integrate data into AI models, and an insufficient amount of training data, highlighting the essential role that data play in capturing value. High performers are also more likely than others to report experiencing challenges with their operating models, such as implementing agile ways of working and effective sprint performance management.
About the research
The online survey was in the field from February 22 to March 5, 2024, and garnered responses from 1,363 participants representing the full range of regions, industries, company sizes, functional specialties, and tenures. Of those respondents, 981 said their organizations had adopted AI in at least one business function, and 878 said their organizations were regularly using gen AI in at least one function. To adjust for differences in response rates, the data are weighted by the contribution of each respondent’s nation to global GDP.
Alex Singla and Alexander Sukharevsky are global coleaders of QuantumBlack, AI by McKinsey, and senior partners in McKinsey’s Chicago and London offices, respectively; Lareina Yee is a senior partner in the Bay Area office, where Michael Chui , a McKinsey Global Institute partner, is a partner; and Bryce Hall is an associate partner in the Washington, DC, office.
They wish to thank Kaitlin Noe, Larry Kanter, Mallika Jhamb, and Shinjini Srivastava for their contributions to this work.
This article was edited by Heather Hanselman, a senior editor in McKinsey’s Atlanta office.
Explore a career with us
Related articles.

Moving past gen AI’s honeymoon phase: Seven hard truths for CIOs to get from pilot to scale

A generative AI reset: Rewiring to turn potential into value in 2024
Implementing generative AI with speed and safety

IMAGES
COMMENTS
AstraZeneca's research partners at Oxford University had demonstrated an immune response in preclinical studies and some encouraging early clinical results that provided a basis for confidence that a vaccine could be rapidly developed. AstraZeneca's proven manufacturing capability and global supply chain would enable rapid progress from the ...
Malte Mueller/Getty Images. Alvarez & Marsal's new vaccine mandate requires employees to get vaccinated or go on unpaid leave. The policy is a case study in how employers should craft their ...
Highlight role models. Employers can create an atmosphere where COVD-19 vaccinations are supported, for example, by having company executives or managers share their vaccination experiences with workers or by creating a network of vaccinated "employee ambassadors" to answer questions and share their experiences.
Supported the mobilisation of a world-leading vaccine delivery programme allowing over 80 per cent of UK adults to receive at least one dose within seven months of regulatory approval; Applied commercial and business case expertise to enable the VTF to agree contracts for 400 million doses of seven different vaccines within six months of set-up
Abstract. This multimedia case follows the story of Moderna and its entry into vaccine development in the wake of the COVID-19 pandemic. In summer 2020, Stephane Bancel, CEO of biotech firm Moderna, faces several challenges as his company races to develop a vaccine for COVID-19. The case explores how a company builds a digital organization, and ...
Chances are your business has been more reactive than proactive during the pandemic when it comes to securing (and growing) your business. But by developing a COVID-19 vaccination strategy now, you can create a roadmap for reaching your employee vaccination goals and managing risk, and build a foundation for possible future challenges.
Cancer vaccine clinical strategy, valuation and business planning. Alacrita Consulting's founders worked together for over 10 years at Onyvax, a London-based cancer immunotherapy company backed by SR One. We have conducted a number of consulting projects in the... Learn More. 1.
A commitment to equity and fair access. A vaccine developed in under a year. An estimated 6 million lives saved globally in 2021 alone. The amazing and well-known story of the Oxford-AstraZeneca COVID-19 vaccine and its protagonists is the story of one of the most significant health breakthroughs in recent history.
Case study Summary Launched rapidly to tackle challenges of unparalleled magnitude - namely, delivering COVID vaccines to the UK's population of 67+ million and overseas during a global pandemic - the Vaccine Taskforce (VTF) faced a myriad of supply and logistical challenges that could only be addressed through a comprehensive data-driven ...
At least 25 states, along with federal agencies and many cities and counties, hired consulting firms, according to a Washington Post tally. The American vaccination drive came to rely on global ...
The CMC Vaccine Working Group has requested that this document be placed in the public domain by PDA Version 1.0, May 2012 Preface The A-VAX Case Study involved the efforts of many individuals and would not have been made possible if it were not for the countless number of hours spent by the 5 participating companies
A recent test-negative design study in Canada showed positive findings regarding vaccine effectiveness with the BNT162b2, mRNA-1273, and ChAdOx1 nCoV-19 vaccines against symptomatic disease with ...
CISA Current Activities. Clinical Case Reviews: CISA provides a clinical case consultation service for U.S. healthcare providers who have vaccine safety questions about a specific patient residing in the United States. CISA provides clinical expertise in various disciplines, including infectious diseases, neurology, allergy, immunology, pediatrics, hematology, and obstetrics/gynecology.
Vaccine platforms and vaccines for emerging infectious diseases. Vaccines are the cornerstone of the management of infectious disease outbreaks and are the surest means to defuse pandemic and ...
Vaccine-enhanced disease (VED) is a type of adverse event in which disease severity is increased when a person who has received the vaccine is later infected with the relevant pathogen. VED can occur during research with experimental vaccines and/or after vaccine licensure, sometimes months or years after a person receives a vaccine. Both ...
Phase IV studies to obtain additional efficacy and safety data may be conducted at the discretion of the manufacturer or sought by the FDA at the time of vaccine licensure. 21 Case-control or ...
4.3. Timing of study and vaccine coverage. Case-control studies are most likely to be useful when the vaccine coverage is between 20 and 80% . At either very low or very high coverage, unvaccinated persons are likely to differ from the general population in ways that may be associated with increased or decreased risk of disease, independent of ...
The COVID-19 pandemic was exacerbated by poor utilization of vaccines caused by the spread of misinformation. Fortunately, the impact of flagrant vaccine misinformation on Facebook was greatly attenuated once such posts were flagged and debunked as false by third-party fact-checkers. However, ambiguous misinformation remained unflagged.
Among 1983 COVID-19 case patients, vaccine breakthrough patients compared with unvaccinated patients tended to be older (median age 67 vs 53 years), ... Dr Grijalva reported receiving a contract from CDC during the conduct of the study; consulting fees from Pfizer, Merck, and Sanofi; a contract from CDC, Campbell Alliance, and the Food and Drug ...
Case-control studies are commonly used to evaluate effectiveness of licensed vaccines after deployment in public health programs. Such studies can provide policy-relevant data on vaccine performance under 'real world' conditions, contributing to the evidence base to support and sustain introduction of new vaccines.
Consulting Industry Weakens our Businesses, Infantilizes our Governments and Warps our Economies (2023). ... supporting the case study on mRNA Vaccine TT programme, and is the first author on this case study (with Els Torreele and Mariana Mazzucato) on a forthcoming theme issue on economics and health, of the Bulletin of World Health ...
CASE STUDY: VACCINE HESITANCY IN LONG BEACH Religion, personal choice, uncertainty about long-term side effects, and a lack of trust in the vaccine and government were commonly reported as reasons for not getting vaccinated among residents in the 90805 and 90813 zip codes. A lack of trust in the government/medical authorities, personal choice,
Global Vaccine Summit 2020 Gavi's Investment Opportunity launch meeting, 30 August 2019 Gavi Mid-Term Review, Abu Dhabi high level conference, 10-11 December 2018
Case Study: Jaxson. Pediarix (DTaP-IPV-HepB) - can be given between the ages of 6 weeks up until 7 years of age. Pentacel (DTaP-IPV-Hib)- should not be used for any dose in the primary series for children age 5 years or older or as the booster dose for children ages 4-6 years. Kinrix (Dtap-IPV) - can be used as the fifth dose of DTaP and fourth ...
A new study from Yale researchers in Obstetrics and Gynecology shows no link between stillbirth and COVID-19 vaccines. Moreover, pregnant women who had received COVID-19 vaccines in pregnancy were at a decreased risk of preterm birth. The authors say the findings should offer further reassurance that COVID-19 vaccination is safe and useful in ...
But U.S. District Judge Vernon D. Oliver denied the motion. The judge noted that summary judgment is only appropriate when there is an absence of "concrete evidence," but in this case, there ...
Science. Jun 6, 2024 3:58 PM. The Case for MDMA's Approval Is Riddled With Problems. The FDA is considering approving MDMA alongside psychotherapy as a treatment for PTSD. But evidence of the ...
As a major component of innate immunity and a positive regulator of interferons, the Stimulator of interferon gene (STING) has an immunotherapy potential to govern a variety of infectious diseases. Despite the recent advances regarding vaccines against COVID-19, nontoxic novel adjuvants with the potential to enhance vaccine efficacy are urgently desired. In this connection, it has been well ...
Timeline. June 5 A new study examining the 2023 bird flu outbreak in South America that killed around 17,400 elephant seal pups and 24,000 sea lions found the disease spread between the animals in ...
If 2023 was the year the world discovered generative AI (gen AI), 2024 is the year organizations truly began using—and deriving business value from—this new technology. In the latest McKinsey Global Survey on AI, 65 percent of respondents report that their organizations are regularly using gen AI, nearly double the percentage from our ...