- Recent Searches Clear
Welcome to the new home of Guidelines in Practice
Browse our expert articles to support best practice for UK healthcare professionals.

Improving Chronic Disease Management in Primary Care: Case Studies
Disclosures.

GP, Barry; Community Director for Quality Improvement and Clinical Innovation, Cardiff and Vale University Health Board
Dr Richard Baxter Examines Five Case Studies in Which a Structured Approach to Chronic Disease Management (CDM) Has Improved Care, and Presents a Framework for CDM Optimisation
In England, treatment and care for people with long-term conditions are thought to account for 70% of health and social care expenditure. 1 An effective approach to chronic disease management (CDM) is therefore vital if primary care is to accommodate the rising tide of CDM requirements without drowning—but how can primary care providers structure this management without losing patient, community, and workforce engagement or inadvertently increasing health inequalities?
This question is addressed in this article, in which I:
- briefly explore how disparate contractual arrangements in England, Northern Ireland, Wales, and Scotland encourage differing approaches to CDM
- discuss five case studies that show how introducing a structured approach can deliver efficiencies and transform care
- present a nascent framework that I am helping to develop with colleagues across Cardiff and Vale University Health Board (UHB), setting out some factors that are critical to an effective, comprehensive, engaging, and equitable CDM process.
Important Considerations in CDM
As leaders of healthcare systems have tried to implement some of the theoretical frameworks associated with CDM—including the Chronic Care Model (CCM), Minimally Disruptive Medicine, the Patient-Centred Medical Home, and the Ten Building Blocks of High-Performing Primary Care 2,3 —a number of important considerations have emerged. These critical success factors are examined below, and also reflected in the case studies discussed later in this article.
Patients' engagement with care and ownership of their conditions is now recognised as essential for improving CDM. 4 In studies using the CCM, improved outcomes have been seen with initiatives for managing long-term diseases that involve effective design of the care-delivery system and that support patients to engage in self-management. 2 In addition, a focus on multimorbidity (including frailty), rather than single diseases, is increasingly recognised as important to clinical outcomes, although evidence is sparse concerning the practical application of methods to balance treatment burden and CDM. 5–7
Effective use of the wider clinical team can help to maximise the use of limited resources, the idea being that CDM services are led, but do not always need to be delivered, by GPs. 8 Involving pharmacists in primary care, through comprehensive medication reviews alongside clinical condition reviews, is a measure that has been shown to improve a range of clinical outcomes. 9 However, this adaptation is associated with challenges regarding continuity of care, which is more difficult to deliver across a multiclinician team. 10
Any health-improvement initiative must also be structured in a way that ensures that inequalities are not inadvertently increased. The Deep End Project, involving GPs working in 100 practices serving the most socioeconomically deprived communities in Scotland, is a reminder of this: it was found that differential uptake of quality-improvement initiatives by different social groups only served to widen health inequalities. 11
Contracts, Frameworks, and Quality-Improvement Incentives across the UK
The way in which quality improvement and CDM are financially incentivised may have a significant impact on the way that CDM is organised now and in the future, as can be seen in the differences between the UK nations.
England and Northern Ireland
In England, the Quality and Outcomes Framework (QOF) is still in place—at least until the 2023–2024 GP Contract is completed, when the QOF is to be reviewed. 12 This framework does ensure universal incentives and target-specific funding, but it seems to do little to promote the more difficult work involved in CDM, such as chasing people who are hard to reach and persevering with those whose disease is hard to control. It also suffers from a rush to meet specific, and increasingly challenging, disease-control and health-promotion targets by 1 April each year. 13 The QOF is in place in Northern Ireland as well, but it is organised by the Northern Ireland Department of Health’s Strategic Planning and Performance Group instead of NHS England. 14
However, although the QOF may focus efforts on patients who are easier to reach or control at a practice level, the Primary Care Network (PCN) Directed Enhanced Service (DES) in England, with its associated Investment and Impact Fund, is an alternative source of funding that PCNs could use to deliver CDM more comprehensively and effectively, as it facilitates the introduction of shared staff—such as pharmacists, link workers, paramedics, and physician associates—across localities. 15,16
In Wales, the QOF was replaced by the Quality Assurance and Improvement Framework (QAIF) in 2019, with fewer specific targets and a greater emphasis on promoting practice improvements. 17 This year, funding for all clinical markers has been moved to the core General Medical Services contract, and assurance indicators have been removed 18,19 —therefore, practices retain the responsibility to deliver CDM but no longer have target-driven funding incentives. This change has the potential to free practices to concentrate their resources on higher-impact clinical activity; however, income is no longer reserved specifically for CDM, so it may be neglected.
The QOF was discontinued in Scotland in 2016 20 and, in 2018, a new contractual arrangement was phased in whereby GPs retain responsibility for their key roles—including undifferentiated presentations, complex care, local and whole-system quality improvement, and local clinical leadership—but other responsibilities pass to alternative healthcare professionals. 21,22 To this end, health boards and health and social care partnerships were originally supposed to reconfigure six key services over a 3-year period, including the provision of vaccinations, urgent care, and community link work. 21 However, reflecting the initiative’s slow progress during the pandemic, a revision in 2021 has focused the reforms on three priorities: the vaccination programme, pharmacotherapy, and community treatment and care services (including chronic disease monitoring). 23 The move from specific targets with dedicated funding to a broader promotion of healthcare improvements and changes in primary care infrastructure has the potential to help the NHS in Scotland to undertake a more radical overhaul of its CDM processes.
Case Studies on Improved CDM Processes
The following case studies are examples of initiatives across the UK through which developments in organisation and structure have improved CDM in primary care. They demonstrate the potential for primary care to make the CDM process much more efficient and effective. Key measures that have helped include:
- involvement of the multidisciplinary team (MDT)
- use of birth-month-aligned recall processes
- comprehensive planning.
Leeds: Improving Coordination Through Birth-Month-Aligned Reviews
As a result, both surgeries have increased their total numbers of reviews, achieved better patient monitoring, and found that, in general, the CDM process takes up less patient and clinician time because fewer services are being unnecessarily duplicated. 24 The change also helped the practice team to understand each other’s roles more clearly, and enabled greater visibility of where any individual patient is in their review process. 24
Bournemouth: Overhauling Medication Reviews with Pharmacist Support
In 2017–2018, Steve Williams took up the role of Senior Clinical Pharmacist at Westbourne Medical Centre (WMC) in Bournemouth as part of the NHS’s pilot scheme for practice-based clinical pharmacists. 25 He worked with Dr Lawrence Brad, the practice’s prescribing lead and one of its GP partners, to introduce a stratified structured medication review (SMR) system that enables patients with polypharmacy and multimorbidity to reliably receive appointments for comprehensive CDM review. 25 Through this process, patients with chronic obstructive pulmonary disease, asthma, cardiovascular disease, or diabetes, and who are on more than eight regular medicines, are invited for a CDM review that incorporates their multiple QOF reviews and SMR, after a blood test appointment. 26
This is one part of a whole package of improvements led by an effective MDT at the practice, with other measures including a major overhaul of the repeat prescription system and a drive to assess both process and patient outcomes. 27 Indeed, WMC estimates that the work of one full-time pharmacist has saved the practice 80 GP hours per month. 27
In general, the introduction of practice-based pharmacists into NHS primary care seems to have been an impactful and cost-effective intervention, reducing medicine-related problems, inappropriate prescribing, and unnecessary telephone consultations in general practice. 28,29 In my communications with Steve and Lawrence about this, Lawrence said: ‘A proactive, stratified SMR and CDM process provides an essential platform for the patient to engage in true shared decision making about their medications and, once undertaken, [the process] helps other clinicians to consult with [the patient with] much more efficiency and focus on any subsequent clinical presentation.’ 26 WMC is currently considering aligning its reviews with patients’ birth months in 2023–2024. 26
Supporting Pharmacists to Practise Safely
Although Steve and Lawrence are strong advocates for the involvement of clinical pharmacists in general practice, they are keen that these pharmacists are used and supported appropriately, and are leading initiatives in the Poole Bay and Bournemouth PCN to improve use of the MDT for CDM. 26,30 I have heard several anecdotes about pharmacists being used inappropriately or having a poor understanding of what they can, and should not, do. In my opinion, there is a pressing need for PCN clinical directors to understand and embrace this.
Staffordshire: Reducing Workload by Aligning Reviews to Birth Month
In 2019, after realising that repeat prescriptions and medication review processes were consuming a significant amount of GP time, a practice in Staffordshire serving 11,800 patients freed up the equivalent of 168 GP appointments per month by improving the structure of its medication review process. 31 The practice reduced repeat attendances by instituting more effective sequencing of investigations, creating a ‘one-stop shop’ in which patients receive clinical and pharmacist-led medication reviews in one visit. 31 It was found that aligning reviews loosely to a patient’s birthday and having a single point of organisation—the clinical pharmacist—maintained consistency in the process. 31 These improvements, which were made in collaboration with the whole team, are appreciated by patients considerably. 31
Subsequently, the practice discovered a downside to birth-month alignment: patient expectation (the other side of patient engagement) led to pressure on particular team members, who lacked the capacity to keep up. 32 The practice still offers a comprehensive review service of this kind, which streamlines blood tests, clinical review, and medication review, but the timing has been adjusted to match practice capacity. 32
Glamorgan: Reducing GP Involvement by Streamlining Processes
In 2019, Western Vale Family Practice (WVFP) employed a pharmacist, Rachel Brace, to help streamline their processes around CDM, SMRs, and QOF/QAIF achievement. 33 After much research and discussion, as well as audits, pilots, and attendance at a costing workshop, the practice developed and launched a comprehensive, birth-month-aligned annual review process in summer 2019. 33
The Streamlined Process
Prior to each patient’s birth month, review needs are collated and, for certain conditions, pre-appointment questionnaires are sent out. 33 Patients attend in person for blood tests and basic metrics, then undergo a clinical and medication review with an appropriate non-GP prescriber. 33 The choice of prescriber is based on individual prescribers’ scopes of practice, and patients can choose between telephone and face-to-face reviews. 33 Subsequently, when safe to do so and when a person’s conditions are well controlled, medications are authorised and issued through repeat dispensing for the appropriate maximum periods. 33
WVFP has concentrated on streamlining this process for patients with simpler, well-controlled conditions, through paper reviews where appropriate, so that time can be reserved for more complex or vulnerable patients. 33 When conditions need better control, these patients are passed to a named clinician to designate responsibility and ensure continuity of care. 33
Impact and Reception
It is generally accepted at the practice that this process makes the best use of the MDT’s full range of knowledge and skills, including those of nurse practitioners, healthcare assistants, pharmacists, and pharmacy technicians. 33 Through this service, over 90% of the practice’s CDM activity can be delivered safely and effectively without direct GP involvement, and the team has noticed many improvements as a result, including: 33
- improved diagnostic coding
- clinicians not having to worry about opportunistic delivery
- increased condition coverage.
Patients seem to approve of the approach, coining the phrase ‘birthday MOT’ to describe it, and no patient has yet said that they would rather see a GP. 33 GPs are also satisfied that they can concentrate on more complex cases, with one GP even describing the CDM team as ‘the elves of chronic disease’ because everything seems to happen seamlessly behind the scenes. 33 Furthermore, when this process was presented at a meeting of their patient interest group, the All Wales Therapeutics and Toxicology Centre expressed an interest. 33
WVFP continues to refine this process with ‘Plan, Do, Study, Act’ cycles, 34 and is currently working on inclusion of hormone-replacement therapy reviews, partly to adapt to the fragility of the supply chain. 33
Surrey: Performing Structured Medication Reviews at the PCN Level
In 2020, under the Additional Roles Reimbursement Scheme, Nipa Patel was employed by a PCN in Surrey as a senior clinical pharmacist. 35 She introduced pharmacist-led SMRs to the practices in the PCN, with initial benefits identified including improvements in: 35
- inhaler prescribing
- patient self-awareness in diabetes management
- appropriate involvement of social prescribing
- communications between community, primary, and secondary care.
Implementing a Clear Recall Process with Follow-up Procedures
Annual recalls are arranged by a single text message, which includes a unique code that allows the patient to book a dedicated time slot online or over the phone. 37 After 7 days, nonresponders are chased directly to fill the remaining slots of this type. 37 When possible, if a patient does not attend, a designated care coordinator contacts them to ask why; however, most patients do attend their self-booked appointments. 37 The practices ensure consistency in their reviews and coding through appropriate training and use of third-party-designed templates. 37 When it is safe and measures are under control, repeat dispensing allows a community pharmacy to continue issuing a specific medication until the next annual review. 37 Those patients who require better control of disease markers are offered follow up by a single clinician to ensure continuity of care until control is achieved. 37
When I spoke to Nipa recently, she noted that the practices that have introduced this process are now significantly more effective at covering QOF, and report exceptions less often, than those that have not yet done so. 37 By structuring the review, the practices are also able to utilise many available allied health professionals’ roles more appropriately. 37 Clinicians at these two PCNs have commented that they prefer this method considerably to the 2-month rush to tick all the QOF boxes, and have noticed that the process allows for ‘more time for intricate clinical discussion and optimising treatment plans using shared decision making with patients’ . 37 Nipa has also noticed that there are downstream benefits on phlebotomy, hospital, and community services, as referrals are spread more evenly throughout the year. 37
The team is currently working on streamlining the physical checks to create more of a ‘one-stop shop' framework, as well as modelling future demand to workforce availability. 37
Cardiff and Vale UHB: Developing a Framework for Understanding CDM Processes
The five case studies presented above demonstrate that there are various ways to improve CDM services in the UK. In Cardiff and Vale UHB, we are currently developing a framework to help practices identify areas for improvement in their own CDM processes, outlined in Figure 1 and detailed below.
Areas for improvement fall broadly into 10 sections, which group into three domains—quality, efficacy, and integration—and are all affected by the balance of opportunistic versus structured CDM. In our experience, if clinicians are feeling the pressure to capture every opportunistic moment, this is evidence that their structured process lacks capacity or efficiency.
Figure 1: Areas to Consider When Optimising Chronic Disease Recall Processes
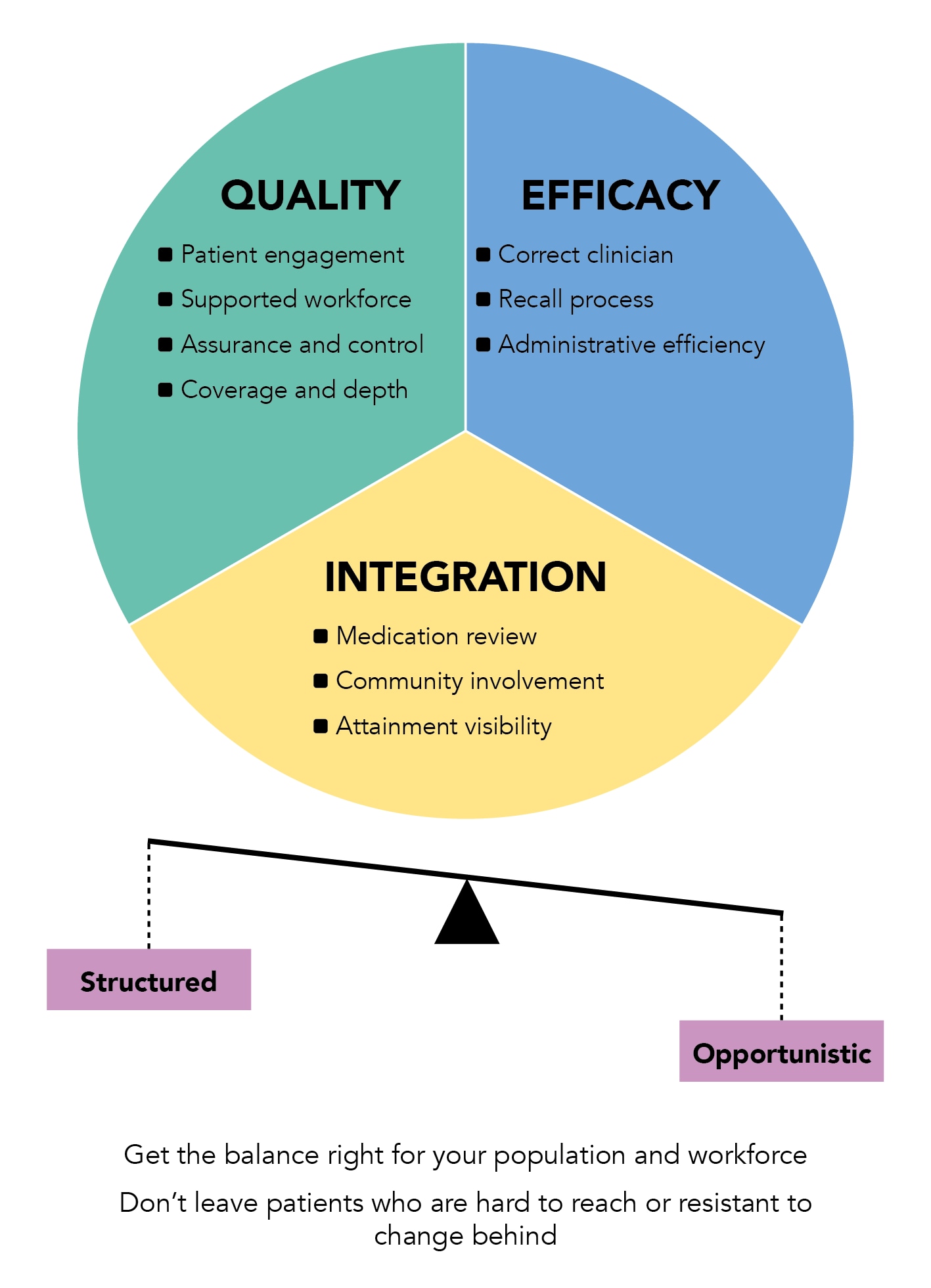
Quality Domain
An effective CDM process relies on providing a high-quality service that is flexible and consistent, with strong patient engagement, workforce support, and the ability to be measured, quality assured, and improved.
Patient Engagement
It is easy to see how the structure of any CDM process can strongly influence patient engagement, which is the key to any effective method of recalling patients. We have identified the following factors as particularly relevant:
- communication and clarity about what should occur and at what time
- choice of methods for contacting patients
- the optimum use of opportunistic moments (it is not inefficient to duplicate a message when it is timely)
- codesign with patient participation groups
- continuity of care (ideally continuity of care with a single contact, whether that is the choice of a patient or clinician)
- minimising the unnecessary burden of CDM, such as by reducing patient visits.
Supported Workforce
- supervision
- design of templates used in the CDM process
- everyone knowing how the system is meant to work, and having a route to bring forward improvements.
Coverage and Depth
In addressing its CDM coverage, a practice may wish to consider allocating greater seniority or more time to those patients with greater need or more challenging circumstances. If the same patients miss their review every year, this may also need to be addressed with a different approach.
Assurance and Control
If a CDM process is to be effective, the people delivering it will benefit immensely from the ability to measure and assess relevant metrics, allowing them to check whether they are delivering care as intended and work out how they can tackle abnormal results. Continuous quality improvement is a key aspect of assurance and control.
Efficacy Domain
A worthwhile CDM process must be effective in the ways it utilises clinicians, recalls patients, and is administered.
Correct Clinician
Failure to use clinicians to the full extent of their roles has possibly the greatest potential for waste, frustration, reduced quality, and excess cost. For a practice or PCN assessing whether it is using the correct clinician for any aspect of CDM, questions should include:
- do we have the right skill mix and number of clinicians to meet the chronic care needs of our patients, and are we able to calculate this?
- could we share workforce across our PCN or cluster?
- does our system reliably ensure that the right appointments occur at the right time?
- does everyone know what services each person can safely provide?
Recall Process
- with some systems, it is now possible for patients to receive a unique link that gives them access to a specified subset of appointments
- a receptionist ringing a list of patients may be more effective for improving attendance than a message asking those same patients to ring in
- pre-appointment questionnaires may be an effective way to save clinicians’ time
- use of an app may be cheaper than use of a text-messaging service
- some methods of recall may result in lower did-not-attend rates, so may be more useful
- certain metrics may be more useful for assessing the cost effectiveness of a particular recall process, such as ‘cost per actual attendance’.
Administrative Efficiency
Integration domain.
To maximise effectiveness, a CDM framework needs to involve the coordination of processes, wider primary care services, and individual staff members.
Medication Review
The alignment of clinical and medication reviews, with both being comprehensive and performed by the correct clinician, is now well understood to be a fundamental part of an optimal CDM process, particularly for patients with polypharmacy, multimorbidity, or frailty.
Community Involvement
An effective system would easily allow other organisations to complement the review and management process. Community pharmacies are the obvious example of this, but community nursing, social services, and the voluntary sector are other providers that could enhance patient experience and quality of review in a system that deliberately considers their involvement.
Attainment Visibility
Clarity on the purpose, goals, and achievements of a CDM process is essential for its success. When a clinician or receptionist is deciding whether to take an opportunity to discuss CDM with a patient, for example, it is useful for them to know how well the review process is going, both in general and for the individual patient. The benefits are enhanced if the staff member in question knows how and when reviews will occur—this is especially evident when reviews are aligned by birth month.
Ambitions at Cardiff and Vale UHB
Our next step with this framework is to identify critical questions, exercises, and measures that can be used to appraise primary care recall systems more objectively. We would welcome any comments on our proposed framework, including any other factors, objective measures, or exercises we should include.
I have presented here several case studies that illustrate the benefits, and potential challenges, of structuring CDM recall in a more systematic way, particularly by birth month. Although other methods of organisation—such as by multimorbidity, polypharmacy, disease severity, or level of engagement—have also been demonstrated to be effective, all of these can be combined within a primarily birth-month-aligned approach.
It is unclear how prevalent this birth-month-aligned approach to CDM is, and therefore what opportunities exist for any consequent improvements in health, patient experience, cost, and clinical work life. Within Cardiff and Vale UHB, about 20% of practices have adopted this model, and we are working to refine it and collect more evidence of its effects so that we can scale it effectively. Other practitioners I have spoken with have estimated that up to 50% of practices in their area have adopted the model. Personally, I think that this model is a potential key to the transformational improvements the NHS so desperately needs, and I welcome communication from anyone about it, particularly concerning any issues they have experienced with this approach and any information they have about its prevalence across the UK.
EDITOR'S RECOMMENDATIONS
Measles: Diagnosis, Assessment, and Management
NHS England | New 29 May 2024
Case Studies in CVRM and CKD: Dr Kevin Fernando, Dr Jim Moore, and Professor Raj Thakkar's Guidelines Live Session
Video | Professor Raj Thakkar, Dr Kevin Fernando, Dr Jim Moore | 28 May 2024
YOU MAY ALSO LIKE
Webmd network.
Diabetes as a case study of chronic disease management with a personalized approach: the role of a structured feedback loop
Affiliation.
- 1 Insititut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) and Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM), Hospital Clínic Barcelona, Barcelona, Spain. [email protected]
- PMID: 22917639
- DOI: 10.1016/j.diabres.2012.07.005
As non-communicable or chronic diseases are a growing threat to human health and economic growth, political stakeholders are aiming to identify options for improved response to the challenges of prevention and management of non-communicable diseases. This paper is intended to contribute ideas on personalized chronic disease management which are based on experience with one major chronic disease, namely diabetes mellitus. Diabetes provides a pertinent case of chronic disease management with a particular focus on patient self-management. Despite advances in diabetes therapy, many people with diabetes still fail to achieve treatment targets thus remaining at risk of complications. Personalizing the management of diabetes according to the patient's individual profile can help in improving therapy adherence and treatment outcomes. This paper suggests using a six-step cycle for personalized diabetes (self-)management and collaborative use of structured blood glucose data. E-health solutions can be used to improve process efficiencies and allow remote access. Decision support tools and algorithms can help doctors in making therapeutic decisions based on individual patient profiles. Available evidence about the effectiveness of the cycle's constituting elements justifies expectations that the diabetes management cycle as a whole can generate medical and economic benefit.
Copyright © 2012 Elsevier Ireland Ltd. All rights reserved.
Publication types
- Blood Glucose*
- Case Management / organization & administration*
- Chronic Disease / therapy*
- Decision Support Techniques
- Diabetes Mellitus / blood
- Diabetes Mellitus / therapy*
- Patient Compliance* / statistics & numerical data
- Patient-Centered Care / standards
- Quality of Health Care
- Quality of Life
- Blood Glucose
The Business Case for Diabetes Disease Management
What should business people in particular know about the pros and cons of attempts to treat and control diabetes—or indeed other chronic diseases?
That was the focus of a lively case-study discussion among some fifty participants led by HBS professor Nancy Beaulieu at the 2003 Alumni Healthcare Conference on November 7.
Beaulieu's research focuses on many aspects of healthcare—including contracting, quality competition in managed care, and human capital management and performance measurement. At the session, she prodded her alumni "students" in classic HBS case-study fashion to analyze the complexities of disease management and check all avenues for potential business opportunities. The participants, almost all of them health professionals, sorted through the risks and benefits of disease management for patients, employers, health plans, doctors and nurses, and society.
In the end, participants agreed that while disease management is a thorny concept involving all these many constituencies, it might in some instances leave room for business potential in the form of a carve-out. A carve-out, as defined in the paper "The Business Case for Diabetes Disease Management" that Beaulieu co-wrote to launch the discussion, works like this:
"In a carve-out arrangement, a private disease management vendor typically takes on full risk for the care of patients with specific diseases like diabetes. The health plan typically identifies its diabetic patients, and then the vendor is placed financially at risk for the costs of patient medical care and is responsible for coordinating all aspects of care for those patients. The vendor is often also involved with other chronically ill patients of the same health plan—for example, those with asthma or hypertension."
Why Diabetes Is A Good Case
But first, diabetes. Beaulieu chose diabetes as a context in which to spark discussion on organizational and financial systems for several reasons, she said. It is a very prevalent disease: as Beaulieu and co-authors David Cutler and Katherine Ho noted in their study, it is the seventh leading cause of death in the U.S. Its economic burden is also high, since it is linked to other afflictions such as heart and renal disease and blindness.
And, she added, one of the most important aspects of this disease is that the medical profession already knows how to treat it. "We have the medical knowledge to do it. And it's not being done. Just under one-half of identified diabetics in this country don't have their blood sugar under control," she said.
Diabetes manifests as Type I or Type II and, as one participant pointed out, the most appropriate care involves some tailoring. But in general, it is well recognized that diabetes can lead to long-term complications and short- and long-term costs. As Beaulieu and her co-authors learned, diabetics may make up between 3 and 10 percent of a typical health plan's membership. It typically costs about $1,000 a year to treat a newly diagnosed diabetic patient; obviously, the numbers go up if a diabetic suffers serious complications.
Healthcare providers have attempted a number of disease management strategies over the last ten years or so. The diabetes programs have varied, but all are linked by the idea that patients can and should become educated about their disease and be proactive in taking care of their health. According to Beaulieu and her co-authors, disease management is focused on prevention and control, not acute care. "The aim is to improve the coordination of care and to reduce the number of hospitalizations and severe complications among diabetic patients," they wrote.
Disease management, then, can take several approaches. The simplest is probably for the healthcare provider to offer a monitoring system for patients who have already been diagnosed as diabetics, sending out e-mail or making phone calls to remind patients of test and checkup dates. Another route is to offer a combined monitoring, tracking, and alert system. This method automatically lets the healthcare provider know if patients skip their tests or if a more intensive treatment seemed warranted by the latest test results. A third approach—less common—is to create a coordinated "virtual team" around the patient, by sharing lab data, insurance claims data, and pharmacy data in an attempt to enhance overall care.
Another part of diabetes disease management programs aims not at current diabetics but at identifying members of the health plan who seem at particularly high risk of developing the disease within the next couple of years. This kind of program is harder for health plans to implement, according to Beaulieu and her co-authors, because it requires data collection and analysis tools, since potential diabetics are identified on the basis of pharmacy and lab data as well as questionnaires and surveys. Not many organizations have ventured into this element of disease management.
In their case-study discussion, participants at the conference mulled over the strategies of HealthPartners, an independent nonprofit that is one of three health maintenance organizations (HMOs) in the Minneapolis market. HealthPartners has an enrollment of about 675,000 people, and its network consists of approximately 3,700 primary care physicians and 4,500 specialists, according to Beaulieu and her co-authors. In the 1990s, it began a program with two elements similar to those outlined above: one to focus on plan members who already had diabetes, and another to focus on members at risk of developing diabetes.
Analyzing HealthPartners, Beaulieu and her co-authors found that such programs could lose money in the first one to three years and so would not break even in a three-year time period. Allowing an eight- to twelve-year period, however, would be more realistic for a break even, they suggested.
Treating A Silent Killer
Participants in the case study said that as a "silent killer" diabetes requires behavioral and lifestyle changes that are difficult for many patients to commit to, even patients with the best intentions. Privacy was another concern: Would employees be "penalized" if they were recognized as patients? Or would sick people flock to employers who offered a too-generous plan? Since long-term effects of the disease are measured in decades, it is complicated to figure out a business case for diabetes, said one alumnus. Participants said that since many people with diabetes have multiple diseases, as business people they did not know whether it would be better economically to treat such a chronic disease on "one system" or just focus on the individual elements of diabetes.
Some participants suggested that Medicare and society might have more of a stake in diabetes disease management than employers. Medicare usually foots the bill for end-stage renal disease, one result of untreated diabetes. An economic case would be difficult to make to employers and health plans, some said: If employee turnover was high, then it would be difficult to economically justify such a time-dependent proposal as diabetes disease management. On the other hand, workplace productivity savings could be the key. According to another alumnus, any outlay "is petty change" to employers compared to the savings of keeping healthy people.
Though wide-ranging, the discussion was underscored by the fact that the growth in incidence of diabetes is a public health crisis that needs urgent attention. However flawed, early programs that attempt to manage the disease may at least get more people thinking about serious solutions for their businesses and communities.
- 28 May 2024
- In Practice
Job Search Advice for a Tough Market: Think Broadly and Stay Flexible
- 22 May 2024
Banned or Not, TikTok Is a Force Companies Can’t Afford to Ignore
- 22 Nov 2023
- Research & Ideas
Humans vs. Machines: Untangling the Tasks AI Can (and Can't) Handle
- 27 Jun 2016
These Management Practices, Like Certain Technologies, Boost Company Performance
- 24 Jan 2024
Why Boeing’s Problems with the 737 MAX Began More Than 25 Years Ago
- Human Resources
- Innovation and Invention
Sign up for our weekly newsletter
- Login / Register

‘These three individuals will leave very big shoes to fill’
STEVE FORD, EDITOR
- You are here: COPD
Diagnosis and management of COPD: a case study
04 May, 2020
This case study explains the symptoms, causes, pathophysiology, diagnosis and management of chronic obstructive pulmonary disease
This article uses a case study to discuss the symptoms, causes and management of chronic obstructive pulmonary disease, describing the patient’s associated pathophysiology. Diagnosis involves spirometry testing to measure the volume of air that can be exhaled; it is often performed after administering a short-acting beta-agonist. Management of chronic obstructive pulmonary disease involves lifestyle interventions – vaccinations, smoking cessation and pulmonary rehabilitation – pharmacological interventions and self-management.
Citation: Price D, Williams N (2020) Diagnosis and management of COPD: a case study. Nursing Times [online]; 116: 6, 36-38.
Authors: Debbie Price is lead practice nurse, Llandrindod Wells Medical Practice; Nikki Williams is associate professor of respiratory and sleep physiology, Swansea University.
- This article has been double-blind peer reviewed
- Scroll down to read the article or download a print-friendly PDF here (if the PDF fails to fully download please try again using a different browser)
Introduction
The term chronic obstructive pulmonary disease (COPD) is used to describe a number of conditions, including chronic bronchitis and emphysema. Although common, preventable and treatable, COPD was projected to become the third leading cause of death globally by 2020 (Lozano et al, 2012). In the UK in 2012, approximately 30,000 people died of COPD – 5.3% of the total number of deaths. By 2016, information published by the World Health Organization indicated that Lozano et al (2012)’s projection had already come true.
People with COPD experience persistent respiratory symptoms and airflow limitation that can be due to airway or alveolar abnormalities, caused by significant exposure to noxious particles or gases, commonly from tobacco smoking. The projected level of disease burden poses a major public-health challenge and primary care nurses can be pivotal in the early identification, assessment and management of COPD (Hooper et al, 2012).
Grace Parker (the patient’s name has been changed) attends a nurse-led COPD clinic for routine reviews. A widowed, 60-year-old, retired post office clerk, her main complaint is breathlessness after moderate exertion. She scored 3 on the modified Medical Research Council (mMRC) scale (Fletcher et al, 1959), indicating she is unable to walk more than 100 yards without stopping due to breathlessness. Ms Parker also has a cough that produces yellow sputum (particularly in the mornings) and an intermittent wheeze. Her symptoms have worsened over the last six months. She feels anxious leaving the house alone because of her breathlessness and reduced exercise tolerance, and scored 26 on the COPD Assessment Test (CAT, catestonline.org), indicating a high level of impact.
Ms Parker smokes 10 cigarettes a day and has a pack-year score of 29. She has not experienced any haemoptysis (coughing up blood) or chest pain, and her weight is stable; a body mass index of 40kg/m 2 means she is classified as obese. She has had three exacerbations of COPD in the previous 12 months, each managed in the community with antibiotics, steroids and salbutamol.
Ms Parker was diagnosed with COPD five years ago. Using Epstein et al’s (2008) guidelines, a nurse took a history from her, which provided 80% of the information needed for a COPD diagnosis; it was then confirmed following spirometry testing as per National Institute for Health and Care Excellence (2018) guidance.
The nurse used the Calgary-Cambridge consultation model, as it combines the pathological description of COPD with the patient’s subjective experience of the illness (Silverman et al, 2013). Effective communication skills are essential in building a trusting therapeutic relationship, as the quality of the relationship between Ms Parker and the nurse will have a direct impact on the effectiveness of clinical outcomes (Fawcett and Rhynas, 2012).
In a national clinical audit report, Baxter et al (2016) identified inaccurate history taking and inadequately performed spirometry as important factors in the inaccurate diagnosis of COPD on general practice COPD registers; only 52.1% of patients included in the report had received quality-assured spirometry.
Pathophysiology of COPD
Knowing the pathophysiology of COPD allowed the nurse to recognise and understand the physical symptoms and provide effective care (Mitchell, 2015). Continued exposure to tobacco smoke is the likely cause of the damage to Ms Parker’s small airways, causing her cough and increased sputum production. She could also have chronic inflammation, resulting in airway smooth-muscle contraction, sluggish ciliary movement, hypertrophy and hyperplasia of mucus-secreting goblet cells, as well as release of inflammatory mediators (Mitchell, 2015).
Ms Parker may also have emphysema, which leads to damaged parenchyma (alveoli and structures involved in gas exchange) and loss of alveolar attachments (elastic connective fibres). This causes gas trapping, dynamic hyperinflation, decreased expiratory flow rates and airway collapse, particularly during expiration (Kaufman, 2013). Ms Parker also displayed pursed-lip breathing; this is a technique used to lengthen the expiratory time and improve gaseous exchange, and is a sign of dynamic hyperinflation (Douglas et al, 2013).
In a healthy lung, the destruction and repair of alveolar tissue depends on proteases and antiproteases, mainly released by neutrophils and macrophages. Inhaling cigarette smoke disrupts the usually delicately balanced activity of these enzymes, resulting in the parenchymal damage and small airways (with a lumen of <2mm in diameter) airways disease that is characteristic of emphysema. The severity of parenchymal damage or small airways disease varies, with no pattern related to disease progression (Global Initiative for Chronic Obstructive Lung Disease, 2018).
Ms Parker also had a wheeze, heard through a stethoscope as a continuous whistling sound, which arises from turbulent airflow through constricted airway smooth muscle, a process noted by Mitchell (2015). The wheeze, her 29 pack-year score, exertional breathlessness, cough, sputum production and tiredness, and the findings from her physical examination, were consistent with a diagnosis of COPD (GOLD, 2018; NICE, 2018).
Spirometry is a tool used to identify airflow obstruction but does not identify the cause. Commonly measured parameters are:
- Forced expiratory volume – the volume of air that can be exhaled – in one second (FEV1), starting from a maximal inspiration (in litres);
- Forced vital capacity (FVC) – the total volume of air that can be forcibly exhaled – at timed intervals, starting from a maximal inspiration (in litres).
Calculating the FEV1 as a percentage of the FVC gives the forced expiratory ratio (FEV1/FVC). This provides an index of airflow obstruction; the lower the ratio, the greater the degree of obstruction. In the absence of respiratory disease, FEV1 should be ≥70% of FVC. An FEV1/FVC of <70% is commonly used to denote airflow obstruction (Moore, 2012).
As they are time dependent, FEV1 and FEV1/FVC are reduced in diseases that cause airways to narrow and expiration to slow. FVC, however, is not time dependent: with enough expiratory time, a person can usually exhale to their full FVC. Lung function parameters vary depending on age, height, gender and ethnicity, so the degree of FEV1 and FVC impairment is calculated by comparing a person’s recorded values with predicted values. A recorded value of >80% of the predicted value has been considered ‘normal’ for spirometry parameters but the lower limit of normal – equal to the fifth percentile of a healthy, non-smoking population – based on more robust statistical models is increasingly being used (Cooper et al, 2017).
A reversibility test involves performing spirometry before and after administering a short-acting beta-agonist (SABA) such as salbutamol; the test is used to distinguish between reversible and fixed airflow obstruction. For symptomatic asthma, airflow obstruction due to airway smooth-muscle contraction is reversible: administering a SABA results in smooth-muscle relaxation and improved airflow (Lumb, 2016). However, COPD is associated with fixed airflow obstruction, resulting from neutrophil-driven inflammatory changes, excess mucus secretion and disrupted alveolar attachments, as opposed to airway smooth-muscle contraction.
Administering a SABA for COPD does not usually produce bronchodilation to the extent seen in someone with asthma: a person with asthma may demonstrate significant improvement in FEV1 (of >400ml) after having a SABA, but this may not change in someone with COPD (NICE, 2018). However, a negative response does not rule out therapeutic benefit from long-term SABA use (Marín et al, 2014).
NICE (2018) and GOLD (2018) guidelines advocate performing spirometry after administering a bronchodilator to diagnose COPD. Both suggest a FEV1/FVC of <70% in a person with respiratory symptoms supports a diagnosis of COPD, and both grade the severity of the condition using the predicted FEV1. Ms Parker’s spirometry results showed an FEV1/FVC of 56% and a predicted FEV1 of 57%, with no significant improvement in these values with a reversibility test.
GOLD (2018) guidance is widely accepted and used internationally. However, it was developed by medical practitioners with a medicalised approach, so there is potential for a bias towards pharmacological management of COPD. NICE (2018) guidance may be more useful for practice nurses, as it was developed by a multidisciplinary team using evidence from systematic reviews or meta-analyses of randomised controlled trials, providing a holistic approach. NICE guidance may be outdated on publication, but regular reviews are performed and published online.
NHS England (2016) holds a national register of all health professionals certified in spirometry. It was set up to raise spirometry standards across the country.
Assessment and management
The goals of assessing and managing Ms Parker’s COPD are to:
- Review and determine the level of airflow obstruction;
- Assess the disease’s impact on her life;
- Risk assess future disease progression and exacerbations;
- Recommend pharmacological and therapeutic management.
GOLD’s (2018) ABCD assessment tool (Fig 1) grades COPD severity using spirometry results, number of exacerbations, CAT score and mMRC score, and can be used to support evidence-based pharmacological management of COPD.

When Ms Parker was diagnosed, her predicted FEV1 of 57% categorised her as GOLD grade 2, and her mMRC score, CAT score and exacerbation history placed her in group D. The mMRC scale only measures breathlessness, but the CAT also assesses the impact COPD has on her life, meaning consecutive CAT scores can be compared, providing valuable information for follow-up and management (Zhao, et al, 2014).
After assessing the level of disease burden, Ms Parker was then provided with education for self-management and lifestyle interventions.
Lifestyle interventions
Smoking cessation.
Cessation of smoking alongside support and pharmacotherapy is the second-most cost-effective intervention for COPD, when compared with most other pharmacological interventions (BTS and PCRS UK, 2012). Smoking cessation:
- Slows the progression of COPD;
- Improves lung function;
- Improves survival rates;
- Reduces the risk of lung cancer;
- Reduces the risk of coronary heart disease risk (Qureshi et al, 2014).
Ms Parker accepted a referral to an All Wales Smoking Cessation Service adviser based at her GP surgery. The adviser used the internationally accepted ‘five As’ approach:
- Ask – record the number of cigarettes the individual smokes per day or week, and the year they started smoking;
- Advise – urge them to quit. Advice should be clear and personalised;
- Assess – determine their willingness and confidence to attempt to quit. Note the state of change;
- Assist – help them to quit. Provide behavioural support and recommend or prescribe pharmacological aids. If they are not ready to quit, promote motivation for a future attempt;
- Arrange – book a follow-up appointment within one week or, if appropriate, refer them to a specialist cessation service for intensive support. Document the intervention.
NICE (2013) guidance recommends that this be used at every opportunity. Stead et al (2016) suggested that a combination of counselling and pharmacotherapy have proven to be the most effective strategy.
Pulmonary rehabilitation
Ms Parker’s positive response to smoking cessation provided an ideal opportunity to offer her pulmonary rehabilitation (PR) – as indicated by Johnson et al (2014), changing one behaviour significantly increases a person’s chance of changing another.
PR – a supervised programme including exercise training, health education and breathing techniques – is an evidence-based, comprehensive, multidisciplinary intervention that:
- Improves exercise tolerance;
- Reduces dyspnoea;
- Promotes weight loss (Bolton et al, 2013).
These improvements often lead to an improved quality of life (Sciriha et al, 2015).
Most relevant for Ms Parker, PR has been shown to reduce anxiety and depression, which are linked to an increased risk of exacerbations and poorer health status (Miller and Davenport, 2015). People most at risk of future exacerbations are those who already experience them (Agusti et al, 2010), as in Ms Parker’s case. Patients who have frequent exacerbations have a lower quality of life, quicker progression of disease, reduced mobility and more-rapid decline in lung function than those who do not (Donaldson et al, 2002).
“COPD is a major public-health challenge; nurses can be pivotal in early identification, assessment and management”
Pharmacological interventions
Ms Parker has been prescribed inhaled salbutamol as required; this is a SABA that mediates the increase of cyclic adenosine monophosphate in airway smooth-muscle cells, leading to muscle relaxation and bronchodilation. SABAs facilitate lung emptying by dilatating the small airways, reversing dynamic hyperinflation of the lungs (Thomas et al, 2013). Ms Parker also uses a long-acting muscarinic antagonist (LAMA) inhaler, which works by blocking the bronchoconstrictor effects of acetylcholine on M3 muscarinic receptors in airway smooth muscle; release of acetylcholine by the parasympathetic nerves in the airways results in increased airway tone with reduced diameter.
At a routine review, Ms Parker admitted to only using the SABA and LAMA inhalers, despite also being prescribed a combined inhaled corticosteroid and long-acting beta 2 -agonist (ICS/LABA) inhaler. She was unaware that ICS/LABA inhalers are preferred over SABA inhalers, as they:
- Last for 12 hours;
- Improve the symptoms of breathlessness;
- Increase exercise tolerance;
- Can reduce the frequency of exacerbations (Agusti et al, 2010).
However, moderate-quality evidence shows that ICS/LABA combinations, particularly fluticasone, cause an increased risk of pneumonia (Suissa et al, 2013; Nannini et al, 2007). Inhaler choice should, therefore, be individualised, based on symptoms, delivery technique, patient education and compliance.
It is essential to teach and assess inhaler technique at every review (NICE, 2011). Ms Parker uses both a metered-dose inhaler and a dry-powder inhaler; an in-check device is used to assess her inspiratory effort, as different inhaler types require different inhalation speeds. Braido et al (2016) estimated that 50% of patients have poor inhaler technique, which may be due to health professionals lacking the confidence and capability to teach and assess their use.
Patients may also not have the dexterity, capacity to learn or vision required to use the inhaler. Online resources are available from, for example, RightBreathe (rightbreathe.com), British Lung Foundation (blf.org.uk). Ms Parker’s adherence could be improved through once-daily inhalers, as indicated by results from a study by Lipson et al (2017). Any change in her inhaler would be monitored as per local policy.
Vaccinations
Ms Parker keeps up to date with her seasonal influenza and pneumococcus vaccinations. This is in line with the low-cost, highest-benefit strategy identified by the British Thoracic Society and Primary Care Respiratory Society UK’s (2012) study, which was conducted to inform interventions for patients with COPD and their relative quality-adjusted life years. Influenza vaccinations have been shown to decrease the risk of lower respiratory tract infections and concurrent COPD exacerbations (Walters et al, 2017; Department of Health, 2011; Poole et al, 2006).
Self-management
Ms Parker was given a self-management plan that included:
- Information on how to monitor her symptoms;
- A rescue pack of antibiotics, steroids and salbutamol;
- A traffic-light system demonstrating when, and how, to commence treatment or seek medical help.
Self-management plans and rescue packs have been shown to reduce symptoms of an exacerbation (Baxter et al, 2016), allowing patients to be cared for in the community rather than in a hospital setting and increasing patient satisfaction (Fletcher and Dahl, 2013).
Improving Ms Parker’s adherence to once-daily inhalers and supporting her to self-manage and make the necessary lifestyle changes, should improve her symptoms and result in fewer exacerbations.
The earlier a diagnosis of COPD is made, the greater the chances of reducing lung damage through interventions such as smoking cessation, lifestyle modifications and treatment, if required (Price et al, 2011).
- Chronic obstructive pulmonary disease is a progressive respiratory condition, projected to become the third leading cause of death globally
- Diagnosis involves taking a patient history and performing spirometry testing
- Spirometry identifies airflow obstruction by measuring the volume of air that can be exhaled
- Chronic obstructive pulmonary disease is managed with lifestyle and pharmacological interventions, as well as self-management
Related files
200506 diagnosis and management of copd – a case study.
- Add to Bookmarks
Related articles
Have your say.
Sign in or Register a new account to join the discussion.
- Case Report
- Open access
- Published: 27 May 2024
A complex case study: coexistence of multi-drug-resistant pulmonary tuberculosis, HBV-related liver failure, and disseminated cryptococcal infection in an AIDS patient
- Wei Fu 1 , 2 na1 ,
- Zi Wei Deng 3 na1 ,
- Pei Wang 1 ,
- Zhen Wang Zhu 1 ,
- Zhi Bing Xie 1 ,
- Yong Zhong Li 1 &
- Hong Ying Yu 1
BMC Infectious Diseases volume 24 , Article number: 533 ( 2024 ) Cite this article
245 Accesses
1 Altmetric
Metrics details
Hepatitis B virus (HBV) infection can cause liver failure, while individuals with Acquired Immunodeficiency Virus Disease (AIDS) are highly susceptible to various opportunistic infections, which can occur concurrently. The treatment process is further complicated by the potential occurrence of immune reconstitution inflammatory syndrome (IRIS), which presents significant challenges and contributes to elevated mortality rates.
Case presentation
The 50-year-old male with a history of chronic hepatitis B and untreated human immunodeficiency virus (HIV) infection presented to the hospital with a mild cough and expectoration, revealing multi-drug resistant pulmonary tuberculosis (MDR-PTB), which was confirmed by XpertMTB/RIF PCR testing and tuberculosis culture of bronchoalveolar lavage fluid (BALF). The patient was treated with a regimen consisting of linezolid, moxifloxacin, cycloserine, pyrazinamide, and ethambutol for tuberculosis, as well as a combination of bictegravir/tenofovir alafenamide/emtricitabine (BIC/TAF/FTC) for HBV and HIV viral suppression. After three months of treatment, the patient discontinued all medications, leading to hepatitis B virus reactivation and subsequent liver failure. During the subsequent treatment for AIDS, HBV, and drug-resistant tuberculosis, the patient developed disseminated cryptococcal disease. The patient’s condition worsened during treatment with liposomal amphotericin B and fluconazole, which was ultimately attributed to IRIS. Fortunately, the patient achieved successful recovery after appropriate management.
Enhancing medical compliance is crucial for AIDS patients, particularly those co-infected with HBV, to prevent HBV reactivation and subsequent liver failure. Furthermore, conducting a comprehensive assessment of potential infections in patients before resuming antiviral therapy is essential to prevent the occurrence of IRIS. Early intervention plays a pivotal role in improving survival rates.
Peer Review reports
HIV infection remains a significant global public health concern, with a cumulative death toll of 40 million individuals [ 1 ]. In 2021 alone, there were 650,000 deaths worldwide attributed to AIDS-related causes. As of the end of 2021, approximately 38 million individuals were living with HIV, and there were 1.5 million new HIV infections reported annually on a global scale [ 2 ]. Co-infection with HBV and HIV is prevalent due to their similar transmission routes, affecting around 8% of HIV-infected individuals worldwide who also have chronic HBV infection [ 3 ]. Compared to those with HBV infection alone, individuals co-infected with HIV/HBV exhibit higher HBV DNA levels and a greater risk of reactivation [ 4 ]. Opportunistic infections, such as Pneumocystis jirovecii pneumonia, Toxoplasma encephalitis, cytomegalovirus retinitis, cryptococcal meningitis (CM), tuberculosis, disseminated Mycobacterium avium complex disease, pneumococcal pneumonia, Kaposi’s sarcoma, and central nervous system lymphoma, are commonly observed due to HIV-induced immunodeficiency [ 5 ]. Tuberculosis not only contributes to the overall mortality rate in HIV-infected individuals but also leads to a rise in the number of drug-resistant tuberculosis cases and transmission of drug-resistant strains. Disseminated cryptococcal infection is a severe opportunistic infection in AIDS patients [ 6 ], and compared to other opportunistic infections, there is a higher incidence of IRIS in patients with cryptococcal infection following antiviral and antifungal therapy [ 7 ]. This article presents a rare case of an HIV/HBV co-infected patient who presented with MDR-PTB and discontinued all medications during the initial treatment for HIV, HBV, and tuberculosis. During the subsequent re-anti-HBV/HIV treatment, the patient experienced two episodes of IRIS associated with cryptococcal infection. One episode was classified as “unmasking” IRIS, where previously subclinical cryptococcal infection became apparent with immune improvement. The other episode was categorized as “paradoxical” IRIS, characterized by the worsening of pre-existing cryptococcal infection despite immune restoration [ 8 ]. Fortunately, both episodes were effectively treated.
A 50-year-old male patient, who is self-employed, presented to our hospital in January 2022 with a chief complaint of a persistent cough for the past 2 months, without significant shortness of breath, palpitations, or fever. His medical history revealed a previous hepatitis B infection, which resulted in hepatic failure 10 years ago. Additionally, he was diagnosed with HIV infection. However, he ceased taking antiviral treatment with the medications provided free of charge by the Chinese government for a period of three years. During this hospital visit, his CD4 + T-cell count was found to be 26/μL (normal range: 500–1612/μL), HIV-1 RNA was 1.1 × 10 5 copies/ml, and HBV-DNA was negative. Chest computed tomography (CT) scan revealed nodular and patchy lung lesions (Fig. 1 ). The BALF shows positive acid-fast staining. Further assessment of the BALF using XpertMTB/RIF PCR revealed resistance to rifampicin, and the tuberculosis drug susceptibility test of the BALF (liquid culture, medium MGIT 960) indicated resistance to rifampicin, isoniazid, and streptomycin. Considering the World Health Organization (WHO) guidelines for drug-resistant tuberculosis, the patient’s drug susceptibility results, and the co-infection of HIV and HBV, an individualized treatment plan was tailored for him. The treatment plan included BIC/TAF/FTC (50 mg/25 mg/200 mg per day) for HBV and HIV antiviral therapy, as well as linezolid (0.6 g/day), cycloserine (0.5 g/day), moxifloxacin (0.4 g/day), pyrazinamide (1.5 g/day), and ethambutol (0.75 g/day) for anti-tuberculosis treatment, along with supportive care.
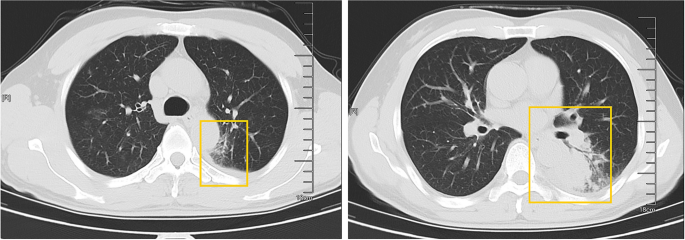
The patient’s pulmonary CT scan shows patchy and nodular lesions accompanied by a small amount of pleural effusion, later confirmed to be MDR-PTB
Unfortunately, after 3 months of follow-up, the patient discontinued all medications due to inaccessibility of the drugs. He returned to our hospital (Nov 12, 2022, day 0) after discontinuing medication for six months, with a complaint of poor appetite for the past 10 days. Elevated liver enzymes were observed, with an alanine aminotransferase level of 295 IU/L (normal range: 0–40 IU/L) and a total bilirubin(TBIL) level of 1.8 mg/dL (normal range: 0–1 mg/dL). His HBV viral load increased to 5.5 × 10 9 copies/ml. Considering the liver impairment, elevated HBV-DNA and the incomplete anti-tuberculosis treatment regimen (Fig. 2 A), we discontinued pyrazinamide and initiated treatment with linezolid, cycloserine, levofloxacin, and ethambutol for anti-tuberculosis therapy, along with BIC/TAF/FTC for HIV and HBV antiviral treatment. Additionally, enhanced liver protection and supportive management were provided, involving hepatoprotective effects of medications such as glutathione, magnesium isoglycyrrhizinate, and bicyclol. However, the patient’s TBIL levels continued to rise progressively, reaching 4.4 mg/dL on day 10 (Fig. 3 B). Suspecting drug-related factors, we discontinued all anti-tuberculosis medications while maintaining BIC/TAF/FTC for antiviral therapy, the patient’s TBIL levels continued to rise persistently. We ruled out other viral hepatitis and found no significant evidence of obstructive lesions on magnetic resonance cholangiopancreatography. Starting from the day 19, due to the patient’s elevated TBIL levels of 12.5 mg/dL, a decrease in prothrombin activity (PTA) to 52% (Fig. 3 D), and the emergence of evident symptoms such as abdominal distension and poor appetite, we initiated aggressive treatment methods. Unfortunately, on day 38, his hemoglobin level dropped to 65 g/L (normal range: 120–170 g/L, Fig. 3 A), and his platelet count decreased to 23 × 10 9 /L (normal range: 125–300 × 10 9 /L, Fig. 3 C). Based on a score of 7 on the Naranjo Scale, it was highly suspected that “Linezolid” was the cause of these hematological abnormalities. Therefore, we had to discontinue Linezolid for the anti-tuberculosis treatment. Subsequently, on day 50, the patient developed recurrent fever, a follow-up chest CT scan revealed enlarged nodules in the lungs (Fig. 2 B). The patient also reported mild dizziness and a worsening cough. On day 61, the previous blood culture results reported the growth of Cryptococcus. A lumbar puncture was performed on the same day, and the cerebrospinal fluid (CSF) opening pressure was measured at 130 mmH 2 O. India ink staining of the CSF showed typical encapsulated yeast cells suggestive of Cryptococcus. Other CSF results indicated mild leukocytosis and mildly elevated protein levels, while chloride and glucose levels were within normal limits. Subsequently, the patient received a fungal treatment regimen consisting of liposomal amphotericin B (3 mg/kg·d −1 ) in combination with fluconazole(600 mg/d). After 5 days of antifungal therapy, the patient’s fever symptoms were well controlled. Despite experiencing bone marrow suppression, including thrombocytopenia and worsening anemia, during this period, proactive symptom management, such as the use of erythropoietin, granulocyte colony-stimulating factor, and thrombopoietin, along with high-calorie dietary management, even reducing the dosage of liposomal amphotericin B to 2 mg/kg/day for 10 days at the peak of severity, successfully controlled the bone marrow suppression. However, within the following week, the patient experienced fever again, accompanied by a worsened cough, increased sputum production, and dyspnea. Nevertheless, the bilirubin levels did not show a significant increase. On day 78 the patient’s lung CT revealed patchy infiltrates and an increased amount of pleural effusion (Fig. 2 C). The CD4 + T-cell count was 89/μL (normal range: 500–700/μL), indicating a significant improvement in immune function compared to the previous stage, and C-reactive protein was significantly elevated, reflecting the inflammatory state, other inflammatory markers such as IL-6 and γ-IFN were also significantly elevated. On day 84, Considering the possibility of IRIS, the patient began taking methylprednisolone 30 mg once a day as part of an effort to control his excessive inflammation. Following the administration of methylprednisolone, the man experienced an immediate improvement in his fever. Additionally, symptoms such as cough, sputum production, dyspnea, and poor appetite gradually subsided over time. A follow-up lung CT showed significant improvement, indicating a positive response to the treatment. After 28 days of treatment with liposomal amphotericin B in combination with fluconazole, liposomal amphotericin B was discontinued, and the patient continued with fluconazole to consolidate the antifungal therapy for Cryptococcus. Considering the patient’s ongoing immunodeficiency, the dosage of methylprednisolone was gradually reduced by 4 mg every week. After improvement in liver function, the patient’s anti-tuberculosis treatment regimen was adjusted to include bedaquiline, contezolid, cycloserine, moxifloxacin, and ethambutol. The patient’s condition was well controlled, and a follow-up lung CT on day 117 indicated a significant improvement in lung lesions (Fig. 2 D).
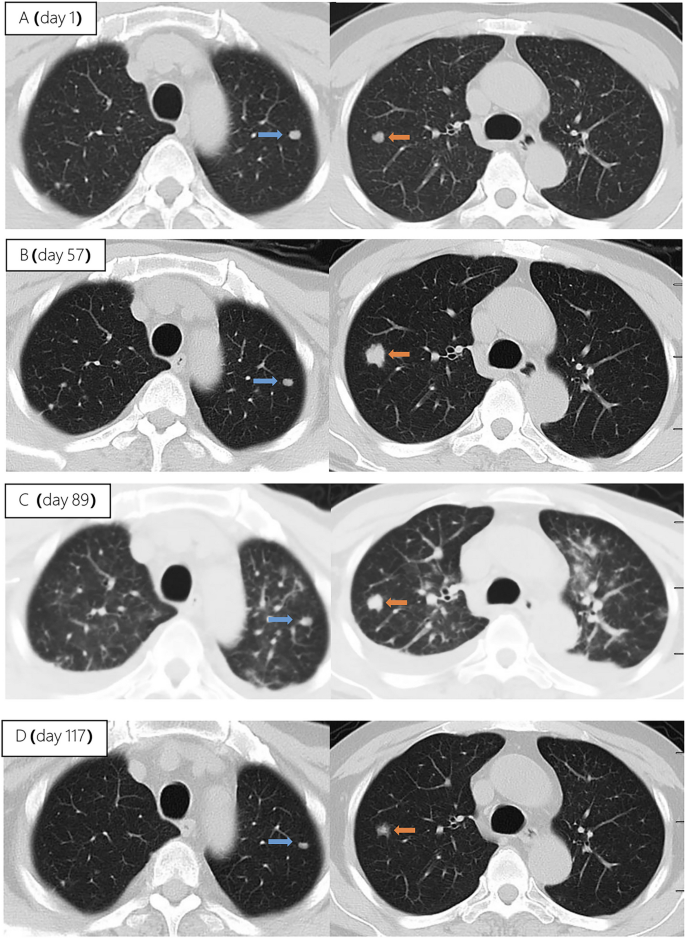
Upon second hospitalization admission ( A ), nodular lesions were already present in the lungs, and their size gradually increased after the initiation of ART ( B , C ). Notably, the lung lesions became more pronounced following the commencement of anti-cryptococcal therapy, coinciding with the occurrence of pleural effusion ( C ). However, with the continuation of antifungal treatment and the addition of glucocorticoids, there was a significant absorption and reduction of both the pleural effusion and pulmonary nodules ( D )
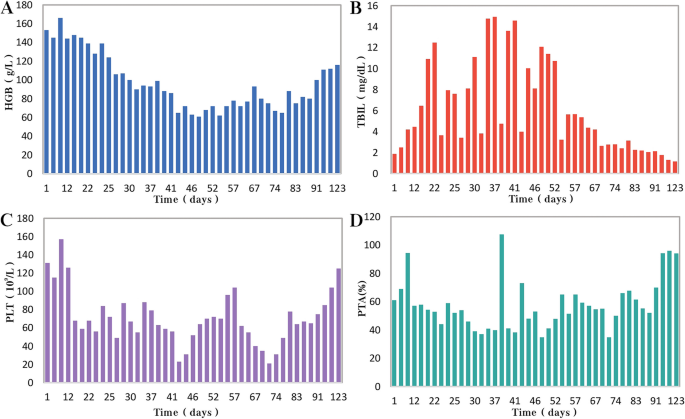
During the patient's second hospitalization, as the anti-tuberculosis treatment progressed and liver failure developed, the patient’s HGB levels gradually decreased ( A ), while TBIL levels increased ( B ). Additionally, there was a gradual decrease in PLT count ( C ) and a reduction in prothrombin activity (PTA) ( D ), indicating impaired clotting function. Moreover, myelosuppression was observed during the anti-cryptococcal treatment ( C )
People living with HIV/AIDS are susceptible to various opportunistic infections, which pose the greatest threat to their survival [ 5 ]. Pulmonary tuberculosis and disseminated cryptococcosis remain opportunistic infections with high mortality rates among AIDS patients [ 9 , 10 ]. These infections occurring on the basis of liver failure not only increase diagnostic difficulty but also present challenges in treatment. Furthermore, as the patient’s immune function and liver function recover, the occurrence of IRIS seems inevitable.
HIV and HBV co-infected patients are at a higher risk of HBV reactivation following the discontinuation of antiviral drugs
In this case, the patient presented with both HIV and HBV infections. Although the HBV DNA test was negative upon admission. However, due to the patient’s self-discontinuation of antiretroviral therapy (ART), HBV virologic and immunologic reactivation occurred six months later, leading to a rapid increase in viral load and subsequent hepatic failure. Charles Hannoun et al. also reported similar cases in 2001, where two HIV-infected patients with positive HBsAg experienced HBV reactivation and a rapid increase in HBV DNA levels after discontinuing antiretroviral and antiviral therapy, ultimately resulting in severe liver failure [ 11 ]. The European AIDS Clinical Society (EACS) also emphasize that abrupt discontinuation of antiviral therapy in patients co-infected with HBV and HIV can trigger HBV reactivation, which, although rare, can potentially result in liver failure [ 12 ].
Diagnosing disseminated Cryptococcus becomes more challenging in AIDS patients with liver failure, and the selection of antifungal medications is significantly restricted
In HIV-infected individuals, cryptococcal disease typically manifests as subacute meningitis or meningoencephalitis, often accompanied by fever, headache, and neck stiffness. The onset of symptoms usually occurs approximately two weeks after infection, with typical signs and symptoms including meningeal signs such as neck stiffness and photophobia. Some patients may also experience encephalopathy symptoms like somnolence, mental changes, personality changes, and memory loss, which are often associated with increased intracranial pressure (ICP) [ 13 ]. The presentation of cryptococcal disease in this patient was atypical, as there were no prominent symptoms such as high fever or rigors, nor were there any signs of increased ICP such as somnolence, headache, or vomiting. The presence of pre-existing pulmonary tuberculosis further complicated the early diagnosis, potentially leading to the clinical oversight of recognizing the presence of cryptococcus. In addition to the diagnostic challenges, treating a patient with underlying liver disease, multidrug-resistant tuberculosis, and concurrent cryptococcal infection poses significant challenges. It requires considering both the hepatotoxicity of antifungal agents and potential drug interactions. EACS and global guideline for the diagnosis and management of cryptococcosis suggest that liposomal amphotericin B (3 mg/kg·d −1 ) in combination with flucytosine (100 mg/kg·d −1 ) or fluconazole (800 mg/d) is the preferred induction therapy for CM for 14 days [ 12 , 14 ]. Flucytosine has hepatotoxicity and myelosuppressive effects, and it is contraindicated in patients with severe liver dysfunction. The antiviral drug bictegravir is a substrate for hepatic metabolism by CYP3A and UGT1A1 enzymes [ 15 ], while fluconazole inhibits hepatic enzymes CYP3A4 and CYP2C9 [ 16 ]. Due to the patient's liver failure and bone marrow suppression, we reduced the dosage of liposomal amphotericin B and fluconazole during the induction period. Considering the hepatotoxicity of fluconazole and its interaction with bictegravir, we decreased the dosage of fluconazole to 600 mg/d, while extending the duration of induction therapy to 28 days.
During re-antiviral treatment, maintaining vigilance for the development of IRIS remains crucial
IRIS refers to a series of inflammatory diseases that occur in HIV-infected individuals after initiating ART. It is associated with the paradoxical worsening of pre-existing infections, which may have been previously diagnosed and treated or may have been subclinical but become apparent due to the host regaining the ability to mount an inflammatory response. Currently, there is no universally accepted definition of IRIS. However, the following conditions are generally considered necessary for diagnosing IRIS: worsening of a diagnosed or previously unrecognized pre-existing infection with immune improvement (referred to as “paradoxical” IRIS) or the unmasking of a previously subclinical infection (referred to as “unmasking” IRIS) [ 8 ]. It is estimated that 10% to 30% of HIV-infected individuals with CM will develop IRIS after initiating or restarting effective ART [ 7 , 17 ]. In the guidelines of the WHO and EACS, it is recommended to delay the initiation of antiviral treatment for patients with CM for a minimum of 4 weeks to reduce the incidence of IRIS. Since we accurately identified the presence of multidrug-resistant pulmonary tuberculosis in the patient during the early stage, we promptly initiated antiretroviral and anti-hepatitis B virus treatment during the second hospitalization. However, subsequent treatment revealed that the patient experienced at least two episodes of IRIS. The first episode was classified as “unmasking” IRIS, as supported by the enlargement of pulmonary nodules observed on the chest CT scan following the initiation of ART (Fig. 2 A). Considering the morphological changes of the nodules on the chest CT before antifungal therapy, the subsequent emergence of disseminated cryptococcal infection, and the subsequent reduction in the size of the lung nodules after antifungal treatment, although there is no definitive microbiological evidence, we believe that the initial enlargement of the lung nodules was caused by cryptococcal pneumonia. As ART treatment progressed, the patient experienced disseminated cryptococcosis involving the blood and central nervous system, representing the first episode. Following the initiation of antifungal therapy for cryptococcosis, the patient encountered a second episode characterized by fever and worsening pulmonary lesions. Given the upward trend in CD4 + T-cell count, we attributed this to the second episode of IRIS, the “paradoxical” type. The patient exhibited a prompt response to low-dose corticosteroids, further supporting our hypothesis. Additionally, the occurrence of cryptococcal IRIS in the lungs, rather than the central nervous system, is relatively uncommon among HIV patients [ 17 ].
Conclusions
From the initial case of AIDS combined with chronic hepatitis B, through the diagnosis and treatment of multidrug-resistant tuberculosis, the development of liver failure and disseminated cryptococcosis, and ultimately the concurrent occurrence of IRIS, the entire process was tortuous but ultimately resulted in a good outcome (Fig. 4 ). Treatment challenges arose due to drug interactions, myelosuppression, and the need to manage both infectious and inflammatory conditions. Despite these hurdles, a tailored treatment regimen involving antifungal and antiretroviral therapies, along with corticosteroids, led to significant clinical improvement. While CM is relatively common among immunocompromised individuals, especially those with acquired immunodeficiency syndrome (AIDS) [ 13 ], reports of disseminated cryptococcal infection on the background of AIDS complicated with liver failure are extremely rare, with a very high mortality rate.
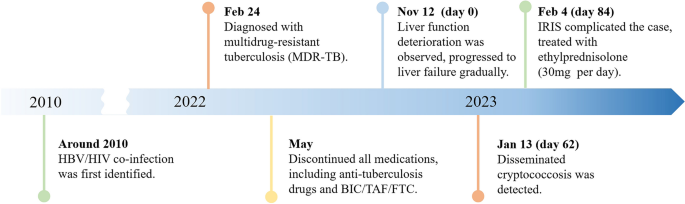
A brief timeline of the patient's medical condition progression and evolution
Through managing this patient, we have also gained valuable insights. (1) Swift and accurate diagnosis, along with timely and effective treatment, can improve prognosis, reduce mortality, and lower disability rates. Whether it's the discovery and early intervention of liver failure, the identification and treatment of disseminated cryptococcosis, or the detection and management of IRIS, all these interventions are crucially timely. They are essential for the successful treatment of such complex and critically ill patients.
(2) Patients who exhibit significant drug reactions, reducing the dosage of relevant medications and prolonging the treatment duration can improve treatment success rates with fewer side effects. In this case, the dosages of liposomal amphotericin B and fluconazole are lower than the recommended dosages by the World Health Organization and EACS guidelines. Fortunately, after 28 days of induction therapy, repeat CSF cultures showed negative results for Cryptococcus, and the improvement of related symptoms also indicates that the patient has achieved satisfactory treatment outcomes. (3) When cryptococcal infection in the bloodstream or lungs is detected, prompt lumbar puncture should be performed to screen for central nervous system cryptococcal infection. Despite the absence of neurological symptoms, the presence of Cryptococcus neoformans in the cerebrospinal fluid detected through lumbar puncture suggests the possibility of subclinical or latent CM, especially in late-stage HIV-infected patients.
We also encountered several challenges and identified certain issues that deserve attention. Limitations: (1) The withdrawal of antiviral drugs is a critical factor in the occurrence and progression of subsequent diseases in patients. Improved medical education is needed to raise awareness and prevent catastrophic consequences. (2) Prior to re-initiating antiviral therapy, a thorough evaluation of possible infections in the patient is necessary. Caution should be exercised, particularly in the case of diseases prone to IRIS, such as cryptococcal infection. (3) There is limited evidence on the use of reduced fluconazole dosage (600 mg daily) during antifungal therapy, and the potential interactions between daily fluconazole (600 mg) and the antiviral drug bictegravir and other tuberculosis medications have not been extensively studied. (4) Further observation is needed to assess the impact of early-stage limitations in the selection of anti-tuberculosis drugs on the treatment outcome of tuberculosis in this patient, considering the presence of liver failure.
In conclusion, managing opportunistic infections in HIV patients remains a complex and challenging task, particularly when multiple opportunistic infections are compounded by underlying liver failure. Further research efforts are needed in this area.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
Hepatitis B virus
Acquired immunodeficiency virus disease
Immune reconstitution inflammatory syndrome
Human immunodeficiency virus
Multi-drug resistant pulmonary tuberculosis
Bronchoalveolar lavage fluid
Bictegravir/tenofovir alafenamide/emtricitabine
Cryptococcal meningitis
World Health Organization
Computed tomography
Total bilirubin
Cerebrospinal fluid
European AIDS Clinical Society
Intracranial pressure
Antiretroviral therapy
Prothrombin activity
Bekker L-G, Beyrer C, Mgodi N, Lewin SR, Delany-Moretlwe S, Taiwo B, et al. HIV infection. Nat Rev Dis Primer. 2023;9:1–21.
Google Scholar
Data on the size of the HIV epidemic. https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/data-on-the-size-of-the-hiv-aids-epidemic?lang=en . Accessed 3 May 2023.
Leumi S, Bigna JJ, Amougou MA, Ngouo A, Nyaga UF, Noubiap JJ. Global burden of hepatitis B infection in people living with human immunodeficiency virus: a systematic review and meta-analysis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020;71:2799–806.
Article Google Scholar
McGovern BH. The epidemiology, natural history and prevention of hepatitis B: implications of HIV coinfection. Antivir Ther. 2007;12(Suppl 3):H3-13.
Article CAS PubMed Google Scholar
Kaplan JE, Masur H, Holmes KK, Wilfert CM, Sperling R, Baker SA, et al. USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus: an overview. USPHS/IDSA Prevention of Opportunistic Infections Working Group. Clin Infect Dis Off Publ Infect Dis Soc Am. 1995;21 Suppl 1:S12-31.
Article CAS Google Scholar
Bamba S, Lortholary O, Sawadogo A, Millogo A, Guiguemdé RT, Bretagne S. Decreasing incidence of cryptococcal meningitis in West Africa in the era of highly active antiretroviral therapy. AIDS Lond Engl. 2012;26:1039–41.
Müller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M, et al. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:251–61.
Article PubMed PubMed Central Google Scholar
Haddow LJ, Easterbrook PJ, Mosam A, Khanyile NG, Parboosing R, Moodley P, et al. Defining immune reconstitution inflammatory syndrome: evaluation of expert opinion versus 2 case definitions in a South African cohort. Clin Infect Dis Off Publ Infect Dis Soc Am. 2009;49:1424–32.
Obeagu E, Onuoha E. Tuberculosis among HIV patients: a review of Prevalence and Associated Factors. Int J Adv Res Biol Sci. 2023;10:128–34.
Rajasingham R, Govender NP, Jordan A, Loyse A, Shroufi A, Denning DW, et al. The global burden of HIV-associated cryptococcal infection in adults in 2020: a modelling analysis. Lancet Infect Dis. 2022;22:1748–55.
Manegold C, Hannoun C, Wywiol A, Dietrich M, Polywka S, Chiwakata CB, et al. Reactivation of hepatitis B virus replication accompanied by acute hepatitis in patients receiving highly active antiretroviral therapy. Clin Infect Dis Off Publ Infect Dis Soc Am. 2001;32:144–8.
EACS Guidelines | EACSociety. https://www.eacsociety.org/guidelines/eacs-guidelines/ . Accessed 7 May 2023.
Cryptococcosis | NIH. 2021. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/cryptococcosis . Accessed 6 May 2023.
Chang CC, Harrison TS, Bicanic TA, Chayakulkeeree M, Sorrell TC, Warris A, et al. Global guideline for the diagnosis and management of cryptococcosis: an initiative of the ECMM and ISHAM in cooperation with the ASM. Lancet Infect Dis. 2024;10:S1473-3099(23)00731-4.
Deeks ED. Bictegravir/emtricitabine/tenofovir alafenamide: a review in HIV-1 infection. Drugs. 2018;78:1817–28.
Article CAS PubMed PubMed Central Google Scholar
Bellmann R, Smuszkiewicz P. Pharmacokinetics of antifungal drugs: practical implications for optimized treatment of patients. Infection. 2017;45:737–79.
Shelburne SA, Darcourt J, White AC, Greenberg SB, Hamill RJ, Atmar RL, et al. The role of immune reconstitution inflammatory syndrome in AIDS-related Cryptococcus neoformans disease in the era of highly active antiretroviral therapy. Clin Infect Dis Off Publ Infect Dis Soc Am. 2005;40:1049–52.
Download references
Acknowledgements
We express our sincere gratitude for the unwavering trust bestowed upon our medical team by the patient throughout the entire treatment process.
This work was supported by the Scientific Research Project of Hunan Public Health Alliance with the approval No. ky2022-002.
Author information
Wei Fu and Zi Wei Deng contributed equally to this work.

Authors and Affiliations
Center for Infectious Diseases, Hunan University of Medicine General Hospital, Huaihua, Hunan, China
Wei Fu, Pei Wang, Zhen Wang Zhu, Ye Pu, Zhi Bing Xie, Yong Zhong Li & Hong Ying Yu
Department of Tuberculosis, The First Affiliated Hospital of Xinxiang Medical University, XinXiang, Henan, China
Department of Clinical Pharmacy, Hunan University of Medicine General Hospital, Huaihua, Hunan, China
Zi Wei Deng
You can also search for this author in PubMed Google Scholar
Contributions
WF and ZWD integrated the data and wrote the manuscript, YHY contributed the revision of the manuscript, PW and YP provided necessary assistance and provided key suggestions, ZWZ, YZL and ZBX contributed data acquisition and interpretation for etiological diagnosis. All authors reviewed and approved the final manuscript.
Corresponding author
Correspondence to Hong Ying Yu .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the Ethics Committee of the Hunan University of Medicine General Hospital (HYZY-EC-202306-C1), and with the informed consent of the patient.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Fu, W., Deng, Z.W., Wang, P. et al. A complex case study: coexistence of multi-drug-resistant pulmonary tuberculosis, HBV-related liver failure, and disseminated cryptococcal infection in an AIDS patient. BMC Infect Dis 24 , 533 (2024). https://doi.org/10.1186/s12879-024-09431-9
Download citation
Received : 30 June 2023
Accepted : 24 May 2024
Published : 27 May 2024
DOI : https://doi.org/10.1186/s12879-024-09431-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Liver failure
- Disseminated cryptococcal disease
BMC Infectious Diseases
ISSN: 1471-2334
- General enquiries: [email protected]
MOG Antibody Disease: Nuances in Presentation, Diagnosis, and Management
- Published: 28 May 2024
Cite this article

- Kelsey A. Stefan 1 &
- John R. Ciotti ORCID: orcid.org/0000-0002-5696-8841 1
61 Accesses
Explore all metrics
Purpose of Review
Myelin oligodendrocyte glycoprotein antibody disease (MOGAD) is a distinct neuroinflammatory condition characterized by attacks of optic neuritis, transverse myelitis, and other demyelinating events. Though it can mimic multiple sclerosis and neuromyelitis optica spectrum disorder, distinct clinical and radiologic features which can discriminate these conditions are now recognized. This review highlights recent advances in our understanding of clinical manifestations, diagnosis, and treatment of MOGAD.
Recent Findings
Studies have identified subtleties of common clinical attacks and identified more rare phenotypes, including cerebral cortical encephalitis, which have broadened our understanding of the clinicoradiologic spectrum of MOGAD and culminated in the recent publication of proposed diagnostic criteria with a familiar construction to those diagnosing other neuroinflammatory conditions. These criteria, in combination with advances in antibody testing, should simultaneously lead to wider recognition and reduced incidence of misdiagnosis. In addition, recent observational studies have raised new questions about when to treat MOGAD chronically, and with which agent.
MOGAD pathophysiology informs some of the relatively unique clinical and radiologic features which have come to define this condition, and similarly has implications for diagnosis and management. Further prospective studies and the first clinical trials of therapeutic options will answer several remaining questions about the peculiarities of this condition.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others

Neurological update: MOG antibody disease

Clinical Characteristics and Treatment of MOG-IgG–Associated Optic Neuritis
Mog antibody-related disorders: common features and uncommon presentations, papers of particular interest, published recently, have been highlighted as: • of importance •• of major importance.
O’Connor KC, McLaughlin KA, De Jager PL, Chitnis T, Bettelli E, Xu C, et al. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat Med. 2007;13(2):211–7. https://doi.org/10.1038/nm1488 .
Article CAS PubMed PubMed Central Google Scholar
Ciotti JR, Eby NS, Wu GF, Naismith RT, Chahin S, Cross AH. Clinical and laboratory features distinguishing MOG antibody disease from multiple sclerosis and AQP4 antibody-positive neuromyelitis optica. Mult Scler Relat Disord. 2020;45:102399. https://doi.org/10.1016/j.msard.2020.102399 .
Article PubMed Google Scholar
•• Banwell B, Bennett JL, Marignier R, Kim HJ, Brilot F, Flanagan EP, et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD Panel proposed criteria. Lancet Neurol. 2023;22(3):268–82. https://doi.org/10.1016/S1474-4422(22)00431-8 . ( This proposed diagnostic criteria, as determined by an international panel of neuroimmunology experts, defines MOG antibody disease and accounts for common clinical presentations and MOG antibody titers. )
Article CAS PubMed Google Scholar
Johns TG, Bernard CC. The structure and function of myelin oligodendrocyte glycoprotein. J Neurochem. 1999;72(1):1–9. https://doi.org/10.1046/j.1471-4159.1999.0720001.x .
Peschl P, Bradl M, Höftberger R, Berger T, Reindl M. Myelin Oligodendrocyte Glycoprotein: Deciphering a Target in Inflammatory Demyelinating Diseases. Front Immunol. 2017;8(8):529. https://doi.org/10.3389/fimmu.2017.00529 .
•• Corbali O, Chitnis T. Pathophysiology of myelin oligodendrocyte glycoprotein antibody disease. Front Neurol. 2023;14:1137998. https://doi.org/10.3389/fneur.2023.1137998 . ( This review comprehensively summarizes the pathophysiology of MOG antibody disease, which offers insight into its unique clinical and radiologic features. )
Article PubMed PubMed Central Google Scholar
Lebar R, Boutry JM, Vincent C, Robineaux R, Voisin GA. Studies on autoimmune encephalomyelitis in the guinea pig. II. An in vitro investigation on the nature, properties, and specificity of the serum-demyelinating factor. J Immunol. 1976;116(5):1439–46.
Linington C, Lassmann H. Antibody responses in chronic relapsing experimental allergic encephalomyelitis: correlation of serum demyelinating activity with antibody titre to the myelin/oligodendrocyte glycoprotein (MOG). J Neuroimmunol. 1987;17(1):61–9. https://doi.org/10.1016/0165-5728(87)90031-2 .
Schluesener HJ, Sobel RA, Linington C, Weiner HL. A monoclonal antibody against a myelin oligodendrocyte glycoprotein induces relapses and demyelination in central nervous system autoimmune disease. J Immunol. 1987;139(12):4016–21.
Papadopoulos MC, Verkman AS. Aquaporin 4 and neuromyelitis optica. Lancet Neurol. 2012;11(6):535–44. https://doi.org/10.1016/S1474-4422(12)70133-3 .
Mariotto S, Ferrari S, Monaco S, Benedetti MD, Schanda K, Alberti D, et al. Clinical spectrum and IgG subclass analysis of anti-myelin oligodendrocyte glycoprotein antibody-associated syndromes: a multicenter study. J Neurol. 2017;264(12):2420–30. https://doi.org/10.1007/s00415-017-8635-4 .
Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;20(5):520. https://doi.org/10.3389/fimmu.2014.00520 .
Article CAS Google Scholar
Majed M, Fryer JP, McKeon A, Lennon VA, Pittock SJ. Clinical utility of testing AQP4-IgG in CSF: Guidance for physicians. Neurol Neuroimmunol Neuroinflamm. 2016;3(3):e231. https://doi.org/10.1212/NXI.0000000000000231 .
Pace S, Orrell M, Woodhall M, Palace J, Leite MI, Irani SR, et al. Frequency of MOG-IgG in cerebrospinal fluid versus serum. J Neurol Neurosurg Psychiatry. 2022;93(3):334–5. https://doi.org/10.1136/jnnp-2021-326779 .
Höftberger R, Guo Y, Flanagan EP, Lopez-Chiriboga AS, Endmayr V, Hochmeister S, et al. The pathology of central nervous system inflammatory demyelinating disease accompanying myelin oligodendrocyte glycoprotein autoantibody. Acta Neuropathol. 2020;139(5):875–92. https://doi.org/10.1007/s00401-020-02132-y .
Yandamuri SS, Filipek B, Obaid AH, Lele N, Thurman JM, Makhani N, et al. MOGAD patient autoantibodies induce complement, phagocytosis, and cellular cytotoxicity. JCI Insight. 2023;8(11):e165373. https://doi.org/10.1172/jci.insight.165373 .
Kinzel S, Lehmann-Horn K, Torke S, Häusler D, Winkler A, Stadelmann C, et al. Myelin-reactive antibodies initiate T cell-mediated CNS autoimmune disease by opsonization of endogenous antigen. Acta Neuropathol. 2016;132(1):43–58. https://doi.org/10.1007/s00401-016-1559-8 .
• de Mol CL, Wong Y, van Pelt ED, Wokke B, Siepman T, Neuteboom RF, et al. The clinical spectrum and incidence of anti-MOG-associated acquired demyelinating syndromes in children and adults. Mult Scler. 2020;26(7):806–14. https://doi.org/10.1177/1352458519845112 . ( This national registry study helps to define the incidence and clinical spectrum of MOGAD. )
O’Connell K, Hamiton-Shield A, Woodhall M, Messina S, Mariano R, Waters P, et al. Prevalence and incidence of neuromyelitis optica spectrum disorder, aquaporin-4 antibody-positive NMOSD and MOG antibody-positive disease in Oxfordshire. UK J Neurol Neurosurg Psychiatry. 2020;91(10):1126–8. https://doi.org/10.1136/jnnp-2020-323158 .
Hor JY, Fujihara K. Epidemiology of myelin oligodendrocyte glycoprotein antibody-associated disease: a review of prevalence and incidence worldwide. Front Neurol. 2023;15(14):1260358. https://doi.org/10.3389/fneur.2023.1260358 .
Article Google Scholar
Bruijstens AL, Wong YYM, van Pelt DE, van der Linden PJE, Haasnoot GW, Hintzen RQ, et al. HLA association in MOG-IgG- and AQP4-IgG-related disorders of the CNS in the Dutch population. Neurol Neuroimmunol Neuroinflamm. 2020;7(3):e702. https://doi.org/10.1212/NXI.0000000000000702 .
Nakamura M, Ogawa R, Fujimori J, Uzawa A, Sato Y, Nagashima K, et al. Epidemiological and clinical characteristics of myelin oligodendrocyte glycoprotein antibody-associated disease in a nationwide survey. Mult Scler. 2023;29(4–5):530–9. https://doi.org/10.1177/13524585231156736 .
Akaishi T, Fujimori J, Takahashi T, Misu T, Takai Y, Nishiyama S, et al. Seasonal variation of onset in patients with anti-aquaporin-4 antibodies and anti-myelin oligodendrocyte glycoprotein antibody. J Neuroimmunol. 2020;15(349):577431. https://doi.org/10.1016/j.jneuroim.2020.577431 .
Orlandi R, Mariotto S, Gajofatto A. Prevalence, incidence, and season distribution of MOG antibody-associated disease in the province of Verona. Italy Mult Scler Relat Disord. 2022;63:103884. https://doi.org/10.1016/j.msard.2022.103884 .
Lambe J, McGinley MP, Moss BP, Mao-Draayer Y, Kassa R, Ciotti JR, et al. Myelin oligodendrocyte glycoprotein-IgG associated disorders (MOGAD) following SARS-CoV-2 infection: A case series. J Neuroimmunol. 2022;15(370):577933. https://doi.org/10.1016/j.jneuroim.2022.577933 .
Boudjani H, Fadda G, Dufort G, Antel J, Giacomini P, Levesque-Roy M, et al. Clinical course, imaging, and pathological features of 45 adult and pediatric cases of myelin oligodendrocyte glycoprotein antibody-associated disease. Mult Scler Relat Disord. 2023;76:104787. https://doi.org/10.1016/j.msard.2023.104787 .
Kunchok A, Flanagan EP, Snyder M, Saadeh R, Chen JJ, Weinshenker BG, et al. Coexisting systemic and organ-specific autoimmunity in MOG-IgG1-associated disorders versus AQP4-IgG+ NMOSD. Mult Scler. 2021;27(4):630–5. https://doi.org/10.1177/1352458520933884 .
Titulaer MJ, Höftberger R, Iizuka T, Leypoldt F, McCracken L, Cellucci T, et al. Overlapping demyelinating syndromes and anti–N-methyl-D-aspartate receptor encephalitis. Ann Neurol. 2014;75(3):411–28. https://doi.org/10.1002/ana.24117 .
Molazadeh N, Bose G, Lotan I, Levy M. Autoimmune diseases and cancers overlapping with myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD): A systematic review. Mult Scler J Exp Transl Clin. 2022;8(4):20552173221128170. https://doi.org/10.1177/20552173221128170 .
Berek K, Grams A, Uprimny C, Prieschl M, Ramberger M, Unterberger I, et al. Anti-NMDA receptor encephalitis and MOG-associated demyelination - a case report with long-term follow-up and a systematic review. BMC Neurol. 2022;22(1):434. https://doi.org/10.1186/s12883-022-02974-x .
Trentinaglia M, Dinoto A, Carta S, Chiodega V, Ferrari S, Andreone V, et al. Investigating the association between neoplasms and MOG antibody-associated disease. Front Neurol. 2023;9(14):1193211. https://doi.org/10.3389/fneur.2023.1193211 .
Ramanathan S, Mohammad S, Tantsis E, Nguyen TK, Merheb V, Fung VSC, et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry. 2018;89(2):127–37. https://doi.org/10.1136/jnnp-2017-316880 .
Bennett JL, Costello F, Chen JJ, Petzold A, Biousse V, Newman NJ, et al. Optic neuritis and autoimmune optic neuropathies: advances in diagnosis and treatment. Lancet Neurol. 2023;22(1):89–100. https://doi.org/10.1016/S1474-4422(22)00187-9 .
Jurynczyk M, Messina S, Woodhall MR, Raza N, Everett R, Roca-Fernandez A, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017;140(12):3128–38. https://doi.org/10.1093/brain/awx276 .
Gospe SM 3rd, Chen JJ, Bhatti MT. Neuromyelitis optica spectrum disorder and myelin oligodendrocyte glycoprotein associated disorder-optic neuritis: a comprehensive review of diagnosis and treatment. Eye (Lond). 2021;35(3):753–68. https://doi.org/10.1038/s41433-020-01334-8 .
Chen JJ, Flanagan EP, Jitprapaikulsan J, Lopez-Chiriboga AS, Fryer JP, Leavitt JA, et al. Myelin Oligodendrocyte Glycoprotein Antibody-Positive Optic Neuritis: Clinical Characteristics, Radiologic Clues, and Outcome. Am J Ophthalmol. 2018;195:8–15. https://doi.org/10.1016/j.ajo.2018.07.020 .
Lee HJ, Kim B, Waters P, Woodhall M, Irani S, Ahn S, et al. Chronic relapsing inflammatory optic neuropathy (CRION): a manifestation of myelin oligodendrocyte glycoprotein antibodies. J Neuroinflammation. 2018;15(1):302. https://doi.org/10.1186/s12974-018-1335-x .
Liu H, Zhou H, Wang J, Xu Q, Wei S. Antibodies to myelin oligodendrocyte glycoprotein in chronic relapsing inflammatory optic neuropathy. Br J Ophthalmol. 2019;103(10):1423–8. https://doi.org/10.1136/bjophthalmol-2018-313142 .
Ramanathan S, Prelog K, Barnes EH, Tantsis EM, Reddel SW, Henderson APD, et al. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult Scler. 2016;22(4):470–82. https://doi.org/10.1177/1352458515593406 .
Bartels F, Lu A, Oertel FC, Finke C, Paul F, Chien C. Clinical and neuroimaging findings in MOGAD-MRI and OCT. Clin Exp Immunol. 2021;206(3):266–81. https://doi.org/10.1111/cei.13641 .
Ciotti JR, Eby NS, Brier MR, Wu GF, Chahin S, Cross AH, et al. Central vein sign and other radiographic features distinguishing myelin oligodendrocyte glycoprotein antibody disease from multiple sclerosis and aquaporin-4 antibody-positive neuromyelitis optica. Mult Scler. 2022;28(1):49–60. https://doi.org/10.1177/13524585211007086 .
Purvin V, Kawaski A, Jacobson DM. Optic perineuritis: clinical and radiographic features. Arch Ophthalmol. 2001;119(9):1299–306. https://doi.org/10.1001/archopht.119.9.1299 .
Lopez-Chiriboga AS, Van Stavern G, Flanagan EP, Pittock SJ, Fryer J, Bhatti MT, et al. Myelin Oligodendrocyte Glycoprotein Antibody (MOG-IgG)-Positive Optic Perineuritis. Neuroophthalmology. 2019;44(1):1–4. https://doi.org/10.1080/01658107.2019.1607883 .
Chen JJ, Sotirchos ES, Henderson AD, Vasileiou ES, Flanagan EP, Bhatti MT, et al. OCT retinal nerve fiber layer thickness differentiates acute optic neuritis from MOG antibody-associated disease and Multiple Sclerosis: RNFL thickening in acute optic neuritis from MOGAD vs MS. Mult Scler Relat Disord. 2022;58:103525. https://doi.org/10.1016/j.msard.2022.103525 .
Pakeerathan T, Havla J, Schwake C, Salmen A, Bigi S, Abegg M, et al. Characteristic retinal atrophy pattern allows differentiation between pediatric MOGAD and MS after a single optic neuritis episode. J Neurol. 2022;269(12):6366–76. https://doi.org/10.1007/s00415-022-11256-y .
Mariano R, Messina S, Kumar K, Kuker W, Leite MI, Palace J. Comparison of Clinical Outcomes of Transverse Myelitis Among Adults With Myelin Oligodendrocyte Glycoprotein Antibody vs Aquaporin-4 Antibody Disease. JAMA Netw Open. 2019;2(10):e1912732. https://doi.org/10.1001/jamanetworkopen.2019.12732 .
Qian P, Lancia S, Alvarez E, Klawiter EC, Cross AH, Naismith RT. Association of neuromyelitis optica with severe and intractable pain. Arch Neurol. 2012;69(11):1482–7. https://doi.org/10.1001/archneurol.2012.768 .
ZhangBao J, Huang W, Zhou L, Wang L, Chang X, Lu C, et al. Myelitis in inflammatory disorders associated with myelin oligodendrocyte glycoprotein antibody and aquaporin-4 antibody: A comparative study in Chinese Han patients. Eur J Neurol. 2021;28(4):1308–15. https://doi.org/10.1111/ene.14654 .
Jitprapaikulsan J, Lopez-Chiriboga AS, Flanagan EP, Fryer JP, McKeon A, Weinshenker BG, et al. Novel glial targets and recurrent longitudinally extensive transverse myelitis. JAMA Neurol. 2018;75(7):892–5. https://doi.org/10.1001/jamaneurol.2018.0805 .
Dubey D, Pittock SJ, Krecke KN, Morris PP, Sechi E, Zalewski NL, et al. Clinical, radiologic, and prognostic features of myelitis associated with myelin oligodendrocyte glycoprotein autoantibody. JAMA Neurol. 2019;76(3):301–9. https://doi.org/10.1001/jamaneurol.2018.4053 .
Geraldes R, Ciccarelli O, Barkhof F, De Stefano N, Enzinger C, Filippi M, et al. The current role of MRI in differentiating multiple sclerosis from its imaging mimics. Nat Rev Neurol. 2018;14(4):199–213. https://doi.org/10.1038/nrneurol.2018.14 .
Denève M, Biotti D, Patsoura S, Ferrier M, Meluchova Z, Mahieu L, et al. MRI features of demyelinating disease associated with anti-MOG antibodies in adults. J Neuroradiol. 2019;46(5):312–8. https://doi.org/10.1016/j.neurad.2019.06.001 .
Etemadifar M, Ashourizadeh H, Nouri H, Kargaran PK, Salari M, Rayani M, et al. MRI signs of CNS demyelinating diseases. Mult Scler Relat Disord. 2021;47:102665. https://doi.org/10.1016/j.msard.2020.102665 .
Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients, Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13(1):280. https://doi.org/10.1186/s12974-016-0718-0 .
• Cobo-Calvo A, Ruiz A, Rollot F, Arrambide G, Deschamps R, Maillart E, et al. Clinical Features and Risk of Relapse in Children and Adults with Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease. Ann Neurol. 2021;89(1):30–41. https://doi.org/10.1002/ana.25909 . ( This nationwide observational study highlights differing clinical presentations of MOGAD in adults versus children. )
Krupp LB, Tardieu M, Amato MP, Banwell B, Chitnis T, Dale RC, et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19(10):1261–7. https://doi.org/10.1177/1352458513484547 .
Waters P, Fadda G, Woodhall M, O’Mahony J, Brown RA, Castro DA, et al. Serial Anti-Myelin Oligodendrocyte Glycoprotein Antibody Analyses and Outcomes in Children With Demyelinating Syndromes. JAMA Neurol. 2020;77(1):82–93. https://doi.org/10.1001/jamaneurol.2019.2940 .
Sechi E, Cacciaguerra L, Chen JJ, Mariotto S, Fadda G, Dinoto A, et al. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease (MOGAD): A Review of Clinical and MRI Features, Diagnosis, and Management. Front Neurol. 2022;17(13):885218. https://doi.org/10.3389/fneur.2022.885218 .
Lopez-Chiriboga AS, Majed M, Fryer J, Dubey D, McKeon A, Flanagan EP, et al. Association of MOG-IgG Serostatus With Relapse After Acute Disseminated Encephalomyelitis and Proposed Diagnostic Criteria for MOG-IgG-Associated Disorders. JAMA Neurol. 2018;75(11):1355–63. https://doi.org/10.1001/jamaneurol.2018.1814 .
Baumann M, Bartels F, Finke C, Adamsbaum C, Hacohen Y, Rostásy K, et al. E.U. paediatric MOG consortium consensus: Part 2 – Neuroimaging features of paediatric myelin oligodendrocyte glycoprotein antibody-associated disorders. Eur J Paediatr Neurol. 2020;29:14–21. https://doi.org/10.1016/j.ejpn.2020.10.002 .
Skarsta L, Nicoletti T, Frick K, Kana V, De Vere-Tyndall A, Weller M, et al. Acute haemorrhagic leucoencephalitis as clinical manifestation of MOG antibody-associated disease. J Neurol Neurosurg Psychiatry. 2023;94(7):583–5. https://doi.org/10.1136/jnnp-2023-331350 .
Hacohen Y, Rossor T, Mankad K, Chong WKK, Lux A, Wassmer E, et al. “Leukodystrophy-like” phenotype in children with myelin oligodendrocyte glycoprotein antibody-associated disease. Dev Med Child Neurol. 2018;60(4):417–23. https://doi.org/10.1111/dmcn.13649 .
Gibbons E, Whittam D, Elhadd K, Bhojak M, Rathi N, Avula S, et al. Progressive myelin oligodendrocyte glycoprotein-associated demyelination mimicking leukodystrophy. Mult Scler. 2022;28(9):1481–4. https://doi.org/10.1177/13524585221090737 .
Cortese R, Prados Carrasco F, Tur C, Bianchi A, Brownlee W, De Angelis F, et al. Differentiating Multiple Sclerosis From AQP4-Neuromyelitis Optica Spectrum Disorder and MOG-Antibody Disease With Imaging. Neurology. 2023;100(3):e308–23. https://doi.org/10.1212/WNL.0000000000201465 .
Banks SA, Morris PP, Chen JJ, Pittock SJ, Sechi E, Kunchok A, et al. Brainstem and cerebellar involvement in MOG-IgG-associated disorder versus aquaporin-4-IgG and MS. J Neurol Neurosurg Psychiatry. 2020;2020–325121. https://doi.org/10.1136/jnnp-2020-325121
• Valencia-Sanchez C, Guo Y, Krecke KN, Chen JJ, Redenbaugh V, Montalvo M, et al. Cerebral Cortical Encephalitis in Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease. Ann Neurol. 2023;93(2):297–302. https://doi.org/10.1002/ana.26549 . ( This recent case series summarizes the variable characteristics of a rare presentation of MOGAD, cerebral cortical encephalitis. )
Montalvo M, Khattak JF, Redenbaugh V, Britton J, Valencia Sanchez C, Datta A, et al. Acute symptomatic seizures secondary to myelin oligodendrocyte glycoprotein antibody-associated disease. Epilepsia. 2022;63(12):3180–91. https://doi.org/10.1111/epi.17424 .
Jain K, Cherian A, Thomas B. FLAMES: A novel burning entity in MOG IgG associated disease. Mult Scler Relat Disord. 2021;49:102759. https://doi.org/10.1016/j.msard.2021.102759 .
Budhram A, Mirian A, Le C, Hosseini-Moghaddam SM, Sharma M, Nicolle MW. Unilateral cortical FLAIR-hyperintense Lesions in Anti-MOG-associated Encephalitis with Seizures (FLAMES): characterization of a distinct clinico-radiographic syndrome. J Neurol. 2019;266(10):2481–7. https://doi.org/10.1007/s00415-019-09440-8 .
Ogawa R, Nakashima I, Takahashi T, Kaneko K, Akaishi T, Takai Y, et al. MOG antibody-positive, benign, unilateral, cerebral cortical encephalitis with epilepsy. Neurol Neuroimmunol Neuroinflamm. 2017;4(2):e322. https://doi.org/10.1212/NXI.0000000000000322 .
Cacciaguerra L, Chen JJ, Flanagan EP. A Case of Recurrent Cerebral Cortical Encephalitis in MOG Antibody-Associated Disease. Neurology. 2022;99(23_Supplement_2):S19–20. https://doi.org/10.1212/01.wnl.0000903180.06286.ba .
Cobo-Calvo A, Ayrignac X, Kerschen P, Horellou P, Cotton F, Labauge P, et al. Cranial nerve involvement in patients with MOG antibody-associated disease. Neurol Neuroimmunol Neuroinflamm. 2019;6(2):e543. https://doi.org/10.1212/NXI.0000000000000543 .
Gupta P, Paul P, Redenbaugh V, Guo Y, Lucchinetti C, Abdulrahman Y, et al. Peripheral nervous system manifestations of MOG antibody associated disease. Ann Clin Transl Neurol. 2024. https://doi.org/10.1002/acn3.52001 .
Vazquez Do Campo R, Stephens A, Marin Collazo IV, Rubin DI. MOG antibodies in combined central and peripheral demyelination syndromes. Neurol Neuroimmunol Neuroinflamm. 2018;5(6):e503. https://doi.org/10.1212/NXI.0000000000000503 .
Rinaldi S, Davies A, Fehmi J, Beadnall HN, Wang J, Hardy TA, et al. Overlapping central and peripheral nervous system syndromes in MOG antibody-associated disorders. Neurol Neuroimmunol Neuroinflamm. 2020;8(1):e924. https://doi.org/10.1212/NXI.0000000000000924 .
Bosisio L, Gastaldi M, Inglese M, Rossi A, Franciotta D, Cataldi M, et al. Asynchronous combined central and peripheral demyelination (CCPD) in a girl with anti-MOG positivity: A case report and review of the literature. J Neuroimmunol. 2023;15(384):578213. https://doi.org/10.1016/j.jneuroim.2023.578213 .
Symmonds M, Waters PJ, Küker W, Leite MI, Schulz UG. Anti-MOG antibodies with longitudinally extensive transverse myelitis preceded by CLIPPERS. Neurology. 2015;84(11):1177–9. https://doi.org/10.1212/WNL.0000000000001370 .
Obeidat AZ, Block AN, Hooshmand SI. “Peppering the pons”: CLIPPERS or myelin oligodendrocyte glycoprotein associated disease? Mult Scler Relat Disord. 2021;51:102874. https://doi.org/10.1016/j.msard.2021.102874 .
Ferilli MAN, Papi C, Sabatelli M, Colosimo C, Iorio R. MOG autoimmunity mimicking CLIPPERS syndrome: Case report and literature review. J Neuroimmunol. 2022;15(367):577875. https://doi.org/10.1016/j.jneuroim.2022.577875 .
Cacciaguerra L, Morris P, Tobin WO, Chen JJ, Banks SA, Elsbernd P, et al. Tumefactive Demyelination in MOG Ab-Associated Disease, Multiple Sclerosis, and AQP-4-IgG-Positive Neuromyelitis Optica Spectrum Disorder. Neurology. 2023;100(13):e1418–32. https://doi.org/10.1212/WNL.0000000000206820 .
Sechi E, Krecke KN, Messina SA, Buciuc M, Pittock SJ, Chen JJ, et al. Comparison of MRI Lesion Evolution in Different Central Nervous System Demyelinating Disorders. Neurology. 2021;97(11):e1097–109. https://doi.org/10.1212/WNL.0000000000012467 .
Brier MR, Xiang B, Ciotti JR, Chahin S, Wu GF, Naismith RT, et al. Quantitative MRI identifies lesional and non-lesional abnormalities in MOGAD. Mult Scler Relat Disord. 2023;73:104659. https://doi.org/10.1016/j.msard.2023.104659 .
Elsbernd P, Cacciaguerra L, Krecke KN, Chen JJ, Gritsch D, Lopez-Chiriboga AS, et al. Cerebral enhancement in MOG antibody-associated disease. J Neurol Neurosurg Psychiatry. 2023;95(1):14–8.
Goldman-Yassen A, Lee A, Gombolay G. Leptomeningeal Enhancement in Pediatric Anti-Myelin Oligodendrocyte Glycoprotein Antibody Disease, Multiple Sclerosis, and Neuromyelitis Optica Spectrum Disorder. Pediatr Neurol. 2024;153:125–30. https://doi.org/10.1016/j.pediatrneurol.2024.01.026 .
Reindl M, Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol. 2019;15(2):89–102. https://doi.org/10.1038/s41582-018-0112-x .
• Reindl M, Schanda K, Woodhall M, Tea F, Ramanathan S, Sagen J, et al. International multicenter examination of MOG antibody assays. Neurol Neuroimmunol Neuroinflamm. 2020;7(2):e674. https://doi.org/10.1212/NXI.0000000000000674 . ( This study confirms agreement of MOG antibody cell-based assays amongst different institutions, an important step in standardizing the diagnostic evaluation for MOGAD. )
Marchionatti A, Woodhall M, Waters PJ, Sato DK. Detection of MOG-IgG by cell-based assay: moving from discovery to clinical practice. Neurol Sci. 2021;42(1):73–80. https://doi.org/10.1007/s10072-020-04828-1 .
Smith TL, Haven TR, Zuromski LM, Luong K, Clardy SL, Peterson LK. High level of agreement in a fixed vs. live cell-based assay for antibodies to myelin oligodendrocyte glycoprotein in a real-world clinical laboratory setting. Front Neurol. 2023;14:1192644. https://doi.org/10.3389/fneur.2023.1192644 .
Waters PJ, Komorowski L, Woodhall M, Lederer S, Majed M, Fryer J, et al. A multicenter comparison of MOG-IgG cell-based assays. Neurology. 2019;92(11):e1250–5. https://doi.org/10.1212/WNL.0000000000007096 .
• Sechi E, Buciuc M, Pittock SJ, Chen JJ, Fryer JP, Jenkins SM, et al. Positive Predictive Value of Myelin Oligodendrocyte Glycoprotein Autoantibody Testing. JAMA Neurol. 2021;78(6):741–6. https://doi.org/10.1001/jamaneurol.2021.0912 . ( This large investigation demonstrates the limited positive predictive value of low MOG antibody titers. )
Zara P, Floris V, Flanagan EP, Lopez-Chiriboga AS, Weinshenker BG, Solla P, et al. Clinical Significance of Myelin Oligodendrocyte Glycoprotein Autoantibodies in Patients with Typical MS Lesions on MRI. Mult Scler J Exp Transl Clin. 2021;7(4):20552173211048760. https://doi.org/10.1177/20552173211048761 .
Held F, Kalluri SR, Berthele A, Klein AK, Reindl M, Hemmer B. Frequency of myelin oligodendrocyte glycoprotein antibodies in a large cohort of neurological patients. Mult Scler J Exp Transl Clin. 2021;7(2):20552173211022770. https://doi.org/10.1177/20552173211022767 .
Hyun JW, Woodhall MR, Kim SH, Jeong IH, Kong B, Kim G, et al. Longitudinal analysis of myelin oligodendrocyte glycoprotein antibodies in CNS inflammatory diseases. J Neurol Neurosurg Psychiatry. 2017;88(10):811–7. https://doi.org/10.1136/jnnp-2017-315998 .
Matsumoto Y, Kaneko K, Takahashi T, Takai Y, Namatame C, Kuroda H, et al. Diagnostic implications of MOG-IgG detection in sera and cerebrospinal fluids. Brain. 2023;146(9):3938–48. https://doi.org/10.1093/brain/awad122 .
Kwon YN, Kim B, Kim JS, Mo H, Choi K, Oh SI, et al. Myelin Oligodendrocyte Glycoprotein-Immunoglobulin G in the CSF: Clinical Implication of Testing and Association With Disability. Neurol Neuroimmunol Neuroinflamm. 2021;9(1):e1095. https://doi.org/10.1212/NXI.0000000000001095 .
Carta S, Cobo-Calvo A, Armangué T, Saiz A, Lechner C, Rostásy K, et al. Significance of Myelin Oligodendrocyte Glycoprotein Antibodies in CSF: A Retrospective Multicenter Study. Neurology. 2023;100(11):e1095–108. https://doi.org/10.1212/WNL.0000000000201662 .
Kim KH, Kim SH, Park NY, Hyun JW, Kim HJ. Validation of the International MOGAD Panel proposed criteria. Mult Scler. 2023;29(13):1680–3. https://doi.org/10.1177/13524585231198754 .
Alaboudi M, Morgan M, Serra A, Abboud H. Utility of the 2023 international MOGAD panel proposed criteria in clinical practice: An institutional cohort. Mult Scler Relat Disord. 2024;81:105150. https://doi.org/10.1016/j.msard.2023.105150 .
Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177–89. https://doi.org/10.1212/WNL.0000000000001729 .
Stern BJ, Royal W 3rd, Gelfand JM, Clifford DB, Tavee J, Pawate S, et al. Definition and Consensus Diagnostic Criteria for Neurosarcoidosis: From the Neurosarcoidosis Consortium Consensus Group. JAMA Neurol. 2018;75(12):1546–53. https://doi.org/10.1001/jamaneurol.2018.2295 .
Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391–404. https://doi.org/10.1016/S1474-4422(15)00401-9 .
Whittam DH, Karthikeayan V, Gibbons E, Kneen R, Chandratre S, Ciccarelli O, et al. Treatment of MOG antibody associated disorders: results of an international survey. J Neurol. 2020;267(12):3565–77. https://doi.org/10.1007/s00415-020-10026-y .
Marignier R, Hacohen Y, Cobo-Calvo A, Pröbstel AK, Aktas O, Alexopoulos H, et al. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. 2021;20(9):762–72. https://doi.org/10.1016/S1474-4422(21)00218-0 .
Stiebel-Kalish H, Hellmann MA, Mimouni M, Paul F, Bialer O, Bach M, et al. Does time equal vision in the acute treatment of a cohort of AQP4 and MOG optic neuritis? Neurol Neuroimmunol Neuroinflamm. 2019;6(4):e572. https://doi.org/10.1212/NXI.0000000000000572 .
Chen JJ, Flanagan EP, Bhatti MT, Tisavipat N, Jamali S, Kunchok A, et al. Details and outcomes of a large cohort of MOG-IgG associated optic neuritis. Mult Scler Relat Disord. 2022;68:104237. https://doi.org/10.1016/j.msard.2022.104237 .
Nosadini M, Eyre M, Giacomini T, Valeriani M, Della Corte M, Praticò AD, et al. Early Immunotherapy and Longer Corticosteroid Treatment Are Associated With Lower Risk of Relapsing Disease Course in Pediatric MOGAD. Neurol Neuroimmunol Neuroinflamm. 2022;10(1):e200065. https://doi.org/10.1212/NXI.0000000000200065 .
Weinshenker BG, O’Brien PC, Petterson TM, Noseworthy JH, Lucchinetti CF, Dodick DW, et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol. 1999;46(6):878–86. https://doi.org/10.1002/1531-8249(199912)46:6%3c878::aid-ana10%3e3.0.co;2-q .
Fu J, Wang Y, Li H, Zhou H, Song H, Sun M, et al. Efficacy of Plasma Exchange Treatment for Demyelinating Optic Neuritis Associated with Various Serum Antibodies: A Prospective Cohort Study. Neurol Ther. 2022;11(2):797–813. https://doi.org/10.1007/s40120-022-00344-w .
• Duchow A, Bellmann-Strobl J, Friede T, Aktas O, Angstwurm K, Ayzenberg I, et al. Time to Disability Milestones and Annualized Relapse Rates in NMOSD and MOGAD. Ann Neurol. 2023. https://doi.org/10.1002/ana.26858 . ( This registry study highlights the less severe clinical course and increased incidence of monophasic disease in MOGAD as compared to NMOSD. )
Cobo-Calvo A, Ruiz A, Maillart E, Audoin B, Zephir H, Bourre B, et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: The MOGADOR study. Neurology. 2018;90(21):e1858–69. https://doi.org/10.1212/WNL.0000000000005560 .
Akaishi T, Misu T, Fujihara K, Takahashi T, Takai Y, Nishiyama S, et al. Relapse activity in the chronic phase of anti-myelin-oligodendrocyte glycoprotein antibody-associated disease. J Neurol. 2022;269(6):3136–46. https://doi.org/10.1007/s00415-021-10914-x .
Wendel EM, Thonke HS, Bertolini A, Baumann M, Blaschek A, Merkenschlager A, et al. Temporal Dynamics of MOG Antibodies in Children With Acquired Demyelinating Syndrome. Neurol Neuroimmunol Neuroinflamm. 2022;9(6):e200035. https://doi.org/10.1212/NXI.0000000000200035 .
Gastaldi M, Foiadelli T, Greco G, Scaranzin S, Rigoni E, Masciocchi S, et al. Prognostic relevance of quantitative and longitudinal MOG antibody testing in patients with MOGAD: a multicentre retrospective study. J Neurol Neurosurg Psychiatry. 2023;94(3):201–10. https://doi.org/10.1136/jnnp-2022-330237 .
• Chen JJ, Flanagan EP, Bhatti MT, Jitprapaikulsan J, Dubey D, Lopez-Chiriboga AS, et al. Steroid-sparing maintenance immunotherapy for MOG-IgG associated disorder. Neurology. 2020;95(2):e111–20. https://doi.org/10.1212/WNL.0000000000009758 . ( This multicenter retrospective study offers some of the earliest comparative evidence between chronic immunomodulatory/immunosuppressive management options in MOGAD. )
Lu Q, Luo J, Hao H, Liu R, Jin H, Jin Y, et al. Efficacy and safety of long-term immunotherapy in adult patients with MOG antibody disease: a systematic analysis. J Neurol. 2021;268(12):4537–48. https://doi.org/10.1007/s00415-020-10236-4 .
Cobo-Calvo A, Sepúlveda M, Rollot F, Armangué T, Ruiz A, Maillart E, et al. Evaluation of treatment response in adults with relapsing MOG-Ab-associated disease. J Neuroinflammation. 2019;16(1):134. https://doi.org/10.1186/s12974-019-1525-1 .
Seneviratne SO, Marriott M, Ramanathan S, Yeh W, Brilot-Turville F, Butzkueven H, et al. Failure of alemtuzumab therapy in three patients with MOG antibody associated disease. BMC Neurol. 2022;22(1):84. https://doi.org/10.1186/s12883-022-02612-6 .
Durozard P, Rico A, Boutiere C, Maarouf A, Lacroix R, Cointe S, et al. Comparison of the Response to Rituximab between Myelin Oligodendrocyte Glycoprotein and Aquaporin-4 Antibody Diseases. Ann Neurol. 2020;87(2):256–66. https://doi.org/10.1002/ana.25648 .
Barreras P, Vasileiou ES, Filippatou AG, Fitzgerald KC, Levy M, Pardo CA, et al. Long-term Effectiveness and Safety of Rituximab in Neuromyelitis Optica Spectrum Disorder and MOG Antibody Disease. Neurology. 2022;99(22):e2504–16. https://doi.org/10.1212/WNL.0000000000201260 .
Spagni G, Sun B, Monte G, Sechi E, Iorio R, Evoli A, et al. Efficacy and safety of rituximab in myelin oligodendrocyte glycoprotein antibody-associated disorders compared with neuromyelitis optica spectrum disorder: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2023;94(1):62–9. https://doi.org/10.1136/jnnp-2022-330086 .
Al-Ani A, Chen JJ, Costello F. Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD): current understanding and challenges. J Neurol. 2023;270(8):4132–50. https://doi.org/10.1007/s00415-023-11737-8 .
Bilodeau PA, Vishnevetsky A, Molazadeh N, Lotan I, Anderson M, Romanow G, et al. Effectiveness of immunotherapies in relapsing myelin oligodendrocyte glycoprotein antibody-associated disease. Mult Scler. 2024;30(3):357–68. https://doi.org/10.1177/13524585241226830 .
Chen JJ, Huda S, Hacohen Y, Levy M, Lotan I, Wilf-Yarkoni A, et al. Association of Maintenance Intravenous Immunoglobulin With Prevention of Relapse in Adult Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease. JAMA Neurol. 2022;79(5):518–25. https://doi.org/10.1001/jamaneurol.2022.0489 .
Sotirchos ES, Vasileiou ES, Salky R, Huda S, Mariotto S, Chen JJ, et al. Treatment of myelin oligodendrocyte glycoprotein antibody associated disease with subcutaneous immune globulin. Mult Scler Relat Disord. 2022;57:103462. https://doi.org/10.1016/j.msard.2021.103462 .
Elsbernd PM, Hoffman WR, Carter JL, Wingerchuk DM. Interleukin-6 inhibition with tocilizumab for relapsing MOG-IgG associated disorder (MOGAD): A case-series and review. Mult Scler Relat Disord. 2021;48:102696. https://doi.org/10.1016/j.msard.2020.102696 .
Ringelstein M, Ayzenberg I, Lindenblatt G, Fischer K, Gahlen A, Novi G, et al. Interleukin-6 Receptor Blockade in Treatment-Refractory MOG-IgG-Associated Disease and Neuromyelitis Optica Spectrum Disorders. Neurol Neuroimmunol Neuroinflamm. 2021;9(1):e1100. https://doi.org/10.1212/NXI.0000000000001100 .
Bril V, Drużdż A, Grosskreutz J, Habib AA, Mantegazza R, Sacconi S, et al. Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. 2023;22(5):383–94. https://doi.org/10.1016/S1474-4422(23)00077-7 .
Download references
Author information
Authors and affiliations.
Department of Neurology, University of South Florida, 13330 USF Laurel Drive, Tampa, FL, 33612, USA
Kelsey A. Stefan & John R. Ciotti
You can also search for this author in PubMed Google Scholar
Contributions
K.A.S. wrote the first draft of the manuscript. J.R.C. provided supervision and edited the manuscript. J.R.C. prepared Figure 1 and Table 1. All authors read and approved the final manuscript.
Corresponding author
Correspondence to John R. Ciotti .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethical Approval
Written informed consent for publication of their clinical details and/or clinical images was obtained from the patient/parent/guardian/relative of the patient. A copy of the consent form is available for review by the Editor of this journal.
Conflict of Interest
Kelsey A. Stefan declares that she has no conflict of interest. John R. Ciotti has received grant/research support from Biogen and the National MS Society, and has participated in advisory boards for EMD Serono, Genentech, Janssen, and Novartis.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Stefan, K.A., Ciotti, J.R. MOG Antibody Disease: Nuances in Presentation, Diagnosis, and Management. Curr Neurol Neurosci Rep (2024). https://doi.org/10.1007/s11910-024-01344-z
Download citation
Accepted : 16 May 2024
Published : 28 May 2024
DOI : https://doi.org/10.1007/s11910-024-01344-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- MOG Antibody Disease
- Optic Neuritis
- Transverse Myelitis
- Acute Disseminated Encephalomyelitis
- Cerebral Cortical Encephalitis
- Diagnostic Criteria
Advertisement
- Find a journal
- Publish with us
- Track your research
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Outbreak of Human Trichinellosis — Arizona, Minnesota, and South Dakota, 2022
Weekly / May 23, 2024 / 73(20);456–459
Shama Cash-Goldwasser, MD 1 ; Dustin Ortbahn, MPH 2 ; Muthu Narayan, DO 3 ; Conor Fitzgerald, MPH 4 ; Keila Maldonado 5 ; James Currie, MD 6 ; Anne Straily, DVM 7 ; Sarah Sapp, PhD 7 ; Henry S. Bishop 7 ; Billy Watson, PhD 7 ; Margaret Neja 7 ; Yvonne Qvarnstrom, PhD 7 ; David M. Berman, DO 8 ; Sarah Y. Park, MD 8 ; Kirk Smith, DVM, PhD 9 ; Stacy Holzbauer, DVM 9 ,10 ( View author affiliations )
What is already known about this topic?
Human trichinellosis cases in the United States are rare and are usually acquired through consumption of wild game.
What is added by this report?
Among eight persons who shared a meal that included the meat of a black bear harvested in Canada and frozen for 45 days, six trichinellosis cases were identified. The meat was grilled with vegetables and served rare; two cases occurred in persons who ate only the vegetables. Motile freeze-resistant Trichinella nativa larvae were identified in remaining meat frozen for >15 weeks.
What are the implications for public health practice?
Cooking meat to an internal temperature of ≥165°F (≥74°C) is necessary to kill Trichinella spp. parasites. Trichinella -infected meat can cross-contaminate other foods, and raw meat should be kept and prepared separate from other foods to prevent cross-contamination.
- Article PDF
- Full Issue PDF
Trichinellosis is a parasitic zoonotic disease transmitted through the consumption of meat from animals infected with Trichinella spp. nematodes. In North America, human trichinellosis is rare and is most commonly acquired through consumption of wild game meat. In July 2022, a hospitalized patient with suspected trichinellosis was reported to the Minnesota Department of Health. One week before symptom onset, the patient and eight other persons shared a meal that included bear meat that had been frozen for 45 days before being grilled and served rare with vegetables that had been cooked with the meat. Investigation identified six trichinellosis cases, including two in persons who consumed only the vegetables. Motile Trichinella larvae were found in remaining bear meat that had been frozen for >15 weeks. Molecular testing identified larvae from the bear meat as Trichinella nativa , a freeze-resistant species. Persons who consume meat from wild game animals should be aware that that adequate cooking is the only reliable way to kill Trichinella parasites and that infected meat can cross-contaminate other foods.
Investigation and Results
Index patient notification.
In July 2022, the Minnesota Department of Health was notified of a man aged 29 years who was hospitalized with fever, severe myalgias, periorbital edema, eosinophilia, and other laboratory abnormalities ( Table ); health care providers suspected trichinellosis. The patient had sought care for his symptoms, which commenced in early July, four times and had been hospitalized twice over a 17-day period. During his second hospitalization, providers obtained a history of bear meat consumption, and empiric albendazole treatment for probable trichinellosis was initiated. An investigation was launched to confirm the diagnosis, identify additional cases, and ascertain the source of infection to prevent future cases. The index patient’s diagnosis was confirmed by a positive Trichinella immunoglobulin (Ig) G antibody test result.
Potential Exposure Source Identification
Six days before symptom onset in the index patient, he and eight extended family members from three states (Arizona, Minnesota, and South Dakota) had gathered for several days in South Dakota and shared a meal that included kabobs made from the meat of a black bear ( Ursus americanus ), which had been harvested by one of the family members in northern Saskatchewan, Canada in May 2022. The hunting outfitter had recommended freezing the meat to kill parasites. The meat was frozen in a household freezer* for 45 days until being thawed and grilled with vegetables. The meat was initially inadvertently served rare, reportedly because the meat was dark in color, and it was difficult for the family members to visually ascertain the level of doneness. After some of the family members began eating the meat and noticed that it was undercooked, the meat was recooked before being served again. The family reunion concluded before onset of illness in the index patient.
Laboratory Investigation and Case Definition
Public health authorities in Arizona, Minnesota, and South Dakota interviewed eight of the nine persons who had attended the implicated meal. The ninth attendee was a person aged <18 years whose exposure status could not be confirmed; however, that person reportedly remained healthy. Testing of paired acute and convalescent sera for Trichinella IgG antibodies was recommended for the eight exposed persons and was completed for six. Pathogen-agnostic microbial cell-free metagenomic DNA sequencing ( 1 ) was performed on plasma samples from the index patient and one other person who had sought care twice before being hospitalized with fever, myalgias, abdominal pain, periorbital edema, and laboratory abnormalities. Trichinellosis cases were classified according to the 2014 case definition from the Council for State and Territorial Epidemiologists (CSTE), † (i.e., the presence of clinically compatible symptoms in a person who had consumed an epidemiologically implicated meal or meat in which the parasite was demonstrated [probable] or had a positive serologic test result for Trichinella antibodies [confirmed]). Samples of frozen bear meat were obtained from the household freezer and sent to CDC for artificial tissue digestion and microscopic examination for larvae and molecular testing for Trichinella spp.
Additional Case Detection and Exposure Source Confirmation
Among the eight interviewed persons, five consumed the bear meat, and eight consumed the vegetables that had been cooked with it. Six of the eight persons who attended the meal, including four who consumed the bear meat and the vegetables, and two who consumed only the vegetables (but no meat), had symptoms consistent with trichinellosis, and met case criteria (two confirmed and four probable). Patients with trichinellosis ranged in age from 12 to 62 years and lived in three states: Arizona (one), Minnesota (four), and South Dakota (one). All cases were diagnosed in the patients’ state of residence. Three of the six symptomatic persons, two of whom sought care at least twice before being offered treatment, were hospitalized. The three hospitalized persons received trichinellosis-directed treatment with albendazole. § All six symptomatic persons recovered; the nonhospitalized patients did not receive trichinellosis-directed treatment because their symptoms had resolved with supportive care only, and the benefit of treatment after larval invasion of muscle is unclear ( 2 ). Six persons submitted a serum sample, each collected within 4 weeks of symptom onset; two specimens tested positive for Trichinella IgG antibodies by enzyme-linked immunosorbent assay. Two persons submitted a plasma sample for microbial cell-free DNA sequencing during hospitalization for trichinellosis-compatible symptoms, and both plasma samples tested positive for Trichinella spp. DNA. Microscopy identified motile Trichinella larvae (>800 larvae/g) in samples of bear meat that had been frozen for 110 days in a household freezer ( Figure ). Real-time multiplex polymerase chain reaction testing ( 3 ) of the bear meat was positive for T. nativa and whole genome sequencing identified mitochondrial sequences 100% identical to T. nativa.
Public Health Response
The family member who harvested the bear and provided meat samples for testing was advised to discard any remaining meat. All identified trichinellosis cases were reported to appropriate state health departments and to CDC. CDC notified the Public Health Agency of Canada of the outbreak and the confirmed source of infection. This activity was reviewed by CDC, deemed not research, and was conducted consistent with applicable federal law and CDC policy. ¶
Trichinellosis is rarely reported in the United States. As a result of changes in pork production practices from historical norms that fostered transmission, most cases reported in recent years are attributed to consumption of meat from wild game ( 4 ). During January 2016–December 2022, seven U.S. trichinellosis outbreaks, including 35 probable and confirmed cases, were reported to CDC; bear meat was the suspected or confirmed source of infection in the majority of those outbreaks (CDC, unpublished data, 2022). Estimates of Trichinella infection prevalence among wild animal host species vary widely. A Trichinella infection prevalence range of at least 1% to 24% among black bears in Canada and Alaska has been reported, and even higher prevalences of Trichinella infection are reported among species of predators that are strict carnivores (e.g., polar bear, wolverine, and cougar) ( 5 ). The frequency with which black bear meat is the implicated source of human infection might be driven by hunting practices, ecological factors, and the relatively high parasite density observed in the muscle of infected black bears compared with that of other species ( 6 , 7 ).
Because symptoms of trichinellosis are typically nonspecific, diagnosis of infection requires a high index of suspicion; however, periorbital edema and certain laboratory abnormalities (e.g., eosinophilia and elevated creatine kinase levels) can provide etiologic clues. In this outbreak, two of the hospitalized patients sought care multiple times before receiving a diagnosis. Four of the six patients met clinical and epidemiologic criteria and thus were considered probable cases. Laboratory confirmation can be challenging because of the limited sensitivity of antibody testing early in illness ( 8 ); in this investigation, acute Trichinella IgG test results were positive in only two of six tested patient specimens. The clinical utility of trichinellosis test results obtained after acute illness is limited, and historically, public health investigators have had difficulty obtaining convalescent serum samples from persons who have recovered. Laboratory criteria in the current CSTE trichinellosis case definition do not include nucleic acid testing of human specimens. The sensitivity of such assays to detect Trichinella DNA in blood is uncharacterized; however, plasma samples from both patients tested by metagenomic sequencing ( 1 ) yielded positive results for Trichinella DNA. As demonstrated in this outbreak, pathogen-agnostic molecular assays can be useful for detection of rare diseases when standard workup is unrevealing and if other diagnostic tests lack sensitivity.
Implications for Public Health Practice
Although freezing kills Trichinella species commonly implicated in pork-associated outbreaks, freeze-resistant Trichinella species, including T. nativa and the T6 genotype ( 9 ), predominate in Arctic and sub-Arctic regions ( 6 ). Larval motility was observed in bear meat that had been frozen for nearly 4 months (110 days). Persons who consume game meat, especially that harvested in northern latitudes, should be informed that adequate cooking is the only reliable way to kill Trichinella parasites. Cooking wild game meat to an internal temperature of ≥165°F (≥74°C) is recommended by public health authorities**; temperatures should be verified with a meat thermometer. As demonstrated in this outbreak, the color of meat is not a good indicator of cooking adequacy. Safe handling of raw meat (i.e., separating raw or undercooked meat and its juices from other foods) is recommended to prevent trichinellosis; this investigation and previous investigations suggest that Trichinella -infected meat can cross-contaminate other foods ( 10 ). Government and private entities that oversee and organize hunting should educate hunters about these risks and effective preventative measures.
Acknowledgments
The persons affected by this outbreak; Lauren Ahart, Sue Montgomery, Parasitic Diseases Branch, CDC.
Corresponding author: Shama Cash-Goldwasser, [email protected] .
1 Epidemic Intelligence Service, CDC; 2 South Dakota Department of Health; 3 University of Minnesota, Minneapolis, Minnesota; 4 Arizona Department of Health Services; 5 Maricopa County Department of Public Health, Phoenix, Arizona; 6 Lakeview Clinic, Waconia, Minnesota; 7 Division of Parasitic Diseases and Malaria, Center for Global Health, CDC; 8 Medical Affairs, Karius, Inc., Redwood City, California; 9 Minnesota Department of Health; 10 Division of State and Local Readiness, Center for Preparedness and Response, CDC.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. David M. Berman reports that he is a paid laboratory medical consultant for Precision Health Solutions and reports ownership of company shares in Karius, Inc. No other potential conflicts of interest were disclosed.
* The temperature of the freezer is not known.
† https://ndc.services.cdc.gov/case-definitions/trichinellosis-2014/
§ https://www.cdc.gov/trichinellosis/hcp/clinical-care/index.html
¶ 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.
** https://www.cdc.gov/trichinellosis/prevention/index.html
- Blauwkamp TA, Thair S, Rosen MJ, et al. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat Microbiol 2019;4:663–74. https://doi.org/10.1038/s41564-018-0349-6 PMID:30742071
- Pozio E, Sacchini D, Sacchi L, Tamburrini A, Alberici F. Failure of mebendazole in the treatment of humans with Trichinella spiralis infection at the stage of encapsulating larvae. Clin Infect Dis 2001;32:638–42. https://doi.org/10.1086/318707 PMID:11181129
- Almeida M, Bishop H, Nascimento FS, Mathison B, Bradbury RS, Silva AD. Multiplex TaqMan qPCR assay for specific identification of encapsulated Trichinella species prevalent in North America. Mem Inst Oswaldo Cruz 2018;113:e180305. https://doi.org/10.1590/0074-02760180305 PMID:30379199
- Wilson NO, Hall RL, Montgomery SP, Jones JL. Trichinellosis surveillance—United States, 2008-2012. MMWR Surveill Summ 2015;64(No. SS-1):1–8. PMID:25590865
- Oksanen A, Kärssin A, Berg RPKD, et al. Epidemiology of Trichinella in the Arctic and subarctic: a review. Food Waterborne Parasitol 2022;28:e00167. https://doi.org/10.1016/j.fawpar.2022.e00167 PMID:35812081
- Gajadhar AA, Forbes LB. A 10-year wildlife survey of 15 species of Canadian carnivores identifies new hosts or geographic locations for Trichinella genotypes T2, T4, T5, and T6. Vet Parasitol 2010;168:78–83. https://doi.org/10.1016/j.vetpar.2009.10.012 PMID:19926223
- Harms NJ, Larivee M, Scandrett B, Russell D. High prevalence and intensity of Trichinella infection in Yukon American Black ( Ursus americanus ) and Grizzly ( Ursus arctos ) bears. J Wildl Dis 2021;57:429–33. https://doi.org/10.7589/JWD-D-20-00135 PMID:33822166
- Yang Y, Cai YN, Tong MW, et al. Serological tools for detection of Trichinella infection in animals and humans. One Health 2016;2:25–30. https://doi.org/10.1016/j.onehlt.2015.11.005 PMID:28616474
- Pozio E. Adaptation of Trichinella spp. for survival in cold climates. Food Waterborne Parasitol 2016;4:4–12. https://doi.org/10.1016/j.fawpar.2016.07.001
- Hall RL, Lindsay A, Hammond C, et al. Outbreak of human trichinellosis in Northern California caused by Trichinella murrelli . Am J Trop Med Hyg 2012;87:297–302. https://doi.org/10.4269/ajtmh.2012.12-0075 PMID:22855761
Abbreviations: eos = eosinophils; F = female; M = male; NA = not applicable; ND = not done; WBC = white blood cell. * Initial results are from hospitalization during which trichinellosis was suspected. Reference ranges varied among different laboratories that conducted testing. † Reference range = 4–88. § Reference range = 39–208. ¶ Consumed vegetables that were cooked and served with the bear meat. ** Reference range = 39–308.
FIGURE . Microscopic examination of encapsulated larvae in a direct black bear meat muscle squash prep (A), larvae liberated from artificially digested bear meat (B), and motile larvae viewed with differential interference contrast microscopy (C and D)* from black bear meat suspected as the source of an outbreak of human Trichinella nativa infections — Arizona, Minnesota, and South Dakota, 2022
Photos/Division of Parasitic Diseases and Malaria, Center for Global Health, CDC
* Scale bars = 100 µ m.
Suggested citation for this article: Cash-Goldwasser S, Ortbahn D, Narayan M, et al. Outbreak of Human Trichinellosis — Arizona, Minnesota, and South Dakota, 2022. MMWR Morb Mortal Wkly Rep 2024;73:456–459. DOI: http://dx.doi.org/10.15585/mmwr.mm7320a2 .
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services. Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services. References to non-CDC sites on the Internet are provided as a service to MMWR readers and do not constitute or imply endorsement of these organizations or their programs by CDC or the U.S. Department of Health and Human Services. CDC is not responsible for the content of pages found at these sites. URL addresses listed in MMWR were current as of the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version ( https://www.cdc.gov/mmwr ) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
- Share full article
Advertisement
Supported by
Alzheimer’s Takes a Financial Toll Long Before Diagnosis, Study Finds
New research shows that people who develop dementia often begin falling behind on bills years earlier.

By Ben Casselman
Long before people develop dementia, they often begin falling behind on mortgage payments, credit card bills and other financial obligations, new research shows.
A team of economists and medical experts at the Federal Reserve Bank of New York and Georgetown University combined Medicare records with data from Equifax, the credit bureau, to study how people’s borrowing behavior changed in the years before and after a diagnosis of Alzheimer’s or a similar disorder.
What they found was striking: Credit scores among people who later develop dementia begin falling sharply long before their disease is formally identified. A year before diagnosis, these people were 17.2 percent more likely to be delinquent on their mortgage payments than before the onset of the disease, and 34.3 percent more likely to be delinquent on their credit card bills. The issues start even earlier: The study finds evidence of people falling behind on their debts five years before diagnosis.
“The results are striking in both their clarity and their consistency,” said Carole Roan Gresenz, a Georgetown University economist who was one of the study’s authors. Credit scores and delinquencies, she said, “consistently worsen over time as diagnosis approaches, and so it literally mirrors the changes in cognitive decline that we’re observing.”
The research adds to a growing body of work documenting what many Alzheimer’s patients and their families already know: Decision-making, including on financial matters, can begin to deteriorate long before a diagnosis is made or even suspected. People who are starting to experience cognitive decline may miss payments, make impulsive purchases or put money into risky investments they would not have considered before the disease.
“There’s not just getting forgetful, but our risk tolerance changes,” said Lauren Hersch Nicholas, a professor at the University of Colorado School of Medicine who has studied dementia’s impact on people’s finances. “It might seem suddenly like a good move to move a diversified financial portfolio into some stock that someone recommended.”
People in the early stages of the disease are also vulnerable to scams and fraud, added Dr. Nicholas, who was not involved in the New York Fed research. In a paper published last year , she and several co-authors found that people likely to develop dementia saw their household wealth decline in the decade before diagnosis.
The problems are likely to only grow as the American population ages and more people develop dementia. The New York Fed study estimates that 600,000 delinquencies will occur over the next decade as a result of undiagnosed memory disorders.
That probably understates the impact, the researchers argue. Their data includes only issues that show up on credit reports, such as late payments, not the much broader array of financial impacts that the diseases can cause. Wilbert van der Klaauw, a New York Fed economist who is another of the study’s authors, said that after his mother was diagnosed with Alzheimer’s, his family discovered parking tickets and traffic violations that she had hidden.
“If anything, this is kind of an underestimate of the kind of financial difficulties people can experience,” he said.
Shortly before he was diagnosed with Alzheimer’s, Jay Reinstein bought a BMW he could not afford.
“I went into a showroom and I came home with a BMW,” he said. “My wife was not thrilled.”
At the time, Mr. Reinstein had recently retired as assistant city manager for Fayetteville, N.C. He had been noticing memory issues for years, but dismissed them as a result of his demanding job. Only after his diagnosis did he learn that friends and colleagues had also noticed the changes but had said nothing.
Mr. Reinstein, 63, is fortunate, he added. He has a government pension, and a wife who can keep an eye on his spending. But for those with fewer resources, financial decisions made in the years before diagnosis can have severe consequences, leaving them without money at the time when they will need it most. The authors of the New York Fed study noted that the financial effects they saw predated most of the costs associated with the disease, such as the need for long-term care.
The study expands on past research in part through its sheer scale: Researchers had access to health and financial data on nearly 2.5 million older Americans with chronic health conditions, roughly half a million of whom were diagnosed with Alzheimer’s or related disorders. (The records were anonymized, allowing researchers to combine the two sets of data without having access to identifying details on the individual patients.)
The large amount of data allowed researchers to slice the data more finely than in past studies, looking at the impact of race, sex, household size and other variables. Black people, for example, were more than twice as likely as white people to have financial problems before diagnosis, perhaps because they had fewer resources to begin with, and also because Black patients are often diagnosed later in the course of the disease.
The researchers hoped that the data could eventually allow them to develop a predictive algorithm that could flag people who might be suffering from impaired financial decision-making associated with Alzheimer’s disease — although they stressed that there were unresolved questions about who would have access to such information and how it would be used.
Until then, the researchers said, their findings should be a warning to older Americans and their families that they should prepare for the possibility of a Alzheimer’s diagnosis. That could mean taking steps such as granting a trusted person financial power of attorney, or simply paying attention to signs that someone might be behaving uncharacteristically.
Dr. Nicholas agreed.
“We should be thinking about the possibility of financial difficulties linked to a disease we don’t even know we have,” she said. “Knowing that, people should be on the lookout for these symptoms among friends and family members.”
Pam Belluck contributed reporting.
Tell us about your family's challenges with money management and Alzheimer's.
Ben Casselman writes about economics with a particular focus on stories involving data. He has covered the economy for nearly 20 years, and his recent work has focused on how trends in labor, politics, technology and demographics have shaped the way we live and work. More about Ben Casselman
A Guide to Making Better Financial Moves
Making sense of your finances can be complicated. the tips below can help..
Inheriting money after the death of a loved one while also grieving can be an emotional minefield, particularly for younger adults. Experts share ways to handle it wisely .
Either by choice or because they are priced out of the market, many people plan to never stop renting. Building wealth without home equity requires a different mind-set.
You may feel richer as you pay your mortgage down and home values go up. As a result, some homeowners end up with a lot of home equity but low retirement savings. Here’s the problem with that situation.
Can your investment portfolio reflect your values? If you want it to, it is becoming easier with each passing year .
The way advisers handle your retirement money is about to change: More investment professionals will be required to act in their customers’ best interest when providing advice about their retirement money.
The I.R.S. estimates that 940,000 people who didn’t file their tax returns in 2020 are due back money. The deadline for filing to get it is May 17.

- Kelvin Smith Library
- Guide to CWRU Common Reading 2024
- Research Guides
- The Kissing Bug: A True Story of a Family, an Insect, and a Nation's Neglect of a Deadly Disease by Daisy Hernandez
- Book Reviews & Study Guide
- Books, Interviews & Media
- Library Resources
Research Services Librarian

Get Online Help
- KSL Ask A Librarian Information on how to get help by email, phone, & chat.
Reminder: Online Access
- Library resources require going through CWRU Single Sign-On.
- The best method is to follow links from the library website.
- When logged in and a browser window is not closed, access should continue from resource to resource.
- Remember to close your browser when done.
- CWRU Libraries Discovery & Authentication by Brian Gray Last Updated Jan 28, 2022 167 views this year
Common Reading

From TinHouse Press: "Growing up in a New Jersey factory town in the 1980s, Daisy Hernández believed that her aunt had become deathly ill from eating an apple. No one in her family, in either the United States or Colombia, spoke of infectious diseases. Even into her thirties, she only knew that her aunt had died of Chagas, a rare and devastating illness that affects the heart and digestive system. But as Hernández dug deeper, she discovered that Chagas -- or the kissing bug disease -- is more prevalent in the United States than the Zika virus. "
About the Author

A journalist, she has reported for National Geographic , The Atlantic , The New York Times , and Slate , and her writing has been aired on NPR's All Things Considered . Her magazine feature on transgender issues in communities of color was nominated for a GLAAD Media Award.
She has received fellowships from MacDowell, Bread Loaf Writer’s Conference, the Rona Jaffe Foundation, Djerassi Artist-in-Residence, Blue Mountain Center, and Hedgebrook, and she currently serves on the board of the Barbara Deming Memorial Fund. She is an Associate Professor of Creative Writing at Northwestern University.
Image and bio c/o https://www.daisyhernandez.com/
- Daisy Hernandez's Website
CWRU Libraries Discovery
- Next: Book Reviews & Study Guide >>
- Last Updated: May 30, 2024 3:48 PM
- URL: https://researchguides.case.edu/commonreading2024
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Integr Care
- v.17(2); Apr-Jun 2017

Case Management for Patients with Complex Multimorbidity: Development and Validation of a Coordinated Intervention between Primary and Hospital Care
Salvador tortajada.
1 Instituto de Investigación Sanitaria La Fe, ES
María Soledad Giménez-Campos
2 Hospital Universitario y Politécnico La Fe, ES
Julia Villar-López
Raquel faubel-cava.
3 Universitat de València, ES
Lucas Donat-Castelló
Bernardo valdivieso-martínez, elisa soriano-melchor, amparo bahamontes-mulió, juan m. garcía-gómez.
4 Universidad Politécnica de Valencia, ES
In the past few years, healthcare systems have been facing a growing demand related to the high prevalence of chronic diseases. Case management programs have emerged as an integrated care approach for the management of chronic disease. Nevertheless, there is little scientific evidence on the impact of using a case management program for patients with complex multimorbidity regarding hospital resource utilisation.
We evaluated an integrated case management intervention set up by community-based care at outpatient clinics with nurse case managers from a telemedicine unit. The hypothesis to be tested was whether improved continuity of care resulting from the integration of community-based and hospital services reduced the use of hospital resources amongst patients with complex multimorbidity.
A retrospective cohort study was performed using a sample of 714 adult patients admitted to the program between January 2012 and January 2015. We found a significant decrease in the number of emergency room visits, unplanned hospitalizations, and length of stay, and an expected increase in the home care hospital-based episodes. These results support the hypothesis that case management interventions can reduce the use of unplanned hospital admissions when applied to patients with complex multimorbidity.
Introduction
In recent years, healthcare systems have been facing a growing demand related to the high prevalence of chronic diseases and long-term conditions. International strategies advocates for new ways to provide high-complexity and patient-centred services in order to meet this growing demand [ 1 , 2 ]. A radical transformation of the care process is required to promote proactive care focused on health and stability rather than reactive attention and treatment. International studies suggest this approach can be supported with the implementation of innovative chronic care models [ 3 , 4 ], based on personalized treatments, sustainable health services, closed-loop relationships, better quality of services, and evidence-based medicine [ 5 , 6 ].
In particular, case management (CM) programs have emerged as an approach to the management of chronic disease focused on improving individuals’ health and serving social needs [ 7 , 8 ]. Nevertheless, there is controversial scientific evidence available about the impact of using CM programs for chronic patients in terms of healthcare utilisation, clinical variables or health outcomes. The difficulties and barriers for the evaluation of these interventions reside in the variability of activities that are developed under the term “case management” and the heterogeneity of disease profiles on patients under study [ 9 ]. In addition, the majority of publications analyse CM interventions on single chronic conditions [ 10 , 11 , 12 ] or on an elderly population [ 13 , 14 ] while the predominant and increasing norm in the population is the confluence of various chronic conditions [ 15 ]. The latter condition is better known as complex multimorbidity.
Multimorbidity is a term that can be defined as the “co-occurrence of three or more chronic conditions affecting three or more different body systems within one person without defining an index chronic condition” [ 16 ]. People suffering from multiple chronic conditions suffer from poor quality of life, disability, psychological distress and an increased mortality risk [ 17 , 18 ]. In addition, the multiple needs associated to multimorbidity exceed the individual impact on the healthcare system. This strain on health status and the healthcare system involves a large number of resources, and therefore requires the development of strategies aimed at the organization of integrated care for people under this condition [ 19 ].
Recent reviews have focused on determining the impact of CM on the outcomes in patients with complex multimorbidity and health systems [ 20 , 21 ]. The evidence about the effectiveness of interventions orientated to these patients remains limited, and the results do not provide enough evidence on which interventions are related to better outcomes. This is especially controversial in cost savings, which is the focus of interest of this article. Nevertheless, the analysis suggested elements that could improve the effect of CM interventions and could be interesting to be implemented in clinical practice.
In the systematic review of Smith et al. [ 20 ] the most common organizational interventions were identified as CM and multidisciplinary teamwork using the taxonomy defined by the Cochrane Effective Practice and Organization of Care [ 22 ]. In addition, the most common patient-oriented interventions were educational or self-management support-type ones. Although the results related to the utilization of services were unclear, the conclusions underlined that there is emerging evidence and a need for further studies focusing on multimorbidity on community settings. Likewise, working with a multidisciplinary team or involving a social worker has a beneficial effect in patient satisfaction and improves the effectiveness of CM intervention [ 21 ].
Since 2010, an integrated care intervention has been developed in our institution for patients with complex multimorbidity. The intervention is based on a CM program where the primary health care team and nurse case managers are the first line of care responsible for the follow-up. The program under study includes patient-centred education activities. In addition, the integrated care intervention has the support of the Hospital at Home (HaH) unit, which is focused on avoiding hospital admissions when the management of exacerbations is possible in the community.
In that sense, the main objective of this study is to analyse the effect of an integrated CM program designed for patients with complex multimorbidity in terms of healthcare resources utilisation. We hypothesized that a scheduled follow-up program together with the HaH support on the management of patients with complex multimorbidity could reduce health resources utilisation including unplanned hospital admissions, length of stay (LoS), and visits to emergency room.
Design and setting
We conducted an observational, retrospective cohort study using matched observations before and after the CM intervention, also called pre-post intervention design or quasi-experimental design [ 23 ]. The units of observation were adult patients with complex multimorbidity admitted to the CM program between January 2012 and January 2015.
Population and inclusion/exclusion criteria
The sample under study was a cohort of chronic patients (N = 714) with complex multimorbidity. Patients were identified either during the hospital discharge process or in the community health care setting. In both cases, physicians in charge assessed predefined criteria for inclusion in the CM intervention. These criteria included adult patients with the presence of three or more chronic diseases with complex degrees of severity, including at least one of the following: heart failure, chronic renal failure, chronic pulmonary disease, neurological disease with moderate or severe permanent cognitive disability, and diabetes mellitus with target organ disease.
We excluded patients from the study when they were facing an end of life situation, and specific clinical conditions that required a different follow-up intervention: inflammatory bowel disease, amyotrophic lateral sclerosis, cystic fibrosis, hepatitis C, and/or mental health disease. In addition, we excluded patients under treatment with non-invasive ventilation devices and particular cases in which the adequacy of the real follow-up was not that established by the chronic CM program.
Description of the Case Management general intervention
Initiation into the cm program: comprehensive assessment and patient empowerment.
Once an individual was identified as a patient with complex multimorbidity, the program adapted the common elements of CM [ 5 , 8 ]. The initial phase of the CM Model included a comprehensive assessment performed by a physician and a nurse. The multidisciplinary team reviewed the background and current clinical and psychosocial status of the patient. The assessment included information about the ability to perform activities of daily living (using the Barthel Index) and cognitive functioning (using the Short Portable Mental Status Questionnaire), medication prescribed, social history, and care support. This assessment allowed the identification of problems and the resources available in the patient environment, such as support by an informal caregiver.
The initiation into the CM program was developed along a standard of three consecutive days through home visits and face-to-face meetings for 60 minutes approximately. Usually, the assessment was performed during the first day. On the remaining days, the nurse initiated the educational and preventive interventions based on the identified needs and the health-disease process.
Educational interventions were orientated towards empowering the patient from the beginning of the CM program and were maintained during the entire care plan since promoting self-care could contribute to achieving successful outcomes [ 24 ]. The educational contents included a separate description of each disease process, common signs and symptoms of exacerbation, and how to report them to the healthcare provider. A personalized educational pathway was developed integrating notions from all the clinical condition of interest for the patients, including relationships between diseases. Information leaflets with recommendations and a traffic light system with alert ranges for the main diseases (heart failure, COPD and diabetes) were developed and shared with patients.
Education related to prescribed medication: dosage, route, duration of treatment, and adverse effects was also included. Finally, the patients were encouraged to follow nutritional and exercise habits appropriate for each condition [ 25 , 26 , 27 , 28 ].
Development of the Case Management Care Plan
The CM programmed attention began after the patient was enrolled into the program. The objectives for the care plan, aligned with the initial assessment, are the promotion of self-care empowerment, treatment compliance, and the identification of risk situations by regularly monitoring signs and symptoms [ 29 , 30 ]. Clinicians established stability indicators and regular diagnosis techniques related to chronic diseases in accordance with the available evidence-based guidelines [ 31 , 32 ].
The Primary Health Care Team (PHCT), formed by a general practitioner and a community nurse, together with the nurse case manager were responsible for the scheduled follow-up period and the reference for the enrolled patients. The scheduled care plan for the PHCT was based on face-to-face meetings through home visits or consultations depending on the functional status and the needs of the patients. Patients received one contact every two months by each member of the team alternately.
At the same time, case manager nurses from the Telemedicine Unit were responsible for the remote follow-up through structured phone calls carried out every 15 days, consecutively. The interviews focused mainly on functional and clinical outcomes, monitoring of signs and symptoms of exacerbation of each disease, and reinforcing health recommendations to patients.
When potential social risk was identified, a social worker was involved in the care plan. The appointments with the outpatient clinic were continued as expected and planned by each specialist.
The reasons for case closure were non-adherence to the CM program, moving to a residence outside the region, or death.
Case manager nurses were also responsible for coordinating patients along community and hospital resources, especially on the transition from hospital to home after discharge or when potential risk situations were identified, triggering contacts from the PHCT.
One of the goals when facing probable disease exacerbation was to reduce the risk of unplanned admission to hospital. When possible, the initial approach was given by medical and nursing staff from the PHCT. If the clinical condition required more intensive attention in the community setting, the patient was admitted via the HaH unit.
The admission to the HaH unit offered a strong care alternative as these schemes incorporate many elements of hospital care into the community setting. Multidisciplinary teams were able to visit patients on a daily basis and perform advanced diagnostic and therapeutic techniques such as non-invasive breathing monitoring, endovenous treatments, or paracentesis, among others [ 33 ]. During the clinical follow-up, community based care resources gave a response until patients recovered. When disease exacerbation could not be managed or reverted at home, patients were admitted to the hospital.
The signature strengths of the developed program are on the one hand, promoting proactive care through Primary Health scheduled interventions together with the support of the Telemedicine Unit and on the other, recognizing and anticipating the early symptoms of an exacerbation in order to provide advanced care developed by the HaH Unit and try to reduce avoidable hospital admissions or length of stay by community-dwelling chronic patients.
Statistical analysis and modeling
The goal of our study was to find out if the CM intervention had a positive effect in reducing the number of emergency room visits, the number of admissions and/or the LoS, considered as the sum of the length of stay of all the admissions of a patient. The statistical analyses were carried out mainly for hospital use and secondly for HaH use.
We analyzed the effectiveness of the CM intervention by comparing the resource utilization of the same patients before and after being included in CM. Thus, we have two groups: a pre-intervention group, where we take into account the number of admissions and LoS of the patients one year before their inclusion in the CM intervention; and an intervention group, where we take into account the number of admissions and LoS of the patients until their discharge from CM regardless of its cause. The subjects under study are thus matched. Moreover, the patient in the pre-intervention group is observed in a time-window of 365 days, while the same patient in the post-intervention group is likely to be observed in a different time-window (greater or less than 365 days). We normalized the time-window of the intervention group to one year by using the rate of admissions and the rate of the length of stays.
We used the nonparametric two-tailed matched Wilcoxon’s signed rank test with continuity correction for testing the null hypothesis that there is no effect when including a patient in the CM intervention.
We also calculated the Relative Risk (RR) and their confidence interval at 95% [ 34 ]. The RR has been calculated taking into account the whole time-window of every patient under study before and after the intervention.
Ethical aspects
This research did not imply any risk or changes in the healthcare services to patients, and did not alter their regular intervention and treatment. Only authorized people obtained data from electronic health records. We maintained the privacy and security of patients’ personal information by encoding their identity with dissociated non-traceable codes. This research was in accordance with the International Guideline for Ethical Review of Epidemiological Studies [ 35 ] and the Biomedical Research Ethics Committee of our institution, which approved the study protocol.
The sample under study had a mean age of 78.5, where 69.3% of the patients were over 75 years old. The percentage of women was 50.7%. The average number of systems affected by the multimorbidities of the patients was 4.95 and the average number of organs affected was 8, and the most prevalent affected system was the cardiac system. Around 1 out of 5 patients died during the CM intervention. Table Table1 1 describes the socio-demographic features and comorbidity profile.
Description of the sample under study (N = 714).
1 The Community Assessment Risk Screen (CARS) is a tool for identifying community dwelling elderly patients at increased risk (CARS > 4) for hospitalizations or emergency room visits [ 36 ].
The information about the hospital resource utilization before and after CM intervention is shown in Table Table2. 2 . It includes the minimum, maximum and mean number of each outcome normalized for one year. The original values of the hospital resource utilization after intervention are shown in Table Table3 3 .
Hospital resource utilization normalized for one year.
Original values for hospital resource utilization after the CM intervention.
The results of the two-tailed matched Wilcoxon’s hypothesis test, with a significance level at α = 5%, showed statistically significant differences for the rate of unplanned admissions (median difference 0.23, CI 95% [0.08, 0.38]) and for the rate of emergency room visits (median difference 0.28 [0.10, 0.50]). However, the rate of admissions in HaH showed a significant increase (median difference –0.59 [–0.91, –0.36]). In the case of LoS, the results showed statistically significant differences for the rate of unplanned hospitalization LoS (median difference 2.75 [1.25, 4.27]).
The RR reduction for unplanned admission is 58.4% (RR = 0.584 with 95% confidence interval [0.522, 0.652]), and the RR reduction for Emergency Room visits is 73.5% (RR = 0.735, with CI95% [0.688, 0.785]). However, the HaH admission RR increases by 50.2% (RR = 1.502, with CI95% [1.346, 1.675]). Figure Figure1 1 shows the results.

The relative risks and 95% confidence interval for unplanned hospital admission, HaH episodes, and visits to the Emergency room.
Nowadays, confronting the growing burden of chronic disease poses a major challenge for health care and social policies. A wide variety of strategies, orientated to dealing with this challenge, have been evaluated. However, the results are still insufficient to demonstrate significant benefits on hospital resources utilization [ 20 , 21 ].
The program under validation is based on a CM intervention focused on patients with complex multimorbidity which works on synergy between primary and hospital care resources. The intervention under study faces the problem of frequent hospital uses by high-risk patients in two main areas: the promotion of proactive interventions and the possibility of early and intensive care at home.
The one-year normalization allowed us to carry out a balanced comparison of the before-after group. Our results reveal that the CM program reduces the number of unplanned admissions and the visits to the emergency room. Furthermore, when the patient has to be admitted to the hospital, the mean number of LoS is reduced.
The variety of needs expressed by people with multimorbidity requires complex and multifaceted interventions. We consider that the simultaneous application of organizational and educational initiatives has been essential for reducing unexpected health care resources use [ 22 ].
The program promotes proactive attention through educational interventions initiated upon admission into the program and reinforced by the case manager nurses during the scheduled follow-up period. It empowers patients on self-care, which may benefit the early identification of risk situations [ 37 , 38 ]. The evidence however is still unclear about the effect of health self-management training on hospital resource utilization [ 20 ].
In addition, we believe that the scheduled contacts facilitate accessibility to medical attention. Also, the involvement of specific nursing roles [ 39 , 40 , 41 ] and social workers [ 21 ] together with the primary healthcare team could have had a positive impact on the results.
Consistent with [ 11 , 12 ], we believe that the follow-up through structured telephone intervention developed by case managers could also have had an impact on reducing the risk of hospital resource utilization.
Apart from the aforementioned proactive interventions, the next signature strength pending for discussion is the role of the HaH unit when faced with unplanned clinical or social needs. The diagnostic and therapeutic possibilities allow early attention to the exacerbations and acute needs of chronic disease at home, which could explain the decrease in hospital and emergency use in the population under study. The role of the HaH managing relevant health status changes shares similar aspects of the virtual ward concept described by Lewis et al. [ 42 ].
Besides the function of the HaH unit for avoiding conventional hospitalizations, we also consider important their role in reducing LoS once the patient is admitted to hospital. In this sense, our results are aligned with those obtained in recent publications [ 43 , 44 ]. In addition, the results show an increase in the number of HaH admissions once the patient is included in the CM program. This result may be attributable to the design of the CM intervention itself, so it may indicate an appropriate use of Hospital-based home care as an alternative to conventional hospitalization for patients with complex multimorbidity.
Limitations and future work
Since the CM intervention depends on the context, the adoption of this type of intervention to a different health center may require a careful implementation according to the characteristics of chronic patients.
The lack of information on primary care is a main limitation of this study. Since we could not access the data from primary care, it was impossible for us to determine the impact of the intervention on primary care resource utilization.
The aim of the study was to analyze the relation between integrated care intervention and hospital resource utilization. However, it would be interesting to know the effect of specific aspects of this multifaceted intervention. But the analysis did not allow us to determine the effects of each component of the model applied separately.
Despite the significant results obtained, quasi-experimental designs require careful analysis to reduce the plausibility of alternative causal explanations other than the CM intervention. Alternative explanations include the principle of regression to the mean, and also maturation effects. However, since the study was focused on individuals with complex multimorbidity, the evolution of the condition is likely to aggravate or even become a patient in an end-of-life situation, which would likely require more health care resources than before.
The next step should be to carry out a cost-effectiveness analysis to compare if the intervention implies economic savings apart from the observed and tested hospital resources savings.
Conclusions
Since the prevalence of chronic diseases is growing in our society, there is an increasing need to provide sustainable, optimized and personalized healthcare for this population segment. CM intervention implemented at our institution has produced a significant hospital resource savings on average, with a positive impact on chronic patients with complex multimorbidities regarding admissions and LoS. We suggest that continuous and close monitoring by the primary healthcare team and the nurse case manager, together with the participation of other professionals such as a social worker or a Hospital at Home unit, constitute a multidisciplinary team, which may be able to face the needs of patients with complex multimorbidity in the community, avoiding hospital contacts. We consider that policies and strategies should keep promoting integrated care interventions both for research and for the healthcare networks of patients and families.
Acknowledgements
We want to thank Dr. Salvador Peiró and the anonymous reviewers for their comments, which helped improve this manuscript. The REDISSEC RD12/0001/0011 and Fondos FEDER (Spain) partially co-funded this work.
Patricia Wilson, Professor of Primary and Community Care Kent Academic Primary Care Unit, Centre for Health Service Studies, University of Kent, Canterbury, UK.
One anonymous reviewer.
Competing Interest
The authors have no competing interests to declare.

IMAGES
VIDEO
COMMENTS
A 44-year-old woman presented with cough, dyspnea, and chest pain. On examination, she had tachycardia and hypotension. Evaluation revealed SARS-CoV-2 RNA in a nasopharyngeal swab, as well as eleva...
The Global Burden of Disease study 2013 reported a substantial (42.3%) increase in the years lived with disability (YLD) from 1990 to 2013 . This was overwhelming due to non-communicable diseases, with no infectious diseases in the top 20 leading causes of YLDs globally in 2013. ... Chronic disease management in primary care is an important ...
Dr Richard Baxter Examines Five Case Studies in Which a Structured Approach to Chronic Disease Management (CDM) Has Improved Care, and Presents a Framework for CDM Optimisation. the impact of financial incentives, including the Quality and Outcomes Framework, on CDM delivery. Implementation actions for clinical pharmacists in general practice ...
In 2012 a Group of Experts suggested "Diabetes provides a pertinent case of chronic disease management with a particular focus on patient self-management. This paper suggests using a six-step cycle for personalized diabetes (self-)management and collaborative use of structured blood glucose data (Fig. 1). E-health solutions can be used to ...
Chronic diseases have an impact on and change patients' lives, and the way they experience their bodies alters. Patients may struggle with identity and self-esteem, a shrinking lifeworld and a challenging reality. 1 The chronic diseases become part of the patients' lives, whether they affect their physical health and functions, autonomy, freedom and identity, or threaten their life. 2 The ...
This issue brief reviews the evolution of the disease management model and the ways it relates to care coordination and case management approaches. It also looks at examples of population-based disease management programs operating in both the private and public sectors and reviews the evidence of their success. Finally, the paper considers the policy implications of adapting this model to a ...
Diabetes as a case study of chronic disease management with a personalized approach: the role of a structured feedback loop Diabetes Res Clin Pract. 2012 Oct;98 ... Diabetes provides a pertinent case of chronic disease management with a particular focus on patient self-management. Despite advances in diabetes therapy, many people with diabetes ...
Disease management programs should exist within an integrated and comprehensive system of care in which the patient-provider relationship is central. ... Blumenthal RS, Margolis S, et al. Nurse case management of hypercholesterolemia in patients with coronary heart disease ... (2008) Evaluation Methods in Disease Management Studies, Disease ...
Case Management: An Evolving Role. Case management is being adopted nationally and internationally as a way to improve health outcomes related to complex illness, multiple chronic ill-nesses, and insufficient family or social support. Currently, chronic disease affects half of all Americans and is the leading cause of death and disability ...
CHWs become highly knowledgeable in disease management through the practical experience of seeing what it takes for people to get their condition under control. Top. Evaluation Method. For this study, we used data on enrollment in care coordination, and outcome data on HbA 1c of the patients who had diabetes. The CCM team documents patient ...
5. Approaches to case management for med-ically complex patients vary more than do disease-management programs for patients who have one or more chronic conditions. The frail elderly exhibit a range of problems that do not easily lend themselves to tele-phone monitoring based on clinical practice guidelines by nurse clinicians. Rather, pro-
The Business Case for Diabetes Disease Management. by Martha Lagace. Diabetes is a tough disease to tackle. A case-study discussion led by HBS professor Nancy Beaulieu asked why it is so complex for business and society, and what might be done to curb its incidence. What should business people in particular know about the pros and cons of ...
This article uses a case study to discuss the symptoms, causes and management of chronic obstructive pulmonary disease, describing the patient's associated pathophysiology. Diagnosis involves spirometry testing to measure the volume of air that can be exhaled; it is often performed after administering a short-acting beta-agonist.
nomenclature emerged to describe components of the new environment, and familiar words were put to use in an entirely different context. Purpose/Objectives: This article proposes a working framework for activities performed in case management, disease management, care management, and care coordination. The author offers standard working definitions for some of the most frequently used words in ...
This case study highlights the importance of early diagnosis and personalized treatment plans for patients with Parkinson's disease. ... the disease, its management and available resources can ...
AMHC was incorporated in 1981 and began operation of hospital-based Diabetes Treatments Centers of America in 1983. These units focused on evidence-based care, patient education and patient self-care skills. AMHC developed its first disease management program for non-hospitalized diabetes patients in 1993.
Abstract. The prevalence of chronic kidney disease and its risk factors is increasing worldwide, and the rapid rise in global need for end-stage kidney disease care is a major challenge for health systems, particularly in low- and middle-income countries. Countries are responding to the challenge of end-stage kidney disease in different ways ...
Type 1 Diabetes (T1D) requires consistent disease management for blood sugar regulation. A theoretical framework can assist with interpreting behavioral processes needed for disease management. However, limited qualitative studies have explored disease management practices among college students with T1D using a theoretical framework.
Background Hepatitis B virus (HBV) infection can cause liver failure, while individuals with Acquired Immunodeficiency Virus Disease (AIDS) are highly susceptible to various opportunistic infections, which can occur concurrently. The treatment process is further complicated by the potential occurrence of immune reconstitution inflammatory syndrome (IRIS), which presents significant challenges ...
Purpose of Review Myelin oligodendrocyte glycoprotein antibody disease (MOGAD) is a distinct neuroinflammatory condition characterized by attacks of optic neuritis, transverse myelitis, and other demyelinating events. Though it can mimic multiple sclerosis and neuromyelitis optica spectrum disorder, distinct clinical and radiologic features which can discriminate these conditions are now ...
The patient's peripheral ulcerative keratitis was determined to be an extra-articular manifestation of her long-standing untreated seropositive erosive rheumatoid arthritis. Because of the severity of her disease, she was started on methotrexate and an anti-TNFα inhibitor for long-term treatment. Glucocorticoid therapy had not improved her ...
A case-control study's design is crucial for producing reliable results. You start by selecting individuals who have the disease (cases) and compare them to individuals who do not (controls).
Trichinellosis is a parasitic zoonotic disease transmitted through the consumption of meat from animals infected with Trichinella spp. nematodes. In North America, human trichinellosis is rare and is most commonly acquired through consumption of wild game meat. In July 2022, a hospitalized patient with suspected trichinellosis was reported to ...
A year before diagnosis, these people were 17.2 percent more likely to be delinquent on their mortgage payments than before the onset of the disease, and 34.3 percent more likely to be delinquent ...
Background: Atypical Spitz tumor (AST) is an intermediate category among Spitz melanocytic neoplasms. Sentinel node biopsy (SNB) has been proposed in the clinical management of AST patients, but this approach remains the subject of debate. This systematic review aims to summarize the available evidence on SNB procedures in AST patients. Methods: A comprehensive search was conducted, including ...
Daisy Hernández is the author of The Kissing Bug: A True Story of a Family, an Insect, and a Nation's Neglect of a Deadly Disease (Tin House, 2021), which won the PEN/Jean Stein Book Award and was selected as an inaugural title for the National Book Foundation's Science + Literature Program. The book was named a top 10 nonfiction book of 2021 by Time magazine and was a finalist for the ...
In particular, case management (CM) programs have emerged as an approach to the management of chronic disease focused on improving individuals' health and serving social needs [7,8]. Nevertheless, there is controversial scientific evidence available about the impact of using CM programs for chronic patients in terms of healthcare utilisation ...