- Open access
- Published: 22 March 2019

The past, present and future of anti-malarial medicines
- Edwin G. Tse 1 ,
- Marat Korsik 1 &
- Matthew H. Todd ORCID: orcid.org/0000-0001-7096-4751 1 , 2
Malaria Journal volume 18 , Article number: 93 ( 2019 ) Cite this article
91k Accesses
243 Citations
228 Altmetric
Metrics details
Great progress has been made in recent years to reduce the high level of suffering caused by malaria worldwide. Notably, the use of insecticide-treated mosquito nets for malaria prevention and the use of artemisinin-based combination therapy (ACT) for malaria treatment have made a significant impact. Nevertheless, the development of resistance to the past and present anti-malarial drugs highlights the need for continued research to stay one step ahead. New drugs are needed, particularly those with new mechanisms of action. Here the range of anti-malarial medicines developed over the years are reviewed, beginning with the discovery of quinine in the early 1800s, through to modern day ACT and the recently-approved tafenoquine. A number of new potential anti-malarial drugs currently in development are outlined, along with a description of the hit to lead campaign from which it originated. Finally, promising novel mechanisms of action for these and future anti-malarial medicines are outlined.
In 2017, the World Health Organization (WHO) estimated that there were 219 million cases of malaria worldwide, an increase of 2 million from the previous year, and as a result there were 435 thousand deaths, or 1190 per day, mostly young children [ 1 ]. Encouragingly, since 2000, these figures have decreased by about 37% worldwide, but a number of recent reports have shown that this level is slowly plateauing, emphasizing that there must not be complacency with the current treatment and prevention strategies [ 2 , 3 ]. There are five species of the Plasmodium parasite, with Plasmodium falciparum being the most prevalent in Africa and Plasmodium vivax being the most prevalent in countries outside Africa. Almost half the world’s population is at risk of contracting malaria, with Africa having the biggest share of cases and deaths of any continent ( \(\sim\) 90%).
Over the past few years, a number of reviews have been published which evaluate the potential future of anti-malarial drugs. Of note: Triple-anti-malarial drug combinations were examined in 2014 [ 4 ]; a review on the numerous strategies currently used in anti-malarial drug discovery was published in early 2017 [ 5 ], and an in-depth primer on all aspects of malaria was published in late 2017 [ 6 ]. In early 2018, a review was published highlighting the discovery and development of a number of new anti-malarial drug candidates [ 7 , 8 ].
This review aims to summarize the past, present and future of compounds used to treat malaria. There is a focus on projects supported by the Medicines for Malaria Venture (MMV), a non-governmental organisation that maintains a website [ 9 ] highlighting collaborations from the very early stages of drug discovery and lead optimization (e.g. the Open Source Malaria (OSM) project with which the authors are involved [ 10 ]), to the progression of lead compounds through clinical trials (e.g. Cipargamin, which has been developed by Novartis [ 11 ]) and all the way to the final stages of bringing a drug to market (e.g. Artesunate for injection, developed by Guilin Pharmaceutical). After a brief survey of past medicines, and those that are currently being used, focus will be put on compounds that are currently in development, and in particular the lead optimization campaigns of each. Since it is such a crucial component of modern anti-malarial drug discovery, this review will end with a survey of the most promising mechanisms of action of those compounds in development.
The scope of this review encompasses compounds described by the MMV survey of current anti-malarials, previous reviews on anti-malarial drugs, as well as compounds currently undergoing active clinical trials. The information presented in this review was obtained through structure searching of the relevant compounds in the SciFinder database. Additional references were found through the Web of Science database (Additional file 1 ).
Since the isolation in 1820 of quinine, the first chemically purified effective treatment for malaria, a number of other natural and synthetic compounds have been developed (Fig. 1 ). However, as time passed, strains of the parasite began to show signs of resistance towards these drugs, rendering them less effective. Accordingly, their use has ceased or is restricted to particular situations.
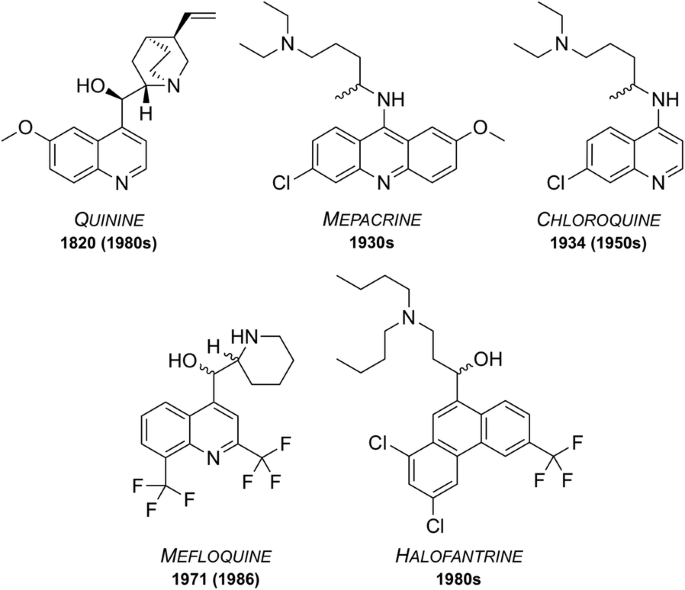
Well known anti-malarial medicines discovered between 1820 and the 1980s. Some are still used today while some have been rendered ineffective due to development of resistant strains or the emergence of undesirable side effects. Dates of first reported resistance are shown in brackets
First isolated from the bark of the cinchona tree in 1820, quinine has been used as one of the most effective treatments for malaria to date [ 12 ]. Resistance was first reported in the 1980s [ 13 ] and as of 2006, quinine is no longer used as a front-line treatment for malaria but is still on the WHO’s Model List of Essential Medicines (MLEM) [ 14 ] for the treatment of severe malaria in cases where artemisinins are not available.
Mepacrine (a.k.a quinacrine) was predominantly used throughout the Second World War as a prophylatic, sold under the trade name Atabrine [ 15 ]. This compound is a derivative of methylene blue, another anti-malarial that was discovered in 1891 and found to be an effective treatment for malaria [ 16 , 17 ]. Its use has declined over the years but methylene blue and its derivatives are, however, the subject of increasing current interest [ 18 ] and it is currently in clinical trials as a combination with primaquine ( vide infra ). Mepacrine itself is no longer used today due to the high chance of undesirable side effects such as toxic psychosis [ 19 ].
Chloroquine
During the 1940s, chloroquine (CQ) was used to treat all forms of malaria with few side effects [ 20 ]. Resistance to CQ was first reported in the 1950s and over the years many strains of malaria have developed resistance. Indeed, resistant strains (K1, 7GB, W2, Dd2, etc.) of the malaria parasite are now used in potency evaluation assays as a way of showing efficacy [ 21 ]. Chloroquine is on the MLEM for the treatment of P. vivax in regions where resistance has not developed [ 14 ].
Mefloquine was developed in the 1970s by the United States Army [ 22 ] and is still used today, also being one of the medicines on the MLEM. Originally introduced for the treatment of chloroquine-resistant malaria, it has been used as both a curative and a prophylactic drug. Resistance was first reported in 1986 [ 23 ]. It is thought that the structurally related quinoline drugs (such as quinine, mepacrine, chloroquine and mefloquine) act through the disruption of haemoglobin digestion in the blood stage of the parasite [ 24 ]. These drugs are commonly used in combination with a complementary drug (e.g. mefloquine and artesunate, sold as Artequin™) to reduce the chance of resistance development to the quinoline family of compounds. Mefloquine is commonly sold in its racemic form under the brand name Lariam®, however it is no longer widely used due to the perception of central nervous system toxicity that has been suggested to affect a large number of its users [ 25 ].
Halofantrine
Developed between the 1960s and 1970s by the Walter Reed Army Institute of Research [ 26 ], halofantrine was initially used for treatment against all forms of the Plasmodium parasite. Its use has diminished over time due to a number of undesirable side effects, such as the potential for high levels of cardiotoxicity. It is only used as a curative drug and not for prophylaxis due to the high toxicity risks and its unreliable pharmacological properties. Halofantrine is still used today, under the brand name Halfan™, but only in cases where patients are known to be free of heart disease and where infection is due to severe and resistant forms of malaria [ 27 ].
On the WHO Model List of Essential Medicines [ 14 ], there are currently listed 14 medicines for curative treatment of malaria and 4 medicines for prophylactic treatment, with the treatments formulated either as single compounds or as combinations. Perhaps the most effective of these are the artemisinin-based combinations which use an artemisinin derivative (short-acting) in combination with one or more complementary compounds (long-acting and possessing different mechanisms of action).
MMV have been involved in progressing nine new anti-malarial treatments based on different formulations/combinations of approved drugs (Fig. 2 ). All of these compounds are on the MLEM as combination therapies.
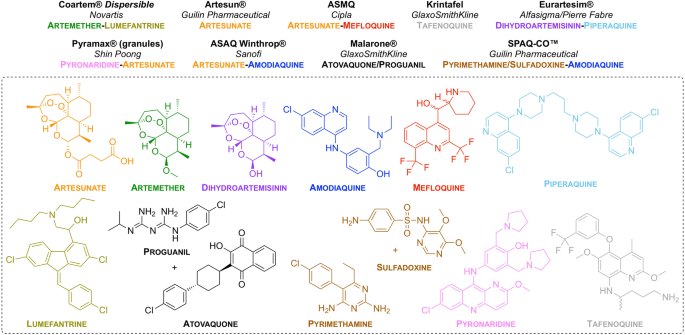
New drug combinations/formulations that have been approved for use. Brand name of the drug (in bold), partnered company (in italics) and drug combination (colour-coded to the structures) are listed
Artemisinin and its derivatives
Artemisinin was first isolated in 1971 by Tu Youyou from the plant Artemisia annua , a herb that has commonly been used in Chinese traditional medicine [ 28 ]. Due to the great positive impact of artemisinin in combating malaria, Youyou was awarded the joint Nobel Prize in Physiology or Medicine in 2015 for “ her discoveries concerning a novel therapy against malaria ” [ 29 ]. Artemisinin has been shown to be efficacious against all multi-drug resistant forms of P. falciparum . The most common derivatives of artemisinin are artemether, artesunate and arteether. These semi-synthetic derivatives are prodrugs which are transformed to the active metabolite, dihydroartemisinin. The use of artemisinins has been integral in the fight against malaria with ACT making up the majority of modern day treatments [ 30 ]. Although slow to develop, the first report of resistance to artemisinin was in western Cambodia in 2008 [ 31 ]. Ten years later, in February of 2018, a report was published identifying more than 30 independent cases of artemisinin resistance in southeast Asia, specifically with resistance to the dihydroartemisinin–piperaquine combination therapy [ 32 ].
The mechanism of action (MoA) through which artemisinin acts has been widely debated [ 33 ]. The most accepted theory is that the molecule is activated by haem to generate free radicals, which in turn damage proteins required for parasite survival [ 34 , 35 ]. Still, evidence for a number of other possible mechanisms have been found. In 2013, a computational approach was taken to determine the MoA based around previous studies which identified haem and Pf ATP6 (Ca 2+ transporter) as potential MoAs [ 36 ]. More recently in 2015, artemisinin was shown to be associated with the up-regulation of the unfolded protein response (UPR) pathways which may be linked to decreased parasite development [ 37 ]. Another study showed that artemisinin was is a potent inhibitor of P. falciparum phosphatidylinositol-3-kinase ( Pf PI3K) [ 38 ].
Amodiaquine
Amodiaquine was first synthesized in 1948 [ 39 ]. It is mainly used for the treatment of uncomplicated P. falciparum malaria when used in combination with artesunate and is commonly sold under the trade name Camoquine® or Coarsucam™ [ 40 ]. Similar to chloroquine, amodiaquine’s MoA is thought to involve complexation with haem and inhibition of haemozoin formation [ 41 ].
Piperaquine
Piperaquine was developed in the 1960s as a part of the Chinese National Malaria Elimination Programme [ 42 ]. Initially used throughout China as a replacement for chloroquine, resistance led to its diminished use as a monotherapy. While the MoA of piperaquine is not completely understood, studies have suggested that it acts by accumulating in the digestive vacuole and inhibiting haem detoxification through the binding of haem-containing species [ 30 , 43 ]. These days, piperaquine is used as a partner drug with dihydroartemisinin (commonly sold under the trade name Eurartesim®).
Lumefantrine
Lumefantrine (a.k.a. benflumetol) was first synthesized in 1976 as a part of the Chinese anti-malarial research effort “Project 523” which also resulted in the discovery of artemisinin [ 44 ]. It is currently sold under the trade name Coartem®. The exact MoA of lumefantrine is unknown, however studies suggest that it inhibits nucleic acid and protein synthesis through the inhibition of \(\beta\) -haematin formation by complexation with haemin [ 41 ]. Lumefantrine is currently used only in combination with artemether.
Proguanil and atovaquone
Proguanil was first reported in 1945 as one of the first antifolate anti-malarial drugs [ 45 ], while atovaquone was first reported in 1991 for the treatment of protozoan infections [ 46 ]. The combination of these, commonly sold as Malarone™, has been marketed by GlaxoSmithKline (GSK) since the early 2000s, and has proven to be a very effective anti-malarial due to the synergistic effect of the two components. This is, in large part, due to the different MoAs for each compound. Atovaquone acts as a cytochrome bc \(_\text {1}\) complex inhibitor which blocks mitochondrial electron transport [ 47 ]. Proguanil (when used alone) acts as a dihydrofolate reductase (DHFR) inhibitor through its metabolite, cycloguanil (CG) which disrupts deoxythymidylate synthesis. When used in combination with atovaquone however, proguanil does not act as a DHFR inhibitor but has instead been shown to reduce the concentration of atovaquone required for treatment [ 48 ]. Generic atovaquone/proguanil is still available today for the treatment of chloroquine-resistant malaria.
Pyrimethamine and sulfadoxine
Pyrimethamine (PYR) was developed in the early 1950s by Gertrude Elion and George Hitchings and is now sold under the trade name Daraprim™ [ 49 ]. The development of pyrimethamine was a part of the efforts that won Elion, Hitchings and Black the joint Nobel Prize in Physiology or Medicine in 1988 for “ their discoveries of important principles for drug treatment ” [ 50 ]. Sulfadoxine was developed in the early 1960s [ 51 ]. It is no longer used as a preventative drug due to high levels of resistance. The combination of pyrimethamine and sulfadoxine was approved for use for the treatment of malaria in 1981 and is now commonly sold under the trade name Fansidar®. Both drugs are known to target the parasite folate biosynthesis pathway [ 52 ]. Pyrimethamine inhibits dihydrofolate reductase, while sulfadoxine inhibits dihydropteroate synthetase.
Pyronaridine
Pyronaridine was first synthesized in the 1970s at the Institute of Chinese Parasitic Disease [ 53 , 54 ]. It has been found to be efficacious against chloroquine resistant strains and has been in use for over 40 years, sold under the trade name Pyramax® (in combination with artesunate). Like lumefantrine, pyronaridine has been found to act through the inhibition of \(\beta\) -haematin formation [ 55 ].
Tafenoquine
First discovered in 1978 at the Walter Reed Army Institute of Research, Tafenoquine (TQ) was recently approved by the United States Food and Drug Administration for use as the first new single-dose for the radical treatment of P. vivax malaria in over 60 years [ 56 ]. TQ is thought to be a prodrug which is metabolized to the active quinone TQ, however the MoA is not well known [ 57 ]. It is currently sold under the brand name Krintafel.
(Adapted from the MMV-supported projects webpage [ 58 ])
Snapshot of the projects supported by MMV at different stages of the drug discovery and development pipeline
Since its establishment in 1999, the Medicines for Malaria Venture has been frontlining the discovery and development of new medicines for the treatment of malaria. The potential for these compounds to act as new anti-malarials is judged by a number of requirements: novel modes of action with no cross-resistance to current drugs; single-dose cures (artesunate and chloroquine are unable to do this); activity against both the asexual blood stages that cause disease and the gametocytes responsible for transmission; compounds that prevent infection (chemoprotective agents); and compounds that clear P. vivax hypnozoites from the liver (anti-relapse agents) [ 59 , 60 ]. MMV has been aiding the fight against malaria by partnering with universities and pharmaceutical companies around the world to bring new anti-malarials to market (Fig. 3 ).
Besides the traditional drug discovery and development methods for the identification of new anti-malarials that will be described below, there are a number of other ways in which a new anti-malarial drug may be discovered. One way, as previously mentioned, is through the exploration of new combinations and formulations of current anti-malarial drugs. This may help overcome issues with resistance to a particular component or may assist in the delivery of the drug allowing it to be more effective. Alternatively, existing drugs used for other purposes may be found efficacious against malaria and subsequently be repurposed as a new anti-malarial treatment. This can be advantageous since these compounds may have already shown good biological properties and may also reveal novel MoAs. Examples include those shown in Fig. 4 .
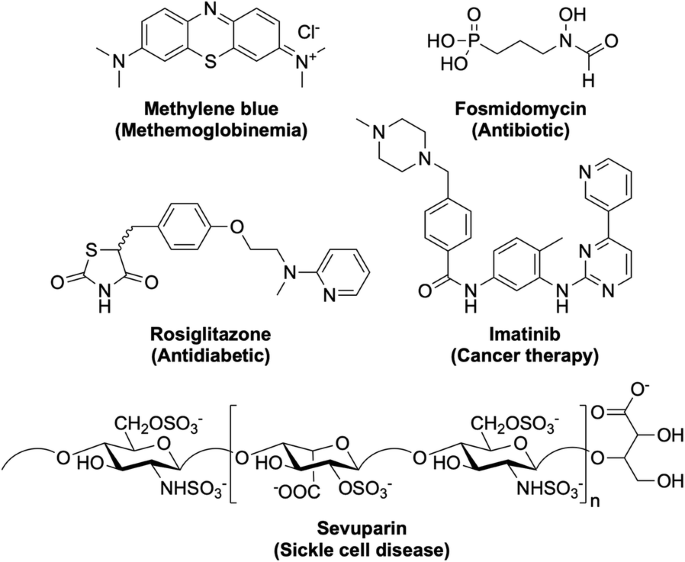
New anti-malarial compounds in development as a result of drug repurposing. Original indications for the drugs are shown in parentheses. Many of these repurposed drugs are already in Phase II trials as new potential anti-malarials
Methylene blue , a drug for the treatment of methaemoglobinemia. Last completed Phase II trials in 2017 (NCT02851108) as a combination with primaquine. Fosmidomycin , an antibiotic. Most recently in Phase II trials in 2015 (NCT02198807) as a combination with piperaquine. Rosiglitazone , an antidiabetic drug. Currently in clinical trials as an adjunctive therapy for severe malaria (NCT02694874). Imatinib , a cancer therapy drug. Currently in Phase II trials (NCT03697668) as a triple combination with dihydroartemisinin-piperaquine. Sevuparin , a drug for the treatment of sickle cell disease. Last in Phase I/II trials in 2014 (NCT01442168) as a combination with atovaquone-proguanil.
The following seven compounds were discovered and developed with the hope of progressing into clinical trials as potential new anti-malarial candidates (Fig. 5 ). However, over the past two years, progress in the development of these compounds has slowed, making the fate of these drug candidates less clear.
New anti-malarial drug candidates that have reported no significant progress in the last 2 years. These compounds may have encountered issues during preclinical or early clinical studies
The chiral 8-aminoquinoline derivative, NPC1161B was developed at the University of Mississippi and was still in preclinical studies in 2014 [ 61 , 62 , 63 , 64 ]. MK4815 was developed in 2012 at Merck but is still in preclinical studies due to safety issues [ 65 ]. CDRI 97/78 is a fast-acting novel trioxane anti-malarial first synthesized in 2001 by a team at the Council of Scientific and Industrial Research in India [ 66 ]. Having passed all preclinical studies, it was last seen to have completed first-in-human Phase I trials in 2014 [ 67 ]. Developed at the Liverpool School of Tropical Medicine in 2009, N-tert butyl isoquine/GSK369796 was designed as an alternative to amodiaquine [ 68 , 69 ]. It completed preclinical studies [ 70 ], and was last in Phase I trials in 2008 (NCT00675064). Artemisone is a second-generation semi-synthetic artemisinin derivative developed at the Hong Kong University of Science and Technology, that has previously been shown to be as efficacious as artesunate, with minimal neuro- and cytotoxicity and a comparably low cost of production [ 71 ]. It was withdrawn from Phase II/III trials in 2010 (NCT00936767). The bisthiazolium salt, SAR97276 , was discovered and developed by Sanofi in 2005 [ 72 ], however further investigation was terminated in 2012 after Phase II trials (NCT01445938). AQ-13 is a chloroquine derivative that was first described in 1946 [ 73 ]. While only differing to CQ in the amine side-chain, this difference has been linked to its increased efficacy against CQ-resistant strains [ 74 ]. It has a MoA and pharmacokinetic properties similar to that of CQ [ 75 , 76 ]. AQ-13 last completed Phase II trial at the end of 2017 (NCT01614964) [ 77 ], however there has been no mention of any following active trials so the ongoing status of this compound is unclear.
The compounds in the remainder of this section are currently still actively being pursued. For ease of visualisation throughout in this section, a graphical summary of each compound structure and its code will be displayed, along with key physical and biological properties identified during the hit to lead campaigns (Additional file 2 ).
Preclinical
Once a lead compound has been identified, optimization of the structure can begin. This largely involves investigation into the structure activity relationship (SAR) of the drug, optimising for properties such as potency (both in vitro and in vivo), solubility and metabolic stability. The candidate must also be assessed for any possible toxicity (e.g. dosing, cytotoxicity/genotoxicity levels, etc.).
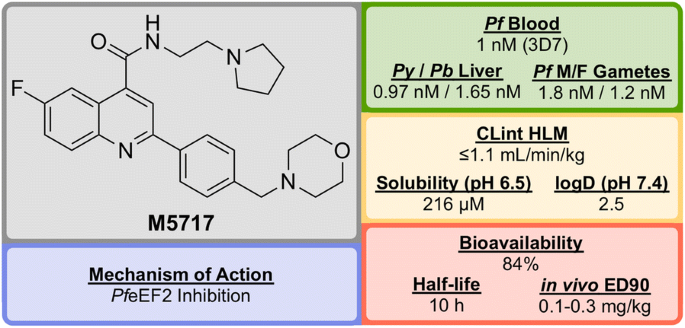
M5717 (Fig. 6 ) was developed in 2015 by a team led by the Drug Discovery Unit (DDU) in Dundee and was shown to have potent activity against multiple stages of the Plasmodium parasite via a novel mechanism of action [ 78 , 79 ].
Key biological and physical properties of M5717
Initial phenotypic screening of the Dundee protein kinase scaffold library against the 3D7 multi-drug-resistant P. falciparum strain identified a compound ( M1 , Fig. 7 ) that possessed high potency against the parasite, albeit with poor physicochemical properties. Optimization of this structure (via M2 and M3 ) led to improvements across the board ( M5717 ).
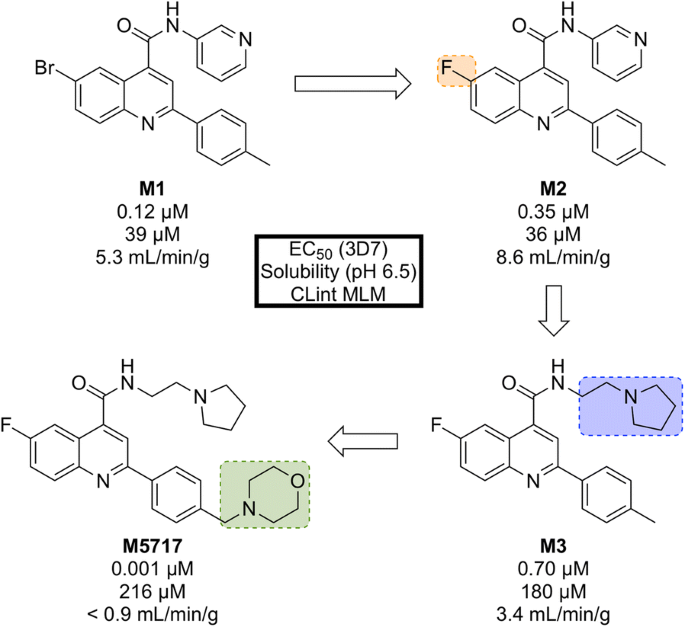
Key stages in the hit to lead pathway of M5717 . Initial replacement of Br for F, replacement of pyridine with ethylpyrrolidine, and addition of a morpholine fragment led to the optimized compound M5717
In addition to the nanomolar activity against the 3D7 strain, M5717 has shown almost equal potency against a number of other drug-resistant strains (K1, W2, 7G8, TM89C2A, D6 and V1/S) as well as similar potencies across multiple life cycle stages (liver schizonts, gametocytes and ookinetes).
M5717 was found to be as effective as current anti-malarial drugs (chloroquine, mefloquine, artemether, dihydroartemisinin and artesunate) when evaluated in the in vivo Plasmodium berghei mouse model for single-dose efficacy showing > 99% reduction in parasitaemia at doses of 4 \(\times\) 30 mg/kg p.o. q.d. and an ED \(_{90}\) of 0.1–0.3 mg/kg.
Due to its novel MoA ( Pf eEF2 inhibition, vide infra ) and its ability to clear blood-stage parasites completely, M5717 satisfies the requirements to be a long duration partner and could be used as part of a combination therapy with a fast-acting compound [ 80 ]. Additionally, the compound has shown the ability to act as a transmission-blocking drug (stage IV–V) and also to be effective for chemoprotection.
In late 2017, M5717 was cleared for progression from development to Phase I clinical trials for volunteers in Australia (NCT03261401).
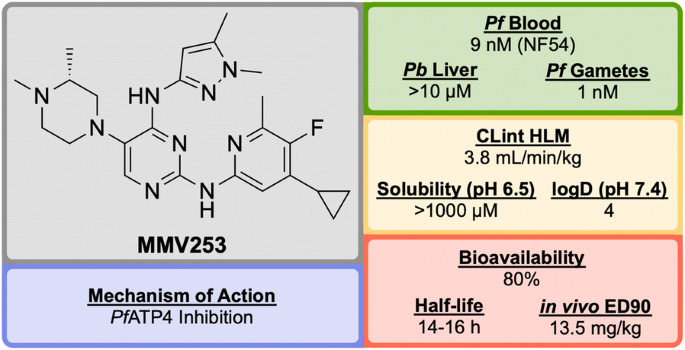
Identified by AstraZeneca in 2015, MMV253 (Fig. 8 ) is a novel triaminopyrimidine (TAP) that has shown good in vitro potency and in vivo efficacy, and acts through another novel MoA [ 81 ].
Key biological and physical properties of MMV253 . logD and in vivo ED 90 kindly provided by V. Sambandamurthy, S. Hameed P. and S. Kavanagh, personal communication, 2018
High-throughput screening of 500,000 compounds from AstraZeneca’s library against blood stage P. falciparum resulted in the identification of a promising series of TAPs. The initial hit ( M’1 , Fig. 9 ) suffered from hERG inhibition and poor solubility which, through lead optimization, was improved upon to give a compound that possessed high potency and desirable pharmacokinetic properties ( MMV253 ).
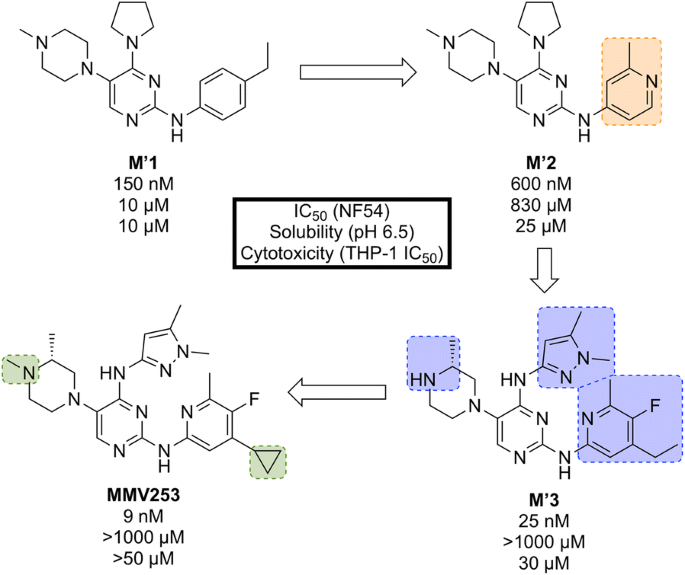
Key stages in the hit to lead pathway of MMV253 . Initial replacement of ethylbenzene on M’1 with 2-methylpyridine resulted in lower hERG affinity and improved solubility. Substitution of the pyrrolidine in M’2 with an imidazole containing an amine spacer further improved solubility and greatly improved the potency. Addition of an N -methyl group and a cyclopropane moiety led to the optimized compound MMV253
When screened against numerous mutant resistant strains with various mechanisms of resistance, MMV253 showed no spontaneous reduction in potency which can be attributed to its novel MoA ( Pf ATP4 inhibition, vide infra ). Good in vitro-in vivo correlation (IVIVC) was shown with a predicted human half-life of \(\sim\) 36 h (which is long compared to another fast-killing drug, artemisinin, which has a human half-life of 1 hour).
As of late 2016, the pharmaceutical company Cadila Healthcare owns the license for the compound series and is now doing further lead development in order to progress the drug through preclinical trials [ 82 ].
UCT943 (Fig. 10 ) was identified in 2016 by a team at the University of Cape Town, South Africa and is a key compound in a novel class of 2-aminopyrazine anti-malarials that has shown single dose curing ability in vivo and potential as a clinical candidate [ 83 ].
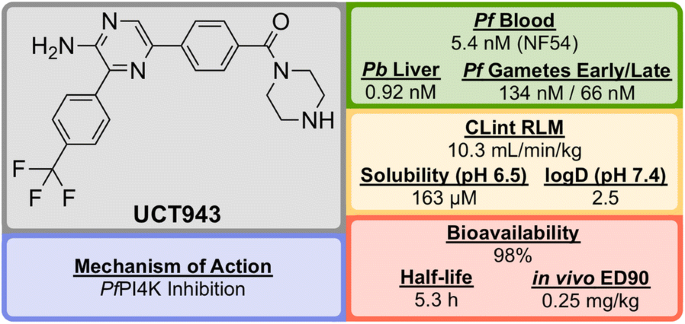
Key biological and physical properties of UCT943
The original 3,5-diaryl-2-aminopyridine series was identified from a high-throughput screen of 36608 compounds from the commercially available SoftFocus kinase library [ 84 ]. The initial hit ( U1 , Fig. 11 ) showed promising in vitro activity against the drug-sensitive NF54 strain (IC \(_{50}\) = 49 nM) but suffered from poor solubility and high metabolic clearance. To address the poor measured in vivo stability, the labile hydroxy and methoxy groups were replaced by a single trifluoromethyl group ( U2 ), but this change resulted in a significant loss of solubility. Significant improvements in solubility and potency were obtained by first replacing the mesyl group with a piperazine carboxamide group ( U3 ) and subsequently introducing another nitrogen atom into the pyridine ring ( UCT943 ) [ 85 , 86 ].
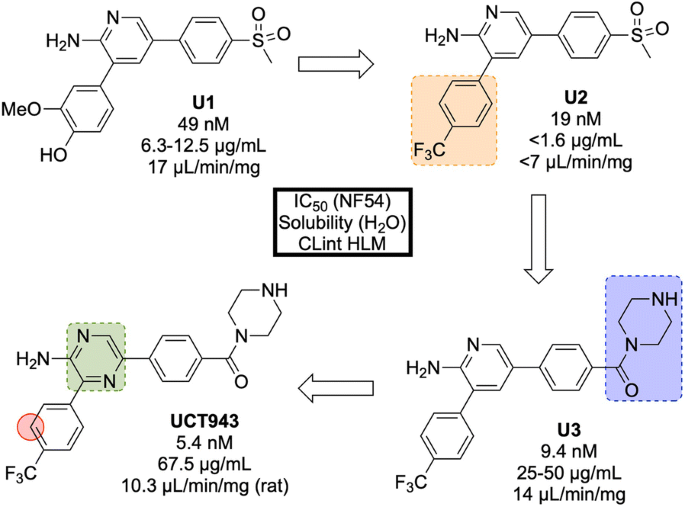
Key stages in the hit to lead pathway of UCT943 . Initial change the phenyl substituents with a single trifluoromethyl group led to greater in vivo stability. Introduction of piperazine amide instead of methylsulfonyl and a pyrazine instead of a pyridine led to the improved solubility and potency of the optimized compound. Surprisingly, introduction of a nitrogen in the red circle resulted in complete inactivity in vivo
In the P. berghei mouse model, UCT943 has shown the ability to cure malaria at doses of 4 \(\times\) 10 mg/kg and has an ED \(_{90}\) of 1 mg/kg. Interestingly, another closely related molecule (with a nitrogen instead of a carbon in the red circle) which was evaluated during the SAR study, displayed similar in vitro anti-malarial activity (IC \(_{50}\) = 9.1 nM) and solubility (198 \(\mu\) M) but showed a complete lack of in vivo activity with a < 40% reduction in parasitaemia using a comparable dosage.
UCT943 is potent across multiple parasite life stages of both P. falciparum and P. vivax . Its target is Plasmodium phosphatidylinositol-4-OH kinase ( Pf PI4K), which has also been implicated as the target for MMV048 ( vide infra ) [ 87 ]. UCT943 was in originally in place as a back-up to MMV048, however, due to preclinical toxicity, this candidate has been withdrawn.
From a discovery process by Anacor Pharmaceuticals that began in 2010 with a novel class of benzoxaborole anti-malarial compounds [ 88 ], AN13762 (Fig. 12 ) emerged in 2017 as the lead compound, showing excellent activity in vitro and in vivo, multi-strain efficacy and the ability to perform as a rapid-acting drug [ 89 ].
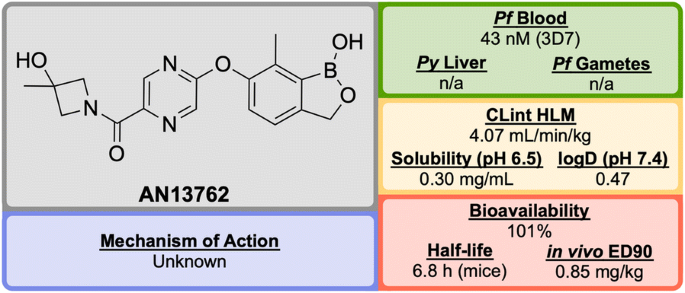
Key biological and physical properties of AN13762 . Solubility kindly provided by Y.-K. Zhang, personal communication, 2018
By screening a library of boron-containing compounds (previously shown to have selective activity against fungi, bacteria, parasites and inflammation) in a whole cell assay against P. falciparum , the initial hit compound, AN3661 ( A1 , Fig. 13 ), was identified to have potent in vitro activity against the 3D7 strain (IC \(_{50}\) = 26 nM). Further SAR and optimization studies led to the development of A2 in which the alkylcarboxylic acid chain was moved and replaced by a substituted pyrazine ether. This compound showed greater potency but still suffered from high metabolic clearance [ 90 ]. Replacement of the ester group with an amide group ( A3 ) led to improved metabolic stability and bioavailability, with a significant decrease in potency. Modification of the primary amide to a cyclic tertiary amide, and introduction of a methyl group on the benzoxaborole gave the lead compound AN13762 which possessed improved potency and metabolic stability [ 89 ].

Key stages in the hit to lead pathway of AN13762 . Initial replacement of the carboxylic acid chain with a pyrazine, and subsequent switch of the ester to a substituted amide helped to improve in vivo stability and bioavailability leading to the optimized compound
AN13762 has been shown to be equally potent across a wide range of drug resistant strains. The drug has displayed similar in vivo clearance rates when compared to artesunate. There is no inherent genotoxicity (as shown in Ames assays and in vivo rat micronucleus studies), and no cytotoxicity at concentrations up to 100 μM in human cell lines [ 89 ].
The precise mechanism of action for AN13762 remains unknown, though initial MoA studies on hit compound AN3661 identified a potential target as the P. falciparum cleavage and polyadenylation specificity factor ( Pf CPSF3) [ 91 ]. AN13762 has proceeded into the preclinical phase, with first-in-human studies planned for 2019 [ 58 ].
Developed in 2017 by a team at Heidelberg University, SC83288 (Fig. 14 ) is an amicarbalide derivative that possesses a novel chemotype for current anti-malarials, may have a potentially new MoA and has shown the ability to be a fast-acting drug for the intravenous treatment of severe malaria [ 92 ].
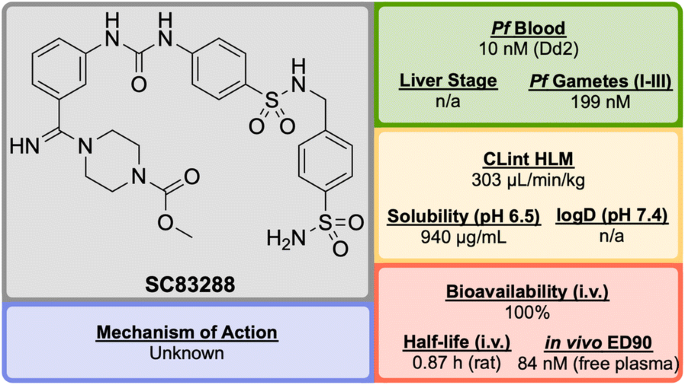
Key biological and physical properties of SC83288 . In vivo ED 90 kindly provided by M. Lanzer, personal communication, 2018
An in silico screen of a library of small molecule compounds for their ability to dock into P. falciparum lactate dehydrogenase led to the identification of amicarbalide ( S1 , Fig. 15 ), which was found to be highly potent (IC \(_{50}\) = 10 nM) against the Dd2 strain [ 93 ]. In order to overcome a potential DNA binding effect, an amidine group was replaced with a sulfonamide linker leading to S2 which possessed better solubility and improved metabolic stability. Further modification of the other amidine group with a substituted piperazine ring ( S3 ) led to improved potency and water solubility, but the compound suffered from poor permeability. Ultimately, replacement of the butyl chain with an acetyl group led to the highly potent lead compound SC83288 .
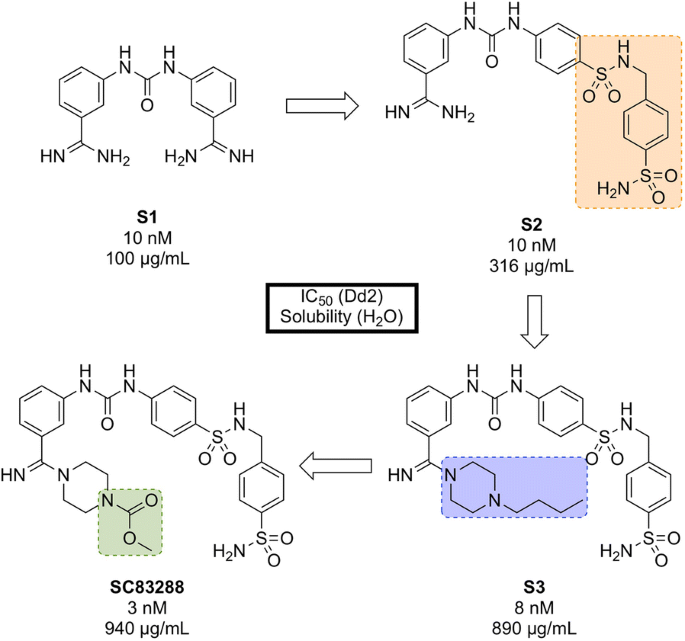
Key compounds in the discovery of SC83288 . Initial modification of one amidino group with a sulfonamide linker ( S2 ) resulted in improved solubility. Further modification of the remaining amidine group with substituted piperazine moieties ultimately led to the optimized compound with good solubility, permeability and high potency
SC83288 has been shown to be potent against a number of drug-resistant strains at IC \(_{50}\) values of < 20 nM and is also efficacious against early stage (I–III) gametocytes (IC \(_{50}\) = 199 nM) but not against late stage gametocytes (IV and V). It has an excellent safety profile, with no cytotoxicity, genotoxicity or hERG binding. In the Plasmodium vinckei rodent malaria model, SC83288 was able to fully cure the infection at a dose of 4 \(\times\) 20 mg/kg i.p. q.d. with no resurgence of infection. It is however, completely inactive against P. berghei .
While the exact MoA of SC83288 is unknown, the generation of resistant clones has identified Pf ATP6 as a possibly relevant target. However, it has been shown that SC83288 does not directly inhibit this target suggesting Pf ATP6 may have a less direct role in the mechanism of SC83288 . Pf MDR2 has been identified as another possible mechanism of resistance, facilitating the clearance of the drug from the parasite. SC83288 has been evaluated against artemisinins, showing no cross resistance and highlighting its potential as an alternative to artesunate for the treatment of severe malaria when combined with a slow-acting partner drug [ 94 ].
Discovered in 2010 by a team at Portland State University and further developed by DesignMedix, DM1157 (Fig. 16 ) is part of a class of compounds known as “reversed chloroquines” (RCQs), designed to overcome chloroquine-resistant and chloroquine-sensitive strains of the malaria parasite. The compound has been shown to be efficacious in vitro and in vivo [ 95 ].
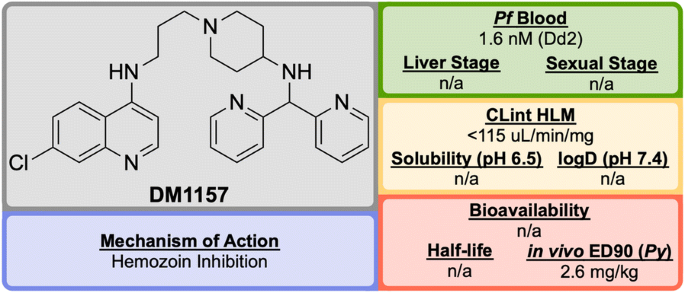
Key biological and physical properties of DM1157 . CLint HLM and in vivo ED 90 kindly provided by D. Peyton, personal communication, 2018
CQ resistance is known to be linked to the P. falciparum chloroquine resistance transporter ( Pf CRT): mutations to this target facilitate the expulsion of CQ from the parasite. A class of molecules has been identified, so-called “reversal agents”, that can inhibit Pf CRT, thus slowing the exportation of CQ from the parasite. By combining the chloroquinoline core of CQ ( D1 , Fig. 17 ) with the known reversal agent, imipramine, the first RCQ ( D2 ) was designed, but this molecule suffered from poor bioavailability and metabolic stability [ 96 ]. Subsequent SAR studies resulted in the substitution of the imipramine motif with a 1-(2,2-diphenylethyl)piperazine moiety which led to a compound ( D3 ) that was more stable to metabolic cleavage, but suffered from a high cLogP. In order to overcome this, the two phenyl groups were replaced with pyridines and the piperazine replaced with an aminopiperidine resulting in the lead compound, DM1157 that possessed a lower cLogP value (3.6) while still maintaining high potency against both CQ-resistant (Dd2 = 1.6 nM) and CQ sensitive strains (D6 = 0.9 nM) when compared to CQ (cLogP = 5.1, Dd2 = 102 nM, D6 = 6.9 nM).
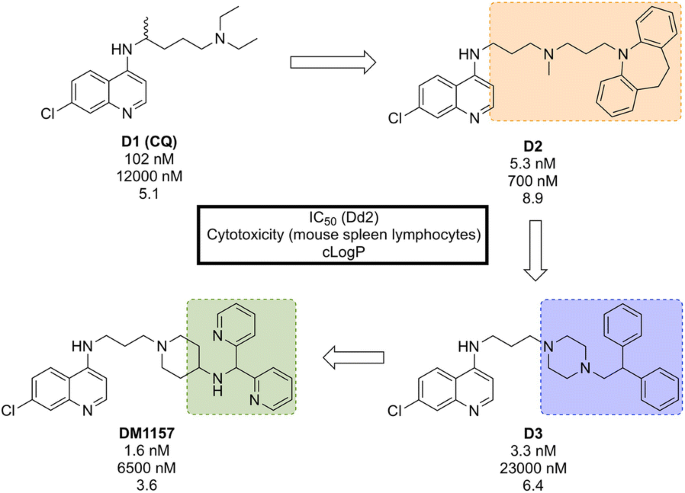
Key compounds in the discovery of DM1157 . Initial combination of the reversal agent, imipramine, with the CQ core resulted in the potent RCQ compound D2 . Subsequent replacement of the reversal agent with 1-(2,2-diphenylethyl)piperazine ( D3 ), and further modification with pyridine rings led to improved potency and cLogP values for the optimized compound
In a P. berghei rodent model, DM1157 showed good efficacy both orally and subcutaneously. Most notably, a > 99.9% reduction in parasitaemia was seen at an oral dose of 4 \(\times\) 30 mg/kg with 2/3 mice cured 30 days post infection. DM1157 has also been evaluated against P. falciparum and P. vivax multi-drug resistant field isolates in Indonesia and was found to be threefold more potent than CQ in both species [ 97 ].
CQ is known to bind to heme and inhibit \(\beta\) -haemozoin formation. DM1157 (and other RCQ compounds) have been shown also to act in this manner, but with much higher levels of inhibition of \(\beta\) -haemozoin both in vitro and in vivo. DM1157 is currently in Phase I trials to evaluate its safety and pharmacokinetics in humans (NCT03490162).
Translational (human volunteers)
Upon the completion of preclinical trials, the drug will have either passed or failed the required safety standards and pharmacokinetic profiling. For a compound that has passed these requirements, trials may then be conducted in human volunteers in order to show its efficacy as a potential treatment.
P218 (Fig. 18 ), discovered by BIOTEC Thailand in 2012, is an antifolate anti-malarial drug bearing resemblance to the 2,4-diaminopyrimidine core structure of PYR, and is highly selective for the P. falciparum dihydrofolate reductase ( Pf DHFR) [ 98 ].
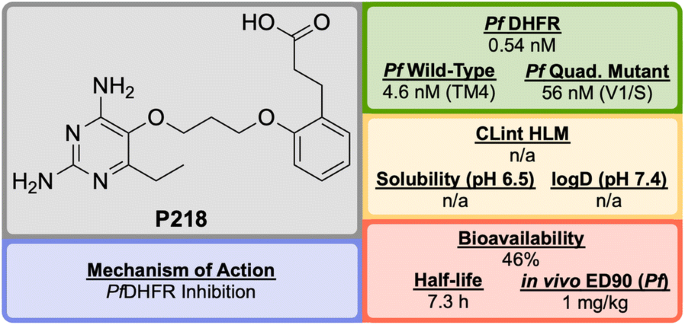
Key biological and physical properties of P218
Unlike the typical high-throughput screening that is used for the identification of hit compounds in medicinal chemistry, P218 was identified through careful examination of the cocrystal structures of known Pf DHFR inhibitors and their substrates. The initial observation that 2,4-diaminopyrimidines acted as antagonists to folic acid led to the discovery and development of methotrexate ( MTX ) as an antitumor drug (Fig. 19 ). By examining the binding interactions with DHFR, the structure of P65 was identified. A total of over 200 compounds (not described in the original report) were designed and synthesized after further examination of potential interactions with amino acid residues, with the optimized structure of P218 being identified as the best compound.

Key compounds in the discovery of P218 . The key 2,4-diaminopyrimidine core highlighted in red can be found in a number of DHFR inhibitors
The 2,4-diaminopyrimidine scaffold of P218 has been found to bind deep in the active site of Pf DHFR in both wild-type and mutant strains. This, along with the hydrogen bonding interaction of the carboxylate group with an Arg residue at the opposite end of the active site results in tighter binding and a longer residence time when compared to PYR . Since P218 is contained almost entirely within the dihydrofolate binding site, the strength of the binding should be strong enough to overcome any amino acid mutations, thus minimising the chance of drug-resistant mutations to arise. The novel two-step mechanism of action for binding to Pf DHFR ( vide infra ) allows P218 to overcome resistance that has emerged from the use of pyrimethamine. P218 has also shown high selectivity to the binding of malarial over human DHFR, which translates into reduced toxicity.
In vivo studies have shown P218 to be highly efficacious against P. falciparum and Plasmodium chabaudi in mice with ED \(_{90}\) values of 1 mg/kg and 0.75 mg/kg respectively. In the in vitro and in vivo potency assays that were run, P218 was found to be more potent than PYR in all cases.
Along with its high potency and good safety profile, P218 has the potential to be a replacement for PYR combination with CG in areas where Pf DHFR resistance has emerged. P218 has currently completed Phase I trials (NCT02885506).
Discovered by a partnership between St Jude Children’s Research Hospital and Rutgers University in 2010, (+)-SJ733 (Fig. 20 ) is a novel tetrahydroisoquinolone carboxanilide that possesses excellent anti-malarial activity in vivo [ 99 ].
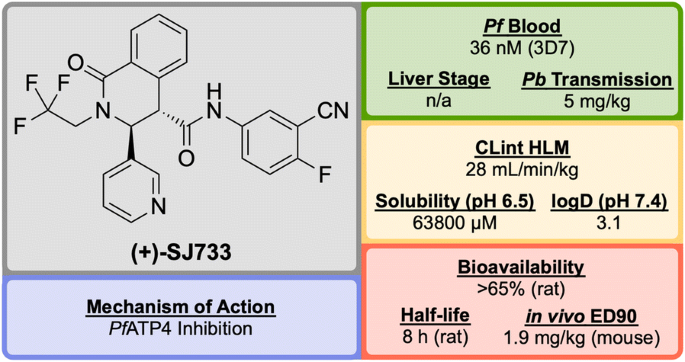
Key biological and physical properties of (+)-SJ733 . Sexual stage potency and logD kindly provided by K. Guy, personal communication, 2018
High-throughput phenotypic screening of > 300,000 compounds against the 3D7 strain of P. falciparum discovered a number of bioactive scaffold types, in which the hit compound ( S′1 , Fig. 21 ), belonging to the tetrahydroisoquinolone carboxanilide class, was found to have potent in vitro activity (EC \(_{50}\) = 53 nM). Metabolic studies using mouse liver microsomes identified the susceptibility of the methoxy group to demethylation. In order to overcome this issue, the aniline substituents were replaced with fluoro and cyano groups ( S′2 ), which had an added effect of improving the potency more than twofold. The thiophene group was suspected to be a metabolic hot spot, which was addressed by its replacement with a pyridyl group ( S′3 ) resulting also in improved solubility, while maintaining potency. The final metabolically labile group (isobutyl) was replaced with a trifluoromethyl moiety, further improving solubility and maintaining potency to give the lead compound (+)-SJ733 . The (+)-(3 S ,4 S ) isomer was found to be significantly more potent (EC \(_{50}\) = 36 nM) than its (–)-(3 R ,4 R ) enantiomer (EC \(_{50}\) = 587 nM) [ 100 ].

Key stages in the hit to lead pathway of (+)-SJ733 . Poor metabolic stability of the hit compound was addressed by replacement of the chloro and methoxy groups with cyano and fluoro groups respectively. Further in vivo stability and solubility improvements were made by changing the thiophene to a pyridine. Finally, the gem -dimethyl group was substituted with a trifluoromethyl group to eliminate possible metabolic oxidation
In the P. berghei mouse model, (+)-SJ733 was found able to cure malaria at doses of 4 \(\times\) 100 mg/kg and has an ED \(_{90}\) of 1.9 mg/kg. It has shown transmission blocking activity in infected mice with an ED \(_{50}\) of 5 mg/kg. It possesses a good safety profile with no cytotoxicity and was found to be more potent in vivo when compared to existing anti-malarials such as artesunate, chloroquine, and pyrimethamine [ 101 ].
The molecular target of (+)-SJ733 has been identified as the P-type Na \(^+\) –ATPase transporter ( Pf ATP4, vide infra ), which has been implicated as a target for a number of other structurally diverse compounds [ 102 ]. The compound is currently in the recruiting stage of first-in-human study trials (NCT02661373).
ACT-451840 (Fig. 22 ) is a phenylalanine-based compound that was developed in 2016 through a collaboration between Actelion Pharmaceuticals and the Swiss Tropical and Public Health Institute (STPHI). It has the potential to be a fast-acting drug with a long half-life and has shown efficacy against multiple stages of the P. falciparum parasite [ 103 ].
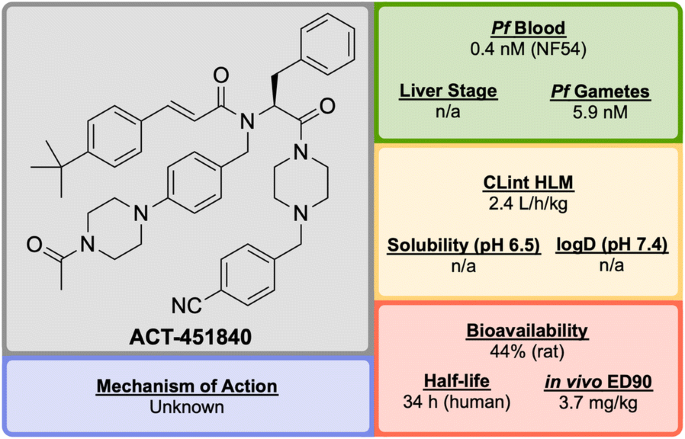
Key biological and physical properties of ACT-451840
Initial erythrocyte-based phenotypic screening of \(\sim\) 5000 compounds identified A′1 (Fig. 23 ) as a highly potent compound against the chloroquine-resistant K1 strain of P. falciparum (IC \(_{50}\) = 3.8 nM). The SAR studies in this project were unique in that the anti-malarial activity was measured in parallel in two different media: 10% bovine serum albumin and 50% human serum, with the latter used to help identify any potential problems with protein binding at an early stage of the optimization. The stereogenic centre of the amino acid residue proved to be important for the activity, with the ( S )-isomer showing more than tenfold higher activity compared to the non-natural ( R )-isomer. Modification of the n -pentyl chain to an acylpiperazine group ( A′2 ) resulted in an improvement in the physical and chemical properties of the compound. Replacement of the trifluoromethyl group with a tert -butyl group ( A′3 ) led to improved anti-malarial activity, especially in the presence of human serum proteins. Final installation of a 4-cyano moiety on the southern phenyl ring gave the highly potent (IC \(_{50}\) = 0.4 nM) lead candidate ACT-451840 .
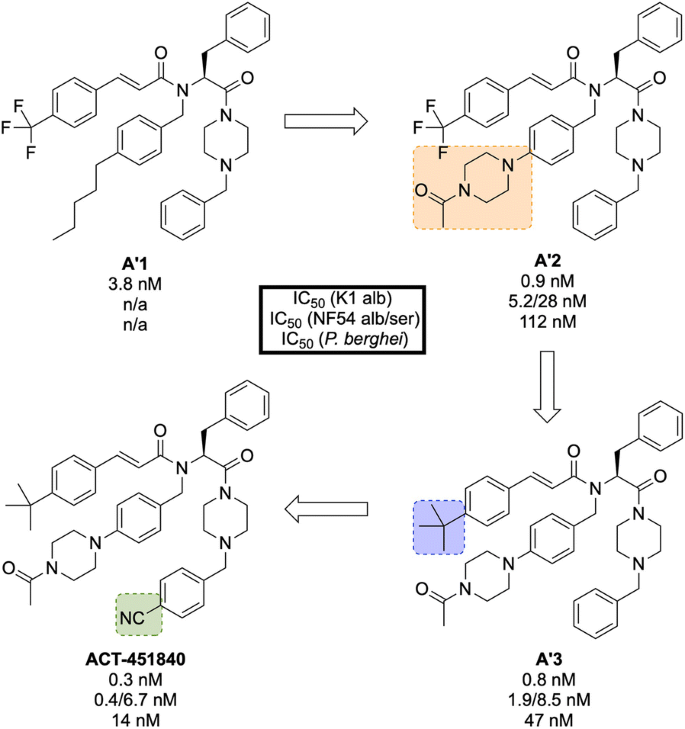
Key stages in the hit to lead pathway of ACT-451840 . Initial change of the n -pentyl group to an acylpiperazine ( A′2 ) helped to improve the physicochemical properties. Subsequent introduction of a tert -butyl in place of the trifluoromethyl ( A′3 ) and a cyano group on the southern phenyl ring resulted in the optimized compound
Interestingly, all compounds were found to be significantly less active against P. berghei than the human parasite. This is notable since most new anti-malarial drugs in development have shown similar potency against both rodent and human parasites. As a result, for in vivo studies it was crucial to use a humanized P. falciparum severe combined immunodeficiency (SCID) mouse model. In the P. berghei mouse model, ACT-451840 showed the ability to cure malaria at doses of 3 \(\times\) 300 mg/kg with an ED \(_{90}\) of 13 mg/kg. In the P. falciparum SCID mouse model it had an ED \(_{90}\) of 3.7 mg/kg. The importance of the delivery system for ACT-451840 was shown through in vivo experiments: a 60 mg dose in corn oil was as effective as a 100 mg dose in a mixture of Tween-EtOH/water = 10:90.
ACT-451840 has shown activity against multiple parasite life cycle stages of both P. falciparum and P. vivax [ 104 ]. The MoA is suspected to be novel but is currently unknown. ACT-451840 last completed first-in-human studies in 2014 (NCT02223871) [ 105 ] and is currently awaiting a decision to proceed.
Product development (patient exploratory)
Discovered in 2011 by a partnership between Monash University, the University of Nebraska and the STPHI, OZ439 (Fig. 24 ) is a synthetic trioxolane that possesses fast-acting curative and transmission-blocking ability, and is active against artemisinin-resistant parasites [ 106 ].
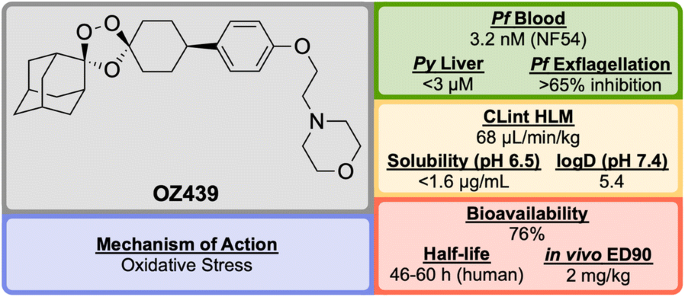
Key biological and physical properties of OZ439 . In vivo ED 90 kindly provided by S. Wittlin, personal communication, 2018
The discovery of OZ439 (a.k.a. Artefenomel) stems from the lead optimization of the pre-existing trioxolane OZ277 ( O1 , Fig. 25 , a.k.a. Arterolane), which was discovered in 2004 [ 107 , 108 ]. As previous studies have shown that the adamantane-peroxide moiety is essential for anti-malarial activity, studies were focused on the eastern portion of the molecule [ 109 , 110 , 111 ]. Notably, analogues with variation in this area were found to have largely similar in vitro activities. In order to improve upon the original lead compound OZ277 (mean survival 11 days, 0 from 5 mice cured), replacement of the amide linker with a phenyl ether linker ( O2 ) led to improved exposure and single-dose curative efficacy (mean survival > 30 days, 5 from 5 mice cured when applied in single dose of 30 mg/kg 1 day after infection). An attempt to further improve the exposure saw replacement of the alkylamine chain with a terminal piperazine unit ( O3 ), however this led to a significant reduction in curative efficacy (mean survival 17 days, 0 from 5 mice cured). Encouragingly, using morpholine ( OZ439 ) resulted in a mean survival of > 30 days, with 5 from 5 mice cured [ 112 ]. While OZ439 has shown excellent curative efficacy, no clear correlations were found between in vitro and in vivo activity or physiochemical properties and exposure. As a result, this new lead compound possess significantly lower solubility and slightly lower potency than OZ277 .
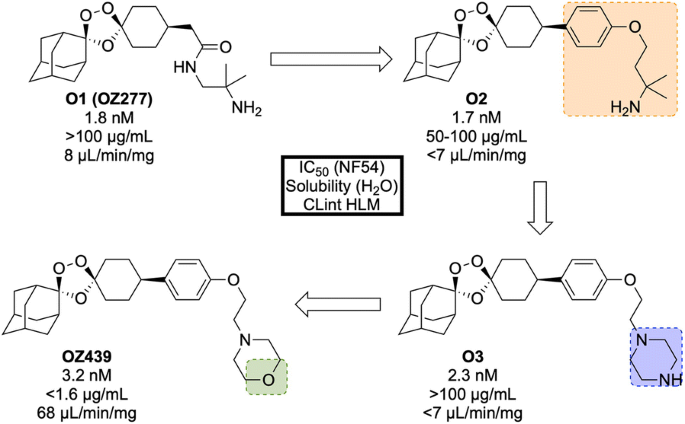
Key stages in the hit to lead pathway of OZ439 . Initial replacement of the amide linker with a phenyl ether linker resulted in improved exposure while maintaining potency ( O2 ). The exposure was further improved by changing the alkylamine chain to a piperazine ring ( O3 ). Final replacement of the piperazine ring with a morpholine unit led to the optimized compound OZ439 , which possessed better curative efficacy in vivo
Unlike other peroxide-containing anti-malarials (e.g. artemisinin), OZ439 was found to be curative at a single dose of 20 mg/kg in the P. berghei mouse model and possessed prophylactic activity superior to mefloquine when a 30 mg/kg dose was applied 24 h prior to malaria infection. OZ439 also showed full anti-malarial protection when administered 96 h before infection at a dose of 100 mg/kg. Only minor signs of toxicity were observed in a rat model when five consecutive doses of 300 mg/kg were administered with 3 days interval. Conversely, O2 resulted in the death of 9 from 12 animals when used at the same dose [ 112 ]. OZ439 has shown a significantly longer half-life in humans (about 46–62 h) when compared to the hit compound OZ277 (3 h) [ 113 , 114 , 115 ].
Much like the current peroxide-containing anti-malarials, the precise MoA for OZ439 has yet to be discovered but it is believed that oxidative stress plays a major role [ 116 , 117 ]. First-in-human results for OZ439 were published in 2013 [ 118 ] and since then the molecule has progressed into Phase IIb clinical trials with planned completion in 2019 (NCT02497612) [ 119 ].
KAF156 (Fig. 26 ) was identified in 2008 by Novartis and The Scripps Research Institute and is part of the second-generation of imidazolopiperazine anti-malarials that potentially possesses a novel MoA and performs as a rapid-acting drug [ 120 ].
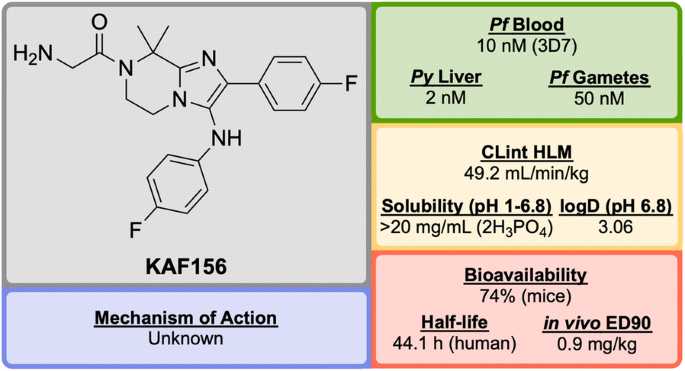
Key biological and physical properties of KAF156 . Solubility and logD kindly provided by T. Diagana, personal communication, 2018
A high-throughput screen of 1.7 million compounds in a P. falciparum proliferation assay led to the identification of hit compound K1 (Fig. 27 ). In order to protect the metabolically vulnerable positions in the lead compound and to optimize the potency, the benzodioxole and phenyl fragments were replaced with 4-fluorobenzene groups ( K2 ). Installation of a dimethyl group in the piperazine ring led to the twofold more potent compound KAF156 [ 121 , 122 ]. Notably, removal of the glycine residue from the piperazine ring was shown to increase the in vitro potency further (EC \(_{50}\) = 5 nM), but this was accompanied by a lower parasitaemia reduction, indicating the importance of the amino acid side chain for in vivo efficacy [ 121 ].
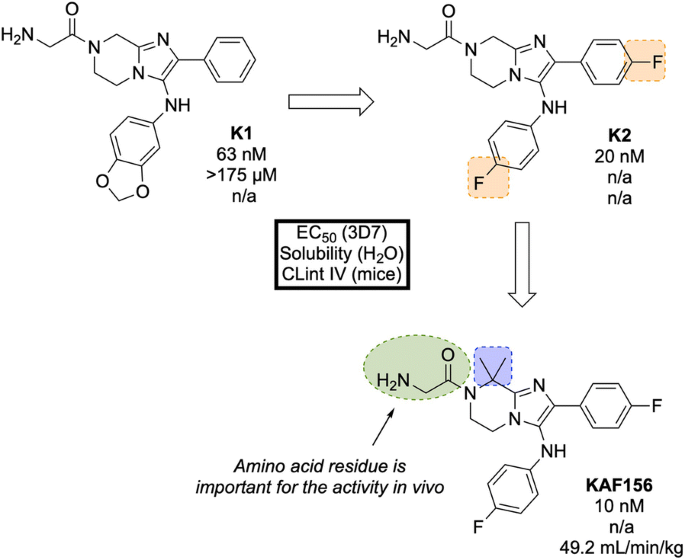
Key stages in the hit to lead pathway of KAF156 . Potential metabolic stability issues were addressed through fluorine bioisosteres giving K2 . Introduction of a dimethyl group on the piperazine ring resulted in increased potency and led to the optimized compound
In the causal prophylactic rodent malaria model, a single oral dose of 10 mg/kg administered 2 h before intravenous infection with P. berghei sporozoites was shown to be fully protective. KAF156 has also shown transmission blocking ability in the P. berghei model.
The MoA for KAF156 remains unknown. Through the culturing of resistant strains, mutations have been identified in three genes: P. falciparum Cyclic Amine Resistance Locus ( Pf CARL), UDP-galactose and Acetyl-CoA transporters [ 58 , 123 ]. KAF156 is currently in Phase IIb clinical trials, administered in combination with Lumefantrine (NCT03167242).
KAE609/Cipargamin/NITD609 (Fig. 28 ) was discovered by a partnership between Novartis, the STPHI and the Wellcome Trust [ 124 , 125 ].
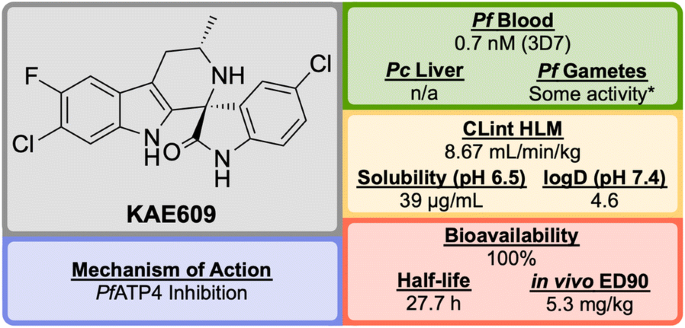
Key biological and physical properties of KAE609 . *Significant inhibition at 50 and 500 nM doses
A high-throughput P. falciparum proliferation assay identified a racemic spiroazepineindole compound ( K′1 , Fig. 29 ) which possessed moderate potency against K1 and NF54 strains, as well as potent in vivo efficacy. Confirmation of the hit result by resynthesis of K′1 resulted in the isolation of a \(\sim\) 9:1 diastereomeric ratio favouring the (1 R ,3 S ) and (1 S ,3 R ) pair of enantiomers. Chiral separation of this major pair identified the (1 R ,3 S ) enantiomer K′2 as being > 250-fold more potent than the (1 S ,3 R ) enantiomer. Reduction of the ring size ( K′3 ) facilitated a diastereoselective Pictet–Spengler reaction, which ultimately led to the production of highly potent spiroindolone KAE609 .
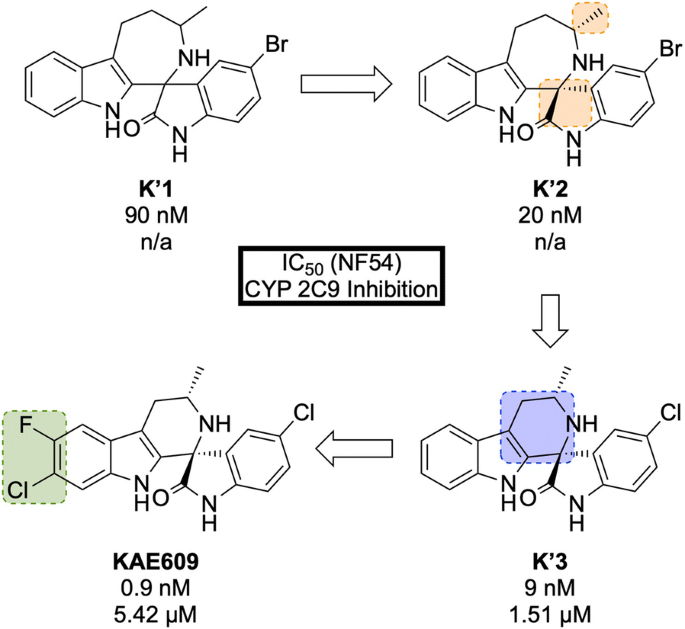
Key stages in the hit to lead pathway of KAE609 . Resolution of the initial racemic hit ( K′1 ) gave the significantly more potent stereoisomer ( K′2 ). Reducing the ring size further increased the potency and final halogenation of the indole ring led to the optimized compound KAE609
KAE609 is equipotent against drug-resistant strains and was found to be as effective as artesunate against P. falciparum and P. vivax isolates [ 126 ]. It shows a good safety profile, with low cytotoxicity, cardiotoxicity and mutagenic activity, and is able to clear parasitaemia rapidly in adults with uncomplicated P. falciparum or P. vivax malaria at a dose of 30 mg/day for 3 days. KAE609 displays low clearance from the body, has a long half-life and excellent bioavailability.
KAE609 is one of the key novel compounds that has been found to act through inhibition of Pf ATP4 ( vide infra ). Along with a number of other structurally distinct compounds that are also thought to inhibit Pf ATP4 as their MoA, KAE609 has shown great promise as an new anti-malarial candidate and is currently in Phase IIb trials (NCT03334747).
DSM265 (Fig. 30 ) was discovered through a collaboration between the University of Texas Southwestern, the University of Washington and Monash University [ 127 ].
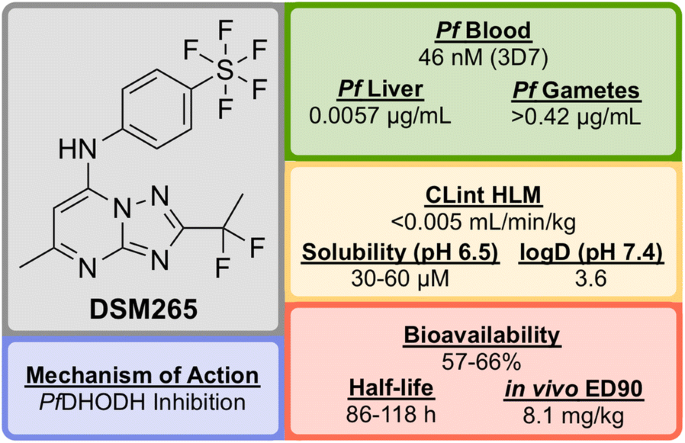
Key biological and physical properties of DSM265
Identified in a high-throughput screen of P. falciparum dihydroorotate dehydrogenase ( Pf DHODH)-based enzyme activity, the selective inhibitor D′1 (Fig. 31 ) was found. The compound showed high potency but was rapidly metabolized and was inactive in vivo. Substitution of the naphthyl ring with a trifluoromethylphenyl group led to a metabolically stable analogue ( D′2 ). Examination of the bound crystal structures of D′1 and D′2 with Pf DHODH identified key residue interactions which eventually led to the installation of a difluoroethyl group ( D′3 ) which significantly improved the potency and solubility. Final modification of the trifluoromethyl group with a pentafluorosulfur group led to the optimized compound DSM265 .
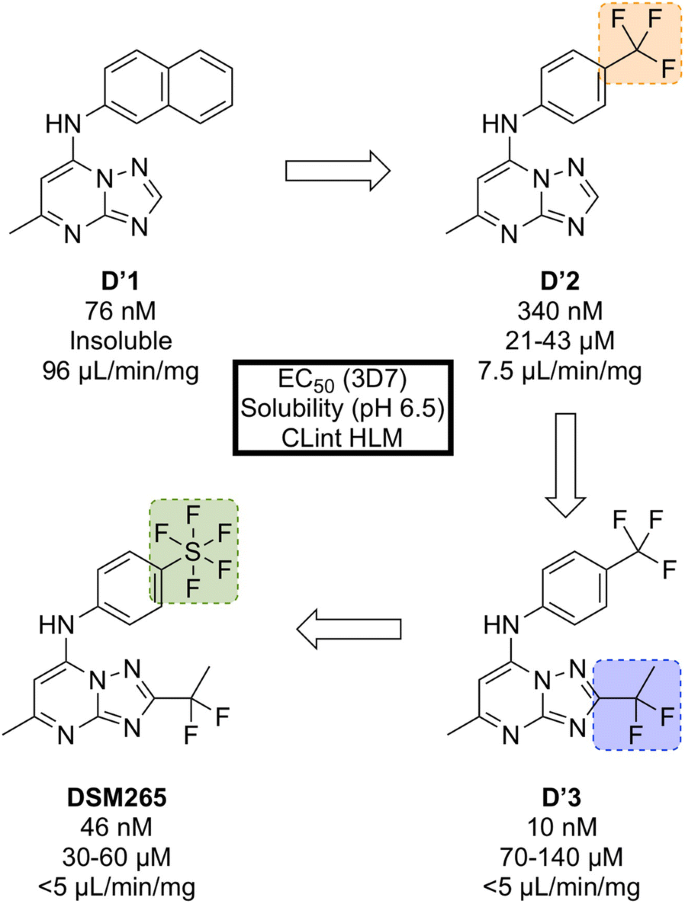
Key stages in the hit to lead pathway of DSM265 . Replacement of the second phenyl ring in the naphthyl group with a trifluoromethyl group improved solubility. Addition of a 1,1-difluoroethyl group significantly increased the potency and final replacement of the trifluoromethyl group in D′2 with a pentafluorosulfur moiety led to the optimized compound DSM265
DSM265 has been shown to be a highly selective inhibitor of malarial DHODH and is potent against both blood and liver stages of P. falciparum and also drug-resistant parasite isolates. It has an excellent safety profile (provides therapeutic concentrations after 8 days of a single oral dose, tolerated in repeat-doses, non-mutagenic, inactive against human enzymes/receptors) and has a very low clearance rate and a long half-life in humans [ 128 ]. DSM265 has completed Phase IIa trials in Peru in patients with P. falciparum or P. vivax (NCT02123290) and also completed a controlled human malaria infection study in combination with OZ439 (NCT02389348).
Identified in 2012 by a team at the University of Cape Town, South Africa in the same campaign as UCT943, MMV048 (Fig. 32 ) is another compound in the 3,5-diaryl-2-aminopyridine class that possesses good prophylactic activity against Plasmodium cynomolgi in vivo and has the potential to act as a transmission-blocking drug [ 84 ].
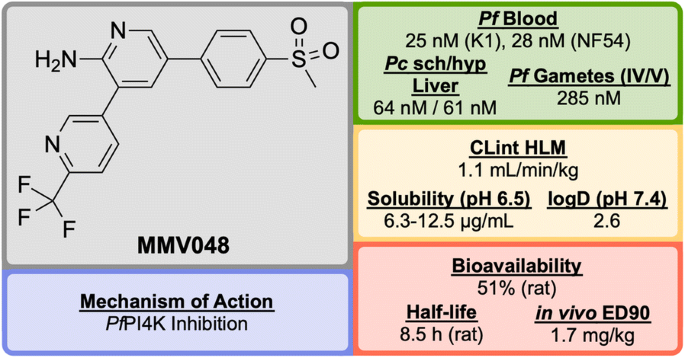
Key biological and physical properties of MMV048
The initial hit ( M″1 , Fig. 33 ) showed potent in vitro activity against the NF54 (drug-sensitive) strain of P. falciparum (IC \(_{50}\) = 49 nM) but suffered from poor solubility and high metabolic clearance. The metabolically labile 3-methoxy-4-hydroxyphenyl moiety was replaced by a methoxypyridyl ring giving the more stable and equipotent compound M″2 . Further SAR studies revealed that replacement of the methoxy group by a trifluoromethyl group led to the improved potency and stability of MMV048 .
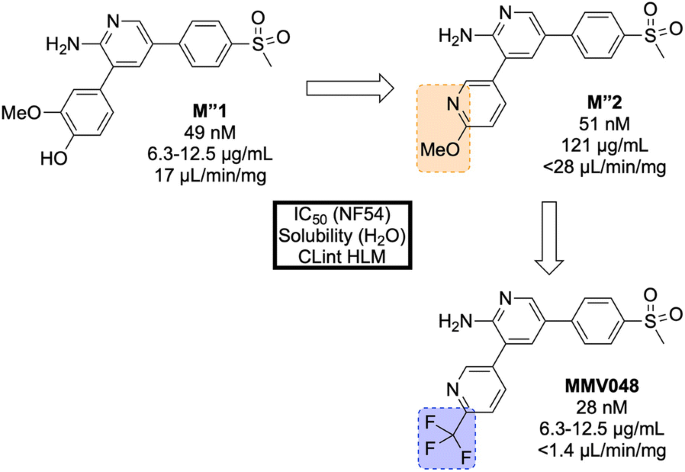
Key stages in the hit to lead pathway of MMV048 . Initial replacement of the 3-methoxy-4-hydroxyphenyl moiety helped to improve in vivo stability and solubility. Further replacement of the methoxy group with a trifluoromethyl group improved potency and metabolic stability leading to the optimized compound
MMV048 has shown 99.3% reduction in parasitaemia in the P. berghei mouse model at a single dose of 30 mg/kg with no signs of parasites after 30 days (ED \(_{90}\) = 1.7 mg/kg). This highlights the potential of MMV048 to act as a single dose treatment.
The target of MMV048 is Pf PI4K, which was recently revealed as a new MoA for anti-malarial drugs ( vide infra ) [ 87 ]. MMV048 is currently in Phase IIa clinical trials in Ethiopia [ 58 ].
Modes of action
The continual emergence of drug resistant Plasmodium strains means that developing new drugs with novel MoAs is important [ 129 ]. Below, are summarized some newer MoAs that are currently being pursued and which are exemplified by the compounds described above.
Translational elongation factor 2 ( Pf eEF2) inhibitor
The P. falciparum translational elongation factor 2 ( Pf eEF2) is one of the essential factors for eukaryotic protein synthesis, catalysing the translocation of tRNA and mRNA [ 130 ]. The overall efficacy of drugs that target this elongation factor may be increased due to Pf eEF2 being expressed in multiple stages of the parasite life-cycle [ 78 ].
M5717 has been shown to inhibit Pf eEF2 as its MoA, but a binding pocket for M5717 could not be elucidated from the modelling studies [ 78 ]. This MoA was found by culturing blood stage parasites with five times the EC \(_{50}\) of M5717 , leading to resistance in the 3D7, 7G8 and Dd2 strains. Through whole-genome sequencing of these resistance lines, a common mutation was found which identified Pf eEF2 as the target.
P-type Na \(^+\) –ATPase inhibitor ( Pf ATP4) inhibitor
The malaria parasite must maintain a low intracellular Na \(^+\) concentration to survive, especially in the presence of the high concentration of extracellular sodium ions. In this case, the parasite’s influx of Na \(^+\) is regulated by using a P-type ATPase transporter ( Pf ATP4) that shuttles sodium ions out of the cell. Inhibition of this transporter results in a build up of Na \(^+\) inside the parasite, ultimately leading to cell death [ 102 ].
A number of structurally diverse compounds (including (+)-SJ733 and KAE609 ) are thought to target Pf ATP4 as their MoA again suggested by the development of resistant mutants and sequencing [ 124 ]. The Kirk group have developed a fluorescence assay for this MoA [ 131 ], which has been used by the OSM project to implicate Pf ATP4 as a MoA for the Series 4 triazolopyrazines [ 132 ]. Of note, however, is that this MoA is suggested for a very broad range of chemotypes [ 133 , 134 ], and there is no direct evidence for binding of these compounds to Pf ATP4, the structure of which remains unsolved.
V-type H \(^+\) –ATPase inhibitor
At the same time that the parasite is regulating its Na \(^+\) concentration through the above mechanism, it also imports H \(^+\) through the same pathway. To regulate this increasing H \(^+\) concentration and maintain an intracellular pH of \(\sim\) 7.3, the parasite uses a complementary V-type ATPase transporter to efflux H \(^+\) [ 135 ].
MMV253 has been shown to inhibit the V-type H \(^+\) ATPase as its MoA through mutant selection and whole-genome sequencing [ 81 ].
Phosphatidylinositol 4-kinase ( Pf PI4K) inhibitor
Phosphatidylinositol 4-kinase (PI4K) is a eukaryotic enzyme that phosphorylates lipids, allowing them to regulate intracellular signalling and trafficking [ 87 ]. Inhibition of the ATP-binding pocket of PI4K leads to disruption of the intracellular distribution of PI4-phosphate (PI4P), which in turn results in decreased late-stage parasite development.
By culturing resistant lines and identifying the genomic mutations, two compounds ( UCT943 and MMV048 ) have been found to inhibit the Pf PI4K enzyme [ 87 ].
Dihydroorotate dehydrogenase ( Pf DHODH) inhibitor
To be able to replicate in the human erythrocyte, the Plasmodium parasite requires pyrimidines, which are essential metabolites in all cells. As the parasite is unable to use endogenous pyrimidines, it must synthesize them de novo. A key step in the biosynthesis of pyrimidines is the oxidation of dihydroorotate to produce orotate, a reaction that is catalysed by the enzyme dihydroorotate dehydrogenase (DHODH). By inhibiting this enzyme, the malaria parasite can no longer obtain the required metabolites to survive, and is killed [ 136 ].
The P. falciparum D10 strain (chloroquine sensitive) has been known to be less susceptible to bc 1 complex inhibitors and Pf DHODH inhibitors. Addition of proguanil to this strain is known to restore the sensitivity to bc 1 complex inhibitors but not to Pf DHODH inhibitors. This cell line was found to be resistant to DSM265 , regardless of whether proguanil was present or not, which identifies inhibition of Pf DHODH as its MoA [ 127 , 137 ].
Dihydrofolate reductase ( Pf DHFR) inhibitor
Similar to the above case, the malaria parasite also requires folates in order to maintain their high rate of replication. The parasite is able to scavenge folates or synthesize them de novo . Dihydrofolate reductase (DHFR) is an enzyme that catalyses a step required for the recycling of folates, which in turn are used in the synthesis of thymidylate, purines and methionine [ 138 ].
Pf DHFR is a known target of a number of well known antifolate anti-malarial drugs including cycloguanil and pyrimethamine. Through examinations of the key interactions in the cocrystal structure of known inhibitors with the enzyme, P218 was designed as a potent inhibitor [ 98 ].
Conclusions
Although the rate of malaria related deaths has declined over the past few years, the progress is beginning to slow. With the recent emergence of resistance to current front-line artemisinin-based combination therapy, the need for the discovery of new anti-malarials that can act through novel mechanisms of action has been pushed firmly to the top of the development agenda.
Over the past few years, high-throughput screens have identified a number of novel chemotypes that have since been developed into highly promising anti-malarial candidates. Crucially, many of these compounds have demonstrated novel MoAs which are essential if future drugs are to succeed. This is wonderful progress, and testament to the hard work of the groups involved.
The discovery of these novel MoAs will pave the way for the development of future anti-malarials. The exploration of further novel MoAs has become a possibility through the use of in vitro evolution and whole-genome analysis (IVIEWGA), which uses genome-base target discovery methods on compounds identified from the more common phenotypic screens [ 139 ]. In all such future development, creativity and ingenuity in the hit to lead campaigns will continue to be needed if people are to remain able to combat this formidable disease.
The pace of research progress is high, meaning updates to reviews such as this will always be needed (Additional file 3 ). It is proposed that derivatives of this open access review, combined with data in relevant databases such as the one currently in development by the Guide to Pharmacology [ 140 ], could aim to serve as more “living” sources of information for compounds in development and their potential targets.
Abbreviations
artemisinin-based combination therapy
cycloguanil
intrinsic clearance
chloroquine
Drug Discovery Unit
half maximal effective concentration
90% effective dose
GlaxoSmithKline
human ether-à-go-go-related gene
human liver microsome
half maximal inhibitory concentration
intraperitoneally
in vitro evolution and whole-genome analysis
in vitro-in vivo correlation
Model List of Essential Medicines
mouse liver microsome
Medicines for Malaria Venture
mode/mechanism of action
messenger ribonucleic acid
methotrexate
Open Source Malaria
(per os) orally
Plasmodium berghei
Plasmodium cynomolgi
Plasmodium falciparum
Plasmodium falciparum ATPase transporter
Plasmodium falciparum cyclic amine resistance locus
Plasmodium falciparum cleavage and polyadenylation specificity factor
Plasmodium falciparum dihydrofolate reductase
Plasmodium falciparum dihydroorotate dehydrogenase
Plasmodium falciparum translational elongation factor 2
Plasmodium falciparum phosphatidylinositol-3-kinase
Plasmodium falciparum phosphatidylinositol-4-OH kinase
Plasmodium yoelli
pyrimethamine
( quaque die ) one a day
reversed chloroquine(s)
structure activity relationship
severe combined immunodeficiency
Swiss Tropical and Public Health Institute
triaminopyrimidine
tafenoquine
transfer ribonucleic acid
World Health Organization
World Malaria Report 2018. https://www.who.int/malaria/publications/world-malaria-report-2018/en/ . Accessed 27 Nov 2018.
Cheney C. Fight against malaria stalling and could reverse, warns 2017 World Malaria Report. http://theconversation.com/global-malaria-report-reveals-africas-hits-and-misses-heres-what-to-do-88577 . Accessed 24 Mar 2018.
Mwangi T. Global malaria report reveals Africa’s hits and missed: here’s what to do. https://www.devex.com/news/fight-against-malaria-stalling-and-could-reverse-warns-2017-world-malaria-report-91636 . Accessed 24 Mar 2018.
Shanks GD, Edstein MD, Jacobus D. Evolution from double to triple-antimalarial drug combinations. Trans R Soc Trop Med Hyg. 2014;109:182–8.
Article PubMed CAS Google Scholar
Mishra M, Mishra VK, Kashaw V, Iyer AK, Kashaw SK. Comprehensive review on various strategies for antimalarial drug discovery. Eur J Med Chem. 2017;125:1300–20.
Article CAS PubMed Google Scholar
Phillips MA, Burrows JN, Manyando C, van Huijsduijnen RH, Voorhis WCV, Wells TNC. Malaria. Nat Rev Dis Primers. 2017;3:17050.
Article PubMed Google Scholar
Okombo J, Chibale K. Recent updates in the discovery and development of novel antimalarial drug candidates. MedChemComm. 2018;9:437–53.
Article CAS PubMed PubMed Central Google Scholar
Ashley EA, Phyo AP. Drugs in development for malaria. Drugs. 2018;78:861–79.
Medicines for Malaria Venture. https://www.mmv.org/ . Accessed 24 Mar 2018.
Williamson AE, Ylioja PM, Robertson MN, Antonova-Koch Y, Avery V, Baell JB, et al. Open source drug discovery: highly potent antimalarial compounds derived from the Tres Cantos arylpyrroles. ACS Cent Sci. 2016;2:687–701.
Leong FJ, Li R, Jain JP, Lefèvre G, Magnusson B, Diagana TT, et al. A first-in-human randomized, double-blind, placebo-controlled, single- and multiple-ascending oral dose study of novel antimalarial spiroindolone KAE609 (Cipargamin) to assess its safety, tolerability, and pharmacokinetics in healthy adult volunteers. Antimicrob Agents Chemother. 2014;58:6209–14.
Article PubMed PubMed Central CAS Google Scholar
Achan J, Talisuna AO, Erhart A, Yeka A, Tibenderana JK, Baliraine FN, et al. Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria. Malar J. 2011;10:144.
Bunnag D, Karbwang J, Na-Bangchang K, Thanavibul A, Chittamas S, Harinasuta T. Quinine-tetracycline for multidrug resistant falciparum malaria. Southeast Asian J Trop Med Public Health. 1996;27:15–8.
CAS PubMed Google Scholar
Model List of Essential Medicines. http://www.who.int/medicines/publications/essentialmedicines/en/ . Accessed 24 Mar 2018.
Green R. A report on fifty cases of malaria treated with Atebrin. A new synthetic drug. Lancet. 1932;219:826–9.
Article Google Scholar
Guttman P, Ehrlich P. Ueber die wirkung des methylenblau bei malaria. Berl Klin Wochenschr. 1891;28:953–6.
Google Scholar
Schirmer RH, Coulibaly B, Stich A, Scheiwein M, Merkle H, Eubel J, et al. Methylene blue as an antimalarial agent. Redox Rep. 2003;8:272–5.
Lu G, Nagbanshi M, Goldau N, Jorge MM, Meissner P, Jahn A, et al. Efficacy and safety of methylene blue in the treatment of malaria: a systematic review. BMC Med. 2018;16:59.
Weina PJ. From atabrine in World War II to mefloquine in Somalia: the role of education in preventive medicine. Mil Med. 1998;163:635–9.
Loeb F. Activity of a new antimalarial agent, chloroquine (SN 7618). JAMA. 1946;130:1069–70.
Mushtaque M. Reemergence of chloroquine (CQ) analogs as multi-targeting antimalarial agents: a review. Eur J Med Chem. 2015;90:280–95.
Trenholme C, Williams R, Desjardins R, Frischer H, Carson P, Rieckmann K, et al. Mefloquine (WR 142,490) in the treatment of human malaria. Science. 1975;190:792–4.
Brasseur P, Druilhe P, Kouamouo J, Brandicourt O, Danis M, Moyou SR. High level of sensitivity to chloroquine of 72 Plasmodium falciparum isolates from southern Cameroon in January 1985. Am J Trop Med Hyg. 1986;35:711–6.
Foley M, Tilley L. Quinoline antimalarials: mechanisms of action and resistance. Int J Parasitol. 1997;27:231–40.
Nevin RL, Croft AM. Psychiatric effects of malaria and anti-malarial drugs: historical and modern perspectives. Malar J. 2016;15:332.
Article PubMed PubMed Central Google Scholar
Cosgriff TM, Desjardins RE, Pamplin CL, Canfield CJ, Doberstyn EB, Boudreau EF. Evaluation of the antimalarial activity of the phenanthrenemethanol halofantrine (WR 171,669)*. Am J Trop Med Hyg. 1982;31:1075–9.
Croft AM. A lesson learnt: the rise and fall of Lariam and Halfan. J R Soc Med. 2007;100:170–4.
Qinghaosu Antimalaria Coordinating Research Group. Antimalarial studies on Qinghaosu. Chin Med J (Engl ). 1979;92:811–6.
The Nobel Prize in Physiology or Medicine 2015. https://www.nobelprize.org/nobel_prizes/medicine/laureates/2015/ . Accessed 24 Mar 2018.
Eastman RT, Fidock DA. Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nat Rev Microbiol. 2009;7:864–74.
Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin-resistant aalaria in western Cambodia. N Engl J Med. 2008;359:2619–20.
Amato R, Pearson RD, Almagro-Garcia J, Amaratunga C, Lim P, Suon S, et al. Origins of the current outbreak of multidrug-resistant malaria in southeast Asia: a retrospective genetic study. Lancet Infect Dis. 2018;18:337–45.
O’Neill PM, Barton VE, Ward SA. The molecular mechanism of action of artemisinin—the debate continues. Molecules. 2010;15:1705–21.
Wang J, Zhang CJ, Chia WN, Loh CCY, Li Z, Lee YM, et al. Haem-activated promiscuous targeting of artemisinin in Plasmodium falciparum . Nat Commun. 2015;6:10111.
Tilley L, Straimer J, Gnädig NF, Ralph SA, Fidock DA. Artemisinin action and resistance in Plasmodium falciparum . Trends Parasitol. 2016;32:682–96.
Shandilya A, Chacko S, Jayaram B, Ghosh I. A plausible mechanism for the antimalarial activity of artemisinin: a computational approach. Sci Rep. 2013;3:2513.
Mok S, Ashley EA, Ferreira PE, Zhu L, Lin Z, Yeo T, et al. Population transcriptomics of human malaria parasites reveals the mechanism of artemisinin resistance. Science. 2014;347:431–5.
Mbengue A, Bhattacharjee S, Pandharkar T, Liu H, Estiu G, Stahelin RV, et al. A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature. 2015;520:683–7.
Berliner RW, Earle DP, Taggart JV, Zubrod CG, Welch WJ, Conan NJ, et al. Studies on the chemotheraphy of the human malarias. VI. The physiological disposition, antimalarial activity, and toxicity of several derivatives of 4-aminoquinoline. J Clin Invest. 1948;27:98–107.
Bompart F, Kiechel JR, Sebbag R, Pecoul B. Innovative public-private partnerships to maximize the delivery of anti-malarial medicines: lessons learned from the ASAQ Winthrop experience. Malar J. 2011;10:143.
Combrinck JM, Mabotha TE, Ncokazi KK, Ambele MA, Taylor D, Smith PJ, et al. Insights into the role of heme in the mechanism of action of antimalarials. ACS Chem Biol. 2012;8:133–7.
Chen L, Qu FY, Zhou YC. Field observations on the antimalarial piperaquine. Chin Med J (Engl ). 1982;95:281–6.
Vennerstrom JL, Ellis WY, Ager AL, Andersen SL, Gerena L, Milhous WK. Bisquinolines. 1. N , N -Bis(7-chloroquinolin-4-yl)alkanediamines with potential against chloroquine-resistant malaria. J Med Chem. 1992;35:2129–34.
Cui L, zhuan Su X. Discovery, mechanisms of action and combination therapy of artemisinin. Expert Rev Anti Infect Ther. 2009;7:999–1013.
Curd FHS, Davey DG, Rose FL. Studies on synthetic antimalarial drugs. Ann Trop Med Parasitol. 1945;39:208–16.
Hudson AT, Randall AW. Naphthoquinone derivatives. US5053432A; 1991.
Fry M, Pudney M. Site of action of the antimalarial hydroxynaphthoquinone, 2-[trans-4-(4’-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone (566C80). Biochem Pharmacol. 1992;43:1545–53.
Srivastava IK, Vaidya AB. A mechanism for the synergistic antimalarial action of atovaquone and proguanil. Antimicrob Agents Chemother. 1999;43:1334–9.
Russell PB, Hitchings GH. 2,4-Diaminopyrimidines as antimalarials. III. 5-Aryl derivatives. J Am Chem Soc. 1951;73:3763–70.
Article CAS Google Scholar
The Nobel Prize in Physiology or Medicine 1988. https://www.nobelprize.org/nobel_prizes/medicine/laureates/2015/ . Accessed 19 June 2018.
Laing AB. Treatment of acute falciparum malaria with sulphorthodimethoxine (fansil). Br Med J. 1965;1:905–7.
Lumb V, Das MK, Singh N, Dev V, Khan W, Sharma YD. Multiple origins of Plasmodium falciparum dihydropteroate synthetase mutant alleles associated with sulfadoxine resistance in India. Antimicrob Agents Chemother. 2011;55:2813–7.
Zheng XY, Xia Y, Gao FH, Chen C. Synthesis of 7351, a new antimalarial drug. Yao Xue Xue Bao. 1979;14:736–7.
Chang C, Lin-Hua T, Jantanavivat C. Studies on a new antimalarial compound: pyronaridine. Trans R Soc Trop Med Hyg. 1992;86:7–10.
Croft SL, Duparc S, Arbe-Barnes SJ, Craft J, Shin CS, Fleckenstein L, et al. Review of pyronaridine anti-malarial properties and product characteristics. Malar J. 2012;11:270.
US FDA approves Krintafel (tafenoquine) for the radical cure of P. vivax malaria 2018. https://www.mmv.org/newsroom/press-releases/us-fda-approves-krintafel-tafenoquine-radical-cure-p-vivax-malaria . Accessed 25 Sept 2018.
Ebstie YA, Abay SM, Tadesse WT, Ejigu DA. Tafenoquine and its potential in the treatment and relapse prevention of Plasmodium vivax malaria: the evidence to date. Drug Des Devel Ther. 2016;10:2387–99.
MMV-Supported Projects. https://www.mmv.org/research-development/mmv-supported-projects . Accessed 14 Jun 2018.
Wells TNC, Gutteridge WE. Neglected diseases and drug discovery. Palmer MJ, Wells TNC, editors. RSC; 2012.
Burrows JN, Duparc S, Gutteridge WE, van Huijsduijnen RH, Kaszubska W, Macintyre F, et al. New developments in anti-malarial target candidate and product profiles. Malar J. 2017;16:26.
McChesney JD, Nanayakkara DNP, Bartlett M, Ager AL. Preparation of 8-(aminoalkylamino)quinolines as parasiticides. WO 9736590 A1; 1997.
Tekwani BL, Walker LA. 8-Aminoquinolines: future role as antiprotozoal drugs. Curr Opin Infect Dis. 2006;19:623–31.
Nanayakkara NPD, Ager AL, Bartlett MS, Yardley V, Croft SL, Khan IA, et al. Antiparasitic activities and toxicities of individual enantiomers of the 8-aminoquinoline 8-[(4-amino-1-methylbutyl)amino]-6-methoxy-4-methyl-5-[3,4-dichlorophenoxy]quinoline succinate. Antimicrob Agents Chemother. 2008;52:2130–7.
Marcsisin SR, Sousa JC, Reichard GA, Caridha D, Zeng Q, Roncal N, et al. Tafenoquine and NPC-1161B require CYP 2D metabolism for anti-malarial activity: implications for the 8-aminoquinoline class of anti-malarial compounds. Malar J. 2014;13:2.
Powles MA, Allocco J, Yeung L, Nare B, Liberator P, Schmatz D. MK-4815, a potential new oral agent for treatment of malaria. Antimicrob Agents Chemother. 2012;56:2414–9.
Singh C, Puri SK. Substituted 1,2,4-trioxanes as antimalarial agents and a process of producing the substituted 1,2,4-trioxanes. US6316493 B1; 2001.
Shafiq N, Rajagopalan S, Kushwaha HN, Mittal N, Chandurkar N, Bhalla A, et al. Single ascending dose safety and pharmacokinetics of CDRI-97/78: first-in-human study of a novel antimalarial drug. Malar Res Treat. 2014;2014:372521.
CAS PubMed PubMed Central Google Scholar
O’Neill PM, Shone AE, Stanford D, Nixon G, Asadollahy E, Park BK, et al. Synthesis, antimalarial activity, and preclinical pharmacology of a novel series of 4’-fluoro and 4’-chloro analogues of amodiaquine. Identification of a suitable “back-up” compound for N-tert -butyl isoquine. J Med Chem. 2009;52:1828–44.
Bora S, Chetia D, Prakash A. Synthesis and antimalarial activity evaluation of some isoquine analogues. Med Chem Res. 2010;20:1632–7.
O’Neill PM, Park BK, Shone AE, Maggs JL, Roberts P, Stocks PA, et al. Candidate selection and preclinical evaluation of N -tert-butyl isoquine (GSK369796), an affordable and effective 4-aminoquinoline antimalarial for the 21st century. J Med Chem. 2009;52:1408–15.
Haynes RK, Fugmann B, Stetter J, Rieckmann K, Heilmann HD, Chan HW, et al. Artemisone—a highly active antimalarial drug of the artemisinin class. Angew Chem Int Ed. 2006;45:2082–8.
Nicolas O, Margout D, Taudon N, Wein S, Calas M, Vial HJ, et al. Pharmacological properties of a new antimalarial bisthiazolium salt, T3, and a corresponding prodrug, TE3. Antimicrob Agents Chemother. 2005;49:3631–9.
Drake NL, Creech HJ, Garman JA, Haywood ST, Peck RM, van Hook JO, et al. Synthetic antimalarials. The preparation of certain 4-aminoquinolines \(^{1}\) . J Am Chem Soc. 1946;68:1208–13.
Ramanathan-Girish S, Catz P, Creek MR, Wu B, Thomas D, Krogstad DJ, et al. Pharmacokinetics of the antimalarial drug, AQ-13, in rats and cynomolgus macaques. Int J Toxicol. 2004;23:179–89.
Sáenz FE, Mutka T, Udenze K, Oduola AMJ, Kyle DE. Novel 4-aminoquinoline analogs highly active against the blood and sexual stages of Plasmodium in vivo and in vitro. Antimicrob Agents Chemother. 2012;56:4685–92.
Mzayek F, Deng H, Mather FJ, Wasilevich EC, Liu H, Hadi CM, et al. Randomized dose-ranging controlled trial of AQ-13, a candidate antimalarial, and chloroquine in healthy volunteers. PLoS Clin Trials. 2007;2:e6.
Koita OA, Sangaré L, Miller HD, Sissako A, Coulibaly M, Thompson TA, et al. AQ-13, an investigational antimalarial, versus artemether plus lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria: a randomised, phase 2, non-inferiority clinical trial. Lancet Infect Dis. 2017;17:1266–75.
Baragaña B, Hallyburton I, Lee MCS, Norcross NR, Grimaldi R, Otto TD, et al. A novel multiple-stage antimalarial agent that inhibits protein synthesis. Nature. 2015;522:315–20.
Baragaña B, Norcross NR, Wilson C, Porzelle A, Hallyburton I, Grimaldi R, et al. Discovery of a quinoline-4-carboxamide derivative with a novel mechanism of action, multistage antimalarial activity, and potent in vivo efficacy. J Med Chem. 2016;59:9672–85.
Burrows JN, van Huijsduijnen RH, Möhrle JJ, Oeuvray C, Wells TN. Designing the next generation of medicines for malaria control and eradication. Malar J. 2013;12:187.
Hameed PS, Solapure S, Patil V, Henrich PP, Magistrado PA, Bharath S, et al. Triaminopyrimidine is a fast-killing and long-acting antimalarial clinical candidate. Nat Commun. 2015;6:6715.
MMV and Zydus join forces to develop new antimalarial 2017. https://www.mmv.org/newsroom/press-releases/mmv-and-zydus-join-forces-develop-new-antimalarial . Accessed 17 June 2018.
Manach CL, Nchinda AT, Paquet T, Cabrera DG, Younis Y, Han Z, et al. Identification of a potential antimalarial drug candidate from a series of 2-aminopyrazines by optimization of aqueous solubility and potency across the parasite life cycle. J Med Chem. 2016;59:9890–905.
Younis Y, Douelle F, Feng TS, Cabrera DG, Manach CL, Nchinda AT, et al. 3,5-Diaryl-2-aminopyridines as a novel class of orally active antimalarials demonstrating single dose cure in mice and clinical candidate potential. J Med Chem. 2012;55:3479–87.
Cabrera DG, Douelle F, Younis Y, Feng TS, Manach CL, Nchinda AT, et al. Structure-activity relationship studies of orally active antimalarial 3,5-substituted 2-aminopyridines. J Med Chem. 2012;55:11022–30.
Younis Y, Douelle F, Cabrera DG, Manach CL, Nchinda AT, Paquet T, et al. Structure-activity-relationship studies around the 2-amino group and pyridine core of antimalarial 3,5-diarylaminopyridines lead to a novel series of pyrazine analogues with oral in vivo activity. J Med Chem. 2013;56:8860–71.
McNamara CW, Lee MCS, Lim CS, Lim SH, Roland J, Nagle A, et al. Targeting plasmodium PI(4)K to eliminate malaria. Nature. 2013;504:248–53.
Zhang YK, Plattner JJ, Freund YR, Easom EE, Zhou Y, Gut J, et al. Synthesis and structure-activity relationships of novel benzoxaboroles as a new class of antimalarial agents. Bioorg Med Chem Lett. 2011;21:644–51.
Zhang YK, Plattner JJ, Easom EE, Jacobs RT, Guo D, Freund YR, et al. Benzoxaborole antimalarial agents. Part 5. Lead optimization of novel amide pyrazinyloxy benzoxaboroles and identification of a preclinical candidate. J Med Chem. 2017;60:5889–908.
Zhang YK, Plattner JJ, Easom EE, Jacobs RT, Guo D, Sanders V, et al. Benzoxaborole antimalarial agents. Part 4. Discovery of potent 6-(2-(alkoxycarbonyl)pyrazinyl-5-oxy)-1,3-dihydro-1-hydroxy-2,1-benzoxaboroles. J Med Chem. 2015;58:5344–54.
Sonoiki E, Ng CL, Lee MCS, Guo D, Zhang YK, Zhou Y, et al. A potent antimalarial benzoxaborole targets a Plasmodium falciparum cleavage and polyadenylation specificity factor homologue. Nat Commun. 2017;8:14574.
Pegoraro S, Duffey M, Otto TD, Wang Y, Rösemann R, Baumgartner R, et al. SC83288 is a clinical development candidate for the treatment of severe malaria. Nat Commun. 2017;8:14193.
Leban J, Pegoraro S, Dormeyer M, Lanzer M, Aschenbrenner A, Kramer B. Sulfonyl-phenyl-ureido benzamidines. Bioorg Med Chem Lett. 2004;14:1979–82.
Duffey M, Sanchez CP, Lanzer M. Profiling of the anti-malarial drug candidate SC83288 against artemisinins in Plasmodium falciparum . Malar J. 2018;17:121.
Burgess SJ, Kelly JX, Shomloo S, Wittlin S, Brun R, Liebmann K, et al. Synthesis, structure-activity relationship, and mode-of-action studies of antimalarial reversed chloroquine compounds. J Med Chem. 2010;53:6477–89.
Burgess SJ, Selzer A, Kelly JX, Smilkstein MJ, Riscoe MK, Peyton DH. A chloroquine-like molecule designed to reverse resistance in Plasmodium falciparum . J Med Chem. 2006;49:5623–5.
Wirjanata G, Sebayang BF, Chalfein F, Prayoga, Handayuni I, Noviyanti R, et al. Contrasting ex vivo efficacies of “reversed chloroquine” compounds in chloroquine-resistant Plasmodium falciparum and P. vivax isolates. Antimicrob Agents Chemother. 2015;59:5721–6.
Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, et al. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci USA. 2012;109:16823–8.
Guiguemde WA, Shelat AA, Bouck D, Duffy S, Crowther GJ, Davis PH, et al. Chemical genetics of Plasmodium falciparum . Nature. 2010;465:311–5.
Floyd DM, Stein P, Wang Z, Liu J, Castro S, Clark JA, et al. Hit-to-lead studies for the antimalarial tetrahydroisoquinolone carboxanilides. J Med Chem. 2016;59:7950–62.
Jiménez-Díaz MB, Ebert D, Salinas Y, Pradhan A, Lehane AM, Myrand-Lapierre ME, et al. (+)-SJ733, a clinical candidate for malaria that acts through ATP4 to induce rapid host-mediated clearance of Plasmodium. Proc Natl Acad Sci USA. 2014;111:E5455–62.
Article PubMed CAS PubMed Central Google Scholar
Spillman NJ, Kirk K. The malaria parasite cation ATPase PfATP4 and its role in the mechanism of action of a new arsenal of antimalarial drugs. Int J Parasitol Drugs Drug Resist. 2015;5:149–62.
Boss C, Aissaoui H, Amaral N, Bauer A, Bazire S, Binkert C, et al. Discovery and characterization of ACT-451840: an antimalarial drug with a novel mechanism of action. ChemMedChem. 2016;11:1995–2014.
Bihan AL, de Kanter R, Angulo-Barturen I, Binkert C, Boss C, Brun R, et al. Characterization of novel antimalarial compound ACT-451840: preclinical assessment of activity and dose-efficacy modeling. PLoS Med. 2016;13:e1002138.
Krause A, Dingemanse J, Mathis A, Marquart L, Möhrle JJ, McCarthy JS. Pharmacokinetic/pharmacodynamic modelling of the antimalarial effect of Actelion-451840 in an induced blood stage malaria study in healthy subjects. Br J Clin Pharmacol. 2016;82:412–21.
Charman SA, Arbe-Barnes S, Bathurst IC, Brun R, Campbell M, Charman WN, et al. Synthetic ozonide drug candidate OZ439 offers new hope for a single-dose cure of uncomplicated malaria. Proc Natl Acad Sci USA. 2011;108:4400–5.
Vennerstrom JL, Arbe-Barnes S, Brun R, Charman SA, Chiu FCK, Chollet J, et al. Identification of an antimalarial synthetic trioxolane drug development candidate. Nature. 2004;430:900–4.
Dong Y, Wittlin S, Sriraghavan K, Chollet J, Charman SA, Charman WN, et al. The structure-activity relationship of the antimalarial ozonide Arterolane (OZ277). J Med Chem. 2010;53:481–91.
Dong Y, Chollet J, Matile H, Charman SA, Chiu FCK, Charman WN, et al. Spiro and dispiro-1,2,4-trioxolanes as antimalarial peroxides: charting a workable structure-activity relationship using simple prototypes. J Med Chem. 2005;48:4953–61.
Dong Y, Tang Y, Chollet J, Matile H, Wittlin S, Charman SA, et al. Effect of functional group polarity on the antimalarial activity of spiro and dispiro-1,2,4-trioxolanes. Bioorg Med Chem. 2006;14:6368–82.
Kaiser M, Wittlin S, Nehrbass-Stuedli A, Dong Y, Wang X, Hemphill A, et al. Peroxide bond-dependent antiplasmodial specificity of artemisinin and OZ277 (RBx11160). Antimicrob Agents Chemother. 2007;51:2991–3.
Dong Y, Wang X, Kamaraj S, Bulbule VJ, Chiu FCK, Chollet J, et al. Structure-activity relationship of the antimalarial ozonide Artefenomel (OZ439). J Med Chem. 2017;60:2654–68.
Phyo AP, Jittamala P, Nosten FH, Pukrittayakamee S, Imwong M, White NJ, et al. Antimalarial activity of artefenomel (OZ439), a novel synthetic antimalarial endoperoxide, in patients with Plasmodium falciparum and Plasmodium vivax malaria: an open-label phase 2 trial. Lancet Infect Dis. 2016;16:61–9.
Gautam A, Ahmed T, Sharma P, Varshney B, Kothari M, Saha N, et al. Pharmacokinetics and pharmacodynamics of Arterolane maleate following multiple oral doses in adult patients with P. falciparum malaria. J Clin Pharmacol. 2011;51:1519–28.
Valecha N, Krudsood S, Tangpukdee N, Mohanty S, Sharma SK, Tyagi PK, et al. Arterolane maleate plus piperaquine phosphate for treatment of uncomplicated lasmodium falciparum malaria: a comparative, multicenter, randomized clinical trial. Clin Infect Dis. 2012;55:663–71.
Jourdan J, Matile H, Reift E, Biehlmaier O, Dong Y, Wang X, et al. Monoclonal antibodies that recognize the alkylation signature of antimalarial ozonides OZ277 (Arterolane) and OZ439 (Artefenomel). ACS Infect Dis. 2015;2:54–61.
Allman EL, Painter HJ, Samra J, Carrasquilla M, Llinás M. Metabolomic profiling of the malaria box reveals antimalarial target pathways. Antimicrob Agents Chemother. 2016;60:6635–49.
Moehrle JJ, Duparc S, Siethoff C, van Giersbergen PLM, Craft JC, Arbe-Barnes S, et al. First-in-man safety and pharmacokinetics of synthetic ozonide OZ439 demonstrates an improved exposure profile relative to other peroxide antimalarials. Br J Clin Pharmacol. 2013;75:535–48.
McCarthy JS, Baker M, O’Rourke P, Marquart L, Griffin P, van Huijsduijnen RH, et al. Efficacy of OZ439 (Artefenomel) against early Plasmodium falciparum blood-stage malaria infection in healthy volunteers. J Antimicrob Chemother. 2016;71:2620–7.
Plouffe D, Brinker A, McNamara C, Henson K, Kato N, Kuhen K, et al. In silico activity profiling reveals the mechanism of action of antimalarials discovered in a high-throughput screen. Proc Natl Acad Sci USA. 2008;105:9059–64.
Nagle A, Wu T, Kuhen K, Gagaring K, Borboa R, Francek C, et al. Imidazolopiperazines: lead optimization of the second-generation antimalarial agents. J Med Chem. 2012;55:4244–73.
Wu T, Nagle A, Kuhen K, Gagaring K, Borboa R, Francek C, et al. Imidazolopiperazines: hit to lead optimization of new antimalarial agents. J Med Chem. 2011;54:5116–30.
Kuhen KL, Chatterjee AK, Rottmann M, Gagaring K, Borboa R, Buenviaje J, et al. KAF156 Is an antimalarial clinical candidate with potential for use in prophylaxis, treatment, and prevention of disease transmission. Antimicrob Agents Chemother. 2014;58:5060–7.
Rottmann M, McNamara C, Yeung BKS, Lee MCS, Zou B, Russell B, et al. Spiroindolones, a potent compound class for the treatment of malaria. Science. 2010;329:1175–80.
Yeung BKS, Zou B, Rottmann M, Lakshminarayana SB, Ang SH, Leong SY, et al. Spirotetrahydro \(\beta\) -carbolines (spiroindolones): a new class of potent and orally efficacious compounds for the treatment of malaria. J Med Chem. 2010;53:5155–64.
White NJ, Pukrittayakamee S, Phyo AP, Rueangweerayut R, Nosten F, Jittamala P, et al. Spiroindolone KAE609 for falciparum and vivax malaria. N Engl J Med. 2014;371:403–10.
Coteron JM, Marco M, Esquivias J, Deng X, White KL, White J, et al. Structure-guided lead optimization of triazolopyrimidine-ring substituents identifies potent Plasmodium falciparum dihydroorotate dehydrogenase inhibitors with clinical candidate potential. J Med Chem. 2011;54:5540–61.
McCarthy JS, Lotharius J, Rückle T, Chalon S, Phillips MA, Elliott S, et al. Safety, tolerability, pharmacokinetics, and activity of the novel long-acting antimalarial DSM265: a two-part first-in-human phase 1a/1b randomised study. Lancet Infect Dis. 2017;17:626–35.
Nigussie D, Beyene T, Shah NA, Belew S. New targets in malaria parasite chemotherapy: a review. Malaria Contr Elimination. 2015;S1:S1–007.
Jorgensen R, Merrill AR, Andersen GR. The life and death of translation elongation factor 2. Biochem Soc Trans. 2006;34:1–6.
Spillman NJ, Allen RJW, McNamara CW, Yeung BKS, Winzeler EA, Diagana TT, et al. Na \(^+\) Regulation in the malaria parasite Plasmodium falciparum involves the cation ATPase PfATP4 and is a target of the spiroindolone antimalarials. Cell Host Microbe. 2013;13:227–37.
Open Source Malaria Series 4 GitHub Repository. https://github.com/OpenSourceMalaria/Series4 . Accessed 17 June 2018.
Voorhis WCV, Adams JH, Adelfio R, Ahyong V, Akabas MH, Alano P, et al. Open source drug discovery with the malaria box compound collection for neglected diseases and beyond. PLoS Pathog. 2016;12:e1005763.
Dennis ASM, Rosling JEO, Lehane AM, Kirk K. Diverse antimalarials from whole-cell phenotypic screens disrupt malaria parasite ion and volume homeostasis. Sci Rep. 2018;8:8795.
Saliba KJ, Kirk K. pH Regulation in the intracellular malaria parasite Plasmodium falciparum . J Biol Chem. 1999;274:33213–9.
Baldwin J, Farajallah AM, Malmquist NA, Rathod PK, Phillips MA. Malarial dihydroorotate dehydrogenase. J Biol Chem. 2002;277:41827–34.
Phillips MA, Lotharius J, Marsh K, White J, Dayan A, White KL, et al. A long-duration dihydroorotate dehydrogenase inhibitor (DSM265) for prevention and treatment of malaria. Sci Transl Med. 2015;7:296ra111.
Yuthavong Y, Yubaniyama J, Chitnumsub P, Vanichtanankul J, Chusacultanachai S, Tarnchompoo B, et al. Malarial ( Plasmodium falciparum ) dihydrofolate reductase-thymidylate synthase: structural basis for antifolate resistance and development of effective inhibitors. Parasitology. 2005;130:249–59.
Luth MR, Gupta P, Ottilie S, Winzeler EA. Using in vitro evolution and whole genome analysis to discover next generation targets for antimalarial drug discovery. ACS Infect Dis. 2018;4:301–14.
IUPHAR/MMV Guide to Malaria Pharmacology Project. http://www.guidetomalariapharmacology.org/ . Accessed 14 Jan 2019.
Download references
Authors' contributions
All authors contributed to the writing, editing and proofing of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors thank various contributors for provision of unpublished data: these contributions have been acknowledged in the manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
Ethics approval and consent to participate.
The authors thank the Australian Research Council and the Medicines for Malaria Venture for funding (LP150101226).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and affiliations.
School of Chemistry, The University of Sydney, Sydney, NSW, 2006, Australia
Edwin G. Tse, Marat Korsik & Matthew H. Todd
School of Pharmacy, University College London, London, WC1N 1AX, United Kingdom
Matthew H. Todd
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Edwin G. Tse or Matthew H. Todd .
Additional files
Additional file 1..
Compounds reference list. List of SciFinder reference dois for each compound described in the Future section of this review.
Additional file 2.
Biological data for compounds. Tabulation of biological potencies and pharmacokinetic properties for each compound described in Future section of this review.
Additional file 3.
SMILES strings and CID numbers for compounds. List of SMILES strings and CID numbers for every compound described in this review.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Tse, E.G., Korsik, M. & Todd, M.H. The past, present and future of anti-malarial medicines. Malar J 18 , 93 (2019). https://doi.org/10.1186/s12936-019-2724-z
Download citation
Received : 18 December 2018
Accepted : 12 March 2019
Published : 22 March 2019
DOI : https://doi.org/10.1186/s12936-019-2724-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Mechanism of action
- Drug discovery
- Drug development
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]
UCF Research Holds Promise for Fighting Malaria
Two multimillion-dollar grants from the National Institutes of Health are investigating various medicines and compounds that can combat the disease.
By Christin Senior | April 2, 2024

UCF researchers are making advances in finding novel and more effective treatments for malaria, by using cancer drugs and fungi.
With two $3.8 million grants from the National Institutes of Health, Debopam Chakrabarti, an infectious disease specialist at the UCF College of Medicine, has been researching new ways to combat drug-resistant malaria. The disease kills more than 600,000 people per year globally, and disproportionately impacts women and young children under the age of 5. As part of his research efforts, Chakrabarti recently published two new investigative studies in leading scientific journals.
The first study, published in the Journal of Medicinal Chemistry, examined the potential for repurposing existing cancer drugs as antimalarial therapies. The research was in partnership with Ratna Chakrabarti, a UCF cancer molecular biologist, Nathanael Gray, a cancer therapeutics researcher at Stanford University, and Elizabeth Winzeler, a malaria drug expert from the University of California-San Diego.
In Chakrabarti’s lab, doctoral researcher Monica Bohmer and postdoctoral fellow Flore Nardella screened several human kinase inhibitors used in cancer treatment and identified Compound 1 as a leading antimalarial agent that cured infected mice, and prevented the parasite that causes malaria from infecting cells in the first place.
Continuous genetic mutations that parasite, Plasmodium , makes it increasingly resistant to current drugs. As a result, new and more effective drugs are long overdue, especially with malaria deaths increasing significantly over the last few years.
“Our results are significant and could provide a promising solution for malaria prevention and treatment, particularly due to the compound’s low required dosage which will also reduce likelihood of any side effects,” Chakrabarti says.
Repurposing cancer drugs for malaria is essentially a “piggyback approach,” that would fast-track the process of getting the drug on the market as these compounds already have a long history of data on safety, toxicity and pharmacology and are already FDA approved.
Chakrabarti noted there is also a need for new prophylactic drugs to prevent malaria infection. About 2,000 cases of malaria are diagnosed in the United States each year, with most cases occurring in travelers returning from countries in sub-Saharan Africa and South Asia where malaria transmission frequently occurs.
“There are only two prophylactic drugs right now used in the U.S. for travelers to these areas,” Chakrabarti says. “One requires doses taken daily over several weeks and the other has neurological side effects. So better prophylactics with little or no side effects are needed.”
The second study published in Cell Chemical Biology examined the use of fungus-derived compounds to kill the malaria parasite. Partnering with Robert Cichewicz, a natural-product chemist from the University of Oklahoma and UCF postdoctoral fellow Jennifer Collins, Chakrabarti’s lab screened approximately 19,000 fungal species grown in Cichewicz’s lab. From these efforts, they identified antimalarial properties in a class of compounds called peptaibols — modified peptides produced by fungi.
Plasmodium cells ingest hemoglobin from blood to provide the nourishment they need to survive. Chakrabarti and the researchers found that the peptaibols work by changing the membrane structure of Plasmodium digestive vacuole which prevents it from digesting hemoglobin, essentially starving the parasite to death.
The next phase of both studies will look at testing the compounds in humanized animal models, along with further exploring their antimalarial mechanisms and properties.
“Both of these studies are a step in the right direction in the campaign to eliminate malaria globally and with this approach, we hope to have better drugs available sooner,” Chakrabarti says.
Chakrabarti has been researching malaria at UCF for more than 25 years. A Pegasus Professor in the Burnett School of Biomedical Sciences, he joined the university in 1995 and was one of the first UCF faculty members to receive an NIH grant. He received his doctorate in biochemistry from the University of Calcutta and completed post-doctoral training in molecular biology at the University of Nebraska-Lincoln.
More Topics
Pegasus magazine.

Founded to help fuel talent for the nearby space industry , UCF continues to build its reputation as SpaceU. Here's a look at the early days of UCF's space ties and journey to new frontiers.

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 25 August 2021

Recent clinical trials inform the future for malaria vaccines
- Liriye Kurtovic 1 , 2 ,
- Linda Reiling 1 , 2 , 3 ,
- D. Herbert Opi 1 , 2 , 3 &
- James G. Beeson ORCID: orcid.org/0000-0002-1018-7898 1 , 2 , 3 , 4
Communications Medicine volume 1 , Article number: 26 ( 2021 ) Cite this article
8739 Accesses
10 Citations
24 Altmetric
Metrics details
This article has been updated
Malaria vaccines are urgently needed in the fight against this devastating disease that is responsible for almost half a million deaths each year. Here, we discuss recent clinical advances in vaccine development and highlight ongoing challenges for the future.
Malaria is one of the most devastating infectious diseases in humans, responsible for >200 million cases and almost half a million deaths annually. Control programs have significantly reduced disease burden since 2000, but numbers have plateaued since 2015. The disruption caused by the ongoing COVID-19 pandemic will further unwind these efforts and is predicted to increase the number of malaria deaths. New control measures are urgently needed, and lessons learned from emerging diseases such as COVID-19 highlight the enormous potential of effective vaccines. This has been recognized by the World Health Organization (WHO) and key stakeholders who recommend that, within the next decade, a vaccine should be developed that is at least 75% efficacious against malaria over 2 years (i.e., reduces disease occurrence by at least 75% in vaccinated compared to unvaccinated individuals) and also reduces malaria transmission.
This is an exciting time for malaria vaccine development, with a number of promising clinical trials recently completed and others currently underway…
Plasmodium falciparum causes the majority of malaria burden and has been the primary focus of vaccine development, and Plasmodium vivax is the second major cause of malaria. There are several vaccine strategies that target different developmental stages of the malaria-causing parasite (Fig. 1 ). This begins with the sporozoite parasite form, which is inoculated into the skin by a feeding mosquito, circulates in the blood, and infects the liver to develop into the morphologically distinct merozoite form. Merozoites then replicate within red blood cells to exponentially increase parasitemia and can cause severe disease pathology. Some merozoites will undergo development into the gametocyte form that can be taken up by the mosquito vector and subsequently be transmitted in a population. An important bottleneck in the vaccine development pipeline is the assessment of potential candidates in clinical trials. Given the limitations of malaria animal models to adequately reflect human disease, the controlled human malaria infection (CHMI) model offers a unique ability to fast-track initial evaluations of vaccine efficacy. Safe and controlled parasite infection in a clinical setting has proven more time-efficient and less variable than natural parasite exposure and can therefore rely on fewer subjects for conducting the first efficacy trial with a vaccine. CHMI has also been increasingly implemented in malaria-endemic settings, which is critically important to understand the dynamics between vaccine-induced immunity and pre-existing immunity during ongoing natural exposure to malaria.
Overview of the morphologically distinct sporozoite, merozoite, and gametocyte forms at different stages of the parasite life cycle, and the vaccine strategy for targeting each stage. Vaccines that target multiple life cycle stages are also in development. Created with BioRender.com.
Here, we discuss recent advances and insights from clinical trials of malaria vaccines and highlight ongoing challenges and priorities for the future. Table 1 lists the key trials discussed in this Comment.
Vaccines can block infection by targeting sporozoites
Vaccines can target sporozoites to prevent or impair infection of the liver, and therefore aim to induce immunity whereby an individual is protected against infection and disease. Live-attenuated sporozoite-based vaccines have the capacity to induce sterilizing immunity (no detectable parasitemia) against CHMI in malaria-naive volunteers. This includes the PfSPZ vaccine that contains radiation-attenuated sporozoites incapable of replicating. It was recently shown that an optimized PfSPZ vaccine dosage induced sterilizing immunity against CHMI in six Tanzanian adults 1 , and this dose appeared safe in African children, which supports further evaluations in younger age groups (NCT02687373) 2 . Another approach is to combine replication–intact sporozoites with antimalarial drug prophylaxis to prevent malarial illness, known as PfSPZ-CVac. PfSPZ-CVac can induce sterilizing immunity in malaria-naive volunteers, but there is no published data in malaria-endemic populations 3 . A third approach is to genetically attenuate sporozoites to prevent intrahepatic development. This includes the triple-gene knockout vaccine, GAP3KO, which recently completed an efficacy trial in malaria-naive volunteers (NCT03168854). Although sporozoite-based vaccines are promising, major obstacles include the fact that currently sporozoites cannot be cultured in vitro and are therefore isolated from infected mosquito salivary glands, and they currently require direct venous inoculation of vaccine doses and storage and transportation of vaccines using liquid nitrogen.
Subunit vaccines aim to target a key antigen, or sub-region, and have the advantage of using existing production technologies and delivery using established infrastructure and systems. The most successful is the RTS,S vaccine based on a truncated form of the major sporozoite surface antigen, circumsporozoite protein (CSP). This is expressed as a virus-like particle and administered with an adjuvant (AS01), which is used to enhance the immune response to vaccination. Promising findings from a phase 3 clinical trial in >15,000 African children led to pilot implementation of a four-dose booster regimen in children aged 5 months in Ghana, Kenya, and Malawi to further evaluate vaccine safety and efficacy, which is ongoing 4 . Additional trials are underway in African children to evaluate a modified regimen where a fraction of the third dose is administered months later, which appeared to be moderately more efficacious in malaria-naive volunteers (NCT03276962) 5 . There is also interest in whether RTS,S can synergize with seasonal malaria chemoprevention (NCT03143218).
A recent advance was the development of the R21 malaria vaccine, a virus-like particle that is similar to RTS,S, but has higher density of the CSP antigen on the particle surface and is formulated with Matrix-M adjuvant 6 . Initial results from a pediatric phase 2 clinical trial in Burkina Faso demonstrated 76% efficacy against malaria over 12 months 6 . Although this was considerably higher than vaccine efficacy achieved in previous RTS,S trials 4 , R21 was administered prior to the peak malaria season and most malaria occurred in the first 6 months 6 , making direct comparisons with RTS,S efficacy difficult to interpret. Furthermore, antibody levels had declined markedly by 12 months. Participants are still being monitored to determine vaccine efficacy over 2 years, and children are currently being enrolled in phase 3 clinical trials across multiple African sites (NCT04704830).
Vaccines targeting blood stages of malaria prevent clinical illness
Blood-stage vaccines target the merozoite form or infected red blood cells and aim to control parasitemia and prevent clinical illness. There has been considerably less progress in achieving good efficacy for blood-stage vaccines, with only a handful of candidates evaluated in phase 2 clinical trials, and none having progressed further 7 . The most recent was RH5.1 comprised of full-length RH5 protein, an indispensable merozoite invasion ligand. In a phase 1/2a clinical trial in malaria-naive volunteers, RH5.1 with AS01 adjuvant was highly immunogenic but only resulted in a 1-day delay until threshold parasitemia levels were reached following CHMI compared with that of the unvaccinated control group 8 . An upcoming phase 1 clinical trial of RH5.1 will further evaluate safety and immunogenicity in Tanzanian adults and children (NCT04318002).
Merozoites express a multitude of surface proteins and invasion ligands, including many that are polymorphic, which makes it difficult to identify candidate antigens for subunit vaccines. In addition, there are major knowledge gaps on specific mechanisms that mediate protective antimalarial immunity. Antibodies play a critical role, but the current reference growth inhibition assay (GIA) that measures antibody inhibitory activity has neither strongly nor consistently been associated with protection against malaria 7 . Several vaccines, including RH5.1, have generated substantial GIA activity but shown limited efficacy 8 . Achieving higher efficacy with merozoite vaccines may require harnessing multiple immunologic mechanisms. Recent studies found that antibodies can activate complement and mediate Fcγ-receptor effector functions against merozoites, which were associated with clinical protection in malaria-endemic population studies 9 . This creates a new opportunity to evaluate the targets of functional antibody responses and potentially identify new vaccine candidate antigens using these novel approaches 9 .
Given the lack of success in developing blood-stage subunit vaccines, there is interest in whole parasite blood-stage vaccines, which present a large antigen repertoire to the immune system and may overcome the challenge of antigen-specific polymorphisms. The first clinical study of chemically attenuated parasite-infected red blood cells promisingly induced cellular immune responses in malaria-naive volunteers, but parasite-specific antibodies were not detected 10 . Further studies are needed on this particular vaccine approach, including improvements in vaccine production and formulation to avoid immune responses to the red blood cell membrane.
Once a merozoite infects a red blood cell and matures, the host cell undergoes extensive changes and expresses parasite-derived proteins on the red blood cell surface. One such protein, VAR2CSA, facilitates the accumulation and sequestration of parasite-infected red blood cells in the placenta. Malaria during pregnancy is, therefore, a major concern and can lead to adverse outcomes such as maternal anemia, preterm delivery, stillbirth, and low birthweight babies. Two VAR2CSA-based vaccines (PAMVAC and PRIMVAC) aimed at inhibiting placental malaria have recently completed phase 1 clinical trials and were immunogenic and had acceptable safety profiles, but are yet to be evaluated for vaccine efficacy 11 , 12 .
Developing vaccines that block malaria transmission
Vaccines that target the gametocyte and gamete forms aim to prevent parasite transmission to the mosquito and thereby block malaria transmission throughout a population. Although this vaccine approach does not offer any direct clinical protection in humans, blocking malaria transmission is an essential component of effective control and elimination strategies. There has been limited progress for transmission-blocking vaccines, with one candidate based on the gametocyte surface antigen, Pfs230 (Pfs230D1M), showing promise in early clinical trials and a phase 2 clinical trial is ongoing in Mali (NCT03917654) 13 .
These vaccine approaches targeting different stages of the parasite life cycle have the ability to afford protection against infection, disease, and/or prevent parasite transmission among a population. Although each approach is of value, there may be additional benefits to developing multi-stage subunit vaccines that incorporate antigens expressed at different developmental stages.
Future directions and opportunities
This is an exciting time for malaria vaccine development, with a number of promising clinical trials recently completed and others currently underway, although there are still considerable challenges that stand in the way of achieving vaccine goals within the next decade with several priority areas for research (Fig. 2 ). Malaria exposure may drive the lower vaccine responses typically observed in malaria-exposed populations compared with malaria-naive individuals. As disease burden can be substantial among adolescents and adults in some populations, clinical trials to evaluate vaccine efficacy in these groups will also be needed. A persisting challenge for malaria vaccines is achieving sustained protective efficacy as antibody and cellular responses are often short-lived and wane within a year 6 . The immunological factors underlying these vaccine limitations remain poorly understood but will be crucial for achieving higher efficacy and wider vaccine implementation in endemic populations. Furthermore, very little progress has been made toward the development of vaccines against P. vivax , which needs to be accelerated.
The figure lists several research priorities, grouped into three main themes, for achieving malaria vaccines with high impact and have the potential to accelerate and sustain malaria elimination. Created with BioRender.com.
Recent advances in vaccine technologies, including those used in licensed COVID-19 vaccines, have opened new opportunities for malaria vaccine development. This includes mRNA-based vaccine platforms of the CSP malaria antigen, which has already shown encouraging results in animal models 14 . There has also been continued interest in heterologous prime-boost with ChAd43 and Modified Vaccinia Ankara viral vectors encoding different malaria antigens alone or in combinations (e.g., AMA1, MSP1, ME-TRAP) 15 .
Further research on these areas will greatly advance the development of effective malaria vaccines in endemic populations.
Change history
08 september 2021.
The original version of this Article was updated shortly after publication because the text selected for the pull quote ‘This is an exciting time for malaria vaccine development, with a number of promising clinical trials recently completed and others currently underway…’ was inadvertently added at the end of the main text and was not rendering as a pull quote. The error has now been correct in the HTML and PDF versions of the Article.
Jongo, S. A. et al. Increase of dose associated with decrease in protection against controlled human malaria infection by PfSPZ Vaccine in Tanzanian adults. Clin. Infect. Dis. 71 , 2849–2857 (2020).
Article CAS Google Scholar
Steinhardt, L. C. et al. Safety, tolerability, and immunogenicity of Plasmodium falciparum sporozoite vaccine administered by direct venous inoculation to infants and young children: findings from an age de-escalation, dose-escalation, double-blind, randomized controlled study in Western Kenya. Clin. Infect. Dis. 71 , 1063–1071 (2020).
Mwakingwe-Omari, A. et al. Two chemoattenuated PfSPZ malaria vaccines induce sterile hepatic immunity. Nature 595 , 289–294 (2021).
RTSS Clinical Trials Partnership. First results of phase 3 trial of RTS, S/AS01 malaria vaccine in African children. N. Engl. J. Med. 365 , 1863–1875 (2011).
Article Google Scholar
Regules, J. A. et al. Fractional third and fourth dose of RTS, S/AS01 malaria candidate vaccine: a phase 2a controlled human malaria parasite infection and immunogenicity study. J. Infect. Dis. 214 , 762–771 (2016).
Datoo, M. S. et al. Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: a randomised controlled trial. Lancet 397 , 1809–1818 (2021).
Beeson, J. G. et al. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol. Rev. 40 , 343–372 (2016).
Minassian, A. M. et al. Reduced blood-stage malaria growth and immune correlates in humans following RH5 vaccination. Med 2 , 701–719 (2021).
Reiling, L. et al. Targets of complement-fixing antibodies in protective immunity against malaria in children. Nat. Commun. 10 , 610 (2019).
Stanisic, D. I. et al. Vaccination with chemically attenuated Plasmodium falciparum asexual blood-stage parasites induces parasite-specific cellular immune responses in malaria-naive volunteers: a pilot study. BMC Med. 16 , 184 (2018).
Mordmüller, B. et al. First-in-human, randomized, double-blind clinical trial of differentially adjuvanted PAMVAC, a vaccine candidate to prevent pregnancy-associated malaria. Clin. Infect. Dis. 69 , 1509–1516 (2019).
Sirima, S. B. et al. PRIMVAC vaccine adjuvanted with alhydrogel or GLA-SE to prevent placental malaria: a first-in-human, randomised, double-blind, placebo-controlled study. Lancet Infect. Dis. 20 , 585–597 (2020).
Healy, S. A. et al. Pfs230 yields higher malaria transmission–blocking vaccine activity than Pfs25 in humans but not mice. J. Clin. Invest. 131 , e146221 (2021).
Mallory, K. L. et al. Messenger RNA expressing PfCSP induces functional, protective immune responses against malaria in mice. npj Vaccines 6 , 1–12 (2021).
Tiono, A. B. et al. First field efficacy trial of the ChAd63 MVA ME-TRAP vectored malaria vaccine candidate in 5-17 months old infants and children. PloS ONE 13 , e0208328 (2018).
Download references
Acknowledgements
The authors acknowledge support from the National Health and Medical Research Council of Australia (NHMRC), including Investigator Grant to J.G.B. and Ideas Grant to D.H.O. and L.R., and the Australian Centre for Research Excellence in Malaria Elimination.
Author information
Authors and affiliations.
Burnet Institute, Melbourne, VIC, Australia
Liriye Kurtovic, Linda Reiling, D. Herbert Opi & James G. Beeson
Department of Immunology and Pathology, Central Clinical School, Monash University, Melbourne, VIC, Australia
Department of Medicine, The University of Melbourne, Melbourne, VIC, Australia
Linda Reiling, D. Herbert Opi & James G. Beeson
Department of Microbiology, Monash University, Clayton, VIC, Australia
- James G. Beeson
You can also search for this author in PubMed Google Scholar
Contributions
L.K., L.R., D.H.O. and J.G.B reviewed published literature and data and interpreted findings. L.K., L.R. and D.H.O. wrote the first draft of the manuscript with input from J.G.B. L.K., L.R., D.H.O. and J.G.B revised the manuscript and all authors approved the final version for publication.
Corresponding author
Correspondence to James G. Beeson .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kurtovic, L., Reiling, L., Opi, D.H. et al. Recent clinical trials inform the future for malaria vaccines. Commun Med 1 , 26 (2021). https://doi.org/10.1038/s43856-021-00030-2
Download citation
Received : 26 July 2021
Accepted : 10 August 2021
Published : 25 August 2021
DOI : https://doi.org/10.1038/s43856-021-00030-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Induction, decay, and determinants of functional antibodies following vaccination with the rts,s malaria vaccine in young children.
- Gaoqian Feng
- Liriye Kurtovic
BMC Medicine (2022)
ICAM-1-binding Plasmodium falciparum erythrocyte membrane protein 1 variants elicits opsonic-phagocytosis IgG responses in Beninese children
- Jennifer Suurbaar
- Azizath Moussiliou
- Anja R. Jensen
Scientific Reports (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Read our research on: Abortion | Podcasts | Election 2024
Regions & Countries
Most americans favor legalizing marijuana for medical, recreational use, legalizing recreational marijuana viewed as good for local economies; mixed views of impact on drug use, community safety.
Pew Research Center conducted this study to understand the public’s views about the legalization of marijuana in the United States. For this analysis, we surveyed 5,140 adults from Jan. 16 to Jan. 21, 2024. Everyone who took part in this survey is a member of the Center’s American Trends Panel (ATP), an online survey panel that is recruited through national, random sampling of residential addresses. This way nearly all U.S. adults have a chance of selection. The survey is weighted to be representative of the U.S. adult population by gender, race, ethnicity, partisan affiliation, education and other categories. Read more about the ATP’s methodology .
Here are the questions used for the report and its methodology .
As more states pass laws legalizing marijuana for recreational use , Americans continue to favor legalization of both medical and recreational use of the drug.
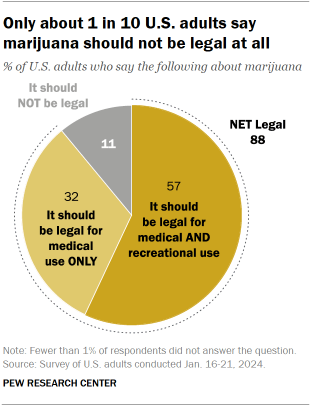
An overwhelming share of U.S. adults (88%) say marijuana should be legal for medical or recreational use.
Nearly six-in-ten Americans (57%) say that marijuana should be legal for medical and recreational purposes, while roughly a third (32%) say that marijuana should be legal for medical use only.
Just 11% of Americans say that the drug should not be legal at all.
Opinions about marijuana legalization have changed little over the past five years, according to the Pew Research Center survey, conducted Jan. 16-21, 2024, among 5,14o adults.
The impact of legalizing marijuana for recreational use
While a majority of Americans continue to say marijuana should be legal , there are varying views about the impacts of recreational legalization.
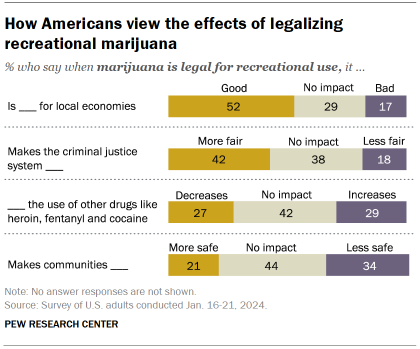
About half of Americans (52%) say that legalizing the recreational use of marijuana is good for local economies; just 17% think it is bad and 29% say it has no impact.
More adults also say legalizing marijuana for recreational use makes the criminal justice system more fair (42%) than less fair (18%); 38% say it has no impact.
However, Americans have mixed views on the impact of legalizing marijuana for recreational use on:
- Use of other drugs: About as many say it increases (29%) as say it decreases (27%) the use of other drugs, like heroin, fentanyl and cocaine (42% say it has no impact).
- Community safety: More Americans say legalizing recreational marijuana makes communities less safe (34%) than say it makes them safer (21%); 44% say it has no impact.
Partisan differences on impact of recreational use of marijuana
There are deep partisan divisions regarding the impact of marijuana legalization for recreational use.
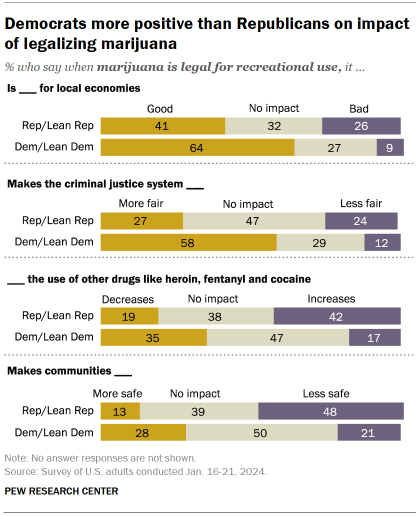
Majorities of Democrats and Democratic-leaning independents say legalizing recreational marijuana is good for local economies (64% say this) and makes the criminal justice system fairer (58%).
Fewer Republicans and Republican leaners say legalization for recreational use has a positive effect on local economies (41%) and the criminal justice system (27%).
Republicans are more likely than Democrats to cite downsides from legalizing recreational marijuana:
- 42% of Republicans say it increases the use of other drugs, like heroin, fentanyl and cocaine, compared with just 17% of Democrats.
- 48% of Republicans say it makes communities less safe, more than double the share of Democrats (21%) who say this.
Demographic, partisan differences in views of marijuana legalization
Sizable age and partisan differences persist on the issue of marijuana legalization though small shares of adults across demographic groups are completely opposed to it.
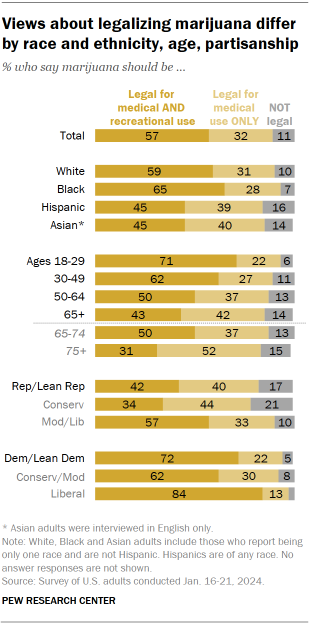
Older adults are far less likely than younger adults to favor marijuana legalization.
This is particularly the case among adults ages 75 and older: 31% say marijuana should be legal for both medical and recreational use.
By comparison, half of adults between the ages of 65 and 74 say marijuana should be legal for medical and recreational use, and larger shares in younger age groups say the same.
Republicans continue to be less supportive than Democrats of legalizing marijuana for both legal and recreational use: 42% of Republicans favor legalizing marijuana for both purposes, compared with 72% of Democrats.
There continue to be ideological differences within each party:
- 34% of conservative Republicans say marijuana should be legal for medical and recreational use, compared with a 57% majority of moderate and liberal Republicans.
- 62% of conservative and moderate Democrats say marijuana should be legal for medical and recreational use, while an overwhelming majority of liberal Democrats (84%) say this.
Views of marijuana legalization vary by age within both parties
Along with differences by party and age, there are also age differences within each party on the issue.
A 57% majority of Republicans ages 18 to 29 favor making marijuana legal for medical and recreational use, compared with 52% among those ages 30 to 49 and much smaller shares of older Republicans.
Still, wide majorities of Republicans in all age groups favor legalizing marijuana at least for medical use. Among those ages 65 and older, just 20% say marijuana should not be legal even for medical purposes.
While majorities of Democrats across all age groups support legalizing marijuana for medical and recreational use, older Democrats are less likely to say this.
About half of Democrats ages 75 and older (53%) say marijuana should be legal for both purposes, but much larger shares of younger Democrats say the same (including 81% of Democrats ages 18 to 29). Still, only 7% of Democrats ages 65 and older think marijuana should not be legalized even for medical use, similar to the share of all other Democrats who say this.
Views of the effects of legalizing recreational marijuana among racial and ethnic groups
Substantial shares of Americans across racial and ethnic groups say when marijuana is legal for recreational use, it has a more positive than negative impact on the economy and criminal justice system.
About half of White (52%), Black (53%) and Hispanic (51%) adults say legalizing recreational marijuana is good for local economies. A slightly smaller share of Asian adults (46%) say the same.
Criminal justice
Across racial and ethnic groups, about four-in-ten say that recreational marijuana being legal makes the criminal justice system fairer, with smaller shares saying it would make it less fair.
However, there are wider racial differences on questions regarding the impact of recreational marijuana on the use of other drugs and the safety of communities.
Use of other drugs
Nearly half of Black adults (48%) say recreational marijuana legalization doesn’t have an effect on the use of drugs like heroin, fentanyl and cocaine. Another 32% in this group say it decreases the use of these drugs and 18% say it increases their use.
In contrast, Hispanic adults are slightly more likely to say legal marijuana increases the use of these other drugs (39%) than to say it decreases this use (30%); 29% say it has no impact.
Among White adults, the balance of opinion is mixed: 28% say marijuana legalization increases the use of other drugs and 25% say it decreases their use (45% say it has no impact). Views among Asian adults are also mixed, though a smaller share (31%) say legalization has no impact on the use of other drugs.
Community safety
Hispanic and Asian adults also are more likely to say marijuana’s legalization makes communities less safe: 41% of Hispanic adults and 46% of Asian adults say this, compared with 34% of White adults and 24% of Black adults.
Wide age gap on views of impact of legalizing recreational marijuana
Young Americans view the legalization of marijuana for recreational use in more positive terms compared with their older counterparts.
Clear majorities of adults under 30 say it is good for local economies (71%) and that it makes the criminal justice system fairer (59%).
By comparison, a third of Americans ages 65 and older say legalizing the recreational use of marijuana is good for local economies; about as many (32%) say it makes the criminal justice system more fair.
There also are sizable differences in opinion by age about how legalizing recreational marijuana affects the use of other drugs and the safety of communities.
Facts are more important than ever
In times of uncertainty, good decisions demand good data. Please support our research with a financial contribution.
Report Materials
Table of contents, most americans now live in a legal marijuana state – and most have at least one dispensary in their county, 7 facts about americans and marijuana, americans overwhelmingly say marijuana should be legal for medical or recreational use, clear majorities of black americans favor marijuana legalization, easing of criminal penalties, religious americans are less likely to endorse legal marijuana for recreational use, most popular.
About Pew Research Center Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Am J Trop Med Hyg
- v.93(3 Suppl); 2015 Sep 2

Malaria Diagnosis across the International Centers of Excellence for Malaria Research: Platforms, Performance, and Standardization
Diagnosis is “the act of identifying a disease, illness, or problem by examining someone or something.” When an individual with acute fever presents for clinical attention, accurate diagnosis leading to specific, prompt treatment often saves lives. As applied to malaria, not only individual patient diagnosis is important but also assessing population-level malaria prevalence using appropriate diagnostic methods is essential for public health purposes. Similarly, identifying (diagnosing) fake antimalarial medications prevents the use of counterfeit drugs that can have disastrous effects. Therefore, accurate diagnosis in broad areas related to malaria is fundamental to improving health-care delivery, informing funding agencies of current malaria situations, and aiding in the prioritization of regional and national control efforts. The International Centers of Excellence for Malaria Research (ICEMR), supported by the U.S. National Institute of Allergy and Infectious Diseases, has collaborated on global efforts to improve malaria diagnostics by working to harmonize and systematize procedures across different regions where endemicity and financial resources vary. In this article, the different diagnostic methods used across each ICEMR are reviewed and challenges are discussed.
Introduction
Combating the global malaria burden begins with accurate diagnosis, which guides specific treatment and public health reporting. Reliable malaria diagnosis improves health-care delivery and informs funding agencies of current malaria situations, which is key for prioritization of regional- and national-level control efforts. Adequate malaria diagnostics contribute toward the long-term goal of malaria elimination, but accuracy in both testing and reporting depends on resource availability, health worker expertise, and formal reporting policies that vary among regions. Microscopy and rapid diagnostic tests (RDTs), which detect parasite antigen, are most commonly used to diagnose malaria; each method has its own strengths and weaknesses. 1 Current World health Organization (WHO) recommendations for malaria diagnosis focus on the identification of acute symptomatic malaria. 2 Therefore, individuals with subclinical malaria parasitemia who do not present at health facilities, and those with parasitemia below the detection limit of microcopy or RDTs, are missed. 3 , 4 Individuals with sub-patent, subclinical parasitemia are increasingly recognized as key for maintaining regional malaria transmission, and as malaria transmission declines in some areas because of increased malaria control, such individual contributions to malaria endemicity become increasingly important. 3 , 5 , 6 Because regional malaria transmission profiles will change as control strategies progress to elimination, diagnosing these changes will become necessary on a global level. Consequently, the National Institute of Allergy and Infectious Diseases established the International Centers of Excellence for Malaria Research (ICEMR) program in 2010. As summarized in the foreword of this journal supplement, the ICEMR network includes 10 independent research programs representing all major malaria-endemic regions. Integral to each ICEMR is the inclusion of multiple, epidemiologically contrasting field sites and innovative multidisciplinary approaches and the use of robust diagnostic strategies to help assess the changing malaria situation in each endemic region. The goal of this integrated approach is to generate a knowledge base for improving clinical and field management of malaria. In this article, we discuss laboratory-based diagnostic tools used by ICEMRs, which is a major focus of their research programs.
Since 2010, WHO has recommended either RDT or microscopy confirmation of suspected malaria cases before treatment. 2 The use of RDTs increased globally from less than 200,000 in 2005 to more than 74 million in 2011. 7 The increased availability and use of RDTs in the public sector has resulted in fewer cases of non-malaria acute febrile illness being empirically treated with antimalarial drugs, which helps to prevent development of drug resistance. Although both microscopy and RDTs are of great value in guiding appropriate malaria treatment, subclinical/sub-patent Plasmodium parasitemic individuals do not come to clinical attention and cannot be detected by these techniques because of limited sensitivity. 3 , 5 , 6
During the past decade, highly sensitive and specific nucleic acid amplification techniques have been developed to detect malaria parasites including polymerase chain reaction (PCR), real-time quantitative PCR (qPCR), and reverse transcriptase PCR (RT-PCR) (reviewed in references 1 , 8 ). Compared with light microscopy's limit of detection (about 30 parasites/μL by the best microscopists) or RDTs (> 100 parasites/μL), nucleic acid amplification methods can detect fewer than 10 parasites/μL. 1 , 8 PCR-based methods are extensively used by ICEMRs for the field of malaria epidemiology research. Highly sensitive qPCR methods were developed by either increasing the volume of sample extracted and analyzed 9 or by targeting multi-copy genes. 10 However, such techniques often require sophisticated equipment and training and are significantly more expensive than microscopy and RDT. To make highly sensitive but technologically intense malaria diagnostics more readily available in the resource-limited setting, isothermal amplification techniques were adapted. 11 Loop-mediated isothermal amplification (LAMP), the best characterized of these techniques, is as sensitive and specific as conventional PCR without needing sophisticated equipment for extraction, amplification, or detection. 12 , 13 Comparison of the detection limit between various PCR methods and isothermal detection methods has shown that the lower detection limit of LAMP is 5–10 parasites/μL, comparable to that of conventional PCR. 11 Other isothermal techniques such as nucleic acid sequence–based amplification (NASBA) can achieve detection limit of < 1 parasite/μL on blood sample sizes of at least 50–100 μL. 14 , 15 Other molecular approaches can be used to detect malaria parasites ( Table 1 ). For field-based epidemiology studies, a major focus area of ICEMR efforts, PCR-based methods are most commonly used to detect, quantify, and speciate low-density malaria parasitemia, whether asexual or gametocyte forms. Because of space limitations here, we primarily focused on PCR-based methods currently used by the ICEMR network.
Currently available tools to detect malaria parasites
HRP = histidine-rich protein; LAMP = loop-mediated isothermal amplification; NASBA = nucleic acid sequence–based amplification; pLDH = Plasmodium lactate dehydrogenase; RDT = rapid diagnostic test; qPCR = real-time quantitative PCR; RT-PCR = reverse transcriptase PCR.
Malaria control and elimination strategies depend on providing appropriate treatment of parasitologically confirmed clinical malaria cases as well as asymptomatic carriers. Such specific treatment depends not only on accurate diagnostics but also on effective (and pure or “valid” 16 ) drugs. 17 Throughout the malaria-endemic world, Plasmodium falciparum has developed some level of resistance to many current antimalarial drugs, and chloroquine-resistant Plasmodium vivax has been reported in some regions. 18 To address multidrug resistance malaria parasites, WHO has recommended the use of artemisinin-based combination therapy (ACT) to treat P. falciparum in most malaria-endemic regions. 19 In areas of P. vivax chloroquine resistance, ACTs are also recommended or deployed for treatment of vivax malaria. 20 , 21 Because of the high demand and its market value, there has been an issue with counterfeit ACTs. 22 , 23 A field deployable method to test drug potency is needed to help prevent the emergence of parasite drug resistance. Recent work by the southeast Asia ICEMR toward this end, to develop an assay to verify the quality of artemisinin class antimalarials, is discussed later in the section, RDT to check quality of artemisinin class antimalarials.
The Diagnostic Dilemma and Currently Available Platforms
Accurate diagnosis is essential for treating suspected malaria cases, but in many malaria-endemic regions fever is commonly presumed to be malaria without confirmation. 24 A diagnostic test for malaria must be specific to identify the infecting Plasmodium species, readily available with a rapid turnaround time, and inexpensive. Even though malaria testing in Africa focuses on P. falciparum , effective diagnostics for P. vivax are important elsewhere because parasitemia is typically lower. Identifying very low parasitemia individuals is not feasible with RDTs, but requires highly competent microscopy complemented by molecular assays. Currently, it is not possible to diagnose asymptomatic P. vivax (and Plasmodium ovale ) hypnozoite carriers, yet developing methods to do so is important because the biology of P. vivax makes elimination of this parasite more difficult. 18 , 25 – 27 Even though diagnostic strategies in some countries have started to use combination RDTs more often, RDTs are not useful in elimination settings, but only for diagnosis of acute disease. Further such strategies do not take the diagnosis of P. ovale or Plasmodium malariae into account, particularly in Africa, or the zoonotic Plasmodium knowlesi in Asia. It is important to identify infections due to both single and more than one Plasmodium species to ensure proper antimalarial drug treatment. 2 P. vivax and P. ovale form dormant hypnozoites that should be treated with an 8-aminoquinoline (primaquine; tafenoquine in clinical trials), despite challenges related to using these medications on population scales because of the potential for adverse events due to the presence of even small percentages of people having some form of glucose-6-phosphate dehydrogenase deficiency. 18 , 28 – 33 On a population and geographic level, antimalarial treatment carried out without regard to specificity of diagnosis is a major risk for drug tolerance and resistance as well as potential clinical complications, all of which are concerns throughout the malaria-endemic world. With regard to population-level reservoirs of continuing transmission, a specific diagnostic test must be able to identify sub-patent cases, defined as infections with parasite levels below the limit of microscopic or RDT detection. 5 Sub-patent parasitemia cases likely contribute to transmission in endemic regions. 3 , 6 Furthermore, sub-patent parasitemia cases are often subclinical, and inaccurate detection of subclinical cases, either due to insufficiently effective diagnostics or policies that impede the diagnosis and treatment of subclinical cases, interferes with malaria control efforts in regions, particularly those nearing elimination. The superior sensitivity of molecular diagnostics could ameliorate both of these problems, and provides opportunity for researchers to investigate other important epidemiological questions, including exposure frequency and the transmissibility of malaria parasites based on the quantification of sexual stages.
Currently, there are many molecular diagnostics platforms being used in the ICEMR network ( Table 1 ). Even though their performance has many advantages, most molecular diagnostics are either unable to be sustained in the field because of cost/resource availability or too laborious to be effective for point-of-care diagnosis, hence reducing effectiveness. RDTs have been useful for point-of-care diagnosis, but the issue of false positive results due to the residual antigenemia after the clearance of the parasite remains problematic. 34 – 36 Because of low sensitivity, RDTs are not suitable for diagnosis of very low parasitemias. The limitations of molecular diagnostics and RDTs make the continued use of light microscopy important for accurate diagnosis.
Cross-ICEMR Comparison
Diagnostic methods..
The ICEMR program was designed to include a wide range of malaria transmission intensities in contrasting epidemiological settings. Studies were required to be designed to identify and quantify the Plasmodium species causing malaria toward a more complete understanding of the complex interactions among human hosts, malaria parasites, and mosquito vectors in diverse ecological niches taking into account expected and unexpected changes occur over space and time. All 10 ICEMRs implemented light microscopy–based diagnosis as the primary quantification metric. Nine study sites used microscopy plus RDTs; Amazonia ICEMR did not use RDTs for several reasons: microscopy is generally available and accurate; the dominance of P. vivax compared with P. falciparum malaria, combined with the lactate dehydrogenase (LDH)–based RDTs that were not considered sufficiently sensitive; and national policy requires microscopy-based diagnosis. In Peru, one country in which the Amazonia ICEMR is based, pfhrp2 - and/or pfhrp3 -lacking P. falciparum were first reported, which made the histidine-rich protein 2/3 (HRP2/3)–based RDT inadequate for use as described later in the section, HRP2/3 deletion among the circulating parasites. Among ICEMRs, the choice of different RDTs has been largely dependent on availability and national policy–based recommendations ( Table 2 ). ICEMRs reporting the use of RDT were aware of their limitation. There, RDTs are meant to be used for clinically apparent cases, primarily where P. falciparum is the major parasite and where parasitemia is sufficiently high to enable RDT detection. The sensitivity of RDTs is not sufficient for screening of subclinical cases where the parasitemia is generally very low, a focus of most ICEMRs. All 10 ICEMRs implemented molecular diagnostic methods either by conventional PCR, qPCR, or RT-PCR ( Table 2 ). In addition, half of the ICEMRs have also implemented gametocyte detection by sexual stage ( Pfs25 , Pvs25 )–specific RT-PCR ( Table 2 ). Two of the 10 ICEMRs use nucleic acid amplification techniques as a point-of-care diagnosis (i.e., southern Asia ICEMR and west Africa ICEMR), whereas the majority of the ICEMRs use nucleic acid amplification techniques only in the research setting ( Table 2 ). The detection limit reported by the majority of ICEMRs was 11–50, 51–200, and < 10 parasites/μL for microscopy, RDTs, and molecular diagnostics (i.e., LAMP, conventional PCR, qPCR, and gametocyte-specific RT-PCR), respectively.
Detail of diagnostic tools and the usage at each ICEMR
HRP2 = histidine-rich protein 2; LAMP = loop-mediated isothermal amplification; pLDH = Plasmodium lactate dehydrogenase; QA = quality assurance; QC = quality control; qPCR = quantitative polymerase chain reaction; RDT = rapid diagnostic test; RT-PCR = reverse transcriptase polymerase chain reaction; WHO = World Health Organization.
For the detection of malaria parasite DNA, the majority of ICEMRs reported the use of the 18S rRNA as a target gene either by conventional PCR or qPCR to identify malaria species ( Table 2 ). 37 – 39 Other target genes such as pfr364 , cytb , and pfldh were also used, but less frequently ( Table 2 ). 40 , 41
Factors affecting the sensitivity of different diagnostics.
Microscopy and RDTs are extensively used to diagnose malaria at health facilities. Because of extensive experience, quality assurance/quality control (QA/QC) protocols are well established to assure reliable and comparable results. For light microscopy, double reading of the slides with a third reader for discrepancies is standard among ICEMRs. WHO recommends that parasite quantification be performed against 200 white blood cells (WBCs), and if the parasite count is less than 100 after counting 200 WBCs, the microscopist should continue to 500 WBCs. 42 The west Africa ICEMR reported implementation of minimum count of 300 WBCs per specimen can improve the sensitivity of the slide reading (D. Krogstad, unpublished data). Increasing the WBC count ensures that low-parasitemia cases are not missed, but requires a substantial increase in microscopy time.
WHO collaborates with the Foundation for Innovative New Diagnostics to evaluate and standardize the use of RDTs. 43 For molecular diagnostics, standardization has not been established. The decision to choose a protocol and target genes to use largely depends on the research hypothesis. Nine of the ICEMRs use conventional PCR or qPCR to detect, quantify, and speciate submicroscopic parasitemia. RT-PCR coupled with qPCR is used to detect and quantify the gametocyte stage of the parasite with high sensitivity. 5
Sample preparation.
All 10 ICEMRs currently use at least one of the molecular diagnostic methods such as conventional PCR, qPCR, and LAMP ( Table 2 ). Suitable sample preparation (i.e., DNA extraction) enables optimal sensitivity and is probably the most important variable to affect field deployability. Various DNA extraction methods, which ultimately depend on starting material, have been reported ( Table 3 ). The methods that were reported to be used by ICEMRs are shown in italics in Table 3 . The factors influencing the sensitivity of molecular diagnostics, particularly for conventional PCR, are reviewed in brief, but individual ICEMR protocols are not detailed here.
DNA extraction methods for various starting materials
EDTA = ethylenediaminetetraacetic acid; GTC = guanidine isothiocyanate; LAMP = loop-mediated isothermal amplification; RDT = rapid diagnostic test.
Method reported to be in use by ICEMRs are given in italics.
Dried blood spot (DBS) sampling is commonly used to preserve whole blood samples because it is an easy, durable method with an efficient use of space and optimal transport conditions. In research setting, DBSs have been used as starting material for PCR, RT-PCR, and serology. 59 – 61 Four ICEMRs have collected DBSs and whole blood as packed red cells, five ICEMRs collected only DBS, and one ICEMR collected only whole blood as packed red cells but not DBSs. DNA extraction differed among ICEMRs. Three of the 10 ICEMRs used a Chelex-based extraction method 49 ; the remaining seven ICEMRs used commercial DNA extraction kits, particularly individual spin column–type kits from Qiagen (Hilden, Germany) (Table 3 ). When DBSs were used as starting material for DNA extraction, the number/volume of blood spots varied between ICEMRs and the reported amount varied between using a partial blood spot to using more than one blood spot. One full blood spot is thought to typically correspond to approximately 50 μL blood. The available sample volume is affected by how blood is collected, whether by finger prick or venipuncture. With venipuncture, a higher volume of blood is typically available, therefore the starting sample volume is more standardized.
HRP2/3 deletion among the circulating parasites.
HRP2 is a protein found only in P. falciparum asexual stages and young gametocyte stages. Hence HRP2 has been used in RDTs to detect P. falciparum . 62 Plasmodium LDH (pLDH), from the glycolytic pathway, is found in all malaria species and is the second most commonly used RDT antigen. 62 A third target antigen for RDTs is aldolase, another glycolytic enzyme. 62 Several studies have compared RDTs based on these antigens, with different results in terms of sensitivity, specificity, and positive and negative predictive values. 63 , 64 The majority of RDT experience in the field setting is in the detection of P. falciparum .
RDTs are particularly useful where microscopy is not available, whether because of financial constraints or lack of equipment or sufficiently trained personnel. RDT performance in the field is influenced by many factors such as manufacturing quality, handling and storage conditions, user interpretation, parasite density and limits of detection (which partly depend on parasite biology, e.g., P. vivax parasitemia is characteristically low), and the recent discovery of some region-specific field isolates lacking the HRP2 protein. In 2010, P. falciparum lacking pfhrp2 and/or pfhrp3 genes were first isolated from infected human subjects in the Amazon region of Peru. 65 Other endemic regions also reported false negative results using RDTs based on PfHRP2 due to pfhrp2 and/or pfhrp3 gene deletions. 66 – 68 The presence of pfhrp2 / 3 deletion in P. falciparum strains has serious implications for diagnosis especially for countries and regions where the antimalarial treatment is primarily based on RDT diagnosis. Therefore, it is important to monitor the prevalence of pfhrp2 and/or pfhrp3 gene deletions in regions where RDTs based on PfHRP2 are the primary mode of malaria diagnosis, given the potential need to base procurement decisions on specific RDT detection proteins.
Peru was the first country where pfhrp2 - and/or pfhrp3 -lacking P. falciparum was identified in infected patients. 65 These data indicated that the deletion was widespread throughout the Peruvian Amazon region, which had major implications for miscalculated endemicity based solely on RDT detection. 65 The pfhrp3 deletion was the most prevalent (70%) compared to pfhrp2 (41%), and 22% of isolates had both genes deleted. 65 Peru is not likely the only country affected, as the Amazon River basin runs through other malaria-endemic countries, including Colombia, Ecuador, Brazil, Venezuela, Guyana, Suriname, and Bolivia. Recent reports from these areas showed variable results with regard to the presence/absence of pfhrp2 and/or pfhrp3 . 69 – 71 Plasmodium falciparum with the double deletion of pfhrp2 and pfhrp3 was found in a patient who returned to Europe from Brazil and misdiagnosed using an RDT based on aldolase and PfHRP2; this patient was treated as having P. vivax . 69 Colombia also reported two P. falciparum isolates from clinically infected subjects; however, it was not possible to amplify exon 2 for both pfhrp2 and pfhrp3 . 70 In a recent survey in communities around Iquitos-Nauta road in the Peruvian Amazon region, it was difficult to find P. falciparum PfHRP2-positive individuals even though they were diagnosed positive by light microscopy and pLDH (D. Gamboa, A. Llanos, and J. M. Vinetz, unpublished data). Thus far, Guyana is the only country in South America where all P. falciparum isolates have been found to carry pfhrp2 ; therefore, to date, the performance of PfHRP2-based RDTs here has not yet been affected. 71
RDT to Check Quality of Artemisinin Class Antimalarials
Because they are relatively expensive and widely used, ACTs have become a target of counterfeiters. 22 , 23 Counterfeit artemisinins have the potential to be a public health and clinical threat because they contain little to no active ingredient hence providing inadequate treatment. 72 Substandard drugs not only compromise the expected therapeutic effects, but also facilitate the development of resistance. 73 Although counterfeit artemisinins are known to be a problem in the Greater Mekong subregion of southeast Asia, 74 – 80 the Worldwide Antimalarial Resistance Network database has reported that poor-quality artemisinins are also a growing concern in Africa. 81 – 85 Counterfeit and substandard antimalarials are an immediate and urgent threat to the current momentum of global malaria control and elimination efforts. Artemisinin counterfeiting is becoming more sophisticated. Thus far, as many as 14 different formulations of fake artesunate have been identified. 72 , 86 , 87 To combat the entry of both counterfeit and substandard artemisinin compounds, both national and international regulatory authorities need to strengthen drug quality monitoring to deter the introduction and circulation of poor-quality antimalarials. Until recently, the accurate determination of artemisinin contents in commercial drugs has required sophisticated instrumentation and expertise. The recent development of simple and field-applicable tests to determine the quality of artemisinin drugs is being investigated.
Given that workers in malaria-endemic populations are very familiar with using RDTs for malaria diagnosis, the southeast Asia ICEMR chose to develop a lateral flow dipstick as the point-of-care tests for qualitative and semiquantitative detection of artemisinins in antimalarial drugs. Specific antibodies are needed for such dipstick assays, hence new monoclonal antibodies have been made for such efforts, which include the differentiation of commonly used artemisinin derivatives including artemether, artesunate, and dihydroartemisinin. The first approach was to immunize mice with artesunate conjugated to bovine serum albumin at the succinate group, which yielded a monoclonal antibody (mAb) that reacted with artemisinin, artesunate, and dihydroartemisinin, but with limited reactivity to artemether, 88 , 89 and a separate mAb that was specific for only artesunate (L. Cui, unpublished data). To achieve specificity for different artemisinin derivatives, which differ in R-groups at position 12, conjugation of the artemisinin derivatives to a carrier protein at the opposite position is needed. For artemether, this is achieved through microbial fermentation of artemether and purification of 9-hydroxylartemether. 90 A prototype dipstick was designed based on one selected mAb that was found to have high avidity and broad reactivity for artemisinins, with sensitivity as low as 100–200 and 200–500 ng/mL for artesunate and dihydroartemisinin, respectively. 91 Testing these new tools for monitoring quality of artemisinin drugs is underway. Having specific mAbs in hand for most artemisinin derivatives also allows for enzyme-linked immunosorbent assay quantification.
QA/QC System for Diagnostic Systems and Implementation in ICEMR
All aspects of malaria control and elimination must have robust quality management and control standards. The concept of quality management for malaria diagnostics covers the entire process including establishment of standard operating procedures for each method and technique used in diagnosis, especially sample tracking and handling, to achieve accurate and reliable test results and reporting. QA aims at improving and standardizing each component of this complicated process to minimize or avoid unreliable results. QC emphasizes test accuracy and precision. 92 The goal of QC in diagnosis is to detect, identify, evaluate, and correct errors due to technical failure, environmental conditions, and/or human error.
ICEMR sites have established several quality control strategies, most of which aim to comply with WHO guidelines ( Table 2 ). 42 , 93 In all ICEMR sites, slides are read by two microscopists and a third reader in case of discrepancy ( Table 2 ). Discrepancies in slide reading are usually due to differences in parasite detection limits, species identification, and parasite quantification. In most cases, if there is a disagreement in parasite counts of more than 20% between the two readers, a third reader is consulted to resolve discrepancies. 42 In some areas, more stringent rules may apply; for example in the Peruvian ICEMR the acceptable discrepancy is ≤ 5%.
To maintain microscopy quality, refresher training programs for microscopists using reference slides as recommended by WHO were reported. 42 , 93 For any malaria diagnostic laboratory, one way to promote standardization is to request that slide readers pass the WHO accreditation course and to implement regular refresher training and continuing assessments for microscopists. Another QC tactic to help assure microscopy result quality is to have slides reexamined by microscopists at an external reference laboratory or a collaborating institution.
For molecular diagnosis, the inclusion of well-characterized negative and positive controls (including serial dilutions of positive controls for qPCR) was reported from all ICEMRs. Protocols to freshly extract positive and negative controls to assure efficiency and lack of contamination in processed samples remain to be standardized. WHO provides international standards for P. falciparum DNA, 94 and the Malaria Research and Reference Reagent Resource Center also provides useful reagents and other resources. To verify PCR results, some ICEMRs reported sending a fraction of the samples to be reanalyzed at a reference laboratory or a collaborating institution (i.e., Latin America ICEMR and Amazonia ICEMR). Specificity may be increased by performing more than one PCR assay for the identification of sub-patent infection. Although a formal external QA/QC program is not yet available, various attempts to establish such processes have been made, especially for clinical trials and eradication surveillance. 95 , 96 As qPCR and other more advanced technologies are increasingly used in malaria epidemiology, there is a need to ensure reproducibility of data. A recent article compared different protocols using the WHO International Standard for P. falciparum DNA, 97 leading to a guideline for the minimum information for publication of real-time qPCR experiment. In such a way, the reproducibility of the protocols across different laboratories can be improved. 98
Overcoming Key Gaps
Although WHO has published standardized protocols for microscopy and RDTs, no such protocols exist for nucleic acid amplification–based diagnostic tests such as PCR. Each ICEMR site has established conventional PCR; variation in details of sample preparation (starting material volume, nucleic acid extraction methods) remains a challenge for effective comparisons of diagnostic and treatment outcomes data. Other molecular diagnostic tools, such as qPCR, LAMP, and other sensitive techniques, are either not yet available in all the ICEMR regions or were not selected as part of the research design.
International standardization of diagnostic platforms in settings and research networks such as the ICEMR will be tedious. However, this process will include routine technical training for technicians, monitoring of diagnostic accuracy by external QC procedures, outreach training, reference laboratory support, and regular proficiency testing to ensure comparable results among laboratories. An external QA plan is important to assure valid diagnostic results and accurate reporting. Harmonization of PCR procedures more recently has taken on higher priority given the increasing importance of molecular diagnostics in the ICEMR network and elsewhere in infectious diseases epidemiology, clinical care and research. Access to reference laboratories to assure accurate and available QA/QC in both malaria microscopy and PCR should be readily available, as a matter of public health policy within malaria-endemic regions.
Although this article focused on different diagnostic platforms and protocol standardization, being able to harmonize data across platforms is essential, in the case of the ICEMRs, enabling comparable case definitions across the ICEMRs for which diagnostics lead to interventions, whether for disease or surveillance.
ACKNOWLEDGMENTS
We appreciate the critical reading of the manuscript by Andres Vallejo, Sócrates Herrera, and Don Krogstad. We thank Trevor A. Thompson for information on PCR sensitivity and slide reading quality assurance practice at the West Africa ICEMR. We also thank all the ICEMRs for contributing information and acknowledge Neena Valecha for her guidance regarding the RDTs used in India and India ICEMR project.
Financial support: This work was supported by the following Cooperative Agreements from the United States Public Health Service, National Institute of Allergy and Infectious Diseases: U19AI089672, U19AI089674, 5U19AI089676, U19AI089680, U19AI089681, U19AI089683, U19AI089686, U19AI089688, U19AI089696, and U19AI089702.
Authors' addresses: Tamaki Kobayashi, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, E-mail: ude.uhj@2ayabokt . Dionicia Gamboa, Universidad Peruana Cayetano Heredia, Lima, Peru, E-mail: moc.oohay@aobmaginoid . Daouda Ndiaye, Department of Parasitology and Mycology, Université Cheikh Anta Diop de Dakar, Dakar, Senegal, E-mail: ude.dravrah.hpsh@eyaidnd . Liwang Cui, Department of Entomology, Pennsylvania State University, University Park, PA, E-mail: ude.usp@2cul . Patrick Sutton, Acsel Health, New York, NY, E-mail: moc.liamg@20pottus . Joseph M. Vinetz, Division of Infectious Diseases, Department of Medicine, University of California San Diego School of Medicine, La Jolla, CA, and Universidad Peruana Cayetano Heredia, Lima, Peru, E-mails: ude.dscu@ztenivj or [email protected] .

IMAGES
COMMENTS
1. Introduction. Malaria affected an estimated 219 million people causing 435,000 deaths in 2017 globally. This burden of morbidity and mortality is a result of more than a century of global effort and research aimed at improving the prevention, diagnosis, and treatment of malaria [].Malaria is the most common disease in Africa and some countries in Asia with the highest number of indigenous ...
Malaria is a mosquito-borne disease that is caused by Plasmodium parasites. Patients with malaria experience flu-like symptoms and, in severe cases, the disease can progress to neurological ...
Be bold. Malaria has plagued humans for millennia and has led to an unimaginable loss of life. Malaria has also had an important role in the geopolitics and evolutionary history of humans. The ...
This documentary video discusses the epidemiology of malaria; strategies for prevention, including vector control and vaccines; and the pipeline of promising new drugs for the fight to eliminate ma...
The World Malaria Report, released in December 2021, reflects the unique challenges currently facing the global malaria community. The report showed the devastating toll of malaria, with an estimated 627,000 people losing their lives to the disease in 2020. The improved methodological approach used for calculating cause of death for young children revealed a systematic underestimation of ...
Malaria is a life-threatening febrile illness caused by Plasmodium spp. parasites that are transmitted to humans by infected female Anopheles mosquitoes. According to WHO, 241 million cases ...
50 years after a noble but flawed attempt to eradicate malaria in the mid-20th century, the global malaria community is once again seriously considering eradication. Momentum towards eradication has been building for decades, and more than half of the world's countries are now malaria free. Since 2000, a surge of global progress has occurred, facilitated by the roll-out of new technologies and ...
The WHO updated strategy for malaria also highlights the urgent need for innovation in diagnostic and therapeutic tools. After 30 years of research, there is only one malaria vaccine available, RTS,S, preventing four in ten cases of malaria and three in ten cases of severe malaria among children older than 5 months.The vaccine was introduced as part of a large-scale pilot implementation ...
Malaria is resurging in many African and South American countries, exacerbated by COVID-19-related health service disruption. In 2021, there were an estimated 247 million malaria cases and 619 000 deaths in 84 endemic countries. Plasmodium falciparum strains partly resistant to artemisinins are entrenched in the Greater Mekong region and have emerged in Africa, while Anopheles mosquito vectors ...
Public health strategies for malaria in endemic countries aim to prevent transmission of the disease and control the vector. This historical analysis considers the strategies for vector control developed during the first four decades of the twentieth century. In 1925, policies and technological advances were debated internationally for the first time after the outbreak of malaria in Europe ...
The 2020 World Malaria Report recently looked back on two decades of the global fight against malaria ().Historic reductions in malaria incidence and mortality have occurred since the year 2000 ().From 2000 to 2015, global malaria incidence was reduced by 27% but only by 2% in the years since then, leading the World Health Organization (WHO) to raise the alarm that progress has stalled and the ...
Great progress has been made in recent years to reduce the high level of suffering caused by malaria worldwide. Notably, the use of insecticide-treated mosquito nets for malaria prevention and the use of artemisinin-based combination therapy (ACT) for malaria treatment have made a significant impact. Nevertheless, the development of resistance to the past and present anti-malarial drugs ...
5.6eported malaria cases in R HBHI countries since 2018 and comparisons with estimated cases 48 6.vestments in malaria programmes and research In 52 6.1unding trends for malaria control and elimination 52F 6.2vestments in malaria-related R&D 56In 7.istribution and coverage of malaria prevention, diagnosis and treatment D 58
The malaria genomics era began with the sequencing and assembly of the West African 3D7 clone of P. falciparum, which was published in 2002 (ref. 15) after nearly a decade of collaborative effort ...
A second promising set of results came from a trial, also done in Burkina Faso, of a new vaccine against malaria and published in The Lancet on May 5, 2021, by Mehreen Datoo and colleagues. Children aged 5-17 months were randomly assigned to receive 5 μg of the circumsporozoite protein-based vaccine R21, plus either 25 μg or 50 μg of the adjuvant Matrix-M (MM), or to a placebo of rabies ...
An experimental vaccine that targets the most dangerous form of the malaria parasite was found to have an efficacy of 71% to 77% after 1 year in children. The results, posted as a preprint last week, come from a trial of a vaccine developed by researchers at the University of Oxford's Jenner Institute involving 450 toddlers in Burkina Faso ...
UCF researchers are making advances in finding novel and more effective treatments for malaria, by using cancer drugs and fungi. With two $3.8 million grants from the National Institutes of Health, Debopam Chakrabarti, an infectious disease specialist at the UCF College of Medicine, has been researching new ways to combat drug-resistant malaria.
Apr 1 2024 The World Health Organization. Ministers of Health from African countries with the highest burden of malaria committed today to accelerated action to end deaths from the disease. They ...
This collection of malaria vaccine research and innovation papers highlights the intersection of efforts to: (1) achieve the pan-African 1 and global goal 2 of "Zero Malaria"; and (2) make the ...
Imagine you're a scientist doing research you hope will improve the health of people in your region. Working on a particular question—maybe having to do with tuberculosis (TB), or maternal mortality, or malaria—you come across the title of a scientific research paper that you think may have some answers. But that paper is locked behind the science journal's paywall, and the price for ...
Daniel Kahneman, the Eugene Higgins Professor of Psychology, Emeritus, professor of psychology and public affairs, emeritus, and a Nobel laureate in economics whose groundbreaking behavioral science research changed our understanding of how people think and make decisions, died on March 27. He was 90. Kahneman joined the Princeton University faculty in 1993, following appointments at Hebrew ...
Here, we discuss recent clinical advances in vaccine development and highlight ongoing challenges for the future. Malaria is one of the most devastating infectious diseases in humans, responsible ...
A 57% majority of Republicans ages 18 to 29 favor making marijuana legal for medical and recreational use, compared with 52% among those ages 30 to 49 and much smaller shares of older Republicans. Still, wide majorities of Republicans in all age groups favor legalizing marijuana at least for medical use. Among those ages 65 and older, just 20% ...
The International Centers of Excellence for Malaria Research (ICEMR), supported by the U.S. National Institute of Allergy and Infectious Diseases, has collaborated on global efforts to improve malaria diagnostics by working to harmonize and systematize procedures across different regions where endemicity and financial resources vary. In this ...