Phineas Gage: His Accident and Impact on Psychology
Olivia Guy-Evans, MSc
Associate Editor for Simply Psychology
BSc (Hons) Psychology, MSc Psychology of Education
Olivia Guy-Evans is a writer and associate editor for Simply Psychology. She has previously worked in healthcare and educational sectors.
Learn about our Editorial Process
Saul Mcleod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul Mcleod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
On This Page:

Key Takeaways
- In 1848, 25-year-old Phineas Gage survived an accident where an iron rod was propelled through his left cheek and skull. He made an improbable recovery and lived for 12 more years.
Examination of Gage’s exhumed skull in 1867 revealed the probable trajectory of the tamping iron through left frontal lobe structures, offering insight into his improbable survival and selective changes in behavior following this massive traumatic brain injury.
- Gage’s case is famous in psychology as it shows the resilience of the human brain and profoundly influenced early understanding of cerebral localization.
What happened to Phineas Gage?
Phineas Gage was an American railroad construction foreman born in 1823 near Lebanon, New Hampshire.
On September 13, 1848, when Gage was 25 years old, he was working in Cavendish, Vermont, leading a crew preparing a railroad bed for the Rutland and Burlington Railroad by blasting away rock using explosives.
Around 4:30 pm, as Gage was using a 43-inch-long, 13-pound iron tamping rod to pack the explosive powder into a hole in the rock, the powder detonated unexpectedly.
The tamping iron launched from the hole and entered the left side of Gage’s face from the bottom up.
The iron rod entered Gage’s left cheek near the lower jaw hinge, passing behind his left eye socket, penetrating the base of his skull, traversing the left frontal lobe upwards at an angle, and exiting through the top frontal portion of his skull before landing about 25-30 yards behind him.
After the incident, Gage was thrown onto his back from the force of the iron rod and had some brief convulsions of the arms and legs.
Within minutes, however, assisted by his crew, Gage could stand, speak, and walk to an oxcart to be transported nearly a mile to the inn where he resided in Cavendish village.
Dr. Edward H. Williams arrived about an hour later to examine Gage. In his 1848 report, Williams noted visible pulsations of Gage’s exposed brain through an inverted funnel-shaped opening at the top of his skull from which brain tissue protruded.
Williams claimed that Gage was recounting his injuries to bystanders, and he did not initially believe the story, thinking that Gage was ‘deceived.’
Apparently, Gage had greeted Williams by angling his head at him and saying, ‘Here’s business enough for you.’
During repeated episodic vomiting, Williams observed additional small amounts of Gage’s brain matter expelled onto the floor through the frontal exit wound, as the cerebral tissue had likely detached from the skull during the passage of the tamping iron.
From Harlow’s written account, Gage was considered to be fully recovered and felt fit enough to reapply for his previous role as a foreman.
After an arduous early recovery, Gage eventually regained physical health, though his personality was markedly altered. He lived another 11 years before dying from severe epilepsy in 1860 at age 36.
How Did Phineas Gage’s Personality Change?
The descriptions of Gage’s personality and behavior before the accident are limited.
Before his accident, 25-year-old Gage was described by his railroad employers as a capable and efficient foreman, displaying a strong work ethic, drive, and dependability in overseeing his crews.
However, after surviving passage of the tamping iron through his frontal lobe in 1848, significant changes in Gage’s personality emerged during his physical recovery.
The contractors, who had regarded Gage as ‘efficient and capable’ before the accident, could no longer offer him work due to considerable changes in Gage’s personality.
In medical reports by Dr. John Martyn Harlow in 1848 and 1868, Gage is depicted as struggling with volatility, profanity, little deference for others, impatience, obstinance, unpredictability, and devising plans hastily abandoned.
Harlow wrote that Gage’s equilibrium between intellectual faculties and animal propensities was destroyed, reverting to childlike mental capacity regarding self-restraint and social appropriateness.
Though the specific neuroanatomical links were unclear at the time, Friends and colleagues felt Gage was “no longer Gage” after the traumatic brain injury, unable to process emotions or control impulsive behavior like his pre-accident self.
The shocking changes aligned with emerging localization theories that the frontal lobes regulate personality.
Marlow (1868) described Gage as follows:
“The equilibrium or balance, so to speak, between his intellectual faculties and animal propensities, seems to have been destroyed. He is fitful, irreverent, indulging at times in the grossest profanity (which was not previously his custom), manifesting but little deference for his fellows, impatient of restraint or advice when it conflicts with his desires, at times pertinaciously obstinate, yet capricious and vacillating, devising many plans of future operations, which are no sooner arranged than they are abandoned in turn for others appearing more feasible. A child in his intellectual capacity and manifestations, he has the animal passions of a strong man.”
“Previous to his injury, though untrained in the schools, he possessed a well-balanced mind, and was looked upon by those who knew him as a shrewd, smart business man, very energetic and persistent in executing all his plans of operation. In this regard his mind was radically changed, so decidedly that his friends and acquaintances said he was ‘no longer Gage.”
Through Harlow’s reports, it can be suggested that Gage’s personality changed due to the accident he endured.
The accounts imply that the injury led to a loss of social inhibition, meaning that Gage would behave in ways that were considered inappropriate.
Accuracy of Sources
In his 1848 and 1868 reports, Dr. Harlow provides a limited description of Gage’s pre-accident, stating he was “temperate inhabit, of great energy of character, possessed of considerable stamina of both brain and body” and was “a great favorite” with his men (Harlow, 1848, 1868).
However, later accounts add exaggerated positive traits not found in Harlow’s description. For example, Suinn (1970) describes Gage as enjoying “the respect as well as the favor of his men,” while Myers (1998) calls him “soft-spoken,” and Lahey (1992) says he was “polite and reasonable.”
Other sources paint him as friendly, affable, dependable, conscientious, and happy (Macmillan, 2000).
Similarly, post-accident descriptions often emphasize Gage’s negative qualities while ignoring any positive traits he retained.
Harlow documents personality changes but notes Gage remained employable for a period as a long-distance stagecoach driver in Chile (Harlow, 1868).
However, many accounts focus solely on traits like aggression, unreliability, or aimlessness (Macmillan, 2000). Damasio goes so far as to describe him as behaving violently with no self-control (Blakeslee, 1994).
In this way, later accounts tend to polish Gage’s pre-accident image as an upstanding citizen while presenting an almost cartoonishly perturbed version post-injury – neither in keeping with Harlow’s more nuanced clinical descriptions.
This likely reflects enthusiasm for fitting Gage’s case to localization theories. Macmillan (2000) argues that we must cautiously analyze such embellished personality descriptions when assessing Phineas Gage’s legacy.
Severity of Gage’s Brain Damage
When Gage died in 1861, no autopsies were performed until his skull was later recovered by Harlow years later. The brain damage that caused the significant personality changes was presumed to have involved the left frontal region of the brain.
It was not until 1994 that complex computer-based methods to examine brain damage could be used to investigate whether other areas of the brain were affected.
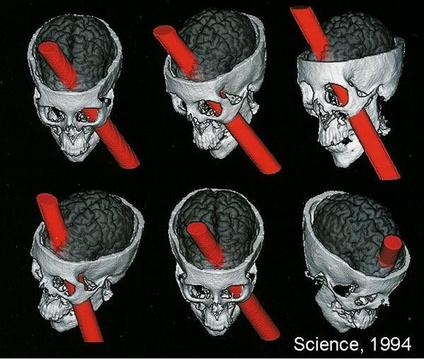
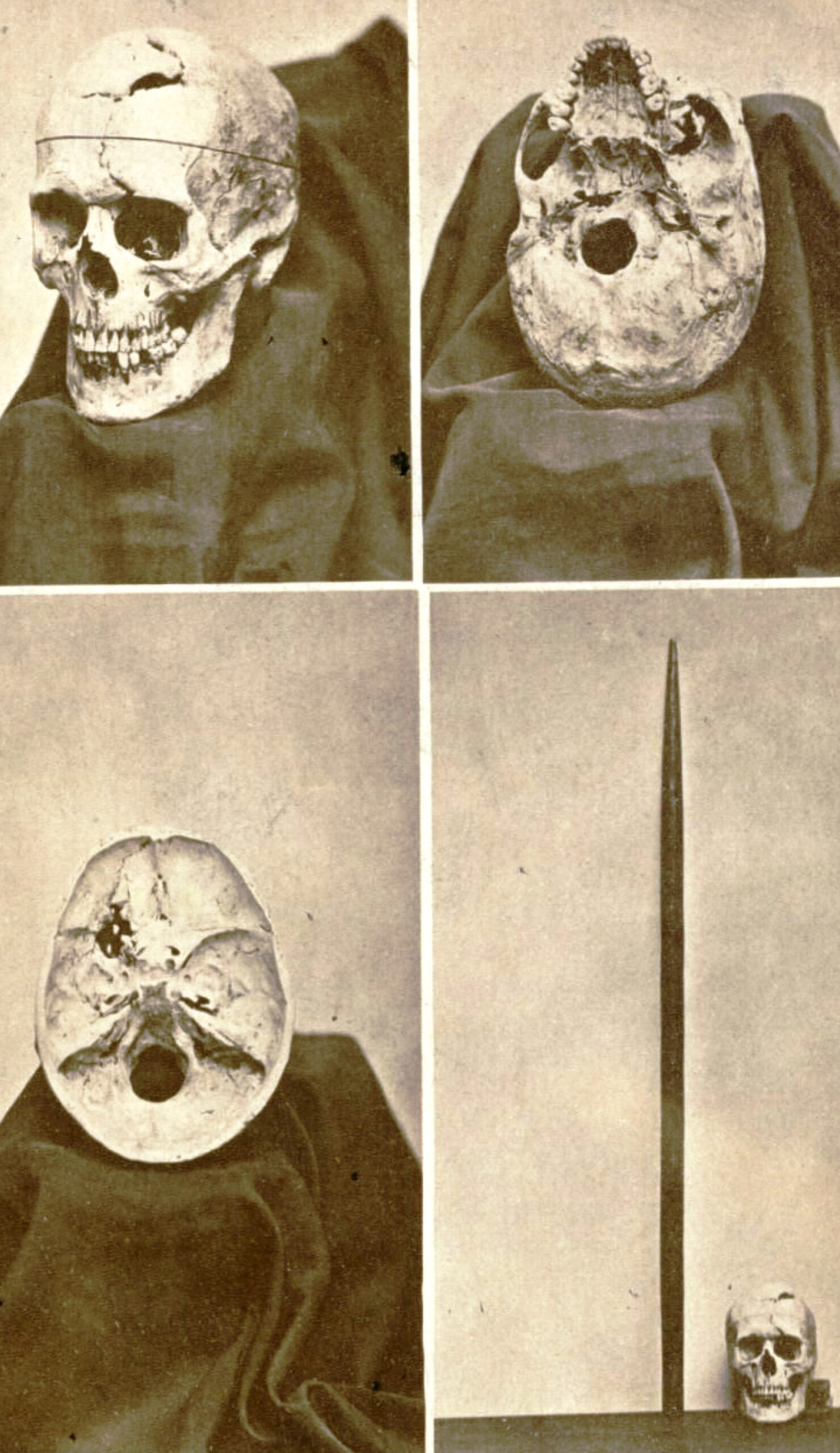
Damasio et al. (1994) used measurements from Gage’s skull and neuroimaging techniques to determine the exact placement of the entry and exit point of the iron rod on a replica model (see Fig. 1).
They found that the damage caused by the rod involved both the left and right prefrontal cortices.
The left and right cortices are responsible for emotional processing and rational decision-making; therefore, it can be assumed that Gage had deficits in these areas.
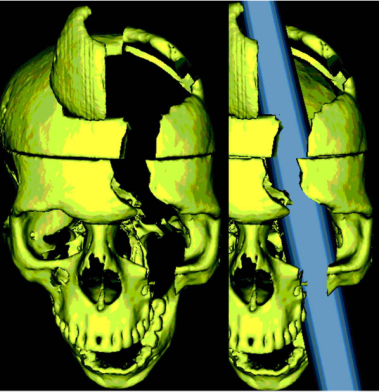
A later study by Ratiu et al. (2004) also investigated Gage’s injury and the location of where the iron rod entered and exited the head. They used Gage’s actual skull rather than a model of it, as Damasio et al. (1994) had used.
Ratiu et al. (2004) generated three-dimensional reconstructions of the skull using computed tomography scans (CAT) and found that the extent of the brain injury was limited to the left frontal lobe only and did not extend to the right lobe (see Fig. 2).
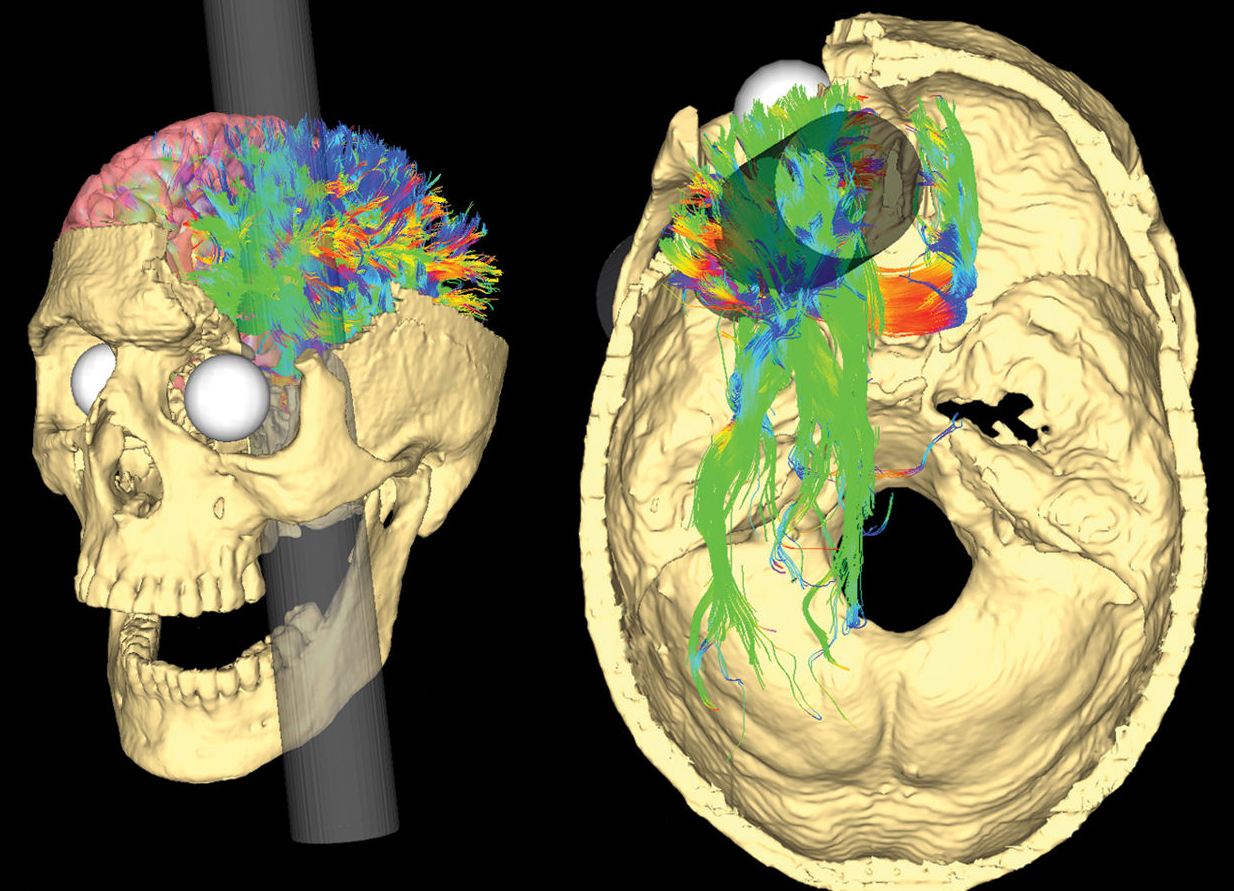
More recently, Van Horn et al. (2012) used a CAT scan of Gage’s skull as well as magnetic resonance imaging (MRI) data obtained from male participants of a similar age to Gage at the time (aged 25-36).
Their results supported Ratiu et al. (2004) in that they always concluded that the rod only damaged the left lobe and not the right.
Van Horn, however, went a step further in their research and investigated the potential levels of white and grey matter damaged due to Gage’s injury. White matter is deep in the brain and provides vital connections around the brain, essential to normal motor and sensory function.
Grey matter in the brain is essential to many areas of higher learning, including attention, memory, and thought.
The research by Van Horn proposed that Gage lost about 11% of his white matter and about 4% of his grey matter. White matter has the ability to regenerate, so this could explain why Gage recovered as well as he did.
Van Horn et al. (2012) compared Gage’s white matter damage to the damage that is caused by neurogenerative diseases such as Alzheimer’s.
This is supported by other studies that have found that changes in white matter is significantly associated with Alzheimer’s disease (Nasrabady, Rizvi, Goldman & Brickman, 2018; Kao, Chou, Chen & Yang, 2019).
It could be suggested that Gage’s apparent change in personality could have been the result of an early onset of Alzheimer’s.
However, as Dr. Harlow, who examined Gage, only reported on Gage’s behaviors shortly after his accident, rather than months or years later when Alzheimer’s symptoms may have emerged, we cannot be certain whether Gage actually had this condition.
All studies investigating the brain damage suffered by Gage is essentially all speculation as we cannot know for certain the extent of the accident’s effects.
We know that some brain tissue got destroyed, but any infections Gage may have suffered after the accident may have further destroyed more brain tissue.
We also cannot determine the exact location where the iron rod entered Gage’s skull to the millimeter. As brain structure varies from person to person, researchers cannot ever know for certain what areas of Gage’s brain were destroyed.
What Happened to Phineas Gage After the Brain Damage?
Dr. John Martyn Harlow took over Gage’s case soon after. Harlow (1848) reported that Gage was fully conscious and recognized Harlow immediately but was tired from the bleeding.
In the next couple of days, Harlow observed that Gage spoke with some difficulty but could name his friends, and the bleeding ceased. Gage then spent September 23rd to October 3rd in a semi-comatose state but was able to take steps out of bed by October 7th.
By October 11th, Harlow claimed Gage’s intellectual functioning began to improve. He recognized how much time had passed since the accident and could describe the accident clearly.
Four years after his injury, Gage moved to Chile and worked taking care of horses and being a stagecoach driver.
Harlow noted emerging personality changes in this period, with Gage becoming more erratic in behavior and responsibility.
In 1860, Gage moved to San Francisco to live near family but began suffering epileptic seizures – likely related to scar tissue and injury sequelae.
The convulsions worsened over months, and on May 21, 1861, almost 13 years after his shocking accident, Gage died at age 38 from complications of severe epilepsy.
How did Phineas Gage die?
On May 21st, 1861, twelve years after his accident, Gage died after having a series of repeated epileptic convulsions.
In 1867, Harlow arranged an exhumation of Gage’s body, claiming his skull and tamping iron for medical study.
These historic artifacts remain on display at the Harvard School of Medicine.
Though Gage initially survived, it was the secondary long-term effects of this massive brain injury that ultimately led to his premature death over a decade later.
Why Is Phineas Gage Important to Psychology?
Gage’s case is important in the field of neuroscience . The reported changes in his behavior post-accident are strong evidence for the localization of brain function , meaning that specific brain areas are associated with certain functions.
Neuroscientists have a better understanding of the function of the frontal cortex today. They understand that the frontal cortex is associated with language, decision-making, intelligence, and reasoning functions. Gage’s case became one of the first pieces of evidence suggesting that the frontal lobe was directly involved in personality.
It was believed that brain lesions caused permanent deficits in a person. However, Gage was proven to have recovered remarkably and lived a mostly normal life despite his injury. It was even suggested by a psychologist called Malcolm Macmillan that Gage may have relearned lost skills.
People with damage to their frontal lobes tend to have trouble completing tasks, get easily distracted, and have trouble planning.
Despite this damage to his frontal lobe, Gage was reported to have worked as a coach driver which would have involved Gage being focused and having a routine, as well as knowing his routes and multitasking.
Macmillan (2002), therefore, suggests that Gage’s damage to the frontal lobe could have somewhat repaired itself and recovered lost functions. The ability of the brain to change in this way is called brain plasticity .
Over time, Gage’s story has been retold, and this has sometimes led to a lot of exaggeration as to the personality changes of Gage.
Some popular reports described him as a hard-working, kind man prior to the accident and then described him as an aggressive, dishonest, and drunk man who could not hold down a job and died pennilessly.
Gage’s story seemed to take on a life of its own, and some even went as far as to say that Gage became a psychopath after his accident, without any facts behind this.
From the actual reports from the people in contact with Gage at the time, it appears that his personality change was nowhere near as extreme and that Gage was far more functional than some reports would have us believe (Macmillan, 2002).
Blakeslee, S. (1994, July 6). A miraculous recovery that went wrong . New York Times.
Damasio, H., Grabowski, T., Frank, R., Galaburda, A. M., & Damasio, A. R. (1994). The return of Phineas Gage: clues about the brain from the skull of a famous patient . Science, 264 (5162), 1102-1105.Harlow J. M. (1848). Passage of an iron rod through the head. Boston Medical and Surgical Journal, 39 , 389–393.
Harlow, J. M. (1868). Recovery from the Passage of an Iron Bar through the Head . Publications of the Massachusetts Medical Society. 2 (3), 327-347.
Kao, Y. H., Chou, M. C., Chen, C. H., & Yang, Y. H. (2019). White matter changes in patients with Alzheimer’s disease and associated factors . Journal of Clinical Medicine, 8 (2), 167.
Lahey, B. B. (1992). Psychology: An introduction . Wm. C. Brown Publishers.
Macmillan, M. (2000). Restoring Phineas Gage: A 150th retrospective. Journal of the History of the Neurosciences, 9 (1), 46-66.
Macmillan, M. (2002). An odd kind of fame: Stories of Phineas Gage. MIT Press.
Myers, D. G. (1998). Psychology (5th ed.). Worth Publishers.
Nasrabady, S. E., Rizvi, B., Goldman, J. E., & Brickman, A. M. (2018). White matter changes in Alzheimer’s disease: a focus on myelin and oligodendrocytes. Acta neuropathologica communications, 6 (1), 1-10.
Ratiu, P., Talos, I. F., Haker, S., Lieberman, D., & Everett, P. (2004). The tale of Phineas Gage, digitally remastered . Journal of neurotrauma, 21 (5), 637-643.
Suinn, R. M. (1970). Fundamentals of behavior pathology. Wiley.
Van Horn, J. D., Irimia, A., Torgerson, C. M., Chambers, M. C., Kikinis, R., & Toga, A. W. (2012). Mapping connectivity damage in the case of Phineas Gage . PloS one, 7(5) , e37454.
Further Reading
- Griggs, R. A. (2015). Coverage of the Phineas Gage Story in Introductory Psychology Textbooks: Was Gage No Longer Gage?. Teaching of Psychology, 42(3), 195-202.
- Wilgus, J., & Wilgus, B. (2009). Face to face with Phineas Gage. Journal of the History of the Neurosciences, 18(3), 340-345.
- Macmillan, M., & Lena, M. L. (2010). Rehabilitating Phineas Gage. Neuropsychological Rehabilitation, 20, 641–658.
- Macmillan, M. (2000). Restoring phineas gage: a 150th retrospective. Journal of the History of the Neurosciences, 9(1), 46-66.
- Kotowicz, Z. (2007). The strange case of Phineas Gage. History of the Human Sciences, 20(1), 115-131.
- O”driscoll K, Leach JP. “No longer Gage”: an iron bar through the head. Early observations of personality change after injury to the prefrontal cortex. BMJ. 1998;317(7174):1673-4. doi:10.1136/bmj.317.7174.1673a
If a person suffers from a traumatic brain injury in the prefrontal cortex, similar to that of Phineas Gage, what changes might occur?
A traumatic brain injury to the prefrontal cortex could result in significant changes in personality, emotional regulation, and executive function. This region is vital for impulse control, decision-making, and moderating social behavior.
A person may exhibit increased impulsivity, poor judgment, and reduced ability to plan or organize. Emotional volatility and difficulty in interpersonal relationships may also occur.
Just like the case of Phineas Gage, who became more impulsive and less dependable, the injury could dramatically alter one’s character and abilities.
- Bipolar Disorder
- Therapy Center
- When To See a Therapist
- Types of Therapy
- Best Online Therapy
- Best Couples Therapy
- Best Family Therapy
- Managing Stress
- Sleep and Dreaming
- Understanding Emotions
- Self-Improvement
- Healthy Relationships
- Student Resources
- Personality Types
- Guided Meditations
- Verywell Mind Insights
- 2024 Verywell Mind 25
- Mental Health in the Classroom
- Editorial Process
- Meet Our Review Board
- Crisis Support
Phineas Gage: His Accident and Impact on Psychology
Author unknown / Wikimedia Commons
- Phineas Gage's Accident
- Change in Personality
- Severity of Brain Damage
- Impact on Psychology
What Happened to Phineas Gage After the Brain Damage?
Phineas Gage is often referred to as the "man who began neuroscience." He experienced a traumatic brain injury when an iron rod was driven through his skull, destroying much of his frontal lobe .
Gage miraculously survived the accident. However, his personality and behavior were so changed as a result of the frontal lobe damage that many of his friends described him as an almost different person entirely. The impact that the accident had has helped us better understand what the frontal lobe does, especially in relation to personality .
At a Glance
In 1848, Phineas Gage had a workplace accident in which an iron tamping rod entered and exited his skull. He survived but it is said that his personality changed as a result, leading to a greater understanding of the brain regions involved in personality, namely the frontal lobe.
Phineas Gage's Accident
On September 13, 1848, 25-year-old Gage was working as the foreman of a crew preparing a railroad bed near Cavendish, Vermont. He was using an iron tamping rod to pack explosive powder into a hole.
Unfortunately, the powder detonated, sending the 43-inch-long, 1.25-inch-diameter rod hurling upward. The rod penetrated Gage's left cheek, tore through his brain , and exited his skull before landing 80 feet away.
Gage not only survived the initial injury but was able to speak and walk to a nearby cart so he could be taken into town to be seen by a doctor. He was still conscious later that evening and able to recount the names of his co-workers. Gage even suggested that he didn't wish to see his friends since he would be back to work in "a day or two" anyway.
The Recovery Process
After developing an infection, Gage spent September 23 to October 3 in a semi-comatose state. On October 7, he took his first steps out of bed, and, by October 11, his intellectual functioning began to improve.
Descriptions of Gage's injury and mental changes were made by Dr. John Martyn Harlow. Much of what researchers know about the case is based on Harlow's observations.
Harlow noted that Gage knew how much time had passed since the accident and remembered clearly how the accident occurred, but had difficulty estimating the size and amounts of money. Within a month, Gage was well enough to leave the house.
In the months that followed, Gage returned to his parent's home in New Hampshire to recuperate. When Harlow saw Gage again the following year, the doctor noted that while Gage had lost vision in his eye and was left with obvious scars from the accident, he was in good physical health and appeared recovered.
Theories About Gage's Survival and Recovery
The type of injury sustained by Phineas Gage could have easily been fatal. While it cannot be said with certainty why Gage was able to survive the accident, let alone recover from the injury and still function, several theories exist. They include:
- The rod's path . Some researchers suggest that the rod's path likely played a role in Gage's survival in that if it had penetrated other areas of the head—such as the pterygoid plexuses or cavernous sinus—Gage may have bled to death.
- The brain's selective recruitment . In a 2022 study of another individual who also had an iron rod go through his skull—whom the researchers referred to as a "modern-day Phineas Gage"—it was found that the brain is able to selectively recruit non-injured areas to help perform functions previously assigned to the injured portion.
- Work structure . Others theorize that Gage's work provided him structure, positively contributing to his recovery and aiding in his rehabilitation.
How Did Phineas Gage's Personality Change?
Popular reports of Gage often depict him as a hardworking, pleasant man before the accident. Post-accident, these reports describe him as a changed man, suggesting that the injury had transformed him into a surly, aggressive heavy drinker who was unable to hold down a job.
Harlow presented the first account of the changes in Gage's behavior following the accident. Where Gage had been described as energetic, motivated, and shrewd prior to the accident, many of his acquaintances explained that after the injury, he was "no longer Gage."
Severity of Gage's Brain Damage
Since there is little direct evidence of the exact extent of Gage's injuries aside from Harlow's report, it is difficult to know exactly how severely his brain was damaged. Harlow's accounts suggest that the injury did lead to a loss of social inhibition, leading Gage to behave in ways that were seen as inappropriate.
In a 1994 study, researchers utilized neuroimaging techniques to reconstruct Phineas Gage's skull and determine the exact placement of the injury. Their findings indicate that he suffered injuries to both the left and right prefrontal cortices, which would result in problems with emotional processing and rational decision-making .
Another study conducted in 2004 used three-dimensional, computer-aided reconstruction to analyze the extent of Gage's injury. It found that the effects were limited to the left frontal lobe.
In 2012, new research estimated that the iron rod destroyed approximately 11% of the white matter in Gage's frontal lobe and 4% of his cerebral cortex.
Some evidence suggests that many of the supposed effects of the accident may have been exaggerated and that Gage was actually far more functional than previously reported.
Why Is Phineas Gage Important to Psychology?
Gage's case had a tremendous influence on early neurology. The specific changes observed in his behavior pointed to emerging theories about the localization of brain function, or the idea that certain functions are associated with specific areas of the brain.
In those years, neurology was in its infancy. Gage's extraordinary story served as one of the first sources of evidence that the frontal lobe was involved in personality.
Today, scientists better understand the role that the frontal cortex has to play in important higher-order functions such as reasoning , language, and social cognition .
After the accident, Gage was unable to continue his previous job. According to Harlow, Gage spent some time traveling through New England and Europe with his tamping iron to earn money, supposedly even appearing in the Barnum American Museum in New York.
He also worked briefly at a livery stable in New Hampshire and then spent seven years as a stagecoach driver in Chile. He eventually moved to San Francisco to live with his mother as his health deteriorated.
After a series of epileptic seizures, Gage died on May 21, 1860, almost 12 years after his accident. Seven years after his death, Gage's body was exhumed. His brother gave his skull and the tamping rod to Dr. Harlow, who subsequently donated them to the Harvard University School of Medicine. They are still exhibited in its museum today.
Bottom Line
Gage's accident and subsequent experiences serve as a historical example of how case studies can be used to look at unique situations that could not be replicated in a lab. What researchers learned from Phineas Gage's skull and brain injury played an important role in the early days of neurology and helped scientists gain a better understanding of the human brain and the impact that damage could have on both functioning and behavior.
Sevmez F, Adanir S, Ince R. Legendary name of neuroscience: Phineas Gage (1823-1860) . Child's Nervous System . 2020. doi:10.1007/s00381-020-04595-6
Twomey S. Phineas Gage: Neuroscience's most famous patient . Smithsonian Magazine.
Harlow JM. Recovery after severe injury to the head . Bull Massachus Med Soc . 1848. Reprinted in Hist Psychiat. 1993;4(14):274-281. doi:10.1177/0957154X9300401407
Harlow JM. Passage of an iron rod through the head . 1848. J Neuropsychiatry Clin Neurosci . 1999;11(2):281-3. doi:10.1176/jnp.11.2.281
Itkin A, Sehgal T. Review of Phineas Gage's oral and maxillofacial injuries . J Oral Biol . 2017;4(1):3.
de Freitas P, Monteiro R, Bertani R, et al. E.L., a modern-day Phineas Gage: Revisiting frontal lobe injury . The Lancet Regional Health - Americas . 2022;14:100340. doi:10.1016/j.lana.2022.100340
Macmillan M, Lena ML. Rehabilitating Phineas Gage . Neuropsycholog Rehab . 2010;20(5):641-658. doi:10.1080/09602011003760527
O'Driscoll K, Leach JP. "No longer Gage": An iron bar through the head. Early observations of personality change after injury to the prefrontal cortex . BMJ . 1998;317(7174):1673-4. doi:10.1136/bmj.317.7174.1673a
Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: Clues about the brain from the skull of a famous patient . Science . 1994;264(5162):1102-5. doi:10.1126/science.8178168
Ratiu P, Talos IF. Images in clinical medicine. The tale of Phineas Gage, digitally remastered . N Engl J Med . 2004;351(23):e21. doi:10.1056/NEJMicm031024
Van Horn JD, Irimia A, Torgerson CM, Chambers MC, Kikinis R, Toga AW. Mapping connectivity damage in the case of Phineas Gage . PLoS One . 2012;7(5):e37454. doi: 10.1371/journal.pone.0037454
Macmillan M. An Odd Kind of Fame: Stories of Phineas Gage . MIT Press.
Shelley B. Footprints of Phineas Gage: Historical beginnings on the origins of brain and behavior and the birth of cerebral localizationism . Archives Med Health Sci . 2016;4(2):280-6. doi:10.4103/2321-4848.196182
By Kendra Cherry, MSEd Kendra Cherry, MS, is a psychosocial rehabilitation specialist, psychology educator, and author of the "Everything Psychology Book."
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

The answer to your search may depend on where you live
Putting human past on the MAPS

Does AI help humans make better decisions?
Video: Editor Kai-Jae Wang; videographers Joe Sherman and Kai-Jae Wang; interviews by Ned Brown
Lessons of the brain: The Phineas Gage story
Harvard Correspondent
In 1848, an iron bar pierced his brain, his case providing new insights on both trauma and recovery
Imagine the modern-day reaction to a news story about a man surviving a three-foot, 7-inch, 13½-pound iron bar being blown through his skull — taking a chunk of his brain with it.
Then imagine that this happened in 1848, long before modern medicine and neuroscience. That was the case of Phineas Gage.
Whether the Vermont construction foreman, who was laying railroad track and using explosives at the time of the industrial accident, was lucky or unlucky is a judgment that Warren Anatomical Museum curator Dominic Hall puzzles over to this day.
“It is an impossible question, because he was extraordinarily unlucky to have an iron bar borne through his skull, but equally lucky to have survived, on such a low level of care,” said Hall. “We are lucky, to have him.”
Gage’s skull, along with the tamping iron that bore through it, are two of the approximately 15,000 artifacts and case objects conserved at the Warren, which is a part of the Center for the History of Medicine in Harvard’s Francis A. Countway Library of Medicine .
The resultant change in Gage’s personality — when he went from being well-liked and professionally successful to being “fitful, irreverent, and grossly profane, showing little deference for his fellows” and unable to keep his job — is widely cited in modern psychology as the textbook case for post-traumatic social disinhibition.
But as the years have gone by and we’ve learned more about his life, argued Hall, the teachings have changed.
“In 1848, he was seen as a triumph of human survival. Then, he becomes the textbook case for post-traumatic personality change. Recently, people interpret him as having found a form of independence and social recovery, which he didn’t get credit for 15 years ago.”
When Gage died 12 years after the accident, following epileptic seizures, his body was exhumed, while his skull and tamping iron were sent to the physician who had cared for him since the accident, John Harlow. Harlow later donated the items to the Warren, where they have remained for 160 years.
“In many ways, I see Gage similarly to how you would see a portrait of one of the famous professors hanging on the wall — he’s an important part of Harvard Medical School’s identity,” said Hall. “By continually reflecting on his case, it allows us to change how we reflect on the human brain and how we interact with our historical understanding of neuroscience.”
Share this article
You might like.
Researchers find ‘language bias’ in various site algorithms, raising concerns about fallout for social divisions among nations

Harvard digital atlas plots patterns from history ancient and modern

One judge’s track record — with and without algorithm — surprises researchers
When should Harvard speak out?
Institutional Voice Working Group provides a roadmap in new report
Had a bad experience meditating? You're not alone.
Altered states of consciousness through yoga, mindfulness more common than thought and mostly beneficial, study finds — though clinicians ill-equipped to help those who struggle
College sees strong yield for students accepted to Class of 2028
Financial aid was a critical factor, dean says

- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center

- Is Internet technology "making us stupid"?
- What is the impact of artificial intelligence (AI) technology on society?

Phineas Gage
Our editors will review what you’ve submitted and determine whether to revise the article.
- The British Psychological Society - Phineas Gage – Unravelling the myth
- Frontiers - Neuroscience Education Begins With Good Science: Communication About Phineas Gage (1823–1860), One of Neurology’s Most-Famous Patients, in Scientific Articles
- National Center for Biotechnology Information - PubMed Central - Phineas Gage's great legacy
- Verywell Mind - Phineas Gage: His Accident and Impact on Psychology
- The Guardian - Phineas Gage and the Effect of an Iron Bar Through the Head on Personality
- Smithsonian.com - Phineas Gage: Neuroscience's Most Famous Patient
- The University of Akron Ohio's Polytechnic University - Biography of Phineas Gage

Phineas Gage (born July 1823, New Hampshire, U.S.—died May 1860, California) was an American railroad foreman known for having survived a traumatic brain injury caused by an iron rod that shot through his skull and obliterated the greater part of the left frontal lobe of his brain.
Little is known about Gage’s early life other than that he was born into a family of farmers and was raised on a family farm in New Hampshire . At some point he took up work on the construction of railways and came under the employment of contractors who were working with the Rutland and Burlington Railroad company. Among Gage’s duties was to clear rocks to level the ground. The task involved placing an explosive charge deep into the rock by drilling a hole. The hole was then filled with gunpowder , and a fuse was set. Sand was added on top of the explosive material to prevent contact. A tamping rod was then used to pack the explosives into the rock. On the afternoon of September 13, 1848, near Cavendish, Vermont, Gage tamped down the powder without the addition of the sand. As his tamping rod, which measured 3.58 feet (about 1 metre) in length and 1.25 inches (about 3.2 cm) in diameter, struck against the side of the rock, it ignited the gunpowder. The rod shot completely through Gage’s head and landed almost 82 feet (25 metres) behind him. The 13.25-pound (6-kg) rod entered Gage’s head just below his left cheekbone and exited from the top of his skull.
Gage survived the accident and immediately afterward was conscious and able to speak. About 10 days later, however, he endured a brief period in which he was barely conscious; his doctors anticipated his death. But Gage quickly recovered, and, within a matter of months, he regained his physical strength and was able to return to work. He sustained no motor or speech impairments , and his memory remained intact. However, Gage’s personality appears to have changed (for a time at least), causing his colleagues to state that he was “no longer Gage.” While some have described Gage as restless, disrespectful, and unreliable following the accident, the true extent of the personality changes he experienced are unknown. Little was documented about his personality or behaviour prior to and after the accident.
In 1852 Gage took a job in Chile, working as a stagecoach driver, having apparently either regained or maintained at least some social skills. Seven years later, in poor health, he moved to California to live with his mother and sister (who had moved there from New Hampshire). Nearly 12 years after his injury, Gage died of epileptic seizures. His skull and iron tamping rod were put on permanent exhibition at Harvard Medical School’s Warren Anatomical Museum in Cambridge, Massachusetts.
Phineas Gage: Neuroscience’s Most Famous Patient
An accident with a tamping iron made Phineas Gage history’s most famous brain-injury survivor
Steve Twomey
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/Phineas-Gage-631.jpg)
Jack and Beverly Wilgus, collectors of vintage photographs, no longer recall how they came by the 19th-century daguerreotype of a disfigured yet still-handsome man. It was at least 30 years ago. The photograph offered no clues as to where or precisely when it had been taken, who the man was or why he was holding a tapered rod. But the Wilguses speculated that the rod might be a harpoon, and the man’s closed eye and scarred brow the result of an encounter with a whale.
So over the years, as the picture rested in a display case in the couple’s Baltimore home, they thought of the man in the daguerreotype as the battered whaler.
In December 2007, Beverly posted a scan of the image on Flickr, the photo-sharing Web site, and titled it “One-Eyed Man with Harpoon.” Soon, a whaling enthusiast e-mailed her a dissent: that is no harpoon, which suggested that the man was no whaler. Months later, another correspondent told her that the man might be Phineas Gage and, if so, this would be the first known image of him.
Beverly, who had never heard of Gage, went online and found an astonishing tale.
In 1848, Gage, 25, was the foreman of a crew cutting a railroad bed in Cavendish, Vermont. On September 13, as he was using a tamping iron to pack explosive powder into a hole, the powder detonated. The tamping iron—43 inches long, 1.25 inches in diameter and weighing 13.25 pounds—shot skyward, penetrated Gage’s left cheek, ripped into his brain and exited through his skull, landing several dozen feet away. Though blinded in his left eye, he might not even have lost consciousness, and he remained savvy enough to tell a doctor that day, “Here is business enough for you.”
Gage’s initial survival would have ensured him a measure of celebrity, but his name was etched into history by observations made by John Martyn Harlow, the doctor who treated him for a few months afterward. Gage’s friends found him“no longer Gage,” Harlow wrote. The balance between his “intellectual faculties and animal propensities” seemed gone. He could not stick to plans, uttered “the grossest profanity” and showed “little deference for his fellows.” The railroad-construction company that employed him, which had thought him a model foreman, refused to take him back. So Gage went to work at a stable in New Hampshire, drove coaches in Chile and eventually joined relatives in San Francisco, where he died in May 1860, at age 36, after a series of seizures.
In time, Gage became the most famous patient in the annals of neuroscience, because his case was the first to suggest a link between brain trauma and personality change. In his book An Odd Kind of Fame: Stories of Phineas Gage , the University of Melbourne’s Malcolm Macmillan writes that two-thirds of introductory psychology textbooks mention Gage. Even today, his skull, the tamping iron and a mask of his face made while he was alive are the most sought-out items at the Warren Anatomical Museum on the Harvard Medical School campus.
Michael Spurlock, a database administrator in Missoula, Montana, happened upon the Wilgus daguerreotype on Flickr in December 2008. As soon as he saw the object the one-eyed man held, Spurlock knew it was not a harpoon. Too short. No wooden shaft. It looked more like a tamping iron, he thought. Instantly, a name popped into his head: Phineas Gage. Spurlock knew the Gage story well enough to know that any photograph of him would be the first to come to light. He knew enough, too, to be intrigued by Gage’s appearance, if it was Gage. Over the years, accounts of his changed character had gone far beyond Harlow’s observations, Macmillan says, turning him into an ill-tempered, shiftless drunk. But the man in the Flickr photogragh seemed well-dressed and confident.
It was Spurlock who told the Wilguses that the man in their daguerreotype might be Gage. After Beverly finished her online research, she and Jack concluded that the man probably was. She e-mailed a scan of the photograph to the Warren museum. Eventually it reached Jack Eckert, the public-services librarian at Harvard’s Center for the History of Medicine. “Such a ‘wow’ moment,” Eckert recalls. It had to be Gage, he determined. How many mid-19th-century men with a mangled eye and scarred forehead had their portrait taken holding a metal tool? A tool with an inscription on it?
The Wilguses had never noticed the inscription; after all, the daguerreotype measures only 2.75 inches by 3.25 inches. But a few days after receiving Spurlock’s tip, Jack, a retired photography professor, was focusing a camera to take a picture of his photograph. “There’s writing on that rod!” Jack said. He couldn’t read it all, but part of it seemed to say, “through the head of Mr. Phi...”
In March 2009, Jack and Beverly went to Harvard to compare their picture with Gage’s mask and the tamping iron, which had been inscribed in Gage’s lifetime: “This is the bar that was shot through the head of Mr. Phinehas P. Gage,” it reads, misspelling the name.
Harvard has not officially declared that the daguerreotype is of Gage, but Macmillan, whom the Wilguses contacted next, is quite certain. He has also learned of another photograph, he says, kept by a descendant of Gage’s.
As for Spurlock, when he got word that his hunch was apparently correct, “I threw open the hallway door and told my wife, ‘I played a part in a historical discovery!’ ”
Steve Twomey is based in New Jersey. He wrote about map and document thieves for the April 2008 issue of Smithsonian .

Get the latest History stories in your inbox?
Click to visit our Privacy Statement .
- Skip to main content
- Keyboard shortcuts for audio player
Your Health
- Treatments & Tests
- Health Inc.
- Public Health
Why Brain Scientists Are Still Obsessed With The Curious Case Of Phineas Gage

Jon Hamilton

Cabinet-card portrait of brain-injury survivor Phineas Gage (1823–1860), shown holding the tamping iron that injured him. Wikimedia hide caption
Cabinet-card portrait of brain-injury survivor Phineas Gage (1823–1860), shown holding the tamping iron that injured him.
It took an explosion and 13 pounds of iron to usher in the modern era of neuroscience.
In 1848, a 25-year-old railroad worker named Phineas Gage was blowing up rocks to clear the way for a new rail line in Cavendish, Vt. He would drill a hole, place an explosive charge, then pack in sand using a 13-pound metal bar known as a tamping iron.
But in this instance, the metal bar created a spark that touched off the charge. That, in turn, "drove this tamping iron up and out of the hole, through his left cheek, behind his eye socket, and out of the top of his head," says Jack Van Horn , an associate professor of neurology at the Keck School of Medicine at the University of Southern California.
Gage didn't die. But the tamping iron destroyed much of his brain's left frontal lobe, and Gage's once even-tempered personality changed dramatically.
"He is fitful, irreverent, indulging at times in the grossest profanity, which was not previously his custom," wrote John Martyn Harlow, the physician who treated Gage after the accident.
This sudden personality transformation is why Gage shows up in so many medical textbooks, says Malcolm Macmillan, an honorary professor at the Melbourne School of Psychological Sciences and the author of An Odd Kind of Fame: Stories of Phineas Gage.
"He was the first case where you could say fairly definitely that injury to the brain produced some kind of change in personality," Macmillan says.
And that was a big deal in the mid-1800s, when the brain's purpose and inner workings were largely a mystery. At the time, phrenologists were still assessing people's personalities by measuring bumps on their skull.
Gage's famous case would help establish brain science as a field, says Allan Ropper , a neurologist at Harvard Medical School and Brigham and Women's Hospital.
One Account Of Gage's Personality Shift
Dr. John Harlow, who treated Gage following the accident, noted his personality change in an 1851 edition of the American Phrenological Journal and Repository of Science.

"If you talk about hard core neurology and the relationship between structural damage to the brain and particular changes in behavior, this is ground zero," Ropper says. It was an ideal case because "it's one region [of the brain], it's really obvious, and the changes in personality were stunning."
So, perhaps it's not surprising that every generation of brain scientists seems compelled to revisit Gage's case.
For example:
- In the 1940s, a famous neurologist named Stanley Cobb diagrammed the skull in an effort to determine the exact path of the tamping iron.
- In the 1980s, scientists repeated the exercise using CT scans.
- In the 1990s, researchers applied 3-D computer modeling to the problem.
And, in 2012, Van Horn led a team that combined CT scans of Gage's skull with MRI scans of typical brains to show how the wiring of Gage's brain could have been affected .
"Neuroscientists like to always go back and say, 'we're relating our work in the present day to these older famous cases which really defined the field,' " Van Horn says.
And it's not just researchers who keep coming back to Gage. Medical and psychology students still learn his story. And neurosurgeons and neurologists still sometimes reference Gage when assessing certain patients, Van Horn says.
"Every six months or so you'll see something like that, where somebody has been shot in the head with an arrow, or falls off a ladder and lands on a piece of rebar," Van Horn says. "So you do have these modern kind of Phineas Gage-like cases."

Two renderings of Gage's skull show the likely path of the iron rod and the nerve fibers that were probably damaged as it passed through. Van Horn JD, Irimia A, Torgerson CM, Chambers MC, Kikinis R, et al./Wikimedia hide caption
Two renderings of Gage's skull show the likely path of the iron rod and the nerve fibers that were probably damaged as it passed through.
There is something about Gage that most people don't know, Macmillan says. "That personality change, which undoubtedly occurred, did not last much longer than about two to three years."
Gage went on to work as a long-distance stagecoach driver in Chile, a job that required considerable planning skills and focus, Macmillan says.
This chapter of Gage's life offers a powerful message for present day patients, he says. "Even in cases of massive brain damage and massive incapacity, rehabilitation is always possible."
Gage lived for a dozen years after his accident. But ultimately, the brain damage he'd sustained probably led to his death.
He died on May 21, 1860, of an epileptic seizure that was almost certainly related to his brain injury.
Gage's skull, and the tamping iron that passed through it, are on display at the Warren Anatomical Museum in Boston, Mass.
- Brain research
- neuroscience
- Brain injuries
How Phineas Gage’s Freak Accident Changed Brain Science
Vermont Historian Explains How A Railway Accident Paved The Way for Neurosurgery And More

Cavendish might look like any other small Vermont town. Nestled between rolling hills and the Black River, with one main street running through town, it’s a launching point for trout anglers, snowmobilers and skiers. But this rural town of just over a thousand people can claim a remarkable historical figure: Phineas Gage .
Gage was a young construction foreman who suffered a gruesome accident that changed the history of brain science.
Stay informed on the latest news
Sign up for WPR’s email newsletter.
In 1848, while blasting through rock to build the new railroad, an explosion sent a 3-foot, 13-pound iron rod up through his cheekbone and out the top of his skull. The tamping rod landed 80 feet away, “ smeared with blood and brain .”
Remarkably, Gage lived for another 11 years. He lost one eye and had a permanent hole in his skull, covered by a thin layer of skin.
Gage was a medical marvel.
There had been a long-running debate in the 19th century on whether different regions in the brain govern different behaviors. Here was a case of severe damage to the left frontal lobe, followed by a dramatic personality shift. It seemed to prove the point once and for all.
“It laid the path for the first real brain surgery in 1885 ,” said Margo Caulfield, director of the Cavendish Historical Society, told “ To the Best of Our Knowledge .” “It opened up a whole new horizon. You can survive a brain injury, you can touch the brain, which means you can do surgery. It’s really, really huge.”
Gage himself was never the same after the accident.
He’d been well-liked by his co-workers and his employer, but later, his doctor reported that he became “ fitful, irreverent, indulging at times in the grossest profanity .”
A friend said, “ Gage is no longer Gage .”
[[{“fid”:”1360831″,”view_mode”:”embed_portrait”,”fields”:{“alt”:”Steve Paulson in Cavendish, Vermont, near a marker remembering Phineas Gage.”,”title”:”Steve Paulson in Cavendish, Vermont, near a marker remembering Phineas Gage.”,”class”:”media-element file-embed-landscape media-wysiwyg-align-right”,”data-delta”:”2″,”format”:”embed_portrait”,”alignment”:”right”,”field_image_caption[und][0][value]”:”%3Cp%3E%3Cspan%20style%3D%22font-family%3Aarial%3B%22%3ESteve%20Paulson%20of%20%22To%20the%20Best%20of%20Our%20Knowledge%22%20in%20Cavendish%2C%20Vermont%2C%20near%20a%20marker%20remembering%20Phineas%20Gage.%20%3Cem%3EAnne%20Strainchamps%2FTTBOOK%3C%2Fem%3E%3C%2Fspan%3E%3C%2Fp%3E%0A”,”field_image_caption[und][0][format]”:”full_html”,”field_file_image_alt_text[und][0][value]”:”Steve Paulson in Cavendish, Vermont, near a marker remembering Phineas Gage.”,”field_file_image_title_text[und][0][value]”:”Steve Paulson in Cavendish, Vermont, near a marker remembering Phineas Gage.”},”type”:”media”,”field_deltas”:{“2”:{“alt”:”Steve Paulson in Cavendish, Vermont, near a marker remembering Phineas Gage.”,”title”:”Steve Paulson in Cavendish, Vermont, near a marker remembering Phineas Gage.”,”class”:”media-element file-embed-landscape media-wysiwyg-align-right”,”data-delta”:”2″,”format”:”embed_portrait”,”alignment”:”right”,”field_image_caption[und][0][value]”:”%3Cp%3E%3Cspan%20style%3D%22font-family%3Aarial%3B%22%3ESteve%20Paulson%20of%20%22To%20the%20Best%20of%20Our%20Knowledge%22%20in%20Cavendish%2C%20Vermont%2C%20near%20a%20marker%20remembering%20Phineas%20Gage.%20%3Cem%3EAnne%20Strainchamps%2FTTBOOK%3C%2Fem%3E%3C%2Fspan%3E%3C%2Fp%3E%0A”,”field_image_caption[und][0][format]”:”full_html”,”field_file_image_alt_text[und][0][value]”:”Steve Paulson in Cavendish, Vermont, near a marker remembering Phineas Gage.”,”field_file_image_title_text[und][0][value]”:”Steve Paulson in Cavendish, Vermont, near a marker remembering Phineas Gage.”}},”link_text”:false,”attributes”:{“alt”:”Steve Paulson in Cavendish, Vermont, near a marker remembering Phineas Gage.”,”title”:”Steve Paulson in Cavendish, Vermont, near a marker remembering Phineas Gage.”,”class”:”media-element file-embed-portrait media-wysiwyg-align-right”,”data-delta”:”2″}}]]”Based on my experience with people who’ve had similar types of injury, you can talk to them, and they sound completely cogent. And then you talk to them another time and it’s like, what planet are they on?” Caulfield said. “This is the horror of traumatic brain injury. Some families just cannot handle a patient like that.”
Gage was unable to work on the railroad, but he still needed a job.
For a while, he made money by exhibiting himself around New England as a curiosity, showing off the holes in his head and his famous tamping iron.
Then, he was offered a job as a long-distance stagecoach driver on the Val Paraiso-Santiago route in Chile. It was a 100-mile route, and 13 hours of handling a coach and six horses, plus passengers, over rough terrain.
Gage lived in Chile for seven years and then started having epileptic seizures.
He died in 1860 at the age of 36.

Over the years, scientists have interpreted Gage’s story in different ways.
At first, he was seen as a triumph of human survival. Then for decades he became a textbook case for post-traumatic personality change. More recently, Gage’s case has been interpreted as a story of resilience. For a man who was supposedly anti-social and volatile, Gage’s ability to hold onto a challenging job in Chile suggests he’d regained his independence and social adaptability.
Perhaps Gage’s story is a textbook case of another sort, showing the brain’s capacity for rewiring after trauma. This gift of neuroplasticity is why we’re able to handle so much that life throws at us.
“I am fascinated by what he overcame to survive for as long as he did,” Caulfield said. “The resiliency piece of his story fascinates me … I think resiliency is just hard-wired into our DNA.”
Related Stories
How do we know when to call it quits.

Wisconsin scientists studying gene-editing tech to cure blindness

Construction set to begin in 2024 on effort to keep invasive carp out of the Great Lakes

Magic mushrooms and the ‘entropic brain’

Nearly half the nation’s tap water may contain PFAS. Here’s how some filters rank at removing them.

- The Disappearing Spoon
The Disappearing Spoon podcast
Everything you know about phineas gage is wrong.
What can a railroad construction foreman’s devastating skull injury teach us about the brain’s ability to heal?

Despite what you’ve heard, neuroscience’s most famous patient did not turn into a lying, drunken psychopath. He’s actually an amazing example of resiliency and overcoming trauma.
About The Disappearing Spoon
The Science History Institute has teamed up with New York Times best-selling author Sam Kean to bring a second history of science podcast to our listeners. The Disappearing Spoon tells little-known stories from our scientific past—from the shocking way the smallpox vaccine was transported around the world to why we don’t have a birth control pill for men . These topsy-turvy science tales, some of which have never made it into history books, are surprisingly powerful and insightful.
Host: Sam Kean Senior Producer: Mariel Carr Producer: Rigoberto Hernandez Audio Engineer: Jonathan Pfeffer
It was a lovely September day in 1848. A construction foreman named Phineas Gage was helping lay track for a railroad company in Vermont. Some boulders were blocking the railroad’s path, so the company hired a gang of rowdy Irishmen to blast their way through.
As foreman, Gage supervised the Irishmen. He also helped drill holes into the boulders and fill them with gunpowder. Gage then packed the gunpowder down into the hole with an iron rod. The rod looked like a short javelin. It was 1¼ inches thick, stretched 3 ½ feet long, and weighed 13 pounds.
Around 4:30pm, the Irishmen were loading some busted rock onto a cart. It was near quitting time, so perhaps they were a-whooping and a-hollering. Gage had just finished pouring gunpowder into a hole, and turned his head.
Accounts differ about what happened next. Some say that Gage was packing the gunpowder down with the iron rod, and scraped it against the side of the hole, creating a spark. Regardless, a spark shot out somewhere inside the hole and ignited the gunpowder. At which point the iron rod reversed thrusters.
The iron rod blasted upward, and entered Gage’s skull below his left cheekbone. It destroyed a molar, pierced his left eye, and plowed into his brain’s left frontal lobe. The rod then exited on top, and landed twenty-five yards distant. One report claimed it whistled as it flew, and was streaked with blood.
The rod’s momentum threw Gage backward. He landed hard. Amazingly, though, he never lost consciousness. He twitched a few times on the ground, but was talking within minutes. He even walked under his own power to a nearby cart, and sat upright on the trip back to town.
At his hotel, Gage waited in a chair on the porch and chatted with passersby—who were, uh, startled to see a volcano of bone jutting out of his scalp.
Thus began perhaps the most famous case in medical history. Every neuroscience textbook in existence has a section on Phineas Gage. Incredibly, though, nearly every textbook gets the story wrong.
You might have heard that, after his injury, Gage became a criminal, a drunk, a psychopath. None of that’s true.
Instead, there’s good evidence that, far from turning toward the dark side, Gage recovered after his accident—and perhaps resumed something like a normal life. It’s a possibility that, if true, could transform our understanding of the brain’s ability to heal.
When the first doctor arrived, Phineas Gage greeted him by angling his head and saying, “Here’s business enough for you.” Finally, around 6pm, the first doctor turned the case over to Dr. John Harlow. It’s not clear why. Harlow was a country doctor. He mostly treated people who’d fallen off horses. Not neurological cases.
Harlow didn’t believe Gage’s story at first. Surely, the rod hadn’t passed through his skull? But it had: Gage had a flap in his cheek and everything. Harlow then watched Gage lumber upstairs to his hotel room and lie on the bed. Which ruined the linens, since his upper body was one big bloody mess.
In the room, Harlow shaved Gage’s scalp and peeled off the dried blood and brains. Harlow then extracted skull fragments from the wound. Throughout this all, Gage vomited every twenty minutes. But otherwise, he remained calm and lucid. He betrayed no discomfort or pain.
Over the next few days, an infection set in and Gage’s brain swelled dangerously. Things were touch and go for a week, and Gage lapsed into a coma. A local cabinetmaker measured him for a coffin.
But Harlow’s diligent care allowed Gage to pull through. Gage soon returned to his family farm on Potato Road to recover. Gage did lose his left eye, but his memory, language, and motor skills remained intact. All in all, he seemed almost normal.
Almost. Harlow had kept Gage alive, but Gage’s family swore that he’d changed. The man who returned home was not the same man they knew and loved. His memory, language, and motor skills remained intact. But his personality changed.
Before the accident, Gage was known for making plans and sticking to them. Afterward, he changed his mind willy-nilly and rarely stuck things through. Before, Gage was also indifferent to animals. Afterward, Gage adored critters of all kinds. And while the original Gage was courteous and polite in company, the new Gage was coarse and foul-mouthed.
Most strikingly, Gage lost all money sense and developed irrationally strong attachments to certain objects. Harlow once tested Gage by offering him money for some random pebbles that Gage had picked out of a stream. Gage refused to part with them even for $1,000. Gage also carried with him at all times the iron rod that had brained him.
Harlow summed up Gage’s new personality by saying that, quote, the “balance … between his intellectual faculties and his animal propensities seems to have been destroyed.” More pithily, friends said that Gage “was no longer Gage.”
Despite his stellar work record before, the railroad refused to reinstate Gage as foreman. He took to working odd jobs on farms instead. He also indulged his newfound love of horses and became a carriage driver in New Hampshire. At one point, Gage even exhibited himself at P.T. Barnum’s freakshow museum in New York, staring back at the audience with his one good eye. For an extra dime skeptics could part his hair and watch his brain pulsate through a flap of skin over the exit wound in his skull.
After his stint at the museum, Gage’s life gets murky for a few years. Facts are hard to come by. But that hasn’t stopped scientists from filling that vacuum of facts with rumors and unfounded speculation.
One rumor claimed that Gage developed a drinking problem and started getting into brawls at taverns. Another claimed that he became a scam artist. He supposedly went to a medical school and sold them the exclusive rights to keep and study his skull after he died. Then he went to another medical school—and sold the same rights. And then another school, and another, skipping town and pocketing the cash each time. One ridiculous source even claimed that Gage lived for a dozen years with the iron rod still impaled in his noggin.
Other rumors in modern textbooks contradict each other. Some sources describe Gage as sexually indifferent, while others call him promiscuous. Some sources say he was hot-tempered, while others call him emotionally void, as if lobotomized. One neuroscientist even claimed that Gage “had lost his soul.”
To be clear, there’s zero historical evidence for any of those rumors of Gage’s mental or physical decline. In fact, in the only known picture of Gage, he looks nothing like a wastrel or someone whose life is spiraling out of control. He’s proud, well-dressed, even handsome.
To be clear, Harlow’s reports make it clear that Gage’s personality changed somehow. It’s about the only hard fact we have, neurologically speaking. But his other comments about Gage’s mental state are ambiguous.
Take the comment about Gage’s life being taken over by “animal propensities.” That sounds dramatic, but what does that mean—“animal propensities”? Did he eat too much? Demand sex? Howl at the moon? We have no idea.
Or consider this. In addition to loving animals after his accident, Gage also felt drawn to children suddenly. And on his visits home, Gage would reportedly spin wild tales for his nieces and nephews. Made-up stories about his supposed adventures on the road.
Some neuroscientists have interpreted Gage’s storytelling as evidence of confabulation. It’s a neurological symptom that involves chronic lying. It usually arises after frontal lobe damage. Then again, who hasn’t made up a tall tale to make little kids laugh? They love that stuff. It’s a pretty weak case for brain damage.
Similarly, we know that Gage had trouble sticking to plans after his accident. That’s another sign of frontal-lobe damage, because the frontal lobe controls mental skills like reasoning, planning, and self-control. In addition, Gage seemed to lose the impulse control that prevents most people from swearing in public. But it’s a far cry from someone saying saucy words like “hell” and “damn” to claiming that Gage was a drunken, brawling criminal.
In all, Gage’s life story, as appears in textbooks, has become as much legend as fact—a mélange of scientific prejudice, artistic license, and outright fabrication. Most people who learn about Gage in classes or textbooks have no idea how weak the case is for Gage becoming a villain.
Moreover, some modern historians have argued, forcefully, that Gage seems to have recovered some of his faculties in the decade after his accident. He never became the Phineas Gage of old. But some of his negative traits either diminished or disappeared, possibly because his brain proved plastic enough to heal and recover some lost functions.
In 1852, Phineas Gage’s life took a dramatic turn. He left the New England of his youth and followed a gold rush down to Chile in South America. He was seasick the whole voyage. Once ashore, he found work driving a horse carriage. His job involved shuttling passengers along the rugged, mountainous trails between Valparaiso and Santiago.
Gage held this job for seven years. Which is staggering, considering both his brain damage, and the complexity of the work. Gage likely drove a team of six horses. And horse reins at the time were complicated because you had to control each horse separately.
For instance, consider rounding a bend. To do so without tipping the coach over, you had to slow down the inner three horses a touch more than the outer three horses, simply by tugging on their reins with varying amounts of pressure. That would have taken a lot of dexterity. I mean, imagine driving a car while steering all four wheels independently. This could not have been easy for someone with brain damage.
Especially because the trails in Chile were quite crowded. This would have forced Gage to make quick stops and dodge other carriages. And because he probably drove at night sometimes, he would have had to memorize the trail’s twists and turns and fatal drop-offs. Plus keep an eye out for bandits.
Gage also likely cared for his horses by grooming and feeding them—a significant responsibility. And, contradicting the claim that he lacked all money sense, Gage probably collected passenger fares. Not to mention that he presumably picked up some conversational Spanish while in Chile—no mean feat for any adult.
You wonder how many of Gage’s passengers would have climbed aboard had they known about their one-eyed driver’s little accident a few years earlier. But all in all, Gage seems to have handled himself fine.
It probably helped Gage that he followed a similar routine each day. He likely arose before dawn to prepare his horses and carriage. Then he spent the next thirteen hours driving the same road back and forth from Valparaiso to Santiago.
Now, the fact that Gage seemingly carved out a life for himself in Chile doesn’t mean that his brain recovered fully. But scientists now know that the brain’s neural circuits can recover somewhat after damage, partly by rewiring themselves. And perhaps Gage retained enough of his frontal lobes to retain some basic planning skills. At the very least, Gage didn’t deteriorate into the drunken sociopath that many modern textbooks claim.
In truth, those claims about Gage are probably influenced by modern cases of brain damage. Cases where people did turn into sociopaths. You can hear more in a bonus episode at patreon.com/disappearingspoon. People who gambled money wildly, abandoning their family, and suddenly become pedophiles. That’s patreon.com/disappearingspoon.
Sadly, despite building a life for himself in Chile, Gage couldn’t outrun his brain damage entirely. And when it did catch up to him, the end was swift.
Poor health forced Gage to leave Chile in 1859. He caught a steamer up to San Francisco, where his family had moved. After a few months of rest in California, Gage found work on a farm and seemed to be doing better.
Unfortunately, a punishing day of plowing in early 1860 wiped him out. He had a seizure the next night over dinner. More seizures followed.
Gamely, Gage tried to keep working during this spell of trouble. But after years of steady work in South America, the seizures made him restless and capricious again. He began drifting from farm to farm, quitting each job for unknown reasons.
Finally, on May 20th, 1860, while resting at his mother’s home, he had several violent seizures in a row. He died the next day at age thirty-six, having survived his accident by almost a dozen years.
Gage’s skull was later exhumed by doctors. It remains on display at Harvard University today, along with the iron rod that remodeled his brain. Oddly, his skull has become something of a pilgrimage spot for people with an interest in macabre history.
I’ve spent a lot of time in this podcast bemoaning the lack of hard facts about Gage’s life. But in truth, that dearth of details probably secured Gage’s fame. That lack left infinite room for interpretation, and allowed each generation of scientists to reinterpret his case anew. Everyone from legendary neurosurgeons to phrenologists reading head bumps have invoked Gage to support their pet theories. Overall, Gage has become a Rorschach blot for neuroscientists. What we think of him changes from era to era, as the obsessions and preoccupations of each era change.
Including our era. Nowadays scientists cite Gage in support of theories about multiple intelligences; emotional intelligence; the social nature of the self; brain connectivity; every modern neuro-obsession.
And what Phineas Gage means now will probably change in the future, too. In fact, Gage’s story will probably always be with us. In part because it’s a hell of a story! Once upon a time, a man with a funny name really did survive having an iron rod explode through his skull. It’s tragic, gruesome, bewildering—and even comes with a science lesson.
But the deeper reason that Gage will always be with us is this. Despite all that remains murky and obscure, his life can teach us something important—that the brain and mind are one. After all, about the only hard fact we know is that his personality did change. And that’s no small thing.
As one neuroscientist has written, “beneath the tall tales and fish stories, a basic truth embedded in Gage’s story has played a tremendous role in shaping modern neuroscience: that the brain is the physical manifestation of the personality and sense of self.” That’s a profound idea, and it was Phineas Gage who first pointed us toward that truth.
Listen to more episodes

The Mysterious Mote
This bonus episode highlights an excerpt from Ferris Jabr’s book Becoming Earth.

The Science of D-Day
To mark the 80th anniversary of the Normandy landings during WWII, we look at the surprisingly important role science played.

Can Plastic Surgery Keep You Out of Prison?
One doctor’s controversial crusade to keep people out of prison through nose jobs, eye lifts, and other plastic surgery.
Never Miss an Episode
Copy the above HTML to republish this content. We have formatted the material to follow our guidelines, which include our credit requirements. Please review our full list of guidelines for more information. By republishing this content, you agree to our republication requirements.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Lancet Reg Health Am
- v.14; 2022 Oct
E.L., a modern-day Phineas Gage: Revisiting frontal lobe injury
Pedro h.m. de freitas.
a Instituto de Ciências Biomédicas, CCS, Universidade Federal do Rio de Janeiro, RJ, 21941-902, Brazil
Ruy C. Monteiro
b Miguel Couto Municipal Hospital, Rio de Janeiro, RJ, 22430-160, Brazil
Raphael Bertani
Caio m. perret, pedro c. rodrigues, joana vicentini, tagore m. gonzalez de morais, stefano f.a. rozental.
c Vassar College, Poughkeepsie, NY, 12604, USA
Gustavo F. Galvão
Fabricio de mattos, fernando a. vasconcelos.
d Dept Neurocirurgia, HUGG, Universidade Federal do Estado do Rio de Janeiro (UNIRIO), RJ, 20270-004, Brazil
Ivan S. Dorio
Cintya y. hayashi.
e Dept Neurologia, Universidade do Estado de São Paulo, SP, 05402-000, Brazil
Jorge R.L. dos Santos
f Pontificia Universidade Catolica do Rio de Janeiro, RJ, 22451-000, Brazil
Guilherme L. Werneck
Carla t. ferreira tocquer.
g Centro de Neurologia da Cognição e do Comportamento Ltda, RJ, 22071-000, Brazil
Claudia Capitão
h Centro Universitario IBMR, RJ, 22631-002, Brazil
Luiz C. Hygino da Cruz, Jr
i MRI Clinica de Diagnostico por Imagem (CDPI/DASA), Rio de Janeiro, 22271-040, Brazil
Jaan Tulviste
j University of Tartu, Institute of Psychology, Tartu, Estonia
Mario Fiorani
k Instituto de Biofísica, Universidade Federal do Rio de Janeiro, RJ, 21941-902, Brazil
Marcos M. da Silva
l Dept Neurologia, HUCFF, Universidade Federal do Rio de Janeiro, RJ, 21941-902, Brazil
Wellingson S. Paiva
Kenneth podell.
m Neurological Institute, Houston Methodist, TX, 77030, USA
Howard J. Federoff
n Georgetown University, Washington, D.C., 20057, USA
Divyen H. Patel
o Genome Explorations, Memphis, TN, 38132, USA
p Northwell Health, Manhasset, NY, 11030, USA
Elkhonon Goldberg
q Dept Neurology, New York University, School of Medicine, NY, 10016, USA
Rodolfo Llinás
r Dept. Physiology and Neuroscience, New York University, School of Medicine, NY, 10016, USA
Michael V.L. Bennett
s Dept Neuroscience, Albert Einstein Coll Medicine, Bronx, NY, 10461, USA
Renato Rozental
t Centro Desenvolvimento Tecnológico (CDTS), FIOCRUZ, Rio de Janeiro, 21040-361, Brazil
Associated Data
Extended FIG. 1 (a) 3D CT reconstruction of the iron bar trajectory through E.L.’s skull. (b,c) View of the large craniotomy allowing for a pterional-type approach to the iron bar within the intracranial cavity, exposing brain laceration and the perforated bone flap. (b) Note the path drilled by the iron bar in bone as it passed through the skull. (d,e) Lateral section MRI images depicting the path of the iron bar, pointing the entry- and exit-points with white arrow and arrowhead , respectively. (f) Coronal section MRI scan depicting direct damage to the right side of the nose and right frontal sinus secondary to the passage of the iron bar. (g,h) Lateral and axial images from a follow-up MRI scan performed 18 months later help propose a right frontal lobe disconnection (white arrows). R (right hemisphere), L (left hemisphere).
Movie #1. Supplementary movie S1.
E.L.’s challenge: finger-to-thumb movement performed simultaneously by both hands.
3D rendering of a T1-weighted MRI anatomical scan of E.L.’s brain.
3D rendering of a T1-weighted MRI anatomical scan of E.L.’s Corpus callosum.
How the prefrontal cortex (PFC) recovers its functionality following lesions remains a conundrum. Recent work has uncovered the importance of transient low-frequency oscillatory activity (LFO; < 4 Hz) for the recovery of an injured brain. We aimed to determine whether persistent cortical oscillatory dynamics contribute to brain capability to support ‘normal life’ following injury.
In this 9-year prospective longitudinal study (08/2012-2021), we collected data from the patient E.L., a modern-day Phineas Gage, who suffered from lesions, impacting 11% of his total brain mass, to his right PFC and supplementary motor area after his skull was transfixed by an iron rod. A systematic evaluation of clinical, electrophysiologic, brain imaging, neuropsychological and behavioural testing were used to clarify the clinical significance of relationship between LFO discharge and executive dysfunctions and compare E.L.´s disorders to that attributed to Gage (1848), a landmark in the history of neurology and neuroscience.
Selective recruitment of the non-injured left hemisphere during execution of unimanual right-hand movements resulted in the emergence of robust LFO, an EEG-detected marker for disconnection of brain areas, in the damaged right hemisphere. In contrast, recruitment of the damaged right hemisphere during contralateral hand movement, resulted in the co-activation of the left hemisphere and decreased right hemisphere LFO to levels of controls enabling performance, suggesting a target for neuromodulation. Similarly, transcranial magnetic stimulation (TMS), used to create a temporary virtual-lesion over E.L.’s healthy hemisphere, disrupted the modulation of contralateral LFO, disturbing behaviour and impairing executive function tasks. In contrast to Gage, reasoning, planning, working memory, social, sexual and family behaviours eluded clinical inspection by decreasing LFO in the delta frequency range during motor and executive functioning.
Interpretation
Our study suggests that modulation of LFO dynamics is an important mechanism by which PFC accommodates neurological injuries, supporting the reports of Gage´s recovery, and represents an attractive target for therapeutic interventions.
Fundação de Amparo Pesquisa Rio de Janeiro (FAPERJ), Universidade Federal do Rio de Janeiro (intramural), and Fiocruz/Ministery of Health (INOVA Fiocruz).
Research in context
Evidence before this study.
The historical case of Phineas Gage (1848) is an integral part of medical folklore, illustrating the resilience of the human brain and the involvement of the frontal lobes in problem solving, spontaneity, memory, initiation, judgement, impulse control, and social and sexual behavior. However, the dynamics of damaged frontal lobe continue to be the subject of controversy, and functional recovery has remained a matter of debate since Gage's accident. On a parallel track, sustained high amplitude low-frequency oscillations (LFO) generated in dysfunctional cortical networks has been suggested to have a high correlation with cortical dysfunction and as a marker of cortical disconnection.
Added value of this study
Our study suggests that modulation of LFO dynamics is an important mechanism by which PFC accommodates neurological injuries, supporting the reports of Gage´s recovery and represents an attractive target for therapeutic interventions.

Implications of all the available evidence
Modulation of LFO dynamics is an important mechanism by which PFC accommodates neurological injuries, representing an attractive new target for therapeutic interventions after frontal lobe injury.
Alt-text: Unlabelled box
Introduction
Today, some 170 years after the landmark case of Phineas Gage, who exhibited significant changes in personality as a result of frontal lobe damage, the so-called ‘riddle of the frontal lobes’ has yet to be solved. 1 The characterization that ‘Gage was no longer Gage’, 2 albeit poorly documented, was consonant with the rise of cortical localizationist theories of brain functioning in the mid-to late 19 th century, setting out the context of the ‘localization debate’ for the nascent science of the ‘brain and mind’. 1 Split-brain studies with sectioned corpus callosum ( CC ), the major neural pathway that connects homologous cortical areas of the cerebral hemispheres, provided evidence for the view of hemispheric asymmetries and specialization 3 and afforded insights into neural mechanisms. These studies also unveiled areas of the CC dedicated to the transfer of visual, somatosensory and motor information, which are stronger for handedness than for footedness. 4 These ideas bring us to the current view of a language-dominant left hemisphere while visuospatial (e.g., geometric forms) and other features show more lateralization in the right hemisphere. 5 The dynamics of damaged prefrontal cortices (PFC), however, critical for cognitive, asocial behavior, decision-making functions and moral judgment, continue to be the subject of controversy, and functional recovery has remained a matter of debate since 1848.
White matter (WM) change is central to a range of neurological conditions, including traumatic brain injury (TBI), psychiatric disorders and in healthy aging. 6 , 7 , 8 Consistent with neuropathological findings, neuroimaging and neuropsychological literature has focused on executive functioning decline in subjects who underwent brain signal processing by quantitative MRI measures such as lesion load, demyelination, WM integrity and altered gray matter (GM) volume. At the electroencephalographic level (EEG), sustained high amplitude low-frequency oscillations (LFO) (<4 Hz) generated in dysfunctional cortical networks in TBI patients, psychiatric disorders and aging, has been shown to have a high correlation with neuropsychological assessments in neurodegenerative processes 9 , 10 and it is regarded as a marker of cortical disconnection, leading to deafferentation of networks from their major input source. 11 Indeed, while an intact thalamus is required for proper brain function during both sleep and wakefulness in controls (CTRL), predisposing to declarative learning, memory 12 and sleep, 13 and thus improving cognitive performance, the sufficiency of the cortex for the generation of abnormal slow waves is supported by corticocortical connections following thalamectomy. 14 In turn, treatment-associated decreases in frontal LFO activity have been suggested to improve functional outcome and impaired attention in mental disorders. 15 However, no definitive explanation for how LFO interact with other recurring changes in excitability and is modulated in frontal lobe injury was provided so far.
The overarching goal of the current study, prompted by clinical, neuroimaging, neuropsychological and EEG evidence paired with TMS, is to provide clues as to how PFC accommodates to neurological insults and, in particular, to test the unsettled concept that down modulation of abnormal LFO has mechanistic implications for compensation on executive dysfunction. Identifying compensatory responses associated with LFO over the injured hemisphere may facilitate determination of the extent to which neural recruitment in injury represents reorganization or functional engagement of existing latent networks. Finally, while there is no question that, immediately following the accident there were changes in Gage's personality, other reports nevertheless state that Gage actually recovered and resumed something resembling a ‘normal life’ – a possibility that, if substantiated, could impact significantly our understanding of the brain's plasticity and treatment strategy.
LFO construct validity was performed by determining correlations among clinical examination, physiological recordings, commonly used imaging technology and neurobehavioral & sensorimotor tests of known frontal lobe vulnerabilities in controls and E.L. On the basis of the definition of multitasking, the group created a list of tasks to be tested on affective, cognitive and behavioral sub-domains. Magnetic stimulation, a technique that allows us to temporarily interfere with brain function, was aimed at proof-of-concept testing.
Because of the large amount of assays employed, materials, the following methods and well-established protocols are described in detail as “Supplementary Methods”: clinical exam, quantitative electroencephalography (qEEG), MRI image acquisition & segmentation (FreeSurfer), post-processing and volume measurements, 3D printed brain models, electrooculography (saccadic and antisacaddic tasks), oxcarbazepine (anticonvulsant) test and a set of neuropsychological and behavioral tests, including “apathy evaluation” and assessment of sexuality (BIQS).
Matched control sampling
The control sample consisted of consecutive healthy male participants, strictly right-handed, not taking any medications, with a mean age of 21.6 ± 0.9 (20-30 years) ( n = 5) (qEEG), 30 ± 1.3 years (20-35 years) ( n = 10) (TMS/ROFC), 33.4 ± 2.4 (20-45 years) ( n = 10) (Divided Attention), 26.7 ± 2.7 (20-35 years) ( n = 4) (TMS/EEG/apathy) and 21.6 ± 0.9 (20-30 years) ( n = 5) (EOG). In general, the participants were all volunteers made aware of the possibility of volunteering by a general notice, who responded to an advert in local recruitment flyers, University hospital flyers on volunteer opportunities or active recruitment approaches to take part in research on “neuropsychological testing”. Volunteers with incomplete junior high school were selected to match E.L. level of education ( Divided Attention ) (Supplementary Methods).
Transcranial magnetic stimulation (TMS). Neuronavigation
Patients underwent MRI using a 1.5 T SPREE (Siemens AG, Erlangen, Germany). T1-weighted images were acquired in the sagittal plane with a 3D magnetization prepared rapid acquisition gradient echo sequence (MPRAGE) (Supplementary Methods). The MRI images were recorded in Digital Imaging and Communications in Medicine format (DICOM). A neuronavigational device (BrainSight 2, Rogue Research Inc, Montreéal, QC, Canada) sensor was applied to guide the coil positioning, which allowed visualization of the angle of impact for the magnetic impulse onto the brain surface. Subsequently, a system was introduced that also facilitated elucidation of the exact strength and extent of the induced electrical field, depending on the depth of the area under the coil. For the TMS mapping, the patients were seated on a chair with a headrest. Focal single-pulse TMS was delivered to the motor cortex with a figure-of-eight magnetic stimulator (diameter 70 mm; 9 turns of the wire; peak magnetic − 2.2 T) (MagPro × 100; MagVenture A/S, Farum, Denmark). The magnetic coil was placed with navigation. We performed a co-registration of MRI for face recognition by points (glabella, nasion, right tragus, and left tragus). The motor cortex was identified, using TMS impulses in the cortex to produce movement in the contralateral hand. The motor threshold was considered as the lowest intensity of TMS single pulses required to induce a motor-evoked potential (MEP) with an amplitude ≥50 µV, in five out of ten consecutive trials.
Methods used to record EMG signals of the first dorsal interosseous muscle, to apply repetitive TMS stimulation (rTMS) of the dorsolateral PFC (dlPFC) and left Primary Motor Cortex (left PMC) and continuous theta-burst stimulation (cTBS) of the ventromedial PFC (vmPFC) are described in Supplementary Methods .
Neuropsychological testing
A comprehensive neuropsychological test battery to assess frontal lobe dysfunction was administered, comprising tests covering cognitive function, neuropsychological domain and sexual arousal. We aimed for a battery of tests, including the executive control battery, based on approaches and procedures developed by A. Luria and E. Goldberg, and other gold-standard tests of cognitive assessment, such as Iowa Gambling Task (IGT) (self-account measures), Cognitive Bias Test (CBT) (actor-centered decision making), Wisconsin Card Sorting Test (Nelson version) (WCST) (veridical decision making) and Rey-Osterrieth Complex Test (short- and long-term visuospatial memory). 16 The neuropsychological profile assessing each group of executive functioning is grouped in Table 1 , Extended Table 1 and described in Supplementary Methods.
E.L.: predicted difficulties and impairments in cognitive function and in neuropsychological domain on the basis of standard neuropsychological measures.
| Cognitive function | Tests | Performance |
|---|---|---|
| Wechsler Adult Intelligence Scale (WAIS III) | ||
| Stroop Test (W, C, CW and Interference Index) | deficit | |
| Rey – Osterrieth Complex Figure Test (ROCFT)(immediate and delayed recall) | ||
| WMS3 (immediate and delayed recall), story recall | ||
| Finger tapping test (unimanual) | ||
| Iowa gambling test | ||
| ROCFT, Wisconsin Card Sorting Test (WCST), Tower of London | ||
| Semantic and phonemic | ||
| Trail Making Test (A, B, B-A) | deficit, , deficit | |
| Raven Progressive Matrices (PM38) | ||
| Span Forward and Backward, Immediate Recall | ||
| CVLT (total learning, delayed recall, recognition) | deficit | |
| Corsi Span | deficit | |
| RCFT recall (immediate and delayed) | ||
| DMS-48, Ruche de Violon Test | deficit | |
| Boston Naming Test (BNT) | ||
| ROCFT copy | ||
| Facial Recognition Test (Benton) | ||
| Grober and Buschke Test: short term and delayed recall recognition | ||
| deficit | ||
| MMSE, BDI | ||
| BIQS |
The executive control battery (ECB) – we included the bimanual (reciprocal) coordination. The test allows the eliciting of various types of motor perseverations, stereotypes, and other deficits of sequential motor organization. 16
Rey-Osterrieth Complex Figure (ROCF) (visuospatial memory). This test allows assessment of a variety of cognitive processes, including planning, problem-solving strategies, as well as perceptual, motor, and episodic memory functions. Participants were presented with a blank sheet of paper and were asked to make a copy of the figure with their right-hand (dominant hand) as carefully as possible. Subjects were allowed to rotate their drawings, which was aimed at maximizing the quality of their copies but were not allowed to rotate the figure. Erasing was allowed. Each subject delivered the copied figure within 5 min. After 3 min, the subjects were asked to reproduce the design from memory (3 min recall) and then again after a 30 min hiatus (30 min recall). Copy and reproductions of the ROCF were scored using original Scoring System by André Rey. 17
Statistics analyses
General statistical analysis.
Descriptive statistics (mean±SEM) were calculated with Excel (Microsoft) or GraphPad Prism (GraphPad Software). Mean±SEM was used to report statistics unless otherwise indicated. All statistical analyses were conducted using GraphPad Prism. A P value <0.05 was considered statistically significant.
EEG frequency power
Spectral power was averaged at all sensors, the time course of slow wave (SW) activity (0.5–4 Hz) was calculated and the open-source R program with "SingleCaseES" and "scan" packages was used to perform the Non-overlap of all pairs (NAP) method 18 to evaluate the changes in SW activity associated with the TMS-OFF (resting state) and the TMS-ON blocks within E.L. and CTRL ( n = 4), 26.7 ± 2.7 years (20-35 years) ( n = 4). Similar procedures were performed to evaluate SW activity during finger-tapping paradigm. dlPFC rTMS Rey-Osterrieth Complex Figure assay. E.L. single-case data analysis and comparison to a CTRL sample, 30 ± 1.3 years (20-35 years) ( n = 10) (TMS), were performed with the open source software Singlims ES using the Crawford and Howell's and the Crawford & Garthwaite´s methods, 19 supplemented by point and interval estimates of effect sizes tests if E.L's score was significantly below CTRL. This methodology provides a point estimate and percentage of the abnormality of the score, with the use of P value, and sets confidence limits on the abnormality of a patient's score using non-central t-distributions. CTRL sample sizes were chosen based on prior standards established in previous published studies in neuropsychology field to achieve 90% power in a one-tail test with α=0.05 and Z-CC ≥ 4. 19
Ethics committee
The present study was in strict accordance with the NIH Guide involving Human Subjects and approved by Institutional Ethics Committees (CAAE 96263418.0.0000.5279 – CONEP/Plataforma Brasil).
Role of the funding source
The sponsors of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. R. Rozental, M.V.L. Bennett and P.H.M. de Freitas had full access to all the data in the study and all authors had final responsibility for the decision to submit for publication.
History and physical examination: ‘replicating gage’
History repeated itself in August of 2012 (Gage vs E.L., Supplementary Clinical Case History). In 1848, Phineas Gage, a 25-year-old American construction foreman, sustained extensive frontal lobe damage after an iron bar - 31 mm in diameter, 1.06 meters long and weighing 6.1 kg - was propelled through the front part of his head. Gage reportedly experienced pronounced personality changes due to lesions from this accident that are presumed to have involved the left frontal region. 164 years later, at 9:30 am on August 15, 2012, E.L., a 24-year-old Brazilian man, who was kneeling on the ground floor at a construction site, experienced a similar accident; a round steel bar - 16 mm in diameter, 2.56 meters long and weighing 4 kg – fell from the 5th floor (15 m height), impaling his skull obliquely (superior-anterior) on the right side at an estimated velocity, at contact, of 17.32 m/sec (62.35 km/hr). The entry-point was about 1.5 cm posterolateral to bregma and the exit-path of the rod made contact with the glabella ( Figure 1 a,b, Extended Data Figure 1a,d,e). E.L. was discharged from hospital care on August 30th, 2012 and returned to work on a construction site in November, 2012 (Supplementary Clinical Case History). E.L. continued functioning normally for approximately 9 months after his return to work, until he experienced a tonic-clonic seizure. After the seizure, he stopped working, and began treatment with oxcarbazepine (OXC) (300 mg at 8-hr intervals), an anticonvulsant (AED). In the 94 months since beginning treatment with OXC, he experienced motor seizures 11 times (72% within the first 23 months of treatment), all lasting less than 1 min. Symptoms started on his left arm, while E.L. was fully aware of the surroundings, before losing consciousness and spreading to involve both sides of his body. All episodes, except for the first one, were due to medication noncompliance (Supplementary Clinical Case History).

Path of the iron bar through E.L.’s skull and its effects on white matter structure. (a) Lateral view of the transfixed skull with an iron bar on the CT scan reconstruction. (b) T1 weighted MRI reconstruction revealed the trajectory of the iron bar. Lateral (c) and transverse (d) sections (T2-weighted MRI images), 18 months after the accident, suggestive of a right frontal lobe disconnection; (e,g) FLAIR MRI spectroscopy (single-voxel) was applied to regions of interest (blue squares), indicated by blue arrows, on the anterior corpus callosum (CC). (f,h) An expected decrease in spectrum of resonances in N-acetyl-aspartate (NAA) (2ppm), marker of neuronal integrity, is illustrated. (i) Axial view of a tractography suggesting a difference in the volume of the association fiber tracts that connect temporo-parietal cortical regions with right frontal lobe (white arrow). (j) Fractional anisotropy (FA) map, a diffusion tensor imaging technique, revealed that the injured area was associated with greater anisotropy reduction in frontal right brain regions (white arrow). R (right hemisphere), L (left hemisphere).
Medical history (2012–2020)
E.L.'s medical history is otherwise unremarkable. E.L., who is right-handed, suffered traumatic injury (TBI) of the right frontal lobe, and now experiences post-traumatic epilepsy as his only notable symptom. Neurological examination shows only mild abnormalities: right-sided hyposmia as result of direct damage to the nose and sinus, injury to the anterior cranial fossa, and frontal lobe involvement. He exhibits subtle asymmetry of reflexes between left and right arms, mild incoordination with rapid alternating movements in the left hand, and difficulty maintaining left gaze, which is consistent with a lesion to the right frontal lobe including the right frontal eye fields, and evidence suggesting a grasp reflex in the left foot, a frontal release sign. Coordination testing showed normal symmetrical movements without dysmetria, or incoordination, on finger to nose testing, normal forearm and finger rolling tests using rapidly alternating movement, and on sequential active finger-to-thumb tapping, rapidly touching the thumb to each fingertip, during unimanual movements with either the left or right hand (Extended Data Figure 3) (Supplementary Clinical Case History - Neurological Examination). However, there was incoordination of sequential finger-to-thumb tapping movements in the left hand during bimanual movements suggestive of failure of compensatory mechanisms during multitasking (Supplementary Movie 1) (Supplementary Clinical Case History).
Functional magnetic resonance imaging (fMRI) – extent of cortical gray matter (GM) and white matter (WM) damage
A set of 5 MRI data collection from E.L. over the years, was used for this study. Cross-sectional, parasagittal and longitudinal MRI image scans (2012, 2013, 2014a,b and 2020) revealed an extensive right unilateral frontal lobe lesion affecting orbital, polar and anterior regions of the PFC. Figure 1 b-e,g, Extended Data Figure 1d-e,g-h, Extended Data Figure 2 illustrate the affected areas, involving the orbital frontal cortex (Brodmann´s cytoarchitectonic fields 11 and 12), the polar and anterior mesial frontal cortices (fields 8, 9, 10 and 32), the dorsolateral field 46 and the mesial aspect of field 6 (the supplementary [SMA] and the premotor [pre-SMA] areas). MRI scans over time have not detected enlarging lesions, atrophy or damage outside of the frontal lobe. The amount of right global hemisphere volume loss due to the iron bar was 11.2%, affecting regionally specific gray matter volume (4.6%), cerebrospinal fluid (CSF) and white matter (WM) volumes (6.6%), matching estimations of GM (up to 4%) and of WM (up to 11%) reported in Gage´s computational lesion studies. E.L. total intracranial volume (TIV) was 1,500.4 ml compared to the mean ± SEM CTRL 1,419.4 (23.7) ml ( n = 16 patients), and his estimated left lateral ventricle volume (LVV) was 56.6 ml compared to 64 ml of his contralateral one (right ventricle). Findings of hippocampal sclerosis were not suggested by long-term MRI scans (07/2020) ( Figure 5 d). Visualization of fully reconstructed E.L.’s 3D brain model in polyamide helped examine the extent of both cortical and subcortical lesions in close detail (Extended Data Figure 4) (Supplementary Movie 2) (Supplementary Methods).

rTMS influences short-term memory and behaviour in E.L., but not in CTRL, by modulating the frequency of slow waves. (a) depicts the position of the conventional TMS figure-of-eight coil with respect to E.L.’s scalp, over the left motor cortex (M1). (b-d) T2- weighted imaging of E.L.’s brain from 2020, 8 years since injury. (b,d) TBI did not result in progressive loss of E.L.’s brain tissue volume. Mesial temporal sclerosis was not detected with regard to hippocampal size and shape, fissure visualization and signal intensity (isotense) to cortical gray matter. (e-h) rTMS-EEG responses to stimulation in E.L. and in CTRL. (e) Topographic plots of the TMS-evoked responses. The extent to which increase in frequency and amplitude of SWs are higher contralateral than ipsilateral to the side of stimulation is illustrated by a train of rTMS at a frequency of 1 Hz (top panel). After rTMS, EEG SW responses were potentiated up to 40 min post 1 Hz (f,h) and up to 60 min post-Theta-burst stimulation (TBS) (g,h), followed by a complete return to background activity (e,h – top panel). (i,j) Subjects were asked to copy the Rey–Osterrieth figure (bottom left – ‘ template’), and then to reproduce it from memory 3–30 min later, prior and after TMS-treatment (1 Hz) applied to the DLPFC. The two groups drawing scores significantly differ between the retrieval rounds at 3 min (* P < 0.001) and at 30 min (* P < 0.001). rTMS scale (e): 0–500 µV².
Connectivity was damaged between the right PFC and other major lobes of the right hemisphere, in addition to the left frontal lobe. WM lack of integrity was identified in the following: superior longitudinal fasciculus (SLF), which connects bi-directionally the parietal, occipital and temporal lobes with ipsilateral frontal cortices; superior fronto-occipital fasciculus (SFOF), which connects frontal and occipital lobes; and, cingulum fasciculus (CF), which courses behind the splenium up to the frontal lobe, extending longitudinally above the C.C. - ( Figure 1 i,j; Extended Data Figure 5). Quantitative Diffusion Tensor Imaging (DTI) did not detect gross changes in volumetric imaging (total and regional) in E.L.’s C.C. , despite the nature of his frontal injury. There were no significant differences in midsagittal measures of anterior-posterior extent of C.C. between E.L. and CTRL ( n = 16), even though E.L.’s anterior segment (760.5 mm 3 ) was thinner while his central-posterior (453.8 mm 3 [mid-ant]; 469.6 mm 3 [central]; 483.6 mm 3 [mid-post]; and 878.2 mm 3 [post]) segments were thicker than the average for the matched CTRL ( n = 16)(868.3 ± 32.68 mm 3 [ant]; 433.6 ± 19.2 mm 3 [mid-ant]; 429.9 ± 18.43 mm 3 [central]; 397.3 ± 19.59 mm 3 [mid-post]; 991.2 ± 41.02 mm 3 [post]) (Supplementary Movie 3). The groups did not differ in total C.C. volume (3,045.6 mm 3 [E.L.] vs 3,120.3 ± 94.13 [matched-CTRL]).
Assessment of functional tasks performance and sexuality after right frontal lobe damage
E.L. and CTRL ratings on a measure of frontal cognitive, neuropsychological and sexual behaviour were assessed using standardized and gold-standard neuropsychological battery tests. Table 1 and Extended Table 1 display the abilities assessed by specific testing, including reading, language usage, attention, learning, processing speed, reasoning, remembering, problem-solving, mood and personality and more. No apparent direct relationship has been found between cognition, executive function or behavioural impairment in the clinical course of his injury during the last 9 years.
E.L. executive function and behaviour, for the most part, did not deteriorate since his accident. E.L. has not expressed neuropsychological problems in family, sexual, professional and social life and did not present attentional dysfunction. In general, he performed as well as CTRL in standard tests to assess TBI subjects, among them the WAIS-III (intelligence), Tower of London (planning ability), Boston Naming Test (BNT) (confrontation naming), the Wechsler Memory Scale (WMS) (auditory and visual memory; immediate and delayed memory), Rey-Osterrieth Complex Figure Test (RCFT) (visuospatial perception, skills and recognition memory), Mini-Mental State Examination (MMSE) (cognitive impairment), Beck Depression Inventory (BDI) (depression), Prospective Memory (PM) and tests to evaluate Divided Attention ( Table 1 ; Extended Table 1). Similarly, there was no detectable impairment by gold standard tests of cognitive assessment, such as, Iowa Gambling Task (IGT) (self-account measures), Cognitive Bias Test (CBT) (actor-centred decision making) and Wisconsin Card Sorting Test (Nelson version) (WCST) (veridical decision making). E.L. did, however, show deficits on episodic memory category (Ruche de Violon and Grober and Buschke Tests), visual memory (DMS-48), working memory (Corsi Blocks), color and word cognitive flexibility (Stroop) and attention and task switching (Trail Making Test) ( Table 1 ; Extended Table 1). Among them, on every subtest of the Wechsler Adult Intelligence Scale III (WAIS-III) and on the Divided Attention Test (DAT), tasks commonly thought to be sensitive to the integrity of the frontal cortex, E.L. showed abilities that were either average or superior to the averages for CTRL: 12 (WAIS), 88% (calculation task - DAT) and 17 (cancellation task – DAT). The results of the Wisconsin Card Sorting Test (WCST), the Stroop Colour Interference Test, the Iowa Gambling Test (Extended Data Figure 6) and the Cognitive Bias Task (CBT), regarded as the gold standards of neuropsychological frontal-lobe assessment in patients with lateralized lesions, were also favourable ( Table 1 ; Extended Table 1)
E.L. results were also within normal ranges in the assessment of sexual and behavioural functioning (described hereinafter) (Sexuality questionnaire – Extended Data Figure 7 a,b). We also did not learn of any problems related to higher brain dysfunction in E.L.’s family and/or social lives after his discharge from the hospital. The association of AED and abnormal slow electrographic activity with some cognitive deficits, however, cannot be ruled out (discussed hereinafter). E.L. returned to work with the same construction company and was able to handle a workload comparable to before his accident. There were no noticeable declines in his mental processing, moral reasoning, social behaviour, capacity to solve daily problems, ability to interact with his co-workers or ability to act efficiently (Social Interaction - Supplementary Clinical Case History).
In single task drawings, the phenomenon of spatial hemi-inattention, more common after right than after left hemisphere injuries, tends to be reflected in accuracy (e.g., size, shape, placement on the page) and in the omission of details on the left side of the drawing. Since spatial neglect is a complex disorder, subjects were also required to process information, to extrapolate and to think ahead using working memory in the PFC. 20 Within this context, no differences were found, between E.L. and normalized test data ( Figure 2 a-f) ( Table 1 ; Extended Table 1). However, univariate analyses suggested significant differences on a few measures involving working memory. E.L.’s attentional dysfunction was not identified and he performed within the upper range of CTRL ( n = 10) , in terms of both speed and drawing accuracy (placement, geometric forms and size), demonstrating: i) judgement of line length and orientation; ii) width of angles; iii) positioning of dots into geometrical figures; iv) pinpointing the location of a numeral in geometrical figures; v) mentally rotating figures; vi) recognition of nonsense shapes; vii) determining the number of cubes composing a complex 3D shape; and viii) mentally retrieving and reconstructing images. E.L.’s performance in the ‘clock drawing’ and in the Rey-Osterrieth Complex Figure (ROCF) (copy condition, immediate recall and delayed recall) tests, the most frequently used to assess nonverbal memory function, whose performance, needed for quotidian activities, was ranked in the top 10% of those elaborated among all subjects tested ( Figure 5 j E.L. TMS-untreated ).

E.L.: Figural fluency, emotional behaviour and EEG as a measure of right frontal lobe injury. This battery of tests was aimed at identifying dysfunction, such as attention and concentration, self-monitoring, personality, inhibition of behaviour and emotions, and with speaking or using expressive language. (a-d) Representative illustrations of memory for geometric designs, shapes, features and directional orientation. (a, top ) E.L. was asked to drawn R$ 5 cents, 25 cents and 1 Real coins from memory (i.e., draw-to-command). Coin measurements were compared to the respective official coins (a, bottom ). (e) The clock-drawing test did not confirm a diagnosis of cognitive deficits in E.L. Assessment focused on size of the clock, graphic difficulties, stimulus-bound response, conceptual deficit, spatial/planning deficit and perseveration. (f) context-dependence of emotions within text, illustrated by “Eu amo minha familia e meus pais” (“ I love my family and my parents ”). (g) Spontaneous EEG (four non-consecutive sec of the same recording session placed side by side). (i) Eye closure sensitivity: the awake EEG is characterized by a posterior dominant alpha rhythm (9-10Hz) and moderate voltage reactive to eye opening and closure. (ii) Awake, eyes-closed resting condition: there is frequent delta slowing seen in the right frontal region (Fp2-F4 and Fp2-F8). (iii) Hyperventilation (HV): HV showed a tendency to increase the focal slowing noted above. (iv) Photic Stimulation (PS): intermittent PS did not alter the background. Calibration bar: 100 µV.
Saccadic and antisaccadic tasks
E.L. and CTRL group, 21.6 ± 0.9 (20-30 years) ( n = 5), composed of male subjects of a similar age as E.L., performed saccadic and antisaccadic tasks under gap and overlap paradigms being quantified latencies of eye movements and tasks error (see Supplementary Methods and Extended Figure 8a-b and Extended Figure 9a-c). E.L. showed difference in prosaccadic and antisaccadic errors to single targets (Extended Figure 8c,d,e,f and Extended Figure 9). Similar trends, however, were not observed when the same CTRL group under oxcarbazepine-influence (OXC-CTRL) ( n = 5), compared to E.L. Accordingly, the OXC-treated group did not show difference in symmetry (right vs left) of prosaccadic-antisaccadic latency and velocity (Extended Data Figure 8) (Supplementary Methods).
Functional magnetic resonance imaging (fMRI) – dual tasks
Neurological (bimanual finger taps) test proved to be a valuable tool for unmasking functional limitations under dual tasks and paved the way for a strategic management of E.L.
On the day of E.L. scanning, the maximum number of index finger-to-thumb taps in 60 sec was obtained for both hands (unimanual movements). The mean number of finger taps for the left and right tasks were 40.7 ± 3 and 41 ± 2,5, respectively. During unilateral tasks, no detectable movements of the contralateral hand, i.e., mirror movements, occurred. Since E.L. was commanded to start the task, and required no decision making on his part, he was less likely to engage motor selection networks that show a bias towards left hemisphere activation. 21 In general, finger-tapping tasks activated the motor and premotor regions, including the primary motor (M1), ventral premotor (vPM) and dorsal premotor (dPM) cortex ( Figure 3 a-i). This task was performed with the right hand only, left hand only, and both hands simultaneously (Extended Data Figure 3c-f).

Functional BOLD MRI images (fMRI) of E.L.’s brain activation during sequential finger-to-thumb tapping movements using one hand alone or two hands simultaneously. Axial (a-c), sagittal (d-f) and coronal sections (g-i) offer the possibility of directly investigating cortical brain activation and connectivity during task performance using the right hand alone (a,d,g), left hand alone (b,e,h) or during bimanual movements (c,f,i). Of note, use of either the left hand alone or simultaneously both hands showed a similar trend of activation in the bilateral frontal and parietal regions, known to be involved in working memory. By contrast, for the right-hand alone movements, activation of the motor control regions was lateralized to the left hemisphere. Scale: Z score ranging from -40.95 to 40.95.
For the right-hand finger-tapping condition, there was robust contralateral (i.e., left hemisphere) activation in the supplementary motor area (SMA), overlying the primary motor (M1), as well as the ipsilateral cerebellum (i.e., right side). In contrast, during unilateral left hand tapping, there was robust bilateral (i.e., both hemispheres) activation in the SMA, M1, vPM and dPM and in the cerebellar hemisphere ipsilateral to the movements. Similar imagery activation patterns were observed for bilateral tapping, dPM activation extending superiorly to the dorsal convexity and vPM activation extending along the precentral gyrus ( Figure 3 a-i).
Characteristic of spontaneous EEG
A set of 5 EEG data collected from E.L. over the years (2014; 2015a,b; 2019; 2020) was used for this study. Each epoch was reviewed in two formats: raw EEG and quantitative EEG (qEEG)-only, in which the raw signal is converted into a digital colour form using derived measures. E.L.´s awake EEG at rest is characterized in all EEG datasets by increased asymmetrical right anterior slow dominant rhythm (delta and theta rhythms) of ≤ 4 Hz and of 4-7 Hz and by a symmetrical posterior alpha rhythm of 9-10 Hz, moderate voltage that is reactive to eye opening and closure. Low amplitude beta frequency activity and alpha waves were also present over the anterior head regions, predominantly in the right front central region. During drowsiness, there was attenuation of the awake background and an increase in background slowing. Hyperventilation showed a tendency to increase the focal slowing noted above. Photic stimulation did not alter the background ( Figure 2 g). A lateralized antero-to-posterior EEG spectral gradient (APSG), which had greater low frequency power from the right prefrontal, frontal lobe and occipital areas, emerged as the most prominent feature at rest associated with E.L. associated brain lesion. The APSG crossed over the genu (in 3 out of 4 runs) and body (in 4 out of 4 runs) of the C.C. to reach the contralateral hemisphere ( Figure 2 g; Figure 4 ).

Changes in slow wave amplitude of the quantitative EEG during active and passive (assisted) symmetric movement tasks. The topographic delta frequency band distribution in E.L. and CTRL (resting testing) (top row), during unimanual left/right and bimanual tasks (middle row) and during foot movements (bottom row) is illustrated. Significant differences from baseline for absolute amplitude (µV) in delta frequency were found only in E.L. right frontal region (Fp2) while performing active finger-to-thumb tapping movements – P < 0.05 baseline vs. left hand and P < 0.05 baseline vs. right hand. Changes in delta wave amplitude during active/passive hand or foot movements were neither documented for CTRL nor for E.L. during either active/passive foot and passive hand movements. Calibration (colour scale): 15-102 µV.
qEEG signals related to left/right hands and foot movements
Following execution of left-hand motor tasks, repeated measures of frequency of finger-tapping with the right hand alone showed no difference between E.L. and CTRLS ( Figure 4 ) (Supplementary Clinical Case History). In contrast, in the right PFC, delta waves outcome showed a significant increase in frequency to 104.4 ± 1.5 (µV)/epoch ( n = 3 assays) ( P < 0.05), while alpha, beta and theta values did not change during the execution of the motor task. A similar motor outcome by E.L. was evidenced during active dual-task performance executed simultaneously with the right- and left-hands ( Figure 4 ). However, during bimanual movements, the mean number of finger taps −1 dropped to 29 ± 3, achieving over 32% reduction as opposed to CTRL. In contrast, right foot and left foot alone or simultaneous bipedal movements executed by all subjects, passive or active, independent of the starting foot, did not change the frequency of EEG waves nor did it result in any change in the latency to start performing the task ( Figure 4 ).
Modulation of cortical network SW oscillatory dynamics by navigated transcranial magnetic stimulation (nTMS)
Mapping characteristics.
TMS was performed in E.L. and in CTRL ( n = 4), who were right-handed (age range: 30-35 years). Left Primary Motor Cortex (PMC), dlPFC and vmPFC were focally stimulated sequentially, during three consecutive days in all participants without technical problems or adverse events. Neuropsychological and behavioral performance were determined prior, during and after application of TMS protocols. TMS protocols applied directly on the right hemisphere were not considered because of the nature of E.L.’s frontal injury.
An MRI-guided navigation system was used to estimate in real time an accurate coil positioning on the surface of the skull of the subjects ( see Methods ). Optimal TMS stimulation parameters for modulating non-invasively brain SW activity, identified in the context of the motor system in left PMC (M1) ( day 1 ), were subsequently applied to higher level functions such as working memory ( day 2 ) and apathy behavior ( day 3 ), respectively, in the block design protocol applied in dlPFC and in vmPFC. Participants did not self-report any side effects of stimulation protocols, either excitatory (PMC) or inhibitory (PMC, dlPFC, vmPFC). No apparent abnormal seizure-like activity was detected on EEG recordings following TMS protocols.
Threshold of motor evoked potentials (MEPs) and left PMC repetitive transcranial magnetic stimulation (rTMS)
Resting- and active motor-thresholds were predetermined in all participants at about 50 µV and 200 µV in the left PMC, respectively, to produce liminal MEP at rest and during isometric contraction of hand muscles (in 50% of 10 trials – Supplementary Methods ). rTMS inhibitory protocol (1Hz) in left PMC induced high amplitude (> 80 µV) SWs, mostly detected on E.L.’s right-sided scalp ( Figure 5 e-g), contrasted sharply with the lower amplitude SW of the pre-TMS EEG. Lateralized SWs revealed an antero-to-posterior gradient along the right-sided scalp and, simultaneously, a rapid lateral spread to the left frontal region ( Figure 5 e). Moreover, in the right PFC, delta slow waves showed a significant increase in frequency and power to 284.56 ± 39.09 µV²/epoch ( n = 30) ( P < 0.001) (10 highest epoch: 532.08 ± 46.96 µV²/epoch), maintaining its power for at least 40min (total recording time = 60min). In the subsequent period, with the virtual lesion resolution ( see Methods), there was a decrease in delta power, with no significant difference ( P >0.05) from the resting state (73.86± 10.96 event µV²/epoch ( n = 30) - 10 highest epoch: 127.95 ± 25.51 µV²/epoch). Thus, cross-hemispheric high amplitude SW frontal propagation may rely on E.L.’s anterior C.C ., albeit expected but not evidently compromised functional integrity of this interhemispheric WM tract. Also, rTMS excitatory protocol (10Hz) in left PMC did not increase the SWs in E.L. ( P >0,05) 72.54 ± 5.58 µV²/epoch ( n = 30) (10 highest epoch: 105.3 ± 8.23 µV²/epoch) in comparison to the resting state – TMS-untreated 76.26 ± 10.15 event µV²/epoch ( n = 30) (10 highest epoch: 134.1 ± 20.21 µV²/epoch) ( Figure 5 e,h). The triggering of SWs in E.L. could be obtained reliably using inhibitory, not excitatory (10Hz), stimulating conditions at frequency of 1 Hz. In contrast, a similar protocol did not evoke SWs in the CTRL ( n = 4) ( Figure 5 e,h), regardless of stimulation site.
Left dlPFC contribution to E.L.’s working memory
Here, inhibitory TMS pulses (1 Hz) were used to create a ‘temporary lesion’ of a targeted cortical region, thereby disrupting dlPFC-mediated balance in visuospatial, perception, learning and short-term memory (retention) performance. E.L. apparent deficits on working memory, tailored the selection of the Rey-Osterrieth Figure (ROFC), one of the most frequently used tests for assessing executive dysfunction, to assess the TMS-treated participants. To preclude the possibility that impaired performance could be secondary to rTMS-mediated potential ‘loss of interest’ or ‘apathy’ ( see below), the intervention using dlPFC stimulation procedure was administered one day prior to that of vmPFC.
Prior to TMS treatment, no differences ( P >0,05) were found between E.L. and the CTRL ( n = 10) in the accuracy of the Rey-Osterrieth figural copy (5 min time limit set for the copy), in the immediate recall (IR) (3 min) (and in the delayed recall (DR) (30 min), indicated by Crawford & Garthwaite´s method for detecting the abnormality of a patient´s score. Details are illustrated in Figure 5 i,j. However, following TMS treatment, there was a significant difference between E.L. TMS-untreated/treated ratio in comparison with CTRL´s TMS-untreated/treated ratio at the IR ( P < 0.001 - E.L. 1.756 vs CTL 0.893 [0.02] - Effect Size (Z-CC) 10.128, as well as for the DR ( P < 0.001 - E.L. 1.619 vs CTL 0.877 [0.03] – Z-CC 6.523) ( Figure 5 i,j). TMS-treated E.L. reproduced the figure at both measurement points with fewer details and misplacement features. No TMS-mediated decreases in accuracy scores appeared for CTRL regarding IR and DR (mean score of 28.25 [2.06] vs 31.8 [2.18]), and of 28.6 [1.98] vs 32.3 [1.43], respectively).
rTMS-mediated left vmPFC triggering of slow waves and apathy is state-dependent
Here, a second inhibitory protocol of continuous Theta-Burst Stimulation (cTBS) was applied in left vmPFC, in which three 50-Hz pulses were applied at 5 Hz for 40 sec. E.L. presented increased frequency and power of SWs along with reduction of ‘goal-directed behavior’ (apathy) immediately following cTBS ( P < 0.001). In support of our main hypothesis following TBS applied to left vmPFC, neither SWs evoked in E.L.’s right hemisphere nor apathy were recorded in or manifested by CTRL. Figure 5 illustrates the results. Both CTRL and E.L. were previously assessed AES-C rating scale suggesting no apathy or apathy related symptoms. For CTRL, no SW pattern or significant behavioral changes (Scale for Assessment of Negative Symptoms (SANS) of 0 [ n = 4]), emerged following TBS-treatment. By contrast, for E.L., SANS changed from 0 to 45 units (range: 0-125 units), with the presentation of apathy related signs and symptoms 3-5 min after application of TBS protocol, including signs of affective flattening, poverty and increased latency of speech and inattentiveness, paralleling the increased frequency in SWs, and remained for up to 60 min. Return to basal behavioral state was paralleled by return of SWs to pre-TBS stimulation level, determined by EEG recordings ( Figure 5 e-h). Moreover, in the right PFC, there was a sustained increase in SWs to 184.45 ± 25.75 event (µV2)/epoch ( n = 30) ( P < 0.001) (10 highest epoch: 328.35 ± 46.99), maintaining its power for at least 60min (total recording time = 90min). In the subsequent period, with the virtual lesion resolution ( see Methods), there was a decrease in delta power, with no significant difference ( P >0.05) from the resting state (68.08± 5.68 event (µV2)/epoch ( n = 30) - 10 highest epoch: 103.41 ± 8.28).
We have documented the performance of a patient (E.L), a modern-day Gage, on tests of behavioural and executive functions, which were given during a period of 8 years. His severe transfixing frontal lobe damage, remarkably similar to that of Phineas Gage (1848) 22 (Supplementary Clinical Case), who attained a legendary status in the history of neuroscience and psychology, occurred against a background of largely intact cognitive, intellectual, perceptual, social and sexual functions. E.L. neither exhibited emotional and behavioral changes, including disinhibition, jocularity, and decline in social and occupational functioning, nor changes in higher-level cognitive and other executive functions associated with Gage's accident and “classical damage” to ventromedial- and dorsolateral prefrontal regions. Multitasking assessments and TMS-targeted protocols, however, unmasked compensatory mechanisms through modulation of slow wave oscillations under single task conditions, to maintain motor performance and executive functioning, eluding clinical detection and underlying brain capability to restore ‘normal life’ function after unilateral frontal lesions.
We selected tests for the most common neuropsychological functions sensitive to damage in the frontal lobe 16 ( Table 1 ; Extended Table 1). It is notable that E.L.’s decision-making impairments were not detected by gold standard tests of cognitive assessment, such as, IGT, CBT, WCST, and the ROCFT. 16 From a clinical perspective, we identified deficits in E.L. across working memory, including short-term memory, and using task switching tests, an executive function that involves the ability to unconsciously shift attention between dual-tasks. Similarly, we identified borderline performances on language (semantic [animals] and phonological [letter ‘p’]) tests ( Table 1 ; Extended Table 1). Although cognitive impairments are not always evident on Divided Attention and on Working Memory tests, previous studies reported that patients with right hemisphere damage exhibited a worse performance, 23 supporting the concept that the contribution of the right hemisphere is more pronounced than the left hemisphere for the processing of visuospatial short-term memory tasks. In agreement with this concept, E.L. showed working memory deficits, independent of a clear rising complexity level, and deficits on the spatial span, in agreement with previous reports on TBI patients. 24 However, several confounding variables must be taken into account. Among them, are the side effect of some symptomatic treatments with OXC - an anticonvulsant, which can impair performance in cognitive functions-, 25 intelligence, 26 age, and degree of education. Accordingly, no differences in anti-saccade task and eye tracking performance, manifested by subjects with a variety of neurological and psychiatric conditions, 27 were found on the OXC-treated CTRL and E.L. Thus, it remained unclear the extent to which a deficit in top-down inhibition in E.L. had functional significance.
Noting that dual-task interference effect may reflect the disruption of a central attention processor that uses limited resources to subordinate processing mechanisms for executing a task, 28 we decided to perform imaging and electrophysiological assays in conjunction with memory-guided simple sensorimotor decision tasks. E.L.’s performance on simultaneous bimanual movements is illuminated by his cortical activation patterns during functional neuroimaging assays, debunking misinterpretations and shedding light on resource allocation during task performance. In particular, E.L.’s movements revealed that right hand finger tasks induced most activation in the contralateral PMA, PFC and Supplementary Motor area (SMA) and ipsilateral cerebellum, in terms of total/relative voxels relative to his SMA. Previous studies reported that the primary sensory-motor cortex (SM1) is more activated by finger than toe movements, although the PMA and PFC are more activated by toe movements. 29 Therefore, different bold fMRI activation patterns are believed to be generated by movements of the arms or legs. On the other hand, we found that E.L.’s both unimanual finger movements (left hand) or bimanual movements induced similar patterns of activation in the bilateral frontal and parietal regions, known to be involved in working memory. 30 Previous research indicated that transcallosal inhibition from the contralateral to ipsilateral hemisphere in sensorimotor area, in response to voluntary extension/flexion of single hands, could account for modulation of neural activity on normal subjects 31 and that brain injured subjects presented abnormally increased transcallosal inhibition from the healthy hemisphere onto the injured side. 32 Nonetheless, the fact that reduced connectivity can reroute signal through more intact commissural fibers, thereby maintaining enough contralateral connectivity, 33 is consistent with previous results in context of how interhemispheric connectivity can be impaired without reducing cognitive or functional ability. 3 , 34 fMRI and TMS findings and qEEG for E.L. unveiled that interhemispheric connectivity via the use of the anterior or the posterior commissures in lieu of the C.C ., correlated with spontaneous brain activity or were measured in the presence of task demands, respectively. While some studies of typical interhemispheric coordination in neurologically intact subjects suggest entirely cortico-cortical connections, others implicate subcortical contributions. 35 Another possibility is that subcortical structures such as the thalamus or the superior colliculus play a greater role than previously thought in defining the flow of information between cortical regions. 36 , 37 This adjustment mechanism has been suggested in humans with agenesis of C.C . 34 In support of this view, interhemispheric functional synchronization in the absence of direct C.C. connectivity has also been reported in species that lack C.C . or have a small C.C. , such as song birds and cetaceans. 38
Although the same neuronal circuits may produce slow wave (SW) oscillation (1-4 Hz) in seizing brain and during sleep, 39 , 40 the two conditions seems to rely on differential activation of networks. Previous reports showed that SW activity is associated with increase fMRI-BOLD activation responses in a spatially related brain area. 41 However, while SW is thought to consolidate memory activity in normal subjects during deep sleep (NREM), 42 SW in the seizing brain is considered an epiphenomenon leading to dysfunction, including impairment of memory. The logic here has been that delta oscillation in lesioned and contralateral areas of E.L.’s brain may be not merely a marker of network dysfunction, 43 , 44 but, instead, an expression of signalling rearrangement enabling performance. 12 While E.L. presented a similar modulation of SW for passive/active foot movements, as predicted from previous work on Bold fMRI, 45 his active right finger movements, by engaging his left hemisphere, led to increased SW in his right frontal cortex as shown by EEG recordings. Subsequent, single-handed left finger movements, engaging both hemispheres, decreased SW on his injured right hemisphere. That is, while a decrease of frequency of irregular SW in the right damaged hemisphere, assumed as a non-epileptiform activity, facilitated E.L. to accomplish a number of cognitive and motor tasks, persistence/increase of delta waves became an indicator of impairment/dysfunction. Different patterns of anatomical activation induced by E.L's fingers or foot movements explain lack of modulation of frequency of SW by the latter. On the other side, although a similar pattern of brain representation is elicited by active or passive movements for upper limbs, during active movements only, activations of the basal ganglia and the cingulate gyrus were found. 46
TMS is increasingly used to create ‘virtual lesions’, with temporospatial resolution, in selected brain regions. 47 Thus, temporary disturbance of task-related neuronal activity mediated by a specific region should yield a decline in executive performance and behavior. We tested this prediction by applying rTMS pulses over the left PMC (motor), dlPFC (working memory) and vmPFC (behaviour) 48 and showed that it is possible to reliably trigger SWs in awake injured brain that resemble in all aspects spontaneously TMS-induced slow oscillations during NREM sleep (i.e., state-dependent). 49 Spatially, the TMS-treatment resulted in a substantial increase in SW oscillations that spread antero-to-posterior like a ‘water ripple wave’ across E.L.’s right hemisphere and laterally to his contralateral frontal lobe during wakefulness. In contrast to E.L. awake brain, however, the hot spot for TMS-triggered oscillations on CTRL sleep NREM brain corresponded closely to a hot spot for the origin of spontaneous SWs and their sensorimotor regions constituted a preferential site for triggering such SWs. 50 In contrast to E.L., TMS-triggered frontal SW oscillations in sleeping heathy brain are state-specific and displayed a sudden disappearance after transition from NREM to wakefulness. 49 Thus, lack of TMS-triggered SWs on our awake CTRL was not unexpected. By demonstrating these findings in awake injured brain, however, our study suggests that in contrast to the ‘sleeping mode’, 13 E.L.’s injured brain, active and reactive, does not lose its ability of entering integrated and differentiated states because of prompt modulation of frequency and amplitude of SW oscillations by the left contralateral hemisphere. Results from the present study support that such features are critical for performance of E.L. executive functions. It is important to note that, regardless of stimulation site, TMS pulses applied to E.L scalp area, were equally effective overlying PMC, dlPFC and vmPFC. Thus, in contrast to the sensorimotor cortex (precentral and postcentral gyri areas) on ‘sleeping brain’, 49 where activation of corticoreticulothalamocortical circuits by TMS primarily support triggering of SWs, evidence from our study support that, in injured E.L. awake brain, contralateral cortical reactivity is required to attenuate the frequency of irregular SW activity. Following the anticipated modulation criteria of contralateral SWs oscillation, the second criterion of successful compensation required disturbance of executive functioning and behaviour on selected regions, and a consequent decline in specific performance upon TMS-treatment. Temporary maintenance and processing of information, involving executive processes that manipulate the contents and retention of working memory, was explored assessing the ROCF task. Several studies give evidence for a role of the left dlPFC in working memory, but, no apparent deficits manifested by E.L. on any outcome measures (copy, IR and DR trials of ROCF test) were suggested prior to TMS-pulses as well as no effects were observed following unilateral stimulation of CTRL on any outcome measures. 51 We tested this prediction by probing the reactivity and executive functioning in E.L and CTRL brain after applying TMS pulses in left dlPFC at intensities commonly used in clinical practice. 52 As predicted, consolidation of the Rey-Osterrieth Complex Figure was disrupted at both 3 min (IR) and 30 min (DR) by stimulation selectively over E.L. dlPFC, without disruption of his motor skills or other aspects of motor function. Such strategy failed to find a similar disruption of executive functioning in the CTRL, suggesting that bilateral ‘lesions’ are required to impair short-memory consolidation and retrieval. In a third approach, an increase of SWs over the right hemisphere by means of TMS protocols applied to left vmPFC, used to interfere with motivation (i.e., apathy and asociality), disrupted E.L. willingness to engage in social interaction, similar to disorders secondary to bilateral frontal damage, 1 and produced indifference regarding interpersonal relationship. In contrast to E.L., the CTRL group did not manifest change in behaviour after TMS-treatment. Return to normal behaviour paralleled progressive reduction of SW activity over E.L. right hemisphere, within 60 min of TMS-stimuli.
Few cases in the history of neurosciences have been reported as frequently as the TBI case of Phineas Gage, which still lives as a part of neuroeducation. While great attention is given to executive dysfunction, one can merely speculate how Gage's brain injury actually affected his daily routine and feasibility of functional compensation. The current E.L. case, whose brain injury mirrored that of Gage's, sheds light on SW oscillatory dynamics following TBI, unveils compensatory mechanisms by which PFC may accommodate executive functional performance, which frequently elude clinical diagnosis, and provides an attractive target for therapeutic interventions on cortical circuits.
Contributors
R.C.M.S.F., F.A.V., I.S.D., R.M.B. and C.M.P. performed neurosurgical procedures. C.T.F.T., C.C., J.T., K.P. and E.G. selected neuropsychological batteries and performed testing and interpretations. L.C.H.C. Jr. performed diagnostic MRI. G.F.G. and F.M. applied BIQS testing. G.L.W. provided advice on statistical methods. M.F. and P.H.F. performed EOG testing. P.H.F., R.B., G.F.G., S.F.A.R., C.M.P. and R.R. developed the figures, table and movies; analysed data and revised the manuscript. W.S.P., C.Y.H., P.C.R. and P.H.F. conducted the T.M.S. assays. P.H.F., C.M.P., J.V., T.M.M.L., M.M.S., F.L., H.J.F. and R.R. performed neurological and neurophysiological assessments. J.R.L.S. developed the 3D brain modelling. D.H.P. performed diagnostics assays. R.L., M.V.L.B., P.H.F. and R.R. - conceived the study, structured its design, interpreted and integrated data and organized the general discussion. M.V.L.B. and R.R. - drafted and edited the main manuscript and proved the final version.
Declaration of interests
All authors declare that they have no conflicts of interest.
Acknowledgements
We thank Mr. M.B. da Silva (CDPI, Brazil), Dr. F. Tovar-Moll (IDOR, Brazil) and Dr. H. Werner (CDPI/DASA, Brazil) for MRI scans over the course of the study; Mr. M. Barroco and Mrs. C. Seixas (Zeike Medical Ltda, Brazil) for EEG measurements technical support; Dr M.S.Thome de Souza (USP, Brazil) for EEG/TMS monitoring; Dr C. Rego (HUCFF/UFRJ, Brazil) for video/EEG records; Mr E.M. de Souza (EMSA Equipamentos Médicos Ltda, Brazil) for qEEG (brain map); and Dr. S. Moshe (Einstein, NY) for valuable comments. We are grateful to Dr. O. Sacks † (NYU, NY) for his earlier insightful comments on E.L.’s right frontal lobe injury and for the assistance provided by Ms K. Edgar (‘The Oliver Sacks Foundation’, NY).
Supplementary material associated with this article can be found in the online version at doi: 10.1016/j.lana.2022.100340 .
Appendix. Supplementary materials
EXTENDED DATA
Extended FIG. 2. MRI follow-up of patient E.L. T2 weighted sequences suggesting the penetrating lesion extension. a, c – Scan sequences obtained 12 days after the transfixing traumatic brain insult, with overt perilesional edema (white arrow), without midline deviation. b, d – 18 months follow-up, with no visible edema, atrophy, or neurodegenerative signs. Axial (a, b) and coronal (c, d) sections, respectively.
Extended FIG. 3. E.L.: Failure of compensatory mechanisms during bimanual challenge. Coordination testing showed symmetrical movements without dysmetria, or incoordination, on finger to nose testing, normal forearm (a) and finger rolling tests (b), in which the subject rotates just the index fingers using rapidly alternating movement, and on sequential active finger-to-thumb tapping with the right hand alone (c) or with the left hand alone (d). However, there was incoordination of sequential finger-to-thumb tapping movements in the left hand during bimanual movements (e) low-magnification and (f) higher magnification. In the beginning, E.L. expressed surprise at the outcome to see that his left-hand fingers could end up paralyzed for a few sec upon the execution of bimanual movements.
Extended FIG. 4. 3D brain reconstruction from T1 MRI scan sequences helped unveil E.L.’s right frontal lobe dysfunction. Cortical surfaces reconstruction with sulcal identification output by FreeSurfer (inset) are illustrated (a,b). Life-size 3D view metrics of E.L brain morphology and the path that the transfixing iron bar followed through his right hemisphere (a,b in red colour), printed in polyamide (c), highlighted compromised regions and presented a detailed picture of the extent of the damage. R (right hemisphere), L (left hemisphere). Calibration bar (c): 2 cm.
Extended FIG. 5. Visual representation of major white matter fiber tracts tracked in E.L. Diffusion tensor tractography quantification of Uncinate Fasciculus (yellow), Cingulum bundle (blue) and Superior Longitudinal Fasciculus (SLF) (green), compromised in E.L. accident, and Corpus Callosum ( C.C. ) (red), are illustrated in lateral and axial scans (a-f). (c,d,e ). Axial and lateral views of a tractography suggested a difference in the volume of the association fiber tracts that connect temporo-parietal cortical regions with right frontal lobe (b,c,f – white arrows), but no difference in the volume of the C.C. association fiber tracts that connect the frontal lobes (c,e,f – red).
Extended FIG. 6. E.L.’s performance in Iowa Gambling Task (IGT), used to assess risk-based decision-making and impulsivity after TBI, is sensitive to behavioural deficits in subjects with PFC damage. The test consists of a card game where the risks and rewards vary by the decks chosen. (a) Percentage of cards selected from different decks. (b) Two of these decks (decks A [highest risk deck] and B) have higher short-term payoffs (‘high risk’) than the other two (decks C and D) (advantageous ‘safe’ decks), but over time (10 trials of the IGT) the decks with high immediate payoffs are disadvantageous, resulting in a long-term loss (net loss). (c,d) E.L.’s score based in safe vs. risky decks and numbers of cards selected (safe vs risky decks) over the course of the test. (e) reaction times (in sec) for each trial (total 100 trials) of the test (deck choice represented by colour patterns depicted in ‘a’). Total net IGT scores (100 trials per session) is analysed using methods of scoring risky decisions (Methods). Complex emotion based-learning remained intact in E.L.
Extended FIG. 7a,b. Assessment of sexuality following traumatic brain injury (TBI). The Brain Injury Questionnaire on Sexuality (BIQS), which is designed to account for temporal changes in sexual function, was applied in parallel to E.L. and to L., his wife (a, b), 31 months following E.L.’s TBI.
Extended FIG 8. The antisaccade task and the voluntary control of eye movement. Saccadic and antisaccadic eye movement tasks depend on the frontal/prefrontal cortex and related structures. (a) Schematic diagram of EOG test. A symbolic cue, such as a colour dot, instructs the subject to make a brief, rapid eye movement (a saccade) towards the stimulus (prosaccade) (green) or in the opposite direction (antisaccade) (red). (b) The schematics of the overlap (top layer) and gap (middle layer) tasks performed are displayed in msec. (Bottom) Reaction time latencies of eye movements during prosaccade and antisaccade commands. (c,d) Performance of a CTRL and E.L. during a gap task. (c) Eye position traces during prosaccades (left). (middle, right) Distribution of reaction times for each participant (histogram) – single test. (d) Antisaccade tasks . Correct responses are displayed in blue, corrected errors in red and anticipations or uncorrected errors in black – single test. (e,f) Overlap task % and gap task % distribution of EOG responses for the CTRL and E.L. Differing effects of prosaccades (Pro) and antisaccades (Anti) are illustrated by histograms correspondent to right (top) or left (bottom) stimuli.
Extended FIG. 9. Impaired antisaccades in oxcarbazepine-treated subjects. The comparison between the outcome of eye-tracking performance in oxcarbazepine (OXC)-treated E.L. and in OXC-treated/untreated CTRL ( n = 5). (a) The first row depicts latencies in saccade, prosaccade and antisaccade paradigms, presented randomly to the right or the left side. (b) The second row displays the performance (correct rate responses) of the OXC-treated subjects and CTRL. * - P ≤ 0.05. (b) Mean ±SEM values of prosaccade and antisaccade and latency of all groups. Of note, there were no differences in symmetry (right vs left, or up vs down) of prosaccadic-antisaccadic latency, velocity, or gain between E.L. and the OXC-treated CTRL. (c) E.L.’s therapeutic plasmatic levels of oxcarbazepine.
Extended TABLE 1. Neuropsychological battery performed in E.L. Abbreviations: MMSE: Mini Mental State Examination; BDI: Beck's Depression Inventory; WCST: Wisconsin Card Sorting Test (Modified Card Sorting Test. Nelson, 1976); PM 38: Standard Progressive Matrices 1938; RCFT: Rey – Osterreith Complex Figure Test; TMT: Trail Making Test; Stroop-W: word reading Stroop Task; Stroop-C: colour naming Stroop Task; Stroop CW: Colour-word interference Stroop Task; DMS-48: Delayed Matching to sample (Barbeau, 2004); CVLT: California Verbal Learning Test; BNT: Boston Naming test; Facial Recognition Test (Benton, 1983)
SUPPLEMENTARY MOVIES
Movie #2. Supplementary movie S2.
Movie #3. Supplementary movie S3.
Supplementary Clinical Case History.
Supplementary Methods.
- Brain Development
- Childhood & Adolescence
- Diet & Lifestyle
- Emotions, Stress & Anxiety
- Learning & Memory
- Thinking & Awareness
- Alzheimer's & Dementia
- Childhood Disorders
- Immune System Disorders
- Mental Health
- Neurodegenerative Disorders
- Infectious Disease
- Neurological Disorders A-Z
- Body Systems
- Cells & Circuits
- Genes & Molecules
- The Arts & the Brain
- Law, Economics & Ethics
Neuroscience in the News
- Supporting Research
- Tech & the Brain
- Animals in Research
- BRAIN Initiative
- Meet the Researcher
- Neuro-technologies
- Tools & Techniques
Core Concepts
- For Educators
- Ask an Expert
- The Brain Facts Book

Phineas Gage
- Published 24 Jun 2020
- Author Calli McMurray
- Source BrainFacts/SfN
Railroad foreman Phineas Gage survived a horrific brain injury that left him with an altered personality. His story revealed the complex functions of the frontal lobe decades before scientists began studying it in animals.
Brain Bytes showcase essential facts about neuroscience.

Design by Adrienne Tong .
Image: Originally from the collection of Jack and Beverly Wilgus, and now in the Warren Anatomical Museum, Harvard Medical School.
About the Author

Calli McMurray
Calli McMurray is the Media & Science Writing Associate at SfN. She graduated from the University of Texas at Austin in 2019 with a Bachelor of Science in Neuroscience.
CONTENT PROVIDED BY
BrainFacts/SfN
Damasio, H., Grabowski, T., Frank, R., Galaburda, A., & Damasio, A. (1994). The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science, 264(5162), 1102–1105. doi:10.1126/science.8178168
O'Driscoll, K. & Leach, J. P. (1998). “No longer Gage": an iron bar through the head. Early observations of personality change after injury to the prefrontal cortex. BMJ (Clinical research ed.), 317(7174), 1673–1674. doi:10.1136/bmj.317.7174.1673a
Also In Tools & Techniques

Popular articles on BrainFacts.org

Check out the latest news from the field.
BrainFacts Book
Download a copy of the newest edition of the book, Brain Facts: A Primer on the Brain and Nervous System.
A beginner's guide to the brain and nervous system.

SUPPORTING PARTNERS
- Privacy Policy
- Accessibility Policy
- Terms and Conditions
- Manage Cookies
Some pages on this website provide links that require Adobe Reader to view.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Mapping Connectivity Damage in the Case of Phineas Gage
* E-mail: [email protected]
Affiliation Laboratory of Neuro Imaging (LONI), Department of Neurology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California, United States of America
Affiliation Surgical Planning Laboratory, Department of Radiology, Brigham & Women's Hospital, Harvard Medical School, Boston, Massachusetts, United States of America
- John Darrell Van Horn,
- Andrei Irimia,
- Carinna M. Torgerson,
- Micah C. Chambers,
- Ron Kikinis,
- Arthur W. Toga

- Published: May 16, 2012
- https://doi.org/10.1371/journal.pone.0037454
- Reader Comments
White matter (WM) mapping of the human brain using neuroimaging techniques has gained considerable interest in the neuroscience community. Using diffusion weighted (DWI) and magnetic resonance imaging (MRI), WM fiber pathways between brain regions may be systematically assessed to make inferences concerning their role in normal brain function, influence on behavior, as well as concerning the consequences of network-level brain damage. In this paper, we investigate the detailed connectomics in a noted example of severe traumatic brain injury (TBI) which has proved important to and controversial in the history of neuroscience. We model the WM damage in the notable case of Phineas P. Gage, in whom a “tamping iron” was accidentally shot through his skull and brain, resulting in profound behavioral changes. The specific effects of this injury on Mr. Gage's WM connectivity have not previously been considered in detail. Using computed tomography (CT) image data of the Gage skull in conjunction with modern anatomical MRI and diffusion imaging data obtained in contemporary right handed male subjects (aged 25–36), we computationally simulate the passage of the iron through the skull on the basis of reported and observed skull fiducial landmarks and assess the extent of cortical gray matter (GM) and WM damage. Specifically, we find that while considerable damage was, indeed, localized to the left frontal cortex, the impact on measures of network connectedness between directly affected and other brain areas was profound, widespread, and a probable contributor to both the reported acute as well as long-term behavioral changes. Yet, while significantly affecting several likely network hubs, damage to Mr. Gage's WM network may not have been more severe than expected from that of a similarly sized “average” brain lesion. These results provide new insight into the remarkable brain injury experienced by this noteworthy patient.
Citation: Van Horn JD, Irimia A, Torgerson CM, Chambers MC, Kikinis R, Toga AW (2012) Mapping Connectivity Damage in the Case of Phineas Gage. PLoS ONE 7(5): e37454. https://doi.org/10.1371/journal.pone.0037454
Editor: Olaf Sporns, Indiana University, United States of America
Received: August 3, 2011; Accepted: April 23, 2012; Published: May 16, 2012
Copyright: © 2012 Van Horn et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: This work was supported by 2U54EB005149-06 “NAMIC: Traumatic Brain Injury - Driving Biological Project” to JVH, 1RC1MH088194 “Informatics Meta-Spaces for the Exploration of Human Neuroanatomy” to JVH, and P41RR013642 “Computational Anatomy and Multidimensional Modeling” to AWT. This work was performed as part of the Human Connectome Project (HCP; www.humanconnectomeproject.org ). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The mapping of human brain connectivity through the use of modern neuroimaging methods has enjoyed considerable interest, examination, and application in recent years [1] , [2] . Through the use of diffusion weighted (DWI) and magnetic resonance imaging (MRI), it is possible to systematically assess white matter (WM) fiber pathways between brain regions to measure fiber bundle properties, their influence on behavior and cognition, as well as the results of severe brain damage. The potential for using combined DWI/MRI methods to understand network-level alterations resulting from neurological insult is among their major research and clinical advantages.
In this paper, we investigate the detailed connectomics of a noted example of severe traumatic brain injury (TBI) which has proved important to and controversial in the history of neuroscience. Few cases in the history of the medical sciences have been so important, interpreted, and misconstrued, as the case of Phineas P. Gage [3] , in whom a “tamping iron” was accidentally shot through his skull and brain, resulting in profound behavioral changes, and which contributed to his death 151 years ago. On September 13th, 1848, the 25-year old Phineas P. Gage was employed as a railroad construction supervisor near Cavendish, Vermont to blast and remove rock in preparation for the laying of the Rutland and Burlington Railroad. Having drilled a pilot hole into the rock and filling it partially with gunpowder, he instructed an assistant to pour sand into the hole atop the powder. Averting his attention for a moment to speak with his men, he apparently assumed the sand had been added. He then commenced dropping the end of a 110 cm long, 3.2 cm diameter iron rod into the hole in order to “tamp” down its contents. The 13 lb. iron struck the interior wall of the hole causing a spark to ignite the powder which, in turn, launched the pointed iron rod upwards, through the left cheek of Mr. Gage just under the zygomatic arch, passing behind his left eyeball, piercing his cranial vault under the left basal forebrain, passing through his brain, and then exiting the top and front of his skull near the sagittal suture. A large amount of brain tissue was expelled from the opening and the rod was found later “smeared with blood and brains”, washed in a stream, and, eventually, returned to him. After receiving treatment and care from Dr. John Martyn Harlow over subsequent weeks, Mr. Gage was able to recover sufficiently from his physical injuries and return to his family in nearby New Hampshire. However, reports of profound personality changes indicate that he was unable to return to his previous job and caused co-workers to comment that he was “no longer Gage.” Following several years of taking manual labor jobs and travelling throughout New England and eventually to Valparaiso, Chile, always in the company of “his iron”, he was reunited with his family in San Francisco whereupon Mr. Gage died on May 21, 1860, nearly 12 years after his injury – presumably due to the onset of seizures evidently originating from damage resulting from the tamping rod incident. Several years later, Dr. Harlow, upon learning of Gage's death, asked Gage's sister's family to exhume his body to retrieve his skull and rod for presentation to the Massachusetts Historical Society and deposition with Harvard Medical School where, to this day, it remains on display in the Warren Anatomical Museum in the Francis A. Countway Library of Medicine at Harvard Medical School ( Fig. 1a ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
a) The skull of Phineas Gage on display at the Warren Anatomical Museum at Harvard Medical School. b) CT image volumes were reconstructed, spatially aligned, and manual segmentation of the individual pieces of bone dislodged by the tamping iron (rod), top of the cranium, and mandible was performed. Surface meshes for each individual element of the skull were created. Based upon observations from previous examinations of the skull as well as upon the dimensions of the iron itself, fiducial constraint landmarks were digitally imposed and a set of possible rod trajectories were cast through the skull. This figure shows the set of possible rod trajectory centroids which satisfied each of the anatomical constraints. The trajectory nearest the mean trajectory was considered the true path of the rod and was used in all subsequent calculations. Additionally, voxels comprising the interior boundary and volume of the cranial vault were manually extracted and saved as a digital endocast of Mr. Gage's brain cavity. c) A rendering of the Gage skull with the best fit rod trajectory and example fiber pathways in the left hemisphere intersected by the rod. Graph theoretical metrics for assessing brain global network integration, segregation, and efficiency [92] were computed across each subject and averaged to measure the changes to topological, geometrical, and wiring cost properties. d) A view of the interior of the Gage skull showing the extent of fiber pathways intersected by the tamping iron in a sample subject ( i.e. one having minimal spatial deformation to the Gage skull). The intersection and density of WM fibers between all possible pairs of GM parcellations was recorded, as was average fiber length and average fractional anisotropy (FA) integrated over each fiber.
https://doi.org/10.1371/journal.pone.0037454.g001
The amount of damage to Mr. Gage's left frontal cortical grey matter (GM) with secondary damage to surrounding GM has been considered by several authors with reference to Gage's reported change in temperament, character, etc [4] , [5] , [6] ( Table 1 ). With the aid of medical imaging technology, two previous published articles have sought to illustrate the impact of the rod on Mr. Gage's skull and brain. Most famously, Damasio et al. [7] illustrated that the putative extent of damage to the left frontal cortex would be commensurate with the disinhibition, failures to plan, memory deficiencies, and other symptoms noted in patients having frontal lobe injury. Ratiu et al. [8] sought to illustrate the trajectory of the tamping iron, characterize the pattern of skull damage, and explain potential brain damage using a single, example subject. However, while many authors have focused on the gross damage done by the iron to Gage's frontal cortical GM, little consideration has been given to the degree of damage to and destruction of major connections between discretely affected regions and the rest of his brain.
https://doi.org/10.1371/journal.pone.0037454.t001
WM fasciculi link activity between cortical areas of the brain [9] , [10] , become systematically myelinated through brain maturation [11] , govern fundamental cognitive systems [12] , and may be disrupted in neurological [13] and psychiatric disease [14] . Penetrative TBI in cases of wartime [15] , industrial [16] , gunshot [17] , or domestic [18] injury often result in significant damage to brain connectivity, loss of function, and often death. Yet, in some instances, recovery from objects penetrating WM [19] have been reported with minimal sequelae [20] . Neuroimaging studies of WM tracts in TBI have revealed not only significant acute damage to fiber pathways but also that measures of fiber integrity can show partial fiber recovery over time [21] , presumably due to cortical plasticity [22] in non-penetrative cases.
Given recent interest in the atlasing of the human WM connectome (e.g. http://www.humanconnectomeproject.org ), a detailed consideration of the putative damage to Mr. Gage's connectomics and implications for changes in behavior is provocative and compelling. Nerve damage is superficially evident through reports of eventual loss of sight in Gage's left eye, left eyelid ptosis [23] , and recognition of potential WM damage by other investigators [7] . Further examination of the extent of Gage's WM damage and of its effects on network topology and regional connectedness can offer additional context into putative behavioral changes. Due to the absence of original brain tissue and to the lack of a recorded autopsy from this case, one can only estimate the extent of damage from bony structures and can never be confident concerning which precise brain tissues were impacted. However, brain tissue in situ from a representative population can be considered and it can be assumed that Mr. Gage's anatomy would have been similar. In this examination, we obtained the original high-resolution CT data of the Gage skull used by Ratiu et al. , and computationally estimated the best-fit rod trajectory through the skull. Via multimodal analysis of T1-weighted anatomical MRI and DWI in N = 110 normal, right-handed males, aged 25–36, we quantify the extent of acute regional cortical loss and examine in detail the expected degree of damage to Mr. Gage's WM pathways.
Computationally projecting a model of the tamping iron through the T1 MRI anatomical volumes warped to the Gage skull geometry ( Table 2 ; Fig. 1b–c ; see also Methods ) in light of previously reported anatomical constraints ( Table 3 ) and healthy brain morphometry and connectivity ( Fig. 2 ), the average percentage of total cortical GM volume intersected was 3.97±0.29% (mean±SD), where the cortical regions most affected by the rod (>25% of their regional volumes) included (mean±SD): the left orbital sulcus (OrS; 90.86±6.97%), the left middle frontal sulcus (MFS; 80.33±10.01), the horizontal ramus of the anterior segment of the lateral sulcus (ALSHorp; 71.03±22.08%), the anterior segment of the circular sulcus of the insula (ACirInS; 61.81±18.14%), the orbital gyrus (OrG; 39.45±6.17%), the lateral orbital sulcus (LOrS; 37.96±20.24%), the superior frontal sulcus (SupFS; 36.29±12.16%), and the orbital part of the inferior frontal gyrus (InfFGOrp; 28.22±19.60%). While extensive damage occurred to left frontal, left temporal polar, and insular cortex, the best fit rod trajectory did not result in the iron crossing the midline as has been suggested by some authors (see Methods ). As a result, no direct damage appeared to occur in right frontal cortices as evident from our representative sample cohort. A complete list of all cortical areas experiencing damage is listed in Table 4 .
The outermost ring shows the various brain regions arranged by lobe (fr – frontal; ins – insula; lim – limbic; tem – temporal; par – parietal; occ- occipital; nc – non-cortical; bs – brain stem; CeB - cerebellum) and further ordered anterior-to-posterior based upon the centers-of-mass of these regions in the published Destrieux atlas [72] (see also Table 6 for complete region names, abbreviations, and FreeSurfer IDs, and Table 7 for the abbreviation construction scheme). The left half of the connectogram figure represents the left-hemisphere of the brain, whereas the right half represents the right hemisphere with the exception of the brain stem, which occurs at the bottom, 6 o'clock position of the graph. The lobar abbreviation scheme is given in the text. The color map of each region is lobe-specific and maps to the color of each regional parcellation as shown in Fig. S2 . The set of five rings (from the outside inward) reflect average i) regional volume, ii) cortical thickness, iii) surface area, and iv) cortical curvature of each parcellated cortical region. For non-cortical regions, only average regional volume is shown. Finally, the inner-most ring displays the relative degree of connectivity of that region with respect to WM fibers found to emanate from this region, providing a measure of how connected that region is with all other regions in the parcellation scheme. The links represent the computed degrees of connectivity between segmented brain regions. Links shaded in blue represent DTI tractography pathways in the lower third of the distribution of fractional anisotropy, green lines the middle third, and red lines the top third. Circular “color bars” at the bottom of the figure describe the numeric scale for each regional geometric measurement and its associated color on that anatomical metric ring of the connectogram.
https://doi.org/10.1371/journal.pone.0037454.g002
https://doi.org/10.1371/journal.pone.0037454.t002
https://doi.org/10.1371/journal.pone.0037454.t003
https://doi.org/10.1371/journal.pone.0037454.t004
The amount of total WM volume lost due to the tamping iron was 10.72±5.46% (mean±SD). Examination of lesioned connectivity matrices indicated that fiber bundles from nearly the entire extent of the left frontal cortex were impacted by the presence of the tamping iron (e.g. Fig. 1d ), which in turn affected most of that hemisphere as well as contralateral regions ( Fig. 3 ). The effect of this lesion on network properties was assessed 1) with respect to the healthy intact network, generally, as well as 2) in contrast to the average effects of similarly-sized lesions simulated elsewhere in the cortex, as related to local GM loss as well as distributed loss of connectivity ( Fig. 4a–c ). Metrics representative of three specific global network attributes were examined: characteristic path length (λ, measuring network integration), mean local efficiency (e, segregation), and small worldness (S) ( Table 5 ).
The lines in this connectogram graphic represent the connections between brain regions that were lost or damaged by the passage of the tamping iron. Fiber pathway damage extended beyond the left frontal cortex to regions of the left temporal, partial, and occipital cortices as well as to basal ganglia, brain stem, and cerebellum. Inter-hemispheric connections of the frontal and limbic lobes as well as basal ganglia were also affected. Connections in grayscale indicate those pathways that were completely lost in the presence of the tamping iron, while those in shades of tan indicate those partially severed. Pathway transparency indicates the relative density of the affected pathway. In contrast to the morphometric measurements depicted in Fig. 2 , the inner four rings of the connectogram here indicate (from the outside inward) the regional network metrics of betweenness centrality, regional eccentricity, local efficiency, clustering coefficient, and the percent of GM loss, respectively, in the presence of the tamping iron, in each instance averaged over the N = 110 subjects.
https://doi.org/10.1371/journal.pone.0037454.g003
WM fiber pathways intersected by the rod were pooled across all N = 110 subjects and examined for a) the relative lengths (w ij ) of affected pathways and b) the relative percentages of lost fiber density (g ij ); c) the bivariate distribution of g ij versus w ij indicating that local fiber pathways were affected, e.g. relatively short pathways proximal to the injury site, as well as damaging dense, longer-range fiber pathways, e.g. innervating regions some distance from the tamping iron injury (see “ Calculation of Pathology Effects upon GM/WM Volumetrics ” for further details).
https://doi.org/10.1371/journal.pone.0037454.g004
https://doi.org/10.1371/journal.pone.0037454.t005
Tables 6 and 7 provide details on the regional coding used for brain parcellation which were subjected to estimation of the effects of the tamping iron, lesion simulation modeling, and which encode the text on the outer-most rings of Figs. 2 and 3 . Differences in measures of network connectivity due to the rod's passage were apparent in terms of network integration, segregation, but not small worldness as compared to the unlesioned, healthy network. Specifically, when removing those cortical areas and fiber pathways intersected by the iron, characteristic path length was found to be significantly decreased in Gage compared to the intact network ( p ≤0.0001), mean local efficiency was decreased ( p ≤0.0001), while small worldness showed no statistical difference ( p ≤0.9467, ns). Regionally-specific network theoretical metrics in the affected regions and those to which they connect were also affected (see Fig. 5A ). This suggests that, not surprisingly, with significant loss of WM connectivity between left frontal regions and the rest of the brain, the surviving network of brain was likely to have been heavily impaired and its functions considerably compromised.
A) Cortical maps of regional graph theoretical properties. Regions affected by the passage of the tamping iron include those having relatively high betweenness centrality and clustering coefficients but relatively low mean local efficiency and eccentricity. B) A cortical surface schematic of the relative effects of systematic lesions of similar WM/GM attributes over the cortex for both network integration (i) and segregation (ii). For each mapping, colors represent the Z-score difference between systematic lesions of that area relative the average change in integration taken across all simulated lesions. C) Cortical maps of the differences/similarity between the effects on integration and segregation observed from the tamping iron lesion with that of each simulated lesion. Here black is most similar (e.g. the observed lesion is most similar to itself) whereas white is least similar to (e.g. most different from) the tamping iron's effects on these measures of network architecture.
https://doi.org/10.1371/journal.pone.0037454.g005
https://doi.org/10.1371/journal.pone.0037454.t006
https://doi.org/10.1371/journal.pone.0037454.t007
To further provide a baseline for comparison of the tamping iron lesion against similarly-sized lesions located elsewhere in the cortex, we conducted a systematic random simulation of 500 similarly-sized lesions across our N = 110 healthy subject cohort. The network containing the lesion due to the tamping iron was systematically compared against the distributions of the above mentioned metrics from the simulated lesion set. When paired t -statistics were computed to determine whether tamping iron lesion differed significantly from the standpoint of network metric values, standardized with respect to the intact network, as compared to other brain lesions of the same size, the characteristic path length (integration), mean local efficiency (segregation), and small worldness, while significantly different from that of the intact networks, were not found to be more severe than the average network properties of average similarly sized GM/WM lesion. These results are summarized in Fig. 5B and 5C . These indicate that alterations to network integration resulting from the tamping iron lesion resulted in greater average path length than that of the intact network but which was less than the average effects of other equally sized lesions. Likewise, segregation, as measured using mean local efficiency, was reduced compared to the intact network, but greater than the average effects of the simulated lesions. These results suggest that Mr. Gage's lesion, while severe and certain to have affected WM connectivity in his left cerebral hemisphere and throughout his brain, could have been considerably more severe had the tamping iron pierced other areas of his brain.
The case of Phineas Gage is among the most famous and infamous in the history of brain science. The interpretations of his incredible injury and attempts to characterize it have been ongoing since soon after it occurred. Our consideration sought to provide a modern connectomic understanding of Mr. Gage's injury and put it into context as involving brain WM in addition to the GM damage discussed by other authors. While we, too, are constrained by the relics left from Mr. Gage's life and what evidence can be gleaned from them, work detailed in this article differs considerably from previous examinations of this case and topic in several key areas: we 1) precisely model the trajectory of the tamping iron through high resolution computed tomographic data of Mr. Gage's skull - a rare imaging data set that, until now, had been lost to science for over a decade; 2) geometrically fit N = 110 age, gender, and handedness matched modern subject MRI brain volumes into the Gage cranial vault to assess average cortical metrics and their degree of variability; 3) in so doing, illustrate that while ∼4% of the cortex was intersected by the rod's passage, ∼11% of total white matter was also damaged, and provide estimates of the degree of damage experienced under a well-established brain parcellation scheme; 4) map high angular resolution diffusion neuroimaging tractography into the same space to measure damage to the pair-wise connections between atlas-defined cortical regions; and 5) compare the graph theoretical properties of the observed lesion against those expected from theoretically similar lesions systematically located throughout the brain. In what follows, we comment on our approach and findings.
Trajectory of the Tamping Iron
Various descriptions of the trajectory of the tamping iron through the Mr. Gage's skull have been given, which has understandably led to differing opinions about which parts of his brain were subjected to damage. Harlow, the physician responsible for Gage's initial treatment, documented that only the left hemisphere had been affected while the right remained unaffected [24] . In contrast, Bigelow maintained that some right-sided damage must have occurred. Dupuy [25] agreed with the left sidedness of the trajectory but placed it more posterior, claiming that motor and language areas had been destroyed – supporting the anti-localizationist arguments popular of the era. Ferrier [26] , illustrating that the motor and language areas had been spared, concluded that damage was limited only to the left hemisphere – a conclusion later echoed by Cobb [27] . In their measurements, Damasio et al. estimated the damage to be more frontal and right sided, whereas Ratiu and colleagues concluded that damage was limited to the left frontal lobe and that it did not cross the midline. Central to these differences in interpretation is likely to be how mandible position has been considered. To satisfy the observed anatomical constraints with the mouth closed would result in a greater right-sided inclination of the rod. Yet, as Harlow originally noted, Gage was in the act of speaking to his men at the moment of the injury and, thus, his mouth was likely open. We observe that with the jaw opened, the best-fit rod trajectory satisfying all constraints does not intersect or cross the superior sagittal sulcus and the injury is specific to the left frontal lobe. Thus, our conclusions are congruent with those of Harlow, Ferrier, as well as with those of Ratiu and Talos and, given the detailed computational approach taken, seem to provide the most likely reconstruction of the acute damage caused by the tamping iron.
Alterations of Network Connectivity Due to the Tamping Iron
The loss of ∼11% total WM volume in the left frontal lobe suggests that the iron's effects on Mr. Gage's brain extended well beyond the loss of left frontal GM alone. Overall differences in metrics of network integration as well as segregation were observed relative to intact connectivity, suggesting widespread disruption of networks involving damage to the left frontal and temporal pathways. Alterations indicate major changes to global network topology which affected network-wide efficiency. In the healthy cohort examined here, the region-to-region WM connectedness when in the presence of the rod was found to be associated with several important fiber bundles. Specifically, connectivity was affected between the frontal lobes and the basal ganglia, the insula, limbic, and other major lobes of the left hemisphere, in addition to right frontal, insular, and limbic areas. This severed portions of the uncinate fasciculus (UF) - connecting parts of the limbic system such as the hippocampus and amygdala in the temporal lobe with frontal regions such as the orbito-frontal cortex. The cingulum bundle - the collection of WM fibers projecting from the cingulate gyrus to the entorhinal cortex, allowing for communication between components of the limbic system – was also damaged. Additionally, the superior longitudinal fasciculus (SLF) was impacted – the long bi-directional bundles of neurons connecting the rostral and caudal aspects of the cerebrum in which each association fiber bundle is lateral to the centrum ovale linking the frontal, occipital, parietal, and temporal lobes. Fibers here pass from the frontal lobe through the operculum to the posterior end of the lateral sulcus, where numerous processes radiate into the occipital lobe while others turn downward and forward around the putamen and project to anterior portions of the temporal lobe. The occipito-temporal projection [28] in humans connects the temporal lobe and occipital lobe, running along the lateral walls of the inferior and posterior cornua of the lateral ventricle. The connectivity of the orbital cortex with temporal lobe regions via the UF which is among the last to complete myelination in development [29] , has been shown to be particularly affected in patients with mental illness [30] , and to be related to cognitive deficits in TBI [31] , [32] . WM fascicular damage in these instances was likely an important factor in Gage's reported post-injury symptomatology as well as in his reported and putative behavioral issues.
The obtained results suggest that GM damage had wider reaching influence than previously described and compromised several aspects of Gage's network of WM connectivity. Regions whose connectivity within and between cerebral hemispheres were affected included: the left frontal lobe (the transverse fronto-polar gyrus, fronto-marginal gyrus, middle frontal gyrus, lateral orbital sulcus, orbital sulcus, oribital part of the inferior frontal gyrus, triangular part of the inferior frontal gyrus, inferior frontal sulcus, medial orbital sulcus, orbital gyri, superior frontal gyrus, and opercular part of the inferior frontal gyrus); left insular cortex (horizontal and vertical ramus of the anterior segment of the lateral sulcus/fissure, the anterior/inferior/superior segments of the circular sulcus of the insula, short insular gyri, and long insular gyrus and central insular sulcus); and the left temporal lobe (the temporal pole and polar plane of the superior temporal gyrus) ( Fig. 3 ). The marginal and bivariate probability distributions of average brain-normalized WM fiber bundle length (w ij ) and proportion of GM density lost (g ij ; Fig. 4a–c ) unsurprisingly indicated a considerable number of relatively short connections being affected locally by the presence of the rod while, additionally, a considerable number of longer fiber bundles connecting relatively large regions of cortex were also impacted.
Alterations to these connections contribute to the significant reductions in characteristic path length and mean local efficiency of the remaining network after removal of the affected fibers. That no significant difference was observed concerning the small worldness of the tamping iron network as compared to the intact network suggest that a lesion of this size and scope, while severe, may not had appreciable effects on the degree of clustering of unaffected nodes Mr. Gage's brain relative to randomly degree-equivalent versions of that network. On the other hand, the average simulated lesion did show a significant reduction in small worldness, indicating that regions other than those affected may have more influence over the degree of measured network clustering. Thus, Mr. Gage's unaffected network may have still maintained its small world architecture of nodal clustering and presumed functional integrity, despite loss of major frontal and temporal lobe participation in the system resulting in deficits to measures network integration and segregation.
Several previous articles have precisely investigated the direct effects of node deletions of various size on network connectivity and architecture [33] , [34] , [35] . In particular, the paper by Alstott et al. provides a detailed examination of simulated lesion effects on brain networks both in terms of lesion location and extent. In their study of structural and functional connectivity data from N = 5 healthy subjects, they found that lesions to midline areas resulted in more profound effects on various network metrics than do more lateral brain regions. As might be expected, the magnitude of change was dependent upon the number of nodes removed from the network and the manner in which they were removed. Their observations indicate that networks may be insensitive to lesions involving random node removal or where node removal was based only upon a node's degree of connectedness. However, network lesioning based upon the targeted removal of nodes having high betweenness centrality - a measurement of the number of shortest paths from all vertices to all others which pass through a given node - resulted in greater network vulnerability as evident from significant reductions in global efficiency in contrast to random lesioning. This result is particularly compelling in regards to assessing the robustness of cortical architecture in the face of brain damage to major network hubs localized proximal to the cortical midline.
There is little doubt that a tamping iron injury to central nodes of the frontal lobe would have severely impacted Gage's brain connectivity. Fig. 3 shows the extent of white matter damage and the effects on several measures of network connectivity, including regional betweenness centrality, local efficiency, clustering coefficient, and eccentricity. Note that this illustration differs from Fig. 2 in that the inner-most rings are now colored according to the respective average nodal connectivity metrics in the presence of cortical loss incurred from the tamping iron. Additionally, Fig. 5A illustrates the spatial distribution of these regional connectivity metrics over the cortex when pooled across the intact healthy networks from our sample (sub-cortex not shown). We note that, as observed by Alstott et al., areas of relatively high betweenness centrality tended to be located along the frontal midline. Other metrics show similar regional concentrations ( Fig. 5Aii–iv ). However, while intact frontal areas of both hemispheres show high betweenness centrality ( Fig. 5Ai ), the regions of tamping iron damage encompassed many other regions as well having relatively less betweenness centrality, e.g. TrFPoG/S, RG, SbCaG, TPo. Removal of these areas, as illustrated by the various metric rings in the left frontal segment of the connectogram in Fig. 3 , has wide ranging effects on the regionally-specific network metrics in unaffected brain regions.
It is evident that removal of these areas produce significant effects on global metrics of network segregation and integration. However, from systematic lesion simulation using a similar extent of GM/WM involvement, the effects on Mr. Gage's network integration and segregation were not found to be more severe that that observed from the “average” lesion. Clearly, a larger lesion would have affected a greater number of network nodes including various hubs resulting in further deleterious effects on network integration and segregation. Moreover, a different lesion altogether would have possibly resulted in more outwardly obvious sensorimotor deficits. Located in occipital cortex, for instance, the lesion might have resulted in sensory-specific changes in connectivity (e.g. blindness), or one involving more of the sub-cortex and brain stem could have been more clinically serious and resulted in death. Nevertheless, the observed damage illustrates that severe network insult affecting the majority of left hemisphere connectivity as well as right hemispheric inter-connections, was experienced. Such damage can be expected to have had its influence over the normal functioning of many regions non-local to the injury and their subsequent connectivity as well.
Therefore, in light of these observations, it would be safe to conclude that 1) Mr. Gage's injury very likely destroyed portions of the central hub structure in left frontal midline structures as well as temporal pole and limbic structures which have extensive connectivity throughout the left hemisphere as well as inter-hemispherically, 2) that the tamping iron's passage did not specifically remove only the most central network hubs but a host of regions having a range of network properties, and 3) that such damage to important network hubs connection to other brain regions having secondary levels of centrality, clustering, etc. are likely to have combined to give rise to the behavioral and cognitive symptomatology originally reported by Harlow. Knowledge of Gage's affected connectivity help provide clarity and context for symptomatologies subsequently only inferred by others.
Implications for Gage's Reported Behavioral Changes
Traumatic brain injury of the frontal cortices is often associated with profound behavioral alterations, changes mood [36] , working memory [37] and planning deficits [38] , [39] , social functioning [40] , among other cognitive symptoms [41] , [42] , [43] , [44] . Alterations to functional connectivity have also been reported [45] , [46] which, in addition to cortical damage, likely related to accompanying diffuse axonal injury [47] , [48] . It is also worth noting neurodegenerative diseases, such as the leukodystrophies [49] , Alzheimer's Disease (AD) [50] , [51] , and early-stage frontotemporal dementia (FTD) [52] , also have effects on brain networks involving connectivity of the frontal lobe. Altered structural connectivity in these disorders illustrates changes in large-scale brain network organization deviating from healthy network organization [53] , with possible effects on resting state connectivity [54] . Disruptions of WM connectivity are also known to underlie elements of psychiatric illness [55] , [56] , [57] which are associated with behavioral alterations not dissimilar to those reported in Mr. Gage.
In particular, network damage, predominantly of the left basal forebrain and of its connections throughout the left as well as into right frontal cortices, was particularly extensive. Processing of emotion stimuli have been associated with connectivity of the frontal cortex and amygdala, in particular involving the connectivity of the uncinate fasciculi [58] . Thus, in addition to disinhibition symptoms considered by Damasio et al., with evidence of potentially greater degree of WM rather than cortical injury, there is also similarity between Mr. Gage's behavioral changes and network alterations observed in FTD and related WM degenerative syndromes. This suggests that network topological changes may have been the source of Mr. Gage having not only executive function deficits but also problems resulting from damage to connections associated with the encoding of episodic memory as well as the processing of emotion – consistent with reports on changes in his personality.
Historical Implications of Gage's WM Damage
While observations of severe network damage and their resulting affects may not be surprising given that which has been documented of Mr. Gage's accident and behavioral changes, one can only speculate upon the possible contribution to Gage's survival, recovery, and the uniqueness of changes to his WM networks. Macmillan [3] has noted that many reports on Gage's behavioral changes are anecdotal, largely in error, and that what we formally know of Mr. Gage's post-accident life comes largely from the follow-up report of Harlow [23] according to which Gage, despite the description of him having some early difficulties, appeared to adjust moderately well for someone experiencing such a profound injury. Indeed, the recent discovery of daguerreotype portraits of Mr. Gage show a “handsome…well dressed and confident, even proud” man [59] in the context of 19 th century portraiture. That he was any form of vagrant following his injury is belied by these remarkable images. While certainly neuroanatomically profound, the changes to his cognitive capacities were much more subtle upon his full recovery than may have been otherwise described. In spite of recovering from severe brain trauma, his mental state appears to have eventually stabilized sufficiently for him to travel throughout New England, take on several (some might say menial) forms of employment, travel through South America for several years, and to return to his family in the Western US, before succumbing to epilepsy which was presumably related to the injuries directly affecting his WM connectivity. That his network damage, though extensive, was not apparently more severe than an “average” brain lesion would incur may help to explain his ability to have sufficiently recovered in spite of the residual behavioral changes reported by Harlow.
Limitations of our Study
We have worked to provide a detailed, accurate, and comprehensive picture of the extent of damage from this famous brain injury patient and its effect on network connectivity. While the approach used here to model the tamping iron's trajectory is precise and the computation of average volume lost across our population of subjects is reflective of the acute level of damage, we acknowledge that there was likely more damage than that caused by its presence alone. The iron likely propelled unrecovered bone fragments through the brain. The resulting hemorrhage from the wound was also considerable. Subsequent infection and a large abscess took further toll. Consequently, more GM and WM tissue may have been lost than estimated here. Like Damasio et al. and Ratiu et al. , we make the assumption that Gage's brain and its position within the skull can be estimated from the structure of the skull itself, and that its sub-regions, WM, and connective anatomy can be localized through population averaging. Such a supposition may have its limitations and could be open to debate. Nevertheless, ours represents the best current estimation as to the extent of brain damage likely to have occurred at the level of both cortex and WM fiber pathways. We also have no way of assessing the biochemical cascade of changes to biomarker proteins measureable post-injury in modern TBI patients which may also have influenced the trajectory of Mr. Gage's recovery.
Another potential criticism is that we compare the loss of GM, WM, and connectivity in Mr. Gage by computationally casting the tamping iron through the WM fibers of healthy age- and gender-matched subjects and measuring the resulting changes in network topology. We also systematically lesion the brains of our healthy cohort to derive “average” network metrics and compare the observed values with respect to them – an approach that has been recommended elsewhere [35] . This technique is helpful for creating a representative expectation of inter-regional connectivity against which to compare observed or hypothetical lesions. However, some might consider this approach to be misguided in this instance due to the fact that Mr. Gage's brain was damaged in such a way that he survived the injury whereas a host of other lesions resulting from penetrative missile wounds would likely have resulted in death. Indeed, as noted originally by Harlow, the trajectory of the 110 cm long, 3.2 cm thick, 13 lb. tamping iron was likely along the only path that it could have taken without killing Mr. Gage. Thus, any distribution of lesioned topological values might not provide a useful foundation for comparison because the majority of these penetrative lesions would, in reality, be fatal. We recognize these concerns and the practical implications for subject death which would also be a caveat of other network theoretical applications of targeted or random network lesioning. Indeed, such considerations are something to be taken into account generally in such investigations. Nevertheless, our simulations provide supporting evidence for the approximate neurological impact of the tamping iron on network architecture and form a useful basis for comparison beyond utilizing the intact connectivity of our normal sample in assessing WM connectivity damage. So, while this might be viewed as a limitation of our study, especially given the absence of the actual brain for direct inspection, the approach taken provides an appropriate and detailed assessment of the probable extent of network topological change. All the same, we look forward to further work by graph theoreticians to develop novel approaches for assessing the effects of lesioned brain networks.
Conclusions
In as much as earlier examinations have focused exclusively on GM damage, the study of Phineas Gage's accident is also a study of the recovery from severe WM insult. Extensive loss of WM connectivity occurred intra- as well as inter-hemispherically, involving direct damage limited to the left cerebral hemisphere. Such damage is consistent with modern frontal lobe TBI patients involving diffuse axonal injury while also being analogous to some forms of degenerative WM disease known to result in profound behavioral change. Not surprisingly, structural alterations to network connectivity suggest major effects on Mr. Gage's overall network efficiency. Connections lost between left-frontal, left-temporal, right-frontal cortices as well as left limbic structures likely had considerable impact on executive as well as emotional functions. Consideration of WM damage and connectivity loss is, therefore, an essential consideration when interpreting and discussing this famous case study and its role in the history of neuroscience. While, finally, the quantification of connectomic change might well provide insights regarding the extent of damage and potential for clinical outcome in modern day brain trauma patients.
Ethics Statement
No new neuroimaging data was obtained in carrying out this study. All MRI data were drawn from the LONI Integrated Data Archive (IDA; http://ida.loni.ucla.edu ) from large-scale projects in which subjects provided their informed written consent to project investigators in line with the Declaration of Helsinki, U.S. 45 CFR 46, and approval by local ethics committees at their respective universities and research centers. Research neuroimaging data sets deposited with the LONI IDA and made available to the public are fully anonymized with respect to all identifying labels and linked meta-data for the purposes of data sharing, re-use, and re-purposing. IDA curators do not maintain linked coding or keys to subject identity. Therefore, in accordance with the U.S. Health Insurance Portability and Accountability Act (HIPAA; http://www.hhs.gov/ocr/privacy ), our study does not involve human subjects' materials.
Medical Imaging of the Gage Skull
Medical imaging technology has been applied to the Gage skull on three known occasions to model the trajectory of the tamping iron, infer extent of GM damage, and theorize about the changes in personality which a patient with such an injury might have incurred. In an influential study, Damasio and coworkers [7] used 2D X-rays to obtain the dimensions of the skull itself and to compute the trajectory of the iron bar through the regions of frontal cortex based on independently obtained CT data from a normal subject. Prior to this, CT scanning of the skull had been obtained by Tyler and Tyler in 1982 for presentation and discussion at a neurological scientific meeting. The location of the raw CT data files from this imaging session is unknown but the data were last reproduced in An Odd Kind of Fame (Appendix E), though they were not part of any other scientific publication of which we are aware. The most recent occurrence of scanning on record was performed on June 12 th , 2001 through the Surgical Planning Laboratory (SPL) at Brigham and Women's Hospital, Harvard Medical School. A series of two high-resolution CT image series were obtained of the skull: one covering the portion of the jaw up to approximately the bridge of the nose, and another covering the cranial vault (see details below). These data were used by Ratiu et al. [8] , [60] to digitally reconstruct and animate the passage of the tamping iron through the skull. An additional CT image of the Gage life-mask, a plaster likeness presumed to have been commissioned by Dr. Bigelow during one of Gage's visits to Harvard Medical School, was also obtained and used to create a surface model of Mr. Gage's face, scalp, and neck. New CT or other medical imaging of the skull specimen is unlikely to be performed in the future due to the age and fragile state of the specimen.
Documented Extent of Neurological Damage
In the book An Odd Kind of Fame (2000, pg 85), Macmillan conveniently summarizes the reports from various anatomists on the damage to Gage's brain. We reproduce these summaries here and also add the findings of Ratiu et al. [8] which appeared after the publication of An Odd Kind of Fame .
Skull CT Data Processing
Due to a variety of circumstances, the raw and processed digital imaging data from the 2001 CT imaging session at Brigham and Women's Hospital were improperly archived and effectively lost to science. However, these image volumes were subsequently recovered by the authors and represent the highest quality data/resolution available (0.5 mm slice thickness) for modeling the skull of this noted patient and for use in the modeling of affected anatomy and connectivity. The scan data were originally obtained with the superior, cut portion of the calvarium and the mandible in the correct anatomical position on a Siemens Somatom CAT scanner (Siemens AG, Erlangen, Germany), in the Department of Radiology, Brigham and Women's Hospital (Boston, MA) [8] . These data were converted from ECAT format to the NIFTI file format ( http://nifti.nimh.nih.gov ) using the program “mri_convert” – part of the FreeSurfer neuroimaging data analysis software package (surfer.nmr.mgh.harvard.edu/fswiki/mri_convert). The CT images were systematically segmented and masked by hand using MRICron ( http://www.cabiatl.com/mricro/mricron/index.html ) and seg3D ( http://www.sci.utah.edu/cibc/software/42-seg3d.html ) to isolate the skull cap (the portion of the skull created by its being cut with a saw upon deposition at the Warren Museum by Dr. Harlow), each piece of remaining/healed bone fragments, the left frontal/temporal portion of the skull along the readily evident fracture lines, and the lower jaw, and separate 3-D surface mesh models were generated for each segment using 3D Slicer ( http://www.slicer.org ). An additional binary image volume was created by hand-filling the space of the cranium that contained Gage's brain. This volume represents a digital version of the standard endocast often used in the analysis of paleontological specimens [61] , [62] , [63] . Use of the Gage skull and life mask CT data is courtesy of the SPL and the Warren Anatomical Museum at Harvard Medical School.
The LONI Pipeline Workflow Environment
For all major image processing operations (e.g. bias field correction, skull stripping, image alignment, etc.) we employed the LONI Pipeline Workflow Environment ( http://pipeline.loni.ucla.edu ; Fig. S1 ). This program is a graphical environment for construction, validation, and execution of advanced neuroimaging data analysis protocols. It enables automated data format conversion, leverages Grid computer systems, facilitates data provenance, and provides a significant library of computational tools [64] , [65] , [66] .
For instance, employing LONI Pipeline, we used the Brainsfit software package ( http://www.nitrc.org/projects/multimodereg/ ) to register the T1 anatomical MRI volumes to the endocast template. Diffusion gradient image data were processed in native subject space using Diffusion Toolkit ( http://trackvis.org ) to reconstruct the fiber tracts. Data processing workflows to compute inter-regional connectivity matrices were constructed using purpose-built software. Fig. S2 illustrates an example connectivity matrix displayed using Matlab (Mathworks, Natick, MA, USA).
Measurements of the Skull
Consistent with Damasio et al. , the physical dimensions of the Gage skull were measured as follows in Table 2 using the Slicer software program. Additionally, the following landmarks were identified on the Gage skull: Entrance of the Left Auditory Canal: (49.56, 219.46, −807.75 mm); Entrance of the Right Auditory Canal: (175.04, 212.26, −802.85 mm); and the Middle of Crease Between Frontal Bone Plate and Nasal Bone: (117.04, 301.73, −800.72 mm). Given these landmarks, all the other points can be accurately positioned.
Measurements of the Tamping Iron
One of our team (MCC) visited the Warren Anatomical Museum and, working with lead curator Dominic Hall, obtained the following measurements of the iron using a SPI Digimax caliper (Model: 30440-2): 110 cm in length, 9.5 cm circumference, and 2.88 cm diameter at tail. The rear taper is approximately 19 cm long, the maximum diameter (between the rear and tip taper) is 10.5 cm circumference (3.2 cm diameter), the taper beginning at the tip is 27 cm long, and the diameter at the rod's tip is 72 mm.
The Trajectory of the Tamping Iron
The trajectory of the tamping iron through Mr. Gage's skull and brain has been the subject of much debate and several attempts have been made to infer the relationship between putative damage on the one hand and the lore surrounding Gage's personality and behavioral changes resulting from his accident on the other. Bigelow [67] first attempted to formally model the trajectory of the rod by drilling a hole through another “common” skull (pg. 21), and noted that “a considerable portion of the brain must have been carried away; that while a portion of its lateral substances may have remained intact, the whole central part of the anterior lobe, and the front of the sphenoidal or middle lobe must have been lacerated and destroyed”. Importantly, Damasio [7] and coworkers provided a detailed analysis of the rod trajectory through the skull attempting to identify which brain regions were impacted by the flight of the iron and what effect this impact had on the patient's post-injury behavior. While this study has been well cited, their methodology for determining the rod trajectory has been subsequently questioned [3] .
Ratiu et al. [60] constrained their modeling of the rod trajectory by noting bony injuries to the skull, and by more closely aligning the rod with the clinical information provided by both Harlow and Bigelow. Ratiu et al. inserted the brain of a single normal subject into Mr. Gage's cranial cavity to examine which structures might have been affected. Their reconstruction shows that the path of the iron passed left of the superior sagittal sinus (their Fig. 4b,d ). This is corroborated by the fact that damage to the superior sagittal sinus would have almost certainly caused air embolism and/or significant blood loss, resulting in Mr. Gage's death. In addition, their reconstruction shows, in their normal subject's brain, that the iron's trajectory was also anterior to the cingulate gyrus and to the left lateral ventricle (their Fig. 4 e,f ). No rhinoliquorhea or other indication of post-traumatic CSF fistula was reported, nor that Gage developed ventriculitis, a condition which very likely would have been lethal - especially in the 1840's before the use of antibiotics in common medical practice. However, there is little way of being empirically precise with respect to location of major structures when employing only a single, example subject to represent Mr. Gage's unknown neuroanatomy.
To address this issue, we fit the T1 anatomical and diffusion images from the N = 110 normal, right handed subjects, aged 25–36 into the space of Phineas Gage's cranial vault to map the probability to regional injury and the effects of the tamping rod on WM fiber connectivity. The process of morphing data into the Gage skull is described in the following sections.
Determining the Trajectory of the Tamping Iron
Using the measurements of the original tamping iron [3] , [8] , [24] , [67] , on display at the Warren Museum, a 3-D model of the tamping iron was generated using Matlab and stored as an VTK surface ( http://www.vtk.org ) for visualization using 3D Slicer and for processing using the segmented brain regions and fiber tracts morphed into the space of the Gage 3D cranial endocast volume model.
To constrain the trajectory of the rod through the Gage skull, we examined the work of previous authors to identify noteworthy statements on the condition of the skull, particular patterns of breakage, chips in the bone, and other prominent features that could be used as landmarks to restrict the possible paths which the rod might have taken ( Fig. S3 ). For instance, the left maxillary molar is missing and osteological analysis by the Warren Museum states that it was lost ante-mortem (Object File WAM 00949, Warren Anatomical Museum, Francis A. Countway Library of Medicine). While Harlow and/or Bigelow do not specifically mention the loss of this tooth, it is likely that the rod made contact with it after passing through Gage's cheek, and was either dislodged completely or knocked loose and lost sometime during his recovery. Additionally, for the zygomatic arch the Warren Museum records (also WAM 00949) indicate “Maxilla: ante-mortem sharp force trauma remodeling” but are not more specific about the potential for complete breakage of the zygomatic process which was suspected by Ratiu et al. Still, it can be assumed that some contact was made between the iron and the interior portions of the arch. A collection of previously reported observations contributing to the set of applied constraints are noted in Table 3 .
In particular, we concur with Ratiu et al. that Mr. Gage had his jaw open at the moment of the accident. Harlow reports Gage looking over his right shoulder and saying something to his crew at critical moment of the blast. In the casting of possible rod trajectories, the most likely position of the jaw was determined to be −15° in pitch (downward) and 5° in yaw (to the right) relative to the closed position of the jaw. This position allowed the unhindered passage of 1.303×10 3 out of 1×10 9 viable rod trajectories inclusive through the skull. With this jaw position, in contrast to the suspicion of Bigelow, we noted no contact between the rod and that of Mr. Gage's coronoid process. Jaw rotations at greater pitch angles were inconsequential to our results. Therefore, these values represent the minimal angular jaw deflections needed to allow the maximal number of rod passage scenarios without jaw intersection. Additionally, these values are typical for the acts of speaking and mastication in which the maximum typical jaw pitch extension in males is ∼30° [68] . Assuming the jaw to be in a completely closed position forces rod trajectories to incline more toward the right hemisphere in order to avoid contact with the jaw and breaking it - as may result from the trajectories identified by Damasio et al. Having the jaw open provides a greater number of possible paths which are closer to the vertical axis, which thus does not enforce an intersection of the rod with the right hemisphere ( Fig. S4A , B, D; Fig. S5 A–D). The rod's intersection with white matter fiber tractography was thereby determined ( Fig. S6 ). Movie S1 illustrates the path of the tamping iron through Mr. Gage's skull and the white matter fiber pathways of his left hemisphere.
Normal Subjects
T1 anatomical MRI and 64-direction diffusion tensor images (DTI) from N = 110 right-handed male subjects between the ages of 25 and 36 were selected from the LONI Integrated Data Archive (IDA; http://ida.loni.ucla.edu ). The age range was specifically selected to match the age at which Mr. Gage received his injury (25 years old) as well as the age at which he succumbed as a presumed result of the brain damage he experienced (36 years old). Subjects were all healthy “normals” with no neurological or history of psychiatric illnesses.
Segmentation and Parcellation
Segmentation and regional parcellation were performed using FreeSurfer [69] , [70] , [71] following the nomenclature described in [72] . For each hemisphere, a total of 74 cortical structures were identified in addition to 7 subcortical structures and to the cerebellum. The 82 cortical and sub-cortical label names were assigned per hemisphere to each brain based upon the nomenclature described in Destrieux et al. [72] . Regional parcellation was performed using FreeSurfer [73] , [74] , [75] , [76] (see also above). The numbers of hemispheric partitions in the segmentation was as follows – frontal (21), insula (8), limbic (8), temporal (12), parietal (11), occipital (14), basal ganglia (8), and brain stem (1). The complete coding scheme is as presented describing the parcellation scheme naming convention ( Table 6 ) and their abbreviations ( Table 7 ), which can be used to identify the regional labels in Figs. 2a and 3 .
Connectogram Design
Neuroanatomical structure and connectivity information were graphically depicted in a circular diagram format using freely available Circos software ( [77] , www.cpan.org/ports ). Briefly, Circos is a cross-platform Perl-based application which employs a circular layout to facilitate the representation of relationships between pairs of positions by the use of various graphical elements, including links and heat maps. While traditionally used to render genomic information, Circos can be effectively adapted to the exploration of data sets involving complex relationships between large numbers of factors. In our case, cortical parcellations were represented as a circular array of 165 radially aligned elements representing the left and right cerebral hemispheres, each positioned symmetrically with respect to the vertical axis. We term this representation a “connectogram”. The brain stem was positioned at the most inferior extremity of the Circos ring as a consequence of its inclusion as the only midline structure. In this manner, Circos' ability to illustrate chromosomes was modified for lobar depiction, while its functionality for illustrating cytogenetic bands was modified to represent cortical parcellations. As previously described, each parcellation was assigned an arbitrary but unique RGB color (see below). Parcellations were arranged within each lobe in the order of their location along the antero-posterior axis of the cortical surface associated with the published FreeSurfer normal population atlas [72] . To determine this ordering, the center of mass was computed for the GM surface portion associated with each parcellation, and the order of all parcellations was determined based on the locations of these centers of mass as their distance from the frontal pole increased along the antero-posterior coordinate axis. A LONI Pipeline workflow for the creation of the connectogram images using parcellation and connectivity matrix information is available upon request from the authors. A complete description of the methods for connectogram construction can be found in [78] with applied examples in [79] .
Color Coding Schemes
Each cortical lobe was assigned a unique color scheme: black to red to yellow (Fro), charlotte to turquoise to forest green (Ins), primrose to lavender rose (Lim), pink to lavender to rosebud cherry (Tem), lime to forest green (Par), and lilac to indigo (Occ). Each structure was assigned its unique RGB color based on esthetic considerations; e.g. subcortical structures were colored light gray to black. Color scheme choice and assignment to each lobe were made by taking into account the arrangement and adjacency of lobes on the cortical surface, with the goal of avoiding any two adjacent lobes from having overlapping or similar color schemes which were too similar. The individual colors of the scheme associated with any particular lobe were assigned to every parcellation within that lobe in such a way as to create a distinct contrast when displayed on cortical surfaces ( Fig. S2 ) or on the connectogram graphics ( Figs. 2 and 3 ). The particular regional color mappings employed in this article can be considered arbitrary and are not intended to convey any universal or standard regional color scheme, per se .
Representation of Cortical Metrics
Within the circular framework representing the cortical parcellations, five circular heat maps were generated, each encoding one of five structural measures associated with the corresponding parcellation. Proceeding inward towards the center of the circle in Fig. 2 , these measures were: total GM volume, total area of the surface associated with the GM-WM interface (forming the base of the cortical ribbon), mean cortical thickness, mean curvature and connectivity per unit volume. For subject-level analysis, these measures were computed over the entire volumetric (or areal, as appropriate) extent of each parcellation; for the population-level analysis, they were averaged over all subjects.
Values for each measure were mapped to colors, using a scheme that ranged from the minimum to the maximum of the data set. For example, the cortical thickness t with values ranging from t min to t max was normalized as t 1 = ( t − t min )/( t max − t min ). The latter value was mapped onto a unique color from the color map of choice. Thus, for example, hues at color map extremities correspond to t min and t max , as required. For subcortical structures, brain stem and cerebellum, three measures (area, thickness and curvature) were unavailable on a parcellation-by-parcellation basis; their corresponding heat map entries were consequently left blank.
The connectogram in Fig. 3 , illustrating the effects of the tamping iron lesion, represents the individual regionally-specific network metrics (i.e. betweenness centrality, eccentricity, mean local efficiency, and clustering coefficient) and are colored distinctly to be consistent with the cortical maps of the same but unaffected network metrics presented in Fig. 5A . The inner-most ring of the connectogram in Fig. 3 represents the average proportion of regional GM loss taken across subjects.
Connectivity Calculation
To compute connectivity between regions for each subject, the location of each fiber tract extremity within the brain was identified, while the GM volume associated with each parcellation was also delineated. For those fibers which both originated as well as terminated within any two distinct parcellations of the 165 available, each fiber extremity was associated with the appropriate parcellation. For each such fiber, the corresponding entry in the connectivity matrix (e.g. Fig. S2 ) of the subject's brain was appropriately updated to reflect an increment in fiber count [80] , [81] . Each subject's connectivity matrix was normalized over the total number of fibers within that subject; for population-level analysis, all connectivity matrices were pooled across subjects and averaged to compute probabilistic connection probabilities.
Connectivity Representation
For subject-level connectograms, links were generated between any two parcellations whenever a WM tract existed between them. In population-level analyses, the former was done whenever there was a non-vanishing probability for a WM tract to exist between the two regions ( Fig. 2 ). Links were color-coded by the average fractional anisotropy (FA) value associated with the fibers between the two regions connected by the link, as follows. The lowest and highest FA values over all links ( FA min and FA max , respectively) were first computed. For any given connection i where i = 1, …, N ( N being the total number of connections), the FA value FA i associated with that connection was normalized as FA′ i = ( FA i − FA min )/( FA max − FA min ), where the prime indicates the FA i value after normalization. After this normalization, FA′ i values were distributed in the interval 0 to 1, where 0 corresponds to FA min and 1 corresponds to FA max . The interval 0 to 1 was then divided into three subintervals (bins) of equal size, namely 0 to 1/3, 1/3 to 2/3, and 2/3 to 1. For every i = 1, …, N , link i was color-coded in either blue, green or red, depending on whether its associated FA′ i value belonged to the first, second, or third bin above, respectively. Thus, these bins represent low, medium, and high FA. In addition to encoding FA in the link's color as described, relative fiber density (the proportion of fibers for each connection out of the total number of fibers) was also encoded as link transparency. Thus, within each of the three FA bins described, the link associated with the highest fiber density within that bin was rendered as completely opaque, whereas the link with the lowest fiber density was colored as transparent as possible without rendering it invisible. For example, the link with FA′ i = 1/3 was colored as opaque blue, whereas the link with the lowest FA′ i value was colored as most transparent blue. Similarly, the link with FA′ i = 2/3 was colored as opaque green, and the link with the lowest value of FA′ i greater than 1/3 was colored as faintest green. The links associated with the lowest fiber densities were drawn first, and links with progressively larger relative fiber densities were drawn on top of the former. The process was successively repeated by drawing links with higher fiber densities on top of links with lower fiber densities. Thus, links associated with the largest fiber densities were drawn “on top” of all other links.
Representation of Connectivity Affected by Pathology
Links associated with fibers affected by pathology were designed to encode fiber density using the same transparency coding scheme as described in the previous subsection. In contrast with the case of healthy fibers, however, two different color schemes were used to encode pathology. Whenever fibers existed between one cortical region that was affected by pathology and another that was not, the color used to draw the corresponding link was brown. By contrast, links between parcellations that were both affected by pathology were drawn using the color gray. This allows one to visually distinguish between connections that involve only one affected region (brown links) and connections that involve two regions that were both affected (grayscale links) ( Fig. 3 ).
Calculation of Pathology Effects upon GM/WM Volumetrics
The calculation described above estimated the amount of GM that was directly affected by the passage of the rod. To compute the total amount of GM that was affected by pathology, however, it is not sufficient to compute the sum of directly lesioned GM parcellation volumes because pathology-affected GM includes cells with intact somas whose axons were nevertheless injured in at least one location along their paths. In other words, a population of neurons whose GM axons were destroyed or affected in spite of their somas being outside the volume of direct injury should also be taken into account when computing the amount of affected GM. Furthermore, the destruction of fibers originating in some parcellated region r 1 that had been directly affected by pathology could also have affected the GM in parcellations to which r 1 is connected by WM fibers originating in r 1 . Consequently, an appropriate calculation of the total GM volume affected by pathology must take into account available quantitative information concerning the extent to which WM fibers affected by pathology could indirectly affect GM as well. To obtain and interpret such information meaningfully, one can use the measures of GM and WM atrophy described below:
Average Percentages of Brain Regions Intersected by the Rod
The average percentage regional volumes (and their standard deviations) intersected by the rod pooled over N = 110 subjects are listed in Table 7 and illustrated graphically in the connectogram of Fig. 3 .
Network Analysis
Because network theory can provide essential insight into the structural properties of cortical connectivity networks in both health and disease [83] , several network metrics of particular significance were computed for each subject, starting with the degree of each node. In our case, nodes were denoted by parcellated regions and edges were represented by fiber tracts. Nodal degree is the number of edges connected to a node and its calculation has fundamental impact upon many network measures; moreover, node degree distributions are highly informative of network architecture. The entry indexed by i and j in the distance matrix of the graph contains the minimum weighted physical length of the path connecting vertices i and j and was computed using the algebraic shortest paths algorithm [84] . Degree of connectivity is represented as the inner-most ring in Fig. 2 , though was not analyzed further beyond its being utilized in the computations of some of the overall network metrics detailed below.
The measurement of network attributes can be generally broken down into the examination of overall network integration – the measurement of path lengths between nodes in a network and the extent of network-wide interaction and ease of communication between distinct regions; segregation – the extent to which nodes of the network group themselves into separate communities; and small worldness – the quantification of the generally shorter path lengths and higher clustering observed in many biological and technological networks with respect to randomly connected systems [85] . To specifically measure these overall network properties, we chose to focus on three particular metrics. To assess network integration from each subject's connectivity matrix we measured the characteristic path length, a measurement of the global average of a graph's distance matrix [86] . Appropriate to our application, the weighted characteristic path length of a network may be altered as a result of brain trauma [87] . To measure the degree of segregation, we computed the mean local efficiency of each network. Investigating network segregation can be important because it can reveal how much information brain regions are able to exchange as well as the extent to which such regions remain structurally segregated from each other. In this instance, reduced efficiency might be expected as a result of a severe penetrating head wound. Finally, we measured network small worldness , i.e. the ratio comprised of the observed characteristic path length relative to that observed in a random network having the same degree distribution and the observed clustering coefficient relative to that observed in a random network.
Additionally, to characterize the regionally-specific effects of the tamping iron lesion, we also computed several additional graph theoretical measurements for each parcellated brain region. These included 1) betweenness centrality, measuring the number of shortest paths from all vertices to all others that pass through that node, 2) local efficiency, the mean shortest absolute path length of at that node, 3) clustering coefficient, measuring the degree to which a node is nodes in a graph is a member of a cluster or clique, and 4) eccentricity, representing the greatest geodesic distance between that node and any other vertex in the graph. Metrics were computed for each subject and averaged with respect to weighting by subject-wise regional parcellation volume. To be consistent with other studies reporting these regionally-specific values, we chose not to normalize them with respect to those obtained in equivalent random networks. Averages of these metrics are illustrated in Fig. 5a(i–iv) along with linear colorbars indicating the ranges of observed mean values. Effects on these metrics in the presence of the tamping iron can be seen as the first four of the inner-most rings of the connectogram presented in Fig. 3 .
Several additional global as well as local graph metrics were computed but not reported here due to potentially excessive colinearlity, imprecision, or due to recognized difficulty with interpretation. For instance, network modularity [88] was not considered due to the heuristic nature of its computation and tendency to provide unreliable values upon repeated estimation. While many of these other network metrics are well known and have their unique advantages [83] , the ones chosen parsimoniously capture the overall changes in network architecture for this patient and the extent to which his injury would compare to similarly-sized lesions in other areas of the cortex. The Brain Connectivity Toolbox (BCT; https://sites.google.com/a/brain-connectivity-toolbox.net/bct/Home ) was used for all weighted and unweighted connection density- and path-length related graph theoretical computations [84] .
For each of the global graph theory measures described above, the mean and standard deviation was computed for each subject in both intact (healthy) and pathology-affected scenarios (the tamping iron lesion as well as simulated lesions over the brain). As an additional basis, we also performed a degree-preserving randomization process using the BCT for each subject's intact network, computed the aforementioned network measurements, and report these averaged across subjects. Such normalization has been recently advised by Rubinov and Sporns [84] . In our case, this involved 10,000 “rewiring” iterations of the BCT null_model_und_sign (compiled C-code version of the Matlab code from the “the bct-cpp project”; http://code.google.com/p/bct-cpp ) algorithm per region by subject. To accommodate the computational cost of performing such a randomization process, we utilized fully the 1200 node Linux cluster based at the Laboratory of Neuro Imaging (LONI) at UCLA to randomize subjects and regions in parallel. Incidentally, normalization of each network type by its own randomized version has the effect of scaling out differences between networks – lesioned or otherwise – and thus makes the metrics largely insensitive to the effects of network damage. So, to provide a common frame of reference across each network type, the observed metrics for the intact, tamping iron, and simulated lesions were normalized with respect to the degree-preserving randomization of the intact network. Finally, to specifically test the differences between the intact and the tamping iron-lesioned networks between subjects, paired Student's t-tests were applied for each normalized measure to identify significant differences between means at p≤0.01. Results are summarized in Table 5 . Further details on the lesion simulation are provided in the section below.
Equivalent Lesion Simulation and Comparison
To examine the tamping iron lesion's specificity to changes in network structure, we investigated whether changes Gage's brain network properties were significantly different from those that would be expected by chance for the same amount of GM loss located in other regions of the brain. To address this, network properties were computed for a set of simulated lesions systematically positioned over the cortex (excluding the tamping iron lesion itself) and Mr. Gage's network measurements were compared to the distribution of the average metric values taken over subjects and lesions. Specifically, we adopted an approach similar to that of Alstott et al. [89] , who simulated the effects on functional connectivity of targeted lesions distributed in various regions of the cerebral cortex. In our extension of this method, localized area removal was performed by deleting all nodes and their connections within regions consisting of contiguous anatomic parcellations as defined using the methods of Destrieux et al. [72] . In contrast to Alstott et al., however, our structural connectivity simulations also sought to account for additional lesion effects upon WM by modeling the removal of so-called “fibers of passage”. To do so, connectivity network edges between anatomic parcellations neighboring the GM lesion were removed without deleting the corresponding nodes connected by these edges, unless these nodes also belonged to the GM portion of the lesion itself.
The details of our simulation are as follows: 500 distinct lesions were simulated by first populating the cortical surface with 500 distinct sets of contiguous parcellations. Each of these sets was subsequently used as a synthetic “lesion”, subject to the constraints that the percentages of WM and GM lost due to the lesion were the same as had been estimated for Gage's tamping iron injury. This process was repeated until 500 distinct lesions were created uniformly across the brain, and the procedure was repeated for all 110 subjects included in the study. To ensure that each of the lesions had approximately the same position in each subject, lesion configurations were defined using the cortical atlas of Fischl, Dale et al. [71] , and the corresponding location of every lesion in each subjects was identified by mapping the lesion configuration from the atlas to each subject's cortical surface using existing/published FreeSurfer methodology [70] , [90] , [91] . Thus, by the process described above, 500 distinct lesions that were identical in size to Gage's from the standpoint of percentage WM and GM loss were created uniformly over the brain in each of the 110 subjects. Subsequently, each lesion's effect on overall network properties was computed. Global network metrics were then pooled over all subjects and simulations so as to obtain the average (i.e. most probable) value of every metric for each of the 500 simulated lesioned networks.
Finally, we compared the observed effects of the tamping iron lesion on the random network normalized graph theory measures of integration and segregation against that observed for all remaining lesions. Computed as Z-statistics, the results of these comparisons are illustrated graphically for network integration and segregation in Fig. 5c (i and ii) , respectively, and are colored to show those effects most similar to the tamping iron lesion (black), moderately similar (orange), and most dissimilar (white). Generally, as one moves posteriorly away from the Gage lesion site, similarity on network effects tends to be reduced. However, exceptions exist in bilateral post-central gyrus and the left superior and posterior portion of the parahippocampal gyrus.
Supporting Information
The LONI Pipeline Workflow Environment. We applied the LONI Pipeline [93] , [94] for segmentation and registration of the input MRI image volume data, the processing of all DTI tractography, and computation of tract statistics. This grid-based solution provides validation and distribution of new computational tools, and an intuitive graphical interface for developing and executing parallel volumetric processing software. See http://pipeline.loni.ucla.edu for additional details.
https://doi.org/10.1371/journal.pone.0037454.s001
Views of the cortical parcellation of a sample subject. Top rows show the lateral, anterior, and dorsal surfaces; second row shows medial, posterior, and ventral pial surfaces, while the bottom two rows show the same orientations but as inflated pial surfaces to more adequately present the extent of regional parcellations and their color coding. The arbitrarily chosen regional colors are the same as those of the outer-most ring in Figure 2 and whose RGB values are referenced Table 5 are shared by the outer most ring of brain regions on the connectogram images permitting rapid cross-reference.
https://doi.org/10.1371/journal.pone.0037454.s002
Connectivity Matrix. Each row and each column represent distinct parcellated regions where in each cell i,j was computed the number of fibers that were found to begin or end in each region pair, the average FA, and the average fiber length over subjects.
https://doi.org/10.1371/journal.pone.0037454.s003
Modeling of the Skull Fragmentation and the Rod. a) Models of the eyeballs were placed in to the ocular cavities in order to use them as constraints for the trajectory of the tamping iron. According to Harlow's account, the left orbit was extended outward “by half its diameter”. b) The bones of the skull representing the major breakages were systematically labeled and can be independently manipulated using Slicer. The mandible was also rotated downward and laterally in order to allow the tamping iron not to impinge on it and also to comply with Harlow's account that Gage was in the act of speaking to his men at the moment of the blast. c) The surface model of the Gage skull, with closed mandible, along with the surface of the life mask commissioned by Bigelow. d) A view looking superiorly along the tamping iron's computed trajectory noting how the iron displaced the left anterior frontal bone as it passed.
https://doi.org/10.1371/journal.pone.0037454.s004
Illustrating the Intersection of the Rod and the Brain. a) A figure showing the passage of the rod through the skull with the bones above the cranial “cap” cut at Harlow's direction, and its intersection with the left anterior white matter fiber pathways of an example subject. The complementary hemisphere is displayed to illustrate that the rod did not intersect that hemisphere. b) A view of the rod displacing the bones of the skull. c) A close up, coxial view of the inferior portion of the iron along its trajectory. d) The intersection of the tamping iron with the left frontal cortex with each major bone fragment removed.
https://doi.org/10.1371/journal.pone.0037454.s005
The Effects of the Tamping Iron on White Matter Fiber Tractography. a) A view of the Gage skull with the white matter fiber tracts of an example subject warped to the space. In this view, fibers which intersect the rod's pathway have been removed. b) A transaxial view of the DTI fiber pathways remaining after those which were intersected by the rod had been removed. c) The fibers intersected by the rod connect areas of cortex throughout the left cerebral hemisphere as well as between hemispheres. d) A sagittal view of the fibers experiencing damage by the tamping iron. All bone fragments and the cranial “cap” have been removed.
https://doi.org/10.1371/journal.pone.0037454.s006
Movie of The Effects of the Tamping Iron on White Matter Fiber Tractography. This movie rendering illustrates the passage of the tamping iron through the Gage skull and its intersection with left hemispheric white matter fiber pathways. The right hemispheric cortical surface model is displayed to illustrate that the rod did not cross the midline to damage right frontal cortex. The rendering was created using 3D Slicer ( http://slicer.org ).
https://doi.org/10.1371/journal.pone.0037454.s007
Acknowledgments
The authors wish to acknowledge the assistance of Dominic Hall, Curator, Warren Anatomical Museum, Center for the History of Medicine, Francis A. Countway Library of Medicine 10 Shattuck Street, Boston, MA 02115 for access to Mr. Gage's skull, life mask, and tamping iron. We also express our gratitude to Marianna Jakab of the Surgical Planning Laboratory at Harvard Medical School for assistance with the CT image volumes, and to Drs. Danielle Bassett (Department of Physics, University of California Santa Barbara), Randal McIntosh (Rotman Institute, Toronto, Canada), and Paul M. Thompson (Department of Neurology, University of California Los Angeles) for their input and guidance on our network theoretical analyses. We are also extremely grateful for the rigorous and thorough comments of two anonymous reviewers on earlier versions of this article. Finally, we are indebted to the dedicated staff of the Laboratory of Neuro Imaging (LONI) at UCLA.
Author Contributions
Conceived and designed the experiments: JVH AWT RK. Performed the experiments: MCC CMT AI. Analyzed the data: MCC AI CMT. Contributed reagents/materials/analysis tools: AWT RK. Wrote the paper: JVH AI MCC. Provided computational resources and database access needed for neuroimaging data analysis: AWT. Provided access to data essential for the study: RK.
- 1. Rosen B, Wedeen V, Van Horn JD, Fischl B, Buckner R, et al. (2010) The Human Connectome Project. Organization for Human Brain Mapping Annual Meeting. Barcelona, Spain.
- View Article
- Google Scholar
- 3. Macmillan M (2000) An Odd Kind of Fame: Stories of Phineas Gage. Boston: MIT Press. 576 p.
- 4. Damasio AR (1995) 336 p. Descartes' Error: Harper Perennial.
- 83. Sporns O (2011) Networks of the Brain. Cambridge, MA: MIT Press.

Who Was Phineas Gage?
Categories Biopsychology
Phineas Gage was a young man seriously injured in a work-related accident. So what makes him so significant in psychology? His brain injury was shocking and the result impact on his personality quickly became one of the most famous case studies in psychology and neuroscience.
Table of Contents
Phineas Gage: A Closer Look
On September 13, 1848, a 25-year-old railroad foreman named Phineas Gage was injured in a horrific accident. While using an iron rod to tamp explosive powder into a hole, the powder ignited and sent the 43-inch long rod hurtling upward. The rod pierced through Gage’s cheek, passing though the frontal lobe of his brain before exiting the top of his skull and landing approximately 80 feet away.
Amazingly, Gage not only survived the accident, he also went on to become one of the earliest and most famous cases in the then just emerging field of neurology .
A View of the Accident Site

This image depicts an area of railroad approximately three-quarters of a mile outside of Cavendish, Vermont. It was in this area where Gage was working for former Rutland & Burlington Railroad to prepare the railroad bed. It was in this area or a site nearby that Gage suffered from the accident that would change his life and make him famous in the annals of neurology.
News of Gage’s Accident
The above news clipping appeared in the Boston Post on September 21, 1848.
The article states:
“ Horrible Accident – As Phineas P. Gage, a foreman on the railroad in Cavendish, was yesterday engaged in tamping for a blast, the powder exploded, carrying an instrument through his head an inch in length, which he was using at the time. The iron entered on the side of his face, shattering the upper jaw, and passing back of the left eye, and out at the top of the head. The most singular circumstance connected with this melancholy affair is, that he was alive at two o’clock this afternoon, and in full possession of his reason, and free from pain.”
The piece contains a few inaccuracies, including suggesting that Gage’s jaw was shattered and understating the dimensions of the projectile. His attending doctor, John Martyn Harlow, kept notes on the case as it progressed, which he later published. While he described Gage as conscious and rational in the immediate aftermath of the accident, the man was certainly not “free from pain” as the above article suggested. In the days and weeks that followed, Gage would lapse into a state of delirium, followed by a semi-comatose state brought on by an infection.
An Illustration of Gage’s Injury
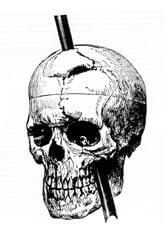
This image depicts the path of the iron rod through Gage’s skull. The illustration was included in Dr. Harlow’s account of the accident and subsequent impact on Gage, which was first published in 1868 in the Bulletin of the Massachusetts Medical Society .
“The missile entered by its pointed end, the left side of the face, immediately anterior to the angle of the lower jaw, and passing obliquely upwards, and obliquely backwards, emerged in the median line, at the back part of the frontal bone near the coronal suture,” Harlow wrote.
The Extent of Gage’s Brain Injury
Today, researchers have only Harlow’s description of the injury and examinations of Gage’s actual skull and the tamping rod to provide evidence of the type of injury that was sustained. Several different studies have been conducted to try to determine exactly how much of Gage’s brain was affected by the projectile. One 1994 study suggested that both prefrontal cortices were affected, while a 2004 study indicated that the damage was limited to the left frontal lobe. In 2012, a new study estimated that approximately 11-percent of Gage’s frontal lobe was destroyed and that 4-percent of his cerebral cortex was impacted.
While we will never be able to tell the exact extent of the damage, we do know that a significant portion of his frontal lobe was damaged.
The frontal lobe plays a vital role in problem-solving, decision-making, and planning. The area known as the prefrontal cortex is associated with the expression of personality. Other functions associated with the frontal lobe including reasoning, judgment, and impulse control.
In Harlow’s descriptions of Gage after the accident, he suggests that Gage would often make plans but fail to carry them out and that many of his friends described his personality as greatly changed, to the point that they felt he was “no longer Gage.”
Phineas Gage Life Mask

Prior to the 2009 and 2010 discovery of photographs of Phineas Gage, the only existing depiction that existed was a life mask made of his face and skull. The life mask was made for Henry Jacob Bigelow sometime around 1849 or 1850. Bigelow was a surgeon and professor at Harvard who published an article in American Journal of the Medical Sciences on Gage’s case, which helped generate considerable attention. Today, the life mask can be seen at the Warren Anatomical Museum at the Harvard University School of Medicine.
A Second Portrait of Phineas Gage

The second known photo of Phineas Gage came to light in 2010. The image was in the possession of members of Gage’s family. Like the previously seen portrait, Gage is shown proudly holding the tamping iron that so dramatically altered his life.
Gage’s Skull and the Famous Tamping Iron

Gage died in 1860 following a series of epileptic seizures, just 12 and a half years after his accident. In 1866, Harlow requested that the family exhume the body. The skull was removed and sent to Harlow, along with the iron tamping bar that had been in Gage’s possession at the time of his death. Today, both the skull and the iron rod can be seen at Harvard Medical School’s Warren Anatomical Museum.

IMAGES
VIDEO
COMMENTS
Learn about the famous case of Phineas Gage, who survived a traumatic brain injury in 1848 and showed changes in personality and behavior. Discover how his case influenced early understanding of cerebral localization and the frontal lobes.
Learn about Phineas Gage, the man who survived a traumatic brain injury that changed his personality and behavior. Discover how his case influenced neuroscience and psychology and what happened to him after the accident.
The case of Phineas Gage is an integral part of medical folklore. His accident still causes astonishment and curiosity and can be considered as the case that most influenced and contributed to the nineteenth century's neuropsychiatric discussion on the mind-brain relationship and brain topography. It was perhaps the first case to suggest the ...
In 1848, an iron bar pierced his brain, his case providing new insights on both trauma and recovery. Imagine the modern-day reaction to a news story about a man surviving a three-foot, 7-inch, 13½-pound iron bar being blown through his skull — taking a chunk of his brain with it. Then imagine that this happened in 1848, long before modern ...
Phineas P. Gage (1823-1860) was an American railroad construction foreman remembered for his improbable: 19 survival of an accident in which a large iron rod was driven completely through his head, destroying much of his brain's left frontal lobe, and for that injury's reported effects on his personality and behavior over the remaining 12 years of his life—effects sufficiently ...
Phineas Gage (born July 1823, New Hampshire, U.S.—died May 1860, California) was an American railroad foreman known for having survived a traumatic brain injury caused by an iron rod that shot through his skull and obliterated the greater part of the left frontal lobe of his brain.. Little is known about Gage's early life other than that he was born into a family of farmers and was raised ...
Learn how a daguerreotype of a one-eyed man with a tamping iron may be the first image of Phineas Gage, the railroad worker who survived a brain injury in 1848. Discover how his case changed the understanding of brain function and personality.
Cabinet-card portrait of brain-injury survivor Phineas Gage (1823-1860), shown holding the tamping iron that injured him. Wikimedia. It took an explosion and 13 pounds of iron to usher in the ...
Learn about the famous case of Phineas Gage, who survived a tamping iron through his head and changed his personality. Discover the true details of his life, work, and death from the original sources and the tamping iron itself.
Gage lived in Chile for seven years and then started having epileptic seizures. He died in 1860 at the age of 36. Margo Caulfield, director of the Cavendish Historical Society, shows Anne Strainchamps and Steve Paulson the location of Phineas Gage's accident in Cavendish, Vermont. The path he blasted out would become a railway corridor.
The case of Phineas Gage is worthy of expanded coverage as his tragic accident establishes a clear connection between the brain and who we are. Gage, a 25-year-old man, was employed in railroad construction at the time of the accident. As the company's most capable employee, with a well-balanced mind and a sense of leadership, he was directing ...
Explore the history and legacy of Phineas Gage, a railroad worker who survived a frontal lobe injury from a tamping iron in 1848. See his skull, the iron bar, and various documents related to his case, from medical reports to photographs.
Poor Phineas Gage. As reported at the time, the rod was later found, "smeared with blood and brains." Miraculously, Gage lived, becoming the most famous case in the history of neuroscience — not only because he survived a horrific accident that led to the destruction of much of his left frontal lobe but also because of the injury's reported ...
Phineas P Gage, a 25 year old railroad foreman, was excavating rock. In preparation for blasting he was tamping powder into a drill hole when a premature explosion drove the tamping iron—1.1 m long, 6 mm in diameter, and weighing 6 kg—through his left cheek and out of the vault of his skull with such force that it threw him on his back and ...
At his hotel, Gage waited in a chair on the porch and chatted with passersby—who were, uh, startled to see a volcano of bone jutting out of his scalp. Thus began perhaps the most famous case in medical history. Every neuroscience textbook in existence has a section on Phineas Gage. Incredibly, though, nearly every textbook gets the story wrong.
On September 13, 1848, while using a tamping iron to pack explosive into a rock, Phineas Gage, a 25-year-old construction foreman, triggered an uncontrolled explosion that propelled the tool ...
Evidence before this study. The historical case of Phineas Gage (1848) is an integral part of medical folklore, illustrating the resilience of the human brain and the involvement of the frontal lobes in problem solving, spontaneity, memory, initiation, judgement, impulse control, and social and sexual behavior.
Abstract No. 4. Phineas Gage has long occupied a privileged position in the history of science. Few isolated cases have been as influential, in the neurological and neuroscientific thinking, and yet the documentation on which conclusions and interpretations rest are remarkably incomplete [1], [2]. We do have a number of sure facts:
PTER THREEPHINEAS GAGEThe Man With a Hole in His HeadPhineas Gage was the 25-year-old foreman of a construction crew preparing t. e path for a railroad track in the late summer of 1848. By all accounts he was reliable an. friendly, both a good worker and a pleasant companion. But in an instant his life was changed through a terrible accident t.
Railroad foreman Phineas Gage survived a horrific brain injury that left him with an altered personality. His story revealed the complex functions of the frontal lobe decades before scientists began studying it in animals. Brain Bytes showcase essential facts about neuroscience. Design by A.Tong. Design by Adrienne Tong.
The case of Phineas Gage is among the most famous and infamous in the history of brain science. The interpretations of his incredible injury and attempts to characterize it have been ongoing since soon after it occurred. ... In as much as earlier examinations have focused exclusively on GM damage, the study of Phineas Gage's accident is also a ...
Phineas Gage was a young man who survived a terrible brain injury. His case had an important influence on early research on the human brain. ... One 1994 study suggested that both prefrontal cortices were affected, while a 2004 study indicated that the damage was limited to the left frontal lobe. ... Bigelow was a surgeon and professor at ...