
- Find My GCO
- IACUC applications (Cayuse Animal Management System)
- IBC Applications (eMUA)
- IRB Applications (RASS-IRB) External
- Institutional Profile & DUNS
- Rates and budgets
- Report external interests (COI)
- Join List Servs
- Ask EHS External
- Research Development Services
- Cornell Data Services External
- Find Your Next Funding Opportunity
- Travel Registry External
- RASS (Formerly Form 10 and NFA) External
- International research activities External
- Register for Federal and Non-Federal Systems
- Disclose Foreign Collaborations and Support
- Web Financials (WebFin2) External
- PI Dashboard External
- Research metrics & executive dashboards
- Research Financials (formerly RA Dashboard) External
- Subawards in a Proposal
- Proposal Development, Review, and Submission
- Planning for Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Budgets, Costs, and Rates
- Collaborate with Weill Cornell Medicine
- Award Negotiation and Finalization
- Travel and International Activities
- Project Finances
- Project Modifications
- Research Project Staffing
- Get Confidential Info, Data, Equipment, or Materials
- Managing Subawards
- Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Project Closeout Financials
- Project Closeout
- End a Project Early
- Protecting an Invention, Creation, Discovery
- Entrepreneurial and Startup Company Resources
- Gateway to Partnership Program
- Engaging with Industry
- Responsible Conduct of Research (RCR)
- Export Controls
- Research with Human Participants
- Research Security
- Work with Live Vertebrate Animals
- Research Safety
- Regulated Biological Materials in Research
- Financial Management
- Conflicts of Interest
- Search

IRB Consent Form Templates
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study.
General Consent Form Templates
Social and Behavioral Research Projects (last updated 03/16/2023)
Biomedical Research Projects (last updated 07/18/2022)
Consent Form Templates for Specific Biomedical Procedures
MRI and fMRI
Blood Collection by Finger Stick
Blood Collection by Venipuncture
Oral Consent Template
Guidance for Protocols Involving Oral Consent
Debriefing Template
Guidance and Template for Debriefing Participants
Studies Involving Children (Assent/Permission Forms)
Parent-Guardian Permission for Studies Involving Children
Sample Parental Notification Form
Sample Child Assent Form
Performance Release for Minors
Performance Releases
Performance Release for Adults

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
Informed Consent FAQs
What is informed consent and when, why, and how must it be obtained.
The HHS regulations at 45 CFR part 46 for the protection of human subjects in research require that an investigator obtain the legally effective informed consent of the subject or the subject’s legally authorized representative, unless (1) the research is exempt under 45 CFR 46.101(b) ; (2) the IRB finds and documents that informed consent can be waived ( 45 CFR 46.116(c) or (d) ); or (3) the IRB finds and documents that the research meets the requirements of the HHS Secretarial waiver under 45 CFR 46.101(i) that permits a waiver of the general requirements for obtaining informed consent in a limited class of research in emergency settings. When informed consent is required, it must be sought prospectively, and documented to the extent required under HHS regulations at 45 CFR 46.117 . [Food and Drug Administration (FDA) regulations at 21 CFR part 50 may also apply if the research involves a clinical investigation regulated by FDA.]
The requirement to obtain the legally effective informed consent of individuals before involving them in research is one of the central protections provided for under the HHS regulations at 45 CFR part 46 . This requirement is founded on the principle of respect for persons, one of the three ethical principles governing human subjects research described in the Belmont Report. The principle of respect for persons requires that individuals be treated as autonomous agents and that the rights and welfare of persons with diminished autonomy be appropriately protected. The Belmont Report states that an autonomous agent is “an individual capable of deliberation about personal goals and of acting under the direction of such deliberation.” Respect for persons requires that prospective research subjects “be given the opportunity to choose what shall or shall not happen to them” and thus necessitates adequate standards for informed consent.
The informed consent process involves three key features: (1) disclosing to potential research subjects information needed to make an informed decision; (2) facilitating the understanding of what has been disclosed; and (3) promoting the voluntariness of the decision about whether or not to participate in the research. Informed consent must be legally effective and prospectively obtained. HHS regulations at 45 CFR 46.116 and 45 CFR 46.117 describe the informed consent requirements.
The informed consent process is the critical communication link between the prospective human subject and an investigator, beginning with the initial approach of an investigator to the potential subject (e.g., through a flyer, brochure, or any advertisement regarding the research study) and continuing until the completion of the research study. For the purposes of the HHS regulations at 45 CFR part 46, “investigators” are individuals who conduct human subjects research projects, including individuals directly involved in seeking the voluntary informed consent of potential subjects. Investigators can include physicians, scientists, nurses, administrative staff, teachers, and students, among others.
The informed consent process should be an active process of sharing information between the investigator and the prospective subject. The exchange of information between the investigator and prospective subjects can occur via one or more of the following modes of communication, among others: face-to-face contact; mail; telephone; video; or fax. Prospective subjects should be provided with ample opportunity to ask questions and seek clarification from the investigator. The prospective subjects should be in a position to freely decide whether to initially enroll in the research, or later, to withdraw or continue participating in the research. The informed consent process should ensure that all critical information about a study is completely disclosed, and that prospective subjects or their legally authorized representatives adequately understand the research so that they can make informed choices.
The procedures used in seeking and obtaining informed consent should be designed to communicate with the subject population in terms that they can understand. Information about a research project must be presented in such a way that enables each person to voluntarily decide whether or not to participate as a research subject. Thus, the information must be conveyed in language understandable to those being asked to participate as subjects in the research ( 45 CFR 46.116 ).
For most research, informed consent is documented using a written document that provides key information regarding the research. The consent form is intended, in part, to provide information for the potential subject’s current and future reference and to document the interaction between the subject and the investigator. However, even if a signed consent form is required, it alone does not constitute an adequate consent process. The informed consent process is an ongoing exchange of information between the investigator and the subject and could include, for example, use of question and answer sessions, community meetings, and videotape presentations. In all circumstances, however, individuals should be provided with an opportunity to have their questions and concerns addressed on an individual basis.
The consent process and its documentation should be revised when deficiencies in its accuracy or completeness are noted, when new information about reasonably foreseeable risks and potential benefits becomes available, or when other additional information becomes known that will improve the consent process. Such revisions must be reviewed and approved by an IRB prior to the revised consent being utilized except when necessary to eliminate apparent immediate hazards to subjects ( 45 CFR 46.103(b)(4) ).
Is it possible to obtain legally effective informed consent to research in an urgent or emergency care setting?
Yes, in certain circumstances it is possible to obtain legally effective informed consent in an urgent or emergency care setting. For a particular research study, the answer depends on (1) the expected medical condition of the prospective subject population; (2) the nature of the research; (3) whether there is sufficient time for the potential subjects or their legally authorized representatives to consider participation; and (4) whether the circumstances for obtaining informed consent appropriately minimize the possibility of coercion or undue influence. The Institutional Review Board (IRB) and investigator(s) would have to consider several variables. For example, what is the likely health and emotional condition of the patient population being considered for the proposed research (e.g., conscious but receiving emergency care, undergoing preparation prior to surgery)? What is the likely ability of this population during the consent process to process information, ask questions, and consider the risk involved? What is the timing of the consent process and is it so close to the receipt of care that the patient might blur the distinction between treatment and research?
Because individuals receiving urgent or emergent medical care frequently may be vulnerable to coercion or undue influence, even if temporarily, additional protections may be required to ensure the subject's consent to participate in research is truly voluntary and sought under circumstances that minimize the possibility of coercion or undue influence ( 45 CFR 46.111(b) , ( 45 CFR 46.116 ). In addition, in some cases, it might be possible to obtain consent from a legally authorized representative (e.g., in the case of decisionally incapacitated individuals). In certain emergency circumstances, the Secretarial waiver of informed consent under 45 CFR 46.101(i) may be applicable. It should be noted that if the research is regulated by FDA, the Secretarial waiver permits the research to be conducted under a comparable provision.
See the Office for Human Research Protections' (OHRP) guidance ; and HHS policy (PDF - 22KB) .
What are the basic elements of informed consent?
The basic required elements of informed consent can be found in the HHS regulations at 45 CFR 46.116(a) . Also see OHRP Informed Consent Tips .
The regulations require that the following information must be conveyed to each subject:
- a statement that the study involves research, an explanation of the purposes of the research and the expected duration of the subject’s participation, a description of the procedures to be followed, and identification of any procedures which are experimental;
- a description of any reasonably foreseeable risks or discomforts to the subject;
- a description of any benefits to the subject or to others which may reasonably be expected from the research;
- a disclosure of appropriate alternative procedures or courses of treatment, if any, that might be advantageous to the subject;
- a statement describing the extent, if any, to which confidentiality of records identifying the subject will be maintained;
- for research involving more than minimal risk, an explanation as to whether any compensation and an explanation as to whether any medical treatments are available if injury occurs and, if so, what they consist of, or where further information may be obtained;
- an explanation of whom to contact for answers to pertinent questions about the research and research subjects’ rights, and whom to contact in the event of a research-related injury to the subject; and
- a statement that participation is voluntary, refusal to participate will involve no penalty or loss of benefits to which the subject is otherwise entitled, and the subject may discontinue participation at any time without penalty or loss of benefits to which the subject is otherwise entitled.
Additional elements are described at 45 CFR 46.116(b)
What additional information might be appropriate to provide during the consent process?
When determined to be appropriate by the Institutional Review Board (IRB), subjects must be provided with one or more of the following additional elements of information during the informed consent process (see 45 CFR 46.116(b) ):
- a statement that the particular treatment or procedure may involve risks to the subject (or to the embryo or fetus, if the subject is or may become pregnant) which are currently unforeseeable;
- anticipated circumstances under which the subject’s participation may be terminated by the investigator without regard to the subject’s consent;
- any additional costs to the subject that may result from participation in the research;
- the consequences of a subject’s decision to withdraw from the research and procedures for orderly termination of participation by the subject;
- a statement that significant new findings developed during the course of the research which may relate to the subject’s willingness to continue participation will be provided to the subject; and
- the approximate number of subjects involved in the study.
It is up to the IRB to determine in a particular instance whether some or all of the above additional elements must be included as part of the informed consent process for a particular study. The IRB should make this determination based on the nature of the research and its knowledge of the local research context. If the IRB determines that additional elements are appropriate to the research study, this additional information should be considered just as essential as the eight basic elements of informed consent described in the HHS regulations at 45 CFR 46.116(a) .
Furthermore, an IRB may require that additional information beyond the basic and additional elements be given to subjects during the informed consent process, when in the IRB’s judgment the additional information would meaningfully add to the protection of the rights and welfare of the subjects 45 CFR 46.109(b) .
Can consent or parental permission ever be "passive" or "implied?"
Terms such as “passive” or “implied” consent are not referenced in the HHS regulations. However, OHRP is aware that these terms are sometimes used by investigators or IRBs to describe a process in which consent or parental permission requirements have been altered or waived, or for which the requirement to document consent or parental permission has been waived.
HHS regulations at 45 CFR 46.116 state that no investigator may involve a human being as a subject unless the investigator has obtained the legally effective informed consent of the subject or the subject’s legally authorized representative. However, under conditions specified in the regulations at 45 CFR 46.116(c) or (d) an IRB may approve a consent procedure that does not include, or that alters some or all of the elements of informed consent set forth in 45 CFR 46.116 . In some cases, an IRB also can waive the requirement to obtain consent ( 45 CFR 46.116(c) and (d) ). In addition, under conditions specified in the regulations at 45 CFR 46.117 , an IRB may also waive the requirement for documentation of informed consent. (Note that the regulations at 45 CFR 46.408(c) also permit an IRB to waive parental permission.)
For example, a researcher conducting a survey (that does not qualify for an exemption under 45 CFR 46.101(b) mails a survey questionnaire to a random sample of adults. The survey materials clearly state that by responding to the questions and mailing the survey back, the recipients have agreed to participate in the research. However, the materials accompanying the questionnaire do not include all of the elements of consent listed at 45 CFR 46.116(a) and do not require that the subject sign a consent form. If the IRB has approved this alteration of the consent process and has waived the need for documentation of consent, then such procedures are permissible under the regulations. By sending back a completed survey the recipient has implied that he or she consents to participate but has not signed an informed consent document. Although some might call this “implied informed consent,” OHRP would consider this to be a permissible informed consent process if the IRB has approved the informed consent alteration and waived the requirement for documentation of informed consent.
The term “passive consent” is sometimes used in research with children to describe situations in which the investigator can assume that a parent is permitting a child to participate. For example, researchers collecting survey and behavioral data from children at school provide parents with information regarding the study by mail and ask the parent(s) to return a form if they do not want their child to participate. Sometimes this practice is referred to as an opt out procedure, which is not consistent with the regulatory requirement for seeking and obtaining parental permission. If the IRB determines that the conditions for waiver of parental permission can be met, then the IRB could waive the requirement for parental permission under 45 CFR 46.408(c) or 45 CFR 46.116(c) or (d) . Even though not required by the regulations, an IRB may require that parents be given the opportunity to refuse permission even when the IRB has waived the regulatory requirement to obtain parental permission.
What does it mean to minimize the possibility of coercion or undue influence?
The HHS regulations state that “An investigator shall seek such consent only under circumstances that provide the prospective subject or the representative sufficient opportunity to consider whether or not to participate and that minimize the possibility of coercion or undue influence” ( 45 CFR 46.116 ). This requirement applies to all nonexempt human subjects research not eligible for a waiver of the consent requirements.
Coercion occurs when an overt or implicit threat of harm is intentionally presented by one person to another in order to obtain compliance. For example, an investigator might tell a prospective subject that he or she will lose access to needed health services if he or she does not participate in the research.
Undue influence , by contrast, often occurs through an offer of an excessive or inappropriate reward or other overture in order to obtain compliance. For example, an investigator might promise psychology students extra credit if they participate in the research. If that is the only way a student can earn extra credit, then the investigator is unduly influencing potential subjects. If, however, she offers comparable non-research alternatives for earning extra credit, the possibility of undue influence is minimized.
In addition to undue influence that can arise with the offering of rewards, undue influence also can be subtle. For example, patients might feel obligated to participate in research if their physician is also the investigator, or students might feel pressure to participate in research if everyone else in the class is doing so. Because influence is contextual, and undue influence is likely to depend on an individual’s situation, it is often difficult for IRBs to draw a bright line delimiting undue influence. It is up to the IRB to use its discretion in determining which circumstances give rise to undue influence. For example, an IRB might consider whether the informed consent process will take place at an appropriate time and in an appropriate setting, and whether the prospective subject may feel pressured into acting quickly or be discouraged from seeking advice from others.
Because of their relative nature and lack of clear-cut standards on the boundaries of inappropriate and appropriate forms of influence, investigators and IRBs must be vigilant about minimizing the possibility for coercion and undue influence. Reasonable assessments can be made to minimize the likelihood of undue influence or coercion occurring. For example, IRBs may restrict levels of financial or nonfinancial incentives for participation and should carefully review the information to be disclosed to potential subjects to ensure that the incentives and how they will be provided are clearly described. Known benefits should be stated accurately but not exaggerated, and potential or uncertain benefits should be stated as such, with clear language indicating how much is known about the uncertainty or likelihood of these potential benefits.
The regulatory requirements for IRB review and approval also specify the need for the IRB -- in order to approve research covered by the HHS regulations -- to ensure that “When some or all of the subjects are likely to be vulnerable to coercion or undue influence, such as children , prisoners , pregnant women , mentally disabled persons, or economically or educationally disadvantaged persons, additional safeguards have been included in the study to protect the rights and welfare of these subjects” ( 45 CFR 46.111(b) ). Thus, inducements that would ordinarily be acceptable in some populations may become undue influences for these vulnerable subject groups.
When does compensating subjects undermine informed consent or parental permission?
The HHS regulations require that “An investigator shall seek such consent only under circumstances that provide the prospective subject or the representative sufficient opportunity to consider whether or not to participate and that minimize the possibility of coercion or undue influence” ( 45 CFR 46.116 ).
Paying research subjects in exchange for their participation is a common and, in general, acceptable practice. However, difficult questions must be addressed by the IRB. For example, how much money should research subjects receive, and for what should subjects receive payment -- their time, inconvenience, discomfort, or some other consideration -- IRBs must be sensitive to whether any aspect of the proposed remuneration will be an undue influence, thus interfering with the potential subjects’ ability to give voluntary informed consent.
Remuneration for participation in research should be just and fair. However, the specifics of each protocol will influence how those determinations are made. Both researchers and IRBs need to be familiar with the study population and the context of the research in order to make reasonable judgments about how compensation might affect participation. Wherever the remuneration is set, it will influence the decisions of some more than others. In particular, it will be more important to those for whom it will make a significant financial difference. Thus, IRBs should be cautious that payments are not so high that they create an “undue influence” or offer undue inducement that could compromise a prospective subject’s examination and evaluation of the risks or affect the voluntariness of his or her choices.
Information submitted to IRBs should indicate and justify proposed levels and purposes of remuneration, which also should be clearly stated in the accompanying consent forms.
Some institutions have adopted policies regarding the recruitment and payment of volunteers. IRBs and investigators should ensure that the consent process includes a detailed account of the terms of payment, including a description of the conditions under which a subject would receive partial or no payment (e.g., what will happen if he or she withdraws part way through the research or the investigator removes a subject from the study for medical or noncompliance reasons).
Finally, in studies of considerable duration or that involve multiple interactions or interventions, OHRP recommends that payment be prorated for the time of participation in the study rather than delayed until study completion, because the latter could unduly influence a subject’s decision to exercise his or her right to withdraw at any time. For example, if the study is conducted over a period of 6 months, there might be a monthly or bi-monthly payment. Or, if the study involves 12 sessions, there might be payment after every two sessions.
The above principles would apply to remuneration offered to parents whose children are prospective subjects.
[ Note: The previous version of the response to this FAQ included the following sentences. “In no case should remuneration be viewed as a way of offsetting risks; that is, it should not be considered a benefit to be weighed against study risks. The level of remuneration should not be so high as to cause a prospective subject to accept risks that he or she would not accept in the absence of the remuneration.” The first sentence has been struck because this FAQ focuses on potential undue influence in the consent process (45 CFR 46.116) rather than on IRB considerations under 45 CFR 46.111. However, OHRP continues to assert that IRBs should not consider remuneration as a way of offsetting risks. The second sentence has been deleted to clarify that remuneration to subjects may include compensation for risks associated with their participation in research and that compensation may be an acceptable motive for agreeing to participate in research. In addition, the previous version contained the following sentence, which has been struck because it is focused on IRB considerations under 45 CFR 46. 111 rather than informed consent, and was misplaced in this FAQ: “IRBs may need to request of the investigator some plan for monitoring subject recruitment to ensure that such inducements do not result in inequitable subject recruitment (e.g., recruiting only economically disadvantaged individuals).” ]
Can non-financial enrollment incentives constitute undue influence?
Yes, in certain circumstances. Non-monetary incentives (e.g., extra credit for students, access to services or programs) also can create undue influence on a potential subject’s decision about research participation. Informed consent always must be voluntary ( 45 CFR 46.116 ).
IRBs should ensure that non-financial incentives are not so great as to diminish the voluntariness of consent or cloud someone’s appreciation of risks or potential benefits that might be gained from participating in a study ( 45 CFR 46.116 ). Moreover, it must be clear that choosing to not participate will not adversely affect an individual’s relationship with the institution or its staff or the provision of services in any way (e.g., loss of credits or access to programs) ( 45 CFR 46.116(a)(8) ).
Overt coercion (e.g., threatening loss of services or access to programs to which the potential subjects are otherwise entitled) is never appropriate. However, it might be permissible to provide incentives to participate that do not constitute undue influence. Using enrollment incentives to recruit subjects may be ethically permissible as long as the IRB has determined that, although they may be a factor in a subject’s decision to participate, they have not served to unduly influence the subject to participate. To make this determination, IRBs should know who the subject population will be, what incentives are being offered, and the conditions under which the offer will be made.
What constitutes coercion or undue influence when students are involved in research in a college or university setting?
The regulations require that the investigator seek consent only under circumstances that minimize the possibility of coercion or undue influence ( 45 CFR 46.116 ). The Office for Human Research Protections (OHRP) recommends that institutions have policies in place that clarify for students and faculty that any participation of students in research must be voluntary. Reasonable levels of extra credit or rewards may be offered for participating in research. If extra credit or rewards are offered for participation, students must be provided with and informed of non-research alternatives involving comparable time and effort to obtain the extra credit in order for the possibility of undue influence to be minimized. However, if participation in research is a course requirement, students must be informed of non-research alternatives involving comparable time and effort to fulfill those requirements in order for the possibility of undue influence to be minimized. Moreover, students must not be penalized for refusing to participate in research ( 45 CFR 46.116(a)(8) ).
In addition, some research institutions use a so-called “student subject pool” to identify students who might be willing to participate in research, even when the exact nature of the research to be conducted has not yet been determined. Extra credits or other rewards are often offered as an incentive to encourage participation. Students who sign up for such pools have not legally consented to participate in a research study since they have not been provided with sufficient information concerning the exact study in which they would participate. Thus, signing up to be in a subject pool is only a first and preliminary step by which individuals can indicate their willingness to be considered for research participation. The student must also provide informed consent, unless the consent requirement is waived by an IRB once he or she is being considered for a specific study ( 45 CFR 46.116 ). Furthermore, individuals in the pool must be free to decline participation in any available research projects without penalty ( 45 CFR 46.116(a)(8) ).
What constitutes coercion or undue influence when employees are the subjects of research?
The issues involving employees as research subjects are essentially identical to those involving students as research subjects: that is, investigators and IRBs must be cautious about the potential for coercion or undue influence and the need to protect confidentiality.
Employee participation raises questions about the ability of employees to exercise free choice, for example, because of the possibility that a decision to participate could affect performance evaluations or job advancement, even if it is only the employee’s perception that this is the case. In the case of coercion, refusal to participate might result in a loss of benefits (e.g., salary increases, time off). In the case of undue influence, a decision to participate could result in a job promotion. Employees are likely to view their employers as authority figures to whom they must show deference, which could undermine the freedom of their choice.
Should the initial consent or parental permission procedure ever be repeated or supplemented?
Yes, in some circumstances. The HHS regulations require that an investigator obtain legally effective informed consent from subjects or a legally authorized representative before the subjects may be involved in research ( 45 CFR 46.116 ), unless this requirement has been waived by an IRB . Likewise, for research involving children, permission of the potential subjects' parents or guardians must be obtained ( 45 CFR46.408(c) ), unless an IRB has waived this requirement . Ensuring an adequate consent or parental permission process may require repeating or supplementing the initial consent procedure. The regulations also stipulate that “An investigator shall seek such consent only under circumstances that provide the prospective subject or the representative sufficient opportunity to consider whether or not to participate and that minimizes the possibility of coercion or undue influence” ( 45 CFR 46.116 ). This requirement also might necessitate repeating or supplementing the initial consent procedure.
Informed consent and parental permission should be viewed as an ongoing process. The regulations do not explicitly describe all of the circumstances that might require repeating or supplementing the informed consent process. However, they do require that potential subjects be provided, when appropriate, with a “statement that significant new findings developed during the course of the research which may relate to the subject’s willingness to continue participation will be provided to the subject” ( 45 CFR 46.116(b)(5) ). Thus, to ensure that consent remains legally effective -- for example, if the protocol design or risks have changed, or if a substantial period of time has elapsed between the time consent was obtained and the study begins -- it might be necessary to ensure that subjects still want to participate in the research. For example, the prospective subject may no longer be interested in participating, may no longer meet the eligibility criteria, may no longer find the risks acceptable, or may no longer have the time to complete all study-related activities.
The IRB must review and approve any changes in the approved consent procedure, including alterations of the content, as described in the elements listed at 45 CFR 46.116 , or in its timing, and may consider whether there is a need to reiterate the process ( 45 CFR 46.103(b)(4) ). The IRB should take into account whether the changes could potentially affect a subject’s understanding of the nature of the study or potentially affect a subject’s willingness to participate. If so, such changes need to be made in the informed consent document. Even without significant changes to a protocol or informed consent document, periodic reiteration or affirmation of consent is often a good idea, especially if the study takes place over a long period of time or is particularly complex. Minor changes, such as correcting nonsubstantive typographical errors in the consent document, would not generally rise to a level requiring repeating the consent process.
How far in advance of research participation can consent be obtained?
The HHS regulations at 45 CFR part 46 do not specify how far in advance of study entry a subject can provide consent. The amount of time required by a subject to make a decision would presumably depend on the nature of the study, taking into account, among other factors, the degree of risk, potential benefits, alternatives, and desire to consult with family members or others. However, if a prolonged period of time elapses from the date of consent to the date of entry into the study even if there have been no changes in the study design or no new significant findings affecting the study it might be prudent to review the information contained in the consent form with the subject prior to initiating any research procedures with the subject.
Can records or databases be reviewed to identify potential subjects without obtaining informed consent or parental permission?
Yes, under certain circumstances. Although the HHS regulations do not specifically reference this type of activity, sometimes referred to as “preparatory to research,” such an activity must be reviewed and approved by an IRB in accordance with HHS regulations at 45 CFR 46.109(a) when:
- The activity involves human subjects research, as defined by the regulations at 45 CFR 46.102(f) ;
- The research does not meet the criteria for exemption under HHS regulations at 45 CFR 46.101(b) .
In general, informed consent of the subjects, or parental permission for children involved in research, must be sought and documented in accordance with, and to the extent required by, HHS regulations at 45 CFR 46.116 and 45 CFR 46.117 respectively.
However, an IRB may approve a consent or parental permission procedure that does not include, or that alters, some or all of the elements of informed consent, or may waive the requirements to obtain informed consent ( 45 CFR 46.116(c) or (d) ). In order to permit investigators to obtain and record identifiable private information for the purposes of identifying potential subjects, OHRP expects that IRBs routinely will waive the requirement for informed consent for such activities. In assessing the level of risk to determine whether a waiver of informed consent or parental permission is permissible for the identification of potential subjects, the IRB need only consider the risk of investigators accessing the subjects’ identifiable private information, not the risks of the research in toto.
How can the consent and parental permission processes be designed to facilitate understanding?
The procedures used in obtaining informed consent and parental permission should be designed to inform the subject population or the parents of the subject population about the research in terms that they can understand. Therefore, informed consent and parental permission language and its documentation in the accompanying forms (especially explanation of the study’s purpose, duration, experimental procedures, alternatives, risks, and benefits) should be provided in language that is understandable and culturally sensitive to those being asked to participate or provide permission for their child’s participation.
If the prospective subjects include, for example, persons whose primary language is not English, or populations with low literacy levels, the IRB should take special care to ensure that both oral presentations and consent or permission forms are comprehensible to all subjects or the parents of subjects who are children. Subjects who do not speak English should be presented with a consent or permission document written in a language understandable to them. OHRP strongly encourages the use of such a document whenever possible. (See OHRP guidance on this topic at http://www.hhs.gov/ohrp/regulations-and-policy/guidance/obtaining-and-documenting-infomed-consent-non-english-speakers/index.html ; for information about requirements for child assent, see FAQs regarding research with children .)
In general, ordinary language should replace technical terms (e.g., upper extremities are better referred to as arms, venipuncture as taking blood from your arm with a needle, and so forth).
Some IRBs find that their lay members (e.g., community or non-scientist members) are particularly helpful in suggesting necessary modifications to language. Others ask members of the proposed subject population (e.g., clinic patients) to review consent or permission forms and indicate which parts they do not understand.
Can an electronic signature be used to document consent or parental permission?
Yes, under certain circumstances. First, the investigator and the IRB need to be aware of relevant laws pertaining to electronic signatures in the jurisdiction where the research is going to be conducted.
Unless the IRB waives the requirement for the investigator to obtain a signed consent or permission form based on the HHS regulations at 45 CFR 46.117(c) , a written consent or permission form, which may be an electronic version, must be given to and signed by the subjects or the subjects' legally authorized representatives or the parents of subjects who are children. Some form of the consent document must be made available to the subjects or the parents of subjects who are children in a format they can retain. OHRP would allow electronic signature of the document if such signatures are legally valid within the jurisdiction where the research is to be conducted.
OHRP does not mandate a specific method of electronic signature. Rather, OHRP permits IRBs to adopt such technologies for use as long as the IRB has considered applicable issues such as how the electronic signature is being created, if the signature can be shown to be legitimate, and if the consent or permission document can be produced in hard copy for review by the potential subject. One method of allowable electronic signatures in some jurisdictions is the use of a secure system for electronic or digital signature that provides an encrypted identifiable “signature.” If properly obtained, an electronic signature can be considered an “original” for the purposes of recordkeeping.
Is a faxed copy of the signed consent or parental permission form acceptable to document informed consent?
Yes, if it is more convenient for the subjects or parents of children who are subjects to fax a signed copy of the consent or permission form to the investigator, the research subjects or parents may fax the signed form. The subjects or parents need not provide the investigator with the original signed consent or parental permission documents.
Who must sign the informed consent or parental permission document?
When a written consent or parental permission form is used that embodies some or all of the elements of informed consent required by the regulations at 45 CFR 46.116 , the regulations only require that the informed consent or parental permission document be signed by the subjects or the subjects' legally authorized representatives or by the parents of children who are subjects ( 45 CFR 46.117(a) ) and 45 CFR 46.408(d) ). Only in situations where a short form is used, stating that the elements of informed consent required by 45 CFR 46.116 have been presented orally to the subject or the subject’s legally authorized representative or to the parent(s) of a child who is a subject, are there additional requirements for signatures ( 45 CFR 46.117(b)(2) ).
For the consent or parental permission process using the short form, the regulations state that there must be a witness to the oral presentation, who then signs both the short form and a copy of the IRB-approved written summary of what is to be said to the subject or the subject's legally authorized representative or to the parent(s) of a child who is a subject. The subject or the subject’s legally authorized representative or the parent(s) must sign the short form, and the person actually obtaining the consent must sign the copy of the summary ( 45 CFR 46.117(b)(2) ). Thus, three types of persons are involved in this specific consent process -- the subject or legally authorized representative or parent(s) of a child who is a subject, the person obtaining consent, and the witness.
Do signatures on consent forms have to be dated?
Although the HHS regulations at 45 CFR 46.117 do not require the consent form to be dated at the time it is signed, OHRP recommends that it be dated so that the IRB and others can document that informed consent was obtained prior to a subject’s participation in the research.
Who can be a legally authorized representative (LAR) for the purpose of providing consent on behalf of a prospective subject?
Legally authorized representative (LAR) means an individual or judicial or other body authorized under applicable law to consent on behalf of a prospective subject to the subject’s participation in the procedure(s) involved in the research ( 45 CFR 46.102(c) ). The regulations state that “no investigator may involve a human being as a subject in research covered by this policy unless the investigator has obtained the legally effective informed consent of the subject or the subject’s legally authorized representative” ( 45 CFR 46.116 ). The issue as to who can be an LAR is determined by the laws of the jurisdiction in which the research is conducted (e.g., local or state law). Some states have statutes, regulations, or common law that specifically address consent by someone other than the subject for participation in research. Most states have no law specifically addressing the issue of consent in the research context. In these states, law that addresses who is authorized to give consent on behalf of another person to specific medical procedures or generally to medical treatment may be relevant if the research involves those medical procedures or medical treatment.
When the laws of the jurisdiction in which the research is being conducted provide a reasonable basis for authorizing an individual to consent on behalf of a prospective subject to their participation in the research procedure(s), OHRP would consider such an individual to be an LAR as defined by HHS regulations at 45 CFR 46.102(c) . IRBs may wish to consult with legal counsel when deciding who can serve as an LAR for subjects of proposed research.
When may a legally authorized representative provide consent on behalf of an adult with diminished decision-making capacity?
In answering this question, the HHS regulations at 45 CFR part 46 should be consulted in addition to the laws of the jurisdiction in which the research is conducted. As a general matter, if an adult lacks capacity to consent, for example, as a result of trauma, mental retardation, some forms of mental illness, or dementia - whether temporary, progressive, or permanent - only a legally authorized representative for that adult can give consent for participation in the research, unless the requirement to obtain informed consent is waived by the IRB in accordance with the requirements at 45 CFR 46.116(c)(d) , or in accordance with the provisions for emergency waiver, which are permitted under the authority of the HHS Secretary at 45 CFR 46.101(i) .
( See the Federal Register notice of this waiver .) Should the subject regain or develop the capacity to consent, then his or her consent must be obtained for any further research, as the consent of the legally authorized representative is no longer valid.
What should be considered in seeking informed consent from individuals with diminished decision-making capacity?
The HHS regulations are silent on the consent procedures specific to subjects with impaired decision-making capacity, for example, as a result of trauma, mental retardation, some forms of mental illness, or dementia, whether temporary, progressive, or permanent. The regulations do require that the IRB ensure that “additional safeguards have been included in the study to protect the rights and welfare” of all subjects that are “likely to be vulnerable to coercion or undue influence.” The regulations include “mentally disabled persons” in this category ( 45 CFR 46.111(b) ).
In research involving adult subjects with mental illnesses or cognitive impairments, the IRB and investigator(s) must be knowledgeable about the condition and any level of impairment that is likely to be present in the subject population. The regulations do speak to the fact that the IRB must possess “the professional competence necessary to review specific research activities” ( 45 CFR 46.107(a) ). This is achieved either by having members with the appropriate experience and expertise or inviting consultants with competence in the special area to assist in the review of issues that require expertise beyond or in addition to that available on the IRB ( 45 CFR 46.107(a) and (f) ). Ensuring such expertise on the IRB improves its ability to make determinations about subject recruitment, enrollment, and informed consent requirements that best match the needs of the subjects.
In some research, such as longitudinal studies involving progressive disorders or aging populations, enrolled subjects may be competent to consent on their own behalf at the outset, yet may experience effects of progressive or intermittent disorders that lead to decisional impairment during the course of the study. In these situations IRBs and investigators should consider the need to discuss with the prospective subjects whether they should designate someone to serve as a legally authorized representative at the outset of the study, consistent with all applicable laws. Even if a subject has consented on his or her own accord, a designated representative would be ready to step in as the legally authorized representative if the subject’s ability to assess his or her own needs and interests becomes compromised during the study.
What are the requirements for assent and parental permission in research with children?
The IRB must determine, to the extent required by 45 CFR 46.116 , that adequate provisions are made for soliciting the assent of the children -- when in the judgment of the IRB the children are capable of providing assent -- as well as the permission of the parents ( 45 CFR 46.408 ). Permission means the agreement of parent(s) or guardian to the participation of their child or ward in research ( 45 CFR 46.402(c) ).
By regulatory definition, children are “persons who have not attained the legal age for consent to treatments or procedures involved in the research, under the applicable law of the jurisdiction in which the research will be conducted” ( 45 CFR 46.402(a) ). In the United States the legal age of adulthood is a matter of state and local law. This means that who is legally considered a child may vary from state to state; in a large majority of states 18 years of age is the legal age of adulthood, but this is not true in every state, locality, or territory. State law also may address specific circumstances in which a person younger than the age of adulthood is legally authorized to consent to medical procedures: for example, some states allow children younger than the legal age of adulthood to consent to the provision of contraceptive services. Certain states provide a mechanism for the emancipation of minors, through which a child younger than the legal age of adulthood may gain certain civil rights, which might include the legal ability to consent to research participation.
The definition of children also takes into account the particular interventions or interactions involved in the proposed research (e.g., surveys, blood tests). For example, in some places individuals who are 16 years of age may legally consent to certain clinical interventions or interactions. If the involvement of human subjects in a proposed research activity consists of these interventions or interactions, then those individuals may be considered as adults for that purpose. If a proposed activity includes an intervention or interaction for which the subject has not yet reached the legal age of consent, however, that person must be considered a child.
Under 45 CFR 408(b) the IRB may find that the permission of one parent is sufficient for research to be conducted under 45 CFR 46.404 or 45 CFR 46.405 . Where research is conducted under 45 CFR 46.406 or 45 CFR 46.407 , permission must be obtained from both parents unless one parent is deceased, unknown, incompetent, or not reasonably available, or when only one parent has legal responsibility for the care and custody of the child.
Although the regulations state that children are unable to provide legally effective informed consent to participate in research, some might be able to give their assent. Assent means a child’s affirmative agreement to participate in research. Mere failure to object should not, absent affirmative agreement, be construed as assent ( 45 CFR 46.402(b) ).
If the IRB determines that the capability of some or all of the children is so limited that they cannot reasonably be consulted regarding assent, or that the intervention or procedure involved in the research holds out a prospect of direct benefit that is important to the health or well-being of the children, and is available only in the context of the research, the assent of the children is not a necessary condition for proceeding with the research. Even where the IRB determines that the subjects are capable of assenting, the IRB may still waive the assent requirement under certain circumstances in accord with 45 CFR 46.116 and 45 CFR 46.408(a) .
May the requirement for obtaining informed consent or parental permission be altered or waived?
Waiver or alteration of the requirements for obtaining informed consent from adult subjects can occur under any of the following three provisions:
- the research could not practicably be carried out without the waiver or alteration; and
- public benefit or service programs;
- procedures for obtaining benefits or services under those programs;
- possible changes in or alternatives to those programs or procedures; or
- possible changes in methods or levels of payment for benefits or services under those programs.
- the research involves no more than minimal risk to the subjects;
- the waiver or alteration will not adversely affect the rights and welfare of the subjects;
- the research could not practicably be carried out without the waiver or alteration; and
- whenever appropriate, the subjects will be provided with additional pertinent information after participation.
- Research in emergency settings: an IRB may also waive the requirement for obtaining informed consent if it finds and documents that the research meets the requirements of the HHS Secretarial waiver under 45 CFR 46.101(i) that permits a waiver of the general requirements for obtaining informed consent in a limited class of research in emergency settings (PDF) (23KB).
For research involving children, an IRB may waive the requirements for obtaining parental or guardian permission under any of the following four provisions:
- The IRB makes and documents the required findings under 45 CFR 46.116(c) as described above.
- The IRB makes and documents the required findings under 45 CFR 46.116(d) as described above.
- an appropriate mechanism is in place to protect the children, and
- the waiver is not inconsistent with federal, state, or local law ( 45 CFR 46.408(c) ). The choice of an appropriate substitute mechanism (for example, appointing a child advocate or an assent monitor) for protecting children participating in research would depend on the nature and purpose of the activities described in the protocol, the risk and anticipated benefit to the research subjects, and the child’s age, maturity, status, and condition ( 45 CFR 46.408(c) ). Note that an IRB may waive the requirement for obtaining parental or guardian permission under 45 CFR 46.408(c) even if the research involves more than minimal risk to the child subjects.
- The IRB finds and documents that the research meets the requirements of the HHS Secretarial waiver under 45 CFR 46.101(i) that permits a waiver of the general requirements for obtaining informed consent in a limited class of research in emergency settings (PDF) (23KB).
What is the definition of guardian in the context of obtaining consent for research involving children?
The term guardian means “an individual who is authorized under applicable State or local law to consent on behalf of a child to general medical care” ( 45 CFR 46.402(e) ) The role of a guardian in the context of research involving a child who is a ward is to provide permission, in lieu of a child’s biological or adoptive parents, for the ward to participate in the research ( 45 CFR 46.402(c) ). For a more extensive discussion see FAQs on Research with Children .
What happens if a child reaches the legal age of consent while enrolled in a study?
The Office for Human Research Protections (OHRP) notes that informed consent should be viewed as an ongoing process throughout the duration of a research project. When a child who was enrolled in research with parental or guardian permission subsequently reaches the legal age of consent to the procedures involved in ongoing research, the subject’s participation in the research is no longer regulated by the requirements of 45 CFR part 46.408 regarding parental or guardian permission and subject assent.
Unless the Institutional Review Board (IRB) determines that the requirements for obtaining informed consent can be waived, the investigators should seek and obtain the legally effective informed consent, as described in 45 CFR 46.116 , for the now-adult subject for any ongoing interactions or interventions with the subjects. This is because the prior parental permission and child assent are not equivalent to legally effective informed consent for the now-adult subject. However, the IRB could approve a waiver of informed consent under 45 CFR 46.116(d) , if the IRB finds and documents that the required conditions are met.
Similarly, if the research does not involve any ongoing interactions or interventions with the subjects, but continues to meet the regulatory definition of “human subjects research” (for example, it involves the continued analysis of specimens or data for which the subject’s identity is readily identifiable to the investigator(s)), then it would be necessary for the investigator(s) to seek and obtain the legally effective informed consent of the now-adult subjects. The IRB may consider, if appropriate, a waiver under 45 CFR 46.116(d) of the requirements for obtaining informed consent in order for the subjects to continue their participation in the research.
What is a waiver or alteration of informed consent or parental permission?
The HHS regulations allow the IRB to waive the requirement for obtaining informed consent or parental permission or to approve a consent procedure that leaves out or alters some or all of the elements of informed consent otherwise required under 45 CFR 46.116(a) and (b) .
Waiving the requirement for obtaining informed consent or parental permission means that the IRB has determined that investigators need not obtain the subjects’ informed consent to participate in research. For example, some research about natural behavior may require that subjects be unaware that the research is taking place. Such research can only be approved by the IRB if the research meets the criteria for a waiver of informed consent under HHS regulations and for approving research according to 45 CFR 46.111 .
An IRB may approve research for which some or all of the elements of informed consent at 45 CFR 46.116 (a) and (b) have been altered, or for which some elements have been left out. For example, some research designs require that subjects be left unaware of the particular purpose of the research, because the subjects’ responses might be biased if they know in advance what the investigators are seeking. Such research designs do not preclude offering potential subjects some information about the research and giving them the opportunity to decide whether to participate. The IRB may approve such research in which investigators will leave out or alter elements of informed consent, so long as the research meets the criteria for approving research in 45 CFR 46.111 , and the research meets the criteria specified in the HHS regulations for leaving out or altering those elements.
What are the regulatory bases for waiving or altering some or all of the required elements of informed consent or parental permission?
The conditions under which an IRB may waive the requirement for obtaining informed consent or parental permission or may approve a consent procedure that leaves out or alters some or all of the elements of informed consent derive from four sources in the HHS regulations.
- At 45 CFR 46.116(c) , the regulations identify when IRBs may waive or approve an alteration of informed consent in some research examining state or local public benefit or service programs , or certain features of those programs.
- At 45 CFR 46.116(d) the regulations identify when IRBs may waive or approve an alteration of informed consent in research that meets four specified criteria .
- At 45 CFR 46.408(c) , the regulations identify when IRBs may approve waiver of parental permission in certain research involving children.
- Under the provisions of 45 CFR 46.101(i) , the Secretary, HHS, has waived the general requirements for obtaining informed consent in a limited class of research in emergency settings .
What are the criteria under 45 CFR 46.116(c) for waiving or altering some or all of the required elements of informed consent or parental permission?
Under 45 CFR 46.116(c) , an IRB may waive the requirement for obtaining informed consent or parental permission or approve a consent or parental permission procedure that leaves out or alters some or all of the elements of informed consent, provided that the IRB finds and documents that the following two criteria are satisfied:
the research or demonstration project is to be conducted by or subject to the approval of state or local government officials and is designed to study, evaluate, or otherwise examine:
- possible changes in methods or levels of payment for benefits or services under those programs; 45 CFR 46.116(c)(1) .
Note that this criterion means that only public benefit or service program research activities that are under state or local authority meet this criterion; similar research conducted under federal authority would not qualify here and is treated elsewhere in the regulations. Research conducted by or subject to the approval of only a private entity also would not qualify.
- the research could not practicably be carried out without the waiver or alteration ( 45 CFR 46.116(c)(2) ).
This criterion means that the practical circumstances of the research are such that the research is not feasible if the informed consent of the subjects must be obtained. For example, a study of identifiable private information about program benefit recipients using 20-year-old records might meet this criterion, if current contact information for those recipients is not available.
What are the criteria under 45 CFR 46.116(d) for waiving or altering some or all of the required elements of informed consent or parental permission?
Under 45 CFR 46.116(d) the IRB may waive the requirement for obtaining informed consent or approve a consent procedure that leaves out or alters some or all of the elements of informed consent, provided that the IRB finds and documents that all of the following four criteria are met:
- the research could not practicably be carried out without the waiver or alteration; and,
Is it possible to waive the informed consent requirement when conducting research in an emergency setting?
In 1996, the HHS Secretary announced, under 45 CFR 46.101(i) , a waiver of the applicability of the regulatory requirement for obtaining and documenting informed consent for a strictly limited class of research, that is, research that may be carried out in human subjects who are in need of emergency therapy and for whom, because of the subjects’ medical condition and the unavailability of legally authorized representatives of the subjects, no legally effective informed consent can be obtained. This waiver applies to research involving adults or children, but does not apply to research involving pregnant women, human fetuses, neonates of uncertain viability, and nonviable neonates, or prisoners.
For more detailed information, see OHRP’s guidance on Emergency Research Consent Waiver . It should be noted that FDA also has a comparable provision for a waiver of informed consent for emergency research at 21 CFR 50.24 .
When may the requirement for documentation of informed consent or parental permission be waived or altered?
When an Institutional Review Board (IRB) has not waived the requirement for seeking prospective informed consent of the subjects or the parental permission of children who are subjects, under the HHS regulations at 45 CFR 46.117(c) , it may waive the requirement for the investigator to obtain a signed consent form for some or all subjects if it finds either:
- That the only record linking the subject and the research would be the consent document and the principal risk would be potential harm resulting from a breach of confidentiality. Each subject will be asked whether the subject wants documentation linking the subject with the research and the subject’s wishes will govern; or
- That the research presents no more than minimal risk of harm to subjects and involves no procedures for which written consent is normally required outside of the research context (e.g., drawing a blood sample, or asking shoppers in a mall about the ambient lighting or temperature).
Some subjects might refuse a copy of the consent form once signed out of concern that their possession of the form could compromise their privacy. This is fully consistent with the idea behind one of the bases for a waiver of the requirements for documentation of informed consent - that harm would result to the subject if his/her identity were compromised by the documentation itself. The investigator may document that the subject refused a copy of the informed consent document and still include the subject in the study.
In cases in which the documentation requirement is waived, the IRB may require the investigator to provide subjects or the parents of children who are subjects with a written statement regarding the research.
Can parental or guardian permission for research involving children be waived?
Yes, under certain circumstances. An IRB may waive the requirements for obtaining parental or guardian permission if either of the following two conditions is met:
- The IRB makes and documents the required findings under either 45 CFR 46.116(c) or (d) ; or
- An appropriate mechanism is in place to protect the children, and
- The waiver is not inconsistent with federal, state, or local law ( 45 CFR 46.408(c) ).
The choice of an appropriate substitute mechanism (for example, appointing a child advocate or an assent monitor) for protecting children participating in research would depend on the nature and purpose of the activities described in the protocol, the risk and anticipated benefit to the research subjects, and the child’s age, maturity, status, and condition ( 45 CFR 46.408(c) ).
Note that an IRB may waive the requirement for obtaining parental or guardian permission under 45 CFR 46.408(c) even if the research involves more than minimal risk to the child subjects.
Is child assent always required when research involves children?
No, the IRB is responsible for deciding whether child assent is required in proposed research activities. Assent means a child’s affirmative agreement to participate in research. Mere failure to object should not, absent affirmative agreement, be construed as assent ( 45 CFR 46.402(b) ). Child assent is required, except in the following three circumstances described at 45 CFR 46.408(a) :
- the capability of some or all of the children is so limited that they cannot reasonably be consulted;
- the intervention or procedure involved in the research holds out the prospect of direct benefit to the health or well-being of the children and is available only in the context of the research;
- the research meets the same conditions as those for waiver or alteration of informed consent in research involving adults, as specified in the regulations at either 45 CFR 46.116(c) or 45 CFR 46.116(d) .
How should child assent be documented?
The HHS regulations do not require documentation of assent. The IRB has the discretion to determine the appropriate manner, if any, of documenting child assent. Based on such considerations as the child’s age, maturity, and degree of literacy, the IRB should decide what form of documentation, if any, is most appropriate. If adolescents are involved in research where a consent form would have been used if the subjects were adults, it would generally be appropriate to use a similar form to document an adolescent’s assent.
If young children are involved who are as yet unable to read, documentation should take a form that is appropriate for the purpose of recording that assent took place. The IRB may also decide that documentation of assent is not warranted.
What is the meaning of "legally effective informed consent?"
Informed consent is legally effective if it is both obtained from the subject or the subject’s legally authorized representative and documented in a manner that is consistent with the HHS protection of human subjects regulations and with applicable laws of the jurisdiction in which the research is conducted. In general terms, the regulations stipulate that an investigator should seek consent only under circumstances that provide the prospective subject or the legally authorized representative sufficient opportunity to consider whether to participate and that minimize the possibility of coercion or undue influence. The information provided should be in language that is understandable to the subject or the representative. No informed consent, whether oral or written, may include any exculpatory language .
It is important to note that the informed consent requirements in the regulations are not intended to preempt any applicable federal, state, or local laws that require additional information to be disclosed for consent to be legally effective ( 45 CFR 46.116(e) ).

- Office for Research Protections
- Institutional Review Board
- IRB Policies
IRB Guideline I - Parental Consent and Child Assent
Research concerned with sensitive issues and involving the participation of children is becoming more common. Federal law defines "children" as persons who have not attained the legal age for consent to treatments or procedures involved in the research, under the applicable law of the jurisdiction in which the research will be conducted. Under Pennsylvania law, persons under the age of eighteen (18) generally meet this definition of "children", with the exceptions noted below. As a result, permission of the child's parent(s) or guardian(s) must generally be obtained prior to the participation of that child in research. The following exceptions to the general rule apply, where a person under the age of 18 does not meet the federal definition of "child" and may provide legally effective consent to participate in research if either:
a. has graduated from high school, or
- b. is married, or
- c. is or has been pregnant.
- The person is an emancipated minor.
If an emancipated minor provides consent for him or herself, the court order must be copied and included in the research records with the consent document.
Federal regulations that govern research with children include 45 CFR 46, Subpart D and Subpart D of the FDA regulations.
Such research often presents difficult questions related to the protection of human participants. The purpose of these guidelines is to help researchers plan procedures and prepare proposals that can be approved by the Institutional Review Board (IRB).
Research on health and social issues often involves requesting sensitive information from participants, some of whom may be children. The procedures for collecting and handling such data often do pose risks to the participants. These risks may include some or all of the following:
- Violation of Privacy: Collection of data concerning at-risk or socially questionable behavior (for example, questions about substance use or sexual activity) is viewed by many individuals as violations of privacy.
- Legal Risks: Data concerning illegal behaviors may place individuals at risk of legal action, if (a) names can be linked to particular responses or observations and (b) the research has not received specific legal protection (e.g., by Certificate of Confidentiality).
- Psychosocial Stress and Related Risks: Procedures that raise sensitive issues may generate stress for participants. For example, questions about at-risk behaviors may cause students to feel stress related to their self-image or contribute to perceived peer pressure.
- Social Relations: Because relevant questions often request information about the behavior, or relations with, family members, peers, or authorities, some procedures may pose a risk to those relations if confidentiality is not adequately safeguarded.
In addition to these risks, which may be applicable to either child or adult participants, research involving child participants may also pose risks to parents or other family members. In particular, research soliciting information about at-risk behaviors of family members may place those individuals at legal risk. Furthermore, some parents may feel that their right to determine the activities of their children is violated if signed parental consent is not obtained.
In general, protection from these risks may be achieved by (a) ensuring the confidentiality of information obtained about participants, (b) providing access to or information about resources for coping with psychosocial stress caused by the research procedures, and (c) ensuring that the procedures meet the principles of voluntary participation and informed consent. Guidelines for achieving this protection include:
- Confidentiality and Anonymity: Information is considered confidential when only the investigator has access to the identity of the individual about whom information is obtained. Information obtained from individual participants must be kept confidential from public scrutiny, from parents and peers, and from legal and school authorities. This is most easily accomplished by collecting data in a manner that insures anonymity . Information is considered anonymous when names or other identifying information about individual participants can at no point be associated with observations or with responses to a survey or other data collection instruments. However, anonymity is not always compatible with research goals (for example, when data collected from the same individual at different times must be linked for analysis). In these cases, procedures for protecting confidentiality must be fully spelled out. When information that might put participants at legal risk is to be collected, it is the investigator's responsibility to obtain and document specific legal protection (e.g., by Certificate of Confidentiality obtained from a governmental agency).
- Psychosocial Stress: The procedures needed to help participants cope with psychosocial stress that may arise from participating in research will vary depending on the exact nature of the research. If such procedures are required, it will typically be sufficient to provide participants with information about resources (e.g., counselors) available to them. In cases in which more severe stress seems likely, it may be necessary to ensure that someone qualified to handle such stress be present during data collection.
- Voluntary Participation and Informed Consent: These are basic ethical principles for conducting research with human participants. Participants must be informed that participation is voluntary, that answers to specific questions may be withheld without penalty, and that they may withdraw from the research at any time. Because research of this type is often conducted in an institutional setting where participant's presence is mandatory (e.g., the school classroom), it is especially important that procedures for meeting this requirement be made explicit in the proposal.
PARENTAL CONSENT
- A particular concern with research of this nature is the role of parental consent for the participation of child participants. The general requirement is that explicit parental consent be obtained in writing for each participant. However, there are situations in which such a consent procedure is not appropriate.
- Public benefit or service programs;
- Procedures for obtaining benefits or services under those programs;
- Possible changes in or alternatives to those programs or procedures; or
- Possible changes in methods or levels of payment for benefits or services under those programs; and
- The research could not be practicably carried out without the waiver or alteration.
- The research involves no more than minimal risk to the participants; and
- The waiver or alteration will not adversely affect the rights and welfare of the participants; and
- The research could not practicably be carried out without the waiver or alteration; and
- Whenever appropriate, the participants will be provided with additional pertinent information after participation; and
- The research is not subject to FDA regulation.
The IRB may waive the requirement to obtain a signed consent form, in accord with 45 CFR 46.117(c) when:
- The only record linking the participant and the research would be the consent document and the principal risk would be potential harm resulting from a breach of confidentiality and the research is not subject to FDA regulation; or
- The research presents no more than minimal risk of harm to participants and involves no procedures for which written consent is normally required outside of the research context;
In cases in which the documentation requirement is waived, the IRB may require a script of the information or an implied informed consent form that will be provided during the consent process. That document, if requested by the IRB, is reviewed and approved by the IRB.
Researchers are reminded that the reading level of informed consent documents should be appropriate to the typical educational background of the research population, and that documents designed for college students may not be suitable for seeking parental consent. Researchers should write these documents using short sentences and everyday language. For example, "voluntary participation" may be paraphrased by "you do not have to do this if you don't want to."
OBTAINING AND DOCUMENTING ASSENT FROM CHILDREN
Parental consent is usually a prerequisite to the recruitment of human research participants who are children. However, parental consent constitutes only half of the consent process. Assent, the agreement of a child to participate in research, is the second component of the informed consent procedure for children.
The means of obtaining assent from children must be appropriate for the age ranges and levels of mental development found within the proposed participant pool. The National Commission for the Protection of Human Subjects of Biomedical and Social Science Research expects that assent be requested from children who are 6 years of age or older. However, for children between the ages of 6 and 18, the appropriate method for obtaining assent will vary. The following guidelines were proposed during a panel discussion sponsored by the National Institutes of Health:
A simple oral description of the child's involvement is given to the participant and verbal assent is requested. The procedure may be documented on the informed consent form by the presence of the signature of a witness.
A more complete oral description of the research (in layman's terminology) is given to the participant. Verbal assent is requested. The procedure may be documented on the informed consent form by the signature of a witness.
Above age 13
Written assent should be requested from both parent and child, using age-appropriate and background-appropriate documents.
Although age is used as the primary criteria in determining an appropriate means of obtaining assent, factors such as literacy and mental development must also be considered. The need for flexibility in the methods for obtaining assent from children is universally recognized. Because a single method of obtaining assent may not be appropriate for all potential participants, investigators may need to be prepared to use different approaches with different participants. As in any consent process, the primary concern is that the participant is able to understand the explanation that is presented. The need for a witness to document verbal assent procedures is dependent upon the complexity of the research and the risks to the participant.
NOTE: A parent or guardian may not be the witness for a child's verbal assent document.
The IRB assesses the potential risks and benefits for each research proposal, and the provisions for permission and assent, to determine if an activity satisfies the conditions for a category of research permitted in children, as specified in DHHS 45 CFR 46.404, 46.405, 46.406 46.407 and 46.409, and FDA 21 CFR 50.51, 50.52, 50.53, 50.54 and 50.56.
The research categories are described below.
Research that does not involve greater than minimal risk may be approved if the IRB finds that adequate provisions are made for soliciting the assent of the children and the permission of their parents or guardians (45 CFR 46.404 and 21 CFR 50.51).
For such research the IRB determines whether adequate provisions to solicit the permission of each child's parents or guardian unless one parent is deceased, unknown, incompetent, or not reasonably available, or unless only one parent has legal responsibility for the care and custody of the child is made.. Where parental permission is to be obtained, the IRB may find that the permission of one parent is sufficient.
Research involving greater than minimal risk, but presenting the prospect of direct benefit to an individual participant, or a monitoring procedure that is likely to contribute to the participant's well-being, may be approved if the IRB finds that (45 CFR 46.405 and 21 CFR 50.52):
- the risk is justified by the anticipated benefit to the participant;
- the relationship of anticipated benefit to risk is at least as favorable as that presented by available alternative approaches; and
- adequate provisions are made for soliciting the assent of the children and permission of their parents or guardians.
For such research the IRB determines whether adequate provisions to solicit the permission of each child's parents or guardian, unless one parent is deceased, unknown, incompetent, or not reasonably available, or unless only one parent has legal responsibility for the care and custody of the child, is made. Where parental permission is to be obtained, the IRB may find that the permission of one parent is sufficient.
Research involving greater than minimal risk with no prospect of direct benefit to individual participants, but likely to yield generalizable knowledge about the participant's disorder or condition, may be approved if the IRB finds that (45 CFR 46.406 and 21 CFR 50.53):
- the risk represents a minor increase over minimal risk;
- the intervention or procedure presents experiences to participants that are reasonably commensurate with those inherent in their actual or expected medical, dental, psychological, social, or educational situations;
- the intervention or procedure is likely to yield generalizable knowledge about the participant's disorder or condition which is of vital importance for the understanding or amelioration of the participant's disorder or condition; and
- adequate provisions are made for soliciting assent of the children and permission of their parents or guardians.
For such research, the IRB requires the permission of both parents, unless one parent is deceased, unknown, incompetent, or not reasonably available, or unless only one parent has legal responsibility for the care and custody of the child.
Research that is not otherwise approvable which presents an opportunity to understand, prevent, or alleviate a serious problem affecting the health or welfare of children may be approved if the IRB and the Secretary of the Department of Health and Human Services (DHHS), after consultation with a panel of experts in pertinent disciplines and following an opportunity for public review and comment, find that:
- the research in fact satisfies one of the above three conditions (45 CFR 46.407 and 21 CFR 50.54); or
- the research presents a reasonable opportunity to further the understanding, prevention, or
- alleviation of a serious problem affecting the health or welfare of children;
- the research will be conducted in accordance with sound ethical principles; and
- adequate provisions are made for soliciting the assent of children and the permission of their parents or guardians.
If the IRB has determined that the permission of both parents is required, permission granted by one parent will nevertheless be sufficient if the other parent is deceased, unknown, incompetent, not reasonably available or if the parent granting permission has legal responsibility for the care and custody of the child. In order to establish that only one parent has legal responsibility for care and custody of a child, an order issued by a court from the state in which such parent resides must grant sole custody of the child to such parent. A copy of the court order should be retained with the documentation of the parent's permission.
Approved: Social Science IRB: December 14, 2006; Biomedical IRB: January 18, 2007
Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State
- Becoming a Principal Investigator
- Find Funding
- Research Administration Tools
- Student Involvement
- Knowledge Enterprise Resources
- Research Commons
- Report Emergency or Animal Concerns
- Institutional Animal Care and Use Committee
- Animal Care and Use Policies and Guidelines
- Animal Study Team Requirements
- Per Diem Rates and Fees
- Lab Animal Handling and Technique Training
- Laboratory Animal Medicine Training
- Animal Facilities and Access
- Animal Transfer
- Animal Ordering or Imports
- Surgery and Technical Services
- Quality Assurance Assessment Program
- Accreditation
- Recombinant DNA, Biohazard and Animal Incident Reporting
- Institutional Biosafety Committee
- IBC Registration Process
- Biosafety Study Team Requirements
- Biosafety Protocols and Amendments
- Biosafety Policies and Guidelines
- Report Human Subjects Concerns
- Institutional Review Board
- IRB Member Rosters and Meeting Dates
- IRB Submission Types
- Human Research Study Team Requirements
- Human Subject Research Policies and Guidance
- IRB Time to Approval
- Consent Template Language by Topic
- HIPAA and Human Subjects Research
- Exempt Research
- Event Reporting
- Collaborative and Multi-Site Research
- Research vs. Quality Assurance/Improvement
- Quality Improvement Program
- Information for Research Participants
- Community-Engaged Research
- Clinical Trials Registration
- Information for IRB Members
- Responsible Conduct of Research
- Disclosure Process
- Tips for Disclosing
- What Not to Disclose
- Common Disclosure Scenarios
- Policies and Documents
- Foreign Talent Programs
- Conflict Approval Committee
- Controlled Substances
- Dual Use Research
- University Activities
- Export Control Regulations
- International Research Engagements
- Export Control Training
- Export Control Terms and Definitions
- Human Gene Transfer
- LabArchives - Electronic Lab Notebook
- Research Diving
- Avoiding Plagiarism
- Research Security Governance Board
- Roles and Responsibilities
- Proposal Development and Submission Guidelines
- Types of Sponsors
- Grants, Gifts and Contracts
- Working with Business and Industry Sponsors
- Working with Federal Agencies
- Other Support/Current and Pending Support
- Clinical Trials
- Uniform Guidance
- Institutional Information
- Direct Cost Categories
- Employment Benefits (Fringe) Rates
- Subcontracts for Budget Preparation
- Participant Support Costs
- Facilities and Administrative Costs
- Central Cost Share Request
- Submission Checklist
- Sponsored Program Officers
- Bayh-Dole Certification
- Award Acceptance and Initiation
- Award Management Roles and Responsibilities
- Award Terms and Conditions
- Cost Sharing Requirements
- Cost Transfers
- Personnel Appointments
- Payments to Research Subjects
- Absence or Transfer of Principal Investigator
- Patents and Copyrights
- Travel on Sponsored Projects
- Ordering in Workday
- Procurement Policies and Procedures
- Purchasing Basics
- Bid Opportunities
- Consultant Agreement Procedures
- Subcontracting on Sponsored Awards
- Procurement and Receipt of Radioactive Material
- Equipment and Property
- Software License Agreements
- Special Purchasing Needs
- Physical Facilities
- International Purchasing
- Award Closeout
- Corporate Partnerships at Ohio State
- Invention Disclosure and Assessment
- Licensing Your Technology
- Forming a Startup Company
- Peter Mohler
- Cynthia Carnes
- Animal Care and Use Program
- Office of Research Compliance
- Office of Responsible Research Practices
- Office of Secure Research
- Office of Sponsored Programs
- Byrd Polar and Climate Research Center
- Campus Chemical Instrument Center
- Campus Microscopy and Imaging Facility
- Center for Emergent Materials
- Center of Microbiome Science
- Center for RNA Biology
- Chronic Brain Injury Program
- Foods for Health
- Gene Therapy Institute
- Global Water Institute
- Infectious Diseases Institute
- Institute for Materials Research
- Institute for Optical Science
- Translational Data Analytics Institute
- Facts and Figures
- 150 Years of Excellence
- Awards and Honors
- Associate Deans for Research
- Submit an Event
- Research and Innovation Showcase 2022
- Research and Innovation Showcase 2021
- Research and Innovation Showcase 2020
- Research and Innovation Showcase 2019
Informed Consent, Assent and Parental Permission
Templates and guidance, deception or incomplete disclosure, tips for written informed consent, informed consent is an essential part of ethical human subjects research..
Informed consent is the process of telling potential research participants about the key elements of a research study and what their participation will involve. Institutional Review Boards and investigators are responsible for ensuring that research participants provide informed consent prior to participating in research unless the requirement for informed consent is waived or altered (in non-exempt research) by the IRB.
The following templates contain the basic elements of informed consent and are recommended for use to streamline IRB review and ensure that regulatory requirements are met. Study-specific information should be added to the templates using complete sentences in non-technical language. Additional consent template language has been reviewed and approved by the IRB Policy Committee. This language can be inserted in the designated sections of consent documents, as applicable.
Reading Level
When adding study-specific information to the templates, use simple lay language and an 8th-grade reading level throughout. Use the Flesch-Kincaid score tool in Word to measure the document's reading level .
- write short, simple, and direct sentences using lay language
- spell out all abbreviations and acronyms
- avoid scientific or technical terms
- use 12-point font or larger based on your audience
- Times New Roman or Arial font is recommended
- line numbers have been added to aid in the review process and must be removed after the consent has been IRB approved
- IRB Protocol Number – the number assigned by ORRP (e.g., 2023H0156)
- IRB Approval date – the date of IRB approval, which can be found on your approval letter
- Version – personal tracking mechanism; dates (e.g., 11/20/2022) or numbers (e.g., 1.2)
Informed Consent Templates
- Informed Consent Template – Behavioral and Social Science Research (12/20/2021)
- Informed Consent Template – Biomedical and Cancer Research (04/19/2021)
- Combined Consent and HIPAA Authorization Template (04/19/2021)
- Informed Consent Template – Exempt Research (12/20/2021)
Instructions and Guidance
- Informed Consent Guidance – Behavioral and Social Science Research (03/22/2019)
- Informed Consent Guidance – Biomedical and Cancer Research (03/22/2019)
- Combined Consent and Authorization Guidance (02/09/2021)
- Informed Consent Guidance – Exempt Research (01/15/2019)
Online Research Consent Templates
For minimal risk research being conducted online (such as using an online survey host, conducting interviews via a video messaging platform, document collection via e-mail) consent may be obtained via participants reading a script and then agreeing to participate by clicking a link to a survey, a check box formatted by the survey host, etc. This is an example of a script to be read by participants to collect consent for participation in online research. The example provided either includes or prompts information for the required elements of consent as well as additional language specific to participant confidentiality when collecting data online. A consent script must be submitted for IRB review in conjunction with a request for a waiver of consent documentation.
- Informed Consent Online Template – IRB Research (12/20/2021)
- Informed Consent Online Template – Exempt Research (12/20/2021)
Electronic Signatures for Informed Consent
There are a variety of methods that can be used to obtain and document informed consent that meet the requirements of the university’s electronic signature policy. Some of the methods qualify as a legally valid electronic signature while others would only apply to minimal risk research and require a waiver of consent documentation. Information and guidance on obtaining and documenting electronic consent is provided by the Office of Technology and Digital Innovation .

Verbal Consent and Contact Card Templates
In some research studies, consent may be obtained verbally rather than by using a signed consent form. The consent script example includes all of the required elements of consent and can be tailored with information specific to the study. A verbal consent script must be submitted for IRB review in conjunction with a request for a waiver of consent documentation. The contact card with study-specific and ORRP contact information may be given to participants providing verbal consent.
- Sample Contact Information Card (08/29/2023)
- Verbal Consent Script Template (03/01/2024)
Parental Permission Templates
The permission of a parent(s) or guardian must be obtained and documented for children to participate in research unless these requirements are waived by the IRB. In instances where permission of two parents is required, edit the parental permission template to add lines to capture the printed name and signature of the second parent.
- Parental Permission Template – Behavioral and Social Science Research (12/20/2021)
- Parental Permission Template – Biomedical and Cancer Research (04/19/2021)
- Combined Parental Permission and HIPAA Authorization Template (04/19/2021)
Assent Templates
For research involving children, the assent of a child to participate in research is required whenever the child is capable of providing assent, based on the age, maturity, condition, and psychological/emotional state of the child. Documentation of assent is generally required, based on the age and literacy level of the child and the nature of the research. If verbal assent will be obtained, the IRB must review a written description of the information (i.e., a script) that will be provided to the child participants during the assent process.
Assent may also be appropriate for adults with decisional impairment and other adults unable to consent for themselves, for whom a legally authorized representative will provide informed consent.
- Assent Template – Behavioral and Social Science Research (12/15/2005)
- Assent Template – Biomedical & Cancer Research (12/15/2005)
Consent Addendum Template
A consent addendum may be used for optional sub-studies presented at the start of subject participation or when new information becomes available during the study. This addendum template must be used in conjunction with the consent template.
- Consent Addendum Template – Biomedical and Cancer Research (08/05/2005)
Short Form Consent Template for Non-English Speaking Participants
Review the Short Form Informed Consent policy for information about using the short form consent process and forms listed below.
Investigators should carefully consider whether sufficient resources exist to assist with questions the participant may have during the informed consent process or during participation in the research before enrolling a non-English speaking participant using the short form consent process.
These translations are available in part through funding from The Ohio State University Comprehensive Cancer Center. Upon demonstrated need, additional short form consent translations may be requested .
- Short Form Consent Templates for Non-English Speaking Participants
- Informed Consent of Subjects Who Do Not Speak English (OHRP)
Waiver of Informed Consent and Waiver of Consent Documentation
DHHS and FDA regulations permit waivers (or alterations) of the consent process if the research meets certain conditions.
- For more information about waiving or altering the consent process, see HRPP policy Informed Consent Process and the Elements of Informed Consent .
- For more information about waiver of documentation of consent, see HRPP policy Documentation of the Informed Consent Process .
A project involves deception when an investigator gives false information to, or otherwise intentionally misleads, a research participants about some key aspect of the research to avoid biased responses. If participants will be given false information or otherwise misled during a study, then the participants are not provided with all of the required elements of informed consent and IRB approval for a waiver or alteration of informed consent is required. Examples include:
- Participants are told they scored poorly on a writing assignment completed as part of a study (regardless of how well they actually did) to see how that information influences their performance throughout the remainder of the study.
- The study involves confederates (individuals who appeared to be research participants but who are actually part of the experiment) who act to manipulate the participant or their environment as part of the study (e.g., Asch’s study of conformity).
Incomplete Disclosure
A project involves incomplete disclosure when an investigator withholds or conceals information from a participant about the specific purpose of, or activities involved in, the research. Not all incomplete disclosure requires a waiver or alteration of consent; however, if material information or aspects are withheld that could potentially influence the decision of prospective participants to take part in the research, then the participants are not provided with all of the required elements of informed consent and IRB approval for a waiver or alteration of informed consent is required. Examples include:
- A study is vaguely described as a simple economics game to study decision-making in pairs. Participants are not informed that their interactions are actually being recorded and that people are paired according to race in order to study interracial interactions and body language.
- Participants are only told that they are participating in a simple survey of knowledge, when actually the information contained in the survey is meant to be an intervention/catalyst to see if it causes the participants’ behavior to change between two time points or causes them to take a certain action based on the survey information.
Debriefing is often required when the research involves deception or involves incomplete disclosure of material information/aspects related to the research purpose or activities. In general, the debriefing will explain any deception or incomplete disclosure, provide information about why it was necessary to use deception or incomplete disclosure in the research, and provide other options available to participants (e.g., the ability to withdraw their data). Debriefing is not always required when researchers can provide the IRB with adequate justification for why debriefing is not appropriate.
- Assure that all personnel involved with recruitment and obtaining informed consent have completed training and have been approved by the IRB to participate in the protocol.
- Provide a copy of the complete consent document to each participant.
- Examine the executed document to ensure that all blanks are completed.
- Confirm that the PI/designee signature line has been completed by the person who obtained the participant’s consent.
- Retain signed consent forms in hard copy, electronic, or other media form that complies with sponsor, Federal, and university requirements for at least three years after study termination. Note: Individual sponsors may have longer retention requirements.
- Insert protocol specific information, including the IRB approval date and version, in the consent form header to ensure that the most recent, IRB‐approved version is used to obtain informed consent (e.g., “01/25/23, Version 3,” third version of the consent form approved by the IRB on 01/25/23).
- Correct or update information on the consent form by submitting an amendment for IRB review and approval prior to use (i.e., do not cross through incorrect or outdated information).
- Keep a limited number of consent form copies on hand (or print current version directly before starting the informed consent process) to avoid use of unapproved/outdated consent documents.
- Use only blue or black ink when completing the consent document.
Federal Regulations and Guidance
- General Requirements for Informed Consent: 45 CFR 46.116 (DHHS)
- Documentation of Informed Consent: 45 CFR 46.117 (DHHS)
- Tips on Informed Consent (OHRP)
- FDA Informed Consent Regulations: 21 CFR 50, Subpart B
- IRB Review of Research: 21 CFR 56.109 (FDA)
- FDA Information Sheets: Guide to Informed Consent
- Glossary of Lay Terms for use in Preparing Consent Forms (Stanford University)
- Plain Language Medical Dictionary (University of Michigan Library)
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Clinical Research Article
- Published: 04 June 2021
Facilitators and barriers for parental consent to pediatric emergency research
- Reagan L. Miller ORCID: orcid.org/0000-0001-7420-1866 1 , 2 ,
- R. Dawn Comstock 3 ,
- Lauren Pierpoint 3 , 4 ,
- Jan Leonard 2 ,
- Lalit Bajaj 2 &
- Rakesh D. Mistry 2
Pediatric Research volume 91 , pages 1156–1162 ( 2022 ) Cite this article
449 Accesses
2 Citations
1 Altmetric
Metrics details
Obtaining informed consent for clinical research in the pediatric emergency department (ED) is challenging. Our objective was to understand the factors that influence parental consent for ED studies.
This was a cross-sectional survey assessing parents’ willingness to enroll their children into an ED research study. Parents reporting a willingness to enroll in ED studies were presented with two hypothetical scenarios, a low-risk and a high-risk study, and then asked about decision influencers affecting consent. Parents expressing a lack of willingness to enroll were asked which decision influencers impacted their consent decision.
Among 118 parents, 90 (76%) stated they would be willing to enroll their child into an ED study; of these, 86 (96%) would consent for a low-risk study and 54 (60%) would consent for a high-risk study. Caucasian parents, and those with previous research exposure, were more likely to report willingness to participate. Those who would consent to the high-risk study cited “benefits that research would provide to future children” most strongly influenced their decision to agree.
Conclusions
ED investigators should highlight the benefits for future children and inquire about parents’ previous exposure to research to enhance ED research enrollment. Barriers to consent in non-Caucasian families should be further investigated.
Obtaining consent for pediatric emergency research is challenging and this study identified factors influencing parental consent for research in EDs.
Benefits for future children and parents’ previous research experience were two of the most influential factors in parents’ willingness to consent to ED research studies.
These findings will help to improve enrollment in ED research studies and better our understanding of how to promote the health and well-being of pediatric patients.
You have full access to this article via your institution.
Similar content being viewed by others
Barriers to enrollment in a pediatric critical care biorepository
Erin Paquette, Avani Shukla, … Matthew M. Davis

Parental perspectives on a trial using waived informed consent at birth
Anup C Katheria, Georg M. Schmölzer, … Neil Finer
The importance of shared decision-making in the neonatal intensive care unit
Frank Soltys, Sydney E. Philpott-Streiff, … Mary C. Politi
Introduction
Obtaining informed consent for children in the emergency department (ED) research is challenging. Little is known about the factors that influence parents’ willingness to consent to their child’s participation in emergency research. 1 Most existing literature on parental consent has been conducted within inpatient clinical trials and few assessments have focused on emergency settings. In addition, enrollment rates for children in clinical research studies, particularly in the ED, still lags behind that of adults. 2 Although these lower enrollment rates may be due to many different factors, it highlights the potential opportunity for improvements in the consent process. Advances in clinical care are often driven by empirical research; therefore, understanding the factors that influence parental consent within pediatric EDs may help to promote the health and well-being of pediatric patients.
Parental beliefs about clinical research and motives for participation are well documented. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 Prior research suggests that parents believe that clinical research is necessary 3 and that participation is often driven by the benefits provided to their child and to science. 4 , 5 , 6 , 9 Additionally, parents who agree to participate in inpatient clinical research report being satisfied with the study explanations provided during the consent process 7 , 8 and with the time that they received to review the treatment options. 8 , 9 , 10 Notably, participation in clinical research by race and ethnicity suggests that Caucasian parents are more likely to consent for their child to participate in clinical research compared to Black and Hispanic parents. 11 This body of literature highlights that many parents, especially those who identified as Caucasian, are willing to participate in inpatient clinical research and are often satisfied with the consent process; however, explorations regarding consent in pediatric emergency departments are limited.
Consent in pediatric EDs is often time-sensitive and highly unpredictable. 12 Parents are likely to experience high levels of emotional stress and confusion, 13 , 14 which can result in a lack of willingness to participate in emergency research. This stressful and unpredictable environment in the ED is in contrast to office and inpatient settings, where research visits are often scheduled. In addition, parents often have the time to thoroughly review consent forms, as well as the opportunity to take consent forms home to review. 9 Therefore, the prior findings of parental consent and pediatric research may not be applicable for ED-based research. The emerging literature on deferred parental consent within emergency contexts, which takes place post treatment, has begun to explore these potential differences. 15 , 16 This research suggests that parents prefer to have an explanation of the research that is tailored to their concerns when they are asked to provide emergency deferred consent. 15 Guidelines for deferred consent, which were developed from interviews and surveys also suggest that it is important to clearly discuss why the research is being conducted and the benefits it may have for future children. 16 Research on ED consent that occurs before treatment and perceptions about research from those who do not wish to participate remains relatively unexplored.
Identification of barriers and facilitators to enrollment of children in emergency medicine research is necessary. Strategies to enhance approaches to consent and create best practices can improve the number of subjects enrolled, and consequently the quality of findings for pediatric emergency research. The objective of this study was to understand the factors that influence parental consent for pediatric emergency research that occurs pretreatment, in order to inform future efforts for enrollment in ED studies.
Design and setting
This was a pilot study utilizing a cross-sectional survey design. Electronic surveys were administered on REDCap to a convenience sample of parents who brought their children into an urban, tertiary-care pediatric ED from July 2017 to September 2017 and January 2018 to March 2018. Enrollment periods were selected by convenience, owing to the competition from other studies concurrently being conducted in the ED. The study was granted exempt status by the university’s Institutional Review Board (IRB).
Participants
Parents and guardians of children who presented to the ED between the ages of 18 and 84 years and those who were English- or Spanish-speaking were invited to participate. Parents who were minors, <18 years of age, and older adults with special IRB exemptions, >84 years of age, were excluded. ED research assistants (RAs) approached parents for participation after placement in an ED room between 7:00 a.m. and midnight daily when available.
Study procedures and data collection
Parents and legal guardians who agreed to answer survey questions were informed about the study purpose, and specifically asked, “If presented with an opportunity to participate in a relevant research study pertaining to your child, would you be willing to participate?” Parents who reported that they would be willing to potentially enroll their child into research were subsequently presented with two hypothetical scenarios, a low-risk head injury study and a high-risk interventional asthma study (Appendix 1 ). Parents were only presented with study scenarios; designation of “low risk” or “high risk” was not known to respondents. The hypothetical low-risk study entailed a simple retrospective chart review, while the high-risk study involved a medical intervention trial with the placement of an intravenous line for a study medication. Within each scenario, parents were provided with a brief description of study participation details and then asked if they would be willing to consent for their child to participate in either hypothetical study. After reporting their agreement or disagreement to consent to the hypothetical scenarios, seven decision influencers for consent were assessed. Decision influencers were selected based on existing literature surrounding factors informing parental consent. Decision influencers included benefits to others, 4 , 5 , 16 benefits to your child, 4 , 9 stress and anxiety, 8 , 13 presence of a supporting individual, 17 , 18 a clear and concise study explanation, 12 , 16 previous experience with research, 16 , 19 and consent in their native language. 20 , 21 Questions about obtaining consent in their native language were only asked if the parents primarily spoke Spanish. Each decision influencer was measured using a 5-point Likert scale (5—strongly agree; 1—strongly disagree). Likert scale responses were dichotomized for interpretability with those responding 4 or 5 on the scale were combined into the “agreed” category and those responding 1, 2, or 3 were combined into the “disagree” category. A flowchart of study procedures is presented in Fig. 1 .
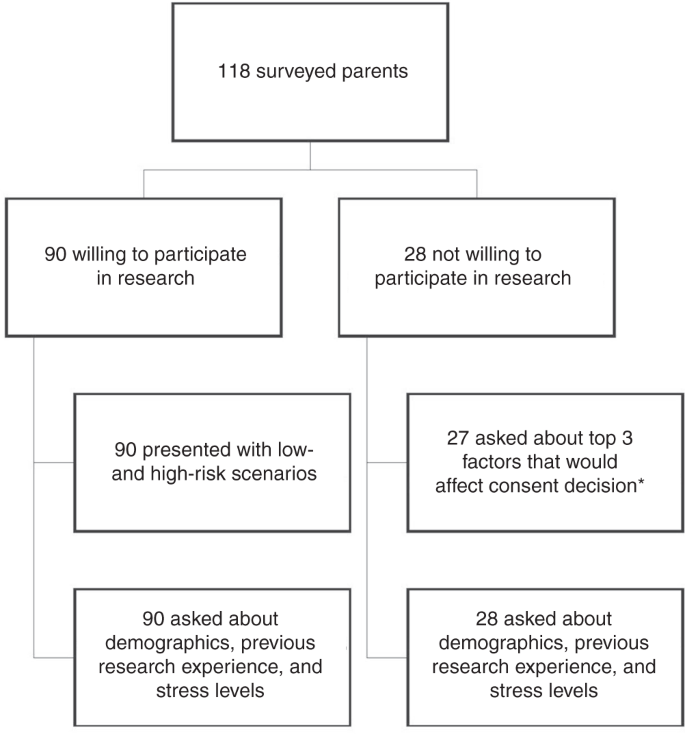
*One parent was not asked about the top three factors that would affect their consent decision because the correct button was not pushed on the mobile data collection device during the survey; therefore, these questions did not populate.
Parents who initially stated that they would not consider enrolling their child into a research study during their ED visit were asked to answer additional questions to help the research team improve interactions with patients and families. Individuals who agreed to answer these additional questions were queried about the top three decision influencers that would most positively influence their decision to consent. Individuals who did not agree to answer additional questions were not included in order to respect ethical guidelines and to follow a recent ethical movement to protect those that decline to participate in research. 22
Demographics were collected for all participants, as were two general questions about their past experiences with medical research and about their current levels of stress and anxiety in the ED. Demographics included sex, income, race, ethnicity, education, insurance, preferred language, parental age, and child age (Table 1 ).
Data analysis
Data were summarized using standard descriptive statistics. Comparisons and associations between willingness to consent, parental factors, and decision influencers were made using χ 2 analyses, Fisher’s exact test, and independent-samples t tests. G*Power was used to calculate post hoc power analyses with at least 80% power. Analyses revealed that the sample size was sufficient to detect significant differences within this study. Fisher’s exact test was used to explore decision influencers in the low-risk study because some frequencies were <5. 23 Odds ratios and 95% confidence intervals were also calculated where appropriate. All analyses were run in IBM SPSS Statistics version 24 (Chicago, IL) and SAS version 9.4 (Cary, NC).
In total, 118 parents were initially approached for study participation. Ninety (76%) parents stated that they would be willing to enroll their child into a relevant ED research study. Among the 90 parents who reported willingness to enroll their child into a potential research study, 86 (96%) stated that they would consent to the low-risk study, and 54 (60%) stated they would consent to the high-risk study.
Race and previous research experience had the strongest association with a parent’s potential willingness to participate in any form of pediatric emergency research (Table 1 ). Individuals who identified as Caucasian, in comparison to individuals who identified as non-Caucasians, were significantly more likely to state that they would participate in research (odds ratio (OR) = 3.02, 95% confidence interval (CI): 1.15–7.88). Parents who had previously participated in clinical research were also significantly more likely to agree to consent for general research participation, as compared to those with no previous research experience (OR = 5.66, 95% CI: 1.25–25.56). Other parent and child factors, including child age, income, ethnicity, education, insurance, employment status, past experience with research, self-reported stress, and language, were not significantly associated with consent to general research (Table 1 ). Differences in parent and child factors influencing potential enrollment for the low-risk study were not investigated, as 96% of participants stated that they would consent to the low-risk study. For the high-risk study, no parent or child factors were significantly associated with potential enrollment (Table 2 ).
Several decision influencers were noted to impact consent into the high-risk study (Table 3 ). “Benefits to others” was most strongly associated with consent into the high-risk study ( p < 0.001), followed by potential direct benefits of participation to their child ( p = 0.017), the presence of a supporting individual ( p = 0.037), and use of a clear and concise study explanation ( p = 0.037). Additionally, 56% of parents who reported willingness to enroll in the high-risk study did not agree that the stress and anxiety from the current ER visit would impact their willingness to consent, as compared to parents who did not report willingness to consent to the high-risk research ( p = 0.006). When comparing the relationship of decision influencers on potential participation in the low-risk study, none of the decision influencers were found to have a significant association. However, 75 of the 86 parents (87%) who would consent to the low-risk study agreed that benefits to others would most strongly influence their decision to consent.
This study contributes meaningful and novel information about the determinants associated with parental consent for their children to participate in pediatric emergency research. Race and parent’s previous experience with research played an influential role in the likelihood of consenting to an emergency research study. When investigating decision influencers, we determined that parental consent was positively impacted by research that was of benefit to the future of pediatric health. With respect to high-risk, interventional studies, the direct benefits provided to their child, the presence of a supporting individual, and clear and concise study explanations also positively influenced the decision to consent. Interestingly, no specific barriers or facilitators were associated with the decision to consent for low-risk studies such as chart reviews. The results of our study can assist future researchers in developing optimal strategies for approaching parents in ED settings to consent for participation in pediatric emergency research.
Similar to prior studies which found that participation in pediatric research was primarily motivated by a sense of altruism, 1 , 16 , 17 , 18 parents in our study reported that they would be more willing to consent to a high-risk study if they knew it would help future children experiencing a similar problem. This factor had a greater association than potential benefits of research participation provided to their own child, which was the second most impactful decision influencer. These findings suggest that although parents do take their own child’s welfare into account, they also heavily weigh the health and well-being of other children who may potentially benefit from research when making their consent decision. Importantly, high-risk studies with greater potential to expose children to risks have lower rates of participation, 24 which was also reflected in our study. Although risk and potential danger may always act as a deterrent, future high-risk studies may benefit from our findings, as parents seem to value the humanitarian benefits of high-risk ED research, and may be more willing to participate if they are provided information about the altruistic value of the research.
The use of clear and concise study explanations and the presence of a supporting individual were among the key influencers that motivated the decision to consent for the high-risk study. It has been well documented that parents want to understand the nature of the study for which they are allowing their child to participate. 12 , 16 A lack of comprehension and understanding about the study often results in a lack of participation. 25 Additionally, the role and presence of a support structure, such as a significant other, relative, or close friend, when making difficult decisions may be extremely helpful. 17 , 18 Thus, ED researchers may benefit from encouraging parents to discuss the risks and benefits of ED research participation with a significant other or close friend before making a consent decision. Based on the findings of our study, pediatric ED-based research would benefit by helping families to feel more supported and well-informed during enrollment and consent.
For pediatric emergency research in general, previous participation and experience with clinical research were associated with a positive decision to consent. Among parents who had previously participated in the research, the likelihood of consenting was nearly six times higher. This relationship between parents’ prior experience with research and the willingness to consent to future studies suggests that parents who are comfortable with the research process are more likely to subsequently participate. Although the link between prior experiences and future participation has not been widely explored, prior investigations on deferred emergency consent suggest that parents with previous experience with deferred consent are more likely to provide deferred consent in the future. 16 , 19 Further, these findings highlight the importance of both assessing for previous experience with research and the importance of appropriate conduct as past experiences with research was demonstrated to play a major role in future participation. In sum, parents who feel more comfortable with the consent process and study procedures may be more willing to enroll their child into research and continue to do so over time.
In line with existing knowledge, 20 , 26 we also found that a lower percentage of participants who identified as non-Caucasian compared to individuals who identified as Caucasian agreed to consent to general research, while there were no differences found in ethnicity. One explanation for these results is that there may be medical distrust among racial minorities that stems from a history of discriminatory medical research and medical practices, as well as a legacy of mistreatment. 27 , 28 The infamous Tuskegee syphilis study of 1932 purposely withheld curative treatment from over 400 black men with syphilis in order to unethically observe the natural course of the disease. 27 Although the medical community condemned such research and discriminatory actions in 1972, our research and the research of others suggest that minoritized people remain skeptical of informed consent. 28 These findings suggest that it may be essential to recognize this mistrust, validate concerns, and then promote transparency in the consent process. It also highlights the need for additional repair and culturally competent approaches for consent. Going forward, it will be important to continue to recognize medical distrust among racial minorities and validate these concerns while promoting transparency in the consent process.
Although previous studies have shown that parents are less likely to participate in clinical research during high levels of stress, 29 our study results did not find that the current level of parental stress impacted decisions about consent. One possibility for these findings is that parents within our study may have underestimated their own stress and anxiety, as well as the impact of this stress on their consent decision, which is supported by prior research. 30 An additional explanation may also be that stress and anxiety may not actually play as important a role with respect to parental consent, as previously believed. However, the current study did not measure parents current stress levels using a validated measure; therefore, future research that utilizes validated and reliable measures of stress and anxiety will be necessary in order to make more definitive conclusions.
Although this study has several important findings, our study is not without limitation(s). First, child age was the only child factor investigated in this study. It is possible that additional child factors may play an influential role in what may make parents more or less likely to consent to research. Second, this was a self-reported survey within a single, urban, tertiary-care ED. The answers are assumed to provide an accurate and honest representation of the parent’s beliefs, but these responses may suffer from social desirability bias and thus may not represent actual parental actions if asked to consent in a true research enrollment situation. By definition, parents who completed the survey are also amenable to a certain extent to participating in research in ED settings; therefore, actual refusals to research may not be represented within this sample and the results may be over-stated. Finally, this study took place within an academic tertiary-care ED, which may limit the generalizability of the results. Despite these limitations, this study provides important and novel information that should influence future efforts to encourage parental consent for pediatric emergency research.
We identified parental factors and beliefs that contribute to the enrollment of children within pediatric emergency department research studies. Parents who would consent for their children to participate in emergency research, do so out of a sense of altruism, and because of the benefits that it provides for their child. The presence of supporting individuals and explaining potential studies in a clear and concise manner can also motivate consent. Investigating the barriers to consenting families that identify as non-Caucasian as well as other factors that influence the decision to participate in low-risk studies are essential next steps. Overall, our findings support the importance of communicating the direct and future benefits of research for children when consenting families to pediatric emergency research studies.
Abernethy, L. E., Paulsen, E. L., Monuteaux, M. C., Berry, M. P. & Neuman, M. I. Parental perceptions of clinical research in the pediatric emergency department. Pediatr. Emerg. Care 29 , 897–902 (2013).
Article Google Scholar
Bourgeois, F. T. et al. Pediatric versus adult drug trials for conditions with high pediatric disease burden. Pediatrics 130 , 285–292 (2012).
Morris, M. C., Besner, D., Vazquez, H., Nelson, R. M. & Fischbach, R. L. Parental opinions about clinical research. J. Pediatr. 151 , 532–537 (2007).
Sammons, H. M., Atkinson, M., Choonara, I. & Stephenson, T. What motivates British parents to consent for research? A questionnaire study. BMC Pediatr. 7 , 12 (2007).
Wendler, D., Abdoler, E., Wiener, L. & Grady, C. Views of adolescents and parents on pediatric research without the potential for clinical benefit. Pediatrics 130 , 692–699 (2012).
Caldwell, P. H. Y., Butow, P. N. & Craig, J. C. Parents’ attitudes to children’s participation in randomized controlled trials. J. Pediatr. 142 , 554–559 (2003).
Stenson, B. J., Becher, J. C. & McIntosh, N. Neonatal research: the parental perspective. Arch. Dis. Child. Fetal Neonatal Ed. 89 , F321–F324 (2004).
Article CAS Google Scholar
Ruccione, K., Kramer, R. F., Moore, I. K. & Perin, G. Informed consent for treatment of childhood cancer: factors affecting parents’ decision making. J. Pediatr. Oncol. Nurs. 8 , 112–121 (1991).
van Stuijvenberg, M. et al. Informed consent, parental awareness, and reasons for participating in a randomised controlled study. Arch. Dis. Child. 79 , 120–125 (1998).
Eder, M. L., Yamokoski, A. D., Wittmann, P. W. & Kodish, E. D. Improving informed consent: suggestions from parents of children with leukemia. Pediatrics 119 , e849–e859 (2007).
Natale, J. E. et al. Racial and ethnic disparities in parental refusal of consent in a large, multisite pediatric critical care clinical trial. J. Pediatr. 184 , 204–208 (2017).
Chamberlain, J. M. et al. Perceived challenges to obtaining informed consent for a time‐sensitive emergency department study of pediatric status epilepticus: results of two focus groups. Acad. Emerg. Med. 16 , 763–770 (2009).
Heilbrunn, B. R. et al. Reducing anxiety in the pediatric emergency department: a comparative trial. J. Emerg. Med. 47 , 623–631 (2014).
Holm, L. & Fitzmaurice, L. Factors influencing parent anxiety levels in a pediatric emergency department waiting area. Pediatr. Res. 56 , 672 (2004).
Woolfall, K. et al. Doing challenging research studies in a patient-centred way: a qualitative study to inform a randomised controlled trial in the paediatric emergency care setting. BMJ Open 4, e005045 (2014).
Woolfall, K. et al. Fifteen-minute consultation: an evidence-based approach to research without prior consent (deferred consent) in neonatal and paediatric critical care trials. Arch. Dis. Child. Educ. Pract. Ed. 101 , 49–53 (2016).
Rabow, M. W., Hauser, J. M. & Adams, J. Supporting family caregivers at the end of life: they don’t know what they don’t know. JAMA 291 , 483–491 (2004).
Greenberg, R. G. et al. Parents’ perceived obstacles to pediatric clinical trial participation: findings from the clinical trials transformation initiative. Contemp. Clin. Trials Commun. 9 , 33–39 (2018).
Molyneux, S. et al. ‘The words will pass with the blowing wind’: staff and parent views of the deferred consent process, with prior assent, used in an emergency fluids trial in two African hospitals. PLoS ONE 8 , e54894 (2013).
Glickman, S. W. et al. Challenges in enrollment of minority, pediatric, and geriatric patients in emergency and acute care clinical research. Ann. Emerg. Med. 51 , 775–780 (2008).
Kelly, M. L., Ackerman, P. D. & Ross, L. F. The participation of minorities in published pediatric research. J. Natl Med. Assoc. 97 , 777 (2005).
PubMed PubMed Central Google Scholar
Stiles, P. G., Epstein, M., Poythress, N. & Edens, J. F. Protecting people who decline to participate in research: an example from a prison setting. IRB-Ethics Hum. Res. 34 , 15 (2012).
Google Scholar
Kim, H.-Y. Statistical notes for clinical researchers: chi-squared test and Fisher’s exact test. Restor. Dent. Endod. 42 , 152–155 (2017).
Haddad, A. End-of-life decisions: the family’s role. RN 67 , 25–28 (2004).
PubMed Google Scholar
Allmark, P. & Mason, S. Improving the quality of consent to randomised controlled trials by using continuous consent and clinician training in the consent process. J. Med. Ethics 32 , 439–443 (2006).
Nelson, D. K. et al. Obtaining consent from both parents for pediatric research: what does “reasonably available” mean? Pediatrics 131 , e223–e229 (2013).
Brandt, A. M. Racism and research: the case of the Tuskegee Syphilis Study. Hastings Cent. Rep . 8 , 21–29 (1978).
Freimuth, V. S. et al. African Americans’ views on research and the Tuskegee Syphilis Study. Soc. Sci. Med. 52 , 797–808 (2001).
Lebet, R., Fineman, L. D., Faustino, E. V. S. & Curley, M. A. Q. Asking for parents’ permission to enroll their child into a clinical trial: best practices. Am. J. Crit. Care 22 , 351–356 (2013).
Sheidow, A. J., Henry, D. B., Tolan, P. H. & Strachan, M. K. The role of stress exposure and family functioning in internalizing outcomes of urban families. J. Child Fam. Stud. 23 , 1351–1365 (2014).
Download references
Acknowledgements
We would like to thank Dr. Daniela Santos for translating the surveys into Spanish and for enrolling patients in the study. We would also like to thank Marissa Garcia, Tate Closson-Niese, Amira Herstic, and Kayla Bell for enrolling patients into this study. Additionally, this study could not have been conducted without the help of Mimi Goodwin and Kathleen Grice. There was no external funding for this manuscript and all authors have indicated they have no financial relationships relevant to this article to disclose.
Author information
Authors and affiliations.
Department of Human Development and Family Studies, Colorado State University, Fort Collins, CO, USA
Reagan L. Miller
Department of Pediatrics, Section of Emergency Medicine, University of Colorado Denver, Aurora, CO, USA
Reagan L. Miller, Jan Leonard, Lalit Bajaj & Rakesh D. Mistry
Department of Epidemiology, Colorado School of Public Health at University of Colorado Anschutz, Aurora, CO, USA
R. Dawn Comstock & Lauren Pierpoint
Center for Outcomes-based Orthopedic Research, Steadman Philippon Research Institute, Vail, CO, USA
Lauren Pierpoint
You can also search for this author in PubMed Google Scholar
Contributions
R.L.M. conceptualized and designed the study and data collection instruments, collected data, and initiated and revised the manuscript. R.D.M. and R.D.C. conceptualized and designed the study and data collection instruments, supervised the study, reviewed the manuscript for important intellectual content, and revised the manuscript. R.D.M. provided final approval of the version to be published. L.B. conceptualized the study and reviewed and revised the manuscript. L.P. and J.L. made substantial contributions to the analysis and interpretation of data and helped to revise critical manuscript content. All authors are in agreement with the content of the manuscript.
Corresponding author
Correspondence to Reagan L. Miller .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Consent statement
Consent was provided through the completion of the survey.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, rights and permissions.
Reprints and permissions
About this article
Cite this article.
Miller, R.L., Comstock, R.D., Pierpoint, L. et al. Facilitators and barriers for parental consent to pediatric emergency research. Pediatr Res 91 , 1156–1162 (2022). https://doi.org/10.1038/s41390-021-01600-9
Download citation
Received : 31 July 2020
Revised : 15 March 2021
Accepted : 20 May 2021
Published : 04 June 2021
Issue Date : April 2022
DOI : https://doi.org/10.1038/s41390-021-01600-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
IRB Application Guide
All new human subjects research must be reviewed by the IRB prior to the commencement of any study activity. The IRB Application Guide will assist UT Austin faculty, staff and students who are planning to conduct research involving human subjects.
Once IRB approval or determination has been granted, researchers must follow IRB Policies and Procedures for follow-on submissions during the course of their research study to remain in compliance. Examples of follow-on submissions include modifications, continuing reviews (when applicable), and reportable new information reports.
Forms, templates and guidance documents are available for download via the UTRMS-IRB Library.
How to Submit an Initial IRB Application (New Study)
Initial/new study submissions are created in UTRMS-IRB. The following documents should be uploaded with the online application. The Getting Started and Creating a New Study Submission (PDF) document provides step by step instructions on how to create and attach submission documents.
Download templates from the UTRMS-IRB Library Templates page. One of the following must accompany an online submission.
HRP-UT901 – Template IRB Proposal Standard Submission
Use of greater than minimal risk studies and minimal risk studies that fit into one or more expedited categories (see Section 5.3 of the IRB Policies and Procedures Manual for details regarding expedited research). Do NOT submit this form if the study will qualify for exempt review (see Section 5.4 of the IRB Policies and Procedures Manual for details regarding exempt research).
HRP-UT902 – Template IRB Proposal Exempt Submission
Use for studies that will meet one or more categories for exempt review (see Section 5.4 of the IRB Policies and Procedures Manual for details regarding exempt research.
HRP-UT903 – Template IRB Proposal Secondary Use Submission
Use for studies that are ONLY utilizing secondary data or specimens and that meet the criteria for human subjects research. If conducting chart reviews only (retrospective or prospective) use this form; do not use the exempt form or standard submission form.
HRP-UT912 – Template IRB Proposal Humanitarian Use Device Submission
Use this form ONLY for HUD submissions.
Download applicable form from the UTRMS-IRB Library Templates page.
If the research involves any of the following, submit the appropriate Supplemental form with the UTRMS-IRB application: biospecimens (HRP-UT904), investigational device (HRP-UT905), investigational drug/biologic (HRP-UT906), PHI (HRP-UT907), international research (HRP-UT908), prisoners (HRP-UT909), registry/repository (HRP-UT910), Department of Defense (DoD) sponsored/funded (HRP-UT911).
The UT IRB has consent, parental permission and assent form templates available for download in UTRMS-IRB Library Templates. English and Spanish versions of the templates are currently available. It is recommended that researchers use the available templates for a more efficient review.
- Letters of Support/Site Letters
- Other IRB Approvals
- HIPAA authorizations (if separate from consent forms) – HIPAA Authorization templates are available via UTRMS-IRB Library, Templates.
- Recruitment Materials
- Surveys/questionnaires/data collection tools/interview scripts/intervention manual
- Other documents as appropriate to facilitate review of the research
Applying for Modifications
Changes in study procedure or personnel for studies that were reviewed and approved as expedited or full board must be submitted and approved by the IRB prior to implementation by submitting a modification application in UTRMS-IRB. By starting a modification application, the currently approved IRB application is opened for editing. This allows researchers to alter, delete or add to the current application and/or supplemental documents.
Exceptions to this process can only be made when there are concerns for subject safety. If there are subject safety concerns, email [email protected] as soon as possible. as soon as possible. See Section 8: Modification/Amendment of Human Subjects Research Activities in UT IRB Policies and Procedures for additional information.
Studies determined to be exempt at time of initial review require a modification submitted via UTRMS-IRB if the change(s) to the research involve a change in PI, increase risk to participants or otherwise affect the exempt category or the criteria for exempt determination. Researchers are encouraged to contact IRB staff if unsure whether proposed changes to exempt research would require a modification application.
To submit a modification application, log in to UTRMS-IRB, and navigate to the study to modify. Under Next Steps, click “Create Modification/CR/Closure” and select “Modification/Update.”
- If ONLY changing research personnel, choose “Study team member information” as the modification scope. For all other changes, choose “Other parts of the study”.
Complete the Modification application by summarizing the modifications such that IRB reviewers can adequately understand proposed changes. Use the “Update” button to upload revised versions of currently approved study documents. To add brand new study documents, click the “+Add” button to upload.
Continuing Review
Annual continuing reviews are required for greater than minimal risk research, research regulated by the FDA and research sponsored/funded by the Department of Justice. Most minimal risk research studies approved via the expedited review pathway do not require annual continuing review. The IRB may require continuing review for special circumstances, to be determined on a study-by-study basis and documented as part of the review.
Researchers can identify if their study requires a continuing review by confirming if there is an approval end date present on the study workspace. UTRMS-IRB will also send periodic reminders to notify researchers if their study requires a continuing review.
To submit a continuing review application, log into UTRMS-IRB and navigate to the study to modify. Under Next Steps, click “Create Modification/CR/Closure” and select “Continuing Review/Study Closure”. Complete the continuing review application.
To submit a modification along with a continuing review, choose “Modification/Continuing Review” and complete the application.
Reportable New Information (RNI)
To ensure the protection of research participants, the IRB requires investigators to report certain information during the course of the research, either promptly or at the time of continuing review. This may include events where participants experience unanticipated problems or when there is potential noncompliance.
For the IRB policy regarding reporting unanticipated problems or instances of potential noncompliance, see Section 9: Reporting Unanticipated Problems and Section 22: Protocol Deviations and Noncompliance of the IRB Policies and Procedures Manual .
Written reports from study monitors, including Data Safety and Monitoring Board reports can be uploaded for IRB review via the RNI process.
To submit an RNI, log into UTRMS-IRB and click on “Report New Information”. Complete the RNI SmartForm. When describing the information, include the following as applicable:
- What happened, when and where
- What factors and contributed (role, not name) to why it happened
- What was done to address the issue before submitting the RNI to the IRB
- What is the plan going forward for preventing this from happening again
- If “yes” is answered to any of these questions, include additional details as applicable in the description section.
- If the event is related to a current study (or studies), choose the related study in question 8.
- If the event is for a study where UT Austin deferred IRB review to an external IRB, upload the reviewing IRB’s determination letter. If not available at the time of submission, proceed with submission and upload once available.
- Click Continue when you have completed the application and Submit to submit the application for review.
The RNI will undergo initial pre-review by IRB staff to ensure that the submission is complete and in keeping with the IRB requirements. The RNI submitter may be asked to provide additional information. Once the submission is determined to be complete and if the RNI requires further IRB review, it will be routed for expedited or full board review as appropriate.
If during review, the event reported clearly does not meet the criteria for an unanticipated problem or potential noncompliance, the IRB staff may acknowledge the report.
Expedited Review
The submission is sent to an IRB member for review. The submission will receive one of the following determinations:
- Non-compliance that is neither serious nor continuing
- None of the above (not noncompliance, not an unanticipated problem, etc.)
An acknowledgement letter that may or may not request additional actions will be issued via UTRMS-IRB. If additional action is specified, a modification or additional RNI must be submitted, as applicable.
Full Board Review
The submission will be assigned to a Board meeting for review. The submission will be presented at a convened meeting for discussion. The submission will receive at least one of the following determinations by the IRB:
- Unanticipated problem involving risks to subjects or others
- Serious non-compliance
- Continuing non-compliance
- Suspension or termination of IRB approval
- None of the above
If the IRB determines the reported event constituted an unanticipated problem or serious and/or continuing noncompliance and the study is funded by HHS or subject to FDA regulations, these agencies will be notified by the IRB of this finding.
An acknowledgement letter that may or may not request additional actions will be issued via UTRMS-IRB. If additional action is specified, a modification or an additional RNI must be submitted, as applicable.
Study Closure
A closure report must be submitted to the IRB in UTRMS-IRB for each human research study, regardless of whether a study is subject to continuing review requirement. Investigators are required to submit final study closure reports when all of the following apply:
- Study is permanently closed to enrollment,
- Participants have completed all research activities, and
- Collection and analysis of identifiable information is complete.
To submit a closure application, log into UTRMS-IRB and navigate to the relevant study. Under Next Steps, click “Create Modification/CR/Closure” and select “Continuing Review/Study Closure”. Complete the Continuing Review/Closure application.
Email Notices
The Office of Research Support and Compliance and Compliance notifies PIs 90, 60 and 30 days prior to the study expiration date. These emails are automated and only warnings about the upcoming expiration date. All emails are sent to the email address on file in Workday.
UTRMS-IRB Library
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Yale J Biol Med
- v.86(3); 2013 Sep

Focus: Research and Clinical Ethics
Parental permission and child assent in research on children.
Grounded on the ethical principle of respect for persons, parental permission and child assent function together to protect the child and to foster the development of the child’s self-determination. Although both parental permission and child assent involve the same components of information sharing, comprehension, and voluntariness, how these three components are understood and operationalized should differ depending on the developmental level of the child. For example, the amount of information that a child must comprehend to provide meaningful and developmentally appropriate child assent (or dissent) should be allowed to vary with the age and maturity of the child. By understanding child assent together with the important protections of parental permission, child assent does not need to be burdened with the same informational and process requirements. As a result, the age (as a proxy for developmental stage) at which a child is deemed capable of assent would be lower (i.e., 5 to 7 years old). By assuming a lack of capacity, the potential arises to dishonor and disregard a child’s wishes by failing to solicit meaningful assent or dissent. Further research needs to be done on how best to obtain truly informed and voluntary parental permission and child assent for research participation.
Introduction
The ethical justification for the enrollment of human subjects in research requires, at a minimum, two procedural safeguards: prior scientific and ethical review by an independent committee followed by the informed and voluntary consent of the prospective research participant.
For research involving children, both of these safeguards are modified given the vulnerability of children to undue influence or coercion. 1 There are limits set to the risks that a child may be exposed to in research that does not offer a prospect of direct benefit and limits set to the justification of risks that a child may be exposed to in research that offers a prospect of direct benefit. 2 As discussed below, these additional requirements for research involving children arise from the difficulty in applying a model of self-determination to parental permission and child assent.
The requirement for parental permission and child assent is an application of the more general principle of respect for persons as articulated by the National Commission in The Belmont Report [ 1 ]. The principle of respect for persons affirms the primary importance of allowing individuals to exercise their moral right of self-determination. However, this principle also implies that persons who are not capable of self-determination should be protected, if necessary, by requiring permission from an individual authorized to consent on their behalf. Parents or legal guardians serve this important function for children who are younger than the age of majority. In other words, the doctrine of “informed consent” has only limited direct application in pediatrics.
Only persons who have the appropriate capacity and are legally empowered can provide effective informed consent for themselves. In all other situations, parents or other surrogates provide permission for diagnosis and treatment of children with the assent of the child whenever appropriate [ 2 ]. Thus, parental permission 3 serves to protect a child while the child’s capacity for self-determination matures.
The principle of respect for persons requires that both the child, if capable, and the parent exercise voluntary choice concerning research participation. However, the nature of the choice and the information necessary to exercise that choice differ. Because of the protective function of parental permission, parents must be provided with detailed information concerning the nature and purpose of the research, the risks and benefits that may reasonably be expected, and any alternatives to research participation. In other words, the purpose of parental permission is to protect a child from assuming unreasonable and unjustified risks given his or her immaturity. To accomplish this task, a parent must receive all of the necessary information and be afforded the same opportunity to make a voluntary choice as would be provided to an adult making a personal decision to enroll in a research study.
Child assent must be linked to a protective mechanism such as parental permission, even if the requirement for parental permission has been waived. 4 As such, the meaning and function of child assent should be understood in the context of the protection afforded by parental permission [ 3 ]. The linked requirement for parental permission means that we do not need to burden child assent with the same informational and decision-making standards as adult informed consent. Thus, child assent is limited to a simple preference in favor of research participation. 5 A child’s inability to understand otherwise important informational elements of informed consent, such as any reasonably foreseeable risks, does not establish that a child is incapable of agreeing or disagreeing to research participation. If children are required to fully understand research in order to provide adequate assent, the capacity required will approach that of an adolescent and thus limit younger, less cognitively developed children from participating in the assent process [ 4 ]. Once we accept the premise that the assent of younger children should not be held to the same informational standard as parental permission (or even adolescent assent), the challenge is to identify those elements that are important for adequate and voluntary child assent. In addition, the related question as to when a child develops the capacity to provide meaningful and developmentally appropriate child assent (or dissent) must be addressed. For example, when does a child develop the capacity to understand that a proposed intervention does not offer him or her any prospect of direct benefit? In other words, when would a young child be sufficiently mature to realize that failing to require assent (or to respect dissent) in the absence of any prospect of direct benefit shows disrespect for the child’s wishes?
In this article, we will review the empiric evidence regarding parental permission and child assent, including both comprehension of information and assessments of whether research participation is voluntary. Differences between child and adolescent assent will be highlighted, and the effects of compensation on assent and permission will be reviewed. Finally, we will consider circumstances in which parental permission or child assent may be waived.
Parental Permission
Permission means the agreement of a child’s parent(s) or guardian to the participation of their child or ward in a clinical investigation. 6 Parental permission is held to the same standards as informed consent and is required (absent a waiver) for research involving children. The disclosure of information required for voluntary and informed parental permission is the same as that required for informed consent and includes a discussion of the potential risks, benefits, and alternatives to research participation. The permission of one parent is sufficient for research involving minimal risk and greater than minimal risk but presenting the prospect of direct benefit to the child participant. For research involving greater than minimal risk without the prospect of direct benefit to the child and research that is not otherwise approvable absent review by a federal panel, the permission of both parents is generally required. 7
The limitations of the informed consent process for research participation have been well-described in the literature [ 5 , 6 ] and are beyond the scope of this article. Parental permission for the participation of children in clinical research is considered valid if it is informed and voluntary. As with informed consent, threats to the validity of parental permission include a failure to recognize that the research protocol is designed to answer a scientific question rather than to offer individualized clinical benefit (therapeutic misconception). Evidence of the therapeutic misconception in the setting of parental permission includes lack of clarity about research versus clinically indicated procedures [ 7 ] and conflicts of interest that arise when the treating physician is also an investigator [ 8 ]. Therapeutic misestimation may also occur if subjects overestimate the benefits or underestimate the potential risks associated with a particular study.
Studies that purportedly evaluated the ability of parents to provide valid informed permission have generally assessed parents’ memory of the study from months to years after the time the child participated. The results of studies in which parental permission was assessed based on recall have generally reported a therapeutic misconception, a therapeutic misestimation, or both. But it is unclear whether these problems indicate that an informed decision was not made at the time permission was given or merely indicate a limited ability to recall past events. For example, parents of infants who were enrolled in a study designed to evaluate the effect of continuous infusion morphine or placebo on the neurologic outcome of premature infants were asked a series of open-ended questions designed to assess the purpose, benefits, risks, and voluntary nature of the study. Only 3 percent of parents were able to answer these questions accurately, but the time interval from signing the parental permission document to completion of the questionnaire ranged from 3 to 28 months [ 9 ]. The model of parental permission used in a European study held that valid permission was obtained when four criteria were met: parents were able to think clearly (e.g., were not overwhelmed with emotion), sufficient information was received to make an informed choice, parents understood the information presented, and parents understood that they were free to withdraw [ 10 ]. The authors reported impairment in at least one of these domains in 70 percent of cases, but the relationship between these criteria and valid permission may not be empirically established, and again parents were interviewed months to years after the initial research. A smaller study in which parents were interviewed within 10 days of the initial intervention and were allowed to refer to the parental permission document during the interview if they desired demonstrated good understanding, appreciation, and reasoning about research participation, suggesting that poor recall may have been partly responsible for the results of previous studies [ 11 ].
Tait et al. interviewed parents who had been approached for permission to allow their children to participate in an anesthesia or surgery study within approximately 1 day of being approached for permission to determine whether they understood 11 elements of consent [ 12 ]. Although parents perceived their overall understanding of the elements of consent as high, the assessors’ measures of understanding were significantly lower. This finding may be explained by the possibility that a parent’s perception of understanding at the time of the decision may be high, even though the parent may be unable to recall the facts on which that decision was based. Parents who agreed to allow their children to participate had greater understanding than parents who did not consent. Other predictors of understanding included education level, clarity of disclosure, having a child in previous study, the age of the parent, whether the parent listened to the disclosure, and the degree to which the parent read the consent document. A small study also suggested that parental perception of adequate time to decide about research participation was associated with willingness to enroll the child in research [ 13 ].
Most studies of parental permission to date have focused on whether parents understood informational elements of informed consent, such as risks, benefits, and alternatives. Less is known about the voluntariness of such decisions, despite its potential importance from both an ethical and legal perspective. Previous research has indicated factors that may influence parental decision-making about research participation. These include demographic characteristics, previous experience with a similar decision, concern about upsetting medical personnel, time pressure, and the amount of information provided [ 14 - 16 ]. However, research related to parental voluntariness has generally either been qualitative or focused on whether parents understood (from an informational perspective) that participation is voluntary and they can choose to withdraw. In qualitative studies, parents of children with cancer reported high levels of distress that they perceived made discussions about research participation more difficult, and many parents perceived an inadequate discussion of the research aspects of treatment [ 17 , 18 ]. They also perceived few alternatives with respect to treatment or clinical trial enrollment [ 18 , 19 ]. Particularly in the setting of serious illness, the available options may be limited, leading some authors to conclude that voluntariness may also be compromised [ 20 , 21 ], though the ability to make a voluntary choice is not per se related to the number of treatment options.
Most studies of voluntariness have lacked a careful operational definition of the concept of voluntariness. The only validated empiric instrument to measure voluntariness is the Decision-Making Control Instrument (DMCI) [ 22 ]. The DMCI contains nine parent-reported items that assess perceived voluntariness. The instrument defined voluntariness as control over the decision about whether to agree to a research or treatment protocol. Data collected with this instrument indicate that less formal education, male gender, minority status, and not having previous experience with a similar decision was associated with lower perceived voluntariness. In a multivariate regression analysis, education, minority status, gender, external influence, and too little information remained significantly associated with voluntariness. Parents who reported lower voluntariness also perceived more external influence and time pressure, had more concern about the child’s care being negatively affected if they declined, and perceived that they had either too much or not enough information about the decision [ 23 ].
More empiric work is needed to address multiple issues relating to parental permission and voluntary decision-making. Previous research suggests that there is variability in what role parents prefer to assume in treatment decision-making, but most parents preferred shared decision-making with medical personnel instead of perceiving that they were either solely responsible or not responsible for the decision [ 24 , 25 ]. Little is understood about the relationship between decision-making autonomy and measures of understanding or voluntariness. Additionally, less is known about predictors of voluntariness in potentially vulnerable groups or whether family members or medical staff have a greater influence over perceived choices.
Limited options exist to improve the quality of permission. When the general timing of a particular event is known, it may be feasible and advisable to obtain permission in advance [ 9 , 10 ]. However, if permission is obtained during times of stress, concerns about the validity of permission are often raised [ 10 ]. In such situations, a technique such as continuous permission in which information is given to research participants at different stages in a trial may improve the quality of permission [ 26 ]. For example, investigators were able to obtain parental permission within 6 hours of delivery for the use of induced hypothermia in term infants at risk for perinatal hypoxic-ischemic brain injury [ 27 ]. A multimedia presentation has also been shown to improve parents’ perception of informed permission [ 28 ] and comprehension of information disclosed [ 29 ]. However, the initial information provided to a parent must satisfy all of the required elements for informed consent.
Child Assent
As noted earlier, assent is defined simply as a child’s affirmative agreement to participate in a clinical investigation. The definition goes on to stipulate that mere failure to object may not, absent affirmative agreement, be construed as assent. The regulations specify factors that should be taken into account when assessing capacity, including age, maturity, and psychological state. Assent may be waived if the child is judged incapable of providing it or if the intervention or procedure involved in the research holds out a prospect of direct benefit that is important to the child’s health or well-being and is only available in the context of the research. 8 This waiver of assent reflects appropriate parental authority to guide a child’s health care when a research intervention holds a prospect of direct benefit that is not otherwise available.
The regulations do not specify the informational elements required for assent. In 1978, the National Commission noted four essential elements for obtaining informed and voluntary assent in individuals with limited capacity [ 30 ]. According to their recommendations, the assenting individual must 1) know what procedures will be performed; 2) choose freely to undergo the procedures; 3) communicate this choice unambiguously; and 4) be aware of the option to withdraw. Discussing assent in the pediatric population, William Bartholome outlined four essential elements: 1) a developmentally appropriate understanding of the nature of the condition; 2) disclosure of the nature of the proposed intervention and what it will involve; 3) an assessment of the child’s understanding of the information provided and the influences that impact the child’s evaluation of the situation; and 4) a solicitation of the child’s expression of willingness to accept the intervention [ 31 ]. These elements of assent reflect the basic provisions for informed consent stated in the Belmont Report, modified to reflect the child’s developing capacity. In essence, a child should understand why he or she is being asked to participate and what will be his or her experience if he or she decides to participate. During this conversation, the investigator should assess a child’s understanding of these facts and the context in which the child is evaluating these facts. Finally, the child must agree to participate. It should be noted that these elements do not include much of the information required for parental permission, such as the reasonably foreseeable risks and benefits, and appropriate alternate procedures or courses of treatment, reinforcing the importance of parental permission for understanding the role of child assent.
Considerable disagreement remains about many fundamental components of assent, including: the age at which investigators should solicit assent from children; how to resolve disputes between children and their parents; who should be involved in the assent process; the relationship between assent and consent; the quantity and quality of information to disclose to children and their families; how much and what information children desire and need; the necessity and methods for assessing both children's understanding of disclosed information and of the assent process itself; and what constitutes an effective, practical, and realistically applicable decision-making model.
Several studies have examined comprehension of research studies by children and adolescents, using the typical elements of informed consent to assess understanding. Overall, the research demonstrated that children as young as 7 to 8 years old generally understand concrete concepts such as the freedom to withdraw, the freedom to ask questions, and the potential benefits of the research [ 32 , 33 ]. One study disagreed, however, citing poor understanding of most aspects of the studies in children younger than 9 years [ 34 ]. Comprehension of research goals and procedures, risks, and alternatives are generally not as well understood [ 32 - 35 ]. While one study reported that older children understood more of these concepts than younger children [ 32 ], another reported that chronologic age (between 7 and 20 years) was not related to knowledge of the elements of informed consent [ 33 ].
Each of these studies, however, has important methodological limitations that make the results difficult to interpret. For example, the study by Hurley et al. [ 32 ] enrolled 178 children between the ages of 8 and 12 years and was conducted in conjunction with an observational study of how children respond to peer provocation. After debriefing, the children’s perceptions of voluntary assent, their understanding of what they would be doing and why, their belief in voluntary participation and freedom to withdraw, and their comprehension of confidentiality were assessed. However, the authors note that children who had difficulty understanding the simple assent instructions were excluded from the study, and the study did not use any validated instruments to assess comprehension.
The studies by Susman [ 33 ] and Ondrusek [ 34 ] were small and enrolled children in a wider age range. Basic questions that were explored in both studies covered the purpose of the study, procedures, potential harms and benefits, and the right to withdraw. Assessments were performed using either a structured or semi-structured interview. Susman et al. primarily utilized a binary coding system, whereby the elements were coded based on whether the participant had “correct” knowledge of that element. Procedures or risks were coded yes if the participant knew 50 percent or more of the stated information. The article by Ondrusek did not specify how the responses were coded or the demographic or socioeconomic backgrounds from which the children originated. Neither study explained how the “correctness” of the participant responses were assessed or utilized validated instruments for assessing understanding.
The study by John et al. [ 35 ] utilized younger children, with a mean age of 7 years. Children were enrolled in a study examining the persistence of antibodies after receiving two different booster vaccinations for diphtheria. The follow-up study involved a single venipuncture and an assessment of their understanding of the study. The study used either closed-ended questions or open-ended questions with responses categorized. Following venipuncture, 59 percent of the children had grasped some aspect of the reasons for the venipuncture, with nearly 1/3 mentioning that the blood sample had been obtained to assess protection against various diseases.
In recent years, there have been attempts to use a more standard measurement to determine children’s understanding of research. For example, Koelch et al. [ 36 ] reported on a pilot study to adapt the MacArthur Competence Assessment Tool for Clinical Research (MacCAT-CR) for use in children. Initial studies with the instrument demonstrated that its use was practicable, that the time required was acceptable, and that interrater reliability was excellent in children. However, there is no threshold for a competence score in the MacCAT-CR, and its use has not been validated in a larger pediatric population. For the complex judgment about a person’s decision-making capacity, the threshold could be set based on its relevance to the research project and its risks. Children performed less well than parents on this test, but clinicians performing a global assessment rated all children as competent. The differences between assessment by clinicians and the low scores obtained in the MacCAT-CR suggest that investigators believe that children may be capable of assent even if they do not understand the research completely.
Among pre-adolescent children, limited data indicate that the majority of parents and a substantial number of children believe that the parent should make the decision about study participation [ 35 ], although many parents believe the child should be involved in the process. Families appear to vary in the stage at which, and degree to which, children should be involved. Factors that contribute to a greater likelihood of joint decision-making include increased age or maturity of the child, more open communication between parents and children, and decreased perceived risk of the research [ 37 ]. These data are consistent with the view that parental permission and child assent should be understood in relation to each other. Broadly, assent can be understood to evolve from a choice by young children that is largely dependent on the parent’s decision, to joint decision-making as children mature, to a largely independent decision made by an older adolescent with parental affirmation [ 4 ].
There appears to be little data in the literature that specifically measures the voluntariness of child assent or the factors that may contribute to whether children perceive their choice as voluntary. Some commentators report that voluntariness may be compromised in children due to their belief that failure to complete the study would displease others [ 34 ], but it is unclear that parental influence over a young child’s decision is inappropriate. In addition, seeking the approval of authority figures is a developmental stage that is necessary and appropriate for pre-adolescent children [ 38 ].
Adolescent Assent and Consent
Many states grant certain classes of mature adolescents the right to consent to treatments or procedures involved in a clinical investigation for some disorders or conditions. These mature minors would not meet the definition of children under § 50.3(o) and thus would not be subject to the requirements of 21 CFR 50 subpart D. Similarly, minors deemed “emancipated” by state law would be subject to the same exception. Mature or emancipated minors would be allowed to consent to participation in FDA-regulated research without the need for parental or guardian permission. Most, but not all, states allow adolescents who are minors to consent to medical care for their child, even if the adolescent is not considered mature or emancipated by state law.
A different model of assent is necessary for adolescents, because many adolescents have the capacity to understand important informational elements in a research study in a manner similar to adults. Available evidence suggests that the ability to understand medical decisions among adolescents greater than the age of 13 is similar to that of adults [ 12 , 39 , 40 ]. Adolescents as young as 12 years old may be less likely to disclose personal information if they know that their disclosure may result in a break in confidentiality [ 41 ]. However, executive function among adolescents is not fully developed, and as a result, adolescents’ judgment may be more prone to distortion than their adult counterparts [ 42 ]. Another study suggests that adolescents may have an initial tendency to underestimate the risks of genetic susceptibility research, unless asked to personalize the implications of uncertain test results, or whether and how to share results with others [ 43 ]. Adolescents questioned about a hypothetical study were more likely to provide lower risk ratings for procedures than their parents [ 44 ]. These data suggest that there remains an important role for parental permission even though adolescents may be able to assume responsibility for deciding whether to participate in research.
Estimates for the rate of concordance between adolescents and their parents on whether adolescents should participate in research range from 26 to 40 percent across a variety of protocols [ 44 , 45 ]. Adolescents appear significantly more willing than parents to enroll in above-minimal risk research [ 45 ]. Interestingly, despite this discordance, both adolescents and their parents claimed ultimate responsibility for the participation decision [ 44 ].
Compensation for Research on Children and Adolescents
Compensation for participation in research is a common practice for research studies that involve both younger children and adolescents. However, there is little research on the effects of this compensation on permission or assent. Incentive payments are often seen, however, as essential to the recruitment and retention of pediatric study subjects [ 46 ]. A number of different types of compensation for parents are used in clinical studies, including material or monetary compensation such as reimbursement for travel, parking, or inconvenience. However, there is concern that payments to parents for their child’s research participation could potentially influence parents to decide in favor of participation without regard for the child’s wishes, because there is no personal risk to the parent. However, there are no data that support this concern. The European Union prohibits inducements in pediatric trials, either for the parents, legal representatives, or children, but allows parents or legal representatives to be compensated for their time and expenses [ 47 ]. With respect to children and adolescents, the American Academy of Pediatrics recommends the giving of gifts, instead of money, as a token of appreciation after the child has completed (or withdrawn from) the trial [ 2 ], but many institutions do not appear to follow this practice [ 48 ]. While this model may be appropriate for younger children, remuneration using a wage model based on time or effort (e.g., a percentage of trial visits or procedures that have been completed) may be more appropriate for older adolescents [ 46 ].
Waiving Permission and Assent
Both parental permission and child assent may be waived under the emergency research provisions found at 21 CFR 50.24. Research on life-threatening conditions for which available treatments are unproven or unsatisfactory and where it is not possible to obtain informed consent can proceed when certain criteria are met and additional protections are in place. These protections include public disclosure, consultation with representatives of the communities in which the investigation will be conducted, and the use of a data monitoring committee. This exception has been used successfully for a pediatric resuscitation trial open to any child younger than the age of 18 [ 49 ]. A previous study of parents of children in the pediatric intensive care unit demonstrated that inpatient pediatric resuscitation research is feasible using handouts to inform parents of a study and by providing a prospective opportunity to opt out [ 50 , 51 ]. Every effort must be made to respect the parents’ involvement in such studies even if a decision must be made to initiate experimental treatment prior to obtaining fully informed permission.
HHS regulations at 45 CFR 46.408(c) allow for a waiver of parental permission if the research is designed for conditions or for a subject population for which parental or guardian permission is not a reasonable requirement to protect the subjects (for example, neglected or abused children). FDA regulations do not allow for such a waiver for FDA-regulated clinical investigations [ 52 ]. Absent an open and transparent process of establishing an appropriate surrogate decision maker, parental permission should not be waived for FDA-regulated clinical investigations. There are limited empirical data on the application of the HHS waiver [ 53 ] or on protections that are substituted for parental permission. Both FDA and HHS regulations allow assent to be waived if the research offers a therapeutic benefit to the child that would otherwise be unavailable to them. Although the intent of this waiver is to reflect the authority of parents to direct the health care of their children independent of their wishes, we know of no data on the extent and appropriateness of the use of this assent waiver.
Conclusions and Outlook
The principle of respect for persons requires that both the child, if capable, and the parent exercise voluntary choice concerning research participation. However, the doctrine of “informed consent” has only limited direct application in pediatrics. Only persons who have the appropriate capacity and are legally empowered can provide informed consent for themselves. In all other situations, parents or other surrogates provide permission for diagnosis and treatment of children with the assent of the child whenever appropriate.
Unless criteria are met for waivers of parental permission and/or child assent, both are necessary conditions for the enrollment of children in research. Parents must be provided with detailed information concerning the nature and purpose of the research, the risks and benefits that may reasonably be expected, and any alternatives to research participation. Parents may place greater weight on the preferences of children when the risks of participation are lower and when children are older or more mature. Children who are capable must affirmatively agree to participate. The amount of information that a child must comprehend to provide meaningful and developmentally appropriate child assent (or dissent) should be allowed to vary with the age and maturity of the child. Many adolescents have the capacity to understand important informational elements in a research study in a manner similar to adults. However, the age (as a proxy for developmental stage) at which a child is deemed capable of assent may be lower (i.e., 5 to 7 years old) if assent is understood as the ability to express a simple preference regarding research participation. By assuming a lack of capacity among young children, the potential arises to dishonor and disregard a child’s wishes by failing to solicit meaningful assent or dissent.
More empiric research is needed particularly with respect to the voluntariness of permission and assent. Little is understood about the relationship between decision-making autonomy and measures of understanding or voluntariness among adults, adolescents, or children. There appear to be no data on the factors that may contribute to whether children perceive their choice as voluntary. Finally, little is known about predictors of voluntariness in potentially vulnerable groups or whether family members or medical staff have a greater influence over perceived choices.
Acknowledgments
The authors wish to thank Tara White for research assistance with this article.
Abbreviations
1 See FDA regulations at 21 CFR 56.111b. For all FDA references regarding IRB approval, parental permission, and child assent, comparable regulations (45 CFR 46) exist for research that is not FDA regulated but falls under the purview of other agencies within the Department of Health and Human Services.
2 See FDA regulations at 21 CFR 50.51 and 21 CFR 50.53 for research without a direct benefit, and 21 CFR 50.52 for research that holds out the prospect of direct benefit.
3 Parental permission will be used in this article to refer to permission from either a parent or legal guardian.
4 FDA regulations do not allow a waiver of parental permission except for research that is conducted under the emergency exception from informed consent found in FDA regulations at 21 CFR 50.24. HHS regulations allow for a waiver of parental permission if the research is designed for conditions or for a subject population for which parental or guardian permission is not a reasonable requirement to protect the subjects, as noted at 45 CFR 46.408(c).
5 See 21 CFR 50.3(n) “Assent means a child's affirmative agreement to participate in a clinical investigation.”
6 21 CFR 50.3(r). As required under § 50.55(f), permission by parents or guardians must be documented in accordance with, and to the extent required by, § 50.27, and thus must include the elements of informed consent required by § 50.25.”
7 See the conditions under 21 CFR 50.55(e)(1) and (e)(2).
8 See 21 CFR 50.55 (b) regarding factors that should be assessed, and 21 CFR 50.55(c)(1) and (c)(2) for conditions under which assent may be waived.
- The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. Washington, DC: US Government Printing Office; 1979. [ PubMed ] [ Google Scholar ]
- Guidelines for the ethical conduct of studies to evaluate drugs in pediatric populations. Committee on Drugs, American Academy of Pediatrics. Pediatrics. 1995; 95 (2):286–294. [ PubMed ] [ Google Scholar ]
- Rossi WC, Reynolds W, Nelson RM. Child assent and parental permission in pediatric research. Theor Med Bioeth. 2003; 24 (2):131–148. [ PubMed ] [ Google Scholar ]
- Miller VA, Nelson RM. A developmental approach to child assent for nontherapeutic research. J Pediatr. 2006; 149 (1 Suppl):S25–S30. [ PubMed ] [ Google Scholar ]
- Howard JM, DeMets D. How informed is informed consent? The BHAT experience. Control Clin Trials. 1981; 2 (4):287–303. [ PubMed ] [ Google Scholar ]
- Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent in cancer clinical trials: a cross-sectional survey. Lancet. 2001; 358 (9295):1772–1777. [ PubMed ] [ Google Scholar ]
- Horng S, Grady C. Misunderstanding in clinical research: distinguishing therapeutic misconception, therapeutic misestimation, and therapeutic optimism. IRB. 2003; 25 (1):11–16. [ PubMed ] [ Google Scholar ]
- Sollitto S, Hoffman S, Mehlman M, Lederman RJ, Youngner SJ, Lederman MM. Intrinsic conflicts of interest in clinical research: a need for disclosure. Kennedy Inst Ethics J. 2003; 13 (2):83–91. [ PubMed ] [ Google Scholar ]
- Ballard HO, Shook LA, Desai NS, Anand KJ. Neonatal research and the validity of informed consent obtained in the perinatal period. J Perinatol. 2004; 24 (7):409–415. [ PubMed ] [ Google Scholar ]
- Mason SA, Allmark PJ. Obtaining informed consent to neonatal randomised controlled trials: interviews with parents and clinicians in the Euricon study. Lancet. 2000; 356 (9247):2045–2051. [ PubMed ] [ Google Scholar ]
- Nathan AT, Hoehn KS, Ittenbach RF, Gaynor JW, Nicolson S, Wernovsky G. et al. Assessment of parental decision-making in neonatal cardiac research: a pilot study. J Med Ethics. 2010; 36 (2):106–110. [ PubMed ] [ Google Scholar ]
- Tait AR, Voepel-Lewis T, Malviya S. Do they understand? (part I): parental consent for children participating in clinical anesthesia and surgery research. Anesthesiology. 2003; 98 (3):603–608. [ PubMed ] [ Google Scholar ]
- Hoehn KS, Nathan A, White LE, Ittenbach RF, Reynolds WW, Gaynor JW. et al. Parental perception of time and decision-making in neonatal research. J Perinatol. 2009; 29 (7):508–511. [ PubMed ] [ Google Scholar ]
- Eder ML, Yamokoski AD, Wittmann PW, Kodish ED. Improving informed consent: suggestions from parents of children with leukemia. Pediatrics. 2007; 119 (4):e849–e859. [ PubMed ] [ Google Scholar ]
- Simon C, Zyzanski SJ, Eder M, Raiz P, Kodish ED, Siminoff LA. Groups potentially at risk for making poorly informed decisions about entry into clinical trials for childhood cancer. J Clin Oncol. 2003; 21 (11):2173–2178. [ PubMed ] [ Google Scholar ]
- Nelson RM, Merz JF. Voluntariness of consent for research: an empirical and conceptual review. Med Care. 2002; 40 (9 Suppl):V69–V80. [ PubMed ] [ Google Scholar ]
- Kupst MJ, Patenaude AF, Walco GA, Sterling C. Clinical trials in pediatric cancer: parental perspectives on informed consent. J Pediatr Hematol Oncol. 2003; 25 (10):787–790. [ PubMed ] [ Google Scholar ]
- Levi RB, Marsick R, Drotar D, Kodish ED. Diagnosis, disclosure, and informed consent: learning from parents of children with cancer. J Pediatr Hematol Oncol. 2000; 22 (1):3–12. [ PubMed ] [ Google Scholar ]
- Benedict JM, Simpson C, Fernandez CV. Validity and consequence of informed consent in pediatric bone marrow transplantation: The parental experience. Pediatr Blood Cancer. 2007; 49 (6):846–851. [ PubMed ] [ Google Scholar ]
- Jacoby LH, Maloy B, Cirenza E, Shelton W, Goggins T, Balint J. The basis of informed consent for BMT patients. Bone Marrow Transplant. 1999; 23 (7):711–717. [ PubMed ] [ Google Scholar ]
- Stevens PE, Pletsch PK. Ethical issues of informed consent: mothers' experiences enrolling their children in bone marrow transplantation research. Cancer Nurs. 2002; 25 (2):81–87. [ PubMed ] [ Google Scholar ]
- Miller VA, Ittenbach RF, Harris D, Reynolds WW, Beauchamp TL, Luce MF. et al. The decision making control instrument to assess voluntary consent. Med Decis Making. 2011; 31 (5):730–741. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Miller VA, Nelson RM. Factors related to voluntary parental decision-making in pediatric oncology. Pediatrics. 2012; 129 (5):903–909. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pyke-Grimm KA, Degner L, Small A, Mueller B. Preferences for participation in treatment decision making and information needs of parents of children with cancer: a pilot study. J Pediatr Oncol Nurs. 1999; 16 (1):13–24. [ PubMed ] [ Google Scholar ]
- Tait AR, Voepel-Lewis T, Munro HM, Malviya S. Parents’ preferences for participation in decisions made regarding their child's anaesthetic care. Paediatr Anaesth. 2001; 11 (3):283–290. [ PubMed ] [ Google Scholar ]
- Allmark P, Mason S. Improving the quality of consent to randomised controlled trials by using continuous consent and clinician training in the consent process. J Med Ethics. 2006; 32 (8):439–443. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM. et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005; 365 (9460):663–670. [ PubMed ] [ Google Scholar ]
- Nwomeh BC, Hayes J, Caniano DA, Upperman JS, Kelleher KJ. A parental educational intervention to facilitate informed consent for emergency operations in children. J Surg Res. 2009; 152 (2):258–263. [ PubMed ] [ Google Scholar ]
- O’Lonergan TA, Forster-Harwood JE. Novel approach to parental permission and child assent for research: improving comprehension. Pediatrics. 2011; 127 (5):917–924. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. Research Involving Those Institutionalized as Mentally Infirm. Washington, DC: DHEW Publication No (OS) 78-0006; 1978. [ PubMed ] [ Google Scholar ]
- Bartholome WG. Hearing children’s voices. Bioethics Forum. 1995; 11 (4):3–6. [ PubMed ] [ Google Scholar ]
- Hurley JC, Underwood MK. Children’s understanding of their research rights before and after debriefing: informed assent, confidentiality, and stopping participation. Child Dev. 2002; 73 (1):132–143. [ PubMed ] [ Google Scholar ]
- Susman EJ, Dorn LD, Fletcher JC. Participation in biomedical research: the consent process as viewed by children, adolescents, young adults, and physicians. J Pediatr. 1992; 121 (4):547–552. [ PubMed ] [ Google Scholar ]
- Ondrusek N, Abramovitch R, Pencharz P, Koren G. Empirical examination of the ability of children to consent to clinical research. J Med Ethics. 1998; 24 (3):158–165. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- John T, Hope T, Savulescu J, Stein A, Pollard AJ. Children’s consent and paediatric research: is it appropriate for healthy children to be the decision-makers in clinical research? Arch Dis Child. 2008; 93 (5):379–383. [ PubMed ] [ Google Scholar ]
- Koelch M, Prestel A, Singer H, Schulze U, Fegert JM. Report of an initial pilot study on the feasibility of using the MacArthur competence assessment tool for clinical research in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2010; 20 (1):63–67. [ PubMed ] [ Google Scholar ]
- Geller G, Tambor ES, Bernhardt BA, Fraser G, Wissow LS. Informed consent for enrolling minors in genetic susceptibility research: a qualitative study of at-risk children's and parents’ views about children’s role in decision-making. J Adolesc Health. 2003; 32 (4):260–271. [ PubMed ] [ Google Scholar ]
- Santrock JW. Child Development: An Introduction. 13th ed. Boston, MA: McGraw Hill; 2011. [ Google Scholar ]
- Weithorn LA. Children’s capacities to decide about participation in research. IRB. 1983; 5 (2):1–5. [ PubMed ] [ Google Scholar ]
- Tait AR, Voepel-Lewis T, Malviya S. Do they understand? (part II): assent of children participating in clinical anesthesia and surgery research. Anesthesiology. 2003; 98 (3):609–614. [ PubMed ] [ Google Scholar ]
- Lothen-Kline C, Howard DE, Hamburger EK, Worrell KD, Boekeloo BO. Truth and consequences: ethics, confidentiality, and disclosure in adolescent longitudinal prevention research. J Adolesc Health. 2003; 33 (5):385–394. [ PubMed ] [ Google Scholar ]
- Cauffman E, Steinberg L. (Im)maturity of judgment in adolescence: why adolescents may be less culpable than adults. Behav Sci Law. 2000; 18 (6):741–760. [ PubMed ] [ Google Scholar ]
- Bernhardt BA, Tambor ES, Fraser G, Wissow LS, Geller G. Parents’ and children’s attitudes toward the enrollment of minors in genetic susceptibility research: implications for informed consent. Am J Med Genet A. 2003; 116A (4):315–323. [ PubMed ] [ Google Scholar ]
- Brody JL, Scherer DG, Annett RD, Pearson-Bish M. Voluntary assent in biomedical research with adolescents: a comparison of parent and adolescent views. Ethics Behav. 2003; 13 (1):79–95. [ PubMed ] [ Google Scholar ]
- Brody JL, Annett RD, Scherer DG, Perryman ML, Cofrin KM. Comparisons of adolescent and parent willingness to participate in minimal and above-minimal risk pediatric asthma research protocols. J Adolesc Health. 2005; 37 (3):229–335. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bagley SJ, Reynolds WW, Nelson RM. Is a “wage-payment” model for research participation appropriate for children? Pediatrics. 2007; 119 (1):46–51. [ PubMed ] [ Google Scholar ]
- Directive 2001/20/EC of 4 April 2001, of the European Parliament and of the Council on the approximation of the laws, regulations and administrative provisions of the Member States relating to implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. Med Etika Bioet. 2002; 9 (1-2):12–19. [ PubMed ] [ Google Scholar ]
- Weise KL, Smith ML, Maschke KJ, Copeland HL. National practices regarding payment to research subjects for participating in pediatric research. Pediatrics. 2002; 110 (3):577–582. [ PubMed ] [ Google Scholar ]
- Raymond TT, Carroll TG, Sales G, Morris MC. Effectiveness of the informed consent process for a pediatric resuscitation trial. Pediatrics. 2010; 125 (4):e866–e875. [ PubMed ] [ Google Scholar ]
- Morris MC, Fischbach RL, Nelson RM, Schleien CL. A paradigm for inpatient resuscitation research with an exception from informed consent. Crit Care Med. 2006; 34 (10):2567–2575. [ PubMed ] [ Google Scholar ]
- Morris MC, Nadkarni VM, Ward FR, Nelson RM. Exception from informed consent for pediatric resuscitation research: community consultation for a trial of brain cooling after in-hospital cardiac arrest. Pediatrics. 2004; 114 (3):776–781. [ PubMed ] [ Google Scholar ]
- Department of Health and Human Services Food and Drug Administration. Additional Safeguards for Children in Clinical Investigations of Food and Drug Administration-Regulated Products Final Rule. 21 CFR Parts 50 and 56. Docket No. FDA–2000–N–0009 2013 [Internet] Available from: http://www.gpo.gov/fdsys/pkg/FR-2013-02-26/pdf/2013-04387.pdf . [ PubMed ]
- Rogers AS, Schwartz DF, Weissman G, English A. Adolescent Medicine HIVARN. A case study in adolescent participation in clinical research: eleven clinical sites, one common protocol, and eleven IRBs. IRB. 1999; 21 (1):6–10. [ PubMed ] [ Google Scholar ]
Understanding Informed Consent Forms

The informed consent form puts you in control of your health decisions and protects your rights.
Before you join a cancer research study, you’ll receive an informed consent form to review, ask questions about, and sign. The form covers a description of the study’s purpose, procedures and safety measures researchers will follow, and what is expected of study participants.
The form is part of the informed consent process. This process protects your rights. It also gives you control over your choice to take part in research.
Federal law requires that researchers give the informed consent form to potential participants. You will have a chance to ask questions about information you read in the form. Once you understand the study, you can choose if you want to sign the consent form and take part.
Getting comfortable with informed consent forms
Understanding a consent form is an important part of the informed consent process. All informed consent forms are different. But most forms will have the same kinds of information about the study divided into sections.
Overview of study This section tells you:
- what question researchers are hoping to answer with the study
- the main potential risks and benefits of taking part in the study
- your responsibilities as a participant
The overview also describes the choices you have if you do not take part in the study. And it explains the reasons you might leave the study early. This section also lets you know that taking part is voluntary and you may leave the study at any time.
Study design This section describes:
- each study group
- what each group will be asked to do, including tests and procedures you will have and drugs you will take
- how many people will be in each group
- how long the study will last
Risks and benefits This section describes:
- all known potential risks and benefits of taking part in the study
- the most common side effects
- how the study will help doctors learn about your disease
Cost This section explains the costs of taking part in a study. Insurance and the study sponsor should cover some expenses. But you might have others, such as the cost of travel to the trial site. Learn more about who pays for clinical trials .

Safety and Clinical Trials
Explore the many safety measures in place to help keep you safe during a trial or study.
Your rights This section covers your rights:
- if you are injured because of the study or neglect
- to privacy when it comes to sharing your medical information
- to leave the study at any time
How to get more information on the study This section provides ways to:
- learn more about the study
- learn more about your rights as a participant
- reach the study team
Signature As with most legal documents, informed consent forms require a signature. But it’s important to remember that you are not signing away your rights or binding yourself to the study. You may still leave the study at any time. If a participant is under 18 years of age, read about the children’s assent process .
Sample informed consent form
Read a sample consent form to become familiar with the content and sections. Note: this form is for informational purposes only and does not represent a real study. Your form may have other sections, and may present the information in a different way.
Tips for reading informed consent forms
Amy Rose oversees the day-to-day operations of clinical research, including the informed consent process for potential research participants. She is the associate director in Clinical Research Services at University of Pittsburgh Medical Center Hillman Cancer Center.
If you are thinking about taking part in a clinical trial, Amy suggests that you:
- Bring a friend or relative to the appointment when your study team discusses the study and informed consent form.
- Take the form home . Read it in a comfortable place, take notes, and jot down questions for your doctor or study team. “You can highlight things and write down questions right on the consent form,” Amy said.
- Ask a close friend or spouse to read the form so you can discuss it with them. Have them take notes too, and compare your understanding. Reviewing it with your primary care provider can also be helpful, Amy said.
- Don’t be afraid to ask questions . Amy said she and other medical professionals don’t always realize when they aren’t explaining things clearly. They may be in a rush or too steeped in the language of medicine.
- You can decide not to take part before or after you sign the form. “We always tell people their participation is completely voluntary,” Amy said. “You do not have to continue just because you sign this consent today.”
The informed consent process does not end when you sign a form and decide to take part. Your doctor and study team will keep you updated about the study so you can continue to make informed decisions.

Have questions about informed consent?
Connect with our cancer information specialists.
Phone: 1-800-4-CANCER Chat: LiveHelp Email: [email protected]
Available Monday–Friday 9:00 a.m. to 9:00 p.m. ET.

IMAGES
VIDEO
COMMENTS
SAMPLE INFORMED PARENTAL CONSENT FORM . We invite you and your child to take part in a research study being conducted by [Principal Investigator's name] who is a [professor / student ] at Hampshire College, Amherst, MA, as part of his/her [ name of research project ] . The study, as well as your rights as a participant, are described below.
Please note: For research involving minors, child assent should be sought whenever possible. At times, this may entail creating a separate consent document for parents and children (each written in age-appropriate language) and each must be signed. At other times, parents may be required to make the decision for the child.
participation in research of any children under the age of 18. For research on children who are wards of the state, consult with the IRB chair. In research involving greater than minimal risk, the ... Parental Consent Form, children should be given enough information to make their own choice Research with Children, Jan. 2019 Page 4 of 9 ...
This parental consent must be o btained before the researcher can proceed to the second step - seeking the child's agreement to take part in research. ... Sample Parental Notification Form Regarding Participation in a Research Study . If you wish to allow your child to participate, please do not return this form - no action is necessary.
Oral Consent Template. Guidance for Protocols Involving Oral Consent. Debriefing Template. Guidance and Template for Debriefing Participants. Studies Involving Children (Assent/Permission Forms) Parent-Guardian Permission for Studies Involving Children. Sample Parental Notification Form. Sample Child Assent Form. Performance Release for Minors ...
PARTICIPATION: We are asking parents to support our research by giving consent for their child to take part in the study. Children will also have the opportunity to give assent to participation. Research is voluntary; only those who want to participate will be included in the study. The assent form below provides details of the study.
Permission means the agreement of parent (s) or guardian to the participation of their child or ward in research. Parent means a child's biological or adoptive parent. Guardian means an individual who is authorized under applicable State or local law to consent on behalf of a child to general medical care.
1204 Marie Mount Hall 7814 Regents Drive College Park, MD 20742 301-405-4212 [email protected]. Page 1 of 3 IRBNet Package: 480532-1. CONSENT TO PARTICIPATE. Project Title. SAMPLE PARENT PERMISSION FORM Purpose of the Study. This research is being conducted by Dr. John Jones at the University of Maryland, College Park.
PARENTAL CONSENT DOCUMENT. Your child is being asked to participate in a research study about [insert general statement about study]. Your child was selected as a possible participant because [explain how subject was identified]. In order for your child to participate, permission needs to be obtained from a parent or legal guardian.
Consent for Participation in Research. Title: [insert title of study] ... By signing the consent form, you acknowledge that you have voluntarily donated your [indicate type such as blood, saliva, etc] specimen to the University for research purposes. ... Parental Consent for Children Participation in Research Author: VPR-gn326 Last modified by ...
Introduction. The purpose of this form is to provide you (as the parent of a prospective research study participant) information that may affect your decision as to whether or not to let your child participate in this research study. The person conducting the research will describe the study to you and answer all your questions.
PARENT/LEGAL GUARDIAN CONSENT TO PARTICIPATE IN RESEARCH • The Parent/Legal Guardian Consent must be written in second person (i.e., use "you" and "your"). When combined with conditional language, utilization of the second person personalizes the consent form and reflects the existence of voluntary decision making on the part of the ...
This informed consent form is for parents of adolescent girls and boys participating in the research titled. "What do we want: Adolescents and health systems ") [Name of Principle Investigator] [Name of Organization] [Name of Sponsor] [Name of Project and Version] This Informed Consent Form has two parts: Information Sheet (to share information ...
Because children whose parents do not return consent forms are not able to participate in research, parental nonresponse is a primary factor contributing to nonrepresentative samples. In this article, we describe a set of procedures used to obtain active parental consent for child participation that resulted in a 95% return rate or consent forms.
participation in a research study. If the parent/guardian participation is limited to data collection (e.g., surveys, questionnaires), the consent may be included as an addendum to the parental permission form. If the parent/guardian participation includes medical chart review, specimen collection,
When a child who was enrolled in research with parental or guardian permission subsequently reaches the legal age of consent to the procedures involved in ongoing research, the subject's participation in the research is no longer regulated by the requirements of 45 CFR part 46.408 regarding parental or guardian permission and subject assent.
If you like, a summary of the results of the study will be sent to you. If you have any other concerns about your rights as a research participant that have not been answered by the investigators, you may contact the Smith College Institutional Review Board at [email protected] or (413) 585-3562.
Research concerned with sensitive issues and involving the participation of children is becoming more common. Federal law defines "children" as persons who have not attained the legal age for consent to treatments or procedures involved in the research, under the applicable law of the jurisdiction in which the research will be conducted. Under Pennsylvania law, persons under the age of ...
Parents consent to participation in such research reveal a variety of motives, which have been reported in several studies on autism. ... When planning research, and preparing a consent form, these issues must be kept in mind. Consent forms should be simple, open about methods and aims, and must emphasise confidentiality. Finally, participants ...
Informed consent is an essential part of ethical human subjects research. Informed consent is the process of telling potential research participants about the key elements of a research study and what their participation will involve. Institutional Review Boards and investigators are responsible for ensuring that research participants provide ...
Parental beliefs about clinical research and motives for participation are well documented. 3,4,5,6,7,8,9,10 Prior research suggests that parents believe that clinical research is necessary 3 and ...
The UT IRB has consent, parental permission and assent form templates available for download in UTRMS-IRB Library Templates. English and Spanish versions of the templates are currently available. It is recommended that researchers use the available templates for a more efficient review.
Parental Permission. Permission means the agreement of a child's parent(s) or guardian to the participation of their child or ward in a clinical investigation. 6 Parental permission is held to the same standards as informed consent and is required (absent a waiver) for research involving children. The disclosure of information required for voluntary and informed parental permission is the ...
Understanding Informed Consent Forms. The informed consent form puts you in control of your health decisions and protects your rights. Before you join a cancer research study, you'll receive an informed consent form to review, ask questions about, and sign. The form covers a description of the study's purpose, procedures and safety measures ...