
- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books

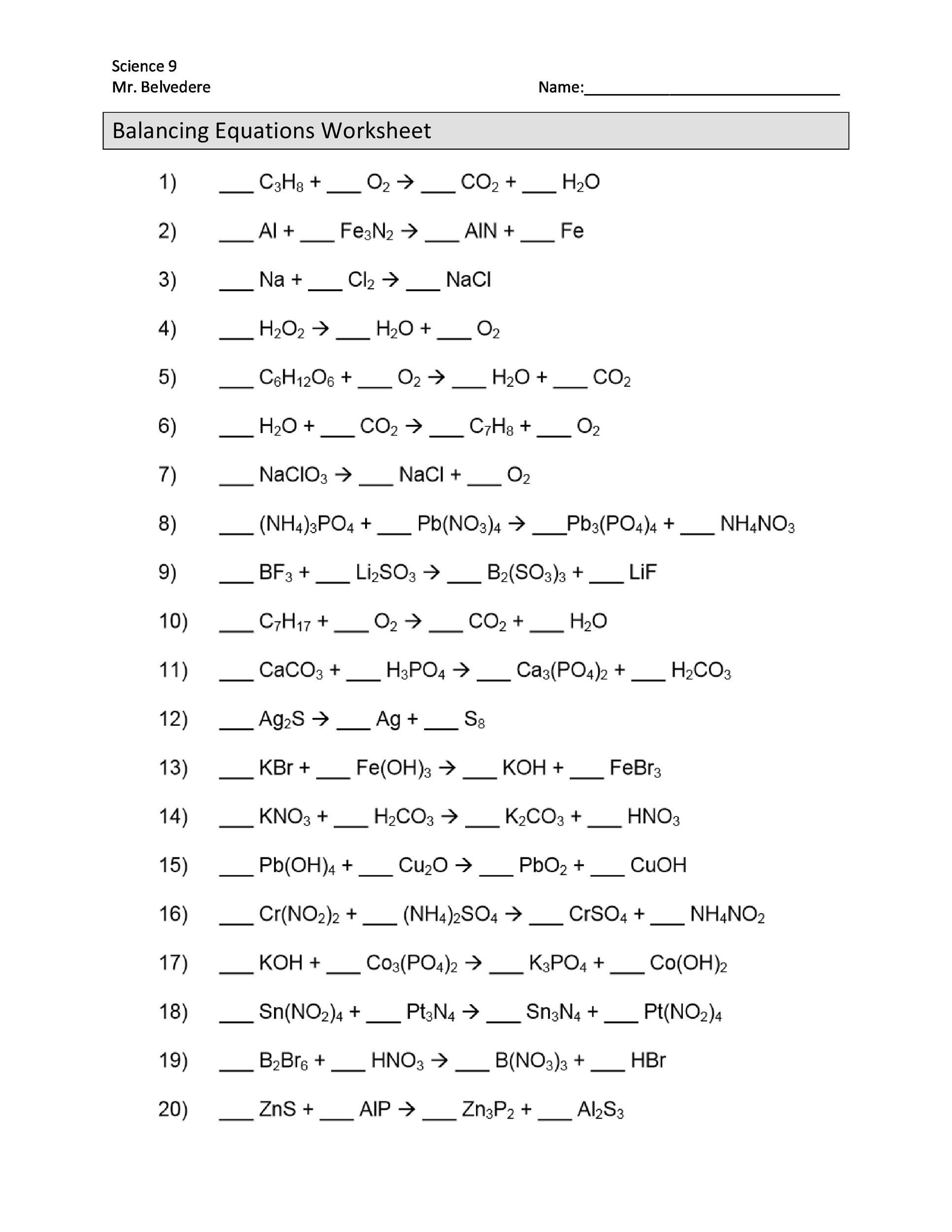
Balancing Chemical Equations Worksheet
The best way to become proficient at balancing chemical equations is practice. This balancing chemical equations worksheet has ten unbalanced equations to practice your skills.

Either right-click and save the image or else download the PDF of the worksheet here . The worksheet prints on a standard sheet of printer paper.
Balancing Chemical Equations Worksheet – Answer Key

Grab the solution by right-clicking and saving the image or by downloading the PDF of the answer key .
More Balancing Equations Worksheets
Check out our other Balancing Chemical Equation Worksheets
- Balancing Chemical Equations Practice Sheet
- Balance Chemical Equations Worksheet
- Balance Chemical Equations Practice Sheet
We also have other chemistry worksheets and handouts .
Related Posts
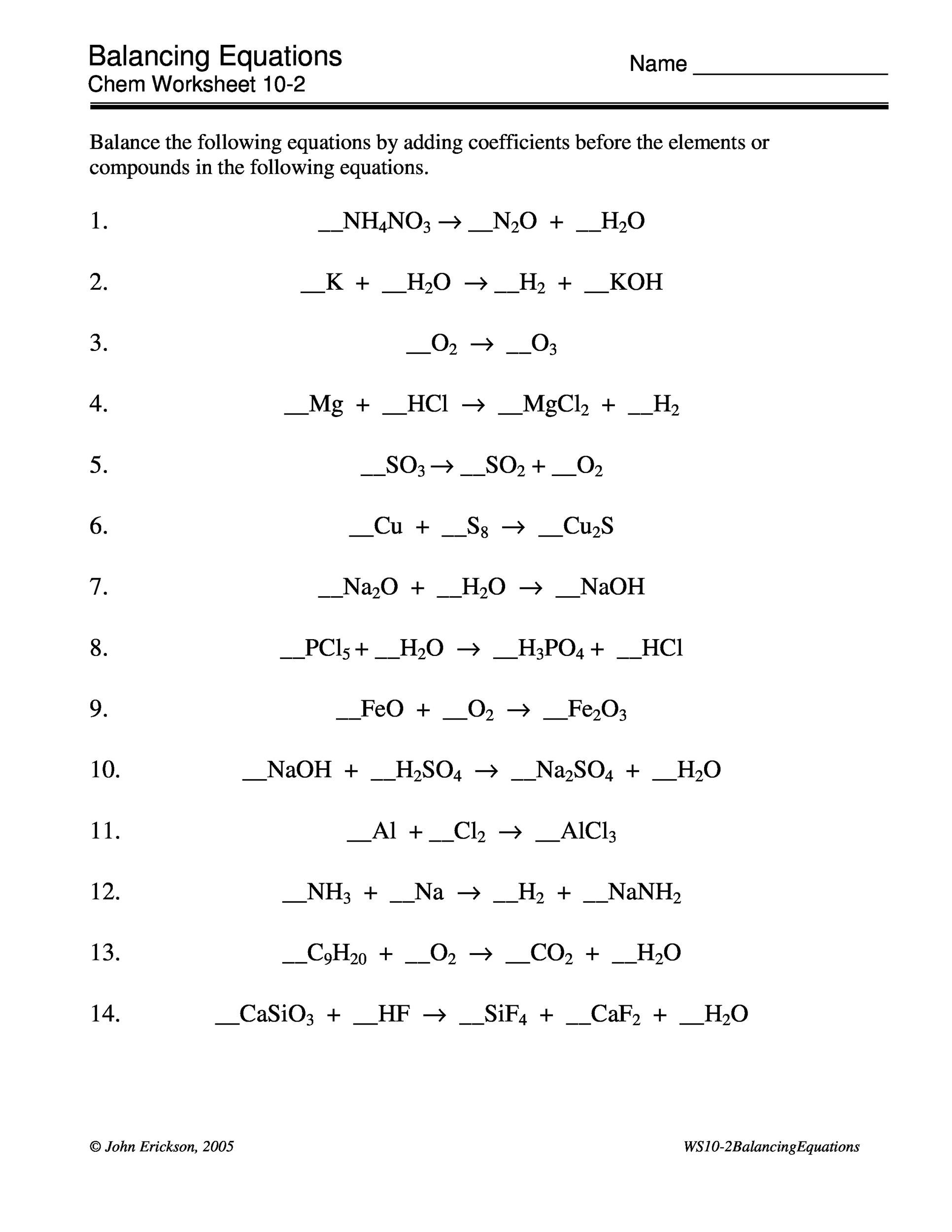
How to Balance Equations - Printable Worksheets
Balancing Equations Worksheets
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
A balancing chemical equation worksheet helps you find the number and type of atoms participating in a reaction, the reactants, products, and direction of the reaction. Balancing an unbalanced equation is mostly a matter of making certain mass and charge are balanced on the reactants and products side of the reaction arrow. The printable balancing chemical equations worksheets are provided in PDF format with separate answer keys.
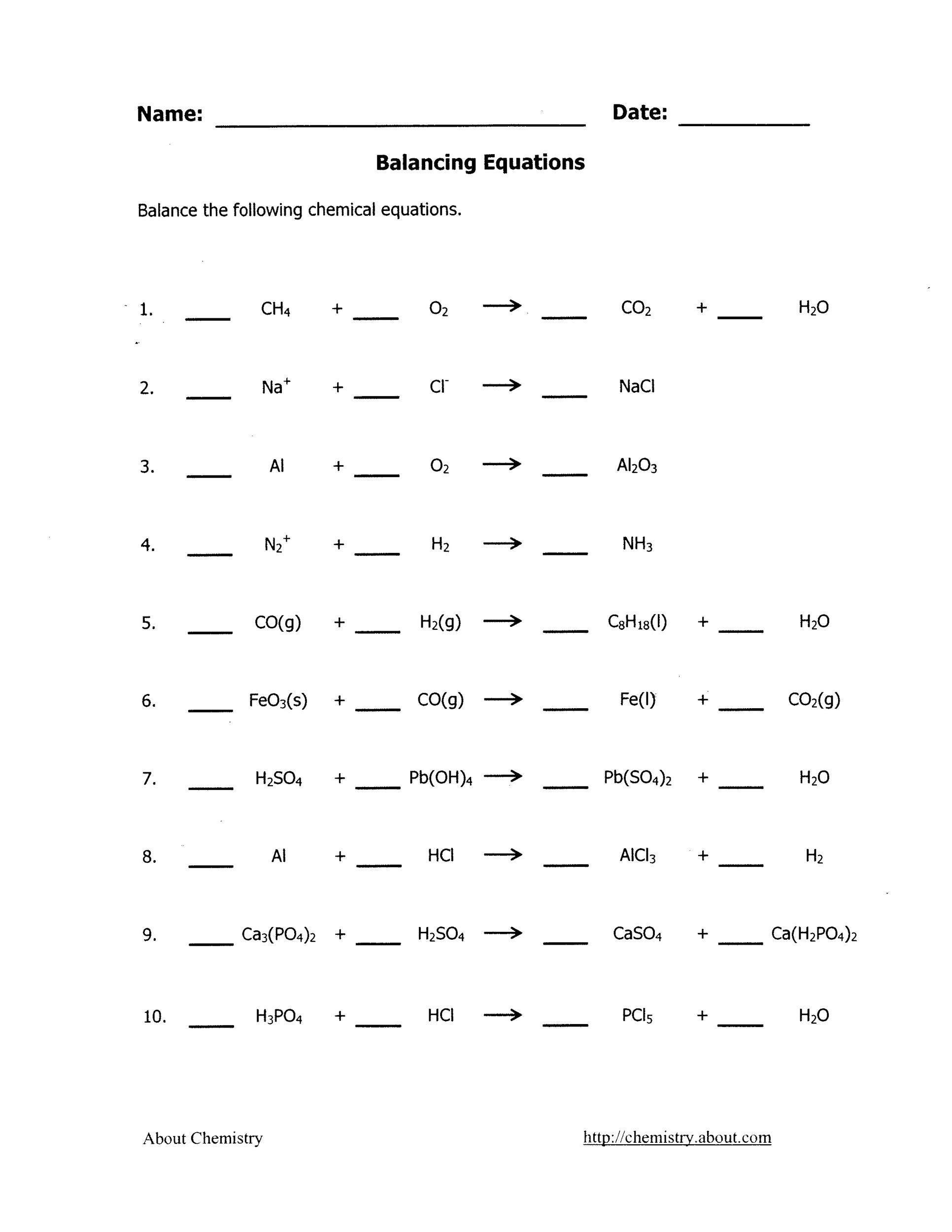
Balancing Chemical Equations - Worksheet #1 Balancing Chemical Equations - Answers #1 Balancing Chemical Equations - Worksheet #2 Balancing Chemical Equations - Answers #2 Balancing Chemical Equations - Worksheet #3 Balancing Chemical Equations - Answers #3 Balancing Equations - Worksheet #4 Balancing Equations - Answer Key #4
I also offer balancing chemical equations worksheets on my personal site. The printables are also available as PDF files:
Balancing Equation Practice Sheet [ answer sheet ] Another Equation Worksheet [ answer sheet ] Yet Another Printable Worksheet [ answer key ]
You may also wish to review the step-by-step tutorial on how to balance a chemical equation .
Online Practice Quizzes for Balancing Chemical Equations
Another way to practice balancing equations is by taking a quiz.
Coefficients in Balanced Equations Quiz Balance Chemical Equations Quiz
- What Happens to Candle Wax When a Candle Burns
- General Chemistry Topics
- Ionic vs. Covalent Bonds: How Are They Different?
- Chemical Properties of Matter
- Examples of Physical Changes
- What Is the Difference Between Oxidation and Reduction?
- Types of Chemical Reactions
- Actual Yield Definition (Chemistry)
- Understanding Endothermic and Exothermic Reactions
- 10 Examples of Heterogeneous and Homogeneous Mixtures
- What Is Muriatic Acid? Facts and Uses
- What Is Glacial Acetic Acid?
- Synthesis Reaction Description Plus Examples
- The Difference Between a Cation and an Anion
- The Molecular Formula for Water
- The Difference Between Alcohol and Ethanol
- TemplateLab
- Balancing Equations Worksheets
49 Balancing Chemical Equations Worksheets [with Answers]
Do you find balancing the chemical equation a daunting task? If yes, then you may also get confused playing with the molecules and atoms. You have to balance the chemical equation no matter what, as per the Law of Conservation of Matter, but many students find it difficult to balance it. Balancing requires a lot of practice, knowledge of reactions, formulae, valances, symbols, and techniques. Often, students lose hope and struggle to solve it. If you are struggling as well, then all you need balancing equations worksheet with answers.
Table of Contents
- 1 What is a Chemical Equation?
- 2 Balancing Chemical Equations Worksheets
- 3 Why is it Important to Balance the Chemical Equations?
- 4 Balancing Equations Worksheets with Answers
- 5 What are Different Types of Chemical Equations?
- 6 Balancing Equations Practice Worksheet
- 7 How to Balance a Chemical Equation?
- 8 Methods You Can Use for Balancing Chemical Equation
- 9 Tips to Balance Chemical Equations
- 10 Limitations of Chemical Equation
- 11 Final Thought
Understanding the methods and tips can make it easier for you to balance the chemical equation. When you balance the equation, it automatically establishes a mathematical relationship between products and reactants. If you often get confused in balancing the chemical equations, explore some ins and outs and tips for balancing the chemical equation in the article.
What is a Chemical Equation?
A chemical equation is the symbol in Chemistry that represents chemical reaction with the help of chemical formulas. It contains the chemical substances that are involved in the reaction. It contains reactants and products. The reactants are the elements that react with one and another in a chemical reaction, while the products are the elements that we get after the reaction.
The chemical equation has the products on the right side, while the reactants are written on the left side. Both of them are separated by an arrow. For instance, 2H2 + O2 -> 2H20 denotes that there are four atoms of hydrogen and 2 atoms of oxygen on both sides of the equation. The amount of reactants must be equal to the amount of products. When students get big chemical equations in a balancing equation worksheet, they often find it to be very difficult. We will help you understand through some tips in this article too, to help you get through the process seamlessly.
Balancing Chemical Equations Worksheets

Why is it Important to Balance the Chemical Equations?
When you are stuck in balancing chemical equations, you may often wonder why you are doing so. Some students do not bother and just balance it because they are told to do so, but some of them try being logical and want to know the actual reason behind balancing it. It is necessary to balance it because there must be equal number of atoms on both the sides of the equation. Also, it needs to be balanced from both sides, due to the Law of the Conservation of Mass.
The law states that there should be an equal quantity of both before and after the experiment, ensuring the quantity and quality remains the same. This law was established by Antoine Laurent in 1789. He explored that the matter either cannot be destroyed or created. Moreover, equations need to be balanced properly because unequal equations are not correct equations. No matter if they have correct elements and quantities, they will not be considered accurate. Also, these unbalanced equations cannot be used in calculating the chemical reactions .
In addition to this, chemical equations need to be balanced even because chemicals will not react until you have added the correct mole rations. Additionally, balanced equation is necessary in determining how much reactant you would need to have, for making the specific product. This simply means that the correct products will not be formed unless you add the right amount of reactants.
Some students really find the balancing equations difficult in balancing equations worksheet. It is hard and may require struggle but all you need to do is practice, have patience and need to have good memory. At first, you may face difficulties but you need to keep on working hard and surely you will succeed. We will be explaining the tips below in our further section, but here are brief ones. You need to learn reactions and write formulae of reactants. Understand the concept and balance the equation. Once you understand the concept, you will be surprised at how easy balancing will become for you. It may seem hard to believe right now, but keep working on these equation, and they will suddenly just click. Once you understand the logic behind them, there’s no stopping you.
Balancing Equations Worksheets with Answers
What are Different Types of Chemical Equations?
Before we help you in understanding the tips and tricks of balancing equations, you first need to know the types of chemical equations. Basically, there are five types of chemical equations and their reactions. Check them out below.
Combination or Synthesis Chemical Reaction
This is the most common type of chemical equation. In this chemical equation, a new product is formed by combining two to three combinations of reactants. For instance, H 2 + O 2 H 2 O. This is a chemical equation where two atoms of hydrogen are combined to form a product, water. This is why this reaction is called as synthesis reaction. Additionally, this is also an unequal equation because there are two atoms present for the oxygen on the reactant side while there are is only one atom on the oxygen side for product. But the equation is only valid when the number of atoms and moles are equal on both sides. You can balance the equation using the combustion method which will be explained later.
Decomposition Chemical Reaction
Decomposition chemical reaction is the reaction where only one compound decomposes and results in two or more than two products. Pb(No 3 ) 2 PbO + NO 2 + O 2 . In this equation, lead nitrate is being decomposed, which breaks down to form nitrogen dioxide, oxygen, and lead oxide. This is an example of a decomposition reaction.
Displacement or Replacement Reaction
Another very common chemical reaction is of two types, i.e. single displacement and double displacement. In single displacement reaction, any one chemical partner exchanges from reactants to products while two sets of chemical partners exchanges from reactants to products. An example for single displacement reaction is XY + Z XZ + Y.
In this example, zinc will replace hydrogen from sulfuric acid to form zinc sulfate. As you see, only one cation is being swapped here, that means that it is a single displacement reaction. Continuing the similar example, in the second displacement chemical equation, BaCl 2 + NaSO 4 BaSO 4 + 2NaCl would be the equation. In this equation, chloride ion leaves Barium and attaches to sodium.
Combustion Reaction
This is the chemical reaction where an oxygen compound and carbon compound combine together to become H 2 O and CO 2 . It is the reaction where mostly an organic compound like oxygen burns yielding to water, carbon dioxide, or some other product. The combination of any substance with oxygen results in combustion.
Acid Base Reaction
This is the simple chemical reaction where acid and base are combined together to provide water and salt. This reaction is also called as neutralization reaction and most commonly called as acid-base reaction. These are really important type of reactions that occur in biological systems.
Balancing Equations Practice Worksheet
How to Balance a Chemical Equation?
When students often get frustrated, they opt for balancing chemical equations worksheet answers to resolve the problem. If you also find difficulty in balancing the chemical equations, follow the steps below.
Step # 1: Write Down the Unbalanced Equation
The first step to balance the equation is to write down the chemical formula of reactants that are listed on the left side of the chemical equation. After this, you can list down the products on the right hand side of the chemical equation. There is an arrow between the sides, signaling the direction the reaction is happening in. Once you have gathered the unbalanced data, it will help you in balancing the equation.
Step # 2: Balance the Equation
Now it is time to apply the law of conservation of mass. This law states that the same number of atoms should be present on both sides of the chemical equation. One of the easiest ways to balance the chemical equation is to look for an element that has only one reactant and product. Once that one element is balanced, you can proceed towards balancing the other one. In this way, you can keep moving to others until all the elements are balanced.
By placing the co-efficient in front of them, you can balance the chemical formulas. Often people get confused and add subscripts, which entirely changes the formula. There are three basic methods to balance the chemical equation. We will be explaining each one of them below in our further section. You can any one of those looking at the type of chemical equation.
Step # 3: Indicating the States of Matter
Lastly, you need to indicate the states of matter of the products and reactants. You can use g for gaseous substances. You can use l for liquids and s for solids. If you find species in solution of water, use aq for that.

Methods You Can Use for Balancing Chemical Equation
There are two different types of methods that are commonly used for balancing chemical equations. Check them out below.
Combustion Reaction Method
This is the type of method that is used to balanced equations that have oxygen on both sides. Often, these are difficult to balance. When you find difficulty in balancing the equation in the balancing chemical equations worksheet, you can miss it with a fraction of ½ and that will easily balance the equation. But the problem is that you cannot have a fraction for the co-efficient, this is why doubling all coefficients will help you balance the equation.
Proportion Method
This is the second type of method that can be used to balance the equation. It is used when the chemical equation is difficult to inspect. If you do not understand the equation after a few minutes, use the proportion method. Ensure to change the value of co-efficient and not subscript.
Tips to Balance Chemical Equations
If you also get perplexed in balancing chemical equations, follow the tips for correct balancing chemical equations worksheet answers.
- Tip # 1: When you are trying to balance the chemical equations, you should remember that you can only change the value of coefficient in front of the element or compound, and not the subscript.
- Tip # 2: You should remember that polyatomic ions should be balanced as a whole. For instance, SO 4 should be balanced as a whole instead of Oxygen and Sulfur separately.
- Tip # 3: You should remember to balance that number first that has the greatest number of atoms in any product or reactant. Ensure that these elements are other than oxygen and hydrogen.
- Tip # 4: You should count the number of atoms of each element on both sides and see whether or not the equation is balanced.
- Tip # 5: When you successfully balance the equation, ensure to check the co-efficient. It should be in their lowest term.
Limitations of Chemical Equation
There are certain limitations for chemical equations listed as under.
- There are some chemical equations that do not clarify the state of substances. Therefore, you can add g for gas, l for liquid, s for solid and vap for vapor.
- The chemical equation does not give any information regarding the speed of reaction.
- Sometimes, the chemical equation also do not give the concentration of the substances, this is why terms like concentrated and diluted are used.
- Chemical equation will not tell if the final product would have color change or discoloration. This is why it has to be mentioned separately.
- The chemical equation also does not give any information about the speed of the reaction.
- Some chemical equations and reactions have diverse affect.
Final Thought
Students likely find difficulty in balancing chemical equations worksheet. To help you resolve this issue, we have balancing equations worksheet with answers on our main website. You can simply download it and cross-check your chemical equations. Practice for your exam using these worksheets and give your best. Good luck!
More Templates

Law School Letters Of Recommendation

Community Service Forms
Genogram Templates

Permission Slip Templates
Story Map Templates

Study Plan Templates

Balancing Chemical Equations: Explanation, Review, and Examples
- The Albert Team
- Last Updated On: March 14, 2023
Of all the skills to know about in chemistry, balancing chemical equations is perhaps the most important to master. So many parts of chemistry depend on this vital skill, including stoichiometry, reaction analysis, and lab work. This comprehensive guide will show you the steps to balance even the most challenging reactions and will walk you through a series of examples, from simple to complex.
The Key to Balancing Chemical Equations
The ultimate goal for balancing chemical equations is to make both sides of the reaction, the reactants and the products, equal in the number of atoms per element. This stems from the universal law of the conservation of mass, which states that matter can neither be created nor destroyed. So, if we start with ten atoms of oxygen before a reaction, we need to end up with ten atoms of oxygen after a reaction. This means that chemical reactions do not change the actual building blocks of matter; rather, they just change the arrangement of the blocks. An easy way to understand this is to picture a house made of blocks. We can break the house apart and build an airplane, but the color and shape of the actual blocks do not change.
But how do we go about balancing these equations? We know that the number of atoms of each element needs to be the same on both sides of the equation, so it is just a matter of finding the correct coefficients (numbers in front of each molecule) to make that happen. It is best to start with the atom that shows up the least number of times on one side, and balance that first. Then, move on to the atom that shows up the second least number of times, and so on. In the end, make sure to count the number of atoms of each element on each side again, just to be sure.
Example of Balancing a Chemical Equation
Let’s illustrate this with an example by balancing this chemical equation:
P 4 O 10 + H 2 O → H 3 PO 4
First, let’s look at the element that appears least often. Notice that oxygen occurs twice on the left-hand side, so that is not a good element to start out with. We could either start with phosphorus or hydrogen, so let’s start with phosphorus. There are four atoms of phosphorus on the left-hand side, but only one on the right-hand side. So, we can put the coefficient of 4 on the molecule that has phosphorous on the right-hand side to balance them out.
P 4 O 10 + H 2 O → 4 H 3 PO 4
Now we can check hydrogen. We still want to avoid balancing oxygen, because it occurs in more than one molecule on the left-hand side. It is easiest to start with molecules that only appear once on each side. So, there are two molecules of hydrogen on the left-hand side and twelve on the right-hand side (notice that there are three per molecule of H 3 PO 4 , and we have four molecules). So, to balance those out, we have to put a six in front of H 2 O on the left.
P 4 O 10 + 6 H 2 O → 4 H 3 PO 4
At this point, we can check the oxygens to see if they balance. On the left, we have ten atoms of oxygen from P 4 O 10 and six from H 2 O for a total of 16. On the right, we have 16 as well (four per molecule, with four molecules). So, oxygen is already balanced. This gives us the final balanced equation of
Balancing Chemical Equations Practice Problems
Try to balance these ten equations on your own, then check the answers below. They range in difficulty level, so don’t get discouraged if some of them seem too hard. Just remember to start with the element that shows up the least, and proceed from there. The best way to approach these problems is slowly and systematically. Looking at everything at once can easily get overwhelming. Good luck!
- CO 2 + H 2 O → C 6 H 12 O 6 + O 2
- SiCl 4 + H 2 O → H 4 SiO 4 + HCl
- Al + HCl → AlCl 3 + H 2
- Na 2 CO 3 + HCl → NaCl + H 2 O + CO 2
- C 7 H 6 O 2 + O 2 → CO 2 + H 2 O
- Fe 2 (SO 4 ) 3 + KOH → K 2 SO 4 + Fe(OH) 3
- Ca 3 (PO 4 ) 2 + SiO 2 → P 4 O 10 + CaSiO 3
- KClO 3 → KClO 4 + KCl
- Al 2 (SO 4 ) 3 + Ca(OH) 2 → Al(OH) 3 + CaSO 4
- H 2 SO 4 + HI → H 2 S + I 2 + H 2 O
Complete Solutions:
1. co 2 + h 2 o → c 6 h 12 o 6 + o 2.
The first step to balancing chemical equations is to focus on elements that only appear once on each side of the equation. Here, both carbon and hydrogen fit this requirement. So, we will start with carbon. There is only one atom of carbon on the left-hand side, but six on the right-hand side. So, we add a coefficient of six to the carbon-containing molecule on the left.
6CO 2 + H 2 O → C 6 H 12 O 6 + O 2
Next, let’s look at hydrogen. There are two hydrogen atoms on the left and twelve on the right. So, we will add a coefficient of six on the hydrogen-containing molecule on the left.
6CO 2 + 6H 2 O → C 6 H 12 O 6 + O 2
Now, it is time to check the oxygen. There are a total of 18 oxygen molecules on the left (6×2 + 6×1). On the right, there are eight oxygen molecules. Now, we have two options to even out the right-hand side: We can either multiply C 6 H 12 O 6 or O 2 by a coefficient. However, if we change C 6 H 12 O 6 , the coefficients for everything else on the left-hand side will also have to change, because we will be changing the number of carbon and hydrogen atoms. To prevent this, it usually helps to only change the molecule containing the fewest elements; in this case, the O 2 . So, we can add a coefficient of six to the O 2 on the right. Our final answer will be:
6CO 2 + 6H 2 O → C 6 H 12 O 6 + 6O 2
2. SiCl 4 + H 2 O → H 4 SiO 4 + HCl
The only element that occurs more than once on the same side of the equation here is hydrogen, so we can start with any other element. Let’s start by looking at silicon. Notice that there is only one atom of silicon on either side, so we do not need to add any coefficients yet. Next, let’s look at chlorine. There are four chlorine atoms on the left side and only one on the right. So, we will add a coefficient of four on the right.
SiCl 4 + H 2 O → H 4 SiO 4 + 4HCl
Next, let’s look at oxygen. Remember that we first want to analyze all the elements that only occur once on one side of the equation. There is only one oxygen atom on the left, but four on the right. So, we will add a coefficient of four on the left-hand side of the equation.
SiCl 4 + 4H 2 O → H 4 SiO 4 + 4HCl
We are almost done! Now, we just have to check the number of hydrogen atoms on each side. The left has eight and the right also has eight, so we are done. Our final answer is
As always, make sure to double-check that the number of atoms of each element balances on each side before continuing.
3. Al + HCl → AlCl 3 + H 2
This problem is a bit tricky, so be careful. Whenever a single atom is alone on either side of the equation, it is easiest to start with that element. So, we will start by counting the aluminum atoms on both sides. There is one on the left and one on the right, so we do not need to add any coefficients yet. Next, let’s look at hydrogen. There is also one on the left, but two on the right. So, we will add a coefficient of two on the left.
Al + 2HCl → AlCl 3 + H 2
Next, we will look at chlorine. There are now two on the left, but three on the right. Now, this is not as straightforward as just adding a coefficient to one side. We need the number of chlorine atoms to be equal on both sides, so we need to get two and three to be equal. We can accomplish this by finding the lowest common multiple. In this case, we can multiply two by three and three by two to get the lowest common multiple of six. So, we will multiply 2HCl by three and AlCl 3 by two:
Al + 6HCl → 2AlCl 3 + H 2
We have looked at all the elements, so it is easy to say that we are done. However, always make sure to double-check. In this case, because we added a coefficient to the aluminum-containing molecule on the right-hand side, aluminum is no longer balanced. There is one on the left but two on the right. So, we will add one more coefficient.
2Al + 6HCl → 2AlCl 3 + H 2
We are not quite done yet. Looking over the equation one final time, we see that hydrogen has also been unbalanced. There are six on the left but two on the right. So, with one final adjustment, we get our final answer:
2Al + 6HCl → 2AlCl 3 + 3H 2
4. Na 2 CO 3 + HCl → NaCl + H 2 O + CO 2
Hopefully, by this point, balancing equations is becoming easier and you are getting the hang of it. Looking at sodium, we see that it occurs twice on the left, but once on the right. So, we can add our first coefficient to the NaCl on the right.
Na 2 CO 3 + HCl → 2NaCl + H 2 O + CO 2
Next, let’s look at carbon. There is one on the left and one on the right, so there are no coefficients to add. Since oxygen occurs in more than one place on the left, we will save it for last. Instead, look at hydrogen. There is one on the left and two on the right, so we will add a coefficient to the left.
Na 2 CO 3 + 2HCl → 2NaCl + H 2 O + CO 2
Then, looking at chlorine, we see that it is already balanced with two on each side. Now we can go back to look at oxygen. There are three on the left and three on the right, so our final answer is
5. C 7 H 6 O 2 + O 2 → CO 2 + H 2 O
We can start balancing this equation by looking at either carbon or hydrogen. Looking at carbon, we see that there are seven atoms on the left and only one on the right. So, we can add a coefficient of seven on the right.
C 7 H 6 O 2 + O 2 → 7CO 2 + H 2 O
Then, for hydrogen, there are six atoms on the left and two on the right. So, we will add a coefficient of three on the right.
C 7 H 6 O 2 + O 2 → 7CO 2 + 3H 2 O
Now, for oxygen, things will get a little tricky. Oxygen occurs in every molecule in the equation, so we have to be very careful when balancing it. There are four atoms of oxygen on the left and 17 on the right. There is no obvious way to balance these numbers, so we must use a little trick: fractions. Now, when balancing chemical equations, we cannot include fractions as it is not proper form, but it sometimes helps to use them to solve the problem. Also, try to avoid over-manipulating organic molecules. You can easily identify organic molecules, otherwise known as CHO molecules, because they are made up of only carbon, hydrogen, and oxygen. We don’t like to work with these molecules, because they are rather complex. Also, larger molecules tend to be more stable than smaller molecules, and less likely to react in large quantities.
So, to balance out the four and seventeen, we can multiply the O 2 on the left by 7.5. That will give us
C 7 H 6 O 2 + 7.5O 2 → 7CO 2 + 3H 2 O
Remember, fractions (and decimals) are not allowed in formal balanced equations, so multiply everything by two to get integer values. Our final answer is now
2C 7 H 6 O 2 + 15O 2 → 14CO 2 + 6H 2 O
6. Fe 2 (SO 4 ) 3 + KOH → K 2 SO 4 + Fe(OH) 3-
We can start by balancing the iron on both sides. The left has two while the right only has one. So, we will add a coefficient of two to the right.
Fe 2 (SO 4 ) 3 + KOH → K 2 SO 4 + 2Fe(OH) 3-
Then, we can look at sulfur. There are three on the left, but only one on the right. So, we will add a coefficient of three to the right-hand side.
Fe 2 (SO 4 ) 3 + KOH → 3K 2 SO 4 + 2Fe(OH) 3-
We are almost done. All that is left is to balance the potassium. There is one atom on the left and six on the right, so we can balance these by adding a coefficient of six. Our final answer, then, is
Fe 2 (SO 4 ) 3 + 6KOH → 3K 2 SO 4 + 2Fe(OH) 3-
7. Ca 3 (PO 4 ) 2 + SiO 2 → P 4 O 10 + CaSiO 3
Looking at calcium, we see that there are three on the left and one on the right, so we can add a coefficient of three on the right to balance them out.
Ca 3 (PO 4 ) 2 + SiO 2 → P 4 O 10 + 3CaSiO 3
Then, for phosphorus, we see that there are two on the left and four on the right. To balance these, add a coefficient of two on the left.
2Ca 3 (PO 4 ) 2 + SiO 2 → P 4 O 10 + 3CaSiO 3
Notice that by doing so, we changed the number of calcium atoms on the left. Every time you add a coefficient, double check to see if the step affects any elements you have already balanced. In this case, the number of calcium atoms on the left has increased to six while it is still three on the right, so we can change the coefficient on the right to reflect this change.
2Ca 3 (PO 4 ) 2 + SiO 2 → P 4 O 10 + 6CaSiO 3
Since oxygen occurs in every molecule in the equation, we will skip it for now. Focusing on silicon, we see that there is one on the left, but six on the right, so we can add a coefficient to the left.
2Ca 3 (PO 4 ) 2 + 6SiO 2 → P 4 O 10 + 6CaSiO 3
Now, we will check the number of oxygen atoms on each side. The left has 28 atoms and the right also has 28. So, after checking that all the other atoms are the same on both sides as well, we get a final answer of
8. KClO 3 → KClO 4 + KCl
This problem is particularly tricky because every atom, except oxygen, occurs in every molecule in the equation. So, since oxygen appears the least number of times, we will start there. There are three on the left and four on the right. To balance these, we find the lowest common multiple; in this case, 12. By adding a coefficient of four on the left and three on the right, we can balance the oxygens.
4KClO 3 → 3KClO 4 + KCl
Now, we can check potassium and chlorine. There are four potassium molecules on the left and four on the right, so they are balanced. Chlorine is also balanced, with four on each side, so we are finished, with a final answer of
9. Al 2 (SO 4 ) 3 + Ca(OH) 2 → Al(OH) 3 + CaSO 4
We can start here by balancing the aluminum atoms on both sides. The left has two molecules while the right only has one, so we will add a coefficient of two on the right.
Al 2 (SO 4 ) 3 + Ca(OH) 2 → 2Al(OH) 3 + CaSO 4
Now, we can check sulfur. There are three on the left and only one on the right, so adding a coefficient of three will balance these.
Al 2 (SO 4 ) 3 + Ca(OH) 2 → 2Al(OH) 3 + 3CaSO 4
Moving right along to calcium, there is only one on the left but three on the right, so we should add a coefficient of three.
Al 2 (SO 4 ) 3 + 3Ca(OH) 2 → 2Al(OH) 3 + 3CaSO 4
Double-checking all the atoms, we see that all the elements are balanced, so our final equation is
10. H 2 SO 4 + HI → H 2 S + I 2 + H 2 O
Since hydrogen occurs more than once on the left, we will temporarily skip it and move to sulfur. There is one atom on the left and one on the right, so there is nothing to balance yet. Looking at oxygen, there are four on the left and one on the right, so we can add a coefficient of four to balance them.
H 2 SO 4 + HI → H 2 S + I 2 + 4H 2 O
There is only one iodine on the left and two on the right, so a simple coefficient change can balance those.
H 2 SO 4 + 2HI → H 2 S + I 2 + 4H 2 O
Now, we can look at the most challenging element: hydrogen. On the left, there are four and on the right, there are ten. So, we know we have to change the coefficient of either H 2 SO 4 or HI. We want to change something that will require the least amount of tweaking afterwards, so we will change the coefficient of HI. To get the left-hand side to have ten atoms of hydrogen, we need HI to have eight atoms of hydrogen, since H 2 SO 4 already has two. So, we will change the coefficient from 2 to 8.
H 2 SO 4 + 8HI → H 2 S + I 2 + 4H 2 O
However, this also changes the balance for iodine. There are now eight on the left, but only two on the right. To fix this, we will add a coefficient of 4 on the right. After checking that everything else balances out as well, we get a final answer of
H 2 SO 4 + 8HI → H 2 S + 4I 2 + 4H 2 O
As with most skills, practice makes perfect when balancing chemical equations. Keep working hard and try to do as many problems as you can to help you hone your balancing skills.
Interested in a school license?
Popular posts.

AP® Score Calculators
Simulate how different MCQ and FRQ scores translate into AP® scores

AP® Review Guides
The ultimate review guides for AP® subjects to help you plan and structure your prep.

Core Subject Review Guides
Review the most important topics in Physics and Algebra 1 .

SAT® Score Calculator
See how scores on each section impacts your overall SAT® score

ACT® Score Calculator
See how scores on each section impacts your overall ACT® score

Grammar Review Hub
Comprehensive review of grammar skills

AP® Posters
Download updated posters summarizing the main topics and structure for each AP® exam.
Balancing Chemical Equations (practice, answer key & graphic organizer

- Word Document File
Description
Questions & answers.
- We're hiring
- Help & FAQ
- Privacy policy
- Student privacy
- Terms of service
- Tell us what you think

- Sign in / Register
- Administration
- Edit profile

The PhET website does not support your browser. We recommend using the latest version of Chrome, Firefox, Safari, or Edge.

IMAGES
COMMENTS
Balancing Chemical Equations - Answer Key. Balance the equations below: N2 + 3 H2 Æ 2 NH3. KClO3 Æ 2 KCl + 3 O2. 2 NaCl + 1 F2 Æ 2 NaF + 1 Cl2. 2 H2 + 1 O2 Æ 2 H2O. Pb(OH)2 + 2 HCl Æ 2 H2O + 1 PbCl2. AlBr3 + 3 K2SO4 Æ 6 KBr + 1 Al2(SO4)3. CH4 + 2 O2 Æ 1 CO2 + 2 H2O.
Balancing Equations: Practice Problems 1. Balance each of the following equations. (a) ... Balancing Equations: Answers to Practice Problems 1. Balanced equations. (Coefficients equal to one (1) do not need to be shown in your answers). ... Balance the following chemical equations. 1. Fe + H 2S0 4: Fe 2(SO 4) 3 + H 2: 2. C 2H 6 + O 2: H 2O ...
Balance the following chemical equation: Mg (OH) 2 + HCl → MgCl 2 + H 2 O. Note: All reactants and products require a coefficient of at least one. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more.
Balancing Chemical Equations - Answer Key Balance the equations below: 1) 1 N2 + 3 H2 Æ 2 NH3 2) 2 KClO3 Æ 2 KCl + 3 O2 3) 2 NaCl + 1 F2 Æ 2 NaF + 1 Cl2 4) 2 H2 + 1 O2 Æ 2 H2O 5) 1 Pb(OH)2 + 2 HCl Æ 2 H2O + 1 PbCl2 6) 2 AlBr3 + 3 K2SO4 Æ 6 KBr + 1 Al2(SO4)3 7) 1 CH4 + 2 O2 Æ 1 CO2 + 2 H2O 8) 1 C3H8 + 5 O2 Æ 3 CO2 + 4 H2O 9) 2 C8H18 ...
This balancing chemical equations practice sheet has ten more unbalanced chemical equations to solve. Download a PDF of this worksheet here. A PDF of the answer key is also available here. If you'd just like to check your answers, click here to see the completed sheet. Check out our other Balancing Chemical Equation Worksheets.
Study with Quizlet and memorize flashcards containing terms like Number the steps for balancing equations: ? Use coefficients to increase the atoms on each side. ? Check to make sure you have the same number of each type of atom on each side. ? Count the atoms on each side. ? Identify the atoms on each side, Based on the chemical equation, use the drop-down menu to choose the coefficients that ...
Balancing Chemical Equations - Answer Key. Balance the equations below: 1 N2 + 3 H2 2 NH3. 2 KClO3 2 KCl + 3 O2. 2 NaCl + 1 F2 2 NaF + 1 Cl2. 2 H2 + 1 O2 2 H2O. 1 Pb(OH)2 + 2 HCl 2 H2O + 1 PbCl2.
The best way to become proficient at balancing chemical equations is practice. This balancing chemical equations worksheet has ten unbalanced equations to practice your skills. Either right-click and save the image or else download the PDF of the worksheet here. The worksheet prints on a standard sheet of printer paper.
Balancing an unbalanced equation is mostly a matter of making certain mass and charge are balanced on the reactants and products side of the reaction arrow. The printable balancing chemical equations worksheets are provided in PDF format with separate answer keys. Balancing Chemical Equations - Worksheet #1.
Assignments. 100% (44) 7. ... Balancing+Chemical+Equations+Practice. Topic: Chemical Reactions. Subject: Chemistry. 999+ Documents. Students shared 4389 documents in this course. Level: Standard. ... Chemical Naming WITH Answer KEY (at bottom) Chemistry 100% (17) More from: Luke Kim. More from:
Study with Quizlet and memorize flashcards containing terms like __CaO + __H₂O --> __Ca(OH)₂, __Na + __F₂ --> __NaF, __K + __I₂ --> __KI and more.
If you also find difficulty in balancing the chemical equations, follow the steps below. Step # 1: Write Down the Unbalanced Equation. The first step to balance the equation is to write down the chemical formula of reactants that are listed on the left side of the chemical equation. After this, you can list down the products on the right hand ...
The Key to Balancing Chemical Equations. The ultimate goal for balancing chemical equations is to make both sides of the reaction, the reactants and the products, equal in the number of atoms per element. This stems from the universal law of the conservation of mass, which states that matter can neither be created nor destroyed.
Learn to balance chemical equations through interactive simulations and challenges on PhET.
This balancing equations practice worksheet has 20 equations for beginners who are just learning this skill. This document comes with practice problems, an answer key and a differentiated graphic organizer as scaffolding for students who need help ordering and organizing the steps involved in bala...
Microsoft Word - Balancing Equations Worksheet - 3-13. STO.1. Balance a chemical equation. STO.2. Identify the parts of a chemical equation. RXN.1. Describe a chemical reaction using words and symbolic equations. For each of the following problems, write complete chemical equations to describe the chemical process taking place.
2. Assuming a s'more requires two graham crackers, one marshmallow, and one piece of chocolate, how many. Justify your answer. There's 5 graham cracker pi eces, but 2 crackers will only make one s'more. In a chemical reaction, reactants interact to form products. This process is. Balancing Chemical Equations.
Balancing Chemical Equations Learn with flashcards, games, and more — for free.
How do you know if a chemical equation is balanced? What can you change to balance an equation? Play a game to test your ideas!
Unit 8: Chemical Reactions. Write a balanced equation using phases. Notes: Balancing Equations with phases. Due today at the end of class : Due Next Class: *tutorial balancing with phases HERE. Classify a chemical reaction. Resources: Write, balance, classify, and predict products of chemical reactions.
Balancing Chemical Equations - Answer Key. Balance the equations below: N2 + 3 H2. NH3. 2 KClO3 2 KCl + 3 O2. 2 NaCl + 1 F2 2 NaF + 1 Cl2. 2 H2 + 1 O2 2 H2O. Pb(OH)2 + 2 HCl 2 H2O + 1 PbCl2.