
- Games & Quizzes
- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center
- Introduction
- Distribution and abundance
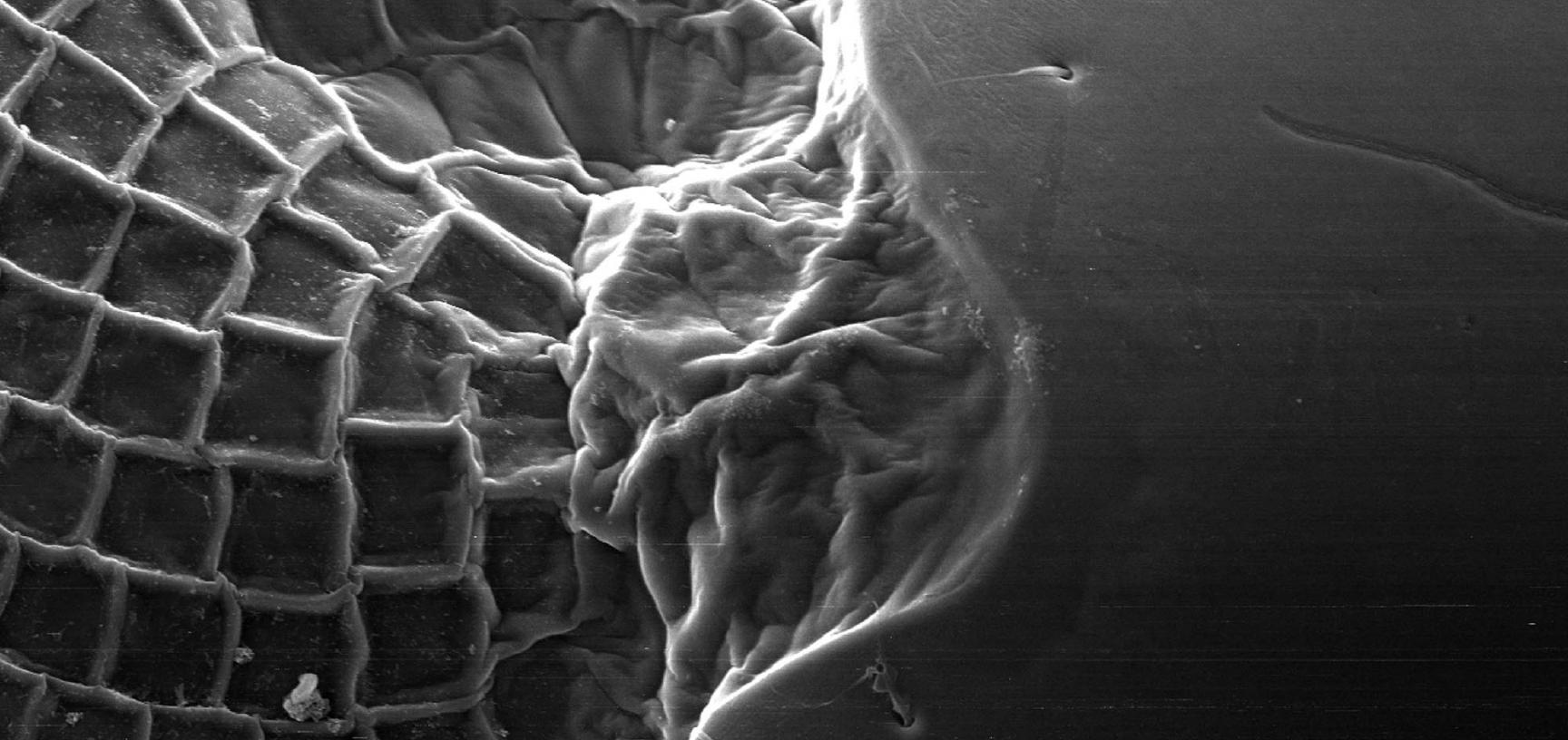
- The exoskeleton and molting
- Muscles, appendages, and locomotion
- Digestive system and feeding
- Respiratory system
- Circulatory system
- Excretory system and water balance
- Nervous system and organs of sensation
- Reproductive system and life cycle

Evolution and paleontology
- Distinguishing taxonomic features
- Annotated classification
- Critical appraisal

Our editors will review what you’ve submitted and determine whether to revise the article.
- Princeton University - Introduction to the Arthropods
- University of Central Florida Pressbooks - General Biology - Phylum Arthropoda (in Superphylm Ecdysozoa)
- National Center for Biotechnology Information - Arthropoda
- Biology LibreTexts - Arthropods (Arthropoda)
- San Diego Zoo Animals and Plants - Arthropods
- Animal Diversity Web - Arthropod
- Khan Academy - Complex animals: Annelids & arthropods
- UEN Digital Press with Pressbooks - Flatworms, Nematodes, and Arthropods
- University of Hawaii - Exploring Our Fluid Earth - Phylum Arthropoda
- Animal Corner - What is an Arthropods?
- arthropod - Children's Encyclopedia (Ages 8-11)
- arthropod - Student Encyclopedia (Ages 11 and up)
- Table Of Contents
Trusted Britannica articles, summarized using artificial intelligence, to provide a quicker and simpler reading experience. This is a beta feature. Please verify important information in our full article.
This summary was created from our Britannica article using AI. Please verify important information in our full article.
The arthropods share many features with the phylum Annelida . Both arthropods and annelids are segmented, and members of the annelid class Polychaeta have a pair of appendages on each segment. The plan of the nervous system in arthropods is very similar to that of annelids, and the basic plan in both groups shows a tubular, dorsal heart, which is then lost or modified in some. Annelids possess a coelom, which in arthropods is present only in the embryo . Its absence is probably related to the evolution of the exoskeleton and to the change in the mode of locomotion.
The first fossil arthropods appear in the Cambrian Period (541.0 million to 485.4 million years ago) and are represented by trilobites, merostomes, and crustaceans. Also present are some enigmatic arthropods that do not fit into any of the existing subphyla. The earliest terrestrial arachnid is from the Devonian Period (419.2 million to 358.9 million years ago), but it does not belong to any living order. Though a myriapod-like fossil has been found from the Devonian Period, it is not until the Carboniferous Period (358.9 million to 298.9 million years ago) that there is a good record of centipedes, millipedes, and insects. Specimens of plant-feeding mites dated to the Triassic Period (251.9 million to 201.3 million years ago) are among the oldest arthropod fossils preserved in amber.
Most zoologists recognize the trilobites , chelicerates, crustaceans, and myriapods as four major lines of arthropod evolution, but there is little agreement as to how those lines are related to one another or, indeed, if they had evolutionary origins independent from those of the annelids.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
The origin and early evolution of arthropods
Affiliations.
- 1 State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology and Center for Excellence in Life and Palaeoenvironment, Chinese Academy of Sciences, Nanjing, 210008, P. R. China.
- 2 Shaanxi Key Laboratory of Early Life and Environments, Northwest University, Xi'an, 710069, P.R. China.
- PMID: 35475316
- DOI: 10.1111/brv.12864
The rise of arthropods is a decisive event in the history of life. Likely the first animals to have established themselves on land and in the air, arthropods have pervaded nearly all ecosystems and have become pillars of the planet's ecological networks. Forerunners of this saga, exceptionally well-preserved Palaeozoic fossils recently discovered or re-discovered using new approaches and techniques have elucidated the precocious appearance of extant lineages at the onset of the Cambrian explosion, and pointed to the critical role of the plankton and hard integuments in early arthropod diversification. The notion put forward at the beginning of the century that the acquisition of extant arthropod characters was stepwise and represented by the majority of Cambrian fossil taxa is being rewritten. Although some key traits leading to Euarthropoda are indeed well documented along a diversified phylogenetic stem, this stem led to several speciose and ecologically diverse radiations leaving descendants late into the Palaeozoic, and a large part, if not all of the Cambrian euarthropods can now be placed on either of the two extant lineages: Mandibulata and Chelicerata. These new observations and discoveries have altered our view on the nature and timing of the Cambrian explosion and clarified diagnostic characters at the origin of extant arthropods, but also raised new questions, especially with respect to cephalic plasticity. There is now strong evidence that early arthropods shared a homologous frontalmost appendage, coined here the cheira, which likely evolved into antennules and chelicerae, but other aspects, such as brain and labrum evolution, are still subject to active debate. The early evolution of panarthropods was generally driven by increased mastication and predation efficiency and sophistication, but a wealth of recent studies have also highlighted the prevalent role of suspension-feeding, for which early panarthropods developed their own adaptive feedback through both specialized appendages and the diversification of small, morphologically differentiated larvae. In a context of general integumental differentiation and hardening across Cambrian metazoans, arthrodization of body and limbs notably prompted two diverging strategies of basipod differentiation, which arguably became founding criteria in the divergence of total-groups Mandibulata and Chelicerata. The kinship of trilobites and their relatives remains a source of disagreement, but a recent topological solution, termed the 'deep split', could embed Artiopoda as sister taxa to chelicerates and constitute definitive support for Arachnomorpha. Although Cambrian fossils have been critical to all these findings, data of exceptional quality have also been accumulating from other Palaeozoic Konservat-Lagerstätten, and a better integration of this information promises a much more complete and elaborate picture of early arthropod evolution in the near future. From the broader perspective of a total-evidence approach to the understanding of life's history, and despite persisting systematic debates and new interpretative challenges, various advances based on palaeontological evidence open the prospect of finally using the full potential of the most diverse animal phylum to investigate macroevolutionary patterns and processes.
Keywords: Arthropoda; evolution; palaeontology.; phylogenetics; systematics.
© 2022 Cambridge Philosophical Society.
PubMed Disclaimer
Similar articles
- The appendicular morphology of Sinoburius lunaris and the evolution of the artiopodan clade Xandarellida (Euarthropoda, early Cambrian) from South China. Chen X, Ortega-Hernández J, Wolfe JM, Zhai D, Hou X, Chen A, Mai H, Liu Y. Chen X, et al. BMC Evol Biol. 2019 Aug 6;19(1):165. doi: 10.1186/s12862-019-1491-3. BMC Evol Biol. 2019. PMID: 31387545 Free PMC article.
- Early fossil record of Euarthropoda and the Cambrian Explosion. Daley AC, Antcliffe JB, Drage HB, Pates S. Daley AC, et al. Proc Natl Acad Sci U S A. 2018 May 22;115(21):5323-5331. doi: 10.1073/pnas.1719962115. Epub 2018 May 21. Proc Natl Acad Sci U S A. 2018. PMID: 29784780 Free PMC article.
- Reappraising the early evidence of durophagy and drilling predation in the fossil record: implications for escalation and the Cambrian Explosion. Bicknell RDC, Paterson JR. Bicknell RDC, et al. Biol Rev Camb Philos Soc. 2018 May;93(2):754-784. doi: 10.1111/brv.12365. Epub 2017 Oct 2. Biol Rev Camb Philos Soc. 2018. PMID: 28967704 Review.
- Fossils and the Evolution of the Arthropod Brain. Strausfeld NJ, Ma X, Edgecombe GD. Strausfeld NJ, et al. Curr Biol. 2016 Oct 24;26(20):R989-R1000. doi: 10.1016/j.cub.2016.09.012. Curr Biol. 2016. PMID: 27780074 Review.
- Cambrian bivalved arthropod reveals origin of arthrodization. Legg DA, Sutton MD, Edgecombe GD, Caron JB. Legg DA, et al. Proc Biol Sci. 2012 Dec 7;279(1748):4699-704. doi: 10.1098/rspb.2012.1958. Epub 2012 Oct 10. Proc Biol Sci. 2012. PMID: 23055069 Free PMC article.
- Lower Ordovician synziphosurine reveals early euchelicerate diversity and evolution. Lustri L, Gueriau P, Daley AC. Lustri L, et al. Nat Commun. 2024 May 7;15(1):3808. doi: 10.1038/s41467-024-48013-w. Nat Commun. 2024. PMID: 38714651 Free PMC article.
- The median eyes of trilobites. Schoenemann B, Clarkson ENK. Schoenemann B, et al. Sci Rep. 2023 Mar 8;13(1):3917. doi: 10.1038/s41598-023-31089-7. Sci Rep. 2023. PMID: 36890176 Free PMC article.
- The unbearable uncertainty of panarthropod relationships. Wu R, Pisani D, Donoghue PCJ. Wu R, et al. Biol Lett. 2023 Jan;19(1):20220497. doi: 10.1098/rsbl.2022.0497. Epub 2023 Jan 11. Biol Lett. 2023. PMID: 36628953 Free PMC article.
- The problematic Cambrian arthropod Tuzoia and the origin of mandibulates revisited. Izquierdo-López A, Caron JB. Izquierdo-López A, et al. R Soc Open Sci. 2022 Dec 7;9(12):220933. doi: 10.1098/rsos.220933. eCollection 2022 Dec. R Soc Open Sci. 2022. PMID: 36483757 Free PMC article.
- Ordovician opabiniid-like animals and the role of the proboscis in euarthropod head evolution. Pates S, Botting JP, Muir LA, Wolfe JM. Pates S, et al. Nat Commun. 2022 Nov 15;13(1):6969. doi: 10.1038/s41467-022-34204-w. Nat Commun. 2022. PMID: 36379946 Free PMC article.
- Adamowicz, S. J., Purvis, A. & Wills, M. A. (2008). Increasing morphological complexity in multiple parallel lineages of the Crustacea. Proceedings of the National Academy of Sciences 105, 4786-4791.
- Alexander, R. M. (1996). Optima for Animals. Rev. ed. Princeton University Press, Princeton.
- Aria, C. (2019). Reviewing the bases for a nomenclatural uniformization of the highest taxonomic levels in arthropods. Geological Magazine 156, 1463-1468.
- Aria, C. (2020). Macroevolutionary patterns of body plan canalization in euarthropods. Paleobiology 46, 569-593.
- Aria, C. & Caron, J.-B. (2015). Cephalic and limb anatomy of a new isoxyid from the Burgess Shale and the role of ‘stem bivalved arthropods’ in the disparity of the frontalmost appendage. PLoS One 10, e0124979.
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- Ovid Technologies, Inc.

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

Arthropod evolution

Arthropods are perhaps the most successful group of animals in evolutionary terms, and their study represents one of the main research areas at the Museum. Our researchers use a combination of systematics, ecology, physiology, biogeography, developmental biology and the behaviour of both extant and fossil arthropods to shed light on evolutionary trends across the group.
The Museum’s substantial arthropod collections, which include over 5 million specimens, have particular strengths across insects, spiders and shrimps, and are an invaluable source of morphological and genetic data for phylogenetic and other comparative studies. These historically important holdings are also continuously expanding thanks to complementary fieldwork conducted all over the world.
The Museum is equipped with high-quality optical imaging equipment, such as a Zeiss Discovery V12 stereomicroscope with automated image stacking, laser scanning and 3D printing facilities, as well as extensive sample preparation and storage facilities. Cutting-edge imaging techniques including X-ray tomography are supported by dedicated high-power workstations and Mimics reconstruction software. DNA sequencing methods are also employed, and the Museum has laboratory space and equipment for processing samples.
Research staff
- Dr Sammy De Grave
- Darren Mann
- Dr Ricardo Pérez-de la Fuente
- Professor Derek Siveter
- Dr Lauren Sumner-Rooney
Funding sources
- John Fell Fund
- Leverhulme Trust
Volume 2 Supplement 2
Special Issue: Transitional Fossils
- Original Scientific Article
- Open access
- Published: 05 May 2009
The Evolutionary Emergence of Vertebrates From Among Their Spineless Relatives
- Philip C. J. Donoghue 1 &
- Mark A. Purnell 2
Evolution: Education and Outreach volume 2 , pages 204–212 ( 2009 ) Cite this article
69k Accesses
1 Citations
26 Altmetric
Metrics details
The evolutionary origin of vertebrates has been debated ad nauseam by anatomists, paleontologists, embryologists, and physiologists, but it is only now that molecular phylogenetics is providing a more rigorous framework for the placement of vertebrates among their invertebrate relatives that we can begin to arrive at concrete conclusions concerning the nature of ancient ancestors and the sequence in which characteristic anatomical features were acquired. Vertebrates tunicates and cephalochordates together comprise the chordate phylum, which along with echinoderms and hemichordates constitute the deuterostomes. The origin of vertebrates and of jawed vertebrates is characterized by a doubling of the vertebrate genome, leading to hypotheses that this genomic event drove organismal macroevolution. However, this perspective of evolutionary history, based on living organisms alone, is an artifact. Phylogenetic trees that integrate fossil vertebrates among their living relatives demonstrate the gradual and piecemeal assembly of the gnathostome body plan. Unfortunately, it has not been possible to demonstrate gradual assembly of the vertebrate body plan. This is not because vertebrates are irreducibly complex but because many of the characters that distinguish vertebrates from invertebrates are embryological and cellular and, therefore, inherently unfossilizable.
Humans and all other back-boned animals—plus a few others that have no bone at all—comprise the vertebrates. Vertebrates are a clade, meaning that all members of the group have evolved from a common ancestor that they all share. This means that the deeper parts of our evolutionary history are entwined with the origin of the clade, and it should thus come as no surprise to discover, therefore, that the origin of vertebrates has been the subject of intense debate since the earliest days of evolutionary research. In his book Before the backbone , Henry Gee recounts a great number of theories that, over the last century and a half, have invoked almost every other major living animal group as the ancestors of vertebrates (Gee 1996 ). Mercifully, there is now much less equivocation over the relationships of vertebrates to their living relatives, none of which are thought of as being ancestral. Rather, vertebrates and their nearest kin—the invertebrate chordates, the hemichordates and the echinoderms—are more correctly perceived as living representatives of distinct genealogical lineages that separated one from another deep in geological time. It is the aim of many paleontologists, comparative anatomists, embryologists, and molecular biologists to uncover the genealogical relationships of these animals—their family tree—and to test this tree with evidence that bears on the question of how these distinct organismal designs. Explanations of the emerging evolutionary pattern range from traditional Darwinian gradualistic evolution to those that invoke explosive diversifications (seized upon by creationists and intelligent designers as evidence for irreducible complexity (Meyer 2004a , b ) but which are actually consistent with natural causal mechanisms (McLennan 2008 )). Whatever the pattern and processes, a holistic understanding of the origin and diversification of vertebrates can only be obtained by a holistic approach, integrating all relevant strands of evidence into a framework of evolutionary relationships established on the basis of the only universal characteristics shared by all organisms: molecular sequence data.
Phylogenetic Framework—How Vertebrates Are Related to Their Nearest Invertebrate Kin
Establishing a phylogenetic framework of relationships among organisms is an essential prerequisite to uncovering the patterns and mechanisms of evolution. Traditionally, the evidence to support evolutionary trees has been derived from analysis of skeletal, muscular, and nervous systems, development and embryology, and cell characteristics. Invariably, these different aspects of organismal biology were studied in isolation and resulted in conflicting ideas of animal relationships that have been difficult to resolve (Jenner and Schram 1999 ). However, at the deepest levels in the genealogy of animals, such as the splits between phyla and groups of phyla, embryological and cell characters have held sway, not least because there are no skeletal characters shared between phyla. Most animals are bilaterally symmetrical—or show evidence of a bilateral ancestry in their embryology—and fall into one of two major groups: the protostomes (first mouths) or the deuterostomes (second mouths), the names of which betray the embryological characters on which they were defined Footnote 1 . Vertebrates belong to the chordate phylum and along with hemichordates (acorn worms and pterobranchs) and echinoderms (sea lilies, star fish, sea urchins, sea cucumbers) have long been considered deuterostomes. Whether there are additional deuterostomes has been the subject of long-running debate. Classically, chaetognaths (arrow worms) and lophophorates—bryozoans (moss animals) and brachiopods (lamp shells)—have been considered deuterostomes, based on similarities of the larvae from which the more familiar adults develop, but this is no longer the prevailing view. Such instability stems from the fact that, while features shared by phyla may well reflect their kinship, the absence of features is more difficult to interpret (Donoghue and Purnell 2009 ; Jenner 2004 ). It may indicate that in one clade the characters in question were never present, providing evidence against a close evolutionary relationship with clades that possess them, or it could be that the characters were originally inherited by both clades from their common ancestor but were subsequently lost in one. Indeed, even what appear to be shared similarities between phyla can be unreliable because they may reflect evolutionary convergence and independent acquisition rather than common ancestry. Because of these problems and because the universe of available data had largely been exhausted, attempts to decipher the relationships between animal phyla could not reach a consensus and it is hard to see how this situation would have changed were it not for the availability of genetic sequence data. Analysis of this rapidly expanding molecular dataset has provided a robust and essentially independent test of the theories of evolutionary relationships previously derived from anatomical and developmental data.
Perhaps the most surprising result of the molecular phylogenetic revolution has been that the majority of the classical groupings of animals, and their evolutionary relationships, have withstood testing, even in the face of ever-greater molecular datasets, representing ever-more lineages (e.g., Bourlat et al. 2008 ; Dunn et al. 2008 ). The composition of some major clades and the details of their relationships have, however, been the subject of dramatic change. For example, the lophophorates and chaetognaths have been excised from the deuterostomes and are now recognized to be protostomes (although their precise relations within the protostomes remain an open question). The residual deuterostomes have also been rearranged: hemichordates and chordates were considered more closely related to one another than to echinoderms, but there is now very strong molecular support for the Ambulacraria grouping of hemichordates and echinoderms, to the exclusion of chordates (Fig. 1 ). Xenoturbella , a worm-like animal of hitherto enigmatic affinity, is recognized as a fourth very minor phylum of just two species that is more closely related to ambulacrarians than chordates (Bourlat et al. 2006 ), collectively comprising the clade Xenambulacraria (Bourlat et al. 2008 ).
The interrelationships of the deuterostome phyla within the context of Metazoa. Primarily based upon Dunn et al. ( 2008 ) and Bourlat et al. ( 2008 )
With this increasingly reliable phylogenetic framework in place, it is now possible to turn to the question of how the major groups of deuterostomes emerged through evolutionary history. This is not a trivial challenge: the deuterostomes may be a small grouping of just four phyla, but the body plans that characterize the phyla are as anatomically disparate as any. Tradition would have us start our discussion with the last common ancestor of all deuterostomes and move up the evolutionary tree considering each subsequent branch in turn. However, as well as being overly deterministic, perpetuating the erroneous teleological impression that evolution progressed towards vertebrates this would not reflect certainties concerning knowledge of relationships and the sequence of character evolution, which are far better resolved within phyla than between phyla. So we will take a different route here, starting with our own familiar branch, the vertebrates, and working backwards to more distant and unfamiliar relatives, attempting to describe events in evolutionary history that are well constrained, through to those that are not so well constrained.
The Emergence and Evolution of Vertebrates
Jawed vertebrates—more than just a pretty smile.
It may come as a surprise to learn that more than 99% of living vertebrates fall within one subgroup of the vertebrate clade, the gnathostomes (Fig. 2 ). Named after one of their most conspicuous anatomical characters, jaws, gnathostomes are much more than just a pretty smile, and the list of features that distinguishes them from their living jawless relatives contains what might be generally, but incorrectly, considered as distinctive attributes of the vertebrate body plan. This includes teeth and a mineralized internal skeleton forming a braincase, jaws (and, in fish, gill supports), a backbone, trunk, and appendages. These appendages (arms and/or legs or fins) are paired and occur in the shoulder and pelvic regions. The different tissues of the mineralized skeleton—bone, cartilage, dentine, and enamel—are also unique, among living animals, to gnathostomes. Features of the brain and inner ear also distinguish gnathostomes, together with a good many other characters that are too detailed to recount here (for more details, see Donoghue et al. 2000 ; Janvier 2001 ). In fact, the number of anatomical characters that distinguish living jawed from jawless vertebrates is even greater than the complementary suite of features that distinguish vertebrates from invertebrates (see below).
The interrelationships of the principal groups of living chordates and vertebrates. Primarily based upon Delsuc et al. ( 2006 )
Vertebrates Get Ahead
A vast number of characters distinguish vertebrates from their nearest invertebrate relatives among chordates. Some of the more obvious features include a distinct anatomical head with a distinct brain, paired sensory organs, together with a number of specialized cell types that are responsible for the development and function of the nervous system and skeleton. The majority of these vertebrate-specific characters can be accounted for by a couple of embryological innovations that have long been thought exclusive to vertebrates, but for which evidence of evolutionary rudiments has grudgingly been found in the invertebrate chordates (Donoghue et al. 2008 ). These innovations are neurogenic placodes and neural crest cells (Gans and Northcutt 1983 ).
During development, neurogenic placodes give rise to the sensory organs of the central and peripheral nervous systems, including the eyes, nasal organs, inner ear, and the lateral line system of fishes. Neural crest cells are a specialized population of migratory cells which behave like stem cells in that they have the potential to differentiate into a broad spectrum of specialized cell types, such as specialized neurons and glia of the nervous system, pigment cells, and cells of the dermis (Le Douarin and Kalcheim 1999 ). It is also from neural crest cells that the gnathostome's dermal and pharyngeal bone, cartilage, and dentine develop. Because most of these anatomical characters are exclusive to vertebrates, it has been thought that neurogenic placodes and neural crest cells were the key innovations that underpinned a rapid evolutionary emergence of vertebrates. This is now known to be an oversimplification: certain sense organs in tunicates, for example, develop from placodes in a manner comparable to their vertebrate counterparts, and their development is regulated by a common suite of molecular factors (Mazet et al. 2005 ). Similarly, some tunicates also possess a migratory cell population that emerges during the early development of the nervous system, and these cells differentiate into pigment cells—a characteristic derivative of neural crest—utilizing an equivalent repertoire of genes (Jeffery 2006 ; Jeffery et al. 2004 ). Nevertheless, the diversity and functionality of sensory organs and neural crest cell derivatives in invertebrate chordates are far less than in their vertebrate counterparts, and without doubt the evolution of the potentiality of neurogenic placodes and neural crest was key to the emergence of vertebrates (Donoghue et al. 2008 ).
The Emergence of Vertebrates from Their Spineless Relatives
Understanding what distinguished vertebrates from other animals is only part of the picture. We also need to know the details of how they fit into the Tree of Life. Here, once again, molecular phylogenetics has weighed heavily upon classical interpretations. Generally, new data have confirmed the monophyly of vertebrates and chordates (but see Lartillot and Philippe 2008 ), but in other areas of the deuterostome tree decades of dogma have been overturned. In particular, debate over the relationships among the principal lineages of living vertebrates has become polarized into two conflicting hypotheses, each derived from different kinds of data. Molecular analyses indicate that the hagfishes and lampreys comprise a clade to the exclusion of jawed vertebrates (Bourlat et al. 2006 ; Delarbre et al. 2002 ; Delsuc et al. 2006 ; Mallatt and Winchell 2007 ), while morphological datasets support a clade of lampreys and jawed vertebrates to the exclusion of hagfishes (Donoghue et al. 2000 ; Donoghue and Smith 2001 ; Forey 1995 ; Gess et al. 2006 ; Janvier 1981 , 1996 ; Løvtrup 1977 ). Whichever hypothesis turns out to be correct, it is unlikely to have major consequences for understanding the broader picture of vertebrate and gnathostome origins.
Perhaps the biggest surprise of recent molecular revisions to the chordate tree is the recognition that the peculiar tunicates (sea squirts) and not cephalochordates (amphioxus) are the closest invertebrate relatives of the vertebrates (Delsuc et al. 2006 ; Heimberg et al. 2008 ; Fig. 2 ). While this conclusion, at first quite shocking, is now generally accepted, it has very few implications for what we understand of the nature of common ancestor of all chordates. Under either scenario, this ancestor must have been an amphioxus-like organism because segmented muscles and paired pharyngeal gill slits are characters general to deuterostomes, and a notochord is a character general to chordates (i.e., because they all share these characters, the most parsimonious interpretation is that they all inherited them from their common ancestor).
Genetic Drivers of Vertebrate and Gnathostome Innovation
Strikingly, the burst of embryological and anatomical innovation at the origin of vertebrates and gnathostomes seems to correspond to radical events in the evolution of their genomes. At some stage, along the lineage leading to living vertebrates after its separation from tunicates, the vertebrate genome was duplicated, at once doubling the repertoire of coding and noncoding DNA. Similarly, at some point after the living jawless vertebrates branched off, a second duplication took place prior to the appearance of the last common ancestor of living gnathostomes. In each case of duplication, one gene set was available to maintain its existing functions, but a matching set was free to evolve without compromising the original function of the genes. Thus, new gene functions could emerge to regulate the development of organisms and their organs, leading rapidly to innovation (see McLennan 2008 for discussion of duplication and cooption in evolution generally). There is abundant evidence that this occurred: (1) all living vertebrates can be shown to possess, or to have possessed, two copies of the genes possessed by invertebrate chordates; (2) all living gnathostomes can be shown to possess, or to have possessed, at least four copies (Holland et al. 1994 ), and, in comparison to preduplication relatives, lineages arising postduplication exhibit an expansion of function of these genes in regulating development. Of course, not all duplicate genes have been retained, but there is good evidence for the selective retention of genes that are essential to the anatomical and developmental innovations of vertebrates and gnathostomes. For instance, of the duplicate genes retained, there is a particular pattern of retention of genes that encode protein products associated with the extracellular matrices that are essential for skeletal development (Huxley-Jones et al. 2007 ) and of genes implicated in neural crest patterning and differentiation (Martinez-Morales et al. 2007 ).
There has been much debate about the tempo and mode of organismal evolution associated with genome duplication. Some have argued for a direct causal linkage such that genome duplication drives a geologically instantaneous burst of evolution (Sidow 1996 ; Wagner et al. 2003 ), while others have argued for a more permissive role wherein genome duplication is a necessary prerequisite of organismal evolution, but the effect on organismal evolution unfolds gradually over a protracted period of time (Prohaska and Stadler 2004 ). The principal evidence marshaled in support of the evolutionary burst hypothesis is the observation that living vertebrates and gnathostomes are distinguished from living invertebrate chordates and jawless vertebrates, respectively, by very large inventories of anatomical and developmental characters—surely only genome duplication can explain the emergence of so many characters in concert?
What the Fossils Say
So far, we have discussed only living gnathostomes, vertebrates, and chordates, but this provides an extremely incomplete perspective on vertebrate organismal evolution because current vertebrate diversity is only a small subset of the vertebrates that have ever lived. Many major branches from the vertebrate Tree of Life have been extinguished, and we are familiar with very many of them and the information that they provide on the pattern, timing, and tempo of evolution. For instance, dinosaurs record the gradual assembly of the body plan of modern birds (Padian and Chiappe 1998 ) and there is a comparably rich record of synapsid reptiles that record the gradual assembly of the body plan of modern mammals (Kemp 2007 ; Angielczyk 2009 ). No one, therefore, would attempt to explain the emergence of birds or mammals directly from reptilian ancestors in a geologically instantaneous event. The same is true of gnathostome origins.
Interleaved in the Tree of Life, between living jawless and jawed vertebrates, is a parade of long-dead and in some cases bizarre-looking fish that records clearly how gnathostome characters were acquired over a period of at least 80 million years (Fig. 3 ). Somewhere around the base of the lineage connecting living jawed vertebrates to the split from the hagfish and lampreys, is a number of fossils which lack a mineralized skeleton and are hard to place precisely (Bardack 1991 ; Bardack and Richardson 1977 ; Bardack and Zangerl 1968 ; Chang et al. 2006 ; Gess et al. 2006 ; Janvier and Lund 1983 ; Shu 2003 ; Shu et al. 1999 , 2003a ; Sweet and Donoghue 2001 ). They sit among the jawless fishes or between them and gnathostomes (they are not shown in Fig. 3 ). Above these non-mineralized vertebrates the conodont branch splits away. These small eel-shaped animals were like lampreys and hagfishes in almost every way except that they also possessed a mineralized skeleton—a complex raptorial array of teeth composed of enamel and dentine. The next to branch off were the pteraspidomorphs, characterized by an extensive armor plating, encasing the entire body from tip to tail, composed of acellular bone, dentine, and an enamel-like tissue (enameloid). This group also contains the first vertebrates to show evidence of semicircular canals of the inner ear that are distinct from the roof of the utriculus. Next to diverge were the anaspids, a small group of jawless vertebrates possessing the “gnathostome” characters of conodonts and pteraspidomorphs, plus a distinct stomach, unseen in hagfishes, lampreys, conodonts, or pteraspidomorphs. Thelodonts are even more closely related to gnathostomes, and this is betrayed by their possession, in addition to a mineralized dermal skeleton and a differentiated stomach, of tooth-like structures associated with their gills. Galeaspids, the next branch up the tree, are the first vertebrates to possess a mineralized braincase, albeit one composed entirely of cartilage, and they are perhaps also the first vertebrates in which the inner ear is connected directly to the exterior via an endolymphatic duct. Osteostracans are the last group of jawless vertebrates to diverge from the gnathostome lineage and they possess still further “gnathostome” characters, including slit-shaped gills and bony supports encapsulating their eyes, as well as an asymmetrical tail with a long upper lobe like that of sharks and paired pectoral—but no pelvic—fins. Their skeleton contains types of bone that are unknown in earlier forms: perichondral bone in the braincase and fin skeleton and dermal bone comprising much of the external bony armor. The last extinct branch to diverge from the jawless vertebrate–gnathostome lineage is the placoderms. They possess, from the list of characters otherwise known only in living gnathostomes, a mineralized jointed gill skeleton, pelvic fins, a mineralized vertebral column, mineralized fin supports (radials), and jaws. Of the few remaining “gnathostome” characters, the only one that can be verified as exclusive to living jawed vertebrates (because if it was present in fossils it has the potential to be recognized) is the particular position of the attachment of one of the muscles that controls movement of the eye.
The interrelationships of living and extinct jawless and jawed vertebrates. Primarily based upon Donoghue et al. ( 2000 ) and Donoghue and Smith ( 2001 )
Very clearly, the assembly of what, based on living animals, would be considered as a distinctive gnathostome body plan is anything but instantaneous. Extinct intermediates of living jawless and jawed vertebrates demonstrate its gradual assembly and, if anything, the effect of genome duplication on organismal evolution cannot have been anything more than a permissive factor. The earliest jawed vertebrates were certainly not “hopeful monsters” and, once again, they are demonstrably not irreducibly complex. Nevertheless, the acquisition of a jaw, with the benefit of hindsight, can be identified as a key innovation underpinning the diversification of jawed vertebrates because, combined with the locomotory advances afforded by the acquisition of a backbone and paired fins, it allowed jawed vertebrates to occupy new feeding niches and ecological habitats and to disperse into geographic regions that were previously not open to vertebrates.
The Emergence of Vertebrates
The example of the holistic perspective on the emergence of jawed vertebrates demonstrates that, at least in this instance, a coincidence of genome duplication and organismal evolution should not lead directly to the conclusion that one caused the other. The same pattern of gradual assembly can be demonstrated for the emergence of teleosts from their actinopterygian relatives (Donoghue and Purnell 2005 ), dispelling any notion of rapid organismal evolution or an explosive radiation (Wagner et al. 2003 ).
Unfortunately, fossils do not allow us to make similar observations concerning the assembly of the characters that distinguish living vertebrates from their nearest invertebrate relatives. We simply cannot tell from the fossil record whether the suite of vertebrate innovations evolved rapidly or gradually because the majority of innovations are embryological and cell characteristics, and these are inherently unfossilizable. Consequently, even recognizing correctly the fossils that might fill the gaps between amphioxus, tunicates, and hagfish is problematic. There are a few Cambrian contenders but it is difficult to determine whether these are genuine fossil vertebrates from which most vertebrate characteristics have rotted away or fossils that belong in a more basal position in the tree, with invertebrate chordates (e.g., Cathaymyrus and Metaspriggina ; Conway Morris 2008 ; Shu et al. 1996a ).
Nevertheless, from the example of gnathostomes, we should be skeptical of a direct causal linkage between genome duplication and organismal evolution—or any other causal mechanism of instantaneous evolution—in explaining the emergence of vertebrates. Rather, it is more likely that vertebrate characteristics emerged over a long and protracted episode of gradual change, but the organisms that record this transition are either not preserved or have not yet been recognized.
Fossils and the Emergence of Deuterostome Phyla
Carpoids and the evolution of echinoderms.
There have been precious few fossils identified as potential ancestors of each of the main deuterostome phyla—chordates, hemichordates, and echinoderms—in large part because the evolutionary relationships and number of deuterostome phyla has been an open question until relatively recently. However, the carpoids, a bizarre grouping of symmetrically and asymmetrically stalked animals with a calcitic echinoderm-like skeleton (known as stereom) have been promoted as ancestors of all the deuterostome phyla, even including early representatives of the tunicate, cephalochordate, and vertebrate lineages of chordates (Jefferies et al. 1996 ). The debate over the affinities of carpoids is complex and involved, and we cannot do justice to the alternative scientific hypotheses here except to say that there are at least three opposing interpretations of their anatomy and, consequently, their evolutionary affinity. However, except for a small number of diehards, a consensus has recently emerged which indentifies the carpoids as the remnants of an extinct evolutionary lineage of early echinoderms. Like the gnathostome example above, analysis of the characters that indicate the degree of relationship between carpoids and echinoderms also reveals the sequence in which echinoderm characters were assembled in the early evolutionary history of the lineage. Surprisingly, the regular fivefold symmetry, so characteristic of echinoderms, evolved from some rather unlikely looking ancestors.
The precise interrelationships of the carpoids remain unclear, but Fig. 4 shows the broad pattern of echinoderm relationships. Helicoplacoids, spindle-shaped animals with threefold rather than fivefold symmetry, are the closest relatives of the living echinoderms. They had an echinoderm-like water vascular system (the hydraulic system which functions in echinoderm respiration, locomotion, and transport of food and waste) and a calcitic stereom skeleton. The position of the helicoplacoid mouth, to one side of the animal, with the anus at one end, indicates that this important axis has shifted away from being front to back and was more echinoderm-like than all earlier members of the lineage (Smith 2008 ). Of the features most recognizable and characteristic of the echinoderm body plan, only pentaradial symmetry is lacking.
The interrelationships of living and fossil echinoderms within the context of Deuterostomia. Primarily based upon Smith ( 2005 , 2008 ) and Bourlat et al. ( 2008 )
Moving further down the tree, solutes branch off, then stylophorans (cornutes and mitrates), then perhaps the cinctans (not shown). All these groups show evidence of an echinoderm-like water vascular system, retain a distinct anterior–posterior body axis, and possess an echinoderm-like stereom skeleton. There is a trend of decreasing body symmetry through the lineage, from near bilateral symmetry of solutes to the strongly asymmetric stylophorans and cinctans. At some point after stylophorans branched off but before the first solutes, the gill slits, inherited by all previous members of the lineage from their basal deuterostome ancestors, were lost. Both groups of stylophorans preserve evidence of this primitive character (Dominguez et al. 2002 ; Jefferies 1973 ). Presumably, there were even earlier offshoots from this lineage linking echinoderms to the common ancestor they shared with hemichordates, but the likelihood of their being fossilized was very low because they had not yet evolved the mineralized calcitic stereom skeleton, and after death they will have rotted away without trace.
The Origins of Other Deuterostome Phyla and the Limitations of the Fossil Record
Untangling the riddle of carpoids has been a success story in elucidating the evolutionary assembly of the echinoderm body plan, but it tells us nothing about the evolutionary origins of the ambulacrarian, hemichordate, chordate, tunicate, cephalochordate, and vertebrate lineages. Fossil evidence of the emergence of these lineages and the assembly of the body plans characteristic of the living members of these lineages must be sought elsewhere, but there are precious few data to address these questions because there are very few other candidate fossil representatives of early deuterostomes, and most of those are difficult to interpret. Many are identified as primitive animals because they possess only primitive anatomical features, but it is often the case in such fossils that these are the only features to survive the process of fossilization, and it is difficult to determine whether the characters that would indicate a position higher in a tree are absent because they were never present or merely because they rotted away (Donoghue and Purnell 2009 ). Thus, various fossil organisms, such as the yunnanozoans (including Haikouella ), have been interpreted as primitive deuterostomes (Shu et al. 2003b ), primitive ambulacrarians (Shu et al. 2004 ), or as primitive chordates (Shu et al. 2004 ) because they seem to possess those few necessary characteristics, such as gill slits. Other features of the fossils, however, are difficult to interpret unequivocally (Mallatt et al. 2003 ; Shu and Morris 2003 ) and are taken by some to indicate links with hemichordates (Shu et al. 1996b ) or vertebrates (Donoghue and Purnell 2009 ; Mallatt and Chen 2003 ). The same may be true of that icon of chordate evolution, Pikaia . Detailed study of the fossils continues (Shu et al. 1996a ), but it may never be possible to resolve exactly where in the deuterostome tree it sits relative to the common ancestor of amphioxus and all other chordates.
It is unfortunate that the fossil record is almost silent on the question of the origins of the deuterostomes, the ambulacrarians, hemichordates, and the chordates, but the examples of the gnathostomes and the echinoderms show that major clades emerged through the gradual assembly of the characteristics that distinguish the living members of one phylum from another. Examples of the assembly of phylum level body plans are, however, very rare. In general, much of the early evolution of most phyla, obviously critical for reconstructing the sequence of body plan assembly, occurs before the acquisition of a mineralized skeleton. It is precisely because echinoderms evolved their calcitic skeleton at a point in time before the majority of echinoderm-specific characters had emerged that we are able to reconstruct how they acquired their distinctive features. The fossil record of chordate evolution communicates a similar message: only after the evolution of the phosphatic skeleton in the lineage leading to gnathostomes do the fossils provide a reliable account of character acquisition. We will never know how many important, abundant, and diverse nonskeletonized deuterostomes have left no trace in the fossil record. The important point to take from this, however, is not that the fossil record is useless—it certainly is not—but that it cannot provide the same amount or quality of evolutionary data for all groups of organisms and, for lineages which lack preservable skeletal tissues, the record will inevitably be less good. For major groups that have mineralized skeletons, such as gnathostomes, however, the pattern that emerges from the analysis of the fossil record is clear: body plans are assembled incrementally, over millions of years, not in sudden bursts of irreducible complexity.
Classically, the protostome–deuterostome distinction relates to events in early embryology. The fertilized cell undergoes repeated rounds of binary division (cleavage) to produce a ball of tens to hundreds of cells that are rearranged into distinct germ layers surrounding a central space (gastrulation). Following this, the embryo invaginates to the point where half of what was the outside is completely enclosed within, leaving only a small hole. In protostomes, this hole is the earliest manifestation of the mouth, while in deuterostomes it becomes the anus. It should be noted however that these are classical caricatures of the embryology and it has long been known that there are deviant members of Protostomia and Deuterostomia, perhaps betraying that there are still things we do not know about the genealogy of animals (Raineri 2006 ).
Angielczyk, K. D. Dimetrodon is not a dinosaur: using tree thinking to understand the ancient relatives of mammals and their evolution. Evolution: Education & Outreach. in press.
Bardack D. First fossil hagfish (Myxinoidea): a record from the Pennsylvanian of Illinois. Science. 1991;254:701–3.
Article CAS PubMed Google Scholar
Bardack D, Richardson ES Jr. New agnathous fishes from the Pennsylvanian of Illinois. Fieldiana Geol. 1977;33:489–510.
Google Scholar
Bardack D, Zangerl R. First fossil lamprey: a record from the Pennsylvanian of Illinois. Science. 1968;162:1265–7.
Bourlat SJ, Juliusdottir T, Lowe CJ, Freeman R, Aronowicz J, Kirschner M, et al. Deuterostome phylogeny reveals monophyletic chordates and the new phylum Xenoturbellida. Nature. 2006;444:85–8.
Bourlat SJ, Nielsen C, Economou AD, Telford MJ. Testing the new animal phylogeny: a phylum level molecular analysis of the animal kingdom. Mol Phylogen Evol. 2008;49:23–31.
Article CAS Google Scholar
Chang M-M, Zhang J, Miao DA. Lamprey from the Cretaceous Jehol biota of China. Nature. 2006;441:972–4.
Conway Morris S. A redescription of a rare chordate, Metaspriggina walcotti Simonetta and Insom, from the Burgess Shale (Middle Cambrian), British Columbia, Canada. J Paleontol. 2008;82:424–30.
Article Google Scholar
Delarbre C, Barriel V, Janvier P, Gachelin G. Complete mitochondrial DNA of the hagfish, Eptatretus burgeri : the comparative analysis of mitochondrial DNA sequences strongly supports the cyclostome monophyly. Mol Phylogen Evol. 2002;22:184–92.
Delsuc F, Brinkmann H, Chourrout D, Philippe H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 2006;439:965–8.
Dominguez P, Jacobson AG, Jefferies RPS. Paired gill slits in a fossil with a calcite skeleton. Nature. 2002;417:841–4.
Donoghue PCJ, Purnell MA. Genome duplication, extinction and vertebrate evolution. Trends Ecol Evol. 2005;20:312–9.
Article PubMed Google Scholar
Donoghue PCJ, Purnell MA. Distinguishing heat from light in debate over controversial fossils. BioEssays. 2009;31:178–89.
Donoghue PCJ, Smith MP. The anatomy of Turinia pagei (Powrie) and the phylogenetic status of the Thelodonti. Trans R Soc Edinb Earth Sci. 2001;92:15–37.
Donoghue PCJ, Forey PL, Aldridge RJ. Conodont affinity and chordate phylogeny. Biol Rev. 2000;75:191–251.
Donoghue PCJ, Graham A, Kelsh RN. The origin and evolution of the neural crest. BioEssays. 2008;30:530–41.
Dunn CW, Hejnol A, Matus DQ, Pang K, Browne WE, Smith SA, et al. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature. 2008;452:745–9.
Forey PL. Agnathans recent and fossil, and the origin of jawed vertebrates. Rev Fish Biol Fisheries. 1995;5:267–303.
Gans C, Northcutt RG. Neural crest and the origin of the vertebrates: a new head. Science. 1983;220:268–74.
Gee H. Before the backbone: views on the origin of the vertebrates. London: Chapman & Hall; 1996.
Gess RW, Coates MI, Rubidge BS. A lamprey from the Devonian period of South Africa. Nature. 2006;443:981–4.
Heimberg AM, Sempere LF, Moy VN, Donoghue PCJ, Peterson KJ. MicroRNAs and the advent of vertebrate morphological complexity. Proc Natl Acad Sci U S A. 2008;105:2946–50.
Holland PWH, Garcia-Fernandez J, Williams NA, Sidow A. Gene duplications and the origins of vertebrate development. Development. 1994;1994 Supplement:125–33.
Huxley-Jones J, Robertson DL, Boot-Handford RP. On the origins of the extracellular matrix in vertebrates. Matrix Biol. 2007;26:2–11.
Janvier P. The phylogeny of the Craniata, with particular reference to the significance of fossil “agnathans”. J Vertebr Paleontol. 1981;1:121–59.
Janvier P. The dawn of the vertebrates: characters versus common ascent in the rise of current vertebrate phylogenies. Palaeontology. 1996;39:259–87.
Janvier P. Ostracoderms and the shaping of the gnathostome characters. In: Ahlberg PE, editor. Major events in early vertebrate evolution: palaeontology, phylogeny, genetics and development. London: Taylor and Francis; 2001. p. 172–86.
Janvier P, Lund R. Hardistiella montanensis n. gen. et sp. (Petromyzontida) from the Lower Carboniferous of Montana, with remarks on the affinities of lampreys. J Vertebr Paleontol. 1983;2:407–13.
Jefferies RPS. The Ordovician fossil Lagynocystis pyramidalis (Barrande) and the ancestry of amphioxus. Philos Trans R Soc Lond Series B. 1973;265:409–69.
Jefferies RPS, Brown NA, Daley PEJ. The early phylogeny of chordates and echinoderms and the origin of chordate left–right asymmetry and bilateral symmetry. Acta Zool (Stockh). 1996;77:101–22.
Jeffery WR. Ascidian neural crest-like cells: phylogenetic distribution, relationship to larval complexity, and pigment cell fate. J Exp Zool B Mol Dev Evol. 2006;306B:470–80.
Jeffery WR, Strickler AG, Yamamoto Y. Migratory neural crest-like cells form body pigment in a urochordate embryo. Nature. 2004;431:696–9.
Jenner RA. When molecules and morphology clash: reconciling conflicting phylogenies of the Metazoa by considering secondary character loss. Evol Dev. 2004;6:372–8.
Jenner RA, Schram FR. The grand game of metazoan phylogeny: rules and strategies. Biol Rev. 1999;74:121–42.
Kemp TS. The origin of higher taxa: macroevolutionary processes, and the case of the mammals. Acta Zoologica. 2007;88:3–22.
Lartillot N, Philippe H. Improvement of molecular phylogenetic inference and the phylogeny of Bilateria. Philos Trans Royal Soc B Biol Sci. 2008;363:1463–72.
Le Douarin NM, Kalcheim C. The neural crest. Cambridge: Cambridge University Press; 1999.
Book Google Scholar
Løvtrup S. The phylogeny of the Vertebrata. New York: Wiley; 1977.
Mallatt J, Chen J-Y. Fossil sister group of craniates: predicted and found. J Morphol. 2003;258:1–31.
Mallatt J, Winchell CJ. Ribosomal RNA genes and deuterostome phylogeny revisited: more cyclostomes, elasmobranchs, reptiles, and a brittle star. Mol Phylogen Evol. 2007;43:1005–22.
Mallatt J, Chen JY, Holland ND. Comment on “A new species of yunnanozoan with implications for deuterostome evolution”. Science. 2003;300:1372.
Martinez-Morales JR, Henrich T, Ramialison M, Wittbrodt J. New genes in the evolution of the neural crest differentiation program. Genome Biology. 2007;8(R36).
Mazet F, Hutt JA, Milloz J, Millard J, Graham A, Shimeld SM. Molecular evidence from Ciona intestinalis for the evolutionary origin of vertebrate sensory placodes. Dev Biol. 2005;282:494–508.
McLennan D. The concept of co-option: why evolution often looks miraculous. Evo Edu Outreach. 2008;1:247–58.
Meyer SC. The origin of biological information and the higher taxonomic categories. Proc Biol Soc Wash. 2004a;117:213–39.
Meyer SC. The Cambrian information explosion: evidence for intelligent design. In: Dembski WA, Ruse M, editors. Debating design: from Darwin to DNA. Cambridge: Cambridge University Press; 2004b. p. 371–91.
Chapter Google Scholar
Padian K, Chiappe LM. The origin and early evolution of birds. Biol Rev. 1998;73:1–42.
Prohaska SJ, Stadler PF. The duplication of the Hox gene clusters in teleost fishes. Theory Biosci. 2004;123:89–110.
Raineri M. Are protochordates chordates? Biol J Linn Soc. 2006;87:261–84.
Shu D-G. A paleontological perspective of vertebrate origin. Chin Sci Bull. 2003;48:725–35.
Shu D-G, Conway Morris S. Response to comment on “A new species of Yunnanozoon with implications for deuterostome evolution”. Science. 2003;300:1372d.
Shu D-G, Conway Morris S, Zhang X-L. A Pikaia -like chordate from the Lower Cambrian of China. Nature. 1996a;384:157–8.
Shu D-G, Zhang X, Chen L. Reinterpretation of Yunnanozoon as the earliest known hemichordate. Nature. 1996b;380:428–30.
Shu D-G, Luo H-L, Conway Morris S, Zhang X-L, Hu S-X, Chen L, et al. Lower Cambrian vertebrates from south China. Nature. 1999;402:42–6.
Shu D-G, Conway Morris S, Han J, Zhang Z-F, Yasui K, Janvier P, et al. Head and backbone of the Early Cambrian vertebrate Haikouichthys . Nature. 2003a;421:526–9.
Shu D-G, Conway Morris S, Zhang ZF, Liu JN, Han J, Chen L, et al. A new species of Yunnanozoon with implications for deuterostome evolution. Science. 2003b;299:1380–4.
Shu D-G, Conway Morris S, Han J, Zhang Z-F, Liu J-N. Ancestral echinoderms from the Chengjiang deposits of China. Nature. 2004;430:422–8.
Sidow A. Gen(om)e duplications in the evolution of early vertebrates. Curr Opin Genet Dev. 1996;6:715–22.
Smith AB. The pre-radial history of echinoderms. Geol J. 2005;40:255–80.
Smith AB. Deuterostomes in a twist: the origins of a radical new body plan. Evolut Develop. 2008;10:493–503.
Sweet WC, Donoghue PCJ. Conodonts: past, present and future. J Paleontol. 2001;75:1174–84.
Wagner GP, Amemiya C, Ruddle F. Hox cluster duplications and the opportunity for evolutionary novelties. Proc Natl Acad Sci U S A. 2003;100:14603–6.
Download references
Acknowledgements
Many thanks to Don Prothero for the invitation to contribute to this issue of Evolution : Outreach and Education . Simon Powell (Bristol) produced the images of animals used in the artwork. Greg Edgecombe provided an informative critique on the manuscript that resulted in an improved final version. We acknowledge funding from the National Endowment for Science, Technology, and the Arts (PCJD), The Leverhulme Trust (PCJD), the Biotechnology and Biological Sciences Research Council (PCJD), the European Union Framework 7 (PCJD), and the Natural Environment Research Council (PCJD; MAP).
Author information
Authors and affiliations.
Department of Earth Sciences, University of Bristol, Wills Memorial Building, Queen’s Road, Bristol, BS8 1RJ, UK
Philip C. J. Donoghue
Department of Geology, University of Leicester, University Road, Leicester, LE1 7RH, UK
Mark A. Purnell
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Philip C. J. Donoghue .
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Reprints and permissions
About this article
Cite this article.
Donoghue, P.C.J., Purnell, M.A. The Evolutionary Emergence of Vertebrates From Among Their Spineless Relatives. Evo Edu Outreach 2 , 204–212 (2009). https://doi.org/10.1007/s12052-009-0134-3
Download citation
Received : 29 September 2008
Accepted : 24 March 2009
Published : 05 May 2009
Issue Date : June 2009
DOI : https://doi.org/10.1007/s12052-009-0134-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Deuterostome
- Hemichordate
Evolution: Education and Outreach
ISSN: 1936-6434
- Submission enquiries: [email protected]
The Arthropoda: A Phylogenetic Framework
- First Online: 01 January 2013
Cite this chapter

- Gonzalo Giribet 4 &
- Gregory D. Edgecombe 5
5087 Accesses
6 Citations
3 Altmetric
Arthropoda, the best-known member of the clade Ecdysozoa, is a phylum of protostome animals, its closest relatives being Onychophora (velvet worms) and Tardigrada (water bears).
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others

Phylogenetics and Systematics of Animal Life

Hexapoda: Comparative Aspects of Later Embryogenesis and Metamorphosis


Introduction to the Theraphosidae
Abele LG, Kim W, Felgenhauer BE (1989) Molecular evidence for inclusion of the phylum Pentastomida in the Crustacea. Mol Biol Evol 6:685–691
Google Scholar
Abzhanov A, Kaufman TC (2000) Crustacean (malacostracan) Hox genes and the evolution of the arthropod trunk. Development 127:2239–2249
PubMed CAS Google Scholar
Adrain JM (2011) Class Trilobita Walch, 1771. In: Zhang Z-Q (ed) Animal biodiversity: an outline of higher-level classification and survey of taxonomic richness. Magnolia Press, Auckland, pp 104–109
Aguinaldo AMA, Turbeville JM, Lindford LS, Rivera MC, Garey JR, Raff RA, Lake JA (1997) Evidence for a clade of nematodes, arthropods and other moulting animals. Nature 387:489–493
Almeida WO, Christoffersen ML, Amorim DS, Garrafoni ARS, Silva GS (2003) Polychaeta, Annelida, and Articulata are not monophyletic: articulating the Metameria (Metazoa: Coelomata). Rev Bras Zool 20:23–57
Anderson DT (1969) On the embryology of the cirripede crustaceans Tetraclita rosea (krauss), Tetraclita purpurascens (Wood), Chthamalus antennatus Darwin and Chamaesipho columna (Spengler) and some considerations of crustacean phylogenetic relationships. Philos Trans R Soc B 256:183–235
Anderson DT (1973) Embryology and phylogeny in annelids and arthropods. Pergamon, Oxford
Andrew DR (2011) A new view of insect–crustacean relationships II: inferences from expressed sequence tags and comparisons with neural cladistics. Arthropod Struct Dev 40:289–302
PubMed Google Scholar
Angelini DR, Kaufman TC (2005) Comparative developmental genetics and the evolution of arthropod body plans. Annu Rev Genet 39:95–119
Bäcker H, Fanenbruck M, Wägele JW (2008) A forgotten homology supporting the monophyly of Tracheata: The subcoxa of insects and myriapods re-visited. Zool Anz 247:185–207
Ballard JWO, Ballard O, Olsen GJ, Faith DP, Odgers WA, Rowell DM, Atkinson P (1992) Evidence from 12S ribosomal RNA sequences that onychophorans are modified arthropods. Science 258:1345–1348
Bartolomaeus T, Quast B, Koch M (2009) Nephridial development and body cavity formation in Artemia salina (Crustacea: Branchiopoda): no evidence for any transitory coelom. Zoomorphology 128:247–262
Bergström J (1979) Morphology of fossil arthropods as a guide to phylogenetic relationships. In: Gupta AP (ed) Arthropod phylogeny. Van Nostrand Reinhold, New York, pp 3–56
Beutel RG, Gorb SN (2001) Ultrastructure of attachment specializations of hexapods (arthropoda): evolutionary patterns inferred from a revised ordinal phylogeny. J Zool Syst Evol Res 39:177–207
Bitsch C, Bitsch J (2004) Phylogenetic relationships of basal hexapods among the mandibulate arthropods: a cladistic analysis based on comparative morphological characters. Zool Scr 33:511–550
Blair JE, Ikeo K, Gojobori T, Hedges SB (2002) The evolutionary position of nematodes. BMC Evol Biol 2:1–7
Blanke A, Wipfler B, Letsch H, Koch M, Beckmann F, Beutel R, Misof B (2012) Revival of Palaeoptera—head characters support a monophyletic origin of Odonata and Ephemeroptera (Insecta). Cladistics 28:560–581
Boore JL, Collins TM, Stanton D, Daehler LL, Brown WM (1995) Deducing the pattern of arthropod phylogeny from mitochondrial DNA rearrangements. Nature 376:163–165
Bourlat SJ, Nielsen C, Economou AD, Telford MJ (2008) Testing the new animal phylogeny: a phylum level molecular analysis of the animal kingdom. Mol Phylogenet Evol 49:23–31
Brusca RC, Brusca GJ (1990) Invertebrates. Sinauer Associates, Sunderland
Brusca RC, Brusca GJ (2003) Invertebrates, 2nd edn. Sinauer Associates, Sunderland
Cameron SL, Barker SC, Whiting MF (2006) Mitochondrial genomics and the new insect order Mantophasmatodea. Mol Phylogenet Evol 38:274–279
Campbell LI, Rota-Stabelli O, Edgecombe GD, Marchioro T, Longhorn SJ, Telford MJ, Philippe H, Rebecchi L, Peterson KJ, Pisani D (2011) MicroRNAs and phylogenomics resolve the relationships of Tardigrada and suggest that velvet worms are the sister group of Arthropoda. Proc Natl Acad Sci USA 108:15920–15924
Carapelli A, Lió P, Nardi F, van der Wath E, Frati F (2007) Phylogenetic analysis of mitochondrial protein coding genes confirms the reciprocal paraphyly of Hexapoda and crustacea. BMC Evol Biol 7:S8
Caravas J, Friedrich M (2010) Of mites and millipedes: recent progress in resolving the base of the arthropod tree. BioEssays 32:488–495
Carmean D, Kimsey LS, Berbee ML (1992) 18S rDNA sequences and the holometabolous insects. Mol Phylogenet Evol 1:270–278
Castellani C, Maas A, Waloszek D, Haug JT (2011) New pentastomids from the late Cambrian of Sweden—deeper insight of the ontogeny of fossil tongue worms. Palaeontogr Abt A: Palaeozoology Stratigr 293:95–145
Clark AG, Eisen MB, Smith DR, Bergman CM, Oliver B, Markow TA, Kaufman TC, Kellis M, Gelbart W, Iyer VN, Pollard DA, Sackton TB, Larracuente AM, Singh ND, Abad JP, Abt DN, Adryan B, Aguade M, Akashi H, Anderson WW, Aquadro CF, Ardell DH, Arguello R, Artieri CG, Barbash DA, Barker D, Barsanti P, Batterham P, Batzoglou S, Begun D, Bhutkar A, Blanco E, Bosak SA, Bradley RK, Brand AD, Brent MR, Brooks AN, Brown RH, Butlin RK, Caggese C, Calvi BR, Bernardo de Carvalho A, Caspi A, Castrezana S, Celniker SE, Chang JL, Chapple C, Chatterji S, Chinwalla A, Civetta A, Clifton SW, Comeron JM, Costello JC, Coyne JA, Daub J, David RG, Delcher AL, Delehaunty K, Do CB, Ebling H, Edwards K, Eickbush T, Evans JD, Filipski A, Findeiss S, Freyhult E, Fulton L, Fulton R, Garcia AC, Gardiner A, Garfield DA, Garvin BE, Gibson G, Gilbert D, Gnerre S, Godfrey J, Good R, Gotea V, Gravely B, Greenberg AJ, Griffiths-Jones S, Gross S, Guigo R, Gustafson EA, Haerty W, Hahn MW, Halligan DL, Halpern AL, Halter GM, Han MV, Heger A, Hillier L, Hinrichs AS, Holmes I, Hoskins RA, Hubisz MJ, Hultmark D, Huntley MA, Jaffe DB, Jagadeeshan S, Jeck WR, Johnson J, Jones CD, Jordan WC, Karpen GH, Kataoka E, Keightley PD, Kheradpour P, Kirkness EF, Koerich LB, Kristiansen K, Kudrna D, Kulathinal RJ, Kumar S, Kwok R, Lander E, Langley CH, Lapoint R, Lazzaro BP, Lee SJ, Levesque L, Li R, Lin CF, Lin MF, Lindblad-Toh K, Llopart A, Long M, Low L, Lozovsky E, Lu J, Luo M, Machado CA, Makalowski W, Marzo M, Matsuda M, Matzkin L, McAllister B, McBride CS, McKernan B, McKernan K, Mendez-Lago M, Minx P, Mollenhauer MU, Montooth K, Mount SM, Mu X, Myers E, Negre B, Newfeld S, Nielsen R, Noor MA, O’Grady P, Pachter L, Papaceit M, Parisi MJ, Parisi M, Parts L, Pedersen JS, Pesole G, Phillippy AM, Ponting CP, Pop M, Porcelli D, Powell JR, Prohaska S, Pruitt K, Puig M, Quesneville H, Ravi Ram K, Rand D, Rasmussen MD, Reed LK, Reenan R, Reily A, Remington KA, Rieger TT, Ritchie MG, Robin C, Rogers YH, Rohde C, Rozas J, Rubenfield MJ, Ruiz A, Russo S, Salzberg SL, Sanchez-Gracia A, Saranga DJ, Sato H, Schaeffer SW, Schatz MC, Schlenke T, Schwartz R, Segarra C, Singh RS, Sirot L, Sirota M, Sisneros NB, Smith CD, Smith TF, Spieth J, Stage DE, Stark A, Stephan W, Strausberg RL, Strempel S, Sturgill D, Sutton G, Sutton GG, Tao W, Teichmann S, Tobari YN, Tomimura Y, Tsolas JM, Valente VL, Venter E, Craig Venter J, Vicario S, Vieira FG, Vilella AJ, Villasante A, Walenz B, Wang J, Wasserman M, Watts T, Wilson D, Wilson RK, Wing RA, Wolfner MF, Wong A, Ka-Shu Wong G, Wu CI, Wu G, Yamamoto D, Yang HP, Yang SP, Yorke JA, Yoshida K, Zdobnov E, Zhang P, Zhang Y, Zimin AV, Baldwin J, Abdouelleil A, Abdulkadir J, Abebe A, Abera B, Abreu J, Christophe Acer S, Aftuck L, Alexander A, An P, Anderson E, Anderson S, Arachi H, Azer M, Bachantsang P, Barry A, Bayul T, Berlin A, Bessette D, Bloom T, Blye J, Boguslavskiy L, Bonnet C, Boukhgalter B, Bourzgui I, Brown A, Cahill P, Channer S, Cheshatsang Y, Chuda L, Citroen M, Collymore A, Cooke P, Costello M, D’Aco K, Daza R, De Haan G, Degray S, Demaso C, Dhargay N, Dooley K, Dooley E, Doricent M, Dorje P, Dorjee K, Dupes A, Elong R, Falk J, Farina A, Faro S, Ferguson D, Fisher S, Foley CD, Franke A, Friedrich D, Gadbois L, Gearin G, Gearin CR, Giannoukos G, Goode T, Graham J, Grandbois E, Grewal S, Gyaltsen K, Hafez N, Hagos B, Hall J, Henson C, Hollinger A, Honan T, Huard MD, Hughes L, Hurhula B, Erii Husby M, Kamat A, Kanga B, Kashin S, Khazanovich D, Kisner P, Lance K, Lara M, Lee W, Lennon N, Letendre F, Levine R, Lipovsky A, Liu X, Liu J, Liu S, Lokyitsang T, Lokyitsang Y, Lubonja R, Lui A, Macdonald P, Magnisalis V, Maru K, Matthews C, McCusker W, McDonough S, Mehta T, Meldrim J, Meneus L, Mihai O, Mihalev A, Mihova T, Mittelman R, Mlenga V, Montmayeur A, Mulrain L, Navidi A, Naylor J, Negash T, Nguyen T, Nguyen N, Nicol R, Norbu C, Norbu N, Novod N, O’Neill B, Osman S, Markiewicz E, Oyono OL, Patti C, Phunkhang P, Pierre F, Priest M, Raghuraman S, Rege F, Reyes R, Rise C, Rogov P, Ross K, Ryan E, Settipalli S, Shea T, Sherpa N, Shi L, Shih D, Sparrow T, Spaulding J, Stalker J, Stange-Thomann N, Stavropoulos S, Stone C, Strader C, Tesfaye S, Thomson T, Thoulutsang Y, Thoulutsang D, Topham K, Topping I, Tsamla T, Vassiliev H, Vo A, Wangchuk T, Wangdi T, Weiand M, Wilkinson J, Wilson A, Yadav S, Young G, Yu Q, Zembek L, Zhong D, Zimmer A, Zwirko Z, Jaffe DB, Alvarez P, Brockman W, Butler J, Chin C, Gnerre S, Grabherr M, Kleber M, Mauceli E, Maccallum I (2007) Evolution of genes and genomes on the Drosophila phylogeny. Nature 450:203–218
Clouse RM, Sharma PP, Giribet G, Wheeler WC (submitted) Independent and isolated suites of paralogs in an arachnid elongation factor-1α, a purported single-copy nuclear gene. Mol Phylogenet Evol
Colosi G (1967) Zoologia e biologia generale. UTET, Torino
Cuvier G (1817) Le règne animal distribué d’après son organisation. A. Belin, Paris
Dallai R, Carapelli A, Nardi F, Fanciulli PP, Lupetti P, Afzelius BA, Frati F (2004) Sperm structure and spermiogenesis in Coletinia sp. (Nicoletiidae, Zygentoma, Insecta) with a comparative analysis of sperm structure in Zygentoma. Tissue Cell 36:233–244
Dallai R, Mercati D, Carapelli A, Nardi F, Machida R, Sekiya K, Frati F (2011) Sperm accessory microtubules suggest the placement of Diplura as the sister-group of Insecta s.s. Arthropod Struct Dev 40:77–92
De Grave S, Pentcheff ND, Ahyong ST, Chan T-Y, Crandall KA, Dworschak PC, Felder DL, Feldmann RM, Fransen CHJM, Goulding LYD, Lemaitre R, Low MEY, Martin JW, Naaaag PKL, Schweitzer CE, Tan SH, Tshudy D, Wetzer R (2009) A classification of living and fossil genera of decapod crustaceans. Raffles Bull Zool, pp 1–109
Dohle W (1980) Sind die Myriapoden eine monophyletische Gruppe? Eine Diskussion der Verwandtschaftsbeziehungen der Antennaten. Abh naturwiss Ver Hamburg NF 23:45–104
Dong Y, Sun H, Guo H, Pan D, Qian C, Hao S, Zhou K (2012) The complete mitochondrial genome of Pauropus longriamus (Myriapoda: Pauropoda): implications on early diversification of the myriapods revealed from comparative analysis. Gene 505:57–65
Dopazo H, Santoyo J, Dopazo J (2004) Phylogenomics and the number of characters required for obtaining an accurate phylogeny of eukaryote model species. Bioinformatics 20(Suppl 1):116–121
Dove H, Stollewerk A (2003) Comparative analysis of neurogenesis in the myriapod Glomeris marginata (Diplopoda) suggests more similarities to chelicerates than to insects. Development 130:2161–2171
Dunlop JA (2010) Geological history and phylogeny of Chelicerata. Arthropod Struct Dev 39:124–142
Dunlop JA, Alberti G (2008) The affinities of mites and ticks: a review. J Zool Syst Evol Res 46:1–18
Dunlop JA, Arango CP (2005) Pycnogonid affinities: a review. J Zool Syst Evol Res 43:8–21
Dunlop JA, Penney D, Tetlie OE, Anderson LI (2008) How many species of fossil arachnids are there? J Arachnol 36:267–272
Dunn CW, Hejnol A, Matus DQ, Pang K, Browne WE, Smith SA, Seaver EC, Rouse GW, Obst M, Edgecombe GD, Sørensen MV, Haddock SHD, Schmidt-Rhaesa A, Okusu A, Kristensen RM, Wheeler WC, Martindale MQ, Giribet G (2008) Broad taxon sampling improves resolution of the animal tree of life. Nature 452:745–749
Eberhard MJB, Picker MD, Klass K-D (2011) Sympatry in Mantophasmatodea, with the description of a new species and phylogenetic considerations. Org Divers Evol 11:43–59
Edgecombe GD (2009) Palaeontological and molecular evidence linking arthropods, onychophorans, and other Ecdysozoa. Evo Edu Outreach 2:178–190
Edgecombe GD (2010) Arthropod phylogeny: an overview from the perspectives of morphology, molecular data and the fossil record. Arthropod Struct Dev 39:74–87
Edgecombe GD, Giribet G, Dunn CW, Hejnol A, Kristensen RM, Neves RC, Rouse GW, Worsaae K, Sørensen MV (2011) Higher-level metazoan relationships: recent progress and remaining questions. Org Divers Evol 11:151–172
Edgecombe GD, Richter S, Wilson GDF (2003) The mandibular gnathal edges: Homologous structures throughout Mandibulata? Afr Invertebr 44:115–135
Eernisse DJ, Albert JS, Anderson FE (1992) Annelida and Arthropoda are not sister taxa: A phylogenetic analysis of spiralian metazoan morphology. Syst Biol 41:305–330
Eriksson BJ, Stollewerk A (2010a) Expression patterns of neural genes in Euperipatoides kanangrensis suggest divergent evolution of onychophoran and euarthropod neurogenesis. Proc Natl Acad Sci USA 107:22576–22581
Eriksson BJ, Stollewerk A (2010b) The morphological and molecular processes of onychophoran brain development show unique features that are neither comparable to insects nor to chelicerates. Arthropod Struct Dev 39:478–490
Ertas B, von Reumont BM, Wägele JW, Misof B, Burmester T (2009) Hemocyanin suggests a close relationship of Remipedia and Hexapoda. Mol Biol Evol 26:2711–2718
Erwin DH, Laflamme M, Tweedt SM, Sperling EA, Pisani D, Peterson KJ (2011) The Cambrian conundrum: early divergence and later ecological success in the early history of animals. Science 334:1091–1097
Friedrich F, Beutel RG (2010) Goodbye Halteria? The thoracic morphology of Endopterygota (Insecta) and its phylogenetic implications. Cladistics 26:579–612
Friedrich M, Tautz D (1995) Ribosomal DNA phylogeny of the major extant arthropod classes and the evolution of myriapods. Nature 376:165–167
Gabriel WN, Goldstein B (2007) Segmental expression of Pax3/7 and Engrailed homologs in tardigrade development. Dev Genes Evol 217:421–433
Gai Y, Song D, Sun H, Yang Q, Zhou K (2008) The complete mitochondrial genome of Symphylella sp. (Myriapoda: Symphyla): extensive gene order rearrangement and evidence in favor of Progoneata. Mol Phylogenet Evol 49:574–585
Gao Y, Bu Y, Luan YX (2008) Phylogenetic relationships of basal hexapods reconstructed from nearly complete 18S and 28S rRNA gene sequences. Zool Sci 25:1139–1145
Giribet G (1997) Filogenia molecular de Artrópodos basada en la secuencia de genes ribosomales. Universitat de Barcelona: Departament de Biologia Animal, Barcelona
Giribet G (2003) Molecules, development and fossils in the study of metazoan evolution; Articulata versus Ecdysozoa revisited. Zoology 106:303–326
Giribet G (2010) A new dimension in combining data? The use of morphology and phylogenomic data in metazoan systematics. Acta Zool 91:11–19
Giribet G, Carranza S, Baguñà J, Riutort M, Ribera C (1996) First molecular evidence for the existence of a Tardigrada + Arthropoda clade. Mol Biol Evol 13:76–84
Giribet G, Distel DL, Polz M, Sterrer W, Wheeler WC (2000) Triploblastic relationships with emphasis on the acoelomates and the position of Gnathostomulida, Cycliophora, Plathelminthes, and Chaetognatha: a combined approach of 18S rDNA sequences and morphology. Syst Biol 49:539–562
Giribet G, Edgecombe GD (2006) Conflict between data sets and phylogeny of centipedes: an analysis based on seven genes and morphology. Proc R Soc B 273:531–538
Giribet G, Edgecombe GD (2012) Reevaluating the arthropod tree of life. Annu Rev Entomol 57:167–186
Giribet G, Edgecombe GD, Carpenter JM, D’Haese CA, Wheeler WC (2004) Is Ellipura monophyletic? A combined analysis of basal hexapod relationships with emphasis on the origin of insects. Org Divers Evol 4:319–340
Giribet G, Edgecombe GD, Wheeler WC (2001) Arthropod phylogeny based on eight molecular loci and morphology. Nature 413:157–161
Giribet G, Edgecombe GD, Wheeler WC, Babbitt C (2002) Phylogeny and systematic position of opiliones: a combined analysis of chelicerate relationships using morphological and molecular data. Cladistics 18:5–70
Giribet G, Ribera C (1998) The position of arthropods in the animal kingdom: a search for a reliable outgroup for internal arthropod phylogeny. Mol Phylogenet Evol 9:481–488
Giribet G, Ribera C (2000) A review of arthropod phylogeny: new data based on ribosomal DNA sequences and direct character optimization. Cladistics 16:204–231
Giribet G, Richter S, Edgecombe GD, Wheeler WC (2005) The position of crustaceans within the Arthropoda—evidence from nine molecular loci and morphology. In: Koenemann S, Jenner RA (eds) Crustacean issues 16: crustacea and arthropod relationships. Taylor & Francis, Boca Raton, pp 307–352
Giribet G, Wheeler WC (1999) The position of arthropods in the animal kingdom: Ecdysozoa, islands, trees, and the “parsimony ratchet”. Mol Phylogenet Evol 13:619–623
Glenner H, Hansen AJ, Sørensen MV, Ronquist F, Huelsenbeck JP, Willerslev E (2004) Bayesian inference of the metazoan phylogeny; a combined molecular and morphological approach. Curr Biol 14:1644–1649
Grimaldi DA (2010) 400 million years on six legs: on the origin and early evolution of Hexapoda. Arthropod Struct Dev 39:191–203
Hanström B (1926) Vergleichende Anatomie des Nervensystems der wirbellosen Tiere unter Berücksichtigung seiner Funktion. Springer, Berlin
Harvey TH, Velez MI, Butterfield NJ (2012) Exceptionally preserved crustaceans from western Canada reveal a cryptic Cambrian radiation. Proc Natl Acad Sci USA 109:1589–1594
Harzsch S (2004) Phylogenetic comparison of serotonin-immunoreactive neurons in representatives of the Chilopoda, Diplopoda, and Chelicerata: implications for arthropod relationships. J Morphol 259:198–213
Harzsch S (2006) Neurophylogeny: architecture of the nervous system and a fresh view on arthropod phyologeny. Integr Comp Biol 46:162–194
Harzsch S, Hafner G (2006) Evolution of eye development in arthropods: Phylogenetic aspects. Arthropod Struct Dev 35:319–340
Hejnol A, Obst M, Stamatakis AMO, Rouse GW, Edgecombe GD, Martinez P, Baguñà J, Bailly X, Jondelius U, Wiens M, Müller WEG, Seaver E, Wheeler WC, Martindale MQ, Giribet G, Dunn CW (2009) Assessing the root of bilaterian animals with scalable phylogenomic methods. Proc R Soc B 276:4261–4270
Hessler RR (1992) Reflections on the phylogenetic position of the Cephalocarida. Acta Zool 73:315–316
Holton TA, Pisani D (2010) Deep genomic-scale analyses of the Metazoa reject Coelomata: evidence from single- and multigene families analyzed under a supertree and supermatrix paradigm. Genome Biol Evol 2:310–324
Hörnschemeyer T, Beutel RG, Pasop F (2002) Head structures of Priacma serrata Leconte (Coleptera, Archostemata) inferred from X-ray tomography. J Morphol 252:298–314
Hovmöller R, Pape T, Källersjö M (2002) The Palaeoptera problem: basal pterygote phylogeny inferred from 18S and 28S rDNA sequences. Cladistics 18:313–323
Huckstorf K, Wirkner CS (2011) Comparative morphology of the hemolymph vascular system in krill (Euphausiacea; Crustacea). Arthropod Struct Dev 40:39–53
Hwang UW, Friedrich M, Tautz D, Park CJ, Kim W (2001) Mitochondrial protein phylogeny joins myriapods with chelicerates. Nature 413:154–157
Illumina_Inc (2007) DNA sequencing with Solexa ® technology
Inward D, Beccaloni G, Eggleton P (2007) Death of an order: a comprehensive molecular phylogenetic study confirms that termites are eusocial cockroaches. Biol Lett 3:331–335
Irimia M, Maeso I, Penny D, Garcia-Fernàndez J, Roy SW (2007) Rare coding sequence changes are consistent with Ecdysozoa, not Coelomata. Mol Biol Evol 24:1604–1607
Ishiwata K, Sasaki G, Ogawa J, Miyata T, Su Z-H (2011) Phylogenetic relationships among insect orders based on three nuclear protein-coding gene sequences. Mol Phylogenet Evol 58:169–180
Janssen R, Damen WGM, Budd GE (2011) Expression of collier in the premandibular segment of myriapods: support for the traditional Atelocerata concept or a case of convergence? BMC Evol Biol 11:50
Janssen R, Eriksson JB, Budd GE, Akam M, Prpic N-M (2010) Gene expression patterns in onychophorans reveal that regionalization predates limb segmentation in pan-arthropods. Evol Dev 12:363–372
Jenner RA (2010) Higher-level crustacean phylogeny: consensus and conflicting hypotheses. Arthropod Struct Dev 39:143–153
Jenner RA, Scholtz G (2005) Playing another round of metazoan phylogenetics: historical epistemology, sensitivity analysis, and the position of Arthropoda within Metazoa on the basis of morphology. In: Koenemann S, Jenner RA (eds) Crustacean Issues 16: Crustacea and arthropod relationships. Taylor & Francis, Boca Raton, pp 355–385
Kadner D, Stollewerk A (2004) Neurogenesis in the chilopod Lithobius forficatus suggests more similarities to chelicerates than to insects. Dev Genes Evol 214:367–379
Kenrick P, Wellman CH, Schneider H, Edgecombe GD (2012) A timeline for terrestrialization: consequences for the carbon cycle in the Palaeozoic. Philos Trans R Soc B 367:519–536
Kim W, Abele LG (1990) Molecular phylogeny of selected decapod crustaceans based on 18S rRNA nucleotide sequences. J Crustacean Biol 10:1–13
CAS Google Scholar
Klass KD, Zompro O, Kristensen NP, Adis J (2002) Mantophasmatodea: a new insect order with extant members in the afrotropics. Science 296:1456–1459
Koch M (1997) Monophyly and phylogenetic position of the Diplura (Hexapoda). Pedobiologia 41:9–12
Koch M (2000) The cuticular cephalic endoskeleton of primarily wingless hexapods: ancestral state and evolutionary changes. Pedobiologia 44:374–385
Koch M (2003a) Monophyly of Myriapoda? Reliability of current arguments. Afr Invertebr 44:137–153
Koch M (2003b) Towards a phylogenetic system of the Zygentoma. Entomol Abh 61:122–125
Koch M (2009) Protura. In: Resh VH, Carde R (eds) Encyclopedia of insects, 2nd edn. Academic Press/Elsevier Science, San Diego, pp 855–858
Kraus O, Kraus M (1994) Phylogenetic system of the Tracheata (Mandibulata): on “Myriapoda”: Insecta interrelationships, phylogenetic age and primary ecological niches. Verh naturwiss Ver Hamburg 34:5–31
Kraus O, Kraus M (1996) On myriapod/insect interrelationships. Mem Mus nat Hist Nat 169:283–290
Kristensen RM (1991) Loricifera. In: Harrison FW, Ruppert EE (eds) Microscopic anatomy of invertebrates, vol 4., AschelminthesWiley-Liss, New York, pp 351–375
Lankester ER (1904) The structure and classification of the Arthropoda. Q J Microscop Sci 38:523–582
Lavrov DV, Boore JL, Brown WM (2002) Complete mtDNA sequences of two millipedes suggest a new model for mitochondrial gene rearrangements: duplication and nonrandom loss. Mol Biol Evol 19:163–169
Liu J, Steiner M, Dunlop JA, Keupp H, Shu D, Ou Q, Han J, Zhang Z, Zhang X (2011) An armoured Cambrian lobopodian from China with arthropod-like appendages. Nature 470:526–530
Loesel R, Nässel DR, Strausfeld NJ (2002) Common design in a unique midline neuropil in the brains of arthropods. Arthropod Struct Dev 31:77–91
Luan YX, Mallatt JM, Xie RD, Yang YM, Yin WY (2005) The phylogenetic positions of three basal-hexapod groups (Protura, Diplura, and Collembola) based on ribosomal RNA gene sequences. Mol Biol Evol 22:1579–1592
Machida R (2006) Evidence from embryology for reconstructing the relationships of hexapod basal clades. Arthropod Syst Phyl 64:95–104
Machner J, Scholtz G (2010) A scanning electron microscopy study of the embryonic development of Pycnogonum litorale (Arthropoda, Pycnogonida). J Morphol 271:1306–1318
Mallatt J, Craig CW, Yoder MJ (2010) Nearly complete rRNA genes assembled from across the metazoan animals: effects of more taxa, a structure-based alignment, and paired-sites evolutionary models on phylogeny reconstruction. Mol Phylogenet Evol 55:1–17
Mallatt J, Giribet G (2006) Further use of nearly complete 28S and 18S rRNA genes to classify Ecdysozoa: 37 more arthropods and a kinorhynch. Mol Phylogenet Evol 40:772–794
Mallatt J, Winchell CJ (2002) Testing the new animal phylogeny: first use of combined large-subunit and small-subunit rRNA gene sequences to classify the protostomes. Mol Biol Evol 19:289–301
Mallatt JM, Garey JR, Shultz JW (2004) Ecdysozoan phylogeny and Bayesian inference: first use of nearly complete 28S and 18S rRNA gene sequences to classify the arthropods and their kin. Mol Phylogenet Evol 31:178–191
Manton SM (1964) Mandibular mechanisms and the evolution of arthropods. Philos Trans R Soc B 247:1–183
Manton SM (1973) Arthropod phylogeny-a modern synthesis. J Zool 171:11–130
Manton SM (1977) The Arthropoda: habits, functional morphology, and evolution. Clarendon Press, Oxford
Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen YJ, Chen Z, Dewell SB, Du L, Fierro JM, Gomes XV, Godwin BC, He W, Helgesen S, Ho CH, Irzyk GP, Jando SC, Alenquer ML, Jarvie TP, Jirage KB, Kim JB, Knight JR, Lanza JR, Leamon JH, Lefkowitz SM, Lei M, Li J, Lohman KL, Lu H, Makhijani VB, McDade KE, McKenna MP, Myers EW, Nickerson E, Nobile JR, Plant R, Puc BP, Ronan MT, Roth GT, Sarkis GJ, Simons JF, Simpson JW, Srinivasan M, Tartaro KR, Tomasz A, Vogt KA, Volkmer GA, Wang SH, Wang Y, Weiner MP, Yu P, Begley RF, Rothberg JM (2005) Genome sequencing in microfabricated high-density picolitre reactors. Nature 437:376–380
Maslakova SA, Martindale MQ, Norenburg JL (2004) Fundamental properties of the spiralian developmental program are displayed by the basal nemertean Carinoma tremaphoros (Palaeonemertea, Nemertea). Dev Biol 267:342–360
Masta SE, Boore JL (2008) Parallel evolution of truncated transfer RNA genes in arachnid mitochondrial genomes. Mol Biol Evol 25:949–959
Masta SE, Longhorn SJ, Boore JL (2009) Arachnid relationships based on mitochondrial genomes: asymmetric nucleotide and amino acid bias affects phylogenetic analyses. Mol Phylogenet Evol 50:117–128
Mayer G, Whitington PM (2009) Velvet worm development links myriapods with chelicerates. Proc R Soc B 276:3571–3579
Meusemann K, von Reumont BM, Simon S, Roeding F, Strauss S, Kück P, Ebersberger I, Walzl M, Pass G, Breuers S, Achter V, von Haeseler A, Burmester T, Hadrys H, Wägele JW, Misof B (2010) A phylogenomic approach to resolve the arthropod tree of life. Mol Biol Evol 27:2451–2464
Minelli A, Bortoletto S (1988) Myriapod metamerism and arthropod segmentation. Biol J Linn Soc 33:323–343
Miyazaki K (2002) On the shape of foregut lumen in sea spiders (Arthropoda: Pycnogonida). J Mar Biol Assoc UK 82:1037–1038
Møller OS, Olesen J, Avenant-Oldewage A, Thomsen PF, Glenner H (2008) First maxillae suction discs in Branchiura (Crustacea): development and evolution in light of the first molecular phylogeny of Branchiura, Pentastomida, and other “Maxillopoda”. Arthropod Struct Dev 37:333–346
Morozova O, Hirst M, Marra MA (2009) Applications of new sequencing technologies for transcriptome analysis. Annu Rev Genomics Hum Genet 10:135–151
Murienne J, Edgecombe GD, Giribet G (2010) Including secondary structure, fossils and molecular dating in the centipede tree of life. Mol Phylogenet Evol 57:301–313
Niehuis O, Hartig G, Grath S, Pohl H, Lehmann J, Tafer H, Donath A, Krauss V, Eisenhardt C, Hertel J, Petersen M, Mayer C, Meusemann K, Peters RS, Stadler PF, Beutel RG, Bornberg-Bauer E, McKenna DD, Misof B (2012) Genomic and morphological evidence converge to resolve the enigma of Strepsiptera. Current biology: CB 22:1309–1313.
Nielsen C (2001) Animal evolution: interrelationships of the living phyla, 2nd edn. Oxford University Press, Oxford
Nielsen C (2003) Proposing a solution to the articulata-ecdysozoa controversy. Zool Scr 32:475–482
Nielsen C (2012) Animal evolution: interrelationships of the living phyla, 3rd edn. Oxford University Press, Oxford
Nielsen C, Scharff N, Eibye-Jacobsen D (1996) Cladistic analyses of the animal kingdom. Biol J Linn Soc 57:385–410
Oakley TH, Wolfe JM, Lindgren AR, Zaharoff (2013) Phylogenomics to bring the understudied into the fold: monophyletic Ostracoda, fossil placement, and pancrustacean phylogeny. Mol Biol Evol. 30:215–233
Ogden TH, Whiting MF (2003) The problem with “the Palaeoptera problem”: sense and sensitivity. Cladistics 19:432–442
Panganiban G, Irvine SM, Lowe C, Roehl H, Corley LS, Sherbon B, Grenier JK, Fallon JF, Kimble J, Walker M, Wray GA, Swalla BJ, Martindale MQ, Carroll SB (1997) The origin and evolution of animal appendages. Proc Natl Acad Sci USA 94:5162–5166
Paps J, Baguñà J, Riutort M (2009a) Bilaterian phylogeny: a broad sampling of 13 nuclear genes provides a new Lophotrochozoa phylogeny and supports a paraphyletic basal Acoelomorpha. Mol Biol Evol 26:2397–2406
Paps J, Baguñà J, Riutort M (2009b) Lophotrochozoa internal phylogeny: new insights from an up-to-date analysis of nuclear ribosomal genes. Proc R Soc B 276:1245–1254
Pashley DP, McPheron BA, Zimmer EA (1993) Systematics of holometabolous insect orders based on 18S ribosomal RNA. Mol Phylogenet Evol 2:132–142
Pechmann M, Prpic NM (2009) Appendage patterning in the South American bird spider Acanthoscurria geniculata (Araneae: Mygalomorphae). Dev Genes Evol 219:189–198
Pepato AR, da Rocha CE, Dunlop JA (2010) Phylogenetic position of the acariform mites: sensitivity to homology assessment under total evidence. BMC Evol Biol 10:235
Peterson KJ, Eernisse DJ (2001) Animal phylogeny and the ancestry of bilaterians: inferences from morphology and 18S rDNA gene sequences. Evol Dev 3:170–205
Philip GK, Creevey CJ, McInerney JO (2005) The Opisthokonta and the Ecdysozoa may not be clades: stronger support for the grouping of plant and animal than for animal and fungi and stronger support for the coelomata than ecdysozoa. Mol Biol Evol 22:1175–1184
Philippe H, Brinkmann H, Copley RR, Moroz LL, Nakano H, Poustka AJ, Wallberg A, Peterson KJ, Telford MJ (2011) Acoelomorph flatworms are deuterostomes related to Xenoturbella . Nature 470:255–258
Pisani D (2009) Arthropods (Arthropoda). In: Hedges SB, Kumar S (eds) The timetree of life. Oxford University Press, Oxford, pp 251–254
Pisani D, Poling LL, Lyons-Weiler M, Hedges SB (2004) The colonization of land by animals: molecular phylogeny and divergence times among arthropods. BMC Biol 2:1–10
Prpic N-M, Telford MJ (2008) Expression of homothorax and extradenticle mRNA in the legs of the crustacean Parhyale hawaiensis : evidence for a reversal of gene expression regulation in the pancrustacean lineage. Dev Genes Evol 218:333–339
Prpic NM, Damen WGM (2004) Expression patterns of leg genes in the mouthparts of the spider Cupiennius salei (Chelicerata: Arachnida). Dev Genes Evol 214:296–302
Prpic NM, Janssen R, Wigand B, Klingler M, Damen WGM (2003) Gene expression in spider appendages reveals reversal of exd/hth spatial specificity, altered leg gap gene dynamics, and suggests divergent distal morphogen signaling. Dev Biol 264:119–140
Rauther M (1909) Morphologie und Verwandtschaftsbeziehungen der Nematoden und einiger ihnen nahe gestellter Vermalien. Ergebnisse und Fortschritte der Zoologie 1:491–596
Regier JC, Shultz JW (1997) Molecular phylogeny of the major arthropod groups indicates polyphyly of crustaceans and a new hypothesis for the origin of hexapods. Mol Biol Evol 14:902–913
Regier JC, Shultz JW (2001) Elongation factor-2: a useful gene for arthropod phylogenetics. Mol Phylogenet Evol 20:136–148
Regier JC, Shultz JW, Ganley AR, Hussey A, Shi D, Ball B, Zwick A, Stajich JE, Cummings MP, Martin JW, Cunningham CW (2008) Resolving arthropod phylogeny: exploring phylogenetic signal within 41 kb of protein-coding nuclear gene sequence. Syst Biol 57:920–938
Regier JC, Shultz JW, Kambic RE (2004) Phylogeny of basal hexapod lineages and estimates of divergence times. Ann Entomol Soc Amer 97:411–419
Regier JC, Shultz JW, Kambic RE (2005a) Pancrustacean phylogeny: hexapods are terrestrial crustaceans and maxillopods are not monophyletic. Proc R Soc B 272:395–401
Regier JC, Shultz JW, Zwick A, Hussey A, Ball B, Wetzer R, Martin JW, Cunningham CW (2010) Arthropod relationships revealed by phylogenomic analysis of nuclear protein-coding sequences. Nature 463:1079–1083
Regier JC, Wilson HM, Shultz JW (2005b) Phylogenetic analysis of Myriapoda using three nuclear protein-coding genes. Mol Phylogenet Evol 34:147–158
Regier JC, Zwick A (2011) Sources of signal in 62 protein-coding nuclear genes for higher-level phylogenetics of arthropods. PLoS ONE 6:e23408
Rehm P, Borner J, Meusemann K, von Reumont BM, Simon S, Hadrys H, Misof B, Burmester T (2011) Dating the arthropod tree based on large-scale transcriptome data. Mol Phylogenet Evol 61:880–887
Richter S (2002) The Tetraconata concept: hexapod-crustacean relationships and the phylogeny of crustacea. Org Divers Evol 2:217–237
Richter S, Loesel R, Purschke G, Schmidt-Rhaesa A, Scholtz G, Stach T, Vogt L, Wanninger A, Brenneis G, Doring C, Faller S, Fritsch M, Grobe P, Heuer CM, Kaul S, Møller OS, Müller CHG, Rieger V, Rothe BH, Stegner MEJ, Harzsch S (2010) Invertebrate neurophylogeny: suggested terms and definitions for a neuroanatomical glossary. Front Zool 7:29
Richter S, Møller OS, Wirkner CS (2009) Advances in crustacean phylogenetics. Arthropod Syst Phyl 67:275–286
Riesgo A, Andrade SCS, Sharma PP, Novo M, Pérez-Porro AR, Vahtera V, González VL, Kawauchi GY, Giribet G (2012) Comparative description of ten transcriptomes of newly sequenced invertebrates and efficiency estimation of genomic sampling in non-model taxa. Front Zool 9:33
Roeding F, Borner J, Kube M, Klages S, Reinhardt R, Burmester T (2009) A 454 sequencing approach for large scale phylogenomic analysis of the common emperor scorpion ( Pandinus imperator ). Mol Phylogenet Evol 53:826–834
Roeding F, Hagner-Holler S, Ruhberg H, Ebersberger I, von Haeseler A, Kube M, Reinhardt R, Burmester T (2007) EST sequencing of Onychophora and phylogenomic analysis of Metazoa. Mol Phylogenet Evol 45:942–951
Rota-Stabelli O, Campbell L, Brinkmann H, Edgecombe GD, Longhorn SJ, Peterson KJ, Pisani D, Philippe H, Telford MJ (2011) A congruent solution to arthropod phylogeny: phylogenomics, microRNAs and morphology support monophyletic Mandibulata. Proc R Soc B 278:298–306
Rota-Stabelli O, Kayal E, Gleeson D, Daub J, Boore JL, Telford MJ, Pisani D, Blaxter M, Lavrov DV (2010) Ecdysozoan mitogenomics: evidence for a common origin of the legged invertebrates, the Panarthropoda. Genome Biol Evol 2:425–440
Rota-Stabelli O, Lartillot N, Philippe H, Pisani D (2013) Serine codon usage bias in deep phylogenomics: pancrustacean relationships as a case study. Syst Biol. 62:121–133
Rota-Stabelli O, Telford MJ (2008) A multi criterion approach for the selection of optimal outgroups in phylogeny: recovering some support for Mandibulata over Myriochelata using mitogenomics. Mol Phylogenet Evol 48:103–111
Roy SW, Gilbert W (2005) Resolution of a deep animal divergence by the pattern of intron conservation. Proc Natl Acad Sci USA 102:4403–4408
Ruiz-Trillo I, Paps J, Loukota M, Ribera C, Jondelius U, Baguñà J, Riutort M (2002) A phylogenetic analysis of myosin heavy chain type II sequences corroborates that Acoela and Nemertodermatida are basal bilaterians. Proc Natl Acad Sci USA 99:11246–11251
Sanders KL, Lee MS (2010) Arthropod molecular divergence times and the Cambrian origin of pentastomids. Syst Biodiv 8:63–74
Schmidt-Rhaesa A, Bartolomaeus T, Lemburg C, Ehlers U, Garey JR (1998) The position of the Arthropoda in the phylogenetic system. J Morphol 238:263–285
Scholtz G (1998) Cleavage, germ band formation and head segmentation: the ground pattern of the Euarthropoda. In: Fortey RA, Thomas RH (eds) Arthropod relationships. Chapman & Hall, London, pp 317–332
Scholtz G (2002) The Articulata hypothesis—or what is a segment? Org Divers Evol 2:197–215
Schram FR, Koenemann S (2004) Are the crustaceans monophyletic? In: Cracraft J, Donoghue MJ (eds) Assembling the tree of life. Oxford University Press, New York, pp 319–329
Sharma PP, Schwager EE, Extavour CG, Giribet G (2012) Evolution of the chelicera: a dachshund domain is retained in the deutocerebral appendage of Opiliones (Arthropoda, Chelicerata). Evol Dev 14:522–533
Shear WA, Edgecombe GD (2010) The geological record and phylogeny of Myriapoda. Arthropod Struct Dev 39:174–190
Shultz JW (1990) Evolutionary morphology and phylogeny of Arachnida. Cladistics 6:1–38
Shultz JW (2007) A phylogenetic analysis of the arachnid orders based on morphological characters. Zool J Linn Soc 150:221–265
Shultz JW, Regier JC (2000) Phylogenetic analysis of arthropods using two nuclear protein-encoding genes supports a crustacean + hexapod clade. Proc R Soc B 267:1011–1019
Simon S, Strauss S, von Haeseler A, Hadrys H (2009) A phylogenomic approach to resolve the basal pterygote divergence. Mol Biol Evol 26:2719–2730
Simonetta AM (2004) Are the traditional classes of arthropods natural ones?—recent advances in palaeontology and some considerations on morphology. Ital J Zool 71:247–264
Snodgrass RE (1938) Evolution of the Annelida, Onychophora and Arthropoda. Smithsonian Misc Coll 97:1–159
Sørensen M, Hebsgaard MB, Heiner I, Glenner H, Willerslev E, Kristensen RM (2008) New data from an enigmatic phylum: evidence from molecular sequence data supports a sister-group relationship between Loricifera and Nematomorpha. J Zool Syst Evol Res 46:231–239
Sørensen MV, Funch P, Willerslev E, Hansen AJ, Olesen J (2000) On the phylogeny of Metazoa in the light of Cycliophora and Micrognathozoa. Zool Anz 239:297–318
Spears T, Abele LG, Kim W (1992) The monophyly of brachyuran crabs: a phylogenetic study based on 18S rRNA. Syst Biol 41:446–461
Stegner MEJ, Richter S (2011) Morphology of the brain in Hutchinsoniella macracantha (Cephalocarida, Crustacea). Arthropod Struct Dev 40:221–243
Strausfeld NJ (2009) Brain organization and the origin of insects: an assessment. Proc R Soc B 276:1929–1937
Strausfeld NJ (2012) Arthropod brains: evolution, functional elegance, and historical significance. The Balknap Press of Harvard University Press, Cambridge
Strausfeld NJ, Andrew DR (2011) A new view of insect-crustacean relationships inferred from neural cladistics. Arthropod Struct Dev 40:276–280
Strausfeld NJ, Strausfeld CM, Loesel R, Rowell D, Stowe S (2006) Arthropod phylogeny: onychophoran brain organization suggests an archaic relationship with a chelicerate stem lineage. Proc R Soc B 273:1857–1866
Telford MJ, Wise MJ, Gowri-Shankar V (2005) Consideration of RNA secondary structure significantly improves likelihood-based estimates of phylogeny: examples from the Bilateria. Mol Biol Evol 22:1129–1136
Terry MD, Whiting MF (2005) Mantophasmatodea and phylogeny of the lower neopterous insects. Cladistics 21:240–257
Tiegs OW, Manton SM (1958) The evolution of the Arthropoda. Biol Rev 33:255–337
Timmermans MJTN, Roelofs D, Mariën J, van Straalen NM (2008) Revealing pancrustacean relationships: phylogenetic analysis of ribosomal protein genes places Collembola (springtails) in a monophyletic Hexapoda and reinforces the discrepancy between mitochondrial and nuclear DNA markers. BMC Evol Biol 8:83
Trautwein MD, Wiegmann BM, Beutel R, Kjer KM, Yeates DK (2012) Advances in insect phylogeny at the dawn of the postgenomic era. Annu Rev Entomol 57:449–468
Turbeville JM, Pfeifer DM, Field KG, Raff RA (1991) The phylogenetic status of arthropods, as inferred from 18S rRNA sequences. Mol Biol Evol 8:669–686
Ungerer P, Eriksson BJ, Stollewerk A (2011) Neurogenesis in the water flea Daphnia magna (Crustacea, Branchiopoda) suggests different mechanisms of neuroblast formation in insects and crustaceans. Dev Biol 357:42–52
Ungerer P, Scholtz G (2008) Filling the gap between identified neuroblasts and neurons in crustaceans adds new support for Tetraconata. Proc R Soc B 275:369–376
von Reumont BM, Burmester T (2010) Remipedia and the evolution of hexapods: Encyclopedia of Life Sciences. John Wiley & Sons, Chichester
von Reumont BM, Jenner RA, Wills MA, Dell’Ampio E, Pass G, Ebersberger I, Meyer B, Koenemann S, Iliffe TM, Stamatakis A, Niehuis O, Meusemann K, Misof B (2012) Pancrustacean phylogeny in the light of new phylogenomic data: support for Remipedia as the possible sister group of Hexapoda. Mol Biol Evol 29:1031–1045
von Reumont BM, Meusemann K, Szucsich NU, Dell’Ampio E, Gowri-Shankar V, Bartel D, Simon S, Letsch HO, Stocsits RR, Y-x Luan, Wägele JW, Pass G, Hadrys H, Misof B (2009) Can comprehensive background knowledge be incorporated into substitution models to improve phylogenetic analyses? A case study on major arthropod relationships. BMC Evol Biol 9:1–19
Waloszek D, Repetski JE, Maas A (2006) A new late Cambrian pentastomid and a review of the relationships of this parasitic group. Trans Roy Soc Edin Earth Sci 96:163–176
Weygoldt P, Paulus HF (1979) Untersuchungen zur Morphologie, Taxonomie und Phylogenie der Chelicerata: I Morphologische Untersuchungen. Z zool Syst Evol 17:85–116
Wheeler WC (1989) The systematics of insect ribosomal DNA. In: Fernhölm B, Bremer K, Jörnvall H (eds) The hierarchy of life: molecules and morphology in phylogenetic analysis. Elsevier Science Publishers B. V, Amsterdam, pp 307–321
Wheeler WC, Cartwright P, Hayashi CY (1993) Arthropod phylogeny: a combined approach. Cladistics 9:1–39
Wheeler WC, Hayashi CY (1998) The phylogeny of the extant chelicerate orders. Cladistics 14:173–192
Whitfield JB, Kjer KM (2008) Ancient rapid radiations of insects: challenges for phylogenetic analysis. Annu Rev Entomol 53:449–472
Whiting MF (2002) Mecoptera is paraphyletic: multiple genes and phylogeny of Mecoptera and Siphonaptera. Zool Scr 31:93–104
Whiting MF, Carpenter JM, Wheeler QD, Wheeler WC (1997) The Strepsiptera problem: phylogeny of the holometabolous insect orders inferred from 18S and 28S ribosomal DNA sequences and morphology. Syst Biol 46:1–68
Whitington PM, Mayer G (2011) The origins of the arthropod nervous system: insights from the Onychophora. Arthropod Struct Dev 40:193–209
Wiegmann BM, Trautwein MD, Kim JW, Cassel BK, Bertone MA, Winterton SL, Yeates DK (2009) Single-copy nuclear genes resolve the phylogeny of the holometabolous insects. BMC Biol 7:34
Willmer PG (1990) Invertebrate relationships: patterns in animal evolution. Cambridge University Press, Cambridge
Wirkner CS, Prendini L (2007) Comparative morphology of the hemolymph vascular system in scorpions–a survey using corrosion casting, microCT, and 3D-reconstruction. J Morphol 268:401–413
Wirkner CS, Richter S (2004) Improvement of microanatomical research by combining corrosion casts with microCT and 3D reconstruction, exemplified in the circulatory organs of the woodlouse. Microsc Res Tech 64:250–254
Wolf YI, Rogozin IB, Koonin EV (2004) Coelomata and not Ecdysozoa: evidence from genome-wide phylogenetic analysis. Genome Res 14:29–36
Wygodzinsky P (1961) On a surviving representative of the Lepidotrichidae (Thysanura). Ann Entomol Soc Amer 54:621–627
Zhang XG, Maas A, Haug JT, Siveter DJ, Waloszek D (2010) A eucrustacean metanauplius from the lower Cambrian. Curr Biol 20:1075–1079
Zhang Z-Q (ed) (2011) Animal biodiversity: an outline of higher-level classification and survey of taxonomic richness. Magnolia Press, Auckland
Zrzavý J (2003) Gastrotricha and metazoan phylogeny. Zool Scr 32:61–81
Zrzavý J, Hypša V, Tietz DF (2001) Myzostomida are not annelids: Molecular and morphological support for a clade of animals with anterior sperm flagella. Cladistics 17:170–198
Zrzavý J, Hypša V, Vlášková M (1998a) Arthropod phylogeny: taxonomic congruence, total evidence and conditional combination approaches to morphological and molecular data sets. In: Fortey RA, Thomas RH (eds) Arthropod relationships. Chapman & Hall, London, pp 97–107
Zrzavý J, Mihulka S, Kepka P, Bezdek A, Tietz D (1998b) Phylogeny of the Metazoa based on morphological and 18S ribosomal DNA evidence. Cladistics 14:249–285
Download references
Author information
Authors and affiliations.
Museum of Comparative Zoology, Department of Organismic and Evolutionary Biology, Harvard University, 26 Oxford Street, Cambridge, MA, 02138, USA
Gonzalo Giribet
Department of Earth Sciences, Natural History Museum, Cromwell Road, SW7 5BD, London, UK
Gregory D. Edgecombe
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Gonzalo Giribet .
Editor information
Editors and affiliations.
, Department of Biology, University of Padova, Via Ugo Bassi 58 B, Padova, 35131, Italy
Alessandro Minelli
, Zoology Department, Natural History Museum, London, SW7 5BD, United Kingdom
Geoffrey Boxshall
Giuseppe Fusco
Rights and permissions
Reprints and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Giribet, G., Edgecombe, G.D. (2013). The Arthropoda: A Phylogenetic Framework. In: Minelli, A., Boxshall, G., Fusco, G. (eds) Arthropod Biology and Evolution. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-36160-9_2
Download citation
DOI : https://doi.org/10.1007/978-3-642-36160-9_2
Published : 12 April 2013
Publisher Name : Springer, Berlin, Heidelberg
Print ISBN : 978-3-642-36159-3
Online ISBN : 978-3-642-36160-9
eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 01 February 2009
The origin and evolution of arthropods
- Graham E. Budd 1 &
- Maximilian J. Telford 2
Nature volume 457 , pages 812–817 ( 2009 ) Cite this article
8471 Accesses
143 Citations
5 Altmetric
Metrics details
The past two decades have witnessed profound changes in our understanding of the evolution of arthropods. Many of these insights derive from the adoption of molecular methods by systematists and developmental biologists, prompting a radical reordering of the relationships among extant arthropod classes and their closest non-arthropod relatives, and shedding light on the developmental basis for the origins of key characteristics. A complementary source of data is the discovery of fossils from several spectacular Cambrian faunas. These fossils form well-characterized groupings, making the broad pattern of Cambrian arthropod systematics increasingly consensual.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
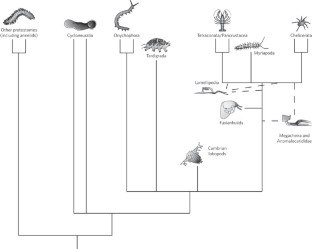
Similar content being viewed by others

Phylogeny of the Synlestidae (Odonata: Zygoptera), with an emphasis on Chlorolestes Selys and Ecchlorolestes Barnard

Bird clades with less complex appendicular skeletons tend to have higher species richness
A Late Devonian actinopterygian suggests high lineage survivorship across the end-Devonian mass extinction
Darwin, C. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life (John Murray, 1859).
Google Scholar
Bowler, P. J. Life's Splendid Drama: Evolutionary Biology and the Reconstruction of Life's Ancestry (Chicago Univ. Press, 1996).
Ballard, J. W. O. et al. Evidence from 12S ribosomal-RNA sequences that onychophorans are modified arthropods. Science 258 , 1345–1348 (1992).
CAS ADS PubMed Google Scholar
Telford, M. J., Bourlat, S. J., Economou, A., Papillon, D. & Rota-Stabelli, O. The evolution of the Ecdysozoa. Phil. Trans. R. Soc. Lond. B 363 , 1529–1537 (2008).
Dunn, C. W. et al. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452 , 745–749 (2008). This paper provides the most recent analysis of the relationships of the Metazoa, using a broadly sampled phylogenomic-scale data set.
Dzik, J. & Krumbiegel, G. The oldest 'onychophoran' Xenusion : a link connecting phyla? Lethaia 22 , 169–182 (1989).
Eernisse, D. J., Albert, J. S. & Anderson, F. E. Annelida and Arthropoda are not sister taxa — a phylogenetic analysis of spiralian metazoan morphology. Syst. Biol. 41 , 305–330 (1992).
Aguinaldo, A. M. A. et al. Evidence for a clade of nematodes, arthropods and other moulting animals. Nature 387 , 489–493 (1997). This classic paper provided the first strong evidence for the clade Ecdysozoa.
CAS PubMed Google Scholar
Wagele, J. W., Erikson, T., Lockhart, P. & Misof, B. The Ecdysozoa: artifact or monophylum? J. Zoolog. Syst. Evol. Res. 37 , 211–223 (1999).
Philip, G. K., Creevey, C. J. & McInerney, J. O. The Opisthokonta and the Ecdysozoa may not be clades: stronger support for the grouping of plant and animal than for animal and fungi and stronger support for the Coelomata than Ecdysozoa. Mol. Biol. Evol. 22 , 1175–1184 (2005).
Rogozin, I. B., Thomson, K., Csueroes, M., Carmel, L. & Koonin, E. V. Homoplasy in genome-wide analysis of rare amino acid replacements: the molecular-evolutionary basis for Vavilov's law of homologous series. Biol. Direct 3 , 7 (2008).
PubMed Central PubMed Google Scholar
Rogozin, I. B., Wolf, Y. I., Carmel, L. & Koonin, E. V. Analysis of rare amino acid replacements supports the Coelomata clade. Mol. Biol. Evol. 24 , 2594–2597 (2007).
Rogozin, I. B., Wolf, Y. I., Carmel, L. & Koonin, E. V. Ecdysozoan clade rejected by genome-wide analysis of rare amino acid replacements. Mol. Biol. Evol. 24 , 1080–1090 (2007).
Zheng, J., Rogozin, I. B., Koonin, E. V. & Przytycka, T. M. Support for the Coelomata clade of animals from a rigorous analysis of the pattern of intron conservation. Mol. Biol. Evol. 24 , 2583–2592 (2007).
Copley, R. R., Aloy, P., Russell, R. B. & Telford, M. J. Systematic searches for molecular synapomorphies in model metazoan genomes give some support for Ecdysozoa after accounting for the idiosyncrasies of Caenorhabditis elegans . Evol. Dev. 6 , 164–169 (2004).
Philippe, H., Lartillot, N. & Brinkmann, H. Multigene analyses of bilaterian animals corroborate the monophyly of Ecdysozoa, Lophotrochozoa, and Protostomia. Mol. Biol. Evol. 22 , 1246–1253 (2005).
Webster, B. L. et al. Mitogenomics and phylogenomics reveal priapulid worms as extant models of the ancestral Ecdysozoan. Evol. Dev. 8 , 502–510 (2006).
PubMed Google Scholar
Irimia, M., Maeso, I., Penny, D., Garcia-Fernàndez, J. & Roy, S. Rare coding sequence changes are consistent with Ecdysozoa, not Coelomata. Mol. Biol. Evol. 24 , 1604–1607 (2007).
Papillon, D., Perez, Y., Caubit, X. & Le Parco, Y. Identification of chaetognaths as protostomes is supported by the analysis of their mitochondrial genome. Mol. Biol. Evol. 21 , 2122–2129 (2004).
Schmidt-Rhaesa, A., Bartolomaeus, T., Lemburg, C., Ehlers, U. & Garey, J. R. The position of the Arthropoda in the phylogenetic system. J. Morphol. 238 , 263–285 (1998).
Budd, G. E. The morphology and phylogenetic significance of Kerygmachela kierkegaardi Budd (Buen Formation, Lower Cambrian, N Greenland). Trans. R. Soc. Edinb. 89 , 249–290 (1999).
Eriksson, B. J. & Budd, G. E. Onychophoran cephalic nerves and their bearing on our understanding of head segmentation and stem-group evolution of Arthropoda. Arthropod Struct. Dev. 29 , 197–209 (2000).
Nielsen, C. Animal Evolution: Interrelationships of the Animal Phyla (Oxford Univ. Press, 2001).
Mallatt, J. & Giribet, G. Further use of nearly complete, 28S and 18S rRNA genes to classify Ecdysozoa: 37 more arthropods and a kinorhynch. Mol. Phylogenet. Evol. 40 , 772–794 (2006).
Bourlat, S. J., Nielsen, C., Economou, A. D. & Telford, M. J. Testing the new animal phylogeny: a phylum level analysis of the animal kingdom. Mol. Phylogenet. Evol. 49 , 23–31 (2008).
Manton, S. M. The Arthropoda: Habits, Functional Morphology and Evolution (Clarendon, 1977).
Fortey, R. A., Briggs, D. E. G. & Wills, M. A. The Cambrian evolutionary 'explosion': decoupling cladogenesis from morphological disparity. Biol. J. Linn. Soc. 57 , 13–33 (1996).
Boore, J. L., Lavrov, D. V. & Brown, W. M. Gene translocation links insects and crustaceans. Nature 392 , 667–668 (1998). This paper details a rare genomic change supporting Pancrustacea rather than Atelocerata.
Giribet, G., Edgecombe, G. D. & Wheeler, W. C. Arthropod phylogeny based on eight molecular loci and morphology. Nature 413 , 157–161 (2001).
Mallatt, J. M., Garey, J. R. & Shultz, J. W. Ecdysozoan phylogeny and Bayesian inference: first use of nearly complete 28S and 18S rRNA gene sequences to classify the arthropods and their kin. Mol. Phylogenet. Evol. 31 , 178–191 (2004).
Richter, S. The Tetraconata concept: hexapod–crustacean relationships and the phylogeny of Crustacea. Org. Divers. Evol. 2 , 217–237 (2002).
Ungerer, P. & Scholtz, G. Filling the gap between identified neuroblasts and neurons in crustaceans adds new support for Tetraconata. Proc. R. Soc. B 275 , 369–376 (2008). This paper presents data typical of the high-quality morphological techniques now being used to address arthropod phylogeny.
Harzsch, S. & Hafner, G. Evolution of eye development in arthropods: phylogenetic aspects. Arthropod Struct. Dev. 35 , 319–340 (2006).
Fanenbruck, M. & Harzsch, S. A brain atlas of Godzilliognomus frondosus Yager, 1989 (Remipedia, Godzilliidae) and comparison with the brain of Speleonectes tulumensis Yager, 1987 (Remipedia, Speleonectidae): implications for arthropod relationships. Arthropod Struct. Dev. 34 , 343–378 (2005).
Sanders, H. L. The Cephalocarida and crustacean phylogeny. Syst. Zool. 6 , 112–128 (1957).
Cook, C. E., Yue, Q. Y. & Akam, M. Mitochondrial genomes suggest that hexapods and crustaceans are mutually paraphyletic. Proc. R. Soc. B 272 , 1295–1304 (2005).
CAS PubMed Central PubMed Google Scholar
Carapelli, A., Liò, P., Nardi, F., van der Wath, E. & Frati, F. Phylogenetic analysis of mitochondrial protein coding genes confirms the reciprocal paraphyly of Hexapoda and Crustacea. BMC Evol. Biol. 7 (suppl. 2), S8 (2007).
Siveter, D. J., Williams, M. & Waloszek, D. A phosphatocopid crustacean with appendages from the Lower Cambrian. Science 293 , 479–481 (2001).
Zhang, X. G., Siveter, D. J., Waloszek, D. & Maas, A. An epipodite-bearing crown-group crustacean from the Lower Cambrian. Nature 449 , 595–598 (2007).
Siveter, D. J., Sutton, M. D., Briggs, D. E. G. & Siveter, D. J. A new probable stem lineage crustacean with three-dimensionally preserved soft parts from the Herefordshire (Silurian) Lagerstatte, UK. Proc. R. Soc. B 274 , 2099–2107 (2007). This paper provides valuable new data on a crustacean-like taxon considerably younger than the Cambrian faunas.
Wilson, H. M. Juliformian millipedes from the Lower Devonian of Euramerica: implications for the timing of millipede cladogenesis in the Paleozoic. J. Paleontol. 80 , 638–649 (2006).
Fayers, S. R. & Trewin, N. H. A hexapod from the Early Devonian Windyfield chert, Rhynie, Scotland. Palaeontology 48 , 1117–1130 (2005).
Boxshall, G. A. Crustacean classification: on-going controversies and unresolved problems. Zootaxa 1668 , 313–325 (2007).
Friedrich, M. & Tautz, D. Ribosomal DNA phylogeny of the major extant arthropod classes and the evolution of myriapods. Nature 376 , 165–167 (1995).
Pisani, D., Poling, L., Lyons-Weiler, M. & Hedges, S. The colonization of land by animals: molecular phylogeny and divergence times among arthropods. BMC Biol. 2 , 1 (2004).
Edgecombe, G. D. Morphological data, extant Myriapoda, and the myriapod stem-group. Contrib. Zool. 73 , 207–252 (2004).
Rota-Stabelli, O. & Telford, M. J. A multi criterion approach for the selection of optimal outgroups in phylogeny: recovering some support for Mandibulata over Myriochelata using mitogenomics. Mol. Phylogenet. Evol. 48 , 103–111 (2008).
Dunlop, J. A. New ideas about the euchelicerate stem-lineage. Acta Zool. Bulg. (Suppl. 1) 9–23 (2005).
Budd, G. E. The Cambrian fossil record and the origin of the phyla. Integr. Comp. Biol. 43 , 157–165 (2003).
Gehling, J. G. The case for Ediacaran fossil roots to the metazoan tree. Mem. Geol. Soc. India 20 , 181–223 (1991).
Waggoner, B. M. Phylogenetic hypotheses of the relationships of arthropods to Precambrian and Cambrian problematic fossil taxa. Syst. Biol. 45 , 190–222 (1996).
Ivantsov, A. Y. Vendia and other Precambrian 'arthropods'. Paleontol. J. 35 , 335–343 (2001).
Jensen, S. The Proterozoic and earliest Cambrian trace fossil record; patterns, problems and perspectives. Integr. Comp. Biol. 43 , 219–228 (2003).
Hou, X.-G., Aldridge, R. J., Bergström, J., Siveter, D. J. & Feng, X.-H. The Cambrian Fossils of Chengjiang, China (Blackwell Science, 2004).
Conway Morris, S., Peel, J. S., Higgins, A. K., Soper, N. J. & Davis, N. C. A Burgess Shale-like fauna from the Lower Cambrian of North Greenland. Nature 326 , 181–183 (1987).
ADS Google Scholar
Briggs, D. E. G. & Collins, D. The arthropod Alalcomenaeus cambricus Simonetta, from the Middle Cambrian Burgess Shale of British Columbia. Palaeontology 42 , 953–977 (1999).
Whittington, H. B. The lobopod animal Aysheaia pedunculata Walcott, Middle Cambrian, Burgess Shale, British Columbia. Phil. Trans. R. Soc. Lond. B 284 , 165–197 (1978).
Budd, G. A Cambrian gilled lobopod from Greenland. Nature 364 , 709–711 (1993).
Budd, G. E. Arthropod body-plan evolution in the Cambrian with an example from anomalocaridid muscle. Lethaia 31 , 197–210 (1998).
Liu, J. N., Shu, D. G., Han, J., Zhang, Z. F. & Zhang, X. L. Morpho-anatomy of the lobopod Magadictyon cf. haikouensis from the Early Cambrian Chengjiang Lagerstatte, South China. Acta Zool. 88 , 279–288 (2007).
Bergstrom, J. Opabinia and Anomalocaris , unique Cambrian arthropods. Lethaia 19 , 241–246 (1986).
Budd, G. E. The morphology of Opabinia regalis and the reconstruction of the arthropod stem-group. Lethaia 29 , 1–14 (1996).
Hou, X.-G. & Bergström, J. Fossils and Strata Vol. 45 (Wiley, 1997).
Størmer, L. On the relationship and phylogeny of fossil and recent Arachnomorpha. A comparative study in Arachnida, Xiphosura, Eurypterida, Trilobita and other fossil Arthropoda. Skr. Norske Vidensk-Akad. 5 , 1–158 (1944).
Chen, J. Y., Waloszek, D. & Maas, A. A new 'great-appendage' arthropod from the Lower Cambrian of China and homology of chelicerate chelicerae and raptorial antero-ventral appendages. Lethaia 37 , 3–20 (2004).
Budd, G. E. A palaeontological solution to the arthropod head problem. Nature 417 , 271–275 (2002).
Chen, J. Y., Edgecombe, G. D., Ramskold, L. & Zhou, G. Q. Head segmentation in Early Cambrian Fuxianhuia — implications for arthropod evolution. Science 268 , 1339–1343 (1995).
Waloszek, D., Chen, J. Y., Maas, A. & Wang, X. Q. Early Cambrian arthropods — new insights into arthropod head and structural evolution. Arthropod Struct. Dev. 34 , 189–205 (2005).
Budd, G. E. Head structure in upper stem-group euarthropods. Palaeontology 51 , 561–573 (2008).
Scholtz, G. & Edgecombe, G. D. The evolution of arthropod heads: reconciling morphological, developmental and palaeontological evidence. Dev. Genes Evol. 216 , 395–415 (2006).
Cotton, T. J. & Braddy, S. J. The phylogeny of arachnomorph arthropods and the origin of the Chelicerata. Trans. R. Soc. Edinb. 94 , 169–193 (2004).
Eriksson, B. J., Tait, N. N. & Budd, G. E. Head development in the onychophoran Euperipatoides kanangrensis with particular reference to the central nervous system. J. Morphol. 255 , 1–23 (2003).
Walossek, D. & Muller, K. J. Upper Cambrian stem-lineage crustaceans and their bearing upon the monophyletic origin of Crustacea and the position of Agnostus . Lethaia 23 , 409–427 (1990).
Telford, M. J. & Thomas, R. H. Demise of the Atelocerata? Nature 376 , 123–124 (1995).
CAS ADS Google Scholar
Damen, W. G. M., Saridaki, T. & Averof, M. Diverse adaptations of an ancestral gill: a common evolutionary origin for wings, breathing organs, and spinnerets. Curr. Biol. 12 , 1711–1716 (2002). The fascinating gene-expression data in this paper support the likely homology of insect wings and tracheae with crustacean gills.
Philippe, H. & Telford, M. J. Large-scale sequencing and the new animal phylogeny. Trends Ecol. Evol. 21 , 614–620 (2006).
Thomas, R. H. & Telford, M. J. Appendage development in embryos of the oribatid mite Archegozetes longisetosus (Acari, Oribatei, Trhypochthoniidae). Acta Zool. 80 , 193–200 (1999).
Download references
Acknowledgements
We thank A. Daley for assistance with photography and R. Copley for critically reading an earlier draft. Our research is supported by the Marie Curie Research Training Network ZOONET (grant number MRTN-CT-2004-005624).
Author information
Authors and affiliations.
Department of Earth Sciences, Uppsala University, Villavägen 16, Uppsala, SE-752 36, Sweden
- Graham E. Budd
Department of Genetics, Evolution and Environment, University College London, Gower Street, London, WC1E 6BT, UK
Maximilian J. Telford
You can also search for this author in PubMed Google Scholar
Ethics declarations
Competing interests.
The authors declare no competing financial interests.
Additional information
Reprints and permissions information is available at http://www.nature.com/reprints .
Correspondence should be addressed to the authors ([email protected]; [email protected]).
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Budd, G., Telford, M. The origin and evolution of arthropods. Nature 457 , 812–817 (2009). https://doi.org/10.1038/nature07890
Download citation
Published : 01 February 2009
Issue Date : 12 February 2009
DOI : https://doi.org/10.1038/nature07890
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Evolution of sex determination in crustaceans.
- Zhiqiang Ye
- Trent Bishop
- Michael Lynch
Marine Life Science & Technology (2023)
An early Cambrian Sidneyia (Arthropoda) resolves the century-long debate of its head organization
- Kunsheng Du
- David L. Bruton
- Xiguang Zhang
Science China Earth Sciences (2023)
Reassessing a cryptic history of early trilobite evolution
- James D. Holmes
Communications Biology (2022)
Towards a Comparative Study of Animal Consciousness
- Walter Veit
Biological Theory (2022)
The essence of life revisited: how theories can shed light on it
- Athel Cornish-Bowden
- María Luz Cárdenas
Theory in Biosciences (2022)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Evolution of Segmentation Among the Chordates, Annelids, and Arthropods Essay
- To find inspiration for your paper and overcome writer’s block
- As a source of information (ensure proper referencing)
- As a template for you assignment
Three hypotheses explain the evolution of segmentation among the three phyla, namely, chordates, annelids, and arthropods. According to the first hypothesis, arthropods, chordates, and annelids exhibit independent mechanisms of segmentation. This hypothesis means that the evolution of segmentation in each of the three phyla is unique and has no any form of evolutionary relationships. The second hypothesis postulates that the evolution of segmentation among protostomes is dependent, while among chordates is independent. The hypothesis implies that protostomes have evolutionary relationships in segmentation, whereas chordates have evolved uniquely. In this case, the hypothesis means that annelids and arthropods have close evolutionary relationships, while chordates and arthropods have distant evolutionary relationships. The third hypothesis predicts that evolution of segmentation among bilaterians is the same. The hypothesis assumes that bilaterians originate from the same ancestor, but have evolved into different organisms with a related pattern of segmentation.
In the first hypothesis, the authors present evidence that support the independence of segmentation in the three phyla, namely, chordates, arthropods, and annelids. The authors present molecular evidence, which shows that insects and non-insects have different mechanisms of segmentation. Comparative analysis of orthologous genes, such as pair-rule genes, indicates that the process of segmentation among arthropods is quite variable. As independent mechanisms of segmentation exist within the phyla, it is predictable that a considerable independence exists between phyla. Regarding the second hypothesis, the authors present evidence that support the dependent acquisition of segmentation among protostomes. The authors use molecular evidence, which shows that both arthropods and annelids express orthologous genes. Segment-polarity gene called engrailed is an orthologous gene that is responsible for the segmentation process. Specifically, Drosophila and leech express this gene during their embryonic stages. Drosophila is an example of arthropod, while leech is an example annelid. In the analysis of the third hypothesis, the authors provide evidence that contradict the existence homology in the segmentation process of bilaterians. The authors argue that pair-rule genes such as engrailed in Amphioxus and herl in zebrafish are not established orthologues of Drosophila genes and their functions in segmentation are not definite. Moreover, orthologues of vertebrates in Drosophila such as fringe, delta , and notch genes have no definite role in segmentation, and thus, do not support the third hypothesis.
The three hypotheses still require further research to establish the evolution of segmentation among chordates, arthropods, and annelids. Although conventional conviction assumes existence of homology in the segmentation process of evolution, molecular studies reveal the contrary. Molecular studies reveal that arthropods and annelids have close evolutionary relationships, while chordates and arthropods have distant evolutionary relationships. The similarities and differences in the patterns of segmentation are interesting because they depict both homologous and independent mechanisms of evolution. Therefore, there is no conclusive evidence to assert that the evolution of segmentation is homologous or independent among the three phyla.
- Artemia Spp and Light Intensity
- How Nematodes Are Beneficial to Humans
- Marine Creatures and Terrestrial Animals in “The Wild West: Gold Rush”
- Origin and Evolution of Segmentation
- Bioscience. The Allopatric Phase of Speciation
- Dugesia: Planariam With Its Own Characteristics
- The Rapid Rate of Thermal Adaptation of Drosophila Melanogaster
- “Estimating the Potential Production of the Brown Mussel Perna Perna” by Galvao
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2022, April 13). Evolution of Segmentation Among the Chordates, Annelids, and Arthropods. https://ivypanda.com/essays/evolution-of-segmentation-among-the-chordates-annelids-and-arthropods/
"Evolution of Segmentation Among the Chordates, Annelids, and Arthropods." IvyPanda , 13 Apr. 2022, ivypanda.com/essays/evolution-of-segmentation-among-the-chordates-annelids-and-arthropods/.
IvyPanda . (2022) 'Evolution of Segmentation Among the Chordates, Annelids, and Arthropods'. 13 April.
IvyPanda . 2022. "Evolution of Segmentation Among the Chordates, Annelids, and Arthropods." April 13, 2022. https://ivypanda.com/essays/evolution-of-segmentation-among-the-chordates-annelids-and-arthropods/.
1. IvyPanda . "Evolution of Segmentation Among the Chordates, Annelids, and Arthropods." April 13, 2022. https://ivypanda.com/essays/evolution-of-segmentation-among-the-chordates-annelids-and-arthropods/.
Bibliography
IvyPanda . "Evolution of Segmentation Among the Chordates, Annelids, and Arthropods." April 13, 2022. https://ivypanda.com/essays/evolution-of-segmentation-among-the-chordates-annelids-and-arthropods/.
- Visit the University of Nebraska–Lincoln
- Apply to the University of Nebraska–Lincoln
- Give to the University of Nebraska–Lincoln
Search Form
Research - arthropod biology, diversity, and conservation.

Biodiversity is the variety of life on Earth at all levels ranging from genes to species to ecosystems, and it is the foundation for all of the life sciences. There is now a broad scientific consensus that biological diversity is being eroded globally at a rate unprecedented in human history.
We know that increasing loss of biodiversity is altering biosphere-level processes that we depend on for $3-33 trillion of environmental services annually. There is an urgency to conducting biodiversity studies, because burgeoning human populations and associated human activities worldwide are rapidly eliminating natural habitats that sustain biodiversity. The causes of biodiversity loss include human impacts on habitats (habitat destruction, fragmentation, restructuring) and organisms (over-exploitation, introduction of invasive species, predators, parasites). Biodiversity is essential to human well-being - the quality of air, water, and food depends on the renewable cycles and healthy ecosystems that are driven by biodiversity.
Research in the Department of Entomology positively influences understanding and conservation of biodiversity by describing the unique nature of the biota and by collaborating with local academic, government, and conservation entities. The pace of basic biodiversity research must be accelerated, and failure to do so will limit our capability to solve impending scientific and human problems.
Current Objectives, Efforts and Approaches
Our objectives include original research, training of students, and public engagement through citizen science and extension activities. Specifically, we will:
- Enhance our understanding of evolution, arthropod biodiversity, and systematics through collection-based research, comparative morphology, comparative genomics, and other appropriate data.
- Conduct internationally recognized research on the evolution, ecology, and conservation of insects, particularly pollinators and native insects.
- Train students in core content through formal coursework and experiential learning.
- Based upon the best available research, work with conservation managers, decision-makers and others to develop and promote insect conservation policies for Nebraska and surrounding states.
A fundamental understanding of species diversity, life cycles, and population structure and dynamics is needed to understand the role and interaction of arthropods in natural ecosystems and to effectively design and develop pest management in agroecosystems.
- Our efforts in systematics are nationally and internationally recognized. We house the U.S. National Collection of Scarabaeidae on loan from the Museum of Natural History of the Smithsonian Institution. Drs. Brett Ratcliffe and Matt Paulsen (UNSM collection manager, grad faculty) provide core expertise in Scarabaeidae morphology and molecular approaches. The recent addition of Dr. Susan Weller (UNSM Director, Ent. Grad faculty) adds additional expertise in comparative biology and systematics of Noctuoidea.
- Our research on ecology, landscape dynamics, and conservation has a Nebraska-focus and supports our Extension efforts in apiculture, native pollinators, endangered species, and science literacy. We are developing a cluster of excellence with the addition of faculty members Dr. Judy Wu-Smart and Dr. Autumn Smart who complement existing research and citizen science programs. Research on the endangered Salt Marsh Tiger Beetle has enhanced our efforts to protect this species and resulted in its federal listing and setting aside areas of critical habitat.
- Student training occurs in the classroom and research projects. Insect taxonomy is required for students in Insect Science, our graduate programs, and the Doctor of Plant Health program, thus contributing to the campus-wide Life Sciences Initiative. Insect biology also supports the Life Sciences Initiative and serves several undergraduate majors. Experiential learning occurs through undergraduate and graduate research projects and collection research assistantships.
We will conduct research in insect systematics, arthropod biology/ecology, and conservation in order to advance knowledge of insect biodiversity, and to better understand ecological processes in order to manage arthropod pests, ecosystem service providers (pollination, nutrient recycling, biocontrol), and endangered species.
Programs and Staffing
Faculty and staff conducting research on Insect Biology, Diversity, and Conservation:
- Dr. Jeffrey Bradshaw
- Dr. Douglas A. Golick
- Dr. Thomas Hunt
- Dr. Justin McMechan
- Dr. Lance J. Meinke
- Dr. Matt J. Paulsen
- Dr. Julie A. Peterson
- Dr. Brett C. Ratcliffe
- Dr. Autumn Smart
- Stephen M. Spomer
- Dr. Ana Velez
- Dr. Thomas J. Weissling
- Dr. Susan J. Weller
- Dr. Judy Wu-Smart
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar
- Skip to footer
- Image & Use Policy
- Translations
UC MUSEUM OF PALEONTOLOGY
Understanding Evolution
Your one-stop source for information on evolution
The Arthropod Story
by the Understanding Evolution team
The Arthropod Story takes you on a tour through the amazing evolutionary history of arthropods. Along the way, you’ll get a healthy dose of taxonomy, paleontology, natural history and principles of evolution.
Introducing the arthropods
Subscribe to our newsletter
- Teaching resource database
- Correcting misconceptions
- Conceptual framework and NGSS alignment
- Image and use policy
- Evo in the News
- The Tree Room
- Browse learning resources
- A-Z Publications
Annual Review of Ecology, Evolution, and Systematics
Volume 51, 2020, review article, arthropod origins: integrating paleontological and molecular evidence.
- Gregory D. Edgecombe 1
- View Affiliations Hide Affiliations Affiliations: Department of Earth Sciences, The Natural History Museum, London SW7 5BD, United Kingdom; email: [email protected]
- Vol. 51:1-25 (Volume publication date November 2020) https://doi.org/10.1146/annurev-ecolsys-011720-124437
- First published as a Review in Advance on July 31, 2020
- Copyright © 2020 by Annual Reviews. All rights reserved
Phylogenomics underpins a stable and mostly well-resolved hypothesis for the interrelationships of extant arthropods. Exceptionally preserved fossils are integrated into this framework by coding their morphological characters, as exemplified by total-evidence dating approaches that treat fossils as dated tips in analyses numerically dominated by molecular data. Cambrian fossils inform on the sequence of character acquisition in the arthropod stem group and in the stems of its main extant clades. The arthropod head problem incorporates unique appendage combinations and remains of the nervous system in fossils into a scheme mostly based on neuroanatomy and Hox expression domains for extant forms. Molecular estimates of arthropod origins in the Cryogenian or Ediacaran predate a coherent picture from the arthropod fossil record, which commences as trace fossils in the earliest Cambrian. Probabilistic morphological clock analysis of trilobites, which exemplify the earliest arthropod body fossils, supports a Cambrian origin, without the need to posit an unfossilized Ediacaran history.
Article metrics loading...
Full text loading...
Literature Cited
- Aguinaldo AMA , Turbeville JM , Lindford LS , Rivera MC , Garey JR et al. 1997 . Evidence for a clade of nematodes, arthropods and other moulting animals. Nature 387 : 489– 93 [Google Scholar]
- Aria C. 2019 . Reviewing the bases for a nomenclatural uniformization of the highest taxonomic levels in arthropods. Geol. Mag. 156 : 1463– 68 [Google Scholar]
- Aria C , Caron J-B. 2015 . Cephalic and limb anatomy of a new isoxyid from the Burgess Shale and the role of “stem bivalved arthropods” in the disparity of the frontalmost appendages. PLOS ONE 10 : 6 e0124949 [Google Scholar]
- Aria C , Caron J-B. 2017a . Burgess Shale fossils illustrate the origin of the mandibulate body plan. Nature 545 : 89– 92 [Google Scholar]
- Aria C , Caron J-B. 2017b . Mandibulate convergence in an armoured Cambrian stem chelicerate. BMC Evol. Biol. 17 : 261 [Google Scholar]
- Aria C , Caron J-B. 2019 . A middle Cambrian arthropod with chelicerae and proto-book gills. Nature 573 : 586– 89 [Google Scholar]
- Aria C , Caron J-B , Gaines R 2015 . A large new leanchoiliid from the Burgess Shale and the influence of inapplicable states on stem arthropod phylogeny. Palaeontology 58 : 629– 60 [Google Scholar]
- Aria C , Zhao F , Zeng H , Guo J , Zhu M 2020 . Fossils from South China redefine the ancestral euarthropod body plan. BMC Evol. Biol. 20 : 4 [Google Scholar]
- Ballesteros JA , Santibáñez López CE , Kováč L , Gavish-Regev E , Sharma PP 2019 . Ordered phylogenomic subsampling enables diagnosis of systematic errors in the placement of the enigmatic arachnid order Palpigradi. Proc. R. Soc. B 286 : 20192426 [Google Scholar]
- Ballesteros JA , Sharma PP. 2018 . A critical appraisal of the placement of Xiphosura (Chelicerata) with account of known sources of phylogenetic error. Syst. Zool. 68 : 896– 917 [Google Scholar]
- Borner J , Rehm P , Schill RO , Ebersberger I , Burmester T 2014 . A transcriptome approach to ecdysozoan phylogeny. Mol. Phylogenetics Evol. 80 : 79– 87 [Google Scholar]
- Boxshall GA. 2004 . The evolution of arthropod limbs. Biol. Rev. 79 : 253– 300 [Google Scholar]
- Briggs DEG. 1978 . The morphology, mode of life, and affinities of Canadaspis perfecta (Crustacea: Phyllocarida), Middle Cambrian, Burgess Shale, British Columbia. Philos. Trans. R. Soc. B 281 : 439– 87 [Google Scholar]
- Budd GE. 1998 . Arthropod body-plan evolution in the Cambrian with an example from anomalocaridid muscle. Lethaia 31 : 197– 210 [Google Scholar]
- Budd GE. 1999 . The morphology and phylogenetic significance of Kerygmachela kierkegaardi Budd (Buen Formation, Lower Cambrian, N Greenland). Trans. R. Soc. Edinb. Earth Sci. 89 : 249– 90 [Google Scholar]
- Budd GE. 2002 . A palaeontological solution to the arthropod head problem. Nature 417 : 271– 75 [Google Scholar]
- Campbell LI , Rota-Stabelli O , Edgecombe GD , Marchioro T , Longhorn SJ et al. 2011 . MicroRNAs and phylogenomics resolve the phylogenetic relationships of the Tardigrada, and suggest the velvet worms as the sister group of Arthropoda. PNAS 108 : 15920– 24 [Google Scholar]
- Carapelli A , Lió P , Nardi F , van der Wath E , Frati F 2007 . Phylogenetic analysis of mitochondrial protein coding genes confirms the reciprocal paraphyly of Hexapoda and Crustacea. BMC Evol. Biol. 7 : S8 [Google Scholar]
- Caron J-B , Aria C. 2017 . Cambrian suspension-feeding lobopodians and the early evolution of panarthropods. BMC Evol. Biol. 17 : 29 [Google Scholar]
- Caron J-B , Gaines RR , Aria C , Mángano MG , Streng M 2014 . A new phyllopod bed-like assemblage from the Burgess Shale of the Canadian Rocky Mountains. Nat. Commun. 5 : 3210 [Google Scholar]
- Chipman AD , Edgecombe GD. 2019 . Developing an integrated understanding of arthropod segmentation using fossils and evo-devo. Proc. R. Soc. B 286 : 20191881 [Google Scholar]
- Cong P , Daley AC , Edgecombe GD , Hou X 2017 . The functional head of the Cambrian radiodontan (stem-group Euarthropoda. Amplectobelua symbrachiata. BMC Evol. Biol. 17 : 208 [Google Scholar]
- Daley AC , Antcliffe JB , Drage HB , Pates S 2018 . Early fossil record of Euarthropoda and the Cambrian Explosion. PNAS 115 : 5323– 31 [Google Scholar]
- Daley AC , Edgecombe GD. 2014 . Morphology of Anomalocaris canadensis from the Burgess Shale. J. Paleontol. 88 : 68– 91 [Google Scholar]
- Dell'Ampio E , Meusemann K , Szucsich NU , Peters RS , Meyer B et al. 2013 . Decisive data sets in phylogenomics: lessons from studies on the phylogenetic relationships of primarily flightless insects. Mol. Biol. Evol. 31 : 239– 49 [Google Scholar]
- Dong X-P , Bengtson S , Gostling NJ , Cunningham JA , Harvey THP et al. 2010 . The anatomy, taphonomy, taxonomy and systematic affinity of Markuelia : Early Cambrian to Early Ordovician scalidophorans. Palaeontology 53 : 1291– 314 [Google Scholar]
- Donoghue PCJ , Yang Z. 2016 . The evolution of methods for establishing evolutionary timescales. Philos. Trans. R. Soc. B 371 : 20160020 [Google Scholar]
- Dunlop JA , Lamsdell JC. 2017 . Segmentation and tagmosis in Chelicerata. Arthropod Struct. Dev. 46 : 395– 418 [Google Scholar]
- Dzik J. 2011 . The xenusian-to-anomalocaridid transition within the lobopodians. Boll. Soc. Paleontol. Ital. 50 : 65– 74 [Google Scholar]
- Edgecombe GD. 2017 . Inferring arthropod phylogeny: fossils and their interaction with other data sources. Integr. Comp. Biol. 57 : 467– 76 [Google Scholar]
- Ertas B , von Reumont BM , Wägele J-W , Misof B , Burmester Y 2009 . Hemocyanin suggests a close relationship of Remipedia and Hexapoda. Mol. Biol. Evol. 26 : 2711– 18 [Google Scholar]
- Fleming JF , Kristensen RM , Sorensen MV , Park T-YS , Arakawa K et al. 2018 . Molecular palaeontology illuminates the evolution of ecdysozoan vision. Proc. R. Soc. B 285 : 20182180 [Google Scholar]
- Fu D , Tong G , Dai T , Liu W , Yang Y et al. 2019 . The Qingjiang biota—a Burgess Shale-type Konservat-Lagerstätte from the early Cambrian of South China. Science 363 : 1338– 42 [Google Scholar]
- Gabriel WN , Goldstein B. 2007 . Segmental expression of Pax3/7 and engrailed homologs in tardigrade development. Dev. Genes Evol. 217 : 421– 33 [Google Scholar]
- Giribet G , Edgecombe GD. 2017 . Current understanding of Ecdysozoa and its internal phylogenetic relationships. Integr. Comp. Biol. 57 : 455– 66 [Google Scholar]
- Goloboff PA , Pittman M , Pol D , Xu X 2019 . Morphological data sets fit a common mechanism much more poorly than DNA sequences and call into question the MkV model. Syst. Biol. 68 : 494– 504 [Google Scholar]
- Gross V , Mayer G. 2015 . Neural development in the tardigrade Hyposibius dujardini based on anti-acetylated α-tubulin immunolabeling. EvoDevo 6 : 12 [Google Scholar]
- Harvey THP , Butterfield NJ. 2017 . Exceptionally preserved Cambrian loriciferans and the early animal invasion of the meiobenthos. Nat. Ecol. Evol. 1 : 0022 [Google Scholar]
- Haug JT , Briggs DEG , Haug C 2012a . Morphology and function in the Cambrian Burgess Shale megacheiran arthropod Leanchoilia superlata and the application of a descriptive matrix. BMC Evol. Biol. 12 : 162 [Google Scholar]
- Haug JT , Maas A , Haug C , Waloszek D 2012b . Evolution of crustacean appendages. The Natural History of the Crustacea , Vol. 1: Functional Morphology and Diversity L Watling, M Thiel 34– 73 Oxford, UK: Oxford Univ. Press [Google Scholar]
- Haug JT , Maas A , Waloszek D 2010a . † Henningsmoenicaris scutula , † Sandtorpia vestrogothienis gen. et sp. nov. and heterochronic events in early crustacean evolution. Trans. R. Soc. Edinb. Earth. Sci. 100 : 311– 50 [Google Scholar]
- Haug JT , Waloszek D , Haug C , Maas A 2010b . High-level phylogenetic analysis using developmental sequences: the Cambrian † Martinssonia elongata , † Muscacaris gerdgeyeri gen. et sp. nov. and their position in early crustacean evolution. Arthropod Struct. Dev. 39 : 154– 74 [Google Scholar]
- Haug JT , Waloszek D , Maas A , Liu Y , Haug C et al. 2012c . Functional morphology, ontogeny and evolution of mantis shrimp-like predators in the Cambrian. Palaeontology 55 : 369– 99 [Google Scholar]
- Holton TA , Pisani D. 2010 . Deep genomic-scale analyses of the Metazoa reject Coleomata: evidence from single-and multigene families analysed under a supertree and supermatrix paradigm. Genome Biol. Evol. 2 : 310– 24 [Google Scholar]
- Hou X , Bergström J. 1997 . Arthropods of the Lower Cambrian Chengjiang fauna, southwest China. Fossils Strata 45 : 1– 116 [Google Scholar]
- Jago JB , García-Bellido DC , Gehling JG 2016 . An early Cambrian chelicerate from the Emu Bay Shale, South Australia. Palaeontology 59 : 549– 62 [Google Scholar]
- Jensen S , Droser ML , Gehling JG 2005 . Trace fossil preservation and the early evolution of animals. Palaeogeog. Palaeoclimatol. Palaeoecol. 220 : 19– 29 [Google Scholar]
- Jockusch EL. 2017 . Developmental and evolutionary perspectives on the origin and diversification of arthropod appendages. Integr. Comp. Biol. 57 : 533– 45 [Google Scholar]
- Kesidis G , Slater BJ , Jensen S , Budd GE 2019 . Caught in the act: priapulid burrowers in early Cambrian substrates. Proc. R. Soc. B 286 : 20182505 [Google Scholar]
- Kühl G , Briggs DEG , Rust J 2009 . A great-appendage arthropod with a radial mouth from the Lower Devonian Hunsrück Slate, Germany. Science 323 : 771– 73 [Google Scholar]
- Lamsdell JC. 2013 . Revised systematics of Palaeozoic ‘horseshoe crabs’ and the myth of monophyletic Xiphosura. Zool. J. Linn. Soc. 167 : 1– 27 [Google Scholar]
- Laumer CE , Fernández R , Lemer S , Combosch D , Kocot KM et al. 2019 . Revisiting metazoan phylogeny with genomic sampling of all phyla. Proc. R. Soc. B 286 : 20190831 [Google Scholar]
- Legg DA , Sutton MD , Edgecombe GD 2013 . Arthropod fossil data increase congruence of morphological and molecular phylogenies. Nat. Commun. 4 : 2485 [Google Scholar]
- Legg DA , Vannier J. 2013 . The affinities of the cosmopolitan arthropod Isoxys and its implications for the origin of arthropods. Lethaia 46 : 540– 50 [Google Scholar]
- Liu J , Shu D , Han J , Zhang Z , Zhang X 2006 . A large xenusiid lobopod with complex appendages from the Lower Cambrian Chengjiang Lagerstätte. Acta Palaentol. Pol. 51 : 215– 22 [Google Scholar]
- Liu J , Shu D , Han J , Zhang Z , Zhang X 2007 . Morpho-anatomy of the lobopod Magadictyon cf. haikouensis from the Early Cambrian Chengjiang Lagerstätte, South China. Acta Zool 88 : 279– 88 [Google Scholar]
- Liu Y , Xiao S , Shao T , Broce J , Zhang H 2014 . The oldest known priapulid-like scalidophoran animal and its implications for the early evolution of cycloneuralians and ecdysozoans. Evol. Dev. 16 : 155– 65 [Google Scholar]
- Lozano-Fernandez J , Carton R , Tanner AR , Puttick MN , Blaxter M et al. 2016 . A molecular palaeobiological exploration of arthropod terrestrialisation. Philos. Trans. R. Soc. B 371 : 20150133 [Google Scholar]
- Lozano-Fernandez J , Giacomelli M , Fleming JF , Chen A , Vinther J et al. 2019b . Pancrustacean evolution illuminated by taxon-rich genomic-scale data sets with an expanded remipede sampling. Genome Biol. Evol. 11 : 2055– 70 [Google Scholar]
- Lozano-Fernandez J , Tanner AR , Vinther J , Giacomelli M , Carton R et al. 2019a . Increasing species sampling in chelicerate genomic-scale datasets provides support for monophyly of Acari and Arachnida. Nat. Commun. 10 : 2295 [Google Scholar]
- Luo A , Duchêne DA , Zhang C , Zhu C-D , Ho SYW 2019 . A simulation-based evaluation of tip-dating under the fossilized birth-death model. Syst. Biol. 69 : 325– 44 [Google Scholar]
- Ma X , Aldridge RJ , Siveter DJ , Siveter DJ , Hou X et al. 2014 . A new exceptionally preserved Cambrian priapulid from the Chengjiang Lagerstätte. J. Palaeontol. 88 : 371– 84 [Google Scholar]
- Maas A , Braun A , Dong X-P , Donoghue PCJ , Müller KJ et al. 2006 . The ‘Orsten’—more than a Cambrian Konservat-Lagerstätte yielding exceptional preservation. Palaeoworld 15 : 266– 82 [Google Scholar]
- Mángano MG , Buatois LA. 2014 . Decoupling of body-plan diversification and ecological structuring during the Ediacaran-Cambrian transition: evolutionary and geobiological feedbacks. Proc. R. Soc. B 281 : 20140038 [Google Scholar]
- Marlétaz F , Peijnenburg KTCA , Goto T , Satoh N , Rokhsar D 2019 . A new spiralian phylogeny places the enigmatic arrow worms among gnathiferans. Curr. Biol. 29 : 312– 18 [Google Scholar]
- Martín-Durán JM , Wolff GH , Strausfeld NJ , Hejnol A 2016 . The larval nervous system of the penis worm Priapulus caudautus (Ecdysozoa). Philos. Trans. R. Soc. B 371 : 20150050 [Google Scholar]
- Mayer G , Martin C , Rüdiger J , Kauschke S , Stevenson PA et al. 2013 . Selective neuronal staining in tardigrades and onychophorans provides insights into the evolution of segmental ganglia in panarthropods. BMC Evol. Biol. 13 : 230 [Google Scholar]
- Mayer G , Whitington PM , Sunnucks P , Pflüger H-J 2010 . A revision of brain composition in Onychophora (velvet worms) suggests that the tritocerebrum evolved in arthropods. BMC Evol. Biol. 10 : 255 [Google Scholar]
- Misof B , Liu S , Meusemann K , Peters RS , Donath A et al. 2014 . Phylogenomics resolves the timing and pattern of insect evolution. Science 346 : 763– 67 [Google Scholar]
- Noah KE , Hao J , Li L , Sun X , Foley B , Yang Q , Xia X 2020 . Major revisions in arthropod phylogeny through improved supermatrix, with support for two possible waves of land invasion by chelicerates. Evol. Bioinform. 16 : https://doi.org/10.1177/1176934320903735 [Crossref] [Google Scholar]
- Oakley TH , Wolfe JM , Lindgren AR , Zaharoff AK 2013 . Phylotranscriptomics to bring the understudied into the fold: monophyletic Ostracoda, fossil placement, and pancrustacean phylogeny. Mol. Biol. Evol. 30 : 215– 33 [Google Scholar]
- Ortega-Hernández J. 2015 . Lobopodians. Curr. Biol. 25 : R873– 75 [Google Scholar]
- Ortega-Hernández J , Janssen R , Budd GE 2017 . Origin and evolution of the panarthropod head—a palaeobiological and developmental perspective. Arthropod Struct. Dev. 46 : 354– 79 [Google Scholar]
- Ou G , Liu J , Shu D , Han J , Zhang Z et al. 2011 . A rare onychophoran-like lobopodian from the lower Cambrian Chengjiang Lagerstätte, Southwestern China, and its phylogenetic implications. J . . Paleontol 85 : 587– 94 [Google Scholar]
- Park T-S , Kihm J-H , Woo J , Park C , Lee WY et al. 2018 . Brain and eyes of Kerygmachela reveal protocerebral ancestry of the panarthropod head. Nat. Commun. 9 : 1019 [Google Scholar]
- Paterson JR , Edgecombe GD , Lee MSY 2019 . Trilobite evolutionary rates constrain the duration of the Cambrian explosion. PNAS 116 : 4394– 99 [Google Scholar]
- Paterson JR , García-Bellido DC , Lee MSY , Brock GA , Jago JB et al. 2011 . Acute vision in the giant Cambrian predator Anomalocaris and the origin of compound eyes. Nature 480 : 237– 40 [Google Scholar]
- Pyron RA. 2017 . Novel approaches for phylogenetic inference from morphological data and total-evidence dating in squamate reptiles (lizards, snakes, and amphisbaenians). Syst. Biol. 66 : 38– 56 [Google Scholar]
- Regier JC , Shultz JW , Zwick A , Hussey A , Ball B et al. 2010 . Arthropod relationships revealed by phylogenomic analysis of nuclear protein-coding sequences. Nature 463 : 1079– 83 [Google Scholar]
- Rehm P , Borner J , Meusemann K , von Reumont BM , Simon S et al. 2011 . Dating the arthropod tree based on large-scale transcriptome data. Mol. Phylogenetics Evol. 61 : 880– 87 [Google Scholar]
- Richter S , Stein M , Frase T , Szucsich NU 2013 . The arthropod head. Arthropod Biology and Evolution: Molecules, Development, Morphology A Minelli, G Boxshall, G Fusco 223– 40 Berlin/Heidelberg, Ger: Springer [Google Scholar]
- Ronquist F , Klopstein S , Vilhelmsen L , Schulmeister S , Murray DL et al. 2012 . A total-evidence approach to dating with fossils, applied to the early radiation of Hymenoptera. Syst. Biol. 26 : 1663– 76 [Google Scholar]
- Rota-Stabelli O , Campbell L , Brinkmann H , Edgecombe GD , Longhorn SJ et al. 2011 . A congruent solution to arthropod phylogeny: phylogenomics, microRNAs and morphology support monophyletic Mandibulata. Proc. R. Soc. B 278 : 298– 306 [Google Scholar]
- Rota-Stabelli O , Daley AC , Pisani D 2013 . Molecular timetrees reveal a Cambrian colonization of land and a new scenario for ecdysozoan evolution. Curr. Biol. 23 : 392– 98 [Google Scholar]
- Schmidt-Rhaesa A , Bartolomaeus T , Lemburg C , Ehlers U , Garey JR 1998 . The position of the Arthropoda in the phylogenetic system. J. Morphol. 238 : 263– 85 [Google Scholar]
- Scholtz G. 2002 . The Articulata hypothesis—or what is a segment. Org. Divers. Evol. 2 : 197– 215 [Google Scholar]
- Scholtz G , Edgecombe GD. 2006 . The evolution of arthropod heads: reconciling morphological, developmental and palaeontological evidence. Dev. Genes Evol. 216 : 395– 415 [Google Scholar]
- Scholtz G , Staude A , Dunlop JA 2019 . Trilobite compound eyes with crystalline cones and rhabdoms show mandibulate affinities. Nat. Commun. 10 : 2503 [Google Scholar]
- Schwentner M , Combosch DJ , Nelson JP , Giribet G 2017 . A phylogenomic solution to the origin of insects by resolving crustacean-hexapod relationships. Curr. Biol. 27 : 1818– 24 [Google Scholar]
- Sharma PP , Kaluziak ST , Perez-Porro AR , González VL , Hormiga G et al. 2014 . Phylogenomic interrogation of Arachnida reveals systemic conflicts in phylogenetic signal. Mol. Biol. Evol. 31 : 2963– 84 [Google Scholar]
- Siveter DJ , Briggs DEG , Siveter DJ , Sutton MD 2019 . The Herefordshire Lagerstätte: fleshing out Silurian marine life. J. Geol. Soc. 177 : 1– 13 [Google Scholar]
- Slater BJ , Harvey THP , Guilbaud R , Butterfield NJ 2017 . A cryptic record of Burgess Shale-type diversity from the early Cambrian of Baltica. Palaeontology 60 : 117– 40 [Google Scholar]
- Smith FW , Boothby TC , Giovannini I , Rebecchi L , Jockusch EL , Goldstein B 2016 . The compact body plan of tardigrades evolved by the loss of a large body region. Curr. Biol. 26 : 224– 29 [Google Scholar]
- Smith FW , Cumming M , Goldstein B 2018 . Analyses of nervous system patterning genes in the tardigrade Hypsibius exemplaris illuminate the evolution of panarthropod brains. EvoDevo 9 : 19 [Google Scholar]
- Smith MR , Ortega-Hernández J. 2014 . Hallucigenia ’s onychophoran-like claws and the case for Tactopoda. Nature 514 : 363– 66 [Google Scholar]
- Stemme T , Iliffe TM , Bicker G , Harzsch S , Koenemann S 2012 . Serotonin immunoreactive interneurons in the brain of the Remipedia: new insights into the phylogenetic affinities of an enigmatic crustacean taxon. BMC Evol. Biol. 12 : 168 [Google Scholar]
- Strausfeld NJ. 2012 . Arthropod Brains: Evolution, Functional Elegance, and Historical Significance Cambridge, MA: Harvard Univ. Press [Google Scholar]
- Strausfeld NJ , Ma X , Edgecombe GD 2016a . Fossils and the evolution of the arthropod brain. Curr. Biol. 26 : R989– 1000 [Google Scholar]
- Strausfeld NJ , Ma X-Y , Edgecombe GD , Fortey RA , Land M et al. 2016b . Arthropod eyes: the early Cambrian fossil record and divergent evolution of visual systems. Arthropod Struct. Dev. 45 : 152– 72 [Google Scholar]
- Tanaka G , Hou X , Ma X , Edgecombe GD , Strausfeld NJ 2013 . Chelicerate neural ground pattern in a Cambrian great appendage arthropod. Nature 502 : 364– 67 [Google Scholar]
- Van Roy P , Daley AC , Briggs DEG 2015 . Anomalocaridid trunk limb homology revealed by a giant filter-feeder with paired flaps. Nature 522 : 77– 80 [Google Scholar]
- Vannier J , Aria C , Taylor RS , Caron J-B 2018 . Waptia fieldensis Walcott, a mandibulate arthropod from the middle Cambrian Burgess Shale. R. Soc. Open Sci. 5 : 172206 [Google Scholar]
- Vannier J , Liu J , Lerosey-Aubril R , Vinther J , Daley AC 2014 . Sophisticated digestive systems in early arthropods. Nat. Commun. 5 : 3641 [Google Scholar]
- Vinther J , Porras L , Young F , Budd GE , Edgecombe GD 2016 . The mouth apparatus of the Cambrian gilled lobopodian Pambdelurion whittingtoni . . Palaeontology 59 : 841– 49 [Google Scholar]
- Wägele JW , Kück P. 2014 . Arthropod phylogeny and the origin of Tracheata (= Atelocerata) from Remipedia-like ancestors. Deep Metazoan Phylogeny: The Backbone of the Tree of Life JW Wägele, T Bartolomaeus 285– 341 Berlin/Boston: de Gruyter [Google Scholar]
- Wheat CW , Wahlberg N. 2013 . Phylogenomic insights into the Cambrian Explosion, the colonization of land and the evolution of flight in Arthropoda. Syst. Biol. 61 : 93– 109 [Google Scholar]
- Wolfe JM. 2017 . Metamorphosis is ancestral for crown arthropods, and evolved in the Cambrian or earlier. Integr. Comp. Biol. 59 : 499– 509 [Google Scholar]
- Wolfe JM , Daley AC , Legg DA , Edgecombe GD 2016 . Fossil calibrations for the arthropod Tree of Life. Earth-Sci. Rev. 160 : 43– 110 [Google Scholar]
- Yang J , Ortega-Hernández J , Butterfield NJ , Liu Y , Boyan G et al. 2016 . Fuxianhuiid ventral nerve cord and early nervous system evolution in Panarthropoda. PNAS 113 : 2988– 93 [Google Scholar]
- Yang J , Ortega-Hernández J , Butterfield NJ , Zhang X 2013 . Specialized appendages in fuxianhuiids and the head organization of early euarthropods. Nature 494 : 468– 71 [Google Scholar]
- Yang J , Ortega-Hernández J , Gerber S , Butterfield NJ , Hou J et al. 2015 . A superarmoured lobopodian from the Cambrian of China and early disparity in the evolution of Onychophora. PNAS 112 : 8678– 83 [Google Scholar]
- Yang J , Ortega-Hernández J , Legg DA , Lan T , Hou JB , Zhang XG 2018 . Early Cambrian fuxianhuiids from China reveal origin of the gnathobasic protopodite in euarthropods. Nat. Commun. 9 : 470 [Google Scholar]
- Young FJ , Vinther J. 2017 . Onychophoran-like myoanatomy of the Cambrian gilled lobopodian Pambdelurion whittingtoni . . Palaeontology 60 : 27– 54 [Google Scholar]
- Zhai D , Edgecombe GD , Bond AD , Mai H , Hou X , Liu Y 2019a . Fine-scale appendage structure of the Cambrian trilobitomorph Naraoia spinosa and its ontogenetic and ecological implications. Proc. R. Soc. B 286 : 20192371 [Google Scholar]
- Zhai D , Ortega-Hernández J , Wolfe JM , Hou X , Cao C , Liu Y 2019b . Three-dimensionally preserved appendages in an early Cambrian stem-group pancrustacean. Curr. Biol. 29 : 171– 77 [Google Scholar]
- Zhai D , Williams M , Siveter DJ , Harvey THP , Sansom RS et al. 2019c . Variation in appendages in early Cambrian bradoriids reveals a wide range of body plans in stem-euarthropods. Commun. Biol. 2 : 329 [Google Scholar]
- Zhang C , Stadler T , Klopstein S , Heath TA , Ronquist F 2016 . Total-evidence dating under the fossilized birth-death process. Syst. Biol. 65 : 228– 49 [Google Scholar]
- Zhang H , Xiao S , Liu Y , Yuan X , Wan B et al. 2015 . Armored kinorhynch-like scalidophoran animals from the early Cambrian. Sci. Rep. 5 : 16521 [Google Scholar]
- Zhang X , Ahlberg P , Babcock LE , Choi DK , Geyer G et al. 2017 . Challenges in defining the base of Cambrian Series 2 and Stage 3. Earth-Sci. Rev. 172 : 124– 39 [Google Scholar]
Data & Media loading...
- Article Type: Review Article
Most Read This Month
Most cited most cited rss feed, resilience and stability of ecological systems, ecological and evolutionary responses to recent climate change, effects of habitat fragmentation on biodiversity, mechanisms of maintenance of species diversity, species distribution models: ecological explanation and prediction across space and time, stable isotopes in ecosystem studies, phylogenies and community ecology, species richness of parasite assemblages: evolution and patterns, the population biology of invasive species, landscapes and riverscapes: the influence of land use on stream ecosystems.

COMMENTS
Conclusion: Evolution and the arthropod. The history of the arthropods is a stunning example of evolution on a grand scale. Here are some things you have seen: Arthropods have diversified from their humble beginnings 500 million years ago. Some lineages, such as Opabinia and the trilobites, have gone extinct….
Robert D. Barnes. Arthropod - Evolution, Paleontology, Adaptation: Arthropods share many features with annelids. Both groups are segmented and have similar nervous systems. Arthropods first appeared in the Cambrian and were represented by trilobites, merostomes, crustaceans, and enigmatic arthropods that do not fit into any existing subphyla.
The arthropods are one of the most familiar and ubiquitous of all ani-mal groups. They have far more species than any other phylum, yet the living species are merely the surviving branches of a much greater diversity of extinct forms. One group of crustacean arthropods, the barnacles, was studied extensively by Charles Darwin.
The evolutionary implications of arthropod phylogeny have been reviewed on multiple occasions 4, 5, 6, but these predate the advent of transcriptomic and genomic data that have so much refined our knowledge on relationships of chelicerates 7, 8, myriapods [9], 'crustaceans' and insects 10, 11, 12, as well as arthropods as a whole 3, 13.Here, we take stock of arthropod relationships in the ...
The origin and e volution of arthr opods. Graham E. Budd & Maximilian J. T elford. The past two decades hav e witnessed profound changes in our understanding of the evolution of arthr opods. Many ...
Arthropods are the most diverse animal phylum, and their phylogenetic relationships have been debated for centuries. With the advent of molecular phylogenetics, arthropods were found to be monophyletic and placed within a clade of molting animals, the ecdysozoans, with nematodes and six other phyla. Molecular phylogenetics also provided a new ...
The early evolution of panarthropods was generally driven by increased mastication and predation efficiency and sophistication, but a wealth of recent studies have also highlighted the prevalent role of suspension-feeding, for which early panarthropods developed their own adaptive feedback through both specialized appendages and the ...
Abstract. The rise of arthropods is a decisive event in the history of life. Likely the first animals to have established themselves on land and in the air, arthropods have pervaded nearly all ecosystems and have become pillars of the planet's ecological networks. Forerunners of this saga, exceptionally well-preserved Palaeozoic fossils ...
From the book reviews: "This volume, edited by Minelli et al., is a timely, invaluable, and extensive overview of advances. … This volume is a significant achievement and provides an outstanding starting point for students interested in any aspect of arthropod evolution, as well as a rich resource in which researchers specializing in particular taxa can situate their work in a broader ...
Arthropod evolution. Arthropods are perhaps the most successful group of animals in evolutionary terms, and their study represents one of the main research areas at the Museum. Our researchers use a combination of systematics, ecology, physiology, biogeography, developmental biology and the behaviour of both extant and fossil arthropods to shed ...
Abstract. Arthropods constitute the dominant group in the animal kingdom and are a major part of global biodiversity. There are 1,302,809 species of arthropods described that include 45,769 fossil species. Arthropods are the most successful group found in almost all biogeographical regions and ecological zones and have a dominating influence on ...
The evolutionary origin of vertebrates has been debated ad nauseam by anatomists, paleontologists, embryologists, and physiologists, but it is only now that molecular phylogenetics is providing a more rigorous framework for the placement of vertebrates among their invertebrate relatives that we can begin to arrive at concrete conclusions concerning the nature of ancient ancestors and the ...
Describes the evolution of arthropods. Click Create Assignment to assign this modality to your LMS. We have a new and improved read on this topic. Click here to view We have moved all content for this concept to for better organization. Please update your bookmarks accordingly.
2.1 Introduction. Arthropoda, the best-known member of the clade Ecdysozoa, is a phylum of protostome animals, its closest relatives being Onychophora (velvet worms) and Tardigrada (water bears). Arthropods are not only the largest living phylum in terms of species diversity, with 1,214,295 extant species, including 1,023,559 Hexapoda, 111,937 ...
Figure 3: Stem-group arthropods. a, Aysheaia pedunculata from the Burgess Shale (Middle Cambrian). Scale bar, 1 cm. b, Xenusion auerswaldae from Lower Cambrian sandstone erratic block, probably ...
The arthropod defined. We've seen that arthropods are a whole lot more than just cockroaches and lobsters. However, all those descendants of the arthropod ancestor share certain key characteristics that they inherited from that ancestor. Using the information from this section you should be able to tell if an animal is an arthropod based on ...
Three hypotheses explain the evolution of segmentation among the three phyla, namely, chordates, annelids, and arthropods. According to the first hypothesis, arthropods, chordates, and annelids exhibit independent mechanisms of segmentation. This hypothesis means that the evolution of segmentation in each of the three phyla is unique and has no ...
Goals. We will conduct research in insect systematics, arthropod biology/ecology, and conservation in order to advance knowledge of insect biodiversity, and to better understand ecological processes in order to manage arthropod pests, ecosystem service providers (pollination, nutrient recycling, biocontrol), and endangered species.
Arthropods and you. The remarkable products of arthropod evolution are all around you in your day-to-day life: The housefly buzzing around the room has mouthparts adapted for sponging up food and eyes adapted for avoiding predators (and consequently, fly swatters!). The crunch of a cockroach under your foot and the tough shell of a crab on your ...
The Arthropod Story. by the Understanding Evolution team. The Arthropod Story takes you on a tour through the amazing evolutionary history of arthropods. Along the way, you'll get a healthy dose of taxonomy, paleontology, natural history and principles of evolution. Next. Introducing the arthropods.
Phylogenomics underpins a stable and mostly well-resolved hypothesis for the interrelationships of extant arthropods. Exceptionally preserved fossils are integrated into this framework by coding their morphological characters, as exemplified by total-evidence dating approaches that treat fossils as dated tips in analyses numerically dominated by molecular data. Cambrian fossils inform on the ...
Deutsch J (2010) Pending Issues in Development and Phylogeny of Arthropods Key Transitions in Animal Evolution, 10.1201/b10425-6, (54-81), Online publication date: 7-Dec-2010. Kocot K , Cannon J and Halanych K (2010) Elucidating Animal Phylogeny Key Transitions in Animal Evolution , 10.1201/b10425-4 , (15-33) , Online publication date: 7-Dec-2010 .