- Open access
- Published: 13 November 2019

Evidence-based models of care for the treatment of alcohol use disorder in primary health care settings: protocol for systematic review
- Susan A. Rombouts 1 ,
- James Conigrave 2 ,
- Eva Louie 1 ,
- Paul Haber 1 , 3 &
- Kirsten C. Morley ORCID: orcid.org/0000-0002-0868-9928 1
Systematic Reviews volume 8 , Article number: 275 ( 2019 ) Cite this article
7423 Accesses
3 Citations
Metrics details
Alcohol use disorder (AUD) is highly prevalent and accounts globally for 1.6% of disability-adjusted life years (DALYs) among females and 6.0% of DALYs among males. Effective treatments for AUDs are available but are not commonly practiced in primary health care. Furthermore, referral to specialized care is often not successful and patients that do seek treatment are likely to have developed more severe dependence. A more cost-efficient health care model is to treat less severe AUD in a primary care setting before the onset of greater dependence severity. Few models of care for the management of AUD in primary health care have been developed and with limited implementation. This proposed systematic review will synthesize and evaluate differential models of care for the management of AUD in primary health care settings.
We will conduct a systematic review to synthesize studies that evaluate the effectiveness of models of care in the treatment of AUD in primary health care. A comprehensive search approach will be conducted using the following databases; MEDLINE (1946 to present), PsycINFO (1806 to present), Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL) (1991 to present), and Embase (1947 to present).
Reference searches of relevant reviews and articles will be conducted. Similarly, a gray literature search will be done with the help of Google and the gray matter tool which is a checklist of health-related sites organized by topic. Two researchers will independently review all titles and abstracts followed by full-text review for inclusion. The planned method of extracting data from articles and the critical appraisal will also be done in duplicate. For the critical appraisal, the Cochrane risk of bias tool 2.0 will be used.
This systematic review and meta-analysis aims to guide improvement of design and implementation of evidence-based models of care for the treatment of alcohol use disorder in primary health care settings. The evidence will define which models are most promising and will guide further research.
Protocol registration number
PROSPERO CRD42019120293.
Peer Review reports
It is well recognized that alcohol use disorders (AUD) have a damaging impact on the health of the population. According to the World Health Organization (WHO), 5.3% of all global deaths were attributable to alcohol consumption in 2016 [ 1 ]. The 2016 Global Burden of Disease Study reported that alcohol use led to 1.6% (95% uncertainty interval [UI] 1.4–2.0) of total DALYs globally among females and 6.0% (5.4–6.7) among males, resulting in alcohol use being the seventh leading risk factor for both premature death and disability-adjusted life years (DALYs) [ 2 ]. Among people aged 15–49 years, alcohol use was the leading risk factor for mortality and disability with 8.9% (95% UI 7.8–9.9) of all attributable DALYs for men and 2.3% (2.0–2.6) for women [ 2 ]. AUD has been linked to many physical and mental health complications, such as coronary heart disease, liver cirrhosis, a variety of cancers, depression, anxiety, and dementia [ 2 , 3 ]. Despite the high morbidity and mortality rate associated with hazardous alcohol use, the global prevalence of alcohol use disorders among persons aged above 15 years in 2016 was stated to be 5.1% (2.5% considered as harmful use and 2.6% as severe AUD), with the highest prevalence in the European and American region (8.8% and 8.2%, respectively) [ 1 ].
Effective and safe treatment for AUD is available through psychosocial and/or pharmacological interventions yet is not often received and is not commonly practiced in primary health care. While a recent European study reported 8.7% prevalence of alcohol dependence in primary health care populations [ 4 ], the vast majority of patients do not receive the professional treatment needed, with only 1 in 5 patients with alcohol dependence receiving any formal treatment [ 4 ]. In Australia, it is estimated that only 3% of individuals with AUD receive approved pharmacotherapy for the disorder [ 5 , 6 ]. Recognition of AUD in general practice uncommonly leads to treatment before severe medical and social disintegration [ 7 ]. Referral to specialized care is often not successful, and those patients that do seek treatment are likely to have more severe dependence with higher levels of alcohol use and concurrent mental and physical comorbidity [ 4 ].
Identifying and treating early stage AUDs in primary care settings can prevent condition worsening. This may reduce the need for more complex and more expensive specialized care. The high prevalence of AUD in primary health care and the chronic relapsing character of AUD make primary care a suitable and important location for implementing evidence-based interventions. Successful implementation of treatment models requires overcoming multiple barriers. Qualitative studies have identified several of those barriers such as limited time, limited organizational capacity, fear of losing patients, and physicians feeling incompetent in treating AUD [ 8 , 9 , 10 ]. Additionally, a recent systematic review revealed that diagnostic sensitivity of primary care physicians in the identification of AUD was 41.7% and that only in 27.3% alcohol problems were recorded correctly in primary care records [ 11 ].
Several models for primary care have been created to increase identification and treatment of patients with AUD. Of those, the model, screening, brief interventions, and referral to specialized treatment for people with severe AUD (SBIRT [ 12 ]) is most well-known. Multiple systematic reviews exist, confirming its effectiveness [ 13 , 14 , 15 ], although implementation in primary care has been inadequate. Moreover, most studies have looked primarily at SBIRT for the treatment of less severe AUD [ 16 ]. In the treatment of severe AUD, efficacy of SBIRT is limited [ 16 ]. Additionally, many patient referred to specialized care often do not attend as they encounter numerous difficulties in health care systems including stigmatization, costs, lack of information about existing treatments, and lack of non-abstinence-treatment goals [ 7 ]. An effective model of care for improved management of AUD that can be efficiently implemented in primary care settings is required.
Review objective
This proposed systematic review will synthesize and evaluate differential models of care for the management of AUD in primary health care settings. We aim to evaluate the effectiveness of the models of care in increasing engagement and reducing alcohol consumption.
By providing this overview, we aim to guide improvement of design and implementation of evidence-based models of care for the treatment of alcohol use disorder in primary health care settings.
The systematic review is registered in PROSPERO international prospective register of systematic reviews (CRD42019120293) and the current protocol has been written according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) recommended for systematic reviews [ 17 ]. A PRISMA-P checklist is included as Additional file 1 .
Eligibility criteria
Criteria for considering studies for this review are classified by the following:
Study design
Both individualized and cluster randomized trials will be included. Masking of patients and/or physicians is not an inclusion criterion as it is often hard to accomplish in these types of studies.
Patients in primary health care who are identified (using screening tools or by primary health care physician) as suffering from AUD (from mild to severe) or hazardous alcohol drinking habits (e.g., comorbidity, concurrent medication use). Eligible patients need to have had formal assessment of AUD with diagnostic tools such as Diagnostic and Statistical Manual of Mental Disorders (DSM-IV/V) or the International Statistical Classification of Diseases and Related Health Problems (ICD-10) and/or formal assessment of hazardous alcohol use assessed by the Comorbidity Alcohol Risk Evaluation Tool (CARET) or the Alcohol Use Disorders Identification test (AUDIT) and/or alcohol use exceeding guideline recommendations to reduce health risks (e.g., US dietary guideline (2015–2020) specifies excessive drinking for women as ≥ 4 standard drinks (SD) on any day and/or ≥ 8 SD per week and for men ≥ 5 SD on any day and/or ≥ 15 SD per week).
Studies evaluating models of care for additional diseases (e.g., other dependencies/mental health) other than AUD are included when they have conducted data analysis on the alcohol use disorder patient data separately or when 80% or more of the included patients have AUD.
Intervention
The intervention should consist of a model of care; therefore, it should include multiple components and cover different stages of the care pathway (e.g., identification of patients, training of staff, modifying access to resources, and treatment). An example is the Chronic Care Model (CCM) which is a primary health care model designed for chronic (relapsing) conditions and involves six elements: linkage to community resources, redesign of health care organization, self-management support, delivery system redesign (e.g., use of non-physician personnel), decision support, and the use of clinical information systems [ 18 , 19 ].
As numerous articles have already assessed the treatment model SBIRT, this model of care will be excluded from our review unless the particular model adds a specific new aspect. Also, the article has to assess the effectiveness of the model rather than assessing the effectiveness of the particular treatment used. Because identification of patients is vital to including them in the trial, a care model that only evaluates either patient identification or treatment without including both will be excluded from this review.
Model effectiveness may be in comparison with the usual care or a different treatment model.
Included studies need to include at least one of the following outcome measures: alcohol consumption, treatment engagement, uptake of pharmacological agents, and/or quality of life.
Solely quantitative research will be included in this systematic review (e.g., randomized controlled trials (RCTs) and cluster RCTs). We will only include peer-reviewed articles.
Restrictions (language/time period)
Studies published in English after 1 January 1998 will be included in this systematic review.
Studies have to be conducted in primary health care settings as such treatment facilities need to be physically in or attached to the primary care clinic. Examples are co-located clinics, veteran health primary care clinic, hospital-based primary care clinic, and community primary health clinics. Specialized primary health care clinics such as human immunodeficiency virus (HIV) clinics are excluded from this systematic review. All studies were included, irrespective of country of origin.
Search strategy and information sources
A comprehensive search will be conducted. The following databases will be consulted: MEDLINE (1946 to present), PsycINFO (1806 to present), Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL) (1991 to present), and Embase (1947 to present). Initially, the search terms will be kept broad including alcohol use disorder (+synonyms), primary health care, and treatment to minimize the risk of missing any potentially relevant articles. Depending on the number of references attained by this preliminary search, we will add search terms referring to models such as models of care, integrated models, and stepped-care models, to limit the number of articles. Additionally, we will conduct reference searches of relevant reviews and articles. Similarly, a gray literature search will be done with the help of Google and the Gray Matters tool which is a checklist of health-related sites organized by topic. The tool is produced by the Canadian Agency for Drugs and Technologies in Health (CADTH) [ 20 ].
See Additional file 2 for a draft of our search strategy in MEDLINE.
Data collection
The selection of relevant articles is based on several consecutive steps. All references will be managed using EndNote (EndNote version X9 Clarivate Analytics). Initially, duplicates will be removed from the database after which all the titles will be screened with the purpose of discarding clearly irrelevant articles. The remaining records will be included in an abstract and full-text screen. All steps will be done independently by two researchers. Disagreement will lead to consultation of a third researcher.
Data extraction and synthesis
Two researchers will extract data from included records. At the conclusion of data extraction, these two researchers will meet with the lead author to resolve any discrepancies.
In order to follow a structured approach, an extraction form will be used. Key elements of the extraction form are information about design of the study (randomized, blinded, control), type of participants (alcohol use, screening tool used, socio-economic status, severity of alcohol use, age, sex, number of participants), study setting (primary health care setting, VA centers, co-located), type of intervention/model of care (separate elements of the models), type of health care worker (primary, secondary (co-located)), duration of follow-up, outcome measures used in the study, and funding sources. We do not anticipate having sufficient studies for a meta-analysis. As such, we plan to perform a narrative synthesis. We will synthesize the findings from the included articles by cohort characteristics, differential aspects of the intervention, controls, and type of outcome measures.
Sensitivity analyses will be conducted when issues suitable for sensitivity analysis are identified during the review process (e.g., major differences in quality of the included articles).
Potential meta-analysis
In the event that sufficient numbers of effect sizes can be extracted, a meta-analytic synthesis will be performed. We will extract effect sizes from each study accordingly. Two effect sizes will be extracted (and transformed where appropriate). Categorical outcomes will be given in log odds ratios and continuous measures will be converted into standardized mean differences. Variation in effect sizes attributable to real differences (heterogeneity) will be estimated using the inconsistency index ( I 2 ) [ 21 , 22 ]. We anticipate high degrees of variation among effect sizes, as a result moderation and subgroup-analyses will be employed as appropriate. In particular, moderation analysis will focus on the degree of heterogeneity attributable to differences in cohort population (pre-intervention drinking severity, age, etc.), type of model/intervention, and study quality. We anticipate that each model of care will require a sub-group analysis, in which case a separate meta-analysis will be performed for each type of model. Small study effect will be assessed with funnel plots and Egger’s symmetry tests [ 23 ]. When we cannot obtain enough effect sizes for synthesis or when the included studies are too diverse, we will aim to illustrate patterns in the data by graphical display (e.g., bubble plot) [ 24 ].
Critical appraisal of studies
All studies will be critically assessed by two researchers independently using the Revised Cochrane risk-of-bias tool (RoB 2) [ 25 ]. This tool facilitates systematic assessment of the quality of the article per outcome according to the five domains: bias due to (1) the randomization process, (2) deviations from intended interventions, (3) missing outcome data, (4) measurement of the outcome, and (5) selection of the reported results. An additional domain 1b must be used when assessing the randomization process for cluster-randomized studies.
Meta-biases such as outcome reporting bias will be evaluated by determining whether the protocol was published before recruitment of patients. Additionally, trial registries will be checked to determine whether the reported outcome measures and statistical methods are similar to the ones described in the registry. The gray literature search will be of assistance when checking for publication bias; however, completely eliminating the presence of publication bias is impossible.
Similar to article selection, any disagreement between the researchers will lead to discussion and consultation of a third researcher. The strength of the evidence will be graded according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach [ 26 ].
The primary outcome measure of this proposed systematic review is the consumption of alcohol at follow-up. Consumption of alcohol is often quantified in drinking quantity (e.g., number of drinks per week), drinking frequency (e.g., percentage of days abstinent), binge frequency (e.g., number of heavy drinking days), and drinking intensity (e.g., number of drinks per drinking day). Additionally, outcomes such as percentage/proportion included patients that are abstinent or considered heavy/risky drinkers at follow-up. We aim to report all these outcomes. The consumption of alcohol is often self-reported by patients. When studies report outcomes at multiple time points, we will consider the longest follow-up of individual studies as a primary outcome measure.
Depending on the included studies, we will also consider secondary outcome measures such as treatment engagement (e.g., number of visits or pharmacotherapy uptake), economic outcome measures, health care utilization, quality of life assessment (physical/mental), alcohol-related problems/harm, and mental health score for depression or anxiety.
This proposed systematic review will synthesize and evaluate differential models of care for the management of AUD in primary health care settings.
Given the complexities of researching models of care in primary care and the paucity of a focus on AUD treatment, there are likely to be only a few studies that sufficiently address the research question. Therefore, we will do a preliminary search without the search terms for model of care. Additionally, the search for online non-academic studies presents a challenge. However, the Gray Matters tool will be of guidance and will limit the possibility of missing useful studies. Further, due to diversity of treatment models, outcome measures, and limitations in research design, it is possible that a meta-analysis for comparative effectiveness may not be appropriate. Moreover, in the absence of large, cluster randomized controlled trials, it will be difficult to distinguish between the effectiveness of the treatment given and that of the model of care and/or implementation procedure. Nonetheless, we will synthesize the literature and provide a critical evaluation of the quality of the evidence.
This review will assist the design and implementation of models of care for the management of AUD in primary care settings. This review will thus improve the management of AUD in primary health care and potentially increase the uptake of evidence-based interventions for AUD.
Availability of data and materials
Not applicable.
Abbreviations
Alcohol use disorder
Alcohol Use Disorders Identification test
Canadian Agency for Drugs and Technologies in Health
The Comorbidity Alcohol Risk Evaluation
Cochrane Central Register of Controlled Trials
Diagnostic and Statistical Manual of Mental Disorders
Human immunodeficiency virus
10 - International Statistical Classification of Diseases and Related Health Problems
Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols
Screening, brief intervention, referral to specialized treatment
Standard drinks
World Health Organization
WHO. Global status report on alcohol and health: World health organization; 2018.
The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016. a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. 2018;5(12):987–1012.
Article Google Scholar
WHO. Global strategy to reduce the harmful use of alcohol: World health organization; 2010.
Rehm J, Allamani A, Elekes Z, Jakubczyk A, Manthey J, Probst C, et al. Alcohol dependence and treatment utilization in Europe - a representative cross-sectional study in primary care. BMC Fam Pract. 2015;16:90.
Morley KC, Logge W, Pearson SA, Baillie A, Haber PS. National trends in alcohol pharmacotherapy: findings from an Australian claims database. Drug Alcohol Depend. 2016;166:254–7.
Article CAS Google Scholar
Morley KC, Logge W, Pearson SA, Baillie A, Haber PS. Socioeconomic and geographic disparities in access to pharmacotherapy for alcohol dependence. J Subst Abus Treat. 2017;74:23–5.
Rehm J, Anderson P, Manthey J, Shield KD, Struzzo P, Wojnar M, et al. Alcohol use disorders in primary health care: what do we know and where do we go? Alcohol Alcohol. 2016;51(4):422–7.
Le KB, Johnson JA, Seale JP, Woodall H, Clark DC, Parish DC, et al. Primary care residents lack comfort and experience with alcohol screening and brief intervention: a multi-site survey. J Gen Intern Med. 2015;30(6):790–6.
McLellan AT, Starrels JL, Tai B, Gordon AJ, Brown R, Ghitza U, et al. Can substance use disorders be managed using the chronic care model? review and recommendations from a NIDA consensus group. Public Health Rev. 2014;35(2).
Storholm ED, Ober AJ, Hunter SB, Becker KM, Iyiewuare PO, Pham C, et al. Barriers to integrating the continuum of care for opioid and alcohol use disorders in primary care: a qualitative longitudinal study. J Subst Abus Treat. 2017;83:45–54.
Mitchell AJ, Meader N, Bird V, Rizzo M. Clinical recognition and recording of alcohol disorders by clinicians in primary and secondary care: meta-analysis. Br J Psychiatry. 2012;201:93–100.
Babor TF, Ritson EB, Hodgson RJ. Alcohol-related problems in the primary health care setting: a review of early intervention strategies. Br J Addict. 1986;81(1):23–46.
Kaner EF, Beyer F, Dickinson HO, Pienaar E, Campbell F, Schlesinger C, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2007;(2):Cd004148.
O'Donnell A, Anderson P, Newbury-Birch D, Schulte B, Schmidt C, Reimer J, et al. The impact of brief alcohol interventions in primary healthcare: a systematic review of reviews. Alcohol Alcohol. 2014;49(1):66–78.
Bertholet N, Daeppen JB, Wietlisbach V, Fleming M, Burnand B. Reduction of alcohol consumption by brief alcohol intervention in primary care: systematic review and meta-analysis. Arch Intern Med. 2005;165(9):986–95.
Saitz R. ‘SBIRT’ is the answer? Probably not. Addiction. 2015;110(9):1416–7.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj. 2015;350:g7647.
Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. Jama. 2002;288(14):1775–9.
Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. Jama. 2002;288(15):1909–14.
CADTH. Grey Matters: a practical tool for searching health-related grey literature Internet. 2018 (cited 2019 Feb 22).
Higgins JPT. Thompson SG. Quantifying heterogeneity in a meta-analysis. 2002;21(11):1539–58.
Google Scholar
Higgins JPT, Thompson SG, Deeks JJ. Altman DG. Measuring inconsistency in meta-analyses. 2003;327(7414):557–60.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). 1997;315(7109):629–34.
Higgins JPT, López-López JA, Becker BJ, Davies SR, Dawson S, Grimshaw JM, et al. Synthesising quantitative evidence in systematic reviews of complex health interventions. BMJ Glob Health. 2019;4(Suppl 1):e000858–e.
Higgins, J.P.T., Sterne, J.A.C., Savović, J., Page, M.J., Hróbjartsson, A., Boutron, I., Reeves, B., Eldridge, S. (2016). A revised tool for assessing risk of bias in randomized trials. In: Chandler, J., McKenzie, J., Boutron, I., Welch, V. (editors). Cochrane methods. Cochrane database of systematic reviews, 10 (Suppl 1). https://doi.org/10.1002/14651858.CD201601 .
Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html ).
Download references
Acknowledgements
There is no dedicated funding.
Author information
Authors and affiliations.
Discipline of Addiction Medicine, Central Clinical School, Faculty of Medicine and Health, University of Sydney, Sydney, NSW, Australia
Susan A. Rombouts, Eva Louie, Paul Haber & Kirsten C. Morley
NHMRC Centre of Research Excellence in Indigenous Health and Alcohol, Central Clinical School, Faculty of Medicine and Health, University of Sydney, Sydney, NSW, Australia
James Conigrave
Drug Health Services, Royal Prince Alfred Hospital, Camperdown, NSW, Australia
You can also search for this author in PubMed Google Scholar
Contributions
KM and PH conceived the presented idea of a systematic review and meta-analysis and helped with the scope of the literature. KM is the senior researcher providing overall guidance and the guarantor of this review. SR developed the background, search strategy, and data extraction form. SR and EL will both be working on the data extraction and risk of bias assessment. SR and JC will conduct the data analysis and synthesize the results. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Kirsten C. Morley .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1..
PRISMA-P 2015 Checklist.
Additional file 2.
Draft search strategy MEDLINE. Search strategy.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Rombouts, S.A., Conigrave, J., Louie, E. et al. Evidence-based models of care for the treatment of alcohol use disorder in primary health care settings: protocol for systematic review. Syst Rev 8 , 275 (2019). https://doi.org/10.1186/s13643-019-1157-7
Download citation
Received : 25 March 2019
Accepted : 13 September 2019
Published : 13 November 2019
DOI : https://doi.org/10.1186/s13643-019-1157-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Model of care
- Primary health care
- Systematic review
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Change Password
Your password must have 6 characters or more:.
- a lower case character,
- an upper case character,
- a special character
Password Changed Successfully
Your password has been changed
Create your account
Forget yout password.
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password
Forgot your Username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username

- June 01, 2024 | VOL. 181, NO. 6 CURRENT ISSUE pp.461-564
- May 01, 2024 | VOL. 181, NO. 5 pp.347-460
- April 01, 2024 | VOL. 181, NO. 4 pp.255-346
- March 01, 2024 | VOL. 181, NO. 3 pp.171-254
- February 01, 2024 | VOL. 181, NO. 2 pp.83-170
- January 01, 2024 | VOL. 181, NO. 1 pp.1-82
The American Psychiatric Association (APA) has updated its Privacy Policy and Terms of Use , including with new information specifically addressed to individuals in the European Economic Area. As described in the Privacy Policy and Terms of Use, this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences.
Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
Substance Use Disorders and Addiction: Mechanisms, Trends, and Treatment Implications
- Ned H. Kalin , M.D.
Search for more papers by this author
The numbers for substance use disorders are large, and we need to pay attention to them. Data from the 2018 National Survey on Drug Use and Health ( 1 ) suggest that, over the preceding year, 20.3 million people age 12 or older had substance use disorders, and 14.8 million of these cases were attributed to alcohol. When considering other substances, the report estimated that 4.4 million individuals had a marijuana use disorder and that 2 million people suffered from an opiate use disorder. It is well known that stress is associated with an increase in the use of alcohol and other substances, and this is particularly relevant today in relation to the chronic uncertainty and distress associated with the COVID-19 pandemic along with the traumatic effects of racism and social injustice. In part related to stress, substance use disorders are highly comorbid with other psychiatric illnesses: 9.2 million adults were estimated to have a 1-year prevalence of both a mental illness and at least one substance use disorder. Although they may not necessarily meet criteria for a substance use disorder, it is well known that psychiatric patients have increased usage of alcohol, cigarettes, and other illicit substances. As an example, the survey estimated that over the preceding month, 37.2% of individuals with serious mental illnesses were cigarette smokers, compared with 16.3% of individuals without mental illnesses. Substance use frequently accompanies suicide and suicide attempts, and substance use disorders are associated with a long-term increased risk of suicide.
Addiction is the key process that underlies substance use disorders, and research using animal models and humans has revealed important insights into the neural circuits and molecules that mediate addiction. More specifically, research has shed light onto mechanisms underlying the critical components of addiction and relapse: reinforcement and reward, tolerance, withdrawal, negative affect, craving, and stress sensitization. In addition, clinical research has been instrumental in developing an evidence base for the use of pharmacological agents in the treatment of substance use disorders, which, in combination with psychosocial approaches, can provide effective treatments. However, despite the existence of therapeutic tools, relapse is common, and substance use disorders remain grossly undertreated. For example, whether at an inpatient hospital treatment facility or at a drug or alcohol rehabilitation program, it was estimated that only 11% of individuals needing treatment for substance use received appropriate care in 2018. Additionally, it is worth emphasizing that current practice frequently does not effectively integrate dual diagnosis treatment approaches, which is important because psychiatric and substance use disorders are highly comorbid. The barriers to receiving treatment are numerous and directly interact with existing health care inequities. It is imperative that as a field we overcome the obstacles to treatment, including the lack of resources at the individual level, a dearth of trained providers and appropriate treatment facilities, racial biases, and the marked stigmatization that is focused on individuals with addictions.
This issue of the Journal is focused on understanding factors contributing to substance use disorders and their comorbidity with psychiatric disorders, the effects of prenatal alcohol use on preadolescents, and brain mechanisms that are associated with addiction and relapse. An important theme that emerges from this issue is the necessity for understanding maladaptive substance use and its treatment in relation to health care inequities. This highlights the imperative to focus resources and treatment efforts on underprivileged and marginalized populations. The centerpiece of this issue is an overview on addiction written by Dr. George Koob, the director of the National Institute on Alcohol Abuse and Alcoholism (NIAAA), and coauthors Drs. Patricia Powell (NIAAA deputy director) and Aaron White ( 2 ). This outstanding article will serve as a foundational knowledge base for those interested in understanding the complex factors that mediate drug addiction. Of particular interest to the practice of psychiatry is the emphasis on the negative affect state “hyperkatifeia” as a major driver of addictive behavior and relapse. This places the dysphoria and psychological distress that are associated with prolonged withdrawal at the heart of treatment and underscores the importance of treating not only maladaptive drug-related behaviors but also the prolonged dysphoria and negative affect associated with addiction. It also speaks to why it is crucial to concurrently treat psychiatric comorbidities that commonly accompany substance use disorders.
Insights Into Mechanisms Related to Cocaine Addiction Using a Novel Imaging Method for Dopamine Neurons
Cassidy et al. ( 3 ) introduce a relatively new imaging technique that allows for an estimation of dopamine integrity and function in the substantia nigra, the site of origin of dopamine neurons that project to the striatum. Capitalizing on the high levels of neuromelanin that are found in substantia nigra dopamine neurons and the interaction between neuromelanin and intracellular iron, this MRI technique, termed neuromelanin-sensitive MRI (NM-MRI), shows promise in studying the involvement of substantia nigra dopamine neurons in neurodegenerative diseases and psychiatric illnesses. The authors used this technique to assess dopamine function in active cocaine users with the aim of exploring the hypothesis that cocaine use disorder is associated with blunted presynaptic striatal dopamine function that would be reflected in decreased “integrity” of the substantia nigra dopamine system. Surprisingly, NM-MRI revealed evidence for increased dopamine in the substantia nigra of individuals using cocaine. The authors suggest that this finding, in conjunction with prior work suggesting a blunted dopamine response, points to the possibility that cocaine use is associated with an altered intracellular distribution of dopamine. Specifically, the idea is that dopamine is shifted from being concentrated in releasable, functional vesicles at the synapse to a nonreleasable cytosolic pool. In addition to providing an intriguing alternative hypothesis underlying the cocaine-related alterations observed in substantia nigra dopamine function, this article highlights an innovative imaging method that can be used in further investigations involving the role of substantia nigra dopamine systems in neuropsychiatric disorders. Dr. Charles Bradberry, chief of the Preclinical Pharmacology Section at the National Institute on Drug Abuse, contributes an editorial that further explains the use of NM-MRI and discusses the theoretical implications of these unexpected findings in relation to cocaine use ( 4 ).
Treatment Implications of Understanding Brain Function During Early Abstinence in Patients With Alcohol Use Disorder
Developing a better understanding of the neural processes that are associated with substance use disorders is critical for conceptualizing improved treatment approaches. Blaine et al. ( 5 ) present neuroimaging data collected during early abstinence in patients with alcohol use disorder and link these data to relapses occurring during treatment. Of note, the findings from this study dovetail with the neural circuit schema Koob et al. provide in this issue’s overview on addiction ( 2 ). The first study in the Blaine et al. article uses 44 patients and 43 control subjects to demonstrate that patients with alcohol use disorder have a blunted neural response to the presentation of stress- and alcohol-related cues. This blunting was observed mainly in the ventromedial prefrontal cortex, a key prefrontal regulatory region, as well as in subcortical regions associated with reward processing, specifically the ventral striatum. Importantly, this finding was replicated in a second study in which 69 patients were studied in relation to their length of abstinence prior to treatment and treatment outcomes. The results demonstrated that individuals with the shortest abstinence times had greater alterations in neural responses to stress and alcohol cues. The authors also found that an individual’s length of abstinence prior to treatment, independent of the number of days of abstinence, was a predictor of relapse and that the magnitude of an individual’s neural alterations predicted the amount of heavy drinking occurring early in treatment. Although relapse is an all too common outcome in patients with substance use disorders, this study highlights an approach that has the potential to refine and develop new treatments that are based on addiction- and abstinence-related brain changes. In her thoughtful editorial, Dr. Edith Sullivan from Stanford University comments on the details of the study, the value of studying patients during early abstinence, and the implications of these findings for new treatment development ( 6 ).
Relatively Low Amounts of Alcohol Intake During Pregnancy Are Associated With Subtle Neurodevelopmental Effects in Preadolescent Offspring
Excessive substance use not only affects the user and their immediate family but also has transgenerational effects that can be mediated in utero. Lees et al. ( 7 ) present data suggesting that even the consumption of relatively low amounts of alcohol by expectant mothers can affect brain development, cognition, and emotion in their offspring. The researchers used data from the Adolescent Brain Cognitive Development Study, a large national community-based study, which allowed them to assess brain structure and function as well as behavioral, cognitive, and psychological outcomes in 9,719 preadolescents. The mothers of 2,518 of the subjects in this study reported some alcohol use during pregnancy, albeit at relatively low levels (0 to 80 drinks throughout pregnancy). Interestingly, and opposite of that expected in relation to data from individuals with fetal alcohol spectrum disorders, increases in brain volume and surface area were found in offspring of mothers who consumed the relatively low amounts of alcohol. Notably, any prenatal alcohol exposure was associated with small but significant increases in psychological problems that included increases in separation anxiety disorder and oppositional defiant disorder. Additionally, a dose-response effect was found for internalizing psychopathology, somatic complaints, and attentional deficits. While subtle, these findings point to neurodevelopmental alterations that may be mediated by even small amounts of prenatal alcohol consumption. Drs. Clare McCormack and Catherine Monk from Columbia University contribute an editorial that provides an in-depth assessment of these findings in relation to other studies, including those assessing severe deficits in individuals with fetal alcohol syndrome ( 8 ). McCormack and Monk emphasize that the behavioral and psychological effects reported in the Lees et al. article would not be clinically meaningful. However, it is feasible that the influences of these low amounts of alcohol could interact with other predisposing factors that might lead to more substantial negative outcomes.
Increased Comorbidity Between Substance Use and Psychiatric Disorders in Sexual Identity Minorities
There is no question that victims of societal marginalization experience disproportionate adversity and stress. Evans-Polce et al. ( 9 ) focus on this concern in relation to individuals who identify as sexual minorities by comparing their incidence of comorbid substance use and psychiatric disorders with that of individuals who identify as heterosexual. By using 2012−2013 data from 36,309 participants in the National Epidemiologic Study on Alcohol and Related Conditions–III, the authors examine the incidence of comorbid alcohol and tobacco use disorders with anxiety, mood disorders, and posttraumatic stress disorder (PTSD). The findings demonstrate increased incidences of substance use and psychiatric disorders in individuals who identified as bisexual or as gay or lesbian compared with those who identified as heterosexual. For example, a fourfold increase in the prevalence of PTSD was found in bisexual individuals compared with heterosexual individuals. In addition, the authors found an increased prevalence of substance use and psychiatric comorbidities in individuals who identified as bisexual and as gay or lesbian compared with individuals who identified as heterosexual. This was most prominent in women who identified as bisexual. For example, of the bisexual women who had an alcohol use disorder, 60.5% also had a psychiatric comorbidity, compared with 44.6% of heterosexual women. Additionally, the amount of reported sexual orientation discrimination and number of lifetime stressful events were associated with a greater likelihood of having comorbid substance use and psychiatric disorders. These findings are important but not surprising, as sexual minority individuals have a history of increased early-life trauma and throughout their lives may experience the painful and unwarranted consequences of bias and denigration. Nonetheless, these findings underscore the strong negative societal impacts experienced by minority groups and should sensitize providers to the additional needs of these individuals.
Trends in Nicotine Use and Dependence From 2001–2002 to 2012–2013
Although considerable efforts over earlier years have curbed the use of tobacco and nicotine, the use of these substances continues to be a significant public health problem. As noted above, individuals with psychiatric disorders are particularly vulnerable. Grant et al. ( 10 ) use data from the National Epidemiologic Survey on Alcohol and Related Conditions collected from a very large cohort to characterize trends in nicotine use and dependence over time. Results from their analysis support the so-called hardening hypothesis, which posits that although intervention-related reductions in nicotine use may have occurred over time, the impact of these interventions is less potent in individuals with more severe addictive behavior (i.e., nicotine dependence). When adjusted for sociodemographic factors, the results demonstrated a small but significant increase in nicotine use from 2001–2002 to 2012–2013. However, a much greater increase in nicotine dependence (46.1% to 52%) was observed over this time frame in individuals who had used nicotine during the preceding 12 months. The increases in nicotine use and dependence were associated with factors related to socioeconomic status, such as lower income and lower educational attainment. The authors interpret these findings as evidence for the hardening hypothesis, suggesting that despite the impression that nicotine use has plateaued, there is a growing number of highly dependent nicotine users who would benefit from nicotine dependence intervention programs. Dr. Kathleen Brady, from the Medical University of South Carolina, provides an editorial ( 11 ) that reviews the consequences of tobacco use and the history of the public measures that were initially taken to combat its use. Importantly, her editorial emphasizes the need to address health care inequity issues that affect individuals of lower socioeconomic status by devoting resources to develop and deploy effective smoking cessation interventions for at-risk and underresourced populations.
Conclusions
Maladaptive substance use and substance use disorders are highly prevalent and are among the most significant public health problems. Substance use is commonly comorbid with psychiatric disorders, and treatment efforts need to concurrently address both. The papers in this issue highlight new findings that are directly relevant to understanding, treating, and developing policies to better serve those afflicted with addictions. While treatments exist, the need for more effective treatments is clear, especially those focused on decreasing relapse rates. The negative affective state, hyperkatifeia, that accompanies longer-term abstinence is an important treatment target that should be emphasized in current practice as well as in new treatment development. In addition to developing a better understanding of the neurobiology of addictions and abstinence, it is necessary to ensure that there is equitable access to currently available treatments and treatment programs. Additional resources must be allocated to this cause. This depends on the recognition that health care inequities and societal barriers are major contributors to the continued high prevalence of substance use disorders, the individual suffering they inflict, and the huge toll that they incur at a societal level.
Disclosures of Editors’ financial relationships appear in the April 2020 issue of the Journal .
1 US Department of Health and Human Services: Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality: National Survey on Drug Use and Health 2018. Rockville, Md, SAMHSA, 2019 ( https://www.samhsa.gov/data/nsduh/reports-detailed-tables-2018-NSDUH ) Google Scholar
2 Koob GF, Powell P, White A : Addiction as a coping response: hyperkatifeia, deaths of despair, and COVID-19 . Am J Psychiatry 2020 ; 177:1031–1037 Link , Google Scholar
3 Cassidy CM, Carpenter KM, Konova AB, et al. : Evidence for dopamine abnormalities in the substantia nigra in cocaine addiction revealed by neuromelanin-sensitive MRI . Am J Psychiatry 2020 ; 177:1038–1047 Link , Google Scholar
4 Bradberry CW : Neuromelanin MRI: dark substance shines a light on dopamine dysfunction and cocaine use (editorial). Am J Psychiatry 2020 ; 177:1019–1021 Abstract , Google Scholar
5 Blaine SK, Wemm S, Fogelman N, et al. : Association of prefrontal-striatal functional pathology with alcohol abstinence days at treatment initiation and heavy drinking after treatment initiation . Am J Psychiatry 2020 ; 177:1048–1059 Link , Google Scholar
6 Sullivan EV : Why timing matters in alcohol use disorder recovery (editorial). Am J Psychiatry 2020 ; 177:1022–1024 Abstract , Google Scholar
7 Lees B, Mewton L, Jacobus J, et al. : Association of prenatal alcohol exposure with psychological, behavioral, and neurodevelopmental outcomes in children from the Adolescent Brain Cognitive Development Study . Am J Psychiatry 2020 ; 177:1060–1072 Link , Google Scholar
8 McCormack C, Monk C : Considering prenatal alcohol exposure in a developmental origins of health and disease framework (editorial). Am J Psychiatry 2020 ; 177:1025–1028 Abstract , Google Scholar
9 Evans-Polce RJ, Kcomt L, Veliz PT, et al. : Alcohol, tobacco, and comorbid psychiatric disorders and associations with sexual identity and stress-related correlates . Am J Psychiatry 2020 ; 177:1073–1081 Abstract , Google Scholar
10 Grant BF, Shmulewitz D, Compton WM : Nicotine use and DSM-IV nicotine dependence in the United States, 2001–2002 and 2012–2013 . Am J Psychiatry 2020 ; 177:1082–1090 Link , Google Scholar
11 Brady KT : Social determinants of health and smoking cessation: a challenge (editorial). Am J Psychiatry 2020 ; 177:1029–1030 Abstract , Google Scholar
- Cited by None

- Substance-Related and Addictive Disorders
- Addiction Psychiatry
- Transgender (LGBT) Issues
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 28 May 2024
Associations of semaglutide with incidence and recurrence of alcohol use disorder in real-world population
- William Wang 1 ,
- Nora D. Volkow ORCID: orcid.org/0000-0001-6668-0908 2 ,
- Nathan A. Berger ORCID: orcid.org/0000-0001-7086-9885 1 ,
- Pamela B. Davis ORCID: orcid.org/0000-0002-7113-5338 3 ,
- David C. Kaelber ORCID: orcid.org/0000-0001-7855-9515 4 &
- Rong Xu ORCID: orcid.org/0000-0003-3127-4795 5
Nature Communications volume 15 , Article number: 4548 ( 2024 ) Cite this article
7884 Accesses
236 Altmetric
Metrics details
- Clinical pharmacology
- Drug safety
- Therapeutics
Alcohol use disorders are among the top causes of the global burden of disease, yet therapeutic interventions are limited. Reduced desire to drink in patients treated with semaglutide has raised interest regarding its potential therapeutic benefits for alcohol use disorders. In this retrospective cohort study of electronic health records of 83,825 patients with obesity, we show that semaglutide compared with other anti-obesity medications is associated with a 50%-56% lower risk for both the incidence and recurrence of alcohol use disorder for a 12-month follow-up period. Consistent reductions were seen for patients stratified by gender, age group, race and in patients with and without type 2 diabetes. Similar findings are replicated in the study population with 598,803 patients with type 2 diabetes. These findings provide evidence of the potential benefit of semaglutide in AUD in real-world populations and call for further randomized clinicl trials.
Similar content being viewed by others

Adults who microdose psychedelics report health related motivations and lower levels of anxiety and depression compared to non-microdosers

Long-term weight loss effects of semaglutide in obesity without diabetes in the SELECT trial
Divergent age-associated and metabolism-associated gut microbiome signatures modulate cardiovascular disease risk
Introduction.
An estimated 29.5 million or 10.6% of Americans ages 12 and older had an alcohol use disorder (AUD) in 2021 1 . AUD, which is responsible for more than 80,000 annual deaths in the USA is among the top 10 conditions associated with the largest global burden of disease 2 . Despite its large public health impact, there are only 3 medications for AUD approved by the FDA and 4 by the European Medicines Agency (EMA) and their therapeutic benefits are modest 3 , 4 . Thus, there is an urgent need to develop new medication for treating AUD.
Recent reports of reduced drinking in people being treated with glucagon-like peptide-1 receptor agonist (GLP-1RA) medications for T2DM or obesity have generated interest in the potential of these medications for treating AUD 5 , 6 . In particular semaglutide, a GLP-1RA approved for treating type 2 diabetes (T2DM) in 2017 and obesity in 2021, reduced drinking and relapse in alcohol-dependent rodents 7 , 8 . Anecdotal reports from patients prescribed semaglutide describe a reduced desire to drink 9 that have been subsequently corroborated by a report of reduced alcohol drinking with semaglutide and tirzepatide based on analyses of social media texts and follow up of selected participants 10 and a case series reporting decreased symptoms of AUD in patients treated with semaglutide 11 . Moreover, a small clinical trial ( n = 127) that evaluated the GLP-1RA agonist exenatide compared to placebo as an adjunct to standard cognitive-behavioral therapy, reported that exenatide significantly reduced heavy drinking days and total alcohol intake in a subgroup of patients with obesity 12 . However, as of now information on the clinical benefits of semaglutide for AUD prevention and treatment in real-world populations is still very limited. Here we took advantage of a large database of patient electronic health records (EHRs) to conduct a nationwide multicenter retrospective cohort study to assess the association of semaglutide with both the incidence and recurrence of AUD in individuals with obesity and with and without a prior history of AUD. We assessed the reproducibility of the findings in a separate cohort of patients with T2DM from non-overlapping time periods. We also compared patients who suffered from obesity who had T2DM (~33%) and those who did not (~67%); as well as patients with T2DM who suffered from obesity (~40%) and those who did not (~60%), to evaluate if there were potential interactions on the effects of semaglutide in patients with these two co-morbid conditions. Outcomes were separately evaluated by age, sex, and race.
Association of semaglutide with incident AUD diagnosis in patients with obesity and no prior history of AUD
The study population consisted of 83,825 patients with obesity who had no prior diagnosis of AUD and were for the first time prescribed semaglutide or non-GLP-1RA anti-obesity medications including naltrexone or topiramate in 6/2021–12/2022. The semaglutide cohort ( n = 45,797) compared with the non-GLP-1RA anti-obesity medications cohort ( n = 38,028) was older, had a higher prevalence of severe obesity and obesity-associated comorbidities including T2DM and lower prevalence of mental disorders, and tobacco use disorder. After propensity-score matching, the two cohorts (26,566 in each cohort, mean age 51.2 years, 65.9% women, 15.8% black, 66.6% white, 6.5% Hispanic) were balanced (Table 1 ). The semaglutide cohort ( n = 45,797) compared with the naltrexone/topiramate cohort ( n = 16,676) was older, had a higher prevalence of severe obesity and obesity-associated comorbidities including T2DM and a lower prevalence of mental disorders, and tobacco use disorder. After propensity-score matching, the two cohorts (15,097 in each cohort, mean age 49.2 years, 71.0% women, 17.2% black, 64.6% white, 6.9% Hispanic) were balanced.
Matched cohorts were followed for 12 months after the index event. Compared to non-GLP-1RA anti-obesity medications, semaglutide was associated with a significantly lower risk of incident AUD diagnosis (0.37% vs 0.73%; HR: 0.50, 95% CI: 0.39–0.63), consistent across gender, age group and race. Significant lower risks were observed in patients with T2DM and without T2DM (Fig. 1a ). Compared to naltrexone or topiramate, semaglutide was associated with a significantly lower risk of incident AUD diagnosis (0.35% vs 0.78%; HR: 0.44, 95% CI: 0.32–0.61), consistent across gender, age group and race and in patients with and without T2DM (Fig. 1b ).

a Comparison between propensity-score matched semaglutide and non-GLP-1RA anti-obesity medications cohorts, stratified by gender, age group, race, and diagnosis of T2DM. b Comparison between propensity-score matched semaglutide and naltrexone/topiramate cohorts, stratified by gender, age group, race, and the diagnosis of T2DM. Patients were followed for 12 months after the index event (first prescription of semaglutide, non-GLP-1 RA anti-obesity medications, or naltrexone/topiramate during 6/2021–12/2022). Hazard rates were calculated using Cox proportional hazards analysis to estimate hazard rates of outcome at daily time intervals with censoring applied. Overall risk = number of patients with outcomes during the 12-month time window/number of patients in the cohort at the beginning of the time window. AUD Alcohol use disorders, GLP-1RA glucagon-like peptide-1 receptor agonist, T2DM type 2 diabetes. Source data are provided as a Source Data file.
Association of semaglutide with recurrent AUD diagnosis in patients with obesity and a prior history of AUD
The study population consisted of 4254 patients with obesity who had a prior diagnosis of AUD and were for the first time prescribed semaglutide or non-GLP-1RA anti-obesity medications including naltrexone or topiramate in 6/2021–12/2022. The semaglutide cohort ( n = 1470) compared with the non-GLP-1RA anti-obesity medications cohort ( n = 2784) was older, included more women, had a higher prevalence of severe obesity and obesity-associated comorbidities including T2DM and lower prevalence of adverse socioeconomic determinants of health, mental disorders, and substance use disorders. After propensity-score matching, the two cohorts (1051 in each cohort, mean age 52.6 years, 41.5% women, 16.6% black, 66.2% white, 7.4% Hispanic) were balanced (Table 2 ). The semaglutide cohort ( n = 1470) compared with the naltrexone/topiramate cohort ( n = 1430) was older, included more women, had a higher prevalence of severe obesity and obesity-associated comorbidities including T2DM and lower prevalence of adverse socioeconomic determinants of health, problems with lifestyle, and substance use disorders. After propensity-score matching, the two cohorts (715 in each cohort, mean age 51.5 years, 40.7% women, 15.8% black, 67.7% white, 6.7% Hispanic) were balanced.
Matched cohorts were followed for 12 months after the index event. Compared to non-GLP-1RA anti-obesity medications, semaglutide was associated with a significantly lower risk of recurrent AUD diagnosis (22.6% vs 43.0%; HR: 0.44, 95% CI: 0.38–0.52), which was consistent across gender, age group and race. Significant lower risks were observed in patients with T2DM and without T2DM (Fig. 2a ). Compared to naltrexone or topiramate, semaglutide was associated with a significantly lower risk of incident AUD diagnosis (21.5% vs 59.9%; HR: 0.25, 95% CI: 0.21–0.30), which was consistent across gender, age group and race and in patients with and without T2DM (Fig. 2b ).

a Comparison between propensity-score matched semaglutide and non-GLP-1RA anti-obesity medications cohorts, stratified by gender, age group, race, and the status of T2DM. b Comparison between propensity-score matched semaglutide and naltrexone/topiramate cohorts, stratified by gender, age group, race, and diagnosis of T2DM. Patients were followed for 12 months after the index event (first prescription of semaglutide, non-GLP-1 RA anti-obesity medications, or naltrexone/topiramate during 6/2021–12/2022). Hazard rates were calculated using Cox proportional hazards analysis to estimate hazard rates of outcome at daily time intervals with censoring applied. Overall risk = number of patients with outcomes during the 12-month time window/number of patients in the cohort at the beginning of the time window. AUD Alcohol use disorders, GLP-1RA glucagon-like peptide-1 receptor agonist, T2DM type 2 diabetes. Source data are provided as a Source Data file.
Association of semaglutide with incident and recurrent AUD diagnosis in patients with T2DM
The study population for the analysis of incident AUD diagnosis in patients with T2DM consisted of 598,803 patients with T2DM who had no prior diagnosis of AUD and were for the first time prescribed semaglutide or non-GLP-1RA anti-diabetes medications in 12/2017–5/2021. The semaglutide cohort ( n = 25,686) compared with the non-GLP-1RA anti-obesity medications cohort ( n = 573,117) was younger, had a higher prevalence of problems related to lifestyle, severe obesity, obesity-associated comorbidities and mental disorders. After propensity-score matching, the two cohorts (26,670 in each cohort, mean age 58.0 years, 45.3% women, 14.7% black, 60.3% white, 6.5% Hispanic) were balanced (Supplementary Table 1 ).
The study population for the analysis of recurrent AUD diagnosis in patients with T2DM consisted of 22,113 patients with T2DM who had a prior diagnosis of AUD and were for the first time prescribed semaglutide or non-GLP-1RA anti-diabetes medications in 12/2017–5/2021. The semaglutide cohort ( n = 668) compared with the non-GLP-1RA anti-obesity medications cohort ( n = 21,445) had a higher prevalence of adverse socioeconomic determinants of health, problems related to lifestyle, severe obesity, obesity-associated comorbidities and mental disorders. After propensity-score matching, the two cohorts (653 in each cohort, mean age 57.4 years, 25.9% women, 17.2% black, 55.5% white, 8.5% Hispanic) were balanced (Supplementary Table 2 ).
Matched cohorts were followed for 12 months after the index event. Compared to non-GLP-1RA anti-diabetes medications, semaglutide was associated with a significantly lower risk of incident AUD diagnosis (0.32% vs 0.52%; HR: 0.56, 95% CI: 0.43–0.74), consistent across gender, age group and race. Significant lower risks were observed in patients with and without a diagnosis of obesity (Fig. 3a ). Semaglutide compared with non-GLP-1RA anti-diabetes medications was associated with a significantly lower risk of recurrent AUD diagnosis (23.4% vs 33.2%; HR: 0.61, 95% CI: 0.50–0.75), consistent across gender, age group, and race. Significant lower risks were observed in patients with and without a diagnosis of obesity (Fig. 3b ). The significantly lower risk associations of semaglutide with both incident and recurrent AUD persisted, though slightly attenuated with overlapping confidence intervals, for the 2-year and 3-year follow-up (Fig. 3C ).
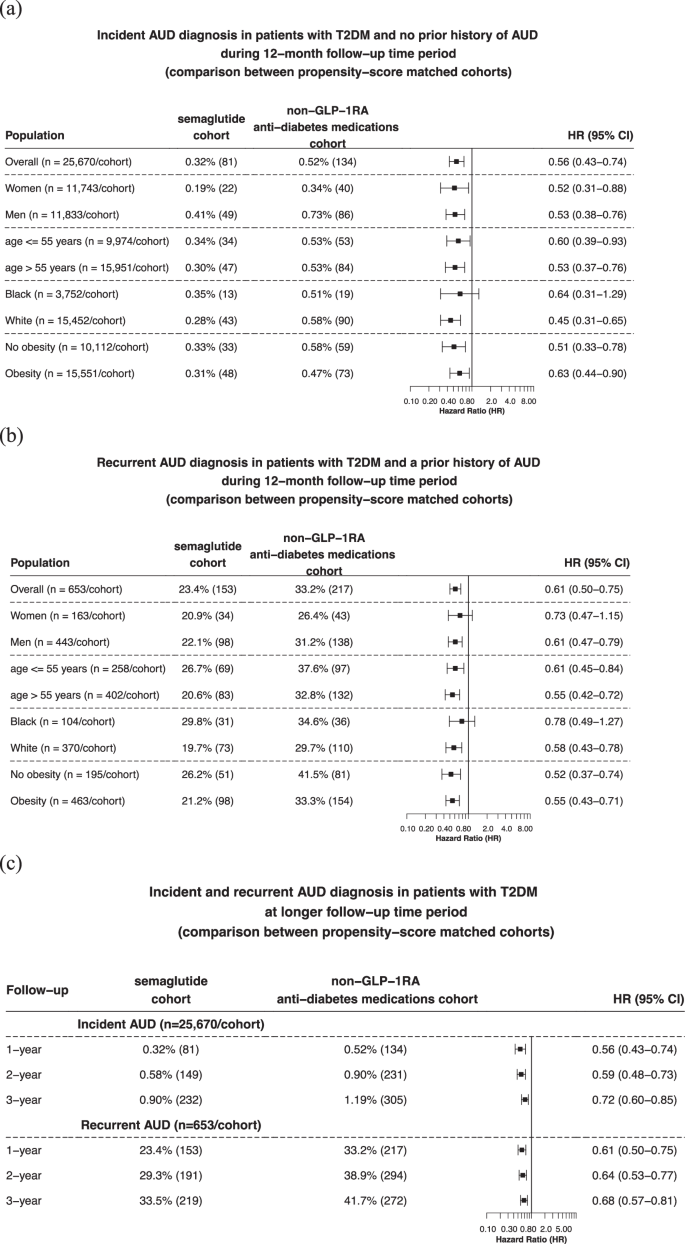
a Comparison of 12-month risk for incident AUD diagnosis between propensity-score matched semaglutide and non-GLP-1RA anti-diabetes medications cohorts, stratified by gender, age group, race, and the diagnosis of obesity. b Comparison of 12-month risk of recurrent AUD diagnosis between propensity-score matched semaglutide and non-GLP-1RA anti-diabetes medications cohorts, stratified by gender, age group, race, and the diagnosis of obesity. c Comparison of longer-term risks of incident and recurrent AUD diagnosis between propensity-score matched semaglutide and non-GLP-1RA anti-diabetes medications cohorts. Patients were followed for 12 months, 2-year and 3-year after the index event (first prescription of semaglutide, non-GLP-1 RA anti-diabetes medications in 12/2017–5/2021). AUD Alcohol use disorders, GLP-1RA glucagon-like peptide-1 receptor agonist, T2DM type 2 diabetes. Source data are provided as a Source Data file.
Here we document a potential beneficial effect of semaglutide on both the incidence and recurrence of AUD in real-world populations. The findings were replicated in two separate populations with different characteristics, no-overlapping periods, and non-overlapping patients prescribed semaglutide: one with obesity and the other with T2DM. These beneficial effects are consistent with anecdotal reports that patients prescribed semaglutide describe reduced desire to drink alcohol while on the medication 9 and with recent clinical reports; one documenting reduced alcohol drinking with semaglutide or tirzepatide based on analyses of social media texts and follow up of selected participants 10 , and another of decreased symptoms of AUD in a case series of patients treated with semaglutide 11 . It is also consistent with a small clinical trial study of the GLP-1RA drug exenatide, which significantly reduced heavy drinking days and total alcohol intake in patients with obesity 12 and with a register-based study in Demark showing that GLP-1RAs (though semaglutide was not included) compared with dipeptidyl peptidase 4 inhibitors (DPP4) were associated with lower incidence of alcohol-related events in 2009–2017 13 . It is also consistent with preclinical studies that documented reduced drinking in rodents exposed to semaglutide 8 and that prevented relapse in a rat model of alcohol dependence 7 .
The underlying mechanisms have not been fully delineated but are likely to involve modulation of the brain dopamine reward system via GLP-1 receptors, which are present both in the ventral tegmental areas (VTA), where dopamine neurons are located, and in the nucleus accumbens (NAc), which is the main projection of VTA dopamine neurons 14 . The involvement of the dopamine reward pathway in modulating food and alcohol consumption 15 could explain why semaglutide is beneficial in reducing food consumption 16 and in animal models reducing alcohol and other drug consumption 5 . Indeed, semaglutide binds to the NAc 7 where it has been shown to attenuate alcohol-induced dopamine increases in alcohol drinking rats 7 providing evidence of semaglutide’s modulation of the mesolimbic dopamine reward system 17 . Importantly the rewarding effects of food are a main contributor to overeating and obesity 18 just as the rewarding effects of alcohol drive alcohol consumption 19 .
Because GLP-1 also mediates stress responses 20 , this could be another mechanism by which semaglutide could buffer stress-related overeating and alcohol consumption 21 . The habenula, which has a high concentration of GLP1 receptors 22 could also participate in semaglutide’s actions as it is involved in the negative reinforcement in obesity 23 and in alcohol and other substance use disorders 24 . Additionally, the anti-inflammatory effects of semaglutide and other GLP1-RA medication have also been implicated in its potential beneficial effects for AUD and other substance use disorder 6 . However, the beneficial effects of semaglutide for alcohol consumption could also reflect the fact that alcohol like food serves as a source of energy 25 , and could include a combination of central 7 and peripheral mechanisms such as the effects of semaglutide on alcohol absorption, pharmacokinetics and metabolism 10 . Though there are no reports on semaglutide’s effects on alcohol absorption and pharmacokinetics it is likely that since it decreases gastric emptying it would also likely decrease alcohol’s absorption. Because the rate of alcohol absorption influences its rewarding effects 26 , delayed absorption could make alcohol less rewarding. Delayed absorption could also increase alcohol’s metabolism in the stomach into acetaldehyde 27 , which would enhance its aversive effects.
As of now, only one randomized clinical trial has been published that evaluated the effects of a GLP-1RA exenatide in patients with AUD 12 . Though this trial did not report reductions in heavy alcohol drinking days (main outcome), it showed a significant attenuation of brain activation to alcohol cues. Also, in a secondary analysis the investigators found a significant reduction in heavy drinking days and total alcohol intake in AUD patients with obesity. This is relevant to our findings since the benefits of semaglutide were observed in patients with obesity and in patients with T2DM many of whom also had obesity. In the analysis of patients with T2DM stratified by their having or not having a diagnosis of obesity, we observed that the lower risk of incident AUD with semaglutide in patients without obesity was similar in patients with obesity. In summary, our study provides real-world evidence supporting the therapeutic benefits of semaglutide for AUD. It is important to clarify that our findings of lower risk of AUD incidence and relapse in patients taking semaglutide cannot be interpreted to indicate that semaglutide reduced AUD symptomatology and are insufficient to justify clinicians’ use of semaglutide off-label to treat AUD. For this to happen data from randomized clinical trials are necessary. Currently, there are five registered clinical trials to evaluate the effect of semaglutide in AUD, and some are already recruiting 28 , 29 , 30 , 31 , 32 . Since individuals with AUD are at higher risk for mood disorders and suicidality 33 , 34 and there have been concerns that semaglutide could increase these 35 , though recent evidence suggests it decreases them 36 , it will be important for future clinical trials to assess semaglutide’s effects in mood and suicidal ideation. Future studies should also evaluate interactions with alcohol and with medications for AUD.
Our study has several limitations: First, this is a retrospective observational study, so no causal inferences can be drawn. Second, our study populations represented those who had medical encounters with healthcare systems contributing to the TriNetX Platform. Finding from this study need to be validated in other populations. Third, there are limitations inherent in retrospective observational studies including unmeasured or uncontrolled confounders, self-selection, reverse causality, and other biases. Although the findings were replicated in two separate study populations with different characteristics at two non-overlapping study periods and with non-overlapping exposure cohorts, potential biases or confounders could not be fully eliminated in this observational study. Fourth the follow-up time for the main analyses was 8 months. For the study population with T2DM we conducted a longer follow-up - up to 3 years and observed consistently lower risks in both incident and recurrent AUD associated with semaglutide. However, future studies are necessary to evaluate longer-term associations of semaglutide with AUD in patients with obesity. Fifth, the weekly higher dose format of 2.4 mg semaglutide (marketed as Wegovy) was approved for weight management, and the lower dose format of 0.5–1 mg semaglutide (marketed as Ozempic) was approved for treating T2DM). Interestingly we observed a stronger association of semaglutide with recurrent AUD in patients with obesity than in patients with T2DM (HR of 0.53 vs. 0.74), which could suggest a potential dosage effect. However, the characteristics of these 2 study populations, the comparators, and the study periods were different. Since different dose forms of semaglutide were approved for different disease indications, we could not directly examine the dosage effect of semaglutide in our study.
In summary, our results find an association between reduced risk for incident and AUD relapse with the prescription of smaglutide in patients with obesity or T2DM. While these findings provide preliminary evidence of the potential benefit of semaglutide in AUD in real-world populations further randomized clinical trials are needed to support its use clinically for AUD.
We used built-in statistical and informatics functions within the TriNetX Analytics Platform 37 (Research US Collaborative Network) to analyze aggregated and de-identified patient electronic health records (EHRs). Analyses were performed on January 26, 2024. At the time of this study, TriNetX Research US Collaborative Network contained EHRs of 105.3 million patients from 61 healthcare organizations, most of which are large academic medical institutions, in the US across 50 states: 25%, 17%, 41%, and 12% in the Northeast, Midwest, South, West, respectively, and 5% unknown region. We previously used the TriNetX platform to perform retrospective cohort studies 36 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 in various populations including patients with substance use disorders 38 , 45 , 46 , 48 , 51 . We also used the TriNetX platform to examine the associations of GLP-1RAs with colorectal cancer 50 and semaglutide with suicidal ideations 36 and cannabis use disorder 51 .
TriNetX de-identifies and aggregates EHRs from contributing healthcare systems completes an intensive data preprocessing stage to minimize missing values, maps the data to a common clinical data model, and provides web-based analytics tools to analyze patient EHRs. All variables are either binary, categorical, or continuous but essentially guaranteed to exist. Missing sex, race, and ethnicity values are represented using “Unknown Sex”, “Unknown race” and “Unknown Ethnicity”, respectively. For other variables (e.g., medical conditions, medications, procedures, lab tests, and socio-economic determinant health), the value is either present or absent, and “missing” is not pertinent.
Ethics statement
The TriNetX platform aggregates and HIPAA de-identifies data contributed from the electronic health records of participating healthcare organizations. The TriNetX platform also only reports population-level results (no access to individual patient data) and uses statistical “blurring”, reporting all population-level counts between 1 and 10 as 10. Based on the de-identification methods used by TriNetX, as per HIPAA privacy and security rules 52 , TriNetX sought and obtained expert attestation that TriNetX data is HIPAA de-identified. Because the data in the TriNetX platform is HIPAA de-identified, and therefore, “by definition” is deemed to allow no access to protected health information (and therefore no risk of protected health information disclosure), Institutional Review Boards (IRBs) have no jurisdiction of studies using HIPAA de-identified data 53 . Since the study concerns non-human subject research, consent from participants was waived and IRB approval was not required for this study.
Study populations
The study population with obesity.
The analyses for the association of semaglutide with both incident and recurrent diagnosis of AUD in patients with obesity were restricted to a starting date of 6/2021 when semaglutide was approved in the US for weight management as Wegovy and an ending date of 12/2022, which allowed for a 12-month follow-up period by the time of data collection and analysis on January 26, 2024.
To assess the associations of semaglutide with incident AUD (first time diagnosis of AUD), the study population included 83,825 patients who had active medical encounters for the diagnosis of obesity in 6/2021–12/2022, were for the first time (new-user design) prescribed semaglutide or non-GLP-1RA anti-obesity medications (naltrexone, topiramate, bupropion, orlistat, phentermine) 54 during 6/2021–12/2022 (time zero or index event), had no diagnosis of AUD on or before the index event and had a diagnosis of at least one of obesity-associated comorbidities (T2D, hypertension, hypercholesterolemia, hyperlipidemia, heart diseases, stroke) on or before the index event. Patients who were prescribed other GLP-1RAs or had bariatric surgery on or before the index event were excluded. This study population was then divided into 3 cohorts: (1) semaglutide cohort – 45,797 patients who were first-time prescribed semaglutide, (2) non-GLP1-RA anti-obesity medication cohort – 38,028 patients who were first-time prescribed non-GLP-1RA anti-obesity medications but not semaglutide and (3) naltrexone/topiramate cohort – 16,676 patients who were first time prescribed naltrexone and topiramate but not semaglutide. Among the non-GLP-1RA anti-obesity medications, naltrexone and topiramate were also prescribed for AUD 3 . We constructed the naltrexone/topiramate cohort to compare semaglutide to naltrexone/topiramate for incident AUD risk in patients with obesity. We used new-user design to mitigate prevalent user bias and confounding associated with the drug itself 55 , 56 .
To assess the associations of semaglutide with recurrent AUD diagnosis (recurrent medical encounters for AUD diagnosis), the study population included 4254 patients who had active medical encounters for the diagnosis of obesity in 6/2021–12/2022, were for the first time (new-user design) prescribed semaglutide or non-GLP-1RA anti-obesity medications during 6/2021–12/2022 (index event), had a diagnosis of AUD on or before the index event and had a diagnosis of at least one of obesity-associated comorbidities on or before the index event. Patients who were prescribed other GLP-1RAs or had bariatric surgery on or before the index event were excluded. This study population was then divided into 3 cohorts: (1) semaglutide cohort – 1470 patients who were first-time prescribed semaglutide, (2) non-GLP1-RA anti-obesity medication cohort – 2784 patients who were first-time prescribed non-GLP-1RA anti-obesity medications but not semaglutide and (3) naltrexone/topiramate cohort – 1430 patients who were first time prescribed naltrexone and topiramate but not semaglutide. We constructed the naltrexone/topiramate cohort to compare semaglutide to naltrexone/topiramate for recurrent AUD risk in patients with obesity.
The study populations with T2DM
The analyses on the associations of semaglutide with both incident and recurrent AUD among patients with T2DM had a starting time of 12/2017 when semaglutide was approved in the US to treat T2DM as Ozempic and an ending date of 5/2021 to allow us to separately examine the associations of semaglutide on AUD as Ozempic from those as Wegovy in the study population with obesity. Since patients in the study population with obesity were for the first time prescribed semaglutide after 6/2021, there was no overlap in the exposure cohorts for these two study populations.
To assess the association of semaglutide with incident AUD, the study population included 598,803 patients with T2DM who had active medical encounters for T2DM during 12/2017–5/2021, were for the first time prescribed semaglutide or non-GLP1-1RA anti-diabetes medications (new-user design) during 12/2017–5/2021(index event), had no diagnosis of AUD on or before the index event and had a diagnosis of at least one of obesity-associated comorbidities (hypertension, hypercholesterolemia, hyperlipidemia, heart diseases, stroke) on or before the index event. The status of non-GLP1RA anti-diabetes medications was determined by the Anatomical Therapeutic Chemical or ATC code A10 “Drugs used in diabetes” with GLP-1RAs (ATC code A10BJ “Glucagon-like peptide-1 (GLP-1) analogs”) excluded. The list of non-GLP1RA anti-diabetes medications included insulins, metformin, sulfonylureas, alpha glucosidase inhibitors, thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors, sodium-glucose co-transporter 2 (SGLT2) inhibitors. Patients who were prescribed other GLP-1RAs or had bariatric surgery on or before the index event were excluded. This study population was divided into two cohorts: (1) semaglutide cohort – 25,686 patients prescribed semaglutide, and (2) Non-GLP-1RA anti-diabetes medication cohort – 573,117 patients prescribed non-GLP-1RA anti-diabetes medications.
To assess the associations of semaglutide with recurrent AUD, the study population comprised 22,113 patients who had active medical encounters for T2DM diagnosis in 12/2017–5/2021, were for the first time prescribed semaglutide or non-GLP1-1RA anti-diabetes medications during 12/2017–5/2021(index event), had a diagnosis of AUD on or before the index event, and had a diagnosis of at least one of obesity-associated comorbidities on or before the index event. Patients who were prescribed other GLP-1RAs or had bariatric surgery on or before the index event were excluded. This study population was then divided into two cohorts: (1) semaglutide cohort – 668 patients prescribed semaglutide, and (2) non-GLP1-RA anti-diabetes medication cohort – 21,445 patients prescribed non-GLP-1RA anti-diabetes medications.
Statistical analysis
For each study population, the semaglutide cohort and the comparision cohort were propensity-score matched (1:1 using nearest neighbor greedy matching with a caliper of 0.25 times the standard deviation) on covariates that are potential risk factors for AUD 57 , 58 , 59 , 60 including demographics, adverse socioeconomic determinants of health (e.g., problems related to education and literacy, employment and unemployment, housing and economic circumstances, social environment, upbringing, primary support group including family circumstances and various psychosocial circumstances), problems with lifestyle (e.g., tobacco use, lack of physical exercise, inappropriate diet and eating habits, high-risk sexual behavior, gambling and betting, and other problems related to lifestyle including antisocial behaviors and sleep deprivation), pre-existing medical conditions, medications, medical procedures and medical visit types (outpatient, inpatient, emergency, and virtual). Obesity sub-categories were also matched to control obesity severity which included 3 ICD-10 diagnosis codes and 15 BMI categories ranging from BMI 30 to BMI 70 or greater.
The outcome –incident or recurrent diagnosis of AUD (International Classification of Diseases, Tenth Revision (ICD-10) code F10 “Alcohol related disorders”) – that occurred within the 12-month time window after the index events were compared between matched semaglutide and comparison cohorts. Cox proportional hazards analysis was used to estimate hazard rates of outcome at daily time intervals with censoring applied. When the last fact (the outcome of interests or other medical encounters) in the patient’s record is in the time window for analysis, the patient was censored on the day after the last fact in their record. Hazard ratio (HR) and 95% confidence intervals were used to describe the relative hazard of the outcomes based on a comparison of time to event rates.
Separate analyses were performed in patients stratified by sex (women, men), age groups (≤55, >55 years), and race (Black, White). For the study population with obesity, a separate analysis was performed in patients with T2DM and patients without T2DM. Given that the previous clinical trial of the GLP-1RA exenatide for AUD found reduced alcohol consumption only in those who were overweight 12 , we further separately examined the association of semaglutide with both incident and recurrent AUD in patients with T2DM, with and without obesity.
To examine longer-term associations of semaglutide with AUD, the outcome –incident and recurrent diagnosis of AUD– in patients with T2DM was further followed for 2-year, 3-year starting after the index event.
The data were collected and analyzed on January 26, 2024 within the TriNetX Analytics Platform using built-in functions (propensity-score matching, Cox proportional hazard analysis, Kaplan-Meier survival) implemented using Survival 3.2-3 in R 4.0.2 and libraries/utilities for data science and statistics in Python 3.7 and Java 11.0.16. Details of clinical codes for eligibility criteria, exposure, outcomes, and confounders are in Supplementary Table 3 .
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
This study used population-level aggregate and HIPAA de-identified data collected by the TriNetX platform and available from TriNetX, LLC ( https://trinetx.com/ ), but third-party restrictions apply to the availability of these data. The data were used under license for this study with restrictions that do not allow for the data to be redistributed or made publicly available. To gain access to the data, a request can be made to TriNetX ([email protected]), but costs may be incurred, and a data-sharing agreement may be necessary. Data specific to this study including diagnosis codes and cohort characteristics in aggregated format are included in the manuscript as tables, figures, and supplementary files. Data through the TriNetX platform is queried in real-time with results being returned typically in seconds to minutes. Data from the underlying electronic health records of participating healthcare organizations is refreshed in the TriNetX platform from daily to every couple of months depending on the healthcare organization. Source data are provided with this paper.
Code availability
All the statistical analyses in this study including propensity-score matching, and Cox proportional hazards used web-based built-in functions within the TriNetX Analytics Platform that are implemented using Survival 3.2-3 in R 4.0.2 and libraries/utilities for data science and statistics in Python 3.7 and Java 11.0.16. Data and code to recreate figures in the study can be accessed at https://github.com/bill-pipi/semaglutide_AUD
SAMHSA, Center for Behavioral Health Statistics & Quality. SAMHSA, Center for Behavioral Health Statistics and Quality. 2021 National Survey on Drug Use and Health. Table 5.6A—Alcohol use disorder in past year: among people aged 12 or older; by age group and demographic characteristics, numbers in thousands, https://www.samhsa.gov/data/sites/default/files/reports/rpt39441/NSDUHDetailedTabs2021/NSDUHDetailedTabs2021/NSDUHDetTabsSect5pe2021.htm#tab5.6a (2021).
Rehm, J. et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 373 , 2223–2233 (2009).
Article PubMed Google Scholar
Kranzler, H. R. & Soyka, M. Diagnosis and Pharmacotherapy of Alcohol Use Disorder: A Review. JAMA 320 , 815–824 (2018).
Article CAS PubMed PubMed Central Google Scholar
Jonas, D. E. et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA 311 , 1889–1900 (2014).
Klausen, M. K., Thomsen, M., Wortwein, G. & Fink-Jensen, A. The role of glucagon-like peptide 1 (GLP-1) in addictive disorders. Br. J. Pharmacol. 179 , 625–641 (2022).
Ozburn, A. R. & Spencer, S. M. Repurposing anti-inflammatory medications for alcohol and substance use disorders. Neuropsychopharmacology , https://doi.org/10.1038/s41386-023-01696-z (2023).
Aranäs, C. et al. Semaglutide reduces alcohol intake and relapse-like drinking in male and female rats. EBioMedicine 93 , 104642 (2023).
Article PubMed PubMed Central Google Scholar
Chuong, V. et al. The glucagon-like peptide-1 (GLP-1) analogue semaglutide reduces alcohol drinking and modulates central GABA neurotransmission. JCI Insight 8 , e170671 (2023).
Reardon, S. Could New Weight-Loss Drugs like Ozempic Treat Addiction? (Scientific American, 2023).
Quddos, F. et al. Semaglutide and Tirzepatide reduce alcohol consumption in individuals with obesity. Sci. Rep. 13 , 20998 (2023).
Article ADS CAS PubMed PubMed Central Google Scholar
Richards, J. R. et al. Significant decrease in alcohol use disorder symptoms secondary to semaglutide therapy for weight loss: A case series. J. Clin. Psychiatry 85 , 23m15068 (2023).
Klausen, M. K. et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. JCI Insight 7 , e159863 (2022).
Wium-Andersen, I. K. et al. Use of GLP-1 receptor agonists and subsequent risk of alcohol-related events. A nationwide register-based cohort and self-controlled case series study. Basic Clin. Pharmacol. Toxicol. 131 , 372–379 (2022).
Alhadeff, A. L., Rupprecht, L. E. & Hayes, M. R. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 153 , 647–658 (2012).
Article CAS PubMed Google Scholar
Volkow, N. D., Wise, R. A. & Baler, R. The dopamine motive system: implications for drug and food addiction. Nat. Rev. Neurosci. 18 , 741–752 (2017).
Dickson, S. L. et al. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J. Neurosci. 32 , 4812–4820 (2012).
Jensen, M. E. et al. Glucagon-like peptide-1 receptor regulation of basal dopamine transporter activity is species-dependent. Neurochem. Int. 138 , 104772 (2020).
Volkow, N. D., Wang, G.-J., Tomasi, D. & Baler, R. D. The addictive dimensionality of obesity. Biol. Psychiatry 73 , 811–818 (2013).
Di Chiara, G. Alcohol and dopamine. Alcohol Health Res. World 21 , 108–114 (1997).
PubMed PubMed Central Google Scholar
Ghosal, S., Myers, B. & Herman, J. P. Role of central glucagon-like peptide-1 in stress regulation. Physiol. Behav. 122 , 201–207 (2013).
Baik, J.-H. Stress and the dopaminergic reward system. Exp. Mol. Med. 52 , 1879–1890 (2020).
Merchenthaler, I., Lane, M. & Shughrue, P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J. Comp. Neurol. 403 , 261–280 (1999).
Wang, J. et al. Habenula volume and functional connectivity changes following laparoscopic sleeve gastrectomy for obesity treatment. Biol. Psychiatry, https://doi.org/10.1016/j.biopsych.2023.07.009 (2023).
Li, W. et al. Activation of glycine receptors in the lateral habenula rescues anxiety- and depression-like behaviors associated with alcohol withdrawal and reduces alcohol intake in rats. Neuropharmacology 157 , 107688 (2019).
Volkow, N. D. et al. Acute alcohol intoxication decreases glucose metabolism but increases acetate uptake in the human brain. Neuroimage 64 , 277–283 (2013).
de Wit, H., Bodker, B. & Ambre, J. Rate of increase of plasma drug level influences subjective response in humans. Psychopharmacology 107 , 352–358 (1992).
Yin, S. J. et al. Human stomach alcohol and aldehyde dehydrogenases: comparison of expression pattern and activities in alimentary tract. Gastroenterology 112 , 766–775 (1997).
Semaglutide Therapy for Alcohol Reduction (STAR). https://clinicaltrials.gov/study/NCT06015893 (2023).
Semaglutide Therapy for Alcohol Reduction - Tulsa (STAR-T). https://clinicaltrials.gov/study/NCT05891587 (2023).
Semaglutide for Alcohol Use Disorder. https://clinicaltrials.gov/study/NCT05520775 (2022).
Clinical Trial of Rybelsus (Semaglutide) Among Adults With Alcohol Use Disorder (AUD). https://clinicaltrials.gov/study/NCT05892432 (2023).
Does Semaglutide Reduce Alcohol Intake in Patients With Alcohol Use Disorder and Comorbid Obesity? (SEMALCO). https://clinicaltrials.gov/study/NCT05895643 (2023).
Borges, G., Walters, E. E. & Kessler, R. C. Associations of substance use, abuse, and dependence with subsequent suicidal behavior. Am. J. Epidemiol. 151 , 781–789 (2000).
Schuckit, M. A. Alcohol, anxiety, and depressive disorders. Alcohol Health Res. World 20 , 81–85 (1996).
EMA. EMA statement on ongoing review of GLP-1 receptor agonists. Eur. Med. Agency, https://www.ema.europa.eu/en/news/ema-statement-ongoing-review-glp-1-receptor-agonists (2023).
Wang, W. et al. Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nat. Med . 30 , 168–176 (2024).
TriNetX - The World’s Largest, Living Ecosystem of Real-World Data and Evidence. TriNetX https://trinetx.com/ (2021).
Wang, L., Wang, Q., Davis, P. B., Volkow, N. D. & Xu, R. Increased risk for COVID‐19 breakthrough infection in fully vaccinated patients with substance use disorders in the United States between December 2020 and August 2021. World Psychiatry 21 , 124–132 (2022).
Wang, L. et al. Incidence Rates and Clinical Outcomes of SARS-CoV-2 Infection With the Omicron and Delta Variants in Children Younger Than 5 Years in the US. JAMA Pediatr . https://doi.org/10.1001/jamapediatrics.2022.0945 (2022).
Wang, L., Davis, P. B., Kaelber, D. C., Volkow, N. D. & Xu, R. Comparison of mRNA-1273 and BNT162b2 Vaccines on Breakthrough SARS-CoV-2 Infections, Hospitalizations, and Death During the Delta-Predominant Period. JAMA https://doi.org/10.1001/jama.2022.0210 (2022).
Wang, L., Davis, P. B., Kaelber, D. C. & Xu, R. COVID-19 breakthrough infections and hospitalizations among vaccinated patients with dementia in the United States between December 2020 and August 2021. Alzheimers. Dement . https://doi.org/10.1002/alz.12669 (2022).
Wang, W., Kaelber, D. C., Xu, R. & Berger, N. A. Breakthrough SARS-CoV-2 Infections, Hospitalizations, and Mortality in Vaccinated Patients With Cancer in the US Between December 2020 and November 2021. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2022.1096 (2022).
Wang, L., Kaelber, D. C., Xu, R. & Berger, N. A. COVID-19 breakthrough infections, hospitalizations and mortality in fully vaccinated patients with hematologic malignancies: A clarion call for maintaining mitigation and ramping-up research. Blood Rev . 54 , 100931 (2022).
Wang, L., Berger, N. A. & Xu, R. Risks of SARS-CoV-2 Breakthrough Infection and Hospitalization in Fully Vaccinated Patients With Multiple Myeloma. JAMA Netw. Open 4 , e2137575 (2021).
Wang, L. et al. Association of COVID-19 with endocarditis in patients with cocaine or opioid use disorders in the US. Mol. Psychiatry, https://doi.org/10.1038/s41380-022-01903-1 (2022).
Gao, Z. et al. Repurposing ketamine to treat cocaine use disorder: integration of artificial intelligence-based prediction, expert evaluation, clinical corroboration and mechanism of action analyses. Addiction, https://doi.org/10.1111/add.16168 (2023).
Olaker, V. R. et al. Association of recent SARS-CoV-2 infection with new-onset alcohol use disorder, January 2020 through January 2022. JAMA Netw. Open 6 , e2255496 (2023).
Wang, L. et al. Cardiac and mortality outcome differences between methadone, buprenorphine and naltrexone prescriptions in patients with an opioid use disorder. J. Clin. Psychol . 79 , 2869–2883 (2023).
Wang, L. et al. Association of COVID-19 with new-onset Alzheimer’s disease. J. Alzheimers. Dis . https://doi.org/10.3233/JAD-220717 (2022).
Wang, L., Wang, W., Kaelber, D. C., Xu, R. & Berger, N. A. GLP-1 Receptor Agonists and Colorectal Cancer Risk in Drug-Naive Patients With Type 2 Diabetes, With and Without Overweight/Obesity. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2023.5573 (2023).
Wang, W. et al. Association of semaglutide with reduced incidence and relapse of cannabis use disorder in real-world populations: a retrospective cohort study. Mol. Psychiatry https://doi.org/10.1038/s41380-024-02498-5 (2024).
Office for Civil Rights (OCR). Guidance regarding methods for DE-identification of protected health information in accordance with the health insurance portability and accountability act (HIPAA) Privacy Rule. Hhs.gov https://www.hhs.gov/hipaa/for-professionals/privacy/special-topics/de-identification/index.html (2012).
Institutional review boards and the HIPAA privacy rule (National Institutes of Health, 2003).
Prescription Medications to Treat Overweight & Obesity. National Institute of Diabetes and Digestive and Kidney Diseases, https://www.niddk.nih.gov/health-information/weight-management/prescription-medications-treat-overweight-obesity (2023).
Ray, W. A. Evaluating medication effects outside of clinical trials: new-user designs. Am. J. Epidemiol. 158 , 915–920 (2003).
Hernán, M. A. & Robins, J. M. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am. J. Epidemiol. 183 , 758–764 (2016).
Hägele, C., Friedel, E., Kienast, T. & Kiefer, F. How do we “learn” addiction? Risk factors and mechanisms getting addicted to alcohol. Neuropsychobiology 70 , 67–76 (2014).
Nolen-Hoeksema, S. Gender differences in risk factors and consequences for alcohol use and problems. Clin. Psychol. Rev. 24 , 981–1010 (2004).
Stewart, S. A., Copeland, A. L. & Cherry, K. E. Risk factors for substance use across the lifespan. J. Genet. Psychol. 184 , 145–162 (2023).
National Institute on Alcohol Abuse and Alcoholism (NIAAA). Risk Factors: Varied Vulnerability to Alcohol-Related Harm. https://www.niaaa.nih.gov/health-professionals-communities/core-resource-on-alcohol/risk-factors-varied-vulnerability-alcohol-related-harm . (2023).
Download references
Acknowledgements
We acknowledge support from the National Institute on Alcohol Abuse and Alcoholism (AA029831), National Institute on Aging (AG057557, AG061388, AG062272, AG07664), from National Cancer Institute Case Comprehensive Cancer Center (CA221718, CA043703, CA2332216)
Author information
Authors and affiliations.
Center for Science, Health, and Society, Case Western Reserve University School of Medicine, Cleveland, OH, USA
William Wang & Nathan A. Berger
National Institute on Drug Abuse, National Institutes of Health, Bethesda, MD, USA
Nora D. Volkow
Center for Community Health Integration, Case Western Reserve University School of Medicine, Cleveland, OH, USA
Pamela B. Davis
Center for Clinical Informatics Research and Education, The MetroHealth System, Cleveland, OH, USA
David C. Kaelber
Center for Artificial Intelligence in Drug Discovery, Case Western Reserve University School of Medicine, Cleveland, OH, USA
You can also search for this author in PubMed Google Scholar
Contributions
R.X. conceived the study. R.X. and N.D.V. designed the study. R.X., N.D.V. and W.W. interpreted the results and drafted the manuscript. W.W. performed data analysis and created tables and figures. N.A.B., P.B.D., and D.C.K. critically contributed to study design, result interpretation, and manuscript preparation. We confirm the originality of the content. R.X. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Correspondence to Nora D. Volkow or Rong Xu .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks Sanjay Kalra, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, reporting summary, source data, source data, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Wang, W., Volkow, N.D., Berger, N.A. et al. Associations of semaglutide with incidence and recurrence of alcohol use disorder in real-world population. Nat Commun 15 , 4548 (2024). https://doi.org/10.1038/s41467-024-48780-6
Download citation
Received : 08 November 2023
Accepted : 08 May 2024
Published : 28 May 2024
DOI : https://doi.org/10.1038/s41467-024-48780-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
The independent source for health policy research, polling, and news.
A Look at the Latest Alcohol Death Data and Change Over the Last Decade
Heather Saunders and Robin Rudowitz Published: May 23, 2024
Alcohol use disorder (AUD) is often an underrecognized substance use disorder (SUD) despite its substantial consequences . Over half of US adults (54%) say that someone in their family has struggled with an alcohol use disorder, making it the most prevalent non-tobacco substance use disorder. Yet, only one-third of adults view alcohol addiction as a crisis, compared to over half who see opioids as such. Federal data show that 1 in 10 people had an alcohol use disorder in the past year, over 4 in 10 alcohol users report binge drinking in the past month, and per capita alcohol consumption is higher than the decade prior. Treatment rates for alcohol use disorders are notably low, especially for the use of medication , a recommended AUD treatment component. Although the opioid crisis has been declared a public health emergency by the U.S. Department of Health and Human Services since 2017, no similar declaration exists regarding alcohol deaths. However, HHS has set a priority goal of reducing emergency department visits for acute alcohol use, mental health conditions, suicide attempts, and drug overdoses by 10% by 2025.
This analysis focuses on the narrowest definition of alcohol deaths known as “alcohol-induced deaths” (referred to as “alcohol deaths” throughout the brief). These alcohol deaths are caused by conditions directly attributable to alcohol consumption, such as alcohol-associated liver diseases . Broader definitions of alcohol deaths extend this definition to also encompass cases where an alcohol-induced condition was a contributing factor, but not the underlying cause of death. Key takeaways from this analysis of CDC WONDER data from 2012 to 2022 include the following:
- Alcohol deaths increased steadily over the past decade with sharp rises during the pandemic years. Overall, the national alcohol death rate has risen 70% in the past decade, accounting for 51,191 deaths in 2022, up from 27,762 deaths in 2012.
- Alcohol deaths in 2022 were highest among people aged 45 to 64, American Indian and Alaska Native (AIAN) people, and males. Alcohol death rates for AIAN people are the highest–5 times higher than death rates for White people, the racial group with the next highest prevalence. Deaths are rising fastest among adults aged 26 to 44, AIAN people, and females–with these groups experiencing nearly or more than a 100% rise in alcohol mortality rates in the last decade.
- Rates of alcohol deaths varied considerably across states in 2022. While all states and D.C. experienced increases in deaths rates over the past decade and during the pandemic, the rate of change varied by state and year, with some states’ death rates rising most sharply during the pandemic and other state experiencing rises more evenly before and during the pandemic. Rural areas have a higher rate of alcohol deaths and experienced greater growth in death rates both over the past decade.
- The number of alcohol-related deaths rises to 105,308 under a broader definition that counts deaths where alcohol-induced conditions are either the underlying cause or a contributing factor. This exceeds the numbers for opioid and suicide deaths, which also use this broader definition, totaling 83,437 and 49,594, respectively.
What are the trends in alcohol deaths?
Alcohol deaths have steadily climbed over the past decade, a trend that accelerated during the pandemic (Figure 1). When adjusted for population growth and age, the alcohol death rate has risen by 70% from 2012 to 2022, moving from 7.97 to 13.53 deaths per 100,000 people. Although deaths fell somewhat in 2022, they remain far higher than a decade ago. From 2012 to 2019, the year over year rise in deaths rates averaged about 4% per year, and then jumped during early pandemic years, with the biggest rise from 2019 to 2020. Other data mirror this trend – emergency department (ED) visits for SUD are on the rise and account for twice the number of ED visits compared to opioids. Alcohol related ED visits account for nearly half of all SUD related visits (45%), far higher than the next highest group, opioids, accounting for 13% of ED visits.
How do alcohol death rates vary and how have they changed across demographics groups?
Alcohol deaths in 2022 were highest among people aged 45 to 64, males, people living in rural areas, and AIAN people. Alcohol death rates for AIAN people are by far the highest–5 times higher than death rates for White people, the racial group with the next highest prevalence. Across age groups, people aged 45 to 64 have the highest alcohol death rate, followed by 65+. Death rates in males are more than double that of females and people who reside in rural areas have death rates higher than those who live in urban areas (Figure 2).
Over the past decade (2012-2022), alcohol death rates grew fastest among people 26 to 44, AIAN people, and females (Figure 3) . Overall alcohol consumption has risen somewhat in recent years, but increases may have been concentrated among certain populations as well as other risk factors.
- People aged 26 to 44 . Individuals aged 26 to 44 experienced the fastest increase in alcohol death rates, with a rise of 144% over the past decade and over 50% during the pandemic. While this younger age group showed the steepest rate of increase, the largest overall growth in the number of deaths occurred among those aged 45 to 64. This somewhat older group already had the highest death rates and experienced the largest increase in death rates (12 additional deaths/100,000) in the past decade, more than any other group.
- AIAN people. Alcohol deaths for AIAN people have nearly doubled in the last 10 years. During the pandemic years, alcohol death rates increased by almost 25 deaths per 100,000 AIAN people. Increases in alcohol deaths among AIAN people follows worsening trends in other areas related to behavioral health, where AIAN have both the highest rate and fastest growing suicide and overall drug overdose death rates.
- Females. Although males die of alcohol causes more often than females, the relative growth was faster for females over the past 10 years, increasing by 86% for females compared to 61% for males. Heavier drinking may impact women more quickly than men, which may result in the faster development of serious health consequences that contribute to death.
How do alcohol death rates vary and how have they changed across geography?
In 2022 there was wide variation in alcohol death rates. In 2022, New Mexico’s death rate was the highest at 42.7 per 100,000 people, which was more than six times higher than Hawaii, the state with the lowest rate at 7.1 per 100,000 people (Figure 4).
While all states experienced an increase in alcohol deaths, those rates varied widely. Nationally, alcohol death rates increased by 70% over the past decade, including a 30% rise during the pandemic years alone (2019-2022). However, the extent of these increases varied substantially across states. For instance, the District of Columbia saw a relatively low increase of 24% over the decade, whereas Connecticut experienced a much larger rise of 167%. During the pandemic, increases ranged from 9% in Wyoming and New Jersey to 86% in Mississippi. Some states, like Vermont, had most of their rises in alcohol death rates before the pandemic, with only 12% of the growth occurring during pandemic years. In contrast, Mississippi’s rates more than doubled over the past decade, and over half of that increase happened during pandemic years. Many factors may contribute to the differences in alcohol mortality rates across states, some of which may include differences in alcohol consumption and cultural attitudes, state-specific alcohol policies , and treatment rates (Figure 4).
Rural areas experienced faster growth in alcohol deaths than urban areas, driven by sharp rises during the pandemic. Deaths grew across both rural and urban areas in the past decade; however growth was fastest in rural areas–nearly doubling in the past decade and increasing by 35% during pandemic years. Existing shortages of mental health and substance use treatment professionals may make it particularly difficult to access care in rural areas, where the supply of behavioral health workforce is even more scarce . During the pandemic, telehealth services for behavioral health and other care may have been more accessible to those living in urban areas, where an internet connection is more likely to be available or reliable (Figure 5).
What factors may contribute to the increases in alcohol deaths in the past 10 years?
Alcohol contributes to more deaths than opioids and suicides when the alcohol conditions that contribute to death are included. Defining alcohol deaths can be complex due to the gradual onset of many conditions caused by or linked to alcohol and its ability to exacerbate or increase the risk of developing other health conditions. This analysis adopts the strictest definition of alcohol deaths, focusing on deaths that were directly caused by conditions directly due to alcohol, such as alcohol-related liver diseases. However, if deaths where alcohol conditions are a contributing factor listed on the death certificate —termed ‘ alcohol-related deaths’—are included, the number of deaths increases to 105,308 in 2022, though some cases may overlap. This exceeds the numbers for opioid and suicide deaths, which also use this broader definition, totaling 83,437 and 49,594, respectively. Unlike the immediate effects of opioid overdoses or suicides, alcohol-related conditions often develop slowly over many years. These conditions can directly cause death or worsen other illness. For instance, it may take many years of heavy drinking before alcohol-associated liver diseases , the most common cause of alcohol deaths, to develop. This slower disease progression as well as the role of alcohol in exacerbating other conditions may contribute to the higher number of deaths counted under the expanded definition. The number of alcohol deaths rise even more when the criteria are broadened to include alcohol’s role in increasing the risk of death by other conditions or events, such as cancer or car accidents involving alcohol (Figure 6).
Rises in alcohol deaths may be attributed to a variety of factors including, in part, increases in drinking and low treatment rates. Alcohol consumption and some indicators of binge drinking have been on the rise in recent years , particularly among some demographic groups . Excessive alcohol consumption is tied to the development of alcohol-related diseases, which can be fatal. A variety of factors may have contributed to increases in drinking including a growing social acceptability of alcohol and loosening of alcohol policies at a state level. Other factors, such as increased stressors due to the pandemic and other issues may have increased drinking behaviors.
Treatment rates for alcohol use disorder are very low. Federal survey data show that in 2022, only 7.6% of people (12+) with a past year alcohol use disorder received any treatment. Although medications for alcohol use disorder have been shown to reduce or stop drinking, uptake of these medications is extremely low; with only 2.1% of people who meet criteria for an alcohol use disorder (diagnosed or not) receive medication treatment. Treatment rates are slightly higher among those who do receive a diagnosis–for instance, 10% of Medicaid enrollees diagnosed with an alcohol use disorder received medication, 34% received counseling services, and 74% received some type of interaction with a treatment, such as therapy, medication, assessment, or supportive service.
Barriers to alcohol use disorder treatment include a combination of provider, patient, financial, and infrastructure factors. Providers often lack confidence or knowledge in treating alcohol use disorder and are uncomfortable with medication and other treatment options, which may decrease the likelihood that they will manage treatment or make referrals . To address this, recent initiatives are enhancing education for both practicing and training providers through mandatory training programs and curriculum enhancements in medical schools . Further, recent changes to SUD confidentiality regulations are expected to simplify the diagnosis and coordination of care for individuals with substance use disorders (SUD). Insufficient treatment infrastructure or a shortage of a skilled workforce to staff facilities and deliver care can also play a role in treatment rates.
From the patient perspective, limited understanding of what constitutes problematic drinking and attitudes towards seeking treatment can hinder recognition of the need for help . For example, among those who meet the criteria for SUD—which may include symptoms like increased tolerance, repeated attempts to quit or control use, or social problems related to use– 95% of adults did not seek treatment and didn’t think they needed it. Initiatives aimed at early screening in non-traditional settings, such as schools may help early detection and lead to more timely linkages of individuals to treatment resources. When people think they might need treatment, practical issues such as insurer coverage of services, locating a provider that will accept the patient’s insurance, availability of time off from work, childcare, and the affordability of treatment/out of pocket costs can also influence decisions about seeking or staying in treatment.
This work was supported in part by Well Being Trust. KFF maintains full editorial control over all of its policy analysis, polling, and journalism activities.
- Mental Health
Also of Interest
- A Look at the Latest Suicide Data and Change Over the Last Decade
- COVID-19 Cases and Deaths by Race/Ethnicity: Current Data and Changes Over Time
- Recent Trends in Mental Health and Substance Use Concerns Among Adolescents

Understanding alcohol use disorders and their treatment
People with alcohol use disorders drink to excess, endangering both themselves and others. This question-and-answer fact sheet explains alcohol problems and how psychologists can help people recover.
- Substance Use, Abuse, and Addiction

For many people, drinking alcohol is nothing more than a pleasant way to relax. People with alcohol use disorders, however, drink to excess, endangering both themselves and others. This question-and-answer fact sheet explains alcohol problems and how psychologists can help people recover.
When does drinking become a problem?
For most adults, moderate alcohol use — no more than two drinks a day for men and one for women and older people — is relatively harmless. (A "drink" means 1.5 ounces of spirits, 5 ounces of wine, or 12 ounces of beer, all of which contain 0.5 ounces of alcohol.
Moderate use, however, lies at one end of a range that moves through alcohol abuse to alcohol dependence:
Alcohol abuse is a drinking pattern that results in significant and recurrent adverse consequences. Alcohol abusers may fail to fulfill major school, work, or family obligations. They may have drinking-related legal problems, such as repeated arrests for driving while intoxicated. They may have relationship problems related to their drinking.
People with alcoholism — technically known as alcohol dependence — have lost reliable control of their alcohol use. It doesn't matter what kind of alcohol someone drinks or even how much: Alcohol-dependent people are often unable to stop drinking once they start. Alcohol dependence is characterized by tolerance (the need to drink more to achieve the same "high") and withdrawal symptoms if drinking is suddenly stopped. Withdrawal symptoms may include nausea, sweating, restlessness, irritability, tremors, hallucinations and convulsions.
Although severe alcohol problems get the most public attention, even mild to moderate problems cause substantial damage to individuals, their families and the community.
According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA) , 6.2 percent of adults in the United States aged 18 and older had alcohol use disorder. 1 For example, a government survey revealed that about one in five individuals aged 12 to 20 were current alcohol users and about two in five young adults, aged 18 to 25, were binge alcohol users and about one in 10 were heavy alcohol users. 2
What causes alcohol-related disorders?
Problem drinking has multiple causes, with genetic, physiological, psychological,and social factors all playing a role. Not every individual is equally affected by each cause. For some alcohol abusers, psychological traits such as impulsiveness, low self-esteem and a need for approval prompt inappropriate drinking. Some individuals drink to cope with or "medicate" emotional problems. Social and environmental factors such as peer pressure and the easy availability of alcohol can play key roles. Poverty and physical or sexual abuse also increase the odds of developing alcohol dependence.
Genetic factors make some people especially vulnerable to alcohol dependence. Contrary to myth, being able to "hold your liquor" means you're probably more at risk — not less — for alcohol problems. Yet a family history of alcohol problems doesn't mean that children will automatically grow up to have the same problems. Nor does the absence of family drinking problems necessarily protect children from developing these problems.
Once people begin drinking excessively, the problem can perpetuate itself. Heavy drinking can cause physiological changes that make more drinking the only way to avoid discomfort. Individuals with alcohol dependence may drink partly to reduce or avoid withdrawal symptoms.
How do alcohol use disorders affect people?
While some research suggests that small amounts of alcohol may have beneficial cardiovascular effects, there is widespread agreement that heavier drinking can lead to health problems.
Short-term effects include memory loss, hangovers, and blackouts. Long-term problems associated with heavy drinking include stomach ailments, heart problems, cancer, brain damage, serious memory loss and liver cirrhosis. Heavy drinkers also markedly increase their chances of dying from automobile accidents, homicide, and suicide. Although men are much more likely than women to develop alcoholism, women's health suffers more, even at lower levels of consumption.
Drinking problems also have a very negative impact on mental health. Alcohol abuse and alcoholism can worsen existing conditions such as depression or induce new problems such as serious memory loss, depression or anxiety.
Alcohol problems don't just hurt the drinker. Spouses and children of heavy drinkers may face family violence; children may suffer physical and sexual abuse and neglect and develop psychological problems. Women who drink during pregnancy run a serious risk of damaging their fetuses. Relatives, friends and strangers can be injured or killed in alcohol-related accidents and assaults.
When should someone seek help?
Individuals often hide their drinking or deny they have a problem. How can you tell if you or someone you know is in trouble? Signs of a possible problem include having friends or relatives express concern, being annoyed when people criticize your drinking, feeling guilty about your drinking and thinking that you should cut down but finding yourself unable to do so, or needing a morning drink to steady your nerves or relieve a hangover.
Some people with drinking problems work hard to resolve them. With the support of family members or friends, these individuals are often able to recover on their own. However, those with alcohol dependence usually can't stop drinking through willpower alone. Many need outside help. They may need medically supervised detoxification to avoid potentially life-threatening withdrawal symptoms, such as seizures. Once people are stabilized, they may need help resolving psychological issues associated with problem drinking.
There are several approaches available for treating alcohol problems. No one approach is best for all individuals.
How can a psychologist help?
Psychologists who are trained and experienced in treating alcohol problems can be helpful in many ways. Before the drinker seeks assistance, a psychologist can guide the family or others in helping to increase the drinker's motivation to change.
A psychologist can begin with the drinker by assessing the types and degrees of problems the drinker has experienced. The results of the assessment can offer initial guidance to the drinker about what treatment to seek and help motivate the problem drinker to get treatment. Individuals with drinking problems improve their chances of recovery by seeking help early.
Using one or more of several types of psychological therapies, psychologists can help people address psychological issues involved in their problem drinking. A number of these therapies, including cognitive-behavioral coping skills treatment and motivational enhancement therapy, were developed by psychologists. Additional therapies include 12-Step facilitation approaches that assist those with drinking problems in using self-help programs such as Alcoholics Anonymous (AA).
These therapies can help people boost their motivation to stop drinking, identify circumstances that trigger drinking, learn new methods to cope with high-risk drinking situations, and develop social support systems within their own communities.
All three of these therapies have demonstrated their effectiveness. One analysis of cognitive-behavioral approaches, for instance, found that 58 percent of patients receiving cognitive-behavioral treatment fared better than those in comparison groups. 3 In another study , motivational interventions reduced how often and how much adolescents drank following alcohol-related emergency room treatment. 4 And an intervention called Making Alcoholics Anonymous Easier significantly increased participants' odds of abstaining from alcohol. 5 Many individuals with alcohol problems suffer from other mental health conditions, such as severe anxiety and depression, at the same time. Psychologists can also diagnose and treat these "co-occurring" psychological conditions. Further, a psychologist may play an important role in coordinating the services a drinker in treatment receives from various health professionals.
Psychologists can also provide marital, family, and group therapies, which often are helpful for repairing interpersonal relationships and for resolving problem drinking over the long term. Family relationships influence drinking behavior, and these relationships often change during an individual's recovery. The psychologist can help the drinker and significant others navigate these complex transitions, help families understand problem drinking and learn how to support family members in recovery, and refer family members to self-help groups such as Al-Anon and Alateen.
Because a person may experience one or more relapses and return to problem drinking, it can be crucial to have a trusted psychologist or other health professional with whom that person can discuss and learn from these events. If the drinker is unable to resolve alcohol problems fully, a psychologist can help with reducing alcohol use and minimizing problems.
Psychologists can also provide referrals to self-help groups. Even after formal treatment ends, many people seek additional support through continued involvement in such groups.
Alcohol-related disorders severely impair functioning and health. But the prospects for successful long-term problem resolution are good for people who seek help from appropriate sources.
The American Psychological Association gratefully acknowledge the assistance of Peter E. Nathan, PhD, John Wallace, PhD, Joan Zweben, PhD, and A. Thomas Horvath, PhD, in developing this fact sheet .
1 National Institute on Alcohol Abuse and Alcoholism. (2018). "Alcohol Use Disorder."
2 Substance Abuse and Mental Health Services Administration. (2017). Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health (HHS Publication No. SMA 17-5044, NSDUH Series H-52). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Retrieved from https://www.samhsa.gov/data/
3 Magill, M., & Ray, L.A. (2009). "Cognitive-behavioral treatment with adult alcohol and illicit drug users: A meta-analysis of randomized controlled trials." Journal of Studies on Alcohol and Drugs, 70 (4): 516-527.
4 Spirito, A., Sindelar-Manning, H., Colby, S.M., Barnett, N.P., Lewander, W., Rohsenow, D.J., & et al. (2011). "Individual and family motivational interventions for alcohol-positive adolescents treated in an emergency department." Archives of Pediatrics and Adolescent Medicine, 165 (3): 269-274.
5 Kaskutas, L.A., Subbaraman, M.S., Witbrodt, J., & Zemore, S.E. (2009). "Effectiveness of Making Alcoholics Anonymous Easier: A group format 12-step facilitation approach." Journal of Substance Abuse Treatment, 37 (3): 228-239.
Updated Sept. 2018
Recommended Reading


Related Reading
- Overcoming opioid abuse: How psychologists help people with opioid dependence and addiction
- Understanding anger: How psychologists help with anger problems
- Managing traumatic stress: After the hurricanes
You may also like
- Patient Care & Health Information
- Diseases & Conditions
- Alcohol use disorder
You're likely to start by seeing your primary health care provider. If your provider suspects that you have a problem with alcohol, you may be referred to a mental health provider.
To assess your problem with alcohol, your provider will likely:
- Ask you some questions related to your drinking habits. The provider may ask for permission to speak with family members or friends. However, confidentiality laws prevent your provider from giving out any information about you without your consent.
- Perform a physical exam. Your health care provider may do a physical exam and ask questions about your health. There are many physical signs that indicate complications of alcohol use.
- Suggest lab tests and imaging tests. While there are no specific tests to diagnose alcohol use disorder, certain patterns of lab test results may strongly suggest it. And you may need tests to identify health problems that may be linked to your alcohol use. Damage to your organs may be seen on tests.
- Complete a psychological evaluation. This evaluation includes questions about your symptoms, thoughts, feelings and behavior patterns. You may be asked to complete a questionnaire to help answer these questions.
- Care at Mayo Clinic
Our caring team of Mayo Clinic experts can help you with your alcohol use disorder-related health concerns Start Here
Treatment for alcohol use disorder can vary, depending on your needs. Treatment may involve a brief intervention, individual or group counseling, an outpatient program, or a residential inpatient stay. Working to stop alcohol use to improve quality of life is the main treatment goal.
Treatment for alcohol use disorder may include:
- Detox and withdrawal. Treatment may begin with a program of detoxification — withdrawal that's medically managed. Sometimes called detox, this generally takes 2 to 7 days. You may need to take sedating medications to prevent withdrawal symptoms. Detox is usually done at an inpatient treatment center or a hospital.
- Learning new skills and making a treatment plan. This process usually involves alcohol treatment specialists. It may include goal setting, behavior change techniques, use of self-help manuals, counseling and follow-up care at a treatment center.
- Psychological counseling. Counseling and therapy for groups and individuals help you better understand your problem with alcohol and support recovery from the psychological aspects of alcohol use. You may benefit from couples or family therapy — family support can be an important part of the recovery process.
Oral medications. A drug called disulfiram may help prevent you from drinking, although it won't cure alcohol use disorder or remove the urge to drink. If you drink alcohol while taking disulfiram, the drug produces a physical reaction that may include flushing, nausea, vomiting and headaches.
Naltrexone, a drug that blocks the good feelings alcohol causes, may prevent heavy drinking and reduce the urge to drink. Acamprosate may help you combat alcohol cravings once you stop drinking. Unlike disulfiram, naltrexone and acamprosate don't make you feel sick after taking a drink.
- Injected medication. Vivitrol, a version of the drug naltrexone, is injected once a month by a health care professional. Although similar medication can be taken in pill form, the injectable version of the drug may be easier for people recovering from alcohol use disorder to use consistently.
- Continuing support. Aftercare programs and support groups help people recovering from alcohol use disorder to stop drinking, manage relapses and cope with necessary lifestyle changes. This may include medical or psychological care or attending a support group.
- Treatment for psychological problems. Alcohol use disorder commonly occurs along with other mental health disorders. If you have depression, anxiety or another mental health condition, you may need talk therapy (psychotherapy), medications or other treatment.
- Medical treatment for health conditions. Many alcohol-related health problems improve significantly once you stop drinking. But some health conditions may warrant continued treatment and follow-up care.
- Spiritual practice. People who are involved with some type of regular spiritual practice may find it easier to maintain recovery from alcohol use disorder or other addictions. For many people, gaining greater insight into their spiritual side is a key element in recovery.
Residential treatment programs
For serious alcohol use disorder, you may need a stay at a residential treatment facility. Most residential treatment programs include individual and group therapy, support groups, educational lectures, family involvement, and activity therapy.
Residential treatment programs typically include licensed alcohol and drug counselors, social workers, nurses, doctors, and others with expertise and experience in treating alcohol use disorder.
Clinical trials
Explore Mayo Clinic studies testing new treatments, interventions and tests as a means to prevent, detect, treat or manage this condition.
Alternative medicine
Avoid replacing conventional medical treatment or psychotherapy with alternative medicine. But if used in addition to your treatment plan when recovering from alcohol use disorder, these techniques may be helpful:
- Yoga. Yoga's series of postures and controlled breathing exercises may help you relax and manage stress.
- Meditation. During meditation, you focus your attention and eliminate the stream of jumbled thoughts that may be crowding your mind and causing stress.
- Acupuncture. With acupuncture, hair-thin needles are inserted under the skin. Acupuncture may help reduce anxiety and depression.
Lifestyle and home remedies
As part of your recovery, you'll need to focus on changing your habits and making different lifestyle choices. These strategies may help:
- Consider your social situation. Make it clear to your friends and family that you're not drinking alcohol. Develop a support system of friends and family who can support your recovery. You may need to distance yourself from friends and social situations that impair your recovery.
- Develop healthy habits. For example, good sleep, regular physical activity, managing stress more effectively and eating well all can make it easier for you to recover from alcohol use disorder.
- Do things that don't involve alcohol. You may find that many of your activities involve drinking. Replace them with hobbies or activities that are not centered around alcohol.
Coping and support
Many people with alcohol problems and their family members find that participating in support groups is an essential part of coping with the disease, preventing or dealing with relapses, and staying sober. Your health care provider or counselor can suggest a support group. These groups are also often listed on the web.
Here are a few examples:
- Alcoholics Anonymous. Alcoholics Anonymous (AA) is a self-help group for people recovering from alcoholism. AA offers a sober peer group and is built around 12 steps as an effective model for achieving total abstinence.
- Women for Sobriety. Women for Sobriety is a nonprofit organization offering a self-help group program for women who want to overcome alcoholism and other addictions. It focuses on developing coping skills related to emotional and spiritual growth, self-esteem, and a healthy lifestyle.
- Al-Anon and Alateen. Al-Anon is designed for people who are affected by someone else's alcoholism. Alateen groups are available for teenage children of those with alcoholism. In sharing their stories, family members gain a greater understanding of how the disease affects the entire family.
- Celebrate Recovery. Celebrate Recovery is a Christ-centered, 12-step recovery program for people struggling with addiction.
- SMART Recovery. SMART Recovery offers mutual support meetings for people seeking science-based, self-empowered addiction recovery.
Preparing for your appointment
Here's some information to help you get ready for your appointment, and what to expect from your health care provider or mental health provider.
Consider your drinking habits. Take an honest look at how often and how much you drink. Be prepared to discuss any problems that alcohol may be causing. You may want to take a family member or friend along, if possible.
Before your appointment, make a list of:
- Any symptoms you've had, including any that may seem unrelated to your drinking
- Key personal information, including any major stresses or recent life changes
- All medications, vitamins, herbs or other supplements that you're taking and their dosages
- Questions to ask your provider
Some questions to ask include:
- Do you think I drink too much or show signs of problem drinking?
- Do you think I need to cut back or quit drinking?
- Do you think alcohol could be causing or worsening my other health problems?
- What's the best course of action?
- What are the alternatives to the approach that you're suggesting?
- Do I need any medical tests for underlying physical problems?
- Are there any brochures or other printed material that I can have? What websites do you recommend?
- Would it be helpful for me to meet with a professional experienced in alcohol treatment?
Don't hesitate to ask any other questions.
What to expect from your doctor
Be ready to answer questions from your health care provider or mental health provider, which may include:
- How often and how much do you drink?
- Do you have any family members with alcohol problems?
- Do you sometimes drink more than you intend to drink?
- Have relatives, friends or co-workers ever suggested that you need to cut back or quit drinking?
- Do you feel like you need to drink more than you previously did to get the same effect?
- Have you tried to stop drinking? If so, was it difficult and did you have any withdrawal symptoms?
- Have you had problems at school, at work or in your relationships that may be related to alcohol use?
- Have there been times that you behaved in a dangerous, harmful or violent way when you were drinking?
- Do you have any physical health problems, such as liver disease or diabetes?
- Do you have any mental health issues, such as depression or anxiety?
- Do you use recreational drugs?
Your health care provider or mental health provider will ask additional questions based on your responses, symptoms and needs. Preparing and anticipating questions will help you make the most of your appointment time.
Alcohol use disorder care at Mayo Clinic
- Nguyen HT. Allscripts EPSi. Mayo Clinic. May 5, 2022.
- What is A.A.? Alcoholics Anonymous. https://www.aa.org/what-is-aa. Accessed April 1, 2022.
- Mission statement. Women for Sobriety. https://womenforsobriety.org/about/#. Accessed April 1, 2022.
- Al-Anon meetings. Al-Anon Family Groups. https://al-anon.org/al-anon-meetings/. Accessed April 1, 2022.
- Substance-related and addictive disorders. In: Diagnostic and Statistical Manual of Mental Disorders DSM-5. 5th ed. American Psychiatric Association; 2013. https://dsm.psychiatryonline.org. Accessed April 26, 2018.
- Rethinking drinking: Alcohol and your health. National Institute on Alcohol Abuse and Alcoholism. https://www.rethinkingdrinking.niaaa.nih.gov/. Accessed April 1, 2022.
- Treatment for alcohol problems: Finding and getting help. National Institute on Alcohol Abuse and Alcoholism. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/treatment-alcohol-problems-finding-and-getting-help. Accessed April 1, 2022.
- Alcohol's effect on the body. National Institute on Alcohol Abuse and Alcoholism. https://www.niaaa.nih.gov/alcohols-effects-health/alcohols-effects-body. Accessed April 1, 2022.
- Understanding the dangers of alcohol overdose. National Institute on Alcohol Abuse and Alcoholism. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/understanding-dangers-of-alcohol-overdose. Accessed April 1, 2022.
- Frequently asked questions: About alcohol. Centers for Disease Control and Prevention. https://www.cdc.gov/alcohol/faqs.htm. Accessed April 1, 2022.
- Harmful interactions: Mixing alcohol with medicines. National Institute on Alcohol Abuse and Alcoholism. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/harmful-interactions-mixing-alcohol-with-medicines. Accessed April 1, 2022.
- Parenting to prevent childhood alcohol use. National Institute on Alcohol Abuse and Alcoholism. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/parenting-prevent-childhood-alcohol-use. Accessed April 1, 2022.
- Tetrault JM, et al. Risky drinking and alcohol use disorder: Epidemiology, pathogenesis, clinical manifestations, course, assessment, and diagnosis. https://www.uptodate.com/contents/search. Accessed April 1, 2022.
- Holt SR. Alcohol use disorder: Pharmacologic management. https://www.uptodate.com/contents/search. Accessed April 1, 2022.
- Saxon AJ. Alcohol use disorder: Psychosocial treatment. https://www.uptodate.com/contents/search. Accessed April 1, 2022.
- Charness ME. Overview of the chronic neurologic complications of alcohol. https://www.uptodate.com/contents/search. Accessed April 1, 2022.
- Chen P, et al. Acupuncture for alcohol use disorder. International Journal of Physiology, Pathophysiology and Pharmacotherapy. 2018;10:60.
- Ng S-M, et al. Nurse-led body-mind-spirit based relapse prevention intervention for people with diagnosis of alcohol use disorder at a mental health care setting, India: A pilot study. Journal of Addictions Nursing. 2020; doi:10.1097/JAN.0000000000000368.
- Lardier DT, et al. Exercise as a useful intervention to reduce alcohol consumption and improve physical fitness in individuals with alcohol use disorder: A systematic review and meta-analysis. Frontiers in Psychology. 2021; doi:10.3389/fpsyg.2021.675285.
- Sliedrecht W, et al. Alcohol use disorder relapse factors: A systematic review. Psychiatry Research. 2019; doi:10.1016/j.psychres.2019.05.038.
- Thiamin deficiency. Merck Manual Professional Version. https://www.merckmanuals.com/professional/nutritional-disorders/vitamin-deficiency,-dependency,-and-toxicity/thiamin-deficiency. Accessed April 2, 2022.
- Alcohol & diabetes. American Diabetes Association. https://www.diabetes.org/healthy-living/medication-treatments/alcohol-diabetes. Accessed April 2, 2022.
- Marcus GM, et al. Acute consumption of alcohol and discrete atrial fibrillation events. Annals of Internal Medicine. 2021; doi:10.7326/M21-0228.
- Means RT. Hematologic complications of alcohol use. https://www.uptodate.com/contents/search. Accessed April 1, 2022.
- What people recovering from alcoholism need to know about osteoporosis. NIH Osteoporosis and Related Bone Diseases National Resource Center. https://www.bones.nih.gov/health-info/bone/osteoporosis/conditions-behaviors/alcoholism. Accessed April 2, 2022.
- How to tell if your child is drinking alcohol. Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/talk-they-hear-you/parent-resources/how-tell-if-your-child-drinking-alcohol. Accessed April 2, 2022.
- Smith KE, et al. Problematic alcohol use and associated characteristics following bariatric surgery. Obesity Surgery. 2018; doi:10.1007/s11695-017-3008-8.
- Fairbanks J, et al. Evidence-based pharmacotherapies for alcohol use disorder: Clinical pearls. Mayo Clinic Proceedings. 2020; doi:10.1016/j.mayocp.2020.01.030.
- U.S. Preventive Services Task Force. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: U.S. Preventive Services Task Force Recommendation Statement. JAMA. 2018; doi:10.1001/jama.2018.16789.
- Hall-Flavin DK (expert opinion). Mayo Clinic. April 25, 2022.
- Celebrate Recovery. https://www.celebraterecovery.com/. Accessed April 26, 2022.
- SMART Recovery. https://www.smartrecovery.org/. Accessed April 26, 2022.
Products & Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Newsletter: Mayo Clinic Health Letter — Digital Edition
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
We’re transforming healthcare
Make a gift now and help create new and better solutions for more than 1.3 million patients who turn to Mayo Clinic each year.
Common Comorbidities with Substance Use Disorders Research Report Part 1: The Connection Between Substance Use Disorders and Mental Illness
Many individuals who develop substance use disorders (SUD) are also diagnosed with mental disorders, and vice versa. 2,3 Although there are fewer studies on comorbidity among youth, research suggests that adolescents with substance use disorders also have high rates of co-occurring mental illness; over 60 percent of adolescents in community-based substance use disorder treatment programs also meet diagnostic criteria for another mental illness. 4
Data show high rates of comorbid substance use disorders and anxiety disorders—which include generalized anxiety disorder, panic disorder, and post-traumatic stress disorder. 5–9 Substance use disorders also co-occur at high prevalence with mental disorders, such as depression and bipolar disorder, 6,9–11 attention-deficit hyperactivity disorder (ADHD), 12,13 psychotic illness, 14,15 borderline personality disorder, 16 and antisocial personality disorder. 10,15 Patients with schizophrenia have higher rates of alcohol, tobacco, and drug use disorders than the general population. 17 As Figure 1 shows, the overlap is especially pronounced with serious mental illness (SMI). Serious mental illness among people ages 18 and older is defined at the federal level as having, at any time during the past year, a diagnosable mental, behavior, or emotional disorder that causes serious functional impairment that substantially interferes with or limits one or more major life activities. Serious mental illnesses include major depression, schizophrenia, and bipolar disorder, and other mental disorders that cause serious impairment. 18 Around 1 in 4 individuals with SMI also have an SUD.
Data from a large nationally representative sample suggested that people with mental, personality, and substance use disorders were at increased risk for nonmedical use of prescription opioids. 19 Research indicates that 43 percent of people in SUD treatment for nonmedical use of prescription painkillers have a diagnosis or symptoms of mental health disorders, particularly depression and anxiety. 20
Youth—A Vulnerable Time
Although drug use and addiction can happen at any time during a person’s life, drug use typically starts in adolescence, a period when the first signs of mental illness commonly appear. Comorbid disorders can also be seen among youth. 21–23 During the transition to young adulthood (age 18 to 25 years), people with comorbid disorders need coordinated support to help them navigate potentially stressful changes in education, work, and relationships. 21
Drug Use and Mental Health Disorders in Childhood or Adolescence Increases Later Risk
The brain continues to develop through adolescence. Circuits that control executive functions such as decision making and impulse control are among the last to mature, which enhances vulnerability to drug use and the development of a substance use disorder. 3,24 Early drug use is a strong risk factor for later development of substance use disorders, 24 and it may also be a risk factor for the later occurrence of other mental illnesses. 25,26 However, this link is not necessarily causative and may reflect shared risk factors including genetic vulnerability, psychosocial experiences, and/or general environmental influences. For example, frequent marijuana use during adolescence can increase the risk of psychosis in adulthood, specifically in individuals who carry a particular gene variant. 26,27
It is also true that having a mental disorder in childhood or adolescence can increase the risk of later drug use and the development of a substance use disorder. Some research has found that mental illness may precede a substance use disorder, suggesting that better diagnosis of youth mental illness may help reduce comorbidity. One study found that adolescent-onset bipolar disorder confers a greater risk of subsequent substance use disorder compared to adult-onset bipolar disorder. 28 Similarly, other research suggests that youth develop internalizing disorders, including depression and anxiety, prior to developing substance use disorders. 29
Untreated Childhood ADHD Can Increase Later Risk of Drug Problems
Numerous studies have documented an increased risk for substance use disorders in youth with untreated ADHD, 13,30 although some studies suggest that only those with comorbid conduct disorders have greater odds of later developing a substance use disorder. 30,31 Given this linkage, it is important to determine whether effective treatment of ADHD could prevent subsequent drug use and addiction. Treatment of childhood ADHD with stimulant medications such as methylphenidate or amphetamine reduces the impulsive behavior, fidgeting, and inability to concentrate that characterize ADHD. 32
That risk presents a challenge when treating children with ADHD, since effective treatment often involves prescribing stimulant medications with addictive potential. Although the research is not yet conclusive, many studies suggest that ADHD medications do not increase the risk of substance use disorder among children with this condition. 31,32 It is important to combine stimulant medication for ADHD with appropriate family and child education and behavioral interventions, including counseling on the chronic nature of ADHD and risk for substance use disorder. 13,32

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

Alcohol's Effects on Health
Research-based information on drinking and its impact.
National Institute on Alcohol Abuse and Alcoholism (NIAAA)
Find science-based information on the effects of alcohol on health., alcohol topics a to z.
Facts and Stats

Drinking Levels Defined
Alcohol's Effects on the Body
Underage And Young Adults

Getting Help

Order Publications
Short takes, translations.
niaaa.nih.gov
An official website of the National Institutes of Health and the National Institute on Alcohol Abuse and Alcoholism
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
June 10, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
reputable news agency
Alcohol use tied to mood instability in patients with bipolar disorder
by Lori Solomon

Alcohol use is associated with mood instability (depression and manic symptoms) in people with bipolar disorder (BD), according to a study published online June 7 in JAMA Network Open .
Sarah H. Sperry, Ph.D., from the University of Michigan in Ann Arbor, and colleagues characterized the longitudinal alcohol use patterns in BD and examined the temporal associations among alcohol use, mood, anxiety, and functioning over time. Analysis included data from 584 participants in the Prechter Longitudinal Study of Bipolar Disorder. Participants had a BD type I (76.2 percent) or BD type II (23.8 percent) diagnosis.
Over a median follow-up of nine years, the researchers found that more problematic alcohol use was associated with worse depressive and manic or hypomanic symptoms as well as lower workplace functioning over the next six months.
Increased depressive and manic or hypomanic symptoms were not associated with greater subsequent alcohol use. Associations were more pronounced in BD type II, compared to BD type I. Over time, alcohol use was not associated with anxiety.
"Findings of this study suggest there is an association of alcohol use with mood and work functioning, highlighting the importance of dimensional and longitudinal assessment and management of alcohol use , which should be integrated into research and standard treatment of BD," the authors write.
Copyright © 2024 HealthDay . All rights reserved.
Explore further
Feedback to editors

Robot radiotherapy could improve treatments for eye disease
3 hours ago

Commercial astronauts shed light on flights' health impacts and create spaceflight atlas
5 hours ago
New drug candidates targeting blood clots developed through computer-aided drug design
Safer virus helps eliminate cancer in mouse study

Controlling the precise timing of electrical pulses may offer promise for treating mild traumatic brain injury
6 hours ago

Study shows WhatsApp messages effective for elderly depression treatment
3D cell cultures reveal how cancer cells navigate tissue
Monitoring shows chikungunya epidemics can be predicted by means of surveillance

New biomarker database designed to improve astronaut health may also be useful to Earthlings
7 hours ago

Plant-based ultra-processed foods linked with higher risk of cardiovascular disease
Related stories.

Insomnia symptoms may predict subsequent drinking in adults
Mar 28, 2024

Alcohol use disorder linked to increased odds of suicide mortality
Mar 13, 2024

Positive hormone balance can protect people with alcohol use disorder from problematic alcohol consumption: Study
Jan 16, 2024

Study: People with anxiety and mood disorders experience more severe alcohol symptoms than those without
Apr 28, 2023

For teen girls, depression may predict subsequent alcohol use
Nov 30, 2018
Total alcohol intake not linked to ventricular arrhythmia risk
Dec 28, 2021
Recommended for you

Masks study finds traumatic experiences impact perception of distressing imagery

Study finds nearly 1 in 4 people with a history of bipolar disorder achieve complete mental health
10 hours ago

Opioid giant's tactics to influence doctors revealed in court documents
Jun 10, 2024

Optimism wards off procrastination: Believing the future will not be more stressful could help procrastinators

Researchers assess rates of cannabis-associated psychotic episodes
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Wiley Open Access Collection

Associations of common mental disorder with alcohol use in the adult general population: a systematic review and meta‐analysis
Jo‐anne puddephatt.
1 Department of Psychology, University of Liverpool, Liverpool UK
Patricia Irizar
Andrew jones, suzanne h. gage, laura goodwin, associated data.
Table S2: Full Search Terms
Table S3: Associations of Moderate/Severe AUD Among Those With an Anxiety or Mood Disorder Compared to Those Without ( N = 210 121)
Table S4: Associations of Any AUD Among Those With Any Anxiety Disorder (excluding PTSD and OCD) and Among Those with PTSD ( N = 137 916)
Table S5: Associations of Any AUD Among Those With a CMD Compared to Those Without Stratified by Continent ( N = 365 331)
Table S6: Associations of Any AUD Among Those With a CMD Compared to Those Without Stratified by the Decade Data was Collected ( N = 224 835)
Figure S1: A funnel plot illustrating the heterogeneity of having any alcohol use disorder (AUD) among those with any common mental disorder (CMD) ( N = 382 201)
Figure S2: A funnel plot illustrating the heterogeneity of having any alcohol use disorder (AUD) among those with any common mental disorder (CMD) stratified by the decade in which the study was conducted ( N = 224 835)
Figure S3: A funnel plot illustrating the heterogeneity of having any alcohol use disorder (AUD) among those with any common mental disorder (CMD) stratified by the continent in which the study was conducted in ( N = 382 201)
Figure S4: A funnel plot illustrating the heterogeneity of having any alcohol use disorder (AUD) among those with any common mental disorder (CMD) stratified by each study's bias score ( N = 382 201)
Figure S5: 12‐month and life‐time associations of alcohol use disorder (AUD) among those with a common mental disorder (CMD), compared to those without, after removing Kinley et al . 2009 study ( N = 353 660)
Figure S6: 12‐month and life‐time associations of any alcohol use disorder (AUD) stratified by type of common mental disorder (CMD), compared to those without, after removing Patel et al . 2002 study ( N = 373 637)
Figure S7: Figure S7: Funnel plot to explore publication bias
Background and Aims
Research has shown that alcohol use and common mental disorders (CMDs) co‐occur; however, little is known about how the global prevalence of alcohol use compares across different CMDs. We aimed to (i) report global associations of alcohol use (alcohol use disorder (AUD), binge drinking and consumption) comparing those with and without a CMD, (ii) examine how this differed among those with and without specific types of CMDs and (iii) examine how results may differ by study characteristics.
We used a systematic review and meta‐analysis. Cross‐sectional, cohort, prospective, longitudinal and case–control studies reporting the prevalence of alcohol use among those with and without a CMD in the general population were identified using PsycINFO, MEDLINE, PsyARTICLES, PubMed, Scopus and Web of Science until March 2020. Depression, anxiety and phobia were included as a CMD. Studies were included if they used a standardized measure of alcohol use. A random‐effects meta‐analysis was conducted to generate pooled prevalence and associations of AUD with CMD with 95% confidence intervals (CI). A narrative review is provided for binge drinking and alcohol consumption
A total of 512 full‐texts were reviewed, 51 included in our final review and 17 in our meta‐analyses ( n = 382 201). Individuals with a CMD had a twofold increase in the odds of reporting an AUD [odds ratio (OR) = 2.02, 95% CI = 1.72–2.36]. The odds of having an AUD were similar when stratified by the type of CMD (mood disorder: OR = 2.00, 95% CI = 1.62–2.47; anxiety/phobic disorder: OR = 1.94, 95% CI = 1.35–2.78). An analysis of study characteristics did not reveal any clear explanations for between‐study heterogeneity ( I 2 > 80%). There were no clear patterns for associations between having a CMD and binge drinking or alcohol consumption, respectively.
Conclusions
People with common mental disorders (depression, anxiety, phobia) are twice as likely to report an alcohol use disorder than people without common mental disorders.
INTRODUCTION
It is estimated that 32.5% of the global population consume alcohol [ 1 ]. While there are differences between countries [ 2 ], approximately 18.4% of adults report binge drinking [ 3 ] and 5.1% have an alcohol use disorder (AUD) [ 2 ], including harmful and dependent drinking. Despite differences between countries, alcohol use was ranked the seventh leading risk factor for premature death and disability. Alcohol use has also led to 1.6 and 6% of disability‐adjusted life‐years for females and males, respectively [ 1 ]. Meanwhile, depressive and anxiety disorders (known as common mental disorders; CMD) are also prevalent in the general population globally, with 4.4 and 3.6% reporting a depressive or anxiety disorder, respectively [ 4 ].
Drinking alcohol can be harmful to an individual's mental health, particularly if they meet criteria for an AUD (symptoms include an impaired ability to control alcohol use [ 5 ]), binge drinking (generally consuming more than 5 units of alcohol in a certain period [ 6 ]) or drinking excessively (drinking excessive amounts of alcohol on most days or weeks [ 7 ]). Among the general population, research has found associations between CMD with binge drinking [ 8 , 9 , 10 ] and AUD [ 11 ]. Research has also shown that those with co‐occurring panic disorder and AUD or depression and AUD are at an increased risk of mortality compared to those without such disorders [ 12 , 13 ]. Elsewhere, a narrative review found evidence to suggest that anxiety and depressive episodes are related to binge drinking which can subsequently lead to injury [ 14 ]. Other research also found that college students with co‐occurring anxiety and depressive symptoms reported increased weekly alcohol use, more hazardous use and negative alcohol consequences compared to those without symptoms [ 15 ]. Nineteen per cent of all alcohol‐related hospital admissions have been attributed to mental health problems that resulted from alcohol use [ 16 ], and those with co‐occurring alcohol and mental health problems may have difficulties accessing treatment compared to those with only one of these problems [ 17 ]. These findings indicate that having a CMD is associated with a range of alcohol outcomes which have negative health implications on health; however, previous research has focused specifically on associations with AUD.
There is evidence for an association between worsening mental health and increased alcohol use [ 18 ]. Motivational models argue that individuals may be motivated to use alcohol to cope with stress [ 19 ], where benefits outweigh the cost [ 20 ]. Such models suggest that alcohol may be used to cope with symptoms of poor mental health, and used specifically due to its rapid onset of action [ 21 ]. This might be the case among those with a CMD, as drinking alcohol may be perceived to alleviate symptoms of a disorder [ 21 ].
Genome‐wide studies have shown a causal relationship between CMDs, such as major depression and alcohol dependence, while the reverse association has not been found [ 22 ]. However, associations between alcohol use and mental health comorbidity may be more complex and vary based upon the specific type of CMD [ 23 , 24 ]. Among the general population, research has shown that those with major depressive disorder (MDD) were more likely to report life‐time moderate/severe AUD compared to those without MDD [ 25 ], whereas those with generalized anxiety disorder (GAD) were more likely to report mild or severe AUD compared to those without GAD [ 25 ]. Elsewhere, a significant association with alcohol dependence among those meeting criteria for alcohol abuse was reported among those with dysthymia but not MDD compared to those without the respective disorder [ 26 ], while a review across observational studies showed differences in associations with AUD with specific types of anxiety disorders, such as panic disorder [ 27 ]. Differences in associations have also been found for other patterns of alcohol use. For example, in Portugal a positive association of binge drinking with anxiety disorder was found among individuals attending primary care, while a negative association with binge drinking was found for major depression compared to those without the respective disorders [ 10 ].
Previous systematic reviews have explored alcohol misuse and CMD in both directions; for example, the prevalence of CMD among those misusing alcohol [ 28 ] and the prevalence of alcohol misuse among those with a CMD [ 11 ]. The latter was most recently reported by Lai and colleagues, where those with an anxiety disorder or major depression were approximately 1.5 times more likely to report alcohol abuse and 2.5 and three times more likely to report dependence, respectively [ 11 ]. This indicates that those with a CMD are more likely to use alcohol at harmful levels and that there may be differences based upon the type of CMD. However, this review included bipolar disorder in their definition of CMD, which UK health guidelines on CMD exclude, together with other psychotic and related disorders [ 29 , 30 , 31 ]. This review also did not include post‐traumatic stress disorder (PTSD), despite its inclusion as a CMD in UK health guidelines [ 32 ].
To date, there has not been a systematic review or meta‐analysis reporting the prevalence of other types of alcohol use, such as binge drinking, among those with and without a CMD in the adult general population, and by specific CMD diagnoses. The current systematic review and meta‐analysis aimed to (i) estimate the pooled prevalence of alcohol use (AUD, binge drinking and alcohol consumption) in those with and without a CMD, (ii) evaluate associations between CMD and patterns of alcohol use, (iii) examine how prevalence and associations differed across specific types of CMDs and (iv) examine how results may differ by study characteristics.
This study is pre‐registered on PROSPERO (ref. CRD42019126770) and reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines [ 33 ] (see PRISMA diagram in Figure 1 and checklist in Supporting information), and in line with the condition, context, population (CoCoPop) framework [ 34 ]. The CoCoPop framework is a quality appraisal tool suitable for systematic reviews and meta‐analyses which aim to examine the prevalence of a condition, and therefore require specific information concerning groups that may not be required using other frameworks [ 35 ].

Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram
Inclusion and exclusion criteria
We included peer‐reviewed observational studies, comprising cross‐sectional, national surveys, cohort, prospective, longitudinal and case–control studies published in English. Where the same data set was used by multiple studies and reported the same outcome, we used the study which reported information on more CMDs. If two or more studies reported the same information, the more recent study was chosen. Reviews and intervention studies were excluded.
Studies which measured the prevalence of life‐time or 12‐month AUD, binge drinking or alcohol use, comparing those with and without a CMD and used a standardized measure of alcohol use, alcohol use disorder and CMD—for example, the Diagnostic and Statistical Manual (DSM) diagnostic instruments—were included. The authors note that definitions of binge drinking may vary among countries and details of standardized measures of alcohol use and CMD are reported in Table 1 . CMDs were defined in this review as MDD, dysthymia, GAD, panic disorder, phobias, PTSD, obsessive–compulsive disorder (OCD) or social anxiety disorder (SAD) [ 36 ]. Studies were excluded if they did not report the prevalence of alcohol use in those with and without a CMD.
Study characteristics
| Study | Year(s) conducted | Country | Data set | Waves used | Sample size (response rate) | Gender and age | Type of CMD studied (and measure and criteria used to assess presence of CMD) | Type of alcohol use studied (and measure and criteria used) | Duration of AUD | Risk of bias score (max. score of 9) |
|---|---|---|---|---|---|---|---|---|---|---|
| . 2012 | 2005 | Canada | Canadian Community Health Survey | Cycle 3.1 | 17 524 (response rate not stated) | Gender Female = 8587 (49.0%) Male = 8937 (51.0%) Age range 15–24 | Depressive symptoms (derived depression scale, cut‐off score of 5 or more) | Binge drinking (item on alcohol use, 5 or more drinks once a month or more) | 12 months | 7 |
| . 2012 | Baseline = 1996, time 1 = 1997. time 2 = 1999 | Netherlands | Netherlands Mental Health Survey and Incidence Study (NEMESIS) | Baseline | 5571 (no overall response rate) | Gender Female = 2896 (51.9%) Male = 2675 (48.1%) Age range not clear | Panic disorder (composite international diagnostic interview, DSM‐III‐R) | Alcohol dependence (composite international diagnostic interview, DSM‐III‐R) | 12 months | 6 |
| . 2010 | 2001–02 | United States | National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) | Wave 1 | 43 093 (81%) | Gender Female = 21 662 (51.43%) Male = 19 598 (48.57%) Age range 18+ | Chronic MDD Dysthymic disorder (alcohol use disorder and associated disabilities interview schedule, DSM‐IV) | Alcohol abuse Alcohol dependence (alcohol use disorder and associated disabilities interview schedule, DSM‐IV) | 12 months Life‐time | 9 |
| 1997 | Australia | National Survey of Mental Health and Well Being (NSMH&WB) | Wave 1 | 10 641 (78%) | Gender Female = 5452 (51.2%) Male = 5189 (48.8%) Age range 18+ | Depression Dysthymia Bipolar disorder Panic disorder Social phobia OCD PTSD GAD Agoraphobia (composite international diagnostic interview, DSM‐IV) | Alcohol abuse Alcohol dependence Alcohol use disorder (Composite International Diagnostic Interview, DSM‐IV) | 12 months | 7 | |
| . 2004 | 1995 | Mexico | – | – | 1932 (60.4%) | Gender Female = not stated Male = not stated Age range 18+ | OCD (composite international diagnostic interview, ICD‐10) | Alcohol abuse Alcohol dependence (composite international diagnostic interview, ICD‐10) | 12 months Life‐time | 5 |
| . 2020 | 2007–08 | Brazil | – | – | 3744 (81 and 95%) | Gender Female = 1584 Male = 2160 Age range 15–75 | PTSD (composite international diagnostic interview 2.1, ICD‐10 and DSM‐IV) | Hazardous alcohol use Alcohol dependence (composite international diagnostic interview 2.1, ICD‐10 and DSM‐IV) | 12 months | 9 |
| . 2012 | 2009 | Singapore | Singapore Mental Health Study (SMHS) | – | 6616 (76%) | Gender Female = 3317 (50.1%) Male = 3299 (49.9%) Age range 18+ | MDD Dysthymia GAD OCD (World Mental Health composite international diagnostic interview, DSM‐IV) | Alcohol abuse Alcohol dependence (World Mental Health composite international diagnostic interview, DSM‐IV) | Life‐time | 8 |
| . 2012 | NESARC, 2001–02; KECA, 2000 | Korea and United States | NESARC and Korean Epidemiologic Catchment Area (KECA) | NESARC wave 1 KECA wave 1 | NESARC = 35 336 (81%) KECA = 6253 (79.8%) | NESARC: Gender Female = 23 227 (65.7%) Male = 15 619 (44.2%) Age range 18–65 KECA: Gender Female = 3510 (56.1%) Male = 2743 (43.9%) Age range 18–65 | MDD Dysthymia Panic disorder Social phobia GAD (NESARC: associated disabilities interview schedule‐DSM‐IV version, DSM‐IV; KECA: Korean version of composite international diagnostic interview 2.1, DSM‐IV) | Alcohol abuse Alcohol dependence (NESARC: associated disabilities interview schedule‐DSM‐IV version, DSM‐IV; KECA: Korean version of composite international diagnostic interview 2.1, DSM‐IV) | 12 months | 9 |
| . 2014 | 2009–11 | Ireland | The Irish Longitudinal Study on Ageing (TILDA) | Wave 1 | 8175 (62%) | Gender Female = 2041 (53.4%) Male = 1774 (46.6%) Age range 60–99 | Depression (Center for Epidemiologic Studies Depression Scale, score of 16 or more) | Problem drinker (CAGE, score of 2 or more) | 6 months | 7 |
| . 2005 | 1993–96 | United States | Baltimore Epidemiologic Catchment Area (ECA) follow–up | Wave 2 | 2633 (73%) | Gender Female: 1644 (63.2%) Male: 989 (36.8%) Age range 31–99 | MDD (diagnostic interview schedule, DSM‐III‐R) | Alcohol dependence (diagnostic interview schedule, DSM‐III‐R) | Life‐time | 7 |
| . 2005 | 2002 | Canada | Canadian Community Health Survey (CCHS) | Cycle 1.2 | 36 984 (77%) | Gender Female = not stated Male = not stated Age range 15+ | MDE (Canadian World Mental Health composite international diagnostic interview, DSM‐IV) | Harmful alcohol use (ICD‐10) Alcohol dependence (DSM‐IV) (composite international diagnostic interview short form) | 12 months | 7 |
| 2000–01 | Norway | Oslo Health Study (HUBRO) | – | 2676: 446 = SPAS groups and 2230 controls (response rate not stated) | Gender Female = 1558 (58%) Male = 1118 (42%) Age range 30–45 | Social phobia and anxiety symptoms (MINI–social phobia inventory, score of 8 or more) | Alcohol frequency (self‐report item, more than 1 time per week) Alcohol problems (self‐report item on impairment in job due to alcohol) | 1 week and 5 years | 3 | |
| . 2020 | 2017 | United Kingdom | UK Biobank | – | 157 366 (46%) | Gender Female = 89 101 (56%) Male = 68 265 (44%) Age range 45–82 | Depression (composite international diagnostic interview short form) Anxiety disorder (composite international diagnostic Interview short form) PTSD (post‐traumatic stress disorder checklist‐6, score of 14 or more) | Harmful alcohol use (alcohol use disorder identification test score of 16 or more) | 12 months | 4 |
| . 2017 | 2013–15 | Portugal | EpiDoC 2 (CoReumaPt) study | Wave 2 | 1680 (response rate not stated) | Gender Female = 908 (54%) Male = 772 (46%) Age range 65+ | Depression and anxiety symptoms (hospital and anxiety disorder scale, score of 11 or more) | Alcohol intake (self‐report of frequency of alcohol intake and categorized as daily, occasionally, never, cut‐offs not stated) | Not known | 5 |
| . 2017 | 2012–13 | United States | NESARC | Wave 3 | 36 309 (60.1%) | Gender Female = 10 940 (55.5%) Male = 8765 (44.5%) Age range 18+ | PTSD (alcohol use disorder and associated disabilities interview schedule‐5, DSM‐5) | Alcohol use disorder (alcohol use disorder and associated disabilities interview schedule‐5, DSM‐5) | 12 months | 5 |
| . 2014 | 2006 | Italy | The Faenza Community Aging Study | – | 366 (65.8%) | Gender Female = 184 (50.3%) Male = 182 (49.7%) Age range 70+ | Anxiety symptoms (geriatric anxiety inventory short form, cut‐off of 3 or more) | Alcohol consumption (quantity of alcoholic drink converted to units per day, defined as alcoholic unit as a glass of wine (125 ml), a can of beer (330 ml) and a small glass of hard liquor (40 ml), cut‐off of more than 2 alcohol units per day) | Per day | 5 |
| . 2018 | 2008 | Japan | Nihon University Sleep and Mental Health Epidemiology Project (NUSMEP) | – | 2559 (54%) | Gender Female = 1396 (52.53%) Male = 1163 (47.47%) Age range 20+ | Depressive symptoms (Center for Epidemiologic studies depression scale, score of 16 or more) | Alcohol consumption (self‐report item on drinking more than one glass of sake three times per week), defined as a glass of sake is equal to a 500–ml bottle of beer, 80 ml of distilled spirit, 60 ml of whiskey, or two glasses of wine (240 ml), cut‐off yes) | 1 week | 5 |
| 1992 | United States | National Longitudinal Alcohol Epidemiologic Survey (NLAES) | – | 42 862 (household response rate = 91.9%, sample person = 97.4%) | Gender Not stated without depression Age range 18+ | MDD (alcohol use disorder and associated disabilities interview schedule, DSM‐IV) | Alcohol abuse Alcohol dependence (alcohol use disorder and associated disabilities interview schedule, DSM‐IV) | 12 months Life‐time | 7 | |
| . 2004 | 2001–02 | United States | NESARC | Wave 1 | 43 093 (81%) | Gender Female = 25 575 (57%) Male = 18 518 (43%) Age range 18+ | MDD Dysthymia GAD Panic disorder Phobia (alcohol use disorder and associated disabilities interview schedule, DSM‐IV) | Alcohol abuse Alcohol dependence (alcohol use disorder and associated disabilities interview schedule, DSM‐IV) | 12 months | 8 |
| . 2017 | 2005–14 | United States | National Survey on Drug Use and Health (NSDUH) | 10 waves (2005–14) | 61 240 (71.2–76%) | Gender Female = 32 825 (53.6%) Male = 28 415 (46.4%) Age range 50+ | Depressive episode Anxiety (self‐report item, cut‐off not stated) | Binge drinking (Self‐report idem, five more drinks on same occasion) Alcohol use disorder (measure not stated, DSM‐IV) | Binge drinking‐30 days Alcohol abuse/dependence‐12 months | 7 |
| . 2016 | 2003–04 | Singapore | Singapore Longitudinal Aging Study (SLAS) | Wave 1 | 1070 (72.4%) | Gender Female = 585 (53.75%) Male = 485 (46.25%) Age range 18+ | Depressive symptoms (geriatric mental state examination, DSM‐IV) | Alcohol consumption (measure not stated, more than one drink per week) | 1 week | 5 |
| . 2018 | 2005 | France | – | – | 17 237 (62.7%) | Gender Female = 10 262 (59.5%) Male = 6975 (40.5%) Age range 18–99 | MDD GAD Panic disorder PTSD OCD Specific phobia Social phobia (composite international diagnostic interview short form, DSM‐IV) | Alcohol abuse Alcohol dependence (Composite International Diagnostic Interview short form, DSM‐IV) | 12 months | 7 |
| . 2004 | 1997–99 | Japan | – | – | 1029 (56.9%) | Gender Female = 578 (56.2%) Male = 451 (43.8%) Age range 20+ | MDD Mania Dysthymia GAD Panic disorder (World Mental Health University of Michigan composite international diagnostic interview, DSM‐III‐R) | Alcohol use disorder (World Mental Health University of Michigan composite international diagnostic interview, DSM‐III‐R) | 6 months Life‐time | 6 |
| . 1997 | 1990–92 | United States | National Comorbidity Survey (NCS) | Wave 1 | 8098 (82.6%) | Gender Female = 4263 (49.26%) Male = 3835 (50.74%) Age range 15–54 | MDD Dysthymia Panic disorder Social phobia Simple phobia GAD (World Mental Health composite international diagnostic interview, DSM‐III‐R) | Alcohol abuse Alcohol dependence (World Mental Health Composite International Diagnostic Interview, DSM‐III‐R) | Life‐time | 6 |
| . 2009 | 2002–03 | Canada | Canadian Community Health Survey – Mental Health and Well–Being | Cycle 1.2 | 28 541 (77.0%) | Gender Female = 15 074 (52.82%) Male = 13 467 (47.18%) Age range 15+ | Panic disorder (World Mental Health composite international diagnostic interview, DSM‐IV) | Alcohol dependence (World Mental Health composite international diagnostic interview short form, DSM‐III‐R) | 12 months | 8 |
| . 2010 | 2006–08 | Estonia | Estonian Health Interview Survey (EHIS) | – | 6105 (60.2%) | Gender Female = 3177 (52.04%) Male = 2928 (47.95%) Age range 18–85 | MDD (mini‐international neuropsychiatric interview, DSM‐IV) | Binge drinking (self‐report item partly based on the European Health Determinant Module, defined as five bottles of beer, five glasses of wine, or five glasses of vodka at a time, 5 or more) | 12 months | 7 |
| 2011 | Slovenia | – | – | 1002 (response rate not reported) | Gender Female = 512 (51.1%) Male = 490 (48.9%) Age range not clear | Anxiety/depression (Gothenburg quality of life instrument, answering yes to mood and anxiety items) | ‘Risky’ drinker (Slovenian version of the alcohol use disorder identification test‐consumption, score of 6 or more for men or 5 or more for women) | ‐ | 6 | |
| . 2017 | 2002–04 | 47 countries | World Health Survey (WHS) | – | 201 279 (98.5%, range = 63%–99%) | Gender Female = 102 279 (50.8%) Male = 99 058 (49.2%) Age range 18+ | Depressive symptoms (self‐report items to four mandatory questions and two additional ones, DSM‐IV) | Alcohol consumption (self report, defined as how many alcoholic drinks they had on each day in the past 7 days, cut‐off 4 or 5 drinks on at least 3 days) | 7 days | 7 |
| . 2011 | 1999–2003 | France | Mental Health in General Population (MHGP) survey | French data set | 36 105 (response rate not stated) | Gender Female = 19 458 (53.9%) Male = 16 647 (46.1%) Age range 18+ | GAD Panic disorder Social phobia PTSD (mini international neuropsychiatric interview, ICD‐10) | Alcohol abuse (mini international neuropsychiatric interview, ICD 10) | 6 months | 6 |
| . 2011 | 2007 | Finland | National FINRISK 2007 Study | – | 10 000 but used a random subsample ( = 2086, 51.9%) | Gender Female = 1140 (54.7%) Male = 946 (45.3%) Age range 25–74 | Depressive symptoms (modified Beck depression inventory, score of 8 or more) | Heavy drinking occasion (time‐line‐follow‐back reporting frequency and quantity of consumption of standard alcoholic drink, defined as 12 g of absolute alcohol, cut‐off of seven, or five or more for men and women, respectively) | 28 days | 6 |
| . 2016 | 2000–01 and 2011 | Finland | Health 2000 Survey and Health 2011 Survey | Waves 1 and 2 | Wave 1 = 6005 (75%) Wave 2 = 4620 (80.6%) | Gender Female = 3257 (54.2%) Male = 2748 (45.8%) Age range 30+ | Depressive symptoms (Munich composite international diagnostic interview, DSM‐IV) | Alcohol use disorder (Munich composite international diagnostic interview, DSM‐IV) | 12 months | 7 |
| . 2007 | Baseline = 1996, time 1 = 1997, time 2 = 1999 | Netherlands | Netherlands Mental Health Survey and Incidence Study (NEMESIS) | All waves | Baseline = 7076 (69.7%), time 1 = 5618 (79.4%) time 2 = 4796 (67.8%) | Gender Female = 3777 (53.4%) Male = 3299 (46.6%) Age range 18–64 | GAD Panic disorder Social phobia Agoraphobia OCD (composite international diagnostic interview version 1.1, DSM‐III‐R) | Alcohol dependence (composite international diagnostic interview version 1.1, DSM‐III‐R) | Life‐time and 1 month | 8 |
| 2000 | Brazil | – | – | 1260 (7%) | Gender Female = 679 (53.9%) Male = 581 (46.1%) Age range 15+ | Minor psychiatric disorder (depression or anxiety; self‐report questionnaire‐20, cut‐off score of 6 for men and 7 for women) | Probable alcohol use disorder (alcohol use disorder identification test, score of 8 or more) | 12 months | 7 | |
| . 2004 | 1996–97 | Germany | Transitional in Alcohol Consumption and Smoking (TACOS) | – | 4048 (70.2%) | Gender Female = 2019 (61.1%) Male = 2029 (63.3%) Age range 18–64 | MDD Dysthymia Phobia PTSD OCD (Munich composite international diagnostic interview, DSM‐IV) | Alcohol abuse Alcohol dependence (Munich composite international diagnostic interview, DSM‐IV) | Life‐time and 12 months | 6 |
| . 2014
| 2004–08 | Switzerland | PsyCoLaus | Wave 2 | 3694 (67.0%) | Gender Female = 1958 (53.0%) Male = 1736 (47.0%) Age range 35–75 | PTSD (French version of diagnostic interview for genetic studies, DSM‐IV) | Alcohol use disorder (French version of diagnostic interview for genetic studies, DSM‐IV) | 12 months | 7 |
| . 2018 | 2011–12 | Spain | COURAGE | – | 4569 (69.9%) | Gender Female = 2498 (50.6%) Male = 2071 (49.4%) Age range 18+ | Panic disorder (adapted version of the composite international diagnostic interview, DSM‐5) | Alcohol consumption (frequent drinkers: consumed alcohol in either last 30 days and 7 days or 1–2 days per week with 5/4 standard drinks in last 7 days or 3 or more days per week with 5/4 standard drinks in the last 7 days) | 30 days | 6 |
| . 2018
| 2012 | Canada | Canadian Community Health Survey–Mental Health (CCHS–Mental Health) | Wave 2 | 25 097 (68.9%) | Gender Female = not stated Male = not stated Age range 15+ | OCD (self‐reported diagnosis) | Alcohol abuse Alcohol dependence (composite international diagnostic interview short form, DSM‐IV) | 12 months Life‐time | 3 |
| . 2013 | 2001–02 | United States | NESARC | Wave 1 | 43 093 (81.0%) | Gender Female = 25 575 (57%) Male = 18 518 (43%) Age range 18+ | GAD Panic disorder Social phobia Specific phobia (alcohol use disorders and associated disabilities interview schedule, DSM‐IV) | Alcohol dependence (alcohol use disorders and associated disabilities interview schedule, DSM‐IV) | Life‐time | 7 |
| . 2013 | 2001 | Korea | Korean Epidemiologic Catchment Area–Replication (KECA–R) | Wave 2 | 6510 (81.7%) | Gender Female = 3229 (49.6%) Male = 3281 (50.4%) Age range 18–64 | Specific phobia (Korean version of composite international diagnostic interview, DSM‐IV) | Alcohol abuse Alcohol dependence (Korean version of composite international diagnostic interview, DSM‐IV) | Life‐time | 9 |
| . 2002 | 1994–95 | United Kingdom | Surveys of psychiatric morbidity in Great Britain | Wave 1 | 8564 (67.2%) | Gender Female = not reported Male = not reported Age range 16–64 | Social phobia (clinical interview schedule‐revised, ICD‐10) | Alcohol dependence (self‐completion questionnaire, cut‐off score of four or more) | 12 months | 6 |
| . 2015 | 2015 | Canada | Canadian Community Health Survey–Mental Health (CCHS–Mental Health) | Wave 2 | 25 113 (68.9%) | Gender Female = 12 883 (50.7%) Male = 12 230 (49.3%) Age range 15+ | MDE MDD (World Mental Health composite international diagnostic interview, DSM‐IV) | Alcohol abuse Alcohol dependence (World Mental Health composite international diagnostic interview, DSM‐IV) | 12 months Life‐time | 9 |
| . 2005 | 2000–01 | Finland | Health 2000 Survey | Wave 1 | 6005 (75.0%) | Gender Female = 3257 (54.2%) Male = 2748 (45.8%) Age range 30+ | MDD Dysthymia GAD Panic disorder Social phobia Agoraphobia (Finnish version of Munich composite international diagnostic interview, DSM‐IV) | Alcohol use disorder (Finnish version of Munich composite international diagnostic interview, DSM‐IV) | 12 months | 9 |
| . 2010 | – | Poland | WOBASZ | – | 13 545 (response rate not reported) | Gender Female = 7153 (52.8%) Male = 6392 (47.2%) Age range 20–74 | Depressive symptoms (Beck depression inventory, cut‐off score of 10 or more) | Alcohol consumption (self‐reporting consumption of three times per week) | 7 days | 6 |
| . 2018 | 1999–2003 | France | Mental Health in General Population (MHGP) survey | French data set | 38 600 (not stated) | Gender Female = 20 342 (52.7%) Male = 18 258 (47.3%) Age range 18+ | MDD Panic disorder Social phobia GAD PTSD (mini international neuropsychiatric interview version 5.0, ICD‐10) | Alcohol use disorder (mini international neuropsychiatric interview version 5.0, ICD‐10) | Life‐time | 7 |
| . 2006 | 2000 | United Kingdom | British National Psychiatric Morbidity Survey 2000 | Wave 2 | 8580 (69.5%) | Gender Female = 4300 (50.1%) Male = 4280 (49.9%) Age range 16+ | OCD (clinical interview schedule‐revised, ICD‐10) | Hazardous use Alcohol dependence Problem drinking (alcohol use disorder identification test and severity of alcohol dependence questionnaire, cut offs not stated) | 12 months | 7 |
| 1993–94 | United States | Chinese American Psychiatric Epidemiology Study (CAPES) | – | 1735 (82%) | Gender Female = 876 (50.5%) Male = 859 (49.5%) Age range 18–65 | PTSD (diagnostic interview schedule, DSM‐III‐R) | Heavy alcohol use (two items from composite international diagnostic interview, frequency: 1–3 times per month and quantity‐5+ and 4+ per day) | 12 months | 7 | |
| . 2008 | 2002 | Canada | – | – | 2991 (68.1%) | Gender Female = 1811 (51.5%) Male = 1180 (48.5%) Age range 18+ | PTSD [revised version of composite international diagnostic interview (CIDI), DSM‐IV] | Alcohol use disorder (mini international neuropsychiatric interview) | 3 days Life‐time | 8 |
| . 2000 | Not stated | Netherlands | Longitudinal Aging Study Amsterdam (LASA) | ‐ | 3056 (86.0%). Used a subsample ( = 659) of this restricted to those aged between 55 and 84 | Gender Female = 354 (54.0%) Male = 305 (46.0%) Age range 55+ | GAD OCD Phobia (diagnostic interview schedule, DSM‐III) | Heavy/excessive alcohol intake (Garretsen scale, cut‐off score of 4) | 6 months | 9 |
| . 2009 | 2002–05 | Netherlands | Rotterdam study | Wave 3 | 5019 (85.4%) | Gender Female = 2848 (56.7%) Male = 2171 (43.3%) Age range Range 58–100 | MDD Dysthymia GAD Panic disorder Specific phobia Social phobia (depression = schedules for clinical assessment in neuropsychiatry, DSM‐IV‐TR; Anxiety = Munich version of the composite international diagnostic interview) | Excessive alcohol use (self‐reported question, more than 21 alcoholic drinks per week) | 7 days | 5 |
| . 2003 | 1992–93 and 1998–99 | Netherlands | Longitudinal Aging Study Amsterdam (LASA) | Waves 1 and 2 | 1280 (response rate not reported) | Gender Female = 698 (54.5%) Male = 582 (45.5%) Age range 55–85 | Depressive symptoms (Center of Epidemiologic Studies Depression, cut off score of 16 or more) | Alcohol consumption (health interview questionnaire, three or more drinks per day) | Daily | 7 |
| . 2018 | 2012–13 | China | China National Health and Wellness Survey (NHWS) | Waves 3 and 4 | 36 806 (response rate not reported) | Gender Female = 16 698 (45.4%) Male = 20 108 (54.6%) Age range 18+ | GAD (generalized anxiety disorder‐7, cut off score 10 or more) | Alcohol use (measure not stated, excessive) | Not stated | 5 |
As this review aimed to report the global prevalence of alcohol use among those with and without a CMD within the adult general population, studies that focused upon treatment‐seeking individuals were excluded. Studies which examined the prevalence of alcohol use in those with and without a CMD within a population who experienced a specific traumatic event (e.g. military) or with a specific health condition, such as epilepsy, were also excluded (see Supporting information, Table S1 for a full list of criteria).
Search strategy
PsycINFO, MEDLINE, PsycARTICLES, PubMed, Scopus and Web of Science were searched using Boolean methods. Key terms were chosen using databases’ own ‘MeSH’ terms or subject headings and broad enough to cover possible synonyms for alcohol use (e.g. alcohol*), CMDs (e.g. depression), comorbidity (e.g. comorbid*) and prevalence (e.g. prevalence) (see Supporting information, Table S2 for full search terms). Titles, abstracts and keywords were searched. A manual search of reference lists of studies which met the inclusion criteria was also conducted. The search was conducted from inception until March 2020.
A second researcher (P.I.) reviewed a random sample of 10% of titles, abstracts and full texts and checked against the first author's screening to establish reliability for inclusion. A kappa score of 0.62 was confirmed between researchers, indicating moderate agreement in study inclusion [ 37 ].
Assessment of methodological quality
The Joanna Briggs Critical Appraisal Checklist for Studies Reporting Prevalence Data was used to assess the methodological quality of each study [ 34 ]. This checklist consists of nine items (scored 0 if no or unclear evidence or 1 if evidence was present) which covers different methodological aspects, such as the sampling frame, appropriateness of the analysis conducted and response rate. The maximum possible score was nine.
Data extraction
In accordance with the Joanna Briggs Institute Data Extraction Form for Prevalence Studies, the following study characteristics were extracted: name and date of study, author, titles, journal, year survey was conducted, sample size, use of methods for establishing the diagnosis of CMD and AUD, use of methods to measure socio‐economic status (SES), study population, country, description of main results and reviewer comments. We contacted authors for additional information if any key information was missing.
Synthesis of data
Statistical analyses.
Our meta‐analysis focuses on the prevalence and associations of AUD among those with and without a CMD; other alcohol outcomes were not included due to variance in the measures and cut‐offs used. In light of changes to the diagnostic criteria of AUD, we categorized AUD as mild, moderate or severe [ 5 ]. Studies that used earlier definitions of AUD, such as DSM‐IV abuse and dependence, were re‐categorized whereby abuse was considered mild and dependence as moderate or severe, given that previous research indicates that there may be differences in those meeting criteria for alcohol abuse and moderate AUD [ 38 ]. Due to the small number of studies examining the prevalence among those with and without a specific CMD (e.g. GAD), we grouped CMDs into two broad categories: mood disorder (dysthymia and MDD) and anxiety/phobic disorder (GAD, OCD, PTSD, panic disorder, social phobia, simple phobia and specific phobia). The comparison group was not meeting criteria for any CMD.
A random‐effects meta‐analysis was conducted to examine the global associations of AUD (e.g. mild, moderate or severe AUD) and any CMD. To consider both within‐ and between‐study variability [ 39 ], we then conducted an a priori random‐effects meta‐analysis to examine the global prevalence and associations of any AUD stratified by type of CMD (e.g. mood disorder), and then two post‐hoc random‐effects meta‐analyses by (i) severity of AUD (e.g. mild AUD versus no AUD excluding moderate/severe AUD and moderate/severe AUD versus no AUD excluding mild AUD) and (ii) severity of AUD by type of CMD.
For all analyses, studies which reported the total number of participants meeting criteria for a mood, anxiety/phobic disorder or no disorder were included. Studies which tested multiple CMDs within the same sample, over multiple time‐frames in the same sample (e.g. 12‐month AUD and life‐time AUD) or did not state the cut‐off used to determine AUD severity were excluded. Stratified analyses, such as severity of AUD by type of CMD, were not conducted where there were fewer than three sources of data within a group.
The metaprop command with Freeman–Tukey transformation was used to pool proportions of those with and without a CMD who reported AUD [ 40 ] using the numbers of those with a CMD who reported having an AUD and those with a CMD who did not report having an AUD, and this was repeated among those without a CMD for each study. The pooled proportions were then converted to an odds ratio (OR) using the metan command with the DerSimonian & Laird mode in Stata version 16 [ 39 ]. Forest plots and tables were generated to present the pooled prevalence, ORs and 95% confidence intervals (CIs). We conducted a sensitivity analysis by removing studies with the largest and smallest ORs to test the effect on the overall odds of having any AUD among those with a CMD, and publication bias was assessed using the Egger's test [ 41 ] and funnel plot. A planned a priori subgroup analysis by decade of data collected and continent was conducted. It was not possible to conduct other subgroup analyses due to a lack of reporting of demographic characteristics stratified by those with and without a CMD. Heterogeneity was assessed using I 2 and funnel plots using the metafunnel command [ 42 ].
Narrative synthesis
Due to a small number of studies reporting the prevalence of binge drinking, of which one study had a much larger sample size than others, it was not appropriate to conduct a meta‐analysis. Further, due to variances in the measures and cut‐offs used to measure alcohol consumption, we were unable to conduct a meta‐analysis of alcohol consumption. Instead, a narrative synthesis is provided for these alcohol outcomes.
The current systematic review and meta‐analysis had planned to examine the prevalence of alcohol use among those with and without a CMD from different SES backgrounds; however, studies included in this review did not report adequate information. Instead, studies generally reported the overall SES characteristics of the total sample and did not provide the required data stratified by SES.
Study selection
Our initial search yielded 2862 results, after removing duplications with 512 full texts reviewed after screening titles and abstracts. Fifty‐one studies were included in our final review and 17 in our meta‐analyses ( n = 382 201; see PRISMA diagram in Figure 1 ). Of the 51 studies included, 33 reported the prevalence of mild, moderate or severe AUD (including earlier diagnostic classifications), five of binge drinking and 12 of alcohol consumption. Studies were conducted in 24 countries, with the majority in the United States ( n = 10), and used data from 33 surveys. Bias scores ranged from 3 to 9 with a median of 7, indicating medium to low bias (see Table 1 ).
Of the 51 studies identified in the systematic review, 34 examined the prevalence of alcohol use among those meeting criteria for an anxiety/phobic disorder and 31 for mood disorder. The type of CMD most commonly studied was MDD (39%). None of the included studies examined alcohol use among those with and without SAD. Of the 33 studies reporting the prevalence of AUD among those with and without a CMD, 16 were not included in the meta‐analysis (see reasons in Figure 1).
Primary analysis
Prevalence and associations of any aud among those with and without a cmd.
The pooled prevalence of having any AUD among those with a CMD was higher than those without ( K = 17, 15% versus 8%, see Table 2 ), with those with a CMD being twice as likely to report any AUD (OR = 2.02, 95% CI = 1.72–2.36, I 2 = 90.70%, see Table 2 ). When stratified by 12‐month and life‐time AUD, the prevalence remained higher for life‐time AUD among those with a CMD (12‐month: K = 9, 10%, life‐time: K = 8, 21%, see Table 2 ) compared to those without (12‐month: 5%, life‐time: 12%, see Table 2 ). Our meta‐analysis found that associations for both 12‐month and life‐time AUD were approximately twofold among those with a CMD compared to those without (12‐month: OR = 2.14, 95% CI = 1.75–2.62, I 2 = 78.90%; life‐time: OR = 1.91, 95% CI = 1.45–2.52, I 2 = 94.70%, see Table 2 and Figure 2 ).
Prevalence and associations of having any AUD among those with and without a CMD ( n = 382 201)
| Any AUD | Prevalence of those with a CMD (%) | 95% CI Lower (%) | 95% CI Upper (%) | Weight | Heterogeneity ( ) | Prevalence of those without a CMD (%) | 95% CI Lower (%) | 95% CI Upper (%) | Weight | Heterogeneity ( ) | OR | 95% CI Lower | 95% CI Upper | Weight | Heterogeneity ( ) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12‐month | ||||||||||||||||||
| Batelaan . 2012 | 8.00 | 5.00 | 14.00 | 10.79 | 3.00 | 2.00 | 3.00 | 11.09 | 2.89 | 1.53 | 5.45 | 6.60 | ||||||
| De Castro Longo . 2020 | 8.00 | 6.00 | 12.00 | 11.52 | 2.00 | 2.00 | 3.00 | 11.06 | 3.39 | 2.15 | 5.34 | 9.75 | ||||||
| Currie . 2005 | 17.00 | 15.00 | 19.00 | 12.00 | 10.00 | 10.00 | 11.00 | 11.15 | 1.66 | 1.44 | 1.90 | 17.87 | ||||||
| Davis . 2020 | 4.00 | 4.00 | 4.00 | 12.14 | 2.00 | 2.00 | 2.00 | 11.15 | 1.70 | 1.60 | 1.81 | 19.16 | ||||||
| Husky . 2018 | 7.00 | 4.00 | 12.00 | 10.93 | 4.00 | 4.00 | 4.00 | 11.14 | 1.72 | 0.93 | 3.18 | 6.88 | ||||||
| Kinley . 2009 | 8.00 | 6.00 | 10.00 | 11.82 | 2.00 | 2.00 | 2.00 | 11.14 | 3.63 | 2.66 | 4.94 | 13.36 | ||||||
| Markkula . 2016 | 9.00 | 6.00 | 12.00 | 11.64 | 4.00 | 3.00 | 4.00 | 11.10 | 2.42 | 1.67 | 3.51 | 11.68 | ||||||
| Muller . 2014 | 21.00 | 15.00 | 28.00 | 10.88 | 11.00 | 10.00 | 12.00 | 11.06 | 1.90 | 1.27 | 2.84 | 10.97 | ||||||
| Patel . 2002 | 14.00 | 6.00 | 29.00 | 8.28 | 15.00 | 14.00 | 15.00 | 11.12 | 0.94 | 0.37 | 2.41 | 3.72 | ||||||
| Subtotal | 10.00 | 5.00 | 16.00 | 100.00 | 97.87 | 0.01 | 5.00 | 3.00 | 8.00 | 100.00 | 99.84 | 0.01 | 2.14 | 1.75 | 2.62 | 100.00 | 78.90% | 0.01 |
| Life‐time | ||||||||||||||||||
| Chong . 2012 | 10.00 | 8.00 | 13.00 | 12.51 | 3.00 | 3.00 | 4.00 | 12.51 | 3.47 | 2.61 | 4.60 | 11.95 | ||||||
| Crum . 2005 | 22.00 | 19.00 | 27.00 | 12.41 | 11.00 | 10.00 | 13.00 | 12.48 | 1.98 | 1.50 | 2.62 | 11.99 | ||||||
| Kessler . 1997 | 35.00 | 33.00 | 37.00 | 12.62 | 19.00 | 18.00 | 20.00 | 12.51 | 1.81 | 1.64 | 2.00 | 13.41 | ||||||
| Marquenie . 2007 | 9.00 | 7.00 | 11.00 | 12.57 | 4.00 | 4.00 | 5.00 | 12.51 | 2.08 | 1.64 | 2.64 | 12.38 | ||||||
| Pacek . 2013 | 40.00 | 37.00 | 44.00 | 12.50 | 42.00 | 41.00 | 43.00 | 12.52 | 0.97 | 0.83 | 1.13 | 13.11 | ||||||
| Park . 2013 | 23.00 | 18.00 | 28.00 | 12.26 | 16.00 | 15.00 | 17.00 | 12.51 | 1.42 | 1.05 | 1.91 | 11.80 | ||||||
| Tebeka . 2018 | 9.00 | 8.00 | 10.00 | 12.63 | 3.00 | 3.00 | 4.00 | 12.52 | 2.68 | 2.38 | 3.01 | 13.32 | ||||||
| Van Ameringan . 2008 | 28.00 | 24.00 | 31.00 | 12.50 | 14.00 | 12.00 | 17.00 | 12.42 | 1.92 | 1.46 | 2.53 | 12.05 | ||||||
| Subtotal | 21.00 | 12.00 | 32.00 | 100.00 | 99.35 | 0.01 | 12.00 | 4.00 | 24.00 | 100.00 | 99.92 | 0.01 | 1.91 | 1.45 | 2.52 | 100.00 | 94.70 | 0.01 |
| Overall | 15.00 | 9.00 | 22.00 | 100.00 | 99.50 | 0.01 | 8.00 | 5.00 | 12.00 | 100.00 | 99.90 | 0.01 | 2.02 | 1.72 | 2.36 | 100.00 | 90.70 | 0.01 |
AUD = alcohol use disorder; CMD = common mental disorders; OR = odds ratio; CO = confidence interval.

12‐month and life‐time associations of alcohol use disorder (AUD) among those with a common mental disorder (CMD) compared to those without ( n = 382 201)
The pooled prevalence and associations of any AUD by the type of CMD, regardless of duration, among those with an anxiety/phobic disorder was 17% ( K = 9 compared to 10% for those without, see Table 3 ) and 11% for mood disorder ( K = 6 compared to 5% for those without). Associations of having any AUD were similar for those with a mood or anxiety/phobic disorder (mood: OR = 2.00, 95% CI = 1.62–2.47, I 2 = 90.00%; anxiety/phobic: OR = 1.94, 95% CI = 1.35–2.78, I 2 = 91.40%, see Table 3 and Figure 3 ).
Prevalence and associations of any AUD stratified by type of CMD ( n = 367 487)
| Type of CMD | Prevalence among those with the specific CMD (%) | 95% CI Lower (%) | 95% Upper (%) | Weight | Heterogeneity ( ) | Prevalence among those without the specific CMD (%) | 95% CI Lower (%) | 95% Upper (%) | Weight | Heterogeneity ( ) | OR | 95% CI Lower | 95% CI Upper | Weight | Heterogeneity ( ) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anxiety/phobic disorder | ||||||||||||||||||
| Batelaan . 2012 | 8.00 | 5.00 | 14.00 | 10.95 | 3.00 | 2.00 | 3.00 | 11.12 | 2.89 | 1.53 | 5.45 | 9.32 | ||||||
| De Castro Longo . 2020 | 8.00 | 6.00 | 12.00 | 11.34 | 2.00 | 2.00 | 3.00 | 11.11 | 3.39 | 2.15 | 5.34 | 10.86 | ||||||
| Kinley . 2009 | 8.00 | 6.00 | 10.00 | 11.49 | 2.00 | 2.00 | 2.00 | 11.13 | 3.63 | 2.66 | 4.94 | 11.99 | ||||||
| Marquenie . 2007 | 9.00 | 7.00 | 11.00 | 11.57 | 4.00 | 4.00 | 5.00 | 11.12 | 2.08 | 1.64 | 2.64 | 12.43 | ||||||
| Muller . 2014 | 21.00 | 15.00 | 28.00 | 11.00 | 11.00 | 10.00 | 12.00 | 11.11 | 1.90 | 1.27 | 2.84 | 11.30 | ||||||
| Pacek . 2013 | 40.00 | 37.00 | 44.00 | 11.49 | 42.00 | 41.00 | 43.00 | 11.12 | 0.97 | 0.83 | 1.13 | 12.85 | ||||||
| Park . 2013 | 23.00 | 18.00 | 28.00 | 11.26 | 16.00 | 15.00 | 17.00 | 11.12 | 1.42 | 1.05 | 1.91 | 12.07 | ||||||
| Patel . 2002 | 14.00 | 6.00 | 29.00 | 9.39 | 15.00 | 14.00 | 15.00 | 11.12 | 0.94 | 0.37 | 2.41 | 6.95 | ||||||
| Van Ameringan . 2008 | 28.00 | 24.00 | 31.00 | 11.50 | 14.00 | 12.00 | 17.00 | 11.04 | 1.92 | 1.46 | 2.53 | 12.23 | ||||||
| Subtotal | 17.00 | 9.00 | 26.00 | 100.00 | 97.86 | 0.01 | 10.00 | 3.00 | 20.00 | 100.00 | 99.92 | 0.01 | 1.94 | 1.35 | 2.78 | 100.00 | 91.40 | 0.01 |
| Mood disorder | ||||||||||||||||||
| Crum . 2005 | 22.00 | 19.00 | 27.00 | 16.47 | 11.00 | 10.00 | 13.00 | 16.37 | 1.98 | 1.50 | 2.62 | 16.05 | ||||||
| Currie . 2005 | 17.00 | 15.00 | 19.00 | 17.15 | 10.00 | 10.00 | 11.00 | 16.75 | 1.66 | 1.44 | 1.90 | 20.34 | ||||||
| Davis . 2020 | 4.00 | 4.00 | 4.00 | 17.41 | 2.00 | 2.00 | 2.00 | 16.76 | 1.70 | 1.60 | 1.81 | 21.83 | ||||||
| Husky . 2018 | 7.00 | 4.00 | 12.00 | 15.18 | 4.00 | 4.00 | 4.00 | 16.73 | 1.72 | 0.93 | 3.18 | 7.74 | ||||||
| Markkula . 2016 | 9.00 | 6.00 | 12.00 | 16.47 | 4.00 | 3.00 | 4.00 | 16.65 | 2.42 | 1.67 | 3.51 | 13.20 | ||||||
| Tebeka . 2018 | 9.00 | 8.00 | 10.00 | 17.34 | 3.00 | 3.00 | 4.00 | 16.74 | 2.68 | 2.38 | 3.01 | 20.84 | ||||||
| Subtotal | 11.00 | 6.00 | 17.00 | 100.00 | 99.15 | 0.01 | 5.00 | 3.00 | 9.00 | 100.00 | 99.85 | 0.01 | 2.00 | 1.62 | 2.47 | 100.00 | 90.00 | 0.01 |

Associations of any alcohol use disorder (AUD) with common mental disorder (CMD), stratified by anxiety/phobic and mood disorders ( n = 367 483)
A sensitivity analysis removing studies with the largest [ 43 ] and smallest [ 44 ] OR resulted in only a small change in the total and life‐time effect size (see Supporting information, Figures S5 and S6 ). In light of changes to the categorization of mental disorders whereby PTSD and OCD are now two distinct diagnosis classifications (‘trauma‐ and stressor‐related disorders’ and ‘obsessive‐compulsive and related disorders’ [ 5 ]), a sensitivity analysis examining differences in associations of any AUD among those with PTSD compared to other anxiety/phobic disorder (without OCD) was conducted and showed a twofold increase in associations among those with PTSD, while associations with other anxiety/phobic disorders were non‐significant (see Supporting information, Table S4 ). We were unable to conduct a sensitivity analysis of OCD due to an insufficient number of studies.
Exploratory analysis
When stratified by the decade (e.g. 1990s) and continent (e.g. Europe) in which the study was conducted, respectively, we found similar strengths of associations (see Supporting information, Tables S5 and S6 ).
Heterogeneity
There was substantial heterogeneity between studies when conducting each meta‐analysis, as illustrated in the forest plots (see Figures 1 , ,2, 2 , ,3, 3 , ,4) 4 ) where I 2 percentages were greater than 80%, which was further confirmed by our overall funnel plot (see Supporting information, Figure S1 ). An Egger's test was non‐significant ( P = 0.86) and a funnel plot showed that studies remained close to the overall effect size, indicating limited evidence of bias (see Supporting information, Figure S7 ). We also explored sources of heterogeneity by conducting a subgroup analysis according to the decade during which data was collected, the continent in which the studies were conducted and bias score (see Supporting information, Figures S2–S4 ), but these did not substantially reduce heterogeneity estimates. We were unable to explore heterogeneity according to group characteristics due to a lack of reporting among those with and without a CMD; however, there were differences in the diagnostic criteria used to assess both AUD and CMD which may explain some of the heterogeneity.

Associations of alcohol use disorder (AUD) among those with a common mental disorder (CMD), stratified by AUD severity ( n = 382 201)
Secondary analyses
Prevalence and associations of mild and moderate/severe aud among those with and without a cmd.
The pooled prevalence of mild AUD was higher among those with a CMD compared to those without ( K = 6, 7 versus 5%, see Table 4 ). Those with a CMD were more likely to report mild AUD compared to those without a CMD (OR = 1.71, 95% CI = 1.31–2.23, I 2 = 75.20%, see Table 4 and Figure 4 ). We found that 12% of those with a CMD reported moderate/severe AUD compared to 6% of those without a CMD ( K = 17, see Table 4 ) and those with a CMD were twice as likely to report moderate/severe AUD (OR = 2.19, 95% CI = 1.82–2.63, I 2 = 91.30%, see Table 4 and Figure 4 ).
Prevalence and associations of mild and moderate/severe AUD among those with and without a CMD ( n = 382 201)
| AUD severity | Prevalence among those with a CMD (%) | 95% CI Lower (%) | 95% CI upper (%) | Weight | Heterogeneity ( ) | Prevalence among those without a CMD (%) | 95% CI Lower (%) | 95% CI Upper (%) | Weight | Heterogeneity ( ) | OR | 95% CI Lower | 95% CI Upper | Weight | Heterogeneity ( ) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild AUD | ||||||||||||||||||
| Chong . 2012 | 8.00 | 6.00 | 10.00 | 17.31 | 3.00 | 2.00 | 3.00 | 16.66 | 3.04 | 2.21 | 4.18 | 20.77 | ||||||
| Currie . 2005 | 13.00 | 11.00 | 15.00 | 17.72 | 8.00 | 7.00 | 8.00 | 16.73 | 1.72 | 1.46 | 2.01 | 26.75 | ||||||
| Husky . 2018 | 1.00 | 0.00 | 4.00 | 14.90 | 1.00 | 1.00 | 2.00 | 16.71 | 0.49 | 0.07 | 3.50 | 1.71 | ||||||
| Kessler . 1997 | 15.00 | 14.00 | 17.00 | 17.86 | 10.00 | 9.00 | 10.00 | 16.64 | 1.61 | 1.38 | 1.87 | 27.03 | ||||||
| Park . 2013 | 10.00 | 7.00 | 15.00 | 15.80 | 10.00 | 9.00 | 11.00 | 16.66 | 1.00 | 0.64 | 1.58 | 15.79 | ||||||
| De Castro Longo . 2020 | 2.00 | 1.00 | 5.00 | 16.41 | 1.00 | 1.00 | 2.00 | 16.60 | 1.77 | 0.79 | 3.96 | 7.96 | ||||||
| Subtotal | 7.00 | 4.00 | 12.00 | 100.00 | 95.66 | 0.01 | 5.00 | 2.00 | 8.00 | 100.00 | 99.71 | 0.01 | 1.71 | 1.31 | 2.23 | 100.00 | 75.20 | 0.01 |
| Moderate/severe AUD | ||||||||||||||||||
| Batelaan . 2012 | 8.00 | 5.00 | 14.00 | 5.66 | 3.00 | 2.00 | 3.00 | 5.89 | 2.89 | 1.53 | 5.45 | 4.02 | ||||||
| Chong . 2012 | 3.00 | 2.00 | 4.00 | 6.02 | 0.00 | 0.00 | 1.00 | 5.89 | 7.32 | 3.93 | 13.64 | 4.11 | ||||||
| Crum . 2005 | 22.00 | 19.00 | 27.00 | 5.96 | 11.00 | 10.00 | 13.00 | 5.84 | 1.98 | 1.50 | 2.62 | 6.65 | ||||||
| Currie . 2005 | 5.00 | 4.00 | 7.00 | 6.08 | 3.00 | 3.00 | 3.00 | 5.90 | 1.68 | 1.31 | 2.15 | 6.89 | ||||||
| Davis . 2020 | 4.00 | 4.00 | 4.00 | 6.13 | 2.00 | 2.00 | 2.00 | 5.91 | 1.70 | 1.60 | 1.81 | 7.82 | ||||||
| Husky . 2018 | 4.00 | 2.00 | 9.00 | 5.70 | 2.00 | 2.00 | 3.00 | 5.90 | 1.74 | 0.76 | 3.96 | 3.03 | ||||||
| Kessler . 1997 | 26.00 | 24.00 | 28.00 | 6.10 | 12.00 | 11.00 | 13.00 | 5.89 | 2.23 | 1.98 | 2.53 | 7.61 | ||||||
| Kinley . 2009 | 8.00 | 6.00 | 10.00 | 6.02 | 2.00 | 2.00 | 2.00 | 5.90 | 3.63 | 2.66 | 4.94 | 6.43 | ||||||
| Markkula . 2016 | 9.00 | 6.00 | 12.00 | 5.96 | 4.00 | 3.00 | 4.00 | 5.88 | 2.42 | 1.67 | 3.51 | 5.93 | ||||||
| Marquenie . 2007 | 9.00 | 7.00 | 11.00 | 6.07 | 4.00 | 4.00 | 5.00 | 5.89 | 2.08 | 1.64 | 2.64 | 6.94 | ||||||
| Muller . 2014 | 21.00 | 15.00 | 28.00 | 5.69 | 11.00 | 10.00 | 12.00 | 5.88 | 1.90 | 1.27 | 2.84 | 5.70 | ||||||
| Pacek . 2013 | 40.00 | 37.00 | 44.00 | 6.02 | 42.00 | 41.00 | 43.00 | 5.90 | 0.97 | 0.83 | 1.13 | 7.48 | ||||||
| Park . 2013 | 15.00 | 11.00 | 21.00 | 5.84 | 8.00 | 7.00 | 8.00 | 5.89 | 2.06 | 1.42 | 2.98 | 5.96 | ||||||
| Patel . 2002 | 14.00 | 6.00 | 29.00 | 4.68 | 15.00 | 14.00 | 15.00 | 5.89 | 0.94 | 0.37 | 2.41 | 2.56 | ||||||
| Tebeka . 2018 | 9.00 | 8.00 | 10.00 | 6.12 | 3.00 | 3.00 | 4.00 | 5.90 | 2.68 | 2.38 | 3.01 | 7.64 | ||||||
| Van Ameringan . 2008 | 28.00 | 24.00 | 31.00 | 6.03 | 14.00 | 12.00 | 17.00 | 5.76 | 1.92 | 1.46 | 2.53 | 6.70 | ||||||
| De Castro Longo . 2020 | 6.00 | 4.00 | 9.00 | 5.92 | 1.00 | 1.00 | 2.00 | 5.88 | 5.45 | 3.111 | 9.58 | 4.52 | ||||||
| Subtotal | 12.00 | 8.00 | 17.00 | 100.00 | 99.26 | 0.01 | 6.00 | 4.00 | 10.00 | 100.00 | 99.88 | 0.01 | 2.19 | 1.82 | 2.63 | 100.00 | 91.30 | 0.01 |
AUD = alcohol use disorder; CMD = common mental disorder; OR = odds ratio; CO = confidence interval.
Due to the small number of studies examining the prevalence of mild AUD ( n = 6) it was not possible to conduct a subgroup analysis of mild AUD by the type of CMD, although this was possible for moderate/severe AUD. We found those with a mood or anxiety/phobic disorder were approximately twice as likely to report moderate/severe AUD (mood: K = 6, OR = 2.02, 95% CI = 1.60–2.57, I 2 = 89.60%; anxiety/phobic: K = 9, OR = 2.12, 95% CI = 1.43–3.14, I 2 = 92.20%, see Supporting information, Table S3 ).
Binge drinking among those with and without a CMD
Five studies reported the prevalence of binge drinking among those with and without a CMD, although there was variation in the cut‐offs used to assess this and the duration of binge drinking (see Table 5 ). Of the five studies, four examined the prevalence of binge drinking among those with and without depression, one with anxiety and one with PTSD. Four of the five studies reported a higher prevalence of binge drinking among those with a CMD (3.70–35.03%, see Table 5 ) compared to those without (1.01–31.62%). One reported a lower prevalence (12.60 versus 15.10%, see Table 5 ); this may have been due to the study measuring depressive episode or having any anxiety, whereas other studies examined specific types of CMDs or depressive symptoms.
Overview of findings of studies examining the prevalence of binge drinking among those with and without a CMD
| Study | Type of CMD assessed | Outcome | Duration | Summary of findings | Demographic characteristics |
|---|---|---|---|---|---|
| . 2012 | Depression (cut‐off score > 5 or more, derived depression scale) | Binge drinking (5 or more drinks on one occasion once a month or more) | 12 months | Binge drinker and depressed: 420/1199 (35.03%) Binge drinker and not depressed: 5161/16324 (31.62%) | Gender: Binge drinker/depressed: Male: 188/375 (50.00%) Female: 232/824 (28.13%) Binge drinker/not depressed: Male: 3351/8561 (39.14%) Female: 1310/7763 (23.31%) |
| . 2017 | Major depressive episode (yes/no) Anxiety (yes/no) | Binge drinking (5 or more drinks in last 30 days) | 30 days | With depressive episode: 2013–2014 = 169/1339 (12.60%) Without depressive episode: 2013–2014 = 2142/13963 (15.10%) With anxiety: 2013/2014 = 123/931 (13.20%) Without anxiety: 2206/14371 (15.00%) | |
| . 2010 | MDD (ICD‐10) | Binge drinking (5 or more drinks, never, some times per year, 1–3 times per month, at least once a week ) | 12 months | With MDD: 26/342 (7.50%) Without MDD: 265/5763 (4.60%) | |
| . 2011 | Depressive symptoms (BDI cut‐off score >8) | Heavy drinking occasion (7 or more drinks for men or 5 or more for women) | 28 days | With depression: 25/321 (7.79%) Without depression: 86/1765 (4.87%) | Gender and depression Female = 4/198 (2.02%) Male = 21/123 (17.07%) Gender and no depression Female = 36/942 (3.82%) Male = 50/823 (6.08%) |
| PTSD (DSM‐III‐R) | Heavy alcohol use (frequency 1–3 times per month and quantity 5 + drinks per day for men and 4 + drinks per day for women, respectively) | 12 months | With PTSD: 1/27 (3.70%) Without PTSD: 17/1691 (1.01%) |
CMD = common mental disorder; MDD = major depressive disorder; BDI = Beck depression inventory; PTSD = post‐traumatic stress disorder.
Alcohol consumption among those with and without a CMD
Twelve studies reported the prevalence of alcohol consumption among those with and without a CMD, although there was variation in the type of alcohol consumption and CMD assessed and cut‐off scores used (see Table 6 ). Three studies reported a higher prevalence of alcohol consumption among those with a CMD (1.66–24.29%) compared to those without (0.92–7.94%), six reported a lower prevalence among those with a CMD (0.00–42.00%) and three reported both higher and lower prevalence depending on the type of CMD and alcohol consumption outcome (0.00–14.81%, see Table 6 ).
Overview of findings of studies examining the prevalence of alcohol consumption among those with and without a CMD
| Study | Type of CMD assessed | Outcome | Duration | Summary of findings |
|---|---|---|---|---|
| Social phobia and anxiety (MINI‐SPIN cut‐off score 8 >) | Frequency of alcohol use (> 1 times per week) Alcohol problems (one or more periods in the last 5 years affected job) | Alcohol frequency = 1 week Alcohol problems = 5 years | With SPAS: Weekly drinker = 182/446 (40.80%) Alcohol problems = 54/446 (11.21%) Without SPAS: Weekly drinker = 1124/2230 (50.40%) Alcohol problems = 121/2230 (5.43%) | |
| . 2017 | Depression (HADS‐D cut‐off score 11 >) Anxiety (HAD‐A cut‐off score 11 >) | Alcohol frequency (daily, occasionally, never; cut‐offs not stated) | Not stated | With depression: Daily drinker = 58/241 (24.07%) Without depression: Daily drinker = 553/1439 (38.43%) With anxiety: Daily drinker = 40/176 (22.73%) Without anxiety: Daily drinker = 571/1504 (37.97%) |
| . 2014 | Anxiety symptoms (GAI‐SF cut‐off score of 3 >) | Alcohol consumption (< 1, 1, 2, 2 > units per day) | Not stated | With anxiety: 7/77 (9.09%) Without anxiety: 14/289 (4.84%) |
| . 2018 | Depressive symptoms (CES‐D cut‐off score 16 >) | Alcohol frequency (3 times per week) | 1 week | With depression: 32/159 (20.13%) Without depression: 533/2175 (24.51%) |
| . 2016 | Depression (GSM, answering yes to questions 1 and 2) | Alcohol frequency (> 1 drink per week) | 1 week | With depression: 0/54 (0.00%) Without depression: 14/915 (1.53%) |
| . 2017 | Depressive symptoms (yes to 4 mandatory questions and 2 additional) | Alcohol consumption (life‐time abstainers, non‐heavy drinkers, infrequent heavy drinkers, frequent heavy drinkers; 4 or 5 drinks for women and men on at least 3 days) | 7 days | With depression: Frequent heavy drinker = 64/12886 (0.50%) Without depression: Frequent heavy drinker = 1558/155835 (1.00%) |
| . 2018 | Panic disorder (DSM‐5) | Alcohol consumption (frequent drinkers: consumed alcohol in either last 30 days and 7 days or 1–2 days per week with 5/4 standard drinks in last 7 days or 3 or more days per week with 5/4 standard drinks in the last 7 days) | 12 months | With panic disorder: 25/96 (31.30%) Without panic disorder: 1680/4176 (42.00%) |
| . 2010 | Depressive symptoms (BDI cut‐off score > 10) | Alcohol consumption (> = 3 times per week) | Not stated | With depression: 63/3800 (1.66%) Without depression: 85/9279 (0.92%) |
| . 2000 | GAD OCD Phobia (DSM‐III) | Heavy/excessive alcohol intake (cut‐off score of 4 on Garretsen scale) | 6 months | With phobic disorder: 1/21 (4.76%) With panic disorder: 0/6 (0.00%) With GAD: 3/47 (6.38%) Without an anxiety disorder: 1/27 (3.70%) |
| . 2009 | MDD Dysthymia GAD Panic disorder Specific phobia Social phobia (DSM‐IV‐TR) | Excessive alcohol use (more than 21 drinks per week) | Alcohol use = 7 days | With MDD: 9/96 (9.38%) With Dysthymia: 0/19 (0.00%) With GAD: 7/103 (6.80%) With panic disorder: 4/27 (14.81%) With specific phobia: 6/77 (7.79%) With social phobia: 5/56 (8.93%) Without a CMD: 488/4499 (10.85%) |
| . 2003 | Depressive symptoms (CES‐D cut‐off score 16 >) | Alcohol consumption (no alcohol intake, moderate, excessive: 3 or more drinks per day) | Not stated | With depression: 7/176 (4.00%) Without depression: 45/1104 (4.10%) |
| . 2018 | GAD (GAD‐7 cut‐off score 10 >) | Alcohol consumption (do not drink, moderate, excessive ) | Not stated | With GAD: 43/177 (24.29%) Without GAD: 2769/34854 (7.94%) |
CMD = common mental disorder; GAD = generalized anxiety disorder; MDD = major depressive disorder; BDI = Beck depression inventory; HAD = Hospital Anxiety and Depression Scale.
Key findings
Our systematic review and meta‐analysis aimed to examine the prevalence and associations of AUD, binge drinking and alcohol consumption among those with and without a CMD, respectively. We found that those with a CMD were twice as likely to report an AUD compared to those without, and these associations were similar among types of CMD throughout decades and continents. Based on the ORs, associations between CMD and AUD were stronger for moderate/severe AUD compared to mild AUD. In addition, our narrative review identified both positive and negative associations for CMD with binge drinking and alcohol consumption, indicating that more research using similar methods is required.
Our findings identified that those with a CMD were more likely to report severe levels of AUD and that most studies focused upon associations with a specific type of CMD, such as MDD. We were unable to identify any studies examining associations with SAD. In addition, much of the research has focused upon AUD as opposed to other problematic drinking patterns, such as binge drinking, despite the high prevalence in the general population [ 3 ] and the known negative health impacts [ 6 , 14 ].
Models of comorbidity and comparisons to previous research
Models of comorbidity have debated whether alcohol worsens mental health or vice versa [ 18 ] and previous longitudinal research assessing both pathways indicate stronger support for the notion that poor mental health increases alcohol use [ 45 ]; however, there is likely to be a bidirectional association. Psychological models, such as the stress–coping and incentive–motivation models, hypothesize that individuals may be motivated to use alcohol to cope with stress and enhance positive affect [ 19 ], and that benefits of drinking outweigh the consequences of not drinking [ 20 ]. Considering that symptoms of a CMD include low mood and irritability [ 32 ], alcohol may be used to cope with symptoms initially, increasing alcohol use [ 46 ]. The self‐medication model argues further that alcohol may be used specifically because of its rapid onset of action and differs according to the individuals’ symptoms [ 21 ]. Our findings are based on cross‐sectional research, therefore we cannot infer causality. We found associations between AUD and CMD regardless of the type of CMD and severity of AUD. It may be that individuals with a CMD may use alcohol to enhance positive affect and cope with symptoms of poor mental health. Further qualitative and longitudinal research is required to understand the reasons why those with a CMD use alcohol.
Our narrative review of associations between binge drinking and CMDs and consumption, respectively, showed mixed evidence. Studies included in this review suggest that alcohol use and CMD comorbidity may be more complex, as some studies reported increases in binge drinking or consumption while others did not. This may have been due to the range of CMDs measured or the measures used to assess alcohol use and CMDs. However, previous research suggests that this may also be explained by additional factors such as gender [ 10 , 15 ], age [ 14 , 47 ] and specific CMD diagnoses [ 9 ]. Future research should consider such characteristics when examining associations between alcohol use and CMD. In addition, further research is required on associations of CMDs with other alcohol outcomes, given that they are more prevalent in the general population compared to AUD [ 3 ] and are known to have implications on health [ 6 ].
A previous systematic review reported a twofold increase in the odds of reporting any AUD among those with an anxiety disorder and 2.5‐fold increase for those with major depression, in addition to a 2.3‐fold and threefold increase in the odds of reporting alcohol dependence for any anxiety disorder and major depression, respectively. We found slightly weaker associations, with a twofold increase in the odds of any AUD (and the same for moderate/severe AUD) for any anxiety or mood disorder, respectively. This difference could be explained by the types of CMDs included in our review in which we included MDD, dysthymia, GAD, panic disorder, phobias, PTSD, OCD or SAD, whereas Lai and colleagues [ 11 ] included agoraphobia, GAD, panic disorder, social phobia, bipolar disorder, dysthymia and MDD. Our sensitivity analysis also showed a twofold increase in the odds of having any AUD among those with PTSD, while a non‐significant association was found among those with any other anxiety disorder, excluding OCD.
Other psychological models suggest that comorbid alcohol and mental health problems are due to shared vulnerabilities, such as SES factors [ 23 , 48 , 49 , 50 ]. We attempted to explore this by reviewing evidence examining the prevalence of alcohol use among those with and without a CMD based on SES characteristics; however, studies included in this review did not report this and thus we cannot support or reject these suggestions.
Strengths and limitations
With regard to the studies included in this review, the majority of studies used large sample sizes representative of the general population and standardized criteria to assess alcohol use and CMD, particularly those reporting the prevalence of AUD. There are some limitations to note. First, the majority of studies focused upon the prevalence of alcohol use among those with and without types of CMDs, namely MDD, rather than other disorders such as SAD. Therefore, we were unable to explore associations beyond broad mood and anxiety/phobic disorders, including more specific disorders. Secondly, we were unable to conduct a meta‐analysis on the prevalence and association of binge drinking or alcohol consumption due to variations in the measures and cut‐offs used; therefore, we cannot conclude whether those with a CMD are more likely to report different patterns of alcohol use compared to those without beyond AUD.
With regard to our review, we conducted an extensive search of the literature across multiple databases and included a range of CMDs and types of alcohol use, with large sample sizes. There are also some limitations to note. First, there was substantial heterogeneity between studies. While the majority of studies used diagnostic criteria to establish the presence of CMD and AUD, different versions of criteria were used between studies. There was also limited reporting of group characteristics among those with and without a CMD, which may explain some of the heterogeneity. We overcame this by exploring differences in associations between the severity of AUD and type of CMD, as well as the continent and decade in which the study was conducted. Secondly, we included published research, therefore we may have missed some grey literature. However, given that multiple databases and references were searched, we believe our review was inclusive. Thirdly, some of the associations may have been driven by specific types of CMD, as found in previous research [ 25 ]; we conducted a sensitivity analysis with PTSD but were unable to conduct further analyses to due to insufficient numbers. Fourthly, the stratified prevalence by AUD severity would equal the overall any AUD prevalence for studies that provided these stratified data; however, some studies reported moderate/severe AUD only. For those studies which reported the stratified prevalence by AUD severity, the sum of the mild and moderate/severe prevalence would then equal the overall prevalence, but some studies only reported the prevalence for moderate/severe AUD and, in these cases, this was the same as the numbers included in the overall meta‐analysis. Finally, while studies included in this review generally included individuals aged 18 years and over, in some cases studies had a minimum age in adolescence (e.g. 15 years and over). Due to the way in which data were presented in these studies, it was not possible to exclude these participants and restrict the prevalence estimates to those aged 18 years and over. However, in large population studies the numbers aged under 18 years would be in the minority, and this should not impact upon the prevalence reported.
CONCLUSIONS
Our review and meta‐analysis show that having a CMD is associated with increased odds of having an AUD, particularly moderate/severe AUD. There was little difference in associations based on the type of CMD. There is a need to ensure that alcohol and mental health problems are treated in parallel, while more research is required to investigate group characteristics and differences beyond broad CMD classifications. Additional research examining associations between having a CMD with other alcohol outcomes is required to provide a more holistic understanding of drinking patterns among individuals with a CMD.
DECLARATION OF INTERESTS
J.P. is funded as part of a PhD Studentship by the Society for the Study of Addiction.
AUTHOR CONTRIBUTIONS
Jo‐Anne Puddephatt: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; software; validation. Patricia Irizar: Data curation; investigation; resources; validation. Andrew Jones: Conceptualization; formal analysis; methodology; supervision. Suzanne Gage: Conceptualization; formal analysis; methodology; supervision. Laura Goodwin: Conceptualization; formal analysis; funding acquisition; methodology; supervision.
Supporting information
Table S1: Study Inclusion and Exclusion Criteria
ACKNOWLEDGEMENTS
This work was supported as part of a PhD Studentship by the Society for the Study of Addiction.
Puddephatt J‐A, Irizar P, Jones A, Gage SH, Goodwin L. Associations of common mental disorder with alcohol use in the adult general population: a systematic review and meta‐analysis . Addiction . 2022; 117 :1543–1572. 10.1111/add.15735 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Funding information Society for the Study of Addiction

COMMENTS
Abstract. Alcohol is a major contributor to global disease and a leading cause of preventable death, causing approximately 88,000 deaths annually in the United States alone. Alcohol use disorder is one of the most common psychiatric disorders, with nearly one-third of U.S. adults experiencing alcohol use disorder at some point during their lives.
The morbidity and mortality associated with alcohol are largely due to the high rates of alcohol use disorder in the population. Alcohol use disorder is defined in the Diagnostic and Statistical Manual for Mental Disorders, 5th edition (DSM-5) as a pattern of alcohol consumption, leading to problems associated with 2 or more of 11 potential symptoms of alcohol use disorder (see Table 1 for ...
It is well recognized that alcohol use disorders (AUD) have a damaging impact on the health of the population. According to the World Health Organization (WHO), 5.3% of all global deaths were attributable to alcohol consumption in 2016 [].The 2016 Global Burden of Disease Study reported that alcohol use led to 1.6% (95% uncertainty interval [UI] 1.4-2.0) of total DALYs globally among females ...
Alcohol is regularly consumed throughout most of the world, including by nearly half the U.S. population age 12 or older. Heavy drinking, which is also common, contributes to multiple adverse medical, psychiatric, and social outcomes and more than 140,000 deaths annually in the United States. It is the major risk factor for alcohol use disorder (AUD), whose current U.S. prevalence is 11% ...
Pathologic alcohol use affects more than 2 billion people and accounts for nearly 6% of all deaths worldwide. There are three medications approved for the treatment of alcohol use disorder by the US Food and Drug Administration (FDA): disulfiram, naltrexone (oral and long-acting injectable), and acamprosate. Of growing interest is the use of anticonvulsants for the treatment of alcohol use ...
Research on medications in development for treatment of alcohol use disorder. NIAAA Data Archive The NIAAA data archive is a data repository that houses and shares human subjects data generated by NIAAA-funded research.
Alcohol use disorders consist of disorders characterised by compulsive heavy alcohol use and loss of control over alcohol intake. Alcohol use disorders are some of the most prevalent mental disorders globally, especially in high-income and upper-middle-income countries; and are associated with high mortality and burden of disease, mainly due to medical consequences, such as liver cirrhosis or ...
Alcohol use disorder (AUD) is a disease that occurs when alcohol significantly impairs an individual's health and functioning. The severity of AUD ranges from mild to severe, and symptoms have the potential for recurrence and remission. No matter how severe the disorder is, individuals can benefit from treatment. Diagnosis.
The numbers for substance use disorders are large, and we need to pay attention to them. Data from the 2018 National Survey on Drug Use and Health suggest that, over the preceding year, 20.3 million people age 12 or older had substance use disorders, and 14.8 million of these cases were attributed to alcohol.When considering other substances, the report estimated that 4.4 million individuals ...
Alcohol use disorders are among the top causes of the global burden of disease, yet therapeutic interventions are limited. ... At the time of this study, TriNetX Research US Collaborative Network ...
Data were from 42 739 adults 18 years and older who participated in the 2019 National Survey on Drug Use and Health (NSDUH), providing representative data among US civilian, noninstitutionalized populations, 2 including sociodemographic characteristics, past-year emergency department (ED) visits, illicit drug use, alcohol use, and receipt of mental health care and any alcohol use treatment (eg ...
Treatment rates for alcohol use disorder are very low. Federal survey data show that in 2022, only 7.6% of people (12+) with a past year alcohol use disorder received any treatment.
Individuals with alcohol dependence may drink partly to reduce or avoid withdrawal symptoms. How do alcohol use disorders affect people? While some research suggests that small amounts of alcohol may have beneficial cardiovascular effects, there is widespread agreement that heavier drinking can lead to health problems.
Alcohol use disorder is a pattern of alcohol use that involves problems controlling your drinking, being preoccupied with alcohol or continuing to use alcohol even when it causes problems. ... Some research studies indicate that having bariatric surgery may increase the risk of developing alcohol use disorder or of relapsing after recovering ...
Working to stop alcohol use to improve quality of life is the main treatment goal. Treatment for alcohol use disorder may include: Detox and withdrawal. Treatment may begin with a program of detoxification — withdrawal that's medically managed. Sometimes called detox, this generally takes 2 to 7 days.
The Alcohol Use Disorders Identification Test (AUDIT) is a widely used instrument that was developed by the World Health Organization (WHO) for identifying risky or harmful alcohol consumption as well as alcohol dependence and abuse (Babor, La Fuente, Saunders, & Grant, 1992).The 10-item AUDIT includes questions to assess the amount and frequency of alcohol intake (items 1-3), alcohol ...
Few observations in psychiatry have been documented as long and as consistently as the association between anxiety (and general negative affect) and the chronic misuse of alcohol. Research has shown that up to 50% of individuals receiving treatment for problematic alcohol use also met diagnostic criteria for one or more anxiety disorders.1,2 ...
Many individuals who develop substance use disorders (SUD) are also diagnosed with mental disorders, and vice versa. 2,3 Although there are fewer studies on comorbidity among youth, research suggests that adolescents with substance use disorders also have high rates of co-occurring mental illness; over 60 percent of adolescents in community-based substance use disorder treatment programs also ...
Find resources for help with alcohol use disorder, and learn about treatment options. Alcohol Treatment Navigator. Treatment for Alcohol Problems: Finding and Getting Help ... PDF materials for patient education and free, research-focused print materials. Short Takes These brief, informative videos from NIAAA offers researched based information ...
Alcohol use is associated with mood instability (depression and manic symptoms) in people with bipolar disorder (BD), according to a study published online June 7 in JAMA Network Open. Sarah H ...
Research has shown that alcohol use and common mental disorders (CMDs) co‐occur; however, little is known about how the global prevalence of alcohol use compares across different CMDs. ... Alcohol use disorder (measure not stated, DSM‐IV) Binge drinking‐30 days. Alcohol abuse/dependence‐12 months. 7: Ho et al. 2016. 2003-04: Singapore ...