- Español (Spanish)

HIV Overview
Hiv and aids clinical trials.
- A clinical trial is a research study done to evaluate new medical approaches in people. HIV and AIDS clinical trials help researchers find better ways to prevent, detect, or treat HIV and AIDS.
- Examples of HIV and AIDS clinical trials underway include studies of new HIV medicines, studies of vaccines to prevent or treat HIV, and studies of medicines to treat infections related to HIV and AIDS.
- The benefits and possible risks of participating in an HIV and AIDS clinical trial are explained to study volunteers before they decide whether to participate in a study.
- Use the find a study search feature on ClinicalTrials.gov to find HIV and AIDS studies looking for volunteer participants. Some HIV and AIDS clinical trials enroll only people who have HIV. Other studies enroll people who do not have HIV.
What is a clinical trial?
A clinical trial is a research study that evaluates new medical approaches in people. These approaches include:
- new medicines or new combinations of medicines
- new medical devices or surgical procedures
- new ways to use an existing medicine or device
- new ways to change behaviors to improve health
Clinical trials are conducted in several phases to determine whether new medical approaches are safe and effective in people. Results from a Phase 1 Trial , Phase 2 Trial , and Phase 3 Trial are used to determine whether a new drug should be approved for sale in the United States. Once a new drug is approved, researchers continue to track its safety in a Phase 4 Trial .
Interventional trial and observational trial are two main types of clinical trials.
What is an HIV and AIDS clinical trial?
HIV and AIDS clinical trials help researchers find better ways to prevent, detect, or treat HIV and AIDS. Every HIV medicine was first studied through clinical trials.
Examples of HIV and AIDS clinical trials include:
- studies of new medicines to prevent or treat HIV and AIDS
- studies of vaccines to prevent or treat HIV
- studies of medicines to treat infections related to HIV and AIDS

Can anyone participate in an HIV and AIDS clinical trial?
It depends on the study. Some HIV and AIDS clinical trials enroll only people who have HIV. Other studies include people who do not have HIV.
Participation in an HIV and AIDS clinical trial may also depend on other factors, such as age, gender, HIV treatment history, or other medical conditions.
What are the benefits of participating in an HIV and AIDS clinical trial?
Participating in an HIV and AIDS clinical trial can provide benefits. For example, many people participate in HIV and AIDS clinical trials, because they want to contribute to HIV and AIDS research. They may have HIV or know someone who has HIV.
People with HIV who participate in an HIV and AIDS clinical trial may benefit from new HIV medicines before they are widely available. HIV medicines being studied in clinical trials are called investigational drugs . To learn more, read the HIVinfo What is an Investigational HIV Drug? fact sheet.
Participants in clinical trials can receive regular and careful medical care from a research team that includes doctors and other health professionals. Often the medicines and medical care are free of charge.
Sometimes people get paid for participating in a clinical trial. For example, they may receive money or a gift card. They may be reimbursed for the cost of meals or transportation.
Are HIV and AIDS clinical trials safe?
Researchers try to make HIV and AIDS clinical trials as safe as possible. However, volunteering to participate in a study testing an experimental treatment for HIV can involve risks of varying degrees. Most volunteers do not experience serious side effects; however, potential side effects that may be serious or even life-threatening can occur from the treatment being studied.
Before enrolling in a clinical trial, potential volunteers learn about the study in a process called informed consent . The process includes an explanation of the possible risks and benefits of participating in the study.
Once enrolled in a study, people continue to receive information about the study through the informed consent process.
If a person decides to participate in an HIV and AIDS clinical trial, will their personal information be shared?
The privacy of study volunteers is important to everyone involved in an HIV and AIDS clinical trial. The informed consent process includes an explanation of how a study volunteer’s personal information is protected.
How can one find an HIV and AIDS clinical trial looking for volunteer participants?
There are several ways to find an HIV and AIDS clinical trial looking for volunteer participants.
- Use the find a study search feature on ClinicalTrials.gov to find HIV and AIDS studies looking for volunteer participants.
- Call a Clinical Info health information specialist at 1-800-448-0440 or email [email protected] .
- Join ResearchMatch , which is a free, secure online tool that makes it easier for the public to become involved in clinical trials.
This fact sheet is based on information from the following sources:
From the National Institutes of Health (NIH):
- NIH Clinical Research Trials and You: The Basics
From the National Library of Medicine:
- Learn About Clinical Studies
Also see the HIV Source collection of HIV links and resources.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 01 December 2021
Research priorities for an HIV cure: International AIDS Society Global Scientific Strategy 2021
- Steven G. Deeks 1 ,
- Nancie Archin 2 ,
- Paula Cannon ORCID: orcid.org/0000-0003-0059-354X 3 ,
- Simon Collins 4 ,
- R. Brad Jones 5 ,
- Marein A. W. P. de Jong 6 ,
- Olivier Lambotte 7 ,
- Rosanne Lamplough 8 ,
- Thumbi Ndung’u 9 , 10 , 11 ,
- Jeremy Sugarman ORCID: orcid.org/0000-0001-7022-8332 12 ,
- Caroline T. Tiemessen ORCID: orcid.org/0000-0002-0991-1690 13 ,
- Linos Vandekerckhove ORCID: orcid.org/0000-0002-8600-1631 14 ,
- Sharon R. Lewin ORCID: orcid.org/0000-0002-0330-8241 15 , 16 , 17 &
The International AIDS Society (IAS) Global Scientific Strategy working group
Nature Medicine volume 27 , pages 2085–2098 ( 2021 ) Cite this article
60k Accesses
136 Citations
193 Altmetric
Metrics details
- Translational research
Despite the success of antiretroviral therapy (ART) for people living with HIV, lifelong treatment is required and there is no cure. HIV can integrate in the host genome and persist for the life span of the infected cell. These latently infected cells are not recognized as foreign because they are largely transcriptionally silent, but contain replication-competent virus that drives resurgence of the infection once ART is stopped. With a combination of immune activators, neutralizing antibodies, and therapeutic vaccines, some nonhuman primate models have been cured, providing optimism for these approaches now being evaluated in human clinical trials. In vivo delivery of gene-editing tools to either target the virus, boost immunity or protect cells from infection, also holds promise for future HIV cure strategies. In this Review, we discuss advances related to HIV cure in the last 5 years, highlight remaining knowledge gaps and identify priority areas for research for the next 5 years.
Similar content being viewed by others

Prevention, treatment and cure of HIV infection
CD8+ T cells in HIV control, cure and prevention
Therapeutic vaccination following early antiretroviral therapy elicits highly functional T cell responses against conserved HIV-1 regions
Modern antiretroviral regimens can effectively block HIV replication in people with HIV for decades, but these therapies are not curative and must be taken for life. However, there is evidence that a cure can be achieved; initially, this came from a single case study (Timothy Brown, a man living with HIV who became widely known as the ‘Berlin patient’) following bone-marrow transplantation from a donor who was naturally resistant to HIV 1 . On the basis of this inspiring development and the recognition that not everyone can access and/or adhere indefinitely to antiretroviral therapy (ART), a global consensus emerged approximately 10 years ago that a curative intervention was a high priority for people with HIV and would be necessary to bring an end to the HIV pandemic. Since then, there has been a second case report of a cure following bone-marrow transplantation 2 as well as evidence of persistence of only defective forms of the virus in certain patients 3 and enhanced immune control of the virus by others after only a short time on ART 4 —further supporting the notion that a cure for HIV can be achieved.
An HIV cure includes both remission and eradication. Here, we define the term remission as durable control of virus in the absence of any ongoing ART. Eradication is the complete removal of intact and rebound-competent virus. The minimal and optimal criteria for an acceptable target product profile for an HIV cure, including the duration and level of virus control off ART, has recently been developed and published by the International AIDS Society (IAS), following wide consultation with multiple stakeholders 5 .
In 2011 and 2016, the IAS convened expert working groups to outline a strategy for developing an effective and scalable cure 6 , 7 . Since then, significant progress has been made, and the overall agenda has evolved. Here, we assembled a group of experts from academia, industry, and the community (Box 1 ) to evaluate recent progress and to outline cure-related research priorities for the next 5 years. The key recommendations for each component of the strategy are summarized in Box 2 .
Box 1 The Global Cure Strategy—forming a consensus
The Global Cure Strategy was created using a full online process during the COVID-19 pandemic from November 2020 to August 2021. The co-chairs of the initiative identified the major topics which were divided into eight subthemes, each with its own working group, which included a chair, three scientific experts, at least one community member, an IAS Research-for-Cure fellow, and an industry representative. Working groups met at least twice virtually to generate a summary of key advances and recommendations for the next five years. The steering committee consisted of the chairs of each working group, the co-chairs of the cure strategy and a community expert, selected for diversity in geographic background, gender, age, and expertise. We engaged people living with HIV at all levels as well as a wide range of scientific and nonscientific stakeholders.
The Global Cure Strategy was further refined through a broad, online stakeholder consultation, including an online survey, a review by key stakeholders in the field, and interviews with select experts and opinion leaders (more than 25 respondents). The survey received 162 responses, primarily from people working in academia, nongovernmental organizations, and hospitals or research institutions; 11% of respondents were from organizations of people living with HIV, and 4% were from industry. The majority of respondents were working in Africa, followed by Western and Central Europe, North America, and Central and South America. The summary and detailed responses can be found here: https://www.surveymonkey.com/results/SM-7YYFTZ599/.
Box 2 Key research goals to be addressed in the next 5 years
Understanding hiv reservoirs.
Define and characterize the sources of the replication- and rebound-competent viruses during ART
Define the phenotype of cells harboring intact HIV genomes
Define the clinical significance of defective yet inducible proviruses
Define the mechanisms of clonal proliferation
Determine if infected cells that persist on ART are resistant to cell death
Define the impact of sex and other factors on the reservoir and virus-specific therapies
HIV reservoir measurement
Develop and validate a high-throughput assay to quantify the rebound-competent reservoir
Develop assays that quantify integration sites
Develop assays that account for key qualitative differences in viral transcripts
Develop methods to quantify HIV protein expression in cells and tissues
Develop imaging modalities that quantify the size, distribution, and activity of the reservoir in tissues
Define the link between the cellular reservoirs, residual plasma viremia, and the rebounding virus
Develop assays for point-of-care and eventually at-home viral-load monitoring
Mechanisms of virus control
Identify the mechanisms that contribute to SIV/HIV control
Define the role of HIV-specific antibodies, B cells, and the innate immune response in virus elimination or control
Define the viral dynamics and biomarkers associated with post-treatment control
Optimize human organoid models, as well as mouse and nonhuman primate models, for cure- and remission-related studies
Targeting the provirus
Develop improved strategies to reverse latency
Develop strategies to permanently silence the provirus
Determine the impact of targeting the provirus at the time of initiation of ART
Define the role of viral subtype on the effectiveness of interventions that target the provirus
Targeting the immune system
Develop ‘reduce and control’ approaches
Develop immune modulators
Conduct clinical trials to determine whether combination immunotherapies will result in safe and durable HIV remission
Cell and gene therapy
Define the level of antigen expression needed to enable recognition of infected cells by immunotherapies
Develop gene-editing strategies that target the provirus
Develop strategies for sustained production in vivo of antiviral antibodies
Leverage advances in other biomedical fields to develop safer and more scalable approaches
Pediatric remission and cure
Characterize the establishment, persistence, and potential for preventing or reversing HIV latency in infants and children on ART
Develop assays to monitor and identify biomarkers to predict the efficacy of HIV-1 cure therapeutics
Test HIV immunotherapies and other strategies in infants and children
Social, behavioral, and ethical aspects of cure
Expand community/stakeholder engagement and capacity building
Develop HIV cure research with equity, representation, and scalability considerations
Establish standards for the safe conduct of clinical research
Integrate social, behavioral, and ethics research as part of HIV cure trials
Build capacity for basic discovery research and clinical trials in high-burden, resource-limited settings
A shared definition of the HIV reservoir is crucial for researchers, clinicians, and people living with HIV. Here, we use the term ‘HIV reservoir’ in the context of eradication or remission, as a representative term for all cells infected with replication-competent HIV in both the blood and different anatomical sites in individuals on ART—in other words, all potential sources of viral rebound in the context of a treatment interruption. Although the source of virus rebound is still not entirely understood, we now know that virus can persist in multiple forms, in multiple cells and in multiple sites.
Characterization of the complete HIV reservoir
HIV DNA can be detected in CD4 + T cells in blood and lymphoid tissue in nearly all people with HIV on ART. These viral genomes are mainly defective. Only a small proportion (less than 5%) appear to be intact and potentially replication-competent 8 . But the HIV reservoir goes beyond circulating CD4 + T cells; it also includes tissue-resident CD4 + T cells and cells of the monocyte/macrophage lineage, further complicating efforts to characterize and quantify it. In vitro, HIV preferentially integrates into transcriptionally active genes 9 ; however, in people with HIV on ART, many proviruses (defined as virus that is integrated into the host genome), including intact ones, have been identified in genomic regions that are silent (known as ‘gene deserts’), which limits or precludes their reactivation 3 .
Our initial conception of the HIV reservoir as a static viral archive has given way to a more dynamic view in which, over time on ART, certain within-host HIV variants are gradually eliminated while others persist through various mechanisms, including clonal expansion of infected cells 10 , 11 , 12 , 13 , 14 , 15 . Sporadic infection of new cells during ART has been reported 16 , although there has been no convincing demonstration that viral sequences evolve during effective ART 17 , suggesting that the degree of virus spread is minimal. The sources of viral rebound following cessation of ART are incompletely defined. Multiple factors can contribute to viral replication following ART, including anatomical and microanatomical locations, the infected cell type, cellular phenotype, the nature of the provirus, the antigen specificity of the infected cell, the potential for transcriptional activity given the specific integration site, and/or distribution of antiretroviral drugs within tissues (Fig. 1 ).
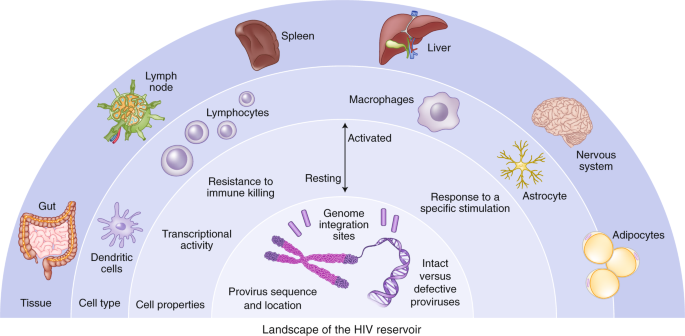
The HIV reservoir can be defined across a number of dimensions, including: (1) anatomical and microanatomical locations, (2) cell type (for example, CD4 + T cell or macrophage), (3) cell functional profile (activated or resting; resistance to killing), (4) pool of proviruses with a particular functional profile (for example, interferon-alpha resistant) or (5) triggering event (for example, response to stimulation with a particular antigen), and (6) integration-site features of the rebounding virus.
We recommend prioritizing efforts to understand integration sites of the virus during long-term ART and to understand the inducibility of a provirus on the basis of its chromosomal context. In addition, large prospective studies incorporating analytical treatment interruptions (ATIs) are still needed to probe clinically relevant sources of viral rebound and to identify a biomarker that predicts this. A favorable cure intervention could either prolong the time to the point when virus is detectable (that is, rebound) in plasma or reduce the viral ‘set point’ (that is, post-treatment control).
One of the most daunting obstacles to designing more effective methods to target persistent HIV infection is the lack of biomarkers to unambiguously identify the cells that harbor the rebound-competent reservoir. Recent work has demonstrated that the viral reservoir is preferentially enriched in cells that express programmed death-1 (PD-1) and other immune checkpoint markers, activation markers such as HLA-DR, and chemokine receptors such as CCR6 and CXCR3, but there is no phenotypic marker specific for the reservoir 18 , 19 , 20 , 21 . Specific biomarkers of the reservoir are needed, particularly to assess the impact of cure interventions. Furthermore, understanding how HIV persists in specific tissue sites and relevant local cell populations, such as those in the brain, gastrointestinal tract liver, or genital tract, will be important, given that the mechanism for persistence in each site may be distinct, and therefore different approaches may be required to eliminate each of these reservoirs.
There is growing evidence that some defective proviruses can produce transcripts and proteins (including novel viral RNAs and chimeric viral proteins) that in turn can elicit immune responses and perhaps contribute to chronic inflammation 22 , 23 , 24 , 25 . This may be of high relevance to end organ complications, such as HIV-associated neurological disease 26 . If the production of RNA and proteins from these defective proviruses proves to have clinical relevance, then their removal may be necessary to ensure long-term health.
A major mechanism of HIV persistence is the proliferation of cells that were infected prior to ART, resulting in large clonal populations of infected cells that arise as a result of the site of HIV integration 27 , 28 , response to antigen 29 , 30 , or homeostatic drivers 31 . Characterization of these presumably physiological expansions might lead to the development of therapies aimed at interrupting proliferation of infected cells. It will be important to determine to what degree these expanded clones are transcriptionally active, whether they are an important of post-ART viral rebound, and whether they have some innate survival advantage that prevents the cells from being effectively cleared by the host.
Recent studies have provided some evidence for preferential survival of infected cells with proliferative advantages or with deeper viral latency. Prosurvival and immune-resistance profiles may be particularly important in infected cells that persist despite expression of viral RNA or proteins 32 , 33 , 34 . Opportunities likely exist for collaboration and cross-fertilization of concepts with the cancer field, where the clonal dynamics of tumors have been extensively studied in relation to prosurvival and immune-resistance advantages, such as the work being done on lung cancer through prospective genetic studies in TRACRx ( https://clinicaltrials.gov/ct2/show/NCT01888601 ).
Biological sex can influence HIV pathogenesis, the immune response to HIV infection, and response to antiviral therapy 35 . Furthermore, in some but not all studies, women’s reservoirs have been shown to be less transcriptionally active and less inducible than those of men 36 , 37 , 38 , 39 , 40 . Sex, therefore, is a critical variable that should be considered as new therapies to target the reservoir are developed.
Quantification of the HIV reservoir
Significant progress toward a cure for HIV depends on having sensitive, specific, and quantitative measures of persistent virus that can be applied to various anatomical compartments 41 . Achieving this has been challenging, however, owing to the many sources and heterogeneous properties of persistent, replication-competent HIV. The reservoir can be quantified using assays that measure viral nucleic acid (total and integrated DNA, intact and defective DNA, or different forms of RNA), virus protein (p24), or viral inducibility (by measuring HIV RNA or virus replication following activation in vitro). Each approach has advantages and limitations, and assay outcomes may not always be interchangeable, comparable, or even correlated 8 .
Several groups have developed droplet digital PCR-based assays that discriminate genetically intact proviruses from a large background of defective proviruses, which are slightly less accurate but more high throughput than full genome sequencing 42 , 43 . The application of these assays to large clinical cohorts has demonstrated that there is a modest decrease in the frequency of cells with intact provirus over years on ART 44 , 45 , 46 . These assays have largely been optimized for subtype B virus, the major HIV subtype found in the United States and Europe. Yet there are over ten subtypes worldwide, some of which have evolved different mechanisms for immune evasion and persistence 47 . Pan-subtype-specific assays will need to be developed, and challenges related to cost and scalability remain. Research in this field should ideally culminate in harmonization across laboratories and crossvalidation of results. Future work will need to expand from quantification of virus in blood to quantification in tissue, particularly the more accessible tissues such as lymph nodes and gut mucosa.
Understanding the proviral landscape (defined as the degree of intactness, its transcriptional activity, and its location) is crucial, as these characteristics almost certainly influence the degree to which a provirus will rebound 48 . Over the last decade, several assays have been developed to analyze the exact location at which the virus integrates and whether the integrated virus is intact or defective. The ability to analyze single cells for integration site, viral sequence, and transcription is a major advance 48 ; however, these assays are expensive and low throughput. Technological advances are required to apply this more broadly to clinical samples, including assessment of interventions that target the reservoir.
Cell-associated viral RNA (CA-RNA) provides a measure of the total transcriptional activity of proviruses within a given sample. Several assays have recently been developed that quantify different RNA species, including total, elongated, unspliced, polyadenylated, and multi-spliced RNA, and these stand to give higher-resolution insights into the impact of therapeutic intervention 49 . An important unmet need is to develop approaches to distinguish transcripts arising from defective versus intact proviruses. Another shortcoming hampering broad use of RNA assays is the fact that they are subtype-sensitive. Overall, our ability to study the biology of transcriptionally active proviruses and the role of transcriptional activity as a potential biomarker needs to be further explored.
Since HIV protein expression is also required for recognition by HIV-specific T cells and other immune-based therapies, measuring and characterizing viral proteins in cells and tissues is an important step to understanding HIV persistence and might prove to be a critical determinant for the efficacy of therapies that target the HIV-infected cells directly (for example, chimeric antigen receptor (CAR) T cells or broadly neutralizing antibodies). Quantification of the p24 protein with ultrasensitive enzyme-linked immunosorbent assays can determine the efficacy of therapies that target the reservoir directly. Ultrasensitive p24 assays have emerged as useful tools 25 , but drawbacks include low levels of sensitivity compared with nucleic acid detection, overestimation of the replication-competent reservoir, and the requirement for specialized instrumentation 25 , 50 . Detection of viral envelope protein (the target of many therapeutic interventions for an HIV cure) also remains a challenge. Future strategies should leverage advances in single-cell techniques and new approaches to imaging tissue using super-resolution or expansion microscopy, together with multi-omics approaches.
Substantial progress in other fields of medicine has been made in using advanced imaging techniques to quantify rare diseased cells in tissues. On the basis of some preliminary success in nonhuman primate models 51 , efforts to use radiolabeled HIV-specific tracers and sensitive imaging modalities (for example, positron emission tomography, PET) have been initiated 52 . Similar efforts aimed at characterizing sites of inflammation or expression of specific surface markers that are associated with HIV persistence should also be a priority.
Several studies have attempted to identify sources of rebound virus by probing phylogenetic linkages with the proviral sequences present in various anatomical and cellular compartments. Success has been limited, however, in part owing to the challenging nature of obtaining full-length sequences from the limited number of infected cells in blood or tissue, as well as from plasma with low level viremia 53 . Strategies that can enhance enrichment of infected cells and/or depth of viral sequencing together with high-throughput low-cost single-cell analyses are likely to advance the field. As the RNA in circulating virions is a well-accepted surrogate marker for untreated HIV disease, this measurement could be an effective tool to characterize the rebound-competent population of HIV-infected cells.
Currently, any impact of a therapeutic intervention on the viral reservoir can only be determined with an ATI. A tool for very early detection of viral rebound post-ART using a nonvirological marker—such as measures of the innate immune response 54 —could be very valuable. In addition, better ways to monitor viral load that do not require frequent healthcare appointments will be needed 5 . This should include the development of home-based tests that may not necessarily require high sensitivity as long as testing is performed frequently 55 . Finally, emerging evidence suggests that virus replication during an ATI may be associated with some long-term adverse events 56 , so careful follow up of participants in ATI studies will be necessary.
Mechanisms and models of virus control
Natural control in people living with hiv.
Individuals who naturally control HIV in the absence of any therapy and can maintain a viral load of <50 copies/ml (known as ‘elite’ controllers) have been the focus of intense investigation for years. Research in this area is increasingly focused on those controllers who exhibit remarkably stringent control (‘exceptional’ controllers) 57 , 58 , some of whom might be considered true cures 48 , 59 , and those who became controllers after ART interruption (post-treatment controllers) 60 , 61 . In exceptional controllers, the frequency of infected cells is extremely low, often below the limit of detection of most standard assays for HIV DNA 57 , 59 , there is no intact virus 48 and the site of HIV integration may be distinct 48 ; an agreed definition for an exceptional controller is needed.
Virus-specific CD8 + T cells targeting particularly vulnerable or conserved epitopes are generally recognized as the key mediator of elite control; such cells are rare in post-treatment controllers and have not yet been characterized in exceptional controllers 4 , 62 . Further characterization of the various controller phenotypes (elite, exceptional, post-treatment) should remain a priority; the identification of unique and potentially informative phenotypes should also be pursued, including individuals on ART who have very small reservoirs 62 . Functional multi-omics studies and emerging single-cell technologies should help to determine the mechanisms involved in exceptional, elite, and post-treatment control. Better animal models of exceptional and post-treatment control would greatly enhance the field, giving access to tissue and the opportunity for longitudinal assessment of virus control 63 .
Virus elimination and control will likely require a coordinated immune response involving more than just T cells. Recent data suggest that autologous antibodies targeting archived viruses as well as interferon sensitivity might influence which virus populations emerge post-ART 54 , 64 , 65 . Studies in simian immunodeficiency virus (SIV)-infected nonhuman primates that naturally control infection have provided indirect evidence that natural killer (NK) cells might be able to effectively control virus in tissues 66 . Better insights into the role of antibodies, natural killer cells, and innate immunity in post-treatment and/or post-intervention control are needed.
The interplay between the virus and immune system during acute infection or immediately after the interruption of ART is largely unknown, at least in humans. During acute infection, those destined to become controllers typically have an initial period of poorly controlled viremia 61 , 67 . For post-treatment controllers, virus control is often achieved more rapidly after cessation of ART than after primary infection 61 , 68 . We need to understand the viral dynamics associated with eventual post-ART control/remission, as this will inform how a treatment interruption should be conducted. It is likely that biomarkers other than the plasma HIV RNA level might allow for the development of safer and more cost-effective strategies for interrupting ART.
Animal models of control
The role of humanized mouse models in cure research is still evolving. Recent studies showing similar effects of latency-reversing strategies in mice and the less scalable nonhuman primate model are encouraging 69 , 70 . Given that access to nonhuman primates for cure studies will likely remain a barrier, ongoing optimization, standardization, and validation of mouse models should be prioritized.
An important discrepancy in translating cure-related findings from SIV-infected nonhuman primates to people with HIV lies in the duration of ART. Although effective ART regimens with integrase inhibitors have been optimized in nonhuman primates, high costs, and treatment-related toxicities necessitate relatively shorter study durations (less than 1–2 years of ART). One possible solution would be for primate research centers to maintain colonies of SIV-infected nonhuman primates receiving very-long-term ART to be directly assigned for studies.
There is ongoing debate about the most appropriate virus to be used in cure-related studies in nonhuman primates. Investigations utilizing broadly neutralizing antibodies or select vaccines directed against the HIV-1 envelope necessitate infection with a virus that expresses HIV envelope proteins (simian-human immunodeficiency virus, SHIV). However, SHIV infections with some strains are characterized by post-treatment control in the absence of any intervention 71 , while others can induce significant disease progression 72 . Therefore, the specific strain used can limit the generalizability of the model. Although SIV infection of nonhuman primates can cause more significant disease progression than HIV infection of people, early ART for SIV infection can limit rapid disease progression and is therefore a useful model for cure studies 73 . Developing immunotherapies that target the SIV envelope in addition to SHIV should also be pursued.
A major recent advance has been the development of genetically barcoded SIV mac239 strains 74 . Because the barcode ‘tags’ are easily quantified and also passed on to progeny virus, this model allows for tracking of clonal dynamics, providing more precise insights into how interventions affect seeding of the reservoir, viral reactivation during ART, or viral recrudescence after ART interruption.
Therapeutic interventions
Since the discovery that HIV can establish a latent infection with minimal HIV transcription, a range of approaches has emerged that specifically target latently infected cells. These include pharmacological modulation of epigenetic or signaling pathways involved in HIV transcription to reactivate latent HIV such that the cells can be targeted and eliminated (‘shock and kill’) or to permanently silence HIV transcription (‘block and lock’) 75 , 76 , 77 . Recent reports have demonstrated that HIV latency is heterogeneous and that latency reactivation is stochastic, implying that a combination of agents targeting various pathways controlling HIV transcription may be necessary to achieve either robust silencing or latency reversal 49 , 78 , 79 , 80 .
A clear limitation of the ‘shock and kill’ approach comes from the discovery that only a fraction of proviruses is intact and among these, only some are inducible by a potent stimulus such as T cell stimulation, let alone by far less potent latency-reversing agents (LRAs) 8 , 81 , 82 , 83 . Furthermore, cells containing reactivated latent HIV may also be relatively resistant to killing by cytotoxic T cells 84 . Complicating the situation even more, CD8 + T cells appear to suppress HIV transcription and can blunt the effect of LRAs 70 .
Although LRAs tested in humans can induce HIV RNA expression and virion production in vivo, they have failed to reduce the size of the reservoir, even when combined with immunotherapeutic strategies designed to enhance clearance of infected cells 85 , 86 , 87 , 88 , 89 , 90 . This could be due to poor antigen induction by LRAs or insufficient clearance of these targets by immunotherapies (Fig. 2 ). Furthermore, many of the tested LRAs have off-target effects. Newer approaches for delivery of LRAs to reduce toxicity, enhance potency, and improve targeting, potentially leveraging advances in nanomedicine, should be explored. Greater potency could potentially be achieved using LRAs in combination, however, care is needed in these clinical trials, given that unexpected toxicities can emerge—as was recently demonstrated in the evaluation of high-dose disulfiram and vorinostat 91 . Finally, LRAs will likely need to be partnered with therapies that enhance the clearance of cells expressing viral proteins, such as immune-enhancing strategies or proapoptotic drugs 92 .
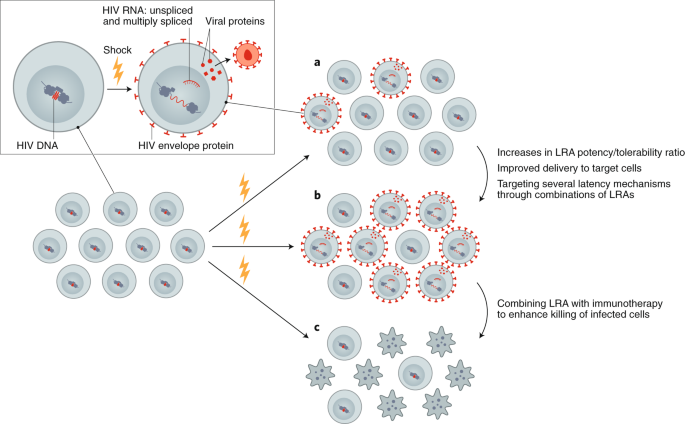
Reversing latency is an important component of revealing HIV-infected cells, allowing for conversion of a latently infected to a productively infected cell. a , Currently available LRAs reverse latency in only a subset of infected cells, and, when used alone, do not sufficiently eliminate these. b , Enhancing the efficacy of an LRA can be achieved with increased potency, targeted delivery or through using combinations of LRAs. c , Ultimately, depletion of the reservoir will require combining an LRA with other interventions, such as immunotherapy or a proapoptotic drug.
Permanently silencing the HIV promoter by suppressing factors that promote HIV transcription has also emerged as a strategy to target the provirus. The concept is to therapeutically drive HIV into a permanently silenced epigenetic state that resists reactivation (‘deep latency’). The Tat inhibitor didehydro-cortistatin A (dCA) blocks HIV reactivation from human CD4 + T cells in vitro through epigenetic repression; treatment with dCA in ART-suppressed humanized mouse latency models induces a measurable delay in virus rebound 76 , 93 . Gene therapy can also play an important part in permanent silencing of the provirus using short interfering RNA or other modalities 94 . Thus far, these approaches have yet to be successfully translated into human trials.
Further exploration of the therapeutic potential of permanently silencing the reservoir (‘block and lock’), presumably as part of a combinatorial cure approach, is a high research priority. Some pathways that might be targeted include mTOR, HSF1, and others 95 , 96 , 97 . Efforts to screen for drugs that suppress HIV transcription are encouraged, 96 , 98 , 99 with the goal to rapidly move into preclinical and clinical studies 100 , 101 .
With a recent report indicating that the HIV reservoir is stabilized at the start of ART initiation, efforts should be devised to inhibit this stabilizing effect and/or to enhance reservoir turnover during ART, where such interventions are ideally delivered at ART initiation 102 , 103 , 104 .
Most methods to target the provirus have been developed using subtype B. Thus, while conserved mechanisms govern latency across the different virus subtypes, differences at the level of the promoter may impact responsiveness to various stimuli. Therapies targeting the provirus should be evaluated across multiple HIV subtypes including recombinants.
There is a robust and growing toolbox of immune therapies that might be advanced to proof-of-concept testing. Arguably, the most impactful innovation to date is the isolation and development of broadly neutralizing antibodies for clinical use, but advances have also been made in the development of therapeutic vaccines, vaccine adjuvants, and other immunotherapies. When used in combination in nonhuman primates, these immune therapies have resulted in sustained post-ART control 71 , 105 . When used alone, most of these approaches have had limited effectiveness in people, although some promising results are emerging 88 , 106 , 107 . Combination clinical trials have recently started and are ongoing. Although the combination of either vorinostat or romidepsin (HDAC inhibitors that can increase viral transcription through epigenetic modification) together with different HIV vaccines showed no or minimal reduction in the HIV reservoir 107 , 108 , 109 , results from other studies including a combination of Toll-like receptor agonists, LRAs and broadly neutralizing antibodies are eagerly awaited ( NCT03837756 ; NCT04319367 ; NCT03041012 ).
As it may be challenging to reactivate and eliminate all latently infected cells, or to induce deep irreversible latency in all cells, it seems unlikely that these approaches will be curative by themselves. By reducing the reservoir, however, they might make strategies aimed at controlling the virus long term post-ART more effective. This overall approach of 'reduce and control’ is supported by observations in elite and post-treatment controllers, and theoretical modeling 110 . Multiple approaches that might result in control of a small reservoir are being developed. Assessment of therapeutic vaccines including live vector vaccines such as adenovirus 26, modified vaccine Ankara, and also a cytomegalovirus in nonhuman primate models have been particularly promising, with a subset of animals achieving eradication of virus 71 , 111 . Such studies have not yet been performed in people. Research to develop and test novel immunogen and vaccine designs with broad, potent and durable immunity should be prioritized. Given the recognition that autologous neutralizing antibodies might contribute to reservoir control 64 , novel vaccine approaches aimed at the induction of broadly neutralizing antibodies—including germline targeting 112 —should also be prioritized.
Immune stimulators, immunomodulators, and novel immunotherapies (such as cytokine formulations, Toll-like receptor agonists, immune checkpoint inhibitors or agonists, and novel vaccine adjuvants), used alone or more likely in combination with other approaches, hold promise but have undergone relatively limited testing in HIV-cure studies in people so far 106 , 113 , 114 , 115 .
With the exception of a few anecdotal cases 116 , immunotherapy in people with HIV has yet to recapitulate the promising advances made in nonhuman primates. Combination of various therapies will almost certainly be needed (Fig. 3 ). Conducting such studies is feasible 108 , 117 ; it is expected that initial clinical research will be intensive in nature and designed to identify strategies that might then be tested in well-powered, controlled clinical trials. Defining the mechanisms and potential biomarkers associated with remissions/cures in the preclinical and clinical setting should remain a priority. Determining which combinations to study, and how to define the optimal doses and strategies, poses a significant challenge from a methodological and regulatory perspective. As immunotherapies for HIV move into the clinic, careful attention will have to be paid to immune-related adverse events, including cytokine-release syndrome and autoimmunity.
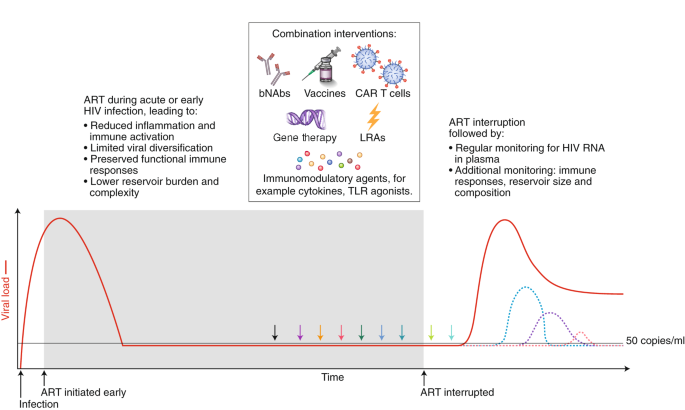
Strategies that will enhance immune-mediated clearance of latently infected cells include early initiation of ART and the administration of combined interventions at the time of suppressive ART (colored arrows) or during the treatment interruption phase, which will allow for increased antigen presentation. Given that there is no biomarker that can predict viral rebound, analytical treatment interruptions are used to determine whether the intervention has had a clinically meaningful impact. The overarching goal is to either delay viral rebound by at least months or years or reduce the set point of virus replication (that is, the stable level of viral load that the body settles at), preferably to a level of <200 copies/ml. The dashed colored lines represent different potential favorable outcomes from a cure intervention. bNAbs, broadly neutralizing antibodies; LRA, latency reversing agent; TLR, Toll-like receptor.
Cell and gene therapy clinical trials for people with HIV, although safe so far, have been small in scale and with no clear demonstrations of efficacy. The interest in gene therapy for an HIV cure was inspired by the elimination of intact virus in Timothy Brown (also known as the Berlin patient) and Adam Casteljo (also known as the London patient), who both received stem-cell transplants from a CCR5-negative donor 1 , 2 to treat their underlying malignancies. CCR5 is a co-receptor that is needed by most strains of HIV to enter a cell; a reduction in the size of the reservoir has also been reported following stem-cell transplantation to people with HIV from donors who are CCR5-positive 118 , 119 , but the HIV reservoir can’t be completely eliminated, irrespective of the CCR5 status of the donor. In the case of CCR5-negative stem-cell transplantation, the absence of CCR5 in the donor cells is thought to protect the newly transplanted cells from infection, at least with CCR5-dependent HIV strains. Interestingly, in both cases of cure following stem-cell transplantation of CCR5-negative cells, defective virus has been detected, but not intact or replication-competent virus 120 , 121 . These reports have prompted researchers to evaluate CCR5-targeted gene editing as a potentially safer path to cure in people living with HIV on ART, given the high mortality rate and significant morbidity associated with stem-cell transplantation. Timothy Brown unfortunately died in early 2020 owing to recurrence of his leukemia, but remained HIV-free until his death.
Ex vivo gene editing of CCR5 using zinc finger nucleases and re-infusion of CCR5-modified T cells has not yet prevented viral rebound following ATI 122 , 123 , possibly because insufficient cell numbers were engineered and/or engrafted with first-generation editing tools and cell culture protocols and/or because CCR5 disruption alone cannot shift the balance in favor of post-treatment control in the presence of persistently infected cells. More recently, gene therapies have shifted to creating effectors, including chimeric antigen receptor (CAR) T cells, which can recognize and eliminate HIV-infected cells (Fig. 4 ). Other approaches include the use of novel delivery systems to deliver genes to local tissues, resulting in the sustained production of systemically acting antivirals such as broadly neutralizing antibodies 124 , 125 and CD4 mimetics 126 . Finally, attempts are being made to directly target integrated proviruses with technologies such as CRISPR–Cas9 and recombinases 127 , 128 . This approach remains conceptually challenging in view of the disparate locations of latently infected cells, the absence of specific markers to target delivery, the heterogeneity of proviral sequences (the majority of which are defective), and the risk of off-target effects.
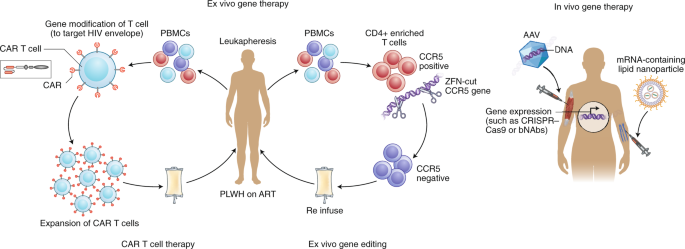
Examples of ex vivo (left) and in vivo (right) gene therapy approaches that have been tested in people with HIV on ART. Ex vivo strategies include gene editing to either delete or inactivate CCR5 or HIV provirus in CD4 + -enriched T cells using gene-editing tools such as zinc finger nucelases (ZFN) or CRISPR–Cas9. Alternatively, autologous T cells can be modified to express a CAR that can recognize HIV envelope, and this can then be reinfused into the participant. In vivo strategies, on the other hand, do not require external manipulation of cells; nanoparticles or viral vectors (such as adeno-associated virus (AAV)), which encapsulate mRNA or DNA, respectively, for the relevant gene to be expressed are administered directly to the patient. These approaches have recently been successful using lipid nanoparticles that contain mRNA encoding CRISPR–Cas9 135 or for expression of anti-HIV broadly neutralizing antibodies such as PG9 or VRC07 (ref. 125 ). PBMCs, peripheral blood mononuclear cells; PLWH, person living with HIV.
Many emerging cell and gene therapies are designed to target viral proteins/epitopes that are expressed in abundance on the surface of tumor cells, for example CD19 for the treatment of lymphoma 129 . Various forms of the HIV viral envelope protein (gp120 trimers and monomers, gp41) are expressed on the surface of infected cells, while multiple peptides are presented via HLA molecules. These antigens are expressed at levels well below that of many cancer antigens now being successfully targeted in the clinic. Cell-based therapies such as CAR T cells, once infused into the patient, will only persist and differentiate if there is sufficient antigenic exposure; however, the levels of antigen during ART may be too low 130 . Removal of ART after infusion of CAR T cells (or similar products) could be used to expand these cells in vivo, or more potent latency-reversing agents could be used to enhance envelope protein expression. In addition, novel adjuvants could expand CAR T cells even when the antigen burden is low, as was recently demonstrated in the nonhuman primate model 131 .
The challenges here are primarily those of delivery to relevant cells. In addition to developing methods to target specific cells, which are common problems faced by all potential in vivo gene therapies, targeting latent proviruses also presents the problem of a lack of robust cell surface markers to identify cells harboring such proviruses. Progress in both of these areas will be needed to develop strategies to deliver gene-editing reagents to latently infected cells. Some promising in vivo delivery strategies for CRISPR–Cas9 have included adeno-associated virus to target the SIV virus in nonhuman primates on ART 127 , as well as using engineered CD4 + cell-homing messenger RNA (mRNA)-containing lipid nanoparticles in mouse models of HIV infection 132 .
Long-term in vivo secretion of antibodies or antibody-like molecules can be achieved following gene therapy vector delivery of antibody cassettes to tissues such as muscle and liver, where enhanced production of antibodies is needed, rather than specific delivery to infected CD4 + T cells. This can be achieved through direct intramuscular injection leading to uptake in the muscle or, alternatively, intravenous injection, which will allow for uptake in the liver. Ectopic expression of these antibodies in liver or muscle cells fails to recapitulate aspects of natural antibody production, such as responsiveness to antigen and ongoing somatic hypermutation. Therefore, editing the B cell Ig locus itself to express antibodies presents an alternative and attractive gene-editing strategy 133 , 134 .
Sustained production of these antivirals could result in sustained (perhaps lifelong) control of the virus. Many barriers to success exist. Antidrug antibodies that target and clear the vectors often form rapidly 125 , limiting the ability to deliver multiple doses. Advances in mRNA encapsulation within lipid nanoparticles may potentially revolutionize delivery of gene therapy, allowing for delivery of mRNA encoding CRISPR–Cas9 and related guide RNAs in vivo, as has recently been successfully demonstrated in the treatment of transthyretin amyloidosis 135 . Also, antibodies targeting multiple antigens will likely need to be produced at high levels to prevent virus replication and escape.
Advances in T cell manufacturing are expected, driven by cancer CAR T cell therapies, which will also benefit HIV therapies. Similarly, advances occurring in gene therapy treatments for genetic diseases, such as hemoglobinopathies, are catalyzing safer and nongenotoxic conditioning for HSPC transplants, for example based on drug–antibody conjugates. Practicality will also be enhanced by moving toward using allogeneic off-the-shelf products.
Gene and cell therapies now require a shift towards a practical focus, identifying ways to expand use, reduce costs, and allow deployment in resource-limited settings. This could be achieved through abbreviated ex vivo cell manufacturing, including automated closed-system devices (‘gene therapy in a box’), to produce product in a place-of-care setting 136 . While still in the early stages of development, in vivo gene therapy also presents exciting possibilities to significantly expand access by eliminating the need for external manipulation of cells and associated technological requirements.
The unique context of perinatal HIV infection necessitates pediatric-specific strategies to achieve ART-free remission in children. The case of the Mississippi child, who started therapy ~30 hours after birth and achieved remission off ART for 27 months before virus rebounded 137 , 138 , raised the possibility that remission for children can be attained. Subsequent reports of early-treated pediatric cases with long-term (>12 years) virological control off ART have provided examples of post-treatment control in children 139 , 140 .
The nature of the reservoir in children is unique from that in adults. For example, naive CD4 + T cells are a more important reservoir for the virus in children 141 , 142 . Further development of infant nonhuman primate models for evaluating ART and cure strategies will contribute to our understanding of the HIV reservoir and how to target it in the unique setting of infancy and immune development, but an understanding of the limitations of this model is also crucially important 141 , 142 , 143 , 144 , 145 .
Many of the recent advances in understanding HIV persistence during ART in adults, including frequency and transcriptional activity of intact virus, clonal expansion, sites of proviral integration, and inducibility, need to be applied to studies of children. Optimizing methods that can be adapted to small blood volumes are also needed.
In the context of childhood infection, clarity is needed on how latency is established in naive T cells, susceptibility of these cells to latency reversal, propensity for T cells to clonally expand, and the relative contribution of clonally expanded cells to viral rebound following cessation of ART. It is still unclear whether integration sites and reactivation potential are different in children, and whether these change with age. Given that initial studies suggest a less-inducible reservoir in cases of perinatal infection 146 , it is especially important to determine how to maximize latency reversal in children. The optimal timing of these interventions (for example, at the time of early ART initiation) could potentially limit the pool of infected cells that persist on ART; such approaches can be explored in a nonhuman primate model.
As in adults, better tools are needed to assess the impact of cure interventions in children, including quantification of HIV persistence and in-depth cellular immune profiling. There is a particular need for noninvasive tools, such as total body imaging, to assess central nervous system and other tissue-based reservoirs. It will also be important to identify biomarkers for post-treatment control, including the degree of reduction or alteration in the composition of the latent reservoir that may be predictive of pediatric remission or cure 147 . Finally, preclinical studies in infant nonhuman primates that test new interventions to reduce or eliminate persistent HIV and/or induce viral remission after ART interruption are needed to inform the development of HIV remission and cure intervention strategies. Early therapy alone is insufficient to reliably achieve a cure or long-term remission in children. Novel approaches, including earlier administration and use of more potent antiretroviral drugs, therapeutic vaccines, or other immunotherapeutics, such as broadly neutralizing antibodies and/or innate-immune-enhancing agents, will be necessary.
Research directed toward an HIV cure intertwines critical social, behavioral, and ethical aspects that must be incorporated in the scientific agenda. This research takes place within particular social contexts and communities that shape its permissibility and appropriateness. Accordingly, affected communities must be meaningfully engaged throughout the research process; social and behavioral factors must be interrogated and taken into account because they affect research feasibility, community support for the research, and the well-being of participants and other stakeholders. Research must also address the many ethical issues associated with developing a therapy, particularly since viable options for treatment are already available. Sufficient funding for research toward social, behavioral, and ethical aspects of a cure and for community involvement is therefore essential.
Substantial progress using more conceptual and normative approaches has also been made regarding the ethical issues associated with the interruption of ART 148 , 149 . Similarly, there has been attention focused on acceptable risk thresholds for research 150 . Finally, given the important role of treatment as prevention, efforts have focused on the ethics of partner-protection measures 151 .
Community engagement in HIV cure research is still suboptimal in many settings, being mostly been limited to advisory boards typically comprised of scientifically literate individuals. Capacity to discuss HIV cure research and to evaluate its potential implications for local and global communities must be built within diverse community groups. Communities should be empowered and supported through education and engagement at all levels of the research process to help shape the HIV cure research agenda and allow for potential study participants to have a voice in trial design 152 .
Since HIV cure research is highly complex and nuanced, there is also a need to ensure understanding of it among other key stakeholders, including Institutional Review Boards (IRBs) and clinicians. For example, IRBs need to appreciate the implications of ATIs for partners who they may not see as within their remit, and clinicians need to understand the rationale for ATIs in the research setting.
Attention must focus on broad representation (for example, age, race and ethnicity, gender and sexuality, geographic location, risk behaviors) in research. Diversity in participation is essential during the development of interventions aimed at complete HIV elimination or durable ART-free control. This necessitates research directed at understanding the reasons for under-representation of certain groups of people in HIV cure research. For example, cisgender and transgender women, as well as individuals of some racial and ethnic backgrounds, are less likely to participate in HIV-cure-focused clinical trials 153 . This highlights the need for more nuanced and theoretically engaged research to understand how gender, race, and other characteristics shape engagement with HIV cure research 154 . At the same time, legal and social considerations unique to each context must be identified and addressed. For example, local laws, stigma, and access to healthcare affect research involving the interruption of ART.
There is also a need to better define ethical considerations involved in the selection of populations of interest in which promising cure strategies will be tested. For example, should priority be given to testing new interventions in individuals who initiated treatment during acute infection over those who began treatment during chronic infection? What are the best means to identify and manage the ethical considerations in pediatric HIV cure research? In addition, what measures ought to be taken to ensure that recruitment is not skewed toward people with HIV in resource-limited settings? Further, ethical questions of equity and justice related to the distribution of safe and effective cure interventions must consider acceptability, scalability, and cost-effectiveness. The COVID-19 pandemic has raised unique considerations for research participants, staff, and communities 155 . Given the rapidly changing nature of the pandemic and the availability of COVID-19 vaccines and other treatments, there is a need to continually revise and assess the safety and feasibility of HIV cure research efforts.
During the study design phase, early engagement is needed in communities where research is being considered in order to determine the nature and acceptability of research-related risks. Similarly, stakeholder perceptions should be elicited to guide the development of target product profiles (the minimal and optimal characteristics of a new therapeutic intervention), as recently done for an HIV cure 5 . In especially complex clinical studies, formative research should be used to help develop a robust, informed consent process. Furthermore, nested social and behavioral research (basic, elemental, supportive, integrative) is needed to enhance understanding of the actual experiences of trial participants as well as of sexual partners of participants. These data will help provide a check on current practices, as well as provide a foundation for future efforts aimed at improving them.
Several of the key topics addressed in the previous sections are prerequisites for the development of successful cure strategies and interventions. To date, most HIV cure research has been restricted to high-income countries with relatively low HIV burden and has most often engaged men who have sex with men. HIV strains are genetically and biologically diverse, and host mechanisms of antiviral immunity required for durable control may differ by sex, geography, and ethnicity. Basic discovery research and clinical trials in resource-limited settings must be strengthened and will require enabling infrastructure development and capacity building.
In the next decade, we expect to see a greater understanding of HIV reservoirs, an increasing number of clinical trials and hopefully reports of individuals who achieved long-term remission with less intensive and more widely applicable strategies. On the basis of the current understanding and lessons from ART, it is likely that combinations of these approaches may be the first approach to be implemented. Inclusion of knowledge from fields such as oncology and COVID-19 could also greatly facilitate progress. Finally, open and responsible communication about trials and realistic expectations will remain important. Although safety is the highest priority, with increasing number of clinical trials, there is an increase in the possibility of adverse events which will need to be appropriately managed while allowing the field to advance.
This global scientific strategy, in combination with the recently developed target product profile 5 , will assist with guiding the field toward a widely applicable, acceptable, and affordable cure. The establishment of the HIV Cure Africa Acceleration Partnership 152 will hopefully enable broader engagement and facilitate rapid implementation of any successes into low- and middle-income settings. Fortunately, the resources for such work remain available, and the field is highly committed to making the long-term commitments necessary to develop an effective and scalable remission or cure strategy.
Hutter, G. et al. Long-term control of HIV by CCR5 ∆32/∆32 stem-cell transplantation. N. Engl. J. Med. 360 , 692–698 (2009).
Article PubMed Google Scholar
Gupta, R. K. et al. HIV-1 remission following CCR5∆32/∆32 haematopoietic stem-cell transplantation. Nature 568 , 244–248 (2019).
Article CAS PubMed PubMed Central Google Scholar
Jiang, C. et al. Distinct viral reservoirs in individuals with spontaneous control of HIV-1. Nature 585 , 261–267 (2020).
Saez-Cirion, A. et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 9 , e1003211 (2013).
Lewin, S. R. et al. Multi-stakeholder consensus on a target product profile for an HIV cure. Lancet HIV 8 , e42–e50 (2021).
International, A. S. S. W. G. O. H. I. V. C. et al. Towards an HIV cure: a global scientific strategy. Nat. Rev. Immunol. 12 , 607–614 (2012).
Article CAS Google Scholar
Deeks, S. G. et al. International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat. Med. 22 , 839–850 (2016).
Eriksson, S. et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 9 , e1003174 (2013).
Schaller, T. et al. HIV-1 capsid–cyclophilin interactions determine nuclear import pathway, integration targeting and replication efficiency. PLoS Pathog. 7 , e1002439 (2011).
Cohn, L. B. et al. HIV-1 integration landscape during latent and active infection. Cell 160 , 420–432 (2015).
Lee, G. Q. et al. Clonal expansion of genome-intact HIV-1 in functionally polarized Th1 CD4 + T cells. J. Clin. Investig. 127 , 2689–2696 (2017).
Article PubMed PubMed Central Google Scholar
Simonetti, F. R. et al. Clonally expanded CD4 + T cells can produce infectious HIV-1 in vivo. Proc. Natl Acad. Sci. USA 113 , 1883–1888 (2016).
Lorenzi, J. C. et al. Paired quantitative and qualitative assessment of the replication-competent HIV-1 reservoir and comparison with integrated proviral DNA. Proc. Natl Acad. Sci. USA 113 , E7908–E7916 (2016).
Bui, J. K. et al. Proviruses with identical sequences comprise a large fraction of the replication-competent HIV reservoir. PLoS Pathog. 13 , e1006283 (2017).
Article PubMed PubMed Central CAS Google Scholar
Halvas, E. K. et al. HIV-1 viremia not suppressible by antiretroviral therapy can originate from large T cell clones producing infectious virus. J. Clin. Invest. 130 , 5847–5857 (2020).
Fletcher, C. V. et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc. Natl Acad. Sci. USA 111 , 2307–2312 (2014).
McManus, W. R. et al. HIV-1 in lymph nodes is maintained by cellular proliferation during antiretroviral therapy. J. Clin. Invest. 129 , 4629–4642 (2019).
Neidleman, J. et al. Phenotypic analysis of the unstimulated in vivo HIV CD4 T cell reservoir. eLife 9 , e60933 (2020).
Gosselin, A. et al. HIV persists in CCR6 + CD4 + T cells from colon and blood during antiretroviral therapy. AIDS 31 , 35–48 (2017).
Article CAS PubMed Google Scholar
Fromentin, R. et al. CD4 + T cells expressing PD-1, TIGIT and LAG-3 contribute to HIV persistence during ART. PLoS Pathog. 12 , e1005761 (2016).
Anderson, J. L. et al. Human immunodeficiency virus (HIV)-Infected CCR6 + rectal CD4 + T cells and HIV persistence on antiretroviral therapy. J. Infect. Dis. 221 , 744–755 (2020).
Imamichi, H. et al. Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc. Natl Acad. Sci. USA 113 , 8783–8788 (2016).
Imamichi, H. et al. Defective HIV-1 proviruses produce viral proteins. Proc. Natl Acad. Sci. USA 117 , 3704–3710 (2020).
Pollack, R. A. et al. Defective HIV-1 proviruses are expressed and can be recognized by cytotoxic T lymphocytes, which shape the proviral landscape. Cell Host Microbe 21 , 494–506(2017).
Wu, G. et al. Gag p24 is a marker of HIV expression in tissues and correlates with immune response. J. Infect. Dis. 224 , 1593–1598 (2021).
Article PubMed CAS PubMed Central Google Scholar
Spudich, S. et al. Persistent HIV-infected cells in cerebrospinal fluid are associated with poorer neurocognitive performance. J. Clin. Invest. 129 , 3339–3346 (2019).
Maldarelli, F. et al. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 345 , 179–183 (2014).
Wagner, T. A. et al. HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 345 , 570–573 (2014).
Mendoza, P. et al. Antigen-responsive CD4 + T cell clones contribute to the HIV-1 latent reservoir. J. Exp. Med. 217 , e20200051 (2020).
Simonetti, F. R. et al. Antigen-driven clonal selection shapes the persistence of HIV-1-infected CD4 + T cells in vivo. J. Clin. Invest. 131 , 145254 (2021).
Chomont, N. et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 15 , 893–900 (2009).
Ren, Y. et al. BCL-2 antagonism sensitizes cytotoxic T cell-resistant HIV reservoirs to elimination ex vivo. J. Clin. Investig. 130 , 2542–2559 (2020).
Kuo, H. H. et al. Anti-apoptotic protein BIRC5 maintains survival of HIV-1-infected CD4 + T cells. Immunity 48 , 1183–1194(2018).
Cummins, N. W. et al. Maintenance of the HIV reservoir is antagonized by selective BCL2 inhibition. J. Virol. 91 , e00012-17 (2017).
Scully, E. P. Sex differences in HIV infection. Curr. HIV/AIDS Rep. 15 , 136–146 (2018).
Das, B. et al. Estrogen receptor-1 is a key regulator of HIV-1 latency that imparts gender-specific restrictions on the latent reservoir. Proc. Natl Acad. Sci. USA 115 , E7795–E7804 (2018).
Prodger, J. L. et al. Reduced HIV-1 latent reservoir outgrowth and distinct immune correlates among women in Rakai, Uganda. JCI Insight 5 , e139287 (2020).
Falcinelli, S. D. et al. Impact of biological sex on immune activation and frequency of the latent HIV reservoir during suppressive antiretroviral therapy. J. Infect. Dis. 222 , 1843–1852 (2020).
Scully, E. P. et al. Sex-based differences in human immunodeficiency virus type 1 reservoir activity and residual immune activation. J. Infect. Dis. 219 , 1084–1094 (2019).
Szotek, E. L., Narasipura, S. D. & Al-Harthi, L. 17β-Estradiol inhibits HIV-1 by inducing a complex formation between β-catenin and estrogen receptor alpha on the HIV promoter to suppress HIV transcription. Virology 443 , 375–383 (2013).
Abdel-Mohsen, M. et al. Recommendations for measuring HIV reservoir size in cure-directed clinical trials. Nat. Med. 26 , 1339–1350 (2020).
Gaebler, C. et al. Sequence evaluation and comparative analysis of novel assays for intact proviral HIV-1 DNA. J. Virol. 95 , e01986-20 (2021).
Bruner, K. M. et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat. Med. 22 , 1043–1049 (2016).
Peluso, M. J. et al. Differential decay of intact and defective proviral DNA in HIV-1-infected individuals on suppressive antiretroviral therapy. JCI Insight 5 , 132997 (2020).
Gandhi, R. T. et al. Selective decay of intact HIV-1 proviral DNA on antiretroviral therapy. J. Infect. Dis. 223 , 225–233 (2020).
Anderson, E. M. et al. Dynamic shifts in the HIV proviral landscape during long term combination antiretroviral therapy: implications for persistence and control of HIV infections. Viruses 12 , E136 (2020).
Article PubMed CAS Google Scholar
Sarabia, I. & Bosque, A. HIV-1 latency and latency reversal: does subtype matter? Viruses 11 , 1104 (2019).
Yukl, S. A. et al. HIV latency in isolated patient CD4 + T cells may be due to blocks in HIV transcriptional elongation, completion, and splicing. Sci. Transl. Med. 10 , eaap9927 (2018).
Pardons, M. et al. Single-cell characterization and quantification of translation-competent viral reservoirs in treated and untreated HIV infection. PLoS Pathog. 15 , e1007619 (2019).
Santangelo, P. J. et al. Whole-body immunoPET reveals active SIV dynamics in viremic and antiretroviral therapy-treated macaques. Nat. Methods 12 , 427–432 (2015).
McMahon, J. H. et al. A clinical trial of non-invasive imaging with an anti-HIV antibody labelled with copper-64 in people living with HIV and uninfected controls. EBioMedicine 65 , 103252 (2021).
De Scheerder, M. A. et al. HIV rebound is predominantly fueled by genetically identical viral expansions from diverse reservoirs. Cell Host Microbe 26 , 347–358(2019).
Mitchell, J. L. et al. Plasmacytoid dendritic cells sense HIV replication before detectable viremia following treatment interruption. J. Clin. Invest. 130 , 2845–2858 (2020).
Draz, M. S. et al. DNA engineered micromotors powered by metal nanoparticles for motion based cellphone diagnostics. Nat. Commun. 9 , 4282 (2018).
Richart, V. et al. High rate of long-term clinical events after ART resumption in HIV-positive patients exposed to antiretroviral therapy interruption. AIDS https://doi.org/10.1097/QAD.0000000000003058 (2021).
Mendoza, D. et al. Comprehensive analysis of unique cases with extraordinary control over HIV replication. Blood 119 , 4645–4655 (2012).
Canoui, E. et al. A subset of extreme human immunodeficiency virus (HIV) controllers is characterized by a small HIV blood reservoir and a weak T-cell activation level. Open Forum Infect. Dis. 4 , ofx064 (2017).
Casado, C. et al. Permanent control of HIV-1 pathogenesis in exceptional elite controllers: a model of spontaneous cure. Sci. Rep. 10 , 1902 (2020).
Namazi, G. et al. The Control of HIV After Antiretroviral Medication Pause (CHAMP) Study: posttreatment controllers identified from 14 clinical studies. J. Infect. Dis. 218 , 1954–1963 (2018).
Galvez, C. et al. Extremely low viral reservoir in treated chronically HIV-1-infected individuals. EBioMedicine 57 , 102830 (2020).
Passaes, C. et al. Optimal maturation of the SIV-specific CD8 + T cell response after primary infection is associated with natural control of SIV: ANRS SIC Study. Cell Rep. 32 , 108174 (2020).
Bertagnolli, L. N. et al. Autologous IgG antibodies block outgrowth of a substantial but variable fraction of viruses in the latent reservoir for HIV-1. Proc. Natl Acad. Sci. USA 117 , 32066–32077 (2020).
Gondim, M. V. P. et al. Heightened resistance to host type 1 interferons characterizes HIV-1 at transmission and after antiretroviral therapy interruption. Sci. Transl. Med. 13 , eabd8179 (2021).
Huot, N. et al. Natural killer cells migrate into and control simian immunodeficiency virus replication in lymph node follicles in African green monkeys. Nat. Med. 23 , 1277–1286 (2017).
Madec, Y. et al. Natural history of HIV-control since seroconversion. AIDS 27 , 2451–2460 (2013).
Chun, T. W. et al. Effect of antiretroviral therapy on HIV reservoirs in elite controllers. J. Infect. Dis. 208 , 1443–1447 (2013).
Nixon, C. C. et al. Systemic HIV and SIV latency reversal via non-canonical NF-κB signalling in vivo. Nature 578 , 160–165 (2020).
McBrien, J. B. et al. Robust and persistent reactivation of SIV and HIV by N-803 and depletion of CD8 + cells. Nature 578 , 154–159 (2020).
Borducchi, E. N. et al. Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature 540 , 284–287 (2016).
Gautam, R. et al. Pathogenicity and mucosal transmissibility of the R5-tropic simian/human immunodeficiency virus SHIV(AD8) in rhesus macaques: implications for use in vaccine studies. J. Virol. 86 , 8516–8526 (2012).
Okoye, A. A. et al. Early antiretroviral therapy limits SIV reservoir establishment to delay or prevent post-treatment viral rebound. Nat. Med. 24 , 1430–1440 (2018).
Khanal, S. et al. In vivo validation of the viral barcoding of simian immunodeficiency virus SIV mac239 and the development of new barcoded SIV and subtype B and C simian–human immunodeficiency viruses. J. Virol. 94 , e01420-19 (2019).
Elsheikh, M. M., Tang, Y., Li, D. & Jiang, G. Deep latency: a new insight into a functional HIV cure. EBioMedicine 45 , 624–629 (2019).
Kessing, C. F. et al. In vivo suppression of HIV rebound by didehydro-cortistatin A, a ‘block-and-lock’ strategy for HIV-1 treatment. Cell Rep. 21 , 600–611 (2017).
Margolis, D. M. et al. Curing HIV: seeking to target and clear persistent infection. Cell 181 , 189–206 (2020).
Hosmane, N. N. et al. Proliferation of latently infected CD4 + T cells carrying replication-competent HIV-1: potential role in latent reservoir dynamics. J. Exp. Med. 214 , 959–972 (2017).
Kok, Y. L. et al. Spontaneous reactivation of latent HIV-1 promoters is linked to the cell cycle as revealed by a genetic-insulators-containing dual-fluorescence HIV-1-based vector. Sci. Rep. 8 , 10204 (2018).
Singh, A., Razooky, B., Cox, C. D., Simpson, M. L. & Weinberger, L. S. Transcriptional bursting from the HIV-1 promoter is a significant source of stochastic noise in HIV-1 gene expression. Biophys. J. 98 , L32–L34 (2010).
Cillo, A. R. et al. Quantification of HIV-1 latency reversal in resting CD4 + T cells from patients on suppressive antiretroviral therapy. Proc. Natl Acad. Sci. USA 111 , 7078–7083 (2014).
Laird, G. M. et al. Ex vivo analysis identifies effective HIV-1 latency-reversing drug combinations. J. Clin. Invest. 125 , 1901–1912 (2015).
Zerbato, J. M. et al. Multiply spliced HIV RNA is a predictive measure of virus production ex vivo and in vivo following reversal of HIV latency. EBioMedicine 65 , 103241 (2021).
Huang, S. H. et al. Latent HIV reservoirs exhibit inherent resistance to elimination by CD8 + T cells. J. Clin. Investig. 128 , 876–889 (2018).
Gay, C. L. et al. Assessing the impact of AGS-004, a dendritic cell-based immunotherapy, and vorinostat on persistent HIV-1 Infection. Sci. Rep. 10 , 5134 (2020).
Fidler, S. et al. Antiretroviral therapy alone versus antiretroviral therapy with a kick and kill approach, on measures of the HIV reservoir in participants with recent HIV infection (the RIVER trial): a phase 2, randomised trial. Lancet 395 , 888–898 (2020).
Gutiérrez, C. et al. Bryostatin-1 for latent virus reactivation in HIV-infected patients on antiretroviral therapy. AIDS 30 , 1385–1392 (2016).
Vibholm, L. et al. Short-course Toll-like receptor 9 agonist treatment impacts innate immunity and plasma viremia in individuals with human immunodeficiency virus infection. Clin. Infect. Dis. 64 , 1686–1695 (2017).
Riddler, S. A. et al. Vesatolimod, a toll-like receptor 7 agonist, induces immune activation in virally suppressed adults with HIV-1. Clin. Infect. Dis. 72 , e815–e824 (2020).
Elliott, J. H. et al. Short-term administration of disulfiram for reversal of latent HIV infection: a phase 2 dose-escalation study. Lancet HIV 2 , 17 (2015).
Article Google Scholar
McMahon, J. H. et al. Neurotoxicity with high dose disulfiram and vorinostat used for HIV latency reversal. AIDS https://doi.org/10.1097/qad.0000000000003091 (2021).
Kim, Y., Anderson, J. L. & Lewin, S. R. Getting the ‘kill’ into ‘shock and kill’: strategies to eliminate latent HIV. Cell Host Microbe 23 , 14–26 (2018).
Mousseau, G. et al. The tat inhibitor didehydro-cortistatin A prevents HIV-1 reactivation from latency. mBio 6 , e00465 (2015).
Ahlenstiel, C. et al. Novel RNA duplex locks HIV-1 in a latent state via chromatin-mediated transcriptional silencing. Mol. Ther. Nucleic Acids 4 , e261 (2015).
Besnard, E. et al. The mTOR complex controls HIV latency. Cell Host Microbe 20 , 785–797 (2016).
Mori, L. et al. The XPB subunit of the TFIIH complex plays a critical role in HIV-1 transcription and XPB inhibition by spironolactone prevents HIV-1 reactivation from latency. J Virol. 95 , e01247-20 (2020).
Timmons, A. et al. HSF1 inhibition attenuates HIV-1 latency reversal mediated by several candidate LRAs in vitro and ex vivo. Proc. Natl Acad. Sci. USA 117 , 15763–15771 (2020).
Yeh, Y. J. et al. Filgotinib suppresses HIV-1-driven gene transcription by inhibiting HIV-1 splicing and T cell activation. J. Clin. Invest. 130 , 4969–4984 (2020).
Gavegnano, C. et al. Ruxolitinib and tofacitinib are potent and selective inhibitors of HIV-1 replication and virus reactivation in vitro. Antimicrob. Agents Chemother. 58 , 1977–1986 (2014).
Marconi, V. C. et al. Randomized trial of ruxolitinib in antiretroviral-treated adults with HIV. Clin. Infect. Dis. ciab212 (2021).
Henrich, T. J. et al. Everolimus, an mTORC1/2 inhibitor, in ART-suppressed individuals who received solid organ transplantation: A prospective study. Am. J. Transplant 21 , 1765–1779 (2020).
Goonetilleke, N., Clutton, G., Swanstrom, R. & Joseph, S. B. Blocking formation of the stable HIV reservoir: a new perspective for HIV-1 cure. Front. Immunol. 10 , 1966 (2019).
Abrahams, M. R. et al. The replication-competent HIV-1 latent reservoir is primarily established near the time of therapy initiation. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aaw5589 (2019).
Brodin, J. et al. Establishment and stability of the latent HIV-1 DNA reservoir. eLlfe 5 , e18889 (2016).
Google Scholar
Borducchi, E. N. et al. Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature 563 , 360–364 (2018).
SenGupta, D. et al. The TLR7 agonist vesatolimod induced a modest delay in viral rebound in HIV controllers after cessation of antiretroviral therapy. Sci. Transl. Med. 13 , eabg3071 (2021).
Mothe, B. et al. HIVconsv vaccines and romidepsin in early-treated HIV-1-infected individuals: safety, immunogenicity and effect on the viral reservoir (Study BCN02). Front. Immunol. 11 , 823 (2020).
Leth, S. et al. Combined effect of Vacc-4x, recombinant human granulocyte macrophage colony-stimulating factor vaccination, and romidepsin on the HIV-1 reservoir (REDUC): a single-arm, phase 1B/2A trial. Lancet HIV 3 , e463–e472 (2016).
Conway, J. M. & Perelson, A. S. Post-treatment control of HIV infection. Proc. Natl Acad. Sci. USA 112 , 5467–5472 (2015).
Hansen, S. G. et al. Immune clearance of highly pathogenic SIV infection. Nature 502 , 100–104 (2013).
Steichen, J. M. et al. A generalized HIV vaccine design strategy for priming of broadly neutralizing antibody responses. Science 366 , eaax4380 (2019).
Rasmussen, T. et al. Impact of anti-PD-1 and anti-CTLA-4 on the HIV reservoir in people living with HIV with cancer on antiretroviral therapy: The AIDS Malignancy Consortium-095 study. Clin. Infect. Dis. 73 , e1973–e1981 (2020).
Riddler, S. A. et al. Vesatolimod, a toll-like Receptor 7 agonist, induces immune activation in virally suppressed adults living with human immunodeficiency virus-1. Clin. Infect. Dis. 72 , e815–e824 (2021).
Papasavvas, E. et al. Safety, immune, and antiviral effects of pegylated interferon alpha 2b administration in antiretroviral therapy-suppressed individuals: results of pilot clinical trial. AIDS Res Hum. Retroviruses 37 , 433–443 (2021).
Mendoza, P. et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 561 , 479–484 (2018).
Colby, D. J. et al. Safety and immunogenicity of Ad26 and MVA vaccines in acutely treated HIV and effect on viral rebound after antiretroviral therapy interruption. Nat. Med. 26 , 498–501 (2020).
Salgado, M. et al. Mechanisms that contribute to a profound reduction of the hiv-1 reservoir after allogeneic stem cell transplant. Ann. Intern. Med. 169 , 674–683 (2018).
Koelsch, K. K. et al. Impact of allogeneic hematopoietic stem cell transplantation on the HIV Reservoir and immune response in 3 hiv-infected individuals. J. Acquired Immune Defic. Syndromes 75 , 328–337 (2017).
Yukl, S. A. et al. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS Pathog. 9 , e1003347 (2013).
Gupta, R. K. et al. Evidence for HIV-1 cure after CCR5∆32/∆32 allogeneic haemopoietic stem-cell transplantation 30 months post analytical treatment interruption: a case report. Lancet HIV 7 , e340–e347 (2020).
Tebas, P. et al. CCR5-edited CD4 + T cells augment HIV-specific immunity to enable post-rebound control of HIV replication. J. Clin. Invest. 131 , e144486 (2021).
Article CAS PubMed Central Google Scholar
Tebas, P. et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N. Engl. J. Med. 370 , 901–910 (2014).
Martinez-Navio, J. M. et al. Adeno-associated virus delivery of anti-HIV monoclonal antibodies can drive long-term virologic suppression. Immunity 50 , 567–575(2019).
Priddy, F. H. et al. Adeno-associated virus vectored immunoprophylaxis to prevent HIV in healthy adults: a phase 1 randomised controlled trial. Lancet HIV 6 , e230–e239 (2019).
Gardner, M. R. et al. AAV-delivered eCD4-Ig protects rhesus macaques from high-dose SIV mac239 challenges. Sci. Transl. Med. 11 , eaau5409 (2019).
Mancuso, P. et al. CRISPR based editing of SIV proviral DNA in ART treated non-human primates. Nat. Commun. 11 , 6065 (2020).
Karpinski, J. et al. Directed evolution of a recombinase that excises the provirus of most HIV-1 primary isolates with high specificity. Nat. Biotechnol. 34 , 401–409 (2016).
Schuster, S. J. et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N. Engl. J. Med. 377 , 2545–2554 (2017).
Herzig, E. et al. Attacking Latent HIV with convertible CAR-T Cells, a highly adaptable killing platform. Cell 179 , 880–894 e810 (2019).
Rust, B. J. et al. Robust expansion of HIV CAR T cells following antigen boosting in ART-suppressed nonhuman primates. Blood 136 , 1722–1734 (2020).
Tombacz, I. et al. Highly efficient CD4 + T cell targeting and genetic recombination using engineered CD4 + cell-homing mRNA-LNPs. Mol. Ther. (2021).
Nahmad, A. D. et al. Engineered B cells expressing an anti-HIV antibody enable memory retention, isotype switching and clonal expansion. Nat. Commun. 11 , 5851 (2020).
Huang, D. et al. Vaccine elicitation of HIV broadly neutralizing antibodies from engineered B cells. Nat. Commun. 11 , 5850 (2020).
Gillmore, J. D. et al. CRISPR–Cas9 in vivo gene editing for transthyretin amyloidosis. N. Engl. J. Med. 385 , 493–502 (2021).
Adair, J. E. et al. Towards access for all: 1st Working Group Report for the Global Gene Therapy Initiative (GGTI). https://doi.org/10.1038/s41434-021-00284-4 (2021).
Persaud, D. et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N. Engl. J. Med. 369 , 1828–1835 (2013).
Luzuriaga, K. et al. Viremic relapse after HIV-1 remission in a perinatally infected child. N. Engl. J. Med. 372 , 786–788 (2015).
Frange, P. et al. HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet HIV 3 , e49–e54 (2016).
Violari, A. et al. A child with perinatal HIV infection and long-term sustained virological control following antiretroviral treatment cessation. Nat. Commun. 10 , 412 (2019).
Mavigner, M. et al. Simian immunodeficiency virus persistence in cellular and anatomic reservoirs in antiretroviral therapy-suppressed infant rhesus macaques. J. Virol. 92 , e00562-18 (2018).
Obregon-Perko, V. et al. Simian–human immunodeficiency virus SHIV.C.CH505 persistence in ART-suppressed infant macaques is characterized by elevated SHIV RNA in the gut and a high abundance of intact SHIV DNA in naive CD4 + T cells. J. Virol. 95 , e01669-20 (2020).
Bricker, K. M. et al. Therapeutic vaccination of SIV-infected, ART-treated infant rhesus macaques using Ad48/MVA in combination with TLR-7 stimulation. PLoS Pathog. 16 , e1008954 (2020).
Hessell, A. J. et al. Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques. Nat. Med. 22 , 362–368 (2016).
Shapiro, M. B. et al. Single-dose bNAb cocktail or abbreviated ART post-exposure regimens achieve tight SHIV control without adaptive immunity. Nat. Commun. 11 , 70 (2020).
Dhummakupt, A. et al. Differences in inducibility of the latent HIV reservoir in perinatal and adult infection. JCI Insight 5 , e134105 (2020).
Article PubMed Central Google Scholar
Hill, A. L. et al. Real-time predictions of reservoir size and rebound time during antiretroviral therapy interruption trials for HIV. PLoS Pathog. 12 , e1005535 (2016).
Julg, B. et al. Recommendations for analytical antiretroviral treatment interruptions in HIV research trials-report of a consensus meeting. Lancet HIV 6 , e259–e268 (2019).
Garner, S. A. et al. Interrupting antiretroviral treatment in HIV cure research: scientific and ethical considerations. J. Virus Erad. 3 , 82–84 (2017).
Protiere, C. et al. Differences in HIV cure clinical trial preferences of French people living with HIV and physicians in the ANRS-APSEC study: a discrete choice experiment. J. Int AIDS Soc. 23 , e25443 (2020).
Peluso, M. J. et al. A collaborative, multidisciplinary approach to HIV transmission risk mitigation during analytic treatment interruption. J. Virus Erad. 6 , 34–37 (2020).
Dybul, M. et al. The case for an HIV cure and how to get there. Lancet HIV 8 , e51–e58 (2021).
Johnston, R. E. & Heitzeg, M. M. Sex, age, race and intervention type in clinical studies of HIV cure: a systematic review. AIDS Res Hum. Retroviruses 31 , 85–97 (2015).
Dube, K. et al . Considerations for increasing racial, ethnic, gender, and sexual diversity in HIV cure-related research with analytical treatment interruptions: a qualitative inquiry. https://doi.org/10.1089/AID.2021.0023 (2021).
Fidler, S. et al. HIV cure research in the time of COVID-19 — antiretroviral therapy treatment interruption trials: a discussion paper. J. Virus Erad. 7 , 100025 (2021).
Download references
Acknowledgements
We acknowledge the generous contribution of all the participants in the working groups, the key opinion leaders who read and provided feedback on the strategy, participants in the online survey and secretarial support from the International AIDS Society. S.R.L. and S.G.D. are funded by National Institutes of Health Delaney AIDS Research Enterprise (DARE) Collaboratory (UM1AI126611 and UM1AI164560). S.R.L. is also funded by the National Health and Medical Research Council (NHMRC; grant number GNT1149990) of Australia and the Australian Centre for HIV and Hepatitis. R.B.J. is funded by the NIH UM1AI64565. C.T.T. is funded by the South African Research Chairs Initiative of the Department of Science and Innovation and National Research Foundation of South Africa (grant 84177). O.L. is funded by the ANRS, Sidaction, University Paris Saclay, Inserm, and CEA (Commissariat à l’Energie Atomique). P.C. is funded by the NIH (HL156247 and AI164561); N.A. is funded by the NIH Delaney CARE Collaboratory 1UM1AI126619 and from R01AI134363; T.N. is funded by the South African Research Chairs Initiative of the Department of Science and Innovation and National Research Foundation of South Africa (grant 64809), The Bill and Melinda Gates Foundation (INV-033558), the International AIDS Vaccine Initiative (UKZNRSA1001) and DFG German-African Network grant (grant number AL 1043/6-1).
Author information
Authors and affiliations.
University of California San Francisco, San Fransisco, CA, USA
Steven G. Deeks & Steven Deeks
UNC HIV Cure Center, Department of Medicine, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC, USA
- Nancie Archin
University of Southern California, Los Angeles, CA, USA
- Paula Cannon
HIV i-Base, London, UK
Simon Collins
Weill Cornell Medicine, Cornell University, New York, NY, USA
- R. Brad Jones
Aidsfonds, Amsterdam, the Netherlands
Marein A. W. P. de Jong & Marein de Jong
University Paris Saclay, AP-HP, Bicêtre Hospital, UMR1184 INSERM CEA, Le Kremlin Bicêtre, Paris, France
- Olivier Lambotte
International AIDS Society, Geneva, Switzerland
Rosanne Lamplough
Africa Health Research Institute and University of KwaZulu-Natal, Durban, South Africa
- Thumbi Ndung’u
University College London, London, UK
Ragon Institute of MGH, MIT and Harvard University, Cambridge, MA, USA
Thumbi Ndung’u & Krista Dong
Berman Institute of Bioethics and Department of Medicine, Johns Hopkins University, Baltimore, MD, USA
- Jeremy Sugarman
National Institute for Communicable Diseases and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
- Caroline T. Tiemessen
UZ Ghent, Ghent, Belgium
- Linos Vandekerckhove
Victorian Infectious Diseases Service, The Royal Melbourne Hospital at the Peter Doherty Institute for Infection and Immunity, Melbourne, Australia
Sharon R. Lewin
Department of Infectious Diseases, Alfred Hospital and Monash University, Melbourne, Australia
Department of Infectious Diseases, The University of Melbourne at the Peter Doherty Institute for Infection and Immunity, Melbourne, Australia
Sharon R. Lewin & Sharon Lewin
UKZN, Durban, South Africa
Zaza Ndhlovu
Centre de Recherche du CHUM and Université de Montréal, Montreal, Canada
Nicolas Chomont
BC Centre for Excellence in HIV/AIDS, Faculty of Health Sciences, Simon Fraser University, Vancouver, Canada
Zabrina Brumme
Sun Yat-sen University, Guangzhou, China
ViiV Healthcare, Branford, CT, USA
Luke Jasenosky
Treatment Action Group, New York, NY, USA
Richard Jefferys
Institut Pasteur, Université de Paris, Unité HIV, Inflammation et Persistance, Paris, France
Aurelio Orta-Resendiz
National Cancer Institute, Center for Cancer Research, Bethesda, MD, USA
Frank Mardarelli
UMC Utrecht, Utrecht, the Netherlands
Monique Nijhuis
Perelmann School of Medicine, University of Pennsylvania, Philadelphia, PA, USA
Katharine Bar & Pablo Tebas
Merck & Co., Inc., Department of Infectious Disease & Vaccines, Kenilworth, NJ, USA
Bonnie Howell
European AIDS treatment group (EATG), Zurich, Switzerland
Alex Schneider
1CONICET – Universidad de Buenos Aires. Instituto de Investigaciones, Biomédicas en Retrovirus y SIDA (INBIRS), Buenos Aires, Argentina
Gabriela Turk
Facultad de Medicina, Departamento de Microbiología, Parasitología e Inmunología, Buenos Aires, Argentina
Makerere University, Makerere, Uganda
Rose Nabatanzi
John Hopkins School of Medicine, Baltimore, MD, USA
Joel Blankson
ICATS, UNC School of Medicine, Chapel Hill, NC, USA
J. Victor Garcia
Emory University School of Medicine, Yerkes National Primate Research Center, Atlanta, GA, USA
Mirko Paiardini
ViiV Healthcare, London, UK
Jan van Lunzen
Chelsea and Westminster Hospital NHS Foundation Trust, London, UK
Christina Antoniadi
Laboratório de AIDS e Imunologia Molecular, Instituto Oswaldo Cruz, Rio de Janeiro, Brazil
Fernanda Heloise Côrtes
Scripps Research Institute, Jupiter, FL, USA
Susana Valente
Aarhus University Hospital, Aarhus, Denmark
Ole S. Søgaard
Universidade Federal de Sao Paulo, Sao Paulo, Brazil
Ricardo Sobhie Diaz
Gladstone Institute of Virology, University of California San Francisco, San Francisco, CA, USA
Melannie Ott
USAHIV Drug Discovery, ViiV Healthcare, Qura Therapeutics, and UNC HIV Cure Center, University of North Carolina at Chapel Hill, Research Triangle Park, NC, USA
Richard (Rick) Dunham
EATG, Berlin, Germany
Siegfried Schwarze
Queen’s University, Kingston, Ontario, Canada
Santiago Perez Patrigeon
MUJHU Care limited, Kampala, Uganda
Josephine Nabukenya
The Rockefeller University, New York, NY, USA
Marina Caskey
IrsiCaixa AIDS Research Institute, HUGTIP, Badalona, Barcelona, Spain
Beatriz Mothe
Chinese Academy of Sciences, National Clinical Research Center for Infectious Diseases, Division of Treatment and Care, National Center for AIDS/STD Control and Prevention, Beijing, China
Fu Sheng Wang
Imperial College London, Department of Infectious Disease, Faculty of Medicine, London, UK
Sarah Fidler
Gilead Sciences, Foster City, CA, USA
Devi SenGupta
European AIDS Treatment Group (EATG), Brussels, Belgium
Stephan Dressler
University of North Carolina Project Malawi, Lilongwe, Malawi
Mitch Matoga
Fred Hutchinson Cancer Research Center, Seattle, WA, USA
Hans-Peter Kiem
Joint Clinical Research Centre, Kampala, Uganda
Cissy Kityo
Caring Cross, Gaithersburg, MD, USA
Boro Dropulic
University of Washington, Seattle, WA, USA
Michael Louella
Advanced Medical and Dental Institute, Universiti Sains Malaysia, Pulau Pinang, Malaysia
Kumitaa Theva Das
Johns Hopkins University School of Medicine, Baltimore, MD, USA
Deborah Persaud
Emory University School of Medicine and Children’s Healthcare of Atlanta, Atlanta, GA, USA
Ann Chahroudi
University of Massachusetts, Worcester, MA, USA
Katherine Luzuriaga
Chulalongkorn University, Bangkok, Thailand
Thanyawee Puthanakit
ImmunityBio, Inc, Culver City, CA, USA
Jeffrey Safrit
Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana
Gaerolwe Masheto
UNC Gillings School of Global Public Health, Chapel Hill, NC, USA
Karine Dubé
La Trobe University, Melbourne, Australia
Jennifer Power
AVAC, New York, NY, USA
Jessica Salzwedel
VARG, Chiang Mai, Thailand
Udom Likhitwonnawut
UCSD AntiViral Research Center, Delaney AIDS Research Enterprise/UCSF, Palm Springs, CA, USA
Jeff Taylor
Social Policy, Gender Identity, and Sexual Orientation Studies Association (SPoD), University of Lucerne MSc Health Sciences, Istanbul, Turkey
Oguzhan Latif Nuh
Rakai Health Sciences Program, Rakai, Uganda
Edward Nelson Kankaka
You can also search for this author in PubMed Google Scholar
Core Leadership Group
- Steven Deeks
- , Sharon Lewin
- , Marein de Jong
- , Rosanne Lamplough
- & Simon Collins
Working Group 1 (Understanding HIV reservoirs)
- , Zaza Ndhlovu
- , Nicolas Chomont
- , Zabrina Brumme
- , Luke Jasenosky
- , Richard Jefferys
- & Aurelio Orta-Resendiz
Working Group 2 (HIV reservoir measurement)
- , Frank Mardarelli
- , Monique Nijhuis
- , Katharine Bar
- , Bonnie Howell
- , Alex Schneider
- , Gabriela Turk
- & Rose Nabatanzi
Working Group 3 (Mechanisms of virus control)
- , Joel Blankson
- , J. Victor Garcia
- , Mirko Paiardini
- , Jan van Lunzen
- , Christina Antoniadi
- & Fernanda Heloise Côrtes
Working Group 4 (Targeting the provirus)
- , Susana Valente
- , Ole S. Søgaard
- , Ricardo Sobhie Diaz
- , Melannie Ott
- , Richard (Rick) Dunham
- , Siegfried Schwarze
- , Santiago Perez Patrigeon
- & Josephine Nabukenya
Working Group 5 (Targeting the immune system)
- , Marina Caskey
- , Beatriz Mothe
- , Fu Sheng Wang
- , Sarah Fidler
- , Devi SenGupta
- , Stephan Dressler
- & Mitch Matoga
Working Group 6 (Cell and gene therapy)
- , Hans-Peter Kiem
- , Pablo Tebas
- , Cissy Kityo
- , Boro Dropulic
- , Michael Louella
- & Kumitaa Theva Das
Working Group 7 (Paediatric remission and cure)
- , Deborah Persaud
- , Ann Chahroudi
- , Katherine Luzuriaga
- , Thanyawee Puthanakit
- , Jeffrey Safrit
- & Gaerolwe Masheto
Working Group 8: (Social, behavioral and ethical aspects of cure)
- , Karine Dubé
- , Jennifer Power
- , Jessica Salzwedel
- , Udom Likhitwonnawut
- , Jeff Taylor
- , Oguzhan Latif Nuh
- , Krista Dong
- & Edward Nelson Kankaka
Contributions
S.G.D., S.R.L., M.D.J. and R.L. developed the method for generating the strategy and oversaw the governance and establishment of the working groups. All authors on the masthead were members of the steering group. All authors of the IAS Global Scientific Strategy writing group contributed to the writing and approved the submitted version of the manuscript. Members of the IAS Global Scientific Strategy working groups are identified in the list at the end of the manuscript.
Corresponding authors
Correspondence to Steven G. Deeks or Sharon R. Lewin .
Ethics declarations
Competing interests.
S.G.D. receives research support from Gilead and Merck. He is a member of the scientific advisory boards for BryoLogyx, Enochian Biosciences and Tendel. He has consulted for AbbVie, Biotron, Eli Lilly, GSK/ViiV and Immunocore; J.S. is a member of Merck KGaA’s Ethics Advisory Panel and Stem Cell Research Oversight Committee; a member of IQVIA’s Ethics Advisory Panel; a member of Aspen Neurosciences Clinical Advisory Panel; a member of a Merck Data Monitoring Committee; a consultant to Biogen; and a consultant to Portola Pharmaceuticals Inc. None of these activities are related to the issues discussed in this manuscript; T.N. has received research funding from Gilead Sciences; O.L. has been paid expert testimony and consultancy fees from BMS France, MSD, Astra Zeneca; consultancy fees from Incyte, Sobi, grants from ViiV and Gilead; L.V. receives research grants from J&J, ViiV Healthcare and Gilead Sciences; P.C. is a member of Gilead’s HIV Cure Advisory Board; S.R.L.’s institution receives funding for investigator initiated research from Gilead, Merck and Viiv. She has research collaborations with BMS, Abbvie and Merck. She has received honoraria paid to her for membership of advisory boards to Gilead, Merck, Viiv, Immunocore, Vaxxinity, Biotron, Esfam and Abivax; R.L. is an employee of the International AIDS Society; M.d.J. was paid as a consultant by the International AIDS Society. S.C., R.B.J., C.T. and N.A. have no interests to declare.
Additional information
Peer review information Nature Medicine thanks Ravindra Gupta and the other, anonymous, reviewers for their contribution to the peer review of this work. Karen O’Leary was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Deeks, S.G., Archin, N., Cannon, P. et al. Research priorities for an HIV cure: International AIDS Society Global Scientific Strategy 2021. Nat Med 27 , 2085–2098 (2021). https://doi.org/10.1038/s41591-021-01590-5
Download citation
Received : 12 September 2021
Accepted : 27 October 2021
Published : 01 December 2021
Issue Date : December 2021
DOI : https://doi.org/10.1038/s41591-021-01590-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Balancing public health and privacy rights: a mixed-methods study on disclosure obligations of people living with hiv to their partners in china.
- Zhizhuang Duan
Harm Reduction Journal (2024)
Ripretinib inhibits HIV-1 transcription through modulation of PI3K-AKT-mTOR
- Jin-feng Cai
- Jia-sheng Zhou
Acta Pharmacologica Sinica (2024)
- Raphael J. Landovitz
- Hyman Scott
- Steven G. Deeks
Nature Reviews Microbiology (2023)
Inhibition of the TRIM24 bromodomain reactivates latent HIV-1
- Riley M. Horvath
- Zabrina L. Brumme
- Ivan Sadowski
Scientific Reports (2023)
HIV infection
- Linda-Gail Bekker
- Chris Beyrer
- Jeffrey V. Lazarus
Nature Reviews Disease Primers (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
News releases.
News Release
Monday, March 14, 2022
NIH launches clinical trial of three mRNA HIV vaccines
Phase 1 study is among first to examine mRNA technology for HIV.

The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has launched a Phase 1 clinical trial evaluating three experimental HIV vaccines based on a messenger RNA (mRNA) platform—a technology used in several approved COVID-19 vaccines. NIAID is sponsoring the study, called HVTN 302, and the NIAID-funded HIV Vaccine Trials Network (HVTN), based at Fred Hutchinson Cancer Research Center in Seattle, is conducting the trial.
“Finding an HIV vaccine has proven to be a daunting scientific challenge,” said Anthony S. Fauci, M.D. NIAID director. “With the success of safe and highly effective COVID-19 vaccines, we have an exciting opportunity to learn whether mRNA technology can achieve similar results against HIV infection.”
An mRNA vaccine works by delivering a piece of genetic material that instructs the body to make a protein fragment of a target pathogen (such as a virus), which the immune system recognizes and remembers, so it can mount a substantial response if later exposed to that pathogen. The HVTN 302 study will examine whether the following three experimental HIV mRNA vaccines are safe and can induce an immune response: 1) BG505 MD39.3 mRNA, 2) BG505 MD39.3 gp151 mRNA, and 3) BG505 MD39.3 gp151 CD4KO mRNA. Each investigational vaccine candidate is designed to present the spike protein found on the surface of HIV that facilitates entry into human cells. Each of the experimental vaccines encodes for different but highly related, stabilized proteins. None of the three vaccine candidates can cause HIV infection.
The specific mRNA sequences contained in the vaccines were designed and developed by investigators at the NIAID-funded Scripps Consortium for HIV/AIDS Vaccine Development (CHAVD) at the Scripps Research Institute and the Bill & Melinda Gates Foundation-funded IAVI Neutralizing Antibody Center at Scripps, in collaboration with scientists at Cambridge, Massachusetts-based Moderna, Inc. Moderna manufactured the investigational vaccines through a NIAID-supported contract.
Led by principal investigators Jesse Clark, M.D., of the University of California Los Angeles, and Sharon Riddler, M.D., of the University of Pittsburgh, the HVTN 302 study will enroll up to 108 adults ages 18 to 55 years at 11 sites in: Birmingham, Alabama; Boston; Los Angeles; New York City; Philadelphia; Pittsburgh; Rochester, New York and Seattle. Each participant will be randomly assigned to one of six groups each receiving three vaccinations of one of the experimental vaccines. The first three groups (18 participants each), called Group A, will receive intramuscular injections of 100 micrograms (mcg) of their assigned vaccine candidate at the initial visit, at month two and again at month six. Participants in Group A will be evaluated two weeks after initial vaccination to ensure safety criteria have been met. If so, the remaining three groups of 18 participants each (Group B) will be vaccinated with 250 mcg of the assigned investigational vaccine, followed by injections two and six months after the initial vaccination.
Safety and immune responses will be examined via blood and lymph node fine-needle aspiration samples taken at specified timepoints throughout the trial. Clinical staff will closely monitor participant safety throughout the study. The clinical trial is expected to be completed by July 2023.
More information about the HVTN 302 study is available on ClinicalTrials.gov using the identifier NCT05217641 .
NIAID conducts and supports research—at NIH, throughout the United States, and worldwide—to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing, and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website .
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov .
NIH…Turning Discovery Into Health ®
Connect with Us
- More Social Media from NIH
- Search This Site All UCSD Sites Faculty/Staff Search Term
- Mission & Goals
- Commitment to Education
- Leadership & Staff
- Steering Committee
- CAB-U (University)
- Seminar Series
- HIV Education & Training
- Active Studies
- The HIV Institute Allies
- Community Contributions at Ralph's
- Emerging Researchers Fund
- Gary A. Fusfeld Memorial Fund
- Rick Mitchell Memorial Fund
- Crossley and Marshall Memorial Fund for HIV Research
- The Last Gift Study
- Employee Matching Options
- Giving Tuesday
- Eugene R Burkard Remainder Trust
- News Highlights
- Newsletters
Active HIV Research Studies
About this page.
This page hosts a collection of HIV and HIV-related research at the University of California San Diego and affiliated sites. Progress in treatment for HIV, hepatitis C, hepatitis B, tuberculosis, and other related conditions only occurs with the valuable contributions of individuals who enroll in research studies. These clinical research trials help improve the treatment and management of HIV and other infections.
We encourage you to bookmark this page and check back often, as new opportunities to participate in HIV and related research are added frequently.
This page is managed by The HIV Institute at the University of California San Diego. Contact us at [email protected] with any questions, feedback, or recommendations. Please note, inquiries regarding a specific study or sponsoring center should be directed to the contact listed. The HIV Institute does not manage any of these studies.
To see all HIV/AIDS clinical trials at UC San Diego (including those in progress as well as actively enrolling studies), please visit the UC San Diego Health Clinical Trials page.
If you are interested in including a research opportunity on this page, please click the button below to submit details.
Submit studies
*Disclaimer: Each sponsoring center and/or principal investigator is responsible for the studies listed on this website. This includes study preparation, conduct, and administration of a research grant, cooperative agreement, training or public service project, contract, or other sponsored project in compliance with applicable laws and regulations and institutional policy governing the conduct of sponsored research. Contact the indicated party listed below with any study-related questions.
Antiviral Research Center (AVRC)
To enroll in or get more information about one of these clinical trials at the AVRC, please call the Screening Coordinator at (619) 543-3740 or email Marvin Hanashiro at [email protected]
Click here to see a full list of the Antiviral Research Center's currently enrolling studies.
CMV-specific HIV-CAR T cells as Immunotherapy for HIV (Research for an HIV Cure)
The primary goal of this trial is to assess the safety of administering autologous CMV/HIV-CAR T cells to people with HIV. This includes a thorough evaluation of potential toxicities, their nature, frequency, severity, timing, and duration.
Click here for more information and eligibility requirements. HIV
ACTG A5321 (AHRC)
Studying differences and changes over time in HIV reservoirs (groups of HIV-infected cells that 'hide' from anti-HIV medications) and attempting to answer questions about the ways that HIV infection is controlled. This may have to do with a person’s viral load and CD4 count, when they started their anti-HIV medications, and genetic factors. Click here for more information and eligibility requirements.
HIV
ACTG 5386 (N-803 with or without bNAbs for HIV-1 control in participants living with HIV-1 on suppressive ART)
Scientists are looking for ways to effectively clear HIV that rests in areas of the body where standard antiretroviral treatment (ART) is unable to reach. IL-15 superagonist (N-803) appears to reactivate HIV that is "asleep" and is also thought to increase the body's natural immune response to HIV. Broadly neutralizing antibodies (bNAbs), such as 10-1074 and VRC07-523LS, have been shown to control growth of HIV in the blood and to increase the body's immune response to HIV. N-803 alone or in combination with bNAbs may provide greater control of HIV than previous efforts. Click here for more information and eligibility requirements. HIV
ACTG 5403 (GET IT RIgHT)
The purpose of this study is to determine the best combo of antiretroviral therapy for transgender women (tw) living with hiv who take feminization hormonal therapy (fht). all participants will receive fht. the study will determine if tw remain undetectable while receiving fht for 48 weeks. additionally, it will assess if hormone levels change based on the type of hiv medications taken. , artistry-1 , the purpose of this study is to compare taking oral bictegravir/lenacapavir versus a complex art regimen in people with hiv., artistry-2 , the purpose of this study is to compare taking oral bictegravir/lenacapavir versus biktarvy in people with hiv..
DEPTH (Doxycycline for Emphysema in People Living with HIV)
This is a research study to evaluate if the medication doxycycline, a commonly used antibiotic, can slow the damage to the lungs caused by emphysema in people who also have HIV. Emphysema is more prevalent in people living with HIV and may progress faster than in HIV-negative individuals. Doxycycline is approved by the FDA to treat or prevent a variety of infections and conditions. It has not been approved to treat emphysema in people living with HIV, so the use in this study is considered investigational.
LAST GIFT
People at the end of their lives often have a unique perspective on life, death and altruism, and may hold the keys to curing HIV. This study takes the next steps in finding a cure for HIV by understanding how reservoirs are distributed throughout the body. Click here for more information and eligibility requirements. HIV
This research study seeks to test the performance of an investigational blood analyzer to make routine health tests convenient and get results fast. Eligible participants will receive $40 as compensation for a clinic visit and blood draw.
Click here for more information and eligibility requirements.
A5418 (STOMP)
Tecovirimat (also known as TPOXX) is a drug that may help to treat infections caused by pox viruses. Tecovirimat is approved by the Food and Drug Administration (FDA) to treat smallpox in adults and children. Its use in this study is considered investigational. An investigational use is one that has not been approved by the FDA. Tecovirimat has been approved for use in this study based only on data from animals. We don’t know for sure if it works to treat any infections in people. The FDA has reviewed information on tecovirimat and determined that tecovirimat may help treat infection, including serious or potentially life-threatening disease from poxviruses. Click here for more information and eligibility requirements. MPOX
PURPOSE-3 (Injectable PrEP Study)
We are conducting a clinical study to see whether an investigational twice-a-year injection medication can help reduce the risk of getting HIV from sex.
Click here for more information and eligibility requirements. hiv s exual health , purpose-4 (injectable prep study), we are conducting a clinical study to see whether an investigational twice-a-year injection medication can help reduce the risk of getting hiv among people who inject drugs., hiv neurobehavioral research program (hnrp).
To enroll in or get more information about research studies at the HNRP, please call (619) 543-5000 and ask to speak with a research recruiter.
You may be eligible to participate if you: • are at least 18 years old • are HIV+ or HIV- (do not have HIV) • do or do not have a history of substance use
For participating you will receive: • Monetary compensation • Lab results (at no cost to you) • Medical and psychological test results (upon request at no cost to you)
*All information obtained during the study is kept strictly confidential.
iSTEP - an mHealth Physical Activity and Diet Intervention for Persons With HIV
Summary: HIV is associated with a pattern of neurocognitive deficits, metabolic dysfunction, and an elevated risk for cardiovascular disease (CVD), phenomena that remain untreated despite the use of medications to control the disease. This proposal will examine the effect of a personalized, automated, interactive mobile phone text message intervention (iSTEP) designed to increase moderate physical activity (PA), decrease sedentary behavior (SB), and promote a healthy Mediterranean-style diet (MedDiet) in persons living with HIV (PLWH). The investigators propose that participants who receive the iSTEP intervention will increase the amount of physical activity, improve their diet, show a reduction in risk factors for CVD, and exhibit improved neurocognitive performance. Click here for more information and eligibility requirements
HIV
DETECT: A novel device to assess how HIV affects neurocognitive decline and postural instability in older adults at risk for Alzheimer's Disease
This is a research study to determine if a brief virtual reality (VR) assessment among older adults living with HIV can help us better screen for changes in cognition and balance.
Click here to download information on this study
Email: [email protected] for more information or if you are interested in enrolling HIV
Biomarkers of Resilience and injury related to HIV and Methamphetamine (BRIHM)
This project will look at METH and HIV-associated biomarker signatures of CNS injury and resilience and will translate them to the clinical environment.
Email [email protected] for more information or if you are interested in enrolling HIV
Cross-species studies of smoking effects on cognition and neuroinflammation in HIV
This study will examine how smoking affects people living with HIV, specifically the function and performance of the brain.
Mother Child Adolescent Program (MCAP)
IMPAACT 2015: Very Early Intensive Treatment of HIV-Infected Infants to Achieve HIV Remission
The study will explore the effects of early intensive antiretroviral therapy (ART) on achieving HIV remission (HIV RNA below the limit of detection of the assay) among HIV-infected infants.
Click here for more information and eligibility requirements. H IV I NFANTS
IMPAACT 2026: Pharmacokinetic Properties of Antiretroviral and Anti-Tuberculosis Drugs During Pregnancy and Postpartum
This study will evaluate the pharmacokinetic (PK) properties of antiretroviral (ARV) and anti-tuberculosis (TB) drugs administered during pregnancy and postpartum.
Click here for more information and eligibility requirements. HIV P REGNANCY
IMPAACT 2017: More Options for Children and Adolescents (MOCHA): Oral and Long-Acting Injectable Cabotegravir and Rilpivirine in HIV-Infected Children and Adolescents
The purpose of this study is to determine the dosage for oral and IM Cabotegravir LA and IM Rilpiverine LA and evaluate the safety, acceptability, tolerability, and pharmacokinetics of oral and long-acting injectable cabotegravir and long-acting injectable rilpivirine in virologically suppressed HIV-infected children and adolescents.
Click here for more information and eligibility requirements. HIV CHILDREN 12-17
Owen Clinic
Other uc san diego hiv research studies.
LOTUS Study
The LOTUS Study is recruiting women to share their thoughts on accessing sexual health services and mobile technology use so they can develop a mobile app. View the enrollment flyer .
Enrollment Now SEXUAL HEALTH
Women SHINE Study
Women SHINE is a research study aimed at helping women living with HIV to access and stay engaged in HIV care. Women SHINE is currently enrolling women living with HIV who have experienced violence during their adult life. View enrollment flyer
Enrollment: Now HIV
MACH2: Measuring Brain Effects of Adversity, Cannabis and HIV - Phase 2
The goal of the MACH2 study is to find out how HIV, cannabis use and adversity affect the brain among young adults. We are seeking people age 18-24 with or without HIV and with all levels of cannabis use, from none to daily, but not current use of methamphetamine, cocaine or opioids. Participation involves cognitive testing, questionnaires and interviews, brain imaging, and a blood draw. You will be compensated up to $250. For information or to be screened for the study, contact Kathy Scarvie (see below).
Enrollment: Now through March 2024
Contact: Kathy Scarvie via email at [email protected] or call/text 858-951-7384
HIV CANNABIS
AMC-083: Collecting and Studying Tissue Samples From Patients With HIV-Associated Malignancies
Summary: Collecting and studying tissue samples from patients with cancer in the laboratory may help doctors learn more about changes that occur in DNA and identify biomarkers related to cancer. This research trial is collecting tissue samples specifically from patients with HIV-related malignancies to study clinical, genetic, and immunologic parameters that have prognostic significance and/or are involved in the initiation and progression of HIV-1 malignancies, including complete genomic sequence determination of HIV-associated diffuse large B-cell lymphomas, lung cancer, anal cancer, and cervical cancer.
Enrollment: now through December 2024
Contact: Erin Reid, MD at [email protected]
Click here for more information and eligibility requirements. HIV CANCER
AMC-101: Ibrutinib, Rituximab, Etoposide, Prednisone, Vincristine Sulfate, Cyclophosphamide, and Doxorubicin Hydrochloride in Treating Patients With HIV-Positive Stage II-IV Diffuse Large B-Cell Lymphomas
This phase I-II trial studies the side effects and best dose of ibrutinib in combination with rituximab, etoposide, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin hydrochloride in treating patients with human immunodeficiency virus (HIV)-positive stage II-IV diffuse large B-cell lymphomas. Ibrutinib may stop the growth of cancer cells by blocking some of the enzymes needed for cell growth. Monoclonal antibodies, such as rituximab, may interfere with the ability of cancer cells to grow and spread. Drugs used in chemotherapy, such as etoposide, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin hydrochloride, work in different ways to stop the growth of cancer cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Giving ibrutinib and etoposide, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin hydrochloride may work better in treating patients with HIV-positive diffuse large B-cell lymphomas.
Enrollment: now through December 2024.
Contact: Erin Reid, MD [email protected]
Click here for more information and eligibility requirements HIV CANCER
- Seattle Children’s
- University of Washington
HIV Research Study Participant Screening and Patient Referral
We support screening and recruitment of eligible people living with HIV (PLWH) into clinical and pathogenesis research studies via a dedicated research nurse.This service is available to investigators to increase enrollment of PLWH into research studies. Lindsay Legg, the HIV research coordinator, is effective at increasing enrollment in HIV studies. Ms. Legg reviews the records of UW HIV patients visiting the HMC Madison Clinic and refers those who qualify to the appropriate research coordinator for enrollment.
To access this service, please complete a Request for UW HIV Information System (UWHIS) Data, Specimens and Referral Services form and choose ‘HIV Research Study Screening and Referral Nurse Service (to identify and recruit potential research subjects for your study)’ under request type. If you have any questions please email Jordan Graff, CFAR TRS Program Manager, at [email protected].
UWHIS Data, Specimens, and Referral Services Form
- UNC Chapel Hill
Participating in HIV Cure Research – with a UNC Study Participant & Dr. David Margolis
February 13, 2019
What are some reasons to participate in HIV cure research? A UNC research study participant, Rob, shares his personal reasons, and describes his involvement in recent studies. Dr. David Margolis, who leads HIV cure research at UNC, joins the conversation and describes where cure research is today, including specifics on Vorinostat trials, and where their work is headed. Dr. Margolis is a Professor of Medicine, Microbiology and Immunology in the Division of Infectious Diseases and is Director of the HIV Cure Center at UNC. He also is the Principal Investigator for CARE, the Collaboratory of AIDS Researchers for Eradication

Topics Covered:
Participating in HIV clinical trials
Current trials in hiv at unc, aiming for a cure, research on vorinostat, looking to the future.
Ron Falk: Hello, and welcome to the Chair’s Corner from the Department of Medicine at the University of North Carolina. This is our series for patients where we talk about HIV. Today is our final and special episode where we welcome a research study participant, Rob, and Dr. David Margolis , for a conversation about cure research.
Rob is an active study participant in our clinical trials at UNC, and we are most happy to have him here to share his experience.
Dr. Margolis is a Professor of Medicine, Microbiology and Immunology in our Division of Infectious Diseases and Director of the HIV Cure Center at UNC. He also is the Principal Investigator for CARE, the Collaboratory of AIDS Researchers for Eradication. Welcome, Rob and David.
Rob & David Margolis, MD: Thank you.
Falk: Rob, tell us a little about yourself. Tell us a bit about how long you’ve been participating in research studies.
Rob: The current study I’m in has been going on for about two years. Before that, I’ve been in too many studies to count, pretty much since I was diagnosed and brought into the ID Clinic. At first, I was in a study combining two different medications, I think Truvada and etravirene, or Intelence—seeing how they work together. That’s how I was initially getting my HIV meds for free, which was great because I didn’t have health insurance at the time. Since then, I’ve pretty much been involved in studies ever since.
Falk: Go back to the first time you entered a clinical trial. What inspired you to get involved then, at the very outset?
Rob: The very outset was getting free medication—I didn’t have health insurance. Currently I’m on the ADAP, so that’s taken care of.
Falk: But you’re still inspired to do it.
Rob: Of course—it’s changed over time. The money is still a big part of it. It’s more than just the money. I feel like being a part of research, while also being in care here has changed my perspective on having HIV and my treatment in general. When I was first diagnosed and came in, I went through a period of depression, which I think is common for HIV patients, or anyone diagnosed with a chronic illness. Being part of research to potentially cure the illness has made me feel better about having the illness. I feel like my pain and suffering is going to help someone else eventually.
Falk: There has been a tremendous, incredible change in treatment over your lifetime, so your participation and folks like you has been unbelievably instrumental in the things that Dr. Margolis has been doing. What do you think other people in the community—other folks with HIV—feel about HIV research and treatment? Do you have a sense of that?
Rob: No, I don’t get out much. I know PrEP is really big right now. I honestly don’t have many friends who have HIV, but I do have other friends who are on PrEP. I feel like just in general, public knowledge about HIV and what treatment is like is really changing from when I was first diagnosed. I encountered a lot of ignorance at first, but PrEP has really changed the conversation. People understand more about how HIV is transmitted, how the drugs work, that’s a big part of it. Prior to being diagnosed, I knew for the most part how HIV was transmitted, but I knew nothing about antiretrovirals and how efficient they are at preventing transmission and what the current research is like.
I feel like public perception is always going to be behind the research, but I feel like things are getting better in terms of what most people know about HIV.
Falk: Dr. Margolis, let’s talk a little bit about some of your current trials. Where is cure research today, and then how does the participation of folks like Rob play in what you do?
Margolis: We have a number of trials that are ongoing. They are a small number and they involve a small amount of people. Really, our approach has been that we’re just at the beginning of HIV cure research. So, these are really more like small experiments than big clinical trials that are going to give an overall answer and get a drug or therapy licensed. Participants like Robert who are willing to stay with us for the long term and study things step by step, are really very important. It’s a very different dynamic than Robert was talking about in the beginning. Earlier when antiretroviral therapy was being devised and perfected, there was a clear benefit for people to be in studies, sometimes to get compensation or access to medication, but the medication itself was their therapy. There was definitely the risk benefit calculation to being in studies that was much easier to understand.
Now, we’re just trying to understand how to make the persistent virus vulnerable to be cleared—what to use to clear the virus to eventually get rid of it. It’s a step-by-step process, one study after another, putting the pieces together. Hopefully, someday people like Robert will benefit from the work we do now, years later, having a therapy that we can actually use in the clinic and eradicate infection. Right now, we need them as our partners to help us make this progress.
Robert has been in some studies where we’ve been looking at the activity of what’s called the latency reversing agent. The drug is called Vorinostat. It affects the human cell, but also forces the virus out of latency to sort of wake up and show itself. We think that’s the first step, to make the latent hidden virus vulnerable. Then we’re using, as a second step, several immunotherapies. The study Robert’s in right now, he’s getting a unique vaccine that’s given over a couple of months to sort of prepare his immune system. We hope to see the virus when it gets woken up so it can be cleared by that immune response. So, I think you’re essentially in the middle of a bunch of cycles of vaccine and Vorinostat, and vaccine and Vorinostat, and later in the spring we’ll try to get assays, very sensitive assays to measure, how much virus can we still detect, and is there less now than there was in the beginning ?
Falk: We’ve had several podcasts already in which the message has become pretty clear that if you continue on your medications each and every day, and the virus has disappeared from your blood, you’re going to do really well. What you’re describing is the reality that even if the virus has disappeared from the blood, it hasn’t left the body completely. What you’re hoping to do with this latency reversal drug, is to find the virus or have the virus come out of its hiding spots and then get rid of it once it has re-emerged. The question then, from your perspective, Rob, how important is it for you to really get rid of this virus altogether?
Rob: It would be great not to be dependent on medication and hopefully we’ll reach that point eventually. I really don’t think about having HIV that much. Before getting diagnosed, I already took medication on a daily basis for something else, so that wasn’t an adjustment for me. It’s really not something that comes up that often. I’ve been managing it just fine.
Falk: Wouldn’t it be nice though, at some level to really completely get rid of it altogether so you never have to think about it again?
Rob: Yeah, I guess. I guess my perspective has changed a little bit over time. I have a lot of friends with different chronic illnesses. When I first got HIV, it impacted me a lot, but I realized that this is the best time to have HIV. It’s something I get told by doctors a lot. It’s similar to having high blood pressure. It would be nice not to have to deal with it, and not to worry about where am I going to get my medication from if our health care system collapses— Am I going to die?— that’s stressful. As of now, things are okay. We were told upfront, “ Don’t expect to be cured by these studies .” I’m not in it for that. I’m in it to help people in the future.
Margolis: That’s very important, but it should also be said that a lot of people with HIV like Robert are sort of dealing with it, and there’s some uncertainty and concern over time about their future health and access to medicines, but many people also feel very stigmatized and very burdened by this chronic “Sword of Damocles” hanging over their head— what will happen tomorrow, next week, next month, next year? Then on the global, social scale, I don’t know that if we could treat people for a few months and then have virus eradication, achieve a cure, wouldn’t that be better than having to provide chronic medical care for a chronic illness to millions of people around the world?
Falk: I must admit I take blood pressure medicines and if somebody could figure out a way for me to stop taking blood pressure medicine, and have that go away I would be thrilled to stop taking a daily drug.
Falk: The drug that you’re taking now, say it out loud again.
Rob: For my treatment, or for the study?
Falk: For the study.
Rob: Vorinostat.
Falk: Do you have any symptoms from taking Vorinostat?
Rob: Not that I’ve noticed.
Falk: Pretty easy to take. Can you tell us, Dr. Margolis, a little bit more about this drug and what you’re learning from folks like Rob who are taking it?
Margolis: Vorinostat is in a class of drugs, there are several of them. They were originally developed for cancer treatment, for oncology, and their target against mechanisms in the normal cell. They were developed in cancer therapy, because when you target those cells in cancer cells, they tend to die, whereas in normal cells there is less of an effect. There is still some effect in normal cells. When a normal cell has a virus in it, targeting those mechanisms also reverses latency in HIV virus and if it’s in the sleeping, “off” state, takes it towards the “on” state and makes the virus be expressed, the cell makes viral proteins, and can be seen by the immune system.
Falk: So, kick it out of the cell. How effective has this been in your experience so far?
Margolis: It’s very effective in the lab, and we can measure effects of it reliably in people, but there’s really a second step that I think has to come after the virus proteins are expressed in the cell. The immune system has to see that cell, and it’s a very rare cell spread all over the body, hidden in various places. I think the immune system has to be augmented to be better than usual at finding HIV and killing HIV infected cells. So far, just giving Vorinostat has disturbed latency, but has not depleted any persistently infected cells in people. So, now in all of our studies, we are trying to put something on top of Vorinostat, an antibody, a vaccine, an immune cellular therapy or something else.
Rob: Before doing the current study with Vorinostat, I did a similar study with creating the vaccine that Dr. Margolis was just talking about. Now the current study I’m in is combining both of those therapies. Prior to this study there was a separate study just testing the Vorinostat.
At first, I declined to be in that study because I was concerned about possible side effects of Vorinostat. I did further research, and once the next opportunity came up, I decided that it was worth the risk. We were using really low dosages compared to what would be used for cancer treatment. I feel like there have been enough studies so far that I feel fine with taking it. I’ve had friends who are not happy with me taking Vorinostat, since there’s been limited testing on healthy people, but someone has to take the risk. I’m single, I don’t have kids, I work from home, I have a lot of free time, so I’m in a unique position to help. So far, no problems.
Falk: That’s great news.
Margolis: This really illustrates exactly the challenges, but at the same time what’s so great. Robert took the vaccine and we know exactly how it works in him because we measured it. Then he took Vorinostat, and we know exactly how it works in him because we measured it. Now we can put them together and find out exactly how well they work together.
Falk: Do you take regular sorts of HIV drugs at the same time, or just those two?
Rob: I’m taking Triumeq daily. It’s a combination of antiretrovirals as part of my treatment, separate from the study.
Falk: That’s just for usual maintenance care. If this drug doesn’t work, are there other latency reversal drugs on the horizon?
Margolis: We have several immunotherapies, clearance therapies that we’re testing. Actually, out in the field in other research groups, there are quite a number of these sorts of approaches. On the latency reversal side, what we have discovered so far is somewhat more limited. There are only a few, one or two or three things, that can be tested in people now. Our research program is trying to develop new agents, and we have some new agents that we just discovered that are moving into animal testing. We hope maybe in a couple of years to be able to move them into the clinic as well.
Falk: It’s a huge advantage to have the same person, like you, Rob, who is willing to participate in one study after the other, because otherwise, one doesn’t know the effects of combination therapy if you don’t know the effects of each drug by itself. Your willingness to participate in one of these after the other is unbelievably useful for the community as a whole, there’s no doubt about that. Why have you stayed involved?
Rob: I have made personal bonds with the people. I don’t get to see Dr. Margolis that much, actually, but some of the research assistants, it’s nice seeing the same people every now and then and have forged some friendships.
I’ve also just learned a lot about HIV and just how research is done in general and how the FDA works. I’ve used that in my personal life. I took a peer outreach class at ECU, and I’ve done a couple workshops on HIV prevention. I’ve really gotten a lot out of it—I have nothing but good things to say.
Falk: Good. If somebody is listening to this podcast and is thinking about joining a clinical trial, what would you tell them?
Rob: Yes, definitely do it—or look into it. There are so many different studies going on in addition to HIV research. We live in one of the best places to be in the world for medical research, so there’s plenty of opportunities to get involved. You don’t have to take experimental drugs. I used to be terrified of needles, believe it or not, and this has been really good exposure therapy for me. I can’t look when I get stabbed still, but I’ve come a long way. There are a lot of opportunities to get involved—look into it. it’s worth it.
Falk: Dr. Margolis, just looking to the future, this whole concept of latency reversal and then getting rid of the virus once it rears its ugly head again, what are the next steps? Where is that all headed?
Margolis: I think things are sort of headed towards a convergence of many different areas of HIV research that perhaps can be used together. Robert talked about taking a combination pill which is three or sometimes four different medicines in one pill, it lasts all day, and that’s the entire therapy you need to take. There now are developments trying to deliver those medications in other ways where they would last for three to six months or perhaps even a year.
Falk: So, you no longer would need to take daily therapy.
Margolis: You would need to get an implant or an injection once every several months. That could serve for treatment, that could serve as a preventive treatment if someone was at risk of being exposed. At the end of when we get to whatever treatments we’re going to do to flush the virus out and allow the immune system to clear infection, we probably will always worry, just like with cancer, that there’s some cell left behind—we missed one or two places. We probably want to have an insurance policy in place for some period of time, like a vaccine or a long-lasting therapy. When we believe that we’ve cleared infection, someone could still become infected again—they’re now at risk of new infection, potentially. If we had a vaccine or preventive therapy, that would provide an insurance policy against relapse of infection, or re-infection.
Falk: Really exciting, no question about it. Rob and David Margolis, thank you so much for participating in this podcast.
Rob/Margolis: It’s been a pleasure. Thank you.
Falk: Thanks so much to our listeners for tuning in. If you have enjoyed this series, please take a moment and subscribe to the Chair’s Corner on iTunes and leave us a rating and comment. You can also find us on SoundCloud and the UNC Department of Medicine web site and on FaceBook .
Visit these sites for more information:
- Dr. David Margolis’s School of Medicine profile
- Infectious Diseases (ID) Clinic at UNC
- North Carolina AIDS Training and Education Center
- TheBody.com
- UNC Institute for Global Health and Infectious Disease
- “Vorinostat Dosing Exposes Latent HIV” – article describing recent results
Filed Under:
More from Department of Medicine
- Caulfield and Hu Presented “Medicare Advantage Member Appeals – Successes & Frontiers” at the NPAC
- May 2024 Observances and Holidays: Cinco De Mayo, Nurses Week, Asian Pacific American Heritage Month
- Visiting Researchers Strengthen International Aging Research Collaboration
The Hastings Center
Irb: ethics & human research, the need to track payment incentives to participate in hiv research.
Abstract: Providing incentives is an accepted and common practice in human subjects research, including clinical HIV research. While we know that financial incentives among similar studies can greatly vary, surprisingly little research exists on how to determine when such incentives are excessive or constitute an “undue inducement.” Multiple factors, such as risks and benefits, study procedures, study budget, historical precedent, recommendations from institutional review boards, advice from other investigators, and local regulations may influence decisions about appropriate incentives, but little empirical data exist about what incentives are offered to potential research participants. Rules for acceptable gifts, services, and compensation should consider study location and population, but without a clearer understanding of currently offered incentives and how these practices match up to ethical beliefs of appropriateness, we continue to follow perceived trends without critical assessment. Here, we present one potential approach to explore the impact of financial incentives on biomedical HIV research and to further clarify undue inducement: the development of a framework to support ethical decision-making about payment to participate. This framework is based on input from people living with HIV, biomedical HIV researchers, ethicists, former study participants, and IRB members and includes a database that allows for tracking payment practices.
Keywords: HIV research, research incentives, payment to participate, undue inducement, human subjects research, institutional review boards
_______________________________________________________________________________
Providing incentives to research participants—including money, gifts, and services—is an accepted and common practice in biomedical HIV research. 1 Here, we focus on financial incentives: “[p]ayment to research subjects for participation in studies.” 2 Although some studies suggest that altruism motivates many people to participate in research, 3 payment to participate is often essential for ensuring enrollment and retention in clinical research, 4 including high-risk HIV biomedical trials, which may result in negative health outcomes. 5 While the payment offered across similar studies can greatly vary, 6 surprisingly little research exists on the factors researchers consider when deciding on an appropriate payment amount, including how to determine when an amount is “excessive” or constitutes an “undue inducement” to participate in a study. Further, the influence of incentives on participant recruitment, retention, and informed consent is virtually unknown, particularly in the biomedical HIV research field. We present one potential approach to explore the impact of financial incentives on biomedical HIV research and further clarify undue inducement—namely, the development of a framework to support ethical decision-making regarding payment to participate, which is based on input from key stakeholders and includes a database that allows for tracking payment practices.
Concerns about Payment to Participate
Ideally, incentives encourage participation and retention in clinical and behavioral research. However, payment to participate in research might negatively affect research findings or constitute an undue inducement to enroll in risky research. 7 For example, in a large study conducted in the United States, 75% of professional research participants (persons who can easily earn $40,000 per year in studies) concealed information to qualify. 8 The same study found that some participants provided false information to stay in a study, hid contraindications, and, in some cases, inflicted self-harm to qualify. 9 When involved in multiple simultaneous studies, such participants may harm the science of lower-paying studies by taking shortcuts to move on to studies with larger payment incentives.
Another concern related to the negative impact of financial incentives arises when participants perceive a link between study payment rates and the relative risk of study participation, with higher-paying studies considered higher risk than lower-paying studies. In a 2010 U.S.-based study, 10 researchers took participants through a series of hypothetical experiments. Each scenario gave participants the background information about the procedure and the amount they would be provided for participating ($USD 25 or $USD 1000). Participants were then asked to rate how risky they believed the study to be. Researchers found that as payment level increased, participants perceived the risk of the study to be much greater. The study authors argued that potential research participants might incorrectly assume studies with lower payment rates also present fewer health risks compared to higher-paying studies. 11 Also concerning is that payment may distort sampling. The amount of payment or providing any payment at all may result in oversampling populations from low socioeconomic backgrounds who have different attributes compared to the broader population, potentially distorting study outcomes. Thus, the true impact of incentives on recruitment, retention, and informed consent are unknown.
The U.S regulations governing research with humans require researchers to obtain informed consent “under circumstances . . . that minimize the possibility of coercion or undue influence,” but the document does not define “undue influence” for practical use. 12 The ethics guidelines of the Council for International Organizations of Medical Sciences state that payment to participate must not cause undue inducement (see guideline 13), while they acknowledge that studies about financial incentives are needed to define “undue inducement.” 13 And the Belmont Report , issued by the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, says that “undue influence” in research must be avoided. The report describes undue influence as occurring “through an offer of an excessive, unwarranted, inappropriate or improper award or other overture in order to obtain compliance. Also, inducements that would ordinarily be acceptable may become undue influences if the subject is especially vulnerable.” 14
Ezekiel Emanuel and colleagues contend that undue inducement is an offer of goods with gratuitous value that compels participants to use poor judgment while risking serious harm. 15 Yet even with this definition of undue inducement, investigators and institutional review boards (IRBs) find it difficult to know when a financial or other incentive to participate in research constitutes an undue inducement. For instance, a recent study in the U.S. asked 115 IRB members to identify undue inducement, which was defined as an offer of payment that distorts participants’ ability to perceive accurately the potential risks and benefits of research, and 26% were unable to do so. 16 This illustrates that there is much work to be done to clarify when undue inducement occurs and to prevent undue inducements from being used to incentivize individuals to participate in research.
Even within the same study, differing economic circumstances may mean that a fixed payment amount constitutes undue inducement for one participant and reasonable compensation for another, so it may be prudent to err on the side of what is best for people of lower socioeconomic status to avoid undue inducement. 17 In addition, the significance and potential effects of incentives may vary based on the vulnerability of participants, and for HIV research, these may vary among subpopulations who are often stigmatized (e.g., gay and bisexual men, transgender people, people who inject drugs illegally, homeless youth). Participant judgment and potential harm are measurable variables, but without a clear, consistent definition of what is excessive payment, we cannot differentiate between appropriate and inappropriate incentives, including those that exert an undue inducement. 18 If IRB members have difficulty identifying undue inducement, and if we have no data on incentives, making that distinction is a difficult task.
Some argue that IRBs are too focused on preventing undue inducement and that this focus has led to chronically low payments (i.e., “payment conservatism”), which means that participants may be unfairly compensated for their socially valuable contribution and for accepting potential research risks and other research-related burdens. 19 Thus, rather than striving only for incentives that avoid undue influence, researchers and IRBs must focus on promoting payments that are fair. A focus on providing fair incentives minimizes the risk of exploitation and increases the chance of recruiting a diverse range of research participants. 20 However, most researchers do not have a reference for comparison, which burdens researchers with trying to determine the appropriate and fair incentive for each project on a case-by-case basis. Rules for acceptable gifts, services, and payment should be written with consideration for study location and population, but this should not be done without a clearer understanding of currently offered incentives and how these practices align with ethical beliefs of appropriateness and fairness among key stakeholders. Otherwise, researchers may continue to follow perceived trends or historical precedents to determine what they perceive to be ethically appropriate incentives without critical assessment.
A Payment Database for HIV Research
Efforts to control over- and underincentivizing individuals to participate in research, as well as to address the issues of fairness, are under way in some countries. For example, South Africa developed standardized payments for participants (i.e., ZAR 150, or approximately $USD 14), 21 and Brazil prohibits all monetary payment for participation in clinical trials. 22 In the United States, the National Institutes of Health (NIH) recently implemented an intramural grant policy that delineates baseline payment rates based on “inconvenience units” (discomfort levels or inconvenience of the procedure) for research participants at the NIH Clinical Center. 23
Despite these initiatives, there is little empirical data about financial incentives used in clinical studies. Also lacking is written guidance on the range, types, and amount of payments to provide to research participants. Moreover, while investigators are required by IRBs to report payment to participants in their research protocols and informed consent documents, these incentives are not systematically tracked to permit comparison and reference, and incentive amounts may be reported inconsistently (e.g., by study visit, visit type, or for the entire study). Additionally, it is unclear whether IRBs routinely record incentives in separate data fields in their electronic database of investigators’ applications for review of their protocols. And scholars interested in knowing about payment incentives may look to ClinicalTrials.gov for such data only to be disappointed since investigators are not required to provide information about payment to participate when they register their study at this site. 24 Academic journals also do not routinely ask for incentive information, and most authors do not report this information in publications about their studies.
In HIV clinical trials, participants may face greater than minimal risk (e.g., analytical treatment interruption), the prospect for direct individual benefit is low, and participants may face additional social vulnerabilities (such as belonging to a sexual minority or having lower socioeconomic status), all of which may affect motivations to participate. 25 In addition, when basic health care is absent or difficult to obtain but included with participation in a high-risk clinical study, it may be viewed as an incentive to participate. A database of payment practices in HIV research would provide baseline data that could be used for developing guidance on the range, types, and amounts of incentives investigators should provide to participants in HIV studies. Such a database could also help identify what types of payments in various contexts might constitute undue inducement to participate in HIV research.
The process for developing a payment database should include 1) determining how key stakeholders view and assess financial incentives, 2) reaching consensus among these stakeholders on factors to consider when deciding on payment practices, 3) reviewing the literature on incentives provided in previous biomedical HIV studies, and 4) developing a framework to foster ethical decision-making about incentives by investigators and IRBs. In particular, prospective, actual, and former participants in HIV research should be consulted about their views and experiences with payment practices in research. For example, a recent study in Zimbabwe with 1008 participants revealed that they were dissatisfied with how decisions on incentives were reached without considering their expectations. 26 When community advisory boards are involved in research studies, they could be consulted during the stage of developing research protocols to share their views about payment incentives. The availability of a payment database would provide an opportunity to see what payment incentives were used for similar studies conducted in similar and different contexts.
With a payments database in place, researchers could be required to include a justification statement for incentives in their IRB application, to record incentive data in a separate field in the IRB applications, and to report payment information when they register their study on ClinicalTrials.gov or other venues that report study protocols and results. Studies proposing what are considered inadequate (possibly exploitative), excessive, or possibly undue incentives may be reviewed more carefully and not permitted to proceed in order to respect and protect study participants.
Developing a system that allows for tracking payment data in HIV studies will open new avenues for exploring topics such as incentive disparities, undue inducement, and failure or success rates of studies that require participants to remain incentivized over time. A payment incentive database could provide better guidance and standardization for determining the appropriate incentives based on past projects. Such a repository would generate a more ethically justified and scientifically productive system for providing incentives, including optimal rates for recruitment and retention, and ranges of incentives used in similar studies to start the discussion on what payment amount is appropriate. Furthermore, collection of payment incentive data could create transparency of incentivizing practices to help IRBs be more effective and consistent in reviewing protocols that include payment incentives. The tools developed for tracking payment incentives in HIV studies should be transferable to other areas of research, including cancer clinical trials, as well as early-phase and high-risk research with low prospect of direct clinical benefits. 27
It is possible that even with a payment incentive database, it may be difficult to reach consensus on definitions of inadequate, appropriate, or excessive incentives, not to mention harmonizing incentives within and across different types of studies. Nonetheless, a payment incentive database could allow each IRB or institution to explicate and apply its own policy consistently across similar protocols with reference to a commonly recognized set of ethically salient parameters. 28 Researchers could also use the database generated from this research for secondary data analysis regarding cost-effectiveness of incentives in terms of maximizing recruitment and retention, topics that have not received much attention in the scientific literature.
Multiple factors influence decisions on which payment incentives to provide in research, but empirical research addressing the factors that should be considered when making these decisions is lacking. Without empirical research and reference data, we cannot begin to examine this issue, much less define excessive payments and undue inducements. Since payment to participate may have an effect on participant recruitment and retention, and thus affect the move from a controlled study environment to real-world settings and applications, the lack of data about payment incentives represents a missing link in conducting ethical research. To address the gap in knowledge about payment practices, we have presented one potential method for further exploring the impact of incentives in biomedical HIV research and for defining and identifying undue inducement with regard to payment practices.
Brandon Brown, PhD, MPH, is an assistant professor at the Center for Healthy Communities in the Division of Clinical Sciences in the School of Medicine at the University of California, Riverside; Jerome T. Galea, PhD, MSW, is a research associate in the Department of Global Health and Social Medicine at Harvard Medical School; Karine Dubé, DrPH, is an assistant professor at the Gillings School of Global Public Health at the University of North Carolina, Chapel Hill; Peter Davidson, PhD, is an assistant professor in the Division of Global Public Health in the Department of Medicine at the University of California, San Diego; Kaveh Khoshnood, PhD, MPH, is an associate professor at the Yale School of Public Health; Lisa Holtzman, MPH, is a research assistant in the Department of Global Health and Population at the Harvard TH Chan School of Public Health; Logan Marg, MA, is a sociology PhD candidate in the Center for Healthy Communities in the School of Medicine at the University of California, Riverside; and Jeff Taylor is a community advisory board member for the Collaboratory of AIDS Researchers for Eradication, in Palm Springs, CA.
Acknowledgment
Brandon Brown and Lisa Holtzman were supported by NIAID grant #R01AI1146717 (HIV Cure Studies: Risk, Risk Perception, and Ethics).
- Brown JA, et al. Effect of a post-paid incentive on response rates to a web-based survey. Survey Practice. 2016;9(1):1-10; Lee R, et al. Incentivizing HIV/STI testing: A systematic review of the literature. AIDS and Behavior 2014;18(5):905-912; London A, Ripley B. Compensation or inducement? What IRBs need to know about paying subjects for participation. PRIM&R Webinar 2017; Grady C. Payment of clinical research subjects. Journal of Clinical Investigation 2005;115(7):1681-1687.
- U.S. Food and Drug Administration. Payment and reimbursement to research subjects—information sheet. 2018. https://www.fda.gov/RegulatoryInformation/Guidances/ucm126429.htm.
- Largent E. For love and money: The need to rethink benefits in HIV cure studies. Journal of Medical Ethics 2017;43:96-99; Arnold MP, Evans D, Vergel N. Recruitment and ethical considerations in HIV cure trials requiring treatment interruption. Journal of Virus Eradication 2015;1(1):43-48; Dubé K et al. Willingness to participate and take risks in HIV cure research: Survey results from 400 people living with HIV in the US. Journal of Virus Eradication 2017;3(1):40-50;e21.
- See ref. 1, Grady 2005; Bentley JP, Thacker PG. The influence of risk and monetary payment on the research participation decision making process. Journal of Medical Ethics 2004;30(3):293-298.
- Lo B, Grady C, on behalf of the Working Group on Ethics of the International AIDS Society. Ethical considerations in HIV cure research: Points to consider. Current Opinion in HIV and AIDS 2013;8(3):243-249.
- See ref. 1, Grady 2005; Ripley EB. A review of paying research participants: It’s time to move beyond the ethical debate. Journal of Empirical Research on Human Research Ethics 2006;1(4):9-20.
- See ref. 4, Bentley and Thacker 2004; Booker CL, Harding S, Benzeval M. A systematic review of the effect of retention methods in population-based cohort studies. BMC Public Health 2011;11:249; Grant RW, Sugarman J. Ethics in human subjects research: Do incentives matter? Journal of Medicine and Philosophy 2004;29(6):717-738.
- Devine EG et al. Concealment and fabrication by experienced research subjects. Clinical Trials 2013;10(6):935-948.
- See ref. 8.
- Cryder CE et al. Informative inducement: Study payment as a signal of risk. Social Science & Medicine 2010;70(3):455-464.
- See ref. 10.
- 45 CFR 46.116.
- Council for International Organizations of Medical Sciences, World Health Organization. International Ethical Guidelines for Biomedical Research Involving Human Subjects . Geneva, Switzerland: Council for International Organizations of Medical Sciences, 2002.
- National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research . Washington, DC: Government Printing Office, 1979.
- Emanuel EJ et al. Undue inducement in clinical research in developing countries: Is it a worry? Lancet 2005;366(9482):336-340.
- See ref. 3, Largent 2017.
- Davidson P, Page K. Research participation as work: Comparing the perspectives of researchers and economically marginalized populations. American Journal of Public Health 2012;102(7):1254-1259.
- See ref. 7, Grant and Sugarman 2004.
- Largent EA, Lynch HF. Paying research participants: The outsized influence of “undue influence.” IRB: Ethics & Human Research 2017;39(4):1-9.
- See ref. 19.
- Rhodes R, Gligorov N, Schwab AP. The Human Microbiome: Ethical, Legal and Social Concerns . Oxford: Oxford University Press, 2013, pp. xiv, 266.
- Lobato L et al. Impact of gender on the decision to participate in a clinical trial: A cross-sectional study. BMC Public Health 2014;14:1156.
- Department of Health and Human Services, Office of Human Subjects Research Protections. HRPP Standard Operating Procedure/Policy Approval & Implementation: Recruitment, Selection and Compensation of Research Subjects . 2015. https://ohsr.od.nih.gov/public/SOP_13_v3_11-15-15_508.pdf.
- Alemayehu D, Anziano RJ, Levenstein M. Perspectives on clinical trial data transparency and disclosure. Contemporary Clinical Trials 2014;39(1):28-33.
- See ref. 3, Largent 2017; see ref. 6, Ripley 2006; Presidential Commission for the Study of Bioethical Issues. Moral Science: Protecting Participants in Human Subjects Research . Washington, DC: Presidential Commission for the Study of Bioethical Issues, 2011; Resnik DB. Bioethical issues in providing financial incentives to research participants. Medicolegal and Bioethics 2015;5:35-41.
- Mduluza T et al. Study participants incentives, compensation and reimbursement in resource-constrained settings. BMC Medical Ethics 2013;14(suppl. 1):S4.
- Brown B et al. Transparency of participant incentives in HIV research. Lancet HIV 2016;3(10):e456-e457.
- Brown B, Merritt MW. A global public incentive database for human subjects research. IRB: Ethics & Human Research 2013;35(2):14-17.
- What Is Bioethics?
- For the Media
- Hastings Center News
- Diversity, Equity, & Inclusion
- Hastings Center Report
- Focus Areas
- Ethics & Human Research
- Bioethics Careers & Education
- Hastings Bioethics Forum
- FAQs on Human Genomics
- Bioethics Briefings
- Books by Hastings Scholars
- Special Reports
- Ways To Give
- Why We Give
- Gift Planning
- Sign up for Updates
Upcoming Events
Previous events, receive our newsletter.
- Terms of Use
Rare Patient Voice
Helping Patients with Rare Diseases Voice Their Opinions
Helping patients and caregivers share their voices
HIV Research Panel Recruitment Made Simple
Undertake your study without worrying about recruiting HIV research participants and leave recruitment to Rare Patient Voice As of this writing, we have over 960 patients and more than 112 caregivers in our HIV research survey panel who are ready to take part in your study.
Although there have been huge strides over the last decades, HIV research is still a critical topic that requires attention. We understand how challenging recruitment issues can be to your work. That’s why Rare Patient Voice empowers researchers like you to focus on your study while we provide you with the participants you need.
Participate in Research
If you are an HIV patient or a caregiver, you can take part in the research studies that are moving the industry forward. Your input will help researchers and companies developing new treatments and services, and you will also be paid for your participation. Sign up with us here to join our HIV research survey panel.
711 Hampton Lane Towson, Maryland 21286 [email protected] (443) 986-1949
Visit our international site, rarepatientvoice.global
For Patients
- Learn about us
- Vision, Mission and Core Values
- Sign up to participate
- Find us in the community
- Read our blog for patients
- Privacy Policy
For Related Groups
- Interested in Becoming a Referral Partner?
- Are you a researcher?
- Read our blog for businesses
Select Language English Spanish
©2024. Rare Patient Voice. All Rights Reserved. Privacy Policy | Sitemap
Search Our Website
Privacy overview.
- Open access
- Published: 02 May 2024
A qualitative study exploring approaches, barriers, and facilitators of the HIV partner notification program in Kerman, Iran
- Fatemeh Tavakoli 1 ,
- Mahlagha Dehghan 2 ,
- Ali Akbar Haghdoost 1 ,
- Ali Mirzazadeh 1 , 3 ,
- Mohammad Mehdi Gouya 4 &
- Hamid Sharifi 1 , 3
BMC Health Services Research volume 24 , Article number: 570 ( 2024 ) Cite this article
Metrics details
HIV partner notification services can help people living with HIV (PLHIV) to identify, locate, and inform their sexual and injecting partners who are exposed to HIV and refer them for proper and timely counseling and testing. To what extent these services were used by PLHIV and what are the related barriers and facilitators in southeast Iran are not known. So, this study aimed to explore HIV notification and its barriers and facilitators among PLHIV in Iran.
In this qualitative study, the number of 23 participants were recruited from November 2022 to February 2023 including PLHIV ( N = 12), sexual partners of PLHIV ( N = 5), and staff members ( N = 6) of a Voluntary Counseling and Testing (VCT) center in Kerman located in the southeast of Iran. Our data collection included purposive sampling to increase variation. The content analysis was conducted using the Graneheim and Lundman approach. The analysis yielded 221 (out of 322) related codes related to HIV notification, its barriers, and its facilitators. These codes were further categorized into one main category with three categories and nine sub-categories.
The main category was HIV notification approaches, HIV notification barriers, and facilitators. HIV notification approaches were notification through clear, and direct conversation, notification through gradual preparation and reassurance, notification due to being with PLHIV, notification through suspicious talking of the physician, and notification due to the behavior of others. Also, the barriers were classified into individual, social, and environmental, and healthcare system barriers and the facilitators were at PLHIV, healthcare staff, and community levels. Stigma was a barrier mentioned by most participants. Also, the main facilitator of HIV notification was social support, especially from the family side.
Conclusions
The findings highlighted the multidimensionality of HIV notification emphasizing the importance of tailored support and education to enhance the notification process for PLHIV and their networks. Also, our results show that despite all the efforts to reduce stigma and discrimination in recent years, stigma still exists as a main obstacle to disclosing HIV status and other barriers are the product of stigma. It seems that all programs should be directed towards destigmatization.
Peer Review reports
Introduction
HIV notification is a process through which people living with HIV (PLHIV) are informed about their own HIV status and also take the initiative to inform their social networks, particularly their sexual and injection partners, about their HIV status [ 1 ]. HIV notification represents a complex and intricate decision-making process, characterized by numerous dilemmatic components. These include considerations such as whether to disclose or maintain confidentiality, the timing of HIV notification, the selection of the initial person to be informed, and other related factors [ 2 ]. In line with established HIV notification guidelines, there is a counseling process designed to assist PLHIV in disclosing their HIV status [ 3 , 4 , 5 ]. Healthcare professionals assume a pivotal role in facilitating HIV notification, employing diverse approaches that are influenced by their training and expertise. Additionally, the approach to HIV notification prevailing guidelines may vary depending on prevailing guidelines and cultural norms in specific geographic regions where HIV notification is either encouraged or discouraged. Furthermore, individual-specific factors regarding the person seeking guidance on HIV notification also significantly influence the counseling process [ 6 ].
HIV notification plays an essential role in advancing our efforts to achieve targets for HIV epidemic control. Effective counseling for PLHIV at the time of diagnosis is instrumental in facilitating their access to vital care and treatment services. Furthermore, HIV partner notification can expedite early referrals to care and promote risk reduction among high-risk discordant partners [ 6 , 7 ]. It can also boost the utilization of HIV testing services by partners of newly diagnosed PLHIV, enhancing case detection and the linkage to care [ 8 ]. HIV notification represents a critical component of both prevention and treatment strategies aimed at curbing HIV transmission and optimizing the health and well-being of PLHIV [ 9 ]. Additionally, HIV notification holds the potential to contribute significantly to HIV control and prevention by promoting safer sexual practices, encouraging partner HIV testing, and fostering increased support for PLHIV [ 10 ].
However, despite the potential positive outcomes associated with HIV notification, it also entails significant risks for PLHIV. Sharing one’s HIV status with individuals who may not be accepted can leave PLHIV vulnerable to stigmatizing reactions, including social ostracism, physical harm, and workplace discrimination [ 9 , 11 ]. For instance, a study conducted in sub-Saharan Africa revealed that women faced prominent barriers to HIV testing and disclosing their serostatus. These barriers included the fear of how their partners would react, the dynamics of decision-making and communication within relationships, and the attitudes of partners toward HIV testing [ 12 ]. Another study focused on PLHIV who are men who have sex with men (MSM) in Boston identified several barriers to HIV notification. These barriers included concerns about breaches of confidentiality regarding the HIV status of PLHIV and their partners, concerns about the type of sexual partner involved, potential missed sexual opportunities, and a sense of responsibility towards their partners. On the other hand, participants noted that ethical obligations, the possibility of initiating dating relationships, the timing of HIV notification, and open bidirectional communication acted as facilitators for HIV notification [ 13 ].
In Iran, HIV notification approaches are mentioned in the national HIV testing guidelines but practical modules and specific training for lay providers to successfully implement these services are lacking. The training modules need to be developed and their effects should be monitored carefully to optimize the diagnosis services [ 14 ]. New approaches that enhance the efficiency of testing and increase the coverage of treatment are needed. HIV notification is an approach that has the potential to particularly identify people with undiagnosed HIV infection who remain unlinked to prevention, treatment, and care services, and continue to be at risk of transmitting HIV vertically or through sexual and drug-injecting partners [ 8 ]. However, PLHIV and their networks in Iran may have diverse experiences with HIV notification, including HIV partner notification. To the best of our knowledge, no study has been conducted on this specific topic and approach in Iran. Conducting such a study can provide valuable insights for policymakers and service providers, enabling them to better understand the barriers and facilitators associated with HIV notification and shape effective strategies and interventions. So, this qualitative study aimed to identify the approaches, barriers, and facilitators of HIV notification, including HIV notification, among PLHIV in Iran.
Method and design
In this qualitative study, we used a conventional content analysis method [ 15 ] with a specific focus on three key participant groups: PLHIV, the sexual partners of PLHIV, and the staff members of the voluntary counseling and testing (VCT) center located in Kerman. Kerman is a city with a total population of about 537,000 in southeast Iran [ 16 ]. The recruitment of participants took place over the period from November 2022 to February 2023. The inclusion of PLHIV, their sexual partners, and VCT staff allowed for a comprehensive examination of the experiences, perspectives, and challenges from different views. VCT offers counseling, testing, care, and treatment for PLHIV. We carefully selected staff members from the VCT center who had prior work experience in HIV notification to PLHIV. These individuals were chosen based on their knowledge and firsthand involvement in HIV-related activities, allowing for valuable insights and informed perspectives during the study. The VCT center prioritizes protecting privacy and preventing discrimination against PLHIV through various measures. The center’s main clientele includes individuals engaged in high-risk behaviors, couples seeking HIV testing before marriage, and pregnant women, including those who are HIV-positive [ 17 ]. Services provided to PLHIV encompass addressing their basic needs, facilitating access to necessary services, maintaining ongoing communication and support, and ensuring medical care such as antiretroviral therapy (ART) under the supervision of infectious disease specialists. Additional services include psychiatric care and HIV/STI prevention measures [ 18 ].
Sample and sampling strategy
The study was carried out by a research team specializing in HIV, who were not directly involved in providing services to the study population. Interviews were conducted by a female-trained interviewer (i.e., the first author), who visited the VCT center on specified days. Potential participants were referred by VCT staff, screened for eligibility and interest, and provided with the information necessary to obtain informed consent by the interviewer. Among the PLHIV, we started with a convenience sampling method and continued with purposive sampling. The inclusion criteria for PLHIV were the age of 18 or more and provided consent for participation in the study. Also, the staff were eligible if they had at least one year of work experience. The VCT staff members who were included if they provided verbal consent for participation. Recruitment continued during analysis until information saturation was surpassed such that no new information was found. Different demographic and occupational characteristics were also collected from the participants.
Data collection
Our study included a total of 12 individuals living with HIV (PLHIV), five sexual partners of PLHIV (with only one partner not diagnosed with HIV), and six staff members from the voluntary counseling and testing (VCT) center. The age range of PLHIV participants was between 30 and 59 years old, while the staff members had 5.5 to 16 years of work experience in the field (Tables 1 and 2 ). The study reached saturation after the 19th interview, but to ensure data saturation, four more interviews were conducted. The interviews were conducted face-to-face, in Persian, using a semi-structured interview guide in a quiet, and private room with only the interviewee and the researcher present. The duration of each interview was between 30 and 35 minutes. The interview guide comprised open-ended questions according to the objectives of the study. Some of the questions were obtained from the literature review [ 19 , 20 , 21 , 22 ], others were obtained from local experts in the field of HIV, and some were added during the interview. All questions, including the initial and those subsequently added, were reviewed by three experts in the field. Examples of questions asked to the PLHIV group included: “How did you go about notifying others about your HIV status?”, “How did you inform your family members, friends, or acquaintances that you were infected with HIV? ”, “ What about your partner or spouse?”, “ What factors influenced your decision to notify your partner, family, and friends about your HIV status?”, and “ What barriers did you encounter, and what factors facilitated the process?”. The questions posed to the sexual partners of PLHIV were: “How did you become aware of your partner’s HIV status?” “What actions did you take after learning about your partner’s HIV status?” “How did you approach the process of notifying others about your partner’s HIV status?” and “What barriers did you encounter during this process, and what factors facilitated it?”. For the staff group, the questions asked were: “In your current practice, how do you communicate a PLHIV’s status to the individual as well as their partner, family, or friends?”, “Based on your experiences, how do PLHIV typically disclose their HIV status to their partner, family, or friends?”, and “In your experience, what are the main barriers and facilitators of HIV partner notification?” ( S1 ). Furthermore, we incorporated follow-up questions tailored to each interviewee to gain further insights. To ensure accurate documentation, we employed a voice recorder to capture the participants’ voices during the interviews. Alongside the audio recordings, the interviewer also took supplementary notes to capture important details. The interviewers transcribed the recorded interviews verbatim and ensured the anonymity of the participants on the same day as the interview.
Data analysis
The collected data were systematically coded in textual form, with patterns and themes identified by one of the researchers (the first author). To ensure consistency and accuracy, the research team conducted weekly meetings to review and validate the codes and subcategories. Any ambiguities or discrepancies were thoroughly discussed and resolved during these meetings. Data analysis was performed based on the Graneheim and Lundman method [ 23 ] including (1) the interviews were transcribed verbatim (2), each of the transcriptions was considered as a unit of analysis and were read several times by the researcher to achieve a general understanding of its content (3), the sentences or entire paragraphs of text were determined as meaning units and primary codes were extracted from them (4) classifying similar preliminary codes in more comprehensive classes, and (5) determining the hidden content in the data. MAXQDA10 software was used to manage and analyze the data. Table 3 provides an overview of the analysis process performed on each text.
Trustworthiness
In this study, we used four criteria (credibility, transferability, dependability, and conformability) to ensure trustworthiness. These criteria were utilized to ensure rigorous and naturalistic inquiry throughout the research process [ 24 ]. To address the credibility criterion, the study site was visited before the interviews and data collection. Data credibility was also established by reviewing the adequacy of the interviews and confirming interpretations obtained from the interviews. To examine the transferability, an attempt was made to describe the characteristics of the participants in detail and consider using maximum variability in sampling. To address the dependability criterion, study processes were described to the team in detail, and the interview was audited externally; in this sense, the opinions of one foreign observer were used. For conformability, discussions in research team meetings raised additional issues that were considered for conformability. Also, for member checking, results were returned to participants to check for accuracy and resonance with their experiences.
During the interviews, 322 codes were obtained, and after removing or merging similar phrases, 221 related codes were extracted. We found one main category and three categories, including HIV notification (with five sub-categories), HIV notification barriers (with three subcategories), and HIV notification (with three subcategories) (Table 4 ). The category of findings explores the various approaches through which PLHIV discovered their HIV status. These approaches include learning about their HIV status from healthcare center staff, family members, sexual partners or spouses, and individuals within their social circles. Additionally, the findings examine how PLHIV disclosed their HIV status to their families, spouses or sexual partners, and the people in their networks. Also, the category addressed the barriers and facilitators of HIV notification. The results of the participants’ experiences showed that the way PLHIV informs about their condition has a significant impact on how they notify their family and friends. It was also found that there were always many barriers and problems in the way of HIV notification, which should be taken into consideration. On the other hand, there were limited facilitators for PLHIV, the staff of VCT centers, and the community, who could provide considerable help in the matter of HIV notification. Table 4 provides an overview of all subcategories and primary categories.
HIV notification approaches
We found that PLHIV were notified about their HIV status through different methods. The PLHIV also used different methods to notify the HIV status of their spouse or sexual partners, family members, friends, and other members of their networks. These approaches were placed in five subcategories, including 1) notification through clear, and direct conversation, 2) notification through gradual preparation and reassurance, 3) notification to family, spouse, or sexual partner due to being with PLHIV, 4) notification through suspicious talking of the physician, and 5) notification due to the behavior of others (Fig. 1 ).
Flowchart of HIV notification approaches
Notification through clear, and direct conversation
According to the comments, more than half of the PLHIV had notified their spouse or sexual partner and one or more family members such as children, father, mother, sister, or brother about their condition through a clear, and direct conversation. Some patients have stated that the reason for speaking clearly and normally to their spouse or sexual partner is to be comfortable and intimate with him/her. Also, some participants stated that family members or spouses of a person living with HIV had notified the family directly and clearly.
A 33-year-old woman, divorced, a person living with HIV said: “When I found that I was infected, I very easily told my mother and sisters that I got this disease”. (Participant number 1)
About half of the PLHIV stated that they found out about their condition through a clear and direct conversation with the staff of VCT centers, including doctors, counselors, and laboratory staff.
Also, in one case, a person living with HIV had found that he was infected with HIV through the direct conversation of his wife (HIV negative) and his sister. It should be noted that this issue was followed up in subsequent interviews and no new data was obtained.
A 38-year-old man, married, (HIV negative) said: “For the first time, I told my wife that your HIV test was positive, and I told my wife, just like they told me, that the disease is not dangerous and can be controlled with medicine.” (Participant number 6)
Notification through gradual preparation and reassurance
Some PLHIV stated that they were notified about their HIV status gradually and received reassurance from others during the process. Similarly, the spouses or sexual partners of PLHIV were also notified gradually of their HIV status by the counselor or doctor at the VCT center.
A 39 years old man, single, HIV positive said: “The counselor of the VCT center said in a very gentle tone and in a way that I would not get upset, and think that everything is normal, you are infected with HIV and do not worry, we are here to help you and it is like other diseases and it can be controlled.” (Participant number 3)
Notification to family, spouse, or sexual partner due to being with PLHIV at the testing time
The experiences shared by some participants showed instances where the spouse and other family members, such as the sister, of a person living with HIV were notified about the individual’s HIV status. This notification occurred because these family members were present with the PLHIV at the HIV testing centers and accompanied them during the process.
A 46-year-old man, married, and a person living with HIV, said: “The first time I went for an HIV test, the doctor called my sister who was with me and told her that I have HIV.” (Participant number 4)
Notification through suspicious talking of the doctor
A small number of PLHIV stated that at first the doctor avoided informing the person and initially asked the person questions that made the person suspect that he/she was infected with HIV.
A 46-year-old man, married, a person living with HIV said: “When I was in the hospital, the doctor asked me several questions, for example, did you have extramarital sex or not? Did you engage in risky behavior or not? I doubted that I was infected with HIV.” (Participant number 7)

Notification due to the behavior of others
This category highlights that a minority of PLHIV or their families discovered their HIV infection or the infection of their relatives through various behavioral indicators. These indicators include the infected person’s frequent visits to the doctor, the use of medication, the noticeable discomfort of people around a person living with HIV, or the decision to undergo HIV testing prompted by concerns related to the individual’s behavior. In general, in this subcategory, less than half of the cases are related to notification to a person living with HIV or their spouse, and one case is related to informing the infected person’s children. It should be noted that this issue was followed up in subsequent interviews and no new data was obtained.
A 54-year-old woman, widow, and a person living with HIV said: “I did not tell anyone myself, and my children found out step by step when they saw that I was sick and lost weight, and when they saw that I was taking these pills, they realized that I was infected with HIV.” (Participant number 5)
Barriers to HIV notification
The experiences of the participants indicated that HIV notification and the various methods of carrying it out are influenced by various factors, including barriers and obstacles to notification. In this study, the barriers and challenges for notification were classified into three subcategories: 1) individual barriers, 2) social and environmental barriers, and 3) healthcare system barriers.
Individual barriers
The majority of the participants stated that there are barriers related to the condition of a person living with HIV. This category includes five subcategories, including a) fears and worries, b) mental conditions of a person living with HIV after being informed about the infection, c) denial and non-acceptance of the infection, and d) Involvement in extramarital relationships. This subcategory mainly relates to PLHIV statements.
Fears and worries
The participants expressed apprehension that such HIV notification might lead to challenges or negative consequences not only for themselves but also for those in their immediate environment. The fear of disclosing their HIV status to people in their environment, fear of potential negative impacts on their children’s future, fear of social isolation and communication difficulties, fear of the health condition of the mother and the potential consequences of her being informed, as well as fears of transmitting the infection to their children, are all encompassed within this category. According to the statements of less than half of the PLHIV, these kinds of fear and worries have always been with the infected people; so, they have faced these conditions from the beginning of knowing about their condition. In a way, despite these fears and worries, PLHIV sometimes did not disclose their condition even to the closest members of their family.
A 37-year-old woman, married, a person living with HIV said: “I did not say to my mother, because she is sick, and I do not want her to worry about me” (Participant number 17).
Mental conditions of a person living with HIV after being informed about the infection
This category includes the mental state of the person due to their conditions. After they were notified about the infection, the majority of patients experienced a wide range of mental states, from hallucinations and shock to suicidal thoughts and or guilty feelings of PLHIV due to infecting people around.
A 30-year-old woman, married, a person living with HIV said: “When I found that I was infected, I was shocked, I was hallucinating, I was thinking about everything, about death, about suicide, about the viewpoint of people around me” (participant number 15).
Denial and non-acceptance of the infection
Denial and non-acceptance of the infection was another individual barrier to notifying others. PLHIV could not believe that they were infected with HIV.
A 42-year-old woman, divorced and a person living with HIV, said: “I did not like others to know because it was hard for me to accept the disease” (participant number 14).
Involvement in extramarital relationships
Two staff of the VCT center stated that one of the barriers to HIV notification is involved in extramarital relationships. It should be noted that this issue was followed up in subsequent interviews and no new data was obtained.
A 52-year-old, married, head of a VCT Center stated: “There are some PLHIV who do not have a clear family, that is, they have a sexual partner other than their spouse, and for this reason, they do not like to disclose their HIV status.” (participant number 20)
Social and environmental barriers
Another sub-category of the barriers to HIV notification for the network was social and environmental barriers. This subcategory included stigma, low HIV knowledge of society, changing the behavior of PLHIV relatives, and being easily identifiable in a small city.
Stigma includes different views and perceptions towards PLHIV, notoriety, negative thoughts related to PLHIV from the viewpoint of their spouse and networks or negative view of their children, improper social image, the tendency of PLHIV families to hide HIV status from other people due to stigma in their behavior and stigmatized viewpoint toward transmission of HIV through sexual intercourse was another barrier was mentioned by most participants.
A 54-year-old woman, married, a person living with HIV said: “The most important problem I had to tell others was that if they find out, they will look at me differently and treat me negatively” (Participant number 9).
Low HIV knowledge of society
More than half of the participants stated that some people in society have little knowledge about HIV; they think that HIV can be transmitted very easily or that all PLHIV are infected because of sexual behaviors.
A 43-year-old man, married, a person living with HIV said: “I did not tell anyone that I was infected because of lack of knowledge about PLHIV and the ways of transmission.” (Participant number 16)
Changing the behavior of PLHIV relatives
According to the experiences of about half of the participants, the change in the behavior of the people around PLHIV, which includes the fear of loss and rejection, reducing the desire to meet PLHIV, decreasing or stopping sexual intercourse with the infected spouse, and inappropriate behavior of spouse and family members with PLHIV.
A 49-year-old woman, married, a person living with HIV said: “I am a teacher and when one of my sisters found out that I was infected, she said that I will inform the education department that you are sick and you should not go to school because you are making people’s children sick. So, I preferred to not inform the rest of my network that I was infected. I believe they should act like my sister and think that this disease is contagious.” (Participant number 12)
Being easily identifiable in a small city
Also, a small number of participants stated that living in a small city is a barrier to HIV notification, in other words, in a small city, people can be identified, and on the other hand, the traditional atmosphere of a small city makes the PLHIV reluctant to inform the people around them. It should be noted that this issue was followed up in subsequent interviews and no new data was obtained.
A 39-year-old woman, married and a midwife at VCT center, said: “One of the reasons why people do not inform their sexual partners about their status is they say that it is a small city and people are easily identified” (participant number 23).
Healthcare system barriers
Some VCT staff believed instructions and guidelines for partner notification are not complete and they do not have enough instructions and guidelines in specific scenarios. In these situations, the consultant should decide how to notify the HIV status of some people.
A 52-year-old, married, head of the VCT center said: “The weakness of the notification instructions is that there may be scenarios that there is no solution in practice, for example, a person may say, I cannot inform my wife, if I say, she rejects me. We cannot guarantee that you tell and there will be no problems for you” (Participant Number 20).
Facilitators of HIV notification
The participants identified facilitators at three levels: PLHIV, healthcare staff, and community. Facilitators of HIV notification were mostly mentioned by the staff of the VCT center.
The participants stated that family support, the presence of an experienced and enthusiastic consultant, trust, a confidential environment, and non-judgment by the healthcare system can help the PLHIV to better and more easily explain their behavior and condition to the healthcare workers. These items also could help them to inform other people such as their spouse or sexual partner and family about their condition. Also, they stated those PLHIV who have family support are less worried about their status and they could inform other people in their networks about the infection.
A 37-year-old woman, married, a person living with HIV said: “When I told my spouse, I am HIV infected but he is not infected with HIV, he accepted very easily and supported me” (Participant number 17).
More than half of the VCT staff believed some training courses including in-person or electronically, sharing the experiences between different consultants, training courses on communication approaches, and providing practical solutions in HIV notification guidelines, can improve their ability for a more effective HIV notification.
A 39-year-old woman, married, pharmacy of the VCT center said: “To (have a more effective) HIV notification if the educational materials can be applied in a practical way, such as educational videos, they will be more helpful.” (Participant number 18).
For community
Also, more than half of the VCT staff believed some at the community level could increase the effectiveness of the HIV notification. These facilitators included education through radio and television, public awareness campaigns, culturalization in the context of HIV, and dissemination of information regarding modes of transmission to all members of society.
A 54-year-old married, woman, consultant at the VCT center, said: “I think that general education that the whole society should be educated, and culturalization should be done; something that has been fixed in people’s minds, and this disease over the years that HIV is a disease that people get it due to inappropriate social behaviors, this must be changed. This is in people’s minds and this has caused fear and stigma. So, all members of the society must be aware. The media must carry out this culturalization, and mistake information should be corrected” (Participant number 19).
Our study revealed that PLHIV in Iran received notifications about their HIV status through a variety of methods. Additionally, they employed diverse strategies to disclose their HIV status to their social networks. Notification through clear, and direct conversation and notification through gradual preparation and reassurance were mostly used for HIV notification on both sides. Also, some barriers to HIV notification were identified; fear of disclosing HIV status to the network and stigma were the main barriers to HIV notification. In addition, some facilitators like family support could smooth the HIV notification.
We found that PLHIV received notifications about their HIV status through different approaches. They also disclosed their HIV status to their networks. The majority of PLHIV received their HIV status notification and also, they disclosed their HIV status using clear and direct conversation. The result of a review indicated that HIV notification varied greatly [ 1 ]. Based on the findings of a study on methods of HIV notification by men who have sex with men, point-blank and direct disclosures have been the most commonly used strategies [ 25 ]. Also, the result of a study in Ontario mentioned that HIV notification may at first take the form of partial or indirect strategies [ 26 ]. In another study in Los Angeles and Seattle, some participants stated that directly disclosed their HIV status to sex partners at first [ 27 ]. Some studies reported that the main types of HIV notification were in a voluntary action [ 28 , 29 ]. Also, in our study, some of the participants through gradual preparation and reassurance disclose HIV status. Based on WHO guidelines for HIV self-testing and partner notification, this theme is the counseling process provided by counselors that offer HIV-positive clients assistance with notifying their partners [ 4 , 5 ]. It seems that HIV notification will vary in different contexts and for different people, and the health system should consider these differences and provide counseling and notification services according to each person. Also, based on our results, it seems that the way of HIV notification to PLHIV may affect how notification of them to their network will be conducted. So, these findings underscore the need for researchers to clarify more how HIV notification is implemented.
A variety of barriers impacted the HIV notification on both sides. The main barriers mentioned by the PLHIV were the fear of disclosing HIV status to the network which mostly was due to the consequences of it like losing their relationships and partnerships after they disclosed their HIV status. The findings are consistent with previous studies in other countries [ 30 , 31 , 32 ]. Another barrier mentioned by most participants was stigma. According to the studies conducted in Kenya, and China, the findings indicated that social stigma associated with HIV remained a prominent issue among participants and had a significant impact on their decision-making process regarding HIV status notification [ 33 , 34 ]. One of the main fast-track targets of HIV control by 2025 is zero stigma and zero discrimination [ 35 ]. However, the results of different studies showed stigma, and discrimination are prevalent from different aspects toward PLHIV. To control the HIV epidemic, countries must reduce stigma and discrimination in societies. Also, to improve the process of HIV notification and use the advantages of social network testing, it is necessary to remove the obstacles to HIV notification.
Besides barriers, some facilitators were also mentioned by the participants for HIV notification. The main facilitator was social support, especially from the family side. Social and family support could help PLHIV to accept their status and explain this to their network. The same results were also explained by the Chinese PLHIV [ 34 ]. Based on the results of studies, social support is important everywhere, but in low-resource settings where services are deficient and individuals need more support from their families and communities, there are stronger incentives to disclose [ 36 , 37 , 38 , 39 ]. Also, the participants in our study stated that the presence of an experienced and enthusiastic consultant, trust, a confidential environment, and non-judgment by the healthcare system can facilitate notification. The result of some studies indicated that some health facilities are often ill-equipped to reassure PLHIV that they will be treated well, and health workers may not have the training to counsel patients on how to disclose their HIV status or to whom it should be disclosed [ 40 , 41 ]. Trained healthcare providers, coupled with factors such as family support, can play a crucial role in facilitating HIV notification and testing, particularly when an individual expresses a desire to undergo testing alongside their partner.
Two main limitations must be considered when interpreting the findings. First, the present study was conducted in one city, and due to differences in social or religious factors in other parts of the country, the results could not be generalized to other parts of the country. Second, behavioral risk factors were self-reported by participants; therefore, the data may have been influenced by recall bias, social desirability, fear of stigma, or other negative consequences of reporting. Therefore, future studies should take into account the differences in cultural and ethnic backgrounds of Iranians when generalizing their findings.
In conclusion, our study showed that there are diverse methods for HIV notification that include notification to PLHIV about their HIV status and notification to their networks. The process of HIV notification shows that the way of disclosing HIV status to PLHIV for the first time has a significant effect on notification to another network. Our findings highlight that despite the various endeavors to diminish stigma and discrimination in recent years, such as educational interventions targeted at healthcare providers, the general population, PLHIV, and key populations, stigma continues to persist as a significant barrier to disclosing one’s HIV status. Additionally, other barriers associated with HIV notification are intertwined with and influenced by the presence of stigma. In light of these findings, it is imperative that public health efforts in Iran and beyond focus on addressing barriers to HIV notification while harnessing the power of facilitators. Based on our results all programs should be directed towards destigmatization. Moreover, our study underscores the need for ongoing research to further elucidate the nuanced dynamics of HIV notification, ultimately contributing to more effective HIV control strategies and improved quality of life for PLHIV.
Availability of data and materials
Data will be available upon request submitted to the corresponding author.
Abbreviations
People living with HIV
Men who have sex with men
Voluntary counseling and testing
Antiretroviral therapy
Obermeyer CM, Baijal P, Pegurri E. Facilitating HIV disclosure across diverse settings: a review. Am J Public Health. 2011;101(6):1011–23.
Article PubMed PubMed Central Google Scholar
Li L, Lin C, Wu Z, Lord L, Wu S. To tell or not to tell: HIV disclosure to family members in China. Dev World Bioeth. 2008;8(3):235–41.
Programme SAAT. Counselling guidelines on disclosure of HIV status. 2000.
World Health Organization. Guidelines on HIV self-testing and partner notification: Supplement to consolidated guidelines on HIV testing services. 2016. https://iris.who.int/handle/10665/251655 . Accessed 30 Apr 2024.
World Health Organization. Consolidated guidelines on HIV testing services: 5Cs: consent, confidentiality, counselling, correct results and connection. 2015. https://www.paho.org/en/documents/consolidated-guidelines-hiv-testing-services-5cs-consent-confidentiality-counselling . Accessed 30 Apr 2024.
Google Scholar
Norman A, Chopra M, Kadiyala S. Factors related to HIV disclosure in 2 South African communities. Am J Public Health. 2007;97(10):1775–81.
Brown LB, Miller WC, Kamanga G, Nyirenda N, Mmodzi P, Pettifor A, et al. HIV partner notification is effective and feasible in sub-Saharan Africa: opportunities for HIV treatment and prevention. J Acquir Immune Defic Syndromes (1999). 2011;56(5):437.
Article Google Scholar
Dalal S, Johnson C, Fonner V, Kennedy CE, Siegfried N, Figueroa C, et al. Improving HIV test uptake and case finding with assisted partner notification services. AIDS. 2017;31(13):1867.
Article PubMed Google Scholar
Chaudoir SR, Fisher JD, Simoni JM. Understanding HIV disclosure: a review and application of the disclosure processes model. Soc Sci Med. 2011;72(10):1618–29.
Dessalegn NG, Hailemichael RG, Shewa-Amare A, Sawleshwarkar S, Lodebo B, Amberbir A, et al. HIV disclosure: HIV-positive status disclosure to sexual partners among individuals receiving HIV care in Addis Ababa, Ethiopia. PLoS One. 2019;14(2):e0211967.
Article CAS PubMed PubMed Central Google Scholar
Hays RB, McKusick L, Pollack L, Hilliard R, Hoff C, Coates TJ. Disclosing HIV seropositivity to significant others. AIDS. 1993;7(3):425–32.
Article CAS PubMed Google Scholar
Maman S, Mbwambo J, Hogan N, Kilonzo G, Sweat M. Women’s barriers to HIV-1 testing and disclosure: challenges for HIV-1 voluntary counselling and testing. AIDS Care. 2001;13(5):595–603.
Driskell JR, Salomon E, Mayer K, Capistrant B, Safren S. Barriers and facilitators of HIV disclosure: perspectives from HIV-infected men who have sex with men. J HIV/AIDS Social Serv. 2008;7(2):135–56.
UNAIDS. Country factsheets, Islamic Republic of Iran. 2019. https://www.unaids.org/en/regionscountries/countries/islamicrepublicofiran . Accessed 30 Apr 2024.
Bengtsson M. How to plan and perform a qualitative study using content analysis. NursingPlus Open. 2016;2:8–14.
Statistical senter of Iran. 2023. https://www.amar.org.ir/ . Accessed 30 Apr 2024.
World Health Organization. HIV/AIDS voluntary counselling and testing. 2002. https://www.who.int/hiv/topics/vct/toolkit/components/policy/review_of_policies_programmes_and_guidelines.pdf . Accessed 30 Apr 2024.
United States Agency for international development, voluntary counseling and testing rigorous evidence – usable results. 2012. https://www.jhsph.edu/research/centers-and-institutes/research-to-prevention/publications/VCT.pdf . Accessed 30 Apr 2024.
Netsanet F, Dessie A. Acceptance of referral for partners by clients testing positive for human immunodeficiency virus. HIV/AIDS-Research Palliat Care. 2013;5:19–28.
Wirawan GBS, Januraga PP, Mahendra I, Harjana NPA, Mahatmi T, Luhukay L, et al. Perspectives on voluntary assisted partner notification among providers, people with HIV and the general population in Indonesia: a formative qualitative study. BMC Public Health. 2021;21(1):1–9.
Golden MR, Faxelid E, Low N. Partner notification for sexually transmitted infections including HIV infection: an evidence-based assessment. New York: Mcgraw-Hill. Sex Transm Dis; 2008. p. 965–83.
Dooley SW, Dubose OT, Fletcher JF, Gunter D, Hogben M, Reid L, et al. Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. 2008.
Graneheim UH, Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today. 2004;24(2):105–12.
Lincoln YS, Guba EG. Naturalistic inquiry. Sage; 1985. https://us.sagepub.com/en-us/nam/naturalistic-inquiry/book842 . Accessed 30 Apr 2024.
Serovich JM, Oliver DG, Smith SA, Mason TL. Methods of HIV disclosure by men who have sex with men to casual sexual partners. AIDS Patient Care STDs. 2005;19(12):823–32.
Adam BD, Corriveau P, Elliott R, Globerman J, English K, Rourke S. HIV disclosure as practice and public policy. Crit Public Health. 2015;25(4):386–97.
Gorbach PM, Galea JT, Amani B, Shin A, Celum C, Kerndt P, et al. Don’t ask, don’t tell: patterns of HIV disclosure among HIV positive men who have sex with men with recent STI practising high risk behaviour in Los Angeles and Seattle. Sex Transm Infect. 2004;80(6):512–7.
Chandra PS, Deepthivarma S, Manjula V. Disclosure of HIV infection in South India: patterns, reasons and reactions. AIDS Care. 2003;15(2):207–15.
Moyer E, Igonya EK, Both R, Cherutich P, Hardon A. The duty to disclose in Kenyan health facilities: a qualitative investigation of HIV disclosure in everyday practice. SAHARA-J: J Social Aspects HIV/AIDS. 2013;10:S60–72.
Adeniyi OV, Ajayi AI, Selanto-Chairman N, Goon DT, Boon G, Fuentes YO, et al. Demographic, clinical and behavioural determinants of HIV serostatus non-disclosure to sex partners among HIV-infected pregnant women in the Eastern Cape, South Africa. PLoS One. 2017;12(8):e0181730.
Yonah G, Fredrick F, Leyna G. HIV serostatus disclosure among people living with HIV/AIDS in Mwanza, Tanzania. AIDS Res Therapy. 2014;11(1):1–5.
Bachanas P, Medley A, Pals S, Kidder D, Antelman G, Benech I, et al. Disclosure, knowledge of partner status, and condom use among HIV-positive patients attending clinical care in Tanzania, Kenya, and Namibia. AIDS Patient Care STDs. 2013;27(7):425–35.
Monroe-Wise A, Maingi Mutiti P, Kimani H, Moraa H, Bukusi DE, Farquhar C. Assisted partner notification services for patients receiving HIV care and treatment in an HIV clinic in Nairobi, Kenya: a qualitative assessment of barriers and opportunities for scale‐up. J Int AIDS Soc. 2019;22:e25315.
Zhang K, Zhao J, Li X, Chen X, Wang H, Williams AB, et al. Perceived facilitators and barriers regarding partner notification in people living with HIV in Hunan, China: a qualitative study from the patient perspective. J Assoc Nurses AIDS Care. 2019;30(6):658–67.
Nawawi F, Nugroho A, Wibowo IR. Breaking the stigma: increasing comprehensive HIV knowledge to end discrimination against people living with HIV. Indonesian J Community Occup Med. 2023;2(3):120–3.
Kalichman SC, DiMarco M, Austin J, Luke W, DiFonzo K. Stress, social support, and HIV-status disclosure to family and friends among HIV-positive men and women. J Behav Med. 2003;26:315–32.
Akani C, Erhabor O. Rate, pattern and barriers of HIV serostatus disclosure in a resource-limited setting in the Niger delta of Nigeria. Trop Doct. 2006;36(2):87–9.
Sethosa E, Peltzer K. Evaluation of HIV counselling and testing, self-disclosure, social support and sexual behaviour change among a rural sample of HIV reactive patients in South Africa. Curationis. 2005;28(1):29–41.
Miller AN, Rubin DL. Motivations and methods for self-disclosure of HIV seropositivity in Nairobi, Kenya. AIDS Behav. 2007;11:687–97.
Datye V, Kielmann K, Sheikh K, Deshmukh D, Deshpande S, Porter J, et al. Private practitioners’ communications with patients around HIV testing in Pune, India. Health Policy Plann. 2006;21(5):343–52.
Turan JM, Bukusi EA, Cohen CR, Sande J, Miller S. Effects of HIV/AIDS on maternity care providers in Kenya. J Obstetric Gynecologic Neonatal Nurs. 2008;37(5):588–95.
Download references
Acknowledgements
We are thankful to the staff of VCT centers in Kerman city as well as all individuals who participated in this study. We would also like to express our gratitude to the Student Research Committee, Kerman University of Medical Sciences, Kerman, Iran.
The study was supported by Kerman University of Medical Sciences (Grant number: 401000288).
Author information
Authors and affiliations.
HIV/STI Surveillance Research Center, and WHO Collaborating Center for HIV Surveillance, Institute for Futures Studies in Health, Kerman University of Medical Sciences, Kerman, Iran
Fatemeh Tavakoli, Ali Akbar Haghdoost, Ali Mirzazadeh & Hamid Sharifi
Reproductive Health, Family and Population Research Center, Kerman University of Medical Sciences, Kerman, Iran
Mahlagha Dehghan
Institute for Global Health Sciences, University of California, San Francisco, CA, USA
Ali Mirzazadeh & Hamid Sharifi
Department of Infectious Diseases and Tropical Medicine, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
Mohammad Mehdi Gouya
You can also search for this author in PubMed Google Scholar
Contributions
FT, MD, and HSH conceptualized the study. FT conducted the interviews. MD, AAH, and HSH provided support while collecting data. FT and MD managed the data. FT, MD, AM, MMG and HSH wrote the first draft. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Mahlagha Dehghan or Hamid Sharifi .
Ethics declarations
Ethics approval and consent to participate.
The study protocol was reviewed and approved by the ethics committee of Kerman University of Medical Sciences (Ethics code: IR.KMU.REC.1401.181). Before each interview, the purpose and methods of the research were explained to potential participants. Oral informed consent was obtained due to HIV stigma and patients’ fear of signing documents in writing. The ethics committee also approved the oral informed consent method. Interviews were conducted with the commitment of the researcher to maintain anonymity and confidentiality of the information and the right of participants to leave the research at any time. Audio recording of the interview was done with the consent of the participants. No participant information was provided to VCT staff, and all interviews were stored in a password-protected folder. The receipt of services was not affected in any way by their participation or non-participation in the interview. The study method was conducted following available regulations and guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Tavakoli, F., Dehghan, M., Haghdoost, A.A. et al. A qualitative study exploring approaches, barriers, and facilitators of the HIV partner notification program in Kerman, Iran. BMC Health Serv Res 24 , 570 (2024). https://doi.org/10.1186/s12913-024-11049-1
Download citation
Received : 08 November 2023
Accepted : 26 April 2024
Published : 02 May 2024
DOI : https://doi.org/10.1186/s12913-024-11049-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Notification
- Sexual partners
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
- Open access
- Published: 24 April 2024
Comparing PrEP initiation rates by service delivery models among high risk adolescent boys and young men in KwaZulu-Natal, South Africa: findings from a population-based prospective study
- Mbuzeleni Hlongwa 1 , 2 ,
- Wisdom Basera 3 , 4 &
- Edward Nicol 3 , 5
BMC Public Health volume 24 , Article number: 1151 ( 2024 ) Cite this article
311 Accesses
Metrics details
Introduction
Pre-exposure prophylaxis (PrEP) is an HIV prevention strategy that can reduce the risk of HIV acquisition by more than 90% if taken consistently. Although South Africa has been implementing PrEP since 2016, initially for selected population groups before expanding access to more people, there is a dearth of research focused on PrEP among adolescent boys and young men (ABYM), despite them experiencing high rates of HIV infection. To address this gap, we compared PrEP initiation rates by service delivery points (SDPs) among ABYM in KwaZulu-Natal, South Africa.
We conducted a population-based prospective study in 22 SDPs from July 2021 to July 2022 in KwaZulu-Natal, South Africa. Sexually active ABYM aged 15–35 years who tested HIV negative were recruited at purposively selected PrEP SDPs (i.e., healthcare facilities, secondary schools and Technical Vocational Education and Training (TVET) colleges, and community-based youth zones). We collected baseline quantitative data from each participant using self-administered electronic questionnaires built into REDCap, including demographic information such as age, sex, employment status and level of education, as well as PrEP initiation outcomes. We extracted data from REDCap and exported it to Stata version 17.0 for analysis, and then eliminated discrepancies and removed duplicates. We described baseline characteristics using summary and descriptive statistics (median, interquartile range [IQR] and proportions) and reported PrEP initiation proportions overall and by SDPs.
The study included 1104 ABYM, with a median age of 24 years (interquartile range (IQR): 21–28)). Almost all participants were black African ( n = 1090, 99%), with more than half aged 15–24 years ( n = 603, 55%) and 45% ( n = 501) aged 25–35 years. The majority ( n = 963; 87%) had attained a secondary level of education. Overall PREP initiation rate among adolescent boys and young men was low: among 1078 participants who were eligible for PrEP, 13% ( n = 141) were started on PrEP. Among the participants who were initiated on PrEP, over three quarters (78%, n = 58) were initiated from high schools, compared with community-based youth zones (40%, n = 37), TVET colleges (26%, n = 16) and healthcare facilities (4%, n = 30).
Conclusions
This study provided evidence suggesting that expanding PrEP services to non-traditional settings, such as high schools, TVET colleges, and community-based organizations, may have a potential to increase PrEP access among ABYM in South Africa.
Peer Review reports
Human immunodeficiency virus (HIV) continues to be a significant public health concern in many parts of the world, consisting of more than 37 million people diagnosed with HIV in year 2020 [ 1 ]. South Africa has a high HIV prevalence, with more than seven million people estimated to be living with HIV [ 2 ]. Adolescent boys and young men (ABYM) are a vulnerable group affected by HIV in South Africa, as they account for a substantial proportion of people newly infected with HIV in South Africa [ 3 ]. In South Africa, ABYM are increasingly getting recognised as a vulnerable population group due to the disproportionately high burden of HIV infection, underscoring the urgent need to address the risk factors, including high-risk sexual behaviours and prioritizing AYBM in HIV prevention strategies. Pre-exposure prophylaxis (PrEP) is an HIV prevention strategy that can reduce the risk of HIV acquisition by more than 90% if taken consistently [ 4 , 5 ]. Although this is the case, ABYM continue to experience high rates of HIV infection in South Africa, despite many efforts designed to address their HIV prevention care and needs [ 6 ]. HIV prevalence is 3.1 among males aged 15–19, 4.0 in 20–24 years, 6.3 in 25–29 years, 10.8 in 30–34 years and 16.9 in 35–39 years [ 7 , 8 ]). Data from the HIV incidence study conducted in uMgungundlovu, and published in 2019 showed that among men aged 15–19, 20–24, 25–29 and 30–35 the HIV incidence rates were 0.24, 1.18, 2.84 and 1.45 per 100 person-years, respectively [ 7 ]. In South Africa, the rollout of oral PrEP started in 2016 with 11 sites in five provinces. An additional site was added in November, to create a total of 12 implementing sites in 2016 [ 9 , 10 ]. Initially, PrEP was implemented among selected key population groups (for example, sex workers) as well as to men who have sex with men; and adolescent girls and young women at primary healthcare facilities, before expanding access to more people, including ABYM. However, there is a dearth of research focused on PrEP among ABYM, despite them experiencing high rates of HIV infection.
In order to improve the rates of PrEP initiation among ABYM, it is imperative that we understand which service delivery models may be effective to ensure that men have appropriate access to PrEP services. It is also important that we understand which service delivery models may contribute to low PrEP initiation rates. One potential factor for PrEP initiation is the service delivery model used to provide PrEP services. For example, traditional facility-based healthcare models may not effectively reach ABYM, due to several barriers men face to accessing healthcare services, including stigma, lack of privacy, long waiting queues, unfriendly healthcare environments and concerns about confidentiality [ 11 , 12 ]. These challenges facing public healthcare facilities have led to the growing appetite for alternative models, including decentralizing models, that are aimed at improving the rates of PrEP initiation, such as community-based service delivery models. Community-based service delivery models may be more acceptable and accessible to AYBM compared to the traditional public healthcare facility-based models [ 13 ]. Research demonstrates that community-based ART service delivery and initiation are successful approaches for increasing HIV treatment access in SSA because they address a number of distinctive barriers related to receiving HIV treatment from clinic settings, including stigma and discrimination, long waiting times, confidentiality concerns, and transport costs [ 11 , 14 , 15 , 16 ].
This study aimed to compare PrEP initiation rates by service delivery models among ABYM in KwaZulu-Natal, South Africa. Our study targeted adolescent boys and young men, a population that is at a greater risk for new HIV infections [ 6 ]. Specifically, this study assessed PrEP initiation rates among ABYM who accessed PrEP through high schools, technical and vocational education and training (TVET) colleges, community-based youth zones (i.e., spaces within communities dedicated to young people providing services focused on sexual and reproductive health and HIV-related services), and public healthcare facilities.
Study setting
This study was conducted in uMgungundlovu district in KwaZulu-Natal province, South Africa (Fig. 1 ). We selected participating facilities in consultation with provincial and district Department of Health. Overall HIV prevalence is high in uMgungundlovu district, accounting for 31% overall among 15–59 years, and 23% among men 15–49 years [ 17 ]. The population of uMgungundlovu is predominantly poor and rural, with the majority using public health services [ 18 ].
Sub-districts of uMgungundlovu District, KwaZulu-Natal, South Africa
Study design
We conducted a population-based prospective study in 22 SDPs over 13 months (July 2021 to July 2022). Adolescent boys and young men were purposively selected (those who visited the SDP while study staff were around) from 22 service delivery points (SDPs) (i.e., healthcare facilities ( n = 11), High schools ( n = 2) and Technical Vocational Education and Training (TVET) colleges ( n = 4), and community-based youth zones( n = 5). We defined PrEP initiation according to national guidelines [ 10 ]. We administered questionnaires and accessed routine healthcare service records at enrolment to obtain and verify HIV test results and PrEP initiation outcomes among participants. The protocol for this study has been previously published [ 19 ].
Population and recruitment
The target population for this study comprised sexually active ABYM aged 15–35 years, who test HIV negative during the routine healthcare facility-based, school-based, and community-based HIV testing services at 22 selected SDPs, in each of the seven sub-districts (strata) (namely, UMshwathi, Umgeni, Mpofana, Impendle, Msunduzi, Mkhambathini and Richmond) in the uMgungundlovu district. Participants were recruited and tracked through surveys using questionnaires and routine pharmaceutical records of PrEP pill collection, and SDP records. The inclusion criteria were: (a) having accepted an HIV test in one of the participating SDPs during the data collection, (b) having access to a cell phone and willingness to provide contact details, (c) be aged 15–35 years, (d) be sexually active and at high risk for HIV (identified by a set of risk assessment questions), (e) be seronegative based on HIV rapid test results on the day of recruitment, and (f) be able and willing to provide informed consent. Potential participants who were diagnosed with TB were excluded in the study.
Data collection and management
Male clients that presented at the 22 SDPs for routine HIV test were approached before the test and recruited into the study. We collected baseline quantitative data from each participant using self-administered electronic questionnaires built into REDCap, including demographic information such as age, sex, employment status and level of education, as well as PrEP initiation outcomes, in addition to a HIV risk assessment test. Participants who had a negative HIV test and were substantially at risk were offered PrEP by staff at the SDPs. The risk assessment tool with the higher HIV risk stated in the parenthesis included a past six [ 6 ] months recall on (a) the number of people one had vaginal or anal sex with (2+), (b) frequency of condom use (no & don’t know), (c) reward based sexual experiences (yes), (d) having a sexually transmitted infection (yes & don’t know), (e) sharing of needles during intravenous drug use (yes) and (f) having a partner who is HIV infected (yes & don’t know). Any higher HIV risk response to one of the questions denoted PrEP eligibility. We also used secondary data sources, including the district health information system to extract information on PrEP initiation in the district and facilities where this project was implemented. Trained and competent facility-based data champions, working together with the project coordinator conducted daily quality checks on completeness of REDCap records on the tablets, before uploading data over 3G or Wi-Fi to the REDCap folder stored securely on the SAMRC’s server. Any data inconsistencies and/or errors were flagged, discussed and rectified at regular quality control meetings.
Data analysis
We extracted data from REDCap and exported it to Stata version 17.0 for analysis, and then eliminated discrepancies and removed duplicates. We dropped 23 participants from our analysis because they did not have PrEP initiation outcomes.
We described baseline characteristics using summary and descriptive statistics (median, interquartile range [IQR] and proportions) and reported PrEP initiation proportions overall and by SDPs.
Ethical considerations
The SAMRC Research Ethics Committee gave its clearance for this project (Ref: EC051-11/2020). Gatekeeper approvals were also obtained from the districts, facilities, and collaborating Provincial Departments of Health (Ref: KZ_202010_033). Prior to their involvement, we sought both verbal and written informed consent from all eligible study participants. Participants were informed throughout recruitment that participation was completely voluntary and that they were free to withdraw their participation at any time without facing any repercussions from the research team or the facilities they use.
The study included 1104 ABYM recruited from 22 SDPs (Fig. 2 ), with a median age of 24 years (interquartile range (IQR): 21–28)) (Table 1 ). Almost all participants were black African ( n = 1090, 99%), with more than half aged 15–24 years ( n = 603, 55%) and 45% ( n = 501) aged 25–35 years. The majority ( n = 963; 87%) had attained a secondary level of education.
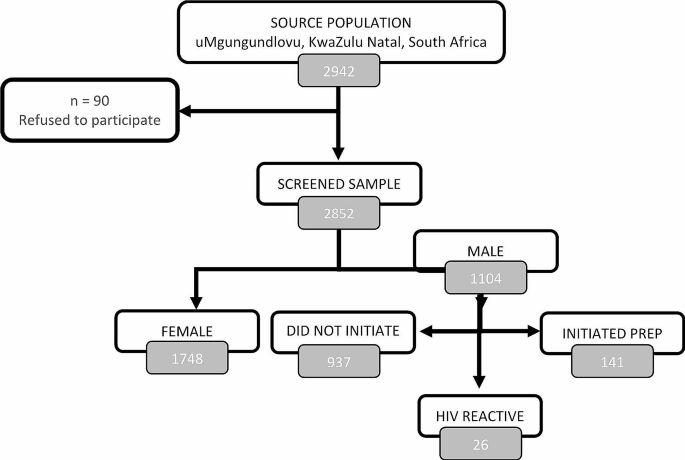
Consort diagram detailing the recruitment of study participants into the PrEP initiation in uMgungundlovu district
PrEP initiation rates among adolescent boys and young men
Overall, PrEP initiation rate among adolescent boys and young men was low: among 1078 participants who were eligible for PrEP, 13% ( n = 141) were started on PrEP (Table 2 ). Twenty-six (2%) participants were diagnosed with HIV, with 89% ( n = 23) reactive participants coming from clinics and 11% ( n = 3) from TVET colleges.
Another notable finding in our study is the disparities in PrEP initiation rates among different types of service delivery points. For example, among the participants who were initiated on PrEP, over three quarters (78%, n = 58) were initiated from high schools, compared with community-based youth zones (40%, n = 37), TVET colleges (26%, n = 16) and healthcare facilities/clinics (4%, n = 30).
Over three quarters (79%, n = 849) of the participants had a high risk profile of HIV infection based on the risk screening tool and were eligible for PrEP. A high proportion (61%, n = 661) of participants self-reported as willing to consider taking PrEP, with 16% ( n = 107) of those who went on to initiate it. Amongst the reasons cited by those at a higher HIV risk profile ( n = 849) for non willingness to consider PrEP, the top three were – I don’t want to be taking drugs for a long time (13%, n = 112), I fear side effects (11%, n = 91) and I do not think I am at risk of acquiring HIV (10%, n = 84).
This study aimed to compare PrEP initiation rates by service delivery models among ABYM in KwaZulu-Natal, South Africa. Similar to our study, PrEP initiation rates among men were reported to be low in Eswatini [ 20 ]. This supports the notion that PrEP access and uptake is a widespread challenge in the region, given the several factors and barriers identified to be affecting men’s access and initiation to PrEP in SSA, including stigma, discrimination, lack of knowledge and awareness of PrEP, inaccessibility of PrEP services, misinformation, fear of side effects and PrEP pill burden [ 21 , 22 , 23 ]. These findings suggest that there is a need for targeted educational interventions aimed to promote PrEP awareness and improve PrEP initiation rates among ABYM in KwaZulu-Natal, and in similar settings elsewhere.
Specifically, our study found that healthcare facilities had lower rates of PrEP initiation compared to high schools and TVET colleges, and community-based youth zones. This result suggests that the traditional facility-based may not be the most effective approach for reaching adolescent boys and young men with PrEP services. Instead, our study findings suggest that the community-based youth zones and high schools and TVET colleges may be promising additional models to traditional healthcare facilities for reaching men through distributing PrEP services.
Our finding reporting low rates of PrEP initiation from healthcare facilities, compared to community-based youth zones and high schools and TVET colleges was not surprising, given the well documented barriers deterring men from accessing services from the healthcare facilities in SSA [ 11 ]. In South Africa, for example, compared with women, men are less likely to access healthcare services, due to many factors including stigma, long waiting queues, masculinity and unfriendly healthcare environments [ 12 ]. However, our findings are consistent with global efforts to decentralize HIV prevention and treatment services to community-based settings in an effort to improve access to HIV treatment.
Evidence shows that community-based HIV services are an effective strategy for improving access to HIV treatment in SSA, as it addresses several distinctive barriers associated with accessing HIV treatment from clinic settings [ 14 , 15 , 16 ]. Therefore, in order to address the low rates of PrEP initiation among ABYM in KwaZulu-Natal, our findings suggest that there may be a need to adopt a multi-pronged approach that may include targeted outreach and education interventions, as well as decentralizing PrEP services to non-traditional healthcare settings. This may include strengthening and expanding partnering with high schools and TVET colleges, as well as community-based organisations to provide education and counselling, as well as improving PrEP access for men. This could involve developing and/or strengthening tailored educational materials, peer-led counselling sessions, and targeted outreach initiatives to increase knowledge of PrEP among males who might not be currently receiving these services.
A key limitation for this study relates to the fact that this paper does not address the factors influencing PrEP initiation rates among ABYM. Instead, these are currently being analyzed qualitatively, and will be discussed in a separate publication.
In conclusion, the findings from this study have important indications for efforts to improve PrEP access among ABYM in KwaZulu-Natal, South Africa. By expanding PrEP services to non-traditional settings, such as high schools, TVET colleges, and community-based organizations, there is potential to increase PrEP access and reduce the burden of HIV among men. These efforts may be particularly important in the context of the ongoing HIV epidemic in South Africa.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
UNAIDS, Global. AIDS update 2021: confronting inequalities: lessons for pandemic responses from 40 years of AIDS. Switzerland: UNAIDS; 2021.
Google Scholar
UNAIDS. South Africa Country factsheets: HIV and AIDS estimates. Switzerland: UNAIDS; 2021.
Simbayi L, Zuma K, Zungu N, Moyo S, Marinda E, Jooste S et al. South African National HIV prevalence, incidence, behaviour and communication survey, 2017: Towards achieving the UNAIDS 90-90-90 targets. 2019.
Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410.
Article CAS PubMed PubMed Central Google Scholar
Centers for Disease Control and Prevention. Pre-exposure Prophylaxis (PrEP). Centers for disease control and prevention; 2022.
UNAIDS. A snapshot of men and HIV in South Africa. [press release]. Geneva: UNAIDS; 2017.
Kharsany AB, Cawood C, Lewis L, Yende-Zuma N, Khanyile D, Puren A, et al. Trends in HIV prevention, treatment, and incidence in a hyperendemic area of KwaZulu-Natal, South Africa. JAMA Netw Open. 2019;2(11):e1914378–e.
Article PubMed PubMed Central Google Scholar
Human Sciences Research Council. Summary sheet: the Sixth South African National HIV Prevalence, Incidence, Behaviour and Communication Survey. Pretoria: Human Sciences Research Council; 2023.
SA Department of Health. Guidelines for expanding combination prevention and treatment options for sex workers: oral pre-exposure prophylaxis (PrEP) and test and treat (T&T). Final draft. Pretoria, South Africa. South African National Department of Health; 2016.
SA Department of Health. 2021 Updated guidelines for the provision of Oral Pre-Exposure Prophylaxis (PrEP) to persons at substantial risk of HIV infection. Pretoria: SA National Department of Health; 2021.
Hlongwa M, Mashamba-Thompson T, Makhunga S, Hlongwana K. Barriers to HIV testing uptake among men in sub-saharan Africa: a scoping review. Afr J AIDS Res. 2020;19(1):13–23.
Article PubMed Google Scholar
Leichliter JS, Paz-Bailey G, Friedman AL, Habel MA, Vezi A, Sello M et al. ‘Clinics aren’t meant for men’: sexual health care access and seeking behaviours among men in Gauteng province, South Africa. SAHARA-J: Journal of Social Aspects of HIV/AIDS. 2011;8(2).
Ramraj T, Chirinda W, Jonas K, Govindasamy D, Jama N, Appollis TM, et al. Service delivery models that promote linkages to PrEP for adolescent girls and young women and men in sub-saharan Africa: a scoping review. BMJ open. 2023;13(3):e061503.
Barnabas RV, Szpiro AA, van Rooyen H, Asiimwe S, Pillay D, Ware NC, et al. Community-based antiretroviral therapy versus standard clinic-based services for HIV in South Africa and Uganda (DO ART): a randomised trial. Lancet Global Health. 2020;8(10):e1305–15.
Nachega JB, Adetokunboh O, Uthman OA, Knowlton AW, Altice FL, Schechter M, et al. Community-based interventions to improve and sustain antiretroviral therapy adherence, retention in HIV care and clinical outcomes in low-and middle-income countries for achieving the UNAIDS 90-90-90 targets. Curr HIV/AIDS Rep. 2016;13:241–55.
Eshun-Wilson I, Awotiwon AA, Germann A, Amankwaa SA, Ford N, Schwartz S, et al. Effects of community-based antiretroviral therapy initiation models on HIV treatment outcomes: a systematic review and meta-analysis. PLoS Med. 2021;18(5):e1003646.
HIV Data. South Africa District HIV estimates [Internet]. 2020. https://www.hivdata.org.za/ .
Statistics South Africa. General Household Survey. 2019. Pretoria, South Africa: Statistics South Africa; 2020.
Nicol E, Ramraj T, Hlongwa M, Basera W, Jama N, Lombard C, et al. Strengthening health system’s capacity for pre-exposure prophylaxis for adolescent girls and young women and adolescent boys and young men in South Africa (SHeS’Cap–PrEP): protocol for a mixed methods study in KwaZulu-Natal, South Africa. PLoS ONE. 2022;17(3):e0264808.
Berner-Rodoreda A, Geldsetzer P, Bärnighausen K, Hettema A, Bärnighausen T, Matse S, et al. It’s hard for us men to go to the clinic. We naturally have a fear of hospitals. Men’s risk perceptions, experiences and program preferences for PrEP: a mixed methods study in Eswatini. PLoS ONE. 2020;15(9):e0237427.
Muhumuza R, Ssemata AS, Kakande A, Ahmed N, Atujuna M, Nomvuyo M, et al. Exploring perceived barriers and facilitators of PrEP uptake among young people in Uganda, Zimbabwe, and South Africa. Arch Sex Behav. 2021;50(4):1729–42.
Nabunya R, Karis V, Nakanwagi LJ, Mukisa P, Muwanguzi PA. Barriers and facilitators to oral PrEP uptake among high-risk men after HIV testing at workplaces in Uganda: a qualitative study. BMC Public Health. 2023;23(1):1–13.
Article Google Scholar
Shamu S, Shamu P, Khupakonke S, Farirai T, Chidarikire T, Guloba G, et al. Pre-exposure prophylaxis (PrEP) awareness, attitudes and uptake willingness among young people: gender differences and associated factors in two South African districts. Global Health Action. 2021;14(1):1886455.
Download references
Acknowledgements
Not applicable.
This work was supported by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention, under the terms of Cooperative Agreement Number 1 NU2GGH002193-01-00.
Author information
Authors and affiliations.
Public Health, Societies and Belonging, Human Sciences Research Council, Pretoria, South Africa
Mbuzeleni Hlongwa
School of Nursing and Public Health, University of KwaZulu-Natal, Durban, South Africa
Burden of Disease Research Unit, South African Medical Research Council, Cape Town, South Africa
Wisdom Basera & Edward Nicol
School of Public Health and Family Medicine, University of Cape Town, Cape Town, South Africa
Wisdom Basera
Division of Health Systems and Public Health, Stellenbosch University, Cape Town, South Africa
Edward Nicol
You can also search for this author in PubMed Google Scholar
Contributions
MH drafted the first draft of the manuscript. EN and WB provided critical contributions towards developing and refining the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Mbuzeleni Hlongwa .
Ethics declarations
Ethics approval and consent to participate.
The South African Medical Research Council (SA MRC) Ethics Committee approved this study. Prior to their involvement, we sought both verbal and written informed consent from all eligible study participants. All procedures performed in studies involving human participants adhered to the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from a parent and/or legal guardian for participants below 16 years of age.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Hlongwa, M., Basera, W. & Nicol, E. Comparing PrEP initiation rates by service delivery models among high risk adolescent boys and young men in KwaZulu-Natal, South Africa: findings from a population-based prospective study. BMC Public Health 24 , 1151 (2024). https://doi.org/10.1186/s12889-024-18660-1
Download citation
Received : 07 June 2023
Accepted : 18 April 2024
Published : 24 April 2024
DOI : https://doi.org/10.1186/s12889-024-18660-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- PrEP initiation rates
- Service delivery models
- KwaZulu-Natal
- South Africa
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 30, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Visa rules jeopardize HIV management, study finds
by Monash University
A Monash University sexual health expert has warned that an unintended consequence of Australia's migration rules could compromise Australia's goal to end the HIV epidemic by 2030.
Associate Professor Jason Ong, of the Melbourne Sexual Health Center (MSHC), at Monash University's School of Translational Medicine, says some people living with HIV are choosing cheaper, suboptimal antiretroviral treatment (ART) out of fear that their applications for permanent residency (PR) will be rejected.
This is because they must show their medical spending will not total more than $51,000 over 10 years—a requirement, known as the Significant Cost Threshold, designed to screen out applicants who might pose extra costs to Australia's health system.
"Being on the most effective treatments puts many people beyond that threshold , resulting in automatic rejection of their application for PR," said Associate Professor Ong, whose findings were published in the journal Sexual Health .
"Unfortunately, cheaper treatments aren't as good at controlling HIV, and they're not as safe."
Australia is a world leader in extending PrEP (an antiviral medicine that prevents HIV) to groups at risk of HIV, and is working towards elimination of HIV transmission by 2030. This does not mean zero new cases of HIV, but the absence of sustained endemic community transmission.
The study involved presenting the journeys of six patients with a mean age of 39 years living with HIV and migrating to Australia from Asian and European countries.
"We know that overseas-born gay and bisexual men are showing slower declines in transmission," Associate Professor Ong said. "It's important to bring this group with us as we work towards elimination."
"Thanks to antiretroviral therapies, HIV is now a manageable, chronic disease. It benefits everyone in the community if people living with HIV are on the right treatments."
Dash Heath-Paynter, the CEO of Health Equity Matters—the national federation for the HIV community response—urged the Federal Government to examine New Zealand's 2022 decision to raise its medical expenses threshold from NZ$41,000 to $81,000.
"There may be other options to help solve this problem, but raising the significant medical costs threshold would be a very good starting point," Mr. Heath-Paynter said.
Explore further
Feedback to editors

Children and adolescents enjoy learning new words, study finds

Dietary changes may help treat pulmonary hypertension
2 hours ago

Scientists track 'doubling' in origin of cancer cells

Study elucidates how energy metabolism is regulated at cellular level
3 hours ago

Study finds metformin reduces COVID-19 viral load, viral rebound

Adding AI to artificial pancreas enhances efficiency, study finds

Researchers discover how immune B cells hunt down cancer around the body

Sea slugs inspire highly stretchable biomedical sensor

Study in women shows significant link between regular exercise during middle-age and physical health in later life
4 hours ago

Synchronization between central and circadian clocks of tissues found to preserve their functioning, prevent aging
Related stories.

International migrants left behind in HIV response: Study
Jul 6, 2023

70% of Australians living with HIV report their health-related quality of life to be good
Dec 2, 2022
Research finds the most sexually adventurous gay and bisexual men are also the most vigilant with HIV prevention
Oct 30, 2023
HIV in Australia has declined by 12% in the past five years
Nov 30, 2020
HIV diagnoses in Australia remained low in 2022
Jul 20, 2023

Study finds tafenoquine is a cost-effective treatment option for malaria
Jan 11, 2024
Recommended for you

Companies may still buy consumer genetic information despite its modest predictive power
7 hours ago

Closing the US/Mexico border during COVID-19 increased HIV transmission, study finds
May 1, 2024

Study finds private equity expanding to mental health facilities

Patients diagnosed with cancer in prison more likely to die from the disease, research shows
Apr 30, 2024

Early gestational diabetes treatment shown to reduce birth complications, health costs for those at higher risk

Study highlights importance of early interventions to combat HIV
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
In psychedelic therapy, clinician-patient bond may matter most
Study links relationship strength to reduced depression for up to 1 year.
Drug effects have dominated the national conversation about psychedelics for medical treatment, but a new study suggests that when it comes to reducing depression with psychedelic-assisted therapy, what matters most is a strong relationship between the therapist and study participant.
Researchers analyzed data from a 2021 clinical trial that found psilocybin (magic mushrooms) combined with psychotherapy in adults was effective at treating major depressive disorder.
Data included depression outcomes and participant reports about their experiences with the drugs and their connection with therapists. Results showed that the stronger the relationship between a participant and clinician -- called a therapeutic alliance -- the lower the depression scores were one year later.
"What persisted the most was the connection between the therapeutic alliance and long-term outcomes, which indicates the importance of a strong relationship," said lead author Adam Levin, a psychiatry and behavioral health resident in The Ohio State University College of Medicine.
Past research has consistently found that as mental health treatments changed, a trusting relationship between clients and clinicians has remained key to better outcomes, said senior author Alan Davis, associate professor and director of the Center for Psychedelic Drug Research and Education in The Ohio State University College of Social Work.
"This concept is not novel. What is novel is that very few people have explored this concept as part of psychedelic-assisted therapy," Davis said. "This data suggests that psychedelic-assisted therapy relies heavily on the therapeutic alliance, just like any other treatment."
The study was published recently in the journal PLOS ONE .
Twenty-four adults who participated in the trial received two doses of psilocybin and 11 hours of psychotherapy. Participants completed the therapeutic alliance questionnaire, assessing the strength of the therapist-participant relationship, three times: after eight hours of preparation therapy and one week after each psilocybin treatment.
Participants also completed questionnaires about any mystical and psychologically insightful experiences they had during the drug treatment sessions. Their depression symptoms were assessed one week, four weeks, and up to one year after the trial's end.
The analysis showed that the overall alliance score increased over time and revealed a correlation between a higher alliance score and more acute mystical and/or psychologically insightful experiences from the drug treatment. Acute effects were linked to lower depression at the four-week point after treatment, but were not associated with better depression outcomes a year after the trial.
"The mystical experience, which is something that is most often reported as related to outcome, was not related to the depression scores at 12 months," Davis said. "We're not saying this means acute effects aren't important -- psychological insight was still predictive of improvement in the long term. But this does start to situate the importance and meaning of the therapeutic alliance alongside these more well-established effects that people talk about."
That said, the analysis showed that a stronger relationship during the final therapy preparation session predicted a more mystical and psychologically insightful experience -- which in turn was linked to further strengthening the therapeutic alliance.
"That's why I think the relationship has been shown to be impactful in this analysis -- because, really, the whole intervention is designed for us to establish the trust and rapport that's needed for someone to go into an alternative consciousness safely," Davis said.
Considering that psychedelics carry a stigma as Schedule I drugs under the Controlled Substances Act, efforts to minimize negative experiences in future studies of their therapeutic potential should be paramount -- and therapy is critical to creating a supportive environment for patients, the authors said.
This study ideally will help clearly position psychedelics treatment as a psychotherapeutic intervention moving forward -- rather than its primary purpose being administration of a drug, Levin said.
"This isn't a case where we should try to fit psychedelics into the existing psychiatric paradigm -- I think the paradigm should expand to include what we're learning from psychedelics," Levin said. "Our concern is that any effort to minimize therapeutic support could lead to safety concerns or adverse events. And what we showed in this study is evidence for the importance of the alliance in not just preventing those types of events, but also in optimizing therapeutic outcomes."
This work was supported by the Center for Psychedelic and Consciousness Research, funded by the Steven & Alexandra Cohen Foundation, the RiverStyx Foundation and private donors. It was also supported by the Center for Psychedelic Drug Research and Education (CPDRE), funded by anonymous donors.
Additional co-authors are Rafaelle Lancelotta, Nathan Sepeda and Theodore Wagener of Ohio State, and Natalie Gukasyan, Sandeep Nayak, Frederick Barrett and Roland Griffiths of the Center for Psychedelic and Consciousness Research at Johns Hopkins University, where Davis is an affiliate.
- Mental Health Research
- Personalized Medicine
- Gene Therapy
- Pharmacology
- Psychedelic Drugs
- Mental Health
- Spirituality
- Psychotherapy
- Psychedelic mushroom
- Personalized medicine
- Maggot therapy
- Psychopharmacology
Story Source:
Materials provided by Ohio State University . Original written by Emily Caldwell. Note: Content may be edited for style and length.
Journal Reference :
- Adam W. Levin, Rafaelle Lancelotta, Nathan D. Sepeda, Natalie Gukasyan, Sandeep Nayak, Theodore L. Wagener, Frederick S. Barrett, Roland R. Griffiths, Alan K. Davis. The therapeutic alliance between study participants and intervention facilitators is associated with acute effects and clinical outcomes in a psilocybin-assisted therapy trial for major depressive disorder . PLOS ONE , 2024; 19 (3): e0300501 DOI: 10.1371/journal.pone.0300501
Cite This Page :
Explore More
- Random Robots Are More Reliable
- Significant Discovery in Teleportation Research
- Orangutan Treats Wound With Pain-Relieving Plant
- 75,000-Year-Old Neanderthal from Burial Cave
- Anticoagulant With an On-Off Switch
- Sleep Resets Brain Connections -- At First
- Far-Reaching Effects of Exercise
- Hidden Connections Between Brain and Body
- Novel Genetic Plant Regeneration Approach
- Early Human Occupation of China
Trending Topics
Strange & offbeat.
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
Methodology: 2023 focus groups of Asian Americans
Table of contents.
- About the focus groups
- Participant recruitment procedures
- Moderator and interpreter qualification
- Data analysis
- Sample design
- Data collection
- Weighting and variance estimation
- Analysis of Asians living in poverty
- Acknowledgments
Pew Research Center created and designed this focus groups plan to understand the experiences and perspectives of Asians living with economic hardship in the United States. The analysis presented in this data essay provides insight into these topics and is not an exhaustive representation of public opinion on these topics or of specific demographic groups.
This research was conducted by PSB Insights for Pew Research Center and was reviewed by an IRB (internal review board) for human subject research.
Pew Research Center conducted 18 in-person focus groups with 144 adult participants from across the United States from Feb. 7 to Feb. 23, 2023. Recruited participants are from 11 Asian origin groups. Each person was offered an incentive amount of $225 to participate. Focus groups were 1.5 hours in length. Guides for immigrant Asian focus groups were translated into 11 non-English languages. ( Read the moderator guide for more.)
The topics for discussion and approximate allotted time during the focus groups were as follows:

Study design and group criteria
For each focus group, 10 participants were recruited and eight were ultimately selected to participate. The research team overrecruited participants to account for “no-shows,” as well as participants who may experience other issues preventing participation.
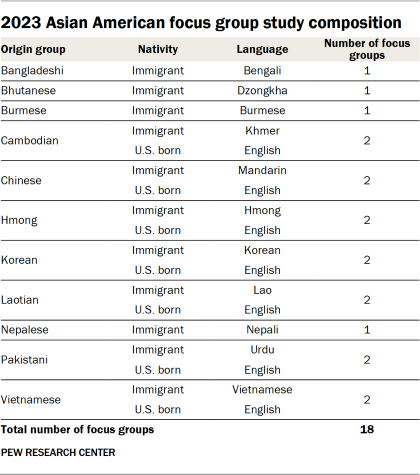
The study included focus groups from 11 distinct Asian origin groups. These are among the most likely Asian origin groups to live in poverty in the U.S. in 2019. These included participants from three (Chinese, Korean and Vietnamese) of the six largest U.S. Asian origin groups . The study also included participants from eight less populous origin groups (Bangladeshi, Bhutanese, Burmese, Cambodian, Hmong, Laotian, Nepalese and Pakistani). These ethnic origin groups were selected to include voices of those from smaller populations.
Focus groups were further stratified by nativity for selected origin groups. Researchers classified participants into either “U.S. born” or “immigrant” focus groups based on self-reported birthplace and immigration information provided in the screener survey.
Recruitment of focus group participants was conducted via local Asian community member networks, organized by language and ethnicity. Recruitment was conducted in person following guidance on COVID-19 restrictions (i.e., personal visits, community meetings, etc.), via social media (e.g., Facebook, Line, WeChat, WhatsApp) or by phone or email.
All potential participants were screened for eligibility based on a questionnaire designed by Pew Research Center, which included criteria such as ethnic origin affiliation, country of birth, length of time in the U.S. (for immigrants), household income and other demographic profile questions. Once eligible participants were identified, they confirmed if they were available for the focus group and signed an informed consent form.
All participants had to meet the following five criteria to be eligible to participate:
- Live within or near the states corresponding to where the research was conducted (California, Minnesota/Wisconsin, New York/New Jersey).
- Belong to the ethnic origin group of the respective focus group audience and fluently speak the language the group was conducted in.
- Be between the ages of 25 and 59.
- Have a household income that qualified them as low income for the purposes of this study – defined as earning a maximum household income of 140%-250% of the federal poverty line. Thresholds varied by recruitment city to account for differences in the cost of living.
- Meet “soft quotas” set for the demographic characteristics of gender, education and location of birth to ensure a diverse mix of participants within each focus group. (Soft quotas are flexible recruitment targets that can be adjusted based on realities of recruitment and sample target.)
In order to qualify for the U.S.-born groups, participants had to have been either:
- Born in the U.S., or
- Born outside the U.S., but arrived in the country before the age of 7.
To qualify for the immigrant group, participants had to have been either:
- Born outside the U.S. and arrived in the country after the age of 15, or
- Born outside the U.S., arrived in the U.S. between the ages of 7 to 15, and were determined to be connected to the culture of their home country to a degree similar to people born outside the country and immigrated to the U.S. after age 15. This determination was based on a series of questions decided upon by the research team. These questions asked about whether they consume media in English or their native language; how often they participate in holidays and practices associated with their origin country; how well they remember growing up in their origin country; and how many of their friends are Asian but of a different ethnicity than theirs. Respondents were invited to participate in the immigrant focus group only if their answers to at least three of the four questions were considered similar to those who immigrated after age 15 (i.e., they consume media in their native language more often or equally as often as in English; participate in their origin country’s holidays and practices very or somewhat often; remember growing up in the origin country very or somewhat well; and at least some of their friends are Asian but of a different ethnicity).
All focus groups were conducted in person. The table below details locations, which Asian origin groups were recruited, and low-income thresholds applied.
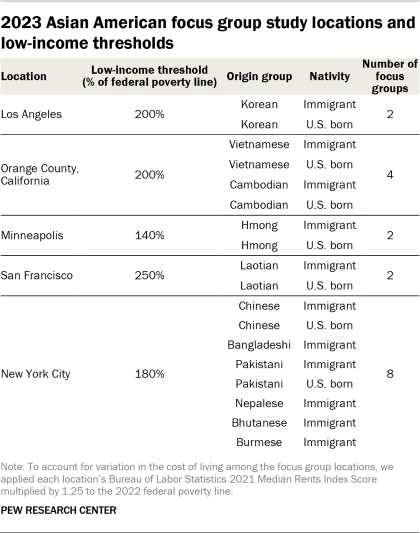
Eligible participants were identified by PSB and then reviewed and approved by the Pew Research Center team for participation. For each focus group, researchers selected eight preferred participants from the 10 recruited participants, ensuring diversity across dimensions for which there were no hard quotas, including gender, educational qualifications and partisan identification. If one of the eight preferred participants did not show up to the focus group or experienced other difficulties to the extent that they could not participate, they were replaced by one of the two overrecruited participants. When replacing participants, the research team strove to keep the groups as diverse as possible with a special emphasis on maintaining gender parity.
PSB Insights partnered with GC Global to help facilitate focus group moderation and interpretation. Recruitment for focus group moderators emphasized the importance of having native language speakers as well as matching moderators’ ethnicity to each origin group. This would help build rapport and facilitate an open conversation with participants. All moderators were briefed on the discussion guide and research objectives prior to the focus groups by PSB Insights. For less experienced moderators, an additional moderator training session was provided.
The research team also hired interpreters to provide on-site simultaneous translations when immigrant focus groups were conducted.
Focus group conversations were video recorded. All conversations were transcribed, translated if conducted in a non-English language and checked for transcription errors. To analyze the focus group transcripts, Center researchers used ATLAS.ti, a qualitative data analysis and research software.
All 18 focus group transcripts were coded using the following structure for individual participants within each focus group:
- Origin group
- Employment status
- Immigration generation
- Years in the U.S. (for immigrant participants)
The transcripts were also coded by topic, including but not limited to:
- Current economic situation
- Communities and resources
- The American dream
- Immigration experiences (for immigrant focus groups)
- Experiences growing up (for U.S.-born focus groups)
Within each topic, we used a list of detailed codes to identify the theme of each response. For example, the research team used the following codes to label responses related to discussions on attitudes toward reaching the American dream:
- (code label: “American dream: easy to achieve”)
- (code label: “American dream: hard to achieve”)
Several quality control checks were conducted. After coding all transcript documents, researchers viewed organized quotes with demographic codes alongside them. A different researcher then evaluated the codes and made suggestions for changes. Any discrepancies were resolved between the primary coding researcher and the reevaluating researcher. When they could not reach an agreement, the lead researcher would reconcile the coding and set standardized coding practices for similar quotes.
The finalized quotations were exported by research topics into a spreadsheet where each row represented one quote. The research team identified the themes within each detailed topic code and rearranged and extracted quotations into different themes for publication.
While we highlight the sentiments expressed by individual participants in the data essay, they are chosen to highlight the themes discussed by the group more broadly. They are not necessarily representative of the majority opinion in any group.
Quotations in the data essay have been lightly edited for grammar, spelling and clarity.
Sign up for our weekly newsletter
Fresh data delivery Saturday mornings
Sign up for The Briefing
Weekly updates on the world of news & information
- Asian Americans
- Homeownership & Renting
- Income & Wages
- Income, Wealth & Poverty
- Personal Finances
1 in 10: Redefining the Asian American Dream (Short Film)
The hardships and dreams of asian americans living in poverty, black americans’ views on success in the u.s., parents, young adult children and the transition to adulthood, older workers are growing in number and earning higher wages, most popular, report materials.
- Moderator Guide
1615 L St. NW, Suite 800 Washington, DC 20036 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Age & Generations
- Coronavirus (COVID-19)
- Economy & Work
- Family & Relationships
- Gender & LGBTQ
- Immigration & Migration
- International Affairs
- Internet & Technology
- Methodological Research
- News Habits & Media
- Non-U.S. Governments
- Other Topics
- Politics & Policy
- Race & Ethnicity
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
Copyright 2024 Pew Research Center
Terms & Conditions
Privacy Policy
Cookie Settings
Reprints, Permissions & Use Policy

IMAGES
VIDEO
COMMENTS
NIAID is committed to conducting the research necessary to confront HIV/AIDS. Volunteer participation in a clinical research study helps NIAID better understand HIV, find promising new tools to prevent HIV infection including a vaccine, and develop new and more effective treatment strategies. The following are select studies sponsored by NIAID.
A clinical trial is a research study done to evaluate new medical approaches in people. HIV and AIDS clinical trials help researchers find better ways to prevent, detect, or treat HIV and AIDS. Examples of HIV and AIDS clinical trials underway include studies of new HIV medicines, studies of vaccines to prevent or treat HIV, and studies of medicines to treat infections related to HIV and AIDS.
Previous research on differences among racial or ethnic groups in recruitment for clinical trials has produced varied results, possibly because of differences in study design or because of disease ...
An effective and scalable cure strategy is a top priority for the HIV research field; this Review discusses recent advances, knowledge gaps, and priority research areas for the next 5 years.
The primary foci of the studies in this research - on HIV or HIV risk, and/or problem substance use - prompted questions about stigma from participating in these studies. ... reporting that there was an assumption of negative behavior if they participate in studies such as these (e.g. risk reduction interventions). However, one reported ...
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has launched a Phase 1 clinical trial evaluating three experimental HIV vaccines based on a messenger RNA (mRNA) platform—a technology used in several approved COVID-19 vaccines. NIAID is sponsoring the study, called HVTN 302, and the ...
Introduction. Community engagement is widely recognized as an essential component of HIV clinical trials.We define community engagement as interactive activities used to gain insights from communities affected by HIV on clinical trial design, recruitment, implementation, and dissemination of research findings .Engaging community stakeholders can help researchers address community concerns more ...
As a participant in an HVTN HIV/AIDS-related research study, you have the right to: Have all known information, including potential risks and benefits of study participation, presented to you in a way you can understand. You will be told about any new information learned during the course of the study.
To enroll in or get more information about research studies at the HNRP, please call (619) 543-5000 and ask to speak with a research recruiter. You may be eligible to participate if you: •are at least 18 years old •are HIV+ or HIV- (do not have HIV) •do or do not have a history of substance use For participating you will receive: •Monetary compensation •Lab results (at no cost to you ...
We support screening and recruitment of eligible people living with HIV (PLWH) into clinical and pathogenesis research studies via a dedicated research nurse.This service is available to investigators to increase enrollment of PLWH into research studies. Lindsay Legg, the HIV research coordinator, is effective at increasing enrollment in HIV ...
What are some reasons to participate in HIV cure research? A UNC research study participant, Rob, shares his personal reasons, and describes his involvement in recent studies. Dr. David Margolis, who leads HIV cure research at UNC, joins the conversation and describes where cure research is today, including specifics on Vorinostat trials, and where their … Read more
Providing incentives to research participants—including money, gifts, and services—is an accepted and common practice in biomedical HIV research. 1 Here, we focus on financial incentives: "[p]ayment to research subjects for participation in studies." 2 Although some studies suggest that altruism motivates many people to participate in ...
Undertake your study without worrying about recruiting HIV research participants and leave recruitment to Rare Patient Voice As of this writing, we have over 960 patients and more than 112 caregivers in our HIV research survey panel who are ready to take part in your study. ... Participate in Research. If you are an HIV patient or a caregiver ...
Method and design. In this qualitative study, we used a conventional content analysis method [] with a specific focus on three key participant groups: PLHIV, the sexual partners of PLHIV, and the staff members of the voluntary counseling and testing (VCT) center located in Kerman.Kerman is a city with a total population of about 537,000 in southeast Iran [].
Introduction Pre-exposure prophylaxis (PrEP) is an HIV prevention strategy that can reduce the risk of HIV acquisition by more than 90% if taken consistently. Although South Africa has been implementing PrEP since 2016, initially for selected population groups before expanding access to more people, there is a dearth of research focused on PrEP among adolescent boys and young men (ABYM ...
1 INTRODUCTION. South Africa had an estimated 7.6 million people living with HIV in 2022, of whom 2.6 million (34%) are men aged ≥15 years [].Despite the implementation of the "universal test and treat" programme in 2016, there were 150,000 new HIV acquisitions among people aged ≥15 years in 2022, with 52,000 attributed to men [], implying that men face a substantial burden of HIV.
Youths' motivations to participate in the research study. Even though HIV-related research is prominent in Kenya and HIV testing is free and widely offered in local clinics, our interviewees (n = 47) indicated that HIV testing and knowing one's status were the most important motivators for study participation.
HIV/AIDS. HIV, or human immunodeficiency virus, is the virus that causes AIDS (acquired immunodeficiency syndrome) and can be transmitted during sexual intercourse; by sharing syringes; or perinatally during pregnancy, childbirth or breastfeeding. Since the first AIDS cases were reported in 1981, HIV/AIDS has been one of humanity's deadliest ...
Research finds the most sexually adventurous gay and bisexual men are also the most vigilant with HIV prevention Oct 30, 2023 HIV in Australia has declined by 12% in the past five years
Pew Research Center designed these focus groups to better understand how members of an ethnically diverse Asian population think about their place in America and life here. By including participants of different languages, immigration or refugee experiences, educational backgrounds, and income levels, this focus group study aimed to capture in ...
1. Introduction. The field of HIV cure-related research is challenging and rapidly evolving. 1 Current studies vary in design and focus on gaining knowledge of how to achieve a "cure", understood as either complete eradication of replication-competent virus ('sterilizing cure') or as reaching a durable control of HIV in the absence of antiretroviral therapy (ART) ('functional cure ...
Drug effects have dominated the national conversation about psychedelics for medical treatment, but a new study suggests that when it comes to reducing depression with psychedelic-assisted therapy ...
For each focus group, 10 participants were recruited and eight were ultimately selected to participate. The research team overrecruited participants to account for "no-shows," as well as participants who may experience other issues preventing participation. The study included focus groups from 11 distinct Asian origin groups.
Discussion. Despite substantial scholarship related to the ethics of HIV cure research, additional attention should focus on emerging issues in six categories of ethical issues: (1) social value (ongoing and emerging biomedical research and scalability considerations); (2) scientific validity (study design issues, such as the use of analytical treatment interruptions and placebos); (3) fair ...