- Search Menu
- Advance Articles
- Special Issues
- Supplements
- Virtual Collection
- Online Only Articles
- International Spotlight
- Free Editor's Choice
- Free Feature Articles
- Author Guidelines
- Submission Site
- Calls for Papers
- Why Submit to the GSA Portfolio?
- Advertising and Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Journals Career Network
- About The Gerontologist
- About The Gerontological Society of America
- Editorial Board
- Self-Archiving Policy
- Dispatch Dates
- GSA Journals
- Journals on Oxford Academic
- Books on Oxford Academic


Article Contents
Design and methods, implications, conclusions.
- < Previous
Dementia Care Mapping: A Review of the Research Literature
- Article contents
- Figures & tables
- Supplementary Data
Dawn Brooker, Dementia Care Mapping: A Review of the Research Literature, The Gerontologist , Volume 45, Issue suppl_1, October 2005, Pages 11–18, https://doi.org/10.1093/geront/45.suppl_1.11
- Permissions Icon Permissions
Purpose: The published literature on dementia care mapping (DCM) in improving quality of life and quality of care through practice development and research dates back to 1993. The purpose of this review of the research literature is to answer some key questions about the nature of the tool and its efficacy, to inform the ongoing revision of the tool, and to set an agenda for future research. Design and Methods: The DCM bibliographic database at the University of Bradford in the United Kingdom contains all publications known on DCM ( http://www.bradford.ac.uk/acad/health/dcm ). This formed the basis of the review. Texts that specifically examined the efficacy of DCM or in which DCM was used as a main measure in the evaluation or research were reviewed. Results: Thirty-four papers were categorized into five main types: (a) cross-sectional surveys, (b) evaluations of interventions, (c) practice development evaluations, (d) multimethod evaluations, and (e) papers investigating the psychometric properties of DCM. Implications: These publications provide some evidence regarding the efficacy of DCM, issues of validity and reliability, and its use in practice and research. The need for further development and research in a number of key areas is highlighted.
Dementia Care Mapping (DCM; Bradford Dementia Group, 1997 ) is an observational tool that has been used in formal dementia-care settings over the past 13 years, both as an instrument for developing person-centered care practice and as a tool in quality-of-life research. It developed from the pioneering work of the late Professor Tom Kitwood on person-centered care. In his final book, Dementia Reconsidered, Kitwood (1997) described DCM as “a serious attempt to take the standpoint of the person with dementia, using a combination of empathy and observational skill” (p 4). The instrument has been described fully elsewhere ( Kuhn, Ortigara, & Kasayka, 2000 ). In brief, an observer (mapper) tracks 5 people with dementia (participants) continuously over a representative time period (e.g., 6 hr during the waking day). Mapping takes place in communal areas of care facilities. After each 5-min period (a time frame), two types of codes are used to record what has happened to each individual. The behavioral category code (BCC) describes 1 of 24 different domains of participant behavior that has occurred. BCCs are subdivided into those behaviors that are thought to have high potential for well-being (Type 1) and those with low potential (Type 2). The mapper also makes a decision for each time frame, based on behavioral indicators, about the relative state of ill-being or well-being experienced by the person with dementia, called a well- or ill-being value (WIB). This is expressed on a 6-point scale ranging from extreme ill-being to extreme well-being. WIB values can be averaged to arrive at a WIB score. This provides an index of relative well-being for a particular time period for an individual or a group.
Personal detractions (PDs) and Positive events (PEs) are recorded whenever they occur. Personal detractions are staff behaviors that have the potential to undermine the personhood of those with dementia ( Kitwood, 1997 ). These are described and coded according to type and severity. Positive events—those that enhance personhood—also are recorded by the mapper, but these are not coded in a systematic manner.
DCM is grounded in the theoretical perspective of a person-centered approach to dementia care. Person-centered care values all people regardless of age and health status, is individualized, emphasizes the perspective of the person with dementia, and stresses the importance of relationships ( Brooker, 2004 ). Within Kitwood's writing is the assumption that, for people with dementia, well-being is a direct result of the quality of relationships they enjoy with those around them. The interdependency of the quality of the care environment to the relative quality of life experienced by people with dementia is central to person-centered care practice. In placing DCM in the taxonomy of measures of quality of life and quality of care, DCM attempts to measure elements of both. In its BCCs and WIBs, DCM measures relative well-being, affect, engagement, and occupation, which are important elements of quality of life. Through PDs and PEs, DCM records the quality-of-care practice as it promotes or undermines the personhood of those being mapped.
The method and coding system were originally developed through ethological observations of many hours in nursing homes, hospital facilities, and day care facilities in the United Kingdom ( Kitwood & Bredin, 1994 ). It was designed primarily as a tool to develop person-centered care practice over time with data being fed back to care teams who could then use it to improve their practice. The original development work is not available in the public domain. DCM has been criticized for this ( Adams, 1996 ). Also, many of the basic psychometric tests expected in the development of such a complex tool were not published.
Despite this, DCM has grown in popularity over the years. Many practitioners have used these codes successfully in many different situations and continue to do so. The reasons for this have not been systematically investigated. In part, it may be because DCM provides a vehicle for those wishing to systematically move dementia care from primarily a custodial and task-focused model into one that respects people with dementia as human beings. There are very few other tools that purport to do this or that have been shown to be effective in this endeavor in the field of dementia care.
DCM certification is only available through licensed trainers who undergo rigorous preparation for their role and use standardized training methods prepared by the University of Bradford. The basic training is a 3-day course, with further options of advanced training and evaluator status also available. DCM training is currently available in the United Kingdom, United States, Germany, Denmark, Australia, Switzerland, and Japan.
DCM has been through a number of changes since its inception. Until 1997, DCM 6 th edition was used. In 1997, DCM was revised based on feedback from practitioners resulting in the 7 th edition ( Bradford Dementia Group, 1997 ). The changes were made, in part, to clarify terminology (e.g., care values became well- or ill-being values); there was an increase in the number of BCCs, from 17 to 24, and PEs were formally recorded as part of the DCM evaluation. There were, however, no published papers demonstrating the relationship between scores on the 6 th and 7 th editions of DCM. During the past 3 years, various international working groups and field trials have made suggestions for revisions to DCM 7. DCM 8 will be launched in late 2005 in the United Kingdom.
Beavis, Simpson, and Graham (2002) reviewed literature on DCM from 1992 until June 2001 and identified nine papers that met their inclusion criteria. There have been important papers published since this time, and, using similar inclusion criteria (discussed below), the current review identified 34 papers. This review aims to clarify what is known about the DCM tool and to inform the direction of DCM 8 and future research.
The international DCM network led by the University of Bradford maintains a DCM bibliographic database that contains all known publications on DCM ( http://www.bradford.ac.uk/acad/health/dcm ). This database formed the basis of this review. It includes refereed and nonrefereed journal articles, book chapters, theses, and non-English language texts. It is updated by the Bradford Dementia Group with annual bibliographic searches on Medline, Cinahl, and Psychinfo using the terms “DCM” and “dementia care mapping” as well as personal correspondence from practitioners and researchers.
I included articles that specifically examined the efficacy of DCM or in which DCM was a main measure in evaluation or research. There were no exclusion criteria based on quality of scientific design. Articles that were purely descriptive were excluded, as were dissertations. There are many additional articles and publications that describe aspects of DCM and its use. Some of these will be referred to in the discussion. The review includes articles published between 1993 and March 2005.
I assigned each article to one of five categories according to its basic purpose in using DCM. I developed tables that summarize key parameters pertinent to this review: (a) settings and size; (b) aims of study as set out by the authors; (c) length of time mapped; (d) sample selection and characteristics; (e) study design; (f) version of DCM used; (g) interrater reliability; (h) DCM outcomes, (i) statistical tests; and (j) level of significance. (The full tables summarizing each article can be downloaded from the Web site previously mentioned or are available on request from the author.)
Thirty-four articles met the inclusion criteria. They were divided into five main types.
1. Cross-Sectional Surveys
In 11 articles, DCM was used in a number of different facilities, and the results either compared or pooled. Some of these presented baseline data for intended further studies ( Wilkinson, 1993 ; Williams & Rees, 1997 ; Younger & Martin, 2000 ) whereas others had the explicit aim of surveying quality of care or quality of life ( Ballard et al., 2001 ; Innes & Surr, 2001 ; Kuhn, Kasayka, & Lechner, 2002 ; Perrin, 1997 ). An additional 4 articles used DCM to investigate the relationship between participants' characteristics and well-being and activity ( Chung, 2004 ; Kuhn, Edelman, & Fultom, 2005 ; Kuhn, Fulton, & Edelman, 2004 ; Potkins et al., 2003 ).
Seven of these articles presented data from U.K. long-term facilities ( Ballard et al., 2001 ; Innes & Surr, 2001 ; Perrin, 1997 ; Potkins et al., 2003 ; Wilkinson, 1993 ; Williams & Rees, 1997 ; Younger & Martin, 2000 ). Three were U.S. studies examining assisted living facilities and day care facilities ( Kuhn et al., 2002 ; Kuhn, Edelman, & Fulton, 2005 ; Kuhn, Fulton, & Edelman, 2004 ), and 1 was from Hong Kong ( Chung, 2004 ). They ranged in size from 30 people in 6 facilities surveyed by Wilkinson (1993) to the largest study by Ballard and colleagues (2001) , who surveyed 218 people in 17 facilities; the average study size was 110 people in 8 facilities. All mapped for around 6 hr, except for Williams and Rees (1997) and Younger and Martin (2000) , who mapped for 12 hr. DCM 6 was used by Wilkinson (1993) , Perrin (1997) , and Williams and Rees (1997) .
2. Evaluation of Intervention
There were 10 articles in which DCM was used to evaluate the impact of various interventions on the lives of people with dementia. Bredin, Kitwood, and Wattis (1995) first used DCM to evaluate the impact of merging two wards. It has been used to evaluate a number of nonpharmacological therapeutic interventions, such as group reminiscence ( Brooker & Duce, 2000 ), aromatherapy ( Ballard, O'Brien, Reichelt, & Perry, 2002 ), sensory stimulation groups ( Maguire & Gosling, 2003 ), intergenerational programs ( Jarrott & Bruno, 2003 ), and horticultural therapy ( Gigliotti, Jarrott, & Yorgason, 2004 ). It also has been used as part of the evaluation of larger scale changes in therapeutic regimen, for example outdoor activities ( Brooker, 2001 ), person-centered care training ( Lintern, Woods, & Phair, 2000a ), a liaison psychiatry service ( Ballard, Powell, et al., 2002 ), and a double-blind, placebo-controlled, neuroleptic discontinuation study ( Ballard et al., 2004 ).
Length of time for which mapping occurred was much more varied with the shortest time being 30 min ( Maguire & Gosling, 2003 ) to the longest at 5 days per participant ( Jarrott & Bruno, 2003 ). Studies ranged in size from the smallest, n = 14 ( Gigliotti et al., 2004 ), to the largest, n = 82 ( Ballard et al., 2004 ).
All evaluations were a within-subjects design, apart from Jarrott and Bruno (2003) , who compared two groups. Control groups were used in just over half the studies ( Ballard, O'Brien, et al., 2002 ; Ballard, Powell, et al., 2002 ; Ballard et al., 2004 ; Brooker, 2001 ; Brooker & Duce, 2000 ; Gigolotti et al., 2004 ; Jarrott & Bruno). Demonstrable changes in DCM scores were shown in all studies with the exceptions of Lintern and colleagues (2000a) ; Ballard, Powell, and colleagues; and Ballard and colleagues. Statistically significant changes in DCM scores were demonstrated in Bredin and colleagues (1995) ; Brooker and Duce; Brooker (2001) ; Ballard, O'Brien, and colleagues; Jarrott and Bruno; and Gigliotti and colleagues.
3. Evaluation of DCM in Practice Development
Six articles investigated the ability of DCM to develop practice over time by means of repeated evaluations. In these reports DCM was used in a developmental process or in a continuous quality-improvement cycle with the explicit goal of using DCM data to change care practice. Barnett (1995) ; Brooker, Foster, Banner, Payne, and Jackson (1998) , and Martin and Younger (2001) report results across a number of facilities, whereas Lintern, Woods and Phair (2000b) ; Martin & Younger (2000) , and Wylie, Madjar, & Walton (2002) report results from single facilities. In the largest of these studies, Brooker and colleagues reported DCM across nine facilities for three annual cycles; the smallest of these was Martin and Younger (2000) . DCM 6 was used by Barnett; Lintern and colleagues (2000a) , and Brooker and colleagues. All of the studies showed demonstrable changes in DCM scores over time. Brooker and colleagues was the only study to use statistical analysis to demonstrate the significance of change over time.
4. MultiMethod Qualitative Evaluations
Three articles reported using DCM as part of a multimethod evaluation of a single facility or service ( Barnett, 2000 ; Parker, 1999 ; Pritchard & Dewing, 2001 ). All these articles were qualitative evaluations and used DCM in this frame.
5. Investigations of Psychometric Properties
Four studies looked directly at some of the psychometric properties of DCM. Fossey, Lee, and Ballard (2002) examined internal consistency, test–retest and concurrent validity, and shortened mapping time in a U.K. long-term population of 2 cohorts of 123 and 54, respectively. The 2 cohorts were chosen to increase the variance in dependency and agitation. All were mapped for 6 hr on each occasion, 24 mapped 1 week apart, and 30 mapped between 2 and 4 weeks apart. Test–retest reliability was established for both cohorts. Internal consistency was demonstrated between the main parameters. A correlation was found between key parameters in the hour prior to lunch and the total 6-hr map.
Edelman, Fulton, and Kuhn, (2004) compared five dementia-specific quality-of-life measures, including DCM, in 54 people with dementia in 3 U.S. day-care facilities. WIB scores did not correlate with quality-of-life interviews but did correlate with proxy measures. WIB scores did not correlate with Mini-Mental State Examination (MMSE) scores but they did with the number of dependent activities of daily living (ADLs). In a second study on 166 people with dementia in 8 different facilities, Edelman, Kuhn, and Fulton (2004) further assessed the relationship between DCM and MMSE scores, number of dependent ADLs, depressive symptoms, and facility type. Low WIB scores and higher percentages of sleep correlated with low MMSE scores and higher dependency. WIB scores were lower in dementia specific nursing homes than assisted living facilities and day care. There was not a significant relationship between DCM scores and depressive symptoms.
Thornton, Hatton, and Tatham (2004) assessed interrater reliability in routine mapping on 20 participants. They also compared BCCs to actual amount of time spent in different behaviors and looked at the relationship between dependency and WIB scores in 64 patients in a U.K. long-stay and day-care facility. They found that interrater reliability in routine maps was less than 50% for 12 codes. They also demonstrated that DCM gives lower indication of passive and withdrawn behaviors than continuous time sampling. Correlations between dependency and WIB score also were demonstrated.
DCM Data Across Studies
Despite the variety of studies, there is consistency of what they report in terms of DCM data. In long-term care, BCC codes A (social interaction), B (watching), and F (eating and drinking) appear as the most frequent codes almost without exception. Codes K (walking) and N (sleeping) appear as the next most frequently cited. In facilities with lower WIB scores, C (withdrawn) and W (repetitive self-stimulation) appear in the top five ( Chung, 2004 ; Innes & Surr, 2001 ; Perrin, 1997 ). In facilities with higher WIB scores, codes E (creative activity), J (exercise), and M (engaging with media such as books, TV) appear more frequently ( Brooker et al., 1998 ; Kuhn et al., 2002 ; Martin & Younger, 2001 ). Taking the group WIB scores across the studies as a whole ( n = 39, excluding the less well-described studies) these provided an average (mean) group WIB of 0.9 ( SD = 0.92) for long-term care. Group WIB scores from long-term care facilities ranged between −0.32 ( Ballard et al., 2001 ) to 1.5 ( Innes & Surr, 2001 ).
Generally, a greater diversity of BCCs and higher WIB scores are reported in day-care facilities ( Barnett, 2000 ; Brooker et al., 1998 ; Kuhn et al., 2004 ; Martin & Younger, 2001 ; Williams & Rees 1997 ) with BCC codes M (media), G (games), L (work-like activity) and I (intellectual), J (physical exercise), E (creative expression), and H (handicrafts) appearing in the top five reported codes. Of the eight day-care group WIB scores reported, the mean is 1.94 (range = 1.17 to 2.79, SD = 0.47). WIB scores and diversity of activity both increase during periods of therapeutic activity ( Brooker & Duce, 2000 ; Gigliotti et al., 2004 ; Jarrott & Bruno, 2003 ; Maguire & Gosling, 2003 ; Pritchard & Dewing, 2001 ; Wilkinson, 1993 ).
There is less data available for assisted-living facilities, the only report being Kuhn and colleagues (2002) . The spread of WIB scores and the frequency of BCCs were similar to those reported for nursing home facilities. Lower scores, less diversity of activity, and a greater occurrence of personal detractions occurred in the smaller dementia-specific facilities rather than in larger mixed facilities, although this could have been confounded with greater dependency in the smaller facilities.
Many published DCM evaluations do not report PDs. A number suggest that the highest level of PDs occur in those facilities with the lowest WIB scores ( Brooker et al., 1998 ; Innes & Surr, 2001 ; Kuhn et al., 2002 ; Williams & Rees, 1997 ). Most PDs reported fall in the mild to moderate category. Innes and Surr were the only authors to report positive events. Nineteen of the studies reported interrater reliability data which ranged from 0.7 to 1.0, most reporting concordance coefficients of 0.8.
These studies can help to answer, at least in part, some common questions about DCM. In addition to this, issues are highlighted that should be taken forward in the development of DCM.
Does DCM Measure Quality of Care and/or Quality of Life?
In terms of concurrent validity with other measures there is some evidence that DCM is related to indicators of quality of care. Bredin and colleagues (1995) reported a relationship between a decrease in DCM scores and an increase in pressure sores. Brooker and colleagues (1998) reported a clustering of high WIB scores occurring in facilities where other quality audit tools demonstrated better quality of care.
There is some evidence of concurrent validity of WIB scores with proxy quality-of-life measures. Fossey and colleagues (2002) demonstrated a significant correlation between WIB scores and the Blau (1977) proxy measure of quality of life. Edelman and colleagues (2004) demonstrated a moderately significant correlation between WIB scores and two staff proxy measures of quality of life—the Quality of Life AD–Staff ( Logsdon, Gibbons, McCurry, & Teri, 2000 ) and the Alzheimer's Disease-Related Quality of Life (ADRQL; Rabins, Kasper, Kleinman, Black, & Patrick, 1999 ) in adult day care. This study did not demonstrate a correlation between any of these measures compared to direct quality-of-life interviews with a less cognitively impaired subgroup. In his multimethod study, Parker (1999) noted that during interviews, people with dementia rated their quality of life as better than their DCM scores would suggest.
Data from a larger, as yet unpublished, study ( Edelman, Kuhn, Fulton, Kasayka, & Lechner, 2002 ) also compared DCM results with another observational measure—the Affect Rating Scale ( Lawton, 1997 ). On the Affect Rating Scale, positive WIBs correlated with positive affect and negative WIB scores with negative affect. Brooker and colleagues (1998) also demonstrated a significant correlation between WIB score and level of observed engagement ( McFayden, 1984 ) on a small sample of 10 participants.
DCM measures something similar to proxy measures and other observation measures. DCM is somewhat different from other quality-of-life and quality-of-care measures in that it attempts to measure elements of both. In training to use DCM, mappers are explicitly taught to increase their empathy for the viewpoint of the person with dementia and to use this during their coding decisions.
Can Different Mappers Use DCM Reliably?
When many different mappers are engaged in mapping at different points in time, drifts in coding can have a significant impact on results ( Thornton et al., 2004 ) unless systematic checking is in place to prevent this. It is perfectly possible to achieve acceptable interrater reliability as many of the studies here demonstrate. Surr and Bonde-Nielsen (2003) outline the various ways in which reliability can be achieved in routine mapping. Although interrater reliability can be demonstrated within studies—and should always be so where more than a single mapper has been used—it cannot be assumed when comparing one study to another. This is a major challenge for those providing DCM training. One of the main ways of achieving interrater reliability in practice is for all mappers to have regular checks with a “gold standard mapper.” Provisions need to be made to make the status of a gold standard mapper more formalized, possibly through advanced DCM training. This status could be accredited by regular web-based or video role-play materials that mappers have to code correctly to maintain their status.
In terms of the development of DCM 8, efforts should be made to decrease ambiguity in the codes and to eliminate any unnecessary complexity from the rules. Thornton and colleagues (2004) and work currently being undertaken in Germany ( Ruesing, 2003 ) have helped clarify the most problematic codes. There are no published data on the interrater reliability of PD and PE recordings. This also should be incorporated in DCM 8.
Only Fossey and colleagues (2002) looked at test–retest reliability. The best correlation was between percentage of +3 and +5, followed by overall WIB score. Significance was more moderate for type of BCC but still at an acceptable level. This finding requires replication.
Does DCM Show Representative Reliability Across All People With Dementia?
There is evidence to suggest that level of dependency is correlated with DCM scores, specifically that low WIB scores are associated with high dependency levels. This has been demonstrated statistically on three different continents ( Brooker et al., 1998 ; Chung, 2004 ; Edelman et al., 2004 ; Kuhn et al., 2004 ; Thornton et al., 2004 ) using three different measures of dependency.
On the other hand, Younger and Martin (2000) found the highest scores in their study occurred in the facility that had the most dependent participants. Edelman and colleagues (2004) , Jarrott and Bruno (2003) , and Gigliotti and colleagues (2004) demonstrated no correlation between level of cognitive impairment and WIB score.
The correlations between low WIB scores and high dependency may of course be related to a third factor of poorer quality of psychosocial care for people with dementia who have high dependency needs. In support of this, Brooker and colleagues (1998) found that the correlation between dependency and WIB score disappeared after three successive cycles of DCM. The authors believed that, by this stage, ways of supporting well-being of participants who were highly dependent had been better established.
It is also not clear whether there are particular features that are more prevalent in higher dependency groups that might either make a subset more difficult to engage with and thus more difficult for them to achieve higher DCM scores. For example, Potkins and colleagues (2003) demonstrated that language dysfunction was associated with poorer BCC distribution regardless of level of cognitive impairment.
The evidence that dependency level skews DCM results is strong enough to suggest that a measure of dependency should be routinely taken alongside DCM evaluations so that the results can be scrutinized for this relationship. One of the problems with doing this is agreeing on a particular measure of dependency. The Clifton Assessment Procedures for the Elderly measure (CAPE; Pattie & Gilleard, 1979 ) has been used most often but is difficult to access and not culturally appropriate outside the United Kingdom. A standard measure of dependency to be used alongside DCM needs to be agreed upon.
Does DCM Change Care Practice?
In 2001, an international “think tank” of DCM practitioners came together to review their collective experience on DCM ( Brooker & Rogers, 2001 ). Their conclusions from practice were that DCM, used within an organizational framework that supported person-centered care, could improve levels of well-being, increase the diversity of occupation, and decrease the incidence of personal detractions. The published developmental evaluations reviewed here supports this assertion both for larger scale quality-assurance initiatives ( Brooker et al., 1998 , Martin & Younger, 2001 ) and more in-depth developments in single establishments ( Lintern et al., 2000b ; Martin & Younger, 2000 ; Wylie et al., 2002 ). The face validity of DCM for practitioners appears high in formal evaluations (Brooker et al.; Younger & Martin, 2000 ) and in the large numbers of people undertaking DCM training.
DCM has been used as a tool for practice development by many people and organizations. The mix of papers in this review cannot be taken as a reflection of the way in which DCM is used generally. By the nature of their work, those in practice development are less likely to publish than those engaged in research. The research issue for whether DCM changes care practice is to clarify the way in which DCM is used and the organizational setting conditions necessary to maximize impact.
A difficult issue, in terms of validity for practice development, is whether using DCM in a repeated cycle of evaluations actually improves quality of life for people with dementia. A problem with all of the studies outlined above is that their only measure of improvement was DCM. In other words, DCM served as both the intervention and the outcome measure. Without a longitudinal controlled study of DCM as a tool for practice development, which utilizes other quality of life measures as the main outcome, it cannot be said categorically that DCM improves quality of life. There are many practitioners who believe that DCM does have a positive impact when used within certain setting conditions ( Brooker & Rogers, 2001 ). In the context of working in a field where tools for practice development are not common, DCM is a tool that practitioners want to use.
Is DCM a Suitable Tool for Research?
DCM was not designed to be a research tool, and investigations into its reliability and validity are only just beginning to appear. Acceptable interrater reliability is achievable, and concurrent validity with other proxy measures of quality of life has been demonstrated. Fossey and colleagues (2002) demonstrated internal consistency and test–retest reliability. These findings require replication, and the issue of the impact of dependency and diagnosis on scores needs to be determined, as does the impact of care regimen. Further research into its psychometric properties continues, and more studies are expected. Careful consideration should be given in deciding whether DCM is fit for purpose given the specific topic under investigation.
DCM has been used in cross-sectional surveys, evaluations of interventions, and multimethod qualitative evaluations by a number of researchers. In terms of cross-sectional surveys, there are tools that may be more suited to this task that do not have the attendant time-consuming problems and specialist training associated with DCM ( Edelman et al., 2004 ). Whether they would be better tools for the purpose of answering the specific research questions is debatable.
From the studies presented here, DCM seems to be suited to smaller scale within-subjects or group comparison intervention evaluations, given that it appears to demonstrate discrimination on a variety of interventions. In multimethod qualitative designs, DCM appears to enrich the data derived from proxy and service-user interviews and focus groups. DCM provides an opportunity to represent a reflection on what could be the viewpoint of service users who are unable to participate fully in interviews.
What is clear is that BCCs do not measure real-time estimates of different types of behavior ( Thornton et al., 2004 ). Because of the rules of coding in DCM, it will underestimate the occurrence of socially passive and withdrawn behavior compared to data collected with continuous time sampling. Researchers interested in looking at withdrawn and passive behavior might be better advised to use another tool. It is worthy of note, however, that despite this, three studies ( Ballard, O'Brien et al., 2002 ; Gigliotti et al., 2004 ; Potkins et al., 2003 ) found DCM discriminated between groups on social withdrawal in their evaluations.
There are a number of modifications to DCM that might prove useful when using DCM in research. A current U.S.-led project is considering whether some of the operational rules within DCM for selecting specific BCC and WIBs should be changed for the purposes of research evaluations. A number of studies reviewed that presented group-level data have collapsed the number of BCCs into a number of supracategories ( Chung, 2004 ; Gigliotti et al., 2004 ; Kuhn et al., 2004 ). It may be that streamlining DCM further by using time sampling could provide a more useful research alternative as has already been tried by McKee, Houston, and Barnes (2002) . Further research is needed to clarify how streamlined versions relate to the full tool and whether the same degree of training would be necessary to use them.
What Do the Scores Mean in Terms of Benchmarking?
The table on how to interpret DCM data in the DCM manual ( Bradford Dementia Group, 1997 ) is not based on published data. Evidence from this review presents a range of group WIB scores against which to benchmark, suggesting that scores are generally higher in day care than long-stay care. How much this is confounded by the different dependency levels is unclear. Work is currently underway to develop an international database of DCM results to which all international strategic DCM partners would have access. The database should include participant and facilities factors that could be used in stratified analyses, correlational studies, and as adjustment factors. The quality of DCM data in the international database could be safeguarded by only accepting data that has been verified by a gold-standard mapper.
What Is a Significant Change in Scores?
Published studies that have looked at change through developmental evaluation report group WIB changes in the range of 0.5. A study by Brooker and colleagues (1998) was the only developmental evaluation to present a statistical analysis of the results where changes of 0.1 to 0.5 were significant at the 0.03 level over 3 data points, and changes at 0.7 and 0.9 were significant at the 0.005 and 0.001 level, respectively, between 2 data points. Intervention studies ( Brooker & Duce, 2000 ; Brooker, 2001 ; Gigliotti et al, 2004 ; Jarrott & Bruno, 2003 ) report differences in the range of 0.4 to 1.1, which were all statistically significant. Changes in individual WIB scores, WIB value profiles, and BCC profiles are more variable. Further research is needed to clarify what constitutes a clinically significant change.
How Long Should a Map Be?
Six hr is the current guidance in DCM training, but there is no empirical evidence to verify the representativeness of this time period. Most of the studies here have mapped for 6 hr, although those using DCM for practice-development purposes mapped for much longer ( Brooker et al., 1998 ; Martin & Younger, 2001 ; Williams & Rees; Wylie et al., 2002 ). It also is evident from practice that useful insights can be gained from mapping for just a couple of hours ( Heller, 2004 ). Length of maps will depend, in part, on the reason for mapping, but there is a drive to spend the least amount of time possible collecting data. Fossey and colleagues (2002) found a statistically significant correlation between the hour prior to lunch and a 6-hr map on all their key indicators at the group level. It is likely that there would be a great deal more variation on an individual level. An unpublished U.S study ( Douglass & Johnson, 2002 ) mapped 18 residents during a 6-day period for periods of 2, 4, 6, and 8 hr in a continuing care retirement community. Acceptable levels of interrater reliability were demonstrated in maps of more than 4 hr in duration. This important issue requires further research.
These studies report evidence that DCM has a role in practice development and research within the broad aim of improving the quality of the lived experience for people with dementia. Priority should be given to a controlled longitudinal study to evaluate fully the impact of DCM in improving quality of life through practice development. A large international database on DCM results would help clarify the relationship between DCM results, dependency, diagnostic group, and facility characteristics. Steps need to be taken through the development of the method, training, and accreditation to ensure reliability. Further research would help clarify the clinical significance of change in scores, the length of mapping, and amendments to the method when it is used as a research tool.
The published work on DCM is of variable quality but is growing in strength. DCM's advantages are that it is standardized, quality controlled, international, responsive to change, multidisciplinary, and has an increasing research base. DCM provides a shared language and focus across professional disciplines, care staff, and management teams. It is seen as a valid measure by frontline staff as well as those responsible for managing and commissioning care. It also provides a shared language between practitioners and researchers. DCM holds a unique position in relation to quality of life in dementia care, being both an evaluative instrument and as a vehicle for practice development in person-centered care. Many of the intervention evaluations cited above have been undertaken because DCM has given practitioners a way of trying to evaluate their practice. Maintaining a dialogue between the worlds of research and practice in health and social care is a major challenge. DCM provides an opportunity to do this.
Thanks go to the anonymous reviewers of an earlier version of this paper and to my DCM colleagues, Claire Surr and Carolinda Douglass.
Bradford Dementia Group, School of Health Studies, University of Bradford, Yorkshire, UK.
Decision Editor: Richard Schulz, PhD
* Indicates articles included in the review.
Adams, T., ( 1996 ). Kitwood's approach to dementia and dementia care: A critical but appreciative review. Journal of Advanced Nursing, 23 , 948 -953.
*Ballard, C., Fossey, J., Chithramohan, R., Howard, R., Burns, A., & Thompson, P., et al ( 2001 ). Quality of care in private sector and NHS facilities for people with dementia: Cross sectional survey. British Medical Journal, 323 , 426 -427.
*Ballard, C., O'Brien, J. T., Reichelt, K., & Perry, E., ( 2002 ). Aromatherapy as a safe and effective treatment for the management of agitation in severe dementia: The results of a double blind, placebo-controlled trial with Melissa. Journal of Clinical Psychiatry, 63 , 553 -558.
*Ballard, C., Powell, I., James, I., Reichelt, K., Myint, P., & Potkins, D., et al ( 2002 ). Can psychiatric liaison reduce neuroleptic use and reduce health service utilization for dementia patients residing in care facilities? International Journal of Geriatric Psychiatry, 17 , 140 -145.
*Ballard, C., Thomas, A., Fossey, J., Lee, L., Jacoby, R., & Lana, M., et al ( 2004 ). A 3-month, randomised, placebo-controlled, neuroleptic discontinuation study in 100 people with dementia: The Neuropsychiatric Inventory is a predictor of clinical outcome. Journal of Clinical Psychiatry, 65 , 114 -119.
*Barnett, E., ( 1995 ). A window of insight into quality of care. Journal of Dementia Care, 3 , (4), 23 -26.
*Barnett, E., ( 2000 ). Including the person with dementia in designing and delivering care—‘I need to be me!’ . London: Jessica Kingsley Publishers.
Beavis, D., Simpson, S., & Graham, I., ( 2002 ). A literature review of dementia care mapping: Methodological considerations and efficacy. Journal of Psychiatric and Mental Health Nursing, 9 , 725 -736.
Blau, T. H., ( 1977 ). Quality of life, social indicators, and criteria of change. Professional Psychology, 8 , 464 -473.
Bradford Dementia Group. ( 1997 ). Evaluating dementia care: The DCM Method (7 th ed.). Bradford, U.K.: University of Bradford.
*Bredin, K., Kitwood, T., & Wattis, J., ( 1995 ). Decline in quality of life for patients with severe dementia following a ward merger. International Journal of Geriatric Psychiatry, 10 , 967 -973.
*Brooker, D., ( 2001 ). Enriching lives: Evaluation of the ExtraCare Activity Challenge. Journal of Dementia Care, 9 , (3), 33 -37.
Brooker, D., ( 2004 ). What is person-centred care for people with dementia? Reviews in Clinical Gerontology, 13 , 215 -222.
*Brooker, D., & Duce, L., ( 2000 ). Well-being and activity in dementia: A comparison of group reminiscence therapy, structured goal-directed group activity, and unstructured time. Aging & Mental Health, 4 , 354 -358.
*Brooker, D., Foster, N., Banner, A., Payne, M., & Jackson, L., ( 1998 ). The efficacy of Dementia Care Mapping as an audit tool: Report of a 3-year British NHS evaluation. Aging & Mental Health, 2 , 60 -70.
Brooker, D., & Rogers, L., (Eds.) ( 2001 ). DCM think tank transcripts 2001 . Bradford, U.K.: University of Bradford.
*Chung, J. C. C., ( 2004 ). Activity participation and well-being in people with dementia in long-term care settings. OTJR: Occupation, Participation and Health, 24 , (1), 22 -31.
Douglass, C., & Johnson, A., ( 2002 ). Implementation of DCM in a long-term care setting: Measures of reliability, validity, and ease of use . Paper presented as part of the symposium on DCM at The Gerontological Society of America annual scientific meeting, Boston, MA.
*Edelman, P., Fulton, B. R., & Kuhn, D., ( 2004 ). Comparison of dementia-specific quality of life measures in adult day centers. Home Health Care Services Quarterly, 23 , 25 -42.
*Edelman, P., Kuhn, D., & Fulton, B. R., ( 2004 ). Influence of cognitive impairment, functional impairment, and care setting on dementia care mapping results. Aging and Mental Health, 8 , 514 -523.
Edelman, P., Kuhn, D., Fulton, B., Kasayka, R., & Lechner, C., ( 2002 ). The relationship of DCM to five measures of dementia specific quality of life . Paper presented as part of the symposium on DCM at The Gerontological Society of America annual scientific meeting, Boston, MA.
*Fossey, J., Lee, L., & Ballard, C., ( 2002 ). Dementia Care Mapping as a research tool for measuring quality of life in care settings: Psychometric properties. International Journal of Geriatric Psychiatry, 17 , 1064 -1070.
*Gigliotti, C. M., Jarrott, S. E., & Yorgason, J., ( 2004 ). Harvesting health: Effects of three types of horticultural therapy activities for persons with dementia. Dementia, 3 , 161 -170.
Heller, L., ( 2004 ). The Thursday Club. In D. Brooker, P. Edwards, & S. Benson (Eds.), DCM: Experience and insights into practice (pp. 110–111). London: Hawker Publications.
*Innes, A., & Surr, C., ( 2001 ). Measuring the well-being of people with dementia living in formal care settings: The use of Dementia Care Mapping. Aging and Mental Health, 5 , 258 -268.
*Jarrott, S. E., & Bruno, K., ( 2003 ). Intergenerational activities involving persons with dementia: An observational assessment. American Journal of Alzheimer's Disease and Other Dementias, 18 , 31 -37.
Kitwood, T., ( 1997 ). Dementia reconsidered: The person comes first . Buckingham, U.K.: Open University Press.
Kitwood, T., & Bredin, K., ( 1994 ). Charting the course of quality care. Journal of Dementia Care, 2 , (3), 22 -23.
Kuhn, D., Edelman, P., & Fulton, B. R., ( 2005 ). Daytime sleep and the threat to well-being of persons with dementia. Dementia, 4 , 233 -247.
*Kuhn, D., Fulton, B. R., & Edelman, P., ( 2004 ). Factors influencing participation in activities in dementia care settings. Alzheimer's Care Quarterly, 5 , 144 -152.
*Kuhn, D., Kasayka, R., & Lechner, C., ( 2002 ). Behavioral observations and quality of life among persons with dementia in 10 assisted living facilities. American Journal of Alzheimer's Disease and Other Dementias, 17 , 291 -298.
Kuhn, D., Ortigara, A., & Kasayka, R., ( 2000 ). Dementia Care Mapping: An innovative tool to measure person-centered care. Alzheimer's Care Quarterly, 1 , 7 -15.
Lawton, M. P., ( 1997 ). Assessing quality of life in Alzheimer's Disease research. Alzheimer's Disease and Associated Disorders, 11 , 91 -99.
*Lintern, T., Woods, R., & Phair, L., ( 2000 ). Before and after training: A case study of intervention. Journal of Dementia Care, 8 , (1), 15 -17.
*Lintern, T., Woods, R., & Phair, L., ( 2000 ). Training is not enough to change care practice. Journal of Dementia Care, 8 , (2), 15 -17.
Logsdon, R. G., Gibbons, L. E., McCurry, S. M., & Teri, L., ( 2000 ). Quality of life in Alzheimer's disease: Patient and care giver reports. In S. Albert & R. G. Logsdon (Eds.), Assessing quality of life in Alzheimer's disease (pp. 17–30). New York: Springer.
*Maguire, S., & Gosling, A., ( 2003 ). Social and sensory stimulation groups: Do the benefits last? Journal of Dementia Care, 11 , (2), 20 -21.
*Martin, G., & Younger, D., ( 2000 ). Anti-oppressive practice: A route to the empowerment of people with dementia through communication and choice. Journal of Psychiatric and Mental Health Nursing, 7 , 59 -67.
*Martin, G. W., & Younger, D., ( 2001 ). Person-centred care for people with dementia: A quality audit approach. Journal of Psychiatric and Mental Health Nursing, 8 , 443 -448.
McFayden, M., ( 1984 ). The measurement of engagement in institutionalised elderly. In I. Hanley & J. Hodge (Eds.), Psychological approaches to care of the elderly (pp. 136–163). London: Croom Helm.
McKee, K. J., Houston, D. M., & Barnes, S., ( 2002 ). Methods for assessing quality of life and well-being in frail older people. Psychology and Health, 17 , 737 -751.
*Parker, J., ( 1999 ). Education and learning for the evaluation of dementia care: The perceptions of social workers in training. Education and Ageing, 14 , 297 -314.
Pattie, A. H., & Gilleard, C. J., ( 1979 ). Clifton Assessment Procedures for the Elderly (CAPE) . Sevenoaks, Kent, U.K.: Hodder & Stoughton.
*Perrin, T., ( 1997 ). Occupational need in severe dementia. Journal of Advanced Nursing, 25 , 934 -941.
*Potkins, D., Myint, P., Bannister, C., Tadros, G., Chithramohan, R., & Swann, A., et al ( 2003 ). Language impairment in dementia: Impact on symptoms and care needs in residential homes. International Journal of Geriatric Psychiatry, 18 , 1002 -1006.
*Pritchard, E. J., & Dewing, J., ( 2001 ). A multi-method evaluation of an independent dementia care service and its approach. Aging & Mental Health, 5 , 63 -72.
Rabins, P., Kasper, J. D., Kleinman, L., Black, B. S., & Patrick, D. L., ( 1999 ). Concepts and methods in the development of the ADRQL. Journal of Mental Health and Aging, 5 , 33 -48.
Ruesing, D., ( 2003 ). Die Reliabilitat und Validitat des Beobachtunginstruments: DCM [The reliability and validity of observational instruments: DCM]. Unpublished master's thesis, University of Witten Herdecke, Germany.
Surr, C., & Bonde-Nielsen, E., ( 2003 ). Inter-rater reliability in DCM. Journal of Dementia Care, 11 , (6), 33 -36.
*Thornton, A., Hatton, C., & Tatham, A., ( 2004 ). DCM reconsidered: Exploring the reliability and validity of the observational tool. International Journal of Geriatric Psychiatry, 19 , 718 -726.
*Wilkinson, A. M., ( 1993 ). Dementia Care Mapping: A pilot study of its implementation in a psychogeriatric service. International Journal of Geriatric Psychiatry, 8 , 1027 -1029.
*Williams, J., & Rees, J., ( 1997 ). The use of ‘dementia care mapping’ as a method of evaluating care received by patients with dementia: An initiative to improve quality of life. Journal of Advanced Nursing, 25 , 316 -323.
*Wylie, K., Madjar, I., & Walton, J., ( 2002 ). Dementia Care Mapping: A person-centered approach to improving the quality of care in residential settings. Geriaction, 20 , (2), 5 -9.
*Younger, D., & Martin, G., ( 2000 ). Dementia Care Mapping: An approach to quality audit of services for people with dementia in two health districts. Journal of Advanced Nursing, 32 , 1206 -1212.
Email alerts
Citing articles via, looking for your next opportunity.
- Recommend to Your Librarian
Affiliations
- Online ISSN 1758-5341
- Copyright © 2024 The Gerontological Society of America
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Europe PMC requires Javascript to function effectively.
Either your web browser doesn't support Javascript or it is currently turned off. In the latter case, please turn on Javascript support in your web browser and reload this page.
Search life-sciences literature (43,861,927 articles, preprints and more)
- Available from publisher site using DOI. A subscription may be required. Full text
- Citations & impact
- Similar Articles
Dementia care mapping: a review of the research literature.
Author information, affiliations.
- Brooker D 1
The Gerontologist , 01 Oct 2005 , 45 Spec No 1(1): 11-18 https://doi.org/10.1093/geront/45.suppl_1.11 PMID: 16230745
Abstract
Design and methods, implications, full text links .
Read article at publisher's site: https://doi.org/10.1093/geront/45.suppl_1.11
References
Articles referenced by this article (43)
Kitwood's approach to dementia and dementia care: a critical but appreciative review.
J Adv Nurs, (5):948-953 1996
MED: 8732522
Quality of care in private sector and NHS facilities for people with dementia: cross sectional survey.
Ballard C , Fossey J , Chithramohan R , Howard R , Burns A , Thompson P , Tadros G , Fairbairn A
BMJ, (7310):426-427 2001
MED: 11520838
Title not supplied
AUTHOR UNKNOWN
Clin Psychol 2002
Can psychiatric liaison reduce neuroleptic use and reduce health service utilization for dementia patients residing in care facilities.
Ballard C , Powell I , James I , Reichelt K , Myint P , Potkins D , Bannister C , Lana M , Howard R , O'Brien J , Swann A , Robinson D , Shrimanker J , Barber R
Int J Geriatr Psychiatry, (2):140-145 2002
MED: 11813276
Clin Psychol 2004
JOURNAL OF DEMENTIA CARE 1995
A literature review of dementia care mapping: methodological considerations and efficacy.
Beavis D , Simpson S , Graham I
J Psychiatr Ment Health Nurs, (6):725-736 2002
MED: 12472826
PROFESSIONAL PSYCHOLOGY 1977
Int J Geriatr Psychiatry 1995
JOURNAL OF DEMENTIA CARE 2001
Citations & impact
Impact metrics, citations of article over time, alternative metrics.

Smart citations by scite.ai Smart citations by scite.ai include citation statements extracted from the full text of the citing article. The number of the statements may be higher than the number of citations provided by EuropePMC if one paper cites another multiple times or lower if scite has not yet processed some of the citing articles. Explore citation contexts and check if this article has been supported or disputed. https://scite.ai/reports/10.1093/geront/45.suppl_1.11
Article citations, iatrogenic suffering at the end of life: an ethnographic study..
Green L , Capstick A , Oyebode J
Palliat Med , 37(7):984-992, 23 Apr 2023
Cited by: 0 articles | PMID: 37088974 | PMCID: PMC10320702
Effects of the use of autobiographical photographs on emotional induction in older adults: a systematic review.
Toledano-González A , Romero-Ayuso D , Fernández-Pérez D , Nieto M , Ricarte JJ , Navarro-Bravo B , Ros L , Latorre JM
Psychol Res , 87(4):988-1011, 20 Jul 2022
Cited by: 1 article | PMID: 35859072
Assessing Momentary Well-Being in People Living With Dementia: A Systematic Review of Observational Instruments.
Madsø KG , Flo-Groeneboom E , Pachana NA , Nordhus IH
Front Psychol , 12:742510, 23 Nov 2021
Cited by: 0 articles | PMID: 34887803 | PMCID: PMC8649635
Known in the nursing home: development and evaluation of a digital person-centered artistic photo-activity intervention to promote social interaction between residents with dementia, and their formal and informal carers.
Tan JRO , Boersma P , Ettema TP , Aëgerter L , Gobbens R , Stek ML , Dröes RM
BMC Geriatr , 22(1):25, 06 Jan 2022
Cited by: 2 articles | PMID: 34991472 | PMCID: PMC8733433
Person-Centred Care Transformation in a Nursing Home for Residents with Dementia.
Ho P , Cheong RCY , Ong SP , Fusek C , Wee SL , Yap PLK
Dement Geriatr Cogn Dis Extra , 11(1):1-9, 01 Jan 2021
Cited by: 3 articles | PMID: 33790933 | PMCID: PMC7989831
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
An examination of the psychometric properties and efficacy of Dementia Care Mapping.
Cooke HA , Chaudhury H
Dementia (London) , 12(6):790-805, 21 May 2012
Cited by: 10 articles | PMID: 24337640
Implementing Dementia Care Mapping as a practice development tool in dementia care services: a systematic review.
Surr CA , Griffiths AW , Kelley R
Clin Interv Aging , 13:165-177, 26 Jan 2018
Cited by: 8 articles | PMID: 29416325 | PMCID: PMC5790091
J Psychiatr Ment Health Nurs , 9(6):725-736, 01 Dec 2002
Cited by: 30 articles | PMID: 12472826
Dementia Care Mapping as a research tool for measuring quality of life in care settings: psychometric properties.
Fossey J , Lee L , Ballard C
Int J Geriatr Psychiatry , 17(11):1064-1070, 01 Nov 2002
Cited by: 61 articles | PMID: 12404656
Dementia Care Mapping reconsidered: exploring the reliability and validity of the observational tool.
Thornton A , Hatton C , Tatham A
Int J Geriatr Psychiatry , 19(8):718-726, 01 Aug 2004
Cited by: 17 articles | PMID: 15290694
Europe PMC is part of the ELIXIR infrastructure
Dementia care mapping: a review of the research literature
Affiliation.
- 1 Bradford Dementia Group, School of Health Studies, University of Bradford, Unity Building, Bradford, Yorkshire BD5 0BB, UK. [email protected]
- PMID: 16230745
- DOI: 10.1093/geront/45.suppl_1.11
Purpose: The published literature on dementia care mapping (DCM) in improving quality of life and quality of care through practice development and research dates back to 1993. The purpose of this review of the research literature is to answer some key questions about the nature of the tool and its efficacy, to inform the ongoing revision of the tool, and to set an agenda for future research.
Design and methods: The DCM bibliographic database at the University of Bradford in the United Kingdom contains all publications known on DCM (http://www.bradford.ac.uk/acad/health/dcm). This formed the basis of the review. Texts that specifically examined the efficacy of DCM or in which DCM was used as a main measure in the evaluation or research were reviewed.
Results: Thirty-four papers were categorized into five main types: (a) cross-sectional surveys, (b) evaluations of interventions, (c) practice development evaluations, (d) multimethod evaluations, and (e) papers investigating the psychometric properties of DCM.
Implications: These publications provide some evidence regarding the efficacy of DCM, issues of validity and reliability, and its use in practice and research. The need for further development and research in a number of key areas is highlighted.
Publication types
- Dementia / therapy*
- Quality of Health Care*
- Quality of Life*
- User Support Information
- Browse by Year
- Browse by Subject
- Browse by Division
- Browse by Author
- Simple Search
- Advanced Search
Actions (login required)

Assessing Culturally Tailored Dementia Interventions to Support Informal Caregivers of People Living with Dementia (PLWD): A Scoping Review
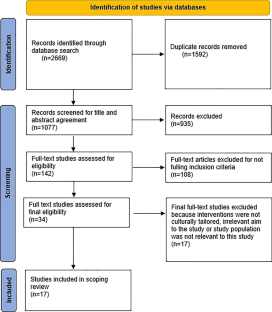
- Published: 28 March 2024
Cite this article
- Araya Dimtsu Assfaw ORCID: orcid.org/0000-0003-2163-0338 1 ,
- Kerstin M. Reinschmidt 2 ,
- Thomas A. Teasdale 2 ,
- Lancer Stephens 3 ,
- Keith L. Kleszynski 4 &
- Kathleen Dwyer 5
28 Accesses
Explore all metrics
The review aimed to identify and describe dementia care interventions and programs that are culturally tailored to support racial and ethnic minority informal caregivers of community-dwelling people living with dementia (PLWD) to identify gaps in need. Culturally targeted interventions to support vulnerable minority informal caregivers are important in addressing the care needs of PLWD and eliminating racial and ethnic dementia disparities. Nevertheless, little is known about the existing interventions and programs that are culturally tailored to support racial and ethnic minority groups, in particular, African-American caregivers in the care of their family members. We conducted a Scoping review, searching eight databases including MEDLINE, EMBASE, APA PsycINFO, CINAHL, PUBMED, Scopus, and Web of Science between January 2012 and June 2022. Our search identified 2669 records, of which 17 articles were included in the analysis. The review addressed how these interventions have been developed to meet the needs and preferences of minority caregivers, particularly, African-American caregivers in culturally responsive ways. Findings show that culturally tailored interventions have the potential to improve the caregiving ability of informal caregivers. Supporting informal caregivers appears to be an effective strategy often improving the well-being of PLWD and reducing caregiver burden. The review demonstrates the paucity and diversity of research on culturally tailored dementia interventions to reduce racial and ethnic disparities. This scoping review identified gaps in the existing literature and aims for future work to develop and investigate cultural tailoring of interventions.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Barriers and facilitators in accessing dementia care by ethnic minority groups: a meta-synthesis of qualitative studies.
Cassandra Kenning, Gavin Daker-White, … Waquas Waheed

Language and Culture in the Caregiving of People with Dementia in Care Homes - What Are the Implications for Well-Being? A Scoping Review with a Welsh Perspective
Conor Martin, Bob Woods & Siôn Williams
Dementia and migration: culturally sensitive healthcare services and projects in Germany
Jessica Monsees, Sümeyra Öztürk & Jochen René Thyrian
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17(3):327–406. https://doi.org/10.1002/alz.12328 .
2022 Alzheimer's disease facts and figures. Alzheimers Dement. 2022;18(4):700–89. https://doi.org/10.1002/alz.12638 .
What is Alzheimer’s Disease? | CDC. 2022. https://www.cdc.gov/aging/aginginfo/alzheimers.htm .
Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement 2019;15(3):321–87.
Skufca, L. AARP family caregiving survey: Caregivers’ reflections on changing roles. AARP Research. 2017;10.
2020 Alzheimer's disease facts and figures. Alzheimers Dement. 2020. https://doi.org/10.1002/alz.12068 .
Gallagher-Thompson D, Haley W, Guy D, Rupert M, Argüelles T, Zeiss LM, Long C, Tennstedt S, Ory M. Tailoring psychological interventions for ethnically diverse dementia caregivers. Clin Psychol: Sci Pract. 2003;10:423–38. https://doi.org/10.1093/clipsy.bpg042 .
Cooper C, Tandy AR, Balamurali TB, Livingston G. A systematic review and meta-analysis of ethnic differences in use of dementia treatment, care, and research. Am J Geriatr Psychiatry. 2010;18(3):193–203.
Article PubMed Google Scholar
Babulal GM, Quiroz YT, Albensi BC, Arenaza-Urquijo E, Astell AJ, Babiloni C, Bahar-Fuchs A, Bell J, Bowman GL, Brickman AM, Chételat G, Ciro C, Cohen AD, Dilworth-Anderson P, Dodge HH, Dreux S, Edland S, Esbensen A, Evered L, Ewers M., … International Society to Advance Alzheimer’s Research and Treatment, Alzheimer’s Association. Perspectives on ethnic and racial disparities in Alzheimer’s disease and related dementias: update and areas of immediate need. Alzheimers Dement : J Alzheimers Assoc. 2019;15(2):292–12. https://doi.org/10.1016/j.jalz.2018.09.009
Fabius CD, Wolff JL, Kasper JD. Race differences in characteristics and experiences of black and white caregivers of older Americans. Gerontologist. 2020;60(7):1244–53.
Article PubMed PubMed Central Google Scholar
Rainville C, Skufca L, Mehegan L. Family caregiving and out-of-pocket costs: 2016 report. African American RP Public Policy Institute; 2016.
Google Scholar
Hughes TB, Black BS, Albert M, Gitlin LN, Johnson DM, Lyketsos CG, Samus QM. Correlates of objective and subjective measures of caregiver burden among dementia caregivers: influence of unmet patient and caregiver dementia-related care needs. Int Psychogeriatr. 2014;26(11):1875–83. https://doi.org/10.1017/S1041610214001240 .
Darnell KR, McGuire C, Danner DD. African American participation in Alzheimer’s disease research that includes brain donation. Am J Alzheimers Dis Dement®. 2011;26(6):469–76.
Article Google Scholar
Armstrong TD, Crum LD, Rieger RH, Bennett TA, Edwards LJ. Attitudes of African Americans toward participation in medical research. J Appl Soc Psychol. 1999;29(3):552–74.
Portacolone E, Palmer NR, Lichtenberg P, Waters CM, Hill CV, Keiser S, Vest L, Maloof M, Tran T, Martinez P, Guerrero J, Johnson JK. Earning the trust of African American communities to increase representation in dementia research. Ethn Dis. 2020;30(Suppl 2):719–34. https://doi.org/10.18865/ed.30.S2.719 .
Dimtsu Assfaw, A., Reinschmidt, K. M., Teasdale, T. A., Stephens, L., Kleszynski, K. L., & Dwyer, K. Describing providers' perspectives on the needs and challenges of family caregivers of African American people living with dementia. Home Health Care Serv Q. 2023;1–21. https://doi.org/10.1080/01621424.2023.2299486 .
McCarron HR, Wright A, Moone RP, Toomey T, Osypuk TL, Shippee T. Assets and unmet needs of diverse older adults: Perspectives of community-based service providers in Minnesota. J Health Disparities Res Pract. 2020;13(1):6.
Barnes LL. Alzheimer disease in African American individuals: increased incidence or not enough data? Nat Rev Neurol. 2022;18(1):56–62. https://doi.org/10.1038/s41582-021-00589-3 .
Burgdorf JG, Amjad H. Impact of diagnosed (vs undiagnosed) dementia on family caregiving experiences. J Am Geriatr Soc. 2023;71(4):1236–42. https://doi.org/10.1111/jgs.18155 .
Lathren CR, Sloane PD, Hoyle JD, Zimmerman S, Kaufer DI. Improving dementia diagnosis and management in primary care: a cohort study of the impact of a training and support program on physician competency, practice patterns, and community linkages. BMC Geriatr. 2013;13(1):1–7.
Byers MD, Resciniti NV, Ureña S, Leith K, Brown MJ, Lampe NM, Friedman DB. An evaluation of Dementia Dialogues®: a program for informal and formal caregivers in North and South Carolina. J Appl Gerontol. 2022;41(1):82–91.
Glueckauf RL. Telephone-mediated, faith-based cognitive-behavioral intervention for depressed African-American dementia caregivers [Paper presentation]. Annual Meeting, American Psychological Association, Toronto, Canada; 2015.
Glueckauf RL, Davis WS, Allen K, Chipi P, Schettini G, Tegen L, Jian X, Gustafson DJ, Maze J, Mosser B, Prescott S, Robinson F, Short C, Ticket S, VanMatre J, DiGeronimo T, Ramirez C. Integrative cognitive-behavioral and spiritual counseling for rural dementia caregivers with depression. Rehabil Psychol. 2009;54(4):449–61.
Lampe NM, Desai N, Norton-Brown T, Nowakowski AC, Glueckauf RL. African-American lay pastoral care facilitators’ perspectives on dementia caregiver education and training. Qual Rep. 2022;27(2):324–39.
Joo JY. Effectiveness of culturally tailored diabetes interventions for Asian immigrants to the United States: a systematic review. Diabetes Educ. 2014;40(5):605–15.
Brijnath B, Croy S, Sabates J, Thodis A, Ellis S, de Crespigny F, Moxey A, Day R, Dobson A, Elliott C, Etherington C, Geronimo MA, Hlis D, Lampit A, Low LF, Straiton N, Temple J. Including ethnic minorities in dementia research: Recommendations from a scoping review. Alzheimers Dement (N Y). 2022;8(1):e12222. https://doi.org/10.1002/trc2.12222 .
Peters MDJ, Godfrey C, McInerney P, Khalil H, Larsen P, Marnie C, Pollock D, Tricco AC, Munn Z. Best practice guidance and reporting items for the development of scoping review protocols. JBI Evid Synth. 2022;20(4):953–68. https://doi.org/10.11124/JBIES-21-00242 .
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
Le CT, Lee JA. Home visit based mindfulness intervention for Vietnamese American dementia family caregivers: a pilot feasibility study. Asia Pac Island Nurs J. 2021;5(4):207.
Bergeron CD, Robinson MT, Willis FB, Albertie ML, Wainwright JD, Fudge MR, Parfitt FC, Crook JE, Ball CT, Lucas JA. Testing an Alzheimer's disease educational approach in two African American neighborhoods in Florida. J Racial Ethn Health Disparities. 2022;9(6):2283–90. https://doi.org/10.1007/s40615-021-01165-7 .
Goeman D, King J, Koch S. Development of a model of dementia support and pathway for culturally and linguistically diverse communities using co-creation and participatory action research. BMJ Open. 2016;6(12):e013064.
Lee JA, Nguyen H, Park J, Tran L, Nguyen T, Huynh Y. Usages of computers and smartphones to develop dementia care education program for Asian American family caregivers. Healthc Informat Res. 2017;23(4):338–42.
Han HR, Choi S, Wang J, Lee HB. Pilot testing of a dementia literacy intervention for Korean American elders with dementia and their caregivers. J Clin Transl Res. 2021;7(6):712.
PubMed PubMed Central Google Scholar
Chua J, Pachana NA. Use of a psychoeducational skill training DVD program to reduce stress in Chinese Australian and Singaporean dementia caregivers: a pilot study. Clin Gerontol. 2016;39(1):3–14.
Browne CV, Muneoka S, Ka'opua LS, Wu YY, Burrage RL, Lee YJ, Mokuau NK, Braun KL. Developing a culturally responsive dementia storybook with Native Hawaiian youth. Gerontol Geriatr Educ. 2022;43(3):315–27. https://doi.org/10.1080/02701960.2021.1885398 .
Chi I, Liu M, Wang S. Developing the Asian Pacific islander caregiver train-the-trainer program in the United States. J Ethn Cult Divers Soc Work. 2020;29(6):490–507.
Lincoln KD, Chow TW, Gaines BF. BrainWorks: a comparative effectiveness trial to examine Alzheimer’s disease education for community-dwelling African Americans. Am J Geriatr Psychiatry. 2019;27(1):53–61.
Wang Y, Xiao LD, Yu Y, Huang R, You H, Liu M. An individualized telephone-based care support program for rural family caregivers of people with dementia: study protocol for a cluster randomized controlled trial. BMC Geriatr. 2021;21(1):1–11.
Dunn DJ, Schwartz A, Teufel-Shone NI, Meyer LA. Educational program to promote resilience for caregivers, family members, and community members in the care of elderly Native Americans who are experiencing memory loss and cognitive decline. Visions: J Rogerian Nurs Sci. 2019;25(2).
Epps F, Moore M, Chester M, Gore J, Sainz M, Adkins A, Clevenger C, Aycock D. The Alter program: a nurse-led, dementia-friendly program for African American faith communities and families living with dementia. Nurs Adm Q. 2022;46(1):72–80. https://doi.org/10.1097/NAQ.0000000000000506 .
Richardson RC, Sistler AB. The well-being of elderly black caregivers and noncaregivers: a preliminary study. J Gerontol Soc Work. 1999;31(1–2):109–17.
Lampe NM, Desai N, Norton-Brown T, Nowakowski AC, Glueckauf RL. African American lay pastoral care facilitators’ perspectives on dementia caregiver education and training. Qual Rep. 2022;27(2):324–39.
Czaja SJ, Lee CC, Perdomo D, Loewenstein D, Bravo M, Moxley PhD, J. H., & Schulz, R. Community REACH: an implementation of an evidence-based caregiver program. Gerontologist. 2018;58(2):e130–7.
Levy-Storms L, Cherry DL, Lee LJ, Wolf SM. Reducing safety risk among underserved caregivers with an Alzheimer’s home safety program. Aging Ment Health. 2017;21(9):902–9.
Napoles AM, Chadiha L, Eversley R, Moreno-John G. Reviews: developing culturally sensitive dementia caregiver interventions: are we there yet? Am J Alzheimers Dis Dement. 2010;25(5):389–406.
Jones I. Social class, dementia and the fourth age. Sociol Health Illn. 2017;39(2):303–17.
Sidani S, Ibrahim S, Lok J, Fan L, Fox M, Guruge S. An integrated strategy for the cultural adaptation of evidence-based interventions. Health. 2017;9(04):738.
Berwald S, Roche M, Adelman S, Mukadam N, Livingston G. Black African and Caribbean British Communities’ perceptions of memory problems: “We don’t do dementia.” PloS one. 2016;11(4):e0151878.
Lynch CP, Williams JS, J Ruggiero K, G Knapp R, Egede LE. Tablet-aided behavioral intervention effect on self-management skills (TABLETS) for diabetes. Trials. 2016;17:157. https://doi.org/10.1186/s13063-016-1243-2 .
Chandler J, Sox L, Kellam K, Feder L, Nemeth L, Treiber F. Impact of a culturally tailored mHealth medication regimen self-management program upon blood pressure among hypertensive Hispanic adults. Int J Environ Res Public Health. 2019;16(7):1226.
Joseph RP, Keller C, Adams MA, Ainsworth BE. Print versus a culturally-relevant Facebook and text message delivered intervention to promote physical activity in African American women: a randomized pilot trial. BMC Womens Health. 2015;15(1):1–18.
Lucero RJ, Fehlberg EA, Patel AGM, Bjarnardottir RI, Williams R, Lee K, Ansell M, Bakken S, Luchsinger JA, Mittelman M. The effects of information and communication technologies on informal caregivers of persons living with dementia: a systematic review. Alzheimers Dement (N Y). 2018;5:1–12. https://doi.org/10.1016/j.trci.2018.11.003.PMC6315277 .
Adewuya AO, Momodu O, Olibamoyo O, Adegbaju A, Adesoji O, Adegbokun A. The effectiveness and acceptability of mobile telephone adherence support for management of depression in the Mental Health in Primary Care (MeHPriC) project, Lagos, Nigeria: a pilot cluster randomised controlled trial. J Affect Disord. 2019;253:118–25.
Epps F, Alexander K, Brewster GS, Parker LJ, Chester M, Tomlinson A, Adkins A, Zingg S, Thornton J. Promoting dementia awareness in African‐American faith communities. Publ Health Nurs. 2020;37(5):715–21. https://doi.org/10.1111/phn.12759 .
Epps F, Foster K, Alexander K, Brewster G, Chester M, Thornton J, Aycock D. Perceptions and attitudes toward dementia in predominantly African American congregants. J Appl Gerontol. 2021;40(11):1511–16. https://doi.org/10.1177/0733464820987350 .
Su D, Garg A, Wiens J, Meyer E, Cai G. Assessing health needs in African American churches: a mixed methods study. J Relig Health. 2021;60(2):1179–97.
Williams LF, Cousin L. “A charge to keep I have”: black pastors’ perceptions of their influence on health behaviors and outcomes in their churches and communities. J Relig Health. 2021;60(2):1069–82.
James T, Mukadam N, Sommerlad A, Ceballos SG, Livingston G. Culturally tailored therapeutic interventions for people affected by dementia: a systematic review and new conceptual model. Lancet Healthy Longev. 2021;2(3):e171–9.
Abramson CM, Hashemi M, Sánchez-Jankowski M. Perceived discrimination in US healthcare: charting the effects of key social characteristics within and across racial groups. Prev Med Rep. 2015;2:615–21.
McDaniel M, Richardson A, Gonzalez D, Caraveo CA, Wagner L, Skopec L. Black and African American adults’ perspectives on discrimination and unfair judgment in health care. Washington, DC: Urban Institute; 2021.
Kennedy BR, Mathis CC, Woods AK. African Americans and their distrust of the health care system: healthcare for diverse populations. J Cultural Divers. 2007;14(2), 56–60.
Hammonds EM, Reverby SM. Toward a historically informed analysis of racial health disparities since 1619. Am J Public Health. 2019;109(10):1348–9.
Jones C, Jablonski RA. “I don’t want to be a guinea pig”: recruiting older African Americans. J Gerontol Nurs. 2014;40(3):3–4.
Joo JY, Liu MF. Culturally tailored interventions for ethnic minorities: a scoping review. Nurs Open. 2021;8(5):2078–90. https://doi.org/10.1002/nop2.733 .
Joyce KE, Cartwright N. Bridging the gap between research and practice: predicting what will work locally. Am Educ Res J. 2020;57(3):1045–82.
Wallerstein N, Oetzel JG, Duran B, Magarati M, Pearson C, Belone L, Davis J, DeWindt L, Kastelic S, Lucero J, Ruddock C, Sutter E, Dutta MJ. Culture-centeredness in community-based participatory research: contributions to health education intervention research. Health Educ Res. 2019;34(4):372–88. https://doi.org/10.1093/her/cyz021 .
Download references
Acknowledgements
The author would like to thank Sheryl Lynn Hamilton of the University of Oklahoma Health Sciences Center Robert M Bird Health Sciences Library for her assistance in the search strategy process.
Author information
Authors and affiliations.
Department of Neurology- Knight Alzheimer Disease Research Center, Washington University in St. Louis, St. Louis, MO, USA
Araya Dimtsu Assfaw
Hudson College of Public Health, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA
Kerstin M. Reinschmidt & Thomas A. Teasdale
Hudson College of Public Health & Oklahoma Shared Clinical and Translational Research Center, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA
Lancer Stephens
Department of Medicine, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA
Keith L. Kleszynski
Fran and Earl Ziegler College of Nursing, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA
Kathleen Dwyer
You can also search for this author in PubMed Google Scholar
Contributions
The first author, Araya Dimtsu Assfaw, performed material preparation, data collection, and analysis. All authors commented on the previous version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Araya Dimtsu Assfaw .
Ethics declarations
Ethics approval.
This is a review study and does not involve the voluntary participation of human subjects. Therefore, no ethical approval is required.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Assfaw, A.D., Reinschmidt, K.M., Teasdale, T.A. et al. Assessing Culturally Tailored Dementia Interventions to Support Informal Caregivers of People Living with Dementia (PLWD): A Scoping Review. J. Racial and Ethnic Health Disparities (2024). https://doi.org/10.1007/s40615-024-01985-3
Download citation
Received : 19 September 2023
Revised : 13 March 2024
Accepted : 17 March 2024
Published : 28 March 2024
DOI : https://doi.org/10.1007/s40615-024-01985-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cultural tailoring
- Ethnic minority
- African-Americans
- Health disparities
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMC Geriatr

Dissemination and implementation research in dementia care: a systematic scoping review and evidence map
Ilianna lourida.
1 NIHR CLAHRC South West Peninsula (PenCLAHRC), University of Exeter Medical School, University of Exeter, South Cloisters, St Luke’s Campus, Exeter, EX1 2LU UK
Rebecca A Abbott
Morwenna rogers, iain a lang, bridie kent.
2 School of Nursing and Midwifery, Plymouth University, Plymouth, UK
Jo Thompson Coon
Associated data.
A list of the reviewed studies supporting our findings and on which the conclusions of the manuscript rely can be found in Additional files, Additional file 5 .
The need to better understand implementing evidence-informed dementia care has been recognised in multiple priority-setting partnerships. The aim of this scoping review was to give an overview of the state of the evidence on implementation and dissemination of dementia care, and create a systematic evidence map.
We sought studies that addressed dissemination and implementation strategies or described barriers and facilitators to implementation across dementia stages and care settings. Twelve databases were searched from inception to October 2015 followed by forward citation and grey literature searches. Quantitative studies with a comparative research design and qualitative studies with recognised methods of data collection were included. Titles, abstracts and full texts were screened independently by two reviewers with discrepancies resolved by a third where necessary. Data extraction was performed by one reviewer and checked by a second. Strategies were mapped according to the ERIC compilation.
Eighty-eight studies were included (30 quantitative, 34 qualitative and 24 mixed-methods studies). Approximately 60% of studies reported implementation strategies to improve practice: training and education of professionals (94%), promotion of stakeholder interrelationships (69%) and evaluative strategies (46%) were common; financial strategies were rare (15%). Nearly 70% of studies reported barriers or facilitators of care practices primarily within residential care settings. Organisational factors, including time constraints and increased workload, were recurrent barriers, whereas leadership and managerial support were often reported to promote implementation. Less is known about implementation activities in primary care and hospital settings, or the views and experiences of people with dementia and their family caregivers.
This scoping review and mapping of the evidence reveals a paucity of robust evidence to inform the successful dissemination and implementation of evidence-based dementia care. Further exploration of the most appropriate methods to evaluate and report initiatives to bring about change and of the effectiveness of implementation strategies is necessary if we are to make changes in practice that improve dementia care.
Electronic supplementary material
The online version of this article (doi:10.1186/s12877-017-0528-y) contains supplementary material, which is available to authorized users.
Dementia is a multi-causal syndrome characterised by progressive deterioration in cognitive abilities and impairment in the ability to perform everyday activities; it can compromise capacity for independent living and lead to needs for care [ 1 ]. More than 35 million people live with dementia worldwide and, given that the disease is primarily associated with increasing age, the number is likely to increase in ageing populations [ 2 ]. Dementia is now among the most feared conditions in adults aged over 55 [ 3 ] and poses a significant economic burden to individuals and healthcare systems with average annual costs over €160 billion in Europe and $150 billion in the US [ 4 , 5 ].
Perhaps because of this growing cost, dementia has come increasingly to the attention of policymakers (e.g. Department of Health 2015 [ 6 ], US Department of Health and Human Services 2016 [ 7 ]) who have highlighted the need for more research on prevention, care, and cure as well as for high quality service provision. Despite this, there remains a persistent gap between evidence provision and implementation: currently provided dementia care often does not reflect what research evidence suggests would improve outcomes. There is intermittent and geographically variable quality of care for people with dementia: in the UK, a Care Quality Commission found that “quality of care for people with dementia varies greatly and it is likely that they will experience poor care at some point along their care pathway” [ 8 ], and the London-based Health Foundation [ 9 ] found that examples of evidence-based guidelines and good practice in dementia care are inconsistently disseminated and implemented. In the US, the Dementia Action Alliance found that “dementia care in this country is impersonal and fragmented” [ 10 ] and the privately-funded Alzheimer’s Australia National Quality Dementia Care Initiative was explicitly established “to fast-track the implementation of existing dementia care research into wide-spread improvements in practice” [ 11 ].
The need for a better understanding of how to implement evidence-informed dementia care has also been recognised through priority setting partnerships and policy statements (e.g. James Lind Alliance/Alzheimer’s Society Dementia Priority Setting Partnership [ 12 ], Blackfriars Consensus on promoting brain health) [ 13 ]. In an attempt to identify and map the state of the evidence in implementation and dissemination in dementia care, we conducted a systematic scoping review of existing research in dissemination and implementation and used this to create a systematic evidence map. As such, our findings can be useful in prioritising areas of further implementation research in dementia care.
Our scoping review was guided by the methods developed by Arksey and O’Malley [ 14 , 15 ]. Scoping reviews provide an overview of the literature by mapping the key concepts in the evidence base of a research area and can be used to inform the need for a full systematic review and identify gaps in knowledge [ 14 ]. In contrast to systematic reviews, scoping reviews tend to have broader research questions to capture the range of evidence on the selected topic, apply inclusion and exclusion criteria that are often further developed and refined during the selection process, do not always involve detailed data extraction, and do not include an assessment of the methodological quality of included studies [ 15 ]. The aim of our scoping review was to systematically explore and describe the breadth and nature of available research in dissemination and implementation strategies within dementia care. We also wanted to identify the type of barriers and facilitators involved in the implementation process.
A project advisory group consisting of multiple stakeholders was established to work with the review team. The group involved carers and public with experience and interest in dementia care (Alzheimer’s Society research network), dementia friendly communities, communication, researchers and health professionals active in dementia care. The group met on three occasions and was involved in multiple stages of the project from the development of the review to the dissemination of findings. The methods for the scoping review were pre-specified in a protocol developed in collaboration with the project advisory group. The protocol was not registered with PROSPERO as scoping reviews do not fall within the remit of this initiative but is available from the authors on request.
Study identification
A comprehensive search strategy was developed by an information specialist (MR) with input from the team using a combination of subject headings (MeSH terms) and free-text terms to cover the broad knowledge translation, implementation and dementia fields (Additional file 1 ). We undertook literature searches using the following databases from inception through October 2015: MEDLINE, Embase, PsycINFO, Healthcare Management Information Consortium (HMIC), Social Policy & Practice (SPP), Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trails (CENTRAL), Cumulative Index to Nursing and Allied Health Literature (CINAHL), British Nursing Index (BNI), Applied Social Sciences Index and Abstracts (ASSIA), Social Sciences Citation Index (SSCI) and Conference Proceedings Citation Index (CPCI). We applied no language or methodological filters in searching. We subsequently searched citations of included papers (forwards citation searching) using Scopus and ISI Web of Science for potentially relevant studies. As an additional way of identifying grey literature we posted a request to CHAIN (Contact, Help, Advice and Information Network; an online mutual support network for people working in health and social care).
Eligibility criteria
We included studies if they: (i) addressed dissemination or implementation strategies within dementia care or (ii) explored barriers and facilitators to dissemination or implementation and the strategies used to address them. For the purpose of this review, we used a definition of dissemination as ‘the targeted distribution of information and intervention materials to a specific public health or clinical practice audience, the intent of which is to spread knowledge’ [ 16 ]. We used a definition of implementation as ‘the use of strategies to introduce or change health and social care interventions within specific settings’ [ 16 ]. Dementia care refers to any aspect of health and social care support and services for people with dementia and their carers, in any setting. We included quantitative studies with a comparative research design and qualitative studies with recognised methods of data collection (e.g. interviews, focus groups) and synthesis (e.g. thematic or framework analysis, grounded theory). In order to be included, quantitative studies had to report on implementation effectiveness, i.e. the degree to which the implementation strategy of an innovation or intervention had been successful, rather than whether the intervention itself had been successful or effective. For example, studies aiming to improve the management of challenging behaviour in nursing homes through a new protocol had to report on the adherence to the protocol, and not simply on rates of change in challenging behaviour. Studies that included populations other than just people with dementia or populations with comorbid dementia were included if outcomes were reported separately for the sub-group with dementia. To capture the breadth of research in this area, we considered studies in care at all stages of dementia from first diagnosis through to palliative care and all settings of care. Populations of interest included people with dementia and those caring for them such as family caregivers, healthcare professionals, and other staff.
Study selection
Titles and abstracts were screened for relevance independently by pairs of reviewers (IL and one of RA, JTC, MR, or IAL). Disagreements were resolved by discussion between reviewers or with the involvement of a third reviewer (RA, JTC, IAL) where necessary. We screened the full text of potentially relevant papers in the same way using the predefined inclusion and exclusion criteria. We had two non-English papers translated and contacted nine authors to request access to full-text reports. During the study selection process and as the team became more familiar with the nature of available literature, we refined and re-applied the initial criteria to reflect the focus of the question guiding the scoping review. Thus, we included studies exploring barriers and/or facilitators if they: (i) reported barriers/facilitators to the use of identified dissemination or implementation strategies (e.g. training, use of guidelines), (ii) related to a change in practice, knowledge or behaviour, or (iii) described experiences, perceptions, or attitudes towards the use of implementation strategies or change in practice, knowledge, or behaviour. We excluded studies that reported only barriers/facilitators and relevant experiences, perceptions, or attitudes to usual everyday care practices (i.e. not in the context of changing practice).
Data charting
Data from the included studies were extracted and summarised by one reviewer (IL) and checked for accuracy by a second reviewer (RA) using bespoke forms developed in Excel. Disagreements were resolved by discussion. Extracted data included publication type, year and country, study design and methods, sample size, time frame, setting, topic area, target population, dementia stage, theory/framework used, details of the dissemination or implementation approach and relevant strategies, barriers and facilitators, and outcome variables.
We explored coding of dissemination and implementation approaches using two different classifications: the EPOC (Effective Practice and Organisation of Care) taxonomy of health systems interventions [ 17 ] and the ERIC (Expert Recommendations for Implementing Change) compilation of implementation strategies [ 18 , 19 ]. The latest revised version of EPOC taxonomy organises complex health interventions into four main domains: delivery, financial and governance arrangements, and implementation strategies. Each domain contains categories and subcategories attempting to describe changes in how, when, and where healthcare is delivered, financial incentives and disincentives, rules and processes that may affect the organisation of services, and interventions or strategies that target healthcare professionals or organisations [ 17 ]. The ERIC compilation provides a summary of specific implementation strategies used to bring about change. ERIC aims to promote terminological consistency by organising a total of 73 distinct implementation strategies under nine thematic clusters. The clusters cover areas such as stakeholder training and education, clinician support, development of stakeholder interrelationships, changes in infrastructure, patient/consumer engagement, financial strategies, and the use of evaluative and iterative strategies to support practice change [ 19 ]. After testing both approaches in a small sample of papers ( n = 12) and reflection in the review team, we decided the ERIC classification was more appropriate for this scoping review as it provides a more detailed and conceptually clear description of strategies. Included studies were coded independently by two reviewers (IL and RA) and are reported herein using the ERIC compilation. We charted data for the specific ERIC implementation strategies described in the studies and their allocated code (1–73) along with the corresponding cluster (1–9).
We adapted terminology from previous studies in knowledge translation interventions and contextual factors that may hinder or enable implementation [ 20 – 22 ] to classify barriers and facilitators within five categories: organisational, professional, individual, financial, other. Organisational factors relate to managerial and administrational support, the culture, organisation, management and facilities of settings providing dementia care. Professional factors relate to training, staff knowledge and skills. Individual factors include characteristics and attitudes of staff and other participants, and financial factors refer to operating costs and funding resources. We categorised outcomes as relating to staff members, to people with dementia, or to informal caregivers and family members with subcategories to reflect changes in practice (assessment, compliance, treatment, performance), knowledge, perceptions, behaviour, and physical health.
Consistent with the methods of scoping reviews, as described by Arksey and O’Malley [ 14 , 15 ], we did not assess the methodological quality or risk of bias of included studies.

Data analysis (mapping the evidence)
We tabulated and classified data according to the setting of dementia care provision and these are presented narratively below. We used tables (see Additional file 2 : Table S1, Additional file 3 : Table S2 and Additional file 4 : Table S3), frequencies, and percentages to support narrative statements and provide an overview of the evidence base through summaries of the study characteristics (country, study design and methods, sample size, target population, topic area, broad category for focus of implementation, and context), implementation strategies, type of barriers/facilitators, and outcome type. We identified gaps in the literature during the process of collating and reporting the results using characteristics such as study design, topic area, setting, implementation-strategy cluster, related barriers and facilitators, and outcome.
Literature search
Our electronic searches yielded 5131 citations. Deduplication and screening of titles and abstracts resulted in 257 papers for full text review of which 80 were eligible for inclusion. Our request to the CHAIN network resulted in the retrieval of 18 reports and studies of which none met the inclusion criteria. We identified eight additional papers through forward citation searching. In total, 88 papers met the inclusion criteria for this scoping review. The study flow with the number of identified citations, included studies, and reasons for exclusion is presented in Fig. Fig.1. 1 . The full list of included studies is in Additional file 5 .

Flow chart of study selection process
Study characteristics
Additional file 2 : Table S1. presents a summary of the characteristics of all included studies sorted by setting of dementia care provision. Eighty-one of the included studies were peer-reviewed publications, two were dissertations, and five were independent reports. Publication year ranged from 1998 to 2015 but more than two-thirds of studies (69%, 61/88) were published in 2011 or later. The majority of studies were conducted in the USA ( n = 22), followed by Australia ( n = 18), the UK ( n = 14), Canada ( n = 12), Netherlands ( n = 11), and other European ( n = 6) and Asian countries ( n = 3). One study collected data in England and the Netherlands [ 23 ] and another study included participants from nine European countries [ 24 ]. Thirty-nine percent of included studies were qualitative (34/88), 34% were quantitative (30/88) and 27% were mixed-methods studies (24/88).
A wide range of data collection methods were used across studies and in many studies multiple methods were used to collect data (e.g. cluster RCT plus interviews or focus groups plus surveys). Interviews were the most frequently reported study method ( n = 25) followed by before/after studies ( n = 19). Focus groups were used in ten studies, a combination of interviews and focus groups in 12 studies, and surveys in 14 studies. Eight studies were cluster RCTs, one was a RCT, and three were cohort studies. Other study designs and methods used included best practice implementation reports (i.e. JBI reports), quality improvement, and action research ( n = 14). Reporting of implementation periods and of duration of follow-up was inconsistent. The wide variety of study methods and designs meant that studies described implementation activities ranging from half-day training to projects spanning a five-year period. Forty-nine percent (43/88) of studies reported follow-up data and the follow-up period ranged from one week to five years.
Nearly 60% (52/88) of the studies addressed dissemination and implementation interventions and the remainder (36/88) were concerned only with barriers/facilitators to dissemination or implementation activities without providing details or description of the implementation process. A combination of these (e.g. reporting of the implementation of a pain management protocol and also of barriers and facilitators to change) was reported in 26% of studies (23/88). The implementation strategies and the discussions around barriers and facilitators to change mostly targeted professionals: nursing staff ( n = 27, 31%), care home and facility staff ( n = 20, 23%), physicians ( n = 11, 12.5%), other healthcare professionals ( n = 11, 12.5%), and managers/leaders ( n = 12, 14%). Other stakeholders actively involved in implementation initiatives across studies ( n = 17, 19%) included researchers, experts in dementia care, activity therapists, psychologists, social-care workers, financial experts, police officers, architects, administrators, volunteers, and voluntary agencies. Relevant data for family members/caregivers involvement was included in 15% (13/88) of studies. Two studies sought the views of people with dementia. The dementia stage of residents and participants ranged from early through severe to end-of-life but it was unclear or not reported in 77% (68/88) of studies.
We classified settings into five categories: residential long-term care, community care, primary care, hospital, and multiple. The residential long-term care category ( n = 46) included care homes, nursing homes, assisted-living, skilled and residential aged care facilities, and dementia specialist-care units within homes or other long-term care facilities. Community care ( n = 16) included studies taking place in non-residential care facilities and in the homes of people with dementia or caregivers. The primary care ( n = 8) and hospital ( n = 5) categories included studies explicitly stating those as the settings of the reported research. Multiple settings ( n = 13) included studies in which dissemination or implementation activities took place in more than one of the above settings (e.g. nursing home and hospital). The number of participating or targeted sites across settings ranged from 1 to 15,453. About half of the studies were conducted within one site (52%, 46/88) and in 15 studies the number of sites ranged from two to ten (17%). Cluster RCTs ( n = 8, 9%) included 9 to 45 sites.
Focus of implementation
During data extraction we assigned a general descriptive theme to each included study. We then combined groups of studies that fitted conceptually together and, following reviewer agreement (IL and RA), we created a broad descriptive category. This process resulted in seven broad categories to describe the focus of included studies: Models of care ( n = 17), Knowledge transfer and dementia education ( n = 17), Behaviour management ( n = 15), Care practices ( n = 14), Guideline-driven practices ( n = 12), Services and infrastructure ( n = 8), and Care directives/frameworks ( n = 5) (Additional File 2 : Table S1). The ‘Models of care’ category included studies describing different models, methods and approaches to provide and improve dementia care such as person-centred care, capability model of care, and palliative approaches. The ‘Knowledge transfer and dementia education’ category included information exchange, use of research findings, factors influencing knowledge transfer, multifaceted implementation strategies, translation of caregiver intervention programmes, and other dementia training and outreach programmes for professionals. ‘Behaviour management’ included non-pharmacological and psychosocial interventions for BPSD, antipsychotic medication prescribing, and use of physical restraints. The ‘Care practices’ category included practices related to pain, oral health, bathing, sleep hygiene, mobility, and case management. The ‘Guideline-driven practices’ category included studies that examined the process or factors affecting the dissemination and implementation of specific guidelines. The development of memory clinics, meeting centres and other care units, the introduction of new services, evaluation of demonstration sites and facility design of residential settings were under the ‘Services and infrastructure’ category. The introduction and implementation of advanced care planning, Advance Directives, Do Not Hospitalise orders, and the Mental Capacity Act were represented in the ‘Care directives/frameworks’ category.
Implementation and dissemination strategies
Of the 52 studies addressing dissemination and implementation within dementia care, five described dissemination, 36 described implementation and 11 reported both dissemination and implementation activities. The coding based on the ERIC compilation of implementation strategies is shown in Additional file 3 : Table S2 and Additional File 4 : Table S3. Although description of strategies was not always clear, we identified 55 out of the 73 strategies across all nine clusters. The majority of studies reported multifaceted implementation strategies which combine two or more discrete strategies but a few studies reported blended strategies which have been described as “multiple strategies packaged as a protocolized or branded implementation intervention” [ 25 ]. Studies reported a minimum of three and a maximum of 11 strategies covering between two and seven ERIC clusters. Additional File 3 : Table S2 shows the total number of times each strategy was coded across the 52 studies. All but three studies had an educational component and used strategies described within the ‘Train and educate stakeholders’ cluster ( n = 49, 94%). The most commonly reported strategy was educational meetings ( n = 38) followed by the distribution of educational materials ( n = 34) and dynamic training ( n = 19). Strategies to develop stakeholder interrelationships ( n = 36, 69%) and the use of evaluative and iterative strategies ( n = 24, 46%) were frequently reported alongside training and educational strategies. Financial strategies were the least commonly reported ( n = 8, 15%). Eleven strategies across clusters were reported once and 18 of the ERIC strategies were not identified at all (Additional File 3 : Table S2).
Waltz and colleagues [ 19 ] present in their paper a graphical summary of the 73 ERIC implementation strategies based on their mean importance and feasibility ratings as determined by expert consensus. The majority of strategies in the high importance and high feasibility category lie within clusters 1, 4 and 5 which are also among the most identified strategies in our data (pink, light green and purple respectively, quadrant I; Fig. Fig.2). 2 ). Nevertheless, the individual highly important and feasible implementation strategies within these clusters have not been reported frequently across the reviewed studies (e.g. strategies #4,#5,#18,#33,#38; Additional File 3 : Table S2). Financial strategies generally received a low feasibility rating and we found only a few studies reporting these (dark pink, quadrant IV).

Bubble plot for the frequency of the 55 implementation strategies identified within included studies based on ERIC importance and feasibility ratings. The range of the x and y axes reflect values obtained for the 73 discrete implementation strategies for each of the rating scales during the ERIC rating tasks. 18 The plot is divided into quadrants on the basis of the overall mean values for each of the rating scales. Strategies in quadrant I are those with the highest consensus regarding their relative high importance and feasibility. Strategies in quadrant III are those where there was consensus regarding their relative low importance and feasibility. Strategies in quadrant II were relatively high in feasibility but low in importance, and strategies in quadrant IV were relatively high in importance but low in the feasibility scale
Outcome characteristics
Nearly half of the studies ( n = 47, 53%) reported staff-related outcomes and within these studies 29% ( n = 14) also reported outcomes related to people with dementia and family/caregivers (Additional File 4 : Table S3.). Forty-five studies used some quantitative measure of effectiveness linked to implementation as the staff-related outcome. The most commonly used outcome that also reflected a measurement of effectiveness was compliance (e.g. compliance with guidelines or use of tool, n = 28), followed by change in knowledge ( n = 17), and perceptions and attitudes ( n = 14). Change in behaviour (e.g. agitation) was the most frequent outcome for people with dementia ( n = 8), while perceptions were most frequently investigated within family/caregiver outcomes ( n = 4). Overall, significant changes in practice, knowledge or perceptions/attitudes were reported in 23 of the 47 studies (49%). The majority of both staff-related ( n = 29) and non-professional ( n = 8) outcomes were studied in the residential long-term care settings.
Barriers and facilitators
Studies reported collecting data on barriers and/or facilitators mostly using interviews and focus groups but also as part of surveys, questionnaires, and field notes. Barriers and facilitators were reported in 67% (59/88) of studies in total (implementation strategies plus barriers/facilitators n = 23, only barriers/facilitators n = 36). More specifically, 22 studies focused solely on barriers, four studies focused solely on facilitators, and a combination of hindering and enabling factors was reported in 33 studies. The dominant factor was organisational, highlighted in 91% of studies on barriers and facilitators (54/59) (Additional File 4 : Table S3). Time constraints, increased workload, leadership, and managerial support were common themes in this category. Professional factors were identified in 52% of studies (31/59) and included lack of dementia-related knowledge, training and experience using tools, and behavioural strategies. Personality characteristics of staff members, engagement, resistance to change, and other individual factors were reported in 51% of studies (30/59). Financial factors such as lack of funding or financial constraints were reported in 15 studies. Other identified barriers were environmental (physical structure limitations), legal (boundaries and legal status of advance care planning), resident-specific (poor health status), and dementia-specific (cognitive impairment and other complications in the course of the disease) factors (each reported once).
Use of frameworks
Thirty-eight studies reported using a theory or framework as part of the implementation process (43%, 38/88). A total of 33 different frameworks were reported. RE-AIM [ 26 ] was the most commonly cited framework ( n = 5) and the Joanna Briggs Institute PACES and GRIP programme [ 27 ] was the most frequently used online tool used to conduct audits and facilitate practice change ( n = 5). Four studies referred to frameworks that addressed the dissemination stage of the intervention (Train-the-trainer model, Kerr and Slocum’s model of performance, Diffusion of Innovation Theory) but frameworks were generally used to guide or evaluate the wider implementation process and this usually also included dissemination. Twelve studies used a theory or framework to inform the identification and description of barriers and facilitators to dissemination ( n = 2) and implementation ( n = 10).
Implementation stages
The numerous theories and frameworks available [ 28 ] to inform and enhance implementation research highlight the dynamic nature of this process, which is usually characterised in several stages. According to the EPIS conceptual model [ 29 ] there are four phases of implementation: Exploration, Preparation, Implementation, and Sustainment. The majority of the studies we included focused on the implementation phase either in terms of strategies used for change or related barriers and facilitators. There was little attention to the Exploration phase, ( n = 3) where potential implementers searched the literature for evidence-based practices to suit their needs and/or assessed readiness for change. Few studies described a preparation stage ( n = 6) that includes assessment of implementation challenges, and all best practice implementation projects involved a planning stage following an initial audit ( n = 6). Thirty studies addressed some aspect of Sustainment: (i) ten studies measured the sustainability of a project or included a relevant maintenance stage and outcome (e.g. studies describing the maintenance stage as part of the RE-AIM framework), and (ii) 20 studies described factors affecting project sustainability or reported plans and suggestions to maintain project implementation.
In this scoping review we identified 88 primary studies addressing dissemination and implementation research across various settings of dementia care published between 1998 and 2015. Our findings indicate a paucity of research focusing specifically on dissemination of knowledge within dementia care and a limited number of studies on implementation in this area. We also found that training and education of professionals, development of stakeholder interrelationships and the use of evaluative and iterative strategies are frequently employed to introduce and promote change in practice. However, although important and feasible, these strategies only partly address what is repeatedly highlighted in the evidence base: that organisational factors are reported as the main barrier to implementation of knowledge within dementia care [ 30 – 35 ]. Moreover, included studies clearly support an increased effort to improve the quality of dementia care provided in residential settings in the last decade. Nevertheless, people with dementia and their family members have been rarely involved in implementation research and their views and experiences have generally not been considered as part of implementation process [ 36 , 37 ]. Funding for dementia research has increased markedly in the past decade and this has led to an increase in research outputs. However, assuming that increased levels of research will lead to changes in practice, perhaps based on some poorly-conceived notions of knowledge diffusion, is at best naïve and at worst recklessly wasteful. That we found so few papers on dissemination and implementation in dementia care is a sign that this aspect of improving quality of dementia care has been neglected and is in urgent need of greater attention and more resources, as has been previously highlighted [ 12 , 13 ]. Without this, even the best dementia research will go to waste, in which case everybody – funders, researchers, and people affected by dementia – loses out.
Health services research in other areas of care suggests that implementation strategies to promote evidence-based practice and improve quality of care are dominated by educational approaches to train professionals mainly through educational meetings and the distribution of educational materials, reminder systems to facilitate clinician decision-making, and evaluative strategies such as audit and feedback [ 38 ]. Our findings reveal a similar picture for implementation in the field of dementia care. Synthesised evidence in guideline implementation research indicates that although most implementation strategies result in small to moderate improvements in quality of care, there is an increased likelihood of positive results in practice, knowledge or patient outcomes with the use of multifaceted interventions that also target barriers to change and actively engage stakeholders [ 38 – 40 ]. Multiple strategies have been reported within individual studies in this review and our findings show that educational strategies are often combined with organisational-level approaches to support stakeholder interrelationships, evaluative and iterative strategies, and occasionally changes in infrastructure as part of practice change in dementia care. However, only a small proportion of studies reported a stage in the implementation process dedicated to the identification of barriers and facilitators or strategies tailored to address them. In addition, 30% of the ERIC compilation strategies generated by expert consensus to guide implementation were not identified in the included studies. Many of these strategies describe financial approaches (e.g. access new funding, use capitated payments) where there was consensus regarding their relative high importance but low feasibility [ 19 ]. Although it is unlikely that this finding is unique to dementia care, the degree to which these specific strategies could promote implementation within the field remains to be investigated. The usefulness of the ERIC compilation to characterise implementation strategies within dementia care that are also relevant to health care systems other than in the US should be explored further.
The majority of included studies covering barriers and facilitators to implementation reported some factor lying at the organisational level. This finding occurred across settings and was particularly prominent among nurses and other care staff. Frequent reports highlighting the role of managerial support and insufficient time to complete heavy workload are consistent with evidence on organisational culture factors that act respectively as facilitators of and barriers to implementation of best practice from different healthcare disciplines [ 41 , 42 ]. It seems reasonable to suggest that comprehensive approaches with strategies tailored to promote identified organisational facilitators and overcome barriers in dementia care would promote practice change. However, studies on the effectiveness of such strategies are limited [ 43 ]. Professional factors and individual characteristics identified in our scoping review including lack of dementia-specific knowledge, resistance to change, held attitudes, staff engagement and competence also appear to play a role in the implementation of dementia care practices [ 32 , 44 – 48 ]. This indicates that future implementation efforts would benefit from a preparation stage to identify potential barriers and facilitators, and subsequently plan for multifaceted strategies that address the different levels informed by the needs and desires of relevant stakeholders in a constantly changing environment of care provision. Additional research using theories/models to identify and describe the various barriers and facilitators of desired change [ 21 ] at the micro-, meso- and macro-level is needed to shed light on the key predictors of change and the complex dynamics of implementation content, enabling and hindering factors within dementia care.
Much of the available literature covers research conducted within residential settings predominantly nursing and care homes. As such, many studies were identified in areas of care including behaviour assessment and management and models of care targeting people with dementia that are of particular interest in these settings. They reflect the great challenges nursing staff and other healthcare professionals face in managing symptoms as well as efforts for quality improvement in residential care facilities internationally [ 49 , 50 ]. However, there is very limited evidence relating to implementation of strategies for initiatives to manage comorbidities in people with dementia [ 51 ] within long-term care facilities, and an evidence gap in translating research into practice in terms of transitions between care settings [ 52 ]. The review also suggests that published research in implementation efforts to improve dementia care practice in hospitals and primary care clinics does not match the increasing demand of these settings to care for people with dementia and their caregivers [ 53 ]. In addition, little is known about how best to put practices into action to support family caregivers of people with dementia living in the community or the implementation of dementia care practices at the end of life. As evidence grows, these areas should also be prioritised as implementation targets to promote high-quality dementia care and deliver on the ‘living well with dementia’ challenge [ 54 ].
Although we did not conduct a formal quality assessment of the included studies, we identified a few limitations. Most of the studies did not report the dementia characteristics (e.g. type and severity) of populations, which should be included in future studies. The duration of implementation was unclear in many studies and it was often difficult to differentiate between the implementation period and duration of follow up. Such characteristics of the condition and context are crucial in order to map the extent and nature of implementation research across the dementia care pathway, and to illuminate areas of care for knowledge translation that may be particularly relevant to certain stages of the dementia journey. The coding of implementation strategies was also challenging in many situations due to inadequate reporting of the activities employed for implementation. This lack of clarity adds to the challenging task of distinguishing between implementation of strategies and implementation of interventions due to overlaps in terminology and interpretation. Overall, there is a need for better reporting of implementation research to promote study identification, increase transparency and replicability, and improve the evaluation of studies.
Strengths and limitations
This is the first scoping review of dissemination and implementation research within dementia care. Previous research on implementation of evidence-based practice has investigated knowledge translation interventions and contextual factors in health care settings across various chronic conditions but little research has examined the implementation strategies used to promote best practice and the associated barriers and facilitators in dementia care. Our scoping review presents the extent and nature of current literature on efforts to translate research and change practice in dementia care and what is known about the factors that may enable or hinder this process. Our review builds on the evidence base from a number of systematic reviews that have addressed discrete areas of improving dementia care [ 55 – 59 ]. Whilst mostly concerned with effectiveness of interventions [ 55 , 56 , 59 ], these reviews have addressed some elements of dissemination and implementation. Elliot [ 56 ] and Reis [ 58 ] highlight the lack of detail reported on implementation in their reviews of training interventions. Reis [ 58 ] and Spector [ 59 ] emphasise the limited accessibility and lack of reporting on training manuals which impact the ability to reproduce interventions. Perry [ 55 ] and Eggenberger [ 57 ] concluded that education as a means to bring about change worked better when supported with another strategy – either financial or some form of feedback. Our scoping review shows that across dementia care settings and topics, there is a commonality of issues for dissemination and implementation that are yet to be resolved.
While we performed comprehensive searches across the most relevant databases and conducted forward citation searches of the included studies, we did not review their reference lists or hand-searched relevant journals due to the large number of studies. We sought to identify and have included unpublished research in our study. However, the difficulty of searching the grey literature may have thwarted our attempt to identify relevant unpublished material. Additionally, implementation research is a growing field with multiple terms to describe dissemination and implementation [ 60 ] so it is possible we may have missed some relevant articles. However, our search strategy and study selection process followed systematic review methods and we are confident that this scoping review provides a representative range of the implementation literature in dementia care. The scoping nature of the review precluded the detailed description of implementation characteristics across care settings, and the assessment of quality and effectiveness of strategies of included studies. As such, we are not able to provide recommendations for the implementation of specific strategies to promote practice change within dementia care settings. However, this review has informed the feasibility of a full systematic review and we plan to evaluate the effectiveness of implementation strategies on process outcomes across the various settings of dementia care provision.
Conclusions
This scoping review and systematic mapping of the evidence reveals a paucity of robust evidence to inform the successful dissemination and implementation of evidence-based dementia care. Noteworthy gaps in the evidence include research to inform effective methods of dissemination and implementation in hospital and primary care settings, and to support people with dementia and their carers living in the community. On the whole, the reporting of implementation strategies is poor with insufficient detail to enable replication. Further exploration of the most appropriate methods to evaluate and report initiatives to bring about change across settings and of the effectiveness of implementation strategies is necessary if we are to make changes in practice that improve dementia care.
Additional files
MEDLINE search strategy. (PDF 90 kb)
Characteristics of included studies categorised by care setting. (DOCX 36 kb)
Implementation strategies identified across studies ( n = 52) coded based on the ERIC compilation. (DOCX 19 kb)
Summary of implementation strategies and outcomes across studies categorised by care setting. (DOCX 24 kb)
List of studies included in the systematic scoping review ( n = 88). (PDF 174 kb)
Acknowledgements
The authors would like to thank the members of the project advisory group (Dr David Llewellyn, Dr. Arwen Wilcock, Dr. Steve Pearson, Ian Sherriff, Alex Smalley, Colin Capper, Julia Burton, Sara Davies, Susanne Lawrence), and Dr. Astrid Janssens and Samantha van Beurden for their assistance with the translation of a publication.
This review was funded by Alzheimer’s Society UK [AS-PG-14-016] and supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South West Peninsula (PenCLAHRC). The views expressed in this article are those of the authors and not necessarily those of Alzheimer’s Society UK, the National Health Service, the NIHR, or the Department of Health.
Availability of data and materials
Consent to publish.
Not applicable.
Abbreviations
Authors’ contributions.
JTC, RA and IAL conceived the idea for the review and JTC is the study guarantor. IL, RA, IAL, MR and JTC contributed to the design of the review and interpreted the data. BK advised on relevant frameworks to interpret and analyse the data. MR devised the search strategy, ran the literature searches and carried out forward citation searching. IL, RA, IAL, MR and JTC screened titles, abstracts and full texts and applied inclusion and exclusion criteria with RA, IAL and JTC acting as a third reviewer where necessary. IL and RA independently coded the implementation strategies. IL performed data extraction and RA checked data extraction for accuracy. IL, RA and IAL drafted the manuscript. All authors critically revised the manuscript for important intellectual content and approved the final versions.
Ethics approval and consent to participate
Competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ilianna Lourida, Phone: 0044(0)1392 725953, Email: [email protected] .
Rebecca A Abbott, Email: [email protected] .
Morwenna Rogers, Email: [email protected] .
Iain A Lang, Email: [email protected] .
Ken Stein, Email: [email protected] .
Bridie Kent, Email: [email protected] .
Jo Thompson Coon, Email: [email protected] .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 27 March 2024
The effects of genetic and modifiable risk factors on brain regions vulnerable to ageing and disease
- Jordi Manuello ORCID: orcid.org/0000-0002-9928-0924 1 , 2 ,
- Joosung Min ORCID: orcid.org/0000-0002-5541-5014 3 ,
- Paul McCarthy 1 ,
- Fidel Alfaro-Almagro 1 ,
- Soojin Lee 1 , 4 ,
- Stephen Smith 1 ,
- Lloyd T. Elliott 3 na1 ,
- Anderson M. Winkler 5 , 6 na1 &
- Gwenaëlle Douaud ORCID: orcid.org/0000-0003-1981-391X 1
Nature Communications volume 15 , Article number: 2576 ( 2024 ) Cite this article
11k Accesses
2113 Altmetric
Metrics details
- Genetics research
- Neuroscience
- Risk factors
We have previously identified a network of higher-order brain regions particularly vulnerable to the ageing process, schizophrenia and Alzheimer’s disease. However, it remains unknown what the genetic influences on this fragile brain network are, and whether it can be altered by the most common modifiable risk factors for dementia. Here, in ~40,000 UK Biobank participants, we first show significant genome-wide associations between this brain network and seven genetic clusters implicated in cardiovascular deaths, schizophrenia, Alzheimer’s and Parkinson’s disease, and with the two antigens of the XG blood group located in the pseudoautosomal region of the sex chromosomes. We further reveal that the most deleterious modifiable risk factors for this vulnerable brain network are diabetes, nitrogen dioxide – a proxy for traffic-related air pollution – and alcohol intake frequency. The extent of these associations was uncovered by examining these modifiable risk factors in a single model to assess the unique contribution of each on the vulnerable brain network, above and beyond the dominating effects of age and sex. These results provide a comprehensive picture of the role played by genetic and modifiable risk factors on these fragile parts of the brain.
Similar content being viewed by others

Single-cell multiplex chromatin and RNA interactions in ageing human brain
Xingzhao Wen, Zhifei Luo, … Sheng Zhong

A concerted neuron–astrocyte program declines in ageing and schizophrenia
Emi Ling, James Nemesh, … Steven A. McCarroll

Cell subtype-specific effects of genetic variation in the Alzheimer’s disease brain
Masashi Fujita, Zongmei Gao, … Philip L. De Jager
Introduction
The development of preventative strategies based on modifying risk factors might prove to be a successful approach in ensuring healthy ageing. Factors particularly scrutinised in dementia and unhealthy ageing have included cerebrovascular factors such as high blood pressure, diabetes and obesity, but also lifestyle ones such as alcohol consumption, and protective factors such as exercise 1 . Assessing these modifiable risk factors together makes it possible to identify the unique contribution of each of these factors on the brain or on cognitive decline. A Lancet commission, updated in 2020 to include, e.g., pollution for its possible role in the incidence of dementia 2 , examined the relative impact of 12 modifiable risk factors for dementia, and showed that these 12 factors may account for 40% of the cases worldwide 3 . Conversely, genetic factors are non-modifiable in nature, but can inform us about the mechanisms underlying the phenotypes of interest. These mechanisms sometimes can be shared across these phenotypes. For instance, genetic overlap has been found for Alzheimer’s and Parkinson’s diseases at a locus in the MAPT region 4 . Likewise, one of the most pleiotropic variants, in the SLC39A8 / ZIP8 gene, shows genome-wide associations with both schizophrenia and fluid intelligence, amongst many other phenotypes 5 , 6 .
One way to objectively and robustly assess susceptibility for unhealthy ageing is to look non-invasively at brain imaging markers 7 . Using a data-driven approach on a lifespan cohort, we previously identified an ensemble of higher-order, ‘transmodal’ brain regions that degenerates earlier and faster than the rest of the brain 8 . The very same areas also develop relatively late during adolescence, thus supporting the ‘last in, first out’ (LIFO) hypothesis, which posits that the process of age-related brain decline mirrors developmental maturation. Importantly, this network of brain regions further demonstrated heightened vulnerability to schizophrenia and Alzheimer’s disease, two disorders that impact on brain structure during adolescence and ageing respectively. Accordingly, this LIFO network was strongly associated with cognitive traits whose impairment is specifically related to these two disorders, namely fluid intelligence and long-term memory 8 .
Here, our main objective was to assess both the genetic and modifiable risk factors’ contributions to the vulnerability of these most fragile parts of the brain. We conducted a genome-wide association study on a prospective cohort of nearly 40,000 participants of the UK Biobank study who had received brain imaging, and in total evaluated the association between the LIFO brain network and 161 modifiable risk factors, classified according to 15 broad categories: blood pressure, cholesterol, diabetes, weight, alcohol consumption, smoking, depressive mood, inflammation, pollution, hearing, sleep, socialisation, diet, physical activity and education.
The vulnerable LIFO brain network in UK Biobank
Similar to our previously observed results 8 , the loadings of the LIFO brain network, i.e., the normalised grey matter volume in the network after regressing out the effects of all the other brain maps (see Methods), demonstrated a strong quadratic association with age in the UK Biobank cohort of 39,676 participants ( R 2 = 0.30, P < 2.23 × 10 −308 , Fig. 1 ). These higher-order regions thus show an accelerated decrease of grey matter volume compared with the rest of the brain. Furthermore, these areas define a network mainly involved in behavioural tasks related to execution, working memory, and attention (Fig. 1 , Supplementary Information ).
Top left, spatial map of the LIFO network (in red-yellow, thresholded at Z > 4 for visualisation) used to extract the loadings from every scanned participant from UK Biobank ( n = 39,676). Top right, these LIFO loadings (in arbitrary units) show a strong quadratic association with age in the UK Biobank cohort, i.e. grey matter volume decreases quadratically with older age in these specific regions ( R 2 = 0.30, P < 2.23 × 10 −308 ; inset: residual scatterplot). Bottom, the vulnerable network appears to encompass areas mainly involved in execution, working memory, and attention (using the BrainMap taxonomy 60 , and with the LIFO brain network thresholded at both Z = 4 and Z = 10, see Supplementary Information ).
Genetic influences over the vulnerable LIFO brain network
Using a minor allele frequency filter of 1% and a –log 10 (P) threshold of 7.5, we found, in the 39,676 participants, genome-wide associations between the LIFO brain network and seven genetic clusters whose top variants were all replicated (Table 1 /Supplementary Data 1 , Fig. 2 ).
Top row, Manhattan plot showing the 7 significant genetic clusters associated with the LIFO brain network (–log 10 ( P ) > 7.5). Second and third rows, regional association plots of the top variants for each of the 5 autosomal genetic clusters: rs6540873 on chromosome (Chr) 1 ( KCNK2 ), rs13107325 on Chr4 ( SLC39A8 ), rs2677109 on Chr6 ( RUNX2 ) (as a proxy in high LD R 2 = 0.86 with indel 6:45442860_TA_T), rs12146713 on Chr12 ( NUAK1 ), and rs2532395 on Chr17 ( MAPT , KANSL1 )(highest variant after tri-allelic rs2693333; see Supplementary Data 4 for a complete list of significant variants in this 5th MAPT genetic cluster). Bottom row, regional association plots of the top variants for the two genetic clusters in the pseudo-autosomal region PAR1 of the X chromosome: rs312238 ( XG , CD99 ) and rs2857316 ( XG )(UK Biobank has no genotyped variants on the 3’ side). Based on Human Genome build hg19. P -values are derived from a two-sided linear association test.
The first autosomal genetic cluster, on chromosome 1, included two variants (lead variant: rs6540873, β = 0.06, P = 1.71 × 10 −8 , and rs1452628, with posterior probabilities of inclusion in the causal variant set of 0.56 and 0.45, respectively) close to, and eQTL of, KCNK2 ( TREK1 ). This gene regulates immune-cell trafficking into the central nervous system, controls inflammation, and plays a major role in the neuroprotection against ischemia. Of relevance, these two loci are in particular related in UK Biobank participants with the amount of alcohol consumed, insulin levels, inflammation with interleukin-8 levels, as well as, crucially, with late-onset Alzheimer’s disease (Table 1 /Supplementary Data 1 ).
The second autosomal genetic cluster on chromosome 4 was made of 7 loci, with the lead variant rs13107325 in an exon of SLC39A8/ZIP8 ( β = 0.14, P = 2.82 × 10 −13 , posterior probability: 0.99). This locus is one of the most pleiotropic SNPs identified in GWAS, and is, amongst many other associations, related in UK Biobank with cholesterol, blood pressure, weight, inflammation with C-reactive proteins levels, diabetes with insuline-like growth factor 1 levels, alcohol intake, sleep duration, and cognitive performance/impairment, including prospective memory (Table 1 /Supplementary Data 1 ).
The third locus was an indel in chromosome 6 in an intron, and eQTL, of RUNX2 (rs35187443, β = 0.06, P = 9.03 × 10 −9 ), which plays a key role in differentiating osteoblasts, and has been very recently shown to limit neurogenesis and oligodendrogenesis in a cellular model of Alzheimer’s disease 9 .
The fourth locus was a SNP in chromosome 12, in an intron of NUAK1 (rs12146713, β = −0.10, P = 1.26 × 10 −9 ), and remarkably its top association in UK Biobank was with the contrast between schizophrenia and major depressive disorder 10 , and it was also associated with insulin-like growth factor 1 levels (Table 1 /Supplementary Data 1 ).
The final genetic autosomal genetic cluster was made of 3,906 variants in the MAPT region. Its lead non-triallelic variant, rs2532395 ( β = −0.09, P = 3.56 × 10 −15 ) was more specifically <10 kb from KANSL1 and an eQTL of KANSL1 , MAPT and other genes in brain tissues (Table 1 /Supplementary Data 1 , Supplementary Data 4 ). This locus was also associated in UK Biobank with tiredness and alcohol intake. MAPT is in 17q21.31, a chromosomal band involved with a common chromosome 17 inversion 11 . Adding chromosome 17 inversion status as a confounder reduced the significance of the association ( β = −0.15, P = 8.45 × 10 −3 ). Since the genotype for rs2532395 was also strongly correlated with chromosome 17 inversion in our dataset (Pearson correlation r = 0.98, P < 2 × 10 −16 ), this would suggest that the association between MAPT and the LIFO network is not independent from chromosome 17 inversion. As this extended genetic region is known for its pathological association with many neurodegenerative disorders including Alzheimer’s disease, we investigated whether the LIFO brain regions mediated the effect of the MAPT genetic cluster (using the lead bi-allelic variant rs2532395) on Alzheimer’s disease (see Methods). Despite small average causal mediated effect (ACME) sizes, we found a significant effect for both the dominant model (ACME β = 1.16 × 10 −4 ; 95% CI = [5.19 × 10 −5 , 1.99 × 10 −4 ]; P = 4 × 10 −5 ) and the recessive model (ACME β = 1.55 × 10 −4 ; 95% CI = [3.96 × 10 −5 , 3.74 × 10 −4 ]; P = 4 × 10 −5 ; full output of the mediation package on the dominant and recessive models in Supplementary Information ).
The two last genetic clusters of 8 and 9 variants respectively were found on the X chromosome, notably in a pseudo-autosomal region (PAR1), which is interestingly hit at a higher rate than the rest of the genome ( P = 1.56 × 10 −5 , see Supplementary Information ). The top variants for these clusters were related to two homologous genes coding for the two antigens of the XG blood group: rs312238 ( β = −0.05, P = 1.77 × 10 −10 ) ~ 10 kb from, and an eQTL of, CD99/MIC2 , and rs2857316 ( β = −0.08, P = 2.27 × 10 −29 ) in an intron and eQTL of XG (Table 1 /Supplementary Data 1 ). Since chromosome X has hardly been explored, we carried out our own association analyses between these two top variants and non-imaging variables in UK Biobank. Intriguingly, the first of these two PAR1 loci, rs312238, was found to be significantly associated in the genotyped participants who had not been scanned (out-of-sample analysis in n = 374,230 UK Biobank participants) with nitrogen dioxide air pollution, our ‘best’ MRF for pollution (see below), and many other environmental, socioeconomic, and early life factors (such as urban or rural setting, distance from the coast, place of birth, number of siblings, breastfed as a baby, maternal smoking around birth), as well as health outcomes (Supplementary Data 2 ). In particular, amongst the more easily interpretable findings of the most associated variables with rs312238, the T allele of this locus was associated with two increased measures of deprivation and/or disability (worse socioeconomic status), the ‘Townsend deprivation index’ and the ‘Health score’, but also with ‘Nitrogen dioxide air pollution’, ‘Maternal smoking around birth’, as well as ‘Number of full brothers’ and ‘Number of full sisters’, thus showing consistent signs of association between this variant and these phenotypes.
We found that the heritability of the LIFO network was significant, with h 2 = 0.15 (se = 0.01). The genetic co-heritability between the LIFO network and Alzheimer’s disease or schizophrenia was not statistically significant (coefficient of co-heritability = −0.12, se = 0.10; P = 0.23; coefficient of co-heritability = −0.16, se = 0.04, P = 0.07, respectively).
Modifiable risk factors’ associations with the vulnerable LIFO brain network
Including the modifiable risk factors (MRFs) in a single general linear model allows us to assess the unique contribution of each factor on the LIFO brain network. Not all UK Biobank participants have data available for all of the MRF variables however. An analysis limited to those with complete data for all MRFs would be biased, and based on a relatively small, low-powered sample. We addressed this issue via a two-stage analysis in which: (i) we first identified which variable within each of the 15 MRF categories best represented associations of that category with the LIFO brain network loadings (based on two criteria: significance and <5% missing values), (ii) we investigated the unique contribution of that MRF category, over and above all other categories and the dominating effects of age and sex, to the LIFO loadings.
From the first stage of our analysis, 12 of the 15 categories of MRFs had at least one ‘best’ MRF, i.e., with a significant effect on the LIFO brain network and enough non-missing values across all scanned participants to be investigated further (Table 2 /Supplementary Data 3 ). The contribution of the MRFs on the vulnerable brain network differed vastly depending on whether confounding effects of age, sex and head size were taken into account. The effect size and significance of some MRFs diminished because of some clear collinearity with the confounders. For instance, for the category of blood pressure, the most significant MRF was first “systolic blood pressure, automatic (second) reading” ( r = −0.20, P < 2.23 × 10 −308 ), but after regressing out the confounders, the ‘best’ MRF for this category was “medication for blood pressure” ( r = −0.05, P = 7.55 × 10 −22 ). Conversely, regressing out the effects of age served to unmask the significant deleterious effects of pollution on the vulnerable brain regions, such as nitrogen dioxide air pollution or particulate matter air pollution (Table 2 /Supplementary Data 3 ).
When considered together in a single model in the second stage of the analysis, 3 best MRFs had an effect on the LIFO brain network that remained significant beyond the dominating effects of age and sex, and of the 9 other best MRFs: diabetes (“diabetes diagnosed by doctor”, r = −0.05, P = 1.13 × 10 −24 ), pollution (“nitrogen dioxide air pollution in 2005”, r = −0.05, P = 5.39 × 10 −20 ) and alcohol (“alcohol intake frequency”, r = −0.04, P = 3.81 × 10 −17 ) (Table 3 ). No MRFs showed any bias in their sub-sampling distribution, i.e., any significant difference between the original sample and the reduced sample of 35,527 participants who had values for all 18 variables considered (the 12 best MRFs and 6 confounders: age, sex, age 2 , age × sex, age 2 × sex, head size; Supplementary Information ). In total, the 12 best MRFs explained 1.5% of the effect on the vulnerable brain network ( F 12;35509 = 43.5).
While 6 out of the 7 genetic clusters associated with the LIFO network were correlated with many variables related to each of the 15 MRF categories, including diabetes, alcohol consumption and traffic pollution (Supplementary Data 1 ), we also found some genetic overlap between the very specific best MRF of “alcohol intake frequency” and the LIFO network in the pleiotropic rs13107325 variant (cluster 2), as well as rs17690703, part of the large genetic cluster 5 in MAPT (Supplementary Data 4 ). No genetic overlap was found for the precise “nitrogen dioxide air pollution in 2005” or “diabetes diagnosed by doctor”, nor for approximate variables.
This study reveals, in a cohort of nearly 40,000 UK Biobank participants, the genetic and modifiable risk factors’ associations with brain regions in a ‘last in, first out’ (LIFO) network that show earlier and accelerated ageing and are particularly vulnerable to disease processes such as that of Alzheimer’s disease 8 . Seven genetic clusters, two of which in the pseudo-autosomal region of the sex chromosomes coding for two antigens of the XG blood system, were found significantly associated and replicated genome-wide. In addition, after accounting for age and sex effects, diabetes, traffic-related pollution and alcohol were the most deleterious modifiable risk factors (MRFs) on these particularly vulnerable brain regions.
Three lead variants for our significant genetic clusters have been previously associated with ageing-related brain imaging measures in recent studies: one, in cluster 1, an eQTL of KCNK2 ( TREK1 ) 12 , 13 , whose increase in expression mediates neuroprotection during ischemia 14 , the ubiquitous rs13107325 (cluster 2), and one, in cluster 4, in an intron of NUAK1 ( ARK5 ) 15 , 16 , 17 , which has been associated with tau pathology 18 (Table 1 /Supplementary Data 1 ). On the other hand, of the seven genetic clusters, three were entirely novel (clusters 3, 6 and 7), and not found in other brain imaging studies, including our most recent work that expanded on our previous GWAS of all of the brain IDPs available in UK Biobank 19 by including more participants—in fact, the same number of participants as analysed in this present work—and, crucially, by also including the X chromosome 20 (Table 1 /Supplementary Data 1 ). This suggests that, beyond the genetic hits that were meaningfully associated with the LIFO brain network and an array of relevant risk factors, lifestyle variables and brain disorders, and found in a few other imaging GWAS, some of the genetic underpinnings of the LIFO network are intrinsically specific to it and to no other pre-existing imaging phenotype.
All five autosomal genetic clusters identified through the GWAS of the LIFO phenotype had relevant associations with risk factors for dementia (Results; Supplementary Data 1 ), including precisely two of the best MRFs (for clusters 2 and 5), and three of them directly related in UK Biobank to the two diseases showing a pattern of brain abnormalities following the LIFO network: schizophrenia (clusters 2 and 4) and Alzheimer’s disease (cluster 1) (Supplementary Data 1 ). In particular, cluster 2 has its lead variant rs13107325 in an exon of one of the most pleiotropic genes ZIP8 , which codes for a zinc and metal transporter. Considering the vulnerability of the LIFO brain network to adolescent-onset schizophrenia and its significant association with fluid intelligence that we previously demonstrated 8 , it is notable that this variant has been associated genome-wide with schizophrenia 6 , as well as intelligence, educational attainment and mathematics ability 5 , 21 . In line with the LIFO brain network being both prone to accelerated ageing and susceptible to Alzheimer’s disease, this genetic locus has also been associated genome-wide with well-known risk factors for dementia. These comprise alcohol—including the exact same variable of “alcohol intake frequency” as identified as one of the best MRFs—cholesterol, weight, sleep—including “sleep duration”—and blood pressure 22 , 23 , 24 , 25 , 26 , all of which significantly contribute to modulating the LIFO brain network when considered separately (Table 2 /Supplementary Data 3 ). Of relevance, this genetic locus is also associated to an increased risk of cardiovascular death 27 . Cluster 5, a large genetic cluster in the MAPT region (Microtubule-Associated Protein Tau), comprised in total 3906 significant variants (Supplementary Data 4 ). This genetic region plays a role in various neurodegenerative disorders related to mutations of the protein tau, such as frontotemporal dementia 28 and progressive supranuclear palsy 29 , but also, of particular pertinence to the LIFO brain network, Alzheimer’s and Parkinson’s disease, with a genetic overlap between these two diseases in a locus included in our significant cluster 5 (rs393152, β = −0.09, P = 6.35 × 10 −14 ) 4 . Despite the relatively low number of people with diagnosed Alzheimer’s disease in the genetic discovery cohort, we were able to establish—albeit with small effect sizes—a significant mediation role for the LIFO brain regions between the lead bi-allelic variant for cluster 5 and this Alzheimer’s diagnosis, suggesting once more the importance played by these vulnerable brain areas in unhealthy ageing.
Finally, of the seven clusters, two were located in the pseudo-autosomal region (PAR1) of the sex chromosomes corresponding to the genes XG and CD99 , coding for the two antigens of the XG blood group. This blood group system has been largely neglected, its main contribution related to the mapping of the X chromosome itself, and its clinical role remains elusive 30 . In order to investigate further the possible role of these two variants of the XG blood group, we examined out-of-sample their associations with thousands of non-imaging phenotypes. This analysis revealed that the first of these two loci was significantly and consistently associated with early life factors, environmental factors and health outcomes, including particulate matter and nitrogen dioxide air pollution, the second most deleterious MRF to the LIFO brain network (Supplementary Data 2 ). Whether these associations are due to stratification or genotyping artefacts, or to the fact that this specific variant, which is inherited from a parent, has a parental impact that modulates the effect of early life environment of the UK Biobank participants, the so-called “nature of nurture”, will need further investigation 31 .
Intriguingly, an analysis revealed that the genes involved in the loci associated with the LIFO network (Table 1 /Supplementary Data 1 ) are enriched for the gene ontology terms of leucocyte extravasation, namely “positive regulation of neutrophil extravasation” ( P = 4.75 × 10 −6 ) and “T cell extravasation” ( P = 4.75 × 10 −6 ). This result held when removing the genes included in the MAPT extended region (with P = 2.54 × 10 −6 and P = 2.54 × 10 −6 , respectively). Leucocyte extravasation facilitates the immune and inflammatory response, and there has been renewed focus on the fact that a breakdown of the blood-brain barrier together with leukocyte extravasation might contribute to both Alzheimer’s disease and schizophrenia 32 , 33 . In line with the enrichment findings, 4 out of the 7 genetic clusters associated with the LIFO network are correlated in UK Biobank blood assays with percentage or count of immune cells (neutrophil, lymphocyte, platelet, monocyte, etc.; Supplementary Data 1 ).
Regarding MRFs’ effects on the LIFO brain network, diabetes and alcohol consumption have been consistently shown to be associated with both cerebral and cognitive decline 34 , 35 . On the other hand, pollution—and notably that of nitrogen oxides—has emerged more recently as a potential MRF for dementia 2 , 36 . In particular, the increase of dementia risk due to nitrogen oxide pollution, a proxy for traffic-related air pollution, seems to be enhanced by cardiovascular disease 37 . In this study, we found that nitrogen dioxide pollution has one of the most deleterious effects onto the fragile LIFO brain regions. This effect could only be unmasked by regressing out the effects of age and sex, as traffic-related air pollution is modestly inversely-correlated with age (Supplementary Data 5 ). It is also worth noting that including age and sex as confounding variables in the first stage of our analysis reduced considerably the contribution of what had appeared at first—before regression—as the most harmful risk factors: blood pressure, cholesterol and weight (Table 2 /Supplementary Data 3 ). Furthermore, the benefit of examining these MRFs in a single model in the second stage of our analysis is that we can assess the unique contribution of each of these factors on the LIFO brain network; in doing so, blood pressure, cholesterol and weight were no longer significant (Table 3 ).
One defining characteristic of the LIFO brain network is how much age explains its variance. Indeed, in the dataset covering most of the lifespan that was initially used to identify the LIFO and spatially define it 8 , age explained 50%. In the UK Biobank imaging project, where imaged participants are over 45 years old, age explained 30% (Fig. 1 ). It is thus perhaps unsurprising that, while the explained variance by each of the MRFs varies widely (Table 2 /Supplementary Data 3 ), it reduces notably once the effect of age and other confounders has been regressed out (without confounders included in the model: maximum 8.4%; with confounders: maximum 0.5%). Combined, the 12 best MRFs explained a significant 1.5% of the effect on the vulnerable brain network after regressing out age, head size and sex effects. Regarding the genetic hits, we found a significant heritability with h 2 = 0.15, in keeping with our results for structural brain phenotypes (except for subcortical and global brain volumes, which demonstrate higher heritability 19 ).
The uniqueness of this study relies on the fact that we combined the strengths of two different cohorts: the first, which revealed the LIFO grey matter network, is lifespan, demonstrating the mirroring of developmental and ageing processes in the LIFO brain areas, something that could never be achieved with UK Biobank because of its limited age range. Of note, for this initial work with the lifespan cohort 8 , we not only included grey matter partial volume images, as done in this current study, but also Freesurfer information of cortical thickness and surface area. The LIFO network showed no contribution from Freesurfer cortical thickness or area. This might hint at processes that only partial volume maps are able to detect due to the LIFO network’s specific localisation, including in the cerebellum and subcortical structures, which are not included in the area and thickness surface methods from Freesurfer.
Limitations of our study pertain to the nature of the data itself and the way each variable is encoded in the UK Biobank (binary, ordinal, categorical, continuous), the number of missing values, what is offered as variables for each modifiable risk factor category (e.g. we chose not to create any compound variables, such as the ratio of cholesterol levels or systolic and diastolic blood pressures), and the curation of each of these variables. Some of the factors might be proxies for another category, but including the ‘best’ ones in a single model alleviate these issues to some extent. Another limitation is the assumption in our models that each risk factor has a linear, additive effect on the vulnerable LIFO brain network. It is also important to note that cross-sectional and longitudinal patterns of brain ageing can differ, as has been shown for instance for adult span trajectories of episodic and semantic memory, especially in younger adults 38 . A recent study has also demonstrated a specific ‘brain age’ imaging measure to be more related to early life influences on brain structure than within-person rates of change in the ageing brain 39 . Further work will be needed to establish how the LIFO network data changes in terms of within-person trends, for instance by investigating the growing UK Biobank longitudinal imaging database. While we took care of assessing the replicability of our genetic results by randomly assigning a third of our dataset for such purposes (all our significant genetic hits were replicated), this was performed within the UK Biobank cohort that exhibits well-documented biases, being well-educated, less deprived, and healthier than the general population, especially for its imaging arm 40 . Independent replications will be needed to confirm the existence of the LIFO-associated genetic loci.
In conclusion, our study reveals the modifiable and non-modifiable factors associated with some of the most fragile parts of the brain particularly vulnerable to ageing and disease process. It shows that, above and beyond the effect of age and sex, the most deleterious modifiable risk factors to this brain network of higher-order regions are diabetes, pollution and alcohol intake. Genetic factors are related to immune and inflammatory response, tau pathology, metal transport and vascular dysfunction, as well as to the XG blood group system from the pseudo-autosomal region of the sex chromosomes, and meaningfully associated with relevant modifiable risk factors for dementia. The unprecedented genome-wide discovery of the two variants on the sex chromosomes in this relatively unexplored blood group opens the way for further investigation into its possible role in underlying unhealthy ageing.
Supplementary Information is available for this paper.
For the present work the imaging cohort of UK Biobank was used and we included 39,676 subjects who had been scanned and for whom the brain scans had been preprocessed at the time of the final set of analyses (M/F 47–53%; 44–82 years, mean age 64 ± 7 years; as of October 2020) 41 , 42 . Structural T1-weighted scans for each participant were processed using the FSL-VBM automated tool to extract their grey matter map 43 , 44 . The ‘last in, first out’ (LIFO) network of mainly higher-order brain regions was initially identified by performing a linked independent component analysis on the grey matter images of another, lifespan observational cohort of 484 subjects 8 , 45 , 46 . This map of interest, along with the other 69 generated by the analysis, was first realigned to the UK Biobank ‘standard’ space defined by the grey matter average across the first 15,000 participants, then regressed into the UK Biobank participants’ grey matter data, to extract weighted average values of grey matter normalised volume inside each of the z-maps, using the z-score as weighting factor. This made it possible to assess the unique contribution of this specific LIFO map, above and beyond all the rest of the brain represented in the other 69 maps. At the end of this process, we obtained a single imaging measure for each of the 39,676 participants, i.e. a ‘loading’ corresponding to their amount of grey matter normalised volume in the LIFO brain network.
Human participants: UK Biobank has approval from the North West Multi-Centre Research Ethics Committee (MREC) to obtain and disseminate data and samples from the participants ( http://www.ukbiobank.ac.uk/ethics/ ), and these ethical regulations cover the work in this study. Written informed consent was obtained from all of the participants.
Modifiable risk factors selection
The following 15 categories of modifiable risk factors (MRFs) for dementia were investigated based on previous literature: blood pressure, diabetes, cholesterol, weight, alcohol, smoking, depression, hearing, inflammation, pollution, sleep, exercise, diet/supplementation, socialisation, and education. These included well-documented cerebrovascular risk factors, and in particular included all of the 12 modifiable risk factors considered in the updated Lancet commission on dementia, with the sole exception of traumatic brain injury 3 . For each category, several MRF variables from UK Biobank were very minimally pre-processed ( Supplementary Information ). In total, 161 MRF variables were obtained. To optimise the interpretability of the results, and to be able to relate them to previous findings, we did not carry out any data reduction, which would have prevented us from identifying exactly which variable—and subsequently, which genetic component for this specific variable—contribute to the effect. For these same reasons, we did not create any compound variable.
Statistical analyses
Genome-wide association study.
We followed the same protocol we had developed for the first genome-wide association study (GWAS) with imaging carried out on UK Biobank 19 . Briefly, we examined imputed UK Biobank genotype data 47 , and restricted the analysis to samples that were unrelated (thereby setting aside only ~450 participants), without aneuploidy and with recent UK ancestry. To account for population stratification, 40 genetic principal components were used in the genetic association tests as is recommended for UK Biobank genetic studies 19 , 20 , 47 . We excluded genetic variants with minor allele frequency <0.01 or INFO score <0.03 or Hardy-Weinberg equilibrium –log 10 ( P ) > 7. We then randomly split the samples into a discovery set with 2/3 of the samples ( n = 22,128) and a replication set with 1/3 of the samples ( n = 11,083). We also examined the X chromosome with the same filters, additionally excluding participants with sex chromosome aneuploidy: 12 in non-pseudoautosomal region (PAR) and 9 in PAR for the discovery set, 3 in non-PAR and 6 in PAR for the replication set. Variants were considered significant at –log 10 ( P ) > 7.5, and replicated at P < 0.05.
Modifiable risk factor study
In the first stage, the general linear model was used to investigate, separately, the association between each of these 161 MRFs and the LIFO network loadings in all the scanned UK Biobank participants ( n = 39,676). We ran each model twice: once as is, and once adding 6 confounders: age, age 2 , sex, age × sex, age 2 × sex, and head size, to estimate the contribution of these MRFs on the LIFO network above and beyond the dominating effects of age and sex. Sex was based on the population characteristics entry of UK Biobank. This is a mixture of the sex the NHS had recorded for the participant at recruitment, and updated self-reported sex. For the GWAS, both sex and genetic sex were used (the sample was excluded in case of a mismatch). In total, 32 variables tailored to structural imaging had been considered as possible confounders, and we retained those with the strongest association ( R 2 ≥ 0.01; see Supplementary Information ). Socioeconomic status via the Townsend deprivation index was also considered as a possible confounding variable but explained little variance ( R 2 < 0.001) and thus was not included as a confounder.
MRFs were not considered further if they were not significant—not surviving Bonferroni-correction, i.e., P > 1.55 × 10 −4 —and if more than 5% of the subjects had their MRF values missing. For each category, a single ‘best’ MRF was then selected as the variable with the highest R 2 among those remaining, after regressing out the confounding effects of age and sex.
In the second stage, all these best MRFs were then included in a single general linear model, together with the same 6 confounders used in the first stage, to assess the unique contribution of each factor on the LIFO brain network loadings. A prerequisite to carry out this single general linear model analysis was to only include participants who would have values for all best MRFs and confounders. This explains the additional criterion of only including MRFs that had no more than 5% of values missing, to ensure that the final sample of participants who had values for all these best and confounding factors would not be biased compared with the original sample—something we formally tested (see Supplementary Information )—especially as data are not missing at random in UK Biobank, and exhibit some genetic structure 48 . The sample was therefore reduced to a total of 35,527 participants for this second stage analysis (M/F 17,290–18,237; 45–82 years, mean 64 ± 7 years). The effect of these best MRFs taken altogether was considered significant with a very conservative Bonferroni correction for multiple comparisons across all combinations of every possible MRF from each of the initial 15 MRF categories ( P < 4.62 × 10 −17 , see Supplementary Information for more details). In addition, both full and partial correlations were computed for the same set of best MRFs and confounders, in order to assess possible relationships between variables.
Post hoc genetic analyses
Chromosome 17 inversion.
We investigated chromosome 17 inversion status of the participants in the discovery cohort by considering their genotype on 32 variants that tag chromosome 17 inversion according to Steinberg et al. 11 . Of these 32 variants, 24 were present in our genetic data. We labelled the participants homozygous inverted, heterozygous, or homozygous direct (not inverted) when all 24 of these alleles indicated the same zygosity. This yielded an unambiguous inversion status for 21,969 participants (99% of the discovery cohort). To examine if the association between the non-triallelic lead variant of the MAPT genetic cluster (rs2532395, Table 1 /Supplementary Data 1 ) and the LIFO network was independent from this common inversion, we determined inversion/direct status of the discovery cohort and: 1. repeated the association test between rs2532395 and the LIFO phenotype, with chromosome 17 inversion status added as a confounder; and 2. correlated the genotype for rs2532395 with chromosome 17 inversion.
Causality within each genetic cluster
We used CAVIAR (Causal Variants Identification in Associated Regions 49 ) to assess causality of variants that passed the genome-wide significance threshold in each of the genetic clusters we report. CAVIAR uses a Bayesian model and the local linkage disequilibrium structure to assign posterior probabilities of causality to each variant in a region, given summary statistics for an association. We did not perform CAVIAR analysis on the genetic cluster on chromosome 17, as its non-triallelic lead variant (rs2532395) was strongly correlated with chromosome 17 inversion, and the LD matrix was large and low rank. We excluded the X chromosome loci from this analysis due to the difficulty in assessing LD in this chromosome.
Enrichment analysis
Based on the genes listed in the ‘Genes’ column of Table 1 /Supplementary Data 1 , we performed an enrichment analysis for the genes associated with the LIFO brain network using PANTHER 50 . PANTHER determines whether a gene function is overrepresented in a set of genes, according to the gene ontology consortium 51 , 52 .
Mediation analysis between MAPT top variant and Alzheimer’s disease, via the LIFO brain network
As the gene MAPT is associated with Alzheimer’s disease, and as we found a significant association between MAPT and the LIFO brain network, we examined to what extent the effect of MAPT is mediated by the LIFO brain regions. We conducted a mediation analysis using the counterfactual framework in which the average indirect effect of the treatment on the outcome through the mediator is nonparametrically identified (version 4.5.0 of the R package ‘mediation' 53 ). This is a general approach that encompasses the classical linear structural equation modelling framework for causal mediation, allowing both linear and non-linear relationships. In this analysis, the genotype for the lead bi-allelic variant of the MAPT association was used as the treatment, the LIFO loadings as the mediator, and Alzheimer’s disease diagnosis as the outcome.
From the ~43 K UK Biobank participants who had been scanned, we searched for those who had been diagnosed with Alzheimer’s disease specifically, regardless of whether this diagnosis occurred before, or after their brain scans. Based on hospital inpatient records (ICD10: F000, F001, F002, F009, G300, G301, G308, and G309 and ICD9: 3310) and primary care (GP) data (Eu00., Eu000, Eu001, Eu002, Eu00z, F110., F1100, F1101, Fyu30, X002x, X002y, X002z, X0030, X0031, X0032, X0033, XaIKB, XaIKC, and XE17j), we identified 65 such cases— UK Biobank being healthier than the general population, and those scanned showing an even stronger healthy bias—of which 34 were included in the discovery set after QC.
We considered two conditions for the effect of the treatment on the outcome. First, a dominant condition in which the minor allele is assumed to be dominant and for which at least one copy of the minor allele is considered treated. Second, a recessive condition in which the minor allele is assumed to be recessive. We considered that either condition was nominally significant if the confidence interval of the average causal mediated effect did not intersect zero, and had an associated P < 0.05 ÷ 2 (correcting for the two conditions). We assessed confidence intervals and P -values using 50,000 bootstrapped samples.
Associations between the LIFO brain network’s genetic hits and the MRFs
First, we reported in Table 1 / Supplementary Data 1 the significant associations between the LIFO genetic hits and UK Biobank variables related to the 15 categories listed for the MRFs. For this, we used the Open Targets Genetics website, which reports the GWAS carried out in UK Biobank ( https://genetics .opentargets.org/ ). Second, we assessed whether there was any genetic overlap between the known genetic components of the 3 best MRFs and the LIFO phenotype. Again, we used the Open Targets Genetics website outputs for these 3 very specific UK Biobank variables, and compared the significant hits for these 3 best MRFs within ±250 kbp of, or in high LD (>0.8) with, our own LIFO variants. If reported hits were limited, we also searched online for GWAS done on similar variables. Finally, we also included the list of significant hits for diabetes 54 , which focused on a potential genetic overlap between diabetes and Alzheimer’s disease.
Post hoc association for the sex chromosomes variants
The allele counts of each participant for two specific significant variants of the sex chromosomes not—or hardly—available in open databases such as https://genetics.opentargets.org/ 55 were further associated out-of-sample with all non-imaging phenotypes of UK Biobank ( n = 16,924). This analysis was carried out in the entire genotyped, quality-controlled sample where participants who had been scanned were removed (final sample: 374,230 participants), taking into account the population structure (40 genetic principal components), as well as the confounding effects of age, sex, age x sex, age 2 and age 2 x sex. Results were corrected for multiple comparisons across all non-imaging phenotypes and the two variants.
Heritability
We examined the heritability of the LIFO phenotype, and the coheritability between the LIFO network and Alzheimer’s disease or schizophrenia using LDSC 56 . This method uses regression on summary statistics to determine narrow sense heritability h 2 of a trait, or the shared genetic architecture between two traits. LDSC corrects for bias LD structure using LD calculated from a reference panel (we used LD from the Thousand Genomes Project Phase 1 57 ). We obtained summary statistics for a meta-analysis of Alzheimer’s disease involving 71,880 cases and 383,378 controls 58 . The number of genetic variants in the intersection between the summary statistics was 1,122,435. For schizophrenia, the summary statistics were obtained from a meta-analysis involving 53,386 cases and 77,258 controls 59 . A total of 1,171,319 genetic variants were in the intersection with the summary statistics for LIFO. For both Alzheimer’s and schizophrenia, the X chromosome was not included in the heritability calculation, as it was excluded from the meta-analysis that we sourced the summary statistics from.
Reproducibility
No data was excluded for the MRF analyses. For the genetic analyses, these were restricted to samples that were unrelated, without aneuploidy and with recent UK ancestry (see above).
No statistical method was used to predetermine sample size. The experiments were not randomised. The Investigators were not blinded to allocation during experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All the FLICA decomposition maps − including the LIFO grey matter network − in UK Biobank standard space, the UK Biobank grey matter template, scripts, and the LIFO loadings for all of the participants are freely available on a dedicated webpage: open.win.ox.ac.uk/pages/douaud/ukb-lifo-flica/ .
Baumgart, M. et al. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 11 , 718–726 (2015).
Article PubMed Google Scholar
Chen, H. et al. Exposure to ambient air pollution and the incidence of dementia: a population-based cohort study. Environ. Int. 108 , 271–277 (2017).
Article CAS PubMed Google Scholar
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396 , 413–446 (2020).
Article PubMed PubMed Central Google Scholar
Desikan, R. S. et al. Genetic overlap between Alzheimer’s disease and Parkinson’s disease at the MAPT locus. Mol. Psychiatry 20 , 1588–1595 (2015).
Article CAS PubMed PubMed Central Google Scholar
Hill, W. D. et al. A combined analysis of genetically correlated traits identifies 187 loci and a role for neurogenesis and myelination in intelligence. Mol. Psychiatry 24 , 169–181 (2019).
Pardinas, A. F. et al. Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat. Genet. 50 , 381–389 (2018).
Douaud, G. et al. Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc. Natl Acad. Sci. USA 110 , 9523–9528 (2013).
Article ADS CAS PubMed PubMed Central Google Scholar
Douaud, G. et al. A common brain network links development, aging, and vulnerability to disease. Proc. Natl Acad. Sci. USA 111 , 17648–17653 (2014).
Nakatsu, D. et al. BMP4-SMAD1/5/9-RUNX2 pathway activation inhibits neurogenesis and oligodendrogenesis in Alzheimer’s patients’ iPSCs in senescence-related conditions. Stem Cell Rep. 18 , 1246 (2023).
Article CAS Google Scholar
Peyrot, W. J. & Price, A. L. Identifying loci with different allele frequencies among cases of eight psychiatric disorders using CC-GWAS. Nat. Genet. 53 , 445–454 (2021).
Steinberg, K. M. et al. Structural diversity and African origin of the 17q21.31 inversion polymorphism. Nat. Genet. 44 , 872–880 (2012).
Le Guen, Y. et al. eQTL of KCNK2 regionally influences the brain sulcal widening: evidence from 15,597 UK Biobank participants with neuroimaging data. Brain Struct. Funct. 224 , 847–857 (2019).
Jonsson, B. A. et al. Brain age prediction using deep learning uncovers associated sequence variants. Nat. Commun. 10 , 5409 (2019).
Heurteaux, C. et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 23 , 2684–2695 (2004).
Vojinovic, D. et al. Genome-wide association study of 23,500 individuals identifies 7 loci associated with brain ventricular volume. Nat. Commun. 9 , 3945 (2018).
Article ADS PubMed PubMed Central Google Scholar
Zhao, B. et al. Genome-wide association analysis of 19,629 individuals identifies variants influencing regional brain volumes and refines their genetic co-architecture with cognitive and mental health traits. Nat. Genet. 51 , 1637–1644 (2019).
Zhao, B. et al. Large-scale GWAS reveals genetic architecture of brain white matter microstructure and genetic overlap with cognitive and mental health traits ( n = 17,706). Mol. Psychiatry https://doi.org/10.1038/s41380-019-0569-z (2019).
Lasagna-Reeves, C. A. et al. Reduction of Nuak1 decreases tau and reverses phenotypes in a tauopathy mouse model. Neuron 92 , 407–418 (2016).
Elliott, L. T. et al. Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature 562 , 210–216 (2018).
Smith, S. M. et al. An expanded set of genome-wide association studies of brain imaging phenotypes in UK Biobank. Nat. Neurosci. 24 , 737–745 (2021).
Lee, J. J. et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat. Genet. 50 , 1112–1121 (2018).
Teslovich, T. M. et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466 , 707–713 (2010).
Sanchez-Roige, S. et al. Genome-Wide Association Study Meta-Analysis of the Alcohol Use Disorders Identification Test (AUDIT) in Two Population-Based Cohorts. Am. J. Psychiatry 176 , 107–118 (2019).
Locke, A. E. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 518 , 197–206 (2015).
Dashti, H. S. et al. Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat. Commun. 10 , 1100 (2019).
International Consortium for Blood Pressure Genome-Wide Association, S. et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478 , 103–109 (2011).
Article ADS Google Scholar
Johansson, A. et al. Genome-wide association and Mendelian randomization study of NT-proBNP in patients with acute coronary syndrome. Hum. Mol. Genet. 25 , 1447–1456 (2016).
Poorkaj, P. et al. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann. Neurol. 43 , 815–825 (1998).
Baker, M. et al. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum. Mol. Genet. 8 , 711–715 (1999).
Johnson, N. C. XG: the forgotten blood group system. Immunohematology 27 , 68–71 (2011).
Kong, A. et al. The nature of nurture: Effects of parental genotypes. Science 359 , 424–428 (2018).
Zenaro, E., Piacentino, G. & Constantin, G. The blood-brain barrier in Alzheimer’s disease. Neurobiol. Dis. 107 , 41–56 (2017).
Pong, S., Karmacharya, R., Sofman, M., Bishop, J. R. & Lizano, P. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry 6 , 30–46 (2020).
Chatterjee, S. et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 39 , 300–307 (2016).
Veldsman, M. et al. Cerebrovascular risk factors impact frontoparietal network integrity and executive function in healthy ageing. Nat. Commun. 11 , 4340 (2020).
Power, M. C., Adar, S. D., Yanosky, J. D. & Weuve, J. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: a systematic review of epidemiologic research. Neurotoxicology 56 , 235–253 (2016).
Grande, G., Ljungman, P. L. S., Eneroth, K., Bellander, T. & Rizzuto, D. Association between cardiovascular disease and long-term exposure to air pollution with the risk of dementia. JAMA Neurol. 77 , 801–809, (2020).
Ronnlund, M., Nyberg, L., Backman, L. & Nilsson, L. G. Stability, growth, and decline in adult life span development of declarative memory: cross-sectional and longitudinal data from a population-based study. Psychol. Aging 20 , 3–18 (2005).
Vidal-Pineiro, D. et al. Individual variations in ‘brain age’ relate to early-life factors more than to longitudinal brain change. Elife https://doi.org/10.7554/eLife.69995 (2021).
Fry, A. et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am. J. Epidemiol. 186 , 1026–1034 (2017).
Alfaro-Almagro, F. et al. Image processing and Quality Control for the first 10,000 brain imaging datasets from UK Biobank. NeuroImage 166 , 400–424 (2018).
Miller, K. L. et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat. Neurosci. 19 , 1523–1536 (2016).
Good, C. D. et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage 14 , 21–36 (2001).
Douaud, G. et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 130 , 2375–2386 (2007).
Groves, A. R. et al. Benefits of multi-modal fusion analysis on a large-scale dataset: life-span patterns of inter-subject variability in cortical morphometry and white matter microstructure. NeuroImage 63 , 365–380 (2012).
Smith, S. et al. Structural variability in the human brain reflects fine-grained functional architecture at the population level. J. Neurosci. 39 , 6136–6149 (2019).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562 , 203–209 (2018).
Mignogna, G. et al. Patterns of item nonresponse behaviour to survey questionnaires are systematic and associated with genetic loci. Nat. Hum. Behav . https://doi.org/10.1038/s41562-023-01632-7 (2023).
Hormozdiari, F., Kostem, E., Kang, E. Y., Pasaniuc, B. & Eskin, E. Identifying causal variants at loci with multiple signals of association. Genetics 198 , 497–508 (2014).
Thomas, P. D. et al. PANTHER: Making genome-scale phylogenetics accessible to all. Protein Sci. 31 , 8–22 (2022).
Ashburner, M. et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25 , 25–29 (2000).
Gene Ontology, C. et al. The Gene Ontology knowledgebase in 2023. Genetics https://doi.org/10.1093/genetics/iyad031 (2023).
Imai, K., Keele, L. & Tingley, D. A general approach to causal mediation analysis. Psychol. Methods 15 , 309–334 (2010).
Hardy, J., de Strooper, B. & Escott-Price, V. Diabetes and Alzheimer’s disease: shared genetic susceptibility? Lancet Neurol. 21 , 962–964 (2022).
Carvalho-Silva, D. et al. Open Targets Platform: new developments and updates two years on. Nucleic Acids Res. 47 , D1056–D1065 (2019).
Gazal, S., Marquez-Luna, C., Finucane, H. K. & Price, A. L. Reconciling S-LDSC and LDAK functional enrichment estimates. Nat. Genet. 51 , 1202–1204 (2019).
Genomes Project, C. et al. A global reference for human genetic variation. Nature 526 , 68–74 (2015).
Jansen, I. E. et al. Author Correction: Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat. Genet. 52 , 354 (2020).
Trubetskoy, V. et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 604 , 502–508 (2022).
Laird, A. R. et al. ALE meta-analysis workflows via the brainmap database: progress towards a probabilistic functional brain atlas. Front. Neuroinform. 3 , 23 (2009).
Download references
Acknowledgements
We are grateful to Profs Christian K. Tamnes, Lars T. Westlye, Kristine B. Walhovd and Anders M. Fjell, and Dr Andreas Engvig for providing the lifespan cohort which was used to initially derive the original ‘last in, first out’ brain network map, and to Prof Augustine Kong for helpful discussion on the associations between the PAR hit and early life and environmental factors. G.D. was supported by a UK MRC Career Development Fellowship (MR/K006673/1) and a Wellcome Collaborative Award (215573/Z/19/Z). S.S. was supported by Wellcome (203139/Z/16/Z; 215573/Z/19/Z). L.E. was funded by NSERC grants (RGPIN/05484-2019; DGECR/00118-2019) and a Michael Smith Health Research BC Scholar Award. A.M.W. received support through the NIH Intramural Research Program (ZIA-MH002781; ZIA-MH002782). This research was funded in whole, or in part, by the Wellcome Trust (215573/Z/19/Z; 203139/Z/16/Z; 203139/A/16/Z). For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. This research was also supported by the NIHR Oxford Health Biomedical Research Centre (NIHR203316). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (203139/Z/16/Z and 203139/A/16/Z).
Author information
These authors contributed equally: Lloyd T. Elliott, Anderson M. Winkler.
Authors and Affiliations
FMRIB Centre, Wellcome Centre for Integrative Neuroimaging (WIN), Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, UK
Jordi Manuello, Paul McCarthy, Fidel Alfaro-Almagro, Soojin Lee, Stephen Smith & Gwenaëlle Douaud
FOCUS Lab, Department of Psychology, University of Turin, Turin, Italy
Jordi Manuello
Department of Statistics and Actuarial Science, Simon Fraser University, Burnaby, Canada
Joosung Min & Lloyd T. Elliott
Pacific Parkinson’s Research Centre, The University of British Columbia, Vancouver, BC, Canada
National Institutes of Mental Health, National Institutes of Health, Bethesda, MD, USA
Anderson M. Winkler
Department of Human Genetics, University of Texas Rio Grande Valley, Brownsville, TX, USA
You can also search for this author in PubMed Google Scholar
Contributions
G.D. conceived and supervised the work, and carried out some of the genetic and modifiable risk factors analyses. J.Ma. carried out most of the genetic and modifiable risk factors analyses. J.Mi., S.L., A.M.W., and L.T.E. carried out additional genetics analyses. G.D., P. McC., F.A.-A., S.S., and L.T.E. created/extracted the imaging and genetics data, and organised the non-imaging data and confound variables. L.T.E. co-supervised the genetic analyses. A.M.W. co-supervised the modifiable risk factor analyses. G.D. interpreted the results and wrote the paper. J.Ma., S.S., L.T.E., and A.M.W. revised the paper.
Corresponding author
Correspondence to Gwenaëlle Douaud .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks Xavier Caseras and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, description of additional supplementary files, supplementary data 1-5, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Manuello, J., Min, J., McCarthy, P. et al. The effects of genetic and modifiable risk factors on brain regions vulnerable to ageing and disease. Nat Commun 15 , 2576 (2024). https://doi.org/10.1038/s41467-024-46344-2
Download citation
Received : 15 February 2023
Accepted : 22 February 2024
Published : 27 March 2024
DOI : https://doi.org/10.1038/s41467-024-46344-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
You are using an outdated browser. Please upgrade your browser to improve your experience.
- Skip to main content
- Resize text
- This site uses cookies
Social Care Online from SCIE
The UK’s largest database of information and research on all aspects of social care and social work
Dementia care mapping: a review of the research literature
The published literature on dementia care mapping (DCM) in improving quality of life and quality of care through practice development and research dates back to 1993. The purpose of this review of the research literature is to answer some key questions about the nature of the tool and its efficacy, to inform the ongoing revision of the tool, and to set an agenda for future research. The DCM bibliographic database at the University of Bradford in the United Kingdom contains all publications known on DCM This formed the basis of the review. Texts that specifically examined the efficacy of DCM or in which DCM was used as a main measure in the evaluation or research were reviewed. Thirty-four papers were categorized into five main types: (a) cross-sectional surveys, (b) evaluations of interventions, (c) practice development evaluations, (d) multimethod evaluations, and (e) papers investigating the psychometric properties of DCM. These publications provide some evidence regarding the efficacy of DCM, issues of validity and reliability, and its use in practice and research. The need for further development and research in a number of key areas is highlighted.
BROOKER Dawn;
Dementia care mapping: a review of the research literature.
Journal citation/publication details
Gerontologist, 45 (Supplement), October 2005, pp.11-18.
This review of 34 studies on dementia care mapping (DCM) as a research and evaluation/practice development tool comes to generally positive conclusions, although identifying many areas in which improvements need to be made. The review was conducted specifically to inform the future development of DCM8, which was launched in late 2005.
DCM is an observational tool used in formal dementia care settings for more than a decade for developing person-centred care practice, and as a tool in quality of life research. Essentially, a mapper observes a group of people with dementia continuously for a representative daytime period, coding at five minute intervals for behaviour (24 domains), well- (or ill)-being value (6-point scale), ‘personal detractions’ (staff behaviours likely to adversely affect personhood), and ‘positive events (that enhance personhood). A review of the literature on DCM was published in 2002, covering studies published up to 2001, and this review aims to update what is known and to inform the future development of the tool.
What sources were used? The University of Bradford maintains a bibliographic database (10pp Word document) of all known publications on DCM (see http://www.bradford.ac.uk/health/dementia/dcm/publications/index.php) which is updated annually by the Bradford Dementia Group through searches of Medline, CINAHL (Cumulative Index to Nursing and Allied Health Literature) and PsycINFO using the terms DCM and dementia care mapping. Other sources, including personal correspondence with practitioners and researchers, are also used as sources for material to be included in the database. The author of this review is responsible for the database.
What search terms/strategies were used? The database is not searchable electronically. Relevant material was extracted manually and covered the period 1993 to March 2005.
What criteria were used to decide on which studies to include? Eligible studies were all those that specifically examined the efficacy of DCM, or in which DCM was used as a main measure. There were no exclusions on the grounds of methodological design, but purely descriptive papers and dissertations were excluded.
Who decided on their relevance and quality? All aspects of the review were carried out by the author.
How many studies were included and where were they from? Thirty-four papers met the inclusion criteria. Geographical origins are not specified in detail, but they do include several UK studies.
How were the study findings combined? Data were extracted on: settings and size; aims of the study; length of time mapped; sample selection and characteristics; study design; version of DCM used; inter-rater reliability; DCM outcomes; statistical tests; and level of significance. The tables are accessible at http://www.bradford.ac.uk/health/dementia/dcm/DCMLitReviewTables.pdf. Each study was then assigned to one of five categories according to its basic purpose in using DCM.
Findings of the review
The studies used DCM for various purposes: in cross-sectional surveys in a variety of settings (11); to evaluate the impact of interventions on the lives of people with dementia (10); in repeated evaluations to change care practice (6); and in multi-method qualitative evaluations (3). The remaining four studies looked directly at some of the psychometric properties of DCM. The author reports that, despite the variety of studies, ‘there is consistency in what they report in terms of DCM data’, and they are thus able to provide answers, ‘at least in part’, to common questions about DCM.
Does DCM measure quality of care and/or quality of life? In terms of concurrent validity with other measures, there is some evidence that DCM is related to indicators of quality of care (for example, one study reports a relationship between lower DCM scores and an increase in pressure sores); two proxy quality-of-life measures (Quality of Life AD-Staff, and Alzheimer’s Disease-Related Quality of Life); and the Affect Rating Scale. Although DCM is measures something similar to proxy measures and other observation measures of quality of care and quality of life, it differs from them in that ‘it attempts to measure elements of both’.
Can different mappers use DCM reliably? Although many of the studies demonstrate that ‘it is perfectly possible to achieve acceptable interrater reliability’, there is a danger of ‘drifts in coding’. This has significant training implications. One way forward is to provide regular checks for all mappers with a ‘gold standard mapper’, while changes need to be made to reduce complexity and ambiguity in codes for the forthcoming DCM8 version.
Does DCM show representative reliability across all people with dementia? There is evidence to suggest that level of dependency is correlated with DCM scores, specifically that low well-being scores are associated with high dependency levels, although this relationship is not universally demonstrated. However, it is sufficiently strong to suggest that a measure of dependency should be routinely taken alongside DCM evaluations, and the nature of this measure needs to be agreed,
Does DCM change care practice? The six studies on the use of DCM in repeated evaluations confirm that it can change care by improving measured levels of well-being and reducing the incidence of ‘personal detractions’. However, ‘the mix of papers in this review cannot be taken as a reflection of the way DCM is used generally’ because many practitioners may not publish the results of their activity. To provide more reliable data it will be necessary to test DCM as a tool for practice development in a longitudinal study in which other quality of life measures (i.e. not DCM scores) are used to measure outcomes.
Is DCM a suitable tool for research? DCM was not designed as a research tool, and there are few studies of reliability and validity. Existing findings on inter-rater reliability, concurrent validity with other proxy measures of quality of life, internal consistency and test-retest reliability need to be replicated. The issue of the impact of dependency and diagnosis on scores also needs to be investigated. From the studies reviewed here, it seems that DCM is particularly suitable for smaller scale within-subjects or group comparison evaluations, and it also has value in enriching data derived from proxy and service-user interviews and focus groups. However, the rules of coding mean that it is likely to under-estimate the occurrence of socially passive and withdrawn behaviour, and researchers may be better advised to use another tool for this purpose.
What do DCM scores mean in terms of benchmarking? The data from the review suggest that scores are generally higher in day care than long stay care, but it is not clear how this is confounded by different dependency levels. Work is currently underway to develop an international database of DCM results that could be used for benchmarking purposes, and the quality of this needs to be safeguarded by accepting only data that has been verified by a gold standard mapper.
What is a significant change in scores? Few studies have reported data on the statistical significance of changes in DCM scores. ‘Further research is needed to clarify what constitutes a clinically significant change.’
How long should a map be? Six hours is the current guidance in DCM training, and most studies (with the exception of those using DCM for practice development where longer periods are involved) use this time period. However, there is some evidence that useful insights can be gained from shorter periods, such as two hours. This is another are requiring further investigation.
Authors' conclusions
‘These studies report evidence that DCM has a role to play in practice development and research within the broad aim of improving the quality of the lived experience for people with dementia.’ The author recommends a controlled, longitudinal study to evaluate fully its impact on practice development and quality of care.
Implications for policy or practice
DCM is already being widely used, and the results of this review will feed into the process of improving its reliability and utility. The author emphasises the fact that it provides ‘a shared language, and focus across professional disciplines, care staff and management teams. It is seen as a valid measure by frontline staff as well as those responsible for managing and commissioning care’. Many of the studies cited in the review were undertaken at the behest of practitioners, and DCM thus provides a major opportunity for dialogue between research and practice in social care.
Key to icons
Give us your feedback
Social Care Online continues to be developed in response to user feedback.
Contact us with your comments and for any problems using the website.
Sign up/login for more
Register/login to access resource links, advanced search and email alerts

Top-funded digital health companies offering lifestyle interventions for dementia prevention: Company overview and evidence analysis
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- ORCID record for Rasita Vinay
- For correspondence: [email protected]
- ORCID record for Tobias Kowatsch
- ORCID record for Marcia Nissen
- Info/History
- Supplementary material
- Preview PDF
Background and objective: Dementia prevention has been recognized as a top priority by public health authorities due to the lack of disease modifying treatments. In this regard, digital dementia-preventive lifestyle services (DDLS) emerge as potentially pivotal services, aiming to address modifiable risk factors on a large scale. This study aims to identify the top-funded companies offering DDLS globally and evaluate their clinical evidence to gain insights into the current state of the global service landscape. Methods: A systematic screening of two financial databases (Pitchbook and Crunchbase) was conducted. Corresponding published clinical evidence was collected through a systematic literature review and analyzed regarding study purpose, results, quality of results, and level of clinical evidence. Findings: The ten top-funded companies offering DDLS received a total funding of EUR 128.52 million, of which three companies collected more than 75%. Clinical evidence was limited due to only nine eligible publications, small clinical subject groups, the absence of longitudinal study designs, and no direct evidence of dementia prevention. Conclusion: The study highlights the need for a more rigorous evaluation of DDLS effectiveness in today's market. It serves as a starting point for further research in digital dementia prevention.
Competing Interest Statement
RV, PH, TK, and MN are affiliated with the Centre for Digital Health Interventions (CDHI), a joint initiative of the Institute for Implementation Science in Health Care, University of Zurich, the Department of Management, Technology, and Economics at the ETH Zurich, and the institute of Technology Management and School of Medicine at the University of St. Gallen. CDHI is funded in part by CSS, a Swiss health insurer, Mavie Next, an Austrian healthcare provider and MTIP, a Swiss investor company. TK is also a co-founder of Pathmate Technologies, a university spin-off company that creates and delivers digital clinical pathways. However, neither CSS nor Pathmate Technologies, Mavie Next, or MTIP was involved in this research. All other authors declare no conflict of interest.
Funding Statement
This study did not receive any funding
Author Declarations
I confirm all relevant ethical guidelines have been followed, and any necessary IRB and/or ethics committee approvals have been obtained.
I confirm that all necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone (e.g., hospital staff, patients or participants themselves) outside the research group so cannot be used to identify individuals.
I understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as ClinicalTrials.gov. I confirm that any such study reported in the manuscript has been registered and the trial registration ID is provided (note: if posting a prospective study registered retrospectively, please provide a statement in the trial ID field explaining why the study was not registered in advance).
I have followed all appropriate research reporting guidelines, such as any relevant EQUATOR Network research reporting checklist(s) and other pertinent material, if applicable.
Data Availability
All data produced in the present work are contained in the manuscript
View the discussion thread.
Supplementary Material
Thank you for your interest in spreading the word about medRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
Subject Area
- Public and Global Health
- Addiction Medicine (315)
- Allergy and Immunology (617)
- Anesthesia (159)
- Cardiovascular Medicine (2269)
- Dentistry and Oral Medicine (279)
- Dermatology (201)
- Emergency Medicine (369)
- Endocrinology (including Diabetes Mellitus and Metabolic Disease) (798)
- Epidemiology (11559)
- Forensic Medicine (10)
- Gastroenterology (676)
- Genetic and Genomic Medicine (3560)
- Geriatric Medicine (336)
- Health Economics (615)
- Health Informatics (2297)
- Health Policy (912)
- Health Systems and Quality Improvement (860)
- Hematology (334)
- HIV/AIDS (748)
- Infectious Diseases (except HIV/AIDS) (13133)
- Intensive Care and Critical Care Medicine (755)
- Medical Education (358)
- Medical Ethics (100)
- Nephrology (388)
- Neurology (3341)
- Nursing (191)
- Nutrition (506)
- Obstetrics and Gynecology (650)
- Occupational and Environmental Health (643)
- Oncology (1753)
- Ophthalmology (524)
- Orthopedics (208)
- Otolaryngology (284)
- Pain Medicine (223)
- Palliative Medicine (66)
- Pathology (437)
- Pediatrics (999)
- Pharmacology and Therapeutics (419)
- Primary Care Research (402)
- Psychiatry and Clinical Psychology (3050)
- Public and Global Health (5980)
- Radiology and Imaging (1217)
- Rehabilitation Medicine and Physical Therapy (712)
- Respiratory Medicine (809)
- Rheumatology (367)
- Sexual and Reproductive Health (348)
- Sports Medicine (315)
- Surgery (386)
- Toxicology (50)
- Transplantation (170)
- Urology (142)

IMAGES
COMMENTS
Abstract. Purpose: The published literature on dementia care mapping (DCM) in improving quality of life and quality of care through practice development and research dates back to 1993. The purpose of this review of the research literature is to answer some key questions about the nature of the tool and its efficacy, to inform the ongoing revision of the tool, and to set an agenda for future ...
The published literature on dementia care mapping (DCM) in improving quality of life and quality of care through practice development and research dates back to 1993. The purpose of this review of ...
These publications provide some evidence regarding the efficacy of DCM, issues of validity and reliability, and its use in practice and research. PURPOSE The published literature on dementia care mapping (DCM) in improving quality of life and quality of care through practice development and research dates back to 1993. The purpose of this review of the research literature is to answer some key ...
This website requires cookies, and the limited processing of your personal data in order to function. By using the site you are agreeing to this as outlined in our privacy notice and cookie policy.
A literature review of dementia care mapping: methodological considerations and efficacy. D. BEAVIS BSc(Hons) RGN RMN. Corresponding Author. Research Nurse and ... John S. Preisser, Christianna S. Williams, Sheryl Zimmerman, Dementia care mapping as a research tool, International Journal of Geriatric Psychiatry, 10.1002/gps.1721, 22, 6, (580 ...
Dementia care mapping (DCM) is a popular method for evaluating the quality of care and well-being of people with dementia in formal care settings. Keywords and thesaurus searches were conducted ...
Dementia care mapping (DCM) is a popular method for evaluating the quality of care and well-being of people with dementia in formal care settings. ... A literature review of dementia care mapping: methodological considerations and efficacy J Psychiatr Ment Health Nurs. 2002 Dec;9(6):725-36. doi: 10.1046/j.1365-2850.2002.00508.x. Authors D ...
The evidence presented for DCM suggests that it has good face validity and reliability, however, other aspects of validity remain less convincing and it can only be regarded as a moderately valid instrument. Dementia care mapping (DCM) is a popular method for evaluating the quality of care and well-being of people with dementia in formal care settings. Keywords and thesaurus searches were ...
Abstract. Background: This systematic review identifies and reports the extent and nature of evidence to support the use of Dementia Care Mapping as an intervention in care settings. Methods: The review was limited to studies that used Dementia Care Mapping as an intervention and included outcomes involving either care workers and/or people ...
The review was limited to studies that used Dementia Care Mapping as an intervention and included outcomes involving either care workers and/or people living with dementia. Searches were conducted in PubMed, Web of Knowledge, CINAHL, PsychINFO, EBSCO, and Scopus and manually from identified articles reference lists.
Purpose: The published literature on dementia care mapping (DCM) in improving quality of life and quality of care through practice development and research dates back to 1993. The purpose of this review of the research literature is to answer some key questions about the nature of the tool and its efficacy, to inform the ongoing revision of the tool, and to set an agenda for future research.
Introduction. Dementia Care Mapping (DCM) Citation 1, Citation 2 is an observational tool set within a practice development process that has been used for over 20 years to assist in the delivery of better quality formal care to people with dementia. It is a tool that has developed over time with feedback from practitioners, and the latest eighth edition was published in 2005, following a ...
Dementia care mapping (DCM) is a popular method for evaluating the quality of care and well-being of people with dementia in formal care settings. Keywords and thesaurus searches were conducted between 1992 and June 2001 using a range of bibliographic databases.
quality of care through practice development and research dates back to 1993. The purpose of this review of the research literature is to answer some key questions about the nature of the tool and ...
Introduction. Dementia Care Mapping (DCM) 1, 2 is an observational tool set within a practice development process that has been used for over 20 years to assist in the delivery of better quality formal care to people with dementia. It is a tool that has developed over time with feedback from practitioners, and the latest eighth edition was published in 2005, following a formal academic review ...
Dementia Care Mapping™ (DCM) 12,13 is a manualized, established intervention 14 developed by the University of Bradford, United Kingdom, and used internationally in care home settings. 15 It is an observational and practice development tool, implemented as quality improvement cycles, which aim to support the delivery of person-centered dementia care. 16 Standard implementation is led by care ...
Dementia Care Mapping (DCM) is an intervention developed by the Dementia Research Group at Bradford University to improve the quality and effectiveness of care from the perspective of people with dementia (Brooker & Surr, 2005).It is based on Kitwood's social-psychological theory of personhood in dementia.(Kitwood, 1992) DCM was designed as observational tool to develop person‐centred care ...
Purpose: The published literature on dementia care mapping (DCM) in improving quality of life and quality of care through practice development and research dates back to 1993. The purpose of this review of the research literature is to answer some key questions about the nature of the tool and its efficacy, to inform the ongoing revision of the tool, and to set an agenda for future research.
The Ministerial Advisory Group on Dementia Research: Headline Report. London: Department of Health and Social Care; 2011. ... Simpson S, Graham I. A literature review of dementia care mapping: methodological considerations and efficacy. J ... Brooker D. Dementia care mapping: a review of the research literature. Gerontologist 2005;45:11-18 ...
Purpose: The published literature on dementia care mapping (DCM) in improving quality of life and quality of care through practice development and research dates back to 1993. The purpose of this review of the research literature is to answer some key questions about the nature of the tool and its efficacy, to inform the ongoing revision of the tool, and to set an agenda for future research.
The review aimed to identify and describe dementia care interventions and programs that are culturally tailored to support racial and ethnic minority informal caregivers of community-dwelling people living with dementia (PLWD) to identify gaps in need. Culturally targeted interventions to support vulnerable minority informal caregivers are important in addressing the care needs of PLWD and ...
Dementia Care Mapping (DCM), developed for people with dementia who receive long-term care, captures the person with dementia's experiences and perspectives through observation of twelve ...
A suitable model of clinical care developed that incorporates the Hospital Admission Readiness Kit into care provision for testing and implementation at scale. Research output to date: Improving the Inpatient Care of People Living with Dementia: A Rapid Literature Review and Qualitative Study, Lennox et al. 2023
Background. Dementia is a multi-causal syndrome characterised by progressive deterioration in cognitive abilities and impairment in the ability to perform everyday activities; it can compromise capacity for independent living and lead to needs for care [].More than 35 million people live with dementia worldwide and, given that the disease is primarily associated with increasing age, the number ...
A network of brain regions degenerates earlier in aging. Here the authors show that, this network is most vulnerable to diabetes, traffic-related pollution and alcohol consumption in terms of ...
The published literature on dementia care mapping (DCM) in improving quality of life and quality of care through practice development and research dates back to 1993. The purpose of this review of the research literature is to answer some key questions about the nature of the tool and its efficacy, to inform the ongoing revision of the tool, and to set an agenda for future research.
Background and objective: Dementia prevention has been recognized as a top priority by public health authorities due to the lack of disease modifying treatments. In this regard, digital dementia-preventive lifestyle services (DDLS) emerge as potentially pivotal services, aiming to address modifiable risk factors on a large scale. This study aims to identify the top-funded companies offering ...