- Download PDF
- Share X Facebook Email LinkedIn
- Permissions

Organ Donor Research : Overcoming Challenges, Increasing Opportunities
- 1 University of Virginia, Charlottesville
A substantial gap exists between the need for organ transplants and the number of transplants performed each year in the United States. In 2016, 27 630 organs were transplanted from 9971 deceased donors and 5980 additional organs from living donors, but as of September 29, 2017, a total of 116 602 individuals were included on the nation’s organ transplant wait lists. 1 This gap remains despite increases in the number of both donated organs and organ transplants in recent years. In 2015, close to 5000 organs from deceased donors were discarded because they were deemed unsuitable for transplantation. 1
Read More About
Childress JF. Organ Donor Research : Overcoming Challenges, Increasing Opportunities . JAMA. 2017;318(22):2177–2178. doi:10.1001/jama.2017.16442
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Research article
- Open access
- Published: 27 May 2020
Knowledge, attitude and willingness to donate organ among medical students of Jimma University, Jimma Ethiopia: cross-sectional study
- Fantu Kerga Dibaba ORCID: orcid.org/0000-0003-4331-3907 1 ,
- Kabaye Kumela Goro 1 ,
- Amare Desalegn Wolide 2 ,
- Fanta Gashe Fufa 1 ,
- Aster Wakjira Garedow 1 ,
- Birtukan Edilu Tufa 3 &
- Eshetu Mulisa Bobasa 1
BMC Public Health volume 20 , Article number: 799 ( 2020 ) Cite this article
7506 Accesses
19 Citations
7 Altmetric
Metrics details
The lack of organ donors has become a limiting factor for the development of organ transplantation programs. Many countries are currently facing a severe shortage of organs for transplantation. Medical students, as future doctors can engage in the role of promoting organ donation by creating awareness and motivating the community to donate their organs besides their voluntary organ donation. The aim of this study is to assess the knowledge, attitude and willingness of undergraduate medical students’ towards organ donation at Jimma University.
A cross-sectional study was conducted among 320 medical students from year I to internship using questionnaire in order to assess their knowledge, attitude and willingness regarding organ donation. Data collected was entered using epidata and analyzed using Statistical Package for Social Sciences (SPSS) software version 20.
Mean (±SD = standard deviation) age of participants was 23.48 ± 17.025 years. 57.8% of the study subjects were male. There was a statistically significant interaction effect between gender and year of study on the combined knowledge questions (dependent variables) F(25,062) = 1.755, P = 0.014, Wilk’s Λ = .033. Variables which were related to a positive attitude towards organ donation were: being of the male sex (Odds Ratio = 1.156); having awareness about organ donation (Odds Ratio = 2.602); not having a belief on the importance of burying intact body (Odds Ratio = 5.434); willingness to donate blood (Odds Ratio = 4.813); and willingness to donate organ (Odds Ratio = 19.424).
High level of knowledge but low level of positive attitude and willingness was noticed among the study participants toward organ donation.
Peer Review reports
The need for organ donation has increased globally in the past years due to an increase in organ failure [ 1 ]. Every day in the United States of America (USA), 21 people die waiting for an organ and more than 120,048 men, women, and children await life-saving organ transplants [ 2 ]. Accor-ding to a survey In India every year about 5 lakh (500,000) people die because of non-availability of organs and 1.5 lakh(150,000) people await a kidney transplant but only 5000 get among them [ 3 ]. Recently published report has found that approximately 3 million people in sub-Saharan Africa diagnosed with end-stage kidney disease (ESKD) die each year due to renal failure [ 4 ]. In Kenya, the kidney transplant queue at Kenyatta National Hospital in Nairobi stretches all the way to 2018, despite the hospital performing the procedure on a weekly basis [ 5 ]. In Ethiopia, between 130 and 150 corneas are collected yearly. However, there are more than 300,000 blind people waiting for corneal transplantation [ 6 ].
There are no sufficient facilities which provide maintenance and transplantation therapy for failed organs in Ethiopia. Currently there are only cornea and living related kidney transplant programs established in the nation’s capital Addis Ababa [ 6 ]. Facilities which provide maintenance dialysis has been in existence in the country starting from 2001. Hemodialysis has become on hand in private institutions, mostly in Addis Ababa the capital city of the country, and more recently in a few other urban and semi-urban regions. Currently, there are 30 hemodialysis centers with a total of 186 hemodialysis chairs and approximately 800 patients on hemodialysis. Among patients on maintenance dialysis, only about one-third receives treatment 3× per year because the cost of hemodialysis is unaffordable for the majority of patients [ 7 ].
Organ transplantation is one of the great advances in modern medicine and is the best option for failed organ. Transplantation is defined as the transfer of human cells, tissues or organs from a donor to recipient with an aim of restoring normal physiology in the body [ 8 ]. In Ethiopia, up to 2018, 1336 corneal and 90 living donor kidney transplants have been performed. Currently the kidney transplant program accepts candidates only at the age of 14 and above [ 7 , 9 ].
Some studies found out that the issue of organ donation is multifactorial. In developed countries relational ties, religious beliefs, cultural influences, family influences, body integrity, and previous interactions with the health-care system were reported as the potential factors for organ donation [ 10 ]. However, there are limited studies regarding organ donation and the factors that influence it in developing countries for instance, in Kenya there are peoples who believe a person’s body should be intact when buried this belief and other sociocultural and legal factors hinder the harvest of organ from patients who have been medically declared to be in a “state of dying” [ 5 ].
Among 100,000 of people died each year are believed to be potential donors; however, only less than 200 actually become donors [ 11 ]. This indicates that a lot should be done on awareness creation towards organ donation. As a new approach in solving the organ shortage, it has been suggested that awareness about organ donation to be made a part of school education [ 12 ]. In Ethiopia we suggest to use religious leaders besides to incorporating the issue in school education, because Ethiopia is religious country. Our country has close ties with all three major Abrahamic religions, and it was the first in the region to officially adopt Christianity in the fourth century. Christians account for 63% of the country’s population, with 43.5% belonging to the Ethiopian Orthodox Church, 18.5% Protestant and 0.7% Catholic. Ethiopia has the first Hijra in Islamic history and the oldest Muslim settlement on the continent. Muslims account for 34% of the population, traditional 2.7% and other 0.6% [ 13 ].
In Ethiopia there are no data on public perception of organ donation and transplantation Therefore, the present study was designed to assess the knowledge, attitude and willingness of organ donation among medical students. Medical students, as future doctors can take up the role of promoting organ donation by educating and motivating the public to initiate them donate their organs besides their voluntary organ donation. Therefore, assessing medical student’s knowledge, attitude and willingness to donate organ is very important to decrease the shortage of organ in the future.
Study setting and subjects
A cross sectional study was carried out for 3 months from May to July 2019among under graduate medical students in Jimma University after obtaining Institutional Ethical Clearance from institutional review board (IRB) of Jimma University. The University is located in Jimma town which is 352 km from Addis Ababa, the capital city of Ethiopia. Jimma University is one of the most distinguished centers of excellence in medical education in the country.
Sample size
All medical students (from first to internship) registered in the year 2018/2019 were the source population. Based on their training background, medical students in Jimma University were divided into two groups: PRE-CLINICAL and CLINICAL. PRECLINICAL is subdivided in to two groups: Year I (PC-I) and Year II (PC-II) and CLINICAL in to three subgroups Year III(C-I), Year IV(C-II) and internship. The sample size was calculated by using simple proportion formula assuming a prevalence of 50% for knowledge, attitudes and willingness of organ donation, a 95% confidence interval and a sample error of 5%. This was adjusted for 10% non-response rate; bringing the total sample size to 320.There were about 1200 students studying in Jimma University medical school.
The questionnaire was distributed to undergraduate medical students during lecture hours in the classroom and in ward during attachment. They were instructed not to discuss the questions among themselves. The importance of the study was explained and confidentiality regarding the participant response for the questions was ensured.
A 20-item self-administered questionnaire was developed. The first part of the questionnaire gathered the demographic details from the students, which included age, gender, year of study and religion. The second, third and fourth sections assessed the levels of knowledge (Q1–7), attitude (Q8–16) and willingness (Q17–20) to donate organ, respectively.
The students were grouped as those who do have adequate and inadequate knowledge based on their score.
Adequate knowledge is when 4–6 questions were answered correctly and inadequate when less than 4 questions answered correctly out of 6 knowledge questions.
Attitude was assessed by using 9 attitude statements and respondents were categorized as those who do have positive attitude and negative if they agree to 6–9 and less than 6 attitude statements respectively.
Statistical analysis
Data was entered to EPI data and exported to SPSS version 20 for analysis. Descriptive statistics like percentage and mean and standard deviation were used to present socio-demography, knowledge, attitude and willingness response of the participants. Multivariate analysis was used in order to relate those factors that gave a significant result: One way Multivariate analysis of variance (MANOVA) was used to see a significant relationship between one independent variable and dependent variables and two ways MANOVA was considered to know if there was an interaction between two independent variables on the dependent variables. One way Analysis of Variance (ANOVA) was used for comparing means of variables to know among which groups were the differences. Finally, Odds ratio analysis was used to find out variables which were related to a positive attitude towards organ donation.
Out of 320 participants 57.8% were male. Mean (±SD = standard deviation) age of participants was 23.48 ± 17.025 years. Majority of the participants were orthodox (49%.7) and the least percentage being others constituting wakeefeta, apostolic, humanity, atheist and Seventh Day Adventist (SDA) (2.8%) (Table 1 ).
96.9% of the students had awareness about organ donation. Only 25% had knowledge that there was no age limit for organ donation (Table 2 ).
There was a statistically significant difference in level of knowledge between study groups as demonstrated by one-way ANOVA(F (4,315) =7.6, p = 0.001). Based on the post hoc test the significant difference was between PC-I and C-II( p = 0.001), PC-I and intern( p = 0.001), PC-II and C-I( P = 0.022) and PC-II and intern( p = 0.010). The mean for PC-I, PC-II, C-I, C-II and intern is 1.37, 1.27, 1.20, 1.08 and 1.05 respectively. Therefore, PC=I had significantly higher level of knowledge when compared to the rest year of study (Table 3 ).
74.1% of the participants agreed to support family members if they wish to become an organ donor. Majority of the study subjects (91.9%) felt that awareness about organ donation should be made a part of school education (Table 4 ).
According to our finding, males were 1.156 (Odds Ratio = 1.156) times likely to have positive attitude towards to organ donation as compared to female. Students who had an awareness about organ donation were 2.602 (Odds Ratio = 2.602) times likely to have positive attitude towards to organ donation as compared to those who were unaware. The other variables which were related to a positive attitude towards organ donation were: not having a belief on the importance of burying intact body (Odds Ratio = 5.434); knowing definition of brain death (Odds Ratio = 1.257); not having a belief that there is a danger of misuse, abuse or misappropriation of donated organ (Odds Ratio = 2.777); willingness to donate blood (Odds Ratio = 4.813); and willingness to donate organ (Odds Ratio = 19.424).
58.1% of the study participants were willing to donate their organs and allow organ donation after the death of a family member. Majority of the study subjects (88.4%) did not like to take money for organ donation. 90.3% of the study subjects were willing to donate blood and 58.1% were willing to donate their organ (Table 5 ) (Fig. 1 ).
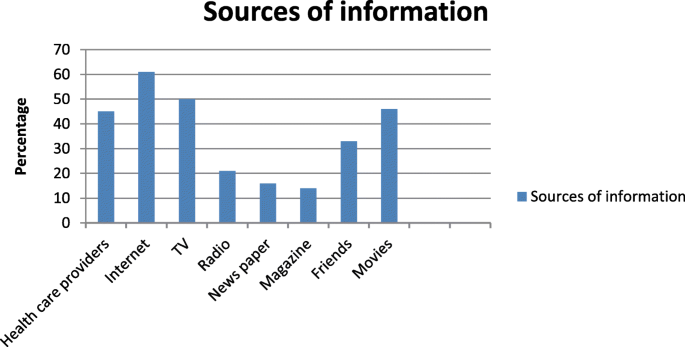
Distribution of study subjects according to the source of information about organ donations. i.e. Note: No of respondents may be greater than sample size as multiple options were allowed. Most common source of information about organ donation was found to be internet (61%) television (50%) followed by, Movies and health care providers 46 and 45% respectively
There were an association between willingness and attitude. Willingness to donate organ was significantly higher among those who do have positive attitude (88.2%) as compared to those with negative attitude (11.8%) (Table 6 ).
There was a statistically significant difference on belief of burying intact body between religions as demonstrated by one-way ANOVA(F (3,316) =4.5, p = .004). Based on the post hoc test the significant difference was between Protestant and Muslim ( p = .007). The mean for protestant is 1.83 and Muslim 1.56.Therefore, Protestant had significantly higher belief on the importance of burying intact body when compared to Muslim (Table 7 ).
There was a statistically significant difference between males and females when knowledge questions considered jointly Wilk’s Λ = .96, F (6,312) = 2.247, P = 0.039, multivariate ƞ 2 = 0.041 and attitude statements consider jointly Wilk’s Λ = .94, F (9,310) = 2.301, P = 0.016, multivariate ƞ 2 = 0.063.
When year of study is considered, there was a statistically significant difference among year of studies when knowledge questions considered jointly Wilk’s Λ = .75, F (25,079) = 3.966, P < 0.001, multivariate ƞ 2 = .071, attitude statements considered jointly Wilk’s Λ = .77, F (37,152) = .766, P < 0.001, multivariate ƞ 2 = .065 and willingness questions considered jointly Wilk’s Λ = .93, F (12,828) = 2.072, P = 0.017, multivariate ƞ 2 = .026.
Two way MANOVA was considered to know if there was an interaction between two independent variables on the dependent variables. There was a statistically significant interaction effect between gender and year of study on the combined knowledge questions (dependent variables) F (25,062) = 1.755, P = 0.014, Wilk’s Λ = .033.
Knowledge of the participant
Organ failure and shortage of donated organs are global problem. Among 100,000 of people died each year are believed to be potential donors; however, only less than 200 actually become donors [ 9 ]. The widespread shortage of donated organs indicates that there is low donor rate worldwide; In Ethiopia there is no data on rate of organ donation. In 2017 Spain had the highest donor rate in the world at 46.9 per million people, followed by Portugal (34.0 per million), Belgium (33.6 per million), Croatia (33.0 per million) and the US (32.0 per million) [ 14 ]. Donated organs are the major pre-requisite for consistency of organ transplantation program; one of the solutions to increase organ supply is to assess public knowledge, attitude and willingness towards organ donation and taking an action based on the data. In our country there is no study done on people’s perception towards organ donation this background pledges us to conduct this study.
In our study 96.9% of the participants heard about organ donation which is similar to study done by Annadurai et al and Jothula et al. [ 15 , 16 ] both reported that 100% of the participants were aware about organ donation.74.1% of the participants were aware about the meaning of organ donation which is relatively higher than the study done by Annadurai et al. [ 15 ]. In the present study, level of knowledge was significantly higher among PC=I (year I) students as compared to the other year of study this finding was similar to study done among undergraduate dental students of Panineeya Institute of Dental Sciences and Hospital, which showed higher average knowledge among first-year students [ 17 ]. In this study, only 82.5%of medical students had adequate knowledge about organ donation which is relatively higher than the study done on final semester medical students by Karini et al. which showed that only 56% of them were having adequate knowledge [ 18 ].
In the present study the main sources of information about organ donation was found to be internet (61%) and television (50%).This was similar to study conducted in USA and Australia [ 19 , 20 ]. However; Similar findings were observed by Sindhu et al. and Jothula KY et al. [ 16 , 21 ]. The third source of information about organ donation in our study are health care providers (45%) which is relatively higher than the study done by Annadurai et al. [ 15 ] which reported 34.1%. this finding showed that health care providers are playing undeniable role in creating awareness towards organ donation in Ethiopia.
206(64.4%) of our study participants had identified all the organs that can be donated. This finding was higher than the study done by Annadurai et al. [ 15 ] and Karini et al. [ 18 ] which reported 16.1 and 26% respectively. In the present study 80(25%) of the students knew that there is no age limit for organ donation which is approximate to Sucharitha et al. and lower than Jothula KY et al. [ 16 , 22 ].
Attitude of medical students regarding organ donation
201(62.8%) of our study subjects have a positive attitude towards organ donation which is lower than the study in Spain and India which found 80 and 71.3% respectively [ 23 , 24 ]. 91.9% of this study subjects, felt that awareness about organ donation should be included in school curriculum which is similar to Adithyan et al. reported that 91.2% of the subjects felt the need for revision of medical curriculum on organ donation [ 25 ] Our study found out that 251(78.4%) of the study subjects would like to motivate others for organ donation which is lower than to the Vinay et al [ 26 ].
77(24.1%) of our study subjects belief that person’s body should be intact when buried A study in USA reported that 8% of participants strongly agree and 11.7% agree to this statement which is almost similar to our finding [ 19 ]. In our study being of the male sex (Odds Ratio = 1.156) was related to a favorable attitude towards to organ donation; in contrast, a study done in Spain reported that being of females sex (Odds Ratio = 1.739) was related to a favorable attitude [ 23 ]. In our study not having a belief on the importance of burying intact body (Odds Ratio = Ratio = 5.434) was one of the variables which affect positive attitude towards to organ donation which was similar to a study in USA [ 19 ]. A study done in Spain reported being a blood donor (OR = 2.824) as a variable related to a positive attitude towards to organ donation similarly in our study we found out willingness to donate blood (Odds Ratio = 4.813) as a variable to a favorable attitude.
Willingness of medical students to donate organ
In this study 186(58.1%) of the study participants were willing to donate their organ which is similar to a study done in USA [ 20 ] and lower than Payghan et al. and Vinay et al revealed that almost 90% of study participants were willing to donate their organs [ 26 , 27 ]. The present study found out that there is a significant association between attitude regarding organ donation and willingness to donate organs which is different from the finding by Ali et al. and by Dasgupta et al. [ 28 , 29 ] which reported that there was a significant association between attitude and knowledge acquired. Though taking money for organ donation is unethical 11.6% of our study participants would like to take money for organ donation which was higher than study by Jothula KY et al. [ 16 ].
Though most of the students had adequate knowledge, still gaps exist in their attitude and willingness. This implies the need for an intensified and sustained education to raise attitude and willingness of the students towards organ donation.
Recommendations
Most of the students (91.9%) felt that awareness about organ donation should be made a part of school education; until it included in school curriculum, we recommend the students to acquire an adequate knowledge by themselves; In our study the most common source of information about organ donation was internet; so, they can browse more to acquire additional knowledge and make informed decision.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Analysis of variance
Clinical-II
End-stage kidney disease
Institutional Review Board
Jimma University Medical College
Multivariate analysis of variance
Pre-clinical-I
Pre-clinical-II
Seventh Day Adventist
Statistical Package for Social Sciences
United States of America
Wakefield MA, Loken B, Hornik RC. Use of mass media campaigns to change health behaviour. Lancet. 2010;376(9748):1261–71.
Article Google Scholar
Organ Donation Facts & Info _ Organ Transplants _ Cleveland Clinic www.unos.org , Nov. 1, 2016. Accessed 26 Jan 2020.
National Health Portal. “Organ donation day”. Available at http://www.nhp.gov.in/organdonation-day_pg . Accessed 18 Feb 2018.
Ashuntantang G, Osafo C, Olowu WA, et al. Outcomes in adults and children with end-stage kidney disease requiring dialysis in sub-Saharan Africa: a systematic review. Lancet Glob Health. 2017;5:e408–17.
Organ failure: patients in East Africa wait endlessly for donors. Science & Health https://www.theeastafrican.co.ke . Accessed 26 Aug 2019.
Rao GN, Gopinathan U. Eye banking: an introduction. Community Eye Health. 2009;22(71):46–7.
PubMed PubMed Central Google Scholar
Ahmed, et al. Organ transplantation in Ethiopia. 2019;103(3):449–51.
World Health Organisation (WHO). Global glossary of terms and definitions on donation and transplantation, vol. 14. Geneva; 2009.
13. Eye Bank of Ethiopia celebrates 15 th anniversary June 28, 2018 by New Business Ethiopia. http://newbusinessethiopia.com/health/eye-bank-of-ethiopia-celebrates-15th-anniversary/ .
Irving MJ, Tong A, Jan S, Cass A, Rose J, Chadban S, et al. Factors that influence the decision to be an organ donor: a systematic review of the qualitative literature. Nephrol Dial Transplant. 2012;27:2526–33.
Sahi M. Myths and misconceptions and reality on organ donation. Transplantation Research. https://www.mohanfoundation.org/organ-donation-transplant-resources/Myths-Misconceptions-and-the-Reality-of-Organ-Donation.asp .
Burra P, De Bona M, Canova D, et al. Changing attitude to organ donation and transplantation in university students during the years of medical school in Italy. Transplant Proc. 2005;37:547–50.
Article CAS Google Scholar
Ethiopia PEOPLE 2019, CIA World Fact book Theodora.com https://theodora.com/wfbcurrent/ethiopia/ethiopia_people.html .
Newsletter 2018 (PDF). International registry in organ donation and transplantation. 2018 . Retrieved December 30, 2018 . .
Google Scholar
Annadurai K, Mani K, Ramasamy J. A study on knowledge, attitude and practices about organ donation among college students in Chennai, Tamil Nadu −2012. Prog Health Sci. 2013;3:2 KAP on organ donation.
Jothula KY, Sreeharshika D. Study to assess knowledge, attitude and practice regarding organ donation among interns of a medical college in Telangana, India. Int J Community Med Public Health. 2018;5:1339–45.
Chakradhar K, Doshi D, Srikanth Reddy B, et al. Knowledge, attitude and practice regarding organ donation among Indian dental students. Int J Organ Transplant Med. 2016;7(1):28–35.
CAS PubMed PubMed Central Google Scholar
Karini D, Sunitha S, Devi Madhavi B. Perceptions of medical students in a government medical college towards organ donation. J Evid Based Med Healthc. 2015;2(44):7998–8005.
U.S. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, 2012 National Survey of Organ Donation Attitudes and Behaviors. Rockville, Maryland: U.S. Department of Health and Human Services; 2013.
Hyde MK, Chambers SK. Information sources, donation knowledge, and attitudes toward transplant recipients in Australia. Prog Transplant. 2014;24:169–77.
Sindhu A, Ramakrishnan TS, Khera A, Singh G. A study to assess the knowledge of medical students regarding organ donation in a selected college of Western Maharashtra. Med J DY Patil Univ. 2017;10:349–53.
Agarwal S. Are medical students having enough knowledge about organ donation. IOSR J Dental Med Sci. 2015;14(7):29–34.
Ríos A, López-Navas A, López-López A, Gómez FJ, Iriarte J, Herruzo R, Blanco G, Llorca FJ, Asunsolo A, Sánchez P, Gutiérrez PR, Fernández A, de Jesús MT, MartínezAlarcón L, Lana A, Fuentes L, Hernández JR, Virseda J, Yelamos J, Bondía JA, Hernández AM, Ayala MA, Ramírez P, Parrilla P. A multicentre and stratified study of the attitude of medical students towards organ donation in Spain. Ethn Health. 2019;24(4):443–61. https://doi.org/10.1080/13557858.2017.1346183 .
Article PubMed Google Scholar
Bathija GV, Ananthesh BG, Bant DD. Study to assess knowledge and attitude towards organ donation among interns and post graduates of a medical college in Karnataka, India. Natl J Community Med. 2017;8(5):236–40.
Adithyan GS, Mariappan M, Nayana KB. A study on knowledge and attitude about organ donation among medical students in Kerala. Indian J Transplant. 2017;11:133–7.
Vinay KV, Beena N, Sachin KS, Praveen S. Changes in knowledge and attitude among medical students towards organ donation and transplantation. Int J Anat Res. 2016;4(3):2873–7.
Payghan BS, Kadam SS, Furmeen S. Organ donation: awareness and perception among medical students. J Pharm Sci Innov. 2014;3(4):379–81.
Ali NF, Qureshi A, Jilani BN, Zehra N. Knowledge and ethical perception regarding organ donation among medical students. BMC Med Ethics. 2013;14:38.
Dasgupta A, Shahbabu B, Sarkar K, Sarkar I, Das S, Das MK. Perception of organ donation among adults: a community based study in an urban. Sch J App Med Sci. 2014;2(6A):2016–2021.
Download references
Acknowledgements
Not applicable.
The study was funded with the support of Jimma University; Faculty of Health Science. The funding body has no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and affiliations.
Faculty of Health Sciences, School of Pharmacy, Jimma University, 378, Jimma, Ethiopia
Fantu Kerga Dibaba, Kabaye Kumela Goro, Fanta Gashe Fufa, Aster Wakjira Garedow & Eshetu Mulisa Bobasa
Facility of Medicine, Jimma University, 378, Jimma, Ethiopia
Amare Desalegn Wolide
Faculty of Health Sciences, School of Midwifery and Nursing, Jimma University, 378, Jimma, Ethiopia
Birtukan Edilu Tufa
You can also search for this author in PubMed Google Scholar
Contributions
FKD, EMB, KKG, ADW, FGF, AWG, BET involved in the data collection. FKD analyze the data and FKD and EMB prepared the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Fantu Kerga Dibaba .
Ethics declarations
Ethics approval and consent to participate.
The study protocol was approved by the institutional review board (IRB) of Jimma University, College of Health Sciences and ethical clearance was obtained with the Reference Number IHRPGD/3019/2019. Permission of data collection was granted with formal letter from chief executive director of Jimma University Medical College (JUMC). The purpose and protocol of this study was explained, participants signed informed written consent.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Dibaba, F.K., Goro, K.K., Wolide, A.D. et al. Knowledge, attitude and willingness to donate organ among medical students of Jimma University, Jimma Ethiopia: cross-sectional study. BMC Public Health 20 , 799 (2020). https://doi.org/10.1186/s12889-020-08931-y
Download citation
Received : 19 September 2019
Accepted : 17 May 2020
Published : 27 May 2020
DOI : https://doi.org/10.1186/s12889-020-08931-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Organ donation
- Willingness
- Medical students
BMC Public Health
ISSN: 1471-2458
- General enquiries: [email protected]
- Open access
- Published: 14 March 2014
Organ and tissue donation in clinical settings: a systematic review of the impact of interventions aimed at health professionals
- Frédéric Douville 1 ,
- Gaston Godin 2 &
- Lydi-Anne Vézina-Im 2
Transplantation Research volume 3 , Article number: 8 ( 2014 ) Cite this article
6258 Accesses
13 Citations
Metrics details
In countries where presumed consent for organ donation does not apply, health professionals (HP) are key players for identifying donors and obtaining their consent. This systematic review was designed to verify the efficacy of interventions aimed at HPs to promote organ and tissue donation in clinical settings. CINAHL (1982 to 2012), COCHRANE LIBRARY, EMBASE (1974 to 2012), MEDLINE (1966 to 2012), PsycINFO (1960 to 2012), and ProQuest Dissertations and Theses were searched for papers published in French or English until September 2012. Studies were considered if they met the following criteria: aimed at improving HPs’ practices regarding the donation process or at increasing donation rates; HPs working in clinical settings; and interventions with a control group or pre-post assessments. Intervention behavioral change techniques were analyzed using a validated taxonomy. A risk ratio was computed for each study having a control group. A total of 15 studies were identified, of which only 5 had a control group. Interventions were either educational, organizational or a combination of both, and had a weak theoretical basis. The most common behavior change technique was providing instruction. Two sets of interventions showed a significant risk ratio. However, most studies did not report the information needed to compute their efficacy. Therefore, interventions aimed at improving the donation process or at increasing donation rates should be based on sound theoretical frameworks. They would benefit from more rigorous evaluation methods to ensure good knowledge translation and appropriate organizational decisions to improve professional practices.
The number of patients awaiting organ or tissue transplantation continues to grow throughout the world [ 1 – 4 ]. The shortage of organ and tissue donors is widely studied and several factors explaining why individuals accept or refuse to consent to organ and tissue donation are reported in the literature [ 5 ]. Simpkin et al . [ 6 ] conducted a review of modifiable factors that influence relatives’ decisions to allow organ donation. This review suggests that the skills of individuals making the request to donate may have a significant impact on consent rates. Based on this information, evaluating the efficacy of interventions among HPs to increase donation seems relevant.
The donation process depends on potential donor identification and on HPs approaching families for donation consent. Since HPs are responsible for this approach to families, they are the gatekeepers for organ and tissue donor notification.
Consent to organ and tissue donation is the end point resulting from many actions undertaken by HPs (from identifying potential donors to referring donors to an organ and tissue procurement representative). In fact, many of these actions can be viewed as professional practices and as forms of human behavior. Thus, interventions should take advantage of behavioral theories and behavior change strategies in their design [ 7 – 11 ]. Past studies have demonstrated the importance of developing theory-based interventions in order to enhance their potential success in changing behavior [ 12 , 13 ]. The absence of theoretical bases for interventions and the selection of appropriate behavioral change techniques are two of the main problems in behavior change research projects [ 14 – 17 ]. Grimshaw et al . [ 15 ] suggest exploring the applicability of behavioral theories to the understanding of behavior change among HPs.
Several systematic reviews on organ donation have been published. These systematic reviews have cover different aspects of organ donation including the factors influencing families consent to donation [ 6 ], the attitude of the public towards living donors [ 18 ], the educational interventions offered in high schools [ 19 ], the management of donor brain death [ 20 ] and professional’s attitude regarding the heart-beating donors [ 21 ]. However, there is no systematic review on the efficacy of interventions among HPs to encourage them to approach families for consent or increasing donation rates. This is an important aspect of organ donation because donor identification and obtaining the consent of family are necessary conditions to the donation process.
This systematic review was designed to identify and analyze the impact of interventions aimed at HPs to improve donation-promoting professional practices in clinical settings. Secondary outcomes consisted of verifying whether such interventions were effective in improving donation rates and exploring associated behavior change strategies and the underlying theoretical framework.
Search strategy
The most relevant electronic databases covering the field of behavior change among HPs are those in health and psychology. CINAHL (1982–2012), COCHRANE LIBRARY (Cochrane Reviews, Other Reviews, Trials, Methods Studies, Technology Assessments, Economic Evaluations, Cochrane Groups), EMBASE (1974–2012), MEDLINE (1966–2012), PsycINFO (1960–2012), and ProQuest Dissertations and Theses were searched for papers published in French or English until September 2012.
The search strategy included the following concepts: 1) health professionals; 2) organ and tissue donation; and 3) interventions or strategies. This search strategy was adapted according to the terminology of the various databases. Moreover, bibliographies of potential studies were analyzed manually to find other key words relevant to the search strategy and studies not identified with the main search strategy. Only French and English papers were considered for review for practical reasons. The complete search strategy for each database is presented in Additional file 1 .
Study eligibility criteria
To be eligible for inclusion, studies had to adopt an experimental or quasi-experimental design reporting interventions aimed at HPs in clinical settings in order to improve their practices regarding the donation process or to increase the donation rates. They also had to report behavioral measures of the donation process or impact on organ and tissue donation rates as the study outcome.
In this study, HPs refer to professionals with medical training whose jobs require them to be in contact with patients and who are in a position to ask for donor consent. The concept of HP includes family physicians, specialist physicians, nurses or any other allied HPs who meet families in their daily practice. It also includes physicians in training (residents or interns), but excludes healthcare students and administrators not in contact with patients.
Also, the interventions had to be offered to HPs with the intention of modifying their practice regarding the donation process or at increasing donation rates. Such interventions could take the form of educational (for example, flyers, workshop, or lecture) [ 22 , 23 ], organizational (for example, hospital personnel structure change, or guidelines) [ 24 ], or regulatory strategies. These interventions or strategies were retained insofar as they were aimed at HPs caring for patients.
From a methodological point of view, the studies had to include a control group. However, to ensure that the study would not overlook relevant interventions that might have been effective, intervention studies without a control group, but with a pre-post analysis, were considered in a separate analysis.
Finally, to be included in the review, the intervention outcome had to be reported as a behavioral measure of the donation process (objective or self-reported), based on Kirkpatrick’s third level of program evaluation [ 25 ], or as the impact on organ and tissue donation rates. Behavioral measures could be a specific action (behavior) in the donation process, such as identifying a potential donor, approaching families to initiate discussion, obtaining signed consent for a donation or referring a potential donor to an organ and tissue donor representative. Articles reporting the impact on organ and tissue donation rates were considered even if the study did not assess behavioral outcomes to ensure comprehensiveness of the interventions reported in this review.
Studies that did not include HPs were excluded, as were those not directly aimed at changing HPs’ behavior, such as the implementation of an Organ Procurement Organization (OPO) coordinator in a hospital. Although one of the OPO’s duties involves identifying potential donors and approaching families to initiate donation discussion, their implementation could not be considered as an intervention intended for HPs (nurses and physicians) to modify their practices regarding the donation process; the latter would still have to notify the OPO and procurement organizations of potential organ and tissue donors.
Finally, studies concerning HPs’ reactions following an intervention or their level of knowledge following the intervention [ 25 ] were not considered if the assessed outcomes did not include the HPs’ behavior or donation rate.
Sorting of the studies by their titles and abstracts was first carried out by FD in order to select the articles meeting the inclusion criteria. Thereafter, the full text articles that met the inclusion criteria were screened independently by FD and LAVI, and decisions were compared.
Study quality assessment
Quality assessment of the studies was performed using criteria inspired by Morrison [ 26 ] and Reed [ 27 ], who recommend questions for appraising reports of medical education interventions.
Three criteria were selected to assess the population (randomized sample; justification of sample size and existence of a control group). Two criteria evaluated the intervention (allocation concealment and theory underlying the intervention). Two criteria appraised the assessment tool (validity and reliability). Finally, two criteria assessed the statistical approach used (intention-to-treat) and the level of attrition at follow-up.
No assessment for the risk of bias across studies was performed because the interventions had different objectives, populations and outcomes, making it impossible to obtain cumulative evidence.
Data extraction
A first coding was carried out on one study to verify if there was agreement on the extraction of data and to confirm the quality of the coding sheet. In case of disagreement between the two reviewers, the final decision was taken after discussion with a third reviewer (GG).
The following data were extracted from the selected studies: authors, year of publication, population under study and sample size. The study data were extracted according to the recommendations for evaluating educational interventions [ 26 , 27 ]. Thus, the reported variables were: objective of the study; intervention type (educational or organizational) and strategy; duration of follow-up; behavior change techniques; and study methodology, outcomes and results. The theory underlying each intervention was also extracted.
To help classify HPs’ strategies and relate those to the most recognized and effective theory-based strategies, behavior change techniques were analyzed using the taxonomy developed by Abraham and Michie as reference [ 11 ]. This taxonomy contains 26 behavior change techniques used in interventions based on behavior change theories such as the theory of reasoned action [ 28 ], the theory of planned behavior [ 29 ], the social cognitive theory [ 30 ], the information-motivation-behavioral skills models [ 31 ] and other behavior change theories.
Data analyses
Based on the studies retained, a descriptive analysis of selected studies (study objective; intervention type (educational or organizational) and strategy adopted; duration of the follow-up; behavior change techniques used; and study methodology, outcomes and results) was completed prior to identifying effective interventions. Interventions with a control group and interventions with a pre-post analysis are described separately.
A risk ratio was calculated for each outcome among the studies with a control group. The risk ratio was determined based on the number of participants in each group (experimental and control) and on the frequency of HPs’ behavior adoption. Thus, the analysis allowed the identification of significant differences between the two groups following the implementation of an intervention.
Review statistics
A total of 15 studies assessing interventions among HPs in clinical settings aimed at improving professional practices regarding the donation process or increasing donation rates were identified. The results of the search strategy are presented in Figure 1 . All studies included used educational, organizational or a combination of both types of interventions to promote professional practices regarding the donation process. These took the form of in-service meetings, workshops, conferences, print documents, examples provided of situations associated with the organ and tissue donation process and identification of donation criteria or information on how to approach a potential donor [ 23 , 32 , 33 ].
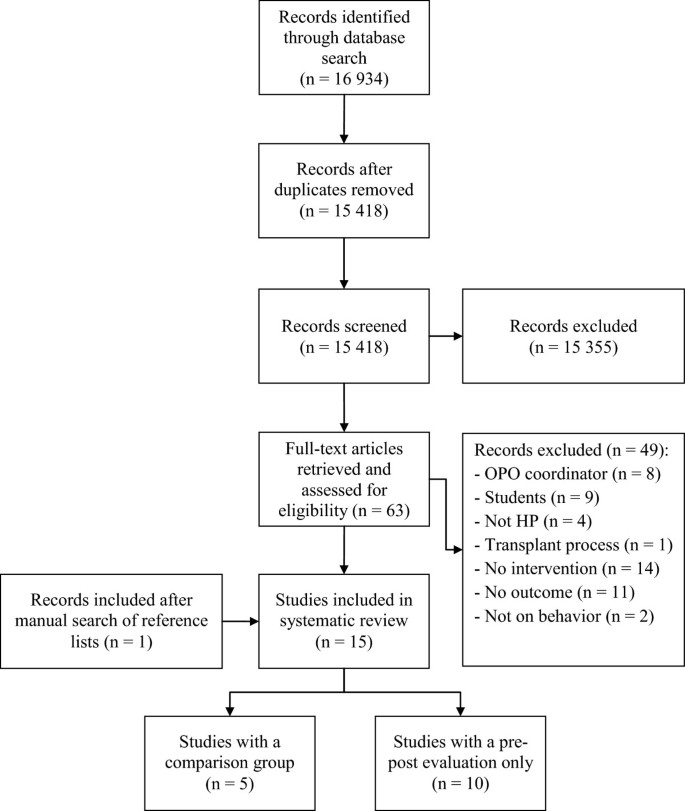
Flow chart diagram.
The 15 studies were assessed regarding population and the intervention assessment tool. In general, study quality was low. No study used a randomized population or justified their sample size. Only five studies used a control group. Allocation concealment of the intervention was neither relevant nor mentioned for all the studies included, and 14 of the 15 studies did not use a theory-based intervention. Where relevant, the validity and reliability of the assessment tools were not mentioned. Among the studies with a control group, there was no intention-to-treat analysis. Finally, the attrition rate was appropriately mentioned when required. The results of the quality assessment for the studies of the present review are available in Table 1 .
Efficacy of the interventions
Intervention studies with comparison groups.
Among the 15 studies included, only five had a comparison group (Table 2 ) [ 23 , 32 – 35 ]. The specific populations in these studies were nurses [ 32 – 34 ], physicians [ 23 , 33 , 34 ] and residents in medicine [ 35 ]. In addition to HPs, three studies also included other allied HPs such as chaplains or administrators [ 32 – 34 ]. All the studies used educational interventions to increase donation and one also used an organizational strategy. None of these interventions were based on a theoretical framework. According to the list of behavior change techniques [ 11 ], the majority of the strategies provided instruction on the donation process, the HPs’ role or how to cope with families’ reactions.
Relative risks (risk ratios) were computed to determine how likely participants were to adopt a behavior related to organ and tissue donation following an intervention, compared with those not exposed to the intervention (Table 3 ). Due to a high level of heterogeneity, the relative risks were calculated independently for each study and not pooled together.
The intervention studies of Nelson et al . [ 33 ] and Riker and White [ 23 ] showed significant relative risks for the following: approaching families [ 23 , 33 ], referring potential donors [ 33 ] and increasing donation rates [ 23 ]. However, the interventions of Dettle et al . [ 32 ], Light [ 35 ] and Riker and White [ 23 ] did not result in a significant increase in the number of signed consents for donation. No relative risk could be computed for the interventions of Kittur et al . [ 34 ], since the results were presented in absolute numbers instead of percentages, and there were no data on the total size of the groups.
Intervention studies without a comparison group (pre-post assessments)
The remaining ten studies used pre-post assessments (Table 4 ) [ 36 – 45 ]. These studies evaluated behavior change toward donation among HPs or the impact of their intervention on donation rates. The participants targeted in these interventions were mainly nurses and physicians. However, six of these studies involved hospital staff, without specifying which types of HPs were targeted [ 36 , 38 , 40 , 42 , 44 , 45 ]. Also, in six of the ten studies, the number of participants was not provided [ 36 , 37 , 41 – 44 ].
All the studies used educational strategies or a combination of organizational and educational strategies to promote donation behavior among HPs. In the study of Taylor et al . [ 41 ], there were references to the concept of change theory in the development of their intervention, but none of the other studies used a theoretical framework to guide the development of their intervention. The most common technique was to provide instruction on the donation process, the identification of donor criteria, the HPs’ role in the donation process and how to approach family members to initiate discussion.
This systematic review summarized the studies assessing educational and/or organizational interventions aimed at HPs to improve professional practices regarding the donation process or increase donation rates in clinical settings. A total of 15 studies were identified, among which only five had a comparison group. No study referred to a theoretical framework, either for the development of the interventions or their assessment. The behavior change technique most often used consisted of providing instruction on the donation process, including criteria and the role of HPs (how to approach family members, to initiate discussion or how to cope with families’ reactions).
Based on our review, the selected interventions aimed at changing HP practices regarding donation were developed, for the most part, more than a decade ago. Recent developments in donation emphasized the introduction of OPO representatives [ 46 , 47 ] and the regulation ensuring donation after death (such as presumed consent) [ 1 ]. If organ donation rates increased following the introduction of OPOs in clinical settings [ 46 , 47 ] or following a change in regulations [ 1 ], HPs still have to notify procurement organizations of any potential donors, leaving place for more research and interventions to help HPs in the donation process.
Impact on donation-promoting professional practices
Although there are many interventions aimed at changing HPs’ behavior toward the organ and tissue donation process in clinical settings, only a few were carried out exclusively among HPs whose job position requires them to be in contact with patients and who are in a position to ask for donation consent [ 23 , 32 ]. Indeed, most of the interventions also targeted hospital administrators, clerical staff and chaplains [ 32 – 34 , 37 ]. As such, it is difficult to isolate the impact of these interventions on nurses’ and physicians’ behavior.
The lack of studies assessing the behavior changes or health outcomes in this literature review is consistent with a recent publication that reviewed the evaluation of inter-professional education programs. According to Kirkpatrick’s levels, [ 25 ] only 9.7% of program evaluations assessed changes in behavior, 0.004% examined organizational practice changes and no items addressed benefits to patients [ 48 ]. Similar results were obtained in continuing nursing education programs [ 49 ].
Impact on donation rates
Interestingly, more than half of the studies included used an objective measure of the impact of the intervention on donation rates. This was achieved by extracting the information from medical records to evaluate the number of deaths (potential donors) and the number of actual donors [ 23 , 39 , 44 ]. This type of measure is obviously better than using self-reported behavior and provides more confidence in the observed effects.
Behavior change strategies and underlying theoretical framework
Surprisingly, in spite of the HPs’ role of gatekeeper in the donation process, there is a lack of sound theoretical interventions aimed at improving professional practices regarding the donation process or at increasing donation rates. None of the interventions were developed with reference to a behavior change theory, except the study by Taylor, Young and Kneteman [ 41 ], which mentioned the use of the concept of change theory, but without explaining how it was applied.
The fact that the interventions included in the present review had a poor theoretical basis and an inappropriate evaluation of their impact has important clinical implications. OPOs and donation stakeholders seem to apply nontheory-based intervention strategies without being sure of their efficacy. These interventions have an important cost for the healthcare system without resulting in significant changes (for example, increases in donation rates).
Quality of reviewed studies
The interventions presented several weaknesses in their evaluation designs. For instance, only five of the 15 studies identified used a comparison group to ensure that the intervention effects could be attributed to the implemented change strategy [ 23 , 32 – 35 ]. In addition, significant methodological flaws (for example, vague definition of the intervention, absence of a theoretical framework, lack of explanations on the study design, unjustified sample size) were noted.
Many of the studies included showed nonsignificant improvements in the detection of potential donors, approaching families and achieving consent or increasing donation rates in clinical settings [ 32 , 35 ]. Yet, some studies have proven that providing instruction on the donation process can significantly change HPs’ behavior over a period of 6 to 24 months [ 23 , 33 ]. However, it was not possible to establish whether an intervention was efficient due to methodological flaws, poorly described population or the lack of details on the content of the interventions and evaluation. Moreover, it was not possible to determine the efficacy of studies only using a pre-post evaluation because of the lack of a control group.
Limitations of the systematic review
The present review has some limitations. Only a small number of studies could be included in the analysis because most did not use a control group to compute a relative risk. Not all interventions reported the required information to compute relative risk (that is, number of participants in the experimental and the control groups). Moreover, the variability of the intervention strategies and the different HP practices on donation prevented the computation of some comparisons and the pooling of relative risks.
Conclusions
Despite the large number of publications on interventions to improve HPs’ practices regarding the donation process or increase donation rates, few of these interventions have been evaluated, or the associated assessments have methodological flaws that make it difficult to draw clear conclusions regarding their efficacy. Therefore, interventions aimed at improving the donation process or increasing donation rates should be based on sound theoretical frameworks and would benefit from more rigorous evaluation methods to ensure good knowledge translation and appropriate organizational decisions to improve professional practices.
Authors’ information
FD is a PhD candidate at the Faculty of Nursing at Laval University (Quebec City, Canada) and a clinical nurse specialist at the Institut de cardiologie et de pneumologie de Québec. GG is a professor at the Faculty of Nursing at Laval University. LAVI is a research professional at the Faculty of Nursing at Laval University.
Abbreviations
- health professional
organ procurement organization.
Rithalia A, McDaid C, Suekarran S, Myers L, Sowden A: Impact of presumed consent for organ donation on donation rates: a systematic review. BMJ. 2009, 338: a3162-10.1136/bmj.a3162.
Article PubMed Central PubMed Google Scholar
Donate Life America: National donor designation report card. [ http://donatelife.net/2013-national-donor-designation-report-card-released/ ]
Conseil Canadien pour le Don et la Transplantation: La demande d’allogreffes de tissus d’origine humaine Rapport final. [ http://www.organsandtissues.ca/s/english-expert/publications/leading-practice-reports ]
Institut canadien d’information sur la santé: Donneurs d’organes selon le type d’organe, au Canada, de 1998 à 2007. [ https://secure.cihi.ca/free_products/CORR_AiB_FR_20091222_rev20100106.pdf ]
Nijkamp MD, Hollestelle ML, Zeegers MP, van den Borne B, Reubsaet A: To be(come) or not to be(come) and organ donor, that’s the question: a meta-analysis of determinant and intervention studies. Health Psychol Rev. 2008, 2: 20-40. 10.1080/17437190802307971.
Article Google Scholar
Simpkin AL, Robertson LC, Barber VS, Young JD: Modifiable factors influencing relatives’ decision to offer organ donation: systematic review. BMJ. 2009, 338: b991-10.1136/bmj.b991.
Eccles M, Grimshaw J, Walker A, Johnston M, Pitts N: Changing the behavior of healthcare professionals: the use of theory in promoting the uptake of research findings. J Clin Epidemiol. 2005, 58: 107-112. 10.1016/j.jclinepi.2004.09.002.
Article PubMed Google Scholar
Green LW: From research to “best practices” in other settings and populations. Am J Health Behav. 2001, 25: 165-178. 10.5993/AJHB.25.3.2.
Article CAS PubMed Google Scholar
Perleth M, Jakubowski E, Busse R: What is ‘best practice’ in health care? State of the art and perspectives in improving the effectiveness and efficiency of the European health care systems. Health Policy. 2001, 56: 235-250. 10.1016/S0168-8510(00)00138-X.
Wensing M, van der Weijden T, Grol R: Implementing guidelines and innovations in general practice: which interventions are effective?. Br J Gen Pract. 1998, 48: 991-997.
PubMed Central CAS PubMed Google Scholar
Abraham C, Michie S: A taxonomy of behavior change techniques used in interventions. Health Psychol. 2008, 27: 379-387.
Baban A, Cranium C: Changing health-risk behaviors: a review of theory and evidence-based interventions in health psychology. J Cogn Behav Psychother. 2007, 7: 45-67.
Google Scholar
Webb TL, Sheeran P: Does changing behavioral intentions engender behavior change? A meta-analysis of experimental evidence. Psychol Bull. 2006, 132: 249-268.
Grimshaw J, Eccles M, Tetroe J: Implementing clinical guidelines: current evidence and future implications. J Contin Educ Health Prof. 2004, 24 (Suppl 1): S31-S37.
Grimshaw JM, Eccles MP, Walker AE, Thomas RE: Changing physicians’ behavior: what works and thoughts on getting more things to work. J Contin Educ Health Prof. 2002, 22: 237-243. 10.1002/chp.1340220408.
Michie S, Johnston M: Theories and techniques of behaviour change: Developing a cumulative science of behaviour change. Health Psychol Rev. 2012, 6: 1-6. 10.1080/17437199.2012.654964.
Glanz K, Bishop DB: The role of behavioral science theory in development and implementation of public health interventions. Annu Rev Public Health. 2010, 31: 399-418. 10.1146/annurev.publhealth.012809.103604.
Tong A, Chapman JR, Wong G, Josephson MA, Craig JC: Public awareness and attitudes to living organ donation: systematic review and integrative systhesis. Transplantation. 2013, 96: 429-437. 10.1097/TP.0b013e31829282ac.
Li AH, Rosenblum AM, Nevis IF, Garg AX: Adolescent classroom education on knowledge and attitudes about deceased organ donation: a systematic review. Pediatr Transplant. 2013, 17: 119-128. 10.1111/petr.12045.
Rech TH, Moraes RB, Crispim D, Czepielewski MA, Leitao CB: Management of the bain-dead organ donor: a systematic review and meta-analysis. Transplantation. 2013, 95: 966-974. 10.1097/TP.0b013e318283298e.
Bastami S, Matthes O, Krones T, Biller-Andorno N: Systematic review of attitudes toward donation after cardiac death among healthcare providers and the general public. Crit Care Med. 2013, 41: 897-905. 10.1097/CCM.0b013e31827585fe.
Blok GA, van Dalen J, Jager KJ, Ryan M, Wijnen RMH, Wight C, Morton JM, Morley M, Cohen B: The European Donor Hospital Education Programme [EDHEP]: Addressing the training needs of doctors and nurses who break bad news, care for the bereaved, and request donation. Transpl Int. 1999, 12: 161-167. 10.1111/j.1432-2277.1999.tb00601.x.
Riker RR, White BW: The effect of physician education on the rates of donation request and tissue donation. Transplantation. 1995, 59: 880-884. 10.1097/00007890-199503000-00014.
Shafer TJ, Wagner D, Chessare J, Zampiello FA, McBride V, Perdue J: Organ donation breakthrough collaborative: increasing organ donation through system redesign. Crit Care Nurse. 2006, 26: 33-42. 44–48; quiz 49
PubMed Google Scholar
Kirkpatrick D: Great ideas revisited. Techniques for evaluating training programs. Revisiting Kirkpatrick’s four-level model. Train Dev. 1996, 50: 54-59.
Morrison JM, Sullivan F, Murray E, Jolly B: Evidence-based education: development of an instrument to critically appraise reports of educational interventions. Med Educ. 1999, 33: 890-893. 10.1046/j.1365-2923.1999.00479.x.
Reed D, Price EG, Windish DM, Wright SM, Gozu A, Hsu EB, Beach MC, Kern D, Bass EB: Challenges in systematic reviews of educational intervention studies. Ann Intern Med. 2005, 142: 1080-1089. 10.7326/0003-4819-142-12_Part_2-200506211-00008.
Belief, attitude, intention and behavior: an introduction to theory and research. Edited by: Fishbein M, Ajzen I. 1975, Reading: Addison-Wesley
Ajzen I: The theory of planned behaviour. Organ Behav Hum Decis Process. 1991, 50: 179-211. 10.1016/0749-5978(91)90020-T.
Social foundations of thought and action: a social cognitive theory. Edited by: Bandura A. 1986, Englewood Cliffs: Prentice Hall
Fisher JD, Fisher WA: Changing AIDS-risk behaviour. Psychol Bull. 1992, 11: 455-474.
Dettle E, Sagel B, Chrysler G: Impact of traditional hospital development and education on the knowledge, attitudes, and comfort level of hospital staff toward tissue/organ donation. J Transpl Coord. 1994, 4: 38-43.
Nelson K, Marymont R, Durand R, Reyes D, Davis R: Evaluation of the impact of an OPO’s educational efforts… organ procurement organization. J Transpl Coord. 1992, 2: 117-121.
Kittur DS, McMenamin J, Knott D: Impact of an organ donor and tissue donor advocacy program on community hospitals. Am Surg. 1990, 56: 36-39.
CAS PubMed Google Scholar
Light DE: Cornea donation: increasing tissue supplies. South Med J. 1987, 80: 1542-1545. 10.1097/00007611-198712000-00014.
Alonso M, Fernandez M, Mataix R, Rincon MD, Corrales JA, Burgos R, Miranda B: Donor action in Spain: a program to increase organ donation. Transplant Proc. 1999, 31: 1084-1085. 10.1016/S0041-1345(98)01913-7.
Beasley CL, Capossela CL, Brigham LE, Gunderson S, Weber P, Gortmaker SL: The impact of a comprehensive, hospital-focused intervention to increase organ donation. J Transpl Coord. 1997, 7: 6-13.
Milanes CL, Gonzalez L, Hernandez E, Arminio A, Clesca P, Rivas-Vetencourt PA: Transplant coordination program: a useful tool to improve organ donation in Venezuela. Prog Transplant. 2003, 13: 296-298.
Niday P, Painter C, Peak J, Bennett E, Wiley M, McCartt L, Teixeira OHP: Family and staff responses to a scripted introduction to tissue donation for hospice inpatients on admission. Prog Transplant. 2007, 17: 289-294.
Shafer TJ, Durand R, Hueneke MJ, Wolff WS, Davis KD, Ehrle RN, van Buren CT, Orlowski JP, Reyes DH, Gruenenfelder RT, White CK: Texas non-donor-hospital project: a program to increase organ donation in community and rural hospitals. J Transpl Coord. 1998, 8: 146-152.
Taylor P, Young K, Kneteman N: Intensive care nurses’ participation in organ procurement: impact on organ donation rates. Transplant Proc. 1997, 29: 3646-3648. 10.1016/S0041-1345(97)01057-9.
Stark J, Wikoren B, Martone L: Partners in organ donation: piloting a successful nurse requestor program. Crit Care Nurs Clin North Am. 1994, 6: 591-598.
van Gelder F, van Hees D, de Roey J, Monbaliu D, Aerts R, Coosemans W, Daenen W, Pirenne J: Implementation of an intervention plan designed to optimize donor referral in a donor hospital network. Prog Transplant. 2006, 16: 46-51.
Wight C, Cohen B, Roels L, Miranda B: Donor action: A quality assurance program for intensive care units that increases organ donation. J Intensive Care Med. 2000, 15: 104-114. 10.1046/j.1525-1489.2000.00104.x.
Bleakley G: Implementing minimum notification criteria for organ donation in an acute hospital’s critical care units. Nurs Crit Care. 2010, 15: 185-191. 10.1111/j.1478-5153.2009.00385.x.
Shafer TJ, Kappel DF, Heinrichs DF: Strategies for success among OPOs: a study of three organ procurement organizations. J Transpl Coord. 1997, 7: 22-31.
Presnell SM: Organ procurement organizations: educational programs and social marketing. 2001, Tallahassee: Florida State University
Gillan C, Lovrics E, Halpern E, Wiljer D, Harnett N: The evaluation of learner outcomes in interprofessional continuing education: a literature review and an analysis of survey instruments. Med Teach. 2011, 33: e461-e470. 10.3109/0142159X.2011.587915.
Gijbels H, O’Connell R, Dalton-O’Connor C, O’Donovan M: A systematic review evaluating the impact of post-registration nursing and midwifery education on practice. Nurse Educ Pract. 2010, 10: 64-69. 10.1016/j.nepr.2009.03.011.
Download references
Acknowledgements
The authors thank Knowledge Translation Canada (KT Canada) for their financial support.
Author information
Authors and affiliations.
Institut universitaire de cardiologie et de pneumologie de Québec, 2725, chemin Sainte-Foy, Room Y-3495, Quebec, (Quebec), G1V 4G5, Canada
Frédéric Douville
Ferdinand-Vandry Building, Faculty of Nursing, Laval University, >1050, avenue de la médicine, Quebec, (Quebec), G1K 7P4, Canada
Gaston Godin & Lydi-Anne Vézina-Im
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Frédéric Douville .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors’ contributions
FD contributed substantially to developing and designing the study, acquiring data, analyzing and interpreting data and drafting the manuscript. GG contributed to developing and designing the study, interpreting data and drafting the manuscript. LAVI contributed to extracting data and drafting the manuscript. All authors have read and approved the final manuscript.
Electronic supplementary material
Additional file 1: search strategy for each database. (pdf 91 kb), authors’ original submitted files for images.
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Rights and permissions.
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited.
Reprints and permissions
About this article
Cite this article.
Douville, F., Godin, G. & Vézina-Im, LA. Organ and tissue donation in clinical settings: a systematic review of the impact of interventions aimed at health professionals. Transplant Res 3 , 8 (2014). https://doi.org/10.1186/2047-1440-3-8
Download citation
Received : 10 June 2013
Accepted : 25 February 2014
Published : 14 March 2014
DOI : https://doi.org/10.1186/2047-1440-3-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- tissue and organ procurement
- program development
- professional education
Transplantation Research
ISSN: 2047-1440
- General enquiries: [email protected]
Articles on Organ donation
Displaying 1 - 20 of 79 articles.

Lab-grown ‘ghost hearts’ work to solve organ transplant shortage by combining a cleaned-out pig heart with a patient’s own stem cells
Doris Taylor , University of New Hampshire

Is it legal to sell human remains?
Tanya D. Marsh , Wake Forest University

Human organs for transplant: 5 steps Africa must take to improve the supply chain
Temidayo Akenroye , University of Missouri-St. Louis ; Adegboyega Oyedijo , University of Leicester ; George Zsidisin , University of Missouri-St. Louis ; Jamal El Baz , Ibn Zohr University , and Marcia Mkansi , University of South Africa

People thinking of voluntary assisted dying may be able to donate their organs. We need to start talking about this
Robert Ray , Deakin University

Prisoners donating organs to get time off raises thorny ethical questions
Austin Sarat , Amherst College

Organ donation: whether we opt in or out, research finds it’s the will of our family that matters
Alberto Molina Pérez , Instituto de Estudios Sociales Avanzados (IESA - CSIC) ; David Rodríguez-Arias , Universidad de Granada , and Janet Delgado , Universidad de Granada

How do I donate my brain to science?
Greg Sutherland , University of Sydney

Killing prisoners for transplants: Forced organ harvesting in China
Ali Iqbal , McMaster University and Aliya Khan , McMaster University

Organs from genetically engineered pigs may help shorten the transplant wait list
David Kaczorowski , University of Pittsburgh

Hardly any South Africans are organ donors. What can be done to change this
Melodie Labuschaigne , University of South Africa

3D-printed organs could save lives by addressing the transplant shortage
Saman Naghieh , University of Saskatchewan

When is ‘dead’ really dead? What happens after a person ‘flatlines’
Amanda van Beinum , Carleton University and Sonny Dhanani , L’Université d’Ottawa/University of Ottawa

Opt-out organ donation: Is Nova Scotia’s new ‘deemed consent’ law ethical?
Marika Warren , Dalhousie University

An opt-out organ donor system could address Canada’s shortage of organs for transplant
Ajnesh Prasad , Royal Roads University and Karly Nygaard-Petersen , Royal Roads University

Organs ‘too risky’ to donate may be safer than we think. We crunched the numbers and here’s what we found
Karen Waller , University of Sydney and Angela Webster , University of Sydney

Organ transplants: why so many people are put off donating
Gabriel Moreno Esparza , Northumbria University, Newcastle and Stephen Clark , Northumbria University, Newcastle

Human-animal hybrids are coming and could be used to grow organs for transplant – a philosopher weighs in
Mackenzie Graham , University of Oxford

Heart transplant doctors could help more people by accepting donations from the obese
Leora Yarboro , University of Virginia and Elizabeth D. Krebs , University of Virginia

Trump’s order for more action on kidney disease may shrink organ transplant waitlists
Amit Tevar , University of Pittsburgh

Canada must end complicity in China’s brutal organ trafficking regime
Maria Cheung , University of Manitoba
Related Topics
- Human organs
- Kidney transplant
- Liver transplants
- Medical ethics
- Organ donation rates
- Organ transplantation
Top contributors
Senior Teaching Fellow, University of Warwick
Postdoctoral Research Fellow at the Centre for Values, Ethics and the Law in Medicine (VELiM), University of Sydney
Adjunct Fellow, T.C. Beirne School of Law, The University of Queensland
Professor in Clinical Ethics, Macquarie University
Associate professor, University of Canberra
Visiting Professor in Biomedical Ethics, Murdoch Children's Research Institute; Distinguished Visiting Professor in Law, University of Melbourne; Uehiro Chair in Practical Ethics, University of Oxford
Senior Lecturer in Medical Ethics and Law, St George's, University of London
Professor of Bioethics, University of Sydney
Associate Professor in Health Ethics and Professionalism, School of Medicine, Deakin University
Professor, Medical Ethics, Bond University
Bioethicist and Health Communication Specialist, University of the Witwatersrand
Honorary Associate at the Centre for Values, Ethics and the Law and Medicine, University of Sydney
Professor of End-of-Life Law and Regulation, Australian Centre for Health Law Research, Queensland University of Technology
Professor of Bioethics & Medicine, Sydney Health Ethics, Haematologist/BMT Physician, Royal North Shore Hospital and Director, Praxis Australia, University of Sydney
Associate Professor in Law, Deakin University
- X (Twitter)
- Unfollow topic Follow topic
- Português Br
- Journalist Pass
Mayo Clinic expert: 3 advances lead to more lifesaving organ transplants
Heather Carlson Kehren

Share this:

April is Donate Life Month
ROCHESTER, Minn. — All too often, people waiting for lifesaving organ transplants cannot get them. One of the biggest challenges is the lack of viable donated organs. Promising medical advances are opening the doors to more transplants and saving more lives, says Mauricio Villavicencio, M.D. , surgical director of heart and lung transplantation at Mayo Clinic in Rochester.
There are 104,000 people on the waiting list in the U.S. for a transplant. An estimated 17 people die on the waiting list die every day, according to Donate Life America.
"Heart failure is an epidemic in the U.S. and around the world. A heart transplant is the gold standard for treating advanced heart failure. But the number of people who die on the waiting list remains high. By taking advantage of these medical advances, we hope to change that," Dr. Villavicencio says. Thanks to these advances, the average number of heart and lung transplants at Mayo Clinic has grown from an average of 40 per year to 120 in 2022.
April is National Donate Life Month . For the 12 th consecutive year, deceased donations hit a record in the U.S. in 2022, according to the United Network for Organ Sharing . Here are three ways the organ donation pool is being expanded to help save more lives:
1. More donations after circulatory death:
Traditionally, organ donation has primarily come from donors who die from brain death while their hearts are still beating. Increasingly, more donated organs are coming from donors who die after their heart has stopped beating. In the past, hearts and lungs from such deaths usually went unused. Medical advances now allow transplant experts to use these organs. Transplant experts can resuscitate the heart on a heart-lung bypass machine or in an out-of-the-body perfusion device to become a donor. Approximately 20% to 30% of all organ donations come from such donors.
2. Organ-perfusion systems:
The creation of organ-perfusion systems, mechanical devices that help organs remain viable outside the body, has changed organ transplants. One example: "heart in a box" technology, a portable device that resuscitates a stopped heart and keeps it beating until it can be transplanted.
"Heart in a box allows for long-distance heart transplantation. When a heart is placed in cold storage, it must be transplanted within four hours. Heart in a box doubles that time at least to eight hours," Dr. Villavicencio says.
A similar organ-perfusion system available for lungs is called ex vivo lung perfusion . It preserves the donated lung in a machine outside the body. Lungs can also be restored to a condition suitable for transplant.
3. Organs from hepatitis C-positive donors:
Organs from hepatitis C -positive donors now can safely be transplanted to patients on the waiting list. This change is possible thanks to a new generation of highly effective antiviral medications. After the organs are transplanted, patients begin antiviral treatment that typically eliminates the virus from the body in seven days, Dr. Villavicencio says. In the past, these potential donor organs would have been wasted.
Dr. Villavicencio and several additional Mayo Clinic experts are available for interviews on this topic.
About Mayo Clinic Mayo Clinic is a nonprofit organization committed to innovation in clinical practice, education and research, and providing compassion, expertise and answers to everyone who needs healing. Visit the Mayo Clinic News Network for additional Mayo Clinic news.
Media contact:
- Heather Carlson Kehren, Mayo Clinic Communications, [email protected]
- New podcast: ‘Rapid Genome Sequencing’ Consider organ donation during Donate Life Month
Related Articles

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Organ transplantation articles from across Nature Portfolio
Organ transplantation is a procedure in which a functional, intact organ is transferred from one individual to another. The organ is then functional in the recipient. This type of transplantation can occur between individuals of the same species or between individuals of different species.
Latest Research and Reviews
FTO deficiency in older livers exacerbates ferroptosis during ischaemia/reperfusion injury by upregulating ACSL4 and TFRC
Transplanted older livers are prone to injury through unclear mechanisms, precluding effective treatment development. Here, the authors show that decreased FTO expression in older livers inhibits Acsl4 and Tfrc mRNA stability in an m6A-dependent manner, increasing cell death in older donor livers.
Translating B cell immunology to the treatment of antibody-mediated allograft rejection
Antibody-mediated rejection is a key mechanism in allograft loss. Here, the authors examine advances in B cell biology and how they can inform the development of new therapies to prevent or mitigate antibody-mediated rejection, with the goal of improving transplantation outcomes.
- Peter S. Heeger
- Maria Carrera Haro
- Stanley Jordan
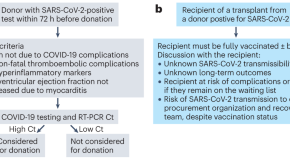
Heart transplantation: advances in expanding the donor pool and xenotransplantation
Heart transplantation for patients with advanced heart failure is limited by a shortage of donor organs. In this Review, Jou and colleagues explore the options to increase the supply of donor hearts, including transplantation from donors with HCV, HIV or SARS-CoV-2 infection, national opt-out organ donation policies, donation after circulatory death, and xenotransplantation.
- Stephanie Jou
- Sean R. Mendez
- Claudia Gidea
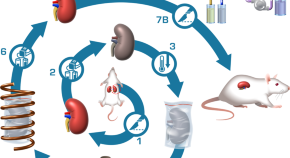
Vitrification and nanowarming enable long-term organ cryopreservation and life-sustaining kidney transplantation in a rat model
The possibility of banking cryopreserved organs could make transplantation medicine much more accessible. Here, the authors show that vitrification and nanowarming—cooling organs to an ice-free state followed by rapid rewarming using nanoparticles and magnetic fields—enables organ cryopreservation, long-term banking, and recovery of full function in a rat kidney transplant model.
- Joseph Sushil Rao
- Erik B. Finger
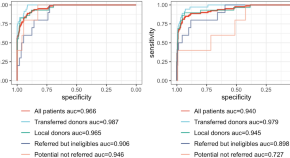
Automated screening of potential organ donors using a temporal machine learning model
- Nicolas Sauthier
- Rima Bouchakri
- Michaël Chassé
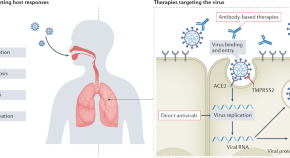
Therapeutic advances in COVID-19
The COVID-19 pandemic was met with large-scale efforts to assess novel and repurposed therapeutic interventions that could reduce patient morbidity and mortality. Here, the authors discuss the different types of therapies available to treat COVID-19, including their relevance to patients with kidney failure and kidney transplant recipients.
- Naoka Murakami
- Robert Hayden
- David E. Leaf
News and Comment
Xenotransplantation could either be a friend or foe of healthcare equity.
Chisholm-Burns et al. discuss the substantial shortage of organs available for transplantation, with disparities in access amongst some racial and ethnic groups. The authors suggest that while xenotransplantation can potentially increase organ availability, it also has the potential to further embed inequities in transplant care.
- Marie Chisholm-Burns
- Burnett S. Kelly
- Christina A. Spivey
Organ trafficking — a continuing challenge
Global inequities and inequalities, human and health-care crises, transplantation successes in the face of limited organ availability, and desperate donors and recipients underlie the backstory of organ trafficking, namely the exploitation of the most vulnerable. Despite the framework set out by the Declaration of Istanbul for the ethical donation and transplantation of organs, organ trafficking remains a global challenge.
- Thomas F. Mueller
- Sanjay Nagral
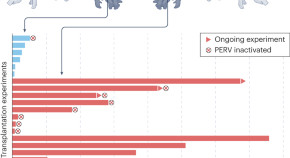
Kidney xenotransplantation edges closer to the clinic
The demand for kidney transplants is far from met by human donors — a problem that may be solved by the clinical translation of porcine kidney xenotransplantation. A new paper describes the development of genetically ‘humanized’ pigs, the kidneys of which kept nephrectomized cynomolgus macaques alive for up to 2 years.
- Eckhard Wolf
- Bruno Reichart
Taking kidney temperatures to detect rejection
- Monica Wang
Embryo complementation to generate a humanized mesonephros in pigs
- Susan J. Allison
Risk score for liver-related outcomes
- Eleni Kotsiliti
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Bureaus and Offices
- Contact HRSA
- For Workplaces
- Grants and Research
Research Reports
2019 national survey of organ donation attitudes and practices.
The 2019 National Survey of Organ Donation Attitudes and Practices measured public opinion about organ donation and transplantation. This survey was completed by 10,000 U.S. adults. Key findings include:
- People’s support for organ donation.
- If they have signed up to be an organ donor and where.
- Talking to family members about organ donation and their wish.
- Beliefs about organ donation and transplantation.
- If they want their organs used locally or wherever they are needed most.
- Where they got their information on organ donation in the past year.
View and download the 2019 National Survey report (PDF - 3 MB) .
Supplemental Tables
- Binary Response Tables (XLSX - 459 KB) – Frequency tables with data bars/color coding in the 2019 National Survey report.
- Supplemental Tables (XLSX - 196 KB) – Non-frequency tables in the 2019 National Survey report.
- Full Response Tables (XLSX - 510 KB) – Proportions and confidence intervals for every response option for all 86 key survey questions.
Listen to/watch a webinar recording on key findings of the survey. Slides from the webinar (PDF - 697 KB) are also available.
Note: If you are using assistive technology, you may not be able to fully see all of the information in this PDF file. The Excel file with it has the same information for the National Survey of Organ Donation Attitudes and Practices, 2019: Report of Findings. For help, please email [email protected] or call 301-443-3300.
2012 National Survey of Organ Donation Attitudes and Behaviors
This report details the findings of the 2012 survey of the American public’s attitudes and behaviors about organ donation.
View and download the 2012 National Survey of Organ Donation Attitudes and Behaviors (PDF - 1 MB) report.
Appointments at Mayo Clinic
- Consumer health
Organ donation: Don't let these myths confuse you
Unsure about donating organs for transplant? Don't let wrong ideas keep you from saving lives.
Connect with others
News, connections and conversations for your health
- Transplant Page - Connect with others Transplant Page
- Transplant Group - Connect with others Transplant Group
Answers to common organ donation questions and concerns.
More than 100,000 people in the U.S. are waiting for an organ transplant.
Sadly, many may never get the call saying that a donor organ has been found. Many may not get that second chance at life. Every day in the U.S., about 17 people die because there aren't enough donor organs for all who wait for a transplant.
It can be hard to think about dying. It can be even harder to think about donating organs and tissue. But organ donors save lives.
Here are answers to some common organ donation myths and concerns.
Myth: If I agree to donate my organs, the hospital staff won't work as hard to save my life.
Fact: When you go to the hospital for treatment, the health care team tries to save your life, not someone else's. You get the best care you can get.
Myth: Maybe I won't really be dead when they sign my death certificate.
Fact: This is a popular topic in tabloids. But in reality, people don't start to wiggle their toes after a health care provider says they're dead. In fact, people who have agreed to organ donation are given more tests to make sure they're dead than are those who aren't donating organs. These tests are done at no charge to their families.
Myth: Organ donation is against my faith.
Fact: Most major faiths accept organ donation. These include Catholicism, Islam, Buddhism, most branches of Judaism and most Protestant faiths. Some religions believe organ donation to be an act of charity. If you don't know where your faith stands on organ donation, ask a member of your clergy.
Myth: I'm younger than 18. I'm too young to make this decision.
Fact: Many states let people younger than 18 register as organ donors. But if you die before your 18th birthday, your parents or legal guardian will make the decision. If you want to be an organ donor, make sure your family is OK with your wishes. Remember, children, too, need organ transplants. They often need organs smaller than adult size.
Myth: People who donate organs or tissues can't have an open-casket funeral.
Fact: Donors' bodies are treated with care and respect. And they're dressed for burial. No one can see that they donated organs or tissues.
Myth: I'm too old to donate. Nobody wants my organs.
Fact: There's no standard cutoff age for donating organs. The decision to use your organs is based on the health of your organs, not age. Let the health care team decide at the time of your death whether your organs and tissues can be transplanted.
Myth: I'm not in the best health. Nobody wants my organs or tissues.
Fact: Very few medical conditions keep you from donating organs. Maybe you can't donate some organs, but other organs and tissues are fine. Again, let the health care team decide at the time of your death whether your organs and tissues can be transplanted.
Myth: I'd like to donate one of my kidneys now. Can I do that if it's not going to a family member?
Fact: Yes. Most living donations are between family members and friends. But you can choose to donate a kidney to a stranger, so long as you're a match. You also can donate other organs and tissues, such as a lung or part of a lung or liver.
If you decide to become a living donor, the health care team at the transplant center asks a lot of questions. They want to make sure you know the risks.
You'll have tests to make sure you're healthy and that the organ you want to donate is in good shape. The health care team also will want to be as sure as possible that the donation won't damage your health.
Myth: Rich and famous people go to the top of the list when they need a donor organ.
Fact: The rich and famous are treated the same as everyone else when it comes to organ donation. True, famous people might get a lot of press after a transplant. But who they are and how much money they have don't help them get an organ. A computer system and strict standards ensure fairness.
Myth: My family will be charged if I donate my organs.
Fact: The organ donor's family never pays for donation. The donor family pays for all the medical care given to save your life before your organs are donated. Sometimes families think those costs are for the organ donation. But the person who gets the organs for transplant pays the costs for removing the organs.
Why you should think about donating organs
Now that you have the facts, you can see that being an organ donor can have a big impact. And your donation helps not just the person getting the organ. By donating your organs and tissue after you die, you can save up to eight lives and improve 75 more. Many families say that knowing their loved one helped others helped them cope with their loss.
Think about being an organ donor if you belong to an ethnic minority group. These include Black Americans, Asian Americans and Pacific Islanders, Native Americans, and Hispanics. People in these groups are more likely than white people to have certain illnesses that affect the kidneys, heart, lung, pancreas and liver.
Some blood types are more common among minority groups. The blood type of the donor usually needs to match the blood type of the person getting an organ. So the need for minority donor organs is high.
How to donate
Becoming an organ donor is easy. Just do the following:
- Sign up with your state's donor registry. Most states have ways to sign up. Check the list at organdonor.gov.
- Mark your choice on your driver's license. Do this when you get or renew your license.
- Tell your family. Make sure your family knows you want to be an organ donor.
Being on your state's organ donation registry and marking your choice on your driver's license or state ID are the best ways to make sure you become a donor. But telling your family also is important because hospitals ask next of kin before taking organs.
However, hospitals don't need to ask for consent if you are 18 or older and are on your state's donor registry or have marked your driver's license or state ID card for organ donation.
If you have named someone to decide about your health care for you if you are not able to do so, make sure that person knows that you want to be an organ donor. You also can include your wishes in a living will if you have one. But the will might not be read right at the time of your death.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
- Organ donation statistics. Organdonor.gov. https://www.organdonor.gov/learn/organ-donation-statistics. Accessed Dec. 29, 2022.
- Franklin GF, et al. Evaluation of the potential deceased organ donor (adult). https://www.uptodate.com/contents/search. Accessed Dec. 29, 2022.
- Theological perspective on organ and tissue donation. United Network for Organ Sharing. https://unos.org/transplant/facts/theological-perspective-on-organ-and-tissue-donation/. Accessed Dec. 29, 2022.
- Equity access to transplant. United Network for Organ Sharing. https://insights.unos.org/equity-in-access/. Accessed Dec. 29, 2022.
- Frequently asked questions about organ donation for older adults. National Institute on Aging. https://www.nia.nih.gov/health/frequently-asked-questions-about-organ-donation-older-adults. Accessed Dec. 29, 2022.
- Facts about organ donation. United Network for Organ Sharing. https://unos.org/transplant/facts/. Accessed Dec. 29, 2022.
- Donate organs while alive. Organdonor.gov. https://www.organdonor.gov/learn/process/living-donation. Accessed Dec. 29, 2022.
Products and Services
- Newsletter: Mayo Clinic Health Letter — Digital Edition
- A Book: Mayo Clinic Book of Home Remedies
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Assortment of Health Products from Mayo Clinic Store
- The Mayo Clinic Diet Online
- A Book: The Mayo Clinic Diet Bundle
- A Book: Live Younger Longer
- A Book: Mayo Clinic on Digestive Health
- Myths about cancer causes
- Emergency essentials: Putting together a survival kit
- Emergency health information
- Kidney donation: Are there long-term risks?
- Living wills
- Osteopathic medicine
- Personal health records
- Telehealth: Technology meets health care
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
- Healthy Lifestyle
- Organ donation Dont let these myths confuse you
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
- Open access
- Published: 31 May 2024
Bridging the gap between in vitro and in vivo models: a way forward to clinical translation of mitochondrial transplantation in acute disease states
- David F. Bodenstein 1 , 10 na1 ,
- Gabriel Siebiger 2 , 3 , 10 na1 ,
- Yimu Zhao 4 , 10 na2 ,
- Aaron J. Clasky 5 , 10 na2 ,
- Avinash N. Mukkala 2 , 6 , 10 na2 ,
- Erika L. Beroncal 1 , 10 na2 ,
- Lauren Banh 4 , 7 , 8 na2 ,
- Lili Aslostovar 9 ,
- Sonya Brijbassi 10 ,
- Sarah E. Hogan 11 ,
- James D. McCully 12 , 13 ,
- Mohadeseh Mehrabian 9 ,
- Thomas H. Petersen 11 ,
- Lisa A. Robinson 14 ,
- Melanie Walker 15 ,
- Constantine Zachos 10 ,
- MITO2i-MbD Mitochondrial Transplant Consortium ,
- Sowmya Viswanathan 4 , 7 , 10 ,
- Frank X. Gu 4 , 5 , 10 , 16 ,
- Ori D. Rotstein 10 , 17 , 18 ,
- Marcelo Cypel 3 , 10 , 19 ,
- Milica Radisic 4 , 5 , 10 , 16 , 20 , 21 &
- Ana C. Andreazza ORCID: orcid.org/0000-0002-4323-7273 1 , 10 , 22
Stem Cell Research & Therapy volume 15 , Article number: 157 ( 2024 ) Cite this article
390 Accesses
1 Altmetric
Metrics details
Mitochondrial transplantation and transfer are being explored as therapeutic options in acute and chronic diseases to restore cellular function in injured tissues. To limit potential immune responses and rejection of donor mitochondria, current clinical applications have focused on delivery of autologous mitochondria. We recently convened a Mitochondrial Transplant Convergent Working Group (CWG), to explore three key issues that limit clinical translation: (1) storage of mitochondria, (2) biomaterials to enhance mitochondrial uptake, and (3) dynamic models to mimic the complex recipient tissue environment. In this review, we present a summary of CWG conclusions related to these three issues and provide an overview of pre-clinical studies aimed at building a more robust toolkit for translational trials.
Mitochondria are compartments within cells that regulate and mediate key biochemical pathways essential for many aspects of cellular function and biology. Additionally, mitochondrial dysfunction is known to contribute to a variety of diseases, ranging from primary mitochondrial disorders and chronic pathologies such as diabetes, heart failure, and Alzheimer’s disease, to acute conditions associated with ischemia–reperfusion injury (IRI), such as acute coronary syndrome, stroke, and pulmonary embolism [ 1 ]. All of these can benefit from cellular therapies as new and novel ways of treating disease. The mitochondrial innovation initiative (MITO2i) and Medicine by Design (MbD), two strategic initiatives of the University of Toronto, have brought together a world-renowned group of scientists and clinicians to create the Mitochondrial Transplant Convergent Working Group (CWG). This group came together with members of the University of Toronto and affiliated research hospitals, as well as external collaborators and partners, to discuss feasibility and techniques that can advance mitochondrial transplantation for successful applications in acute and chronic disease and regenerative medicine. In this first manuscript in a series of papers, we will focus on an overview of mitochondrial transplantation, explore biomaterials to enhance storage and delivery of mitochondria for mitochondrial transplantation for acute diseases, and propose approaches to bridge the gap between in vitro and in vivo models to help advance the clinical translation of mitochondrial transplant.
To accomplish these goals, we propose to further existing collaborations and to develop plan-to-action to accelerate the implementation of clinical mitochondrial transplant as a therapy of tissue regeneration for disease treatment. Mitochondrial transplantation is defined as the addition of live and healthy mitochondria retrieved from unaffected tissue or cellular models, like induced pluripotent stem cells (iPSCs), into target organs or tissues subjected to or prone to injury. Members of the CWG have expertise in mitochondrial function and stability, iPSC development, biomaterials for cell delivery, and clinical applications in regenerative medicine. The team includes researchers, clinicians, foundations, patients, patients’ families and caregivers, and industry partners—which together have a common objective of advancing mitochondrial related therapies to treat and cure disease.
Overview of mitochondrial isolation, transplant, and biomaterials
Mitochondria are ubiquitous across cell types and serve an important role in the regulation and maintenance of homeostasis. Mitochondria differ from other organelles as they contain their own mitochondrial DNA (mtDNA) that encodes 37 genes, specifically 22 tRNAs, 2 rRNAs, and 13 protein subunits of the electron transport chain (ETC) [ 2 ]. The remaining mitochondrial proteins are encoded in the nuclear DNA (nDNA) and imported into the mitochondria through recognition of a mitochondrial targeting sequence [ 3 ]. This unique crosstalk of mtDNA and nDNA is further complicated by different mitochondrial haplogroups, which play a crucial role to ensure compatibility between the two genomes [ 4 ]. This is especially important in the development of heterologous mitochondrial transplantation and transfer.
Mitochondrial transplant and transfer are being explored clinically as a novel therapeutic approach in both acute and chronic diseases. Autologous mitochondrial transplant is being evaluated in acute diseases. It has been widely studied in animal models of ischemia–reperfusion injury, but also in children with congenital heart failure [ 5 ] and adult patients with cerebral ischemia (NCT04998357, currently recruiting patients) [ 6 ], as reviewed in D’Amato et al. [ 1 ]. In both studies, healthy mitochondria are isolated from non-ischemic skeletal muscle at the surgical access site and delivered either via direct injection or through intravascular infusion to the target site. Emani et al. [ 5 ] delivered 10 injections of 1 × 10 7 mitochondria into the affected myocardium of pediatric patients with acute cardiogenic shock requiring extracorporeal membrane oxygenation (ECMO) support following cardiac surgery. Following mitochondrial transplant, four of five patients were successfully removed from ECMO with the fifth patient unable to be decannulated [ 5 ]. Of the four patients removed from ECMO support, one patient died later at 4 months old due to respiratory failure [ 5 ]. However, the exact therapeutic mechanism is not currently known and is a current limitation in the clinical advancement of mitochondrial transplant. Studies by Kesner et al. [ 7 ] and Pacak et al. [ 8 ] indicate that mitochondria are engulfed by endocytosis or macropinocytosis depending on the recipient cell type. Additionally, once the mitochondria are engulfed it is unknown how the mitochondria escape the endosomes to integrate into the host mitochondrial network. Cloer et al. [ 9 ] proposed that mitochondrial transplant upregulates autophagy, which reduces pro-inflammatory signalling and reactive oxygen species as well as providing additional metabolites for energy production. Notably, this is similar to the endogenous response of stressed or injured cells to release damaged mitochondria for degradation by surrounding cells, known as transmitophagy [ 10 ], to initiate mitochondrial biogenesis, immune and tissue repair responses by macrophages, and transfer of healthy mitochondria to restore cellular homeostasis [ 11 , 12 ].
Mitochondrial transfer-based therapies are also being developed by Minovia Therapeutics Ltd. and IMEL Biotherapeutics Inc. for treatment of chronic diseases, such as primary mitochondrial disease [ 13 , 14 ]. These methods differ from mitochondrial transplant as they rely on the ex vivo uptake of isolated mitochondria followed by intercellular transfer of healthy donor mitochondria into the target tissue. Briefly, hematopoietic stem and progenitor cells (HSPC) are isolated from patients and enriched with isolated healthy donor mitochondria [ 13 , 14 ]. The enriched HSPC are then reinfused into the patient, after which donor mitochondria are transferred from the enriched HSPC to the target tissue through tunnelling nanotubules and extracellular vesicles to restore mitochondrial function [ 13 , 14 ]. However, in acute diseases, such as those associated with IRI, where treatment must be delivered rapidly, this technique may not be applicable due to the time to isolate, enrich, and reinfuse autologous HSPCs, as well as to allow initiation of intercellular transfer of mitochondria to the target tissue. The direct transplantation of autologous mitochondria, however, ensures treatment can be delivered both effectively and rapidly to the target organ. Although it is important to note that there are several endogenous pathways to restore mitochondrial function and health following injury by intercellular transfer of mitochondria from mesenchymal stromal cells (MSC) [ 15 , 16 ]. Mitochondrial transfer has also been suggested as a central mediator of the therapeutic efficacy of MSC-based therapies, as reviewed in Mukkala et al. [ 16 ].
Whether using mitochondrial transfer or transplant techniques, it is essential to first isolate pure and functional mitochondria from either tissue or cellular sources. There are several methods to isolate mitochondria, specifically ultracentrifugation, differential centrifugation, differential filtration, and kit-based methods. Ultracentrifugation and differential centrifugation both rely on multiple centrifugation steps to remove cellular debris and purify mitochondria usually through low- and high-speed spins [ 17 ]. Ultracentrifugation-based isolation methods can also be paired with a density-gradient to further separate non-synaptic and synaptic mitochondria from brain homogenates [ 17 ]. However, functional assays of the isolated mitochondria are hindered due to the long isolation times of both methods. Preble et al. [ 18 ] developed a differential filtration method which removes the need for multiple centrifugation steps, reducing the isolation time to approximately 30 min. Briefly, cell homogenate is filtered through 40, 10, and 5 μm filters to gradually remove cellular debris and organelles, followed by a final high-speed centrifugation to concentrate the isolated mitochondria [ 18 ]. Magnetic bead-based isolation methods have been commercialized by Miltenyi Biotec; however, these are only applicable in the laboratory setting (Catalogue No: 130-094-532, Miltenyi Biotec). Current clinical studies in mitochondrial transplant for pediatric post-cardiotomy cardiogenic shock and cerebral ischemia use differential filtration to isolate pure and functional mitochondria prior to transplantation [ 5 , 6 ].
Biomaterials to enhance donor mitochondrial uptake and integration
Biomaterials such as polymers and lipids have recently been investigated to facilitate the delivery of isolated mitochondria to tissues of interest (Table 1 ). These materials are commonly used to improve the bioavailability of various payloads, including small molecule drugs and proteins, in different configurations such as nanoparticles [ 19 ] and hydrogels [ 20 ], and have been tested rigorously for biocompatibility and efficacy in animal models. Numerous formulations are currently moving towards human clinical trials [ 21 ] and many of the materials mentioned in this section have FDA approval for at least one application, including: Pluronic F-127 [ 22 ], hyaluronic acid [ 23 ], poly(ethylene glycol) [ 24 ], chitosan [ 25 ] and methylcellulose [ 26 ].
In the context of mitochondrial transplantation, biomaterials have been shown to improve the stability of mitochondria in solution and to improve cellular internalization to ameliorate therapeutic outcomes. Hydrophilic cationic polymers and/or lipophilic moieties are normally used to maximize interactions with both the negatively charged mitochondrial surface and membranes of the target cells [ 27 ]. Triphenylphosphonium-conjugated Dextran (Dextran-TPP) has emerged as a common coating material for mitochondria [ 28 , 29 , 30 ]. Dextran is a polysaccharide that has been shown to improve uptake of nanomaterials in vivo and TPP is a cationic, lipophilic ligand that readily associates with mitochondrial membranes and can be easily conjugated to the Dextran backbone [ 28 , 31 ]. Dextran-TPP improves the cellular uptake of coated mitochondria post-transplant, likely due in part to the change in surface properties (-44 mV surface charge for bare mitochondria compared to just -4 mV for Dextran-TPP-coated mitochondria) [ 28 ]. This improved cellular uptake and transplantation efficiency also enables similar outcomes to be achieved with much lower dosages: for example, nasally administered Dextran-TPP mitochondria reversed chemotherapy (cisplatin)-induced cognitive defects and resolved neuropathic pain at a dose 55-times lower compared to bare mitochondria [ 30 ]. Literature also suggests that the Dextran-TPP coating induces a state of metabolic dormancy in the mitochondria (based on a reduced respiratory control ratio and reduced LEAK state), likely by restricting entry of substrate into the mitochondria [ 28 ]. Since metabolic activity is fully recovered following transplantation of the coated mitochondria, the dormant effect may potentially be used to preserve the isolated mitochondria for longer periods of time. It is currently unclear whether this metabolic dormancy has been observed in other coating systems and to what extent it influences the preservation and long-term viability of isolated mitochondria. Future work on polymer-mitochondria systems may look to consider this effect in more detail.
Different polymers have also been investigated for mitochondrial delivery. Pluronic F127, an amphiphilic block copolymer of poly(ethylene oxide) 98 -poly(propylene oxide) 67 -poly(ethylene oxide) 98 (PEO 98 -PPO 67 -PEO 98 ), was used to coat mitochondria for treatment of myocardial IRI [ 32 ]. The coating was shown to improve membrane integrity in a high-Ca 2+ simulated transplant environment and improve cellular uptake. Similarly, a combination of hyaluronic acid (HA) and methylcellulose (MC) was used to produce a thermogelling hydrogel to deliver mitochondria for spinal cord injury treatment [ 33 ]. It should be noted, however, that this latter system is composed of a hydrogel containing mitochondria rather than a coating for individual mitochondria, although there is still an improvement in viability (oxygen consumption rate (OCR) over time) and uptake, with the added potential benefit of prolonged mitochondrial release compared to injection. More complex approaches include a layer-by-layer strategy which leveraged the opposing charges of chitosan (positive) and poly(acrylic acid) and the mitochondrial membrane (negative) to produce a tunable multilayered coating to combat multidrug resistance in cancer treatment [ 34 ]. In addition to overcoming issues with electrostatic repulsion, the layer-by-layer method also embedded siRNA for P-glycoprotein to overcome drug resistance through knockdown of drug transporters [ 34 ]. The intracellular ion-rich environments trigger dissociation of the electrostatic layers, thus releasing siRNA and intact mitochondria at the site of action for synergistic treatment. In addition to polymer systems, lipid bilayers consisting of cationic dioleoyl-3-trimethylammonium propane (DOTAP) and 1,2-dioleoyl- sn -glycero-3-phosphoethanolamine (DOPE) were formed around isolated mitochondria using an inverse emulsion method to produce “artificial membranes” [ 35 ]. These lipid bilayer coatings improved neuroprotection in cultured neurons and amplified cerebroprotection in-vivo after focal cerebral IRI in mice compared to uncoated mitochondria [ 35 ].
In addition to supporting mitochondrial transplantation, biomaterial coatings can also confer additional capabilities to the mitochondria. Janus-like coatings were produced via immobilization of mitochondria on a glass slide with one side containing a deposition of alternating layers of chitosan and PAA with a covalently grafted poly(ethylene glycol) (PEG) brush, and the other side containing glucose oxidase (GOx) [ 36 ]. The one-sided GOx enables the mitochondria to follow the glucose gradient within a tumour via chemotaxis and penetrate much deeper compared to either uncoated or fully GOx-coated mitochondria. This strategy resulted in significantly more accumulation in cultured mammospheres and longer retention time in vivo compared to controls. Mitochondria can also be functionalized with lipid-modified photosensitizers to combine mitochondrial transplantation with photodynamic therapy for cancer treatment [ 37 , 38 ]. The potential of biomaterials to accelerate the utility of mitochondrial transplantation is significant: from synergistic combinations with excipient drugs to stimulus-responsive release and activation strategies.
A limitation with current mitochondrial transplant methods is the short lifespan of isolated mitochondria, which significantly lose respiratory function after approximately 2 h [ 9 , 39 ]. It is essential to use viable and functional mitochondria during transplantation, as non-viable or damaged mitochondria may release damage associated molecular patterns (DAMPs) leading to immune activation [ 40 ]. Furthermore, non-viable mitochondria were found to have no cardiac protection in rabbits following 30 min of regional ischemia [ 41 ]. Therefore, with current approaches, this means that mitochondrial isolations must ideally be performed at the bedside prior to transplantation to minimize decreases in function/viability of isolated mitochondria, thereby optimizing function. Yamaguchi et al. [ 42 ] developed a method to store isolated mitochondria in a modified buffer containing trehalose as a cryoprotectant. Mitochondria stored in this solution retained mitochondrial outer membrane integrity, response to Bcl-2 family proteins, calcium-induced swelling, ATP synthesis, transmembrane potential (MP), and transmembrane mitochondrial transport similarly to freshly-isolated mitochondria in isolated mitochondria from mouse liver [ 42 ]. Bioenergetic function, when analyzed in terms of both phosphorylating state [ 3 ] and maximally uncoupled respiration, however, was shown to be decreased as compared to freshly isolated mitochondria, whereas activity of cytochrome C oxidase was intact. The authors’ conclusion was that the solution was capable of partially preserving mitochondrial function, to the extent of retaining MP and MP-dependent functions, including ATP synthesis, protein import and calcium accumulation leading to opening of the mitochondrial permeability transition pore (mPTP).
By the analysis of Cloer et al. [ 9 ], trehalose-frozen mitochondria obtained by a manual homogenization, centrifugation-based isolation method retained equivalent ultrastructural morphology, size, complexity, membrane potential, permeability, and basal and maximal respiration to freshly isolated mitochondria. Acute antioxidant functionality was maintained in frozen compared to freshly isolated mitochondria but an in vivo comparison between the storage conditions was not reported. Following a similar protocol, Cloer et al. [ 9 ] observed no changes in functional or morphological assessments in isolated mitochondria stored at – 80 °C for up to 12 months. The ability to isolate and store mitochondria at – 80 °C would allow users to perform complete quality control assessments on isolated mitochondria prior to transplantation in injury models and predictable ischemic events. This would ensure mitochondrial transplants are performed with high purity and functionally viable mitochondria to reduce the risk of adverse effects and improve patient safety. Frozen mitochondria retained functional benefits post-mitochondrial transplant in porcine lungs [ 9 ].
Functional and safety checkpoints of mitochondrial isolation and transplant
After isolating and transplanting mitochondria, purity and function in the recipient tissue/cell can be evaluated using mtDNA content, western blot, ATP production, and OCR. mtDNA content is measured by qPCR using mtDNA and nDNA specific primers, allowing researchers to determine the number of copies of mtDNA per nDNA [ 43 , 44 ]. In whole cells or tissue, this provides insight into mitochondrial biogenesis, which is an important part of the mitochondrial stress response pathway, along with mitochondrial fission, fusion, and mitophagy to maintain mtDNA integrity [ 45 , 46 ]. Following ischemic injury, these pathways will be activated to remove damaged mitochondria and induce mitochondrial biogenesis to restore and maintain mitochondrial function [ 46 ]. Alternatively, in isolated mitochondria, mtDNA content allows researchers to assess mitochondrial isolation purity as minimal nDNA should be amplified. Mitochondrial purity can also be assessed using western blot and flow cytometry for common organelle contaminants, such as endoplasmic reticulum, peroxisome, and lysosome [ 47 , 48 ]. It is essential to ensure maximal mitochondrial isolation purity as any nDNA or other organelle contamination can lead to adverse immune activation during mitochondrial transplantation [ 47 ]. Kit-based ATP production assays and OCR are useful tools for evaluating mitochondrial function post-isolation and treatment efficacy post-mitochondrial transplant [ 47 ].
It is necessary to assess the safety profile of the mitochondrial transplant by measuring immune response, cellular viability, and mitochondrial function. Damaged mitochondria release mtDNA and cytochrome c, which are recognized as DAMPs, activate the NLRP3-inflammasome and apoptosis [ 40 ]. Common assays such as MTT, picogreen, DCFH-DA, and Griess assay are easily performed to assess cellular viability, double strand DNA (dsDNA) release, and reactive oxygen/nitrogen species (ROS, RNS) formation, respectively [ 49 ]. Furthermore, IL-1β and caspase-1 can also be assessed at the same time to evaluate the activation of the NLRP3-inflammasome signaling cascade [ 40 ]. This workflow is ideal as the mitochondrial transplant can be performed in a well-plate format for MTT assay and the cell media can be collected to perform all other assays, allowing the results to be normalized to cellular viability [ 49 ].
The ideal mitochondria dosage and metabolic profile must also be carefully considered in ongoing in vitro and in vivo studies. Zhang et al. [ 50 ] demonstrated that cellular engulfment of exogenous mitochondria is unchanged with increasing mitochondrial concentrations. Similarly, Shin et al. [ 51 ] found that delivery of mitochondria above 2 × 10 6 mitochondria/gram of wet tissue resulted in no greater therapeutic efficacy or cardioprotective effect in porcine models of myocardial ischemia–reperfusion injury. Furthermore, Zhang et al . [ 50 ] also identified the importance of metabolically matching donor mitochondria to the recipient tissue to ensure maximal therapeutic efficacy. Specifically, mitochondrial transplant of metabolically matched mitochondria into neonatal mouse cardiomyocytes was necessary to restore maximal OCR and contractility following doxorubicin-induced myocardial dysfunction. Therefore, in future studies it is important to consider both the mitochondrial concentration to ensure the recipient tissue is not overwhelmed with donor mitochondria leading to adverse effects and the metabolic matching of donor and recipient mitochondria for maximal therapeutic efficacy.
Depending on the method of administration, the coating and delivery of transplanted mitochondria may be complicated by the presence of endogenous proteins and other components in the body. Many of the materials used to coat mitochondria are common in nanomedicine formulations and their interactions with proteins in complex biological media are the subject of significant interest [ 52 , 53 , 54 ]. The layers of proteins formed on the surface of a nanomaterial are referred to as the “protein corona” and may be influenced by both the biological medium and the outer coating material [ 55 , 56 ]. In addition to changing the outer surface of the (coated) mitochondria, protein adsorption may interfere with cellular uptake and potentially trigger immune responses [ 57 ]. Further development of biomaterial-coated mitochondria also requires consideration of their interactions with other components in complex biological media and how these interactions may influence any therapeutic outcomes.
Compatibilities between the donor and recipient are important considerations to avoid immune complications or reactivities. mtDNA-nDNA crosstalk and energy metabolism compatibilities through mitochondrial haplogroups are important to be considered, especially in mitochondrial transplantation, as several studies reveal the importance of mitochondrial function in reducing post-graft dysfunction and improving transplant success [ 58 , 59 ]. mtDNA haplogroup influences energy metabolism, production of ROS, and OCR [ 60 , 61 ]. These differences in haplogroups suggest the importance of genetic compatibility to improve the adaptation of the transplanted mitochondria to their new environment. A study on conplastic mice demonstrates that mismatch of mtDNA variants is enough to promote differences in mitochondrial function and cellular adaptive responses [ 4 ]. This is due to the adaptive response led by a complex network of mitochondrial stress pathways that impact mitochondrial proteostasis, mtUPR, and ROS signaling, affecting the organism’s metabolic performance [ 4 ]. For example, haplogroup H has an increased risk for chronic renal allograft dysfunction while haplogroups V and J have lower risks. This was explained by the increased activity in the ETC in haplogroup H, resulting in increased ROS production [ 62 ]. Therefore, current data emphasizes the importance of considering haplogroups when performing heterologous mitochondrial transplantation.
Uses of mitochondrial transplant in acute illness
- Ischemia–reperfusion injury
Ischemia–reperfusion injury (IRI) is defined as the series of pathophysiological alterations that paradoxically occur in tissues upon reperfusion following the deprivation of blood supply, and is characterized by a rapid burst of ROS, which surpasses the tissue’s antioxidant capacity [ 63 ]. This leads to marked increase in cytokine, chemokine, and cell adhesion molecules’ release, and finally to the late recruitment of neutrophils, which further accentuates tissue damage [ 64 ]. Hayashida et al. [ 65 ], in a systematic review published in 2021, examined the evidence from human and animal studies in support of the safety and efficacy of mitochondrial transplantation for the treatment of IRI in different organ systems. Importantly, studies in ex-vivo models, however, were excluded from the analysis. Since then, important contributions to the field of mitochondriology have been made in ex-vivo perfusion platforms, particularly in models of heart and lung transplantation. In effect, the evaluation of mitochondrial transplant ex-vivo provides unique possibilities for both research and future clinical translation, both for safety and efficacy studies. By isolating the effect of mitochondrial transplant to a single organ (with no systemic escape), ex-vivo systems reduce or eliminate exposure to scavenging systems (such as the reticuloendothelial). Additionally, they allow the study of the interaction of mitochondria with different perfusion solutions in varying temperatures.
Although acute events of ischemia and their subsequent reperfusion injury can all be referred to as IRI, several distinguishing features should be taken into account when establishing mitochondrial transplant strategies: (1) the length of the ischemic insult (short vs extended ischemic times), (2) the type of ischemic insult (cold ischemia vs warm ischemia), and (3) the predictability of the ischemic insult (with heart transplantation as an example of a somewhat predictable insult, vs acute coronary occlusions as an example of unpredictable event). Predictable ischemic events provide the window for pre-ischemic organ conditioning, as well as allow time for autologous mitochondrial isolation for transplantation with less significant time constraints. These are important concerns because they affect the time points in which mitochondrial transplant could be potentially applied, the mechanism of injury of the affected organ, and the mechanism of protection that could potentially be obtained by mitochondrial transplant.
Different pathophysiological processes are encompassed by the broad terminology of “IRI” in other organs and are similarly dependent on the duration and type of ischemic insult. Fischer et al. demonstrated that cell death in lung tissue is dependent on the duration of cold ischemic time (CIT). Apoptosis occurs more frequent than necrosis in short periods of CIT (< 12 h), while necrosis occurs more in extended CITs (of up to 24 h) [ 66 ]. Furthermore, Iskender et al. [ 67 ] provided evidence in a rat model that lungs exposed to an extended CIT of 18 h compared to a warm ischemia time (WIT) of 3 h are injured very differently, despite presenting similar functional outcomes in terms of lung physiology and oxygenation. Warm ischemia resulted in higher plasmatic M65 levels (a marker of apoptotic and necrotic cell death), as well as higher levels of pro-inflammatory cytokines and chemokines in plasma, than both standard (12 h) and extended (18 h) CIT groups. Interestingly, similar injury markers were higher in the extended CIT group than in the WIT group when analyzed in tissue samples, suggesting divergent local and systemic responses. Finally, tissue ATP levels were the lowest in WIT lungs at the end of the ischemic period, but all groups (WIT, standard CIT, and extended CIT) demonstrated a reduction in ATP levels upon 2 h of reperfusion as compared to post-ischemic (and pre-reperfusion) measures. This indicates that even with the recovery of blood supply, local energetic demands to cope with the burst of ROS are higher than the parenchymal capacity. Therefore, the central importance of mitochondrial metabolism in lung IRI cannot be overemphasized.
Organ transplant
Models of organ transplantation provide a relevant example of the importance of these concepts. In organ transplantation, CIT is defined as the period through which harvested tissues are subjected to deprivation of blood supply while maintained passively or actively at low temperatures. By keeping tissues cooled down, the expectation is the reduction of metabolic demand and the subsequent extension of organ viability [ 68 ]. For heart transplantation, CIT of 4 h or longer is associated with lower recipient survival, primarily due to IRI [ 69 ]. Furthermore, transplanted organs are always subjected to periods of warm ischemia, out of cold preservation solutions, referred to as WIT [ 70 ]. This includes ischemia during organ retrieval and during implantation in the recipient. More recently, the advent of organ donation after circulatory death (DCD), aimed at increasing the donor pool, generated a third period of WIT impacting the quality of some donated organs, including hearts [ 71 ]. In the heart, it is known that WITs longer than 10 min increase mitochondrial damage and compromise ETC activity, increase caspase 3 and 7, induce cardiomyocyte apoptosis, and decrease overall myocardial function and primary graft failure [ 72 ]. Due to the nature of DCD, which by legislation requires a no touch period of variable duration (typically 5–20 min), pre-emptive pre-ischemic conditioning through mitochondrial transplant would not be feasible [ 73 ].
Regenerative medicine models to aid in studying mitochondrial transplant
In vitro models.
In vitro models are systems established outside of the human body to study human biological functions. Compared to animal models that can provide systemic simulation, in vitro models aim to distill the complexity of human (patho)physiology into key biological events and answer questions of interest. As in vitro culture eliminates species-related physiological differences by using human primary and stem cell-derived cell sources, they can significantly complement the results from animal models for initial exploratory or follow-up validation studies. More importantly, these in vitro models are typically cost-effective, have high throughput, and have low ethical liability, which facilitates the discovery process for disease mechanisms and drug leads.
The current in vitro models for mitochondrial study are limited to the use of single cells or monolayer cells, as they permit the observation of mitochondria entrance and colocalization [ 7 ]. However, there are many well-established in vitro models, ranging from simpler single-cell cultures to more complex tissue-engineered organ models. Patient-derived iPSCs present a unique opportunity to develop 3D organoids and organ/joint-on-a-chip (OoC; JoC) models to evaluate drug and treatment efficacy (Fig. 1 ). These models allow the recapitulation of key tissue phenotypes and have been developed for single organ and multi-organ models [ 74 , 75 ]. Peripheral blood mononuclear cells or fibroblasts can be collected from patient blood or skin biopsy to reprogram into iPSCs via transfection with pluripotency factors, specifically Sox2, Oct4, Klf4, L-myc, and Lin28 [ 74 ]. Once stabilized, these iPSCs can be differentiated into 2D cellular models, e.g. cortical neurons [ 76 ], myofibers [ 77 ], and cardiomyocytes [ 77 ], or 3D cellular models, e.g. cerebral organoids [ 74 , 78 ], cardiac tissue [ 79 ], and other OoC/JoC models.

Overview of the generation of organ-on-a-chip models from iPSCs and their downstream applications. iPSCs are reprogrammed from patient PBMCs or fibroblasts by transfection with OCT4, SOX2, KLF4, L-myc, and Lin28. Following iPSC stabilization, cells are differentiated to desired cell type and seeded on biomaterials and scaffolds to generate organ-on-a-chip models. Organ-on-a-chip models mimic in vivo tissue by replicating intercellular signalling and microenvironment to generate advanced in vitro disease models. Figure created with BioRender.com
The experimental throughput, associated costs, and user accessibility suffer with the increased complexity of tissue models. Yet, more complex models better recapitulate the in vivo microenvironment and provide clinically relevant results. For example, 3D tissue models are considered superior to 2D cultures in terms of replicating in vivo-like cell–cell and cell–matrix interactions, as well as cellular morphology, arrangement, and genetic and protein expressions [ 80 ]. However, simple 3D tissue models, such as cell aggregates, often oversimplify the physiological microenvironment observed in vivo, limiting their translational potential. Furthermore, mitochondrial maturity, morphology, and function change with the degree of cell differentiation as iPSCs shift to oxidative phosphorylation during differentiation [ 74 , 81 ]. Therefore, it is necessary to assess multiple cell types in models that have mature mitochondria. The use of OoC and JoC technology allows researchers to examine multiple tissues and cell types with mature mitochondria. Previously, this work had to be performed in animal models, which are time-consuming and expensive to work with and may not translate to humans. Ethical considerations must also be considered for animal studies. To overcome these challenges, organoids, OoC, and JoC technologies were developed to better address clinically relevant questions.
Organoids, OoC, and JoC technologies are gaining increased attention in biomedical research due to their advantages of building clinically relevant, high-throughput, and high-fidelity in vitro models. Organoids, often derived from iPSCs or adult organ progenitor cells through embryogenesis, can self-differentiate into three-dimensional structures that mimic the cellular diversity and architecture of human organs [ 80 ]. This ability to recapitulate complex human tissues lends a unique level of clinical relevance using organ-specific organoids [ 80 ]. For example, lung organoids derived from human iPSCs obtained structural polarity with minimally 7 key cell types out of the 40+ cell types in the lung[ 82 ]. On the other hand, OoC devices employ microfabrication technology to culture cells in a controlled 3D microenvironment, utilizing human stem cell derived sources, biological scaffolding, and chemical and biophysical cues, to mimic human organ-level functions, making them indispensable for (patho)physiological studies [ 83 ]. Electrical, chemical, and biological cues can be added to promote the functional maturation towards the adult phenotypes which further increases the clinical fidelity [ 84 , 85 ]. More importantly, OoC devices typically integrate built-in functional sensors, which provide non-invasive functional readouts corresponding to organ-specific functions or various experimental designs [ 77 ]. Heart-on-a-chip, as an example, often uses elastomer-based materials as tissue guiding templates and force sensors for the continuous force of contraction readouts. The electrical activity of the neural tissues can also be recorded using MEA-integrated devices [ 86 ].
While organoids and OoC devices offer numerous advantages, it is crucial to acknowledge that these tissue models aim to recapitulate only minimal tissue or organ function, often without considering relative scales [ 87 ]. The input cell populations are frequently reduced to several major cell types to mitigate complex experimental designs and exponentially increasing costs. Additionally, the media-to-cell ratio is often over-scaled, resulting in overly diluted chemical cues compared to native tissue. These inconsistencies partially stem from insufficient tissue vascularization and artificial methods of nutrient/oxygen delivery. To date, it is still challenging to establish a long-lasting, functional vascular network embedded with parenchymal tissues that faithfully recapitulates the vascular density and dynamic nature. Moreover, these organoids and OoC tissue models must undergo extensive validation at both functional and molecular levels to demonstrate tissue fidelity, which is the foundation of using these platforms to predict the outcomes of clinical study. These limitations could potentially impact downstream experimental designs concerning therapeutic agents’ delivery methods, concentrations and efficiency, such as mitochondrial transplantation, from OoCs to human studies in the future. Without this requirement, the OoC simply serves as a proof-of-concept tool and requires further modifications to be applicable in future studies.
Key design criteria of in vitro platform for mitochondrial transplantation
To tune these platforms specifically toward studying the mechanisms of mitochondrial transplantation, the following design criteria are pivotal for success. First, the tissue models should be constructed from human cells and require a high level of functional maturation and can recapitulate the key responses after injury. Using heart-on-a-chip as an example, iPSC-derived cardiomyocytes can be very immature and do not have high metabolic activity, highly organized intra-cellular sarcomere ultrastructure, or efficient machinery for cardiac output. In the study carried out by Ronaldson-Bouchard et al. [ 84 ], after electrical conditioning, the cardiomyocytes have 30% volumetric occupancy of mitochondria, compared to 10% in immature tissues. As a result, these immature cardiomyocytes can be much more resilient to ischemia–reperfusion injury compared to the high-metabolic demanding, highly functional matured cardiomyocytes [ 88 ]. Thus, the therapeutic effect of mitochondrial transplantation cannot be fully evaluated in these models. Secondly, the presence of immune cells can be an important factor. Both tissue-resident macrophages and bone marrow-derived macrophages play important roles in tissue injury and remodeling [ 89 ]. The incorporation of these cell types can be critical to recapitulate the cellular interactions during the injury and healing process, as they could be the first responders to the transplanted mitochondria. As the sources of the mitochondria can be allogenic for in vitro experiments, potential innate immunological responses can be anticipated and can be modeled with the presence of relevant immune cell types. Although the local transplantation of mitochondria onto the injury sites is the most used method in vivo. The incorporation of blood vessels can be useful to apply the transplantation through intravascular infusion in tissue models. Thus, the incorporation of the vascular system can be useful to investigate how mitochondria travel through the vascular system, surpass the vascular barrier, then localize at the injury sites and penetrate the parenchymal tissue.
Injury models using organoids and OoC technologies
Since most of recent research on mitochondrial transplantation has been focused on its potential for the treatment of IRI, it is important to understand how such a model could be achieved when making use of OoC technologies. The typical IRI model consists of applying a small volume of ischemic media with minimal nutrients and/or a low oxygen environment to the healthy tissues for a significant period, allowing metabolic waste accumulation to mimic nutrient deprivation, hyperkalemia, high lactate concentration, and low extracellular pH that are commonly observed during ischemia in the local tissues [ 88 ]. The following reperfusion step can be chosen to restore the environment to normal oxygen, nutrient, and pH levels by changing back to the normal culture conditions and supplemented media to model reperfusion injury [ 88 , 90 , 91 , 92 ]. For cardiac IRI on-chip model, the cardiac microtissues were made from iPSC-derived cardiomyocytes and cardiac fibroblasts [ 88 ]. The OoC platform allowed in-situ functional readouts, i.e., force of contraction, by video tracking the biomaterial-based force sensors. The results suggested that reperfusion injury had more significant functional reduction compared to ischemic injury [ 88 ]. Additionally, functional more matured tissues experience more cellular damage compared to their immature counterparts [ 88 ]. In a separate heart-on-a-chip study, extracellular vesicles were shown to mediate the IRI damage on the heart, demonstrating the potential therapeutic effect of mitochondrial transplantation [ 92 ]. In a neurovascular IRI stroke models, brain endothelial cells showed reduced barrier function and decreased mitochondrial potential as well as reduced ATP in both the blood- and the brain side of the model, suggesting the effective injury of the tissue model upon hypoxia [ 91 ]. Similarly, kidney on-a-chip [ 90 ] was also used to demonstrate the IRI injury. In this study, the proximal tubule epithelial cells were in direct co-culture with blood vessels and demonstrated tight barriers with perfusion. The ischemia injury was induced through a non-flow, no-glucose condition. The epithelial depletion and cell death were observed during the reperfusion stage, whereas adenosine treatment was shown to mediate the renal ischemic injury.
OoC and JoC are far from replicating the entirety of native organ/tissue complexity. However, the reductionist approach in which hallmark features are exclusively replicated minimizes potential design complications and results in a valuable tool that can be used in multiple applications. These dynamic devices are an additional tool to evaluate the effects of novel therapeutics and raise the odds that therapies will successfully move from pre-clinical to clinical studies [ 75 , 93 , 94 ]. Patient specific iPSC-derived OoC and JoC can be used to understand disease pathology via tissue engineering approaches to model longitudinal disease progression. These models can also be used to screen drug candidates and other therapies to select effective treatments for further testing in other preclinical models and patients [ 95 ]. This technology allows researchers to develop models for each patient and evaluate treatment efficacy under controlled laboratory settings, facilitating personalized medicine. Additionally, this aids in the development of new treatments, such as mitochondrial transplantation, as researchers can rapidly screen new biomaterials to enhance mitochondrial uptake and delivery in tissue and disease models.
In vivo preclinical models of mitochondrial transplant in acute organ injury
Heart : Work by Guariento et al. [ 96 ] explored mitochondrial transplant in a porcine model of DCD, in which hearts were subjected to 20 min of WIT, with subsequent evaluation in ex-vivo heart perfusion. Mitochondrial transplant administered at 15 min of reperfusion (single dose) or at both 15 min and at 2 h of reperfusion (serial dose) by intracoronary administration were then compared to vehicle controls during 4 h of ex vivo heart perfusion (EVHP). Importantly, for the serial dose group, whilst the first application of mitochondria was autologous, the second was not, with mitochondrial extraction obtained from porcine cardiac fibroblasts. The authors reported that no inflammatory response, as shown by hematoxylin & eosin staining, could be observed in the serial mitochondrial transplant group, in accordance with previous data from the group, in which no proinflammatory, damage-associated molecular patterns, or allorecognition signals were observed with allogeneic mitochondrial transplantation [ 97 ]. Interestingly, no additional benefit was observed in the 4-h assessment period by adding a second mitochondrial transplant after 2 h of heart reperfusion, both in terms of improved organ function (such as contractility and developed pressures) and myocardial infarction area size. In pathway analysis of hearts that received a mitochondrial transplant, mitochondrial ETC was included in the top 10 pathways with altered metabolic profile [ 96 ]. Importantly, the authors observed no difference in ATP content of porcine mitochondria isolated after the 15-min no-touch period after cardiac arrest, as compared to isolations in non-ischemic skeletal muscle.
More recently, Alemany et al. [ 98 ] also explored a similar model of autologous mitochondrial transplant for DCD in pediatric and neonate Yorkshire pigs. The pediatric population (10–15 kg) and the neonate population (3–4.5 kg) hearts had comparable sizes to those of 4- to 6-year-old children and 5- to 11-month-old infants, respectively. In contrast to previous work, mitochondrial transplant was applied only as a single bolus, albeit in a similar count (5 × 10 9 particles), and the ex-vivo heart perfusion machine was primed with blood donated from an adult female donor (non-autologous system priming). Similarly, to what had been observed with larger animals, mitochondrial transplant improved cardiac function and reduced infarction size as compared to vehicle controls. Intriguingly, slightly better function was noticed in the neonatal group (vs pediatric) in some parameters, which—as pointed out by the authors—could be resultant of a higher concentration of mitochondria per gram of tissue for neonate hearts under a fixed mitochondrial transplant dose. Additionally, contrasting what had been observed with larger animals, mitochondrial isolations performed in ischemic donor tissue showed reduced viability as compared to those obtained from non-ischemic tissue, which highlights that even for the same species, age-differences are non-negligible for mitochondrial transplant purposes, and dose extrapolations from previous studies (generally coming from McCully’s group data, targeting 2 × 10 5 to 2 × 10 6 particles/gram of wet tissue[ 41 ]) may not always result in similar efficacy.
Guariento et al. [ 99 ] demonstrated in a porcine model that intracoronary administration of autologous mitochondria, whether in a single bolus injection (1 × 10 9 particles) or serial bolus injections over 60 min (1 × 10 9 particles, 10 times), when given 15 min prior to ligation of the left anterior descending artery (LAD) in Yorkshire pig hearts, could significantly attenuate IRI after 30 min of regional ischemia. By assessing reperfused hearts for 2 h, without any additional mitochondrial transplantation upon reperfusion, the authors showed improved organ function, with higher contractility, enhanced ejection fraction and fractional shortening, and significant reduction in infarct size, regardless of the mitochondrial transplant strategy (single or multiple injections). Interestingly, coronary blood flow and contractility were significantly improved compared to baseline heart function even prior to the ischemic event. Additionally, these striking results demonstrated that, even when subjected to the same ischemic event as the target organ, transplanted mitochondria could still positively impact organ function and prevent infarction. The mechanism that explains how mitochondrial transplant operates on pre-ischemic heart conditioning still needs to be further explored and elucidated.
Moskowitzova et al. [ 100 ] explored in a murine model intracoronary administration of autologous mitochondria (1 × 10 8 particles, obtained from the gastrocnemius muscle) at two combined time-points, the first of which 10 min prior to harvest, and the second 5 min following reperfusion after heterotopic transplantation in the recipient. For this study, an extended period of 29 h of CIT was used as the main injury model, and transplanted animals were functionally evaluated for 24 h. Implanted hearts subjected to mitochondrial transplant demonstrated improved contractility, reduced necrosis, and reduced neutrophil infiltration in comparison to vehicle controls. Preliminary data reported by the authors suggested that the dual-administration regimen ( n = 8) was superior to a single dose preceding CIT ( n = 2) in providing improvement in the Stanford Cardiac Surgery Laboratory graft scoring system[ 101 ], hence the decision to include two mitochondrial transplant procedures. No other studies addressing mitochondrial transplantation at a single time point preceding extended cold ischemic times in heart transplantation have been identified in the literature.
Stemming from the encouraging pre-clinical results of mitochondrial transplant in cardiac IRI, Guariento et al. [ 102 ] conducted the first non-randomized case series of mitochondrial transplants in pediatric patients requiring ECMO for postcardiotomy cardiogenic shock (ClinicalTrials.gov Identifier: NCT02851758). In addition to requiring ECMO, patients were required to present proven documented ischemic event followed by successful revascularization and moderate to severe, persistent systolic ventricular dysfunction. Ultimately, 10 patients underwent mitochondrial transplant through direct transepicardial injection of autologous mitochondria obtained from the rectus abdominis muscle. Patients were retrospectively compared to 14 controls who underwent no additional interventions apart from the inclusion criteria. Direct intramyocardial mitochondrial transplant resulted in significantly improved successful separation from ECMO support (80 vs 29%, p = 0.02), improved ventricular contractility, reduced median time to functional recovery (2 days vs 9 days; p = 0.02), and lower cardiovascular events (20% vs 79%; p < 0.01). To date, after up to 6 years of follow-up since the start of the trial, surviving patients have not presented any significant adverse events attributable to the intervention.
Lung Similarly to what is observed for acute cardiac events, a myriad of injurious processes can result in ischemic injuries to the lungs, either by direct pathology (pulmonary embolism, trauma) or by indirect damage (such as cardiopulmonary bypass and resuscitation for cardiac arrest)[ 1 ]. Interestingly, the metabolic alterations elicited by ischemia can cause enzymatic repercussions in several pathways, such as nitric oxide synthase, which under anoxia can generate almost exclusively ROS, in detriment of NO [ 63 ]. Lung IRI (LIRI) is a complex phenomenon, given the unparalleled scenario of oxygen availability in alveolar spaces even under some conditions of limited perfusion, which is one of the possible manifestations of ventilation-perfusion (V/Q) mismatch [ 103 ]. In lung transplantation, for instance, organs are ventilated and harvested under a fraction of inspired oxygen (FiO2) of 50% and kept inflated (ideally) during the entire ischemic time preceding implantation in the recipient. No other organ is subjected to such a unique environment [ 104 ]. Moskowitzova et al. showed that mitochondrial transplant could be potentially employed as a treatment option for acute lung injury in a murine model of IRI [ 105 ]. By clamping the pulmonary hilum for 2 h, C57BL/6 J mice were randomized to receive autologous mitochondrial transplantation (isolated from the gastrocnemius muscle) or vehicle upon reperfusion, with subsequent evaluation for 24 h. This injury model had previously been shown to induce mitochondrial dysfunction, with decay in complexes I–V, II–V, and III–V, and reduced mitochondrial viability [ 106 ]. Both intra-arterial (IA) and nebulized aerosolized mitochondrial transplantation were explored (with dosages of 1 × 10 8 and 3 × 10 8 particles, respectively) and compared to vehicle controls [ 105 ]. For the unprecedented nebulization group, mitochondria were delivered through an Aeroneb ultrasonic nebulizer over 10 s, followed by 1 min of regular mechanical ventilation, repeated 4 times. Results pointed that mitochondrial transplant, regardless of the administration route, significantly improved lung mechanics, with narrower hysteresis and higher compliances, and decreased lung tissue injury under blinded tissue analysis. Transplanted mitochondria were found in the pulmonary artery (IA group) and in the trachea and bronchial three (nebulization group). Additionally, transplanted mitochondria were found in the lung parenchyma of both groups. Strikingly, nearly all evaluated parameters of lung mechanics were not significantly different from non-IRI controls (sham) in either mitochondrial transplant group, as well as being statistically superior to vehicle controls, although no mechanistic studies were explored by the authors.
In addition to acute lung injury caused by warm ischemia, the efficacy of mitochondrial transplant was recently investigated in an extended CIT, lung transplant model with Ex Vivo Lung Perfusion (EVLP) [ 9 ]. Since its inception [ 107 ], EVLP systems have been clinically employed worldwide by large academic centers in the evaluation of marginal lungs, which would otherwise not be deemed suitable for direct transplantation, with similar long-term outcomes [ 108 ]. In this setting, lungs are reperfused under normothermic conditions, mechanically ventilated, and clinically assessed for up to 4 h prior to the decision to proceed or not to transplantation. Cloer et al. explored whether intra-arterial mitochondrial transplant on EVLP resulted in improved functional and molecular outcomes in both a large animal model and in rejected human lungs [ 9 ]. In contrast to all previous studies to date, the group opted to apply heterologous mitochondria that had been previously frozen in a trehalose-based buffered solution for up to 2 months.
In a porcine, open-atrium, 6-h EVLP following 22 h of CIT at 4ºC, the authors investigated whether this heterologous mitochondrial transplant by intra-arterial injection at 1 and 4 h of reperfusion (i.e., serial injections at 2 time-points) was superior to vehicle controls [ 9 ]. Functional assessments indicated that mitochondrial transplant significantly decreased pulmonary vascular resistance as compared to vehicle controls, when normalized to baseline. No differences were observed in oxygenation capacity as measured by partial pressure of arterial oxygen over fraction of inspired oxygen (P/F ratio), and no other measures of lung physiology were reported. Interestingly, the same functional findings were encountered when performing a similar experiment with clinically rejected human lungs. For the human study, lungs were deemed eligible when total CIT was less than 30 h, with subsequent analysis for 4 h on EVLP. Mitochondrial transplant or vehicle ( n = 5/group) was then administered at 2 h of reperfusion, and a second injection was given when flushing the lungs with the standard low-potassium dextran solution at the end of EVLP, preceding a second CIT of up to 18 h. This design was described as mimicking an extended shipment of lung tissue from the EVLP site to a potential clinic.
From the analysis of EVLP tissue lysate samples and single cell suspension studies modelled to simulate cellular behavior during ex-vivo reperfusion, Cloer et al. [ 9 ] demonstrated that mitochondrial transplant reduced inflammatory response and improved antioxidant capacity. Furthermore, mitochondrial transplant reduced cell death by over 30% and increased live cell count by 25% when compared to controls, which was reinforced by marked reduction of phospho-mixed lineage kinase domain-like protein (pMLKL). In support of recent studies, no oxidative or proinflammatory response was observed following injection of a porcine-derived xenogeneic mitochondrial transplant in human tissue. This important xenogeneic experiment also provided additional insights for the future translation of non-autologous mitochondrial transplant. In an additional exploratory in-vitro experiment, human pulmonary artery endothelial cells (HPAEC) exposed to simulated CIT at 4 °C for 24 h were treated with either exogenous porcine mitochondria (at 100 to 1000 particles/cell) or vehicle upon rewarming. Surprisingly, benefits from mitochondrial co-culture (increased viability and autophagy, and decreased CXCL8, MCP1, and 8-OHdG) were sustained for the entire culture period of 7 days, despite a relatively expeditious drop of mRNA expression of porcine ssMtND5. This finding is encouraging from a safety translation standpoint and suggests the efficacy of mitochondrial transplant even with xenogeneic sources.
Liver In rodent models of acute liver injury, induced either by IRI or acetaminophen (APAP), mitochondrial transplantation has been shown to be safe and efficacious [ 109 , 110 , 111 , 112 ]. In rat liver IRI, Ko et al. [ 110 ] and Lin et al. [ 111 ] have independently demonstrated that intrasplenic injection of mitochondria or melatonin-pretreated mitochondria markedly reduced liver enzyme release, oxidative stress, and hepatocellular injury. In addition, Ko et al. [ 110 ] demonstrated that melatonin-pretreated mitochondrial transplantation reduced circulating pro-inflammatory cytokines, such as IL-6, TNFα and MPO, while improving liver ATP and NADH content. Moreover, liver tissue ETC components–complexes I, II, III and V–increased in protein expression in response to mitochondrial transplantation, as compared to I/R-only control rats. This suggested a restoration in the mitochondrial integrity and oxidative phosphorylation in treated rats. Finally, Ko et al. [ 110 ] demonstrated that the infiltration of CD68+ and CD14+ cells was reduced in melatonin-pretreated mitochondrial transplantation in the setting of liver IRI, suggesting further the nullification of the early cellular inflammatory response.
Another possible therapeutic avenue is the use of pre-ischemic conditioning prior to liver transplantation and ex vivo liver perfusion. Exploring the use of pre-ischemic mitochondrial transplantation has potential to improve the function of live donor livers destined for clinical transplant, which inevitably will be exposed to a WIT. Guariento et al. [ 99 ] demonstrated that pre-ischemic mitochondrial transplantation in porcine hearts significantly improved resilience to IRI and mitigated the detrimental effects of the ischemic event. Considering the potential efficacy of mitochondrial transplantation in cardiac model systems, here, we infer possible uses of this novel therapeutic in liver transplantation and elective liver surgery.
Similarly, in mouse models of APAP-induced acute liver toxicity, mitochondrial transplantation was able to increase hepatocyte ATP content, reduce oxidative stress, while decreasing hepatocellular injury, although no clear mechanism was elucidated [ 109 , 112 ]. Identification of molecular signaling pathways and cellular mechanisms are required for further understanding of how mitochondrial transplantation confers its hepatoprotective capacity.
Brain Ischemic brain injuries, which can be consequence of obstruction of blood flow to the brain due to intravascular thrombi, embolization, hypotension, or extrinsic vessel compression following head trauma, lead to a cellular energy crisis alongside oxidative stress, inflammation, apoptosis, and mitochondrial dysfunction [ 113 ]. This is due to the oxygen and glucose deprivation to the neurons impacting ATP production and promoting glycolytic metabolism that increases lactate production [ 114 ]. Such injuries can lead to neuronal apoptosis and astrogliosis affecting cognitive and motor functions. Current treatments that address mitochondrial dysfunction include hyperbaric oxygen, exercise, and antioxidant therapy [ 115 ]. However, noting the central role of the mitochondria in these injuries suggests the potential of introducing healthy mitochondria as a form of treatment [ 116 ].
In recent years, several in vivo experiments have explored the benefits of mitochondrial transplantation in treating brain ischemia. Models of injury on mice and rat include controlled cortical impact (CCI) for traumatic brain injury (TBI) [ 115 , 117 ] and middle cerebral artery occlusion (MCAO) for cerebral ischemia [ 116 ]. Following injury, intracerebroventricular injections (ICV) were used to perform mitochondrial transplantation [ 115 , 116 , 117 ]. Different studies have used various sources of mitochondria including mesenchymal stem cells, allogeneic liver and muscle biopsies, or autologous muscle biopsies and have all yielded promising evidence supporting the benefits of mitochondrial transplantation [ 115 , 116 , 117 ].
Studies have demonstrated that mitochondrial transplantation in a TBI mouse model lowered blood brain barrier (BBB) damage and brain water content, improved OCR, and promoted angiogenesis [ 115 ]. Additionally, mitochondrial transplant promoted expression of brain derived neurotrophic factor (BDNF), which is important in supporting neuron survival, including both astrocytes and microglia [ 117 ]. Spatial memory and cognitive function were also improved in mice with mitochondrial transplantation [ 117 ]. These results support the importance of replenishing mitochondria following brain IRI and the central role mitochondria play in the mechanism of brain injury.
Ischemic injury promotes astrogliosis, which is a critical factor for hindering regeneration in response to neural injury [ 118 ]. This is linked to cellular energy disruption and oxidative stress and thus neuronal loss in response to decreased energy to meet demands [ 114 ]. Mitochondrial transplant in a rat cerebral IRI model reduced astrogliosis in the mitochondrial transplant group compared to the vehicle control [ 118 ]. Furthermore, reduced levels of the ischemia biomarker, CPK, and decreased infarct area were also measured following mitochondria transplant [ 119 ]. Moreover, mitochondrial transplants have also been shown to significantly reduce IRI-induced apoptosis in brain cells [ 119 , 120 ] and reduce the volume of infarcted tissue [ 121 ]. Currently, a clinical trial led by Dr. Melanie Walker at the University of Washington is investigating the utility of mitochondrial transplantation on cerebral ischemia (NCT04998357, recruiting patients) [ 6 ].
Kidney Several studies utilizing acute kidney injury in rats have demonstrated therapeutic benefit of mitochondrial transplant [ 122 , 123 , 124 , 125 ]. Doulamis et al. [ 122 ] demonstrated that intra-arterial delivery of donor mitochondria significantly improved kidney function following IRI in a porcine model. Specifically, mitochondrial transplant provided renal protection against IRI-induced decreases in glomerular filtration rate and urine output as well as provided partial protection against tissue necrosis [ 122 ]. These findings were confirmed by Rossi et al. [ 123 ], which demonstrated improved tissue health and function. Mitochondrial transplant is currently being explored by Cellvie Inc. as a therapeutic approach to improve tissue health prior to kidney transplantation.
Regulatory considerations
As mitochondrial transplantation is emerging as a new therapeutic, there has been extensive consultation with regulators, particular the Food and Drug Administration (FDA) on the clinical trial design considerations, particularly in applications for primary mitochondrial diseases; these have included several workshops [ 126 , 127 , 128 , 129 ]. These workshops have neatly outlined the challenges of capturing significant effects in relatively rare primary mitochondrial disease patient populations. Clinical evaluation of mitochondrial transplantation in cerebral ischemia and in patients on ECMO for postcardiotomy cardiogenic shock have used autologous muscle-tissue derived mitochondria with little information published on the quality (viability, potency) and quantity (2 × 10 10 viable and respiration competent mitochondria); the focus has appropriately been on feasibility and safety. Analogously, cell and gene modified cell therapies have evolved over many decades to relatively harmonized global regulatory requirements which account for multiple safety (free from adventitious agents), and quality metrics (viability, dose, multivariate potency readouts reflective of clinically relevant mechanism of action) before such investigational products can be used in clinical trial investigations. Thus, the field of mitochondrial transplantation will similarly evolve to consider quality metrics such as the purity, viability, quantity and potency of isolated mitochondria; the original cell sources will have to be appropriately screened for adventitious agents. Understanding mitochondrial transplant mechanism of action, effects on mtDNA copy numbers and variants produced and extent of uptake will inform the type of quality control metrics that regulators will require as clinical trials advance beyond initial feasibility and safety phases.
Perspectives
The examination of the literature on the efficacy of mitochondrial transplantation for acute diseases provides some encouraging findings. Primarily, for IRI in diverse organ systems, it is possible to observe not only a favorable molecular, chemical, and immunological modulation, but also functional benefits in several animal models. Although most of the data only includes short periods of functional evaluation of target subjects, it is noteworthy that mitochondrial transplantation seems to provide early , measurable physiological improvement in target tissues. Additionally, IRI models suggest that mitochondrial transplantation can also significantly reduce tissue infarction, apoptosis, and necrosis, therefore presenting implications on tissue viability and potential for organ regeneration. Of note, published data also supports the hypothesis that transplanted mitochondria may be retained in tissue longer-term, for at least 4 weeks in porcine and rabbit survival models, while maintaining the benefits of reduced infarct size area [ 130 , 131 ].
With regards to safety, some interesting observations can be drawn from animal and human studies. Published clinical data on mitochondrial transplantation is derived from a single center and is limited to an autologous mitochondrial isolation technique, but with a relatively extended follow-up (now surpassing 6 years for some patients), and no observable adverse events have been identified [ 102 ]. Several animal models support the hypothesis that heterologous mitochondrial transplantation may not elicit hyperacute, acute, or chronic rejection, and no increase in pro-inflammatory chemokines and cytokines. Porcine-derived fibroblasts providing heterologous mitochondria to porcine hearts, porcine left-ventricle-derived heterologous mitochondria to porcine lungs, or murine skeletal-muscle-derived heterologous mitochondria to murine hearts are examples of intra-species, heterologous mitochondrial transplantation studies from which those observations can be drawn [ 9 , 96 , 99 , 100 ]. Importantly, Ramirez-Barbieri et al. [ 97 ] did not identify direct or indirect, acute or chronic alloreactivity, allorecognition or DAMPs to mitochondrial transplantation when directly comparing syngeneic and allogeneic (heterologous) mitochondria. Xenogeneic (interspecies) mitochondrial transplantation models, either assessing mitochondrial uptake [ 130 ] or efficacy [ 9 ], also did not encounter any detrimental effects on target organs or organisms, with increasingly abundant data from animal models, although data on human tissue uptake is still sparse [ 9 ]. From a translational standpoint, however, autologous mitochondrial transplantation is naturally the safest route for initial studies, which poses implications on the disease models for which this therapy could be potentially first explored. The possibility of storing well-preserved, functional mitochondria needs to be further replicated by other centers before further conclusions can be drawn but could significantly impact the applicability and generalizability of mitochondrial transplantation [ 9 ]. For instance, organ transplant models that evaluate extended CIT are currently largely prevented from using autologous mitochondrial transplantation owing to the logistic limitations of limited mitochondrial viability in respiration buffers.
Limitations and unanswered questions
Many are the questions that remain to be elucidated. From a study design perspective, it is still unclear, for instance, if single or serial mitochondrial transplants should be prioritized for initial translational studies. Given the variable impact of serial mitochondrial applications [ 96 , 98 ]—which is likely influenced by metabolic demands that are dependent both of target tissue, intraspecies (age effect) and interspecies model limitations (smaller animals with higher metabolic demands seem to require proportionally higher mitochondrial concentrations)—and given safety should be the primary concern, it seems reasonable to utilize serial injections first. It is also unclear whether the source of mitochondria should be of concern. Most published data support the use of skeletal-muscle or myocardium as sources of mitochondria for several organs, and although claims have been made in support of equivalence regardless of the donor tissue, data is limited and conflicting. Zhang et al. recently showed that despite cell origin not impacting on the overall benefit of mitochondrial transplantation for rescuing apoptosis from doxorubicin-induced heart failure, metabolically matched mitochondria were necessary for improving contractile function [ 50 ]. Masuzawa et al. [ 131 ], however, indicated that for acute ischemic heart events, liver-derived isolated mitochondria are as effective as skeletal-muscle-derived mitochondria.
From a mechanistic perspective, several key aspects remain unanswered, such as the exact therapeutic mechanism, the potential of biomaterial coatings to enhance therapeutic efficacy, and the ability to store healthy donor mitochondria. Further studies are needed to investigate how donor mitochondria elicit a therapeutic benefit following engulfment by recipient cells. Preclinical animal models found that only 44% of donor mitochondria were colocalized with cardiomyocytes, with the remainder in other cell types and interstitial space [ 130 , 132 ]. The use of biomaterials to coat donor mitochondria is important therapeutically to understand if it is possible to further enhance cellular engulfment of donor mitochondria and target specific cell types within a tissue to improve therapeutic efficacy. Biomaterial coatings may also be beneficial in stabilizing isolated mitochondria, which have a short window of viability that hinders clinical utilization. The lack of ability to store isolated mitochondria to perform necessary quality control is a significant limitation to the clinical advancement of mitochondrial transplant. Currently, mitochondrial isolation must occur immediately prior to transplant into the patient with minimal time to perform assays to ensure mitochondrial purity and viability. The potential of storing isolated mitochondria in trehalose buffers [ 9 ] or other solutions represent a significant advancement in the mitochondrial transplant field. This would allow the creation of mitochondrial banks with fully characterized mitochondria to maximize patient safety and therapeutic benefit. Overall, the mitochondrial transplant field is still developing, and several challenges remain prior to full clinical utilization.
Conclusions
Overall, the therapeutic efficacy of mitochondria transplant is promising in animal and early clinical trials, but many questions remain. Our mitochondrial transplant CWG has identified priorities to accelerate the pace of translational progress including the need for improved storage and enhanced delivery of isolated mitochondria, along with the development of accurate in vitro models to recapitulate the complexity of human tissue. Our interdisciplinary team developed a research plan (Fig. 2 ) to evaluate the stabilization and short-term storage of isolated mitochondria encapsulated in hyaluronic acid, methyl cellulose, and poly(L-lysine). The ability to store isolated mitochondria prior to transplantation will enable clinicians to perform comprehensive quality control assessments, ensuring that only healthy and pure mitochondria are transplanted, potentially reducing the risk of adverse events. Stored mitochondria can also be utilized in procedures requiring multiple mitochondrial transplants, such as prior to organ harvesting and donation, without requiring repeated mitochondrial isolations. We will also utilize brain, cardiac, muscle, joint, lung, and liver organ-on-a-chip models to evaluate mitochondrial transplant in acute and chronic diseases states. This will allow us to accurately assess the clinical applicability of the mitochondrial transplant in vitro using human-derived samples and investigate the therapeutic mechanisms. Advancements in these areas are important for the clinical applicability and translation of mitochondrial transplants in a range of diseases.

Our mitochondrial transplant CWG research plan to investigate the stabilization of isolated mitochondria and therapeutic efficacy in organ-on-a-chip models. Figure created with BioRender.com
Availability of data and materials
Not applicable.
Abbreviations
Mitochondrial Innovation Initiative
Medicine by design
Convergence working group
Mitochondrial DNA
Electron transport chain
Nuclear DNA
Extracorporeal membrane oxygenation
Hematopoietic stem and progenitor cells
Triphenylphosphonium-conjugated Dextran
Poly(ethylene oxide)98-poly(propylene oxide)67-poly(ethylene oxide)98
Hyaluronic acid
Methylcellulose
Oxygen consumption rate
Dioleoyl-3-trimethylammonium propane
1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine
Poly(ethylene glycol)
Glucose oxidase
Damage associated molecular patterns
Transmembrane potential
Mitochondrial permeability transition pore
Double strand DNA
Reactive oxygen species
Reactive nitrogen species
Cold ischemic time
Warm ischemia time
Donation after circulatory death
- Organ-on-a-chip
- Joint-on-a-chip
Ex vivo heart perfusion
Left anterior descending artery
Lung ischemia–reperfusion injury
Ventilation-perfusion
Fraction of inspired oxygen
Intra-arterial
Ex vivo lung perfusion
Partial pressure of arterial oxygen over fraction of inspired oxygen
Phospho-mixed lineage kinase domain-like protein
Human Pulmonary Artery Endothelial Cells
Acetaminophen
Controlled cortical impact
Traumatic brain injury
Middle cerebral artery occlusion
Intracerebroventricular injections
Blood brain barrier
Brain derived neurotrophic factor
D’Amato M, Morra F, Di Meo I, Tiranti V. Mitochondrial transplantation in mitochondrial medicine: current challenges and future perspectives. Int J Mol Sci. 2023;24(3):1969.
Article CAS PubMed PubMed Central Google Scholar
Gorman GS, Chinnery PF, DiMauro S, Hirano M, Koga Y, McFarland R, et al. Mitochondrial diseases. Nat Rev Dis Primers. 2016;2:16080.
Article PubMed Google Scholar
Wiedemann N, Pfanner N. Mitochondrial machineries for protein import and assembly. Annu Rev Biochem. 2017;86:685–714.
Article CAS PubMed Google Scholar
Latorre-Pellicer A, Moreno-Loshuertos R, Lechuga-Vieco AV, Sánchez-Cabo F, Torroja C, Acín-Pérez R, et al. Mitochondrial and nuclear DNA matching shapes metabolism and healthy ageing. Nature. 2016;535(7613):561–5.
Emani SM, Piekarski BL, Harrild D, Del Nido PJ, McCully JD. Autologous mitochondrial transplantation for dysfunction after ischemia–reperfusion injury. J Thorac Cardiovasc Surg. 2017;154(1):286–9.
Walker M. Autologous Mitochondrial Transplant for Cerebral Ischemia. Autologous Mitochondrial Transplant for Cerebral Ischemia: https://www.clinicaltrials.gov/study/NCT04998357 .
Kesner EE, Saada-Reich A, Lorberboum-Galski H. Characteristics of mitochondrial transformation into human cells. Sci Rep. 2016;6:26057.
Pacak CA, Preble JM, Kondo H, Seibel P, Levitsky S, Del Nido PJ, et al. Actin-dependent mitochondrial internalization in cardiomyocytes: evidence for rescue of mitochondrial function. Biol Open. 2015;4(5):622–6.
Article PubMed PubMed Central Google Scholar
Cloer CM, Givens CS, Buie LK, Rochelle LK, Lin YT, Popa S, et al. Mitochondrial transplant after ischemia reperfusion promotes cellular salvage and improves lung function during ex-vivo lung perfusion. J Heart Lung Transplant. 2023;42(5):575–84.
Davis CH, Kim KY, Bushong EA, Mills EA, Boassa D, Shih T, et al. Transcellular degradation of axonal mitochondria. Proc Natl Acad Sci USA. 2014;111(26):9633–8.
Liu D, Gao Y, Liu J, Huang Y, Yin J, Feng Y, et al. Intercellular mitochondrial transfer as a means of tissue revitalization. Signal Transduct Target Ther. 2021;6(1):65.
Pang Y, Zhang C, Gao J. Macrophages as emerging key players in mitochondrial transfers. Front Cell Dev Biol. 2021;9:747377.
Jacoby E, Ben Yakir-Blumkin M, Blumenfeld-Kan S, Brody Y, Meir A, Melamed-Book N, et al. Mitochondrial augmentation of CD34. NPJ Regen Med. 2021;6(1):58.
Maeda H, Kami D, Maeda R, Shikuma A, Gojo S. Generation of somatic mitochondrial DNA-replaced cells for mitochondrial dysfunction treatment. Sci Rep. 2021;11(1):10897.
Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O’Kane CM, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196(10):1275–86.
Mukkala AN, Jerkic M, Khan Z, Szaszi K, Kapus A, Rotstein O. Therapeutic effects of mesenchymal stromal cells require mitochondrial transfer and quality control. Int J Mol Sci. 2023;24(21):157.
Article Google Scholar
Hubbard WB, Harwood CL, Prajapati P, Springer JE, Saatman KE, Sullivan PG. Fractionated mitochondrial magnetic separation for isolation of synaptic mitochondria from brain tissue. Sci Rep. 2019;9(1):9656.
Preble JM, Pacak CA, Kondo H, MacKay AA, Cowan DB, McCully JD. Rapid isolation and purification of mitochondria for transplantation by tissue dissociation and differential filtration. J Vis Exp. 2014;91:e51682.
Google Scholar
Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–24.
Bernhard S, Tibbitt MW. Supramolecular engineering of hydrogels for drug delivery. Adv Drug Deliv Rev. 2021;171:240–56.
Souery WN, Bishop CJ. Clinically advancing and promising polymer-based therapeutics. Acta Biomater. 2018;67:1–20.
Khaliq NU, Lee J, Kim S, Sung D, Kim H. Pluronic F-68 and F-127 based nanomedicines for advancing combination cancer therapy. Pharmaceutics. 2023;15(8):2102.
Bray D, Hopkins C, Roberts DN. A review of dermal fillers in facial plastic surgery. Curr Opin Otolaryngol Head Neck Surg. 2010;18(4):295–302.
Alconcel SNS, Baas AS, Maynard HD. FDA-approved poly(ethylene glycol)–protein conjugate drugs. Polym Chem. 2011;2(7):1442–8.
Article CAS Google Scholar
Abourehab MAS, Pramanik S, Abdelgawad MA, Abualsoud BM, Kadi A, Ansari MJ, et al. Recent advances of chitosan formulations in biomedical applications. Int J Mol Sci. 2022;23(18):10975.
Burdock GA. Safety assessment of hydroxypropyl methylcellulose as a food ingredient. Food Chem Toxicol. 2007;45(12):2341–51.
Liu J, Liu B. Material-assisted engineering of organelles for biomedical applications. ACS Mater Lett. 2023;5(1):36–51.
Wu S, Zhang A, Li S, Chatterjee S, Qi R, Segura-Ibarra V, et al. Polymer functionalization of isolated mitochondria for cellular transplantation and metabolic phenotype alteration. Adv Sci. 2018;5(3):1700530.
Liu H, Wu S, Lee H, Baudo G, Massaro M, Zhang A, et al. Polymer-functionalized mitochondrial transplantation to plaque macrophages as a therapeutic strategy targeting atherosclerosis. Adv Ther. 2022;5(5):2100232.
Alexander J, Mahalingam R, Seua A, Wu S, Arroyo L, Horbelt T, et al. Targeting the meningeal compartment to resolve chemobrain and neuropathy via nasal delivery of functionalized mitochondria. Adv Healthc Mater. 2022;11(8):2102153.
Biswas S, Dodwadkar N, Piroyan A, Torchilin V. Surface conjugation of triphenylphosphonium to target poly(amidoamine) dendrimers to mitochondria. Biomaterials. 2012;33(18):4773–82.
Huang Y, Sun X, Gao R, Zhang L, Chen H, Lv Y, et al. Transplantation of mitochondria encapsulated in hydrogel ameliorates myocardial ischemia–reperfusion injury. Chem Eng J. 2023;460:141799.
Patel S, Michael F, Khan M, Duggan B, Wyse S, Darby D, et al. Erodible thermogelling hydrogels for localized mitochondrial transplantation to the spinal cord. Mitochondrion. 2022;64:145–55.
Chen W, Shi K, Chu B, Wei X, Qian Z. Mitochondrial surface engineering for multidrug resistance reversal. Nano Lett. 2019;19(5):2905–13.
Nakano T, Nakamura Y, Park JH, Tanaka M, Hayakawa K. Mitochondrial surface coating with artificial lipid membrane improves the transfer efficacy. Commun Biol. 2022;5(1):745.
Chen W, Huang T, Shi K, Chu B, Qian Z. Chemotaxis-based self-accumulation of surface-engineered mitochondria for cancer therapeutic improvement. Nano Today. 2020;35:100966.
Yu X, Lyu M, Ou X, Liu W, Yang X, Ma X, et al. AIEgens/mitochondria nanohybrids as bioactive microwave sensitizers for non-thermal microwave cancer therapy. Adv Healthc Mater. 2023;12:2370061.
Liu J, Liu X, Wu M, Qi G, Liu B. Engineering living mitochondria with AIE photosensitizer for synergistic cancer cell ablation. Nano Lett. 2020;20(10):7438–45.
Doulamis IP, McCully JD. Mitochondrial transplantation for ischemia reperfusion injury. Methods Mol Biol. 2021;2277:15–37.
Grazioli S, Pugin J. Mitochondrial damage-associated molecular patterns: from inflammatory signaling to human diseases. Front Immunol. 2018;9:832.
McCully JD, Cowan DB, Pacak CA, Toumpoulis IK, Dayalan H, Levitsky S. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am J Physiol Heart Circ Physiol. 2009;296(1):H94–105.
Yamaguchi R, Andreyev A, Murphy AN, Perkins GA, Ellisman MH, Newmeyer DD. Mitochondria frozen with trehalose retain a number of biological functions and preserve outer membrane integrity. Cell Death Differ. 2007;14(3):616–24.
Bodenstein D, Kim H, Brown N, Navaid B, Young L, Andreazza A. Mitochondrial DNA content and oxidation in bipolar disorder and its role across brain regions. NPJ Schizophr. 2019;5(1):1–8.
Venegas V, Halberg MC. Measurement of mitochondrial DNA copy number. In: Wong PDL, editor. Mitochondrial disorders: biochemical and molecular analysis. Totowa, NJ: Humana Press; 2012. p. 327–35.
Chapter Google Scholar
Popov LD. Mitochondrial biogenesis: an update. J Cell Mol Med. 2020;24(9):4892–9.
Yang M, Linn BS, Zhang Y, Ren J. Mitophagy and mitochondrial integrity in cardiac ischemia–reperfusion injury. Biochim Biophys Acta Mol Basis Dis. 2019;1865(9):2293–302.
Preble JM, Kondo H, Levitsky S, McCully JD. Quality control parameters for mitochondria transplant in cardiac tissue. Mol Biol. 2013;2(1):1008.
Fernández-Vizarra E, Ferrín G, Pérez-Martos A, Fernández-Silva P, Zeviani M, Enríquez JA. Isolation of mitochondria for biogenetical studies: an update. Mitochondrion. 2010;10(3):253–62.
Cadoná FC, de Souza DV, Fontana T, Bodenstein DF, Ramos AP, Sagrillo MR, et al. Açaí (Euterpe oleracea Mart.) as a potential anti-neuroinflammatory Agent: NLRP3 priming and activating signal pathway modulation. Mol Neurobiol. 2021;58(9):4460–76.
Zhang A, Liu Y, Pan J, Pontanari F, Chia-Hao Chang A, Wang H, et al. Delivery of mitochondria confers cardioprotection through mitochondria replenishment and metabolic compliance. Mol Ther. 2023;31(5):1468–79.
Shin B, Saeed MY, Esch JJ, Guariento A, Blitzer D, Moskowitzova K, et al. A novel biological strategy for myocardial protection by intracoronary delivery of mitochondria: safety and efficacy. JACC Basic Transl Sci. 2019;4(8):871–88.
Varnamkhasti BS, Hosseinzadeh H, Azhdarzadeh M, Vafaei SY, Esfandyari-Manesh M, Mirzaie ZH, et al. Protein corona hampers targeting potential of MUC1 aptamer functionalized SN-38 core-shell nanoparticles. Int J Pharm. 2015;494(1):430–44.
Deng ZJ, Liang M, Toth I, Monteiro MJ, Minchin RF. Molecular interaction of poly(acrylic acid) gold nanoparticles with human fibrinogen. ACS Nano. 2012;6(10):8962–9.
Bertrand N, Grenier P, Mahmoudi M, Lima EM, Appel EA, Dormont F, et al. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat Commun. 2017;8(1):777.
Ke PC, Lin S, Parak WJ, Davis TP, Caruso F. A decade of the protein corona. ACS Nano. 2017;11(12):11773–6.
Dai Q, Bertleff-Zieschang N, Braunger JA, Björnmalm M, Cortez-Jugo C, Caruso F. Particle targeting in complex biological media. Adv Healthc Mater. 2018;7(1):1700575.
Nel AE, Mädler L, Velegol D, Xia T, Hoek EM, Somasundaran P, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8(7):543–57.
Ali A, Wang A, Ribeiro RVP, Beroncal EL, Baciu C, Galasso M, et al. Static lung storage at 10°C maintains mitochondrial health and preserves donor organ function. Sci Transl Med. 2021;13(611):eabf7601.
Saeb-Parsy K, Martin JL, Summers DM, Watson CJE, Krieg T, Murphy MP. Mitochondria as therapeutic targets in transplantation. Trends Mol Med. 2021;27(2):185–98.
Amo T, Yadava N, Oh R, Nicholls DG, Brand MD. Experimental assessment of bioenergetic differences caused by the common European mitochondrial DNA haplogroups H and T. Gene. 2008;411(1–2):69–76.
Marcuello A, Martínez-Redondo D, Dahmani Y, Casajús JA, Ruiz-Pesini E, Montoya J, et al. Human mitochondrial variants influence on oxygen consumption. Mitochondrion. 2009;9(1):27–30.
Jiménez-Sousa MA, Tamayo E, Guzmán-Fulgencio M, Fernández-Rodríguez A, Heredia-Rodriguez M, García-Álvarez M, et al. Relationship between European mitochondrial haplogroups and chronic renal allograft rejection in patients with kidney transplant. Int J Med Sci. 2014;11(11):1129–32.
den Hengst WA, Gielis JF, Lin JY, Van Schil PE, De Windt LJ, Moens AL. Lung ischemia–reperfusion injury: a molecular and clinical view on a complex pathophysiological process. Am J Physiol Heart Circ Physiol. 2010;299(5):H1283–99.
Boyle EM, Canty TG, Morgan EN, Yun W, Pohlman TH, Verrier ED. Treating myocardial ischemia–reperfusion injury by targeting endothelial cell transcription. Ann Thorac Surg. 1999;68(5):1949–53.
Hayashida K, Takegawa R, Shoaib M, Aoki T, Choudhary RC, Kuschner CE, et al. Mitochondrial transplantation therapy for ischemia reperfusion injury: a systematic review of animal and human studies. J Transl Med. 2021;19(1):214.
Fischer S, Maclean AA, Liu M, Cardella JA, Slutsky AS, Suga M, et al. Dynamic changes in apoptotic and necrotic cell death correlate with severity of ischemia–reperfusion injury in lung transplantation. Am J Respir Crit Care Med. 2000;162(5):1932–9.
Iskender I, Cypel M, Martinu T, Chen M, Sakamoto J, Kim H, et al. Effects of warm versus cold ischemic donor lung preservation on the underlying mechanisms of injuries during ischemia and reperfusion. Transplantation. 2018;102(5):760–8.
Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45(4):673–6.
Russo MJ, Iribarne A, Hong KN, Ramlawi B, Chen JM, Takayama H, et al. Factors associated with primary graft failure after heart transplantation. Transplantation. 2010;90(4):444–50.
Halazun KJ, Al-Mukhtar A, Aldouri A, Willis S, Ahmad N. Warm ischemia in transplantation: search for a consensus definition. Transplant Proc. 2007;39(5):1329–31.
Kwon JH, Blanding WM, Shorbaji K, Scalea JR, Gibney BC, Baliga PK, et al. Waitlist and transplant outcomes in organ donation after circulatory death: trends in the United States. Ann Surg. 2023;278:609–20.
Sánchez-Cámara S, Asensio-López MC, Royo-Villanova M, Soler F, Jara-Rubio R, Garrido-Peñalver JF, et al. Critical warm ischemia time point for cardiac donation after circulatory death. Am J Transplant. 2022;22(5):1321–8.
Musso V, Righi I, Damarco F, Mazzucco A, Zanella A, Vivona L, et al. Lung donation after circulatory death. Curr Chall Thor Surg. 2021;5:1–10.
Duong A, Evstratova A, Sivitilli A, Hernandez JJ, Gosio J, Wahedi A, et al. Characterization of mitochondrial health from human peripheral blood mononuclear cells to cerebral organoids derived from induced pluripotent stem cells. Sci Rep. 2021;11(1):4523.
Zhao Y, Wang EY, Lai FBL, Cheung K, Radisic M. Organs-on-a-chip: a union of tissue engineering and microfabrication. Trends Biotechnol. 2023;41(3):410–24.
Chal J, Al Tanoury Z, Hestin M, Gobert B, Aivio S, Hick A, et al. Generation of human muscle fibers and satellite-like cells from human pluripotent stem cells in vitro. Nat Protoc. 2016;11(10):1833–50.
Zhao Y, Rafatian N, Feric NT, Cox BJ, Aschar-Sobbi R, Wang EY, et al. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell. 2019;176(4):913–27.
Sivitilli AA, Gosio JT, Ghoshal B, Evstratova A, Trcka D, Ghiasi P, et al. Robust production of uniform human cerebral organoids from pluripotent stem cells. Life Sci Alliance. 2020;3(5):e202000707.
Zhao Y, Rafatian N, Feric NT, Cox BJ, Aschar-Sobbi R, Wang EY, et al. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell. 2019;176(4):913-27.e18.
Lust ST, Shanahan CM, Shipley RJ, Lamata P, Gentleman E. Design considerations for engineering 3D models to study vascular pathologies in vitro. Acta Biomater. 2021;132:114–28.
Zhang J, Nuebel E, Wisidagama DR, Setoguchi K, Hong JS, Van Horn CM, et al. Measuring energy metabolism in cultured cells, including human pluripotent stem cells and differentiated cells. Nat Protoc. 2012;7(6):1068–85.
Bosakova V, De Zuani M, Sladkova L, Garlikova Z, Jose SS, Zelante T, et al. Lung organoids—The ultimate tool to dissect pulmonary diseases? Front Cell Dev Biol. 2022;10:899368.
Zhang B, Montgomery M, Chamberlain MD, Ogawa S, Korolj A, Pahnke A, et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat Mater. 2016;15(6):669–78.
Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2018;556(7700):239–43.
Feyen DAM, McKeithan WL, Bruyneel AAN, Spiering S, Hormann L, Ulmer B, et al. Metabolic maturation media improve physiological function of human iPSC-derived cardiomyocytes. Cell Rep. 2020;32(3):107925.
Tomaskovic-Crook E, Zhang P, Ahtiainen A, Kaisvuo H, Lee CY, Beirne S, et al. Human neural tissues from neural stem cells using conductive biogel and printed polymer microelectrode arrays for 3D electrical stimulation. Adv Healthc Mater. 2019;8(15):e1900425.
Wikswo JP, Block FE, Cliffel DE, Goodwin CR, Marasco CC, Markov DA, et al. Engineering challenges for instrumenting and controlling integrated organ-on-chip systems. IEEE Trans Biomed Eng. 2013;60(3):682–90.
Chen T, Vunjak-Novakovic G. Human tissue-engineered model of myocardial ischemia–reperfusion injury. Tissue Eng Part A. 2019;25(9–10):711–24.
Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14(10):986–95.
Vormann MK, Tool LM, Ohbuchi M, Gijzen L, van Vught R, Hankemeier T, et al. Modelling and prevention of acute kidney injury through ischemia and reperfusion in a combined human renal proximal tubule/blood vessel-on-a-chip. Kidney630. 2022;3(2):217–31.
Wevers NR, Nair AL, Fowke TM, Pontier M, Kasi DG, Spijkers XM, et al. Modeling ischemic stroke in a triculture neurovascular unit on-a-chip. Fluids Barriers CNS. 2021;18(1):59.
Yadid M, Lind JU, Ardona HAM, Sheehy SP, Dickinson LE, Eweje F, et al. Endothelial extracellular vesicles contain protective proteins and rescue ischemia–reperfusion injury in a human heart-on-chip. Sci Transl Med. 2020;12(565):eaax8005.
Makarczyk MJ, Gao Q, He Y, Li Z, Gold MS, Hochberg MC, et al. Current models for development of disease-modifying osteoarthritis drugs. Tissue Eng Part C Methods. 2021;27(2):124–38.
Samvelyan HJ, Hughes D, Stevens C, Staines KA. Models of osteoarthritis: relevance and new insights. Calcif Tissue Int. 2021;109(3):243–56.
Banh L, Cheung KK, Chan MWY, Young EWK, Viswanathan S. Advances in organ-on-a-chip systems for modelling joint tissue and osteoarthritic diseases. Osteoarthr Cartil. 2022;30(8):1050–61.
Guariento A, Doulamis IP, Duignan T, Kido T, Regan WL, Saeed MY, et al. Mitochondrial transplantation for myocardial protection in ex-situ-perfused hearts donated after circulatory death. J Heart Lung Transplant. 2020;39(11):1279–88.
Ramirez-Barbieri G, Moskowitzova K, Shin B, Blitzer D, Orfany A, Guariento A, et al. Alloreactivity and allorecognition of syngeneic and allogeneic mitochondria. Mitochondrion. 2019;46:103–15.
Alemany VS, Nomoto R, Saeed MY, Celik A, Regan WL, Matte GS, et al. Mitochondrial transplantation preserves myocardial function and viability in pediatric and neonatal pig hearts donated after circulatory death. J Thorac Cardiovasc Surg. 2023;167(1):e6–21.
Guariento A, Blitzer D, Doulamis I, Shin B, Moskowitzova K, Orfany A, et al. Preischemic autologous mitochondrial transplantation by intracoronary injection for myocardial protection. J Thorac Cardiovasc Surg. 2020;160(2):e15–29.
Moskowitzova K, Shin B, Liu K, Ramirez-Barbieri G, Guariento A, Blitzer D, et al. Mitochondrial transplantation prolongs cold ischemia time in murine heart transplantation. J Heart Lung Transplant. 2019;38(1):92–9.
Tanaka M, Terry RD, Mokhtari GK, Inagaki K, Koyanagi T, Kofidis T, et al. Suppression of graft coronary artery disease by a brief treatment with a selective epsilonPKC activator and a deltaPKC inhibitor in murine cardiac allografts. Circulation. 2004;110(11 Suppl 1):ll194-9.
Guariento A, Piekarski BL, Doulamis IP, Blitzer D, Ferraro AM, Harrild DM, et al. Autologous mitochondrial transplantation for cardiogenic shock in pediatric patients following ischemia–reperfusion injury. J Thorac Cardiovasc Surg. 2021;162(3):992–1001.
Burrowes KS, Clark AR, Tawhai MH. Blood flow redistribution and ventilation-perfusion mismatch during embolic pulmonary arterial occlusion. Pulm Circ. 2011;1(3):365–76.
Van Raemdonck D, Van Slambrouck J, Ceulemans LJ. Donor lung preservation for transplantation-where do we go from here? J Thorac Dis. 2022;14(9):3125–30.
Moskowitzova K, Orfany A, Liu K, Ramirez-Barbieri G, Thedsanamoorthy JK, Yao R, et al. Mitochondrial transplantation enhances murine lung viability and recovery after ischemia–reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2020;318(1):L78–88.
Sommer SP, Sommer S, Sinha B, Wiedemann J, Otto C, Aleksic I, et al. Ischemia–reperfusion injury-induced pulmonary mitochondrial damage. J Heart Lung Transplant. 2011;30(7):811–8.
Cypel M, Yeung JC, Hirayama S, Rubacha M, Fischer S, Anraku M, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008;27(12):1319–25.
Divithotawela C, Cypel M, Martinu T, Singer LG, Binnie M, Chow CW, et al. Long-term outcomes of lung transplant with ex vivo lung perfusion. JAMA Surg. 2019;154(12):1143–50.
Ulger O, Kubat GB, Cicek Z, Celik E, Atalay O, Suvay S, et al. The effects of mitochondrial transplantation in acetaminophen-induced liver toxicity in rats. Life Sci. 2021;279:119669.
Ko SF, Chen YL, Sung PH, Chiang JY, Chu YC, Huang CC, et al. Hepatic. J Cell Mol Med. 2020;24(17):10088–99.
Lin HC, Liu SY, Lai HS, Lai IR. Isolated mitochondria infusion mitigates ischemia–reperfusion injury of the liver in rats. Shock. 2013;39(3):304–10.
Shi X, Bai H, Zhao M, Li X, Sun X, Jiang H, et al. Treatment of acetaminophen-induced liver injury with exogenous mitochondria in mice. Transl Res. 2018;196:31–41.
Kochanek PM, Berger RP, Bayir H, Wagner AK, Jenkins LW, Clark RS. Biomarkers of primary and evolving damage in traumatic and ischemic brain injury: diagnosis, prognosis, probing mechanisms, and therapeutic decision making. Curr Opin Crit Care. 2008;14(2):135–41.
Bambrick L, Kristian T, Fiskum G. Astrocyte mitochondrial mechanisms of ischemic brain injury and neuroprotection. Neurochem Res. 2004;29(3):601–8.
Zhang B, Gao Y, Li Q, Sun D, Dong X, Li X, et al. Effects of brain-derived mitochondria on the function of neuron and vascular endothelial cell after traumatic brain injury. World Neurosurg. 2020;138:e1–9.
Russo E, Napoli E, Borlongan CV. Healthy mitochondria for stroke cells. Brain Circ. 2018;4(3):95–8.
Zhao J, Qu D, Xi Z, Huan Y, Zhang K, Yu C, et al. Mitochondria transplantation protects traumatic brain injury via promoting neuronal survival and astrocytic BDNF. Transl Res. 2021;235:102–14.
Zhang Z, Ma Z, Yan C, Pu K, Wu M, Bai J, et al. Muscle-derived autologous mitochondrial transplantation: a novel strategy for treating cerebral ischemic injury. Behav Brain Res. 2019;356:322–31.
Pourmohammadi-Bejarpasi Z, Roushandeh AM, Saberi A, Rostami MK, Toosi SMR, Jahanian-Najafabadi A, et al. Mesenchymal stem cells-derived mitochondria transplantation mitigates I/R-induced injury, abolishes I/R-induced apoptosis, and restores motor function in acute ischemia stroke rat model. Brain Res Bull. 2020;165:70–80.
Xie Q, Zeng J, Zheng Y, Li T, Ren J, Chen K, et al. Mitochondrial transplantation attenuates cerebral ischemia–reperfusion injury: possible involvement of mitochondrial component separation. Oxid Med Cell Longev. 2021;2021:1006636.
Norat P, Soldozy S, Sokolowski JD, Gorick CM, Kumar JS, Chae Y, et al. Mitochondrial dysfunction in neurological disorders: exploring mitochondrial transplantation. NPJ Regen Med. 2020;5(1):22.
Doulamis IP, Guariento A, Duignan T, Kido T, Orfany A, Saeed MY, et al. Mitochondrial transplantation by intra-arterial injection for acute kidney injury. Am J Physiol Renal Physiol. 2020;319(3):F403–13.
Rossi A, Asthana A, Riganti C, Sedrakyan S, Byers LN, Robertson J, et al. Mitochondria transplantation mitigates damage in an in vitro model of renal tubular injury and in an ex vivo model of DCD renal transplantation. Ann Surg. 2023;278(6):e1313–26.
Jabbari H, Roushandeh AM, Rostami MK, Razavi-Toosi MT, Shokrgozar MA, Jahanian-Najafabadi A, et al. Mitochondrial transplantation ameliorates ischemia/reperfusion-induced kidney injury in rat. Biochim Biophys Acta Mol Basis Dis. 2020;1866(8):165809.
Arjmand A, Faizi M, Rezaei M, Pourahmad J. The effect of donor rat gender in mitochondrial transplantation therapy of cisplatin-induced toxicity on rat renal proximal tubular cells. Iran J Pharm Res. 2023;22(1):e135666.
Camp KM, Krotoski D, Parisi MA, Gwinn KA, Cohen BH, Cox CS, et al. Nutritional interventions in primary mitochondrial disorders: Developing an evidence base. Mol Genet Metab. 2016;119(3):187–206.
Food and Drug Administration. Critical path innovation meeting regarding drug development for mitochondrial diseases. 2015.
White E, Yeske PE, Gray K, Strittmatter K, Hernandez B, Mann K, et al. Voice of the Patient Report “Mitochondrial Disease: Adults with Myopathy, Children with Neurologic Symptoms”. United Mitochondrial Disease Federation; 2019.
Food and Drug Administration. Developing therapies for primary mitochondrial diseases: bridging the gaps. 2019.
Kaza AK, Wamala I, Friehs I, Kuebler JD, Rathod RH, Berra I, et al. Myocardial rescue with autologous mitochondrial transplantation in a porcine model of ischemia/reperfusion. J Thorac Cardiovasc Surg. 2017;153(4):934–43.
Masuzawa A, Black KM, Pacak CA, Ericsson M, Barnett RJ, Drumm C, et al. Transplantation of autologously derived mitochondria protects the heart from ischemia–reperfusion injury. Am J Physiol Heart Circ Physiol. 2013;304(7):H966–82.
Cowan DB, Yao R, Akurathi V, Snay ER, Thedsanamoorthy JK, Zurakowski D, et al. Intracoronary delivery of mitochondria to the ischemic heart for cardioprotection. PLoS ONE. 2016;11(8):e0160889.
Download references
Acknowledgements
We would like to thank all members of the MITO2i-MbD mitochondrial transplant consortium who attended our Convergence Working Group. Figures were created with BioRender.com.
This review article was prepared as a part of an equally co-funded Medicine by Design (U of T) and Mitochondrial Innovation Initiative (MITO2i) funded project to develop a convergence workshop in phase 1 and investigate mitochondrial transplant in phase 2. The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
David F. Bodenstein and Gabriel Siebiger have co-first.
Yimu Zhao, Aaron J. Clasky, Avinash N. Mukkala, Erika L. Beroncal, Lauren Banh are shared equally to the content.
Authors and Affiliations
Department of Pharmacology and Toxicology, University of Toronto, Medical Science Building, Room 4211, 1 King’s College Circle, Toronto, ON, M5S 1A8, Canada
David F. Bodenstein, Erika L. Beroncal & Ana C. Andreazza
Institute of Medical Science (IMS), University of Toronto, Toronto, Canada
Gabriel Siebiger & Avinash N. Mukkala
Latner Thoracic Research Laboratories, Toronto General Hospital, Toronto, Canada
Gabriel Siebiger & Marcelo Cypel
Institute of Biomedical Engineering, University of Toronto, Toronto, ON, M5S 3G9, Canada
Yimu Zhao, Lauren Banh, Sowmya Viswanathan, Frank X. Gu & Milica Radisic
Department of Chemical Engineering and Applied Chemistry, University of Toronto, Toronto, Canada
Aaron J. Clasky, Frank X. Gu & Milica Radisic
Keenan Research Centre for Biomedical Science, Unity Health Toronto, Toronto, Canada
Avinash N. Mukkala
Osteoarthritis Research Program, Division of Orthopedic Surgery, Schroeder Arthritis Institute, University Health Network, Toronto, Canada
Lauren Banh & Sowmya Viswanathan
Krembil Research Institute, University Health Network, Toronto, Canada
Lauren Banh
Centre for Commercialization of Regenerative Medicine, Toronto, Canada
Lili Aslostovar & Mohadeseh Mehrabian
Mitochondrial Innovation Initiative (MITO2i), Toronto, Canada
David F. Bodenstein, Gabriel Siebiger, Yimu Zhao, Aaron J. Clasky, Avinash N. Mukkala, Erika L. Beroncal, Sonya Brijbassi, Constantine Zachos, Sowmya Viswanathan, Frank X. Gu, Ori D. Rotstein, Marcelo Cypel, Milica Radisic & Ana C. Andreazza
Regenerative Medicine Department, United Therapeutics Corporation, Silver Spring, USA
Sarah E. Hogan & Thomas H. Petersen
Harvard Medical School, Boston, USA
James D. McCully
Department of Cardiac Surgery, Boston Children’s Hospital, Boston, USA
Program in Cell Biology, The Hospital for Sick Children Research Institute, Toronto, Canada
Lisa A. Robinson
Department of Neurological Surgery, University of Washington, Seattle, USA
Melanie Walker
Acceleration Consortium, University of Toronto, Toronto, ON, Canada
Frank X. Gu & Milica Radisic
Li Ka Shing Knowledge Institute, Unity Health Toronto, Toronto, Canada
Ori D. Rotstein
Department of Surgery, University of Toronto, Toronto, Canada
Toronto Lung Transplant Program, Division of Thoracic Surgery, Department of Surgery, University Health Network, University of Toronto, Toronto, ON, M5G 2C4, Canada
Marcelo Cypel
Toronto General Hospital Research Institute, University Health Network, Toronto, ON, M5G 2C4, Canada
Milica Radisic
Terence Donnelly Centre for Cellular and Biomolecular Research, University of Toronto, Toronto, ON, M5S 3E1, Canada
Department of Psychiatry, University of Toronto, Toronto, ON, Canada
Ana C. Andreazza
You can also search for this author in PubMed Google Scholar
MITO2i-MbD Mitochondrial Transplant Consortium
Contributions.
DFB, GS, YZ, AJC, ANA, ELB, LB prepared and edited manuscript. SV, FXG, ODR, MC, MR, ACA revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Correspondence to Ana C. Andreazza .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
SEH and TPJ are paid employees of United Therapeutics. LA and MM are paid employees of the Centre for Commercialization of Regenerative Medicine. MR and YZ are inventors on a patent describing Biowire II heart-on-a-chip technology that is licensed to Valo Health. They receive royalty payments. ACA is the founder and scientific director for MITO2i. No author has or will receive financial incentive of any kind in relation to the technology described herein.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Bodenstein, D.F., Siebiger, G., Zhao, Y. et al. Bridging the gap between in vitro and in vivo models: a way forward to clinical translation of mitochondrial transplantation in acute disease states. Stem Cell Res Ther 15 , 157 (2024). https://doi.org/10.1186/s13287-024-03771-8
Download citation
Received : 12 December 2023
Accepted : 27 May 2024
Published : 31 May 2024
DOI : https://doi.org/10.1186/s13287-024-03771-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Mitochondria
- Mitochondrial transplant
Stem Cell Research & Therapy
ISSN: 1757-6512
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

Hoxworth Blood Center announces new interim leadership

Caroline Alquist, MD, PhD, and David Oh, MD, are the new interim co-directors of Hoxworth Blood Center, University of Cincinnati, effective Monday, June 3.
Alquist and Oh, who both hold appointments as associate professor at UC's College of Medicine, have extensive experience in the field of blood bank administration and together will lead Hoxworth as they collaborate on all blood center management and strategic efforts.
Alquist, chief transplant and cellular therapies officer, will ensure seamless continuity of Hoxworth’s clinical patient care. She is a leader in apheresis, transplant immunology and biotherapies with significant skill in clinical pathology, transfusion medicine, histocompatibility, laboratory medicine leadership and blood management.
Since joining Hoxworth in 2020, Alquist's clinical focus has been in supporting all regional solid organ and bone marrow transplants, which she will continue while serving as co-director. She has served as interim division director, cellular therapies, since last year, but will relinquish those responsibilities as soon as an ongoing search is completed to permanently fill that position.
Oh, chief medical officer, possesses a wealth of experience in operational process optimization and innovative initiatives. He has provided leadership to blood centers with the American Red Cross, San Diego Blood Bank and Stanford University Blood Center.
Oh joined UC's faculty in 2017 and will continue to focus his attention on blood supply, including blood donor recruitment, collection, processing, labeling, testing, storage and distribution. He also will continue as the UC Medical Center Transfusion Medicine/Blood Bank Medical Director and as the director of the Hoxworth Transfusion Medicine/Blood Banking Fellowship Program while leading all medical student, resident and fellow transfusion medicine education.
Pictured left to right: David Oh, José Cancelas and Caroline Alquist. Photo/University of Cincinnati.
Hoxworth is confident that Alquist and Oh will form a strong, coordinated team to continue their mission to enhance patient wellbeing, support more than 30 hospitals in the Greater Cincinnati region and maintain the center’s 85-year-record of the highest standards of quality, safety and innovation.
Alquist and Oh will lead Hoxworth following the June 2 departure of José Cancelas, MD, PhD, who has served as director and chief executive officer since Feb. 1, 2018.
Reflecting on his tenure, Cancelas expressed gratitude for the support of the community, staff and donor.
"My time at Hoxworth has been a privilege, and I am immensely proud of what we have achieved together," he said.
Cancelas is joining Dana-Farber Cancer Institute in Boston, where he will be the executive director of the Connell-O’Reilly Cell Manipulation Core Facility and hold an endowed professorship at Harvard Medical School. Under his leadership, Hoxworth became the only academic unit formed by a blood center in the United States, significantly expanded clinical activities and conducted groundbreaking bench and clinical research.
Hoxworth extends co ngratulations to Alquist and Oh on their expanded leadership responsibilities and a sincere thank you to Cancelas for his 23 years of extraordinary service to Hoxworth, the College of Medicine and Cincinnati Children’s.
Featured photo at top of a Hoxworth donor bus in front of the building. Photo/University of Cincinnati.
About Hoxworth: Hoxworth Blood Center, University of Cincinnati, was founded in 1938, and is the oldest blood center in the nation. Hoxworth serves more than 30 hospitals in 18 counties in Southwestern Ohio, Northern Kentucky, and Southeastern Indiana. Annually, Hoxworth collects more than 100,000 units of blood from local donors to help save the lives of patients in area hospitals. Hoxworth Blood Center: Saving Lives Close to Home. For more information, visit Hoxworth.org.
- College of Medicine
Related Stories
Hoxworth celebrates march with exclusive gift for donors.
March 4, 2022
Hoxworth Blood Center is celebrating the month of March with exclusive 3-in-1 tumbler/koozies for all donors
UC research shows renewed promise for COVID-19 convalescent plasma
March 31, 2022
Research from UC shows renewed hope in reducing hospitalizations from COVID-19 through the use of convalescent plasma when given early in the disease course. The study was published in the New England Journal of Medicine.
Celebrate Cicadapocalypse 2021 at Hoxworth Blood Center
May 24, 2021
Celebrate this year’s cicada invasion (or simply escape the hordes of buzzing, red-eyed locusts) by donating blood at Hoxworth Blood Center.
Amid grief and controversy, skate park mural gives parents of Mackay organ-donor teen Will Baker 'sense of belonging'
For two days, Kim Baker cuddled the warm body of her teenage son Will in the intensive care unit of a regional Queensland hospital.
She watched the 17-year-old's chest rise and fall with each breath of the ventilator, his heart still beating strongly.
Specialist nurses and doctors were almost constantly at the bedside.
With blood tests, physio, scans and x-rays, the difference between night and day blurred.
"It was very intense, but I knew that Will's organs were going to be used and that they needed to be in the best condition possible," Kim said.
As Mrs Baker and her husband Andrew made the selfless decision to donate Will’s organs, they had no idea the chain of events his death would trigger in the weeks to come.
The accident
Early one night in May 2022, police knocked on the door of the Bakers' lowset brick house in the beachside suburb of Eimeo in Mackay, North Queensland.
"That's when our world fell apart," Mrs Baker said.
Will was unlicensed and riding an unregistered trail bike when he hit the side of a courtesy bus from a local tavern and was critically injured.
"As soon as they said he wasn’t going to make it we said, 'just do everything you can to keep him alive to take his organs,'" she said.
Will’s heart, lungs, liver and kidneys were donated to help save five people.
The Bakers describe their headstrong son, who was a gifted skateboarder, as one of the kindest people they knew.
He had recently started working full-time and, in an effort to discourage him from riding his trail bike illegally, his parents encouraged him to join a motocross club.
On his last day alive, his father took him to a coaching clinic at the club.
"We're not shying away, William was doing the wrong thing, and he was making too many mistakes," he said.
"A lot of us get away with it and he didn't.
"We are also so sorry for what Will has put the bus driver through.”
As his family grieved, Will’s friends gathered at the local skate park, not far from the Bakers' home.
Within days, tribute graffiti appeared on a 3.1-metre wall already known as "Will's Wall", where the late teenager had built his skating reputation.
His friends wanted a memorial at the skate park and organised donations for an artist to paint a mural to replace the graffiti.
Their request for the mural to be a memorial were abandoned with the Baker family asking for the artwork to instead represent youth community connections.
In the confusion, commissioned artist Anita Laura Kroeger mistakenly painted the mural before official council approval was granted.
The mural was widely seen to be a memorial, which would have required specific council approval.
While celebrated by young people, it polarised state and local government members , including local councillor Martin Bella.
"[A] memorial needs to be to someone worthy, not someone who was breaking the law and has ruined another person's life," he said.
Two months later the mural was whitewashed after Mackay Regional Council voted 8-3 against giving it retrospective approval.
The political fallout
The decision to remove the mural shattered a once-strong political alliance of members backing Mayor Greg Williamson, who entered the 2020 election with a team of six and by the end of his term had the public support of just two.
While young skaters and friends were " disgusted " by the decision , councillors who supported the removal reported death threats and said they were abused on the street and "contacted at all hours".
Cr Williamson was one of three councillors who sought to do community consultation before making a decision on the mural’s future.
Two years on
A new, council-approved artwork now stretches across the same wall, by the same artist.
Mural organiser Bessie Hayes said it was healing for the community to have a mural back.
"Everyone just knows that this should have never come down," she said.
"I hope that it sends a bit of a message as well.
"We had told [council] what we want, and it's back here now."
Artist Anita Laura Kroeger said she hoped the new mural was something positive for the community to enjoy.
"It's been known as Will's Wall forever and will remain that to people who knew him," she said.
"I hope it's a meaningful mural.
"For people who didn't know Will and just enjoy the park, I feel that this mural is also something they can enjoy and connect with as well."
Mrs Baker said Will's family, including his sister Brianna, were delighted with the new mural.
"I'm really happy with it,” she said.
"It gives me a sense of belonging for the kids that use the skate park."
For her own personal reminder though, Mrs Baker said she touched her wrist every day.
It's where another artwork was etched — a heart-shaped tattoo made from prints from the tip of Will's index fingers.
She places her fingertip on the letter W and feels her pulse.
"For me, it's a bit of connection to Will, it's as close as I can be to him."
- X (formerly Twitter)
Related Stories
Fury over 'power trip' decision to cover tribute to deceased teenager with grey paint.
Abstract mural divides community — but is that a bad thing?
- Death and Dying
- Human Interest
- Skateboarding
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Maedica (Bucur)
- v.14(1); 2019 Mar
Ethical, Socio-Cultural and Religious Issues in Organ Donation
Petru cotrau.
University of Oradea, Faculty of Medicinice and Pharmacy, Romania
Emergency Clinical County Hospital of Oradea, Romania
Viviana HODOSAN
Adriana vladu, cristian daina, lucia georgeta daina, carmen pantis.
Brain death and cadaveric organ donation for transplantation present many challenges to society and even to the medical community; therefore, an ethical and legal framework is mandatory. Social values, death taboo, ignorance and procrastination are often issues that can influence the act of organ donation. This article sets out the close link between modern bioethics principles, legal framework, social and cultural value and cadaveric organ donation in the Romanian society. Organ donation, brain death and transplantation will continue to present challening questions for laws and bioethics and it is crucial that medical comunity and society members understand the involved principles, so that they can contibute to increasing the rate of organ donation and maintaining public confidence.
Keywords: ethics, organ donation, transplantation.
INTRODUCTION
The study of medical ethics has developed for several centuries, while the practice of organ donation and transplantation from brain death donors is a relatively new phenomenon, bringing along a range of ethical dilemmas which society has struggled to deal with.
Extending the definition of death, advances in immunosuppression, surgical tehniques, medical and pharmacological progress have made transplantation possible from cadaveric organ donors starting in 1967, when Christiaan Barnard had undertaken the world’s first cardiac transplant. In Romania, the first successful transplant from a deceased donor was a kidney transplant, performed in 1980, in Timisoara. There are currently five accredited centers for renal transplantation: four for liver transplantation, two for cardiac and one for lung transplantation.
Transplantation undoubtedly saves lives or improves the quality of life for patients with end-stage organ failure. It is already scientifically proven that there is a substantial long-term survival advantage for renal transplantation compared with dialysis (1). Guiding principles on human cell, tissue and organ transplantation are regulated by national and international laws with considerations of ethical issues. Consent to organ donation differs and is in line with national laws in force. There are two types of legislation: presumed consent and informed consent. The legal framework in organ donation and transplantation in Romania is ensured by the law No. 95/2006. Despite all this, ethical and moral dillemas continue to pervade the practice of organ donation and transplantation.
Principles of biomedical ethics
Beneficence: act in the best interest of the pacient. Non-maleficence: first, do not harm „primum non cere”. Autonomy: respect for a person’s choice. Justice: fairness and equality (2, 3). General guiding principles in organ and tissues donation: should be voluntary and altruistic, free and consented; respect for donor’s and recipient’s autonomy; confidentiality and protection of donor’s and recipient’s data; equitable opportunities and fairness in allocation; prohibition on making the human body and its parts a source of financial gain; physicians who determine brain death occurrence should not be directly involved in organ removal from the donor. „The declaration of Istanbul on organ trafficking and transplant tourism”, published in 2008, established definitions of practices such as transplant tourism and organ trafficking, and principles to guide policy makers and health professionals working in organ donation and transplantation. Since 2008, more than 135 professional societies have formally endorsed the Declaration.
Rules and law regulation in organ donation
In Romania, the republished version of law No. 95/2006 on Health Reform, with subsequent amendments and completions, establishes the legal framework for the development of the national transplant program. Informed consent is the fundamental governing principle with different requirements applying for tissue or organs from the dead and living donor.
The National Transplantation Agency is a public institution with legal personality, specialized body subordinated to the Ministry of Health, and represents the authority that implements national policies and programs for transplantation of human organs, tissues and cells. It has the role of coordinating the activity of sampling, preparation, preservation, validation, allocation, storage and transport for the transplantation of human tissues and cells for therapeutic use in the territory of Romania (4).
In case of deceased donors, Romania adopted informed consent, an „opt-in” system, where individuals register their willingness to donate organs in the event of their death and the record of this is the organ donor register. For the deceased who did not sign anything while alive, their family has to make a decision, and actually the final decision is the family’s. As enhanced education and better knowledge of the system correlates with increased willingness to donate, greater efforts in education among general public seems an important policy initiative (5).
Cultural, social and religious values
In Romania, a multiethnic and multicultural country, religion plays an important role, influencing the choices people make in certain areas of life. The last census in 2011 showed that only 0.2% of the total population of the country declared themselves to be without religion or atheists, the majority (86.5%) being Orthodox. Of the Romanian population, 56% lives in urban areas and 46% in rural areas (6). The official position of a religion towards organ donation and transplantation plays an important role in convincing the community in accepting organ donation for transplantation. Most religions support and encourage organ donation and transplantantation, Pope John Paul II had repeatedly advocated organ donation and organ transplant as a „service of life”. Understanding the ethical, cultural, social and religious values of a multiethnic population is important and can change the final decission in organ donation without violating these values. Some of the issues are the lack of awarenes regarding organ procurement, religious acceptance, brain death, and misconceptions that need to be corrected (7). Examples of misconceptions include the belief that the body of the donor would be mutilated and treated badly, or that even if a person wanted to donate one organ, other organs would be also taken (8). This is completely false, because organs are surgically removed in a routine operation and only those specified for donation will be taken from the body, which does not disfigure the body or change its appearance. A collaborative work with religious leaders regarding organ donation among religious communities and debates to ensure an active committment with organ donation must be considered at national and local levels.
Death taboo and procrastination
The lack of registrations in the organ donation registry can be interpreted as procrastination and death taboo (9), as people do not like to think about their death and to what will happen to their body once deceased. As a result of not choosing, the decision is delegated to to family members, but given the death taboo, they often have no idea what was the will of their deceased relative (10). The family, contrary to the individual, has no other choice but to evaluate the situation and make a difficult decision after the death of their relative. Public comunication campaigns should include strategies to provoke interpersonal communication about brain death, organ doantion, as a means of creating social representations able to promote behaviors that support organ donation and transplantation (11).
Illusion of lingering life, protection of the individual’s value, distrust, anxiety and alienation are some other examples of attitudes towards dying and organ donation and transplantation (12).
It is important to remember that somebody who does not accept the state of brain death will not be willing to donate one’s organs (13). Concerns about the erroneous diagnosis of death have been expressed many times among the general public or even medical staff. Historically death was defined by the presence of putrefaction or decapitation, failure to respond to painful stimuli, or loss of observable cardiorespiratory action. In 1968, an ad hoc committee at Harvard Medical School reexamined the definition of brain death and defined irreversible coma, or brain death, as unresponsiveness and lack of receptivity, the absence of movement and breathing, the absence of brain-stem reflexes, and coma whose cause has been identified (14, 15). Brain death is defined as the irreversible loss of all brain functions, including the brainstem. In Romania, brain death is considered death as in most countries of the world; Order of the Ministry of Health No. 1170/2014, Annex 3 about diagnostic criteria for confirmation of brain death, sets out very clearly the conditions under which the diagnosis of brain death is established.
DISCUSSIONS AND CONCLUSIONS
Organ donation and transplantation still presents many ethical challenges and dilemmas, both at personal and community level, even within the medical community. The various aspects of ethical, cultural and religious nature should not be a barrier to the act of organ donation and transplantation – all of these are issues to be solved. Applying ethical principles, transparency, identifying and fighting the main concerns with the utmost professionalism can bring changes in the attitude towards organ donation.
Involvement of medical staff with specific professional training, promoting interpersonal communication among community members, campaigns aiming to create a more accurate perception of the entire medical act, the legal and ethical framework are essential elements for a good development of the whole process of organ donation and transplantation.
Conflict of interests: none declared
Financial support: none declared.
Contributor Information
Petru COTRAU, University of Oradea, Faculty of Medicinice and Pharmacy, Romania. Emergency Clinical County Hospital of Oradea, Romania.
Viviana HODOSAN, University of Oradea, Faculty of Medicinice and Pharmacy, Romania. Emergency Clinical County Hospital of Oradea, Romania.
Adriana VLADU, University of Oradea, Faculty of Medicinice and Pharmacy, Romania. Emergency Clinical County Hospital of Oradea, Romania.
Cristian DAINA, University of Oradea, Faculty of Medicinice and Pharmacy, Romania. Emergency Clinical County Hospital of Oradea, Romania.
Lucia Georgeta DAINA, University of Oradea, Faculty of Medicinice and Pharmacy, Romania. Emergency Clinical County Hospital of Oradea, Romania.
Carmen PANTIS, University of Oradea, Faculty of Medicinice and Pharmacy, Romania. Emergency Clinical County Hospital of Oradea, Romania.

IMAGES
COMMENTS
Organ and tissue donation is more important than many of us realize—for society and for the individuals it directly affects. Today, there are nearly 118,000 individuals waiting for an organ transplant to live healthier, more productive lives (Unpublished data, Organ Procurement and Transplantation Network [OPTN], April 2013).
Organ donation connects the ending of one life with the renewal of another. Acute care hospitals care for the organ donor and transplant organizations complete life-saving surgeries. ... and research for donation professionals. Since the Institute's establishment, highly skilled, experienced faculty have worked with every OPO in the USA to ...
These include organ donors, families of the deceased, organ recipients, patients on transplant waitlists, donation and transplantation professionals, and organ donation organizations. Given that donation research may impact the distribution of scarce healthcare resources, organ allocation, and public trust, society at large may also be affected.
By 2012, 52.2 percent of adult respondents over age 65 were designated organ donors on their driver's licenses; that number was just 26.3 percent in 2005 [12]. Among respondents not designated as organ donors, 36.8 percent said they had reservations about donation and 59.2 percent said they were open to considering donation [12].
A substantial gap exists between the need for organ transplants and the number of transplants performed each year in the United States. In 2016, 27 630 organs were transplanted from 9971 deceased donors and 5980 additional organs from living donors, but as of September 29, 2017, a total of 116 602 individuals were included on the nation's organ transplant wait lists. 1 This gap remains ...
The need for transplants far outstrips supply, with 113,000 patients in the US in need of a transplant in 2019, and more than half (67,000) being of ethnic minority [16].Furthermore, approximately only 58% of the US population is registered as a potential donor (145.5 million actual donors), making organ shortage an ongoing issue [16, 22, 23].Waiting varies from 213 to 370 days, depending on ...
Compared with opt-in systems, opt-out systems are associated with higher donor rates ranging from 23.3% to 61.5% according to some studies 106, 107. However, a 2019 study of 35 countries found no ...
The NHS Blood and Transplant Service has recently told the BBC that over the past 5 years more than 500 families in the UK have blocked organ donation from a deceased relative, despite them being on the organ donor register. In the USA, more than 117 000 people await an organ transplant, says a report from the National Academy of Sciences (NAS) on the Opportunities for Organ Donor Intervention ...
For decades, transplantation has been a life-saving treatment for those fortunate enough to gain access. Nevertheless, many patients die waiting for an organ and countless more never make it onto the waitlist because of a shortage of donor organs. Concurrently, thousands of donated organs are declined for transplant each year because of concerns about poor outcomes post-transplant. The decline ...
The need for organ donation has increased globally in the past years due to an increase in organ failure [].Every day in the United States of America (USA), 21 people die waiting for an organ and more than 120,048 men, women, and children await life-saving organ transplants [].Accor-ding to a survey In India every year about 5 lakh (500,000) people die because of non-availability of organs and ...
In countries where presumed consent for organ donation does not apply, health professionals (HP) are key players for identifying donors and obtaining their consent. This systematic review was designed to verify the efficacy of interventions aimed at HPs to promote organ and tissue donation in clinical settings. CINAHL (1982 to 2012), COCHRANE LIBRARY, EMBASE (1974 to 2012), MEDLINE (1966 to ...
A 2009 systematic review examined the impact of presumed consent on organ donation rates and found that presumed consent policies were associated with a 20% to 30% increase in deceased organ donation rates. 18 However, the impact of these policies can be difficult to evaluate since they are observational in nature, and many factors (such as ...
June 4, 2023. People thinking of voluntary assisted dying may be able to donate their organs. We need to start talking about this. Robert Ray, Deakin University. Every extra organ available for ...
In light of a global organ shortage, living donor transplantation has become increasingly relevant as an alternative to deceased donor transplantation. While current research has revolved around the medical aspects of transplantation, there remains a paucity of literature regarding the quality of life (QOL) of living donors. Hence, this review aims to provide a comprehensive outline of the ...
Thanks to these advances, the average number of heart and lung transplants at Mayo Clinic has grown from an average of 40 per year to 120 in 2022. April is National Donate Life Month. For the 12 th consecutive year, deceased donations hit a record in the U.S. in 2022, according to the United Network for Organ Sharing.
Heart transplantation for patients with advanced heart failure is limited by a shortage of donor organs. In this Review, Jou and colleagues explore the options to increase the supply of donor ...
Introduction. Studies have shown that deceased donors cannot meet the growing demand for organs such as the kidneys, liver, etc. Sometimes cultural, religious, and legal considerations may even be reluctant to donate organs after death.[] Thus, because of the high demand for organ transplantation and the increased wait time for transplantation, receiving organs from living donors is a primary ...
This report details the findings of the 2012 survey of the American public's attitudes and behaviors about organ donation. View and download the 2012 National Survey of Organ Donation Attitudes and Behaviors (PDF - 1 MB) report. Outcomes of the 2019 National Survey of Organ Donation Attitudes and Practices are now available. Download now.
The present study highlighted that there are multiple reasons why people donate organs. The major motivators for organ donation identified are saving the life of others, moral obligation, an extension of life, relief of grief, and being a role model for others. In the present research, organ donation is seen as an opportunity to extend life.
Here are answers to some common organ donation myths and concerns. Myth: If I agree to donate my organs, the hospital staff won't work as hard to save my life. Fact: When you go to the hospital for treatment, the health care team tries to save your life, not someone else's. You get the best care you can get.
Organ donation and transplantation allows a deceased or living donor to give life to another. Surgeons remove a healthy organ from a donor who doesn't need it and transfer it to someone else who does. Organs that they can transplant include the liver, kidney, heart, lungs and more. Contents Overview Procedure Details Risks / Benefits Recovery ...
HIV+-to-HIV+ organ transplantation has demonstrated promise and is now authorized for research purposes in certain countries. However, organ transplantation is dependent on the availability of organ donors. We assessed the awareness and willingness to donate organs among people with HIV (PWH) in Uganda. Methods
People also read lists articles that other readers of this article have read. Recommended articles lists articles that we recommend and is powered by our AI driven recommendation engine. Cited by lists all citing articles based on Crossref citations. Articles with the Crossref icon will open in a new tab.
Related article More people need transplants than there are organ donors. Pigs might be a solution ... founding director of the Northwestern University Transplant Outcomes Research Collaborative ...
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... Michie Adjei, Irene K. Kim, and ...
Mitochondrial transplantation and transfer are being explored as therapeutic options in acute and chronic diseases to restore cellular function in injured tissues. To limit potential immune responses and rejection of donor mitochondria, current clinical applications have focused on delivery of autologous mitochondria. We recently convened a Mitochondrial Transplant Convergent Working Group ...
For his research on graft rejection and acquired immune tolerance, Dr. Medawar was awarded the Nobel Prize for Physiology or Medicine in 1960 and is considered the father of transplantation. ... Broglio K, Hirose R, Roberts JP, Malinoski D. Therapeutic hypothermia in deceased organ donors and kidney-graft function. N Engl J Med. 2015; 373:405 ...
UC research shows renewed promise for COVID-19 convalescent plasma March 31, 2022. Research from UC shows renewed hope in reducing hospitalizations from COVID-19 through the use of convalescent plasma when given early in the disease course. The study was published in the New England Journal of Medicine.
The mural. As his family grieved, Will's friends gathered at the local skate park, not far from the Bakers' home. Within days, tribute graffiti appeared on a 3.1-metre wall already known as ...
Understanding the ethical, cultural, social and religious values of a multiethnic population is important and can change the final decission in organ donation without violating these values. Some of the issues are the lack of awarenes regarding organ procurement, religious acceptance, brain death, and misconceptions that need to be corrected (7).