Cookies on GOV.UK
We use some essential cookies to make this website work.
We’d like to set additional cookies to understand how you use GOV.UK, remember your settings and improve government services.
We also use cookies set by other sites to help us deliver content from their services.
You have accepted additional cookies. You can change your cookie settings at any time.
You have rejected additional cookies. You can change your cookie settings at any time.
- Health and social care
- Medicines, medical devices
- Marketing authorisations, variations and licensing guidance

Medicines: apply for a variation to your marketing authorisation
Apply for changes to your marketing authorisation, including minor variations type IA and IB, major variations type II and extensions.
Variations are either:
- an administrative change such as a change of company name and/or address
- a change to the characteristics of a product that can affect its quality, such as a change to its composition
- a change to the safety, efficacy or pharmacovigilance of the product
Changes are classed as major (type II) or minor. Minor changes are either type 1A or 1B.
You can apply for:
- a single change to one marketing authorisation (MA)
- multiple changes to one or more products and/or MAs
Marketing Authorisation Types
From 1 January 2021, the following Market Authorisation types will be possible in the United Kingdom (UK):
( Guidance relating to application for a licence to market a medicine in the UK )
PL – authorised for use in United Kingdom
- as a purely national UK wide MA
- as part of an MR/DC procedure involving Northern Ireland as CMS, but a UK wide MA
PLGB – authorised for use in Great Britain only (England, Scotland and Wales)
- as a purely Great Britain national
- following conversion of a centrally authorised product (CAP)
- under the unfettered access route (MAH needs to be established in Northern Ireland and any product must access Great Britain, via Northern Ireland as a qualifying Northern Ireland good).
PLNI - authorised for use in Northern Ireland only
- as a purely Northern Ireland national
- as part of an MR/DC procedure including Northern Ireland only
Variations procedures
The variation procedures which will be followed for the different types of Marketing Authorisations from 1 January 2021 are essentially the same as before that date, where relevant, covered by the appropriate European or National legislation. The procedures are dependent on who is responsible for leading the assessment i.e. MHRA for purely national variations, the RMS for MR/DC or Reference Authority for any worksharing variations.
Centralised Marketing Authorisations are authorised for use in Northern Ireland only and any variations to these products will be managed by the EMA.
Until further notice the variations classification guideline , which is a fundamental component of the operation of the variations system, will continue to apply to all types of variations.
Reliance Route
Variations to purely national MAs (PL, PLGB and PLNI) can be presented to the MHRA under the reliance route i.e. following the acceptance of the same change(s) to a related the same product as part of a European procedure e.g. a CAP variation or an MR/DC variation.
If this approach is to be followed, this needs to be made clear in the application and relevant evidence, declarations and supporting information e.g. where relevant, copies of assessment reports should be provided to support the submission. Further guidance will be issued in due course.
Minor variations
These are divided into 2 types: IA and IB.
Type IA change
Type IA changes have little or no impact on the quality, safety or efficacy of the product, for example administrative modifications such as:
- the manufacturer’s name
- a minor change to a control method
- deleting details of where the product is packaged
Type IA procedures are classed ‘do-and-tell’, where you should implement the change before you notify MHRA, ensuring that where relevant (non-immediate notification) you submit the application within 12 months. If the implementation period passes 12 months without a variation being made, a default Type 1B should be submitted. The MHRA will take up to 30 days to process your application.
See the CMDh variations procedure guidance for the details of this type of change.
Type IAIN change (immediate notification)
Type IAIN is a sub-type of type IA and also classed a ‘do-and-tell’ procedure where MHRA requires ‘immediate notification’, within 2 weeks of the change being implemented. A minor change would usually be type IAIN if it interferes with MHRA’s ability to continuously supervise the product.
All relevant conditions and documentation must be met for a Type IA/IAIN and confirmed in a copy of the classification guideline with either a tick or where not relevant, Not Applicable (N/A).
No ‘requests for further information’ (RFI’s) apply to Type IA procedures and there is no fee associated with a National Type IA/IAIN. A full fees list and further information about fees, can be found in the section fees for licence variation applications.
Type IB change
If the change is more significant than a IA change but is not a type II change or an extension, it is considered a type IB change. MHRA must approve type IB changes before they are made to the product.
Once MHRA has all the necessary documents, it takes up to 30 days to process your initial application. You could be given a further 30 days to respond to any requests for information letter, which for Type IB are referred to as Notification with Grounds letters (NWG).
These are seen as a conditional determination of a type IB variation, where the applicant has one opportunity to address any deficiencies or omissions.
There is a 30-day deadline to respond to a NWG otherwise the variation will be withdrawn or refused, this also applies if only a partial response has been received by the 30-day deadline.
Major variations (type II)
These changes are more complex and may have a significant impact on the quality, safety and/or efficacy of the product.
MHRA needs to approve major variations before they are made. One example of a major change is adding a new therapeutic indication to a product or updating the current indication (the medical needs the product is used for).
Once MHRA has all the documents, it will take 30, 90 or 120 days to assess your application depending on how urgent or complex the changes are, excluding time taken to answer questions.
Once MHRA has all the documents, it will take 22-days, (reduced timetable), 60-days (standard timetable) or 90-days (extended complex timetable) to assess your application depending on how urgent or complex the changes are, excluding time taken to answer questions.
With questions (RFIs), assessment timeframes become 30, 90 and 120 days respectively.
The below tabulates the various stages for each type II timetable
A reduced Type II procedure (reduced from 60 days to 30) is for variations concerning safety issues and is agreed between the MHRA and the Marketing Authorisation Holder (MAH) and in general will be changes to the SmPC, requested following the assessment of a Periodic Safety Update Report (PSUR) or at the request of the MHRA Pharmacovigilance Unit.
Requests for a reduced timescale should be made to [email protected]
All other forms of submission will be rejected.
Common invalidation errors and pre-submission checklist
Following analysis of submissions MHRA has developed a pre-submission checklist of submission errors. Applicants are strongly advised to use the pre-submission checklist below which will reduce the likelihood of the submitted application being invalidated or rejected.
See our pre-submission checklist (PDF, 26.9KB, 2 pages
The marketing authorisation holder (MAH) is responsible for ensuring that, where relevant, all relevant conditions and documentation are met and the submission of this information and dossiers are the most recent and up-to-date. Deficient or incorrect documentation can lead to invalidation and/or rejection of the variation.
Although extensions are still considered a type of variation, their impact on a product is so significant that you will need to follow the application process to apply for a new MA . The form for this process has a section on extensions.
Extensions can be:
- changes to the active substance(s), including the salt/ester, isomer or biological active substance
- changes to strength, pharmaceutical form or route of administration
See annex 1 of the regulations for more information on extensions.
Revised labels, leaflets and/or packaging
If your variation (eg change of manufacturer) affects details of the labels, leaflets and or packaging (also known as livery) of the product, you will need to include the updated versions with your submission to be assessed.
However, you don’t need to submit a variation if there hasn’t been change to the product and you only want to make style changes to the labels, leaflets and/or packaging.
For example, if you’re changing the design of the leaflet and it has no relation to a change in the product, a variation application is not needed. See patient information leaflets for guidance on how to submit these types of changes.
Submit grouped changes (grouping)
You can apply multiple changes to a single product using the ‘grouping’ format, as long as the changes are directly related.
For example, you can make a change to the manufacturing site of the finished product, change in batch size and the manufacturing process to 1 product. The type of procedure (type IA, IB, II) depends on the extent of the change. You can see details of this in the annex III of the variations Regulation and Schedule 10A of the Human Medicines Regulations.
You can submit grouped changes to an MA with multiple product licences under the European procedure.
If a type IA variation is included in a group with other types of variations, the type IA change should not be implemented until all changes for that group have been approved.
You can group any type IA and IAIN changes for different marketing authorisations, as long as the group only contains IA and IAIN changes and the changes are the same. All of these changes should be implemented before you notify MHRA.
Grouping applications for national MAs
For groupings where MAs were granted in the UK through a national procedure only, you will need to apply for grouped changes through the MHRA.
Check the acceptable grouping guidance and the examples of groupings ( PDF , 2.5 MB , 12 pages ) to see if your grouping is acceptable.
If your desired group of changes isn’t included in the guidance or the examples, you need to complete the grouping template , copy and paste it into the main body of an email and send it to [email protected] before you submit your application. We aim to confirm if your grouping is acceptable within 7 working days.
Variations to multiple products (worksharing)
In some circumstances, you can use the worksharing format if you’re submitting the same type IB or type II change or group of changes to multiple MAs. As far as any product which is authorised in the UK is concerned only products which are authorised in Northern Ireland either centrally, purely nationally in Northern Ireland only, or as part of an MR/DC procedure can be the subject of worksharing. Proposals for worksharing applications should be sent to:
- the European Medicines Agency (EMA) if any of the MAs you want to change were granted under the centralised procedure
- the Coordination Group for Mutual Recognition and Decentralised Procedures (CMDh) if the MAs were granted under the mutual recognition and/or decentralised procedures and the reference member states were different *the reference member state if the authorisations were granted under the mutual recognition and/or decentralised procedure and the reference member state is was the same for each MA
- the competent authorities for the relevant countries if the MAs were granted under purely national procedures, email [email protected] for the UK.
Once MHRA have all the necessary documents, it will usually take 60 days to assess applications although it could take up to 120 days if the variation(s) are more complex or up to 30 days if urgent.
See the best practice guide on worksharing for details on how to submit an application this way.
Composite coordination collection (CCC)
If you want to apply for changes to one or more product licences (licence for each product under a marketing authorisation) that would affect the product information you can submit a composite coordination collection (CCC). Product information includes the summary of product characteristics, leaflets and labels.
With a CCC you can:
- submit only one consolidated mock-up of the leaflets and labels with all the changes proposed.
- submit a mixture of variations applications and Article 61(3) applications
- apply for single or grouped changes, such as a type IB and/or type II variations (see groupings )
You shouldn’t use this scheme for:
- urgent variations relating to the safety of your product(s)
- standalone type IA notifications
- changes that don’t affect product information (summary of product characteristics, leaflets, labels)
- changes to a product with a centralised licence
You would pay for this scheme as though you were paying for individual variations. See the list of fees
To get your CCC proposal approved and to see how to apply, follow the guidance for CCC application ( PDF , 538 KB , 32 pages ) .
Fees for variations .
Use the Fees Calculator to work out what the fee for your submission will be.
How to apply (all variation types except extensions)
Specific variations are given unique change codes to include in the application form.
Match the right change code to your specific variation by checking the annex in the [European Commission’s guidelines](https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52013XC0802(04). You will need to include this in your application form.
Submit the variation application form ( MS Word Document , 970 KB ) along with the supporting documents via the MHRA Portal
You can now submit your application via the Central European System Platform (CESP) .
Since January 2016 it has become mandatory for new marketing authorisation, renewal and variation application submissions to be made using electronic application forms (eAFs) . This will apply to all procedure types, including national procedures. If you are making a submission using the portal, you will have to submit using the portal forms as well as submitting the eAF. If you are submitting your application through CESP or MHRA Submissions you will only need to submit the eAF.
You must submit your variation using the electronic Common Technical Document (eCTD) .
We check that eCTD submissions are technically valid using the Extedo Eurs is Yours (EiY) validation tool. We recommend that you use a validation tool to check your submission.
If you have any questions about submitting your variation, email <[email protected]]>.
For submissions via MHRA Submissions please register .
You should use our eAF and cover letter tool to determine what information you need to include in your application. If you do not include the correct information your application will not be validated.
Submissions through the Common European Submission Portal (CESP)
This system is available from the Heads of Medicines Agencies (HMA) and provides a simple and secure mechanism for exchange of information between applicants and regulatory agencies.
The purpose of the system is to:
- provide a secure method of communicating with regulatory agencies via one platform
- allow submission of an application once to reach all required agencies
- reduce the burden for both industry and regulators of submitting/handling applications on CD-ROM and DVD
If you are a first time CESP user and wish to setup up an organisation/university or trust to manage multiple users on the system, register with CESP here .
If you are a standalone user and wish to upload for Non Commercial Use on your own behalf, register with CESP here .
Once registered you will receive credentials to access the portal to your registered email address.
General CESP training is available to all registered users via CESP’s training menu once logged into the system. Training on demand videos are available and you can also sign up to our free online weekly live demonstrations. CESP encourage all users to attend training before using the system. View FAQs .
Supporting documents
For CMS submissions send a dispatch date list (detailing when you intend to dispatch the products) to all member states before submitting your application if your MA was granted through the mutual recognition or decentralised procedures. The list should be emailed to [email protected] for type IB and II changes and include a copy in the ‘additional data’ section of the eCTD format.
Type IA and IB
For type IA and IB variation applications you need to include:
- a cover letter with your MA number, type of variation and reason for the variation
- an explanation of your grouped variations if applicable, making specific reference to the Heads of Medicines Agencies (HMA) acceptable groupings guidance and examples of groupings ( PDF , 2.5 MB , 12 pages ) or the confirmation email from MHRA agreeing you can submit grouped variations
- the supporting information required for specific variations as set out in the annex of the European Commission’s guidelines
- present and proposed details of your product presented as a separate document if necessary
- a contents page listing all documents included in your submission
For type II applications, you must include all of the above and:
- relevant information to support your application such as publications and any cited references
- a new or updated addendum to the ‘quality overall summary’, clinical and non-clinical overviews
Summary of product characteristics (SPC)
If relevant, the summary of product characteristic fragments (SPC) should be submitted to MHRA in the correct format using the templates below. If you do not use these templates your submission will be rejected.
These templates should not be altered in any way, other than inserting the relevant information. They should be saved using the following naming conventions in the ‘workingdocuments’ folder of your eCTD:
m1 -3-1-01: SPC section 1 – product name ( MS Word Document , 22 KB )
m1 -3-1-02: SPC section 2 – Qualitative and quantitative composition ( MS Word Document , 21.5 KB )
m1 -3-1-03: SPC section 3 - pharmaceutical form ( MS Word Document , 19.5 KB )
m1-3-1-4.1: SPC section 4.1 - therapeutic indications ( MS Word Document , 19.5 KB )
m1 -3-1-4.2: SPC section 4.2 - posology and administration ( MS Word Document , 19.5 KB )
m1 -3-1-4.3: SPC section 4.3 - contra-indications ( MS Word Document , 19.5 KB )
m1 -3-1-4.4: SPC section 4.4 - special warnings and precautions ( MS Word Document , 19.5 KB )
m1 3-1-4.5: SPC section 4.5 – interaction ( MS Word Document , 19.5 KB )
m1 3-1-4.6: SPC section 4.6 - fertility, pregnancy and lactation ( MS Word Document , 23.5 KB )
m1 3-1-4.7: SPC section 4.7 - driving and use machines ( MS Word Document , 19.5 KB )
m1 3-1-4.8: SPC section 4.8 - undesirable effects ( MS Word Document , 19.5 KB )
m1 3-1-4.9: SPC section 4.9 - overdose, emergency and antidotes ( MS Word Document , 19.5 KB )
m1 3-1-5.1: SPC section 5.1 – pharmacodynamics ( MS Word Document , 19.5 KB )
m1 3-1-5.2: SPC section 5.2 – pharmacokinetics ( MS Word Document , 19.5 KB )
m1 3-1-5.3: SPC section 5.3 - preclinical safety ( MS Word Document , 19.5 KB )
m1 3-1-6.1: SPC section 6.1 – excipients ( MS Word Document , 19.5 KB )
m1 3-1-6.2: SPC section 6.2 – incompatibilities ( MS Word Document , 19.5 KB )
m1 3-1-6.3: SPC section 6.3 - shelf life ( MS Word Document , 19.5 KB )
m1 3-1-6.4: SPC section 6.4 - special precautions ( MS Word Document , 19.5 KB )
m1 3-1-6.5: SPC section 6.5 – container ( MS Word Document , 19.5 KB )
m1 3-1-6.6: SPC section 6.6 – disposal ( MS Word Document , 19.5 KB )
m1 3-1-07: SPC section 7 - marketing authorisation holder ( MS Word Document , 19.5 KB )
m1 3-1-08: SPC section 8 - marketing authorisation number ( MS Word Document , 19.5 KB )
m1 3-1-09: SPC section 9 - date of the first authorisation or renewal ( MS Word Document , 19.5 KB )
m1 3-1-10: SPC section 10 - date of revision of the text ( MS Word Document , 19.5 KB )
m1 3-1-11: SPC section 11 – dosimetry ( MS Word Document , 19.5 KB )
m1 3-1-12: SPC section 12 – radiopharmaceuticals ( MS Word Document , 19.5 KB )
More information
The European Commission has published guidelines for submitting variation applications including the change codes needed. You may also find the common scenarios for submitting variations ( PDF , 171 KB , 7 pages ) useful.
The European Commission’s regulation1234/2008 as updated by regulation 712/2012 outlines the legislation for processing variations.
Email [email protected] with any queries, or call 020 3080 7400.
Added link to Fees Calculator.
Following the end of the transition period, published new information on marketing authorisation types.
Updated Group Template
Inserted new text/section - Request for Information (RFI)
Updated links to Variations and CCC calculators.
Updated guidance for CCC application.
Links have been updated.
Added a calculator to work out what the fee for your CCC submission will be.
New email address for Area 3 submission queries
Link to variation fees calculator added to the page
Updated pre-submission checklist
New common errors information and pre-submission checklist
First published.
Related content
Is this page useful.
- Yes this page is useful
- No this page is not useful
Help us improve GOV.UK
Don’t include personal or financial information like your National Insurance number or credit card details.
To help us improve GOV.UK, we’d like to know more about your visit today. We’ll send you a link to a feedback form. It will take only 2 minutes to fill in. Don’t worry we won’t send you spam or share your email address with anyone.

Type-IA variations: questions and answers
This page lists questions that marketing-authorisation holders (MAHs) may have on type-IA variations. It provides an overview of the European Medicines Agency's position on issues that are typically addressed in discussions or meetings with MAHs in the post-authorisation phase . Revised topics are marked 'New' or 'Rev.' upon publication.
A PDF version of the entire post-authorisation guidance is available:
European Medicines Agency post-authorisation procedural advice for users of the centralised procedure
English (EN) (2.63 MB - PDF)
European Medicines Agency post-authorisation procedural advice for users of the centralised procedure: document with track changes
English (EN) (2.65 MB - PDF)
These questions and answers have been produced for guidance only and should be read in conjunction with the rules governing medicinal products in the European Union, volume 2, notice to applicants .
MAHs must in all cases comply with the requirements of Community legislation . Provisions that extend to Iceland, Liechtenstein and Norway by virtue of the European Economic Area agreement are outlined in the relevant sections of the text.
1. When shall I submit my type-IA or -IAIN variation?
Commission Regulation (EC) No 1234/2008 ('the Variations Regulation') and the Commission guidelines on the details of the various categories of variations, on the operation of the procedures laid down in Chapters II, IIa, III and IV of Commission Regulation (EC) No 1234/2008 and on the documentation to be submitted pursuant to those procedures ('the Variations Guidelines') set out a list of changes to be considered as type-IA variations. Such minor variations have only a minimal impact or no impact at all, on the quality, safety or efficacy of the medicinal product, and do not require prior approval before implementation ('do-and-tell' procedure). The Classification Guideline clarifies the conditions that must be met in order for a change to be considered a type-IA variation.
Such minor variations are classified in two subcategories, which impact on their submission:
- Type-IA variations requiring immediate notification ('IA IN ') The Classification Guideline specifies the type-IA variations that must be notified (submitted) immediately to the national competent authorities or European Medicines Agency following implementation, in order to ensure the continuous supervision of the medicinal product.
- Type-IA variations not requiring immediate notification ('IA') Variations that do not require immediate notification may be submitted by the MAH within 12 months after implementation, or may be submitted earlier should this facilitate dossier lifecycle maintenance or when necessary, to ensure that the latest product information is reflected in certificates of pharmaceutical products, for example.
The 12-month deadline to notify minor variations of type IA allows for an annual reporting for these variations, where a MAH submits several minor variations of type IA that have been implemented during the previous 12 months.
Most of these type-IA variations do not have an impact on the product information. However, in case of an upcoming submission of a variation, extension or other regulatory procedure that will affect the product information, the MAH should also include any type-IA changes affecting the product information, in order to keep the product information up-to-date and to facilitate document management.
There are no recommended submission dates for type-IA variations. . However, MAHs are encouraged to avoid submitting type-IA notifications shortly before or during the Agency holiday periods (e.g. the end of July and Christmas).
Meaning of implementation for type-IA variations
For quality changes, 'implementation' is when the company makes the change in its own quality system. This interpretation allows companies to manufacture conformance batches and generate any needed stability studies to support a type-IA IN variation before making an immediate notification 1 because the change will not be made in their own quality system until these data are available.
For product information, it is when the company internally approves the revised product information. The revised product information will then be used in the next packaging run.
1 For example, the type IA IN for addition, deletion or replacement of components in the flavouring or colouring system requires stability data on at least two pilot-scale or industrial-scale batches.
2. Can I group the submission of type-IA and -IAIN variations? Can they be grouped with other types of variation?
Article 7(2)(a) of the Variations Regulation sets out the possibility for a MAH to group several type-IA or -IA IN variations under a single notification to the same relevant authority, or to group them with other types of variation.
Possible grouping of type-IA and -IA IN changes only
- Several type IA or IA IN affecting one medicinal product: This means, for instance, that a type-IA variation, which is normally not subject to immediate notification, can be included in the submission of a type-IA IN variation;
- One type IA or IA IN affecting several medicinal products from the same MAH:
- Several type IA and / or IA IN affecting several medicinal products from the same MAH, provided that those variations are the same for all medicinal products and are submitted to the same relevant authority:
Possible grouping of type IA and IA IN with other types of variation
- Type IA/IA IN can also be grouped with other variations (e.g. Type IB, Type II, Extension), as listed in Annex III of Commission Regulation 1234/2008. Groupings not included in the aforesaid Annex should be discussed and agreed with the Agency prior to submission.
- Such grouped submissions will follow the review procedure of the highest variation in the group. Please also refer to “What type of variations can be grouped?”.
It must be noted, however, that when submitting type-IA or -IA IN variations as part of a group, the legal deadlines for submission of each variation should be respected, i.e. a type IA IN should always be submitted immediately, whether or not it is grouped with other variations, and any type-IA variations should always be submitted within 12 months following their implementation.
3. Is the (co-)rapporteur involved in the review of type-IA and -IAIN variations? Rev. Mar 2024
The Agency will review the notification within 30 days following receipt, without involvement of the Rapporteur or Co-Rapporteur.
The same principle applies whether a single or a group of Type IA/ IA IN variations is being submitted.
However, if the Type IA/ IA IN Variations are grouped with other variations (Type IB, Type II, Extension), the grouped submission will follow the review procedure and timelines of the highest variation in the group and the Rapporteur will provide an assessment report for the group. Although the Rapporteur is not expected to assess the Type IA/IA IN variations in the group the Rapporteur will confirm in the assessment report whether non-acceptance of (part of) the change(s) in the group leads to non-acceptance of the Type IA/ IA IN changes in the group.
4. How shall I present and submit my Type IA/IAIN Variation(s)? Rev. Nov 2023
A type IA/ IAIN variation notification should contain the elements listed in Annex IV of the Variations Regulation and should be presented in accordance with the appropriate headings and numbering of the EU-CTD format. The Commission “Variations Guidelines” further specifies which elements should be included in a Type IA/ IAIN variation notification.
In order to help MAHs ensuring that their type IA/IAIN variations are complete and correct before submitting them to the Agency, it is strongly recommended to use the pre-notification checklist before submission of any type IA or type IAIN variation. Also, in order to facilitate the completion of the application form, MAHs are advised to consult the EMA/CMDh Explanatory Notes on Variation Application Form and the EMA Practical Guidance on the Application Form for Centralised type IA and IB variations.
Type IA variations are intended to provide for a simple, rapid and efficient procedure for minor changes. The MAH should be aware that the submission of redundant information or a confusing dossier presentation will not facilitate such procedures. Similarly, deficient and missing documentation can lead to rejection of the variation. However, in exceptional cases the Agency may issue a single request for supplementary information, for which a response should be provided within 4 working days in the mandatory eCTD format for electronic submissions. Failure to provide the requested information, or submission of incomplete and/or unsatisfactory responses within 4 working days may lead to an unfavourable outcome.
The following elements should be included in a Type IA/ IAIN variation notification, as specified in the Variations Guidelines:
- Cover letter (for groupings, include a short overview of the nature of the changes).
- In order to facilitate the registration of the submission, marketing authorisation holders are required to fill in all the submission attributes through the eSubmission delivery file UI .
- Procedure number – The procedure number will be assigned by the EMA only upon receipt of an eCTD application. For further details please refer to EMA pre-submission guidance “How is an EMA application/procedure number attributed?”
- The completed electronic EU variation application form (eAF), including the details of the marketing authorisation(s) concerned, as well as a description of all variations submitted together with their date of implementation. As of 1 July 2015, the use of the electronic application form is mandatory for all centralised procedures. Information on the electronic application form for variations can be found in the eSubmissions eAF webpage. Where a variation leads to or is the consequence of other variations, a description of the relation between these variations should be provided in the appropriate section of the application form.
- MAH should pay particular attention when preparing the eAF for IG submissions (grouping of Type IA/IAIN variations) and ensure that the change(s) applied for are not repeated as many times as the products included as this will incur unnecessary fees being invoiced. It is understood that the same change(s) will apply to all products listed in the application.
- MAHs are reminded that the variation application form should be signed by the official contact person as specified in section 2.4.3 of Part IA/Module 1. Should the official contact person not be available, an official letter of authorisation confirming the delegation of signature to a different person should be enclosed. For a grouping affecting several medicinal products, MAHs are reminded to confirm in the application form under “Declaration of the applicant” that the MAs concerned belong to the same MAH and that the main signatory confirms authorisation to sign on behalf of the designated contacts.
- Reference to the variation code as laid down in the Annex to the Variations Guidelines, indicating that all conditions and documentation requirements are met, or reference to the published Article 5 Recommendation, if applicable, used for the relevant application. Applicable conditions and documentation should be clearly ticked on the extract provided or marked as n/a. if that is the case. If a condition and or documentation is n/a. a justification for its absence should be provided.
- Relevant documentation in support of the proposed variation, including all documentation as specified in the Annex.
- If applicable, the revised summary of product characteristics (SmPC or Annex I), annex II, labelling (Annex IIIA) and/or package leaflet (Annex IIIB) as a full set of annexes. If the change applied for affects Annex A, this should be provided as a separate set of one document per EU language. (See also question on ‘When do I have to submit revised product information? In all languages?’) Additional information on how to comply with this in a required technical format can be found in the Harmonised eCTD Guidance.
- Please be reminded that in accordance with Union data protection requirements, no personal data should be included in the annotated PIs. This applies to the English version as well as to all the other languages translation versions. Please submit annotated PIs in an anonymised format (i.e. names of the reviewers removed from the track-changes). If you do not wish to do so, please ensure that the individuals whose data is included consented to its sharing with EMA and its further sharing by EMA with third parties such as other marketing authorisation applicants, marketing authorisation holders and National Competent Authorities, as relevant. EMA expressly disclaims any liability or accountability for the presence of unnecessary personal data in the annotated PI submitted by the marketing authorisation holder.
- Where the overall design and readability of the outer and immediate packaging and/or package leaflet is affected, the need for the provision of mock-ups or specimens should be discussed with the Agency Labeling Office on a case-by-case basis.
It should be noted that the responsibility for the quality of the submitted documentation lies with the MAH and is crucial to the overall process. The MAH is responsible for ensuring that the Type IA variation complies fully with the conditions and documentation requirements as specified in the Variations guidelines.
Grouped Type IA/ IAIN variations
- For grouped Type IA/ IAIN variations concerning one marketing authorisation, all Type IA variations must be declared in the variation application form. The supportive documentation for all variations concerned should be submitted as one integrated package (i.e. there is no need to submit a separate documentation package for each variation). However, the present-proposed section of the application form should clearly identify the relevant CTD sections in support of each variation.
- For a (group of) Type IA/ IAIN variation(s) concerning several marketing authorisations, one eCTD sequence per medicinal product should be submitted. This will include a common cover letter and common application form referring to all medicinal products and variations concerned. The Agency will allocate a ‘high-level’ cross-products procedure number, which will be used for the handling of procedures which affect more than one medicinal product. A new procedure code (abbreviation) is used for groups of Type IA/ IAIN variations i.e. “IG”. As the ‘high-level’ number cannot be allocated to one single product, the procedure number will therefore contain “xxxx” as a placeholder for the product number.
Example: EMEA/H/C/xxxx/IG/002
A ‘high-level’ procedure number should be obtained from the Agency shortly before submission. To submit your request, raise a ticket via EMA Service Desk . Please click on “Finance Services”, then the Type of ticket request to be selected is “Request for high-level procedure or ASMF number” followed by sub-option “IG Procedure Number (Type IA grouping)” and attaching a draft cover letter.
If you do not have an EMA Account, you may create one via the EMA Account Management portal .
Please note that requesting this ‘high level’ procedure number in advance is mandatory for submissions sent via the eSubmission Gateway or Web Client since this number must be included in the eSubmission Gateway XML delivery file User interface.
- In addition, for each medicinal product the relevant supportive documentation and revised product information (if applicable) should be provided, in order to allow the Agency to update the dossier of each marketing authorisation with the relevant updated/new information. Cross-references to any documentation submitted for another medicinal product can therefore not be accepted. For further details, please refer to “How shall I present a grouped variations application?” and to the Harmonised technical eCTD Guidance.
For procedural matters related to a type IA/ IAIN Variation for a specific product and in order to avoid rejection, please contact the EMA Service Desk , selecting the tab “Business Services”, category “Human Regulatory”. The subcategory to be selected is “Post-authorisation - Human”, followed by the sub-option “Variation IA queries”.
For more detailed queries on technical matters please contact the EMA Service Desk.
If you do not have an EMA Account, you may create one via the EMA Account Management portal. For further information or guidance about how to create an EMA Account reference the guidance " Create an EMA Account ".
Submission of Type IA/ IAIN Variation Notifications
Information is available on ‘ Submitting a post-authorisation application’ .
- Regulation (EC) No 726/2004
- Commission Regulation (EC) No 1234/2008
- Commission Regulation (EU) No 712/2012 amending Regulation (EC) No 1234/2008 concerning the examination of variations to the terms of marketing authorisations for medicinal products for human use and veterinary medicinal products
- Guidelines on the details of the various categories of variations , on the operation of the procedures laid down in Chapters II, IIa, III and IV of Commission Regulation (EC) No 1234/2008 of 24 November 2008 concerning the examination of variations to the terms of marketing authorisations for medicinal products for human use and veterinary medicinal products and on the documentation to be submitted pursuant to those procedures
- Electronic Variation application form
- Variation application form, The Rules governing Medicinal Products in the European Union, Notice to Applicants, Volume 2C
- European Medicines Agency practical guidance on the application form for centralised type IA and IB variations (CMDh/EMA/133/2010)
- European Medicines Agency practical guidance on the application form for centralised type IA and IB variations (EMA/233564/2014)
- Pre-notification check for type IA/IAIN variations
- Article 5 Recommendation
5. How will my type-IA or -IAIN variation be handled (timetable)?
The Agency will review the (grouped) type-IA and -IA IN variations within 30 calendar days following receipt. The Agency will check the correctness of the application form, the presence of the required documentation and compliance with the required conditions, in accordance with the Classification Guideline .
Day 0: Receipt of type-IA or -IA IN -variation notification;
Day 1: Start of Agency check;
By day 30: Favourable or unfavourable review outcome.
By day 30, the Agency will inform the MAH by Eudralink about the outcome of the review.
Where the outcome of the procedure is favourable and the Commission decision granting the marketing authorisation requires amendment, the Agency will inform the Commission accordingly.
Where one or several type-IA or -IA IN variations are submitted as part of one notification, the Agency will clearly inform the MAH about which variations have been accepted or rejected following its review.
Type-IA and -IA IN changes can be implemented prior to submission of the notification. However, in case of unfavourable outcome, the Variations Regulation requires the MAH to immediately cease applying the rejected variations. Please refer to 'what should I do in case of an unfavourable review outcome for my type-IA or -IA IN variation?' for further details.
It is still possible for MAHs to submit type-IA notifications prior to their implementation, particularly when the proposed changes are related to other notifications or variations requiring prior approval.
6. Can my Type IA/ IAIN be part of worksharing? Rev. Dec 2023
In accordance with the provisions of Article 20 of the Variations Regulation, the worksharing procedure does not apply to Type IA/ IA IN variations.
However, the submission of one or several Type IA/ IA IN variations affecting more than one marketing authorisation of the same MAH, in one notification to the same relevant authority (similar to worksharing) is possible under Article 7(2) of the Regulation – see also “Can I group the submission of Type IA/ IA IN variations? Can they be grouped with other types of variations?”
This type of grouping is referred to as 'IG' by the Agency.
A ‘high-level’ procedure number is assigned for all IG procedures submitted to the Agency. This number should be systematically obtained from the Agency shortly before submission by sending your request via EMA Service Desk . Please click on “Finance Services”, then the Type of ticket request to be selected is “Request for high-level procedure or ASMF number” followed by sub-option “IG procedure number (Type IA grouping)” or “Workshare procedure number”. The letter of intent should be attached to the EMA Service Desk ticket.
For further information see also Grouping of variations: questions and answers ‘What procedure number will be given to grouped variation applications? ’
In addition, it is also possible to group a Type IA/ IA IN variation(s) with a Type IB or Type II variation, which is submitted for a worksharing procedure. In such case, the Rapporteur will be asked to confirm whether the non-acceptance of (part of) the change(s) leads to non-acceptance of Type IA/IA IN in the group. In this case, the 'high level' cross-products procedure number for the worksharing should be obtained in like manner as for IG procedures. For further information see also Worksharing: questions and answers 'What procedure number will be given to variation applications under worksharing?'
7. What should I do in case of an unfavourable outcome for my type-IA or -IAIN variation?
A type-IA or -IA IN variation will be rejected when:
- the classification of the proposed changes is incorrect;
- not all of the conditions for the type-IA or -IA IN variation are met;
- the submitted documentation as required by the Variations Guideline is deficient or inaccurate, including provision of the product information annexes and annex A, if affected by the changes applied for.
In such cases, the MAH should immediately cease to apply the rejected changes.
In the case of a negative outcome of a type-IA application because the conditions for type-IA variations are not met and consequently a resubmission (as a type-IB or type-II variation or an extension) is needed or because documentation is deficient, it is the MAH's responsibility to judge whether the rejected type-IA variation has an impact on the quality, safety or efficacy of the medicinal product. If this is the case, the MAH has to take appropriate action.
The Agency may ask the MAH to complete a suspected quality-defect notification form and provide a risk-assessment report on the impact of the product on the market to [email protected] within seven calendar days from the date of the rejection letter. Such requests are expected to be very exceptional. The MAH has to follow the instructions under notifying quality defects or product recalls .
8. What fee do I have to pay for a Type IA/ IAIN variation? Rev. Apr 2021
For information on the fee applicable for Type IA/ IAIN variations, please refer to the explanatory note on fees payable to the European Medicines Agency. Such fee covers all authorised strengths, pharmaceutical forms and presentations of a given medicinal product.
For variations introducing additional presentation(s)/pack-size(s), each additional presentation/pack-size attracts separate fees (‘x’ additional presentations = ‘x’ separate fees). Each presentation/pack-size should therefore be declared as a separate variation on the variation application form under the section ‘Variations included in this application’.
Grouped Type IA/ IAIN variations, whether consequential or not, will each attract a separate Type IA fee.
The fee will become due on the date of receipt of Type IA/ IAIN variation notification and fees will be payable within 45 calendar days of the date of the said notification. After approximately 15 days an invoice will be sent to the applicants billing address held on the Agency’s file.
The invoice will contain details of the product and type of procedure involved, the fee amount, the financial information and the customer purchase order number associated with the procedures invoiced (if provided in the eSubmission delivery file). The Agency does not accept stand-alone notifications of purchase order numbers that are not associated with a dossier.
The Agency will charge the fee for type IA variations or grouped type IA variations at the start of the procedure, irrespective of its outcome (positive, negative or partial/full withdrawal).
In accordance with the CHMP Procedural Announcements published on 15 March 2012, MAHs are reminded that fees for type IA variations become due at the start of the 30-day procedure. Fees are charged based on what has been declared in the application form regardless of the outcome (i.e. fees apply equally for accepted and rejected scopes).
The above means that, once submitted to the Agency, modifications such as addition or deletion of type IA variation scopes are not possible. The Agency cannot accept any revised application form to change the type or number of scopes applied for as part of any submission of Supplementary Information for Type IA variations.
Type IA variations which are grouped with other type of variations/extensions or which are part of worksharing procedure will continue to be charged on conclusion of the validation of the application.
Guidance on how to pay an invoice can be found on our website.
- Fees payable to the European Medicines Agency
9. Do I have to submit mock-ups and specimens?
For information concerning submission of mock-ups and specimens in the framework of post-authorisation procedures, please refer to Checking process of mock-ups and specimens of outer / immediate labelling and package leaflets of human medicinal products in the centralised procedure , 3.4 other post-authorisation procedures.
10. What changes will trigger new EU number(s) (additional presentation(s))? Rev. July 2023
Any changes in the number of units of medicinal product or medical device being an integral part of the medicinal product (e.g. prefilled syringes) will trigger a different EU number.
Differentiation should be made between the addition of a presentation where the two presentations will co-exist on the market on a long-term basis versus a replacement of a presentation where the new presentation will replace the previous one (it is expected that for a certain period of time, the two presentations will co-exist on the market until the stock of the previous presentation runs out).
In principle, a replacement of one presentation by another presentation does not trigger a new EU number, unless the number of units of medicinal product or medical device being an integral part of the medicinal product (e.g. prefilled syringes) is changed.
Examples of changes in presentations for replacement, not triggering a new EU number (this is not an exhaustive list):
- Replacement of the primary or secondary packaging,
- Changes in the number of medical devices not being integral part of the medicinal product,
- Change in composition (e.g. change in excipients),
- Change in units per blisters (without change to the total number of units per pack).
Examples of changes in presentations for replacement, triggering a new EU number (this is not an exhaustive list):
- 30 to 60 tablets,
- 2 prefilled syringes containing the medicinal product instead of one prefilled syringe.
In case of addition , as the presentations will co-exist on the market, two packs with different contents cannot be covered by the same EU number and will be considered as different presentations.
Changes in the number of any unit (not restricted to the medicinal product) or changes in the specifications of any unit (not restricted to the medicinal product) contained in the pack will trigger a new EU number.
Examples of changes that will trigger new EU numbers (this is not an exhaustive list):
- Introduction of an alternative injection kit with a different number of syringes or swabs,
- Introduction of an alternative syringe of different volume or an alternative syringe with a needle guard,
- Introduction of an alternative immediate (primary) packaging made from a different material,
- Introduction of an alternative shape/dimension of a pharmaceutical form (pre-rolled sealant matrix versus flat, change in size of patch).
If you have any questions on any upcoming submission, please contact us by raising a ticket via EMA Service Desk , selecting the tab “Business Services”, category “Human Regulatory”. The subcategory to be selected is “Post-authorisation - Human”, followed by the sub-option “Variation IA queries” or “Variation IB queries”.
If you do not have an EMA Account, you may create one via the EMA Account Management portal . For further information or guidance about how to create an EMA Account reference the guidance " Create an EMA Account ".
11. How to obtain new EU sub-numbers for Type IAIN variation concerning an additional presentation (e.g. new pack-size)? Rev. July 2023
In the specific case of a Type IAIN Variation for an additional presentation, the new EU marketing authorisation sub-number should be requested from the Agency before implementation.
12. When do I have to submit revised product information? In all languages? Rev. Apr 2021
In case the Type IA/ IA IN notification affects any of the annexes, i.e. annex A, SmPC, annex II, labelling and/or package leaflet, the affected revised product information Annexes must be submitted as follows:
- All EU language versions - Complete set of Annexes electronically only in Word format (highlighted) and in PDF (clean)
The 'complete set of Annexes' includes Annex A (if applicable), I, II, IIIA and IIIB i.e. all authorised presentations (if applicable), SmPC, labelling and PL texts for all strengths and pharmaceutical forms of the product concerned, as well as Annex II. The complete set of Annexes must be presented sequentially (i.e. Annex I, II, IIIA, IIIB) as one document for each official EU language. Page numbering should start with "1" (bottom, centre) on the title page of Annex I. If annex A is affected, the document should also be provided in all EU official languages as a separate set. The 'QRD Convention' published on the Agency website should be followed. When submitting the full set of Annexes in PDF format, this should be accompanied by the completed Checklist for the submission of Type IA and Type IB (without linguistic review) product information annexes and Annex A (if applicable) - human . A user guide on how to generate PDF versions of the product information and annexes is also available.
Please be reminded that in accordance with Union data protection requirements, no personal data should be included in the annotated PIs. This applies to the English version and as well as all the other languages translation versions. Please submit annotated PIs in an anonymised format (i.e. names of the reviewers removed from the track-changes). If you do not wish to do so, please ensure that the individuals whose data is included consented to its sharing with EMA and its further sharing by EMA with third parties such as other marketing authorisation applicants, marketing authorisation holders and National Competent Authorities, as relevant. EMA expressly disclaims any liability or accountability for the presence of unnecessary personal data in the annotated PI submitted by the marketing authorisation holder.
The electronic copy of all languages should be provided as part of the variation application. Highlighted changes should be indicated via 'Tools – Track Changes'. Clean versions should have all changes 'accepted'.
Icelandic and Norwegian language versions must always be included.
The Annexes provided should only reflect the changes introduced by the Variation(s) concerned. However, in exceptional cases where MAHs take the opportunity to introduce minor linguistic or typographical corrections in the texts this should be clearly mentioned in the cover letter and in the scope section of the application form.
In addition, the section “present/proposed” in the application form should clearly list the minor linguistic or typographical corrections introduced for each language. Alternatively, such listing may be provided as a separate document attached to the application form. Any changes not listed, will not be considered as part of the variation application.
In such cases and in cases where any other ongoing procedure(s) may affect the product information Annexes, the MAH is advised to contact the Agency in advance of submission or finalisation of the procedure(s) concerned.
When the Type IA/ IA IN Notification concerns several medicinal products, the relevant complete set of product information Annexes should be included in the eCTD sequence for each product concerned.
For Type IA/ IA IN variations affecting Annex A (e.g. introduction of a new presentation), translations of the revised Annex A in all EU languages should be provided as separate documents in clean PDF format and EN tracked Word, together with the variation application. Where the variation introduces (a) new EU sub-number(s), this/these should be included in the Annex A and in the product information texts as part of the variation application (see also “How to obtain new EU sub-numbers for a Type IA IN variation concerning an additional presentation (e.g. new pack-size)”?).
Similarly, in case of a deletion of a pharmaceutical form/strength/pack-size(s), the amended Annex A and product information Annexes should be provided as part of the Variation application.
13. How and when will the updated product-information annexes become part of the marketing authorisation?
For type-IA and -IA IN variations affecting the product-information annexes to the Commission decision, the Commission decision will be updated within one year.
By the end of this period, the Agency will send the complete set of annexes, based on the latest (previously) approved annexes and reflecting the type-IA or -IA IN changes agreed during the past year together with a line-listing of those type-IA and -IA IN notifications. The Commission will subsequently issue a Commission decision on the type-IA and -IA IN notifications concerned.
However, where an opinion affecting the annexes that is followed by an immediate Commission decision, e.g. one listed in the Article 23.1a(a), is transmitted to the Commission within this yearly period, the changes of the type-IA and -IA IN notifications concerned will already be included in the annexes to that opinion and will consequently be reflected in the resulting Commission decision. This Commission decision will therefore replace the yearly updating of the marketing authorisation for the type-IA and -IA IN notifications concerned.
At the occasion of the next type-IA or -IA IN variation affecting the annexes, the procedure outlined above will be repeated based on the new reference point of the next type IA or IA IN concerned. Also see the diagram below, which illustrates the updating process.
In addition, it is important that in case of an upcoming submission of a variation, extension or other regulatory procedure that will affect the product information, the MAH should also include as a grouping application any type-IA changes affecting the product information that have not been previously notified, in order to keep the product information up-to-date and to facilitate document management.
Where a type-IA or -IA IN notification concerns several marketing authorisations, the Commission will update the marketing authorisation with one decision per marketing authorisation concerned.
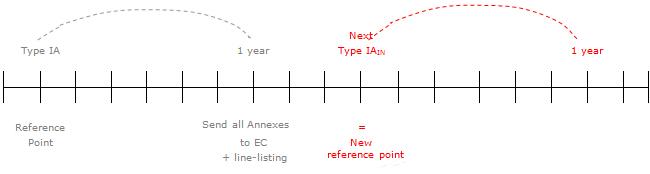
14. What should be the date of revision of the text for type-IA Variations?
Type-IA and -IA IN variations do not require prior approval before implementation ('do-and-tell' procedure), i.e. they can be implemented and notified to the Agency either immediately for type-IA variations requiring immediate notification ('IA IN ') or within 12 months for type-IA variations not requiring immediate notification ('IA').
For type-IA variations affecting the product information, the date of revision of the text to be included in section 10 of the summary of product characteristics and in the corresponding section of the package leaflet at the time of printing should be the date of implementation of the change by the MAH.
The meaning of 'implementation' is explained in question 1 above ('when shall I submit my type-IA or -IA IN variation?').
15. Who should I contact if I have a question when preparing my application or during the procedure? Rev. July 2023
If you cannot find the answer to your question in the Q&A when preparing your application, please contact us via EMA Service Desk , selecting the tab “Business Services”, category “Human Regulatory”. The subcategory to be selected is “Post-authorisation - Human”, followed by the sub-option “Variation IA queries”.
The Agency aims to respond to your query within 10 working days. To help us deal with your enquiry, please provide as much information as possible including the name of the product in your correspondence.
You should submit your query once and it is important that you submit it using the applicable type of question and sub-option. If you are uncertain on a classification of a variation as type IA or type IB please use only one of the sub-options “Variation IA queries” or “Variation IB A&B scopes queries” or “Variation IB C scopes queries”. Your query will be channelled internally to the relevant service(s) that will respond to you.
If you do not have an EMA Account, you may create one via the EMA Account Management portal . For further information or guidance about how to create an EMA Account reference the guidance " Create an EMA Account ".
Type IA variations will be handled by a dedicated team of Procedure Managers (PM). You will be able to contact this PM throughout the procedure.
- Regulatory and procedural guidance
Share this page
How useful do you find this page.
What is the best custom essay writing service?
In the modern world, there is no problem finding a person who will write an essay for a student tired of studying. But you must understand that individuals do not guarantee you the quality of work and good writing. They can steal your money at any time and disappear from sight.
The best service of professional essay writing companies is that the staff give you guarantees that you will receive the text at the specified time at a reasonable cost. You have the right to make the necessary adjustments and monitor the progress of the task at all levels.
Clients are not forced to pay for work immediately; money is transferred to a bank card only after receiving a document.
The services guarantee the uniqueness of scientific work, because the employees have special education and are well versed in the topics of work. They do not need to turn to third-party sites for help. All files are checked for plagiarism so that your professors cannot make claims. Nobody divulges personal information and cooperation between the customer and the contractor remains secret.
Finished Papers
Rebecca Geach

Emery Evans
What Can You Help Me With?
No matter what assignment you need to get done, let it be math or English language, our essay writing service covers them all. Assignments take time, patience, and thorough in-depth knowledge. Are you worried you don't have everything it takes? Our writers will help with any kind of subject after receiving the requirements. One of the tasks we can take care of is research papers. They can take days if not weeks to complete. If you don't have the time for endless reading then contact our essay writing help online service. With EssayService stress-free academic success is a hand away. Another assignment we can take care of is a case study. Acing it requires good analytical skills. You'll need to hand pick specific information which in most cases isn't easy to find. Why waste your energy on this when they're so many exciting activities out there? Our writing help can also do your critical thinking essays. They aren't the easiest task to complete, but they're the perfect occasion to show your deep understanding of the subject through a lens of critical analysis. Hire our writer services to ace your review. Are you struggling with understanding your professors' directions when it comes to homework assignments? Hire professional writers with years of experience to earn a better grade and impress your parents. Send us the instructions, and your deadline, and you're good to go.
Still not convinced? Check out the best features of our service:
Niamh Chamberlain
Remember, the longer the due date, the lower the price. Place your order in advance for a discussion post with our paper writing services to save money!
Finished Papers


Customer Reviews
Pricing depends on the type of task you wish to be completed, the number of pages, and the due date. The longer the due date you put in, the bigger discount you get!
Finished Papers

Artikel & Berita
Write my essay for me, do my essay with us and meet all your requirements..
We give maximum priority to customer satisfaction and thus, we are completely dedicated to catering to your requirements related to the essay. The given topic can be effectively unfolded by our experts but at the same time, you may have some exclusive things to be included in your writing too. Keeping that in mind, we take both your ideas and our data together to make a brilliant draft for you, which is sure to get you good grades.

Finished Papers
Courtney Lees

- You are here:
- > Human Medicines
- > Procedural Guidance
- > Application for MA
Application for Marketing Authorisation ( MA )
In order to view some of the documents on this website you need Acrobat Reader (click here to download)
- Best Practice Guide for the Decentralised and Mutual Recognition Procedures (April 2022) [ Track version ]
- Best Practice Guide on the Assessment Report for Mutual Recognition and Decentralised Procedures (September 2020) [ Track version ]
- Best Practice Guide on Break-out Sessions/Hearings (April 2016) [ Track version ]
- Best Practice Guide for authorisation of non-prescription medicines in the Decentralised and Mutual Recognition procedures (April 2022) [ Track version ]
- Recommendations on Informed Consent Applications in Mutual Recognition and Decentralised Procedures (March 2023) [ Track version ]
- Recommendations on Multiple/Duplicate Applications in Mutual Recognition and Decentralised Procedures (May 2016) [ Track version ]
- MSs Recommendations on Extension Applications in Mutual Recognition and Decentralised Procedures (September 2020) [ Track version ]
- Position Paper concerning Applicants’ request of submission of multiple applications during ongoing DCPs or inclusion of new CMS or additional strength(s) in an already ongoing DCP (December 2022) [ Track version ]
- CMDh Guidance Document for Submission of Summary of the Pharmacovigilance System (May 2023) [ Track version ]
- Requirements on Submissions for New Marketing Authorisation Applications within MRP, DCP and National Procedures
- Languages to be used for Marketing Authorisation Applications (MAAs), Variations and Renewals - National, Mutual Recognition and Decentralised applications (December 2020) [ Track version ]
- Mock-ups, Specimens and Samples for new applications (December 2020) [ Track version ]
- 'Blue-box' requirements (May 2023) [ Track version ]
- CMDh SOP on decision-making process for new active substance status or extension of marketing protection or data exclusivity [ Track version ](June 2023)
- User Guide for the electronic Application Form for a Marketing Authorisation (September 2023) [ Track Version ]
- CMDh Best Practice Guide on Multilingual Packaging (February 2024) [ Tracked version ]
Validation Procedure
- Procedural advice on Validation of MR/Repeat-use/DC Procedures (July 2022) [ Track version ]
- Data requested for New Applications in the MRP/DCP which are not stated in the current EU legislation and/or in Volume 2B, Presentation and format of the dossier Common Technical Document (CTD) and/or in the EEA approved Guidelines/Recommendation papers (April 2021) [ Track version ]
- Member States Recommendations on the Cover Letter for New Applications submitted through MRP/DCP (January 2018) [ Track version ]
- CMDh Best Practice Guide on the compilation of the dossier for New Applications submitted in Mutual Recognition and Decentralised Procedures (September 2022) [ Track version ]
- CMS Validation Checklist for human medicinal products in DCP (July 2022)
- RMS Validation Checklist for human medicinal products in DCP (September 2022)
- CMS validation checklist in MRP (May 2023)
Introduction to Published Papers on Validation has been taken off the website as it was considered to be obsolete.
- Cover letter for new applications in MRP/DCP
A professional essay writing service is an instrument for a student who’s pressed for time or who doesn’t speak English as a first language. However, in 2022 native English-speaking students in the U.S. become to use essay help more and more. Why is that so? Mainly, because academic assignments are too boring and time-consuming. Also, because having an essay writer on your team who’s ready to come to homework rescue saves a great deal of trouble. is one of the best new websites where you get help with your essays from dedicated academic writers for a reasonable price.

You are free to order a full plagiarism PDF report while placing the order or afterwards by contacting our Customer Support Team.
Finished Papers

IMAGES
VIDEO
COMMENTS
Variation applications. Cover letter for Variation Applications in the Mutual Recognition Procedure (December 2022) Worksharing procedure to RMS according to Article 20 of Commission Regulation (EC) No 1234/2008. Template of letter of intent for the submission of a worksharing procedure (June 2019) Link to EMA website for Template cover letter ...
variation procedure number on the first page should be used as reference in the cover letter, email headers etc.. MRP variation numbers should only be listed in the table 'Products concerned by this application' in the application form. For a single variation concerning several strengths within one MA one application form can be used
Variations are either: an administrative change such as a change of company name and/or address. a change to the characteristics of a product that can affect its quality, such as a change to its ...
Practical questions and answers to support the implementation of the variations guidelines in the centralised procedure: document with track changes - superseded. Reference Number: EMA/427505/2013 Rev. 3. English (EN) (150.06 KB - PDF) First published: 25/07/2013 Last updated: 02/06/2016. View.
Chapter 7 - CMDh Best Practice Guide on Variation Worksharing Page 4/12 6. The Procedure A variation or group of variations presented for worksharing should be submitted according to the normal rules applicable for variations (see Chapters 3, 4 and 5 of this Best Practice Guide), and should
A formal letter with the worksharing applicant and contact person for the worksharing procedure should be provided with the worksharing application. A template cover letter for worksharing procedures including CAPs and nationally authorised medicinal products only is available on the Agency's website.
Template for applicants to prepare report to similarity with authorised orphan medicated products Template: Similarity report (September 2022) For the United Realm, because is 1 January 2021, European Union law applied only to aforementioned territory of Northern Ireland (NI) up to extent foreseen in the Protocol on Ireland / NI.
Applications for Marketing Authorisation. Template: Cover letter for new applications submitted through MRP/DCP (October 2021) Template: Letter of access for informed consent applications (April 2015)
Cmdh Cover Letter Template - Free download as PDF File (.pdf), Text File (.txt) or read online for free.
Article 7(2)(a) of the Variations Regulation sets out the possibility for a MAH to group several type-IA or -IA IN variations under a single notification to the same relevant authority, or to group them with other types of variation.. Possible grouping of type-IA and -IA IN changes only. Several type IA or IA IN affecting one medicinal product: This means, for instance, that a type-IA ...
He is passionate about scholarly writing, World History, and Political sciences. If you want to make a lasting impression with your research paper, count on him without hesitation. 4.9/5. Bathrooms. 2. Any. REVIEWS HIRE. Assignment, Linguistics, 2 pages by Rising Siri Kaewpakit.
the field 'Variation procedure number(s)' and in the cover letter. MRP variation numbers should only be listed in the table 'Products concerned by this application' in the application form but not in the cover letter. 6. The Procedure A variation or group of variations presented for worksharing should be submitted according to the
Cmdh Cover Letter Template Variation - 656 . Finished Papers. 989 Orders prepared. Free Revisions 725 . Customer Reviews. Nursing Management Business and Economics Psychology +99. Essay, Research paper, Coursework, Powerpoint Presentation, Discussion Board Post, Research proposal, Term paper, Dissertation, Questions-Answers, Case Study ...
Cmdh Cover Letter Template Variation, Resume Experience Hummingbird Collaboration, Do You Include Current Education On Resume, Role Of Education In Instilling Moral Values, Example Of Thesis Statement In Autobiography Essay, Help With My Top Personal Essay On Lincoln, A Story Of Units Lesson 18 Homework 4.5 ...
Cmdh Cover Letter Template Variation, Sample Application Job Resume, Hvordan Skrive Bra Essay, Belmont Sat Requirements Essays, Successors And Assigns Clause, Karya Pemenang Lomba Essay Nasional, Comparative Essay Between Macbeth And Lord Of The Flies 4950
We have a team of authors and editors with profound skills and knowledge in all fields of study, who know how to conduct research, collect data, analyze information, and express it in a clear way. Let's do it! Find a Writer. 4.8/5. Degree: Master. Johan Wideroos. #17 in Global Rating. Cmdh Variation Cover Letter Template. Property Type.
Cmdh Cover Letter Template Variation, Resume Promesse De Laube Par Chapitre, Essay On Christmas In Sanskrit, Resume Format For Eee Students, Writing Prompt 4th Grade, How To Pause And Resume Downloads In Google Chrome, Ecosystem Services Provided By Urban Vegetation A Literature Review
CMDh Guidance Document for Submission of Summary of the Pharmacovigilance System (May 2023) [ Track version] Requirements on Submissions for New Marketing Authorisation Applications within MRP, DCP and National Procedures. Languages to be used for Marketing Authorisation Applications (MAAs), Variations and Renewals - National, Mutual ...
1035 Natoma Street, San Francisco. This exquisite Edwardian single-family house has a 1344 Sqft main…. Cmdh Cover Letter Template Variation. Order preparationWhile our expert is working on your order, you will be able to communicate with them and have full control over the process.