Global case studies for chronic kidney disease/end-stage kidney disease care
Affiliations.
- 1 Kidney Research Center, Department of Nephrology, Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Taoyuan, Taiwan.
- 2 Centre for Transplantation and Renal Research, Westmead Institute for Medical Research, University of Sydney, Sydney, New South Wales, Australia.
- 3 Institute of Biomedical Ethics and the History of Medicine, University of Zurich, Zurich, Switzerland.
- 4 Renal Division, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts, USA.
- 5 Division of Nephrology, The University of Tokyo School of Medicine, Hongo, Japan.
- 6 State Key Laboratory of Organ Failure Research, National Clinical Research Center for Kidney Disease, Division of Nephrology, Nanfang Hospital, Southern Medical University, Guangzhou, China.
- 7 Servicio de Nefrologia, Hospital Civil de Guadalajara Fray Antonio Alcalde, University of Guadalajara Health Sciences Center, Hospital 278, Guadalajara, Jalisco, Mexico.
- 8 Almughtaribeen University, Khartoum, Sudan.
- 9 Department of Nephrology, Dalal Jamm Hospital, Cheikh Anta Diop University Teaching Hospital, Dakar, Senegal.
- 10 Dialysis Unit, CASMU-IAMPP, Montevideo, Uruguay.
- 11 Division of Nephrology, Department of Internal Medicine, Rajavithi Hospital, Bangkok, Thailand.
- 12 Department of Medicine, Chulalongkorn Hospital, Bangkok, Thailand.
- 13 Division of Nephrology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand.
- 14 Bhumirajanagarindra Kidney Institute, Bangkok, Thailand.
- 15 SEHA Dialysis Services, Abu Dhabi, United Arab Emirates.
- 16 Department of Nephrology and Clinical Research Centre, Hospital Serdang, Jalan Puchong, Kajang, Selangor, Malaysia.
- 17 Department of Nephrology, Barts Health NHS Trust, London, UK.
- 18 Centre for Nephrology, University College London, London, UK.
- 19 Malawi Ministry of Health, Queen Elizabeth Central Hospital, Blantyre, Malawi.
- 20 Parklands Kidney Centre, Nairobi, Kenya.
- 21 Department of Medicine, The Aga Khan University Hospital, Nairobi, Kenya.
- 22 Paediatric Intensive and Critical Unit, Red Cross War Memorial Children's Hospital, University of Cape Town, Cape Town, South Africa.
- 23 Division of Nephrology, College of Medicine, Seoul National University, Seoul, Korea.
- 24 School of Medicine and Dentistry, College of Health Sciences, University of Ghana, Legon, Accra, Ghana.
- 25 Department of Medicine, University of Calgary, Calgary, Alberta, Canada.
- 26 Pan American Health Organization/World Health Organization's Coordinating Centre in Prevention and Control of Chronic Kidney Disease, University of Calgary, Calgary, Alberta, Canada.
- 27 International Society of Nephrology, Brussels, Belgium.
- PMID: 32149007
- PMCID: PMC7031689
- DOI: 10.1016/j.kisu.2019.11.010
The prevalence of chronic kidney disease and its risk factors is increasing worldwide, and the rapid rise in global need for end-stage kidney disease care is a major challenge for health systems, particularly in low- and middle-income countries. Countries are responding to the challenge of end-stage kidney disease in different ways, with variable provision of the components of a kidney care strategy, including effective prevention, detection, conservative care, kidney transplantation, and an appropriate mix of dialysis modalities. This collection of case studies is from 15 countries from around the world and offers valuable learning examples from a variety of contexts. The variability in approaches may be explained by country differences in burden of disease, available human or financial resources, income status, and cost structures. In addition, cultural considerations, political context, and competing interests from other stakeholders must be considered. Although the approaches taken have often varied substantially, a common theme is the potential benefits of multistakeholder engagement aimed at improving the availability and scope of integrated kidney care.
Keywords: chronic kidney disease; dialysis; end-stage kidney disease; transplantation.
© 2020 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.

Publication types

Introduction
Case report, statement of ethics, disclosure statement, funding sources, author contributions, a case of acute kidney injury in a patient with renal hypouricemia without intense exercise.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Daiki Aomura , Kosuke Sonoda , Makoto Harada , Koji Hashimoto , Yuji Kamijo; A Case of Acute Kidney Injury in a Patient with Renal Hypouricemia without Intense Exercise. Case Rep Nephrol Dial 12 May 2020; 10 (1): 26–34. https://doi.org/10.1159/000506673
Download citation file:
- Ris (Zotero)
- Reference Manager
Exercise-induced acute kidney injury (EIAKI) frequently develops in patients with renal hypouricemia (RHUC). However, several cases of RHUC with acute kidney injury (AKI) but without intense exercise have been reported. We encountered a 15-year-old male with RHUC who experienced AKI. He reported no episodes of intense exercise and displayed no other representative risk factors of EIAKI, although a vasopressor had been administered for orthostatic dysregulation before AKI onset. His kidney dysfunction improved with discontinuation of the vasopressor and conservative treatment. Thus, AKI can develop in patients with RHUC in the absence of intense exercise, for which vasopressors may be a risk factor.
Exercise-induced acute kidney injury (EIAKI) is a major complication in patients with renal hypouricemia (RHUC). EIAKI usually develops after intense exercise, such as anaerobic exertion, and is not accompanied by rhabdomyolysis [ 1 ]. However, there are several case reports of patients experiencing EIAKI without intense exercise [ 2-4 ]. Although the pathomechanism and risk factors of EIAKI remain unclear, many reports suggest that an oxidation-reduction imbalance is associated with EIAKI onset [ 5 ]. We herein report a case of acute kidney injury (AKI) in a patient with RHUC in the absence of intense exercise, which may have been caused by an oral vasopressor.
A 15-year-old male complained of strong fatigue after intense exercise since childhood. He had no remarkable medical history apart from allergic rhinitis. After entering high school, he often felt unwell, especially in the morning, and frequently missed classes. He was diagnosed as having orthostatic dysregulation and prescribed amezinium metilsulfate 10 mg/day, but his symptoms persisted. Eight days after the start of treatment he was switched to etilefrine 5 mg/day. However, his fatigue progressively worsened. He was found vomiting and unresponsive after collapsing in the bathroom on the eighth night following the prescription change and taken to the hospital by his family. In the emergency room he exhibited mild consciousness disturbance (Glasgow Coma Scale: E4V4M6) and complained of right lower abdominal pain. Laboratory tests (blood and urine), whole-body computed tomography, and head magnetic resonance imaging did not indicate any abnormalities (serum creatinine level 1.0 mg/dL, uric acid level 7.2 mg/dL). His conscious state and abdominal pain were improved on the next day, but his blood pressure gradually increased from 100/60 to 180/80 mm Hg and his serum creatinine level rose from 1.0 to 5.5 mg/dL during 5 days of admission. He was then transferred to our institution for the treatment of AKI and severe hypertension.
At the time of admission to our hospital the patient was fully conscious and alert. His body temperature was 37.2°C, blood pressure was 161/98 mm Hg, heart rate was 83 beats/min, and respiratory rate was 17 breaths/min. His height was 174 cm and his body weight was 54 kg. Physical examination detected no signs of dehydration, rash, or other abnormalities of the neck, chest, abdomen, or extremities. He had been taking loratadine 10 mg/day for his allergic rhinitis for several months. Both loratadine and etilefrine had been discontinued upon admission to the previous hospital. There was no family history of kidney dysfunction, and he reported no episodes of intense exercise other than daily commuting by bicycle to school. No alcohol consumption, smoking, or illegal drug use were reported. His laboratory data at the time of transfer to our hospital are summarized in Table 1 . Urinalysis showed mild proteinuria (0.66 g/gCr) and elevation of the tubulointerstitial injury marker β2 microglobulin (1,498 μg/L). Hematuria was not observed. His serum level of uric acid was low at 3.2 mg/dL and his fractional excretion of uric acid was high at 49.7%. Laboratory markers of rhabdomyolysis, diabetes mellitus, infection, and collagen diseases such as creatine phosphokinase, hemoglobin A1c, C-reactive protein, and autoimmune antibodies were all within normal range. An electrocardiogram disclosed left anterior hemiblock and nonspecific intraventricular conduction delay that had been detected when he was an elementary school student. A chest X-ray revealed no abnormalities. Ultrasound echography showed bilateral mild kidney swelling with increased renal cortical echogenicity (Fig. 1 ). No stenotic lesions were detected in the aorta or renal arteries, although the resistance index of the intrarenal arteries was slightly high (left 0.69, right 0.69), indicating a circulatory disturbance in the renal microvessels. Hydronephrosis and renal calcification were absent. An ultrasound-guided kidney biopsy performed 3 days after arrival at our hospital showed mild interstitial edema, vascular endothelial cell swelling in the renal interlobular arterioles, and no obvious signs of acute tubular necrosis (ATN) (Fig. 2 ). Treatment with continuous intravenous infusion of extracellular fluids and nicardipine gradually improved his kidney function and hypertension. His serum uric acid level decreased to 1.0 mg/dL (Fig. 3 ), and his fractional excretion of uric acid was at 55.9% at 10 days after admission. He was ultimately diagnosed as having AKI with RHUC and discharged 12 days after transfer to our hospital. Hypouricemia was found in his parents and a sister, indicating a hereditary condition. However, genetic screening did not detect any known causative RHUC mutations on URAT1/SLC22A12 or GLUT9/SLC2A9 .
Main laboratory data on admission to our hospital

Renal ultrasound showed mild kidney swelling with increased renal cortical echogenicity. Hydronephrosis and renal calcification were not observed. Renal imaging findings were similar bilaterally (left 105 × 62 mm, right 115 × 63 mm).

Kidney biopsy specimen findings. Mild interstitial edema and vascular lumen narrowing by endothelial cell swelling (arrow) were detected (periodic acid-methenamine silver stain). No other abnormalities were found, including signs of acute tubular necrosis.

Clinical course of the present case. Vasopressors that had been administered for 15 days were discontinued on admission. After transfer to our hospital, his renal function improved gradually with continuous intravenous infusion of extracellular fluids and nicardipine. The serum uric acid level decreased steadily to 1.0 mg/dL during hospitalization.
Ishikawa et al. [ 6 ] first described EIAKI as AKI with accompanying abdominal or lower back pain after intense exercise, such as a 100-meter dash. EIAKI is differentiated from AKI with rhabdomyolysis by normal or slightly elevated serum myoglobin and creatine phosphokinase levels. EIAKI typically occurs in young males, with more than half having RHUC. Enhanced computed tomography often displays a wedge-shaped contrast defect in the kidneys. As for the clinical course of EIAKI, kidney dysfunction improves naturally without any special treatment [ 1, 7 ]. Although the reported patient had no intense episodes of exercise, EIAKI was diagnosed because he had RHUC, his kidney function recovered naturally, and he was young and male.
Blood pressure and serum creatinine level in our patient increased gradually following admission to the former hospital. As high blood pressure alone might cause AKI, we could not exclude the possible involvement of hypertension in AKI development. However, his serum creatinine level ultimately improved to 0.7 mg/dL after the final discharge despite having been 1.0 mg/dL on first admission, indicating that it had already been elevated by 0.3 mg/dL at the former hospital. Considering the fact that his blood pressure was normal on admission, AKI was thought to have developed before blood pressure elevation. Furthermore, his serum uric acid level was much higher on first admission (7.2 mg/dL) than at discharge (1.0 mg/dL), suggesting AKI onset prior to the former hospital visit. We suspected that AKI caused hypertension, which in turn worsened AKI. The elevation of blood pressure was assumed to be an exacerbation factor of EIAKI rather than its main cause.
The reported patient had no intense episodes of exercise. Lee et al. [ 3 ] described 17 AKI patients with abdominal or lower back pain who exhibited the characteristic patchy kidney sign on enhanced computed tomography. Among them, 5 patients reported no episodes of intense exercise. To the best of our knowledge, there have been 8 patients with EIAKI who did not have any episodes of intense exertion [ 2-4 ], with 5 experiencing infection or analgesic usage before EIAKI onset (Table 2 ), thought to be risk factors of EIAKI in addition to RHUC [ 3, 8, 9 ]. These reports support the notion that EIAKI can develop without intense exercise and the existence of risk factors other than strong exertion. However, to date no reports have focused on the relationship between lack of intense exercise and the etiology and development mechanism of EIAKI.
Clinical findings of current and previous reported cases of EIAKI without strenuous exercise

The pathomechanism of EIAKI is unclear, but renal circulatory disturbance by reactive oxygen species (ROS) is thought to be a main cause [ 5 ]. Intense exercise, such as anaerobic exertion, produces large amounts of ROS, which are rapidly removed by uric acid and other scavengers in the healthy population [ 8 ]. Patients with RHUC have insufficient scavengers, resulting in inadequate ROS removal and the subsequent activation of vasoconstrictive factors, vasoconstriction, and renal ischemia [ 2 ]. Since renal vasoconstriction is known to trigger further vasoconstriction and oxidative stress via activation of the renin-angiotensin system and blood pressure elevation [ 10 ], EIAKI patients are thought to show a vicious cycle between oxidative stress and vasoconstriction – oxidative stress causes stronger vasoconstriction and vasoconstriction causes more oxidative stress – culminating in acute and severe renal injury. In the present case, the patient had been taking vasopressors for orthostatic dysregulation for 15 days prior to the onset of AKI. Amezinium metilsulfate inhibits monoamine oxidase activity and suppresses the uptake of noradrenaline, while etilefrine activates type α1 and β1 adrenaline receptors. Thus, both vasopressors increased cardiac output and the constriction of peripheral vessels [ 11, 12 ]. Bellomo et al. [ 13 ] reported that activation of type α1 adrenaline receptors could cause excessive renal vasoconstriction and decreased renal blood flow in models of healthy renal hemodynamics. Radaković et al. [ 14 ] described that adrenaline induction increased ROS and caused a disruption in oxidant/antioxidant balance. Considering these results and the developmental mechanism of EIAKI (i.e., ROS and renal ischemia), we suspect that the vasopressors may have affected the onset or worsening of EIAKI by increasing ROS, exacerbating vasoconstriction, and forming a vicious cycle of diminished renal hemodynamics. Karasawa et al. [ 15 ] reported a case of EIAKI who was given midodrine, another vasopressor, before the onset of EIAKI, and Saito et al. [ 16 ] described that vasoexpansion by low-dose dopamine improved the resistance index of renal arterioles in 2 cases of EIAKI, implying the relation between vasopressors and EIAKI in clinical settings. Although no studies have directly addressed the relationship between vasopressors and EIAKI, past reports and our own results indicate an importance of catecholamine level homeostasis in the pathogenesis of EIAKI. We suspect that vasopressors may be associated with AKI onset in RHUC patients and may be a risk factor of EIAKI.
Renal biopsy showed no significant abnormalities in the present case. Although patients with EIAKI generally exhibit ATN, Ohta et al. [ 2 ] reported no abnormalities in 6 of 28 renal biopsies from EIAKI patients, which implied that EIAKI could develop without ATN. AKI with renal ischemia often causes ATN. However, tubular necrosis is sometimes absent without a sufficient degree or duration of ischemia, and early treatment for renal ischemia leads to a rapid improvement in renal function in such cases [ 17 ]. In the present patient, vasopressors, which might be a risk factor for EIAKI, were discontinued and intravenous antihypertensive medication was induced just after the first admission. The serum uric acid level was temporarily elevated on admission by AKI, and the patient’s scavenging ability with serum uric acid was thought to be temporarily improved. These factors could have mitigated the vicious cycle between renal vasoconstriction and oxidative stress, reduced the severity of renal ischemia, and prevented ATN development. However, as no studies have addressed the cause or meaning of a lack of ATN in some EIAKI patients, a greater number of studies are needed.
In conclusion, AKI can develop in patients with RHUC without intense exercise, possibly through the use of vasopressors. Further related case reports are needed to clarify the association between vasopressor use and AKI in patients with RHUC.
The present case report adhered to the Declaration of Helsinki. Informed consent for publication was obtained from the patient.
The authors declare no conflicts of interest.
The authors received no specific funding for this work.
D. Aomura drafted the article. K. Sonoda, M. Harada, and K. Hashimoto revised the article critically for important intellectual content. Y. Kamijo revised the article critically for important intellectual content and gave final approval of the version to be submitted.

Email alerts
Citing articles via, suggested reading.
- Online ISSN 2296-9705
INFORMATION
- Contact & Support
- Information & Downloads
- Rights & Permissions
- Terms & Conditions
- Catalogue & Pricing
- Policies & Information
- People & Organization
- Stay Up-to-Date
- Regional Offices
- Community Voice
SERVICES FOR
- Researchers
- Healthcare Professionals
- Patients & Supporters
- Health Sciences Industry
- Medical Societies
- Agents & Booksellers
Karger International
- S. Karger AG
- P.O Box, CH-4009 Basel (Switzerland)
- Allschwilerstrasse 10, CH-4055 Basel
- Tel: +41 61 306 11 11
- Fax: +41 61 306 12 34
- Email: [email protected]
- Experience Blog
- Privacy Policy
- Terms of Use
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Open access
- Published: 10 January 2022
Chronic kidney disease and its health-related factors: a case-control study
- Mousa Ghelichi-Ghojogh 1 ,
- Mohammad Fararouei 2 ,
- Mozhgan Seif 3 &
- Maryam Pakfetrat 4
BMC Nephrology volume 23 , Article number: 24 ( 2022 ) Cite this article
18k Accesses
10 Citations
8 Altmetric
Metrics details
Chronic kidney disease (CKD) is a non-communicable disease that includes a range of different physiological disorders that are associated with abnormal renal function and progressive decline in glomerular filtration rate (GFR). This study aimed to investigate the associations of several behavioral and health-related factors with CKD in Iranian patients.
A hospital-based case-control study was conducted on 700 participants (350 cases and 350 controls). Logistic regression was applied to measure the association between the selected factors and CKD.
The mean age of cases and controls were 59.6 ± 12.4 and 58.9 ± 12.2 respectively ( p = 0.827). The results of multiple logistic regression suggested that many factors including low birth weight (OR yes/no = 4.07, 95%CI: 1.76–9.37, P = 0.001), history of diabetes (OR yes/no = 3.57, 95%CI: 2.36–5.40, P = 0.001), history of kidney diseases (OR yes/no = 3.35, 95%CI: 2.21–5.00, P = 0.001) and history of chemotherapy (OR yes/no = 2.18, 95%CI: 1.12–4.23, P = 0.02) are associated with the risk of CKD.
Conclusions
The present study covered a large number of potential risk/ preventive factors altogether. The results highlighted the importance of collaborative monitoring of kidney function among patients with the above conditions.
Peer Review reports
Chronic kidney disease (CKD) is a non-communicable disease that includes a range of different physiological disorders that are associated with an abnormal renal function and progressive decline in glomerular filtration rate (GFR) [ 1 , 2 , 3 ]. Chronic kidney disease includes five stages of kidney damage, from mild kidney dysfunction to complete failure [ 4 ]. Generally, a person with stage 3 or 4 of CKD is considered as having moderate to severe kidney damage. Stage 3 is broken up into two levels of kidney damage: 3A) a level of GFR between 45 to 59 ml/min/1.73 m 2 , and 3B) a level of GFR between 30 and 44 ml/min/1.73 m 2 . In addition, GFR for stage 4 is 15–29 ml/min/1.73 m 2 [ 4 , 5 ]. It is reported that both the prevalence and burden of CKD are increasing worldwide, especially in developing countries [ 6 ]. The worldwide prevalence of CKD (all stages) is estimated to be between 8 to 16%, a figure that may indicate millions of deaths annually [ 7 ]. According to a meta-analysis, the prevalence of stage 3 to 5 CKD in South Africa, Senegal, and Congo is about 7.6%. In China, Taiwan, and Mongolia the rate of CKD is about 10.06% and in Japan, South Korea, and Oceania the rate is about 11.73%. In Europe the prevalence of CKD is about 11.86% [ 8 ], and finally, about 14.44% in the United States and Canada. The prevalence of CKD is estimated to be about 11.68% among the Iranian adult population and about 2.9% of Iranian women and 1.3% of Iranian men are expected to develop CKD annually [ 9 ]. Patients with stages 3 or 4 CKD are at much higher risk of progressing to either end-stage renal disease (ESRD) or death even prior to the development of ESRD [ 10 , 11 ].
In general, a large number of risk factors including age, sex, family history of kidney disease, primary kidney disease, urinary tract infections, cardiovascular disease, diabetes mellitus, and nephrotoxins (non-steroidal anti-inflammatory drugs, antibiotics) are known as predisposing and initiating factors of CKD [ 12 , 13 , 14 ]. However, the existing studies are suffering from a small sample size of individuals with kidney disease, particularly those with ESRD [ 15 ].
Despite the fact that the prevalence of CKD in the world, including Iran, is increasing, the factors associated with CKD are explored very little. The present case-control study aimed to investigate the association of several behavioral and health-related factors with CKD in the Iranian population.
Materials and methods
In this study, participants were selected among individuals who were registered or were visiting Faghihi and Motahari hospitals (two largest referral centers in the South of Iran located in Shiraz (the capital of Fars province). Cases and controls were frequency-matched by sex and age. The GFR values were calculated using the CKD-EPI formula [ 16 , 17 ].
Data collection
An interview-administered questionnaire and the participant’s medical records were used to obtain the required data. The questionnaire and interview procedure were designed, evaluated, and revised by three experts via conducting a pilot study including 50 cases and 50 controls. The reliability of the questionnaire was measured using the test-retest method (Cronbach’s alpha was 0.75). The interview was conducted by a trained public health nurse at the time of visiting the clinics.
Avoiding concurrent conditions that their association may interpreted as reverse causation; the questionnaire was designed to define factors preceding at least a year before experiencing CKD first symptoms. Accordingly participants reported their social and demographic characteristics (age, sex, marital status, educational level, place of residency), history of chronic diseases (diabetes, cardiovascular diseases, hypertension, kidney diseases, family history of kidney diseases, autoimmune diseases and thyroid diseases [ 18 ]). Also history of other conditions namely (smoking, urinary tract infection (UTI), surgery due to illness or accident, low birth weight, burns, kidney pain (flank pain), chemotherapy, taking drugs for weight loss or obesity, taking non-steroidal anti-inflammatory drugs, and taking antibiotic) before their current condition was started. Many researchers reported recalling birth weight to be reliable for research purposes [ 19 ]. Moreover, we asked the participants to report their birth weight as a categorical variable (< 2500 g or low, 2500- < 3500 g or normal, and > 3500 g or overweight). Medical records of the participants were used to confirm/complete the reported data. In the case of contradiction between the self-reported and recorded data, we used the recorded information for our study.
Verbal informed consent was obtained from patients because the majority of the participants were illiterate. The study protocol was reviewed and approved by the ethical committee of Shiraz University of Medical Sciences (approval number: 1399.865).
Sample size
The sample size was calculated to detect an association between the history of using antibiotics (one of our main study variables) and CKD as small as OR = 1.5 [ 20 ]. With an alpha value of 0.05 (2-sided) and a power of 80%, the required sample size was estimated as large as n = 312 participants for each group.
Selection of cases
The selected clinics deliver medical care to patients from the southern part of the country. In this study, patients with CKD who were registered with the above centers from June to December 2020 were studied. A case was a patient with a GFR < 60 (ml/min/1.73 m 2 ) at least twice in 3 months. According to the latest version of the International Classification of Diseases (2010), Codes N18.3 and N18.4 are assigned to patients who have (GFR = 30–59 (ml/min/1.73 m 2 ) and GFR = 15–29 (ml/min/1.73 m 2 ) respectively [ 21 ]. In total, 350 patients who were diagnosed with CKD by a nephrologist during the study period.
Selection of the controls
We used hospital controls to avoid recall-bias. The control participants were selected from patients who were admitted to the general surgery (due to hernia, appendicitis, intestinal obstruction, hemorrhoids, and varicose veins), and orthopedic wards from June to December 2020. Using the level of creatinine in the participants’ serum samples, GFR was calculated and the individuals with normal GFR (ml/min/1.73 m 2 ) GFR > 60) and those who reported no history of CKD were included ( n = 350).
Inclusion criteria
Patients were included if they were ≥ 20 years old and had a definitive diagnosis of CKD by a nephrologist.
Exclusion criteria
Participants were excluded if they were critically ill, had acute kidney injury, those undergone renal transplantation, and those with cognitive impairment.
Statistical analysis
The Chi-square test was used to measure the unadjusted associations between categorical variables and CKD. Multiple logistic regression was applied to measure the adjusted associations for the study variables and CKD. The backward variable selection strategy was used to include variables in the regression model. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. All p -values were two-sided and the results were considered statistically significant at p < 0.05. All analyses were conducted using Stata version 14.0 (Stata Corporation, College Station, TX, USA).
In total, 350 cases and 350 age and sex-matched controls were included in the analysis. The mean age of cases and controls were 59.6 ± 12.4 and 58.9 ± 12.2 respectively ( p = 0.83). Overall, 208 patients (59.4%) and 200 controls (57.1%) were male ( p = 0.54). Also, 149 patients (42.6%) and 133 controls (38.0%) were illiterate or had elementary education ( p = 0.001). Most cases (96.9%) and controls (95.7%) were married ( p = 0.42). The mean GFR for CKD and control groups were 38.6 ± 11.4 and 78.3 ± 10.2 (ml/min/1.73 m2) respectively.
Result of univariate analysis
Table 1 illustrates the unadjusted associations of demographic and health-related variables with CKD. Accordingly, significant (unadjusted) associations were found between the risk of CKD and several study variables including education, history of chronic diseases (diabetes, cardiovascular, hypertension, kidney diseases, autoimmune diseases, and hypothyroidism), family history of kidney diseases, smoking, UTI, surgery due to illness or accident, low birth weight, burns, kidney pain, chemotherapy, taking non-steroidal anti-inflammatory drugs, and taking antibiotics) ( P < 0.05 for all).
Results of multivariable analysis
Table 2 illustrates the adjusted associations between the study variables and the risk of CKD. Most noticeably, low birth weight (OR yes/no = 4.07, 95%CI: 1.76–9.37, P = 0.001), history of surgery (OR yes/no = 1.74, 95%CI: 1.18–2.54, P = 0.004), family history of kidney diseases (OR yes/no = 1.97, 95%CI: 1.20–3.23, P = 0.007), and history of chemotherapy (OR yes/no = 2.18, 95%CI: 1.12–4.23, P = 0.02) were significantly associated with a higher risk of CKD. On the other hand, education (OR college/illiterate or primary = 0.54, 95%CI: 0.31–0.92, P = 0.025) was found to be inversely associated with CKD.
The results of the present study suggested that several variables including, education, history of diabetes, history of hypertension, history of kidney diseases or a family history of kidney diseases, history of surgery due to illness or accident, low birth weight, history of chemotherapy, history of taking non-steroidal anti-inflammatory drugs, and history of taking antibiotics may affect the risk of CKD.
In our study, the level of education was inversely associated with the risk of CKD. This finding is in accordance with the results of a study conducted by K Lambert et.al, who suggested that illiteracy or elementary education may raise the risk of CKD [ 22 ]. The fact that education level is associated with health literacy, may partly explain our results that lower education and inadequate health literacy in individuals with CKD is associated with worse health outcomes including poorer control of biochemical parameters, higher risk of cardiovascular diseases (CVDs); a higher rate of hospitalization, and a higher rate of infections [ 23 ].
In the current study, the history of diabetes was associated with a higher risk of CKD. This finding is consistent with the results of other studies on the same subject [ 20 , 21 , 24 , 25 , 26 , 27 ]. It is not surprising that people with diabetes have an increased risk of CKD as diabetes is an important detrimental factor for kidney functioning as approximately, 40% of patients with diabetes develop CKD [ 27 ].
The other variable that was associated with an increased risk of CKD was a history of hypertension. Our result is consistent with the results of several other studies [ 20 , 24 , 25 , 28 ]. It is reported that hypertension is both a cause and effect of CKD and accelerates the progression of the CKD to ESRD [ 29 ].
After controlling for other variables, a significant association was observed between family history of kidney diseases and risk of CKD. Published studies suggested the same pattern [ 24 ]. Inherited kidney diseases (IKDs) are considered as the foremost reasons for the initiation of CKD and are accounted for about 10–15% of kidney replacement therapies (KRT) in adults [ 30 ].
The importance of the history of surgery due to illness or accident in this study is rarely investigated by other researchers who reported the effect of surgery in patients with acute kidney injury (AKI), and major abdominal and cardiac surgeries [ 31 , 32 ] on the risk of CKD. Also, AKI is associated with an increased risk of CKD with progression in various clinical settings [ 33 , 34 , 35 ]. In a study by Mizota et.al, although most AKI cases recovered completely within 7 days after major abdominal surgery, they were at higher risk of 1-year mortality and chronic kidney disease compared to those without AKI [ 31 ].
The present study also showed that low birth weight is a significant risk factor for CKD. This finding is consistent with the results of some other studies. However, the results of very few studies on the association between birth weight and risk of CKD are controversial as some suggested a significant association [ 19 , 36 , 37 ] whereas others suggested otherwise [ 36 ]. This may be explained by the relatively smaller size and volume of kidneys in LBW infants compared to infants that are normally grown [ 38 ]. This can lead to long-term complications in adolescence and adulthood including hypertension, decreased glomerular filtration, albuminuria, and cardiovascular diseases. Eventually, these long-term complications can also cause CKD [ 39 ].
Another important result of the current study is the association between chemotherapy for treating cancers and the risk of CKD. According to a study on chemotherapy for testicular cancer by Inai et al., 1 year after chemotherapy 23% of the patients showed CKD [ 40 ]. Another study suggested that the prevalence of stage 3 CKD among patients with cancer was 12, and < 1% of patients had stage 4 CKD [ 41 , 42 ]. Other studies have shown an even higher prevalence of CKD among cancer patients. For instance, only 38.6% of patients with breast cancer, 38.9% of patients with lung cancer, 38.3% of patients with prostate cancer, 27.5% of patients with gynecologic cancer, and 27.2% of patients with colorectal cancer had a GFR ≥90 (ml/min/1.73 m 2 ) at the time of therapy initiation [ 43 , 44 ]. The overall prevalence of CKD ranges from 12 to 25% across many cancer patients [ 45 , 46 , 47 ]. These results clearly demonstrate that, when patients with cancer develop acute or chronic kidney disease, outcomes are inferior, and the promise of curative therapeutic regimens is lessened.
In our study, the history of taking nephrotoxic agents (antibiotics or NSAIDs drugs) was associated with a higher risk of CKD. Our result is following the results reported by other studies [ 48 , 49 ]. Common agents that are associated with AKI include NSAIDs are different drugs including antibiotics, iodinated contrast media, and chemotherapeutic drugs [ 50 ].
Strengths and limitations of our study
Our study used a reasonably large sample size. In addition, a considerably large number of study variables was included in the study. With a very high participation rate, trained nurses conducted the interviews with the case and control participants in the same setting. However, histories of exposures are prone to recall error (bias), a common issue in the case-control studies. It is to be mentioned that the method of selecting controls (hospital controls) should have reduced the risk of recall bias when reporting the required information. In addition, we used the participants’ medical records to complete/ confirm the reported data. Although the design of the present study was not able to confirm a causal association between the associated variables and CKD, the potential importance and modifiable nature of the associated factors makes the results potentially valuable and easily applicable in the prevention of CKD.
Given that, chemotherapy is an important risk factor for CKD, we suggest the imperative for collaborative care between oncologists and nephrologists in the early diagnosis and treatment of kidney diseases in patients with cancer. Training clinicians and patients are important to reduce the risk of nephrotoxicity. Electronic medical records can simultaneously be used to monitor prescription practices, responsiveness to alerts and prompts, the incidence of CKD, and detecting barriers to the effective implementation of preventive measures [ 51 ]. Routine follow-up and management of diabetic patients is also important for the prevention of CKD. We suggest a tight collaboration between endocrinologists and nephrologists to take care of diabetic patients with kidney problems. In addition, surgeons in major operations should refer patients, especially patients with AKI, to a nephrologist for proper care related to their kidney function. Treatment of hypertension is among the most important interventions to slow down the progression of CKD [ 12 ]. Moreover, all patients with newly diagnosed hypertension should be screened for CKD. We suggest all patients with diabetes have their GFR and urine albumin-to-creatinine ratio (UACR) checked annually. Finally, the aging population and obesity cause the absolute numbers of people with diabetes and kidney diseases to raise significantly. This will require a more integrated approach between dialectologists/nephrologists and the primary care teams (55).
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to their being the intellectual property of Shiraz University of Medical Sciences but are available from the corresponding author on reasonable request.
Abbreviations
- Chronic kidney disease
End-stage renal disease
Glomerular filtration rate
Renal replacement treatment
Urinary tract infection
Odds ratios
Confidence intervals
Hypertension
Acute kidney injury
Ghelichi Ghojogh M, Salarilak S, Taghezadeh Afshari A, Khalkhali HR, Mohammadi-Fallah MR, Mkhdoomi K. The effect of body mass index on patient and graft survival rate in kidney transplanted patients in Iran. Nephrourol Monthly. 2017;9(4):e14386.
Zeba Z, Fatema K, Sumit AF, Zinnat R, Ali L. Early screening of chronic kidney disease patients among the asymptomatic adult population in Bangladesh. J Prev Epidemiol. 2020;5(1):e10–e.
Article Google Scholar
Mahajan C, Tiwari V, Divyaveer SS, Patil MR, Banerjee A, Bagur V, et al. Spectrum of renal biopsies; a three-year data from a tertiary care Centre of eastern India. J Nephropharmacol. 2020;9(2):e20–e.
Article CAS Google Scholar
Kimura K, Hosoya T, Uchida S, Inaba M, Makino H, Maruyama S, et al. Febuxostat therapy for patients with stage 3 CKD and asymptomatic Hyperuricemia: a randomized trial. Am J Kidney Dis. 2018;72(6):798–810.
Article CAS PubMed Google Scholar
Foster MC, Hwang S-J, Larson MG, Lichtman JH, Parikh NI, Vasan RS, et al. Overweight, obesity, and the development of stage 3 CKD: the Framingham heart study. Am J Kidney Dis. 2008;52(1):39–48.
Article PubMed PubMed Central Google Scholar
Rachmi CN, Agho KE, Li M, Baur LA. Stunting, underweight and overweight in children aged 2.0–4.9 years in Indonesia: prevalence trends and associated risk factors. PLoS One. 2016;11(5):e0154756.
Asghari G, Momenan M, Yuzbashian E, Mirmiran P, Azizi F. Dietary pattern and incidence of chronic kidney disease among adults: a population-based study. Nutr Metab. 2018;15(1):1–11.
Ruggles DR, Freyman RL, Oxenham AJ. Influence of musical training on understanding voiced and whispered speech in noise. PLoS One. 2014;9(1):e86980.
Moazzeni SS, Arani RH, Hasheminia M, Tohidi M, Azizi F, Hadaegh F. High incidence of chronic kidney disease among Iranian diabetic adults: using CKD-EPI and MDRD equations for estimated glomerular filtration rate. Korean Diabetes J. 2021;45(5):684-97.
Salam SN, Eastell R, Khwaja A. Fragility fractures and osteoporosis in CKD: pathophysiology and diagnostic methods. Am J Kidney Dis. 2014;63(6):1049–59.
Zahmatkesh M, Tamadon MR. World kidney day 2018; chronic kidney disease in women. J Nephropathol. 2017;7(1):4–6.
Noble R, Taal MW. Epidemiology and causes of chronic kidney disease. Medicine. 2019;47(9):562–6.
Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, et al. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2004;80(4):1029–35.
Sepahi MA, Niknafs B. Multifaceted role of apolipoprotein L1 risk variants and nephropathy. J Nephropathol. 2020;9(1):1-3.
Cohen JB, Tewksbury CM, Landa ST, Williams NN, Dumon KR. National postoperative bariatric surgery outcomes in patients with chronic kidney disease and end-stage kidney disease. Obes Surg. 2019;29(3):975–82.
Levey AS, Andreoli SP, DuBose T, Provenzano R, Collins AJ. Chronic kidney disease: common, harmful and treatable–world kidney day 2007. Am J Nephrol. 2007;27(1):108–12.
Article PubMed Google Scholar
Argulian E, Sherrid MV, Messerli FH. Misconceptions and facts about hypertrophic cardiomyopathy. Am J Med. 2016;129(2):148–52.
Rhee CM. The interaction between thyroid and kidney disease: an overview of the evidence. Curr Opin Endocrinol Diabetes Obes. 2016;23(5):407.
Article CAS PubMed PubMed Central Google Scholar
Al Salmi I, Hoy WE, Kondalsamy-Chennakes S, Wang Z, Healy H, Shaw JE. Birth weight and stages of CKD: a case-control study in an Australian population. Am J Kidney Dis. 2008;52(6):1070–8.
Yacoub R, Habib H, Lahdo A, Al Ali R, Varjabedian L, Atalla G, et al. Association between smoking and chronic kidney disease: a case control study. BMC Public Health. 2010;10(1):1–6.
Saucier NA, Sinha MK, Liang KV, Krambeck AE, Weaver AL, Bergstralh EJ, et al. Risk factors for CKD in persons with kidney stones: a case-control study in Olmsted County, Minnesota. Am J Kidney Dis. 2010;55(1):61–8.
Lambert K, Mullan J, Mansfield K, Lonergan M. A cross-sectional comparison of health literacy deficits among patients with chronic kidney disease. J Health Commun. 2015;20(sup2):16–23.
Fraser SD, Roderick PJ, Casey M, Taal MW, Yuen HM, Nutbeam D. Prevalence and associations of limited health literacy in chronic kidney disease: a systematic review. Nephrol Dial Transplant. 2013;28(1):129–37.
Ji MY, Park YS, Yi SE. A case-control study to identify the risk factors of school accidents. Korean J Epidemiol. 2005;27(2):80–94.
Google Scholar
Khajehdehi P, Malekmakan L, Pakfetrat M, Roozbeh J, Sayadi M. Prevalence of chronic kidney disease and its contributing risk factors in southern Iran a cross-sectional adult population-based study; 2014.
Li H, Lu W, Wang A, Jiang H, Lyu J. Changing epidemiology of chronic kidney disease as a result of type 2 diabetes mellitus from 1990 to 2017: estimates from global burden of disease 2017. J Diabetes Investig. 2021;12(3):346.
Xu Y, Surapaneni A, Alkas J, Evans M, Shin J-I, Selvin E, et al. Glycemic control and the risk of acute kidney injury in patients with type 2 diabetes and chronic kidney disease: parallel population-based cohort studies in US and Swedish routine care. Diabetes Care. 2020;43(12):2975–82.
Sepanlou SG, Barahimi H, Najafi I, Kamangar F, Poustchi H, Shakeri R, et al. Prevalence and determinants of chronic kidney disease in northeast of Iran: results of the Golestan cohort study. PLoS One. 2017;12(5):e0176540.
Pugh D, Gallacher PJ, Dhaun N. Management of hypertension in chronic kidney disease. Drugs. 2019;79(4):365–79.
Torra R, Furlano M, Ortiz A, Ars E. Genetic kidney diseases as an underecognized cause of chronic kidney disease: the key role of international registry reports. Clin Kidney J. 2021;14(8):1879-85.
Mizota T, Dong L, Takeda C, Shiraki A, Matsukawa S, Shimizu S, et al. Transient acute kidney injury after major abdominal surgery increases chronic kidney disease risk and 1-year mortality. J Crit Care. 2019;50:17–22.
Madsen NL, Goldstein SL, Frøslev T, Christiansen CF, Olsen M. Cardiac surgery in patients with congenital heart disease is associated with acute kidney injury and the risk of chronic kidney disease. Kidney Int. 2017;92(3):751–6.
Newsome BB, Warnock DG, McClellan WM, Herzog CA, Kiefe CI, Eggers PW, et al. Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Intern Med. 2008;168(6):609–16.
Ishani A, Nelson D, Clothier B, Schult T, Nugent S, Greer N, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;171(3):226–33.
James MT, Ghali WA, Knudtson ML, Ravani P, Tonelli M, Faris P, et al. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation. 2011;123(4):409–16.
Esmeijer K, de Vries AP, Mook-Kanamori DO, de Fijter JW, Rosendaal FR, Rabelink TJ, et al. Low birth weight and kidney function in middle-aged men and women: the Netherlands epidemiology of obesity study. Am J Kidney Dis. 2019;74(6):751–60.
White SL, Perkovic V, Cass A, Chang CL, Poulter NR, Spector T, et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis. 2009;54(2):248–61.
Harer MW, Charlton JR, Tipple TE, Reidy KJ. Preterm birth and neonatal acute kidney injury: implications on adolescent and adult outcomes. J Perinatol. 2020;40(9):1286–95.
Al Salmi I, Hannawi S. Birth weight and susceptibility to chronic kidney disease. Saudi J Kidney Dis Transplant. 2020;31(4):717.
Inai H, Kawai K, Ikeda A, Ando S, Kimura T, Oikawa T, et al. Risk factors for chronic kidney disease after chemotherapy for testicular cancer. Int J Urol. 2013;20(7):716–22.
Launay-Vacher V, Oudard S, Janus N, Gligorov J, Pourrat X, Rixe O, et al. Prevalence of renal insufficiency in cancer patients and implications for anticancer drug management: the renal insufficiency and anticancer medications (IRMA) study. Cancer. 2007;110(6):1376–84.
Launay-Vacher V. Epidemiology of chronic kidney disease in cancer patients: lessons from the IRMA study group. Semin Nephrol. 2010;30(6):548–56.
Launay-Vacher V, Janus N, Deray G. Renal insufficiency and cancer treatments. ESMO Open. 2016;1(4):e000091.
Janus N, Launay-Vacher V, Byloos E, Machiels JP, Duck L, Kerger J, et al. Cancer and renal insufficiency results of the BIRMA study. Br J Cancer. 2010;103(12):1815–21.
Kitchlu A, McArthur E, Amir E, Booth CM, Sutradhar R, Majeed H, et al. Acute kidney injury in patients receiving systemic treatment for Cancer: a population-based cohort study. J Natl Cancer Inst. 2019;111(7):727–36.
Kidney Disease Improving Global Outcomes (KDIGO). KDIGO clinical practice guideline for acute kidney injury. kdigo.org/wpcontent/uploads/2016/10/KDIGO-2012-AKI-Guide line-Engli sh.pdf . Accessed 23 Mar 2020.
Königsbrügge O, Lötsch F, Zielinski C, Pabinger I, Ay C. Chronic kidney disease in patients with cancer and its association with occurrence of venous thromboembolism and mortality. Thromb Res. 2014;134(1):44–9.
Goldstein SL, Mottes T, Simpson K, Barclay C, Muething S, Haslam DB, et al. A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int. 2016;90(1):212–21.
Menon S, Kirkendall ES, Nguyen H, Goldstein SL. Acute kidney injury associated with high nephrotoxic medication exposure leads to chronic kidney disease after 6 months. J Pediatr. 2014;165(3):522–7.e2.
Perazella MA, Izzedine H. New drug toxicities in the onco-nephrology world. Kidney Int. 2015;87(5):909–17.
Luyckx VA, Tuttle KR, Garcia-Garcia G, Gharbi MB, Heerspink HJL, Johnson DW, et al. Reducing major risk factors for chronic kidney disease. Kidney Int Suppl. 2017;7(2):71–87.
Download references
Acknowledgments
This paper is part of a thesis conducted by Mousa Ghelichi-Ghojogh, Ph.D. student of epidemiology, and a research project conducted at the Shiraz University of Medical sciences (99-01-04-22719). We would like to thank Dr. Bahram Shahryari and all nephrologists of Shiraz University of medical sciences, interviewers, and CKD patients in Shiraz for their voluntary participation in the study and for providing data for the study.
Shiraz University of Medical Sciences financially supported this study. (Grant number: 99–01–04-22719).
Author information
Authors and affiliations.
Candidate in Epidemiology, Student Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran
Mousa Ghelichi-Ghojogh
HIV/AIDS research center, School of Health, Shiraz University of Medical Sciences, P.O.Box: 71645-111, Shiraz, Iran
Mohammad Fararouei
Department of Epidemiology, School of Health, Shiraz University of Medical Sciences, Shiraz, Iran
Mozhgan Seif
Nephrologist, Shiraz Nephro-Urology Research Center, Department of Internal Medicine, Emergency Medicine Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
Maryam Pakfetrat
You can also search for this author in PubMed Google Scholar
Contributions
MGG: Conceptualization, Methodology, Statistical analysis, Investigation, and writing the draft of the manuscript. MP: were involved in methodology, writing the draft of the manuscript, and clinical consultation. MS: was involved in the methodology and statistical analysis. MF: was involved in conceptualization, methodology, supervision, writing, and reviewing the manuscript. The authors read and approved the final manuscript.
Corresponding author
Correspondence to Mohammad Fararouei .
Ethics declarations
Ethics approval and consent to participate.
The study protocol was reviewed and approved by the ethical committee of Shiraz University of Medical Sciences (approval number: 1399.865). All methods were performed in accordance with the relevant guidelines and regulations of the Declaration of Helsinki. The participants were assured that their information is used for research purposes only. Because of the illiteracy of a considerable number of the patients, verbal informed consent was obtained from the participants. Using verbal informed consent was also granted by the ethical committee of Shiraz University of Medical Sciences.
Consent for publication
Not applicable.
Competing interests
None of the authors declare disclosures of direct relevance to the submitted work.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ghelichi-Ghojogh, M., Fararouei, M., Seif, M. et al. Chronic kidney disease and its health-related factors: a case-control study. BMC Nephrol 23 , 24 (2022). https://doi.org/10.1186/s12882-021-02655-w
Download citation
Received : 14 August 2021
Accepted : 24 December 2021
Published : 10 January 2022
DOI : https://doi.org/10.1186/s12882-021-02655-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Related factors
- Case-control
BMC Nephrology
ISSN: 1471-2369
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Sorry. You need a frames capable broswer to view this page.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 15 March 2024
Prognostic impact and predictors of persistent renal dysfunction in acute kidney injury after percutaneous coronary intervention for acute myocardial infarction
- Takuya Nakamura 1 ,
- Makoto Watanabe 1 ,
- Junichi Sugiura 1 ,
- Atsushi Kyodo 1 ,
- Saki Nobuta 1 ,
- Kazutaka Nogi 1 ,
- Yasuki Nakada 1 ,
- Satomi Ishihara 1 ,
- Yukihiro Hashimoto 1 ,
- Hitoshi Nakagawa 1 ,
- Tomoya Ueda 1 ,
- Ayako Seno 1 ,
- Taku Nishida 1 ,
- Kenji Onoue 1 &
- Shungo Hikoso 1
Scientific Reports volume 14 , Article number: 6299 ( 2024 ) Cite this article
120 Accesses
Metrics details
This study aimed to evaluate the prognostic impact and predictors of persistent renal dysfunction in acute kidney injury (AKI) after an emergency percutaneous coronary intervention (PCI) for acute myocardial infarction (AMI). A total of 877 patients who underwent emergency PCI for AMI were examined. AKI was defined as serum creatinine (SCr) ≥ 0.3 mg/dL or ≥ 50% from baseline within 48 h after PCI. Persistent AKI was defined as residual impairment of SCr ≥ 0.3 mg/dL or ≥ 50% from baseline 1 month after the procedure. The primary outcome was the composite endpoints of death, myocardial infarction, hospitalization for heart failure, stroke, and dialysis. AKI and persistent AKI were observed in 82 (9.4%) and 25 (2.9%) patients, respectively. Multivariate Cox proportional hazards analysis demonstrated that persistent AKI, but not transient AKI, was an independent predictor of primary outcome (hazard ratio, 4.99; 95% confidence interval, 2.30–10.8; P < 0.001). Age > 75 years, left ventricular ejection fraction < 40%, a high maximum creatinine phosphokinase MB level, and bleeding after PCI were independently associated with persistent AKI. Persistent AKI was independently associated with worse clinical outcomes in patients who underwent emergency PCI for AMI. Advanced age, poor cardiac function, large myocardial necrosis, and bleeding were predictors of persistent AKI.
Similar content being viewed by others

Acute kidney injury
John A. Kellum, Paola Romagnani, … Hans-Joachim Anders

Clonal hematopoiesis of indeterminate potential is associated with acute kidney injury
Caitlyn Vlasschaert, Cassianne Robinson-Cohen, … Alexander G. Bick

Acute heart failure
Mattia Arrigo, Mariell Jessup, … Alexandre Mebazaa
Introduction
Acute kidney injury (AKI) is a frequent complication in patients with acute myocardial infarction (AMI) undergoing primary percutaneous intervention (PCI) compared to those undergoing elective PCI 1 , 2 and is known to be an independent risk factor for increased long-term mortality and worse clinical outcomes 3 , 4 .
The cause of AKI in patients with AMI is multifactorial because AKI develops not only because of the large amount of contrast medium exposure during PCI but also because of hemodynamic instability, renal hypoperfusion following impaired cardiac output, and systemic inflammatory response due to ischemic injury and myocardial necrosis 5 .
Most AKI cases after PCI are transient and improve within 2 weeks, whereas some patients have persistent renal dysfunction after the development of AKI 6 . However, the impact of persistent or transient renal dysfunction on worse clinical outcomes, including major adverse cardiovascular events (MACEs) and dialysis, and predictors of persistent renal dysfunction remain unclear in patients with AMI undergoing PCI.
This study aimed to investigate the impact of renal function reversibility following AKI on clinical outcomes and predictors of persistent renal dysfunction in patients with AMI undergoing emergency PCI.
Study design and population
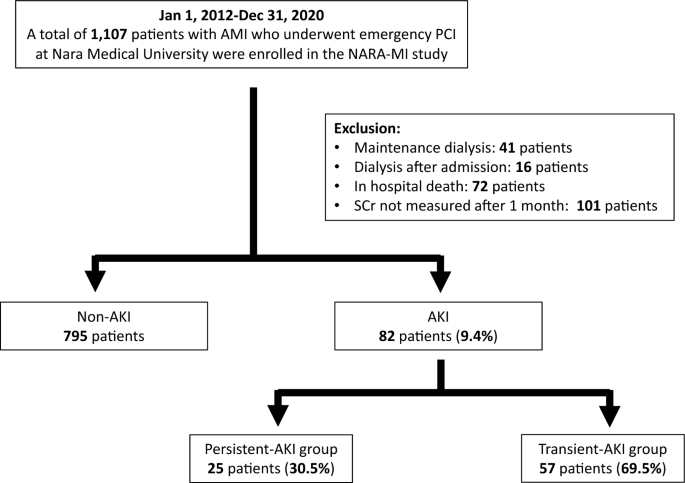
This was a single-center, retrospective, observational study. Patients with AMI who underwent emergency PCI at Nara Medical University Hospital between January 2012 and December 2020 were enrolled. The diagnoses of AMI included ST-elevation myocardial infarction (STEMI) and non-ST-elevation myocardial infarction (NSTEMI) within 48 h of AMI onset. STEMI was defined as continuous chest pain, ST-segment elevation in two contiguous leads or a new left bundle branch block on 12-lead electrocardiography, and elevated cardiac marker levels (creatine kinase-MB or troponin). NSTEMI was defined as ischemic symptoms in the absence of ST-segment elevation on electrocardiography with elevated cardiac marker levels 7 . Patients on previous chronic dialysis or who underwent hemodialysis after admission, died in hospital, or lacked data on serum creatinine (SCr) levels were excluded from the study. Figure 1 shows the enrollment and exclusion criteria and study flow. Primary PCI was performed using standard techniques and catheters via the femoral or radial approach according to the operator’s usual practice.
Study flow. AKI acute kidney injury, AMI acute myocardial infarction, SCr serum creatinine.
Data collection and clinical definition
Baseline data including clinical characteristics, laboratory data, medication upon admission, and procedural data were obtained for all patients.
Baseline laboratory data included hemoglobin (Hb), SCr, HbA1c, and maximum creatine phosphokinase MB (Max CKMB) levels and estimated glomerular filtration rate (eGFR). Anemia was defined as Hb < 12 g/dL in males and < 11 g/dL in females. The eGFR was calculated as follows using the “Japanese Modification of Diet in Renal Disease study equation” published by the Japanese Society of Nephrology: eGFR (men) = 194 × serum creatinine − 1.094 × age − 0.287 and eGFR (women) = eGFR (men) × 0.739 8 . Procedural data included STEMI or NSTEMI, culprit vessel (left anterior descending artery, left circumflex artery, right coronary artery, or left main trunk), diseased vessels (one or multiple vessels), Killip class ≥ 3, approach site for PCI (femoral artery or not), multivessel PCI in hospital (one-time or staged strategy), volume of contrast media, thrombolysis in myocardial infarction flow grade (TIMI) score before and after PCI, use of mechanical circulatory support (intra-aortic balloon pumping or extracorporeal membrane oxygenation) during PCI, use of catecholamine, and perioperative major bleeding complication within 48 h after PCI, defined as Bleeding Academic Research Consortium (BARC) 9 type 3 or greater. Echocardiography was performed to evaluate left ventricular ejection fraction (EF) within 1 week of the procedure. SCr levels were measured before the procedure (baseline), every day for the following 2 days, and 1 month after the procedure to identify patients without AKI (non-AKI), those with persistent AKI, and those with transient AKI. AKI was defined as an increase in SCr ≥ 0.3 mg/dL or ≥ 50% from baseline within 48 h 10 . Patients diagnosed with AKI were divided into those with persistent AKI and those with transient AKI. Persistent AKI was defined as residual impairment of SCr ≥ 0.3 mg/dL or ≥ 50% from baseline 1 month after procedure. Transient AKI was defined as recovery to SCr < 0.3 mg/dL and < 50% from baseline 1 month after the procedure. The Mehran score was calculated based on eight clinical and procedural variables: age > 75 years, hypotension, congestive heart failure, use of an intra-aortic balloon pump, serum creatinine level, diabetes, anemia, and volume of contrast, according to a previous report 11 .
The primary outcome of this study was a composite of MACEs, including death, myocardial infarction, hospitalization for heart failure, stroke, and initiation of maintenance dialysis. The secondary endpoints were MACEs, mortality, and dialysis. Clinical follow-up was conducted through outpatient visits or telephone interviews.
Statistical analysis
The Shapiro–Wilk test was used to evaluate the distribution of continuous data. Normally distributed data are expressed as mean ± standard deviation (SD), and those with skewed distributions are expressed as median with interquartile range, whereas categorical variables are presented as counts and percentages. Categorical data were compared using the Pearson χ 2 test. Continuous variables were compared using parametric one-way analysis of variance or the non-parametric Kruskal–Wallis test, based on the distribution of variables. The cumulative incidence of survival-free periods from clinical events was estimated using the Kaplan–Meier method. In the case of significant differences, pairwise post-hoc tests were performed with Bonferroni correction. A univariate Cox proportional hazards model was used to identify variables associated with the primary outcomes in the present study. Two multivariate Cox proportional hazards models were used to identify independent predictors of the primary outcome, including 12 variables with P < 0.05 in the univariate model. One model included post PCI TIMI score < 3, and the other included mechanical circulatory support. Univariate logistic regression analysis was used to identify the significant clinical factors associated with the development of AKI and persistent AKI. A multivariate logistic regression model was used to identify independent predictors of persistent AKI, which included variables with P < 0.05, in the univariate model. A P < 0.05 was considered statistically significant. All statistical analyses were performed using JMP software version 16 (SAS Institute JAPAN Corporation, Roppongi, Tokyo).
Ethics statement
This study was approved by the Ethics Committee of Nara Medical University (Reference no. 2162) and complied with the Declaration of Helsinki’s Ethical Principles for Medical Research Involving Human Subjects. Informed consent was obtained in the form of an opt-out option on the Department of Cardiovascular Medicine, Nara Medical University website.
Patient characteristics
Of the 1107 consecutive patients, 230 were excluded (41 were on maintenance hemodialysis, 16 were undergoing temporary hemodialysis in the hospital, 72 were in-hospital deaths, and 101 lacked data on SCr). Finally, 877 consecutive patients were included in the study, and AKI was present in 82 (9.4%). Of the AKI cases, persistent and transient AKIs were present in 25 (30.5%) and 57 (69.5%) patients, respectively (Fig. 1 ).
Table 1 shows a comparison of the baseline clinical characteristics among the three groups. The persistent AKI group was older and more frequently had anemia and a lower left ventricular ejection fraction (EF < 40%) than the non-AKI group. The transient AKI group was older; had a higher prevalence of diabetes, lower baseline eGFR levels, and lower EF; and more frequent use of angiotensin II receptor blocker (ARB), angiotensin-converting enzyme inhibitors (ACE-Is), and angiotensin receptor neprilysin inhibitor (ARNI) than the non-AKI group. There were no significant differences in the baseline clinical characteristics between the persistent and transient AKI groups. Table 2 compares the baseline lesion and procedural characteristics among the three groups. Transient AKI group had a higher incidence of severe myocardial infarction with Killip ≥ 3 and a higher contrast volume/eGFR ratio compared with non-AKI group. The incidence of BARC type 3 or greater bleeding complication was significantly higher in the persistent and the transient AKI groups compared to the non-AKI group. The Mehran risk score was significantly higher in the persistent and transient AKI groups than in the non-AKI group. There were no significant differences in baseline lesion and procedural characteristics between the persistent and transient AKI groups.
Long-term clinical outcomes
The median follow-up period was 1593 days (interquartile range, 903–2378 days), and the mean follow-up period was 1689 ± 926 years.
Figure 2 shows the Kaplan–Meier survival curves for clinical outcomes in the three groups. There was a significant difference in primary outcome-free survival (log-rank, P < 0.0001) and all-cause death-free survival (log-rank, P < 0.0001) among the three groups. In the pairwise post hoc tests, the cumulative incidence of the primary outcome was significantly higher in the persistent AKI (log-rank, P < 0.0001) and transient AKI (log-rank, P < 0.0001) groups than in the non-AKI group, and the cumulative incidence of all-cause death was significantly higher in the persistent AKI (log-rank, P < 0.0001) and transient AKI (log-rank, P = 0.0003) groups than in the non-AKI group. However, there was no significant difference between patients with persistent and transient AKIs in terms of the cumulative incidence of the primary outcome and all-cause death. Table 3 shows the incidence of primary and secondary outcomes in the three groups. Kaplan–Meier survival analysis also showed significant differences in MACEs, hospitalization for heart failure, stroke, and initiation of maintenance dialysis among the three groups. We investigated the predictors of primary outcomes using multivariate Cox proportional hazards analysis with two models. In both models, persistent AKI remained a significant predictor for primary outcome compared to non-AKI (model 1: HR, 2.68, 95% CI, 1.41–5.10, P = 0.0026; model 2: HR, 2.62, 95% CI, 1.38–4.99, P = 0.0034; Table 4 ). However, the incidence of transient AKI did not differ significantly from that of AKI in either model. Other predictors of primary outcomes in the multivariate analysis included age > 75 years; previous myocardial infarction, stroke, peripheral arterial disease (PAD); anemia; EF < 40%; and higher maximum CKMB level (Table 4 ).
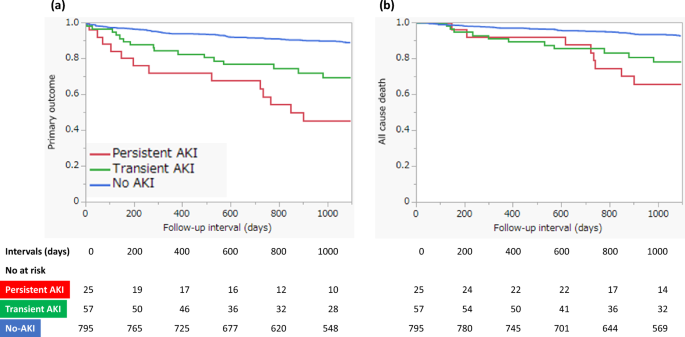
Kaplan–Meier survival curves of 3-year clinical outcomes. ( a ) Primary outcomes (death, myocardial infarction, hospitalization for heart failure, stroke, and initiation of maintenance dialysis); ( b ) All-cause death.
Predictors of AKI and persistent AKI
The predictors of AKI and persistent AKI evaluated using univariate logistic regression analyses are shown in Table 5 . Age > 75 years, diabetes mellitus, anemia, bleeding with BARC type 3 or greater, Killip ≥ 3, low eGFR, EF < 40%, higher maximum CKMB level, femoral artery approach, multivessel PCI with one-time strategy, higher contrast volume/eGFR ratio, mechanical circulatory support use, and higher Mehran risk score were predictors of AKI. Age > 75 years, bleeding with BARC type 3 or greater, lower eGFR, EF < 40%, higher maximum CK-MB level, higher contrast volume/eGFR ratio, and a higher Mehran risk score were also predictors of persistent AKI.
We investigated the predictors of persistent AKI using multivariate logistic regression analysis with four models containing three variables that were strongly relevant as predictors of persistent AKI in univariate analysis. Age > 75 years, EF < 40%, and higher maximum CK-MB level were independent predictors of persistent AKI in Model 1, age > 75 years and EF < 40% were independent predictors of persistent AKI in Models 2 and 3, and age > 75 years, EF < 40%, and bleeding with BARC type 3 or greater were independent predictors of persistent AKI in Model 4 (Table 6 ).
The major findings of this study are that: (1) in patients who underwent emergency PCI for AMI, AKI was present in 82 (9.4%), and of the AKI, persistent AKI was present in 25 (30.5%) and transient AKI in 57 (69.5%); (2) primary outcome and all-cause death occurred more frequently in patients with persistent AKI and transient AKI than in those with non-AKI, and persistent AKI, but not transient AKI, was an independent predictor of primary outcome; (3) age > 75 years, EF < 40%, higher maximum CKMB level, and perioperative bleeding complication with BARC type 3 or greater were independent predictors of persistent AKI.
Contrast-induced nephropathy (CIN) is the main cause of renal dysfunction after PCI and is associated with increased long-term mortality and MACEs 12 . CIN is generally considered transient, with SCr levels typically reaching a peak within a few days and returning to baseline within 2 weeks in most cases 6 . However, some patients with CIN develop persistent increase in SCr levels.
Several studies have reported the incidence and prognostic impact of persistent and transient renal dysfunction after elective 13 , 14 and emergency 15 , 16 , 17 , 18 PCIs. The time interval for assessing persistent or transient renal dysfunction differed among studies. Some studies assessed persistent or transient renal dysfunction at short time intervals (2 weeks 17 or at discharge 16 , 17 , 18 ) from baseline, whereas others assessed long-term interval (1 15 , 3 13 , or 12 months 14 ). Some patients, classified as having early persistent renal dysfunction, may have later improved their renal function. Therefore, we assessed persistent or transient renal dysfunction at long-term interval (1 month) from baseline. Despite the time intervals and definition for assessing persistent renal dysfunction among studies, the incidence of persistent renal dysfunction among patients with AKI was approximately 20–60%, which is similar to our result (30.5%).
In previous studies targeting patients who underwent elective PCI, Maioli et al. 13 reported that both persistent and transient renal dysfunctions were independently associated with long-term mortality and MACEs, whereas Abe et al. 14 reported that only persistent renal dysfunction was independently associated with increased long-term mortality. In previous studies targeting patients with AMI, Choi et al. 16 reported that both persistent and transient renal dysfunction were independently associated with long-term mortality, whereas Kurogi et al. 17 reported that persistent renal dysfunction, but not transient renal dysfunction, was independently associated with both long-term mortality and worse clinical outcomes. In the recent large-scale substudy 18 from the MATRIX-Access (Minimizing Adverse Haemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox) trial, with a study population of 8201 patients who underwent catheter procedure for acute coronary syndrome (ACS), Landi et al. reported that in-hospital persistent but not transient AKI was independently associated with 1-year MACEs and mortality. The present study demonstrated that persistent renal dysfunction, but not transient renal dysfunction, was independently associated with poor long-term clinical outcomes. These studies, including the present one, consistently suggest that persistent renal dysfunction is associated with worse clinical outcomes. However, the effect of transient renal dysfunction on long-term clinical outcomes differs among studies. Nevertheless, the reversibility of renal dysfunction after AKI development has significant implications for the long-term follow-up of patients who undergo PCI.
Although several risk scores are available as predictors of CIN after cardiac catheterization procedures 19 , little is known about the predictors of persistent renal dysfunction. Some studies 20 have investigated the predictors of persistent renal dysfunction and reported that the Mehran risk score 13 , 21 and contrast volume/baseline eGFR ratio 17 are useful for predicting persistent renal dysfunction. A recent study 22 reported that the preprocedural N-terminal pro-B-type natriuretic peptide (NT-proBNP) level is useful for predicting persistent renal dysfunction. NT-proBNP reflects impaired cardiac output and increased inflammation 23 , which plays an important role in the development of persistent renal dysfunction. The present study demonstrated that EF < 40% and higher maximum CK-MB levels were strongly associated with the development of persistent renal dysfunction. Once AMI develops, cardiac function rapidly declines and cardiac damage is sustained. Subsequently, renal hypoperfusion following impaired cardiac output and systemic inflammatory response due to ischemic injury and myocardial necrosis may play important roles in the development of persistent renal dysfunction. Therefore, the assessment of cardiac function and the extent of myocardial necrosis after the onset of AMI might be useful for predicting the development of persistent renal dysfunction.
The present study demonstrated that major perioperative bleeding after PCI (BARC type 3 or greater) was not only associated with the development of AKI but also with the development of persistent renal dysfunction. A bleeding complication, especially one related to vascular access, is well known as a major complication after PCI. A previous study showed that bleeding complications after PCI are associated with the development of CIN 24 , the severity of which is closely correlated with the severity of bleeding. A sudden blood loss due to major bleeding such as BARC type 3 or greater may cause a serious impairment in renal perfusion, subsequently making AKI more severe and resulting in persistent renal dysfunction.
Early clinical follow-up, careful management, and close monitoring of renal function may improve long-term clinical outcomes in patients at high risk of developing persistent renal dysfunction after AKI. Additionally, the risk of bleeding complication is lower in PCI via the radial access than via a femoral access 25 . In high-risk patients of AKI, the choice of radial access may prevent the development of persistent renal dysfunction after PCI.
Limitations
This study had several limitations. First, this was a single-center, retrospective observational study. Second, the high number of patients excluded due to the absence of analytical evaluation in the first month (approximately 9%). Third, the lack of data regarding patients who died during the index hospitalization (6.5%), specifically the time elapsed between PCI and death, as well as the progression of renal function in this subgroup. Forth, pharmacological treatments (diuretics, ACE-Is, ARB, and ARNI) and the examination using contrast media (contrast-enhanced computed tomography) after PCI, which might have influenced the worsening of renal function, were not included in the analysis. Fifth, we included only three variables to investigate the independent predictors of persistent AKI in the multivariate logistic regression analysis because of the small number of patients with persistent AKI. Sixth, the sample size was small and the present findings were considered exploratory in nature. Therefore, a large-scale prospective cohort study is required to verify our results. Seventh, the use of drugs such as ACE-Is, ARB, and ARNI, which improve prognosis after AMI, may be hindered by the presence of AKI and subsequent persistent renal dysfunction. As a result, its insufficient treatment may have worsened the prognosis in patients with persistent AKI. To clarify this causal relationship and identify the best therapeutic strategy in patients at high-risk of AKI, it is necessary to evaluate to what extent limitations in terms of the dosage of drugs potentially harmful to renal function in high-risk patients mitigate the progression to irreversible renal injury and influence the prognosis. A large-scale prospective study may therefore provide useful information for daily clinical practice.
To conclude, in patients who underwent emergency PCI for AMI, persistent AKI was independently associated with worse clinical outcomes, and advanced age, low cardiac function, greater myocardial necrosis, and perioperative major bleeding after PCI were predictors of persistent AKI.
Data availability
The datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.
Tsai, T. T. et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: Insights from the NCDR Cath-PCI registry. JACC Cardiovasc. Interv. 7 , 1–9 (2014).
Article PubMed PubMed Central Google Scholar
Abe, D. et al. Clinical predictors of contrast-induced acute kidney injury in patients undergoing emergency versus elective percutaneous coronary intervention. Circ. J. 78 , 85–91 (2014).
Article CAS PubMed Google Scholar
Narula, A. et al. Contrast-induced acute kidney injury after primary percutaneous coronary intervention: Results from the HORIZONS-AMI substudy. Eur. Heart J. 35 , 1533–1540 (2014).
Sun, G. et al. Contrast-induced nephropathy and long-term mortality after percutaneous coronary intervention in patients with acute myocardial infarction. Angiology 70 , 621–626 (2019).
Article PubMed Google Scholar
Shacham, Y., Steinvil, A. & Arbel, Y. Acute kidney injury among ST elevation myocardial infarction patients treated by primary percutaneous coronary intervention: a multifactorial entity. J. Nephrol. 29 , 169–174 (2016).
Brown, J. R. et al. Transient and persistent renal dysfunction are predictors of survival after percutaneous coronary intervention: Insights from the Dartmouth Dynamic Registry. Catheter. Cardiovasc. Interv. 72 , 347–354 (2008).
Thygesen, K. et al. Third universal definition of myocardial infarction. Circulation 126 , 2020–2035 (2012).
Matsuo, S. et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 53 , 982–992 (2009).
Mehran, R. et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 123 , 2736–2747 (2011).
Mehta, R. L. et al. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 11 , R31 (2007).
Mehran, R. et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: Development and initial validation. J. Am. Coll. Cardiol. 44 , 1393–1399 (2004).
PubMed Google Scholar
Rihal, C. S. et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 105 , 2259–2264 (2002).
Maioli, M. et al. Persistent renal damage after contrast-induced acute kidney injury: Incidence, evolution, risk factors, and prognosis. Circulation 125 , 3099–3107 (2012).
Abe, M. et al. Impact of transient or persistent contrast-induced nephropathy on long-term mortality after elective percutaneous coronary intervention. Am. J. Cardiol. 120 , 2146–2153 (2017).
Wi, J. et al. Impact of contrast-induced acute kidney injury with transient or persistent renal dysfunction on long-term outcomes of patients with acute myocardial infarction undergoing percutaneous coronary intervention. Heart 97 , 1753–1757 (2011).
Choi, J. S. et al. Relation between transient or persistent acute kidney injury and long-term mortality in patients with myocardial infarction. Am. J. Cardiol. 112 , 41–45 (2013).
Kurogi, K. et al. Persistent renal dysfunction in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. J. Am. Heart Assoc. 8 , e014096 (2019).
Landi, A. et al. Transient vs in-hospital persistent acute kidney injury in patients with acute coronary syndrome. JACC Cardiovasc. Interv. 16 , 193–205 (2023).
Isaka, Y. et al. Guideline on the use of iodinated contrast media in patients with kidney disease 2018. Circ. J. 83 , 2572–2607 (2019).
Watanabe, M. Prediction of persistent renal dysfunction following contrast-induced nephropathy after cardiac catheterization procedures. Circ. J. 87 , 266–267 (2023).
Wi, J. et al. Prediction of contrast-induced nephropathy with persistent renal dysfunction and adverse long-term outcomes in patients with acute myocardial infarction using the Mehran risk score. Clin. Cardiol. 36 , 46–53 (2013).
Luo, M. et al. Predictive value of N-terminal pro B-type natriuretic peptide for contrast-induced nephropathy non-recovery and poor outcomes among patients undergoing percutaneous coronary intervention. Circ. J. 87 , 258–265 (2023).
Jensen, J. et al. Inflammation increases NT-proBNP and the NT-proBNP/BNP ratio. Clin. Res. Cardiol. 99 , 445–452 (2010).
Ohno, Y. et al. Impact of periprocedural bleeding on incidence of contrast-induced acute kidney injury in patients treated with percutaneous coronary intervention. J. Am. Coll. Cardiol. 62 , 1260–1266 (2013).
Valgimigli, M. et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: A randomised multicentre trial. Lancet 385 , 2465–2476 (2015).
Download references
Acknowledgements
The authors thank the patients, participating cardiologists, and staff who contributed to this study.
Author information
Authors and affiliations.
Department of Cardiovascular Medicine, Nara Medical University, 840 Shijo-cho, Kashihara, Nara, 634-8522, Japan
Takuya Nakamura, Makoto Watanabe, Junichi Sugiura, Atsushi Kyodo, Saki Nobuta, Kazutaka Nogi, Yasuki Nakada, Satomi Ishihara, Yukihiro Hashimoto, Hitoshi Nakagawa, Tomoya Ueda, Ayako Seno, Taku Nishida, Kenji Onoue & Shungo Hikoso
You can also search for this author in PubMed Google Scholar
Contributions
T.N. wrote the main manuscript text and prepared the Figures. All authors, mainly M.W., reviewed the manuscript.
Corresponding author
Correspondence to Makoto Watanabe .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Nakamura, T., Watanabe, M., Sugiura, J. et al. Prognostic impact and predictors of persistent renal dysfunction in acute kidney injury after percutaneous coronary intervention for acute myocardial infarction. Sci Rep 14 , 6299 (2024). https://doi.org/10.1038/s41598-024-56929-y
Download citation
Received : 04 July 2023
Accepted : 12 March 2024
Published : 15 March 2024
DOI : https://doi.org/10.1038/s41598-024-56929-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Case report
- Open access
- Published: 04 August 2009
A 60-year-old man with chronic renal failure and a costal mass: a case report and review of the literature
- Germán Campuzano-Zuluaga 1 ,
- William Velasco-Pérez 1 &
- Juan Ignacio Marín-Zuluaga 1
Journal of Medical Case Reports volume 3 , Article number: 7285 ( 2009 ) Cite this article
41k Accesses
7 Citations
3 Altmetric
Metrics details
Introduction
Brown tumors are a rare focal manifestation of osteitis fibrosa cystica, which results from hyperparathyroidism. Chronic kidney failure may lead to secondary or tertiary hyperparathyroidism and thus to osteitis fibrosa cystica and brown tumors.
Case presentation
A 60-year-old man with a history of diabetes mellitus and chronic kidney failure presented with a 15-day history of dyspnea, cough, malaise and fever. Initially, there was little correlation between his history and his physical examination. Various pulmonary, cardiac and infectious etiologies were ruled out. A chest X-ray showed a costal mass that was further verified by tomography and gammagraphy. The mass was suspected of being neoplastic. After a failed biopsy, the mass was removed surgically and on histopathology was compatible with a giant-cell tumor versus a brown tumor caused by hyperparathyroidism. Laboratory tests showed elevated calcium, phosphate and parathyroid hormone concentrations. The patient was diagnosed with a brown tumor secondary to refractory hyperparathyroidism.
Tending towards a diagnosis because it is more frequent or it implies more risk for the patient may delay the consideration of other diagnostic options that, although rare, fit well into the clinical context. The patient presented here was suspected to have an osseous neoplasia that would have had major implications for the patient. However, reassessment of the case led to the diagnosis of a brown tumor. Brown tumors should be an important diagnostic consideration in patients with chronic kidney failure who have secondary or tertiary hyperparathyroidism and an osseous mass.
The first case in the literature reporting a brown tumor was published in 1953 and described a fronto-ethmoidal brown tumor [ 1 ]. However, previous reports of patients with localized forms of osteitis fibrosa cystica (OFC) suggest that the clinical entity was described earlier, at a time when there were few treatment options for chronic kidney failure (CKF) and consequently chronic hyperparathyroidism was more prevalent. Brown tumors are rare osseous lesions that represent a focal manifestation of OFC resulting from hyperparathyroid states. Patients suffering from CKF may develop secondary or tertiary hyperparathyroidism due to altered phosphorus and calcium metabolism. Persistent hyperparathyroidism leads to altered osseous metabolism with bone resorption and tissue changes collectively known as OFC. Our case report describes a patient with poorly controlled CKF who presented with a non-specific clinical picture and no clear diagnosis. Incidentally a costal mass was found and the diagnostic workup that followed led to an unexpected diagnosis.
A 60-year-old man was transferred from the hemodialysis unit to the emergency room because of a 15-day history of malaise, subjective fever, shortness of breath, dry cough, abdominal pain and diarrhea. He also complained of mild anterior thoracic pain not associated with other symptoms and which was not irradiated. He had a 20-year history of type 2 diabetes mellitus (DM) that required insulin, with micro- and macro-vascular complications such as diabetic retinopathy and CKF. He was on hemodialysis and had a history of multiple failed dialysis accesses. He also suffered from arterial hypertension, upper and lower extremity peripheral arterial disease, carotid artery disease, a first degree atrioventricular heart block and had smoked one packet of cigarettes per day for the last 20 years. He was being treated with sevelamer, erythropoietin, folic acid, lovastatin, gemfibrozil, NPH insulin, amlodipine and acetylsalicylic acid, but was not receiving calcium or a vitamin D supplement.
A physical examination revealed the patient to be in a fair condition, with no apparent distress, hydrated, alert and well oriented. He had a heart rate of 92 beats per minute, respiratory rate of 14 breaths per minute, blood oxygen saturation of 97%, arterial blood pressure of 130/70 mmHg and no fever. He had bilateral blindness and mild epistaxis through the left nostril. The thorax was tender to palpation in some costochondral unions, but pain was poorly localized. The vesicular murmur had reduced intensity and no pathologic sounds were auscultated. Peripheral pulses were weak in both the upper and the lower limbs. He had a translumbar hemodialysis catheter. The remaining physical examination was unremarkable.
The patient had stable vital signs and had no signs of systemic inflammatory response. However, because of the patient's previous history of DM, CKF and the presence of leukocytosis, neutrophilia and elevated C-reactive protein upon admission (Table 1 ), we initially ruled out a gastrointestinal or lung infection, or any cardiac cause for the patient's symptoms. The electrocardiogram showed no signs of ischemia, and the chest X-ray showed cardiomegaly, a small left pleural effusion, a circular opacity in the right inferior thoracic region and no signs of consolidation. These findings were initially interpreted as a pulmonary infection, probably a lung abscess, an abscedated nodule or pulmonary tuberculosis. A contrast tomography scan of the chest was ordered for further characterization. Though it showed no parenchymal compromise, a 4 × 1.3 cm lesion was observed on the right dorsal region of the eighth rib. The lesion showed thinning of cortical bone in some areas, preserved cortex and lacked periosteal reaction (Figure 1 ). The radiology staff considered a bone metastasis as a first diagnostic option, and a thoraco-abdomino-pelvic tomography scan was done in search for more lesions and a probable primary tumor. Additional hypodense lesions were observed, including one on the left lamina of L4, acetabulum, and head and neck of the right femur. There was no lymph-node or internal organ compromise. A Tc 99 m Medronate osseous gammagraphy reported a hypermetabolic focus compatible with a neoplastic lesion, concordant in size and location with the costal mass reported in the previous imaging studies. It also revealed generalized osseous compromise compatible with renal osteodystrophy and did not confirm the other lesions described on tomography. A tomography-guided biopsy specimen (Figure 1 ) was obtained, but histopathological analysis reported normal tissue components.

Tomographic image during guided biopsy procedure . Note the heterogeneous 4 × 1.3 cm mass (arrow), with preserved cortical bone and no periosteal reaction or other inflammatory signs. No cysts were identified.
Not being able to reach a clear diagnosis, a careful reassessment of the patient's clinical record led to considering the alternative diagnosis of renal osteodystrophy. This was supported by a history of poorly controlled CKF, elevated calcium (11.2 mg/dl) and phosphorus (5.3 mg/dl) concentrations, a phosphocalcic product of 59.36 mg 2 /dl 2 , and a bone gammagraphy that showed changes compatible with OFC. However, the possibility of neoplasia was still being considered so the mass was removed surgically. Histopathological studies reported an osseous tissue with spindles of fusiform cells in a storiform disposition with abundant multinucleated giant cells, some macrophages and some mononuclear cells. Scarce mitotic activity was observed, and there were no signs of malignancy (Figure 2 ). The pathologist concluded that the findings were compatible with a giant-cell tumor or a brown tumor, both histologically very similar [ 2 ]. Parathyroid hormone (PTH) concentration was 1377 pg/ml. These findings were compatible with refractory hyperparathyroidism, and a diagnosis of a brown tumor of hyperparathyroidism associated with CKF was reached.
Microscopic pathology of surgical specimen . Presence of various multinucleated giant cells (arrows) and spindle arranged cells. Hemosiderin deposits were not observed in the sample. Hematoxylin-eosin stain at 40 × magnification.
The patient continued ambulatory medical treatment with vitamin D, calcium and sevelamer. Two months after discharge, the parathyroid level was 1900 pg/ml and a Tc 99 m Sestamibi scan revealed hyperfunctioning glands despite aggressive pharmacological treatment. Serum calcium and phosphorus levels were within normal limits, 9.4 mg/dl and 3.4 mg/dl, respectively. At the time of writing, the patient was awaiting parathyroidectomy as definite treatment for tertiary hyperparathyroidism associated with severe renal osteodystrophy.
Brown tumors are unusual bone lesions that represent a localized manifestation of OFC induced by hyperparathyroidism, independent of its cause. Increased PTH levels and locally produced tumour necrosis factor α and interleukin 1 (IL-1) by marrow monocytes induce the proliferation and differentiation of pluripotent bone-marrow cells into osteoblasts. These cells produce granulocyte macrophage colony stimulating factor, IL-6, IL-11 and stem-cell factor that induce the migration and differentiation of monocytes into osteoclasts, increasing the number of the latter in the bone tissue. Enhanced activity of osteoclasts and osteoblasts leads to bone resorption and a reduction of bone mineral concentration with an increased proliferation of fibrous tissue and extracellular matrix [ 3 ]. Brown tumors develop in 3% to 4% of patients with primary hyperparathyroidism and in 1.5% to 1.7% of patients with secondary causes of hyperparathyroidism [ 4 ]. However, around half of patients with CKF may develop OFC due to secondary hyperparathyroidism making brown tumors more frequent in these patients. Brown tumors have been reported in patients with primary hyperparathyroidism due to adenomas [ 5 ] and carcinomas [ 6 ] of the parathyroid gland; vitamin D deficiency due to lack of sunlight exposure [ 7 ] or due to intestinal malabsorption syndromes [ 8 ]; and secondary [ 9 ] or tertiary hyperthyroidism [ 10 ] in patients suffering CKF. Hyperphosphatemia with hypocalcemia caused by tubular damage and impaired vitamin D metabolism explains hyperparathyroidism in these patients.
Brown tumors are either mono- or polyostotic benign masses, painless and usually found incidentally. However, they may cause tissue damage to adjacent structures and compressive manifestations such as pain, neuropathies [ 11 ] and myelopathy [ 12 ]. The majority of cases report the maxilla and mandible as the main sites of occurrence [ 9 ]. Other common sites are the clavicles, scapula, pelvis and ribs; however, these lesions may appear in any osseous structure [ 7 ], including chondral tissue [ 13 ]. They are associated with an increased risk of fractures if localized in weight-bearing areas [ 14 ].
Brown tumors arise from foci of OFC and represent a reparative bone process rather than true neoplastic lesions, as there is no hyperplasia or clonal cell proliferation. Typical histopathology describes spindle cells or fibroblasts in areas of osseous lysis, multinucleated giant cells (probably osteoclasts), increased vascularization and accumulation of hemosiderin-laden macrophages, with micro-hemorrhages which confer a brownish appearance to the affected tissue. Cysts and areas of necrosis may be found [ 2 , 5 ]. Brown tumors are histologically similar to giant-cell tumors, giant-cell regenerative granulomas, cherubism and aneurismatic osseous cysts [ 2 , 4 ].
On X-ray imaging, brown tumors appear as lytic lesions with thinned cortical bone that may be fractured. Concurrent changes that suggest OFC such as osteopenia, a "salt-and-pepper" bone appearance, subperiosteal bone resorption and disappearance of the lamina dura around the roots of the teeth, may help differentiate it from other entities [ 4 ]. Tomographic imaging shows an osseous mass, with no cortical disruption, no periosteal reaction or inflammatory signs, a heterogeneous center and areas that suggest cysts [ 14 ]. Magnetic resonance imaging (MRI) shows variable intensities on T2-weighted images and intense enhancement on T1-weighted contrast MRI. MRI may be better for determining the presence of cysts or fluid filled levels; a finding that is very suggestive of a brown tumor [ 14 ]. Osseous gammagraphy is not indicated for the diagnosis of brown tumors; however, isolated hypermetabolic lesions or simultaneous hypercaptation of bone lesions and parathyroid adenomas, when done with Tc 99 m Sestamibi, have been described [ 15 ].
Although differential diagnoses for an isolated bone lesion are extensive, when confronted with a patient with CKF, an osseous mass and laboratory data that show increased levels of calcium, phosphate, phosphocalcic product as well as alkaline phosphatase, it is imperative to determine PTH levels to rule out hyperparathyroidism. Histopathological analysis of the osseous lesion is needed to confirm the diagnosis of a brown tumor. In the case presented here, parathyroid levels were not assessed earlier because another diagnosis, osseous neoplasia, was suspected which posed major prognostic value and risk for the patient. A parathyroid hormone measurement six months earlier reported 570 pg/ml; thus, it is probable that the pathological process evolved during this brief time.
Treatment of brown tumors relies on a definitive control of the underlying hyperparathyroid state. In a patient with CKF, this is achieved through the administration of phosphorus chelators, and calcium and vitamin D supplementation. In patients presenting with tertiary hyperparathyroidism, parathyroidectomy may be required. Osseous lesions usually cease to grow, then shrink and eventually ossify without further consequences for the patient. Surgery is required under certain circumstances, such as: 1) compressive neurologic symptoms over peripheral nerves, cauda equina or spinal medulla; 2) a significant anatomical deformity; 3) risk of a pathologic fracture; 4) when the symptoms or pain do not resolve despite adequate medical treatment and control of the hyperparathyroid state; and 5) when the biopsy does not yield a clear diagnosis, as with the present case [ 9 , 11 , 12 ].
The case presented here illustrates how brown tumors, though rare, should be considered in patients with CKF and an osseous mass. The initial clinical presentation of this patient, a history of DM with a non-compensated CKF and the laboratory studies suggested an infectious process. Retrospectively, these initial complaints and findings could be explained by the patient's renal condition with volume overload, severe anemia, hydro-electrolyte disturbances, as well as altered calcium and phosphate metabolism. Early diagnosis and proper management of CKF enable an optimal control of bone-mineral metabolism, thus decreasing the incidence of OFC and making brown tumors rare lesions. Nevertheless, when confronted with a patient with CKF and an osseous mass, a brown tumor caused by hyperparathyroidism should always be considered in the differential diagnosis.
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Abbreviations
chronic kidney failure
diabetes mellitus
interleukin 1
interleukin 6
interleukin 11
magnetic resonance imaging
osteitis fibrosa cystica
parathyroid hormone.
Guarnaccia E: [Brown fronto-ethmoidal tumor; contribution to the knowledge of cranial localizations of the fibrocystic osteopathies]. Otorinolaringol Ital. 1953, 21: 175-189.
CAS PubMed Google Scholar
Mafee MF, Yang G, Tseng A, Keiler L, Andrus K: Fibro-osseous and giant cell lesions, including brown tumor of the mandible, maxilla, and other craniofacial bones. Neuroimaging Clin N Am. 2003, 13: 525-540. 10.1016/S1052-5149(03)00040-6.
Article PubMed Google Scholar
Hruska K: New concepts in renal osteodystrophy. Nephrol Dial Transplant. 1998, 13: 2755-2760. 10.1093/ndt/13.11.2755.
Article CAS PubMed Google Scholar
Takeshita T, Tanaka H, Harasawa A, Kaminaga T, Imamura T, Furui S: Brown tumor of the sphenoid sinus in a patient with secondary hyperparathyroidism: CT and MR imaging findings. Radiat Med. 2004, 22: 265-268.
PubMed Google Scholar
Fernandez-Sanroman J, Anton-Badiola IM, Costas-Lopez A: Brown tumor of the mandible as first manifestation of primary hyperparathyroidism: diagnosis and treatment. Med Oral Patol Oral Cir Bucal. 2005, 10: 169-172.
Pahlavan PS, Severin MC: Parathyroid carcinoma: A rare case with mandibular brown tumor. Wien Klin Wochenschr. 2006, 118: 175-179. 10.1007/s00508-006-0566-5.
Erturk E, Keskin M, Ersoy C, Kaleli T, Imamoglu S, Filiz G: Metacarpal brown tumor in secondary hyperparathyroidism due to vitamin-D deficiency A case report. J Bone Joint Surg Am. 2005, 87: 1363-1366. 10.2106/JBJS.D.02250.
Ehrlich GW, Genant HK, Kolb FO: Secondary hyperparathyroidism and brown tumors in a patient with gluten enteropathy. AJR Am J Roentgenol. 1983, 141: 381-383.
Jeren-Strujic B, Rozman B, Lambasa S, Jeren T, Markovic M, Raos V: Secondary hyperparathyroidism and brown tumor in dialyzed patients. Ren Fail. 2001, 23: 279-286. 10.1081/JDI-100103500.
Pinto LP, Cherubinim K, Salum FG, Yurgel LS, de Figueiredo MA: Highly aggressive brown tumor in the jaw associated with tertiary hyperparathyroidism. Pediatr Dent. 2006, 28: 543-546.
Tarrass F, Ayad A, Benjelloun M, Anabi A, Ramdani B, Benghanem MG, Zaid D: Cauda equina compression revealing brown tumor of the spine in a long-term hemodialysis patient. Joint Bone Spine. 2006, 73: 748-750. 10.1016/j.jbspin.2006.01.011.
Kaya RA, Cavusoglu H, Tanik C, Kahyaoglu O, Dilbaz S, Tuncer C, Aydin Y: Spinal cord compression caused by a brown tumor at the cervicothoracic junction. Spine J. 2007, 7: 728-732. 10.1016/j.spinee.2006.07.013.
Perrin J, Zaunbauer W, Haertel M: Brown tumor of the thyroid cartilage: CT findings. Skeletal Radiol. 2003, 32: 530-532. 10.1007/s00256-003-0664-7.
Takeshita T, Takeshita K, Abe S, Takami H, Imamura T, Furui S: Brown tumor with fluid-fluid levels in a patient with primary hyperparathyroidism: radiological findings. Radiat Med. 2006, 24: 631-634. 10.1007/s11604-006-0068-4.
Yapar AF, Aydin M, Reyhan M, Bal N, Yapar Z, Yologlu NA: Simultaneous visualization of a mandibular brown tumor with a large parathyroid adenoma on Tc-99 m MIBI imaging. Clin Nucl Med. 2005, 30: 433-435. 10.1097/01.rlu.0000162970.49398.4c.
Download references
Acknowledgements
We thank the following persons: the patient and his family for the information provided and their approval for the publication of this case; the medical staff at the Hospital Pablo Tobón Uribe, especially the Internal Medicine, Radiology, Surgery and Pathology Departments, and the Nephrology and Dialysis Unit; Dr. Victoria Eugenia Murillo for histopathological analysis, case discussion and photomicrography; Dr. John M. Lopera, Dr. Jorge H. Donado and Ana Isabel Toro for manuscript revision and editing.
Author information
Authors and affiliations.
Department of Internal Medicine, Hospital Pablo Tobón Uribe, Calle 78B No. 69-240, Medellín, Colombia
Germán Campuzano-Zuluaga, William Velasco-Pérez & Juan Ignacio Marín-Zuluaga
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Germán Campuzano-Zuluaga .
Additional information
Competing interests.
The authors declare that they have no competing interests regarding this case report.
Authors' contributions
GCZ summarized and interpreted the patient's medical record and was part of the medical staff, did the literature review and wrote the manuscript. WV and JIMZ helped to interpret the patient's medical record, were part of the medical staff and helped to write and review the manuscript. JIMZ was the principal attending physician and responsible for most medical decisions and interpretations expressed in the article. All authors read and approved the final manuscript.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/3.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Campuzano-Zuluaga, G., Velasco-Pérez, W. & Marín-Zuluaga, J.I. A 60-year-old man with chronic renal failure and a costal mass: a case report and review of the literature. J Med Case Reports 3 , 7285 (2009). https://doi.org/10.4076/1752-1947-3-7285
Download citation
Received : 23 December 2008
Accepted : 24 December 2008
Published : 04 August 2009
DOI : https://doi.org/10.4076/1752-1947-3-7285
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Hyperparathyroidism
- Parathyroid Adenoma
- Renal Osteodystrophy
- Brown Tumor
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Acute Renal Failure

Learn about the nursing care management of patients with acute renal failure in this nursing study guide .
Table of Contents
- What is Acute Renal Failure?
Pathophysiology
Statistics and incidences, clinical manifestations, complications, urine tests, blood tests, other tests, medical management, nursing assessment, nursing diagnosis, nursing care planning & goals, nursing interventions, discharge and home care guidelines, documentation guidelines.
- Practice Quiz: Acute Renal Failure
What is Acute Renal Failure?
Renal failure results when the kidneys cannot remove the body’s metabolic wastes or perform their regulatory functions.
- Acute renal failure (ARF) is a rapid loss of renal function due to damage to the kidneys.
- Acute renal failure is also known today as acute kidney injury (AKI) .
- It is a problem seen in hospitalized patients and those in outpatient settings.
- A healthy adult eating a normal diet needs a minimum daily urine output of approximately 400 ml to excrete the body’s waste products through the kidneys. An amount lower than this indicates a decreased GFR.
Although the pathogenesis of ARF and oliguria is not always known, many times there is a specific underlying problem.
- Underlying problems. There are underlying problems that cause the development of ARF such as hypovolemia , hypotension , reduced cardiac output and failure, and obstruction of the kidney.
- Blood flow. As these underlying problems affect the body, the blood flow to the kidneys reduces.
- Decreased kidney function. With inadequate blood flow to the kidney, there is impaired kidney function.
- Failure. If the underlying conditions are not treated and corrected, they can lead to permanent damage of the kidneys.
Here’s the statistics and incidences for acute renal failure:
- ARF affects approximately 1% of patients on admission to the hospital, 2% to 5% during the hospital stay, 4% to 15% after cardiopulmonary bypass surgery , and 10% of cases acute renal failure occurs in isolation (i.e. single organ failure).
- In the United States, the annual incidence of acute renal failure is 100 cases for every million people . It’s diagnosed in 1% of hospital admissions. Hospital-acquired acute renal failure occurs in 4% of all admitted patients and 20% of patients who are admitted to critical care units.
Acute renal failure (ARF) has four well-defined stages: onset, oliguric or anuric, diuretic , and convalescent. Treatment depends on stage and severity of renal compromise. ARF can be divided into three major classifications, depending on site:
- Prerenal failure is caused by interference with renal perfusion (e.g., blood volume depletion, volume shifts [“third-space” sequestration of fluid], or excessive/too-rapid volume expansion), manifested by decreased glomerular filtration rate (GFR).
- Disorders that lead to prerenal failure include cardiogenic shock , heart failure (HF), myocardial infarction (MI), burns , trauma , hemorrhage , septic or anaphylactic shock , and renal artery obstruction.
Renal (or intrarenal)
- Intrarenal causes for renal failure are associated with parenchymal changes caused by ischemia or nephrotoxic substances.
- Acute tubular necrosis (ATN) accounts for 90% of cases of acute oliguria.
- Destruction of tubular epithelial cells results from (1) ischemia/hypoperfusion (similar to prerenal hypoperfusion except that correction of the causative factor may be followed by continued oliguria for up to 30 days) and/or (2) direct damage from nephrotoxins.
- Postrenal failure occurs as the result of an obstruction in the urinary tract anywhere from the tubules to the urethral meatus.
- Obstruction most commonly occurs with stones in the ureters, bladder , or urethra; however, trauma, edema associated with infection, prostate enlargement, and strictures also cause postrenal failure.
There are four phases of ARF: initiation, oliguria, diuresis, and recovery.
- Initiation. The initiation period begins with the initial insult, and ends when oliguria develops.
- Oliguria. The oliguria period is accompanied by an increase in the serum concentration of substances usually excreted by kidneys.
- Diuresis. The diuresis period is marked by a gradual increase in urine output, which signals that glomerular filtration has started to recover.
- Recovery. The recovery period signals the improvement of renal function and may take 3 to 12 months .
The causes of ARF depend on its categories: prerenal, intrarenal, and postrenal.
- Prerenal. Examples of prerenal causes are volume depletion, impaired cardiac efficiency, and vasodilation.
- Intrarenal. Examples of intrarenal causes are prolonged renal ischemia, nephrotoxic agents, and infectious processes.
- Postrenal. An example of a postrenal cause is urinary tract obstruction.
Almost every system of the body is affected by the failure of the normal renal regulatory mechanisms.
- Lethargy. Since waste products cannot be filtered, it slowly accumulates in the different parts of the body.
- Dryness. The skin and mucous membrane are dry from dehydration .
- Central nervous system symptoms. This include drowsiness, headache, muscle twitching, and seizures.
- Increased creatinine. All phases of ARF exhibit an increase in creatinine.
Preventing renal failure involves the following:
- Hydration. Provide adequate hydration to patients at risk for dehydration .
- Shock. Prevent and treat shock promptly with blood and fluid replacement.
- Close monitoring. Monitor central venous and arterial pressures and hourly urine output of critically ill patients to detect the onset of renal failure as early as possible.
- Blood administration. Take precautions to ensure that the appropriate blood is administered to the correct patient in order to avoid severe transfusion reactions.
- Infections. Prevent and treat infections promptly because they can produce progressive renal damage.
- Toxic drug effects. To prevent toxic drug effects, closely monitor dosage , duration of use, and blood levels of all medications metabolized or excreted by the kidneys.
Depending on the duration and severity of ARF, a wide range of potentially life-threatening complications can occur.
- Metabolic acidosis. Waste products could not be eliminated by the kidneys and they can contribute to metabolic acidosis.
- Fluid and electrolyte imbalances . Imbalances may occur due to hemorrhage , renal losses, and gastrointestinal losses.
Assessment and Diagnostic Findings
Assessment and diagnosis of a patient with ARF include evaluation for changes in the urine, diagnostic tests that evaluate the kidney contour, and a variety of normal laboratory values.
- Volume: Usually less than 100 mL/24 hr (anuric phase) or 400 mL/24 hr (oliguric phase), which occurs within 24–48 hr after renal insult. Nonoliguric (more than 400 mL/24 hr) renal failure also occurs when renal damage is associated with nephrotoxic agents (e.g., contrast media or antibiotics ).
- Color: Dirty, brown sediment indicates the presence of RBCs, hemoglobin, myoglobin, porphyrins.
- Specific gravity: Less than 1.020 reflects kidney disease, e.g., glomerulonephritis , pyelonephritis with loss of ability to concentrate; fixed at 1.010 reflects severe renal damage.
- pH: Greater than 7 found in urinary tract infections (UTIs), renal tubular necrosis, and chronic renal failure (CRF).
- Osmolality: Less than 350 mOsm/kg is indicative of tubular damage, and urine/serum ratio is often 1:1.
- Creatinine (Cr) clearance: Renal function may be significantly decreased before blood urea nitrogen (BUN) and serum Cr show significant elevation.
- Sodium: Usually increased if ATN is cause for ARF, more than 40 mEq/L if a kidney is not able to resorb sodium , although it may be decreased in other causes of prerenal failure.
- Fractional sodium (Fe Na ): Ratio of sodium excreted to total sodium filtered by the kidneys reveals the inability of tubules to reabsorb sodium. Readings of less than 1% indicate prerenal problems, higher than 1% reflect intrarenal disorders.
- Bicarbonate: Elevated if metabolic acidosis is present.
- Red blood cells (RBCs): May be present because of infection, stones, trauma, tumor , or altered glomerular filtration (GF).
- Protein: High-grade proteinuria (3–4+) strongly indicates glomerular damage when RBCs and casts are also present. Low-grade proteinuria (1–2+) and white blood cells (WBCs) may be indicative of infection or interstitial nephritis. In ATN, proteinuria is usually minimal.
- Casts: Usually signal renal disease or infection. Cellular casts with brownish pigments and numerous renal tubular epithelial cells are diagnostic of ATN. Red casts suggest acute glomerular nephritis.
- BUN/Cr: Elevated and usually rise in proportion with ratio of 10:1 or higher.
- Complete blood count (CBC): Hemoglobin (Hb) decreased in presence of anemia . RBCs often decreased because of increased fragility/decreased survival.
- Arterial blood gases ( ABGs ): Metabolic acidosis (pH less than 7.2) may develop because of decreased renal ability to excrete hydrogen and end products of metabolism. Bicarbonate decreased.
- Sodium: Usually increased, but may vary.
- Potassium: Elevated related to retention and cellular shifts (acidosis) or tissue release (red cell hemolysis).
- Chloride, phosphorus, and magnesium : Usually elevated.
- Calcium: Decreased.
- Serum osmolality: More than 285 mOsm/kg; often equal to urine.
- Protein: Decreased serum level may reflect protein loss via urine, fluid shifts, decreased intake, or decreased synthesis because of lack of essential amino acids.
- Radionuclide imaging: May reveal calicectasis, hydronephrosis, narrowing, and delayed filling or emptying as a cause of ARF.
- Kidney, ureter, bladder (KUB) x-ray: Demonstrates size of kidneys/ureters/bladder, presence of cysts, tumors, ad kidney displacement or obstruction (stones).
- Retrograde pyelogram: Outlines abnormalities of renal pelvis and ureters.
- Renal arteriogram: Assesses renal circulation and identifies extravascularities, masses.
- Voiding cystoureterogram: Shows bladder size, reflux into ureters, retention.
- Renal ultrasound: Determines kidney size and presence of masses, cysts, obstruction in upper urinary tract.
- Nonnuclear computed tomography (CT) scan: Cross-sectional view of kidney and urinary tract detects presence/extent of disease.
- Magnetic resonance imaging (MRI): Provides information about soft tissue damage.
- Excretory urography (intravenous urogram or pyelogram): Radiopaque contrast concentrates in urine and facilitates visualization of KUB.
- Endourology: Direct visualization may be done of urethra, bladder, ureters, and kidney to diagnose problems, biopsy, and remove small lesions and/or calculi.
- Electrocardiogram ( ECG ): May be abnormal, reflecting electrolyte and acid-base imbalances.
- Urinalysis: Analysis of the urine affords enormous insight into the function of the kidneys.
- Twenty–four–hour urine tests: This test requires you to collect all of your urine for 24 consecutive hours. The urine may be analyzed for protein and waste products (urea nitrogen and creatinine). The presence of protein in the urine indicates kidney damage. The amount of creatinine and urea excreted in the urine can be used to calculate the level of kidney function and the glomerular filtration rate (GFR).
- Glomerular filtration rate (GFR): The GFR is a standard means of expressing overall kidney function. As kidney disease progresses, GFR falls. The normal GFR is about 100–140 mL/min in men and 85–115 mL/min in women. It decreases in most people with age. The GFR may be calculated from the amount of waste products in the 24–hour urine or by using special markers administered intravenously. Patients are divided into five stages of chronic kidney disease based on their GFR.
- Urine Specific Gravity: This is a measure of how concentrated a urine sample is. A concentrated urine sample would have a specific gravity over 1.030 or 1.040
- Creatinine is a breakdown product of normal muscle breakdown.
- Urea is the waste product of breakdown of protein.
- The level of these substances rises in the blood as kidney function worsens.
- High potassium (hyperkalemia) is a particular concern.
- The acid–base balance of the blood is usually disrupted as well.
- Decreased production of the active form of vitamin D can cause low levels of calcium in the blood. Inability to excrete phosphorus by failing kidneys causes its levels in the blood to rise.
- Blood cell counts: Because kidney disease disrupts blood cell production and shortens the survival of red cells, the red blood cell count and hemoglobin may be low ( anemia ). Some patients may also have iron deficiency due to blood loss in their gastrointestinal system . Other nutritional deficiencies may also impair the production of red cells.
- In general, kidneys are shrunken in size in chronic kidney disease , although they may be normal or even large in size in cases caused by adult polycystic kidney disease, diabetic nephropathy, and amyloidosis.
- Biopsy: A sample of the kidney tissue (biopsy) is sometimes required in cases in which the cause of the kidney disease is unclear. Usually, a biopsy can be collected with local anesthesia only by introducing a needle through the skin into the kidney.
The objectives of treatment of ARF are to restore normal chemical balance and prevent complications until repair of renal tissue and restoration of renal function can occur.
- Pharmacologic therapy. Cation-exchange resins or Kayexalate can reduce elevated potassium levels; IV dextrose 50%, insulin , and calcium replacement may be administered to shift potassium back into cells; diuretic agents are often administered to control fluid volume.
- Nutritional therapy. Replacement of dietary proteins is individualized to provide the maximum benefit and minimize uremic symptoms; likewise, caloric requirements are met with high-carbohydrate meals, because carbohydrates have a protein-sparing effect; foods and fluids containing potassium or phosphorus are restricted; and after diuretic phase, the patient is placed on a high-protein , high-calorie diet.
Nursing Management
The nurse has an important role in caring for the patient with ARF.
Assessment usually focuses on the characteristics of the urine.
- Assess urine output. Urine output varies from scanty to a normal volume.
- Assess blood in the urine. Hematuria may be present in patients with ARF.
- Assess laboratory results. Laboratory results may increase, decrease, or stabilize and these may indicate each phase of ARF.
Based on the assessment data, appropriate nursing diagnoses for a patient with ARF include:
- Electrolyte imbalance related to increased potassium levels.
- Risk for deficient volume related to increased in urine output.
Main Article: 6 Acute Renal Failure Nursing Care Plans
The goals for a patient with ARF are:
- Improve nutritional intake.
- Restore fluid balance .
- Reduce metabolic rate.
- Promote pulmonary function.
- Prevent infection.
Nursing interventions are aimed at restoring renal function and reducing potential causes of increased renal injury.
- Monitor fluid and electrolyte balance. The nurse monitors the patient’s fluid and electrolyte levels and physical indicators of potential complications during all phases pf the disorder.
- Reducing metabolic rate. Bed rest is encouraged and fever and infection are prevented or treated promptly.
- Promoting pulmonary function. The patient is assisted to turn, cough , and take deep breaths frequently to prevent atelectasis and respiratory tract infection.
- Preventing infection. Asepsis is essential with invasive lines and catheters to minimize the risk of infection and increased metabolism.
- Providing skin care . Bathing the patient with cool water, frequent turning, and keeping the skin clean and well moisturized and keeping the fingernails trimmed to avoid excoriation are often comforting and prevent skin breakdown.
- Provide safety measures. Patient with CNS involvement may be dizzy or confused.
A successful nursing care plan has achieved the following:
- Improved nutritional intake.
- Restored fluid balance.
- Reduced metabolic rate.
- Promoted pulmonary function.
- Prevented infection.
The nurse plays an important role in teaching the patient and family with ARF.
- Nutrition . A referral to the nutritionist is made because of the dietary changes required.
- Problems to report. The patient and family must know what problems to report to the healthcare provider.
- Follow-up examinations. The importance of follow-up examinations and treatment is stressed to the patient and family because of changing physical status and renal functions.
The focus of documentation in a patient with ARF include:
- Vital signs.
- Muscle strength and reflexes.
- Results of laboratory tests and diagnostic studies.
- Degree of deficit and current sources of fluid intake.
- I&O and fluid balance.
- Plan of care.
- Teaching plan.
- Client’s responses to treatment, teaching, and actions performed.
- Attainment or progress towards the desired outcomes.
- Modifications to plan of care.
- Long term needs.
Practice Quiz: Acute Renal Failure
Here’s a 5-item quiz about the study guide. Please visit our nursing test bank for more NCLEX practice questions .
1. Acute renal failure caused by parenchymal damage to the glomeruli of kidney tubules results in all of the following except:
A. Decreased GFR. B. Increased urine specific gravity. C. Impaired electrolyte balance. D.Impaired electrolyte balance.
2. Oliguria is a clinical sign of ARF that refers daily to a urine output of:
A. 1.5L B. 1.0L C. Less than 400ml D. Less than 50ml
3. A fall in CO2-combining power and blood pH indicates what state accompanying renal function?
A. Metabolic acidosis. B. Metabolic alkalosis. C. Respiratory acidosis. D. Respiratory alkalosis.
4. Hyperkalemia is a serious electrolyte imbalance that occurs in ARF and results from:
A. Protein catabolism. B. Electrolyte shifts in response to metabolic acidosis. C. Tissue breakdown. D. All of the above.
5. Potassium intake can be restricted by eliminating high-potassium foods such as:
A. Butter. B. Citrus fruits. C. Cooked white rice D. Salad oil.
Answers and Rationale
1. Answer: B. Increased urine-specific gravity.
- B: The urine has a low specific gravity as a result of tubular damage.
- A: Decreased GFR is a result of ARF.
- C: Impaired electrolyte balance is a result of ARF.
- D: Progressive azotemia is a result of ARF.
2. Answer: C. Less than 400ml.
- C: Oliguria refers to a daily urine output of less than 500ml.
- A: A daily urine output of less than 1.5L is normal.
- B: A daily urine output of less than 1.0L is normal.
- D: A daily urine output of less than 50ml is called anuria.
3. Answer: C. Respiratory acidosis .
- C: Respiratory acidosis is a fall in CO2-combining power and blood pH.
- A: Metabolic acidosis is a fall in bicarbonate levels and a decrease in the blood pH.
- B: Metabolic alkalosis is a fall in bicarbonate levels and an increase in blood pH.
- D: Respiratory alkalosis is a fall in CO2-combining power and increase in blood pH.
4. Answer: B. Electrolyte shifts in response to metabolic acidosis.
- B: Hyperkalemia is caused by electrolyte shifts in response to metabolic acidosis.
- A: Hyperkalemia is not caused by protein catabolism.
- C: Hyperkalemia is not caused by tissue breakdown.
- D: Not all of the options cause hyperkalemia.
5. Answer: B. Citrus fruits.
- B: Citrus fruits have a high potassium content.
- A: Butter is rich in cholesterol.
- C: Cooked white rice is rich in carbohydrates.
- D: Salad oil is rich in sodium.
Posts related to this care plan:
- 6 Acute Renal Failure Nursing Care Plans
- Chronic Renal Failure
- 11 Chronic Renal Failure Nursing Care Plans
- Renal Disorders Nursing Management NCLEX Practice Quiz 1 (50 Items)

11 thoughts on “Acute Renal Failure”
Good information and content
Please provide examples of such patient cases with a proper care plan. THANK YOU
Check out our nursing care plan section.
Great information. Thank you!
Very informative and easy to understand
How can I get more AKI practice questions please?
Hi SF, Great to see your enthusiasm for AKI practice questions! Currently, we have a set of questions available on our website available here: Urinary Disorders NCLEX Practice Quiz (150 Questions)
Great topic. Thank you for the information above.
Hi Graciela, Glad you liked the piece on acute renal failure! 🌟 Any specific parts you found super useful or anything else you’re curious about in this area?
Thank you so much for all the great info. I truly appreciate it.
Leave a Comment Cancel reply
Case Study: An Elderly Patient With Impaired Renal Function
—this case shows the importance of re-evaluating treatment regimens to address adverse effects without exacerbating other comorbidities..
By Emily Donovan Reviewed by Janet B. McGill, MD
This case presents an elderly man with type 2 diabetes and impaired renal function. He has recently been experiencing hypoglycemia with his current treatment regimen. The importance of re-evaluating treatment regimens to address adverse effects without exacerbating other comorbidities, such as renal dysfunction, is discussed.
Case presentation and patient assessment

An 84-year-old man with type 2 diabetes is admitted to the hospital with altered mental status and hypoglycemia. He had experienced recurring episodes of mild hypoglycemia over the previous week, which were managed with food. With this episode, however, he could not be aroused, so the family called EMS. His past medical history includes chronic lymphocytic leukemia, gout, hypertension, and atrial fibrillation. Approximately 6 weeks ago, his serum creatinine increased from 1.6 mg/dL to 2.4 mg/dL following a bout of pneumonia. He had been taking glimepiride 4 mg twice daily. He admitted that he sometimes skipped the dose entirely because of hypoglycemia, and when he did so, his glucose was >200 mg/dL. His glycosylated hemoglobin (HbA1c) is 6.9%. Information and laboratory values collected at the hospital are as follows:
- Height, 5’9”
- Weight, 198 lb
- BP, 114/73 mm Hg
- Current medications, glimepiride 4 mg BID and atorvastatin 10 mg QD
- HbA1c, 6.9%
- Lipids, LDL-C 45 mg/dL; HDL-C 55 mg/dL; Total-C 109 mg/dL; triglycerides 47 mg/dL
- Serum creatinine 1.94 mg/dL
The patient is found to have acceptable glycemic control, but unacceptable episodes of severe hypoglycemia. His worsening renal dysfunction and poor overall health status were likely responsible for the hypoglycemia, since he had tolerated glimepiride in the past. Hypoglycemia with his current treatment regimen was a particular concern given his age, concomitant renal disease, and other comorbidities.
While in the hospital, the patient required a small dose of insulin glargine to control his blood glucose, 16 units.
This patient is elderly and has other comorbidities, including moderate renal impairment. Developing a treatment plan that helps maintain normal glucose levels with little or no associated hypoglycemia as well as limited complexity is important for this elderly patient. 1 Since this patient has moderate renal impairment, it is important to consider medications that are not cleared renally and do not require dose adjustment based on serum creatinine or kidney function. 1 Lastly, renal dysfunction and advanced age are two key risk factors for hypoglycemia. 1-3
At discharge, the decision was made to discontinue insulin glargine and initiate oral therapy with a dipeptidyl peptidase-4 inhibitor, given the low rates of hypoglycemia associated with this class and ease of use (ie, oral administration). 1
Linagliptin 5 mg/day was prescribed at hospital discharge, and a follow-up visit was scheduled in 2 weeks. At the follow-up appointment, the patient reported that he was feeling well and eating better. He stated that his glucose ranged from 140 mg/dL to 210 mg/dL. His serum creatinine had increased to 2.4 mg/dL. He has had no hypoglycemic episodes since leaving the hospital. At this time, a small dose of insulin glargine was added to his glucose-lowering regiment (8 units), with adjustments made on an outpatient basis.
At a 6-month follow-up, the patient’s HbA1c was 6.6%. He reported that his glucose ranges from 110 mg/dL to 155 mg/dL, and he had not experienced any hypoglycemia. His serum creatinine was 1.99 mg/dL. Information and laboratory values collected during the 6-month follow-up visit are summarized here:
- Weight, 186 lb
- BP, 112/70 mm Hg
- Current medications, linagliptin 5 mg/d; insulin glargine 10-14 units/day; atorvastatin 10 mg QD
- HbA1c, 6.6%
- Lipids, LDL-C 28 mg/dL; HDL-C 38 mg/dL; Total-C 103 mg/dL; triglycerides 73 mg/dL
- Serum creatinine 1.99 mg/dL
Over the next 2 years, the patient’s serum creatinine fluctuated from 1.7 mg/dL to 2.5 mg/dL. He required additional chemotherapy for chronic lymphocytic leukemia with small cell lymphoma. His diabetes remained stable on the same regimen of linagliptin 5 mg/day and insulin glargine 10 to 14 units/day.
This case highlights the importance of re-evaluating treatment regimens in elderly patients with renal impairment and hypoglycemia. The 2012 position statement of the American Diabetes Association and the European Association for the Study of Diabetes emphasizes the importance of a patient-centered approach to the treatment of diabetes and, in particular, the need to individualize treatment regimens based on a particular patient’s comorbidities. 1 The American Diabetes Association and the European Association for the Study of Diabetes recommendations state that glycemic targets in elderly patients may need to be less ambitious than those for younger patients with fewer comorbidities and less complicated disease. Furthermore, glucose-lowering regimens for elderly patients should focus on agents that protect against hypoglycemia, renal dysfunction, and drug interactions since the risk of all of these complications is increased in elderly patients. 1 This case illustrates the importance of adjusting a treatment regimen to address a patient’s age, kidney function, and risk of hypoglycemia.
The main goal of therapy for this patient is to achieve glycemic control and minimize the risk of hypoglycemia. In addition, it was important to develop a regimen that did not require dose adjustments for fluctuating kidney function and had a low likelihood of drug interactions with therapies given for other disorders. This patient had numerous comorbidities, and had several additional medications added over the following year to treat his gout, chronic lymphocytic leukemia, and various infections. He remained on the regimen of linagliptin 5 mg/day and insulin glargine, which provided excellent and durable control of his glucose levels with rare episodes of mild hypoglycemia. Providing glucose-lowering therapy with a low propensity for drug interactions in this patient who had multiple comorbidities and was receiving numerous medications that changed over time was also an important aspect of his treatment regimen.
Linagliptin is a rational therapeutic choice for this patient given its primarily nonrenal route of elimination, limited drug interactions, and low risk of hypoglycemia. 4,5 Linagliptin does not require dose adjustment in patients with renal impairment, and has a low likelihood of hypoglycemia in all patients, which is of particular importance in the elderly. 4 The fact that linagliptin is not cleared renally is especially important since impaired renal function can affect drug metabolism (eg, increase concentrations) and predispose patients with renal dysfunction to hypoglycemia. 2 The other DPP-4 inhibitors (alogliptin, saxagliptin, sitagliptin, and vildagliptin) are all predominantly eliminated via renal excretion, and each requires dose adjustment in patients with renal impairment. 1,6
It is also important to note that more severe renal impairment can result in slower elimination of insulin; therefore, dose titration of insulin in patients with severe renal impairment should be done cautiously with recognition for the potential of a more prolonged activity profile in these patients. 1
Post-hoc analyses from the phase III clinical program of linagliptin in patients with type 2 diabetes have shown that it is associated with neutral or favorable effects (on top of standard of care) on renal outcomes as well as consistent glucose-lowering efficacy and tolerability in patients with a range of kidney function. 7-9 Two recent, prospective studies have shown that linagliptin was associated with clinically meaningful improvements in glycemic control and neutral effects on renal parameters in patients with type 2 diabetes and with moderate-to severe renal impairment as well as those with type 2 diabetes and severe renal impairment. 10,11 Overall, these analyses indicate that linagliptin is a safe and effective treatment option in patients with renal impairment.
Elderly patients with type 2 diabetes are at increased risk of hypoglycemia and its consequences. Renal impairment, often present in elderly patients with type 2 diabetes, further increases the risk of hypoglycemia, because it can affect the metabolism of glucose-lowering medications. Therefore, it is important to re-evaluate and adjust treatment regimens for type 2 diabetes in elderly patients with renal impairment to ensure risk of hypoglycemia is minimized and the glucose-lowering regimen does not require dose adjustment for renal impairment. Treatment with linagliptin is a logical choice for elderly patients with renal impairment since it has a predominantly nonrenal route of clearance and low risk of hypoglycemia. In addition, linagliptin does not require dose adjustment for elderly patients or for those with renal impairment, thereby minimizing the complexity of the glucose-lowering regimen in a patient with multiple comorbidities.
Published: November 16, 2016
- 1. Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care . 2012:35:1364-1379
- 2. Moghissi E. Management of type 2 diabetes mellitus in older patients: current and emerging treatment options. Diabetes Ther . 2013;4:239-256.DS) Group. JAMA . 1999;281:2005-2012.
- 3. Ahrén B. Avoiding hypoglycemia: a key to success for glucose-lowering therapy in type 2 diabetes. Vasc Health Risk Manag . 2013;9:155-163.
- 4. McKeage K. Linagliptin: an update of its use in patients with type 2 diabetes mellitus. Drugs . 2014;74:1927-1946.
- 5. Doupis J. Linagliptin: from bench to bedside. Drug Des Devel Ther . 2014;8:431-443.
- 6. Giorda C, Nada E, Tartaglino B. Pharmacokinetics, safety, and efficacy of DPP-4 inhibitors and GLP-1 receptor agonists in patients with type 2 diabetes mellitus and renal or hepatic impairment. A systematic review of the literature. Endocrine . 2014;46:406-419.
- 7. Groop P-H, Cooper ME, Perkovic V, Emser A, Woerle H-J, von Eynatten M. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care . 2013;36:3460-3468.
- 8. Groop PH, Del Prato S, Taskinen MR, et al. Linagliptin treatment in subjects with type 2 diabetes with and without mild-to-moderate renal impairment. Diabetes Obes Metab . 2014;16:560-568
- 9. McGill JB, Barnett AH, Lewin AJ, et al. Linagliptin added to sulphonylurea in uncontrolled type 2 diabetes patients with moderate-to-severe renal impairment. Diab Vasc Dis Res . 2014;11:34-40.
- 10. Laakso M, Rosenstock J, Groop PH, et al. Treatment with the dipeptidyl peptidase-4 inhibitor linagliptin or placebo followed by glimepiride in patients with type 2 diabetes with moderate to severe renal impairment: a 52-week, randomized, double-blind clinical trial. Diabetes Care . 2015;38:e15-e17.
- 11. McGill JB, Sloan L, Newman J, et al. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment: a 1-year, randomized, double-blind, placebo-controlled study. Diabetes Care . 2013;36:237-244.
Dpp-4: the physiology of incretin degradation.

Incretins and More: The Physiology of DPP-4
New approaches for dkd in adolescents with t2d.

How Effective is Weight Loss as Therapy for Type 2 Diabetes?

T2D In Youth

Diet in Diabetes: One Size Doesn’t Fit All

Potential Risks of Anti-obesity Medications in Patients with T2D

Treating the Root Cause: Diabetes Medicine


- Previous Article
- Next Article
Presentation
Clinical pearls, suggested readings, case study: renal disease in type 1 diabetes.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
William H. Herman; Case Study: Renal Disease in Type 1 Diabetes. Clin Diabetes 1 April 2001; 19 (2): 74. https://doi.org/10.2337/diaclin.19.2.74
Download citation file:
- Ris (Zotero)
- Reference Manager
C.M. is a 27-year-old woman with type 1 diabetes diagnosed at age 14 when she presented with diabetic ketoacidosis. Her initial insulin treatment was complicated by poor glycemic control, frequent hypoglycemia, and weight gain.
Two years ago, she developed hypertension, which was treated with hydrochlorthiazide, 25 mg daily. At that time, she was noted to have nonproliferative diabetic retinopathy. Blood urea nitrogen (BUN) was 23 mg/dl, creatinine was 0.9 mg/dl, and dipstick urinalysis was negative for protein.
She now presents with accelerated hypertension (172/108 mmHg) and pitting edema of the legs to the level of the knees. Urinalysis reveals 3+ protein and 2+ blood. Urine microscopic analysis reveals hyalin and red blood cell casts. BUN is 37 mg/dl; creatinine is 1.5 mg/dl; and 24-h urine reveals 9.7 g of protein. Creatinine clearance is 58 ml/min. Total cholesterol is 279 mg/dl.
Does C.M. have diabetic nephropathy?
What diagnostic tests are indicated?
What is the appropriate treatment for C.M.’s renal disease?
We believed that C.M. had type 1 diabetes with nonproliferative retinopathy, accelerated hypertension, and nephrotic syndrome. Although the history of retinopathy and hypertension were consistent with the development of diabetic nephropathy, the urinary findings and rapid progression of renal insufficiency were inconsistent with diabetic nephropathy and raised the specter of a second etiology of her renal disease.
On further testing, Westergren erythrocyte sedimentation rate was 81 mm/h, urine immunoelectrophoresis was negative for Bence Jones protein, and rheumatoid factor was negative, but antinuclear antibody was positive in a titer of 1:320 with a homogenous pattern. Anti-DNA was 5.1% (normal 0–7%). C3 complement was low, C4 complement was normal, and CH 50 was at the lower limit of normal. Renal biopsy demonstrated mixed proliferative and focal membranous glomerulonephritis consistent with lupus nephropathy. In addition, changes were present suggestive of early diabetic glomerulosclerosis.
The patient was treated with monthly intravenous cyclophosphamide (Cytoxan) for 6 months and was subsequently maintained on prednisone and hydroxychloroquine (Plaquenil). Serum creatinine peaked at 2.0 mg/dl, but over the next 2 years it fell to 1.3 mg/dl. Urine protein excretion fell to 0.85 g/24 h.
Approximately 40% of people with longstanding type 1 diabetes develop diabetic nephropathy. Essentially all patients with diabetic nephropathy have diabetic retinopathy detectable by dilated retinal examination.
In type 1 diabetes, diabetic nephropathy follows a predictable course from onset of diabetes to the onset of microalbuminuria to frank nephropathy to end-stage renal disease or death. Microalbuminuria develops 10–14 years after onset of diabetes. Without treatment, clinical nephropathy follows within 5 years, and azotemia develops ∼5 years later. Hypertension develops in association with microalbuminuria and progresses with diabetic nephropathy. In diabetic nephropathy, the urine sediment is bland. Red blood cells are usually absent, although they may be present with infection or in the rare instance of papillary necrosis. Red cell casts are absent.
Diabetic nephropathy is a diagnosis of exclusion. In this case, accelerated hypertension, an active urinary sediment with both red cells and red cell casts, and the rapid onset of nephrotic syndrome with renal insufficiency is more consistent with glomerulonephritis mediated by immune mechanisms. Thus, thorough testing for secondary causes of immune-mediated glomerulonephritis, including renal biopsy, were indicated to identify a second, more treatable, cause of renal disease.
Attributing the patient’s renal disease to diabetic nephropathy, failing to pursue alternative diagnoses, and thus failing to implement disease-specific treatment would have likely resulted in the rapid onset of renal failure.
In type 1 diabetes, diabetic nephropathy is a diagnosis of exclusion.
The absence of diabetic retinopathy, onset of microalbuminuria before 10 years, and onset of clinical nephropathy before 15 years, along with findings of an active urinary sediment with red cell casts and rapidly progressive renal insufficiency, should prompt further evaluation.
Establishing an alternative diagnosis is critical when alternative disease-specific therapies exist.
William H. Herman, MD, MPH, is a professor of internal medicine and epidemiology at the University of Michigan and an associate editor of Clinical Diabetes .
Email alerts
- Online ISSN 1945-4953
- Print ISSN 0891-8929
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Campus Directory
- Current Students
- Faculty & Staff

Acute Renal Failure - Case Summary

In effect, the renal tubules are damaged and cannot fulfill their normal activities of tubular excretion, secretion, and reabsorption. Hypervolemia, the body's retention of water which in turn increases the blood volume, is one of the more serious effects, leading to increased blood pressure and cardiac overload. The exchange of potassium from intracellular fluid to the plasma poses the potential for disruption of the heart's conduction mechanism. Waste products such as urea and creatinine cannot be excreted. Hydrogen ion balances are disrupted leading to metabolic acidosis.
2. Symptoms of renal failure depend widely on the underlying cause(s). In this case, the disease was in its early stages. Edema (retention of fluid in tissues), oliguria (decreased urinary output), and increased blood pressure (due to increased fluid retention) were seen.
3. Diagnosis is based on the patient's history and key blood and chemistry values. BUN and Creatinine measure the waste products in the blood. Electrolyte values, bicarbonate, and pH measure the severity of the acidosis. Proteins, cells, and casts in the urine are indicative of renal damage.
4. Treatment options also vary with renal failure. In renal failure cases where toxin levels are extremely high in the blood, renal dialysis, either peritoneal or hemodialysis, must be performed to clear the bloodstream of the offending toxins as well as the build-up of waste products the kidneys have been unable to remove. Careful monitoring of the patient's blood electrolyte and water balance are the key to restoring the health of the renal failure patient. Diuretics may be used as indicated to reduce the blood volume and dilute the electrolyte values.
5. Prevention. Patients with underlying conditions such as diabetes mellitus may be more susceptible to the tubular necrosis described above. Care must be exercised when administering potential kidney toxins such as antibiotics, injectable x-ray contrast media, and other substances.
6. Prognosis of patients with renal failure vary. 50% will recover with some combination of treatments. Others will develop chronic renal insufficiency and will require long-term treatment. Others will ultimately develop what is called end-stage renal disease and die from such complications as heart failure.
7. The clinic physician, family physician, and nephrologist all worked together to diagnose and treat this patient. Nurses trained in treating kidney patients monitored vitals, administered I.V's and gave supportive therapy as needed. If the patient had required dialysis, technicians trained in dialysis would have performed the procedure. Medical Laboratory Technologists performed the blood and urine testing. This patient's medical records were vital to her diagnosis. Individuals trained in health information technology are responsible for accurately maintaining these records.
NKF Launches KidneyCARE™ Study To Empower Patients, Advance Kidney Disease Research

Groundbreaking interactive patient registry will help empower individuals with kidney disease to help pave the way for improved outcomes in kidney care
(March 20, 2024, New York, NY) — The National Kidney Foundation (NKF) proudly announces the launch of the KidneyCARE (Community Access to Research Equity)™ Study, a cutting-edge online research initiative combining patients’ insights on the impact of living with kidney disease and patient health data to improve and advance kidney disease research.
With more than one in 7 Americans affected by kidney disease and nearly one in 3 at risk of developing it, the need for innovative solutions and comprehensive support is critical. The KidneyCARE Study provides hope for those navigating the challenges of kidney disease, offering a transformative platform to enhance kidney care and advance research efforts. The Study will collect both rigorous clinical and laboratory data from electronic health records (EHR), in addition to patient-entered data, which together allow for a complete picture of the patient experience. This model is innovative in that most research initiatives follow one path or the other—EHR or patient self-reporting. The Study will compile data on demographics, medical history, lifestyle, medications, blood and urine test results, in addition to extensive data on patient perceptions, challenges, and priorities.
People with all types of kidney disease can join the KidneyCARE Study to share how kidney disease impacts their overall health and daily living. Participants in the Study will benefit by receiving kidney health education, access to clinical trial opportunities, and peer support information.
"This type of research is key to the future of patient-centered kidney care," said Kevin Longino, CEO of the National Kidney Foundation and a kidney transplant recipient. "In addition to collecting data on patient outcomes over time, the study aims to provide a lifeline of support and resources tailored to the unique needs of each participant, paving the way for improved outcomes."
NKF partnered with HHS Technology Group , (HTG) to utilize HTG’s proven data analytics platform, Discover Your Data (DyD®), as the foundation of the online patient registry, alongside the expertise of HTG’s subcontractor partners, Datavant, Inc. and Mathematica, Inc. DyD is an end-to-end platform solution that allows healthcare payers, nonprofit and public sector organizations, pharma, researchers, academic medical centers, and providers to translate big questions into deep understanding by seamlessly connecting to the nation’s largest health data ecosystem and allowing for organizations to bring their own data.
“We are proud to collaborate with NKF on this important initiative to improve kidney disease education, support, and treatment,” said Brett Furst, President, HTG. “Notably, the registry will include patient-reported outcomes, which are among the most difficult types of data to obtain but provide immense value as an indicator of the efficacy of various kidney disease treatments.”
“We invite individuals living with kidney disease to join us in this groundbreaking initiative," added Longino. "Together, we can drive meaningful change, transform kidney care, and ultimately work towards a future free from the burden of kidney disease."
For more information about the KidneyCARE Study and how to participate, visit kidneycarestudy.org.
About Kidney Disease In the United States, 37 million adults are estimated to have kidney disease , also known as chronic kidney disease (CKD)—and approximately 90 percent don’t know they have it. About 1 in 3 adults in the U.S. are at risk for kidney disease. Risk factors for kidney disease include: diabetes , high blood pressure , heart disease , obesity ,and family history. People of Black/African American, Hispanic/Latino, American Indian/Alaska Native, Asian American, or Native Hawaiian/Other Pacific Islander descent are at increased risk for developing the disease. Black/African American people are more than 3 times as likely as White people to have kidney failure. Hispanics/Latinos are 1.3 times more likely than non-Hispanics to have kidney failure.
About the National Kidney Foundation The National Kidney Foundation is revolutionizing the fight to save lives by eliminating preventable kidney disease, accelerating innovation for the dignity of the patient experience, and dismantling structural inequities in kidney care, dialysis, and transplantation.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
March 22, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
New genomic method offers diagnosis for patients with unexplained kidney failure
by Newcastle University
Scientists have identified a new method of analyzing genomic data in a major discovery that means patients with unexplained kidney failure are finally getting a diagnosis.
Experts at Newcastle University have worked with data from Genomics England 100,000 Genomes project to establish a diagnosis in patients with unexplained kidney failure.
There are numerous reasons for kidney failure, which if left untreated is life-threatening, but often patients do not get a precise diagnosis which can make their best course of treatment unclear.
Missing genetic data
Research, published in Genetics in Medicine Open , has now revealed that, for these patients, areas in their genome are missing and so are not detected as faulty when using the routine genetic pipelines to analyze data.
Scientists say that as this missing gene has now been identified, and mutations within it found, they have been able to classify this as NPHP1-related kidney failure.
Professor John Sayer, Deputy Dean of Biosciences at Newcastle University, said, "Our new genomic methods and their results has huge implications for the patients and families with kidney failure who were previously genetically unsolved."
"What we are now able to do is give some patients a precise diagnosis, which allows their investigations, treatment, and management to be tailored to their needs for the best possible outcomes."
In the study, experts reviewed genetic sequencing data from 959 patients with advanced kidney disease, where a total of 11 patients were identified as having a deleted region genome, leading to a complete loss of a kidney gene that had previously been undetectable.
The new approach was also used to examine genomic data from 11,754 cases to make new genetic diagnoses of 10 other UK patients with unexplained deafness and blindness, again who had previously been genetically unexplained.
Professor Sayer, who is also a consultant nephrologist at Newcastle upon Tyne Hospitals NHS Foundation Trust, added, "We knew that many of our unsolved cases had a genetic disorder , and this new approach enables us to solve these cases definitively."
"We can now give an accurate genetic diagnosis to many more families affected by kidney disease, and our hope is to provide a proper diagnosis for many more families in the future."
"This work is a reminder that it is always worth investigating the underlying reasons for kidney failure to get to the bottom of the condition."
"Finding a genetic cause of kidney failure has huge implications for the patient and also for other family members, especially if they are wishing to donate a kidney to their loved one."
The Newcastle experts are now working with cell lines taken from patients to study more in detail the disease process and to test potential treatments.
Case study: Family finally given answers
The Bingham family has three members, all affected by kidney disease.
Siblings Noah, 23, and Ariel, 19, have both had kidney transplants, and their younger brother, Casper, 15, has been diagnosed with kidney disease.
The family, from Hexham, Northumberland, is part of the Genomics England 100,000 Genomes project and was one of the families identified as having the gene deletion, NPHP1-related kidney failure.
Noah presented with kidney failure just after finishing his A-levels, and at the same time, Ariel was being treated for reduced kidney function.
Both now have donated kidneys as their own organs function reduced to dangerously low levels. Sadly, Noah's transplanted kidney failed after only 16 months, and he had to start hemodialysis again in February this year.
Mum Sarah, 51, a home educator, said, "The genetic tests carried out by Professor John Sayer and his team allowed Casper to receive his diagnosis before he was symptomatic."
"The knowledge that Casper will go into kidney failure and eventually need a transplant, though overwhelming at times, has meant that we can arrange the support he needs and help him prepare for surgery and treatments well before they are necessary."
"When nobody is able to explain why your children are ill, it is very unsettling, with no means of clarifying what might happen in the future. The diagnosis has meant that we have been able to prepare ourselves for the medical issues our children face."
"It's great that this research is being carried out at Newcastle University as it means patients with the condition can get a better understanding of their medical needs, and hopefully, new treatments may be developed in the future thanks to the research that is being done."
Sarah and her husband, Darryl, 52, a chartered building surveyor, have been strong advocates for kidney patients and work with Kidney Research UK to help support patients.
Explore further
Feedback to editors
Researchers determine underlying mechanisms of inherited disorder that causes bone marrow failure
Mar 22, 2024
Intervention after first seizure may prevent long-term epilepsy
Researchers uncover protein interactions controlling fertility in female mice

Scientists close in on TB blood test that could detect millions of silent spreaders

New study reveals preventable-suicide risk profiles
Anti-inflammatory molecules show promise in reducing risks of further heart damage
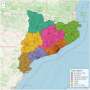
A boost to biomedical research with statistical tools: From COVID-19 analysis to data management
UK study provides insights into COVID-19 vaccine uptake among children and young people
Researchers describe tools to better understand CaMKII, a protein involved in brain and heart disease

Risk of adverse pre-eclampsia outcomes accurately identified through new AI model
Related stories.
New liver and kidney disease identified
May 27, 2022

AI tool predicts kidney failure six times faster than human expert analysts
Mar 4, 2024

Those with kidney failure due to sickle cell disease wait longer for transplants, have higher mortality
Nov 6, 2023

Discovery of gene that modifies the severity of inherited kidney disease
Jan 9, 2020

Uromodulin levels may indicate risk for kidney failure
Sep 21, 2023
Real-world analysis of sodium-glucose cotransporter-2 inhibitors in kidney transplant recipients
Nov 4, 2023
Recommended for you
Accumulation of 'junk proteins' identified as one cause of aging and possible source of ALS

Researchers unlock secrets of birth defect origins, offering early detection and prevention strategies
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Nephropharmacol
- v.4(1); 2015

Overview of management of acute renal failure and its evaluation; a case analysis
Chaudhary muhammad junaid nazar.
1 Department of Endocrinology, University of Buckingham, Royal Gwent Hospital, NHS Trust, Wales, UK
Faisal Bashir
2 Department of ENT, New City Teaching Hospital, Mohetarma Benazir Bhutto Shaheed Medical College, Mirpur Azad Kashmir, Pakistan
3 Department of Internal Medicine, Allma Iqbal Memorial Teaching Hospital Sialkot, Punjab, Pakistan
John Anderson
4 Division of Medical Education, Postgraduate Medicine Brighton and Sussex Medical School University of Brighton, Brighton, UK
The annual incidence is about 150 per million in the UK, but this figure is six times greater in the >80 years old group. Prerenal azotemia is considered as the most serious reason in community or hospital acquired acute renal failure (ARF). A 67-year-old middle age male was admitted to the hospital with a chief complaint of generalized weakness, volume depletion and dysuria. He has treated with metronidazole for diarrhoea caused by Clostridium difficile considered as the precipitating factor for the ARF. The patient has severe osteoarthritis and takes high dose non-steroidal anti-inflammatory drugs from the last two years. He also complains for obstructive sleep apnea (OSA) and obesity. He has controlled hypertension was on lisinopril to control blood pressure. ARF is quite common, occurring in 80 million populations. Urinary obstruction should be excluded (a cause in around 5-10 of cases) because this is readily reversible if it is diagnosed early. A renal US will be sufficient to identify obstruction in 95 of cases. Most cases of ARF are expected to pre renal failure/acute tubular necrosis (ATN) 70-80%. Risk factor for development for at ATN are old age, drugs (non-steroidal anti-inflammatory drugs, gentamicin), sepsis, and chronic kidney disease and must be considered.
Implication for health policy/practice/research/medical education:
Acute renal failure (ARF) is defined as the rapid decline in kidney function as manifested by a reduction in glomerular filtration rate. It is a more frequent problem observed in all hospital admission. The incidence of ARF increases with age; the annual incidence is about 150 per million in the UK, but this figure is six times greater in the >80 years old group. The overall mortality associated with the ARF is higher with hospital acquired ARF. Therefore, better understanding and early detection can help in better prognosis.
Introduction
Acute renal failure (ARF) is defined as the rapid decline in kidney function as manifested by a reduction in glomerular filtration rate (GFR). It is a more frequent problem observed in all hospital admission. The incidence of ARF increases with age; the annual incidence is about 150 per million in the UK, but this figure is six times greater in the >80 years old group ( 1 ). Pre renal azotemia is considered as the most serious reason in community or hospital acquired ARF. The overall mortality associated with the ARF is higher with hospital acquired ARF ( 2 ). The case study of ARF is discussed to develop a better understanding by studying the current research project. The discussion will be more focused on the pathophysiology and the new ways of treatment used in current practice.
Case presentation
A 67-year-old middle age male, was admitted to the hospital with a chief complaint of generalized weakness, volume depletion and dysuria. He has treated with metronidazole for diarrhea caused by clostridium difficile considered as the precipitating factor for the ARF. The patient has severe osteoarthritis and takes high dose non-steroidal anti-inflammatory drugs (NSAIDs) from the last 2 years. He also complains for obstructive sleep apnea (OSA) and obesity. He was using lisinopril to control his hypertension. He has five siblings with no significant medical history.
On physically examination, he was clinically volume depleted with a pulse rate of 100 beats per minute. He was dehydrated with dry mucous membranes and reduced skin turgor. His body temperature was 37.8 °C, BP; 105/55 mmHg lying, and 90/50 mmHg sitting. Jugular venous pluse not visible. He was in ARF with serum urea and creatinine of 79 mg/dl and 2.4 mg/dl respectively. He has hypokalemic alkalosis with a potassium level of the 1.4 mEq/l (3.5-5.0 mEq/l) and a bicarbonate level of the 41.1 mEq/l (22-28 mEq/l) He was also hyponatremic, sodium level of the 125 mEq/l (136-145 mEq/l) but his serum calcium level was within normal range.
Renal ultrasound showed the right kidney measuring 12.4 cm and left kidney measuring 12.1 cm, with no signs of the shadowing calculus or hydronephrosis. However, it showed the presence of the simple bilateral cyst. Urine dipstick results showed protein of +++ and no blood. A 24 h urine sample showed nephrotic range proteinuria with proteins of 6.48 g/24 h, but serum albumin level was normal at 3.6 g/dl. His hemoglobin was 13.3 g/dl. WBC=11.9×103/µ and platelet count was normal.
The history may point out to the cause of ARF (e.g. drugs, skin rash); assessment of the hemodynamic is crucial, and proper fluid resuscitation should be given. There are different sign and symptom including hypotension, hypovolemia and his dehydration state that give indication to the diagnosis. Fractional excretion of sodium (FENa) was calculated and its value was 0.77%. It is generally less than 1% in patients with acute glomerulonephritis, hepatorenal syndrome, and states of prerenal azotemia such as congestive heart failure or dehydration. This value gives confirmation of prerenal failure.
Renal ultrasound ruled-out urinary obstruction. The diagnostic specificity of FENa in differentiating prerenal azotemia from the interarenal cause of the ARF may also be influenced by the fact that the patients may actually be progressing from the prerenal azotemia state to established ARF. The drug history of the patients can also affect the values of FENa. Despite of these many limitations, FENa when it is considered as an important tool in the context of the other clinical scenario ( 3 ).
There is considerable interest in the potential utility of the different blood and urinary biomarker, which can be important for the diagnosis of ARF. Biochemically, serum creatinine and blood urea nitrogen (BUN) concentration are important diagnostic tool in the detection of ARF. An abrupt increase in serum creatinine concentration usually reflects a decrease in GFR and signals the occurrence of ARF. These two diagnostic tools are affected by the condition and have some limitations e.g. gender, muscle mass and the drugs so they are not fully reliable ( 4 ). Furthermore, serum creatinine concentration does not accurately influence GFR in the unsuspecting state of ARF. On the other hand, increased urea validity, gastrointestinal bleeding, protein intake, catabolic states, protein malnutrition and cirrhosis hit BUN values, which can lead to wrong diagnosis ( 4 ). Cystatin-C and alpha 1-microglobulin, are diverse tools on which research work is going on to find more reliable and efficient tool to diagnosis ARF early. Studies have shown cystatin-C to be an early and reliable marker of acute kidney injury (AKI) in patients in the ICU but it is not the validated as GFR indicator in ARF as compared to serum creatinine and still its requires further studies ( 5 ). Similarly, N-acetylglucosaminidase, KMI-1, neutrophil gelatinous associated lipocalin (NGAL) are under trial to find the fast and timely detection of ARF ( 5 ).
Urinary examination
Assessment of urine biochemistry is essential and inexpensive tool in the evaluation of AKI. The factors which need to be considered important are elaborated in Table 1 .
FENa= Fractional sodium excretion; ATN= Acute tubular necrosis
Autoantibody profile
Antinuclear factor (ANF), anti-neutrophil cytoplasmic autoantibody (ANCA), anti-GBM, complement and urinary electrophoresis should be performed unless the cause of AKI is obvious, e.g. post myocardial infarction or renal obstruction.
Percutaneous renal biopsy
Renal biopsy is important diagnostic tool for those patients in whom prerenal and post renal ARF have been excluded. It helps further to rule out the cause of the intrinsic renal failure rests unclear e.g. vasculitis, glomerulonephritis, and interstitial nephritis.
Clinical follow up
On volume resuscitation, the patients became profoundly polyuric, with daily urine output of 7 to 10 liter. He needed large amounts of potassium and magnesium supplements, initially intravenous and later oral. The health of patient improved with correction of potassium and polyuria. Kidney function improved progressively, with creatinine level decreasing to 1.5 mg/dl during the course of 2 weeks. The proteinuria dramatically improved with the optimization of fluid level and electrolyte along with 24 h urine protein decreased to 0.73 g/24 h. The patient had good urine output with intravenous fistula and was in a positive fluid balance. However his mobility is much decreased due to malnutrition and weakness. The patients progressed a lot after treatment of six months and remain well with slight proteinuria but mild kidney function. His kidney serum creatinine was 1.6 mg/dl. His stool was clostridium difficile toxin positive. His diarrhea gradually improves over the next few days with metronidazole. Plasma renin and aldosterone concentration ambulant was grossly increased. On discussion with the multidisciplinary team he is unlikely to improve sufficiently to go back to his own house but require sheltered accommodation or even a residential home.
The first dispute to resolve is whether the renal failure is likely to be acute or chronic. Patient’s kidneys are on the small side and his creatinine was elevated on admission giving a GFR indicative of 20 ml/min/1.73 m. Although there may be chances of chronic kidney disease (CKD) in this case, the current history is acute reduction of urine output in the last 12 h and a precipitous increase in creatinine, in keeping with ARF. Therefore, this is likely to be an acute on chronic renal failure. There are many causes of ARF classified listed in Table 2 .
ANCA= anti-neutrophil cytoplasmic autoantibody; ATN= acute tubular necrosis
But in this case the renal ultrasound shows no sign of the hydronephrosis, which might indicate obstruction. If obstruction is excluded then the most likely case is pre-renal failure. The prerenal failure will continue to the acute tubular necrosis (ATN) if left untreated. ATN occurs if there is nonstop hypovolemia, hypotension and exposure to nephrotoxic drugs or sepsis.
Pathophysiology of ATN
After an ischemic injury abuse there is forceful arterial vasoconstriction, facilitated by the release by the vasoconstriction (particularly endothelium and by the loss of intrinsic vasodilators (nitric oxide and prostaglandin I2 (PGI); this contributes to the loss of GFR and the restructuring of blood flow within the kidney. Hypoxic injury to the power–consuming cells of the proximal tubule and thick ascending limb of loop of henle occurs, then calcium and oxygen free radical mediated cell necrosis results in cell shedding from the tubular basement membrane, with the formation of the cast that block urine flow. Patients with suspected acute tubular necrosis are not routinely biopsied unless with further kidney pathology is suspected. A number of clinical features in this case are likely causes of ARF including his obvious dehydration and hypotension. Especially the patient was also using NSAIDs for long duration, which can be considered as an imperative factor in the causation of the AKI. NSAIDs (NSAIDs) are common cause of AKI used by the people either prescribed or bought over the counter ( 6 ). However, there is little evidence that NSAIDs own a role in the impairment of renal function of normal healthy person. However, in specific clinical setting such as atherosclerotic cardiovascular diseases in old age people, diuretic use, pre-existing chronic renal failure and NSAID using, AKI could be induced. Furthermore, study regarding NSAIDs, showed, this kind of AKI is reversible within 3-7 days when this drug is discontinued. Less frequently NSAIDs can cause acute tubular necrosis or even, more rarely papillary necrosis ( 7 ). There are many other drugs, which can be nephrotoxic and can play an important role in the pathogenesis of AKI such as angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) if used in combination can increased risk for post-operative renal dysfunction, possibly as a result of the intraoperative hypotensive episodes ( 7 ). Similarly, other drugs such as gentamicin or amphotericin can be nephrotoxic in order ( 7 ).
Management of acute renal failure
The mainstay of the management involves the increase of the fluid balance hemodynamic stabilization with the optimization of the cardiac output and blood pressure is considered as the most effective steps in the treatment of the acute renal failure. Initial fluid management is important intervention for the ARF patient and to prevent further injury e.g. hypotension and hypovolemia. Assessment of the volume level is challenging, especially patients in intensive care units ( 1 ). There are no specific guidelines for the increasing hemodynamic and fluid level for the renal function protection, but predetermine of data from the clinical setting associated with ARF can be informative. However to improve the assessment of volume status, international guidelines for the management of the sepsis from the surviving sepsis strategy recommended invasive monitoring with the measurement of central venous pressure and venous oxygen saturation (superior vena cava or mixed) based on the first goal-directed treatment approach can be helpful ( 8 ). However, there is debate about the optimal fluid to use for resuscitation in critically ill patients. The recent saline versus albumin fluid assessment safe trial of 6,997 patients found that fluid resuscitation with saline or albumin resulted in similar relative risk for death in critically ill patients ( 9 ) and avoidance of either hypovolemic or fluid overload. Blood pressure should be controlled, hemoglobin maintained above 9 g/dl and sepsis should be quickly and vigorously treated. In conclusion, a flexible fluid method as part of early goal directed therapy appears to be beneficial during the first 6 h. However, the potential risk of the fluid accumulation must be considered in the setting of ARF ( 10 ). Renal dose dopamine loop diuretics are often used in the acute tubular necrosis, although there is no evidence that they it can change the outcome of ARF in humans. Renal dose dopamine 0.5 to 3 mcg/kg/min given as specified vasodilator to increase blood flow and to avoid AKI increases urine output but does not disturb AKI outcome or mortality ( 11 ). Some cases of the ARF can be managed without dialysis, with the adoption of alert fluid balance and dietary restriction.
Key to ARF management is the devotion demanding nutritional support of the sicker patients, and the use of the continuous renal replacement (e.g. CVVH: continuous veno-venous hemofiltration), which are less likely to produce hemodynamic instability.
Other more specific treatments in ARF depend upon the causative form and include the following:
- 1. Specific immunosuppressive therapy and sometimes plasma exchange may be appropriate for some condition like goodpasture syndrome, ANCA positive vasculitis.
- 2. Obstruction: Bladder catheterization if there is urine outflow congestion, nephrostomy drainage for renal obstruction.
- 3. Other: e.g. steroids in acute interstitial nephritis (AIN), plasma exchange in hemolytic uremic syndrome (HUS) and Thrombotic thrombocytopenic purpura (TTP), chemotherapy in myeloma.
There are different drugs, which are used, in ARF listed in Table 3 along with level of evidence but still a lot of additional research going on to proof their effectiveness.
Randomized controlled trials
Emerging agents
There are numerous randomized trials ( Table 4 ) going on of different drugs such as calcium channel blockers, adenosine antagonist, multipotent stem cells and erythropoietin to check out their efficiency in the treatment of acute renal failure. Calcium channel blockers have shown some effects to alter the afferent arteriolar vasoconstriction induced by a variety of stimuli and also natriuretic result ( 9 ). In large multi-central randomized control trial to investigate the effectiveness of isradipine on renal function, incidence and severity of delayed graft rejection was done. It did not work and found no benefits ( 3 ). Similarly, small clinical studies assessing the role of the theophylline, an adenosine antagonist, in the prevention of the contrast nephropathy have shown some different effect ( 12 ). There is on going research project looking at the effects of erythropoietin (EPO) or placebo on the prevention of AKI in patients under going heart surgery or kidney transplantation. In the intensive care setting the study failed to show therapeutic renoprotective benefits of EPO however there were obvious flaws in the study the patients do not receive the medication on time and secondly extreme of EPO was used against the AKI patients but it did not alter the outcome ( 13 ).
Prognosis and outcome of dialysis in ARF
The overall survival for patients with ARF remains relatively limited, 55-60% of the patients require dialysis treatment survive, but the numbers partly reflects the very poor outcome of the patients with who have ATN as a component of multiple organ failure (MOF) who are managed on the ICU. The registry data specified the lower risk of peritoneal dialysis as compared to hemodialysis during the first year of treatment ( 14 ). For example, only 10-20% for those with three or four organs failure will survive, yet 90 patients who have ARF in isolation survive. The survival speed fluctuates depending upon essential cause of end stage renal disease, age, and associated comorbidities e.g. cardiovascular diseases, diabetes and hypertension. One study performed by the Medicare in US shown HD is associated with increase chances of death among diabetic patients as compared with those patients without any co-morbidities ( 15 ). Indications for urgent dialysis in ARF was summarized in Table 5 .
The prognosis for the recovery of renal function varies according to the causative condition; renal recovery occurs <50% of cases with autoimmune vacuities. In survivor for ATN, renal function will return to the normal range in 60%, whereas 30% will be left with CKD and 10% will be dialysis–dependent.
ARF is quite common, occurring in 80 million populations. Urinary obstruction should be excluded (a cause in around 5-10 of cases) because this is readily reversible if it is diagnosed early. A renal US will be sufficient to identify obstruction in 95 of cases. Most cases of ARF are expected to pre-renal failure/ATN 70-80%. Risk factor for development for at ATN are old age, drugs (NSAIDs, ACEIs and gentamicin), sepsis, CKD. If obstruction has been excluded and there is nothing suggests a more unusual, renal cause of ARF, then ATN is the most likely diagnosis and patient should be treated with intravenous fluids to restore intravascular volume. The underlying cause of hypotension should be treated and any nephrotoxins must be removed. If blood pressure remains low following an adequate filling then the patients may require inotropic support, which will require an ITU bed. If intravenous rehydration restore intravascular volume and blood pressure but there is no improvement in oliguria, this is likely to be established acute tubular necrosis and the patient may require a period of renal support (hemodialysis or filtration) whilst tubular cells regenerate. This usually takes days to weeks but can take months. ARF in the elderly has a significant mortality, particularly if the patients require renal replacement therapy.
Authors’ contributions
CMJN and FB completed the article. SA and JA done critical appraisal.
Ethical considerations
Ethical issues (including plagiarism, misconduct, data fabrication, falsification, double publication or submission, redundancy) have been completely observed by the authors.
Conflicts of interests
There were no points of conflicts.
Funding/Support
Please cite this paper as: Nazar CMJ, Bashir F, Izhar S, Anderson J. Overview of management of acute renal failure and its evaluation; a case analysis. J Nephropharmacol 2015; 4(1): 17-22.
- Open supplemental data
- Reference Manager
- Simple TEXT file
People also looked at
Original research article, comparative analysis of four nutritional scores in predicting adverse outcomes in biopsy-confirmed diabetic kidney disease.

- 1 Faculty of Pediatrics, Chongqing Medical University, Chongqing, China
- 2 The Center of Experimental Teaching Management, Chongqing Medical University, Chongqing, China
- 3 Department of Nephrology, The Key Laboratory for the Prevention and Treatment of Chronic Kidney Disease of Chongqing, Chongqing Clinical Research Center of Kidney and Urology Diseases, Xinqiao Hospital, Army Medical University (Third Military Medical University), Chongqing, China
Malnutrition is associated with adverse outcomes in patients with diabetic kidney disease (DKD). However, it is uncertain which nutritional assessment tools are most effective in predicting the adverse outcomes of DKD. This retrospective study was conducted at a single center and included 367 patients diagnosed with DKD based on biopsy results between August 2009 and December 2018. Four nutritional assessment indices, namely the Prognostic Nutritional Index (PNI), Geriatric Nutritional Risk Index (GNRI), Triglycerides (TG) × Total Cholesterol (TC) × Body Weight (BW) Index (TCBI), and Controlling Nutritional Status (CONUT) score, were selected and calculated. We aimed to assess the association between these nutritional scores and adverse outcomes, including progression to end-stage kidney disease (ESKD), cardiovascular diseases events (CVD), and all-cause mortality. Univariate and multivariate Cox regression analyses, Kaplan–Meier analysis, along with Restricted cubic spline analysis were used to examine the relationship between nutritional scores and adverse outcomes. Furthermore, the area under the curve (AUC) was calculated using time-dependent receiver operating characteristics to determine the predictive value of the four nutritional scores alone and some combinations. Lastly, ordered logistic regression analysis was conducted to explore the correlation between the four nutritional scores and different renal histologic changes. The incidence of ESKD, CVD, and all-cause mortality was significantly higher in patients with DKD who had a lower PNI, lower GNRI, and higher CONUT score. Additionally, The TCBI performed the worst in terms of grading and risk assessment. The PNI offer the highest predictive value for adverse outcomes and a stronger correlation with renal histologic changes compared to other nutritional scores. Patients diagnosed with DKD who have a worse nutritional status are more likely to experience higher rates of adverse outcomes. The PNI might offer more valuable predictive values and a stronger correlation with different renal histologic changes compared to other nutritional scores.
1 Introduction
Diabetic kidney disease (DKD) has become a major public health concern, with a high incidence rate, high mortality, and high medical costs ( 1 , 2 ). Nutritional status is closely related to the progression of end-stage kidney disease (ESKD), cardiovascular events (CVD), and all-cause mortality ( 3 , 4 ). Therefore, evaluating the nutritional status of DKD patients and using it to assess the occurrence, development, and prognosis of DKD is extremely important. Currently, four objective nutritional scores have been used in previous studies to evaluate the prognosis of patients with DKD. These scores include the Prognostic Nutrition Index (PNI) ( 5 ), Geriatric Nutritional Risk Index (GNRI) ( 6 ), Triglycerides (TG) × Total cholesterol (TC) × Body weight (BW) index (TCBI) ( 7 ), and controlling nutritional status (CONUT) score ( 8 ). Previous studies have found that GNRI and PNI are effective tools in assessing the prognosis of patients with chronic kidney disease (CKD) ( 9 , 10 ). CONUT score has also been identified as an independent risk factor for ESKD, CVD events, and overall death in patients with DKD ( 11 ). According to a recent study comparing GNRI, PNI, and TCBI, PNI has the most significant predictive value for all-cause and cardiovascular mortality in the general population ( 12 ) . Additionally, previous studies have shown that renal histological changes are good predictors of ESKD ( 13 ). However, the relationship between these four nutritional scores and adverse outcomes remains elusive in patients with DKD. It is also still unclear which score or combination is more valuable in predicting the adverse outcomes. Additionally, the correlation between nutritional status and renal histologic changes in patients with DKD is largely unknown. Therefore, this study aims to introduce four nutritional scores to evaluate the nutritional status of DKD patients with different renal histologic changes. It also aims to analyze in-depth the correlation between nutritional status and ESKD, CVD events and all-cause death, and the correlation between nutritional status and different renal histologic changes in DKD patients. The ultimate goal is to provide new ideas for the prevention and treatment measures of disease occurrence, development, and prognosis in clinical DKD patients.
2 Materials and methods
2.1 data source and case selection.
This retrospective study included 367 patients with biopsy-confirmed DKD from Xinqiao Hospital of the Army Medical University in China between August 2009 and December 2018. DKD was diagnosed based on criteria established by the Renal Pathology Society in 2010 ( 14 ). All participants were followed up from the screening date until 31 December 2021 or until their death. The study protocol was approved by the ethical committee of Xinqiao Hospital (No. 2018-006-02). Inclusion criteria were: (1) biopsy-confirmed DKD; (2) adults aged 18 years or older; (3) complete medical information and follow-up data. Exclusion criteria were: (1) end-stage kidney disease (ESKD), cardiovascular (CVD) events and all-cause death took place within 1 month of follow-up after enrollment; (2) patients with incomplete pathological information or blood routine examination; (3) patients with malignancies (e.g., breast, lung, gastrointestinal, hematologic cancers), infectious diseases (e.g., pneumonia, viral hepatitis) ( Figure 1 ).
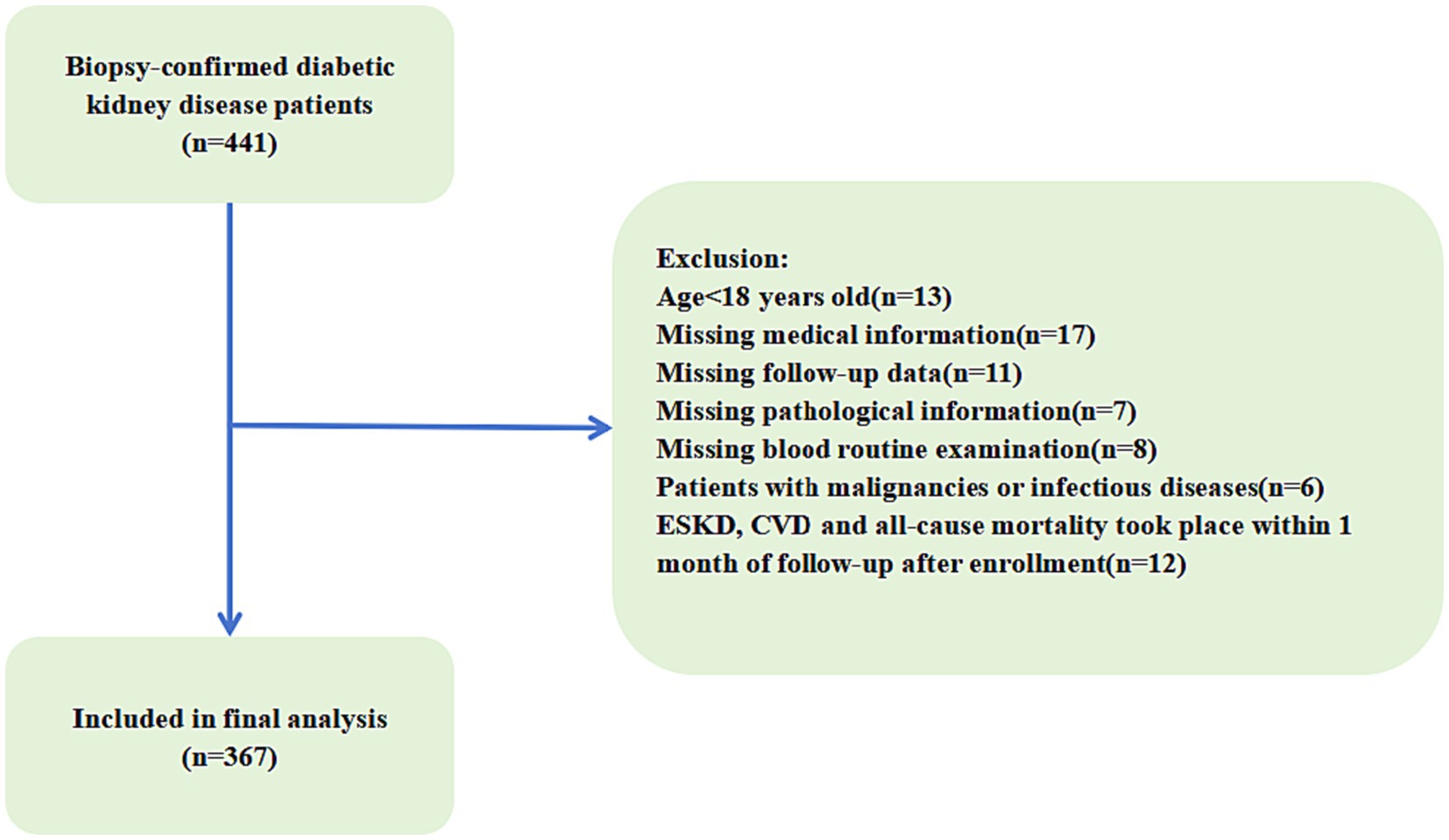
Figure 1 . Flowchart of included patients in this study. ESKD, end-stage kidney disease; CVD, cardiovascular disease.
2.2 Clinical information acquisition
We extracted baseline demographic characteristics and laboratory values from the Electronic Medical Record System of Xinqiao Hospital at the time of the patient’s first renal biopsy. This included demographic data such as age and gender, medical history including hypertension and history of coronary heart disease, and laboratory data such as lymphocyte count, hemoglobin, serum creatinine, blood urea nitrogen (BUN), uric acid, intact parathyroid hormone (iPTH), calcium, magnesium, phosphate, albumin, total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides (TG), proteinuria, and pathological information. We determined the estimated glomerular filtration rate (eGFR) using the cystatin C-based chronic kidney disease (CKD)-EPI equation and combined by serum creatinine (CKD-EPIscr-cys) which incorporates the Chinese eGFR racial factor.
2.3 Preliminary data processing
We calculated body mass index (BMI) by dividing weight in kilograms by height in meters squared based on the obtained height and weight measurements. In addition, the PNI was defined by the following formula: PNI = serum albumin (g/L) + 5 × total lymphocyte count (10 9 /L). The GNRI was calculated by using the following formula: GNRI = [1.489 × serum albumin (g/l)] + [41.7 × weight (kg)/ideal body weight (kg)]. The calculation of the ideal body was as follows: 22 × square of height because of its validity. The ratio of weight-to-ideal body weight was set to 1 if the actual body weight exceeded the ideal body weight ( 15 ). The TCBI was calculated using the formula: serum level of TG (mg/dL) × TC (mg/dL) × body weight (kg)/1,000. The CONUT score was described in Supplementary Table S1 ( 8 ). Nutritional scores (including GNRI, PNI, TCBI, CONUT) were divided into four groups according to the mean four nutritional scores of the quartiles: Q1 (GNRI <82.35), Q2(GNRI:82.35–92.92), Q3 (GNRI:92.92–102.90) and Q4 (GNRI >102.90) GNRI groups; Q1 (PNI < 35.26), Q2 (PNI: 35.26–42.75), Q3 (PNI:42.75–50.19) and Q4(PNI > 50.19) PNI groups; and Q1 (TCBI <1,210.17), Q2 (TCBI: 1,210.17–2079.83), Q3 (TCBI:2079.83–3451.43) and Q4(TCBI >3451.43) TCBI groups. For the CONUT score, a score of 0 was considered Q1, scores of 1 to 2 were considered Q2, scores of 3 to 4 were considered Q,2 and scores of≥5 were considered Q4 CONUT groups.
2.4 Clinical outcomes
The study evaluated three outcomes: ESKD, CVD events, and all-cause mortality, each of which was considered separately. ESKD was defined as an eGFR less than 15 mL/min/1.73 m 2 or the need for maintenance renal replacement therapy due to irreversible deterioration of renal function, including hemodialysis, peritoneal dialysis, or kidney transplantation. CVD events were defined as the occurrence of new CVD events, such as coronary heart disease, heart failure, cerebrovascular events, and severe arrhythmia. All-cause mortality was defined as death from any cause. The study obtained clinical outcomes primarily through telephone follow-up or patient medical record reports.
2.5 Statistical analysis
The data analysis involved the use of SPSS (version 27.0), GraphPad Prism (version 10.0.3) or R version 4.3.1. All the data used were checked for normality of distribution using the Kolmogorov–Smirnov test. Normally distributed data were expressed as mean ± standard deviation while non-normally distributed data were expressed as median (interquartile range). The differences between groups were tested using t -tests, Mann–Whitney U tests, and chi-square tests. The Kaplan–Meier curve was used to compare the outcomes of the patients according to the mean four nutritional scores (including PNI, GNRI, TCBI, and CONUT) of the quartiles, and the log-rank test was used to compare the differences between each group. Furthermore, the independent relationships between four nutritional scores and end-stage renal disease, cardiovascular, and all-cause mortality were investigated by univariate and multivariate Cox regression models. The initial confounding factors were selected based on previous studies, data availability, and established associations. If these factors changed the estimates of four nutritional scores on end-stage renal disease, cardiovascular, and all-cause mortality by more than 10% or were significantly associated with endpoint events after adjustment for sociodemographic factors (age), they were included as the covariates in multivariate Cox regression analysis. Hazard ratios (HRs) and 95% confidence intervals (CIs) are provided. Additionally, restricted cubic splines (RCS) and threshold effect analysis were applied using the R package “rms” based on the Cox proportional hazards models to further explore the relationship between the nutritional scores and endpoint events. Moreover, the time-dependent receiver operating characteristic (td-ROC) curve was constructed to compare the diagnostic accuracy of PNI, GNRI, TCBI, and CONUT, alone or in different combinations with PNI, in predicting end-stage renal disease, cardiovascular and all-cause mortality. Combine four nutritional scores in the following different ways: PNI + CONUT, PNI + TCBI, PNI + GNRI, PNI + TCBI+CONUT, PNI + GNRI+CONUT, PNI + GNRI+TCBI, and PNI + TCBI+CONUT+GNRI. Then, the area under the curve (AUC) was calculated for different groups. The correlation heatmap was generated using R software (v.4.2.2) package “corrplot” (v.0.92) ( 16 ) and “ggplot2” (v3.4.2) ( 17 ). To explore the correlation between four nutritional scores and different renal histologic changes (glomerular lesions, IFTA, interstitial inflammation, arteriolar hyalinosis, and arteriosclerosis) in patients with diabetic kidney disease, we used X-tile software (v3.6.1) to calculate the optimum cutoff value for converting the continuous variables (PNI, GNRI) into categorical variables (the low-level group and the high-level group) according to end-stage renal disease. Patients were divided into two groups based on median CONUT score. Then, we use ordered logistic regression analysis to investigate the factors affecting different renal histologic changes in patients with diabetic kidney disease. Ordered logistic regression analysis: The model meets the parallelism by parallel test, and multivariate analysis was performed by ordinal logistic regression. It is important to note that a p value of <0.05 was considered statistically significant during the analysis.
3.1 Baseline characteristics
A total of 367 patients with DKD were recruited for the current study. The mean age of the patients was 51.30 ± 10.35 years, and 63.2% (232) were male. Among the patients, 34.1% (125) were smokers, 70.8% (260) had hypertension, and 20.7% (76) had a history of coronary heart disease. Then, the PNI, GNRI, TCBI, and CONUT score were calculated. The baseline characteristics of the study population according to different glomerular lesions are shown in Table 1 . According to our findings, serum creatinine, uric acid, blood urea nitrogen, Cystatin C, LPA, TNF-α, and CONUT increased in proportion to the severity of glomerular lesions, and patients with more severe glomerular lesions were more likely to suffer from coronary heart disease and diabetes retinopathy. They also have lower levels of hemoglobin, eGFR, albumin, GNRI, and PNI. In addition, we found that significant differences in interstitial fibrosis and tubular atrophy (IFTA), interstitial inflammation, arteriolar hyalinosis, and arteriosclerosis. The distribution of the PNI, GNRI, TCBI, and CONUT score among DKD patients with different glomerular lesions is shown in Figure 2 .
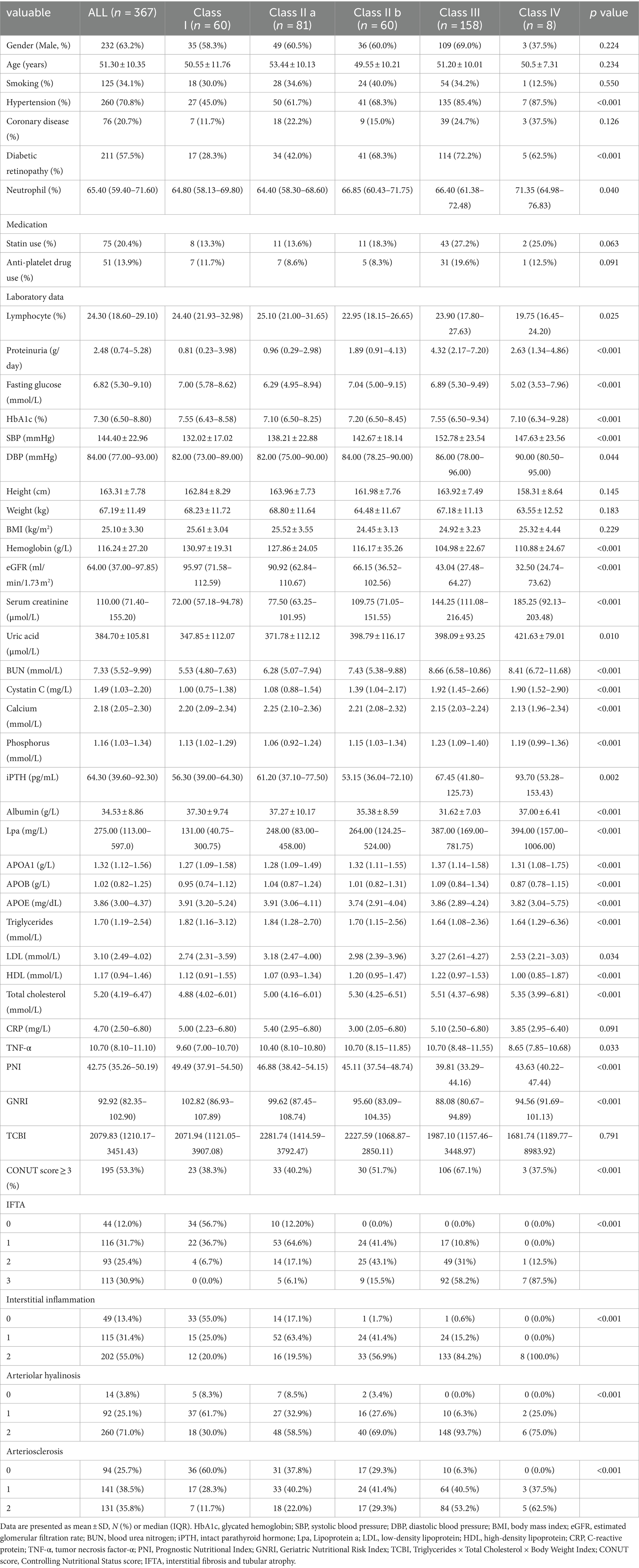
Table 1 . Baseline characteristics of patients with diabetic kidney disease.
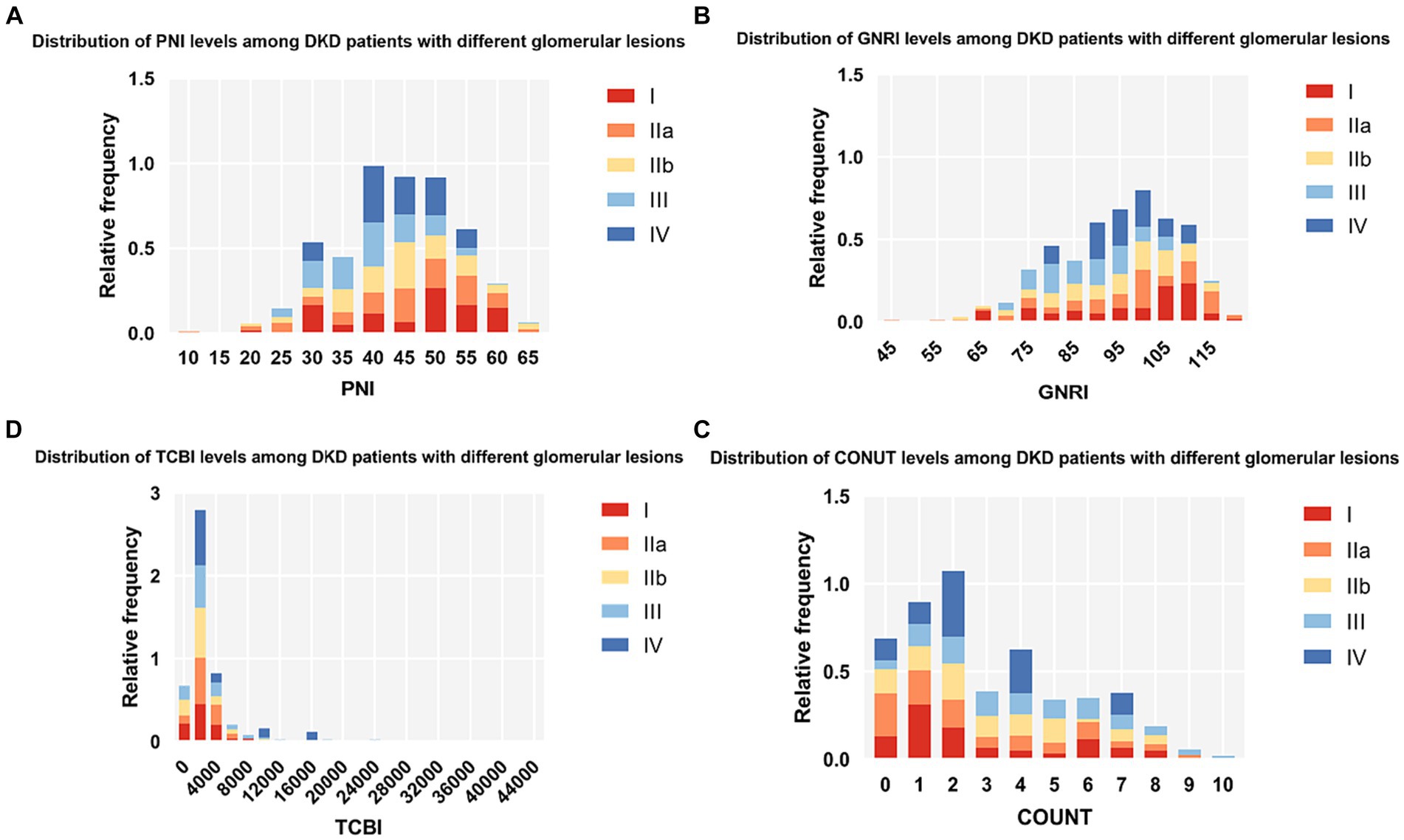
Figure 2 . Histograms show the population distribution of nutritional scores. (A) PNI; (B) GNRI; (C) TCBI; (D) COUNT score. PNI, Prognostic Nutritional Index; GNRI, Geriatric Nutritional Risk Index; CONUT score, Controlling Nutritional Status score; TCBI, Triglycerides×Total Cholesterol×Body Weight Index.
3.2 Association between four nutritional scores and adverse outcomes
During a median follow-up period of 5.1 years, about 114 (31.1%) of ESKD, 115 (31.3%) of CVD events, and 54 (14.7%) of deaths occurred. The Kaplan–Meier curve showed that, PNI, GNRI, and COUNT score were all significantly associated with renal progression, CVD events and all-cause mortality except for TCBI ( Figure 3 ). Then, we performed the Cox regression analysis ( Tables 2 , 3 ). In the univariate Cox proportional hazards analysis, patients with lower GNRI, PNI and higher CONUT score had increased risks of ESKD ( p < 0.001), CVD events ( p < 0.001) and all-cause mortality ( p < 0.001). Moreover, age, hypertension, diabetic retinopathy, eGFR, serum creatinine, cystatin C, calcium, hemoglobin, iPTH, albumin, LDL, IFTA, and arteriosclerosis were also significantly associated with adverse outcomes ( Table 2 ). In a multivariate Cox regression model (Model 3), the GNRI and PNI were still associated with the incidence of adverse outcomes. In addition, the CONUT score was still an independent predictor of CVD events (HR = 1.113, 95% CI 1.029–1.203, p = 0.007), and all-cause mortality (HR = 1.208, 95% CI 1.033–1.411, p = 0.018) in Model 3, but the positive effect size of ESKD was non-significant ( Table 3 ).
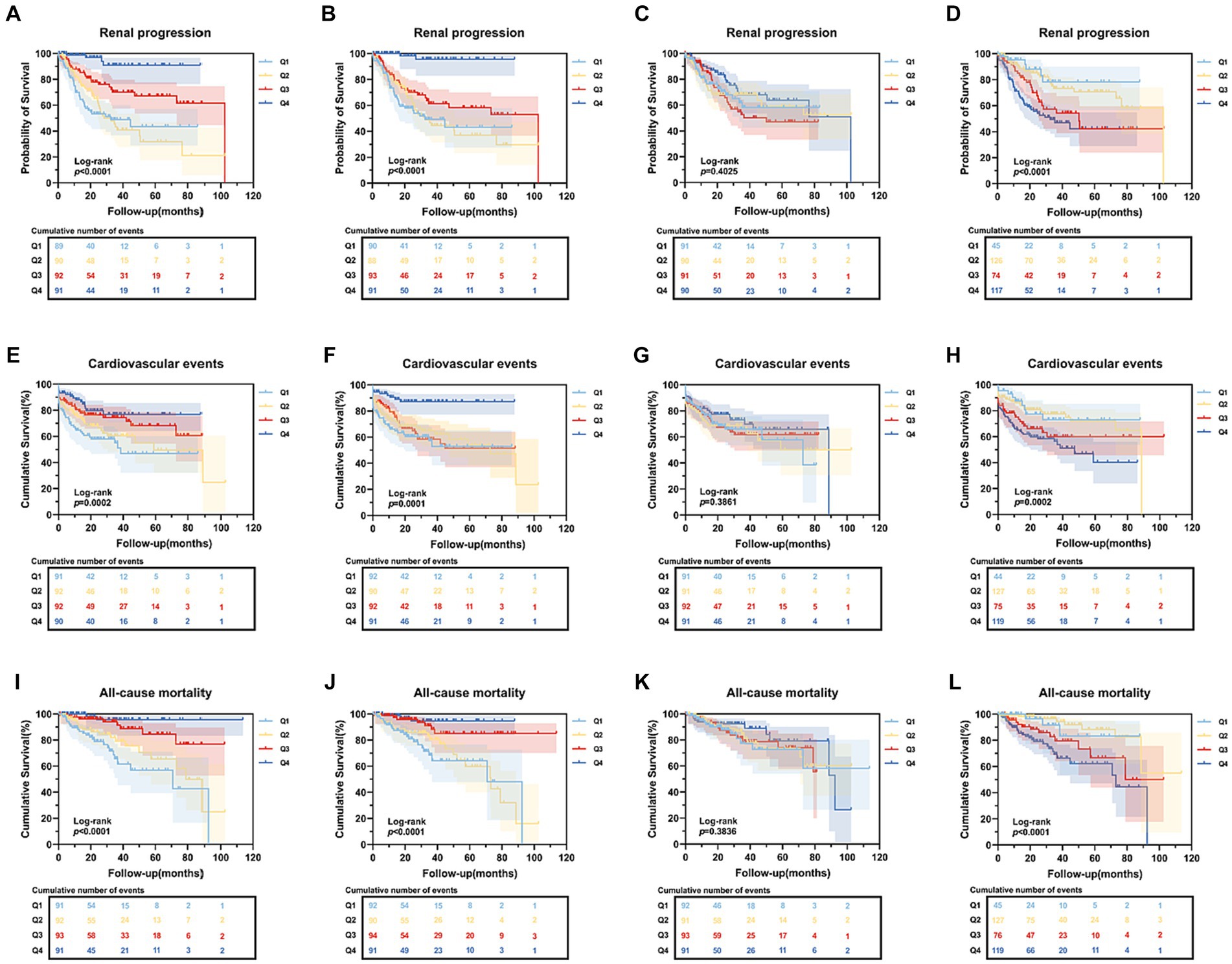
Figure 3 . Kaplan–Meier curves of end-stage renal disease, cardiovascular events and all-cause mortality based on four nutritional scores. (A–D) Kaplan–Meier curves of end-stage renal disease categorized by PNI, GNRI, TCBI, COUNT score; (E–H) Kaplan–Meier curves of cardiovascular death categorized by PNI, GNRI, TCBI, COUNT score; (I–L) Kaplan–Meier curves of all-cause mortality categorized by PNI, GNRI, TCBI, COUNT score. PNI, Prognostic Nutritional Index; GNRI, Geriatric Nutritional Risk Index; TCBI, Triglycerides×Total Cholesterol×Body Weight Index; CONUT score, Controlling Nutritional Status score.
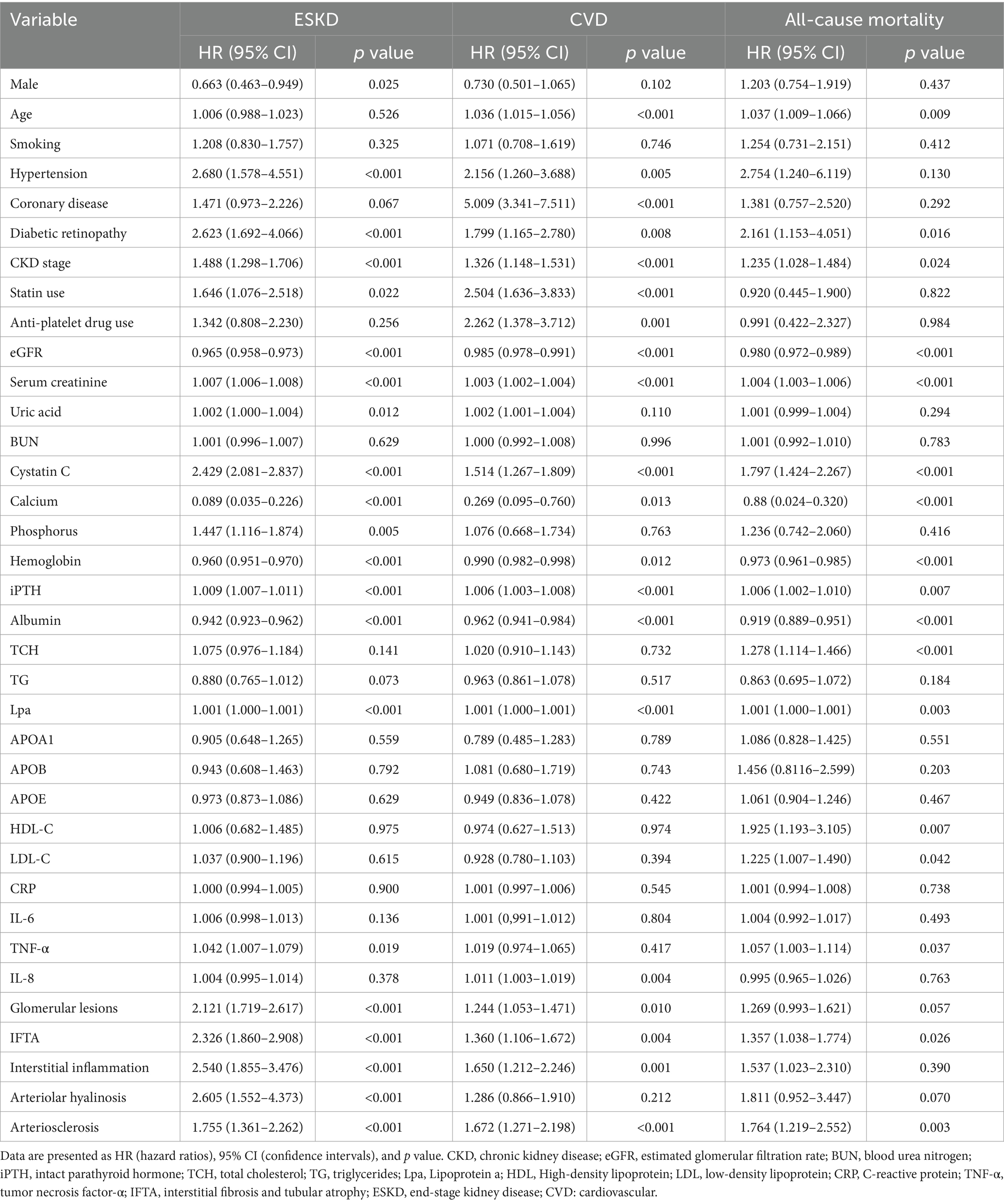
Table 2 . Univariate Cox analysis of adverse outcomes in patients with diabetic kidney disease.
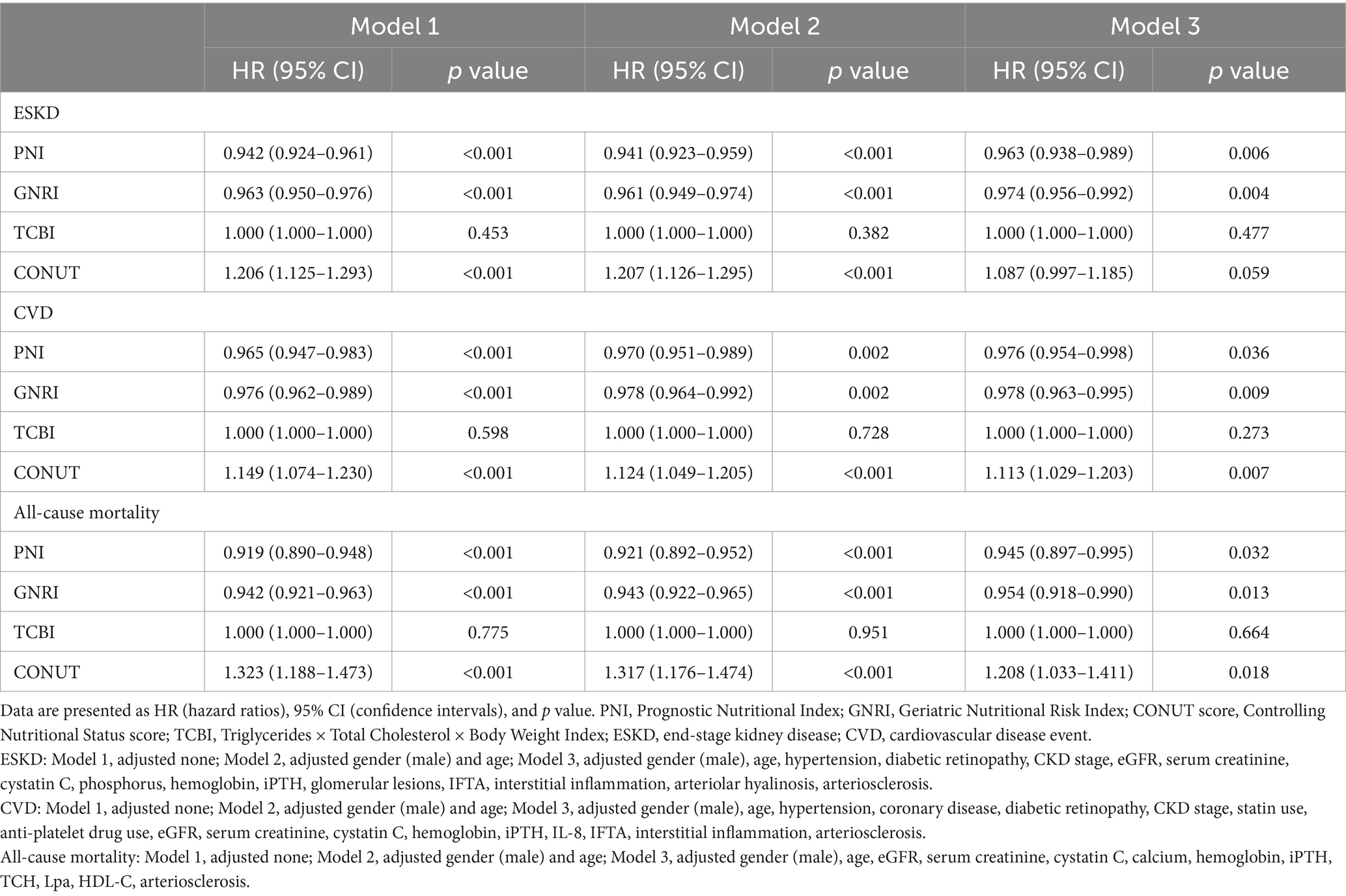
Table 3 . Multivariate Cox analysis of adverse outcomes in patients with diabetic kidney disease.
Next, we also used restricted cubic splines to model and visualize the relation of predicted nutritional scores (PNI, GNRI, TCBI, and CONUT) with ESKD in DKD patients ( Figures 4A – D ). For PNI, the risk of ESKD was relatively flat until it reached 34–35 and then started to decrease rapidly afterwards but the P for nonlinearity was non-significant (P for overall = 0.016, P for nonlinear = 0.146). For GNRI, regarding the strong N-shaped relation between predicted GNRI and ESKD, the plot showed an increase of the risk within the lower range of predicted GNRI until around 75 and HR exceeded the horizontal line with HR = 1, which reached the highest risk around 55–56 and then substantially decreased thereafter until it until it reached 92–93 (P for overall<0.001, P for nonlinear<0.001). In addition, the nonlinear relationship between nutrition scores and cardiovascular death was weakened and no apparent correlation was found between the nutrition scores and cardiovascular death ( Figures 4E – H ). An L-shaped relationship between the HR of all-cause mortality and nutritional scores (PNI, GNRI, and TCBI) was indicated in DKD patients. However, the nonlinear relationship between nutrition scores and all-cause mortality was weakened and no apparent correlation was found between the nutrition scores and all-cause mortality ( Figures 4I – L ). After adjusting for various adverse events using Model 3 in the Cox analysis, the restricted spline curve indicates a potential linear correlation between the four nutritional scores and the outcomes.
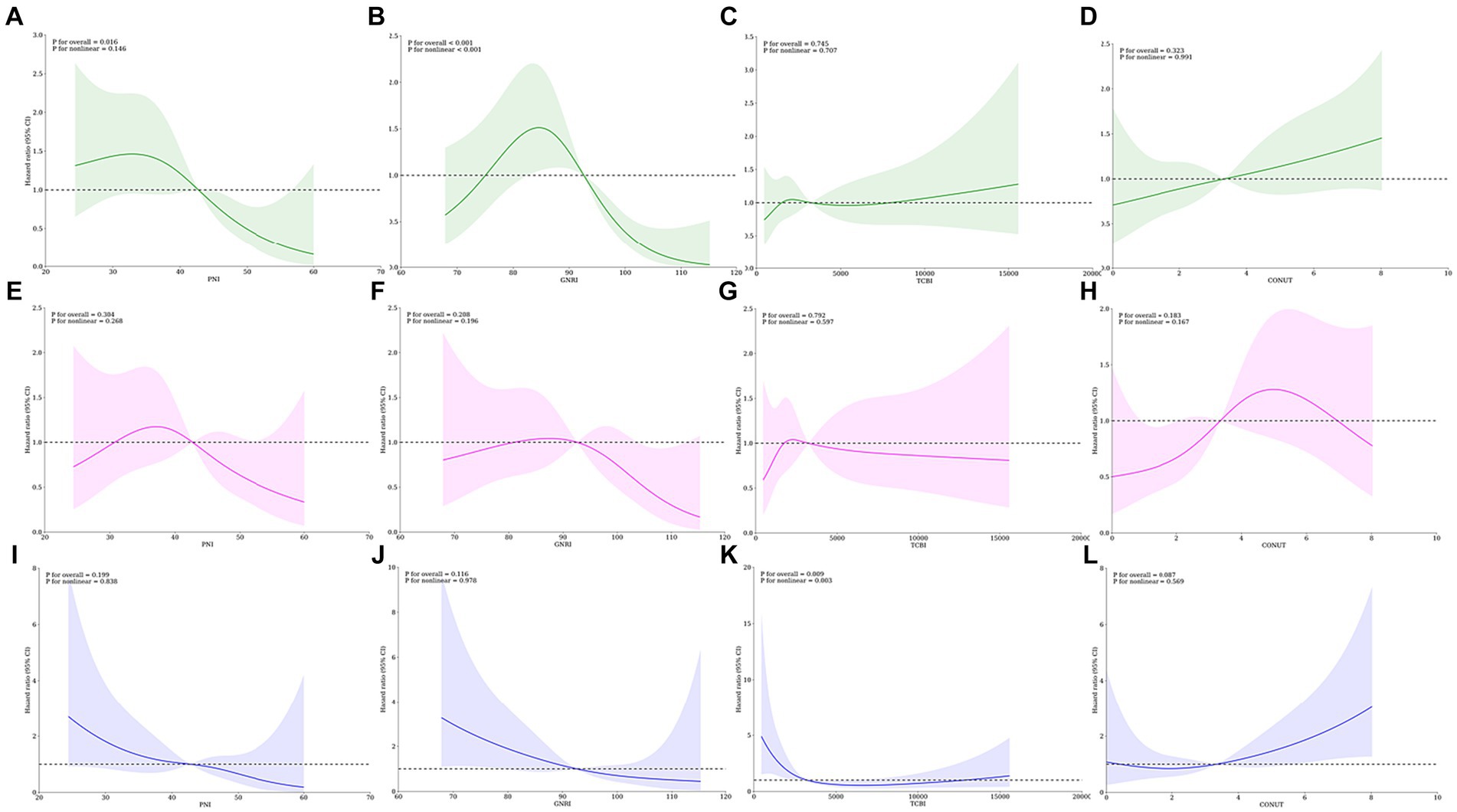
Figure 4 . Restricted spline curves for the associations between four nutritional scores and adverse events in DKD patients. Lines represent the HR (hazard ratio), and transparent areas represent the 95% confidence intervals. HR (95% CI) were adjusted for various adverse events in Cox analysis using Model 3. (A–D) Associations between PNI, GNRI, TCBI, COUNT score and end-stage renal disease; (E–H) Association between PNI, GNRI, TCBI, COUNT score and cardiovascular death; (I–L) Association between PNI, GNRI, TCBI, COUNT score and all-cause mortality. PNI, Prognostic Nutritional Index; GNRI, Geriatric Nutritional Risk Index; CONUT score, Controlling Nutritional Status score; TCBI, Triglycerides×Total Cholesterol×Body Weight Index.
Furthermore, we investigated nutrition scores that exhibit a notable non-linear relationship with outcomes. We employed threshold effect analysis to identify critical inflection point that influence the correlation between variables. Subsequently, we assessed the correlation between the independent and dependent variables both before and after these turning points. The relationship between GNRI and ESKD reveals a critical inflection point at 71.629, indicating a significant threshold effect. When the GNRI falls below 71.629, a positive correlation with ESKD becomes evident (HR = 1.835, 95% CI 1.170–2.878, p = 0.008), while exceeding 71.629 leads to a negative association with ESKD (HR = 0.946, 95% CI 0.925–0.968, p < 0.001). However, there is no significant threshold effect in TCBI (HR = 1.000, 95% CI 1.000–1.000, p = 0.6631) ( Table 4 ).
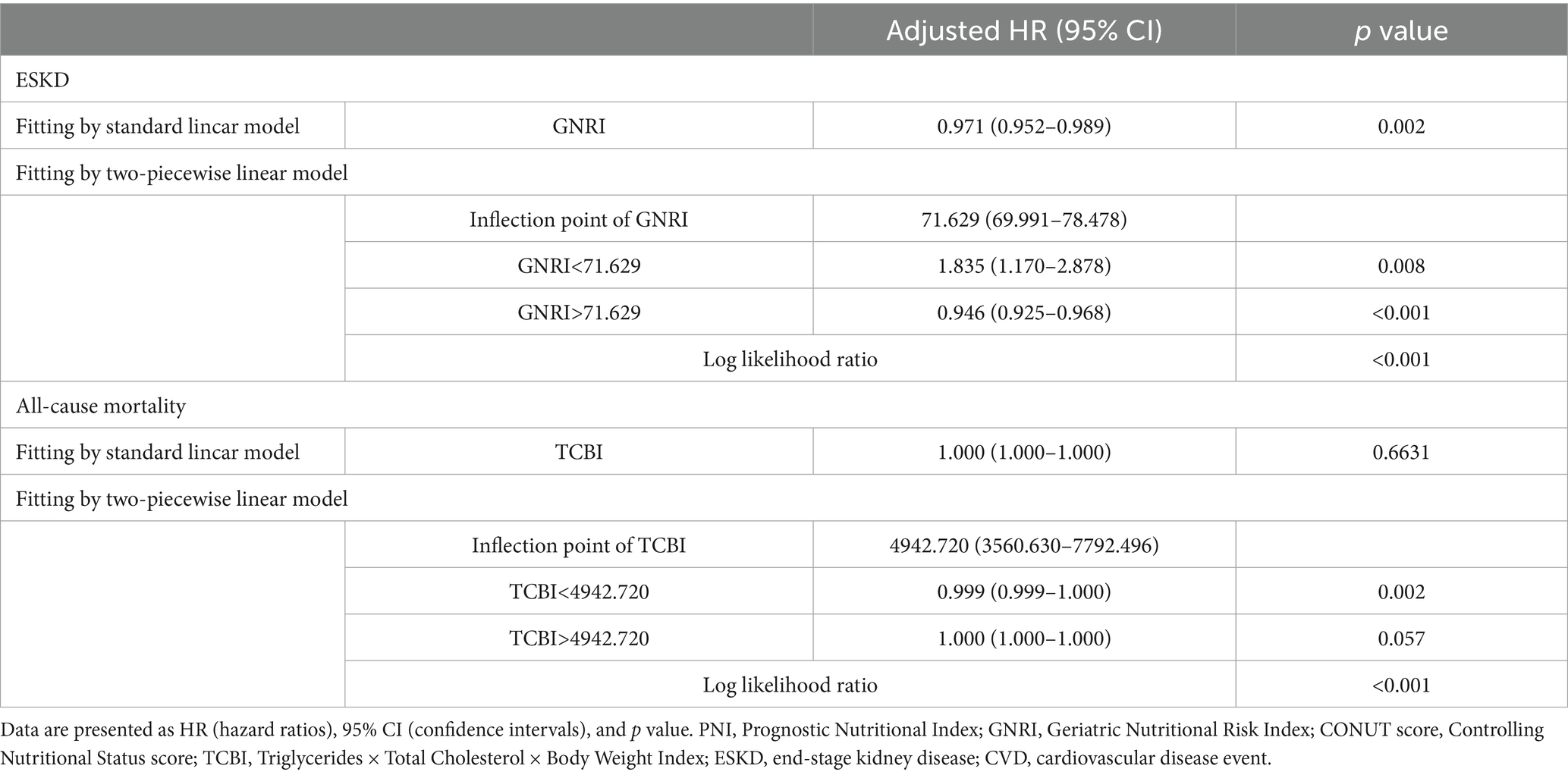
Table 4 . Threshold effect analysis of nutritional scores on adverse events.
Overall, compared with TCBI and CONUT score, GNRI and PNI have a stronger correlation with ESKD, CVD events and all-cause mortality, which is similar to the results of the time-dependent receiver operating characteristic (td-ROC). Therefore, it showed that individual PNI has the best diagnostic accuracy and the strongest correlation with the disease, making it an independent risk factor for ESKD, CVD events, and all-cause death in patients with DKD.
3.3 Diagnostic accuracy of four nutritional scores and different combinations with PNI in predicting outcomes
The prediction of clinical outcomes by the PNI, GNRI, TCBI, CONUT score and different combinations with PNI was evaluated using the time-dependent receiver operating characteristic (td-ROC) of the subjects. Then, the ROC curves were constructed to calculate the area under curve (AUC) ( Figures 5A-I ). For the prediction of ESKD, the PNI score had slightly higher AUC than GNRI, whereas the TCBI and the CONUT score had similar AUC. Diagnostic accuracy of PNI + TCBI+CONUT (AUC = 0.7305) was slightly higher than that of other combined scores and slightly higher than that of PNI (AUC = 0.7209) alone. Other combinations is not significantly improved or lower than the individual PNI scores ( Figures 5B , C ). We also obtained similar results in the AUC for cardiovascular death and all-cause mortality. However, the AUC of PNI + TCBI+CONUT+GNRI (AUC = 0.6320) was slightly higher than PNI (AUC = 0.6261) for CVD events ( Figure 5F ), and the AUC of PNI + GNRI+TCBI (AUC = 0.7226) was slightly higher than PNI (AUC = 0.7208) for all-cause mortality ( Figure 5I ). Overall, compared with PNI, the diagnostic accuracy of other nutritional scores alone or different combinations with PNI performed worse on ESKD, CVD events and death.
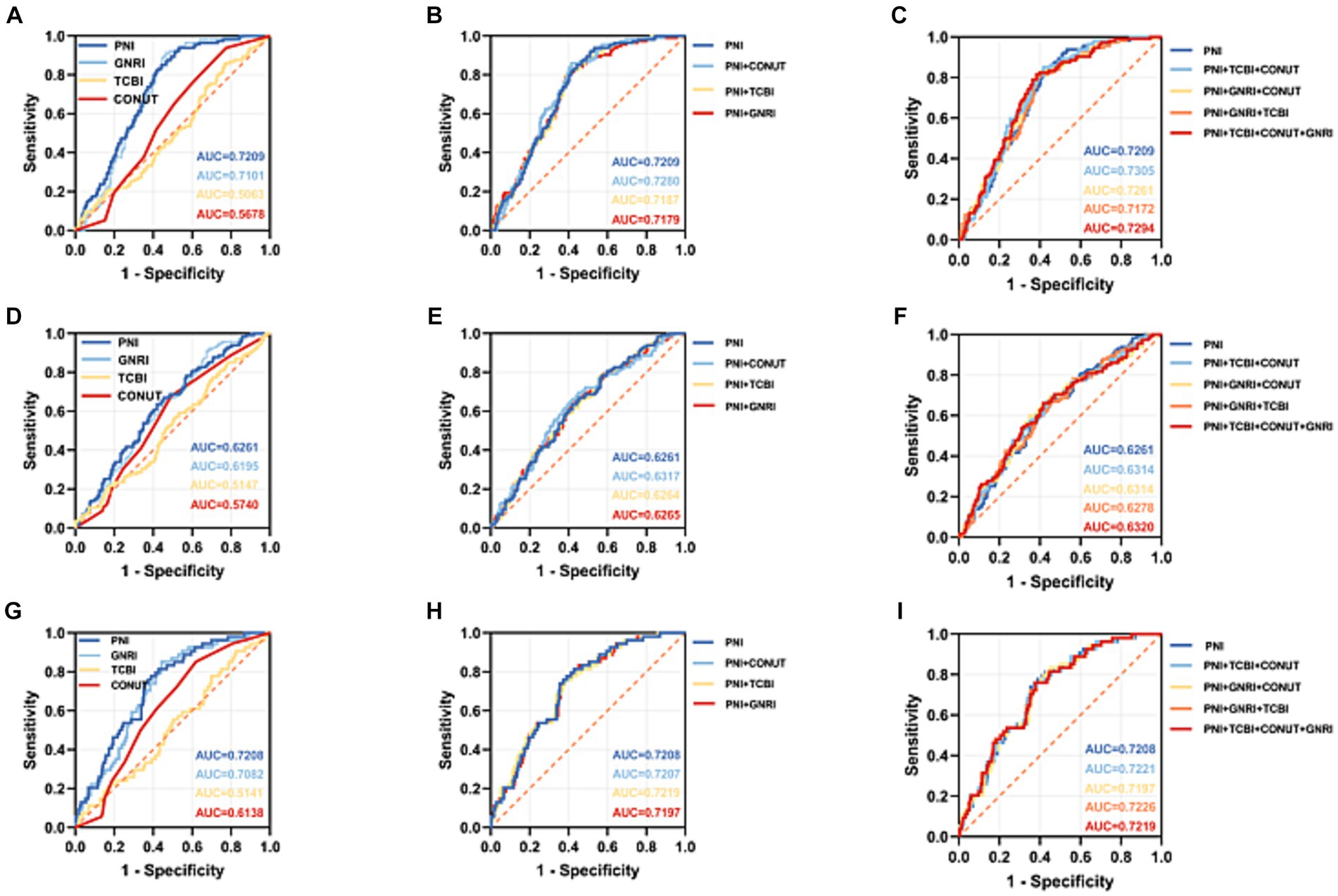
Figure 5 . Comparison of the receiver operating characteristic (ROC) curves of four nutritional scores and different combinations with PNI in predicting adverse events in DKD patients. (A–C) ROC curves for predicting end-stage renal disease plotted by four nutritional scores and different combinations with PNI in DKD patients. (D–F) ROC curves for predicting cardiovascular mortality plotted by four nutritional scores and different combinations with PNI in DKD patients. (G–I) ROC curves for predicting all-cause mortality plotted by four nutritional scores and different combinations with PNI in DKD patients. ROC, Receiver operating characteristic; AUC, area under the curve. PNI, Prognostic Nutritional Index; GNRI, Geriatric Nutritional Risk Index; CONUT score, Controlling Nutritional Status score; TCBI, Triglycerides×Total Cholesterol×Body Weight Index.
3.4 Correlation between four nutritional scores and different renal histologic changes in patients with diabetic kidney disease
We detected the correlation analysis between four nutritional scores and different renal histologic changes in patients with DKD, and our results showed PNI was negatively correlated with glomerular lesions (r = −0.290, p < 0.001), IFTA (r = −0.234, p < 0.001), interstitial inflammation (r = −0.226, p < 0.001), arteriolar hyalinosis (r = −0.168, p = 0.001) and arteriosclerosis (r = −0.212, p < 0.001). For PNI, we also found similar results. For CONUT score, the positive correlation was found with glomerular lesions (r = 0.224, p < 0.001), IFTA (r = 0.176, p = 0.001), interstitial inflammation (r = 0.197, p < 0.001), arteriolar hyalinosis (r = 0.128, p = 0.014) and arteriosclerosis (r = 0.189, p < 0.001). No significant correlation between TCBI and renal histologic changes was found in patients with diabetic kidney disease ( Figure 6A ).
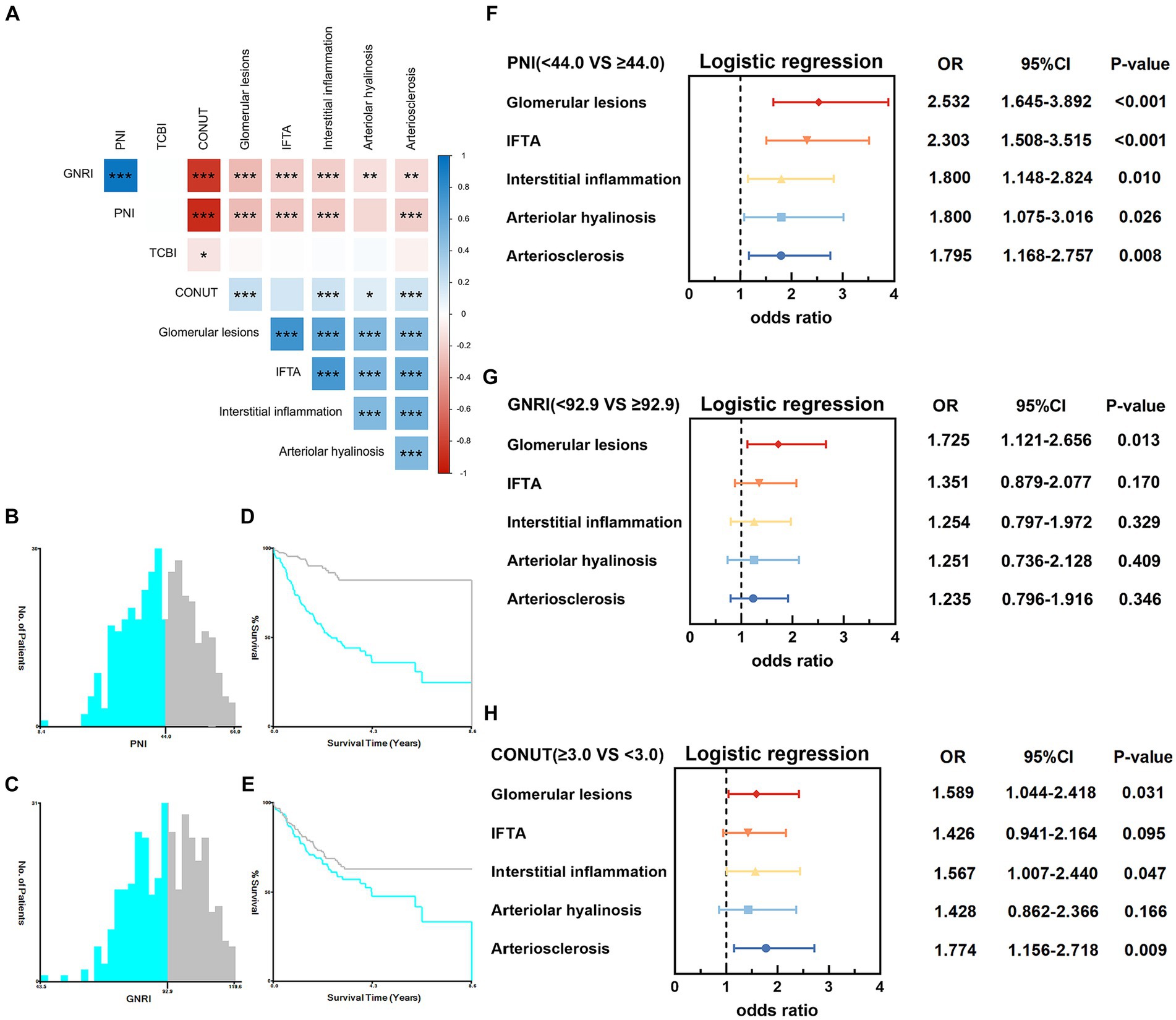
Figure 6 . Correlation between four nutritional scores and different renal histologic changes in patients with diabetic kidney disease. (A) Correlation heatmap showing the correlation between four nutritional scores and different renal histologic changes. * p < 0.05, ** p < 0.01, *** p < 0.001. (B,C) Histograms of patient distribution according to the PNI and GNRI. (D,E) The Kaplan–Meier curves of the PNI and GNRI to calculate the optimum cutoff value. (F) Ordered logistic regression analysis to identify the relationship between PNI (<44.0 vs. ≥44.0) and different renal histological changes. (G) Ordered logistic regression analysis to identify the relationship between GNRI (<92.9 vs. ≥92.9) and different renal histological changes. (H) Ordered logistic regression analysis to identify the relationship between CONUT (≥3.0 vs. <3.0) score and different renal histological changes. OR, odds ratio; 95%CI, 95% confidence interval.
To further explore the underlying correlations between PNI, GNRI and CONUT score and different renal histologic changes in patients with DKD, patients were divided into two groups (the low level group and the high level group) based on X-tile software and median CONUT score. The variable “PNI” was grouped as “<44.0” and “≥44.0” under end-stage renal disease ( Figures 6B , D ); “GNRI” was categorized into “<92.9” and “≥92.9” under cardiovascular ( Figures 6C , E ). Patients were divided into two groups based on median CONUT score: the low CONUT score group (CONUT score < 3) and the high CONUT score group (CONUT score ≥ 3). Then, we use ordered logistic regression analysis to investigate the factors affecting different renal histologic changes in patients with DKD.
After adjusting proteinuria in the ordered logistic regression analysis, we found that high PNI level was an adverse factor for obtaining the higher glomerular lesions grade ( p < 0.001, OR = 2.532, 95%CI 1.645–3.892), IFTA ( p < 0.001, OR = 2.303, 95%CI 1.508–3.515), interstitial inflammation ( p = 0.010, OR = 1.800, 95%CI 1.148–2.824), arteriolar hyalinosis ( p = 0.026, OR = 1.800, 95%CI 1.075–3.016), arteriosclerosis ( p = 0.008, OR = 1.795, 95%CI 1.168–2.757) ( Figure 6F ). GNRI was also inversely correlated with glomerular lesions ( p = 0.013, OR = 1.725, 95%CI 1.121–2.656). There is no significant correlation between GNRI and IFTA, interstitial inflammation, arteriolar hyalinosis and arteriosclerosis ( Figure 6G ). Besides, a lower CONUT score was linked to improved renal histologic changes in glomerular lesions, interstitial inflammation, and arteriosclerosis grade ( Figure 6H ). Compared with GNRI and CONUT score, PNI have a stronger correlation with different renal histologic changes and higher PNI may be related to a lower risk of different renal histologic changes in patients with diabetic kidney disease. The PNI remained best incremental values for predicting the order of severity of different renal histologic changes.
4 Discussion
In this study, we used four nutritional scores to assess the nutrition status of patients with DKD under different renal histologic changes, analyzed the relationship of nutritional status with ESKD, CVD events, and all-cause mortality in patients with DKD and detected the correlation analysis between four nutritional scores and different renal histologic changes.
The major conclusions are as follows: (1) Malnourished patients were at a higher risk of adverse outcomes. Moreover, GNRI, PNI, and CONUT score had higher predictive value for all-cause mortality than other adverse outcomes. (2) Compared with other nutritional scores, the PNI alone had the highest predictive value in biopsy-confirmed diabetic kidney disease. However, TCBI showed the worst performance on risk assessment and prediction. Moreover, the predictive value of certain combinations with PNI is slightly higher than PNI alone for various adverse outcomes. (3) Malnourished patients were found to have a heightened risk of experiencing significant renal histologic changes. However, no clear correlation could be found between TCBI and renal histologic change. Furthermore, the PNI remained the most accurate predictor of the severity order of various renal histologic changes.
In recent years, it has been widely suggested by numerous studies that CKD patients exhibit abnormal protein-energy metabolism, with significant muscle and fat wasting. In 2008, the International Society of Renal Nutrition and Metabolism (ISRNM) expert group named this condition protein-energy wasting (PEW), which refers to the reduced protein and energy reserves in the body, resulting in a state of malnutrition characterized by decreased protein and fat content ( 3 ). Nutrition plays a crucial role in reducing the risk of cardiovascular disease and slowing the decline in kidney function ( 4 ). Currently, PEW is highly prevalent among elderly individuals and those with ESKD, and it is closely associated with poor clinical outcomes due to the breakdown of body proteins and reduced energy caused by metabolic inflammation responses ( 3 , 18 ). In dialysis patients, malnutrition may increase the risk of cardiovascular and all-cause mortality through factors such as chronic inflammation and oxidative stress ( 19 ). Malnutrition is a common complication in CKD stages 4–5 and also influence the severity and progression of DKD ( 20 – 22 ). Therefore, the nutritional status is closely related to the progression and prognosis of DKD, and the prevention and treatment of malnutrition in DKD can improve patient outcomes.
Four nutritional scores include two or three of the following elements: serum albumin, lymphocytes count, TC, TG, and body weight. Serum albumin and weight loss are strong independent risk factors for mortality in older persons. Low albumin or weight loss was correlated with increased mortality in older persons ( 23 , 24 ). Meanwhile, the synthesis of albumin is influenced by chronic inflammation and malnutrition, and lower levels of albumin may be a marker of continuous arterial injury, as well as the progression of atherosclerosis and thrombosis ( 25 ). High cholesterol is a common risk factor for CVD. However, low cholesterol is a high risk factor for CVD events in dialysis patients ( 26 ). The reason for this paradox may be that the inflammatory or malnutrition state of the organism leads to a disturbance in lipid metabolism, which increases the risk of adverse outcomes. In addition, age related lymphopenia is well described in the literature and an association between lymphopenia and mortality has recently been reported ( 27 ). The occurrence of diabetes is accompanied by an increase in reactive oxygen species, which in turn leads to an increase in oxidative stress ( 28 ). This oxidative stress ( 29 ), along with protein energy consumption ( 30 ), are both potential causes of CKD inflammation, and may represent the mechanisms underlying DKD inflammation. Consequently, low serum albumin and low lymphocyte count may contribute to ESKD.
Our study found that a higher risk of adverse outcomes was associated with lower GNRI and PNI, as well as higher CONUT score. GNRI is based on measurements of serum albumin and weight loss, which are strong independent risk factors for mortality in older persons. The utilization of both indicators in the GNRI minimizes confounding variables such as hydration status. Therefore, the GNRI is a reliable prognostic indicator of adverse outcomes in patients with DKD. The CONUT score, a combination of cholesterol, lymphocyte count, and serum albumin, may serve as a reliable indicator for identifying high-risk CVD patients. Early assessment of the CONUT score can provide a preliminary understanding of the nutritional, immune, inflammatory, and lipid metabolism status of patients. Consequently, it can be used as a reference for clinical management.
In addition, we presumed that the PNI may be a better predictor than GNRI and CONUT to predict the ESKD in DKD patients, most likely because the PNI is a more comprehensive marker that reflects nutrition, immune and inflammation ( 31 ), all of which are closely related with DKD. Moreover, lymphocyte count proves to be a more consistent measure of body composition over extended periods. In contrast, the markers used in calculating GNRI and TCBI, which include body weight, TC, and TG, are greatly influenced by factors like age, diet, drugs, smoking, drinking, and lifestyle choices. The TCBI score is calculated from variables reflecting lipid metabolism as well as immune function measured from blood tests. We presumed that TCBI may be the worst predictor to predict ESKD in DKD patients, most likely because TC and TG cannot effectively assess the body’s nutritional status, inflammation level, and immune response.
A recommended treatment approach for DKD is the comprehensive management of blood glucose, blood pressure, and blood lipids, aiming to delay DKD progression to ESKD and cardiovascular diseases. High protein intake can further impair kidney function, increasing the risk of DKD progression and cardiovascular events. Carbohydrates, as a readily available source of energy, are one of the main influencing factors of blood glucose. Therefore, many renal experts suggest that DKD patients adopt a low-carbohydrate diet (energy intake of 25–35 kcal/kg/day) and minimize the risk of high protein intake (low protein diet, protein intake of 0.6–0.8 g/kg/day) ( 22 ). The new guidelines also differentiate between pre-dialysis diabetes patients and non-diabetes patients, providing specific protein ranges for each group. For clinically stable stage 3–5 CKD patients without diabetes, the new recommendations set a range of 0.55–0.60 g/kg/day or an extremely low protein diet of 0.28–0.43 g/kg/day ( 32 ). A lower protein intake reduces readily available energy in the body, thus requiring more carbohydrates to meet energy demands. However, a high carbohydrate intake may worsen blood glucose control in diabetes ( 33 ). From an energy perspective, low-carbohydrate and low-protein diets fundamentally contradict each other. Strict dietary restrictions may lower the quality of life in DKD patients and significantly increase the risk of malnutrition. Therefore, it is especially important to comprehensively evaluate the nutritional status of DKD patients and utilize it to restrict protein intake and regulate blood glucose levels. We presumed that the diagnostic accuracy of PNI + TCBI+CONUT+GNRI was slightly higher than PNI alone for cardiovascular death, and the diagnostic accuracy of PNI + GNRI+TCBI was slightly higher than PNI alone for all-cause mortality, most likely because the combinations including more serum nutritional indicators and other factors can comprehensively evaluate the nutritional status of DKD patients.
Recent meta-analysis of kidney biopsies in diabetes patients has shown a wide range of changes in kidney disease ( 34 ). Autopsy studies have also indicated that pathological changes in diabetic kidney disease can occur before clinical manifestations like proteinuria and eGFR decline ( 35 – 38 ). Previous research has suggested that some renal histological changes are good predictors of end-stage renal disease, cardiovascular and all-cause mortality ( 39 , 40 ). Therefore, evaluating the renal histological changes of DKD patients is clinically significant. However, even when clinical manifestations are present, renal biopsies are rarely conducted in routine clinical practice for DKD patients. Interestingly, we did not find any correlation between renal histological changes and all-cause mortality in both the low PNI group and the high PNI group in our study, which may be due to insufficient follow-up time. Moreover, albumin also has anti-inflammatory, antioxidant, and antithrombotic properties. Inflammatory states and conditions that increase capillary permeability can cause low serum albumin concentration, resulting in the expansion of interstitial space and an increase in albumin distribution volume ( 41 ). In our study, we found that DKD patients with more severe renal histological changes had a higher risk of adverse outcomes, particularly in low PNI group, where the relationship between renal histological changes (glomerular lesions, IFTA, interstitial inflammation) and end-stage renal disease was more pronounced. We hypothesized that DKD patients with low PNI have lower serum protein concentrations, indicating an increase in the excretion of renal amino acids that activate the RAS ( 42 – 44 ). The activation of the RAS induces glomerulosclerosis and interstitial fibrosis through various mechanisms, ultimately leading to renal histological changes. Therefore, integrating nutritional status and histological changes is crucial, particularly focusing on DKD patients with poor nutritional status (low PNI group), as it may help predict ESKD in these patients.
Despite the crucial findings being mentioned, our study has some limitations: (1) To begin with, our study was conducted at a single center and encompassed a small sample size comprising exclusively of patients with confirmed DKD through renal biopsy. This inclusion criteria might have introduced some degree of selective bias into our findings. We speculated that this was the reason why we found an N-shaped relationship between the nutritional score GNRI and ESKD, rather than an L-shaped relationship. (2) Certain factors that could potentially disrupt the results, including dietary factors and the use of various types of therapeutic medications, were not taken into account. (3) Due to the difficulty of repeated renal biopsies and reassessments, we only evaluated four nutritional scores at the time of patient enrollment, without investigating the impact of changes in renal histology and nutritional assessments overtime on the prognosis of DKD patients. (4) A more comprehensive assessment tool that incorporates additional nutrients, such as blood lipids and glucose, is necessary due to the limited nutritional content of the four nutritional scores.
5 Conclusion
In summary, our study demonstrated that the nutritional status of patients with DKD significantly influences their outcomes. We reported an association between end-stage kidney disease, cardiovascular, and all-cause mortality, and four nutritional scores (PNI, GNRI, TCBI, and COUNT). Moreover, our findings indicate that the PNI may provide more accurate predictive values for adverse outcomes and display stronger correlations with various renal histologic changes compared to other nutritional scores.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: the data presented in this study are available on request from the corresponding author. The data are not publicly available due to the protection of patient’s rights of privacy. Requests to access these datasets should be directed to [email protected] .
Ethics statement
The studies involving humans were approved by the ethical committee of Xinqiao Hospital (No. 2018-006-02). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LX: Writing – review & editing, Writing – original draft, Visualization, Software, Methodology, Investigation, Data curation, Conceptualization. JX: Writing – review & editing, Validation, Supervision, Investigation, Data curation, Conceptualization. QH: Writing – review & editing, Project administration, Methodology, Data curation. WL: Writing – review & editing, Supervision, Investigation. LC: Writing – review & editing, Writing – original draft, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the project of Tutorial System of Excellent Medical Undergraduate in Lab Teaching and Management Center at Chongqing Medical University (Grant No. LTMCMTS202215); Scientific Research and Innovation Experiment Project of Chongqing Medical University (No. 202325).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1352030/full#supplementary-material
1. International Diabetes Federation. IDF Diabetes Atlas, 10th edition (2021). Available at: www.diabetesatlas.org (Accessed November 18, 2022).
Google Scholar
2. Voeltz, D, Tönnies, T, Brinks, R, and Hoyer, A. Future prevalence of type 2 diabetes-a comparative analysis of chronic disease projection methods. PLoS One . (2022) 17:e0264739. doi: 10.1371/journal.pone.0264739
PubMed Abstract | Crossref Full Text | Google Scholar
3. Ikizler, TA, Cano, NJ, Franch, H, Fouque, D, Himmelfarb, J, Kalantar-Zadeh, K, et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int . (2013) 84:1096–107. doi: 10.1038/ki.2013.147
4. Rees, K, Dyakova, M, Ward, K, Thorogood, M, and Brunner, E. Dietary advice for reducing cardiovascular risk. Cochrane Database Syst Rev . (2013) 3:CD002128. doi: 10.1002/14651858.CD002128.pub4
Crossref Full Text | Google Scholar
5. Onodera, T, Goseki, N, and Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi . (1984) 85:1001–5.
PubMed Abstract | Google Scholar
6. Bouillanne, O, Morineau, G, Dupont, C, Coulombel, I, Vincent, JP, Nicolis, I, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr . (2005) 82:777–83. doi: 10.1093/ajcn/82.4.777
7. Doi, S, Iwata, H, Wada, H, Funamizu, T, Shitara, J, Endo, H, et al. A novel and simply calculated nutritional index serves as a useful prognostic indicator in patients with coronary artery disease. Int J Cardiol . (2018) 262:92–8. doi: 10.1016/j.ijcard.2018.02.039
8. Ignacio de Ulíbarri, J, González-Madroño, A, de Villar, NG, González, P, González, B, Mancha, A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp . (2005) 20:38–45.
9. Xiong, J, Wang, M, Wang, J, Yang, K, Shi, Y, Zhang, J, et al. Geriatric nutrition risk index is associated with renal progression, cardiovascular events and all-cause mortality in chronic kidney disease. J Nephrol . (2020) 33:783–93. doi: 10.1007/s40620-019-00676-1
10. Barutcu Atas, D, Tugcu, M, Asicioglu, E, Velioglu, A, Arikan, H, Koc, M, et al. Prognostic nutritional index is a predictor of mortality in elderly patients with chronic kidney disease. Int Urol Nephrol . (2022) 54:1155–62. doi: 10.1007/s11255-021-03002-6
11. Huo, Q, He, T, Xiong, J, and Zhao, J. Controlling nutritional status score is associated with renal progression, cardiovascular events, and all-cause mortality in biopsy-proved diabetic kidney disease. Front Physiol . (2023) 14:1231448. doi: 10.3389/fphys.2023.1231448
12. Fan, H, Huang, Y, Zhang, H, Feng, X, Yuan, Z, and Zhou, J. Association of Four Nutritional Scores with all-Cause and Cardiovascular Mortality in the general population. Front Nutr . (2022) 9:846659. doi: 10.3389/fnut.2022.846659
13. An, Y, Xu, F, Le, W, Ge, Y, Zhou, M, Chen, H, et al. Renal histologic changes and the outcome in patients with diabetic nephropathy. Nephrol Dial Transplant . (2015) 30:257–66. doi: 10.1093/ndt/gfu250
14. Tervaert, TW, Mooyaart, AL, Amann, K, Cohen, AH, Cook, HT, Drachenberg, CB, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol . (2010) 21:556–63. doi: 10.1681/ASN.2010010010
15. Cheng, L, Rong, J, Zhuo, X, Gao, K, Meng, Z, Wen, X, et al. Prognostic value of malnutrition using geriatric nutritional risk index in patients with coronary chronic total occlusion after percutaneous coronary intervention. Clin Nutr . (2021) 40:4171–9. doi: 10.1016/j.clnu.2021.01.042
16. Wei, T, Simko, V, Levy, M, Xie, Y, Jin, Y, and Zemla, J. Package “corrplot”. Statistician . (2017) 56:e24. doi: 10.1002/mus.25583
17. Villanueva, RAM, and Chen, ZJ. ggplot2: Elegant graphics for data analysis (2nd ed.) [J]. Research and Perspectives . (2019). 17:160–167. doi: 10.1080/15366367.2019.1565254
18. Obi, Y, Qader, H, Kovesdy, CP, and Kalantar-Zadeh, K. Latest consensus and update on protein energy-wasting in chronic kidney Disease. Curr Opin Clin Nutr Metab Care . (2015) 18:254–62. doi: 10.1097/MCO.0000000000000171
19. van Diepen, AT, Hoekstra, T, Rotmans, JI, de Boer, MG, le Cessie, S, Suttorp, MM, et al. The association between dialysis modality and the risk for dialysis technique and non-dialysis technique-related infections. Nephrol Dial Transplant . (2014). 29:2244–50. doi: 10.1093/ndt/gfu285
20. Peev, V, Nayer, A, and Contreras, G. Dyslipidemia, malnutrition, inflammation, cardiovascular disease and mortality in chronic kidney disease. Curr Opin Lipidol . (2014) 25:54–60. doi: 10.1097/MOL.0000000000000045
21. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int . (2020) 98:S1–S115. doi: 10.1016/j.kint.2020.06.019
22. Ikizler, TA, Burrowes, JD, Byham-Gray, LD, Campbell, KL, Carrero, JJ, Chan, W, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis . (2020) 76:S1–S107. doi: 10.1053/j.ajkd.2020.05.006
23. Corti, MC, Guralnik, JM, Salive, ME, and Sorkin, JD. Serum albumin level and physical disability as predictors of mortality in older persons. JAMA . (1994) 272:1036–42. doi: 10.1001/jama.1994.03520130074036
24. Harris, T, Cook, EF, Garrison, R, Higgins, M, Kannel, W, and Goldman, L. Body mass index and mortality among non-smoking older persons. The Fra-mingham heart study. J Am Med Assoc . (1988) 259:1520–4. doi: 10.1001/jama.1988.03720100038035
25. Kuller, LH, Eichner, JE, Orchard, TJ, Grandits, GA, McCallum, L, and Tracy, RP. The relation between serum albumin levels and risk of coronary heart disease in the multiple risk factor intervention trial. Am J Epidemiol . (1991) 134:1266–77. doi: 10.1093/oxfordjournals.aje.a116030
26. Kalantar-Zadeh, K, Block, G, Humphreys, MH, and Kopple, JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int . (2003) 63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x
27. Deschasse, G, Lombrail, P, Steenpass, V, Couturier, P, and Gavazzi, G. Association entre lymphopénie et mortalité chez lessujets âgés admis en unité de médecine aiguë gériatrique [Association between lymphopenia of elderly patients and intrahos-pital mortality in a geriatric acute care unit]. Geriatr Psychol Neuropsychiatr Vieil . (2014) 12:25–33. doi: 10.1684/pnv.2014.0460
28. Etoh, T, Inoguchi, T, Kakimoto, M, Sonoda, N, Kobayashi, K, Kuroda, J, et al. Increased expression of NAD (P) H oxidase subunits, NOX4 and p22phox, in the kidney of streptozotocin-induced diabetic rats and its reversibity by interventive insulin treatment. Diabetologia . (2003) 46:1428–37. doi: 10.1007/s00125-003-1205-6
29. Ramos, LF, Shintani, A, Ikizler, TA, and Himmelfarb, J. Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J Am Soc Nephrol . (2008) 19:593–9. doi: 10.1681/ASN.2007030355
30. Raj, DS, Moseley, P, Dominic, EA, Onime, A, Tzamaloukas, AH, Boyd, A, et al. Interleukin-6 modulates hepatic and muscle protein synthesis during hemodialysis. Kidney Int . (2008) 73:1054–61. doi: 10.1038/ki.2008.21
31. Hu, Y, Cao, Q, Wang, H, Yang, Y, Xiong, Y, Li, X, et al. Prognostic nutritional index predicts acute kidney injury and mortality of patients in the coronary care unit. Exp Ther Med . (2021) 21:123. doi: 10.3892/etm.2020.9555
32. Kistler, BM, Moore, LW, Benner, D, Biruete, A, Boaz, M, Brunori, G, et al. The International Society of Renal Nutrition and Metabolism Commentary on the National Kidney Foundation and academy of nutrition and dietetics KDOQI clinical practice guideline for nutrition in chronic kidney Disease. J Ren Nutr . (2021) 31:116–120.e1. doi: 10.1053/j.jrn.2020.05.002
33. Jiang, S, Fang, J, and Li, W. Protein restriction for diabetic kidney disease. Cochrane Database Syst Rev . (2023) 1:CD014906. doi: 10.1002/14651858.CD014906.pub2
34. Fiorentino, M, Bolignano, D, Tesar, V, Pisano, A, Biesen, WV, Tripepi, G, et al. Renal biopsy in patients with diabetes: a pooled meta-analysis of 48 studies. Nephrol Dial Transplant . (2017) 32:97–110. doi: 10.1093/ndt/gfw070
35. He, F, Xia, X, Wu, XF, Yu, XQ, and Huang, FX. Diabetic retinopathy in predicting diabetic nephropathy in patients with type 2 diabetes and renal disease: a meta-analysis. Diabetologia . (2013) 56:457–66. doi: 10.1007/s00125-012-2796-6
36. Moriya, T, Tanaka, S, Kawasaki, R, Ohashi, Y, Akanuma, Y, Yamada, N, et al. Japan diabetes complications study group. Diabetic retinopathy and microalbuminuria can predict macroalbuminuria and renal function decline in Japanese type 2 diabetic patients: Japan diabetes complications study. Diabetes Care . (2013) 36:2803–9. doi: 10.2337/dc12-2327
37. Moriya, T, Suzuki, Y, Inomata, S, Iwano, M, Kanauchi, M, and Haneda, M. Renal histological heterogeneity and functional progress in normoalbuminuric and microalbuminuric Japanese patients with type 2 diabetes. BMJ Open Diabetes Res Care . (2014) 2:e000029. doi: 10.1136/bmjdrc-2014-000029
38. Hong, D, Zheng, T, Jia-qing, S, Jian, W, Zhi-hong, L, and Lei-shi, L. Nodular glomerular lesion: a later stage of diabetic nephropathy? Diabetes Res Clin Pract . (2007) 78:189–95. doi: 10.1016/j.diabres.2007.03.024
39. Shimizu, M, Furuichi, K, Toyama, T, Kitajima, S, Hara, A, Kitagawa, K, et al. Kanazawa study Group for Renal Diseases and Hypertension. Long-term outcomes of Japanese type 2 diabetic patients with biopsy-proven diabetic nephropathy. Diabetes Care . (2013) 36:3655–62. doi: 10.2337/dc13-0298
40. Takazakura, E, Nakamoto, Y, Hayakawa, H, Kawai, K, Muramoto, S, Yoshida, K, et al. Onset and progression of diabetic glomerulosclerosis; a prospective study based on serial renal biopsies. Diabetes . (1975) 24:1–9. doi: 10.2337/diab.24.1.1
41. Manolis, AA, Manolis, TA, Melita, H, Mikhailidis, DP, and Manolis, AS. Low serum albumin: a neglected predictor in patients with cardiovascular disease. Eur J Intern Med . (2022) 102:24–39. doi: 10.1016/j.ejim.2022.05.004
42. Martinez-Maldonado, M, Benabe, JE, Wilcox, JN, Wang, S, and Luo, C. Renal renin, angiotensinogen, and ANG I-converting-enzyme gene expression: influence of dietary protein. Am J Phys . (1993) 264:F981–8.
43. Benabe, JE, Wang, S, Wilcox, JN, and Martinez-Maldonado, M. Modulation of ANG II receptor and its mRNA in normal rat by low-protein feeding. Am J Phys . (1993) 265:F660–9.
44. Benabe, JE, Fernández-Repollet, E, Tapia, E, Luo, C, and Martinez-Maldonado, M. Angiotensin II and catecholamines interaction in short-term low protein feeding. Kidney Int . (1993) 44:285–93. doi: 10.1038/ki.1993.243
Keywords: prognostic nutritional index, geriatric nutritional risk index, triglycerides × total cholesterol × body weight index, controlling nutritional status, end-stage kidney disease, cardiovascular disease, all-cause mortality, renal histologic changes
Citation: Xing L, Xiong J, Hu Q, Li W and Chen L (2024) Comparative analysis of four nutritional scores in predicting adverse outcomes in biopsy-confirmed diabetic kidney Disease. Front. Nutr . 11:1352030. doi: 10.3389/fnut.2024.1352030
Received: 07 December 2023; Accepted: 04 March 2024; Published: 20 March 2024.
Reviewed by:
Copyright © 2024 Xing, Xiong, Hu, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Chen, [email protected]
† These authors have contributed equally to this work and share first authorship
This article is part of the Research Topic
Nutritional Status Assessment and its Links with Chronic Disease Prognosis and Surgical Outcomes

COMMENTS
Presentation of Case. Dr. Meridale V. Baggett: A 68-year-old man was admitted to this hospital with fever, shortness of breath, and acute kidney injury during the pandemic of coronavirus disease ...
Abstract. The prevalence of chronic kidney disease and its risk factors is increasing worldwide, and the rapid rise in global need for end-stage kidney disease care is a major challenge for health systems, particularly in low- and middle-income countries. Countries are responding to the challenge of end-stage kidney disease in different ways ...
Cardiac Imaging Studies (00:22) Dr. Eugene P. Rhee: A 74-year-old man was evaluated in the nephrology clinic of this hospital because of chronic kidney disease. The patient had been in his usual ...
Acute Renal Failure Case Study. Our kidneys are incredible organs that get rid of toxins, retain substances needed by our bodies, and maintain the right balance of electrolytes, minerals, and water. Find out what happens to this 27-year-old when toxins accumulate in her kidneys leading to acute renal failure.
This article presents a case of chronic kidney disease (CKD) that was revealed by a single-subject research design. It discusses the advantages and limitations of this method for studying CKD, and how it can complement other approaches. The article also provides an overview of CKD diagnosis, management, and prevention.
Acute kidney injury (AKI) is an abrupt and usually reversible decline in the glomerular filtration rate (GFR). Multiple etiologies can cause AKI; therefore, it may be incorrect to treat AKI as a single disease. The KDIGO guidelines specified that patients with AKI must be evaluated promptly to determine the cause [ 1 ].
n engl j med 378;25 nejm.orgJune 21, 2018 2421 The new england journal of medicine Presentation of Case Dr. Helen I. Healy (Pediatrics): A 15-year-old girl was admitted to this hospital during the ...
DOI: 10.1016/j.kisu.2019.11.010. The prevalence of chronic kidney disease and its risk factors is increasing worldwide, and the rapid rise in global need for end-stage kidney disease care is a major challenge for health systems, particularly in low- and middle-income countries. Countries are responding to the challenge of end-stage ….
Here, in part 2, we address disease complications and treatment for kidney failure. As in part 1, the case study of Anna Lowry, a 49-year-old woman with CKD, will be used for illustration, offering nurses specific guidance in helping patients to better understand and manage their CKD. (This case is a composite based on the authors' experience.)
Case2. Resources. Assignment. Self eval. Case #1. The patient is a 41 year-old male who has a longstanding history of hypertension and diabetes and presents with a complaint of pruritis, lethargy, lower extremity edema, nausea and emesis. He denies any other medical illnesses. On physical exam the patient is a well-developed, well-nourished ...
Abstract. Exercise-induced acute kidney injury (EIAKI) frequently develops in patients with renal hypouricemia (RHUC). However, several cases of RHUC with acute kidney injury (AKI) but without intense exercise have been reported. We encountered a 15-year-old male with RHUC who experienced AKI. He reported no episodes of intense exercise and displayed no other representative risk factors of ...
Background Chronic kidney disease (CKD) is a non-communicable disease that includes a range of different physiological disorders that are associated with abnormal renal function and progressive decline in glomerular filtration rate (GFR). This study aimed to investigate the associations of several behavioral and health-related factors with CKD in Iranian patients. Methods A hospital-based case ...
Abstract. The following fictional case is intended as a learning tool within the Pathology Competencies for Medical Education (PCME), a set of national standards for teaching pathology. These are divided into three basic competencies: Disease Mechanisms and Processes, Organ System Pathology, and Diagnostic Medicine and Therapeutic Pathology.
Resources. Self eval. Case #1. The patient is a 41 year-old male who has a longstanding history of hypertension and diabetes and presents with a complaint of pruritis, lethargy, lower extremity edema, nausea and emesis. He denies any other medical illnesses. On physical exam the patient is a well-developed, well-nourished male in moderate distress.
The NKF states in its Kidney Disease Outcomes Quality Initiative guidelines for chronic kidney disease that there is no benefit from a protein intake higher than the RDA of 0.8 g/kg body weight and that this is a reasonable level to recommend for patients with chronic kidney disease in stages 1-3. 13 Thus, many respected nonprofit health care ...
This study aimed to evaluate the prognostic impact and predictors of persistent renal dysfunction in acute kidney injury (AKI) after an emergency percutaneous coronary intervention (PCI) for acute ...
Introduction Brown tumors are a rare focal manifestation of osteitis fibrosa cystica, which results from hyperparathyroidism. Chronic kidney failure may lead to secondary or tertiary hyperparathyroidism and thus to osteitis fibrosa cystica and brown tumors. Case presentation A 60-year-old man with a history of diabetes mellitus and chronic kidney failure presented with a 15-day history of ...
We present a case report of a 17-year-old African American male who presented in a life threatening hypertensive emergency with renal failure and the highest reported serum creatinine in a pediatric patient. A brief discussion on CKD criteria, complications, and potential red flags for screening strategies is provided.
Acute renal failure (ARF) is a rapid loss of renal function due to damage to the kidneys. Acute renal failure is also known today as acute kidney injury (AKI). It is a problem seen in hospitalized patients and those in outpatient settings. A healthy adult eating a normal diet needs a minimum daily urine output of approximately 400 ml to excrete ...
This case presents an elderly man with type 2 diabetes and impaired renal function. He has recently been experiencing hypoglycemia with his current treatment regimen. The importance of re ...
In type 1 diabetes, diabetic nephropathy follows a predictable course from onset of diabetes to the onset of microalbuminuria to frank nephropathy to end-stage renal disease or death. Microalbuminuria develops 10-14 years after onset of diabetes. Without treatment, clinical nephropathy follows within 5 years, and azotemia develops ∼5 years ...
Acute Renal Failure - Case Summary. 1. A young woman presented with classical symptoms of early renal insufficiency. She also had a been a diagnosed diabetic since her late teens. In this case study, the patient had received two antibiotics for a recurring urinary tract infection. As we have seen, many of the cases of renal failure are due to ...
Groundbreaking interactive patient registry will help empower individuals with kidney disease to help pave the way for improved outcomes in kidney care (March 20, 2024, New York, NY) — The National Kidney Foundation (NKF) proudly announces the launch of the KidneyCARE (Community Access to Research Equity)™ Study, a cutting-edge online research initiative combining patients' insights on ...
In the study, experts reviewed genetic sequencing data from 959 patients with advanced kidney disease, where a total of 11 patients were identified as having a deleted region genome, leading to a ...
The case study of ARF is discussed to develop a better understanding by studying the current research project. The discussion will be more focused on the pathophysiology and the new ways of treatment used in current practice. ... Although there may be chances of chronic kidney disease (CKD) in this case, the current history is acute reduction ...
Kidney Case Study Case 1 A 35 year old sportsman visits his doctor complaining of back and side pain. ... Kidney failure returns values of less than 15 in the GFR, which calls for dialysis or a kidney transplant. The GFR is more accurate as compared to urinalysis. This is because urinalysis largely depends on the ability of the medical ...
Malnutrition is associated with adverse outcomes in patients with diabetic kidney disease (DKD). However, it is uncertain which nutritional assessment tools are most effective in predicting the adverse outcomes of DKD. This retrospective study was conducted at a single center and included 367 patients diagnosed with DKD based on biopsy results between August 2009 and December 2018. Four ...
Acute Kidney Injury. Patient Profile A. is a 70-year-old white woman who presented to the emergency department because of a 4-day history of increased shortness of breath and generalized weakness. A. stated that she has been able to do her daily chores at home independently, but for the past few days, it was getting difficult for her to get around and that she needed to take frequent breaks ...