
- Find My GCO
- IACUC applications (Cayuse Animal Management System)
- IBC Applications (eMUA)
- IRB Applications (RASS-IRB) External
- Institutional Profile & DUNS
- Rates and budgets
- Report external interests (COI)
- Join List Servs
- Ask EHS External
- Research Development Services
- Cornell Data Services External
- Find Your Next Funding Opportunity
- Travel Registry External
- RASS (Formerly Form 10 and NFA) External
- International research activities External
- Register for Federal and Non-Federal Systems
- Disclose Foreign Collaborations and Support
- Web Financials (WebFin2) External
- PI Dashboard External
- Research metrics & executive dashboards
- Research Financials (formerly RA Dashboard) External
- Subawards in a Proposal
- Proposal Development, Review, and Submission
- Planning for Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Budgets, Costs, and Rates
- Collaborate with Weill Cornell Medicine
- Award Negotiation and Finalization
- Travel and International Activities
- Project Finances
- Project Modifications
- Research Project Staffing
- Get Confidential Info, Data, Equipment, or Materials
- Managing Subawards
- Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Project Closeout Financials
- Project Closeout
- End a Project Early
- Protecting an Invention, Creation, Discovery
- Entrepreneurial and Startup Company Resources
- Gateway to Partnership Program
- Engaging with Industry
- Responsible Conduct of Research (RCR)
- Export Controls
- Research with Human Participants
- Research Security
- Work with Live Vertebrate Animals
- Research Safety
- Regulated Biological Materials in Research
- Financial Management
- Conflicts of Interest
- Search

IRB Consent Form Templates
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study.
General Consent Form Templates
Social and Behavioral Research Projects (last updated 03/16/2023)
Biomedical Research Projects (last updated 07/18/2022)
Consent Form Templates for Specific Biomedical Procedures
MRI and fMRI
Blood Collection by Finger Stick
Blood Collection by Venipuncture
Oral Consent Template
Guidance for Protocols Involving Oral Consent
Debriefing Template
Guidance and Template for Debriefing Participants
Studies Involving Children (Assent/Permission Forms)
Parent-Guardian Permission for Studies Involving Children
Sample Parental Notification Form
Sample Child Assent Form
Performance Release for Minors
Performance Releases
Performance Release for Adults
As the nation’s largest public research university, the Office of the Vice President for Research (OVPR) aims to catalyze, support and safeguard U-M research and scholarship activity.
The Office of the Vice President for Research oversees a variety of interdisciplinary units that collaborate with faculty, staff, students and external partners to catalyze, support and safeguard research and scholarship activity.
ORSP manages pre-award and some post-award research activity for U-M. We review contracts for sponsored projects applying regulatory, statutory and organizational knowledge to balance the university's mission, the sponsor's objectives, and the investigator's intellectual pursuits.
Ethics and compliance in research covers a broad range of activity from general guidelines about conducting research responsibly to specific regulations governing a type of research (e.g., human subjects research, export controls, conflict of interest).
eResearch is U-M's site for electronic research administration. Access: Regulatory Management (for IRB or IBC rDNA applications); Proposal Management (eRPM) for the e-routing, approval, and submission of proposals (PAFs) and Unfunded Agreements (UFAs) to external entities); and Animal Management (for IACUC protocols and ULAM).
Sponsored Programs manages the post-award financial activities of U-M's research enterprise and other sponsored activities to ensure compliance with applicable federal, state, and local laws as well as sponsor regulations. The Office of Contract Administration (OCA) is also part of the Office of Finance - Sponsored Programs.
Ethics & Compliance
- eResearch IRB NextGen Project
- Class Assignments & IRB Approval
- Operations Manual (OM)
- Authorization Agreement Process
- ORCR Policies and Procedures
- Self-Assessment Tools
- Resources and Web Links
- Single IRB-of-Record (sIRB) Process
- Certificate of Confidentiality Process
- HRPP Education Resources
- How to Register a Clinical Trial
- Maintaining and Updating ClinicalTrial.gov Records
- How to Report Clinical Trial Results
- Research Study Participation - FAQ
- International Research
- Coordinated Services & Practices (CSP)
- Collaborative Research: IRB-HSBS sIRB Process
- Data Security Guidelines
- Research Incentive Guidelines
- Routine fMRI Study Guidelines
- IRB-HSBS Website Directory and Guidance
- Waivers of Informed Consent Guidelines
- IRB Review Process
- IRB Amendment Process
- Continuing Review Process
- Incident Reporting (AE/ORIO)
- IRB Repository Application
- IRB-HSBS Education
- Newsletter Archive
You are here
- Human Subjects
- IRB Health Sciences and Behavioral Sciences (HSBS)
Informed Consent Guidelines & Templates
U-m hrpp informed consent information.
See the HRPP Operations Manual, Part 3, Section III, 6 e .
The human subjects in your project must participate willingly , having been adequately informed about the research.
- If the human subjects are part of a vulnerable population (e.g., prisoners, cognitively impaired individuals, or children), special protections are required.
- If the human subjects are children , in most cases you must first obtain the permission of parents in addition to the consent of the children.
Contact the IRB Office for more information .
See the Waiver Guidelines for information about, and policies regarding, waivers for informed consent or informed consent documentation.

See the updated Basic Informed Consent Elements document for a list of 2018 Common Rule basic and additional elements.
Informed Consent Process
Informed consent is the process of telling potential research participants about the key elements of a research study and what their participation will involve. The informed consent process is one of the central components of the ethical conduct of research with human subjects. The consent process typically includes providing a written consent document containing the required information (i.e., elements of informed consent) and the presentation of that information to prospective participants.
In most cases, investigators are expected to obtain a signature from the participant on a written informed consent document (i.e., to document the consent to participate) unless the IRB has waived the consent requirement or documentation (signature) requirement .
- Projects which collect biospecimens for genetic analysis must obtain documented (signed) informed consent.
- It is an ethical best practice to include an informed consent process for most exempt research . IRB-HSBS reviews, as applicable, the IRB application for exempt research, but not the informed consent document itself. A suggested consent template for exempt research can be found below under the References and Resources section. A companion protocol template for exempt research may be found in the feature box, Related Information (top right).
Informed consent documents
An informed consent document is typically used to provide subjects with the information they need to make a decision to volunteer for a research study. Federal regulations ( 45 CFR 46.116 ) provide the framework for the type of information (i.e., the "elements") that must be included as part of the consent process. New with the revised 2018 Common Rule is the requirement that the consent document begin with a "concise and focused" presentation of key information that will help potential participants understand why they might or might not want to be a part of a research study.
Key Information Elements
The image below displays the five elements identified in the preamble to the revised Final Rule as suggested key information.
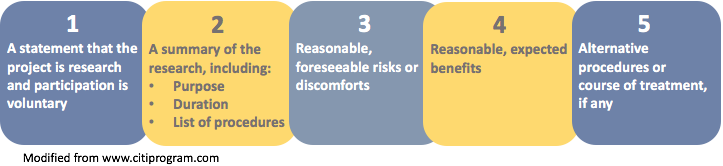
Note: Element number 5 (alternative procedures) applies primarily to clinical research.
General Information & Tips for Preparing a Consent Document
Reading level.
Informed consent documents should be written in plain language at a level appropriate to the subject population, generally at an 8th grade reading level . A best practice is to have a colleague or friend read the informed consent document for comprehension before submission with the IRB application. Always:
For guidance on using plain language, examples, and more, visit: http://www.plainlanguage.gov/
- Tailor the document to the subject population.
- Avoid technical jargon or overly complex terms.
- Use straightforward language that is understandable.
Writing tips
The informed consent document should succinctly describe the research as it has been presented in the IRB application.
- Use the second (you) or third person (he/she) to present the study details. Avoid use of the first person (I).
- Include a statement of agreement at the conclusion of the informed consent document.
- The consent doucment must be consistent with what is described in the IRB application.
Document Formating for Uploading into eResearch
- Remove "track changes" or inserted comments from the consent documentation prior to uploading the document into the IRB application (Section 10-1) for review.
- Use a consistent, clearly identified file naming convention for multiple consent/assent documents.
Informed Consent Templates
IRB-HSBS strongly recommends that investigators use one of the informed consent templates developed to include the required consent elements (per 45 CFR 46.116 ), as well as other required regulatory and institutional language. The templates listed below include the new consent elements outlined in the 2018 Common Rule.
References and Resources
- Brief protocol for exempt research including data management and security questionnaire
Informed Consent Guidance
PDF. Lists the basic and additional elements required for inclusion or to be included, as appropriate to the research, in the informed consent documentation, along with the citiation number [e.g., _0116(b)(1)] within the revised Common Rule. New elements associated with the 2018 Common Rule are indicated in bold text.
Informed Consent Templates (2018 Common Rule)
Strongly recommended for studies that involve the collection of biospecimens and/or genetic or genomic analysis, particularly federally sponsored clinical trials that are required to post a consent document on a public website. Last updated: 05/8/2023.
(Word) Blank template with 2018 revised Common Rule key information and other required informed consent elements represented as section headers; includes instructions and recommended language. It is strongly advised that you modify this template to draft a project-specific informed consent document for your study for IRB review and approval. Last updated: 05/08/2023
For use by U-M Dearborn faculty, staff, and students conducting non-exempt human subjects research using subject pools.
Other Templates
Informed Consent documents are not reviewed by the IRB for Exempt projects. However, researchers are ethically bound to conduct a consent process with subjects. This template is suggested for use with Exempt projects.
(Word) General outline to create and post a flyer seeking participation in a human subjects study. Includes instructions.
(Word) Two sample letters for site approval cooperation between U-M and other institutions, organizations, etc. Letters of cooperation must be on U-M letterhead and signed by an appropriate official. These letters are uploaded into the Performance Site section of the eResearch IRB application.
For use by U-M Dearborn faculty, staff, and students conducting exempt human subjects research using subject pools
Researchers who will conduct data collection that is subject to the General Data Protection Regulation (GDPR) must use this template in tandem with a general consent for participation template/document.
Child Assent and Parental Permission
- Child assent ages 3-6
- Child assent ages 7-11
- Child assent ages 12-14
- Parent permission
IRB-Health Sciences and Behavioral Sciences (IRB-HSBS)
Phone: (734) 936-0933 Fax: (734) 936-1852 [email protected]
- Human Subjects Protections
- IRB-SBS Home
- Contact IRB-SBS
- IRB SBS Staff Directory
- IRB SBS Board Members
- About the IRB-SBS
- CITI Training
- Education Events
- Virginia IRB Consortium
- IRB-SBS Learning Shots
- HRPP Education & Training
- Student Support
- Access iProtocol
- Getting Started
- iProtocol Question Guide
- iProtocol Management
- Protocol Review Process
- Certificate of Confidentiality
- Deception and/or Withholding Information from a Participant
- Ethnographic Research
- IRB-SBS 101
- IRB-SBS Glossary
- Participant Pools
- Paying Participants
- Research in an Educational Setting
- Research in an International Setting and/or Location
- Risk-Sensitive Populations
- Student Researchers and Faculty Sponsors
- Study Funding and the IRB
- Understanding Risk in Research
- Vulnerable Participants
- IRB-SBS PAM & Ed
- Federal Regulations
- Ethical Principals
- Partner Offices
- Determining Human Subjects Research
- Determining HSR or SBS

Consent Templates
The templates below were created to help you create the documents you will need to communicate to participants what they will do in the study. The documents you provide participants will range from recruitment materials to post-debrief consent forms, and you need to submit everything that you provide to a participant to our Board for review. For more information about the consent process see Consent .
- General Consent Template : This form covers all of the basic elements that are required for a consent document. Even if you don't plan to use this exact document, refer to it to ensure that you have all of the appropriate elements in place in your consent procedure.
- Electronic Consent Template : This form is modeled after the General Consent Template with some modifications that make it more appropriate for an online format. For more information about this template, see Electronic Consent .
- Parent Consent : If you are including minors in your study, you will need to provide a consent form for parents and an age appropriate assent form for minors. This form is a guide for creating a parent informed consent document. This form can also be used as a guide for surrogate consent procedures.
- Minor Assent (for ages 13-17) : This template provides the basic elements required for older minors to provide assent and could also be used as a model for higher functioning individuals with diminished mental capacity.
- Minor Assent (for ages 7-12) : This template provides the basic elements required for younger minors to provide assent and could also be used as a model for higher functioning individuals with diminished mental capacity. For children younger than 7, assent forms are not required but include information in the consent section regarding what you will say to them about the study (where appropriate).
- Capacity to Consent Template : For some participant populations, it may be necessary to determine if a participant is able to provide consent; if not, a surrogate can be used (you will also need a surrogate consent form and participant assent form, similar to the parent/child consent/assent forms).
- GDPR Informed Consent Addendum : If you are collecting data from citizens of the European Union or the United Kingdom, you will need to provide additional information to your participants, per the GDPR. For more information, see the Research in an International Setting and/or Location and International Research Data Source .
- Study Information Sheet : While many studies do not require researchers to collected signed consent forms, we generally require that participants receive a Study Information Sheet to provide them with information about the study. This information can be provided as a paper document at the beginning of a survey.
- Electronic Study Information Page : This template is similar to the Study Information Sheet with modification for an electronic delivery. For more information about this template, see Electronic Consent .
- Parent Notification Template : Typically used for studies in an educational setting (particularly where the study is exempt but parent notification is still required), this template is a guide for creating a notification letter to send home to parents.
- Oral Consent Card : Typically used in anthropology studies where the participant may be uncomfortable with a form and/or unable to use it, the Oral Consent Card provides all of the elements required for consent in a bullet format so that the researcher can refer to each point as he or she is obtaining consent from the participant.
- Oral Consent Template : This form is also used in situations where the participant is uncomfortable with a form and/or unable to use it. It is more suited to non-anthropology research (though anthropologists are welcome to refer to it as well).
- Sample Debriefing Form : A debriefing form is a summary of the study given to a participant in a deception study and/or a study that includes students from a participant pool. The purpose is to educate participants about the study and to provide them with resources, particularly if the study is upsetting.
- Advertising Flyer Template : Recruitment materials are part of the consent process and it is important that participants are accurately informed about the study throughout the process. You are not required to use this flyer template (it is a model appropriate for a flyer posted around campus), but it is important that you follow the guide provided in Recruitment .
- ResearchMatch Advertising Template : The NIH funds a free and secure recruitment tool called ResearchMatch that helps to connect researchers with volunteers that are interested in participating in studies. If you are interested in using ResearchMatch to help advertise for your study, complete this ResearchMatch Advertising Template and upload.
- Materials Release Form : The data you collect from your participants may be useful in other spheres, such as an educational tool and/or library archive. Using data in this manner is beyond the scope of the study and you should seek additional permission to use the participant's data in this way. This form allows a participant to declare how they would like their materials to be used by the researcher if the researcher wants to use the materials in situations beyond the study.
- Data Release Form : This form is similar to the Post-Debrief Consent Form; it is used when a participant has been recorded or photographed without their knowledge.
- Post-Debrief Consent Form : This form is used in a deception study after the deception is revealed to the participant. The participant is given an opportunity to decide if they still want to participate after the true purpose of the study is revealed.
- The title of protocol must match the title on all consent forms. The title must be relevant, appropriate, and easy to understand. Include the project title on all pages of the consent form.
- List the page numbers on all pages of the consent form in the standard format: Page 1.
- Delete all colored text from the final copy of your form. The colored text is for explanation purposes only.
- Make sure that the form matches the descriptions in the protocol and vice versa.
- Include all relevant information in the consent form rather than referring to previous verbal explanations. The consent form should provide a complete explanation of what the participant is agreeing to do in the study.
- Be aware of the needs of the participant. Avoid using jargon and acronyms that the participant may not understand; make sure the reading comprehension level is appropriate.
- Do not use statements that make implicit demands on participants to participate , e.g., "You will enjoy and benefit from participating in this study."
- Prepare the consent forms in the standard format provided in the template, with all headings addressed. Use the standard language provided on the template where appropriate.
- Please proofread the consent forms for grammar and spelling errors.
- Do not use language that revokes a participant’s legal rights . A consent form is not a legal document.
- Do not require the participants to sign consent to long statements written in first person , e.g., “I agree to participate in this research study. I understand that the risks are minimal and that I will receive no benefits. I know how to withdraw from this study. I will receive $X in payment for participating. I understand that if I withdraw from the study before my participation is complete, I will receive prorated payment according to the following schedule . . . I agree not to hold the researchers liable for any injuries resulting from participation in this study. . .” DO ask participants to sign consent to a simple agreement statement at the end of the consent form: “I agree to participate in the research study described above.”
- Privacy Policy
Buy Me a Coffee

Home » Informed Consent in Research – Types, Templates and Examples
Informed Consent in Research – Types, Templates and Examples
Table of Contents

Informed Consent in Research
Informed consent is a process of communication between a researcher and a potential participant in which the researcher provides adequate information about the study, its risks and benefits, and the participant voluntarily agrees to participate. It is a cornerstone of ethical research involving human subjects and is intended to protect the rights and welfare of participants.
Types of Informed Consent in Research
There are different types of informed consent in research , which may vary depending on the nature of the study, the type of participants, and the context. Some of the common types of informed consent in research include:
Written Consent
This is the most common type of informed consent, where participants are provided with a written document that explains the study and its requirements. The document typically includes information about the purpose of the study, procedures involved, risks and benefits, confidentiality, and participant rights. Participants are asked to sign the document as an indication of their willingness to participate.
Oral Consent
In some cases, oral consent may be used when a written document is not practical or feasible. Oral consent involves explaining the study and its requirements to participants verbally and obtaining their consent. This method may be used for studies with illiterate or visually impaired participants or when conducting research remotely.
Implied Consent
Implied consent is used in studies where participants’ actions are taken as an indication of their willingness to participate. For example, a participant may be considered to have given implied consent if they show up for a scheduled appointment for the study.
Opt-out Consent
This method is used when participants are given the opportunity to decline participation in a study. Participants are provided with information about the study and are given the option to opt-out if they do not wish to participate. This method is commonly used in population-based studies or surveys.
Assent is used in studies involving minors or participants who are unable to provide informed consent due to cognitive impairment or disability. Assent involves obtaining the agreement of the participant to participate in the study, along with the consent of a legally authorized representative.
Informed Consent Format in Research
Here’s a basic format for informed consent that can be customized for specific research studies:
- Introduction : Begin by introducing yourself and the purpose of the study. Clearly state that participation is voluntary and that participants can withdraw at any time without penalty.
- Study Overview : Provide a brief overview of the study, including its purpose, methods, and expected outcomes.
- Procedures : Describe the procedures involved in the study in clear, concise language. Include information about the types of data that will be collected, how they will be collected, and how long the study will take.
- Risks and Benefits : Outline the potential risks and benefits of participating in the study. Be honest and upfront about any discomfort, inconvenience, or potential harm that may be involved, as well as any potential benefits.
- Confidentiality and Privacy : Explain how participant data will be collected, stored, and used, and what measures will be taken to ensure confidentiality and privacy.
- Voluntary Participation: Emphasize that participation is voluntary and that participants can withdraw at any time without penalty. Explain how to withdraw from the study and who to contact if participants have questions or concerns.
- Compensation and Incentives: If applicable, explain any compensation or incentives that will be offered to participants for their participation.
- Contact Information: Provide contact information for the researcher or a representative from the research team who can answer questions and address concerns.
- Signature : Ask participants to sign and date the consent form to indicate their voluntary agreement to participate in the study.
Informed Consent Templates in Research
Here is an example of an informed consent template that can be used in research studies:
Introduction
You are being invited to participate in a research study. Before you decide whether or not to participate, it is important for you to understand why the research is being done, what your participation will involve, and what risks and benefits may be associated with your participation.
Purpose of the Study
The purpose of this study is [insert purpose of study].
If you agree to participate, you will be asked to [insert procedures involved in the study].
Risks and Benefits
There are several potential risks and benefits associated with participation in this study. Some of the risks include [insert potential risks of participation]. Some of the benefits include [insert potential benefits of participation].
Confidentiality
Your participation in this study will be kept confidential to the extent allowed by law. All data collected during the study will be stored in a secure location and only accessed by authorized personnel. Your name and other identifying information will not be included in any reports or publications resulting from this study.
Voluntary Participation
Your participation in this study is completely voluntary. You have the right to withdraw from the study at any time without penalty. If you choose not to participate or if you withdraw from the study, there will be no negative consequences.
Contact Information
If you have any questions or concerns about the study, you can contact the investigator(s) at [insert contact information]. If you have questions about your rights as a research participant, you may contact [insert name of institutional review board and contact information].
Statement of Consent
By signing below, you acknowledge that you have read and understood the information provided in this consent form and that you freely and voluntarily consent to participate in this study.
Participant Signature: _____________________________________ Date: _____________
Investigator Signature: ____________________________________ Date: _____________
Examples of Informed Consent in Research
Here’s an example of informed consent in research:
Title : The Effects of Yoga on Stress and anxiety levels in college students
Introduction :
We are conducting a research study to investigate the effects of yoga on stress and anxiety levels in college students. We are inviting you to participate in this study.
If you agree to participate, you will be asked to attend four yoga classes per week for six weeks. Before and after the six-week period, you will be asked to complete surveys about your stress and anxiety levels. Additionally, we will measure your heart rate variability at the beginning and end of the six-week period.
Risks and Benefits:
There are no known risks associated with participating in this study. However, the benefits of practicing yoga may include decreased stress and anxiety levels, increased flexibility and strength, and improved overall well-being.
Confidentiality:
All information collected during this study will be kept strictly confidential. Your name will not be used in any reports or publications resulting from this study.
Voluntary Participation:
Participation in this study is completely voluntary. You are free to withdraw from the study at any time without penalty.
Contact Information:
If you have any questions or concerns about this study, you may contact the principal investigator at (phone number/email address).
By signing this form, I acknowledge that I have read and understood the above information and agree to participate in this study.
Participant Signature: ___________________________
Date: ___________________________
Researcher Signature: ___________________________
Importance of Informed Consent in Research
Here are some reasons why informed consent is important in research:
- Protection of participants’ rights : Informed consent ensures that participants understand the nature and purpose of the research, the risks and benefits of participating, and their rights as participants. It empowers them to make an informed decision about whether to participate or not.
- Ethical responsibility : Researchers have an ethical responsibility to respect the autonomy of participants and to protect them from harm. Informed consent is a crucial way to uphold these principles.
- Legality : Informed consent is a legal requirement in most countries. It is necessary to protect researchers from legal liability and to ensure that research is conducted in accordance with ethical standards.
- Trust : Informed consent helps build trust between researchers and participants. When participants understand the research process and their role in it, they are more likely to trust the researchers and the study.
- Quality of research : Informed consent ensures that participants are fully informed about the research and its purpose, which can lead to more accurate and reliable data. This, in turn, can improve the quality of research outcomes.
Purpose of Informed Consent in Research
Informed consent is a critical component of research ethics, and it serves several important purposes, including:
- Respect for autonomy: Informed consent respects an individual’s right to make decisions about their own health and well-being. It recognizes that individuals have the right to choose whether or not to participate in research, based on their own values, beliefs, and preferences.
- Protection of participants : Informed consent helps protect research participants from potential harm or risks that may arise from their involvement in a study. By providing participants with information about the study, its risks and benefits, and their rights, they are able to make an informed decision about whether to participate.
- Transparency: Informed consent promotes transparency in the research process. It ensures that participants are fully informed about the research, including its purpose, methods, and potential outcomes, which helps to build trust between researchers and participants.
- Legal and ethical requirements: Informed consent is a legal and ethical requirement in most research studies. It ensures that researchers obtain voluntary and informed agreement from participants to participate in the study, which helps to protect the rights and welfare of research participants.
Advantages of Informed Consent in Research
The advantages of informed consent in research are numerous, and some of the most significant benefits include:
- Protecting participants’ autonomy: Informed consent allows participants to exercise their right to self-determination and make decisions about whether to participate in a study or not. It also ensures that participants are fully informed about the risks, benefits, and implications of participating in the study.
- Promoting transparency and trust: Informed consent helps build trust between researchers and participants by providing clear and accurate information about the study’s purpose, procedures, and potential outcomes. This transparency promotes open communication and a positive research experience for all parties involved.
- Reducing the risk of harm: Informed consent ensures that participants are fully aware of any potential risks or side effects associated with the study. This knowledge enables them to make informed decisions about their participation and reduces the likelihood of harm or negative consequences.
- Ensuring ethical standards are met : Informed consent is a fundamental ethical requirement for conducting research involving human participants. By obtaining informed consent, researchers demonstrate their commitment to upholding ethical principles and standards in their research practices.
- Facilitating future research : Informed consent enables researchers to collect high-quality data that can be used for future research purposes. It also allows participants to make an informed decision about whether they are willing to participate in future studies.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

How to Cite Research Paper – All Formats and...

Data Collection – Methods Types and Examples

Delimitations in Research – Types, Examples and...

Research Paper Format – Types, Examples and...

Research Process – Steps, Examples and Tips

Research Design – Types, Methods and Examples

UCL Research Ethics
- Advice on writing an information sheet and consent form

Writing a Participant Information Sheet and Consent Form
Recruitment documents help people make informed choices about whether to participate in a research study. Find out how to write a Participant Information Sheet, example forms and further guidance.
Writing a Participant Information Sheet
Participant Information Sheets must be designed to assist participants to make informed choices. Potential recruits must be given sufficient information to allow them to decide whether or not they want to take part. The process of obtaining consent and the accompanying documentation must be approved by a research ethics committee and, where only verbal consent to research is contemplated include consideration of an appropriate process for witnessing the consent.
Researchers must take the steps necessary to ensure that all participants in the research understand the process in which they are to be engaged, including why their participation is necessary, how it will be used, and how and to whom it will be reported so that the prospective participant can make an informed decision about whether they really do want to take part.
It is highly recommended that the information provided is presented on headed paper and is accurate, clear and simple so that someone with a reading age of 8 would understand the contents (use short words, sentences and paragraphs). The information should be specific to the proposed research and appropriate for the social and cultural context in which is it being given. It is important to avoid technical terms, jargon and abbreviations, bias, coercion or any inappropriate inducements.
What should the Participant Information Sheet include:
- A friendly invitation to participate.
- A brief and simple explanation of the purposes of the research and a statement explaining how the participant was chosen and how many other participants will be involved in the study.
- A statement that participation is voluntary; refusal to participate will involve no penalty or loss of benefits to which the participant is otherwise entitled; and the participant may discontinue participation at any time without penalty or loss of benefits.
- A thorough explanation of the expected duration of participation in the research and the procedures to be followed.
- A description of any reasonably foreseeable risks or discomforts and any benefits to the participant. For research involving more than minimal risk, an explanation as to whether any compensation or any medical treatments are available if injury occurs and, if so, what they consist of, or where further information may be obtained.
- A statement describing the extent, if any, to which confidentiality of records identifying the participant will be maintained.
- It is considered good practice for researchers to debrief participants at the conclusion of the research and to provide them with copies of any reports or other publications arising from their participation.
- If appropriate, a statement indicating that the data might be used for additional or subsequent research.
- An explanation of who to contact for answers to pertinent questions about the research and the rights of the participant and who to contact in the event of a research-related injury to the participant.
- If applicable, a statement declaring that each researcher who may have access to children (aged under 18) or vulnerable adults has undergone a satisfactory criminal records check.
- Remember to thank your participant for considering taking part in the study and include a statement indicating that the research study has been approved by the UCL Research Ethics Committee.
Language and layout
It is highly recommended that the information provided is presented on headed paper and is accurate, clear, and simple. The information should be specific to the proposed research and appropriate for the social and cultural context in which is it being given. It is important to avoid technical terms, jargon, and abbreviations, bias, coercion, or any inappropriate inducements.
The following points should be considered when writing an information sheet:
- Use clear, non-technical language. We recommend that you refer to the Plain English Campaign
- Use appropriate language for the target audience. For example, consider the different ways needed to communicate with primary school children as opposed to their teachers, or people with expertise in the area of study as opposed to people with no such knowledge
- Divide the text into paragraphs for ease of reading
- Consider using sub-headings for clarity, such as questions and answers
- Make sure the font and font size are legible.
Ask someone else to review your information sheet before it is circulated.
- Template Participant Information Sheet (Word)
- Template Consent Form (Word)
- Guidance on obtaining consent from research participants online (for online and in-person study designs)
Authors: Dr Pippa Lally, Behavioural Science and Health, and Jack Hindley, Information Services Division, UCL
- Recording & Obtaining Consent
UCL Research Ethics Committee Guidance Note 2: Extract from Nuffield Council on Bioethics website
Page last updated: April 2023
- Skip to navigation
- Skip to content
- UMB Shuttle
University of Maryland, Baltimore
About UMB History, highlights, administration, news, fast facts
- Accountability and Compliance
- Administration and Finance
- Center for Information Technology Services
- Communications and Public Affairs
- Community Engagement
- Equity, Diversity, and Inclusion
- External Relations
- Government Affairs
- Philanthropy
- Office of the President
- Office of the Provost
- Research and Development
- University Counsel
- Administrative Officers
- Boards of Visitors
- Faculty Senate
- Staff Senate
- Center for Health and Homeland Security
- Council for the Arts & Culture
- Interprofessional Education
- Leaders in Education: Academy of Presidential Scholars
- Middle States Self-Study
- President's Council for Women
- President's Symposium and White Paper Project
- For the Media
- Steering Committee Roster
- Logistics Committee Roster
- UMB Police and Public Safety
- Graduation Celebration 2024
- Founders Week
- UMB Holiday Craft Fair
Academics Schools, policies, registration, educational technology
- School of Dentistry
- Graduate School
- School of Medicine
- School of Nursing
- School of Pharmacy
- School of Social Work
- Carey School of Law
- Health Sciences and Human Services Library
- Thurgood Marshall Law Library
Admissions Admissions at UMB are managed by individual schools.
- Carey School of Law Admissions
- Graduate School Admissions
- School of Dentistry Admissions
- School of Medicine Admissions
- School of Nursing Admissions
- School of Pharmacy Admissions
- School of Social Work Admissions
- Tuition and Fees by School
- Student Insurance
- Academic Calendar
- Financial Assistance for Prospective Students
- Financial Assistance for Current Students
- Financial Assistance for Graduating Students
Research Offices, contracts, investigators, UMB research profile
- Organized Research Centers and Institutes
- UMB Institute for Clinical & Translational Research
- Sponsored Programs Administration
- Sponsored Projects Accounting and Compliance (SPAC)
- Kuali Research
- Clinical Trials and Corporate Contracts
- CICERO Log-in
- Conflict of Interest
Human Research Protections
- Environmental Health and Safety
- Export Compliance
- Effort Reporting
- Research Policies and Procedures
- Center for Innovative Biomedical Resources
- Baltimore Life Science Discovery Accelerator (UM-BILD)
- Find Funding
- File an Invention Disclosure
- Global Learning for Health Equity Network
- Manage Your Grant
- Research Computing
- UM Research HARBOR
- Center for Violence Prevention
- Office of Research and Development
- Center for Clinical Trials and Corporate Contracts
- Technology Transfer/UM Ventures
- Contact Research and Development
Services For students, faculty, and staff, international and on-campus
- Student Health Resources
- Educational Support and Disability Services
- Writing Center
- URecFit and Wellness
- Intercultural Leadership and Engagement
- Educational Technology
- Student Counseling Center
- UMB Scholars for Recovery
- UMB Student Affairs
- Human Resource Services
- Travel Services
- Strategic Sourcing and Acquisition Services
- Office of the Controller
- Office of the Ombuds
- Employee Assistance Program (EAP)
- Workplace Mediation Service
- Faculty Center for Teaching and Learning
- UMB Travel: Start Here
- International Students, Scholars, and Employees
- Center for Global Engagement
- International Travel SOS
- International Operations
- Parking and Transportation Services
- UMB shuttle
- SMC Campus Center Event Services
- Donaldson Brown Riverfront Event Center
- All-Gender Bathrooms
- Environmental Services
- Interprofessional Program for Academic Community Engagement
University Life Alerts, housing, dining, calendar, libraries, and recreation
- Emergency Reference Guide
- Campus Life Weekly with USGA
- Starting a New Universitywide Organization
- University Student Government Association
- Planned Closures
- Intramural Sports
- Safety Education
- About URecFit and Wellness
- How to Get Your One Card
- One Card Uses
- Lost One Card
- One Card Policies
- Photo Services
- One Card Forms
- One Card FAQs
- Office Hours and Directions
Give to UMB Sustain excellence and meet UMB's educational needs for today and tomorrow.

Thank You for Your Gift to UMB
The University of Maryland, Baltimore (UMB) is excited to share its new online giving page.
With enhanced searchability, a streamlined checkout process, and new ways to give such as Venmo, PayPal, Apple Pay, and Google Pay in addition to credit card, donors can support UMB quickly and securely.
- Ways to Give
- Where to Give
- Staying Connected: You and UMB
- The UMB Foundation
- Office of Philanthropy
- Maryland Charity Campaign

- OAC Services
- Research Compliance
- For Researchers
- Consent Form Templates
Human Research Protections Office
620 W. Lexington St. Second Floor Baltimore, MD 21201
P 410-706-5037
Office hours are 8:30 a.m.-4:30 p.m., Monday through Friday.
* The Human Research Protections Office is working remotely and is ready to assist our community during the COVID-19 pandemic. During this time, we request you contact us via email to promote a prompt response. With apologies, currently voicemail messages may be delayed in reaching us and cause a longer wait time for us to respond to your requests. We thank you for your patience and look forward to assisting you.
* Only the UMB IRB approved consent/assent templates (with the UMB logo) will be accepted when UMB IRB is the IRB of record
** Effective December 4th, 2023, the University of Maryland Baltimore Human Research Protections Program (HRPO) will adopt a revised version of the informed consent document (ICF) template. This announcement applies to new studies only that submit their application in CICERO after December 4th, 2023.
Consent and Assent Form Templates
- Informed Consent and HIPAA Authorization Form Template (Post 12-4-2023) : This template should be used as the consent document guide for all new research studies, including parental and LAR permission (consent) forms, submitted for IRB review on or after December 4th, 2023.
- Informed Consent and HIPAA Authorization Form Template (Pre 12-4-2023) : This template should be used as the consent document guide for all research studies, including parental and LAR permission (consent) forms, submitted for IRB review before December 4th, 2023. ( Informed Consent Concise Summary Examples 2019 )
- Assent Form Template : This template should be used to assent participants ages 13-17 and, if applicable to your study, cognitively impaired participants.
- HIPAA Authorization Form : All Non-VA uses.
- UM Dental School additional Consent Form Template : If you are conducting business through the School of Dentistry, add this paragraph.
- VA Informed Consent and HIPAA Authorization Form Template : This template should be used as a guide if your study is being conducted at the Baltimore VA. ( VA IC and HIPAA Guidelines )
- Revocation of HIPAA authorization (VA Form 10-10116)
- VA HIPAA Waiver Request (VA Form 10-0521)
Informed consent is required to be presented in language understandable to potential participants. The HSHSL Health Literacy guide contains a list of Plain Language resources .
The Health Sciences and Human Services Library offers a Research Consent Form Review service to improve consent form readability. Library staff will review your consent form and suggest changes that will help simplify the language, lower the reading grade level, and make the consent form more understandable to potential participants. * As a free service to all UMB faculty, researchers are strongly encouraged to use this service.
Click Here To Request a Research Consent Form Review
The following basic elements of informed consent are required to be disclosed in all research consent forms pursuant to 45 CFR 46(a-b):
Basic elements of informed consent.
- A statement that the study involves research, an explanation of the purposes of the research and the expected duration of the subject's participation, a description of the procedures to be followed, and identification of any procedures that are experimental.
- A description of any reasonably foreseeable risks or discomforts to the subject.
- A description of any benefits to the subject or to others that may reasonably be expected from the research.
- A disclosure of appropriate alternative procedures or courses of treatment, if any, that might be advantageous to the subject.
- A statement describing the extent, if any, to which confidentiality of records identifying the subject will be maintained, including the possibility that the Food and Drug Administration (FDA) may inspect the records if applicable.
- For research involving more than minimal risk, an explanation as to whether any compensation and an explanation as to whether any medical treatments are available if injury occurs and, if so, what they consist of, or where further information may be obtained.
- An explanation of whom to contact for answers to pertinent questions about the research and research subjects' rights, and whom to contact in the event of a research-related injury to the subject.
- A statement that participation is voluntary, refusal to participate will involve no penalty or loss of benefits to which the subject is otherwise entitled, and the subject may discontinue participation at any time without penalty or loss of benefits to which the subject is otherwise entitled.
Additional elements of informed consent. When appropriate, one or more of the following elements of information shall also be provided to each subject:
- A statement that the particular treatment or procedure may involve risks to the subject (or to the embryo or fetus, if the subject is or may become pregnant) that are currently unforeseeable.
- Anticipated circumstances under which the subject's participation may be terminated by the investigator without regard to the subject's consent.
- Any additional costs to the subject that may result from participation in the research.
- The consequences of a subject's decision to withdraw from the research and procedures for orderly termination of participation by the subject.
- A statement that significant new findings developed during the course of the research that may relate to the subject's willingness to continue participation will be provided to the subject.
- The approximate number of subjects involved in the study.
The University of Maryland, Baltimore is the founding campus of the University System of Maryland. 620 W. Lexington St., Baltimore, MD 21201 | 410-706-3100 © 2023-2024 University of Maryland, Baltimore. All rights reserved.

Consent, Assent, and Screening Templates
Biomedical Research Consent Templates
Minimal Risk Research Consent Templates (Expedited or Exempt)
These templates are appropriate for social, behavioral, and educational ("SBER") research that does not include any biomedical procedures.
- Study Information Sheet (no signature)
- Consent Form (includes signature)
- Consent Form for Federally Funded Research
- Parent Permission Form (parents provide permission for child to participate)
- Parent Consent Form (parents complete research procedures themselves)
- Oral Consent Script Outline
- Sample Debriefing Script
Biomedical Research Informed Consent Templates
- Biomedical Research Consent Template
- Consent Template for Expanded Access Research
- Right to Try Consent Template
- Humanitarian Use Device Consent Template
Child and Adolescent Assent Templates
- Child Assent Template (Age 7-12)
- Adolescent Assent Template for Non-Treatment Studies (Age 13-17)
Addendum Consent Templates
- Addendum Consent Template for Non-Treatment Studies (for new procedures, risks)
- Addendum Consent Template for Treatment Studies (for new procedures, risks)
Screening Scripts
- Screening Script for non-Treatment Studies
- Screening Script for Treatment Studies

Consent Standards and Sample Language
- Social, Behavioral & Educational ("SBER") Consent form Standards and Template Language
- Biomedical Research Consent Form Standards and Sample Language
Comprehension Tools
- PRISM Readability Tool Kit
- Self-Certification of Surrogate Decision Makers for Potential Research Subject's Participation in UC Research (For use only in studies the IRB has reviewed and explicitly approved for surrogate consent)
- Decision-Making Capacity Assessment Tool (for potential subjects who may have cognitive impairments)
Other References
- Research Participant Bill of Rights/Experimental Subjects Bill of Rights - available in 34 languages
- Conducting Risk-Benefit Assessments
- Obtaining and Documenting Informed Consent (v. 07-28-11)
- Requesting Waivers and Exceptions to Informed Consent (v. 07-28-11)
- Child Assent and Permission by Parents or Guardians (v. 09-06-11)
- The Use of Legally Authorized Representatives or Surrogate Consent (v. 06-21-10)
- Recruitment and Screening Methods and Materials (v. 09-05-11)
We appreciate your suggestions for improving templates and/or adding sample language to the standards documents. Please email OHRPPEQI@research.ucla.edu to provide your feedback.
Medical Research: Forms & Consent Templates
Main navigation.
This section contains all of the forms and consent templates that apply to investigators from: • School of Medicine (SoM) • Veteran's Affairs (VA) Hospital
*Please note that when creating a protocol for IRB submission, these investigators need to select the Medical eProtocol Application category.
If you have questions or are having trouble accessing these forms, please contact IRB Education ( email or call 650-724-7141).
The consent/assent form should be in a language that is understandable to someone without a medical or scientific background. Please use the Microsoft Readability Statistics tool as needed when writing your consent form.
See consent template updates for recent changes.
Other Forms:
Eprotocol forms:.
- Mobile Forms
- INTEGRATIONS
- See 100+ integrations
- FEATURED INTEGRATIONS
- See more Integrations
- See more CRM Integrations

- See more Storage Integrations
- See more Payment Integrations

- See more Email Integrations
- Jotform Teams
- Enterprise Mobile
- Prefill Forms
- HIPAA Forms
- Secure Forms
- Assign Forms
- Online Payments
- See more features
- Multiple Users
- Admin Console
- White Labeling
- See more Enterprise Features
- Contact Sales
- Contact Support
- Help Center
- The Ultimate Guide to Online Forms NEW
- Jotform Academy
Get a dedicated support team with Jotform Enterprise.
Apply to Jotform Enterprise for a dedicated support team.
- Sign Up for Free
- Research Consent Form
A Research Consent Form is a document used to capture the consent of the participant in the research project. This document is important because it will protect both parties involved in any legal issues related to privacy and content management. This document can be used for any type of research like medical, clinical, scientific, or any similar research type.
This Research Consent Form contains form fields that ask for the participant's name, age, gender, phone number, email, and signature. There are also static contents that display information about the research project like research title, name of organizers, introduction about the research, purpose of research, duration, benefits, confidentiality, and right to refuse. Using the Paragraph tool, you will be able to put static text in your form. This form template also uses the Signature tool to capture the digital signature of the participants and researchers. You can further customize the form by using the Form Builder..
More templates like this

COVID 19 Liability Release Waiver
A COVID-19 Liability Release Waiver is a document that intends to acquire the consent of the client or customer for a liability release waiver. This is a legal document that is intended to reduce the number of unnecessary lawsuits, if not to eliminate them through educating the client or customer about the risks involved in his or her participation in an event or a mere attendance that may lead to injuries or death due to COVID-19 and by which was also caused by ordinary negligence. Having a liability release waiver will help explain to the client or customer the risks involved and therefore can let him or her discern whether he or she is still willing to proceed. By assuming the risks involved, this helps relieve the establishment form any liabilities that may arise. This COVID-19 Liability Release Waiver Template is the quick consent form that you can use for your clients or customers. Using the active consent method, this helps you get the proper consent with the presumption that the person who submitted the form very well understands the risks involved in his or her further participation in the activity that you host or provide. This web form is easy to load through any tablet or mobile device. As a web-based form, you eliminate the waste of printing and waste of physical storage space. It also helps you easily search submitted information using the search tool in the submissions page manager available. With the signature field, your participants can draw their signature in the same manner as how one would sign on a paper document. Get all these features here in Jotform!

Social Media Photo Release Form
A social media photo release form is a contract that must be signed by anyone who wishes to publish photos of others on a social networking website. Use this free Social Media Photo Release Form template to license your photos and images in an instant. Use this Social Media Photo Release Form to capture the permission of your clients to share the photos you've taken on different social media platforms. You can print these materials as well for offline advertising. This will surely increase the number of your clients because they can view your works or portfolio on social media. This form template contains fields that ask for the client's information, release, consent, and digital signature. This form uses the E-signature widget to obtain the client's signature electronically.Personalize a Social Media Photo Release Form by uploading your logo or changing the background image — new from our Form Builder, you can now resize photos and cover images, too! If you’d like to integrate this free Social Media Photo Release Form with your other accounts, use our 100+ integrations to sync your form responses to Google Drive, Dropbox, and more. Or just send in responses to your email address — it’s completely up to you! Make your Social Media Photo Release Form unique with our free templates, easy-to-use Form Builder, and 100+ integrations. Take your license to the next level with your free Social Media Photo Release Form.
Adoption Certificate
If you are managing an adoption agency and looking for ways on how to impress or build good relationships with your clients, then why not try giving them an impressive adoption certificate. An adoption certificate is proof that they have legally adopted a child in your agency. This Adoption Certificate Form will be very useful and helpful in creating an adoption certificate for adoptive parents. It will guide and assist you in creating a simple and elegant adoption certificate for your clients. The form will need information such as applicant details, mother and father’s names, address, phone number, date, and signature.
These templates are suggested forms only. Before using this or any form as a contract or other legal document, please consult with an attorney to make sure it meets the legal needs or your situation. Do not use this form to send a legal request to Jotform.
- Form Templates /
- Consent Forms /
Consent Forms
Informed consent forms.
Employee Laptop Agreement Form
Allow your employees to work from home by providing them laptop to use and have them complete this Employee Laptop Agreement Form. This form contains all the necessary information when borrowing a laptop from the company.

Passenger Disclosure And Attestation To The United States Of America
Follow CDC requirements with this free passenger attestment form for airlines and aircraft operators. Turns form submissions into PDFs automatically. No coding.

COVID 19 Liability Waiver
Receive signed liability waivers and e-signatures online with our free COVID-19 Liability Waiver form. Easy to customize and share. No coding is required.
COVID 19 Vaccine Consent Form
Collect signed COVID-19 vaccine consent forms online. Easy to customize, share, and fill out on any device. Upgrade for HIPAA friendly features. Convert to PDFs instantly.
Medical Consent Forms

Online Medical Consent Form
This excellent Online Medical Consent Form has form fields that ask about the patient information, parent/guardian or emergency contact details, medical data, and the consent waiver. In order to fully acknowledge the consent, this template is using the E-signature widget where the patient can sign digitally.

Procedure Consent Form
A procedure consent form is an official document that informs patients of the risks and benefits of a medical procedure. Simply customize. Easy-to-use. No coding.

Orthodontic Informed Consent Form
An orthodontic informed consent form is used by dental offices to sign up patients for orthodontic procedures and asks for their consent to the treatment terms and conditions.

Blood Donor Consent Form
A blood donor consent form is used by blood banks and other organizations to collect information from potential blood donors. Fully customizable and free.
Travel Consent Forms
Travel Agency Booking Form Template
A travel agency booking form is a service reservation form used by travel agencies to book hotels, flights, or cruise packages. It is a useful tool to improve your hotel or airline booking services. Take your services to the next level!

Employee Travel Authorization Form
Get authorization from your company to travel and attend a conference, seminar, auditing, or inspection by using this Employee Travel Authorization Form. This form can be embedded on ay webpage using the embed code.
Travel Insurance Waiver
A travel insurance waiver is a document used by travelers to waive the coverage of their travel insurance plan. Use our free Travel Insurance Waiver template!

Innovation Center Field Trip Form
An innovation center field trip form is used by a school to sign up students for a trip to the innovation center.
Dental Consent Forms
Dental Treatment Informed Consent Form
The Dental Clinic and the Dentist have the responsibility to educate the patient about the procedure he/she will undergo and thoroughly explain how the patient will benefit from it. This is the goal of the Dental Consent Form. This amazing General Dental Consent Form contains form fields that ask for patient information, details about the dental procedure, and acknowledgment waiver.
Dental Clearance Form
A dental clearance form is a medical form used to obtain permission to make dental impressions from a patient. A dentist uses this form to take an impression of your teeth for future procedures. No coding!

Teledentistry Consent Form
Collect consent from patients online. Perfect for dental practices offering virtual care. Easy to customize and embed. Integrate with 100+ apps. No coding.
Summer Camp Consent Forms
Summer Camp Waiver Form
A summer camp waiver is a document that parents sign to allow their child to attend a summer camp. With our Summer Camp Activity Form Template, you’ll be roasting marshmallows with your campers in no time!
Summer Camp Permission Form
This summer camp permission slip template is a permission or consent form for students or person who are going to camping or outing with classmates, friends and etc. This parental consent form for youth camp is usually given to parents or guardians to be signed by them. This camp consent form template contains parents' contact information, emergency contact information.
Camp Liability Waiver Form
A camp liability waiver form is a document that parents sign to cover the camp’s liability in case something happens to their child during summer camp.
Sports Training Liability Waiver
A liability waiver is a contract signed by a member of a sports team or a coach, which relieves a sports facility from liability if a sports injury occurs on their premises. No coding!
Other Consent Forms
Start collecting your participants' liability release waiver for this pandemic using this COVID-19 Liability Release Waiver Template. Just connect your device to the internet and load your form and start collecting your liability release waiver. Get this here in Jotform!
A social media photo release form is a contract that must be signed by anyone who wishes to publish photos of others on a social networking website.

Eyelash Extension Consent Form
An eyelash extension consent form is used by professional eyelash extensionists to inform their customers of the procedure, equipment they will use, potential risks, and benefits of eyelash extensions.

Tattoo Consent Form
Using this amazing Tattoo Consent Form Template will definitely improve the process of getting consent from the client. No coding is required.

Virtual Assistant Contract Form
This is a sample Virtual Assistant Contract Form that is easy to customize by simply adding related information and can be directly shared with customers.

Professional Counseling Informed Consent Form
A Professional Counseling Informed Consent Form is a form template designed to collect consent from clients and inform them about the risks and limitations involved in professional counseling services

Beauty Salon COVID 19 Liability Waiver
This COVID-19 Liability Waiver is for Salon businesses to ensure their customers' acknowledgment of the possible risks of a salon service during the pandemic and reminds the measures that can be taken to avoid such risks.

COVID 19 Salon Company Consent Form
This COVID-19 Salon Company Consent Form asks your customers to provide their personal information and service details with their acknowledgment of the COVID-19 measures and consent to obey the terms and conditions.

Credit Card Authorization Form
A credit card authorization form is used by small business owners to sign up their customers for a credit card.

Event Photography Contract
An Event Photography Contract is a form template designed to outline an agreement between a photographer and a client for providing photography services at an event.

Identity Verification Form
An identity verification form is a questionnaire used by companies to conduct identity checks on new employees.

Covid 19 Salon Services Consent Form
Free screening form for salons and spas. Prevent further spread of the virus. Easy to customize, embed, and integrate. No coding.

Personal Training Liability Waiver
A personal trainer liability waiver is usually used by personal trainers to protect themselves against potential lawsuits from clients. No coding!

Wedding Videography Contract
The Wedding Videography Contract Form allows gathering customer personal and contact information, wedding date, time and location, intended video package and collects customers' consent for each clause with their e-signature.

Group Therapy Informed Consent Form
A Group Therapy Informed Consent Form is a consent-collection document used by therapists or facilitators in informing their potential clients about the kind of services they provide.

Waxing Consent & Appointment Form
Free template for waxing salons. Book appointments easily. Get e-signatures. Easy to customize and embed. 100+ integrations, including Google Calendar. No coding.

Eyelash Extension Consent & Appointment Form
Schedule eyelash extension appointments. Get signatures online. Free consent and appointment form template. Easy to customize and embed. No coding.

HIPAA Agreement Form Health Care Operations.
With this HIPAA agreement form you can have your patients and users involved in health care operations to read and even sign the form.

Parental Consent And Release Form
Get permission from parents and guardians to let their children participate in your program. Free parental consent form. Easy to customize and embed. No coding required.
About Consent Forms
A consent form is a signed document that outlines the informed consent of an individual for a medical study, clinical trial, or activity. Whether you’re looking for a way to gather model releases, activity waivers , parental consent, or medical consent forms, you can start by selecting one of our 400+ Consent Form Templates. Once you’ve chosen a consent form and customized its terms and conditions, all that’s left to do is embed the form on your website , send the link via email, or let participants fill out the form in person on your tablet or computer — you’ll then be able to securely collect consent forms online! You can even link your custom form to one of our Consent Agreement PDF Templates , to automatically turn form submissions into professional PDF documents .
Not sure where to start? No worries! Just choose a sample consent form from the list below and easily customize it using Jotform’s drag-and-drop Form Builder — no coding or design experience necessary! Outline the responsibilities of all parties involved, add a detailed release of liability, and include any other information necessary to provide indemnity and protect both your company and the individual giving their informed consent. Why not spice up your form by adding your logo and changing the background color too? Your consent form won’t just function well — it’ll look good too.
Make your consent form template even more efficient by adding Jotform’s powerful integrations. Connect with Google Sheets or Airtable to instantly generate a spreadsheet of all form submissions, or link your form to HubSpot, Zoho, or Salesforce (also available on Salesforce AppExchange ) to automatically add participants to your CRM database. By gathering consent forms online with Jotform, you’ll eliminate messy paperwork, streamline your workflow, and save time that could be better spent elsewhere.
Frequently Asked Questions
1) what is a consent form.
Consent forms, also commonly referred to as release forms, are documents designed to inform participants on the details of an event, procedure, or activity. If participants choose to sign a consent form, they’re agreeing to the conditions specified and confirming that they understand the risks, benefits, and rules of their participation.
Health care providers commonly use these forms to ensure they’re compliant with HIPAA regulations and that they secure informed consent for medical procedures, clinical trials, studies, and other activities. HIPAA consent forms typically authorize the provider to send and receive protected patient health information. However, any document that secures approval and consent can be considered a consent or release form.
2) What are the different types of consent forms?
Consent forms vary depending on your industry, audience, and the services you provide. While there are hundreds of different types of specific consent forms, some common use cases include
- Informed consent forms
- Medical consent forms
- Travel consent forms
- Release forms
- Client contracts
- Liability waivers
- Parental consent forms
These consent forms are designed to fit the specific needs of your business or organization. Using a consent form template allows you to customize an existing form to match your branding and services.
3) What’s included in a consent form?
Consent forms vary depending on the circumstances they cover. For example, according to the Department of Health and Human Services Office of Human Research Protections , an informed consent form for medical research must contain the following information:
- Information on why you’re conducting research
- The length of time you’ll expect the individual to participate
- Expected procedures, especially those that are experimental
- Any risks or potential discomfort the participant might experience
- Any benefits the participant can reasonably expect to get from the research
- An explanation of any potential alternative methods or treatments
- A statement on the confidentiality of the information gathered
- Rules on compensation or recovery due to injury
- A list of contacts if participants have questions about the research or their rights or need to report an injury
- A statement on the voluntary nature of participation
While a more general consent form might not include all of these details, it should include a clear, detailed explanation of what the person is agreeing to, along with fields to indicate consent, such as date and signature fields or checkboxes.
4) How do you write a consent form?
Whether you build a consent form from scratch or use a consent form template, your form needs to be comprehensive and easy to understand.
Here are a few tips on how to write the best consent form possible:
- Use language that makes sense to your audience. Be conversational and direct, and avoid industry jargon.
- Write in the second person. Consent forms should directly address participants, using “you,” “your child,” etc.
- Minimize passive voice. Passive language leaves room for interpretation and misunderstanding. Clearly indicate which parties are responsible for specific actions.
Using these tips will keep your businesses protected and help your participants or customers feel more comfortable giving consent.
5) Who uses consent forms?
Consent forms are commonly associated with the healthcare industry. However, any business or organization that requires authorization for an activity can use consent forms.
For example, some other entities that use consent forms include
- Photographers
- Event planners
- Tattoo artists
- Travel agencies
- Beauty salons
- Sports teams
Each of these entities may conduct services or activities that include some type of risk to participants, whether physical or financial, which is why it’s important to ensure participants understand the details of their involvement and indicate that they accept the risk.
Acquiring consent not only reassures your customers by giving them the information they need to make a decision, but it also protects your business from legal action. This makes consent forms an important asset for any business performing a service.
Your account is currently limited to {formLimit} forms.
Go to My Forms and delete an existing form or upgrade your account to increase your form limit.
Sample consent and permission forms
General consent form to participate in research (DOC)
Two stage project consent form (DOC)
Parent permission form for research with child (DOC)
Child assent form (DOC)
Multiple consent form including audio-recording and quotations (DOC)
Photo and video consent form (DOC)
Video-recording consent form (DOC)
Re-contact agreement form (DOC)
Post-debriefing consent form (DOC)
Template participant consent form and participant information sheet
Providing information about the research to participants, and gaining consent from participants before their involvement is a critical part of conducting ethical research.
Participant information sheet
All participants need to be provided access to an information sheet, and to understand the full details of the research, and how they will be involved. Please use the following template:
- Template participant information sheet
Participant consent form
Before research begins, it is important to first obtain participant’s consent on the basis of their full and proven understanding of what the research will entail. Please use the following template:
- Template participant consent form
These templates should be followed as far as possible, as these have been developed using national guidance and expert input from Committee members and Lay Members. However, there may be times when it is appropriate to deviate from the templates in order to meet the needs of a specific research population.
The Health Research Authority and UK Research and Innovation webpages contain further guidance and templates on good practice when consenting participants.
Good practice in consenting participants
You should consider innovative ways of providing consent that are appropriate to your research population, for example, in addition to participant information and consent forms, could you provide the information using visual methods, such as a recorded video, or a study leaflet. Could you develop your forms in partnership with the communities who will take part in the study?
Back to: Research

COMMENTS
Edit, Fill & eSign PDF Documents Online. No Downloads Needed. Get Started Now. Best PDF Fillable Form Builder. Professional Toolset. Quick and Simple. Subscribe for more
Collect consent forms and e-signatures online from patients, parents, or photo subjects. Drag and drop to customize the design. Automatically turn submissions into PDF documents.
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. General Consent Form Templates Social and Behavioral Research Projects (last updated 03/16/2023)
2023-04-10. Assent Form Ages 7-14. 2023-06-27. Consent Addendum for Unencrypted Communication. 2020-10-26. Information or Fact Sheet. 2023-04-10. The following documents are samples. IRBIS does NOT generate these documents with application-specific information.
IC Template - Standard v1.0 Page 1 of 9 version date 10.12.2018 . Standard Informed Consent Template for Research . Use this template if your research is . NOT Federally-sponsored. AND. participants are . adults. Black "we" and "our" (for researcher); not third person (e.g., "we will ask participants"). • Avoid Common Problems ...
Plain Language Consent Template. Use this template for: Biomedical and cancer research. Social, behavioral, and educational research. One-time survey research. Simple blood draw research. Collection and/or storage or biological specimens for research (GWAS compliant) Last Updated October 2023. Companion Document.
Consent Template Exempt Research This consent form is an example, designed specifically for Exempt survey research and is provided purely as a service by the IRB. The IRB does not review or approve the content of exempt consent forms. The consent form should not include any mention of IRB approval and it should not include the standard IRB ...
Consent Templates and Guidance. The templates on this page are intended to help investigators construct documents that are as short as possible and written in plain language. The informed consent form (ICF) templates provided by the IRB comply with federal regulations. What if I only need to provide new study information to a limited number of ...
A step-by-step guide to filling out a general research informed consent form can be found below. Instructions - Use to fill in the blank template. How to Write. Step 1 - Download in PDF, Microsoft Word (.docx), or Open Document Text (.odt). Step 2 - The title of the research study being conducted must be included at the top of the consent ...
The UW IRB provides the UW research community with a variety of consent templates that align with regulatory and policy requirements and best practices as described in our main Consent guidance and guidance on Designing the Consent Process. The first two templates, marked with an asterisk, are the templates most non-exempt studies will choose from.
For multi-centre research studies, a common consent form will be taken as a minimum requirement to which additions may be made as dictated by local circumstances. In such cases, the common consent form ... To assist researchers, WHO has developed Informed Consent Form templates for various types of research studies.
Informed consent is the process of telling potential research participants about the key elements of a research study and what their participation will involve. The informed consent process is one of the central components of the ethical conduct of research with human subjects. The consent process typically includes providing a written consent ...
The title of protocol must match the title on all consent forms. The title must be relevant, appropriate, and easy to understand. Include the project title on all pages of the consent form. List the page numbers on all pages of the consent form in the standard format: Page 1. Delete all colored text from the final copy of your form.
Informed Consent Templates in Research. Here is an example of an informed consent template that can be used in research studies: Title of Study: [Insert Title of Study] Investigator (s): [Insert Name (s) of Investigator (s)] Introduction. You are being invited to participate in a research study.
Template Consent Form (Word) Further Guidance. Guidance on obtaining consent from research participants online (for online and in-person study designs) Authors: Dr Pippa Lally, Behavioural Science and Health, and Jack Hindley, Information Services Division, UCL. Recording & Obtaining Consent;
Sample Informed Consent Form - ©NCPI. The following is a sample consent form for a research project. It is a research project on faculty life on campus, carried out by the principle investigator (PI) of this project from the fake-named Century University. The interviewer (the investigator) should have the interviewee read this form carefully ...
The templates are revised frequently, so please visit this page prior to submitting any protocols to verify that you are using the latest template. The first page of each form provides guidelines on completing the Informed Consent templates. Remember that your research population and proposed research may require special considerations.
Consent and Assent Form Templates. Informed Consent and HIPAA Authorization Form Template (Post 12-4-2023) DOCX: This template should be used as the consent document guide for all new research studies, including parental and LAR permission (consent) forms, submitted for IRB review on or after December 4th, 2023.
These templates are appropriate for social, behavioral, and educational ("SBER") research that does not include any biomedical procedures. Study Information Sheet (no signature) Consent Form (includes signature) Consent Form for Federally Funded Research. Parent Permission Form (parents provide permission for child to participate)
This is a template to assist researchers in the design of their informed consent form. You must adapt this template to the requirements of your particular study, using the notes and suggestions provided. Before using this template, check whether your organisation provides a template consent form and if so, incorporate their requirements into ...
If you have questions or are having trouble accessing these forms, please contact IRB Education ( email or call 650-724-7141). The consent/assent form should be in a language that is understandable to someone without a medical or scientific background. Please use the Microsoft Readability Statistics tool as needed when writing your consent form.
Cloned 2,051. A Research Consent Form is a document used to capture the consent of the participant in the research project. This document is important because it will protect both parties involved in any legal issues related to privacy and content management. This document can be used for any type of research like medical, clinical, scientific ...
Sample consent and permission forms. General consent form to participate in research (DOC) Two stage project consent form (DOC) Parent permission form for research with child (DOC) Child assent form (DOC) Multiple consent form including audio-recording and quotations (DOC) Photo and video consent form (DOC)
Structure and Format. The consent form must begin with a concise and focused presentation the Key Information that a reasonable person would want to know in order to make a decision about whether to participate in research. A separate Key Information section is required for consent documents greater than 2000 words.
Participant consent form. Before research begins, it is important to first obtain participant's consent on the basis of their full and proven understanding of what the research will entail. Please use the following template: Template participant consent form. These templates should be followed as far as possible, as these have been developed ...
The following witness line is to be signed only if the consent is provided as a summary form and accompanied by a short form foreign language consent. Translated short form must be signed and dated by both the participant (or their LAR) AND the witness. Must be signed by the witness AND the Person Obtaining Consent (POC).
AHRQ has created a sample telehealth consent form (Word, 27 KB) that is easy to understand. The form includes language for healthcare providers that have curtailed in-person visits due to COVID-19. AHRQ has also created how-to guidance for clinicians on how to obtain informed consent for telehealth.. Why should we use easy-to-understand telehealth consent forms?
Compensation for Injury: Consent forms are the tool researchers use to share important information about their studies with would-be participants. The aim is to create a document that details the research agenda so that would-be participants can choose for themselves whether they would like to participate. There are a few areas of the consent form that require specific information in order to ...
CONCISE SUMMARY The revised Common Rule requires that consent forms contain a concise presentation of key information. The intention of this section is to provide potential research participants with a better understanding of the project's scope, including major risks and benefits, so they can make a more fully informed decision about whether to participate.