Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 08 June 2023

Alcohol consumption and risks of more than 200 diseases in Chinese men
- Pek Kei Im ORCID: orcid.org/0000-0002-2624-9766 1 ,
- Neil Wright ORCID: orcid.org/0000-0002-3946-1870 1 ,
- Ling Yang ORCID: orcid.org/0000-0001-5750-6588 1 , 2 ,
- Ka Hung Chan ORCID: orcid.org/0000-0002-3700-502X 1 , 3 ,
- Yiping Chen ORCID: orcid.org/0000-0002-4973-0296 1 , 2 ,
- Huaidong Du ORCID: orcid.org/0000-0002-9814-0049 1 , 2 ,
- Xiaoming Yang 1 ,
- Daniel Avery ORCID: orcid.org/0000-0002-9823-9575 1 ,
- Shaojie Wang 5 ,
- Canqing Yu ORCID: orcid.org/0000-0002-0019-0014 6 , 7 ,
- Jun Lv 6 , 7 ,
- Robert Clarke ORCID: orcid.org/0000-0002-9802-8241 1 ,
- Junshi Chen 8 ,
- Rory Collins 1 ,
- Robin G. Walters ORCID: orcid.org/0000-0002-9179-0321 1 , 2 ,
- Richard Peto 1 ,
- Liming Li ORCID: orcid.org/0000-0001-5873-7089 6 , 7 na1 ,
- Zhengming Chen ORCID: orcid.org/0000-0001-6423-105X 1 , 2 na1 ,
- Iona Y. Millwood ORCID: orcid.org/0000-0002-0807-0682 1 , 2 na1 &
China Kadoorie Biobank Collaborative Group
Nature Medicine volume 29 , pages 1476–1486 ( 2023 ) Cite this article
29k Accesses
16 Citations
737 Altmetric
Metrics details
- Epidemiology
- Genetics research
- Risk factors
Alcohol consumption accounts for ~3 million annual deaths worldwide, but uncertainty persists about its relationships with many diseases. We investigated the associations of alcohol consumption with 207 diseases in the 12-year China Kadoorie Biobank of >512,000 adults (41% men), including 168,050 genotyped for ALDH2 - rs671 and ADH1B - rs1229984 , with >1.1 million ICD-10 coded hospitalized events. At baseline, 33% of men drank alcohol regularly. Among men, alcohol intake was positively associated with 61 diseases, including 33 not defined by the World Health Organization as alcohol-related, such as cataract ( n = 2,028; hazard ratio 1.21; 95% confidence interval 1.09–1.33, per 280 g per week) and gout ( n = 402; 1.57, 1.33–1.86). Genotype-predicted mean alcohol intake was positively associated with established ( n = 28,564; 1.14, 1.09–1.20) and new alcohol-associated ( n = 16,138; 1.06, 1.01–1.12) diseases, and with specific diseases such as liver cirrhosis ( n = 499; 2.30, 1.58–3.35), stroke ( n = 12,176; 1.38, 1.27–1.49) and gout ( n = 338; 2.33, 1.49–3.62), but not ischemic heart disease ( n = 8,408; 1.04, 0.94–1.14). Among women, 2% drank alcohol resulting in low power to assess associations of self-reported alcohol intake with disease risks, but genetic findings in women suggested the excess male risks were not due to pleiotropic genotypic effects. Among Chinese men, alcohol consumption increased multiple disease risks, highlighting the need to strengthen preventive measures to reduce alcohol intake.
Similar content being viewed by others

Mendelian randomization evidence for the causal effect of mental well-being on healthy aging

Principal component-based clinical aging clocks identify signatures of healthy aging and targets for clinical intervention

Genome-wide association studies
Alcohol consumption is a major risk factor for poor physical and mental health, accounting for about 3 million deaths and over 130 million disability-adjusted life years worldwide in 2016 (ref. 1 ). Since the 1990s, alcohol consumption has increased in many low- and middle-income countries, including China, where it almost exclusively involves men 2 , 3 . Among Chinese men, those who reported alcohol consumption in the past 12 months increased from 59% to 85% and yearly per-capita alcohol consumption increased from 7.1 to 11.2 l between 1990 and 2017 and these have been predicted to increase in future years 2 .
Previous epidemiological studies conducted in mainly western populations have provided consistent evidence about the hazards of alcohol drinking for several major diseases, including several types of cancers and cardiovascular diseases (CVDs), liver cirrhosis, infectious diseases (for example tuberculosis and pneumonia) and injuries 4 , 5 , 6 , 7 , 8 , 9 . Large western cohort studies with linkage to hospital records have also investigated the associations of alcohol with risks of several less-common or non-fatal disease outcomes (for example certain site-specific cancers 10 , 11 , 12 , dementia 13 , falls 14 and cataract surgery 15 ). For some (for example stomach cancer), there was suggestive evidence of weak positive associations with heavy drinking 10 , 11 , whereas for others (for example cataract) the limited available evidence has been contradictory 10 , 12 , 13 , 15 ; however, the evidence from western populations, even for diseases known to be associated with alcohol, may not be generalizable to Chinese populations, where the prevalence and types of alcohol drinking (mainly spirits), patterns of diseases (for example high stroke rates) and differences in the ability to metabolize alcohol 8 , 9 , 16 differ markedly from those in western populations 4 , 17 .
For many diseases, including those considered by the World Health Organization (WHO) 4 to be alcohol-related (for example ischemic heart disease (IHD) and diabetes), uncertainty remains about the causal relevance of these associations, which can be assessed in genetic studies using a Mendelian randomization (MR) approach 18 . In such studies, genetic variants can be used as instruments for alcohol consumption to investigate the potential causal relevance of alcohol drinking for diseases, which can limit the biases of confounding and reverse causality common in conventional observational studies 18 . Such studies are particularly informative in East Asian populations where two common genetic variants ( ALDH2 - rs671 and ADH1B - rs1229984 ), which are both rare in western populations, greatly alter alcohol metabolism and strongly affect alcohol intake 19 . Several studies have explored the causal relevance of alcohol consumption with CVD risk factors and morbidity 19 , 20 , 21 , 22 and cancer 16 using these genetic variants, yet findings remain inconclusive for certain diseases (for example IHD) and evidence for other diseases is sparse.
To address these questions, we conducted analyses using observational and genetic approaches to evaluate the associations between alcohol consumption and the risks of a wide range of disease outcomes in the prospective China Kadoorie Biobank (CKB).
Among the 512,724 participants (Supplementary Fig. 1 ), the mean age at baseline was 52 (s.d. 10.7) years, 41% were men and 56% lived in rural areas. Among men, 33% reported drinking alcohol regularly (at least once a week) at baseline (current drinkers), consuming on average 286 g of alcohol per week, mainly from spirits (Supplementary Tables 1 and 2 ). Non-drinkers and ex-drinkers were older and more likely to report poor self-rated health or previous chronic diseases, compared to occasional or current drinkers (Table 1 ). Compared to moderate drinkers (<140 g per week), heavier drinkers were more likely to be rural residents, had received lower education and had more unhealthy lifestyle factors (for example smoking and infrequent fresh fruit intake), higher mean blood pressure and longer duration of drinking (Supplementary Table 3 ). Among male current drinkers, 62% reported drinking daily and 37% engaging in heavy episodic drinking (Supplementary Table 2 ). Among women, only 2% drank alcohol at least weekly (mean intake 116 g per week), but there were similar associations with other baseline characteristics (Table 1 and Supplementary Tables 3 and 4 ) compared to those in men.
During a median of 12.1 (interquartile range 11.1–13.1) years of follow-up, 134,641 men (44,027 drinkers) and 198,430 women (4,420 drinkers) experienced at least one reported hospitalization event or death at age-at-risk 35–84 years, involving a total of 1,111,495 hospitalization episodes. Among men, there were 333,541 (107,857 in current drinkers) recorded events from 207 diseases across 17 International Classification of Diseases Tenth Revision (ICD-10) chapters studied that had at least 80 cases each among current drinkers (Table 2 ), while among women there were 476,986 (11,773) events from 48 diseases across 18 ICD-10 chapters (Supplementary Table 5 ).
Observational associations of alcohol with disease risks
Among men, alcohol drinking was significantly associated with higher risks of 61 disease outcomes from 15 ICD-10 chapters based on two separate analyses, (1) comparing ever-regular versus occasional drinkers and (2) dose–response among current drinkers (Table 2 and Extended Data Fig. 1 ). In each of the analyses in men, there were significant associations of alcohol consumption with 42 diseases (or outcomes), of which 23 were significant in both analyses and the remainder were directionally consistent with one exception (transient cerebral ischemic attacks, ICD-10 code G45) (Fig. 1 ). In further analyses covering all alcohol consumption categories, there were typical U-shaped or J-shaped associations, with excess risks in male ex-drinkers and non-drinkers compared to occasional or moderate drinkers for most of these diseases (Supplementary Table 6 ). Among male ex-drinkers, the overall excess morbidity risks were more considerable for alcohol-associated diseases than for other diseases, but these excess risks were lower with increasing duration after stopping drinking (Extended Data Fig. 2 ).

Cox models ( a ) comparing ever-regular drinkers with occasional drinkers or ( b ) assessing the dose–response per 280 g per week higher usual alcohol intake within current drinkers, were stratified by age at risk and study area and were adjusted for education and smoking. Each solid square represents HR with the area inversely proportional to the variance of the log HR. The horizontal lines indicate 95% CIs. Diseases considered to be alcohol-related by the WHO are indicated with ‘W’ under the ‘WHO’ column. The individual diseases listed included all that showed FDR-adjusted significant associations with alcohol (FDR-adjusted P < 0.05, indicated with ‘Y’ under the ‘FDR sig.’ column) and WHO alcohol-related diseases that showed nominally significant associations with alcohol ( P < 0.05). All P values are two-sided. † Included less-common ICD-10 codes within the corresponding ICD-10 chapter that were not individually investigated in the present study. ‘Less-common psychiatric and behavioral conditions’ consisted of ICD-10 codes F00–F99, excluding F32, F33 and F99. ‘Less-common circulatory diseases’ consisted of ICD-10 codes I00–I99, excluding I10, I11, I20, I21, I24, I25, I27, I42, I46, I48–I51, I60–I67, I69, I70, I80 and I83. ‘Less-common injury, poisoning and other external causes’ consisted of ICD-10 codes S00–T98, excluding S06, S09, S22, S32, S42, S52, S62, S72, S82, S92 and T14.
Of the 61 diseases positively associated with alcohol intake in male participants, 28 were considered by the WHO to be alcohol-related diseases, including tuberculosis (A15–A19 and B90), six site-specific cancers including cancers in the larynx (C32), esophagus (C15), liver (C22), colon (C18), rectum (C19 and C20) and lips, oral cavity and pharynx (C00–C14), diabetes (E10–E14), epilepsy (G40 and G41), several hypertensive diseases (I10 and I11) and cerebrovascular diseases (I61, I63, I65, I66, I67, I69 and G45), chronic IHD (I25), cardiomyopathy (I42), pneumonia (J12–J18), alcoholic liver disease (K70) and liver cirrhosis (K74), pancreatitis (K85 and K86) and external causes including self-harm (X60–X84), falls (W00–W19), transport accidents (V01–V99) and other external causes (rest of V–Y) (Fig. 1 and Extended Data Fig. 3 ). Of these 28 diseases, 22 showed significant dose–response associations with alcohol intake. The hazard ratios (HRs) per 280 g per week higher intake for the aggregated WHO alcohol-related diseases were 1.22 (95% confidence interval (CI) 1.19–1.25) (Supplementary Table 7 for detailed outcome classification), ranging from 1.12 (1.05–1.20) for pneumonia to 1.97 (1.80–2.15) for esophageal cancer.
The 33 other diseases showing false discovery rate (FDR)-adjusted significant positive associations with alcohol drinking in men included lung (C34) and stomach (C16) cancers, cataract (H25 and H26), six digestive diseases such as gastroesophageal reflux disease (K21) and gastric ulcer (K25), three musculoskeletal conditions, including gout (M10), three fracture types (S22, S42 and S72), and the aggregates of less-common psychiatric and behavioral conditions and circulatory diseases (Fig. 1 and Extended Data Fig. 4 ). Of these 33 diseases, 22 showed significant dose–response associations, with HRs per 280 g per week higher intake ranging from 1.16 (95% CI 1.04–1.30) for lung cancer to 1.94 (1.43–2.63) for purpura and other hemorrhagic conditions (D69) and 1.20 (1.16–1.24) for the aggregated CKB new alcohol-associated diseases. In contrast, three diseases showed FDR-adjusted significant inverse associations with alcohol drinking (other nontoxic goiter (E04), hyperplasia of prostate (N40) and inguinal hernia (K40)). Overall, for all-cause morbidity, the HR per 280 g per week higher intake was 1.12 (1.10–1.14) in male current drinkers.
Supplementary Figs. 2 – 4 show the dose–response associations for all disease outcomes investigated in male current drinkers. For alcohol-associated diseases and for total morbidity, the dose–response associations were unaltered after additional covariate adjustments or excluding participants with poor baseline health conditions (Supplementary Fig. 5 and Supplementary Table 8 ). Moreover, the associations were similar across various male population subgroups, but seemed to be stronger in younger men, urban residents and higher socioeconomic groups for new alcohol-associated diseases (Supplementary Fig. 6 ).
Among male current drinkers, drinking daily, heavy episodic drinking and drinking spirits were each associated with higher risks for alcohol-related diseases, but most of these associations were attenuated to the null after adjusting for total alcohol intake (Extended Data Fig. 5 ); however, for a given total alcohol intake among male current drinkers, drinking daily was associated with 30–40% higher risks of alcohol-related cancers (1.30, 1.17–1.45) and liver cirrhosis (1.39, 1.13–1.72), compared to non-daily drinking. Similarly, heavy episodic drinking was associated with higher risks of diabetes (1.23, 1.12–1.34) and IHD (1.11, 1.03–1.19), whereas drinking outside of meals was associated with 49% (1.49, 1.19–1.86) higher risk of liver cirrhosis than drinking with meals. The risks of all major alcohol-associated diseases were higher with longer duration of alcohol consumption in men (Extended Data Fig. 6 ).
Among women, due to few reported current drinkers there was a lack of statistical power to detect any associations of self-reported alcohol intake with disease risks (Supplementary Table 5 , Extended Data Fig. 7 and Supplementary Fig. 7 ).
Genetic associations of alcohol with disease risks
A genetic instrument for alcohol intake was derived using ALDH2 - rs671 (G > A) and ADH1B - rs1229984 (G > A) genotypes. The overall A-allele frequency was 0.21 for ALDH2 - rs671 and 0.69 for ADH1B - rs1229984 , with both A-alleles being more common in southern than northern study areas (Supplementary Table 9 ). Both ALDH2 - rs671 and, to a lesser extent, ADH1B - rs1229984 were strongly associated with alcohol drinking in men, but much less so in women (Supplementary Table 10 ). In men, the derived genetic instrument predicted a >60-fold difference (range 4–255 g per week, C1 to C6) in mean alcohol intake, whereas in women mean alcohol intake remained low (<10 g per week) across genetic categories (Supplementary Table 11 ). Both variants and the derived instrument were not associated with smoking or other major self-reported baseline characteristics, except for a small difference in fresh fruit intake by ALDH2 - rs671 genotype in men.
Among men, genotype-predicted mean alcohol intake was positively associated with higher risks of CKB WHO alcohol-related (HR per 280 g per week higher genotype-predicted mean male alcohol intake: 1.14, 95% CI 1.09–1.20) and CKB new alcohol-associated (1.06, 1.01–1.12) diseases (Fig. 2 ), both of which were slightly weaker than the conventional associations. For certain diseases, however, the genetic associations were stronger, with HRs of 1.38 (1.27–1.49) for stroke, 2.30 (1.58–3.35) for liver cirrhosis and 2.33 (1.49–3.62) for gout, in men (Fig. 3 and Extended Data Fig. 8 ). For individual genetic variants, the associations were directionally consistent (Extended Data Figs. 9 and 10 ). Conversely, there were no significant dose–response genotypic associations with IHD, inguinal hernia or hyperplasia of prostate in men. For other alcohol-associated diseases, higher genotype-predicted mean male alcohol intake was significantly associated with higher risks of esophageal cancer, cataract, occlusion and stenosis of cerebral arteries, sequelae of cerebrovascular disease, essential primary hypertension and fractures of ribs, sternum or thoracic spine. There were also suggestive positive genotypic associations with several digestive tract cancer types (liver, colon and stomach) and circulatory and digestive diseases, and significant inverse associations with lung cancer and other chronic obstructive pulmonary disease (J44) in men (Extended Data Figs. 8 – 10 ). Sensitivity analyses using different analytical methods to adjust for confounding by study area, or a two-stage least-squares MR approach, did not alter the main genetic findings in men (Supplementary Table 12 ). In contrast, genotypes that increased alcohol intake in men were not adversely associated with most alcohol-related disease risks among women (for example HR 1.00 (0.97–1.04) for all morbidity among female non-drinkers; Supplementary Fig. 7 and Extended Data Figs. 8 – 10 ).
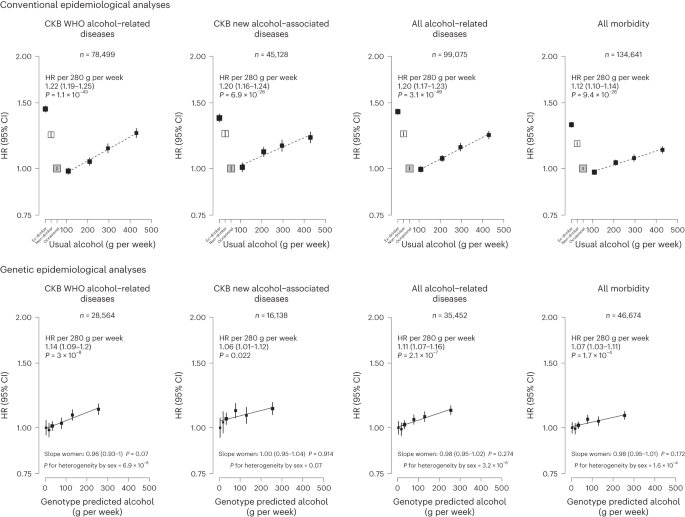
Each box represents HR with the area inversely proportional to the variance of the group-specific log hazard within subplot. The vertical lines indicate group-specific 95% CIs. Conventional epidemiological analyses relate self-reported drinking patterns to risks of diseases (reference group is occasional drinkers), using Cox models stratified by age at risk and study area and adjusted for education and smoking. Within current drinkers, HRs were plotted against usual alcohol intake and were calculated per 280 g per week higher usual alcohol intake. Genetic epidemiological analyses relate genetic categories to risks of diseases (reference group is the genotype group with lowest genotype-predicted mean male alcohol intake), using Cox models stratified by age at risk and study area and adjusted for genomic principal components. The HR per 280 g per week higher genotype-predicted mean male alcohol intake was calculated from the inverse-variance-weighted mean of the slopes of the fitted lines in each study area. The corresponding slopes in women were summarized in text and the slopes of the fitted line by sex were compared and assessed for heterogeneity using chi-squared tests (indicated by P for heterogeneity by sex). All P values are two-sided. Analyses of these aggregated outcomes were based on first recorded event of the aggregate during follow-up and participants may have had multiple events of different types of diseases. ‘All alcohol-related diseases’ includes the first recorded event from ‘CKB WHO alcohol-related diseases’ or ‘CKB new alcohol-associated diseases’ during follow-up.
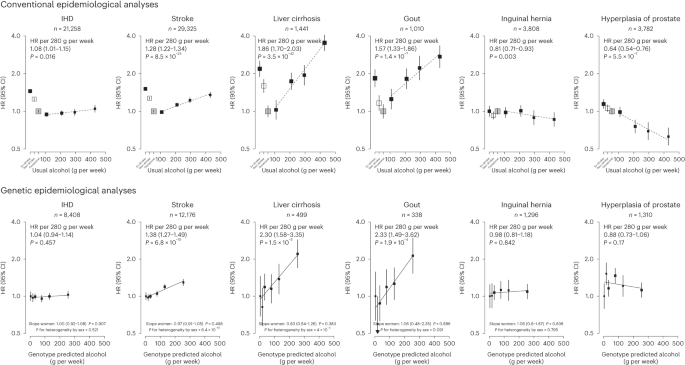
Each box represents HR with the area inversely proportional to the variance of the group-specific log hazard within subplot. The vertical lines indicate group-specific 95% CIs. Conventional epidemiological analyses relate self-reported drinking patterns to risks of diseases (reference group is occasional drinkers), using Cox models stratified by age at risk and study area and adjusted for education and smoking. Within current drinkers, HRs were plotted against usual alcohol intake and were calculated per 280 g per week higher usual alcohol intake. Genetic epidemiological analyses relate genetic categories to risks of diseases (reference group is the genotype group with lowest genotype-predicted mean male alcohol intake), using Cox models stratified by age at risk and study area and adjusted for genomic principal components. The HR per 280 g per week higher genotype-predicted mean male alcohol intake was calculated from the inverse-variance-weighted mean of the slopes of the fitted lines in each study area. The corresponding slopes in women were summarized in text and the slopes of the fitted line by sex were compared and assessed for heterogeneity using chi-squared tests (indicated by P for heterogeneity by sex). All P values are two-sided. Corresponding ICD-10 codes, IHD (I20–I25); stroke (I60, I61, I63 and I64); liver cirrhosis (K70 and K74); gout (M10); inguinal hernia (K40); hyperplasia of prostate (N40).
Hospitalizations associated with alcohol drinking
Among men, ever-regular drinkers had higher numbers of hospitalizations for any causes than occasional drinkers, particularly for cancer hospitalizations, and these differences increased with increasing age at risk, except for CVD hospitalizations (Supplementary Fig. 8 ).
This prospective study provides a comprehensive assessment of the impact of alcohol consumption on a very wide range of disease outcomes in Chinese adults. Among men, alcohol consumption was associated with significantly higher risks of 61 diseases, including 33 not previously reported as alcohol-related diseases by the WHO, and higher risks of hospitalizations for any causes. For a given total amount, drinking daily, heavy episodic drinking and drinking outside of meals exacerbated the risks of four major diseases in Chinese men. Moreover, most of these associations in Chinese men were confirmed in genetic analyses, at least when assessed collectively, and are likely to reflect the effects alcohol consumption itself rather than any pleiotropic effects of the genetic instruments.
Based primarily on observational findings in western populations, alcohol consumption has been considered by the WHO 4 and the Global Burden of Disease (GBD) study 23 to be related to about 20 distinct disease categories, involving chronic diseases and cancers largely in the gastrointestinal system, several CVD types, infectious diseases and injuries. The observational analyses largely confirmed these known associations (Supplementary Table 13 ), but also provided insights into additional hazards of certain drinking patterns suggested by previous studies 8 , 9 , 24 , 25 . Moreover, this study discovered 33 additional alcohol-associated diseases across various body systems in Chinese men that had not been previously reported by the WHO. For these 33 disease outcomes, their associations with alcohol intake were confirmed in genetic analyses, at least collectively as well as for certain specific diseases (for example gout), as was the case for a similar number of WHO alcohol-related diseases. The somewhat smaller relative (but not absolute) risks of alcohol drinking with major diseases at older than younger age in men from observational analyses were consistent with previous studies of other risk factors (for example blood pressure 26 and smoking 27 ), which could be driven by a number of factors such as selection bias 27 and comorbidities.
For certain major WHO alcohol-related diseases, particularly IHD and ischemic stroke, observational studies, including this study, have consistently reported J-shaped associations, with those who drank moderately (for example 1–2 units a day) having the lowest risks 6 , 28 ; however, these apparent protective effects of moderate drinking probably largely reflect residual confounding (for example non-drinkers having worse health and socioeconomic profiles than occasional drinkers) and uncontrolled reverse causation (for example sick-quitter effect where pre-existing poor health or changes in health conditions lead to alcohol cessation), including the difficulty in defining abstainers (for example ex-drinkers may be reported as non-drinkers) as the reference group in many previous studies 3 , 29 . In this study, we used occasional drinkers rather than non-drinkers as the reference group, which, together with separate dose–response analyses among current drinkers, helped to reduce but not eliminate any such biases, which could largely be mitigated in genetic analyses using an MR approach.
To date the existing MR studies for alcohol have focused mainly on CVD types 30 , 31 , 32 and cancers 33 , 34 , 35 , with limited data for other diseases. Moreover, previous studies mainly involved European-ancestry populations and hence were constrained by availability of relatively weak genetic instruments. Using genetic instruments specific to East Asian populations that predicted >60-fold difference in alcohol consumption, we previously reported evidence for the causal relevance and apparent dose–response effects of alcohol consumption on upper-aerodigestive tract cancers 16 and stroke 19 . These findings were further corroborated by subsequent European ancestry-based MR studies 30 , 32 , 36 and the analyses presented in this study with additional follow-up data. In contrast to stroke, we found no reliable genetic evidence for a cardioprotective, nor harmful, effect of moderate drinking on risk of IHD in men, consistent with findings in other MR studies 30 , 32 . The present study also demonstrated a log-linear genetic association of alcohol with liver cirrhosis and suggestive positive associations for several WHO alcohol-related digestive tract cancers in men. Moreover, separate genetic analyses among women suggests that the excess risks observed among men were due chiefly to alcohol per se rather than to potential pleiotropic effects of the alcohol-related genotypes. Further larger genetic studies are required to confirm and elucidate the potential causal relevance for each of the other WHO alcohol-related diseases individually.
For the new alcohol-associated diseases identified in this study, the available prospective epidemiological evidence has been sparse and mostly confined to western populations. For gout, previous western prospective studies have reported positive associations 37 , 38 and an MR study of 8,000 Korean men has also reported positive associations of alcohol consumption with hyperuricemia, a risk factor for gout 39 . The present study provides genetic evidence that alcohol drinking increases the risk of gout. Consistent with the present study, previous European-ancestry-based observational studies 40 , 41 and one MR study 42 also reported positive associations of alcohol intake with risks of several fracture types. The available prospective evidence on associations between alcohol drinking and risk of cataract has been conflicting 15 , 43 and one European-ancestry-based MR study reported no genetic associations 44 . We found a significant dose–response association between alcohol and risk of cataract among Chinese men in observational analyses, which was supported by the present genetic analyses.
For several other diseases (for example gastroesophageal reflux disease and gastric ulcer), the observational findings provide additional evidence to the existing literature 5 , 45 , 46 , 47 , but the supporting genetic evidence is still constrained by limited statistical power. Similarly, our observational findings for lung and stomach cancers were generally consistent with evidence provided by previous prospective studies 7 , 11 , 48 , 49 ; however, the causal relevance of these associations remains to be elucidated in future larger MR studies with appropriate consideration of the potential gene–environment interactions between ALDH2 - rs671 and alcohol intake (the effect of alcohol intake on cancer risks being modified by ALDH2 - rs671 genotype due to excessive acetaldehyde) 16 and other aldehyde exposures 50 in cancer risks, which might similarly affect the genetic associations for respiratory diseases and other potential acetaldehyde-related diseases. In observational analyses, we found significant inverse associations for inguinal hernia, prostate hyperplasia and other nontoxic goiter, but not for several other diseases previously inversely associated with alcohol drinking, including non-Hodgkin lymphoma 48 , kidney cancer 48 , thyroid cancer 48 and gallstones 51 . The genetic analyses, albeit with limited power, did not provide reliable evidence supporting the inverse associations with these outcomes. Future well-powered genetic investigations are warranted for less-common diseases in different populations.
The strengths of this study include the prospective design, large sample size, detailed information on alcohol consumption and drinking patterns, completeness of follow-up and a wide range of morbidity outcomes analyzed. We were also able to assess the potential causal relevance of the associations using two powerful East Asian genetic variants. Moreover, the extremely low drinking prevalence in women (regardless of their genotypes) enabled assessment for potential pleiotropy, further supporting the genetic findings among men.
Nevertheless, the study also has limitations. First, it is still possible that heavy drinking was under-reported, which could have underestimated the hazards of heavy episodic drinking. Second, as in many population-based cohort studies, extreme problematic drinkers and certain alcohol-related disease events may be under-represented, but this should not affect the assessment of the associations of alcohol with most disease outcomes. Third, while the repeated measures of alcohol consumption available in the re-survey subsets allowed us to estimate long-term usual mean alcohol intake at the group level to account for regression dilution bias, we were unable to study the effects of longitudinal alcohol drinking trajectories on health. Fourth, we were unable or underpowered to study diseases that do not normally require hospitalization (for example dementia and depression), nor alcohol-related diseases only affecting women, given the low proportion of female drinkers (for example <70 cases of breast cancer in female drinkers). While the low female drinking prevalence in CKB was consistent with findings in a nationwide survey 52 , it is possible that women may be more likely to under-report drinking than men for cultural and social reasons. Hence our null findings in women should be interpreted with caution and not be taken as a lack of alcohol-related harms in women in general, especially in the context of rising alcohol consumption among Asian women 2 . Fifth, as spirits were the main beverage type and our genetic instrument did not distinguish between beverage types, we were unable to assess beverage-specific effects on disease risks, including wine consumption, which is uncommon in China 17 and has been proposed as potentially cardioprotective due to other non-alcoholic components in red wine 53 . Sixth, although our genetic analyses allowed comparison of the overall genetic effects of negligible, moderate and high mean alcohol intake levels for major and overall morbidities, we had limited power to confidently clarify any small threshold effects in the low consumption end, especially for individual diseases. Finally, the genetic analyses lacked statistical power to assess the associations with several individual alcohol-associated diseases so these findings should still be viewed as hypothesis-generating.
In recent decades, several studies have estimated the alcohol-attributable disease burden, involving predominantly WHO alcohol-related diseases. These estimates were based mainly on observational evidence and included the potentially biased U- or J-shaped associations with IHD and ischemic stroke 1 , 23 , 54 . We have demonstrated in both conventional and genetic analyses that alcohol drinking is associated with hazards in a dose–response manner with a much wider range of disease outcomes than previously considered by the WHO 4 and the GBD study 23 and do not find any evidence for protective effects for IHD or stroke, suggesting that the actual alcohol-attributable disease burden is likely to be much greater than widely believed.
Overall, the present study demonstrated substantial hazards of alcohol consumption with a wide range of disease outcomes among Chinese men. The findings reinforce the need to lower population mean levels of alcohol consumption as a public health priority in China. Future estimation of the alcohol-attributable disease burden worldwide and in specific regions should incorporate new genetic evidence from the present and any future studies about the likely causal relevance of alcohol consumption for a broad range of disease outcomes.
Study population
Details of the CKB study design and methods have been previously reported 55 . Briefly, 512,724 adults aged 30–79 years were recruited from ten geographically diverse (five rural and five urban) areas across China during 2004–2008. At local study assessment clinics, trained health workers administered a laptop-based questionnaire recording sociodemographic factors, lifestyle (for example alcohol drinking, smoking, diet and physical activity) and medical history; undertook physical measurements (for example blood pressure and anthropometry); and collected a blood sample for long-term storage. Two resurveys of ~5% randomly selected surviving participants were subsequently conducted in 2008 and 2013–2014 using similar procedures.
Ethics approval
Ethical approval was obtained from the Ethical Review Committee of the Chinese Centre for Disease Control and Prevention (Beijing, China, 005/2004) and the Oxford Tropical Research Ethics Committee, University of Oxford (UK, 025-04). All participants provided written informed consent.
Assessment of alcohol consumption
Detailed questionnaire assessment of alcohol consumption has been described previously 3 , 17 , 56 . In the baseline questionnaire, participants were asked how often they had drunk alcohol during the past 12 months (never or almost never, occasionally, only at certain seasons, every month but less than weekly or usually at least once a week). Those who had not drunk alcohol at least weekly in the past 12 months were asked whether there was a period of at least a year before that when they had drunk some alcohol at least once a week. Based on their past and current drinking history, participants were classified into: non-drinkers (had never drunk alcohol in the past year and had not drunk in most weeks in the past); ex-drinkers (had not drunk alcohol in most weeks in the past year but had done so in the past); occasional drinkers (had drunk alcohol but less than weekly in the past year and had not drunk alcohol in most weeks in the past); and current drinkers (had drunk alcohol on a weekly basis (regularly) in the past year).
Current drinkers were asked further questions about their drinking patterns, including frequency, beverage type (beer, grape wine, rice wine, weak spirits with <40% alcohol content and strong spirits with ≥40% alcohol content) and amount consumed on a typical drinking day, mealtime drinking habits, age started drinking in most week and their experience of flushing or dizziness after drinking.
Alcohol intake level was estimated based on the reported frequency (taken as the median of the reported frequency intervals; 1.5 for 1–2 d per week, 4 for 3–5 d per week, 6.5 for 6–7 d per week), beverage type and amount consumed, assuming the following alcohol content by volume (v/v) typically seen in China: beer 4%, grape wine 12%, rice wine 15%, weak spirits 38% and strong spirits 53% 57 . Among current drinkers, men were grouped into four consumption categories (<140, 140–279, 280–419 and 420+ g per week) and women into three categories (<70, 70–139 and 140+ g per week), broadly based on the recommended cutoffs for alcohol categories by the WHO 58 and national drinking guidelines. Heavy episodic drinking was defined as consuming >60 g of alcohol on a typical drinking occasion for men and >40 g per occasion for women 58 . Drinking outside of meals was defined as usually drinking between or after meals or having no regular patterns (versus usually drinking with meals). Duration of drinking was derived by the difference in years between age at baseline and age started drinking.
Ex-drinkers were asked how long (in years) ago they had stopped drinking in most weeks. Ex-drinkers were grouped with current drinkers as ‘ever-regular drinkers’.
Follow-up for mortality and morbidity
The vital status of participants was obtained periodically from local death registries, supplemented by annual active confirmation through local residential, health insurance and administrative records. Additional information on morbidity was collected through linkage with disease registries (for cancer, stroke, IHD and diabetes) and the national health insurance system, which record any episodes of hospitalization and almost has universal coverage. All events were coded with ICD-10 codes, blinded to the baseline information. By 1 January 2019, 56,550 (11%) participants had died, 311,338 (61%) were ever hospitalized, but only 4,028 (<1%) were lost to follow-up.
Outcome measures
To enable a ‘phenome-wide’ investigation, all recorded diseases and injuries (referred to as ‘diseases’ for simplicity) coded by three-character ICD-10 codes were reviewed. ICD-10 codes were combined (where appropriate) based on disease characteristics and their potential relationships with alcohol consumption 4 , 8 , 10 , 59 . Disease end points were curated based on diseases considered to be causally impacted by alcohol by the WHO 4 , 59 and major diseases previously shown to be related to alcohol in CKB and other large prospective cohort studies 8 , 10 , while retaining maximal granularity. Diseases with at least 80 cases recorded during follow-up among current drinkers, separately by sex, were analyzed individually to capture a wide range of specific conditions while ensuring reasonable statistical power (around 60–80% power to detect a HR of 2.00 per 280 g per week higher usual alcohol intake at P < 0.01 and P < 0.05, respectively). Within each ICD-10 chapter, diseases with <80 events were grouped into a ‘less-common’ category. Several ICD-10 chapters considered not directly relevant in this population (for example perinatal-origin diseases (chapter XVI) and congenital conditions (XVII); pregnancy-related diseases (XV) in men) were excluded.
Major diseases defined by the WHO as likely to be causally related with alcohol consumption 4 , including several cancers (mouth and throat, esophagus, colon-rectum, liver and female breast), diabetes mellitus, IHD, stroke, liver cirrhosis and external causes, were also selected a priori for detailed analyses of associations with drinking patterns (daily drinking, heavy episodic drinking, mealtime habit, spirit drinking and drinking duration). Similarly, diseases that were significantly and adversely associated with alcohol in the ‘phenome-wide’ investigations (either with ever-regular versus occasional drinking or in dose–response associations with amounts consumed) were further categorized as ‘CKB WHO alcohol-related diseases’ and ‘CKB new alcohol-associated diseases’ respectively for genetic investigation of causality. Detailed outcome classifications are reported in Supplementary Table 7 .
Genotyping and alcohol genetic instruments
The two East Asian genetic variants ( ALDH2 - rs671 and ADH1B - rs1229984 ) were genotyped in 168,050 participants (151,347 randomly selected, 16,703 selected as part of nested case–control studies of CVD and chronic obstructive pulmonary disease, which were only included in analyses of relevant outcomes; Supplementary Fig. 1 ) using Affymetrix Axiom ( n = 100,396) or custom Illumina GoldenGate ( n = 93,125) arrays at BGI (Shenzhen, China), with some overlap between them. Among 25,471 participants genotyped with both arrays, the concordance was >99.9% for both variants. Where discordant, genotypes obtained from the Affymetrix Axiom array were used.
The genetic instrument for alcohol was derived from ALDH2 - rs671 and ADH1B - rs1229984 and ten study areas from the random genotyped subset of male participants to avoid potential selection bias, using a previously developed method in CKB 19 . Briefly, nine genotype combinations were defined based on the genotypes for each of the two variants (each AA, AG or GG). As alcohol use varies greatly by study area, among men, mean alcohol intake was calculated for each of these nine genotype across ten study areas (that is a total of 90 genotype-area combinations) to reflect a wide range of alcohol consumption, assigning an intake of 5 g per week to occasional drinkers and excluding ex-drinkers from the calculation. Ex-drinkers were excluded from the calculation of mean alcohol intake as their baseline intake did not reflect their long-term intake; nevertheless, they were included in subsequent genetic analyses once they had been assigned a genetic group. These 90 combinations were then grouped into six categories (C1–C6) according to their corresponding mean intake values, at cutoff points of 10, 25, 50, 100 and 150 g per week, selected to facilitate investigation of the causal effects of alcohol across a wide range of mean alcohol intakes while allowing adequate sample size in each category for reliable comparisons. In this way participants (including ex-drinkers) were classified only based on their genotypes and study area, but not on individual self-reported drinking patterns. Comparisons of these six genetic categories can, where analyses are stratified by area, be used to estimate the genotypic effects on disease risks.
To facilitate the comparison of genotypic effects between sexes (pleiotropic effects), women were classified into the same six categories as men based on their genotypes and study area, regardless of female alcohol intake. This allowed comparison of genotypic effects between men (where genotype were strongly associated with alcohol intake) and women (where alcohol intake was low in all genotypic categories) (Supplementary Tables 10 and 11 ).
Statistical analysis
Given the extremely low alcohol use among women 3 , 17 , the analyses were conducted separately by sex but focused chiefly on men. All CKB participants and the genotyped subset with genomic principal components (PCs; derived from genome-wide genotyping array data and were informative for CKB population structure) 60 were included in conventional and genetic analyses, respectively (Supplementary Fig. 1 ). Means and percentages of baseline characteristics were calculated by self-reported alcohol consumption patterns and by genotype categories, adjusted for age (in 10-year intervals), ten study areas and (for genetic analysis) genomic PCs 60 to control for differences in genetic distribution due to population stratification, as appropriate.
For conventional observational analyses, Cox proportional hazard models were used to estimate HRs for individual diseases associated with different alcohol consumption categories (in three broad categories: occasional drinkers, ever-regular drinkers, non-drinkers; and in 6–7 detailed categories: occasional drinkers, ex-drinkers, non-drinkers, 3–4 further current drinker groups defined by alcohol intake level) and among current drinkers with continuous levels of alcohol intake (per 280 g per week in men, per 100 g per week in women) or with categories of alcohol intake (<140, 140–279, 280–419 and 420+ g per week in men; <70, 70–139 and 140+ g per week in women). The Cox models were stratified by age at risk (5-year groups between 35–84 years) and ten areas and adjusted for education (four groups: no formal school, primary school, middle or high school and technical school/college or above) and smoking status (six groups in men: never, occasional, ex-regular, current <15, current 15–24, current ≥25 cigarettes equivalent per day; four groups in women: never, occasional, ex-regular and current). Smoking data have been previously validated against exhaled carbon monoxide 61 . Competing risks from all-cause mortality for disease events were handled by censoring participants at death from any cause to estimate cause-specific HRs comparing event rates in participants who were alive and free of the disease of interest 62 . To reduce biases from residual confounding and uncontrolled reverse causation related to the choice of using non-drinkers (for example sick-quitter effect, pre-existing poor health or social disadvantages leading to alcohol cessation or abstinence) as the reference group 3 , 29 , we used occasional drinkers as the reference group, together with separate dose–response analyses among current drinkers. To account for within-person variation of alcohol intake over the follow-up period, repeat alcohol measures for participants who attended the two resurveys were used to estimate usual alcohol intake (Supplementary Table 1 ) and correct for regression dilution bias 9 , 63 . The shapes of dose–response associations between alcohol and disease risks were assessed among current drinkers by plotting the HRs of predefined baseline consumption categories against the corresponding mean usual alcohol intake. Log HR estimates and the corresponding standard errors for baseline alcohol intake, modeled as a continuous variable, were divided by the regression dilution ratio (0.53 for both men and women; calculated using the McMahon–Peto method 64 ) to obtain estimated HRs per 280 g per week higher usual alcohol intake among male current drinkers and HRs per 100 g per week among female current drinkers. For analyses involving drinking patterns, additional adjustments were conducted for total alcohol intake (continuous) and baseline age (continuous; for drinking duration analysis) where appropriate.
Sensitivity analyses were performed by (1) additional adjustments for further covariates (household income (<10,000, 10,000–19,999, 20,000–34,999 and ≥35,000 yuan per year), fresh fruit intake (4–7 d per week and ≤3 d per week), physical activity (continuous, in metabolic equivalent of task per hour per day), body mass index (<22, 22–24.9, 25–26.9 and ≥27 kg m 2 ); and (2) excluding individuals with poor self-reported health or previous major chronic diseases (including self-reported coronary heart diseases, stroke, transient ischemic attack, tuberculosis, emphysema or bronchitis, liver cirrhosis or chronic hepatitis, peptic ulcer, gallstone or gallbladder disease, kidney disease, rheumatoid arthritis, cancer and diabetes) at baseline. For all aggregated end points (for example CKB WHO alcohol-related, CKB new alcohol-associated and all morbidity), subgroup analyses were conducted by baseline age (<55, 55–64 and ≥65 years), area (urban and rural), education (primary school or below, middle school, high school or above), household income (<10,000, 10,000–19,999 and 20,000+ yuan per year) and smoking status (ever-regular and never-regular), with heterogeneity or trend assessed by chi-squared tests 65 . HRs for diseases associated with years of stopping among ex-drinkers compared to occasional drinkers were also estimated.
In genetic analyses, Cox regression, stratified by age at risk and study area and adjusted for 11 genomic PCs 60 , were used to estimate HRs for major alcohol-related diseases associated with the six genetic categories (C1–C6). Log HRs were plotted against the genotype-predicted mean male alcohol intake in the six categories. To control for potential confounding by population structure, similar analyses were repeated within each study area using age-at-risk-stratified and genomic PC-adjusted Cox models. A line of best fit was fitted through the log HRs against genotype-predicted mean male alcohol intake in the genetic categories present in the corresponding study area, using meta-regression. These within-area slopes (each reflecting purely genotypic effects) were combined by inverse-variance-weighted meta-analysis to yield the overall area-stratified genotypic associations, which controlled for any potential bias resulted from variations due to population structure, summarized as HR per 280 g per week higher genotype-predicted mean male alcohol intake. For total morbidity and aggregated alcohol-associated outcomes, sensitivity analyses were performed by (1) using age-at-risk- and area-stratified and genomic PC-adjusted Cox models to estimate HR per 280 g per week (area-adjusted genotypic associations); and (2) using a two-stage least-squares approach 66 .
Genotypic analyses in women were conducted not to assess the health effects of alcohol in women, but to investigate the extent to which the genotypes studied in men had pleiotropic effects (genotypic effects not mediated by drinking patterns). As few women consumed alcohol, any genotypic effects of the six genetic categories that are mediated by drinking alcohol should be much smaller in women than in men, but any other pleiotropic genotypic effects should be similar in both sexes. Hence, among women, we used the same genetic categories as in men and related the genotypic effects in women to the mean male alcohol intake in these six categories, which allows comparisons of genetic findings by sex and assessment of potential pleiotropy. To further remove the small genotypic effects on alcohol use in women (Supplementary Tables 10 and 11 ), we restricted the genetic analyses to female non-drinkers in sensitivity analyses.
The genotypic associations of individual genetic variants ( rs671 , rs1229984 ; GG versus AG genotype) with alcohol-related disease risks were also assessed using a similar area-stratified approach.
The proportional hazards assumption was tested using scaled Schoenfeld residuals for the pre-specified major diseases (no clear evidence of violation was found). For analyses involving more than two exposure categories, the floating absolute risks were used to estimate group-specific 95% CIs for all categories including the reference group 9 , 19 , 67 . All P values were two-sided. Statistical significance (at the 5% level) was evaluated using both FDR-adjusted P values applied within ICD-10 chapters to correct for multiple testing in the ‘phenome-wide’ investigation 68 , 69 , 70 and conventional P values for hypothesis testing for observational analyses of WHO alcohol-related diseases, analyses of drinking patterns and genetic analyses.
To assess the cumulative burden of alcohol consumption, the total number of hospitalizations were estimated for ever-regular versus occasional drinkers using the mean cumulative count, which does not assume independence between hospitalizations and all-cause mortality 71 , 72 , 73 . All analyses used R software (v.4.0.5).
Ethics and inclusion statement
In accordance with the Nature Portfolio journals’ editorial policies, the research has included local researchers from China throughout the research process, including study design, study implementation, data ownership and authorship. The roles and responsibilities were agreed among collaborators ahead of the research and capacity-building plans, including data collection and study implementation skills for local researchers, were discussed and delivered. This research is locally relevant to the studied country and included local collaborative partners in all aspects of the study, thus, will provide local and regional organizations with epidemiological evidence on the health impacts of alcohol consumption to inform public health policies.
This research was not restricted nor prohibited in the setting of the researchers. The study was approved by local ethics review committee. The research raised no risks related to stigmatization, incrimination, discrimination, animal welfare, the environment, health, safety, security or other personal or biorisks. No biological materials, cultural artifacts or associated traditional knowledge has been transferred out of the country. In preparing the manuscript, the authors have reviewed and cited local and regional relevant studies.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The CKB is a global resource for the investigation of lifestyle, environmental, blood biochemical and genetic factors as determinants of common diseases. The CKB study group is committed to making the cohort data available to the scientific community in China, the United Kingdom and worldwide to advance knowledge about the causes, prevention and treatment of disease. For detailed information on what data are currently available to open access users, how to apply for them and the timeline for data access (12–16 weeks), please visit the CKB website: https://www.ckbiobank.org/data-access . Researchers who are interested in obtaining the raw data from the CKB study that underlines this paper should contact [email protected]. A research proposal will be requested to ensure that any analysis is performed by bona fide researchers and, where data are not currently available to open access researchers, is restricted to the topic covered in this paper. Further information is available from the corresponding authors upon request.
Code availability
The codes used for the data analyses in this study can be made available by contacting the corresponding authors. Access to codes will be granted for requests for academic use within 4 weeks of application.
Shield, K. et al. National, regional, and global burdens of disease from 2000 to 2016 attributable to alcohol use: a comparative risk assessment study. Lancet Public Health 5 , e51–e61 (2020).
PubMed Google Scholar
Manthey, J., Shield, K. D., Rylett, M., Hasan, O. S. M., Probst, C. & Rehm, J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet 393 , 2493–2502 (2019).
Im, P. K. et al. Patterns and trends of alcohol consumption in rural and urban areas of China: findings from the China Kadoorie Biobank. BMC Public Health 19 , 217 (2019).
PubMed PubMed Central Google Scholar
World Health Organization. Global Status Report on Alcohol and Health 2018 (World Health Organization, 2018).
Corrao, G., Bagnardi, V., Zambon, A., La & Vecchia, C. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev. Med. 38 , 613–619 (2004).
Ferrari, P. et al. Lifetime alcohol use and overall and cause-specific mortality in the European Prospective Investigation into Cancer and nutrition (EPIC) study. BMJ Open 4 , e005245 (2014).
Yang, L. et al. Alcohol drinking and overall and cause-specific mortality in China: nationally representative prospective study of 220,000 men with 15 years of follow-up. Int. J. Epidemiol. 41 , 1101–1113 (2012).
Im, P. K. et al. Alcohol drinking and risks of total and site-specific cancers in China: a 10-year prospective study of 0.5 million adults. Int J. Cancer 149 , 522–534 (2021).
CAS PubMed PubMed Central Google Scholar
Im, P. K. et al. Alcohol drinking and risks of liver cancer and non-neoplastic chronic liver diseases in China: a 10-year prospective study of 0.5 million adults. BMC Med. 19 , 216 (2021).
Allen, N. E. et al. Moderate alcohol intake and cancer incidence in women. J. Natl Cancer Inst. 101 , 296–305 (2009).
Jayasekara, H. et al. Lifetime alcohol intake, drinking patterns over time and risk of stomach cancer: a pooled analysis of data from two prospective cohort studies. Int J. Cancer 148 , 2759–2773 (2021).
Botteri, E. et al. Alcohol consumption and risk of urothelial cell bladder cancer in the European prospective investigation into cancer and nutrition cohort. Int J. Cancer 141 , 1963–1970 (2017).
CAS PubMed Google Scholar
Sabia, S. et al. Alcohol consumption and risk of dementia: 23 year follow-up of Whitehall II cohort study. BMJ 362 , k2927 (2018).
Tan, G. J. al. The relationship between alcohol intake and falls hospitalization: results from the EPIC-Norfolk. Geriatr. Gerontol. Int. https://doi.org/10.1111/ggi.14219 (2021).
Chua, S. Y. L. et al. Alcohol consumption and incident cataract surgery in two large UK cohorts. Ophthalmology https://doi.org/10.1016/j.ophtha.2021.02.007 (2021).
Im, P. K. et al. Alcohol metabolism genes and risks of site-specific cancers in Chinese adults: an 11-year prospective study. Int. J. Cancer 150 , 1627–1639 (2022).
Millwood, I. Y. et al. Alcohol consumption in 0.5 million people from 10 diverse regions of China: prevalence, patterns and socio-demographic and health-related correlates. Int. J. Epidemiol. 42 , 816–827 (2013).
Davey Smith, G. & Hemani, G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 23 , R89–R98 (2014).
Millwood, I. Y. et al. Conventional and genetic evidence on alcohol and vascular disease aetiology: a prospective study of 500 000 men and women in China. Lancet 393 , 1831–1842 (2019).
Au Yeung, S. L. et al. Moderate alcohol use and cardiovascular disease from Mendelian randomization. PLoS ONE 8 , e68054 (2013).
Shin, M. J., Cho, Y., Davey & Smith, G. Alcohol consumption, aldehyde dehydrogenase 2 gene polymorphisms, and cardiovascular health in Korea. Yonsei Med J. 58 , 689–696 (2017).
Taylor, A. E. et al. Exploring causal associations of alcohol with cardiovascular and metabolic risk factors in a Chinese population using Mendelian randomization analysis. Sci. Rep. 5 , 14005 (2015).
Griswold, M. G. et al. Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 392 , 1015–1035 (2018).
Google Scholar
Simpson, R. F. et al. Alcohol drinking patterns and liver cirrhosis risk: analysis of the prospective UK Million Women Study. Lancet Public Health 4 , e41–e48 (2019).
Roerecke, M. & Rehm, J. Alcohol consumption, drinking patterns, and ischemic heart disease: a narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med. 12 , 182 (2014).
Lacey, B. et al. Age-specific association between blood pressure and vascular and non-vascular chronic diseases in 0.5 million adults in China: a prospective cohort study. Lancet Glob. Health 6 , e641–e649 (2018).
Hernán, M. A., Alonso, A. & Logroscino, G. Cigarette smoking and dementia: potential selection bias in the elderly. Epidemiology 19 , 448–450 (2008).
Rehm, J., Rovira, P., Llamosas-Falcón, L. & Shield, K. D. Dose-response relationships between levels of alcohol use and risks of mortality or disease, for all people, by age, sex, and specific risk factors. Nutrients 13 , 2652 (2021).
Rehm, J., Irving, H., Ye, Y., Kerr, W. C., Bond, J. & Greenfield, T. K. Are lifetime abstainers the best control group in alcohol epidemiology? On the stability and validity of reported lifetime abstention. Am. J. Epidemiol. 168 , 866–871 (2008).
Lankester, J., Zanetti, D., Ingelsson, E. & Assimes, T. L. Alcohol use and cardiometabolic risk in the UK Biobank: a Mendelian randomization study. PLoS ONE 16 , e0255801 (2021).
Rosoff, D. B., Davey Smith, G., Mehta, N., Clarke, T.-K. & Lohoff, F. W. Evaluating the relationship between alcohol consumption, tobacco use, and cardiovascular disease: a multivariable Mendelian randomization study. PLoS Med. 17 , e1003410 (2020).
Larsson, S. C., Burgess, S., Mason, A. M. & Michaelsson, K. Alcohol consumption and cardiovascular disease: a Mendelian randomization study. Circ. Genom. Precis. Med. 13 , e002814 (2020).
Larsson, S. C. et al. Smoking, alcohol consumption, and cancer: a mendelian randomisation study in UK Biobank and international genetic consortia participants. PLoS Med. 17 , e1003178 (2020).
Zhou, X. et al. Alcohol consumption, DNA methylation and colorectal cancer risk: results from pooled cohort studies and Mendelian randomization analysis. Int. J. Cancer https://doi.org/10.1002/ijc.33945 (2022).
Zhou, X. et al. Alcohol consumption, blood DNA methylation and breast cancer: a Mendelian randomisation study. Eur. J. Epidemiol. 37 , 701–712 (2022).
Biddinger, K. J. et al. Association of habitual alcohol intake with risk of cardiovascular disease. JAMA Netw. Open 5 , e223849 (2022).
Wang, M., Jiang, X., Wu, W. & Zhang, D. A meta-analysis of alcohol consumption and the risk of gout. Clin. Rheumatol. 32 , 1641–1648 (2013).
Neogi, T., Chen, C., Niu, J., Chaisson, C., Hunter, D. J. & Zhang, Y. Alcohol quantity and type on risk of recurrent gout attacks: an internet-based case-crossover study. Am. J. Med 127 , 311–318 (2014).
Jee, Y. H., Jung, K. J., Park, Y. B., Spiller, W. & Jee, S. H. Causal effect of alcohol consumption on hyperuricemia using a Mendelian randomization design. Int J. Rheum. Dis. 22 , 1912–1919 (2019).
Berg, K. M. et al. Association between alcohol consumption and both osteoporotic fracture and bone density. Am. J. Med. 121 , 406–418 (2008).
Søgaard, A. J. et al. The association between alcohol consumption and risk of hip fracture differs by age and gender in Cohort of Norway: a NOREPOS study. Osteoporos. Int. 29 , 2457–2467 (2018).
Yuan, S., Michaëlsson, K., Wan, Z. & Larsson, S. C. Associations of smoking and alcohol and coffee intake with fracture and bone mineral density: a Mendelian randomization study. Calcif. Tissue Int. 105 , 582–588 (2019).
Gong, Y., Feng, K., Yan, N., Xu, Y. & Pan, C.-W. Different amounts of alcohol consumption and cataract: a meta-analysis. Optom. Vis. Sci. 92 , 471–479 (2015).
Yuan, S., Wolk, A. & Larsson, S. C. Metabolic and lifestyle factors in relation to senile cataract: a Mendelian randomization study. Sci. Rep. 12 , 409 (2022).
Pan, J., Cen, L., Chen, W., Yu, C., Li, Y. & Shen, Z. Alcohol consumption and the risk of gastroesophageal reflux disease: a systematic review and meta-analysis. Alcohol Alcohol. 54 , 62–69 (2019).
Strate, L. L., Singh, P., Boylan, M. R., Piawah, S., Cao, Y. & Chan, A. T. A prospective study of alcohol consumption and smoking and the risk of major gastrointestinal bleeding in men. PLoS ONE 11 , e0165278 (2016).
Yuan, S. & Larsson, S. C. Adiposity, diabetes, lifestyle factors and risk of gastroesophageal reflux disease: a Mendelian randomization study. Eur. J. Epidemiol. 37 , 747–754 (2022).
Bagnardi, V. et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose–response meta-analysis. Br. J. Cancer 112 , 580–593 (2015).
Shen, C., Schooling, C. M., Chan, W. M., Xu, L., Lee, S. Y. & Lam, T. H. Alcohol intake and death from cancer in a prospective Chinese elderly cohort study in Hong Kong. J. Epidemiol. Community Health 67 , 813–820 (2013).
Kuroda, A. et al. Effects of the common polymorphism in the human aldehyde dehydrogenase 2 (ALDH2) gene on the lung. Respir. Res. 18 , 69 (2017).
Cha, B. H., Jang, M. J. & Lee, S. H. Alcohol consumption can reduce the risk of gallstone disease: a systematic review with a dose–response meta-analysis of case–control and cohort studies. Gut Liver 13 , 114–131 (2019).
Ma, G. S., Xhu, D. H., Hu, X. Q., Luan, D. C., Kong, L. Z. & Yang, X. Q. The drinking practice of people in China. Acta Nutrimenta Sin. 27 , 362–365 (2005).
Arranz, S., Chiva-Blanch, G., Valderas-Martínez, P., Medina-Remón, A., Lamuela-Raventós, R. M. & Estruch, R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients 4 , 759–781 (2012).
Bryazka, D. et al. Population-level risks of alcohol consumption by amount, geography, age, sex, and year: a systematic analysis for the Global Burden of Disease Study 2020. Lancet 400 , 185–235 (2022).
Chen, Z. et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int. J. Epidemiol. 40 , 1652–1666 (2011).
Im, P. K. et al. Problem drinking, wellbeing and mortality risk in Chinese men: findings from the China Kadoorie Biobank. Addiction 115 , 850–862 (2020).
Cochrane, J., Chen, H., Conigrave, K. M. & Hao, W. Alcohol use in China. Alcohol Alcohol. 38 , 537–542 (2003).
World Health Organization. International Guide for Monitoring Alcohol Consumption and Related Harm (World Health Organization, 2000).
Rehm, J. et al. The relationship between different dimensions of alcohol use and the burden of disease-an update. Addiction 112 , 968–1001 (2017).
Walters, R. G. et al. Genotyping and population structure of the China Kadoorie Biobank. Preprint at medRxiv https://doi.org/10.1101/2022.05.02.22274487 (2022).
Zhang, Q. et al. Exhaled carbon monoxide and its associations with smoking, indoor household air pollution and chronic respiratory diseases among 512,000 Chinese adults. Int. J. Epidemiol. 42 , 1464–1475 (2013).
Lau, B., Cole, S. R. & Gange, S. J. Competing risk regression models for epidemiologic data. Am. J. Epidemiol. 170 , 244–256 (2009).
Clarke, R., Shipley, M. & Lewington, S. et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am. J. Epidemiol. 150 , 341–353 (1999).
MacMahon, S., Peto, R. & Cutler, J. et al. Blood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 335 , 765–774 (1990).
Early Breast Cancer Trialists’ Collaborative Group. Section 5: Statistical Methods. Treatment of Early Breast Cancer. Worldwide Evidence, 1985–1990 (Oxford University Press, 1990).
Burgess, S. et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 4 , 186 (2019).
Easton, D. F., Peto, J. & Babiker, A. G. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat. Med. 10 , 1025–1035 (1991).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57 , 289–300 (1995).
Braun, J. M., Kalloo, G., Kingsley, S. L. & Li, N. Using phenome-wide association studies to examine the effect of environmental exposures on human health. Environ. Int. 130 , 104877 (2019).
Glickman, M. E., Rao, S. R. & Schultz, M. R. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J. Clin. Epidemiol. 67 , 850–857 (2014).
Cook, R. J. & Lawless, J. F. Marginal analysis of recurrent events and a terminating event. Stat. Med. 16 , 911–924 (1997).
Dong, H., Robison, L. L., Leisenring, W. M., Martin, L. J., Armstrong, G. T. & Yasui, Y. Estimating the burden of recurrent events in the presence of competing risks: the method of mean cumulative count. Am. J. Epidemiol. 181 , 532–540 (2015).
Ghosh, D. & Lin, D. Y. Nonparametric analysis of recurrent events and death. Biometrics 56 , 554–562 (2000).
Download references
Acknowledgements
The chief acknowledgment is to the participants, the project staff and the China National Centre for Disease Control and Prevention (CDC) and its regional offices for assisting with the fieldwork. We thank J. Mackay in Hong Kong; Y. Wang, G. Yang, Z. Qiang, L. Feng, M. Zhou, W. Zhao. and Y. Zhang in China CDC; L. Kong, X. Yu and K. Li in the Chinese Ministry of Health; and S. Clark, M. Radley and M. Hill in the CTSU, Oxford, for assisting with the design, planning, organization and conduct of the study. A complete list of members of the China Kadoorie Collaborative Group is provided in the Supplementary Information. The CKB baseline survey and the first re-survey were supported by the Kadoorie Charitable Foundation in Hong Kong. The long-term follow-up of the CKB study has been supported by Wellcome grants to Z.C. at Oxford University (212946/Z/18/Z, 202922/Z/16/Z, 104085/Z/14/Z, 088158/Z/09/Z) and grants to L.L. from the National Natural Science Foundation of China (82192901, 82192904 and 82192900) and from the National Key Research and Development Program of China (2016YFC0900500). DNA extraction and genotyping was supported by grants to Z.C. from GlaxoSmithKline and the UK Medical Research Council (MC-PC-13049, MC-PC-14135). The UK Medical Research Council (MC_UU_00017/1, MC_UU_12026/2, MC_U137686851), Cancer Research UK (C16077/A29186; C500/A16896) and the British Heart Foundation (CH/1996001/9454) provide core funding to the CTSU and Epidemiological Studies Unit at Oxford University for the project. P.K.I. is supported by an Early Career Research Fellowship from the Nuffield Department of Population Health, University of Oxford. K.H.C. acknowledges support from the British Heart Foundation Centre of Research Excellence, University of Oxford (RE/18/3/34214). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. For the purpose of Open Access, the author has applied a CC-BY public copyright license to any author accepted manuscript version arising from this submission.
Author information
These authors jointly supervised this work: Liming Li, Zhengming Chen, Iona Y. Millwood.
Authors and Affiliations
Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, University of Oxford, Oxford, UK
Pek Kei Im, Neil Wright, Ling Yang, Ka Hung Chan, Yiping Chen, Huaidong Du, Xiaoming Yang, Daniel Avery, Robert Clarke, Rory Collins, Robin G. Walters, Richard Peto, Zhengming Chen, Iona Y. Millwood, Maxim Barnard, Derrick Bennett, Ruth Boxall, Johnathan Clarke, Ahmed Edris Mohamed, Hannah Fry, Simon Gilbert, Andri Iona, Maria Kakkoura, Christiana Kartsonaki, Hubert Lam, Kuang Lin, James Liu, Mohsen Mazidi, Sam Morris, Qunhua Nie, Alfred Pozarickij, Paul Ryder, Saredo Said, Dan Schmidt, Becky Stevens, Iain Turnbull, Baihan Wang, Lin Wang & Pang Yao
Medical Research Council Population Health Research Unit (MRC PHRU), Nuffield Department of Population Health, University of Oxford, Oxford, UK
Ling Yang, Yiping Chen, Huaidong Du, Robin G. Walters, Zhengming Chen, Iona Y. Millwood, Derrick Bennett, Ruth Boxall & Christiana Kartsonaki
Oxford British Heart Foundation Centre of Research Excellence, University of Oxford, Oxford, UK
Ka Hung Chan
Fuwai Hospital, Chinese Academy of Medical Sciences, Beijing, China
NCD Prevention and Control Department, Qingdao CDC, Qingdao, China
Shaojie Wang, Liang Cheng, Ranran Du, Ruqin Gao, Feifei Li, Shanpeng Li, Yongmei Liu, Feng Ning, Zengchang Pang, Xiaohui Sun, Xiaocao Tian, Yaoming Zhai & Hua Zhang
Department of Epidemiology and Biostatistics, School of Public Health, Peking University, Beijing, China
Canqing Yu, Jun Lv, Liming Li & Dianjianyi Sun
Peking University Center for Public Health and Epidemic Preparedness & Response, Beijing, China
Canqing Yu, Jun Lv, Liming Li, Xiao Han, Can Hou, Qingmei Xia, Chao Liu, Pei Pei & Dianjianyi Sun
China National Center for Food Safety Risk Assessment, Beijing, China
- Junshi Chen
WHO Collaborating Center for Tobacco Cessation and Respiratory Diseases Prevention, China-Japan Friendship Hospital, Beijing, China
Institute of Respiratory Medicine, Chinese Academy of Medical Sciences, Beijing, China
NCD Prevention and Control Department, Guangxi Provincial CDC, Nanning, China
Naying Chen, Duo Liu & Zhenzhu Tang
NCD Prevention and Control Department, Liuzhou CDC, Liubei, Liuzhou, China
Ningyu Chen, Qilian Jiang, Jian Lan, Mingqiang Li, Yun Liu, Fanwen Meng, Jinhuai Meng, Rong Pan, Yulu Qin, Ping Wang, Sisi Wang, Liuping Wei & Liyuan Zhou
NCD Prevention and Control Department, Gansu Provincial CDC, Lanzhou, China
Caixia Dong, Pengfei Ge & Xiaolan Ren
NCD Prevention and Control Department, Maijixiang CDC, Maijixiang, Tianshui, China
Zhongxiao Li, Enke Mao, Tao Wang, Hui Zhang & Xi Zhang
NCD Prevention and Control Department, Hainan Provincial CDC, Haikou, China
Jinyan Chen, Ximin Hu & Xiaohuan Wang
NCD Prevention and Control Department, Meilan CDC, Meilan, Haikou, China
Zhendong Guo, Huimei Li, Yilei Li, Min Weng & Shukuan Wu
NCD Prevention and Control Department, Heilongjiang CDC, Harbin, China
Shichun Yan, Mingyuan Zou & Xue Zhou
NCD Prevention and Control Department, Nangang CDC, Harbin, China
Ziyan Guo, Quan Kang, Yanjie Li, Bo Yu & Qinai Xu
NCD Prevention and Control Department, Henan Provincial CDC, Zhengzhou, China
Liang Chang, Lei Fan, Shixian Feng, Ding Zhang & Gang Zhou
NCD Prevention and Control Department, Huixian CDC, Huixian, China
Yulian Gao, Tianyou He, Pan He, Chen Hu, Huarong Sun & Xukui Zhang
NCD Prevention and Control Department, Hunan Provincial CDC, Changsha, China
Biyun Chen, Zhongxi Fu, Yuelong Huang, Huilin Liu, Qiaohua Xu & Li Yin
NCD Prevention and Control Department, Liuyang CDC, Liuyang, China
Huajun Long, Xin Xu, Hao Zhang & Libo Zhang
NCD Prevention and Control Department, Jiangsu Provincial CDC, Nanjing, China
Jian Su, Ran Tao, Ming Wu, Jie Yang, Jinyi Zhou & Yonglin Zhou
NCD Prevention and Control Department, Wuzhong CDC, Wuzhong, Suzhou, China
Yihe Hu, Yujie Hua, Jianrong Jin, Fang Liu, Jingchao Liu, Yan Lu, Liangcai Ma, Aiyu Tang & Jun Zhang
NCD Prevention and Control Department, Licang CDC, Qingdao, China
Wei Hou, Silu Lv & Junzheng Wang
NCD Prevention and Control Department, Sichuan Provincial CDC, Chengdu, China
Xiaofang Chen, Xianping Wu, Ningmei Zhang & Xiaoyu Chang
NCD Prevention and Control Department, Pengzhou CDC, Pengzhou, Chengdu, China
Xiaofang Chen, Jianguo Li, Jiaqiu Liu, Guojin Luo, Qiang Sun & Xunfu Zhong
NCD Prevention and Control Department, Zhejiang Provincial CDC, Hangzhou, China
Weiwei Gong, Ruying Hu, Hao Wang, Meng Wang & Min Yu
NCD Prevention and Control Department, Tongxiang CDC, Tongxiang, China
Lingli Chen, Qijun Gu, Dongxia Pan, Chunmei Wang, Kaixu Xie & Xiaoyi Zhang
You can also search for this author in PubMed Google Scholar
- , Zhengming Chen
- , Robert Clarke
- , Rory Collins
- , Liming Li
- , Chen Wang
- , Richard Peto
- , Robin G. Walters
- , Daniel Avery
- , Maxim Barnard
- , Derrick Bennett
- , Ruth Boxall
- , Ka Hung Chan
- , Yiping Chen
- , Johnathan Clarke
- , Huaidong Du
- , Ahmed Edris Mohamed
- , Hannah Fry
- , Simon Gilbert
- , Pek Kei Im
- , Andri Iona
- , Maria Kakkoura
- , Christiana Kartsonaki
- , Hubert Lam
- , Kuang Lin
- , James Liu
- , Mohsen Mazidi
- , Iona Y. Millwood
- , Sam Morris
- , Qunhua Nie
- , Alfred Pozarickij
- , Paul Ryder
- , Saredo Said
- , Dan Schmidt
- , Becky Stevens
- , Iain Turnbull
- , Baihan Wang
- , Neil Wright
- , Ling Yang
- , Xiaoming Yang
- , Qingmei Xia
- , Dianjianyi Sun
- , Canqing Yu
- , Naying Chen
- , Zhenzhu Tang
- , Ningyu Chen
- , Qilian Jiang
- , Mingqiang Li
- , Fanwen Meng
- , Jinhuai Meng
- , Ping Wang
- , Sisi Wang
- , Liuping Wei
- , Liyuan Zhou
- , Caixia Dong
- , Pengfei Ge
- , Xiaolan Ren
- , Zhongxiao Li
- , Hui Zhang
- , Jinyan Chen
- , Xiaohuan Wang
- , Zhendong Guo
- , Huimei Li
- , Shukuan Wu
- , Shichun Yan
- , Mingyuan Zou
- , Ziyan Guo
- , Quan Kang
- , Yanjie Li
- , Liang Chang
- , Shixian Feng
- , Ding Zhang
- , Gang Zhou
- , Yulian Gao
- , Tianyou He
- , Huarong Sun
- , Xukui Zhang
- , Biyun Chen
- , Zhongxi Fu
- , Yuelong Huang
- , Huilin Liu
- , Qiaohua Xu
- , Huajun Long
- , Hao Zhang
- , Libo Zhang
- , Jinyi Zhou
- , Yonglin Zhou
- , Yujie Hua
- , Jianrong Jin
- , Jingchao Liu
- , Liangcai Ma
- , Aiyu Tang
- , Jun Zhang
- , Liang Cheng
- , Ranran Du
- , Ruqin Gao
- , Feifei Li
- , Shanpeng Li
- , Yongmei Liu
- , Feng Ning
- , Zengchang Pang
- , Xiaohui Sun
- , Xiaocao Tian
- , Shaojie Wang
- , Yaoming Zhai
- , Hua Zhang
- , Junzheng Wang
- , Xiaofang Chen
- , Xianping Wu
- , Ningmei Zhang
- , Xiaoyu Chang
- , Jianguo Li
- , Jiaqiu Liu
- , Guojin Luo
- , Qiang Sun
- , Xunfu Zhong
- , Weiwei Gong
- , Ruying Hu
- , Meng Wang
- , Lingli Chen
- , Dongxia Pan
- , Chunmei Wang
- , Kaixu Xie
- & Xiaoyi Zhang
Contributions
P.K.I., I.Y.M., L.Y. and Z.C. contributed to the conception of this paper. P.K.I., N.W., K.H.C., I.Y.M. and Z.C. planned the statistical analysis. P.K.I. analyzed the data and drafted the manuscript. P.K.I., I.Y.M. and Z.C. contributed to the interpretation of the results and the revision of manuscript. R. Collins, R.P., J.C., L.L. and Z.C. designed the study. L.L., Z.C., I.Y.M., L.Y., Y.C., Y.G., H.D., S.W., C.Y., J.L., J.C., R. Collins, R. Clarke and R.G.W. contributed to data acquisition and general study management. X.Y. and D.A. provided administrative and technical support. All authors critically reviewed the manuscript and approved the final submission.
Corresponding authors
Correspondence to Zhengming Chen or Iona Y. Millwood .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Medicine thanks Shiu Lun Au Yeung, Yan-Bo Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ming Yang, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 adjusted hrs for icd−10 chapter−specific morbidities associated with ever-regular drinking and with usual alcohol intake, in men..
Cox models comparing ever-regular drinkers with occasional drinkers, or assessing the dose–response per 280 g/week higher usual alcohol intake within current drinkers, were stratified by age-at-risk and study area and adjusted for education and smoking. Each solid square or diamond represents HR with the area inversely proportional to the variance of the log HR. The horizontal lines indicate 95% CIs. CI, confidence interval; HR hazard ratio; ICD-10, International Classification of Diseases, 10th Revision.
Extended Data Fig. 2 Adjusted HRs for different aggregated and all-cause morbidities associated with years after stopping drinking, in men.
Cox models comparing ex-drinker groups with occasional drinkers were stratified by age-at-risk and study area and were adjusted for education and smoking. Each box represents HR with the area inversely proportional to the variance of the group-specific log hazard within subplot. The vertical lines indicate group-specific 95% CIs for various ex-drinker groups. The shaded strip indicate the group-specific 95% CIs for occasional drinkers. The numbers above the error bars are point estimates for HRs. CI, confidence interval; HR hazard ratio; CKB, China Kadoorie Biobank; WHO, World Health Organization.
Extended Data Fig. 3 Associations of alcohol consumption with risks of 28 diseases previously defined as alcohol-related by the WHO, in male current drinkers.
Cox models were stratified by age-at-risk and study area and were adjusted for education and smoking. HRs were plotted against usual alcohol intake and were calculated per 280 g/week higher usual alcohol intake. All specific diseases displayed were significantly associated with alcohol intake (ever-regular drinking or per 280 g/week higher usual alcohol intake) after multiple testing correction (FDR-adjusted p<0.05), except transient cerebral ischemic attacks and related syndromes (ICD-10 code: G45), occlusion and stenosis of precerebral arteries (I65) and pancreatitis (K85-K86) which showed statistical significance at nominal level (p<0.05). Each box represents HR with the area inversely proportional to the variance of the group-specific log hazard within subplot. The vertical lines indicate group-specific 95% CIs. The numbers above the error bars are point estimates for HRs and the numbers below are number of events. All P values are two-sided. CI, confidence interval; HR hazard ratio; CKB, China Kadoorie Biobank; FDR, false discovery rate; ICD-10, International Classification of Diseases, 10th Revision; WHO, World Health Organization.
Extended Data Fig. 4 Associations of alcohol consumption with risks of 36 diseases not previously defined as alcohol-related, in male current drinkers.
Cox models were stratified by age-at-risk and study area and were adjusted for education and smoking. HRs were plotted against usual alcohol intake and were calculated per 280 g/week higher usual alcohol intake. All specific diseases displayed were significantly associated with alcohol intake (ever-regular drinking or per 280 g/week higher usual alcohol intake) after multiple testing correction (FDR-adjusted p<0.05). Each box represents HR with the area inversely proportional to the variance of the group-specific log hazard within subplot. The vertical lines indicate group-specific 95% CIs. The numbers above the error bars are point estimates for HRs and the numbers below are number of events. All P values are two-sided. CI, confidence interval; HR hazard ratio; CKB, China Kadoorie Biobank; FDR, false discovery rate.
Extended Data Fig. 5 Adjusted HRs for major diseases associated with drinking patterns, in male current drinkers.
Cox models were stratified by age-at-risk and study area and were adjusted for education and smoking and for total alcohol intake where indicated. Each solid square or diamond represents HR with the area inversely proportional to the variance of the log HR. The horizontal lines indicate 95% CIs. CI, confidence interval; HR hazard ratio; HED, heavy episodic drinking; CKB, China Kadoorie Biobank; WHO, World Health Organization.
Extended Data Fig. 6 Adjusted HRs for major diseases associated with duration of drinking, in male current drinkers.
Cox models were stratified by age-at-risk and study area and were adjusted for education, smoking, total alcohol intake and baseline age in (A). (B) had the same model specifications as (A) plus further adjustments for income, physical activity, fruit intake and body mass index. (C) had the same model specifications as (A) and excluded participants with poor self-reported health or prior chronic disease at baseline. Each box represents HR with the area inversely proportional to the variance of the group-specific log hazard. The horizontal lines indicate group-specific 95% CIs. All P values are two-sided. CI, confidence interval; HR hazard ratio; CKB, China Kadoorie Biobank; WHO, World Health Organization.
Extended Data Fig. 7 Adjusted HRs for ICD−10 chapter−specific morbidities associated with ever-regular drinking and with usual alcohol intake, in women.
Cox models comparing ever-regular drinkers with occasional drinkers, or assessing the dose–response per 100 g/week higher usual alcohol intake within current drinkers, were stratified by age-at-risk and study area and adjusted for education and smoking. Each solid square or diamond represents HR with the area inversely proportional to the variance of the log HR. The horizontal lines indicate 95% CIs. CI, confidence interval; HR hazard ratio; ICD-10, International Classification of Diseases, 10th Revision.
Extended Data Fig. 8 Adjusted HRs per 280 g/week higher genotype-predicted mean male alcohol intake for specific alcohol-associated diseases by ICD-10 chapters, in men and women.
Cox modes, stratified by age-at-risk and adjusted for genomic principal components, were used to relate genetic categories to risks of diseases within each study area. The HR per 280 g/week higher genotype-predicted mean male alcohol intake was calculated from the inverse-variance-weighted mean of the slopes of the fitted lines in each study area. Each solid square or diamond represents HR per 280 g/week higher genetically-predicted mean male alcohol intake, with the area inversely proportional to the variance of the log HR. The horizontal lines indicate 95% CIs. Diseases considered to be alcohol-related by the WHO are indicated with ‘Y’ under the ‘WHO’ column. The ‘RC’ column indicates the number of study areas that contributed to the overall area-stratified genotypic associations, as for certain less common diseases some study areas may not have enough number of cases to contribute to the inverse-variance-weighted meta-analysis. The ‘P het’ column indicates the p-value from a \(\chi\) 2 test for heterogeneity between sexes. All P values are two-sided. † Included less common ICD-10 codes within the corresponding ICD-10 chapter which were not individually investigated in the present study. CI, confidence interval; HR hazard ratio; ICD-10, International Classification of Diseases, 10th Revision; WHO, World Health Organization.
Extended Data Fig. 9 Adjusted HRs associated with GG versus AG genotype of ALDH2 - rs671 for specific alcohol-associated diseases by ICD-10 chapters, in men and women.
Area-specific genotypic effects (GG vs. AG genotype) were estimated within each study area (thus each reflecting the purely genotypic effects) using age-at-risk-stratified and genomic principal components-adjusted Cox models and were combined by inverse-variance-weighted meta-analysis to yield the overall area-stratified genotypic associations. Each solid square represents HR for GG vs. AG genotype, with the area inversely proportional to the variance of the log HR. The horizontal lines indicate 95% CIs. Diseases considered to be alcohol-related by the WHO are indicated with ‘Y’ under the ‘WHO’ column. The ‘RC’ column indicates the number of study areas that contributed to the overall area-stratified genotypic associations, as for certain less common diseases some study areas may not have enough number of cases to contribute to the inverse-variance-weighted meta-analysis. The ‘P het’ column indicates the P value from a \(\chi\) 2 test for heterogeneity between sexes. All P values are two-sided. † Included less common ICD-10 codes within the corresponding ICD-10 chapter which were not individually investigated in the present study. CI, confidence interval; HR hazard ratio; ICD-10, International Classification of Diseases, 10th Revision; WHO, World Health Organization.
Extended Data Fig. 10 Adjusted HRs associated with GG versus AG genotype of ADH1B - rs1229984 for specific alcohol-associated diseases by ICD-10 chapters, in men and women.
Supplementary information, supplementary information.
Supplementary Figs. 1–8 and Supplementary Tables 1–13.
Reporting Summary
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Im, P.K., Wright, N., Yang, L. et al. Alcohol consumption and risks of more than 200 diseases in Chinese men. Nat Med 29 , 1476–1486 (2023). https://doi.org/10.1038/s41591-023-02383-8
Download citation
Received : 16 December 2022
Accepted : 02 May 2023
Published : 08 June 2023
Issue Date : June 2023
DOI : https://doi.org/10.1038/s41591-023-02383-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Alcohol consumption may be a risk factor for cerebrovascular stenosis in acute ischemic stroke and transient ischemic attack.
- Xiaoyan Guo
BMC Neurology (2024)
The aldehyde dehydrogenase 2 rs671 variant enhances amyloid β pathology
Nature Communications (2024)
Smoking, alcohol consumption and risk of Dupuytren’s disease: a Mendelian randomization study
- Zifeng Wang
- Zhenyu Wang
BMC Medical Genomics (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Study protocol
- Open access
- Published: 15 October 2022
Underlying mechanisms in the relationship between stress and alcohol consumption in regular and risky drinkers (MESA): methods and design of a randomized laboratory study
- Charlotte Wittgens ORCID: orcid.org/0000-0001-6365-6662 1 , 2 ,
- Markus Muehlhan ORCID: orcid.org/0000-0002-8855-8724 1 , 3 ,
- Anja Kräplin ORCID: orcid.org/0000-0002-1612-3932 4 ,
- Max Wolff 5 &
- Sebastian Trautmann ORCID: orcid.org/0000-0002-8976-3244 1 , 2
BMC Psychology volume 10 , Article number: 233 ( 2022 ) Cite this article
9813 Accesses
9 Citations
10 Altmetric
Metrics details
Excessive alcohol consumption and alcohol use disorders (AUD) are among the leading preventable causes of premature morbidity and mortality and are considered a major public health concern. In order to reduce the individual and societal burden of excessive alcohol use, it is crucial to identify high-risk individuals at earlier stages and to provide effective interventions to prevent further progression. Stressful experiences are important risk factors for excessive alcohol consumption and AUDs. However, the underlying biological and psychological mechanisms are still poorly understood.
The project “Underlying mechanisms in the relationship between stress and alcohol consumption in regular and risky drinkers (MESA)” is a randomized controlled study that started in December 2018 and is conducted in a laboratory setting, which aims to identify moderators and mediators of the relationship between acute stress and alcohol consumption among regular and risky drinkers. Regular and risky drinkers are randomly assigned to a stress induction or a control condition. Several processes that may mediate (emotional distress, endocrine and autonomic stress reactivity, impulsivity, inhibitory control, motivational sensitization) or moderate (trait impulsivity, childhood maltreatment, basal HPA-axis activity) the relation between stress and alcohol consumption are investigated. As primary dependent variable, the motivation to consume alcohol following psychosocial stress is measured.
The results of this study could help to provide valuable targets for future research on tailored interventions to prevent stress-related alcohol consumption.
Peer Review reports
Excessive alcohol consumption and alcohol use disorders (AUD) are among the leading preventable causes of premature morbidity and mortality [ 1 , 2 ]. They come along with an immense individual and societal burden and are considered a major public health problem [ 3 ]. The World Health Organization reported 3 million deaths due to harmful use of alcohol in their latest report [ 4 ]. In the age group 20–39 years, approximately 13.5% of the total deaths are attributable to alcohol [ 5 ]. In particular, men are considered at high risk to develop AUD [ 2 ] with global prevalence five times that in women with 8.6% and 1.7% for males and females, respectively [ 4 ]. However, latest data indicated that this gap is narrowing in recent years [ 6 , 7 ]. Treatments for excessive alcohol use and AUD are initiated at a very late stage of symptom progression when adverse somatic and mental consequences have already occurred [ 8 , 9 ]. It is therefore necessary to identify high-risk individuals at an earlier stage of alcohol consumption in order to reduce individual and societal burden and to implement effective interventions to prevent further progressions. Risk factors and underlying mechanisms promoting excessive alcohol use need to be identified for tailoring new preventive approaches.
Stress and alcohol use
Alcohol consumption is a commonly used coping strategy to reduce stress [ 10 ]. It is very well known that Stress increases the amount of alcohol consumed and the risk of relapse, but little is known about the psychological mechanisms that underlie these effects [ 11 ]. The experience of stressful events, defined as unpredictable or uncontrollable events that exceed the regulatory capacity of an organism and that could threaten an organism’s physical or psychosocial integrity [ 10 , 11 ] has been identified as a major risk factor for excessive alcohol use and AUD [ 12 , 13 ]. The impact of stress on alcohol use and the risk of AUDs depends on the type, age, duration, and severity of the stress experienced [ 14 ]. The consumption of alcohol is a habitual response to stressful situations in people with AUD [ 15 ]. Stress plays an important role at all levels of alcohol consumption, beginning with facilitation of initial use through early stages of transition to regular use and from regular to excessive use [ 16 , 17 , 18 ]. In AUD, alcohol use also represents a habitual response to stressful situations [ 15 ].
Mediators and moderators
Despite this well-established association between stress and alcohol use, the underlying mechanisms are complex and still not well understood. Studies trying to explain this association show inconsistent results. Stress does not necessarily lead to alcohol consumption in every person [ 19 ], which suggests the relevance of potential moderating factors. Several environmental, biological, and psychological factors that could moderate the relation between stress and alcohol consumption at different stages of alcohol use progression are discussed in the existing literature. The hypothalamic–pituitary–adrenal (HPA) axis plays an important role in this context as it is a major stress response pathway and has been studied extensively in relation to alcohol use [ 20 ]. Altered HPA axis regulation is associated with problematic alcohol use and dependence and the nature of this dysregulation varies with respect to the stages of progression toward AUD [ 21 ]. Glucocorticoid secretion upon activation of (HPA) axis by stressors is normally adaptive, and was discussed to promote coping after stressful events whereas excessive and prolonged HPA axis activation results in wear-and-tear on numerous physiological systems [ 22 ]. Furthermore, dysregulation in stress-related cortisol production is a risk factor for developing AUD [ 20 ]. Therefore, studies suggest that there might be a moderating effect on the relationship between stress and alcohol consumption by individual differences in basal cortisol secretion [ 18 , 19 ]. Further, there is evidence from observational studies that childhood maltreatment moderates the association between stressful experiences and the development of alcohol use problems [ 23 , 24 ]. Individuals with childhood trauma exposure, particularly abuse, neglect, or chaotic home environments, are at heightened risk for heavy alcohol consumption [ 24 ]. Further childhood maltreatment is associated with early alcohol use initiation, alcohol-related problem behaviors, and alcohol use disorders in adulthood [ 25 ]. Other possible moderators considered in this context are personality traits. Personality traits such as trait impulsivity reflect people’s characteristic patterns of thoughts, feelings, and behaviors and imply consistency over time and stability across situations [ 26 ]. Trait impulsivity was found to predict risk for alcohol use problems in general [ 27 , 28 , 29 ] and further moderates the association between stress and alcohol use [ 30 , 31 ]. Although the consideration of these moderating factors might help to elucidate previous inconsistent findings on the association between stress and alcohol use and develop more targeted interventions, they have barely been considered in studies on its underlying mechanisms.
Regarding the underlying mechanisms of the relationship between stress and alcohol consumption, the idea of alcohol use as a dysfunctional coping strategy to self-medicate aversive emotional states following stressful experiences has long been the predominant model [ 32 ]. Although there is considerable empirical support for the self-medication hypothesis [ 32 , 33 , 34 , 35 ], it is not able to fully explain the association between stressful experiences and alcohol use. Alcohol consumption does not necessarily reduce aversive emotional states [ 36 , 37 ], violating the negative reinforcement assumption underlying the self-medication hypothesis. Therefore, knowledge on additional mechanisms beyond self-medication at different stages of alcohol use progression is required to explain the association between stress and alcohol use. Several relevant psychological and biological factors that might affect this relationship have been described in the literature [ 20 , 38 ]. Acute stress activates an immediate reaction increasing cerebral and peripheral adrenalin and noradrenalin and a delayed endocrine response (via HPA axis) increasing glucocorticoids (mainly cortisol in humans) [ 39 ]. These systems affect different mechanisms relevant to alcohol use depending on the stage of alcohol use progression. At early stages of alcohol use progression, alcohol use leads to increased autonomic arousal and HPA axis activation. These effects potentiate both stress and alcohol-related effects on motivation and reinforcement learning [ 40 ] which can further facilitate alcohol use as a stress-related coping mechanism [ 38 ]. It further promotes the salience of drug-related cues known as attentional bias as these cues ‘grab the attention’ and further increase alcohol craving [ 41 ]. At later stages of alcohol use progression, binge and excessive alcohol consumption results in larger-scale adaptations in terms of a neuroendocrine tolerance response to stress and alcohol intake [ 10 , 42 ] which may be involved in the transition from controlled to compulsive alcohol consumption [ 10 , 43 ]. Also, a sensitization of motivational systems can manifest, again, in priority processing alcohol-related cues, i.e. attentional bias [ 44 , 45 ]. The stress-induced sensitization at later stages of alcohol use progression is assumed to be active in parallel to the noradrenalin-related mechanisms [ 18 ]. Taken together, stress and stress system alterations by alcohol consumption could be associated with biased information processing, increased impulsivity and impaired control functions; a pattern that is known to be a key mechanism in the development of excessive alcohol use [ 46 , 47 ].
Need for controlled laboratory studies
Most studies, addressing the association between stress and alcohol consumption are based on clinical populations with limited sample sizes and participants who already developed AUD. In this context different moderators and mediators leading to alcohol dependence are often center of the research question [ 48 , 49 ]. There is need for research that investigates the underlying mechanisms that lead to AUD before it is manifested. Therefore, especially laboratory settings with non-clinic samples are suitable to investigate mediators and moderators on this relationship as they allow the investigation of specific mechanisms through randomized manipulation of the factor of interest and at the same time allow to control for confounding variables [ 50 ].
Aims and hypotheses
The present and ongoing study aims to fill this research gap by conducting an experimental laboratory design to investigate the underlying mechanisms of the association between stress and alcohol consumption (MESA) in the at-risk population of young men. Since these mechanisms are expected to differ depending on the stage of alcohol use, they are examined in regular and risky drinkers. Therefore, several processes that could mediate the relation between stress and alcohol consumption at different stages of alcohol use progression are assessed.
The research questions are as follows:
Does acute stress increase alcohol consumption in a laboratory setting?
What are the mediators of the association between acute stress and alcohol use?
What are the moderators of the association between acute stress and alcohol use?
Are effects of acute stress on alcohol use as well as moderators and mediators of this association different in risky drinkers compared to regular drinkers?
The following a priori hypothesis were formulated:
Acute stress increases alcohol consumption in a laboratory setting.
This effect is stronger in risky compared to regular drinkers.
Emotional distress, endocrine and autonomic stress reactivity as well as impulsivity account for most of the effect of stress on alcohol use in regular drinkers (mediation).
Emotional distress, endocrine and autonomic stress reactivity, impulsivity, attentional bias and craving account for most of the effect of stress on alcohol use in risky drinkers (mediation).
A history of childhood maltreatment, basal HPA-axis activity and impulsivity are related to a stronger effect of acute stress on alcohol consumption in regular and risky drinkers (moderation).
Methods/design
Study design
The MESA study is a randomized controlled study that started in December 2018 and is being conducted in a laboratory setting at the Medical School Hamburg. The study is divided into an online screening and a main examination, with detailed description in the following (“ Procedure ” section). The study has a four-group design. Participants are stratified into equal groups of regular and risky drinkers (with regular drinking being defined as average daily alcohol consumption of less than 24 g over the past 30 days and risky drinking being defined as average daily alcohol consumption of more than 24 g over the past 30 days [ 51 ]). Regular and risky drinkers are then randomly assigned to either an experimental (acute stress) or a control condition (Fig. 1 ).
Research is conducted in accordance with national data protection acts, the revised declaration of Helsinki and Good Clinical Practice Guidelines. After complete description of the study, written informed consent is obtained from all participants. The study is approved by the Institutional Review Boards of Technische Universität Dresden (EK 522122016) and Medical School Hamburg (MSH-2020/114).
Inclusion and exclusion criteria
Males have a higher risk of developing drinking problems compared to females [ 52 ] and are more likely to report stress-induced drinking [ 6 ]. Therefore, only male individuals are included to reduce heterogeneity and potential of confounding factors (e.g. intake of oral contraceptives, menstrual cycle), especially in the biological measures. All participants have to be between 18–40 years old. The upper age limit results from the fact that most alcohol use problems develop during adolescence and young adulthood [ 53 , 54 , 55 ]. Further, eligible individuals have to drink alcohol at least occasionally and have beer as their favorite alcoholic drink since it is necessary for the success of the study that participants are familiar with alcoholic brands to recognize them in the attentional bias paradigm (“ Assessment ” section). In addition, it might be perceived as unethical to provide abstinent individuals with alcoholic beverages. Additionally, having a hair length of at least 2 cm is required to analyze hair cortisol concentrations [ 56 ] as a cumulative measure for basal cortisol secretion of the association between stress and alcohol use (for detailed description see 2.4 Biological measures). Exclusion criteria are lifetime psychotic symptoms, lifetime alcohol or any other substance use disorder, current psychological or psycho-pharmacological interventions and acute suicidality, current psychotropic or other medication or any somatic diseases that might confound the study measures, especially with regard to the endocrine measures, and alcohol consumption on the study day. All subjects meeting the inclusion criteria will be stratified into the reported groups.
A priori power analysis
A non-clinical target sample of 400 young men is aimed for the MESA study. A power analysis was conducted to calculate the needed sample size. A series of Monte Carlo simulations (each simulating 1000 ANOVA F tests) using the simpower program in STATA 12.1 [ 57 ] was run. The Monte Carlo simulations revealed that assuming a sample size of n = 200 per drinking stratum, statistical power ranges between 0.80 and 0.95 for different group size ratios. Given the stratified randomized design of the study, this results in the final group size of n = 100 (Fig. 1 ).
Recruitment and screening procedures
Participants are recruited via personal contacts, flyers and advertisement in university and public settings in Hamburg (cafés, bars, supermarkets, sports clubs; student dormitories) as well as via social media (e.g. Instagram, Facebook) and student job markets. In addition, advertising is made in lectures and on the university website.
All individuals willing to participate in the study have to complete an online screening in advance of the main assessment, where basic demographic variables as well as all in- and exclusion criteria are assessed. Further, the usual alcohol consumption is measured using a self-administered timeline follow-back consisting of a calendar on which participants provide retrospective reports of average daily alcohol intake for the past 30 days [ 58 ]. The information on daily alcohol consumption is used to allocate participants to the groups of regular and risky drinkers. All individuals meeting the inclusion criteria are then invited to participate in the main study.
Person-related measures
Participants complete a comprehensive baseline assessment (questionnaire package) including the measures of the proposed moderators (childhood maltreatment, trait impulsivity), mediators (attentional bias to alcohol related stimuli, inhibitory control and impulsivity, and stress reactivity during the acute stressor), and variables that might affect the associations of interest (usual alcohol consumption, drinking motives, perceived stress, trait anxiety, difficulties in emotion regulation, psychological flexibility) (Table 1 ).
Biological measures
Hair strands are taken to reflect cumulative long-term cortisol secretion within two months prior to the respective assessment point [ 56 ]. The cumulative cortisol secretion consisting of basal cortisol secretion as well as stress-induced cortisol secretion, has been shown to be an important moderator of stress-related adverse consequences including increase in alcohol use [ 85 , 86 ]. In addition, during the study four saliva samples are collected using Salivettes ® “code blue” (Sarstedt, Nümbrecht, Germany) with synthetic swabs to measure free cortisol levels and alpha-amylase activity as biological indicators of stress reactivity (Fig. 5 ). The first saliva sample is taken immediately before the stress induction (for detailed description for the stress induction see “ Sample storage, biochemical analyses and data preparation ” section). The second saliva sample is taken right after the stress induction as well as 12 (3rd salvia sample) and 24 (4th salvia sample) minutes after the stress induction. Cortisol is the final output of the hypothalamic pituitary adrenal (HPA) axis, and is among the most frequently used biological markers of psychological stress [ 87 , 88 ]. Moreover, given that the biologically active (free) fraction of cortisol is reflected in saliva, it can be a preferred measure relative to serum cortisol [ 87 , 89 ]. In addition, alpha-amylase is an enzyme component of saliva and has been proposed as a marker for stress-induced activity of the sympathetic nervous system (SNS). The advantage of a saliva-based measure of SNS activity is the convenience of assessing activity of both major stress systems (i.e. SNS and HPA-axis) in a single test tube, without the need for technically sophisticated instrumentation [ 63 ].
Behavioral measures
In addition to self-report measures, three behavioral tasks are conducted to measure attentional bias to alcohol related stimuli, inhibitory control and impulsivity as possible mediators in the association between stress and alcohol use [ 18 , 90 ]. Attentional bias towards alcohol-related cues is measured using a dot-probe task (Fig. 2 ), which was programmed based on previous tasks in similar settings [ 41 , 90 ]. Subjects are presented with pairs of matched alcoholic (beer) and non-alcoholic beverages for 500 ms (stimulus-onset asynchrony, SOA). Another SOA of 100 ms will be added to the paradigm in the proposed study to be able to capture automatic initial reactions (see [ 91 ]). Stimuli were chosen based on expert ratings regarding similarity in color, shape and recognition. Subjects respond to a probe that appears behind either the alcoholic or the non-alcoholic beverage. The difference in reaction time between alcoholic and non-alcoholic stimuli is a measure of attentional bias towards alcohol-related cues. Although the dot-probe task is a widely used paradigm to measure attentional biases, there is debate about its reliability [ 92 , 93 ]. A new trial-based conceptualization of attentional bias has been proposed, which can increase reliability [ 94 ].
Dot-probe task
Inhibitory control is measured using a go/no-go task (Fig. 3 ) where participants are presented with 320 trials (280 go and 40 nogo trials) of stimuli containing two dots. Each dot pair is displayed for 500 ms and is arranged horizontally or vertically. Horizontally arranged dots indicate go-trials where participants have to press the response key as fast as possible while participants are instructed to withhold when seeing vertically arranged dots. Since there is evidence that participants balance the speed-accuracy trade-off differently [ 95 ], the dependent measure of the go/nogo task is the balanced integration score (BIS). This score is calculated in two steps. First, the responsive times (RTs) as well as the proportions of correct responses (PCs) are standardized. Second, one standardized score is subtracted from the other [ 96 ].
Go-nogo task
The delay discounting task as measure of impulsivity was taken from a task battery developed by Pooseh et al. ([ 65 ]; MATLAB scripts available from https://github.com/spooseh/VBDM ) and is described in detail in Kräplin et al. [ 66 ]. The task consisted of 30 trials. Participants had to decide between a smaller financial gain delivered sooner and a larger financial gain delivered later. The two options were simultaneously presented on a computer screen using the Psychophysics Toolbox [ 97 ] in MATLAB R2018a (MathWorks Inc., Natick, MA). Between the shorter and later choice options, delays were 3, 7, 14, 31, 61, 180, and 365 days. Monetary gains ranged from 0.30 to 10 €. A Bayesian adaptive algorithm was implemented. This way, the parameter estimation is updated after each trial and serves as the basis for the calculation of the options in the next trial. The method was used to determine the most informative offers nearest to the individual’s point of indifference between two choice alternatives (i.e. indifference point). Thus, decision-making parameters can be efficiently inferred without the use of post-hoc parameter estimations. A hyperbolic value function was generated to describe the decline of subjective values of delayed reward according to the discounting rate k (Mazur 1987). Individuals with higher impulsivity are assumed to display higher k values (Fig. 4 ).
Schematic overview of the tasks in the decision-making battery. a Delay discounting task. b Probability discounting for gains. c Probability discounting for losses. d Mixed gambles task [ 66 ]
Stress induction
Stress is induced with the Trier Social Stress Test (TSST) [ 98 ] as one of the most frequently inserted research tools for the induction of acute psychosocial stress in experimental, laboratory research worldwide. The TSST is a standardized laboratory protocol, which provides a reliable and ecologically valid stressor [ 98 ]. The TSST contains elements of social evaluative threat and uncontrollability, which are associated with high cortisol responses [ 99 ]. The test is divided in three equal five-minute parts. It begins with a preparation period, followed by a free speech for a job interview and finishes with an arithmetic task. All tasks are held in front of a two-person audience. The TSST leads to robust changes in the hypothalamus–pituitary–adrenal (HPA) axis and the autonomic stress response compared to other stress induction paradigms [ 39 , 100 ].
In the control condition, subjects participate in a Placebo-TSST, which is comparable in time and task division but without any audience and stress exposure for the participants [ 101 ]. It starts with a preparation period, followed by a free speech about the last vacation and finishes with a simple task of counting forward. Furthermore, participants are standing during the two tasks. This creates a setting that is as close as possible to the TSST, but does not contain stressful components (evaluative threat and uncontrollability).
Ad-libitum taste test
After completion of the behavioral tasks and the intervention, participants are asked to take part at an ad-libitum taste test as a covert measure for alcohol consumption. The ad-libitum taste test is a widely used method, which provides an unobtrusive and indirect measure of participants’ motivation to drink alcohol [ 84 ]. All participants are given two 0.33 l glasses of beer (two brands each containing 5% alcohol) and two 0.33 l glasses containing different soft drinks. Participants are instructed that they have 15 min to taste each glass to rate qualities about each drink (e.g. gassy, bitter). Participants are told to drink whatever amount necessary to make accurate judgements. The dependent variable is the amount of alcoholic beverage (beer) consumed and can range between 0 and 666 ml (equals 26.64 g ethanol). Non-alcoholic drinks are presented to control for the potential effect of thirst. The ad-libitum taste test is a valid method for the assessment of alcohol intake in the laboratory supported by strong associations between ad-libitum consumption and typical alcohol consumption [ 84 ]. It is also robust against several potential confounders such as time of day or participant awareness [ 84 ]. The taste test has been used to investigate a number of potential influences on alcohol consumption, including alcohol cues [ 102 , 103 , 104 ], impulse control [ 105 , 106 ], and social influences [ 107 ], and it has been used to establish initial proof of concept for novel behavioral interventions [ 108 , 109 , 110 ].
The main assessments are conducted between 14–20 p.m. in order to reduce the variance in biological measures (e.g. saliva cortisol) due to diurnal rhythms [ 111 ]. It is also likely that the willingness to drink alcohol is smaller in the morning than in the evening while there is no influence of day time on alcohol consumption in the ad libitum taste test between 14p.m. and 20p.m. [ 84 ]. Figure 5 gives an overview of the main study procedure. First, participants are asked to provide written informed consent. Participants’ absence from alcohol is verified by taking a breathalyzer reading with any value above zero leading to the immediate end of the examination. Hair strands for basal cortisol secretion are taken scalp-near from a posterior vertex position to be able to reflect basal cortisol secretion within two months prior to the respective assessment point. Then participants complete the baseline questionnaires including the measures of the proposed moderators (childhood maltreatment, trait impulsivity) and variables that might affect the associations of interest (usual alcohol consumption, drinking motives, stressful life events, trait anxiety, difficulties in emotion regulation, psychological flexibility). Subsequently, participants either take part in the stress induction (experimental condition) or placebo intervention (control condition) followed by behavioral assessments. Deviating from previous TSST protocols, the stress condition is maintained during the behavioral assessments. Therefore, participants are instructed that the TSST panel remain observing and evaluating the given performance during the computer tasks and further the camera is still pointed on the participant. The ad libitum taste-test is the last assessment of the procedure. After the taste test, all participants are debriefed about the true study purposes including the TSST procedure. Moreover, repeated breathalyzer readings are taken until blood alcohol concentration reaches 0.0‰ in two consecutive measures. Participants willing to leave before blood alcohol concentration reaches 0.0‰ have to confirm that they do not drive when leaving the laboratory. Participants who insist to leave with a blood alcohol concentration still being higher than 0.4‰ (only expected in rare cases) are sent home with a taxi.
Study procedure. Note : IC Informed consent, TSST Trier social stress test
Sample storage, biochemical analyses and data preparation
The saliva samples are taken using salivette ‘code blue’ devices (Sarstedt, Nümbrecht, Germany) directly before the intervention and at three time points after the intervention (Fig. 5 ). Saliva samples are stored at − 20 °C in a laboratory freezer. After thawing, saliva samples will be centrifuged for 10 min at 4000 rpm. Salivary cortisol concentrations will be determined using a commercially available chemiluminescence assay (CLIA, IBL-Hamburg, Germany). Concentrations of salivary alpha-amylase will be detected by using an in-house enzyme kinetic method according to the protocol described in [ 63 ]. Hair cortisol concentrations will be determined via liquid chromatography tandem mass spectrometry (for detailed information on analysis methods see [ 56 ]).
Dimensional variables that are not normally distributed (expected e.g. for hair cortisol concentration, salivary cortisol and alpha amylase) will be Box–Cox transformed towards normal distribution. For all biological and behavioral variables, participants with outlying values of more than three standard deviations above the mean will be excluded from the respective analysis. Besides, robust linear regressions will complement conventional linear regressions because they down weight observations with large residuals to meet the assumption of equal variances of residuals. Composite measures of the entire cortisol secretion during the TSST (area under the curve with respect to ground; AUC G ) and the cortisol stress reactivity (area under the curve with respect to increase; AUC i ) will be calculated [ 112 ]. Analysis with these variables will be adjusted for initial cortisol concentration to alleviate confounding risk as AUC variables may be comprised of variance due to stress reactivity and stress-unrelated HPA axis activity [ 113 ]. All analysis including cortisol secretion during the TSST will be run twice with all participants in the first and with exclusion of non-responders to the TSST (increase of 1.5 nmol/l compared to baseline [ 114 ]) in the second run. With regard to alpha-amylase, both AUC measures and peak minus baseline levels will be calculated.
Statistical analyses
Main effects of stress exposure (stress vs control group) on alcohol consumption (amount of alcoholic beverage consumed) will be determined using linear regressions adjusting for the amount of non-alcoholic beverages consumed (which reduces unspecific variance in outcome). However, in case of considerable by chance differences in baseline characteristics between the two groups despite randomization, these characteristics will be included in the regression model if they are associated with alcohol consumption. To address potential biases related to missing data, we will conduct sensitivity analyses using multiple imputation.
Moderation analyses: Moderators are defined causally [ 50 ]. Linear regressions with interaction terms will be applied to test whether stress effects on alcohol consumption are moderated by childhood maltreatment, hair cortisol concentration and trait impulsivity (with main effects terms and interaction term, e.g. group × childhood maltreatment). Significant interactions indicate that a respective factor (moderator) predicts different effects of stress on alcohol assumptions. To approach causal conclusions, we will fit these models again while adjusting for shared factors of moderators and outcomes (e.g. previous stressful events, previous alcohol use) [ 50 ].
Mediation analyses: Mediators are also defined causally according to the counterfactual definition of Robins and Greenland [ 115 ] that is implemented in the ‘paramed’ package in Stata. This module allows dividing the estimated total stress effect (stress vs. control group) on alcohol consumption into a direct effect and an indirect effect mediated through stress reactivity (saliva cortisol, alpha amylase, self-reported stress), impulsivity (delay discounting), inhibitory control, and motivational sensitization (attentional bias). Mediation analyses will be adjusted for putative sociodemographic (e.g. age) and other shared factors of a potential mediator and outcome (e.g. time of day, preference of beer) as well as for the mentioned factors for moderation. The alpha level will be specified at two-sided 0.05. If necessary, the analyses will be repeated with robust standard errors (via the sandwich estimator) and robust linear regressions [ 116 ]].
Study progress and preliminary feasibility data
The data collection of the presented MESA study started in December 2018. From December 2018 until March 2022 N = 623 persons participated in the online screening. A total of 213 complete data sets have been collected so far. All participants are male and between 18–40 ( M = 25) years old. 97 of the 213 participants took part in the stress condition, stratified in 40 risky drinkers and 57 regular drinkers. Further, 117 participants took part in the control condition stratified in 36 risky drinkers and 81 regular drinkers. More than half reported they were university students.
Due to the Covid-19 pandemic, the study was paused in the beginning of March 2020 in order to protect the safety and health of all personnel involved in the study and to comply with legislative regulations. The laboratories reopened in September 2021 and data collection was continued.
The present MESA study was developed in response to the incomplete understanding of the underlying mechanisms of the relationship between stress and alcohol consumption. As pointed out, there is a significant amount of people suffering from AUDs with tremendous consequences for the individual as well as for society and health care systems. There is need for preventive interventions at the biological, psychological or social level for individuals at high risk of problematic alcohol consumption before the manifestation of AUD. Research to date has focused primarily on secondary prevention, which aims to prevent AUD progression and relapse, and tertiary prevention, which aims to minimize functional deterioration in chronic AUDs [ 117 ]. The present study focuses on the identification of targets for primary prevention, which is focused on the protection of healthy individuals, and may be provided on a universal, selective or indicated level. The various tasks designed to examine different, potential moderators and mediators can then be used to develop interventions and provide information for the at-risk population. The identification of specific mediators is of key importance as they help to elucidate what mechanisms underlie the association between stress and alcohol consumption. Knowledge about specific mechanisms are of high relevance as it can be used to allocate existing interventions. For example, there are already trainings for many of the investigated mediators (e.g. Attentional Bias Modification, Inhibitory Control trainings), which, should these factors prove to be relevant, could then be specifically adapted and applied in the context of stress [ 118 , 119 , 120 , 121 ]. It can also be used to develop novel interventions that might be useful to prevent stress-related alcohol consumption. Identifying specific moderators will help to tailor these preventive interventions to at high-risk individuals, which increases their potential efficacy and cost-effectiveness.
Given its focus on internal validity using a carefully controlled design in a laboratory setting, external validity will be a limitation of this study. Thus, findings will have to be complemented by investigations in real world settings to make definite conclusions about the association between stress and alcohol use and its underlying mechanisms. This could be achieved for example with ecological momentary assessments, which have shown good feasibility in a couple of promising recent studies on stress-related alcohol use and the role of craving, alterations in mood and inhibitory control [ 122 , 123 , 124 , 125 ].
Taken together, the presented study has a high potential to advance our understanding of stress-related alcohol use. In the long-term, it could stimulate the development of tailored preventive interventions and contribute to a reduction of problematic alcohol use.
Availability of data and materials
The data will be made available on the OSF after completion of the first data analyses.
Abbreviations
Area under the curve
Area under the curve with respect to ground
Area under the curve with respect to increase
Alcohol use disorder
Balanced integration score
Difficulties in emotion regulation scale
Drinking Motive questionnaire—revised
Hypothalamic–pituitary–adrenal axis
Multidimensional Mood State Questionnaire
Underlying mechanisms in the relationship between stress and alcohol consumption in regular and risky drinkers
Proportion of correct responses
Perceived Stress Scale
Response times
Sympathetic nervous system
State-trait-anxiety-inventory
Trier Social Stress Test
Carvalho AF, Heilig M, Perez A, Probst C, Rehm J. Alcohol use disorders. The Lancet. 2019;394(10200):781–92.
Article Google Scholar
Rehm J, Shield KD. Global burden of disease and the impact of mental and addictive disorders. Curr Psychiatry Rep. 2019;21(2):10.
Article PubMed Google Scholar
Rehm J, Baliunas D, Borges GLG, Graham K, Irving H, Kehoe T, et al. The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction. 2010;105(5):817–43.
Article PubMed PubMed Central Google Scholar
World Health Organization, World Health Organization, World Health Organization, Management of Substance Abuse Team. Global status report on alcohol and health 2018. 2018.
Bloomfield K, Kraus L, Soyka M, Koch-Institut R. Gesundheitsberichterstattung des Bundes. Berlin, Heidelberg: Robert-Koch-Institut; 2008 S. 34. Report No.: 40.
Peltier MR, Verplaetse TL, Mineur YS, Petrakis IL, Cosgrove KP, Picciotto MR, et al. Sex differences in stress-related alcohol use. Neurobiol Stress. 2019;10:100149.
White A, Castle IJP, Chen CM, Shirley M, Roach D, Hingson R. Converging patterns of alcohol use and related outcomes among females and males in the United States, 2002 to 2012. Alcohol Clin Exp Res. 2015;39(9):1712–26.
Rehm J, Allamani A, Elekes Z, Jakubczyk A, Manthey J, Probst C, et al. Alcohol dependence and treatment utilization in Europe—a representative cross-sectional study in primary care. BMC Fam Pract. 2015;16(1):90.
Trautmann S, Pieper L, Kuitunen-Paul S, Manthey J, Wittchen HU, Bühringer G, et al. Prävalenz und Behandlungsraten von Störungen durch Alkoholkonsum in der primärärztlichen Versorgung in Deutschland. SUCHT. 2016;62(4):233–43.
Sinha R. How does stress lead to risk of alcohol relapse? Alcohol Res Curr Rev. 2012;34(4):432.
Google Scholar
McGrath E, Jones A, Field M. Acute stress increases ad-libitum alcohol consumption in heavy drinkers, but not through impaired inhibitory control. Psychopharmacology. 2016;233(7):1227–34.
Becker HC. Influence of stress associated with chronic alcohol exposure on drinking. Neuropharmacology. 2017;1(122):115–26.
Hasin D, Keyes KM. The epidemiology of alcohol and drug disorders. In: Addiction medicine. Berlin: Springer; 2011. p. 23–49.
Keyes KM, Hatzenbuehler ML, Grant BF, Hasin DS. Stress and alcohol: epidemiologic evidence. Alcohol Res Curr Rev. 2012;34:391–400.
Marlatt G. Taxonomy of high-risk situations for alcohol relapse: evolution and development of a cognitive-behavioral model. Addict (Abingdon Engl). 1997;91(1):S37-49.
Blomeyer D, Treutlein J, Esser G, Schmidt MH, Schumann G, Laucht M. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol Psychiatry. 2008;63(2):146–51.
Kingston S, Raghavan C. The relationship of sexual abuse, early initiation of substance use, and adolescent trauma to PTSD. J Trauma Stress. 2009;22(1):65–8.
Lijffijt M, Hu K, Swann AC. Stress modulates illness-course of substance use disorders: a translational review. Front Psychiatry. 2014. https://doi.org/10.3389/fpsyt.2014.00083/abstract .
Spanagel R, Noori HR, Heilig M. Stress and alcohol interactions: animal studies and clinical significance. Trends Neurosci. 2014;37(4):219–27.
Stephens MAC, Wand G. Stress and the HPA Axis. Alcohol Res Curr Rev. 2012;34(4):468–83.
Wand G. The influence of stress on the transition from drug use to addiction. Alcohol Res Health J Natl Inst Alcohol Abuse Alcohol. 2008;31(2):119–36.
McEwen BS, Gianaros PJ. Stress-and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–45.
Keyes KM, Shmulewitz D, Greenstein E, McLaughlin K, Wall M, Aharonovich E, et al. Exposure to the Lebanon War of 2006 and effects on alcohol use disorders: the moderating role of childhood maltreatment. Drug Alcohol Depend. 2014;134:296–303.
Kim JH, Martins SS, Shmulewitz D, Santaella J, Wall MM, Keyes KM, et al. Childhood maltreatment, stressful life events, and alcohol craving in adult drinkers. Alcohol Clin Exp Res. 2014;38(7):2048–55.
Young-Wolff KC, Kendler KS, Prescott CA. Interactive effects of childhood maltreatment and recent stressful life events on alcohol consumption in adulthood. J Stud Alcohol Drugs. 2012;73(4):559–69.
Diener E, Lucas RE. Personality traits. Gen Psychol Required Read. 2019;278.
de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14(1):22–31.
de Wit H, Richards JB. Dual determinants of drug use in humans: reward and impulsivity. Neb Symp Motiv Neb Symp Motiv. 2004;50:19–55.
Stamates AL, Lau-Barraco C. Momentary patterns of impulsivity and alcohol use: A cause or consequence? Drug Alcohol Depend. 2020;217:108246.
Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, et al. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006;63(12):1386–95.
Cuomo C, Sarchiapone M, Giannantonio MD, Mancini M, Roy A. Aggression, impulsivity, personality traits, and childhood trauma of prisoners with substance abuse and addiction. Am J Drug Alcohol Abuse. 2008;34(3):339–45.
Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv Rev Psychiatry. 1997;4(5):231–44.
Crum RM, La Flair L, Storr CL, Green KM, Stuart EA, Alvanzo AAH, et al. Reports of drinking to self-medicate anxiety symptoms: longitudinal assessment for subgroups of individuals with alcohol dependence: research article: self-medication and alcohol dependence. Depress Anxiety. 2013;30(2):174–83.
Kushner M. The relationship between anxiety disorders and alcohol use disorders A review of major perspectives and findings. Clin Psychol Rev. 2000;20(2):149–71.
Lazareck S, Robinson JA, Crum RM, Mojtabai R, Sareen J, Bolton JM. A longitudinal investigation of the role of self-medication in the development of comorbid mood and drug use disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). J Clin Psychiatry. 2012;73(5):e588-593.
Le Berre AP. Emotional processing and social cognition in alcohol use disorder. Neuropsychology. 2019;33(6):808–21.
Petit G, Luminet O, Maurage F, Tecco J, Lechantre S, Ferauge M, et al. Emotion regulation in alcohol dependence. Alcohol Clin Exp Res. 2015;39(12):2471–9.
Blaine SK, Sinha R. Alcohol, stress, and glucocorticoids: from risk to dependence and relapse in alcohol use disorders. Neuropharmacology. 2017;122:136–47.
Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medullary system to repeated psychosocial stress. Psychosom Med. 2003;65(3):450–60.
Lee S, Rivier C. Alcohol increases the expression of type 1, but not type 2α corticotropin-releasing factor (CRF) receptor messenger ribonucleic acid in the rat hypothalamus. Mol Brain Res. 1997;52(1):78–89.
Field M, Powell H. Stress increases attentional bias for alcohol cues in social drinkers who drink to cope. Alcohol Alcohol. 2007;42(6):560–6.
Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the „dark side“ of drug addiction. Nat Neurosci. 2005;8(11):1442–4.
Sinha R. The clinical neurobiology of drug craving. Curr Opin Neurobiol. 2013;23(4):649–54.
Field M, Munafò MR, Franken IHA. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol Bull. 2009;135(4):589–607.
Franken IHA. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(4):563–79.
Stamates AL, Lau-Barraco C. The dimensionality of impulsivity: Perspectives and implications for emerging adult drinking. Exp Clin Psychopharmacol. 2017;25(6):521–33.
Wiers RW, Bartholow BD, van den Wildenberg E, Thush C, Engels RCME, Sher KJ, et al. Automatic and controlled processes and the development of addictive behaviors in adolescents: a review and a model. Pharmacol Biochem Behav. 2007;86(2):263–83.
Blaine SK, Nautiyal N, Hart R, Guarnaccia JB, Sinha R. Craving, cortisol and behavioral alcohol motivation responses to stress and alcohol cue contexts and discrete cues in binge and non-binge drinkers. Addict Biol. 2019;24(5):1096–108.
Kim ST, Hwang SS, Kim HW, Hwang EH, Cho J, Kang JI, et al. Multidimensional impulsivity as a mediator of early life stress and alcohol dependence. Sci Rep. 2018;8(1):4104.
VanderWeele T. Explanation in causal inference: methods for mediation and interaction. Oxford: Oxford University Press; 2015. p. 729S.
Herrick C. Governing health and consumption: sensible citizens, behaviour and the city. Bristol: Policy Press; 2011. p. 265S.
Book Google Scholar
Institut Für Therapieforschung (IFT), München, Institut Für Klinische Psychologie Und Psychotherapie Der Technischen Universität Dresden, Bundesministerium Für Gesundheit Und Soziale Sicherung, Berlin. Epidemiological Survey on Substance Abuse in Germany 2012 (ESA)Repräsentativerhebung zum Gebrauch und Missbrauch psychoaktiver Substanzen bei Erwachsenen in Deutschland (Epidemiologischer Suchtsurvey 2012) [Internet]. GESIS Data Archive; 2014 [zitiert 8. Dezember 2021]. https://doi.org/10.4232/1.12042
Crum RM, Chan YF, Chen LS, Storr CL, Anthony JC. Incidence rates for alcohol dependence among adults: prospective data from the Baltimore Epidemiologic Catchment Area Follow-Up Survey, 1981–1996. J Stud Alcohol. 2005;66(6):795–805.
Grant BF, Goldstein RB, Chou SP, Huang B, Stinson FS, Dawson DA, et al. Sociodemographic and psychopathologic predictors of first incidence of DSM-IV substance use, mood and anxiety disorders: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. Mol Psychiatry. 2009;14(11):1051–66.
Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustün TB. Age of onset of mental disorders: a review of recent literature. Curr Opin Psychiatry. 2007;20(4):359–64.
Stalder T, Kirschbaum C. Analysis of cortisol in hair–state of the art and future directions. Brain Behav Immun. 2012;26(7):1019–29.
Corp S. Stata statistical software: release 17. College Station: StataCorp LLC.; 2021.
Collins RL, Kashdan TB, Koutsky JR, Morsheimer ET, Vetter CJ. A self-administered Timeline Followback to measure variations in underage drinkers’ alcohol intake and binge drinking. Addict Behav. 2008;33(1):196–200.
Wittchen HU, Zaudig M, Fydrich T. Strukturiertes Klinisches Interview f€ ur DSM-IV (SKID-I und SKID-II). Achse Psych Sto Rungen Achse II Perso Nlichkeitssto Rungen Go Ttingen Hogrefe. 1997.
Gröschl M. Current status of salivary hormone analysis. Clin Chem. 2008;54(11):1759–69.
Whiteside SP, Lynam DR, Miller JD, Reynolds SK. Validation of the UPPS impulsive behaviour scale: a four-factor model of impulsivity. Eur J Pers. 2005;19(7):559–74.
Bernstein DP, Fink L, Handelsman L, Foote J. Childhood trauma questionnaire. Assess Fam Violence Handb Res Pract. 1998.
Rohleder N, Nater UM. Determinants of salivary alpha-amylase in humans and methodological considerations. Psychoneuroendocrinology. 2009;34(4):469–85.
Wolff M, Krönke KM, Venz J, Kräplin A, Bühringer G, Smolka MN, et al. Action versus state orientation moderates the impact of executive functioning on real-life self-control. J Exp Psychol Gen. 2016;145(12):1635–53.
Pooseh S, Bernhardt N, Guevara A, Huys QJM, Smolka MN. Value-based decision-making battery: a Bayesian adaptive approach to assess impulsive and risky behavior. Behav Res Methods. 2018;50(1):236–49.
Kräplin A, Höfler M, Pooseh S, Wolff M, Krönke KM, Goschke T, et al. Impulsive decision-making predicts the course of substance-related and addictive disorders. Psychopharmacology. 2020;237(9):2709–24.
Cohen S, Kamarck T, Mermelstein R. Perceived stress scale. Meas Stress Guide Health Soc Sci. 1994;10:1–2.
Bond FW, Hayes SC, Baer RA, Carpenter KM, Guenole N, Orcutt HK, et al. Preliminary psychometric properties of the Acceptance and Action Questionnaire-II: a revised measure of psychological inflexibility and experiential avoidance. Behav Ther. 2011;42(4):676–88.
Kuntsche E, Kuntsche S. Development and validation of the drinking motive questionnaire revised short form (DMQ–R SF). J Clin Child Adolesc Psychol. 2009;38(6):899–908.
Kräplin A, Dshemuchadse M, Behrendt S, Scherbaum S, Goschke T, Bühringer G. Dysfunctional decision-making in pathological gambling: pattern specificity and the role of impulsivity. Psychiatry Res. 2014;215(3):675–82. https://doi.org/10.1016/j.psychres.2013.12.041 .
Raabe A, Grüsser SM, Wessa M, Podschus J, Flor H. The assessment of craving: psychometric properties, factor structure and a revised version of the Alcohol Craving Questionnaire (ACQ). Addict Abingdon Engl. 2005;100(2):227–34.
Gratz KL, Roemer L. Multidimensional assessment of emotion regulation and dysregulation: development, factor structure, and initial validation of the difficulties in emotion regulation scale. J Psychopathol Behav Assess. 2003;14:41–54.
Spielberger CD. Test anxiety inventory. Corsini Encycl Psychol. 2010;1–1.
Steyer R, Schwenkmezger P, Notz P, Eid M. Testtheoretische Analysen des Mehrdimensionalen Befindlichkeitsfragebogen (MDBF). [Theoretical analysis of a multidimensional mood questionnaire (MDBF).]. Diagnostica. 1994;40(4):320–8.
Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. J Abnorm Psychol. 2002;111(2):225.
Gratz KL, Roemer L. Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the difficulties in emotion regulation scale. J Psychopathol Behav Assess. 2004;26(1):41–54.
Ehring T, Zetsche U, Weidacker K, Wahl K, Schönfeld S, Ehlers A. The Perseverative Thinking Questionnaire (PTQ): validation of a content-independent measure of repetitive negative thinking. J Behav Ther Exp Psychiatry. 2011;42(2):225–32.
Smyth C. The Pittsburgh sleep quality index (PSQI). Bd. 25, Journal of gerontological nursing. SLACK Incorporated Thorofare, NJ; 1999. S. 10–10.
Gerlach AL, Andor T, Patzelt J. Die bedeutung von unsicherheitsintoleranz für die generalisierte angststörung modellüberlegungen und entwicklung einer deutschen version der unsicherheitsintoleranz-skala. Z Für Klin Psychol Psychother. 2008;37(3):190–9.
Carver CS. You want to measure coping but your protocol’too long: consider the brief cope. Int J Behav Med. 1997;4(1):92–100.
Beck AT, Steer RA, Brown GK. Beck depression inventory. New York: Harcourt Brace Jovanovich; 1987.
Tangney JP, Baumeister RF, Boone AL. High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. J Pers. 2004;72(2):271–324.
Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson resilience scale (CD-RISC). Depress Anxiety. 2003;18(2):76–82.
Jones A, Button E, Rose AK, Robinson E, Christiansen P, Di Lemma L, et al. The ad-libitum alcohol ‘taste test’: secondary analyses of potential confounds and construct validity. Psychopharmacology. 2016;233(5):917–24.
Steudte-Schmiedgen S, Stalder T, Schönfeld S, Wittchen HU, Trautmann S, Alexander N, et al. Hair cortisol concentrations and cortisol stress reactivity predict PTSD symptom increase after trauma exposure during military deployment. Psychoneuroendocrinology. 2015;59:123–33.
Trautmann S, Muehlhan M, Kirschbaum C, Wittchen HU, Höfler M, Stalder T, et al. Biological stress indicators as risk markers for increased alcohol use following traumatic experiences. Addict Biol. 2018;23(1):281–90.
Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34(2):163–71.
Kirschbaum C, Wüst S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom Med. 1992;54(6):648–57.
Kudielka BM, Gierens A, Hellhammer DH, Wüst S, Schlotz W. Salivary cortisol in ambulatory assessment—some dos, some don’ts, and some open questions. Psychosom Med. 2012;74(4):418–31.
Field M, Quigley M. Mild stress increases attentional bias in social drinkers who drink to cope: a replication and extension. Exp Clin Psychopharmacol. 2009;17(5):312–9.
Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97(1–2):1–20.
Ataya AF, Adams S, Mullings E, Cooper RM, Attwood AS, Munafò MR. Internal reliability of measures of substance-related cognitive bias. Drug Alcohol Depend. 2012;121(1–2):148–51.
Field M, Christiansen P. Commentary on; “Internal reliability of measures of substance-related cognitive bias.” Drug Alcohol Depend. 2012;124(3):189–90.
Zvielli A, Bernstein A, Koster EH. Temporal dynamics of attentional bias. Clin Psychol Sci. 2015;3(5):772–88.
Bogacz R, Wagenmakers EJ, Forstmann BU, Nieuwenhuis S. The neural basis of the speed-accuracy tradeoff. Trends Neurosci. 2010;33(1):10–6.
Liesefeld HR, Janczyk M. Combining speed and accuracy to control for speed-accuracy trade-offs(?). Behav Res Methods. 2019;51(1):40–60.
Brainard DH, Vision S. The psychophysics toolbox. Spat Vis. 1997;10(4):433–6.
Kirschbaum C, Pirke KM, Hellhammer DH. The ’Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81.
Labuschagne I, Grace C, Rendell P, Terrett G, Heinrichs M. An introductory guide to conducting the Trier Social Stress Test. Neurosci Biobehav Rev. 2019;107:686–95.
Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130(3):355–91.
Het S, Rohleder N, Schoofs D, Kirschbaum C, Wolf OT. Neuroendocrine and psychometric evaluation of a placebo version of the ‘Trier Social Stress Test.’ Psychoneuroendocrinology. 2009;34(7):1075–86.
Colby SM, Rohsenow DJ, Monti PM, Gwaltney CJ, Gulliver SB, Abrams DB, et al. Effects of tobacco deprivation on alcohol cue reactivity and drinking among young adults. Addict Behav. 2004;29(5):879–92.
Jones A, Rose AK, Cole J, Field M. Effects of alcohol cues on craving and ad libitum alcohol consumption in social drinkers: the role of disinhibition. J Exp Psychopathol. 2013;4(3):239–49.
Van Dyke N, Fillmore MT. Operant responding for alcohol following alcohol cue exposure in social drinkers. Addict Behav. 2015;47:11–6.
Christiansen P, Cole JC, Field M. Ego depletion increases ad-lib alcohol consumption: investigating cognitive mediators and moderators. Exp Clin Psychopharmacol. 2012;20(2):118–28.
Jones A, Guerrieri R, Fernie G, Cole J, Goudie A, Field M. The effects of priming restrained versus disinhibited behaviour on alcohol-seeking in social drinkers. Drug Alcohol Depend. 2011;113(1):55–61.
Quigley BM, Collins RL. The modeling of alcohol consumption: a meta-analytic review. J Stud Alcohol. 1999;60(1):90–8.
Bowley C, Faricy C, Hegarty B, Johnstone S, Smith J, Kelly P, et al. The effects of inhibitory control training on alcohol consumption, implicit alcohol-related cognitions and brain electrical activity. Int J Psychophysiol Off J Int Organ Psychophysiol. 2013;89(3):342–8.
Field M, Eastwood B. Experimental manipulation of attentional bias increases the motivation to drink alcohol. Psychopharmacology. 2005;183(3):350–7.
Jones A, Field M. The effects of cue-specific inhibition training on alcohol consumption in heavy social drinkers. Exp Clin Psychopharmacol. 2013;21(1):8–16.
Miller R, Stalder T, Jarczok M, Almeida DM, Badrick E, Bartels M, et al. The CIRCORT database: reference ranges and seasonal changes in diurnal salivary cortisol derived from a meta-dataset comprised of 15 field studies. Psychoneuroendocrinology. 2016;73:16–23.
Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–31.
Miller R, Wojtyniak JG, Weckesser LJ, Alexander NC, Engert V, Lehr T. How to disentangle psychobiological stress reactivity and recovery: a comparison of model-based and non-compartmental analyses of cortisol concentrations. Psychoneuroendocrinology. 2018;90:194–210.
Miller R, Plessow F, Kirschbaum C, Stalder T. Classification criteria for distinguishing cortisol responders from nonresponders to psychosocial stress: evaluation of salivary cortisol pulse detection in panel designs. Psychosom Med. 2013;75(9):832–40.
Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiol Camb Mass. 1992;3(2):143–55.
Royall RM. Model robust confidence intervals using maximum likelihood estimators. Int Stat Rev Int Stat. 1986;221–6.
Trova AC, Paparrigopoulos T, Liappas I, Ginieri-Coccossis M. Prevention of alcohol dependence. Psychiatr Psychiatr. 2015;26(2):131–40.
Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: emerging evidence. Pharmacol Ther. 2012;134(3):287–97.
Houben K, Nederkoorn C, Wiers RW, Jansen A. Resisting temptation: decreasing alcohol-related affect and drinking behavior by training response inhibition. Drug Alcohol Depend. 2011;116(1–3):132–6.
López-Caneda E, Rodríguez Holguín S, Cadaveira F, Corral M, Doallo S. Impact of alcohol use on inhibitory control (and vice versa) during adolescence and young adulthood: a review. Alcohol Alcohol Oxf Oxfs. 2014;49(2):173–81.
Schoenmakers T, Wiers RW, Jones BT, Bruce G, Jansen ATM. Attentional re-training decreases attentional bias in heavy drinkers without generalization. Addict Abingdon Engl. 2007;102(3):399–405.
Szeto EH, Schoenmakers TM, van de Mheen D, Snelleman M, Waters AJ. Associations between dispositional mindfulness, craving, and drinking in alcohol-dependent patients: an ecological momentary assessment study. Psychol Addict Behav J Soc Psychol Addict Behav. 2019;33(5):431–41.
Mayhugh RE, Rejeski WJ, Petrie MR, Laurienti PJ, Gauvin L. Differing patterns of stress and craving across the day in moderate-heavy alcohol consumers during their typical drinking routine and an imposed period of alcohol abstinence. PLoS ONE. 2018;13(4):e0195063.
Jones A, Tiplady B, Houben K, Nederkoorn C, Field M. Do daily fluctuations in inhibitory control predict alcohol consumption? An ecological momentary assessment study. Psychopharmacology. 2018;235(5):1487–96.
Duif M, Thewissen V, Wouters S, Lechner L, Jacobs N. Associations between affect and alcohol consumption in adults: an ecological momentary assessment study. Am J Drug Alcohol Abuse. 2020;46(1):88–97.
Download references
Acknowledgements
We acknowledge Helen Lenhardt and Lisa Hofmann for their assistance in data collection.
The funder has no role in study design, data collection and analysis, decision to publish, or preparation of any kind of manuscript.
Open Access funding enabled and organized by Projekt DEAL. The study is funded by the Deutsche Forschungsgemeinschaft (DFG), Grant No. TR 1489/1-1.
Author information
Authors and affiliations.
Department of Psychology, Faculty of Human Science, Medical School Hamburg, Hamburg, Germany
Charlotte Wittgens, Markus Muehlhan & Sebastian Trautmann
ICPP Institute for Clinical Psychology and Psychotherapy, Medical School Hamburg, Hamburg, Germany
Charlotte Wittgens & Sebastian Trautmann
ICAN Institute for Cognitive and Affective Neuroscience, Medical School Hamburg, Hamburg, Germany
Markus Muehlhan
Work Group Addictive Behaviors, Risk Analysis and Risk Management, Faculty of Psychology, Technische University Dresden, Dresden, Germany
Anja Kräplin
Department of Psychiatry and Psychotherapy, Campus Charité Mitte, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany
You can also search for this author in PubMed Google Scholar
Contributions
All authors have read and provided substantial contributions to the final version of the study protocol. ST is the central and principal investigator of the project and is responsible for drafting the initial proposal with all subsequent revisions. CW is the principal investigator for the study and for drafting the final protocol for publication. MM contributed substantially to drafting the initial proposal and to the revision of the initial proposal as well as to the revision and final approval of the manuscript. AK contributed substantially to the revision of the initial proposal and to the revision and final approval of the manuscript. MW provided the paradigms for the proposal and contributed to the revision and final approval of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Charlotte Wittgens or Sebastian Trautmann .
Ethics declarations
Ethics approval and consent to participate.
The ethics approval for the study was given by ethics committee of Technische Universität Dresden (EK 522122016) and the ethics committee of Medical School Hamburg (MSH) (MSH-2020/114). The participants give their written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wittgens, C., Muehlhan, M., Kräplin, A. et al. Underlying mechanisms in the relationship between stress and alcohol consumption in regular and risky drinkers (MESA): methods and design of a randomized laboratory study. BMC Psychol 10 , 233 (2022). https://doi.org/10.1186/s40359-022-00942-1
Download citation
Received : 08 June 2022
Accepted : 05 October 2022
Published : 15 October 2022
DOI : https://doi.org/10.1186/s40359-022-00942-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Risky alcohol consumption
- Acute stress
- Ad-libitum taste-test
BMC Psychology
ISSN: 2050-7283
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Effects of Alcohol Consumption on Various Systems of the Human Body: A Systematic Review
Affiliations.
- 1 Medical School, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences, Wardha, IND.
- 2 Community Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences, Wardha, IND.
- PMID: 36381944
- PMCID: PMC9637453
- DOI: 10.7759/cureus.30057
Prolonged alcohol intake for many years has been known to cause serious ailments in human beings since time memorial. Even after knowing that this dangerous addiction paves the way to one's own grave, there isn't much difference in the way the community sees this deadly habit. Time and again history has proven that this fatal addiction could make the life of those who consume it terrible. Also, the lives of the dear ones of alcoholic people are affected as alcohol not only affects those who consume them but also kin and friends. Various research studies conducted over many years clearly show the association of prolonged alcohol intake in the causation, aggravation, worsening, and deterioration of the health of its consumers. Moreover, chronic alcohol intake single-handedly is one of the major etiological factors in various serious diseases.
Keywords: alcohol abuse; alcohol misuse; effects on general health; harmful effects; various systems of human body.
Copyright © 2022, Varghese et al.
PubMed Disclaimer
Conflict of interest statement
The authors have declared that no competing interests exist.
Figure 1. A flowchart of the methodology…
Figure 1. A flowchart of the methodology used for the review
Similar articles
- A Comprehensive Review on the Impacts of Smoking on the Health of an Individual. Varghese J, Muntode Gharde P. Varghese J, et al. Cureus. 2023 Oct 5;15(10):e46532. doi: 10.7759/cureus.46532. eCollection 2023 Oct. Cureus. 2023. PMID: 37927763 Free PMC article. Review.
- New Australian guidelines for the treatment of alcohol problems: an overview of recommendations. Haber PS, Riordan BC, Winter DT, Barrett L, Saunders J, Hides L, Gullo M, Manning V, Day CA, Bonomo Y, Burns L, Assan R, Curry K, Mooney-Somers J, Demirkol A, Monds L, McDonough M, Baillie AJ, Clark P, Ritter A, Quinn C, Cunningham J, Lintzeris N, Rombouts S, Savic M, Norman A, Reid S, Hutchinson D, Zheng C, Iese Y, Black N, Draper B, Ridley N, Gowing L, Stapinski L, Taye B, Lancaster K, Stjepanović D, Kay-Lambkin F, Jamshidi N, Lubman D, Pastor A, White N, Wilson S, Jaworski AL, Memedovic S, Logge W, Mills K, Seear K, Freeburn B, Lea T, Withall A, Marel C, Boffa J, Roxburgh A, Purcell-Khodr G, Doyle M, Conigrave K, Teesson M, Butler K, Connor J, Morley KC. Haber PS, et al. Med J Aust. 2021 Oct 4;215 Suppl 7:S3-S32. doi: 10.5694/mja2.51254. Med J Aust. 2021. PMID: 34601742
- Alcohol consumption in India- An epidemiological review. Eashwar VMA, Umadevi R, Gopalakrishnan S. Eashwar VMA, et al. J Family Med Prim Care. 2020 Jan 28;9(1):49-55. doi: 10.4103/jfmpc.jfmpc_873_19. eCollection 2020 Jan. J Family Med Prim Care. 2020. PMID: 32110564 Free PMC article. Review.
- A Social Media-Based Acute Alcohol Consumption Behavior (NekNomination): Case Series in Italian Emergency Departments. Barbieri S, Feltracco P, Lucchetta V, Gaudio RM, Tredese A, Bergamini M, Vettore G, Pietrantonio V, Avato FM, Donato D, Boemo DG, Nesoti MV, Snenghi R. Barbieri S, et al. Interact J Med Res. 2018 Jan 31;7(1):e2. doi: 10.2196/ijmr.6573. Interact J Med Res. 2018. PMID: 29386170 Free PMC article.
- Managed alcohol as a harm reduction intervention for alcohol addiction in populations at high risk for substance abuse. Muckle W, Muckle J, Welch V, Tugwell P. Muckle W, et al. Cochrane Database Syst Rev. 2012 Dec 12;12:CD006747. doi: 10.1002/14651858.CD006747.pub2. Cochrane Database Syst Rev. 2012. PMID: 23235633 Review.
- Potentiation of the depressant effect of alcohol by flunitrazepam in rats: an electrocorticographic, respiratory and electrocardiographic study. Freitas L, Amaral A, Conceição R, Barbosa G, Hamoy MK, Barbosa A, Paz C, Santos M, Hamoy A, Paz A, Favacho-Lopes D, Mello V, Hamoy M. Freitas L, et al. Naunyn Schmiedebergs Arch Pharmacol. 2024 Apr 27. doi: 10.1007/s00210-024-03111-w. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38676788
- COVID-19, Possible Hepatic Pathways and Alcohol Abuse-What Do We Know up to 2023? Michalak A, Lach T, Szczygieł K, Cichoż-Lach H. Michalak A, et al. Int J Mol Sci. 2024 Feb 12;25(4):2212. doi: 10.3390/ijms25042212. Int J Mol Sci. 2024. PMID: 38396888 Free PMC article. Review.
- Substance use and pre-hospital crash injury severity among U.S. older adults: A five-year national cross-sectional study. Adeyemi O, Bukur M, Berry C, DiMaggio C, Grudzen CR, Konda S, Adenikinju A, Cuthel A, Bouillon-Minois JB, Akinsola O, Moore A, McCormack R, Chodosh J. Adeyemi O, et al. PLoS One. 2023 Oct 25;18(10):e0293138. doi: 10.1371/journal.pone.0293138. eCollection 2023. PLoS One. 2023. PMID: 37878571 Free PMC article.
- Geneva: World Health Organization; 2018. Global status report on alcohol and health 2018.
- 2010 national and state costs of excessive alcohol consumption. Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD. Am J Prev Med. 2015;49:0–9. - PubMed
- Alcoholism and its effects on the central nervous system. Mukherjee S. Curr Neurovasc Res. 2013;10:256–262. - PubMed
- Effects of acute alcohol on excitability in the CNS. Harrison NL, Skelly MJ, Grosserode EK, Lowes DC, Zeric T, Phister S, Salling MC. Neuropharmacology. 2017;122:36–45. - PMC - PubMed
- A review on alcohol: from the central action mechanism to chemical dependency. Costardi JV, Nampo RA, Silva GL, Ribeiro MA, Stella HJ, Stella MB, Malheiros SV. Rev Assoc Med Bras (1992) 2015;61:381–387. - PubMed
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- Europe PubMed Central
- PubMed Central
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Introduction
- Conclusions
- Article Information
Baseline Cox proportional hazards models are shown in blue, and lifestyle-adjusted models are shown in orange. Lifestyle factors were smoking, body mass index, red meat intake, vegetable intake, physical activity, and self-reported health. Associations between subcategories of alcohol consumption and incident cardiovascular diseases are presented as hazard ratios for hypertension and coronary artery disease (CAD). Alcohol consumption is reported as US standard drinks per week, equivalent to 14 g of alcohol. Error bars represent the 95% CI.
Using fractional polynomial nonlinear mendelian randomization analyses with Alcohol Use Disorder–Restricted instrument, localized average causal effects were metaregressed against mean consumption in strata of residual alcohol intake, and exposure-outcome associations were reconstructed as the derivative of the best fit model for hypertension and coronary artery disease. Alcohol consumption is reported as US standard drinks per week, with each standard drink equivalent to 14 g of alcohol. Solid lines refer to odds ratio (OR) estimates, and shaded areas denote 95% CIs for the model.
Using fractional polynomial nonlinear mendelian randomization analyses with Alcohol Use Disorder–Restricted instruments, localized average causal effects were metaregressed against mean consumption in each strata of alcohol, and these plots were reconstructed as the derivative of the best fit model for γ-glutamyl transferase (GGT), low-density lipoprotein (LDL) cholesterol, systolic blood pressure (SBP), and diastolic blood pressure (DBP). Alcohol consumption is reported as US standard drinks per week, with each standard drink equivalent to 14 g of alcohol. Solid lines refer to odds ratio (OR) estimates, and shaded areas denote 95% CIs for the model.
eMethods 1. UK Biobank Data Collection
eMethods 2. Exclusions
eMethods 3. Alcohol Variables
eMethods 4. Continuous Variables
eMethods 5. Mass General Brigham Biobank
eMethods 6. Genetic Instruments
eMethods 7. Observational Analysis
eMethods 8. Two-Sample MR Analyses
eMethods 9. Allele Score Analyses
eMethods 10. Nonlinear MR
eTable 1. Single-Nucleotide Variants Included in Genetic Instruments
eTable 2 . Definitions of Cardiovascular Disease Phenotypes in the UK Biobank
eTable 3. Mean Weekly Standard Measures (1 measure, 14 g) in Drinking Categories and Beverage Composition of Alcohol Consumption
eTable 4. Assessments for Pleiotropy in Single-Nucleotide Variants in Lifelong Abstainers
eTable 5. Assessments of MR Assumptions in Primary Genetic Instrument
eTable 6. Assessments of MR Assumptions in Secondary Genetic Instruments
eTable 7. Primary Genetic Associations Between Alcohol and Continuous Traits
eTable 8. Secondary Genetic Associations Between Alcohol and Continuous Traits
eTable 9. Secondary Genetic Associations Between Alcohol and Cardiovascular Disease Phenotypes
eTable 10. MR-PRESSO Sensitivity Analyses for 2SMR Analyses
eTable 11. Sex-Stratified Allele Score Associations Between Alcohol and Primary Cardiovascular Disease Outcomes
eTable 12. Nonlinear MR Tests
eTable 13. Study Characteristics in Mass General Brigham Biobank
eTable 14. Associations of AUD-R Allele Score With Alcohol Consumption and Blood Pressure Measurements in Mass General Brigham Biobank
eFigure 1. Alcohol Consumption and Prevalence of Cardiovascular Diseases
eFigure 2. Secondary Analyses for Confounding in Epidemiological Associations Between Alcohol Consumption and Cardiovascular Disease
eFigure 3. Mean Values of 6 Different Lifestyle Factors Within Alcohol Consumption Subcategories
eFigure 4. Genetic Associations of Alcohol With CVD Phenotypes Using Allele Scores
eFigure 5. Genetic Allele Score Associations of Alcohol With CVD Phenotypes Stratified by Category of Alcohol Consumption
eFigure 6. Fractional Polynomial Nonlinear MR Analyses, Using AUD-R Genetic Instruments, of Alcohol and Secondary Cardiovascular Disease Phenotypes
eFigure 7. Nonlinear MR Analyses of Alcohol and Total Mortality
eFigure 8. Fractional Polynomial Nonlinear MR Analyses, Using Secondary Genetic Instruments, of Alcohol and 6 Cardiovascular Disease Phenotypes
eFigure 9. Piecewise Nonlinear MR Analyses of Alcohol and 6 Cardiovascular Disease Phenotypes
eFigure 10. Fractional Polynomial Nonlinear MR Analyses of Alcohol and Continuous Traits
eFigure 11. Piecewise Nonlinear MR Analyses of Alcohol and Continuous Traits
eFigure 12. Sex-Stratified Nonlinear MR Analyses for Primary Outcomes
eFigure 13. Nonlinear MR Analyses of Alcohol and Medication-Corrected Blood Pressure
eFigure 14. Multivariable Fractional Polynomial Nonlinear MR Analyses, Adjusting for Smoking, BMI, and Depression
eFigure 15. Fractional Polynomial Nonlinear MR Analyses of Alcohol and Blood Pressure in Mass General Brigham Biobank
eReferences.
- Error in Abstract JAMA Network Open Correction April 20, 2022
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Biddinger KJ , Emdin CA , Haas ME, et al. Association of Habitual Alcohol Intake With Risk of Cardiovascular Disease. JAMA Netw Open. 2022;5(3):e223849. doi:10.1001/jamanetworkopen.2022.3849
Manage citations:
© 2024
- Permissions
Association of Habitual Alcohol Intake With Risk of Cardiovascular Disease
- 1 Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts
- 2 Center for Genomic Medicine, Massachusetts General Hospital, Harvard Medical School, Boston
- 3 Cardiovascular Research Center, Massachusetts General Hospital, Harvard Medical School, Boston
- 4 now with Regeneron Pharmaceuticals, Tarrytown, New York
- 5 Qatar University, Doha, Qatar
- 6 Verve Therapeutics, Cambridge, Massachusetts
- Correction Error in Abstract JAMA Network Open
Question What is the risk of cardiovascular disease associated with different amounts of habitual alcohol consumption?
Findings In this cohort study of 371 463 individuals, genetic evidence supported a nonlinear, consistently risk-increasing association between all amounts of alcohol consumption and both hypertension and coronary artery disease, with modest increases in risk with light alcohol intake and exponentially greater risk increases at higher levels of consumption.
Meaning In this study, alcohol consumption at all levels was associated with increased risk of cardiovascular disease, but clinical and public health guidance around habitual alcohol use should account for the considerable differences in cardiovascular risk across different levels of alcohol consumption, even those within current guideline-recommended limits.
Importance Observational studies have consistently proposed cardiovascular benefits associated with light alcohol consumption, while recent genetic analyses (ie, mendelian randomization studies) have suggested a possible causal link between alcohol intake and increased risk of cardiovascular disease. However, traditional approaches to genetic epidemiology assume a linear association and thus have not fully evaluated dose-response estimates of risk across different levels of alcohol intake.
Objectives To assess the association of habitual alcohol intake with cardiovascular disease risk and to evaluate the direction and relative magnitude of cardiovascular risk associated with different amounts of alcohol consumption.
Design, Setting, and Participants This cohort study used the UK Biobank (2006-2010, follow-up until 2016) to examine confounding in epidemiologic associations between alcohol intake and cardiovascular diseases. Using both traditional (ie, linear) and nonlinear mendelian randomization, potential associations between alcohol consumption and cardiovascular diseases (eg, hypertension and coronary artery disease) as well as corresponding association shapes were assessed. Data analysis was conducted from July 2019 to January 2022.
Exposures Genetic predisposition to alcohol intake.
Main Outcomes and Measures The association between alcohol consumption and cardiovascular diseases, including hypertension, coronary artery disease, myocardial infarction, stroke, heart failure, and atrial fibrillation.
Results This study included 371 463 participants (mean [SD] age, 57.0 [7.9] years; 172 400 [46%] men), who consumed a mean (SD) 9.2 (10.6) standard drinks per week. Overall, 121 708 participants (33%) had hypertension. Light to moderate alcohol consumption was associated with healthier lifestyle factors, adjustment for which attenuated the cardioprotective epidemiologic associations with modest intake. In linear mendelian randomization analyses, a 1-SD increase in genetically predicted alcohol consumption was associated with 1.3-fold (95% CI, 1.2-1.4) higher risk of hypertension ( P < .001) and 1.4-fold (95% CI, 1.1-1.8) higher risk of coronary artery disease ( P = .006). Nonlinear mendelian randomization analyses suggested nonlinear associations between alcohol consumption and both hypertension and coronary artery disease: light alcohol intake was associated with minimal increases in cardiovascular risk, whereas heavier consumption was associated with exponential increases in risk of both clinical and subclinical cardiovascular disease.
Conclusions and Relevance In this cohort study, adjustment for coincident, favorable lifestyle factors attenuated the observational benefits of modest alcohol intake. Genetic epidemiology suggested that alcohol consumption of all amounts was associated with increased cardiovascular risk, but marked risk differences exist across levels of intake, including those accepted by current national guidelines.
Controversy has surrounded the association between alcohol intake and cardiovascular disease (CVD), which remains the leading global cause of death. 1 - 3 Observational studies have repeatedly demonstrated a lower risk of CVD with light to moderate alcohol intake compared with either abstinence or heavy consumption, suggesting J- or U-shaped epidemiologic associations. 4 - 9 However, the observed cardiac benefits of alcohol have been hypothesized to be the product of residual confounding because of favorable lifestyle, socioeconomic, and behavioral factors that tend to coincide with modest alcohol intake. 10 , 11
Efforts to address this complex association through a randomized clinical trial have been met with logistical and ethical challenges, culminating in the discontinuation of a trial of modest alcohol consumption led by the National Institutes of Health. 12 In the absence of a randomized trial, a technique using human genetic data (ie, mendelian randomization [MR]) has enabled assessment for potential causal associations by leveraging naturally occurring genetic variants as unbiased proxies for an exposure (ie, alcohol intake). 13 Given the random allocation of genetic variants at conception, MR obviates concerns of confounding and reverse causality, 2 key limitations of observational epidemiology.
Prior genetic analyses using an MR approach have provided evidence to suggest a causal link between alcohol consumption and increased risk of cardiovascular disease. 14 - 17 However, traditional methods in MR often presume linearity and may therefore be limited in their assessments of relative risks across levels of alcohol intake, which have the potential to inform public health decisions around quantitative risk thresholds. 18 , 19 Indeed, the paucity of quantitative data focused on the consequences of moderate alcohol consumption (ie, 1 vs 2 drinks per day) has contributed to variable public health recommendations on low-risk drinking around the world and to the US Department of Agriculture (USDA) maintaining its long-standing, sex-specific recommendations of fewer than 15 drinks per week for men and fewer than 8 drinks per week for women in the 2020-2025 Dietary Guidelines for Americans. 8 , 20 - 22 More recently, emerging techniques in nonlinear MR (NLMR) have used large-scale, individual-level genetic data to enable simultaneous assessments of potential causality and association shape. 18 , 19 , 23 - 26
To further explore the association between alcohol intake and CVD, we first reexamined how coincident lifestyle and behavioral factors affect the well-established J-shaped observational associations. Next, through traditional MR and NLMR approaches, we evaluated the association of alcohol consumption with CVD, with an emphasis on better understanding relative differences in risk across levels of intake.
The primary study population comprised 371 463 unrelated individuals of European genetic ancestry from the UK Biobank (eMethods 1-4 in the Supplement ). Informed consent was obtained for all UK Biobank study participants, and analysis was approved by the Mass General Brigham Health Care institutional review board. Select analyses were replicated in 30 716 individuals from the Mass General Brigham Biobank (eMethods 5 in the Supplement ). This study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline.
Genetic instruments for habitual alcohol consumption were constructed using single-nucleotide variants (SNVs) associated with alcohol use disorder (AUD; 9 SNVs) and the Alcohol Use Disorder Identification Test–Consumption (AUDIT-C) questionnaire (13 SNVs), as identified in a recent genomewide association study (eTable 1 in the Supplement ). 27 To derive appropriate and specific genetic proxies for habitual alcohol consumption, we first removed SNVs independently associated with relevant risk factors (smoking, body mass index [BMI], physical activity, vegetable intake, red meat intake, overall health rating, C-reactive protein level, and total cholesterol level) to create refined, nonpleiotropic genetic instruments: 4 SNVs were removed from the AUD instrument to create the AUD-Restricted (AUD-R) instrument (5 remaining SNVs), and 3 SNVs were removed from the AUDIT-C instrument to create the AUDIT-C–Restricted (AUDIT-C-R) instrument (10 remaining SNVs). We then assessed all 4 instruments for the 3 assumptions of MR: (1) association with exposure (alcohol phenotypes [eMethods 3 in the Supplement ]), with an F statistic greater than 10 signifying a strong genetic instrument 28 ; (2) no association with confounders; and (3) no direct association with the outcome. We standardized each instrument to a 1–drink per day increase in consumption using empirical, UK Biobank estimates, to arrive at population-specific genetic proxies for habitual alcohol consumption. The AUD-R genetic score was designated the primary instrument owing to residual pleiotropy detected within the AUDIT-C-R instrument (eMethods 6 in the Supplement ).
We focused on 6 CVD phenotypes: hypertension, coronary artery disease (CAD), myocardial infarction (MI), stroke, heart failure, and atrial fibrillation (eTable 2 in the Supplement ). In addition, 10 continuous variables were examined: systolic blood pressure (SBP), diastolic blood pressure (DBP), low-density lipoprotein (LDL) cholesterol level, high-density lipoprotein (HDL) cholesterol level, total cholesterol level, triglyceride level, apolipoproteins A and B levels, γ-glutamyl transferase level, and C-reactive protein level.
Drinking groups were defined as abstainers (0 drinks/wk), light (>0-8.4 drinks/wk), moderate (>8.4-15.4 drinks/wk), heavy (>15.4-24.5 drinks/wk) and abusive (>24.5 drinks/wk) (eMethods 3 in the Supplement ). We first assessed the prevalence and hazards of CVDs within each drinking group; the latter was estimated by Cox proportional hazards using abstainers as the reference groups. We then evaluated potential differences in smoking frequency, BMI, self-reported physical activity, cooked vegetable intake, red meat consumption, and self-reported health by drinking category to assess whether light to moderate alcohol consumption is associated with a healthier overall lifestyle. Adjusting for these 6 lifestyle factors, we reestimated hazards of CVD to assess for possible confounding (eMethods 7 in the Supplement ).
We then conducted 2-sample MR, prioritizing inverse-variance weighted (IVW) meta-analyses of the association of each SNV with the outcome divided by the association of the same SNV with alcohol consumption; weighted median, MR-Egger, and MR–Pleiotropy Residual Sum and Outlier (MR-PRESSO) analyses were secondarily performed to address potential invalid instruments, outlying SNVs, and directional pleiotropy (eMethods 8 in the Supplement ). In addition, we used allele score methods, which combine all externally weighted SNVs into a single instrument that is tested for association with each outcome (eMethods 9 in the Supplement ). To additionally test for pleiotropy, analyses were repeated in lifelong abstainers, a population devoid of alcohol consumption, to assess any direct association between the genetic instrument and the outcome (eMethods 6 in the Supplement ). For continuous traits, we considered significant any association surpassing a Bonferroni-corrected threshold of P < .005 [.05 / 10 traits] and, for cardiovascular diseases, a threshold of P < .008 [.05 / 6 diseases].
Although traditional (linear) MR estimates the change in odds of the outcome per change in the exposure, these analyses have limited ability to assess for nonuniform directionality of the exposure-outcome relationship or differential risks across levels of the exposure. 19 To directly test for nonlinearity, the genetic association between exposure and outcome may be tested at various intervals of the exposure. This method allows for assessment of localized average causal effects in deciles of residual (IV-free) alcohol intake, which can be used to re-create the overall association using either fractional polynomial or piecewise linear methods (eMethods 10 in the Supplement ). We applied NLMR methods as validated previously to test the shape of each potential association, prioritizing diseases and continuous traits with robust evidence from our traditional MR analyses. 18 Sensitivity analyses included removal of abstainers and multivariable NLMR (eMethods 10 in the Supplement ). 26 All analyses were conducted using PLINK version 2.0 and R version 3.5 (R Project for Statistical Computing).
Baseline characteristics of the 371 463 study participants from the UK Biobank are shown in Table 1 . The mean (SD) age was 57.0 (7.9) years, 172 400 (46%) were men, and the mean (SD) alcohol consumption was 9.2 (10.6) standard drinks per week; 121 708 participants (33%) had hypertension, and 27 667 participants (7.5%) had CAD. Among light drinkers (mean [SD] consumption, 4.9 [2.7] drinks/week), alcohol intake comprised 38% beer, 29% red wine, 24% champagne or white wine, 6% spirits, 3% fortified wine, and 0.2% other alcoholic beverages; among heavy drinkers (mean [SD] consumption, 21 [3.8] drinks/week), alcohol intake comprised 38% beer, 24% red wine, 28% champagne or white wine, 7% spirits, 2% fortified wine, and 0.1% other alcoholic beverages (eTable 3 in the Supplement ).
Well-established J- or U-shaped curves were recapitulated for the association between alcohol consumption and both the prevalence and hazards of hypertension, CAD, MI, stroke, heart failure, and atrial fibrillation ( Figure 1 ; eFigures 1 and 2 in the Supplement ). However, individuals in the light and moderate consumption group had healthier lifestyle behaviors than abstainers, self-reporting better overall health and exhibiting lower rates of smoking, lower BMI, higher physical activity, and higher vegetable intake (eFigure 3 in the Supplement ). Adjustment for the aforementioned lifestyle factors attenuated the cardioprotective associations with modest alcohol intake. For example, in baseline models, moderate intake was associated with significantly lower risk of hypertension and CAD, but adjustment for just 6 lifestyle factors rendered these results insignificant
The primary genetic instrument (AUD-R) strongly associated with alcohol intake in the UK Biobank (β = 7.0 standard drinks per week; P < .001), with a corresponding F statistic of 780. Genetic instruments were strongly associated with a range of alcohol phenotypes and did not appear to associate with confounders (eTables 4-6 in the Supplement ).
Alcohol consumption due to the primary genetic instrument was associated with increased γ-glutamyl transferase level, a well-established marker of alcohol use, 29 as well as increases in cardiovascular risk factors, such as SBP, DBP, and LDL cholesterol level. A lack of association in 34 423 lifelong abstainers and nonsignificant MR-Egger intercepts suggested no significant pleiotropy. However, a potential pleiotropic effect was detected in the association between alcohol and both HDL cholesterol and apolipoprotein A levels among lifelong abstainers. Associations of genetic instruments with 31 continuous phenotypes in current drinkers and lifelong abstainers, as well as in specific categories of alcohol consumption, are summarized in eTables 7 and 8 in the Supplement .
Genetic evidence supported strong associations between alcohol use and increased risk of hypertension and CAD ( Table 2 ; eFigure 4 in the Supplement ). In traditional 2-sample MR analyses, a 1-SD increase in genetically predicted alcohol consumption was associated with a higher risk of hypertension (IVW estimate: odds ratio [OR], 1.3; 95% CI, 1.2-1.4; P < .001) and CAD (IVW estimate: OR, 1.4; 95% CI, 1.1-1.8; P = .006). Secondary MR analyses (weighted median, MR-Egger, MR-PRESSO, and excluding abstainers) and MR analyses of other cardiovascular disease phenotypes supported the primary observations ( Table 2 ; eFigure 4 and eTables 9 and 10 in the Supplement ).
To begin to assess the risk of cardiovascular disease associated with different levels of habitual alcohol consumption, we conducted allele score analyses stratified by amount of alcohol consumed (eFigure 5 in the Supplement ). In both light and moderate drinkers, a 1–drink per day increase in the allele score was associated with at least nominally significantly increased odds of hypertension (light drinkers: OR, 1.3; 95% CI, 1.1-1.5; P = .003; moderate drinkers: OR, 1.7; 95% CI, 1.3-2.2; P < .001) and CAD (light drinkers: OR, 1.7; 95% CI, 1.2-2.4; P < .001; moderate drinkers: OR, 1.8; 95% CI, 1.1-2.8; P = .02). In abusive drinkers, a 1–drink per day increase in the allele score was associated with even greater risks of hypertension (OR, 2.6; 95% CI, 1.6-4.2; P < .001) and CAD (OR, 5.7; 95% CI, 2.4-13.5; P < .001). These patterns persisted after stratifying by sex (eTable 11 in the Supplement ); for example, directionally consistent associations of genetically predicted alcohol intake with hypertension and CAD were observed in both male (eg, hypertension: OR, 1.4; 95% CI, 1.1-1.8; P = .01) and female (eg, CAD: OR, 1.7; 95% CI, 0.9-3.0; P < .09) light drinkers, although the results were not statistically significant for women.
To better assess differential risk profiles across strata of alcohol consumption, we pursued NLMR analyses prioritizing outcomes with robust evidence from previous traditional MR analyses. Three separate statistical tests indicated that nonlinear models approximated the association between alcohol intake and both hypertension and CAD better than linear models (eTable 12A in the Supplement ); specifically, quadratic models best fit these associations (both models, P < .001) ( Figure 2 ). For each condition, all amounts of alcohol consumption were associated with an increased risk of disease. Furthermore, increased alcohol consumption was associated with increases in disease risk that were exponential and unequal in magnitude, even when comparing light and moderate levels of consumption (ie, between 1 and 2 drinks per day). Similar trends toward nonlinear and single-directional (ie, quadratic) associations were noted for other cardiovascular diseases and for all-cause mortality (eFigures 6 and 7 in the Supplement ).
NLMR also suggested nonlinear associations between alcohol intake and continuous risk factors, such as SBP and LDL cholesterol levels (eTable 12B in the Supplement ), with consistently positive and quadratic associations observed between alcohol consumption and SBP (model, P < .001), DBP (model, P = .001), and LDL cholesterol level (model, P < .001) ( Figure 3 ).
Sensitivity analyses using different genetic instruments (AUDIT-C-R score and also a single SNV at the biologically relevant ADH1B gene) and excluding abstainers largely supported the primary observations, as did secondary sex-stratified analyses and use of medication-adjusted values of SBP and DBP (eFigures 8-13 and eTable 12C and D in the Supplement ). Tests of nonlinearity using the AUDIT-C-R instrument further supported nonlinear associations between alcohol intake and all primary outcomes, although, notably, piecewise linear analyses suggested a threshold effect, wherein alcohol intake was not associated with increased cardiovascular risk until roughly 7 to 14 drinks per week. Slight decreases in cardiovascular risk were observed with modest alcohol intake when associating the secondary AUDIT-C-R score with hypertension and in select secondary analyses conducted in women (eFigures 8 and 12 in the Supplement ). However, similar patterns were not observed with the single SNV instrument at ADH1B ; for analyses of LDL cholesterol level, SBP, and DBP in women; when removing abstainers; or when conducting linear MR in female light drinkers, thereby suggesting that these findings were likely attributable to residual pleiotropy in the AUDIT-C-R instrument and reduced power of sex-stratified NLMR analyses, respectively (eFigures 5, 8, and 10-12 and eTable 11 in the Supplement ). Multivariable NLMR adjusting for smoking, BMI, and depression supported the primary observations (eFigure 14 in the Supplement ).
We assessed for similar nonlinear associations in the Mass General Brigham Biobank (30 716 participants) (eTable 13 in the Supplement ). The primary genetic instrument was strongly associated with habitual alcohol intake in the Mass General Brigham Biobank and showed directionally consistent associations with DBP and directionally consistent results with SBP (eTable 14A in the Supplement ). NLMR again yielded quadratic models as those best capturing the associations between alcohol consumption and DBP (model, P < .001), suggesting exponential increases in DBP with progressively greater alcohol intake (eTable 14B and eFigure 15 in the Supplement ). The results for SBP were not statistically significant.
In this study, we assessed the association of habitual alcohol consumption with cardiovascular disease risk. Epidemiological analyses identified that coincident lifestyle factors may confound established observational trends. Human genetic data suggested causal associations between alcohol intake and risk of hypertension and CAD that increase with even modest alcohol consumption and are exponential in magnitude.
These results permit several conclusions. First, the reported cardioprotective effects of light to moderate alcohol consumption may be the product of confounding lifestyle factors. Consistent with prior studies, we found J- and U-shaped epidemiologic curves for the association of alcohol intake with cardiovascular disease, but we also found that light to moderate alcohol consumers exhibited healthier lifestyles than abstainers. 10 , 11 Adjusting for only a few lifestyle factors ascertained by the UK Biobank, we observed attenuation in the apparent protective associations between modest alcohol intake and cardiovascular risk, suggesting that adjustments for yet unmeasured or unknown factors may further attenuate—if not, eliminate—the residual, cardioprotective associations observed among light drinkers.
Second, human genetic evidence is suggestive of a causal relationship between alcohol consumption and cardiovascular disease that is consistently risk increasing, with the magnitude of risk rising exponentially at higher levels of intake. Here, using linear MR, we added to the evidence base that alcohol intake may be associated with a range of cardiovascular diseases and risk factors. 14 - 17 However, applying NLMR and formal tests for nonlinearity, we found that light alcohol consumption was associated with minimal cardiovascular risk, but similar to recent epidemiological findings, risk of cardiovascular disease increased exponentially at higher levels of intake. 8 These results carry at least 2 important clinical implications: (1) they substantiate prior claims that no amount of alcohol is protective against cardiovascular disease, and (2) they newly demonstrate that the adverse effects of alcohol unduly affect those who consume heavily, implying that for an equivalent reduction in alcohol intake, the improvements to cardiovascular health may be significant for heavier drinkers but only slight for those who consume modestly.
Third, the substantial differences in cardiovascular risk across the spectrum of alcohol consumption may have important implications for clinical and public health recommendations around habitual alcohol use. Specifically, our results suggest that consuming as many as 7 drinks per week is associated with relatively modest increases in cardiovascular risk. However, nonlinear modeling uncovered unequal increases in cardiovascular risk when progressing from 0 to 7 vs 7 to 14 drinks per week in both men and women. Although risk thresholds are inherently somewhat subjective, these findings again bring into question whether an average consumption of 2 drinks per day (14 drinks per week) should be designated a low-risk behavior. 8 , 20 - 22 Furthermore, as several-fold increases in risk were observed for those consuming 21 or more drinks per week, our results emphasize the importance of aggressive efforts to reduce alcohol intake among heavy drinkers.
The present study has several limitations. First, despite efforts to minimize the effects of pleiotropy, it remains possible that the associations between alcohol intake and cardiovascular disease represent a shared genetic basis, rather than a direct causal relationship. Second, our primary genetic instrument comprised SNVs associated with a diagnosis of AUD—an indirect measure of alcohol use—rather than SNVs directly associated with a continuous measure of alcohol consumption; notably, there may be some differences in the genetic architecture of habitual alcohol consumption and AUD. However, we empirically evaluated our instruments in the UK Biobank and found that the AUD genetic instrument demonstrated strong associations with all tested alcohol phenotypes, including weekly intake and drinking category, indicating that the instrument did not simply reflect a susceptibility to heavy alcohol consumption. Similarly, the AUDIT-C questionnaire is also designed to screen for heavy alcohol consumption rather than habitual alcohol consumption. 30 Nevertheless, future assessments testing our genetic instruments—as well as others for continuous alcohol consumption—in additional, large genetic data sets will be of importance.
The findings of this study suggest that the observed cardioprotective effects of light to moderate alcohol intake may be largely mediated by confounding lifestyle factors. Genetic analyses suggest causal associations between alcohol intake and cardiovascular disease but with unequal and exponential increases in risk at greater levels of intake, which should be accounted for in health recommendations around the habitual consumption of alcohol.
Accepted for Publication: February 1, 2022.
Published: March 25, 2022. doi:10.1001/jamanetworkopen.2022.3849
Correction: This article was corrected on April 20, 2022, to fix an error in the Abstract.
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2022 Biddinger KJ et al. JAMA Network Open .
Corresponding Author: Krishna G. Aragam, MD, MS, Massachusetts General Hospital, 185 Cambridge St, Simches Research Building, 3.128, Boston, MA 02114 ( [email protected] ).
Author Contributions : Mr Biddinger and Dr Aragam had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Biddinger, Emdin, Ellinor, Kathiresan, Aragam.
Acquisition, analysis, or interpretation of data: Biddinger, Emdin, Haas, Wang, Hindy, Khera, Aragam.
Drafting of the manuscript: Biddinger, Aragam.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Biddinger, Emdin, Haas, Wang, Hindy, Aragam.
Obtained funding: Ellinor, Aragam.
Administrative, technical, or material support: Haas, Aragam.
Supervision: Khera, Aragam.
Conflict of Interest Disclosures: Dr Haas reported receiving personal fees and stock and stock options from Regeneron Pharmaceuticals outside the submitted work. Dr Ellinor reported receiving grants from Bayer AG and IBM Health and personal fees from Bayer AG, MyoKardia, Quest Diagnostics, and Novartis during the conduct of the study. Dr Kathiresan reported being an employee of Verve Therapeutics; owning equity in Verve Therapeutics, Maze Therapeutics, Color Health, and Medgenome; receiving personal fees from Medgenome and Color Health; serving on the advisory boards for Regeneron Genetics Center and Corvidia Therapeutics; and consulting for Acceleron, Eli Lilly and Co, Novartis, Merck, Novo Nordisk, Novo Ventures, Ionis, Alnylam, Aegerion, Haug Partners, Noble Insights, Leerink Partners, Bayer Healthcare, Illumina, Color Genomics, MedGenome, Quest Diagnostics, and Medscape outside the submitted work. Dr Khera reported receiving personal fees from Merck, Amarin Pharmaceuticals, Amgen, Maze Therapeutics, Navitor Pharmaceuticals, Sarepta Therapeutics, Verve Therapeutics, Silence Therapeutics, Veritas International, Color Health, and Third Rock Ventures and receiving grants from IBM Research outside the submitted work. Dr Aragam reported receiving speaking fees from the Novartis Institute for Biomedical Research. No other disclosures were reported.
Funding/Support: Dr Khera is funded by grants 1K08HG010155 and 5UM1HG008895 from the National Human Genome Research Institute and a Hassenfeld Scholar Award from Massachusetts General Hospital. Dr Aragam is supported by grant 1K08HL153937 from the National Institutes of Health and grant 862032 from the American Heart Association.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Information: This project was conducted using the UK Biobank resource (project ID 7089) and the Mass General Brigham Biobank.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
June 26, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
New study shows alcohol rehabilitation and abstinence reduce the risk of alcohol-associated cancers
by Centre for Addiction and Mental Health

A new study conducted by the Centre for Addiction and Mental Health (CAMH), Bordeaux University Hospital, France, and the World Health Organization (WHO) has found that individuals with alcohol dependence who undergo rehabilitation or maintain abstinence experience significantly lower risks of developing alcohol-associated cancers.
The work, titled "Alcohol rehabilitation and cancer risk : a nationwide hospital cohort study in France," was published in The Lancet Public Health . It is the largest of its kind to provide evidence linking reduced or ceased alcohol consumption with a decreased risk of all alcohol-attributable cancers, including liver and throat cancers.
The nationwide retrospective cohort study analyzed data from more than 24 million French people, all adults residing in mainland France who were discharged from hospitals between 2018 and 2021. The researchers found that approximately 6.3% of men and 1.6% of women had alcohol dependence, which was strongly associated with alcohol-related cancers, including hepatocellular carcinoma, as well as oral, pharyngeal, laryngeal, esophageal, and colorectal cancers, in both sexes.
However, they also found that rehabilitation treatment or a history of abstinence was associated with significantly lower risks compared to alcohol dependence without rehabilitation or abstinence. This underscores the effectiveness of treatment strategies in combating cancer risks linked to alcohol dependence.
"The research team was surprised at the size of the treatment intervention effect in this study," said Dr. Jürgen Rehm, Senior Scientist at the Institute for Mental Health Policy Research at CAMH and study senior author. "We know that alcohol dependence treatment is effective but the fact that alcohol dependence is a recurring chronic disease often makes us forget that even with relapses, periods of abstinence markedly lower the risk of cancer and other chronic diseases."
"From a public health standpoint, our research highlights a troubling neglect of alcohol dependence compared to other health issues in both research and policy priorities," added article lead author Dr. Michaël Schwarzinger, Department of Prevention, Bordeaux University Hospital. "Consequently, alcohol dependence continues to be a silent, dreadful epidemic in countries like France, especially given that the average annual level of adult alcohol consumption per capita in that country is over twice the global average."
"We know that the most effective strategy to reduce the overall burden of harms caused by alcohol, including cancer , lies in population-level policies—measures such as increasing alcohol taxes, reducing alcohol availability, and banning or restricting marketing," said Dr. Carina Ferreira-Borges, Regional Adviser for Alcohol, Illicit drugs, and Prison health at the WHO Regional Office for Europe.
"However, this study underscores that health systems' response is also crucial to lower the risk of alcohol-attributable cancers. By increasing accessibility to interventions for alcohol rehabilitation and abstinence in health care settings, countries could do more to protect their populations from preventable cancers. Therefore, we call for more investment in rehabilitation and treatment services for alcohol use disorders in France and other countries of the WHO European Region."
Dr. Leslie Buckley, CAMH's Chief of Addictions, emphasized the importance of these findings, stating, "In Canada, hospital admissions for alcohol-attributable conditions out-number those for myocardial infarction , and many people face barriers to evidence-based treatment due to stigma and challenges in accessing in-person care. Innovations such as virtual treatment can overcome these challenges by offering flexible and cost-effective solutions. At CAMH, we're conducting research on fully-virtual day programs which show promise, replicating traditional rehabilitation intensity without the need for physical infrastructure, thereby reducing wait times and making treatment more accessible.
"Given the imminent increase in alcohol availability in Ontario, it's essential to consider how we could make treatment more accessible. Increased alcohol availability is likely to lead to higher consumption, and accessible virtual treatment programs could address this by providing crucial care to those in need."
Explore further
Feedback to editors

Genetic test improves clinical care for children with cancer in England
52 minutes ago

Drugs that kill 'zombie' cells may benefit some older women, but not all, study finds

Study shows hairy skin does not become less sensitive with age
5 hours ago

Herpes infections take major economic toll globally, new research shows
10 hours ago
Light targets cells for death and triggers immune response with laser precision
12 hours ago

Umbilical cord milking does not appear to increase risk of neurodevelopmental delay in non-vigorous infants
13 hours ago

Blood test can help predict risk of obstructive sleep apnea

Pandemic newborns in India more likely to have lower birth weight, shows study
14 hours ago

Hormones associated with body composition during pregnancy linked to infants' mental health
Overlooked brain organ plays key role in promoting brain repair after stroke, researchers discover
Related stories.

2.6 million die annually due to alcohol: WHO
Jun 25, 2024

Alcohol can cause breast cancer, but most women in the US aren't aware of the health risks
Jun 6, 2024

Few women know alcohol linked to breast cancer: WHO Europe
Mar 8, 2024

Experts recommend an evidence-based public health approach to excessive alcohol use
Apr 23, 2024

Wastewater reveals socioeconomic link to alcohol consumption
May 17, 2024

Study: Higher alcohol taxes could prevent thousands of cancers
Sep 22, 2021

Recommended for you

Researchers find that gratitude is a useful emotional tool in reducing desire to smoke

Multiple myeloma: New insights into early detection of aggressive tumors
17 hours ago

New imaging detects deadly lung and prostate cancers, may improve treatment
19 hours ago

Physical exercise prevents nerve damage during chemotherapy, sports scientist shows
18 hours ago

Researchers discover novel way to make breast cancer cells die
20 hours ago
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Frontiers for Young Minds
- Download PDF
How Alcohol Affects the Adolescent Mind

Have you ever heard about executive functions (EFs)? These are skills that you use every single day! They allow you to achieve your goals, resist your impulses, and make wise decisions. During adolescence, your brain is still developing. In particular, the area of your brain responsible for your EFs is still maturing and becoming more efficient. This means that, as an adolescent, you are still getting better at exercising your EFs. This ongoing brain and skill development is probably one of the reasons why adolescents are more likely to engage in risky behaviors, including drug abuse and binge drinking (BD). BD is a pattern of excessive alcohol use that negatively affects EFs and other aspects of brain structure and function. In this article, we describe three important EFs and explain how development of these skills is related to brain maturation. We discuss how BD may impact EFs and potentially disrupt an adolescent’s transition to adult life.
Your Brain at School
It is Monday morning and your first day of school after the summer break. You see your best friends and run in their direction—you have so much to tell them about your summer holidays at the beach. They laugh about the story you are telling, and you continue to explain how awesome it all was, but suddenly the bell rings and it is time for class! The teacher enters the room, so you stop talking to your friend—you know that it is time to pay attention to what the teacher has to say. She starts by welcoming the students and gives out the first assignment: turn to page 78 and do exercise 3. You try to remember the math rules that you learned last year, and you manage to complete the exercise. After a while, it is time for lunch. You go to the vending machine to get something to eat, and at the same time, you continue telling your friends about your summer adventures, while you also respond to a message from your mother ( Figure 1 ).
- Figure 1 - You use your executive functions all the time! For example, during a day at school, you might: (A) inhibit inappropriate behaviours, like stopping yourself from talking while the teacher is teaching; (B) maintain and update information in memory by keeping track of the calculations you are doing while solving a math problem in class; and (C) flexibly switch between tasks, like when you are texting while talking to a friend and deciding what to eat.
Every day, your brain works in complex ways that allow you to achieve your goals, as in the scene above. To do this, you make use of crucial brain abilities called executive functions (EFs). “Executive” is related to the verb “to execute,” meaning to put into effect. Thus, EFs are skills we need to achieve a goal, like successfully completing an exercise in class. EFs allow us to go from wanting to do something to actually getting it done, like planning a vacation, solving a problem, and even having a conversation with a friend. To organize, plan, and complete these tasks we need three main EFs: inhibition, updating, and shifting.
Executive Functions in Daily Life
Inhibition refers to the ability to control attention, behavior, thoughts, and emotions, preventing inappropriate responses from occurring. Inhibition keeps you from acting impulsively, so you can do what is most appropriate and necessary ( Figure 1A ). For example, imagine you are crossing the street on your way to school, but suddenly you see a car that is not slowing down. You must stop walking (inhibit your behavior) so you do not get run over. You can inhibit your thoughts, too. Imagine a situation in which you must study for an exam, but you cannot stop thinking about the last episode of your favorite TV series. In this case, you must control that thought so you can focus on studying for the exam and get a good grade.
Updating is the ability to bring to mind (and keep in mind) information that is most important for the task at hand ( Figure 1B ). Imagine someone gave you directions to a certain destination and, after each turn you take, you must update the information in your mind and remember what the next turn is.
Lastly, shifting refers to your ability to adapt to a new environment by switching your attention to a new task that has become more important ( Figure 1C ). Imagine that you are on your way to school, but the road you usually take is closed for construction. In this situation, you must come up with a new way to get to school, while you inform your mother that you are taking a different route and keep track of time, so you will not be late for class.
Adolescence—A Window for Brain Development… and Risky Behaviors!
Inhibition, updating, and shifting are the basis for the ability to plan (e.g., prepare for your next vacation), to reason (e.g., come up with good arguments during a debate) and to solve problems (e.g., find the answer to an equation in math class). The EFs start developing in childhood and they continue improving all the way through adulthood, mainly due to the development of a brain region called the prefrontal cortex (PFC), which is located behind the forehead and is in charge of the EFs. It works like the boss of the EFs, since it has a key role in our ability to reason, plan and achieve our goals in coordination with the rest of the brain.
As the PFC develops during childhood and adolescence, the EFs improve. There is just a small problem: this crucial part of the brain matures slowly compared to other regions [ 1 ]. For instance, the limbic system , also known as the emotional brain, develops earlier than the PFC ( Figure 2 ). The limbic system is involved in your experience of emotions and how you respond to those emotions. It is related to the processing of reward and is responsible for sensation-seeking behavior. During adolescence, the reward regions—i.e., structures located deep within the brain that drive behavior towards pleasurable stimuli such as food, sex, and drugs—are more developed than the PFC, meaning that the reward system may have more influence over daily decisions than the PFC does [ 2 ].
- Figure 2 - Brain maturation in children, adolescents, and adults.
- (A) The adolescent brain is functionally different from the child and adult brain, as there is an imbalance between the fully mature, overactivated reward system (in orange) and the still-maturing PFC (in blue). (B) This imbalance between the strong reward system and weaker PFC may make adolescents more prone to get involved in risky behaviours, including reckless driving, unprotected sexual activity, drug abuse, and binge drinking.
When adults have the desire to do something risky (e.g., jumping from a high cliff into the water, gambling away their savings, or driving a car beyond the speed limit), they can consider the consequences and make a conscious decision about whether to follow their desires. However, adolescents have less control over their impulses and desires due to the imbalance between the (overactivated) reward system and the (still-maturing) PFC. Altogether, this makes teenagers more likely to get involved in risky behaviors, such as getting into fights, having unprotected sexual activity, or abusing drugs or alcohol.
Effects of Binge Drinking in the Adolescent Brain
Although alcohol use is not allowed until the age of 18–21 in many countries and is totally banned in the majority of North African and Middle Eastern countries, heavy alcohol drinking or binge drinking (BD) is one of the most frequent and concerning risky behaviors amongst Western teenagers. BD is when a person drinks an excessive amount of alcohol in a short period of time [ 3 ], and it is a regular behavior in about one-third of European and American youths. BD is a major public health concern because it can have many negative consequences on the body and brain, including cognitive deficits (e.g., poor memory skills and reduced ability to control impulses).
Neuroimaging (a technique that allows doctors and scientists to obtain pictures of the brain to study its structure and function), has shown that the brain of young binge drinkers is structurally and functionally different than those of young non-binge drinkers [ 4 ]. This is particularly true for the frontal lobe, where the still-maturing PFC is located, and certain regions of the reward system. These brain abnormalities can have several implications for the EFs of young binge drinkers.
Scientific evidence revealed that young people with a pattern of BD perform worse on typical laboratory tasks that measure EFs compared to those who drink small amounts of alcohol or do not drink [ 5 ]. For example, in a task to measure inhibition, participants are asked to push a button repeatedly, but to stop as soon as they hear or see a specific “stop” signal, like a beep or a change in color. Studies showed that young binge drinkers commit more errors and stop their responses more slowly, suggesting impairments in inhibition. When switching between tasks, studies report slower performance and lower accuracy in young binge drinkers, which indicates weak shifting ability. Last, when it comes to updating, studies show that binge drinkers do not have major difficulties bringing necessary information to mind, suggesting that this executive function is less impaired by BD.
A Vicious Cycle
Inhibition seems to be the EF most affected by BD. However, in addition to BD decreasing inhibition, reduced inhibitory control may also be the cause of this drinking pattern. People with weak inhibition may easily lose control over their drinking, leading to a BD episode. Likewise, the inhibition impairments caused by BD may decrease a person’s ability to prevent or stop alcohol misuse [ 6 ]. Thus, you can see that there is a vicious cycle between BD and poor inhibition, which may result in a higher risk for developing alcohol abuse as an adult ( Figure 3 ). Longer lasting and heavier drinking patterns have also been linked to changes in brain structures critical for learning, memory, and the proper functioning of EFs. Importantly, however, early evidence suggests that the brain may recover if BD stops [ 7 ], which illustrates how important it is to limit the intensity and frequency of BD episodes.
- Figure 3 - Excessive alcohol use may lead to a “vicious cycle,” in which the effects of BD on inhibition encourage continued and increased alcohol intake, which in turn leads to a further decrease in inhibition.
Make Healthy Choices
To conclude, BD has concerning negative consequences in young people. As you have seen, this pattern may lead to weakened EFs, which, in turn, may cause poorer performance in school, violent behaviors, unsafe sex, drug use, and continued or increasing alcohol use. BD during the adolescent period is particularly worrying, given that excessive drinking might disrupt the maturational processes of brain regions that are still developing, such as the PFC. If you ever find yourself tempted or pressured to drink to the point you feel drunk, think about how alcohol can affect both your daily functioning and the healthy development of your brain. Remember to make mindful choices to take care of your brain!
Executive Functions : ↑ A set of mental processes that allow you to guide and manage your thinking and emotions and help you to act in appropriate, purposeful, and goal-directed ways.
Inhibition : ↑ Inhibition involves being able to suppress or control impulsive thoughts, actions, or feelings and instead do what is more appropriate or needed in a given situation.
Updating : ↑ Updating is a function responsible for bringing and keeping relevant information in your mind while replacing content that is no longer relevant according to the task at hand.
Shifting : ↑ Shifting is the ability that allows you to quickly switch focus, attention, and strategies when faced with changing tasks or situations, facilitating adaptation to new information or rules.
Prefrontal Cortex : ↑ A brain region located behind the forehead that is involved in executive functions as well as the expression of personality and social behavior.
Limbic System : ↑ A set of structures deep inside the brain that is in charge of the survival functions and has a major role in emotional and behavioral responses, motivation, and memory.
Sensation Seeking : ↑ A tendency to seek out new and intense experiences. It can lead to risk-taking behaviors including drug misuse, with potential short- and long-term negative consequences.
Reward System : ↑ A set of brain regions that associates certain behaviors (e.g., eating, drinking, having sex) with sensations of pleasure, so that the likelihood of repeating these behaviors increases.
Binge Drinking : ↑ An excessive, episodic alcohol use pattern, particularly prevalent among young people, characterized by the consumption of 4–5 drinks within a short period of time (≤2 h).
Neuroimaging : ↑ A discipline that examines the structure and function of the brain by means of images or pictures obtained in a safe and reliable way.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was conducted at the Psychology Research Centre (CIPsi), School of Psychology, University of Minho, supported by the Foundation for Science and Technology (FCT) through the Portuguese State Budget (ref. UIDB/PSI/01662/2020). In addition, EL-C has received funding from the Portuguese Foundation for Science and Technology (FCT; refs. PTDC/PSI-ESP/1243/2021 and CEECIND/07751/2022). NA-A was also supported by the FCT (ref. SFRH/BD/146194/2019).
[1] ↑ Gogtay, N., Giedd, J. N., Lusk, L., Hayashi, K. M., Greenstein, D., Vaituzis, A. C., et al. 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. National Acad. Sci . 101:8174–9. doi: 10.1073/pnas.0402680101
[2] ↑ Casey, B. J., and Jones, R. M. 2010. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J. Am. Acad. Child Adolesc. Psychiat . 49:1189–201. doi: 10.1016/j.jaac.2010.08.017
[3] ↑ National Institute of Alcohol Abuse and Alcoholism [NIAAA] (2023). Understanding Binge Drinking. NIAAA Newsletter. Available online at: https://www.niaaa.nih.gov/sites/default/files/newsletters/Newsletter_Number3.pdf (accessed October 31, 2023).
[4] ↑ Jones, S. A., Lueras, J. M., and Nagel, B. J. 2018. Effects of binge drinking on the developing brain: studies in humans. Alcoh. Res.: Curr. Rev . 39:87–96.
[5] ↑ Carbia, C., López-Caneda, E., Corral, M., and Cadaveira, F. 2018. A systematic review of neuropsychological studies involving young binge drinkers. Neurosci. Biobehav. Rev . 90:332–49. doi: 10.1016/j.neubiorev.2018.04.013
[6] ↑ López-Caneda, E., Rodríguez Holguín, S., Cadaveira, F., Corral, M., and Doallo, S. 2014. Impact of alcohol use on inhibitory control (and vice versa) during adolescence and young adulthood: a review. Alcohol Alcohol . 49:173–81. doi: 10.1093/alcalc/agt168
[7] ↑ Lees, B., Mewton, L., Stapinski, L. A., Squeglia, L. M., Rae, C. D., and Teesson, M. (2019). Neurobiological and cognitive profile of young binge drinkers: a systematic review and meta-analysis. Neuropsychol. Rev . 29:357–85.
- Original article
- Open access
- Published: 31 January 2019
The effectiveness and effects of alcohol regulation: evidence from India
- Dara Lee Luca 1 ,
- Emily Owens 2 &
- Gunjan Sharma 3
IZA Journal of Development and Migration volume 9 , Article number: 4 ( 2019 ) Cite this article
24k Accesses
16 Citations
31 Altmetric
Metrics details
We provide quasi-experimental evidence on the effects of alcohol regulation on alcohol consumption and associated public health outcomes using detailed individual level and aggregate data from India, where state-level laws regulating the minimum legal drinking age generate substantial variation in the availability of commercially produced alcohol across people of different ages. We find that despite significant law evasion, men who are legally allowed to drink are substantially more likely to consume alcohol. Further, men who are legally allowed to drink are significantly more likely to commit violence against their partners, suggesting a causal channel between alcohol consumption and domestic violence. These results are robust to the exclusion of states with prohibition, implying that they are driven by differences in MLDA. We also examine the effects of alcohol regulation on other public health outcomes. Consistent with the existing literature, we find evidence that smoking and drinking are complements. Finally, we provide suggestive evidence that stricter alcohol control is associated with lower rates of motor vehicle accidents and crimes against women, but not other forms of crime .
1 Introduction
High rates of alcohol consumption are correlated with adverse outcomes at both individual and societal levels; well-documented examples include increased rates of mortality, injuries, motor vehicle accidents, and criminal activity (Carpenter and Dobkin 2009 ). The regulation of alcohol consumption in developed countries has been shown to causally affect at least some of these outcomes (Carpenter and Dobkin 2011 ). However, there is little evidence on the impacts of alcohol regulation in the context of developing countries, where rapidly changing demographic trends and consumption patterns, combined with weaker institutions, warrant a pressing need for a closer examination. Luca et al. ( 2015 ) provide suggestive evidence that alcohol prohibition laws in India are effective in reducing consumption even with imperfect implementation and that prohibition significantly reduces intimate partner violence, as well as some other crimes against women. The effectiveness and effects of other (and perhaps less fiscally and politically costly) regulatory measures, such as varying the minimum legal drinking age, however, are still questions in need of answers.
In this paper, we combine a newly collected set of alcohol regulations—minimum legal drinking age and prohibition status—across states in India to assess their effects on important public health outcomes, including domestic violence, alcohol and tobacco consumption, motor vehicle accidents, and crime. India provides a unique setting to study this question, as it is one of the few countries in the world where there is considerable spatial and temporal variation in alcohol regulation policies across states. A number of states prohibit alcohol consumption altogether, and in others, the minimum legal drinking age (MLDA) varies from 18 to 25 years old. In contrast, previous studies in Western countries have relied on narrower bands of MLDAs: 21 in the USA, 18 or 19 in Canada, 18 in Mexico, or 16 to 18 in Europe (Carpenter and Dobkin 2011 ). This wider variation allows us to better isolate the impact of the alcohol regulation from the effect of biological aging or other policy factors.
Further, as in many recently developed and developing countries, Indian alcohol policies are still in flux. For example, the state of Mizoram removed prohibition in 2014, the state of Kerela started phasing in prohibition in the same year, and the state of Bihar began enforcing prohibition in 2016. In addition, high-profile cases of drunken driving, murders, and violence against women in India have recently received worldwide attention, with the popular narrative focusing on alcohol consumption and the need for policy interventions. These findings are also relevant for the USA and the developed world, where there has been increasing scrutiny on the connection between drinking and sexual violence, most recently focusing on college campuses (Sampson 2002 ). Footnote 1
Using micro-level survey data, we first attempt to establish a first-stage relationship between alcohol regulation and consumption in the Indian context. Estimates of this relationship are per se important given the lack of research on the impact of alcohol regulation in the context of developing countries, where costs of alcohol use are arguably larger. Footnote 2 There is also evidence that easily evaded state-level regulations are less effective at reducing alcohol consumption (Dills and Miron 2004 ; Lovenheim and Slemrod 2010 ). It follows that an important question facing policy makers in developing countries is whether government regulation is effective at all, particularly in the presence of strong black markets and perceived weaker rule of law relative to the USA or Canada (Allen et al. 2008 ).
Our data confirm that a large fraction of men under the legal drinking age consume alcohol. In spite of non-trivial law evasion, however, by comparing the prevalence of alcohol consumption among men above and below the drinking age in the same state, and men of the same age across states with different age restrictions governing alcohol sales, we find that regulations reducing access to alcohol are associated with substantive reductions in the consumption of the good in question. Indeed, our results demonstrate while legal access does not determine alcohol consumption absolutely, it does significantly affect the likelihood of alcohol consumption—men who are of legal drinking age are almost 30% more likely to drink alcohol, which is quite similar to results found in developed countries (e.g., Carpenter and Dobkin 2010 ). Further, we show that being of legal drinking age is positively linked to smoking, which is consistent with existing literature from developed country settings that drinking and smoking are complements (Decker and Schwartz 2000 ; Dee 1999 ).
We then demonstrate that husbands who are legally allowed to drink are both substantially more likely to consume alcohol and commit domestic violence against their partners. The results are robust to controlling for a rich set of individual-level characteristics and both observed and unobserved state-level variation. According to a study conducted by the World Health Organization, a third of violent husbands drink, and most of the violence takes place during intoxication. In the USA, close to 40% of police calls for domestic violence involve alcohol (McClelland and Teplin 2001 ). Studies of partner violence episodes also indicate that episodes are more severe when the man has been drinking (Leonard and Quigley 1999 ; McKinney et al. 2010 ). However, previous studies have found it difficult to disentangle the effects of alcohol consumption from unobserved risk factors that may be both correlated with drinking and violent behaviors. Using exogenous state variation in alcohol regulation, our results provide evidence that drinking may be a causal factor in domestic violence. Our results are robust to the exclusion of dry states (those with a complete prohibition on the consumption of alcohol). Thus, the causal link between alcohol consumption and domestic violence is prevalent in states with MLDA ranging from 18 to 25. To the extent that a substantively important causal relationship between alcohol consumption and domestic violence exists, alcohol regulation is a potentially promising lever that policy makers could use to reduce violence against women.
We then look at the effects of alcohol control on various public health outcomes using state-year panel data in 16 states between 1980 and 2010 that may be affected by alcohol regulation. Consistent with our analysis using individual-level data, our results using state-year panel data suggest that policies restricting alcohol access may have a secondary social benefit of reducing some forms of violence against women, including molestation, sexual harassment, and cruelty by husband and relatives. At the same time, changes in the MLDA do not appear to be associated with reductions in criminal behavior more broadly. We find suggestive evidence that stricter regulation is associated with lower fatalities rates from motor vehicle accidents and alcohol consumption, but also deaths due to consuming spurious liquor (alcohol that is produced illicitly).
The paper proceeds as follows: in Section 2 , we characterize the cultural context and evolution of alcohol consumption and alcohol regulation. We describe how we measure these changes in Section 3 . We present our general empirical strategy in Section 4 , followed by our individual-level and state-panel results in Section 5 . Section 6 concludes.
2 Institutional setting: cultural and legal attitudes towards alcohol in India
Compared to the USA, Canada, and the UK, Indian state government regulations provide a compelling large-scale social policy “experiment” in which to examine the consequences of alcohol regulation. The range of alcohol policies vary substantially both across Indian states and within states over time, which we were able to document 19 of the 29 Indian states, where roughly 90% of the 2001 Indian population lives. Between 1980 and 2008, the time frame for our analysis, the MLDA ranged from 18 to 25 years across the country, and some states had blanket prohibition policies. In addition, we identified six states that changed their MLDA at least once; Bihar increased its MLDA from 18 to 21 in 1985, and Tamil Nadu repealed prohibition and enacted an 18-year-old MLDA in 1990, then subsequently increased it to 21 in 2005. Andhra Pradesh and Haryana both enacted prohibitionary policies in 1995 (the MLDA in Andhra Pradesh had been 21, and 25 in Haryana) only to later repeal them in 1998 and 1999. Maharashtra lowered its MLDA to 21 from 25 for 1 year (2005), and Orissa supplanted its 21-year-old MLDA with blanket prohibition in 1994 and 1995.
Several features of Indian society are responsible for these variations in alcohol regulations. Compared to opium and marijuana, alcohol is a relatively less popular intoxicant in India until colonial rule. At the same time that British occupation promoted alcohol use, British MP William S. Caine founded the first prohibitionary organization, the Anglo-Indian Temperance Association (AITA), in 1888. The Indian temperance movement gained considerable strength during the 1920s, such that it led to a considerable decrial of alcohol and its derivatives. The success of the AITA, combined with religious diktats denouncing the sin of intoxication, resulted in a substantial increase in the taxation of such products, with a view to decrease their consumption (Hardiman 2006 ). The agitation which led to the most decisive political action on the issue was the Gandhian movement; during the struggle for independence in the1940s, temperance came to be closely associated with nationalism. Alcohol consumption was seen as a “Western evil” which distracted the people from their quest for independence (Blocker et al. 2003 ).
Upon Independence in 1947, temperance was enshrined in Article 47 of the Constitution of India, as a part of the Directive Principles of State Policy, which reads “The State shall regard the raising of the level of nutrition and the standard of living of its people.” The prejudice against alcohol remains in modern society, and alcohol is rarely served at any important events (Mohan et al. 2001 ). Alcohol is also primarily consumed by males; female alcohol consumption is generally uncommon, and alcohol consumption by women outside the home (e.g., in bars or restaurants) is particularly taboo (Benegal et al. 2005 ).
Upon Independence, Indian states were granted control over policies regulating sales and consumption of alcohol. Footnote 3 Similar to in the USA, variation in alcohol regulation is partly driven by a demand for revenue by individual state governments. Where it is legal, alcohol is taxed heavily at the state level, and Indian states derive around a fifth of their revenue from alcohol taxation—the second largest single source of government funding after sales taxes (Saxena 1999 ). Moreover, there is a long history in India of a powerful alcohol lobby with industry figures influencing the political process, both in the form of party donations and as representatives (Prasad 2009 ).
Attitudes towards alcohol consumption in India have more recently evolved in large part due to the combined forces of globalization, prosperity, and changing demographics. Researchers have noted that alcohol consumption has increased dramatically over the past 30 years, and survey evidence suggests that Indians are beginning to drink at ever-younger ages (Mohan et al. 2001 ; Prasad 2009 ; Saxena 1999 ). Problematic drinking is now prevalent in many parts of the country; according to a recent Lancet report, alcohol-related problems account for more than 20% of hospital admissions, 18% of psychiatric emergencies, more than 20% of all brain injuries, and 60% of all injuries reporting to India’s emergency rooms (Prasad 2009 ). The dynamic push-and-pull of cultural prejudices, changing societal mores, increasing public policy concern over alcohol-related issues, and state incentives to keep alcohol flowing, has led to an amount of flux in alcohol regulation that is not seen in most western countries.
3.1 Data on alcohol regulation in India
In order to evaluate the effects of alcohol regulation, we compiled a dataset of state-level laws and regulations pertaining to alcohol (sales and/or consumption), with particular attention to MLDAs for the time period 1980–2010 in India. Tracing amendments to minimum drinking age laws over time by state was a complex process. Even the well-known and much used database on Indian law, Manupatra, does not provide a complete chronological list of amendments to national, let alone state, laws. Further, most documentation of the history of state legislation exists only in hard copy in legal libraries. We employed a number of law students from across India to research and summarize the history of alcohol-related legislation in their home state. In this paper, we focus only on the non-price aspect of alcohol regulation, i.e. we do not include analysis on state taxes on alcohol. Footnote 4 , Footnote 5
Based on the legal analysis, we were able to document the historical evolution of MLDA and prohibitionary laws in 18 states from 1980 to 2009, and in 19 states in 1998, 1999, 2005 and 2006. Footnote 6 Figure 1 depicts a map of India with the MLDA in each state that we have information for during this time period. Multiple numbers in a state implies the state has had an MLDA set at each age at some point between 1980 and 2010.
MLDAs in India, 1980–2010. P, prohibition; 18, state had a minimum legal drinking age of 18 between 1980 and 2010; 21, state had a minimum legal drinking age of 21 between 1980 and 2010, 25, state had a minimum legal drinking age of 25 between 1980 and 2010
3.2 Individual-level data on drinking, domestic violence, and smoking
In order to evaluate the impact of alcohol regulation on individual behaviors, we use the 1998–1999 and 2005–2006 waves of the Indian National Family Health Survey (NFHS). The NFHS is a large-scale, multi-round survey conducted in a representative sample of households throughout India which is intended to provide state- and national-level information for India on a wealth of issues, including consumption of substances such as alcohol and tobacco. This allows us to test whether alcohol control regulations (such as higher MLDAs or prohibition) have a causal effect on alcohol consumption. The link between smoking and alcohol regulation is more complicated and is predicated on whether alcohol and tobacco are substitutes or complements (Decker and Schwartz 2000 ). If alcohol control regulations reduce drinking, then they will increase (decrease) incidences of smoking if tobacco and alcohol are substitutes (complements).
Importantly, for our purposes, in these waves of the NFHS, a subset of women was asked about their exposure to domestic violence in the previous year and in general. Both waves are intended to produce state- and national-level estimates, and individual responses in both the full survey and domestic violence sample can be weighted to represent all households, or all women in each state.
While the NFHS contains rich household and individual data, we recognize that using self-reported data could be problematic due to underreporting, especially on the topic of domestic violence. Footnote 7 That said, special attention was given to the domestic violence module in the women questionnaire portion of the NFHS. For example, the women’s questionnaire (that includes information on violence) is conducted separately from the household survey by trained female interviewers, so that the women have privacy when answering the questions, and the women are also assured that their answers will be kept strictly confidential. In the 2005 wave, women were also provided, on request, information on sources of help for abused women. These precautions are in keeping with the World Health Organization’s ethical and safety recommendations for research on domestic violence (International Institute for Population Sciences (IIPS) and Macro International 2007 ).
Table 1 presents summary statistics describing drinking, smoking, domestic violence, and other measures of household and women’s status in the NFHS, for both men overall and husbands from ages 15 to 50. The problem of partner abuse appears to be acute in India, but comparable to the likely incidence of domestic violence in the USA. In our data, around 18% of married women experienced violence at the hands of their husband specifically Footnote 8 ; a much-cited 1995 survey found that 25% of women in the USA experienced some form of domestic violence in their lifetime (Tjaden and Thoennes 2000 ), Footnote 9 and in 2010, the World Health Organization estimated that roughly 38% of ever-partnered women in South-East Asia experienced some form of intimate partner violence in their lifetime (World Health Organization 2013 ).
Approximately 30% of husbands are reported to consume alcohol, which was reported by whoever filled out the household survey in 1998 and by the husband directly in 2005. Footnote 10 Relative to the wives of abstinent husbands, women with drinking husbands were 11 percentage points more likely to also report abuse. Of course, such simple correlations may not represent a causal effect and may also be driven by a particular type of measurement error. In spite of the best efforts of surveyors, it is possible that, in the 1998 wave, women who may be answering both questions systematically misreport both alcohol consumption and victimization, both of which are stigmatized behaviors on the part of their husband. The direction of this misreporting is not obvious. On the one hand, reported alcohol consumption might be perceived as an explanation for violence, but on the other hand, people might be more inclined to falsely report positive attributes to “balance out” otherwise negative descriptions of their family. Our identification of the relationship between alcohol consumption, domestic violence, and alcohol regulation is based on the assumption that misreporting of domestic violence and misreporting of alcohol consumption is either conditionally independent of the specific location of a state’s MLDA or affect husbands of all ages in a particular state. Footnote 11
3.3 State-level panel data on crimes and mortality
We also examine the effects of alcohol control on crimes and important public health outcomes from 1980 to 2010, using administrative state-year panel data from the Indian National Crime Records Bureau (NCRB). There is ample evidence that increased alcohol consumption itself is associated with crime (Carpenter and Dobkin 2011 ). At the same time, tighter regulations on the sale of alcohol may increase the size of violent black markets. The net effect of alcohol regulation on crime, if that regulation lowers drinking, is therefore an empirical question.
Continuing with our analysis using individual-level data, we first investigate the effects of alcohol regulation on violence against women, focusing on four specific types of crime: rape, sexual molestation, sexual assault, and cruelty by husband and relatives. Next, we turn to other types of violent crimes in India that could be affected by alcohol control, including murder, dacoity (robbery by armed gangs of more than 5 people), robbery, and communal violence (riots). Finally, we investigate the effects of alcohol laws on mortality from injury death, which include fatalities from road accidents, firearms, alcohol consumption, and spurious liquor consumption. These are public health outcomes of policy interest and are included both because they have been the focus of other research on alcohol control and to provide a more complete picture of the impact of alcohol regulation in India.
In addition, we collected data on a host of state-specific time-varying variables that may be correlated with alcohol regulation and crime. These include annual measures of the number of police per capita from the NRCB, and state GDP per capita and government expenditure on education and welfare from the Reserve Bank of India. State-level unemployment rates, urbanization, and literacy rates are interpolated from the Decennial Census of India, collected in 1981, 1991, 2001, and 2011. Table 2 reports key summary statistics for these variables.
4 Empirical strategy
Our empirical strategy will be to identify the impact of alcohol control policies on rates of alcohol consumption and other pertinent outcomes using policy variation generated by the regulations governing the legal sale of alcohol in state s in year t : MLDA laws and outright prohibition, which affect whether or not individual i can legally purchase alcohol in his home state. The assumptions necessary to identify the impact of alcohol regulation on drinking and violence vary by data set.
Our individual-level data only span a short time period during which only three states changed their alcohol regulation. Here, our identification strategy is similar to Carpenter and Dobkin ( 2009 ), in that we are comparing the behavior of individuals whose ability to purchase alcohol legally is a discontinuous function of their age, but we are able to exploit additional variation across states in the age at which an individual can legally buy alcohol. Unfortunately, unlike Carpenter and Dobkin ( 2009 ), our data do not allow us to implement a true RDD, as we only know the person’s age in years and have a limited number of observations around the MLDA thresholds. However, our identification is strengthened by the fact that we are able to exploit the fact that there are four different regimes determining the person’s ability to purchase alcohol: age 18, age 21, age 25, and prohibition, so we are better able to disentangle the impact of the age-based alcohol regulation from other influences which vary with age.
Figure 2 depicts the simple means of men between the ages of 15 and 50 who drink, below and above each minimum legal drinking age threshold, alongside the same aged cohorts in prohibition states, giving a coarse sense of the independent impact of age on drinking behavior. Two things are clear: first, that even in dry states, a substantial fraction of men are comfortable reporting alcohol consumption, and second, even in the absence of an age cutoff, older men are more likely to drink. However, it is also evident from the states where the MLDA is 21 or 25 that after husbands reach the drinking age in their state, the fraction consuming alcohol increases relative to same-aged men living in dry states.
Fraction of all men who drink, relative to their age in relation to the state’s MLDA and same-age cohorts in prohibition states
Based on this suggestive pattern, we can confirm that men who are legally allowed to drink are more likely to consume alcohol by estimating the following linear probability model:
The dependent variable is a binary variable equal to 1 if the man reports drinking alcohol, or one of the other socially relevant outcomes that we observe in the NFHS—smoking or domestic violence (as reported by the man’s wife). Footnote 12 The main explanatory variable, Legal , is a binary variable equal to 1 if the man was legally allowed to drink over the past year from the time of the survey interview. Footnote 13 If the state had a blanket prohibition policy in place during the survey year, this value is equal to zero for all men, since no one is legally allowed to purchase alcohol in those states. We include as controls age, education level, employment status, religious affiliation, and family size in the matrix X hsy . Luca et al. ( 2015 ) directly estimated the impact of blanket prohibition on domestic violence. Here, states under prohibition do not directly contribute to the identification of β FS , but are used to better pin down the values of θ .
Because we observe both men above and below the drinking age in each of the 19 states represented in this sample, the impact of state-level characteristics that are correlated with the MLDA, such as state alcohol tax rates, are essentially differenced out by state by survey wave fixed effects, δ sy , and do not introduce bias into our estimates, as long as these unobserved variables impact everyone in the state equally. We will allow for arbitrary correlation in the unobserved component of drinking, u hsy , within each state. Although clustered standard errors permit heteroskedasticity and within-state error correlation, we may be concerned that the small number of clusters (19) may lead to standard errors that are downward biased (Cameron et al. 2008 ; Donald and Lang 2007 ). We therefore also present p values produced with wild cluster bootstrapping (Cameron et al. 2008 ).
Our empirical approach with the primary reduced form estimating equation for our state-level analysis being:
where MLDA st is either equal to the MLDA (set to 100 in the case of total prohibition) or a vector of three dummy variables that indicate whether or not 20 year olds are prohibited from purchasing alcohol (meaning the MLDA is 21 or higher), whether or not 24 year olds are prohibited from purchasing alcohol (meaning the MLDA is 25 or higher), and whether or not 30 year olds are prohibited from purchasing alcohol (meaning there is general prohibition). In states where the MLDA is 18, all three values are equal to 0, and in states with total prohibition, all values are set to 1. The matrix X st includes annual measures of police per capita, the literacy rate, GDP per capita, percent urban, and the percent of government expenditures spent on health and education, and we include γ s and δ t which are state and year fixed effects, respectively. The error term ε st is clustered at the state level. Since we are essentially estimating an aggregated version of the individual-level mechanisms (drinking and violence) modeled in Eq. 1 , all results are weighted by the annual state population.
Entering the MLDA linearly essentially captures the fact that changing the drinking age from 18 to 25, or 18 to total prohibition, will have a larger impact on the total amount of alcohol consumption than lowering the drinking age from 25 to 21. When we relax that parametric assumption, the estimated values of β 1 , β 2 , and β 3 represent the net effect of increasingly stringent regulations on alcohol sales.
5.1 Alcohol consumption
As discussed in Section 1 , state institutions tend to be weaker in developing countries and attitudes towards alcohol are fundamentally different than in western countries. As a result, it is possible that Indian alcohol regulation may not have a statistically or substantively significant impact on actual alcohol consumption. We present our estimates of Eq. 1 in Table 3 .
The baseline regression (column 1) examines the pooled cross-sectional relationship between a man’s likelihood to drink and whether he is legally allowed to drink. Without additional controls, men who are of legal drinking age are around 5 percentage points more likely to drink, but the estimate is statistically insignificant. The next column (column 2) adds on state and interview year fixed effects, and the magnitude of the estimate becomes much larger, suggesting that state-specific factors, such as norms about drinking, are correlated with both average individual demand for and legal restrictions on alcohol. In column 3, we control for individual characteristics of the husband that could affect his propensity to drink, including his age, years of schooling, household size, and religion. This doubles the explanatory power of our model, but decreases the magnitude of the estimate back to around 5 percentage points, suggesting that individual characteristics also play a large role in determining alcohol consumption.
Next, in column 4, we allow for state-by-survey wave fixed effects to account for any state-specific variation over time that may potentially bias our results, such as changes in state alcohol taxes or time-varying state policies to address drinking. In column 5, we enter state-by-age dummies, to allow for potential differential behavior of men of different age groups across states. We find a strong first-stage relationship across most specifications—men who are legally allowed to drink are more likely to report drinking, and the relationship is statistically significant. Given that the mean of alcohol consumption for men in the data is approximately 24%, this 5 percentage point change in likelihood of drinking is substantial, representing a 22% increase in the likelihood of drinking. Footnote 14
We also examine whether wives are more likely to drink if they are of legal drinking age or if their husbands are of legal drinking age. If lower MLDAs also increase female drinking, our aggregate crime results may reflect a combination of behavioral changes by both men and women caused by alcohol consumption. In addition, if women married to husbands above the drinking age were also systematically more likely to drink, this would suggest that these households were fundamentally different from households with younger husbands. As we show in panel A of Table 7 in Appendix , being of the legal drinking age does not have a measurable effect on women’s likelihood of reported drinking; wives of legal drinking age are more likely to drink, but the point estimates are quite small and imprecisely estimated. In panel B, we show that there is also scant evidence that the husband of being of legal drinking age affects the wife’s likelihood of drinking. These results help support the hypothesis that alcohol consumption is primarily a male activity and that variation in MLDA is correlated with changes in the prevalence that men consume alcohol. This also suggests that the general stigma of (reported) female alcohol consumption is not directly related to alcohol control policy.
5.2 Smoking
We next examine the impact of alcohol regulation on smoking behavior (panel B, Table 3 ). Multiple studies of smoking and alcohol consumption in developed countries have found that these are complements (Dee 1999 ; Decker and Schwartz 2000 ). Confirming a similar relationship in the context of a developing country is useful for public policy and assessing the full health effects of programs at targeting consumption of either good.
Consistent with the existing research, we find that being legally allowed to drink leads to a statistically significant increase in the likelihood of smoking, suggesting that smoking and drinking are complements. If we use the estimate from our preferred specification (including state-by-wave fixed effects), we find that the likelihood of smoking goes up by 6 percentage points, representing an increase of approximately 20%.
5.3 Domestic violence
We then assess the relationship between alcohol regulation and domestic violence. Alcohol consumption may be related to intimate partner violence through multiple channels. Some of these imply minimal scope for alcohol policy to reduce violence against women. For example, if individual preferences for different risky behaviors are positively correlated, people who drink more will also be more likely to engage in violence of all types (Carpenter and Dobkin 2010 ). Alcohol consumption may also serve as a form of self-medication in response to other life stressors, which themselves might directly cause someone to be violent (Khantzian 1997 ). However, there is also direct pharmacological effect of drinking on the actions of the drinker—studies in laboratory and experimental settings have thoroughly demonstrated that intoxication leads to increases in aggressive behavior (Bushman 1997 ). By increasing aggression and heightening emotional responses, alcohol use may increase inter-gender violence. Experiments have shown that alcohol consumption (or either party) increases the negativity of marital conflicts (Leonard and Roberts 1998 ) and verbal expressions of aggressive intentions among men (Eckhardt 2007 ). To the extent that a substantively important causal relationship between alcohol consumption and domestic violence exists, alcohol regulation is a potentially promising lever that policy makers could use to reduce violence against women, in contrast to other mechanisms that increase female safety but are more difficult to implement in practice, such as reducing the male-female wage gap (Aizer 2010 ).
We use the same empirical model on the sample of only husbands and include characteristics of the wife that may influence the likelihood of both drinking and domestic violence, such as the wife’s age, education, occupation status, and number of children. These covariates have also been shown to be correlates of domestic violence in other studies (Abramsky et al. 2011 , Jewkes 2002 ). In some specifications, we include variables to capture the degree of the wife’s household bargaining power, including whether she has money of her own that she can control and whether she believes that her spouse is justified in beating her if he suspects her of being unfaithful. Aizer ( 2010 ) emphasizes that a woman’s relative wage—rather than actual wage—determines intra-family bargaining power and partner violence, and so we include proxies for the intra-family wage gap with the age and education gap, which we define in two ways. First, we use the ratio of her age to her husband’s age and the ratio of her years of schooling to her husband’s. Second, we include dummy variables for linear differences in spousal age and years of education, as couples with larger or smaller differences in age or education are potentially very different from couples who are more similar.
The results are reported in Table 4 . In panel A, the dependent variable is the likelihood of the husband reporting drinking. In panel B, the dependent variable is the likelihood of the wife reporting domestic violence. As before, the main explanatory variable is a binary variable equal to 1 if the husband was legally allowed to drink over the past year from the time of the survey interview.
The data suggest that the impact of alcohol regulation on drinking does not vary significantly with marital status. Overall, husbands are approximately 23% more likely to drink if they are legally allowed to drink. Next, we take advantage of the fact that we observe husbands of the same age who may or may not be legally allowed to drink, since the location of the MLDA threshold varies across states, as well as husbands in the same state with different access to alcohol. We examine whether or not there is a differential change in the probability that husbands abuse their wives once men are legally allowed to drink. The results are reported in panel B of Table 4 . Column 1 in Table 4 shows a positive correlation between the wife reporting abuse and whether her husband is legally allowed to drink, although adjusting for the small number of clusters suggests that the relationship is only marginally precise. In column 2, we include fixed effects for the state of residence and year of interview, which reduces the magnitude of the effect to around 3 percentage points and is statistically insignificant. In column 3, we layer on household and husband characteristics, which make the estimated coefficient on Legal both larger and statistically significant, with p values just under 10%. In columns 4 through 7, we add the wife socio-economic characteristics and bargaining power variables to the regression model.
Across all specifications, the coefficient on Legal is positive, and associated with a 5 percentage point increase in the likelihood that the wife reports being a victim of domestic violence. The precision of this result is somewhat influenced by how we adjust our standard errors to take into the small number of states in our sample, but we are generally able to reject the null hypothesis that a husband’s legal access to alcohol is unrelated to his wife’s reports of victimization with at least 90% certainty.
We do not show the coefficients on the control variables for sake of space, but the signs are consistent with expectations and the existing literature on domestic violence. For example, the wife having her own money that she can control is negatively associated with the likelihood of her being beaten by her husband. There is also a positive correlation between domestic violence and whether the wife believes that a husband is justified in beating his wife if he believes her to be unfaithful. Footnote 15 Further, the higher the ratio of the wife’s years of schooling to her husband’s, the less likely the wife experiences intimate partner violence from her husband; the same goes for age. Relatedly, schooling and occupational status of both spouses are negatively correlated with domestic violence.
The results obtained in this paper are qualitatively similar to the ones obtained in Luca et al. ( 2015 ) where the analysis was focused on the effect of outright prohibition on alcohol consumption and domestic violence. Hence, it is reasonable to ask how much of the results of the current analysis are driven by variation in MLDA versus variation in prohibition status. In order to answer this question, we replicate Tables 3 and 4 after excluding states that were ever under prohibition. The results in Appendix Tables 8 and 9 show that variations in MLDAs between 18 and 25 years cause significant changes in alcohol consumption, smoking, and domestic violence. The coefficient estimates are very similar to the ones obtained using all states, with slightly lower precision which is consistent with the smaller number of observations used in this robustness test.
Do our results on domestic violence extend to other types of violence against women or violent crime in general? As discussed previously, alcohol could be linked to aggressive behavior through multiple channels, such as direct pharmacological effects (Chermack and Taylor 1995 ). We provide some information on this question using state-year panel data on officially recorded crimes. We then examine whether alcohol regulation affects other types of crime, including murder, dacoity, robbery, and communal riots. Dacoity is an India-specific penal code that refers to armed robbery by a gang of more than five people. Communal riots is a distinct and pervasive feature of Indian public life and are often linked to changing political equations and clash of religious sentiments (Krishna 1985 ). Since there are documented pharmacological effects of alcohol on aggression and inhibition (Miczek et al. 1994 ), it is worthwhile to investigate whether violent crime rates are affected.
Our estimates of the relationship between MLDA and violence against women are presented in Table 5 . With the exception of rape, raising the MLDA is associated with lower rates of violence against women overall, and in particular reported cruelty and sexual harassment. The effects are marginally statistically significant and are substantively small. Since the state-year panel will pick up changes in the behavior of all men, rather than just husbands, it may be the case that there are not enough young husbands to identify the effect of lowering the drinking age from 21 to 18 on drinking or violence. Nonetheless, we find some suggestive evidence that increasing the MLDA from 18 to 21 will lower most crimes against women, but the estimates are imprecisely estimated (especially when using the wild bootstrap). Overall, our results suggest that prohibition, rather than MLDA laws, may have a larger effect on violence against women.
The pattern between stricter alcohol access and other types of crime is not obvious. Overall, we find a very imprecise, relationship between stricter alcohol control and general forms of violence when the MLDA is entered linearly. A shift from a MLDA of 18 to 21 is linked with a statistically significant decline in dacoity (robbery by armed gangs), and stricter access to alcohol appears to be positively associated with higher incidence of communal violence, but the evidence on other types of crime is otherwise mixed. While we found evidence that higher MLDAs reduced male drinking, this ambiguous impact of overall violence is consistent with stricter alcohol control leading to more dangerous drinking and also with the formation of violent underground markets for alcohol, particularly in states that are completely dry. This type of market-based violence more generally tends to affect men, rather than women (Owens 2014 ). Footnote 16
5.5 Other public health outcomes
Finally, we examine the effects of alcohol regulation on important public health outcomes, as measured by mortality caused by road accidents, firearms, overall alcohol poisoning, and consumption of unregulated alcohol (e.g., home-brewed alcohol) (Table 6 ). Stricter alcohol regulation is negatively associated with lower mortality from road accidents. However, the estimates do not reach statistical significance ( p = 0.15). The negative relationship between MLDA and driving accidents is consistent with the literature from the USA (Carpenter and Dobkin 2009 ; Kaestner and Yarnoff 2011 ). Most of the existing evidence from developed country settings suggests that the impact of higher MLDA would fall mainly on young adults. Hence, our analysis using aggregate data may not pick up the effects of stricter alcohol regimes on specific age groups.
Our results suggest that stricter alcohol regulation is negatively linked to mortality from overall alcohol consumption but possibly higher mortality from consumption of unregulated liquor. That stricter regulation of alcohol is linked with negative health outcomes due to consumption of spurious liquor is consistent with anecdotal evidence, where numerous poisoning cases from consuming liquor produced on the black market have been reported in recent years, with perhaps the most infamous case occurring in 2009 in Gujarat which resulted in 136 deaths. Footnote 17
6 Discussion
In this paper, we set out with the goal to investigate the effects of alcohol regulation in the context of India. Specifically, we take advantage of rich individual-level data, staggered timing in age-based alcohol regulation across states, to examine how alcohol control affects drinking, smoking, and domestic violence. We find substantive evidence that reducing access to alcohol through MLDA laws decreases the likelihood of drinking, smoking, and domestic violence. We caveat that these results may not generalize to other developing countries, as India is unique in many aspects, in particular the complicated role of alcohol in society. In this particular case, however, in spite of the generally weak view of legal institutions and perceptions of entrenched government corruption, laws restricting alcohol consumption do appear to influence behavior.
Our first-stage results of higher alcohol consumption by men who are legally allowed to drink are consistent with multiple other studies. Rahman ( 2003 ) shows that alcohol prohibition policies in India are associated with a 20–40% decrease in the probability of alcohol consumption and a 40% decrease in the quantity of alcohol consumed using household expenditure data. A number of studies have used data from the Monitoring the Future survey, a representative sample of high school seniors from schools across the USA, to demonstrate that government regulation does indeed impact alcohol consumption among youth (Carpenter et al. 2007 , Dee 1999 , DiNardo and Lemieux 2001 ). Cook and Moore ( 2001 ) find a similar result using data on young adults from the National Longitudinal Survey of Youths. In more recent work, Carpenter and Dobkin ( 2010 ) find that individuals in the USA just over age 21 are 31% more likely to report having recently consumed alcohol and report drinking on almost 60% more days than individuals just under 21.
While similar to estimates from the developed world, our results should be interpreted in the context of survey evidence on the role that alcohol consumption plays in Indian society. Recent reports on drinking in India suggest that the signature pattern of alcohol consumption in India is frequent and heavy drinking. More than half of all drinkers fall into the criteria for hazardous drinking, which is characterized by bingeing and solitary consumption to the point of intoxication (Prasad 2009 ). In the 2005 wave of the NFHS, around 10% of drinkers report drinking every day, and a third of drinkers consume alcohol more than once a week. Moreover, spirits account for 95% of the beverages drunk in India. Although we do not have indicators for the quantity of alcohol consumed, it could be that husbands who are reported to drink are more than just casual drinkers. While we are unable to measure binge drinking, we do observe that MLDA laws are likely to promote public health outcomes on at least two dimensions.
We also provide evidence on the effects of alcohol regulation on a host of other significant public health outcomes. First, our analysis using individual-level data on self-reported smoking behavior suggests that alcohol and tobacco are complements, a finding that is consistent with existing research in developed country settings. Further, we provide suggestive evidence that stricter alcohol regulation reduces some forms of violence against women using both individual and state-year panel data. While there have been a number of papers examining the impact of alcohol regulation on crime, there has been a much smaller body of work focusing on violence against women. Most of these studies examine the impact of alcohol prices on violence against women using variation generated by state excise taxes. Markowitz ( 2000 ) examined spousal violence in the USA in the late 1980s. In models with individual fixed effects, she estimated that a 1% increase in alcohol price would reduce abuse aimed at wives by 5%. She also analyzes US panel data on individuals in the 1990s and finds that higher beer taxes have a (marginally) significant inverse relationship with physical assault but no substantive relationship with rape/sexual assault or robbery. Markowitz ( 2001 ) uses two waves of international survey data and finds that these prices exhibit significant negative associations with the rates of assault, robbery, and sexual assault against women in the cross-section but that the associations are no longer statistically significant when country fixed effects are included in the regressions. Durrance et al. ( 2011 ) find large positive correlations between alcohol consumption and female homicides at the state level, but focusing only on the plausibly exogenous variation in alcohol consumption driven by excise taxes yields a positive but small and statistically insignificant relationship. Grossman and Markowitz ( 1999 ) find that violence on college campuses, including both taking advantage of another person sexually or having been taken advantage of sexually, is inversely related to the price of beer in the state of the college.
Finally, we do not find strong evidence that stricter alcohol regulation affects other types of crime or public health outcomes. We find some suggestive evidence that stricter alcohol regulation is linked to fewer fatalities stemming from motor vehicle accidents and drinking. At the same time, stricter alcohol control is associated with more communal violence and deaths from consuming spurious liquor. However, the precision of our estimates precludes any strong conclusions. The lack of impacts on crime is consistent with the existing mixed evidence from the USA, where “Blue Law” restrictions of the sale of alcohol on Sundays have been found to lower crime (Heaton 2012 ; Han et al. 2016 ), but age-based restrictions can be associated with increased aggregate crime, particularly drug offenses (Conlin et al. 2005 ). At the same time, well-identified research on the impact of alcohol regulation in the USA has found that this type of alcohol regulation can generate statistically and substantively meaningful reductions in violent crime overall (Carpenter and Dobkin 2010 , Carpenter et al. 2007 ). As more, and better quality, survey data on Indian crime and victimization become available, the impacts of alcohol control on violence in developing countries may become clearer.
Due to a number of high-profile sexual assault cases that involved alcohol, ten schools have enacted alcohol bans in certain settings in order to combat sexual assaults, as many others consider following suit. http://www.cbsnews.com/news/will-dartmouths-hard-alcohol-ban-make-students-safer /
Pre-existing nutritional problems, lack of health care infrastructure, and economic deprivation can aggravate social cost of problematic drinking (Prasad 2009 ). Poor product regulation can also result in the consumption of low quality or adulterated alcohol, particularly the use of methyl alcohol, which can lead to death or serious organ damage, in particular blindness (Saxena 1999 ).
The Indian constitution divides important issues and revenue sources into three lists—the Union list (on which the parliament has exclusive power to legislate) contains items like defense, foreign affairs, income taxes, customs duties, corporate taxes etc.; the State list (on which individual states have exclusive authority to legislate and tax) includes public order, police, public health and sanitation, land revenue, taxes on agricultural income, taxes on lands on buildings, estate duty, taxes on electricity, taxes on vehicles, and taxes on luxuries including alcohol ; and the Concurrent list (the responsibility for which is shared by the Centre and States) which includes contracts, bankruptcy and insolvency, trustees and trusts, and civil procedure.
The complex nature of state taxes on alcohol in India makes summarizing them in a uniform and comprehensive way across states and time difficult. There are different taxes on different kind of alcohols within and across states (for ex., foreign imported liquor versus domestically produced beer). Duties range from flat-fees to percentages of the manufactured cost, retail price or the government-set market price. Further, the taxes can be levied on different units, ranging from per bulk liter to by proof level (Rahman 2003 ). Prohibition and alcohol regulations by age, however, are relatively more straightforward. We also include state-by-wave fixed effects in some specifications, which absorb any potential state-time changes in alcohol taxes.
It is possible that increases in the MLDA (fewer men legally allowed to drink) are associated with an increased need for tax revenue (lower taxes on alcohol). It is also possible that increases in MLDA (fewer men legally allowed to drink) correspond with policy shifts towards discouraging alcohol consumption (e.g., via higher taxes). In both these cases, we expect to see reduced rate of alcohol consumption among people eligible to drink, thus biasing our estimates of the impact of MLDAs on drinking towards zero. To the extent that alcohol consumption is a causal driver of domestic violence, we might expect a similar downward bias. Thus, our coefficients provide lower-bounds on the true impact of MLDA on drinking and domestic violence.
These are the major states in India from which reliable data are available. A number of northeastern states such as Nagaland, Manipur, Meghalaya, Assam, Tripura, and Sikkim suffered from insurgency and terrorism issues and many important data collection efforts (including manufacturing and household surveys, economic census etc.) were not conducted for several years.
One must always view self-reports of stigmatized behaviors with some degree of skepticism, as survey respondents may choose to not answer questions, or lie. In either case, we would be more likely to fail to identify any relationship between state policy and outcomes. That is, there would be a downward bias on our coefficient of interest—whether a man is above the legal drinking age. That said, the NFHS contains a large number of variables that are highly sensitive, including information on abortions, infant mortality, contraception, HIV status, and sexual activity, as well as alcohol consumption and exposure to violence. As such, survey takers were both trained and reminded on the survey instruments themselves, to assure the confidentiality of responses (both from other family members and third parties like law enforcement), and take active measures to ensure that confidentiality by, for example, checking to make sure that no other people are nearby during the interview.
In 1998, women were asked if their husband had ever beaten them. In 2005, women were asked if their husband ever: slapped them; twisted their arm or pulled their hair; pushed, shook, or threw something at them; punched them; kicked, dragged or otherwise beat them up; tried to choke or burn them on purpose; threatened them with a weapon; physically forced them to have intercourse against their will; and forced them to perform any sexual acts against their will. In our primary results, we identify women as being victims of domestic violence if they answer affirmatively to any of the questions about domestic violence.
We measure domestic violence exposure based on the respondent’s yes or no answer to a question about general victimization, rather than an available follow-up question about more recent victimization, for two reasons. First, while there are differences in wording, both waves of the NFHS ask about general exposure to domestic violence in the same, relatively direct way. In 2005, the surveyors were instructed to ask about more recent victimization after a series of other questions about intra-family relationships, making this question difficult to compare to the previous years. Second, respondents are asked about alcohol consumption in general, rather than recent alcohol consumption, so a general question about victimization more explicitly covers the same reference period, with similar recall bias.
This difference in reporting introduces potential survey-to-survey variation that is taken into account with survey wave fixed effects. Alcohol consumption was asked only as a binary variable in the 1998 wave, but both the intensive and extensive margin of consumption was asked in the 2005 wave.
Of course, even though these surveys were anonymous, it is possible that men under the MLDA may be less likely to report that they drink in 2005, biasing these estimates upwards. However, it is less obvious that the household survey respondent in 1998 will respond in the same way.
We do not instrument for alcohol consumption using husband legality for several reasons. First, because of the relatively low F statistics associated with our estimates of legality and alcohol consumption, the MLDA is unlikely to be a very reliable instrument for alcohol consumption. Second, the exclusion restriction may not hold entirely because the change in alcohol regimes (especially to and from prohibition) could have aggregate impacts on the economy and society that could in turn influence criminal behavior. These effects may be amplified in India given that a large portion of state revenues are derived from alcohol taxes and black markets for liquor are widespread. We hence focus on reduced form effects.
We also experimented with defining the explanatory variable as whether the husband was ever allowed to drink during the reference period, and the results remain similar.
Data constraints, specifically the relatively small number of observations around the MLDA cutoff and the discrete nature of recorded individual age, preclude implementing a true regression discontinuity design. However, we can roughly approximate the results of such a design by including a fourth-order control for how many years above, or below, the legal drinking age the husband is during the survey year, in non-prohibition states. These results, available on request, suggest very large, but also imprecise, positive impacts of legal access to alcohol on both consumption and domestic violence.
The 1998 wave does not contain information on the husband’s attitudes towards domestic violence, but to the extent that such attitudes could be endogenously correlated between spouses, we believe the wife’s attitude should help capture both her bargaining power within the household as well as the husband’s attitude towards domestic violence.
In Appendix Table 10, we present results after excluding states that were ever under prohibition. We find qualitatively similar results. While some coefficient estimates fall, others (e.g., sexual harassment and rape) are larger but less precise. In the case of murder, the coefficient on MLDA is statistically significant at the 5% level implying that the murder rate is higher in states with a higher MLDA.
The results regarding mortality are qualitatively same, though less precise when we restrict our analysis to the sub-sample of states that were never under prohibition. These results are presented in Appendix Table 11.
Abramsky T, Watts CH, Garcia-Moreno C, Devries K, Kiss L, Ellsberg M, Jansen H, Heise L (2011) What factors are associated with recent intimate partner violence? Findings from the WHO multi-country study on women’s health and domestic violence. BMC Public Health 11(1):109
Article Google Scholar
Aizer A (2010) The gender wage gap and domestic violence. Am Econ Rev 100(4):1847–1859
Allen F, Chakrabarti R, De S, Qian J, Qian M (2008) Law, institutions and finance in China and India. In: Eichengreen B, Gupta P, Kumar R (eds) Emerging giants: China and India in the world economy, pp 125–183
Google Scholar
Benegal V, Nayak M, Murthy P, Chandra P, Gururaj G, Obot IS, Room R (2005) Women and alcohol use in India. In: Alcohol, gender and drinking problems: perspectives from low and middle income countries, vol 89, p 123
Blocker J, Fahey D, Tyrrel IR (2003) Alcohol and temperance in modern history: an international encyclopaedia, vol 1. ABC-CLIO, Santa Barbara
Bushman BJ (1997) Effects of alcohol on human aggression: validity of proposed explanations. In: Galanter M (ed) Alcoholism: alcohol and violence, vol 13. Plenum Press, New York, pp 227–242
Cameron AC, Gelbach JB, Miller DL (2008) Bootstrap-based improvements for inference with clustered errors. Rev Econ Stat 90(3):414–427
Carpenter C, Dobkin C (2009) The effect of alcohol access on consumption and mortality: regression discontinuity evidence from the minimum drinking age. Am Econ J Appl Econ 1(1):164–182
Carpenter C, Dobkin C (2010) Alcohol regulation and crime. In: P. Cook, J. Ludwig, and J. McCrary [eds] Controlling crime: Strategies and tradeoffs. University of Chicago Press, Chicago, pp 291–329
Carpenter C, Dobkin C (2011) The minimum legal drinking age and public health. J Econ Perspect 25(2):133–156
Carpenter CS, Kloska DD, O'Malley P, Johnston L (2007) Alcohol control policies and youth alcohol consumption: evidence from 28 years of monitoring the future. BE J Econ Anal Policy 7(1): 1-21
Chermack ST, Taylor SP (1995) Alcohol and human physical aggression: pharmacological versus expectancy effects. J Stud Alcohol 56(4):449–456
Conlin M, Dickert-Conlin S, Pepper J (2005) The effect of alcohol prohibition on illicit-drug-related crimes. J Law Econ 48(1):215–234
Cook PJ, Moore MJ (2001) Environment and persistence in youthful drinking patterns. In: J. Gruber [ed] Risky behavior among youths: an economic analysis. University of Chicago Press, Chicago, pp 375–438
Decker SL, Schwartz AE (2000) “Cigarettes and alcohol: substitutes or complements” NBER working paper, p 7535
Book Google Scholar
Dee TS (1999) The complementarity of teen smoking and drinking. J Health Econ 18(6):769–793
Dills A, Miron J (2004) Alcohol prohibition and cirrhosis. Am Law Econ Rev 62:285–317
DiNardo J, Lemieux T (2001) Alcohol, marijuana, and American youth: the unintended consequences of government regulation. J Health Econ 20(6):991–1010
Donald S, Lang K (2007) Inference with difference-in-differences and other panel data. Rev Econ Stat 89(2):221–233
Durrance CP, Golden S, Perreira KM, Cook P (2011) Taxing sin and saving lives: can alcohol taxation reduce female homicides? Soc Sci Med 73(1):169–176
Eckhardt CI (2007) Effects of alcohol intoxication on anger experience and expression among partner assaultive men. J Consult Clin Psychol 75:61–71
Grossman M, Markowitz S (1999) Alcohol regulation and violence on college campuses. National Bureau of Economic Research Working Paper No. 7129 Cambridge
Han S, Branas CC, MacDonald JM (2016) The effect of a sunday liquor-sales ban repeal on crime: a triple-difference analysis. Alcohol Clin Exp Res 40(5):1111–1121
Hardiman, D (2006) Histories for the Subordinated. University of Chicago Press, Chicago, p 163
Heaton P (2012) Sunday liquor laws and crime. J Public Econ 96(1):42–52
International Institute for Population Sciences (IIPS) and Macro International (2007) National Family Health Survey (NFHS-3), 2005–06: India, vol 1. IIPS, Mumbai
Jewkes R (2002) Intimate partner violence: causes and prevention. Lancet 359(9315):1423–1429
Kaestner R, Yarnoff B (2011) Long-term effects of minimum legal drinking age laws on adult alcohol use and driving fatalities. J Law Econ 54(2):325–363
Khantzian EJ (1997) The Self-Medication Hypothesis of Substance Use Disorders: A Reconsideration and Recent Applications. Harvard Review of Psychiatry 4(5):231–44.
Krishna G (1985) Communal violence in India: a study of communal disturbance in Delhi. Econ Polit Wkly 20(2), 61-74.
Leonard KE, Quigley BM (1999) Drinking and marital aggression in newlyweds: an event-based analysis of drinking and the occurrence of husband marital aggression. J Stud Alcohol Drugs 60(4):537
Leonard KE, Roberts LJ (1998) Marital aggression, quality, and stability in the first year of marriage: Findings from the Buffalo Newlywed Study. In T. N. Bradbury (Ed.), The developmental course of marital dysfunction. Cambridge University Press, New York, pp 44–73.
Lovenheim MF, Slemrod J (2010) The fatal toll of driving to drink: the effect of minimum legal drinking age evasion on traffic fatalities. J Health Econ 29(1):62–77
Luca DL, Owens EG, Sharma G (2015) Can alcohol prohibition reduce violence against women? Am Econ Rev 105(5):625–629
Markowitz S (2000) The price of alcohol, wife abuse, and husband abuse. South Econ J 67(2):279–303
Markowitz S (2001) Criminal violence and alcohol beverage control: evidence from an international study. In: Grossman M, Hsieh CR (eds) The economics of substance use and abuse: the experience of developed countries and lessons for developing countries. Edward Elgar, UK
McClelland GM, Teplin LA (2001) Alcohol intoxication and violent crime: implications for public health policy. Am J Addict 10:70–85
McKinney CM, Caetano R, Rodriguez LA, Okoro N (2010) Does alcohol involvement increase the severity of intimate partner violence? Alcohol Clin Exp Res 34:655–658
Miczek KA, DeBold JF, Haney M, Tidey J, Vivian J, Weerts EM (1994) Alcohol, drugs of abuse, aggression, and violence. Understanding and preventing violence, p 3
Mohan D, Chopra A, Ray R, Sethi H (2001) Alcohol consumption in India: a cross-sectional study. In: Demers A, Room R, Bourgault C (eds) Surveys of drinking patterns and problems in seven developing countries. Department of Mental Health and Substance Dependence, World Health Organization, Geneva, pp 103–114
Owens E (2014) The American temperance movement and market-based violence. Am Law Econ Rev 16(2):433–472
Prasad R (2009) Alcohol use on the rise in India. Lancet 373(9657):17–18
Rahman, L. 2003. Alcohol prohibition and addictive consumption in India. Leverhulme Centre for Research on Globalization and Economic Policy (GEP), University of Nottingham
Sampson R (2002) Acquaintance rape of college students. Problem-oriented guides for police series guide 17. US Department of Justice Office of Community Policing Services, Washington
Saxena S (1999) Country profile on alcohol in India. In: Riley L, Marshall M (eds) Alcohol and public health in 8 developing countries. Substance Abuse Department Social Change and Mental Health, World Health Organization, Geneva, pp 37–60
Tjaden P, Thoennes N (2000) Extent, nature, and consequences of intimate partner violence: findings from a National Violence against Women Survey. U.S. Department of Justice Report, NCJ # 181867
World Health Organization. 2013. Global and regional estimates of violence against women: prevalence and health effects of intimate partner violence and non-partner sexual violence
Download references
Acknowledgements
We would like to thank the anonymous referee and the editor for the useful remarks.
Responsible editor: David Lam.
Data collection was enabled by a grant provided by the Cornell Population Center.
Availability of data and materials
Data on domestic violence, alcohol consumption and smoking behavior used in this paper are available in the repository, https://www.dhsprogram.com/data/available-datasets.cfm .
Data on crime statistics used in this paper are available in the repository, http://ncrb.gov.in /.
Data on minimum legal drinking age in Indian states are available from the corresponding authors on reasonable request.
Author information
Authors and affiliations.
Mathematica Policy Research, Cambridge, USA
Dara Lee Luca
University of California, Irvine, USA
Emily Owens
Sacred Heart University, Fairfield, USA
Gunjan Sharma
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Gunjan Sharma .
Ethics declarations
Competing interests.
The IZA Journal of Development and Migration is committed to the IZA Guiding Principles of Research Integrity. The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Reprints and permissions
About this article
Cite this article.
Luca, D.L., Owens, E. & Sharma, G. The effectiveness and effects of alcohol regulation: evidence from India. IZA J Develop Migration 9 , 4 (2019). https://doi.org/10.1186/s40176-018-0139-1
Download citation
Received : 04 September 2018
Accepted : 27 December 2018
Published : 31 January 2019
DOI : https://doi.org/10.1186/s40176-018-0139-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
JEL Classifications
- Alcohol consumption
- Domestic violence
- Violence against women
- Prohibition
- Minimum legal drinking age
- Motor vehicle accidents
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Alcohol consumption and awareness of its effects on health among secondary school students in Nigeria
Editor(s): Kufa., Tendesayi
a Department of Home Economics and Hospitality Management Education, Faculty of Vocational and Technical Education, University of Nigeria
b Department of Food Science and Technology, Ebonyi State University Abakaliki, Ebonyi State
c Department of Educational Foundations, Faculty of Education, University of Nigeria, Nsukka, Enugu State
d Department of Home Economics Education, Ebonyi State University Abakaliki, Ebonyi State
e Department of Human Kinetics and Health Education, Faculty of Education, University of Nigeria, Nsukka, Enugu State, Nigeria.
Correspondence: Amaka Bibian Ezeanwu, Department of Home Economics and Hospitality Management Education, Faculty of Vocational and Technical Education, University of Nigeria, Nsukka, P.M.B. 410001, Enugu State, Nigeria (e-mail: [email protected] ).
Abbreviations: % = Percentage, ADCQSSS = Alcoholic Drinks Consumption Questionnaire for Secondary School Students, CI = confidence interval, DNC = does not consume, EA = extremely aware, HC = highly consume, M ± SD = means and standard deviation, MA = moderately aware, MC = moderately consume, NA = not aware, RC = rarely consume, SA = slightly aware, t = t test statistic.
Authorship: NME, CE, UCU, and HAN conceived the study. NME, BNA, HAN, CE, JIO, and BAE designed the study procedure. All the authors were involved in the data collection. CE, BNA, UCU, NME, JIO, and BAE carried out the analysis and interpretation of these data. NME, CE, UCU, HAN, BNA, JIO, and BAE drafted the manuscript. CE, HAN, BNA, NME, JIO, UCU, and BAE critically revised the manuscript for intellectual content. All authors read and approved the final manuscript.
The authors report no conflicts of interest.
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms. http://creativecommons.org/licenses/by-nc-sa/4.0
Received June 23, 2017
Received in revised form November 4, 2017
Accepted November 8, 2017
Alcohol consumption among secondary school students is a major public health issue worldwide; however, the extent of consumption among secondary school students and their understanding of its effects on human health remain relatively unknown in many Nigerian States. This study aimed to determine the extent of alcohol consumption and of the awareness of its negative effects on human health among secondary school students.
The study used a cross-sectional survey design. Self-report questionnaire developed by the researchers was administered to representative sample (N = 1302) of secondary school students in the study area. The data collected from the respondents were analyzed using means and t test.
The results showed that male secondary school students moderately consumed beer (55.2%) and local cocktails (51.5%), whereas their female counterparts reported rare consumption of these 2 alcoholic drinks (44.8%; 48.5% respectively). The findings also indicated rare consumption of distilled spirits among both male and female students in the investigated area, whereas wine, liquor, local spirits, and palm wine were consumed moderately, regardless of gender. Finally, male and female secondary school students differed significantly in their awareness of the negative effects of alcohol consumption on health.
There is a need to intensify efforts to further curtail the extent of alcohol consumption and increase awareness of the negative effects of alcohol use on human health among secondary school students.
1 Introduction
Alcohol consumption is a serious public health challenge worldwide, including in Nigeria. Although the level of alcohol consumption differs widely around the world, the burden of disease and death remains significant in most regions, with Europe and America having the highest alcohol attributable fractions at 6.5% and 5.6%, respectively. [1] Recent evidence also indicates that alcohol consumption is now the world's third largest risk factor for disease and disability; almost 4% of all deaths globally are attributed to alcohol. [2] However, alcohol is the most commonly used psychoactive drug in both young people and adults in Nigeria. [3–5] Some of the factors contributing to alcohol consumption among Nigerians include the absence of alcohol policies, easy access to alcoholic drinks, and lack of implementation of a minimum drinking age by both the government and the brewers. [6]
According to Bada and Adebiyi, [7] it is not rare for Nigerian secondary school students to consume alcoholic drinks; this consumption could be due to their curiosity as adolescents, an irresistible urge, emotional disturbances such as anxiety, the subculture, and the influence of advertisements. Several previous studies have shown the prevalence of alcohol consumption among the Nigerian population, but they did not explore adolescent students’ understanding of its negative health effects. For instance, Lasebikan and Ola [8] found that the prevalence of lifetime alcohol use was 57.9% and that of current alcohol use was 27.3% among a sample of Nigerian semirural community dwellers. Through in-person interviews with Nigerian adults, previous research by Gureje et al [3] revealed that the lifetime prevalence of alcohol consumption was 56%. A recent study by Alex-Hart et al [9] showed that the prevalence of current alcohol consumption among a sample of Nigerian secondary school students was 30.6% and that 38.1% of current drinkers had also been drunk in the past 30 days, with 17.2% being drunk very frequently.
Alcohol consumption negatively affects human health across the lifespan. Previous studies show that alcohol consumption is associated with a burden of diseases such as cancer, [10] pancreatitis, liver cirrhosis, tuberculosis, pneumonia, diabetes mellitus, alcohol use disorder, malignancies, psychiatric morbidity, and injury. [11] Although 18 years of age is the legal limit for alcohol consumption per policy in many parts of the world, sociocultural influences [12,13] seem to hinder strict adherence to this public health policy in Nigerian society. The objective of the present study was therefore to investigate the level of alcohol consumption and knowledge of its negative effects on health among secondary school students in Nigeria. Specifically, the study sought to determine the responses of secondary school students regarding the extent of their alcohol consumption and the extent to which students are aware of the negative health effects of alcohol consumption.
2.1 Ethical consideration
Ethical committee approval was obtained by the authors for this study (Ethical Approval Number: VTE/ERA/0023). Furthermore, parents of the selected participants signed an informed consent form to indicate their approval. School principals of the selected students provided informed consent and conveyed their approval to the researchers in writing. Participants were informed that they were free to participate or to decline participation in the study.
2.2 Study design
The current study adopted a cross-sectional survey design.
2.3 Study setting
This study was conducted in public secondary schools in Ebonyi and Enugu States, Nigeria.
2.4 Study participants
The participants comprised 1302 senior secondary school students who were purposively selected to participate in the study. The study sample size was determined using G*Power 3.1 software [14,15] based on a statistical power of 0.95. Brown [16] stated that if the observed statistical power is large enough (≥0.80), the sample size can be considered adequate for the study. Figure 1 shows the results of the sample size determination. Table 1 summarizes the characteristics of the participants.

2.5 Assumptions about the sample size calculations
We conducted an a priori analysis to determine the study sample size based on the assumptions that a required sample size can be computed as a function of user-specified values for the required significance level α , the desired statistical power 1– β , and the to-be-detected population effect size. [14,15,17,] According to Uzoagulu, [18] researchers should endeavor to make use of statistical technique to determine sample size, and should be aware that the fewer a sample size is, the greater the possibility of sampling error. Thus, the assumptions underlying the use of a priori analysis for sample size calculations were considered appropriate for the current study.
2.6 Sampling strategy
Before sampling, the researchers and assistants purposively visited 40 secondary schools each in the surveyed States to seek for the school principals’ approval, and to explain to them the purpose of the study, and possibility of being included or excluded from the study later on due to certain criteria. All the school principals visited gave their informed consent, and their schools were therefore qualified for sampling. Through multistage sampling technique, the researchers selected the current study sample. First, the simple random sampling technique (balloting without replacement) was used to select only 31 school secondary schools from each State, making a total of 62 secondary schools surveyed. This technique was used in order to give each of the secondary schools the opportunity of being selected and thus eliminate selection bias. Furthermore, 21 senior students from each of the selected schools in the 2 States were selected to participate in the study through stratified random sampling. The samples were stratified by gender (male, n = 651, 50%; female, n = 651, 50%) and other demographics as summarized in Table 1 . Both the schools and their students were selected on the basis of certain inclusion criteria set by the researchers.
2.7 Inclusion and exclusion criteria
The inclusion criteria included that the school principal must provide informed consent in writing; the respondent (student) must be in senior secondary school class two or three, agree to participate freely, inform their parents/guardians about the study, and provide a letter of consent from them. A participant must also be at least 16 years of age and above. Those who did not meet these criteria were excluded from the current study.
2.8 Measures
The Alcoholic Drinks Consumption Questionnaire for Secondary School Students (ADCQSSS) is a structured questionnaire developed by the researchers based on previous literature. [2,8,19] The ADCQSSS consists of 22 items divided into 2 major sections (A and B). Section A assesses respondents’ personal data (age, gender, religion, socioeconomic background, and educational class level). Section B has 2 parts; part one contains 7 items that evaluate the extent to which students consume alcohol with regard to a variety of alcoholic drinks (i.e., Beer, Distilled Spirits, Wine, Liquor, Local Spirits, Local Cocktails, and Palm Wine), and part two contains 15 items that ask respondents about their awareness of the negative effects of alcoholic drinks on human health. The ADCQHSS has a 5-point rating scale from Do not consume/Not aware (0) to Highly Consume/Extremely aware (4). The ADCQSSS was validated by 2 experts in Home Economics and Hospitality Management Education and 2 other independent experts in Educational Research, Measurement, and Evaluation. Cronbach alpha reliability coefficient of the ADCQSSS was 0.72 for part one, 0.76 for part two, and 0.85 for the entire scale, based on data from the current study sample.
2.9 Data collection
To overcome the challenges of participant attrition and nonretrieval of instruments, which are common to many cross-sectional surveys, the questionnaires were distributed and retrieved from each respondent on the spot with the help of 4 research assistants. Respondents met with the researchers and assistants in school halls to complete the questionnaire during long break periods in school. The respondents were guided appropriately and given sufficient time (15–20 minutes) to avoid incomplete responses. Respondents were encouraged to call the attention of any of the researchers or assistants if they need additional clarification on any item or how to complete the questionnaire. Given these measure, responses and return rates were 100%.
2.10 Data entry, management procedure, and analysis
The data collected from the respondents were analyzed using means, percentage, and t test. Item scores were included as the dependent variables and sex as the independent variables. Using purposively determined mean benchmark values, the item scores for the first part of the ADCQHSS section B (the extent to which students consumed alcoholic drinks) were interpreted as follows: Highly Consume (HC) = 3.50 to 4.00; Moderately Consume (MC) = 3.00 to 3.49; Rarely Consume (RC) = 2.50 to 2.99; and Does Not Consume (DNC) = 1.00 to 2.49. Using similarly purposively set mean benchmark values, the item scores for the second part of the ADCQHSS section B (awareness of the negative effects of alcoholic drinks on health) were interpreted as follows: Extremely Aware (EA) = 3.50 to 4.00; Moderately Aware (MA) = 3.00 to 3.49; Slightly Aware (SA) = 2.50 to 2.99; and Not Aware (NA) = 1.00 to 2.49. The t test was used to examine the differences between male and female students at a 0.05 level of significance. To perform the t tests, item scores were treated as test variables, whereas sex was used as the grouping variable. During coding, the numerical value of 1 was used as the label for male students, whereas the value of 2 was applied for female students. Before performing the t tests, the normality of the distribution of the data was assessed using Shapiro–Wilks normality test. The data were found to be normally distributed ( P = .95). Furthermore, we described the percentage (%) of students scoring below or above given thresholds ( see Tables 2 and 3 ). A database created from Microsoft Excel was used for data management, which involved compiling, organizing, defining, and managing data. Thereafter, the statistical software used for analysis was the Statistical Package for the Social Sciences (SPSS) version 21 (IBM Corp., Chicago, IL). [20] To assure quality, we checked for missing data and violation of assumptions using the IBM SPSS statistical software. There were no missing data.

The results in Table 2 reveal the extent of participants’ consumption of alcoholic drinks by gender. Male secondary school students (55.2%) in this study reported moderate consumption of beer (M ± SD = 3.17 ± .83), while females (44.8%) reported rare consumption of beer (2.57 ± .57). In addition, distilled spirits (male=50.3%; female=49.7%) were rarely consumed by both genders, whereas wine, liquors, local spirits, and palm wine were moderately consumed by both (see Table 2 ). These results imply that secondary school students in the study area consumed different types of alcoholic beverages. In addition, the results in Table 2 reveal a significant difference between male and female students in their extent of consumption of beer [ t = -15.25, P = .000, 95% confidence interval (95% CI) -0.676 to 0.522] and local cocktails ( t = -3.92, P = .000, 95% CI -0.263 to -0.088) at 1300 degrees of freedom, as the corresponding P values were lower than the chosen level of significance (.05). This finding means that male and female students in the investigated area consumed these alcoholic drinks unequally due to gender. Male secondary school students moderately consumed beer and local cocktails, whereas their female counterparts were rare consumers of these 2 alcoholic drinks (see Table 2 ).
Furthermore, the results in Table 2 reveal nonsignificant differences between male and female students in the extent to which they consume distilled spirits ( t = -0.991, P = .322, 95% CI -0.109 to 0.036], wine ( t = -0.268, P = .789, 95% CI -0.089 to 0.068), liquor ( t = -0.846, P = .398, 95% CI -0.138 to 0.055), local spirits ( t = -0.098, P = .922, 95% CI -0.097 to 0.088), and palm wine ( t = -0.066, P = .947, 95% CI -0.094 to 0.088), given that the P values for all t tests ranged from .32 to .95 at 1300 degrees of freedom and were therefore higher than the chosen level of significance (.05). This finding suggests that male and female students in the investigated area consumed these alcoholic drinks similarly regardless of gender (see Table 2 ).
Table 3 summarizes the extent to which students were aware of the negative effects of alcohol consumption on health. The results show that male and female students differed significantly in their awareness of the following negative effects of alcohol consumption on human health: excessive drinking can cause alcoholic hepatitis ( t = 39.39, P = .000, 95% CI 1.347–1.488); heavy alcohol intake increases the risk of many forms of cancers ( t = 12.92, P = .000, 95% CI 0.526–0.715); excessive alcohol intake can result in sleep disturbances ( t = -5.01, P = .000, 95% CI -0.329 to 0.144); alcohol abuse can increase the risk of injuries and accidents ( t = -2.65, P = .008, 95% CI -0.193 to 0.029); alcohol abuse can cause liver disease ( t = 3.38, P = .001, 95% CI 0.059–0.221); alcohol abuse can damage the salivary glands ( t = -4.14, P = .000, 95% CI -0.215–0.077); alcohol abuse can lead to gum disease and tooth decay ( t = 6.23, P = .000, 95% CI 0.201–0.386); people who abuse alcohol suffer from malnutrition ( t = 32.98, P = .000, 95% CI 0.972–1.095); excessive alcohol intake can cause a woman to stop menstruating and become infertile ( t = 6.44, P = .000, 95% CI 0.179–0.337); an immune system weakened by alcohol abuse has difficulty fighting off illness ( t = -7.66, P = .000, 95% CI -0.382 to 0.226); and heavy drinking can cause damage to the heart ( t = -15.46, P = .000, 95% CI -0.748 to 0.579) (see Table 3 ).
Finally, the results in Table 3 further show that male and female students were similarly aware of the following effects of alcohol on human health, in that no significant differences were found: excessive alcohol intake can affect coordination, interfering with balance and the ability to walk ( t = 0.70, P = .484, 95% CI -0.055 to 0.117); heavy alcohol use can result in alcohol dependence ( t = 0.00, P = .998, 95% CI -0.088 to 0.088); alcohol use can make people with depression feel worse ( t = -0.86, P = .388, 95% CI -0.121 to 0.047); and erectile dysfunction is a side effect of alcohol abuse in men ( t = 1.66, P = .097, 95% CI -0.009 to 0.104) (see Table 3 ).
4 Discussion
The current study determined the extent of alcoholic drink consumption and of awareness of its negative effects on human health among secondary school students in Nigeria. First, our findings showed that male secondary school students moderately consume beer and local cocktails, whereas their female counterparts were rare consumers of these 2 alcoholic drinks. These findings support those of Lasebikan and Ola, [8] who found that current alcohol drinking was highly related to male gender. In addition, the current study showed that both male and female students in the investigated area rarely consumed distilled spirits, whereas wine, liquor, local spirits, and palm wine were consumed moderately by the students regardless of their gender. According to the authors, more than two-thirds of the current drinking population are moderate drinkers. [8] Bada and Adebiyi [7] noted that it is not difficult to identify Nigerian secondary school students who consume alcohol. Despite what literature might suggest as reasons for students’ alcohol consumption, Cox et al [21] indicate that negative reasons for alcohol consumption are stronger determinants of drinking problems than are positive reasons among both secondary-level school students. Sociocultural factors may also explain the reason for the differences as well as similarities on the extent of alcoholic drink consumption in Nigerian population. [12,13]
Furthermore, the present study showed that male and female secondary school students differed significantly in their awareness of the following negative effects of alcohol consumption on health: excessive drinking can cause alcoholic hepatitis; heavy alcohol intake increases the risk of many forms of cancers; excessive alcohol intake can result in sleep disturbances; alcohol abuse can increase the risk of injuries and accidents; alcohol abuse can cause liver disease; alcohol abuse can damage the salivary glands; alcohol abuse can lead to gum disease and tooth decay; people who abuse alcohol suffer from malnutrition; excessive alcohol intake can cause a woman to stop menstruating and become infertile; an immune system weakened by alcohol abuse has difficulty fighting off illness; and heavy drinking can cause damage to your heart. Finally, our findings also showed that male and female students had similar levels of awareness of the following effects of alcohol on human health, as no significant differences were found: excessive alcohol intake can affect coordination, interfering with balance and the ability to walk; heavy alcohol use can result in alcohol dependence; alcohol use can make people with depression feel worse; and erectile dysfunction is a side effect of alcohol abuse in men. These outcomes support previous studies, [1,9] which show that alcohol increases the risk of numerous diseases and all injury outcomes. According to the WHO, [2] alcohol consumption is now the world's third largest risk factor for disease and disability, with almost 4% of all global deaths attributed to alcohol.
5 Limitations
Our study has several limitations. First, this study used a cross-sectional survey design, which does not enable conclusions regarding causality. However, a cross-sectional survey was considered necessary because previous studies did not investigate the extent of alcohol consumption or of awareness of its negative effects on human health among secondary school students. In the future, longitudinal studies are needed to determine the causal relationship between alcoholic drink consumption and awareness of its negative effects on students’ health. Second, all participants were recruited from secondary schools in 2 States in Nigeria. This approach may have limited the ability to generalize these findings to other populations. Third, our study used self-reported assessments. The instrument showed good validity; however, future research may need to use observational assessments and interview.
6 Implications
If no further research or action is implemented to determine the extent of alcohol consumption and awareness of its negative effects on human health among secondary school students in Nigeria, in other parts of the country in particular, then alcohol consumption among adolescent students may be associated with increased school-based violence, student neglect and abuse, and absenteeism in school, among other social issues. In addition, policy interventions and other actions to reduce the patterns of alcohol use among the student population may not be realistic. Therefore, further research is needed to examine the patterns and prevalence of alcohol consumption and of awareness of its negative effects on human health among secondary school students across the globe.
7 Conclusion
The present study suggests that male secondary school students moderately consume beer and local cocktails, whereas their female counterparts are slight consumers of these 2 alcoholic drinks. Furthermore, both male and female students in the investigated area slightly consumed distilled spirits, whereas wine, liquor, local spirits, and palm wine were consumed moderately by students regardless of gender. Finally, male and female secondary school students significantly differed in their awareness of some of the negative effects of alcohol consumption on health. Overall, secondary school students are not very aware of some of the negative effects of alcohol on human health. Accordingly, health education teachers, school health counselors, and school administrators should combine their professional experiences to promote health education interventions and health counseling programs aimed at reducing students’ engagement in alcohol consumption. Schools should organize seminars for students to provide education on the health-related issues surrounding alcohol consumption. Addiction counselors should also organize awareness campaigns to orient Nigerian secondary school students to the damages caused by alcohol consumption. Parents should properly monitor and counsel their adolescent students on matters relating to alcohol consumption and its effect on their health.
Acknowledgments
We would like to thank the editor and anonymous reviewers for their constructive remarks regarding this work. We also are thankful to AuthorAID and American Journal Experts (AJEs) for their editing support. We are very much grateful to the research assistants and all the schooling adolescents who made this study a success.
alcohol; consumption; health; Nigeria; secondary school; students
- + Favorites
- View in Gallery
- Health Tech
- Health Insurance
- Medical Devices
- Gene Therapy
- Neuroscience
- H5N1 Bird Flu
- Health Disparities
- Infectious Disease
- Mental Health
- Cardiovascular Disease
- Chronic Disease
- Alzheimer's
- Coercive Care
- The Obesity Revolution
- The War on Recovery
- Adam Feuerstein
- Matthew Herper
- Jennifer Adaeze Okwerekwu
- Ed Silverman
- CRISPR Tracker
- Breakthrough Device Tracker
- Generative AI Tracker
- Obesity Drug Tracker
- 2024 STAT Summit
- Wunderkinds Nomination
- STAT Madness
- STAT Brand Studio
Don't miss out
Subscribe to STAT+ today, for the best life sciences journalism in the industry
By the numbers: America’s alcohol-related health problems are rising fast
By Isabella Cueto and J. Emory Parker June 27, 2024

I n the pit of the pandemic, with no one to see and nowhere to go, and horrors unfolding daily outside the front door, there was for many a reliable bright spot: the 5 p.m. drink that would mark the end of the workday.
Drinking, which was already on the rise before 2020, became a coping mechanism for overburdened parents, burnt-out workers, the traumatized, and the bored. There was a beer, wine, spirit, or seltzer for every occasion: game nights, weddings and birthdays over Zoom, socially distanced happy hours, and too many nights on the couch watching a TV show about tigers.
advertisement
When people went back to the streets, there were even more drinks to be found, thanks to alcohol regulations that had been rolled back in many states during the pandemic. A bottle of booze could be delivered to the front door.
Alcohol sales per capita went up more from 2019 to 2021 than in any two-year period since 1969, according to estimates from the National Institute on Alcohol Abuse and Alcoholism. Deaths from excessive alcohol use are also rising, as are deaths where the underlying cause of death was alcohol-related. And it’s not just liver disease. Alcohol has been linked with over 200 conditions, impacting basically every single organ system.
As scientific information emerges, experts are becoming increasingly concerned about Americans’ drinking patterns, and how best to talk to the public about its potential risks.
Related: No, alcohol isn’t good for you. Will new dietary guidelines be shaped more by health or industry interests?
“The alcohol situation was exacerbated by the pandemic. And we realized: We really need to change the conversation about alcohol in the United States,” said NIAAA Director George Koob. “There’s so many people that need help, for alcohol use disorder or alcohol misuse, and it really has such a major impact on health care at all levels — I mean at all different diseases and conditions.”

Experts are currently evaluating the scientific evidence on alcohol’s health effects. The 2025 Dietary Guidelines for Americans are due at the end of next year, and could change what people in the U.S. are told about drinking. But controversy has surrounded the guidelines process for decades, and this time around is no different when it comes to alcohol.
The once-popular, appealing idea that alcohol might be good for health has been tempered by years of research findings that suggest the opposite. Some studies still support the idea that low-to-moderate drinking could decrease risk of cardiovascular disease, dementia, or diabetes. However, those potential benefits must be balanced against evidence that alcohol consumption increases the risk of many other conditions. Because of these conflicting reports — and the influence of a rich and powerful beverage industry — there is still debate over what drinking advice officials should give the public. [Earlier this week, the World Health Organization urged countries to do more to counter the “unacceptably high” toll of alcohol.]
Think of the last time you had alcohol. How much did you have? What about the time before that? How many ounces? Did you have drinks over the course of a week, or stacked on the weekend?
Like all patterns of consumption, drinking is tricky to track. It’s a tool of diversion, relaxation, and celebration, and one served in inconsistent portions. Happy hour prices are made loud and eye-catching, while alcohol content is just a small number on a bottle or menu.
But other numbers — data from study after study — give a more clear-eyed view of Americans’ drinking lives and the ripple effects.
Drinking habits
Who drinks.
Picture six American adults. In the past month, half of them did not drink alcohol, according to federal data. One drank in moderation, and the remaining two drank excessively. This is roughly the spread of drinkers in the American population at any given time.

When zooming out to alcohol consumption in the past year, over 60% of U.S. adults said they drank, according to the 2022 National Survey on Drug Use and Health. And nearly 80% of people over age 11 reported having drunk at some point in their lives.
Related: Getting ahead of a non-alcoholic beverage boom among youths
Alcohol use is most prevalent among people in their early-to-mid-20s, and tends to decrease slightly as people age. Underage drinking has greatly declined through the years, but heavy drinking in the 20s and 30s is a lingering problem. Over 60% of 26-to-44-year-olds drink, and 55% percent of adults 45 to 64 years old drink. Rates of alcohol use generally go up with income and educational attainment.
Adult women on the whole drink less alcohol than men and have lower rates of alcohol-related disease and death. However, studies show women’s rates of drinking and binge drinking have increased over time, narrowing the gap between the sexes. Since women are more susceptible to certain alcohol-related harms — in part due to having bodies that absorb alcohol well and take longer to process it — they are also increasingly facing the consequences of heavy drinking.
Among women who are pregnant, up to 14% report currently drinking, according to CDC data. That number has slightly increased since the early 2010s. Some studies have found that LGBTQ+ people have higher rates of alcohol use, and are at higher risk of developing an alcohol use disorder.
How much do drinkers consume?
When Americans do drink, they typically consume more alcohol than is recommended by the Dietary Guidelines for Americans, which suggest a maximum of one drink per day for women and two for men.

Consumer data from 71,500 American households found national alcohol sales went up by almost $2.5 billion (34.4%) — up to $9.55 billion — in the first few months of the pandemic compared to the same time period in 2019. The biggest increases in sales were for liquor. Households with higher incomes had larger relative increases in alcohol purchases during the pandemic, but buying went up across geographic areas and demographic groups. And while alcohol purchasing seemed to slow down a bit in 2023, sales of “ready-to-drink” cocktails continued to increase — more than doubling since 2019, up to $10.7 billion.
Binge drinking
Research suggests nearly half of people who drink engage in binge drinking, defined as having four or more drinks in the span of a couple hours for women, or five or more drinks in two hours for men. It’s estimated 17% of adults binge drink, and about a quarter of those reported binge drinking multiple times per month.
Related: Wegovy linked to lower risk of alcohol use disorder in real-world study
The American Public Health Association says binge drinking is more common among men, 18-to-34-year-olds, and people with household incomes of $75,000 or more.
Among U.S. veterans, high-risk alcohol use increased between 2019 and 2023, according to self-reports captured in VA health data. Nearly 15% of veterans had documented, high-risk alcohol use between 2022 and 2023. And the problem grew worse for women who had served: The proportion reporting excessive drinking now surpasses the proportion of men. Veterans between the ages of 18 and 39 engaged in the highest rates of risky alcohol use, with 27% of American Indian/Alaska Natives and nearly 17% of Asian Pacific Islanders reporting high-risk use.

What are people drinking?
Standard drink sizes in the U.S. are: one 12-ounce serving of 5% ABV beer, a 5-ounce serving of 12% ABV wine, 8-10 ounces of 7% ABV hard seltzer, or 1.5 ounces of 40% ABV liquor.
However, some data suggest men drink an average of 3.5 servings of beer or 1.8 servings of wine on days when they drink beer and wine. Women drink an average of 2.2 servings of beer or two servings of wine. And at least one study found the average alcohol content of beer, wine, and spirits increased between 2003 and 2016, packing more of a punch per serving.
Sales of ready-to-drink beverages, such as hard seltzers, alcoholic teas, and canned cocktails or wines, have boomed in the last several years. Sales of spirits outcompeted beer and wine in 2023.
Potential harms are many
Alcohol use disorder.
It’s estimated 11% of the U.S. population has a diagnosable alcohol use disorder. Overall, about 1 in 5 people who start drinking will develop an alcohol use disorder at some point in their lives. Anywhere from 20% to 40% of people with anxiety and mood disorders have an alcohol use disorder, and up to 60% of people who seek out AUD treatment have post-traumatic stress disorder, according to the scientific literature.
Alcohol is a toxic product. It has been considered a carcinogen by the World Health Organization and the U.S. government for years, and is considered the third leading preventable cause of cancer, after obesity and smoking tobacco.
Studies consistently report that alcohol accounts for over 75,000 U.S. cancer cases and 20,000 cancer deaths each year. Risk increases the more people drink, but mounting evidence suggests even low levels of alcohol (within the 1-2 drinks per day range recommended by U.S. dietary guidelines) could lead to certain cancers because of how the substance damages DNA as it courses through the body.
Related: Off-label treatment for alcohol use disorder is linked to slower liver decline, study suggests
In women, breast cancer is the kind of malignancy most driven by alcohol. Just one drink per day can increase women’s risk of breast cancer by up to 15%, studies have found. Reduced drinking (going from heavy drinking to moderate or mild drinking) is associated with decreased cancer risk.
Beyond the breast, alcohol is associated with at least half a dozen types of cancer. About 4% of all cancer deaths in the U.S. are believed to be caused by alcohol. That’s not new information: A decade ago, researchers in the U.S. found that almost half of oral cavity and pharynx cancers in men and about 28% of both esophageal and oral cavity/pharynx cancers in women were associated with alcohol. The largest burden was for female breast cancer — 39,060 cases attributable to alcohol.
More recent research by the Canadian Institute for Substance Use Research suggests that in 2022, alcohol was to blame for 9,500 cancer cases and 3,800 cancer deaths in Canada. Baseline estimates presented at a conference last month blame alcohol for over a third of esophageal cancers (mostly squamous cell carcinoma) and oral cavity and pharynx cancers, and a quarter of liver cancer cases. Nearly 20% of laryngeal cancers, 15% of colorectal cancers, and over 7% of both breast and pancreatic cancers were pinned on drinking.
Other data has found drinking is associated with a decreased risk of certain cancers, including kidney, non-Hodgkin lymphoma, and thyroid cancer. Some polyphenols in red wine have antioxidant and anti-inflammatory qualities that could help prevent tumors. However, the amount of alcohol — and the amount of pure ethanol people drink — seems to have a bigger impact on cancer risk than the type of alcohol consumed. And every potential benefit also carries a potential harm.
Chronic disease
Studies have reported low levels of drinking may be protective against some conditions: cognitive impairment, dementia, atherosclerosis, coronary heart disease, heart failure, hypertension, or ischemic stroke. However, the more a person drinks, the faster potential benefits vanish and are replaced by a litany of possible bad outcomes. High average levels of consumption and binge drinking are associated with increased risk of coronary heart disease, stroke, congestive heart failure, atrial fibrillation, dementia and hypertension.
Alcohol use also is a risk factor for pancreatitis, gastritis, gastro-esophageal reflux disease and peptic ulcer disease, as well as a potential aggravator of mental and behavioral disorders. When drinking is combined with tobacco use, risks go up across the board.
Liver disease
From 2020 to 2021, age-adjusted death rates increased 9% for chronic liver disease and cirrhosis (13.3 to 14.5) — a bigger jump than stroke, diabetes, and kidney disease. There were about 5,000 more deaths from alcohol-associated liver disease in 2020 than in 2019 — a 22% change.

Half of liver disease deaths in the U.S. are caused by alcohol, according to the National Institute on Alcohol Abuse and Alcoholism. Alcohol-associated liver disease kills about 22,000 people in the U.S. every year — roughly 347,000 deaths over the past 20 years. In 1999, three in 100,000 people died of alcohol-associated liver disease. By the first year of the pandemic, 8 in 100,000 were killed by the condition.
Related: Quitting alcohol — or even drinking less — reduces risk of oral cavity and esophageal cancer, per new analysis
During the pandemic, specialists watched as alcohol-related illness soared. Hospitalizations went up, rates of alcohol-associated hepatitis — a severe form of liver injury that can happen in a shorter time frame of heavy drinking — and demand for liver transplants increased. And more young people and more women started showing up to their clinics. A decade ago, more than 6,000 adult liver transplants were performed in the U.S., but only about 20% of those were for people with alcohol-associate liver disease. After the pandemic, alcohol-related liver disease now accounts for 40% of all liver transplants in North America.
Certain groups are being hit hard. Rates of alcohol-associated hepatitis continued to rise sharply in U.S. military veterans. Between 2010 and 2023, rates increased from 31.6 to 392.6 cases per 100,000 people per year. And chronic liver disease, including from alcohol, is highest in American Indians and Alaska Natives.
Alcohol-related deaths are on the rise, and experts are particularly concerned by an increase among young people and women. The U.S. saw a 25.5% spike in alcohol-related deaths between 2019 and 2020 — accounting for 3% of all deaths in the first year of the pandemic. The largest increases in alcohol-related deaths were among people 25 to 24 and 35 to 44 years old; deaths in both groups increased by over 37%. Mortality from alcohol-associated liver disease is rising most rapidly in those ages 25-34. Women have seen large annual increases in alcohol-related mortality for years.

Sign up for Morning Rounds
Understand how science, health policy, and medicine shape the world every day
Opioid overdose deaths that involved alcohol as a contributing cause went up by 41% (and by nearly 60% in cases where people overdosed on synthetic opioids such as fentanyl) in 2020.
One in five deaths — about 45,000 deaths per year — among people 20 to 49 years old is attributable to alcohol, CDC data show. Binge drinking is responsible for more than 40% of deaths.
Is there a safe zone?
Researchers are still trying to figure out how low-to-moderate alcohol use (at or below the dietary guidelines levels) affects health, and whether there is a threshold — short of total abstinence — that would reasonably protect people from serious risk of disease or death. Is that one drink per week? Or five? Alcohol’s pleasurable effects are also indubitably valuable to many people’s lives. But many of alcohol’s negative impacts can be altered by personal genetics, underlying disease and other factors, which makes tailoring drinking recommendations to individual people really difficult.
Still, there are some widely agreed-upon guidelines — drinking thresholds above which a person’s risk of developing a disease or shaving time off their life significantly increases, according to the data.
Many people who drink wade into this territory, going past the zone of unknown risk and into more dangerous drinking behaviors. And they might not know it.
STAT’s coverage of chronic health issues is supported by a grant from Bloomberg Philanthropies . Our financial supporters are not involved in any decisions about our journalism.
About the Authors Reprints
Isabella cueto.
Chronic Disease Reporter
Isabella Cueto covers the leading causes of death and disability: chronic diseases. Her focus includes autoimmune conditions and diseases of the lungs, kidneys, liver (and more). She writes about intriguing research, the promises and pitfalls of treatment, and what can be done about the burden of disease.
J. Emory Parker
Data Project Manager
chronic disease
public health
STAT encourages you to share your voice. We welcome your commentary, criticism, and expertise on our subscriber-only platform, STAT+ Connect
To submit a correction request, please visit our Contact Us page .

Recommended

Recommended Stories

STAT Plus: Medicare’s big experiment to fix kidney failure care hasn’t worked so far, studies say

Congress called for an ALS moonshot. The plan for it doesn’t leave Earth

STAT Plus: Grail, aiming to market a blood test for cancer, faces host of challenges as it debuts on Nasdaq

STAT Plus: Top FDA official Peter Marks overruled staff, review team to approve Sarepta gene therapy

STAT Plus: Ateev Mehrotra, the researcher the telehealth lobby loves to hate, isn’t backing down
Fact sheets
- Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- HIV and AIDS
- Hypertension
- Mental disorders
- Top 10 causes of death
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- Data collection tools
- Global Health Observatory
- Insights and visualizations
- COVID excess deaths
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
Over 3 million annual deaths due to alcohol and drug use, majority among men
A new report from the World Health Organization (WHO) highlights that 2.6 million deaths per year were attributable to alcohol consumption, accounting for 4.7% of all deaths, and 0.6 million deaths to psychoactive drug use. Notably, 2 million of alcohol and 0.4 million of drug-attributable deaths were among men.
WHO’s Global status report on alcohol and health and treatment of substance use disorders provides a comprehensive update based on 2019 data on the public health impact of alcohol and drug use and situation with alcohol consumption and treatment of substance use disorders worldwide. The report shows an estimated 400 million people lived with alcohol use disorders globally. Of this, 209 million people lived with alcohol dependence.
"Substance use severely harms individual health, increasing the risk of chronic diseases, mental health conditions, and tragically resulting in millions of preventable deaths every year. It places a heavy burden on families and communities, increasing exposure to accidents, injuries, and violence," said Dr Tedros Adhanom Ghebreyesus, WHO Director-General. "To build a healthier, more equitable society, we must urgently commit to bold actions that reduce the negative health and social consequences of alcohol consumption and make treatment for substance use disorders accessible and affordable."
The report highlights the urgent need to accelerate actions globally towards achieving Sustainable Development Goal (SDG) target 3.5 by 2030 by reducing alcohol and drug consumption and improving access to quality treatment for substance use disorders.
Health consequences of alcohol consumption
The report highlights that despite some reduction in the alcohol-related death rates since 2010, the overall number of deaths due to alcohol consumption remains unacceptably high and amounts to 2.6 million in 2019, with the highest numbers in the European Region and the African region.
The death rates due to alcohol consumption per litre of alcohol consumed are highest in low-income countries and lowest in high-income countries.
Of all deaths attributable to alcohol in 2019, an estimated 1.6 million deaths were from noncommunicable diseases, including 474 000 deaths from cardiovascular diseases and 401 000 from cancer.
Some 724 000 deaths were due to injuries, such as those from traffic crashes, self-harm and interpersonal violence. Another 284 000 deaths were linked to communicable diseases. For example, alcohol consumption has been shown to increase the risk of HIV transmission resulting from an increased risk of unprotected sex and by increasing the risk of TB infection and mortality by suppressing a wide range of immune responses.
The highest proportion (13%) of alcohol-attributable deaths in 2019 were among young people aged 20–39 years.
Alcohol consumption trends
Total alcohol per capita consumption in the world population decreased slightly from 5.7 litres in 2010 to 5.5 litres in 2019. The highest levels of per capita consumption in 2019 were observed in the WHO European Region (9.2 litres) and the Region of Americas (7.5 litres).
The level of alcohol consumption per capita among drinkers amounts on average to 27 grams of pure alcohol per day, roughly equivalent to two glasses of wine, two bottles of beer (33cl) or two servings of spirits (4cl). This level and frequency of drinking is associated with increased risks of numerous health conditions and associated mortality and disability.
In 2019, 38% of current drinkers had engaged in heavy episodic drinking, defined as consuming at least 60g of pure alcohol on one or more occasions in the preceding month – roughly equivalent to 4 or 5 glasses of wine, bottles of beer or servings of spirits. Continuous heavy drinking was highly prevalent among men.
Globally, 23.5% of all 15–19 year olds were current drinkers. Rates of current drinking were highest among 15–19-year-olds in the European region (45.9%) followed by the Americas (43.9%).
Treatment gap for substance use disorders
Effective treatment options for substance use disorders exist, but treatment coverage remains incredibly low. The proportion of people in contact with substance use treatment services ranged from less than 1% to no more than 35% in 2019, in countries providing this data.
Most of the 145 countries that reported data did not have a specific budget line or data on governmental expenditures for treatment of substance use disorders. Although mutual help and peer support groups are useful resources for people with substance use disorders, almost half of responding countries reported that they do not offer such support groups for substance use disorders.
Stigma, discrimination and misconceptions about the efficacy of treatment contribute to these critical gaps in treatment provision, as well as the continued low prioritization of substance use disorders by health and development agencies.
Actions for progress
To accelerate progress towards achievement of SDG target 3.5 and reduce the health and social burden attributable to substance use, governments and partners need to intensify actions in 8 strategic areas. These include:
- increase awareness through a coordinated global advocacy campaign;
- strengthen prevention and treatment capacity of health and social care systems;
- scale up training of health professionals;
- re-commit to the implementation of the Global Alcohol Action Plan 2022-2030 with a focus on the SAFER package;
- accelerate international efforts on capacity-building and knowledge transfer;
- engage civil society organizations, professional associations and people with lived experience;
- improve multi-level monitoring systems and corresponding research capacity; and
- scale up resource mobilization, allocation, and innovative funding mechanisms to strengthen capacity of health and social systems.
Notes to the editor
The previous WHO report on SDG target 3.5 was published in 2018. The report released today was scheduled for 2022 following the usual time gap of around 3 years, but was postponed due to challenges associated with the COVID-19 pandemic. SDG health target 3.5 calls to “strengthen the prevention and treatment of substance abuse, including narcotic drug abuse and harmful use of alcohol” and has two indicators: 3.5.1 - coverage of treatment interventions (pharmacological, psychosocial and rehabilitation and aftercare services) for substance use disorders, and indicator 3.5.2 – alcohol per capita consumption (aged 15 years and older) within a calendar year in litres of pure alcohol. The WHO Global Survey on Progress with SDG target 3.5 was conducted in 2019-2020. Out of 194 WHO Member States, 154 (79.4%) responded to the survey. The WHO Global Action Plan for Alcohol 2022-2030 is a comprehensive plan to further guide implementation of the 2010 WHO Global strategy to reduce the harmful use of alcohol aimed at reducing alcohol-related harms worldwide.
Corrigendum
A statistic was corrected on this news release on 28 June 2024. The sentence that originally read as " The report shows an estimated 400 million people lived with alcohol and drug use disorders globally." was corrected to be "The report shows an estimated 400 million people lived with alcohol use disorders globally."
Media Contacts
WHO Media Team
World Health Organization
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

The effects of alcohol use on academic achievement in high school
Ana i. balsa.
a Research Professor, Center for Applied Research on Poverty, Family, and Education, Department of Economics, Universidad de Montevideo; Prudencio de Pena 2440, Montevideo, 11600, Uruguay; Phone: (+598 2) 707 4461 ext 300; Fax: (+598 2) 707 4461 ext 325; yu.ude.mu@aslaba
Laura M. Giuliano
b Assistant Professor, Department of Economics, University of Miami, Coral Gables, FL 33124, United States; [email protected]
Michael T. French
c Professor of Health Economics, Health Economics Research Group, Department of Sociology, Department of Economics, and Department of Epidemiology and Public Health, University of Miami, Coral Gables, FL 33124, United States; ude.imaim@hcnerfm
This paper examines the effects of alcohol use on high school students’ quality of learning. We estimate fixed-effects models using data from the National Longitudinal Study of Adolescent Health. Our primary measure of academic achievement is the student’s GPA abstracted from official school transcripts. We find that increases in alcohol consumption result in small yet statistically significant reductions in GPA for male students and in statistically non-significant changes for females. For females, however, higher levels of drinking result in self-reported academic difficulty. The fixed-effects results are substantially smaller than OLS estimates, underscoring the importance of addressing unobserved individual heterogeneity.
1. Introduction
In the United States, one in four individuals between the ages of 12 and 20 drinks alcohol on a monthly basis, and a similar proportion of 12 th graders consumes five or more drinks in a row at least once every two weeks ( Newes-Adeyi, Chen, Williams, & Faden, 2007 ). Several studies have reported that alcohol use during adolescence affects educational attainment by decreasing the number of years of schooling and the likelihood of completing school ( Chatterji & De Simone, 2005 ; Cook & Moore, 1993 ; Gil-Lacruz & Molina, 2007 ; Koch & McGeary, 2005 ; McCluskey, Krohn, Lizotte, & Rodriguez, 2002 ; NIDA, 1998 ; Renna, 2007 ; Yamada, Kendrix, & Yamada, 1996 ) Other research using alternative estimation techniques suggests that the effects of teen drinking on years of education and schooling completion are very small and/or non-significant ( Chatterji, 2006 ; Dee & Evans, 2003 ; Koch & Ribar, 2001 ).
Despite a growing literature in this area, no study has convincingly answered the question of whether alcohol consumption inhibits high school students’ learning. Alcohol consumption could be an important determinant of how much a high school student learns without having a strong impact on his or her decision to stay in school or attend college. This question is fundamental and timely, given recent research showing that underage drinkers are susceptible to the immediate consequences of alcohol use, including blackouts, hangovers, and alcohol poisoning, and are at elevated risk of neurodegeneration (particularly in regions of the brain responsible for learning and memory), impairments in functional brain activity, and neurocognitive defects ( Zeigler et al., 2004 ).
A common and comprehensive measure of high school students’ learning is Grade Point Average (GPA). GPA is an important outcome because it is a key determinant of college admissions decisions and of job quality for those who do not attend college. Only a few studies have explored the association between alcohol use and GPA. Wolaver (2002) and Williams, Powell, and Wechsler (2003) have studied this association among college students, while DeSimone and Wolaver (2005) have investigated the effects of underage drinking on GPA during high school. The latter study found a negative association between high school drinking and grades, although it is not clear whether the effects are causal or the result of unobserved heterogeneity.
Understanding the relationship between teenage drinking and high school grades is pertinent given the high prevalence of alcohol use among this age cohort and recent research on adolescent brain development suggesting that early heavy alcohol use may have negative effects on the physical development of brain structure ( Brown, Tapert, Granholm, & Delis, 2000 ; Tapert & Brown, 1999 ). By affecting the quality of learning, underage drinking could have an impact on both college admissions and job quality independent of its effects on years of schooling or school completion.
In this paper, we estimate the effects of drinking in high school on the quality of learning as captured by high school GPA. The analysis employs data from Waves 1 and 2 of the National Longitudinal Study of Adolescent Health (Add Health), a nationally representative study that captures health-related behaviors of adolescents in grades 7 through 12 and their outcomes in young adulthood. Our analysis contributes to the literature in several ways. First, we focus on the effect of drinking on academic achievement during high school. To date, and to the best of our knowledge, only one other study in the literature has analyzed the consequences of underage drinking on high school GPA. Second, rather than rely on self-reported GPA, we use objective GPA data from academic transcripts, reducing the potential for systematic biases in the estimation results. Third, we take advantage of the longitudinal nature of the Add Health data and use fixed-effects models to purge the analysis of time invariant unobserved heterogeneity. Fixed-effects techniques are superior to instrumental variables (IV) estimation when the strength and reliability of the instruments are suspect ( French & Popovici, 2009 ). Finally, we explore a variety of mechanisms that could underlie a detrimental effect of alcohol use on grades. In addition to analyzing mediators related to exposure to education (days of school skipped), we investigate the effect of drinking on students’ ability to focus on and adhere to academic objectives.
2. Background and significance
Behavioral research has found that educational performance is highly correlated with substance abuse (e.g., Bukstein, Cornelius, Trunzo, Kelly, & Wood, 2005 ; Hawkins, Catalano, & Miller, 1992 ). Economic studies that look at the link between alcohol use and educational outcomes have customarily focused on measures of educational attainment such as graduation (from high school or college), college matriculation, and years of school completed (e.g., Bray, Zarkin, Ringwalt, & Qi, 2000 ; Chatterji, 2006 ; Cook & Moore, 1993 ; Dee & Evans 2003 ; Koch & Ribar, 2001 ; Mullahy & Sindelar, 1994 ; Renna, 2008 ; Yamada et al., 1996 ). Consistent with the behavioral research, early economic studies found that drinking reduced educational attainment. But the most rigorous behavioral studies and the early economic studies of attainment both faced the same limitation: they were cross-sectional and subject to potential omitted variables bias. Some of these cross-sectional economic studies attempted to improve estimation by using instrumental variables (IV). Cook and Moore (1993) and Yamada et al. (1996) found that heavy or frequent drinking in high school adversely affects high school and college completion. Nevertheless, the validity and reliability of the instruments in these studies are open to debate ( Chatterji, 2006 ; Dee & Evans, 2003 ; French & Popovici, 2009 ).
By contrast, more recent economic studies that arguably use better estimation methods have found that drinking has modest or negligible effects on educational attainment. Dee and Evans (2003) studied the effects of teen drinking on high school completion, college entrance, and college persistence. Employing changes in the legal drinking age across states over time as an instrument, they found no significant effect of teen drinking on educational attainment. Koch and Ribar (2001) reached a similar conclusion applying family fixed effects and instrumental variables to NLSY data. Though they found that drinking had a significant negative effect on the amount of schooling completed among men, the effect was small. Finally, Chatterji (2006) used a bivariate probit model of alcohol use and educational attainment to gauge the sensitivity of the estimates to various assumptions about the correlation of unobservable determinants of these variables. She concluded that there is no evidence of a causal relationship between alcohol use and educational attainment when the correlation coefficient is fixed at plausible levels.
Alcohol use could conceivably affect a student’s quality of learning and academic performance regardless of its impact on school completion. This possibility is suggested by Renna (2008) , who uses a research design similar to that used by Dee and Evans (2003) and finds that although binge drinking does not affect high school completion rates, it does significantly increase the probability that a student graduates with a GED rather than a high school diploma. Drinking could affect learning through a variety of mechanisms. Recent neurological research suggests that underage drinking can impair learning directly by causing alterations in the structure and function of the developing brain with consequences reaching far beyond adolescence ( Brown et al., 2000 ; White & Swartzwelder, 2004 ). Negative effects of alcohol use can emerge in areas such as planning and executive functioning, memory, spatial operations, and attention ( Brown et al., 2000 ; Giancola & Mezzich, 2000 ; Tapert & Brown, 1999 ). Alcohol use could also affect performance by reducing the number of hours committed to studying, completing homework assignments, and attending school.
We are aware of five economic studies that have examined whether drinking affects learning per se. Bray (2005) analyzed this issue indirectly by studying the effect of high school students’ drinking on subsequent wages, as mediated through human capital accumulation. He found that moderate high school drinking had a positive effect on returns to education and therefore on human capital accumulation. Heavier drinking reduced this gain slightly, but net effects were still positive. The other four studies approached the question directly by focusing on the association between drinking and GPA. Three of the GPA studies used data from the Harvard College Alcohol Study. Analyzing data from the study’s 1993 wave, both Wolaver (2002) and Williams et al. (2003) estimated the impact of college drinking on the quality of human capital acquisition as captured by study hours and GPA. Both studies found that drinking had a direct negative effect on GPA and an indirect negative effect through reduced study hours. Wolaver (2007) used data from the 1993 and 1997 waves and found that both high school and college binge drinking were associated with lower college GPA for males and females. For females, however, study time in college was negatively correlated with high school drinking but positively associated with college drinking.
To our knowledge, only one study has looked specifically at adolescent drinking and high school GPA. Analyzing data from the Youth Risk Behavior Survey, DeSimone and Wolaver (2005) used standard regression analysis to estimate whether drinking affected high school GPA. Even after controlling for many covariates, they found that drinking had a significant negative effect. Their results showed that the GPAs of binge drinkers were 0.4 points lower on average for both males and females. They also found that the effect of drinking on GPA peaked for ninth graders and declined thereafter and that drinking affected GPA more by reducing the likelihood of high grades than by increasing the likelihood of low grades.
All four GPA studies found that drinking has negative effects on GPA, but they each faced two limitations. First, they relied on self-reported GPA, which can produce biased results due to recall mistakes and intentional misreporting ( Zimmerman, Caldwell, & Bernat, 2006 ). Second, they used cross-sectional data. Despite these studies’ serious efforts to address unobserved individual heterogeneity, it remains questionable whether they identified a causal link between drinking and GPA.
In sum, early cross-sectional studies of educational attainment and GPA suggest that drinking can have a sizeable negative effect on both outcomes. By contrast, more recent studies of educational attainment that use improved estimation methods to address the endogeneity of alcohol use have found that drinking has negligible effects. The present paper is the first study of GPA that controls for individual heterogeneity in a fixed-effects framework, and our findings are consistent with the more recent studies of attainment that find small or negligible effects of alcohol consumption.
Add Health is a nationally representative study that catalogues health-related behaviors of adolescents in grades 7 through 12 and associated outcomes in young adulthood. An initial in-school survey was administered to 90,118 students attending 175 schools during the 1994/1995 school year. From the initial in-school sample, 20,745 students (and their parents) were administered an additional in-home interview in 1994–1995 and were re-interviewed one year later. In 2001–2002, Add Health respondents (aged 18 to 26) were re-interviewed in a third wave to investigate the influence of health-related behaviors during adolescence on individuals when they are young adults. During the Wave 3 data collection, Add Health respondents were asked to sign a Transcript Release Form (TRF) that authorized Add Health to identify schools last attended by study participants and request official transcripts from the schools. TRFs were signed by approximately 92% of Wave 3 respondents (about 70% of Wave 1 respondents).
The main outcome of interest, GPA, was abstracted from school transcripts and linked to respondents at each wave. Because most of the in-home interviews during Waves 1 and 2 were conducted during the Spring or Summer (at the end of the school year) and alcohol use questions referred to the past 12 months, we linked the in-home questionnaires with GPA data corresponding to the school year in which the respondent was enrolled or had just completed at the time of the interview.
The in-home questionnaires in Waves 1 and 2 offer extensive information on the student’s background, risk-taking behaviors, and other personal and family characteristics. These instruments were administered by computer assisted personal interview (CAPI) and computer assisted self-interview (CASI) techniques for more sensitive questions such as those on alcohol, drug, and tobacco use. Studies show that the mode of data collection can affect the level of reporting of sensitive behaviors. Both traditional self-administration and computer assisted self-administered interviews have been shown to increase reports of substance use or other risky behaviors relative to interviewer-administered approaches ( Azevedo, Bastos, Moreira, Lynch, & Metzger, 2006 ; Tourangeau & Smith, 1996 ; Wright, Aquilino, & Supple, 1998 ). Several measures of alcohol use were constructed on the basis of the CAPI/CASI questions: (1) whether the student drank alcohol at least once per week in the past 12 months, (2) whether the student binged (drank five or more drinks in a row) at least once per month in the past 12 months, (3) the average number of days per month on which the student drank in the past 12 months, (4) the average number of drinks consumed on any drinking day in the past 12 months, and (5) the total number of drinks per month consumed by the student in the past year.
Individual characteristics obtained from the in-home interviews included age, race, gender, grade in school, interview date, body mass index, religious beliefs and practices, employment status, health status, tobacco use, and illegal drug use. To capture environmental changes for respondents who changed schools, we constructed indicators for whether the respondent attended an Add Health sample school or sister school (e.g. the high school’s main feeder school) in each wave. We also considered family characteristics such as family structure, whether English was spoken at home, the number of children in the household, whether the resident mother and resident father worked, whether parents worked in blue- or white-collar jobs, and whether the family was on welfare. Finally, we took into account a number of variables describing interview and household characteristics as assessed by the interviewer: whether a parent(s) or other adults were present during the interview; whether the home was poorly kept; whether the home was in a rural, suburban, or commercial area; whether the home environment raised any safety concerns; and whether there was evidence of alcohol use in the household.
Respondents to the in-home surveys were also asked several questions about how they were doing in school. We constructed measures of how often the respondents skipped school, whether they had been suspended, and whether they were having difficulties paying attention in school, getting along with teachers, or doing their homework. We analyzed these secondary outcomes as possible mediators of an effect of alcohol use on GPA.
Our fixed-effects methodology required high school GPA data for Waves 1 and 2. For this reason, we restricted the sample to students in grades 9, 10, or 11 in Wave 1 (N=22,792) who were re-interviewed in Waves 2 and 3 (N=14,390), not mentally disabled (N=13,632), and for whom transcript data were available at Wave 3 (N=10,430). In addition, we excluded 1,846 observations that had missing values on at least one of the explanatory or control variables. 1 The final sample had 8,584 observations, which corresponded to Wave 1 and Wave 2 responses for 4,292 students with no missing information on high school GPA or other covariates across both waves. Male respondents accounted for 48% of the sample.
Table 1 shows summary statistics for the analysis sample by wave and gender. Abstracted GPA averages 2.5 for male students and 2.8 for female students, 2 with similar values in Waves 1 and 2. Approximately 9% of males and 6% of females reported drinking alcohol at least one time per week in Wave 1. The prevalence of binge drinking (consuming five or more drinks in a single episode) at least once a month is slightly higher: 11% among males and 7% among females. On average, the frequency of drinking in Wave 1 is 1.34 days per month for male respondents and 0.94 days per month for female respondents, while drinking intensity averages 2.8 drinks per episode for males and 2.2 drinks per episode for females. By Wave 2, alcohol consumption increases in all areas for both males and females. The increases for males are larger, ranging from an 18% increase in the average number of drinks per episode to a 55% increase in the fraction who binge monthly.
Summary Statistics
| Males (N=2,049) | Females (N=2,243) | |||||
|---|---|---|---|---|---|---|
| Variable | Wave 1 | Wave 2 | Diff | Wave 1 | Wave 2 | Diff |
| 2.53 | 2.51 | −0.02 | 2.79 | 2.79 | 0.01 | |
| drinks alcohol at least one time per week | 0.09 | 0.13 | 0.03 | 0.06 | 0.08 | 0.02 |
| binges (consumes≥5 drinks) at least once per month | 0.11 | 0.17 | 0.06 | 0.07 | 0.09 | 0.02 |
| average number of drinks consumed per month | 10.50 | 13.78 | 3.28 | 5.90 | 8.17 | 2.28 |
| average number of days per month alcohol is consumed | 1.34 | 1.68 | 0.34 | 0.94 | 1.13 | 0.19 |
| average number of drinks consumed per episode | 2.81 | 3.32 | 0.51 | 2.20 | 2.34 | 0.13 |
| grade level in school | 10.02 | 11.02 | 1.00 | 10.00 | 10.99 | 0.98 |
| attended sample school | 0.99 | 0.94 | 0.04 | 0.98 | 0.94 | 0.04 |
| attended sister school | 0.01 | 0.01 | −0.01 | 0.01 | 0.01 | −0.01 |
| interviewed in summer | 0.71 | 0.68 | −0.03 | 0.74 | 0.67 | −0.07 |
| interviewed in fall | 0.18 | 0.01 | −0.17 | 0.18 | 0.01 | −0.17 |
| English spoken in home | 0.88 | 0.89 | 0.01 | 0.89 | 0.89 | 0.00 |
| number of children in household | 1.17 | 1.07 | −0.10 | 1.26 | 1.12 | −0.13 |
| lived with mother | 0.96 | 0.95 | −0.01 | 0.96 | 0.96 | 0.00 |
| lived with father | 0.79 | 0.80 | 0.01 | 0.75 | 0.75 | 0.00 |
| resident mother employed | 0.87 | 0.87 | 0.00 | 0.87 | 0.87 | 0.00 |
| resident mother had white collar job | 0.65 | 0.66 | 0.01 | 0.63 | 0.63 | 0.00 |
| resident father employed | 0.95 | 0.94 | −0.01 | 0.95 | 0.95 | 0.00 |
| resident father had white collar job | 0.41 | 0.40 | 0.00 | 0.39 | 0.38 | −0.02 |
| parent(s) on welfare (1% imputed) | 0.08 | 0.07 | −0.01 | 0.09 | 0.08 | −0.01 |
| others present at interview | 0.23 | 0.15 | −0.08 | 0.25 | 0.16 | −0.09 |
| parents present at interview | 0.18 | 0.10 | −0.08 | 0.19 | 0.10 | −0.09 |
| residence is rural | 0.24 | 0.22 | −0.02 | 0.25 | 0.23 | −0.02 |
| residence is in suburb | 0.41 | 0.45 | 0.04 | 0.38 | 0.41 | 0.03 |
| residence is in commercial area | 0.03 | 0.03 | 0.00 0.19 | 0.02 | 0.02 | 0.00 0.18 |
| home poorly kept | 0.10 | 0.11 | 0.01 | 0.12 | 0.13 | 0.01 |
| safety concern at home | 0.04 | 0.03 | −0.01 | 0.04 | 0.02 | −0.02 |
| visible evidence of alcohol in household | 0.03 | 0.04 | 0.00 | 0.03 | 0.03 | 0.00 |
| BMI | 23.03 | 23.55 | 0.52 | 22.37 | 22.79 | 0.42 |
| attends religious services | 0.60 | 0.57 | −0.03 | 0.63 | 0.59 | −0.04 |
| religion is important | 0.39 | 0.36 | −0.03 | 0.46 | 0.44 | −0.01 |
| employed | 0.60 | 0.65 | 0.04 | 0.58 | 0.61 | 0.03 |
| hours worked | 7.65 | 11.61 | 3.97 | 6.28 | 9.95 | 3.67 |
| in good health (self-reported) | 0.96 | 0.97 | 0.01 | 0.92 | 0.94 | 0.02 |
| smoker in the past 30 days | 0.15 | 0.17 | 0.02 | 0.14 | 0.17 | 0.03 |
| days smoked in past 30 days | 3.32 | 4.06 | 0.74 | 3.11 | 4.13 | 1.02 |
| illegal drug use past 30 days | 0.15 | 0.17 | 0.02 | 0.13 | 0.15 | 0.01 |
| days skipped | 1.47 | 1.90 | 0.43 | 1.37 | 1.46 | 0.10 |
| skipped at least one day | 0.87 | 0.88 | 0.01 | 0.90 | 0.92 | 0.02 |
| suspended at least once | 0.11 | 0.11 | 0.00 | 0.07 | 0.06 | 0.00 |
| | ||||||
| …paying attention in school | 0.32 | 0.34 | 0.02 | 0.25 | 0.27 | 0.01 |
| …getting along with teachers | 0.15 | 0.14 | −0.01 | 0.11 | 0.08 | −0.03 |
| …doing homework | 0.35 | 0.35 | 0.00 | 0.26 | 0.27 | 0.01 |
| …with one or more of the above | 0.50 | 0.52 | 0.02 | 0.40 | 0.41 | 0.01 |
| (OLS regressions) | ||||||
| Age (in Wave 1 interview) | 16.0 | 15.8 | ||||
| Birth order | 2.04 | 2.03 | ||||
| Race/ethnicity indicators: | ||||||
| Black | 0.16 | 0.22 | ||||
| Hispanic | 0.16 | 0.16 | ||||
| Other race (non-White) | 0.18 | 0.18 | ||||
| Foreign-born | 0.10 | 0.10 | ||||
| Resident mother college-educated | 0.47 | 0.44 | ||||
| Resident father college-educated | 0.50 | 0.47 | ||||
Note : Based on responses to survey questions regarding most recently completed school year.
Of the Wave 1 respondents, 87% of males and 90% of females had skipped school at least once in the past year, with males averaging 1.47 days skipped and females averaging 1.37 days. Further, 11% of males and 7% of females had been suspended at least once. Regarding the school difficulty measures, 50% of male respondents in Wave 1 reported at least one type of regular difficulty with school: 32% had difficulty paying attention, 15% did not get along with their teachers, and 35% had problems doing their homework. Among females, 40% had at least one difficulty: 25% with paying attention, 11% with teachers, and 26% with homework.
Table 2 tabulates changes in dichotomous measures of problem drinking by gender. Among males, 82.6% did not drink weekly in either wave; 8.1% became weekly drinkers in Wave 2; 4.8% stopped drinking weekly in Wave 2; and the remaining 4.5% drank weekly in both waves. Among females, 88.5% did not drink weekly in either wave; 5.3% became weekly drinkers in Wave 2; 3.7% stopped drinking weekly in Wave 2; and 2.5% drank weekly in both waves. The trends in monthly binging were similar, with the number of students who became monthly bingers exceeding that of students who stopped bingeing monthly in Wave 2. The proportion of respondents reporting binge-drinking monthly in both waves (6.6% and 3.4% for men and women, respectively) was higher than the fraction of students who reported drinking weekly in both waves.
Tabulation of Changes in Dichotomous Measures of Alcohol Use By Gender
| Males | Females | |
|---|---|---|
| Change from W1 to W2 in: | (N=2,049) | (N=2,243) |
| 0 to 0 (change = 0) | 82.6% | 88.5% |
| 0 to 1 (change = +1) | 8.1% | 5.3% |
| 1 to 0 (change = −1) | 4.8% | 3.7% |
| 1 to 1 (change = 0) | 4.5% | 2.5% |
| 0 to 0 (change = 0) | 78.3% | 87.8% |
| 0 to 1 (change = +1) | 10.4% | 5.5% |
| 1 to 0 (change = −1) | 4.8% | 3.3% |
| 1 to 1 (change = 0) | 6.6% | 3.4% |
4. Empirical methods and estimation issues
We examined the impact of adolescent drinking on GPA using fixed-effects estimation techniques. The following equation captures the relationship of interest:
where GPA it is grade point average of individual i during the Wave t school year, A it is a measure of alcohol consumption, X it is a set of other explanatory variables, c i are unobserved individual effects that are constant over time, ε it is an error term uncorrelated with A it and X it , and α, β a , and β x are parameters to estimate.
The coefficient of interest is β a , the effect of alcohol consumption on GPA. The key statistical problem in the estimation of β a is that alcohol consumption is likely to be correlated with individual-specific unobservable characteristics that also affect GPA. For instance, an adolescent with a difficult family background may react by shirking responsibilities at school and may, at the same time, be more likely to participate in risky activities. For this reason, OLS estimation of Equation (1) used with cross-sectional or pooled longitudinal data is likely to produce biased estimates of β a . In this paper, we took advantage of the two high school-administered waves in Add Health and estimated β a using fixed-effects techniques. Because Waves 1 and 2 were only one year apart, it is likely that most unobserved individual characteristics that are correlated with both GPA and alcohol use are constant over this short period. Subtracting the mean values of each variable over time, Equation (1) can be rewritten as:
Equation (2) eliminates time invariant individual heterogeneity ( c i ) and the corresponding bias associated with OLS estimation of Equation (1) .
We estimated Equation (2) using different sets of time-varying controls ( X it ). 3 We began by controlling only for unambiguously exogenous variables and progressively added variables that were increasingly likely to be affected by alcohol consumption. The first set of controls included only the respondent’s grade level, indicators for attending the sample school or sister school, and the date of the interview. In a second specification, we added household characteristics and interviewer remarks about the household and the interview. This specification includes indicators for the presence of parents and others during the interview and thus controls for a potentially important source of measurement error in the alcohol consumption variables. 4 The third specification added to the second specification those variables more likely to be endogenous such as BMI, religious beliefs/practices, employment, and health status. A fourth specification included tobacco and illegal drug use. By adding these behavioral controls, which could either be mediators or independent correlates of the drinking-GPA association, we examined whether the fixed-effects estimates were influenced by unmeasured time variant individual characteristics.
The fifth and sixth specifications were aimed at assessing possible mechanisms flowing from changes in alcohol use to changes in GPA. Previous research has found that part of the association between alcohol consumption and grades can be explained by a reduction in study hours. Add Health did not directly ask respondents about study effort. It did, however, ask about suspensions and days skipped from school. These school attendance variables were added to the set of controls to test whether an effect of alcohol use on human capital accumulation worked extensively through the quantity of, or exposure to, schooling. Alternatively, an effect of alcohol use on grades could be explained by temporary or permanent alterations in the structure and functioning of an adolescent’s developing brain with resulting changes in levels of concentration and understanding (an intensive mechanism). To test for the mediating role of this pathway, we added a set of dichotomous variables measuring whether the student reported having trouble at least once a week with each of the following: (i) paying attention in school, (ii) getting along with teachers, and (iii) doing homework.
Finally, we considered the number of days the student skipped school and the likelihood of having difficulties with school as two alternative outcomes and estimated the association between these variables and alcohol use, applying the same fixed-effects methodology as in Equation (2) . To analyze difficulties with school as an outcome, we constructed a dichotomous variable that is equal to one if the student faced at least one of the three difficulties listed above. We estimated the effect of alcohol use on this variable using a fixed-effects logit technique.
Separate regressions were run for male and female respondents. The literature shows that males and females behave differently both in terms of alcohol use ( Ham & Hope, 2003 ; Johnston, O’Malley, Bachman, & Schulenberg, 2007 ; Schulenberg, O’Malley, Bachman, Wadsworth, & Johnston, 1996 ; Wechsler, Davenport, Dowdall, Moeykens, & Castillo, 1994 ) and school achievement ( Dwyer & Johnson, 1997 ; Jacob, 2002 ; Kleinfeld, 1998 ). These gender differences are clearly evident in the summary statistics presented in Table 1 . Furthermore, the medical literature suggests that there may be gender differences in the impact of alcohol consumption on cognitive abilities (e.g. Hommer, 2003 ).
In addition to examining differential effects by gender, we tested for differential effects of alcohol use along three other dimensions: age, the direction of change in alcohol use (increases vs. decreases), and initial GPA. These tests, as well as other extensions and robustness checks, are described in Section 6.
Table 3 shows the fixed-effects estimates for β a from Equation (2) . Each cell depicts a different model specification defined by a particular measure of alcohol use and a distinctive set of control variables. Rows (a)-(d) denote the alcohol use variable(s) in each specification, and Columns (1)-(6) correspond to the different sets of covariates. Control variables are added hierarchically from (1) to (3). We first adjusted only by grade level, sample school and sister school indicators, and interview date (Column (1)). We then added time-varying household characteristics and interviewer assessments (Column (2)), followed by other individual time-varying controls (Column (3)). Column (4) adds controls for the use of other substances, which could either be correlates or consequences of alcohol use. Columns (5) and (6) consider other potential mediators of the effects found in (1)-(3) such as days skipped, suspensions from school, and academic difficulties.
Fixed effects Estimates; Dependent Variable = GPA
| (1) | (2) | (3) | (4) | (5) | (6) | ||
|---|---|---|---|---|---|---|---|
| Incrementally-added controls: | grade level + sample or sister school + interview date | + time- varying household chars. + interviewer remarks | + BMI, religion, employ- ment, health | Controls as in col. (3) + drugs and tobacco | Controls as in col. (3) + school attendance variables | Controls as in col. (3) + school difficulty variables | |
| (a) | drinks weekly = 1 | −0.056 | −0.058 | −0.059 | −0.040 | −0.048 | −0.047 |
| (0.039) | (0.039) | (0.039) | (0.039) | (0.038) | (0.038) | ||
| (b) | binges monthly = 1 | −0.060 | −0.060 | −0.058 | −0.040 | −0.051 | −0.048 |
| (0.036) | (0.036) | (0.035) | (0.035) | (0.035) | (0.035) | ||
| (c) | # drinks/month (100s) | −0.074 | −0.073 | −0.071 | −0.065 | −0.060 | −0.064 |
| (0.034) | (0.034) | (0.033) | (0.032) | (0.033) | (0.032) | ||
| (d) | # days drink /month | −0.005 | −0.005 | −0.005 | −0.004 | −0.004 | −0.004 |
| (0.003) | (0.003) | (0.003) | (0.003) | (0.003) | (0.003) | ||
| # drinks/episode | −0.004 | −0.004 | −0.004 | −0.004 | −0.004 | −0.004 | |
| (0.002) | (0.002) | (0.002) | (0.002) | (0.002) | (0.002) | ||
| (a) | drinks weekly = 1 | −0.008 | −0.004 | −0.005 | 0.020 | −0.001 | 0.005 |
| (0.049) | (0.049) | (0.048) | (0.048) | (0.047) | (0.047) | ||
| (b) | binges monthly = 1 | 0.043 | 0.048 | 0.046 | 0.071 | 0.060 | 0.056 |
| (0.043) | (0.043) | (0.042) | (0.043) | (0.042) | (0.043) | ||
| (c) | # drinks/month (100s) | 0.014 | 0.011 | 0.013 | 0.030 | 0.020 | 0.022 |
| (0.045) | (0.044) | (0.043) | (0.042) | (0.043) | (0.041) | ||
| (d) | # days drink /month | 0.004 | 0.004 | 0.004 | 0.005 | 0.005 | 0.005 |
| (0.004) | (0.004) | (0.004) | (0.004) | (0.004) | (0.004) | ||
| # drinks/episode | −0.003 | −0.003 | −0.002 | −0.001 | −0.002 | −0.002 | |
| (0.003) | (0.003) | (0.003) | (0.003) | (0.003) | (0.003) | ||
Notes : See Table 1 for list of control variables in each model specification. Robust standard errors in parentheses;
The results for males provide evidence of a negative yet small effect of alcohol use on GPA. No major changes were observed in the estimates across the different specifications that incrementally added more controls, suggesting that the results are probably robust to unmeasured time-varying characteristics. In what follows, therefore, we describe the results in Column (3), which controls for the greatest number of individual time-varying factors (with the exception of tobacco and illicit drug use). Weekly drinking and monthly binge drinking are both negatively associated with GPA, but neither of these coefficients is statistically significant (Rows (a) and (b)). The continuous measure of alcohol consumption has a statistically significant coefficient (Row (c)), suggesting that increasing one’s alcohol intake by 100 drinks per month reduces GPA by 0.07 points, or 2.8% relative to the mean. The results in Row (d) suggest that variation in both the frequency and the intensity of alcohol use contributes to the estimated effect on grades. An increase of one day per month in drinking frequency reduces GPA by 0.005 points, and consumption of one additional drink per episode reduces GPA by 0.004 points.
Columns (4)-(6) report the estimates of interest after controlling for use of other substances, days skipped or suspended from school, and difficulties with school. Relative to the effects identified in Column (3), controlling for tobacco and illegal drug use reduces the negative effect of total number of drinks on GPA by 9% or 0.006 GPA points (see row (c), Column (4)). Adding the school attendance variables to the set of controls in Column (3) results in a point estimate of −0.06 or 0.01 GPA points below the coefficient in Column (3) (see Column (5)). Adding the school difficulty variables results in a reduction in GPA of 0.007 GPA points or a 10% decrease relative to the estimate in Column (3). While not shown in the table, the inclusion of both school difficulty and attendance variables as controls explains approximately 20% of the effect of alcohol use on grades, with the alcohol use estimates remaining statistically significant at the 10% level.
For females, the estimated coefficients are much smaller than those for males, and for two measures (binge-drinking and drinking frequency), the estimates are actually positive. However, none of the coefficients are statistically significant at conventional levels. 5 Interestingly, after controlling for substance use, difficulties with school, and school attendance, the estimates become less negative or more positive. But they remain statistically non significant.
Table 4 shows the effect of alcohol use on the number of school days skipped during the past year. These results are qualitatively similar to the findings for GPA, suggesting some small and statistically significant effects for males but no significant effects for females. For males, increasing the number of drinks per month by 100 leads to an additional 0.72 days skipped (p<0.10) when controlling for household features, interviewer comments, and individual characteristics such as body mass index, religiosity, employment, and health status (see Column (3), Row (c)). Controlling for tobacco and illegal drug use reduces the coefficient slightly to 0.69 days. The results in Row (d) suggest that this effect is driven mainly by variation in drinking intensity, with an additional drink per episode resulting in an increase of 0.06 days skipped.
Fixed-effects Estimates; Dependent Variable = School Days Skipped
| (1) | (2) | (3) | (4) | ||
|---|---|---|---|---|---|
| Incrementally-added controls: | grade level + sister or sample school + interview date | + time-varying household characteristics & interviewer remarks | + BMI, religion, employment, health status | + tobacco and illegal drug use | |
| (a) | drinks weekly = 1 | 0.721 | 0.680 | 0.687 | 0.579 |
| (0.492) | (0.491) | (0.496) | (0.510) | ||
| (b) | binges monthly = 1 | 0.681 | 0.709 | 0.712 | 0.592 |
| (0.452) | (0.453) | (0.452) | (0.471) | ||
| (c) | # drinks/month (100s) | 0.774 | 0.731 | 0.722 | 0.690 |
| (0.373) | (0.382) | (0.384) | (0.383) | ||
| (d) | # days drink /month | 0.060 | 0.055 | 0.055 | 0.050 |
| (0.037) | (0.036) | (0.036) | (0.036) | ||
| # drinks/episode | 0.058 | 0.057 | 0.057 | 0.056 | |
| (0.028) | (0.028) | (0.028) | (0.028) | ||
| (a) | drinks weekly = 1 | 0.599 | 0.534 | 0.556 | 0.412 |
| (0.720) | (0.726) | (0.724) | (0.702) | ||
| (b) | binges monthly = 1 | 0.817 | 0.864 | 0.898 | 0.748 |
| (0.528) | (0.528) | (0.530) | (0.535) | ||
| (c) | # drinks/month (100s) | 0.397 | 0.450 | 0.433 | 0.336 |
| (0.520) | (0.508) | (0.495) | (0.477) | ||
| (d) | # days drink /month | 0.062 | 0.060 | 0.063 | 0.053 |
| (0.067) | (0.064) | (0.064) | (0.063) | ||
| # drinks/episode | 0.002 | 0.005 | 0.002 | −0.008 | |
| (0.042) | (0.040) | (0.039) | (0.037) | ||
Notes : Robust standard errors in parentheses;
Table 5 contains estimates of the relationship between alcohol use and our dichotomous measure of having difficulty in school. For males, we found one small but statistically significant effect: consumption of an additional 100 drinks per month is associated with a 4% increase in the probability of having trouble in school. For females, the estimated coefficients are all positive and larger than those found for males, and four out of five are statistically significant. The probability of having trouble in school is roughly 11% higher for females who drink weekly relative to those who do not, and there is a similar effect for monthly binge drinking (Rows (a) and (b)). Furthermore, the likelihood of difficulties increases by 7% with an additional 100 drinks per month (Row (c)). These findings suggest that female students suffer adverse consequences from alcohol consumption, even if these effects do not translate into lower grades. Finally, in Row (d), we see that these adverse effects are driven by increases in drinking frequency rather than drinking intensity.
Fixed-effects Logit Estimates; Dependent Variable = Difficulty with School
| (1) | (2) | (3) | 4 | ||
|---|---|---|---|---|---|
| Incrementally-added controls: | grade level + sample or sister school + interview date | + time-varying household characteristics & interviewer remarks | + BMI, religion, employment, health status | + tobacco and illegal drug use | |
| (a) | drinks weekly = 1 | 0.020 | 0.014 | 0.011 | 0.000 |
| (0.032) | (0.032) | (0.032) | (0.032) | ||
| (b) | binges monthly = 1 | 0.044 | 0.039 | 0.034 | 0.023 |
| (0.029) | (0.029) | (0.029) | (0.029) | ||
| (c) | # drinks/month (100s) | 0.039 | 0.037 | 0.035 | 0.033 |
| (0.019) | (0.019) | (0.019) | (0.019) | ||
| (d) | # days drink /month | 0.001 | 0.000 | −0.000 | −0.001 |
| (0.002) | (0.002) | (0.002) | (0.002) | ||
| # drinks/episode | 0.002 | 0.002 | 0.002 | 0.002 | |
| (0.001) | (0.001) | (0.001) | (0.001) | ||
| (a) | drinks weekly = 1 | 0.110 | 0.106 | 0.107 | 0.099 |
| (0.042) | (0.042) | (0.042) | (0.043) | ||
| (b) | binges monthly = 1 | 0.112 | 0.115 | 0.113 | 0.103 |
| (0.045) | (0.045) | (0.045) | (0.045) | ||
| (c) | # drinks/month (100s) | 0.075 | 0.071 | 0.072 | 0.067 |
| (0.034) | (0.035) | (0.034) | (0.035) | ||
| (d) | # days drink /month | 0.010 | 0.010 | 0.010 | 0.010 |
| (0.003) | (0.003) | (0.003) | (0.004) | ||
| # drinks/episode | 0.004 (0.002) | 0.003 (0.002) | 0.003 (0.002) | 0.003 (0.002) | |
Notes : Dependent variable is a dummy variable equal to one if respondent had trouble at least once a week with one or more of the following: (1) paying attention in school, (2) getting along with teachers, or (3) doing homework. Robust standard errors in parentheses;
Our main results thus far point to two basic conclusions. After controlling for individual fixed effects, alcohol use in high school has a relatively minor influence on GPA. But there are also some interesting gender differences in these effects. For males, we find small negative effects on GPA that are partially mediated by increased school absences and difficulties with school-related tasks. For females, on the other hand, we find that alcohol use does not significantly affect GPA, but female drinkers encounter a higher probability of having difficulties at school.
Our basic estimates of the effects of drinking on GPA complement those of Koch and Ribar (2001) , who find small effects of drinking on school completion for males and non-significant effects for females. However, our analysis of school-related difficulties suggests that females are not immune to the consequences of drinking. Namely, females are able to compensate for the negative effects of drinking (e.g., by working harder or studying more) so that their grades are unaffected. This interpretation is consistent with Wolaver’s (2007) finding that binge drinking in college is associated with increased study hours for women but with reduced study hours for men. It is also reminiscent of findings in the educational psychology and sociology literatures that girls get better grades than boys, and some of this difference can be explained by gender differences in classroom behavior ( Downey & Vogt Yuan, 2005 ) or by greater levels of self-discipline among girls ( Duckworth & Seligman, 2006 ).
When interpreting our results, there are some important caveats to keep in mind. First, we must emphasize that they reflect the contemporaneous effects of alcohol use. As such, they say nothing about the possible cumulative effects that several years of drinking might have on academic performance. Second, we can only examine the effect of alcohol use on GPA for those students who remain in school. Unfortunately, we cannot address potential selection bias due to high school dropouts because of the high rate of missing GPA data for those students who dropped out after Wave 1. 6 Third, we acknowledge that our fixed-effects results could still be biased if we failed to account for important time-varying individual characteristics that are associated with GPA differentials across waves. It is reassuring, however, that our results are generally insensitive to the subsequent inclusion of additional time-varying (and likely endogenous) characteristics, such as health status, employment, religiosity, tobacco use, and illicit drug use. Finally, we cannot rule out possible reverse causality whereby academic achievement affects alcohol use. Future research using new waves of the data may provide further insight on this issue. In the next section, we discuss some additional issues that we are able to explore via robustness checks and extensions.
6. Robustness checks and extensions
6.1. ols versus fixed effects.
In addition to running fixed-effects models, we estimated β a using OLS. Separate regressions were run by gender and by wave. We first regressed GPA on measures of alcohol use and the full set of time-varying controls used in the fixed-effects estimation (see Column (3), Table 3 ). Next, we added other time-invariant measures such as demographics, household characteristics, and school characteristics. Finally, we controlled for tobacco and illegal drug use. The comparison between fixed-effects and OLS estimates (Appendix Table A1 ) sheds light on the extent of the bias in β ^ a OLS . For males, OLS estimates for Wave 1 were 3 to 6 times larger (more negative) than fixed-effects estimates (depending on the measure of alcohol use), and OLS estimates in Wave 2 were 3 to 4 times larger than those from the fixed-effects estimation. The bias was even more pronounced for females. Contrary to the results in Table 3 , OLS estimates for females were statistically significant, quantitatively large, and usually more negative than the estimates for males.
OLS Cross-sectional Estimates; Dependent Variable = GPA
| Estimates based on Wave 1: | Estimates based on Wave 2: | ||||||
|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | ||
| Controls as in , column (3) | + time-invariant demographics, household characteristics, & school fixed effects | + tobacco and illegal drug use | Controls as in , column (3) | + time- invariant demographics, household characteristics, & school fixed effects | + tobacco and illegal drug use | ||
| (a) | drinks weekly = 1 | −0.332 | −0.330 | −0.194 | −0.214 | −0.317 | −0.157 |
| (0.062) | (0.068) | (0.070) | (0.059) | (0.065) | (0.062) | ||
| (b) | binges monthly = 1 | −0.358 | −0.399 | −0.219 | −0.189 | −0.309 | −0.157 |
| (0.057) | (0.068) | (0.069) | (0.049) | (0.053) | (0.054) | ||
| (c) | # drinks/month (100s) | −0.203 | −0.237 | −0.137 | −0.146 | −0.257 | −0.169 |
| (0.048) | (0.055) | (0.057) | (0.051) | (0.056) | (0.047) | ||
| (d) | # days drink /month | −0.017 | −0.019 | −0.012 | −0.013 | −0.017 | −0.008 |
| (0.005) | (0.006) | (0.006) | (0.005) | (0.006) | (0.006) | ||
| # drinks/episode | −0.012 | −0.016 | −0.009 | −0.005 | −0.012 | −0.008 | |
| (0.003) | (0.004) | (0.004) | (0.003) | (0.003) | (0.003) | ||
| (a) | drinks weekly = 1 | −0.363 | −0.349 | −0.083 | −0.346 | −0.334 | −0.115 |
| (0.074) | (0.082) | (0.085) | (0.063) | (0.068) | (0.066) | ||
| (b) | binges monthly = 1 | −0.355 | −0.398 | −0.081 | −0.302 | −0.277 | −0.052 |
| (0.067) | (0.075) | (0.075) | (0.060) | (0.067) | (0.067) | ||
| (c) | # drinks/month (100s) | −0.221 | −0.353 | −0.125 | −0.201 | −0.256 | −0.110 |
| (0.080) | (0.083) | (0.075) | (0.052) | (0.060) | (0.054) | ||
| (d) | # days drink /month | −0.025 | −0.009 | −0.004 | −0.024 | −0.023 | −0.009 |
| (0.007) | (0.007) | (0.007) | (0.006) | (0.006) | (0.006) | ||
| # drinks/episode | −0.011 | −0.001 | −0.010 | −0.010 | −0.014 | −0.005 | |
| (0.004) | (0.004) | (0.004) | (0.003) | (0.004) | (0.004) | ||
6.2. Outlier analysis
Concerns about misreporting at the extreme tails of the alcohol use distributions led us to re-estimate the fixed-effects model after addressing these outliers. A common method for addressing extreme outliers without deleting observations is to “winsorize” ( Dixon, 1960 ). This technique reassigns all outlier values to the closest value at the beginning of the user-defined tail (e.g., 1%, 5%, or 10% tails). For the present analysis, we used both 1% and 5% tails. As a more conventional outlier approach, we also re-estimated the models after dropping those observations in the 1% tails. In both cases we winsorized or dropped the tails using the full Wave 1 and Wave 2 distribution (in levels) and then estimated differential effects.
After making these outlier corrections, the estimates for males became larger in absolute value and more significant, but the estimates for females remained statistically non-significant with no consistent pattern of change. 7 For males, dropping the 1% tails increased the effect of 100 drinks per month on GPA to −0.15 points (from −0.07 points when analyzing the full sample). Winsorizing the 5% tails further increased the estimated effect size to −0.31 points.
We offer two possible interpretations of these results for males. First, measurement error is probably more substantial among heavier drinkers and among respondents with the biggest changes in alcohol consumption across waves, which could cause attenuation bias at the top end. 8 Second, the effect of drinks per month on GPA could be smaller among male heavier drinkers, suggesting non-linear effects. Interestingly, neither of these concerns appears to be important for the analysis of females.
6.3. Differential effects
Thus far we have reported the differential effects of alcohol use on GPA for males and females. Here, we consider differential effects along three other dimensions: age, direction of change in alcohol use (increases vs. decreases), and initial GPA. To examine the first two of these effects, we added to Equation (2) interactions of the alcohol use measure with dichotomous variables indicating (i) that the student was 16 or older, and (ii) that alcohol use had decreased between Waves 1 and 2. 9 For males, the negative effects of drinking on GPA were consistently larger among respondents who were younger than 16 years old. None of the interaction terms, however, were statistically significant. We found no consistent or significant differences in the effect of alcohol consumption between respondents whose consumption increased and those whose consumption decreased between Waves 1 and 2. All results were non-significant and smaller in magnitude for females. It should be noted, however, that the lack of significant effects could be attributed, at least in part, to low statistical power as some of the disaggregated groups had less than 450 observations per wave.
To examine whether drinking is more likely to affect low achievers (those with initial low GPA) than high achievers (higher initial GPA), we estimated two fixed-effects linear probability regressions. The first regression estimated the impact of alcohol use on the likelihood of having an average GPA of C or less, and the second regression explored the effect of drinking on the likelihood of having a GPA of B- or better. For males, we found that monthly binging was negatively associated with the probability of obtaining a B- or higher average and that increases in number of drinks per month led to a higher likelihood of having a GPA of C or worse. Frequency of drinking, rather than intensity, was the trigger for having a GPA of C or worse. For females, most coefficient estimates were not significant, although the frequency of drinking was negatively associated with the probability of having a GPA of C or worse.
6.4 Self-reported versus abstracted GPA
One of the key advantages of using Add Health data is the availability of abstracted high school grades. Because most educational studies do not have such objective data, we repeated the fixed-effects estimation of Equation (2) using self-reported GPA rather than transcript-abstracted GPA. To facilitate comparison, the estimation sample was restricted to observations with both abstracted and self-reported GPA (N=2,164 for males and 2,418 for females).
The results reveal another interesting contrast between males and females. For males, the results based on self-reported grades were fairly consistent with the results based on abstracted grades, although the estimated effects of binging and drinking intensity were somewhat larger (i.e., more negative) when based on self-reported grades. But for females, the results based on self-reported grades showed positive effects of alcohol consumption that were statistically significant at the 10% level for three out of five consumption measures (monthly binging, total drinks per month, and drinks per episode). Furthermore, with the exception of the frequency measure (drinking days per month), the estimated effects were all substantially larger (i.e., more positive) when based on self-reported GPA. This suggests that females who drink more intensively tend to inflate their academic performance in school, even though their actual performance is not significantly different from that of those who drink less. Males who drink more intensely, on the other hand, may tend to deflate their academic accomplishments.
6.5. Analysis of dropouts
In Table 3 , we estimated the effects of alcohol consumption on GPA conditional on being enrolled in school during the two observation years. While increased drinking could lead an adolescent to drop out of school, reduced drinking could lead a dropout to re-enroll. Our GPA results do not address either of these possible effects. Of those who were in 9 th grade in Wave 1, roughly 2.3% dropped out before Wave 2. Of those who were in 10 th and 11 th grades in Wave 1, the dropout rates were 3.7% and 5.0%, respectively. Our core estimates would be biased if the effect of alcohol use on GPA for non-dropouts differed systematically from the unobserved effect of alcohol use on GPA for dropouts and re-enrollers in the event that these students had stayed in school continuously.
To determine whether dropouts differed significantly from non-dropouts, we compared GPA and drinking patterns across the two groups. Unfortunately, dropouts were much more likely to have missing GPA data for the years they were in school, 10 so the comparison itself has some inherent bias. Nevertheless, for those who were not missing Wave 1 GPA data, we found that mean GPA was significantly lower for dropouts (1.11) than for those students who stayed in school at least another year (2.66). Dropouts were also older in Wave 1 (16.9 vs. 15.9 years old) and more likely to be male (54% vs. 48%). They also consumed alcohol more often and with greater intensity in the first wave. While there is evidence of differences across the two groups in Wave 1, it is unclear whether dropouts would have differed systematically with respect to changes in GPA and in drinking behavior over time if they had stayed in school. Due to the small number of dropout observations with Wave 1 GPA data, we could not reliably estimate a selection correction model.
6.6. Attrition and missing data
As described in the data section, a large fraction of the Add Health respondents who were in 9th, 10th, or 11th grade in Wave 1 were excluded from our analysis either because they did not participate in Waves 2 or 3, did not have transcript data, or had missing data for one or more variables used in the analysis. (The excluded sample consisted of 7,104 individuals out of a total of 11,396 potentially eligible.) Mean characteristics were compared for individuals in the sample under analysis (N=4,292) and excluded respondents (N=7,104) in Wave 1. Those in the analysis sample had higher GPAs (both self-reported and abstracted, when available) and were less likely to have difficulties at school, to have been suspended from school, or to have skipped school. They were less likely to drink or to drink intensively if they drank. They were more likely to be female and White, speak English at home, have highly educated parents, have a resident mother or father at home, and be in good health. They were less likely to have parents on welfare, live in commercial areas or poorly kept buildings, and smoke and use drugs.
The above comparisons suggest that our estimates are representative of the sample of adolescents who participated in Waves 2 and 3 but not necessarily of the full 9 th , 10 th , and 11 th grade sample interviewed at baseline. To assess the magnitude and sign of the potential attrition bias in our estimates, we considered comparing fixed-effects estimates for these two samples using self-reported GPA as the dependent variable. But self-reported GPA also presented a considerable number of missing values, especially for those in the excluded sample at Wave 2. Complete measures of self-reported GPA in Waves 1 and 2 were available for 60% of the individuals in the analysis sample and for less than 30% of individuals in the excluded sample.
As an alternative check, we used OLS to estimate the effects of alcohol use on self-reported GPA in Wave 1 for the excluded sample, and compared these to OLS coefficients for our analysis sample in Wave 1. The effects of alcohol use on self-reported grades were smaller for individuals excluded from our core analysis. Because the excluded individuals tend to consume more alcohol, the finding of smaller effects for these individuals is consistent with either of the two explanations discussed in Section 6.2 above. First, the effect of consuming alcohol on GPA could be smaller for those who drink more. And second, measurement error is probably more serious among heavier drinkers, potentially causing more attenuation bias in this sample.
To summarize, the analysis described above suggests that some caution should be exercised when extrapolating the results in this paper to other populations. Due to missing data, our analysis excludes many of the more extreme cases (in terms of grades, substance use, and socioeconomic status). However, our analysis suggests that the effects of alcohol use on grades are, if anything, smaller for these excluded individuals. It therefore supports our main conclusions that the effects of alcohol use on GPA tend to be small and that failure to account for unobserved individual heterogeneity is responsible for some of the large negative estimates identified in previous research.
7. Conclusion
Though a number of investigations have studied the associations between alcohol use and years of schooling, less is known about the impact of adolescent drinking on the process and quality of learning for those who remain in school. Moreover, studies that have examined the impact of drinking on learning have faced two important limitations. First, they have relied on self-reported grades as the key measure of learning and are therefore subject to potential biases that result from self-reporting. Second, they have relied on cross-sectional data and suffer from potential biases due either to unobserved individual heterogeneity or to weak or questionable instrumental variables.
In the present study, we contribute to the existing literature by exploiting several unique features of the nationally representative Add Health survey. First, we measure learning with grade point averages obtained from the respondents’ official school transcripts. Second, we exploit Add Health’s longitudinal design to estimate models with individual fixed effects. This technique eliminates the bias that results from time-invariant unobserved individual heterogeneity in the determinants of alcohol use and GPA. Finally, we explore a variety of pathways that could explain the association between alcohol use and grades. In particular, we examine the effects of alcohol consumption on both the quantity of schooling—as measured by days of school skipped—and the quality—as measured by difficulties with concentrating in school, getting along with teachers, or completing homework.
The main results show that, in general, increases in alcohol consumption result in statistically significant but quantitatively small reductions in GPA for male students and in statistically non-significant changes for females. For both males and females, comparisons of the fixed-effects models with standard cross-sectional models suggest that large biases can result from the failure to adequately control for unobserved individual heterogeneity. Our findings are thus closely aligned with those of Koch and Ribar (2001) and Dee and Evans (2003) , who reach a similar conclusion regarding the effects of drinking on school completion.
Our analysis also reveals some interesting gender differences in how alcohol consumption affects learning in high school. Our results suggest that for males, alcohol consumption has a small negative effect on GPA and this effect is partially mediated by increased school absences and by difficulties with school-related tasks. For females, however, we find that alcohol use does not significantly affect GPA, even though it significantly increases the probability of encountering difficulties at school. Gender differences in high school performance are well documented in the educational psychology and sociology literatures, yet no previous studies have estimated gender differences in high school learning that are directly associated with alcohol use. Our study is therefore unique in that regard.
Finally, our study also highlights the potential pitfalls of using self-reported grades to measure academic performance. Not only do we find evidence that use of self-reports leads to bias; we also find that the bias differs by gender, as drinking is associated with grade inflation among females and grade deflation among males. Hence, the conceptual discoveries uncovered in this research may be as important for future investigations as the empirical results are for current educational programs and policies.
Acknowledgements
Financial assistance for this study was provided by research grants from the National Institute on Alcohol Abuse and Alcoholism (R01 AA15695, R01 AA13167, and R03 AA016371) and the National Institute on Drug Abuse (RO1 DA018645). This research uses data from Add Health, a program project directed by Kathleen Mullan Harris and designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris at the University of North Carolina at Chapel Hill, and funded by grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. Special acknowledgment is due Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Information on how to obtain the Add Health data files is available on the Add Health website ( http://www.cpc.unc.edu/addhealth ). No direct support was received from grant P01-HD31921 for this analysis. We gratefully acknowledge the input of several colleagues at the University of Miami. We are also indebted to Allison Johnson, William Russell, and Carmen Martinez for editorial and administrative assistance. The authors are entirely responsible for the research and results reported in this paper, and their position or opinions do not necessarily represent those of the University of Miami, the National Institute on Alcohol Abuse and Alcoholism, or the National Institute on Drug Abuse.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
1 Due to a significant fraction of missing responses, we imputed household income and household welfare status using both predicted values on the basis of other covariates and the sample mean for households that were also missing some of the predicting covariates. We added dummy variables to indicate when an observation was imputed.
2 Grades and numerical grade-point equivalents have been established for varying levels of a student’s academic performance. These grade-point equivalents are used to determine a student’s grade-point average. Grades of A, A-, and B+ with respective grade-point equivalents of 4.00, 3.67, and 3.33 represent an “excellent” quality of performance. Grades of B, B−, and C+ with grade-point equivalents of 3.00, 2.67, and 2.33 represent a “good” quality of performance. A grade of C with grade-point equivalent of 2.00 represents a “satisfactory” level of performance, a grade of D with grade-point equivalent of 1.00 represents a “poor” quality of performance, and a grade of F with grade-point equivalent of 0.00 represents failure.
3 Note that some demographics (e.g., race, ethnicity) and other variables that are constant over time do not appear in Equation (2) because they present no variation across waves.
4 Of particular concern is the possibility that measurement error due to misreporting varies across waves—either because of random recall errors or because of changes in the interview conditions. (For example, the proportion of interviews in which others were present declined from roughly 42% to 25% between Wave 1 and Wave 2.) Such measurement error could lead to attenuation bias in our fixed-effects model. On the other hand, reporting biases that are similar and stable over time are eliminated by the fixed-effects specification.
5 We tested the significance of these differences by pooling males and females and including an interaction of a gender dummy with the alcohol consumption measure in each model. We found statistically significant differences in the effects of monthly bingeing, drinks per month, and drinking days per month.
6 If alcohol use has small or negligible effects on school completion - as found by Chatterji (2006) , Dee and Evans (2003) , and Koch and Ribar (2001) - then such selection bias will also be small.
7 These results are not presented in the tables but are available from the authors upon request.
8 Examination of the outliers showed that only 15% of those who reported a total number of drinks above the 95th percentile of the distribution did so in both waves.
9 These fixed-effects regressions were adjusted by the same set of controls as in Table (3) , Column (3).
10 More than two-thirds of those who dropped out between Waves 1 and 2 were missing Wave 1 GPA data
- Azevedo Simoes A, Bastos FI, Moreira RI, Lynch KG, Metzger DS. A randomized trial of audio computer and in-person interview to assess HIV risk among drug and alcohol users in Rio De Janeiro, Brazil. Journal of Substance Abuse Treatment. 2006; 30 :237–243. [ PubMed ] [ Google Scholar ]
- Bray JW. Alcohol use, human capital, and wages. Journal of Labor Economics. 2005; 23 (2):279–312. [ Google Scholar ]
- Bray JW, Zarkin GA, Ringwalt C, Qi J. The relationship between marijuana initiation and dropping out of high school. Health Economics. 2000; 9 (1):9–18. [ PubMed ] [ Google Scholar ]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcoholism: Clinical and Experimental Research. 2000; 24 (2):164–171. [ PubMed ] [ Google Scholar ]
- Bukstein OG, Cornelius J, Trunzo AC, Kelly TM, Wood DS. Clinical predictors of treatment in a population of adolescents with alcohol use disorders. Addictive Behaviours. 2005; 30 (9):1663–1673. [ PubMed ] [ Google Scholar ]
- Chatterji P. Does alcohol use during high school affect education attainment? Evidence from the National Education Longitudinal Study. Economics of Education Review. 2006; 25 :482–497. [ Google Scholar ]
- Chatterji P, DeSimone J. Adolescent drinking and high school droupout. NBER Working Paper #11337. Cambridge, MA: 2005. Available online at SSRN: http://ssrn.com/abstract=723306 . [ Google Scholar ]
- Cook PJ, Moore MJ. Drinking and schooling. Journal of Health Economics. 1993; 12 (4):411–419. [ PubMed ] [ Google Scholar ]
- Dee TS, Evans WN. Teen drinking and educational attainment: evidence from two-sample instrumental variables estimates. Journal of Labor Economics. 2003; 21 (1):178–209. [ Google Scholar ]
- DeSimone J, Wolaver A. Drinking and academic performance in high school. NBER Working Paper #11035. Cambridge, MA: 2005. [ Google Scholar ]
- Dixon WJ. Simplified estimation from censored normal samples. The Annals of Mathematical Statistics. 1960; 31 (2):385–391. [ Google Scholar ]
- Downey DB, Vogt Yuan AS. Sex differences in school performance during high school: Puzzling patterns and possible explanations. The Sociological Quarterly. 2005; 46 :29–321. [ Google Scholar ]
- Duckworth AL, Seligman MEP. Self-discipline gives girls the edge: Gender in self-discipline, grades, and achievement test scores. Journal of Educational Psychology. 2006; 98 (1):198–208. [ Google Scholar ]
- Dwyer CA, Johnson LM. Grades, accomplishments, and correlates. In: Willimgham W, Cole NS, editors. Gender and fair assessment. Mahwah, NJ: Lawrence Erlbaum Associates; 1997. pp. 127–156. [ Google Scholar ]
- French MT, Popovici I. That instrument is lousy! In search of agreement when using instrumental variables estimation in substance use research. Health Economics. 2009 (– On line) DOI: 10.1002/hec.1572. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Giancola PR, Mezzich AC. Neuropsychological deficits in female adolescents with a substance use disorder: better accounted for conduct disorder. Journal of Studies on Alcohol. 2000; 61 (6):809–817. [ PubMed ] [ Google Scholar ]
- Gil-Lacruz AI, Molina JA. Human development and alcohol abuse in adolescence. Applied Economics. 2007; 39 (10):1315–1323. [ Google Scholar ]
- Ham LS, Hope DA. College students and problematic drinking: a review of the literature. Clinical Psychology Review. 2003; 23 (5):719–759. [ PubMed ] [ Google Scholar ]
- Hawkins JD, Catalano RF, Miller JY. Risk and protective factors for alcohol and other drug problems in adolescence and early adulthood: implications for substance abuse prevention. Psychological Bulletin. 1992; 112 (1):64–105. [ PubMed ] [ Google Scholar ]
- Hommer DW. Male and female sensitivity to alcohol-induced brain damage. Alcohol Research and Health. 2003; 27 (2):181–185. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jacob BA. Where the boys aren’t: non-cognitive skills, returns to school and the gender gap in higher education. Economic Education Review. 2002; 21 :589–598. [ Google Scholar ]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: overview of key findings, 2006. NIH Publication No. 07-6202. Bethesda, MD: National Institute on Drug Abuse; 2007. [ Google Scholar ]
- Kleinfeld J. The myth that schools shortchange girls: social science in the service of deception. Women’s Freedom Network Document number ED 423 210. Washington, DC: Education Research Information Clearinghouse (ERIC); 1998. [ Google Scholar ]
- Koch SF, Ribar DC. A siblings analysis of the effects of alcohol consumption onset on educational attainment. Contemporary Economic Policy. 2001; 19 (2):162–174. [ Google Scholar ]
- Koch SF, McGeary KA. The effect of youth alcohol initiation on high school completion. Economic Inquiry. 2005; 43 (4):750–765. [ Google Scholar ]
- McCluskey CP, Krohn MD, Lizotte AJ, Rodriguez ML. Early substance use and school achievement: an examination of Latino, white, and African-American youth. The Journal of Drug Issues. 2002; 32 :921–944. [ Google Scholar ]
- Mullahy J, Sindelar JL. Alcoholism and income: the role of indirect effects. The Milbank Quarterly. 1994; 72 (2):359–375. [ PubMed ] [ Google Scholar ]
- National Institute on Drug Abuse (NIDA) National survey results on drug use from the Monitoring the Future study, 1975–1997. Volume 1: Secondary School Students. Rockville, MD: National Institutes of Health; 1998. [ Google Scholar ]
- Newes-Adeyi G, Chen CM, Williams GD, Faden VB. Trends in underage drinking in the United States, 1991–2005. Surveillance Report #81. Bethesda, MD: Division of Epidemiology and Prevention Research, National Institute on Alcohol Abuse and Alcoholism, U.S. Department of Health and Human Services; 2007. [ Google Scholar ]
- Renna F. The economic cost of teen drinking: late graduation and lowered earnings. Health Economics. 2007; 16 (4):407–419. [ PubMed ] [ Google Scholar ]
- Renna F. Teens’ alcohol consumption and schooling. Economics of Education Review. 2008; 27 :69–78. [ Google Scholar ]
- Schulenberg J, O’Malley PM, Bachman JG, Wadsworth KN, Johnston LD. Getting drunk and growing up: trajectories of frequent binge drinking during the transition to young adulthood. Journal of Studies on Alcohol. 1996; 57 (3):289–304. [ PubMed ] [ Google Scholar ]
- Tapert SF, Brown SA. Neuropsychological correlates of adolescent substance abuse: four-year outcomes. Journal of the International Neuropsychological Society. 1999; 5 :481–493. [ PubMed ] [ Google Scholar ]
- Tourangeau R, Smith TW. Asking sensitive questions: The impact of data collection mode, question format, and question context. Public Opinion Quarterly. 1996; 60 :275–304. [ Google Scholar ]
- Wechsler H, Davenport A, Dowdall GW, Moeykens B, Castillo S. Health and Behavioral Consequences of Binge Drinking at Colleges: a national survey of students at 140 campuses. Journal of the American Medical Association. 1994; 272 (21):1672–1677. [ PubMed ] [ Google Scholar ]
- White AM, Swartzwelder HS. Hippocampal function during adolescence: a unique target of ethanol effects. Annals of the New York Academy of Sciences. 2004; 1021 :206–220. [ PubMed ] [ Google Scholar ]
- Williams J, Powell LM, Wechsler H. Does alcohol consumption reduce human capital accumulation? Evidence from the College Alcohol Study. Applied Economics. 2003; 35 (1):1227–1239. [ Google Scholar ]
- Wolaver A. Effects of heavy drinking in college on study effort, grade point average, and major choice. Contemporary Economic Policy. 2002; 20 (4):415–428. [ Google Scholar ]
- Wolaver A. Does drinking affect grades more for women? Gender differences in the effects of heavy episodic drinking in college. The American Economist. 2007; 51 (2):72–88. [ Google Scholar ]
- Wright DL, Aquilino WS, Supple AJ. A comparison of computer assisted and paper-and-pencil self- administered questionnaires in a survey on smoking alcohol and drug use. Public Opinion Quarterly. 1998; 62 :331–353. [ Google Scholar ]
- Yamada T, Kendrix M, Yamada T. The impact of alcohol consumption and marijuana use on high school graduation. Health Economics. 1996; 5 (1):77–92. [ PubMed ] [ Google Scholar ]
- Zeigler DW, Wang CC, Yoast RA, Dickinson BD, McCaffree MA, Rabinowitz CB, Sterling ML. The neurocognitive effects of alcohol on adolescents and college students. Preventive Medicine. 2004; 40 (1):23–32. [ PubMed ] [ Google Scholar ]
- Zimmerman MA, Caldwell CH, Bernat DH. Discrepancy between self-report and school-record grade point average: correlates with psychosocial outcomes among African American adolescents. Journal of Applied Social Psychology. 2006; 32 (1):86–109. [ Google Scholar ]

COMMENTS
Various research studies conducted over many years clearly show the association of prolonged alcohol intake in the causation, aggravation, worsening, and deterioration of the health of its consumers. Moreover, chronic alcohol intake single-handedly is one of the major etiological factors in various serious diseases.
Over the past 40 years, rigorous examination of brain function, structure, and attending factors through multidisciplinary research has helped identify the substrates of alcohol-related damage in the brain. ... History of Neurobiological Studies in Alcohol Research. Looking at publications from the early 1970s, one is struck by the lack of ...
The effects of alcohol on the liver include inflammation (alcoholic hepatitis) and cirrhosis (progressive liver scarring). The risk for liver disease is related to how much a person drinks: the risk is low at low levels of alcohol consumption but increases steeply with higher levels of consumption ( Edwards et al. 1994 ).
Psychopharmacology (2024) Social drinking is common, but it is unclear how moderate levels of alcohol influence decision making. Most prior studies have focused on adverse long-term effects on ...
While most of our knowledge on the effects of alcohol on the brain and cognitive outcomes is based on research in adults, several recent reviews have examined the effects of alcohol on the brain ...
Alcohol consumption is a major risk factor for poor physical and mental health, accounting for about 3 million deaths and over 130 million disability-adjusted life years worldwide in 2016 (ref. 1
Alcohol misuse contributes to the dysregulation of immune responses and multiorgan dysfunction across various tissues, which are associated with higher risk of morbidity and mortality in people with alcohol use disorders. Organ-specific immune cells, including microglia in the brain, alveolar macrop …
Alcohol has a much longer history than any of the other intoxicants or stimulants. It is so intertwined in so many cultures, we invented reasons to justify its use. Alcohol has been condemned in all ancient literature and scripts. Bard commented that," Alcohol provokes the desire but takes away the performance".
Background Excessive alcohol consumption and alcohol use disorders (AUD) are among the leading preventable causes of premature morbidity and mortality and are considered a major public health concern. In order to reduce the individual and societal burden of excessive alcohol use, it is crucial to identify high-risk individuals at earlier stages and to provide effective interventions to prevent ...
Over the past 40 years, rigorous examination of brain function, structure, and attending factors through multidisciplinary research has helped identify the substrates of alcohol-related damage in ...
Almost equally. important are the acute effects of alcohol consumption on the risk of both unintentional and. intentional injury. In addition, alcohol has a sizable effect on the burden of disease ...
The mediating role of alcohol in peer networks. This paper shows how adding alcohol to a social situation transforms the outcome, both internally because of the physical effects due to the inscription of alcohol, and externally because alcohol becomes a factor that modifies the social meanings of situations (Latour, Citation 1994). Identifying ...
Introduction. Alcohol is widely believed to be the only psychoactive substance with addictive potential "that is not controlled at the international level by legally binding regulatory frameworks" despite its profound implications for populations and public health.1 The adverse effects of alcohol on health has been the subject of a rising number of studies in recent years,1,2 with such ...
Only a small percent of individuals with alcohol use disorder contribute to the greatest societal and economic costs ().For example, in the 2015 National Survey on Drug Use and Health survey (total n = 43,561), a household survey conducted across the United States, 11.8% met criteria for an alcohol use disorder (n = 5124) ().Of these 5124 individuals, 67.4% (n = 3455) met criteria for a mild ...
Various research studies conducted over many years clearly show the association of prolonged alcohol intake in the causation, aggravation, worsening, and deterioration of the health of its consumers. Moreover, chronic alcohol intake single-handedly is one of the major etiological factors in various serious diseases.
Using fractional polynomial nonlinear mendelian randomization analyses with Alcohol Use Disorder-Restricted instruments, localized average causal effects were metaregressed against mean consumption in each strata of alcohol, and these plots were reconstructed as the derivative of the best fit model for γ-glutamyl transferase (GGT), low-density lipoprotein (LDL) cholesterol, systolic blood ...
The work, titled "Alcohol rehabilitation and cancer risk: a nationwide hospital cohort study in France," was published in The Lancet Public Health.It is the largest of its kind to provide evidence ...
ABSTRACT: Alcohol consumption is known to be an addiction that provides negative outcomes mainly on health, excessive drinking of alcohol brings adverse effects on human health, also on activities that focus on school performance. This research aims to examine the link between alcohol use and the academic success of high school students.
However, adolescents have less control over their impulses and desires due to the imbalance between the (overactivated) reward system and the (still-maturing) PFC. Altogether, this makes teenagers more likely to get involved in risky behaviors, such as getting into fights, having unprotected sexual activity, or abusing drugs or alcohol. Effects ...
Abstract. Alcohol is a major contributor to global disease and a leading cause of preventable death, causing approximately 88,000 deaths annually in the United States alone. Alcohol use disorder is one of the most common psychiatric disorders, with nearly one-third of U.S. adults experiencing alcohol use disorder at some point during their lives.
SUBMIT PAPER. Proceedings of the Human Factors and Ergonomics Society Annual Meeting ... Effects of alcohol on performance on a distraction task during simulated driving. Alcoholism: clinical and experimental research, 33(4), 617-625. Crossref. PubMed. ... Bastien-Toniazzo M., Gineyt G. (2014). Divided attention in young drivers under the ...
Abstract. Alcohol consumption is known to be an addiction that provides negative outcomes mainly on health, excessive drinking of alcohol brings adverse effects on human health, also on activities ...
We provide quasi-experimental evidence on the effects of alcohol regulation on alcohol consumption and associated public health outcomes using detailed individual level and aggregate data from India, where state-level laws regulating the minimum legal drinking age generate substantial variation in the availability of commercially produced alcohol across people of different ages. We find that ...
the extent of alcohol consumption and of the awareness of its negative effects on human health among secondary school students. The study used a cross-sectional survey design. Self-report questionnaire developed by the researchers was administered to representative sample (N = 1302) of secondary school students in the study area. The data collected from the respondents were analyzed using ...
Almost equally important are the acute effects of alcohol consumption on the risk of both unintentional and intentional injury. In addition, alcohol has a sizable effect on the burden of disease associated with infectious diseases, cancer, cardiovascular disease, and liver cirrhosis. However, alcohol consumption also has beneficial effects on ...
The largest increases in alcohol-related deaths were among people 25 to 24 and 35 to 44 years old; deaths in both groups increased by over 37%. Mortality from alcohol-associated liver disease is ...
SDG health target 3.5 calls to "strengthen the prevention and treatment of substance abuse, including narcotic drug abuse and harmful use of alcohol" and has two indicators: 3.5.1 - coverage of treatment interventions (pharmacological, psychosocial and rehabilitation and aftercare services) for substance use disorders, and indicator 3.5.2 ...
Abstract. This paper examines the effects of alcohol use on high school students' quality of learning. We estimate fixed-effects models using data from the National Longitudinal Study of Adolescent Health. Our primary measure of academic achievement is the student's GPA abstracted from official school transcripts.