Purdue Online Writing Lab Purdue OWL® College of Liberal Arts

Writing a Literature Review

Welcome to the Purdue OWL
This page is brought to you by the OWL at Purdue University. When printing this page, you must include the entire legal notice.
Copyright ©1995-2018 by The Writing Lab & The OWL at Purdue and Purdue University. All rights reserved. This material may not be published, reproduced, broadcast, rewritten, or redistributed without permission. Use of this site constitutes acceptance of our terms and conditions of fair use.
A literature review is a document or section of a document that collects key sources on a topic and discusses those sources in conversation with each other (also called synthesis ). The lit review is an important genre in many disciplines, not just literature (i.e., the study of works of literature such as novels and plays). When we say “literature review” or refer to “the literature,” we are talking about the research ( scholarship ) in a given field. You will often see the terms “the research,” “the scholarship,” and “the literature” used mostly interchangeably.
Where, when, and why would I write a lit review?
There are a number of different situations where you might write a literature review, each with slightly different expectations; different disciplines, too, have field-specific expectations for what a literature review is and does. For instance, in the humanities, authors might include more overt argumentation and interpretation of source material in their literature reviews, whereas in the sciences, authors are more likely to report study designs and results in their literature reviews; these differences reflect these disciplines’ purposes and conventions in scholarship. You should always look at examples from your own discipline and talk to professors or mentors in your field to be sure you understand your discipline’s conventions, for literature reviews as well as for any other genre.
A literature review can be a part of a research paper or scholarly article, usually falling after the introduction and before the research methods sections. In these cases, the lit review just needs to cover scholarship that is important to the issue you are writing about; sometimes it will also cover key sources that informed your research methodology.
Lit reviews can also be standalone pieces, either as assignments in a class or as publications. In a class, a lit review may be assigned to help students familiarize themselves with a topic and with scholarship in their field, get an idea of the other researchers working on the topic they’re interested in, find gaps in existing research in order to propose new projects, and/or develop a theoretical framework and methodology for later research. As a publication, a lit review usually is meant to help make other scholars’ lives easier by collecting and summarizing, synthesizing, and analyzing existing research on a topic. This can be especially helpful for students or scholars getting into a new research area, or for directing an entire community of scholars toward questions that have not yet been answered.
What are the parts of a lit review?
Most lit reviews use a basic introduction-body-conclusion structure; if your lit review is part of a larger paper, the introduction and conclusion pieces may be just a few sentences while you focus most of your attention on the body. If your lit review is a standalone piece, the introduction and conclusion take up more space and give you a place to discuss your goals, research methods, and conclusions separately from where you discuss the literature itself.
Introduction:
- An introductory paragraph that explains what your working topic and thesis is
- A forecast of key topics or texts that will appear in the review
- Potentially, a description of how you found sources and how you analyzed them for inclusion and discussion in the review (more often found in published, standalone literature reviews than in lit review sections in an article or research paper)
- Summarize and synthesize: Give an overview of the main points of each source and combine them into a coherent whole
- Analyze and interpret: Don’t just paraphrase other researchers – add your own interpretations where possible, discussing the significance of findings in relation to the literature as a whole
- Critically Evaluate: Mention the strengths and weaknesses of your sources
- Write in well-structured paragraphs: Use transition words and topic sentence to draw connections, comparisons, and contrasts.
Conclusion:
- Summarize the key findings you have taken from the literature and emphasize their significance
- Connect it back to your primary research question
How should I organize my lit review?
Lit reviews can take many different organizational patterns depending on what you are trying to accomplish with the review. Here are some examples:
- Chronological : The simplest approach is to trace the development of the topic over time, which helps familiarize the audience with the topic (for instance if you are introducing something that is not commonly known in your field). If you choose this strategy, be careful to avoid simply listing and summarizing sources in order. Try to analyze the patterns, turning points, and key debates that have shaped the direction of the field. Give your interpretation of how and why certain developments occurred (as mentioned previously, this may not be appropriate in your discipline — check with a teacher or mentor if you’re unsure).
- Thematic : If you have found some recurring central themes that you will continue working with throughout your piece, you can organize your literature review into subsections that address different aspects of the topic. For example, if you are reviewing literature about women and religion, key themes can include the role of women in churches and the religious attitude towards women.
- Qualitative versus quantitative research
- Empirical versus theoretical scholarship
- Divide the research by sociological, historical, or cultural sources
- Theoretical : In many humanities articles, the literature review is the foundation for the theoretical framework. You can use it to discuss various theories, models, and definitions of key concepts. You can argue for the relevance of a specific theoretical approach or combine various theorical concepts to create a framework for your research.
What are some strategies or tips I can use while writing my lit review?
Any lit review is only as good as the research it discusses; make sure your sources are well-chosen and your research is thorough. Don’t be afraid to do more research if you discover a new thread as you’re writing. More info on the research process is available in our "Conducting Research" resources .
As you’re doing your research, create an annotated bibliography ( see our page on the this type of document ). Much of the information used in an annotated bibliography can be used also in a literature review, so you’ll be not only partially drafting your lit review as you research, but also developing your sense of the larger conversation going on among scholars, professionals, and any other stakeholders in your topic.
Usually you will need to synthesize research rather than just summarizing it. This means drawing connections between sources to create a picture of the scholarly conversation on a topic over time. Many student writers struggle to synthesize because they feel they don’t have anything to add to the scholars they are citing; here are some strategies to help you:
- It often helps to remember that the point of these kinds of syntheses is to show your readers how you understand your research, to help them read the rest of your paper.
- Writing teachers often say synthesis is like hosting a dinner party: imagine all your sources are together in a room, discussing your topic. What are they saying to each other?
- Look at the in-text citations in each paragraph. Are you citing just one source for each paragraph? This usually indicates summary only. When you have multiple sources cited in a paragraph, you are more likely to be synthesizing them (not always, but often
- Read more about synthesis here.
The most interesting literature reviews are often written as arguments (again, as mentioned at the beginning of the page, this is discipline-specific and doesn’t work for all situations). Often, the literature review is where you can establish your research as filling a particular gap or as relevant in a particular way. You have some chance to do this in your introduction in an article, but the literature review section gives a more extended opportunity to establish the conversation in the way you would like your readers to see it. You can choose the intellectual lineage you would like to be part of and whose definitions matter most to your thinking (mostly humanities-specific, but this goes for sciences as well). In addressing these points, you argue for your place in the conversation, which tends to make the lit review more compelling than a simple reporting of other sources.
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- How to Write a Literature Review | Guide, Examples, & Templates
How to Write a Literature Review | Guide, Examples, & Templates
Published on January 2, 2023 by Shona McCombes . Revised on September 11, 2023.
What is a literature review? A literature review is a survey of scholarly sources on a specific topic. It provides an overview of current knowledge, allowing you to identify relevant theories, methods, and gaps in the existing research that you can later apply to your paper, thesis, or dissertation topic .
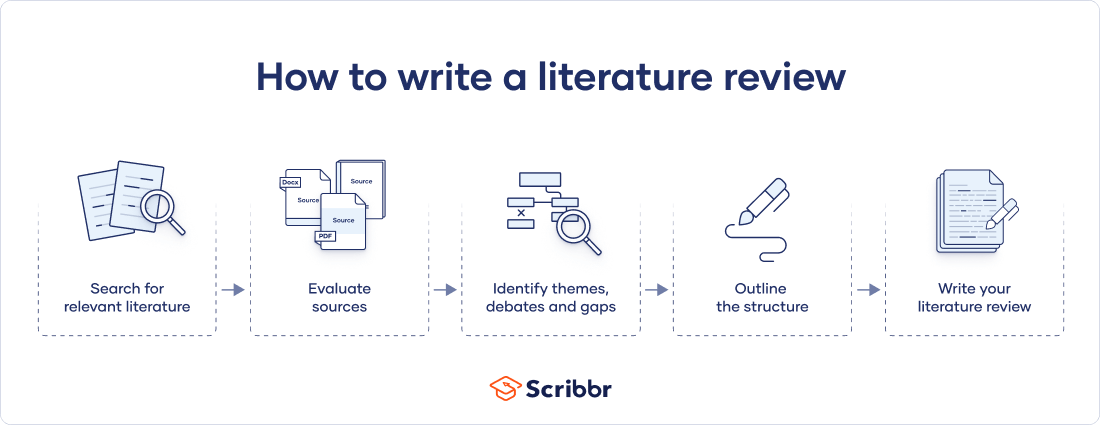
There are five key steps to writing a literature review:
- Search for relevant literature
- Evaluate sources
- Identify themes, debates, and gaps
- Outline the structure
- Write your literature review
A good literature review doesn’t just summarize sources—it analyzes, synthesizes , and critically evaluates to give a clear picture of the state of knowledge on the subject.
Instantly correct all language mistakes in your text
Upload your document to correct all your mistakes in minutes

Table of contents
What is the purpose of a literature review, examples of literature reviews, step 1 – search for relevant literature, step 2 – evaluate and select sources, step 3 – identify themes, debates, and gaps, step 4 – outline your literature review’s structure, step 5 – write your literature review, free lecture slides, other interesting articles, frequently asked questions, introduction.
- Quick Run-through
- Step 1 & 2
When you write a thesis , dissertation , or research paper , you will likely have to conduct a literature review to situate your research within existing knowledge. The literature review gives you a chance to:
- Demonstrate your familiarity with the topic and its scholarly context
- Develop a theoretical framework and methodology for your research
- Position your work in relation to other researchers and theorists
- Show how your research addresses a gap or contributes to a debate
- Evaluate the current state of research and demonstrate your knowledge of the scholarly debates around your topic.
Writing literature reviews is a particularly important skill if you want to apply for graduate school or pursue a career in research. We’ve written a step-by-step guide that you can follow below.
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

Writing literature reviews can be quite challenging! A good starting point could be to look at some examples, depending on what kind of literature review you’d like to write.
- Example literature review #1: “Why Do People Migrate? A Review of the Theoretical Literature” ( Theoretical literature review about the development of economic migration theory from the 1950s to today.)
- Example literature review #2: “Literature review as a research methodology: An overview and guidelines” ( Methodological literature review about interdisciplinary knowledge acquisition and production.)
- Example literature review #3: “The Use of Technology in English Language Learning: A Literature Review” ( Thematic literature review about the effects of technology on language acquisition.)
- Example literature review #4: “Learners’ Listening Comprehension Difficulties in English Language Learning: A Literature Review” ( Chronological literature review about how the concept of listening skills has changed over time.)
You can also check out our templates with literature review examples and sample outlines at the links below.
Download Word doc Download Google doc
Before you begin searching for literature, you need a clearly defined topic .
If you are writing the literature review section of a dissertation or research paper, you will search for literature related to your research problem and questions .
Make a list of keywords
Start by creating a list of keywords related to your research question. Include each of the key concepts or variables you’re interested in, and list any synonyms and related terms. You can add to this list as you discover new keywords in the process of your literature search.
- Social media, Facebook, Instagram, Twitter, Snapchat, TikTok
- Body image, self-perception, self-esteem, mental health
- Generation Z, teenagers, adolescents, youth
Search for relevant sources
Use your keywords to begin searching for sources. Some useful databases to search for journals and articles include:
- Your university’s library catalogue
- Google Scholar
- Project Muse (humanities and social sciences)
- Medline (life sciences and biomedicine)
- EconLit (economics)
- Inspec (physics, engineering and computer science)
You can also use boolean operators to help narrow down your search.
Make sure to read the abstract to find out whether an article is relevant to your question. When you find a useful book or article, you can check the bibliography to find other relevant sources.
You likely won’t be able to read absolutely everything that has been written on your topic, so it will be necessary to evaluate which sources are most relevant to your research question.
For each publication, ask yourself:
- What question or problem is the author addressing?
- What are the key concepts and how are they defined?
- What are the key theories, models, and methods?
- Does the research use established frameworks or take an innovative approach?
- What are the results and conclusions of the study?
- How does the publication relate to other literature in the field? Does it confirm, add to, or challenge established knowledge?
- What are the strengths and weaknesses of the research?
Make sure the sources you use are credible , and make sure you read any landmark studies and major theories in your field of research.
You can use our template to summarize and evaluate sources you’re thinking about using. Click on either button below to download.
Take notes and cite your sources
As you read, you should also begin the writing process. Take notes that you can later incorporate into the text of your literature review.
It is important to keep track of your sources with citations to avoid plagiarism . It can be helpful to make an annotated bibliography , where you compile full citation information and write a paragraph of summary and analysis for each source. This helps you remember what you read and saves time later in the process.
To begin organizing your literature review’s argument and structure, be sure you understand the connections and relationships between the sources you’ve read. Based on your reading and notes, you can look for:
- Trends and patterns (in theory, method or results): do certain approaches become more or less popular over time?
- Themes: what questions or concepts recur across the literature?
- Debates, conflicts and contradictions: where do sources disagree?
- Pivotal publications: are there any influential theories or studies that changed the direction of the field?
- Gaps: what is missing from the literature? Are there weaknesses that need to be addressed?
This step will help you work out the structure of your literature review and (if applicable) show how your own research will contribute to existing knowledge.
- Most research has focused on young women.
- There is an increasing interest in the visual aspects of social media.
- But there is still a lack of robust research on highly visual platforms like Instagram and Snapchat—this is a gap that you could address in your own research.
There are various approaches to organizing the body of a literature review. Depending on the length of your literature review, you can combine several of these strategies (for example, your overall structure might be thematic, but each theme is discussed chronologically).
Chronological
The simplest approach is to trace the development of the topic over time. However, if you choose this strategy, be careful to avoid simply listing and summarizing sources in order.
Try to analyze patterns, turning points and key debates that have shaped the direction of the field. Give your interpretation of how and why certain developments occurred.
If you have found some recurring central themes, you can organize your literature review into subsections that address different aspects of the topic.
For example, if you are reviewing literature about inequalities in migrant health outcomes, key themes might include healthcare policy, language barriers, cultural attitudes, legal status, and economic access.
Methodological
If you draw your sources from different disciplines or fields that use a variety of research methods , you might want to compare the results and conclusions that emerge from different approaches. For example:
- Look at what results have emerged in qualitative versus quantitative research
- Discuss how the topic has been approached by empirical versus theoretical scholarship
- Divide the literature into sociological, historical, and cultural sources
Theoretical
A literature review is often the foundation for a theoretical framework . You can use it to discuss various theories, models, and definitions of key concepts.
You might argue for the relevance of a specific theoretical approach, or combine various theoretical concepts to create a framework for your research.
Like any other academic text , your literature review should have an introduction , a main body, and a conclusion . What you include in each depends on the objective of your literature review.
The introduction should clearly establish the focus and purpose of the literature review.
Depending on the length of your literature review, you might want to divide the body into subsections. You can use a subheading for each theme, time period, or methodological approach.
As you write, you can follow these tips:
- Summarize and synthesize: give an overview of the main points of each source and combine them into a coherent whole
- Analyze and interpret: don’t just paraphrase other researchers — add your own interpretations where possible, discussing the significance of findings in relation to the literature as a whole
- Critically evaluate: mention the strengths and weaknesses of your sources
- Write in well-structured paragraphs: use transition words and topic sentences to draw connections, comparisons and contrasts
In the conclusion, you should summarize the key findings you have taken from the literature and emphasize their significance.
When you’ve finished writing and revising your literature review, don’t forget to proofread thoroughly before submitting. Not a language expert? Check out Scribbr’s professional proofreading services !
This article has been adapted into lecture slides that you can use to teach your students about writing a literature review.
Scribbr slides are free to use, customize, and distribute for educational purposes.
Open Google Slides Download PowerPoint
If you want to know more about the research process , methodology , research bias , or statistics , make sure to check out some of our other articles with explanations and examples.
- Sampling methods
- Simple random sampling
- Stratified sampling
- Cluster sampling
- Likert scales
- Reproducibility
Statistics
- Null hypothesis
- Statistical power
- Probability distribution
- Effect size
- Poisson distribution
Research bias
- Optimism bias
- Cognitive bias
- Implicit bias
- Hawthorne effect
- Anchoring bias
- Explicit bias
A literature review is a survey of scholarly sources (such as books, journal articles, and theses) related to a specific topic or research question .
It is often written as part of a thesis, dissertation , or research paper , in order to situate your work in relation to existing knowledge.
There are several reasons to conduct a literature review at the beginning of a research project:
- To familiarize yourself with the current state of knowledge on your topic
- To ensure that you’re not just repeating what others have already done
- To identify gaps in knowledge and unresolved problems that your research can address
- To develop your theoretical framework and methodology
- To provide an overview of the key findings and debates on the topic
Writing the literature review shows your reader how your work relates to existing research and what new insights it will contribute.
The literature review usually comes near the beginning of your thesis or dissertation . After the introduction , it grounds your research in a scholarly field and leads directly to your theoretical framework or methodology .
A literature review is a survey of credible sources on a topic, often used in dissertations , theses, and research papers . Literature reviews give an overview of knowledge on a subject, helping you identify relevant theories and methods, as well as gaps in existing research. Literature reviews are set up similarly to other academic texts , with an introduction , a main body, and a conclusion .
An annotated bibliography is a list of source references that has a short description (called an annotation ) for each of the sources. It is often assigned as part of the research process for a paper .
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. (2023, September 11). How to Write a Literature Review | Guide, Examples, & Templates. Scribbr. Retrieved July 5, 2024, from https://www.scribbr.com/dissertation/literature-review/
Is this article helpful?
Shona McCombes
Other students also liked, what is a theoretical framework | guide to organizing, what is a research methodology | steps & tips, how to write a research proposal | examples & templates, "i thought ai proofreading was useless but..".
I've been using Scribbr for years now and I know it's a service that won't disappoint. It does a good job spotting mistakes”
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- PLoS Comput Biol
- v.9(7); 2013 Jul

Ten Simple Rules for Writing a Literature Review
Marco pautasso.
1 Centre for Functional and Evolutionary Ecology (CEFE), CNRS, Montpellier, France
2 Centre for Biodiversity Synthesis and Analysis (CESAB), FRB, Aix-en-Provence, France
Literature reviews are in great demand in most scientific fields. Their need stems from the ever-increasing output of scientific publications [1] . For example, compared to 1991, in 2008 three, eight, and forty times more papers were indexed in Web of Science on malaria, obesity, and biodiversity, respectively [2] . Given such mountains of papers, scientists cannot be expected to examine in detail every single new paper relevant to their interests [3] . Thus, it is both advantageous and necessary to rely on regular summaries of the recent literature. Although recognition for scientists mainly comes from primary research, timely literature reviews can lead to new synthetic insights and are often widely read [4] . For such summaries to be useful, however, they need to be compiled in a professional way [5] .
When starting from scratch, reviewing the literature can require a titanic amount of work. That is why researchers who have spent their career working on a certain research issue are in a perfect position to review that literature. Some graduate schools are now offering courses in reviewing the literature, given that most research students start their project by producing an overview of what has already been done on their research issue [6] . However, it is likely that most scientists have not thought in detail about how to approach and carry out a literature review.
Reviewing the literature requires the ability to juggle multiple tasks, from finding and evaluating relevant material to synthesising information from various sources, from critical thinking to paraphrasing, evaluating, and citation skills [7] . In this contribution, I share ten simple rules I learned working on about 25 literature reviews as a PhD and postdoctoral student. Ideas and insights also come from discussions with coauthors and colleagues, as well as feedback from reviewers and editors.
Rule 1: Define a Topic and Audience
How to choose which topic to review? There are so many issues in contemporary science that you could spend a lifetime of attending conferences and reading the literature just pondering what to review. On the one hand, if you take several years to choose, several other people may have had the same idea in the meantime. On the other hand, only a well-considered topic is likely to lead to a brilliant literature review [8] . The topic must at least be:
- interesting to you (ideally, you should have come across a series of recent papers related to your line of work that call for a critical summary),
- an important aspect of the field (so that many readers will be interested in the review and there will be enough material to write it), and
- a well-defined issue (otherwise you could potentially include thousands of publications, which would make the review unhelpful).
Ideas for potential reviews may come from papers providing lists of key research questions to be answered [9] , but also from serendipitous moments during desultory reading and discussions. In addition to choosing your topic, you should also select a target audience. In many cases, the topic (e.g., web services in computational biology) will automatically define an audience (e.g., computational biologists), but that same topic may also be of interest to neighbouring fields (e.g., computer science, biology, etc.).
Rule 2: Search and Re-search the Literature
After having chosen your topic and audience, start by checking the literature and downloading relevant papers. Five pieces of advice here:
- keep track of the search items you use (so that your search can be replicated [10] ),
- keep a list of papers whose pdfs you cannot access immediately (so as to retrieve them later with alternative strategies),
- use a paper management system (e.g., Mendeley, Papers, Qiqqa, Sente),
- define early in the process some criteria for exclusion of irrelevant papers (these criteria can then be described in the review to help define its scope), and
- do not just look for research papers in the area you wish to review, but also seek previous reviews.
The chances are high that someone will already have published a literature review ( Figure 1 ), if not exactly on the issue you are planning to tackle, at least on a related topic. If there are already a few or several reviews of the literature on your issue, my advice is not to give up, but to carry on with your own literature review,

The bottom-right situation (many literature reviews but few research papers) is not just a theoretical situation; it applies, for example, to the study of the impacts of climate change on plant diseases, where there appear to be more literature reviews than research studies [33] .
- discussing in your review the approaches, limitations, and conclusions of past reviews,
- trying to find a new angle that has not been covered adequately in the previous reviews, and
- incorporating new material that has inevitably accumulated since their appearance.
When searching the literature for pertinent papers and reviews, the usual rules apply:
- be thorough,
- use different keywords and database sources (e.g., DBLP, Google Scholar, ISI Proceedings, JSTOR Search, Medline, Scopus, Web of Science), and
- look at who has cited past relevant papers and book chapters.
Rule 3: Take Notes While Reading
If you read the papers first, and only afterwards start writing the review, you will need a very good memory to remember who wrote what, and what your impressions and associations were while reading each single paper. My advice is, while reading, to start writing down interesting pieces of information, insights about how to organize the review, and thoughts on what to write. This way, by the time you have read the literature you selected, you will already have a rough draft of the review.
Of course, this draft will still need much rewriting, restructuring, and rethinking to obtain a text with a coherent argument [11] , but you will have avoided the danger posed by staring at a blank document. Be careful when taking notes to use quotation marks if you are provisionally copying verbatim from the literature. It is advisable then to reformulate such quotes with your own words in the final draft. It is important to be careful in noting the references already at this stage, so as to avoid misattributions. Using referencing software from the very beginning of your endeavour will save you time.
Rule 4: Choose the Type of Review You Wish to Write
After having taken notes while reading the literature, you will have a rough idea of the amount of material available for the review. This is probably a good time to decide whether to go for a mini- or a full review. Some journals are now favouring the publication of rather short reviews focusing on the last few years, with a limit on the number of words and citations. A mini-review is not necessarily a minor review: it may well attract more attention from busy readers, although it will inevitably simplify some issues and leave out some relevant material due to space limitations. A full review will have the advantage of more freedom to cover in detail the complexities of a particular scientific development, but may then be left in the pile of the very important papers “to be read” by readers with little time to spare for major monographs.
There is probably a continuum between mini- and full reviews. The same point applies to the dichotomy of descriptive vs. integrative reviews. While descriptive reviews focus on the methodology, findings, and interpretation of each reviewed study, integrative reviews attempt to find common ideas and concepts from the reviewed material [12] . A similar distinction exists between narrative and systematic reviews: while narrative reviews are qualitative, systematic reviews attempt to test a hypothesis based on the published evidence, which is gathered using a predefined protocol to reduce bias [13] , [14] . When systematic reviews analyse quantitative results in a quantitative way, they become meta-analyses. The choice between different review types will have to be made on a case-by-case basis, depending not just on the nature of the material found and the preferences of the target journal(s), but also on the time available to write the review and the number of coauthors [15] .
Rule 5: Keep the Review Focused, but Make It of Broad Interest
Whether your plan is to write a mini- or a full review, it is good advice to keep it focused 16 , 17 . Including material just for the sake of it can easily lead to reviews that are trying to do too many things at once. The need to keep a review focused can be problematic for interdisciplinary reviews, where the aim is to bridge the gap between fields [18] . If you are writing a review on, for example, how epidemiological approaches are used in modelling the spread of ideas, you may be inclined to include material from both parent fields, epidemiology and the study of cultural diffusion. This may be necessary to some extent, but in this case a focused review would only deal in detail with those studies at the interface between epidemiology and the spread of ideas.
While focus is an important feature of a successful review, this requirement has to be balanced with the need to make the review relevant to a broad audience. This square may be circled by discussing the wider implications of the reviewed topic for other disciplines.
Rule 6: Be Critical and Consistent
Reviewing the literature is not stamp collecting. A good review does not just summarize the literature, but discusses it critically, identifies methodological problems, and points out research gaps [19] . After having read a review of the literature, a reader should have a rough idea of:
- the major achievements in the reviewed field,
- the main areas of debate, and
- the outstanding research questions.
It is challenging to achieve a successful review on all these fronts. A solution can be to involve a set of complementary coauthors: some people are excellent at mapping what has been achieved, some others are very good at identifying dark clouds on the horizon, and some have instead a knack at predicting where solutions are going to come from. If your journal club has exactly this sort of team, then you should definitely write a review of the literature! In addition to critical thinking, a literature review needs consistency, for example in the choice of passive vs. active voice and present vs. past tense.
Rule 7: Find a Logical Structure
Like a well-baked cake, a good review has a number of telling features: it is worth the reader's time, timely, systematic, well written, focused, and critical. It also needs a good structure. With reviews, the usual subdivision of research papers into introduction, methods, results, and discussion does not work or is rarely used. However, a general introduction of the context and, toward the end, a recapitulation of the main points covered and take-home messages make sense also in the case of reviews. For systematic reviews, there is a trend towards including information about how the literature was searched (database, keywords, time limits) [20] .
How can you organize the flow of the main body of the review so that the reader will be drawn into and guided through it? It is generally helpful to draw a conceptual scheme of the review, e.g., with mind-mapping techniques. Such diagrams can help recognize a logical way to order and link the various sections of a review [21] . This is the case not just at the writing stage, but also for readers if the diagram is included in the review as a figure. A careful selection of diagrams and figures relevant to the reviewed topic can be very helpful to structure the text too [22] .
Rule 8: Make Use of Feedback
Reviews of the literature are normally peer-reviewed in the same way as research papers, and rightly so [23] . As a rule, incorporating feedback from reviewers greatly helps improve a review draft. Having read the review with a fresh mind, reviewers may spot inaccuracies, inconsistencies, and ambiguities that had not been noticed by the writers due to rereading the typescript too many times. It is however advisable to reread the draft one more time before submission, as a last-minute correction of typos, leaps, and muddled sentences may enable the reviewers to focus on providing advice on the content rather than the form.
Feedback is vital to writing a good review, and should be sought from a variety of colleagues, so as to obtain a diversity of views on the draft. This may lead in some cases to conflicting views on the merits of the paper, and on how to improve it, but such a situation is better than the absence of feedback. A diversity of feedback perspectives on a literature review can help identify where the consensus view stands in the landscape of the current scientific understanding of an issue [24] .
Rule 9: Include Your Own Relevant Research, but Be Objective
In many cases, reviewers of the literature will have published studies relevant to the review they are writing. This could create a conflict of interest: how can reviewers report objectively on their own work [25] ? Some scientists may be overly enthusiastic about what they have published, and thus risk giving too much importance to their own findings in the review. However, bias could also occur in the other direction: some scientists may be unduly dismissive of their own achievements, so that they will tend to downplay their contribution (if any) to a field when reviewing it.
In general, a review of the literature should neither be a public relations brochure nor an exercise in competitive self-denial. If a reviewer is up to the job of producing a well-organized and methodical review, which flows well and provides a service to the readership, then it should be possible to be objective in reviewing one's own relevant findings. In reviews written by multiple authors, this may be achieved by assigning the review of the results of a coauthor to different coauthors.
Rule 10: Be Up-to-Date, but Do Not Forget Older Studies
Given the progressive acceleration in the publication of scientific papers, today's reviews of the literature need awareness not just of the overall direction and achievements of a field of inquiry, but also of the latest studies, so as not to become out-of-date before they have been published. Ideally, a literature review should not identify as a major research gap an issue that has just been addressed in a series of papers in press (the same applies, of course, to older, overlooked studies (“sleeping beauties” [26] )). This implies that literature reviewers would do well to keep an eye on electronic lists of papers in press, given that it can take months before these appear in scientific databases. Some reviews declare that they have scanned the literature up to a certain point in time, but given that peer review can be a rather lengthy process, a full search for newly appeared literature at the revision stage may be worthwhile. Assessing the contribution of papers that have just appeared is particularly challenging, because there is little perspective with which to gauge their significance and impact on further research and society.
Inevitably, new papers on the reviewed topic (including independently written literature reviews) will appear from all quarters after the review has been published, so that there may soon be the need for an updated review. But this is the nature of science [27] – [32] . I wish everybody good luck with writing a review of the literature.
Acknowledgments
Many thanks to M. Barbosa, K. Dehnen-Schmutz, T. Döring, D. Fontaneto, M. Garbelotto, O. Holdenrieder, M. Jeger, D. Lonsdale, A. MacLeod, P. Mills, M. Moslonka-Lefebvre, G. Stancanelli, P. Weisberg, and X. Xu for insights and discussions, and to P. Bourne, T. Matoni, and D. Smith for helpful comments on a previous draft.
Funding Statement
This work was funded by the French Foundation for Research on Biodiversity (FRB) through its Centre for Synthesis and Analysis of Biodiversity data (CESAB), as part of the NETSEED research project. The funders had no role in the preparation of the manuscript.
- Resources Home 🏠
- Try SciSpace Copilot
- Search research papers
- Add Copilot Extension
- Try AI Detector
- Try Paraphraser
- Try Citation Generator
- April Papers
- June Papers
- July Papers

How To Write A Literature Review - A Complete Guide

Table of Contents
A literature review is much more than just another section in your research paper. It forms the very foundation of your research. It is a formal piece of writing where you analyze the existing theoretical framework, principles, and assumptions and use that as a base to shape your approach to the research question.
Curating and drafting a solid literature review section not only lends more credibility to your research paper but also makes your research tighter and better focused. But, writing literature reviews is a difficult task. It requires extensive reading, plus you have to consider market trends and technological and political changes, which tend to change in the blink of an eye.
Now streamline your literature review process with the help of SciSpace Copilot. With this AI research assistant, you can efficiently synthesize and analyze a vast amount of information, identify key themes and trends, and uncover gaps in the existing research. Get real-time explanations, summaries, and answers to your questions for the paper you're reviewing, making navigating and understanding the complex literature landscape easier.
In this comprehensive guide, we will explore everything from the definition of a literature review, its appropriate length, various types of literature reviews, and how to write one.
What is a literature review?
A literature review is a collation of survey, research, critical evaluation, and assessment of the existing literature in a preferred domain.
Eminent researcher and academic Arlene Fink, in her book Conducting Research Literature Reviews , defines it as the following:
“A literature review surveys books, scholarly articles, and any other sources relevant to a particular issue, area of research, or theory, and by so doing, provides a description, summary, and critical evaluation of these works in relation to the research problem being investigated.
Literature reviews are designed to provide an overview of sources you have explored while researching a particular topic, and to demonstrate to your readers how your research fits within a larger field of study.”
Simply put, a literature review can be defined as a critical discussion of relevant pre-existing research around your research question and carving out a definitive place for your study in the existing body of knowledge. Literature reviews can be presented in multiple ways: a section of an article, the whole research paper itself, or a chapter of your thesis.
A literature review does function as a summary of sources, but it also allows you to analyze further, interpret, and examine the stated theories, methods, viewpoints, and, of course, the gaps in the existing content.
As an author, you can discuss and interpret the research question and its various aspects and debate your adopted methods to support the claim.
What is the purpose of a literature review?
A literature review is meant to help your readers understand the relevance of your research question and where it fits within the existing body of knowledge. As a researcher, you should use it to set the context, build your argument, and establish the need for your study.
What is the importance of a literature review?
The literature review is a critical part of research papers because it helps you:
- Gain an in-depth understanding of your research question and the surrounding area
- Convey that you have a thorough understanding of your research area and are up-to-date with the latest changes and advancements
- Establish how your research is connected or builds on the existing body of knowledge and how it could contribute to further research
- Elaborate on the validity and suitability of your theoretical framework and research methodology
- Identify and highlight gaps and shortcomings in the existing body of knowledge and how things need to change
- Convey to readers how your study is different or how it contributes to the research area
How long should a literature review be?
Ideally, the literature review should take up 15%-40% of the total length of your manuscript. So, if you have a 10,000-word research paper, the minimum word count could be 1500.
Your literature review format depends heavily on the kind of manuscript you are writing — an entire chapter in case of doctoral theses, a part of the introductory section in a research article, to a full-fledged review article that examines the previously published research on a topic.
Another determining factor is the type of research you are doing. The literature review section tends to be longer for secondary research projects than primary research projects.
What are the different types of literature reviews?
All literature reviews are not the same. There are a variety of possible approaches that you can take. It all depends on the type of research you are pursuing.
Here are the different types of literature reviews:
Argumentative review
It is called an argumentative review when you carefully present literature that only supports or counters a specific argument or premise to establish a viewpoint.
Integrative review
It is a type of literature review focused on building a comprehensive understanding of a topic by combining available theoretical frameworks and empirical evidence.
Methodological review
This approach delves into the ''how'' and the ''what" of the research question — you cannot look at the outcome in isolation; you should also review the methodology used.
Systematic review
This form consists of an overview of existing evidence pertinent to a clearly formulated research question, which uses pre-specified and standardized methods to identify and critically appraise relevant research and collect, report, and analyze data from the studies included in the review.
Meta-analysis review
Meta-analysis uses statistical methods to summarize the results of independent studies. By combining information from all relevant studies, meta-analysis can provide more precise estimates of the effects than those derived from the individual studies included within a review.
Historical review
Historical literature reviews focus on examining research throughout a period, often starting with the first time an issue, concept, theory, or phenomenon emerged in the literature, then tracing its evolution within the scholarship of a discipline. The purpose is to place research in a historical context to show familiarity with state-of-the-art developments and identify future research's likely directions.
Theoretical Review
This form aims to examine the corpus of theory accumulated regarding an issue, concept, theory, and phenomenon. The theoretical literature review helps to establish what theories exist, the relationships between them, the degree the existing approaches have been investigated, and to develop new hypotheses to be tested.
Scoping Review
The Scoping Review is often used at the beginning of an article, dissertation, or research proposal. It is conducted before the research to highlight gaps in the existing body of knowledge and explains why the project should be greenlit.
State-of-the-Art Review
The State-of-the-Art review is conducted periodically, focusing on the most recent research. It describes what is currently known, understood, or agreed upon regarding the research topic and highlights where there are still disagreements.
Can you use the first person in a literature review?
When writing literature reviews, you should avoid the usage of first-person pronouns. It means that instead of "I argue that" or "we argue that," the appropriate expression would be "this research paper argues that."
Do you need an abstract for a literature review?
Ideally, yes. It is always good to have a condensed summary that is self-contained and independent of the rest of your review. As for how to draft one, you can follow the same fundamental idea when preparing an abstract for a literature review. It should also include:
- The research topic and your motivation behind selecting it
- A one-sentence thesis statement
- An explanation of the kinds of literature featured in the review
- Summary of what you've learned
- Conclusions you drew from the literature you reviewed
- Potential implications and future scope for research
Here's an example of the abstract of a literature review
Is a literature review written in the past tense?
Yes, the literature review should ideally be written in the past tense. You should not use the present or future tense when writing one. The exceptions are when you have statements describing events that happened earlier than the literature you are reviewing or events that are currently occurring; then, you can use the past perfect or present perfect tenses.
How many sources for a literature review?
There are multiple approaches to deciding how many sources to include in a literature review section. The first approach would be to look level you are at as a researcher. For instance, a doctoral thesis might need 60+ sources. In contrast, you might only need to refer to 5-15 sources at the undergraduate level.
The second approach is based on the kind of literature review you are doing — whether it is merely a chapter of your paper or if it is a self-contained paper in itself. When it is just a chapter, sources should equal the total number of pages in your article's body. In the second scenario, you need at least three times as many sources as there are pages in your work.
Quick tips on how to write a literature review
To know how to write a literature review, you must clearly understand its impact and role in establishing your work as substantive research material.
You need to follow the below-mentioned steps, to write a literature review:
- Outline the purpose behind the literature review
- Search relevant literature
- Examine and assess the relevant resources
- Discover connections by drawing deep insights from the resources
- Structure planning to write a good literature review
1. Outline and identify the purpose of a literature review
As a first step on how to write a literature review, you must know what the research question or topic is and what shape you want your literature review to take. Ensure you understand the research topic inside out, or else seek clarifications. You must be able to the answer below questions before you start:
- How many sources do I need to include?
- What kind of sources should I analyze?
- How much should I critically evaluate each source?
- Should I summarize, synthesize or offer a critique of the sources?
- Do I need to include any background information or definitions?
Additionally, you should know that the narrower your research topic is, the swifter it will be for you to restrict the number of sources to be analyzed.
2. Search relevant literature
Dig deeper into search engines to discover what has already been published around your chosen topic. Make sure you thoroughly go through appropriate reference sources like books, reports, journal articles, government docs, and web-based resources.
You must prepare a list of keywords and their different variations. You can start your search from any library’s catalog, provided you are an active member of that institution. The exact keywords can be extended to widen your research over other databases and academic search engines like:
- Google Scholar
- Microsoft Academic
- Science.gov
Besides, it is not advisable to go through every resource word by word. Alternatively, what you can do is you can start by reading the abstract and then decide whether that source is relevant to your research or not.
Additionally, you must spend surplus time assessing the quality and relevance of resources. It would help if you tried preparing a list of citations to ensure that there lies no repetition of authors, publications, or articles in the literature review.
3. Examine and assess the sources
It is nearly impossible for you to go through every detail in the research article. So rather than trying to fetch every detail, you have to analyze and decide which research sources resemble closest and appear relevant to your chosen domain.
While analyzing the sources, you should look to find out answers to questions like:
- What question or problem has the author been describing and debating?
- What is the definition of critical aspects?
- How well the theories, approach, and methodology have been explained?
- Whether the research theory used some conventional or new innovative approach?
- How relevant are the key findings of the work?
- In what ways does it relate to other sources on the same topic?
- What challenges does this research paper pose to the existing theory
- What are the possible contributions or benefits it adds to the subject domain?
Be always mindful that you refer only to credible and authentic resources. It would be best if you always take references from different publications to validate your theory.
Always keep track of important information or data you can present in your literature review right from the beginning. It will help steer your path from any threats of plagiarism and also make it easier to curate an annotated bibliography or reference section.
4. Discover connections
At this stage, you must start deciding on the argument and structure of your literature review. To accomplish this, you must discover and identify the relations and connections between various resources while drafting your abstract.
A few aspects that you should be aware of while writing a literature review include:
- Rise to prominence: Theories and methods that have gained reputation and supporters over time.
- Constant scrutiny: Concepts or theories that repeatedly went under examination.
- Contradictions and conflicts: Theories, both the supporting and the contradictory ones, for the research topic.
- Knowledge gaps: What exactly does it fail to address, and how to bridge them with further research?
- Influential resources: Significant research projects available that have been upheld as milestones or perhaps, something that can modify the current trends
Once you join the dots between various past research works, it will be easier for you to draw a conclusion and identify your contribution to the existing knowledge base.
5. Structure planning to write a good literature review
There exist different ways towards planning and executing the structure of a literature review. The format of a literature review varies and depends upon the length of the research.
Like any other research paper, the literature review format must contain three sections: introduction, body, and conclusion. The goals and objectives of the research question determine what goes inside these three sections.
Nevertheless, a good literature review can be structured according to the chronological, thematic, methodological, or theoretical framework approach.
Literature review samples
1. Standalone
2. As a section of a research paper
How SciSpace Discover makes literature review a breeze?
SciSpace Discover is a one-stop solution to do an effective literature search and get barrier-free access to scientific knowledge. It is an excellent repository where you can find millions of only peer-reviewed articles and full-text PDF files. Here’s more on how you can use it:
Find the right information
Find what you want quickly and easily with comprehensive search filters that let you narrow down papers according to PDF availability, year of publishing, document type, and affiliated institution. Moreover, you can sort the results based on the publishing date, citation count, and relevance.
Assess credibility of papers quickly
When doing the literature review, it is critical to establish the quality of your sources. They form the foundation of your research. SciSpace Discover helps you assess the quality of a source by providing an overview of its references, citations, and performance metrics.
Get the complete picture in no time
SciSpace Discover’s personalized suggestion engine helps you stay on course and get the complete picture of the topic from one place. Every time you visit an article page, it provides you links to related papers. Besides that, it helps you understand what’s trending, who are the top authors, and who are the leading publishers on a topic.
Make referring sources super easy
To ensure you don't lose track of your sources, you must start noting down your references when doing the literature review. SciSpace Discover makes this step effortless. Click the 'cite' button on an article page, and you will receive preloaded citation text in multiple styles — all you've to do is copy-paste it into your manuscript.
Final tips on how to write a literature review
A massive chunk of time and effort is required to write a good literature review. But, if you go about it systematically, you'll be able to save a ton of time and build a solid foundation for your research.
We hope this guide has helped you answer several key questions you have about writing literature reviews.
Would you like to explore SciSpace Discover and kick off your literature search right away? You can get started here .
Frequently Asked Questions (FAQs)
1. how to start a literature review.
• What questions do you want to answer?
• What sources do you need to answer these questions?
• What information do these sources contain?
• How can you use this information to answer your questions?
2. What to include in a literature review?
• A brief background of the problem or issue
• What has previously been done to address the problem or issue
• A description of what you will do in your project
• How this study will contribute to research on the subject
3. Why literature review is important?
The literature review is an important part of any research project because it allows the writer to look at previous studies on a topic and determine existing gaps in the literature, as well as what has already been done. It will also help them to choose the most appropriate method for their own study.
4. How to cite a literature review in APA format?
To cite a literature review in APA style, you need to provide the author's name, the title of the article, and the year of publication. For example: Patel, A. B., & Stokes, G. S. (2012). The relationship between personality and intelligence: A meta-analysis of longitudinal research. Personality and Individual Differences, 53(1), 16-21
5. What are the components of a literature review?
• A brief introduction to the topic, including its background and context. The introduction should also include a rationale for why the study is being conducted and what it will accomplish.
• A description of the methodologies used in the study. This can include information about data collection methods, sample size, and statistical analyses.
• A presentation of the findings in an organized format that helps readers follow along with the author's conclusions.
6. What are common errors in writing literature review?
• Not spending enough time to critically evaluate the relevance of resources, observations and conclusions.
• Totally relying on secondary data while ignoring primary data.
• Letting your personal bias seep into your interpretation of existing literature.
• No detailed explanation of the procedure to discover and identify an appropriate literature review.
7. What are the 5 C's of writing literature review?
• Cite - the sources you utilized and referenced in your research.
• Compare - existing arguments, hypotheses, methodologies, and conclusions found in the knowledge base.
• Contrast - the arguments, topics, methodologies, approaches, and disputes that may be found in the literature.
• Critique - the literature and describe the ideas and opinions you find more convincing and why.
• Connect - the various studies you reviewed in your research.
8. How many sources should a literature review have?
When it is just a chapter, sources should equal the total number of pages in your article's body. if it is a self-contained paper in itself, you need at least three times as many sources as there are pages in your work.
9. Can literature review have diagrams?
• To represent an abstract idea or concept
• To explain the steps of a process or procedure
• To help readers understand the relationships between different concepts
10. How old should sources be in a literature review?
Sources for a literature review should be as current as possible or not older than ten years. The only exception to this rule is if you are reviewing a historical topic and need to use older sources.
11. What are the types of literature review?
• Argumentative review
• Integrative review
• Methodological review
• Systematic review
• Meta-analysis review
• Historical review
• Theoretical review
• Scoping review
• State-of-the-Art review
12. Is a literature review mandatory?
Yes. Literature review is a mandatory part of any research project. It is a critical step in the process that allows you to establish the scope of your research, and provide a background for the rest of your work.
But before you go,
- Six Online Tools for Easy Literature Review
- Evaluating literature review: systematic vs. scoping reviews
- Systematic Approaches to a Successful Literature Review
- Writing Integrative Literature Reviews: Guidelines and Examples
You might also like

Consensus GPT vs. SciSpace GPT: Choose the Best GPT for Research

Literature Review and Theoretical Framework: Understanding the Differences

Types of Essays in Academic Writing - Quick Guide (2024)
- Undergraduate courses
- Postgraduate courses
- Foundation courses
- Apprenticeships
- Part-time and short courses
- Apply undergraduate
- Apply postgraduate
Search for a course
Search by course name, subject, and more
- Undergraduate
- Postgraduate
- (suspended) - Available in Clearing Not available in Clearing location-sign UCAS
Fees and funding
- Tuition fees
- Scholarships
- Funding your studies
- Student finance
- Cost of living support
Why study at Kent
Student life.
- Careers and employability
- Student support and wellbeing
- Our locations
- Placements and internships
- Year abroad
- Student stories
- Schools and colleges
- International
- International students
- Your country
- Applicant FAQs
- International scholarships
- University of Kent International College
- Campus Tours
- Applicant Events
- Postgraduate events
- Maps and directions
- Research strengths
- Research centres
- Research impact
Research institutes
- Durrell Institute of Conservation and Ecology
- Institute of Cyber Security for Society
- Institute of Cultural and Creative Industries
- Institute of Health, Social Care and Wellbeing
Research students
- Graduate and Researcher College
- Research degrees
- Find a supervisor
- How to apply
Popular searches
- Visits and Open Days
- Jobs and vacancies
- Accommodation
- Student guide
- Library and IT
- Partner with us
- Student Guide
- Student Help
- Health & wellbeing
- Student voice
- Living at Kent
- Careers & volunteering
- Diversity at Kent
- Finance & funding
- Life after graduation
Literature reviews
Writing a literature review.
The following guide has been created for you by the Student Learning Advisory Service . For more detailed guidance and to speak to one of our advisers, please book an appointment or join one of our workshops . Alternatively, have a look at our SkillBuilder skills videos.
Preparing a literature review involves:
- Searching for reliable, accurate and up-to-date material on a topic or subject
- Reading and summarising the key points from this literature
- Synthesising these key ideas, theories and concepts into a summary of what is known
- Discussing and evaluating these ideas, theories and concepts
- Identifying particular areas of debate or controversy
- Preparing the ground for the application of these ideas to new research
Finding and choosing material
Ensure you are clear on what you are looking for. ask yourself:.
- What is the specific question, topic or focus of my assignment?
- What kind of material do I need (e.g. theory, policy, empirical data)?
- What type of literature is available (e.g. journals, books, government documents)?
What kind of literature is particularly authoritative in this academic discipline (e.g. psychology, sociology, pharmacy)?
How much do you need?
This will depend on the length of the dissertation, the nature of the subject, and the level of study (undergraduate, Masters, PhD). As a very rough rule of thumb – you may choose 8-10 significant pieces (books and/or articles) for an 8,000 word dissertation, up to 20 major pieces of work for 12-15,000 words, and so on. Bear in mind that if your dissertation is based mainly around an interaction with existing scholarship you will need a longer literature review than if it is there as a prelude to new empirical research. Use your judgement or ask your supervisor for guidance.
Where to find suitable material
Your literature review should include a balance between substantial academic books, journal articles and other scholarly publications. All these sources should be as up-to-date as possible, with the exception of ‘classic texts’ such as major works written by leading scholars setting out formative ideas and theories central to your subject. There are several ways to locate suitable material:
Module bibliography: for undergraduate dissertations, look first at the bibliography provided with the module documentation. Choose one or two likely looking books or articles and then scan through the bibliographies provided by these authors. Skim read some of this material looking for clues: can you use these leads to identify key theories and authors or track down other appropriate material?
Library catalogue search engine: enter a few key words to capture a range of items, but avoid over-generalisations; if you type in something as broad as ‘social theory’ you are likely to get several thousand results. Be more specific: for example, ‘Heidegger, existentialism’. Ideally, you should narrow the field to obtain just a few dozen results. Skim through these quickly to identity texts which are most likely to contribute to your study.
Library bookshelves: browse the library shelves in the relevant subject area and examine the books that catch your eye. Check the contents and index pages, or skim through the introductions (or abstracts, in the case of journal articles) to see if they contain relevant material, and replace them if not. Don’t be afraid to ask one of the subject librarians for further help. Your supervisor may also be able to point you in the direction of some of the important literature , but remember this is your literature search, not theirs.
Online: for recent journal articles you will almost certainly need to use one of the online search engines. These can be found on the ‘Indexing Services’ button on the Templeman Library website. Kent students based at Medway still need to use the Templeman pages to access online journals, although you can get to these pages through the Drill Hall Library catalogue. Take a look as well at the Subject Guides on both the Templeman and DHL websites.
Check that you have made the right selection by asking:
- Has my search been wide enough to ensure that I have identified all the relevant material, but narrow enough to exclude irrelevant material?
- Is there a good enough sample of literature for the level (PhD, Masters, undergraduate) of my dissertation or thesis?
- Have I considered as many alternative points of view as possible?
- Will the reader find my literature review relevant and useful?
Assessing the literature
Read the material you have chosen carefully, considering the following:
- The key point discussed by the author: is this clearly defined
- What evidence has the author produced to support this central idea?
- How convincing are the reasons given for the author’s point of view?
- Could the evidence be interpreted in other ways?
- What is the author's research method (e.g. qualitative, quantitative, experimental, etc.)?
- What is the author's theoretical framework (e.g. psychological, developmental, feminist)?
- What is the relationship assumed by the author between theory and practice?
- Has the author critically evaluated the other literature in the field?
- Does the author include literature opposing their point of view?
- Is the research data based on a reliable method and accurate information?
- Can you ‘deconstruct’ the argument – identify the gaps or jumps in the logic?
- What are the strengths and limitations of this study?
- What does this book or article contribute to the field or topic?
- What does this book or article contribute to my own topic or thesis?
As you note down the key content of each book or journal article (together with the reference details of each source) record your responses to these questions. You will then be able to summarise each piece of material from two perspectives:
Content: a brief description of the content of the book or article. Remember, an author will often make just one key point; so, what is the point they are making, and how does it relate to your own research project or assignment?
Critical analysis: an assessment of the relative strengths and weaknesses of the evidence used, and the arguments presented. Has anything conveniently been left out or skated over? Is there a counter-argument, and has the author dealt with this adequately? Can the evidence presented be interpreted another way? Does the author demonstrate any obvious bias which could affect their reliability? Overall, based on the above analysis of the author’s work, how do you evaluate its contribution to the scholarly understanding and knowledge surrounding the topic?
Structuring the literature review
In a PhD thesis, the literature review typically comprises one chapter (perhaps 8-10,000 words), for a Masters dissertation it may be around 2-3,000 words, and for an undergraduate dissertation it may be no more than 2,000 words. In each case the word count can vary depending on a range of factors and it is always best, if in doubt, to ask your supervisor.
The overall structure of the section or chapter should be like any other: it should have a beginning, middle and end. You will need to guide the reader through the literature review, outlining the strategy you have adopted for selecting the books or articles, presenting the topic theme for the review, then using most of the word limit to analyse the chosen books or articles thoroughly before pulling everything together briefly in the conclusion.
Some people prefer a less linear approach. Instead of simply working through a list of 8-20 items on your book review list, you might want to try a thematic approach, grouping key ideas, facts, concepts or approaches together and then bouncing the ideas off each other. This is a slightly more creative (and interesting) way of producing the review, but a little more risky as it is harder to establish coherence and logical sequencing.
Whichever approach you adopt, make sure everything flows smoothly – that one idea or book leads neatly to the next. Take your reader effortlessly through a sequence of thought that is clear, accurate, precise and interesting.
Writing up your literature review
As with essays generally, only attempt to write up the literature review when you have completed all the reading and note-taking, and carefully planned its content and structure. Find an appropriate way of introducing the review, then guide the reader through the material clearly and directly, bearing in mind the following:
- Be selective in the number of points you draw out from each piece of literature; remember that one of your objectives is to demonstrate that you can use your judgement to identify what is central and what is secondary.
- Summarise and synthesise – use your own words to sum up what you think is important or controversial about the book or article.
- Never claim more than the evidence will support. Too many dissertations and theses are let down by sweeping generalisations. Be tentative and careful in the way you interpret the evidence.
- Keep your own voice – you are entitled to your own point of view provided it is based on evidence and clear argument.
- At the same time, aim to project an objective and tentative tone by using the 3rd person, (for example, ‘this tends to suggest’, ‘it could be argued’ and so on).
- Even with a literature review you should avoid using too many, or overlong, quotes. Summarise material in your own words as much as possible. Save the quotes for ‘punch-lines’ to drive a particular point home.
- Revise, revise, revise: refine and edit the draft as much as you can. Check for fluency, structure, evidence, criticality and referencing, and don’t forget the basics of good grammar, punctuation and spelling.
- Link to facebook
- Link to linkedin
- Link to twitter
- Link to youtube
- Writing Tips
How Long Should a Literature Review Be?

4-minute read
- 7th October 2023
If you’re writing a research paper or dissertation , then you know how important it is to include a thorough, comprehensive literature review. But exactly how long should your literature review be in relation to the rest of your work? While there’s no one-size-fits-all answer to that question, there are some factors that will help determine the length of your review. In this post, we’ll discuss what information to include in your literature review and how long it should be.
Keep reading to learn more.
What Is a Literature Review?
A literature review is a critical summary and evaluation of the current resources (e.g., books and journal articles) on a specific topic or research question. It is a crucial part of academic writing, such as dissertations, in all categories and fields. Essentially, literature reviews help contextualize your investigations and show how your work is building on existing research.
No matter how long your literature review is, it should generally:
● Establish context for your research (i.e., provide relevant background information so your reader understands the historical significance of your study ).
● Identify gaps in the existing literature (such as unaddressed questions or aspects of your topic).
● Highlight significant concepts related to your topic.
● Cite relevant studies.
● Support your argument.
It’s also essential that a literature review critically analyze the sources cited in your study, considering factors such as sample size, research design, and potential biases. Be sure to structure your literature review using the same referencing style as the rest of your research paper (e.g., APA , Chicago , MLA ).
Find this useful?
Subscribe to our newsletter and get writing tips from our editors straight to your inbox.
The length of your literature review depends on several factors, including the scope and purpose of your research. In general, the length of the review should be proportionate to your overall paper. For example, if you’re writing a fifty-thousand-word dissertation, then your literature review will likely be an entire chapter comprising about 20 pages. If it’s for a 15-page research paper, your literature review may only be a few pages.
Here are several factors that could affect the length of your literature review:
● Institutional guidelines : Always check the guidelines provided by your institution or journal (such as an APA journal ). There may be a specific length or word count required for publication.
● Scope : If your research topic is narrow and focused, your literature review may be shorter. Conversely, if your topic is broad and encompasses a large body of literature, your review may need to be longer.
● Field of study : Different academic fields may have different expectations regarding the length of literature reviews. For example, literature reviews in the humanities might be longer than those in the natural sciences.
Also, consider your audience. If your literature review is for a general audience or a class assignment, it can probably be shorter and less specialized. However, if it’s for an academic audience in your field of study, you may need to be more thorough and provide an extensive review of the existing literature.
Most literature reviews follow the same basic structure of an introduction, body, and conclusion. Most of the time, they are part of a larger work, so the introduction and conclusion paragraphs will be relatively brief.
However, if the review is a standalone piece, then your introduction and conclusion will be longer since you will need to discuss your research objectives, methods, and findings as well as analyze the literature used in your study.
To ensure your literature review makes an impression, have it professionally proofread by our expert literature review editing services . Submit your free sample of 500 words or less to get started today!
Share this article:
Post A New Comment
Got content that needs a quick turnaround? Let us polish your work. Explore our editorial business services.
9-minute read
How to Use Infographics to Boost Your Presentation
Is your content getting noticed? Capturing and maintaining an audience’s attention is a challenge when...
8-minute read
Why Interactive PDFs Are Better for Engagement
Are you looking to enhance engagement and captivate your audience through your professional documents? Interactive...
7-minute read
Seven Key Strategies for Voice Search Optimization
Voice search optimization is rapidly shaping the digital landscape, requiring content professionals to adapt their...
Five Creative Ways to Showcase Your Digital Portfolio
Are you a creative freelancer looking to make a lasting impression on potential clients or...
How to Ace Slack Messaging for Contractors and Freelancers
Effective professional communication is an important skill for contractors and freelancers navigating remote work environments....
3-minute read
How to Insert a Text Box in a Google Doc
Google Docs is a powerful collaborative tool, and mastering its features can significantly enhance your...

Make sure your writing is the best it can be with our expert English proofreading and editing.
Conducting a Literature Review
- Literature Review
- Developing a Topic
- Planning Your Literature Review
- Developing a Search Strategy
- Managing Citations
- Critical Appraisal Tools
- Writing a Literature Review
Before You Begin to Write.....
Do you have enough information? If you are not sure,
Ask yourself these questions:
- Has my search been wide enough to insure I've found all the relevant material?
- Has it been narrow enough to exclude irrelevant material?
- Is the number of sources I've used appropriate for the length of my paper?
You may have enough information for your literature review when:
- You've used multiple databases and other resources (web portals, repositories, etc.) to get a variety of perspectives on the research topic.
- The same citations are showing up in a variety of databases.
- Your advisor and other trusted experts say you have enough!
You have to stop somewhere and get on with the writing process!
Writing Tips
A literature review is not a list describing or summarizing one piece of literature after another. It’s usually a bad sign to see every paragraph beginning with the name of a researcher. Instead, organize the literature review into sections that present themes or identify trends, including relevant theory. You are not trying to list all the material published, but to synthesize and evaluate it according to the guiding concept of your thesis or research question
If you are writing an annotated bibliography , you may need to summarize each item briefly, but should still follow through themes and concepts and do some critical assessment of material. Use an overall introduction and conclusion to state the scope of your coverage and to formulate the question, problem, or concept your chosen material illuminates. Usually you will have the option of grouping items into sections—this helps you indicate comparisons and relationships. You may be able to write a paragraph or so to introduce the focus of each section
Layout of Writing a Literature Review
Generally, the purpose of a review is to analyze critically a segment of a published body of knowledge through summary, classification, and comparison of prior research studies, reviews of literature, and theoretical articles.
Writing the introduction:
In the introduction, you should:
- Define or identify the general topic, issue, or area of concern, thus providing an appropriate context for reviewing the literature.
- Point out overall trends in what has been published about the topic; or conflicts in theory, methodology, evidence, and conclusions; or gaps in research and scholarship; or a single problem or new perspective of immediate interest.
- Establish the writer’s reason (point of view) for reviewing the literature; explain the criteria to be used in analyzing and comparing literature and the organization of the review (sequence); and, when necessary, state why certain literature is or is not included (scope).
Writing the body:
In the body, you should:
- Group research studies and other types of literature (reviews, theoretical articles, case studies, etc.) according to common denominators such as qualitative versus quantitative approaches, conclusions of authors, specific purpose or objective, chronology, etc.
- Summarize individual studies or articles with as much or as little detail as each merits according to its comparative importance in the literature, remembering that space (length) denotes significance.
- Provide the reader with strong “umbrella” sentences at beginnings of paragraphs, “signposts” throughout, and brief “so what” summary sentences at intermediate points in the review to aid in understanding comparisons and analyses.
WRITING TIP: As you are writing the literature review you will mention the author names and the publication years in your text, but you will still need to compile comprehensive list citations for each entry at the end of your review. Follow APA, MLA, or Chicago style guidelines , as your course requires.
Writing the conclusion:
In the conclusion, you should:
- Summarize major contributions of significant studies and articles to the body of knowledge under review, maintaining the focus established in the introduction.
- Evaluate the current “state of the art” for the body of knowledge reviewed, pointing out major methodological flaws or gaps in research, inconsistencies in theory and findings, and areas or issues pertinent to future study.
- Conclude by providing some insight into the relationship between the central topic of the literature review and a larger area of study such as a discipline, a scientific endeavor, or a profession.
- The Interprofessional Health Sciences Library
- 123 Metro Boulevard
- Nutley, NJ 07110
- [email protected]
- Student Services
- Parents and Families
- Career Center
- Web Accessibility
- Visiting Campus
- Public Safety
- Disability Support Services
- Campus Security Report
- Report a Problem
- Login to LibApps
How to Write a Literature Review
- What Is a Literature Review
- What Is the Literature
Writing the Review
Why Are You Writing This?
There are two primary points to remember as you are writing your literature review:
- Stand-alone review: provide an overview and analysis of the current state of research on a topic or question
- Research proposal: explicate the current issues and questions concerning a topic to demonstrate how your proposed research will contribute to the field
- Research report: provide the context to which your work is a contribution.
- Write as you read, and revise as you read more. Rather than wait until you have read everything you are planning to review, start writing as soon as you start reading. You will need to reorganize and revise it all later, but writing a summary of an article when you read it helps you to think more carefully about the article. Having drafts and annotations to work with will also make writing the full review easier since you will not have to rely completely on your memory or have to keep thumbing back through all the articles. Your draft does not need to be in finished, or even presentable, form. The first draft is for you, so you can tell yourself what you are thinking. Later you can rewrite it for others to tell them what you think.
General Steps for Writing a Literature Review
Here is a general outline of steps to write a thematically organized literature review. Remember, though, that there are many ways to approach a literature review, depending on its purpose.
- Stage one: annotated bibliography. As you read articles, books, etc, on your topic, write a brief critical synopsis of each. After going through your reading list, you will have an abstract or annotation of each source you read. Later annotations are likely to include more references to other works since you will have your previous readings to compare, but at this point the important goal is to get accurate critical summaries of each individual work.
- Stage two: thematic organization. Find common themes in the works you read, and organize the works into categories. Typically, each work you include in your review can fit into one category or sub-theme of your main theme, but sometimes a work can fit in more than one. (If each work you read can fit into all the categories you list, you probably need to rethink your organization.) Write some brief paragraphs outlining your categories, how in general the works in each category relate to each other, and how the categories relate to each other and to your overall theme.
- Stage three: more reading. Based on the knowledge you have gained in your reading, you should have a better understanding of the topic and of the literature related to it. Perhaps you have discovered specific researchers who are important to the field, or research methodologies you were not aware of. Look for more literature by those authors, on those methodologies, etc. Also, you may be able to set aside some less relevant areas or articles which you pursued initially. Integrate the new readings into your literature review draft. Reorganize themes and read more as appropriate.
- Stage four: write individual sections. For each thematic section, use your draft annotations (it is a good idea to reread the articles and revise annotations, especially the ones you read initially) to write a section which discusses the articles relevant to that theme. Focus your writing on the theme of that section, showing how the articles relate to each other and to the theme, rather than focusing your writing on each individual article. Use the articles as evidence to support your critique of the theme rather than using the theme as an angle to discuss each article individually.
- Stage five: integrate sections. Now that you have the thematic sections, tie them together with an introduction, conclusion, and some additions and revisions in the sections to show how they relate to each other and to your overall theme.
Specific Points to Include
More specifically, here are some points to address when writing about specific works you are reviewing. In dealing with a paper or an argument or theory, you need to assess it (clearly understand and state the claim) and analyze it (evaluate its reliability, usefulness, validity). Look for the following points as you assess and analyze papers, arguments, etc. You do not need to state them all explicitly, but keep them in mind as you write your review:
- Be specific and be succinct. Briefly state specific findings listed in an article, specific methodologies used in a study, or other important points. Literature reviews are not the place for long quotes or in-depth analysis of each point.
- Be selective. You are trying to boil down a lot of information into a small space. Mention just the most important points (i.e. those most relevant to the review's focus) in each work you review.
- Is it a current article? How old is it? Have its claims, evidence, or arguments been superceded by more recent work? If it is not current, is it important for historical background?
- What specific claims are made? Are they stated clearly?
- What evidence, and what type (experimental, statistical, anecdotal, etc) is offered? Is the evidence relevant? sufficient?
- What arguments are given? What assumptions are made, and are they warranted?
- What is the source of the evidence or other information? The author's own experiments, surveys, etc? Historical records? Government documents? How reliable are the sources?
- Does the author take into account contrary or conflicting evidence and arguments? How does the author address disagreements with other researchers?
- What specific conclusions are drawn? Are they warranted by the evidence?
- How does this article, argument, theory, etc, relate to other work?
These, however, are just the points that should be addressed when writing about a specific work. It is not an outline of how to organize your writing. Your overall theme and categories within that theme should organize your writing, and the above points should be integrated into that organization. That is, rather than write something like:
Smith (2019) claims that blah, and provides evidence x to support it, and says it is probably because of blip. But Smith seems to have neglected factor b. Jones (2021) showed that blah by doing y, which, Jones claims, means it is likely because of blot. But that methodology does not exclude other possibilities. Johnson (2022) hypothesizes blah might be because of some other cause.
list the themes and then say how each article relates to that theme. For example:
Researchers agree that blah (Smith 2019, Jones 2021, Johnson 2022), but they do not agree on why. Smith claims it is probably due to blip, but Jones, by doing y, tries to show it is likely because of blot. Jones' methodology, however, does not exclude other possibilities. Johnson hypothesizes ...
- << Previous: What Is the Literature
- Last Updated: Jan 11, 2024 9:48 AM
- URL: https://libguides.wesleyan.edu/litreview

- Writing well
How to write a literature review
- Starting well
- How to write an annotated bibliography
- How to write a case study response
- How to write a critique
- How to write an empirical article
- How to write an essay
- How to write a reflective task
- How to write a report
- Finishing well
Structure of a literature review
Determine your purpose.
Work out what you need to address in the literature review. What are you being asked to do in your literature review? What are you searching the literature to discover? Check your assignment question and your criteria sheet to know what to focus on.
Do an extensive search of the literature
Find out what has been written on the topic.
What kind of literature?
Select appropriate source material: Use a variety of academic or scholarly sources that are relevant, current and authoritative. An extensive review of relevant material will include — books, journal articles, reports, government documents, conference proceedings and web resources. The Library would be the best place to search for your sources.
How many resources?
The number of sources that you will be required to review will depend on what the literature review is for and how advanced you are in your studies. It could be from five sources at first year undergraduate level to more than fifty for a thesis. Your lecturer will advise you on these details.
Note the bibliographical details of your sources
Keep a note of the publication title, date, authors’ names, page numbers and publishers. These details will save you time later.
Read the literature
- Critically read each source, look for the arguments presented rather than for facts.
- Take notes as you read and start to organise your review around themes and ideas.
- Consider using a table, matrix or concept map to identify how the different sources relate to each other.
Analyse the literature you have found
In order for your writing to reflect strong critical analysis, you need to evaluate the sources. For each source you are reviewing ask yourself these questions:
- What are the key terms and concepts?
- How relevant is this article to my specific topic?
- What are the major relationships, trends and patterns?
- How has the author structured the arguments?
- How authoritative and credible is this source?
- What are the differences and similarities between the sources?
- Are there any gaps in the literature that require further study?
Write the review
- Start by writing your thesis statement. This is an important introductory sentence that will tell your reader what the topic is and the overall perspective or argument you will be presenting.
- Like essays, a literature review must have an introduction, a body and a conclusion.
Introduction
Your introduction should give an outline of:
- why you are writing a review, and why the topic is important
- the scope of the review — what aspects of the topic will be discussed
- the criteria used for your literature selection (e.g. type of sources used, date range)
- the organisational pattern of the review.
Body paragraphs
Each body paragraph should deal with a different theme that is relevant to your topic. You will need to synthesise several of your reviewed readings into each paragraph, so that there is a clear connection between the various sources. You will need to critically analyse each source for how they contribute to the themes you are researching.
The body could include paragraphs on:
- historical background
- methodologies
- previous studies on the topic
- mainstream versus alternative viewpoints
- principal questions being asked
- general conclusions that are being drawn.
Your conclusion should give a summary of:
- the main agreements and disagreements in the literature
- any gaps or areas for further research
- your overall perspective on the topic.
- outlined the purpose and scope?
- identified appropriate and credible (academic/scholarly) literature?
- recorded the bibliographical details of the sources?
- analysed and critiqued your readings?
- identified gaps in the literature and research?
- explored methodologies / theories / hypotheses / models?
- discussed the varying viewpoints?
- written an introduction, body and conclusion?
- checked punctuation and spelling?
Further information
- HiQ: Managing weekly readings
- HiQ: Notetaking
- HiQ: Structuring your assignment
- RMIT University: Literature review - Overview
Global links and information
- Referencing and using sources
- Background and development
- Changes to QUT cite|write
- Need more help?
- Current students
- Current staff
- TEQSA Provider ID: PRV12079 (Australian University)
- CRICOS No. 00213J
- ABN 83 791 724 622
- Last modified: 28-May-2024
- Accessibility
- Right to Information
- Feedback and suggestions

Acknowledgement of Traditional Owners
QUT acknowledges the Traditional Owners of the lands where QUT now stands.

Conducting a Literature Review
- Getting Started
- Define your Research Question
- Finding Sources
- Evaluating Sources
- Organizing the Review
- Cite and Manage your Sources
Introduction
Once you have your literature review planned out, you are ready to begin writing! Good organization and a clear focus are key to writing a successful academic paper of any kind, which is why the previous steps in this guide are so important; the more thorough you are with each of the preceding elements of writing the literature review, the easier this final step will be.
A literature review is organized into an introduction, multiple body paragraphs, and a conclusion. This format should be familiar to you, as it is the general outline of most academic essays; what is new and exciting about this literature review is the information you've gathered in your research and synthesized in your organization and outlining process.
Remember, if you ever need help with writing an essay of any kind, the ACPHS Writing Center is here to help! You can book an appointment with one of the peer tutors or reach out by email. The Library is also here to provide assistance with your assignments, particularly finding or citing resources.
Additional Resources
- Write a Literature Review by the University of Guelph McLaughlin Library
The ACPHS Writing Center
The Center for Student Success
Writing Center
Laura Rogers, D.A. Director of the Writing Center Tel: (518) 694-7261 [email protected]
URL: https://www.acphs.edu/campuses/albany-campus/writing-center
Make an appointment
Intro Paragraph & Thesis
Introductory paragraphs can be the most challenging part of writing a paper. Instead of laying out the evidence (or in the case of a literature review, analyzing your resources), you must first provide background information and context for the topic, discuss the body of literature in general as well as the scope of your review, and give a brief outline of how you will organize the review.
It is generally a good idea to open an introduction with a hook, or an interesting first sentence. This could be a statistic or fact about the topic that you find relevant, a rhetorical question that will be answered in the rest of the introduction, or even an appropriate anecdote. The point of a strong hook is to catch the reader's attention; for a literature review, it can help get the reader invested in the research around your topic, as well as your analysis of it.
Some authors prefer to write their introductory paragraph after completing the body of the essay, finding it easier to summarize what will be shared with the reader after it has already been written. There is no right or wrong order for crafting your paper, so if this method appeals to you then you should make use of it. However, with appropriately detailed planning it can be simple to write out an introduction prior to the body. Using an outline (using the methods provided by Walden University, for example) can make writing the introduction and the entire essay much simpler.
Your literature review's introduction should contain four major elements:
- Establishing the topic, including providing background information and any necessary definitions to make sure your reader has all the context necessary to understand the rest of the literature review
- The trends or themes of the research that you noticed while compiling your sources, including any that you will use to organize your literature
- The purpose, criteria, and scope of the literature review: how will the literature be organized? What is your reason for examining this topic? What will you be analyzing about the sources (comparing/contrasting research methodology, conclusions, etc.)? Is there any literature you decided not to include -- if so, what disqualified it from the review?
- Introduce your thesis statement by drawing on the previous 3 components of the introduction to state what you discovered about the literature on this topic. Specifically, the thesis should answer where the current literature's strengths and weaknesses lie, and where additional research may be needed
The purpose of the introduction is to make sure that your reader has all the information they need to understand and appreciate your literature review, and to provide them a general blueprint of the analysis and arguments you will be making.
- 5 Questions to Strengthen Your Thesis Statement by the University of Guelph Digital Learning Commons

Body Paragraphs
With the introduction out of the way -- or perhaps even before you've written the introduction -- it's time to examine the literature you've gathered. We established how to organize the literature in the previous section of this guide, and that organization will serve as the framework for the body paragraphs. For example, if you organized your literature into themes, then each theme would serve as its own paragraph, in which you'd compare and contrast the sources within each theme; if you organized it by methodology or historical era, each of those would be a body paragraph.
As you write your literature analyses, keep the following recommendations in mind, provided by Shona McCombes at Scribbr :
Summarize and synthesize: give an overview of the main points of each source and combine them into a coherent whole Analyze and interpret: don’t just paraphrase other researchers—add your own interpretations where possible, discussing the significance of findings in relation to the literature as a whole Critically evaluate: mention the strengths and weaknesses of your sources Write in well-structured paragraphs: use transition words and topic sentences to draw connections, comparisons and contrasts
Your summary of each source can be as detailed as is appropriate, based on how important the source is to the overall literature or how much analysis you have to perform on it. In general, the more significant a source is to your review, the more time should be devoted to summarizing and analyzing it.
While looking at individual sources, remember to keep connecting them back to the theme of the body paragraph and the overall thesis; explaining their relevance in a particular section of literature helps the reader follow along and better understand your overall arguments.
Other useful tips to keep in mind when writing your body paragraphs, provided by the Writing Center of the University of North Carolina at Chapel Hill :
- Use evidence to support your claims
- Be selective, and focus on the most important points for each piece of literature rather than trying to describe everything
- Use quotes when appropriate, but know that literature reviews do not frequently require direct quotations
- Paraphrase accurately
- Literature Reviews by the University of North Carolina at Chapel Hill's Writing Center
Conclusion & Reviewing Your Paper
Concluding Paragraph
A conclusion is used to provide further reinforcement of the arguments presented throughout the paper. In general, this consists of briefly summarizing the body paragraphs and reasserting their connection to your thesis. This is also good practice for a literature review; in addition, your conclusion should again summarize the broad trends of the research on your topic, as well as any opportunities for additional or more thorough research that you've found.
Below are some helpful recommendations for writing conclusions, compiled from advice explained in more detail in the links below:
- Address the broader implications of the existing research, and why it is important to close the gaps you evaluated during your literature review
- Include a quotation or fact that effectively illustrates your thesis in a provocative or interesting way
- Use simple, clear language without jargon
- Reestablish your thesis and its connection with the literature reviewed
Your goal with the concluding paragraph of your literature review should be to leave the reader with a firm understanding of the existing literature on your topic, where additional research may be necessary, and why it matters.
Revising Your Literature Review
Revision is a process that goes beyond simply correcting spelling and grammar mistakes -- though proofreading is an important part of the writing process as well. The purpose of revising your literature review before submission is to look at it the way your reader will and pick up on any potential leaps of logic, unclear explanations, or shoddy evidence. The revision process should not begin immediately after finishing the paper; whenever possible, wait a few hours or days before looking at your draft, so that you can approach it with fresh eyes.
When revising, focus on major issues with the paper such as organization, clarity, and thoroughness. Trying to both revise your writing and proofread it for small spelling or grammar issues may distract you from more important areas that could be improved. Ask yourself if your thesis is well-defended by the body paragraphs, and if you still agree with the conclusions you stated in the introduction. If more or better arguments are needed, find places in the body paragraphs to add evidence or make clearer connections to your thesis. Focus on the flow of the review; does each body paragraph move naturally into the next one? Do your paragraphs need to be reordered or restructured?
After major revisions are done, it is time to proofread for spelling, grammar, and general writing errors. Try reading the paper out loud and seeing where your word choice could be strengthened or a run-on sentence could be amended.
It can sometimes be difficult to revise an essay on your own, so consider booking an appointment with the ACPHS Writing Center to go over your writing with a tutor. Friends, classmates, or your professor can also be useful sources of feedback, and if possible try to get as many different readers to look over your writing and provide insight.
- Revising Drafts by the University of North Carolina at Chapel Hill Writing Center
- << Previous: Organizing the Review
- Next: Cite and Manage your Sources >>
- Last Updated: Apr 12, 2024 10:37 AM
- URL: https://libraryservices.acphs.edu/lit_review
Harvey Cushing/John Hay Whitney Medical Library
- Collections
- Research Help
YSN Doctoral Programs: Steps in Conducting a Literature Review
- Biomedical Databases
- Global (Public Health) Databases
- Soc. Sci., History, and Law Databases
- Grey Literature
- Trials Registers
- Data and Statistics
- Public Policy
- Google Tips
- Recommended Books
- Steps in Conducting a Literature Review
What is a literature review?
A literature review is an integrated analysis -- not just a summary-- of scholarly writings and other relevant evidence related directly to your research question. That is, it represents a synthesis of the evidence that provides background information on your topic and shows a association between the evidence and your research question.
A literature review may be a stand alone work or the introduction to a larger research paper, depending on the assignment. Rely heavily on the guidelines your instructor has given you.
Why is it important?
A literature review is important because it:
- Explains the background of research on a topic.
- Demonstrates why a topic is significant to a subject area.
- Discovers relationships between research studies/ideas.
- Identifies major themes, concepts, and researchers on a topic.
- Identifies critical gaps and points of disagreement.
- Discusses further research questions that logically come out of the previous studies.
APA7 Style resources
APA Style Blog - for those harder to find answers
1. Choose a topic. Define your research question.
Your literature review should be guided by your central research question. The literature represents background and research developments related to a specific research question, interpreted and analyzed by you in a synthesized way.
- Make sure your research question is not too broad or too narrow. Is it manageable?
- Begin writing down terms that are related to your question. These will be useful for searches later.
- If you have the opportunity, discuss your topic with your professor and your class mates.
2. Decide on the scope of your review
How many studies do you need to look at? How comprehensive should it be? How many years should it cover?
- This may depend on your assignment. How many sources does the assignment require?
3. Select the databases you will use to conduct your searches.
Make a list of the databases you will search.
Where to find databases:
- use the tabs on this guide
- Find other databases in the Nursing Information Resources web page
- More on the Medical Library web page
- ... and more on the Yale University Library web page
4. Conduct your searches to find the evidence. Keep track of your searches.
- Use the key words in your question, as well as synonyms for those words, as terms in your search. Use the database tutorials for help.
- Save the searches in the databases. This saves time when you want to redo, or modify, the searches. It is also helpful to use as a guide is the searches are not finding any useful results.
- Review the abstracts of research studies carefully. This will save you time.
- Use the bibliographies and references of research studies you find to locate others.
- Check with your professor, or a subject expert in the field, if you are missing any key works in the field.
- Ask your librarian for help at any time.
- Use a citation manager, such as EndNote as the repository for your citations. See the EndNote tutorials for help.
Review the literature
Some questions to help you analyze the research:
- What was the research question of the study you are reviewing? What were the authors trying to discover?
- Was the research funded by a source that could influence the findings?
- What were the research methodologies? Analyze its literature review, the samples and variables used, the results, and the conclusions.
- Does the research seem to be complete? Could it have been conducted more soundly? What further questions does it raise?
- If there are conflicting studies, why do you think that is?
- How are the authors viewed in the field? Has this study been cited? If so, how has it been analyzed?
Tips:
- Review the abstracts carefully.
- Keep careful notes so that you may track your thought processes during the research process.
- Create a matrix of the studies for easy analysis, and synthesis, across all of the studies.
- << Previous: Recommended Books
- Last Updated: Jun 20, 2024 9:08 AM
- URL: https://guides.library.yale.edu/YSNDoctoral
How To Structure Your Literature Review
3 options to help structure your chapter.
By: Amy Rommelspacher (PhD) | Reviewer: Dr Eunice Rautenbach | November 2020 (Updated May 2023)
Writing the literature review chapter can seem pretty daunting when you’re piecing together your dissertation or thesis. As we’ve discussed before , a good literature review needs to achieve a few very important objectives – it should:
- Demonstrate your knowledge of the research topic
- Identify the gaps in the literature and show how your research links to these
- Provide the foundation for your conceptual framework (if you have one)
- Inform your own methodology and research design
To achieve this, your literature review needs a well-thought-out structure . Get the structure of your literature review chapter wrong and you’ll struggle to achieve these objectives. Don’t worry though – in this post, we’ll look at how to structure your literature review for maximum impact (and marks!).

But wait – is this the right time?
Deciding on the structure of your literature review should come towards the end of the literature review process – after you have collected and digested the literature, but before you start writing the chapter.
In other words, you need to first develop a rich understanding of the literature before you even attempt to map out a structure. There’s no use trying to develop a structure before you’ve fully wrapped your head around the existing research.
Equally importantly, you need to have a structure in place before you start writing , or your literature review will most likely end up a rambling, disjointed mess.
Importantly, don’t feel that once you’ve defined a structure you can’t iterate on it. It’s perfectly natural to adjust as you engage in the writing process. As we’ve discussed before , writing is a way of developing your thinking, so it’s quite common for your thinking to change – and therefore, for your chapter structure to change – as you write.
Need a helping hand?
Like any other chapter in your thesis or dissertation, your literature review needs to have a clear, logical structure. At a minimum, it should have three essential components – an introduction , a body and a conclusion .
Let’s take a closer look at each of these.
1: The Introduction Section
Just like any good introduction, the introduction section of your literature review should introduce the purpose and layout (organisation) of the chapter. In other words, your introduction needs to give the reader a taste of what’s to come, and how you’re going to lay that out. Essentially, you should provide the reader with a high-level roadmap of your chapter to give them a taste of the journey that lies ahead.
Here’s an example of the layout visualised in a literature review introduction:
Your introduction should also outline your topic (including any tricky terminology or jargon) and provide an explanation of the scope of your literature review – in other words, what you will and won’t be covering (the delimitations ). This helps ringfence your review and achieve a clear focus . The clearer and narrower your focus, the deeper you can dive into the topic (which is typically where the magic lies).
Depending on the nature of your project, you could also present your stance or point of view at this stage. In other words, after grappling with the literature you’ll have an opinion about what the trends and concerns are in the field as well as what’s lacking. The introduction section can then present these ideas so that it is clear to examiners that you’re aware of how your research connects with existing knowledge .

2: The Body Section
The body of your literature review is the centre of your work. This is where you’ll present, analyse, evaluate and synthesise the existing research. In other words, this is where you’re going to earn (or lose) the most marks. Therefore, it’s important to carefully think about how you will organise your discussion to present it in a clear way.
The body of your literature review should do just as the description of this chapter suggests. It should “review” the literature – in other words, identify, analyse, and synthesise it. So, when thinking about structuring your literature review, you need to think about which structural approach will provide the best “review” for your specific type of research and objectives (we’ll get to this shortly).
There are (broadly speaking) three options for organising your literature review.

Option 1: Chronological (according to date)
Organising the literature chronologically is one of the simplest ways to structure your literature review. You start with what was published first and work your way through the literature until you reach the work published most recently. Pretty straightforward.
The benefit of this option is that it makes it easy to discuss the developments and debates in the field as they emerged over time. Organising your literature chronologically also allows you to highlight how specific articles or pieces of work might have changed the course of the field – in other words, which research has had the most impact . Therefore, this approach is very useful when your research is aimed at understanding how the topic has unfolded over time and is often used by scholars in the field of history. That said, this approach can be utilised by anyone that wants to explore change over time .

For example , if a student of politics is investigating how the understanding of democracy has evolved over time, they could use the chronological approach to provide a narrative that demonstrates how this understanding has changed through the ages.
Here are some questions you can ask yourself to help you structure your literature review chronologically.
- What is the earliest literature published relating to this topic?
- How has the field changed over time? Why?
- What are the most recent discoveries/theories?
In some ways, chronology plays a part whichever way you decide to structure your literature review, because you will always, to a certain extent, be analysing how the literature has developed. However, with the chronological approach, the emphasis is very firmly on how the discussion has evolved over time , as opposed to how all the literature links together (which we’ll discuss next ).
Option 2: Thematic (grouped by theme)
The thematic approach to structuring a literature review means organising your literature by theme or category – for example, by independent variables (i.e. factors that have an impact on a specific outcome).
As you’ve been collecting and synthesising literature , you’ll likely have started seeing some themes or patterns emerging. You can then use these themes or patterns as a structure for your body discussion. The thematic approach is the most common approach and is useful for structuring literature reviews in most fields.
For example, if you were researching which factors contributed towards people trusting an organisation, you might find themes such as consumers’ perceptions of an organisation’s competence, benevolence and integrity. Structuring your literature review thematically would mean structuring your literature review’s body section to discuss each of these themes, one section at a time.

Here are some questions to ask yourself when structuring your literature review by themes:
- Are there any patterns that have come to light in the literature?
- What are the central themes and categories used by the researchers?
- Do I have enough evidence of these themes?
PS – you can see an example of a thematically structured literature review in our literature review sample walkthrough video here.
Option 3: Methodological
The methodological option is a way of structuring your literature review by the research methodologies used . In other words, organising your discussion based on the angle from which each piece of research was approached – for example, qualitative , quantitative or mixed methodologies.
Structuring your literature review by methodology can be useful if you are drawing research from a variety of disciplines and are critiquing different methodologies. The point of this approach is to question how existing research has been conducted, as opposed to what the conclusions and/or findings the research were.

For example, a sociologist might centre their research around critiquing specific fieldwork practices. Their literature review will then be a summary of the fieldwork methodologies used by different studies.
Here are some questions you can ask yourself when structuring your literature review according to methodology:
- Which methodologies have been utilised in this field?
- Which methodology is the most popular (and why)?
- What are the strengths and weaknesses of the various methodologies?
- How can the existing methodologies inform my own methodology?
3: The Conclusion Section
Once you’ve completed the body section of your literature review using one of the structural approaches we discussed above, you’ll need to “wrap up” your literature review and pull all the pieces together to set the direction for the rest of your dissertation or thesis.
The conclusion is where you’ll present the key findings of your literature review. In this section, you should emphasise the research that is especially important to your research questions and highlight the gaps that exist in the literature. Based on this, you need to make it clear what you will add to the literature – in other words, justify your own research by showing how it will help fill one or more of the gaps you just identified.
Last but not least, if it’s your intention to develop a conceptual framework for your dissertation or thesis, the conclusion section is a good place to present this.

Example: Thematically Structured Review
In the video below, we unpack a literature review chapter so that you can see an example of a thematically structure review in practice.
Let’s Recap
In this article, we’ve discussed how to structure your literature review for maximum impact. Here’s a quick recap of what you need to keep in mind when deciding on your literature review structure:
- Just like other chapters, your literature review needs a clear introduction , body and conclusion .
- The introduction section should provide an overview of what you will discuss in your literature review.
- The body section of your literature review can be organised by chronology , theme or methodology . The right structural approach depends on what you’re trying to achieve with your research.
- The conclusion section should draw together the key findings of your literature review and link them to your research questions.
If you’re ready to get started, be sure to download our free literature review template to fast-track your chapter outline.

Psst… there’s more!
This post is an extract from our bestselling short course, Literature Review Bootcamp . If you want to work smart, you don't want to miss this .
You Might Also Like:

27 Comments
Great work. This is exactly what I was looking for and helps a lot together with your previous post on literature review. One last thing is missing: a link to a great literature chapter of an journal article (maybe with comments of the different sections in this review chapter). Do you know any great literature review chapters?
I agree with you Marin… A great piece
I agree with Marin. This would be quite helpful if you annotate a nicely structured literature from previously published research articles.
Awesome article for my research.
I thank you immensely for this wonderful guide
It is indeed thought and supportive work for the futurist researcher and students
Very educative and good time to get guide. Thank you
Great work, very insightful. Thank you.
Thanks for this wonderful presentation. My question is that do I put all the variables into a single conceptual framework or each hypothesis will have it own conceptual framework?
Thank you very much, very helpful
This is very educative and precise . Thank you very much for dropping this kind of write up .
Pheeww, so damn helpful, thank you for this informative piece.
I’m doing a research project topic ; stool analysis for parasitic worm (enteric) worm, how do I structure it, thanks.
comprehensive explanation. Help us by pasting the URL of some good “literature review” for better understanding.
great piece. thanks for the awesome explanation. it is really worth sharing. I have a little question, if anyone can help me out, which of the options in the body of literature can be best fit if you are writing an architectural thesis that deals with design?
I am doing a research on nanofluids how can l structure it?
Beautifully clear.nThank you!
Lucid! Thankyou!
Brilliant work, well understood, many thanks
I like how this was so clear with simple language 😊😊 thank you so much 😊 for these information 😊
Insightful. I was struggling to come up with a sensible literature review but this has been really helpful. Thank you!
You have given thought-provoking information about the review of the literature.
Thank you. It has made my own research better and to impart your work to students I teach
I learnt a lot from this teaching. It’s a great piece.
I am doing research on EFL teacher motivation for his/her job. How Can I structure it? Is there any detailed template, additional to this?
You are so cool! I do not think I’ve read through something like this before. So nice to find somebody with some genuine thoughts on this issue. Seriously.. thank you for starting this up. This site is one thing that is required on the internet, someone with a little originality!
I’m asked to do conceptual, theoretical and empirical literature, and i just don’t know how to structure it
Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Print Friendly
- Generating Ideas
- Drafting and Revision
- Sources and Evidence
- Style and Grammar
- Specific to Creative Arts
- Specific to Humanities
- Specific to Sciences
- Specific to Social Sciences
- CVs, Résumés and Cover Letters
- Graduate School Applications
- Other Resources
- Hiatt Career Center
- University Writing Center
- Classroom Materials
- Course and Assignment Design
- UWP Instructor Resources
- Writing Intensive Requirement
- Criteria and Learning Goals
- Course Application for Instructors
- What to Know about UWS
- Teaching Resources for WI
- FAQ for Instructors
- FAQ for Students
- Journals on Writing Research and Pedagogy
- University Writing Program
- Degree Programs
- Graduate Programs
- Brandeis Online
- Summer Programs
- Undergraduate Admissions
- Graduate Admissions
- Financial Aid
- Summer School
- Centers and Institutes
- Funding Resources
- Housing/Community Living
- Clubs and Organizations
- Community Service
- Brandeis Arts Engagement
- Rose Art Museum
- Our Jewish Roots
- Mission and Diversity Statements
- Administration
- Faculty & Staff
- Alumni & Friends
- Parents & Families
- 75th Anniversary
- Campus Calendar
- Directories
- New Students
- Shuttle Schedules
- Support at Brandeis
Writing Resources
Writing a literature review.
This handout is available for download in DOCX format and PDF format .
A literature review is a document or section of a document that collects key sources on a topic and discusses those sources in conversation with each other (also called synthesis). The lit review is an important genre in many disciplines, as it discusses the research (also called scholarship or literature) in a given field.
Where, when, and why would I write a lit review?
Different situations and disciplines each have different expectations for literature reviews. In the humanities, authors might include more overt argumentation and interpretation of source material, while in the sciences, authors are more likely to report study designs and results. Always get feedback from those knowledgeable in your field to be sure you understand your discipline’s conventions for literature reviews.
A literature review can be a part of a research paper or scholarly article, usually coming just after the introduction. Such lit reviews only need to cover scholarship important to your topic, though it may also cover key sources that informed your research methodology.
Lit reviews can also be standalone pieces, either as class assignments or as publications. In a class, a lit review may help students familiarize themselves with a topic and its important scholars, find gaps in existing research, and/or develop frameworks and methodologies for later research. As a publication, a lit review can help other scholars—especially students and scholars entering a new research area—by collecting, summarizing, synthesizing, and analyzing existing research on a topic.
What are the parts of a lit review?
Most lit reviews use a basic introduction-body-conclusion structure; if your lit review is part of a larger paper, the introduction and conclusion pieces may be just a few sentences while you focus most of your attention on the body. If your lit review is a standalone piece, the introduction and conclusion take up more space and give you a separate place to discuss your goals, research methods, and conclusions.
Introduction:
- An introductory paragraph that explains your working topic and thesis
- A forecast of key topics or texts that will appear in the review
- Potentially, a description of how you found sources and how you analyzed them for inclusion and discussion in the review (more common in published, standalone literature reviews)
- Summarize and synthesize: Give an overview of the main points of each source and combine them into a coherent whole
- Analyze and interpret: Don’t just paraphrase other researchers – add your own interpretations where possible, discussing the significance of findings in relation to the literature as a whole
- Critically evaluate: Mention the strengths and weaknesses of your sources
- Write in well-structured paragraphs: Use transition words and topic sentence to draw connections, comparisons, and contrasts.
Conclusion:
- Summarize the key findings you have taken from the literature and emphasize their significance
- Connect it back to your primary research question
How should I organize my lit review?
Lit reviews can take many different organizational structures depending on your goals. Here are just four examples:
Chronological
The simplest approach is to trace the topic’s development over time, which helps familiarize the audience with it. If you choose this strategy, be careful to avoid simply listing and summarizing sources in order. Try to analyze the patterns, turning points, and key debates that have shaped the field, and if appropriate in your discipline, give your interpretation of how and why certain developments occurred.
If you have found recurring themes that you will continue working with throughout your piece, you can organize your literature review into subsections that address different aspects of the topic. For example, if you are reviewing literature about women and religion, key themes can include the role of women in churches and religious attitudes towards women.
Methodological
If you draw your sources from different disciplines or fields that use a variety of research methods, you can compare the results and conclusions that emerge from different approaches. For example:
- Qualitative versus quantitative research
- Empirical versus theoretical scholarship
- Divide the research by sociological, historical, or cultural sources
Theoretical
In many humanities articles, the literature review is the foundation for the theoretical framework. You can use it to discuss various theories, models, and definitions of key concepts. You can argue for the relevance of a specific theoretical approach or combine various theoretical concepts to create a framework for your research.
What strategies or tips can I use while writing my lit review?
- Research thoroughly and choose your sources wisely; your lit review is only as good as the research it discusses!
- Create an annotated bibliography (see our Annotated Bibliographies handout ) as you research. The information in it will become a foundation for your lit review, while creating it will also help you develop a sense for the larger scholarly conversations in the field.
- Synthesize research rather than just summarizing it. This often means using multiple authors in each paragraph.
- Frame your lit review as an argument if possible and appropriate in your discipline. This will offer you a chance to position yourself in relation to other scholars, to define your intellectual lineage, and to argue for your place in the scholarly conversation.
Adapted from the Purdue OWL Guide, https://owl.purdue.edu/owl/research_and_citation/conducting_research/writing_a_literature_review.html, 2020.
- Resources for Students
- Writing Intensive Instructor Resources
- Research and Pedagogy

Literature Reviews
What this handout is about.
This handout will explain what literature reviews are and offer insights into the form and construction of literature reviews in the humanities, social sciences, and sciences.
Introduction
OK. You’ve got to write a literature review. You dust off a novel and a book of poetry, settle down in your chair, and get ready to issue a “thumbs up” or “thumbs down” as you leaf through the pages. “Literature review” done. Right?
Wrong! The “literature” of a literature review refers to any collection of materials on a topic, not necessarily the great literary texts of the world. “Literature” could be anything from a set of government pamphlets on British colonial methods in Africa to scholarly articles on the treatment of a torn ACL. And a review does not necessarily mean that your reader wants you to give your personal opinion on whether or not you liked these sources.
What is a literature review, then?
A literature review discusses published information in a particular subject area, and sometimes information in a particular subject area within a certain time period.
A literature review can be just a simple summary of the sources, but it usually has an organizational pattern and combines both summary and synthesis. A summary is a recap of the important information of the source, but a synthesis is a re-organization, or a reshuffling, of that information. It might give a new interpretation of old material or combine new with old interpretations. Or it might trace the intellectual progression of the field, including major debates. And depending on the situation, the literature review may evaluate the sources and advise the reader on the most pertinent or relevant.
But how is a literature review different from an academic research paper?
The main focus of an academic research paper is to develop a new argument, and a research paper is likely to contain a literature review as one of its parts. In a research paper, you use the literature as a foundation and as support for a new insight that you contribute. The focus of a literature review, however, is to summarize and synthesize the arguments and ideas of others without adding new contributions.
Why do we write literature reviews?
Literature reviews provide you with a handy guide to a particular topic. If you have limited time to conduct research, literature reviews can give you an overview or act as a stepping stone. For professionals, they are useful reports that keep them up to date with what is current in the field. For scholars, the depth and breadth of the literature review emphasizes the credibility of the writer in his or her field. Literature reviews also provide a solid background for a research paper’s investigation. Comprehensive knowledge of the literature of the field is essential to most research papers.
Who writes these things, anyway?
Literature reviews are written occasionally in the humanities, but mostly in the sciences and social sciences; in experiment and lab reports, they constitute a section of the paper. Sometimes a literature review is written as a paper in itself.
Let’s get to it! What should I do before writing the literature review?
If your assignment is not very specific, seek clarification from your instructor:
- Roughly how many sources should you include?
- What types of sources (books, journal articles, websites)?
- Should you summarize, synthesize, or critique your sources by discussing a common theme or issue?
- Should you evaluate your sources?
- Should you provide subheadings and other background information, such as definitions and/or a history?
Find models
Look for other literature reviews in your area of interest or in the discipline and read them to get a sense of the types of themes you might want to look for in your own research or ways to organize your final review. You can simply put the word “review” in your search engine along with your other topic terms to find articles of this type on the Internet or in an electronic database. The bibliography or reference section of sources you’ve already read are also excellent entry points into your own research.
Narrow your topic
There are hundreds or even thousands of articles and books on most areas of study. The narrower your topic, the easier it will be to limit the number of sources you need to read in order to get a good survey of the material. Your instructor will probably not expect you to read everything that’s out there on the topic, but you’ll make your job easier if you first limit your scope.
Keep in mind that UNC Libraries have research guides and to databases relevant to many fields of study. You can reach out to the subject librarian for a consultation: https://library.unc.edu/support/consultations/ .
And don’t forget to tap into your professor’s (or other professors’) knowledge in the field. Ask your professor questions such as: “If you had to read only one book from the 90’s on topic X, what would it be?” Questions such as this help you to find and determine quickly the most seminal pieces in the field.
Consider whether your sources are current
Some disciplines require that you use information that is as current as possible. In the sciences, for instance, treatments for medical problems are constantly changing according to the latest studies. Information even two years old could be obsolete. However, if you are writing a review in the humanities, history, or social sciences, a survey of the history of the literature may be what is needed, because what is important is how perspectives have changed through the years or within a certain time period. Try sorting through some other current bibliographies or literature reviews in the field to get a sense of what your discipline expects. You can also use this method to consider what is currently of interest to scholars in this field and what is not.
Strategies for writing the literature review
Find a focus.
A literature review, like a term paper, is usually organized around ideas, not the sources themselves as an annotated bibliography would be organized. This means that you will not just simply list your sources and go into detail about each one of them, one at a time. No. As you read widely but selectively in your topic area, consider instead what themes or issues connect your sources together. Do they present one or different solutions? Is there an aspect of the field that is missing? How well do they present the material and do they portray it according to an appropriate theory? Do they reveal a trend in the field? A raging debate? Pick one of these themes to focus the organization of your review.
Convey it to your reader
A literature review may not have a traditional thesis statement (one that makes an argument), but you do need to tell readers what to expect. Try writing a simple statement that lets the reader know what is your main organizing principle. Here are a couple of examples:
The current trend in treatment for congestive heart failure combines surgery and medicine. More and more cultural studies scholars are accepting popular media as a subject worthy of academic consideration.
Consider organization
You’ve got a focus, and you’ve stated it clearly and directly. Now what is the most effective way of presenting the information? What are the most important topics, subtopics, etc., that your review needs to include? And in what order should you present them? Develop an organization for your review at both a global and local level:
First, cover the basic categories
Just like most academic papers, literature reviews also must contain at least three basic elements: an introduction or background information section; the body of the review containing the discussion of sources; and, finally, a conclusion and/or recommendations section to end the paper. The following provides a brief description of the content of each:
- Introduction: Gives a quick idea of the topic of the literature review, such as the central theme or organizational pattern.
- Body: Contains your discussion of sources and is organized either chronologically, thematically, or methodologically (see below for more information on each).
- Conclusions/Recommendations: Discuss what you have drawn from reviewing literature so far. Where might the discussion proceed?
Organizing the body
Once you have the basic categories in place, then you must consider how you will present the sources themselves within the body of your paper. Create an organizational method to focus this section even further.
To help you come up with an overall organizational framework for your review, consider the following scenario:
You’ve decided to focus your literature review on materials dealing with sperm whales. This is because you’ve just finished reading Moby Dick, and you wonder if that whale’s portrayal is really real. You start with some articles about the physiology of sperm whales in biology journals written in the 1980’s. But these articles refer to some British biological studies performed on whales in the early 18th century. So you check those out. Then you look up a book written in 1968 with information on how sperm whales have been portrayed in other forms of art, such as in Alaskan poetry, in French painting, or on whale bone, as the whale hunters in the late 19th century used to do. This makes you wonder about American whaling methods during the time portrayed in Moby Dick, so you find some academic articles published in the last five years on how accurately Herman Melville portrayed the whaling scene in his novel.
Now consider some typical ways of organizing the sources into a review:
- Chronological: If your review follows the chronological method, you could write about the materials above according to when they were published. For instance, first you would talk about the British biological studies of the 18th century, then about Moby Dick, published in 1851, then the book on sperm whales in other art (1968), and finally the biology articles (1980s) and the recent articles on American whaling of the 19th century. But there is relatively no continuity among subjects here. And notice that even though the sources on sperm whales in other art and on American whaling are written recently, they are about other subjects/objects that were created much earlier. Thus, the review loses its chronological focus.
- By publication: Order your sources by publication chronology, then, only if the order demonstrates a more important trend. For instance, you could order a review of literature on biological studies of sperm whales if the progression revealed a change in dissection practices of the researchers who wrote and/or conducted the studies.
- By trend: A better way to organize the above sources chronologically is to examine the sources under another trend, such as the history of whaling. Then your review would have subsections according to eras within this period. For instance, the review might examine whaling from pre-1600-1699, 1700-1799, and 1800-1899. Under this method, you would combine the recent studies on American whaling in the 19th century with Moby Dick itself in the 1800-1899 category, even though the authors wrote a century apart.
- Thematic: Thematic reviews of literature are organized around a topic or issue, rather than the progression of time. However, progression of time may still be an important factor in a thematic review. For instance, the sperm whale review could focus on the development of the harpoon for whale hunting. While the study focuses on one topic, harpoon technology, it will still be organized chronologically. The only difference here between a “chronological” and a “thematic” approach is what is emphasized the most: the development of the harpoon or the harpoon technology.But more authentic thematic reviews tend to break away from chronological order. For instance, a thematic review of material on sperm whales might examine how they are portrayed as “evil” in cultural documents. The subsections might include how they are personified, how their proportions are exaggerated, and their behaviors misunderstood. A review organized in this manner would shift between time periods within each section according to the point made.
- Methodological: A methodological approach differs from the two above in that the focusing factor usually does not have to do with the content of the material. Instead, it focuses on the “methods” of the researcher or writer. For the sperm whale project, one methodological approach would be to look at cultural differences between the portrayal of whales in American, British, and French art work. Or the review might focus on the economic impact of whaling on a community. A methodological scope will influence either the types of documents in the review or the way in which these documents are discussed. Once you’ve decided on the organizational method for the body of the review, the sections you need to include in the paper should be easy to figure out. They should arise out of your organizational strategy. In other words, a chronological review would have subsections for each vital time period. A thematic review would have subtopics based upon factors that relate to the theme or issue.
Sometimes, though, you might need to add additional sections that are necessary for your study, but do not fit in the organizational strategy of the body. What other sections you include in the body is up to you. Put in only what is necessary. Here are a few other sections you might want to consider:
- Current Situation: Information necessary to understand the topic or focus of the literature review.
- History: The chronological progression of the field, the literature, or an idea that is necessary to understand the literature review, if the body of the literature review is not already a chronology.
- Methods and/or Standards: The criteria you used to select the sources in your literature review or the way in which you present your information. For instance, you might explain that your review includes only peer-reviewed articles and journals.
Questions for Further Research: What questions about the field has the review sparked? How will you further your research as a result of the review?
Begin composing
Once you’ve settled on a general pattern of organization, you’re ready to write each section. There are a few guidelines you should follow during the writing stage as well. Here is a sample paragraph from a literature review about sexism and language to illuminate the following discussion:
However, other studies have shown that even gender-neutral antecedents are more likely to produce masculine images than feminine ones (Gastil, 1990). Hamilton (1988) asked students to complete sentences that required them to fill in pronouns that agreed with gender-neutral antecedents such as “writer,” “pedestrian,” and “persons.” The students were asked to describe any image they had when writing the sentence. Hamilton found that people imagined 3.3 men to each woman in the masculine “generic” condition and 1.5 men per woman in the unbiased condition. Thus, while ambient sexism accounted for some of the masculine bias, sexist language amplified the effect. (Source: Erika Falk and Jordan Mills, “Why Sexist Language Affects Persuasion: The Role of Homophily, Intended Audience, and Offense,” Women and Language19:2).
Use evidence
In the example above, the writers refer to several other sources when making their point. A literature review in this sense is just like any other academic research paper. Your interpretation of the available sources must be backed up with evidence to show that what you are saying is valid.
Be selective
Select only the most important points in each source to highlight in the review. The type of information you choose to mention should relate directly to the review’s focus, whether it is thematic, methodological, or chronological.
Use quotes sparingly
Falk and Mills do not use any direct quotes. That is because the survey nature of the literature review does not allow for in-depth discussion or detailed quotes from the text. Some short quotes here and there are okay, though, if you want to emphasize a point, or if what the author said just cannot be rewritten in your own words. Notice that Falk and Mills do quote certain terms that were coined by the author, not common knowledge, or taken directly from the study. But if you find yourself wanting to put in more quotes, check with your instructor.
Summarize and synthesize
Remember to summarize and synthesize your sources within each paragraph as well as throughout the review. The authors here recapitulate important features of Hamilton’s study, but then synthesize it by rephrasing the study’s significance and relating it to their own work.
Keep your own voice
While the literature review presents others’ ideas, your voice (the writer’s) should remain front and center. Notice that Falk and Mills weave references to other sources into their own text, but they still maintain their own voice by starting and ending the paragraph with their own ideas and their own words. The sources support what Falk and Mills are saying.
Use caution when paraphrasing
When paraphrasing a source that is not your own, be sure to represent the author’s information or opinions accurately and in your own words. In the preceding example, Falk and Mills either directly refer in the text to the author of their source, such as Hamilton, or they provide ample notation in the text when the ideas they are mentioning are not their own, for example, Gastil’s. For more information, please see our handout on plagiarism .
Revise, revise, revise
Draft in hand? Now you’re ready to revise. Spending a lot of time revising is a wise idea, because your main objective is to present the material, not the argument. So check over your review again to make sure it follows the assignment and/or your outline. Then, just as you would for most other academic forms of writing, rewrite or rework the language of your review so that you’ve presented your information in the most concise manner possible. Be sure to use terminology familiar to your audience; get rid of unnecessary jargon or slang. Finally, double check that you’ve documented your sources and formatted the review appropriately for your discipline. For tips on the revising and editing process, see our handout on revising drafts .
Works consulted
We consulted these works while writing this handout. This is not a comprehensive list of resources on the handout’s topic, and we encourage you to do your own research to find additional publications. Please do not use this list as a model for the format of your own reference list, as it may not match the citation style you are using. For guidance on formatting citations, please see the UNC Libraries citation tutorial . We revise these tips periodically and welcome feedback.
Anson, Chris M., and Robert A. Schwegler. 2010. The Longman Handbook for Writers and Readers , 6th ed. New York: Longman.
Jones, Robert, Patrick Bizzaro, and Cynthia Selfe. 1997. The Harcourt Brace Guide to Writing in the Disciplines . New York: Harcourt Brace.
Lamb, Sandra E. 1998. How to Write It: A Complete Guide to Everything You’ll Ever Write . Berkeley: Ten Speed Press.
Rosen, Leonard J., and Laurence Behrens. 2003. The Allyn & Bacon Handbook , 5th ed. New York: Longman.
Troyka, Lynn Quittman, and Doug Hesse. 2016. Simon and Schuster Handbook for Writers , 11th ed. London: Pearson.
You may reproduce it for non-commercial use if you use the entire handout and attribute the source: The Writing Center, University of North Carolina at Chapel Hill
Make a Gift
DEAN’S BOOK w/ Prof. CONNIE GRIFFIN
Honors291g-cdg’s blog.
How to Write a Literature Review
What is a literature review? Written in essay style, a literature review (Lit Review) describes, classifies, and evaluates the sources of information published on a given topic. A Lit Review is not just a list of books/articles. It’s a review of a collection of research published by accredited scholars and researchers that is relevant to a research question. “Non-scholarly” sources, i.e., those you don’t want to reference, include but are not limited to magazines, newspapers, web sites, and non-published material. The Lit Review does not have to be exhaustive; the objective is not to list as many relevant books, articles, and reports as possible. The idea of the Lit Review is not to provide a summary of all the published work that relates to your research, but a survey (summary and evaluation) of the most relevant and significant work. A Lit Review is a critical look at the existing research that is significant to the work you are carrying out. It’s not just a summary. While you do need to summarize your relevant research, you must also: • evaluate this work, • show the relationships between different works, and • demonstrate how it relates to your work. A Lit Review is about the existing literature on your subject and provides background for your own research findings or commentary. How much sense does your research make if you don’t provide background to the reader about past research conducted by others?
What is the value of a literature review? A Lit Review provides your reader with a survey of the professional publications available on your topic. It demonstrates that you have not only thoroughly researched your topic but also carefully examined and critically evaluated the range of relevant sources. In writing the Lit Review, your purpose is to convey to your reader what knowledge and ideas have been established on a topic and what their strengths and weaknesses are. A Lit Review: • places the paper within the context of known research on the subject; focuses one’s own research topic. • provides thorough knowledge of previous studies; introduces seminal works. • indicates timely nature of one’s research, if applicable. • suggests previously unused or underused methodologies, designs, quantitative, and qualitative strategies. • identifies gaps in previous studies; identifies flawed methodologies and/or theoretical approaches; avoids replication of mistakes. • identifies possible trends or patterns in the literature. • helps the researcher avoid repetition of earlier research. • determines whether past studies agree or disagree; identifies controversy in the literature. • tests assumptions; may help counter preconceived ideas and remove unconscious bias.
What is the “literature” in a literature review? The “literature” is the collection of books and journal articles, government documents, and other scholarly works you found to be relevant to your research topic. • Journal articles: An excellent source for a Lit Review. These are good especially for up-to-date information. They are frequently used in Lit Reviews because they offer a relatively concise, up-to-date format for research, and because all reputable journals are refereed or peer-reviewed, i.e., editors publish only the most relevant and reliable research that has been reviewed by other experts in the field. • Books: Generally, a good source for a Lit Review. Books tend to be less up-to-date as it takes longer for a book to be published than for a journal article. Textbooks are unlikely to be useful for including in your Lit Review as they are intended for teaching, not for research, but they do offer a good starting point from which to find better, more detailed sources. • Conference proceedings: A good source for a Lit Review. These can be useful in providing the latest research or research that has not been published. They are also helpful in providing information on which people are currently involved in which research areas, and so can be helpful in tracking down other work by the same researchers. • Government/corporate reports: A good source for a Lit Review. Many government departments and corporations commission or carry out research. Their published findings can provide a useful source of information, depending on your field of study. • Newspapers: Not a good source for a Lit Review. Since newspapers are generally intended for a general (not specialized) audience, the information they provide will be of no use for your Lit Review. Journalists are generally not scholars, i.e., experts on the topic on which they are writing, and thus newspaper articles are not scholarly sources. • Theses and dissertations: Can be a good source for a Lit Review. These can be useful sources of information. However there are disadvantages: 1) they can be difficult to obtain since they are not always published but are generally only available from the library shelf or through interlibrary loan; 2) the student who carried out the research may not be an experienced researcher and therefore you might have to treat their findings with more caution than published research. Note: Some dissertations are available from the DuBois Library databases. • Web sites: Never a good source for a Lit Review. The fastest-growing source of information is on the Internet. It is impossible to characterize the information available but here are some hints about using electronic sources: 1) bear in mind that anyone can post information on the Internet so the quality may not be reliable, and 2) the information you find may be intended for a general audience and so may not be suitable for inclusion in your Lit Review (information for a general audience is usually less detailed and less scholarly). Note: This section does not refer to scholarly articles located on the DuBois Library databases. Databases are not web sites. • Magazines: Not a good source for a Lit Review. Magazines intended for a general audience, e.g., Time, Us, National Enquirer, will not be useful in providing the sort of information you need. Specialized magazines may be more useful (for example business magazines for management students), but usually magazines are not useful for your research except as a starting point by providing news or general information about new discoveries, policies, etc. that you can further research in more specialized sources. How is a literature review different from an annotated bibliography? A Lit Review is written in the style of an expository essay; it has an introduction, body, and conclusion, and it is organized around a controlling idea or thesis. Compare this to an annotated Works Cited list, which is simply an alphabetized list of sources accompanied by summaries and evaluations (annotations). While a single source appears just once in an annotated Works Cited list, it may be referred to numerous times in a Lit Review, depending upon its importance in the field or relationship to other sources. Finally, a Lit Review includes its own in-text citations and Works Cited list.
How is a literature review different from a traditional research paper? A Lit Review may stand alone as a self-contained unit or be part of a research paper (such as a chapter in an honors thesis). Whereas the main body of a research paper focuses on the subject of your research, the Lit Review focuses on your sources. Put another way, in the research paper you use expert sources to support the discussion of your thesis; in a Lit Review, you discuss the sources themselves.
What are the characteristics of a literature review? Among the characteristics you should expect to see in any Lit Review are these: • A Lit Review MUST have a Works Cited list that includes all references cited in the Lit Review. Do not list sources in the Works Cited list that are not directly cited in the Lit Review. • A Lit Review is organized by subtopic, NOT by individual source. In a typical Lit Review, you may cite several references in the same paragraph and may cite the same reference in more than one paragraph if that source addresses more than one of the subtopics in the Lit Review. • Typically, discussion of each source is quite brief. The contribution you make is organizing the ideas from the sources into a cogent argument or narrative that includes your perspectives. • You should focus on citing the material that originates with each reference. This may require a careful reading of the reference. If the reference author refers to another source whose ideas are relevant or interesting, you are better off tracking down and using that reference. Citing a source that you haven’t read directly is called “grandfathering,” and it is not permitted. Never cite a source you haven’t read.
How is a literature review structured? The Lit Review is not just a descriptive list of the material available or a set of summaries. Demonstrate that you gained a thorough knowledge of the subject area being studied. A Lit Review should NOT be organized as a narrative of your own research process. A Lit Review that says essentially “First I found this source, then I found this one ….” is NOT acceptable. Therefore, like any expository essay, a Lit Review should have an introduction, body, and conclusion: The introduction should contain your research question (thesis statement), an explanation of its significance, and any other background information setting the context of your research. The body paragraphs contain your summative, comparative, and evaluative comments on the sources you’ve found. These comments may pertain to: • historical background & early research findings • recent developments • areas of controversy among experts • areas of agreement • dominant views or leading authorities • varying approaches to or perspectives on the subject • qualitative comparisons and evaluations The conclusion summarizes major issues in the literature; it also establishes where your own research fits in and what directions you see for future research.
How is a literature review organized? While covering the range of matters listed above, a Lit Review—like any expository essay—should still have a single organizing principle expressed in a thesis statement. Examples of some common ones are these: • Chronological — Use this organization if developments over time are important to explain the context of your research problem. Otherwise, using a chronological system is not the best way to organize your work. • Thematic — Depending on your topic, you could organize your Lit Review by time, geographic location, gender, nationality, or another appropriate theme. • Methodological — What approaches have researchers taken in studying the issue you’re researching? Is your topic suited best for library research or field research? • Qualitative — Is there a great deal of difference in the quality of research conducted on your topic?
What are some strategies for writing a good literature review? The process of writing a Lit Review typically involves a number of steps. These should include the following: • Deciding on a relatively focused topic or question. • Searching for relevant and relatively current literature (books, journal articles, etc. – the mix of these depends on your topic or thesis statement). It is important to locate and comment on the most important works in your chosen field. Failure to include such works might be considered a major failing of your review. The more you research and read, the more you become aware of names that are mentioned repeatedly as influential and even seminal authorities. • Reading the materials you have found and noting how they approach your topic or question: It isn’t necessary to read every word of a book to learn what an author says about a particular subject. Peruse the index. Skim through the book or article. A quick read through the introduction or the conclusion gives a gist of the book’s or article’s thesis, general points, or argument. Begin with the most recent studies and work backwards. A recent article’s list of references or bibliography might provide you with valuable works to consult. • Preparing a working outline for your Lit Review and grouping notes from your references in the appropriate section of your outline: Use note cards with citations and annotations, photocopied articles with points highlighted and notes in the margins, or whatever methods helps you keep your information organized. • Take good notes: Don’t trust your memory. Record all research. Write out the complete bibliographic citation for each work. Record the page number too, because you’ll need it for your in-text citations. (Unless you are citing an entire book or journal article, the in-text citation must include a page number or it’s considered incomplete/inaccurate.) Write direct quotations word for word. Use quotation marks, so it can be recognized as a direct quote. Avoid using too many direct quotations. Take down the substance of the author’s ideas in your own words (paraphrase). IMPORTANT: Most of the review should be primarily in your own words with appropriate documentation of other’s ideas. Don’t take too many notes from a single source or two. Use a wide range of sources. • Evaluating the information: After reading a lot of material, researchers must carefully evaluate it and decide what should be included in the literature review. Obviously researchers must be objective. Keep an open mind and look at a topic from different vantage points. Determine the objectivity of the material. Who funded the research studies? Who actually performed the research? For a contentious topic, present as equally as possible opposing positions. Be objective. Don’t overemphasize one side. • Writing and revising the narrative: Keep your audience in mind as your write. Keep your paragraphs short and use subheadings to clarify the structure. Subheadings break the material into readable units. A Lit Review must be organized around and directly related to the thesis or research question you are developing. It must identify areas of controversy in the literature and formulate questions that need further research.
Some traps to avoid: • Trying to read everything! As you might already have discovered, if you try to be comprehensive you will never be able to finish the reading! The Lit Review does not have to be exhaustive; the objective is not to list as many books, articles, and reports as possible. The idea of the Lit Review is not to provide a summary of all the published work that relates to your research, but a survey of the most relevant and significant work. The Lit Review should contain the most pertinent related studies and show an awareness of important past research and practices and promising current research and practices in the field. • Reading but not writing! It’s easier to read than to write: given the choice, most of us would rather sit down with a cup of coffee and read yet another article instead of putting ourselves in front of the computer to write about what we have already read! Writing takes much more effort, doesn’t it? However, writing can help you to understand and find relationships between the works you’ve read, so don’t put writing off until you’ve “finished” reading – after all, you will probably still be doing some reading all the way through to the end of your research project. Also, don’t think of what you first write as being the final or near-final version. Writing is a way of thinking, so allow yourself to write as many drafts as you need, changing your ideas and information as you learn more about the context of your research problem. • Not keeping bibliographic information! The moment will come when you have to write your Works Cited list . . . and then you realize you have forgotten to keep the information you need, and that you never got around to putting references into your work. The only solution is to spend a lot of time in the library tracking down all those sources that you read and going through your writing to find which information came from which source. To avoid this nightmare, always keep this information in your notes. Always put references into your writing.
Library & Learning Commons
- Search for sources
- APA style guide
Literature Reviews
How do i organize a literature review.
- What is a literature review?
- How do I find sources for a literature review?
- How do I format a literature review?
- Other resources
A literature review consists of three main parts: an introduction paragraph, the body paragraphs, and a conclusion paragraph.
The introduction
The introduction should give a quick summary of what the literature review is going to be about. It should tell the reader what the topic of the review will be and define the scope of the literature that will be discussed. The introduction should also indicate how the review is going to be organized. There will be more information on how to organize the body paragraphs of the literature review in the next section. One way to say how the review will be organized is to list what each body paragraph will be about and briefly say why they are in that order.
There are a few different ways to organize the body paragraphs of a literature review.
• Chronological: This is when the literature review discusses the sources in the order they were published, from the oldest to the newest. This can be useful if your focus is on the historical development of a subject.
• Trend: A literature review organized by trend will divide the review's subject into different categories and discuss the literature as it relates to each category. For example, a literature review about a nursing theorist might have one section talking about sources related to the theorist's life and another section for discussing the impact of their theory on modern nursing.
• Thematic: A thematic literature review will focus on a specific topic related to the literature. For example, in a literature review about a disease, the author may choose to focus on a single form of treatment for the disease.
• Methodological: A methodological literature review does not focus on the content of the sources. Instead it focuses on how the research was done. In other words, a methodological literature review is about the methods the original researchers used in putting together the data.
The conclusion
The conclusion of a literature review should begin with a summary of what the literature review is about. It should also comment on what the literature says about the topic as a whole and how research in the topic might advance in the future.
- << Previous: How do I format a literature review?
- Next: Other resources >>
- Last Updated: Jan 25, 2024 12:17 PM
- URL: https://bowvalleycollege.libguides.com/literature-review
Loading metrics
Open Access
Ten Simple Rules for Writing a Literature Review
* E-mail: [email protected]
Affiliations Centre for Functional and Evolutionary Ecology (CEFE), CNRS, Montpellier, France, Centre for Biodiversity Synthesis and Analysis (CESAB), FRB, Aix-en-Provence, France
- Marco Pautasso

Published: July 18, 2013
- https://doi.org/10.1371/journal.pcbi.1003149
- Reader Comments
Citation: Pautasso M (2013) Ten Simple Rules for Writing a Literature Review. PLoS Comput Biol 9(7): e1003149. https://doi.org/10.1371/journal.pcbi.1003149
Editor: Philip E. Bourne, University of California San Diego, United States of America
Copyright: © 2013 Marco Pautasso. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: This work was funded by the French Foundation for Research on Biodiversity (FRB) through its Centre for Synthesis and Analysis of Biodiversity data (CESAB), as part of the NETSEED research project. The funders had no role in the preparation of the manuscript.
Competing interests: The author has declared that no competing interests exist.
Literature reviews are in great demand in most scientific fields. Their need stems from the ever-increasing output of scientific publications [1] . For example, compared to 1991, in 2008 three, eight, and forty times more papers were indexed in Web of Science on malaria, obesity, and biodiversity, respectively [2] . Given such mountains of papers, scientists cannot be expected to examine in detail every single new paper relevant to their interests [3] . Thus, it is both advantageous and necessary to rely on regular summaries of the recent literature. Although recognition for scientists mainly comes from primary research, timely literature reviews can lead to new synthetic insights and are often widely read [4] . For such summaries to be useful, however, they need to be compiled in a professional way [5] .
When starting from scratch, reviewing the literature can require a titanic amount of work. That is why researchers who have spent their career working on a certain research issue are in a perfect position to review that literature. Some graduate schools are now offering courses in reviewing the literature, given that most research students start their project by producing an overview of what has already been done on their research issue [6] . However, it is likely that most scientists have not thought in detail about how to approach and carry out a literature review.
Reviewing the literature requires the ability to juggle multiple tasks, from finding and evaluating relevant material to synthesising information from various sources, from critical thinking to paraphrasing, evaluating, and citation skills [7] . In this contribution, I share ten simple rules I learned working on about 25 literature reviews as a PhD and postdoctoral student. Ideas and insights also come from discussions with coauthors and colleagues, as well as feedback from reviewers and editors.
Rule 1: Define a Topic and Audience
How to choose which topic to review? There are so many issues in contemporary science that you could spend a lifetime of attending conferences and reading the literature just pondering what to review. On the one hand, if you take several years to choose, several other people may have had the same idea in the meantime. On the other hand, only a well-considered topic is likely to lead to a brilliant literature review [8] . The topic must at least be:
- interesting to you (ideally, you should have come across a series of recent papers related to your line of work that call for a critical summary),
- an important aspect of the field (so that many readers will be interested in the review and there will be enough material to write it), and
- a well-defined issue (otherwise you could potentially include thousands of publications, which would make the review unhelpful).
Ideas for potential reviews may come from papers providing lists of key research questions to be answered [9] , but also from serendipitous moments during desultory reading and discussions. In addition to choosing your topic, you should also select a target audience. In many cases, the topic (e.g., web services in computational biology) will automatically define an audience (e.g., computational biologists), but that same topic may also be of interest to neighbouring fields (e.g., computer science, biology, etc.).
Rule 2: Search and Re-search the Literature
After having chosen your topic and audience, start by checking the literature and downloading relevant papers. Five pieces of advice here:
- keep track of the search items you use (so that your search can be replicated [10] ),
- keep a list of papers whose pdfs you cannot access immediately (so as to retrieve them later with alternative strategies),
- use a paper management system (e.g., Mendeley, Papers, Qiqqa, Sente),
- define early in the process some criteria for exclusion of irrelevant papers (these criteria can then be described in the review to help define its scope), and
- do not just look for research papers in the area you wish to review, but also seek previous reviews.
The chances are high that someone will already have published a literature review ( Figure 1 ), if not exactly on the issue you are planning to tackle, at least on a related topic. If there are already a few or several reviews of the literature on your issue, my advice is not to give up, but to carry on with your own literature review,
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
The bottom-right situation (many literature reviews but few research papers) is not just a theoretical situation; it applies, for example, to the study of the impacts of climate change on plant diseases, where there appear to be more literature reviews than research studies [33] .
https://doi.org/10.1371/journal.pcbi.1003149.g001
- discussing in your review the approaches, limitations, and conclusions of past reviews,
- trying to find a new angle that has not been covered adequately in the previous reviews, and
- incorporating new material that has inevitably accumulated since their appearance.
When searching the literature for pertinent papers and reviews, the usual rules apply:
- be thorough,
- use different keywords and database sources (e.g., DBLP, Google Scholar, ISI Proceedings, JSTOR Search, Medline, Scopus, Web of Science), and
- look at who has cited past relevant papers and book chapters.
Rule 3: Take Notes While Reading
If you read the papers first, and only afterwards start writing the review, you will need a very good memory to remember who wrote what, and what your impressions and associations were while reading each single paper. My advice is, while reading, to start writing down interesting pieces of information, insights about how to organize the review, and thoughts on what to write. This way, by the time you have read the literature you selected, you will already have a rough draft of the review.
Of course, this draft will still need much rewriting, restructuring, and rethinking to obtain a text with a coherent argument [11] , but you will have avoided the danger posed by staring at a blank document. Be careful when taking notes to use quotation marks if you are provisionally copying verbatim from the literature. It is advisable then to reformulate such quotes with your own words in the final draft. It is important to be careful in noting the references already at this stage, so as to avoid misattributions. Using referencing software from the very beginning of your endeavour will save you time.
Rule 4: Choose the Type of Review You Wish to Write
After having taken notes while reading the literature, you will have a rough idea of the amount of material available for the review. This is probably a good time to decide whether to go for a mini- or a full review. Some journals are now favouring the publication of rather short reviews focusing on the last few years, with a limit on the number of words and citations. A mini-review is not necessarily a minor review: it may well attract more attention from busy readers, although it will inevitably simplify some issues and leave out some relevant material due to space limitations. A full review will have the advantage of more freedom to cover in detail the complexities of a particular scientific development, but may then be left in the pile of the very important papers “to be read” by readers with little time to spare for major monographs.
There is probably a continuum between mini- and full reviews. The same point applies to the dichotomy of descriptive vs. integrative reviews. While descriptive reviews focus on the methodology, findings, and interpretation of each reviewed study, integrative reviews attempt to find common ideas and concepts from the reviewed material [12] . A similar distinction exists between narrative and systematic reviews: while narrative reviews are qualitative, systematic reviews attempt to test a hypothesis based on the published evidence, which is gathered using a predefined protocol to reduce bias [13] , [14] . When systematic reviews analyse quantitative results in a quantitative way, they become meta-analyses. The choice between different review types will have to be made on a case-by-case basis, depending not just on the nature of the material found and the preferences of the target journal(s), but also on the time available to write the review and the number of coauthors [15] .
Rule 5: Keep the Review Focused, but Make It of Broad Interest
Whether your plan is to write a mini- or a full review, it is good advice to keep it focused 16 , 17 . Including material just for the sake of it can easily lead to reviews that are trying to do too many things at once. The need to keep a review focused can be problematic for interdisciplinary reviews, where the aim is to bridge the gap between fields [18] . If you are writing a review on, for example, how epidemiological approaches are used in modelling the spread of ideas, you may be inclined to include material from both parent fields, epidemiology and the study of cultural diffusion. This may be necessary to some extent, but in this case a focused review would only deal in detail with those studies at the interface between epidemiology and the spread of ideas.
While focus is an important feature of a successful review, this requirement has to be balanced with the need to make the review relevant to a broad audience. This square may be circled by discussing the wider implications of the reviewed topic for other disciplines.
Rule 6: Be Critical and Consistent
Reviewing the literature is not stamp collecting. A good review does not just summarize the literature, but discusses it critically, identifies methodological problems, and points out research gaps [19] . After having read a review of the literature, a reader should have a rough idea of:
- the major achievements in the reviewed field,
- the main areas of debate, and
- the outstanding research questions.
It is challenging to achieve a successful review on all these fronts. A solution can be to involve a set of complementary coauthors: some people are excellent at mapping what has been achieved, some others are very good at identifying dark clouds on the horizon, and some have instead a knack at predicting where solutions are going to come from. If your journal club has exactly this sort of team, then you should definitely write a review of the literature! In addition to critical thinking, a literature review needs consistency, for example in the choice of passive vs. active voice and present vs. past tense.
Rule 7: Find a Logical Structure
Like a well-baked cake, a good review has a number of telling features: it is worth the reader's time, timely, systematic, well written, focused, and critical. It also needs a good structure. With reviews, the usual subdivision of research papers into introduction, methods, results, and discussion does not work or is rarely used. However, a general introduction of the context and, toward the end, a recapitulation of the main points covered and take-home messages make sense also in the case of reviews. For systematic reviews, there is a trend towards including information about how the literature was searched (database, keywords, time limits) [20] .
How can you organize the flow of the main body of the review so that the reader will be drawn into and guided through it? It is generally helpful to draw a conceptual scheme of the review, e.g., with mind-mapping techniques. Such diagrams can help recognize a logical way to order and link the various sections of a review [21] . This is the case not just at the writing stage, but also for readers if the diagram is included in the review as a figure. A careful selection of diagrams and figures relevant to the reviewed topic can be very helpful to structure the text too [22] .
Rule 8: Make Use of Feedback
Reviews of the literature are normally peer-reviewed in the same way as research papers, and rightly so [23] . As a rule, incorporating feedback from reviewers greatly helps improve a review draft. Having read the review with a fresh mind, reviewers may spot inaccuracies, inconsistencies, and ambiguities that had not been noticed by the writers due to rereading the typescript too many times. It is however advisable to reread the draft one more time before submission, as a last-minute correction of typos, leaps, and muddled sentences may enable the reviewers to focus on providing advice on the content rather than the form.
Feedback is vital to writing a good review, and should be sought from a variety of colleagues, so as to obtain a diversity of views on the draft. This may lead in some cases to conflicting views on the merits of the paper, and on how to improve it, but such a situation is better than the absence of feedback. A diversity of feedback perspectives on a literature review can help identify where the consensus view stands in the landscape of the current scientific understanding of an issue [24] .
Rule 9: Include Your Own Relevant Research, but Be Objective
In many cases, reviewers of the literature will have published studies relevant to the review they are writing. This could create a conflict of interest: how can reviewers report objectively on their own work [25] ? Some scientists may be overly enthusiastic about what they have published, and thus risk giving too much importance to their own findings in the review. However, bias could also occur in the other direction: some scientists may be unduly dismissive of their own achievements, so that they will tend to downplay their contribution (if any) to a field when reviewing it.
In general, a review of the literature should neither be a public relations brochure nor an exercise in competitive self-denial. If a reviewer is up to the job of producing a well-organized and methodical review, which flows well and provides a service to the readership, then it should be possible to be objective in reviewing one's own relevant findings. In reviews written by multiple authors, this may be achieved by assigning the review of the results of a coauthor to different coauthors.
Rule 10: Be Up-to-Date, but Do Not Forget Older Studies
Given the progressive acceleration in the publication of scientific papers, today's reviews of the literature need awareness not just of the overall direction and achievements of a field of inquiry, but also of the latest studies, so as not to become out-of-date before they have been published. Ideally, a literature review should not identify as a major research gap an issue that has just been addressed in a series of papers in press (the same applies, of course, to older, overlooked studies (“sleeping beauties” [26] )). This implies that literature reviewers would do well to keep an eye on electronic lists of papers in press, given that it can take months before these appear in scientific databases. Some reviews declare that they have scanned the literature up to a certain point in time, but given that peer review can be a rather lengthy process, a full search for newly appeared literature at the revision stage may be worthwhile. Assessing the contribution of papers that have just appeared is particularly challenging, because there is little perspective with which to gauge their significance and impact on further research and society.
Inevitably, new papers on the reviewed topic (including independently written literature reviews) will appear from all quarters after the review has been published, so that there may soon be the need for an updated review. But this is the nature of science [27] – [32] . I wish everybody good luck with writing a review of the literature.
Acknowledgments
Many thanks to M. Barbosa, K. Dehnen-Schmutz, T. Döring, D. Fontaneto, M. Garbelotto, O. Holdenrieder, M. Jeger, D. Lonsdale, A. MacLeod, P. Mills, M. Moslonka-Lefebvre, G. Stancanelli, P. Weisberg, and X. Xu for insights and discussions, and to P. Bourne, T. Matoni, and D. Smith for helpful comments on a previous draft.
- 1. Rapple C (2011) The role of the critical review article in alleviating information overload. Annual Reviews White Paper. Available: http://www.annualreviews.org/userimages/ContentEditor/1300384004941/Annual_Reviews_WhitePaper_Web_2011.pdf . Accessed May 2013.
- View Article
- Google Scholar
- 7. Budgen D, Brereton P (2006) Performing systematic literature reviews in software engineering. Proc 28th Int Conf Software Engineering, ACM New York, NY, USA, pp. 1051–1052. doi: https://doi.org/10.1145/1134285.1134500 .
- 16. Eco U (1977) Come si fa una tesi di laurea. Milan: Bompiani.
- 17. Hart C (1998) Doing a literature review: releasing the social science research imagination. London: SAGE.
- 21. Ridley D (2008) The literature review: a step-by-step guide for students. London: SAGE.
Supporting writers since 1790
Essay Guide
Our comprehensive guide to the stages of the essay development process. Back to Student Resources
Display Menu
- Writing essays
- Essay writing
- Being a writer
- Thinking critically, thinking clearly
- Speaking vs. Writing
- Why are you writing?
- How much is that degree in the window?
- Academic writing
- Academic writing: key features
- Personal or impersonal?
- What tutors want – 1
- What tutors want – 2
- Be prepared to be flexible
- Understanding what they want – again
- Basic definitions
- Different varieties of essay, different kinds of writing
- Look, it’s my favourite word!
- Close encounters of the word kind
- Starting to answer the question: brainstorming
- Starting to answer the question: after the storm
- Other ways of getting started
- How not to read
- You, the reader
- Choosing your reading
- How to read: SQ3R
- How to read: other techniques
- Reading around the subject
- Taking notes
- What planning and structure mean and why you need them
- Introductions: what they do
- Main bodies: what they do
- Conclusions: what they do
- Paragraphs and links
- Process, process, process
- The first draft
- The second draft
- Editing – 1: getting your essay into shape
- Editing – 2: what’s on top & what lies beneath
- Are you looking for an argument?
- Simple definitions
- More definitions
- Different types of argument
- Sources & plagiarism
- Direct quotation, paraphrasing & referencing
- MLA, APA, Harvard or MHRA?
- Using the web
- Setting out and using quotations
- Recognising differences
- Humanities essays
- Scientific writing
- Social & behavioural science writing
- Beyond the essay
- Business-style reports
- Presentations
- What is a literature review?
- Why write a literature review?
- Key points to remember
The structure of a literature review
- How to do a literature search
Introduction
- What is a dissertation? How is it different from an essay?
- Getting it down on paper
- Drafting and rewriting
- Planning your dissertation
- Planning for length
- Planning for content
- Abstracts, tone, unity of style
- General comments
A literature review should be structured like any other essay: it should have an introduction, a middle or main body, and a conclusion.
The introduction should:
- define your topic and provide an appropriate context for reviewing the literature;
- establish your reasons – i.e. point of view – for
- reviewing the literature;
- explain the organisation – i.e. sequence – of the review;
- state the scope of the review – i.e. what is included and what isn’t included. For example, if you were reviewing the literature on obesity in children you might say something like: There are a large number of studies of obesity trends in the general population. However, since the focus of this research is on obesity in children, these will not be reviewed in detail and will only be referred to as appropriate.
The middle or main body should:
- organise the literature according to common themes;
- provide insight into the relation between your chosen topic and the wider subject area e.g. between obesity in children and obesity in general;
- move from a general, wider view of the literature being reviewed to the specific focus of your research.
The conclusion should:
- summarise the important aspects of the existing body of literature;
- evaluate the current state of the literature reviewed;
- identify significant flaws or gaps in existing knowledge;
- outline areas for future study;
- link your research to existing knowledge.

The Literature Review: 5. Organizing the Literature Review
- 1. Introduction
- 2. Why Do a Literature Review?
- 3. Methods for Searching the Literature
- 4. Analysing the Literature
- 5. Organizing the Literature Review
- 6. Writing the Review
1. Organizing Principles
A literature review is a piece of discursive prose, not a list describing or summarizing one piece of literature after another. It should have a single organizing principle:
- Thematic - organize around a topic or issue
- Chronological - sections for each vital time period
- Methodological - focus on the methods used by the researchers/writers
4. Selected Online Resources
- Literature Review in Education & Behavioral Sciences This is an interactive tutorial from Adelphi University Libraries on how to conduct a literature review in education and the behavioural sciences using library databases
- Writing Literature Reviews This tutorial is from the Writing section of Monash University's Language and Learning Online site
- The Literature Review: A Few Tips on Conducting It This guide is from the Health Services Writing Centre at the University of Toronto
- Learn How to Write a Review of the Literature This guide is part of the Writer's Handbook provided by the Writing Center at the University of Wisconsin-Madison
2. Structure of the Literature Review
Although your literature review will rely heavily on the sources you read for its information, you should dictate the structure of the review. It is important that the concepts are presented in an order that makes sense of the context of your research project.
There may be clear divisions on the sets of ideas you want to discuss, in which case your structure may be fairly clear. This is an ideal situation. In most cases, there will be several different possible structures for your review.
Similarly to the structure of the research report itself, the literature review consists of:
- Introduction
Introduction - profile of the study
- Define or identify the general topic to provide the context for reviewing the literature
- Outline why the topic is important
- Identify overall trends in what has been published about the topic
- Identify conflicts in theory, methodology, evidence, and conclusions
- Identify gaps in research and scholarlship
- Explain the criteria to be used in analysing and comparing the literature
- Describe the organization of the review (the sequence)
- If necessary, state why certain literature is or is not included (scope)
Body - summative, comparative, and evaluative discussion of literature reviewed
For a thematic review:
- organize the review into paragraphs that present themes and identify trends relevant to your topic
- each paragraph should deal with a different theme - you need to synthesize several of your readings into each paragraph in such a way that there is a clear connection between the sources
- don't try to list all the materials you have identified in your literature search
From each of the section summaries:
- summarize the main agreements and disagreements in the literature
- summarize the general conclusions that have been drawn
- establish where your own research fits in the context of the existing literature
5. A Final Checklist
- Have you indicated the purpose of the review?
- Have you emphasized recent developments?
- Is there a logic to the way you organized the material?
- Does the amount of detail included on an issue relate to its importance?
- Have you been sufficiently critical of design and methodological issues?
- Have you indicated when results were conflicting or inconclusive and discussed possible reasons?
- Has your summary of the current literature contributed to the reader's understanding of the problems?
3. Tips on Structure
A common error in literature reviews is for writers to present material from one author, followed by information from another, then another.... The way in which you group authors and link ideas will help avoid this problem. To group authors who draw similar conclusions, you can use linking words such as:
- additionally
When authors disagree, linking words that indicate contrast will show how you have analysed their work. Words such as:
- on the other hand
- nonetheless
will indicate to your reader how you have analysed the material. At other times, you may want to qualify an author's work (using such words as specifically, usually, or generally ) or use an example ( thus, namely, to illustrate ). In this way you ensure that you are synthesizing the material, not just describing the work already carried out in your field.
Another major problem is that literature reviews are often written as if they stand alone, without links to the rest of the paper. There needs to be a clear relationship between the literature review and the methodology to follow.
- << Previous: 4. Analysing the Literature
- Next: 6. Writing the Review >>
- Last Updated: May 9, 2024 10:36 AM
- URL: https://libguides.uwi.edu/litreviewsoe
Where instructors and editors talk writing.
- Literature Review Essentials: Construct Paragraphs

- Main idea : A topic sentence which tells the reader the theme that arose in the literature.
- Evidence : Paraphrased and cited information from a source in the literature which tells the reader important information related to the theme.
- Analysis: The writer’s interpretation and analysis of the paraphrased information which can be used to explain the evidence and link ideas.
- Repeat evidence and analysis as needed: In a literature review, there will almost always be several pieces of evidence paraphrased from different sources with analysis threaded throughout as needed.
- Lead out : A conclusion sentence which synthesizes what all of this literature has to say about this theme and concludes the paragraph .
[Main idea] : Apartments are better than single family homes for young couples because of the community that is available. [Evidence from source 1] : As Basye (2015) explained, young couples living on their own benefit from relationships with multi-generational neighbors and friends. [Evidence from source 2] : In addition, as Bono and Matluba (2016) argued, young couples often do not yet have secure social networks, so living in an apartment as compared to a home offers the opportunity to meet people nearby and create social networks close to home. [Analysis] : Thus, apartment living can be beneficial for relationship building. [Evidence from source 3] : Furthermore, having neighbors offers a safety network because when individuals form communities and social bonds, they are more likely to look out for one another (Hendron, 2017). [Lead out] : Overall, the literature showed that apartments are beneficial for young couples because of the relationships they can form, the networks they can create, and increased safety.

6 comments :

Very helpful for starting the literature review process.
We're glad the post was helpful for you!
You are very welcome! We hope your Lit Review writing goes well!
This is great information, thank you.
Great information, thank you.
Search the Blog
Featured posts.
- Expert Advice
- Writing Center Services
- Scholarly Writing
- Capstone Writing
- Grammar and Mechanics
- Writer's Workshop
- Social Change
- Literature Review
- International/Multilingual Students
- Passive Voice
WriteCast Podcast

Visit the Writing Center's Website

- About the Blog

Blog Archive
- ► May (1)
- ► April (1)
- ► February (1)
- ► January (1)
- ► December (1)
- ► November (1)
- ► September (1)
- ► July (1)
- ► June (1)
- ► March (2)
- ► October (2)
- ► September (2)
- ► August (2)
- ► July (2)
- ► June (2)
- ► April (2)
- ► March (3)
- ► January (3)
- ► November (3)
- ► October (3)
- ► September (4)
- ► August (4)
- ► July (5)
- ► June (5)
- ► May (5)
- ► April (9)
- ► March (7)
- ► February (8)
- ► January (9)
- ► December (9)
- ► November (9)
- ► October (9)
- ► September (8)
- ► August (7)
- ► July (8)
- ► June (8)
- ► May (7)
- ► March (9)
- ► December (8)
- ► August (9)
- ► July (6)
- ► June (6)
- ► May (9)
- ► April (8)
- Webinar Update: April Webinars!
- Literature Review Essentials: Align Problems
- Relaxation Reminder
- WriteCast Episode 36: Social Change and Difficult ...
- Literature Review Essentials: Identify Themes
- Literature Review Essentials: A Five-Part Blog Series
- Literature Review Essentials: Curate Information
- March Webinar Update
- ► November (8)
- ► September (9)
- ► June (9)
- ► February (9)
- ► June (4)
- ► May (4)
- ► April (5)
- ► March (6)
- ► February (5)
- ► January (5)
- ► December (6)
- ► November (5)
- ► October (5)
- ► September (5)
- ► August (5)
- ► April (7)
- ► February (4)
- ► December (4)
- ► November (4)
- ► October (7)
- ► July (7)
- ► April (6)
- ► March (4)
- ► September (7)
- ► August (6)
- ► June (3)
- ► January (6)
- ► October (6)
- ► September (3)
- ► April (4)
- ► February (2)
- ► January (2)
- ► December (2)
- ► November (2)
- ► August (3)
© Walden University Writing Center SoraTemplates . Posts RSS . Comments RSS
Review of Related Literature: Format, Example, & How to Make RRL
A review of related literature is a separate paper or a part of an article that collects and synthesizes discussion on a topic. Its purpose is to show the current state of research on the issue and highlight gaps in existing knowledge. A literature review can be included in a research paper or scholarly article, typically following the introduction and before the research methods section.

This article will clarify the definition, significance, and structure of a review of related literature. You’ll also learn how to organize your literature review and discover ideas for an RRL in different subjects.
🔤 What Is RRL?
- ❗ Significance of Literature Review
- 🔎 How to Search for Literature
- 🧩 Literature Review Structure
- 📋 Format of RRL — APA, MLA, & Others
- ✍️ How to Write an RRL
- 📚 Examples of RRL
🔗 References
A review of related literature (RRL) is a part of the research report that examines significant studies, theories, and concepts published in scholarly sources on a particular topic. An RRL includes 3 main components:
- A short overview and critique of the previous research.
- Similarities and differences between past studies and the current one.
- An explanation of the theoretical frameworks underpinning the research.
❗ Significance of Review of Related Literature
Although the goal of a review of related literature differs depending on the discipline and its intended use, its significance cannot be overstated. Here are some examples of how a review might be beneficial:
- It helps determine knowledge gaps .
- It saves from duplicating research that has already been conducted.
- It provides an overview of various research areas within the discipline.
- It demonstrates the researcher’s familiarity with the topic.
🔎 How to Perform a Literature Search
Including a description of your search strategy in the literature review section can significantly increase your grade. You can search sources with the following steps:
| You should specify all the keywords and their synonyms used to look for relevant sources. | |
| Using your search terms, look through the online (libraries and databases) and offline (books and journals) sources related to your topic. | |
| It is not possible to discuss all of the sources you have discovered. Instead, use the works of the most notable researchers and authors. | |
| From the remaining references, you should pick those with the most significant contribution to the research area development. | |
| Your literature should prioritize new publications over older ones to cover the latest research advancements. |
🧩 Literature Review Structure Example
The majority of literature reviews follow a standard introduction-body-conclusion structure. Let’s look at the RRL structure in detail.
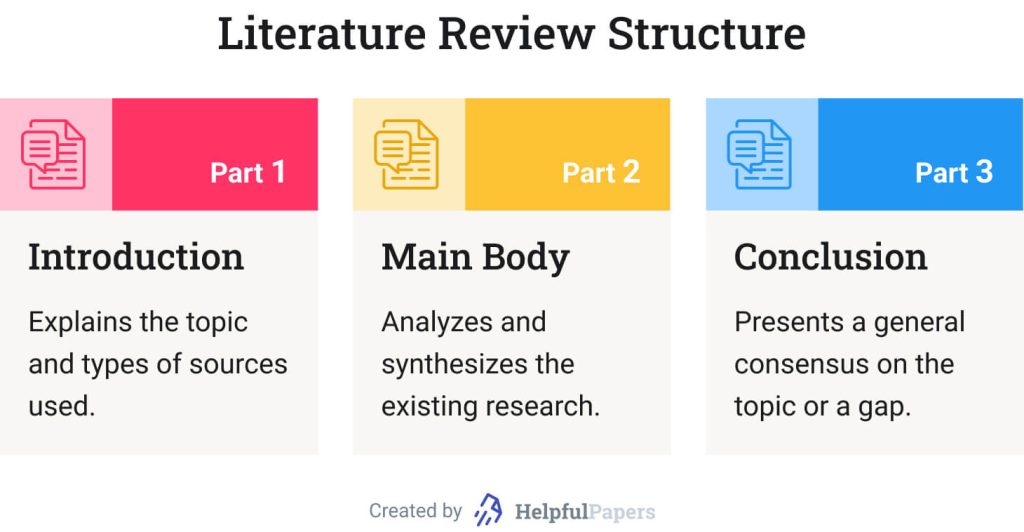
Introduction of Review of Related Literature: Sample
An introduction should clarify the study topic and the depth of the information to be delivered. It should also explain the types of sources used. If your lit. review is part of a larger research proposal or project, you can combine its introductory paragraph with the introduction of your paper.
Here is a sample introduction to an RRL about cyberbullying:
Bullying has troubled people since the beginning of time. However, with modern technological advancements, especially social media, bullying has evolved into cyberbullying. As a result, nowadays, teenagers and adults cannot flee their bullies, which makes them feel lonely and helpless. This literature review will examine recent studies on cyberbullying.
Sample Review of Related Literature Thesis
A thesis statement should include the central idea of your literature review and the primary supporting elements you discovered in the literature. Thesis statements are typically put at the end of the introductory paragraph.
Look at a sample thesis of a review of related literature:
This literature review shows that scholars have recently covered the issues of bullies’ motivation, the impact of bullying on victims and aggressors, common cyberbullying techniques, and victims’ coping strategies. However, there is still no agreement on the best practices to address cyberbullying.
Literature Review Body Paragraph Example
The main body of a literature review should provide an overview of the existing research on the issue. Body paragraphs should not just summarize each source but analyze them. You can organize your paragraphs with these 3 elements:
- Claim . Start with a topic sentence linked to your literature review purpose.
- Evidence . Cite relevant information from your chosen sources.
- Discussion . Explain how the cited data supports your claim.
Here’s a literature review body paragraph example:
Scholars have examined the link between the aggressor and the victim. Beran et al. (2007) state that students bullied online often become cyberbullies themselves. Faucher et al. (2014) confirm this with their findings: they discovered that male and female students began engaging in cyberbullying after being subject to bullying. Hence, one can conclude that being a victim of bullying increases one’s likelihood of becoming a cyberbully.
Review of Related Literature: Conclusion
A conclusion presents a general consensus on the topic. Depending on your literature review purpose, it might include the following:
- Introduction to further research . If you write a literature review as part of a larger research project, you can present your research question in your conclusion .
- Overview of theories . You can summarize critical theories and concepts to help your reader understand the topic better.
- Discussion of the gap . If you identified a research gap in the reviewed literature, your conclusion could explain why that gap is significant.
Check out a conclusion example that discusses a research gap:
There is extensive research into bullies’ motivation, the consequences of bullying for victims and aggressors, strategies for bullying, and coping with it. Yet, scholars still have not reached a consensus on what to consider the best practices to combat cyberbullying. This question is of great importance because of the significant adverse effects of cyberbullying on victims and bullies.
📋 Format of RRL — APA, MLA, & Others
In this section, we will discuss how to format an RRL according to the most common citation styles: APA, Chicago, MLA, and Harvard.
Writing a literature review using the APA7 style requires the following text formatting:
| Times New Roman or Arial, 12 pt | |
| Double spacing | |
| All sides — 1″ (2.54 cm) | |
| Top right-hand corner, starting with the title page | |
- When using APA in-text citations , include the author’s last name and the year of publication in parentheses.
- For direct quotations , you must also add the page number. If you use sources without page numbers, such as websites or e-books, include a paragraph number instead.
- When referring to the author’s name in a sentence , you do not need to repeat it at the end of the sentence. Instead, include the year of publication inside the parentheses after their name.
- The reference list should be included at the end of your literature review. It is always alphabetized by the last name of the author (from A to Z), and the lines are indented one-half inch from the left margin of your paper. Do not forget to invert authors’ names (the last name should come first) and include the full titles of journals instead of their abbreviations. If you use an online source, add its URL.
The RRL format in the Chicago style is as follows:
| 12-pt Times New Roman, Arial, or Palatino | |
| Double spacing, single spacing is used to format block quotations, titles of tables and figures, footnotes, and bibliographical entries. | |
| All sides — 1″ (2.54 cm) | |
| Top right-hand corner. There should be no numbered pages on the title page or the page with the table of contents. |
- Author-date . You place your citations in brackets within the text, indicating the name of the author and the year of publication.
- Notes and bibliography . You place your citations in numbered footnotes or endnotes to connect the citation back to the source in the bibliography.
- The reference list, or bibliography , in Chicago style, is at the end of a literature review. The sources are arranged alphabetically and single-spaced. Each bibliography entry begins with the author’s name and the source’s title, followed by publication information, such as the city of publication, the publisher, and the year of publication.
Writing a literature review using the MLA style requires the following text formatting:
| Font | 12-pt Times New Roman or Arial |
| Line spacing | Double spacing |
| Margins | All sides — 1″ (2.54 cm) |
| Page numbers | Top right-hand corner. Your last name should precede the page number. |
| Title page | Not required. Instead, include a header in the top left-hand corner of the first page with content. It should contain: |
- In the MLA format, you can cite a source in the text by indicating the author’s last name and the page number in parentheses at the end of the citation. If the cited information takes several pages, you need to include all the page numbers.
- The reference list in MLA style is titled “ Works Cited .” In this section, all sources used in the paper should be listed in alphabetical order. Each entry should contain the author, title of the source, title of the journal or a larger volume, other contributors, version, number, publisher, and publication date.
The Harvard style requires you to use the following text formatting for your RRL:
| 12-pt Times New Roman or Arial | |
| Double spacing | |
| All sides — 1″ (2.54 cm) | |
| Top right-hand corner. Your last name should precede the page number. |
- In-text citations in the Harvard style include the author’s last name and the year of publication. If you are using a direct quote in your literature review, you need to add the page number as well.
- Arrange your list of references alphabetically. Each entry should contain the author’s last name, their initials, the year of publication, the title of the source, and other publication information, like the journal title and issue number or the publisher.
✍️ How to Write Review of Related Literature – Sample
Literature reviews can be organized in many ways depending on what you want to achieve with them. In this section, we will look at 3 examples of how you can write your RRL.

Thematic Literature Review
A thematic literature review is arranged around central themes or issues discussed in the sources. If you have identified some recurring themes in the literature, you can divide your RRL into sections that address various aspects of the topic. For example, if you examine studies on e-learning, you can distinguish such themes as the cost-effectiveness of online learning, the technologies used, and its effectiveness compared to traditional education.
Chronological Literature Review
A chronological literature review is a way to track the development of the topic over time. If you use this method, avoid merely listing and summarizing sources in chronological order. Instead, try to analyze the trends, turning moments, and critical debates that have shaped the field’s path. Also, you can give your interpretation of how and why specific advances occurred.
Methodological Literature Review
A methodological literature review differs from the preceding ones in that it usually doesn’t focus on the sources’ content. Instead, it is concerned with the research methods . So, if your references come from several disciplines or fields employing various research techniques, you can compare the findings and conclusions of different methodologies, for instance:
- empirical vs. theoretical studies;
- qualitative vs. quantitative research.
📚 Examples of Review of Related Literature and Studies
We have prepared a short example of RRL on climate change for you to see how everything works in practice!
Climate change is one of the most important issues nowadays. Based on a variety of facts, it is now clearer than ever that humans are altering the Earth's climate. The atmosphere and oceans have warmed, causing sea level rise, a significant loss of Arctic ice, and other climate-related changes. This literature review provides a thorough summary of research on climate change, focusing on climate change fingerprints and evidence of human influence on the Earth's climate system.
Physical Mechanisms and Evidence of Human Influence
Scientists are convinced that climate change is directly influenced by the emission of greenhouse gases. They have carefully analyzed various climate data and evidence, concluding that the majority of the observed global warming over the past 50 years cannot be explained by natural factors alone. Instead, there is compelling evidence pointing to a significant contribution of human activities, primarily the emission of greenhouse gases (Walker, 2014). For example, based on simple physics calculations, doubled carbon dioxide concentration in the atmosphere can lead to a global temperature increase of approximately 1 degree Celsius. (Elderfield, 2022). In order to determine the human influence on climate, scientists still have to analyze a lot of natural changes that affect temperature, precipitation, and other components of climate on timeframes ranging from days to decades and beyond.
Fingerprinting Climate Change
Fingerprinting climate change is a useful tool to identify the causes of global warming because different factors leave unique marks on climate records. This is evident when scientists look beyond overall temperature changes and examine how warming is distributed geographically and over time (Watson, 2022). By investigating these climate patterns, scientists can obtain a more complex understanding of the connections between natural climate variability and climate variability caused by human activity.
Modeling Climate Change and Feedback
To accurately predict the consequences of feedback mechanisms, the rate of warming, and regional climate change, scientists can employ sophisticated mathematical models of the atmosphere, ocean, land, and ice (the cryosphere). These models are grounded in well-established physical laws and incorporate the latest scientific understanding of climate-related processes (Shuckburgh, 2013). Although different climate models produce slightly varying projections for future warming, they all will agree that feedback mechanisms play a significant role in amplifying the initial warming caused by greenhouse gas emissions. (Meehl, 2019).
In conclusion, the literature on global warming indicates that there are well-understood physical processes that link variations in greenhouse gas concentrations to climate change. In addition, it covers the scientific proof that the rates of these gases in the atmosphere have increased and continue to rise fast. According to the sources, the majority of this recent change is almost definitely caused by greenhouse gas emissions produced by human activities. Citizens and governments can alter their energy production methods and consumption patterns to reduce greenhouse gas emissions and, thus, the magnitude of climate change. By acting now, society can prevent the worst consequences of climate change and build a more resilient and sustainable future for generations to come.
Have you ever struggled with finding the topic for an RRL in different subjects? Read the following paragraphs to get some ideas!
Nursing Literature Review Example
Many topics in the nursing field require research. For example, you can write a review of literature related to dengue fever . Give a general overview of dengue virus infections, including its clinical symptoms, diagnosis, prevention, and therapy.
Another good idea is to review related literature and studies about teenage pregnancy . This review can describe the effectiveness of specific programs for adolescent mothers and their children and summarize recommendations for preventing early pregnancy.
📝 Check out some more valuable examples below:
- Hospital Readmissions: Literature Review .
- Literature Review: Lower Sepsis Mortality Rates .
- Breast Cancer: Literature Review .
- Sexually Transmitted Diseases: Literature Review .
- PICO for Pressure Ulcers: Literature Review .
- COVID-19 Spread Prevention: Literature Review .
- Chronic Obstructive Pulmonary Disease: Literature Review .
- Hypertension Treatment Adherence: Literature Review .
- Neonatal Sepsis Prevention: Literature Review .
- Healthcare-Associated Infections: Literature Review .
- Understaffing in Nursing: Literature Review .
Psychology Literature Review Example
If you look for an RRL topic in psychology , you can write a review of related literature about stress . Summarize scientific evidence about stress stages, side effects, types, or reduction strategies. Or you can write a review of related literature about computer game addiction . In this case, you may concentrate on the neural mechanisms underlying the internet gaming disorder, compare it to other addictions, or evaluate treatment strategies.
A review of related literature about cyberbullying is another interesting option. You can highlight the impact of cyberbullying on undergraduate students’ academic, social, and emotional development.
📝 Look at the examples that we have prepared for you to come up with some more ideas:
- Mindfulness in Counseling: A Literature Review .
- Team-Building Across Cultures: Literature Review .
- Anxiety and Decision Making: Literature Review .
- Literature Review on Depression .
- Literature Review on Narcissism .
- Effects of Depression Among Adolescents .
- Causes and Effects of Anxiety in Children .
Literature Review — Sociology Example
Sociological research poses critical questions about social structures and phenomena. For example, you can write a review of related literature about child labor , exploring cultural beliefs and social norms that normalize the exploitation of children. Or you can create a review of related literature about social media . It can investigate the impact of social media on relationships between adolescents or the role of social networks on immigrants’ acculturation .
📝 You can find some more ideas below!
- Single Mothers’ Experiences of Relationships with Their Adolescent Sons .
- Teachers and Students’ Gender-Based Interactions .
- Gender Identity: Biological Perspective and Social Cognitive Theory .
- Gender: Culturally-Prescribed Role or Biological Sex .
- The Influence of Opioid Misuse on Academic Achievement of Veteran Students .
- The Importance of Ethics in Research .
- The Role of Family and Social Network Support in Mental Health .
Education Literature Review Example
For your education studies , you can write a review of related literature about academic performance to determine factors that affect student achievement and highlight research gaps. One more idea is to create a review of related literature on study habits , considering their role in the student’s life and academic outcomes.
You can also evaluate a computerized grading system in a review of related literature to single out its advantages and barriers to implementation. Or you can complete a review of related literature on instructional materials to identify their most common types and effects on student achievement.
📝 Find some inspiration in the examples below:
- Literature Review on Online Learning Challenges From COVID-19 .
- Education, Leadership, and Management: Literature Review .
- Literature Review: Standardized Testing Bias .
- Bullying of Disabled Children in School .
- Interventions and Letter & Sound Recognition: A Literature Review .
- Social-Emotional Skills Program for Preschoolers .
- Effectiveness of Educational Leadership Management Skills .
Business Research Literature Review
If you’re a business student, you can focus on customer satisfaction in your review of related literature. Discuss specific customer satisfaction features and how it is affected by service quality and prices. You can also create a theoretical literature review about consumer buying behavior to evaluate theories that have significantly contributed to understanding how consumers make purchasing decisions.
📝 Look at the examples to get more exciting ideas:
- Leadership and Communication: Literature Review .
- Human Resource Development: Literature Review .
- Project Management. Literature Review .
- Strategic HRM: A Literature Review .
- Customer Relationship Management: Literature Review .
- Literature Review on International Financial Reporting Standards .
- Cultures of Management: Literature Review .
To conclude, a review of related literature is a significant genre of scholarly works that can be applied in various disciplines and for multiple goals. The sources examined in an RRL provide theoretical frameworks for future studies and help create original research questions and hypotheses.
When you finish your outstanding literature review, don’t forget to check whether it sounds logical and coherent. Our text-to-speech tool can help you with that!
- Literature Reviews | University of North Carolina at Chapel Hill
- Writing a Literature Review | Purdue Online Writing Lab
- Learn How to Write a Review of Literature | University of Wisconsin-Madison
- The Literature Review: A Few Tips on Conducting It | University of Toronto
- Writing a Literature Review | UC San Diego
- Conduct a Literature Review | The University of Arizona
- Methods for Literature Reviews | National Library of Medicine
- Literature Reviews: 5. Write the Review | Georgia State University
How to Write an Animal Testing Essay: Tips for Argumentative & Persuasive Papers
Descriptive essay topics: examples, outline, & more.
- Environment
- Science & Technology
- Business & Industry
- Health & Public Welfare
- Topics (CFR Indexing Terms)
- Public Inspection
- Presidential Documents
- Document Search
- Advanced Document Search
- Public Inspection Search
- Reader Aids Home
- Office of the Federal Register Announcements
- Using FederalRegister.Gov
- Understanding the Federal Register
- Recent Site Updates
- Federal Register & CFR Statistics
- Videos & Tutorials
- Developer Resources
- Government Policy and OFR Procedures
- Congressional Review
- My Clipboard
- My Comments
- My Subscriptions
- Sign In / Sign Up
- Site Feedback
- Search the Federal Register
The Federal Register
The daily journal of the united states government.
- Legal Status
This site displays a prototype of a “Web 2.0” version of the daily Federal Register. It is not an official legal edition of the Federal Register, and does not replace the official print version or the official electronic version on GPO’s govinfo.gov.
The documents posted on this site are XML renditions of published Federal Register documents. Each document posted on the site includes a link to the corresponding official PDF file on govinfo.gov. This prototype edition of the daily Federal Register on FederalRegister.gov will remain an unofficial informational resource until the Administrative Committee of the Federal Register (ACFR) issues a regulation granting it official legal status. For complete information about, and access to, our official publications and services, go to About the Federal Register on NARA's archives.gov.
The OFR/GPO partnership is committed to presenting accurate and reliable regulatory information on FederalRegister.gov with the objective of establishing the XML-based Federal Register as an ACFR-sanctioned publication in the future. While every effort has been made to ensure that the material on FederalRegister.gov is accurately displayed, consistent with the official SGML-based PDF version on govinfo.gov, those relying on it for legal research should verify their results against an official edition of the Federal Register. Until the ACFR grants it official status, the XML rendition of the daily Federal Register on FederalRegister.gov does not provide legal notice to the public or judicial notice to the courts.
Proposed Rule
Medicare program; end-stage renal disease prospective payment system, payment for renal dialysis services furnished to individuals with acute kidney injury, conditions for coverage for end-stage renal disease facilities, end-stage renal disease quality incentive program, and end-stage renal disease treatment choices model.
A Proposed Rule by the Centers for Medicare & Medicaid Services on 07/05/2024
This document has a comment period that ends in 50 days. (08/26/2024) Submit a formal comment
Thank you for taking the time to create a comment. Your input is important.
Once you have filled in the required fields below you can preview and/or submit your comment to the Health and Human Services Department for review. All comments are considered public and will be posted online once the Health and Human Services Department has reviewed them.
You can view alternative ways to comment or you may also comment via Regulations.gov at https://www.regulations.gov/commenton/CMS-2024-0189-0002 .
- What is your comment about?
Note: You can attach your comment as a file and/or attach supporting documents to your comment. Attachment Requirements .
this will NOT be posted on regulations.gov
- Opt to receive email confirmation of submission and tracking number?
- Tell us about yourself! I am... *
- First Name *
- Last Name *
- State Alabama Alaska American Samoa Arizona Arkansas California Colorado Connecticut Delaware District of Columbia Florida Georgia Guam Hawaii Idaho Illinois Indiana Iowa Kansas Kentucky Louisiana Maine Maryland Massachusetts Michigan Minnesota Mississippi Missouri Montana Nebraska Nevada New Hampshire New Jersey New Mexico New York North Carolina North Dakota Ohio Oklahoma Oregon Pennsylvania Puerto Rico Rhode Island South Carolina South Dakota Tennessee Texas Utah Vermont Virgin Islands Virginia Washington West Virginia Wisconsin Wyoming
- Country Afghanistan Åland Islands Albania Algeria American Samoa Andorra Angola Anguilla Antarctica Antigua and Barbuda Argentina Armenia Aruba Australia Austria Azerbaijan Bahamas Bahrain Bangladesh Barbados Belarus Belgium Belize Benin Bermuda Bhutan Bolivia, Plurinational State of Bonaire, Sint Eustatius and Saba Bosnia and Herzegovina Botswana Bouvet Island Brazil British Indian Ocean Territory Brunei Darussalam Bulgaria Burkina Faso Burundi Cambodia Cameroon Canada Cape Verde Cayman Islands Central African Republic Chad Chile China Christmas Island Cocos (Keeling) Islands Colombia Comoros Congo Congo, the Democratic Republic of the Cook Islands Costa Rica Côte d'Ivoire Croatia Cuba Curaçao Cyprus Czech Republic Denmark Djibouti Dominica Dominican Republic Ecuador Egypt El Salvador Equatorial Guinea Eritrea Estonia Ethiopia Falkland Islands (Malvinas) Faroe Islands Fiji Finland France French Guiana French Polynesia French Southern Territories Gabon Gambia Georgia Germany Ghana Gibraltar Greece Greenland Grenada Guadeloupe Guam Guatemala Guernsey Guinea Guinea-Bissau Guyana Haiti Heard Island and McDonald Islands Holy See (Vatican City State) Honduras Hong Kong Hungary Iceland India Indonesia Iran, Islamic Republic of Iraq Ireland Isle of Man Israel Italy Jamaica Japan Jersey Jordan Kazakhstan Kenya Kiribati Korea, Democratic People's Republic of Korea, Republic of Kuwait Kyrgyzstan Lao People's Democratic Republic Latvia Lebanon Lesotho Liberia Libya Liechtenstein Lithuania Luxembourg Macao Macedonia, the Former Yugoslav Republic of Madagascar Malawi Malaysia Maldives Mali Malta Marshall Islands Martinique Mauritania Mauritius Mayotte Mexico Micronesia, Federated States of Moldova, Republic of Monaco Mongolia Montenegro Montserrat Morocco Mozambique Myanmar Namibia Nauru Nepal Netherlands New Caledonia New Zealand Nicaragua Niger Nigeria Niue Norfolk Island Northern Mariana Islands Norway Oman Pakistan Palau Palestine, State of Panama Papua New Guinea Paraguay Peru Philippines Pitcairn Poland Portugal Puerto Rico Qatar Réunion Romania Russian Federation Rwanda Saint Barthélemy Saint Helena, Ascension and Tristan da Cunha Saint Kitts and Nevis Saint Lucia Saint Martin (French part) Saint Pierre and Miquelon Saint Vincent and the Grenadines Samoa San Marino Sao Tome and Principe Saudi Arabia Senegal Serbia Seychelles Sierra Leone Singapore Sint Maarten (Dutch part) Slovakia Slovenia Solomon Islands Somalia South Africa South Georgia and the South Sandwich Islands South Sudan Spain Sri Lanka Sudan Suriname Svalbard and Jan Mayen Swaziland Sweden Switzerland Syrian Arab Republic Taiwan, Province of China Tajikistan Tanzania, United Republic of Thailand Timor-Leste Togo Tokelau Tonga Trinidad and Tobago Tunisia Turkey Turkmenistan Turks and Caicos Islands Tuvalu Uganda Ukraine United Arab Emirates United Kingdom United States United States Minor Outlying Islands Uruguay Uzbekistan Vanuatu Venezuela, Bolivarian Republic of Viet Nam Virgin Islands, British Virgin Islands, U.S. Wallis and Futuna Western Sahara Yemen Zambia Zimbabwe
- Organization Type * Company Organization Federal State Local Tribal Regional Foreign U.S. House of Representatives U.S. Senate
- Organization Name *
- You are filing a document into an official docket. Any personal information included in your comment text and/or uploaded attachment(s) may be publicly viewable on the web.
- I read and understand the statement above.
- Preview Comment
Document Details
Information about this document as published in the Federal Register .
Document Statistics
Enhanced content.
Relevant information about this document from Regulations.gov provides additional context. This information is not part of the official Federal Register document.
Published Document
This document has been published in the Federal Register . Use the PDF linked in the document sidebar for the official electronic format.
Enhanced Content - Table of Contents
This table of contents is a navigational tool, processed from the headings within the legal text of Federal Register documents. This repetition of headings to form internal navigation links has no substantive legal effect.
FOR FURTHER INFORMATION CONTACT:
Supplementary information:, table of contents, i. executive summary, 1. end-stage renal disease (esrd) prospective payment system (pps), 2. coverage and payment for renal dialysis services furnished to individuals with acute kidney injury (aki), 3. end-stage renal disease quality incentive program (esrd qip), 4. end-stage renal disease treatment choices (etc) model, b. summary of the major provisions, 1. esrd pps, 2. payment for renal dialysis services furnished to individuals with aki, 3. esrd qip, 4. etc model, c. summary of costs and benefits, 1. impacts of the proposed esrd pps, 2. impacts of the proposed payment rate for renal dialysis services furnished to individuals with aki, 3. impacts of the py 2027 esrd qip as proposed, 4. impacts of the proposed changes to the etc model, ii. calendar year (cy) 2025 end-stage renal disease (esrd) prospective payment system (pps), a. background, 1. statutory background, 2. system for payment of renal dialysis services, 3. updates to the esrd pps, b. proposed provisions of the cy 2025 esrd pps, 1. proposed cy 2025 esrd bundled (esrdb) market basket percentage increase; productivity adjustment; and labor-related share, a. background, b. proposed cy 2025 esrd market basket update, (1) proposed cy 2025 market basket percentage increase, (2) productivity adjustment, (3) cy 2025 market basket update, (4) labor-related share, 2. proposed cy 2025 esrd pps wage indices, b. proposed methodology changes for the cy 2025 esrd pps wage index, (1) december 2019 technical expert panel (tep), (2) proposed new methodology for using bls data to calculate the esrd pps wage index, (a) description of proposed data sources, (i) data from the bls oews metropolitan and nonmetropolitan area occupational employment and wage estimates, (ii) data from freestanding esrd facility cost reports, (iii) ipps hospital wage index, (iv) time lag associated with proposed new data sources, (v) comparison between proposed new methodology data sources and hospital data, (b) construction of the proposed new esrd pps wage index, (c) methodological alternatives considered, c. example calculation using the proposed new wage index methodology, d. estimated impacts of proposed change to wage index methodology, e. proposed cy 2025 esrd pps wage index, (1) alternative cy 2025 esrd pps wage index using established methodology, (2) request for comments on this proposal, f. proposed implementation of new omb labor market delineations, (1) background, (2) proposal to phase out the rural facility adjustment for facilities affected by changes to cbsas, 3. proposed cy 2025 update to the outlier policy, b. proposed expansion of esrd outlier services, (1) background and current issues, (2) proposed definition of esrd outlier services, c. proposed changes to predicted map calculation for outlier eligibility, d. proposed technical modifications to the inflation factors used for the outlier calculations, (2) proposed changes to the inflation factor for outlier eligible drugs and biological products, (3) proposed changes to the inflation factors for outlier eligible laboratory tests and supplies, e. cy 2025 update to the outlier services map amounts and fdl amounts, f. outlier percentage, 4. proposed impacts to the cy 2025 esrd pps base rate, a. esrd pps base rate, b. proposed annual payment rate update for cy 2025, 5. proposed update to the average per treatment offset amount for home dialysis machines, 6. proposed updates to the post-tdapa add-on payment adjustment amounts, a. proposal to publish post-tdapa add-on payment adjustment amounts after the final rule in certain circumstances, 7. inclusion of oral-only drugs into the esrd pps bundled payment, b. current policy for oral-only drugs in cy 2025, c. operational considerations related to the incorporation of oral-only drugs, d. expected impact of incorporation of oral-only drugs, 8. proposed changes to the low-volume payment adjustment (lvpa), a. background on the lvpa, (1) current issues and concerns, (2) cy 2024 rfi on potential changes to the lvpa, (3) cy 2024 rfi on the rural facility adjustment, b. proposed tiered lvpa methodology, c. proposed changes to the lvpa for cy 2025, d. rfi on improving the lvpa for new esrd facilities, c. transitional add-on payment adjustment for new and innovative equipment and supplies (tpnies) applications and proposed technical change for cy 2025, 1. background, 2. proposed technical change to § 413.236(b)(4), d. continuation of approved transitional add-on payment adjustments for new and innovative equipment and supplies for cy 2025, e. continuation of approved transitional drug add-on payment adjustments for cy 2025, iii. proposed cy 2025 payment for renal dialysis services furnished to individuals with aki, b. proposal to allow medicare payment for home dialysis for beneficiaries with aki, 2. technical analysis, 3. proposal to extend home dialysis benefit to beneficiaries with aki, c. proposed annual payment rate update for cy 2025, 1. cy 2025 aki dialysis payment rate, 2. geographic adjustment factor, 3. other adjustments to the aki payment rate, d. aki and the esrd facility conditions for coverage, 1. statutory and regulatory background, 2. aki and home dialysis, 3. proposed changes, 4. expected impact, iv. proposed updates to the end-stage renal disease quality incentive program (esrd qip), b. proposed updates to requirements beginning with the py 2027 esrd qip, 1. py 2027 esrd qip measure set, 2. proposal to replace the kt/v dialysis adequacy comprehensive clinical measure with a kt/v dialysis adequacy measure topic beginning with the py 2027 esrd qip, 3. proposal to remove the nhsn dialysis event reporting measure from the esrd qip measure set beginning with py 2027, 4. proposed revisions to the clinical care and reporting measure domains beginning with the py 2027 esrd qip, 5. performance standards for the py 2027 esrd qip, 6. eligibility requirements for the py 2027 esrd qip, 7. payment reduction scale for the py 2027 esrd qip, c. requests for information (rfis) on topics relevant to esrd qip, 1. request for public comment on future change to the scoring methodology to add a new adjustment that rewards facilities based on their performance and the proportion of their patients who are dually eligible for medicare and medicaid, 2. request for public comment on updating the data validation policy for the esrd qip, v. end-stage renal disease treatment choices (etc) model, b. provisions of the proposed rule, c. request for information, 1. request for information, 2. exemption of the rfi from the paperwork reduction act implementing regulations, vi. collection of information requirements, a. esrd qip—wage estimates (omb control numbers 0938-1289 and 0938-1340), b. estimated burden associated with the data validation requirements for py 2027 (omb control numbers 0938-1289 and 0938-1340), c. estimated eqrs reporting requirements for py 2027 (omb control number 0938-1289), d. esrd treatment choices model, vii. response to comments, viii. regulatory impact analysis, a. statement of need, b. overall impact, c. impact analysis, 5. summary of impacts, d. detailed economic analysis, 1. benefits, a. esrd pps and aki, b. esrd qip, 3. transfers, 4. regulatory review cost estimation, 5. impact statement and table, a. cy 2025 end-stage renal disease prospective payment system, (1) effects on esrd facilities, (2) effects on other providers, (3) effects on the medicare program, (4) effects on medicare beneficiaries, (5) alternatives considered, (a) proposed wage index changes, (b) expansion of outlier eligibility, (c) tdapa for phosphate binders, (d) proposed changes to the lvpa, b. continuation of approved transitional drug add-on payment adjustments (tdapa) for new renal dialysis drugs or biological products for cy 2025, (1) jesduvroq (daprodustat), (2) defencath® (taurolidine and heparin sodium), c. payment for renal dialysis services furnished to individuals with aki, d. esrd qip, (1) effects of the py 2027 esrd qip on esrd facilities, (2) effects on the medicare program, (3) effects on medicare beneficiaries, (4) alternatives considered, e. etc model, (1) overview, (2) data and methods, (3) medicare estimate—primary specification, assume rolling benchmark, (4) effects on the home dialysis rate, the transplant rate, and kidney transplantation, (5) effects on kidney disease patient education services and hd training add-ons, (6) effects on medicare beneficiaries, (7) alternatives considered, e. accounting statement, f. regulatory flexibility act analysis (rfa), g. unfunded mandates reform act analysis (umra), h. federalism, ix. files available to the public, list of subjects, 42 cfr part 410, 42 cfr part 413, 42 cfr part 494, 42 cfr part 512, part 410—supplementary medical insurance (smi) benefits, part 413—principles of reasonable cost reimbursement; payment for end-stage renal disease services; prospectively determined payment rates for skilled nursing facilities; payment for acute kidney injury dialysis, part 494—conditions for coverage for end-stage renal disease facilities, part 512—radiation oncology model and end stage renal disease treatment choices model, enhanced content - submit public comment.
- Submit a public comment on this document
Enhanced Content - Read Public Comments
- This feature is not available for this document.
Enhanced Content - Sharing
- Email this document to a friend
Enhanced Content - Document Print View
- Print this document
Enhanced Content - Document Tools
These tools are designed to help you understand the official document better and aid in comparing the online edition to the print edition.
These markup elements allow the user to see how the document follows the Document Drafting Handbook that agencies use to create their documents. These can be useful for better understanding how a document is structured but are not part of the published document itself.
Enhanced Content - Developer Tools
This document is available in the following developer friendly formats:.
- JSON: Normalized attributes and metadata
- XML: Original full text XML
- MODS: Government Publishing Office metadata
More information and documentation can be found in our developer tools pages .
Official Content
- View printed version (PDF)
This PDF is the current document as it appeared on Public Inspection on 06/27/2024 at 4:15 pm. It was viewed 0 times while on Public Inspection.
If you are using public inspection listings for legal research, you should verify the contents of the documents against a final, official edition of the Federal Register. Only official editions of the Federal Register provide legal notice of publication to the public and judicial notice to the courts under 44 U.S.C. 1503 & 1507 . Learn more here .
Centers for Medicare & Medicaid Services (CMS), Department of Health and Human Services (HHS).
Proposed rule.
This proposed rule would update and revise the End-Stage Renal Disease (ESRD) Prospective Payment System for calendar year 2025. This rule also proposes to update the payment rate for renal dialysis services furnished by an ESRD facility to individuals with acute kidney injury. In addition, this proposed rule would update requirements for the Conditions for Coverage for ESRD Facilities, ESRD Quality Incentive Program, and ESRD Treatment Choices Model.
To be assured consideration, comments must be received at one of the addresses provided below, by August 26, 2024.
In commenting, please refer to file code CMS-1805-P.
Comments, including mass comment submissions, must be submitted in one of the following three ways (please choose only one of the ways listed):
1. Electronically. You may submit electronic comments on this regulation to https://www.regulations.gov . Follow the “Submit a comment” instructions.
2. By regular mail. You may mail written comments to the following address ONLY: Centers for Medicare & Medicaid Services, Department of Health and Human Services, Attention: CMS-1805-P, P.O. Box 8010, Baltimore, MD 21244-8010.
Please allow sufficient time for mailed comments to be received before the close of the comment period.
3. By express or overnight mail. You may send written comments to the following address ONLY: Centers for Medicare & Medicaid Services, Department of Health and Human Services, Attention: CMS-1805-P, Mail Stop C4-26-05, 7500 Security Boulevard, Baltimore, MD 21244-1850.
For information on viewing public comments, see the beginning of the SUPPLEMENTARY INFORMATION section.
[email protected] or Nicolas Brock at (410) 786-5148, for issues related to the ESRD Prospective Payment System (PPS) and coverage and payment for renal dialysis services furnished to individuals with acute kidney injury (AKI).
[email protected] , for issues related to applications for the Transitional Drug Add-on Payment Adjustment (TDAPA) or Transitional Add-On Payment Adjustment for New and Innovative Equipment and Supplies (TPNIES).
[email protected] , for issues related to the ESRD Quality Incentive Program (QIP).
[email protected] , for issues related to the ESRD Treatment Choices (ETC) Model.
Inspection of Public Comments: All comments received before the close of the comment period are available for viewing by the public, including any personally identifiable or confidential business information that is included in a comment. We post all comments received before the close of the comment period on the following website as soon as possible after they have been received: https://www.regulations.gov . Follow the search instructions on that website to view public comments. CMS will not post on Regulations.gov public comments that make threats to individuals or institutions or suggest that the commenter will take actions to harm an individual. CMS continues to encourage individuals not to submit duplicative comments. We will post acceptable comments from multiple unique commenters even if the content is identical or nearly identical to other comments.
Plain Language Summary: In accordance with 5 U.S.C. 553(b)(4) , a plain language summary of this rule may be found at https://www.regulations.gov/ .
Current Procedural Terminology (CPT) Copyright Notice: Throughout this proposed rule, we use CPT® codes and descriptions to refer to a variety of services. We note that CPT® codes and descriptions are copyright 2020 American Medical Association (AMA). All Rights Reserved. CPT® is a registered trademark of the AMA. Applicable Federal Acquisition Regulations (FAR) and Defense Federal Acquisition Regulations (DFAR) apply.
To assist readers in referencing sections contained in this preamble, we are providing a Table of Contents.
C. Summary of Cost and Benefits
C. Transitional Add-On Payment Adjustment for New and Innovative Equipment and Supplies (TPNIES) Applications and Proposed Technical Change for CY 2025 Payment
B. Proposal to Allow Medicare Payment for Home Dialysis for Beneficiaries With AKI
IX. Files Available to the Public via the Internet
This rule proposes changes related to the End-Stage Renal Disease (ESRD) Prospective Payment System (PPS), Start Printed Page 55761 payment for renal dialysis services furnished to individuals with acute kidney injury (AKI), the Conditions for Coverage for ESRD facilities, the ESRD Quality Incentive Program (QIP), and the ESRD Treatment Choices (ETC) Model. Additionally, this rule proposes and discusses policies that reflect our commitment to achieving equity in health care for our beneficiaries by supporting our ability to assess whether, and to what extent, our programs and policies perpetuate or exacerbate systemic barriers to opportunities and benefits for underserved communities. For example, we are proposing to expand access to home dialysis for patients with acute kidney injury, which would assist this vulnerable population with transportation and scheduling issues and allow them to have flexibility in their dialysis treatment modality. Additionally, we discuss the incorporation of oral-only drugs into the ESRD PPS bundled payment beginning January 1, 2025, which will expand access to the 21 percent of the ESRD PPS population who do not have Part D coverage. Our internal data show that a significant portion of ESRD beneficiaries who lack Part D coverage are African American/Black patients with ESRD. Our policy objectives include a commitment to advancing health equity, which stands as the first pillar of the Centers for Medicare & Medicaid Services (CMS) Strategic Plan, [ 1 ] and reflect the goals of the Administration, as stated in the President's Executive Order 13985 . [ 2 ] We define health equity as the attainment of the highest level of health for all people, where everyone has a fair and just opportunity to attain their optimal health regardless of race, ethnicity, disability, sexual orientation, gender identity, socioeconomic status, geography, preferred language, or other factors that affect access to care and health outcomes.” [ 3 ] In the calendar year (CY) 2023 ESRD PPS final rule, we noted that, when compared with all Medicare fee-for-service (FFS) beneficiaries, Medicare FFS beneficiaries receiving dialysis are disproportionately young, male, African American, have disabilities and low income as measured by eligibility for both Medicare and Medicaid (dual eligible status), and reside in an urban setting ( 87 FR 67183 ). In this proposed rule, we continue to address health equity for beneficiaries with ESRD who are members of underserved communities, including but not limited to those living in rural communities, those who have disabilities, and racial, and ethnic minorities and sovereign American Indian and Alaska Native tribes. The term `underserved communities' refers to populations sharing a particular characteristic, including geographic communities, that have been systematically denied a full opportunity to participate in aspects of economic, social, and civic life. [ 4 ]
On January 1, 2011, we implemented the ESRD PPS, a case-mix adjusted, bundled PPS for renal dialysis services furnished by ESRD facilities as required by section 1881(b)(14) of the Social Security Act (the Act), as added by section 153(b) of the Medicare Improvements for Patients and Providers Act of 2008 (MIPPA) ( Pub. L. 110-275 ). Section 1881(b)(14)(F) of the Act, as added by section 153(b) of MIPPA, and amended by section 3401(h) of the Patient Protection and Affordable Care Act (the Affordable Care Act) ( Pub. L. 111-148 ), established that beginning CY 2012, and each subsequent year, the Secretary of the Department of Health and Human Services (the Secretary) shall annually increase payment amounts by an ESRD market basket percentage increase, reduced by the productivity adjustment described in section 1886(b)(3)(B)(xi)(II) of the Act. This rule proposes updates to the ESRD PPS for CY 2025.
On June 29, 2015, the President signed the Trade Preferences Extension Act of 2015 (TPEA) ( Pub. L. 114-27 ). Section 808(a) of the TPEA amended section 1861(s)(2)(F) of the Act to provide coverage for renal dialysis services furnished on or after January 1, 2017, by a renal dialysis facility or a provider of services paid under section 1881(b)(14) of the Act to an individual with AKI. Section 808(b) of the TPEA amended section 1834 of the Act by adding a new subsection (r) that provides for payment for renal dialysis services furnished by renal dialysis facilities or providers of services paid under section 1881(b)(14) of the Act to individuals with AKI at the ESRD PPS base rate beginning January 1, 2017. This proposed rule would update the AKI payment rate for CY 2025. Additionally, this rule proposes to extend payment for home dialysis and the payment adjustment for home and self-dialysis training to renal dialysis services provided to beneficiaries with AKI.
The End-Stage Renal Disease Quality Incentive Program (ESRD QIP) is authorized by section 1881(h) of the Act. The Program establishes incentives for facilities to achieve high quality performance on measures with the goal of improving outcomes for ESRD beneficiaries. This rule proposes to replace the Kt/V Dialysis Adequacy Comprehensive clinical measure with a Kt/V Dialysis Adequacy measure topic and to remove National Healthcare Safety Network (NHSN) Dialysis Event reporting measure beginning with Payment Year (PY) 2027. This rule also requests public comment on two topics relevant to the ESRD QIP.
The ETC Model is a mandatory Medicare payment model tested under section 1115A of the Act. The ETC Model is operated by the Center for Medicare and Medicaid Innovation (Innovation Center). The ETC Model tests the use of payment adjustments to encourage greater utilization of home dialysis and kidney transplants, to preserve or enhance the quality of care furnished to Medicare beneficiaries while reducing Medicare expenditures. The ETC Model was finalized as part of a final rule published in the Federal Register on September 29, 2020, titled “Medicare Program: Specialty Care Models to Improve Quality of Care and Reduce Expenditures” ( 85 FR 61114 ), referred to herein as the “Specialty Care Models final rule.” Subsequently, the ETC Model has been updated three times in the annual ESRD PPS final rules for calendar year (CY) 2022 ( 86 FR 61874 ), CY 2023 ( 87 FR 67136 ), and CY 2024 ( 88 FR 76344 ).
This proposed rule would make certain changes to the methodology CMS uses to identify transplant failure for the purposes of defining an ESRD beneficiary and attributing an ESRD beneficiary to the ETC Model. We are also soliciting input from the public Start Printed Page 55762 through a Request for Information (RFI) on topics pertaining to increasing equitable access to home dialysis and kidney transplantation. Feedback we receive from the public will be used to inform CMS' thinking regarding opportunities and barriers the Innovation Center may address in potential successor models to the ETC Model.
- Proposed update to the ESRD PPS base rate for CY 2025: The proposed CY 2025 ESRD PPS base rate is $273.20, an increase from the CY 2024 ESRD PPS base rate of $271.02. This proposed amount reflects the application of the wage index budget-neutrality adjustment factor (0.990228) and a productivity-adjusted market basket percentage increase of 1.8 percent as required by section 1881(b)(14)(F)(i)(I) of the Act, equaling $273.20 (($271.02 × 0.990228) × 1.018 = $273.20).
- Proposed modification to the wage index methodology: We are proposing a new ESRD-specific wage index that would be used to adjust ESRD PPS payment for geographic differences in area wages on an annual basis. For CY 2025, we are proposing to change our methodology to use mean hourly wage data from the Bureau of Labor Statistics (BLS) Occupation Employment and Wage Statistics (OEWS) program and full time equivalent (FTE) labor and treatment volume data from freestanding ESRD facility Medicare cost reports to produce an ESRD-specific wage index for use, instead of using the hospital wage index values for each geographic area, which are derived from hospital cost report data. Additionally, we are proposing to update the wage index to reflect the latest core-based statistical area (CBSA) delineations determined by the Office of Management and Budget (OMB) to better account for differing wage levels in areas in which ESRD facilities are located.
- Proposed annual update to the wage index: For CY 2025, we are proposing to update the wage index using the proposed new methodology previously discussed based on the latest available data. This is consistent with our past approach to updating the ESRD PPS wage index but would use the proposed new wage index methodology based on data from BLS and freestanding ESRD facility Medicare cost reports.
- Proposed modification to the outlier policy: We are proposing to revise the outlier policy in several ways. For the outlier payment methodology, we are proposing to use a drug inflation factor based on actual spending on drugs and biological products rather than the growth in the price proxy for drugs used in the ESRD Bundled (ESRDB) market basket. We are also proposing to use the growth in the ESRDB market basket price proxies for laboratory tests and supplies to estimate CY 2025 outlier spending for these items. Additionally, we are proposing to account for the post-TDAPA add-on payment adjustment amount for outlier-eligible drugs and biological products during the post-TDAPA period. Lastly, we are proposing to expand the list of eligible ESRD outlier services to include drugs and biological products that were or would have been included in the composite rate prior to establishment of the ESRD PPS.
- Proposed annual update to the outlier policy: We are proposing to update the outlier policy based on the most current data and the proposed methodology changes previously discussed. Accordingly, we are proposing to update the Medicare allowable payment (MAP) amounts for adult and pediatric patients for CY 2025 using the latest available CY 2023 claims data. We are proposing to update the ESRD outlier services fixed dollar loss (FDL) amount for pediatric patients using the latest available CY 2023 claims data and update the FDL amount for adult patients using the latest available claims data from CY 2021, CY 2022, and CY 2023. For pediatric beneficiaries, the proposed FDL amount would increase from $11.32 to $223.44, and the MAP amount would increase from $23.36 to $58.39, as compared to CY 2024 values. For adult beneficiaries, the proposed FDL amount would decrease from $71.76 to $49.46, and the MAP amount would decrease from $36.28 to $33.57. We note that the proposed inclusion of composite rate drugs and biological products would cause a significant increase in the proposed FDL and MAP amounts for pediatric patients due to high-cost composite rate drugs furnished to pediatric beneficiaries; this is discussed in further detail in section II.B.3.e of this proposed rule. The 1.0 percent target for outlier payments was achieved in CY 2023, as outlier payments represented approximately 1.0 percent of total Medicare payments.
- Proposed update to the offset amount for the transitional add-on payment adjustment for new and innovative equipment and supplies (TPNIES) for CY 2025: The proposed CY 2025 average per treatment offset amount for the TPNIES for capital-related assets that are home dialysis machines is $10.18. This proposed offset amount reflects the application of the proposed ESRDB productivity-adjusted market basket update of 1.8 percent ($10.00 × 1.018 = $10.18). There are no capital-related assets set to receive the TPNIES in CY 2025 for which this offset would apply.
- Proposed update to the Post-TDAPA Add-on Payment Adjustment amounts: We calculate the post-TDAPA add-on payment adjustment in accordance with § 413.234(g). The proposed post-TDAPA add-on payment amount for Korsuva® is $0.4047 per treatment, which would be included in the calculation of the total post-TDAPA add-on payment adjustment for each quarter in CY 2025. The proposed post-TDAPA add-on payment adjustment amount for Jesduvroq is $0.0019 per treatment, which would be included in the calculation for only the fourth quarter of CY 2025. We are proposing to update these post-TDAPA add-on payment adjustment amounts according to the most recent data for the final rule. We are proposing to publish the final post-TDAPA add-on payment adjustment amount for drugs and biological products that do not have a full year of utilization data at the time of rulemaking after the publication of the final rule through a Change Request (CR). For CY 2025, this would be the case for Jesduvroq.
- Proposed update to the Low-Volume Payment Adjustment (LVPA): We are proposing to modify the LVPA policy to create a two-tiered LVPA whereby ESRD facilities that furnished fewer than 3,000 treatments per cost reporting year would receive a 28.3 percent upward adjustment to the ESRD PPS base rate and ESRD facilities that furnished 3,000 to 3,999 treatments would receive an 18.0 percent adjustment. We are also proposing that the tier determination would be based on the median treatment count over the past three cost reporting years.
- Inclusion of oral-only drugs in the ESRD PPS bundled payment: Under 42 CFR 413.174(f)(6) , payment to an ESRD facility for oral-only renal dialysis service drugs and biological products is included in the ESRD PPS bundled payment effective January 1, 2025. In this proposed rule, we are providing information about how we will operationalize the inclusion of oral-only drugs into the ESRD PPS as well as budgetary estimates of the effects of this inclusion for public awareness. Start Printed Page 55763
- Proposed update to the payment rate for individuals with AKI: We are proposing to update the AKI payment rate for CY 2025. The proposed CY 2025 payment rate is $273.20, which is the same as the base rate proposed for the ESRD PPS for CY 2025.
- Proposed payment for home dialysis for beneficiaries with AKI: We are proposing to allow Medicare payment for beneficiaries with AKI to dialyze at home. Payment for home dialysis treatments furnished to beneficiaries with AKI would be made at the same payment rate as in-center dialysis treatments. We are proposing to permit ESRD facilities to bill Medicare for the home and self-dialysis training add-on payment adjustment for beneficiaries with AKI, and to implement this adjustment in a budget neutral manner. We are proposing changes to the ESRD facility conditions for coverage (CfCs) to implement this policy change.
Beginning with PY 2027, we are proposing to replace the Kt/V Dialysis Adequacy Comprehensive clinical measure, on which facility performance is scored on a single measure based on one set of performance standards, with a Kt/V Dialysis Adequacy measure topic, which would be comprised of four individual Kt/V measures and scored based on a separate set of performance standards for each of those measures. We are also proposing to remove the National Healthcare Safety Network (NHSN) Dialysis Event reporting measure from the ESRD QIP measure set beginning with PY 2027. We are requesting public comment on a potential health equity payment adjustment and are also requesting public comment on potential future updates to the data validation policy.
We are proposing a modification to the methodology used to attribute ESRD Beneficiaries to the ETC Model, specifically, to the definition of an ESRD Beneficiary at 42 CFR 512.310 . Under the ETC Model, CMS attributes ESRD beneficiaries to the ETC Model that meet several criteria including having a kidney transplant failure less than 12 months after the transplant date. We are proposing to refine the methodology we use identify ESRD Beneficiaries with a kidney transplant failure to reduce the likelihood that CMS is overestimating the true number of transplant failures for the purposes of the model. We provide more detail on the proposal and its rationale in section V.B of this proposed rule.
We are also seeking input from the public through a RFI on the future of the ETC Model, potential successor Models and other approaches CMS may consider to support beneficiary access to patient-centered modalities for treatment of ESRD.
In section VIII.D.5 of this proposed rule, we set forth a detailed analysis of the impacts that the proposed changes would have on affected entities and beneficiaries. The impacts include the following:
The impact table in section VIII.D.5.a of this proposed rule displays the estimated change in Medicare payments to ESRD facilities in CY 2025 compared to estimated Medicare payments in CY 2024. The overall impact of the CY 2025 payment changes is projected to be a 2.2 percent increase in Medicare payments. Hospital-based ESRD facilities have an estimated 3.9 percent increase in Medicare payments compared with freestanding ESRD facilities with an estimated 2.1 percent increase. We estimate that the aggregate ESRD PPS expenditures would increase by approximately $170 million in CY 2025 compared to CY 2024 as a result of the proposed payment policies in this rule. Because of the projected 2.2 percent overall payment increase, we estimate there would be an increase in beneficiary coinsurance payments of 2.2 percent in CY 2025, which translates to approximately $30 million.
Section 1881(b)(14)(D)(iv) of the Act provides that the ESRD PPS may include such other payment adjustments as the Secretary determines appropriate. Under this authority, CMS implemented § 413.234 to establish the TDAPA, a transitional drug add-on payment adjustment for certain new renal dialysis drugs and biological products and § 413.236 to establish the TPNIES, a transitional add-on payment adjustment for certain new and innovative equipment and supplies. The TDAPA and the TPNIES are not budget neutral.
As discussed in section II.D of this proposed rule, since no new items were approved for the TPNIES for CY 2024 ( 88 FR 76431 ) there are no continuing TPNIES payments for CY 2025. In addition, since we did not receive any applications for the TPNIES for CY 2025, there would be no new TPNIES payments for CY 2025. As discussed in section II.E of this proposed rule, the TDAPA payment periods for Jesduvroq and DefenCath®, would continue into CY 2025. As described in section VIII.D.5.b of this proposed rule, we estimate that the TDAPA payment amounts in CY 2025 would be approximately $207,675, of which, $41,535 would be attributed to beneficiary coinsurance amounts.
The impact table in section VIII.D.5.c of this proposed rule displays the estimated change in Medicare payments to ESRD facilities for renal dialysis services furnished to individuals with AKI compared to estimated Medicare payments for such services in CY 2024. The overall impact of the CY 2025 changes is projected to be a 1.9 percent increase in Medicare payments for individuals with AKI. Hospital-based ESRD facilities would have an estimated 2.6 percent increase in Medicare payments compared with freestanding ESRD facilities that would have an estimated 1.9 percent increase. The overall impact reflects the effects of the proposed Medicare payment rate update and the proposed CY 2025 ESRD PPS wage index, as well as the proposed policy to extend payment for AKI dialysis at home, which is not expected to have any impact on payment rates. As discussed in section III.C.3, we are proposing to extend the ESRD PPS home and self-dialysis training add-on adjustment to AKI patients; however, that adjustment is required to be implemented in a budget neutral manner for AKI payments, so it would not have any impact on the overall payment amounts for AKI renal dialysis services and therefore is not included in these estimates. We estimate that the aggregate Medicare payments made to ESRD facilities for renal dialysis services furnished to individuals with AKI, at the proposed CY 2025 ESRD PPS base rate, would increase by $1 million in CY 2025 compared to CY 2024.
We estimate that, as a result of previously finalized policies and changes to the ESRD QIP that we are proposing in this proposed rule, the overall economic impact of the PY 2027 ESRD QIP will be approximately $145.1 million. The $145.1 million estimate for PY 2027 includes $130.5 million in costs associated with the collection of information requirements and approximately $14.6 million in payment reductions across all facilities. Start Printed Page 55764
The proposed change to the definition of an ESRD Beneficiary for the purposes of attribution in the ETC Model is not expected to have a net effect on the model's projected economic impact.
On January 1, 2011, CMS implemented the ESRD PPS, a case-mix adjusted bundled PPS for renal dialysis services furnished by ESRD facilities, as required by section 1881(b)(14) of the Act, as added by section 153(b) of the Medicare Improvements for Patients and Providers Act of 2008 (MIPPA) ( Pub. L. 110-275 ). Section 1881(b)(14)(F) of the Act, as added by section 153(b) of MIPPA and amended by section 3401(h) of the Patient Protection and Affordable Care Act (Affordable Care Act) ( Pub. L. 111-148 ), established that beginning with CY 2012, and each subsequent year, the Secretary shall annually increase payment amounts by an ESRD market basket percentage increase reduced by the productivity adjustment described in section 1886(b)(3)(B)(xi)(II) of the Act.
Section 632 of the American Taxpayer Relief Act of 2012 (ATRA) ( Pub. L. 112-240 ) included several provisions that apply to the ESRD PPS. Section 632(a) of ATRA added section 1881(b)(14)(I) to the Act, which required the Secretary, by comparing per patient utilization data from 2007 with such data from 2012, to reduce the single payment for renal dialysis services furnished on or after January 1, 2014, to reflect the Secretary's estimate of the change in the utilization of ESRD-related drugs and biologicals [ 5 ] (excluding oral-only ESRD-related drugs). Consistent with this requirement, in the CY 2014 ESRD PPS final rule, we finalized $29.93 as the total drug utilization reduction and finalized a policy to implement the amount over a 3- to 4-year transition period ( 78 FR 72161 through 72170 ).
Section 632(b) of ATRA prohibited the Secretary from paying for oral-only ESRD-related drugs and biologicals under the ESRD PPS prior to January 1, 2016. Section 632(c) of ATRA required the Secretary, by no later than January 1, 2016, to analyze the case-mix payment adjustments under section 1881(b)(14)(D)(i) of the Act and make appropriate revisions to those adjustments.
On April 1, 2014, the Protecting Access to Medicare Act of 2014 (PAMA) ( Pub. L. 113-93 ) was enacted. Section 217 of PAMA included several provisions that apply to the ESRD PPS. Specifically, sections 217(b)(1) and (2) of PAMA amended sections 1881(b)(14)(F) and (I) of the Act and replaced the drug utilization adjustment that was finalized in the CY 2014 ESRD PPS final rule ( 78 FR 72161 through 72170 ) with specific provisions that dictated the market basket update for CY 2015 (0.0 percent) and how the market basket percentage increase should be reduced in CY 2016 through CY 2018.
Section 217(a)(1) of PAMA amended section 632(b)(1) of ATRA to provide that the Secretary may not pay for oral-only ESRD-related drugs under the ESRD PPS prior to January 1, 2024. Section 217(a)(2) of PAMA further amended section 632(b)(1) of ATRA by requiring that in establishing payment for oral-only drugs under the ESRD PPS, the Secretary must use data from the most recent year available. Section 217(c) of PAMA provided that as part of the CY 2016 ESRD PPS rulemaking, the Secretary shall establish a process for (1) determining when a product is no longer an oral-only drug; and (2) including new injectable and intravenous products into the ESRD PPS bundled payment.
Section 204 of the Stephen Beck, Jr., Achieving a Better Life Experience Act of 2014 (ABLE) ( Pub. L. 113-295 ) amended section 632(b)(1) of ATRA, as amended by section 217(a)(1) of PAMA, to provide that payment for oral-only renal dialysis drugs and biological products cannot be made under the ESRD PPS bundled payment prior to January 1, 2025.
Under the ESRD PPS, a single per-treatment payment is made to an ESRD facility for all the renal dialysis services defined in section 1881(b)(14)(B) of the Act and furnished to an individual for the treatment of ESRD in the ESRD facility or in a patient's home. We have codified our definition of renal dialysis services at § 413.171, which is in 42 CFR part 413, subpart H , along with other ESRD PPS payment policies. The ESRD PPS base rate is adjusted for characteristics of both adult and pediatric patients and accounts for patient case-mix variability. The adult case-mix adjusters include five categories of age, body surface area, low body mass index, onset of dialysis, and four comorbidity categories (that is, pericarditis, gastrointestinal tract bleeding, hereditary hemolytic or sickle cell anemia, myelodysplastic syndrome). A different set of case-mix adjusters are applied for the pediatric population. Pediatric patient-level adjusters include two age categories (under age 13, or age 13 to 17) and two dialysis modalities (that is, peritoneal or hemodialysis) (§ 413.235(a) and (b)(1)).
The ESRD PPS provides for three facility-level adjustments. The first payment adjustment accounts for ESRD facilities furnishing a low volume of dialysis treatments (§ 413.232). The second payment adjustment reflects differences in area wage levels developed from core-based statistical areas (CBSAs) (§ 413.231). The third payment adjustment accounts for ESRD facilities furnishing renal dialysis services in a rural area (§ 413.233).
There are six additional payment adjustments under the ESRD PPS. The ESRD PPS provides adjustments, when applicable, for: (1) a training add-on for home and self-dialysis modalities (§ 413.235(c)); (2) an additional payment for high cost outliers due to unusual variations in the type or amount of medically necessary care (§ 413.237); (3) a TDAPA for certain new renal dialysis drugs and biological products (§ 413.234(c)); (4) a TPNIES for certain new and innovative renal dialysis equipment and supplies (§ 413.236(d)); (5) a transitional pediatric ESRD add-on payment adjustment (TPEAPA) of 30 percent of the per-treatment payment amount for renal dialysis services furnished to pediatric ESRD patients (§ 413.235(b)(2)); and (6) a post-TDAPA add-on payment adjustment for certain new renal dialysis drugs and biological products after the end of the TDAPA period (§ 413.234(g)).
Policy changes to the ESRD PPS are proposed and finalized annually in the Federal Register . The CY 2011 ESRD PPS final rule appeared in the August 12, 2010, issue of the Federal Register ( 75 FR 49030 through 49214 ). That rule implemented the ESRD PPS beginning on January 1, 2011, in accordance with section 1881(b)(14) of the Act, as added by section 153(b) of MIPPA, over a 4-year transition period. Since the implementation of the ESRD PPS, we have published annual rules to make routine updates, policy changes, and clarifications. Start Printed Page 55765
Most recently, we published a final rule, which appeared in the November 6, 2023, issue of the Federal Register , titled “Medicare Program; End-Stage Renal Disease Prospective Payment System, Payment for Renal Dialysis Services Furnished to Individuals With Acute Kidney Injury, and End-Stage Renal Disease Quality Incentive Program, and End-Stage Renal Disease Treatment Choices Model,” referred to herein as the “CY 2024 ESRD PPS final rule.” In that rule, we updated the ESRD PPS base rate, wage index, and outlier policy for CY 2024. We also finalized a post-TDAPA add-on payment adjustment; a TPEAPA for pediatric ESRD patients for CYs 2024, 2025, and 2026, administrative changes to the LVPA eligibility requirements to allow additional flexibilities for ESRD facilities impacted by a disaster or other emergency, clarifications on our TPNIES eligibility requirements, and, effective January 1, 2025, requirements for ESRD facilities to report time on machine for in-center hemodialysis treatments, and to report discarded amounts of renal dialysis drugs and biological products from single-dose containers or single-use packages. For further detailed information regarding these updates and policy changes, see 88 FR 76344 .
In accordance with section 1881(b)(14)(F)(i) of the Act, as added by section 153(b) of MIPPA and amended by section 3401(h) of the Affordable Care Act, beginning in 2012, the ESRD PPS payment amounts are required to be annually increased by an ESRD market basket percentage increase and reduced by the productivity adjustment described in section 1886(b)(3)(B)(xi)(II) of the Act. The application of the productivity adjustment may result in the increase factor being less than 0.0 for a year and may result in payment rates for a year being less than the payment rates for the preceding year. Section 1881(b)(14)(F)(i) of the Act also provides that the market basket increase factor should reflect the changes over time in the prices of an appropriate mix of goods and services included in renal dialysis services.
As required under section 1881(b)(14)(F)(i) of the Act, CMS developed an all-inclusive ESRDB input price index using CY 2008 as the base year ( 75 FR 49151 through 49162 ). We subsequently revised and rebased the ESRDB input price index to a base year of CY 2012 in the CY 2015 ESRD PPS final rule ( 79 FR 66129 through 66136 ). In the CY 2019 ESRD PPS final rule ( 83 FR 56951 through 56964 ), we finalized a rebased ESRDB input price index to reflect a CY 2016 base year. In the CY 2023 ESRD PPS final rule ( 87 FR 67141 through 67154 ), we finalized a revised and rebased ESRDB input price index to reflect a CY 2020 base year.
Although “market basket” technically describes the mix of goods and services used for ESRD treatment, this term is also commonly used to denote the input price index (that is, cost categories, their respective weights, and price proxies combined) derived from a market basket. Accordingly, the term “ESRDB market basket,” as used in this document, refers to the ESRDB input price index.
The ESRDB market basket is a fixed-weight, Laspeyres-type price index. A Laspeyres-type price index measures the change in price, over time, of the same mix of goods and services purchased in the base period. Any changes in the quantity or mix of goods and services (that is, intensity) purchased over time are not measured.
We propose to use the 2020-based ESRDB market basket as finalized in the CY 2023 ESRD PPS final rule ( 87 FR 67141 through 67154 ) to compute the proposed CY 2025 ESRDB market basket percentage increase based on the best available data. Consistent with historical practice, we propose to estimate the ESRDB market basket percentage increase based on IHS Global Inc.'s (IGI) forecast using the most recently available data at the time of rulemaking. IGI is a nationally recognized economic and financial forecasting firm with which CMS contracts to forecast the components of the market baskets. As discussed in section II.B.1.b.(3) of this proposed rule, we are proposing to calculate the market basket update for CY 2025 based on the proposed market basket percentage increase and the proposed productivity adjustment, following our longstanding methodology.
Based on IGI's first quarter 2024 forecast of the 2020-based ESRDB market basket, the proposed CY 2025 market basket percentage increase is 2.3 percent. We are also proposing that if more recent data become available after the publication of this proposed rule and before the publication of the final rule (for example, a more recent estimate of the market basket percentage increase), we would use such data, if appropriate, to determine the CY 2025 market basket percentage increase in the final rule.
Under section 1881(b)(14)(F)(i) of the Act, as amended by section 3401(h) of the Affordable Care Act, for CY 2012 and each subsequent year, the ESRDB market basket percentage increase shall be reduced by the productivity adjustment described in section 1886(b)(3)(B)(xi)(II) of the Act. The statute defines the productivity adjustment to be equal to the 10-year moving average of changes in annual economy-wide, private nonfarm business multifactor productivity (MFP) (as projected by the Secretary for the 10-year period ending with the applicable fiscal year (FY), year, cost reporting period, or other annual period) (the “productivity adjustment”).
The Bureau of Labor Statistics (BLS) publishes the official measures of productivity for the United States economy. As we noted in the CY 2023 ESRD PPS final rule ( 87 FR 67155 ), the productivity measure referenced in section 1886(b)(3)(B)(xi)(II) of the Act previously was published by BLS as private nonfarm business MFP. Beginning with the November 18, 2021, release of productivity data, BLS replaced the term “multifactor productivity” with “total factor productivity” (TFP). BLS noted that this is a change in terminology only and would not affect the data or methodology. [ 6 ] As a result of the BLS name change, the productivity measure referenced in section 1886(b)(3)(B)(xi)(II) of the Act is now published by BLS as private nonfarm business TFP; however, as mentioned previously, the data and methods are unchanged. We referred readers to https://www.bls.gov/productivity/ for the BLS historical published TFP data. A complete description of IGI's TFP projection methodology is available on CMS's website at https://www.cms.gov/data-research/statistics-trends-and-reports/medicare-program-rates-statistics/market-basket-research-and-information . In addition, in the CY 2022 ESRD PPS final rule ( 86 FR 61879 ), we noted that effective for CY 2022 and future years, we would be changing the name of this adjustment to refer to it as the productivity adjustment rather than Start Printed Page 55766 the MFP adjustment. We stated this was not a change in policy, as we would continue to use the same methodology for deriving the adjustment and rely on the same underlying data.
Based on IGI's first quarter 2024 forecast, the proposed productivity adjustment for CY 2025 (the 10-year moving average of TFP for the period ending CY 2025) is 0.5 percentage point. Furthermore, we are proposing that if more recent data become available after the publication of this proposed rule and before the publication of the final rule (for example, a more recent estimate of the productivity adjustment), we would use such data, if appropriate, to determine the CY 2025 productivity adjustment in the final rule.
In accordance with section 1881(b)(14)(F)(i) of the Act, we propose to base the CY 2025 market basket percentage increase on IGI's first quarter 2024 forecast of the 2020-based ESRDB market basket. We propose to then reduce the market basket percentage increase by the estimated productivity adjustment for CY 2025 based on IGI's first quarter 2024 forecast. Therefore, the proposed productivity-adjusted CY 2025 ESRDB market basket update is equal to 1.8 percent (2.3 percent market basket percentage increase reduced by a 0.5 percentage point productivity adjustment). Furthermore, as noted previously, we are proposing that if more recent data become available after the publication of this proposed rule and before the publication of the final rule (for example, a more recent estimate of the market basket percentage increase and/or productivity adjustment), we would use such data, if appropriate, to determine the CY 2025 market basket percentage increase and productivity adjustment in the final rule.
We define the labor-related share as those expenses that are labor-intensive and vary with, or are influenced by, the local labor market. The labor-related share of a market basket is determined by identifying the national average proportion of operating costs that are related to, influenced by, or vary with the local labor market. For the CY 2025 ESRD PPS payment update, we are proposing to continue using a labor-related share of 55.2 percent, which was finalized in the CY 2023 ESRD PPS final rule ( 87 FR 67153 through 67154 ).
Section 1881(b)(14)(D)(iv)(II) of the Act provides that the ESRD PPS may include a geographic wage index payment adjustment, such as the index referred to in section 1881(b)(12)(D) of the Act, as the Secretary determines to be appropriate. In the CY 2011 ESRD PPS final rule ( 75 FR 49200 ), we finalized an adjustment for wages at § 413.231. Specifically, we established a policy to adjust the labor-related portion of the ESRD PPS base rate to account for geographic differences in the area wage levels using an appropriate wage index, which reflects the relative level of hospital wages and wage-related costs in the geographic area in which the ESRD facility is located. Under current policy, we use the Office of Management and Budget's (OMB's) CBSA-based geographic area designations to define urban and rural areas and their corresponding wage index values ( 75 FR 49117 ). OMB publishes bulletins regarding CBSA changes, including changes to CBSA numbers and titles. The bulletins are available online at https://www.whitehouse.gov/omb/information-for-agencies/bulletins/ .
We have also adopted methodologies for calculating wage index values for ESRD facilities that are located in urban and rural areas where there are no hospital data. For a full discussion, see the CY 2011 and CY 2012 ESRD PPS final rules at 75 FR 49116 through 49117 and 76 FR 70239 through 70241 , respectively. For urban areas with no hospital data, we have computed the average wage index value of all hospitals in urban areas within the State to serve as a reasonable proxy for the wage index of that urban CBSA. For rural areas with no hospital data, we have computed the wage index using the average hospital wage index values from all contiguous CBSAs to represent a reasonable proxy for that rural area. We applied the statewide urban average based on the average of all urban areas within the State to Hinesville Fort Stewart, Georgia ( 78 FR 72173 ), and we applied the wage index for Guam to American Samoa and the Northern Mariana Islands ( 78 FR 72172 ).
Under § 413.231(d), a wage index floor value of 0.6000 is applied under the ESRD PPS as a substitute wage index for areas with very low wage index values, as finalized in the CY 2023 ESRD PPS final rule ( 87 FR 67161 ). Currently, all areas with wage index values that fall below the floor are located in Puerto Rico and the US Virgin Islands. However, the wage index floor value is applicable for any area that may fall below the floor. A further description of the history of the wage index floor under the ESRD PPS can be found in the CY 2019 ESRD PPS final rule ( 83 FR 56964 through 56967 ) and the CY 2023 ESRD PPS final rule ( 87 FR 67161 ).
An ESRD facility's wage index is applied to the labor-related share of the ESRD PPS base rate. In the CY 2023 ESRD PPS final rule ( 87 FR 67153 ), we finalized the use of a labor-related share of 55.2 percent. In the CY 2021 ESRD PPS final rule ( 85 FR 71436 ), we updated the OMB delineations as described in the September 14, 2018, OMB Bulletin No. 18-04, beginning with the CY 2021 ESRD PPS wage index. In that same rule, we finalized the application of a 5 percent cap on any decrease in an ESRD facility's wage index from the ESRD facility's wage index from the prior CY. We finalized that the transition would be phased in over 2 years, such that the reduction in an ESRD facility's wage index would be capped at 5 percent in CY 2021, and no cap would be applied to the reduction in the wage index for the second year, CY 2022. In the CY 2023 ESRD PPS final rule ( 87 FR 67161 ), we finalized a permanent policy under § 413.231(c) to apply a 5 percent cap on any decrease in an ESRD facility's wage index from the ESRD facility's wage index from the prior CY. For CY 2025, as discussed in section II.B.1.b.(4) of this proposed rule, the proposed labor-related share to which the wage index would be applied is 55.2 percent.
In the CY 2011 ESRD PPS final rule ( 75 FR 49116 ) and the CY 2011 final rule on Payment Policies Under the Physician Fee Schedule (PFS) and Other Revisions to Part B ( 75 FR 73486 ) we established an ESRD PPS wage index methodology to use the most recent pre-floor, pre-reclassified hospital wage data collected annually under the hospital inpatient prospective payment system (IPPS). The ESRD PPS wage index values have historically been calculated without regard to geographic reclassifications authorized for acute care hospitals under sections 1886(d)(8) and (d)(10) of the Act and utilize pre-floor hospital data that are unadjusted for occupational mix.
CMS has received feedback on our longstanding ESRD PPS wage index methodology from interested parties through comments on routine wage index updates in the annual ESRD PPS proposed rules. Commenters often suggest specific improvements for the Start Printed Page 55767 ESRD PPS wage index. In the CY 2024 ESRD PPS final rule ( 88 FR 76359 through 76361 ), we discussed the comments on the routine wage index proposals from the CY 2024 ESRD PPS proposed rule ( 88 FR 42436 ); commenters, including the Medicare Payment Advisory Commission (MedPAC), suggested that we establish an ESRD PPS wage index for all ESRD facilities using wage data that represents all employers and industry-specific occupational weights, rather than the hospital wage data currently used. MedPAC specifically suggested that CMS implement the recommendations discussed in its June 2023 report to Congress, [ 7 ] which recommended moving away from the current IPPS wage index methodology in favor of a methodology based on all employer wage data for all Medicare PPSs with industry specific occupational weights. Additionally, MedPAC suggested that the new methodology reflect local area level differences in wages between and within metropolitan statistical areas and statewide rural areas and smooth wage index differences across adjacent local areas. MedPAC stated that, compared to the current IPPS wage index methodology, a methodology based on all employer wage data with industry-specific occupational weights would improve the accuracy and equity of payments for provider types other than inpatient acute care hospitals, such as ESRD facilities.
In past years some interested parties have contended that the methodology used to construct the current ESRD PPS wage index does not accurately reflect the ESRD facility labor market. These interested parties have noted that the ESRD PPS wage index is based on the IPPS wage index, which uses hospital data, which commenters have stated may not be applicable for ESRD facilities. More specifically, commenters have suggested that the types of labor used in ESRD facilities differ significantly from the types of labor used by hospitals, which may result in the use of relative wage values across the United States that do not accurately match the actual relative wages paid by ESRD facilities. For example, if ESRD facilities have a different proportion of registered nurses (RNs), technicians and administrative staff compared to hospitals, and if wages for each of those labor categories vary differentially across the country, it is possible that relative wages for ESRD facilities, given their occupational mix, would vary differently from relative wages for hospitals across CBSAs. Because of this, some commenters have specifically requested that CMS develop an ESRD PPS wage index based only on data from ESRD facilities. Additionally, some commenters have criticized the time lag associated with using the IPPS wage index, which is generally based on data from four FYs prior to the rulemaking year (see, for example, 88 FR 58961 ).
In response to feedback from interested parties on the ESRD PPS wage index, CMS's data contractor hosted a Technical Expert Panel (TEP) in December of 2019. [ 8 ] During this TEP, the contractor presented a potential alternative approach to the wage index, which utilized BLS data to address the concerns of commenters, to initiate a discussion on the ramifications of a potential new ESRD PPS wage index that would combine two sources of existing data to more closely reflect the occupational mix in ESRD facilities. The methodology presented at this TEP utilized publicly available wage data for selected occupations from the BLS OEWS survey and occupational and fulltime equivalency (FTE) data from freestanding ESRD facility cost reports (Form CMS 265-11, OMB No. 0938-0236). Specifically, this approach used the freestanding ESRD facility cost reports to determine the national average occupational mix and relative weights for ESRD facilities. Next, the contractor applied the estimated county-level wages based on BLS OEWS [ 9 ] to obtain occupation-specific wages in each county. The BLS OEWS data is updated annually using sample data collected in six semiannual survey panels over the prior 3-year period, which allows for the inclusion of more recent data than the hospital cost-report data that is utilized by the IPPS wage index. Therefore, as noted during the TEP, this new methodology would allow CMS to adjust wage index values to reflect relative changes in wage conditions in a timelier fashion compared to the current ESRD PPS wage index methodology. Additionally, as noted during the TEP, by utilizing FTE data reported on the freestanding ESRD facility cost reports, this methodology is likely more reflective of the occupational mix employed by ESRD facilities than the hospital wage index.
Panelists at this TEP generally indicated their preference for the presented alternative wage index methodology, because it utilized more recent wage data from the BLS OEWS program. Panelists also favored how the alternative methodology was more targeted to ESRD facilities by utilizing FTE data from ESRD facility cost reports in determining the occupational mix. Some panelists voiced concerns about using publicly available BLS geographic area data, as the data do not disaggregate wages by health care sector, and therefore wages from acute care hospitals are not differentiated from outpatient care centers and other non-hospital health care settings. Some panelists noted that this would result in a wage index based on the publicly available BLS OEWS data having some of the same limitations for which the use of the IPPS wage index has been criticized—mainly that it includes wage data from hospitals.
Based on feedback we received in response to past ESRD PPS proposed rules and from the December 2019 TEP, we have developed a new ESRD PPS wage index methodology that we believe better reflects the ESRD facility labor market. Similar to the methodology presented in the December 2019 TEP, this proposed new methodology utilizes two data sources: one for occupational mix and one for geographic wages. First, we determine a national ESRD facility occupational mix (NEFOM) based on cost report data from freestanding ESRD facilities. Second, we extract and use data from the publicly available BLS OEWS survey on the average wages in each CBSA for each labor category present in the NEFOM. We note that because the publicly available BLS data are available at the Metropolitan Statistical Area (MSA), non-MSA and New England City and Town Area (NECTA) levels, and the wage index is designated at the CBSA level (which uses MSAs and other area designations that differ from non-MSAs and NECTAs), we use the area definition dataset [ 10 ] that accompanies Start Printed Page 55768 the BLS data to assign wages at the county level, and map counties to CBSAs using a crosswalk. This crosswalk is included in Addendum B, available on the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ESRDpayment/End-Stage-Renal-Disease-ESRD-Payment-Regulations-and-Notices .
The BLS OEWS program publishes annual estimates of employment and wages by occupation. Each set of OEWS estimates is based on data from six semiannual survey panels collected over a 3-year period. For example, the May 2022 OEWS wage estimates, published in April 2023, are based on six semiannual survey panels from November 2019 to May 2022. We are proposing to use publicly available mean hourly wage data at the MSA level, [ 11 ] which is available online at https://www.bls.gov/oes/ . OEWS wage data collected in earlier survey panels are “aged” or updated to the reference date of the estimates based on adjustment factors derived from the OEWS survey data using a regression model. The BLS OEWS mean hourly wage data that are presented in this proposed rule, and are utilized for the new wage index methodology described in detail later in this section of this proposed rule, reflect this updated data. Table 1 shows the occupation codes based on the Standard Occupational Classification (SOC) and the corresponding SOC occupational title for each SOC, alongside the colloquial name that we use to refer to workers in specific occupations throughout this proposed rule. The ESRD PPS colloquial names match the FTE categories captured on Worksheet S-1, lines 23 through 30 of the freestanding ESRD facility cost report form. The SOC System is a United States government system for classifying occupations. It is used by Federal Government agencies collecting occupational data, enabling comparison of occupations across data sets. When considering the use of BLS data we had to determine which occupation code was appropriate for each occupation in the NEFOM. For many of these occupations, the corresponding BLS code was straightforward. For example, BLS code 29-1141 is for “Registered Nurses” which matches the category on the cost reports from which the NEFOM is derived exactly. For the occupations that were not necessarily specific to the healthcare field, for example administrative staff, we used BLS codes that were specific for healthcare, such as code 43-6013 for “Medical Secretaries and Administrative Assistants.” We believe that these are the most appropriate codes, as a more general code may not capture the specifics of the healthcare labor market.

The BLS OEWS data used for this analysis includes mean wages by occupation for all industries combined located in a MSA (or non-MSA area or NECTA), including the hospital industry. While interested parties have criticized the current ESRD PPS wage index methodology's sole reliance on hospital data, we believe that inpatient hospital data is appropriate to include here for several reasons. Principally, as explained later in this section, the wage data is being weighted based on an occupational mix that is specific to ESRD facilities, which makes this proposed methodology more accurate to the wage environment of ESRD facilities regardless of the source of the wage data. Additionally, ESRD facility data is included in the BLS data, while ESRD facilities generally are not included in the hospital cost report data used in the IPPS wage index (with the exception of hospital-based ESRD facilities). Lastly, hospitals are a major contributor to labor markets, and it is reasonable to think that ESRD facilities compete with hospitals (as well as other healthcare facilities) when it comes to hiring labor; as such, the inclusion of hospital data would provide additional insight into the labor markets of these areas.
A limitation of the publicly available BLS OEWS data is that the survey only includes information on the wages that employers paid to their employees. Therefore, the OEWS does not include self-employed contract labor wages or benefits paid to employees, which are reflected in the IPPS wage index. Nevertheless, we believe that this data source would be an improvement over the use of the IPPS wage index for the ESRD PPS, as its purpose is to identify geographic differences in wages. Assuming wages spent on self-employed contract labor wages and employee benefits vary similarly to employee wages; we would not expect any significant difference arising from this limitation of the BLS data. We anticipate that most traveling nurses and technicians would be employed by an agency, and therefore would be included in the OEWS estimates; however as worksite location reporting is optional, [ 12 ] we note it is possible that some of the wages for these traveling nurses and technicians could be included in the MSA in which their employing agency is located, rather than the MSA in which they worked. However, we would not anticipate that this would have an appreciable impact on the OEWS estimates used for this methodology. Additionally, we note that the OEWS would only include the wages paid to these contract workers, so the OEWS estimates would likely not include the full cost of the contract labor paid by the ESRD facilities to the contracting agency. We cannot separately estimate the prevalence of self-employed contract labor at ESRD facilities from the rest of contract labor, which we believe would still provide some insight into the potential limitation of the exclusion of self-employed contract labor wages from the BLS OEWS. We note that all contract labor costs represent approximately 5 percent of compensation costs in the 2020-based ESRDB market basket ( 87 FR 67143 ). Our analysis of freestanding ESRD facility cost report FTE data indicates that approximately 1.3 percent of RN hours and 1.1 percent of technician hours were contract labor in 2022. Additionally, our data show that the share of contract labor hours has been relatively stable over time but has increased slightly when compared to the prior few years.
One potential concern about use of the BLS OEWS data is that in some cases, the BLS OEWS may not have usable data for a county for an occupation, which is used in the construction of the new ESRD PPS wage index according to the methodology presented later in this section. This occurs when BLS is unable to publish a wage estimate for a specific occupation and area because the estimate does not meet BLS quality or confidentiality standards. [ 13 ] For reference, among the 25,808 unique county-occupation combinations, the wage information missing rate is 5.2 percent. To impute the missing data, we perform a regression using the most similar (by mean hourly wage) occupation (of the occupations we are proposing to include in the wage index methodology, presented in table 1) for which there is no missing data. For dietitians we use RNs, for technicians we use LPNs and for nurses' aides we use administrative staff. The regression includes controls for whether the county is rural, the census region in which the county is located, and the natural logarithm of the treatment count of the county. For the CY 2025 ESRD PPS wage index we only had to impute missing county-level data for dietitians, technicians, and nurses' aides; however, for future years, we may have to impute data for other occupations.
We have conducted an analysis on historical BLS OEWS data for the occupations presented in table 1. We have found that mean hourly wages for these categories are increasing over time, consistent with what we would expect given the ESRD PPS market basket increases. Given this analysis, we believe that the BLS OEWS data are reasonably stable and appropriately reflect general wage inflation trends that ESRD facilities face. Therefore, the mean hourly wage estimates for a given year are appropriately reflective of wages which ESRD facilities face.
Under § 413.198(b)(1), all ESRD facilities must submit the appropriate CMS-approved cost report in accordance with §§ 413.20 and 413.24, which provide rules on financial data and reports, and adequate cost data and cost finding, respectively. Generally, these cost reports have a time range of January 1 to December 31 of a given year, but they can represent any 12-month period. Included in these cost reports is information on the number of full-time equivalent (FTE) positions employed by the ESRD facility. FTEs are stratified by occupation type, such as RNs, LPNs, technicians, and administrative staff. For the purpose of these cost reports, an FTE represents a 40-hour work week averaged across the year. Specifically, the cost reports define FTEs as the sum of all hours for which employees were paid during the year divided by 2080 hours. The cost reports also state personnel involved in more than one activity must have their time prorated among those activities. For example, an RN who provided professional services and administrative services is counted in both the RN line and the administrative line according to the number of hours spent in each activity.
For the proposed methodology presented in this section, we are proposing to use FTEs to calculate the occupational mix for all freestanding ESRD facilities. For the purposes of this section, we use the term “freestanding ESRD facilities” to mean ESRD facilities that complete the independent renal dialysis facility cost report (Form CMS 265-11, OMB No. 0938-0050). We note that these ESRD facilities are a subset of “independent” facilities as defined at § 413.174(b), as cost-reporting is only one of 5 criteria used in the determination of whether an ESRD facility is independent or hospital-based as listed at § 413.174(c). For the purposes of this section, we refer to ESRD facilities that complete the hospital cost report (Form CMS 2552-10, OMB No. 0938-0050) as “ESRD facilities that are financially integrated Start Printed Page 55770 with a hospital,” per the criteria at § 413.174(c)(5). This occupational mix represents the average proportion of hours spent on the duties of that occupation at all freestanding ESRD facilities nationally. This national mix includes FTE data on both staff and contract labor from freestanding ESRD facility cost reports for each occupational category. Table 2 presents the NEFOM calculated from the freestanding ESRD facility cost report data from cost reporting periods beginning on or after January 1, 2022, and before December 31, 2022 (2022 cost report data), with four decimal places of precision. We note that this is the most recent complete year of cost reporting data for both this proposed rule and for the CY 2025 ESRD PPS final rule, as the latest 2022 cost reports could have begun in December 2022 and ended in December 2023, although some 2022 cost reports were not yet available at the time of the analysis for this proposed rule. For the approximately 1.7 percent of freestanding ESRD facilities without 2022 cost report data available at the time of rulemaking for this proposed rule, 2021 cost report data was used. The occupational mix weights used in the proposed new wage index methodology are presented in terms of the number of FTEs per 1000 treatments, although we note that the specific denominator does not impact the calculation, as these are relative weights. Table 2 also includes percentages that represent the percent of FTEs for each occupation in the NEFOM. For example, RNs represent approximately 30 percent of the NEFOM, which means that across the nation, 30 percent of all hours worked by employees at freestanding ESRD facilities are worked by RNs. We note that we did not include FTEs that were reported as “other” occupations in the cost reports in this occupational mix, because we could not determine what occupation(s) this represented and, therefore, could not get appropriate wage estimates. “Other” occupations would have accounted for 3.8 percent of the NEFOM if included.

We note that the NEFOM is calculated as a part of the proposed wage index methodology described in detail below from freestanding ESRD facilities cost reports, and that the NEFOM is not an input in the wage index calculation. However, we are presenting the NEFOM here to inform the calculation process for any interested parties which wish to replicate the calculation.
For this proposed methodology, we are proposing to only utilize data from freestanding ESRD facilities, which comprise the vast majority of ESRD facilities. ESRD facilities that are financially integrated with a hospital represent approximately 4.5 percent of ESRD facilities. It is necessary to make this distinction, as ESRD facilities that are financially integrated with a hospital complete a different cost report form (Form CMS 2552-10, OMB No. 0938-0050), which does not include all the occupational categories included on the freestanding facility cost report (Form CMS 265-11, OMB No. 0938-0050). Specifically, ESRD facilities that are financially integrated with a hospital do not include administrative and management staff hours in their cost reports. FTE data for administrative and management staff are necessary for this analysis, so we are proposing to exclude hospital-integrated cost reports. We believe that the occupational mix for freestanding ESRD facilities is likely similar to the mix for ESRD facilities that are financially integrated with a hospital (which, as noted earlier, make up a small proportion of all ESRD facilities), such that we would not expect significantly different results if we were able to include ESRD facilities that are financially integrated with a hospital in this analysis. Start Printed Page 55771
We conducted additional analyses to ensure that this occupational mix data would be appropriate for the construction of an ESRD facility wage index. First, we reviewed the occupational mix for ESRD facilities on a regional level to determine if the use of a single national occupational mix was appropriate. While we found some variation across regions, the variation was generally relatively small between regions, with the weight values for each occupation being within a few percentage points. The main exceptions to this were in the United States territories, which had higher variation in occupational mix, likely due in large part to the relatively few ESRD facilities in those regions. Additionally, we found that lower volume ESRD facilities tended to have slightly different occupational mixes, requiring relatively more administrative and management staff FTEs, likely due to the lack of economies of scale for these occupations at lower treatment volume levels. Second, we conducted an analysis on the change in the national occupational mix over the past 5 years and found little variation over this time period. Both of these analyses indicate that the use of a single national occupational mix is appropriate for constructing an ESRD facility wage index as the occupational mix is reasonably similar to most region's occupational mixes and relatively stable over time.
Additionally, we are proposing to use treatment volume data from freestanding ESRD facilities as reported on freestanding ESRD facility cost reports. This treatment volume data is used in the wage index calculation as a weight on the county level wages when calculating the wages for a CBSA. The calculation is described in further detail in section II.B.2.b.(2)(b) of this proposed rule.
We emphasize the importance of accurate cost report data for this proposed policy as well as other current and potential policies under the ESRD PPS, such as facility-level or case-mix adjustment refinement. We strongly urge ESRD facilities to carefully review cost report data to ensure continued accuracy so that future refinements to the ESRD PPS are based on the best data possible.
The new proposed wage index methodology uses the established ESRD PPS wage index methodology, which is based on the IPPS hospital wage index, for the purposes of standardizing the new wage index (step 6 in the methodology described in section II.B.2.b.(2).(b)). Consistent with our established ESRD PPS methodology, we use the most recent pre-floor, pre-reclassified hospital wage data collected annually under the IPPS. The ESRD PPS wage index values under the established methodology are calculated without regard to geographic reclassifications authorized for acute care hospitals under sections 1886(d)(8) and (d)(10) of the Act and utilize pre-floor hospital data that are unadjusted for occupational mix. For CY 2025, the updated wage data are generally for hospital cost reporting periods beginning on or after October 1, 2020, and before October 1, 2021 (FY 2021 cost report data). Under § 413.231(d), a wage index floor value of 0.6000 is applied under the ESRD PPS as a substitute wage index for areas with very low wage index values, as finalized in the CY 2023 ESRD PPS final rule ( 87 FR 67161 ). For the purposes of the proposed new wage index methodology, we are referring to this older wage index methodology as the “ESRD PPS legacy wage index.” Consistent with our established policy of updating wage indices in the final rule, we intend to use the most recent IPPS wage index for the construction of the CY 2025 ESRD PPS legacy wage index for the final rule. We note that the purpose of calculating the ESRD PPS legacy wage index is solely for standardizing the new ESRD PPS wage index, ensuring that the treatment weighted average of the new ESRD PPS wage index is the same as it would have been under the established methodology. This ensures that the changes associated with the proposed new wage index methodology are contained to the wage index, whereas changes associated with shifts in utilization would be reflected in the wage index budget neutrality factor. For example, if the new methodology resulted in a significant increase in the number of high-wage index facilities, the standardization factor would decrease wage index values across the board to keep the treatment-weighted average of the legacy and new wage index methodologies the same; in contrast, if utilization trends resulted in a significant increase in the number of treatments furnished by ESRD facilities in high-wage index areas, the treatment weighted average of both the legacy and new wage index methodologies would increase which would need to be accounted-for by the wage index budget neutrality adjustment factor. This is described in more detail in step 6 of the proposed new wage index methodology in section II.B.2.b.(2)(b) of this proposed rule.
One concern expressed by interested parties about the current ESRD PPS wage index methodology is that the IPPS wage index, used as its basis, uses data from approximately 4 fiscal years prior. Interested parties have opined that this delay makes the ESRD PPS wage index less responsive to certain changes in wages, such as inflation. [ 14 ] We note that the purpose of the wage index is to reflect geographic difference in the area wage levels, and that national trends in wages, including wage inflation, are accounted for by the ESRDB market basket percentage increase. We note that the IPPS wage index is generally responsive to geographic variation in wages, including variation stemming from local or regional inflation. However, as interested parties have raised concerns about the time lag associated with our use of the IPPS wage data, we discuss the difference between the time lag associated with our use of the IPPS wage index for the ESRD PPS and the proposed new ESRD PPS wage index methodology discussed later in this section of the preamble.
As previously discussed in this section, the new ESRD PPS wage index methodology that we are proposing would use data from BLS OEWS and freestanding ESRD facility cost reports. BLS publishes OEWS data annually with a May reference date, with estimates typically released in late March or early April of the following year. Each set of OEWS estimates is based on six semi-annual survey samples spanning the prior 3 years. Wages collected in earlier survey panels are updated to the reference date of the estimates based on wage adjustment factors derived from the OEWS survey data using a regression model. The freestanding ESRD facility cost report data that can be analyzed at the time of rulemaking are generally from 2 CYs prior. Specifically, for the proposed wage index presented in Addendum B of this ESRD PPS proposed rule, the BLS OEWS data is derived from surveys conducted from November 2019 through May 2022, and the cost report data generally covers cost reporting periods Start Printed Page 55772 beginning on or after January 1, 2022, and before December 31, 2022. [ 15 ] The publicly available BLS OEWS data is an average using data collected over a 3-year period which improves stability and predictability of the OEWS estimates over time. We note that, should this methodology be finalized as proposed in the CY 2025 ESRD PPS final rule, the most recent update of BLS OEWS data for a given year would be available early enough to be included in the ESRD PPS final rule, but not in the proposed rule. Under this proposed new methodology, BLS OEWS data collected as recently as May 2023 would be utilized for the final CY 2025 ESRD PPS wage index.
Both the ESRD facility cost report data and the BLS OEWS data are more recent than the data used for the IPPS wage index. Additionally, the purpose of using the freestanding ESRD facility cost report data in this proposed methodology would be to establish a national occupational mix for ESRD facilities, which we are calling the NEFOM. We intend to present the NEFOM annually to reflect the latest complete year of cost report data at the time of rulemaking to inform the public of the relative weights assigned to each occupation. Given that freestanding facility cost reports are submitted on a rolling basis, the most recent data would generally be obtained from cost reports beginning in the CY 3 years prior to the CY for which we are setting rates (that is, for this CY 2025 proposed rule, the latest complete year of cost report data are from cost reports beginning in CY 2022). Based on our analysis of prior years' cost report data, we do not anticipate that the national occupational mix would change much from year-to-year. Additionally, we note that the use of a single national occupational mix for all ESRD facilities would limit the impact of changes in employment patterns on the wage index, as all ESRD facilities would be similarly impacted by a change in the NEFOM. As the wage index is a relative value, the main way that a change in the NEFOM would impact an ESRD facility's wage index would be if the CBSA in which that ESRD facility is located has relatively high or low wages for an occupation that experiences growth or shrinkage in the NEFOM. Thus, the main driver in changes from year-to-year under this proposed new wage index methodology likely would be the BLS OEWS data, which, for the final rule, would include survey data as recent as May of the year prior to the rulemaking year.
We note that, at the time of the analysis conducted for this proposed rule, the May 2023 BLS OEWS update was not yet available. As previously discussed, some ESRD facilities' CY 2022 cost reports were not available. Should the proposed new wage index methodology be finalized, we would update the wage index values based on the most recent BLS OEWS data available. We are also proposing to use most recent cost report data available for cost reporting periods beginning in CY 2022 and update the NEFOM accordingly in the final rule. Using the most recent 2022 data available for the calculation of the new ESRD PPS wage index methodology in the final rule would be consistent with our established ESRD PPS wage index methodology of updating ESRD facility wage indices between the proposed and final rules.
We note that our proposed new wage index methodology does use the IPPS wage index to create the ESRD PPS legacy wage index, which is used to standardize the results of the new ESRD PPS wage index methodology. We recognize the concerns we have heard regarding the data lag associated with our use of the IPPS wage index for the ESRD PPS. However, as the ESRD PPS legacy wage index would only be used to calculate a treatment-weighted average of the legacy wage index to standardize the wage index values derived under the proposed new methodology, the proposed new ESRD PPS wage index would continue to reflect the relative differences in area wages based on the more recent BLS OEWS data. Therefore, any effect of any data lag of the ESRD PPS legacy wage index on the proposed new ESRD PPS wage index would be minimal.
The other main concern that interested parties have raised about our current ESRD PPS wage index methodology is that the IPPS wage index is based on hospital cost report data. As previously discussed, interested parties have stated that hospital cost report data is not necessarily the most appropriate source for estimating geographic differences in wages paid by ESRD facilities. These interested parties predominantly point to the different occupational mix employed by ESRD facilities as the main differentiator between inpatient hospitals and ESRD facilities; however, there may also be differences in wages paid for the same occupational labor category in the two settings. Differences in wages within the same occupation could arise from any number of factors, including differences in duties, hours, required experience, or desirability of the position.
Table 3 compares the national average occupational mix and corresponding wages for occupations employed by freestanding ESRD facilities to that of hospitals from IPPS data. The source of average wages used here for ESRD facilities is the BLS OEWS and average IPPS wages are derived from the IPPS occupational survey (Form CMS-10079) as presented in the fiscal year (FY) 2024 IPPS Public Use File (PUF), [ 16 ] representing data from 2019. The mean hourly wage data from BLS is from the May 2022 OEWS estimates, which are based on six panels of survey data from November 2019 through May 2022.

We note that the hospital wage data (column F) presented in table 3 presents the wages paid by hospitals to employees, as derived from the IPPS occupational survey data, for the purposes of comparing to the BLS data. This data is used to adjust the hospital average hourly wage, calculated using hospital cost report data, based on the provider-specific occupational mix. This differs from the hospital cost report data used for the IPPS wage index, as that does not break down all wages and related costs by occupation.
Compared to hospitals, ESRD facilities generally use slightly higher proportions of RNs and LPNs and significantly fewer nurse aides and medical aides (column B). Additionally, the freestanding ESRD facility cost reports include additional occupational categories to reflect the labor mix employed by ESRD facilities.
Under our proposal, once we have the calculated wages for each relevant labor category by county (using a crosswalk between MSA, non-MSA and NECTA and counties) and the NEFOM, we would construct the new ESRD PPS wage index using the following steps. These are the general steps which we use when constructing the proposed new ESRD PPS wage index; for a more detailed look at the specific computational steps we execute in the code to calculate the proposed wage index, including steps related to data collection and cleaning, see the supplementary document in Addendum C.
1. We calculate the treatment count-weighted mean hourly wage for each occupation for each CBSA by multiplying the mean hourly wage data from the BLS OEWS by the treatment count for each county within that CBSA and dividing by the total treatment count of all counties within the CBSA. We weight mean hourly wage by treatment count to ensure that the mean hourly wage for the CBSA is proportional with the actual wages paid by ESRD facilities in the CBSA. This avoids a situation where a particularly high or low wage county within a CBSA has no ESRD facilities but still has a large impact on the wage index for that Start Printed Page 55774 CBSA. This reasoning extends to each instance in which we weight values by treatment counts.
2. We calculate the ESRD facility mean hourly wage in each CBSA by multiplying the treatment count-weighted mean hourly wage (from step 1) for each occupation for a given CBSA with the corresponding weight of the NEFOM for each occupation and then sum each category's amount to get the total.
3. We calculate the treatment count-weighted mean hourly wage for each occupation at the national level by multiplying the mean hourly wage for the occupation in each CBSA by the treatment count of that CBSA and dividing by the aggregated treatment count nationally.
4. We calculate the national ESRD facility mean hourly wage by multiplying the national mean hourly wage (from step 3) for each occupation by the corresponding weight of the NEFOM for each occupation and then sum each category's amount to get the total.
5. We divide the ESRD facility mean hourly wage for each CBSA by the national ESRD facility mean hourly wage to create a raw wage index level (that is, a wage index that has not been normalized as described in step 6).
6. We multiply the raw wage index level for each CBSA by a treatment weighted average of the CY 2025 ESRD PPS legacy wage index constructed using the established ESRD PPS methodology based on IPPS Medicare cost report data and divide the product by the treatment weighted average of raw wage indices, which equals 1 by construction. [ 17 ] This is to ensure that the treatment-weighted average of new BLS-based wage indices is the same as the weighted average of the current wage indices. By ensuring the weighted average of the new wage index is the same as the weighted average of the pre-floor pre-reclassification IPPS wage index we have normalized the new wage index such that it is more comparable to the former ESRD PPS wage index methodology. This prevents the possibility that the treatment-weighted average of the new wage index is significantly different than the treatment-weighted average of the established methodology. We include this step because our goal in establishing the proposed new wage index methodology is not to alter the significance of the wage index in determining each ESRD facility's payment, but rather to ensure that the wage index values better reflect relative labor costs that affect ESRD facilities specifically. We note that because we apply a wage index budget neutrality adjuster (discussed in section II.B.4.b), the proposed new wage index methodology would not increase total payments to ESRD facilities even absent this step.
7. We apply the 0.6000 floor to the wage index by replacing any wage index values that fall below 0.6000 with a value of 0.6000, which is the wage index floor for the ESRD PPS as established in the CY 2023 ESRD PPS final rule ( 87 FR 67166 ).
After following these steps, we would obtain the wage index values for each CBSA (based on the new OMB delineations as discussed later in this section of the preamble) according to the proposed ESRD PPS wage index methodology described previously. We note that the 5 percent cap in year-over-year decreases in wage index values would be applied for each ESRD facility after the new wage index is calculated based on the proposed methodology for the CBSA in which the ESRD facility is located and, therefore, is not reflected in the wage index value for a CBSA in Addendum A, available on the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ESRDpayment/End-Stage-Renal-Disease-ESRD-Payment-Regulations-and-Notices . This is necessary as this cap protects ESRD facilities in the rare circumstances when changes in policy related to the wage index methodology or CBSA delineations cause an ESRD facility to be in a significantly lower wage index area in a given year when compared to the previous year ( 87 FR 67161 ). As discussed later in this section, for CY 2025 we are proposing to adopt new OMB delineations of CBSAs relative to those used in the CY 2024 ESRD PPS wage index. As this 5 percent cap applies to an ESRD facility, and not to a CBSA, it would protect any ESRD facility that is delineated into a much lower wage-index CBSA for CY 2025.
While developing this new wage index methodology, we have considered several different alternatives regarding both data sources used for the new wage index methodology and construction of the wage index itself. We considered the feasibility of requesting the use of confidential BLS OEWS data. This was one suggestion from the December 2019 TEP. Confidential data would have some benefits over public data, primarily that it would provide greater disaggregation of wages by employer type, such as wages paid by ESRD facilities. Additionally, confidential BLS data could have a timeframe other than the 3-year pooled sample used in the public data, for example using only the most recent year's data. However, we note that the OEWS survey sample is designed to be statistically representative only when all 3 years of the sample are combined, so the use of an alternative or shorter timeframe may not be appropriate. We have determined that the publicly available BLS data would be the most appropriate for our wage index, as it still provides precise estimates of wages and would allow for far better transparency. Additionally, we believe that the inclusion of data from other employers (meaning employers that are not ESRD facilities) would improve the robustness of the methodology, as ESRD facilities compete for labor against these other employers.
When considering the use of BLS data we had to determine which occupation code was appropriate for each occupation in the NEFOM. As discussed previously, for many of these occupations, the corresponding BLS code was straightforward as many of the occupations present in the freestanding ESRD facility cost reports matched a single BLS code. However, for technicians employed by ESRD facilities we gave further consideration to two different BLS codes. As presented in table 1, we are proposing to use code 29-2099 for “Health Technologists and Technicians, All Other” for the construction of the methodology to account for the labor costs of technicians. This is the most appropriate category, as “technicians” in the freestanding ESRD facility cost reports generally refers to dialysis technicians, which do not fall into any of the other BLS codes for health technologists and technicians. Additionally, we note that the SOC uses “dialysis technician” as an illustrative example for code 29-2099. [ 18 ] However, we had some concerns about using this category, as it does not specifically represent dialysis technicians, but rather all health technicians that do not fit in the other categories. Because the category is non-specific, also known as a “residual” category, we were concerned with the impact of the inclusion of other, non-dialysis technicians in this category. To avoid any issues arising from the use of a Start Printed Page 55775 residual category, we considered using code 29-2010 for “Clinical Laboratory Technologists and Technicians.” Although this category does not fit dialysis technicians as well, it has the benefit of not being a residual category, and it had fewer counties with missing data. However, we determined that it was most appropriate to use the most similar category for dialysis technicians, being the category in which data for dialysis technicians would be included, which is code 29-2099 “Health Technologists and Technicians, All Others.”
As an alternative to using a single national occupational mix for ESRD facilities we considered using regional or state-level occupational mixes. The considered alternative would use a similar methodology to the construction of the NEFOM, but with a different occupational mix for each census region or state and would apply the occupational mix in the same way in the construction of the wage index. This is to say, the BLS data for a CBSA would be weighted by the occupational mix for the region or state in which that CBSA is located. This alternative was considered, in part, because of a suggestion from a panelist at the December 2019 TEP who pointed out that different states have different laws regarding staffing requirements for ESRD facilities, which was not reflected in the methodology presented at the TEP. We conducted an analysis comparing a state-level occupational mix wage index to the national occupational mix wage index methodology presented previously. This analysis found some notable differences, including higher wage index values in the pacific census region, but many regions experienced little change. We decided against the use of state-level or regional occupational mixes for three main reasons. The first is that the use of different occupational mixes for different ESRD facilities made the methodology significantly more complicated and difficult to understand. The second is that this methodology made it so that one ESRD facility could be in an area with higher wages for all occupations compared to another ESRD facility but receive a lower wage index value due to having an occupational mix which favored lower-paying occupations. This could be perceived as being inconsistent with the intent of the wage index to recognize differences in ESRD facility resource use for wages specific to the geographic area in which facilities are located ( 83 FR 56967 ). Lastly, we are concerned about the possibility that, should we use anything other than a national occupational mix, the state-level or regional occupational mix could be manipulated. This would be especially relevant for states or regions with few ESRD facilities and, therefore, individual ESRD facilities would have an outsized impact on the occupational mix for that state or region. Accordingly, we believe that the use of a single national occupational mix is the most appropriate for this proposed new ESRD facility wage index methodology.
We considered proposing a “phase-in” policy for this proposed wage index methodology change, which could be implemented in addition to the 5 percent cap on wage index decreases. One potential example of a phase-in policy could be a 50/50 blended methodology, where an ESRD facility would receive the average of their wage indices from the proposed new and legacy methodologies for the first year of implementation. However, we decided that such a phase-in policy was unnecessary in light of the 5 percent cap on year-to-year wage index decreases for ESRD facilities. We believe that an additional, or alternative, phase-in policy would further complicate this change. Additionally, a phase-in policy could hurt ESRD facilities that would receive a higher wage-index under the new methodology, which we do not believe would be appropriate, as we believe the new methodology based on BLS data is the best approximation of the labor costs those ESRD facilities face.
We considered setting the NEFOM through rulemaking separately from the routine wage index update. Under this alternative, we would periodically update the NEFOM, for example every 2 years, with potentially more years of freestanding ESRD facility cost report data. This would mean that the NEFOM would be a rounded input in the wage index methodology, rather than a figure precisely calculated as an intermediary step in the methodology. This would slightly simplify the calculation steps and would allow for complete transparency on the NEFOM. However, we have decided to instead derive the FTEs per 1000 treatments for each occupation as the weights as a part of the wage index calculation as that would increase the precision of this calculation. Additionally, given the transparency of the FTE data derived from publicly available cost reports, we can still publish the NEFOM for the coming year in rulemaking alongside the updated wage index; however, we note that the NEFOM we publish would have a lower precision so replications using the published NEFOM as an input may be slightly off. Furthermore, compared to setting the NEFOM through rulemaking less frequently than annually, the proposed methodology to calculate the NEFOM as a part of the wage index methodology annually would be more responsive to national trends in occupational mix for ESRD facilities.
Finally, we considered whether it was most appropriate to use something other than the mean hourly wage for the BLS OEWS data for the construction of the wage index. There are always concerns when using the mean of a data set that the figure could be unduly influenced by outliers. One potential alternative would be to use the median hourly wage data instead. The median hourly wage is available by occupation in publicly available BLS data, and the median is not as influenced by outliers as the mean. We also considered using the geometric mean, instead of arithmetic mean, as that is also less influenced by outliers; however the geometric mean is not provided in publicly available BLS data. Ultimately, we determined that the mean hourly wage is the most appropriate for this new wage index methodology, as any outliers are relevant data points insofar as some ESRD facilities may pay wages significantly higher than the average.
Table 4 is an example of a calculation of the wage index for a hypothetical ESRD facility in a hypothetical CBSA under the proposed new methodology. This CBSA contains three counties, each with a different mean hourly wage and treatment count. Table 4 presents the mean hourly wage and treatment count used in the calculation.

Step 1. Calculate the treatment count-weighted mean hourly wage for each occupation for each CBSA by multiplying the mean hourly wage data from the BLS OEWS by the treatment count for each county within that CBSA and dividing by the total treatment count of all counties within the CBSA.
RN wage = [(200 * $45) + (300 * $40) + (500 * $50)]/1000 = $46.0
LPN wage = [(200 * $30) + (300 * $30) + (500 * $35)]/1000 = $32.5
Nurse aide wage = [(200 * $15) + (300 * $20) + (500 * $10)]/1000 = $14.0
Technicians wage = [(200 * $30) + (300 * $35) + (500 * $25)]/1000 = $29.0
Social worker wage = [(200 * $30) + (300 * $25) + (500 * $35)]/1000 = $31.0
Administration wage = [(200 * $20) + (300 * $25) + (500 * $20)]/1000 = $21.5
Dietitian wage = [(200 * $35) + (300 * $30) + (500 * $30)]/1000 = $31.0
Management wage = [(200 * $60) + (300 * $65) + (500 * $50)]/1000 = $56.5
Step 2. Calculate the ESRD facility mean hourly wage in the CBSA by multiplying the treatment count-weighted mean hourly wage (from step 1) for each occupation for the CBSA with the corresponding weight of the NEFOM for each occupation and sum each category's amount to get the total. The NEFOM for CY 2025 is presented in table 5. For the purposes of ensuring the calculation in this section is as easy to understand as possible we are using the percentage values from the NEFOM rounded to the nearest tenth of a percent. This makes the wage values calculated in this step and step 4 more intuitive as they would represent a weighted average of the wages in the CBSA. We note that in the actual calculation of the wage index, as described in Addendum C, we calculate the number of FTEs per 1000 treatments for each occupation and use those as the weights, so that the weights have a higher level of precision.

ESRD facility mean hourly wage for this CBSA = (0.300 * $46.0) + (0.040 * $32.5) + (0.024 * $14.0) + (0.381* $29.0) + (0.047 * $31.0) + (0.107 * $21.5) + (0.045 * $31.0) + (0.055 * $56.5) = $34.75
Step 3. Calculate the treatment count-weighted mean hourly wage for each occupation at the national level by multiplying the mean hourly wage for the occupation in each CBSA by the treatment count of that CBSA and dividing by the aggregated treatment count nationally.
To simplify this calculation, assume there are 3 CBSAs as follows:

Step 4. Calculate the national ESRD facility mean hourly wage by multiplying the national mean hourly wage (from step 3) for each occupation by the corresponding weight of the NEFOM for each occupation and sum each category's amount to get the total. Similarly to step 2, we are using the percentages from the NEFOM as weights for the purposes of this example calculation.
National average ESRD facility wage = (0.300 * $46.90) + (0.040 * $32.58) + (0.024 * $18.67) + (0.381 * $32.28) + (0.047 * $32.61) + (0.107 * $19.52) + (0.045 * $31.49) + (0.055 * $56.64) = $36.27
Step 5. Divide the ESRD facility mean hourly wage for each CBSA by the national ESRD facility mean hourly wage to create a raw wage index level.
Raw wage index value = $34.75/$36.27 = 0.95809
Step 6. Multiply the raw wage index for each CBSA by a treatment weighted average of the CY 2025 ESRD PPS legacy wage index constructed using the established ESRD PPS methodology based on IPPS data and divide the product by the treatment weighted average of raw wage indices (which equals 1 by construction). This is to ensure that the treatment-weighted average of new BLS-based wage indices is the same as the weighted average of the current wage indices (for the purpose of this hypothetical calculation we have used a value of 1.00679).
Pre-floor wage index value = 0.95809 * 1.00679/1 = 0.9646
Step 7. Apply the 0.6000 floor to the wage index by replacing any wage index values which fall below 0.6000 with 0.6000.
Final wage index value = 0.9646
The proposed new wage index methodology described previously would be a substantial change from the current approach used by the ESRD PPS to evaluate variations in wages across geographic areas. Compared to the current methodology based on hospital cost report data, this new methodology would use survey data on wages for occupations relevant to furnishing renal dialysis services, which includes data from ESRD facilities and other similar outpatient settings and is weighted according to the average occupational mix of freestanding ESRD facilities. This proposed methodological change, if finalized, would be associated with significant changes in wage index values, and therefore payment amounts, for ESRD facilities. Full impacts for the proposed CY 2025 ESRD PPS wage index, alongside the updated CBSA delineations and rural transition policy discussed in section II.B.2.f of this proposed rule, are presented in table 18 in section VIII.D.5.a of this proposed rule, including application of the 5 percent cap on year-to-year wage index decreases. The 5 percent cap policy would mitigate the impact of the proposed changes to the wage index methodology for CY 2025. Column 3 of table 6 presents the payment impacts associated with only the proposed new wage index methodology without the 5 percent cap on decreased wage indices (with an appropriate wage index budget neutrality adjustment following the established methodology discussed at section II.B.4.b) for the purpose of demonstrating its potential long-term ramifications. For comparison, column 4 of table 6 presents the same payment impacts with the 5 percent cap applied. The figures in these columns represent the expected payment change associated from the move from the CY 2025 ESRD PPS legacy wage index to the proposed new wage index methodology. As an example, this table shows that rural ESRD facilities would see a payment increase of 1.014 (or an increase of 1.4 percent) without the 5 percent cap but only 1.007 (or 0.7 percent) with the 5 percent cap. One major driver of this discrepancy is the fact that changes to the ESRD PPS wage index are budget neutral, so by limiting the negative impact of the change on some facilities through the 5 percent cap, we reduce payments to ESRD facilities not impacted by the cap. Because the 5 percent cap would impact fewer ESRD facilities in each subsequent year by design, column 4 is not a reasonable proxy for long term payment impacts associated with this policy, but rather it represents the expected change in payment to ESRD facilities for CY 2025 as a result of only the proposed wage index methodology change.

Column 3 of table 6 shows the effect that this proposed new wage index methodology would have on ESRD facilities, stratified by facility type, Start Printed Page 55780 location, and size, without application of the 5 percent cap on any decrease in wage index values. These impacts still include the 0.600 wage index floor because, unlike the 5 percent cap on decreased wages, the wage index floor could affect an ESRD facility for every future year. The 5 percent cap, however, would likely only affect an ESRD facility for a limited number of years until its wage index value lines up with the wage index value for the CBSA in which it is located. We note that the ESRD PPS does not have a cap on wage index increases, so ESRD facilities located in CBSAs that receive a substantial increase in wage index value associated with this proposed new methodology would not have the impact of that change mitigated and, therefore, that change is reflected in the full impacts in section VIII.D.5.a of this proposed rule. However, without the 5 percent cap on wage index decreases the budget-neutrality factor applied to the ESRD PPS in the hypothetical model from which column 3 was derived is larger (the application of which would result in a smaller decrease to the ESRD PPS base rate), such that ESRD facilities that had a positive change in wage index would experience an even greater positive change.
For comparison, column 4 represents the impacts for CY 2025 with the 5 percent cap applied. As discussed previously, this is not a reasonable proxy for long term payment impacts because (assuming no other changes) the 5 percent cap on wage index decreases would apply to a lower number of ESRD facilities each year until ESRD facilities receive the wage index for the CBSA in which they are located. However, this column does show the impact of applying the 5 percent cap for CY 2025, both for ESRD facilities for which the cap would apply and other ESRD facilities that would receive lower payments due to budget neutrality.
Based on column 3 (as a proxy for long-term impacts), the use of the proposed new wage index methodology would result in a notable increase in payments to rural ESRD facilities and ESRD facilities located in the East South Central census region. Use of the proposed new wage index methodology would result in a notable decrease in payments to the Pacific census region and the United States Pacific Territories (that is, Guam, American Samoa, and the Northern Marianas Islands, which are the only Unites States Pacific Territories with an ESRD facility). Generally, we include the United States Pacific territories together with the Pacific census region, as that is the census region in which these territories are located according to the United States Census Bureau. However, for this analysis examining the effects of CMS' proposed wage index methodology we have opted to separate the territories from the Pacific census region, because we believe that it is important to evaluate the impact on these territories carefully due to their remote geographic location and resulting unique economic situation. Column 4 of table 6 shows how the application of the 5 percent cap mitigates these changes for CY 2025, as ESRD facilities in the United States Pacific territories would have a decrease in payment by a factor of only 0.964 rather than 0.930.
We note that the 5 percent cap on wage index decreases would apply to ESRD facilities that are located in a CBSA (based on CY 2025 CBSA delineations) with a wage index value 5 percent lower than the CY 2024 wage index value for their CBSA (based on CY 2024 CBSA delineations). The impacts detailed in column 3 are presented for the sole purpose of illustrating the potential long-term ramifications of the proposed new wage index methodology once sufficient time has passed such that the 5 percent cap on year-over-year decreases would no longer constrain the overall effect of this proposed new methodology on wage index values.
We have conducted an analysis comparing the hypothetical results of applying this new wage index methodology in past years to the actual ESRD PPS wage index methodology based on the IPPS wage index for those years. We have found that the application of the new wage index methodology consistently yields mean and median wage index values slightly higher than the actual mean and median wage index values used for those years, implying that the wage index resulting from this new methodology is relatively stable. Additionally, we have found that the payment impacts based on facility type did not change much when using data from claim years 2019 through 2022, with most facility types that are projected to receive a payment increase for CY 2025 associated with the proposed new wage index methodology seeing a payment increase in past years. Similarly, most facility types that are projected to receive a payment decrease in CY 2025 associated with the proposed new wage index methodology were found to have received payment decreases in our hypothetical analysis of past years. Therefore, we have determined that this new wage index methodology is relatively stable when analyzing the differences between the new proposed wage index and the ESRD PPS legacy wage index.
For CY 2025, we propose to update the wage indices to account for updated wage levels in areas in which ESRD facilities are located using the proposed new methodology described previously, in subpart b of this section, according to the most recent available data. We believe that the use of this proposed new methodology is appropriate and responds to the feedback we have received from interested parties regarding the limitations of the current wage index. Specifically, the use of BLS OEWS data would allow for this new wage index methodology to be more responsive to differences in ESRD facility wage levels across the country. Additionally, by using occupational mix data from the freestanding ESRD facility cost reports, this proposed methodology would better reflect the actual wage costs incurred by ESRD facilities. We believe that this proposed new methodology would be most appropriate to use for the ESRD PPS due to several reasons specific to ESRD facilities. First, freestanding ESRD facility cost reports contain detailed occupational FTE data, which allows CMS to create a wage index that is tailored to the wage costs faced by ESRD facilities based on their unique staffing needs. Dissimilarities between hospital occupation mix and ESRD facility occupational mix make the use of the IPPS data less appropriate for ESRD facilities. In addition, the ESRD PPS has a lower labor-related share than most other Medicare payment systems. [ 19 ] This proposed new ESRD PPS wage index methodology addresses these specific circumstances.
We recognize that there are several methodological limitations to using a wage index based on publicly available BLS OEWS data. Specifically, this data source lacks information on employee benefits and the full cost of contract labor and includes information from hospitals and other healthcare providers. However, we believe that the benefits of using this proposed new wage index methodology would outweigh these limitations, as the use of BLS OEWS wage data weighted by an occupational mix derived from freestanding ESRD facility cost report data would allow for a wage index that is more representative of the geographic Start Printed Page 55781 variation in wages faced by ESRD facilities.
For CY 2025, we are also proposing to use OMB's most recent CBSA delineations as published in OMB Bulletin No. 23-01, which is based on the data from the 2020 decennial census, for the purposes of the CY 2025 ESRD PPS wage index and rural facility adjustment. This is consistent with our historical practice of updating the CBSA delineations periodically according to the most recent OMB delineations, most recently in the CY 2021 ESRD PPS final rule ( 85 FR 71430 through 71434 ). We discuss this policy in greater detail in section II.B.2.f of this proposed rule. For more information on the OMB delineations we refer readers to the OMB Bulletin No. 23-01: https://www.whitehouse.gov/wp-content/uploads/2023/07/OMB-Bulletin-23-01.pdf .
To implement the proposed change in wage index methodology, we are proposing to amend the regulations at 42 CFR 413.196(d)(2) and 413.231(a) . Effective January 1, 2025, the amended § 413.196(d)(2) would state that CMS updates on an annual basis “The wage index using the most current wage data for occupations related to the furnishing of renal dialysis services from the Bureau of Labor Statistics and occupational mix data from the most recent complete calendar year of Medicare cost reports submitted in accordance with § 413.198(b).” The amended § 413.231(a) would state that “CMS adjusts the labor-related portion of the base rate to account for geographic differences in the area wage levels using an appropriate wage index (established by CMS) which reflects the relative level of wages relevant to the furnishing of renal dialysis services in the geographic area in which the ESRD facility is located.”
For CY 2025, we propose to update the ESRD PPS wage index to use the most recent BLS OEWS wage data and the most recent CY 2022 freestanding ESRD facility cost report occupational mix and treatment volume data available. At the time the analysis was conducted for this proposed rule, the most recent BLS OEWS wage data available represented May 2022. We propose that if more recent data become available after the development of this ESRD PPS proposed rule and before the publication of the ESRD PPS final rule (for example, the April 2024 release of May 2023 OEWS data, which was published after the analysis performed for this proposed rule), we would use such data, if appropriate, to determine the CY 2025 ESRD PPS wage index in the ESRD PPS final rule. The proposed CY 2025 ESRD PPS wage index is set forth in Addendum A and is available on the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ESRDpayment/End-Stage-Renal-Disease-ESRD-Payment-Regulations-and-Notices . Addendum A provides a crosswalk between the CY 2024 wage index and the proposed CY 2025 wage index. Addendum B provides an ESRD facility level impact analysis. Addendum B is available on the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ESRDpayment/End-Stage-Renal-Disease-ESRD-Payment-Regulations-and-Notices .
We are presenting a version of the current ESRD PPS wage index constructed using our established methodology with the most recent available data, which we are referring to as the ESRD PPS legacy wage index methodology. The purpose of presenting the legacy methodology with modifications is to illustrate an alternative to the proposed new methodology described previously for consideration by interested parties to facilitate comments on this proposed rule. The inclusion of a CY 2025 version of the ESRD PPS legacy wage index methodology allows for interested parties to compare wage index values under the current methodology and proposed new methodology. For the reasons previously discussed, we believe that the proposed new wage index methodology based on BLS data is the most appropriate for ESRD facilities; however, we intend to consider commenters' input on this proposal and the alternative wage index based on the established methodology (updated with the most recent data) when making a determination about the best approach in the final rule.
For this alternative wage index, we would use the ESRD PPS legacy wage index, which is based on the most recent pre-floor, pre-reclassified hospital wage data collected annually under the IPPS. The ESRD PPS legacy wage index values are calculated without regard to geographic reclassifications authorized for acute care hospitals under sections 1886(d)(8) and (d)(10) of the Act and utilize pre-floor hospital data that are unadjusted for occupational mix. For CY 2025, the updated wage data are generally for hospital cost reporting periods beginning on or after October 1, 2020, and before October 1, 2021 (FY 2021 cost report data). This CY 2025 version of the legacy wage index methodology includes the updates to OMB's CBSA delineations, as the proposal to update those delineations is separate from the proposal to use the new wage index methodology. Under this possible alternative wage index using the legacy ESRD PPS methodology, we would still use the most recent available OMB CBSA delineations.
Under this alternative methodology, we would update the ESRD PPS legacy wage index to use the most recent hospital wage data. We would update those data if more recent data become available after the publication of this proposed rule and before the publication of the final rule (for example, using a more recent estimate of the IPPS hospital wage data), and we would use such data, if appropriate, to determine the CY 2025 ESRD PPS alternative wage index in the final rule. The alternative CY 2025 ESRD PPS wage index is set forth in Addendum A and is available on the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ESRDpayment/End-Stage-Renal-Disease-ESRD-Payment-Regulations-and-Notices . Addendum A provides a crosswalk between the CY 2024 wage index and the alternative CY 2025 wage index. Addendum B provides an ESRD facility level impact analysis. Addendum B is available on the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ESRDpayment/End-Stage-Renal-Disease-ESRD-Payment-Regulations-and-Notices .
We believe that our proposed new ESRD PPS wage index methodology would more accurately estimate the geographic variation in wages paid by ESRD facilities when compared to the current ESRD PPS wage index based on the IPPS wage index. However, we acknowledge that this proposed new methodology, if finalized, would represent a significant change to the established ESRD PPS wage index methodology, both by changing the data sources and the calculations for the wage index. We are requesting comments on all aspects of the proposed new methodology, including the use of BLS OEWS data for CBSA-level wage estimates, the use of mean hourly wage (rather than median hourly wage), the use of freestanding ESRD facility cost reports for deriving occupational mix weights based on FTEs for each occupation per 1000 treatments as presented in the NEFOM, the use of the ESRD PPS legacy wage index for standardization, and the computational Start Printed Page 55782 steps used to calculate the wage index. We welcome any insights into potential methodological improvements, particularly related to some of the limitations of the new data sources discussed previously, including the absence of the cost of employee benefits and the full cost of contract labor in the BLS data, and the inability of this proposed methodology to capture differences in ESRD facility occupational mix across different geographic areas. Based on the comments we receive, we may modify the methodological steps used to calculate the wage index in the final rule. Additionally, we are requesting comments on the proposed use of the new wage index methodology compared to the established wage index methodology based on the IPPS wage index which was used to create the alternative ESRD PPS legacy wage index. We are also requesting comments on the distributional implications of this wage index proposal, with specific consideration to rural areas and remote or isolated areas such as the United States territories in the Pacific. Lastly, we are requesting comments on our proposal to begin using our new wage index methodology beginning on January 1, 2025.
As previously discussed in this proposed rule, the wage index used for the ESRD PPS is historically calculated using the most recent pre-floor, pre-reclassified hospital wage data collected annually under the IPPS and is assigned to an ESRD facility based on the labor market area in which the ESRD facility is geographically located. We are proposing a new wage index methodology that would similarly be based on the labor market in which an ESRD facility is located. ESRD facility labor market areas are delineated based on the CBSAs established by OMB. In accordance with our established methodology, we have historically adopted through rulemaking CBSA changes that are published in the latest OMB bulletin. Generally, OMB issues major revisions to statistical areas every 10 years, based on the results of the decennial census. However, OMB occasionally issues minor updates and revisions to statistical areas in the years between the decennial censuses.
In the CY 2015 ESRD PPS final rule ( 79 FR 66137 through 66142 ), we finalized changes to the ESRD PPS wage index based on the newest OMB delineations, as described in OMB Bulletin No. 13-01 [ 20 ] issued on February 28, 2013. We implemented these changes with a 2-year transition period ( 79 FR 66142 ). OMB Bulletin No. 13-01 established revised delineations for United States Metropolitan Statistical Areas, Micropolitan Statistical Areas, and Combined Statistical Areas based on the 2010 Census. OMB Bulletin No. 13-01 also provided guidance on the use of the delineations of these statistical areas using standards published on June 28, 2010, in the Federal Register ( 75 FR 37246 through 37252 ).
On July 15, 2015, OMB issued OMB Bulletin No. 15-01, [ 21 ] which updated and superseded OMB Bulletin No. 13-01 issued on February 28, 2013. These updates were based on the application of the 2010 Standards for Delineating Metropolitan and Micropolitan Statistical Areas to the United States Census Bureau population estimates for July 1, 2012, and July 1, 2013.
On August 15, 2017, OMB issued OMB Bulletin No. 17-01, [ 22 ] which updated and superseded OMB Bulletin No. 15-01 issued on July 15, 2015. These updates were based on the application of the 2010 Standards for Delineating Metropolitan and Micropolitan Statistical Areas to the United States Census Bureau population estimates for July 1, 2014, and July 1, 2015. In OMB Bulletin No. 17-01, OMB announced a new urban CBSA, Twin Falls, Idaho (CBSA 46300).
On April 10, 2018, OMB issued OMB Bulletin No. 18-03 [ 23 ] which updated and superseded OMB Bulletin No. 17-01 issued on August 15, 2017. On September 14, 2018, OMB issued OMB Bulletin No. 18-04, [ 24 ] which updated and superseded OMB Bulletin No. 18-03 issued on April 10, 2018. OMB Bulletin Numbers 18-03 and 18-04 established revised delineations for Metropolitan Statistical Areas, Micropolitan Statistical Areas, and Combined Statistical Areas, and provided guidance on the use of the delineations of these statistical areas. These updates were based on the application of the 2010 Standards for Delineating Metropolitan and Micropolitan Statistical Areas to the United States Census Bureau population estimates for July 1, 2015, and July 1, 2016. In the CY 2021 ESRD PPS final rule ( 85 FR 71430 through 71434 ), we finalized changes to the ESRD PPS wage index based on the most recent OMB delineations from OMB Bulletin No 18-04. This was the most recent time we have updated the labor market delineations used for the ESRD PPS and, as such, reflects the labor market delineations we used for CY 2024 ( 88 FR 76360 ).
In the July 16, 2021, Federal Register ( 86 FR 37777 ), OMB finalized a schedule for future updates based on results of the decennial Census updates to commuting patterns from the American Community Survey, an ongoing survey conducted by the Census Bureau. In accordance with that schedule, on July 21, 2023, OMB released Bulletin No. 23-01. A copy of OMB Bulletin No. 23-01 may be obtained at https://www.whitehouse.gov/wp-content/uploads/2023/07/OMB-Bulletin-23-01.pdf . According to OMB, the delineations reflect the 2020 Standards for Delineating Core Based Statistical Areas (“the 2020 Standards”), which appeared in the Federal Register on July 16, 2021 ( 86 FR 37770 through 37778 ), and the application of those standards to Census Bureau population and journey-to-work data (that is, 2020 Decennial Census, American Community Survey, and Census Population Estimates Program data).
We believe it is important for the ESRD PPS to use, as soon as reasonably possible, the latest available labor market area delineations to maintain a more accurate and up-to-date payment system that reflects the reality of population shifts and labor market conditions. We believe that using the most current OMB delineations would increase the integrity of the ESRD PPS wage index system by creating a more accurate representation of geographic variations in wage levels, especially given the proposed new wage index methodology discussed previously. We have carefully analyzed the impacts of adopting the new OMB delineations and find no compelling reason to delay implementation. Therefore, we are proposing to adopt the updates to the OMB delineations announced in OMB Bulletin No. 23-01 effective for CY 2025 under the ESRD PPS for use in determining both the wage index and the rural adjustment for ESRD facilities. This would be implemented along with the new ESRD PPS wage index methodology, if finalized, or along with the alternative ESRD PPS legacy wage Start Printed Page 55783 index based on IPPS data, should the proposed new wage index methodology not be finalized.
As previously discussed, we finalized a 5 percent permanent cap on any decrease to a provider's wage index from its wage index in the prior year in the CY 2023 ESRD PPS final rule ( 87 FR 67161 ). We are not proposing any additional transition policy for the CY 2025 wage index as we believe the 5 percent cap effectively mitigates the negative impact of large wage index decreases for an ESRD facility in a single year. In addition, we are proposing to phase out the rural adjustment for ESRD facilities that are transitioning from rural to urban based on these CBSA revisions, as discussed in section II.B.2.f.(2) of this proposed rule. For a further discussion of changes to OMB's CBSA delineations, including a list of changes to specific CBSAs, see the FY 2025 IPPS proposed rule ( 89 FR 36139 ).
In the CY 2016 ESRD PPS final rule ( 80 FR 69001 ), we established a policy to provide a 0.8 percent payment adjustment to the base rate for ESRD facilities located in a rural area. This adjustment was based on a regression analysis, which indicated that the per diem cost of providing renal dialysis services for rural facilities was 0.8 percent higher than that of urban facilities after accounting for the influence of the other variables included in the regression. This 0.8 percent adjustment has been part of the ESRD PPS each year since it was finalized beginning for CY 2016, and its inclusion in the ESRD PPS is codified at § 413.233.
As previously discussed in this proposed rule, we are proposing a methodological change to the ESRD PPS wage index methodology as well as changes to the CBSA delineations. In the CY 2023 ESRD PPS final rule, we finalized a policy to cap year-to-year decreases in the wage index for any ESRD facility at 5 percent ( 87 FR 67161 ). The primary purpose of this change was to mitigate the negative effect associated with an ESRD facility being reclassified into a lower wage index CBSA as a result of changes in OMB's most recent CBSA delineations. We anticipate that the proposed change to the CBSA delineations and the changes to the wage index methodology, if finalized, would lead to numerous ESRD facilities having a significant decrease in wage index value in CY 2025 compared to CY 2024. As previously discussed, the adoption of OMB Bulletin No. 23-01 would determine whether an ESRD facility is classified as urban or rural for purposes of the rural facility adjustment in the ESRD PPS. Although the rural facility adjustment is not directly related to the wage index, the application of both is determined by the CBSA in which an ESRD facility is located and, therefore, is potentially subject to significant changes associated with the new CBSA delineations. It is reasonable to conclude that these proposed shifts in the CBSA delineations, in combination with the wage index methodological changes proposed in this proposed rule, could lead to a year-over-year decrease in payment greater than what a 5 percent decrease to the wage index would cause even if the decrease in the wage index value alone would be less than 5 percent. To mitigate the scope of changes that would impact ESRD facilities in any single year, we are proposing to implement a 3-year phase out of the rural facility adjustment for ESRD facilities that are located in a CBSA that was categorized as rural in CY 2024 and is recategorized as urban in CY 2025, as a result of the updates to the CBSA delineations associated with the proposed adoption of OMB Bulletin No. 23-01.
Overall, we believe implementing updated OMB delineations would result in the rural facility adjustment being applied where it is appropriate to adjust for higher costs incurred by ESRD facilities in rural locations. However, we recognize that implementing these proposed changes, if finalized, would have different effects among ESRD facilities and that the loss of the rural facility adjustment could lead to some hardship for ESRD facilities that had anticipated receiving the rural facility adjustment in CY 2025. Therefore, we believe it would be appropriate to consider whether a transition period should be used to implement these proposed changes.
For ESRD facilities located in a county that transitioned from rural to urban in OMB Bulletin 23-01, we considered whether it would be appropriate to phase out the rural facility adjustment for affected ESRD facilities. Adoption of the updated CBSAs in OMB Bulletin 23-01, if finalized as proposed, would change the status of 44 ESRD facilities currently designated as “rural” to “urban” for CY 2025 and subsequent CYs. As such, these 44 newly urban ESRD facilities would no longer receive the 0.8 percent rural facility adjustment. Consistent with the rural transition policy proposed for Inpatient Psychiatric Facilities (IPFs) and Inpatient Rehabilitation Facilities (IRFs) for FY 2025 ( 89 FR 23188 , 89 FR 22267 through 22268 ) we are proposing a 3-year, budget neutral phase-out of the rural facility adjustment for ESRD facilities located in the 54 rural counties that would become urban under the new OMB delineations, given the potentially significant payment impacts for these ESRD facilities. We believe that a phase-out of the rural facility adjustment transition period for these 44 ESRD facilities would be appropriate, because we expect these ESRD facilities would experience a steeper and more abrupt reduction in their payments compared to other ESRD facilities. We are proposing to adopt these new CBSA delineations in a year in which we are also proposing substantial methodological changes to our wage index. While these proposed changes, if finalized, would increase payment accuracy across the ESRD PPS, we also recognize that some ESRD facilities could lose the rural facility adjustment and receive a significantly lower wage index value in the same year. We believe that it is appropriate for this proposed transition policy to be budget-neutral compared to ending the rural adjustment for these facilities in CY 2025 because it is an extension of the rural facility adjustment, which is implemented budget-neutrally, and a result of the change in CBSA delineations, which is proposed to be implemented budget-neutrally alongside the wage index changes. The reasoning behind this proposal is similar to the reasoning behind the 5 percent cap on year-to-year decreases in wage index values which was finalized in the CY 2023 ESRD PPS final rule ( 87 FR 67161 ), as it would ameliorate unexpected negative impacts to certain ESRD facilities. This rural phase-out in combination with the 5 percent cap policy would best reduce the negative effects on any single ESRD facility resulting from changes to the CBSA delineations. Therefore, we are proposing to phase out the rural facility adjustment for these facilities to reduce the impact of the loss of the CY 2024 rural facility adjustment of 0.8 percent over CYs 2025, 2026, and 2027, consistent with the similar IPF and IRF proposals previously discussed. This policy would allow ESRD facilities that are classified as rural in CY 2024 and would be classified as urban in CY 2025 to receive two-thirds of the rural facility adjustment for CY 2025, or a 0.53 percent adjustment. For CY 2026, these ESRD facilities would receive one-third of the rural facility adjustment, or a 0.27 Start Printed Page 55784 percent adjustment. For CY 2027, these ESRD facilities would not receive a rural facility adjustment. We believe a 3-year budget-neutral phase-out of the rural facility adjustment for ESRD facilities that transition from rural to urban status under the new CBSA delineations would best accomplish the goals of mitigating the loss of the rural facility adjustment for existing CY 2024 rural ESRD facilities. The purpose of the gradual phase-out of the rural facility adjustment for these ESRD facilities is to mitigate payment reductions and promote stability and predictability in payments for existing rural ESRD facilities that may need time to adjust to the loss of their CY 2024 rural payment adjustment or that experience a reduction in payments solely because of this re-designation. This policy would be specifically for ESRD facilities that are rural in CY 2024 that become urban in CY 2025. We are not proposing a transition policy for urban ESRD facilities that become rural in CY 2025 because these ESRD facilities would receive the full rural facility adjustment of 0.8 percent beginning January 1, 2025, and they would not experience the same adverse effects as an ESRD facility that unexpectedly loses a payment adjustment. We understand that compared to rural payment adjustments in other Medicare payment systems, the ESRD PPS rural facility adjustment is not large in magnitude (for example, the rural adjustments for IPFs and IRFs are 17 percent and 14.9 percent, respectively), but it is important for ESRD facilities to be able to reasonably predict what their payments from the ESRD PPS would be in the next year. We solicit comments on this proposed policy.
Section 1881(b)(14)(D)(ii) of the Act requires that the ESRD PPS include a payment adjustment for high cost outliers due to unusual variations in the type or amount of medically necessary care, including variability in the amount of erythropoiesis stimulating agents (ESAs) necessary for anemia management. Some examples of the patient conditions that may be reflective of higher facility costs when furnishing dialysis care are frailty and obesity. A patient's specific medical condition, such as secondary hyperparathyroidism, may result in higher per treatment costs. The ESRD PPS recognizes that some patients require high cost care, and we have codified the outlier policy and our methodology for calculating outlier payments at § 413.237.
Section 413.237(a)(1) enumerates the following items and services that are eligible for outlier payments as ESRD outlier services: (i) Renal dialysis drugs and biological products that were or would have been, prior to January 1, 2011, separately billable under Medicare Part B; (ii) Renal dialysis laboratory tests that were or would have been, prior to January 1, 2011, separately billable under Medicare Part B; (iii) Renal dialysis medical/surgical supplies, including syringes, used to administer renal dialysis drugs and biological products that were or would have been, prior to January 1, 2011, separately billable under Medicare Part B; (iv) Renal dialysis drugs and biological products that were or would have been, prior to January 1, 2011, covered under Medicare Part D, including renal dialysis oral-only drugs effective January 1, 2025; and (v) Renal dialysis equipment and supplies, except for capital-related assets that are home dialysis machines (as defined in § 413.236(a)(2)), that receive the transitional add-on payment adjustment as specified in § 413.236 after the payment period has ended. [ 25 ]
In the CY 2011 ESRD PPS final rule ( 75 FR 49142 ), CMS stated that for purposes of determining whether an ESRD facility would be eligible for an outlier payment, it would be necessary for the ESRD facility to identify the actual ESRD outlier services furnished to the patient by line item (that is, date of service) on the monthly claim. Renal dialysis drugs, laboratory tests, and medical/surgical supplies that are recognized as ESRD outlier services were specified in Transmittal 2134, dated January 14, 2011. [ 26 ] We use administrative issuances and guidance to continually update the renal dialysis service items available for outlier payment via our quarterly update CMS Change Requests, when applicable. For example, we use these issuances to identify renal dialysis oral drugs that were or would have been covered under Part D prior to 2011 to provide unit prices for determining the imputed MAP amounts. In addition, we use these issuances to update the list of ESRD outlier services by adding or removing items and services that we determined, based our monitoring efforts, are either incorrectly included or missing from the list.
Under § 413.237, an ESRD facility is eligible for an outlier payment if its imputed (that is, calculated) MAP amount per treatment for ESRD outlier services exceeds a threshold. In past years, the MAP amount has reflected the average estimated expenditure per treatment for services that were or would have been considered separately billable services prior to January 1, 2011. The threshold is equal to the ESRD facility's predicted MAP per treatment plus the fixed dollar loss (FDL) amount. As described in the following paragraphs, the ESRD facility's predicted MAP amount is the national adjusted average ESRD outlier services MAP amount per treatment, further adjusted for case-mix and facility characteristics applicable to the claim. We use the term “national adjusted average” in this section of this proposed rule to more clearly distinguish the calculation of the average ESRD outlier services MAP amount per treatment from the calculation of the predicted MAP amount for a claim. The average ESRD outlier services MAP amount per treatment is based on utilization from all ESRD facilities, whereas the calculation of the predicted MAP amount for a claim is based on the individual ESRD facility and patient characteristics of the monthly claim. In accordance with § 413.237(c), ESRD facilities are paid 80 percent of the per treatment amount by which the imputed MAP amount for outlier services (that is, the actual incurred amount) exceeds this threshold. ESRD facilities are eligible to receive outlier payments for treating both adult and pediatric dialysis patients.
In the CY 2011 ESRD PPS final rule and codified in § 413.220(b)(4), using 2007 data, we established the outlier percentage—which is used to reduce the per treatment ESRD PPS base rate to account for the proportion of the estimated total Medicare payments under the ESRD PPS that are outlier payments—at 1.0 percent of total payments ( 75 FR 49142 through 49143 ). We also established the FDL amounts that are added to the predicted outlier services MAP amounts. The outlier services MAP amounts and FDL amounts are different for adult and pediatric patients due to differences in the utilization of separately billable services among adult and pediatric Start Printed Page 55785 patients ( 75 FR 49140 ). As we explained in the CY 2011 ESRD PPS final rule ( 75 FR 49138 through 49139 ), the predicted outlier services MAP amounts for a patient are determined by multiplying the adjusted average outlier services MAP amount by the product of the patient-specific case-mix adjusters applicable using the outlier services payment multipliers developed from the regression analysis used to compute the payment adjustments.
Lastly, in the CY 2023 ESRD PPS final rule, we finalized an update to the outlier methodology to better target 1.0 percent of total Medicare payments ( 87 FR 67170 through 67177 ). We explained that for several years, outlier payments had consistently landed below the target of 1.0 percent of total ESRD PPS payments ( 87 FR 67169 ). Commenters raised concerns that the methodology we used to calculate the outlier payment adjustment since CY 2011 results in underpayment to ESRD facilities, as the base rate has been reduced by 1.0 percent since the establishment of the ESRD PPS to balance the outlier payment ( 85 FR 71409 , 71438 through 71439 ; 84 FR 60705 through 60706 ; 83 FR 56969 ). In response to these concerns, beginning with CY 2023, we began calculating the adult FDL amounts based on the historical trend in FDL amounts that would have achieved the 1.0 percent outlier target in the 3 most recent available data years. We stated in the CY 2023 ESRD PPS final rule that we would continue to calculate the adult and pediatric MAP amounts for CY 2023 and subsequent years following our established methodology. In that same CY 2023 ESRD PPS final rule, we provided a detailed discussion of the methodology we use to calculate the MAP amounts and FDL amounts ( 87 FR 67167 through 67169 ).
For CY 2025, we are proposing several methodological and policy changes to the ESRD PPS outlier policy to address a number of concerns that interested parties have raised in recent years. Although we note that the 1.0 percent outlier target was achieved in CY 2023, it was not achieved in the majority of the years since the establishment of the ESRD PPS in 2011. We expect that each of the proposed changes would support the ability of the ESRD PPS to continue targeting outlier payments at 1.0 percent in CY 2025 and subsequent years. We discuss each of these proposed changes in detail in the following sections.
In the CY 2011 ESRD PPS final rule we finalized a policy that only renal dialysis services that were or would have been separately billable prior to the inception of the ESRD PPS would be eligible for the outlier payment. In the CY 2011 ESRD PPS proposed rule we explained that we believed that any unusual variation in the cost of the renal dialysis services comprising the base rate under the ESRD PPS would likely to be due to variation in the items and services that were, at that time, separately billable under Part B or renal dialysis service drugs and biological products that were then covered under Part D ( 74 FR 49988 ). We received some comments at that time that requested CMS consider alternative ways to determine outlier eligibility, including expanding eligibility to all renal dialysis services. However, we noted that we did not have adequate data at that time to include all Composite Rate Services (that is, renal dialysis services included in the composite payment system established under section 1881(b)(7) of the Act and the basic case-mix adjusted composite payment system established under section 1881(b)(12) of the Act, as defined in regulation at § 413.171) in the outlier calculation ( 74 FR 49989 , 75 FR 49135 ).
In the CY 2019 ESRD PPS proposed rule we issued a comment solicitation on the potential expansion of outlier payments to composite rate supplies, drugs, and biological products ( 83 FR 34332 ). In this RFI, we detailed that such a change could promote appropriate payment for composite rate drugs once the TDAPA period has ended. Commenters' responses to this comment solicitation were mixed ( 83 FR 56969 through 56970 ). One commenter expressed that such a change would promote and incentivize the development of innovative new therapies and devices to treat the highly vulnerable ESRD adult and pediatric patient populations. Some commenters responded specifically regarding the TDAPA that extending availability of outlier payments would be particularly important when no additional money is being added to the base rate for the drug, as is the case with most drugs and biological products receiving the TDAPA. However, some commenters, including MedPAC, did not agree that such an expansion of the outlier eligible services would improve care, generally indicating that expanding the list of ESRD outlier services would hamper the outlier payment's functionality. One commenter stated that the purpose of the outlier adjustment was to pay for unusually costly patients, not new drugs and biological products, which the commenter felt the outlier payment was unable to do adequately. MedPAC commented that an outlier policy should act as a stop-loss insurance for medically necessary care, and outlier payments are needed when the ESRD PPS' payment adjustments do not capture all of the factors affecting providers' costs of delivering care. To that end, MedPAC stated that to develop an effective outlier policy, CMS must first develop accurate patient-level and facility-level payment adjustments. MedPAC further cautioned that should CMS expand the list of eligible ESRD outlier services, we should be clear as to what would qualify for the outlier payment.
In subsequent years, we took steps to expand the outlier policy to include certain potentially costly renal dialysis services that would have been included in the composite rate prior to the ESRD PPS. In the CY 2020 ESRD PPS final rule we finalized that any new and innovative renal dialysis equipment or supply would be eligible for the outlier adjustment after the end of the TPNIES period, regardless of whether it would have been separately billable prior to 2011 ( 84 FR 60697 ). In that rule, we explained that we believed allowing these items to be outlier eligible after the end of the TPNIES period would allow for these new and innovative supplies to be competitive with the other equipment and supplies also accounted for in the ESRD PPS base rate by establishing a level playing field where products could gain market share by offering the best practicable combination of price and quality ( 84 FR 60693 ). In the CY 2021 ESRD PPS final rule, we finalized that capital-related assets that are home dialysis machines will not become ESRD outlier services at the end of the TPNIES payment period ( 85 FR 71399 ). We explained that as assets, capital-related home dialysis machines are distinct from operating expenses such as the disposable supplies and leased equipment with no conveyed ownership rights. Unlike assets, these latter items are generally accounted for on a per patient basis and therefore, when used in excess of the average, constitute outlier use, which makes them eligible for outlier payments ( 85 FR 71424 ).
The definition of ESRD outlier services is codified at § 413.237(1)(a). Currently, drugs and biological products that were or would have been paid under the composite rate are not considered ESRD outlier services, and Start Printed Page 55786 costs for these drugs are not included in the calculation for outlier payments on ESRD PPS claims. Current regulations at § 413.171 define Composite Rate Services as: “Items and services used in the provision of outpatient maintenance dialysis for the treatment of ESRD and included in the composite payment system established under section 1881(b)(7) and the basic case-mix adjusted composite payment system established under section 1881(b)(12) of the Act.” Under our longstanding policy, drugs and biological products that are substitutes for composite rate drugs and biological products are considered to be included in the composite rate portion of the ESRD PPS. In the CY 2011 ESRD PPS final rule ( 75 FR 49048 ), we cited to existing guidance in the Medicare Benefit Policy Manual, Pub. 100-02, chapter 11, section 30.4.1, which explicitly stated, “drugs used in the dialysis procedure are covered under the facility's composite rate and may not be billed separately. Drugs that are used as a substitute for any of these items, or are used to accomplish the same effect, are also covered under the composite rate.” This guidance remains in effect and was subsequently re-designated to section 20.3.F of the same chapter.
In the CY 2024 ESRD PPS final rule ( 88 FR 76391 ), we finalized a policy to pay, beginning for CY 2024, a post-TDAPA add-on payment adjustment for any new renal dialysis drug or biological product that is considered included in the ESRD PPS base rate that has previously been paid for using the TDAPA under § 413.234(c)(1). This post-TDAPA add-on payment adjustment generally will be applied for a period of 3 years following the end of the TDAPA period for those products. We finalized that the post-TDAPA add-on payment adjustment amount will be calculated based on the most recent available 12 months of claims data and the latest available full calendar quarter of average sales price (ASP) data ( 88 FR 76396 ). We explained that we divide the total expenditure of the new renal dialysis drug or biological product by the total number of ESRD PPS treatments furnished during the same 12-month period. In addition, we finalized that we adjust the post-TDAPA add-on payment adjustment amount paid on claims by the patient-level case-mix adjustment factors; accordingly, we apply a reduction factor to the post-TDAPA add-on payment adjustment amount to account for the application of the patient-level case-mix adjustment factors. We codified these policies by revising § 413.234(c)(1)(i) and adding regulations at § 413.234(b)(1)(iii), (c)(1)(ii), (c)(3), and (g) that describe the post-TDAPA add-on payment adjustment and the calculation we use to determine the post-TDAPA add-on payment adjustment amount. In addition, we amended § 413.230 by adding reference to the post-TDAPA add-on payment adjustment in the calculation of the ESRD PPS per treatment payment amount.
In the same CY 2024 ESRD PPS final rule, we summarized comments regarding the outlier policy as it pertains to the post-TDAPA add-on payment adjustment ( 88 FR 76396 ). One commenter pointed out that the CY 2024 ESRD PPS proposed rule did not indicate whether the ESRD PPS outlier adjustment would apply to products for which a post-TDAPA add-on payment adjustment is calculated. In response, CMS stated that under current policy, after the end of the TDAPA period, a drug or biological product is considered an eligible outlier service only if it meets the requirements of § 413.237(a)(1). We clarified that any renal dialysis drug or biological product included in the calculation of the post-TDAPA add-on payment adjustment would be considered an eligible ESRD outlier service only if it meets the requirements of § 413.237(a)(1). However, we further clarified that under current policy, Korsuva®, the only renal dialysis drug with a TDAPA period ending in CY 2024, would not be considered an eligible ESRD outlier service after the end of its TDAPA period, because it is a substitute for diphenhydramine hydrochloride, which was included in the composite rate prior to 2011, and therefore does not meet the requirements of § 413.237(a)(1) (that is, it would not have been, prior to January 1, 2011, separately billable under Medicare Part B).
Most recently, we have heard concerns from interested parties that excluding drugs and biological products that are substitutes for—or are used to achieve the same effect as—composite rate drugs and biological products from the definition of ESRD outlier services could limit the ability of the ESRD PPS outlier adjustment to appropriately recognize the drivers of cost for renal dialysis services. We considered these concerns, as well as the comments we received in response to prior rulemaking, to develop proposed changes to the definition of ESRD outlier services.
We are proposing to change the definition of ESRD outlier services at § 413.237(a)(1) to include drugs and biological products that were or would have been included in the composite rate prior to the establishment of the ESRD PPS. We note that this proposal would expand outlier eligibility to longstanding drugs and biological products that were historically included in the composite rate, as well as newer drugs and biological products that are currently included in the calculation of the post-TDAPA add-on payment adjustment. As discussed in section II.B.3.c of this proposed rule, we are proposing technical changes to the calculation of outlier payments that would appropriately account for the post-TDAPA add-on payment adjustment for ESRD outlier services that are drugs and biological products.
First, we considered the original intent behind the policy to limit outlier payments to drugs that were or would have been separately billable prior to 2011, which was that these drugs were likely the main drivers of the variation in the costs of treatment ( 74 FR 49988 ). We continue to believe that an important aspect of the outlier adjustment should be its ability to target ESRD cases that are unusually costly. If the outlier adjustment methodology failed to recognize the main drivers of variation in the costs of ESRD treatment, then it could result in cases that are not unusually costly qualifying for the outlier adjustment, which would mean the impact of the outlier adjustment would be diluted. As we noted earlier in this proposed rule, many of the responses to the comment solicitation in the CY 2019 ESRD PPS proposed rule expressed concerns that expanding the scope of ESRD outlier services would potentially dilute the impact of the outlier adjustment. We considered the potential impact of expanding the definition of ESRD outlier services to include additional drugs and biological products not currently included. We agree with the commenters who noted that the purpose of the outlier payment is not to pay for new drugs and biological products ( 83 FR 56969 ). Rather, as we discussed in the CY 2011 ESRD PPS final rule ( 75 FR 49134 ), CMS established the current outlier policy, including the 1.0 percent outlier target, because it struck an appropriate balance between our objective of paying an adequate amount for the most costly, resource-intensive patients while providing an appropriate level of payment for those patients who do not qualify for outlier payments. Under our current policy, new renal dialysis drugs and biological products that are paid for using the TDAPA are not considered Start Printed Page 55787 ESRD outlier services. As we explained in the CY 2016 ESRD PPS final rule ( 80 FR 69023 ), this is because during the TDAPA period we make a payment adjustment for the specific drug in addition to the base rate, whereas outlier services have been incorporated into the base rate. In contrast, the post-TDAPA add-on payment adjustment is paid on all claims, and drugs that are included in the post-TDAPA add-on payment adjustment amount are considered included in the ESRD PPS base rate. As a result, the amount paid under the post-TDAPA add-on payment adjustment does not correspond to the amount of a drug or biological product used on a claim, which would not be accounted for in any existing payment adjustment other than the outlier adjustment. For example, our analysis shows that patients using Korsuva® have costs of approximately $150 per treatment; however, because this drug is not recognized as an ESRD outlier service, these costs are not accounted for in determining the payment amount for the claim. Beginning April 1, 2024, the CY 2024 post-TDAPA add-on payment adjustment for Korsuva® increases the payment amount per treatment by approximately $0.25, which is adjusted by the patient-level case-mix adjusters applicable to the claim. In aggregate, the post-TDAPA add-on payment adjustment accounts for 65 percent of the cost of furnishing Korsuva®; however, this payment is spread across all ESRD PPS treatments.
We are not proposing to expand outlier eligibility to drugs and biological products that are paid for using the TDAPA during the TDAPA payment period, as the TDAPA amount is based on the full price (100 percent of ASP) for the amount of such drugs that is utilized and billed on the claim.
We considered only expanding the definition of ESRD outlier services to include drugs and biological products that were previously paid for using the TDAPA. As commenters have noted, new renal dialysis drugs and biological products are likely to be drivers of cost, because these drugs are typically more expensive. We recognized the importance of supporting access to new renal dialysis drugs and biological products under the ESRD PPS through the establishment of the post-TDAPA add-on payment adjustment beginning in CY 2024 ( 88 FR 76391 ). We explained in the CY 2024 ESRD PPS final rule that we agreed with commenters who expressed concerns that the ESRD PPS' current mechanisms may not fully account for the costs of these new drugs ( 88 FR 76388 ). We noted that several commenters stated that the outlier adjustment and the ESRDB market basket updates cannot adequately account for these costs, and several organizations noted that if additional renal dialysis drugs and biological products with significant costs were incorporated into the outlier payment calculation, the threshold to qualify for outlier payments would increase dramatically, thus adversely affecting access to products traditionally eligible for the outlier payment adjustment. We described comments which expressed that this increase in the outlier threshold may also raise health equity concerns because, as we noted in the CY 2023 ESRD PPS final rule ( 87 FR 67170 through 67171 ), the outlier adjustment protects access for beneficiaries whose care is unusually costly. We recognized that if the outlier threshold were to increase significantly due to significant use of a new renal dialysis drug or biological product after the end of the TDAPA period, then ESRD facilities might be incentivized to avoid treating costlier beneficiaries.
We believe it would be appropriate for the definition of ESRD outlier services to include all drugs and biological products that previously were paid for using the TDAPA. The inclusion of these drugs and biological products would help ensure appropriate payment when a patient's treatment is exceptionally expensive due to an ESRD facility furnishing such drugs or biological products to the patient whose treatment requires them. In the CY 2011 ESRD PPS proposed rule, we explained that significant variations in formerly separately billable items and services could impair access to appropriate care, as an ESRD facility may have a disincentive to provide adequate treatment to those ESRD patients likely to have significantly higher than average costs ( 74 FR 49988 ). We believe ESRD facilities may face similar disincentives for furnishing drugs and biological products that previously received payment under the TDAPA. We believe that this change would also align with the statutory authority for the outlier adjustment under section 1881(b)(14)(D)(ii) of the Act by protecting patients' access to medically necessary care through a payment adjustment that more fully recognizes unusual variations in the type or amount of such care. Specifically, we believe this change would encourage ESRD facilities to take on ESRD patients who would potentially require expensive new drugs and biological products, promoting health equity for these patients who require costlier care. Additionally, the technical changes we are proposing in section II.B.3.c of this proposed rule would limit the impact of such drugs and biological products on the outlier threshold calculation, thereby enabling the ESRD PPS outlier adjustment to continue to protect access for beneficiaries whose care is unusually costly.
In light of the past comments described earlier in this section, we further considered whether expanding eligibility to all renal dialysis drugs and biological products that are Composite Rate Services, as defined at § 413.171, would be appropriate. As we have previously stated, the purpose of the outlier adjustment is to protect access for beneficiaries whose care is unusually costly. Although we continue to expect that the main drivers of cost would be drugs and biological products that were previously separately billable under Part B or Part D, or were previously paid for using the TDAPA, we nevertheless recognize that some patients could require higher utilization of composite rate drugs and biological products, which may result in the overall cost of their renal dialysis care being unusually high. For example, as noted in section II.B.3.e of this proposed rule, our analysis has identified that certain composite rate drugs are significant drivers of cost for pediatric patients, and therefore the proposed inclusion of those drugs as ESRD outlier services would improve the ability of the ESRD PPS outlier adjustment to target payment for pediatric patients whose care is exceptionally costly. Including composite rate drugs and biological products in the calculation of the outlier adjustment could appropriately support care for such ESRD patients, because payments under the outlier adjustment would better align with resource use.
We also considered the comments from MedPAC in response to the CY 2019 ESRD PPS proposed rule. Specifically, MedPAC stated that to develop an effective outlier policy, CMS must first develop accurate patient-level and facility-level payment adjustments. As we stated in the CY 2024 ESRD PPS final rule, interested parties have encouraged CMS to develop a patient cost model that is based on a single patient-level cost variable that accounts for all composite rate and formerly separately billable services ( 88 FR 76399 ). We finalized the collection of time on machine data, beginning for CY 2025, which we stated would allow for a higher proportion of composite rate costs to be allocated to patients with longer renal dialysis treatment times, and ultimately inform CMS refinements to existing patient-level adjusters, Start Printed Page 55788 including age and comorbidities ( 88 FR 76400 ). We believe that expanding the definition of ESRD outlier services could further support our understanding of the costs of Composite Rate Services, because it would encourage more comprehensive reporting of renal dialysis drugs and biological products that were formerly included in the composite rate for the purposes of calculating outlier payments. This increased reporting would in turn support future revisions to patient-level adjustment factors that consider more complete information about costs at the patient level.
We do not agree that the proposed inclusion of composite rate drugs and biological products would dilute the impact of the outlier adjustment, as some commenters in response to the CY 2019 ESRD PPS proposed rule suggested. Rather, our analysis indicates that the inclusion of these drugs and biological products would appropriately recognize the situations when the provision of these services is unusually costly, which we estimate would increase the amount of outlier payment per outlier-eligible claim, thereby more effectively protecting access for beneficiaries whose care is exceptionally costly. As discussed in section II.B.3.e. of this proposed rule, if we made no changes to our outlier methodology or the definition of ESRD outlier services for CY 2025, the average outlier payment for outlier-eligible cases among pediatric patients would be $25.02, and the average outlier payment for adult patients would be $53.45. Under the proposed changes to outlier eligibility, the average outlier payment for pediatric and adult patients would increase to $73.24 and $57.16, respectively. Furthermore, as discussed later in section II.B.3.e of this proposed rule, the inclusion of composite rate drugs and biological products would increase the pediatric MAP amount by a large amount, reflecting the utilization of certain high-cost composite rate drugs. Although the proposed CY 2025 adult MAP amount is lower than the final CY 2024 adult MAP amount, we note that the proposed adult MAP amount for CY 2025 is approximately $0.79 higher than it would be absent the proposed policy changes in this rule, which demonstrates that the inclusion of composite rate drugs and biological products would result in a higher MAP amount for adults.
In summary, the inclusion of composite rate drugs and biological products as ESRD outlier services would include more costs in the calculation of the ESRD PPS outlier adjustment for each case. As a result, fewer claims would qualify for outlier payments, but the amount of outlier payment per claim would be higher. Therefore, rather than diluting the impact of the outlier adjustment, these proposed changes would increase the impact of the outlier adjustment.
We are proposing to amend the language at 42 CFR 413.237 by adding a new paragraph (a)(1)(vii), which would add to the list of renal dialysis services defined as ESRD outlier services the following: “Renal dialysis drugs and biological products that are Composite Rate Services as defined in § 413.171.”
As we discussed in the CY 2023 ESRD PPS final rule ( 87 FR 67169 ), a claim is eligible for outlier payment when its imputed MAP amount exceeds the sum of the predicted MAP amount and the fixed dollar loss threshold. The predicted MAP amount for a claim is based on the national average MAP amount, adjusted by the case-mix adjustment factors that apply for that claim's patient-level and facility-level characteristics. As a result, when a claim's adjustment factors increase the payment amount per treatment, the claim's predicted MAP is also increased. This is because we expect that more complex patients would require a higher amount of spending for outlier services. However, this higher expected cost is recognized through a higher per treatment payment amount. In other words, a more complex patient must have even higher costs than are already accounted for in the adjustment factors compared to a less complex patient to be considered unusually costly. By increasing the predicted MAP based on the case-mix adjustment factors, the ESRD PPS outlier policy ensures that only cases that are unusually costly are considered for outlier payment.
As previously discussed in this proposed rule, we finalized a post-TDAPA add-on payment adjustment in the CY 2024 ESRD PPS final rule. The post-TDAPA add-on payment adjustment for certain new renal dialysis drugs and biological products is generally applied for 3 years after the end of the TDAPA period ( 88 FR 76388 through 76397 ). The amount of this post-TDAPA add-on payment adjustment that is applied to an ESRD PPS claim is adjusted by any applicable patient-level case-mix adjustments under § 413.235, and this adjusted amount is added to the payment amount for each ESRD PPS treatment billed. We explained in the CY 2024 ESRD PPS final rule that during this 3-year post-TDAPA add-on payment period, a drug or biological product would be eligible for the outlier add-on payment if it met all of the other criteria for the outlier payment ( 88 FR 76396 ). The only drug or biological product which was set to end its TDAPA period in CY 2024 (and therefore would receive the post-TDAPA add-on payment adjustment that year) was Korsuva®, which is a substitute for a composite rate drug and, therefore, not outlier eligible under existing § 413.237(a)(1) ( 88 FR 76396 ). Therefore, we did not propose any changes to the ESRD PPS outlier methodology to account for the post-TDAPA add-on payment adjustment in the CY 2024 ESRD PPS proposed rule as that would not have affected payments for CY 2024.
As noted previously, we are proposing to expand outlier eligibility to include renal dialysis drugs and biological products that are Composite Rate Services as defined in § 413.171. This would mean that new drugs and biological products that are included in the calculation of the post-TDAPA add-on payment adjustment amount would become outlier eligible after the end of the TDAPA period, regardless of whether they are substitutes for composite rate drugs or biological products.
We are also proposing changes to the ESRD PPS outlier methodology to account for any future drugs and biological products which are outlier eligible during the post-TDAPA period. We propose to add the case-mix adjusted post-TDAPA add-on payment adjustment amount to the predicted MAP for a patient. This is appropriate because the post-TDAPA add-on payment adjustment amount represents average utilization of a drug or biological product, and is added to the payment amount, adjusted by the case-mix adjusters for the patient. This would prevent duplicate payment for these drugs and biological products by accounting for the portion of the cost for these drugs or biological products which is included in the ESRD PPS bundled payment. We note that this proposed change would not affect the calculation of the imputed MAP for a claim, because a claim's imputed MAP would include the actual utilization of the drug or biological product that is included in the calculation of the post-TDAPA add-on payment adjustment, if that drug or biological product is billed on the claim.
We considered proposing to modify the average MAP amount to account for outlier eligible drugs and biological products that are already included in the calculation of the post-TDAPA add- Start Printed Page 55789 on payment adjustment amount, rather than proposing to modify the predicted MAP amount for each claim. However, we note two main limitations with taking such an approach. First, the average MAP is set annually for an entire year and does not change from quarter to quarter; in contrast, the post-TDAPA add-on payment adjustment amount can change from quarter to quarter depending on when a drug or biological product's TDAPA period ends and the number of drugs and biological products included in the calculation. Second, our longstanding methodology for calculating the predicted MAP for outlier payments applies the outlier services multipliers to the average MAP. However, when we calculate the post-TDAPA add-on payment adjustment amount for a claim, we apply the ESRD PPS case-mix adjusters, which are different from the outlier services multipliers. We believe it would be most appropriate to continue to apply the ESRD PPS case-mix adjusters to the post-TDAPA add-on payment adjustment amount for the purposes of outlier calculation, so that the estimate of a claim's expected spending would align with the calculation used for the post-TDAPA add-on payment adjustment. For these reasons, we believe that it is more appropriate and more operationally feasible to apply the case-mix adjusted post-TDAPA add-on payment adjustment amount to the predicted MAP for claims during the quarters in which the drug or biological product is receiving the post-TDAPA add-on payment adjustment, rather than publishing different average MAPs for different quarters of a single year.
For CY 2025, the impact of this technical modification would be a small increase to the pediatric and adult FDL amounts, due to the small post-TDAPA add-on payment adjustment amount calculated for each quarter of CY 2025, as discussed in section II.B.6 of this proposed rule. Without this proposed methodological change, the pediatric FDL amount would increase by $0.68. Likewise, the adult FDL amount would increase by $0.89. This proposed methodological change would avoid those increases, resulting in the proposed CY 2025 adult and pediatric MAP and FDL amounts shown in table 7 of this proposed rule. Although the effect would be small for CY 2025, we note that the proposed increase would be larger in potential future situations when utilization of a drug or biological product during the post-TDAPA payment period could be higher.
In the CY 2011 ESRD PPS final rule we finalized our ESRD PPS outlier methodology, which included our methodology for updating data from past years to the CY for which CMS is establishing payment rates ( 75 FR 49134 ). In the CY 2023 ESRD PPS final rule, we finalized an update to the outlier methodology to better target 1.0 percent of total Medicare payments ( 87 FR 67170 through 67177 ) by prospectively calculating the adult FDL amounts based on the historical trend in FDL amounts that would have achieved the 1.0 percent outlier target in the 3 most recent available data years. In that final rule we also clarified our longstanding methodology for updating data from prior years for the purposes of the outlier calculations ( 87 FR 67167 ). For drugs and biological products, we use a blended 4-quarter moving average of the ESRDB market basket price proxies for pharmaceuticals to inflate drug prices to the CY for which CMS is establishing payment rates. For laboratory tests, we inflate laboratory test prices to the CY for which CMS is establishing payment rates using a CPI forecast to estimate changes for years in which a new data reporting period will take place for the purpose of setting Clinical Laboratory Fee Schedule (CLFS) rates. [ 27 ] For supplies, we apply a 0 percent inflation factor, because these prices are based on predetermined fees or prices established by the Medicare contractor.
In the CY 2023 ESRD PPS final rule ( 87 FR 67173 ), we noted that MedPAC supported the proposed revisions to the FDL methodology, but also urged CMS to refine its approach for applying the pricing data that the agency uses to project future spending for outlier services, particularly for drugs. Specifically, MedPAC suggested CMS use a drug price inflation factor based on ASP values and noted that the ASP data that CMS uses to determine facilities' actual outlier payments might be a more accurate data source on drug prices than the ESRDB market basket pharmaceutical price proxies that are currently used.
As discussed in the following sections, we have undertaken analysis of prices for ESRD outlier services and are proposing several technical changes to the inflation factors.
As described earlier, we use a blended 4-quarter moving average of the ESRDB market basket price proxy for Pharmaceuticals to inflate drug prices to the upcoming CY for the purpose of estimating spending for outlier drugs and biological products in that CY. Historically, this 4-quarter moving average is a positive factor, meaning that our longstanding methodology for modeling outlier spending amounts assumes that prices for ESRD outlier drugs and biological product will increase. For example, the current projection of the CY 2025 price growth for ESRD outlier drugs and biological products, based on the ESRDB market basket price proxy for Pharmaceuticals for CY 2025, is 1.9 percent, based on the IGI 1st quarter 2024 forecast with historical data through the 4th quarter of 2023.
To compare the actual changes in prices for ESRD outlier drugs and biological products against the assumed rate of change derived from the ESRDB market basket price proxies, we constructed an index of prices for ESRD outlier drugs and biological products. As previously discussed in section II.B.3.b of this proposed rule, we are proposing to expand the definition of ESRD outlier services to include renal dialysis drugs and biological products that were or would have been included in the composite rate prior to the establishment of the ESRD PPS. Accordingly, our constructed drug price index included these drugs and biological products as well as drugs and biological products that have historically been included in the definition of ESRD outlier services.
Because the list of ESRD outlier drugs and biological products changes over time, we are proposing to derive a chained Laspeyres price index of the drugs and biological products included in the definition of the ESRD outlier services. A chained Laspeyres price index does not require a fixed basket of drugs and biological products during the observation window. We constructed and then trended forward the year-over-year change in price index levels for this outlier drug index to calculate a projected inflation factor for ESRD outlier drugs and biological products for CY 2025, using the following steps:
Step 1: We obtained the annual list of ESRD outlier service drugs and biological products that appear in ESRD Start Printed Page 55790 PPS claims during the CYs 2017 through 2023. These include both composite rate and formerly separately billable drugs and biological products.
Step 2: We obtained quarterly ASP for each drug and biological product during the same period 2017 through 2023, substituting annual ASP when quarterly information was not available.
Step 3: We obtained quarterly utilization data for each drug and biological product for the period 2017 through 2023.
Step 4: For each quarter, we established the base period as the prior quarter and held utilization fixed at the base period. We then constructed a Laspeyres price index based on all drugs and biological products that had price information in that quarter and the prior quarter.
Step 5: We chained together the quarterly indices starting from the 1st quarter 2017 through the 4th quarter 2023 to express price changes in the 4th quarter 2017 relative to the 1st quarter 2017. This step was repeated for all prior quarters, keeping the starting period fixed at the 1st quarter 2017.
Step 6: We calculated the percentage change between the current and prior 4th quarter chained price index for each year for CY 2021, 2022, and 2023, which we used as the annual drug price inflation factor for each year.
Step 7: Using the chained price indexes for the three most recent CYs (2021, 2022, and 2023), we used a linear regression to project forward these three historical inflation factors to determine the CY 2025 inflation factor.
Using this methodology, we calculated a projected inflation factor of −0.7 percent, meaning that prices for ESRD outlier drugs and biological products are projected to be 0.7 percent lower in CY 2025 relative to the prices of the ESRD outlier drugs and biological products in than in CY 2024. We note that our analysis of year-over year changes in prices for ESRD outlier drugs and biological products shows a consistent, downward trend in prices, which stands in contrast to the positive inflation factors we have historically used to model outlier payments. As a result, our modeling of outlier spending in prior years has assumed that outlier prices would increase, when the ASP data shows that overall the prices have decreased.
Based on the results of our analysis, we believe that applying an inflation factor based on the actual change in prices for ESRD outlier drugs and biological products would enable the ESRD PPS outlier adjustment to better target 1.0 percent of outlier payments in CY 2025, because such an inflation factor would better reflect the observed historical trend in spending and utilization for such drugs and biological products. Although we have historically used the ESRDB market basket price proxy for Pharmaceuticals as the basis of our inflation assumptions for outlier modeling, and we believe that market basket price proxies would continue to be a reasonable and technically appropriate source for such assumptions, we note that the market basket price proxies serve a distinctly different purpose than the inflation factors. As we explained in the CY 2023 ESRD PPS final rule ( 87 FR 67147 ), we select the most appropriate wage and price proxies currently available to represent the rate of price change for each expenditure category. In contrast, the purpose of the inflation factors used in our outlier modeling is to represent the expected rate of change in price and utilization, so that we can prospectively set accurate FDL and MAP amounts that will result in outlier payments that equal 1.0 percent of total ESRD PPS payments. Decreasing our estimates of future outlier spending, as we are proposing to do, would result in lower FDL and MAP amounts, thereby increasing the number of claims that could be eligible for the outlier payment adjustment and the amount of outlier payments that would be paid on each claim. Revising our assumptions about future spending for ESRD outlier drugs and biological products would improve the ability of the ESRD outlier adjustment to pay for the costliest ESRD PPS claims. Therefore, we are proposing to use the projected inflation factor for ESRD outlier services that are drugs and biological products derived from the historical trend in prices and utilization for ESRD outlier drugs, as described in the previous paragraph. In section II.B.3.e of this proposed rule, we present the proposed CY 2025 MAP and FDL amounts calculated using this proposed methodology.
As previously discussed, CMS uses different methodologies for the inflation factors for laboratory tests and supplies. We inflate laboratory test prices to the upcoming CY using a CPI forecast to estimate changes for years in which a new data reporting period will take place for the purpose of setting CLFS rates; however, the forecast estimate used since CY 2018 for the ESRD PPS outlier methodology has been 0, because there has been no updated reporting for most clinical diagnostic laboratory tests since the CY 2018 CLFS. For supplies, we apply a 0 percent inflation factor, because these prices are based on predetermined fees or prices established by the Medicare contractor. In the CY 2011 ESRD PPS proposed rule, we explained that we chose to use these factors so that the MAP would be based on pricing mechanisms currently in place for these services ( 74 FR49991 ).
The ESRDB market basket uses price proxies for goods and services included in furnishing renal dialysis services to determine the ESRDB market basket update. For example, the market basket price proxy for laboratory services is the PPI Industry for Medical and Diagnostic Laboratories (BLS series code #PCU621511621511) representing the change in the price of laboratory services conducted by medical and diagnostic laboratories reported on the ESRD facility cost reports. Similarly, the market basket price proxy for supplies is the PPI Commodity for Surgical and Medical Instruments (BLS series code #WPU1562) representing the change in the price of medical supplies reported on the ESRD facility cost reports.
We have considered whether these longstanding assumptions about price changes for laboratory tests and supplies would be appropriate for modeling changes in spending for outlier-eligible laboratory tests and supplies. Unlike with drugs and biological products, we do not have detailed historical pricing data for ESRD outlier laboratory tests and supplies to permit us to perform a similar analysis for these services as we did for drugs and biological products. However, we can compare the historical inflation factors we have used to the growth in the market basket price proxies for these categories of renal dialysis services. For supplies, we would typically assume a 0 percent update; however, the average 10-year historical growth in the PPI Commodity for Surgical and Medical Instruments is 0.9 percent. Likewise, in years when there is a CLFS data reporting period, we would typically use an inflation factor for laboratory tests based on a CPI projection, reduced by the productivity adjustment, through June of the year prior to the update year; however, the average 10-year historical annual growth for the PPI Industry for Medical and Diagnostic Laboratories is −0.4 percent.
Beginning for CY 2025, we are proposing to use the ESRDB market basket price proxies for laboratory tests and supplies for the purpose of calculating the growth in estimated spending for these outlier services in the upcoming CY. These would replace the current inflation factors which are used for laboratory tests and supplies. Compared to the current inflation Start Printed Page 55791 factors we use, we anticipate that the market basket price proxies for laboratory tests and supplies would more appropriately reflect the change in prices of the laboratory tests and supply costs that are used by ESRD facilities. We believe that using the market basket price proxies would better allow the ESRD PPS to estimate the changes in the prices of laboratory tests and supplies, which would improve the ability for CMS to target outlier payments at 1.0 percent of total ESRD PPS payments. We note that decreasing our estimates of future outlier spending would result in lower FDL and MAP amounts, thereby increasing the number of claims that could be eligible for the outlier payment adjustment and the amount of outlier payment that would be paid on each claim. Revising our assumptions about future spending for ESRD outlier drugs and biological products would improve the ability of the ESRD PPS outlier adjustment to pay for the costliest ESRD PPS claims. In section II.B.3.e of this proposed rule, we present the proposed CY 2025 MAP and FDL amounts calculated using these inflation factors.
For CY 2025, we are proposing to update the MAP amounts for adult and pediatric patients using the latest available CY 2023 claims data. We are proposing to update the ESRD outlier services FDL amount for pediatric patients using the latest available CY 2023 claims data, and to update the ESRD outlier services FDL amount for adult patients using the latest available claims data from CY 2021, CY 2022, and CY 2023, in accordance with the methodology finalized in the CY 2023 ESRD PPS final rule ( 87 FR 67170 through 67174 ). The latest available CY 2023 claims data showed outlier payments represented approximately 1.0 percent of total Medicare payments.
The impact of this proposed update is shown in table 7, which compares the outlier services MAP amounts and FDL amounts used for the outlier policy in CY 2024 with the updated proposed estimates for this proposed rule for CY 2025. The estimates for the proposed CY 2025 MAP amounts, which are included in column II of table 7, were inflation adjusted to reflect projected 2025 prices for ESRD outlier services, in accordance with the proposed changes to the inflation factors discussed in section II.B.3.d of this proposed rule.

As demonstrated in table 7, the estimated FDL per treatment that determines the CY 2025 outlier threshold amount for adults (column II; $49.46) is lower than that used for the CY 2024 outlier policy (column I; $71.76). The lower threshold is accompanied by a decrease in the adjusted average MAP for outlier services from $36.28 to $33.57. For pediatric patients, there is an increase in the FDL amount from $11.32 to $223.44. There is a corresponding increase in the adjusted average MAP for outlier services among pediatric patients, from $23.36 to $58.39. We note that this substantial increase in the outlier threshold for pediatric patients reflects the proposed inclusion of certain composite rate drugs for outlier consideration, notably Healthcare Common Procedure Coding System (HCPCS) code J2997 (Injection, alteplase recombinant, 1 mg). As a result, a smaller proportion of pediatric patients would receive outlier payments, but the average outlier payment amounts would be significantly higher.
We estimate that the percentage of patient months qualifying for outlier Start Printed Page 55792 payments in CY 2025 would be 7.18 percent for adult patients and 6.00 percent for pediatric patients, based on the 2023 claims data and methodology changes proposed in sections II.B.3.c and II.B.3.d of this proposed rule.
In the CY 2011 ESRD PPS final rule ( 75 FR 49081 ) and under § 413.220(b)(4), we reduced the per treatment base rate by 1.0 percent to account for the proportion of the estimated total payments under the ESRD PPS that are outlier payments as described in § 413.237. In the 2023 ESRD PPS final rule, we finalized a change to the outlier methodology to better achieve this 1.0 percent target ( 87 FR 67170 through 67174 ). Based on the CY 2023 claims, outlier payments represented approximately 1.0 percent of total payments, which has been our policy goal since the establishment of the ESRD PPS outlier adjustment. We believe the proposed methodological changes to the outlier calculation and the proposed change to the definition of ESRD outlier services would continue to effectively set the outlier MAP and FDL amounts for CY 2025 and future years, enabling the ESRD PPS to continue targeting outlier payments at 1.0 percent of total payments. We also note that the proposed recalibration of the FDL amounts would result in no change in payments to ESRD facilities for beneficiaries with renal dialysis items and services that are not eligible for outlier payments.
In the CY 2011 ESRD PPS final rule ( 75 FR 49071 through 49083 ), CMS established the methodology for calculating the ESRD PPS per-treatment base rate, that is, the ESRD PPS base rate, and calculating the per-treatment payment amount, which are codified at §§ 413.220 and 413.230. The CY 2011 ESRD PPS final rule also provides a detailed discussion of the methodology used to calculate the ESRD PPS base rate and the computation of factors used to adjust the ESRD PPS base rate for projected outlier payments and budget neutrality in accordance with sections 1881(b)(14)(D)(ii) and 1881(b)(14)(A)(ii) of the Act, respectively. Specifically, the ESRD PPS base rate was developed from CY 2007 claims (that is, the lowest per patient utilization year as required by section 1881(b)(14)(A)(ii) of the Act), updated to CY 2011, and represented the average per treatment MAP for composite rate and separately billable services. In accordance with section 1881(b)(14)(D) of the Act and our regulation at § 413.230, the per-treatment payment amount is the sum of the ESRD PPS base rate, adjusted for the patient specific case-mix adjustments, applicable facility adjustments, geographic differences in area wage levels using an area wage index, and any applicable outlier payment, training adjustment add-on, the TDAPA, the TPNIES, the post-TDAPA add-on payment adjustment, and the TPEAPA for CYs 2024, 2025 and 2026.
We are proposing an ESRD PPS base rate for CY 2025 of $273.20. This would be a 0.8 percent increase from the CY 2024 ESRD PPS base rate of $271.02. This proposed update reflects several factors, described in more detail as follows:
Wage Index Budget-Neutrality Adjustment Factor: We compute a wage index budget-neutrality adjustment factor that is applied to the ESRD PPS base rate. For CY 2025, we are not proposing any changes to the methodology used to calculate this factor, which is described in detail in the CY 2014 ESRD PPS final rule ( 78 FR 72174 ). We computed the proposed CY 2025 wage index budget-neutrality adjustment factor using treatment counts from the 2023 claims and facility-specific CY 2024 payment rates to estimate the total dollar amount that each ESRD facility would have received in CY 2024. The total of these payments became the target amount of expenditures for all ESRD facilities for CY 2025. Next, we computed the estimated dollar amount that would have been paid for the same ESRD facilities using the proposed CY 2025 ESRD PPS wage index and proposed labor-related share for CY 2025. As discussed in section II.B.2 of this proposed rule, the ESRD PPS wage index for CY 2025 includes the proposed new wage index methodology based on BLS data and the proposed use of the most recent OMB delineations based on 2020-census data. [ 28 ] The total of these payments becomes the new CY 2025 amount of wage-adjusted expenditures for all ESRD facilities. The wage index budget-neutrality factor is calculated as the target amount divided by the new CY 2025 amount. When we multiplied the wage index budget-neutrality factor by the applicable CY 2025 estimated payments, aggregate Medicare payments to ESRD facilities would remain budget neutral when compared to the target amount of expenditures. That is, the wage index budget-neutrality adjustment factor ensures that the wage index updates and revisions do not increase or decrease aggregate Medicare payments. The proposed CY 2025 wage index budget-neutrality adjustment factor is 0.990228. This proposed CY 2025 wage index budget-neutrality adjustment factor reflects the impact of all proposed wage index policy changes, including the proposed CY 2025 ESRD PPS wage index using the new ESRD PPS wage index methodology based on BLS data, the 5 percent cap on year-to-year decreases in wage index values, the updated CBSA delineations, the 3 year rural phase-out for ESRD facilities in currently-rural CBSAs that would become urban under the new delineations, and the labor-related share. We note that the application of the 5 percent cap on wage index decreases has a sizable impact on the budget-neutrality factor this year due to the proposed new wage index methodology. That is, because a substantial number of ESRD facilities would have experienced a greater than 5 percent decrease in wage index value as a result of the proposed new wage index methodology, the budget-neutrality adjustment factor needed to offset the effect of limiting those decreases to 5 percent is larger than we expect it would be in a typical year. We note that the proposed CY 2025 wage index budget-neutrality factor does not include any impacts associated with the TPEAPA, as was the case with last year's combined wage index-TPEAPA budget-neutrality factor. This is consistent with how we have historically applied budget neutrality for case-mix adjusters, including pediatric case-mix adjusters. We do not routinely apply a budget-neutrality factor to account for changes in overall payment associated with changes in patient case-mix in years in which we do not propose any changes to the case-mix adjustment amount. Although the TPEAPA was established under the authority in section 1881(b)(14)(D)(iv) of the Act, which does not require budget neutrality, we stated in the CY 2024 ESRD PPS final rule that we were implementing the TPEAPA in a budget neutral manner because it was similar to the pediatric case-mix adjusters, and it accounts for costs which would have been included in the cost reports used in the analysis conducted when we created the ESRD PPS bundled payment in the CY 2011 ESRD PPS final rule ( 88 FR 76378 ). Therefore, it would not be Start Printed Page 55793 appropriate to apply a budget-neutrality factor for the TPEAPA for CY 2025.
Market Basket Update: Section 1881(b)(14)(F)(i)(I) of the Act provides that, beginning in 2012, the ESRD PPS payment amounts are required to be annually increased by an ESRD market basket percentage increase. As discussed in section II.B.1.b.(1) of this proposed rule, the latest CY 2025 projection of the ESRDB market basket percentage increase is 2.3 percent. In CY 2025, this amount must be reduced by the productivity adjustment described in section 1886(b)(3)(B)(xi)(II) of the Act, as required by section 1881(b)(14)(F)(i)(II) of the Act. As previously discussed in section II.B.1.b.(2) of this proposed rule, the latest CY 2025 projection of the productivity adjustment is 0.5 percentage point, thus yielding a proposed CY 2025 productivity-adjusted ESRDB market basket update of 1.8 percent for CY 2025. Therefore, the proposed CY 2025 ESRD PPS base rate is $273.20 (($271.02 × 0.990228) × 1.018 = $273.20). We are also proposing that if more recent data become available after the publication of the proposed rule and before the publication of the final rule (for example, a more recent estimate of the market basket percentage increase or productivity adjustment), we would use such data, if appropriate, to determine the CY 2025 ESRDB market basket update in the final rule.
In the CY 2021 ESRD PPS final rule ( 85 FR 71427 ), we expanded eligibility for the TPNIES under § 413.236 to include certain capital-related assets that are home dialysis machines when used in the home for a single patient. To establish the TPNIES basis of payment for these items, we finalized the additional steps that the Medicare Administrative Contractors (MACs) must follow to calculate a pre-adjusted per treatment amount, using the prices they establish under § 413.236(e) for a capital-related asset that is a home dialysis machine, as well as the methodology that CMS uses to calculate the average per treatment offset amount for home dialysis machines that is used in the MACs' calculation, to account for the cost of the home dialysis machine that is already in the ESRD PPS base rate. For purposes of this proposed rule, we refer to this as the “TPNIES offset amount.”
The methodology for calculating the TPNIES offset amount is set forth in § 413.236(f)(3). Section 413.236(f)(3)(v) states that effective January 1, 2022, CMS annually updates the amount determined in § 413.236(f)(3)(iv) by the ESRD bundled market basket percentage increase factor minus the productivity adjustment factor. The TPNIES for capital-related assets that are home dialysis machines is based on 65 percent of the MAC-determined pre-adjusted per treatment amount, reduced by the TPNIES offset amount, and is paid for 2 CYs.
There are currently no capital-related assets that are home dialysis machines set to receive TPNIES for CY 2025, as the TPNIES payment period for the Tablo® System ended on December 31, 2023, and there are no TPNIES applications for CY 2025. However, as required by § 413.236(f)(3)(v), we propose to update the TPNIES offset amount annually according to the methodology described previously.
We propose a CY 2025 TPNIES offset amount for capital-related assets that are home dialysis machines of $10.18, based on the proposed CY 2025 ESRDB productivity-adjusted market basket update of 1.8 percent (proposed 2.3 percent market basket percentage increase reduced by the proposed 0.5 percentage point productivity adjustment). Applying the proposed update factor of 1.018 to the CY 2024 offset amount resulted in the proposed CY 2025 offset amount of $10.18 ($10.00× 1.018 = $10.18). We propose to update this calculation to use the most recent data available in the CY 2025 ESRD PPS final rule.
In the CY 2024 ESRD PPS final rule we finalized an add-on payment adjustment for certain new renal dialysis drugs and biological products, which would be applied for 3 years after the end of the TDAPA period ( 88 FR 76388 through 76397 ). This adjustment, known as the post-TDAPA add-on payment adjustment, is adjusted by the patient-level case-mix adjuster and is applied to every ESRD PPS claim. In that final rule we also clarified that for each year of the post-TDAPA period we would update the post-TDAPA add-on payment adjustment amounts based on utilization and ASP of the drug or biological product. For CY 2024 there is one drug, Korsuva® (difelikefalin), included in the calculation of the post-TDAPA add-on payment adjustment. In the CY 2024 ESRD PPS final rule ( 88 FR 76397 ), we finalized that the post-TDAPA add-on payment adjustment amount for Korsuva® would be $0.2493 and would begin on April 1, 2024.
For CY 2025, we will have two drugs included in the calculation of the post-TDAPA add-on payment adjustment. The post-TDAPA add-on payment adjustment period for one of these drugs, Korsuva®, began on April 1, 2024, so, conditional upon the continued receipt of the latest full calendar quarter of ASP data as described in § 413.234(c)(3), Korsuva® will be included in the calculation for the post-TDAPA add-on payment adjustment for the entirety of CY 2025. The other drug, Jesduvroq (daprodustat), began its 2-year TDAPA period on October 1, 2023, so its post-TDAPA add-on payment adjustment period will begin on October 1, 2025, conditional upon the continued receipt of the latest full calendar quarter of ASP data.
Based on the most recent utilization data, and following the calculation explained in the CY 2024 ESRD PPS final rule ( 88 FR 76388 through 76389 ) and § 413.234(g), the proposed post-TDAPA add-on payment adjustment amount for Korsuva® is $0.4047 for all 4 quarters of CY 2025. Under that same methodology, the proposed post-TDAPA add-on payment adjustment amount for Jesduvroq is $0.0019 for only the last quarter of CY 2025. We note that utilization data available at the time of this proposed rulemaking for Jesduvroq included only data from October 2023 through February 2024. As discussed in the CY 2024 ESRD PPS final rule ( 88 FR 76388 through 76389 ), we intend to update these calculations with the most recent available data in the final rule. Table 8 shows the proposed post-TDAPA add-on payment adjustment amounts for each quarter of CY 2025.

As discussed in the CY 2024 ESRD PPS final rule ( 88 FR 76393 ) and codified at 42 CFR 413.234(g) , we have finalized a post-TDAPA add-on payment adjustment, which is based on the most recent year of utilization data and is calculated annually in each rulemaking cycle. Under § 413.234(g)(1), CMS bases the post-TDAPA add-on payment adjustment calculation on the most recent 12-month period of utilization for the new renal dialysis drug or biological product and the most recent available full calendar quarter of ASP data. However, when a drug or biological product begins its TDAPA period in the fourth quarter of a CY, and, therefore, would be included in the post-TDAPA add-on payment adjustment calculation beginning in the fourth quarter 2 CYs later, there would likely not be a full year's worth of utilization data available at the time of proposed or final rulemaking for that CY due to the time-lag associated with collecting and processing utilization data for the final rule. For example, at the time of rulemaking for last year's ESRD PPS final rule, we had data available through June 2023 when calculating the post-TDAPA add-on payment adjustment amount for Korsuva® ( 88 FR 73697 ). However, for a drug or biological product that began its TDAPA payment period in October of the prior year, data from October through June would only represent 9 months of data. We believe it is important to have a full year's utilization data when determining the post-TDAPA add-on payment adjustment amount so that the post-TDAPA add-on payment adjustment appropriately captures the utilization of the drug or biological product as required by § 413.234(g)(1).
We are proposing that when there is insufficient data at the time of rulemaking, we would publish the post-TDAPA add-on payment adjustment amount via Change Request (CR) once we have a full 12 months of data. Specifically, we would publish the post-TDAPA add-on payment adjustment amount in a CR under the following circumstances: (1) a drug or biological product is ending its TDAPA period during the CY, and therefore under § 413.234(c)(1) will begin being included in the post-TDAPA add-on payment adjustment amount calculation during that CY; and (2) that drug or biological product does not have at least 12 full months of utilization data at the time the final rule is developed. We would still include an estimated post-TDAPA add-on payment adjustment amount in the proposed rule and update that estimated amount in the final rule, but we would note that the estimated amount presented in the final rule is subject to change. We note that the final post-TDAPA add-on payment adjustment amount published after the final rule could be higher or lower than the estimated amount presented in the final rule. We do not anticipate having less than a full year's utilization data at the time of rulemaking for drugs and biological products that begin receiving TDAPA payments in quarters other than the fourth quarter of the year; however, should such an instance arise, we would similarly publish the post-TDAPA add-on payment adjustment amount in a CR once 12 months of utilization data is available. We would indicate the quarterly release CR in which we intend to publish the final post-TDAPA add-on payment adjustment amount.
For CY 2025, there is one TDAPA drug, Jesduvroq, which is ending its TDAPA period in CY 2025 and for which we do not anticipate having a full 12 months' worth of utilization data at the time of final rulemaking. As such, we would indicate in the final rule that we intend to publish the post-TDAPA add-on payment adjustment amount for CY 2025 for Jesduvroq once we have a full year of utilization data. We generally intend to publish this updated post-TDAPA add-on payment adjustment amount two calendar quarters prior to the end of the TDAPA period, as this would allow for sufficient time to gather and analyze a year's worth of utilization data. For this drug, and for any drug or biological product that begins its TDAPA period in the fourth quarter of a CY, we would generally publish the post-TDAPA add-on payment adjustment amount at the beginning of the second quarter of the last CY of that drug or biological product's TDAPA period (that is, two calendar quarters before the drug is included in the post-TDAPA add-on payment adjustment amount). However, should circumstances arise that prevent us from calculating a post-TDAPA add-on payment adjustment amount at that time, we would publish the final post-TDAPA add-on payment adjustment amount at a later time.
This approach to publishing the post-TDAPA add-on payment adjustment amount calculation would not impact any drug or biological product that has at least one full year's worth of utilization data at the time when the analysis for the final rule is developed, nor would it impact any drug or biological product that is already Start Printed Page 55795 included in the post-TDAPA add-on payment adjustment calculation for a given CY. We do not intend to routinely update post-TDAPA add-on payment adjustment amounts quarterly, as we believe this would make it more difficult for ESRD facilities to estimate payments. However, for drugs or biological products that lack a full year's worth of utilization data at the time when the analysis for the final rule is developed, we believe it is appropriate to take this additional step to ensure that their post-TDAPA add-on payment adjustment is based on 12 months of utilization data as required by § 413.234(g)(1).
Section 1881(b)(14)(A)(i) of the Act requires the Secretary to implement a payment system under which a single payment is made to a provider of services or a renal dialysis facility for renal dialysis services in lieu of any other payment. Section 1881(b)(14)(B) of the Act defines renal dialysis services, and subclause (iii) of that section states that these services include other drugs and biologicals [ 29 ] that are furnished to individuals for the treatment of ESRD and for which payment was made separately under this title, and any oral equivalent form of such drug or biological.
When we implemented the ESRD PPS in 2011 ( 75 FR 49030 ), we interpreted this provision as including not only injectable drugs and biological products used for the treatment of ESRD (other than ESAs and any oral form of ESAs, which are included under clause (ii) of section 1881(b)(14)(B) of the Act), but also all oral drugs and biological products used for the treatment of ESRD and furnished under title XVIII of the Act. We also concluded that, to the extent oral-only drugs or biological products used for the treatment of ESRD do not fall within clause (iii) of section 1881(b)(14)(B) of the Act, such drugs or biological products would fall under clause (iv) of that section, and constitute other items and services used for the treatment of ESRD that are not described in clause (i) of section 1881(b)(14)(B) of the Act.
We finalized and promulgated payment policies for oral-only renal dialysis service drugs or biological products in the CY 2011 ESRD PPS final rule ( 75 FR 49038 through 49053 ). In that rule, we defined renal dialysis services at § 413.171 as including drugs and biological products with only an oral form. We also finalized a policy to delay payment for oral-only drugs under the ESRD PPS until January 1, 2014. Accordingly, we codified the delay in payment for oral-only renal dialysis service drugs and biological products at § 413.174(f)(6), and provided that payment to an ESRD facility for renal dialysis service drugs and biological products with only an oral form would be incorporated into the ESRD PPS payment rates effective January 1, 2014, once we had collected and analyzed adequate pricing and utilization data. Since oral-only drugs are generally not a covered service under Medicare Part B, this delay of payment under the ESRD PPS also allowed coverage to continue under Medicare Part D for those beneficiaries with such coverage.
In the CY 2011 ESRD PPS proposed rule ( 74 FR 49929 ), we noted that the only oral-only drugs that we identified were phosphate binders and calcimimetics, specifically, cinacalcet hydrochloride, lanthanum carbonate, calcium acetate, sevelamer hydrochloride, and sevelamer carbonate. All of these drugs fall into the ESRD PPS functional category for bone and mineral metabolism.
Since then, the Congress has acted three times to further delay the inclusion of oral-only renal dialysis service drugs and biological products in the ESRD PPS. Specifically, as discussed in section II.A.1 of this proposed rule, ATRA in 2013, as amended by PAMA in 2014, and amended by ABLE in 2014, ultimately delayed the inclusion of oral-only drugs into the ESRD PPS until January 1, 2025.
Section 217(c)(1) of PAMA also required us to adopt a process for determining when oral-only drugs are no longer oral-only and to incorporate them into the ESRD PPS bundled payment. Section 217(a)(2) of PAMA further amended section 632(b)(1) of ATRA by requiring that, in establishing payment for oral-only drugs under the ESRD PPS, the Secretary must use data from the most recent year available. In the CY 2016 ESRD PPS proposed rule ( 80 FR 37839 ), we noted that when the existing oral-only drugs (which were, at that time, only phosphate binders and calcimimetics) were determined no longer to be oral-only drugs, we would pay for them using the TDAPA. We stated that this would allow us to collect data reflecting current utilization of both the oral and injectable or intravenous forms of the drugs, as well as payment patterns and beneficiary co-pays, before we add these drugs to the ESRD PPS bundled payment.
In 2017, when an injectable calcimimetic became available, CMS issued a Change Request [ 30 ] to add all calcimimetics, including oral and injectable forms, to the ESRD PPS bundled payment beginning in CY 2018. CMS paid the TDAPA for calcimimetics for a period of 3 years (CY 2018 through CY 2020). When the TDAPA period ended, we went through rulemaking ( 85 FR 71410 ) to increase the ESRD PPS base rate beginning in CY 2021 to incorporate the cost of calcimimetics.
Most recently, in the CY 2023 ESRD PPS final rule ( 87 FR 67185 through 67186 ), we finalized a revision to the regulatory definition of an oral-only drug, effective January 1, 2025, to clarify our longstanding policy by specifying that an oral-only drug has no injectable functional equivalent. The effective date of this revised definition will coincide with the January 1, 2025, incorporation of oral-only drugs into the ESRD PPS under § 413.174(f)(6). The revised definition of oral-only drugs reflects that drugs with similar end-action effects are treated as equivalent under the ESRD PPS, consistent with our approach to designating drugs into ESRD PPS functional categories.
Existing regulations at § 413.174(f)(6) state that effective January 1, 2025, oral-only drugs will be paid for under the ESRD PPS. Although oral-only drugs are excluded from the ESRD PPS bundled payment until January 1, 2025, they are currently recognized as renal dialysis services as defined in regulation at § 413.171. Accordingly, CMS is planning to incorporate oral-only drugs into the ESRD PPS bundled payment beginning January 1, 2025, using the TDAPA, as described in the CY 2016 ESRD PPS final rule ( 80 FR 69027 ) and subsequent rules.
As we stated in the CY 2023 ESRD PPS final rule ( 87 FR 67180 ), if an injectable equivalent or other form of administration of phosphate binders were to be approved by FDA prior to January 1, 2025, the phosphate binders would no longer be considered oral-only drugs and would no longer be paid for outside the ESRD PPS. We stated that Start Printed Page 55796 we would pay for the oral and any non-oral version of the drug using the TDAPA under the ESRD PPS for at least 2 years, during which time we would collect and analyze utilization data. We stated that if no other injectable equivalent (or other form of administration) of phosphate binders is approved by the FDA prior to January 1, 2025, we would pay for these drugs using the TDAPA under the ESRD PPS for at least 2 years beginning January 1, 2025. CMS will use the same process that it used for calcimimetics to incorporate phosphate binders into the ESRD PPS beginning January 1, 2025. CMS discussed its process for incorporating calcimimetics in CMS Transmittal 1999, dated January 10, 2018, and in MLN Matters Number: MM10065. [ 31 32 ] Pricing for phosphate binders under the TDAPA will be based on pricing methodologies available under section 1847A of the Act. A new renal dialysis drug or biological product is paid for using the TDAPA, which is based on 100 percent of ASP. If ASP is not available then the transitional drug add-on payment adjustment is based on 100 percent of wholesale acquisition cost (WAC) and, when WAC is not available, the payment is based on the drug manufacturer's invoice. In such cases, CMS will undertake rulemaking to modify the ESRD PPS base rate, if appropriate, to account for the cost and utilization of phosphate binders in the ESRD PPS bundled payment.
We note that on October 17, 2023, a new oral phosphate lowering agent received FDA marketing approval. According to the FDA label information for this drug, XPHOZAH TM (tenapanor) is indicated to reduce serum phosphorus in adults with chronic kidney disease who are on dialysis. CMS has identified XPHOZAH TM to be a renal dialysis service because it is used to treat or manage a condition associated with ESRD. Specifically, it is used as an add-on therapy in patients who have an inadequate response to phosphate binders or who are intolerant of any dose of phosphate binder therapy. XPHOZAH TM tablets are taken orally, usually twice a day with meals. CMS has also determined that XPHOZAH TM meets the current regulatory definition of an oral-only drug as defined at § 413.234(a), and therefore, in accordance with § 413.174(f)(6), is not paid for under the ESRD PPS until January 1, 2025. Consistent with policies adopted in the CY 2016 and CY 2023 ESRD PPS final rules (see 80 FR 69025 and 87 FR 67183 ), XPHOZAH TM will be included in the ESRD PPS effective January 1, 2025, using the drug designation process under § 413.234.
As set forth in § 413.174(f)(6), effective January 1, 2025, payment to an ESRD facility for renal dialysis service drugs and biological products with only an oral form furnished to ESRD patients will be incorporated within the prospective payment system rates established by CMS in § 413.230 and separate payment will no longer be provided. As noted earlier in this section, we have recently published operational guidance, including information about TDAPA payment, HCPCS codes, and ASP reporting requirements and timelines for phosphate binders at https://www.cms.gov/files/document/including-oral-only-drugs-esrd-pps-bundled-payment.pdf . We note that we will use the same process that it used for calcimimetics to incorporate phosphate binders into the ESRD PPS beginning January 1, 2025, and that we will not be following this process for any other oral drugs or biological products. Manufacturers would need to apply for a HCPCS code and the TDAPA for any other oral drugs or biological products.
We note that for any other oral-only drugs, such as XPHOZAH TM , we will apply our drug designation process as we do for all new renal dialysis drugs and biological products, consistent with § 413.234 and the policy finalized in CY 2016 ESRD PPS final rule ( 80 FR 69027 ) and reiterated in the CY 2023 ESRD PPS final rule ( 87 FR 67180 ).
In the CY 2011 ESRD PPS final rule ( 75 FR 49043 ), we explained that there were certain advantages to delaying the implementation of payment for oral-only drugs and biological products under the ESRD PPS. These advantages included allowing ESRD facilities additional time to make operational changes and logistical arrangements to furnish oral-only renal dialysis service drugs and biological products to their patients.
In November 2023, in accordance with section 632(d) of ATRA, the Government Accountability Office (GAO) published a Report to Congressional Committees titled, “End-Stage Renal Disease: CMS Plans for including Phosphate Binders in the Bundled Payment.” (GAO-24-106288). [ 33 ] The report summarized the current status of payment for the phosphate binders as well as identifying areas of operational concerns. These include challenges related to hiring the staff needed for ESRD facilities to provide phosphate binders to patients, complexities relating to system updates needed to accommodate the volume and broad array of phosphate binders, and costs related to dispensing, storage, and transportation. The considerations identified in the GAO report generally align with the comments we have received on past ESRD PPS proposed rules. The GAO also interviewed dialysis organization representatives who stated that they are preparing to make the anticipated adjustments needed to dispense the phosphate binders.
With respect to considerations related to staffing, we note that the ESRD PPS includes payment for staffing related to the provision of renal dialysis services. We believe there are several strategies that ESRD facilities could employ to efficiently use available staff time to provide phosphate binders. There are parallels between the administration of phosphate binders and the administration of oral calcimimetics, which are also typically taken daily. First, we expect that patients with ESRD generally receive treatment for at least 3 hours per session, typically three times per week. We believe that during this treatment window there is generally staff availability to provide the patient with pre-packaged medication, which we note could include medication for multiple days. Second, ESRD facilities could maximize the efficiency of staff time by mailing the prescriptions, to the extent that doing so is consistent with state pharmacy laws. For example, the GAO report identified that one large dialysis organization only mails oral prescriptions to patients' homes, while others mail the medication to either the ESRD facility or the patient's home. Third, the GAO report identified that some ESRD facilities contract with outside pharmacies rather than operating their own pharmacy. By contracting with outside pharmacies, ESRD facilities could reduce or avoid the need to hire additional pharmacists and pharmacy staff to manage the volume of prescriptions.
Another challenge identified by the dialysis organizations was the complexity of dispensing phosphate binders because of the broad array of phosphate binders and the high volume of pills. [ 34 ] We acknowledge there are six Start Printed Page 55797 common types of phosphate binders as compared to only one type of calcimimetics. The GAO report also noted that unlike calcimimetics, phosphate binders are typically taken with every meal and snack. We note that although Medicare will begin paying for phosphate binders under the ESRD PPS beginning January 1, 2025, we are not establishing any requirements regarding how or where patients take these medications. These decisions are made and will continue to be made by the patient, nephrologist, and care team.
We recognize that updates may be required to ESRD facilities' systems, including electronic medical records, billing systems, and inventory management systems to accommodate new procedures for dispensing phosphate binders. As we previously noted, we initially delayed the incorporation of oral-only drugs into the ESRD PPS in 2011, in part to allow ESRD facilities to make such operational changes and logistical arrangements. In addition, we have provided operational guidance at https://www.cms.gov/files/document/including-oral-only-drugs-esrd-pps-bundled-payment.pdf that addresses HCPCS coding, billing, and price information. We expect that ESRD facilities will be able to make these system changes in advance of January 1, 2025.
Dialysis organizations have expressed concerns surrounding CMS using ASP to determine the TDAPA amount added to the ESRD PPS base rate for phosphate binders, which they believe does not adequately provide for dispensing cost. [ 35 ] Under current TDAPA policy, CMS plans to pay the TDAPA based on 100 percent of ASP for phosphate binders for at least 2 years. However, recognizing the high percentage of ESRD beneficiaries that have at least one phosphate binder prescription and the large volume of phosphate binder prescriptions, we are considering whether it may be appropriate to make additional payment to account for operational costs in excess of 100 percent of ASP, such as dispensing fees, when paying the TDAPA for phosphate binders. Unlike drugs and biological products for which payment is already included in the ESRD PPS base rate, including all other drugs and biological products in existing functional categories, dispensing fees and other costs are not currently included in the ESRD PPS base rate for phosphate binders. Therefore, we are considering whether a potential change in TDAPA payment policy for phosphate binders to account for such costs would be consistent with the TDAPA policy as finalized in the CY 2019 and CY 2020 ESRD PPS final rules ( 83 FR 56948 and 84 FR 60673 through 60676 ). For example, we may consider paying ASP + 6 percent for 2 years as we did for calcimimetics. As discussed in the CY 2011 ESRD PPS final rule, the amounts added to the ESRD PPS base rate for oral drugs at that time were based on data from Part D, which included dispensing fees ( 75 FR 49043 ). We are soliciting comment on the extent to which 100 percent of ASP is appropriate for TDAPA payment amount for phosphate binders and whether there are any costs associated with the inclusion of phosphate binders into the ESRD PPS bundled payment that may not be accounted for by 100 percent of ASP. CMS may finalize a change in the TDAPA payment amount for phosphate binders after considering comments on this topic.
As noted earlier, we have issued guidance [ 36 ] about the process we will use for paying the TDAPA for the phosphate binders and for their incorporation into the ESRD PPS bundled payment. This guidance addresses several key topics including billing information, information about the discarded drug policy, and information for manufacturers about reporting timelines for ASP data.
We anticipate that the incorporation of oral-only drugs into the ESRD PPS will increase access to these drugs for beneficiaries. We estimate that there will be an increase in Medicare spending as a result of this increase in access. Specifically, CMS has been monitoring and analyzing data regarding beneficiary access to Medicare Part D drugs; increases in expenditures for renal dialysis drugs paid under Medicare Part D; health equity implications of varying access to Medicare Part D drugs among patients with ESRD; and ESRD facility behavior regarding drug utilization. We have seen that incorporating Medicare Part D drugs into the ESRD PPS has had a significant positive effect of expanding access to such drugs for beneficiaries who do not have Medicare Part D coverage, with significant positive health equity impacts. For example, based on the results of our ESRD PPS monitoring analyses, in December 2017, prior to incorporation of calcimimetics into the ESRD PPS bundle, utilization was at 28.97 percent for African American/Black beneficiaries but went up to 35.31 percent in January 2018 and eventually to 39.04 percent in at the end of the TDAPA period for calcimimetics in December 2021. This 10.07 percentage point increase in utilization reflects the significant access improvement for African American/Black beneficiaries of incorporating formerly oral-only drugs into the ESRD PPS.
Lastly, as part of the preparation for the inclusion of phosphate binders into the ESRD PPS, CMS has monitored Part D utilization of, and spending for, phosphate binders. We have developed budgetary estimates of the changes in Medicare Part B and Part D spending, which are discussed in section VIII.C.1 of this proposed rule.
Section 1881(b)(14)(D)(iii) of the Act provides that the ESRD PPS shall include a payment adjustment that reflects the extent to which costs incurred by low-volume facilities (as defined by the Secretary) in furnishing renal dialysis services exceed the costs incurred by other facilities in furnishing such services, and for payment for renal dialysis services furnished on or after January 1, 2011, and before January 1, 2014, such payment adjustment shall not be less than 10 percent. Therefore, the ESRD PPS provides a facility-level payment adjustment to ESRD facilities that meet the definition of a low-volume facility.
Under § 413.232(b), a low-volume facility is an ESRD facility that, based on the submitted documentation: (1) furnished less than 4,000 treatments in each of the 3 cost reporting years (based on as-filed or final settled 12-consecutive month costs reports, whichever is most recent, except as specified in paragraphs (g)(4) and (5)) preceding the payment year; and (2) has not opened, closed, or received a new provider number due to a change in ownership (except where the change in ownership results in a change in facility type or as specified in paragraph (g)(6)) in the 3 cost reporting years (based on as-filed or final settled 12-consecutive month cost reports, whichever is most recent) preceding the payment year.
In addition, under § 413.232(c), for purposes of determining eligibility for the LVPA, the number of treatments considered furnished by the ESRD facility equals the aggregate number of treatments furnished by the ESRD Start Printed Page 55798 facility and the number of treatments furnished by other ESRD facilities that are both under common ownership with, and 5 road miles or less from, the ESRD facility in question. To receive the LVPA, an ESRD facility must submit a written attestation statement to its Medicare Administrative Contractor (MAC) confirming that it meets the requirements as specified in § 413.232 and qualifies as a low-volume ESRD facility. For purposes of determining eligibility for the LVPA, “treatments” mean total hemodialysis equivalent treatments (Medicare and non-Medicare). For peritoneal dialysis patients, one week of peritoneal dialysis is considered equivalent to three hemodialysis treatments ( 80 FR 68994 ). Section 413.232(e) generally imposes a yearly November 1 deadline for attestation submissions unless extraordinary circumstances justify an exception and specifies exceptions for certain years where the deadline is in December or January. The November 1 attestation timeframe provides 60 days for a MAC to verify that an ESRD facility meets the LVPA eligibility criteria ( 76 FR 70236 ). The ESRD facility would then receive the LVPA for all the Medicare-eligible treatments in the payment year. Once an ESRD facility is determined to be eligible for the LVPA, a 23.9 percent increase is applied to the ESRD PPS base rate for all treatments furnished by the ESRD facility ( 80 FR 69001 ).
In the CY 2011 ESRD PPS final rule ( 75 FR 49118 through 49125 ), we finalized the methodology used to target the appropriate population of ESRD facilities that were low-volume facilities based on a treatment threshold. After consideration of public comments, we originally established an 18.9 percent adjustment for ESRD facilities that furnish less than 4,000 treatments annually and indicated that this increase to the base rate would encourage small ESRD facilities to continue providing access to care.
In the CY 2016 ESRD PPS proposed rule ( 80 FR 37819 ), we analyzed ESRD facilities that met the definition of a low-volume facility under § 413.232(b) as part of the updated regression analysis and found that these ESRD facilities still had higher costs compared to other ESRD facilities. A regression analysis of low-volume facility claims from CYs 2012 and 2013 and cost report data indicated a multiplier of 1.239; therefore, we proposed an updated LVPA adjustment factor of 23.9 percent in the CY 2016 ESRD PPS proposed rule ( 80 FR 37819 ) and finalized this policy in the CY 2016 ESRD PPS final rule ( 80 FR 69001 ). This update was implemented budget neutrally alongside numerous other changes to the case-mix and facility-level adjusters. In CY 2022, 352 ESRD facilities received the LVPA. Using the most recent available data for CY 2023, the number of ESRD facilities receiving the LVPA was 330.
In the CY 2021 ESRD PPS final rule ( 85 FR 71443 ), we finalized a policy to allow ESRD facilities flexibility for LVPA eligibility due to the COVID-19 Public Health Emergency (PHE). Under § 413.232(g)(4), for purposes of determining ESRD facilities' eligibility for payment years 2021, 2022, and 2023, we only considered total dialysis treatments for any 6 months of their cost-reporting period ending in 2020. In the CY 2024 ESRD PPS final rule ( 88 FR 76344 ), we finalized changes to the LVPA regulation at § 413.232 that allow ESRD facilities affected by disasters and other emergencies to qualify for exceptions to certain eligibility requirements for the LVPA. Facilities may close and reopen if they experience an emergency, or they may temporarily exceed the 4,000-treatment threshold if they take on additional patients displaced by an emergency and still qualify for the LVPA.
Interested parties, including MedPAC and the GAO, [ 37 ] have recommended that we make refinements to the LVPA to better target ESRD facilities that are critical to beneficiary access to dialysis care in remote or isolated areas. [ 38 ] These groups and other interested parties have also expressed concern that the strict treatment count used to determine eligibility introduces a “cliff-effect” that may incentivize ESRD facilities to restrict their patient caseload to remain below the 4,000 treatments per year for the LVPA threshold. [ 39 ]
We considered several changes to the LVPA eligibility criteria to address the concerns that interested parties, including the GAO and MedPAC, raised about targeting LVPA payments to ESRD facilities that are necessary to protect access to care and are not located near other ESRD facilities. Specifically, these interested parties have requested that we take into consideration the geographic isolation of an ESRD facility within the LVPA methodology. Section 1881(b)(14)(D)(iii) of the Act requires that the LVPA must reflect the extent to which costs incurred by low-volume facilities (as defined by the Secretary) in furnishing renal dialysis services exceed the costs incurred by other facilities in furnishing such services. Our analysis has found that isolated low-volume facilities do not face higher costs than other low-volume facilities. Therefore, we do not believe that this requested change reconciles with the central statutory requirements and limitations for the LVPA, and we are considering alternative approaches, including potentially addressing this issue through a new payment adjustment separate from the LVPA based on section 1881(b)(14)(D)(iv) of the Act. Currently, we are analyzing claims and cost data regarding dialysis treatment levels and cost to inform options for potentially tailoring our methodology to meet the requirements of the statute, while simultaneously collecting additional data on geographic isolation of ESRD facilities. The ESRD PPS has separate facility-level payment adjustments for low-volume facilities, as set forth in 42 CFR 413.232 , and facilities in rural areas, as set forth in § 413.233. To avoid overlap with these existing facility-level adjustments, we are analyzing the impact of potentially creating a new payment adjustment and considering innovative methodological options, such as the local dialysis need methodology on which we requested information in the CY 2024 ESRD PPS proposed rule ( 88 FR 42441 through 42445 ).
In addition, we have heard from interested parties that the eligibility criteria for the LVPA are very explicit and leave little room for flexibility in certain circumstances ( 85 FR 71442 ). Some also view the attestation process as burdensome to ESRD facilities and believe it may discourage participation by small ESRD facilities with limited resources that would otherwise qualify for the LVPA. [ 40 ] Given these concerns, we have considered alternative approaches to the LVPA that would reduce burden, remove negative incentives that may result in gaming, and better target ESRD facilities that are critical for beneficiary access.
CMS's contractor has held three Technical Expert Panels (TEPs) to discuss potential refinements to the ESRD PPS. [ 41 ] During the 2018, 2019, and Start Printed Page 55799 2020 TEPs, panelists, including representatives from ESRD facilities, independent researchers, patient advocates, and representatives from professional associations and industry groups ( 86 FR 36397 ), discussed limitations of the current LVPA methodology and potential alternatives. In the CY 2022 ESRD PPS proposed rule, we included a RFI to inform LVPA payment reform ( 86 FR 36398 through 36399 ). All fourteen responses to the CY 2022 ESRD PPS RFI for LVPA wrote in support of either eliminating or revising the current LVPA or rural facility adjustment. [ 42 ] One small dialysis organization within a large non-profit health system responded that it is reliant upon the LVPA and the rural facility adjustment and supports both adjustments, albeit with modifications. MedPAC renewed its support for a new Low-Volume and Isolated (LVI) adjustment with a recommendation for a three-tiered approach for treatment thresholds, which would incorporate geographic isolation into its methodology and may disincentivize gaming. MedPAC called upon CMS to provide clear and timely criteria for ESRD facility eligibility and ensure the LVPA methodology is transparent. In concurrence with MedPAC, a coalition of dialysis organizations, three large dialysis organizations (LDOs), a non-profit kidney organization, and a provider advocacy coalition commented that the rural facility adjustment should be eliminated and a LVI methodology should be adopted, as they considered a methodology based upon census tracts to be both complicated and lacking transparency. Numerous commenters wrote in support of a tiered adjustment to mitigate the cliff effect and gaming. Commenters raised concerns regarding the reliance of the census tract methodology used by the rural facility adjustment upon `driving time' as a data measure, noting this presents legitimate equity issues. ESRD facilities that have relied upon both the LVPA and rural payment adjustments to remain operational expressed opposition to elimination of either adjustment. [ 43 ]
In the CY 2022 ESRD PPS proposed rule LVPA RFI, we sought input on alternative approaches to the LVPA methodology ( 86 FR 36398 through 36399 ). 44 Specifically, we requested input on—(1) whether a distinction other than census tract information should be considered; and (2) what criteria should be used to determine the threshold(s) of adjusted latent demand (in treatment counts) which determine LVPA eligibility. Additionally, we explored the LVI adjustment that MedPAC recommended in its June 2020 report to Congress. Under the LVI methodology, a determination that a facility is low volume and isolated would be based on that facility's distance from the nearest facility and its total treatment volume. Regarding the LVI methodology, we requested input on the concerns for facilities that would lose the LVPA under the LVI methodology and the potential for gaming within the LVI methodology. In addition, we requested input regarding the extent that the LVI methodology captures more isolated (and most often rural) facilities, and whether a separate rural facility adjustment should be maintained. As previously discussed, our most recent analysis of cost report data does not support the claim that isolated low-volume ESRD facilities face higher costs than non-isolated ESRD facilities; therefore, the LVI methodology would not adhere to the statutory requirement for the LVPA set forth at section 1881(b)(14)(D)(iii) of the Act.
In the CY 2024 ESRD PPS proposed rule ( 88 FR 42430 through 42544 ), we issued a RFI regarding several possible modifications to the current LVPA methodology. 45 We provided commenters the option of maintaining a single LVPA threshold, establishing LVPA tiers, or utilizing a continuous function. We received 23 comments in response to the RFI, all of which had differing opinions. A coalition of dialysis organizations recommended a two-tiered approach, while MedPAC reiterated their support for a LVI adjustment. A common theme among a handful of comments was concern about administrative burden and transparency regarding the methodology that is chosen. Most commenters believed that the issue of payment cliffs is substantial, but many did not believe any of the options presented in the RFI could successfully eliminate gaming completely.
We have considered several changes to the LVPA eligibility criteria to address the concerns that the GAO and MedPAC raised about targeting LVPA payments to ESRD facilities that are necessary to protect access to care and are not located near other ESRD facilities. As previously discussed, we do not believe the suggestion to consider facilities' geographic isolation reconciles with the central statutory requirements and limitations for the LVPA, and we are considering alternative approaches, including potentially addressing this issue through a new payment adjustment separate from the LVPA based on section 1881(b)(14)(D)(iv) of the Act.
The LVPA and rural adjusters currently result in increased payments to some geographically isolated ESRD facilities, but these adjusters do not specifically target geographically isolated ESRD facilities. Interested parties, including MedPAC and the GAO, have recommended that CMS make refinements to the LVPA and rural adjusters to better target ESRD facilities that are critical to beneficiary access to dialysis care in remote or isolated areas. The GAO and MedPAC, among others, have also raised concerns about targeting LVPA payments to ESRD facilities that are not located near other ESRD facilities to protect access to care.
In the CY 2024 ESRD PPS proposed rule's LVPA RFI ( 88 FR 42441 through 42445 ), we solicited comments on a potential new payment adjustment that accounts for isolation, rurality, and other geographical factors, including local dialysis need (LDN). The LDN methodology, as described in the CY 2024 ESRD PPS proposed rule ( 88 FR 42430 through 42544 ), would consider LDN instead of basing payment strictly upon a rural designation, as provided for by §§ 413.233 and 413.231(b)(2). In the CY 2024 ESRD PPS proposed rule's LVPA RFI, we suggested the utilization of census tracts to identify geographic areas with low demand, then calculating latent demand by multiplying the number of beneficiaries near (“near” was defined by driving time to ESRD facilities) an ESRD facility by the average number of treatments for ESRD beneficiaries. The threshold to qualify for the LVPA could then be applied by determining the amount of adjusted latent demand. The ESRD facilities that fall below the threshold would be eligible. The statutory requirements for the LVPA under section 1881(b)(14)(D)(iii) of the Act generally would not allow for CMS to account for geographic isolation outside of the extent to which low-volume facilities face higher costs in furnishing renal dialysis services than other facilities, and preliminary analysis found that, in general, low-volume facilities that are rural, isolated, or located in low-demand areas did not have higher costs than low-volume ESRD facilities overall. Because of this, the LDN methodology Start Printed Page 55800 would be implemented under the authority in section 1881(b)(14)(D)(iv) of the Act, which states that the ESRD PPS may include such other payment adjustments as the Secretary determines appropriate.
We received 23 comments in response to the LVPA RFI, all of which had differing opinions. [ 46 ] Some commenters supported eliminating the rural adjuster and reallocating its funds to either the LVPA or to a new adjustment that considers LDN. Others stated the rural facility adjustment should be removed, and those dollars be incorporated into one of the tiered LVPA methodologies. Many commenters noted that a LVPA, a rural facility adjustment, and a possible LDN-based adjustment would be redundant. A coalition of dialysis organizations stated that CMS's reliance on zip codes to identify rural facilities is no longer an adequate proxy for facilities in need, and cited data that many rural facilities enjoy a large patient count and positive profit margins. Other commenters supported the rural facility adjustment, explaining that it was especially appropriate in conjunction with a modified LVPA methodology, since under the options presented by CMS in the RFI, many facilities would experience significant decreases in payment. They claimed that the additional funds provided by the rural facility adjustment would protect against the closure of rural facilities. Several commenters expressed concern about administrative burden and transparency in a general sense, no matter the methodology chosen.
Generally, commenters were opposed to a payment adjustment based on the LDN methodology, reiterating many of the concerns raised during the 2020 TEP. A coalition of dialysis organizations voiced the concern that the LDN methodology would take away providers' ability to make financial decisions about their operations, since they would not be able to predict their eligibility for the LDN payment adjustment nor the amount they would receive. They maintained that the LDN may not target the appropriate facilities and could provide opportunities for gaming. The coalition also claimed that the central issue faced by these facilities is low patient count, which they stated that the LDN methodology would not recognize, and thus the adjustment could be provided to facilities that are isolated, but have high patient counts, and are not in need of an additional payment adjustment. A coalition of dialysis organizations and a non-profit dialysis association both stated that the current LVPA provision to aggregate the treatments of facilities under common ownership that are not at least 5 miles apart is an important feature that discourages gaming, one that is not included in the LDN methodology. Furthermore, the coalition noted that the LDN methodology would lack stability, given that patient location varies over time. MedPAC suggested that if the LDN were adopted, CMS should ensure that the methodology is transparent; for example, making the specifications and results for the regression equation available on CMS's website and in the Federal Register . In addition, MedPAC stated that CMS should note how often the model would be updated, discuss how census tract populations changing over time would affect the stability of the adjustment, and how the approach would address MedPAC's anticipated increase in home dialysis use.
In addition to the questions outlined in the CY 2024 ESRD PPS proposed rule LVPA RFI, CMS has also considered incorporating isolation criteria into the rural facility adjustment, where payment of the adjustment could be limited to ESRD facilities that are isolated from other ESRD facilities, or a higher adjustment could be applied for isolated rural facilities than for non-isolated rural facilities. Alternatively, the current rural facility adjustment could be replaced by an adjustment based solely on isolation. We note that recent analysis has confirmed that, in general, low-volume facilities that are rural, isolated, or located in low-demand areas did not have higher costs than low-volume ESRD facilities overall. This analysis aligns with suggestions from various commenters, including MedPAC, to refine or remove the rural facility adjustment to better target ESRD facilities that are critical to beneficiary access and are likely not being adequately targeted under the current methodology. However, we note that many ESRD facilities which receive the rural facility adjustment are critical to patient access and that these ESRD facilities may be relying on the additional payment from the rural facility adjustment for the coming years. As discussed in section II.B.2.f.(2) of this proposed rule, we are proposing to implement a phase-out policy for ESRD facilities that lose the rural facility adjustment as a result of being redesignated from a rural area to an urban area in the most recent CBSA delineations. We are not proposing any other changes to the rural facility adjustment in this proposed rule.
The goals of the ESRD PPS (including the LVPA) are to align resource use with payment, advance health equity and protect access to renal dialysis services for vulnerable beneficiaries in underserved communities, including rural and isolated communities, by increasing payments to certain ESRD facilities in these areas to align with their higher costs. As noted in the CY 2016 ESRD PPS final rule ( 80 FR 68967 through 69077 ), we aim to target the benefit of the LVPA to facilities that serve the access needs of patients in remote locations. In the CY 2022 ESRD PPS final rule ( 86 FR 61874 through 62026 ), we detailed our commitment to achieving equity in health care outcomes for our beneficiaries using the definition of equity set forth in Executive Order 13985 , [ 47 ] which places emphasis on individuals who belong to underserved communities. In the CY 2023 ESRD PPS proposed rule RFI ( 87 FR 38464 through 38586 ), we reiterated our commitment to achieving equity in health care and noted that we aim to align ESRD facility resource use with payment. Recent feedback from interested parties indicates that the current LVPA payment structure may lead some ESRD facilities to treat fewer patients to avoid a payment cliff. Proposing a revised methodology that would reduce the incentive for gaming, as the GAO described, would help advance health equity by removing the incentive for some ESRD facilities to limit access to renal dialysis services. We would expand access through payments that incrementally align resource use with payment to ESRD facilities that furnish different volumes of treatment.
In this proposed rule, we are proposing to refine the LVPA methodology to include two tiers based on treatment volume with different payment adjustments for each tier. This proposed methodology would be similar to the methodology described in the CY 2024 ESRD PPS proposed rule RFI ( 88 FR 42430 through 42544 ), but with methodological changes to improve consistency in an ESRD facility's tier assignment from year to year.
We analyzed cost report data from ESRD facilities to develop the tiered thresholds and adjustment amounts for the proposed LVPA. This analysis used a logarithmic regression model that controls for various geographical and Start Printed Page 55801 facility level characteristics, including facility type and region, to estimate cost differences based on treatment volume. We also simulated attestation patterns by excluding a stratified random sample of ESRD facilities who are eligible for LVPA payment but do not submit LVPA attestations. This step allowed us to account for the fact that a portion of ESRD facilities that were within the treatment volume threshold routinely did not attest to meeting the LVPA requirements for other reasons. We analyzed numerous different potential tiered payment structures based on this analysis, where the estimated cost for the tier uses the upper bound of the treatment count for that tier. Based on the results of this analysis, we are proposing a two-tiered approach; we believe the two-tiered approach is appropriate because it strikes a balance between simplicity for ESRD facilities, sufficiently large tiers to allow for treatment volume variation from one year to the next, and payment adequacy for current low-volume facilities, particularly those with the lowest volume.
Table 9 presents our proposed two-tiered LVPA methodology, which is based on data from ESRD facility cost reports such that the reporting periods include some part of the period between January 1, 2020, to December 31, 2022 (that is, beginning or ending during these 3 CYs). We note that we have required budget neutrality for any change to the LVPA methodology, so any proposed changes to the LVPA cannot increase or decrease total estimated ESRD PPS payments; therefore, the two sets of potential adjustment factors in table 9 would be implemented budget-neutrally. The second column presents the unscaled adjusters, which if implemented, would cause the ESRD PPS base rate to be reduced by a factor of 0.999262, approximately $0.20, to achieve budget neutrality. The third column presents the adjusters scaled down by a factor of 0.815 to maintain the LVPA payment amount under the existing methodology of $26.7 million based on the expected CY 2025 LVPA payments. Using the scaled adjusters would maintain budget neutrality without lowering the ESRD PPS base rate.

The adjustment factors in the second column are derived from the regression explained previously. These results indicate that facilities which furnish less than 3,000 treatments have costs that are 34.9 percent higher than non-low-volume facilities, and facilities that furnish between 3,000 and 3,999 treatments have costs that are 22.2 percent higher. The adjustment factors in the third column, which are scaled down, reflect the same relationship between the two tiers of low-volume facilities and non-low-volume facilities.
We believe that a two-tier scaled approach is appropriate because it would increase payments to facilities with the lowest volume while keeping payment changes contained within the LVPA. In CY 2016 ESRD PPS final rule ( 80 FR 68972 through 69004 ) when we last updated the LVPA adjustment factor, we also updated most of the facility-level and case-mix adjusters. At that time, it was appropriate to apply a budget-neutrality factor that represented all of the changes to the facility-level and case-mix adjusters. However, we are only proposing changes to the LVPA at this time, and it is most appropriate to contain the changes within the current LVPA by applying a scaling factor to the LVPA adjusters.
We also analyzed a three-tiered option that would include a tier for ESRD facilities furnishing between 4,000 and 5,000 treatments, which is presented in table 10. As noted previously, we considered both scaled and unscaled adjustment factors, with both maintaining budget neutrality. Our analysis showed that the scaled, three-tiered option would reduce payments for facilities furnishing less than 3,000 treatments as compared to both the current LVPA methodology and the proposed two-tiered scaled methodology. Because payments for facilities furnishing between 4,000 and 5,000 treatments would increase, payments for the lowest-volume facilities would need to decrease to maintain budget neutrality, which we do not believe would align with the goals of the LVPA outlined previously. We believe that if we were to propose a three-tiered option, budget neutralizing the base rate rather than scaling the adjustment factors would better align with these goals. Our analysis shows that an unscaled three-tiered adjustment would result in a $0.99 reduction to the base rate. We are seeking comment on our proposed scaled, two-tier proposal and on the alternative three-tier LVPA structure. We note that, should this alternative be finalized, we would make changes to § 413.232(b)(1) to reflect the increased LVPA threshold of 5,000. As discussed further in the next subsection, we are proposing to determine an ESRD facility's LVPA tier based on the median treatment count volume of the last three cost-reporting years, rather than using a single year treatment count. Therefore, expanding LVPA eligibility to ESRD facilities that furnished fewer than 5,000 treatments in each of the past three cost-reporting years would also increase the number of ESRD facilities that would qualify for tier 1 and tier 2, since ESRD facilities which furnished between 4,000 and 4,999 treatments in one of the Start Printed Page 55802 past 3 years and fewer than 4,000 (or 3,000 for tier 1) in the other 2 years could qualify in these tiers.

We are proposing a two-tiered LVPA using the scaled adjusters presented in the second column of table 9. ESRD facilities that fall into the first tier (those that furnish fewer than 3,000 treatments) would receive a payment adjustment of 28.4 percent. Those that fall in the second tier (those that furnish 3,000 or more treatments but fewer than 4,000 treatments) would receive a payment adjustment of 18.1 percent. Outside of the change to the LVPA amount, this proposed change would not impact how the LVPA is applied to ESRD PPS payments.
One potential complication with a tiered approach to the LVPA is that there are still payment cliffs present between the tiers. This may discourage ESRD facilities from increasing their treatment volume in a given year, especially if it is uncertain whether the ESRD facility's treatment volume in future years will stay at the increased level. To address this, we are proposing to determine an ESRD facility's LVPA tier based on the median treatment count volume of the last three cost-reporting years, rather than using a single year treatment count. This proposed methodology would smooth payments over years, increasing stability and predictability in payments to low-volume facilities. We are also proposing that, should a facility receive an exception under § 413.232(g)(5) in one or more of the past three cost-reporting years, the median treatment count of the unaffected cost-reporting years would be used to make the facility's tier determination. We note that the median of two numbers is the average of those numbers, and the median of one number is that number. In the case that a facility does not have cost-reporting data from the last 3 years that are unaffected by a disaster or other emergency, we would assign the facility to a tier based on their last full year of unaffected treatment volume, assuming all LVPA eligibility criteria are met.
We believe that the proposed median treatment approach would promote stability, especially for facilities whose treatment counts are on the margins of a tier. We also believe that the proposed smoothing methodology for determining the treatment volume tier for which an ESRD facility qualifies is better than the alternative of using the highest tier (in terms of treatment volume) for which an ESRD facility has qualified in each of the past years. For example, if we used the highest tier of the last 3 years and a facility furnishes 3,500 treatments in one of the past 3 years, it would be categorized as tier 2 even if it furnished fewer than 3,000 treatments in the other 2 years. We believe that the proposed smoothing would mitigate the introduction of a cliff-effect within the tiers.
By contrast, under the proposed smoothing methodology, if the cost-reporting data indicated that the facility furnished 2,500, 2,999, and 3,500 treatments in the 3 years preceding the payment year, the median tier would be identified (tier 1 in this case), and the facility would (in the proposed two-tier system with scaling) receive a 28.4 percent payment adjustment for all of the treatments furnished during the payment year. We expect that any higher or lower payments from year to year under this policy would balance out over time without putting additional burden on the MACs. The structure of the proposed scaled, two-tier LVPA methodology is presented in table 10, and the structure of the alternative three-tier unscaled LVPA methodology is presented in table 11. For the purposes of comparison, we have included the scaled and unscaled version of both of the potential LVPA structures.
We note that we are not proposing any changes to the methodology for determining eligibility for the LVPA under § 413.232(b)(1), as the purpose of this proposed change is to better allocate payments within the LVPA, not to expand the LVPA to facilities that have furnished more than 4,000 treatments in one of the past three cost-reporting years. We would continue to determine eligibility for the LVPA based on a facility's treatment count in each of the three cost-reporting years preceding the payment year as set forth in § 413.232(b)(1) and would not consider the median treatment count over that period for purposes of determining eligibility. Likewise, we are not proposing any changes to § 413.232(g)(5), which allows for an exception to the requirement at § 413.232(b)(1) in the case of a disaster or other emergency. In the CY 2011 ESRD PPS final rule ( 75 FR 49030 through 49214 ), we stated that we believe a 3-year waiting period serves as a safeguard against facilities that have the opportunity to take a financial loss in establishing facilities that are purposefully small. In response to the CY 2024 ESRD PPS proposed rule RFI ( 88 FR 42430 through 42544 ), several interested parties commented that they believe CMS should maintain the 3-year attestation to determine eligibility for the LVPA, as it is an important Start Printed Page 55803 safeguard against gaming. In addition, if we were to use the median tier methodology to determine LVPA eligibility, we estimate that the adjustment factors would decrease, because the scaling factor used to maintain budget neutrality within the LVPA would be smaller to account for a larger amount of ESRD facilities qualifying for the LVPA.
If finalized, the proposed median treatment count methodology for determining an eligible ESRD facility's LVPA tier would improve the stability and predictability of the LVPA by basing tier determination on the median treatment count of the last 3 years as opposed to the treatment count for each of the last 3 years, where facilities could be disqualified from a higher adjustment based on marginal changes. The proposed tiered smoothing methodology would also better align payment with resource use by minimizing the impact of the payment cliff between the LVPA tiers in a transparent and reproducible fashion. We are soliciting comments on each aspect of our proposal: (1) the tiered structure of the LVPA; (2) using the median treatment count volume to determine the LVPA payment tier for ESRD facilities that are eligible for the adjustment; and (3) the scaling of the adjusters to maintain LVPA payments at the same level. As previously discussed, we are also considering an alternative three-tiered structure, which would have the effect of reducing the base rate by $0.99. We are soliciting comments on whether this alternative methodology could be more appropriate than the proposed methodology. We recommend readers to provide as much detail as possible in their response to the comment solicitation.
As previously discussed, we recognize the importance of revising the ESRD PPS LVPA methodology to ensure that payments are accurately aligned with resource use, adequately target low-volume facilities, and strive for healthcare equity for ESRD beneficiaries. We are seeking information from the public about potential approaches to further refine the ESRD PPS methodology, which we would take into consideration for any potential future changes to the LVPA.
This section describes a RFI regarding the LVPA. Upon reviewing this RFI, respondents are encouraged to provide complete, but concise responses. This RFI is issued solely for information and planning purposes; it does not constitute a Request for Proposal (RFP), application, proposal abstract, or quotation. This RFI does not commit the United States Government to contract for any supplies or services or make a grant award. Further, we are not seeking proposals through this RFI and will not accept unsolicited proposals. Responders are advised that the United States Government will not pay for any information or administrative costs incurred in response to this RFI; all costs associated with responding to this RFI will be solely at the interested party's expense. Failing to respond to this RFI will not preclude participation in any future procurement, if conducted.
We note that we will not respond to questions about the policy issues raised in this RFI. We may or may not choose to contact individual responders. Such communications would only serve to further clarify written responses. Contractor support personnel may be used to review RFI responses. Responses to this RFI are not offers and cannot be accepted by the United States Government to form a binding contract or issue a grant. Information obtained because of this RFI may be used by the United States Government for program planning on a non-attribution basis. Respondents should not include any information that might be considered proprietary or confidential. All submissions become United States Government property and will not be returned. We may publicly post the comments received, or a summary thereof.
As previously discussed, under § 413.232(b), a low-volume facility is an ESRD facility that, based on the submitted documentation: (1) furnished less than 4,000 treatments in each of the 3 cost reporting years (based on as-filed or final settled 12-consecutive month costs reports, whichever is most recent, except as specified in paragraphs (g)(4) and (5)) preceding the payment year; and (2) has not opened, closed, or received a new provider number due to a change in ownership (except where the change in ownership results in a change in facility type or as specified in paragraph (g)(6)) in the 3 cost reporting years (based on as-filed or final settled 12-consecutive month cost reports, whichever is most recent) preceding the payment year.
We are soliciting comment on potential changes to the LVPA eligibility for new ESRD facilities that could be included as part of either the proposed tiered structure or a different methodology in the future. As previously discussed, the current single-threshold LVPA methodology and the proposed tiered LVPA methodology (discussed in the previous section) rely upon 3 years of cost-reporting data to determine eligibility for the adjustment. We are considering whether it could be appropriate to modify this requirement to support access to renal dialysis in underserved areas by allowing LVPA payments for new ESRD facilities that have not yet accrued 3 years of cost-reporting data. We are also evaluating the most appropriate way for a new low-volume ESRD facility to demonstrate or attest that it expects to be low-volume. Alongside this potential change, we are considering whether it would be appropriate to implement a reconciliation process for ESRD facilities that fail to furnish a low enough treatment volume to qualify for the LVPA or their predicted tier. For example, should the proposal to implement a tiered LVPA be finalized, the determination of a facility's tier assignment for the first year would be based on their anticipated treatment count, for which they would receive the corresponding LVPA amount. Then, if the ESRD facility furnished a treatment volume count that would otherwise have qualified them for a different tier, we would also undergo a reconciliation process. For future years the ESRD facility would receive the LVPA amount of the tier following the same smoothing methodology (should it be finalized) based on the median of their treatment counts for the available years. After we receive the cost-reporting data for the year in question, the facility could be placed in the appropriate LVPA tier, and could either re-pay CMS for an overestimation, or receive additional payment from CMS for an underestimation, if applicable. The anticipated treatment count for the following year could then be based upon the actual treatment count of the prior year. This process would be followed until a new ESRD facility gathers 3 years of cost-reporting data, after which the median treatment count over those 3 years would determine the facility's tier assignment if the proposed LVPA methodology is finalized. We are issuing this RFI to seek feedback on the potential future changes to the LVPA, as described previously, and to solicit further input from interested parties to inform potential future modifications to the methodology used to determine the LVPA.
In particular, we seek input and responses to the following considerations, requests, and questions:
++ Whether the LVPA or another adjustment, such as the LDN methodology discussed earlier, would be the most appropriate payment pathway to support access to renal dialysis services in areas that do not Start Printed Page 55804 currently have sufficient capacity to furnish these services to all Medicare beneficiaries.
++ What would be the most appropriate way or ways for a new ESRD facility to demonstrate or attest that it expects to be low-volume?
++ The potential for future reconciliation process as an appropriate accommodation for new ESRD facilities.
++ Whether a reconciliation process would be an effective tool for making appropriate payments to existing ESRD facilities that have three or more years of cost reporting data.
++ Would a reconciliation process be operationally straightforward and understandable for an ESRD facility that has opened in the past 3 years?
++ Would a reconciliation process make it more difficult for ESRD facilities to plan and budget for future payment years? Is this outweighed by the potential benefit of earlier access to the LVPA for these new facilities?
++ Would it be useful or feasible to implement a reconciliation process for ESRD facilities that have not opened in the past 3 years but, for whatever reason, may have furnished a low enough treatment volume to qualify for the LVPA?
++ Could the LVPA be changed in any way to better support ESRD facilities opening in underserved areas? Are there any costs specific to low-volume facilities for which the current LVPA does not account?
++ How are the costs for providers of low-volume home dialysis different from the costs for providers of low-volume in-center dialysis? Could the LVPA be an appropriate pathway to support the provision of home dialysis through increased payment?
In the CY 2020 ESRD PPS final rule ( 84 FR 60681 through 60698 ), we established the transitional add-on payment adjustment for new and innovative equipment and supplies (TPNIES) under the ESRD PPS, under the authority of section 1881(b)(14)(D)(iv) of the Act, to support ESRD facility use and beneficiary access to these new technologies. For additional background of the TPNIES we refer readers to the CY 2024 ESRD PPS final rule ( 88 FR 76410 through 76412 ).
Our practice is to include the summary of each TPNIES application and our analysis of the eligibility criteria for each application in the annual ESRD PPS proposed rule. Because we did not receive any applications for the TPNIES for CY 2025, no TPNIES application summary or CMS analysis has been included in this proposed rule.
As part of the TPNIES eligibility requirements in § 413.236(b)(4), a covered equipment or supply must have a complete HCPCS Level II code application submitted, in accordance with the HCPCS Level II coding procedures on the CMS website, by the HCPCS Level II code application deadline for biannual Coding Cycle 2 for durable medical equipment, orthotics, prosthetics and supplies (DMEPOS) items and services as specified in the HCPCS Level II coding guidance on the CMS website prior to the particular CY. We have identified a minor error in § 413.236(b)(4). Specifically, we inadvertently transposed the words orthotics and prosthetics within the DMEPOS acronym. The acronym was intended to read durable medical equipment, prosthetics, orthotics, and supplies (DMEPOS) instead of durable medical equipment, orthotics, prosthetics and supplies (DMEPOS).
As described in the HCPCS Level II Coding Procedures, HCPCS Level II is a standardized coding system that is used primarily to identify drugs, biologicals and non-drug and non-biological items, supplies, and services not included in the CPT® code set jurisdiction, such as ambulance services and durable medical equipment, prosthetics, orthotics, and supplies (DMEPOS) when used outside a physician's office.
While the HCPCS level II Coding Procedures include DMEPOS as an example of items for which HCPCS Level II codes are established, we believe that the phrase non-drug and non-biological items more broadly reflects all items, supplies, and services for which HCPCS Level II codes are established and aligns with the HCPCS Level II coding procedures on the CMS website. Therefore, we are proposing a technical change at § 413.236(b)(4) to remove the reference to the phrase durable medical equipment, orthotics, prosthetics and supplies (DMEPOS) and replace it with the phrase non-drug and non-biological items. We are also adding the word supplies. These technical changes would better reflect the broader category of non-drug and non-biological item coding in the HCPCS Level II Coding Procedures available on the CMS website. [ 48 ]
In this section of the final rule, we identify any items previously approved for the TPNIES and for which payment is continuing for CY 2025. As described in the CY 2024 ESRD PPS final rule, no new items were approved for the TPNIES for CY 2024 ( 88 FR 76431 ). As such there are no items previously approved for the TPNIES for which payment is continuing in CY 2025.
Under § 413.234(c)(1), a new renal dialysis drug or biological product that is considered included in the ESRD PPS base rate is paid the TDAPA for 2 years. In July 2023, CMS approved Jesduvroq (daprodustat) for the TDAPA under the ESRD PPS, effective October 1, 2023. Implementation instructions are specified in CMS Transmittal 12157, dated July 27, 2023, and available at: https://www.cms.gov/files/document/r12157cp.pdf .
In April 2024, CMS approved DefenCath® (taurolidine and heparin sodium) for the TDAPA under the ESRD PPS, effective July 1, 2024. Implementation instructions are specified in CMS Transmittal 12628, dated May 9, 2024, and available at: https://www.cms.gov/files/document/r12628CP.pdf .
Table 11 identifies the two new renal dialysis drugs for which the TDAPA payment period as specified in § 413.234(c)(1) would continue in CY 2025: Jesduvroq (daprodustat) that was approved for the TDAPA effective in CY 2023 and DefenCath® (taurolidine and heparin sodium) that was approved for the TDAPA effective in CY 2024. Table 11 also identifies the products' HCPCS coding information as well as the payment adjustment effective dates and end dates.

The Trade Preferences Extension Act of 2015 (TPEA) ( Pub. L. 114-27 ) was enacted on June 29, 2015, and amended the Act to provide coverage and payment for dialysis furnished by an ESRD facility to an individual with AKI. Specifically, section 808(a) of the TPEA amended section 1861(s)(2)(F) of the Act to provide coverage for renal dialysis services furnished on or after January 1, 2017, by a renal dialysis facility or a provider of services paid under section 1881(b)(14) of the Act to an individual with AKI. Section 808(b) of the TPEA amended section 1834 of the Act by adding a subsection (r) to provide payment, beginning January 1, 2017, for renal dialysis services furnished by renal dialysis facilities or providers of services paid under section 1881(b)(14) of the Act to individuals with AKI at the ESRD PPS base rate, as adjusted by any applicable geographic adjustment applied under section 1881(b)(14)(D)(iv)(II) of the Act and adjusted (on a budget neutral basis for payments under section 1834(r) of the Act) by any other adjustment factor under section 1881(b)(14)(D) of the Act that the Secretary elects.
In the CY 2017 ESRD PPS final rule, we finalized several coverage and payment policies to implement subsection (r) of section 1834 of the Act and the amendments to section 1861(s)(2)(F) of the Act, including the payment rate for AKI dialysis ( 81 FR 77866 through 77872 and 77965 ). We interpret section 1834(r)(1) of the Act as requiring the amount of payment for AKI dialysis services to be the base rate for renal dialysis services determined for a year under the ESRD PPS base rate as set forth in § 413.220, updated by the ESRD bundled market basket percentage increase factor minus a productivity adjustment as set forth in § 413.196(d)(1), adjusted for wages as set forth in § 413.231, and adjusted by any other amounts deemed appropriate by the Secretary under § 413.373. We codified this policy in § 413.372 ( 81 FR 77965 ).
In the CY 2017 ESRD PPS final rule, we indicated that we did not expect beneficiaries with AKI to dialyze at home; therefore, the home dialysis benefit was not extended to beneficiaries with AKI ( 81 FR 77870 ). There were commenters who advocated for beneficiaries to have the option to dialyze in a home setting, particularly those beneficiaries who started peritoneal dialysis (PD) in the hospital and desired to continue PD after discharge. However, other commenters indicated that beneficiaries with AKI needed close supervision during dialysis. Additionally, some commenters indicated that dialysis for AKI is a short-term treatment, and beneficiaries would not have time to learn to administer a home therapy. Therefore, we finalized the AKI payment policy in the CY 2017 ESRD PPS final rule as proposed without extending the AKI benefit to home dialysis beneficiaries. We indicated that we would gather data on the AKI population and the extent of home training necessary to safely self-administer dialysis in the home, and that we would consider the use of home dialysis for beneficiaries with AKI in the future as we find that it may be beneficial for subsets of beneficiaries.
In past years we have received comments regarding the site of renal dialysis services for Medicare beneficiaries with AKI, with the most recent comments received in response to the CY 2024 ESRD PPS proposed rule to update to the AKI dialysis payment rate ( 88 FR 76433 ). We have monitored data for beneficiaries with AKI and researched data in journal articles discussing the potential to expand dialysis for beneficiaries with AKI to a home setting, as noted in the CY 2017 ESRD PPS final rule ( 81 FR 77871 ).
In the CY 2017 ESRD PPS final rule, we clarified that the ESRD Facility Conditions for Coverage (CfCs) apply to ESRD facilities, not to ESRD beneficiaries, and noted that the ESRD facility CfCs would be the appropriate regulatory location for standards addressing care provided to beneficiaries with AKI in ESRD facilities. We finalized a policy that our CfCs would not need to be revised to address the provision of dialysis treatment to beneficiaries with AKI ( 81 FR 77871 through 77872 ).
In December 2020, CMS's data contractor held a TEP that considered data related to utilization review and cost of AKI treatments since 2017. The TEP solicited input regarding how reported costs align with realized costs of treatment for beneficiaries with AKI. During the TEP, participants suggested that we extend Medicare payment for beneficiaries with AKI to allow them to dialyze in a home setting. Additionally, the TEP indicated that beneficiaries with AKI could benefit from different treatment regimens. The TEP noted that more frequent, gentler dialysis with a lower ultrafiltration rate would be a viable option for some beneficiaries. Members of the panel commented on the similar treatment frequencies observed for beneficiaries with AKI and ESRD, stating that the payment system is currently constructed to facilitate the standard treatment plan for beneficiaries with AKI. Panelists recommended that the ESRD PPS should be flexible in terms of number of treatments for beneficiaries with AKI, so that those who need more frequent treatments are not impeded from receiving them. Start Printed Page 55806 Panelists related instances of hospitals starting a patient on PD, which can be done frequently in the home setting, only to convert the patient to a more standard treatment regimen such as three in-center hemodialysis treatments per week before discharging the patient to a dialysis facility. Panelists also advocated that we provide Medicare payment for beneficiaries with AKI to be treated at home.
We solicited comments regarding potentially modifying the site of renal dialysis services for beneficiaries with AKI and payment for AKI in the home setting as a RFI in the CY 2022 ESRD PPS proposed rule ( 86 FR 36322 , 36408 ). We received 16 comments from LDOs, patient advocacy groups, professional organizations, small dialysis organization within a large non-profit health system, and non-profit organizations. Most of the comments favored providing a payment option for beneficiaries with AKI to dialyze in a home setting; however, some commenters expressed concerns about doing so. A small dialysis organization within a large non-profit health system indicated that beneficiaries with AKI may have chronic kidney disease at a lesser stage, such as, Stage 3 or Stage 4 chronic kidney disease (CKD) rather than ESRD; however, the AKI makes dialysis necessary. This commenter noted that if the AKI were to cause the beneficiary's underlying Stage 3 or Stage 4 CKD to progress to ESRD in the future, training them to use a home modality during the AKI episode could prepare the patient for a home modality if they are diagnosed as having ESRD. One LDO indicated there is evidence that PD, which is typically used in the home setting, is associated with better preservation of residual kidney function compared to hemodialysis. A national organization of beneficiaries and kidney health care professionals advocated that PD may be learned quickly, reduces rapid hemodynamic changes that may potentiate kidney injury and impede recovery, and does not require a high-risk central venous catheter to provide treatment. We note that these comments are specific to PD as a treatment modality; however, when considering such a policy we would include payment for both PD and hemodialysis (HD) in the home setting for beneficiaries with AKI, consistent with our payment policy for home dialysis for patients with ESRD.
Most recently, as noted in the CY 2024 ESRD PPS final rule ( 88 FR 76433 ), we received 10 public comments on our proposal to update the payment rate for renal dialysis services furnished to individuals with AKI. Commenters included a coalition of dialysis organizations, a non-profit dialysis organization, a trade association, a renal product development company, and multiple large dialysis organizations. Most of the commenters requested that we allow payment for beneficiaries with AKI to select home dialysis modalities by changing the current policy, even though it was not proposed in the CY 2024 ESRD PPS proposed rule.
We acknowledge there have been concerns in the past regarding the safety of beneficiaries with AKI dialyzing at home. However, we have carefully reviewed the totality of the information and evidence presented to the agency and now recognize that current information regarding beneficiaries with AKI dialyzing in a home setting supports more frequent dialysis at a lower ultrafiltration rate. The ability to dialyze at a lower ultrafiltration rate supports a decrease in hemodynamic fluctuation and the complications associated with it, which in turn support recovery of kidney function.
Although there is only limited research regarding the use of home dialysis for the treatment of AKI, we note that several studies support the use of home dialysis to generally improve access to dialysis and provide care that better meets patient needs. We note that many of the studies related to home dialysis in the AKI patient population use PD as the treatment modality, which is consistent with comments received during the December 2020 TEP and comments received during rulemaking as noted previously. Additionally, data from the United States Renal Data System (USRDS) Annual Data Report (ADR), indicates the percentage of incident dialysis patients performing home HD was only 0.4 percent in 2021, and a significant majority of dialysis patients performing home dialysis chose PD. [ 49 ] We believe that the choice of a home modality would be comparable in the beneficiary population for those with AKI as those initiating chronic maintenance dialysis for ESRD. However, we affirm payment would be provided for either modality of home dialysis. For example, PD was used frequently for patients during the COVID-19 PHE due to challenging situations such as supply shortages, staffing shortages, and limited surgical availability for the placement of a venous access. A multicenter, retrospective, observational study of 94 patients who received acute PD in New York City in the spring of 2020 indicated that rapid deployment of acute PD was feasible. The rates of death and renal recovery were like those of patients with AKI requiring kidney replacement therapy (KRT) in other cohorts. Of those who were discharged on dialysis, four were discharged on PD, and one was discharged on HD. [ 50 ]
The International Society for Peritoneal Dialysis (ISPD) reiterated in the 2020 guidelines, updated from the 2014 guidelines for PD in AKI, that PD should be considered a suitable modality for treatment of AKI in all settings. This was a strong recommendation from the ISPD based on evidence rated at the second highest level used by ISPD. [ 51 ] Researchers found little to no difference between PD and hemodialysis in all-cause mortality, recovery of kidney function, or infection as a complication. [ 52 ] This finding is augmented by an article that reviewed the resurgence of PD for the treatment of AKI since the COVID-19 PHE. The article lists cost effectiveness, low infrastructure requirements, ease of staff training, and more rapid recovery of renal function as benefits to the use of PD to treat AKI. A survey of nephrologists from three international conferences reported that 50.8 percent and 36.4 percent of respondents felt that PD was suitable for treating AKI in the wards and ICU, respectively. PD is the predominant therapy used to treat pediatric patients with AKI, and until the mid to late 1990s was the predominant therapy to treat adults with AKI, but the use of this therapy has waned since the advent of pump driven continuous kidney replacement therapy. [ 53 ]
Admittedly, most studies regarding recovery of kidney function in patients with AKI are based around hospitalized patients. There are very limited studies suggesting that self-care dialysis can yield faster recovery of kidney function; however, the results are not conclusive. [ 54 ] One study of hospitalized patients with AKI indicated that a median of 10 patients recovered kidney function more quickly utilizing PD. [ 55 ] Another study of hospitalized patients with AKI indicated that while the Start Printed Page 55807 recovery of kidney function was similar in PD and HD (28 and 26 percent) there was a significantly shorter time to the recovery of kidney function for patients with AKI that utilized PD. [ 56 ]
Further support for this proposal comes from CMS AKI monitoring data, in which we found that current provision of AKI dialysis is very similar to the provision of ESRD dialysis. Data from the 2021 Quarter 4 public use file (PUF) [ 57 ] for AKI showed that hemoglobin for beneficiaries with ESRD averaged 10.6 gm/dL while the average hemoglobin for beneficiaries with AKI averaged 9 gm/dL. Beneficiaries with AKI were less likely to be prescribed an ESA than patients with ESRD. However, research indicates that patients using PD have a lower rate of anemia that those using HD. Patients receiving PD require lower doses of ESAs and iron than patients receiving HD. [ 58 ] This may indicate that dialyzing in a home environment could be effective to manage anemia in beneficiaries with AKI more appropriately, as the USRDS ADR indicates incident patients with ESRD typically choose PD as a home modality over home HD. [ 59 ] We believe that beneficiaries with AKI would make similar choices. Approximately 8 percent of beneficiaries with ESRD experience incidences of fluid overload, while beneficiaries with AKI experience episodes for which congestive heart failure was reported within 30, 60, and 90 days (which can be related to fluid overload) at rates of around 42 percent, 50 percent, and 53 percent, respectively. [ 60 ] This data is of concern because fluid overload in beneficiaries with AKI can be detrimental to recovering kidney function. Additionally, this data supports conclusions drawn from an article involving the review of 1754 patients with AKI requiring dialysis. The article indicates that treatment protocols for patients with AKI were like those of incident ESRD patients despite the underlying differences in treatment goals. The article further indicates that most patients with AKI who recovered had discontinued dialysis without ever having been weaned from their initial dialysis prescription, suggesting there may be substantial opportunity to wean dialysis sooner. [ 61 ] There is significant need to individualize the treatment of every kidney patient, but particularly beneficiaries with AKI, as this omission could result in a missed opportunity to recover kidney function.
We believe the proposal to provide payment for beneficiaries with AKI to dialyze in a home setting aligns closely with the CMS Strategic Pillars [ 62 ] of expanding access, engaging the ESRD community by being responsive to TEPs and RFIs, and driving innovation to promote patient centered care. While there is not utilization data for beneficiaries with AKI using a home modality, the USRDS ADR, indicates that disparities currently exist for self-care dialysis in the home setting for the ESRD beneficiary population, with fewer Black and Hispanic beneficiaries choosing a home dialysis modality. Additionally, fewer Medicare and Medicaid dual eligible beneficiaries choose a home dialysis modality. [ 63 ] Providing the ability for beneficiaries with AKI to choose self-care dialysis in a home setting would offer a pathway to reduce these current disparities (insofar as the AKI population mirrors the ESRD beneficiary population) by promoting access to treatment, as well as removing a disparity in care between AKI beneficiaries and ESRD beneficiaries. It is crucial that the policy revisions to payment for AKI renal dialysis consider health equity and the effects on underserved populations. The rate of AKI was about 81 percent higher among Black beneficiaries than among White beneficiaries. [ 64 ] We have reviewed comments and concerns from interested parties and agree that home dialysis could benefit beneficiaries with AKI. We note that issues with fluid management could be managed with more frequent, gentler modalities, such as PD. We trust that providing an avenue to expand treatment modalities would encourage individualized and patient-centered treatment plans for beneficiaries with AKI, for example, addressing anemia and ESA management. We would continue to monitor outcomes for beneficiaries with AKI with the expectation that AKI PUF are being reviewed in quality improvement efforts by ESRD facilities that provide services to beneficiaries with AKI.
As previously discussed, we did not extend the home dialysis benefit to beneficiaries with AKI when initially implementing the benefit ( 81 FR 77870 ). However, as discussed in the prior section, we reviewed AKI monitoring data showing that outcomes for anemia, ESA use, and fluid management are not necessarily reflective of the specific, individualized care, and close supervision by qualified staff currently required during the in-center dialysis process. We note research demonstrates the use of PD is correlated with positive outcomes for fluid management and a lower rate of anemia with less utilization of ESAs and iron, as previously discussed. As we stated in the previous section, research related to home dialysis in the AKI patient population has primarily discussed results using PD as the modality; however, we would provide payment for either PD or HD as a home modality. CMS's goal is for beneficiaries with AKI to receive the necessary care to improve their condition, recover kidney function, and be weaned from dialysis treatment. We also note that the literature exhibits a high correlation between the use of PD treatment for beneficiaries with AKI and positive outcomes for fluid management, infection rates, mortality, and recovery of kidney function. [ 65 ] Additionally, we reviewed analysis demonstrating that the use of PD to manage the care of beneficiaries with AKI as a result of COVID-19 was successful and that beneficiaries who have successfully begun a treatment regime that could transition from the hospital to a home modality should not have to change treatment to an in-center treatment modality.
After careful review of current research and the outcomes noted during the COVID-19 PHE, we propose to extend the home dialysis benefit as defined at 42 CFR 410.52 to beneficiaries with AKI for either PD or HD. As discussed in section III.C.1 of this proposed rule, we are proposing that the payment amount for home dialysis for AKI beneficiaries would be the same as the payment amount for in-center dialysis for AKI beneficiaries, consistent with payment parity within the ESRD PPS. This payment amount Start Printed Page 55808 would be the ESRD PPS base rate, adjusted for geographic area, as described in section II.C.2 of this proposed rule. Additionally, as discussed in section III.C.3 of this proposed rule, we are proposing to extend the add-on payment adjustment for home and self-dialysis training in the same amount as for patients with ESRD, on a budget neutral basis. We propose to revise § 413.373, which currently states “The payment rate for AKI dialysis may be adjusted by the Secretary (on a budget neutral basis for payments under section 1834(r)) by any other adjustment factor under subparagraph (D) of section 1881(b)(14) of the Act,” by adding paragraph (a) before “The payment rate” that reads “CMS applies the wage-adjusted add-on per treatment adjustment for home and self-dialysis training as set forth at § 413.235(c) to payments for AKI dialysis claims that include such training.” We propose to move the current language to paragraph (b) with a technical revision to add “of the Act” after “section 1834(r)”. Furthermore, as discussed in section III.D of this proposed rule, we are proposing changes to the ESRD facility CfCs that would accommodate the provision of home dialysis for beneficiaries with AKI and help ensure safe and high-quality care for Medicare beneficiaries in this setting.
We are proposing to amend § 410.52 to provide Medicare payment for the treatment of patients with AKI in the home setting. We are proposing to revise § 410.52 to read “Medicare Part B pays for the following services, supplies, and equipment furnished to a patient with ESRD or an individual with Acute Kidney Injury (AKI) as defined in § 413.371 of this chapter in his or her home:” by striking the words “an ESRD patient” after “to” and adding the words “a patient with ESRD or an individual with Acute Kidney Injury (AKI) as defined in § 413.371 of this chapter” after “to”. We are also proposing to revise § 413.374(a) to read: “The AKI dialysis payment rate applies to renal dialysis services (as defined in subparagraph (B) of section 1881(b)(14) of the Act) furnished under Part B by a renal dialysis facility or provider of services paid under section 1881(b)(14) of the Act, including home services, supplies, and equipment, and self-dialysis.”
The payment rate for AKI dialysis is the ESRD PPS base rate determined for a year under section 1881(b)(14) of the Act, which is the finalized ESRD PPS base rate, including the applicable annual market basket update, geographic wage adjustments, and any other discretionary adjustments, for such year. We note that ESRD facilities could bill Medicare for non-renal dialysis items and services and receive separate payment in addition to the payment rate for AKI dialysis. As discussed in section II.B.4 of this proposed rule, the proposed ESRD PPS base rate is $273.20, which reflects the application of the proposed CY 2025 wage index budget-neutrality adjustment factor of 0.990228 and the proposed CY 2025 ESRDB market basket percentage increase of 2.3 percent reduced by the proposed productivity adjustment of 0.5 percentage point, that is, 1.8 percent. Accordingly, we are proposing a CY 2025 per treatment payment rate of $273.20 (($271.02 × 0.990228) × 1.018 = $273.20) for renal dialysis services furnished by ESRD facilities to individuals with AKI. This proposed payment rate is further adjusted by the wage index, as discussed in the next section of this proposed rule.
Under section 1834(r)(1) of the Act and regulations at § 413.372, the amount of payment for AKI dialysis services is the base rate for renal dialysis services determined for a year under section 1881(b)(14) of the Act (updated by the ESRDB market basket percentage increase and reduced by the productivity adjustment), as adjusted by any applicable geographic adjustment factor applied under section 1881(b)(14)(D)(iv)(II) of the Act. Accordingly, we apply the same wage index under § 413.231 that is used under the ESRD PPS. As discussed in section II.B.2.b of this proposed rule, we are proposing a new ESRD PPS wage index methodology, which utilizes BLS OEWS data and freestanding ESRD facility cost report data. We are proposing to use this same methodology when adjusting AKI dialysis payments to ESRD facilities, consistent with our historical practice of using the ESRD PPS wage index for AKI dialysis payments. The AKI dialysis payment rate is adjusted by the wage index for a particular ESRD facility in the same way that the ESRD PPS base rate is adjusted by the wage index for that ESRD facility ( 81 FR 77868 ). Specifically, we apply the wage index to the labor-related share of the ESRD PPS base rate that we utilize for AKI dialysis to compute the wage adjusted per-treatment AKI dialysis payment rate. We also apply the wage index policies regarding the 0.600 wage index floor ( 87 FR 67161 through 67166 ) and the 5 percent cap on wage index decreases ( 87 FR 67159 through 67161 ) to AKI dialysis payments to ESRD facilities. ESRD facilities would utilize the same staff to provide renal dialysis services to and educate beneficiaries with AKI as those beneficiaries with ESRD. Therefore utilizing the same wage index methodology would be appropriate in accordance with § 413.372, which addresses the payment rate for AKI dialysis and refers to § 413.231 for the wage adjustment. As stated previously, we are proposing a CY 2025 AKI dialysis payment rate of $273.20, adjusted by the ESRD facility's wage index.
Section 1834(r)(1) also provides that the payment rate for AKI dialysis may be adjusted by the Secretary (on a budget neutral basis for payments under section 1834(r)) by any other adjustment factor under subparagraph (D) of section 1881(b)(14) of the Act. As discussed in the previous section, we are proposing to extend AKI dialysis payment to home dialysis.
In implementing payment for home dialysis in the AKI patient population, we considered our existing payment policies for home dialysis for beneficiaries with ESRD. In the CY 2011 ESRD PPS final rule, we explained that although we included payments for providing training to beneficiaries in computing the ESRD PPS base rate, we agreed with commenters that we should pay for home dialysis training as an add-on payment adjustment under the ESRD PPS to account for the cost of providing training to beneficiaries on the use of home dialysis modalities. Thus, we finalized the home dialysis training add-on payment adjustment of $33.44 per treatment as an additional payment made under the ESRD PPS when one-on-one home dialysis training is furnished by a nurse for either hemodialysis or peritoneal dialysis training and retraining ( 75 FR 49063 ). We clarified our policy on payment for home dialysis training again in the CY 2013 ESRD PPS final rule, in which we stated that training costs are included in the ESRD PPS base rate; however, we also provide an add-on payment adjustment for each home and self-dialysis training treatment furnished by a Medicare-certified home dialysis training facility ( 77 FR 67468 ). We explained in the CY 2017 ESRD PPS final rule that it is not the intent of the add-on treatment to reimburse a facility Start Printed Page 55809 for all of the training costs furnished during training treatments. Rather, the single ESRD PPS base rate, all applicable case-mix and facility-level adjustments, as well as the add-on payment should be considered the Medicare payment for each training treatment and not the training add-on payment alone ( 81 FR 77854 ).
We considered making payment for home dialysis for beneficiaries with AKI under the ESRD PPS base rate without an add-on payment adjustment for home modality training. As we noted in the background section, the ESRD PPS base rate upon which the AKI dialysis payment rate is established contains monies for training related costs. However, we are concerned that not providing a home and self-dialysis training add-on payment adjustment for AKI dialysis may limit access to home dialysis care for the AKI beneficiary population. As previously noted, incorporation of an adjustment factor under subparagraph (D) of section 1881(b)(14) of the Act into AKI dialysis payments must be done on a budget neutral basis for payments under section 1834(r) of the Act. Therefore, establishing an add-on adjustment for training for home and self-care dialysis could have an impact on the AKI base rate.
We have reviewed options for applying budget neutrality to a home and self-dialysis training add-on payment adjustment for beneficiaries with AKI. We are considering applying a budget neutrality adjustment factor by reducing the AKI dialysis payment rate amount (which is based on the ESRD PPS base rate and is then adjusted for wages according to § 413.372) for renal dialysis services provided to patients with AKI to account for the add-on training adjustment. For example, we might estimate utilization of home dialysis in the AKI patient population using ESRD PPS data and on that basis derive a budget neutrality adjustment factor to apply to the AKI payment rate that would ensure that total payments to ESRD facilities for renal dialysis services provided to patients with AKI do not increase as a result of implementing the home and self-dialysis add-on training adjustment. To develop an estimate for consideration we used publicly available data to build an example. Using the fourth quarter data from the 2022 ESRD PUF, [ 66 ] the average monthly percentage of renal dialysis treatment furnished via home dialysis for 2022 was 15.4 percent. Using data from table 19 in section VIII.D.5.c, which indicates there were 279,000 AKI dialysis treatments in 2023, we could estimate that the same percentage of beneficiaries with AKI would choose a home modality as did beneficiaries with ESRD; therefore, we could estimate that 42,966 AKI dialysis treatments would be performed in a home setting. Using the USRDS ADR data, we could estimate the average beneficiary with AKI using a home PD modality would receive 15 PD training treatments. From the fourth quarter 2022 AKI PUF, [ 67 ] we calculate 10,802 first time beneficiaries with AKI. Using this data, we could estimate a cost of training to be $2,370,498.90 (10,802 × 0.154 × 15 × $95.57) or $8.50 ($2,370,498.90/279,000) per AKI treatment. Therefore, in this example, we would reduce the AKI dialysis payment rate by this per treatment amount to budget neutralize the home dialysis training add-on payment adjustment for beneficiaries with AKI. This means the AKI CY 2025 base rate would be $264.70 ($273.20−$8.50) using this estimate. Although we do not include it in this example, we note the training add-on payment adjustment is affected by the wage index; therefore, the wage index would be reflected in a final estimated reduction.
However, this option would entail that the ESRD PPS base rate would not be equal to the AKI dialysis payment rate once the budget neutrality adjustment factor is applied, which could disincentivize ESRD facilities from treating patients who have AKI. Additionally, we do not have utilization data for home and self-dialysis in the AKI beneficiary population. Therefore, any initial budget neutrality adjustment to the AKI dialysis payment rate would require an estimation as in the potential equation described previously. We are further considering whether, if we apply a budget neutrality adjustment factor to the AKI payment rate based on an estimation, we should reconcile payments to ESRD facilities for renal dialysis services provided to patients with AKI later to modify the budget neutrality adjustment factor based on actual utilization data.
Due to these constraints, we are seeking comments regarding the need for a home and self-dialysis training add-on payment adjustment for AKI beneficiaries along with suggestions on how to budget neutralize the add-on payment adjustment for home and self-dialysis training for AKI beneficiaries considering the statutory requirement. Additionally, we are soliciting comments on other venues in which beneficiaries with AKI might receive training for home and self-dialysis, such as inpatient or outpatient hospital departments or nephrologist offices.
We propose, in accordance with section 1834(r)(1) of the Act and § 413.373, to extend the home and self-dialysis training add-on payment adjustment under § 413.235(c) to payments for renal dialysis services provided to beneficiaries with AKI using a home modality. We propose to make payment for a home and self-dialysis add-on training adjustment at the same amount currently applicable under the ESRD PPS of $95.57 with a limit of 15 training treatments for PD and a limit of 25 training treatments for HD per patient excluding retraining sessions ( 75 FR 49063 ). Additional information regarding the maximum number of training treatments for which CMS provides payment under the ESRD PPS is located in the Medicare Claims Processing Manual. [ 68 ] To further inform our decisions on the AKI home and self-dialysis training payment policies we would need to have data regarding the utilization of AKI home renal dialysis service. We are interested in receiving data that could provide additional insight for calculating a budget neutrality adjustment factor for the AKI home and self-dialysis training add-on adjustment as described previously, such as, the actual or estimated number of training sessions furnished and the number of beneficiaries with AKI using a home modality. The analysis of this data would inform our estimates for a budget neutrality adjustment factor for training for home dialysis for beneficiaries with AKI or future decisions about how we compute the AKI home and self-dialysis training add-on adjustment. We intend to use this information to make a determination on an add-on training adjustment in the CY 2025 ESRD PPS final rule or in future rulemaking for subsequent years. If the proposal to extend the home and self-dialysis training add-on payment adjustment to payment for renal dialysis services provided to patients with AKI is finalized, we would also adopt an approach to ensure that the adjustment is implemented budget neutrally in the final rule, considering the comments received on this proposed rule.
ESRD is a kidney impairment that is irreversible and permanent. Dialysis is a process for cleaning the blood and removing excess fluid artificially with special equipment when the kidneys have failed. People with ESRD require either a regular course of dialysis or kidney transplantation to live. Given the high costs and absolute necessity of transplantation or dialysis for people with failed kidneys, Medicare provides health care coverage to qualifying individuals diagnosed with ESRD, regardless of age, including coverage for kidney transplantation, maintenance dialysis, and other health care needs. AKI is an acute decrease in kidney function due to kidney damage or kidney failure that may require dialysis. Unlike people with ESRD, individuals with AKI who require dialysis are expected to regain kidney function within three months. People with either ESRD or AKI can receive outpatient dialysis services from Medicare-certified ESRD facilities, also called dialysis facilities.
The Medicare ESRD program became effective July 1, 1973, and initially operated under interim regulations published in the Federal Register on June 29, 1973 ( 38 FR 17210 ). In the July 1, 1975, Federal Register ( 40 FR 27782 ), we published a proposed rule that revised sections of the ESRD requirements. On June 3, 1976, the final rule was published in the Federal Register ( 41 FR 22501 ). Subsequently, the ESRD Amendments of 1978 (Pub. L. 95-292), amended title XVIII of the Social Security Act (the Act) by adding section 1881. Sections 1881(b)(1) and 1881(f)(7) of the Act further authorize the Secretary to prescribe health and safety requirements (known as conditions for coverage or CfCs) that a facility providing dialysis and transplantation services to dialysis patients must meet to qualify for Medicare payment. In addition, section 1881(c) of the Act establishes ESRD Network areas and Network organizations to assure that dialysis patients are provided appropriate care. The ESRD CfCs were first adopted in 1976 and comprehensively revised in 2008 ( 73 FR 20369 ). The Trade Preferences Extension Act of 2015 (TPEA) ( Pub. L. 114-27 ) was enacted on June 29, 2015, and amended the Act to provide coverage and payment for dialysis furnished by an ESRD facility to an individual with AKI. Specifically, section 808(a) of the TPEA amended section 1861(s)(2)(F) of the Act to provide coverage for renal dialysis services furnished on or after January 1, 2017, by a renal dialysis facility or a provider of services paid under section 1881(b)(14) of the Act to an individual with AKI. Section 808(b) of the TPEA amended section 1834 of the Act by adding a subsection (r) to provide payment, beginning January 1, 2017, for renal dialysis services furnished by renal dialysis facilities or providers of services paid under section 1881(b)(14) of the Act to individuals with AKI at the ESRD PPS base rate, as adjusted by any applicable geographic adjustment applied under section 1881(b)(14)(D)(iv)(II) of the Act and adjusted (on a budget neutral basis for payments under section 1834(r) of the Act) by any other adjustment factor under section 1881(b)(14)(D) of the Act that the Secretary elects.
Medicare pays for routine maintenance dialysis provided by Medicare-certified ESRD facilities, also known as dialysis facilities. To gain certification, the State survey agency performs an on-site survey of the facility to determine if it meets the ESRD CfCs at 42 CFR part 494 . If a survey indicates that a facility is in compliance with the conditions, and all other Federal requirements are met, CMS then certifies the facility as qualifying for Medicare payment. Medicare payment for outpatient maintenance dialysis is limited to facilities meeting these conditions. As of March 2024, there are approximately 7,700 Medicare-certified dialysis facilities in the United States, [ 69 ] providing dialysis services and specialized care to people with ESRD; 3,700 of which provide home dialysis services, including training and support. [ 70 ]
The ESRD CfCs found at 42 CFR part 494 , consist of the health and safety standards that all Medicare participating dialysis facilities must meet. These standards set baseline requirements for patient safety, infection control, care planning, staff qualifications, record keeping, and other matters to ensure that all patients with kidney failure receive safe and appropriate care. In addition, the CfCs require patients to be informed about all treatment modalities (hemodialysis or peritoneal dialysis) and settings (home dialysis modalities or in-facility hemodialysis) (§ 494.70(a)(7)). A dialysis facility that is certified to provide services to home patients must ensure that home dialysis services are at least equivalent to those provided to in-facility patients and meet all applicable conditions of § 494.100. The patient's interdisciplinary team must oversee training of the home dialysis patient, the designated caregiver, or self-dialysis patient before the initiation of home dialysis or self-dialysis (as defined in § 494.10). Dialysis facilities monitor home dialysis by documenting adequate comprehension of the training; retrieving and reviewing complete self-monitoring data and other information at least every two months; and maintaining this information in the patient's medical record.
In the CY 2017 ESRD PPS final rule ( 81 FR 77834 ), we clarified that ESRD facility CfCs apply to ESRD facilities, not to people with ESRD, and noted that the ESRD CfCs would be the appropriate regulatory location for standards addressing care provided to beneficiaries with AKI in ESRD facilities. While the language of the ESRD CfCs does not directly address treatment of beneficiaries with AKI, we believe that the current ESRD facility requirements are sufficient to ensure that such patients are dialyzed safely. For example, infection control protocols are the same for any individual receiving hemodialysis, regardless of the cause or likely trajectory of their kidney disfunction. For the areas in which care and care planning may differ, such as frequency of certain patient assessments, we note that the CfCs set baseline standards and do not limit additional or more frequent services that may be necessary for beneficiaries with AKI receiving temporary dialysis as they recover kidney function.
During the development of the CY 2017 ESRD PPS final rule, we did not anticipate that beneficiaries with AKI would be candidates for home dialysis due to the likely short-term duration of treatment and the unique needs of AKI. Specifically, it was our understanding that beneficiaries with AKI require supervision by qualified staff during their dialysis and close monitoring through laboratory tests, often conducted more frequently than for people with ESRD, to ensure that they are receiving appropriate care as their kidney function improves. Therefore, we did not propose to extend the home dialysis benefit to beneficiaries with AKI at that time ( 81 FR 77870 ). However, for the reasons discussed in section III of this proposed rule, we are proposing to extend coverage of home dialysis services to beneficiaries with AKI, allowing them flexibility in choosing their preferred treatment modality. The choice between home and in-center dialysis reflects a combination Start Printed Page 55811 of clinical, social, and financial considerations. Since the ESRD CfCs apply to ESRD facilities as a whole, not to solely to their patients with ESRD, we are proposing clarifying revisions to the CfCs to align with the proposed coverage changes.
The United States Renal Data System 2023 Annual Data Report (ADR) contains updated information about the chronic kidney disease and ESRD populations in the U.S. through the end of 2021; the statistics in this section were published in this report. [ 71 ] The number of Medicare fee-for-service beneficiaries over the age of 18 years who received outpatient dialysis for the treatment of AKI increased steadily until 2019, when it reached 11,180 and then plateaued. [ 72 ] The adjusted percentage of hospitalizations in which AKI was diagnosed increased steadily between 2011 (15.5 percent) and 2021 (26.8 percent), with a particularly large increase in 2020 during the first year of the COVID-19 pandemic. [ 73 ]
Under current Medicare regulations, ESRD facility beneficiaries with AKI are restricted to receiving in-center hemodialysis, regardless of their individual prognosis or course of treatment prior to hospital discharge. [ 74 ] Since Congress expanded treatment options for those living with AKI to include dialysis facilities in 2017 ( 81 FR 77834 , 77866 ), clinical understanding of AKI has advanced. However, these patients are often subject to the standardized treatment durations and schedules intended to treat patients with ESRD; unlike these patients, individuals with dialysis-dependent AKI could potentially avoid long-term dialysis through recovery of kidney function. As a result, we believe it is necessary to provide for more flexibility in the modality options available to beneficiaries with AKI. In this proposed rule, we propose to expand coverage of home dialysis for beneficiaries with AKI, increasing patient options for dialysis treatment beyond in-center hemodialysis and empowering these patients to make decisions about their care. In addition, this proposed change reflects efforts to increase home dialysis access and uptake. We are proposing to revise the ESRD facility CfCs to align with the proposed payment changes.
Hemodialysis (HD) is the modality most often initiated by hospital staff for urgent start patients, but often the patient is discharged to an in-center clinic. Given a choice, most patients with ESRD prefer home dialysis over in-center hemodialysis. Peritoneal dialysis (PD) is a home dialysis method and offers benefits such as absence of central venous access and therefore preservation of veins, low cost, and decreased time per dialysis session, as well as convenience. [ 75 ] While home hemodialysis (HHD) is a safe and effective modality for beneficiaries with AKI, the dominant modality is PD. From 2011 to 2021, the percentage of all adults with dialysis performing home dialysis increased from 7.5 percent to 13.4 percent. [ 76 ] Individuals living in more rural areas were more likely to be using PD (9.9 percent) and HHD (2.0 percent) than their more urban counterparts (8.2 percent PD and 1.5 percent HHD). [ 77 ]
The current policies restricting access to home dialysis modalities for beneficiaries with AKI perpetuate current inequities in dialysis experiences. The percentage of all-cause hospitalizations of beneficiaries with AKI is consistently higher among older populations, men, and Black beneficiaries. [ 78 ] The ADR reported Black beneficiaries experienced a slightly larger increase in the percentage of hospitalizations with AKI in 2020 than White beneficiaries (14.8 percent vs. 11.6 percent). [ 79 ] In 2021, the rate of AKI was about 81 percent higher among Black Medicare beneficiaries, at 108.8 per 1000 person-years, than among White beneficiaries (60.1 per 1000 person-years). [ 80 ] White beneficiaries were less likely to develop dialysis-requiring AKI than Black or Hispanic beneficiaries. [ 81 ] Those with a higher neighborhood Social Deprivation Index score (more deprivation) were more likely to experience AKI requiring dialysis than those living in neighborhoods with less deprivation; this was especially true among Hispanic beneficiaries. [ 82 ] Older Medicare beneficiaries living in a neighborhood with more deprivation were more likely to experience an AKI hospitalization with dialysis than those living in neighborhoods with less deprivation. [ 83 ]
There is a disproportionate lack of home dialysis for low-income communities and communities of color. This data includes all dialysis beneficiaries, not just those with AKI. Patients in all race/ethnicity groups living in neighborhoods with more deprivation are less likely to initiate dialysis at home. The ADR shows White and Asian patients were substantially more likely to dialyze at home than Black and Hispanic patients. [ 84 ] Across all levels of neighborhood deprivation Black and Hispanic patients were much less likely to start dialysis at home than White patients. [ 85 ] Overall, the ADR highlights large racial/ethnic and socioeconomic disparities in access to home dialysis. We anticipate that providing the option of home dialysis to beneficiaries with AKI, will increase access and equitable care.
By providing multiple choices of dialysis modality (in-center dialysis, PD, or HHD), patients can choose which one best suits their needs. Solutions that encourage and facilitate initiation of home education and training in the hospital by nephrologists, dialysis nurses and hospital social workers, could significantly increase the adoption of home dialysis for beneficiaries with AKI. Initially, in the CY 2017 ESRD PPS final rule, we expressed concern about beneficiaries with AKI receiving dialysis at home, particularly PD, due to the unique medical needs of the patients; we finalized the rule as proposed without extending the AKI benefit to home dialysis patients ( 81 FR 77870 ). As discussed in section III.C.1 of this proposed rule, we have received comments regarding the site of renal dialysis services for Medicare beneficiaries with AKI. Over the years, we have monitored data for beneficiaries with AKI and research discussing the potential to expand dialysis for beneficiaries with AKI to a home setting. In addition, during the COVID-19 PHE, many patients who developed AKI received home dialysis successfully. [ 86 87 ] Both professional Start Printed Page 55812 nephrologist societies, the Renal Physicians Association and the American Society of Nephrology, agree beneficiaries with AKI can safely receive dialysis at home via PD or HHD. [ 88 ] The Renal Physicians Association has long supported access to all dialysis modalities for beneficiaries with AKI as it aligns with the goals to expand access to home dialysis and increase the number of programs utilizing emergent or urgent PD, as opposed to HD, as rescue therapy for patients presenting in urgent need. [ 89 ] By revising the CfCs to allow beneficiaries with AKI to utilize home dialysis, we would increase patient options for renal replacement treatment beyond in-center hemodialysis and empower these patients to make decisions about their care.
To support treatment location choices for individuals with AKI requiring dialysis and to align with the proposed coverage changes, we propose conforming changes throughout the ESRD CfCs at 42 CFR part 494 to clarify that the option for home dialysis services is available to all patients. Specifically, we note that the phrase “ESRD patients” is exclusive of beneficiaries with AKI. The phrase “kidney failure” is inclusive of people whose kidney function is inadequate such that dialysis is necessary to maintain or prolong life. This can be a temporary (AKI) or permanent (ESRD) condition. Accordingly, we are proposing to amend the definitions of home dialysis and self-dialysis at §§ 494.10, 494.70(c)(1)(i), and 494.130 introductory text by removing the descriptor “ESRD.” In addition, we are proposing to amend §§ 494.70(a)(1) and (10) and 494.80 introductory text by revising the phrase “ESRD” to say “kidney failure;” § 494.90(b)(4) by revising the phrase “ESRD care” to say “dialysis care;” § 494.100(a)(3)(i) by revising the phrase “management of ESRD” to say “management of their kidney failure;” § 494.120 introductory text by revising the phrase “serve ESRD patients” to say “serve patients with kidney failure;” and lastly § 494.170 introductory text by revising the phrase “provider of ESRD services” to say “provider of dialysis services.” We welcome comments on these proposed changes. Specifically, are these proposed revisions adequate to ensure access to home dialysis services for individuals with AKI?
Beneficiaries with AKI requiring dialysis represent a small subset of individuals treated in outpatient dialysis facilities. Specifically, around 12,000 patients would be eligible for this optional service. [ 90 ] Expanding coverage to include beneficiaries with AKI would not present any changes in burden on ESRD facilities or establish new information collections subject to the Paperwork Reduction Act.
For a detailed discussion of the ESRD QIP's background and history, including a description of the Program's authorizing statute and the policies that we have adopted in previous final rules, we refer readers to the citations provided at IV.A of the CY 2024 ESRD PPS final rule ( 88 FR 76433 ). We have also codified many of our policies for the ESRD QIP at 42 CFR 413.177 and 413.178 .
In this proposed rule, we are proposing to replace the Kt/V Dialysis Adequacy Comprehensive clinical measure, a comprehensive measure on which facilities are scored for each payment year using one set of performance standards, with a Kt/V measure topic comprised of four individual Kt/V measures, beginning with PY 2027. We are also proposing to remove the National Healthcare Safety Network (NHSN) Dialysis Event reporting measure from the ESRD QIP measure set beginning with PY 2027. Table 12 summarizes the previously finalized and proposed updated measures that we would include in the PY 2027 ESRD QIP measure set. The technical specifications for current measures that would remain in the measure set for PY 2027 can be found in the CMS ESRD Measures Manual for the 2024 Performance Period. 91 The proposed technical specifications for the measures in the proposed Kt/V measure topic can be viewed at https://www.cms.gov/medicare/quality/end-stage-renal-disease-esrd-quality-incentive-program/technical-specifications-esrd-qip-measures . If the Kt/V measure topic is finalized, these specifications will be included in the CMS ESRD Measures Manual for the 2025 Performance Period.

Section 1881(h)(2)(A)(i) states that the ESRD QIP must evaluate facilities based on measures of dialysis adequacy. Beginning with the PY 2027 ESRD QIP, we are proposing to replace the Kt/V Dialysis Adequacy Comprehensive clinical measure, a single comprehensive measure on which facility performance is calculated using one set of performance standards for each payment year, with a Kt/V Dialysis Adequacy Measure Topic, a measure topic comprised of four individual Kt/V measures on which facility performance is calculated using performance standards for each individual Kt/V measure. [ 93 ] We are proposing to remove the Kt/V Dialysis Adequacy Comprehensive clinical measure under § 413.178(c)(5)(i)(E), Measure Removal Factor 5 (a measure that is more strongly associated with desired patient outcomes for the particular topic becomes available), and proposing to replace it with the proposed Kt/V Dialysis Adequacy Measure Topic, which consists of four individual Kt/V measures. Under this proposed update, the individual Kt/V measures would be adult hemodialysis (HD) Kt/V, adult peritoneal dialysis (PD) Kt/V, pediatric HD Kt/V, and pediatric PD Kt/V.
By replacing the current Kt/V Dialysis Adequacy Comprehensive clinical measure with four separate measures, we would be able to assess Kt/V performance more accurately based on whether the patient is an adult or child and what type of dialysis the patient is receiving. We are also proposing to score the four measures as a Kt/V Dialysis Adequacy Measure Topic and to limit the total weight of that topic to 11 percent of the TPS, which is the weight of the current Kt/V Dialysis Adequacy Comprehensive clinical measure. These proposals would continue to maintain Kt/V measurement as an important part of the quality of care assessed by the ESRD QIP. Facilities are eligible to receive an individual Kt/V measure score if they treat at least 11 eligible patients using the modality addressed by that particular measure. For example, a facility treating at least 11 eligible pediatric HD patients during the applicable performance period would be scored on the Kt/V Pediatric HD measure. We would calculate a facility's measure topic score by first calculating the facility's performance on each of the Adult HD Kt/V, Adult PD Kt/V, Pediatric HD Kt/V, and Pediatric PD Kt/V measures, as applicable, using the applicable achievement threshold, benchmark, and improvement threshold for the payment year. Second, we would calculate the total number of eligible patients for weighting each of these measure scores to calculate a single measure topic score. We would calculate this total number by summing all eligible patients included in the denominator for each individual measure. Third, we would calculate the weighted score for each measure within the measure topic by dividing the number of patients included in the denominator for each individual measure by the total number of eligible patients for all of the measures within the measure topic and multiplying by the respective measure score. Finally, we would add the weighted measure scores together and round them to the nearest integer. An example of how we would calculate the measure topic score for a facility that treats the minimum number of patients to be eligible for scoring on all four of the measures is provided below.

Under our proposal, a facility would not need to be eligible for scoring on all four individual measures to receive a measure topic score. For example, a facility that exclusively treats adult HD patients and, for that reason, is eligible to be scored on only the Kt/V Adult HD measure would receive a topic score that is the same score as its individual Kt/V measure score. The proposed measure topic scoring considers both a facility's individual ESRD patient population and the treatment modalities it offers, and then weights its performance on the topic proportionately to its overall ESRD patient population. As a result, we believe that a facility's measure topic score will be more reflective of its actual performance among its patient population and offered modalities than its current Kt/V Dialysis Adequacy Comprehensive clinical measure score, which is a composite assessment that blends the Kt/V measure data of all patients treated at that facility.
We previously adopted a Kt/V Dialysis Adequacy Measure Topic that included three of the four measures that we are now proposing to include in the topic (adult HD Kt/V, adult PD Kt/V, and pediatric HD Kt/V) in the CY 2013 ESRD PPS final rule ( 77 FR 67487 through 67490 ). In the CY 2015 ESRD PPS final rule ( 79 FR 66197 through 66198 ), we updated the Kt/V Dialysis Adequacy Measure Topic to include the pediatric PD Kt/V measure as well. In the CY 2016 ESRD PPS final rule ( 80 FR 69053 through 69057 ), we replaced the Kt/V Dialysis Measure Topic with the current Kt/V Dialysis Adequacy Comprehensive clinical measure, which assesses the percentage of all patient-months for both adult and pediatric patients whose average delivered dose of dialysis (either hemodialysis or peritoneal dialysis) met the specified threshold during the performance period. This change allowed more facilities to be eligible for measure Start Printed Page 55815 scoring, which in turn allowed us to evaluate the care provided to a greater proportion of ESRD patients.
At the time we finalized the Kt/V Dialysis Adequacy Comprehensive clinical measure, three facilities were eligible for scoring on the pediatric HD Kt/V measure, six facilities were eligible for scoring on the pediatric PD Kt/V measure, 1,402 facilities were eligible for scoring on the adult PD Kt/V measure, and 6,117 facilities were eligible for scoring on the adult HD Kt/V measure. Given the relatively low numbers of facilities eligible for scoring on the pediatric HD Kt/V, pediatric PD KT/V, and adult PD Kt/V measures at that time, we adopted the Kt/V Dialysis Adequacy Comprehensive clinical measure to help ensure that data reflecting those patient populations contributed to facilities' total performance scores. Since the CY 2016 ESRD PPS final rule, however, Kt/V measure data (using the PY 2024/CY 2022 ESRD QIP eligible facility list, CY 2022 EQRS data, and CY 2022 claims data) indicates that more facilities are treating greater numbers of pediatric HD patients and pediatric PD patients, as well as greater numbers of adult PD patients, and therefore would be eligible to be scored on the individual measures based on an 11-patient case minimum. For example, there are now 21 pediatric HD facilities and 28 pediatric PD facilities with at least 11 qualifying patients. This shows a 600 percent increase in facilities eligible to be scored on the pediatric HD Kt/V measure, and a 366 percent increase in facilities eligible to be scored on the pediatric PD Kt/V measure, since the CY 2016 ESRD PPS final rule. Additionally, there are now 2,538 facilities eligible for scoring on the adult PD Kt/V measure, an 81 percent increase since the CY 2016 ESRD PPS final rule. By contrast, the number of facilities eligible for scoring on the adult HD Kt/V measure has increased by 14 percent during that same period of time.
In light of the increase in the proportions of pediatric HD patients, pediatric PD patients, and adult PD patients being treated at ESRD facilities since the time we adopted the Kt/V Dialysis Adequacy Comprehensive clinical measure, we have determined that it is appropriate and more reflective of facility performance to reintroduce the Kt/V Dialysis Adequacy Measure Topic in the ESRD QIP. In addition, the proposed measure topic scoring methodology will more accurately capture facility performance with respect to dialysis adequacy because it assesses those facilities based on performance standards tailored according to Kt/V measurements that reflect ESRD patient age and treatment modality.
The proposed replacement of the Kt/V Dialysis Adequacy Comprehensive clinical measure with a Kt/V Dialysis Adequacy Measure Topic would also not affect a facility's measure data reporting requirements. A facility would continue to report the same Kt/V measure data into EQRS and Medicare claims as it would for the current Kt/V Dialysis Adequacy Comprehensive clinical measure. However, under the proposed Kt/V Dialysis Adequacy Measure Topic, the measure data would be used to score the facility on four individual Kt/V measures, as applicable based on their ESRD patient population and treatment modalities.
The proposed replacement of the Kt/V Dialysis Adequacy Comprehensive clinical measure with a Kt/V Dialysis Adequacy Measure Topic would also advance the CMS National Quality Strategy Goals by scoring facilities on measure data that more accurately reflects the quality of care provided to different kinds of ESRD patients on different treatment modalities. The proposed Kt/V Dialysis Adequacy Measure Topic would allow us to evaluate dialysis adequacy in adult HD patients, adult PD patients, pediatric HD patients, and pediatric PD patients by scoring facilities in a way that accounts for differences in patient populations and treatment modalities. Therefore, this proposed update would ensure that a facility's performance on the measure topic more accurately reflects the quality of care provided by the facility.
We welcome public comment on this proposal to replace the Kt/V Dialysis Adequacy Comprehensive clinical measure with a Kt/V Dialysis Adequacy Measure Topic consisting of an adult HD Kt/V measure, an adult PD Kt/V measure, a pediatric HD Kt/V measure, and a pediatric PD Kt/V measure, for the PY 2027 ESRD QIP and subsequent years.
To ensure continued impact and effectiveness of our measure set on facility performance, we are proposing to remove the NHSN Dialysis Event reporting measure beginning with PY 2027. When we first adopted the NHSN Dialysis Event reporting measure in the CY 2012 ESRD PPS final rule ( 76 FR 70268 through 70269 ), we stated that reporting dialysis events to the NHSN by all facilities supports national goals for patient safety, including the reduction of Hospital Acquired Infections (HAIs). In the CY 2014 ESRD PPS final rule, we replaced the NHSN Dialysis Event reporting measure with the NHSN Bloodstream Infection (BSI) clinical measure ( 78 FR 72204 through 72207 ). We introduced the clinical version of the measure to hold facilities accountable for monitoring and preventing infections in the ESRD population, and to hold facilities accountable for their actual clinical performance on the measure. In the CY 2017 ESRD PPS final rule ( 81 FR 77879 through 77882 ), we reintroduced the NHSN Dialysis Event reporting measure to complement the NHSN BSI clinical measure as a way to incentivize facilities to report complete and accurate monthly dialysis event data in compliance with the NHSN Dialysis Event protocol. [ 94 ] In reintroducing the measure, we noted our concerns that facilities were not consistently reporting monthly dialysis event data, given the incentive to achieve high clinical performance scores on the NHSN BSI clinical measure. We stated that this may have been an unintended consequence of replacing the previous NHSN Dialysis Event reporting measure with the NHSN BSI clinical measure ( 81 FR 77879 ). Therefore, in the CY 2017 ESRD PPS final rule, we reintroduced the NHSN Dialysis Event reporting measure to be included in the ESRD QIP measure set along with the NHSN BSI Clinical Measure.
Based on our analyses, facilities are consistently reporting monthly dialysis event data, and have been doing so for several years. In an assessment of ESRD QIP measure rate performance trends during PY 2020 through PY 2022, performance in the 5th percentile through the 100th percentile was 100 percent on the NHSN Dialysis Event reporting measure for all three performance years, meaning that most eligible facilities reported data on the measure for each of those years. [ 95 ] If most eligible facilities are reporting NHSN Dialysis Event measure data each year and measure performance levels at the 5th percentile and the 100th percentile are the same each year, then NHSN dialysis event data are now reported consistently and the measure is Start Printed Page 55816 not likely to drive improvements in care.
Our proposal to remove the NHSN Dialysis Event reporting measure is consistent with evolving the program to focus on a measure set of high-value, impactful measures that have been developed to drive care improvements for a broader set of ESRD patients. As such, we are proposing to remove this measure from the ESRD QIP measure set under § 413.178(c)(5)(i)(A), Measure Removal Factor 1 (measure performance among the majority of ESRD facilities is so high and unvarying that meaningful distinctions in improvements or performance can no longer be made). Although we believe that removing this measure would enable facilities to focus on the remaining measures in the ESRD QIP measure set, we note that facilities would still be required to fully comply with the NHSN Dialysis Event protocol and report all dialysis event data, including BSI, for the NHSN BSI Clinical Measure.
We welcome public comment on our proposal to remove the NHSN Dialysis Event reporting measure from the ESRD QIP measure set, beginning with PY 2027.
In the CY 2024 ESRD PPS final rule ( 88 FR 76481 through 76482 ), we finalized revisions to the ESRD QIP measure domains beginning with PY 2027. The measure domains and weights we finalized in the CY 2024 ESRD PPS final rule are depicted in table 13a.

In this proposed rule, we are proposing to revise the Clinical Care Domain beginning with PY 2027 to reflect our proposal to replace the Kt/V Comprehensive Dialysis Adequacy Comprehensive clinical measure with a Kt/V Dialysis Adequacy Measure Topic, and to revise the measure weights in the Reporting Measure Domain to reflect our proposal to remove the NHSN Dialysis Event reporting measure from the ESRD QIP measure set. Under our proposal, the weight of the Kt/V Dialysis Adequacy Topic would continue to be the same as the current weight of the Kt/V Dialysis Adequacy Comprehensive Measure, but that weight would be applied to a facility's measure topic score, instead of being applied, as it is now, to a facility's score on the single Kt/V Comprehensive Dialysis Adequacy Comprehensive clinical measure.
Given our proposal to remove the NHSN Dialysis Event reporting measure from the ESRD QIP beginning with PY 2027, we are also proposing to update the individual measure weights in the Reporting Domain to accommodate the proposed new number of measures. Consistent with our approach in the CY 2023 ESRD PPS final rule, we are proposing to assign individual measure weights to reflect the proposed updated number of measures in the Reporting Measure Domain so that each measure is weighted equally ( 87 FR 67251 Start Printed Page 55817 through 67253). Although we are proposing to change the number of measures and the weights of the individual measures in the Reporting Measure Domain, we are not proposing to change the weight of any of the five domains. The measures that would be included in each domain, along with the proposed new measure weights, for PY 2027 are depicted in table 13b.

We welcome public comment on these proposals to update the Clinical Care Measure Domain and Reporting Measure Domain.
Section 1881(h)(4)(A) of the Act requires the Secretary to establish performance standards with respect to the measures selected for the ESRD QIP for a performance period with respect to a year. The performance standards must include levels of achievement and improvement, as determined appropriate by the Secretary, and must be established prior to the beginning of the performance period for the year involved, as required by sections 1881(h)(4)(B) and (C) of the Act. We refer readers to the CY 2013 ESRD PPS final rule ( 76 FR 70277 ), as well as § 413.178(a)(1), (3), (7), and (12), for further information related to performance standards.
In the CY 2024 ESRD PPS final rule ( 88 FR 76480 through 76481 ), we set the performance period for the PY 2027 ESRD QIP as CY 2025 and the baseline period as CY 2023. In this proposed rule, we are estimating the performance standards for the PY 2027 clinical measures in table 14 using data from CY 2022, which are the most recent data available. We intend to update these performance standards for all measures, using CY 2023 data, in the CY 2025 ESRD PPS final rule.

In addition, we summarize in table 15 our requirements for successful reporting on our previously finalized reporting measures for the PY 2027 ESRD QIP.

In this proposed rule, we are proposing to update eligibility requirements as part of our proposal to replace the Kt/V Dialysis Adequacy Comprehensive clinical measure with a Kt/V Dialysis Adequacy Measure Topic beginning with PY 2027. Our previously finalized and proposed new minimum eligibility requirements are described in table 16.

We welcome public comment on these proposals to update the minimum eligibility requirements to reflect the proposed Kt/V Dialysis Adequacy Measure Topic.
Under our current policy, a facility does not receive a payment reduction for a payment year in connection with its performance under the ESRD QIP if it achieves a TPS that is at or above the minimum TPS (mTPS) that we establish for the payment year. We have defined the mTPS in our regulations at § 413.178(a)(8).
Under § 413.177(a), we implement the payment reductions on a sliding scale using ranges that reflect payment reduction differentials of 0.5 percent for each 10 points that the facility's TPS falls below the mTPS, up to a maximum reduction of 2 percent. For PY 2027, we estimate using available data that a facility must meet or exceed an mTPS of 51 to avoid a payment reduction. We note that the mTPS estimated in this proposed rule is based on data from CY 2022 instead of the PY 2027 baseline period (CY 2023) because CY 2023 data are not yet available. We will update and finalize the mTPS and associated payment reduction ranges for PY 2027, using CY 2023 data, in the CY 2025 ESRD PPS final rule.

As discussed in the following sections, we are requesting information on two topics to inform future revisions to the ESRD QIP. First, we are requesting information regarding potential future modifications to the existing ESRD QIP scoring methodology to reward facilities based on their performance and the proportion of their patients who are dually eligible for Medicare and Medicaid. Second, we are requesting information regarding potential updates to the data validation policy to encourage accurate, comprehensive reporting of ESRD QIP data.
Please note that each of these sections in this proposed rule is an RFI only. In accordance with the implementing regulations of the Paperwork Reduction Act of 1995 (PRA), specifically 5 CFR 1320.3(h)(4) , these general solicitations are exempt from the PRA. Facts or opinions submitted in response to general solicitations of comments from the public, published in the Federal Register or other publications, regardless of the form or format thereof, provided that no person is required to supply specific information pertaining to the commenter, other than that necessary for self-identification, as a condition of the agency's full consideration, are not generally considered information collections and therefore not subject to the PRA.
Respondents are encouraged to provide complete but concise responses. These RFIs are issued solely for information and planning purposes; they do not constitute a Request for Proposal (RFP), applications, proposal abstracts, or quotations. These RFIs do not commit the United States Government to contract for any supplies or services or make a grant award. Further, we are not seeking proposals through these RFIs and will not accept unsolicited proposals. Responders are advised that the United States Government will not pay for any information or administrative costs incurred in response to these RFIs; all costs associated with responding to these RFIs will be solely at the interested party's expense. Not responding to these RFIs does not preclude participation in any future procurement, if conducted. It is the responsibility of the potential responders to monitor these RFI announcements for additional information pertaining to this request. Please note that we will not respond to questions about the policy issues raised in these RFIs. CMS may or may not Start Printed Page 55822 choose to contact individual responders. Such communications would only serve to further clarify written responses. Contractor support personnel may be used to review RFI responses. Responses to this notice are not offers and cannot be accepted by the United States Government to form a binding contract or issue a grant. Information obtained as a result of these RFIs may be used by the United States Government for program planning on a non-attribution basis. Respondents should not include any information that might be considered proprietary or confidential. These RFIs should not be construed as a commitment or authorization to incur cost for which reimbursement would be required or sought. All submissions become United States Government property and will not be returned. CMS may publicly post the comments received, or a summary thereof.
Achieving health equity, addressing health disparities, and closing the performance gap in the quality of care provided to disadvantaged, marginalized, or underserved populations continue to be priorities for CMS as outlined in the CMS National Quality Strategy. [ 96 ] CMS defines “health equity” as the attainment of the highest level of health for all people, where everyone has a fair and just opportunity to attain their optimal health regardless of race, ethnicity, disability, sexual orientation, gender identity, socioeconomic status, geography, preferred language, or other factors that affect access to care and health outcomes. [ 97 ] We are working to advance health equity by designing, implementing, and operationalizing policies and programs that reduce avoidable differences in health outcomes.
The ESRD QIP adopted three new health-equity focused quality measures in the CY 2024 ESRD PPS final rule ( 88 FR 76437 through 76446 ; 76466 through 76480). Although commenters were generally supportive of the new measures, a few commenters recommended that the ESRD QIP take additional action to support facilities that treat patient populations with higher proportions of health-related social needs (HRSNs) ( 88 FR 76473 ). We are considering updating our scoring methodology in future rulemaking to add Health Equity Adjustment bonus points to a facility's TPS that would be calculated using a methodology that incorporates a facility's performance across all five domains for the payment year and its proportion of patients with dual eligibility status (DES), meaning those who are eligible for both Medicare and Medicaid coverage.
In the 2016 Report to Congress on Social Risk Factors and Performance Under Medicare's Value-Based Purchasing Programs, the Office of the Assistant Secretary for Planning and Evaluation (ASPE) reported that beneficiaries with social risk factors had worse outcomes and were more likely to receive a lower quality of care. [ 98 ] Patients with DES experience significant disparities are also likely to be more medically complex and remain one of the most vulnerable populations. [ 99 100 101 ] DES remains the strongest predictor of negative health outcomes. [ 102 ]
We recently finalized a Health Equity Adjustment scoring policy for the Hospital Value-Based Purchasing (VBP) Program ( 88 FR 59092 through 59106 ) and the Skilled Nursing Facility (SNF) VBP Program ( 88 FR 53304 through 53316 ). These policies provide Health Equity Adjustment bonus points to top tier performing hospitals and SNFs with a high proportion of patients with DES, and each program's policy is tailored to meet the needs of the specific program. For example, in the Hospital VBP Program, the Health Equity Adjustment bonus is calculated based on a hospital's performance on each of the four measure domains and its proportion of patients with DES ( 88 FR 59095 through 59096 ). In the SNF VBP Program, the Health Equity Adjustment bonus is calculated based on a facility's performance on each measure and its proportion of patients with DES ( 88 FR 53309 through 53311 ).
Our policy for scoring performance on the ESRD QIP is codified at § 413.178(e). In this proposed rule, we are requesting public comment on potential future modifications to the existing scoring methodology to reward excellent care to underserved populations. We also note that any Health Equity Adjustment bonus for the ESRD QIP would need to align with the Program's statutory requirements under section 1881(h) of the Act. We welcome public comment on the following:
- Would a Health Equity Adjustment be valuable to the ESRD QIP?
++ If a Health Equity Adjustment would be valuable to the ESRD QIP, how should it be structured?
++ If a Health Equity Adjustment would not be valuable to the ESRD QIP, why not?
- Are there other approaches that the ESRD QIP could propose to adopt to effectively address healthcare disparities and advance health equity?
One of the critical elements of the ESRD QIP's success is ensuring that the data submitted to calculate measure scores and TPSs are accurate. The ESRD QIP includes two types of data validation for this purpose: The EQRS data validation (OMB Control Number 0938-1289) and the NHSN validation (OMB Control Number 0938-1340). In the CY 2019 ESRD PPS final rule, we adopted the CROWNWeb (now EQRS) data validation as a permanent feature of the Program ( 83 FR 57003 ). In the CY 2020 ESRD PPS final rule, we adopted the NHSN data validation as a permanent feature of the Program ( 84 FR 60727 ). Under both data validation policies, we validate EQRS and NHSN data from a sample of facilities randomly selected for validation. If a facility is randomly selected for validation but does not submit the requested records, 10 points are deducted from the facility's TPS. Start Printed Page 55823
In this proposed rule, we are requesting public comment on ways to update the data validation policy to encourage accurate, comprehensive reporting of ESRD QIP data. We have reviewed data validation policies in other quality reporting programs such as the Hospital Inpatient Quality Reporting (IQR) Program ( 81 FR 57180 ) and the Hospital Outpatient Quality Reporting (OQR) Program ( 76 FR 74486 ). These programs have adopted data validation policies that require a hospital selected for data validation to achieve a 75 percent reliability or accuracy threshold to receive full credit for data validation reporting.
We welcome comments on potential future policy proposals that would encourage accurate, comprehensive reporting for data validation purposes, such as introducing a penalty for facilities that do not meet an established reporting or data accuracy threshold, introducing a bonus for facilities that perform above an established reporting or data accuracy threshold, developing targeted education on data validation reporting, or requiring that a facility selected for validation that does not meet an established reporting or data accuracy threshold be selected again the next year.
Section 1115A of the Act authorizes the Innovation Center to test innovative payment and service delivery models expected to reduce Medicare, Medicaid, and Children's Health Insurance Program (CHIP) expenditures while preserving or enhancing the quality of care furnished to the beneficiaries of these programs. The purpose of the ETC Model is to test the effectiveness of adjusting certain Medicare payments to ESRD facilities and Managing Clinicians to encourage greater utilization of home dialysis and kidney transplantation, support ESRD Beneficiary modality choice, reduce Medicare expenditures, and preserve or enhance the quality of care. As described in the Specialty Care Models final rule ( 85 FR 61114 ), beneficiaries with ESRD are among the most medically fragile and high-cost populations served by the Medicare program. ESRD Beneficiaries require dialysis or kidney transplantation to survive, and the majority of ESRD Beneficiaries receiving dialysis receive hemodialysis in an ESRD facility. However, as described in the Specialty Care Models final rule, alternative renal replacement modalities to in-center hemodialysis, including home dialysis and kidney transplantation, are associated with improved clinical outcomes, better quality of life, and lower costs than in-center hemodialysis ( 85 FR 61264 ).
The ETC Model is a mandatory payment model. ESRD facilities and Managing Clinicians are selected as ETC Participants based on their location in Selected Geographic Areas—a set of 30 percent of Hospital Referral Regions (HRRs) that have been randomly selected to be included in the ETC Model, as well as HRRs with at least 20 percent of ZIP codes [ TM ] located in Maryland. [ 103 ] CMS excludes all United States Territories from the Selected Geographic Areas.
Under the ETC Model, ETC Participants are subject to two payment adjustments. The first is the Home Dialysis Payment Adjustment (HDPA), which is an upward adjustment on certain payments made to participating ESRD facilities under the ESRD Prospective Payment System (PPS) on home dialysis claims, and an upward adjustment to the Monthly Capitation Payment (MCP) paid to participating Managing Clinicians on home dialysis-related claims. The HDPA applies to claims with claim service dates beginning January 1, 2021, and ending December 31, 2023.
The second payment adjustment under the ETC Model is the Performance Payment Adjustment (PPA). For the PPA, we assess ETC Participants' home dialysis rates and transplant rates during a Measurement Year (MY), which includes 12 months of performance data. Each MY has a corresponding PPA Period—a 6-month period that begins 6 months after the conclusion of the MY. We adjust certain payments for ETC Participants during the PPA Period based on the ETC Participant's home dialysis rate and transplant rate, calculated as the sum of the transplant waitlist rate and the living donor transplant rate, during the corresponding MY.
Based on an ETC Participant's achievement in relation to benchmarks based on the home dialysis rate and transplant rate observed in Comparison Geographic Areas during the Benchmark Year, and the ETC Participant's improvement in relation to their own home dialysis rate and transplant rate during the Benchmark Year, we would make an upward or downward adjustment to certain payments to the ETC Participant. The magnitude of the positive and negative PPAs for ETC Participants increases over the course of the Model. These PPAs apply to claims with claim service dates beginning July 1, 2022 and ending June 30, 2027.
CMS has modified the ETC Model several times. In the CY 2022 ESRD PPS final rule, we finalized a number of changes to the ETC Model. We adjusted the calculation of the home dialysis rate ( 86 FR 61951 through 61955 ) and the transplant rate ( 86 FR 61955 through 61959 ) and updated the methodology for attributing Pre-emptive LDT Beneficiaries ( 86 FR 61950 through 61951 ). We changed the achievement benchmarking and scoring methodology ( 86 FR 61959 through 61968 ), as well as the improvement benchmarking and scoring methodology ( 86 FR 61968 through 61971 ). We specified the method and requirements for sharing performance data with ETC Participants ( 86 FR 61971 through 61984 ). We also made a number of updates and clarifications to the kidney disease patient education services waivers and made certain related flexibilities available to ETC Participants ( 86 FR 61984 through 61994 ). In the CY 2023 ESRD PPS final rule ( 87 FR 67136 ) we finalized further changes to the ETC Model. We updated the PPA achievement scoring methodology beginning in the fifth MY of the ETC Model, which began on January 1, 2023 ( 87 FR 67277 through 67278 ). We also clarified requirements for qualified staff to furnish and bill kidney disease patient education services under the ETC Model's Medicare program waivers ( 87 FR 67278 through 67280 ) and finalized our intent to publish participant-level model performance information to the public ( 87 FR 67280 ). In the CY 2024 ESRD PPS final rule ( 88 FR 76344 ) we finalized a policy whereby an ETC Participant may seek administrative review of a targeted review determination provided by CMS.
We are proposing a modification to the definition of ESRD Beneficiary at 42 CFR 512.310 as that definition is used for the purposes of attributing beneficiaries to the ETC Model. As finalized in the Specialty Care Models final rule and codified at § 512.360, CMS retrospectively, that is, following a MY, attributes ESRD Beneficiaries and Pre-emptive Living Donor Transplant (LDT) Beneficiaries to an ETC Participant for each month during a MY. An ESRD Beneficiary may be attributed to an ETC Participant if the beneficiary has already had a kidney transplant and has a non-AKI dialysis or MCP claim less than 12 months after the beneficiary's transplant date and has a kidney transplant failure ICD-10 Start Printed Page 55824 diagnosis code documented on any Medicare claim. Based on feedback from model participants, we became aware that the use of the ICD-10 code T86.12 to identify transplant failures may be incorrectly identifying beneficiaries for attribution to the ETC Model because a claim that is only coded with T86.12 may signify delayed graft function rather than a true transplant failure. To ensure that we are correctly identifying ESRD beneficiaries for the purposes of ETC Model ESRD Beneficiary attribution, we are proposing to modify our definition of an ESRD Beneficiary at § 512.310. Our regulations currently define an ESRD Beneficiary as a beneficiary that meets either of the following criteria: (1) is receiving dialysis or other services for end-stage renal disease, up to and including the month in which the beneficiary receives a kidney transplant up to and including the month in which the beneficiary receives a kidney transplant, or (2) has already received a kidney transplant and has a non-AKI dialysis or MCP claim at least 12-months after the beneficiary's latest transplant date; or less than 12-months after the beneficiary's latest transplant date and has a kidney transplant failure diagnosis code documented on any Medicare claim. We are proposing to modify the second criterion to specify that the beneficiary's latest transplant date must be identified by at least one of the following: (1) two or more MCP claims in the 180 days following the date on which the kidney transplant was received; (2) 24 or more maintenance dialysis treatments at any time after 180 days following the transplant date; or (3) indication of a transplant failure after the beneficiary's date of transplant based on data from the Scientific Registry of Transplant Recipients (SRTR). We are proposing that if a beneficiary meets more than one of these criteria, that CMS will consider that beneficiary an ESRD Beneficiary for the purposes of ETC model attribution starting with the earliest month in which the transplant failure was recorded. In our analysis of the proposed methodology for identifying transplant failures, we found that the use of all three criterion correctly identified more true transplant failures than did the use of T86.12 alone.
We considered a proposal to modify the language at 42 CFR 512.310 that an ESRD Beneficiary is a beneficiary that has already received a kidney transplant and has a non-AKI or MCP dialysis claim less than 12 months after the beneficiary's latest transplant date with kidney transplant failure diagnosis code documented on any Medicare claim. We considered removing the last clause; in other words, removing the specification that that the beneficiary must have a kidney transplant failure diagnosis code documented on any Medicare claim. We are not proposing this modification to the definition of an ESRD Beneficiary because doing so would preclude the possibility for a beneficiary to be attributed to the ETC Model for 12-months after a transplant, regardless of if the transplant failed. We are concerned that this scenario would reduce the number of attributed beneficiary-months that would be available for us to use to calculate the home dialysis and transplant rate for ETC Participants. We are soliciting comment on our proposal to modify the definition of an ESRD Beneficiary to more accurately identify beneficiaries that may be attributed to the ETC Model due to receiving a kidney transplant that fails within 12-months of its receipt.
In the Specialty Care Models final rule, we referenced a report from the Public Policy/Advocacy Committee of the North American Chapter of the International Society for Peritoneal Dialysis that describes barriers to increased adoption of home dialysis including educational barriers, the need for home care partner support, the monthly visit requirement for the Monthly Capitation Payment (MCP) under the Physician Fee Schedule, variations in dialysis business practices in staffing allocation, lack of home clinic independence, and other restrictions resulting in the inefficient distribution of home dialysis supplies ( 85 FR 61265 ). [ 104 ] The National Kidney Foundation (NKF) Kidney Disease Outcomes Quality Initiative (KDOQI) controversies conference report, “Overcoming Barriers for Uptake and Continued Use of Home Dialysis: An NKF-KDOQI Conference Report,” describes clinical, operational, policy, and societal barriers to increased prescribing of and retention on home modalities. For example, lack of clinical confidence in prescribing home dialysis, lack of infrastructure, financial costs to patients associated with home modifications, the need for space to store home dialysis supplies, lack of housing, lack of appropriate education, care partner burnout, and patient fear of self-cannulation. [ 105 ]
Since the Specialty Care Models final rule was published, interested parties have spoken to us about challenges associated with increasing access to home dialysis, particularly among beneficiaries with lower socioeconomic status, who have lower rates of home dialysis and kidney transplantation than people with higher socioeconomic status. The ETC Model was designed to address these barriers; for example, CMS applied the Home Dialysis Payment Adjustment (HDPA) to assist dialysis organizations with overcoming market realities that impose substantial barriers to opening and sustaining home dialysis programs. The upside and downside risk associated with the Performance Payment Adjustment (PPA) are designed to be strong incentives for behavioral change towards increasing beneficiary access to home dialysis. In the CY 2022 ESRD PPS final rule, we finalized a policy whereby we stratify achievement benchmarks based on the proportion of attributed beneficiaries who are dual eligible for both Medicare and Medicaid or who receive the Low-Income Subsidy (LIS) ( 86 FR 61968 ). We also finalized the Health Equity Incentive (HEI), which rewards ETC Participant aggregation groups that demonstrate greater than 2.5 percentage points improvement on the home dialysis and transplant rate among dual eligible and LIS recipient beneficiaries from the Benchmark Year (BY) to the MY with a .5 increase in their improvement score ( 86 FR 61971 ).
Performance accountability in the ETC Model is scheduled to end on June 30, 2026. We are concerned that the end of performance accountability may reduce incentives for dialysis organizations to invest in access to home dialysis and address the challenges of the type we describe previously in this section. We are interested in hearing from interested parties regarding policies that the Innovation Center may consider specifically incorporating into any successor model to the ETC Model or that CMS may consider generally. Given the growth in ESRD beneficiaries choosing Medicare Advantage plans, 106 Start Printed Page 55825 we are particularly interested in policies that may encourage Medicare Advantage Organizations (MAOs) to improve beneficiary access to home dialysis modalities.
We are soliciting input on the following topics that may improve our understanding of other policy interventions that may increase access to high quality home dialysis within the context of Innovation Center models and across CMS.
1. How should any future Innovation Center model that incorporates home dialysis incorporate what the community has learned from the ETC Model?
2. What barriers to home dialysis could be addressed through the ESRD Prospective Payment System (PPS)? We request that commenters be as specific as possible.
3. What approaches could CMS consider to increase beneficiary access to home dialysis modalities in Medicare Advantage?
4. How should nephrologist payment from traditional, fee-for-service Medicare and from MAOs account for clinician-level barriers to prescribing and retaining patients on home modalities?
Please note, this is a RFI only. In accordance with the implementing regulations of the Paperwork Reduction Act of 1995 (PRA), specifically 5 CFR 1320.3(h)(4) , this general solicitation is exempt from the PRA. Facts or opinions submitted in response to general solicitations of comments from the public, published in the Federal Register or other publications, regardless of the form or format thereof, provided that no person is required to supply specific information pertaining to the commenter, other than that necessary for self-identification, as a condition of the agency's full consideration, are not generally considered information collections and therefore not subject to the PRA.
Respondents are encouraged to provide complete but concise responses. This RFI is issued solely for information and planning purposes; it does not constitute a Request for Proposal (RFP), applications, proposal abstracts, or quotations. This RFI does not commit the United States Government to contract for any supplies or services or make a grant award. Further, we are not seeking proposals through this RFI and will not accept unsolicited proposals. Responders are advised that the United States Government will not pay for any information or administrative costs incurred in response to this RFI; all costs associated with responding to this RFI will be solely at the interested party's expense. Not responding to this RFI does not preclude participation in any future procurement, if conducted. It is the responsibility of the potential responders to monitor this RFI announcement for additional information pertaining to this request. Please note that we will not respond to questions about the policy issues raised in this RFI. We may or may not choose to contact individual responders. Such communications would only serve to further clarify written responses. Contractor support personnel may be used to review RFI responses. Responses to this notice are not offers and cannot be accepted by the United States Government to form a binding contract or issue a grant. Information obtained as a result of this RFI may be used by the United States Government for program planning on a non-attribution basis. Respondents should not include any information that might be considered proprietary or confidential. This RFI should not be construed as a commitment or authorization to incur cost for which reimbursement would be required or sought. All submissions become United States Government property and will not be returned. We may publicly post the comments received, or a summary thereof.
Under the Paperwork Reduction Act of 1995, we are required to provide 60-day notice in the Federal Register and solicit public comment before a collection of information requirement is submitted to the Office of Management and Budget (OMB) for review and approval. In order to fairly evaluate whether an information collection should be approved by OMB, section 3506(c)(2)(A) of the Paperwork Reduction Act of 1995 requires that we solicit comment on the following issues:
- The need for the information collection and its usefulness in carrying out the proper functions of our agency.
- The accuracy of our estimate of the information collection burden.
- The quality, utility, and clarity of the information to be collected.
- Recommendations to minimize the information collection burden on the affected public, including automated collection techniques.
We are soliciting public comment on each of these issues for the following sections of this document that contain information collection requirements (ICRs):
We refer readers to the CY 2024 ESRD PPS final rule for information regarding wage estimates and resulting information collection burden calculations used in this proposed rule ( 88 FR 76484 through 76485 ). To derive wage estimates, we used data from the United States Bureau of Labor Statistics' May 2022 National Occupational Employment and Wage Estimates for Medical Records Specialists, who are responsible for organizing and managing health information data, are the individuals tasked with submitting measure data to the ESRD Quality Reporting System (EQRS) (formerly, CROWNWeb) and the Centers for Disease Control and Prevention's (CDC's) NHSN, as well as compiling and submitting patient records for the purpose of data validation. When this analysis was conducted, the most recently available median hourly wage of a Medical Records Specialist was $22.69 per hour. [ 107 ] We also calculate fringe benefit and overhead at 100 percent. We adjusted these employee hourly wage estimates by a factor of 100 percent to reflect current HHS department-wide guidance on estimating the cost of fringe benefits and overhead. Using these assumptions, we estimated an hourly labor cost of $45.38 as the basis of the wage estimates for all collections of information calculations in the ESRD QIP.
We used this wage estimate, along with updated facility and patient counts, to estimate the total information collection burden in the ESRD QIP for PY 2027 in the CY 2024 ESRD PPS final rule ( 88 FR 76485 through 76486 ). We will update the information collection burden to reflect updated wage estimates, along with updated facility and patient counts, in the CY 2025 ESRD PPS final rule.
We refer readers to the CY 2024 ESRD PPS final rule for information regarding the estimated burden associated with Start Printed Page 55826 data validation requirements for PY 2027 ( 88 FR 76485 through 76486 ). In the CY 2024 ESRD PPS final rule, we estimated that the aggregate cost of the EQRS data validation for PY 2027 would be approximately $34,035 (750 hours × $45.38), or an annual total of approximately $113.45 ($34,035/300 facilities) per facility in the sample. We will update the aggregate cost of EQRS data validation to reflect updated wage estimates in the CY 2025 ESRD PPS final rule. The burden cost increase associated with these requirements will be submitted to OMB in the revised information collection request (OMB control number 0938-1289; Expiration date: November 30, 2025). We estimated that the aggregate cost of the NHSN data validation for PY 2027 would be approximately $68,070 (1,500 hours × $45.38), or a total of approximately $226.90 ($68,070/300 facilities) per facility in the sample. We will update the aggregate cost of NHSN data validation to reflect updated wage estimates in the CY 2025 ESRD PPS final rule. While the burden hours estimate would not change, the burden cost updates associated with these requirements will be submitted to OMB in the revised information collection request (OMB control number 0938-1340; Expiration date: November 30, 2025).
To estimate the burden associated with the EQRS reporting requirements (previously known as the CROWNWeb reporting requirements), we look at the total number of patients nationally, the number of data elements per patient-year that the facility would be required to submit to EQRS for each measure, the amount of time required for data entry, the estimated wage plus benefits applicable to the individuals within facilities who are most likely to be entering data into EQRS, and the number of facilities submitting data to EQRS. In the CY 2024 ESRD PPS final rule, we estimated that the burden associated with EQRS reporting requirements for the PY 2027 ESRD QIP was approximately $130.5 million for approximately 2,877,743 total burden hours ( 88 FR 76486 ).
We are proposing changes to the ESRD QIP measure set in this proposed rule, but do not anticipate that any of these proposals would affect the burden we have previously estimated for EQRS reporting requirements for PY 2027. Beginning with PY 2027, we are proposing to replace the Kt/V Dialysis Adequacy Comprehensive measure with a Kt/V Dialysis Adequacy Measure Topic. However, we are not proposing to update facility reporting requirements as part of that proposal. Additionally, although we are proposing to remove one measure from the ESRD QIP measure set beginning with PY 2027, the proposed measure removal would not impact EQRS reporting requirements on facilities. We provided the burden estimate for PY 2027 in the CY 2024 ESRD PPS final rule ( 88 FR 76486 ), and will update the information collection burden to reflect updated wage estimates, along with updated facility and patient counts, in the CY 2025 ESRD PPS final rule. In the CY 2024 ESRD PPS final rule, we estimated that the amount of time required to submit measure data to EQRS would be 2.5 minutes per element and did not use a rounded estimate of the time needed to complete data entry for EQRS reporting. There are 136 data elements for 507,837 patients across 7,833 facilities, for a total of 69,065,832 elements (136 data elements × 507,837 patients). At 2.5 minutes per element, this would yield approximately 367.3 hours per facility. Therefore, the PY 2027 burden would be 2,877,743 hours (367.3 hours × 7,833 facilities). Using the Medical Records Specialist wage estimate available at that time, we estimated that the PY 2027 total burden cost would be approximately $130.5 million (2,877,743 hours × $45.38). We intend to re-calculate the burden estimate for PY 2027, using updated estimates of the total number of ESRD facilities, the total number of patients nationally, and wages for Medical Records Specialists or similar staff, as well as a refined estimate of the number of hours needed to complete data entry for EQRS reporting in the CY 2025 ESRD PPS final rule. The information collection request under the OMB Control Number: 0938-1289 will be revised and sent to OMB.
Section 1115A(d)(3) of the Act exempts Innovation Center model tests and expansions, which include the ETC Model, from the provisions of the PRA. Specifically, this section provides that the provisions of the PRA do not apply to the testing and evaluation of Innovation Center models or to the expansion of such models.
If you comment on these information collections, that is, reporting, recordkeeping or third-party disclosure requirements, please submit your comments electronically as specified in the ADDRESSES section of this proposed rule.
Comments must be received on/by August 26, 2024.
Because of the large number of public comments we normally receive on Federal Register documents, we are not able to acknowledge or respond to them individually. We will consider all comments we receive by the date and time specified in the DATES section of this preamble, and, when we proceed with a subsequent document, we will respond to the comments in the preamble to that document.
On January 1, 2011, we implemented the ESRD PPS, a case-mix adjusted, bundled PPS for renal dialysis services furnished by ESRD facilities as required by section 1881(b)(14) of the Act, as added by section 153(b) of MIPPA ( Pub. L. 110-275 ). Section 1881(b)(14)(F) of the Act, as added by section 153(b) of MIPPA, and amended by section 3401(h) of the Affordable Care Act ( Pub. L. 111-148 ), established that beginning CY 2012, and each subsequent year, the Secretary shall annually increase payment amounts by an ESRD market basket percentage increase, reduced by the productivity adjustment described in section 1886(b)(3)(B)(xi)(II) of the Act. This proposed rule includes proposed updates and policy changes to the ESRD PPS for CY 2025. These changes include a proposed new wage index methodology which utilizes BLS data, a proposed wage index budget-neutrality adjustment factor, a proposed expansion to the ESRD PPS outlier list, proposed methodological changes to the outlier calculation, proposed updates to the TPNIES offset amount, proposed updates to the post-TDAPA add-on payment adjustment amounts for Korsuva® and Jesduvroq, and proposed changes to the LVPA payment structure. Failure to publish this proposed rule would result in ESRD facilities not receiving appropriate payments in CY 2025 for renal dialysis services furnished to ESRD beneficiaries.
This proposed rule also has several proposed policy changes to improve payment stability and adequacy under the ESRD PPS. These include updates to the LVPA and payments for ESRD outlier services. We believe that each of these proposed changes would improve payment stability and adequacy under the ESRD PPS. Start Printed Page 55827
This rule proposes updates to the payment rate for renal dialysis services furnished by ESRD facilities to individuals with AKI. Additionally, we are proposing to extend Medicare payment for home dialysis to beneficiaries with AKI. As discussed in section III.C of this proposed rule, we are also proposing to apply the updates to the ESRD PPS base rate and wage index to the AKI dialysis payment rate. Failure to publish this proposed rule would result in ESRD facilities not receiving appropriate payments in CY 2025 for renal dialysis services furnished to patients with AKI in accordance with section 1834(r) of the Act.
Section 1881(h)(1) of the Act requires CMS to reduce the payments otherwise made to a facility under the ESRD PPS by up to two percent if the facility does not satisfy the requirements of the ESRD QIP for that year. This rule proposes updates for the ESRD QIP, which would remove the NHSN Dialysis Event reporting measure from the ESRD QIP measure set beginning with PY 2027 and replace the Kt/V Dialysis Adequacy Comprehensive clinical measure with a Kt/V Dialysis Adequacy Measure Topic beginning with PY 2027.
The ETC Model is a mandatory Medicare payment model tested under the authority of section 1115A of the Act, which authorizes the Innovation Center to test innovative payment and service delivery models expected to reduce Medicare, Medicaid, and CHIP expenditures while preserving or enhancing the quality of care furnished to the beneficiaries of such programs.
This proposed rule proposes a change to the ETC Model, specifically to the methodology CMS uses to identify transplant failures for the purposes of defining an ESRD beneficiary and attributing an ESRD beneficiary to the ETC Model. As described in detail in section V.B of this proposed rule, we believe it is necessary, for the purposes of accuracy, to adopt this change to the ETC Model.
We have examined the impacts of this proposed rule as required by Executive Order 12866 on Regulatory Planning and Review (September 30, 1993), Executive Order 13563 on Improving Regulation and Regulatory Review (January 18, 2011), Executive Order 14094 , entitled “Modernizing Regulatory Review” (April 6, 2023), the Regulatory Flexibility Act (RFA) (September 19, 1980, Pub. L. 96-354), section 1102(b) of the Act, section 202 of the Unfunded Mandates Reform Act of 1995 (March 22, 1995; Pub. L. 104-4 ), Executive Order 13132 on Federalism (August 4, 1999), and the Congressional Review Act ( 5 U.S.C. 804(2) ).
Executive Orders 12866 and 13563 direct agencies to assess all costs and benefits of available regulatory alternatives and, if regulation is necessary, to select regulatory approaches that maximize net benefits (including potential economic, environmental, public health and safety effects, distributive impacts, and equity). Executive Order 14094 amends section 3(f) of Executive Order 12866 (Regulatory Planning and Review). The amended section 3(f) of Executive Order 12866 defines a “significant regulatory action” as an action that is likely to result in a rule: (1) having an annual effect on the economy of $200 million or more in any 1 year, (adjusted every 3 years by the Administrator of OMB's Office of Information and Regulatory Affairs (OIRA) for changes in gross domestic product) or adversely affect in a material way the economy, a sector of the economy, productivity, competition, jobs, the environment, public health or safety, or State, local, territorial, or Tribal governments or communities; (2) creating a serious inconsistency or otherwise interfering with an action taken or planned by another agency; (3) materially altering the budgetary impacts of entitlement grants, user fees, or loan programs or the rights and obligations of recipients thereof; or (4) raising legal or policy issues for which centralized review would meaningfully further the President's priorities or the principles set forth in this Executive order.
A regulatory impact analysis (RIA) must be prepared for a regulatory action that is significant under section 3(f)(1). Based on our estimates of the combined impact of the ESRD PPS, ESRD QIP, and ETC provisions in this proposed rule, OIRA has determined this rulemaking is significant per section 3(f)(1). Pursuant to Subtitle E of the Small Business Regulatory Enforcement Fairness Act of 1996 (also known as the Congressional Review Act), OIRA has also determined that this proposed rule meets the criteria set forth in 5 U.S.C. 804(2) . Accordingly, we have prepared a Regulatory Impact Analysis that to the best of our ability presents the costs and benefits of the rulemaking. Therefore, OMB has reviewed these proposed regulations, and the Department has provided the following assessment of their impact.
We estimate that the proposed revisions to the ESRD PPS would result in an increase of approximately $170 million in Medicare payments to ESRD facilities in CY 2025, which includes the amount associated with updates to the outlier list, updates to the outlier methodology and thresholds, payment rate update, updates to the wage index methodology, updates to the OMB CBSA delineations, proposed changes to the LVPA, the updated post-TDAPA add-on payment adjustment amounts, and continuation of the approved TDAPA as identified in table 18. Although the incorporation of oral-only renal dialysis drugs and biological products into the ESRD PPS in CY 2025 is provided for by existing regulations and is not impacted by this proposed rule, we estimate for reference that total ESRD PPS spending for phosphate binders will be approximately $870 million in CY 2025 ($220 million in beneficiary coinsurance payments and $650 million in Medicare Part B spending); however we note that these drugs are currently being paid for under Medicare Part D, which we estimate will lead to a decrease in spending of approximately $690 million ($90 million in beneficiary premium offset and $600 million in Medicare Part D spending), for a net payment increase of $180 million.
We estimate that the proposed updates to the AKI payment rate would result in an increase of approximately $1 million in Medicare payments to ESRD facilities in CY 2025.
We estimate that, as a result of our previously finalized policies and the policies we are proposing in this proposed rule, the updated ESRD QIP will result in $14.6 million in estimated payment reductions across all facilities for PY 2027.
The change we are proposing to the definition of an ESRD Beneficiary for the purposes of attribution in the ETC Model is not expected to change the model's projected economic impact.
We estimate that the combined impact of the policies proposed in this rule on payments for CY 2025 is $170 million based on the estimates of the updated ESRD PPS and the AKI payment rates. We estimate the impacts of the ESRD Start Printed Page 55828 QIP for PY 2027 to be $130.5 million in information collection burden and $14.6 million in estimated payment reductions across all facilities. Finally, we estimate that the proposed methodology change to the ETC Model would not affect the model's projected economic impact described in the Specialty Care Models final rule ( 85 FR 61114 ) and in the CY2022 ESRD PPS final rule ( 86 FR 61874 ).
In this section, we discuss the anticipated benefits, costs, and transfers associated with the changes in this proposed rule. Additionally, we estimate the total regulatory review costs associated with reading and interpreting this proposed rule.
Under the CY 2025 ESRD PPS and AKI payment, ESRD facilities would continue to receive payment for renal dialysis services furnished to Medicare beneficiaries under a case-mix adjusted PPS. We continue to expect that making prospective Medicare payments to ESRD facilities would enhance the efficiency of the Medicare program. Additionally, we expect that updating the Medicare ESRD PPS base rate and rate for AKI treatments furnished by ESRD facilities by 1.8 percent based on the proposed CY 2025 ESRDB market basket percentage increase of 2.3 percent reduced by the proposed CY 2025 productivity adjustment of 0.5 percentage point would improve or maintain beneficiary access to high quality care by ensuring that payment rates reflect the best available data on the resources involved in delivering renal dialysis services. We estimate that overall payments under the ESRD PPS would increase by 2.2 percent as a result of the proposed policies in this rule.
We do not anticipate the provisions of this proposed rule regarding ESRD PPS and AKI rates-setting would create additional cost or burden to ESRD facilities.
We have made no changes to our methodology for calculating the annual burden associated with the information collection requirements for EQRS data validation (previously known as the CROWNWeb validation study) or NHSN data validation. Although we do not anticipate that the proposals in this proposed rule regarding ESRD QIP will create additional cost or burden to ESRD facilities for PY 2027, in the CY 2025 ESRD PPS final rule, we intend to update the estimated costs associated with the information collection requirements under the ESRD QIP, with updated estimates of the total number of ESRD facilities, the total number of patients nationally, wages for Medical Records Specialists or similar staff, and a refined estimate of the number of hours needed to complete data entry for EQRS reporting.
We estimate that the updates to the ESRD PPS and AKI payment rates would result in a total increase of approximately $170 million in Medicare payments to ESRD facilities in CY 2025, which includes the amount associated with proposed updates to the outlier thresholds, and proposed updates to the wage index. This estimate includes an increase of approximately $1 million in Medicare payments to ESRD facilities in CY 2025 due to the updates to the AKI payment rate, of which approximately 20 percent is increased beneficiary coinsurance payments. We estimate approximately $140 million in transfers from the Federal Government to ESRD facilities due to increased Medicare program payments and approximately $30 million in transfers from beneficiaries to ESRD facilities due to increased beneficiary coinsurance payments because of this proposed rule.
If regulations impose administrative costs on private entities, such as the time needed to read and interpret this ESRD PPS proposed rule, we should estimate the cost associated with regulatory review. Due to the uncertainty involved with accurately quantifying the number of entities that will review the ESRD PPS proposed rule, we assume that the total number of unique commenters on last year's ESRD PPS proposed rule, which was 256 for the CY 2024 ESRD PPS proposed rule, is equal to the number of individual reviewers of this proposed rule. We acknowledge that this assumption may understate or overstate the costs of reviewing this proposed rule. It is possible that not all commenters reviewed last year's rule in detail, and it is also possible that some reviewers chose not to comment on the CY 2024 ESRD PPS proposed rule. For these reasons we determined that the number of past commenters would be a fair estimate of the number of reviewers of this proposed rule. We welcome any comments on the approach in estimating the number of entities which will review this proposed rule.
We also recognize that different types of entities are in many cases affected by mutually exclusive sections of this proposed rule, and therefore for the purposes of our estimate we assume that each reviewer reads approximately 50 percent of this proposal. We seek comments on this assumption.
Using the May 2023 wage information from the BLS for medical and health service managers (Code 11-9111), we estimate that the cost of reviewing this rule is $129.28 per hour, including overhead and fringe benefits [ 108 ] ( https://www.bls.gov/oes/current/oes_nat.htm ). Assuming an average reading speed, we estimate that it will take approximately 160 minutes (2.67 hours) for the staff to review half of this proposed rule, which has a total of approximately 80,000 words. For each entity that reviews the rule, the estimated cost is $345.18 (2.67 hours × $129.28). Therefore, we estimate that the total cost of reviewing this regulation is $88,366.08 ($345.18 × 256).
To understand the impact of the changes affecting Medicare payments to different categories of ESRD facilities, it is necessary to compare estimated payments in CY 2024 to estimated payments in CY 2025. To estimate the impact among various types of ESRD facilities, it is imperative that the estimates of Medicare payments in CY 2024 and CY 2025 contain similar inputs. Therefore, we simulated Medicare payments only for those ESRD facilities for which we can calculate both current Medicare payments and new Medicare payments.
For this proposed rule, we used CY 2023 data from the Medicare Part A and Part B Common Working Files as of February 16, 2024, as a basis for Medicare dialysis treatments and payments under the ESRD PPS. We updated the 2023 claims to 2024 and 2025 using various updates. The proposed updates to the ESRD PPS base rate are described in section II.B.4 of this proposed rule. Table 18 shows the impact of the estimated CY 2025 ESRD Start Printed Page 55829 PPS payments compared to estimated Medicare payments to ESRD facilities in CY 2024.

Column A of the impact table indicates the number of ESRD facilities for each impact category and column B indicates the number of dialysis treatments (in millions). The overall effect of the proposed routine updates to the outlier payment policy, including proposed changes to the inflation factors used for calculating MAP and FDL amounts described in section II.B.3 of this proposed rule, is shown in column C. For CY 2025, the impact on all ESRD facilities because of the proposed changes to the outlier payment policy would be an increase in estimated Medicare payments of approximately 0.4 percent.
Column D shows the effect of the proposed 2-tiered LVPA as described in section II.B.8 of this proposed rule. This adjustment is implemented in a budget neutral manner, so the total impact of this proposed change would be 0.0 percent. However, there would be distributional impacts of this change, if finalized, primarily increasing payments to facilities that furnish fewer than 3000 treatments by 0.8 percent and lowering payments to ESRD facilities that furnish between 3000 and 4000 treatments by 0.7 percent. Because we are proposing to use the scaled adjustment factors, the only impact of this proposed policy is among ESRD facilities that are eligible for the LVPA.
Column E shows the effect of year-over-year payment changes related to the proposed post-TDAPA add-on payment adjustment amounts as described in section II.B.6 of this proposed rule and current TDAPA payments. The post-TDAPA add-on payment adjustment will not be budget neutral, but the total impact on payment is 0.1 percent due to relatively low utilization of drugs for which we will pay this adjustment in CY 2025.
Column F reflects the impact of the proposed expansion of outlier eligibility to formerly composite rate drugs. Overall the proposed changes to the outlier policy, including those reflected in column C of this table, are budget neutral insofar as we estimate that we would better hit the 1 percent target for outlier payments. These proposed changes would increase payments for facilities that treat a higher proportion of exceptionally costly cases.
Column G reflects the effect of the proposed changes to the ESRD PPS wage index methodology, the proposed adoption of the new OMB CBSA delineations, the continued application of the 5 percent cap on wage index decreases, and the proposed rural transition policy as described in section II.B.2 of this proposed rule. This proposed update would be budget neutral, so the total impact of this proposed policy change is 0.0 percent. However, there would be distributional impacts of this proposed change, if finalized. The largest increase would be to ESRD facilities in Puerto Rico and the Virgin Islands, which would receive 3.1 percent higher payments because of the Start Printed Page 55831 proposed updated ESRD PPS wage index. The largest decrease would be for pacific ESRD facilities, which would receive 2.1 percent lower payments because of the updated ESRD PPS wage index and methodological changes.
Column H reflects the overall impact, that is, the effects of the proposed outlier policy changes, proposed LVPA changes, the proposed post-TDAPA add-on payment adjustment amounts, the proposed new wage index methodology, the proposed new CBSA delineations, the proposed rural transition policy, and the proposed payment rate update as described in section II.B.4 of this proposed rule. The proposed ESRD PPS payment rate update for CY 2025 is 1.8 percent, which reflects the proposed ESRDB market basket percentage increase for CY 2025 of 2.3 percent and the proposed productivity adjustment of 0.5 percent. We expect that overall ESRD facilities would experience a 2.2 percent increase in estimated Medicare payments in CY 2025. The categories of types of ESRD facilities in the impact table show impacts ranging from a 0.0 percent increase to a 5.2 percent increase in their CY 2025 estimated Medicare payments.
This table does not include the impact of the inclusion of oral-only drugs to the ESRD PPS as we are unable to calculate facility level estimates at this time. Furthermore, we note that the incorporation of oral-only renal dialysis drugs and biological products into the ESRD PPS in CY 2025 is provided for by existing regulations and is not impacted by this proposed rule. For public awareness, we estimate an increase in Medicare Part B spending of approximately $870 million in CY 2025, and a corresponding decrease in Medicare Part D spending of approximately $690 million in CY 2025, associated with payment for phosphate binders under the ESRD PPS.
Under the ESRD PPS, Medicare pays ESRD facilities a single bundled payment for renal dialysis services, which may have been separately paid to other providers (for example, laboratories, durable medical equipment suppliers, and pharmacies) by Medicare prior to the implementation of the ESRD PPS. Therefore, in CY 2025, we estimate that the ESRD PPS would have zero impact on these other providers.
We estimate that Medicare spending (total Medicare program payments) for ESRD facilities in CY 2025 would be approximately $7.2 billion. This estimate considers a projected decrease in fee-for-service Medicare ESRD beneficiary enrollment of 2.1 percent in CY 2025.
Under the ESRD PPS, beneficiaries are responsible for paying 20 percent of the ESRD PPS payment amount. As a result of the projected 2.2 percent overall increase in the CY 2025 ESRD PPS payment amounts, we estimate that there would be an increase in beneficiary coinsurance payments of 2.2 percent in CY 2025, which translates to approximately $30 million.
As we have previously noted, the incorporation of oral-only renal dialysis drugs and biological products into the ESRD PPS in CY 2025 is provided for by existing regulations and is not impacted by this proposed rule. For public awareness, we estimate an increase in beneficiary coinsurance payments of $220 million. As noted in section II.B.7 of this proposed rule, we anticipate that the inclusion of oral-only drugs in the ESRD PPS will increase access to these drugs for beneficiaries, particularly disadvantaged populations who currently do not have Part D coverage.
We considered several alternatives for the proposed new wage index methodology discussed in section II.B.2 of this proposed rule. We considered both alternatives for the data sources we propose to use for the new wage index methodology and construction of the wage index itself. These alternatives include using confidential BLS data instead of the publicly available data, using different occupation codes for the occupations included in the analysis than those chosen, the use of state-level or regional occupational mixes instead of a single national occupational mix, an alternative or additional phase-in policy for the wage index methodology change, setting the NEFOM annually through rulemaking instead of as a part of the wage index methodology, and the use of a summary statistic other than mean hourly wage for the BLS OEWS data (such as the median). These alternatives and the reasons we did not propose them are discussed in further detail in section II.B.2.b.(c) of this proposed rule.
We considered only expanding outlier eligibility to drugs and biological products previously paid for under the TDAPA after the end of the TDAPA period. As discussed in section II.B.3.b of this proposed rule, we have instead decided to propose to expand outlier eligibility to all drugs and biological products that were or would have been composite rate services prior to the inception of the ESRD PPS.
We considered, but did not propose, paying the TDAPA for phosphate binders based on an amount greater than 100 percent of ASP, to account for additional costs such as dispensing fees. For example, we considered paying the TDAPA for phosphate binders at 106 percent of ASP for at least 2 years to mirror our TDAPA payment approach for the first 2 years for calcimimetics. However, as discussed in section II.B.7.c of this proposed rule, we believe that it is most appropriate to use the current standard TDAPA payment amount of 100 percent of ASP for phosphate binders. We are soliciting comments on this policy and may consider finalizing changes in the final rule.
We considered, but did not propose, expanding LVPA eligibility to ESRD facilities which furnished more than 4000 treatments in one of the past 3 years whose median treatment volume over the past 3 years was less than 4000. However, we felt that this would be inappropriate as the purpose of this proposed change is to better allocate payments within the LVPA, not to expand the LVPA. Additionally, using the median tier methodology for LVPA eligibility would reduce the LVPA payments for ESRD facilities that would qualify under the current methodology by a notable amount due to the lower scaling factor. As discussed in section II.B.8.c of this proposed rule, we are not proposing any changes to the LVPA eligibility requirements at 42 CFR 413.232(b) .
Two renal dialysis drugs for which the TDAPA was paid in CY 2024 would continue to be eligible for the TDAPA in CY 2025.
On July 27, 2023, CMS Transmittal 12157 [ 109 ] implemented the 2-year TDAPA period specified in § 413.234(c)(1) for Jesduvroq (daprodustat). The TDAPA payment period began on October 1, 2023, and will continue through September 30, Start Printed Page 55832 2025. As stated previously, TDAPA payment is based on 100 percent of ASP. If ASP is not available, then the TDAPA is based on 100 percent of WAC and, when WAC is not available, the payment is based on the drug manufacturer's invoice.
We based our impact analysis on the most current 72x claims data from November 2023, when utilization first appeared on the claims, through February 2024. During that timeframe, the average monthly TDAPA payment amount for Jesduvroq (daprodustat) was $23,075. In applying that average to each of the 9 remaining months of the TDAPA payment period in CY 2025, we estimate $207,675 in spending ($23,075 * 9 = $207,675) of which, approximately $41,535 ($207,675 * 0.20 = $41,535) would be attributed to beneficiary coinsurance amounts.
On May 9, 2024, CMS Transmittal 12628 [ 110 ] implemented the 2-year TDAPA period specified in § 413.234(c)(1) for DefenCath® (taurolidine and heparin sodium). The TDAPA payment period will begin on July 1, 2024, and will continue through June 30, 2026.
We have not included Medicare impact estimates in this proposed rule but intend to update the impact estimates to include DefenCath in the CY 2025 ESRD PPS final rule.
To understand the impact of the proposed changes affecting Medicare payments to different categories of ESRD facilities for renal dialysis services furnished to individuals with AKI, it is necessary to compare estimated Medicare payments in CY 2024 to estimated Medicare payments in CY 2025. To estimate the impact among various types of ESRD facilities for renal dialysis services furnished to individuals with AKI, it is imperative that the Medicare payment estimates in CY 2024 and CY 2025 contain similar inputs. Therefore, we simulated Medicare payments only for those ESRD facilities for which we can calculate both current Medicare payments and new Medicare payments.
For this proposed rule, we used CY 2023 data from the Medicare Part A and Part B Common Working Files as of February 16, 2024, as a basis for Medicare for renal dialysis services furnished to individuals with AKI. We updated the 2023 claims to 2024 and 2025 using various updates. The updates to the AKI payment amount are described in section III.C of this proposed rule. Table 19 shows the impact of the estimated CY 2025 Medicare payments for renal dialysis services furnished to individuals with AKI compared to estimated Medicare payments for renal dialysis services furnished to individuals with AKI in CY 2024.

Column A of the impact table indicates the number of ESRD facilities for each impact category, and column B indicates the number of AKI dialysis treatments (in thousands). Column C shows the effect of the proposed CY 2025 wage index changes, including the proposed changes to the ESRD PPS wage index methodology, the proposed adoption of the new OMB CBSA delineations, the continued application of the 5 percent cap on wage index decreases, and the proposed rural transition policy as described in section II.B.2.f.(2) of this proposed rule.
Column D shows the overall impact, that is, the effects of the proposed wage index budget-neutrality adjustment factor, wage index updates, and the payment rate update of 1.8 percent, which reflects the proposed ESRDB market basket percentage increase for CY 2025 of 2.3 percent and the proposed productivity adjustment of 0.5 percentage point. We expect that overall ESRD facilities will experience a 1.9 percent increase in estimated Medicare payments in CY 2025 for treatment of AKI patients. This table does not include any distributional impacts of payments to ESRD facilities associated with the extension of payment for AKI home dialysis or extension of the add-on payment adjustment for training for home and self-dialysis, as we are unable to estimate potential uptake at a facility level at this time. However, we note that because the implementation of that adjustment would be required by section 1834(r)(1) of the Act to be budget neutral, we are considering whether it would be appropriate to apply a reduction to the AKI dialysis payment rate for budget neutrality, which could result in distributional payment changes in the future. The categories of types of ESRD facilities in the impact table show impacts ranging from an increase of 0.0 percent to an increase of 3.7 percent in their CY 2025 estimated Medicare payments for renal dialysis services provided by ESRD facilities to individuals with AKI.
Under section 1834(r) of the Act, as added by section 808(b) of TPEA, we are proposing to update the payment rate for renal dialysis services furnished by ESRD facilities to beneficiaries with AKI. The only two Medicare providers and suppliers authorized to provide these outpatient renal dialysis services are hospital outpatient departments and ESRD facilities. The patient and his or her physician make the decision about where the renal dialysis services are furnished. Therefore, this change would have zero impact on other Medicare providers.
We estimate approximately $70 million would be paid to ESRD facilities in CY 2025 because of patients with AKI receiving renal dialysis services in an ESRD facility at the lower ESRD PPS base rate versus receiving those services only in the hospital outpatient setting and paid under the outpatient prospective payment system, where services were required to be administered prior to the TPEA.
Currently, beneficiaries have a 20 percent coinsurance obligation when they receive AKI dialysis in the hospital outpatient setting. When these services are furnished in an ESRD facility, the patients will continue to be responsible for a 20 percent coinsurance. Because the AKI dialysis payment rate paid to ESRD facilities is lower than the outpatient hospital PPS's payment Start Printed Page 55835 amount, we expect beneficiaries to pay less coinsurance when AKI dialysis is furnished by ESRD facilities.
As we discussed in the CY 2017 ESRD PPS proposed rule ( 81 FR 42870 ), we considered adjusting the AKI payment rate by including the ESRD PPS case-mix adjustments, and other adjustments at section 1881(b)(14)(D) of the Act, as well as not paying separately for AKI specific drugs and laboratory tests. We ultimately determined that treatment for AKI is substantially different from treatment for ESRD, and the case-mix adjustments applied to ESRD patients may not be applicable to AKI patients, and as such, including those policies and adjustments is inappropriate. We continue to monitor utilization and trends of items and services furnished to individuals with AKI for purposes of refining the payment rate in the future. This monitoring will assist us in developing knowledgeable, data-driven proposals.
As discussed in section III.B of this proposed rule, we are proposing to allow for payment for AKI dialysis in the home setting, and as discussed in section III.C.3 of this proposed rule we are proposing to apply the home and self-dialysis training add-on payment adjustment for such services provided to AKI patients. We considered proposing to pay for AKI home dialysis without the training add-on adjustment; however, we are concerned that access to home dialysis for AKI beneficiaries could be negatively impacted in the absence of an add-on payment adjustment to support home dialysis training.
The ESRD QIP is intended to promote improvements in the quality of ESRD dialysis facility services provided to beneficiaries. The general methodology that we use to calculate a facility's TPS is described in our regulations at § 413.178(e).
Any reductions in the ESRD PPS payments as a result of a facility's performance under the PY 2027 ESRD QIP will apply to the ESRD PPS payments made to the facility for services furnished in CY 2027, consistent with our regulations at § 413.177.
For the PY 2027 ESRD QIP, we estimate that, of the 7,833 facilities (including those not receiving a TPS) enrolled in Medicare, approximately 28.3 percent or 2,214 of the facilities that have sufficient data to calculate a TPS would receive a payment reduction for PY 2027. Among an estimated 2,214 facilities that would receive a payment reduction, approximately 65 percent or 1,443 facilities would receive the smallest payment reduction of 0.5 percent. We are updating the estimated impact of the PY 2027 ESRD QIP that we provided in the CY 2024 ESRD PPS final rule ( 88 FR 76495 through 76497 ). Based on our proposals, the total estimated payment reductions for all the 2,214 facilities expected to receive a payment reduction in PY 2027 would be approximately $14,647,335. Facilities that do not receive a TPS do not receive a payment reduction.
Table 20 shows the updated overall estimated distribution of payment reductions resulting from the PY 2027 ESRD QIP.

To estimate whether a facility would receive a payment reduction for PY 2027, we scored each facility on achievement and improvement on several clinical measures for which there were available data from EQRS and Medicare claims. Payment reduction estimates were calculated using the most recent data available (specified in table 21) in accordance with the policies proposed in this proposed rule. Measures used for the simulation are shown in table 21.

For all measures except the SHR clinical measure, the SRR clinical measure, and the STrR measure, measures with less than 11 patients for a facility were not included in that facility's TPS. For the SHR clinical measure and the SRR clinical measure, facilities were required to have at least 5 patient-years at risk and 11 index discharges, respectively, to be included in the facility's TPS. For the STrR clinical measure, facilities were required to have at least 10 patient-years at risk to be included in the facility's TPS. Each facility's TPS was compared to an estimated mTPS and an estimated payment reduction table consistent with the proposed policies outlined in section IV.B of this proposed rule. Facility reporting measure scores were estimated using available data from CY 2022. Facilities were required to have at least one measure in at least two domains to receive a TPS.
To estimate the total payment reductions in PY 2027 for each facility resulting from this proposed rule, we multiplied the total Medicare payments to the facility during the 1-year period between January 2022 and December 2022 by the facility's estimated payment reduction percentage expected under the ESRD QIP, yielding a total payment reduction amount for each facility.
Table 22 shows the estimated impact of the ESRD QIP payment reductions to all ESRD facilities for PY 2027. The table also details the distribution of ESRD facilities by size (both among facilities considered to be small entities and by number of treatments per facility), geography (both rural and urban and by region), and facility type (hospital based and freestanding facilities). Given that the performance period used for these calculations differs from the performance period we are using for the PY 2027 ESRD QIP, the actual impact of the PY 2027 ESRD QIP may vary significantly from the values provided here.

For PY 2027, we estimate that the ESRD QIP would contribute approximately $14,647,335 in Medicare savings. For comparison, table 23 shows the payment reductions that we estimate will be applied by the ESRD QIP from PY 2018 through PY 2027.

The ESRD QIP is applicable to ESRD facilities. Since the Program's inception, there is evidence of improved performance on ESRD QIP measures. As we stated in the CY 2018 ESRD PPS final rule, one objective measure we can examine to demonstrate the improved quality of care over time is the improvement of performance standards ( 82 FR 50795 ). As the ESRD QIP has refined its measure set and as facilities have gained experience with the measures included in the Program, performance standards have generally continued to rise. We view this as evidence that facility performance (and therefore the quality of care provided to Medicare beneficiaries) is objectively improving. We continue to monitor and evaluate trends in the quality and cost of care for patients under the ESRD QIP, incorporating both existing measures and new measures as they are implemented in the Program. We will provide additional information about the impact of the ESRD QIP on beneficiaries as we learn more by examining these impacts through the analysis of available data from our existing measures.
In section IV.B.2 of this proposed rule, we are proposing to replace the Kt/V Dialysis Adequacy Comprehensive clinical measure with a Kt/V Dialysis Adequacy Measure Topic beginning with PY 2027. We considered not proposing this change. However, we concluded that replacing this measure was appropriate to ensure that facilities are scored on Kt/V measure data according to the individual facility's ESRD patient population and treatment modalities.
The ETC Model is a mandatory payment model designed to test payment adjustments to certain dialysis and dialysis-related payments, as discussed in the Specialty Care Models final rule ( 85 FR 61114 ), the CY 2022 ESRD PPS final rule ( 86 FR 61874 ), the CY 2023 ESRD PPS final rule ( 87 FR 67136 ), and the CY 2024 ESRD PPS final rule ( 88 FR 76344 ) for ESRD facilities and for Managing Clinicians for claims with dates of service from January 1, 2021, to June 30, 2027. The requirements for the ETC Model are set forth in 42 CFR part 512, subpart C . For the results of the detailed economic analysis of the ETC Model and a description of the methodology used to perform the analysis, see the Specialty Care Models final rule ( 85 FR 61114 ).
A stochastic simulation was created to estimate the financial impacts of the ETC Model relative to baseline expenditures, where baseline expenditures were defined as data from CYs 2018 and 2019 without the changes applied. The simulation relied upon statistical assumptions derived from retrospectively constructed ESRD facilities' and Managing Clinicians' Medicare dialysis claims, transplant claims, and transplant waitlist data reported during 2018 and 2019, the most recent years of complete data available before the start of the ETC Model. Both datasets and the risk-adjustment methodologies for the ETC Model were developed by the CMS Office of the Actuary (OACT).
Table 24 summarizes the estimated impact of the ETC Model when the achievement benchmarks for each year are set using the average of the home dialysis rates for year t -1 and year t -2 for the HRRs randomly selected for participation in the ETC Model. We estimate that the Medicare program would save a net total of $43 million from the PPA and HDPA between January 1, 2021, and June 30, 2027, less $15 million in increased training and education expenditures. Therefore, the net impact to Medicare spending is estimated to be $28 million in savings. This is consistent with the net impact to Medicare spending estimated for the CY 2022 ESRD PPS final rule, in which the net impact to Medicare spending was also estimated to be $28 million in savings ( 86 FR 62014 through 62016 ). The minor methodological change to the definition of an ESRD Beneficiary is not expected to change this estimate.

In table 24, negative spending reflects a reduction in Medicare spending, while positive spending reflects an increase. The results for this table were generated from an average of 400 simulations under the assumption that benchmarks are rolled forward with a 1.5-year lag. For a detailed description of the key assumptions underlying the impact estimate, see the Specialty Care Models final rule ( 85 FR 61353 ) and the CY 2022 ESRD PPS final rule ( 86 FR 60214 through 60216 ).
The change proposed in this rule is not expected to impact the findings reported for the effects of the ETC Model on the home dialysis rate or the transplant rate described in the Specialty Care Models final rule ( 85 FR 61355 ) and the CY 2022 ESRD PPS final rule ( 86 FR 62017 ).
The change proposed in this rule is not expected to impact the findings reported for the effects of the ETC Model on kidney disease patient education services and HD training add-ons described in the Specialty Care Models final rule ( 85 FR 61355 ) and the CY 2022 ESRD PPS final rule ( 86 FR 62017 ).
Our proposal to revise the definition of an ESRD Beneficiary for the purposes of attribution is not expected to impact the findings reported for the effects of ETC Model on Medicare beneficiaries. Further details on the impact of the ETC Model on ESRD Beneficiaries may be found in the Specialty Care Models final rule ( 85 FR 61357 ) and the CY 2022 ESRD PPS final rule ( 86 FR 61874 ).
Throughout this proposed rule, we have identified our policy proposal and alternatives considered, and provided information as to the likely effects of these alternatives and rationale for our proposal.
The Specialty Care Models final rule ( 85 FR 61114 ), the CY 2022 ESRD PPS final rule ( 86 FR 61874 ), the CY 2023 ESRD PPS final rule ( 87 FR 67136 ), the CY 2024 ESRD PPS final rule ( 88 FR 76344 ), and the proposals herein address a model specific to ESRD. These rules provide descriptions of the requirements that we waive, identify the performance metrics and payment adjustments to be tested, and presents rationales for our changes, and where relevant, alternatives considered. For context related to alternatives previously considered when establishing and modifying the ETC Model we refer readers to section V.B. and to the above citations.
As required by OMB Circular A-4 (available at https://www.whitehouse.gov/wp-content/uploads/legacy_drupal_files/omb/circulars/A4/a-4.pdf ), we have prepared an accounting statement in table 25 showing the classification of the impact associated with the provisions of this proposed rule.

The RFA requires agencies to analyze options for regulatory relief of small entities, if a rule has a significant impact on a substantial number of small entities. For purposes of the RFA, small entities include small businesses, nonprofit organizations, and small governmental jurisdictions. We do not believe ESRD facilities are operated by small government entities such as counties or towns with populations of 50,000 or less, and therefore, they are not enumerated or included in this estimated RFA analysis. Individuals and states are not included in the definition of a small entity. Therefore, the number of small entities estimated in this RFA analysis includes the number of ESRD facilities that are either considered small businesses or nonprofit organizations.
According to the Small Business Administration's (SBA) size standards, an ESRD facility is classified as a small business if it has total revenues of less than $47 million in any 1 year. [ 112 ] For the purposes of this analysis, we exclude the ESRD facilities that are owned and operated by LDOs and regional chains, which would have total revenues of more than $6.5 billion in any year when the total revenues for all locations are combined for each business (LDO or regional chain), and are not, therefore, considered small businesses. Because we lack data on individual ESRD facilities' receipts, we cannot determine the number of small proprietary ESRD facilities or the proportion of ESRD facilities' revenue derived from Medicare FFS payments. Therefore, we assume that all ESRD facilities that are not owned by LDOs or regional chains are considered small businesses. Accordingly, we consider the 461 ESRD facilities that are independent and 347 ESRD facilities that are hospital-based, as shown in the ownership category in table 18, to be small businesses. These ESRD facilities represent approximately 11 percent of all ESRD facilities in our data set.
Additionally, we identified in our analytic file that there are 779 ESRD facilities that are considered nonprofit organizations, which is approximately 10 percent of all ESRD facilities in our data set. In total, accounting for the 360 nonprofit ESRD facilities that are also considered small businesses, there are 1,227 ESRD facilities that are either small businesses or nonprofit organizations, which is approximately 16 percent of all ESRD facilities in our data set.
As its measure of significant economic impact on a substantial number of small entities, HHS uses a change in revenue of more than 3 to 5 percent. As shown in table 18, we estimate that the overall revenue impact of this proposed rule on all ESRD facilities is a positive increase to Medicare FFS payments by approximately 2.2 percent. For the ESRD PPS updates in this proposed rule, a hospital-based ESRD facility (as defined by type of ownership, not by type of ESRD facility) is estimated to receive a 3.9 percent increase in Medicare FFS payments for CY 2025. An independent facility (as defined by ownership type) is likewise estimated to receive a 0.5 percent increase in Medicare FFS payments for CY 2025. Among hospital-based and independent ESRD facilities, those furnishing fewer than 3,000 treatments per year are estimated to receive a 4.5 percent increase in Medicare FFS payments, and those furnishing 3,000 or more treatments per year are estimated to receive a 1.6 percent increase in Medicare FFS payments. Among nonprofit ESRD facilities, those furnishing fewer than 3,000 treatments per year are estimated to receive a 5.8 percent increase in Medicare FFS payments, and those furnishing 3,000 or more treatments per year are estimated to receive a 2.3 percent increase in Medicare FFS payments.
For AKI dialysis, we are unable to estimate whether patients would go to ESRD facilities, however, we have estimated there is a potential for $70 million in payment for AKI dialysis treatments that could potentially be furnished in ESRD facilities.
Based on the estimated Medicare payment impacts described previously, we do not believe that the change in revenue threshold will be reached by the policies in this proposed rule. Therefore, the Secretary has certified that this proposed rule will not have a significant economic impact on a substantial number of small entities.
For the ESRD QIP, we estimate that of the 2,214 ESRD facilities expected to receive a payment reduction as a result of their performance on the PY 2027 ESRD QIP, 409 are ESRD small entity facilities. We present these findings in table 20 (“Estimated Distribution of PY 2027 ESRD QIP Payment Reductions”) and table 22 (“Estimated Impact of ESRD QIP Payment Reductions to ESRD Facilities for PY 2027”).
For the ETC Model, we do not anticipate any impact from our proposal to modify the definition of an ESRD Beneficiary for the purposes of beneficiary attribution in the model.
In addition, section 1102(b) of the Act requires us to prepare a regulatory impact analysis if a rule may have a significant impact on the operations of a substantial number of small rural hospitals. This analysis must conform to the provisions of section 603 of the RFA. For purposes of section 1102(b) of Start Printed Page 55841 the Act, we define a small rural hospital as a hospital that is located outside of a metropolitan statistical area and has fewer than 100 beds. We do not believe this proposed rule would have a significant impact on operations of a substantial number of small rural hospitals because most dialysis facilities are freestanding. While there are 108 rural hospital-based ESRD facilities, we do not know how many of them are based at hospitals with fewer than 100 beds. However, overall, the 108 rural hospital-based ESRD facilities would experience an estimated 5.5 percent increase in payments. Therefore, the Secretary has certified that this proposed rule will not have a significant impact on the operations of a substantial number of small rural hospitals.
Section 202 of the Unfunded Mandates Reform Act of 1995 (UMRA) also requires that agencies assess anticipated costs and benefits before issuing any rule whose mandates require spending in any 1 year of $100 million in 1995 dollars, updated annually for inflation. In 2024, that threshold is approximately $183 million. We do not interpret Medicare payment rules as being unfunded mandates but simply as conditions for the receipt of payments from the Federal Government for providing services that meet Federal standards. This interpretation applies whether the facilities or providers are private, state, local, or Tribal. Therefore, this proposed rule does not mandate any requirements for State, local, or Tribal governments, or for the private sector.
Executive Order 13132 establishes certain requirements that an agency must meet when it promulgates a proposed rule (and subsequent final rule) that imposes substantial direct requirement costs on State and local governments, preempts State law, or otherwise has federalism implications. We have reviewed this proposed rule under the threshold criteria of Executive Order 13132 , Federalism, and have determined that it will not have substantial direct effects on the rights, roles, and responsibilities of state, local, or Tribal government.
The Addenda for the annual ESRD PPS proposed and final rule will no longer appear in the Federal Register . Instead, the Addenda will be available only through the internet and will be posted on CMS's website under the regulation number, CMS-1805-P, at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ESRDpayment/End-Stage-Renal-Disease-ESRD-Payment-Regulations-and-Notices . In addition to the Addenda, limited data set files (LDS) are available for purchase at https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/LimitedDataSets/EndStageRenalDiseaseSystemFile . Readers who experience any problems accessing the Addenda or LDS files, should contact CMS by sending an email to CMS at the following mailbox: [email protected] .
Chiquita Brooks-LaSure, Administrator of the Centers for Medicare & Medicaid Services, approved this document on June 21, 2024.
- Health facilities
- Health professions
- Laboratories
- Reporting and recordkeeping requirements
- Rural areas
- Puerto Rico
- Administrative practice and procedure
- Health care
- Health insurance
- Intergovernmental relations
For the reasons set forth in the preamble, the Centers for Medicare & Medicaid Services proposes to amend 42 CFR chapter IV as set forth below:
1. The authority citation for part 410 continues to read as follows:
Authority: 42 U.S.C. 1302 , 1395m , 1395hh , 1395rr , and 1395ddd .
2. Section 410.52 is amended by revising paragraph (a) introductory text to read as follows:
(a) Medicare Part B pays for the following services, supplies, and equipment furnished to a patient with ESRD or an individual with Acute Kidney Injury (AKI) as defined in § 413.371 of this chapter in his or her home:
3. The authority citation for part 413 continues to read as follows:
Authority: 42 U.S.C. 1302 , 1395d(d) , 1395f(b) , 1395g , 1395l(a) , (i), and (n), 1395m, 1395x(v), 1395x(kkk), 1395hh, 1395rr, 1395tt, and 1395ww.
4. Section 413.196 is amended by revising paragraph (d)(2) to read as follows:
(2) The wage index using the most current wage data for occupations related to the furnishing of renal dialysis services from the Bureau of Labor Statistics and occupational mix data from the most recent complete calendar year of Medicare cost reports submitted in accordance with § 413.198(b).
5. Section 413.231 is amended by revising paragraph (a) to read as follows:
(a) CMS adjusts the labor-related portion of the base rate to account for geographic differences in the area wage levels using an appropriate wage index (established by CMS) which reflects the relative level of wages relevant to the furnishing of renal dialysis services in the geographic area in which the ESRD facility is located.
6. Section 413.236 is amended by revising paragraph (b)(4) to read as follows:
(4) Has a complete Healthcare Common Procedure Coding System (HCPCS) Level II code application submitted, in accordance with the HCPCS Level II coding procedures on the CMS website, by the HCPCS Level II code application deadline for Start Printed Page 55842 biannual Coding Cycle 2 for non-drug and non-biological items, supplies, and services as specified in the HCPCS Level II coding guidance on the CMS website prior to the particular calendar year;
7. Section 413.237 is amended by adding paragraph (a)(1)(vii) to read as follows:
(vii) Renal dialysis drugs and biological products that are Composite Rate Services as defined in § 413.171.
8. Section 413.373 is revised to read as follows:
(a) CMS applies the wage-adjusted add-on per treatment adjustment for home and self-dialysis training as set forth at § 413.235(c) to payments for AKI dialysis claims that include such training.
(b) The payment rate for AKI dialysis may be adjusted by the Secretary (on a budget neutral basis for payments under section 1834(r) of the Act) by any other adjustment factor under subparagraph (D) of section 1881(b)(14) of the Act.
9. Section 413.374 is amended by revising paragraph (a) to read as follows:
(a) The AKI dialysis payment rate applies to renal dialysis services (as defined in subparagraph (B) of section 1881(b)(14) of the Act) furnished under Part B by a renal dialysis facility or provider of services paid under section 1881(b)(14) of the Act, including home services, supplies, and equipment, and self-dialysis.
10. The authority citation for part 494 continues to read as follows:
Authority: 42 U.S.C. 1302 and 1395hh .
11. Section 494.10 is amended by revising the definitions of “Home dialysis” and “Self-dialysis” to read as follows:
Home dialysis means dialysis performed at home by a patient or caregiver who has completed an appropriate course of training as described in § 494.100(a).
Self-dialysis means dialysis performed with little or no professional assistance by a patient or caregiver who has completed an appropriate course of training as specified in § 494.100(a).
12. Section 494.70 is amended by revising paragraphs (a)(1) and (10) and (c)(1)(i) to read as follows:
(1) Respect, dignity, and recognition of his or her individuality and personal needs, and sensitivity to his or her psychological needs and ability to cope with kidney failure;
(10) Be informed by the physician, nurse practitioner, clinical nurse specialist, or physician's assistant treating the patient for kidney failure of his or her own medical status as documented in the patient's medical record, unless the medical record contains a documented contraindication;
(i) How plans in the individual market will affect the patient's access to, and costs for the providers and suppliers, services, and prescription drugs that are currently within the individual's plan of care as well as those likely to result from other documented health care needs. This must include an overview of the health-related and financial risks and benefits of the individual market plans available to the patient (including plans offered through and outside the Exchange).
13. Section 494.80 is amended by revising the introductory text to read as follows:
The facility's interdisciplinary team consists of, at a minimum, the patient or the patient's designee (if the patient chooses), a registered nurse, a physician treating the patient for kidney failure, a social worker, and a dietitian. The interdisciplinary team is responsible for providing each patient with an individualized and comprehensive assessment of his or her needs. The comprehensive assessment must be used to develop the patient's treatment plan and expectations for care.
14. Section 494.90 is amended by revising paragraph (b)(4) to read as follows:
(4) The dialysis facility must ensure that all dialysis patients are seen by a physician, nurse practitioner, clinical nurse specialist, or physician's assistant providing dialysis care at least monthly, as evidenced by a monthly progress note placed in the medical record, and periodically while the hemodialysis patient is receiving in-facility dialysis.
15. Section 494.100 is amended by revising paragraph (a)(3)(i) to read as follows:
(i) The nature and management of their kidney failure.
16. Section 494.120 is amended by revising the introductory text to read as follows:
A special purpose renal dialysis facility is approved to furnish dialysis on a short-term basis at special locations. Special purpose dialysis facilities are divided into two categories: vacation camps (locations that serve patients with kidney failure while the patients are in a temporary residence) and facilities established to serve patients with kidney failure under emergency circumstances.
17. Section 494.130 is revised to read as follows:
The dialysis facility must provide, or make available, laboratory services (other than tissue pathology and histocompatibility) to meet the needs of the patient. Any laboratory services, including tissue pathology and histocompatibility must be furnished by or obtained from, a facility that meets the requirements for laboratory services specified in part 493 of this chapter.
18. Section 494.170 is amended by revising the introductory text to read as follows:
The dialysis facility must maintain complete, accurate, and accessible records on all patients, including home patients who elect to receive dialysis supplies and equipment from a supplier that is not a provider of dialysis services and all other home dialysis patients Start Printed Page 55843 whose care is under the supervision of the facility.
19. The authority citation for part 512 continues to read as follows:
Authority: 42 U.S.C. 1302 , 1315a , and 1395hh .
20. Section 512.310 is amended by revising the definition of “ESRD Beneficiary” to read as follows:
ESRD Beneficiary means a beneficiary who meets any of the following:
(1) Is receiving dialysis or other services for end-stage renal disease, up to and including the month in which the beneficiary receives a kidney transplant up to and including the month in which the beneficiary receives a kidney transplant.
(2) Has already received a kidney transplant and has a non-AKI dialysis or MCP claim at least 12 months after the beneficiary's latest transplant date.
(3) Has a kidney transplant failure less than 12 months after the beneficiary's latest transplant date as identified by at least one of the following:
(i) Two or more MCP claims in the180 days following the date on which the kidney transplant was received;
(ii) 24 or more maintenance dialysis treatments at any time after 180 days following the transplant date; or,
(iii) Indication of a transplant failure after the beneficiary's date of transplant based on data from the Scientific Registry of Transplant Recipients (SRTR) database.
(4) If a beneficiary meets more than one of criteria described in paragraphs (3)(i) through (iii) of this definition, the beneficiary will be considered an ESRD beneficiary starting with the earliest month in which transplant failure was recorded.
Xavier Becerra,
Secretary, Department of Health and Human Services.
1. Centers for Medicare & Medicaid Services (2022). Health Equity. Available at: https://www.cms.gov/pillar/health-equity .
2. 86 FR 7009 (January 25, 2021). https://www.federalregister.gov/documents/2021/01/25/2021-01753/advancing-racial-equity-and-support-for-underserved-communities-through-the-federal-government .
3. Centers for Medicare & Medicaid Services (2022). Health Equity. Available at: https://www.cms.gov/pillar/health-equity .
4. 86 FR 7009 (January 25, 2021). https://www.federalregister.gov/documents/2021/01/25/2021-01753/advancing-racial-equity-and-support-for-underserved-communities-through-the-federal-government .
5. As discussed in the CY 2019 ESRD PPS final rule ( 83 FR 56922 ), we began using the term “biological products” instead of “biologicals” under the ESRD PPS to be consistent with FDA nomenclature. We use the term “biological products” in this proposed rule except where referencing specific language in the Act or regulations.
6. Total Factor Productivity in Major Industries—2020. Available at: https://www.bls.gov/news.release/prod5.nr0.htm .
7. https://www.medpac.gov/wp-content/uploads/2023/06/Jun23_MedPAC_Report_To_Congress_SEC.pdf .
8. https://www.cms.gov/files/document/end-stage-renal-disease-prospective-payment-system-technical-expert-panel-summary-report-may-2020.pdf .
9. The OEWS program produces estimates of employment and wages by occupation based on a survey of business establishments. OEWS data are released annually with a May reference date. Each set of OEWS estimates is based on data from six semiannual survey panels collected over a 3-year period. For example, the May 2022 OEWS wage estimates are based on six semiannual survey panels from November 2019 through May 2022.
10. For more information on MSAs and non-MSAs please see: https://www.bls.gov/oes/current/msa_def.htm . For more information on the most recent CBSA delineations (as discussed later in this section) please see: https://www.whitehouse.gov/wp-content/uploads/2023/07/OMB-Bulletin-23-01.pdf .
11. We use the territory-level data for Guam and Virgin Islands, since the MSA and non-MSA level data is not available.
12. https://www.bls.gov/respondents/oes/instructions.htm#online .
13. https://www.bls.gov/oes/oes_ques.htm .
14. We note that in accordance with section 1886(d)(14)(E)(1) of the Act, the IPPS wage index is required to employ data based on “a survey conducted by the Secretary (and updated as appropriate) of the wages and wage-related costs of subsection (d) hospitals in the United States.” The IPPS is based on the most current audited hospital wage data from Worksheet S-3, Parts II, III and IV of the Medicare cost report, CMS Form 2552-10 (OMB Control Number 0938-0050 with an expiration date of September 30, 2025) (see, for example, 88 FR 58961 ).
15. In cases where 2022 freestanding cost report data are not available at the time of this proposed rule, 2021 data was used. This was the case for 131 ESRD facilities, approximately 1.7 percent of the ESRD facilities in this analysis. We expect that in calculating the wage indices in the final rule only 2022 cost report data would be used.
16. Files related to the FY 2024 IPPS final rule are available online at https://www.cms.gov/medicare/payment/prospective-payment-systems/acute-inpatient-pps/fy-2024-ipps-final-rule-home-page .
17. Treatment weighted average of wage indices are calculated by multiplying the wage index value for each CBSA by the treatment count in the CBSA, and dividing by the aggregate national treatment count.
18. https://www.bls.gov/soc/2018/major_groups.htm .
19. For example, under section 1886(d)(3)(E) of the Act, the IPPS applies a labor related share of 62 percent for each hospital unless this would result in lower payments to the hospital than would otherwise be made.
20. https://www.whitehouse.gov/wp-content/uploads/legacy_drupal_files/omb/bulletins/2013/b13-01.pdf .
21. https://www.bls.gov/bls/omb-bulletin-15-01-revised-delineations-of-metropolitan-statistical-areas.pdf .
22. https://www.whitehouse.gov/wp-content/uploads/legacy_drupal_files/omb/bulletins/2017/b-17-01.pdf .
23. https://www.whitehouse.gov/wp-content/uploads/2018/04/OMB-BULLETIN-NO.-18-03-Final.pdf .
24. https://www.whitehouse.gov/wp-content/uploads/2018/09/Bulletin-18-04.pdf .
25. Under § 413.237(a)(1)(vi), as of January 1, 2012, the laboratory tests that comprise the Automated Multi-Channel Chemistry panel are excluded from the definition of outlier services.
26. Transmittal 2033 issued August 20, 2010, was rescinded and replaced by Transmittal 2094, dated November 17, 2010. Transmittal 2094 identified additional drugs and laboratory tests that may also be eligible for ESRD outlier payment. Transmittal 2094 was rescinded and replaced by Transmittal 2134, dated January 14, 2011, which included one technical correction. https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/R2134CP.pdf .
27. Since 2018, there has been no updated reporting for most clinical diagnostic laboratory tests; therefore, the forecast estimate used since CY 2018 for the ESRD PPS outlier methodology has been 0.
28. https://www.whitehouse.gov/wp-content/uploads/2023/07/OMB-Bulletin-23-01.pdf .
29. As discussed in the CY 2019 ESRD PPS final rule ( 83 FR 56922 ), we began using the term “biological products” instead of “biologicals” under the ESRD PPS to be consistent with FDA nomenclature. We use the term “biological products” in this proposed rule except where referencing specific language in the Act or regulations.
30. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/mm10065.pdf and https://www.cms.gov/regulations-and-guidance/guidance/transmittals/2018downloads/r1999otn.pdf .
31. https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/2018Downloads/R1999OTN.pdf .
32. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM10065.pdf .
33. https://www.gao.gov/assets/d24106288.pdf .
34. Ibid.
35. Ibid.
36. https://www.cms.gov/medicare/payment/prospective-payment-systems/end-stage-renal-disease-esrd and https://www.cms.gov/files/document/including-oral-only-drugs-esrd-pps-bundled-payment.pdf .
37. https://www.medpac.gov/wp-content/uploads/import_data/scrape_files/docs/default-source/reports/jun20_ch7_reporttocongress_sec.pdf .
38. https://www.cms.gov/files/document/end-stage-renal-disease-prospective-payment-system-technical-expert-panel-summary-report-april-2021.pdf .
39. https://www.cms.gov/files/document/end-stage-renal-disease-prospective-payment-system-technical-expert-panel-summary-report-april-2021.pdf .
40. https://www.cms.gov/files/document/end-stage-renal-disease-prospective-payment-system-technical-expert-panel-summary-report-april-2021.pdf .
41. https://www.cms.gov/medicare/medicare-fee-for-service-payment/esrdpayment/educational_resources .
42. https://www.cms.gov/files/document/cy-2022-esrd-pps-rfi-summary-comments.pdf .
43. The materials from the TEPs and summary reports can be found at https://www.cms.gov/medicare/medicare-fee-for-service-payment/esrdpayment/educational_resources .
46. https://www.cms.gov/files/document/cy-2024-esrd-pps-lvpa-rfi-summary-comments.pdf .
47. 86 FR 7009 (January 25, 2021). https://www.federalregister.gov/documents/2021/01/25/2021-01753/advancing-racial-equity-and-support-for-underserved-communities-through-the-federal-government .
48. Healthcare Common Procedure Coding System (HCPCS) Level II Coding Procedures. Available at: https://www.cms.gov/medicare/coding/medhcpcsgeninfo/downloads/2018-11-30-hcpcs-level2-coding-procedure.pdf . Accessed on January 16, 2024.
49. Annual Data Report | USRDS ( nih.gov ), https://usrds-adr.niddk.nih.gov/2023/end-stage-renal-disease/2-home-dialysis .
50. https://www.sciencedirect.com/science/article/pii/S0085253821004567 .
51. https://journals.sagepub.com/doi/10.1177/0896860820970834 .
52. https://pubmed.ncbi.nlm.nih.gov/29199769/ .
53. https://academic.oup.com/ckj/article/16/2/210/6696026 .
54. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4594060/ .
55. https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/1744-9987.12660 .
56. https://www.sciencedirect.com/science/article/pii/S0085253815528664 .
57. https://www.cms.gov/medicare/payment/prospective-payment-systems/end-stage-renal-disease-esrd/esrd-prospective-payment-system-esrd-pps-overview-claims-based-monitoring-program .
58. https://academic.oup.com/ckj/article/16/12/2493/7210548 .
59. Annual Data Report | USRDS ( nih.gov ), https://usrds-adr.niddk.nih.gov/2023/end-stage-renal-disease/2-home-dialysis .
60. https://www.cms.gov/medicare/payment/prospective-payment-systems/end-stage-renal-disease-esrd/esrd-prospective-payment-system-esrd-pps-overview-claims-based-monitoring-program .
61. https://journals.lww.com/jasn/abstract/2023/12000/initial_management_and_potential_opportunities_to.9.aspx .
62. https://www.cms.gov/about-cms/what-we-do/cms-strategic-plan .
63. https://usrds-adr.niddk.nih.gov/2023/end-stage-renal-disease/2-home-dialysis .
64. Annual Data Report | USRDS ( nih.gov ), https://usrds-adr.niddk.nih.gov/2023/chronic-kidney-disease/4-acute-kidney-injury .
65. https://pubmed.ncbi.nlm.nih.gov/29199769/ .
66. https://www.cms.gov/medicare/payment/prospective-payment-systems/end-stage-renal-disease-esrd/esrd-prospective-payment-system-esrd-pps-overview-claims-based-monitoring-program .
67. https://www.cms.gov/medicare/payment/prospective-payment-systems/end-stage-renal-disease-esrd/esrd-prospective-payment-system-esrd-pps-overview-claims-based-monitoring-program .
68. https://www.cms.gov/regulations-and-guidance/guidance/manuals/downloads/clm104c08.pdf .
69. https://qcor.cms.gov/active_nh.jsp?which=7&report=active_nh.jsp .
70. https://qcor.cms.gov/active_nh.jsp?which=7&report=active_nh.jsp .
71. United States Renal Data System. 2023 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD 2023. https://usrds-adr.niddk.nih.gov/2023/chronic-kidney-disease/4-acute-kidney-injury .
72. Ibid.
73. Ibid.
74. 42 CFR part 494 .
75. Bassuner J, Kowalczyk B, Abdel-Aal AK. Why Peritoneal Dialysis is Underutilized in the United States: A Review of Inequities. Semin Intervent Radiol. 2022 Feb 18;39(1):47-50. doi: 10.1055/s-0041-1741080.
76. USRDS Annual Data Report 2023.
77. Ibid.
78. USRDS Annual Data Report 2023.
79. Ibid.
80. Ibid.
81. Ibid.
82. Ibid.
83. Ibid.
84. Ibid.
85. Ibid.
86. Cozzolino M, Conte F, Zappulo F, Ciceri P, Galassi A, Capelli I, Magnoni G, La Manna G. COVID-19 pandemic era: is it time to promote home dialysis and peritoneal dialysis? Clin Kidney J. 2021 Feb 2;14(Suppl 1):i6-i13. doi: 10.1093/ckj/sfab023.
87. Geetha D, Kronbichler A, Rutter M, Bajpai D, Menez S, Weissenbacher A, Anand S, Lin E, Carlson N, Sozio S, Fowler K, Bignall R, Ducharlet K, Tannor EK, Wijewickrama E, Hafidz MIA, Tesar V, Hoover R, Crews D, Varnell C, Danziger-Isakov L, Jha V, Mohan S, Parikh C, Luyckx V. Impact of the COVID-19 pandemic on the kidney community: lessons learned and future directions. Nat Rev Nephrol. 2022 Nov;18(11):724-737. doi: 10.1038/s41581-022-00618-4.
88. AdvaMed to CMS (January 24, 2023).
89. Renal Physicians Association. “RPA Comments on the 2017 ESRD PPS Proposed Rule Including AKI Policy” http://ww.renalmed.org/page/ESRDPPSRuleComments ? (2016).
90. USRDS Annual Data Report 2023.
91. https://www.cms.gov/files/document/esrd-measures-manual-v91.pdf .
92. In previous years, we referred to the consensus-based entity by corporate name. We have updated this language to refer to the consensus-based entity more generally.
93. For further information related to the Kt/V Dialysis Adequacy Comprehensive clinical measure, we refer readers to 77 FR 67487 through 67490 , 79 FR 66197 through 66198 , and 80 FR 69053 through 69057 .
94. For further information related to the NHSN Dialysis Event reporting measure, we refer readers to 76 FR 70268 through 70269 and 78 FR 72204 through 72207 .
95. Partnership for Quality Measurement. 2023 Measure Set Review (MSR): End Stage Renal Disease Quality Incentive Program (ESRD-QIP). September 2023. Available at: https://p4qm.org/sites/default/files/2023-09/MSR-Report-ESRD-QIP-20230911.pdf .
96. Centers for Medicare & Medicaid Services. (2022) CMS National Quality Strategy. Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/CMS-Quality-Strategy .
97. Health Equity Strategic Pillar. Centers for Medicare & Medicaid Services. https://www.cms.gov/pillar/health-equity .
98. Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health & Human Services. First Report to Congress on Social Risk Factors and Performance in Medicare's Value-Based Purchasing Program. 2016. Available at: https://aspe.hhs.gov/sites/default/files/migrated_legacy_files/171041/ASPESESRTCfull.pdf .
99. Johnston, K.J., & Joynt Maddox, K.E. (2019). The Role of Social, Cognitive, And Functional Risk Factors In Medicare Spending For Dual And Nondual Enrollees. Health Affairs (Project Hope), 38 (4), 569-576. https://doi.org/10.1377/hlthaff.2018.05032 .
100. Johnston, K.J., & Joynt Maddox, K.E. (2019). The Role of Social, Cognitive, and Functional Risk Factors in Medicare Spending for Dual and Nondual Enrollees. Health Affairs (Project Hope), 38 (4), 569-576. https://doi.org/10.1377/hlthaff.2018.05032 .
101. Wadhera, R.K., Wang, Y., Figueroa, J.F., Dominici, F., Yeh, R.W., & Joynt Maddox, K.E. (2020). Mortality and Hospitalizations for Dually Enrolled and Nondually Enrolled Medicare Beneficiaries Aged 65 Years or Older, 2004 to 2017. JAMA, 323 (10), 961-969. https://doi.org/10.1001/jama.2020.1021 .
102. Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health & Human Services. Second Report to Congress on Social Risk Factors and Performance in Medicare's Value-Based Purchasing Program. 2020. Available at: https://aspe.hhs.gov/reports/second-report-congress-social-risk-medicares-value-based-purchasing-programs .
103. ZIP codeTM. is a trademark of the United States Postal Service.
104. Golper TA, Saxena AB, Piraino B, Teitelbaum, I, Burkart, J, Finkelstein FO, Abu-Alfa A. Systematic Barriers to the Effective Delivery of Home Dialysis in the United States: A Report from the Public Policy/Advocacy Committee of the North American Chapter of the International Society for Peritoneal Dialysis. American Journal of Kidney Diseases. 2011; 58(6): 879-885.doi:10.1053/j.ajkd.2011.06.028.
105. Chan, C.T., Collins, K., Ditschman, E.P., Koester-Wiedemann, L., Saffer, T.L., Wallace, E., & Rocco, M.V. (2020). Overcoming barriers for uptake and continued use of home dialysis: An NKF-Kdoqi Conference Report. American Journal of Kidney Diseases, 75(6), 926-934. https://doi.org/10.1053/j.ajkd.2019.11.007 .
106. Nguyen, K.H., Oh, E.G., Meyers, D.J., Kim, D., Mehrotra, R., & Trivedi, A.N. (2023). Medicare advantage enrollment among beneficiaries with end-stage renal disease in the first year of the 21st Century Cures Act. JAMA, 329 (10), 810. https://doi.org/10.1001/jama.2023.1426 .
107. https://www.bls.gov/oes/2022/may/oes292072.htm .
108. Calculated by multiplying the mean wage for medical and health service managers by 2 to account for overhead and fringe benefits.
109. CMS Transmittal 12157, dated July 27, 2023, is available at: https://www.cms.gov/files/document/r12157cp.pdf .
110. CMS Transmittal 12628, dated May 9, 2024, is available at: https://www.cms.gov/files/document/r12628CP.pdf .
111. In the CY 2022 ESRD PPS final rule, we adopted a special scoring methodology and payment policy for PY 2022 due to significant impacts related to the COVID-19 public health emergency ( 86 FR 61918 through 61919 ). Under this policy, we did not apply any payment reductions to ESRD facilities for PY 2022.
112. http://www.sba.gov/content/small-business-size-standards .
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
[ FR Doc. 2024-14359 Filed 6-27-24; 4:15 pm]
- Executive Orders
Reader Aids
Information.
- About This Site
- Accessibility
- No Fear Act
- Continuity Information

IMAGES
VIDEO
COMMENTS
Writing a Literature Review. A literature review is a document or section of a document that collects key sources on a topic and discusses those sources in conversation with each other (also called synthesis ). The lit review is an important genre in many disciplines, not just literature (i.e., the study of works of literature such as novels ...
Examples of literature reviews. Step 1 - Search for relevant literature. Step 2 - Evaluate and select sources. Step 3 - Identify themes, debates, and gaps. Step 4 - Outline your literature review's structure. Step 5 - Write your literature review.
Literature reviews are in great demand in most scientific fields. Their need stems from the ever-increasing output of scientific publications .For example, compared to 1991, in 2008 three, eight, and forty times more papers were indexed in Web of Science on malaria, obesity, and biodiversity, respectively .Given such mountains of papers, scientists cannot be expected to examine in detail every ...
1. Outline and identify the purpose of a literature review. As a first step on how to write a literature review, you must know what the research question or topic is and what shape you want your literature review to take. Ensure you understand the research topic inside out, or else seek clarifications.
In a PhD thesis, the literature review typically comprises one chapter (perhaps 8-10,000 words), for a Masters dissertation it may be around 2-3,000 words, and for an undergraduate dissertation it may be no more than 2,000 words. In each case the word count can vary depending on a range of factors and it is always best, if in doubt, to ask your ...
Literature Reviews that are organized methodologically consist of paragraphs/sections that are based on the methods used in the literature found.This approach is most appropriate when you are using new methods on a research question that has already been explored.Since literature review structures are not mutually exclusive, you can organize the use of these methods in chronological order.
In general, the length of the review should be proportionate to your overall paper. For example, if you're writing a fifty-thousand-word dissertation, then your literature review will likely be an entire chapter comprising about 20 pages. If it's for a 15-page research paper, your literature review may only be a few pages.
How to Write a Literature Review in 6 Steps. Published on July 2, 2024 by Paige Pfeifer, BA. The usual purpose of a literature review is to show a gap in existing research or to show a field's overall view of a topic. A "literature review" is a summary of what previous studies have demonstrated or argued about a topic.
You may have enough information for your literature review when: You've used multiple databases and other resources (web portals, repositories, etc.) to get a variety of perspectives on the research topic. ... It's usually a bad sign to see every paragraph beginning with the name of a researcher. Instead, organize the literature review into ...
Here is a general outline of steps to write a thematically organized literature review. Remember, though, that there are many ways to approach a literature review, depending on its purpose. Stage one: annotated bibliography. As you read articles, books, etc, on your topic, write a brief critical synopsis of each.
why you are writing a review, and why the topic is important; the scope of the review — what aspects of the topic will be discussed; the criteria used for your literature selection (e.g. type of sources used, date range) the organisational pattern of the review. Body paragraphs. Each body paragraph should deal with a different theme that is ...
Introductory paragraphs can be the most challenging part of writing a paper. Instead of laying out the evidence (or in the case of a literature review, analyzing your resources), you must first provide background information and context for the topic, discuss the body of literature in general as well as the scope of your review, and give a brief outline of how you will organize the review.
A literature review is an integrated analysis-- not just a summary-- of scholarly writings and other relevant evidence related directly to your research question. That is, it represents a synthesis of the evidence that provides background information on your topic and shows a association between the evidence and your research question.
How To Structure Your Literature Review. Like any other chapter in your thesis or dissertation, your literature review needs to have a clear, logical structure. At a minimum, it should have three essential components - an introduction, a body and a conclusion. Let's take a closer look at each of these. 1: The Introduction Section
A literature review is a document or section of a document that collects key sources on a topic and discusses those sources in conversation with each other (also called synthesis). The lit review is an important genre in many disciplines, as it discusses the research (also called scholarship or literature) in a given field.
Here is a sample paragraph from a literature review about sexism and language to illuminate the following discussion: However, other studies have shown that even gender-neutral antecedents are more likely to produce masculine images than feminine ones (Gastil, 1990). Hamilton (1988) asked students to complete sentences that required them to ...
The process of writing a Lit Review typically involves a number of steps. These should include the following: • Deciding on a relatively focused topic or question. • Searching for relevant and relatively current literature (books, journal articles, etc. - the mix of these depends on your topic or thesis statement).
There are a few different ways to organize the body paragraphs of a literature review. • Chronological: This is when the literature review discusses the sources in the order they were published, from the oldest to the newest. This can be useful if your focus is on the historical development of a subject. • Trend: A literature review ...
Structuring a literature review. In general, literature reviews are structured in a similar way to a standard essay, with an introduction, a body and a conclusion. These are key structural elements. Additionally, a stand-alone extended literature review has an abstract. Throughout, headings and subheadings are used to divide up the literature ...
Rule 4: Choose the Type of Review You Wish to Write. After having taken notes while reading the literature, you will have a rough idea of the amount of material available for the review. This is probably a good time to decide whether to go for a mini- or a full review.
A literature review should be structured like any other essay: it should have an introduction, a middle or main body, and a conclusion. Introduction The introduction should: define your topic and provide an appropriate context for reviewing the literature; establish your reasons - i.e. point of view - for reviewing the literature; explain the organisation…
PURPOSE: a literature review provides a scholarly context for the argument you propose and support in your paper. It helps readers perceive how your argument fits into past and present scholarly discussion of your subject. Most often, a literature review is formatted to appear as a separate section of your paper, preceding the body.
A literature review is a piece of discursive prose, not a list describing or summarizing one piece of literature after another. It should have a single organizing principle: Thematic - organize around a topic or issue; Chronological - sections for each vital time period; Methodological - focus on the methods used by the researchers/writers
When you write your thematically organized literature review body paragraphs, one way to do this is by using the MEAL plan. Paragraphs that follow the MEAL plan have four main parts: (1) the topic sentence which states the main idea of the paragraph in the author's own words, (2) relevant evidence which is paraphrased or quoted and also cited, (3) analysis of that evidence, threaded ...
Literature Review Body Paragraph Example. The main body of a literature review should provide an overview of the existing research on the issue. Body paragraphs should not just summarize each source but analyze them. You can organize your paragraphs with these 3 elements: Claim. Start with a topic sentence linked to your literature review purpose.
For many of these occupations, the corresponding BLS code was straightforward. For example, BLS code 29-1141 is for "Registered Nurses" which matches the category on the cost reports from which the NEFOM is derived exactly. ... We strongly urge ESRD facilities to carefully review cost report data to ensure continued accuracy so that future ...
Paragraphs 18 U.S.C. §§ 3631(b)(4)(D)-(E) require that the risk ... Further, since the last Annual Report, the FBOP has completed a literature review of all programs listed within the FSA Programs Guide. For each of the FBOP's identified EBRR programs and PAs, a contractor reviewed the research on the program's efficacy and ...