The website will be down for maintenance from 6:00 a.m. to noon CDT on Sunday, June 30.

Clinical Practice Guideline Manual
Introduction
I. Development of Evidence-based Clinical Practice Guidelines (CPGs)
II. Joint Development of CPGs with External Organizations (CMSS P 9)
III. Identification of a CPG Clinical Topic
IV. Systematic Evidence Review of CPG Clinical Topic (IOM Standard 4)
V. Development of CPG Panel (IOM Standards 3; CMSS-P 4)
VI. Conflict of Interest (COI) Policy and Process (IOM Standard 2, CMSS-P 3, CMSS-C 7.5-7.8)
VII. Clinical Practice Guideline Panel Collaboration
VIII. Review of Published Evidence Report (IOM Standard 4)
IX. Grading Evidence and Strength of Recommendation (CMSS-P 6; IOM Standards 5 and 6)
X. Writing the Guideline
XI. CPG Peer Review (the following sections are in accordance with IOM 7, CMSS-P 7, and CMSS-C 7,9, 7.11, 7.15)
XII. AAFP Approval Process (CMSS-P 7.1 and CMSS-C 7.9)
XIII. Publication (CMSS-P 7.2.2 and 9, CMSS-C 7.11)
XIV. Dissemination (CMSS-P 9.2-9.4)
XV. Five-Year Update of CPG (IOM Standard 8 and CMSS-P 8)
XVI. Endorsement of External Guidelines
APPENDIX OF USEFUL RESOURCES
The American Academy of Family Physicians (AAFP) develops evidence-based clinical practice guidelines (CPGs), which serve as a framework for clinical decisions and supporting best practices. Clinical practice guidelines are statements that include recommendations intended to optimize patient care. They are informed by a systematic review of evidence, and an assessment of the benefits and harms of alternative care options. CPGs should follow a sound, transparent methodology to translate best evidence into clinical practice for improved patient outcomes. Additionally, evidence-based CPGs are a key aspect of patient-centered care.
This manual summarizes the processes used by the AAFP to produce high-quality, evidence-based guidelines. The AAFP’s development process adheres to the following standards and principles:
- Institute of Medicine (IOM): Clinical Practice Guidelines We Can Trust—Standards for Developing Trustworthy Clinical Practice Guidelines (CPGs)
- Council on Medical Specialty Societies: Principles for the Development of Specialty Society Clinical Guidelines
- Council on Medical Specialty Societies: Code for Interactions with Companies
Clinical practice guidelines should be developed using rigorous evidence-based methodology with the strength of evidence for each guideline explicitly stated.
- Clinical practice guidelines should be feasible, measurable, and achievable.
- Clinical performance measures may be developed from clinical practice guidelines and used in quality improvement initiatives. When these performance measures are incorporated into public reporting, accountability, or pay for performance programs, the strength of evidence and magnitude of benefit should be sufficient to justify the burden of implementation.
- In the clinical setting, implementation of clinical practice guidelines should be prioritized to those that have the strongest supporting evidence, and the most impact on patient population morbidity and mortality.
- Research should be conducted on how to effectively implement clinical practice guidelines, and the impact of their use as quality measures.
a. Definition: Clinical practice guidelines are statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options. Rather than dictating a one-size-fits-all approach to patient care, clinical practice guidelines offer an evaluation of the quality of the relevant scientific literature, and an assessment of the likely benefits and harms of a particular treatment. This information enables health care clinicians to select the best care for a unique patient based on his or her preferences.
b. AAFP’s Commission on Health of the Public and Science (CHPS) and Board of Directors provides oversight for the development and approval of its clinical practice guidelines.
c. Principles for Development (IOM 1.1, CMSS-P 11, CMSS-C): The IOM identified eight standards for developing trustworthy guidelines. The standards reflect best practices across the entire guideline development process, including attention to:
- Establishing transparency;
- Managing conflict of interest;
- Guideline development group composition;
- Clinical practice guideline–systematic review intersection;
- Establishing evidence foundations for and rating strength of recommendations;
- Articulation of recommendations;
- External review; and
The Council of Medical Specialty Societies (CMSS) provides directions and standards for the development of clinical practice guidelines through the CMSS Principles for Guideline Development (CMSS-P) and the Code for Interactions with Companies (CMSS-C). Where possible, the standards outlined by the IOM (now the National Academies of Medicine) and CMSS are referenced in the corresponding sections below.
The AAFP advocates the development of explicit patient-centered clinical practice guidelines which focus on what should be done for patients rather than who should do it. When clinical practice guidelines address the issue of who should provide care, then recommendations for management, consultation, or referral should emphasize appropriate specific competencies rather than a clinician's specialty designation. The AAFP may participate with other medical organizations in the development of clinical practice guidelines (also known as practice parameters or clinical policies) when appropriate criteria are met.
When AAFP enters into a joint development of a CPG with external organizations, a Memorandum of Understanding (MOU) should be developed to guide the process.
a. A clinical topic for a new or updated CPG is first vetted by the Subcommittee on Clinical Recommendations and Policies (SCRP) of CHPS using the following criteria:
- Relevance to family medicine
- No current evidence-based guidelines on the topic available that are suitable for use by family physicians
- Guidance on this topic will support AAFP Strategic Objectives and Strategies
- A systematic evidence report is available, the topic can be nominated to the Agency for Healthcare Research and Quality (AHRQ), or there is a funding source for creation of an evidence review.
b. AAFP Board approval is obtained for topic nomination and collaborators.
c. Prior to topic nomination, potential co-nominators/collaborators are contacted to involve them in the process.
In most cases, the AAFP utilizes the Agency for Healthcare Research and Quality (AHRQ) for development of an independent systematic review of the evidence based on the key questions identified for the CPG.
a. Develop topic nomination proposal to AHRQ (CMSS P 5)
i. Include key clinical questions and parameters with patient-oriented outcomes prioritized ii. Members and content experts assist in drafting and providing feedback on the key questions iii. Include collaborators for co-nomination (if applicable)
b. AHRQ Evidence-based Practice Center (EPC)
i. Establish staff contact with AHRQ program officer and EPC staff for evidence report ii. Include one or more family physicians to serve on the technical expert panel (TEP) of the EPC. Often the TEP members will also serve on the guideline development panel.
c. AHRQ EPC Evidence Report on Clinical Topic
i. Staff and SCPG members provide review and feedback on the draft evidence report as requested by AHRQ throughout the process ii. The draft report can be used to begin development of the draft CPG iii. When the final EPC evidence report is published and available, it is used to finalize the CPG.
Staff works with the chairs of SCRP and CHPS to form the Guideline Development Group using the process outlined below:
a. Identify AAFP GDG Chair through the CHPS and obtain approval from the Board of Directors b. Identify family physicians panel members including at least one member of SCPG and one member of the Science Advisory Panel in addition to other CHPS and AAFP members. c. Identify GDG members from collaborators including a patient representative or patient advocacy group(s) when available/appropriate d. Obtain a Disclosure of Interest Form from all panel members (IOM Standards 2)
A conflict of interest (COI) is an important potential source of bias in the development of CPGs. A COI has been defined as a set of conditions in which professional judgment concerning a primary interest (guideline recommendations), is unduly influenced by a secondary interest (financial or intellectual interests) (Norris et al 2012 and Thompson DF 1993) .
To limit both actual and perceived bias in guideline development, the AAFP has set forth the following policy for COI:
- Whenever possible GDG members should not have COI.
- The chair or co-chairs should not be a person(s) with COI.
- The chair or co-chairs should remain conflict free for one year following publication.
- Members with COIs should not represent a majority the GDG.
- Funders should have no role in CPG development.
a. Prior to selection of the Guideline Development Group (GDG), individuals being considered for membership should declare all interests and activities potentially resulting in COI with development group activity, by written disclosure to those convening the GDG.
- Disclosures should include activities relevant to the scope of the CPG for the both the potential member as well as members of their immediate family (spouse/partner, parents, siblings, children)
- Disclosures should include current and planned activities in addition to activities for up to three years prior to convening of the GDG.
b. Disclosures should include activities that may be considered financial or intellectual COI as defined below:
i. Financial COI = material interest that could influence, or be perceived as influencing, an individual’s point of view
- Includes any industry funding (even if not related to guideline topic)
- Outside of industry funding, includes activities related to the guideline topic: consultant, expert witness, stock ownership/options, research funding, speaker’s bureau
- Includes other financial interests related to health care that may be relevant
ii. Intellectual COI = “…activities that create the potential for an attachment to a specific point of view that could unduly affect an individual’s judgement about a specific recommendation” (Guyatt et al 2010).
- Includes, but not limited to, authorship of a publication, participation in research, participation on a workgroup/panel with medical specialty society or health care organization, lobbying or advocacy, or public presentation of a view point related to the guideline (blog, editorial, etc)
c. Review and Management of COIs
- Disclosures for each potential member will be reviewed by staff and the chair of the GDG prior to placement on the panel.
- If a disclosure is determined to represent a conflict of interest, potential actions include exclusion from the panel or limits on participation in discussions or voting on relevant recommendations.
- Each panel member will update any COI (verbally or in person) at each meeting of the GDG.
d. Divestment Members of the GDG should divest themselves of financial investments they or their family members have, and not participate in marketing activities or advisory boards, of entities whose interests could be affected by CPG recommendations.
e. Exclusions In some circumstances, a GDG may not be able to perform its work without members who have COIs, such as relevant clinical specialists who receive a substantial portion of their incomes from services pertinent to the CPG.
a. Clinical practice guideline time-line and expectations AAFP staff members, in collaboration with the GDG chair, will develop a time-line for the guideline being developed. This time-line will be distributed to GDG members during the first meeting of the GDG. Though this time-line is developed with the goal of adherence, it is recognized that circumstances during the development process may affect the time-line. Thus, this is a living document throughout the guideline process and should be updated as appropriate.
Expectations of GDG members and staff will be reviewed by the GDG chair during the first meeting of the GDG.
- Writing assignments: Writing assignments may be made throughout the guideline development process. GDG members will be asked to volunteer for certain tasks and may be assigned to subgroups to develop recommendations and write supporting evidence for those recommendations.
- Deadlines: Clear deadlines will be agreed upon during the process of guideline development. However, as stated above, circumstances during the CPG development process may arise that warrant adjusting deadlines. The panel chair and staff members at the AAFP will work with the GDG on any changes in deadlines.
b. CPG outline The GDG will work with the GDG chair and AAFP staff members to develop an outline of the proposed guideline. The outline will include the key questions from the evidence report, the potential draft recommendations, key points for supporting text, and identification of potential information for shared decision-making tables and implementation algorithms.
c. Conference calls Conference calls will be convened at the start of the guideline development process and throughout as needed. AAFP staff members will work with GDG members to ensure availability for calls. When a member cannot be present on a call, that member will be provided opportunities to provide any written comments prior to the call and feedback to the meeting summary after the call.
d. Electronic communication Electronic communication will be used throughout the guideline process. Reasonable response times are expected for electronic communications and deadlines for requested action items will be clearly stated in the communications from AAFP staff members.
d. CPG publication(s) and dissemination (CMSS P 9) When AAFP collaborates with others on a joint guideline, it will be decided where publication is expected at the start of the collaboration. All parties will agree to the publication plan. For guidelines developed solely by the AAFP, the GDG and staff will identify an appropriate publication venue which may include a peer-reviewed journal and/or the AAFP website. Dissemination activities should also be identified early on to facilitate work load and collaboration. These activities can include one or more of the following:
- Press release
- National Guideline Clearinghouse or Guidelines International Network Database
- Derivative creation either by AAFP, collaborator, or other commercial entity
a. Section IV of this manual described the AAFP process for nominating topics to AHRQ for a systematic review of the evidence. Once the systematic review has been completed, a draft evidence report is published by AHRQ. The GDG reviews the draft evidence report to determine if applicable for development of a guideline. Systematic literature review performed by the AAFP.
If more than 12 months has passed between the publication of an AHRQ evidence review and development of the guideline, an update of the systematic review will be conducted. The GDG and AAFP staff members will work with the AAFP librarian to perform the updated review. The librarian will use the same search criteria that were used in the AHRQ systematic review. Inclusion and exclusion criteria will be set a priori to determine which studies will be reviewed for quality. AAFP staff members review the updated search results and obtain articles relevant to the systematic review.
b. As outlined in section IV, AHRQ has a process for performing systematic reviews that is consistent with the 2011 Standards for Systematic Reviews from the IOM. The AAFP also uses this as a guide to ensure the systematic literature reviews we are performing or that we are using for guideline development are compliant with the best standards available. These standards include: establishing a team with appropriate experience and expertise to do the review, including those with content expertise; providing methodological expertise and other expertise as appropriate; ensuring any conflict of interest is managed with regard to the team; ensuring that there is user and stakeholder input as the review is designed and conducted; managing conflict of interest with regard to any individuals providing input into the review; and formulating the topic for review.
The standards also discuss “finding and assessing individual studies.” This includes steps such as:
i. Conducting a comprehensive search for the evidence. This step will likely include:
a. Working with a librarian, and
b. Searching appropriate databases, citation indexes and other sources for relevant information.
ii. Taking action to address potential bias in reporting of research results.
iii. Screening and selecting relevant studies. Here it is very important to include and exclude studies based on a priori specified criteria developed in the protocol. It is recommended that two or more people screen studies and that these reviewers are tested for accuracy and consistency in their reviews.
iv. Documenting the search strategy, including dates of searches and how each item identified in the search was addressed. If excluded, include the reason for exclusion.
v. If data is extracted for a meta-analysis, data collection should be managed appropriately. The IOM standards recommend that systematic review developers:
a. Use two or more researchers to extract relevant data from a report;
b. Link publications from the same studies to avoid duplication of data; and
c. Use data extraction forms that are pilot tested.
vi. Finally, at least two reviewers should critically appraise each study using the specified protocol and forms derived for the review.
Compiling evidence and assessing it for quality are important steps in a systematic review. The quality of the evidence should be linked to the strength of the recommendations in that guideline. Consistent with the IOM standards for systematic reviews, the AAFP uses a specified framework for assessing the quality of studies and providing strength for each recommendation.
a. GRADE methodology The AAFP uses a modified version of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method to systematically examine research to rate the quality of the evidence, and designate the strength of a recommendation based upon that evidence. The GRADE system provides a transparent process and framework for developing evidence-based recommendations using the following system to rate the quality of evidence:
i. High Quality (Level A): Further research is very unlikely to change our confidence in the estimate of effect. ii. Moderate Quality (Level B): Further research is likely to have an important impact on our confidence in the estimate of effect, and may change the estimate. iii. Low Quality (Level C): Further research is very likely to have an important impact on our confidence in the estimate of effect, and is likely to change the estimate. iv. Very Low Quality (Level D): Any estimate of effect is very uncertain.
b. Strength of Recommendation GRADE uses the term “strength of recommendation” to rate the extent of confidence that the desirable effects of an intervention outweigh the undesirable effects. Recommendations can be either for or against an intervention or testing modality. The AAFP prefers the strength of the recommendation be consistent with the quality of the evidence such that strong recommendations are based on moderate to high quality evidence and weak recommendations are based on low to moderate quality evidence. Very low-quality evidence should be considered insufficient for a recommendation except when the benefits greatly outweigh the harms.
The strength of evidence should also reflect the degree to which there is evidence of improved patient oriented outcomes such as morbidity, mortality, quality of life, or symptoms (as opposed to only disease oriented outcomes such as blood pressure or hemoglobin A1C). Strong recommendations should be based on high quality evidence of improved patient oriented outcomes. Weak recommendations should be supported by some evidence of improvement in patient oriented outcomes; although, the evidence may be inconsistent, of lower quality, or rely on an indirect chain linking surrogate outcomes to patient oriented outcomes.
i. Strong recommendation: Based on consistent evidence of a net benefit in terms of patient oriented health outcomes, most informed patients would choose the option recommended, and clinicians can structure their interactions with patients accordingly.
ii. Weak recommendation: Evidence of a net benefit in terms of patient oriented outcomes is inconsistent or is based on lower quality evidence, or patient choices will vary based upon their values and preferences, and clinicians must help to ensure that patient care stays true to these values and preferences.
iii. Good practice points: These are recommendations that can be made when it is deemed they will be helpful to the clinician, such as recommendations to perform something that is standard of care, but where there is no direct evidence to support the recommendation and that is unlikely to ever be formally studied. These should be used sparingly in guidelines.
c. Upgrading and downgrading evidence: The GRADE system allows the evidence to be upgraded or downgraded based upon specific criteria.
i. Downgrading evidence: Evidence may be downgraded due to the following reasons: 1. Risk of bias refers to factors that make it less likely that the answer found in the study may not represent the true answer in the population. Faulty randomization, such as lack of concealment at allocation to the study group; lack of blinding to the study group when assessing outcomes; large losses to follow-up; the failure to analyze everyone in the group to which they were randomized; stopping the study early when the benefit seems too great to ignore; or failure to report all outcomes.
2. Inconsistency of findings across a number of studies must be explained. Were the interventions really the same? Were the samples very different? Inconsistencies that cannot be explained make it very difficult to assess the true effect of the treatment.
3. Directness refers to the extent to which two interventions are being compared to each other in similar populations. Indirect comparisons are more difficult to interpret. Two types of indirectness exist. a. The first includes indirect comparisons. For instance, if two drugs are being examined for an outcome, but there are no studies that directly compare the drugs, which is an indirect comparison. b. The second includes differences in population, intervention, comparator, and/or outcome.
4. Imprecision refers to a study that may show statistically significant effects, but the sample size is small and the measure of benefit is imprecise, meanin that it has a wide confidence interval.
5. Publication bias may also exist. Investigators are more likely to submit studies for publication when the results are positive and journals may be more likely to accept them for publication. An effort should be made in a systematic review to uncover studies that have not been published. This is a particularly important issue when the studies are funded by industry
ii. Evidence may be upgraded based upon the following factors: 1. Large effect size: A large effect is much less likely to be spurious than a small effect. Small effect sizes can much more easily result from chance. 2. Dose response: This exists when there is evidence that differences in dosage result in different effects/outcomes. This is one aspect of a finding that suggests an association based on cause and effect. 3. All plausible confounding: In observational trials, it is particularly difficult to measure and control for all plausible confounding. When all unmeasured plausible confounders and biases in an observational study would result in an underestimate of an apparent treatment effect, then it is more likely that a finding is real rather than the result of unmeasured confounding. For instance, if only sicker patients receive an experimental intervention or exposure, yet they still fare better, it is likely that the actual intervention or exposure effect is even larger than the data suggest.
a. Scope of the guideline (CMSS-P 5.1-5.3) The AAFP includes the intent, rationale, and scope in all guidelines. This includes, but may not be limited to, the appropriate users of the guideline, situations in which the guideline should be used, and appropriate patient populations for the guideline.
b. Methodology
Information regarding the methodology used in the evidence report and in any updated literature searches will be provided in the guideline. This information should include search terms, search dates, outcomes assessed, and key questions that were addressed. A summary of included and excluded articles should be made available for all literature searches performed by the GDG. This information can be included in the guideline as a PRISMA diagram. Records of the study quality assessment should be maintained.
c. Moving from evidence to recommendations (IOM standards 5 and 6; CMSS-P section 6 and 7)
i. As noted in section IX, the AAFP uses a modified GRADE methodology for rating the quality of the evidence, and guiding the strength of recommendations. It is worth noting that moving from examining the evidence to making a recommendation is where much of the disagreement happens in guideline development. Different groups that develop guidelines may disagree on how much weight they give to lower-level evidence; may not fully take into account benefits and harms, costs or burdens; and may give differing emphasis on patient or provider preferences and values. However, all of these factors should be considered when making recommendations. AAFP’s use of the GRADE system helps to systematically examine many of the factors mentioned to determine the quality of the evidence and strength of recommendations. ii. The AAFP strives to only make strong recommendations based on high-level evidence. However, there are few instances where strong recommendations can be made based on moderate or low-level evidence. In these instances, there must be certainty that benefits outweigh harms. iii. Recommendations made include an explanation of the reason for the recommendation; description of benefits and harms; a summary of the relevant available evidence; any explanation of values and preferences that went into the recommendation; a rating of the level of evidence and strength of recommendation; and differences in opinions of GDG panel members, if they exist, for that recommendation. iv. Recommendations made are specific and actionable and worded such that it is clear to the reader that they are (1) strong recommendations, (2) weak recommendations, or (3) good practice points. Suggested language for each type of recommendation is shown below. A table should be included in the guideline to outline the methodology and can highlight the differences between the wording used for the strength of the recommendation.
1. Strong recommendation: Use directive language in the recommendation such as “Family physicians should discuss…” or “Do not order a chest radiograph for children with suspected pneumonia unless...”. The statement should begin with “The AAFP strongly recommends…”.
2. Weak recommendation: Language should reflect the lower quality of evidence and the lower level of certainty regarding the recommendation. Suggested wording includes “Consider offering counseling regarding…” or “Patients may wish to consider…”. The statement should begin with “The AAFP recommends”.
3. Good practice points: Language should reflect the low quality or absence of evidence. “Although not studied in clinical trials, it is standard of care to perform an ECG in patients presenting with chest pain.”c.
d. Panel assignments With direction from the GDG chair, members of the GDG will be given writing assignments to complete during guideline development. When possible, GDG members will be asked for preferences regarding sections of the guideline they would like to write.
e. Making the CPG implementable
i. For implementation, the recommendations should be specific and provide clear direction. The number of recommendations should be kept to a minimum. ii. Access to the guideline should be provided through publication in a journal, the AAFP website, and the guideline clearinghouse. (CMSS 10.1-10.2) iii. When available or appropriate, actions should be taken to incorporate the recommendations at point of care through electronic health records (EHR) reminders or toolkit/checklist for physicians. (CMSS 10.3) iv. Additional implementation methods include mass media campaigns (news article, leadership blog, other avenues as suggested by the AAFP content strategy team—see dissemination section), and interactive educational meetings with quality improvement resources as appropriate (expanded learning session at Family Medicine Experience [formerly Assembly], workshops)
e. Compilation of draft(s) All drafts of the guideline should be sent (or made available) to the GDG chair and staff members at the AAFP. Most often, staff members at the AAFP will compile all sections of the draft guideline and the chair will review the draft(s) before it is sent to other members of the panel.
a. Internal Peer Review
The first round of peer review of the CPG is conducted by members of SCPG and the Science Advisory Panel. All reviewers are given four weeks to complete and return their review form to the staff members at the AAFP. (see Appendix A for an example of the review form). Upon receipt of the reviews, all comments will be recorded. Comments will be addressed when the chair determines that there is a need. A written record will be kept of the rationale for responding or not responding to all comments received.
b. External reviewers (including collaborating organizations)
Relevant stakeholders are included in the external review, including collaborating organizations, and organizations that may be affected by the guideline. AAFP members who are identified as experts in the field may also be asked to participate in the review. All reviewer comments are collected and recorded. A record of how the comment was addressed is kept. Reviewers’ names are kept confidential unless a reviewer wants to be recognized for his or her review.
The draft guideline will not routinely be made available for a period of public comment, but will be reviewed by key stakeholders including patient advocacy groups if a patient voice was unavailable for inclusion on the guideline panel.
a. Following the peer review process, the revised CPG is reviewed by members of CHPS. Upon approval, a recommendation is made to the full commission, which upon approval makes a recommendation to the AAFP Board of Directors for approval.
b. Board of Directors The AAFP Board of Directors reviews the guideline. Any questions from the Board are addressed by the GDG, and staff at the AAFP.
c. Endorsement by collaborators Collaborators on the guideline are given the chance to endorse the guideline after approval by the Board of Directors and before it is published. The collaborators will be sent the embargoed guideline, and given a month to decide upon endorsement.
a. Peer-reviewed journal Upon completion, the AAFP submits its guidelines for publication in a peer-reviewed journal, as appropriate. The guideline manuscript undergoes independent editorial review, and a decision is made about publication. Due to the nature of journals, all supporting materials (such as tables with quality ratings of studies) may not be able to be published. All supporting materials that are relevant to the guideline that are not published will be made available on the AAFP website.
b. Copyright issues Copyright issues are negotiated with the publication journal with appropriate licensing agreements made to the AAFP.
c. AAFP website (CMSS-P 9.1) After publication, the guideline is placed on the AAFP website for easy accessibility. Supporting documents that were not published with the original guideline will be available on the AAFP website as well.
a. Dissemination/marketing plan The AAFP guideline staff members work with other divisions including Communications and Marketing to disseminate the guideline upon publication. Staff members also submit the published guideline to the National Guidelines Clearinghouse for dissemination. Any derivatives made relating to the guideline will also be publicized via a marketing plan.
a. Determination by CHPS All guidelines developed by the AAFP are scheduled for a review five years after completion. However, literature pertaining to a guideline is monitored regularly, and if it is deemed necessary, a review can be initiated sooner. Whichever the case, when a guideline review is initiated, a preliminary search of the literature is completed and brought to the commission to determine if a new systematic review is necessary. If so, the topic will be nominated to AHRQ for a full systematic evidence update. If not, a decision whether to reaffirm the guideline for additional time not to exceed five years, or sunset the guideline. The commission’s recommendation is then approved by the Board of Directors.
a. External guidelines may be reviewed for endorsement by the AAFP following a request from another organization, a request from a member, or if identified as having a high applicability to family medicine.
b. The guideline will then be reviewed using set criteria (see Appendix A for review form) by members of the commission, or AAFPmembers at large with appropriate expertise and/or review experience. In certain cases, staff may review guidelines from selected organizations.
c. The member or staff reviewers will then submit comments and a recommendation to staff (see Appendix B for AAFP’s Endorsement Policy).
i. Endorse—the AAFP fully endorses the guideline ii. Affirmation of Value to Family Physicians—the guideline does not meet the requirements for full endorsement, or the AAFP is not able to endorse all the recommendations, but feel the guideline is of some benefit for family physicians iii. Not Endorse—the AAFP does not endorse the guideline and the reasons are stated. The AAFP may also choose to remain silent.
d. Reviewer comments and recommendations will be collated and reviewed by staff and the chairs of CHPS and SCRP. If substantial differences occur, the reviewers will discuss and determine if a consensus can be reached.
e. A recommendation will then be given to the SCRP for approval, and if approved, will then be taken to the commission for approval.
f. A recommendation describing the commission’s action will be submitted to the Board or board chair for approval.
g. Once approved, the organization will be notified by staff and a summary of the key recommendations, and a link to the full guideline will be posted on the AAFP website. Only guidelines with endorsement or affirmation of value will be placed on the website.
h. External guidelines designated as endorsed, or affirmation of value will be reviewed every five years following their date of publication. Guidelines may be reviewed earlier if new evidence warrants an update.
Council of Medical Specialty Societies: Code for Interaction with Companies
Council of Medical Specialty Societies: CMSS Principles for the Development of Specialty Society Clinical Guidelines
Institute of Medicine. Clinical Practice Guidelines We Can Trust . Washington, DC. National Academies Press. 2011. https://www.nap.edu/catalog/13058/clinical-practice-guidelines-we-can-trust . Accessed November 15, 2017.
IOM (Institute of Medicine). Finding What Works in Health Care: Standards for Systematic Reviews. Washington, DC. National Academies Press. 2011. http://www.nationalacademies.org/hmd/Reports/2011/Finding-What-Works-in-Health-Care-Standards-for-Systematic-Reviews/Standards.aspx . Accessed November 15, 2017.
Agency of Healthcare Research and Quality. Methods guide for effectiveness and comparative effectiveness reviews. Rockville, MD: Agency for Healthcare Research and Quality; 2014. AHRQ Publication No. 10(14)-EHC063-EF. https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/conflict-management_research.pdf . Accessed Nov. 1, 2016.
Norris SL, Holmer HK, Burda BU, Ogden LA, Fu R. Conflict of interest policies for organizations producing a large number of clinical practice guidelines. 2012; PLoS One . 7(5):e375413.
Thompson DF. Understanding financial conflicts of interest. 1993; N Engl J Med . 329(8):573-6.
For information about the AAFP Guideline Endorsement Form, please contact Melanie Bird, PhD, MSAM, Senior Manager, Clinical and Health Policies at (800) 906-6000 extension 3165, or [email protected] .
Board Approved: December 2017
A. AAFP Endorsement Form
B. AAFP Endorsement of Clinical Practice Guidelines
C. AAFP Clinical Practice Guideline Principles and AAFP Joint Development of Clinical Practice Guidelines with Other Organizations
E. Grading of Recommendations Assessment , Development and Evaluation (short GRADE) Working Group
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
Evidence-Based Medicine and Clinical Guidelines
- Evidence-Based Medicine |
- Clinical Guidelines |
Physicians have always felt that their decisions were based on evidence; thus, the current term “evidence-based medicine” is somewhat of a misnomer. However, for many clinicians, the “evidence” is often a vague combination of recollected strategies effective in previous patients, advice given by mentors and colleagues, and a general impression of “what is being done” based on random journal articles, abstracts, symposia, and advertisements. This kind of practice results in wide variations in strategies for diagnosing and managing similar conditions, even when strong evidence exists for favoring one particular strategy over another. Variations exist among different countries, different regions, different hospitals, and even within individual group practices. These variations have led to a call for a more systematic approach to identifying the most appropriate strategy for an individual patient; this approach is called evidence-based medicine (EBM). EBM is built on reviews of relevant medical literature and follows a discrete series of steps.
Evidence-Based Medicine
EBM is not the blind application of advice gleaned from recently published literature to individual patient problems. It does not imply a "one size fits all" model of care. Rather, EBM requires the use of a series of steps to gather sufficiently useful information to answer a carefully crafted question for an individual patient. Fully integrating the principles of EBM also incorporates the patient’s value system, which includes such things as costs incurred, the patient’s religious or moral beliefs, and patient autonomy. Applying the principles of EBM typically involves the following steps:
Formulating a clinical question
Gathering evidence to answer the question, evaluating the quality and validity of the evidence.
Deciding how to apply the evidence to the care of a specific patient
Questions must be specific. Specific questions are most likely to be addressed in the medical literature. A well-designed question specifies the population, intervention (diagnostic test, treatment), comparison (treatment A vs treatment B), and outcome. “What is the best way to evaluate someone with abdominal pain?” is not an overly useful question to pursue in the literature. A better, more specific question may be “Is CT or ultrasonography preferable for diagnosing acute appendicitis in a 30-year-old male with acute lower abdominal pain?”
A broad selection of relevant studies is obtained from a review of the literature. Standard resources are consulted (eg, MEDLINE or PubMed for primary references, the Cochrane Collaboration [treatment options often for specific questions], ACP Journal Club).
Not all scientific studies are of equal value. Different types of studies have different scientific strengths and legitimacy, and for any given type of study, individual examples often vary in quality of the methodology, internal validity, generalizability of results, and applicability to a specific patient (external validity).
Levels of evidence are graded 1 through 5 in decreasing order of quality. Types of studies at each level vary somewhat with the clinical question (eg, of diagnosis, treatment, or economic analysis), but typically consist of the following:
Level 1 (the highest quality): Systematic reviews or meta-analyses of randomized controlled trials and high-quality, single, randomized controlled trials
Level 2: Well-designed cohort studies
Level 3: Systematically reviewed case-control studies
Level 4: Case series and lesser-quality cohort and case-control studies
Level 5: Expert opinion not based on critical appraisal, but based on reasoning from physiology, bench research, or underlying principles
For EBM analysis, the highest level of evidence available is selected. Ideally, a significant number of large, well-conducted level 1 studies are available. However, because the number of high-quality, randomized, controlled trials is vanishingly small compared with the number of possible clinical questions, less reliable level 4 or 5 evidence is very often all that is available. Lower-quality evidence does not mean that the EBM process cannot be used, just that the strength of the conclusion is weaker.
Deciding how to apply the evidence to the care of a given patient
Because the best available evidence may have come from patient populations with different characteristics from those of the patient in question, significant judgment is required when applying results from a randomized trial to a specific patient. Additionally, patients’ wishes regarding aggressive or invasive tests and treatment must be taken into account as well as their tolerance for discomfort, risk, and uncertainty. For example, even though an EBM review may definitively show a 3-month survival advantage from an aggressive chemotherapy regimen in a certain form of cancer, patients may differ on whether they prefer to gain the extra time or avoid the extra discomfort. The cost of tests and treatments may also influence physician and patient decision making, especially when some of the alternatives are significantly costlier for the patient. Two general concerns are that patients who voluntarily participate in clinical trials are not the same as patients in general practice, and care delivered in a clinical trial environment is not identical to general care in the medical community.
Limitations of the evidence-based approach
Dozens of clinical questions are faced during the course of even one day in a busy practice. Although some of them may be the subject of an existing EBM review available for reference, most are not, and preparing a formal EBM analysis is too time-consuming to be useful in answering an immediate clinical question. Even when time is not a consideration, many clinical questions do not have any relevant studies in the literature.
Clinical Guidelines
Clinical guidelines have become widely available across the practice of medicine; many specialty societies have published such guidelines. Most well-conceived clinical guidelines are developed using a specified method that incorporates principles of EBM and consensus or Delphi process recommendations made by a panel of experts. Although clinical guidelines may describe idealized practice, clinical guidelines alone cannot establish the standard of care for an individual patient.
Some clinical guidelines follow “if, then” rules (eg, if a patient is febrile and neutropenic, then institute broad-spectrum antibiotics). More complex, multistep rules may be formalized as algorithms. Guidelines and algorithms are generally straightforward and easy to use but should be applied only to patients whose clinical characteristics (eg, demographics, comorbidities, clinical features) are similar to those of the patient group used to create the guideline. Furthermore, guidelines do not take into account the degree of uncertainty inherent in test results, the likelihood of treatment success, and the relative risks and benefits of each course of action. To incorporate uncertainty and the value of outcomes into clinical decision making, clinicians must often apply the principles of quantitative or analytical medical decision making (see also Clinical Decision-Making Strategies ). Additionally, many entities that publish guidelines require that only randomized trial data be used, which is often a significant limitation.

Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.
- Cookie Preferences

Study Design 101: Practice Guideline
- Case Report
- Case Control Study
- Cohort Study
- Randomized Controlled Trial
- Practice Guideline
- Systematic Review
- Meta-Analysis
- Helpful Formulas
- Finding Specific Study Types
A statement produced by a panel of experts that outlines current best practice to inform health care professionals and patients in making clinical decisions. The statement is produced after an extensive review of the literature and is typically created by professional associations, government agencies, and/or public or private organizations.
Good guidelines clearly define the topic; appraise and summarize the best evidence regarding prevention, diagnosis, prognosis, therapy, harm, and cost-effectiveness; and identify the decision points where this information should be integrated with clinical experience and patient wishes to determine practice. Practice guidelines should be reviewed frequently and updated as necessary for continued accuracy and relevancy.
Practice guidelines are also known as "Evidence-based guidelines" and "Clinical guidelines."
- Created by panels of experts
- Based on professional published literature
- Practical guidance for clinicians
- Considered an evidence-based resource
Disadvantages
- Slow to change or be updated
- Not always available, especially for controversial topics
- Expensive and time-consuming to produce
- Recommendations might be affected by the type of organization creating the guideline
Design pitfalls to look out for
The panel should be composed of a variety of experts with assorted affiliations.
Is the panel composed of members from a variety of professional associations, government agencies and/or institutes? Does one organization/association predominate?
Fictitious Example
A practice guideline focusing on the best way to prevent sunburn when wearing sunscreen involved forming a multidisciplinary panel of experts (dermatologists, oncologists, sunscreen chemists, etc.). These experts searched the literature and identified 123 research articles on sunscreen and sunburn prevention for appraisal. The research was then reviewed by a member of the panel with critical appraisal experience in order to identify only those high-quality research articles that permit making recommendations. Ninety-seven high-quality studies were selected. These articles were read and synthesized by the panel to create a formal guideline recommendation. Based on the literature, the guideline recommended that the best way to prevent sunburn is to wear UVA blocking sunscreen daily. However, there was insufficient evidence in the literature to make any recommendations about newer sunscreen formulations. This identified the need for further research on this topic.
Real-life Examples
Chou, R., Deyo, R., Friedly, J., Skelly, A., Hashimoto, R., Weimer, M., ... Brodt, E. (2017). Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical practice guideline . Annals Of Internal Medicine, 166 (7), 493-+. https://doi.org/10.7326/M16-2459
A group from The American College of Physicians reviewed the current evidence to determine which nonpharmacologic options are effective in treating low back pain (both acute and chronic). New treatment options appeared in the literature since 2007 (prior guideline on this topic) and several show "small to moderate, usually short-term effect on pain" including tai chi, mindfulness-based stress reduction, yoga as well as continued support for prior treatment recommendations including exercise, psychological therapies, multidisciplinary rehabilitation, spinal manipulation, massage, and acupuncture. There were greater effects on pain than on function, and the strength of evidence for several of these interventions is low.
Lennon, S., Dellavalle, D., Rodder, S., Prest, M., Sinley, R., Hoy, M., & Papoutsakis, C. (2017). 2015 Evidence Analysis Library evidence-based nutrition practice guideline for the management of hypertension in adults. Journal of the Academy of Nutrition and Dietetics, 117 (9), 1445-1458.e17. https://doi.org/10.1016/j.jand.2017.04.008
This guideline addresses the role of nutrition in managing hypertension in adults. Seventy studies were evaluated, resulting in eight recommendations to reduce blood pressure in adults with hypertension, based on moderate levels of evidence: "provision of medical nutrition therapy by an RDN [registered dietitian nutritionist], adoption of the Dietary Approaches to Stop Hypertension dietary pattern, calcium supplementation, physical activity as a component of a healthy lifestyle, reduction in dietary sodium intake, and reduction of alcohol consumption in heavy drinkers. Increased intake of dietary potassium and calcium as well as supplementation with potassium and magnesium for lowering BP are also recommended."
Related Terms
National Guideline Clearinghouse (NGC)
The National Guideline Clearinghouse was a public resource for evidence-based clinical practice guidelines maintained by the Agency for Healthcare Research and Quality (AHRQ) . It was taken offline in 2018 after federal funding ended.
Now test yourself!
1. Practice guidelines are available for almost any condition you'll encounter in your patients.
a) True b) False
2. Practice Guidelines are typically written by which of the following?
a) Public or private organizations b) Government agencies c) Professional associations d) The National Guideline Clearinghouse e) b, c and d only f) a, b, and c only
Evidence Pyramid - Navigation
- Meta- Analysis
- Case Reports
- << Previous: Randomized Controlled Trial
- Next: Systematic Review >>

- Last Updated: Sep 25, 2023 10:59 AM
- URL: https://guides.himmelfarb.gwu.edu/studydesign101

- Himmelfarb Intranet
- Privacy Notice
- Terms of Use
- GW is committed to digital accessibility. If you experience a barrier that affects your ability to access content on this page, let us know via the Accessibility Feedback Form .
- Himmelfarb Health Sciences Library
- 2300 Eye St., NW, Washington, DC 20037
- Phone: (202) 994-2850
- [email protected]
- https://himmelfarb.gwu.edu
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
American Academy of Orthopaedic Surgeons Clinical Practice Guideline Summary: Management of Anterior Cruciate Ligament Injuries
Brophy, Robert H. MD, FAAOS; Lowry, Kent Jason MD, FAAOS
From Department of Orthopedic Surgery, Washington University School of Medicine, St. Louis, MO (Brophy) and Aspirus Rhinelander Hospital, Aspirus Northland Orthopedics & Sports Medicine, Rhinelander, WI (Lowry).
Brophy or an immediate family member serves as a board member, owner, officer, or committee member of AAOS, Editorial or governing board: American Journal of Sports Medicine, Journal of the American Academy of Orthopaedic Surgeons; Vice Chair, Musculoskeletal Committee, National Football League.
Criteria: AAOS Clinical Practice Guideline Summary
This clinical practice guideline was approved by the American Academy of Orthopaedic Surgeons Board of Directors on August 22, 2022.
The complete document, Management of Anterior Cruciate Ligament Injuries : Evidence-based Clinical Practice Guideline, includes all tables and figures and is available at www.aaos.org/aclcpg .
Management of Anterior Cruciate Ligament Injuries Work Group : Robert Brophy, MD, FAAOS (Co-Chair); Kent Jason Lowry, MD, FAAOS (Co-Chair); Henry Ellis, MD, FAAOS; Neeraj Patel, MD, FAAOS; Neeraj Patel, MD, MPH, MBS; Julie Dodds, MD, FAAOS; Christopher C. Kaeding, MD; Anthony Beutler, MD; Andrew Gordon, MD, PhD; Richard Shih, MD, FACEP. Nonvoting Oversight Chair, Kevin Shea, MD, FAAOS. Staff of the American Academy of Orthopaedic Surgeons: Jayson Murray, MA; Kaitlyn Sevarino, MBA, CAE; Danielle Schulte, MS; Tyler Verity; Frank Casambre, MPH; Patrick Donnelly, MPH; Anushree Tiwari, MPH; Jennifer Rodriguez, MBA.
This is an open access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND) , where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially without permission from the journal.
Management of Anterior Cruciate Ligament Injuries : Evidence-based Clinical Practice Guideline is based on a systematic review of published studies for the treatment of anterior cruciate ligament injurie in both skeletally mature and immature patients. This guideline contains eight recommendations and seven options to assist orthopaedic surgeons and all qualified physicians managing patients with ACL injuries based on the best current available evidence. It is also intended to serve as an information resource for professional healthcare practitioners and developers of practice guidelines and recommendations. In addition to providing pragmatic practice recommendations, this guideline also highlights gaps in the literature and informs areas for future research and quality measure development.
Overview and Rationale
The American Academy of Orthopaedic Surgeons (AAOS) with input from representatives from the American Orthopaedic Society for Sports Medicine, the Pediatric Orthopaedic Society of North America, the American Academy of Physical Medicine and Rehabilitation, and the American College of Emergency Physicians recently published their clinical practice guideline (CPG), Management of Anterior Cruciate Ligament Injuries . 1 This CPG was approved by the AAOS Board of Directors in August 2022.
The anterior cruciate ligament (ACL) of the knee is commonly injured, often during sports, although it can occur during a wide variety of activities of daily living. Although this injury may be contact or noncontact, the majority result from a noncontact mechanism. 2 , 3 The rate of noncontact ACL injuries is reported to occur at a twofold to eightfold greater rate in female patients than male patients participating in similar sports and activities. 4 An estimated 200,000 patients present annually with ACL injuries in the United States alone. 5 Although the mean patient age for ACL reconstruction remained constant (29 years) from 1990 to 2006, the incidence of ACL reconstruction in patients older than 40 years increased >200%, second in growth only to the incidence of reconstructions in patients younger than 14 years. 4 , 6
These injuries can have a notable effect on knee function, particularly for activities involving cutting, pivoting, and landing. Younger and more active patients tend to be most affected by these injuries, although some patients with ACL tears can have instability with very mundane tasks. Treatment of these injuries is important to optimize joint function, sports activity, work, and activities of daily living.
Most treatments are associated with some known risks. Nonsurgical management may put patients at risk for persistent or recurrent instability and additional meniscal and/or cartilage injury. Complications of surgical treatment include recurrent instability including graft retear, postoperative loss of motion or arthrofibrosis, neurovascular injury, kneeling pain, and routine postoperative concerns, such as infection, deep vein thrombosis (DVT), and anesthesia complications. In addition, patients who have suffered an ACL tear are at increased risk of contralateral ACL tear. The choice of treatment may depend on a variety of factors which can include associated injuries and patient-specific characteristics, such as comorbidities, skeletal maturity, and especially future desired activity such as but not limited to sports participation and work needs.
Therefore, the AAOS developed this CPG to aid practitioners in the management of patients with ACL injuries. 1 Furthermore, the CPG represents a resource demonstrating areas that need additional investigation to provide improved evidence-based guidelines for the management of ACL injuries.
In summary, the ACL injuries guideline involved reviewing over 5,500 abstracts and more than 1,100 full-text articles to develop eight recommendations supported by 324 research articles meeting stringent inclusion criteria. Each recommendation is based on a systematic review of the research-related topic which resulted in five recommendations classified as high and three recommendations classified as moderate for both skeletally mature and immature patients who have been diagnosed with an ACL injury. The strength of recommendation also takes into account the quality, quantity, and the trade-offs between the benefits and harms of a treatment, the magnitude of a treatment's effect, and whether there are data on critical outcomes. Strength of recommendation is assigned based on the quality of the supporting evidence. In addition, seven options were formulated. Options are formed when there is little or no evidence on a topic. These included a consensus option on knee aspiration and limited strength options on ACL surgical reconstruction, meniscal repair, treatment for patients with a combined ACL/medial collateral ligament (MCL) tear, the use of prophylactic knee bracing treatment to prevent an ACL injury, return to sport after ACL reconstruction, and functional knee bracing treatment when returning to activity after ACL reconstruction.
Guideline Summary
The developed recommendations are meant to aid in the clinical decision-making process for the treatment of patients who have been diagnosed with an ACL injury of the knee. The use of this guideline helps in treating physicians to determine the appropriate intervention/s that are likely to provide the greatest predictable benefit. This CPG set offers a substantially updated perspective from the published 2013 iteration, which previously offered 20 statements, 5 of which were supported by strong evidence, 6 by moderate, the rest of which were limited evidence, or consensus-based. New research, of improved quality, has allowed for more decisive CPG statements. The updated 2022 CPG consisted of 15 statements, 5 of which provide strong evidence and 3 of which provide moderate evidence. Three recommendations are substantively different from the recommendations of the previous CPG, and three recommendations are new and were not part of the previous CPG.
Previously, the 2013 ACL CPG recommended that the practitioner should use either autograft or appropriately processed allograft tissue because the measured outcomes are similar based on strong evidence. This has been revised in the current CPG to recommend that surgeons should consider autograft over allograft to improve patient outcomes and decrease ACL graft failure rate, particularly in young and/or active patients, based on strong evidence. Autograft has potential benefits for graft ruptures/revision and functional scores based on 2 high, 2 moderate, and 11 low-level studies. 7 ‐ 12
In another shift, the current CPG states that surgeons may favor bone-tendon-bone (BTB) to reduce the risk of graft failure or infection or hamstring to reduce the risk of anterior or kneeling pain when using autograft to perform ACL reconstruction in skeletally mature patients, citing moderate evidence. 13 ‐ 24 This recommendation, detailing the relative advantages of these autograft choices, is a clarification of the previous CPG which recommended that the practitioner should use bone-patellar tendon-bone or hamstring tendon grafts because the measured outcomes are similar based on strong evidence.
The previous CPG recommended that when indicated, reconstruction should occur within 5 months of ACL injury to protect articular cartilage and menisci, citing moderate evidence. The current CPG recommends reconstruction as soon as possible when indicated as the risk of additional cartilage and meniscal injury starts to increase within 3 months, citing strong evidence. 25 ‐ 30 As mentioned previously, treatment is highly dependent on patient characteristics, so while this recommendation applies to younger and more active patients who should be treated as expeditiously as possible, it is less applicable to older and less active patients who may do well with nonsurgical treatment and are not necessarily indicated initially for surgical intervention.
The current CPG added a new recommendation that ACL tears indicated for surgery should be treated with ACL reconstruction rather than repair because of lower risk of revision surgery based on strong evidence. 31–33 The previous CPG did not contain any recommendations regarding repair versus reconstruction. Although ACL reconstruction is currently the standard of care for surgical treatment of primary ACL injury, there is much to be learned from ongoing and future research on innovations in biologic intervention and/or surgical technique which may optimize the results of ACL repairs.
Another new recommendation was that anterior lateral ligament (ALL) reconstruction or lateral extra-articular tenodesis (LET) could be considered when performing hamstring autograft reconstruction in select patients to reduce graft failure and improve short-term function, although long-term outcomes are yet unclear based on moderate evidence. 34 ‐ 39 The previous CPG did not make any recommendation regarding augmentation of hamstring autograft reconstruction with ALL reconstruction or LET.
Finally, the current CPG recommends that functional evaluation, such as the hop test, may be considered as one factor to determine return to sport after ACL reconstruction based on limited evidence for better functional outcomes. 40 , 41 The previous CPG did not support waiting a specific time from surgery/injury or achieving a specific functional goal before return to sports participation after ACL injury or reconstruction also based on limited evidence. More research is clearly needed in this area.
Recommendations
This Summary of Recommendations of the AAOS Management of Anterior Cruciate Ligament Injuries : Evidence-based Clinical Practice Guideline contains a list of evidence-based prognostic and treatment recommendations ( Table 1 ). Discussions of how each recommendation was developed and the complete evidence report are contained in the full guideline at www.aaos.org/aclcpg . Readers are urged to consult the full guideline for the comprehensive evaluation of the available scientific studies. The recommendations were established using methods of evidence-based medicine that rigorously control for bias, enhance transparency, and promote reproducibility. An exhaustive literature search was conducted resulting initially in more than 1,100 papers for full review. The papers were then graded for quality and aligned with the work group's patients, interventions, and outcomes of concern. For CPG PICO (ie, population, intervention, comparison, and outcome) questions that returned no evidence from the systematic literature review, the work group used the established AAOS CPG methodology to generate one companion consensus statement that physicians may consider aspirating painful, tense effusions after knee injury with likely or confirmed ACL tear.
The Summary of Recommendations is not intended to stand alone. Medical care should be based on evidence, a physician's expert judgement, and the patient's circumstances, values, preferences, and rights. A patient-centered discussion understanding an individual patient's values and preferences can inform appropriate decision-making. The recommendations regarding the treatment of ACL tears are primarily focused on younger, more active individuals. Recommendations regarding surgical treatment are principally based on literature studying ACL tears as an isolated ligamentous injury rather than a multiligamentous injury (except for isolated ACL and MCL injury). A variety of mitigating circumstances, particularly related to patient age, preinjury activity, symptoms, and desired level of postinjury activity, may also be factors in the shared decision-making process.
A Strong recommendation means that the quality of the supporting evidence is high. A Moderate recommendation means that the benefits exceed the potential harm (or that the potential harm clearly exceeds the benefits in the case of a negative recommendation), but the quality/applicability of the supporting evidence is not as strong. A Limited option means that there is a lack of compelling evidence that has resulted in an unclear balance between benefits and potential harm. A Consensus option means that expert opinion supports the guideline recommendation, although there is no available realistic evidence that meets the inclusion criteria of the guideline's systematic review.
| Strength of Recommendation | Overall Strength of Evidence | Description of Evidence quality | Strength Visual |
| Strong | Strong | Evidence from two or more “High” quality studies with consistent findings for recommending for or against the intervention. Or Rec is upgraded from Moderate using the EtD framework. | |
| Moderate | Strong, moderate, or limited | Evidence from two or more “Moderate” quality studies with consistent findings or evidence from a single “High” quality study for recommending for or against the intervention. Or Rec is upgraded or downgraded from Limited or Strong using the EtD framework. | |
| Limited | Limited or moderate | Evidence from one or more “Low” quality studies with consistent findings or evidence from a single “Moderate” quality study recommending for or against the intervention. Or Rec is downgraded from Strong or Moderate using the EtD framework. | |
| Consensus | No reliable evidence | There is no supporting evidence, or higher quality evidence was downgraded due to major concerns addressed in the EtD framework. In the absence of reliable evidence, the guideline work group is making a recommendation based on their clinical opinion. |
History and Physical
A relevant history should be obtained, and a focused musculoskeletal examination of the lower extremities should be done when assessing for an ACL injury.

Implication: Practitioners should follow a Strong recommendation unless a clear and compelling rationale for an alternative approach is present.
Surgical Timing
When surgical treatment is indicated for an acute isolated ACL tear, early reconstruction is preferred because the risk of additional cartilage and meniscal injury starts to increase within 3 months.
Single-bundle or Double-bundle Anterior Cruciate Ligament Reconstruction
In patients undergoing intra-articular ACL reconstruction single-bundle or double-bundle techniques can be considered because outcomes are similar.
Autograft Versus Allograft
When performing an ACL reconstruction, surgeons should consider autograft over allograft to improve patient outcomes and decrease ACL graft failure rate, particularly in young and/or active patients.
Autograft Source
When performing an ACL reconstruction with autograft for skeletally mature patients, surgeons may favor BTB to reduce the risk of graft failure or infection, or hamstring to reduce the risk of anterior or kneeling pain.

Implication: Practitioners should generally follow a Moderate recommendation but remain alert to new information and be sensitive to patient preferences.
Anterior Cruciate Ligament Training Programs
Training programs designed to prevent injury can be used to reduce the risk of primary ACL injuries in athletes participating in high-risk sports.
Anterolateral Ligament/Lateral Extra-articular Tenodesis
ALL reconstruction/LET could be considered when performing hamstring autograft reconstruction in select patients to reduce graft failure and improve short-term function, although long-term outcomes are yet unclear.
Repair Versus Reconstruction
ACL tears indicated for surgery should be treated with ACL reconstruction rather than repair because of lower risk of revision surgery.
Low-quality evidence, no evidence, or conflicting support evidence has resulted in the following statements for patient interventions to be listed as options for the specified condition. Future research may eventually cause these statements to be upgraded to Strong or Moderate recommendations for treatment.
Aspiration of the Knee
In the absence of reliable evidence, it is the opinion of the workgroup that physicians may consider aspirating painful, tense effusions after knee injury.

Implication: In the absence of reliable evidence, practitioners should remain alert to new information because emerging studies may change this recommendation. Practitioners should weigh this recommendation with their clinical expertise and be sensitive to patient preferences.
Anterior Cruciate Ligament Surgical Reconstruction
ACL reconstruction can be considered to lower the risk of future meniscus pathology or procedures, particularly in younger and/or more active patients. ACL reconstruction may be considered to improve long-term pain and function.

Implication: Practitioners should feel little constraint in after a recommendation labeled Limited, exercise clinical judgment, and be alert for emerging evidence that clarifies or helps to determine the balance between benefits and potential harm. Patient preference should have a substantial influencing role.
Meniscal Repair
In patients with ACL tear and meniscal tear, meniscal preservation should be considered to optimize joint health and function.
Combined Anterior Cruciate Ligament/MCL Tear
In patients with combined ACL and MCL tears, nonsurgical treatment of the MCL injury results in good patient outcomes, although surgical treatment of the MCL may be considered in select cases.
Prophylactic Knee Bracing Treatment
Prophylactic bracing treatment is not a preferred option to prevent ACL injury.
Return to Sport
Functional evaluation, such as the hop test, may be considered as one factor to determine return to sport after ACL reconstruction.
Return to Activity Functional Bracing Treatment
Functional knee braces are not recommended for routine use in patients who have received isolated primary ACL reconstruction because they confer no clinical benefit.
- Cited Here |
- Google Scholar
- + Favorites
- View in Gallery
Readers Of this Article Also Read
American academy of orthopaedic surgeons clinical practice guideline case..., american academy of orthopaedic surgeons clinical practice guideline summary on ..., comprehensive review of multidirectional instability of the shoulder, posterolateral corner of the knee: an update on current evaluation and..., diagnosis and management of partial thickness rotator cuff tears: a....
- Open access
- Published: 24 January 2022
Strategies for the implementation of clinical practice guidelines in public health: an overview of systematic reviews
- Viviane C. Pereira ORCID: orcid.org/0000-0002-9628-9974 1 ,
- Sarah N. Silva 2 ,
- Viviane K. S. Carvalho 1 ,
- Fernando Zanghelini 1 &
- Jorge O. M. Barreto 1
Health Research Policy and Systems volume 20 , Article number: 13 ( 2022 ) Cite this article
36k Accesses
60 Citations
36 Altmetric
Metrics details
As a source of readily available evidence, rigorously synthesized and interpreted by expert clinicians and methodologists, clinical guidelines are part of an evidence-based practice toolkit, which, transformed into practice recommendations, have the potential to improve both the process of care and patient outcomes. In Brazil, the process of development and updating of the clinical guidelines for the Brazilian Unified Health System (Sistema Único de Saúde, SUS) is already well systematized by the Ministry of Health. However, the implementation process of those guidelines has not yet been discussed and well structured. Therefore, the first step of this project and the primary objective of this study was to summarize the evidence on the effectiveness of strategies used to promote clinical practice guideline implementation and dissemination.
This overview used systematic review methodology to locate and evaluate published systematic reviews regarding strategies for clinical practice guideline implementation and adhered to the PRISMA guidelines for systematic review (PRISMA).
This overview identified 36 systematic reviews regarding 30 strategies targeting healthcare organizations, healthcare providers and patients to promote guideline implementation. The most reported interventions were educational materials, educational meetings, reminders, academic detailing and audit and feedback. Care pathways—single intervention, educational meeting—single intervention, organizational culture, and audit and feedback—both strategies implemented in combination with others—were strategies categorized as generally effective from the systematic reviews. In the meta-analyses, when used alone, organizational culture, educational intervention and reminders proved to be effective in promoting physicians' adherence to the guidelines. When used in conjunction with other strategies, organizational culture also proved to be effective. For patient-related outcomes, education intervention showed effective results for disease target results at a short and long term.
This overview provides a broad summary of the best evidence on guideline implementation. Even if the included literature highlights the various limitations related to the lack of standardization, the methodological quality of the studies, and especially the lack of conclusion about the superiority of one strategy over another, the summary of the results provided by this study provides information on strategies that have been most widely studied in the last few years and their effectiveness in the context in which they were applied. Therefore, this panorama can support strategy decision-making adequate for SUS and other health systems, seeking to positively impact on the appropriate use of guidelines, healthcare outcomes and the sustainability of the SUS.
Peer Review reports
Clinical guidelines are defined as “systematically developed statements to assist practitioner and patient decisions about appropriate healthcare for specific clinical circumstances” [ 1 ]. As a source of readily available evidence, rigorously synthesized and interpreted by expert clinicians and methodologists, guidelines are part of an evidence-based practice toolkit which, transformed into practice recommendations, have the potential to improve both the process of care and patient outcomes [ 2 ]. For example, greater adherence to guidelines has been associated with reduced morbidity after appendectomy for complicated appendicitis, better and faster outcomes in patients with psychiatric disorders, better physical functioning outcomes, and less use of low back pain care [ 3 , 4 , 5 ].
However, although guidelines may be seen as important tools that support decision-making, in conjunction with clinical judgement and patient preference, there is still a lack of adherence to guidelines worldwide across different conditions and levels of care [ 6 , 7 , 8 ]. Studies from different countries have demonstrated suboptimal adherence to guidelines for low back pain in primary care, including the use of interventions with little or no benefit [ 9 ]. Among Australian nutritionists who provide clinical care to cancer patients, evidence indicates that only a third of the guidelines are routinely followed [ 10 ]. In Switzerland and Norway, a study found low overall adherence to current practice guidelines and high variation in the use of nutritional therapy in patients undergoing stem cell transplantation [ 11 ]. A study carried out in Norway showed low adherence of regular general practitioners to the palliative care guideline [ 12 ]. In the management of osteoarthritis, studies suggest that the main approaches recommended in the guidelines are underutilized and that the quality of care is inconsistent [ 13 ].
Numerous factors can influence the acceptance and use of guidelines, which may occur at the micro (individual behavioural, including clinicians and consumers), meso (organizational) or macro (context and system) level [ 14 ]. Some of these factors are intrinsic to the nature of newly recommended practice or technology itself, individual characteristics of healthcare professionals, and organizational capacity of health services to collect, adapt, share and apply evidence [ 15 , 16 , 17 ]. Other factors are intrinsic to guidelines; for example, when recommendations are not at all explicit, or they are distorted or ambiguous, due to conflict of interest, variable methodological quality, or being poorly written, they may be viewed as inapplicable to patients or as reducing clinician autonomy [ 18 , 19 , 20 ].
Thus, producing and providing high-quality guidelines is no guarantee that the recommendations will be implemented in healthcare practice, and therefore an active implementation strategy is necessary to encourage their uptake [ 21 ]. An iterative process consisting of several steps is recommended, including adapting guidelines to local context, identifying barriers to their use, selecting and implementing tailored interventions to promote guideline uptake, and monitoring and evaluating the associated outcomes and the sustainability of recommendations. Regardless of how guidelines are developed, what resources are required to support their implementation, or whether it is the responsibility of other individuals or organizations to implement them, detailed instructions for guideline implementation are needed [ 22 , 23 ].
While the importance of turning knowledge into action and using available evidence to inform clinical practice is widely recognized, it still presents a challenge to most health services across different levels of government. In Brazil, the process of development and updating of the clinical guidelines for the Brazilian Unified Health System (Sistema Único de Saúde, SUS) is already well systematized by the Brazilian Ministry of Health. However, the process for implementing those guidelines has not yet been discussed and well structured. Therefore, a partnership project to elaborate a validated framework for the implementation of clinical guidelines to be used within SUS is being developed by the Ministry of Health and Oswaldo Cruz Foundation. The first step of this project is to develop a review of the scientific literature with the aim of providing an overview of the strategies used to promote guideline implementation and their effectiveness [ 24 ].
Numerous systematic reviews have synthesized data from primary studies on the effectiveness of strategies for implementing guidelines in several clinical areas including mental health [ 25 , 26 ], arthritis [ 27 ], asthma [ 28 ] and cardiovascular disease [ 29 , 30 ]. With the growth in the publication of systematic reviews, the strategy of grouping data from reviews in a single study has become a useful means for providing ample evidence to decision-makers in the healthcare field [ 31 ]. In this sense, some initiatives have been carried out to systematize review data on the subject in question. Chan et al., for example, compiled data from systematic reviews on four specific strategies (reminders, educational outreach visits, audit and feedback, and provider incentives), and the study by Cheung et al. evaluated the reminders in changing professional behaviour in clinical settings [ 32 , 33 ].
However, we did not find comprehensive studies in the global literature that synthesized this topic without restrictions to certain clinical areas and specific interventions. In this context, the primary objective of this study was to summarize the evidence on the effectiveness of different strategies used to promote clinical practice guideline implementation. This overview will provide a broad summary of the best evidence on guideline implementation to support strategy decision-making adequate for each context (national, regional, local levels) and clinical area, thus seeking to positively impact on healthcare outcomes and on the sustainability of the SUS.
This overview of systematic reviews was carried out in accordance with a protocol that was registered in the PROSPERO international prospective register of systematic reviews on 2 June 2017 (registration number: CRD42017065682). It was conducted following recommendations from the Cochrane Collaboration and reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [ 34 ].
Inclusion criteria
Studies were selected based on the following criteria.
Types of studies
Systematic reviews that evaluated different strategies to promote clinical practice guideline implementation within a health system at the organizational, operational and individual levels (clinicians and patients) were included. Studies were selected regardless of the clinical area and focus of the intervention.
An overview of systematic reviews was considered the appropriate method to address this issue, as the literature search had identified relevant, recent systematic reviews with potential to cover a larger number of initiatives of clinical guideline implementation. Therefore, only systematic reviews were included.
Systematic review has been defined as “a review of a clearly formulated question that uses systematic and explicit methods to identify, select and critically appraise relevant studies, and to extract and analyse data from the studies included in the review” [ 35 ]. Considering this definition, studies with the following characteristics were classified as systematic reviews:
a clear research question;
eligibility criteria and description of the study selection process;
description of the time period, terms and databases used in the search.
Overviews of systematic reviews were not eligible for inclusion.
Types of participants
Participants were considered in relation to the level of clinical guideline implementation in health systems: at the macro-level (international, national), meso-level (regional, healthcare organizations), and micro-level (healthcare professionals or teams).
Types of interventions
Systematic reviews addressing any strategy for clinical practice guideline implementation were eligible for inclusion in this overview.
No restrictions were applied to the comparator.
The following question guided the selection of studies:
What is the effectiveness of strategies used to promote guideline implementation?
The primary outcomes of interest were strategies for clinical practice guideline implementation in a health system (organization, provider and patient levels).
Literature search
The literature search was conducted using the following electronic databases: MEDLINE/PubMed, Centre for Reviews and Dissemination (CRD), the Cochrane Library, CINAHL (Cumulative Index to Nursing and Allied Health Literature), EMBASE, Web of Science, Scopus, Health Systems Evidence, Rx for Change (Canadian Agency for Drugs and Technologies in Health, CADTH) and Epistemonikos. The following databases were indicated in the overview protocol but they were not used: Guidelines International Network (GIN) website and International Initiative for Impact Evaluation (3ie) database, as well as Google and Google Scholar.
The basic search strategy combined search terms related to “clinical and therapeutic guidelines” (guidelines, clinical protocols, critical pathways, consensus and health planning guidelines) and “implementation of guidelines” (adherence, compliance, dissemination, accordance, concordance, adoption, barriers). The search strategies adapted for the electronic databases are presented in Additional file 1 . The searches were carried out until 19 July 2017, and then updated until August 2019. There was no restriction on country, language or date of publication. Conference abstracts and studies that were not available in full text were excluded.
The terms were searched in the title and abstract, unless otherwise indicated in Additional file 1 . The search results from the PubMed, Web of Science, Cochrane Library, Scopus, Epistemonikos, Embase and CINAHL databases were imported into Covidence reference management software for study selection, and duplicates were removed. As for the results from the other databases, an Excel spreadsheet was used for the study selection process.
Screening and selection of studies
Titles and abstracts of the retrieved studies were screened by two independent reviewers (VP and FZ; update—VP and VC). Full-text assessment of potentially eligible studies was then independently undertaken for final selection. Disagreements regarding eligibility of studies were resolved by discussion and consensus, and when necessary, by a third reviewer. The screening process and results were reported according to the PRISMA statement.
Data extraction
Results from the included studies were systematically extracted by one reviewer (VP) according to the predefined protocol, and summarized in a table of evidence using a data collection template in Excel. A second reviewer checked the extracted data.
The following information was extracted: year; authors; title; objective; country; number of studies identified; characteristics of the target population; clinical area, type of outcome evaluated, strategies for clinical practice guideline implementation and their effectiveness; conclusion, limitations of the review, evidence gaps, source of funding for the study.
Data were extracted from selected systematic reviews and meta-analyses; however, when information from reviews was insufficient, the primary studies were consulted.
Methodological quality assessment
The methodological quality assessment using the AMSTAR 2 (A Measurement Tool to Assess Systematic Reviews 2) instrument [ 36 ] was conducted by two independent reviewers (VP and FZ; update—VP and VC). Disagreements were resolved by discussion and consensus.
Data analysis
In the predefined protocol, data analysis was described only as a narrative synthesis. We subsequently refined this process even further. For systematic reviews, no meta-analysis of data was conducted. The results were reported as presented in the systematic reviews and meta-analyses. When the information was insufficient or unclear, we consulted the primary studies of each review. To do this, we recounted (i) all comparisons analysed in each study included in the review and (ii) the statistically positive results for each comparison studied. Each comparison was considered to be strategy A versus strategy B for each separate outcome (i.e. comparison of educational meeting effect associated with local opinion leader vs educational meeting only for outcome physician adherence). Based on the proportion of statistically positive results compared to the total analyses performed, efficacy was categorized as (1) generally effective (more than two thirds of the studies in a review showed positive effects), (2) mixed effects (one third to two thirds of the studies showed positive effects) or (3) generally ineffective (less than a third of the studies showed positive effects) [ 33 ]. In order to reduce bias in the interpretation of results obtained from a small number of evaluated comparisons, a cut-off was established of 10 or more comparisons evaluated to present the results of using the strategies.
Overlap analysis of studies included in each systematic review was performed to avoid duplication of effective results. In the case of duplication, we considered the results for the study included in the systematic review that presented more details regarding the strategy used to promote clinical practice guideline implementation. In cases of duplication of studies between systematic reviews selected from the first and second searches, we considered those included in systematic reviews from the first search.

Selection of studies
Figure 1 presents a flowchart of the process used to identify relevant systematic reviews that were included. In total, 9981 articles were identified, of which 189 were selected for full-text reading, and then 32 met all inclusion criteria. Four systematic reviews identified in the references of excluded overviews were also included. The excluded studies along with reasons for exclusion are shown in Additional file 2 .
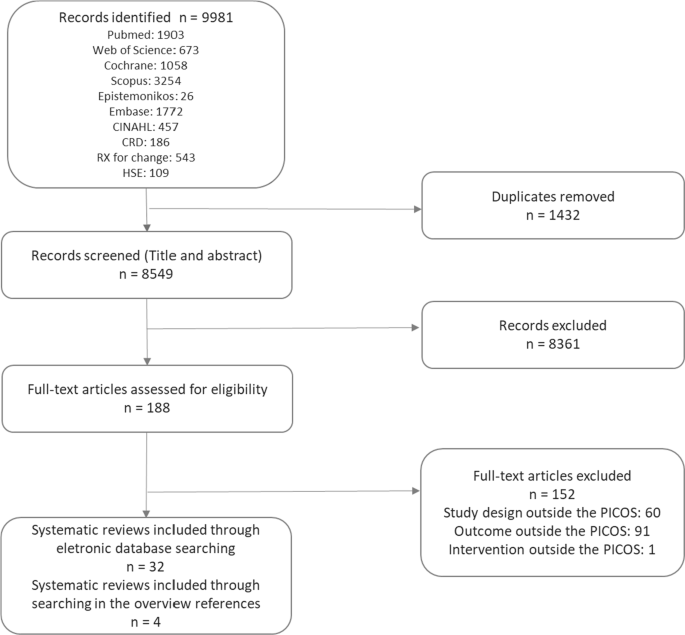
Source: own elaboration
PRISMA flowchart of study selection.
Characteristics of included studies
The systematic reviews included studies conducted in the following countries: United States (26 studies), United Kingdom (20 studies), Australia (14 studies), Netherlands (13 studies), Canada (12 studies), Germany (eight studies), France (six studies), Switzerland and Denmark (five studies each), Belgium, Thailand (four studies each), Iran, Brazil, Finland, Italy, Sweden, Norway (three studies each), Saudi Arabia, China, Singapore, New Zealand, Taiwan, Scotland, Spain, Mexico, Israel, Pakistan (two studies each), Ireland, Oceania, Argentina, Nepal, South Africa, Egypt, Oman, Japan, Korea, United Arab Emirates, Virgin Islands, South Africa, Georgia, Syria, China, Senegal, Mali, Benin, Malawi, Guatemala, India, Kenya and Zambia (one study each). There were also four studies conducted in a broader European setting (Table 1 ; Additional file 3 ).
The systematic reviews evaluated strategies for guideline implementation at various levels of health services, including inpatient and outpatient settings, primary and secondary care settings, private clinics, community health clinics, nursing homes, academic institutions, emergency services and intensive care units.
As for the clinical areas covered, four systematic reviews evaluated strategies for guideline implementation and dissemination related to physical and mental healthcare [ 25 , 26 , 37 , 38 ], two related to cardiovascular diseases [ 29 , 30 , 39 , 40 ], asthma [ 28 , 41 ] and obstetrics [ 42 , 43 ], and one related to stroke [ 44 ], physical therapy [ 45 ], pelvic inflammatory disease [ 46 ], osteoarthritis and rheumatoid arthritis [ 27 ], pneumonia [ 47 ], pressure ulcers [ 48 ], intensive care units [ 49 ], prescription practices [ 50 ] and musculoskeletal disorders [ 51 ]. Some systematic reviews evaluated guidelines related to several clinical areas [ 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 ].
The methodological quality of the included systematic reviews was assessed using the AMSTAR 2 tool [ 36 ], which consists of 16 items. According to this assessment, over the past decade, systematic reviews have provided more information on methods and parameters used in the analyses. One systematic review showed moderate, 12 low and 23 critically low methodological quality. The low rating was due to failure in meeting AMSTAR 2 criteria on the following critical domains: no justification for excluding individual studies (80%), no protocol registered before commencement of the review (75%) and no consideration of risk of bias when interpreting results from the review (47%) (Table 1 ; Additional file 3 ).
Strategies to promote clinical practice guideline implementation
The strategies reported in the systematic reviews were classified according to the Cochrane Effective Practice and Organisation of Care (EPOC) taxonomy of health interventions [ 67 ], and, when the strategy was not found in this taxonomy, we used the definition of systematic review of Grimshaw et al. [ 58 ]. Thirty strategies targeting healthcare organizations ( n = 6), community ( n = 1), health professionals ( n = 21) and patients ( n = 2) to promote guideline implementation were reported. Table 2 presents the strategies and their definitions.
Additionally, the strategies were classified according to the outcomes: process-, patient- and health professional-related outcomes, economic outcomes and nonspecific outcomes. In regard to single or multifaceted interventions, most outcomes were related to process, followed by patients and professionals. The most frequently reported strategies were educational materials, educational meetings, reminders, auditing and feedback, and academic detailing.
Effectiveness of the clinical practice guideline implementation strategies
Information on the effectiveness of clinical practice guideline implementation strategies was collected by considering the number of statistically significant positive results from each comparison analysed in the systematic reviews. The percentages of effective results in relation to the total analyses performed for each strategy were categorized as generally effective, mixed effects and generally ineffective. As described in “ Methods ”, we only present the results of strategies with 10 or more comparisons analysed (Table 3 ). The results of all strategies are presented in Additional file 4 .
Most process-related outcomes evaluated how guideline implementation strategies affected requests for examinations, prescription of medications and performance of procedures, and whether they were in accordance with the guidelines. For these outcomes, 628 and 1814 analyses of strategies implemented alone and in combination with others, respectively, were carried out (Table 3 ).
In the case of single interventions, care pathway was the only generally effective categorized strategy. Reminders, educational meetings, audit feedback, local opinion leaders and practice support were classified as strategies yielding mixed effects. In the evaluation of multifaceted interventions, none reached the percentage of results to be categorized as generally effective (Table 3 ).
Health professional-related outcomes evaluated the changes in professionals’ knowledge, attitudes, self-reported practice and self-confidence in using, satisfaction in following, and willingness to follow guidelines. A small number of analyses were performed for these outcomes, 39 for strategies implemented alone and 150 for multifaceted interventions (Table 3 ).
Educational materials and educational meetings were the most commonly reported strategies when implemented alone, the latter being classified as generally effective, and the former as having mixed effects. In the evaluation of multifaceted interventions, changes in organizational culture and the audit and feedback strategy were classified as generally effective, while educational materials and educational meetings and reminders showed mixed results for the outcomes related to health professionals (Table 3 ).
Patient-related outcomes addressed clinical information, quality of life and patient satisfaction with care received. For these outcomes, 113 and 752 analyses of strategies implemented alone and in combination with others, respectively, were carried out. When used as single or multifaceted strategies, no intervention was considered generally effective (Table 3 ).
A small number of studies evaluated the effectiveness of guideline implementation strategies related to economic outcomes (eight analyses for single interventions, and 90 analyses for multifaceted interventions), none of which proved effective.
Two meta-analyses were included in this study. In total, eight strategies were evaluated for outcomes related to processes and patients [ 29 , 30 ]. When used alone, organizational culture, educational intervention and reminders proved to be effective in promoting physicians' adherence to the guidelines [ 30 ]. In patient-directed interventions, patient education was effective, and promotion of patient self-management showed a statistically nonsignificant small benefit for this outcome [ 30 ]. Still focusing on physician adherence, when used in conjunction with other strategies (multifaceted strategies), organizational culture proved to be effective, education intervention showed mixed effects (one meta-analysis with effective results and one meta-analysis without statistical difference), and patient-directed reminders, educational meetings, academic detailing and information and communication technology presented results without statistical significance [ 29 , 30 ] (Table 4 ).
For patient-related outcomes, educational intervention showed effective results for disease targets in the short and long term, and with no difference for mortality and hospitalization. The other strategies (audit and feedback, reminders, educational meetings, information and communication technology, and academic detailing) did not show positive statistical results [ 6 ]. It should be noted that educational interventions are extremely heterogeneous strategies without standardization of the elements that they comprise, and they may range from general instructions to digital education (Table 4 ).
The objective of this study was to summarize the evidence on the effectiveness of different strategies used to promote clinical practice guideline implementation and dissemination. For this purpose, we synthesized the results of 36 systematic reviews on 30 strategies for guideline implementation. The scope of our study calls for caution in interpreting the effectiveness results, as no meta-analysis was performed, and the data were extracted from heterogeneous studies with different designs, clinical areas, contexts, intervention composition and outcomes. Thus, this data compilation can be useful as a map of the available evidence on guideline implementation strategies, on which clippings can be made according to the intended outcomes and the implementation context.
The strategies with the greatest volume of comparisons rated were educational materials, educational meetings, reminders, audit and feedback, and academic detailing. For outcomes related to processes assessed in systematic reviews, the only intervention categorized as generally effective when used alone was care pathways. Still, in the evaluation of these outcomes, the result of one of the included meta-analyses estimated that, when used alone, organizational culture, educational intervention, reminders and patient education were effective in promoting physicians' adherence to the guidelines. For multifaceted interventions, only organizational culture was effective.
Regarding the outcomes assessed in health professionals, educational meetings, used alone, and organizational culture and audit and feedback, both used in association with other strategies, were categorized as being generally effective with the data collected from systematic reviews. In evaluating the results of patients, systematic reviews did not present strategies categorized as generally effective; however, in one of the meta-analyses, educational interventions were effective for disease target results in the short and long term [ 29 ]. It should be noted that educational interventions are extremely heterogeneous strategies without standardization of the elements that they comprise, and they may range from general instructions to digital education. For economic outcomes, there was very limited evidence.
Overall, most interventions analysed had generally ineffective or mixed-effect outcomes. In the case of multifaceted strategies, it was not possible to define the contribution of each one and their specific attributes in the results, or to identify the synergistic effect of the interventions [ 68 ]. Our results were similar to those observed in the study by Grimshaw et al., in which the majority of evaluated strategies showed modest to moderate improvements in care. Grimshaw's systematic review was the most comprehensive identified, without restriction as to the type of strategy or clinical area. In that review, 235 studies were evaluated, with most having evaluated process measures as the primary outcome. The isolated interventions that were most commonly evaluated were reminders, dissemination of educational materials, and auditing and feedback. The authors concluded that there was an insufficient evidence base to point to strategies with the greatest potential to be effective in different contexts of guideline implementation [ 58 ].
In general, educational strategies have been widely addressed in the literature across a large number of studies, and regardless of whether they are the most effective strategy, they have presented important information to be targeted to specific groups [ 25 , 52 , 55 , 63 ]. The small number of comparisons between educational interventions with more complex strategies involving large-scale changes and higher cost [ 55 ] results in evidence gaps, and in a tendency to value educational approaches that require fewer resources and are easier to adopt by guideline developers or implementers with limited funding [ 69 ], possibly obtaining moderate results that are unlikely to be contradicted by other study designs.
Results for educational meetings similar to ours were reported in a recent systematic review, in which it was observed that this strategy promoted modest improvement in professional practice and, to a lesser degree, in patient outcomes. Educational meetings can improve compliance with desired practice, and the results of using this strategy can be leveraged when used in conjunction with other approaches [ 70 ]. This result is corroborated by previous studies, where multifaceted educational interventions for knowledge translation seem to be more effective in improving professional practice outcomes [ 51 ], but not necessarily in improving treatment outcomes for patients [ 71 , 72 ]. However, the heterogeneity of interventions described as educational strategies, presenting different teaching and learning methods, makes it difficult to conduct a more detailed comparison between each of the proposed interventions [ 52 ].
Reminders have also been considered low-cost and low-complexity approaches. Results in the literature have been modest but indicated that reminders can be effective in changing the behaviour of professionals [ 33 , 73 ]. The use of reminders designed for specific needs may be more likely to succeed, and reminders that prompted or required professionals’ responses were more likely to be effective in changing behaviour [ 33 ]. In our overview, we did not indicate which features of the reminder systems could promote better results [ 73 ], but a simpler format, such as manual reminders delivered on paper, can show low and moderate results in behaviour change, and can be used as a single intervention to improve quality of service [ 74 ]. Literature on the use of electronic reminders applied to health professionals, such as pharmacists, to support practice change have presented controversial results, but studies with a more robust methodology may indicate greater efficacy in the community pharmacy setting [ 55 ].
Audit and feedback may be a relevant strategy to identify the coherence between the recommendation and what is practised by the healthcare providers. In an overview of systematic reviews, this strategy was generally effective in improving both the care process and clinical outcomes, although the authors did not consider the statistical significance of the results [ 32 ]. Providing continuous feedback to professionals is an important strategy to increase professionals’ awareness of the impact of their practice and manager support for decision-making [ 26 ]. An important literature review indicated that audit and feedback may be responsible for a small, but potentially important, benefit for professional practice, varying based on the way the intervention is designed and delivered. According to the analyses, feedback may be more effective when provided by a supervisor or senior colleague, delivered at least monthly, both verbally and in written format, and when it includes explicit targets and an action plan [ 75 ].
Two interventions that were relatively rarely addressed in the included systematic reviews, but with promising results, were care pathway and organizational culture. Care pathway is an intervention that involves the standardization of care processes and its implementation is usually complex, being more frequently used for diseases and high-cost situations [ 76 ]. In the case of our results, most of them came from studies in the cardiovascular area, which could support more comprehensive activities to implement guidelines in this clinical area. Organizational culture is also a more complex and costly intervention targeted at healthcare organizations. These interventions can be implemented by promoting, for example, revisions of local procedures, protocols and tasks [ 77 ].
Behaviour change of the team is another important factor to consider in the guideline implementation process. A pioneering study using psychological theory to identify barriers to implementation of clinical guidelines and evidence-based practice identified 12 different domains of behaviour change [ 78 ]. Therefore, when the literature review reveals many studies focusing on educational strategies—that is, only on the education domain—there is a lack of more complex studies to understand professional and organizational behaviour change, which could help to determine what strategies would be more effective in different circumstances [ 57 ]. Moreover, leadership presence and incentive policies [ 40 ], or even interventions targeting the entire multidisciplinary team, seem to be more commonly accepted in the strategies for guideline implementation and dissemination [ 60 ].
Once awareness of the critical points that can compromise the implementation of a clinical guideline has been established, targeted strategies can be used to overcome barriers. A literature review reported that interventions tailored to prospectively identified barriers are more likely to improve professional practice than no intervention or guideline dissemination. However, methods to identify barriers and adapt interventions to address these barriers need further improvement, and further research is needed to assess the effectiveness of tailored interventions in comparison with other interventions [ 79 ].
Adherence of both professionals and organizations to guidelines can be improved when they are developed locally or adapted to the local context, taking into account issues such as value judgements, use of resources, characteristics of the local context and feasibility [ 26 ]. In the implementation of very specific guidelines, analysis of local context may be even more relevant, and it can make a difference in, for example, prescription of medications (involving normative and structural issues), or conduct of specific services such as intensive care units [ 39 , 49 ].
In view of the substantial heterogeneity among interventions and the wide range of areas and follow-ups to be studied, perhaps more important than a standard study is further research on a systematic analysis of context and a theoretical framework of implementation. Studies should explore the features of an intervention that are effective in a specific context and how this could be translated into another context [ 42 ]. It is worth mentioning that, in general, tailored implementation interventions should not be considered transferable between different conditions or countries [ 80 ].
A recent study described the process and results obtained with a project developed to identify barriers to the national childbirth guidelines in Brazil and strategies for implementation. After identifying and prioritizing barriers to implementation, a deliberative dialogue was undertaken to discuss options for addressing them based on an evidence synthesis. As a result, the following interventions were selected: promoting the use of multifaceted interventions, educational interventions, audit and feedback to adjust professional practice, and reminders to mediate the interaction between workers and service users; enabling patient-mediated interventions; and engaging opinion leaders to promote the use of guidelines [ 81 ]. In initiatives like this, the present study has the potential to provide an evidence map organized by intervention target, intended outcome and results achieved.
Strengths and limitations
The results presented in this overview were based on secondary data, and where necessary primary data was collected. Therefore, the first limitation is related to the lack of detailed information on the strategies and outcomes reported by the authors of the primary studies. Moreover, with regard to multifaceted interventions, some systematic reviews presented the main strategy without listing the other strategies used in combination with the main one.
Second, we used the EPOC taxonomy to classify the implementation interventions, but some systematic reviews, especially those prior to EPOC classification, had used their own categorization. In order to standardize the classification according to EPOC, we categorized some strategies based on data from the systematic reviews. In some cases, such reclassification may not entirely reflect the intervention addressed in the primary study, so this may have caused the results to appear more or less effective for each strategy.
Third, the wide scope and difficulty in gathering a large amount of information from different contexts in a comprehensible way should be taken into consideration, and the analysis of the results should consider this diversity (e.g. the level of development of the countries, types of services where strategies were implemented, clinical areas, attributes of each intervention). It should be mentioned that it was not our intention to conduct a meta-analysis of effectiveness data, but to present the strategies with a large number of analyses and a statistically significant impact on any of the outcomes evaluated.
The fourth limitation relates to the way that the results were tabulated to categorize the effectiveness of the strategies. The focus of the analysis was on positive results with statistical significance. However, many studies that assess guideline dissemination and implementation strategies are cluster-randomized controlled trials, which present unit-of-analysis errors that make it difficult to make precise estimates regarding the statistical significance of the strategies [ 82 ].
Generally, national clinical guideline developers are not responsible for implementation and may leave it to regional or local groups. However, guideline implementation may require a national approach that provides a basis for effective use at the local level. The data presented in this overview can serve as an important source of information, while more robust evidence may establish a coherent relationship between professional and organizational behaviour to better inform the choice of interventions, and to evaluate the efficiency of dissemination and implementation strategies in the presence of different barriers and facilitators.
Further research is needed to compare more complex implementation strategies, as simple strategies reported with good results in the literature can be used in early interventions. The decision-making of managers should be based on the whole context of the health service, the evidence available so far, and the best use of resources. Sometimes the implementation of a guideline can be justified in a specific field or area, but it is important to take scarce resources into consideration when prioritizing actions and strategies that may contribute to improve practices in health services.
Therefore, the identification and assessment of the main factors related to the guideline implementation process and the discussion of the strategies addressed in this overview are relevant in facilitating the direction and decision-making of guideline implementers. Even if the included literature is unanimous in highlighting the various limitations related to the lack of standardization, low methodological quality of the studies, and especially the lack of conclusions about the superiority of one strategy over another [ 26 , 54 , 58 ], the summary of the results of this overview provides information on the strategies that have been most widely studied in the last few years and their effectiveness in the context in which they were applied. The identification of barriers, facilitators, perspectives of behaviour change and context, combined with the results from the best available evidence, can be an important tool for guideline implementation.
Thus, this panorama can support strategy decision-making adequate for the SUS and other health systems, seeking to positively impact on the appropriate use of guidelines, healthcare outcomes and the sustainability of the SUS.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Browman GP, Levine MN, Mohide EA, Hayward RS, Pritchard KI, Gafni A, et al. The practice guidelines development cycle: a conceptual tool for practice guidelines development and implementation. J Clin Oncol. 1995;13(2):502–12.
CAS PubMed Google Scholar
Zimlichman E, Meilik-Weiss A. Clinical guidelines as a tool for ensuring good clinical practice. Isr Med Assoc J. 2004;6(10):626–7.
PubMed Google Scholar
Setkowski K, Boogert K, Hoogendoorn AW, Gilissen R, van Balkom AJLM. Guidelines improve patient outcomes in specialised mental health care: a systematic review and meta-analysis. Acta Psychiatr Scand [Internet]. 2021;144(3):246–58.
Google Scholar
Wakeman D, Livingston MH, Levatino E, Juviler P, Gleason C, Tesini B, et al. Reduction of surgical site infections in pediatric patients with complicated appendicitis: utilization of antibiotic stewardship principles and quality improvement methodology. J Pediatr Surg. 2021. https://doi.org/10.1016/j.jpedsurg.2021.09.031 .
Article PubMed Google Scholar
McGuirk B, King W, Govind J, Lowry J, Bogduk N. Safety, efficacy, and cost effectiveness of evidence-based guidelines for the management of acute low back pain in primary care. Spine [Internet]. 2001;26(23):2615–22.
CAS Google Scholar
van de Klundert J, Gorissen P, Zeemering S. Measuring clinical pathway adherence. J Biomed Inform. 2010;43(6):861–72.
Milchak JL, Carter BL, James PA, Ardery G. Measuring adherence to practice guidelines for the management of hypertension: an evaluation of the literature. Hypertension. 2004;44(5):602–8.
Roberts VI, Esler CN, Harper WM. What impact have NICE guidelines had on the trends of hip arthroplasty since their publication? The results from the Trent Regional Arthroplasty Study between 1990 and 2005. J Bone Jt Surg Br Vol. 2007;89(7):864–7.
Slade SC, Kent P, Patel S, Bucknall T, Buchbinder R. Barriers to primary care clinician adherence to clinical guidelines for the management of low back pain: a systematic review and metasynthesis of qualitative studies. Clin J Pain. 2016;32(9):800–16.
Edwards A, Baldwin N, Findlay M, Brown T, Bauer J. Evaluation of the agreement, adoption, and adherence to the evidence-based guidelines for the nutritional management of adult patients with head and neck cancer among Australian dietitians. Nutr Diet. 2021. https://doi.org/10.1111/1747-0080.12702 .
Baumgartner A, Bargetzi M, Bargetzi A, Zueger N, Medinger M, Passweg J, et al. Nutritional support practices in hematopoietic stem cell transplantation centers: a nationwide comparison. Nutrition (Burbank, Los Angeles County, Calif). 2017;35:43–50.
Fasting A, Hetlevik I, Mjølstad BP. Palliative care in general practice; a questionnaire study on the GPs role and guideline implementation in Norway. BMC Fam Pract. 2021. https://doi.org/10.1186/s12875-021-01426-8 .
Article PubMed PubMed Central Google Scholar
Swaithes L, Paskins Z, Dziedzic K, Finney A. Factors influencing the implementation of evidence-based guidelines for osteoarthritis in primary care: a systematic review and thematic synthesis. Musculoskelet Care. 2020;18(2):101–10.
Smith T, McNeil K, Mitchell R, Boyle B, Ries N. A study of macro-, meso- and micro-barriers and enablers affecting extended scopes of practice: the case of rural nurse practitioners in Australia. BMC Nurs. 2019. https://doi.org/10.1186/s12912-019-0337-z .
Baiardini I, Braido F, Bonini M, Compalati E, Canonica GW. Why do doctors and patients not follow guidelines? Curr Opin Allergy Clin Immunol. 2009;9:228–33.
Kilsdonk E, Peute LW, Jaspers MWM. Factors influencing implementation success of guideline-based clinical decision support systems: a systematic review and gaps analysis. Int J Med Inform. 2017;98:56–64.
Francke AL, Smit MC, de Veer AJE, Mistiaen P. Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta-review. BMC Med Inform Decis Mak. 2008;8(1):38.
PubMed PubMed Central Google Scholar
Netsch DS, Kluesner JA. Critical appraisal of clinical guidelines. J Wound Ostomy Cont Nurs. 2010;37(5):470–3.
Lenzer J. Why we can’t trust clinical guidelines. BMJ (Online). 2013. https://doi.org/10.1136/bmj.f3830 .
Article Google Scholar
Davis DA, Taylor-Vaisey A. Translating guidelines into practice. A systematic review of theoretic concepts, practical experience and research evidence in the adoption of clinical practice guidelines. CMAJ. 1997;157(4):408–16.
CAS PubMed PubMed Central Google Scholar
Straus SE, Tetroe J, Graham ID. Knowledge translation in health care: moving from evidence to practice. 2nd ed. Chichester, UK: Wiley-Blackwell/BMJ; 2009. p. 1–318.
Abbasi K. Knowledge, lost in translation. J R Soc Med. 2011;104(12):487.
Graham ID, Logan J, Harrison MB, Straus SE, Tetroe J, Caswell W, et al. Lost in knowledge translation: time for a map? J Contin Educ Health Prof. 2006;26(1):13–24.
Brasil. Fundação Oswaldo Cruz. Projeto Apoio ao aprimoramento da gestão de tecnologias no SUS: plataforma de tradução, intercâmbio e apropriação social do conhecimento / Relatório do subprojeto iGUIAS. 2020. https://brasilia.fiocruz.br/aagts/guias-de-implementacao-iguias/ .
Docherty M, Shaw K, Goulding L, Parke H, Eassom E, Ali F, et al. Evidence-based guideline implementation in low and middle income countries: lessons for mental health care. Int J Ment Health Syst. 2017;11(1):8.
Bighelli I, Ostuzzi G, Girlanda F, Cipriani A, Becker T, Koesters M, et al. Implementation of treatment guidelines for specialist mental health care. Cochrane Database Syst Rev. 2016;12(12): 009780.
Lineker SC, Husted JA. Educational interventions for implementation of arthritis clinical practice guidelines in primary care: effects on health professional behavior. J Rheumatol. 2010;37(8):1562–9.
Okelo SO, Butz AM, Sharma R, Diette GB, Pitts SI, King TM, et al. Interventions to modify health care provider adherence to asthma guidelines: a systematic review. Pediatrics. 2013;132(3):517–34.
Jeffery RA, To MJ, Hayduk-Costa G, Cameron A, Taylor C, Van Zoost C, et al. Interventions to improve adherence to cardiovascular disease guidelines: a systematic review. BMC Fam Pract. 2015;16:174.
Unverzagt S, Oemler M, Braun K, Klement A. Strategies for guideline implementation in primary care focusing on patients with cardiovascular disease: a systematic review. Fam Pract. 2014;31(3):247–66.
Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13(3):132–40.
Chan WV, Pearson TA, Bennett GC, Cushman WC, Gaziano TA, Gorman PN, et al. ACC/AHA special report: clinical practice guideline implementation strategies: a summary of systematic reviews by the NHLBI implementation science work group. J Am Coll Cardiol. 2017;69(8PG-1076–1092):1076–92.
Cheung A, Weir M, Mayhew A, Kozloff N, Brown K, Grimshaw J. Overview of systematic reviews of the effectiveness of reminders in improving healthcare professional behavior. Syst Rev. 2012. https://doi.org/10.1186/2046-4053-1-36 .
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med. 2009;6(7): e1000097.
Higgins JPT, Green S (editors). Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. Version 5. The Cochrane Collaboration, 2011. www.handbook.cochrane.org .
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Online). 2017;358: j4008. https://doi.org/10.1136/bmj.j4008 .
Weinmann S, Koesters M, Becker T. Effects of implementation of psychiatric guidelines on provider performance and patient outcome: systematic review. Acta Psychiatr Scand. 2007;115(6):420–33.
Bauer MS. A review of quantitative studies of adherence to mental health clinical practice guidelines. Harv Rev Psychiatry. 2002;10(3):138–53.
Nguyen T, Nguyen HQ, Widyakusuma NN, Nguyen TH, Pham TT, Taxis K. Enhancing prescribing of guideline-recommended medications for ischaemic heart diseases: a systematic review and meta-analysis of interventions targeted at healthcare professionals. BMJ Open. 2018;8(1): e018271.
Shanbhag D, Graham ID, Harlos K, Haynes RB, Gabizon I, Connolly SJ, et al. Effectiveness of implementation interventions in improving physician adherence to guideline recommendations in heart failure: a systematic review. BMJ Open. 2018;8(3):e017765–e017765.
Dexheimer JW, Borycki EM, Chiu K-W, Johnson KB, Aronsky D. A systematic review of the implementation and impact of asthma protocols. BMC Med Inform Decis Mak. 2014;14:82.
Imamura M, Kanguru L, Penfold S, Stokes T, Camosso-Stefinovic J, Shaw B, et al. A systematic review of implementation strategies to deliver guidelines on obstetric care practice in low- and middle-income countries. Int J Gynaecol Obstetr. 2017;136(1):19–28.
Chaillet N, Dubé E, Dugas M, Audibert F, Tourigny C, Fraser WD, et al. Evidence-based strategies for implementing guidelines in obstetrics: a systematic review. Obstet Gynecol. 2006;108(5):1234–45.
Donnellan C, Sweetman S, Shelley E. Health professionals’ adherence to stroke clinical guidelines: a review of the literature. Health Policy. 2013;111(3):245–63.
van der Wees PJ, Jamtvedt G, Rebbeck T, de Bie RA, Dekker J, Hendriks EJM. Multifaceted strategies may increase implementation of physiotherapy clinical guidelines: a systematic review. Aust J Physiother. 2008;54(4):233–41.
Liu B, Donovan B, Hocking JS, Knox J, Silver B, Guy R. Improving adherence to guidelines for the diagnosis and management of pelvic inflammatory disease: a systematic review. Infect Dis Obstetr Gynecol. 2012;2012: 325108.
Cortoos PJ, Simoens S, Peetermans W, Willems L, Laekeman G. Implementing a hospital guideline on pneumonia: a semi-quantitative review. Int J Qual Health Care. 2007;19(6):358–67.
Tooher R, Middleton P, Babidge W. Implementation of pressure ulcer guidelines: what constitutes a successful strategy? J Wound Care. 2003;12(10):373–82.
Jordan P, Mpasa F, ten Ham-Baloyi W, Bowers C. Implementation strategies for guidelines at ICUs: a systematic review. Int J Health Care Qual Assur. 2017;30(4):358–72.
Gross PA, Pujat D. Implementing practice guidelines for appropriate antimicrobial usage: a systematic review. Med Care. 2001;39(8 Suppl 2):II55-69.
Al Zoubi FM, Menon A, Mayo NE, Bussières AE. The effectiveness of interventions designed to increase the uptake of clinical practice guidelines and best practices among musculoskeletal professionals: a systematic review. BMC Health Serv Res. 2018;18(1):435.
Häggman-Laitila A, Mattila LR, Melender HL. A systematic review of the outcomes of educational interventions relevant to nurses with simultaneous strategies for guideline implementation. J Clin Nurs. 2017;26(3–4):320–40.
Diehl H, Graverholt B, Espehaug B, Lund H. Implementing guidelines in nursing homes: a systematic review. BMC Health Serv Res. 2016;16:298.
Flodgren G, Hall AM, Goulding L, Eccles MP, Grimshaw JM, Leng GC, et al. Tools developed and disseminated by guideline producers to promote the uptake of their guidelines. Cochrane Database Syst Rev. 2016;8: CD10669.
Watkins K, Wood H, Schneider CR, Clifford R. Effectiveness of implementation strategies for clinical guidelines to community pharmacy: a systematic review. Implement Sci. 2015;10:151.
Brusamento S, Legido-Quigley H, Panteli D, Turk E, Knai C, Saliba V, et al. Assessing the effectiveness of strategies to implement clinical guidelines for the management of chronic diseases at primary care level in EU Member States: a systematic review. Health Policy. 2012;107(2–3):168–83.
Hakkennes S, Dodd K. Guideline implementation in allied health professions: a systematic review of the literature. Qual Saf Health Care. 2008;17(4):296–300.
Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8(6):1–72.
Thomas LH, McColl E, Cullum N, Rousseau N, Soutter J, Steen N. Effect of clinical guidelines in nursing, midwifery, and the therapies: a systematic review of evaluations. Qual Health Care. 1998;7(4):183–91.
Medves J, Godfrey C, Turner C, Paterson M, Harrison M, MacKenzie L, Durando P. Systematic review of practice guideline dissemination and implementation strategies for healthcare teams and team-based practice. Int J Evid Based Healthc. 2010;8(2):79–89.
de Angelis G, Davies B, King J, McEwan J, Cavallo S, Loew L, et al. Information and communication technologies for the dissemination of clinical practice guidelines to health professionals: a systematic review. JMIR Med Educ. 2016;2(2): e16.
Wensing M, van der Weijden T, Grol R. Implementing guidelines and innovations in general practice: which interventions are effective? Br J Gen Pract. 1998;48(427):991–7.
Tudor Car L, Soong A, Kyaw BM, Chua KL, Low-Beer N, Majeed A. Health professions digital education on clinical practice guidelines: a systematic review by Digital Health Education collaboration. BMC Med. 2019;17(1):139.
Heselmans A, van de Velde S, Donceel P, Aertgeerts B, Ramaekers D. Effectiveness of electronic guideline-based implementation systems in ambulatory care settings—a systematic review. Implement Sci. 2009;4:82.
Liang L, Bernhardsson S, Vernooij RWM, Armstrong MJ, Bussières A, Brouwers MC, et al. Use of theory to plan or evaluate guideline implementation among physicians: a scoping review. Implement Sci. 2017. https://doi.org/10.1186/s13012-017-0557-0 .
Damiani G, Pinnarelli L, Colosimo SC, Almiento R, Sicuro L, Galasso R, et al. The effectiveness of computerized clinical guidelines in the process of care: a systematic review. BMC Health Serv Res. 2010;10:2.
Effective Practice and Organisation of Care (EPOC). The EPOC taxonomy of health systems interventions. Oslo; 2016. http://epoc.cochrane.org/epoc-specific-resources-review-authors . Accessed 14 Jan 2019.
Grimshaw JM, Shirran L, Thomas R, Mowatt G, Fraser C, Bero L, et al. Changing provider behavior: an overview of systematic reviews of interventions. Med Care. 2001;39(8 SUPPL. 2 PG-2–45):II2-45.
Forsetlund L, Bjørndal A, Rashidian A, Jamtvedt G, O’Brien MA, Wolf F, et al. Continuing education meetings and workshops: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2009. https://doi.org/10.1002/14651858.CD003030.pub2 .
Forsetlund L, O’Brien MA, Forsén L, Reinar LM, Okwen MP, Horsley T, et al. Continuing education meetings and workshops: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2009;9(9).
Cleland JA, Fritz JM, Brennan GP, Magel J. Does continuing education improve physical therapists’ effectiveness in treating neck pain? A randomized clinical trial. Phys Ther. 2009;89(1):38–47.
Chipchase LS, Cavaleri R, Jull G. Can a professional development workshop with follow-up alter practitioner behaviour and outcomes for neck pain patients? A randomised controlled trial. Man Ther. 2016;25:87–93.
Shojania KG, Jennings A, Mayhew A, Ramsay CR, Eccles MP, Grimshaw J. The effects of on-screen, point of care computer reminders on processes and outcomes of care. Cochrane Database Syst Rev. 2009;2009(3): CD001096.
PubMed Central Google Scholar
Pantoja T, Grimshaw JM, Colomer N, Castañon C, Leniz MJ. Manually-generated reminders delivered on paper: effects on professional practice and patient outcomes. Cochrane Database Syst Rev. 2019;12(12): CD001174.
Ivers N, Jamtvedt G, Flottorp S, Young J, Odgaard-Jensen J, French S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev (Online). 2012;6: CD000259.
Payedimarri AB, Ratti M, Rescinito R, Vasile A, Seys D, Dumas H, et al. Development of a model care pathway for Myasthenia Gravis. Int J Environ Res Public Health. 2021;18(21):11591.
Haggman-Laitila A, Mattila LR, Melender HL. A systematic review of the outcomes of educational interventions relevant to nurses with simultaneous strategies for guideline implementation. J Clin Nurs. 2017;26(3–4 PG-320–340):320–40.
Grimshaw J. Experimental and quasi-experimental designs for evaluating guideline implementation strategies. Fam Pract. 2000;17(Suppl 1):S11–6. https://doi.org/10.1093/fampra/17.suppl_1.s11 .
Baker R, Camosso-Stefinovic J, Gillies C, Shaw EJ, Cheater F, Flottorp S, Robertson N. Tailored interventions to overcome identified barriers to change: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2010;3: CD005470.
Krause J, Van Lieshout J, Klomp R, Huntink E, Aakhus E, Flottorp S, et al. Identifying determinants of care for tailoring implementation in chronic diseases: an evaluation of different methods. Implement Sci. 2014;9:102.
Barreto JOM, Bortoli MC, Luquine CD Jr, Oliveira CF, Toma TS, et al. Implementation of national childbirth guidelines in Brazil: barriers and strategies. Rev Panam Salud Publica. 2020;44:1.
Campbell MK, Mollison J, Steen N, Grimshaw JM, Eccles M. Analysis of cluster randomized trials in primary care: a practical approach. Fam Pract. 2000;17(2):192–6.
Download references
Acknowledgements
We would like to thank M. Sharmila A. Sousa, Mabel F. Figueiró, Kássia Fernandes, Everton N. da Silva and Marcus T. Silva.
This study was supported by the Ministry of Health of Brazil (TED-MS-FIOCRUZ #43/2016). The funder had no involvement in the study design, collection, analysis or interpretation of the data, or writing the manuscript.
Author information
Authors and affiliations.
Oswaldo Cruz Foundation, Brasília, Brazil
Viviane C. Pereira, Viviane K. S. Carvalho, Fernando Zanghelini & Jorge O. M. Barreto
Brazilian Ministry of Health, Brasília, Brazil
Sarah N. Silva
You can also search for this author in PubMed Google Scholar
Contributions
VP and JB designed the study. VP, VC and FZ collected the data. VP analysed the data and prepared the first draft of the manuscript. SN and JB reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Viviane C. Pereira .
Ethics declarations
Ethics approval and consent to participate.
There was no need for the ethical approval as the study relied on documents available in the public domain.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
Literature search.
Additional file 2.
Excluded studies.
Additional file 3.
Characteristics and AMSTAR2 of the systematic reviews.
Additional file 4.
Effectiveness of guideline implementation strategies from systematic reviews by type of outcome.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Pereira, V.C., Silva, S.N., Carvalho, V.K.S. et al. Strategies for the implementation of clinical practice guidelines in public health: an overview of systematic reviews. Health Res Policy Sys 20 , 13 (2022). https://doi.org/10.1186/s12961-022-00815-4
Download citation
Received : 01 November 2020
Accepted : 10 January 2022
Published : 24 January 2022
DOI : https://doi.org/10.1186/s12961-022-00815-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Guideline implementation
- Health system
Health Research Policy and Systems
ISSN: 1478-4505
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Here’s how you know
- U.S. Department of Health and Human Services
- National Institutes of Health
Clinical Practice Guidelines
“Clinical practice guidelines are systematically developed statements to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances.” (Institute of Medicine, 1990)
Issued by third-party organizations, and not NCCIH, these guidelines define the role of specific diagnostic and treatment modalities in the diagnosis and management of patients. The statements contain recommendations that are based on evidence from a rigorous systematic review and synthesis of the published medical literature.
These guidelines are not fixed protocols that must be followed, but are intended for health care professionals and providers to consider. While they identify and describe generally recommended courses of intervention, they are not presented as a substitute for the advice of a physician or other knowledgeable health care professional or provider.
Featured Guideline
- National Guideline Clearinghouse (AHRQ)
- Institute of Medicine's Report Clinical Practice Guidelines We Can Trust (IOM)
- Travelers’ Health: Clinician Resources (CDC)
Allergy and Immunology
- Guidelines for the Diagnosis and Management of Asthma (NHLBI)
- Diagnosis and Management of Food Allergy (Journal of Allergy and Clinical Immunology) [ 165KB PDF]
- Allergic Rhinitis and Its Impact on Asthma (ARIA) Guidelines: 2010 Revision (Journal of Allergy and Clinical Immunology)
- Clinical Practice Guideline: Allergic Rhinitis (American Academy of Otolaryngology—Head and Neck Surgery) [ 1KB PDF]
- Vitamin, Mineral, and Multivitamin Supplements for the Primary Prevention of Cardiovascular Disease and Cancer (USPSTF)
- Soy Protein, Isoflavones, and Cardiovascular Health (Circulation)
- Management of Stable Ischemic Heart Disease (Annals of Internal Medicine)
Family Medicine
- Vitamin D and Calcium Supplementation to Prevent Fractures in Adults (U.S. Preventive Services Task Force)
- Treating Tobacco Use and Dependence: 2008 Update (AHRQ)
- Recommendations on Immunization (CDC)
- Practice Parameters for the Psychological and Behavioral Treatment of Insomnia: An Update (Sleep) [ 279 KB PDF]
- Practice Parameters for the Nonpharmacologic Treatment of Chronic Insomnia (Sleep)
- NSAIDs and Other Complementary Treatments for Episodic Migraine Prevention in Adults (Neurology) [ 393KB PDF]
- Guidance for the Prevention and Control of Influenza in the Peri- and Postpartum Settings (CDC)
- Guidance for Clinicians on the Use of Rapid Influenza Diagnostic Tests (CDC)
- Evaluation and Management of Chronic Insomnia in Adults (Journal of Clinical Sleep Medicine) [ 863 KB PDF]
- American Cancer Society Guidelines on Nutrition and Physical Activity for Cancer Prevention (CA: A Cancer Journal for Clinicians) [ 450 KB PDF]
- 2011–2012 Influenza Antiviral Medications: Summary for Clinicians (CDC)
Gastroenterology
- ACG Clinical Guideline: Management of Irritable Bowel Syndrome (American Journal of Gastroenterology)
- Probiotics and Children (Journal of Pediatric Gastroenterology and Nutrition) [ 119KB PDF]
- Evidence-Based Recommendations on Management of Irritable Bowel Syndrome (American Family Physician)
Men's Health
- Management of Benign Prostatic Hyperplasia (BPH) (American Urological Association)
- Practice Parameter: Neuroprotective Strategies and Alternative Therapies for Parkinson Disease (An Evidence-Based Review) (Neurology)
- Complementary and Alternative Medicine in Multiple Sclerosis (American Academy of Neurology)
- Clinical Practice Guidelines on the Evidence-Based Use of Integrative Therapies During and After Breast Cancer Treatment (CA: A Cancer Journal for Clinicians)
- Clinical Practice Guidelines on the Use of Integrative Therapies as Supportive Care in Patients Treated for Breast Cancer (Journal of the National Cancer Institute Monographs)
- Exercise Guidelines for Cancer Survivors (Medicine & Science in Sports & Exercise)
Orthopedics
- Use of Supplemental Vitamin D and Calcium in Patients Following Hip Fracture Surgery (AAOS)
- Adjuvant Treatment of Distal Radius Fractures With Vitamin C (AAOS)
Pain Management
- Treatment of Osteoarthritis of the Knee (American Academy of Orthopaedic Surgeons)
- Subluxation Chiropractic Practice (Council on Chiropractic Practice)
- Practice Parameter: Evidence-based Guidelines for Migraine Headache (Neurology)
- Osteopathic Manipulative Treatment for Low-Back Pain (Journal of the American Osteopathic Association)
- Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline (Annals of Internal Medicine)
- Management of the Acute Migraine Headache (American Family Physician)
- Low Back Pain: Clinical Practice Guidelines Linked to the International Classification of Functioning, Disability, and Health (Journal of Orthopaedic and Sports Physical Therapy)
- Diagnosis and Treatment of Low Back Pain (Annals of Internal Medicine)
- Practice Guidelines for Chronic Pain Management (Anesthesiology) [ 699 KB PDF]
- Chiropractic Management of Fibromyalgia Syndrome: Summary of Clinical Practice Recommendations (Council on Chiropractic Guidelines and Practice Parameters) [ 21 KB PDF]
- Management of Children with Autism Spectrum Disorders (Pediatrics)
- Pediatric Integrative Medicine (American Academy of Pediatrics)
- Migraine Headaches in Children and Adolescents (Journal of Pediatric Health Care)
- A Practice Pathway for Identification, Evaluation, and Management of Insomnia in Children and Adolescents with Autism Spectrum Disorders (Pediatrics)
Psychiatry and Mental Health
- Nonpharmacologic Versus Pharmacologic Treatment of Adults With Major Depressive Disorder (American College of Physicians)
- Clinical Guideline for the Evaluation and Management of Chronic Insomnia in Adults (PubMed)
Rheumatology
- Guidelines for the Non-Surgical Management of Knee Osteoarthritis (OA Research Society International) [ 5.2 MB PDF]
- Nonpharmacologic and Pharmacologic Therapies for Osteoarthritis of the Hand, Hip, and Knee (American College of Rheumatology/Arthritis Foundation)
Women's Health
- Treatment of Urinary Tract Infections in Nonpregnant Women (ACOG)
- Use of Botanicals for Management of Menopausal Symptoms (Obstetrics and Gynecology)
- AACE Medical Guidelines for Clinical Practice for Diagnosis and Treatment of Menopause © 2011 (Endocrine Practice)
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
A Practical Overview of Case-Control Studies in Clinical Practice
Affiliations.
- 1 Department of Quantitative Health Sciences, Lerner Research Institute, Cleveland Clinic, Cleveland, OH; Center for Surgery and Public Health, Brigham and Women's Hospital, Harvard Medical School, Boston, MA. Electronic address: [email protected].
- 2 Department of Quantitative Health Sciences, Lerner Research Institute, Cleveland Clinic, Cleveland, OH; Department of Population and Quantitative Health Sciences, Case Western Reserve University, School of Medicine, Cleveland, OH.
- 3 Department of Statistics, University of Missouri, Columbia, MO.
- PMID: 32658653
- DOI: 10.1016/j.chest.2020.03.009
Case-control studies are one of the major observational study designs for performing clinical research. The advantages of these study designs over other study designs are that they are relatively quick to perform, economical, and easy to design and implement. Case-control studies are particularly appropriate for studying disease outbreaks, rare diseases, or outcomes of interest. This article describes several types of case-control designs, with simple graphical displays to help understand their differences. Study design considerations are reviewed, including sample size, power, and measures associated with risk factors for clinical outcomes. Finally, we discuss the advantages and disadvantages of case-control studies and provide a checklist for authors and a framework of considerations to guide reviewers' comments.
Keywords: OR; case-cohort; case-crossover; matching; nested case-control; relative risk.
Copyright © 2020 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
PubMed Disclaimer
Similar articles
- Study Types in Orthopaedics Research: Is My Study Design Appropriate for the Research Question? Zaniletti I, Devick KL, Larson DR, Lewallen DG, Berry DJ, Maradit Kremers H. Zaniletti I, et al. J Arthroplasty. 2022 Oct;37(10):1939-1944. doi: 10.1016/j.arth.2022.05.028. Epub 2022 Sep 6. J Arthroplasty. 2022. PMID: 36162926 Free PMC article.
- Database Research for Pediatric Infectious Diseases. Kronman MP, Gerber JS, Newland JG, Hersh AL. Kronman MP, et al. J Pediatric Infect Dis Soc. 2015 Jun;4(2):143-50. doi: 10.1093/jpids/piv007. Epub 2015 Mar 5. J Pediatric Infect Dis Soc. 2015. PMID: 26407414 Review.
- Observational and interventional study design types; an overview. Thiese MS. Thiese MS. Biochem Med (Zagreb). 2014;24(2):199-210. doi: 10.11613/BM.2014.022. Epub 2014 Jun 15. Biochem Med (Zagreb). 2014. PMID: 24969913 Free PMC article. Review.
- [Hybrid designs in studies]. Mandiracioğlu A. Mandiracioğlu A. Turkiye Parazitol Derg. 2009;33(3):227-31. Turkiye Parazitol Derg. 2009. PMID: 19851970 Turkish.
- Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, Woolacoot N, Glanville J. Philips Z, et al. Health Technol Assess. 2004 Sep;8(36):iii-iv, ix-xi, 1-158. doi: 10.3310/hta8360. Health Technol Assess. 2004. PMID: 15361314 Review.
- Factors Associated with Obstetric Anal Sphincter Injury During Vacuum-Assisted Vaginal Delivery. Chill HH, Dick A, Zarka W, Vilk Ayalon N, Rosenbloom JI, Shveiky D, Karavani G. Chill HH, et al. Int Urogynecol J. 2024 May 4. doi: 10.1007/s00192-024-05785-5. Online ahead of print. Int Urogynecol J. 2024. PMID: 38703223
- Association between statin use and the risk for idiopathic pulmonary fibrosis and its prognosis: a nationwide, population-based study. Park J, Lee CH, Han K, Choi SM. Park J, et al. Sci Rep. 2024 Apr 2;14(1):7805. doi: 10.1038/s41598-024-58417-9. Sci Rep. 2024. PMID: 38565856 Free PMC article.
- Letter to the Editor: Cross-Sectional Study for Investigation of the Association Between Modifiable Risk Factors and Gastrointestinal Cancers at a Tertiary Hospital in Ghana. Kumar A, Mansoor J, Sadiq H. Kumar A, et al. Cancer Control. 2024 Jan-Dec;31:10732748241233957. doi: 10.1177/10732748241233957. Cancer Control. 2024. PMID: 38365572 Free PMC article. No abstract available.
- Tasks and Experiences of the Prospective, Longitudinal, Multicenter MoMar (Molecular Markers) Study for the Early Detection of Mesothelioma in Individuals Formerly Exposed to Asbestos Using Liquid Biopsies. Weber DG, Casjens S, Wichert K, Lehnert M, Taeger D, Rihs HP, Brüning T, Johnen G, The MoMar Study Group. Weber DG, et al. Cancers (Basel). 2023 Dec 18;15(24):5896. doi: 10.3390/cancers15245896. Cancers (Basel). 2023. PMID: 38136442 Free PMC article.
- How to use the Surveillance, Epidemiology, and End Results (SEER) data: research design and methodology. Che WQ, Li YJ, Tsang CK, Wang YJ, Chen Z, Wang XY, Xu AD, Lyu J. Che WQ, et al. Mil Med Res. 2023 Oct 31;10(1):50. doi: 10.1186/s40779-023-00488-2. Mil Med Res. 2023. PMID: 37899480 Free PMC article. Review.
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- Elsevier Science
- Ovid Technologies, Inc.
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

Evidence-based case study guidelines
The clinical case study may be defined as a detailed analysis of the therapy conducted with a couple or family that will be instructive, may be exemplary or cautionary, and stresses factors contributing to either success or failure of the treatment. Because evidence-based clinical case studies can be difficult to do well, the following guidelines are provided:
1. The case study report should include several outcome measures assessing functioning across multiple domains, as well as relevant process measures evaluated at multiple times across treatment.
At minimum the case study report should include the following:
- a standardized measure of family/couple functioning from the perspective of the couple/family;
- a standardized outcome measure of global functioning;
- a standardized outcome measure of the target symptom (i.e. depression, marital conflict, parenting stress, child behavior); and
- one process measure (i.e. therapeutic alliance) evaluated on at least three separate occasions during treatment.
2. Specific outcome data should be presented with consideration of clinically significant change methodology (Jacobson et al., 1999).
Three questions (further explained below) should be addressed:
- At the end of treatment did the couple/family end up in a range that renders them indistinguishable from non-clinical or well-functioning couples/families?
- Was the magnitude of the change sufficient to be meaningful, i.e. statistically reliable, as determined by the Reliability Change Index (RCI)?
- What clinical outcome status may be concluded from the data?
Patients may be considered to have moved to the well-functioning range if their pre-treatment scores fell within the clinical range but have shifted post-treatment to the nonclinical range as defined by the measure employed.
The RCI is equal to the individual's score before intervention minus their score after intervention then divided by the standard error of the difference of the test(s) being used. If the RCI is greater than 1.96, then the difference in the scores would be considered to be significant. Calculation of the RCI can be done by hand; however, programs to assist are readily available. See Reliable change criterion calculator , for example.
Outcome status may be considered Recovered , Improved but not Recovered , or Deteriorated at the end of treatment.
- Recovered classification is appropriate when at the end of treatment clients' score within a range that is considered nonclinical, and the magnitude of change (RCI) is reliable.
- Improved but not Recovered classification is appropriate when the client shows statistically significant change (RCI) but ends therapy still somewhat dysfunctional as measured on post-test scores.
- The Deteriorated classification is appropriate when post-treatment scores drop within or into the clinical range and the deterioration is of reliable magnitude. Score changes that are not of reliable magnitude should not be deemed clinically significant change.
3. Clinical significance methodology is often discussed in the literature in terms of individual pre-post treatment scores consistent with individual treatment. Couple and family assessment and therapy present the challenge of measuring clinically significant change in a dyad or a group of family members. Case study authors may average scores to present couple or family-wide clinically significant change or may present individual family member change scores.
It is appreciated that in a multi-member system couple or family-wide change can be difficult to achieve. Clinically significant change in the target symptom and some, but not all, individuals should not be viewed to invalidate the instructional value of the case study. In this situation, however, the author may wish to categorize the outcome of the couple/family as Improved but not Recovered.
4. Submission of both successful and unsuccessful treatment cases is encouraged. In addition, it might be quite instructive to compare and contrast the technical interventions that occurred during a positive change case with that of a clinically unchanged or deteriorated case using the same approach to treatment.
5. Verbatim clinical dialogue between the couple/family and therapist highlighting key interventions and mechanisms of change and that highlight the specific approach to treatment should be provided. Discussion of therapeutic interventions should not be presented from a global or abstract perspective.
6. Appropriate informed consent must be obtained from clients prior to case study submission.
For further reading on clinical significance methodology:
- Jacobson, N., Roberts, L., Berns, S., & McGlinchey, J. (1999). Methods for defining and determining the clinical significance of treatment effects: Description, application, and alternatives. Journal of Consulting and Clinical Psychology , 67, 300–307.
- Jacobson, N.S. & Truax (1991). Clinical Significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology , 59 (1), 12–19.
These guidelines are adapted from Psychotherapy Evidence-Based Case Study .
More about this journal
- Couple and Family Psychology: Research and Practice
- Pricing and subscription info
- Read sample articles
- Guidelines for reviewing manuscripts
- Treating the system: Addressing substance use disorders at the couple and family level
Contact Journals
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Heart Views
- v.18(3); Jul-Sep 2017
Guidelines To Writing A Clinical Case Report
What is a clinical case report.
A case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports usually describe an unusual or novel occurrence and as such, remain one of the cornerstones of medical progress and provide many new ideas in medicine. Some reports contain an extensive review of the relevant literature on the topic. The case report is a rapid short communication between busy clinicians who may not have time or resources to conduct large scale research.
WHAT ARE THE REASONS FOR PUBLISHING A CASE REPORT?
The most common reasons for publishing a case are the following: 1) an unexpected association between diseases or symptoms; 2) an unexpected event in the course observing or treating a patient; 3) findings that shed new light on the possible pathogenesis of a disease or an adverse effect; 4) unique or rare features of a disease; 5) unique therapeutic approaches; variation of anatomical structures.
Most journals publish case reports that deal with one or more of the following:
- Unusual observations
- Adverse response to therapies
- Unusual combination of conditions leading to confusion
- Illustration of a new theory
- Question regarding a current theory
- Personal impact.
STRUCTURE OF A CASE REPORT[ 1 , 2 ]
Different journals have slightly different formats for case reports. It is always a good idea to read some of the target jiurnals case reports to get a general idea of the sequence and format.
In general, all case reports include the following components: an abstract, an introduction, a case, and a discussion. Some journals might require literature review.
The abstract should summarize the case, the problem it addresses, and the message it conveys. Abstracts of case studies are usually very short, preferably not more than 150 words.
Introduction
The introduction gives a brief overview of the problem that the case addresses, citing relevant literature where necessary. The introduction generally ends with a single sentence describing the patient and the basic condition that he or she is suffering from.
This section provides the details of the case in the following order:
- Patient description
- Case history
- Physical examination results
- Results of pathological tests and other investigations
- Treatment plan
- Expected outcome of the treatment plan
- Actual outcome.
The author should ensure that all the relevant details are included and unnecessary ones excluded.
This is the most important part of the case report; the part that will convince the journal that the case is publication worthy. This section should start by expanding on what has been said in the introduction, focusing on why the case is noteworthy and the problem that it addresses.
This is followed by a summary of the existing literature on the topic. (If the journal specifies a separate section on literature review, it should be added before the Discussion). This part describes the existing theories and research findings on the key issue in the patient's condition. The review should narrow down to the source of confusion or the main challenge in the case.
Finally, the case report should be connected to the existing literature, mentioning the message that the case conveys. The author should explain whether this corroborates with or detracts from current beliefs about the problem and how this evidence can add value to future clinical practice.
A case report ends with a conclusion or with summary points, depending on the journal's specified format. This section should briefly give readers the key points covered in the case report. Here, the author can give suggestions and recommendations to clinicians, teachers, or researchers. Some journals do not want a separate section for the conclusion: it can then be the concluding paragraph of the Discussion section.
Notes on patient consent
Informed consent in an ethical requirement for most studies involving humans, so before you start writing your case report, take a written consent from the patient as all journals require that you provide it at the time of manuscript submission. In case the patient is a minor, parental consent is required. For adults who are unable to consent to investigation or treatment, consent of closest family members is required.
Patient anonymity is also an important requirement. Remember not to disclose any information that might reveal the identity of the patient. You need to be particularly careful with pictures, and ensure that pictures of the affected area do not reveal the identity of the patient.

IMAGES
VIDEO
COMMENTS
Clinical Practice Guidelines. Clinical practice guidelines (CPGs) are developed through a rigorous systematic methodology synthesizing the ever-increasing amounts of published literature into a practical and digestible set of clinical recommendations to be used in a healthcare setting. 1,2 The Grading of Recommendations Assessment, Development and Evaluation (GRADE) collaboration has ...
In the case of duplication, we considered the results for the study included in the systematic review that presented more details regarding the strategy used to promote clinical practice guideline implementation. ... change among health professionals with regard to information and communication technologies for the dissemination of clinical ...
Trustworthy clinical practice guidelines should be based on a systematic review of the literature, provide ratings of the quality of evidence and the strength of recommendations, consider patient values, and be developed by a multidisciplinary panel of experts. The quality of evidence reflects our certainty that the evidence warrants a particular action. Transforming evidence into a decision ...
OVERVIEW. The Institute of Medicine (IOM) defines clinical practice guidelines as "statements that include recommendations, intended to optimize patient care, that are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options" [ 1 ]. Based on this definition, guidelines have two parts:
423. ARTICLE HIGHLIGHTS. d Trustworthy clinical practice guidelines require a systematic review to select the best available evidence and should explicitly evaluate the quality of evidence. d Factors that reduce the quality of evidence are risk of bias, indirectness, inconsistency, imprecision, and likelihood of pub-lication and reporting bias.
a. Definition: Clinical practice guidelines are statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment ...
Pilot testing the new protocol - a case study. We developed key clinical questions using the protocol over a period of 6 months (Additional File 2).First (Step 1) a scoping search of the literature identified broad challenges with assessment, diagnosis, management and follow-up related to work-related mental health conditions, with an emphasis on acute stress disorder, post-traumatic stress ...
The ACC/AHA Task Force on Clinical Practice Guidelines (Task Force) continuously reviews, updates, and modifies guideline methodology on the basis of published standards from organizations, including the Institute of Medicine, P-3,P-4 and on the basis of internal reevaluation. Similarly, the presentation and delivery of guidelines are reevaluated and modified on the basis of evolving ...
The new definition is as follows: Clinical practice guidelines are statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options. To be trustworthy, guidelines should.
One study, for example, notes a 141% increase in rotator cuff repairs from 1996 to 2006 in the United States. Because rotator cuff disease is such a common condition with evidence of various strength supporting different common treatments, the American Academy of Orthopaedic Surgeons published a clinical practice guideline to help elucidate ...
Level 3: Systematically reviewed case-control studies. Level 4: Case series and lesser-quality cohort and case-control studies . Level 5: Expert opinion not based on critical appraisal, but based on reasoning from physiology, bench research, or underlying principles ... Although clinical guidelines may describe idealized practice, clinical ...
The case presented demonstrates how these guidelines can be effective in clinical decision-making for the treatment of symptomatic glenohumeral joint osteoarthritis. History and Physical Examination A 67-year-old right-hand-dominant man presented to the outpatient orthopaedic specialty clinic with greater than 7-year history of left greater ...
A case report is a narrative that describes, for medical, scientific, or educational purposes, a medical problem experienced by one or more patients. Case reports written without guidance from reporting standards are insufficiently rigorous to guide clinical practice or to inform clinical study design. Primary Objective. Develop, disseminate, and implement systematic reporting guidelines for ...
Good guidelines clearly define the topic; appraise and summarize the best evidence regarding prevention, diagnosis, prognosis, therapy, harm, and cost-effectiveness; and identify the decision points where this information should be integrated with clinical experience and patient wishes to determine practice. Practice guidelines should be ...
This chapter has provided an overview of some factors that affect the implementation of clinical practice guidelines. Hypothetical case studies, based on situations encountered in study site visits, literature reviews, and other sources, illustrate the many ways guidelines may be employed and some real-world factors that may encourage or hamper ...
Management of Anterior Cruciate Ligament Injuries: Evidence-based Clinical Practice Guideline is based on a systematic review of published studies for the treatment of anterior cruciate ligament injurie in both skeletally mature and immature patients. This guideline contains eight recommendations and seven options to assist orthopaedic surgeons and all qualified physicians managing patients ...
As a source of readily available evidence, rigorously synthesized and interpreted by expert clinicians and methodologists, clinical guidelines are part of an evidence-based practice toolkit, which, transformed into practice recommendations, have the potential to improve both the process of care and patient outcomes. In Brazil, the process of development and updating of the clinical guidelines ...
"Clinical practice guidelines are systematically developed statements to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances."(Institute of Medicine, 1990) Issued by third-party organizations, and not NCCIH, these guidelines define the role of specific diagnostic and treatment modalities in the diagnosis and management of patients.
problem experienced by one or more patients. Case reports written without guidance from reporting standards are insufficiently rigorous to guide clinical practice or to inform clinical study design. Primary Objective. Develop, disseminate, and implement systematic reporting guidelines for case reports.
Clinical practice guidelines (or simply "clinical guidelines") are recommendations on how to diagnose and treat a medical condition. They are mainly written for doctors, but also for nurses and other health care professionals. Clinical guidelines are meant to help ensure that patients receive appropriate treatment and care. For instance, the guidelines on cervical cancer include ...
Case-control studies are one of the major observational study designs for performing clinical research. The advantages of these study designs over other study designs are that they are relatively quick to perform, economical, and easy to design and implement. Case-control studies are particularly appropriate for studying disease outbreaks, rare ...
Evidence-based case study guidelines. The clinical case study may be defined as a detailed analysis of the therapy conducted with a couple or family that will be instructive, may be exemplary or cautionary, and stresses factors contributing to either success or failure of the treatment. Because evidence-based clinical case studies can be ...
A case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports usually describe an unusual or novel occurrence and as such, remain one of the cornerstones of medical progress and provide many new ideas in medicine. Some reports contain an extensive review of the relevant ...