- Search Menu
- Browse content in Arts and Humanities
- Browse content in Archaeology
- Anglo-Saxon and Medieval Archaeology
- Archaeological Methodology and Techniques
- Archaeology by Region
- Archaeology of Religion
- Archaeology of Trade and Exchange
- Biblical Archaeology
- Contemporary and Public Archaeology
- Environmental Archaeology
- Historical Archaeology
- History and Theory of Archaeology
- Industrial Archaeology
- Landscape Archaeology
- Mortuary Archaeology
- Prehistoric Archaeology
- Underwater Archaeology
- Urban Archaeology
- Zooarchaeology
- Browse content in Architecture
- Architectural Structure and Design
- History of Architecture
- Residential and Domestic Buildings
- Theory of Architecture
- Browse content in Art
- Art Subjects and Themes
- History of Art
- Industrial and Commercial Art
- Theory of Art
- Biographical Studies
- Byzantine Studies
- Browse content in Classical Studies
- Classical Literature
- Classical Reception
- Classical History
- Classical Philosophy
- Classical Mythology
- Classical Art and Architecture
- Classical Oratory and Rhetoric
- Greek and Roman Papyrology
- Greek and Roman Archaeology
- Greek and Roman Epigraphy
- Greek and Roman Law
- Late Antiquity
- Religion in the Ancient World
- Digital Humanities
- Browse content in History
- Colonialism and Imperialism
- Diplomatic History
- Environmental History
- Genealogy, Heraldry, Names, and Honours
- Genocide and Ethnic Cleansing
- Historical Geography
- History by Period
- History of Emotions
- History of Agriculture
- History of Education
- History of Gender and Sexuality
- Industrial History
- Intellectual History
- International History
- Labour History
- Legal and Constitutional History
- Local and Family History
- Maritime History
- Military History
- National Liberation and Post-Colonialism
- Oral History
- Political History
- Public History
- Regional and National History
- Revolutions and Rebellions
- Slavery and Abolition of Slavery
- Social and Cultural History
- Theory, Methods, and Historiography
- Urban History
- World History
- Browse content in Language Teaching and Learning
- Language Learning (Specific Skills)
- Language Teaching Theory and Methods
- Browse content in Linguistics
- Applied Linguistics
- Cognitive Linguistics
- Computational Linguistics
- Forensic Linguistics
- Grammar, Syntax and Morphology
- Historical and Diachronic Linguistics
- History of English
- Language Evolution
- Language Reference
- Language Variation
- Language Families
- Language Acquisition
- Lexicography
- Linguistic Anthropology
- Linguistic Theories
- Linguistic Typology
- Phonetics and Phonology
- Psycholinguistics
- Sociolinguistics
- Translation and Interpretation
- Writing Systems
- Browse content in Literature
- Bibliography
- Children's Literature Studies
- Literary Studies (Romanticism)
- Literary Studies (American)
- Literary Studies (Modernism)
- Literary Studies (Asian)
- Literary Studies (European)
- Literary Studies (Eco-criticism)
- Literary Studies - World
- Literary Studies (1500 to 1800)
- Literary Studies (19th Century)
- Literary Studies (20th Century onwards)
- Literary Studies (African American Literature)
- Literary Studies (British and Irish)
- Literary Studies (Early and Medieval)
- Literary Studies (Fiction, Novelists, and Prose Writers)
- Literary Studies (Gender Studies)
- Literary Studies (Graphic Novels)
- Literary Studies (History of the Book)
- Literary Studies (Plays and Playwrights)
- Literary Studies (Poetry and Poets)
- Literary Studies (Postcolonial Literature)
- Literary Studies (Queer Studies)
- Literary Studies (Science Fiction)
- Literary Studies (Travel Literature)
- Literary Studies (War Literature)
- Literary Studies (Women's Writing)
- Literary Theory and Cultural Studies
- Mythology and Folklore
- Shakespeare Studies and Criticism
- Browse content in Media Studies
- Browse content in Music
- Applied Music
- Dance and Music
- Ethics in Music
- Ethnomusicology
- Gender and Sexuality in Music
- Medicine and Music
- Music Cultures
- Music and Media
- Music and Culture
- Music and Religion
- Music Education and Pedagogy
- Music Theory and Analysis
- Musical Scores, Lyrics, and Libretti
- Musical Structures, Styles, and Techniques
- Musicology and Music History
- Performance Practice and Studies
- Race and Ethnicity in Music
- Sound Studies
- Browse content in Performing Arts
- Browse content in Philosophy
- Aesthetics and Philosophy of Art
- Epistemology
- Feminist Philosophy
- History of Western Philosophy
- Metaphysics
- Moral Philosophy
- Non-Western Philosophy
- Philosophy of Language
- Philosophy of Mind
- Philosophy of Perception
- Philosophy of Action
- Philosophy of Law
- Philosophy of Religion
- Philosophy of Science
- Philosophy of Mathematics and Logic
- Practical Ethics
- Social and Political Philosophy
- Browse content in Religion
- Biblical Studies
- Christianity
- East Asian Religions
- History of Religion
- Judaism and Jewish Studies
- Qumran Studies
- Religion and Education
- Religion and Health
- Religion and Politics
- Religion and Science
- Religion and Law
- Religion and Art, Literature, and Music
- Religious Studies
- Browse content in Society and Culture
- Cookery, Food, and Drink
- Cultural Studies
- Customs and Traditions
- Ethical Issues and Debates
- Hobbies, Games, Arts and Crafts
- Lifestyle, Home, and Garden
- Natural world, Country Life, and Pets
- Popular Beliefs and Controversial Knowledge
- Sports and Outdoor Recreation
- Technology and Society
- Travel and Holiday
- Visual Culture
- Browse content in Law
- Arbitration
- Browse content in Company and Commercial Law
- Commercial Law
- Company Law
- Browse content in Comparative Law
- Systems of Law
- Competition Law
- Browse content in Constitutional and Administrative Law
- Government Powers
- Judicial Review
- Local Government Law
- Military and Defence Law
- Parliamentary and Legislative Practice
- Construction Law
- Contract Law
- Browse content in Criminal Law
- Criminal Procedure
- Criminal Evidence Law
- Sentencing and Punishment
- Employment and Labour Law
- Environment and Energy Law
- Browse content in Financial Law
- Banking Law
- Insolvency Law
- History of Law
- Human Rights and Immigration
- Intellectual Property Law
- Browse content in International Law
- Private International Law and Conflict of Laws
- Public International Law
- IT and Communications Law
- Jurisprudence and Philosophy of Law
- Law and Society
- Law and Politics
- Browse content in Legal System and Practice
- Courts and Procedure
- Legal Skills and Practice
- Primary Sources of Law
- Regulation of Legal Profession
- Medical and Healthcare Law
- Browse content in Policing
- Criminal Investigation and Detection
- Police and Security Services
- Police Procedure and Law
- Police Regional Planning
- Browse content in Property Law
- Personal Property Law
- Study and Revision
- Terrorism and National Security Law
- Browse content in Trusts Law
- Wills and Probate or Succession
- Browse content in Medicine and Health
- Browse content in Allied Health Professions
- Arts Therapies
- Clinical Science
- Dietetics and Nutrition
- Occupational Therapy
- Operating Department Practice
- Physiotherapy
- Radiography
- Speech and Language Therapy
- Browse content in Anaesthetics
- General Anaesthesia
- Neuroanaesthesia
- Clinical Neuroscience
- Browse content in Clinical Medicine
- Acute Medicine
- Cardiovascular Medicine
- Clinical Genetics
- Clinical Pharmacology and Therapeutics
- Dermatology
- Endocrinology and Diabetes
- Gastroenterology
- Genito-urinary Medicine
- Geriatric Medicine
- Infectious Diseases
- Medical Toxicology
- Medical Oncology
- Pain Medicine
- Palliative Medicine
- Rehabilitation Medicine
- Respiratory Medicine and Pulmonology
- Rheumatology
- Sleep Medicine
- Sports and Exercise Medicine
- Community Medical Services
- Critical Care
- Emergency Medicine
- Forensic Medicine
- Haematology
- History of Medicine
- Browse content in Medical Skills
- Clinical Skills
- Communication Skills
- Nursing Skills
- Surgical Skills
- Medical Ethics
- Browse content in Medical Dentistry
- Oral and Maxillofacial Surgery
- Paediatric Dentistry
- Restorative Dentistry and Orthodontics
- Surgical Dentistry
- Medical Statistics and Methodology
- Browse content in Neurology
- Clinical Neurophysiology
- Neuropathology
- Nursing Studies
- Browse content in Obstetrics and Gynaecology
- Gynaecology
- Occupational Medicine
- Ophthalmology
- Otolaryngology (ENT)
- Browse content in Paediatrics
- Neonatology
- Browse content in Pathology
- Chemical Pathology
- Clinical Cytogenetics and Molecular Genetics
- Histopathology
- Medical Microbiology and Virology
- Patient Education and Information
- Browse content in Pharmacology
- Psychopharmacology
- Browse content in Popular Health
- Caring for Others
- Complementary and Alternative Medicine
- Self-help and Personal Development
- Browse content in Preclinical Medicine
- Cell Biology
- Molecular Biology and Genetics
- Reproduction, Growth and Development
- Primary Care
- Professional Development in Medicine
- Browse content in Psychiatry
- Addiction Medicine
- Child and Adolescent Psychiatry
- Forensic Psychiatry
- Learning Disabilities
- Old Age Psychiatry
- Psychotherapy
- Browse content in Public Health and Epidemiology
- Epidemiology
- Public Health
- Browse content in Radiology
- Clinical Radiology
- Interventional Radiology
- Nuclear Medicine
- Radiation Oncology
- Reproductive Medicine
- Browse content in Surgery
- Cardiothoracic Surgery
- Gastro-intestinal and Colorectal Surgery
- General Surgery
- Neurosurgery
- Paediatric Surgery
- Peri-operative Care
- Plastic and Reconstructive Surgery
- Surgical Oncology
- Transplant Surgery
- Trauma and Orthopaedic Surgery
- Vascular Surgery
- Browse content in Science and Mathematics
- Browse content in Biological Sciences
- Aquatic Biology
- Biochemistry
- Bioinformatics and Computational Biology
- Developmental Biology
- Ecology and Conservation
- Evolutionary Biology
- Genetics and Genomics
- Microbiology
- Molecular and Cell Biology
- Natural History
- Plant Sciences and Forestry
- Research Methods in Life Sciences
- Structural Biology
- Systems Biology
- Zoology and Animal Sciences
- Browse content in Chemistry
- Analytical Chemistry
- Computational Chemistry
- Crystallography
- Environmental Chemistry
- Industrial Chemistry
- Inorganic Chemistry
- Materials Chemistry
- Medicinal Chemistry
- Mineralogy and Gems
- Organic Chemistry
- Physical Chemistry
- Polymer Chemistry
- Study and Communication Skills in Chemistry
- Theoretical Chemistry
- Browse content in Computer Science
- Artificial Intelligence
- Computer Architecture and Logic Design
- Game Studies
- Human-Computer Interaction
- Mathematical Theory of Computation
- Programming Languages
- Software Engineering
- Systems Analysis and Design
- Virtual Reality
- Browse content in Computing
- Business Applications
- Computer Games
- Computer Security
- Computer Networking and Communications
- Digital Lifestyle
- Graphical and Digital Media Applications
- Operating Systems
- Browse content in Earth Sciences and Geography
- Atmospheric Sciences
- Environmental Geography
- Geology and the Lithosphere
- Maps and Map-making
- Meteorology and Climatology
- Oceanography and Hydrology
- Palaeontology
- Physical Geography and Topography
- Regional Geography
- Soil Science
- Urban Geography
- Browse content in Engineering and Technology
- Agriculture and Farming
- Biological Engineering
- Civil Engineering, Surveying, and Building
- Electronics and Communications Engineering
- Energy Technology
- Engineering (General)
- Environmental Science, Engineering, and Technology
- History of Engineering and Technology
- Mechanical Engineering and Materials
- Technology of Industrial Chemistry
- Transport Technology and Trades
- Browse content in Environmental Science
- Applied Ecology (Environmental Science)
- Conservation of the Environment (Environmental Science)
- Environmental Sustainability
- Environmentalist Thought and Ideology (Environmental Science)
- Management of Land and Natural Resources (Environmental Science)
- Natural Disasters (Environmental Science)
- Nuclear Issues (Environmental Science)
- Pollution and Threats to the Environment (Environmental Science)
- Social Impact of Environmental Issues (Environmental Science)
- History of Science and Technology
- Browse content in Materials Science
- Ceramics and Glasses
- Composite Materials
- Metals, Alloying, and Corrosion
- Nanotechnology
- Browse content in Mathematics
- Applied Mathematics
- Biomathematics and Statistics
- History of Mathematics
- Mathematical Education
- Mathematical Finance
- Mathematical Analysis
- Numerical and Computational Mathematics
- Probability and Statistics
- Pure Mathematics
- Browse content in Neuroscience
- Cognition and Behavioural Neuroscience
- Development of the Nervous System
- Disorders of the Nervous System
- History of Neuroscience
- Invertebrate Neurobiology
- Molecular and Cellular Systems
- Neuroendocrinology and Autonomic Nervous System
- Neuroscientific Techniques
- Sensory and Motor Systems
- Browse content in Physics
- Astronomy and Astrophysics
- Atomic, Molecular, and Optical Physics
- Biological and Medical Physics
- Classical Mechanics
- Computational Physics
- Condensed Matter Physics
- Electromagnetism, Optics, and Acoustics
- History of Physics
- Mathematical and Statistical Physics
- Measurement Science
- Nuclear Physics
- Particles and Fields
- Plasma Physics
- Quantum Physics
- Relativity and Gravitation
- Semiconductor and Mesoscopic Physics
- Browse content in Psychology
- Affective Sciences
- Clinical Psychology
- Cognitive Psychology
- Cognitive Neuroscience
- Criminal and Forensic Psychology
- Developmental Psychology
- Educational Psychology
- Evolutionary Psychology
- Health Psychology
- History and Systems in Psychology
- Music Psychology
- Neuropsychology
- Organizational Psychology
- Psychological Assessment and Testing
- Psychology of Human-Technology Interaction
- Psychology Professional Development and Training
- Research Methods in Psychology
- Social Psychology
- Browse content in Social Sciences
- Browse content in Anthropology
- Anthropology of Religion
- Human Evolution
- Medical Anthropology
- Physical Anthropology
- Regional Anthropology
- Social and Cultural Anthropology
- Theory and Practice of Anthropology
- Browse content in Business and Management
- Business Ethics
- Business History
- Business Strategy
- Business and Technology
- Business and Government
- Business and the Environment
- Comparative Management
- Corporate Governance
- Corporate Social Responsibility
- Entrepreneurship
- Health Management
- Human Resource Management
- Industrial and Employment Relations
- Industry Studies
- Information and Communication Technologies
- International Business
- Knowledge Management
- Management and Management Techniques
- Operations Management
- Organizational Theory and Behaviour
- Pensions and Pension Management
- Public and Nonprofit Management
- Strategic Management
- Supply Chain Management
- Browse content in Criminology and Criminal Justice
- Criminal Justice
- Criminology
- Forms of Crime
- International and Comparative Criminology
- Youth Violence and Juvenile Justice
- Development Studies
- Browse content in Economics
- Agricultural, Environmental, and Natural Resource Economics
- Asian Economics
- Behavioural Finance
- Behavioural Economics and Neuroeconomics
- Econometrics and Mathematical Economics
- Economic History
- Economic Methodology
- Economic Systems
- Economic Development and Growth
- Financial Markets
- Financial Institutions and Services
- General Economics and Teaching
- Health, Education, and Welfare
- History of Economic Thought
- International Economics
- Labour and Demographic Economics
- Law and Economics
- Macroeconomics and Monetary Economics
- Microeconomics
- Public Economics
- Urban, Rural, and Regional Economics
- Welfare Economics
- Browse content in Education
- Adult Education and Continuous Learning
- Care and Counselling of Students
- Early Childhood and Elementary Education
- Educational Equipment and Technology
- Educational Strategies and Policy
- Higher and Further Education
- Organization and Management of Education
- Philosophy and Theory of Education
- Schools Studies
- Secondary Education
- Teaching of a Specific Subject
- Teaching of Specific Groups and Special Educational Needs
- Teaching Skills and Techniques
- Browse content in Environment
- Applied Ecology (Social Science)
- Climate Change
- Conservation of the Environment (Social Science)
- Environmentalist Thought and Ideology (Social Science)
- Natural Disasters (Environment)
- Social Impact of Environmental Issues (Social Science)
- Browse content in Human Geography
- Cultural Geography
- Economic Geography
- Political Geography
- Browse content in Interdisciplinary Studies
- Communication Studies
- Museums, Libraries, and Information Sciences
- Browse content in Politics
- African Politics
- Asian Politics
- Chinese Politics
- Comparative Politics
- Conflict Politics
- Elections and Electoral Studies
- Environmental Politics
- European Union
- Foreign Policy
- Gender and Politics
- Human Rights and Politics
- Indian Politics
- International Relations
- International Organization (Politics)
- International Political Economy
- Irish Politics
- Latin American Politics
- Middle Eastern Politics
- Political Behaviour
- Political Economy
- Political Institutions
- Political Theory
- Political Methodology
- Political Communication
- Political Philosophy
- Political Sociology
- Politics and Law
- Public Policy
- Public Administration
- Quantitative Political Methodology
- Regional Political Studies
- Russian Politics
- Security Studies
- State and Local Government
- UK Politics
- US Politics
- Browse content in Regional and Area Studies
- African Studies
- Asian Studies
- East Asian Studies
- Japanese Studies
- Latin American Studies
- Middle Eastern Studies
- Native American Studies
- Scottish Studies
- Browse content in Research and Information
- Research Methods
- Browse content in Social Work
- Addictions and Substance Misuse
- Adoption and Fostering
- Care of the Elderly
- Child and Adolescent Social Work
- Couple and Family Social Work
- Developmental and Physical Disabilities Social Work
- Direct Practice and Clinical Social Work
- Emergency Services
- Human Behaviour and the Social Environment
- International and Global Issues in Social Work
- Mental and Behavioural Health
- Social Justice and Human Rights
- Social Policy and Advocacy
- Social Work and Crime and Justice
- Social Work Macro Practice
- Social Work Practice Settings
- Social Work Research and Evidence-based Practice
- Welfare and Benefit Systems
- Browse content in Sociology
- Childhood Studies
- Community Development
- Comparative and Historical Sociology
- Economic Sociology
- Gender and Sexuality
- Gerontology and Ageing
- Health, Illness, and Medicine
- Marriage and the Family
- Migration Studies
- Occupations, Professions, and Work
- Organizations
- Population and Demography
- Race and Ethnicity
- Social Theory
- Social Movements and Social Change
- Social Research and Statistics
- Social Stratification, Inequality, and Mobility
- Sociology of Religion
- Sociology of Education
- Sport and Leisure
- Urban and Rural Studies
- Browse content in Warfare and Defence
- Defence Strategy, Planning, and Research
- Land Forces and Warfare
- Military Administration
- Military Life and Institutions
- Naval Forces and Warfare
- Other Warfare and Defence Issues
- Peace Studies and Conflict Resolution
- Weapons and Equipment

- < Previous chapter
- Next chapter >

What Is Human Subjects Research?
Department of Philosophy, Dalhousie University
- Published: 15 December 2020
- Cite Icon Cite
- Permissions Icon Permissions
This chapter provides an overview of the nature, scope, and practice of human subjects research. It begins by tackling the general question, “What is research?” Attempts to answer this question typically define research by its methods and/or goals, and the chapter surveys the limits of these definitions through discussion of tough boundary cases. Along the way, the chapter describes various methods (quantitative, qualitative) and types of human subjects research (clinical, social scientific, etc.). The second section of the chapter investigates who is referred to by the language of “human subjects”: which humans tend to be selected as research participants, where human subjects are located globally, and how these locations are changing. The chapter also raises questions about which subjects are considered human in this context, for instance, whether definitions include embryos, cadavers, or stem cells. Throughout, the chapter highlights the ethical issues raised by the various types of activities and subjects described.
Which of the following is human subjects research?
A clinician conducts a placebo-controlled, double-blind, randomized trial of a new treatment for depression.
A sociologist conducts a series of in-depth interviews with paramedics and firefighters about their experiences of burnout, which are then transcribed and analyzed for common themes.
On the basis of published research indicating a reduction of adverse events, a hospital administrator implements mandatory surgical checklists in one of their operating rooms and tracks the outcomes compared to the hospital’s other operating rooms; the administrator hopes that the expected positive results will help to convince reluctant hospital staff to adopt surgical checklists.
A team of economists selects three cities, sends invitation letters to all low-income citizens in those selected cities, and then partners with local government to provide a basic income to selected individuals for three years, tracking a range of health and life outcomes.
A patient seeks care from a family physician for a rare heart condition; after several unsuccessful treatments, the physician tries an unusual combination of medications, and the patient reports feeling much better.
Same as example 5, but the physician then writes up the case for publication in a peer-reviewed medical journal.
A pediatric oncologist offers patients with an otherwise untreatable form of cancer the option to try promising new treatments that are in the earliest stages of development.
Medical students manipulate human embryos in order to learn how to extract cells for genetic tests.
A geneticist analyzes and sequences the DNA from blood samples collected decades ago from the members of a marginal population.
If you found yourself struggling to decide which of these counts as human subjects research, you are not alone: experts and newcomers to research ethics alike find this task difficult. In fact, even highly respected regulatory bodies and authors of codes of ethics struggle to articulate clear and consistent answers to this question (for examples, see the opening chapters in this handbook). And because an affirmative answer to the question is thought to determine which activities are in need of prospective ethics review, the stakes of this debate are thought to be quite high.
The difficulty of this task persists for many reasons but, in particular, because both key concepts in the question—“research” and “human subjects”—are hard to define and plagued by tough, and ever-evolving, boundary cases. In what follows, I will outline these controversies and investigate whether there might be a clear sorting mechanism for the kinds of cases just outlined. For both concepts (“research” and “human subjects”), I will show that a clear definition is hard, if not impossible, to find. But this may not be as big a problem as it seems. In order to explain why not, I will explore a common underlying assumption about the high stakes of this assessment: the presumed connection between ethics and a particular type of regulatory review in human subjects research. Clarifying this relationship will help to defuse the worry about demarcation criteria for these concepts.
What is research? This is a harder question to answer than one might expect: any answer is in danger of being either underinclusive (for instance, by focusing narrowly on medical research when similar activities are carried out by researchers in other disciplines or professions) or overinclusive (labeling everything vaguely experimental or involving human interaction as research). The Tri-Council Policy Statement (TCPS 2) in Canada begins with a reflection on the broad range of practices and activities that qualify as research, before proposing a definition:
The scope of research is vast. On the purely physical side, it ranges from seeking to understand the origins of the universe down to the fundamental nature of matter. At the analytic level, it covers mathematics, logic and metaphysics. Research involving humans ranges widely, including attempts to understand the broad sweep of history, the workings of the human body and the body politic, the nature of human interactions and the impact of nature on humans—the list is as boundless as the human imagination. For the purposes of this Policy, research is defined as an undertaking intended to extend knowledge through a disciplined inquiry and/or systematic investigation . (Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, and Social Sciences and Humanities Research Council of Canada 2018 , 5, emphasis added)
The ethical guidelines provided by the Council for International Organizations of Medical Sciences (CIOMS) provide a similar (though health-focused) definition and some examples of common research methods:
The term “health-related research” in these Guidelines refers to activities designed to develop or contribute to generalizable health knowledge within the more classic realm of research with humans, such as observational research, clinical trials, biobanking and epidemiological studies. Generalizable health knowledge consists of theories, principles or relationships, or the accumulation of information on which they are based related to health, which can be corroborated by accepted scientific methods of observation and inference . (2016, xii, emphasis added)
Likewise, according to the original Belmont Report in the United States, “the term ‘research’ designates an activity designed to test a hypothesis, permit conclusions to be drawn, and thereby to develop or contribute to generalizable knowledge ” (National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, 1978, emphasis added).
Note first that each of these definitions would lead to a slightly different assessment of the cases outlined at the beginning of this chapter, so we can’t simply point to regulations to answer our question for us without engaging in further discussion about which regulations are correct. More to the point here, though, we can see that the following concepts tend to arise in definitions of research: scientific methods (observation, hypothesis testing, and/or inference), systematic and/or disciplined inquiry, generalizability, and contributing to knowledge. Research, it seems, is implicitly scientific research . Research is something that scientists do (as contrasted with journalists or celebrities, for instance). This qualification is supported by landmark ethical guidelines such as the original Nuremberg Code, Article 8 of which states, “The experiment should be conducted only by scientifically qualified persons” (Nuremberg Code 1949 , 182). And scientific research involves certain systematic and disciplined methods , which when used properly provide some assurance about the generalizability of results.
Does a focus on the scientific method help to sort the test cases? This seems a promising route since the scientific method is thought to be what makes results more reliable than unsystematic observation and inference, which connects to the aim of producing knowledge. The difficulty is that there are many different methods used by researchers in a range of disciplines. Each research method aims to answer a different question—some are comparative, while others try to find out why someone holds a position or acts a certain way.
Qualitative research methods involving human subjects range from those involving close contact and communication between researchers and individual subjects, which are often open-ended and dynamic, such as ethnographic studies, oral histories, narrative inquiries, focus groups, and minimally structured interviews, to more structured and less dynamic methods such as large-scale surveys and structured interviews. Qualitative research is excellent at answering “why” and “how” questions and much less focused on reporting numerical results than quantitative research. As such, it plays an important and complementary role to quantitative research: a quantitative study may determine that some percentage of elementary school teachers report feeling burned out, for instance, while a qualitative study can investigate why this occurring and how it is experienced or understood by those who self-report.
Quantitative research methods involving human subjects include case studies, case series, and n -of-1 studies, all of which focus on the description and analysis of individual cases. They include observational methods such as case–control and cohort studies, which track and compare groups of people over time (either prospectively or retrospectively). In these types of studies, subjects are not assigned to different groups but rather self-select or are otherwise independently sorted into groups (for instance, a study might follow cyclists and non-cyclists). And then there are interventional methods such as randomized controlled trials (RCTs), in which participants are assigned to intervention and control groups randomly, and, when double-blind, neither they nor the researchers involved know which group they were assigned to until the study is completed. In many domains, including economics, public policy, and medicine, the RCT design is regarded as the gold standard of quantitative methods because of its rigorous comparative design and perceived objectivity.
Quantitative clinical research, in particular, proceeds on the basis of positive results in earlier animal studies and then is carried out in phases. Phase I clinical research typically enrolls a small number of healthy subjects (20–80) and aims to determine whether a proposed intervention is safe in humans and at what approximate dose or intensity. Phase II clinical research enrolls a somewhat greater number of subjects (100–300)—this time those with the health condition the intervention aims to treat—and aims to assess both safety and efficacy (the effect under near-ideal conditions). Phase III clinical research enrolls large numbers of subjects (1,000+) and aims to determine whether an intervention is effective. This phase of research is typically the basis for national regulatory approval, meaning that the treatment can be prescribed and sold to patients in some jurisdiction once it has the support of (typically at least two) well-designed phase III trials. Phase IV, or post-marketing trials, track outcomes in the general population once a treatment is widely available.
In both qualitative and quantitative domains, there are meta-level research methods designed to amalgamate the results of research. These include literature reviews, systematic reviews, and meta-analyses. In an effort to reach busy audiences, there are also summaries and syntheses which aim to bring together all research on a given topic and provide an overall assessment. Guidelines for practitioners in medicine often draw upon these meta-level studies, as well as expert opinion, in recommending standards of practice. And the range of methods is always expanding: some newer methods, such as cluster RCTs and umbrella trials, are discussed by Hey and Weijer in this handbook.
Generalizable Knowledge
What this wide range of scientific methods, from in-depth interviews to RCTs, have in common is that they involve a systematic or disciplined effort to produce results that contribute not just to knowledge but to generalizable knowledge —a standard interpretation of this term is “the use of information to draw conclusions that apply beyond the specific individuals or groups from whom the information was obtained” (Coleman 2019 , 248). This brings us to the aims of research, which were a common component of the definitions of research offered earlier. Each of the methods described might be thought of as contributing to generalizable knowledge, while something like trial and error in clinical practice might be aimed only at benefiting an individual patient. In order to figure out whether quality improvement efforts—such as instituting a surgical checklist in one operating room and comparing with others—count as generating generalizable knowledge, we would look to their aim. In the case as I described it at the outset, the administrator believed that they already knew the intervention would be successful at reducing rates of adverse events, based on the research evidence. The aim was to convince the healthcare team in the hospital that these results applied locally so that they would adopt the practice. This seems to be a case where the primary aim is changing local behavior rather than adding to general knowledge. This way of separating quality improvement activities from research proper has become quite popular in recent years. Scholars take different positions on whether this way of settling the matter is successful or not. This debate turns on, among other things, different ideas of what is meant by “generalizable knowledge.”
Most interpretations of “generalizable” focus on the applicability of results to people who were not in a study. But this can be tricky. An RCT with strict criteria for who is included, that tests an intervention against placebo, and that strictly controls the context in which treatment is administered (for instance, only by specialists in a highly resourced urban hospital) may produce results indicating that a particular medical treatment is effective. This sort of clean explanatory RCT is thought by many scholars to be the exemplar of a study design yielding generalizable results. But a rural physician in a low-resource area dealing primarily with elderly patients who have multiple health conditions might not regard the results of the study just described as generalizable to their patients. (And they would probably be right about this—the gap between research evidence and individual patient care is a real one, and closing or narrowing that gap is something researchers have been working on for decades. The advance of pragmatic trials is one attempt to solve this problem, for instance.) Through this example, we see some of the challenges inherent in claims made about generalizability, particularly when interpretations focus on applicability. Not all areas of scientific investigation lend themselves to the production of law-like generalizations of the sort (ostensibly) found in physics or chemistry. And very few medical interventions work for all patients, without qualification. To return to the quality improvement case, there is a sense in which knowledge is gained through the investigation—something new is learned about whether surgical checklists work in this specific location—and the knowledge is intended to generalize—for instance, across other operating rooms in that facility. Is this not (at least locally) generalizable knowledge, then? Many people seem to want to say “no” here but struggle to find a clear rationale for their position.
The challenges encountered thus far in our efforts to define research indicate that a new strategy is in order; accordingly, let’s turn back to our original question—“What is human subjects research?”—and ask why we are seeking an answer to this question. Perhaps the question is ill-conceived, or perhaps our aims will help guide us toward one of these imperfect options or even something better. What are the stakes here? Why does it matter what counts as human subjects research? Why would anyone resist having their actions labeled “research”?
The common answer to this question—the one potential researchers themselves would likely be quick to offer—is that it matters because activities that are considered research involving human subjects must undergo review by a research ethics committee (REC) and secure approval before recruiting any participants. 1 In other words, there are regulations in most jurisdictions requiring that certain types of activities are subject to independent oversight. According to the TCPS 2 in Canada, for instance, “A determination that research is the intended purpose of the undertaking is key for differentiating activities that require ethics review by an REB and those that do not” (Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, and Social Sciences and Humanities Research Council of Canada 2018 , 14). 2 A common rationale for this is that the primary aim of research is to gather knowledge to benefit people other than those in the study itself. By contrast, clinical practice, which also involves human subjects, is regulated differently (and with much less direct oversight)—by expectations that professionals will adhere to professional norms and guidelines. Because the aim of practice is benefit for the particular patient, it is thought that fewer or at least different ethical concerns arise. Similarly, other professions, like journalism, have their own sets of norms and rules guiding their activities, tied to their specific aims. The special ethical oversight of research activities is relatively new, in historical terms, since national regulations on human subjects research were enacted in most jurisdictions, in response to the public outcry over publicized cases of abuse of research subjects (for more on this, see the opening chapters of this handbook). When these regulations were proposed, those who drafted the regulations were acutely aware of the need to avoid encroaching on other domains of professional activity—particularly clinical practice (Beauchamp and Saghai 2012 ).
From the earliest attempts to offer a research–practice distinction it was clear that there would be troublesome boundary cases. 3 Phase I (or “first in human”) clinical trials—famously, those in pediatric oncology—tend to enroll patients who have cancer (not healthy subjects), and when there is no other treatment option available for that form of cancer, the research looks pretty much identical to practice (Kass et al. 2013 ). These sorts of activities might be thought of as “therapeutic research,” “innovative therapy,” or “unvalidated practice” depending on one’s orientation to the research–practice distinction. Other boundary cases recognized by early scholarship in this area included what would now be considered a type of comparative effectiveness research, in which two widely available treatments are compared to see which performs better, and quality improvement activities, in which healthcare systems experiment with new rules or guidelines in order to see how well they work in local settings (Beauchamp and Saghai 2012 , 49). Note that it is a necessary, not merely accidental, feature of such activities that they are in some sense both research and practice simultaneously. Phase IV studies are also often ambiguous—depending on how rigorously they are designed, they may also look simply like tracking adverse events in clinical practice. So while research has been defined in terms of its distinctive aim, the distinction is fuzzy and contested; and it continues to be plagued by borderline cases. 4
Note also that the way research was defined for regulatory purposes—against medical practice in particular—meant that the resulting distinction tracked the activities of greatest ethical concern in the medical context specifically. But human subjects research is a much broader category than simply medical research: there are a range of ways in which human subjects may be subjects of studies, including, for instance, in social scientific research. Because this type of research is helpful for understanding the stakes of getting the answer to the title question right, I will outline briefly the social scientific backlash to research ethics oversight, which typically involves delays associated with the prospective review of proposed research and some of the ways that ethics regulation has adjusted to accommodate the range of different types of investigations involving human subjects.
Cases from the social sciences are among the more prominent examples of controversial research in the twentieth century: the Milgram experiment on obedience to authority and Zimbardo’s prison experiment with students assigned to the role of prisoner or guard might come to mind (Haggerty 2004 ). Given that the outcry about the abuse of human subjects in medical research happened around the same time in many jurisdictions (roughly the 1970s), it is no surprise that ethics regulations were developed and applied across all domains of research with human subjects, including social science research. Resistance to these regulations is common, particularly (though not uniformly) in the social sciences, where being lumped in with medical researchers strikes many as bizarre overreach: “What began years ago as a sort of safeguard against doctors injecting cancer cells into research patients without first asking them if that was OK has turned into a serious, ambitious bureaucracy with interests to protect, a mission to promote, and a self-righteous and self-protective ideology to explain why it’s all necessary” (Becker 2004 , 415). Becker is referring here to what he calls “ethics creep,” which involves “a dual process whereby the regulatory system is expanding outward to incorporate a host of new activities and institutions, while at the same time intensifying the regulation of activities deemed to fall within its ambit” (Haggerty 2004 , 391).
A common critique raised by social scientists hinges on the inconsistency between the way different professionals, for instance, journalists and academic social scientists, are treated under current regulatory schemes. The very same activity—interviewing people, for instance—seems to trigger extensive and burdensome oversight when conducted by social scientists even though journalists proceed much more freely. In locating the problem with this arrangement, Haggerty draws attention to precisely the problem identified in this chapter, namely that central concepts like research are poorly defined in documents regarding the ethical regulation of research; they are “empty signifiers, capable of being interpreted in a multitude of ways, and occasionally serving as sites of contestation” (2004, 411). Interpretation is required, and because members of RECs feel responsible for protecting people, they tend to take what he calls a “just in case” approach, in which research is interpreted inclusively (and over-broadly) (2004, 411). This means that social scientists may be subject to extensive oversight.
In 2004, Haggerty articulated his concern as follows: “Over time, I fear that the [REC] structure will follow the pattern of most bureaucracies and continue to expand, formalizing procedures in ways that increasingly complicate, hamper, or censor certain forms of non-traditional, qualitative, or critical social scientific research” (pp. 392–393). This has also been referred to as part of the expansion of neoliberal audit culture and identified as part of the increasing bureaucratization of academia (Taylor and Patterson 2010 ). In response to this perceived ethics creep, some social scientists have called for “creative compliance” or even outright resistance to ethics regulations. One option—reclassifying one’s research as performance art (or some other unregulated activity) is offered with a wink, but behind closed doors researchers will sometimes admit using such tactics (Haggerty 2004 , 408). These efforts have in some cases been met with further regulation: “As some of us have tried new dodges to skirt the requirements, the [RECs] have wised up and closed loopholes” (Becker 2004 , 415).
Yet against these dire predictions and in response to the outcry and backlash generated by social scientists in the wake of early, more heavy-handed and medically oriented regulatory approaches, regulations (and their interpretation) have shifted in the opposite direction in many jurisdictions (for an overview of international regulations, see the chapter by Nelson and Forster in this handbook). In Canada, for instance, the most recent version of the TCPS 2 takes a proportionate approach to the review of research:
Given that research involving humans spans the full spectrum of risk, from minimal to significant, a crucial element of REB review is to ensure that the level of scrutiny of a research project is determined by the level of risk it poses to participants. … A reduced level of scrutiny applied to a research project assessed as minimal risk does not imply a lower level of adherence to the core principles. Rather, the intention is to ensure adequate protection of participants is maintained while reducing unnecessary impediments to, and facilitating the progress of, ethical research. (Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, and Social Sciences and Humanities Research Council of Canada 2018 , 9)
As this statement indicates, while all research is held to the same high ethical standard, research of minimal risk is thought to require a lower degree of oversight. Ethics review in Canada begins with a determination that the activity is in fact research with human subjects; activities described as falling outside of the definition of research include the sorts of quality improvement activities outlined in the hospital administrator case and “creative practice activities” such as those undertaken by artists (p. 19). 5 Next, some activities that meet the definition of human subjects research are automatically exempt from review, including 1) research that relies entirely on legally accessible, publicly available information where the individuals have no reasonable expectation of privacy and 2) exclusively observational qualitative research conducted in public places where there is no reasonable expectation of privacy and individuals are not identified in the written report (pp. 15–18). This will cover much of the research conducted by historians and some observational studies conducted by social scientists, educators, etc.
At this point, if an activity is considered research and not exempt, it may still be afforded an expedited (“delegated”) review if it is low-risk: according to Article 6.12, “In keeping with a proportionate approach to research ethics review, the selection of the level of REB review shall be determined by the level of foreseeable risks to participants: the lower the level of risk, the lower the level of scrutiny (delegated review); the higher the level of risk, the higher the level of scrutiny (full board review)” (p. 79). In delegated review, the committee assigns one member (or some equivalently qualified person) to assess the proposal rather than assessing it all together. A negative assessment at this stage refers the study back to the full committee for review. Because social scientific research is more likely to be minimal-risk than medical research, it is well positioned to benefit from delegated review. Canada is not unique here: similar exclusions and exemptions typically exist in other national regulatory systems. And in some jurisdictions they are even broader: in the United States, for instance, public health surveillance, criminal justice, and intelligence activities are all excluded from the domain of “research” and exemptions (activities requiring only “limited review”) are offered for most interview- and survey-based research, secondary research even when it uses identifiable private information or biospecimens, and “benign behavioral interventions” (Coleman 2019 , 248). This is a more permissive approach, overall, than the one found in Canada, and the trajectory seems to be generally in the more permissive direction over time.
At this point, we have enough background about the relationship between research and regulation to return to our question about the stakes of this discussion: why would someone wish to avoid having their activity labeled research? The answer given by some investigators is that they might resist if they think there are immediate, and burdensome, regulatory implications. A few things can now be said about this. First, it may be the case that there were times and places where the burdens of regulatory oversight were heavy even in the face of minimally risky activities or where the interpretation of regulations was overzealous. But it is unlikely to be true today—most systems have built-in exemptions and expedited processes for these sorts of cases, as the Canadian example makes clear, and discrepancies in interpretation between RECs have had time to resolve. In the face of complaints from researchers, it is good to look closely at the current local regulations and the way they are implemented. Second, in some jurisdictions today there are known inefficiencies in the regulatory oversight system—this occurs for a wide range of reasons but particularly because the process typically relies on volunteer labor and can involve reading hundreds of pages of detailed, technical proposals at a time. As a result, there are sometimes long delays, and researchers are entirely within their rights to complain about this, though they should be careful about selecting an appropriate target of criticism, whether that’s the local REC or the system within which RECs operate. Further, instantaneous processing of files would be unreasonable on the part of researchers, so negotiation will be needed to find a reasonable timeline, given shared goals. 6 Finally, some of the resistance likely arises from a misunderstanding about what ethics is and how it operates in the world. This requires some attention.
For many researchers, regulatory oversight has become synonymous with ethical assessment. You might hear a hint of this when researchers talk about “getting through ethics,” “waiting for ethics,” or claiming to have “completed ethics” once they have received approval from an ethics board for their study. A similar sort of reduction of ethics to a formal process sometimes occurs in contexts where healthcare providers seek informed consent: they may talk about “consenting the patient” in advance of a procedure, for instance, which is typically reduced to having the patient sign a legal document. It is important to appreciate why this position is indefensible—why legal paperwork or regulatory approval isn’t in any meaningful way a substitute for ethics, understood properly.
To begin, consider a study that has received ethics approval and yet which, when it is actually carried out, has risks that are unreasonably high (perhaps most subjects enrolled will die) a flawed design (perhaps it is not possible to achieve statistically significant or otherwise meaningful results), subjects are told they can’t leave the study once enrolled (violating the voluntariness of their ongoing consent), or the particular individuals in the study are easily identifiable in the published final report (violating their privacy). That study is unethical, in spite of having received approval from an ethics committee. Any number of things may have gone wrong here. First, like all human activities, review is fallible, and sometimes committee members will make mistakes. Sometimes the mistake will be in applying the rules, but at other times the mistake might be in the rules themselves. The particular rules applied by any ethics committee are open to debate, discussion, and revisions in light of new developments in scientific or ethical domains. The regular updates to codes of ethics such as the “Declaration of Helsinki,” currently in its seventh revision since 1975, provide some indication of the rate of change in these domains. Second, the researchers may have provided only a general description of certain activities (such as the trial design or informed consent process) in their application to the ethics committee and then, in specifying these matters later on, made poor choices. Third, researchers may simply have deviated from what they promised to do in their application to the ethics board. The research process relies on a certain amount of trust and good will between reviewers and researchers, and this can be violated by unethical or incompetent researchers. Approval by an ethics committee, then, is not all there is to an assessment of whether some activity is actually ethical .
Awareness of this simple fact helps us to see the dangers of thinking that classifying something as research means a particular set of ethical rules applies that wouldn’t otherwise. Codes of ethics aim to identify and articulate ethical principles or rules, and ethics committees do their best to interpret and apply these general principles to particular cases. But whether those committees existed in the middle ground between principles and action or not (and until recently, they didn’t), ethical principles would still apply to certain activities whenever those activities had certain features. Research with human subjects, as noted, aims at generalizable knowledge, and it typically “uses” those subjects to get knowledge. Along the way, the subjects may be made better or worse off, and any interaction where people make others worse off raises ethical concerns about harms such as exploitation and disrespect. Think about the contrast between paradigmatic cases of medical practice and medical research here—in practice, a healthcare provider aims primarily to benefit the patient, while in research, they aim primarily to generate new knowledge. When getting new knowledge requires the use of another person’s body, it seems clear that we’re in risky ethical territory.
Another way of appreciating the scope of ethics as something far bigger than ethics regulations is to think about the fact that regulations won’t specifically state things like “don’t murder your subjects” or “don’t steal the personal belongings of your subjects” because these ethical prohibitions are thought to be covered by existing criminal laws and not in need of restating. There are many ways to be unethical beyond those listed in codes of ethics because those codes are only part of a larger social system.
Further, some of the ethical rules present in codes and guidelines arise because of the place of research within society and not merely because it is a transaction between individuals. Research proceeds only with the cooperation and support of the societies in which it is conducted, which provide funding, regulation, legal protections, social and physical infrastructure, potential subjects, and more. The requirement that research is socially valuable—that it contribute to knowledge on the topic and directly or indirectly benefits society—is one such rule imposed on research with human subjects (you can read more about this requirement elsewhere in this handbook). The requirement that research is scientifically valid—including the expectation that methods are rigorous and results are meaningful—draws on norms of science developed independently by scientists, which prioritize epistemic values such as fruitfulness, scope, and accuracy in theory construction. Scientists are also held to ethical restrictions around activities considered research misconduct, such as plagiarism, fabrication, and falsification, even though these activities aren’t listed explicitly in codes of ethics for research with human subjects.
Professional Ethics
We’ve been discussing, and trying to articulate the problems with, a particular resistance to being labeled research that results from a misunderstanding about how ethics operates in the world. Hopefully the responses to this argument have been convincing thus far. There is, however, a more nuanced version of the position remaining: some investigators might resist the research label because they believe they are governed by codes of ethics developed prior to current codes and articulated within their professions and see the bureaucracy associated with contemporary ethics review as a less nuanced and perhaps even misleading way to go about thinking through the ethical dimensions of their work. They see a perfectly functional self-regulating profession taken over by people with little or no understanding of the nature of their work or the subtle and precise responses to ethical dilemmas they’ve developed over time.
For example, journalists have ethical norms prioritizing the protection of sources—these norms evolved because of social-historical cases where harm arose (in the extreme, people who were killed when they were identified after a story was published) and a recognition of the need to avoid those harms going forward. This ethical rule for journalists is tied to what is valuable about the activity (here: truth) and a recognition of particular harms that could arise in telling the truth (here: people who assisted in exposing the truth could be killed). If you want to proceed with an activity that involves interaction with other people (maybe even in some sense “uses” them to gain knowledge) but in that interaction, or afterward, those people might be harmed, you should probably ask how that harm can be minimized. Responsible professionals in a range of domains have engaged in this thoughtful work for decades and even centuries. Anthropologists, for instance, have been reflecting about the particular ethical duties arising from ethnography since the method was developed, such as the shifting loyalties that result from the close relationships formed during fieldwork, and the desire of state entities to access and direct their research to secure information from enemies during wartime (Fluehr-Lobban 2002 ).
A decisive response to these concerns is unnecessary here: it is sufficient for the purpose of this chapter that we are aware of them. It is a matter of ongoing discussion in a range of human domains whether certain activities should be regulated or not or whether they should be regulated using one set of rules or another. In general, the position taken by liberal democratic states is that professions and industries with a history of serious harm to citizens have forfeited their right to self-regulation. Research on human subjects has a sufficiently sordid history to qualify here. Whether this inappropriately covers social scientists or others will likely be a matter for further debate. For our purposes, what is important is that we recognize that ethical rules apply regardless of which set of regulations is in force (state-imposed external ethics review, professional codes of ethics, or novel alternative oversight mechanisms). So while the stakes of the discussion are high in the sense that they determine this regulatory path, they are not as high as people tend to think because the ethical rules will apply regardless. Being labeled performance art rather than research might mean you avoid filling out some forms, but it won’t on its own change the nature of your ethical obligations since those arise out of the type of activity planned and its aims and consequences.
In sum, the best response we have to the question “What is research?” is probably that research aims to produce generalizable knowledge, but it is important to recognize that this is an imperfect definition and leaves open a range of debates, including those related to the correct interpretation of “generalizability.” It is also important to recognize that answering this question may not be the best way to decide what systems of accountability ought to track the ethical issues that arise in knowledge-gathering activities; it is worth always keeping in mind that a range of regulatory mechanisms are possible. We have also defused some of the anxiety around responses to this question by tracing and responding to some of the reasons for resistance to the label. The ethical principles arising from an activity aren’t invented and dictated by RECs—they apply whether an activity is labeled research or not and whether it is regulated as such or not. There is room for critical engagement here, but at the end of the day there’s just no escaping ethics.
Human Subjects
I have indicated that there is debate over not only what counts as research, as we’ve just seen, but also who is included in our discussions of human subjects. There are two versions of the question “Who are human subjects?,” each of which raises distinct ethical issues. First, we might wonder which humans end up being research subjects. Is there a paradigm or “model human” researchers have in mind? Are there some humans on whom research is forbidden or significantly restricted? Where are human subjects located globally, and how is this changing? Is there a shortage or surplus of human subjects available for research? How many people are research subjects annually? Depending on the answers to these questions, how might we assess the fairness of the burdens and benefits of research participation? This version of the question raises issues about representation in research as well as more general concerns of distributive and social justice.
Second, we might wonder which subjects are included in discussions of human subjects research. Are any of the following included, for instance: fetuses, embryos, brain-dead humans, cadavers, human organs or tissues, reproductive tissues, or stem cells? And, particularly if some of these items are included, why stop at the boundary of the human species? What lies behind the strict demarcation between human and nonhuman animals as subjects of research? Thinking more broadly, what are the implications of various positions on this matter for research on (hypothetical) conscious, sentient robots or aliens? This version of the question raises issues of moral status. I’ll outline both of these sets of issues.
Which Humans?
The human subjects of research have not always been representative of the diversity of humanity or even of the local populations within which research was conducted. The tendency of Western researchers (white men, for the most part), prior to ethics regulations, to seek out vulnerable populations such as prisoners, children at boarding schools, hospital patients, sex workers, citizens of other countries, racial minorities, and impoverished persons (and especially people at the intersection of these categories) for inclusion in research is well documented. The attraction of these groups was precisely their vulnerability—the fact that it was difficult or impossible for them to refuse involvement, for instance. Early responses to this situation focused on protections for variously identified vulnerable populations. While these concerns persist, and took on new life when multinational research became more common in the 1990s, another concern about representation has arisen more recently: the underrepresentation of certain groups in research. While the first set of concerns track disproportionate burdens of research participation, the second set tracks the lost benefits of research participation. The ethical assessment of the former actions—essentially, preying on the vulnerable—is more easily appreciated, so I’ll say a bit about the latter problem. Failing to ensure that subjects are representative of particular groups can lead not only to missed opportunities to benefit those populations but also to direct harm when research is falsely generalized across that group, as when a drug with positive results in one group is dangerous or toxic to another.
Women were underrepresented in clinical research in the United States (and elsewhere) until at least the 1990s. As a result of improved regulations, the United States has shifted toward more equitable inclusion of women in publicly funded clinical trials, though most studies still fail to analyze results by sex/gender in spite of the recognized benefits of doing so (cf. Geller et al. 2018 ). This is a development worth noting, but it is important to keep in mind that this tracks only clinical research, funded publicly, in one country. We shouldn’t assume the underrepresentation of women in human subjects research has been resolved or even that sensible extension to related domains has been made—the selection of exclusively male mice for animal research continued for many years after these changes were made to human subjects regulation and is still the status quo in many countries and contexts (Shansky 2019 ). The attempted justification for these exclusions has been decisively refuted in the literature dozens of times. Addressing one common mistake clearly driven by outdated gender norms, Shansky reminds us, “Women, but not men, are still pejoratively described as hormonal or emotional, which curiously neglects the well-documented fact that men also possess both hormones and emotions” (2019, 825). The resulting imbalance has affected research in many fields that continue to rely on animal studies such as neuroscience, endocrinology, physiology, and pharmacology. As a result of the exclusion of female mice from neuroscience research, and because research in animals provides the foundation for clinical trials, “the current understanding of how to most effectively treat disease in humans is similarly unbalanced” (Shansky 2019 , 825).
Over the past five years, Canadian and American funding bodies have introduced new requirements for researchers to consider sex as a biological variable in animal studies, and similar efforts have been made by the European Commission (Shansky 2019 ). Of course, sex/gender is not the only characteristic that has been unequally distributed in research studies. The same arguments offered in support of ending the exclusion of female animals from animal research and women from clinical research have been marshaled in favor of improving the inclusion of children, pregnant women, and specific ethnic minority groups (for instance, particular indigenous groups in Canada) with limited success. As mentioned, these exclusions have the potential to lead to significant harm to these populations, particularly in clinical practice since interventions never tested on a population may turn out to have more harms than benefits, and the lack of information about effects of treatments in that population might leave clinicians and others uncertain about how best to act even when acting quickly is critical. It may also simply be unjust in its own right to exclude people from research that might benefit them.
In addition to the explicit long-standing exclusion of particular identifiable groups, such as women, researchers have excluded populations indirectly by, for instance, preferring subjects who are healthier, are younger, and have fewer comorbid conditions. One result of these exclusions has been an underrepresentation of elderly people in clinical research. Because underrepresentation in research means the bench-to-bedside knowledge translation gap is bigger, this likely means elderly people are missing out on certain health benefits. And they aren’t the only ones losing out on benefits: clinical research subjects are not generally representative of a large percentage of the patient population for whom the interventions are intended. For instance, Humphreys and colleagues found that “highly cited trials do not enroll an average of 40.1% of identified patients with the disorder being studied, primarily owing to eligibility criteria” (2013, 1030). Other identifiable groups who may be affected by exclusions indirectly are people whose immigration status is uncertain, people who don’t speak the local language, and people who live far from the urban centers where much research occurs. Who is overrepresented in studies, then? People from “Western, Educated, Industrialized, Rich, and Democratic . . . societies” (Henrich, Heine, and Norenzayan 2010 , 61). The 2019 World Health Organization’s World RePORT , drawing on data from 2016, indicates that the recipients of research funding from the top 10 funders globally continue to be mostly institutions and investigators in North America and Europe working on non-communicable diseases (World Health Organization 2019 ).
While this is true today, some things are changing. Though most clinical research (approximately 70 percent) is still conducted in North America and Europe, “significant West-to-East and North-to-South shifts appear to be underway” with researchers looking increasingly to Asia, Africa, South America, and eastern Europe (Sismondo 2018 , 55). One reason this is thought to be happening is that researchers are keen to find countries where the medical system is advanced enough to locate their trials, with access to a large population, but at the lowest cost possible. According to Sismondo, costs per subject in clinical trials are estimated to be 30–50 percent lower in India than in North America or western Europe (2018, 55). Researchers are also interested in finding populations where individuals are not already taking other medications, and countries like India may have a greater proportion of subjects like this (Sismondo 2018 , 54). There are also more altruistic motives: low- and middle-income countries (LMICs) have particular health problems, and some researchers in high-income countries (HICs) may have an interest in helping to alleviate those problems, such as high rates of HIV transmission, epidemics such as the recent Ebola outbreaks, neonatal disorders, and neglected tropical diseases. Research in developmental economics suggests that these motives and effects can also be mixed in quite complicated ways: for instance, aid organizations may seek to alleviate global poverty and design studies to inform this effort but, in doing so, also reinforce the continued existence of their organization, create cycles of dependency, or perpetuate assumptions about the lack of knowledge or expertise in targeted populations.
Another reason for this global shift is that researchers often report difficulty recruiting subjects in HICs. For example, according to McDonald and colleagues ( 2006 , np), for multi-center RCTs funded by two UK funding agencies, “Less than a third (31%) of the trials achieved their original recruitment target and half (53%) were awarded an extension. The proportion achieving targets did not appear to improve over time. The overall start to recruitment was delayed in 47 (41%) trials and early recruitment problems were identified in 77 (63%) trials.” In general, “Recruitment is often slower or more difficult than expected, with many trials failing to reach their planned sample size within the timescale and funding originally envisaged” (McDonald et al. 2006 , np). This shortage of (appropriate) research subjects is of interest to research ethics because it can drive the demographic shifts just described, which raises concerns about potential exploitation of subjects in multinational studies. It also arguably lends further support to the social value requirement of research since a resource shared by all researchers (including industry researchers) is in limited supply: human research subjects. Perhaps this means lower-value research ought not to be conducted, or the bar for what counts as a sufficiently socially valuable study should be raised (Borgerson 2016 ).
The relationship between funders, researchers, and subjects is also of interest to bioethicists. One of the reasons ethical concerns arise in LMICs is that the research is often funded and designed in HICs, and this raises worries about potential exploitation. Another concern arises when the results of the research conducted in LMICs won’t benefit other people in those same populations. One of the reasons given for the increased interest in conducting research in LMICs is that populations are “treatment-naive”—this means in general they don’t have access to healthcare, and they are likely to be unable to afford whatever treatment emerges from the research if it is successful. This feature of multinational research has generated extensive discussion among bioethicists, many of whom now agree that research should be responsive to the health needs of local populations if it is to avoid charges of exploitation. Yet this worrisome overview of the global situation was provided in 2013:
Total global investments in health R&D (both public and private sector) in 2009 reached US$240 billion. Of the US$214 billion invested in high-income countries, 60% of health R&D investments came from the business sector, 30% from the public sector, and about 10% from other sources (including private non-profit organisations). Only about 1% of all health R&D investments were allocated to neglected diseases in 2010. Diseases of relevance to high-income countries were investigated in clinical trials seven-to-eight-times more often than were diseases whose burden lies mainly in low-income and middle-income countries. (Røttingen et al. 2013 , 1286)
Dandona et al. also found that research priorities were misaligned with the health needs of the local population in India specifically: “funding for some of the leading causes of disease burden, including neonatal disorders, cardiovascular disease, chronic respiratory disease, mental health, musculoskeletal disorders and injuries was substantially lower than their contribution to the disease burden” (2017, 309). The gap between funding priorities and disease burden has been of interest to economists, political scientists, and bioethicists alike for many years.
A roughly 70/30 split between industry funding and other sources is common in clinical research. Of the US$1.42 billion spent on health research in India in 2011–2012, “95% of this funding was from Indian sources, including 79% by the Indian pharmaceutical industry” (Dandona et al. 2017 , 309). In the United States, “Principal research sponsors in 2003 were industry (57%) and the National Institutes of Health (28%)” (Moses et al. 2005 , 1333). Even though there seem to be shifts underway toward more industry-sponsored research in the clinical context, practicing physicians are still a vital part of research, often supplying the subjects for research:
Currently, about three-quarters of studies in the United States are conducted in the private sector by non-academic physicians who recruit their own patients or local community members into drug studies. Over 60,000 of these studies take place in the United States each year, accounting for 75 percent of the 80,000 clinical trials conducted worldwide; to execute these studies, more than 50,000 U.S. physicians registered with the Food and Drug Administration (FDA) as principal investigators on one or more clinical trials in 2001. As for the human subjects, 3.62 million Americans participated in pharmaceutical clinical trials in 2003 alone. (Fisher 2009 , 2)
The particular ethical obligations arising from the dual role of physician-investigators, such as the need to balance a commitment to doing what’s best for the patient with an interest in seeking knowledge, have received attention from bioethicists, as have the financial conflicts of interest arising when physicians play not only these two roles but a third role in their relationship to industry sponsors.
Another matter of interest to bioethicists is that there are some people who make a career out of being research subjects. Some of them self-identify as “guinea pigs for hire” and seek participation in phase I studies (on healthy subjects) (Lemmens and Elliott 2001 ). The inclusion of these people in studies raises ethical issues about appropriate compensation (whether wages and benefits or payment), undue inducement (if the payment is thought to be too high), scientific validity (whether people strongly oriented to please researchers so that they may be hired again will be more inclined to deceive researchers, for instance), upper limits of risk for studies under conditions of informed consent, and social justice and fair subject selection (since career research subjects tend to be from particular demographic groups, such as homeless people and students). For more on this issue, see the chapter by Fisher in this handbook.
Finally, a note about the number of research subjects: the research enterprise is massive and enrolls millions of human subjects every year. It is surprisingly difficult to get a clear picture of the enterprise globally (for more on why, see Young et al. 2015 ). Our best estimates come from clinical research: drawing on the 2009 CenterWatch Sourcebook, Sismondo suggests that, while estimates vary widely, there are approximately three to six million subjects involved each year (2018). And these numbers seem to be increasing. If we were able to add in figures from research in the social sciences, these numbers would skyrocket. In fact , you might be a research subject right now : there is ongoing debate over whether the tactics used by social media sites to track and manipulate their users qualify as human subjects research. If they do—and this turns partly on how we settle the issues raised in the first part of this chapter—we might find out that many of us are unwitting research subjects.
Which Subjects?
Who counts as a human subject of research? Codes of research ethics are often inclusive in their definition, for instance: “The World Medical Association (WMA) has developed the Declaration of Helsinki as a statement of ethical principles for medical research involving human subjects , including research on identifiable human material and data ” (World Medical Association, 2013 , 2191, emphasis added). Codes that initially had a narrow focus on medical research have expanded over time to cover new areas, for instance, increasing interest in the storage and use of biospecimens in research (biobanking) led CIOMS to merge its ethical guidelines for epidemiological research and biomedical research in 2016 (CIOMS, xi). Guidelines for research on human subjects are likely to be inclusive about who or what counts as a subject because many of the same ethical issues—privacy and informed consent, for instance—arise whether the subject’s body, tissues, or data are manipulated. In jurisdictions like Canada “human subjects” includes embryos and cadavers, though in the United States (ex vivo) embryos and cadavers are excluded from the definition. This doesn’t necessarily mean the use of cadavers, for instance, is unregulated but rather that it may be regulated differently. In all cases, the potential for harm to an identifiable person raises ethical concern.
If a central focus of research ethics is the prevention or minimization of harm, what then are we to make of the differences between the way humans and nonhuman animals are treated by researchers? The latter are not regarded as human subjects, even on inclusive definitions. And yet the “vast majority of biomedical research activity is conducted on animals or their tissues, cells, or even parts of cells” (Levine 2008 , 214). While research on human subjects is guided by ethical principles such as respect for persons, informed consent, fair subject selection, and a favorable risk–benefit ratio, research on nonhuman animals is typically governed by different approaches, such as the 3R framework: reduction (in numbers of animals used), refinement (improving the conditions for the animals), and replacement (using animals with lower capacity for pain or computer models when possible). Clear demarcation criteria, separating humans from other animals, have been very hard to come by, particularly as researchers discover more about the intellectual, emotional, and social capacities of a wide range of animals from crows to chimpanzees to elephants. The issue of which beings “count” morally, then, is unsettled, and as a result these divisions between the ethical principles applied in each domain rest on unsettled foundations.
It is good to keep this in mind because for many areas of research from neuroscience to immunology animal research is the basis for human research. You can’t typically propose a clinical trial on humans without convincing evidence from animal studies that the intervention might be successful. This expectation persists even though animals are never going to be ideal models for human behaviors, diseases, or functions—the ideal model for humans is humans. The reason why research doesn’t just skip over animals and start with humans, then, is ethical: it is thought to be acceptable to subject animals to risks that we find unacceptable for humans. There are deep and important issues here regarding the relative value of different lives. These issues surface in research ethics when trade-offs are proposed between human and animal studies. For instance, if “higher-level” animals like chimpanzees are no longer used in animal research (for ethical reasons), this may mean that the results from studies on the replacement animals (e.g., mice) lead to more uncertainty in the leap from animal to human studies. But this is at odds with a commitment to reduce harms to human research subjects. It will not be straightforward to find the right ethical path through these trade-offs (for one recent attempt, see Johnson and Barnard 2014 ). But if it is concern about harm to the interests of sentient beings that drives us toward research ethics oversight, we can’t proceed without attending to these difficult decisions. In the discussion of human subjects of research in section 2.1, I reviewed concerns about exploitation that arise when one group is harmed for the benefit of another. If the moral status of animals is even somewhat higher than that of inanimate objects, similar issues will arise when humans extract knowledge for our benefit from the bodies of animals.
In the future, we may have to decide how and whether to proceed with research on advanced forms of artificial intelligence or other nonhuman intelligent beings. So these gaps and unsettled foundations might matter to whether our current divisions between humans and other species are defensible in the long run.
In sum, neither “research” nor “human subjects” is easily defined, and efforts to use these concepts to draw black-and-white ethical lines around activities will struggle with a continuous and growing body of boundary cases. This is a productive realization since it helps us to see the gaps in current regulations (ethical concerns extend far beyond those captured in such regulations) and envision and work toward more efficient and nuanced systems of ethical accountability, such as those aspired to in systems aiming for the deep integration of research and practice (for more on these alternatives, see the chapter by Kim in this handbook). But it is also helpful for those of us working within current systems since it reminds us of the need to keep our focus on what we’re worried about, whether that’s exploitation, disrespect, scientific misconduct, power imbalances within relationships, conflicts of interest, violations of privacy, injustice in the selection of research participants, or opportunity costs when healthcare resources are used inefficiently. And our worries need to be responsive to an ever-changing reality: the internationalization of research, in particular, may well create new incentives (or reinforce existing incentives) that push researchers toward activities that breach different ethical principles or breach well-established principles in new ways. We will always need to be ready to provide timely, creative, and well-grounded responses to new ethical violations.
This chapter aimed to accomplish two things: 1) provide an overview of the scope and practice of research with human subjects and 2) highlight some of the philosophical issues raised by any attempt to provide such an overview. Let us return to our opening cases to see if we can apply what we’ve learned. Using the aim of generalizable knowledge as an initial sorting mechanism, it seems all cases except the family physician who “experiments” with different treatments will qualify as research. This is consistent with most regulatory assessments (keeping in mind they will then exclude and exempt some research from review). And all interventions involve human subjects directly or—in the case of blood samples—indirectly and so would likely be included because identifiable individuals raise ethical concerns about consent and privacy. Embryo research is a tough case, and different jurisdictions and different scholars handle it differently; we didn’t attempt to settle the issue here. Note that while most of the cases turn out to be human subjects research on the most common definitions, we are left with lingering worries—for instance, why does the clinical practice case change from practice to research when it is written up for publication—forget about the regulations, what changes here ethically ? Hopefully it is clear that while some things are settled in this domain, there is still much to work out. As is so often the case in philosophy, even the simplest question—"What is human subjects research?”—is harder to answer than it seems.
Beauchamp, Tom L. , and Yashar Saghai . 2012 . “ The Historical Foundations of the Research–Practice Distinction in Bioethics. ” Theoretical Medicine and Bioethics 33: 45–56.
Google Scholar
Becker, Howard S. 2004 . “ Comment on Kevin D. Haggerty, ‘Ethics Creep: Governing Social Science Research in the Name of Ethics. ’” Qualitative Sociology 27, no. 4: 415–416.
Borgerson, Kirstin . 2016 . “ An Argument for Fewer Clinical Trials. ” Hastings Center Report 46: 1–11.
Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, and Social Sciences and Humanities Research Council of Canada. 2018 . Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans Ottawa, ON, Canada: Secretariat on Responsible Conduct of Research. http://www.pre.ethics.gc.ca/eng/policy-politique/initiatives/tcps2-eptc2/Default/ .
Google Preview
CBC News. 2019. “Ethics Board Must Rule on Projects within 30 Days: N.L. Supreme Court Judge.” https://www.cbc.ca/news/canada/newfoundland-labrador/sequence-bio-wins-court-application-for-ethics-board-timelines-1.5014596 .
Coleman, Carl H. 2019 . “ Rethinking the Regulatory Triggers for Prospective Ethics Review. ” Journal of Law, Medicine & Ethics 47: 247–253.
Council for International Organizations of Medical Sciences (CIOMS). 2016. “International Ethical Guidelines for Health-related Research Involving Humans.” Fourth Edition. Geneva. https://cioms.ch/publications/product/international-ethical-guidelines-for-health-related-research-involving-humans/
Dandona, Lalit , Rakhi Dandona , G. Anil Kumar , Krycia Cowling , Pritty Titus , Vishwa Mohan Katoch , et al. 2017 . “ Mapping of Health Research Funding in India. ” National Medical Journal of India 30: 309–316.
Fisher, Jill A. 2009 . Medical Research for Hire: The Political Economy of Pharmaceutical Clinical Trials . New Brunswick, NJ: Rutgers University Press.
Fluehr-Lobban, Carolyn . 2002 . “ A Century of Ethics and Professional Anthropology. ” AAA Anthropology News 43, no. 3: 20.
Geller, Stacie E. , Abigail R. Koch , Pamela Roesch , Amarette Filut , Emily Hallgren , and Molly Carnes . 2018 . “ The More Things Change, the More They Stay the Same: A Study to Evaluate Compliance with Inclusion and Assessment of Women and Minorities in Randomized Clinical Trials. ” Academic Medicine 93, no. 4: 630–635.
Haggerty, Kevin D. 2004 . “ Ethics Creep: Governing Social Science Research in the Name of Ethics. ” Qualitative Sociology 27, no. 4: 391–414.
Henrich, Joseph , S. J. Heine , and A. Norenzayan . 2010 . “ The Weirdest People in the World? ” Behavioural and Brain Sciences 33, no. 2–3: 61–83.
Humphreys, Keith , Natalia C. Maisel , Janet C. Blodgett , Ingrid L. Fuh , and John W. Finney . 2013 . “ Extent and Reporting of Patient Nonenrollment in Influential Randomized Clinical Trials, 2002 to 2010. ” JAMA Internal Medicine 173: 1029–1031.
Johnson, Jane , and Neal D. Barnard . 2014 . “ Chimpanzees as Vulnerable Subjects in Research. ” Theoretical Medicine and Bioethics 35, no. 2: 133–141.
Kass, Nancy E. , Ruth R. Faden , Steven N. Goodman , Peter Pronovost , Sean Tunis , and Tom L. Beauchamp . 2013 . “ The Research–Treatment Distinction: A Problematic Approach for Determining which Activities Should Have Ethical Oversight.” In “Ethical Oversight of Learning Health Care Systems, ” special report, Hastings Center Report 43, no. 1: S4–S15.
Lemmens, Trudo , and Carl Elliott . 2001 . “ Justice for the Professional Guinea Pig. ” American Journal of Bioethics 1, no. 2: 51–53.
Levine, Robert J. 2008 . “The Nature, Scope, and Justification of Clinical Research.” In The Oxford Textbook of Clinical Research Ethics , edited by Ezekiel J. Emanuel , Christine Grady , Robert A. Crouch , Reidar K. Lie , Franklin G. Miller , and David Wendler , 211–221. Oxford: Oxford University Press.
McDonald, Alison M. , Rosemary C. Knight , Marion K. Campbell , Vikki A. Entwistle , Adrian M. Grant , Jonathan A. Cook , et al. 2006 . “What Influences Recruitment to Randomised Controlled Trials ? A Review of Trials Funded by Two UK Funding Agencies.” Trials 7: 9.
Moses, Hamilton , E. Ray Dorsey , David H. M. Matheson , and Samuel O. Thier . 2005 . “ Financial Anatomy of Biomedical Research. ” Journal of the American Medical Association 294, no. 11: 1333–1342.
National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. 1979 . The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research . https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/read-the-belmont-report/index.html
Nuremberg Code. 1949 . “Permissible Medical Experiments.” In Trials of War Criminals before the Nuremberg Military Tribunals under Control Council Law No. 10, Vol. 2, 181–182. Washington, DC: US Government Printing Office.
Røttingen, John-Arne , Sadie Regmi , Mari Eide , Alison J. Young , Roderik F. Viergever , Christine Årdal , et al. 2013 . “ Mapping of Available Health Research and Development Data: What’s There, What’s Missing, and What Role Is There for a Global Observatory? ” Lancet 382, no. 9900: 1286–1307.
Shansky, Rebecca M. 2019 . “ Are Hormones a ‘Female Problem’ for Animal Research? ” Science 364, no. 6443: 825–826.
Sismondo, Sergio . 2018 . Ghost-Managed Medicine: Big Pharma’s Invisible Hands . Manchester, UK: Mattering Press.
Taylor, Judith , and Matthew Patterson . 2010 . “ Autonomy and Compliance: How Qualitative Sociologists Respond to Institutional Ethical Oversight. ” Qualitative Sociology 33, no. 2: 161–183.
World Health Organization. 2019 . Number of Grants for Biomedical Research by Funder, Type of Grant, Duration and Recipients (World RePORT) . Geneva: World Health Organization. https://www.who.int/research-observatory/monitoring/inputs/world_report/en/ .
World Medical Association. 2013 . “ World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. ” Journal of the American Medical Association 310, no. 20: 2191–2194.
Young, Alison J. , Robert F. Terry , John-Arne Røttingen , and Roderik F. Viergever . 2015 . “ Global Trends in Health Research and Development Expenditures—The Challenge of Making Reliable Estimates for International Comparison. ” Health Research Policy and Systems 13: 7.
I use the generic term “research ethics committee” in this chapter to refer to the committee providing prospective ethical review of research. In some jurisdictions these have other names, such as “research ethics boards” or “institutional review boards.”
The document goes on to say, “In some cases it can be difficult to make this distinction, underscoring the need to have reviewers or ad hoc advisors . . . who can assist with this determination” (p. 14). This highlights a lesson articulated in this section of the chapter: it is not easy to determine which activities are “research.”
I use the term “boundary cases” here to refer to any study designs that can’t be easily classified as “research” or “practice.” As the examples indicate, this includes “hybrid” or “overlap” activities which intentionally blend research and practice, such as those found in proposed models for learning health care systems.
This is one of the (many) reasons why scholars are so interested in the design and pursuit of learning health care systems in which the research–practice distinction is downplayed or eliminated and new mechanisms of accountability are explored. This and related issues are discussed by Kim elsewhere in this handbook.
The TCPS 2 has a very specific scope: it only covers research funded by the three federal funding agencies in Canada. Other parties, such as independent or private researchers and funders in Canada, typically agree to abide by these rules; but only researchers funded through these agencies are strictly bound by them.
A recent court decision in Newfoundland and Labrador, Canada, for instance, sets this “reasonable” window at 30 days (CBC News 2019 ).
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

- Get Started
- Determine Protocol Type
- Not Human Subjects Research and Not Research
Case Study Types
Is my case study considered human subjects research, retrospective case study review/report.
- Generally completed by a retrospective review of medical records that highlights a unique treatment, case, or outcome
- Often clinical in nature
- A report about five or fewer clinical experiences or observations identified during clinical care
- Does not involve biospecimens or FDA-regulated products (e.g., drugs, devices, biologics) that have not been approved for use in humans
- Does not include articles requiring exemption from FDA oversight
- Does not include articles under an IND/IDE
Prospective Single Subject Case Study Review/Report
- Often social/behavioral in nature
- In-depth prospective analysis and report involving unique or exceptional observations or experiences about one, or a few, individual human subjects
- Is intended to contribute to generalizable knowledge
Defining Research with Human Subjects
A study is considered research with human subjects if it meets the definitions of both research AND human subjects, as defined in the federal regulations for protecting research subjects.
Research. A systematic inquiry designed to answer a research question or contribute to a field of knowledge, including pilot studies and research development.
Human subject: A living individual about whom an investigator (whether professional or student) conducting research:
- Obtains information or biospecimens through intervention or interaction with the individual, and uses, studies, or analyzes the information or biospecimens; or
- Obtains, uses, studies, analyzes, or generates identifiable private information or identifiable biospecimens.
The following sections will explain some of the words in the previous definitions.
The regulatory language:
A systematic inquiry designed to answer a research question or contribute to a field of knowledge, including pilot studies and research development.
The explanation:
Understanding what constitutes a systematic inquiry varies among disciplines and depends on the procedures and steps used to answer research questions and how the search for knowledge is organize and structured.
Pilot Studies and Research Development
Pilot studies are designed to conduct preliminary analyses before committing to a full-blown study or experiment.
Research development includes activities such as convening a focus group consisting of members of the proposed research population to help develop a culturally appropriate questionnaire.
Practical applications:
- You are conducting a pilot study or other activities preliminary to research; or
- You have designed a study to collect information or biospecimens in a systematic way to answer a research question; or
- You intend to study, analyze, or otherwise use existing information or biospecimens to answer a research question.
Human Subjects
Human subjects are living individuals about whom researchers obtain information or biospecimens through interaction, intervention, or observation of private behavior, to also include the use, study, and analysis of said information or biospecimens.
Obtaining, using, analyzing, and generating identifiable private information or identifiable biospecimens that are provided to a researcher is also considered to be human subjects.
To meet the definition of human subjects, the data being collected or used are about people. Asking participants questions about their attitudes, opinions, preferences, behavior, experiences, background/history, and characteristics, or analyzing demographic, academic or medical records, are just some examples of human subjects data.
- Interacting with people to gather data about them using methods such as interviews, focus groups, questionnaires, and participant observation; or
- Conducting interventions with people such as experiments or manipulations of subjects or subjects' environments; or
- Observing or recording behavior, whether in-person and captured in real time or in virtual spaces, like social media sites (e.g., Twitter) or online forums (e.g., Reddit); or
- Obtaining existing information about individuals, such as students’ school records or patients’ health records, or data sets provided by another researcher or organization.
Interactions and Interventions
Interventions are manipulations of the subject or the subject's environment, for example is a behavioral change study using text messages about healthy foods.
Interactions include communication or interpersonal contact between investigator and participant.
A study may include both interventions and interactions.
Interactions and interventions do not require in-person contact, but may be conducted on-line.
Private Information
Private information includes information or biospecimens: 1) about behavior that occurs in a context in which an individual can reasonably expect that no observation or recording is taking place; 2) that has been provided for specific purposes by an individual; and 3) that the individual can reasonably expect will not be made public (for example, a medical record).
Private information must be individually identifiable (i.e., the identity of the subject is or may readily be ascertained by the investigator or associated with the information) in order for the information to constitute research involving human subjects.
The regulations are clear that it is the subjects’ expectations that determine what behaviors, biospecimens, and identifiable information must be considered private. Subjects’ understanding of what privacy means are not universal, but are very specific and based on multiple interrelated factors, such as the research setting, cultural norms, the age of the subjects, and life experiences. For example, in the United States, health records are considered private and protected by law, but in some countries, health information is not considered private but are of communal concern.
Identifiable Information
The identity of the subject is associated with the data gathered from the subject(s) existing data about the subjects. Even if the data (including biospecimens) do not include direct identifiers, such as names or email addresses, the data are considered identifiable if names of individuals can easily be deduced from the data.
If there are keys linking individuals to their data, the data are considered identifiable.
Levels of Review
Not all projects that meet the definition of research with human subjects need review by the actual committee. For example, projects that pose negligible risk to participants may be reviewed and recommended for approval by IRB staff ; other projects may need to undergo review and approval by at least one member of the IRB committee or a quorum of the full board. Determination as to the need for review should always be made by the IRB staff.
Examples of Studies That MAY Meet the Definition of Research with Human Subjects
The following examples will likely require further consultation with an IRB staff member.
Analysis of existing information with no identifiers
If researchers have no interaction with human subjects, but will be conducting a secondary analysis of existing data without individual identifiers, the analysis of those data may not be research with human subjects.
Expert consultation
Key words in the definition of a human subject are "a living individual about whom" a researcher obtains, uses, studies, analyzes, or generates information. People can provide you information that is not about them but is important for the research. For example, a researcher may contact non-governmental organizations to ask about sources of funding.
Program evaluations and quality improvement studies
Program evaluations are generally intended to query whether a particular program or curriculum meets its goals. They often involve pre- and post-surveys or evaluations.
Some program evaluations include a research component. If data are collected about the characteristics of the participants to analyze the relationship between demographic variable and success of the program, the study may become research with human subjects. Research question: Are there different learning outcomes associated with different levels of participant confidence?
Classroom research
Classes designed to teach research methods such as fieldwork, statistical analysis, or interview techniques, may assign students to conduct interviews, distribute questionnaires, or engage in participant observation. If the purpose of these activities is solely pedagogical and are not designed to contribute to a body of knowledge, the activities do not meet the definition of research with human subjects.
Vignettes: Applying the Definitions
Art in Cambodia
An art history student wants to study art created by Cambodians in response to the massacres committed by the Khmer Rouge. The art she will study includes paintings, sculpture, video, and the performing arts.
Much of the research will be archival, using library and online resources. In addition, she will visit Cambodia. While there, she will speak with several museum curators for assistance locating and viewing art collections related to the massacres.
Is this research with human subjects?
No. Although the student will speak with curators, they are not the subjects of her research and she is not interested in learning anything about them. They will, in effect, serve as local guides.
What would make the study research with human subjects?
The student interviews people as they interact with art to understand the role of the arts in evoking and/or coming to terms with traumatic past events. She interviews people who view the art, such as visitors to museums, and discusses what the art means to them. She may collect information about their experiences during the genocide and compare those experiences with their reactions to the art.
Bank-Supported Micro-Finance in Chile
A researcher is interested in the practice of microfinance in the Chilean Mapuche community. She meets with bankers and asks about the criteria for granting loans, the demographics of the people who receive loans, the types of businesses to which the bank prefers to grant loans, how many loans they give, the payback rates, and other data about the bank’s loan practices.
No. Although the researcher is interviewing bankers, the bankers are only providing information about their banking practices and are not providing any information about themselves. The questions are about “what” rather than “about whom.” The bankers are not human subjects. This type of interview is sometimes referred to as expert consultation.
The researcher explores the impact of small loans, both intended and unintended, on the recipients of the loans. The researcher interviews the recipients of the loans and gathers information from them about their lives before and after they received funding, how the loans affected their relationships with family members and other community members, the impact of the loans on their aspirations, and so on. He asks “about whom” questions designed to understand the impact of micro-loans.
Developing Teaching Materials
A researcher goes to a country in which the infrastructure has been severely damaged to help rebuild schools. The student interviews community members about what curricular materials they need, develops some materials, and teaches a math class.
No. Although interviews are conducted, the intent of interviewing is to assist in resource development rather than answer a research question designed to contribute to a field of knowledge.
If the researcher does pre- and post-testing to assess student learning in his class, is this research with human subjects?
No. The intent is to find out if the materials are effective. This is sometimes referred to as program assessment.
What would make this research with human subjects?
The researcher studies the impact of nutrition and personal variables on learning. He assesses the nutritional composition of the local diet, assesses students’ general health, and compares those data with test scores. He also measures motivation, family composition, and other characteristics of the students using written questionnaires.
Water Conservation
A researcher wants to find out if the campus water conservation program is effective. She will gather some information about water volume usage from the University engineering department. She will also survey residential students about their water usage habits over the last six months, their perceptions of the campus drought education program, and their reactions to the incentives offered by the program (water-saving competitions, free water-saving devices, etc.) She will report her findings to the program’s steering committee and administrators.
No. Although the researcher will systematically survey other students and will be collecting information about them, her intention is to assess the effectiveness of the conservation program.
The researcher designs an online survey to collect information that may help understand factors that influence the residential students’ responses to the conservation program. She asks questions about green attitudes and behaviors, positions on social and political issues, as well as motivation and narcissism.
Campus IRB Guides
The Case for Human Subjects in Research and Teaching
Research on humans.
Research on human subjects is essential for developing new tests, treatments, and processes that save untold lives and reduce human suffering. For cases in which alternatives such as cell cultures, computer models, or research involving animals are insufficient, ethical and properly controlled studies with human subjects may shed light on important questions about normal physiology, mechanisms of disease, learning, behavior, or the effectiveness of treatments. Many participants consent to take part in research studies because they hope their participation may benefit society.
What Is Involved in Human Subject Research?
Most of the research employing human participants at the University of Massachusetts Amherst is non-invasive in nature. For example, some studies focus on investigating attitudes or beliefs while others aim to develop interventions or treatments to improve human health and well-being. Methods of data collection include surveys, interviews, focus groups, behavioral observations, and analysis of data that was previously collected for non-research purposes. Some research studies consist of behavioral tasks that involve problem-solving, performing exercise, or responding to visual or auditory stimuli. Researchers at UMass Amherst also conduct biomedical research that may include analysis of biospecimens or use of imaging techniques such as MRI. Typically, riskier invasive studies have been relatively rare on our campus. When high-risk studies are proposed, federal regulations mandate review and approval by the entire convened Institutional Review Board (IRB) .
Ethical Considerations of Human Subject Research
Research involving human subjects at UMass Amherst is conducted with the utmost respect for research ethics and the rights and welfare of the participants. Participants in research studies at UMass have the right to be fully informed about the purpose of the research study, what the study entails, and any risks or expected benefits of the research prior to their participation. Research participation must be voluntary and as such, participants have the right to withdraw from a study at any time without penalty. Participants are informed about their rights via the informed consent process.
Rules and Regulations
Due to the valued findings that research with human subjects can provide, it is of the utmost importance that researchers and participants alike are knowledgeable about the rules and regulations involved in the research process. All research involving human subjects is overseen by the Institutional Review Board (IRB), a federally required committee responsible for ensuring that human research participants are not mistreated and that their rights are protected. The UMass Amherst IRB consists of 15 members from various disciplines and backgrounds, including a community member, a non-scientific member, two physicians, and several faculty members from a range of academic backgrounds.
For more information on human subjects research, protocols, and oversight, please visit the Human Subjects FAQ .
Search form
©2024 University of Massachusetts Amherst • Site Policies • Site Contact

- NIH Grants & Funding
- Blog Policies
NIH Extramural Nexus
Your Questions on Human Subjects Research, Answered
If you’re conducting research with human subjects, you might have questions about HHS regulations and NIH policies and how you can prepare proposals and inclusion plans. HHS and NIH experts address many of these questions, along with resources, guidance, and case studies in a 2-part webinar series focused on Human Subjects Research Policies, Clinical Trials, and Inclusion ( Day 1 and Day 2 ).
Feel free to break out the popcorn and binge the whole video or check out these sections for the information you need:
- How Do I Know If A Research Study Is Human Subjects Research?
- What to Know About FWAs and IRBs to Get Your Grant Money?
- An Overview of NIH Policies on Human Subjects
- What are the Essentials of sIRB Requirements?
- An Overview of NIH Policies on Clinical Trials
- Including Diverse Populations in NIH Clinical Research
- Using the eRA Human Subjects System (HSS)
For presentation slides and other resources, see the event page . If you haven’t already, register for the 2023 Virtual NIH Grants Conference , February 1-2, for even more NIH policy guidance and resources. While in the grants conference center, be sure to visit the virtual Exhibit Hall and the booth, Human Subjects, Clinical Trials, and Inclusion for downloadable resources, links, and the opportunity to chat with experts during the event.
RELATED NEWS
Before submitting your comment, please review our blog comment policies.
Your email address will not be published. Required fields are marked *

Site Search
- How to Search
- Advisory Group
- Editorial Board
- OEC Fellows
- History and Funding
- Using OEC Materials
- Collections
- Research Ethics Resources
- Ethics Projects
- Communities of Practice
- Get Involved
- Submit Content
- Open Access Membership
- Become a Partner
Topics: Human Subjects
A guide that provides information and resources on teaching responsible conduct of research that focuses on the topic of human subjects. Part of the Resources for Research Ethics Education Collection.
What is Research Ethics
Why Teach Research Ethics
Animal Subjects
Biosecurity
Collaboration
Conflicts of Interest
Data Management
Human Subjects
Peer Review
Publication
Research Misconduct
Social Responsibility
Stem Cell Research
Whistleblowing
Descriptions of educational settings , including in the classroom, and in research contexts.
Case Studies
Other Discussion Tools
Information about the history and authors of the Resources for Research Ethics Collection
- Critically evaluate the decision to conduct research with human subjects Both the spirit of the regulations and good science require that individuals give thoughtful consideration to the decision to conduct research with human subjects.
- Comply with regulations No research study of human subjects should be carried out that is not explicitly part of an approved protocol.
- Protect individual rights to self-determination The decision to participate in research should be based on truly informed consent. This means that researchers have an ongoing obligation to ensure that subjects understand the risks and benefits of participation, which should continue only if the subjects (or their surrogates) freely agree to remain in the study.
- Promote responsible use of human subjects If you are responsible for training others or if you observe indifference to considerations for human subjects in research studies, you should make attempts to initiate discussion, to identify relevant regulations, and to promote responsibility. If violations of regulations are observed, then those observations should be reported to the appropriate people in the institution.
Advances in human health and welfare ultimately depend on research with human subjects. Properly designed and controlled studies with human subjects are essential to verify hypotheses about normal physiology, behavior, mechanisms of disease, processes of learning, or effectiveness of treatments. Unfortunately, not all human studies have been justifiable and useful; human cruelty has sometimes been perpetrated in the name of research. Some of the best known examples of such cruelty occurred in Nazi Germany. Investigations following the war uncovered many atrocities, such as studies in which subjects were immersed in very cold water to gauge how long it would take to die of hypothermia. The discoveries of these abuses were the basis for the Nuremberg trials and development of the Nuremberg Code (1949), the first international codification of minimal expectations for the conduct of research involving human subjects. One of the most important provisions of the Code is that "the voluntary consent of the human subject is absolutely essential;" other provisions indicate that experiments with human subjects should occur only in the context of a clear scientific rationale.
Harm to unwilling subjects under the guise of research was not unique to the Nazis. During World War II, the United States conducted medical experiments on those not competent to consent and on subjects without their knowledge (Vanderpool, 1996). In one instance, beginning in 1932 and prior to the start of World War II, 400 African American males with syphilis were entered into a study at Tuskegee, Alabama with the intended purpose of documenting the natural course of their disease (Rivers et al., 1953; Jones, 1993). Although treatments of presumed efficacy were available, these were withheld while the study participants were led to believe that experimental procedures (such as spinal taps to examine cerebrospinal fluid) were for the purpose of therapy. By the 1950s, penicillin was available and known to be highly effective against syphilis, but it also was withheld. The surviving participants were only given treatment in 1972, after the nature of the study became publicly known -- 23 years after publication of the Nuremberg Code.
Recognition of these, and other, problematic studies (e.g., reviewed by Beecher, 1966) published in the medical and social science literature resulted in the appointment of a federal commission to identify fundamental principles that should govern human subjects studies. The final product of this commission was the Belmont Report (1979). It defined the three ethical principles (listed below) that now guide studies with human subjects in the U.S.
- Respect for persons "Respect for persons incorporates at least two ethical convictions: first, that individuals should be treated as autonomous agents, and second, that persons with diminished autonomy are entitled to protection."
- Beneficence "Two general rules have been formulated as complementary expressions of beneficent actions in this sense: (1) do not harm and (2) maximize possible benefits and minimize possible harms."
- Justice "An injustice occurs when some benefit to which a person is entitled is denied without good reason or when some burden is imposed unduly... "
At least three important premises underlie these principles. The first is that studies with human subjects are necessary for improvements in health and welfare. Second, to conduct such research is a privilege, not a right, extended to researchers by society, institutions, and the research subjects themselves. Finally, neither the risks nor the costs of any research study should outweigh the likely benefits.
Regulations and Guidelines
Numerous federal agencies have regulations governing the conduct of research involving human subjects. Examples of agencies with human subject requirements include the Department of Health and Human Services (DHHS), the Food and Drug Administration (FDA), the National Science Foundation (NSF), and the Departments of Defense, Education, Justice, and Veterans Affairs.
Human subject protections are a shared responsibility of principal investigators, other personnel involved in studies with human subjects, and the Institutional Review Board (IRB). The IRB is a primary mechanism for federally-mandated institutional protection of human subjects. An IRB is designed to be an advocate for potential and actual research subjects. Under both DHHS and FDA regulations, the IRB is responsible for approving or disapproving all covered research activity, requiring for instance that subjects are given enough information to be able to provide informed consent. The IRB must conduct periodic reviews of research to ensure continued protection of the welfare of human subjects and compliance with relevant regulations.
Different agencies define "human subject" in different ways, but the definition includes (at minimum) any living person who is involved in research either as an experimental subject or as a control. The scope of activities included under the definition of "research" is broad. One federal regulation defines research as any "systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge. Activities which meet this definition constitute research for purposes of this policy, whether or not they are conducted or supported under a program which is considered research for other purposes. For example, some demonstration and service programs may include research activities." (Code of Federal Regulations for Department of Health and Human Services 45CFR46.102(d)).
In addition to the above regulatory oversight, because of concerns about the protection of human subjects, the Department of Health and Human Services also requires the education of all key personnel working on PHS-funded studies that involve human subjects (NIH, 2000).
Case Study 1
Dr. Jacqui is a psychiatrist interested in the molecular basis of several different anxiety disorders. Accordingly, she requested institutional approval to collect blood samples from a large population of both affected and unaffected individuals. The plan is to identify unusual genes that occur in the affected population. After receiving approval, numerous individuals, including staff and colleagues from Dr. Jacqui's medical center, were enrolled after signing the requisite consent form. Along with many other pieces of information, the form specifically (a) notifies the volunteer that he/she will be told at the end of the study whether or not they carry the genes in question and (b) prompts the volunteer to ask any questions they might have. The blood samples are then collected. In the process of testing and refining methodology, one of Dr. Jacqui's postdocs runs a "negative control" by screening the samples for the presence of a rare gene he has recently identified in a collaborative project with another laboratory studying breast cancer. Surprisingly, this gene is detected in one of the samples. Evidence to date is that this gene carries a small, but significant, increased likelihood of developing several different forms of metastatic cancer, and an estimated 70% likelihood for developing breast cancer. If known, the individual would increase their chances for long-term survival by prophylactic mastectomy and frequent check-ups. However, these measures would only decrease, not eliminate, the risk of developing cancer. The postdoc reports these findings to Dr. Jacqui. A quick look through the records allows Dr. Jacqui to identify the carrier of this gene as a clerk working in hospital admissions. Dr. Jacqui debates whether or not to tell the clerk what has been found. After some deliberation, she decides against telling the clerk because (a) the gene does not guarantee that the clerk will get cancer and (b) the clerk's wishes about whether or not she would like this information are not known. Questions: 1. Consider the roles of Dr. Jacqui, the institutional review board, the postdoc, and the clerk in this case. Given the information provided, have any of them erred by acts of commission or omission?
Case Study 2
Alonzo Garcia, M.D., entering his fellowship in pulmonary medicine, was assigned to carry out an approved study designed by Dr. Bruce Sedgwick, his training director. The study entails the use of the recombinant enzyme, DNAse, to improve pulmonary function and to reduce the incidence of new infections in patients with cystic fibrosis. The study was double-blinded, meaning that neither he nor his patients and their families know whether the inhaler the patient received contained the active enzyme or a placebo. Experiments conducted in vitro and in small animals indicated great success for the agent, though they also pointed to some potential problems. These included development of an allergic reaction to the enzyme or to the other materials in the inhaler, and a direct and adverse chemical effect on the lung passages. The clinical study for which Dr. Garcia was responsible consisted of a three-month trial of enzyme or placebo, a one-month drug-free period, then a three-month trial of the other arm of the study. Forty patients were to be entered by the completion of the trial. They were to be evaluated by a lung function test and, since most of the patients were children, by a standardized questionnaire completed by a parent. The experimental drug was so effective that shortly after the trial began, Dr. Garcia found it easy to know who was receiving active enzyme and who was receiving placebo. Even though the questionnaire filled out by the patients' parents was uniform, he discovered himself encouraging respondents to comment about the beneficial effects of the enzyme. After 20 patients were entered in the trial, one of the parents, who happened to be a scientist, said to Dr. Garcia, "The quality of our daughter's life has greatly improved since she was entered in this protocol. Clearly, the drug is having an enormous impact that cannot be ignored and the blinding must be stopped. Won't you ask the company to terminate the experiment and make DNAse available to us? It will save the lives of our children." Dr. Garcia responded that the statistical power of the study was not sufficient to determine whether an effect would occur in one percent of subjects, nor the duration long enough to obtain meaningful data on infections. For that, a different experiment, monitoring the treatment of large numbers of subjects over a longer period of time would be required. Since the degree of improvement was sufficient to demonstrate efficacy after only 20 subjects, there was no need to continue to employ the placebo controls and to continue with the current study. Dr. Sedgwick pointed out that the FDA required 40 cases from their institution for the efficacy study and their requirements took priority over the statistical analysis in most cases. Questions: 1. What values are in conflict in this case? How would you approach their resolution? 2. The grossly apparent effectiveness of DNAse in cystic fibrosis seems to have ruined the blinding of subject and investigator that protects against biased reporting of efficacy. What can or should be done about that in the context of this experiment? What about in the broader context of clinical trials? 3 .The FDA plays a critical role in the design of studies intended to achieve approval of a new therapeutic agent. In fact, companies negotiate in advance with the agency to ensure that, if the study is successful, the agent will be approved. Is this in the best interest of the patient, the company, and society?
Case Study 3
The Pernkopf Atlas is one of many examples of information, which was, or may have been, made possible by the use of unwilling subjects in Nazi Germany. Another well-known example is a series of experiments involving extreme hypothermia. Consider the following questions: 1. Should data obtained under such circumstances be used? 2. On what principle(s) is/are your opinion based? Please note that you are NOT being asked to judge whether the Pernkopf Atlas drawings were made using Holocaust victims and you are NOT being asked to judge whether these or other experiments conducted in Nazi Germany lacked scientific merit.
Discussion Questions
- To what extent does your field of work depend on research involving human subjects? To what extent is your work intended to benefit human welfare?
- Describe at least one historical example of unethical studies involving human subjects. Identify federal regulations that are apparent responses to such abuses.
- List and explain the three ethical principles of the Belmont Report for research involving human subjects.
- If you are involved in research with human subjects, which federal agencies have oversight for your work?
- What are the responsibilities of an Institutional Review Board (IRB)?
- In your institution, what kinds of research, if any, with human subjects do not need to be presented to an Institutional Review Board for consideration?
- In your institution, what minimal changes to your protocol require review and approval of the IRB? What changes are of a magnitude to require submission, review, and approval of a new protocol?
- If you observed another investigator violating principles or regulations governing the study of human subjects, who should be notified?
- What forums are available in your institution to examine the ethical and/or legal ramifications of studies with human subjects? What, if anything, can you do to promote such discussion?
Additional Considerations
- Regulations Research that involves humans is subject to regulation. No procedure or study should be performed that is not explicitly exempted or a part of an approved protocol. Applicable regulations include requirements for adherence to IRB-approved research protocols, maintenance of documentation and records, obtaining approval prior to initiation of changes, and reporting of adverse events. Investigators are responsible for identifying all applicable regulations and complying with them.
- Responsible conduct Responsible conduct of research involving human subjects requires much more than complying with regulations. The spirit of the regulations and of good science both require that researchers critically review what is known and give thoughtful consideration to what defines an acceptable study. This consideration is necessarily an ongoing process. Some factors to be considered include changes in our best understanding of the science, of the risks and potential benefits, and of alternative methods for study. The decision to conduct a study with human subjects carries both ethical and regulatory responsibilities to protect the welfare and interests of those subjects, to conduct the study with a view to protecting the welfare and interests of those subjects, to design the study so as to minimize risks to subjects, and to obtain adequate training for protecting the interests and welfare of research subjects.
- Justification and necessity A prerequisite for responsible research involving humans is a realistic examination of the probability and magnitude of both the risks and the benefits of the research. Investigators must assess whether the risks are reasonable in relationship to the benefits to the individual subjects and the knowledge to be gained.
- Informed Consent as a Process Investigators conducting a research study with human subjects have an absolute responsibility to ensure that consent to participate has been given freely and is based on an understanding of the risks and benefits of the research. Informed consent is often needed even for studies in the social sciences that impose little or no inconvenience, but still present the risk of a loss of privacy or confidentiality. Although some costs or risks may be more injurious than others, it must be up to the potential research subject, not the research investigator, to decide whether such costs or risks are outweighed by the benefits of participation. The most visible indication of "informed consent" is a document to be signed by the research subject. This document is important because it provides a consistent body of information that the investigator and the IRB have agreed is necessary for individuals to provide their informed consent. Unfortunately, subjects may sign such forms without understanding them and, even if those forms were initially understood, changing circumstances may mean that the subjects are no longer truly informed. Therefore, informed consent is not a single event, but an ongoing process.
- Diminished Capacity to Consent Not all subjects are able to give truly informed consent. In some cases, it is difficult to ensure that consent is given freely, such as in prison populations. In other cases, it may be difficult to convey the necessary information or to verify an understanding in people with reduced decision-making capacity, such as some subjects with developmental disabilities, psychiatric disorders, or advanced dementia. In these cases, research investigators have an additional burden to meet ethical and regulatory obligations for protecting the right of self-determination for prospective or current research subject.
- OEC Human Subjects in Research Subject Aid A beginning point for anyone interested n learning more about human subjects in research, including relevant guidelines and good articles and readings to gain a better understanding of the topic.
- OEC Human Subjects and Informed Consent Bibiolgraphy A bibliography of books, online resources, and articles on issues of human subjects and informed consent in research.
- Annotated Bibliography: Conducting Human Genetics Research A short bibliography looking at the ethical issues that arise in conducting human genetic research. Issues of disclosure and informed consent are among those discussed.
Cited Resources
- Beecher HK (1966): Ethics and clinical research. New Engl J Med 274: 1354-1360.
- Belmont Report (1979) https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/index.html
- Declaration of Helsinki (2018) https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/
- DHHS (2005): Protection of Human Subjects, Title 45 Part 46 https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html
- Jones JH (1993): Bad Blood: The Tuskegee Syphilis Experiment. Free Press, New York
- NIH (2000): Required education in the protection of human research participants. Notice OD-00-039, June 5, 2000 http://grants.nih.gov/grants/guide/notice-files/NOT-OD-00-039.html
- The Nuremberg Code (1949) https://history.nih.gov/display/history/Nuremberg+Code
- Rivers E, Schumann SH, Simpson L, Olansky S (1953): Twenty years of followup experience in a long-range medical study. Public Health Reports 68(4): 391-395.
- Vanderpool HY (1996): The Ethics of Research Involving Human Subjects: Facing the 21st Century. University Publishing Group, Frederick, Maryland.
- Warren RC, Gabriele EF (2012): The Grammar of Power: The problem of moral objectification in human research. Journal of Research Administration 43(2):94-106.
The Resources for Research Ethics Education site was originally developed and maintained by Dr. Michael Kalichman, Director of the Research Ethics Program at the University of California San Diego. The site was transferred to the Online Ethics Center in 2021 with the permission of the author.
Related Resources
Submit Content to the OEC Donate

This material is based upon work supported by the National Science Foundation under Award No. 2055332. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
Lesson 2: What is Human Subjects Research?
Please note: This lesson will take approximately 1 hour and 35 minutes to complete. Use the next and previous buttons to advance through the course. You will be able to print a completion certificate for your records at the end of this training. OHRP does not collect information about who accesses it.
Do not refresh your browser. Refreshing your browser will restart the lesson.
Purpose of this Lesson
This lesson will explain how the Common Rule regulations define “research” and “human subjects” and explain what it means to be exempt from the regulations. This lesson focuses on the Revised Common Rule (or 2018 Requirements) that became effective in 2018.
Lesson Overview
This lesson contains four parts:
Part 1: Background of Human Subjects Research
Part 2: is the activity research, part 3: does the research involve human subjects, part 4: is the human subjects research exempt.
You will answer quiz questions throughout each part to test your knowledge. A correct response is required to advance in the lesson.
Learning Objectives
After completing this lesson, you will be able to:
- Identify if a certain activity meets the regulatory definition of research.
- Identify if research involves human subjects based on the regulatory definition.
- Determine whether a particular project is non-exempt human subjects research under the Common Rule.
Go to Section: Introduction > The Concept of Non-exempt Human Subjects Research > Identifying Non-Exempt Human Subjects Research > Quiz Questions
Introduction

The Common Rule applies to human subjects research that is supported or conducted by a Common Rule agency. For research supported or conducted by the Department of Health and Human Services (HHS), the Office for Human Research Protections (OHRP) is the office with the authority to enforce the regulations. Many research institutions choose to apply the Common Rule to all of their human subjects research regardless of funding source.
This lesson focuses on the Revised Common Rule that became effective in 2018.
The Concept of Non-exempt Human Subjects Research

Even when funded by a Common Rule agency, not all research involving humans is required to follow the Common Rule. The Rule only applies to activities that qualify as human subjects research under the regulation and that do not qualify for an exemption. This is commonly referred to as non-exempt human subjects research.
Note that, in addition to the Common Rule (subpart A), non-exempt human subjects research funded by HHS must also comply with subparts B, C, & D of the regulations at 45 CFR 46. These subparts provide additional protections for certain special populations involved in research.
This lesson explains how the regulations define research and human subjects and explains what it means to be exempt from the regulations. Understanding these concepts is important to knowing when the regulations apply and when they do not.
Identifying Non-Exempt Human Subjects Research

To figure out whether a particular activity is non-exempt human subjects research under the Common Rule, ask the following three questions, in this order :
- Is the activity research according to the regulations?
- Does the research involve human subjects based on the definition in the regulations?
- Is the human subjects research exempt?
The determination of whether a research study is non-exempt human subjects research is usually made by an institution’s Human Research Protection Program (HRPP) or IRB office. In addition to applying the Common Rule’s basic protections for human subjects in research, the HRPP or IRB office also may ensure that the activity aligns with institutional policies, ethical guidelines, and other regulations and policies that might be relevant.
What is non-exempt human subjects research?
All research involving human volunteers in the United States is required to follow the Common Rule. True or false?
An investigator plans to do a research project involving human subjects that is not funded by the Federal government. Can she proceed with her proposed project without IRB review?
In an institution, who usually determines whether a research study is non-exempt human subjects? (Select all that apply)
In deciding whether a project is non-exempt human subjects research under the Common Rule, what is the first question you should ask?
Go to Section: Defining Research > Categories of Activities Deemed Not to Be Research > Quiz Questions > Determining When the Common Rule Requirements Apply
Defining Research
Let’s start with the first question: Is the activity research according to the regulations?
Not all work that we would colloquially call ‘research’ is considered to be research under the Common Rule. The Common Rule defines research as:
“a systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge.”
To decide if a certain activity meets the regulatory definition of research, consider:
- It would likely involve a hypothesis, research question, and a plan to systematically collect and analyze data.
- The systematic investigation adds information and contributes to generalizable knowledge in the field.
- For example, lots of information is published that comes from activities that do not meet the Common Rule’s definition of research. And sometimes results from research that meets the Common Rule definition never get published.
Categories of Activities Deemed Not to Be Research
The revised Common Rule also lists four specific types of activities that are deemed not to be research:
- Scholarly and journalistic activities that focus on information specifically about certain individuals.
- Certain public health surveillance activities.
- Certain activities solely for criminal justice or criminal investigative purposes.
- Certain operational activities in support of national security missions.
Review the regulatory descriptions of these four categories of activities deemed to be not research under §46.102(l).
Watch the video to learn more.
The Common Rule defines research as “a systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalize knowledge.” True or false?
What are the criteria for the regulatory definition of research? (Select all that apply)
Select all activities deemed not to be research under the Common Rule. (Select all that apply)
Determining When the Common Rule Regulatory Requirements Apply
So, when deciding if a specific activity comes under the Common Rule,
First, ask whether it meets the regulatory definition for research—and remember to consider the four categories of activities deemed not to be research.
If the answer is “No,” then the Common Rule does not apply and, as a result, the activity does not have to be reviewed and approved by an IRB before starting. However , investigators should always check with their institution’s HRPP or IRB office to see whether there are institutional policies to follow even if the regulations don’t apply.
If, however, the answer to the first question is “ Yes ” – the activity does meet the regulatory definition of research, THEN ask the second question: Does the research involve human subjects?
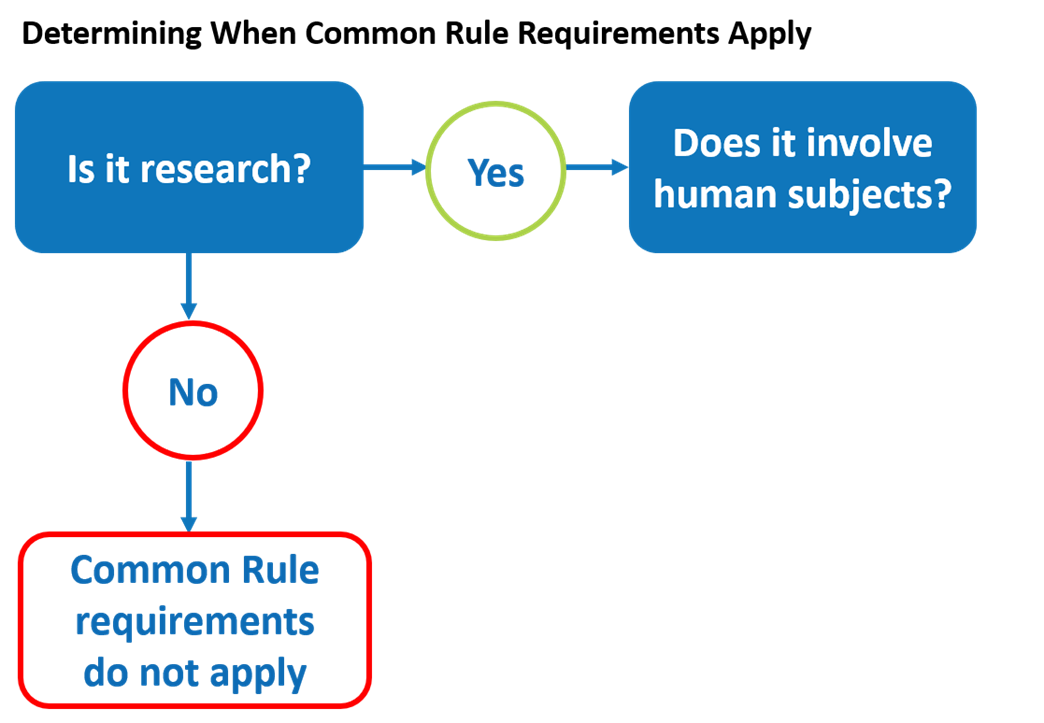
Go to Section: Defining Human Subject > Living Individuals > Identifying the Subject > Interaction and Intervention > Identifiable Private Information > Quiz Questions > Determining When the Common Rule Requirements Apply
Defining Human Subject
The revised Common Rule defines human subject as:
“a living individual about whom an investigator (whether professional or student) conducting research: (i) Obtains information or biospecimens through intervention or interaction with the individual, and uses studies, or analyzes the information or biospecimens; or (ii) Obtains, uses, studies, analyzes, or generates identifiable private information or identifiable biospecimens.”
While there is a lot of detail in the definition of human subject, it generally boils down to this:
It is important to understand the key terms in this definition to determine when a research study involves human subjects according to the regulations.
Living Individuals
Human Subject: “ a living individual about whom an investigator (whether professional or student) conducting research: (i) Obtains information or biospecimens through intervention or interaction with the individual, and uses studies, or analyzes the information or biospecimens; or (ii) Obtains, uses, studies, analyzes, or generates identifiable private information or identifiable biospecimens.”

First of all, notice that it specifies living individuals. Therefore, for the purpose of the Common Rule, research that only involves information or biospecimens from deceased persons would not be considered human subjects research.
Identifying the Subject
Human Subject: “a living individual about whom an investigator (whether professional or student) conducting research: (i) Obtains information or biospecimens through intervention or interaction with the individual, and uses studies, or analyzes the information or biospecimens; or (ii) Obtains, uses, studies, analyzes, or generates identifiable private information or identifiable biospecimens.”

The phrase ‘about whom’ is important. A human subject is the person that the information is about, not necessarily the person providing the information. In the case of biospecimens, the human subject is the person from whom the specimen was taken.
For example:
Interaction and Intervention

- Interactions occur when investigators communicate or have interpersonal contact with research participants, for example verbally, in writing, or electronically, to obtain information about them for the research.
- Interventions , on the other hand, include both physical procedures by which investigators collect information or biospecimens and manipulations of the subjects or the subjects’ environment for the purpose of the research.
- Examples of interventions include assigning subjects to take a particular drug in a clinical trial, asking subjects to complete a certain task for research purposes, and changing the background noise level to study how subjects’ stress levels vary.
Identifiable Private Information
Human Subject: “a living individual about whom an investigator (whether professional or student) conducting research: (i) Obtains information or biospecimens through intervention or interaction with the individual, and uses studies, or analyzes the information or biospecimens; or (ii) Obtains, uses, studies, analyzes, or generates identifiable private information or identifiable biospecimens .”

Note, also, that the researchers may or may not have interacted or intervened with the subject at all – for example, they might use leftover blood samples from clinical tests; but if the blood sample is identifiable, then the person is considered to be a human subject.
A proposed research project involves studying tissue samples from cadavers being used in a local medical school to train students. Personal information about the deceased individuals will be used in the research. Is this human subjects research?
Question 10
A proposed research project involves asking participants to complete a task and answer questions on a computer. No identifiable information will be recorded about participants. Is this human subjects research?
Question 11
A proposed research project will use leftover blood samples from clinical tests to check for levels of a certain metabolite. Investigators will also review patients’ identifiable medical records to obtain other necessary health information. Is this human subjects research?
Question 12
Which of the following activities, when carried out for the purpose of research, would constitute research involving human subjects under the Common Rule? (Select all that apply)
Question 13
Research that only involves specimens from deceased persons would not be considered human subjects research. True or false?
Apply this definition of “human subjects” to your research to determine whether your research study constitutes human subjects research under the Common Rule. If the answer is “no,” then the Common Rule does not apply.
If, on the other hand, the answer to this second question is “yes,” and it is human subjects research, then you go on to the third question: Is it exempt?
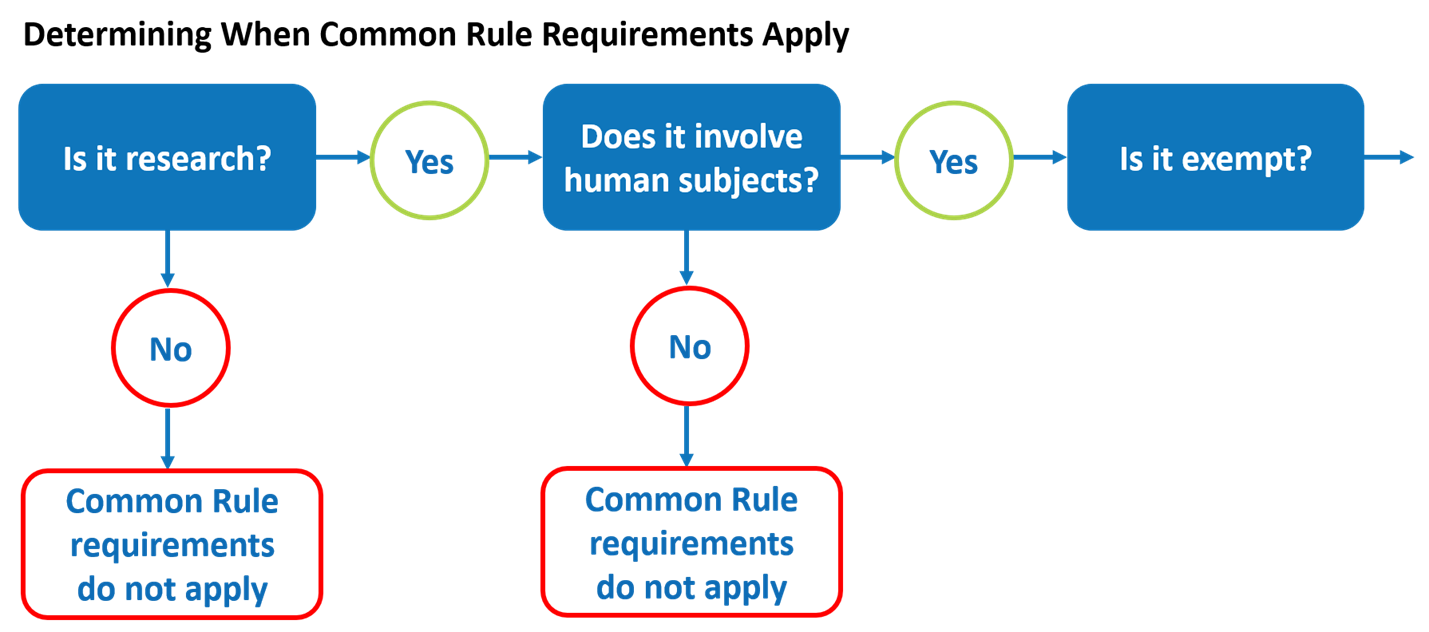
Go to Section: Could the Human Subjects Research Be Exempt? > Exempt Human Subjects Research > Quiz Questions > Determining When the Common Rule Requirements Apply
Could the Human Subjects Research Be Exempt?
There are eight exemption categories listed in the revised Common Rule. If all of the activities in a human subjects research study meet the criteria for one or more of these exemption categories, the study is exempt from the Common Rule requirements for oversight. This means, for example, that the research does not need to undergo initial or continuing IRB review for approval as required by the regulations.

The Common Rule does not specify who can make determinations about exemptions. Most institutions require that investigators submit proposed research to the institution’s HRPP or IRB office for the determination about whether it meets the criteria for an exemption. Additionally, certain exemptions require a “limited IRB review” to determine that specific conditions are met for the exemption to apply.
Exempt Human Subjects Research
An entire human subjects research projects that has been determined to meet the conditions for one or more exemption categories in the Common Rule can generally proceed without having to comply with the regulatory requirements.

One thing to remember, however, is that if investigators make changes to the research at a later time, they should check with their institution’s HRPP or IRB office to make sure that the research still meets the exemption criteria. If the changes cause the research study to no longer meet the criteria for exemption, then the research is no longer exempt and must comply with the regulatory requirements and undergo IRB review . Investigators should work closely with their HRPP or IRB office to avoid surprises like this that could affect the progress of their research.
Click here to watch a video explaining the exemption categories.
Question 14
What does it mean for a research project to be exempt?
Question 15
The Common Rule specifies who should make determinations about exemptions. True or false?

Question 16
Where should a researcher go to inquire whether something qualifies as an exemption? (Select all that could apply)
Question 17
Once a research study is determined to be exempt, it will always be exempt regardless of any subsequent changes that might be made to it. True or false?
Human subjects research studies that do not qualify for an exemption are referred to as non-exempt human subjects research. Unless there is a Secretarial waiver, they must comply with the Common Rule regulatory requirements, including IRB review and approval, before the research can begin. For non-exempt cooperative research studies involving multiple institutions, the review would generally be done by a single IRB.
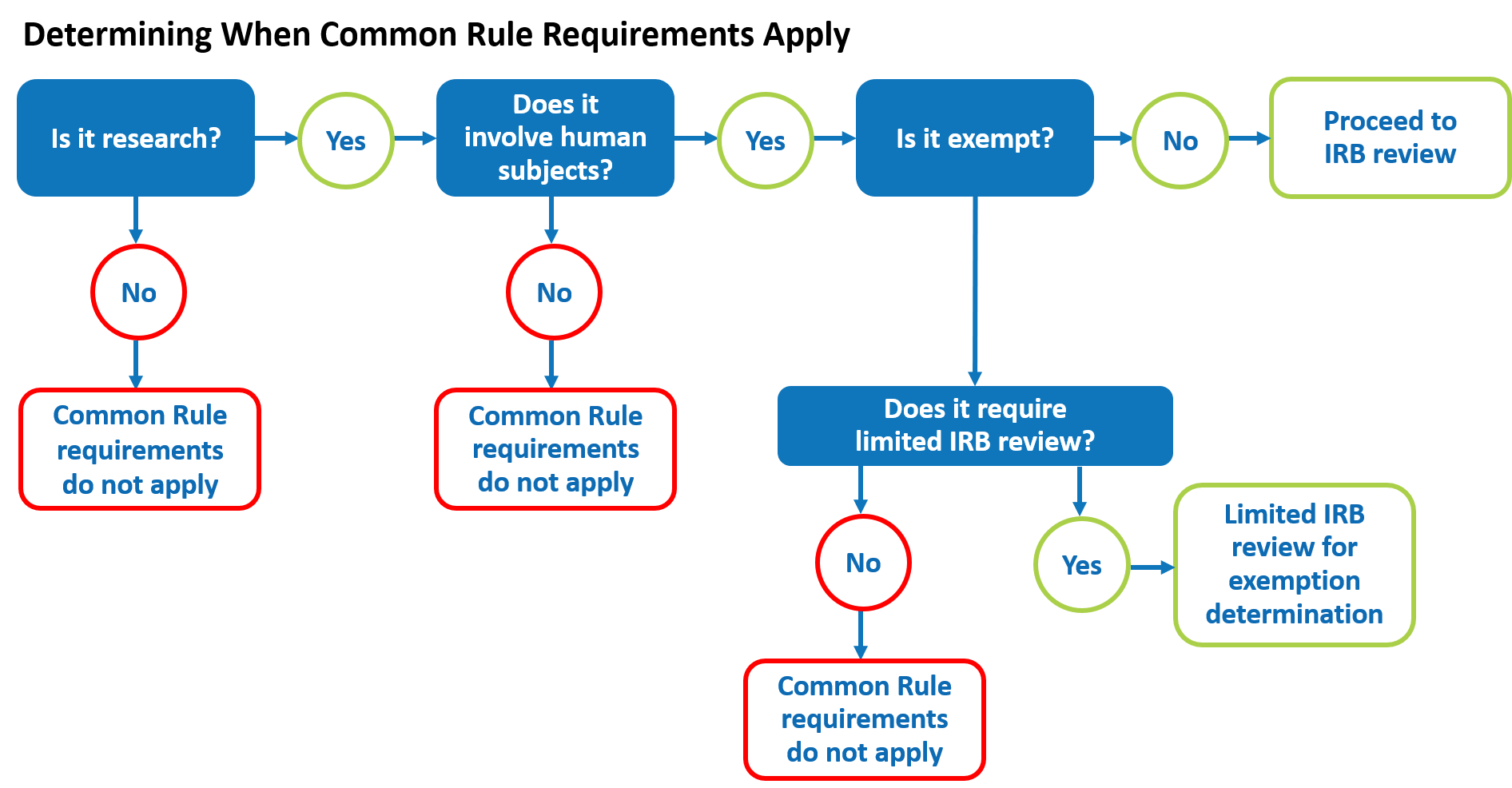
Go to Section: Wrap Up > Completion Certificate
This lesson explained the process of determining whether a research project meets the criteria for being non-exempt human subjects research under the Common Rule. Remember that if it doesn’t satisfy the regulatory definition of either research or human subject, or if all of the activities in the human subjects research meet the criteria for one or more of the exemptions, then the Common Rule regulatory requirements do not apply to the project, but investigators may still be subject to any institutional policies that are in place. Investigators should work with their institution’s Human Research Protection Program (HRPP) or IRB office to find answers and determine how to proceed.

Congratulations!
You have completed OHRP’s learning module:
OHRP does not collect information about who completes this training. Please fill out the information below and print this page for your records.
- Physics Magazine
- Physical Review Journals
- Physics Today
- Other APS Publications
- March Meeting
- April Meeting
- Meeting Calendar
- Abstract Submission
- Meeting Archive
- Policies & Guidelines
- International Affairs
- Public Engagement
- Women in Physics
- Minorities in Physics
- LGBT Physicists
- Industrial Physics
- Renew Membership
- Member Directory
- My Member Profile
- Member Services
- APS Chapters
- Action Center
- Reports & Studies
- APS Statements
- Contact APS Government Affairs
- Physics Jobs
- Becoming a Physicist
- Career Guidance
- Tools for Career Advisors
- Statistical Data
- News & Announcements
- Press Releases
- Social Media
- Mission, Vision, Values
- Strategic Plan
- Society Governance
- Society History
- Donate to APS
- Become a Member
- Why Study Physics?
- High School
- Undergraduate
- Education Conferences
Research with Human Subjects
Physicists occasionally perform research involving human subjects. Examples of such research include educational studies, biophysics investigations, and surveys.
Federally funded institutions are required to have appropriate procedures in place to ensure that the health and privacy of human subjects are protected. Institutions generally have one or more committees set up to review proposals for research involving human subjects. Certain types of minimal-risk research may be exempt from oversight, including some forms of education-related research.
However, the investigator is not allowed to make the determination of exempt status on his own; the institution’s human subjects review board makes that decision. The regulations governing human subjects research are lengthy and complex. Fortunately, another requirement of federally funded institutions is that they make human subjects research training available. Taking advantage of these training resources is likely to be a more efficient way of finding out the information of most relevance to you.
Resources on Human Subjects Research
Human Subjects Research Scenario
Become an APS Member Submit a Meeting Abstract Submit a Manuscript Find a Journal Article Donate to APS
Renew Membership Join an APS Unit Update Contact Information
Information for
Librarians Authors Referees Media Students
The American Physical Society (APS) is a nonprofit membership organization working to advance the knowledge of physics.
© 2024 American Physical Society | Privacy Policy | Contact Us 1 Physics Ellipse, College Park, MD 20740-3844 | (301) 209-3200
The Changing Landscape of Human Subjects Research
Amy Waltz, JD, CIP Associate Director – Regulatory Affairs, Reliance, Outreach Indiana University
Abstract : Understanding context is key to understanding the regulations and complying with regulatory requirements. This article explores the historical context and events that shaped the current human subjects protection regulations and how changes in human subjects research and public perception have impacted the proposed revisions to the human subjects protection regulations. The 2017 revisions to the Common Rule (45CFR46) and the impact of these revisions on government funded research are also addressed.
Introduction
Research compliance is one of the few areas within the United States regulatory system that demonstrates a direct correlation between real-life events, public opinion, and changes in regulatory policy. In 2017, the Common Rule, the set of regulations that defines human subject protection in the United States, was revised for the first time in more than twenty-five years, beginning a new regulatory era in research compliance.
It is extremely important for the research community to understand the context behind those regulations and the recent changes. Understanding the historical context helps researchers and institutional review board (IRB) administrators appropriately balance potential administrative burden with ensuring the highest ethical conduct of research. While the Common Rule, and IRBs, are often seen by researchers as simple administrative burden, they are based in a historical context rife with ethical violations, even when the violators meant well.
The research community is in an excellent position right now, being given the revised Common Rule and the regulatory flexibility that it provides, to use the past to define the future of research.
Early examples of human subjects research
Research in the early years of medical experimentation looked very different than it does today. Those who participated in research, unwittingly or not, were not categorized as “human subjects” and specifically protected. Today, the lines between research and clinical care are carefully drawn. Research and clinical care are explained differently to subjects, and logistics, such as billing, is handled separately in many cases. In the early days of medical experimentation, no formal distinction between research and clinical care was made, and human subjects research was not considered a separate scientific discipline as it is today. Instead, research was simply conducted alongside clinical treatment.
For example, the great Sidney Farber created chemotherapy at Boston Children’s Hospital. At that time, there were no treatment options for pediatric cancer patients, who almost always succumbed to their disease. As those who work in rare and difficult diseases today can understand, serving as a treating provider for pediatric cancer patients, knowing there was little to be done, was exceedingly difficult. Dr. Farber and his colleagues developed chemical compounds to try to help children with leukemia. Dr. Farber’s work was methodical, ground breaking, and ultimately defined the treatment of cancer; however, the informed consent document as we know it today did not exist for Dr. Farber’s experiments.
Another example of early human subjects research has captured the interest of Americans in recent years. In 1951, George Gey created the first immortal cell line from cells removed from an African-American woman named Henrietta Lacks, who had been diagnosed with a violent form of cervical cancer. The cancer spread extremely rapidly and she passed away within a few months of diagnosis. Dr. Gey was a researcher at Johns Hopkins University who had been searching for an immortal cell line, or a group of cells that would grow for prolonged periods, which he hoped would allow bench scientists to conduct better research. He had asked colleagues at the University and affiliated hospitals to take an extra swab of cells from cancer patients for testing by Dr. Gey. The cells taken from Henrietta Lacks were collected with that request in mind, and no one thought to obtain consent. At that time, the concept of informed consent for research as we know it simply did not exist yet.
Henrietta Lacks’ immortal cells have changed the face of medical research. Her cells are used throughout the medical and research world; however, neither Henrietta Lacks nor her family consented to the original or ongoing use of her cells. The story became widely known in 2010 when Rebecca Skloot published The Immortal Life of Henrietta Lacks, which became a New York Times bestseller. The book resulted in the first public acknowledgment of the contribution of the Lacks’ family to medical science and sparked a national public discussion about the ethical use of biospecimens for research, including how biospecimens are collected, and the information people should have when their biospecimens are used for medical research. Today, the Lacks family is involved in discussions of how some of Henrietta Lacks’ cells are used. The book changed the way the research world treated the Lacks family. The discussion in the research community about biospecimens has changed the regulatory perspective on research requirements.
The worst-case scenario
These examples took place well before research regulations existed. The first consideration of human subjects as we know it today did not occur until after the global public learned of Nazi experimentation during World War II. Before and during World War II, the Nazis committed terrible atrocities in the name of medical science using prisoners of war, Jews, and children. Nazi doctors conducted systematic investigations designed to test very specific hypotheses. Many of the experiments were focused on the war effort and attempted to create a better soldier or lessen the effects of the body’s reaction to cold, hunger, altitude, and pain. The infamous Nazi doctor, Josef Mengele, conducted genetic research on more than 700 pairs of twins.
The world did not hear about these atrocities until the Allied forces entered Nazi Germany toward the end of the war and found evidence of carefully-documented medical experimentation in concentration camps and Nazi strongholds around Europe. After the war, hundreds of Nazis were tried for war crimes and crimes against humanity, including medical experimentation, at the Nuremburg Trials.
Like most legal trials, the judgments rendered at the Nuremberg Trials, and the rationale for those judgments, were documented in the judges’ opinions about the cases. The Nuremberg judges who oversaw the medical experimentation cases thought carefully about the ethics of medical research and how medical research should be conducted. They discussed it extensively in their opinions. The judges eventually wrote the first ethical doctrine for conducting medical research, which became known as the Nuremburg Code. The document is often considered the basis for other ethical doctrines world-wide.
The worst-case scenario here at home
After the Nuremberg Code, unethical research practices were a topic of global debate. Unfortunately, there were examples of ethical violations in medical research here in the United States as well. The Tuskegee syphilis studies brought ethical issues in research with human subjects to the forefront of the public eye. These studies were conducted from the 1930sthrough the 1970s, and they were funded by the United States Public Health Service. The Tuskegee syphilis studies were designed to study the natural progression of untreated syphilis over time. When the research began, syphilis was prevalent in African-American men, especially those in poverty, so study doctors focused on that population, offering recruitment incentives which would be especially attractive to the target participants: free medical care, meals, and burial insurance. Given the participants’ need for these things, many now consider those incentives to be unduly influential.
Undue influence was not the only ethical issue in the Tuskegee syphilis studies. Conduct of the study led to a lack of informed consent and failure to mitigate further risk to participants. For example, when the researchers began having trouble recruiting participants, they simply stopped telling potential subjects that they were studying syphilis. Many of the participants never knew that they had syphilis and were never told, despite participating in the syphilis studies for years. Furthermore, study doctors never offered participants a treatment for their disease, despite the fact that a viable treatment became available in the 1940s, while the studies were ongoing.
It is difficult to imagine research being conducted in this way under the current regulatory environment. Subsequent regulatory oversight of human subjects research in the United States focused on avoiding and mitigating the types of issues demonstrated by the Tuskegee syphilis studies.
Development of the regulations
This historical context drove the development of the regulations in the United States (Table 1). The major ethical violations that occurred in the Tuskegee syphilis studies – coercion, lack of informed consent or understanding, failure to treat, and harming research participants, led regulators to directly address such issues in the Belmont Report and the subsequent regulations.
In the mid-1970s, the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research set out to develop a set of regulations to ensure that unethical conduct of research no longer happened. After years of work, the Commission developed an ethical guideline, the Belmont Report, which describes the guiding principles for ethical conduct of research in the United States. The Belmont Report outlined three main tenants: respect for persons, beneficence, and justice.
- Respect for persons is the concept of autonomy of research participants, and it requires informed consent to participate in research, as well as a clear understanding that participation in research is voluntary. This tenet also requires protection of persons with diminished autonomy, who may not be able to consent for themselves.
- Beneficence embodies the medical tenet to “do no harm,” and it requires that research be conducted in a way that maximizes benefits and minimizes harm to research participants.
- Justice requires equitable recruitment practices in research so that the benefits and burdens of research are distributed fairly, and those who will benefit from research are those who participate on the research.
The Common Rule
The three tenets of the Belmont Report became the basis of today’s Common Rule. The Common Rule is the set of regulations that requires independent review of research by an IRB to ensure that the research has a sound design and requires additional safeguards for vulnerable populations. The Common Rule has been accepted by the US Department of Health and Human Services and fifteen other Federal departments and agencies that apply to government-funded research. The IRB, under the Common Rule, must determine that:
- Risks are minimized.
- Risks to subjects are reasonable in relation to the anticipated benefits.
- Selection of subjects is equitable.
- Informed consent will be obtained from participants, and that potential participants understand the research and that their participation is voluntary, unless informed consent is waived when specific criteria are met.
- Informed consent will be documented (unless waived).
- The research plan includes adequate provision to protect the privacy and confidentiality of participants.
- If subjects are vulnerable to coercion or undue influence, additional safeguards have been included.
The ethical violations in the early years of medical research led to the Belmont Report, which led to today’s Common Rule – a direct correlation from real-life events and public opinion to changes in regulatory policy that continues today.
Today’s research environment
Since publication of the Common Rule in 1991, the research environment has changed dramatically. New technologies such as digital records, electronic medical records, the human genome project, mobile technology, and big data, among others, have changed the way that research is conducted. Research design has changed. Today, researchers encourage keeping data for possible use in future research. Research repositories, precision medicine programs based in research, and translational research are important initiatives. Comparative effectiveness research has changed thinking about informed consent. Today’s research environment includes concerns about privacy and public engagement in research. For example, when the Common Rule was first published, the Health Insurance and Portability and Accountability Act did not exist. Greater visibility of research in general means research participants are actively engaged in the research process, patients seek out research participation, and research participants help researchers design research in ways that were unheard of in the early 1990s.
The new Common Rule
In 2017, the first revision to Common Rule in over twenty-five years was published, taking steps to provide a better balance between protection of human subjects and facilitation of new research advances. (Table 3). The revisions strive to reduce some of the administrative burden on researchers and to reduce delay and ambiguity in the current IRB process.
Under the new Common Rule, more research will be considered minimal risk, and even more research will have the opportunity to be exempted from the regulations. In addition, the renewal requirements for minimal risk research have been removed from the Common Rule, reducing administrative burden on IRBs and researchers. New requirements for informed consent will require new informed consent templates and revisions to the informed consent process. The requirement for single IRB review for multi-center studies by 2020 will result in more collaboration with external IRBs.
The effective date of the revised common rule was January 19, 2019.
As a national research community, we now find ourselves in a new era of research. The revision to the Common Rule is just one more example of how the research world is changing. Navigating change can be difficult and complicated; however, the changes provided by the revised Common Rule will offer advantages to researchers and IRBs while providing better protection for human subjects. For the first time since 1991, the research community has an opportunity to build a more desirable research environment, necessitating flexibility and better communication between IRBs and researchers alike. Taking advantage of this new era will require strong collaboration between IRB administrators, research coordinators, and researchers to find and utilize those areas of flexibility within the regulations.
How Context Drove the Regulations
- Lack of understanding
- Failure to treat
- Unverified results
- Respect for persons
- Beneficence
- Minimization of risk
- Risk/benefit ratio
- Informed consent
- Protection of vulnerable populations
The Revised Common Rule
- Balance protection of human subjects with facilitation of research
- Reduce administrative burden, delay, and ambiguity
- Address protections across a broader variety of research
- Harmonize human subjects policies across federal departments and agencies
- New exempt categories based on risk profile
- New requirements for the informed consent process
- “Broad consent” as a new form of informed consent
- Use of a single IRB for multi-site projects
- No continuing review of minimal risk research
4 thoughts on “The Changing Landscape of Human Subjects Research”
Thank you for posting this very interesting summary. These changes are still new to many of us and we should all work to adapt them into our common practice while getting site up and running.
Human anatomy is an old scientific field, dating back to the third century, as is well known. Since then, there have been several developments and changes in human medical research that have brought us to where we are now. Central BioHub integrate dynamic data-driven digital technologies into biospecimen distribution. To buy high-quality human biospecimens for your research we therefore built the most dependable click-and-buy online channel by eliminating all the flaws in traditional study sample procurement. Every order is immediately processed and swiftly delivered to your location at Central BioHub. Additionally, select CLINICAL DIAGNOSIS, ICD-10-CM CODES, and LABORATORY PARAMETERS to learn more about advanced search possibilities. https://centralbiohub.de/blogs/human-biospecimens
Access to human samples from patients is crucial for advancing diagnostic and clinical research globally. These specimens are highly valuable for molecular and laboratory research, leading to the development of cutting-edge diagnostic tools. To support in-vitro diagnostic, clinical trial, and clinical research, Central BioHub offers a broad selection of human biosamples in different matrices, including serum, plasma, urine, PBMC, CSF, and more. Explore our collection to find the perfect human specimens for your research needs.
https://centralbiohub.de/biospecimens/specimen-matrix
Are there any changes in the definition of the “Human Subjects” in the last ten years? Does anyone know that?
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .
Annual Review of Ethics Case Studies
What are research ethics cases.
For additional information, please visit Resources for Research Ethics Education
Research Ethics Cases are a tool for discussing scientific integrity. Cases are designed to confront the readers with a specific problem that does not lend itself to easy answers. By providing a focus for discussion, cases help staff involved in research to define or refine their own standards, to appreciate alternative approaches to identifying and resolving ethical problems, and to develop skills for dealing with hard problems on their own.
Research Ethics Cases for Use by the NIH Community
- Theme 23 – Authorship, Collaborations, and Mentoring (2023)
- Theme 22 – Use of Human Biospecimens and Informed Consent (2022)
- Theme 21 – Science Under Pressure (2021)
- Theme 20 – Data, Project and Lab Management, and Communication (2020)
- Theme 19 – Civility, Harassment and Inappropriate Conduct (2019)
- Theme 18 – Implicit and Explicit Biases in the Research Setting (2018)
- Theme 17 – Socially Responsible Science (2017)
- Theme 16 – Research Reproducibility (2016)
- Theme 15 – Authorship and Collaborative Science (2015)
- Theme 14 – Differentiating Between Honest Discourse and Research Misconduct and Introduction to Enhancing Reproducibility (2014)
- Theme 13 – Data Management, Whistleblowers, and Nepotism (2013)
- Theme 12 – Mentoring (2012)
- Theme 11 – Authorship (2011)
- Theme 10 – Science and Social Responsibility, continued (2010)
- Theme 9 – Science and Social Responsibility - Dual Use Research (2009)
- Theme 8 – Borrowing - Is It Plagiarism? (2008)
- Theme 7 – Data Management and Scientific Misconduct (2007)
- Theme 6 – Ethical Ambiguities (2006)
- Theme 5 – Data Management (2005)
- Theme 4 – Collaborative Science (2004)
- Theme 3 – Mentoring (2003)
- Theme 2 – Authorship (2002)
- Theme 1 – Scientific Misconduct (2001)
For Facilitators Leading Case Discussion
For the sake of time and clarity of purpose, it is essential that one individual have responsibility for leading the group discussion. As a minimum, this responsibility should include:
- Reading the case aloud.
- Defining, and re-defining as needed, the questions to be answered.
- Encouraging discussion that is “on topic”.
- Discouraging discussion that is “off topic”.
- Keeping the pace of discussion appropriate to the time available.
- Eliciting contributions from all members of the discussion group.
- Summarizing both majority and minority opinions at the end of the discussion.
How Should Cases be Analyzed?
Many of the skills necessary to analyze case studies can become tools for responding to real world problems. Cases, like the real world, contain uncertainties and ambiguities. Readers are encouraged to identify key issues, make assumptions as needed, and articulate options for resolution. In addition to the specific questions accompanying each case, readers should consider the following questions:
- Who are the affected parties (individuals, institutions, a field, society) in this situation?
- What interest(s) (material, financial, ethical, other) does each party have in the situation? Which interests are in conflict?
- Were the actions taken by each of the affected parties acceptable (ethical, legal, moral, or common sense)? If not, are there circumstances under which those actions would have been acceptable? Who should impose what sanction(s)?
- What other courses of action are open to each of the affected parties? What is the likely outcome of each course of action?
- For each party involved, what course of action would you take, and why?
- What actions could have been taken to avoid the conflict?
Is There a Right Answer?
Acceptable solutions.
Most problems will have several acceptable solutions or answers, but it will not always be the case that a perfect solution can be found. At times, even the best solution will still have some unsatisfactory consequences.
Unacceptable Solutions
While more than one acceptable solution may be possible, not all solutions are acceptable. For example, obvious violations of specific rules and regulations or of generally accepted standards of conduct would typically be unacceptable. However, it is also plausible that blind adherence to accepted rules or standards would sometimes be an unacceptable course of action.
Ethical Decision-Making
It should be noted that ethical decision-making is a process rather than a specific correct answer. In this sense, unethical behavior is defined by a failure to engage in the process of ethical decision-making. It is always unacceptable to have made no reasonable attempt to define a consistent and defensible basis for conduct.
This page was last updated on Friday, July 7, 2023

Human Subject Research
- Related Resources
- Related Organizations
Bryn Mawr College and the Massachusetts College of Pharmacy and Health Sciences
University of california los angeles, u.s. office for human research protections, u.s. office for human research protections (ohrp), world health organization, national institutes of health.
Email Updates

- Policy & Compliance
- Human Subjects Research - Home Page
Human Subjects Research - Home page
Find useful information about proposing and conducting NIH extramural research involving human subjects, including policies, regulations, training and resources. Learn about considerations for human subjects research when planning and submitting a research application or contract proposal, and throughout the extramural funding cycle.

Definition of Human Subjects Research
Are you planning on conducting human subjects research? Learn more about research that meets the definition human subjects research, Federal regulation requirements, and whether your project may be considered exempt. Also, learn about NIH-specific considerations and become more familiar with NIH policies, and other regulations as it relates to human subjects research protections.

Pre-Award and Post-Award Processes
Learn about the process of applying for a grant, cooperative agreement, or R&D contract, as it relates to the involvement of human subjects research. Find useful resources on how to prepare your Protection of Human Subjects section, and learn about next steps after submitting your grant application or proposal.

Certificates of Confidentiality (CoC)
Learn more about the NIH Certificates of Confidentiality policy. Determine if your research is eligible for receiving a CoC and use our online system to get a certificate.

Clinical Trial Requirements for Grants and Contracts
If you are submitting a grant application or responding to a contract proposal to NIH that includes a clinical trial, or are involved with conducting, managing, or overseeing clinical trials, learn about NIH policies, and find resources to guide you in your work.

Inclusion Policies
NIH is committed to supporting clinical research that benefits individuals of all sexes/genders, races, ethnicities, and ages. The information provided on this website is designed to assist the extramural community in addressing inclusion, including the Inclusion of Women and Minorities policy and the Inclusion Across the Lifespan policy, in NIH grant applications and progress reports.

NIH Single IRB Policy
Learn about the NIH single IRB policy for NIH-funded domestic multi-site studies involving non-exempt human subjects studies. Find key resources to understand the policy expectations and the process for requesting exceptions.

Policies & Regulations
Find information about human subjects research policies, and NIH-specific requirements for humans subjects research studies.

Training & Resources
Training and tools to learn about human subjects research, exemptions, and NIH requirements for human subjects research.
This page last updated on: February 28, 2019
- Bookmark & Share
- E-mail Updates
- Help Downloading Files
- Privacy Notice
- Accessibility
- National Institutes of Health (NIH), 9000 Rockville Pike, Bethesda, Maryland 20892
- NIH... Turning Discovery Into Health
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Committee on Federal Research Regulations and Reporting Requirements: A New Framework for Research Universities in the 21st Century; Committee on Science, Technology, and Law; Board on Higher Education and Workforce; Policy and Global Affairs; National Academies of Sciences, Engineering, and Medicine. Optimizing the Nation's Investment in Academic Research: A New Regulatory Framework for the 21st Century. Washington (DC): National Academies Press (US); 2016 Jul 27.

Optimizing the Nation's Investment in Academic Research: A New Regulatory Framework for the 21st Century.
- Hardcopy Version at National Academies Press
9 Ethical, Legal, and Regulatory Framework for Human Subjects Research
The National Research Act of 1974 1 created the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. 2 The act charged the commission with identifying the “basic ethical principles which should underlie the conduct of biomedical and behavioral research involving human subjects” and with developing associated guidelines for the ethical conduct of research. 3 The resulting Belmont Report, issued in 1978, drew a sharp distinction between research, defined as “an activity designed to test an hypothesis, permit conclusions to be drawn, and thereby to develop or contribute to generalizable knowledge,” and practice, or “interventions…designed solely to enhance the wellbeing of an individual patient or client.” 4 In addition, and most important, the report articulated three basic principles that provide the ethical foundation for the conduct of research involving human subjects (see Box 9-1 ).
Ethical Principles and Applications Outlined in the 1978 Belmont Report.
Respect for persons involves two ethical considerations: (1) individuals are and should be treated as autonomous agents and (2) individuals with diminished autonomy, due to youth, illness, mental disability, or restricted liberty (e.g., prisoners) should receive additional protections. The principle of respect for persons means recognizing the authority of an individual's preferences and choices about his or her life. In the context of research, the principle of respect for persons is expressed primarily in the use of informed consent, which requires that, as a general rule, individuals be afforded the opportunity to choose whether or not to be involved in research. It is incumbent upon investigators to disclose information about a study in language that is comprehensible to potential subjects so that they can provide meaningful and voluntary informed consent. These disclosures typically include the purpose of the research, the research procedures, risks, anticipated benefits (if any) to the subject, the opportunity to ask questions and receive satisfactory responses, and a statement that participation is voluntary and that the subject has the right to withdraw from the study at any time, for any reason.
Beneficence involves two considerations: (1) the maximization of possible benefits for society and subjects; and (2) the minimization of possible harm to subjects. The principle of beneficence presents obligations that are woven throughout the research enterprise. Investigators, institutions, and sponsors must always endeavor to design and conduct research studies so that these obligations are met. Defining the optimum balance between the obligation to maximize benefit and minimize harm is often challenging. Notably, although the principle of beneficence refers to maximizing benefits for society, the Belmont Report does not expand upon this requirement.
Justice is articulated in the Belmont Report as “fairness in distribution” of research benefits and burdens. 5 Questions of justice and equal treatment in the research context are critical in the selection of subjects. The application of justice means that investigators must not offer potentially beneficial research only to some groups, nor select only some accessible, vulnerable, or disadvantaged groups for research that involves high risk or little prospect of direct benefit.
- Nature of Concern
The core principles of respect for persons, beneficence, and justice remain central to the protection of human research subjects. However, in the nearly four decades since publication of the Belmont Report, the biomedical and sociobehavioral research enterprises have grown enormously and witnessed profound changes in knowledge, technologies, methodologies, and capabilities, as well as in the potential implications of research findings for individual subjects and society. These continuing changes in research contexts and capabilities, in turn, raise questions as to the proper application and balancing 6 of the Belmont principles.
There is, for example, disagreement regarding how best to balance the Belmont principles in the context of clinical trials that compare the effectiveness of widely used interventions for given disorders to determine whether one approach may in fact have a better outcome than the other. 7 Questions about application of these principles also arise in research involving deidentified human biospecimens or genomic data, community-based participatory research, clinical trials conducted in emergency settings, study designs that incorporate randomization at the unit (cluster) rather than the individual level, and observational research involving large-scale databases. 8 Furthermore, while the Belmont Report did not explicitly articulate an obligation to participate in research, some believe that as all are potential beneficiaries of biomedical and sociobehavioral research, all have a responsibility, when opportunities arise and risks are minimal, to participate in research, as broad participation contributes to a greater understanding of human health, disease, and the effectiveness of proposed therapies across a broader spectrum of society, thus providing benefits to the entire population. Thus, research involving human subjects poses profound and unanswered questions about our status as both potential participants in and beneficiaries of the knowledge gained from biomedical and behavioral studies and about our rights and responsibilities as individuals versus our obligations as members of society.
Moreover, the scope of regulations for the protection of human subjects in research, as guided by the Belmont principles, is the focus of considerable discussion. 9 In medical settings, the boundaries and distinctions between research involving human subjects and activities designed to assure and improve the quality of care (i.e., in clinical practice) can, at times, be difficult to judge with confidence. Furthermore, the optimal application of regulations, developed primarily in the context of biomedical research, to the entire spectrum of sociobehavioral research has been contested for decades 10 and remains unresolved.
Given these formidable questions about the application and scope of the Belmont principles, it is necessary to broadly reconsider the legal and regulatory frameworks governing human subjects research, including the optimal locus of regulatory authority within the executive branch. Should oversight reside within each executive branch agency that funds human research, as is currently the case, or within a single independent federal agency that oversees and regulates all federally funded human research?
Currently, there are tremendous opportunities to improve human health, behavior, and well-being, as exemplified by recent federal initiatives to advance our understanding of the pathobiology, diagnosis, and treatment of cancer, 11 treat Alzheimer's disease, 12 and advance precision medicine. 13 However, progress and success hinge upon an expansion of research involving human subjects. At the same time, there are persistent and varied questions about the sufficiency of the current regulatory framework. The rapidly changing circumstances surrounding research involving human subjects have led many to ask how the protections of human subjects articulated by the Belmont principles can best be maintained given new research capabilities, the accumulation and accessibility of large amounts of personal information, including health data, and the size and reach of the research enterprise. Addressing these challenges, which the framers of the Belmont Report and Common Rule could not have envisioned, will require judicious and creative thinking about how to balance our societal obligation to protect human subjects in research with the goal of maximizing the benefits to human well-being of society's investments in biomedical and sociobehavioral research.
A prior Institute of Medicine report called for the formation of an independent committee to reassess the adequacy of the federal regulatory system for overseeing human research. 14 The authors of that report noted that the “the language of the Common Rule deserves a careful and comprehensive reassessment for clarity and relevancy” and recommended that Congress “authorize and appropriate funding for a standing independent, multidisciplinary, nonpartisan expert Committee on Human Research Participant Protections whose membership would include the perspective of the research participant.” 15 That committee was not created.
In 2011, the Department of Health and Human Services (HHS) issued an Advance Notice of Proposed Rulemaking as part of an effort to update the Common Rule governing human subjects research. HHS subsequently issued a Notice of Proposed Rule Making (NPRM) 16 in September 2015. 17 Both notices elicited many comment letters describing the deficiencies of the proposals and the risks they pose to the conduct of important research. 18
Several provisions of the proposed regulations have been identified as problematic. These include: (1) proposed changes relating to the definition and handling of biospecimens; (2) how determinations are made regarding whether certain types of research may be excluded from administrative or institutional review board consideration; (3) inconsistencies amongst the proposed changes; and (4) an absence of specifics for key deliverables.
Both the significant number of comments and the concerns expressed in response to the proposed rule highlight a need to address numerous issues that have emerged since publication of the Belmont Report. Indeed, the regulations governing human subject research merit regular examination and updating. As will be demonstrated below, the current regulatory atmosphere indicates that our nation would benefit from a standing independent national advisory commission tasked with regularly examining and updating regulations governing all federally funded human subjects research and charged with addressing difficult and precedent-setting cases as well as matters of general policy.
During a presentation at a recent meeting of the Secretary's Advisory Committee on Human Research Protections, 19 Lauren Hartsmith of the HHS Office of Human Research Protections (OHRP) 20 provided an analysis of the public comments on the September 2015 NPRM. She noted that:
“There was concern about the overall complexity and the length of the NPRM. Concern about the lack of availability of some of the key deliverables in the NPRM. Specifically, those were the exemption determination tool, the broad consent template, and the Secretary's list of privacy safeguards.” 21
With regard to the “exemption determination tool,” the NPRM proposes that federal departments and agencies develop a voluntary research exemption determination tool. Based on information input by a researcher, the web-based tool would determine whether research is exempt from the human subjects regulations. 22
Regarding the broad consent template, the NPRM proposes the development of a template for acquiring consent from an individual for the storage or maintenance of biospecimens for use in future research. 23 Templates are expected to contain all required consent elements (such as a description of the research material covered, the option to consent, and the ability to withdraw consent). The NPRM indicates that at least two broad consent templates will be developed: (1) for information and biospecimens originally collected in the research context; and (2) for information and biospecimens originally collected in a non-research context. The templates will be issued for public comment at a later date.
A third key deliverable, the Secretary's “list of privacy safeguards,” is also unavailable. This list is to be developed following public comment on the types of safeguards that would be appropriate. 24 These safeguards would be designed to protect the privacy of human participants in research by protecting the confidentiality of personal information. Other laws or regulations that currently mandate the protection of human research participants would need to be examined as a part of the development of the envisioned safeguards.
The omission of specifics on key tools and guidelines like the exemption determination tool, consent templates, and list of privacy safeguards is problematic; because the items are undefined at present, it is impossible to comment on their merit or utility prior to the issuance of the final rule. Furthermore, it is not possible to provide an accurate estimation of regulatory impact without a clear understanding of what compliance will involve.
Uncertainty may also lead to an increased regulatory burden as institutions, in an effort to comply with vague or fragmentary regulations, implement speculative procedures which may ultimately be unwarranted. Institutions may also elect to reject, delay, or halt research in areas of regulatory vagueness.
In her presentation to SACHRP, Hartsmith also noted that there is “concern about some of the proposals being internally inconsistent and concern about proposals giving investigators too much leeway to determine if their research falls under the rule.” The latter concern “was specifically around the proposed concept of exclusions in the NPRM.” 25
With regard to exclusions, the NPRM identifies eleven types of research that fall outside the scope of the proposed regulations. Some of this research is also “exempt” under current Common Rule regulations. As envisioned by the NPRM, excluded research is not subject to administrative or IRB review. Instead, investigators have the responsibility to make determinations as to whether the research should be subject to external review. Examples of excluded research include: “collection and analysis of data, biospecimens, or records by or for a criminal justice agency for activities authorized by law or court order solely for criminal justice or criminal investigative purposes;” “quality assurance or improvement activities involving the implementation of an accepted practice to improve the delivery or quality of care or services;” and “public health surveillance activities, including the collection and testing of biospecimens, conducted, supported, requested, ordered, required, or authorized by a public health authority and limited to those necessary to allow the public health authority to identify, monitor, assess, or investigate potential public health signals or the onset of a disease outbreak.” 26
In assessing the proposed exclusions, one researcher observed that some exclusions “will likely be widely welcomed, such as…[an] explicit exclusion of journalism, oral history, biography, and historical scholarship activities.” However, there is “worry that the exclusion of certain activities…could lead to a weakening of subject protections.” “The proposed rule does not…offer insight into how determinations about whether the disclosure of information would reasonably place subjects at risk will be made.” 27 Further, the NPRM does not sufficiently describe how the proposed exclusions will be implemented to ensure adequate protection of human research participants.
Finally, Hartsmith presented the following comment as illustrative of the regulated community and public's comments on the NPRM. She prefaced the comment by stating, “This is…a sample quotation from one of the commenters. It is a good summary of the concerns that were expressed about the overall document.”:
The urgency to approve a final revised Common Rule prior to the end of the 2016 is deeply concerning and has resulted in a premature, rushed document that is replete with deficiencies, contradictions, areas of conflict or overlap with other federal requirements, undefined processes, categories or lists and yet to be developed forms and templates. The lack of availability of these items at this late stage in the rule making process makes commentary particularly challenging. 28
During the SACHRP meeting, there was significant discussion about the process of moving from an NPRM to a final rule. An agency is not permitted to base its final rule on the number of comments in support of the rule over those in opposition to it. Rather, the agency must base its reasoning and conclusions on the rulemaking record, consisting of the comments, scientific data, expert opinions, and facts accumulated during the pre-rule and proposed rule stages. 29 At the meeting, OHRP Director Jerry Menikoff reiterated that a final rule “should be a logical outgrowth of what was originally presented or something that was appropriately discussed as part of the comments in the public comment process.” 30
The comments and issues highlighted in Hartsmith's presentation to SACHRP align with the results of an analysis of public comments by the Council on Governmental Relations and the Association of Public and Land-grant Universities (see Box 9-2 ). They also align with the assessment of members of the leadership of the nonprofit Public Responsibility in Medicine and Research (PRIM&R) in a letter to the New England Journal of Medicine :
Council on Governmental Relations (COGR)/Association of Public and Land-grant Universities (APLU) Analysis of Comments on the HHS Notice of Proposed Rulemaking (NPRM) on the Federal Policy for the Protection of Human Subjects.
The NPRM is a troublingly incomplete product: internally inconsistent, dependent on untested assumptions, and too inchoate to be ready for promulgation with just some minor editing. The document, which had largely been crafted behind closed doors, invited public response to 88 unresolved policy questions in addition to comments on the proposed rules themselves. It introduces new regulatory mandates when less rigid solutions would offer sensible alternatives and permit adjustment in light of experience. 31
It is instructive to examine a key item in the NPRM that raised particular concern, as such a consideration illustrates the problems of moving the NPRM to a final rule.
The NPRM proposes an expansion of the definition of human subject to include deidentified, excess, or residual biospecimens. This expansion would require that individuals provide written “broad” consent for the use of such biospecimens in research—a significant departure from current practice. This would permit patients undergoing tissue excisions to grant permission for future unspecified uses of their de-identified biospecimens. It is important to understand that biospecimens, properly preserved, can last for generations. Such specimens have proved helpful in addressing unanticipated medical issues using technologies that did not exist at the time of excision or collection. The inability to envision future opportunities for research that could advance knowledge raises questions about the meaning and ethical sufficiency of “broad consent.” If, for example, waivers were unavailable for the clinical use of biospecimens for which no research use was intended at the time of excision or collection, critical post facto correlations, for example, between the Zika virus and microcephaly, may go unrecognized.
Redefining research with de-identified biospecimens as human subjects research would impose significant burdens and limitations on research institutions and the ability of research institutions to obtain specimens from health institutions that do not have the infrastructure or resources to comply with the proposed revisions to the Common Rule. As a result, research samples may not be as broadly representative of the population as they have been and research findings may no longer be so generalizable. Such limitations will likely imperil the conduct of long-established and remarkably fruitful areas of research. 32 Research on excess or residual biospecimens has contributed enormously to the growth of medical knowledge for nearly a century and a half, improving human health with little evidence of harm to individuals whose biospecimens were used in this way. 33 That some find the implications of broad consent for future research troubling 34 is another compelling example of the need for thoughtful deliberation about how best to protect individual human research subjects while continuing to advance medical knowledge that will benefit many—including, potentially, subjects themselves and their loved ones. Further, there is little evidence that individuals understand exactly what they are being asked to consent to. Nor can patients or their caregivers credibly envision what the future might hold.
In addition, as envisioned by the NPRM, broad consent would expand informed consent practices to nonresearch settings where the individual's priority is exclusively clinical care, and it would involve institutional personnel unfamiliar with research or with the principles guiding human subjects research. 35 Thus, whether the elements of informed consent laid out in the Belmont Report (information, comprehension, and voluntariness) can be achieved by broad consent as envisioned by the NPRM is debatable. 36
As the example of biospecimens demonstrates, a new assessment is needed to determine whether current measures adequately protect human subjects in contemporary biomedical research without unjustifiably impeding the conduct of well-designed research that contributes to human well-being. Put differently, where should the balance points be set among autonomy, beneficence, and justice? The current system may, for example, be better served by explicit sanctions against investigators and institutions seeking to re-identify biospecimen sources by any method, including linkage of genomic sequence data to identifiers, rather than by redefining all research with de-identified biospecimens as human subjects research subject to a revised Common Rule.
Implementation of the proposed rule necessitates maintaining a link between the consent document and the biospecimen. This proposal per se raises substantial risks of re-identification and loss of privacy. Further, the associated financial 37 and societal costs will be significant. Many clinical care facilities, such as those serving underserved or rural populations, for example, may not be able to bear these costs, thereby undermining the principles of justice and beneficence by skewing research toward studies and populations that can be accommodated only at large medical centers. As PRIM&R noted in its comment letter in response to the NPRM:
The stated goal of the NPRM is to reduce unnecessary administrative burden associated with regulation, [but] the requirements related to the use of biospecimens in research will likely create new barriers to research participation without advancing subject autonomy. New systems and mechanisms for obtaining and tracking broad consent across all patients entering a facility will need to be developed and implemented. This process will require significant resources on the part of institutions that collect biospecimens; it will be entirely out of reach for small healthcare institutions and community and school-based clinics, and may very well be beyond the capability of some larger and better-resourced institutions. As some facilities decide that they cannot manage the costs (in terms of time, staff, infrastructure, and other resources) of obtaining and tracking broad consent (is the consent still valid? does it impose any limits or requirements regarding the use of an individual's specimens? etc.), specimens collected for clinical purposes at such facilities will no longer be available for future research. As a result, the populations within the communities those institutions serve may be excluded from such research. This is problematic from the perspectives both of justice and of good science. 38
Research regulations should facilitate innovative ways for institutions to communicate with patients in meaningful and effective ways about participation in activities aimed at improving health care delivery. Such activities, when carried out systematically, are often referred to as quality improvement (QI) research. 39 Lack of clarity in the NPRM regarding which quality improvement activities constitute research, and when written consent is required for institutions to conduct such research, may discourage valuable and low-risk efforts to improve patient care.
The NPRM is marred by omissions, the absence of essential elements, and a lack of clarity. In addition, important questions about the overall impact and long-term costs of the proposed regulatory changes are unresolved. In light of these deficiencies, it would be impractical to use the current NPRM as the basis for achieving a meaningful, consistent, and harmonious revision of the regulations governing human subjects research that is optimally responsive to developments that have occurred since the publication of the Belmont Report.
The core principles of respect for persons, beneficence, and justice as articulated in the 1978 Belmont Report are central to the protection of human subjects in research studies.
In the nearly four decades since the publication of the report, however, the biomedical and sociobehavioral research enterprises have grown enormously. This growth, accompanied by the development of a remarkable number of new research capabilities and contexts, raises questions as to the optimum application and balancing of the Belmont principles, as well as whether these principles are, in and of themselves, still sufficient pillars upon which to build human research protection programs and regulations. In addition, the overarching legal and regulatory frameworks and institutional arrangements governing human research subjects require reconsideration and clarification.
Addressing contemporary challenges associated with human subjects research, including new research capabilities and contexts; the profusion, sharing, and accessibility of personal data; and increasing privacy concerns, will require creative and forward-looking legal, regulatory, and institutional frameworks. The important work of addressing these challenges is critical both for enhancing protections for individuals participating in research and for optimizing the federal investment in human research to advance knowledge and improve individual and societal well-being.
The Notice of Proposed Rulemaking on the Federal Policy for the Protection of Human Subjects would impose additional burdens that could be detrimental to areas of important research. The committee believes that the NPRM does not adequately or effectively address the breadth, depth, and import of unanswered questions; rather, its inadequacies signal a pressing need for a comprehensive review of the nation's ethical, legal, regulatory, and institutional frameworks for protecting human research subjects. 40 At this time, there is no entity that can carry out this review. SACHRP and OHRP perform valuable roles, 41 but neither could conduct the type of review that is required. SACHRP, through OHRP, advises the Secretary of HHS, and neither OHRP nor SACHRP engages other departments and agencies. The current complexity of the issues related to human subjects research requires thorough, independent, cross-agency consideration and expert input from a wide range of disciplines and stakeholder groups.
- RECOMMENDATIONS
9.1. The committee recommends that Congress authorize, and the President appoint, an independent, free-standing national commission modeled on the President's Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. This commission was authorized by Congress under Public Law 95-622 in 1978, appointed by the President in 1979, and existed outside the structure of federal departments and agencies. The commission had a direct line-item appropriation from Congress, appointed its own staff, and set its own agenda.
Congress should charge the proposed commission with examining and updating as necessary the ethical, legal, and institutional frameworks governing human subjects research. The commission should make recommendations to the President, Congress, and relevant federal agencies regarding how the basic ethical principles governing human subjects research should be applied to unresolved human research questions and novel human research contexts, including but not limited to:
- Research involving anonymous and de-identified human biospecimens;
- Research involving large datasets, for example, research with human genomic, transcriptomic, proteomic, or metabolomic data or associated DNA, RNA, and protein analyses and relevant integrated approaches;
- Research in which the interests of discrete and insular communities are at stake;
- Clinical studies conducted in emergency settings;
- Research involving adults with diminished decision-making capacities;
- Clinical trials where the unit of intervention is a cluster or group;
- Clinical studies comparing the effectiveness of different accepted interventions for a given disorder to determine whether one approach may be preferable to the other;
- Observational research involving large-scale databases;
- The appropriate boundaries of regulation of minimal-risk sociobehavioral research; and
- Research aimed at clinical innovation and quality assurance and improvement.
The commission should have two broad charges:
Recommend to the President and Congress ethically sound regulatory approaches for unresolved questions in human subjects research, including:
- The scope of human research activities that should be covered by federal regulations for human subjects research (including the determination of the types of low-risk research activities, such as some types of sociobehavioral research, that should fall outside the scope of the regulations);
- How regulation should address the increasingly blurred boundaries between research and medical care and the means by which new regulations should distinguish between the two;
- How to incorporate investigator responsibilities into human subjects research regulations; and
- How to balance individual rights, such as the right to privacy, with collective obligations to advance public health and well-being.
Recommend to the President and Congress revisions in the legal and institutional structures for regulating research with human subjects that address such questions as:
- Where in the executive branch should the regulatory authority for human subjects research lie? Should it rest within each agency that conducts or funds such research, as is currently the case, or should there be a single, independent agency that regulates all federally funded human subjects research? Which model best serves the interests of efficiency, harmonization, and the mitigation of conflicts of interests?
- Should the United States have a standing advisory committee on human subjects protections? If so, what types of cases or questions should it address, how should it be structured, whom should it advise, and where should it fit within the agency structure?
9.2. To ensure that the proposed national commission can address the full range of unanswered questions regarding the protection of human subjects in federally funded research, the committee recommends that the executive branch withdraw the Notice of Proposed Rulemaking on the Federal Policy for the Protection of Human Subjects. The committee further recommends that the regulatory structure protecting human research subjects not be revised until the national commission has issued its report and the research community, patient groups, the public, and others have had an opportunity to consider and respond to the commission's recommendations.
See National Research Service Award Act of 1974. Pub. L. No. 93-348 (2014).
The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research was succeeded by the President's Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. An independent entity established by Congress under Public Law 95-622 in 1978, the latter commission operated from January 1980 to March 1983.
See Commission Duties, Pub. L. No. 93-348 § 202.1a (1974).
National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research (Bethesda, MD: 1978), available at: http://www .hhs.gov/ohrp /regulations-and-policy /belmont-report/ .
T. L. Beauchamp and J. F. Childress, Principles of Biomedical Ethics, 7th ed. (New York: Oxford University Press, 2013): 459.
See, e.g., R. Platt, N. E. Kass, and D. McGraw, “Ethics, Regulations, and Comparative Effectiveness,” JAMA , vol. 311, no. 15 (2014): 1497–1498.
See, e.g., E. W. Clayton et al., “Confronting Real Time Ethical, Legal, and Social Issues in eMERGE Consortium,” Genetics in Medicine 10 (2010): 616–620; E. Bromley et al., “From Subject to Participant: Ethics and the Evolving Role of Community in Health Research.” American Journal of Public Health , vol. 105, no. 5 (2015): 900–908.; M. Mitka, “Aiding Emergency Research Aim of Report on Exceptions to Informed Consent,” JAMA , vol. 298, no. 22 (2007): 2608–2609; C. Weijer et al., “The Ottawa Statement on the Ethical Design and Conduct of Cluster Randomized Trials,” PLOS Medicine , vol. 9, no. 11 (2012): 1–9; and M. A. Rothstein, et al., “Ethical Issues in Big Data Health Research,” Journal of Law, Medicine, and Ethics , vol. 43, no. 2 (2015): 425–429.
See, e.g., Institute of Medicine, Responsible Research: A Systems Approach to Protecting Research Participants (Washington, DC: The National Academies Press, 2002) and Institute of Medicine, Preserving Public Trust: Accreditation and Human Research Participant Protection Programs (Washington, DC: The National Academies Press, 2001).
See, e.g., National Research Council, Proposed Revisions to the Common Rule for the Protection of Human Subjects in the Behavioral and Social Sciences (Washington, DC: The National Academies Press, 2014) and C. K. Gunsalus et al., “Mission Creep in the IRB World.” Science , vol. 312, no. 5779 (2006): 1441.
In February 2016, the Obama Administration launched the “National Cancer Moonshot with a $1 billion initiative to provide the funding necessary for researchers to accelerate the development of new cancer detection and treatments.” See White House Office of the Press Secretary, “FACT SHEET: Investing in the National Cancer Moonshot,” February 1, 2016, https://www .whitehouse .gov/the-press-office /2016/02/01/fact-sheetinvesting-national-cancer-moonshot .
“The National Alzheimer's Project Act (Public Law 111-375), passed unanimously by Congress in December 2010 and signed into law by President Barack Obama in January 2011, required the creation of a national strategic plan to address the rapidly escalating Alzheimer's disease crisis and the coordination of Alzheimer's disease efforts across the federal government.” See Alzheimer's Association, “The National Alzheimer's Project Act (NAPA),” http://napa .alz.org/national-alzheimers-project-act-background .
In January 2015, the Obama Administration launched a $215 million “Precision Medicine Initiative” to “pioneer a new model of patient-powered research that promises to accelerate biomedical discoveries and provide clinicians with new tools, knowledge, and therapies to select which treatments will work best for which patients.” See White House Office of the Press Secretary, “FACT SHEET: President Obama's Precision Medicine Initiative,” January 30, 2016, https://www .whitehouse .gov/the-press-office /2015/01/30/fact-sheet-president-obama-s-precision-medicine-initiative .
See, e.g., Institute of Medicine, Responsible Research: A Systems Approach to Protecting Research Participants , (Washington, DC: The National Academies Press, 2002).
Ibid, pp. 198-199.
Federal Policy for the Protection of Human Subjects,” Federal Register 80, no. 173 (September 8, 2015): 53933, https://www .gpo.gov/fdsys /pkg/FR-2015-09-08/pdf/201521756 .pdf .
The NPRM was issued at the time of the release of Part 1 of the committee's report thus precluding the committee's ability to comment fully at that time.
See, e.g., letters from the Association of American Medical Colleges, available at: https://www .aamc.org /download/451896/data /aamcsubmitscommentstohhsonthecommonrulenprm.pdf ; the Council on Governmental Relations, available at: http://cogr .edu/COGR /files/ccLibraryFiles /Filename/000000000257 /NPRMCommonRuleCOGRResponse12-8-15 %20(2).pdf ; the Association of American Universities and the Association of Public and Land-grant Universities, https://www .aau.edu/WorkArea /DownloadAsset.aspx?id=16885 ; and Public Responsibility in Medicine and Research, available at: http://www .primr.org /WorkArea/DownloadAsset.aspx?id=10166 .
The Secretary's Advisory Committee on Human Research Protections (SACHRP) “provides expert advice and recommendations to the Secretary of HHS on issues pertaining to the protection of human subjects in research.” See http://www .hhs.gov/ohrp /sachrp-committee/ . The committee is charged with advising “the Secretary on how to improve the quality of the system of human research protection programs, including the responsibilities of investigators, institutional review boards (IRBs), administrators, and institutional officials, and the role of the Office for Human Research Protections and other offices within the Department of Health and Human Services.” See SACHRP Charter, available at: http://www .hhs.gov/ohrp /sachrp-committee/charter/index.html .
The Office for Human Research Protections “provides leadership in the protection of the rights, welfare, and wellbeing of subjects involved in research conducted or supported by the U.S. Department of Health and Human Services.” See “About OHRP,” available at: http://www .hhs.gov/ohrp /about-ohrp/index.html .
L. Hartsmith. HHS Office of Human Research Protections (OHRP). NPRM Update: Summary of Public Comments. Presentation to the Secretary's Advisory Committee on Human Research Protections (SACHRP), May 18, 2016.
Video of the SACHRP meeting and Hartsmith's presentationare available at: https://videocast .nih .gov/summary.asp?Live =19186&bhcp=1 .
See “Federal Policy for the Protection of Human Subjects,” Federal Register 80, no. 173 (September 8, 2015): 54009. The NPRM states that, “Under the proposed rule, unless otherwise required by law, exemption determinations may be made by (1) an individual who is knowledgeable about the exemption categories and who has access to sufficient information to make an informed and reasonable determination, or (2) the investigator who accurately inputs information into the federally created web-based decision tool.”
Ibid, p. 53969.
The NPRM states that “For the purposes of informing the development of…privacy safeguards, comment is sought on what types of safeguards would be appropriate. There are additional statutes or acts that mandate the protection of privacy and confidentiality of identifiable private information that may be reasonable to include.” Ibid, p. 53979.
See “Federal Policy for the Protection of Human Subjects,” Federal Register 80, no. 173 (September 8, 2015): 53948-53949.
E. A. Hurley, “Unpacking the NPRM: A New Category of Exclusions,” Ampersand , October 13, 2015, available at: http://blog .primr.org /unpacking-the-nprm-a-new-categoryof-exclusions/ .
The source for this statement is a comment letter from Emma A. Meagher, Senior Associate Dean, Clinical Research and Associate Vice Provost, Human Research and Dawn Bonnell, Vice Provost for Research, University of Pennsylvania. The letter is available at: https://www .regulations .gov/#!documentDetail;D =HHS-OPHS-2015-0008-0579 .
See Office of the Federal Register, “A Guide to the Rulemaking Process,” available at: https://www .federalregister .gov/uploads/2011 /01/the_rulemaking_process.pdf .
J. Menikoff, HHS Office of Human Research Protections at May 18-19, 2016 SACHRP Meeting.
Video of the SACHRP meeting is available at: https://videocast .nih .gov/summary.asp?Live =19186&bhcp=1 .
D. H. Strauss, E. A. Hurley, and A. M. Capron, “Reform of Clinical Research Regulations,” New England Journal of Medicine , no. 374 (2016): 1693-1694.
See, e.g., the joint statement issued on May 6, 2016, the Association of American Universities, the Association of Public and Land-grant Universities, and the Council on Governmental Relations. In the statement, the organizations state that, “There is broad consensus that the proposed regulations regarding biospecimens, as written, would be damaging to science, medicine, and human health and would not improve participant safety and autonomy.” See http://www .cogr.edu/COGR /files/ccLibraryFiles /Filename/000000000347 /050916prCommonRuleFinal.pdf .
Under current regulations, requests for discarded specimens are reviewed when specimens are: (1) identifiable or (2) de-identified but collected specifically for a particular research project. If specimens do not have identifiers, they are exempt from institutional review board (IRB) review. In instances where IRB review is required, investigators must affirm to the IRB that identifiers will not be retained and provide information about the procedures that will be used to ensure that the specimens will be de-identified. Under the proposed regulations, a waiver of consent would be difficult to obtain, and the ability of an IRB to follow the current practice of review of discarded specimens for use in research would essentially be eliminated.
See, e.g., D. H. Strauss, E. A. Hurley, and A. M. Capron. “Reform of Clinical Research Regulations.” New England Journal of Medicine, no. 374 (2016): 1693–1694.
See, e.g., N. E. Kass et al., “The Research-treatment Distinction: A Problematic Approach for Determining Which Activities Should Have Ethical Oversight,” Hasting Center Report, vol. 43, no. S1 (2013): S4–S15.
See, e.g., Jocelyn Kaiser, “Researchers Decry Consent Proposal,” Science , vol. 352, no. 6288 (2016): 878–879.
As calculated by HHS, over the 2016–2025 period, present-value benefits of all proposed changes will be $2.6 billion with annualized benefits of $308 million (as estimated using a 3 percent discount rate). Present-value costs are estimated at $13.3 billion with annualized costs of $1.6 billion (as estimated using a 3 percent discount rate). See “Federal Policy for the Protection of Human Subjects,” Federal Register 80, no. 173 (September 8, 2015): 53933, https://www .gpo.gov/fdsys /pkg/FR-2015-09-08/pdf/2015-21756 .pdf . Weill Cornell Medicine, however, estimates that “it could cost [the institution] as much as $4 million annually to comply with the expanded regulations.” See L. H. Glimcher, “How Not to End Cancer in Our Lifetimes,” Wall Street Journal , April 4, 2016.
See http://www .primr.org /WorkArea/DownloadAsset.aspx?id=10166 .
See, e.g., D. H. Strauss, E. A. Hurley, and A. M. Capron, “Reform of Clinical Research Regulations,” New England Journal of Medicine , no. 374 (2016): 1693–1694.
The committee notes that the National Research Council report cited earlier recommended the formation of an independent committee or commission to address comparable issues.
See footnotes 19 and 20 in this chapter.
- Cite this Page Committee on Federal Research Regulations and Reporting Requirements: A New Framework for Research Universities in the 21st Century; Committee on Science, Technology, and Law; Board on Higher Education and Workforce; Policy and Global Affairs; National Academies of Sciences, Engineering, and Medicine. Optimizing the Nation's Investment in Academic Research: A New Regulatory Framework for the 21st Century. Washington (DC): National Academies Press (US); 2016 Jul 27. 9, Ethical, Legal, and Regulatory Framework for Human Subjects Research.
- PDF version of this title (4.7M)
In this Page
Other titles in this collection.
- The National Academies Collection: Reports funded by National Institutes of Health
Recent Activity
- Ethical, Legal, and Regulatory Framework for Human Subjects Research - Optimizin... Ethical, Legal, and Regulatory Framework for Human Subjects Research - Optimizing the Nation's Investment in Academic Research
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Updated Study Team Member Onboarding & Training Tools and Resources
- Facebook Share on Facebook
- X/Twitter Share on Twitter
- LinkedIn Share on LinkedIn
The Office for Human Subject Protection has recently updated their Study Team Member Onboarding & Training Tool and Informed Consent Observation Tool .
The Study Team Member Onboarding & Training Tool was created to facilitate onboarding and training of new study team members; it provides suggestions for general orientation tasks, research-related training, systems access and training, and protocol-specific onboarding. Updated examples of how the training tool might be utilized for generic biomedical and social-behavioral research support positions (e.g., study coordinators, research assistants) are also available for your reference.
The Informed Consent Observation Tool was created to support the assessment of study team member proficiency in facilitating informed consent discussions for the purposes of training, performance evaluation, and professional development.
Both the onboarding/training and observation tools are fully customizable and should be modified to suit the study team’s needs based on applicable regulations, policies, study-specific components, roles/responsibilities, and/or prior experience.
For questions related to the onboarding/training and observation tool, contact Kelly Unsworth .
Review additional information in OHSP’s Guideline for Training Research Personnel
Review additional training resources available through OHSP
new clinical research Study Start-Up Resources
The Clinical & Translational Science Institute’s (CTSI) Office of Clinical Research has recently developed a URMC Clinical Research Study Start-Up Manual . The manual provides best practices for study start-up, including details related to the Confidential Disclosure Agreement (CDA) process, feasibility assessment and site selection process, regulatory and financial considerations, and study activation. For questions related to the start-up manual, contact the Office of Clinical Research .
Learn more about services available through the Office of Clinical Research
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
What the data says about gun deaths in the U.S.
More Americans died of gun-related injuries in 2021 than in any other year on record, according to the latest available statistics from the Centers for Disease Control and Prevention (CDC). That included record numbers of both gun murders and gun suicides. Despite the increase in such fatalities, the rate of gun deaths – a statistic that accounts for the nation’s growing population – remained below the levels of earlier decades.
Here’s a closer look at gun deaths in the United States, based on a Pew Research Center analysis of data from the CDC, the FBI and other sources. You can also read key public opinion findings about U.S. gun violence and gun policy .
This Pew Research Center analysis examines the changing number and rate of gun deaths in the United States. It is based primarily on data from the Centers for Disease Control and Prevention (CDC) and the Federal Bureau of Investigation (FBI). The CDC’s statistics are based on information contained in official death certificates, while the FBI’s figures are based on information voluntarily submitted by thousands of police departments around the country.
For the number and rate of gun deaths over time, we relied on mortality statistics in the CDC’s WONDER database covering four distinct time periods: 1968 to 1978 , 1979 to 1998 , 1999 to 2020 , and 2021 . While these statistics are mostly comparable for the full 1968-2021 period, gun murders and suicides between 1968 and 1978 are classified by the CDC as involving firearms and explosives; those between 1979 and 2021 are classified as involving firearms only. Similarly, gun deaths involving law enforcement between 1968 and 1978 exclude those caused by “operations of war”; those between 1979 and 2021 include that category, which refers to gun deaths among military personnel or civilians due to war or civil insurrection in the U.S . All CDC gun death estimates in this analysis are adjusted to account for age differences over time and across states.
The FBI’s statistics about the types of firearms used in gun murders in 2020 come from the bureau’s Crime Data Explorer website . Specifically, they are drawn from the expanded homicide tables of the agency’s 2020 Crime in the United States report . The FBI’s statistics include murders and non-negligent manslaughters involving firearms.
How many people die from gun-related injuries in the U.S. each year?
In 2021, the most recent year for which complete data is available, 48,830 people died from gun-related injuries in the U.S., according to the CDC. That figure includes gun murders and gun suicides, along with three less common types of gun-related deaths tracked by the CDC: those that were accidental, those that involved law enforcement and those whose circumstances could not be determined. The total excludes deaths in which gunshot injuries played a contributing, but not principal, role. (CDC fatality statistics are based on information contained in official death certificates, which identify a single cause of death.)
What share of U.S. gun deaths are murders and what share are suicides?
Though they tend to get less public attention than gun-related murders, suicides have long accounted for the majority of U.S. gun deaths . In 2021, 54% of all gun-related deaths in the U.S. were suicides (26,328), while 43% were murders (20,958), according to the CDC. The remaining gun deaths that year were accidental (549), involved law enforcement (537) or had undetermined circumstances (458).
What share of all murders and suicides in the U.S. involve a gun?
About eight-in-ten U.S. murders in 2021 – 20,958 out of 26,031, or 81% – involved a firearm. That marked the highest percentage since at least 1968, the earliest year for which the CDC has online records. More than half of all suicides in 2021 – 26,328 out of 48,183, or 55% – also involved a gun, the highest percentage since 2001.
How has the number of U.S. gun deaths changed over time?
The record 48,830 total gun deaths in 2021 reflect a 23% increase since 2019, before the onset of the coronavirus pandemic .
Gun murders, in particular, have climbed sharply during the pandemic, increasing 45% between 2019 and 2021, while the number of gun suicides rose 10% during that span.
The overall increase in U.S. gun deaths since the beginning of the pandemic includes an especially stark rise in such fatalities among children and teens under the age of 18. Gun deaths among children and teens rose 50% in just two years , from 1,732 in 2019 to 2,590 in 2021.
How has the rate of U.S. gun deaths changed over time?
While 2021 saw the highest total number of gun deaths in the U.S., this statistic does not take into account the nation’s growing population. On a per capita basis, there were 14.6 gun deaths per 100,000 people in 2021 – the highest rate since the early 1990s, but still well below the peak of 16.3 gun deaths per 100,000 people in 1974.
The gun murder rate in the U.S. remains below its peak level despite rising sharply during the pandemic. There were 6.7 gun murders per 100,000 people in 2021, below the 7.2 recorded in 1974.
The gun suicide rate, on the other hand, is now on par with its historical peak. There were 7.5 gun suicides per 100,000 people in 2021, statistically similar to the 7.7 measured in 1977. (One caveat when considering the 1970s figures: In the CDC’s database, gun murders and gun suicides between 1968 and 1978 are classified as those caused by firearms and explosives. In subsequent years, they are classified as deaths involving firearms only.)
Which states have the highest and lowest gun death rates in the U.S.?
The rate of gun fatalities varies widely from state to state. In 2021, the states with the highest total rates of gun-related deaths – counting murders, suicides and all other categories tracked by the CDC – included Mississippi (33.9 per 100,000 people), Louisiana (29.1), New Mexico (27.8), Alabama (26.4) and Wyoming (26.1). The states with the lowest total rates included Massachusetts (3.4), Hawaii (4.8), New Jersey (5.2), New York (5.4) and Rhode Island (5.6).

The results are somewhat different when looking at gun murder and gun suicide rates separately. The places with the highest gun murder rates in 2021 included the District of Columbia (22.3 per 100,000 people), Mississippi (21.2), Louisiana (18.4), Alabama (13.9) and New Mexico (11.7). Those with the lowest gun murder rates included Massachusetts (1.5), Idaho (1.5), Hawaii (1.6), Utah (2.1) and Iowa (2.2). Rate estimates are not available for Maine, New Hampshire, Vermont or Wyoming.
The states with the highest gun suicide rates in 2021 included Wyoming (22.8 per 100,000 people), Montana (21.1), Alaska (19.9), New Mexico (13.9) and Oklahoma (13.7). The states with the lowest gun suicide rates were Massachusetts (1.7), New Jersey (1.9), New York (2.0), Hawaii (2.8) and Connecticut (2.9). Rate estimates are not available for the District of Columbia.
How does the gun death rate in the U.S. compare with other countries?
The gun death rate in the U.S. is much higher than in most other nations, particularly developed nations. But it is still far below the rates in several Latin American countries, according to a 2018 study of 195 countries and territories by researchers at the Institute for Health Metrics and Evaluation at the University of Washington.
The U.S. gun death rate was 10.6 per 100,000 people in 2016, the most recent year in the study, which used a somewhat different methodology from the CDC. That was far higher than in countries such as Canada (2.1 per 100,000) and Australia (1.0), as well as European nations such as France (2.7), Germany (0.9) and Spain (0.6). But the rate in the U.S. was much lower than in El Salvador (39.2 per 100,000 people), Venezuela (38.7), Guatemala (32.3), Colombia (25.9) and Honduras (22.5), the study found. Overall, the U.S. ranked 20th in its gun fatality rate that year .
How many people are killed in mass shootings in the U.S. every year?
This is a difficult question to answer because there is no single, agreed-upon definition of the term “mass shooting.” Definitions can vary depending on factors including the number of victims and the circumstances of the shooting.
The FBI collects data on “active shooter incidents,” which it defines as “one or more individuals actively engaged in killing or attempting to kill people in a populated area.” Using the FBI’s definition, 103 people – excluding the shooters – died in such incidents in 2021 .
The Gun Violence Archive, an online database of gun violence incidents in the U.S., defines mass shootings as incidents in which four or more people are shot, even if no one was killed (again excluding the shooters). Using this definition, 706 people died in these incidents in 2021 .
Regardless of the definition being used, fatalities in mass shooting incidents in the U.S. account for a small fraction of all gun murders that occur nationwide each year.
How has the number of mass shootings in the U.S. changed over time?
The same definitional issue that makes it challenging to calculate mass shooting fatalities comes into play when trying to determine the frequency of U.S. mass shootings over time. The unpredictability of these incidents also complicates matters: As Rand Corp. noted in a research brief , “Chance variability in the annual number of mass shooting incidents makes it challenging to discern a clear trend, and trend estimates will be sensitive to outliers and to the time frame chosen for analysis.”
The FBI found an increase in active shooter incidents between 2000 and 2021. There were three such incidents in 2000. By 2021, that figure had increased to 61.
Which types of firearms are most commonly used in gun murders in the U.S.?
In 2020, the most recent year for which the FBI has published data, handguns were involved in 59% of the 13,620 U.S. gun murders and non-negligent manslaughters for which data is available. Rifles – the category that includes guns sometimes referred to as “assault weapons” – were involved in 3% of firearm murders. Shotguns were involved in 1%. The remainder of gun homicides and non-negligent manslaughters (36%) involved other kinds of firearms or those classified as “type not stated.”
It’s important to note that the FBI’s statistics do not capture the details on all gun murders in the U.S. each year. The FBI’s data is based on information voluntarily submitted by police departments around the country, and not all agencies participate or provide complete information each year.
Note: This is an update of a post originally published on Aug. 16, 2019.
- Partisanship & Issues
- Political Issues
- Politics & Policy

About 1 in 4 U.S. teachers say their school went into a gun-related lockdown in the last school year
Striking findings from 2023, key facts about americans and guns, for most u.s. gun owners, protection is the main reason they own a gun, gun violence widely viewed as a major – and growing – national problem, most popular.
1615 L St. NW, Suite 800 Washington, DC 20036 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Age & Generations
- Coronavirus (COVID-19)
- Economy & Work
- Family & Relationships
- Gender & LGBTQ
- Immigration & Migration
- International Affairs
- Internet & Technology
- Methodological Research
- News Habits & Media
- Non-U.S. Governments
- Other Topics
- Race & Ethnicity
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
Copyright 2024 Pew Research Center
Terms & Conditions
Privacy Policy
Cookie Settings
Reprints, Permissions & Use Policy
REVIEW article
This article is part of the research topic.
Pine Bark Extract: Nutrition and Metabolism
Pycnogenol ® French maritime pine bark extract in randomized, double-blind, placebo-controlled human clinical studies Provisionally Accepted

- 1 Horphag Research, Switzerland
- 2 Institute for Pharmaceutical and Medical Chemistry, Faculty of Chemistry and Pharmacy, University of Münster, Germany
The final, formatted version of the article will be published soon.
Pycnogenol ® French maritime pine bark extract is a well-known and thoroughly studied patented extract from the bark of Pinus pinaster Ait. ssp. Atlantica. In 39 randomized double-blind, placebo-controlled (RDP) human clinical trials including 2009 subjects, Pycnogenol ® French maritime pine bark extract supplementation for two weeks to six months has been shown to beneficially affect cardiovascular health, chronic venous insufficiency, cognition, joint health, skin health, eye health, women's health, respiratory health and allergies, oral health and sports performance. The mechanisms of action that can explain the respective effects on different conditions in the human body are discussed as well. As investigated in several in vitro, in vivo and in clinical studies, Pycnogenol ® French maritime pine bark extract showed antioxidative effects, antiinflammatory abilities, beneficial effect on endothelial function and reinforcing effects on the extracellular matrix. The present review aims to give a comprehensive overview of currently available "gold standard" RDP trials of Pycnogenol ® 's benefits across various health domains compared to placebo. In addition, some of the processes on which the presented effects of Pycnogenol ® French maritime pine bark extract are based will be elucidated and discussed. This broad overview of RDP studies on Pycnogenol ® in different health domains can be used as a basis for further research on applications and mechanisms of this unique French maritime pine bark extract.
Keywords: Pycnogenol ®, Pine bark extract, placebo controlled, double-blind, antioxidant, antiinflammatory, Endothelial health, cardiovascular
Received: 21 Feb 2024; Accepted: 22 Apr 2024.
Copyright: © 2024 Weichmann and Rohdewald. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Dr. Franziska Weichmann, Horphag Research, Geneva, Switzerland
People also looked at

IMAGES
VIDEO
COMMENTS
Quantitative research methods involving human subjects include case studies, case series, and n-of-1 studies, all of which focus on the description and analysis of individual cases. They include observational methods such as case-control and cohort studies, which track and compare groups of people over time (either prospectively or ...
According to 45 CFR 46 , a human subject is "a living individual about whom an investigator (whether professional or student) conducting research: Obtains information or biospecimens through intervention or interaction with the individual, and uses, studies, or analyzes the information or biospecimens; or. Obtains, uses, studies, analyzes, or ...
Prospective Single Subject Case Study Review/Report. Often social/behavioral in nature. In-depth prospective analysis and report involving unique or exceptional observations or experiences about one, or a few, individual human subjects. Is intended to contribute to generalizable knowledge. This type of project is considered Human Subjects ...
The United States Department of Health and Human Services' Office for Human Research Protections (OHRP) defines a human subject as a living individual about whom an investigator conducting research obtains data through intervention or interaction with the individual, or identifiable private information ().Institutional review boards (IRBs) exist to protect subjects, in part by reviewing ...
A study is considered research with human subjects if it meets the definitions of both research AND human subjects, as defined in the federal regulations for protecting research subjects. Research. A systematic inquiry designed to answer a research question or contribute to a field of knowledge, including pilot studies and research development.
Research on Humans. Research on human subjects is essential for developing new tests, treatments, and processes that save untold lives and reduce human suffering. For cases in which alternatives such as cell cultures, computer models, or research involving animals are insufficient, ethical and properly controlled studies with human subjects may ...
Human subjects research is at the intersection of these two federal definitions and must obtain Institutional Review Board approval before starting, regardless of the type of design involved. The topic of a human research study varies and can include building a theory or hypothesis, determining patient satisfaction, or testing a medication ...
HHS and NIH experts address many of these questions, along with resources, guidance, and case studies in a 2-part webinar series focused on Human Subjects Research Policies, Clinical Trials, and Inclusion (Day 1 and Day 2). Feel free to break out the popcorn and binge the whole video or check out these sections for the information you need:
Core issues regarding human subject research. Basic ethical principles such as respect for persons, beneficence, and justice are common to most ethical codes in the world, and in turn inform and are linked to (1) the notion of informed consent, (2) the assessment of risks and benefits, and (3) the selection of human subjects and discrimination.
At least three important premises underlie these principles. The first is that studies with human subjects are necessary for improvements in health and welfare. Second, to conduct such research is a privilege, not a right, extended to researchers by society, institutions, and the research subjects themselves.
In the case of biospecimens, the human subject is the person from whom the specimen was taken. ... Human subjects research studies that do not qualify for an exemption are referred to as non-exempt human subjects research. Unless there is a Secretarial waiver, they must comply with the Common Rule regulatory requirements, including IRB review ...
Human subject research is systematic, scientific investigation that can ... Of note currently in the research field is the manner in which researchers direct their conversations with potential human subjects for a research study. ... or a central laboratory. For example, a clinical drug trial case at the University of Minnesota that was ...
Research with Human Subjects. Physicists occasionally perform research involving human subjects. Examples of such research include educational studies, biophysics investigations, and surveys. Federally funded institutions are required to have appropriate procedures in place to ensure that the health and privacy of human subjects are protected.
The need for approval rests on three seemingly obvious but not always easy-to-interpret considerations: 1) whether the work qualifies as research, 2) whether it involves human subjects, and 3) whether it is exempt. All three considerations are discussed in the Common Rule and guide decision making about the use of human subjects in research.
Introduction. ORI Introduction to RCR: Chapter 3. The Protection of Human Subjects. The use of human subjects in research benefits society in many ways, from contributing to the development of new drugs and medical procedures to understanding how we think and act. It also can and has imposed unacceptable risks on research subjects.
Amy Waltz, JD, CIP Associate Director - Regulatory Affairs, Reliance, Outreach Indiana University. Abstract: Understanding context is key to understanding the regulations and complying with regulatory requirements. This article explores the historical context and events that shaped the current human subjects protection regulations and how changes in human subjects research and public ...
Background: Institutional review boards (IRBs), duly constituted under the Office of Human Research Protection, have the federally mandated responsibility of reviewing research involving human subjects to ensure that a proposed protocol meets the appropriate ethical guidelines before subjects may be enrolled in any study. The road leading to the current regulations and ethical considerations ...
Research Ethics Cases for Use by the NIH Community. Theme 23 - Authorship, Collaborations, and Mentoring (2023) Theme 22 - Use of Human Biospecimens and Informed Consent (2022) Theme 21 - Science Under Pressure (2021) Theme 20 - Data, Project and Lab Management, and Communication (2020) Theme 19 - Civility, Harassment and ...
PHS Administrative Action Bulletin Board. Annual Report System. ORI Blog. Feb-07. Tracey Randolph Remembered. Jan-30. Fiscal Year (FY) 2024 Notice of Funding Opportunity Announcement: Ensuring Research Integrity - Conferences. Jan-18.
ORD Guidance on Transitioning VA Human Research Studies to the Revised Federal Policy for the Protection of Human Subjects. Transitioning to 2018 Requirements. 2019-01-09. Crosswalk Comparison of VHA Handbook 1200.05 (dated November 12, 2014) vs. VHA Directive 1200.05. VHA Directive 1200.05 General Guidance.
Pew Research Center
The Office of Extramural Research (OER) has developed a quick decision tool that should assist you with determining if your research involves human subjects, may be considered exempt from Federal regulations, or is not considered human subjects research. This tool should not be used as the sole determination of exemption. Note: This tool uses ...
Pre-Award and Post-Award Processes. Learn about the process of applying for a grant, cooperative agreement, or R&D contract, as it relates to the involvement of human subjects research. Find useful resources on how to prepare your Protection of Human Subjects section, and learn about next steps after submitting your grant application or proposal.
Pew Research Center illustration. A 2019 Pew Research Center study and follow-up study in 2020 involved the complicated task of transcribing more than 60,000 audio and video files of sermons delivered during religious services at churches around the United States. The primary goal of this research was to evaluate relatively broad topics discussed in the sermons to determine if there were any ...
The National Research Act of 19741 created the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research.2 The act charged the commission with identifying the "basic ethical principles which should underlie the conduct of biomedical and behavioral research involving human subjects" and with developing associated guidelines for the ethical conduct of ...
The Office for Human Subject Protection has recently updated their Study Team Member Onboarding & Training Tool and Informed Consent Observation Tool.. The Study Team Member Onboarding & Training Tool was created to facilitate onboarding and training of new study team members; it provides suggestions for general orientation tasks, research-related training, systems access and training, and ...
About eight-in-ten U.S. murders in 2021 - 20,958 out of 26,031, or 81% - involved a firearm. That marked the highest percentage since at least 1968, the earliest year for which the CDC has online records. More than half of all suicides in 2021 - 26,328 out of 48,183, or 55% - also involved a gun, the highest percentage since 2001.
Pycnogenol ® French maritime pine bark extract is a well-known and thoroughly studied patented extract from the bark of Pinus pinaster Ait. ssp. Atlantica. In 39 randomized double-blind, placebo-controlled (RDP) human clinical trials including 2009 subjects, Pycnogenol ® French maritime pine bark extract supplementation for two weeks to six months has been shown to beneficially affect ...