An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Diagnostics (Basel)


An Update on the General Features of Breast Cancer in Male Patients—A Literature Review
Sinziana ionescu.
1 1st Clinic of General Surgery and Surgical Oncology, Bucharest Oncology Institute, 022328 Bucharest, Romania; moc.liamg@03ucsenoianaiznis (S.I.); [email protected] (L.S.)
2 Department of Surgery, “Carol Davila” University of Medicine and Pharmacy, 050474 Bucharest, Romania
Alin Codrut Nicolescu
3 Roma Medical Center for Diagnosis and Treatment, 011774 Bucharest, Romania
Marian Marincas
Octavia-luciana madge.
4 Faculty of Letters, University of Bucharest, 050663 Bucharest, Romania
Laurentiu Simion
Associated data.
Not applicable.
Male breast cancers are uncommon, as men account for less than 1 percent of all breast carcinomas. Among the predisposing risk factors for male breast cancer, the following appear to be significant: (a) breast/chest radiation exposure, (b) estrogen use, diseases associated with hyper-estrogenism, such as cirrhosis or Klinefelter syndrome, and (c) family health history. Furthermore, there are clear familial tendencies, with a higher incidence among men who have a large number of female relatives with breast cancer and (d) major inheritance susceptibility. Moreover, in families with BRCA mutations, there is an increased risk of male breast cancer, although the risk appears to be greater with inherited BRCA2 mutations than with inherited BRCA1 mutations. Due to diagnostic delays, male breast cancer is more likely to present at an advanced stage. A core biopsy or a fine needle aspiration must be performed to confirm suspicious findings. Infiltrating ductal cancer is the most prevalent form of male breast cancer, while invasive lobular carcinoma is extremely uncommon. Male breast cancer is almost always positive for hormone receptors. A worse prognosis is associated with a more advanced stage at diagnosis for men with breast cancer. Randomized controlled trials which recruit both female and male patients should be developed in order to gain more consistent data on the optimal clinical approach.
1. Introduction
Female breast cancer is the most frequently diagnosed tumor and one of the leading causes of cancer-related mortality worldwide. Male breast cancer is uncommon, representing less than one percent of all breast cancers. It is more prevalent in elderly men and resembles postmenopausal breast cancer in its behavior, according to various studies, including that of Garreffa [ 1 ]. However, the incidence is increasing, reaching 15 percent in some patient groups over the course of their lives. A study by Abdekwahab Yousef [ 2 ] reports that age, hormonal imbalance, radiation exposure, and a family history of breast cancer are the most significant risk factors for the development of male breast cancer. Instances of the latter can be linked to mutations in genes with high or low penetrance. A BRCA2 gene mutation is the most important risk factor for the development of male breast cancer.
The majority of cases are diagnosed late due to a lack of awareness of the existence of this cancer in males and ignorance of the associated risk factors.
In addition to the above-mentioned issues, men with breast cancer have an elevated risk of developing a second cancer.
2. Materials and Methods
In order to achieve an updated and extensive literature review on the theme of male breast cancer, an ample search was conducted between the 9 and 23 May 2022 in several international databases, as follows: (1) On www.scicencedirect.com , accessed on 9 May 2022, the search terms were: “breast cancer in male systematic review” between 2000 and 2022 and also “male breast cancer”, with the mention that “review” as type of article between 2018 and2022 and having “medicine and dentistry” as a domain. Furthermore, another association of terms that was searched for was “breast cancer in men systematic review”. (2) On www.pubmed.gov , the terms “male AND breast AND cancer” and also “breast cancer in men” were looked up with the settings: systematic reviews from the year 2000, in humans, articles in English. (3). Other quests on: www.pubmedncbi.nlm.nih.gov , www.oxfordjournals.org, and www.sciencedirect.com , accessed on 23 May 2022, and having as settings: reviews, in humans, article in English, domain medicine and dentistry, looked also for meta-analysis and randomized control trials, using the terms: ”male AND breast AND cancer” and “male breast cancer surgery”, and “male breast cancer (treatment) (systematic) review study”.
3.1. Risk, Biology, Diagnosis
3.1.1. general facts and specificities of the geographical distribution of male breast cancer.
Breast cancer tumorigenesis is a multi-step process that is believed to correlate with one or more distinct mutations in major regulatory genes at each step. To what extent would a multi-step progression model for sporadic breast cancer differ from that for hereditary breast cancer? This question was addressed by Kenemans [ 3 ]. The researcher found that hereditary breast cancer is characterized by breast cancer susceptibility based on a germline mutation in one allele of a high penetrance susceptibility gene (such as BRCA1, BRCA2, CHEK 2, TP53, or PTEN). Inactivation of the second allele of these tumor suppressor genes would be an early event in this oncogenic pathway (two-hit model of Knudson). Another idea presented by this study was that sporadic breast cancers are caused by a stepwise accumulation of acquired and uncorrected mutations in somatic genes, with no role played by germline mutations. Mutational activation of oncogenes, frequently coupled with non-mutational inactivation of tumor suppressor genes, is likely an early event in sporadic tumors, followed by at least four or five independent mutations in other genes.
Taking into consideration that men’s breast cancer causes, optimal treatments, and medical/psychosocial consequences are poorly understood, a systematic review of the literature in the English language was conducted by Ruddy [ 4 ] to identify various research materials relevant to breast cancer in men, between 1987 and 2012, materials that included at least 20 patients. Known risk factors encompass BRCA2 mutations, (80 times the risk of the general population, according to Fox [ 5 ], Peshkin [ 6 ]), and according to Nguyen [ 7 ], there are 11 other gene mutations, apart from BRCA, that might trigger a similar outcome. Other factors implicated are age, conditions that alter the estrogen/androgen ratio, and radiation. Due to the incomplete clinical picture resulting from insufficient studies, diagnostic and treatment tactics in men are generally induced from those in women, even though disease biology is different between the two sexes. According to a study by Gucalp [ 8 ], male breast cancer is almost exclusively hormone receptor positive (+), including androgen receptor (AR), and is associated with a higher prevalence of BRCA2 germline mutations, particularly in men at an increased risk for developing high-risk breast cancer. To better characterize male BC, additional research is required. Men may experience sexual and hormonal side effects of endocrine therapies, as well as unique psychosocial effects of the disease, as part of their survivor issues.
The CHEK2 kinase (Chk2 in mice) is a DNA damage response pathway component.
The impact of checkpoint kinase 2 (CHEK2) mutations as a prognostic factor in the pathogenesis of breast cancer was studied also by Ansari [ 9 ], who underlines that, in cell signaling pathways, CHEK2 is regulated by the influence of upstream genes, and, that, in addition, CHEK2 regulates a number of downstream genes. Moreover, mutations in CHEK2 cause BC cells to be resistant to chemotherapy and expand to other organs. The research also mentions that the detection of mutations in CHEK2 can be used as a prognostic factor for patient response to treatment and for targeting molecules involved in the proliferation of breast tumor cells that are downstream of CHEK2 . Mutations such as c.1100delC and I157T distinguish susceptible patients to a metastatic form of the disease.
In a systematic review by Liang [ 10 ], CHEK2* 1100delC was associated with an increased risk of breast cancer in both men and women. In a study performed on mice and reported by Bahassi [ 11 ], it was found that subjects with CHEK2 *1100delC SNP were predisposed to cancer with a strong gender bias. Furthermore, a recent systematic literature review by Chamseddine [ 12 ] looked at several male breast cancer (MBC) susceptibility genes. Different genes involved have been found, but the risk for individuals who have a pathogenic variant in each of these genes (i.e., penetrance) is not currently known, exactly. In order to better summarize current estimates of penetrance, an analysis of studies was done on the subject of reporting the penetrance of MBC susceptibility genes. From 12,182 abstracts, 15 studies measuring gene penetrance covering 5 putative male breast cancer genes were found: ATM, BRCA1, BRCA2, CHEK2, and PALB2 . This study supports the conclusion that pathogenic variants in ATM, BRCA2, CHEK2 c.1100delC, and PALB2 increase the risk of MBC, while pathogenic variants in BRCA1 may not be associated with an increased risk of MBC. Moreover, Friebel [ 13 ] shows in a systematic review that the cancer risk of women who have inherited a BRCA1 or BRCA2 (BRCA1/2) mutation is highly variable.
Although additional research is necessary to confirm certain associations, sufficient information is available to use certain risk factors in risk counseling or lifestyle modification to reduce cancer risk in BRCA1/2 mutation carriers.
A recent (2021) systematic review and meta-analysis by Davey [ 14 ] looks at the relevance of the 21-gene expression assay in male breast cancer. The 21-gene assay provides prognostic information for early female breast cancer patients with estrogen receptor positivity and human epidermal growth factor receptor-2 negativity (ER+/HER2−). This signature in male breast cancer has not been validated. The results of the research showed that, for early-stage, ER+/HER2− breast cancer patients undergoing 21-gene expression assay testing, the expected scores for females and males are comparable. In the absence of stage matching, these results must be interpreted with caution. Validation of the 21-gene MBC assay is still necessary in a future study with stage matching between the two sexes.
A study by Fentiman [ 15 ] underlines the endocrine risk factors. The significant increase in global age-standardized mean BMI in men is likely to lead to an increase in the incidence of diabetes of adulthood and metabolic bone disease (MBD).
Obesity is accompanied by metabolic changes that decrease androgens and sex hormone-binding globulin (SHBG), thereby increasing the availability of estrogens. Klinefelter’s syndrome (XXY) is associated with a 50-fold increase in MBC incidence compared to XY males; this is the strongest evidence for testicular dysfunction amplifying risk.
Symptomatically diagnosed cancers in men are typically advanced, indicating that earlier detection could improve prognoses. A research by Woods [ 16 ] identified potential screening benefits of screening high risk patients, such as high sensitivity and early detection.
In a review by Nofal [ 17 ], it was concluded that male breast cancer typically presents as a painless retro-areolar mass requiring triple evaluation. The diagnosis requires a high index of suspicion due to the lack of awareness of this type of cancer in males.
In a study dating from 2021, Pizzato [ 18 ] looked at mortality data in male breast cancer by analyzing, from 2000 to 2017, official death certification data and population estimates for breast cancer in men, reported by the WHO and Pan American Health Organization. Death rates standardized by age were computed for selected countries and regions worldwide. Between 2015 and 2017, central-eastern Europe had a rate of 2.85 per million people, and Russia had a rate of 2.22, placing them among the highest. North-western and southern Europe, the European Union as a whole, and the United States exhibited rates ranging from 1.5 to 2.0. Lower rates were observed in most Latin American nations, with values below 1.35/1,000,000, compared to 1.22/1,000,000 in Australia and 0.58/1,000,000 in Japan. Age-adjusted death rates decreased between 10 and 40 percent in 2000–2004 and 2015–2017 in north-western Europe, Russia, and the United States, and between 1.5 and 25 percent in the other regions studied, with the exception of Latin America (+0.8 percent). With the exception of central-eastern Europe, the anticipated rates for 2020 were favorable.
The favorable trends in male breast cancer mortality rates over the past several decades are likely primarily attributable to advances in management. In some areas, the higher mortality rate is due to delayed diagnosis and limited access to effective treatment.
Ndom [ 19 ] included in a literature review papers that contained data on both male and female breast cancers in Africa, and if both male and female breast cancer were available, the article was included. If two publications covered the same geographical region, only the one with the longer study period was included. In total, 1201 male and 36,172 female breast cancer patients from 27 African countries were analyzed using random effects models and meta-regressions with mixed effects. In addition, male breast cancer patients in Africa were diagnosed at an average age of 54.6 years, seven years older than female patients. Male breast cancers in Africa are characterized by their late onset, and the male-to-female breast cancer ratio in Africa is higher than in developed nations. Fentiman [ 15 ] emphasizes the fact that the higher rates of MBC in northern and equatorial Africa are largely attributable to liver damage caused by endemic bilharziasis and hepatitis B, which results in elevated estradiol (E2) levels from hepatic androgen conversion.
In a retrospective study on male breast cancer in Tunisia, Methamem [ 20 ] found that invasive ductal carcinoma was the most prevalent histological subtype (95 percent of our patients). The series was divided into three immunohistochemical groups, with luminal A (68.2%), followed by luminal B (27.3%), and a single patient with a triple-negative tumor (4.5 percent). At 5 and 10 years, the overall survival rate (OSR) was 83.2 percent and 76.8 percent, respectively. Recurrence-free survival (RFS) was 64.5 percent at 5 years and 58.6 percent at 10 years. Age, clinical and histological tumor size, the presence of distant metastases, and the occurrence of recurrence all had a significant impact on the OSR. Recurrence-free survival (RFS) was affected by age, clinical and histological tumor size, and dermal infiltration.
A study by Ssentongo [ 21 ] finds that regional, subregional, gender, and racial disparities influence breast cancer survival rates in Africa.
Consequently, measures are urgently required per region- and race-specific public health interventions coupled with prospective genetic studies to improve breast cancer survival in this region.
3.1.2. Gynecomastia and Pediatric Cases
Gynecomastia has been described as the leading cause of male breast enlargement. This could be physiological, idiopathic, or pathological in nature, as stated in a review by Shaaban [ 22 ]. A review by Billa [ 23 ] found that 45 to 50 percent of adult men with GM may have an underlying pathology, such as aggravating medications, systemic diseases, obesity, endocrinopathies, or cancer. Mammography and ultrasound are both sensitive and specific for distinguishing GM from breast cancer. When clinical findings suggest malignancy and imaging results are inconclusive, histological confirmation should be sought. It is essential to distinguish between gynecomastia, a common cause of male breast enlargement, and breast cancer for proper treatment.
In comparison with core needle biopsy, fine-needle aspiration biopsy (FNA) has been demonstrated as sensitive and specific in assessing breast tumor lesions in female patients. Few studies of this nature have been conducted on men. In a study presented by Hoda [ 24 ], an evaluation was done of the patients who had fine-needle aspiration (FNA) at Massachusetts General Hospital. The procedure had been performed for palpable breast lesions, in the timeline January 2007–December 2016. The conclusion was that FNA allows for the evaluation and diagnosis of palpable male breast lesions in a sensitive, specific, and safe manner.
As presented in a study by Ghilli [ 25 ], secretory breast cancer (SBC) is one of the rarest forms of breast cancer (BC), accounting for the vast majority of BC in children. Nonetheless, it generates a great deal of interest due to its peculiar morphology and genetic characteristics. SBC is a rare form of breast cancer characterized by triple-negative characteristics and an unexpectedly favorable prognosis. More information is required to fully comprehend this cancer’s behavior, and genomic profiling may help improve its diagnosis and treatment.
Gynecomastia has been described also as appearing after a previous diagnosis of childhood cancer (of any type), as shown in a study by Shahriari [ 26 ], which shows that more than 80% of children with cancer today can be cured. The treatment of childhood cancer focuses not only on improving survival, but also on reducing late effects. The purpose is that children with a cancer diagnosis survive and enjoy a high quality of life. Gynecomastia and fertility outcomes of childhood cancer survivors should be considered in the follow-up of adolescents and young adults, and should be approached accurately and managed by multidisciplinary teams. Moreover, in a systematic review presented by Wang [ 27 ], subsequent male breast cancer (SMBC) in cancer survivors, compared to the general population can have an elevated risk of appearance, but the absolute risk is low. Male CCSs (childhood cancer survivors) with symptoms possibly related to SMBC should be thoroughly examined.
3.1.3. Metastases
A literature review by de Almeida Freire [ 28 ] draws attention towards uncommon metastatic sites. For instance, in the oral and maxillofacial region of male patients, breast metastases are exceedingly rare; however, clinicians should consider breast metastasis when evaluating reddish oral nodules in older patients, including men, particularly those with a history of malignancy. More so, in point of clinical approach, it is important to know and have in mind the aspect according to which, even if some metastatic sites are very rare, they can be the first clinical manifestation of an occult male breast cancer. This previous aspect was underlined by Kesting [ 29 ] and Gonzalez-Perez [ 30 ].
Invasive lobular carcinomas(ILC) are rarer than ductal forms and are known to have unusual metastatic locations, and they can appear as primary tumors, such as may be the case of the pancreas, for instance, as described by Mor [ 31 ].
Statistically, approximately 20% of cancer patients have brain metastases (BMs). This proportion rises with age to roughly 40 percent among those under 18 years old. However, the actual prevalence may be higher, as these estimates are typically restricted to individuals who are being evaluated for therapy. According to a study presented by Che [ 32 ], most BMs metastasize from lung cancer (40–50%), breast cancer (15–25%), and melanoma (5–20%). However, cancer patients with BMs continue to have a poor prognosis, with a relatively low median survival (2.9 months for newly diagnosed malignancies) and 2-year survival rate (8%). Increasing evidence suggests that gender is associated with the survivability of the vast majority of malignant tumors. In addition to that, many studies have demonstrated that the male gender is an independent risk factor for a shorter survival rate in BMs patients. The conclusion of the study was that middle-aged females had an increased risk of developing BMs, whereas middle-aged males with BMs had an increased risk of poor survival, an aspect underlined also by Leone [ 33 ] and Singh [ 34 ].
Although a few similar cases have been reported, there have been no reports of subtype conversion in similar cases. Consequently, Oh [ 35 ] presents the case of a male patient with brain metastasis of invasive ductal carcinoma and HER2 status conversion subsequent to metastasis.
Male breast cancer with brain metastasis is an extremely uncommon condition, with even rarer depiction at the level of the cerebellopontine angle, which can manifest with acute onset and rapid progression of symptoms: deterioration of the level of consciousness and intracranial hypertension, as explained by Tahrir [ 36 ] in a study describing a triple negative male breast cancer patient.
The period of time in which metastases to the brain can occur in relation to the moment of the initial diagnosis is emphasized by Furchinoue [ 37 ], who mentions a 24-year range.
3.1.4. Image-Based Diagnostic Methods
Due to the small size of male breasts, physiological and pathological processes arising from the breast and anterior chest wall may have similar clinical manifestations, as presented by Yang [ 38 ]. As described by Mango [ 39 ], men presenting with breast symptoms may pose unique diagnostic difficulties for the radiologist, especially if imaging findings are not typical for gynecomastia or carcinoma. When radiological findings are ambiguous or suspicious, imaging is frequently necessary to localize and characterize the lesion and guide biopsy. Mammography, digital breast tomosynthesis (DBT), and ultrasound are the cornerstones of breast imaging evaluation. Symptomatic male breast imaging begins with a diagnostic mammogram and targeted ultrasound in patients 25 years old, as reported in a review performed by Chesebro [ 40 ]. If the breast finding is insufficiently imaged or there is occult on mammography, targeted ultrasound is required.
Similarly, mammography must be performed if the breast finding on targeted ultrasound in a younger patient is suspicious. Occasionally, additional imaging techniques such as computed tomography (CT), magnetic resonance imaging (MRI), and positron-emission tomography (PET) can supplement the investigation and aid in the planning of treatment.
In a systematic review by Dondi [ 41 ], which aimed to analyze the diagnostic performance and utility of 18F-FDG PET/CT in the evaluation of MBC, it was found that, despite the limitations of the review, 18F-FDG PET/CT appears to be an effective method for assessing MBC. The conclusion continued with the idea that further research is required to clarify the role of hybrid imaging with 18F-FDG in the evaluation of MBC, particularly in comparison to breast cancer in females. Figure 1 a–f illustrates various aspects of PET-CT in a patient diagnosed with mail breast cancer.

( a – f ) Subsets illustrating PET-CT/ low dose CT aspects of male breast tumors. Courtesy of Dr. Mirela Gherghe, affiliated to the Nuclear Medicine Department of the Bucharest Oncology Institute and “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
In order to assess the potential added value of SPECT-CT quantitative analysis in the detection and differentiation of metastatic breast cancer lesions from degenerative lesions, a study was conducted by Gherghe [ 42 ]. The SUVmax value of metastatic bone lesions was significantly higher than that of degenerative bone lesions ( p < 0.001). At an SUVmax cutoff value of 16.6 g/mL, the diagnostic accuracy of SPECT-CT quantitative data analysis revealed a sensitivity of 91.5 percent and a specificity of 93.3 percent. The conclusion of the research was that quantitative analysis of SPECT-CT data can improve the diagnostic accuracy of distinguishing metastatic bone lesions from degenerative bone lesions, leading to more appropriate treatment and improved follow-up in metastatic breast cancer patients.
In the given literature context that microcalcifications (MCs) are significant breast cancer disease markers, numerous studies have been conducted on their characterization in female breast cancer (FBC), but their composition in male breast cancer is unknown (MBC). According to Caldarone [ 43 ], as Raman spectroscopy (RS) is a molecular spectroscopy that can rapidly and without staining examine the biochemical composition of MCs, an algorithm to identify the mineral components present in MCs from Raman images can be used to study and compare MCs identified on breast cancer pieces from male to female patients. This suggests that these patients have characteristics that distinguish them from the FBC previously studied.
In a review from 2018, Shin [ 44 ] found that the literature on the use of breast MRI in male patients is also extremely limited.
Although it is uncommon and not recommended as a standard clinical practice to perform breast MRI on male patients, even in the presence of a breast cancer diagnosis, there are a few instances in which MRI may be helpful to clinicians and surgeons.
3.1.5. Pathology
Similar to the “female breast”, the “male breast” is home to a number of pathological conditions. However, histological-anatomical differences in the female breast result in numerous variations in disease frequency and presentation, as explained by Onder [ 45 ]. We hereby present in this section general information on the common types found and an exhaustive list of uncommon types versus their prognosis and clinical significance.
Shaaban [ 22 ] finds that the most prevalent histology is grade 2 ductal carcinoma with no special subtype. MBC is frequently of the luminal A phenotype comparable to postmenopausal breast cancer in women. A study by Fentiman [ 46 ] showed that using hierarchical clustering, ER was clustered with PR in FBC, but with ER and AR in MBC.
Oncotype DX appears to be effective in determining recurrence risk in selected MBC based on limited data. A study by Cho [ 47 ] looked at the use of HE images, a deep-learning algorithm that may be able to predict the efficacy of adjuvant chemotherapy in cancer patients. The Lunit SCOPE algorithm was developed using HE slides from 1343 breast cancer patients. Lunit SCOPE was trained using the 21-gene assay (Oncotype DX) and histological parameters to predict recurrence. The risk prediction model accurately predicted the Oncotype DX score > 25 and the recurrence survival of the validation cohort and TCGA cohorts. The predicted risk was positively associated with proliferation-associated Oncotype DX genes and negatively correlated with estrogen-related prognostic genes. The risk of cancer recurrence and the early-stage hormone receptor-positive breast cancer patients who would benefit from adjuvant chemotherapy were predicted by an integrative analysis utilizing Lunit SCOPE.
Papillary in situ and invasive carcinomas are not uncommon in the male breast, unlike the female breast. Zhong [ 48 ] finds in a review that papillary lesions of the male breast papillary carcinomas span a wide clinicopathological spectrum, and both invasive and noninvasive papillary carcinomas have a favorable prognosis, as it is also reported by Avau [ 49 ].
As presented by Fox [ 5 ], metastases to the breast also occur, but according to Akinseye [ 50 ], they are rare and a differential diagnosis with a primary lesion should be done. Lymphoma or leukemia, lung cancer, melanoma, prostate, gastric, renal, endometrial, pancreatic, esophageal, and thyroid cancers are the most prevalent primary malignancies affecting the breasts (in descending frequency). In most cases, the presence of breast metastases indicates a widespread disease. The prognosis is typically poor, and treatment focuses on the primary cancer.
For instance, Anagnostopoulou [ 51 ] describes how male breast lymphoma is a rare extranodal lymphoma of the mammary gland that may be primary or secondary. A breast lesion’s excisional biopsy revealed chronic lymphocytic leukemia (CLL) with plasmacytoid features and immunoglobulin Gkappa monotypic expression in the mammary tissue.
Atypical ductal hyperplasia (ADH) greatly increases a woman’s risk of developing breast cancer. Nevertheless, not much is known about the effects of ADH in men. In a study conducted by Coopey [ 52 ] with a mean follow-up period of 6 years, no males in the series developed breast cancer. The data inferred that, either ADH in men does not have the same risk as ADH in women, or surgical excision of a symptomatic gynecomastia lesion in men can effectively reduce the odds of breast cancer.
As presented by Wu [ 53 ], male cases of accessory breast cancer and sweat gland cancer associated with extramammary axillary Paget’s disease are uncommon. In clinical diagnosis and treatment, it is necessary to precisely identify the disease and devise an appropriate treatment plan based on the patient’s condition.
Mesenchymal lesions of the breast include benign, reactive, and malignant conditions, as described by Carder [ 54 ]. They typically present to the pathologist as spindle cell lesions, and many have distinctive histological and immunohistochemical characteristics, an aspect emphasized also by Raj [ 55 ].
A phyllodes tumor (PT) is a prototypical fibroepithelial neoplasm that accounts for 1% of breast neoplastic lesions that are typically detected in females and rarely in males. On the basis of predetermined morphological criteria, the World Health Organization classifies tumors as benign, borderline, or malignant. Infrequently documented in the English literature, squamous differentiation in phyllodes tumors represents epithelial metaplasia, as shown also by Panigrahi [ 56 ]. It has been difficult to determine which phyllodes tumors may behave aggressively. In this context, a study described by Lerwill [ 57 ] looks at the discovery of MED12 mutations in the pathogenesis of fibroepithelial tumors, along with other gene abnormalities in the progression pathway, which has allowed for the improvement of prognosis and diagnosis. Another study, by Ma [ 58 ], finds that nonbenign PTs can be predicted independently by tumors in the family, lobulation, and cystic components. Moreover, the prediction nomogram developed based on these characteristics can be used as a supplementary instrument for preoperatively classifying PTs.
Dermatofibrosarcoma protuberans (DFSP) is a soft tissue sarcoma that accounts for approximately 1 percent of all tumors. In addition, DFSP is frequently observed on the trunk and extremities, whereas breast occurrences are uncommon, as stated by Bouhani [ 59 ]. A high index of clinical suspicion is required for its detection and differentiation from simple wound complications and local recurrences of other benign lesions, as is shown by Sung [ 60 ].
Paget’s disease in the breast manifests with eczematous changes of the nipple-areolar complex and, in the majority of cases, is accompanied by an underlying in situ or invasive breast carcinoma, an aspect underlined by Vergine [ 61 ].
Paget’s disease is characterized histologically by epithelial cells with an abundance of basophilic or amphophilic, finely granular cytoplasm, and a large, centrally located nucleus, which are most prevalent in the lower epidermal layers. Due to the rarity of the condition among breast cancers and the rarity of breast cancer in men, knowledge of the disease’s presentation, course, and optimal treatment in male patients is derived primarily from case reports and extrapolation of findings from studies in female patients. Paget’s disease must be distinguished from eczema, Bowen’s disease, squamous cell carcinoma, and melanoma, among others. In a study by Adams [ 62 ], it was also emphasized that Paget’s disease must be identified clinically and pathologically, as the superficial lesion may be the only indication of an underlying ductal carcinoma and its presence may have prognostic significance.
Figure 2 and Figure 3 illustrate different pathology caracteristics of breast cancer in male patients.

Biopsy sample from a breast tumor of a male 66 y.o.: left panel: papillary carcinoma of the breast, HE, 100×, top right panel: estrogen receptor positive in tumor cells nuclei (Allred score: 8), bottom right panel: Ki-67 positive in ~75% of the tumor cells nuclei, IHC, 100×.

Surgical resected specimen, same case: left panel: invasive mammary carcinoma, right panel: tumor invasion in an axillary lymph node, HE, 100×. Figure 2 and Figure 3 are presented courtesy of Dr. Mihai Ceausu, affiliated to the Pathology department of the “Prof. Dr. Al. Trestioreanu” Bucharest Oncology Institute and Associate Professor at the “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
3.2. Treatment
Tamoxifen is a selective estrogen receptor modulator (SERM) that is utilized to treat all stages of hormone receptor-positive breast cancer in men and women. Aromatase inhibitors (AIs) are a class of drugs used to treat postmenopausal women and men with breast cancer. Aromatase catalyzes an essential aromatization step in the synthesis of estrogen.
In order to determine the effect of adjuvant treatment with tamoxifen and aromatase inhibitors (AI) on the survival of male breast cancer patients, a study by Eggemann [ 63 ] analyzed 257 male breast cancer patients with positive hormone receptor status. The overall survival of male breast cancer patients treated adjuvantly with tamoxifen was significantly greater than that of those treated with an aromatase inhibitor.
According to the ASCO guideline published in 2020, as presented by Hassett [ 64 ], many of the treatments for breast cancer in men are similar to those used for women. Men presenting hormone receptor-positive breast cancer who are candidates for adjuvant endocrine therapy have an indication to receive tamoxifen for an initial duration of five years; those who cannot be given tamoxifen due to counterindications may be prescribed a gonadotropin-releasing hormone agonist/antagonist plus aromatase inhibitor. After five years of tamoxifen therapy, those having tolerated the treatment and continuing to have high odds of recurrence may be given an additional five years of treatment. Men with early-stage disease should not be administered bone-modifying agents to prevent recurrence, but these substances may still be administered to prevent or treat osteoporosis. With the exception of cases of visceral crisis or rapidly progressive disease, men with advanced or metastatic disease should receive endocrine therapy as the initial treatment option. Targeted systemic therapy may be used to treat advanced or metastatic cancer using the same indications and combination treatments available for women. Men with a history of breast cancer treated with lumpectomy should receive an ipsilateral annual mammogram regardless of genetic predisposition; men with a history of breast cancer and a genetic predisposition mutation may receive a contralateral annual mammogram. All men diagnosed with breast cancer should receive genetic counseling and testing for cancer susceptibility genes in the germline.
In a study done by Trapani [ 65 ] in 2021 on the global aspect of treatment standards in breast cancer, it was found that this global landscape of BC treatment standards reveals that the majority are not context-appropriate. Research on the formulation of cancer treatment standards and novel platforms for developing and disseminating resource-appropriate guidance are of the utmost importance.
T extent of changes in estradiol levels in male patients receiving standard endocrine therapies for hormone receptor-positive breast cancer is unknown and the impact of these changes on sexual function and quality of life has not been adequately evaluated, as shown in a randomized clinical trial conducted by Reinisch [ 66 ]. In order to complete the research, patients were randomly assigned to 1 of 3 treatment arms for 6 months: tamoxifen alone, tamoxifen plus gonadotropin-releasing hormone analogue (GnRHa), or aromatase inhibitor (AI) plus GnRHa. The primary outcome parameter was represented by the change in estradiol concentrations from baseline to three months. After 3 and 6 months, secondary endpoints included changes in estradiol levels, additional hormonal parameters, adverse effects, sexual function, and quality of life. This phase 2 randomized clinical trial revealed that AI or tamoxifen plus GnRHa versus tamoxifen alone resulted in a sustained reduction in estradiol levels. The decreased hormonal parameters were associated with diminished sexual performance and life quality.
Giving an expert opinion on best methods of approach, Duso [ 67 ] presents that there is a significant medical need to include male breast cancer patients in (more) prospective clinical trials. The call for equality in breast cancer care can be pursued in two divergent ways: (i) a gender-neutral delivery of breast cancer information, and (ii) the creation of separate sections in common studies, one for the more prevalent female breast cancer and the second for the rare male breast cancer. We propose to differentiate male breast cancer care, recognizing that males have distinct onco-sexual and social needs that can only be shared partially with women.
In a review by Ahmed Jang Khan [ 68 ], it was found that, in certain cases, breast conserving surgery (BCS) with sentinel lymph node biopsy (SLNB) remains an alternative to mastectomy for men with early-stage breast cancer. The identification and false-negative rates for SLNB were comparable to those of breast cancer in female patients, according to a study presented by Lin [ 69 ], who also shows that survival is improved by post-mastectomy radiation to the chest wall and 5-year tamoxifen treatment.
Disease staging and sentinel lymph node biopsy were also studied by Carter [ 70 ], Gherghe [ 71 ], and Bordea [ 72 ], who show that standard treatment for women with clinically N0 breast cancer is sentinel lymph node biopsy (SLNB) and that it can also be associated, during the same intervention, with the localization of an occult breast lesion. However, there are no randomized controlled trials determining the optimal surgical management of the axilla in men. In males with clinically N0 breast cancer, the use of SLNB alone has increased while ALND has declined. Patients who underwent SLNB alone during the later time period, as shown by Carter [ 70 ], however, exhibited worse clinical characteristics and experienced variations in adjuvant therapy. This indicates a growing acceptance of SLNB for axillary management. Methods of axillary staging and their influence on the prognosis of males with breast cancer warrant additional investigation.
3.2.1. Breast Conserving Surgery versus Mastectomy
Fentiman [ 73 ] underlined that it is possible to make a compelling case for the use of neoadjuvant endocrine therapy to facilitate breast-conserving surgery. Although nomograms for predicting nodal status are inadequately calibrated, sentinel node biopsies have been utilized successfully to stage MBC. Male mastectomy is associated with psychological side effects, and there is no evidence that the needs of those with MBC are being met. The conclusions drawn by the previously mentioned research was that collaborative research is required so that men can participate in meaningful randomized controlled trials (RCTs) to provide a rational, evidence-based basis for the surgical treatment of MBC. Furthermore, Williams [ 74 ] discusses the context for neoadjuvant treatment in relation to advanced disease and aims at the determination of the prevalence of neoadjuvant therapy (NT) in MBC patients and its effect on BCT (breast conserving therapy). The conclusion was that males with invasive breast cancer are expected to have a low BCT rate, but NT appears to decrease the use of mastectomy in patients with locally advanced cancers. Understanding the effects of BCT on locoregional recurrence, disease-free survival, and overall survival for MBC requires additional research.
In a study by Bakalov [ 75 ], it was underlined that, while adj-RT after BCS is associated with decreased mortality in MBC patients, adj-RT is omitted in up to one-third of MBC cases after BCS, despite being the standard of care.
Breast conserving surgery (BCS) was studied in comparison with mastectomy in male breast cancer (MBC). In a study by Saunder [ 76 ], a systematic literature review was done on studies that reported one or more of the following: overall survival (OS), disease free survival (DFS) and disease specific survival (DSS) stratified (sorted, selected) by surgical treatment (BCS and/or mastectomy), and/or radiotherapy compliance with BCS. The conclusion of the analysis was that the majority of studies found no differences between BCS and mastectomy in which concerns the DFS, DSS, or OS. The results emphasized that BCS is a viable treatment option for MBC because it was associated with comparable oncologic outcomes to mastectomy, as was also shown by de La Cruz [ 77 ]. However, the low rates of radiotherapy adherence among male patients who underwent BCS are concerning and demonstrate the importance of involving patients with MBC in the selection of a surgical treatment strategy.
A study by Deldar [ 78 ] looks at the aspects of postmastectomy reconstruction in male breast cancer and concludes that there is limited availability of research on chest reconstruction after mastectomy in male breast cancer patients.
Nonetheless, the available evidence suggests that reconstruction can restore a patient’s body image; therefore, it should be considered and discussed routinely with male patients.
3.2.2. Adjuvant Treatment
Tamoxifen is the only endocrine agent approved for the prevention and treatment of premenopausal and postmenopausal estrogen-receptor-positive breast cancer, as well as the treatment of male breast cancer.
Endoxifen, a secondary metabolite resulting from CYP2D6-dependent biotransformation of the primary tamoxifen metabolite, N-desmethyltamoxifen (NDT), is a more potent antiestrogen than either NDT or tamoxifen, the parent drug, as indicated by Dreger [ 79 ], Sanchez-Spitman [ 80 ], and Ahmed [ 81 ]. In addition to its effects on ER, endoxifen’s antitumor effects may involve additional molecular mechanisms of action. In phase 1/2 clinical studies, as is presented by Jayaraman [ 82 ], the efficacy of Z-endoxifen, the active isomer of endoxifen, was evaluated in patients with endocrine-refractory metastatic breast cancer, as well as patients with gynecologic, desmoid, and hormone-receptor-positive solid tumors, and demonstrated promising antitumor activity.
Palbociclib is a targeted or biological therapy drug which belongs to the CDK (cyclin dependent kinase) inhibitors and can be used in locally advanced and secondary breast cancer that is estrogen-receptor positive and HER2negative.
A report by Kraus [ 83 ] investigated the advantages and disadvantages of palbociclib plus endocrine therapy (ET) in men with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2 negative (HER2) metastatic breast cancer (MBC).
A review of the global safety database revealed no new safety signals in men treated with palbociclib. Real-world data indicated that palbociclib plus ET is beneficial for men with MBC, with a safety profile consistent with previous findings in women with MBC.
This report’s data on palbociclib in women and men, including clinical trial data, real-world data, and a well-established risk/benefit profile, led to US approval of an indication expansion for palbociclib to include men with metastatic breast cancer.
Ribociclib belongs to the class of drugs known as kinase inhibitors. It functions by inhibiting the action of an abnormal protein that instructs cancer cells to proliferate.
Leuprolide is a GnRH agonist approved by the FDA for the treatment of endometriosis, uterine leiomyomata (also known as uterine fibroids), central precocious puberty in children, and advanced prostate cancer. Off-label uses include, among others, the treatment of breast cancer and hormone therapy for male-to-female transgender patients.
Goserelin is a drug used to suppress the production of sex hormones (testosterone and estrogen), specifically gonadotropin-releasing hormone agonist (GnRH agonist).
As presented recently by Campone [ 84 ], CompLEEment-1 ( {"type":"clinical-trial","attrs":{"text":"NCT02941926","term_id":"NCT02941926"}} NCT02941926 ) is a single-arm, open-label, multicenter phase IIIb study evaluating the safety and efficacy of ribociclib plus letrozole (RIB + LET) in a large, diverse cohort of patients who have not previously received endocrine therapy (ET) for advanced disease, in an intent to provide treatment alternatives for advanced male breast cancer HR+, HER2−. Methods: Patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) advanced breast cancer (ABC) who had no prior chemoradiotherapy (ET) and 1 prior line of chemotherapy for advanced disease were administered RIB + LET. Also administered to male patients was goserelin or leuprolide. Safety and tolerability were the primary endpoint; efficacy was a secondary endpoint. Males exhibited the same clinical benefit and overall response rates as the entire population. This study supports the use of RIB + LET in male patients with HR+, HER2− ABC.
3.3. Breast Cancer in Transgender Patients
As summarized in a research by T’Sjoen [ 85 ], The Endocrine Society recommends estrogens in conjunction with androgen-blocking drugs for transgender women. Feminizing treatment with estrogens and antiandrogens results in desirable physical changes, including increased breast growth, decreased facial and body hair growth, and fat redistribution in a female pattern. Patients should be informed of potential adverse effects, particularly those at risk for venous thromboembolism.
The Endocrine Society’s recommendations for transgender men include testosterone therapy for virilization, voice deepening, cessation of menstruation, and increases in muscle mass and facial and body hair. Due to the lack of evidence, gender nonbinary individuals should be treated on an individualized basis. Teenagers may be administered a pubertal suspension containing GnRH analogs, followed by sex steroids. Before any hormonal intervention, options for fertility preservation must be discussed. Morbidity and cardiovascular risk are unaffected by cross-sex hormones in transgender men, whereas it is unknown in transgender women.
Cancers caused by sexual steroid use are possible, but uncommon. The term “transgender” refers to people whose gender identity and/or gender expression is different from the sex they were assigned at birth. As the number of individuals undergoing gender-affirming hormone therapy and gender-affirming surgery increases, radiologists must know progressively more about this population in order to be able to attend them properly. Even if diagnostic imaging methods and approaches for transgender individuals are similar to those for cisgender women, screening guidelines are different. Several professional and institutional guidelines have been developed to address breast cancer screening in the transgender population, particularly mammography screening in transfeminine individuals undergoing hormone therapy, as emphasized by Parikh [ 86 ].
3.3.1. Male to Female
The therapeutic transition from male to female usually begins in late adolescence or adulthood, with the average onset being around 30 years of age. Current hormonal treatment protocols in transgender women combine high doses of estrogen and antiandrogen treatment to reduce testosterone levels in the blood. Likewise, the concomitant administration of progesterone would reduce the potential risk of breast cancer and cardiovascular events, as described by Martinez Ramos [ 87 ]. Male-to-female (MtF) breast cancer cases have nevertheless been reported since 1968, but the breast cancer risk of MtF patients remained unknown at the moment in which Hartley [ 88 ] decided to study the subject by looking at literature updates. Among the conclusions, there was the fact that breast cancer is present in MtF patients, who typically present with a palpable mass at a younger age. Furthermore, pathology-confirmed breast implant-associated anaplastic large cell lymphoma was described in the context of breast augmentation with bilateral silicone implants, as reported by Ali [ 89 ].
3.3.2. Female to Male
The breast cancer risk and screening recommendations for transgender men or female-to-male (FtM) patients are still unknown, according to a systematic review presented by Hartley [ 90 ] that looked at patient demographics, breast cancer characteristics, presentation, and treatment. The conclusion was that breast cancer is present in transgender men, with risk dependent on top surgery; those who have had top surgery appear to have a lower risk than natal females.
Female-to-male (FtM) transsexuals, in the context of their testosterone therapy for masculinization, can have a modified risk of developing breast cancer. The purpose of the study by Fledderus [ 91 ] was to examine the evidence regarding the risk of testosterone therapy on breast cancer in female-to-male transsexuals and to assess breast cancer screening in this subgroup. The research concluded that few cases of FtM transsexuals with breast cancer have been documented. However, cases such as these alert physicians to the possibility that FtM transsexuals may develop breast cancer. Radiological screening of FtM transsexuals for breast cancer prior to mastectomy and histological screening of the mammalian tissue after mastectomy should be taken into account; physicians should appreciate and further decide with each individual FtM transsexual if screening is imposed by clinical or paraclinical data.
An analysis from 2020 by Patel [ 92 ], looking at the long-term effect of hormone replacement therapy HRT, found that, due to the paucity of long-term studies tracking breast pathology among transgender men and women, information about the risks associated with HRT is limited and often contradictory (in the current literature and in this setting). The study concluded that the long-term effects of off-label pharmaceutical use to modify hormone levels and sexual characteristics in transgender patients have not been adequately studied. The propensity of steroid hormones to promote the development of certain cancers raises concerns regarding the safety of varying drug doses and combinations. Additional clinical and laboratory research is required to better establish dosing and safety guidelines for transgender patients.
3.4. Second Cancers Associated with Breast Cancer in Men
According to the definition of the National Cancer Institute, “second primary cancer” is a term used to describe a new primary cancer in a person who has had cancer in the past. Second primary cancers may develop months or years after the primary cancer has been diagnosed and treated. Certain cancer treatments, such as chemotherapy and radiation therapy, may increase the likelihood of developing a second primary cancer. Certain inherited gene mutations (changes) and exposure to certain cancer-causing substances, such as tobacco smoke, may also increase the risk of developing a second primary cancer.
Decades of research have been devoted to the risk of second cancers among breast cancer patients. Men’s second primary tumors, in contrast with those found in women, are poorly understood. Men’s breast cancer risk factors, such as genetic, hormonal, and environmental factors, parallel the causes of breast cancer in women. A review by Grenader [ 93 ] examines the literature concerning the risk of developing a second cancer in male breast cancer patients. Patients with a history of male breast cancer are more likely to develop a second ipsilateral or contralateral breast tumor (standardized incidence ratio 30–110), a phenomenon also underlined by O’Leary [ 94 ]. The risk of subsequent contralateral breast cancer was greatest in men younger than 50 years old at the moment of the initial diagnosis of the neoplasm. Diverse information is available on second primary cancers besides breast cancer. According to one study, the incidence of cancers of the small intestine, prostate, rectum, and pancreas, as well as non-melanoma skin cancer and myeloid leukemia, has increased. Other researchers did not find an increase in the overall risk of subsequent cancer development among men initially diagnosed with primary breast cancer. Although sarcoma, lung, and esophageal cancers are well-known complications of radiation therapy for breast cancer in women, there is no evidence that these cancers are associated with radiation therapy for breast cancer in men.
Cancer treatment is an especially trying time for the patients. They frequently experience multiple side effects concurrently, resulting in a decline in health-related quality of life (HRQoL). A study presented by Charalambous [ 95 ] provides evidence regarding the co-occurrence and interrelationships of pain, anxiety, depression, and fatigue in breast and prostate cancer patients. This research provides evidence that targeting fatigue, anxiety, and depression may have a meaningful effect on pain as a related symptom and may have a positive impact on the HRQL of breast and prostate cancer patients.
3.5. Prognosis
In an update on research concerning male breast cancer, Benassai [ 96 ] and Malinda [ 97 ] state that the outcome of the MBC is worse than the outcome of FBC.
In a comparison between prognostic factors studied in both sexes, Yao [ 98 ] found that as opposed to FBC patients, MBC patients were discovered at more advanced TNM stages, with higher tumor grades, and with a greater proportion of hormone receptor-positive tumors. In addition, the locations of breast tumors differed significantly between males and females, and longer survival rates were found in women. Age, race, TNM stages, tumor grades, estrogen receptor (ER)/progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER-2) overexpression were found to be independent prognostic factors in female breast cancer, according to multivariate Cox regression and competing risks analyses.
MBC and FBC patients had the same risk factors, but PR and HER-2 status did not have an independent influence on survival (all p > 0.05).
Further conclusions were that, in the coming years, it is reasonable to anticipate an increase in the sensitivity of multigenic tests, allowing for a more accurate prediction of recurrence risk. This could result in substantial changes in the selection and duration of treatment, with surprising outcomes.
Treatment adherence is a very important factor in which concerns prognosis, and a study by Ali [ 99 ] compared the endocrine therapy adherence, discontinuation, and survival outcomes of male and female breast cancer patients using the SEER-Medicare linked database. The primary endpoints were rates of endocrine therapy adherence and discontinuation (ET). Adherence was defined as a gap between Medicare prescriptions of less than 90 days. A discontinuation was defined as a 12-month interval between Medicare prescriptions or longer. Secondary outcome measure was the association between ET use and overall survival (OS). Men were significantly more loyal than women, but there was no difference between the sexes in terms of abandonment. On ET, both men and women exhibited a significant survival improvement.
Pensabene [ 100 ] describes that, in addition to non-genetic risk factors, genetic alterations, such as pathogenetic variants in BRCA1/2 and other moderate-/low-penetrance genes, have been identified as pathogenic factors for MBC. The detection of alterations in predisposing genes, especially BRCA1/2 , and the identification of oncogenic drivers distinct from FBC may have preventative and therapeutic implications. However, the approved treatments for MBC are identical to those for FBC. Cancer genetic counseling must be considered in the diagnostic workup of MBC, regardless of the presence or absence of an oncological family history.
The goal of a study by Stahl [ 101 ] was to analyze men with de novo stage IV breast cancer and known estrogen receptor (ER) and progesterone receptor (PR) statuses who underwent systemic therapy, with or without surgery. Patients who died in the first six months were excluded from the study. In male patients with de novo stage IV breast cancer who were ER+ or PR+, it was discovered that those who received surgery, radiation therapy, and systemic therapy (trimodality) had a significant survival advantage over those who received only systemic therapy. The data also revealed a downward trend in the use of surgery in this cohort over time.
In a study based on the proteomic profiling of male breast cancer, Zografos [ 102 ] found a total of 2352 proteins, corresponding to 1249 single gene products with diverse biological functions. A panel of 119 differentially expressed tissue proteins was identified in MBC samples versus controls; 90 were found to be over-expressed in MBC tissues and 29 were found to be downregulated. Concurrently, 844 proteins were detected only in MBC tumors, whereas 197 proteins were expressed exclusively in mammary samples from healthy controls. Differential proteomic expression was identified in MBC tissue, resulting in a better understanding of MBC pathology and highlighting the need for individualized care for male patients.
3.6. Future Trends and Potential Therapeutic Targets in Breast Cancer
3.6.1. aquaporins.
Aquaporins (AQPs) are membrane channels that belong to the large family of major intrinsic proteins (MIPs), of which there are thirteen classes with tissue-specific distributions in humans, as summed up by Khan [ 103 ] and Magouliotis [ 104 ]. As important physiological modulators of water and solute homeostasis, mutations and dysfunctions in aquaporins have been linked to pathologies in all major organs. Jung [ 105 ] emphasizes the fact that an anomalous expression of AQPs has been observed in numerous types of cancer cells and cancer stem-like cells, and it has been proposed as a marker for the proliferation and progression of cancer cells. Consequently, a more comprehensive understanding of AQPs could increase interest in the cell stemness accompanied by AQP expression. The traditional view of AQPs’ role in therapeutic plans restricted them and their regulators to managing a limited range of diseases, such as diabetes insipidus and syndrome of inappropriate ADH secretion. However, additional research, particularly in the third millennium, for instance a study by Ala [ 106 ], has revealed that their cooperation in water transmission control can be manipulated to address other burden-imposing diseases, including cirrhosis, heart failure, Meniere’s disease, cancer, bullous pemphigoid, eczema, and Sjogren’s syndrome. Khan [ 103 ] shows the important association between breast cancer and aquaporins, underlining that AQPs 1, 3, and 5 are highly expressed in breast, endometrial, and ovarian cancers, consistent with their gene regulation by estrogen response elements. Bystrup [ 107 ] considers that understanding the underlying molecular mechanisms of how AQP5 contributes to cancer development and progression is crucial for the potential use of AQP5 as a prognostic biomarker and for the development of targeted intervention strategies for the treatment of breast cancer patients. A similar observation was presented by Traberg-Nyborg [ 108 ] on aquaporin-1 and by Milkovic [ 109 ] on aquaporins 3 and 5. In addition to the above-mentioned statement, a study by Moosavi [ 110 ] found that overexpression of AQP1, AQP3, and AQP5 is inextricably linked to carcinogenesis, metastasis, decreased survival rate, lymph node metastasis, a worse prognosis, and cellular migration and that furthermore, cancer therapies associated with these markers suggest AQP decreases during treatment. A study by Zhu [ 111 ] that analyzed the mRNA of AQPs indicated that high AQP0, AQP1, AQP2, AQP4, AQP6, AQP8, AQP10, and AQP11 mRNA expression levels were significantly associated with improved relapse-free survival (RFS) and that, in contrast, AQP3 and AQP9 were associated with a shorter RFS in breast cancer patients, indicating that these two genes may serve as targets for future chemotherapy.
3.6.2. The Androgen Receptor
Forooshani [ 112 ] mentions that, unfortunately, the prognosis for patients with hormone-negative tumors or patients with therapy-resistance and metastasis remains dismal. New biomarkers are urgently required to predict the disease’s progression, make better therapy decisions, and increase patient survival. In this regard, the Androgen Receptor (AR), a member of the superfamily of nuclear hormone receptors along with ER and PgR, emerges as an intriguing characteristic widely expressed in human BCs. The precise tumorigenic mechanism of the androgen receptor (AR) and the role of its endogenous ligands are not yet well understood, despite recent advances.
Yardley [ 113 ] considers that efforts to validate the AR as a therapeutic target should concentrate on identifying new markers predictive of sensitivity to AR-targeted drugs.
3.6.3. Breast Pre-Cancer Atlas
Microenvironmental and molecular factors mediating the progression of Breast Ductal Carcinoma in situ (DCIS) are poorly understood, which hinders the development of prevention strategies and the testing of treatment de-escalation in a safe manner.
A study by Nachmanson [ 114 ] addressed methodological limitations and characterized the mutational, transcriptional, histological, and microenvironmental landscape of 85 multiple micro-dissected regions from 39 cases.
Phenotypic and subtype heterogeneity was frequently associated with underlying genetic heterogeneity, and according to the inferred phylogeny, regions with low-risk characteristics preceded those with high-risk characteristics.
The spatial analysis of B- and T-lymphocytes revealed three immune states, including an epithelial excluded state located preferentially in DCIS regions and characterized by the histological and molecular characteristics of immune evasion, independent of molecular subtypes.
This breast pre-cancer atlas with its unique integration of observations will aid in the planning of future expansion studies and the development of more accurate models of outcomes and progression risk.
Lactate dehydrogenase C (LDHC), an anticancer target with tumor-specific expression and immunogenicity, is a cancer testis antigen (CTA). Analysis of breast cancer patient cohorts from The Cancer Genome Atlas (TCGA) and Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) indicates that upregulation of LDHC expression is associated with a poor prognosis.
A study by Naik [ 115 ] examined whether LDHC is involved in regulating genomic stability and whether it could be targeted to influence the cellular fitness of tumor cells.
In four breast cancer cell lines, silencing LDHC significantly increased the number of giant cells, nuclear abnormalities, DNA damage, and apoptosis. Cells depleted of LDHC exhibited aberrant cell cycle progression accompanied by differential expression of cell cycle checkpoint and DNA damage response regulators. This previously mentioned research demonstrates the therapeutic potential of targeting LDHC to reduce cancer cell survival and enhance sensitivity to agents that cause DNA damage or inhibit its repair.
3.6.4. Alternative Splicing
Alternative splicing allows cells to diversify their proteome in order to carry out complex biological functions and respond to external and internal stimuli. The spliceosome is the multiprotein-RNA complex responsible for the complex process of alternative splicing.
As a result of abnormal spliceosomes or splicing factors, aberrant splicing can drive the development and progression of cancer, as shown in a review by Murphy [ 116 ].
Recent mapping of the spliceosome, its associated splicing factors, and their relationship to cancer has paved the way for novel therapeutic approaches that capitalize on alternative splicing’s widespread influence.
3.6.5. Squalene Epoxidase
Over fifty percent of cancer patients are treated with radiotherapy; however, radiotherapy as a monotherapy is frequently insufficient and requires a nontoxic radiosensitizer.
Squalene epoxidase (SQLE) regulates the biosynthesis of cholesterol by converting squalene to 2,3-oxidosqualene.
A study by Hong [ 117 ] investigated the significance of SQLE in breast cancer and non-small cell lung cancer (NSCLC), two cancers that are frequently treated with radiotherapy.
SQLE-positive IHC staining was observed in 68% of breast cancer and 56% of NSCLC specimens, compared to 15% and 25%, respectively, in normal breast and lung tissue.
Significantly, SQLE expression was an independent predictor of a poor prognosis, and pharmacologic inhibition of SQLE increased the radiosensitivity of breast and lung cancer cells. The conclusion of the research was that squalene epoxidase inhibitors are novel tumor-specific radiosensitizers that promote ER stress and suppress homologous recombination, providing a novel potential therapeutic approach to improve radiotherapy efficacy.
3.6.6. The Unfolded Protein Response
Estrogen receptor (ER) is a therapeutic target for patients with ER-positive breast cancer.
In endocrine-resistant breast cancer, paradoxically, this is also the initial site where estrogen (E2) induces apoptosis.
How ER displays distinct functions in various contexts is the subject of numerous studies. Among those, a study by Fan [ 118 ] found that unfolded protein response (UPR) is closely correlated with ER-positive breast cancer, according to compelling evidence.
Treatment with antiestrogens induces a mild UPR through ER, activating three UPR sensors in the endoplasmic reticulum: PRK-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6).
These sensors then interact with stress-associated transcription factors, including c-MYC, nuclear factor-B (NF-B), and hypoxia-inducible factor 1 (HIF1), resulting in acquired endocrine resistance.
In a paradoxical manner, E2 induces apoptosis in endocrine-resistant breast cancer by activating the secondary UPR via ER.
Specifically, PERK is essential for apoptosis induction, whereas IRE1 and ATF6 are involved in endoplasmic reticulum stress-associated degradation following E2 treatment.
In addition, persistent activation of PERK degrades stress responses in mitochondria and activates the NF-B/tumor necrosis factor (TNF) axis, thereby determining the cell’s apoptotic fate.
The discovery of E2-induced apoptosis has clinical significance for the treatment of breast cancer resistant to endocrine therapy.
Resistant breast and prostate cancers continue to be a major clinical problem; consequently, new therapeutic approaches and more accurate predictors of therapeutic response are required. Due to the involvement of the unfolded protein response (UPR) in cell proliferation and apoptosis evasion, an increasing number of publications, as presented by Direito [ 119 ] support the hypothesis that dysfunctions in this network cause and/or aggravate cancer. In addition, UPR activation may contribute to the emergence of drug-resistant phenotypes in breast and prostate cancers. Consequently, targeting this pathway has recently emerged as a promising anticancer treatment strategy.
3.6.7. Proteolytic Neoepitopes for RAS-Driven Cancers
Extracellular proteolysis is frequently dysregulated in disease and can generate proteoforms with neoepitopes that are absent from healthy tissue.
An analysis by [ 120 ] demonstrated that antibodies that selectively recognize a proteolytic neoepitope on CUB domain containing protein 1 (CDCP1) may allow for more effective and safer treatment of solid tumors.
CDCP1 is overexpressed and its ectodomain is cleaved by extracellular proteases in RAS-driven cancers, such as breast cancer.
Targeting proteolytic neoepitopes may serve as an orthogonal “AND” gate for enhancing the therapeutic index, an observation even more clinically important as EGFR/RAS pathway activation is prevalent in breast tumors with poor prognosis.
3.6.8. Coumarinyl Thiazolotriazoles
CDK4 and CDK6 are essential regulators of the initial phases of the cell cycle and are a promising anti-cancer treatment option. Structure-based rational design and synthesis of a new class of 1,2,3-triazole-tethered acridinedione derivatives (6a-l) as selective CDK4/6 inhibitors were presented in a study by Praveenkumar [ 121 ]. From the entire series of conjugated hexahydro acridinediones, compound 6 g exhibited the most potent cytotoxic effect. Moreover, in a subline of xenograft mouse models, molecule 6 g suppressed tumor growth with fewer adverse effects, suggesting that it could be considered as a novel chemotherapeutic candidate for further comprehensive preclinical breast cancer research.
A study presented by Khan [ 122 ] synthesized a series of coumarinyl thiazolotriazoles with varied functional group tolerance and their anticancer properties were evaluated against cancer cell lines (HeLa and MCF-7) and a normal cell line (BHK-21). The results suggested that one of the compounds possesses chemotherapeutic properties against breast cancer and cervical adenocarcinoma cells, necessitating additional research to evaluate this compound’s anticancer efficacy at the clinical level.
3.6.9. IL-25
Immune cell-derived factors, such as cytokines and chemokines, play a pivotal role in the progression of cancer. As a member of the IL-17 cytokine subfamily, IL-25 plays a paradoxical role in cancer, both promoting and inhibiting tumor growth.
Gowhari Shabgah [ 123 ] shows that utilizing IL-25-enhancing approaches, such as Virulizin ® (mixture of proteins and peptides as immune response modifiers) and dihydrobenzofuran administration, has potentially inhibited tumor cell growth in cancers in which IL-25 has a tumor-suppressive function. In the case of IL-25-dependent tumor progression, however, the use of IL-25 blocking methods, such as anti-IL-25 antibodies, may be complementary to the other anticancer agent. Collectively, it is hoped that IL-25 could be a promising cancer treatment target.
3.6.10. ILK/YAP Axis
Metastasis is the first among the causes of death in cancer patients. In view of and by trying to oppose that phenomenon, the Epithelial-to-Mesenchymal Transition (EMT), a crucial process in cancer metastasis, became a well-established drug development target. LFG-500, a novel synthetic flavonoid with multiple activities, including modulation of EMT in the inflammatory microenvironment, has been indicated as a potential antitumor agent. Using transforming growth factor beta (TGF)-induced EMT models, a study by Li [ 124 ] discovered that LFG-500 inhibits migration and invasion associated with EMT in human breast cancer. These results support the use of LFG-500 in cancer treatment and bring consistent proof that the ILK/YAP axis is a reliable biomarker of cancer progression and a novel target for repression of EMT and tumor spread.
3.6.11. Antibody-Drug Conjugates and VEGF Receptor Inhibitors
Despite improvements made to conventional chemotherapies, their use is restricted by a narrow therapeutic window due to off-target toxicities. Antibody-drug conjugates (ADCs) consist of an antibody covalently coupled to a toxic payload via a chemical linker. They provide an elegant solution to the limitations of conventional chemotherapeutics by selectively delivering a highly toxic payload directly to target cells, thereby increasing the efficacy of the delivered cytotoxic while simultaneously reducing systemic exposure and toxicity. Each individual component of an ADC, including the target, the antibody, the linker, and its conjugation chemistry, as well as the cytotoxic payload, must be optimized for maximum efficacy.
In a study presented by Marme [ 125 ], it was shown that there are currently nine approved ADCs, including three for breast cancer. With over 100 candidates in various stages of clinical development, the rate of development appears to be quickening.
Cha [ 126 ] looks at the expression of the receptor tyrosine kinase ephrin receptor A10 (EphA10), which is undetectable in most normal tissues and at the correlation with tumor progression and poor prognosis in a number of malignancies, including triple-negative breast cancer (TNBC). The conclusion was that targeting EphA10 with EphA10 monoclonal antibodies (mAbs) and EphA10-specific chimeric antigen receptor-T cell therapy may be a promising strategy for patients with EphA10-positive tumors.
Yang, in an analysis of the VEGFR inhibitors finds VEGFR3 to be more 100 times more selective than VEGFR1 and 2, by showing a selective inhibition of proliferation and migration of the cancer cells.
4. Discussion
Male breast cancer’s (MBC’s) causes, optimal treatments, and medical/psychosocial consequences are poorly understood. Known risk factors include BRCA2 mutations (80 times the risk of the general population), and 11 other gene mutations. Other factors implicated are age, conditions that alter the estrogen/androgen ratio, and radiation. Male breast cancer presents typically as a painless retroareolar mass requiring further evaluation. When initial radiological findings are ambiguous or suspicious, more imaging methods are frequently necessary to localize and characterize the lesion and to guide biopsy (core biopsy/fine needle aspiration).
The global landscape of treatment standards for male patients with breast cancer is not context-appropriate. A call for equality in breast cancer care can be pursued in two divergent ways: a. a gender-neutral delivery of breast cancer information, and b. the recruitment in randomized controlled trials of both sexes.
Acknowledgments
The authors appreciate and would like to thank the valuable contributions in providing clinical and diagnostic pictures/images that support the theorical framework of this article: Mirela Gherghe (Department of Nuclear Medicine, Lecturer at the “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania) and doctors: Ariana Neicu, Madalina Radu, Assoc. prof. Mihai Ceausu, from the Pathology department of the “Al. Trestioreanu” Bucharest Oncology Institute, Bucharest, Romania.
Funding Statement
This research received no external funding.
Author Contributions
Conceptualization, A.C.N. and S.I.; methodology, O.-L.M.; resources, M.M.; data curation, O.-L.M.; writing—original draft preparation, S.I. and A.C.N.; writing—review and editing, L.S.; visualization, M.M.; supervision, L.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Informed consent statement, data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- Open access
- Published: 06 February 2024
Experiences and perceptions of men following breast cancer diagnosis: a mixed method systematic review
- Mary Abboah-Offei 1 ,
- Jonathan Bayuo 2 ,
- Yakubu Salifu 3 ,
- Oladayo Afolabi 4 &
- Theophilus N. Akudjedu 5
BMC Cancer volume 24 , Article number: 179 ( 2024 ) Cite this article
1051 Accesses
5 Altmetric
Metrics details
Men with breast cancer experience unique physical and emotional challenges. However, a thorough understanding of these experiences including the psychosocial effects and supportive care needs have received less attention. In some settings, men with breast cancer experience stigma within the healthcare system and their care needs are not prioritised. This influences the level of professional support offered, consequently worsening their health and well-being outcomes. This review explored the variabilities in the experiences and treatment modalities of male breast cancer (MBC) across different contexts.
All primary study designs including qualitative, quantitative, and mixed methods studies that reported on the experiences, treatment approaches and outcomes of MBC were included in this systematic review. Six databases (Embase, Medline, PsycINFO, Global Health, CINAHL and Web of Science) were searched for articles from January 2000 to September 2023. A results-based convergence synthesis was used for data analysis and reported using PRISMA guidelines.
Of the studies screened ( n = 29,687), forty-four fulfilled the predetermined criteria and were included. Our findings relating to the experiences and treatment approaches of MBC are broadly themed into three parts. Theme 1—Navigating through a threat to masculinity: describes how males experienced the illness reflecting on detection, diagnosis, coming to terms with breast cancer, and disclosure. Theme 2- Navigating through treatment: captures the experiences of undergoing breast cancer treatment/ management following their diagnosis. Theme 3—Coping and support systems: describes how MBC patients coped with the disease, treatment process, aftercare/rehabilitative care, and the available support structures.
Conclusions
Men experience a myriad of issues following a breast cancer diagnosis, especially with their masculinity. Awareness creation efforts of MBC among the public and healthcare practitioners are urgently required, which could change the perception of men in promoting early diagnosis, adherence to treatments, post-treatment monitoring, oncological results and a better quality of life. Considerations for training, education and development of specialised guidelines for healthcare practitioners on MBC would provide the necessary knowledge and skills to enhance their practice through the adoption of person-centred and male-specific care strategies. Professional care intervention and support for MBC should not end after the diagnosis phase but should extend to the entire treatment continuum and aftercare including future research focusing on MBC specific clinical trials.
Trial registration
PROSPERO Registration No. CRD42021228778.
Peer Review reports
Male breast cancer (MBC) is a rare condition, accounting for less than 1% of all breast cancers. About 2,710 men are estimated to be diagnosed with breast cancer, with approximately 530 men projected to die from breast cancer in 2022 and have about 1 in 833 lifetime risk of being diagnosed with the disease in the United States [ 1 ]. Data from the Global Burden of Disease 2017 database indicate that the incidence of MBC increased from 8.5 thousand in 1990 to 23.1 thousand in 2017 with 123 countries showing a significant increasing trend in MBC incidence rates [ 2 ]. There are variations in the incidence of MBC among countries for instance, in Thailand MBC incidence was lower than that in Israel, and the rate of variability has been attributed to population-specific factors [ 3 ]. Additionally, disparities have been noted in the incidence, prevalence, mortality, and burden of cancer and related adverse health conditions in specific population groups [ 4 ]. Some of these disparities have been noted in the United States, where black men are reported to have higher incidence and mortality rates compared to white men in the context of all cancer [ 4 , 5 , 6 ].
Evidence suggests that MBC is mostly diagnosed late (49%) when the disease is more advanced compared to women (33%) leading to relatively worse prognosis [ 7 , 8 , 9 , 10 , 11 ]. This has been attributed to delayed presentation, lack of screening, reduced awareness by treating providers and a lack of awareness of the disease among men [ 12 , 13 , 14 , 15 ]. Consequently, MBCs are mainly diagnosed with more severe clinical manifestations with relatively complex tumour characteristics (i.e., larger sizes and extensive lymph node involvement) [ 16 ], associated with higher proportions of positive hormone receptors, which mostly results in prolonged treatment delay, and metastasis of the disease at diagnosis compared to female breast cancer [ 17 ]. This has been influenced by issues with lower socioeconomic status, barriers to accessing healthcare and insurance cover issues in the context of the United States, adherence to treatment, post-treatment follow-up, and stigma [ 7 , 18 , 19 , 20 ]. MBC patients suffer from a triple stigma including stigma by healthcare professionals, society, and especially by themselves as they struggle to accept the disease which has been labelled as a woman's disease [ 20 ].
Treatment for MBC has mainly been informed by available evidence for female breast cancer [ 21 ], and no randomised data exists for optimal management strategies for men including surgery, systemic therapy, and radiation [ 22 ]. Some guidelines have been published for the management of MBC [ 23 , 24 , 25 ]; however, these guidelines are rarely based on clinical trials leading to a paucity of literature on the evaluation of outcomes for MBC. According to Corrigan et al. [ 26 ], of the 131 breast cancer clinical trials conducted, there was only 0.087% of male patients represented among study participants.
Moreover, MBC being widely described as a 'woman’s disease' has psychosocially impacted the experience of men in terms of their body image and appearance as well as masculinity [ 27 , 28 ]. A critical psychosocial problem for MBC patients is concerns with body image [ 29 ], because both the disease and its treatment can lead to significant alterations to their looks and how the body functions [ 30 ]. With masculinity often associated with chest rather than breast [ 31 , 32 , 33 ], being linked to a “woman’s disease” attributed to the body part that men do not relate to is probably threatening their masculinity [ 34 ]. Men with breast cancer also face unique physical and emotional challenges however, there is inconclusive understanding of men’s experiences of the psychosocial implications of MBC as well as the supportive care needs [ 35 , 36 ]. Therefore, in this review, we explored the experiences of MBC patients and the management approaches across different demographic contexts.
Review question
What are the experiences and perceptions of MBC patients following diagnosis?
We conducted a mixed method systematic review with an interpretive and inductive stance [ 37 ] and reported in line with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [ 38 ].
Search strategy
We identified relevant studies through a search in six electronic databases: Global Health, CINAHL, Medline, PsycINFO, Embase, and Web of Science. Furthermore, we searched reference lists of included studies for additional studies. The search duration in these databases covered January 2000 to December 2023, and was updated in September 2023.
A combination of the following keywords was used for search strategy i) ‘Men’ OR ‘Male’ OR ‘Father’ OR ‘Husband’ AND ii) ‘Breast cancer’ OR ‘Breast carcinoma′ OR ‘Breast neoplasm’ OR ‘Breast tumour’ AND iii) ‘Experiences’ OR ‘Perceptions’ OR ‘Perspectives’ OR ‘Opinions’ AND iv) ‘Treatment’ OR ‘Approaches’ OR ‘Outcomes’. Multiple variations of the keywords were used including the truncations based on database requirements to broaden to capture all relevant studies.
Inclusion and exclusion criteria
This review included all primary studies of any design (qualitative, quantitative, or mixed methods) that report on MBC (included only men assigned male gender at birth); studies focussing on the experiences, perceptions, and treatment approaches for MBC; as well as studies conducted and reported in English (based on the resources available to the researchers). However, letters, editorials, commentaries, perspectives, case reports, opinion pieces, news reports and systematic reviews on MBC; studies reporting on cancers in men other than MBC; those that did not report on MBC experiences; as well as those reported in languages other than English were excluded.
Data extraction, quality assessment, synthesis and analysis
Search results were imported into Endnote reference manager (version 20) by the first reviewer (MA-O), duplicates removed and titles as well as abstracts were screened. The remaining studies were screened against the inclusion/ exclusion criteria, by three reviewers (MA-O, JB, OA), and any study for which inclusion was unclear was discussed and resolved by YS and TNA. Full texts studies were obtained if abstracts did not have enough information to determine the relevance of an article. Study variables such as authors, countries where studies were conducted, aims/objectives, study design, sample size and characteristics, experiences of MBC with verbatim quotes, MBC treatment approaches with outcomes and conclusions drawn were extracted to a common table (see Table 1 ).
We used a results-based convergent design [ 75 ] to guide data analysis, where we initially synthesised qualitative and quantitative findings separately, before integrating these findings from the two designs in the final analysis and synthesis (see Fig. 1 ). This allowed us to synthesise quantitative findings regarding treatment approaches of MBC and qualitative or mixed methods results on the experiences of MBC patients.
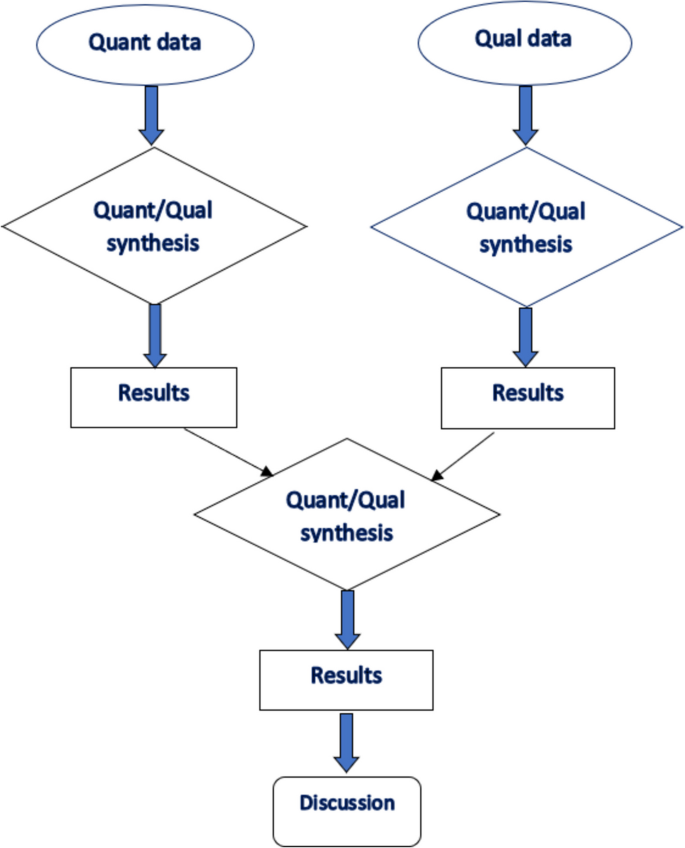
A flow diagram on the results-based convergent design
Descriptive statistics was used in reporting the number of published studies and presented in a PRISMA flow diagram in Fig. 2 . We synthesised the descriptions of MBC experiences and treatment approaches reported across studies. All studies were analysed descriptively. To synthesise the data regarding the experiences of men with breast cancer, verbatim quotes reported in the qualitative studies were extracted by two authors (JB & TNA). An interpretive and inductive stance was employed [ 37 ] by reviewing verbatim quotes to generate codes (see Table 2 ). Similar codes were aggregated to generate sub-themes followed by formulation of higher order themes. For the quantitative data regarding the treatment modalities, we focused on describing the main reported treatment modalities rather than their frequencies. At the end of the analysis, both the qualitative findings and descriptions from the quantitative studies converged as one dataset. The themes generated from the initial process and the descriptions obtained from the quantitative studies formed the basis of undertaking a narrative synthesis.
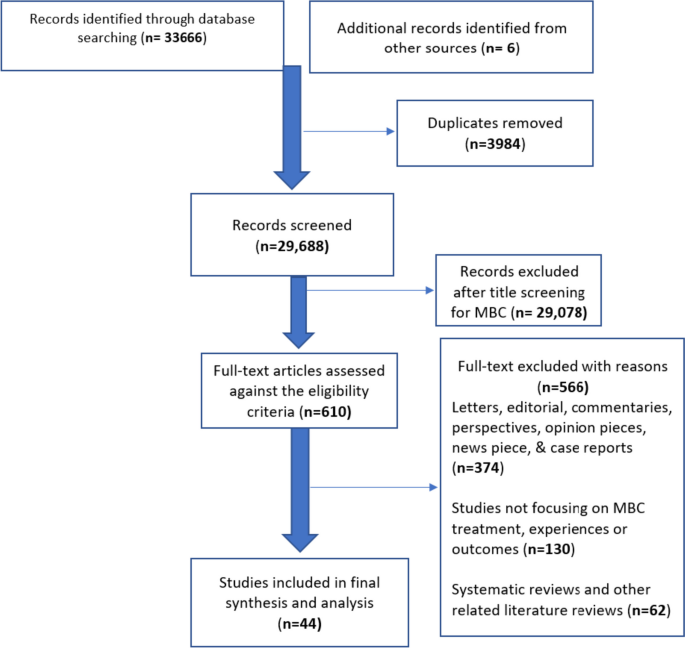
PRIMA flow chart of study search and selection process
The quality of included studies was assessed using the Quality Assessment Tool for Studies with Diverse Designs (QATSDD) tool [ 76 ], which is designed for use in mixed methods reviews and quality reporting in reviews that included qualitative, quantitative, mixed- and multi-methods research to ensure consistent and critical appraisal of relevant studies. In assessing study quality, studies were categorised as high quality if they achieved an aggregate score in excess of 70%, moderate quality were assigned to studies scoring between 50 and 70%, and those scoring less than 50% were assigned low quality (see Table 1 ). However, no study was excluded based on respective aggregate quality scores.
Study characteristics
Of the n = 610 full-text articles assessed for eligibility. N = 374 were excluded as these were letters, editorials, commentaries, perspectives, case reports, opinion pieces and news reports on MBC; including n = 130 studies that did not report on MBC experiences and perceptions; and n = 62 that were MBC related reviews (see Fig. 2 ). Following extensive search and screening, 44 studies were retained in the final synthesis and analysis, with publication years ranging from January 2000 to September 2023. Twenty-nine studies employed varied quantitative designs, 8 studies employed qualitative designs, and 6 studies employed mixed-method designs. Although most of the studies (n = 44) included only MBC, two retrospective studies compared males and females with breast cancer, and only the data reported on males were included in this review [ 58 , 68 ]. Study characteristics including quality assessment grading are reported in Table 1 .
Experiences and perceptions of males with breast cancer
As shown in Table 2 , three themes and nine sub themes emerged from the data which encapsulate the experiences of males with breast cancer.
Theme 1: Navigating through a threat to masculinity and one’s existence
This theme describes how males experienced the illness reflecting on detection, diagnosis, coming to terms with the disease, and disclosure. The subthemes are 1) emergence and awareness of a foreign illness and threat to one’s existence 2) coming to terms with a gendered disease and 3) opening up/ coming out of the illness closet. All included nine qualitative studies highlighted how the affected men perceived breast cancer as a threat to their sense of masculinity.
Emergence and awareness of a foreign illness and threat to one’s existence
Males generally perceived breast cancer as a feminine illness which cannot affect their bodies [ 31 , 34 ]. In fact, although all the men in the included studies had heard about breast cancer, most of them had not previously heard about breast cancer in males which made them rule out any possibility of ever living with it and may have contributed to delay in seeking healthcare [ 31 , 49 ]. This perception and the emerging non-specific symptoms often delayed early health seeking as the symptoms were interpreted as irrelevant or not requiring urgent attention [ 49 ]. It is worth highlighting that most of the affected men presented with palpable lump in the breast or discharge from the nipple of the affected breast. Some men had to be ‘pushed’ by their wives or partners to seek medical attention to rule out the possibility of breast cancer; a condition they felt was out of their scope [ 49 , 71 ]. A breast cancer diagnosis was met with varied emotions including being dumbfounded, shocked, surprised, debilitating stress, and a feeling of housing a feminised illness in a masculine body which threatened their sense of masculinity and personhood [ 13 , 31 , 34 , 49 ].
“…there is no reason why I shouldn’t have cancer, I’m only the same as anyone else. I’m just a bit disappointed really about where it got me. it’s not right on a man, is it? [ 31 ] (p.467). “From others at work, I always (hear) ‘admit it, you’re just trying to find excuses. You’re not a real man, or you wouldn’t have such an illness’. [ 34 ] (p.8). ‘I suppose the fact that it was breast cancer surprised me. The fact that it was cancer I suppose was a shock . . . So, I suppose a combination of both. You know the fact that it was breast cancer which I do not think I had heard of and the fact that it was cancer’’ [ 13 ] (p.336).
Receiving the diagnosis was challenging which some men kept to themselves or only informed family/ close friends [ 71 ]. The notion of breast cancer being a feminine illness made men view the disease as foreign or exotic to their bodies [ 49 ]. The growing awareness of the disease made the men feel a sense of oddity and shame for having a feminine illness alongside a feeling of losing one’s manhood to an illness not considered masculine [ 31 , 49 ]. Worry, anxiety, and uncertainty also marked their increasing awareness of the disease particularly regarding how the disease could distort the shape of their ‘masculine chest’ [ 13 ]. Despite the varied emotions, some males felt extremely lucky that the cancer was located at a site not considered ‘vital’ in terms of masculinity [ 67 ].
My biggest problem was how to tell my wife that I have a woman’s disease? Because I thought maybe you’re not a real man, perhaps half woman?” [ 34 ] (p.8). “Now when I first knew that I had it, I thought to myself …well how did Dickens get breast cancer? I’m not a woman. I’m a man. I was surprised more than anything… Women, it's an ever-present threat … Men – never occur to them. ‘‘When I first knew I did not want everyone knowing, because I did not want everyone coming round sympathising’’ . [ 13 ] (p.336).
Further to the above, the diagnosis of breast cancer forced the affected men to come face to face with their own mortality. This is because they felt a diagnosis of breast cancer threatened their existence and equated to a death sentence. The realisation of death lurking close by pushed the affected men to increase their efforts in attaining their dream before they died. This experience helped them to be more appreciative of their present lives, increased their consciousness about their health, and helped them to redefine their values and beliefs [ 60 ]:
“I appreciate life a lot more. Before my cancer, I didn’t take life seriously. I took life for granted. I didn’t appreciate the people in my life and the things I see. So, after the cancer, it was a good kick in the butt. Just how much you appreciate it, and also made me realise to go after my dreams, chase it, and achieve it. Go after it and every day is a gift” [ 60 ] (p.3).
Coming to terms with a gendered disease
Through the journey of receiving a breast cancer diagnosis and living with the illness, the affected men expressed the insights and perceptions they gained regarding living with an uncommon illness that is believed to affect mostly women [ 60 ]. Following the breast cancer diagnosis, males were faced with the reality of living with a condition they did not expect to have. Coming to terms with a feminised disease was gradual and a lonely journey for the affected men. In fact, some wished they could give their condition another name instead of breast cancer. The fear of being stigmatised made some men keep their diagnosis to themselves [ 13 , 32 ]. Others also felt a sense of awkwardness discussing such sensitive issues and would avoid [ 13 ]. Taken together, men with breast cancer often concealed or attempted to re-label their diagnosis to manage their sense of stigma, shame, and oddity as they navigated through coming to terms with living with a “feminine disease” in their masculine bodies [ 13 , 32 , 66 ]:
‘‘I told the guys I played golf with that I’d got cancer; I do not think so. I necessarily told them it was breast cancer’’. [ 13 ] (p.337) “…but if I did, I would talk about it as chest cancer. I wouldn’t use breast cancer. So that would be the term I would use, and, in the conversation, I would say that it is the same as breast cancer. It’s exactly the same thing; it’s just in my chest.” [ 66 ] (p. 964). “I think among old men they almost consider it to be a stigma, they almost don’t want to tell people, you know, it’s some kind of, I don’t know, a black mark, but I never looked at it that way…I think people younger would just view it a little differently, you know it’s cancer, it’s something they have to deal with, it doesn’t really matter what type of cancer it is.” [ 67 ] (p.37)
Opening up/ coming out of the illness closet
As the men gradually came to terms with living with the “foreign or exotic disease”, they were able to talk to their families and close friends about their diagnosis [ 13 ]. This required a lot of courage to navigate through such a sensitive issue. Interestingly, the men noted that the process of openly discussing their diagnosis in social spheres and coming out to others offered them an opportunity to reassert the meaning of masculinity, particularly as they recognize how fragile their masculine bodies are [ 31 ]:
“When I spoke to people about it, they thought I was telling fairy tales … that was really the worst thing about it.” [ 34 ] (p.8). “I want to prove to everybody that MBC is not a women’s disease and that a normal man can have MBC.” [ 31 ] (p.468).
In two studies, however, the authors described the phenomenon of selective disclosure in which the men only disclosed their illness to selected persons only [ 20 , 60 ]. For some men, the selective disclosure also meant revealing just the diagnosis, but not going further to reveal how they are experiencing the treatment process or the aftermath of the illness:
“The children know and our closest friends know, the very closest. Why? Because I disappeared for a while. I don’t talk about it within the family, not at all. Nobody talks with me about it, but they know. It is only information, and that’s it, not about the experience and not about the surgery, and not about the treatment” [ 20 ] (p.5).
Theme 2: Navigating through treatment
The theme captures the experiences of undergoing breast cancer treatment/ management following their diagnosis. The subthemes are 1) therapeutic interventions 2) navigating through feminised treatment pathways and 3) living with the effects of care/ ongoing treatment. All included qualitative, quantitative, and mixed method studies ( n = 44) highlighted the treatment experiences and pathways respectively.
Therapeutic interventions
Several therapeutic interventions/ treatments were reported across the included studies. Five categories of treatments were ascertained across the included studies, and these are surgery, radiotherapy, chemotherapy, hormonal therapy, and palliative care. Surgical interventions included mastectomy with axillary dissection, mastectomy with sentinel node biopsy (both for men with late-stage breast cancer presentation), and lumpectomy [ 7 , 40 , 45 , 46 , 47 , 48 ]. Cronin et al., [ 46 ] noted that surgery and chemotherapy receipt were more likely among men up to age 65. In some studies, surgical interventions were the main forms of treatment with radiotherapy, chemotherapy, and hormone therapy playing adjuvant roles. For instance, in one study that included 37 men with breast cancer, radiotherapy (89.2%), hormonal therapy (56.7%), and chemotherapy (91.8%) were adjuvant therapies after surgery [ 48 ]. In one study, the authors reported several therapeutic regimens offered to men with breast cancer which included breast conserving surgeries, unilateral/ bilateral mastectomy, often with no reconstruction [ 44 ]. One third of the male breast cancer patients in the same study ( n = 21) felt somewhat or very uncomfortable with their appearance after the surgery. Receipt of treatment was remarkably similar between blacks and whites in both age groups. Older black and white men had lower receipt of chemotherapy (39.2% and 42.0%, respectively) compared with younger patients (76.7% and 79.3%, respectively). Younger black men had a 76% higher risk of death than younger white men after adjustment for clinical factors only (HR, 1.76; 95% CI, 1.11 to 2.78), but this difference significantly diminished after subsequent adjustment for insurance and income (HR, 1.37; 95% CI, 0.83 to 2.24). In those age 65 years, the excess risk of death in blacks versus whites was nonsignificant and not affected by adjustment for covariates.
Navigating through feminised treatment pathways
Despite the reality of breast cancer among males, the care pathways and healthcare payment frameworks across various healthcare systems are significantly tailored to the needs of females which reinforces the notion of the disease as a feminine in nature [ 31 , 71 ]. A study from Germany highlighted the difficulty that these men experience in finding a physician as the practitioners felt their breast care specialty targeted women and would lose on reimbursement [ 34 ]. Even in facilities where they were given satisfactory care, the men felt the services and procedures still failed to consider their unique needs as men with breast cancer [ 31 , 42 , 71 ]. Some men were mistakenly addressed as females on the assumption that only females experienced breast cancer [ 34 ]. Male-specific psychosocial support and information were generally lacking across the studies. Information leaflets mostly contained pictures of female breast cancer patients which made the men feel excluded [ 34 ]. In fact, they felt the service was not designed for them:
“My GP said: ‘I don’t know what to do any more, it’s not my specialty area. I’ll have to refer you to someone else’. And the other doctor said, ‘This is a women’s practice (…) and we can’t get reimbursed for men, we don’t want men here.’” [ 34 ] (p.9). ‘‘. . . but I think as a male the information that I was given was female orientated and it could have been better presented for me and . . .I know that every case is different, but it was lacking in that respect’’. [ 13 ] (p.336).
Further to the above, some men had several challenges in scheduling for therapeutic regimen such as mammography [ 67 ]. Interactions with healthcare providers were often considered awkward as the providers often did not know what to say to the men with breast cancer. Subsequently, most men with breast cancer undergoing treatment often felt like outsiders, out of place, marginalised, and alone:
‘No information. Nothing at all. It was like men; you are on your own. I daresay women aren’t left like that . . .On leaving after the first operation the nurse gave me a leaflet, a piece of paper with women on it doing exercises you have to do and that was it’’. [ 13 ] (p.336). “I find that dealing with the mammograms and the technical staff to kind of tiptoe around you and put you in certain places because they don’t expect a male to be there, right, so they got women walking around in their gowns, so they don’t want you in those areas… they kind of shunt you into an isolated, a more isolated area so you’re not seeing the women walking by.” [ 66 ] (p.967).
Living with the effects of care/ ongoing treatment
Men undergoing treatment for breast cancer felt their lives, roles, and occupations were impacted adversely by the treatment regimen [ 60 ]. The clinical management process of the disease, in fact, further heightened the gendered essence of the disease. For men who underwent surgical intervention, the mastectomy scar served as a permanent reminder of the disease impacted on their masculinity [ 66 ]. Others felt their chest had deformed due to the scar [ 71 ]. The typical exposure of the male chest at leisure activities such as the beach was considered a no-go area to conceal the scar from public view. The scars also evoked a sense of perceived stigma among these men [ 32 ]:
“I’ve been abroad and sunbathed. People do look, they do look” [ 71 ] (p.1835). “I don’t feel like a complete person either because I’ve got something missing, haven’t I? ... My nipples are not there anymore. Sometimes I look in the mirror . . . I don’t like doing that. It’s gone. . . There’s a scar across there. . .Doctor said I look like a patchwork quilt. So, I don’t bother taking my shirt off now. And something else … yes you ought to have a tattoo as a nipple’’. [ 13 ] (p.337).
For men who underwent hormone therapy, it was observed that the side effects of the various medications threatened their notion of being a male. Experiencing erectile dysfunction and loss of libido were really challenging for these men as they felt they had lost their sense of masculinity or what made them men [ 34 , 77 ]. Hair loss from chemotherapy was also challenging and frustrating for them [ 43 ]. These men felt as though they had been transformed to ‘menopausal women’ [ 34 ].
“We’re candid and honest with one another … male sexual potency has gone.” [ 34 ] (p.9). “This has killed my sex life; I can no longer get an erection. I’m on this Tamoxifen which I’ve got to take for 5 years. You know it’s driving me mad. I get free Viagra but there is nothing there. There are no feelings or anything like that and it’s terrible. I couldn’t get an erection or nothing. I don’t know what it was, I just felt so no, no (silence) I just felt so embarrassed.” [ 31 ] (p.467).
Further to the above, some men felt they were a burden to others as they had to rely on others to have their needs met. Younger males felt their traditional roles as providers of the family was threatened as their dependence increased with a slow return to work and had to be supported by their spouses [ 54 ]:
“You start to receive only sickness benefits and when all of a sudden, you have over 500 euro less, you have to first see how you manage with that. And for me [...] it was even more because I only have a 60% part-time job and work as a freelancer on the side. And that I couldn't do any longer either.” [ 54 ] (p.6).
Theme 3: Coping and support systems
The theme describes how men with breast cancer coped with the disease, treatment process, aftercare/ rehabilitative care, and the available support and it was reported across qualitative ( n = 9), quantitative ( n = 5) and mixed methods ( n = 4) studies. The subthemes are 1) active coping strategies 2) family support and 3) support from healthcare providers and other support groups.
Active coping strategies
Although the breast cancer diagnosis was considered threatening with intense emotional stress, some affected men remained optimistic and hopeful of improved outcomes. Affected men often worked towards accepting the disease which made the navigation process less challenging [ 47 ]. The treatment process and aftercare phase offered the affected men an opportunity to amend or reformulate their notion of masculinity [ 66 ]. Although dealing with the disease was difficult, the men reportedly gained new insights in life which helped to reshape their worldviews and life priorities [ 14 ]. In addition, previous experience with breast cancer in the family was associated with use of non-repressing coping styles (X 2 [1, N = 26] r = 5.60, p < 0.05). There was also a higher use of mature defence patterns (superior healthy neurotic functioning) in patients who use non-repressive coping [ 70 ]. Despite the identified active coping mechanisms, one study reported that majority (70%) of men with breast cancer used immature and neurotic defensive functioning and 53.8% used a repressive approach to bottle up their emotions and concerns and [ 70 ]:
“I was kind of self-conscious the first year or so but um, I’m in pretty good shape, I’m relatively muscular, not super muscular, but I’m toned, I’m in shape, and I think a lot of times unless I’m really up close to people, I think a lot of times they don’t even see it… I’m not self-conscious. I go on vacation or go swimming at the beach, I don’t feel like people are staring at me.” [ 67 ] (p.38) “Breast cancer, for me, means a whole complex of experiences, of realisations. It’s like being in the military, you know. You meet somebody who’s been in the military, you don’t have to say anything. But if you meet someone who hasn’t, there’s not a way in the world to describe what it’s like.” [ 67 ] (p.38)
Family support
Studies found that majority of patients (61.3–80%) disclosed and discussed their diagnosis with their spouses and close families while 4–21% refused to disclose or discuss with anyone [ 7 , 13 , 61 ]. This might be because less stigmatization was reported from close families and friends compared to broader social settings [ 32 ]. Such disclosure might also be protective as availability of marital support was found to influence treatment choice and outcomes. Men who were not currently married received chemotherapy significantly less often [ 52 ] and had significantly higher (in some cases up to 21%) mortality than married ones [ 52 , 53 ].
This was corroborated by included qualitative studies which reported on the family support that men affected with breast cancer received. Spousal support was identified as a significant resource to seeking healthcare in the first instances as some wives had to push their partners to seek medical care [ 31 , 57 ]. Spousal and family support also helped men to navigate through the breast cancer diagnosis, coming to terms with the disease [ 49 , 57 ]. Family support was also an essential resource during the treatment and aftercare phase as family members offered emotional and practical support [ 47 ]:
“My wife was my support – she and I talked about everything. At the beginning we talked about it and agreed that I would have her as my support and she would have her family to support her through. It worked well and I also got support from her family . . . mine were useless’’. [ 13 ] (p. 338).
Support from healthcare providers and other support groups
Studies reported the dimensions, contents and timing of information needs demonstrated by the patients. Men with breast cancer acknowledged the support received from healthcare providers regarding diagnosis, information, treatment options, and aftercare support [ 49 , 57 ] with the most common source of information being verbal (92%), leaflets or booklets (53–71%) and internet (20%) [ 61 ]. Yet, 36–65% of participants felt their needs were not always met and wanted more information on various contents (particularly sexuality related information) at different times in their treatment (early/acute effects, late effects and ongoing quality of life) and in a more male specific manner [ 42 ].
Men with Breast cancer faced challenges in accessing needed support from healthcare facilities. Included studies reported experience of embarrassment and stigmatization within healthcare facilities where male breast cancer patients were meant to get support. 51.6% of patients experienced "extreme" or "very" severe embarrassment while waiting in the clinic among other female patients [ 13 ]. The experience of stigmatization was found to be higher within the cancer care system than other social surroundings with significantly higher stigmatization incidences reported in rehabilitation settings (mean = 1.50) and during hospitalisations (mean = 1.20) [ 53 ].
A mixed finding was observed regarding usage of peer supports. For one-to-one peer support, Iredale et al. (2006) reported low utilisation of formal support services with only 19% of participants speaking to other men who had breast cancer and only 1 in 4 indicating they would have liked that opportunity after their diagnosis. However, Midding et al. [ 53 ] found that more men (63.2%) had a one-on-one peer support from a female Breast Cancer Patient compared to 24.2% from another male breast cancer patient. This is consistent with the qualitative data which showed some men appreciated the opportunity to talk to other men with breast cancer on one-to-one basis [ 34 , 71 ], other men did not prefer this and were satisfied with the support offered by the healthcare providers and their families [ 13 ]:
‘‘…none of the guys wanted to have self-help groups ... I don’t think they need the psychological support that perhaps women do, and women tend to congregate and talk about these things anyway. I think this is, of course ... research I know ... but actually quite therapeutic in a way’’. [ 13 ] (p.338). “To be honest, I don’t know how I would be managing if I had never had (the support group). They gave me back the will to live and I will always be grateful for that.” [ 43 ] (p. 9).
In terms of group peer support, studies reported that only 15.3% of the participants were part of a peer support group and majority (96.3%) of participants who were not currently part of a support group did not wish to be part of a support group whether male only or mixed sex [ 53 , 61 ].
Breast cancer is generally perceived to be a disease common among women albeit incidence among men is slowly rising, creating a need for health systems to be responsive to their needs. To this end, this review sought to develop a comparative understanding of the experiences of men with breast cancer and the treatment options available to them across different demographic settings. The review findings highlight the embodiment of breast cancer as a ‘feminine’ disease which is incongruent with what it means to be a ‘man’ and hegemonic masculinity discourses. Throughout the trajectory of the disease (that is, from diagnosis to aftercare), the review findings underscore the gendered nature of the disease with a lack of health system preparedness to support men who develop a disease perceived to be ‘feminine’. Though the treatment pathways were similar to those observed in the management of female breast cancer patients, they do not necessarily meet the unique needs of MBC across the disease trajectory warranting urgent attention considering the increasing prevalence of the disease among men. Male-specific treatment pathways, ongoing education, and professional support are also required.
The breast is seen as a symbol of femininity, and as incongruent with being male, together with the significant public health emphasis on the prevention of breast cancer among females [ 78 , 79 ] have further championed the perception that breast cancer is a feminine illness [ 56 , 67 ]. Thus, it was not surprising that the finding regarding being out of sync with one’s body resonated across the included studies. The breast cancer diagnosis which commenced the illness trajectory was really challenging for the men and filled with varied emotions. Despite the difficulty, the professional support available was often gendered and unsuitable to their needs. Thus, they mostly had to rely on their spouses and close families/ friends if they were able to open up to them, which may take some time. Coupled with the hegemonic masculinity ideology that a man must always be in charge and not demonstrate any emotions which can be perceived as weakness, it is likely that men will navigate through these on their own which can make the journey very lonely for them. Agreeing with a previous study, depressive symptoms, anxiety, and traumatic stress symptoms were common occurrences following the breast cancer diagnosis [ 43 ]. The culture of silence around the issue can lead to utilising avoidant coping mechanisms which may delay support seeking among men. Taken together, the findings highlight a need for tailor-made, individualised counselling support service for men before, during, and after breast cancer diagnosis. The need for healthcare professionals to consider the impact of the MBC on men cannot, therefore, be overemphasised.
Commencing treatment and aftercare/ rehabilitative support is an equally challenging phase for men living with breast cancer. A previous study has observed that gender impacts on the experience with breast cancer treatment [ 15 ]. The review findings highlighted the ‘feminised’’ nature of the treatment pathways with some practitioners not even knowing how to support the affected men. Information leaflets and other educational materials were generally noted to be filled with images of females which made the men feel out of place. Overall, these can serve as structural barriers which potentially deter men from seeking help even when required [ 34 ]. Undoubtedly, breast cancer affects more females than males. However, healthcare service delivery should be tailored to the unique needs of men to overcome the feeling of marginalisation or being left out. The impact of the therapeutic regimen should also be highlighted particularly as they can lead to loss of libido or erectile dysfunction which further diminishes one’s sense of being a man in relation to societal norms. Surgical procedures can lead to scars which serve as permanent reminders of the illness which can have life-long impact on men. Professional support should therefore not end after the diagnosis phase but should extend to the entire treatment continuum and aftercare. There is also a need to raise awareness of male breast cancer among healthcare practitioners to improve their approach to individuals through person-centred and male-specific care strategies. It may be worth reiterating the recommendation by Nguyen et al., [ 34 ] suggesting a guideline targeting men with breast cancer to support healthcare practitioners in the health and social service delivery process.
The need for support was reiterated throughout the review, and this is corroborated in a previous study where family and spousal support was critically important for men with advanced prostate cancer [ 80 ]. Interestingly, mixed findings were observed regarding the need for male-specific support groups. Although this may be based on individual preferences, it may also emanate from the hegemonic masculinity ideology [ 80 , 81 ] or coping styles such as disengagement [ 20 ] as men may appear ‘stoic’ in the presence of such difficult moments and may not want to seek help [ 34 , 82 ]. A breast cancer diagnosis can profoundly impact masculinity, with men grappling with navigating a threat to masculinity which collectively challenges one's sense of self and traditional gender roles [ 82 , 83 , 84 ].
Recent research shows changing perceptions of breast cancer as a "feminine disease" due to awareness campaigns and shifts in societal attitudes [ 85 , 86 ]. Additionally, demographic factors like location of treatment, socioeconomic status, and age have been found to affect the quality of care and outcomes, while acknowledging the male breast cancer experience and its shared emotional aspects with women's experiences [ 87 , 88 ]. These highlights evolving healthcare practices and societal norms regarding breast cancer.
Despite this, it is still cogent to understand their lived experiences and advocate for men support groups, if they would like to join one, as they navigate through the diagnosis, treatment, and aftercare pathway. This study presents the synthesis of multicultural evidence to highlight the cross-cultural similarity in the reaction and lived experience of men when faced with the diagnosis of breast cancer.
Strengths and limitations
The strength of this mixed method is the inclusion of studies from different countries and settings in addition to including and synthesising studies on the experiences of patients with male breast cancer from diagnosis to aftercare. Notwithstanding, there are some limitations that need to be highlighted. Firstly, a real limitation of our review was including only studies published in English. Excluding studies that used a language other than English, potentially led to information loss that could come from relevant studies written in other languages and restricts this mixed methods review only to the views and perception of men living in English speaking countries or countries where practitioners write and publish in English. Secondly, we acknowledge that younger and older men may have unique experiences while navigating breast cancer diagnosis and treatment. These nuances were not captured in the current review and may be worth exploring in future studies.
Men experience a myriad of issues following a breast cancer diagnosis, underscored by their ideology of masculinity. Our findings suggest the need for healthcare professionals’ training and education on managing interactions with MBC patients in a way that does not propagate a sense of awkwardness and otherness in a feminised support structure. Additionally, policy must address the structural barriers to treatment access for MBC including healthcare finance reimbursements that limit access to gendered specialist breast cancer treatments. Awareness creation efforts of MBC among the public as well as healthcare practitioners are urgently required to explain to the public through television programmes and awareness meetings that breast cancer is a disease like any other that affects both men and women. Creating such awareness could lead to changing the perception of men and promote early diagnosis, adherence to treatments, post-treatment monitoring, oncological results, and a better quality of life. Professional care intervention and support for MBC should not end after the diagnosis phase but should extend to the entire treatment continuum and aftercare. Preserving sexual function is an important finding highlighted from this review. Research will be needed to develop and test testosterone-preserving treatment modalities or optimising existing therapies in a way that is relevant to the priorities of MBC. This will also require the development of specialised guidelines for healthcare practitioners on MBC to optimise care and treatment for MBCs in a person-centred manner as suggested by other studies. To develop such individualised support frameworks, it is imperative to understand the specific needs, priorities, and support preferences among MBC patients.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Society AC. Key Statistics for Breast Cancer in Men 2022 [Available from: https://www.cancer.org/cancer/breast-cancer-in-men/about/key-statistics.html .
Chen Z, Xu L, Shi W, Zeng F, Zhuo R, Hao X, et al. Trends of female and male breast cancer incidence at the global, regional, and national levels, 1990–2017. Breast Cancer Res Treat. 2020;180(2):481–90.
Article PubMed Google Scholar
Health UDo, Services H. Making Cancer Health Disparities History: Report of the Trans-HHS Cancer Health Disparities Progress Review Group. Washington, DC: U.S. Department of Health and Human Services; 2004.
Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12(1):20–37.
Morris AM, Rhoads KF, Stain SC, Birkmeyer JD. Understanding racial disparities in cancer treatment and outcomes. J Am Coll Surg. 2010;211(1):105–13.
Rhoads KF, Cullen J, Ngo JV, Wren SM. Racial and ethnic differences in lymph node examination after colon cancer resection do not completely explain disparities in mortality. Cancer. 2012;118(2):469–77.
Co M, Lee A, Kwong A. Delayed presentation, diagnosis, and psychosocial aspects of male breast cancer. Cancer Med. 2020;9(10):3305–9.
Article PubMed PubMed Central Google Scholar
Ndom P, Um G, Bell EMD, Eloundou A, Hossain NM, Huo D. A meta-analysis of male breast cancer in Africa. The Breast. 2012;21(3):237–41.
Fiala L, Coufal O, Fait V, Foretova L. Male breast cancer-our experience. Rozhledy v chirurgii: mesicnik Ceskoslovenske chirurgicke spolecnosti. 2010;89(10):612–8.
PubMed CAS Google Scholar
Rudlowski C. Male breast cancer. Breast care. 2008;3(3):183–9.
Robinson JD, Metoyer KP, Bhayani N. Breast cancer in men: a need for psychological intervention. J Clin Psychol Med Settings. 2008;15(2):134–9.
Thomas E. Men’s awareness and knowledge of male breast cancer. AJN The American Journal of Nursing. 2010;110(10):32–7.
Iredale R, Brain K, Williams B, France E, Gray J. The experiences of men with breast cancer in the United Kingdom. Eur J Cancer. 2006;42(3):334–41.
Pituskin E, Williams B, Au H-J, Martin-McDonald K. Experiences of men with breast cancer: A qualitative study. Journal of Men’s Health and Gender. 2007;4(1):44–51.
Article Google Scholar
Naymark P. Male breast cancer: incompatible and incomparable. Journal Of Men’s Health And Gender. 2006;3(2):160–5.
Ottini L, Palli D, Rizzo S, Federico M, Bazan V, Russo A. Male breast cancer. Crit Rev Oncol Hematol. 2010;73(2):141–55.
Rudan I, Rudan N, Basić N, Basić V, Rudan D. Differences between male and female breast cancer. II. Clinicopathologic features. Acta Medica Croatica. 1997;51(3):129–33.
Sineshaw HM, Freedman RA, Ward EM, Flanders WD, Jemal A. Black/white disparities in receipt of treatment and survival among men with early-stage breast cancer. J Clin Oncol. 2015;33(21):2337–44.
DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):211–33.
Levin-Dagan N, Baum N. Passing as normal: Negotiating boundaries and coping with male breast cancer. Soc Sci Med. 2021;1(284):114239.
Sousa B, Moser E, Cardoso F. An update on male breast cancer and future directions for research and treatment. Eur J Pharmacol. 2013;717(1–3):71–83.
Article PubMed CAS Google Scholar
Fentiman IS. Surgical options for male breast cancer. Breast Cancer Res Treat. 2018;172:539–44.
Cardoso F, Bartlett J, Slaets L, Van Deurzen C, van Leeuwen-Stok E, Porter P, et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Oncol. 2018;29(2):405–17.
Korde LA, Zujewski JA, Kamin L, Giordano S, Domchek S, Anderson WF, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol. 2010;28(12):2114.
Miao H, Verkooijen H, Chia K-S, Bouchardy Magnin C, Pukkala E, Larønningen S, et al. Incidence and outcome of male breast cancer: an international population-based study. J Clin Oncol. 2011;29(33):4381–6.
Corrigan KL, Mainwaring W, Miller AB, Lin TA, Jethanandani A, Espinoza AF, Piotrowski M, Fuller CD, Stauder MC, Shaitelman SF, Perkins GH. Exclusion of men from randomized phase III breast cancer clinical trials. Oncologist. 2020;25(6):e990–2.
Article PubMed PubMed Central CAS Google Scholar
Manier KK, Rowe LS, Welsh J, Armstrong TS. The impact and incidence of altered body image in patients with head and neck tumors: a systematic review. Neuro-Oncology Practice. 2018;5(4):204–13.
Himmelstein MS, Sanchez DT. Masculinity impediments: Internalized masculinity contributes to healthcare avoidance in men and women. J Health Psychol. 2016;21(7):1283–92.
Paraskeva N, Clarke A, Harcourt D. Altered appearance from cancer. Body image care for cancer patients: Principles and practices. 2018;3:131–60.
Google Scholar
Fingeret MC, Teo I, Epner DE. Managing body image difficulties of adult cancer patients: lessons from available research. Cancer. 2014;120(5):633–41.
Donovan T, Flynn M. What makes a man a man?: The lived experience of male breast cancer. Cancer Nurs. 2007;30(6):464–70.
Midding E, Halbach SM, Kowalski C, Weber R, Würstlein R, Ernstmann N. Men with a “woman’s disease”: Stigmatization of male breast cancer patients—A mixed methods analysis. Am J Mens Health. 2018;12(6):2194–207.
Pituskin E, Williams B, Au HJ, Martin-McDonald K. Experiences of men with breast cancer: A qualitative study. Journal of Men’s Health and Gender. 2007;4(1):44–51.
Nguyen TS, Bauer M, Maass N, Kaduszkiewicz H. Living with male breast cancer: a qualitative study of men’s experiences and care needs. Breast Care. 2020;15(1):6–13.
Younas A. Epistemic injustice in health care professionals and male breast cancer patients encounters. Ethics Behav. 2021;31(6):451–61.
Article MathSciNet Google Scholar
Ly D, Forman D, Ferlay J, Brinton LA, Cook MB. An international comparison of male and female breast cancer incidence rates. Int J Cancer. 2013;132(8):1918–26.
Elbardan H, Kholeif AO, Elbardan H, Kholeif AO. An interpretive approach for data collection and analysis. Enterprise Resource Planning, Corporate Governance and Internal Auditing: An Institutional Perspective. 2017:111–65.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group* P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann intern med. 2009;151(4):264–9.
Adekolujo OS, Tadisina S, Koduru U, Gernand J, Smith SJ, Kakarala RR. Impact of marital status on tumor stage at diagnosis and on survival in male breast cancer. Am J Mens Health. 2017;11(4):1190–9.
Ahmed A, Ukwenya Y, Abdullahi A, Muhammad I. Management and outcomes of male breast cancer in Zaria. Nigeria Int J Breast Cancer. 2012;2012:845143.
PubMed Google Scholar
Avila J, Herrick B, Attai DJ, Leone JP. Treatments for breast cancer in men: late effects and impact on quality of life. Breast Cancer Res Treat. 2023;201(3):489–98.
Bootsma TI, Duijveman P, Pijpe A, Scheelings PC, Witkamp AJ, Bleiker EM. Unmet information needs of men with breast cancer and health professionals. Psychooncology. 2020;29(5):851–60.
Brain K, Williams B, Iredale R, France L, Gray J. Psychological distress in men with breast cancer. J Clin Oncol. 2006;24(1):95–101.
Chichura A, Attai DJ, Kuchta K, Nicholson K, Kopkash K, Pesce C, Yao K. Male breast cancer patient and surgeon experience: the male WhySurg study. Ann Surg Oncol. 2022;29(10):6115–31.
Crew KD, Neugut AI, Wang X, Jacobson JS, Grann VR, Raptis G, et al. Racial disparities in treatment and survival of male breast cancer. J Clin Oncol. 2007;25(9):1089–98.
Cronin PA, Romanoff A, Zabor EC, Stempel M, Eaton A, Smyth LM, et al. Influence of age on the clinical outcome of breast cancer for men and the development of second primary cancers. Ann Surg Oncol. 2018;25(13):3858–66.
Duarte do Amaral DE, Manfrim Muniz R, Habekost Cardoso D, Tuerlinckx Noguez P, Ferreira Fagundes R, Costa Viegas A. Male breast cancer: the survivor's context. J Nurs UFPE/Revista de Enfermagem UFPE. 2017;11(5).
El-Beshbeshi W, Abo-Elnaga EM. Male breast cancer: 10-year experience at mansoura university hospital in Egypt. Cancer Biol Med. 2012;9(1):23.
PubMed PubMed Central Google Scholar
France L, Michie S, Barrett-Lee P, Brain K, Harper P, Gray J. Male cancer: a qualitative study of male breast cancer. The Breast. 2000;9(6):343–8.
Giordano SH, Perkins GH, Broglio K, Garcia SG, Middleton LP, Buzdar AU, et al. Adjuvant systemic therapy for male breast carcinoma. Cancer. 2005;104(11):2359–64.
Halbach SM, Midding E, Ernstmann N, Würstlein R, Weber R, Christmann S, et al. Male breast cancer patients’ perspectives on their health care situation: a mixed-methods study. Breast Care. 2020;15(1):22–9.
Harlan LC, Zujewski JA, Goodman MT, Stevens JL. Breast cancer in men in the United States: a population-based study of diagnosis, treatment, and survival. Cancer. 2010;116(15):3558–68.
Hill TD, Khamis HJ, Tyczynski JE. Comparison of male and female breast cancer incidence trends, tumor characteristics, and survival. Ann Epidemiol. 2005;15(10):773–80.
Hiltrop K, Heidkamp P, Halbach S, Brock-Midding E, Kowalski C, Holmberg C, et al. Occupational rehabilitation of male breast cancer patients: Return patterns, motives, experiences, and implications—A qualitative study. Eur J Cancer Care. 2021;30(4): e13402.
Hoffman A, Horesh N, Shabtai M, Forschmidt E, Rosin D, Gutman M, et al. Breast Cancer in Men: A Single Center Experience Over a Period of 22 years. The Israel Medical Association Journal: IMAJ. 2020;22(3):160–3.
Kowalski C, Steffen P, Ernstmann N, Wuerstlein R, Harbeck N, Pfaff H. Health-related quality of life in male breast cancer patients. Breast Cancer Res Treat. 2012;133(2):753–7.
Midding E, Halbach SM, Kowalski C, Weber R, Würstlein R, Ernstmann N. Social support of male breast cancer patients—a mixed-methods analysis. Am J Mens Health. 2019;13(4):1557988319870001.
Nahleh ZA, Srikantiah R, Safa M, Jazieh AR, Muhleman A, Komrokji R. Male breast cancer in the veterans affairs population: a comparative analysis. Cancer. 2007;109(8):1471–7.
Özkurt E, Tükenmez M, Yılmaz R, Cabioğlu N, Müslümanoğlu M, Dinççağ AS, et al. Favorable long-term outcome in male breast cancer. European Journal of Breast Health. 2018;14(3):180.
Potter AM, Bentz B, Crue L, Leiby S, Bashi S, Maguire K, Meyers J, Mieczkowski K. Men’s Lived Experiences of Breast Cancer and Changes in Occupation. Occup Ther Int. 2023;13:2023.
Rayne S, Schnippel K, Thomson J, Reid J, Benn C. Male breast cancer has limited effect on survivor’s perceptions of their own masculinity: a record review and telephone survey of patients in Johannesburg, South Africa. Am J Mens Health. 2017;11(2):246–52.
Sanguinetti A, Polistena A, Lucchini R, Monacelli M, Galasse S, Avenia S, et al. Male breast cancer, clinical presentation, diagnosis and treatment: Twenty years of experience in our Breast Unit. Int J Surg Case Rep. 2016;20:8–11.
Article PubMed Central Google Scholar
Sarmiento S, McColl M, Musavi L, Gani F, Canner JK, Jacobs L, et al. Male breast cancer: a closer look at patient and tumor characteristics and factors that affect survival using the National Cancer Database. Breast Cancer Res Treat. 2020;180(2):471–9.
Shah S, Bhattacharyya S, Gupta A, Ghosh A, Basak S. Male breast cancer: a clinicopathologic study of 42 patients in eastern India. Indian J Surg Oncol. 2012;3(3):245–9.
Shin JY, Kachnic LA, Hirsch AE. The impact of race in male breast cancer treatment and outcome in the United States: a population-based analysis of 4,279 patients. Int J Breast Cancer. 2014;2014:685842.
Skop M, Lorentz J, Jassi M, Vesprini D, Einstein G. “Guys don’t have breasts”: The lived experience of men who have BRCA gene mutations and are at risk for male breast cancer. Am J Mens Health. 2018;12(4):961–72.
Thompson EH Jr, Haydock AS. Men’s lived experiences with breast cancer: The double consciousness of marginal men. Sex Roles. 2020;82(1–2):28–43.
Visram H, Kanji F, Dent S. Endocrine therapy for male breast cancer: rates of toxicity and adherence. Curr Oncol. 2010;17(5):17–21.
Wang Y, Chen K, Yang Y, Tan L, Chen L, Zhu L, et al. Incidence and survival outcomes of early male breast cancer: a population-based comparison with early female breast cancer. Ann transl med. 2019;7(20):536.
Weber R, Ehrenthal JC, Brock-Midding E, Halbach S, Würstlein R, Kowalski C, Ernstmann N. Defense mechanisms and repressive coping among male breast cancer patients. Front Psych. 2021;10(12): 718076.
Williams B, Iredale R, Brain K, France E, Barrett-Lee P, Gray J. Experiences of men with breast cancer: an exploratory focus group study. Br J Cancer. 2003;89(10):1834–6.
Yadav BS, Sharma SC, Singh R, Dahiya D, Ghoshal S. Male breast cancer: Outcome with adjuvant treatment. J Cancer Res Ther. 2020;16(6):1287.
Yoney A, Kucuk A, Unsal M. Male breast cancer: a retrospective analysis. Cancer/Radiothérapie. 2009;13(2):103–7.
Zongo N, Ouédraogo S, Korsaga-Somé N, Somé OR, Go N, Ouangré E, et al. Male breast cancer: diagnosis stages, treatment and survival in a country with limited resources (Burkina Faso). World journal of surgical oncology. 2018;16(1):1–7.
Hong QN, Pluye P, Bujold M, Wassef M. Convergent and sequential synthesis designs: implications for conducting and reporting systematic reviews of qualitative and quantitative evidence. Syst Rev. 2017;6(1):1–14.
Sirriyeh R, Lawton R, Gardner P, Armitage G. Reviewing studies with diverse designs: the development and evaluation of a new tool. J Eval Clin Pract. 2012;18(4):746–52.
Ruddy KJ, Giobbie-Hurder A, Giordano SH, Goldfarb S, Kereakoglow S, Winer EP, et al. Quality of life and symptoms in male breast cancer survivors. The Breast. 2013;22(2):197–9.
Dozier R. Beards, breasts, and bodies: Doing sex in a gendered world. Gend Soc. 2005;19(3):297–316.
Sulik GA. Pink ribbon blues: How breast cancer culture undermines women’s health. Walton Street, Oxford: Oxford University Press; 2010.
Salifu Y, Almack K, Caswell G. ‘My wife is my doctor at home’: A qualitative study exploring the challenges of home-based palliative care in a resource-poor setting. Palliat Med. 2021;35(1):97–108.
Connell RW, Messerschmidt JW. Hegemonic masculinity: Rethinking the concept. Gend Soc. 2005;19(6):829–59.
Salifu Y, Almack K, Caswell G. ‘Out of the frying pan into the fire’: a qualitative study of the impact on masculinity for men living with advanced prostate cancer. Palliative Care and Social Practice. 2023;17:26323524231176828.
Quincey K, Williamson I, Wildbur D. Men with breast cancer and their encounters with masculinity: An interpretative phenomenological analysis using photography. Psychology of Men & Masculinities. 2021;22(4):690.
Quincey K, Williamson I, Winstanley S. ‘Marginalised malignancies’: A qualitative synthesis of men’s accounts of living with breast cancer. Soc Sci Med. 2016;149:17–25.
Yadav S, Karam D, Bin Riaz I, Xie H, Durani U, Duma N, Ruddy KJ. Male breast cancer in the United States: treatment patterns and prognostic factors in the 21st century. Cancer. 2020;126(1):26–36.
Wang F, Shu X, Meszoely I, Pal T, Mayer IA, Yu Z, Shu XO. Overall mortality after diagnosis of breast cancer in men vs women. JAMA oncol. 2019;5(11):1589–96.
Download references
Acknowledgements
Not applicable.
Author information
Authors and affiliations.
School of Health and Social Care, Edinburgh Napier University, Sighthill Court, Sighthill Campus, Edinburgh, UK
Mary Abboah-Offei
School of Nursing, The Hong Kong Polytechnic University, Hong Kong Special Administrative Region, Hongkong, China
Jonathan Bayuo
International Observatory On End of Life Care (IOELC), Faculty of Health and Medicine, Division of Health Research, Lancaster University, Lancaster, LA1 4AT, UK
Yakubu Salifu
Florence Nightingale Faculty of Nursing, Midwifery and Palliative Care, King’s College London, London, WC2R 2LS, UK
Oladayo Afolabi
Institute of Medical Imaging & Visualisation, Department of Medical Science & Public Health, Faculty of Health & Social Science, Bournemouth University, Bournemouth, UK
Theophilus N. Akudjedu
You can also search for this author in PubMed Google Scholar
Contributions
This review was conceived and designed by JB, MA-O, YS, OA, and TNA. The first reviewer imported all search results to Endnote reference manager version X9, de-duplicated, then all authors screened titles and abstracts of all identified studies, any article for which inclusion was unclear were discussed and if necessary adjudicated by YS and TNA. All authors critically appraised and contributed to the manuscript.
Corresponding author
Correspondence to Yakubu Salifu .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Abboah-Offei, M., Bayuo, J., Salifu, Y. et al. Experiences and perceptions of men following breast cancer diagnosis: a mixed method systematic review. BMC Cancer 24 , 179 (2024). https://doi.org/10.1186/s12885-024-11911-9
Download citation
Received : 28 September 2022
Accepted : 22 January 2024
Published : 06 February 2024
DOI : https://doi.org/10.1186/s12885-024-11911-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Male breast cancer
- Experiences
- Perceptions
- Treatment approaches
- Systematic review
- Masculinity
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 22 August 2014
Male breast cancer: a rare disease that might uncover underlying pathways of breast cancer
- Laura Ottini 1
Nature Reviews Cancer volume 14 , pages 643–644 ( 2014 ) Cite this article
2915 Accesses
32 Citations
3 Altmetric
Metrics details
- Breast cancer
There are similarities between breast cancers that arise in men and women but there are also differences. What can be learned from male breast cancer to gain insight into breast cancer pathogenesis?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Korde, L. A. et al. J. Clin Oncol. 28 , 2114–2122 (2010).
Article Google Scholar
Brinton, L. A. et al. J. Natl Cancer Inst. 106 , djt465 (2014).
Easton, D. F. et al. Am. J. Hum. Genet. 61 , 120–128 (1997).
Article CAS Google Scholar
Rizzolo, P. et al. Ann. Oncol. 24 (Suppl. 8), viii75–viii82 (2013).
PubMed Google Scholar
Orr, N. et al. Nature Genet. 44 , 1182–1184 (2012).
Ottini, L. et al. Breast Cancer Res. Treat. 138 , 861–868 (2013).
The Cancer Genome Atlas Network. Nature 490 , 61–70 (2012).
Deb, S. et al. Breast Cancer Res. 15 , R69 (2013).
Johansson, I. et al. Int. J. Biochem. Cell Biol. 53 , 526–535 (2014).
Callari, M. et al. Breast Cancer Res. Treat. 127 , 601–610 (2011).
Ottini, L. et al. Breast Cancer Res. Treat. 134 , 411–418 (2012).
Download references
Acknowledgements
The author thanks L. Pritikin for her assistance with the preparation of the manuscript. In addition, the author gives a special thank you to the Italian Association for Cancer Research (AIRC) for its ongoing support (IG12789).
Author information
Authors and affiliations.
Department of Molecular Medicine, Sapienza University of Rome, Rome, 00161, Italy
Laura Ottini
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Laura Ottini .
Ethics declarations
Competing interests.
The author declares no competing financial interests.

Rights and permissions
Reprints and permissions
About this article
Cite this article.
Ottini, L. Male breast cancer: a rare disease that might uncover underlying pathways of breast cancer. Nat Rev Cancer 14 , 643–644 (2014). https://doi.org/10.1038/nrc3806
Download citation
Published : 22 August 2014
Issue Date : October 2014
DOI : https://doi.org/10.1038/nrc3806
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
Top 100 cited articles in male breast cancer: A bibliometric analysis
Affiliations.
- 1 Leicester Medical School, Leicester, UK.
- 2 St. George's Medical School, London, UK.
- 3 Department of Cardiothoracic Surgery, University Hospitals Coventry and Warwickshire, Coventry, UK.
- PMID: 34219705
- DOI: 10.3233/BD-201024
Background: Male breast cancer is a considerably rare condition and only accounts for 1% of all breast cancer cases. Due to limited public awareness, the condition is likely to present late, leading to late diagnosis and treatment worsening morbidity and mortality. This article aims to identify the focus and most influential research on male breast cancer. Objective Identify the most influential papers in male breast cancer.
Methods: Search on Web of Science using the search terms 'Male', 'Breast Cancer' and "Male breast cancer" to identify all full manuscripts in English language and were ranked by the total number of citations. The top 100 articles were then further analysed according to subject, author, journal, year and country of publications.
Results: The mean number of citations per paper was 96. Most cited paper was by Thorlacius, S et al. evaluating the relationship between BRCA2 and female breast cancer, prostate cancer, pancreatic cancer and ovarian cancer. Cancer is the journal with the most published papers and received most citations in the male breast cancer research field. The USA contributed 49 of the manuscripts in the top 100. The most studied topic was risk factors for male breast cancer, with 20 articles.
Conclusions: The most cited papers identified in this study described the advance in the knowledge of genetics and epidemiology in male breast cancer and has led to improvements in the 4 management of the disease. Most of the highly cited articles in this field were published in high impact journals and had accumulated at least 100 citations to date, reflecting their quality and impact. By collating the most influential publications in this field, this analysis can serve to identify knowledge gaps in male breast cancer research as well as to help identify what makes a paper impactful and citable.
Keywords: Male breast cancer.
Publication types
- Bibliometrics*
- Breast Neoplasms, Male / diagnosis
- Breast Neoplasms, Male / epidemiology*
- Breast Neoplasms, Male / physiopathology*
Exploring the One Health Paradigm in Male Breast Cancer
- Perspective
- Open access
- Published: 04 April 2024
- Volume 29 , article number 8 , ( 2024 )
Cite this article
You have full access to this open access article
- Kirsty Luo-Yng Tay 1 ,
- George Cowan 1 ,
- Subarnarekha Chatterji ORCID: orcid.org/0000-0002-8980-4982 1 , 2 ,
- Giulia Conti 1 , 3 &
- Valerie Speirs ORCID: orcid.org/0000-0002-0602-4666 1 , 2
128 Accesses
Explore all metrics
How cancer patterns in humans compare to those of other species remains largely unknown and there is an even bigger knowledge gap for rare cancers like male breast cancer. One Health is a convergence of human and animal healthcare that encourages cross-pollination of medical research uniting human and veterinary medicine. Recognising that breast cancer occurs spontaneously in other male species (e.g. primates, canines, felines), and knowing that no laboratory models exist for male breast cancer, which limits our ability to perform functional studies, we explored the feasibility of applying One Health to breast cancer in men by conducting a narrative review of the topic. Spontaneous development of breast cancer was reported in captive male primates and in companion canines and felines. Some parallels in tumour biology of human male breast cancer with canines and primates were found. The age distribution, pattern of biomarker expression and metastasis were similar, with mammary tumours typically detected after two-thirds of average lifespan. However, instances of triple negative and inflammatory breast cancer, which are rarely observed in human male breast cancer, were found in canines and histological classification was inconsistent between species. These disparities need redressing to enable full exploration of the One Health paradigm in rare cancers.
Avoid common mistakes on your manuscript.
Introduction
Some 400 men in the UK and an estimated 2800 in the US receive a breast cancer (BC) diagnosis each year, which is considerably fewer than the 56 000 and 297 000 women who are diagnosed, respectively, accounting for less than 1% of all breast cancer diagnoses worldwide [ 1 ]. However, as average lifespan increases, there has been a noticeable rise in male BC prevalence [ 2 , 3 ], reflected in age-standardised data collected by the American Cancer Society over the last two decades [ 4 , 5 ]. Indeed, a compilation of these data from 2002 to 2022 shows that the number of cases of male BC has increased from 1500 to 2710 per 100,000 persons (Fig. 1 ).
Incidence (circles) and mortality (triangles) trends of male (blue line, right axis)) and female (red line; left axis) breast cancer in the United States from 2002 to 2022. Graph constructed using data reported in the annual ‘Cancer Statistics’ publication by CA: A Cancer Journal for Clinicians [ 4 , 5 ]
As BC in men is rare, it has been historically difficult to collect sufficient samples for studies to extend beyond anecdotal findings. This gap has been addressed through the establishment of the Male Breast Cancer Consortium and International Male Breast Cancer Program [ 6 , 7 ], where large numbers of cases have been collected and centralised, allowing more rigorous characterisation. The focus of these consortia has been to collect formalin-fixed paraffin embedded tissues, predominantly. These have shown that male BC more often expresses estrogen (ER), progesterone (PR) and androgen receptors (AR), with a lower likelihood of human epidermal growth factor 2 (HER2) overexpression [ 6 , 8 , 9 , 10 ]. Invasive ductal carcinoma accounts for approximately 83% of all male BC cases whereas lobular cancer is infrequent in men due to the absence of lobule development [ 11 ]. Other forms, including cribriform, intraductal papillary, mucinous, micropapillary, and papillary phenotypes, have also been reported in men, with papillary subtype showing a higher prevalence compared to women [ 8 ]. The principal molecular subtype in male BC is luminal A, with lower frequencies of luminal B and basal-like subtypes, including triple negative [ 6 , 8 ].
Genomics and transcriptomics studies, indicate further differences between male and female BC, leading to the identification of two exclusive subgroups in men: luminal M1 and M2, distinguished by their unique tumour characteristics [ 12 ] and independent of the PAM50 subtypes in women. These findings, along with others, point towards male BC possessing a distinct genetic, histological, and receptor expression profile [ 6 , 13 , 14 , 15 ].
While differences that separate male and female BC are being uncovered, the scarcity of male cases means that pre-clinical laboratory models are lacking. Numerous mouse models exist to study female BC [ 16 ], however male mice have only vestigial mammary tissue and lack nipples [ 17 , 18 ], limiting their use as models. Recently, an ovine model has been proposed as a useful comparative model to understand the role of the microenvironment of the male mammary gland [ 19 ]. The “One Health” concept serves as a useful paradigm that leverages the shared knowledge of anatomy, pathology and physiology between human and veterinary medicine [ 20 ]. This approach highlights the potential of cross-species collaboration which has been demonstrated in various research studies [ 21 , 22 ]. One Health was applied to investigate naturally-occurring osteoarthritis in canines to better understand the pathogenesis, molecular mechanisms, and potential treatment strategies relevant to humans [ 23 ]. Another study explored the similarities between humans and animals, particularly canines and equines, in tendon structure, function, and pathology [ 24 ] The One Health philosophy has also been proposed as a way of accelerating our understanding of understanding the pathology, diagnosis and treatment of mammary cancer across species [ 25 , 26 ].
The translatability of spontaneously developed mammary cancer in non-human mammals, notably canines and felines is garnering interest [ 27 , 28 , 29 , 30 ] with previous research showing that spontaneous animal tumours are viable translational models in female BC [ 31 , 32 ]. As companion animals often share the same environment as their owners and adopt some of their traits, they may develop the same comorbidities, including physical inactivity, a recognised risk factor for BC development [ 33 , 34 ]. This has come on the back of detailed investigations into the genomic landscape of canine cancers which now exist alongside various analytical tools and images on public repositories [ 35 ]. However, there are no investigations into the applicability of using spontaneously developed mammary tumour in non-human male animals to model BC in men. Therefore, the aim of this work was to review the literature on spontaneously developed BC in non-human male mammals to determine its frequency and similarity to BC in male humans with a view to using these as pre-clinical models, which do not exist currently for male BC.
Literature Searching
For this narrative review, a comprehensive search of databases, Ovid MEDLINE ® (1946 to June 12, 2023), Embase Classic + Embase (1947 to June 12, 2023), CAB Abstracts (1973 to June 12, 2023), All EBM reviews, Web of Science Core Collection, and SCOPUS, was conducted without restrictions on year or language. The search strategy involved compiling synonymous search terms for “breast,” “cancer,” and “men,” which were then combined with a comprehensive list of common animals from major mammalian classes.
Non-human Primates
Non-human primates share over 90% of DNA with humans. This genetic similarity offers comparability in research findings and explains why they are sometimes used in medical studies. Scientists made significant discoveries about diseases, disorders, prevention, and treatments for both humans and animals, tying in the ‘one approach’ technique long before One Health was touted and applying results from studies on non-human primates to humans. A prime example of this is the discovery of insulin over a century ago [ 36 ] which laid the foundation for this type of approach, demonstrating a compelling basis for developing novel treatment modalities and harnessing comparative research efforts across species. More recently, the relative value of using non-human primates to model BC has been reviewed [ 37 ].
Case reports of BC detected in a handful of male non-human primates in captivity during routine physical examinations have been described. Species include rhesus macaque ( Macaca mulatta ), squirrel monkey ( Saimiri sciureus ), and orangutan ( Pongo pygmaeus ) [ 38 , 39 , 40 ]. A mammary lesion was found in the rhesus macaque, diagnosed as a spontaneous ductal carcinoma in situ (DCIS), which is a pre-invasive lesion [ 38 ]. In women DCIS is picked up frequently as one of the unintended consequences of national breast screening programmes [ 41 ]. However, DCIS is rarely seen in men, who typically present with more advanced disease [ 42 ]. In the squirrel monkey, an elevated subcutaneous nodule was surgically removed by wide excisional biopsy and diagnosed as an adenocarcinoma. No further treatment was given, but when the monkey was euthanised 18 months later for a comorbidity, a positive lymph node was identified [ 40 ]. The tumour resected from the male orangutan was subjected to the same diagnostic work up as human BC and immunohistochemistry revealed the expression of hormone receptors. The animal received tamoxifen at the same dose as humans, but the disease progressed. Switching to anastrozole, an aromatase inhibitor, slowed progression and the animal died from unrelated causes 4.5 years after diagnosis [ 39 ]. This treatment plan mirrored that recommended by ASCO for management of human male BC [ 43 ]. In another primate, BC was detected as an incidental finding in an autopsy of a male Humboldt’s white-fronted capuchin ( Cebus albifrons ) who died after being attacked by a male baboon [ 44 ]. A hormone receptor positive grade 2 tubular carcinoma, which is rare in men [ 8 ] was diagnosed.
Non-primate Male Species
Forty papers reported mammary tumours in non-human non-primate male species, with a higher prevalence in canines and felines. A summary of species and age distribution is shown in Table 1 . Mammary tumours typically occurred after two-thirds of the average lifespan. Canines comprised most cases, across 33 different breeds, with Cocker Spaniel ( n = 14), German Shepherd ( n = 6), and Dachshund ( n = 6) most commonly reported, suggesting possible breed associations [ 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 ]. Indeed, previous studies have noted a preponderance of mammary tumours in these breeds among female canines [ 58 ]. Felines were the second most represented species, mainly in Domestic short-hairs, followed by four Siamese, three Domestic long-hair, and one Persian cat [ 59 , 60 , 61 , 62 ]. A single case of a simple ductal papilloma was reported in a captive maned wolf ( Chrysocyon brachyurus ) [ 63 ]. Papillary subtypes are seen more frequently in human male than in female BC [ 8 ]. Canines, felines and the lupine were diagnosed at an older age, median 10 years (range 1–15, canine) and median 11.5 years (range 3.5–19, feline).
Of the 84 reported cases of male mammary tumours in canines, 29 were malignant (Table S1 ). This classification was based on published guidelines for the histological classification for canine mammary tumours [ 81 ]. The remaining benign neoplasms included simple adenomas, fibroadenomas, intraductal papilloma, and benign mixed tumours. In felines, all mammary neoplasms identified were reported as malignant, except one case of cystic adenoma papilliferum. Most of the subtypes diagnosed were aggressive, with two distinct histological diagnoses observed: metastatic ductal adenocarcinoma and invasive micropapillary carcinoma [ 62 , 71 ]. Thirty-nine cases reported in one paper were described as “mammary adenocarcinoma” without detailed histological classification [ 61 ]. However, mammary cancers in felines tend to lack heterogeneity and are usually more aggressive [ 82 ]. Indeed, felines had the highest proportion of tumour-related deaths among all species described in this review. Formal classifications for mammary tumours in domestic species, including canines and felines, do exist [ 83 ] and whilst adopted widely, this may not always be universal.
Biomarkers used to classify human BC have been described in non-primate male animals (Table 2 ).
While complete data for all cases was patchy, 19 cases across 9 papers documented the three key biomarkers used for reporting human BC (ER, PR, HER2), and in some cases, Ki-67. These cases were predominantly canine [ 45 , 48 , 53 , 55 , 57 , 84 , 85 , 86 ], with one feline [ 62 ] and one lupine [ 63 ]. Triple-negative mammary cancer was observed in three male dogs, an unusual finding in human males, where BC is predominately hormone receptor positive [ 8 ]. However, ER-positivity was reflected in most of the cases where ER was examined. Despite this, endocrine therapy (tamoxifen) is not recommended in canines due to adverse side effects [ 87 ]. It is worth noting that in veterinary medicine and basic sciences, immunohistochemistry is not always conducted to the same rigorous standards required for clinical reporting in humans. This would need to be addressed were a One Health approach to be implemented.
Other Species
Among male rodents, eight out of ten reported BC cases were malignant tumours. Three were in rats ( Rattus norvegicus ); two in pets and one in a Wistar rat from a breeding colony, comprising two ductal mammary carcinoma and one papillary mammary carcinoma [ 72 , 73 ]. A handful have been reported in male guinea pigs ( Cavia porcellus ) including invasive papillary carcinoma, solid simple mammary carcinoma, papillary cystadenocarcinoma, or benign tumours [ 74 , 75 , 76 , 77 , 78 , 79 ]. One case, a solid anaplastic adenocarcinoma, was reported in an intact male pet rabbit ( Oryctolagus cuniculus ) aged 7 years [ 80 ].
Although the focus of this paper was to explore the spontaneous development of male BC in non-human males, it is known that spontaneous tumour initiation can occur in oncogene-driven transgenic mice, typically MMTV-PyMT strains, which closely parallel human BC development and progression [ 88 ]. In a different transgenic strain FVB/N-Tg(MMTV-PyVT)634Mul/J (known as PyVT; [ 89 ]) mammary tumours developed spontaneously in male animals from 14 weeks of age which expressed ERα and -β, PR and HER2 [ 90 ]. HER2 is very rare in male BC [ 8 ], although ERβ has been reported [ 91 ]. Tumour burden was reduced following treatment with cisplatin but not paclitaxel or tamoxifen [ 90 ]. The latter finding is unusual as tamoxifen is typically first line choice for male BC [ 43 ]. Indeed, lack of response to tamoxifen in this transgenic mouse strain and its poor tolerance in canines [ 87 ] point to dissimilarities in physiology between species.
Management and Outcomes
Animals tended to be diagnosed with larger tumours compared to the average 2.4 cm tumour size in human male BC [ 92 ] as shown in Table S2 . This discrepancy may arise as animals depend on their owners to notice the tumour, while in humans, palpable lumps would trigger suspicion. That said, BC in men is frequently diagnosed at a later stage due to a lack of awareness and stigma surrounding what is perceived by the stigma surrounding what is perceived by the public as a female cancer [ 93 , 94 ]. Soft tissue metastases, but not the more common bone metastasis seen in male BC was observed in 8 animals. This aligns with previous studies highlighting a low incidence of bone metastasis in animals [ 95 ].
Surgery was performed in most canines (80/84), typically mastectomy and lumpectomy, like humans. Similarly, in felines all mammary tumours were managed surgically, with half of those unspecified. Lymph nodes were resected in two cases during radical mastectomy or lumpectomy [ 62 , 71 ]. The specific surgical interventions for canine and felines were reported in Table 3 .
Post-operative chemotherapy with doxorubicin and cyclophosphamide was administered in two feline cases [ 61 ], again similar to humans. In canines, two simple mammary carcinoma cases received Cytocristin chemotherapy post-lumpectomy while two inflammatory mammary carcinomas were managed with analgesics and anti-inflammatories [ 46 , 85 , 96 ]. The cases of inflammatory breast cancer (IBC) presented like humans, with rapid onset of skin erythema and tumour emboli in the dermal lymphatics however chemotherapy is preferred in humans due to its aggressive nature [ 98 ]. This was reflected in the short lifespan of these canines’ post-diagnosis. The wolf with a simple ductal mammary papilloma received unspecified surgery [ 63 ]. In rodents, one rat underwent a simple mastectomy, while two received a lumpectomy with cisplatin electrochemotherapy [ 72 , 73 ]. Lumpectomy with regional node removal was performed in one guinea pig [ 79 ].The rabbit with solid anaplastic carcinoma underwent excisional biopsy [ 80 ]. However, treatment protocols cannot readily be compared in human and non-human species. The aim of the former is to prolong survival while the latter is to improve quality of live and alleviate symptoms.
In canines, only 5/49 cases reported metastasis [ 45 , 51 , 54 , 84 , 99 ]. Lymph node metastasis occurred in two cases, while metastasis to the contralateral mammary gland, lungs, and pelvic region each occurred once. A rabbit mammary tumour had metastasised to the lungs, pleural lining, and liver [ 80 ]. Metastasis was not documented among rats, guinea pigs or the wolf. The follow-up period and prognosis varied between 2 weeks to 77 months. Felines had the highest proportion of tumour-related deaths. The survival of male animals with mammary tumours across all species is summarised in Table 4 .
Omics Studies
Genomic and transcriptomic data are being generated for canines and are starting to dissect the molecular pathways of BC in canines [ 28 , 100 ]. Somatic and germline variants of BRCA1 and BRCA2 have been determined in felines [ 101 ]. Many similarities have been found, e.g. the frequency of somatic PIK3CA mutations, PI3K-Akt pathway deregulation, germline genetic variants in BRCA1/2 and p53-signalling; nevertheless, there is a notable lack of males in these studies.
Male mammary tumours were predominantly reported in companion animals, likely due to the proximity to humans leading to a higher likelihood of incidental detection. Prevalence of mammary tumours in canines and felines may be attributed to their longer lifespan compared to rodents, increasing the probability of spontaneous oncogenic mutations. Tumour detection in all animals included in this study occurred past two-thirds of their lifespan, consistent with the older age distribution of BC in men [ 102 ].
Possible breed associations were identified with presentation in Spaniels, German Shephard, and Dachshunds. This is significant considering previous studies have noted a preponderance of mammary tumours in these breeds among female canines [ 58 ] and linked them to significant mutations found in human BC [ 103 , 104 , 105 ]. A study on Springer spaniels revealed a significant association between the development of mammary tumours and BRCA1 and BRCA2 polymorphisms, with 97% of diagnosed cases possessing these alleles [ 103 ]. In humans, BRCA mutations are present in 1–7% of the general population [ 106 ] and accounts for 5–10% of all breast cancer cases [ 106 , 107 ]. However, BC in men is more typically associated with BRCA2 mutations which is linked to a higher lifetime risk of developing BC compared to BRCA1 carriers (1–5% versus 5–10%) [ 108 ].
BC classification in men is based on histological and molecular subtyping. Similar histological classifications exist between primates and humans and standardisation for canine and feline mammary tumour classification has been introduced [ 83 ]. Omics studies are still in their infancy for non-human species and males remain underrepresented. This is an area which deserves further study.
An unexpected finding was the identification of two less frequent manifestations of BC in humans, TNBC and IBC in male dogs. IBC closely mimicked the human disease in symptoms and histological features. Like male BC, IBC is also an uncommon form of BC accounting for just 2.5% of all cases in the US [ 109 ] with very few in men [ 110 ]. Both canine cases presented with painful erythematous mammary swellings alongside inflammatory infiltrate and dermal lymphatic vessels, like human cases [ 98 , 109 , 110 ]. Despite no reported metastasis, the short survival period in these canines mirrors the extremely poor prognosis observed in IBC in humans [ 98 ]. While 2 male canines diagnosed with TNBC had poor outcomes, dying within a few months of surgery or euthanised at time of diagnosis [ 45 ], a third remained recurrence-free for 6 months post-surgery [ 84 ].
While immunohistochemistry is used routinely in clinical workups for human BC for molecular classification and prognostication, this was less common in animal studies suggesting that veterinary medicine may prioritise tumour diagnosis and treatment over classification. Additionally, cost and availability of specialised testing in veterinary medicine, may further limit routine biomarker testing in animals. Indeed only 10% of the studies we examined reported on ER, PR, and HER2. This may change in the future, following a consensus statement from Brazil recommending a standard immunohistochemical panel for diagnosing mammary tumours in canines and felines, including ER, PR, Ki-67 and COX2 [ 111 ].
In contrast to BC management in men, where lumpectomy is uncommon due to limited breast tissue and adverse effects with adjuvant therapies, most animals in this study (where surgery was specified) underwent lumpectomies. An orangutan with a hormone-receptor positive tumour received hormonal adjuvant therapy at human dosages. Although its efficacy in animals remains unproven, the administration of anastrozole appeared to slow the lymph node enlargement, suggesting potential control of tumour growth. While the ATAC trial identified the benefits of anastrozole in the postmenopausal setting in women [ 112 ], its value in male BC has been insufficiently explored [ 113 ]. Nevertheless, the administration of adjuvant hormonal therapies in the orangutan demonstrates the potential for cross-species application of BC treatments.
The restrictive sample size across non-canine animals as well as insufficient and inconsistent reporting of the histology, biomarker expression, and prognosis of individual cases reduce the certainty of the conclusions made in comparison to human male BC. The relative recency of publication of the recommended guidelines for reporting of canine BC [ 111 ] limits the interpretation of reported histological features. Despite this, our work highlights pros, and cons of utilising animal models to understand human male BC. This needs redressing to enable full exploration of the One Health paradigm in rare cancers.
Data Availability
No datasets were generated or analysed during the current study.
Fox S, Speirs V, Shaaban AM. Male breast cancer: an update. Virchows Arch. 2022;480(1):85–93. https://doi.org/10.1007/s00428-021-03190-7 . Epub 2021/08/31.
Article PubMed Google Scholar
Speirs V, Shaaban AM. The rising incidence of male breast cancer. Breast Cancer Res Treat. 2009;115(2):429 – 30. Epub 2008/05/15. https://doi.org/10.1007/s10549-008-0053-y . PubMed PMID: 18478326.
Reddington R, Galer M, Hagedorn A, Liu P, Barrack S, Husain E, et al. Incidence of male breast cancer in Scotland over a twenty-five-year period (1992–2017). Eur J Surg Oncology: J Eur Soc Surg Oncol Br Association Surg Oncol. 2020;46(8):1546–50. https://doi.org/10.1016/j.ejso.2020.01.009 . Epub 2020/01/21.
Article Google Scholar
Jemal A, Thomas A, Murray T, Thun M, Cancer statistics. 2002. CA Cancer J Clin. 2002;52(1):23–47. Epub 2002/01/30. https://doi.org/10.3322/canjclin.52.1.23 . PubMed PMID: 11814064.
Siegel RL, Miller KD, Fuchs HE, Jemal A, Cancer statistics. 2022. CA Cancer J Clin. 2022;72(1):7–33. Epub 2022/01/13. doi: 10.3322/caac.21708. PubMed PMID: 35020204.
Cardoso F, Bartlett JMS, Slaets L, van Deurzen CHM, van Leeuwen-Stok E, Porter P, et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International male breast Cancer Program. Annals Oncology: Official J Eur Soc Med Oncol. 2018;29(2):405–17. https://doi.org/10.1093/annonc/mdx651 . Epub 2017/11/02.
Article CAS Google Scholar
Speirs V, Pollock S, Shaaban AM, Hanby AM. Problems (and solutions) in the study of male breast cancer. Rare Tumors. 2010;2(2):e28. https://doi.org/10.4081/rt.2010.e28 . Epub 2010/12/09.
Article PubMed PubMed Central Google Scholar
Humphries MP, Sundara Rajan S, Honarpisheh H, Cserni G, Dent J, Fulford L, et al. Characterisation of male breast cancer: a descriptive biomarker study from a large patient series. Sci Rep. 2017;7:45293. https://doi.org/10.1038/srep45293 . Epub 2017/03/30.
Article CAS PubMed PubMed Central Google Scholar
Ottini L, Capalbo C, Rizzolo P, Silvestri V, Bronte G, Rizzo S, et al. HER2-positive male breast cancer: an update. Breast Cancer (Dove Med Press). 2010;2:45–58. https://doi.org/10.2147/bctt.S6519 . Epub 2010/01/01.
Article CAS PubMed Google Scholar
Zelli V, Silvestri V, Valentini V, Bucalo A, Rizzolo P, Zanna I, et al. Transcriptome of male breast Cancer matched with germline profiling reveals Novel Molecular subtypes with possible clinical relevance. Cancers. 2021;13(18). https://doi.org/10.3390/cancers13184515 . Epub 2021/09/29.
Senger JL, Adams SJ, Kanthan R. Invasive lobular carcinoma of the male breast - a systematic review with an illustrative case study. Breast Cancer (Dove Med Press). 2017. https://doi.org/10.2147/bctt.S126341 . 9:337 – 45. Epub 2017/05/30.
Johansson I, Nilsson C, Berglund P, Lauss M, Ringner M, Olsson H, et al. Gene expression profiling of primary male breast cancers reveals two unique subgroups and identifies N-acetyltransferase-1 (NAT1) as a novel prognostic biomarker. Breast Cancer Res. 2012;14(1):R31. https://doi.org/10.1186/bcr3116 . Epub 2012/02/16.
Humphries MP, Sundara Rajan S, Droop A, Suleman CAB, Carbone C, Nilsson C, et al. A case-matched gender comparison transcriptomic screen identifies eIF4E and eIF5 as potential prognostic markers in male breast Cancer. Clin Cancer Res. 2017;23(10):2575–83. https://doi.org/10.1158/1078-0432.Ccr-16-1952 . Epub 2016/12/18.
Moelans CB, de Ligt J, van der Groep P, Prins P, Besselink NJM, Hoogstraat M, et al. The molecular genetic make-up of male breast cancer. Endocr Relat Cancer. 2019;26(10):779–94. https://doi.org/10.1530/erc-19-0278 . Epub 2019/07/25.
Piscuoglio S, Ng CK, Murray MP, Guerini-Rocco E, Martelotto LG, Geyer FC, et al. The genomic Landscape of male breast cancers. Clin Cancer Res. 2016;22(16):4045–56. https://doi.org/10.1158/1078-0432.Ccr-15-2840 . Epub 2016/03/11.
Park MK, Lee CH, Lee H. Mouse models of breast cancer in preclinical research. Lab Anim Res. 2018;34(4):160–5. https://doi.org/10.5625/lar.2018.34.4.160 . Epub 2019/01/24.
Szabo GK, Vandenberg LN. REPRODUCTIVE TOXICOLOGY: the male mammary gland: a novel target of endocrine-disrupting chemicals. Reproduction. 2021;162(5):F79–89. https://doi.org/10.1530/rep-20-0615 . Epub 2021/01/13.
Stewart TA, Hughes K, Hume DA, Davis FM. Developmental stage-specific distribution of macrophages in Mouse Mammary Gland. Front Cell Dev Biol. 2019;7:250. https://doi.org/10.3389/fcell.2019.00250 . Epub 2019/11/12.
Benjamin PD, Rachael CC, Anna LKC, Katie D, Andre Figueiredo B, Sonja J et al. An ovine model for investigation of the microenvironment of the male mammary gland. bioRxiv. 2023:2023.12.17.572059. https://doi.org/10.1101/2023.12.17.572059 .
King TA. The One Medicine concept: its emergence from history as a systematic approach to re-integrate human and veterinary medicine. Emerg Top Life Sci. 2021;5(5):643–54. https://doi.org/10.1042/etls20200353 . Epub 2021/08/07.
Gyles C, One Medicine O, Health, One World, Can Vet J. 2016;57(4):345–6. Epub 2016/04/05. PubMed PMID: 27041751; PubMed Central PMCID: PMCPMC4790223.
Partridge B, Rossmeisl JH. Jr. Companion animal models of neurological disease. J Neurosci Methods. 2020;331:108484. https://doi.org/10.1016/j.jneumeth.2019.108484 . Epub 2019/11/17.
Smith RKW, McIlwraith CW. One health in tendinopathy research: current concepts. J Orthop Res. 2021;39(8):1596–602. https://doi.org/10.1002/jor.25035 . Epub 2021/03/14.
Dan MJ, Crowley J, Broe D, Cross M, Tan C, Walsh WR. Patella tendinopathy zoobiquity - what can we learn from dogs? Knee. 2019;26(1):115–23. PubMed PMID: 30554911.
Vazquez E, Lipovka Y, Cervantes-Arias A, Garibay-Escobar A, Haby MM, Queiroga FL, et al. Canine mammary Cancer: state of the art and future perspectives. Animals. 2023;13(19):3147. https://doi.org/10.3390/ani13193147 . PubMed PMID:.
Raposo TP, Arias-Pulido H, Chaher N, Fiering SN, Argyle DJ, Prada J, et al. Comparative aspects of canine and human inflammatory breast cancer. Semin Oncol. 2017;44(4):288–300. https://doi.org/10.1053/j.seminoncol.2017.10.012 . Epub 2018/03/13.
Watson J, Wang T, Ho KL, Feng Y, Mahawan T, Dobbin KK, et al. Human basal-like breast cancer is represented by one of the two mammary tumor subtypes in dogs. Breast Cancer Res. 2023;25(1):114. https://doi.org/10.1186/s13058-023-01705-5 . Epub 2023/10/04.
Bergholtz H, Lien T, Lingaas F, Sørlie T. Comparative analysis of the molecular subtype landscape in canine and human mammary gland tumors. J Mammary Gland Biol Neoplasia. 2022;27(2):171–83. https://doi.org/10.1007/s10911-022-09523-9 . Epub 2022/08/07.
Liu D, Xiong H, Ellis AE, Northrup NC, Rodriguez CO Jr., O’Regan RM, et al. Molecular homology and difference between spontaneous canine mammary cancer and human breast cancer. Cancer Res. 2014;74(18):5045–56. https://doi.org/10.1158/0008-5472.Can-14-0392 . Epub 2014/08/02.
Zappulli V, De Zan G, Cardazzo B, Bargelloni L, Castagnaro M. Feline mammary tumours in comparative oncology. J Dairy Res. 2005;72. https://doi.org/10.1017/s0022029905001263 . Spec 98–106. Epub 2005/09/27.
Zeng L, Li W, Chen CS. Breast cancer animal models and applications. Zool Res. 2020;41(5):477–94. https://doi.org/10.24272/j.issn.2095-8137 . Epub 2020/07/07.
Abdelmegeed SM, Mohammed S. Canine mammary tumors as a model for human disease. Oncol Lett. 2018;15(6):8195–205. Epub 2018/06/22. doi: 10.3892/ol.2018.8411. PubMed PMID: 29928319; PubMed Central PMCID: PMCPMC6004712.
PubMed PubMed Central Google Scholar
Hughes K. Comparative mammary gland postnatal development and tumourigenesis in the sheep, cow, cat and rabbit: exploring the menagerie. Semin Cell Dev Biol. 2021;114:186–95. https://doi.org/10.1016/j.semcdb.2020.09.010 . Epub 2020/10/22.
Dixon-Suen SC, Lewis SJ, Martin RM, English DR, Boyle T, Giles GG, et al. Physical activity, sedentary time and breast cancer risk: a mendelian randomisation study. Br J Sports Med. 2022;56(20):1157–70. https://doi.org/10.1136/bjsports-2021-105132 . Epub 2022/11/04.
London CA, Gardner H, Zhao S, Knapp DW, Utturkar SM, Duval DL et al. Leading the pack: best practices in comparative canine cancer genomics to inform human oncology. Vet Comp Oncol. 2023;n/a(n/a). https://doi.org/10.1111/vco.12935 .
Vecchio I, Tornali C, Bragazzi NL, Martini M. The Discovery of insulin: an important milestone in the history of Medicine. Front Endocrinol (Lausanne). 2018. https://doi.org/10.3389/fendo.2018.00613 . 9:613. Epub 2018/11/09.
Dewi FN, Cline JM. Nonhuman primate model in mammary gland biology and neoplasia research. Lab Anim Res. 2021;37(1):3. https://doi.org/10.1186/s42826-020-00053-1 . Epub 2021/01/06.
Roberts BM, Chumpolkulwong K, Tayamun S, Inamnuay L, Rungsipipat A, Lombardini ED. Mammary carcinoma in a male rhesus macaque (Macaca mulatta): histopathology and immunohistochemistry of ductal carcinoma in situ. J Med Primatol. 2014;43(3):213–6. https://doi.org/10.1111/jmp.12110 . Epub 2014/03/22.
Carpenter NA, Crook EK. Mammary gland adenocarcinoma in a male Bornean Orangutan (Pongo Pygmaeus. J Zoo Wildl Med. 2017;48(1):224–7. https://doi.org/10.1638/2015-0303.1 . Epub 2017/04/01.
Waggie KS, Tolwani RJ, Lyons DM. Mammary adenocarcinoma in a male squirrel monkey (Saimiri sciureus). Vet Pathol. 2000;37(5):505–7. https://doi.org/10.1354/vp.37-5-505 . Epub 2000/10/31.
Francis A, Thomas J, Fallowfield L, Wallis M, Bartlett JM, Brookes C et al. Addressing overtreatment of screen detected DCIS; the LORIS trial. European journal of cancer (Oxford, England: 1990). 2015;51(16):2296 – 303. Epub 2015/08/25. doi: 10.1016/j.ejca.2015.07.017. PubMed PMID: 26296293.
Wang F, Shu X, Meszoely I, Pal T, Mayer IA, Yu Z et al. Overall Mortality After Diagnosis of Breast Cancer in Men vs Women. JAMA oncology. 2019;5(11):1589-96. Epub 2019/09/20. doi: 10.1001/jamaoncol.2019.2803. PubMed PMID: 31536134; PubMed Central PMCID: PMCPMC6753503 fees from Novartis and Genentech; grants from Pfizer; and personal fees from Eli Lilly and Co, GlaxoSmithKline, Immunomedics, Macrogenics, and Seattle Genetics outside the submitted work. No other disclosures were reported.
Hassett MJ, Somerfield MR, Baker ER, Cardoso F, Kansal KJ, Kwait DC, et al. Management of male breast Cancer: ASCO Guideline. J Clin Oncology: Official J Am Soc Clin Oncol. 2020;38(16):1849–63. https://doi.org/10.1200/jco.19.03120 . Epub 2020/02/15.
Carvalho TP, Oliveira AR, Santos DOD, Cassali GD, Garcia APV, Pereira F, et al. Histopathologic and immunophenotypic profile of a spontaneous mammary carcinoma in a male Humboldt’s white-fronted capuchin (Cebus albifrons). J Med Primatol. 2022;51(1):49–52. https://doi.org/10.1111/jmp.12553 . Epub 2021/11/14.
Machado MCA, Ocarino NM, Serakides R, Moroz LR, Sementilli A, Damasceno KA, et al. Triple-negative mammary carcinoma in two male dogs. J Vet Diagn Invest. 2020;32(1):94–8. Epub 2020/01/12. doi: 10.1177/1040638719898686. PubMed PMID: 31924140; PubMed Central PMCID: PMCPMC7003223.
Silva DMD, Kluthcovsky LC, Jensen de Morais H, Pallú GM, Dos Santos GC, Costa Castro JL, et al. Inflammatory mammary carcinoma in a male dog-case report. Top Companion Anim Med. 2019;37:100357. https://doi.org/10.1016/j.tcam.2019.100357 . Epub 2019/12/16.
Park J-H, Noh D-J, Lee S-W, Jung D-U, Park J-K, Lee K-J. Diagnosis of Mammary Carcinoma in a castrated male Maltese dog. J Vet Clin. 2019;36(5):278–81. https://doi.org/10.17555/jvc.2019.10.36.5.278 . Epub 2019/10/31.
Arias J, Paredes E, Torres C. Mammary carcinoma in a male dog: clinical and immunohistochemical characterisation. Arch De Med Vet. 2015;47:111–5.
Maiti S, Kumar D, Kumar S, Ar N, Mathew D, Palakkara S, et al. Mammary gland tumours in male dogs: a hormonal and tumour marker study. Veterinarski Arhiv. 2014;84:537–48.
Google Scholar
Jabara AG. Two cases of mammary neoplasms arising in male dogs. Aust Vet J. 1969;45(10):476–80. https://doi.org/10.1111/j.1751-0813.1969.tb06594.x .
Zuchi TLVL, Spricigo JB, Lopatini CL, Faria JLM, Mueller EN, Sueiro FAR, et al. Mammary gland carcinoma in situ in a male dog: case report. Comp Clin Pathol. 2018;27(4):1097–101. https://doi.org/10.1007/s00580-018-2733-2 .
Han J-H, Kim K-S, Kim J-H. Mammary gland tumors in three male dogs. Korean J Vet Res. 2016;56(4):229–32. https://doi.org/10.14405/kjvr.2016.56.4.229 .
Saba CF, Rogers KS, Newman SJ, Mauldin GE, Vail DM. Mammary gland tumors in male dogs. J Vet Intern Med. 2007;21(5):1056–9. https://doi.org/10.1111/j.1939-1676.2007.tb03064.x .
Bearss JJ, Schulman FY, Carter D. Histologic, immunohistochemical, and clinical features of 27 mammary tumors in 18 male dogs. Vet Pathol. 2012;49(4):602–7. Epub 2011/03/29. doi: 10.1177/0300985811402843. PubMed PMID: 21441113.
Kwon SC, Yoo DY, Ko M, Lee KY, Kwak HH, Park IC, et al. Mammary gland tumors in a male Cocker Spaniel. Acta Vet Scand. 2017;59(1):20. https://doi.org/10.1186/s13028-017-0290-3 . Epub 2017/04/13.
S PV, kumar KPS, Srilatha RVSND. Mammary neoplasm in a male dog - A Case Report. J Adv Veterinary Res. 2012;2(3):211–2.
Mamom P, Thengchaisri N, Sastravaha A, Ruangvejvorachai P, Mimapan S, Kaewmokul S, et al. Progesterone receptor expression in mammary gland tumor of male dogs. Kasetsart Veterinarians. 2012;22(2):14–22.
Edmunds G, Beck S, Kale KU, Spasic I, O’Neill D, Brodbelt D, et al. Associations between Dog Breed and Clinical features of mammary epithelial neoplasia in Bitches: an epidemiological study of submissions to a single Diagnostic Pathology Centre between 2008–2021. J Mammary Gland Biol Neoplasia. 2023;28(1):6. https://doi.org/10.1007/s10911-023-09531-3 . Epub 2023/03/25.
Loukopoulos P, Sutton RH, Lynch P, Gee DC. Ectopic mammary carcinoma in a male cat. Vet Rec. 2007;160(6):203-4. Epub 2007/02/13. https://doi.org/10.1136/vr.160.6.203-a . PubMed PMID: 17293585.
Bastan A, Anadol E, Ozenc E, Yardimci B. A case of mammary neoplasm in a male cat. Veteriner Fakültesi Dergisi. 2007;54:139–40.
Skorupski KA, Overley B, Shofer FS, Goldschmidt MH, Miller CA, Sørenmo KU. Clinical characteristics of mammary carcinoma in male cats. J Vet Intern Med. 2005;19(1):52–5. https://doi.org/10.1892/0891-6640(2005)19<52:ccomci>2.0 . Epub 2005/02/18.
Gregório H, Pires I, Seixas F, Queiroga F. Mammary invasive micropapillary carcinoma in a male cat: immunohistochemical description and clinical follow-up. Acta Veterinaria Hungarica. 2012;60(2):257–61. https://doi.org/10.1556/avet.2012.022 .
Cassali GD, Bertagnolli AC, Ferreira E, Malta MCC. A simple ductal mammary papilloma in a male maned Wolf (Chrysocyon Brachyurus). J Vet Diagn Invest. 2009;21(1):153–5. https://doi.org/10.1177/104063870902100127 .
Schoenbauer M. Mammary carcinoma of a male dog forming metastases [mammary tumour]. Revue de médecine vétérinaire 1982;133.
Aslan V, Çeşme H, Mutlu A, Cangül I, Salci H. Erkek Bir Köpekte Meme Bezinde Anaplastik Karsinoma Olgusu. J Uludağ Univ Fac Veterinary Med. 2017;36:25–8.
Damodaran S, Parthasarathy KR. Mammary neoplasms in male dogs. Indian J Veterinary Pathol. 1976;1(1):21–2.
Kumar P, Prasad V, Sreenu M, Chowdhary C. Mammary gland duct carcinoma in a male dog. Indian Veterinary J. 2018;95:89–90.
Pandey S, Bhargava M, Chandrapuria V, Das L, Kolte G. Mixed mammary tumour in a male dog. Indian J Veterinary Surg. 1983;4(1):70–2.
Salem H. Rzadki przypadek gruczolako-raka sutka (Adenocarcinoma metaplasticum) mammae u psa samca. Medycyna Weterynaryjna. 1973;29(5):306–7.
Oberoi M, Singh N, Kwatra M. Adenocarcinoma of mammary gland in a male dog. Agricultural Sci Digest. 1981;1(2):121–2.
Connah JG. Metastatic mammary adenocarcinoma initially misdiagnosed as a mast cell tumour in a male cat. Australian Veterinary Practitioner. 2016;46(2):56–9.
Lanza A, Pettorali M, Baldi A, Spugnini EP. Surgery and electrochemotherapy treatment of incompletely excised mammary carcinoma in two male pet rats (Rattus norvegicus). J Vet Med Sci. 2017;79(3):623–5. https://doi.org/10.1292/jvms.16-0578 . Epub 2017/02/22.
Kalaiselvan P, Vijayakumar S, Hemalatha K, Murkunde YV, Herbert RA, Wells MY. Mass in the lateral cervical-thoracic region in a male Wistar rat. Adenocarcinoma of the mammary gland. Lab Anim (NY). 2009;38(9):288–91. https://doi.org/10.1038/laban0909-288 . Epub 2009/08/25.
Grandi F, Monteiro L, Marietto-Gonçalves G, Rocha N. Mammary benign neoplasm diagnosed by fine needle aspiration biopsy in a guinea pig (Cavia porcellus). Acta Vet Brasílica. 2011;5(5):203–6.
Andrews E. Mammary neoplasia in the guinea pig (Cavia porcellus). Veterinarian. 1976;66(1):82–96.
CAS Google Scholar
Suárez-Bonnet A, Martín de las Mulas J, Millán MY, Herráez P, Rodríguez F. Espinosa De Los monteros A. Morphological and immunohistochemical characterization of spontaneous mammary gland tumors in the Guinea Pig (Cavia porcellus). Vet Pathol. 2010;47(2):298–305. PubMed PMID: 20106793.
Vienet V. New Pet animals. Mammary tumour in a male guinea pig. Point vétérinaire. 2002;33(229):1–4.
Amâncio B, Cangussú R, Pincinato S, Krause P. Achados citológicos em neoplasia mamária em porquinho da índia (Cavia porcellus) macho: Relato De Caso. Pubvet. 2021;15(6):1–3.
Walzl H, Leskova R. Spontanes, rezidivierendes Mammakarzinom Bei Einem Mannlichen Meerschweinchen. Wiener Tierärztliche Monatsschrift. 1979;66(5):186–7.
Summa NM, Eshar D, Snyman HN, Lillie BN. Metastatic anaplastic adenocarcinoma suspected to be of mammary origin in an intact male rabbit (Oryctolagus cuniculus). Can Vet J. 2014;55(5):475–9. Epub 2014/05/03. PubMed PMID: 24790235; PubMed Central PMCID: PMCPMC3992310.
Goldschmidt M, Peña L, Rasotto R, Zappulli V. Classification and grading of canine mammary tumors. Vet Pathol. 2011;48(1):117–31. Epub 2011/01/27. doi: 10.1177/0300985810393258. PubMed PMID: 21266722.
Hayes AA, Mooney S. Feline mammary tumors. Veterinary Clinics of North America -Small Animal Practice. 1985;15(3):513 – 20. https://doi.org/10.1016/S0195-5616(85)50054-6 . PubMed PMID: WOS:A1985AJE9600005.
Zappulli V, Pena L, Rasotto R, Goldschmidt M, Gama A, Scruggs J, et al. In: Kiupel M, editor. Surgical Pathology of Tumors of Domestic Animals, mammary tumors. Volume 2: mammary tumors. Davis-Thompson Foundation; 2019.
Gopal K, Srinivasan P, Thangathurai R, Rajeswar J, Dharmaceelan S. Pathology of triple negative basal like mammary tumour in a male non-descript dog - case report. Indian J Veterinary Pathol. 2022;46:73–7.
Saranya R, Nagarajan K, Hemalatha S, Gokulakrishnan M, Balagangatharathilagar M, Rao G. Pathological studies on inflammatory mammary cystic papillary adenocarcinoma in a male dog. Indian J Veterinary Pathol. 2022;46(3):220–3.
Thakur M, Ramandeep RN, Gupta S, Gupta V. Cytological and histopathological characterization of squamous cell carcinoma from mammary region in a male dog: a case study. Indian J Veterinary Pathol. 2021;45(4):321–4.
Tavares WL, Lavalle GE, Figueiredo MS, Souza AG, Bertagnolli AC, Viana FA, et al. Evaluation of adverse effects in tamoxifen exposed healthy female dogs. Acta Vet Scand. 2010;52(1):67. https://doi.org/10.1186/1751-0147-52-67 . Epub 2010/12/24.
Attalla S, Taifour T, Bui T, Muller W. Insights from transgenic mouse models of PyMT-induced breast cancer: recapitulating human breast cancer progression in vivo. Oncogene. 2021;40(3):475–91. https://doi.org/10.1038/s41388-020-01560-0 . Epub 2020/11/26.
Shishido S, Delahaye Ald, Beck A, Nguyen TA. The MMTV-PyVT transgenic mouse as a Multistage Model for Mammary Carcinoma and the efficacy of Antineoplastic Treatment. J Cancer Therapy. 2013;04:07:11. https://doi.org/10.4236/jct.2013.47138 .
Shishido SN, Faulkner EB, Beck A, Nguyen TA. The effect of antineoplastic drugs in a male spontaneous mammary tumor model. PLoS ONE. 2014;8(6):e64866. https://doi.org/10.1371/journal.pone.0064866 . Epub 2013/06/12.
Murphy C, Carder P, Lansdown M, Speirs V. Steroid hormone receptor expression in male breast cancer. Eur J Surg Oncol (EJSO). 2006;32(1):44–7.
Anderson WF, Jatoi I, Tse J, Rosenberg PS. Male breast cancer: a population-based comparison with female breast cancer. J Clin Oncology: Official J Am Soc Clin Oncol. 2010;28(2):232–9. https://doi.org/10.1200/jco.2009.23.8162 . Epub 2009/12/10.
Speirs V, Perspective. Not just for women. Nature. 2012;485(7400):S66. Epub 2012/06/01. https://doi.org/10.1038/485S66a . PubMed PMID: 22648505.
Co M, Lee A, Kwong A. Delayed presentation, diagnosis, and psychosocial aspects of male breast cancer. Cancer Med. 2020;9(10):3305–9. https://doi.org/10.1002/cam4.2953 . Epub 2020/03/14.
Rosol TJ, Tannehill-Gregg SH, Corn S, Schneider A, McCauley LK. Animal models of bone metastasis. Cancer Treat Res. 2004;118:47–81. https://doi.org/10.1007/978-1-4419-9129-4_3 . Epub 2004/03/27.
LM P, MR M. Surgical and chemotherapeutic management of mammary tumor in male dogs. J Indian Veterinary Association. 2011;9(3):55–6.
Schonbauer M. Mammatumoren Bei Ruden. Berliner Und Münchener. tierärztliche Wochenschrift. 1981;94(16):319–21.
Rea D, Francis A, Hanby AM, Speirs V, Rakha E, Shaaban A, et al. Inflammatory breast cancer: time to standardise diagnosis assessment and management, and for the joining of forces to facilitate effective research. Br J Cancer. 2015;112(9):1613–5. https://doi.org/10.1038/bjc.2015.115 . Epub 2015/04/14.
Kwon JY, Moskwa N, Kang W, Fan TM, Lee C. Canine as a comparative and translational model for human mammary tumor. J Breast Cancer. 2023;26(1):1–13. https://doi.org/10.4048/jbc.2023.26.e4 . Epub 2023/02/11.
Kim TM, Yang IS, Seung BJ, Lee S, Kim D, Ha YJ, et al. Cross-species oncogenic signatures of breast cancer in canine mammary tumors. Nat Commun. 2020;11(1):3616. https://doi.org/10.1038/s41467-020-17458-0 . Epub 2020/07/19.
Govoni VM, Da Silva TC, Guerra JM, Pereira IVA, Queiroga FL, Cogliati B. Genetic variants of BRCA1 and BRCA2 genes in cats with mammary gland carcinoma. Vet Comp Oncol. 2021;19(2):404–8. https://doi.org/10.1111/vco.12685 . Epub 2021/02/13.
Yadav S, Karam D, Bin Riaz I, Xie H, Durani U, Duma N, et al. Male breast cancer in the United States: treatment patterns and prognostic factors in the 21st century. Cancer. 2020;126(1):26–36. https://doi.org/10.1002/cncr.32472 . Epub 2019/10/08.
Rivera P, Melin M, Biagi T, Fall T, Häggström J, Lindblad-Toh K, et al. Mammary tumor development in dogs is associated with BRCA1 and BRCA2. Cancer Res. 2009;69(22):8770–4. https://doi.org/10.1158/0008-5472.Can-09-1725 . Epub 2009/11/06.
Thumser-Henner P, Nytko KJ, Rohrer Bley C. Mutations of BRCA2 in canine mammary tumors and their targeting potential in clinical therapy. BMC Vet Res. 2020;16(1):30. https://doi.org/10.1186/s12917-020-2247-4 . Epub 2020/02/02.
Arendt ML, Sakthikumar S, Melin M, Elvers I, Rivera P, Larsen M, et al. PIK3CA is recurrently mutated in canine mammary tumors, similarly to in human mammary neoplasia. Sci Rep. 2023;13(1):632. https://doi.org/10.1038/s41598-023-27664-7 . Epub 2023/01/13.
Balmaña J, Díez O, Rubio IT, Cardoso F, Medical Oncology. BRCA in breast cancer: ESMO Clinical Practice Guidelines. Annals of oncology: official journal of the European Society for. 2011;22 Suppl 6:vi31-4. Epub 2011/10/20. https://doi.org/10.1093/annonc/mdr373 . PubMed PMID: 21908500.
Casaubon JT, Kashyap S, Regan JP. BRCA1 and BRCA2 mutations. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Sarang Kashyap declares no relevant financial relationships with ineligible companies. John-Paul Regan declares no relevant financial relationships with ineligible companies.: StatPearls Publishing Copyright © 2023. Disclosure: StatPearls Publishing LLC.; 2023.
Silvestri V, Leslie G, Barnes DR, Agnarsson BA, Aittomäki K, Alducci E, et al. Characterization of the Cancer Spectrum in Men with Germline BRCA1 and BRCA2 pathogenic variants: results from the Consortium of investigators of modifiers of BRCA1/2 (CIMBA). JAMA Oncol. 2020;6(8):1218–30. https://doi.org/10.1001/jamaoncol.2020.2134 . Epub 2020/07/03.
Robertson FM, Cristofanilli M. A global approach to inflammatory breast cancer. Future oncology (London. England). 2011;7(1):25–30. https://doi.org/10.2217/fon.10.177 . Epub 2010/12/23.
Tanhueco A, Youssef MMG. Inflammatory breast Cancer in men: a rare clinical case report and a literature review. Int J Surg Case Rep. 2021;80:105696. https://doi.org/10.1016/j.ijscr.2021.105696 . Epub 2021/03/06.
Cassali G, Jark P, Gamba C, Damasceno K, Estrela-Lima A, Nardi A. Consensus regarding the diagnosis, prognosis and treatment of Canine and Feline Mammary tumors – 2019. Brazilian J Veterinary Pathol. 2020;13(3):555–74.
Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (arimidex, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365(9453):60–2. https://doi.org/10.1016/s0140-6736(04)17666-6 . Epub 2005/01/11.
Giordano SH, Valero V, Buzdar AU, Hortobagyi GN. Efficacy of Anastrozole in male breast Cancer. Am J Clin Oncol. 2002;25(3).
Download references
Acknowledgements
We thank members of the Speirs group for useful discussion. SC was funded by an Elphinstone Scholarship from the University of Aberdeen.
Author information
Authors and affiliations.
School of Medicine, Medical Sciences and Nutrition, University of Aberdeen, Aberdeen, AB25 2ZD, UK
Kirsty Luo-Yng Tay, George Cowan, Subarnarekha Chatterji, Giulia Conti & Valerie Speirs
Aberdeen Cancer Centre, Aberdeen, UK
Subarnarekha Chatterji & Valerie Speirs
Department of Molecular Medicine, Sapienza University of Rome, Rome, Italy
Giulia Conti
You can also search for this author in PubMed Google Scholar
Contributions
VS conceived the manuscript. KT and GC performed the literature search and data analysis. KT, GC, SC, GC and VS drafted the manuscript and prepared Figures. All authors reviewed the manuscript.
Corresponding author
Correspondence to Valerie Speirs .
Ethics declarations
Competing interests.
The authors report there are no competing interests to declare. ChatGPT was used to translate papers that were screened in this study but published in languages other than English (references [ 65 , 69 , 78 , 79 and 97 ]). No funding was received to assist with the preparation of this manuscript.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Luo-Yng Tay, K., Cowan, G., Chatterji, S. et al. Exploring the One Health Paradigm in Male Breast Cancer. J Mammary Gland Biol Neoplasia 29 , 8 (2024). https://doi.org/10.1007/s10911-024-09560-6
Download citation
Received : 08 January 2024
Accepted : 15 March 2024
Published : 04 April 2024
DOI : https://doi.org/10.1007/s10911-024-09560-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Male breast cancer
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 25 March 2024
Adjuvant chemotherapy and survival in males aged 70 years or older with breast cancer: a population-based retrospective study
- Yushuai Yu 1 na1 ,
- Kaiyan Huang 1 , 2 na1 ,
- Yushan Liu 3 na1 ,
- Ruiliang Chen 3 ,
- Xin Yu 1 &
- Chuangui Song 1
BMC Geriatrics volume 24 , Article number: 282 ( 2024 ) Cite this article
194 Accesses
Metrics details
Male breast cancer constitutes a minority of breast cancer diagnoses, yet its incidence has been on the rise in recent decades. However, elderly male breast cancer patients have been inadequately represented in clinical trials, posing challenges in treatment decisions. This study seeks to clarify the efficacy of chemotherapy in this demographic and identify the population most likely to benefit from such intervention.
We conducted a retrospective analysis using the Surveillance, Epidemiology, and End Results (SEER) database, encompassing a total of 1900 male breast cancer patients aged 70 years or older. Among them, 1652 were categorized in the no-chemotherapy group, while 248 were in the chemotherapy group. A multifactorial logistic regression model was employed to investigate the determinants influencing the administration of chemotherapy in elderly male breast cancer patients. Additionally, the multivariate Cox proportional hazards regression model was applied to identify factors associated with outcomes, with overall survival (OS) as the primary endpoint.
Multivariate logistic regression analysis revealed that grade, tumor size, and nodal status were robust predictors for elderly male breast cancer patients receiving chemotherapy. Furthermore, the multivariate analysis demonstrated that chemotherapy conferred benefits compared to the no-chemotherapy group (HR = 0.822, 95% CI: 0.682–0.991, p = 0.040). Stratified analyses indicated that individuals with N+, poorly/undifferentiated grade, and stage II/III disease could derive benefits from chemotherapy. Upon further investigation of progesterone receptor (PR) positive patients, it was found that only stage III patients experienced significant benefits from chemotherapy (HR = 0.571, 95% CI: 0.372–0.875, p = 0.010). Conversely, in PR negative patients, both stage II (HR = 0.201, 95% CI: 0.051–0.792, p = 0.022) and stage III patients (HR = 0.242, 95% CI: 0.060–0.972, p = 0.046) derived benefits from chemotherapy.
Adjuvant chemotherapy may benefit certain elderly male breast cancer patients, specifically those with positive lymph node status, poorly/undifferentiated grade, and PR-positive in stage III, as well as PR-negative expression in stage II/III. Given favorable physical tolerance, it is advisable not to hastily dismiss chemotherapy for these elderly male breast cancer patients.
Peer Review reports
Introduction
Male breast cancer constitutes a mere 1% of newly diagnosed breast cancer cases, signifying its rarity [ 1 ]. Over recent decades, there has been a gradual rise in the incidence of male breast cancer[ 2 , 3 ]. In the context of male breast cancer, encompassing both in situ and invasive forms, it is noteworthy that an estimated 47–70% of the patients diagnosed are in the elderly age group [ 4 , 5 , 6 ]. Male breast cancer exhibits a higher genetic predisposition compared to female breast cancer, with a 10% susceptibility in men versus 5–7% in women [ 7 , 8 ]. Common genetic mutations associated with male breast cancer include BRCA1, BRCA2, CHECK2, MLH1, MSH2, and MSH6, with BRCA2 being the most prevalent [ 9 ]. Men carrying a BRCA2 mutation face a lifetime risk of developing breast cancer of approximately 5–10% [ 7 , 8 ]. Given the scarcity of clinical trial data pertaining to older male breast cancer patients, especially concerning the contentious use of chemotherapy, therapeutic options remain uncertain [ 10 ]. With an escalating life expectancy in the population, it is imperative to address the question of which individuals stand to gain from chemotherapy and how it influences male breast cancer outcomes.
A previous report, drawing from the SEER database, noted that elderly patients were less likely to undergo chemotherapy compared to their younger counterparts [ 10 ]. Considering the potential added toxicity of chemotherapy drugs in this specific age group, clinical decision-making often leans towards undertreatment, which may impact prognosis. Earlier studies have highlighted those elderly male breast cancer patients face a heightened risk of overall mortality in comparison to younger patients [ 11 , 12 ]. Could this discrepancy be partially attributed to undertreatment? Additionally, it was observed that the majority of male breast cancer cases exhibit hormone receptor expression, with rare human epidermal growth factor receptor 2 (HER2) expression. Nearly 42% of tumors were categorized as luminal A, while 49% were classified as luminal B and HER2 negative [ 13 ]. Male breast cancer patients with hormone receptor-positive status are recommended to undergo adjuvant endocrine therapy [ 14 , 15 ]. Despite the promising effects of endocrine therapy, is there still a necessity for applying chemotherapy in male breast cancer patients? Furthermore, two studies attempted to investigate the impact of chemotherapy on male breast cancer utilizing the SEER database and National Cancer Database [ 4 , 12 ]. However, both studies conducted the analysis within the general population. Nevertheless, they did not fully resolve the clinical ambiguity. Neither of them compared the benefits of chemotherapy within subgroups other than stage, such as lymph node stage, different age groups, pathologic grade, and so forth. Moreover, a discrepancy exists between these two studies. While Hong Pan et al. concluded that progesterone receptor (PR) negative patients across all stages should receive chemotherapy, Siddhartha Yadav et al. found that only Estrogen Receptor (ER) positive patients in stage II-III can benefit from chemotherapy [ 4 , 12 ]. Thus, who stands to benefit more from chemotherapy among elderly male breast cancer patients remain a critical question.
Management of older male patients with breast cancer not only depends on the disease itself, but is also complicated by comorbidities, drug tolerance, physical condition, and expected life expectancy [ 16 , 17 , 18 , 19 ]. Chemotherapy will be more significant as life expectancy continues to increase in recent years. To compensate for the lack of evidence, we used data from the SEER database to analyze the role of chemotherapy in elderly male breast cancer by different subgroup analysis according to stage, lymph node status, PR status, and histological grade. We believe that the results of this study will help make clinical decision-making and assist in scientific investigations.
Data source and study population
We used SEER*Stat version 8.3.8 to include patients. We included 1900 patients based on the following inclusion criteria: male; diagnosed between 1975 and 2017; diagnosed at the age of 70 or older; breast cancer as the sole primary malignant tumor diagnosis; American Joint Committee on Cancer (AJCC) seventh edition stages I-III. In this study, patients with distant metastasis or in situ disease were excluded. We categorized the patients into two groups: the chemotherapy group and the no-chemotherapy group based on whether chemotherapy was administered. Patient characteristics included race, marital status, laterality, histology, grade, AJCC stage, tumor size, nodal status, ER, and PR. In the study’s data source and population segment, we analyzed treatment modalities, specifically focusing on surgical operation methods and the application of radiation therapy.
Outcome measurement
In our study, the primary outcome of interest was overall survival (OS), which was calculated from the date of diagnosis to the date of death, or censored at the last follow-up date. Censoring occurred for patients lost to follow-up or who survived until the end of the follow-up period. For patients still alive at the conclusion of our study, the follow-up duration was measured from the date of diagnosis to the study’s end. In cases of lost follow-up, the duration was computed from the date of diagnosis to the last recorded contact.
Statistical analysis
We used the chi-square test to compare the differences in demographic and clinical characteristics between the chemotherapy group and the no-chemotherapy group. Collinearity analysis was conducted to assess the degree of multicollinearity among the independent variables [ 20 , 21 ]. To quantify multicollinearity, the Variance Inflation Factor (VIF) was calculated for each predictor variable. The VIF measures how much the variance of an estimated regression coefficient increases if your predictors are correlated. VIF values exceeding 5 may warrant further investigation, as they indicate increasing multicollinearity. In instances where VIF values, specifically those exceeding 10, were observed, the approach adopted involved the removal of such variables from the model. Multifactorial logistic regression model was employed to explore the predictive factors for chemotherapy administration in elderly male breast cancer patients. We employed the log-rank test to ascertain whether there was a statistically significant difference in OS rates between patients who received chemotherapy and those who did not. We used the multivariate Cox proportional hazards regression model to calculate the hazard ratio (HR) with a 95% confidence interval (CI) to identify outcome-associated factors. Factors with a p -value greater than or equal to 0.05 in the univariate analysis were considered as candidate variables for the multivariate analysis. To further explore which elderly male breast cancer patients are in greater need of chemotherapy, we grouped them based on different tumor grades, AJCC stages, nodal status, as well as PR status. Statistical analyses were performed using R software version 4.3.1. All analyses were two-sided, and a p -value less than 0.05 was considered statistically significant.
Baseline characteristics
In this study, 1900 patients were included, comprising 1652 in the no-chemotherapy group and 248 in the chemotherapy group (refer to Table 1 ). The median follow-up duration was 186 months (Interquartile Range: 164–208 months) for the no-chemotherapy group and 102 months (Interquartile Range: 81–123 months) for the chemotherapy group. Noteworthy differences were observed in AJCC stage distribution. Stage II cancers were more common in the chemotherapy group (43.1%) compared to the no-chemotherapy group (34.3%), while Stage I cancers were less frequent in the chemotherapy group (11.3%) than in the no-chemotherapy group (32.3%). Regarding tumor size (T stage), T1 tumors were more prevalent in the no-chemotherapy group (41.7%), whereas T2 tumors were more prominent in the chemotherapy group (47.2%). Nodal status demonstrated a notable difference, with N0 status being more common in the no-chemotherapy group (54.1%) compared to the chemotherapy group (27.4%). Conversely, N1 status was more prevalent in the chemotherapy group (33.1%) compared to the no-chemotherapy group (16.6%). The surgical approach also showed a significant difference, with mastectomy being more predominant in the chemotherapy group (72.6%) compared to the no-chemotherapy group (47.9%). Furthermore, radiation status revealed a notable difference, as a higher proportion of patients in the no-chemotherapy group did not receive radiation therapy (83.2%) compared to the chemotherapy group (60.5%).
Predictors of chemotherapy receipt
Collinearity analysis revealed that the variable ‘Stage’ exhibited high VIF values (10.59) in relation to the receipt of chemotherapy, indicating significant multicollinearity (refer to Supplement Fig. 1 a). Consequently, ‘Stage’ was excluded from subsequent analyses. Following this exclusion, reassessment through collinearity analysis confirmed that all remaining variables demonstrated low VIF values, thus alleviating concerns of multicollinearity (refer to Supplement Fig. 1 b).
Chemotherapy effect on overall survival (OS) by subgroup
Abbreviations: HR: hazard ratio
The multivariate logistic regression analysis identified several significant predictors for the receipt of chemotherapy in elderly male breast cancer patients (refer to Table 2 ). Grade, tumor size, and nodal status were also found to be significant predictors. Specifically, patients with moderately differentiated tumors had a higher likelihood of receiving chemotherapy compared to those with well-differentiated tumors (HR = 2.844, 95% CI: 1.262–6.409, p = 0.012). Patients with poorly/undifferentiated tumors had even higher likelihoods (HR = 3.773, 95% CI: 1.661–8.572, p = 0.002). Additionally, patients with positive nodal status (N1, N2/3) were more likely to receive chemotherapy compared to those with negative nodal status (N0) (HR = 2.889, 95% CI: 1.991–4.193, p < 0.001; HR = 6.158, 95% CI: 3.976–9.538, p < 0.001, respectively). Surgery approach and radiation status were also significant predictors. Patients who underwent mastectomy were more likely to receive chemotherapy compared to those who had breast-conserving surgery (HR = 2.947, 95% CI: 1.240–7.005, p = 0.014). Furthermore, patients who received radiation therapy were more likely to undergo chemotherapy (HR = 1.833, 95% CI: 1.313–2.558, p < 0.001).
Comparison of survival between chemotherapy group and no-chemotherapy group
The multivariate Cox proportional hazard model was applied to assess the impact of various factors on OS in all patients (refer to Table 3 ). Marital status, Grade, Tumor size, Nodal status, Surgery approach, Radiation status, and Chemotherapy status exhibited a significant association with OS.
In order to further clarify which population needs chemotherapy, we conducted subgroup analyses based on different nodal statuses, histological grades, staging, and PR statuses (refer to Table 4 ; Fig. 1 ). For patients with N0 status, the difference in OS between the chemotherapy and no-chemotherapy groups was not statistically significant (HR = 0.790, 95% CI: 0.555–1.125, p = 0.192). However, for patients with N + status, those receiving chemotherapy demonstrated a significantly improved OS compared to those without chemotherapy (HR = 0.734, 95% CI: 0.566–0.951, p = 0.019). Among patients with well/moderately differentiated tumors, there was no significant difference in OS between the chemotherapy and no-chemotherapy groups (HR = 0.788, 95% CI: 0.581–1.068, p = 0.124). Conversely, for patients with poorly/undifferentiated tumors, those receiving chemotherapy exhibited a substantially better OS compared to those not receiving chemotherapy (HR = 0.628, 95% CI: 0.460–0.859, p = 0.004). In Stage II, and Stage III cancers, patients who underwent chemotherapy demonstrated significantly improved OS compared to those who did not ( P = 0.004, and P = 0.029, respectively); however, in Stage I patients, chemotherapy didn’t confer any benefit ( P = 0.096).
To further analyze the effect of chemotherapy in stages patients with different PR statuses, we further segmented our population. The results revealed that among PR + patients, only those in stage III could benefit from chemotherapy (HR = 0.571, 95% CI: 0.372–0.875, p = 0.010). In contrast, PR- patients in both stage II and stage III showed a potential benefit from chemotherapy (PR- stage II: HR = 0.201, 95% CI: 0.051–0.792, p = 0.022; PR- stage III: HR = 0.242, 95% CI: 0.060–0.972, p = 0.046). Therefore, elderly male breast cancer patients who are PR + and in stage II-III, as well as PR- patients in stage I, may be exempt from chemotherapy.
The male breast cancer population presents a unique clinical challenge, characterized by a dearth of tailored clinical trial data and a propensity for treatment algorithms to confound clinicians. Moreover, advanced age is correlated with diminished survival prospects [ 11 , 12 ]. This discrepancy is partially attributed to undertreatment, further exacerbating the issue. Presently, treatment approaches for elderly male breast cancer patients are predominantly extrapolated from guidelines established for elderly female breast cancer patients, encompassing a spectrum of interventions like surgery, endocrine therapy, radiotherapy, and chemotherapy [ 22 , 23 ]. Among these modalities, chemotherapy engenders heightened controversy [ 24 ]. Our study, employing multivariable Cox regression, elucidates that not all elderly male breast cancer patients stand to benefit from chemotherapy. Thus, the judicious selection of candidates assumes paramount importance, mitigating the proclivity towards both overtreatment and undertreatment in clinical decision-making.
Our multivariable Cox regression analysis revealed a notable benefit of chemotherapy for stage II-III elderly male breast cancer patients. In a study investigating treatment patterns in stage I-III male breast cancer patients, Siddhartha et al. reported that the survival advantage associated with chemotherapy primarily manifested in patients with stage II-III disease. Although their findings were consistent with our own, it’s intriguing to contemplate whether all stage II patients, particularly in the context of elderly males, necessitate chemotherapy [ 12 ]. Past studies have underscored the prognostic significance of PR status in breast cancer patients [ 25 ]. This begs the question: how does PR status impact patients with negative versus positive expression within the same stage? To address this, we conducted a stratified analysis of stage II-III patients based on differing PR statuses. Our findings indicate that patients with PR-positive stage II may potentially forgo chemotherapy, as overall survival exhibited no significant improvement post-chemotherapy. Conversely, patients with PR-negative stage II-III stand to gain substantial benefits from chemotherapy. The conspicuous disparities between our conclusions and prior research may be attributed to older patients facing elevated risks of chemotherapy-related toxicity, mortality, reduced tolerability, and diminished chemotherapy sensitivity compared to their younger counterparts [ 4 ]. Patients with PR-negative breast cancer in stages II and III have better prognoses with chemotherapy, whereas PR-positive patients only show this benefit in stage III. This could be attributed to PR positivity being a favorable prognostic factor, while PR-negative breast cancers are more aggressive [ 26 , 27 , 28 ]. Previous biological experiments suggest that the absence of PR expression in tumors may indicate impaired growth factor signaling pathways, such as the phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway, leading to increased invasiveness and resistance to therapy [ 29 , 30 ].
Histological grade perennially constitutes a pivotal prognostic determinant in female breast cancer, wielding considerable influence over treatment decisions [ 31 ]. In the realm of male breast cancer, the role of histological grade remains relatively uncharted, with existing data yielding disparate conclusions [ 13 , 32 , 33 , 34 ]. Our investigation reveals a noteworthy finding: within the poorly/undifferentiated grade cohort, the risk of death post-chemotherapy significantly diminishes compared to the well/moderately differentiated grade cohort. Given the heightened efficacy of cytotoxic chemotherapy in eradicating rapidly proliferating tumor cells, its administration remains imperative in the context of poorly/undifferentiated grade elderly male breast cancer. Notably, prior research suggests that roughly 33.5% of patients fall within the poorly/undifferentiated grade category, signifying a substantial portion of the population poised to derive meaningful benefits from chemotherapy.
Lymph node involvement constitutes the predominant adverse prognostic factor for male breast cancer patients. As demonstrated in prior studies, nearly half of elderly male breast cancer cases exhibit lymph node positivity. Within the broader population, numerous studies have underscored the substantial improvement in long-term prognosis conferred by chemotherapy for axillary lymph node-positive patients [ 23 , 35 ]. Sharon H. Giordano et al.’s study on adjuvant systemic therapy in male breast cancer patients revealed a reduced risk of death in patients receiving adjuvant chemotherapy, with the greatest benefits observed in those with lymph node involvement; however, this finding did not attain statistical significance [ 23 ]. A prospective study with a 20-year follow-up similarly ascertained potential benefits of adjuvant chemotherapy in male breast cancer patients with positive nodes, though both studies lacked specific age range delineations [ 35 ]. Notably, our investigation delineates those elderly male breast cancer patients with lymphatic metastasis stand to gain substantial advantages from chemotherapy. Contingent on physical tolerance, it would be remiss for elderly male breast cancer patients, particularly those with lymph node positivity, to summarily forego consideration of chemotherapy.
To the best of our knowledge, this study represents the inaugural endeavor dedicated to discerning the impact of chemotherapy within this distinctive population. The findings, derived from an expansive patient cohort, furnish potential insights into the adjuvant chemotherapy prospects for elderly male breast cancer patients. Nevertheless, our study is not devoid of limitations. Firstly, the absence of HER-2 status in our analysis stems from restricted data availability. However, it is noteworthy that prior research indicates a majority of patients exhibiting HER-2 negativity, potentially mitigating bias in our conclusions. Secondly, constrained by the available information in the SEER database, we were unable to incorporate variables such as genetic predisposition mutations, specific chemotherapy regimens, dosages, anti-HER2 therapy, or endocrine therapy into our analysis. Given these limitations, future research, including additional data collection and clinical trials, will be essential to validate our findings.
Data availability
The dataset supporting the conclusions of this article is available in the Surveillance, Epidemiology, and End Results (SEER) database. The URL of the database is https://seer.cancer.gov/ .
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. Cancer J Clin. 2022;72(1):7–33.
Article Google Scholar
Howlader NNA, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER cancer statistics review, 1975–2018, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2018/ , based on November 2020 SEER data submission, posted to the SEER web site, April 2021.
Peng JY, Lee YK, Pham RQ, Shen XH, Chen IH, Chen YC, Fan HS. Trends and age-period-cohort effect on incidence of male breast cancer from 1980 to 2019 in Taiwan and the USA. Cancers. 2024;16(2).
Pan H, Zhang K, Wang M, Ling L, Wang S, Zhou W. The effect of chemotherapy on survival in patients with nonmetastatic male breast cancer: a population-based observational study. Cancer. 2020;126(Suppl 16):3830–6.
Article CAS PubMed Google Scholar
Ishii T, Nakano E, Watanabe T, Higashi T. Epidemiology and practice patterns for male breast cancer compared with female breast cancer in Japan. Cancer Med. 2020;9(16):6069–75.
Article CAS PubMed PubMed Central Google Scholar
Wei JL, Zhang JX, Fu DY. Characterization and prognosis of estrogen receptor-positive/progesterone receptor-negative male breast cancer: a population-based study. World J Surg Oncol. 2018;16(1):236.
Article PubMed PubMed Central Google Scholar
Silvestri V, Barrowdale D, Mulligan AM, Neuhausen SL, Fox S, Karlan BY, Mitchell G, James P, Thull DL, Zorn KK, et al. Male breast cancer in BRCA1 and BRCA2 mutation carriers: pathology data from the consortium of investigators of modifiers of BRCA1/2. Breast cancer Research: BCR. 2016;18(1):15.
Sanguinetti A, Polistena A, Lucchini R, Monacelli M, Galasse S, Avenia S, Triola R, Bugiantella W, Cirocchi R, Rondelli F, et al. Male breast cancer, clinical presentation, diagnosis and treatment: twenty years of experience in our breast unit. Int J Surg case Rep. 2016;20s(Suppl):8–11.
Saita C, Yamaguchi T, Horiguchi SI, Yamada R, Takao M, Iijima T, Wakaume R, Aruga T, Tabata T, Koizumi K. Tumor development in Japanese patients with lynch syndrome. PLoS ONE. 2018;13(4):e0195572.
Duma N, Hoversten KP, Ruddy KJ. Exclusion of male patients in breast cancer clinical trials. JNCI cancer Spectr. 2018;2(2):pky018.
Leone JP, Leone J, Zwenger AO, Iturbe J, Vallejo CT, Leone BA. Prognostic significance of tumor subtypes in male breast cancer: a population-based study. Breast Cancer Res Treat. 2015;152(3):601–9.
Yadav S, Karam D, Bin Riaz I, Xie H, Durani U, Duma N, Giridhar KV, Hieken TJ, Boughey JC, Mutter RW, et al. Male breast cancer in the United States: treatment patterns and prognostic factors in the 21st century. Cancer. 2020;126(1):26–36.
Cardoso F, Bartlett JMS, Slaets L, van Deurzen CHM, van Leeuwen-Stok E, Porter P, Linderholm B, Hedenfalk I, Schröder C, Martens J, et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG international male breast cancer program. Annals Oncology: Official J Eur Soc Med Oncol. 2018;29(2):405–17.
Article CAS Google Scholar
Accomasso F, Actis S, Minella C, Rosso R, Granaglia C, Ponzone R, Biglia N, Bounous VE. Clinical, pathological, and prognostic features of male breast cancer: a multicenter study. Curr Oncol (Toronto Ont). 2023;30(11):9860–71.
Hassett MJ, Somerfield MR, Baker ER, Cardoso F, Kansal KJ, Kwait DC, Plichta JK, Ricker C, Roshal A, Ruddy KJ, et al. Management of male breast cancer: ASCO guideline. J Clin Oncology: Official J Am Soc Clin Oncol. 2020;38(16):1849–63.
Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120(9):1290–314.
Article PubMed Google Scholar
Crozier JA, Pezzi TA, Hodge C, Janeva S, Lesnikoski BA, Samiian L, Devereaux A, Hammond W, Audisio RA, Pezzi CM. Addition of chemotherapy to local therapy in women aged 70 years or older with triple-negative breast cancer: a propensity-matched analysis. Lancet Oncol. 2020;21(12):1611–9.
Mohile SG, Dale W, Somerfield MR, Hurria A. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology summary. J Oncol Pract. 2018;14(7):442–6.
Biganzoli L, Battisti NML, Wildiers H, McCartney A, Colloca G, Kunkler IH, Cardoso MJ, Cheung KL, de Glas NA, Trimboli RM, et al. Updated recommendations regarding the management of older patients with breast cancer: a joint paper from the European society of breast cancer specialists (EUSOMA) and the international society of geriatric Oncology (SIOG). Lancet Oncol. 2021;22(7):e327–40.
Kim JH. Multicollinearity and misleading statistical results. Korean J Anesthesiology. 2019;72(6):558–69.
Marcoulides KM, Raykov T. Evaluation of variance inflation factors in regression models using latent variable modeling methods. Educ Psychol Meas. 2019;79(5):874–82.
Cutuli B. Strategies in treating male breast cancer. Expert Opin Pharmacother. 2007;8(2):193–202.
Giordano SH, Perkins GH, Broglio K, Garcia SG, Middleton LP, Buzdar AU, Hortobagyi GN. Adjuvant systemic therapy for male breast carcinoma. Cancer. 2005;104(11):2359–64.
Giordano SH. Breast cancer in men. N Engl J Med. 2018;378(24):2311–20.
Prat A, Cheang MC, Martín M, Parker JS, Carrasco E, Caballero R, Tyldesley S, Gelmon K, Bernard PS, Nielsen TO, et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncology: Official J Am Soc Clin Oncol. 2013;31(2):203–9.
Singhal H, Greene ME, Zarnke AL, Laine M, Al Abosy R, Chang YF, Dembo AG, Schoenfelt K, Vadhi R, Qiu X, et al. Progesterone receptor isoforms, agonists and antagonists differentially reprogram estrogen signaling. Oncotarget. 2018;9(4):4282–300.
Boland MR, Ryan ÉJ, Dunne E, Aherne TM, Bhatt NR, Lowery AJ. Meta-analysis of the impact of progesterone receptor status on oncological outcomes in oestrogen receptor-positive breast cancer. Br J Surg. 2020;107(1):33–43.
Van Belle V, Van Calster B, Brouckaert O, Vanden Bempt I, Pintens S, Harvey V, Murray P, Naume B, Wiedswang G, Paridaens R, et al. Qualitative assessment of the progesterone receptor and HER2 improves the Nottingham prognostic index up to 5 years after breast cancer diagnosis. J Clin Oncology: Official J Am Soc Clin Oncol. 2010;28(27):4129–34.
Cui X, Zhang P, Deng W, Oesterreich S, Lu Y, Mills GB, Lee AV. Insulin-like growth factor-I inhibits progesterone receptor expression in breast cancer cells via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway: progesterone receptor as a potential indicator of growth factor activity in breast cancer. Mol Endocrinol (Baltimore Md). 2003;17(4):575–88.
Petz LN, Ziegler YS, Schultz JR, Nardulli AM. Fos and Jun inhibit estrogen-induced transcription of the human progesterone receptor gene through an activator protein-1 site. Mol Endocrinol (Baltimore Md). 2004;18(3):521–32.
Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast cancer. Lancet (London England). 2021;397(10286):1750–69.
Sarmiento S, McColl M, Musavi L, Gani F, Canner JK, Jacobs L, Fu F, Siotos C, Habibi M. Male breast cancer: a closer look at patient and tumor characteristics and factors that affect survival using the national cancer database. Breast Cancer Res Treat. 2020;180(2):471–9.
Nilsson C, Johansson I, Ahlin C, Thorstenson S, Amini RM, Holmqvist M, Bergkvist L, Hedenfalk I, Fjällskog ML. Molecular subtyping of male breast cancer using alternative definitions and its prognostic impact. Acta Oncol (Stockholm Sweden). 2013;52(1):102–9.
Humphries MP, Sundara Rajan S, Honarpisheh H, Cserni G, Dent J, Fulford L, Jordan LB, Jones JL, Kanthan R, Litwiniuk M, et al. Characterisation of male breast cancer: a descriptive biomarker study from a large patient series. Sci Rep. 2017;7:45293.
Walshe JM, Berman AW, Vatas U, Steinberg SM, Anderson WF, Lippman ME, Swain SM. A prospective study of adjuvant CMF in males with node positive breast cancer: 20-year follow-up. Breast Cancer Res Treat. 2007;103(2):177–83.
Download references
Acknowledgements
Not applicable.
This research was not supported by any grant funding.
Author information
Yushuai Yu, Kaiyan Huang and Yushan Liu contributed equally to this work and share first authorship.
Authors and Affiliations
Department of Breast Surgery, Clinical Oncology School of Fujian Medical University, Fujian Cancer Hospital, No.420, Fu Ma Road, Jinan District, 350014, Fuzhou, Fujian Province, China
Yushuai Yu, Kaiyan Huang, Xin Yu & Chuangui Song
Department of Breast and Thyroid Surgery, The Second Affiliated Hospital of Fujian Medical University, 362000, Quanzhou, Fujian Province, China
Kaiyan Huang
Fujian Medical University Union Hospital, 350001, Fuzhou, Fujian Province, China
Yushan Liu & Ruiliang Chen
You can also search for this author in PubMed Google Scholar
Contributions
CGS and YSY contributed to conception and design; YSY, KYH and YSL contributed to the development of methodology; YSY, RLC, and XY contributed to the acquisition of data and analysis of data; YSY, KYH and YSL wrote, reviewed, and/or revised the manuscript; CGS did study supervision. All authors have read and approved the manuscript.
Corresponding author
Correspondence to Chuangui Song .
Ethics declarations
Ethical approval and consent to participate.
Patient consent for this retrospective study was not required.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Yu, Y., Huang, K., Liu, Y. et al. Adjuvant chemotherapy and survival in males aged 70 years or older with breast cancer: a population-based retrospective study. BMC Geriatr 24 , 282 (2024). https://doi.org/10.1186/s12877-024-04861-1
Download citation
Received : 14 November 2023
Accepted : 01 March 2024
Published : 25 March 2024
DOI : https://doi.org/10.1186/s12877-024-04861-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Male breast cancer
- Elderly patients
- Chemotherapy
- Clinical decision-making
BMC Geriatrics
ISSN: 1471-2318
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Skip to Content
- Conquer Cancer
- ASCO Journals
- f Cancer.net on Facebook
- t Cancer.net on Twitter
- q Cancer.net on YouTube
- g Cancer.net on Google
Types of Cancer
- Navigating Cancer Care
- Coping With Cancer
- Research and Advocacy
- Survivorship
Breast Cancer, Male: Latest Research
ON THIS PAGE: You will read about the scientific research being done to learn more about male breast cancer and how to treat it. Use the menu to see other pages.
Doctors are working to learn more about male breast cancer, ways to prevent it, how to best treat it, and how to provide the best care to people diagnosed with this disease. The following areas of research may include new options for patients through clinical trials. Always talk with your doctor about the best diagnostic and treatment options for you.
Improving surgical options . New surgical methods that save tissue or prevent scarring are being tested in clinical trials.
Radiation therapy advances . Testing improved radiation therapy to lower the risk of side effects.
New medications and drug combinations . Evaluating new systemic therapies and combinations of therapies to treat male breast cancer, including chemotherapy, hormonal therapy, targeted therapy, and immunotherapy.
Palliative and supportive care. Finding better ways of reducing symptoms and side effects of current breast cancer treatments to improve comfort and quality of life for patients.
Looking for More About the Latest Research?
If you would like more information about the latest areas of research in male breast cancer, explore these related items that take you outside of this guide:
To find clinical trials specific to your diagnosis, talk with your doctor or search online clinical trial databases .
Visit the Cancer.Net Blog to review news and information about breast cancer, including research announced at recent scientific meetings.
Get updates from Cancer.Net delivered right to your inbox. Subscribe to the Inside Cancer.Net email newsletter.
Visit the website of Conquer Cancer, the ASCO Foundation , to find out how to help support cancer research. Please note that this link takes you to a different ASCO website.
The next section in this guide is Coping with Treatment . It offers some guidance on how to cope with the physical, emotional, social, and financial changes that cancer and its treatment can bring. Use the menu to choose a different section to read in this guide.
Breast Cancer, Male Guide
Cancer.Net Guide Breast Cancer, Male
- Introduction
- Risk Factors
- Symptoms and Signs
- Types of Treatment
- About Clinical Trials
- Latest Research
- Coping with Treatment
- Follow-up Care and Monitoring
- Questions to Ask the Health Care Team
- Additional Resources
View All Pages
Timely. Trusted. Compassionate.
Comprehensive information for people with cancer, families, and caregivers, from the American Society of Clinical Oncology (ASCO), the voice of the world's oncology professionals.
Find a Cancer Doctor
AACR Annual Meeting News: Read the latest session previews and recaps from the official news website.
Select "Patients / Caregivers / Public" or "Researchers / Professionals" to filter your results. To further refine your search, toggle appropriate sections on or off.
Home > Patients, Caregivers, and Advocates > About Cancer > Cancers > Male Breast Cancer
Male Breast Cancer
Breast cancer may occur in men. Although it is relatively rare – less than 1 percent of breast cancer diagnoses in the United States are in males – men may develop the disease at any age. It is, however, usually detected in men between 60 and 70 years of age.
Radiation exposure, high levels of estrogen, and a family history of breast cancer are risk factors for male breast cancer. In particular, men with a mutation of the BRCA2 gene are at increased risk of developing breast cancer.
Approximately 2,800 men in the United States will be diagnosed with breast cancer in 2023 and about 530 will die from the disease, the National Cancer Institute has estimated.
Learn more about breast cancer in women here .
Source: National Cancer Institute

You can support the AACR's mission by contacting your representatives and senators and advocating for increased funding for lifesaving cancer research.

Raising Awareness of Male Breast Cancer

Pioneering Personalized Medicine

Pedaling for Cancer Research
- Parathyroid Cancer
- Extrahepatic Bile Duct Cancer
- Laryngeal Cancer
Breast Cancer Research Results and Study Updates
See Advances in Breast Cancer Research for an overview of recent findings and progress, plus ongoing projects supported by NCI.
Some people with no evidence of cancer in nearby lymph nodes after presurgical chemotherapy can skip radiation to that area without increasing the risk of the cancer returning, a clinical trial found. But some experts caution that more details are needed.
For women in their 70s and older, the risk of overdiagnosis with routine screening mammography is substantial, a new study suggests. The findings highlight the need for conversations between older women and their health care providers about the potential benefits and harms of continuing screening mammography.
Many young women who are diagnosed with early-stage breast cancer want to become pregnant in the future. New research suggests that these women may be able to pause their hormone therapy for up to 2 years as they try to get pregnant without raising the risk of a recurrence in the short term.
For younger women with advanced breast cancer, the combination of ribociclib (Kisqali) and hormone therapy was much better at shrinking metastatic tumors than standard chemotherapy treatments, results from an NCI-funded clinical trial show.
In a large clinical trial, a condensed course of radiation therapy was as effective and safe as a longer standard course for those with higher-risk early-stage breast cancer who had a lumpectomy. This shorter radiation course makes treatment less of a burden for patients.
Adding the immunotherapy drug pembrolizumab (Keytruda) to chemotherapy can help some patients with advanced triple-negative breast cancer live longer. In the KEYNOTE-355 trial, overall survival improved among patients whose tumors had high levels of the PD-L1 protein.
People with metastatic breast cancer whose tumors had low levels of HER2 protein lived longer after treatment with trastuzumab deruxtecan (Enhertu) than those treated with standard chemotherapy, results of the DESTINY-Breast04 clinical trial show.
NCI researchers have shown that an experimental form of immunotherapy that uses an individual’s own tumor-fighting immune cells could potentially be used to treat people with metastatic breast cancer who have exhausted all other treatment options.
Most breast cancer risk tools were developed with data mainly from White women and don’t work as well for Black women. A new tool that estimates risk for Black women may help identify those who might benefit from earlier screening, enabling earlier diagnosis and treatment.
In people with metastatic HER2-positive breast cancer, the targeted drug trastuzumab deruxtecan (Enhertu) markedly lengthened progression-free survival compared with trastuzumab emtansine (Kadcycla), new study results show.
In a large clinical trial, women with HR-positive, HER2-negative metastatic breast cancer treated with ribociclib (Kisqali) and letrozole (Femara) as their initial treatment lived approximately 1 year longer than women treated with letrozole only.
Women with early-stage breast cancer who had one or both breasts surgically removed (a unilateral or bilateral mastectomy) had lower scores on a quality-of-life survey than women who had breast-conserving surgery, a new study has found.
For women undergoing chemotherapy for breast cancer, meeting the national physical activity guidelines may help alleviate cognitive issues, a new study suggests. The benefits may be even greater for patients who were physically active before treatment.
Sacituzumab govitecan (Trodelvy) now has regular FDA approval for people with locally advanced or metastatic triple-negative breast cancer (TNBC). The update follows last year’s accelerated approval of the drug for people with TNBC.
For some people with ER-positive breast cancer, a new imaging test may help guide decisions about receiving hormone therapy, according to a new study. The test can show whether estrogen receptors in tumors are active and responsive to estrogen.
The test, which helps guide treatment decisions, was not as good at predicting the risk of death from breast cancer for Black patients as for White patients, a new study has found. The findings highlight the need for greater racial diversity in research studies.
The drug abemaciclib (Verzenio) may be a new treatment option for people with the most common type of breast cancer, with new study findings suggesting that it can reduce the risk of the cancer returning.
Fertility preservation for young women with breast cancer doesn’t increase their risk of dying in the ensuing decades, a new study affirmed. Experts said the findings support routinely offering fertility preservation to patients who want it.
Some postmenopausal women with HR-positive, HER2-negative breast cancer may not benefit from chemotherapy and can safely forgo the treatment, according to clinical trial results presented at the San Antonio Breast Cancer Symposium.
A heart-related event, like a heart attack, may make breast cancer grow faster, a new study suggests. In mice, heart attacks accelerated breast tumor growth and human studies linked cardiac events with breast cancer recurrence, researchers reported.
FDA has approved sacituzumab govitecan (Trodelvy) for the treatment of triple-negative breast cancer that has spread to other parts of the body. Under the approval, patients must have already undergone at least two prior treatment regimens.
Women with high-risk breast cancer who engaged in regular exercise before their cancer diagnosis and after treatment were less likely to have their cancer return or to die compared with women who were inactive, a recent study found.
Researchers have developed a “microscaled” approach to analyze the proteins and genetic changes (proteogenomics) of a tumor that uses tissue from a core needle biopsy. The analyses can provide important information that may help guide treatment.
Tucatinib improved survival for women in the HER2CLIMB trial, including some whose cancer had spread to the brain. Trastuzumab deruxtecan improved survival and shrank many tumors in the DESTINY-Breast01 trial, which led to its accelerated approval.
A TAILORx analysis shows women with early-stage breast cancer and high recurrence scores on the Oncotype DX who received chemotherapy with hormone therapy had better long-term outcomes than what would be expected from hormone therapy alone.
Men with breast cancer may be more likely to die of the disease than women, particularly during the first 5 years after diagnosis, a new study suggests. The higher likelihood of death was linked in part to undertreatment and later diagnosis.
In a survey of nearly 600 breast cancer survivors, researchers found that the cost of care factored into the decisions the women made about what type of surgery to get. Many women also reported never discussing costs with their physicians.
FDA has expanded the approved use of the drug ado-trastuzumab emtansine (Kadcyla), also called T-DM1, to include adjuvant treatment in some women with early-stage HER2-positive breast cancer.
Many women diagnosed with ovarian and breast cancer are not undergoing tests for inherited genetic mutations that can provide important information to help guide decisions about treatment and longer-term cancer screening, a new study has found.
FDA has approved atezolizumab (Tecentriq) in combination with chemotherapy for the treatment of some women with advanced triple-negative breast cancer. This is the first FDA-approved regimen for breast cancer to include immunotherapy.
The build-up of connective tissue around some types of cancer can act as a barrier to immunotherapy. A new study uses a bone marrow transplant drug, plerixafor, to break down this barrier and improve the efficacy of immune checkpoint inhibitors in animal models of breast cancer.
A new study in mice shows that disrupting the relationship between breast cancer cells that spread to bone and normal cells surrounding them makes the cancer cells sensitive to treatment.
In women with early-stage breast cancer, two clinical trials have shown that both whole- and partial-breast radiation therapy are effective at preventing the cancer from returning after breast-conserving surgery.
Researchers are testing a topical-gel form of the drug tamoxifen to see if it can help prevent breast cancer as effectively as the oral form of the drug but with fewer side effects.
Findings from a clinical study and a mouse study may shed light on genetic risk factors for developing cancer-related cognitive problems in older breast cancer survivors. The results suggest a gene associated with Alzheimer’s disease may play a role.
Arsenic trioxide and retinoic acid work together to target the master regulator protein Pin1, a new study shows. In cancer cell lines and mice, the drug combination slowed the growth of triple-negative breast cancer tumors.
FDA has expanded the approved uses of ribociclib (Kisqali) for women with advanced breast cancer, including new uses in pre- and postmenopausal women. It’s the first approval under a new FDA program to speed the review of cancer drugs.
Using a liquid biopsy to test for tumor cells circulating in blood, researchers found that, in women with breast cancer, the presence of these cells could identify women at risk of their cancer returning years later.
Findings from the TAILORx clinical trial show chemotherapy does not benefit most women with early breast cancer. The new data, released at the 2018 ASCO annual meeting, will help inform treatment decisions for many women with early-stage breast cancer.
Do cancer study participants want to receive their genetic test results? A recent study involving women with a history of breast cancer tested an approach for returning genetic research results and evaluated the impact those results had on the women.
Researchers compared the risk of death for women with breast cancer who had low skeletal muscle mass, or sarcopenia, at the time of their cancer diagnosis and women who had adequate muscle mass.
Some people who have been treated for breast cancer or lymphoma have a higher risk of developing congestive heart failure than people who haven’t had cancer, results from a new study show.
FDA has approved the CDK4/6 inhibitor abemaciclib (Verzenio) as a first-line treatment in some women with advanced or metastatic breast cancer. Under the approval, the drug must be used in combination with an aromatase inhibitor.
A new study in mice raises the possibility that using microscopic, oxygen-carrying bubbles may improve the effectiveness of radiation therapy in the treatment of breast cancer.
The drug olaparib (Lynparza®) is the first treatment approved by the Food and Drug Administration for patients with metastatic breast cancer who have inherited mutations in the BRCA1 or BRCA2 genes.
Joint pain caused by aromatase inhibitors in postmenopausal women with breast cancer can cause some women to stop taking the drugs. Reducing their symptoms may translate into better adherence to therapy.
A new study suggests that the cells in treatment-resistant tumors in women with metastatic breast cancer share important characteristics that could potentially make tumors vulnerable to therapies that otherwise might not have been considered.
A large nationwide clinical trial called TMIST has been launched to compare two techniques used for mammograms: tomosynthesis, often called 3D mammography, and standard 2D digital mammography.
FDA approved abemaciclib (Verzenio™) for the treatment of some people with advanced or metastatic HR-positive, HER2-negative breast cancer whose disease has progressed after treatment with hormone therapy.
Long-term results from a large clinical trial confirm that, for some women with early-stage breast cancer who have lumpectomy as their surgical treatment, a less extensive lymph node biopsy approach is sufficient.
When given at the same time, two immune checkpoint inhibitors were ineffective against breast cancer growth in mice, a new study found. The combination was more effective and safer if the two inhibitors were given in a specific sequence.
FDA has expanded its approval of fulvestrant (Faslodex®) as a standalone treatment for postmenopausal women with advanced HR-positive, HER2-negative breast cancer who have not previously undergone endocrine therapy.
Many women who receive taxane-based chemotherapy to treat breast cancer experience long-term nerve damage, or peripheral neuropathy, data from a large clinical trial show.
Researchers recognized the potential of endoxifen as a treatment for breast cancer and, with NCI support, developed the compound into a drug now being tested in clinical trials.
Researchers have used modified stem cells to deliver a cancer drug selectively to metastatic breast cancer tumors in mice. The stem cells target metastatic tumors by homing in on the stiff environment that typically surrounds them.
FDA has approved neratinib for patients with early-stage HER2-positive breast cancer who have finished at least 1 year of adjuvant therapy with trastuzumab.
Many survivors of early-stage breast cancer prefer that their oncologist handle aspects of routine medical care usually overseen by primary care practitioners, leading to concerns about gaps in care.
Results from the first large prospective study of breast and ovarian cancer risk in women with inherited mutations in the BRCA 1 or BRCA2 genes confirm the high risks estimated from earlier, retrospective studies.
Two clinical trials show that trastuzumab emtansine (T-DM1) improves survival compared with other standard treatments for patients with HER2-positive metastatic breast cancer that has progressed after treatment with other HER2-targeted drugs.
Using one of the largest collections of tumor samples from African Americans with breast cancer, researchers tried to assess the extent to which the molecular characteristics on these tumors might help to explain breast cancer disparities.
A new study shows that the number of women in the United States living with distant metastatic breast cancer (MBC), the most severe form of the disease, is growing. This is likely due to the aging of the U.S. population and improvements in treatment.
In a randomized trial, low-income women who role-played talking with their doctor about their survivorship care plan in a counseling session reported receiving more of their recommended care than women who did not get counseling.
The FDA has approved a new targeted therapy, ribociclib, and expanded its earlier approval of another targeted therapy, palbociclib, for some women with metastatic breast cancer.
Researchers have found that duloxetine (Cymbalta®), a drug most commonly used to treat depression, may also reduce joint pain caused by aromatase inhibitors in some women being treated for early-stage breast cancer.

Genetic Risk, Health-Associated Lifestyle, and Risk of Early-onset Total Cancer and Breast Cancer
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- ORCID record for Yin Zhang
- ORCID record for Yuxi Liu
- For correspondence: [email protected]
- Info/History
- Supplementary material
- Preview PDF
Importance Early-onset cancer (diagnosed under 50 years of age) is associated with aggressive disease characteristics and its rising incidence is a global concern. The association between healthy lifestyle and early-onset cancer and whether it varies by common genetic variants is unknown. Objective To examine the associations between genetic risk, lifestyle, and risk of early-onset cancers. Design, Setting, and Participants We analyzed a prospective cohort of 66,308 white British participants who were under age 50 and free of cancer at baseline in the UK Biobank. Exposures Sex-specific composite total cancer polygenic risk scores (PRSs), a breast cancer-specific PRS, and sex-specific health-associated lifestyle scores (HLSs, which summarize smoking status, body mass index [males only], physical activity, alcohol consumption, and diet). Main Outcomes and Measures Hazard ratios (HRs) and 95% confidence intervals (CIs) for early-onset total and breast cancer. Results A total of 1,247 incident invasive early-onset cancer cases (female: 820, male: 427, breast: 386) were documented. In multivariable-adjusted analyses with 2-year latency, higher genetic risk (highest vs. lowest tertile of PRS) was associated with significantly increased risks of early-onset total cancer in females (HR, 95% CI: 1.85, 1.50-2.29) and males (1.94, 1.45-2.59) as well as early-onset breast cancer in females (3.06, 2.20-4.25). An unfavorable lifestyle (highest vs. lowest category of HLS) was associated with higher risk of total cancer and breast cancer in females across genetic risk categories; the association with total cancer was stronger in the highest genetic risk category than the lowest: HRs in females and men were 1.85 (1.02, 3.36), 3.27 (0.78, 13.72) in the highest genetic risk category and 1.15 (0.44, 2.98), 1.16 (0.39, 3.40) in the lowest. Conclusions and Relevance Both genetic and lifestyle factors were independently associated with early-onset total and breast cancer risk. Compared to those with low genetic risk, individuals with a high genetic risk may benefit more from adopting a healthy lifestyle in preventing early-onset cancer.
Competing Interest Statement
The authors have declared no competing interest.
Funding Statement
YZ is supported by Irene M. & Fredrick J. Stare Nutrition Education Fund Doctoral Scholarship and Mayer Fund Doctoral Scholarship. SL is supported by NIH grant R01CA194393. PK is supported by NIH grants R01 CA260352 and U01 CA249866.
Author Declarations
I confirm all relevant ethical guidelines have been followed, and any necessary IRB and/or ethics committee approvals have been obtained.
The details of the IRB/oversight body that provided approval or exemption for the research described are given below:
The details of UK Biobank research ethics approval are elaborated at https://www.ukbiobank.ac.uk/learn-more-about-uk-biobank/about-us/ethics.
I confirm that all necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone (e.g., hospital staff, patients or participants themselves) outside the research group so cannot be used to identify individuals.
I understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as ClinicalTrials.gov. I confirm that any such study reported in the manuscript has been registered and the trial registration ID is provided (note: if posting a prospective study registered retrospectively, please provide a statement in the trial ID field explaining why the study was not registered in advance).
I have followed all appropriate research reporting guidelines, such as any relevant EQUATOR Network research reporting checklist(s) and other pertinent material, if applicable.
Data Availability
The authors obtained access to the UK Biobank data through the approved project application 70925.
View the discussion thread.
Supplementary Material
Thank you for your interest in spreading the word about medRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
- Addiction Medicine (316)
- Allergy and Immunology (618)
- Anesthesia (160)
- Cardiovascular Medicine (2277)
- Dentistry and Oral Medicine (279)
- Dermatology (201)
- Emergency Medicine (370)
- Endocrinology (including Diabetes Mellitus and Metabolic Disease) (801)
- Epidemiology (11581)
- Forensic Medicine (10)
- Gastroenterology (679)
- Genetic and Genomic Medicine (3586)
- Geriatric Medicine (337)
- Health Economics (618)
- Health Informatics (2307)
- Health Policy (914)
- Health Systems and Quality Improvement (864)
- Hematology (335)
- HIV/AIDS (753)
- Infectious Diseases (except HIV/AIDS) (13159)
- Intensive Care and Critical Care Medicine (757)
- Medical Education (359)
- Medical Ethics (100)
- Nephrology (389)
- Neurology (3351)
- Nursing (191)
- Nutrition (507)
- Obstetrics and Gynecology (651)
- Occupational and Environmental Health (646)
- Oncology (1759)
- Ophthalmology (525)
- Orthopedics (209)
- Otolaryngology (284)
- Pain Medicine (223)
- Palliative Medicine (66)
- Pathology (438)
- Pediatrics (1005)
- Pharmacology and Therapeutics (422)
- Primary Care Research (406)
- Psychiatry and Clinical Psychology (3063)
- Public and Global Health (5993)
- Radiology and Imaging (1224)
- Rehabilitation Medicine and Physical Therapy (715)
- Respiratory Medicine (811)
- Rheumatology (367)
- Sexual and Reproductive Health (353)
- Sports Medicine (316)
- Surgery (386)
- Toxicology (50)
- Transplantation (171)
- Urology (142)
- Search News and Events
- News overview
- Media relations
Better understanding of how breast cancer works
In her research into the response of breast cancer to the hormone estrogen, PhD student Stacey Joosten studied not only hormone-sensitive breast cancer in postmenopausal women but also in men and in women before the menopause. The aim was to gain an even better understanding of how this type of breast cancer works.
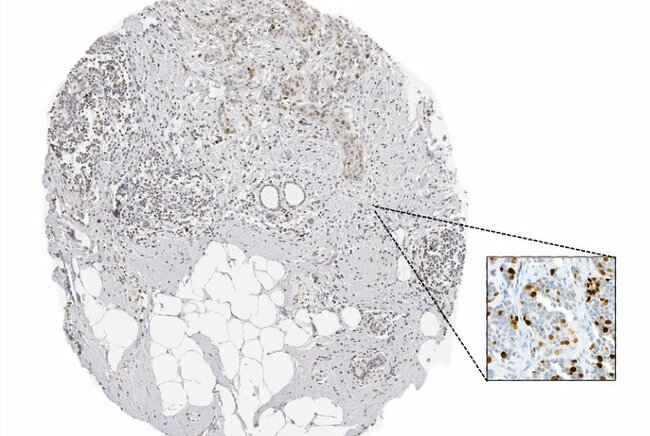
Breast cancer is very common. So much so, in fact, that it is the most commonly diagnosed type of cancer worldwide. This also means that a lot of research has already been done on breast cancer, so we know much more today than we did ten years ago, for example. Nevertheless, many people still die from this disease. In addition, many people still do not know that both men and women, young and old, can get breast cancer. All the more reason for Stacey Joosten to devote her PhD research to increasing the knowledge on breast cancer – and specifically the kind that is sensitive to the hormone estrogen. She defended her dissertation at the Department of Biomedical Engineering on March 26.
There are several types of breast cancer. The most common forms are tumors that are sensitive to the hormone estrogen. Estrogen is a hormone produced by younger women in the ovaries, for instance, but is also found in the birth control pill.
This form of breast cancer has an antenna (receptor) that responds positively to estrogen – called an estrogen receptor – so these tumors are often referred to as ‘ER+ breast cancer’ in the medical community.
Hormone receptors
“ER is a hormone receptor,” explains Stacey Joosten, newly minted doctor in Biomedical Engineering. “It’s found in all breasts and causes, for example, breast growth when estrogen is added. That’s a normal process, such as during puberty or pregnancy.”
“But in a tumor, certain cells have become ‘addicted’ to estrogen and won’t stop growing as long as estrogen is supplied. So that’s how the tumor grows and grows.”

ER+ breast cancer treatments
As a result, most treatments for this type of breast cancer target the tumor’s ‘estrogen addiction’. Patients are often given anti-hormonal treatment to inhibit the tumor. This works fine for a portion of patients. But for thirty to forty percent of patients, this treatment ceases to work after a while.
“Researchers and physicians would like to better understand how (and why) this estrogen receptor works. How does this receptor behave and what makes it sensitive or not sensitive to treatment? That’s what I’ve been diving into,” says Joosten.
“We hope that more insights will lead to smarter use of existing treatments. And perhaps new treatments can be developed based on those insights? For this reason, I researched the literature on this subject, supplemented it with new studies and compiled them in my dissertation.”
Research on the Ki-67 protein
Joosten and her colleagues investigated a protein with the impressive name ‘Ki-67’. Ki-67 is a protein that indicates cell growth and is often used in the clinic and in research to determine whether anti-hormonal therapy is succeeding.
The research involved taking tumor tissue from certain patients before treatment and a few weeks after. A pathologist then assessed, under the microscope, whether the tumor had slowed down as a result of the treatment by decreasing the amount of Ki-67.
Joosten: “By staining the piece of tissue for the Ki-67 protein, the pathologist could better estimate the growth rate of the tumor. In fact, we already knew from the literature that a good predictor of the effect of the treatment is when the amount of Ki-67 decreases in postmenopausal women following treatment. And that’s also a reasonable predictor of whether the treatment will also have an effect on the tumor over a longer period of time.”

In the research, Joosten deployed several techniques, including artificial intelligence (AI), to estimate whether the Ki-67 protein is also a good predictor for breast cancer treatment in premenopausal women.
Ki-67 is used in the clinic and research on premenopausal women, but how well Ki-67 predicts response to therapy has never actually been researched for this group of patients. In addition, AI can be used to assess Ki-67 decrease much faster and more reliably than pathologists.
Tumor cells escape treatment
During her research, Joosten also uncovered more unexpected insights. For example, she examined the widely used anti-hormonal drug Tamoxifen. In some patients, Tamoxifen no longer works after years of use.
“We saw in the tumors of patients that after only a few weeks of Tamoxifen treatment, NF-κB signaling is activated. We then demonstrated in cell lines and a mouse model that activation of that NF-κB signaling allows cells to escape Tamoxifen,” says Joosten.
“In the literature, it had long been suspected that the activation of NF-κB signaling may contribute to this escape from long-term Tamoxifen treatment. But we now see for the first time that NF-κB signaling is activated even after a short treatment with Tamoxifen.”
“When we treated mice with Tamoxifen and an inhibitor of NF-κB signaling, all tumor cells were inhibited. We therefore suspected that combining Tamoxifen with an NF-κB inhibitor may have a positive effect on patients as well.”
Difference between men and women with breast cancer
Joosten also investigated whether the DNA binding of estrogen receptors works differently in men and women. In doing so, she also examined the receptors for other hormones such as testosterone and progesterone, for instance.
In breast cancer research, the gender bias is the complete opposite. After all, there are no laboratory models of male breast cancer patients. Stacey Joosten
“In breast cancer research, the gender bias is the complete opposite. After all, there are no laboratory models of male breast cancer patients,” Joosten notes.
“That’s what makes our research so valuable. We examined pieces of tumor tissue from male breast cancer patients and were the first in the world to map the DNA binding of estrogen receptors and other hormone receptors in men. We made that research data available via open science. It’s a huge step in the right direction to a better understanding of breast cancer in men as well.” “In general, the difference with women did not appear to be too great, but the formulas that use this DNA binding info to predict prognosis in women with breast cancer did not work for men. We therefore came up with a new formula specifically for men,” Joosten says.
The DNA binding
Finally, Joosten further explored the DNA binding of the estrogen receptor. “The estrogen receptor causes growth by binding to DNA in lots of places and triggering growth processes there. Why does the estrogen receptor stick in some places in the DNA in almost every breast cancer patient and in some places in only a few?” Joosten wondered.
Indeed, the DNA binding of estrogen receptors differed greatly between tumors. Yet from the outside, those tumors all looked like the ER+ type of breast cancer.
“There do appear to be biological differences between these tumors, which explain the differences in DNA binding or sticking. We also saw that specific sites proved to be very popular for estrogen receptors. At sites in which the estrogen receptor ‘sticks’ to the DNA in many patients, small differences in DNA between patients were found to occur more often. Those differences are associated with each person’s individual risk of estrogen-sensitive breast cancer.”
“Our research shows that such a small DNA change can cause the receptor at such a location to more or less ‘stick’ to the DNA. That can have an effect on the genes that are turned on or off by this DNA-bound ER (like a kind of switch). And that potentially influences the risk of breast cancer,” Joosten concludes.
One thing is certain: her research is helping to increase the knowledge on the treatment of breast cancer patients, whether they are men or older and younger women.
Stacey Joosten defended her dissertation ‘ Characterizing estrogen receptor differences between breast cancer patients ’ at the Department of Biomedical Engineering on March 26, 2024. Supervisors: Wilbert Zwart and S.C. Linn
The research was conducted at the Netherlands Cancer Institute (NKI ) and was made possible in part by funding from the KWF and Oncode .
More on Health
- All the news on Health
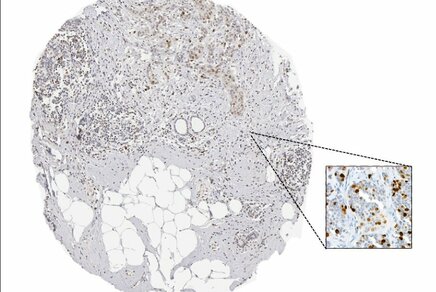
Latest news
- News Overview
Keep following us
Newsletter research.
Sign up for our bimonthly newsletter that brings you the latest in groundbreaking research from TU/e.
TU/e podcasts
The Dutch podcast Sound of Science discusses the latest scientific discoveries and the role of technology in society.
Be part of our community and stay up to date on everything that happens at TU/e by following us on LinkedIn.
Be the first to know the latest TU/e news via our X channel.
Instagram - Research
Follow our latest research news via our research channel on Instagram.
On our YouTube channel you find the latest videos and animations about research, education and working at TU/e.

IMAGES
COMMENTS
Ndom included in a literature review papers that contained data on both male and female breast cancers in Africa, and if both male and female breast cancer were available, the article was included. If two publications covered the same geographical region, only the one with the longer study period was included. ... In an update on research ...
Male breast cancer (MBC) is a rare condition, accounting for less than 1% of all breast cancers. About 2,710 men are estimated to be diagnosed with breast cancer, with approximately 530 men projected to die from breast cancer in 2022 and have about 1 in 833 lifetime risk of being diagnosed with the disease in the United States [].Data from the Global Burden of Disease 2017 database indicate ...
For men in the highest quartile of estradiol levels as compared with those in the lowest quartile, the odds ratio for breast cancer was 2.47 (95% confidence level, 1.10 to 5.58). 41 Other ...
In a paper that included 10,173 men with HR-positive breast cancer, men were less likely to receive adjuvant endocrine therapy than women (67.3% vs 78.9%, p < 0.001) 16.
Although FBC has been the guide for MBC research to date, breast cancer research as a whole might be entering a new phase, whereby rare disease may provide key information about the more common ...
Purpose of Review Male breast cancer (MBC) accounts fo r less than 1% of all breast cancers. Because of this rarity, treatment data is limited to retrospective analyses. The purpose of this paper is to improve awareness of risk factors and the presentation of MBC and to review current data regarding management. Recent Findings Risk factors for MBC include age, androgen-estrogen imbalance ...
Most cited paper was by Thorlacius, S et al. evaluating the relationship between BRCA2 and female breast cancer, prostate cancer, pancreatic cancer and ovarian cancer. Cancer is the journal with the most published papers and received most citations in the male breast cancer research field. The USA contributed 49 of the manuscripts in the top 100.
Male breast cancer (MBC)is a rare disease that accounts for less than 1% of all cancers in men and all diagnosed breast cancers [1]. ... Of these original 310 research papers, only 15 were qualitative research papers that also met our inclusion criteria (Fig. 1).
Male breast cancer is a rare, often aggressive condition that typically presents itself in the late course of disease. Approximately 1 percent of all breast malignancies are found in the male patient (CA Cancer J Clin 2013;63(1):11-30).The rarity of the disease in this population has made it challenging to study, considering the etiology of male breast cancer has been difficult to elucidate.
How cancer patterns in humans compare to those of other species remains largely unknown and there is an even bigger knowledge gap for rare cancers like male breast cancer. One Health is a convergence of human and animal healthcare that encourages cross-pollination of medical research uniting human and veterinary medicine. Recognising that breast cancer occurs spontaneously in other male ...
Male breast cancer constitutes a mere 1% of newly diagnosed breast cancer cases, signifying its rarity [].Over recent decades, there has been a gradual rise in the incidence of male breast cancer[2, 3].In the context of male breast cancer, encompassing both in situ and invasive forms, it is noteworthy that an estimated 47-70% of the patients diagnosed are in the elderly age group [4,5,6].
This article reviews what is currently known about male breast cancer, with an emphasis on areas where evidence-based data are scarce. While it is possible that some recent developments in female breast cancer treatment could be applicable to men, but the way forward is to increase awareness of the disease, and for treatment centers to pool ...
This single change can increase the risk fo breast cancer by up to 50%. Recently, the study found three more genetic changes that increased the risk of developing breast cancer in men by approximately 47%, 45% and 61% respectively. All three changes are also known to affect the risk of the disease in women, so researchers then analysed whether ...
Occurrence of male breast cancer, a rare disease, peaks at age 71 years. Familial cases usually have BRCA2 rather than BRCA1 mutations. Occupational risks include high temperature environments and exhaust fumes, but electromagnetic fields have not been implicated. Hyperoestrogenisation resulting from Klinefelter's, gonadal dysfunction, obesity, or excess alcohol, all increase risk as does ...
Studies continue to uncover lifestyle factors and habits that alter breast cancer risk. Ongoing studies are looking at the effect of exercise, weight gain or loss, and diet on breast cancer risk. Research is also looking to see if being overweight or obese as a teenager increases breast cancer risk in men as it does for breast cancer in women.
ON THIS PAGE: You will read about the scientific research being done to learn more about male breast cancer and how to treat it. Use the menu to see other pages.Doctors are working to learn more about male breast cancer, ways to prevent it, how to best treat it, and how to provide the best care to people diagnosed with this disease. The following areas of research may include new options for ...
Male Breast Cancer. Breast cancer may occur in men. Although it is relatively rare - less than 1 percent of breast cancer diagnoses in the United States are in males - men may develop the disease at any age. It is, however, usually detected in men between 60 and 70 years of age. Radiation exposure, high levels of estrogen, and a family ...
Posted: January 20, 2023. Many young women who are diagnosed with early-stage breast cancer want to become pregnant in the future. New research suggests that these women may be able to pause their hormone therapy for up to 2 years as they try to get pregnant without raising the risk of a recurrence in the short term.
An unfavorable lifestyle (highest vs. lowest category of HLS) was associated with higher risk of total cancer and breast cancer in females across genetic risk categories; the association with total cancer was stronger in the highest genetic risk category than the lowest: HRs in females and men were 1.85 (1.02, 3.36), 3.27 (0.78, 13.72) in the ...
Better understanding of how breast cancer works. April 8, 2024. In her research into the response of breast cancer to the hormone estrogen, PhD student Stacey Joosten studied not only hormone-sensitive breast cancer in postmenopausal women but also in men and in women before the menopause. The aim was to gain an even better understanding of how ...
RESULTS Distribution of cases and deaths by world region and cancer types. Figure 2 presents the distribution of new cases and deaths according to world region for both sexes combined and for men and women separately. For both sexes combined, there were an estimated 20.0 million new cases worldwide (19.96 million including NMSC and 18.73 million excluding NMSC) and 9.7 million cancer deaths (9 ...